Abstract
Background
Maternal over- and undernutrition in pregnancy plays a critical role in fetal brain development and function. The effects of different maternal diet compositions on intrauterine programing of the fetal brain is a lesser-explored area. The goal of this study was to investigate the impact of two chowmaternal diets on fetal brain gene expression signatures, fetal/neonatal growth, and neonatal and adult behavior in a mouse model.
Methods
Throughout pregnancy and lactation, female C57Bl/6J mice were fed one of two standard, commercially available chow diets (pellet versus powder). The powdered chow diet was relatively deficient in micronutrients and enriched for carbohydrates and n-3 long-chain polyunsaturated fatty acids compared to the pelleted chow. RNA was extracted from embryonic day 15.5 forebrains and hybridized to whole genome expression microarrays (N = 5/maternal diet group). Functional analyses of significantly differentially expressed fetal brain genes were performed using Ingenuity Pathways Analysis and Gene Set Enrichment Analysis. Neonatal behavior was assessed using a validated scale (N = 62 pellet-exposed and 31 powder-exposed). Hippocampal learning, locomotor behavior, and motor coordination were assessed in a subset of adults using fear conditioning, open field testing, and Rotarod tests (N = 16 pellet-exposed, 14 powder-exposed).
Results
Comparing powdered to pelleted chow diets, neither maternal weight trajectory in pregnancy nor embryo size differed. Maternal powdered chow diet was associated with 1647 differentially expressed fetal brain genes. Functional analyses identified significant upregulation of canonical pathways and upstream regulators involved in cell cycle regulation, synaptic plasticity, and sensory nervous system development in the fetal brain, and significant downregulation of pathways related to cell and embryo death. Pathways related to DNA damage response, brain immune response, amino acid and fatty acid transport, and dopaminergic signaling were significantly dysregulated. Powdered chow-exposed neonates were significantly longer but not heavier than pelleted chow-exposed counterparts. On neonatal behavioral testing, powdered chow-exposed neonates achieved coordination- and strength-related milestones significantly earlier, but sensory maturation reflexes significantly later. On adult behavioral testing, powdered chow-exposed offspring exhibited hyperactivity and hippocampal learning deficits.
Conclusion
In wild-type offspring, two diets that differed primarily with respect to micronutrient composition had significant effects on the fetal brain transcriptome, neonatal and adult behavior. These effects did not appear to be mediated by alterations in gross maternal nutritional status nor fetal/neonatal weight. Maternal dietary content is an important variable to consider for investigators evaluating fetal brain development and offspring behavior.
Keywords: maternal diet, fetal brain, transcriptome, strength, coordination, sensory, micronutrient, fatty acid
Introduction
In both human epidemiologic and animal model studies, maternal under- and overnutrition in pregnancy has been well-demonstrated to have a deleterious impact on fetal brain development and offspring behavior (Tozuka et al., 2009, 2010; White et al., 2009; Antonow-Schlorke et al., 2011; Brion et al., 2011; Krakowiak et al., 2012; Kang et al., 2014; Edlow et al., 2016a, b; Li et al., 2016; van der Burg et al., 2016; Veena et al., 2016; Edlow, 2017; Winther et al., 2018). The impact of maternal dietary micronutrient composition on the developing fetal brain has not been as well-characterized. Dietary micronutrient deficiency may have broader relevance to conditions such as maternal obesity, in which a relative micronutrient deficiency has been proposed due to poor maternal dietary quality (Pinhas-Hamiel et al., 2003; Kimmons et al., 2006; Laraia et al., 2007; Darnton-Hill and Mkparu, 2015; Jones et al., 2016; Scholing et al., 2018). The relative contribution of maternal micronutrient deficiency compared to maternal pre-pregnancy obesity or maternal high-fat diet in mediating some of the deleterious effects of maternal obesity on the developing brain is unknown. Similarly, to what extent the deleterious impact of maternal undernutrition in pregnancy on the developing fetal brain may be mediated by micronutrient versus macronutrient deficiency in the diet remains to be elucidated.
While the impact of maternal macronutrient and micronutrient intake in pregnancy has been examined in several human observational studies, these focus primarily on the impact of maternal intake on pregnancy outcomes such as preeclampsia and preterm birth, on fetal growth trajectory, and on neonatal outcomes such as small- and large-for-gestational age, and incidence of congenital anomalies (Mousa et al., 2019). Fewer studies have focused directly on the impact of maternal pregnancy and lactational nutrition on fetal brain development and offspring behavior (Prado and Dewey, 2014; Li et al., 2019). There remains a knowledge gap regarding the impact of maternal micro- and macronutrient intake specifically on fetal brain development and offspring behavior.
We sought to address this knowledge gap by evaluating the impact of maternal dietary composition in pregnancy and lactation on fetal brain development and offspring behavior, in the absence of maternal pre-pregnancy obesity or maternal over- or under-nutrition, using two standard commercially available chow diets that differed significantly with respect to their micronutrient content (including vitamins and minerals), and differed in macronutrient content only with respect to carbohydrate. Our objective was to evaluate the impact of maternal pregnancy diet on fetal brain gene expression, neonatal behavior, neonatal growth trajectory, and adult offspring behavior. If standard maternal chow diets themselves have an impact on fetal brain development, the choice of chow diet may be an important variable to consider for neuroscience researchers investigating the impact of maternal exposures on the developing brain.
Materials and Methods
Mouse Strain, Breeding, Pregnancy and Lactation Diets
This study was part of a larger research program examining the impact of maternal nutrient supplementation in pregnancy on fetal and offspring brain development in mouse models of Down syndrome compared to wild-type (Guedj et al., 2018). Female C57Bl/6J mice (Jackson Laboratory, Bar Harbor, ME, United States) were crossed with Ts1Cje males [B6 T(12:16)1Cje/CjeDnJ], to generate pregnancies in which approximately half of the fetuses were affected with Down syndrome and half were wild-type. Only outcomes from wild-type fetuses and offspring (exposed to the intrauterine environment of a wild-type/C57Bl/6J dam) were examined. Breeder pairs received either a standard commercially available pelleted chow (Teklad 2918), or a standard commercially available purified powdered chow diet (Bioserv F3197). The content of each diet is depicted in Table 1.
TABLE 1.
Maternal diet composition.
| Class | Component | Powder chow Bioserv F3197 | Regular chow Teklad 2918 |
| Isoflavone | Daidzein, genistein | Not present | 150–250 mg/kg |
| macronutrients | Crude protein | 18.1% | 18.6% |
| Fat | 7.1% | 6.2% | |
| Carbohydrate | 59.3% | 44.2% | |
| Crude fiber | 4.8% | 3.5% | |
| Nutral detergent fiber | Not present | 14.7% | |
| Ash | 2.2% | 5.3% | |
| Caloric profile | Protein | 0.72 Kcal/g | 0.74 Kcal/g |
| Fat | 0.64 Kcal/g | 0.56 Kcal/g | |
| Carbohydrates | 2.37 Kcal/g | 1.20 Kcal/g | |
| Total | 3.74 Kcal/g | 3.1 Kcal/g | |
| Micronutrients-Minerals | Calcium | 5.1 g/kg | 10 g/kg |
| Phosphorus | 2.8 g/kg | 7 g/kg | |
| Sodium | 1.03 g/kg | 2 g/kg | |
| Potassium | 3.6 g/kg | 6 g/kg | |
| Chloride | 1.6 g/kg | 4 g/kg | |
| Magnesium | 0.51 g/kg | 2 g/kg | |
| Zinc | 37.7 mg/kg | 70 mg/kg | |
| Manganese | 10.5 mg/kg | 100 mg/kg | |
| Copper | 6.0 mg/kg | 15 mg/kg | |
| Iodine | 0.21 mg/kg | 15 mg/kg | |
| Iron | 37.2 mg/kg | 200 mg/kg | |
| Selenium | 0.17 mg/kg | 0.23 mg/kg | |
| Chromium | 1 mg/Kg | Not present | |
| Fluoride | 1 mg/kg | Not present | |
| Sulfur | 301 mg/kg | Not present | |
| Micronutrients-Vitamins | Vit A | 4.14 IU/g | 15 IU/g |
| Vit D3 | 1 IU/g | 1.5 IU/g | |
| Vit E | 0.083 IU/g | 110 IU/g | |
| Vit K3 | Not present | 50 mg/kg | |
| Vit B1 | 6 mg/kg | 17 mg/kg | |
| Vit B2 | 6 mg/kg | 15 mg/kg | |
| Niacin | 30 mg/kg | 70 mg/kg | |
| Vit B6 | 5.8 mg/kg | 18 mg/kg | |
| Pantothenic acid | 14.7 mg/kg | 33 mg/kg | |
| Vit B12 | 0.025 mg/kg | 0.08 mg/kg | |
| Biotin | 0.2 mg/kg | 0.4 mg/kg | |
| Folate | 2 mg/kg | 4 mg/kg | |
| Choline | 1028 mg/kg | 1200 mg/kg | |
| Vit K1 | 0.88 mg/kg | Not present | |
| Amino acids | Alanine | 4.6 g/kg | 11 g/kg |
| Arginine | 6.4 g/kg | 10 g/kg | |
| Aspartic acid | 11.2 g/kg | 14 g/kg | |
| Cystine | 3.5 g/kg | 3 g/kg | |
| Glutamic acid | 35.6 g/kg | 34 g/kg | |
| Glycine | 4.3 g/kg | 8 g/kg | |
| Histidine | 4.8 g/kg | 4 g/kg | |
| Isoleucine | 9.6 g/kg | 8 g/kg | |
| Leucine | 14.6 g/kg | 18 g/kg | |
| Lysine | 13.0 g/kg | 9 g/kg | |
| Methionine | 4.5 g/kg | 4 g/kg | |
| Phenylalanine | 7.8 g/kg | 10 g/kg | |
| Proline | 18.0 g/kg | 16 g/kg | |
| Serine | 10.0 g/kg | 11 g/kg | |
| Threonine | 7.7 g/kg | 7 g/kg | |
| Tryptophan | 2 g/kg | 2 g/kg | |
| Tyrosine | 10 g/kg | 6 g/kg | |
| Valine | 11.4 g/kg | 9 g/kg | |
| Fatty acids | C16: o Palmetic | Not present | 7 g/kg% |
| C18: o Stearic | Not present | 2 g/kg | |
| C18: 1ω9 Oleic | Not present | 12 g/kg | |
| C18:2ω6 (n-6 LC-PUFA or linoleic acid, LA) | 35.7 g/kg | 31 g/kg | |
| C18:3ω3 (n-3 LC-PUFA α-linolenic acid or ALA) | 4.8 g/kg | 3 g/kg | |
| Total saturated | 11 g/kg | 9 g/kg | |
| Total monounsaturated | 15.9 g/kg | 13 g/kg | |
| Total polyunsaturated | 40.4 g/kg | 34 g/kg |
The rationale was to determine the effects of different chow diets on wild-type fetal brain development and offspring behavior, in order to select an optimal control diet for a subsequent set of experiments focused on the impact of isoflavone supplementation during pregnancy on fetal brain development and offspring behavior in Down syndrome (Guedj et al., 2018). The powdered chow was specifically selected for interrogation given the absence of added isoflavones. The absence of isoflavone supplementation at baseline in the powdered chow diet was of interest because this study was a precursor to an intervention study investigating the benefits of maternal dietary supplementation with apigenin, an isoflavone, on brain development in offspring with Down syndrome (Guedj et al., 2018). It was therefore important to examine the effects of a diet that could be supplemented with apigenin and did not already contain other isoflavones that could confound the evaluation of apigenin’s effect.
All fetuses and offspring reported here are wild-type fetuses exposed to the intrauterine environment of wild-type/C57Bl/6J dams that consumed one of two diets during pregnancy without additional isoflavone supplementation. A subsequent study compared brain development in mouse models of Down syndrome versus their wild-type littermates with and without isoflavone (apigenin) supplementation using only powdered chow (Guedj et al., 2018).
Dams were started on the study diet at the time of initial breeding with a sire, in order to isolate the impact of maternal dietary intake in pregnancy and lactation on fetal brain development and offspring behavior. The dams continued on the diet throughout pregnancy and lactation. Offspring were weaned to the same diet they were exposed to during pregnancy and lactation. With respect to the breeding strategy, females were bred with males overnight. Vaginal plugs were checked by 9 am the next day, with the presence of a vaginal plug defined as embryonic day 0.5 (E0.5). To exclude the possibility of daytime mating, males were separated from females during the day. Male sires were consistently fed the same single diet as per their initial breeding pair. Females were weighed at embryonic days 0 (day of mating), 10, and 15.5, just prior to euthanasia. Greater than or equal to 10% weight gain at E10 was used to confirm pregnancy (Johnson et al., 2010). Animals were housed in cages with standard bedding and nestlets. Animals were given ad libitum access to chow and water. The colony was maintained on a standard 12:12 light-dark cycle, with lights on at 07:00. All experiments were approved by the Tufts Medical Center Institutional Animal Care and Use Committee (IACUC, protocol #B2013-20).
Maternal Diet Composition Differences
Table 1 demonstrates the components of the two different chow diets. They differ significantly in several respects. The powdered chow was relatively enriched for carbohydrates compared to the pelleted diet. The powdered chow also contained significantly higher concentrations of n-3 long-chain polyunsaturated fatty acids (LC-PUFAs, linolenic acid) compared to pelleted chow, with a more favorable n-6/n-3 LC-PUFA ratio (7.4 for powdered versus 10.3 for pelleted chow, with an n-6/n-3 ratio of <10 recommended for infant and adult nutrition) (Gerster, 1998; Abedi and Sahari, 2014). The remainder of differences between the two diets favored enrichment of the pelleted diet over the powdered. Compared to the powdered chow, the pelleted chow was enriched for: (1) The isoflavones daidzein and genistein (phytoestrogens known to cross the blood-brain barrier and exert antioxidant effects) (Bang et al., 2004; Zeng et al., 2004); (2) the macroelements calcium, phosphorus, sodium, potassium, chloride, and magnesium (all two- to approximately four-fold higher in pelleted chow); (3) the amino acids alanine, arginine and glycine (all approximately two-fold higher in the pelleted chow); (4) micronutrients zinc, manganese, copper, iodine, iron, and selenium (all higher in pelleted chow, ranging from approximately 2-fold to as high as 70-fold) and vitamin content, with the pelleted chow containing universally higher vitamin concentrations than the powdered chow. With respect to micronutrients, manganese, iodine, and iron have the greatest fold differences between the two diets (9. 5-, 71-, and 5.4-fold higher in pelleted chow, respectively). With respect to vitamins, the difference between the two diets was most notable for Vitamin E (more than 1,000-fold higher in the pelleted chow). Vitamins A, B1, B2, Niacin, B6, pantothenic acid, B12, Biotin, and folate were all two- to four-fold higher in the pelleted chow. In summary, the powdered chow was relatively enriched for carbohydrate content and favorable LC-PUFAs compared to pelleted chow, but was relatively deficient in macroelements, specific amino acids, and micronutrients (including all vitamins and most minerals), compared to the pelleted chow. The experimental paradigm is depicted in Figure 1.
FIGURE 1.
Experimental timeline. e, Embryonic day; P, Postnatal day.
Tissue Collection
On embryonic day 15.5 (E15.5), pregnant dams were euthanized with isoflurane followed by decapitation. Embryos were rapidly dissected from the uterine horns and placed in ice-cold 1X phosphate-buffered saline (PBS) containing RNA preservative (RNALater, Qiagen). Theiler staging was performed to confirm the gestational age of E15.5 (Theiler, 1989, accessed April, 2018). On a cold platform, embryonic brains were rapidly removed from skulls, and forebrain was isolated and snap frozen in liquid nitrogen, prior to storage at −80°C. Tail snips were obtained for Ts1Cje and sex genotyping. Only mice that had the wild-type genotype were used for analysis here.
Fetal Brain Gene Expression Studies
Total RNA was isolated from embryonic forebrains using the NucleoSpin II RNA/protein kits (Machery-Nagel, Duren, Germany) per the manufacturer’s instructions. The isolation included an on-column DNase digestion step to remove genomic DNA. RNA purity, integrity and quantity were assessed using the NanoDrop ND-800 (NanoDrop, Wilmington, DE, United States) and the Bioanalyzer system (Agilent 2100; Agilent Technologies Inc., Palo Alto, CA, United States). RNA was processed and hybridized to Mouse gene 1.0 ST Arrays (Affymetrix, Santa Clara, CA, United States) as previously described (Edlow et al., 2016a; Guedj et al., 2016). Five arrays per experimental group were used, with each array corresponding to one embryonic brain. One embryo per litter from five different litters per diet group was included in microarray analyses, to minimize litter effects. Five biological replicates per group has been demonstrated to be sufficient to detect global gene expression changes in microarray studies (Allison et al., 2006; Tarca et al., 2006).
Normalization was performed using the robust multichip average algorithm (RMA) and the MBNI custom CDF1 version #15 for the Mouse Gene 1.0 ST array. Output consisted of data for 21,225 probe sets each corresponding to unique Entrez Gene IDs. Statistical analyses were performed using R software (version 3.1.2 or later). Fetal brain gene expression data from day 15.5 embryos of dams eating pelleted chow were compared to those from day 15.5 embryos of dams eating powdered chow.
Statistical Analyses: Gene Expression
Student’s t-test was used to identify differentially-expressed genes (DEGs) between the two diet groups. P-values were jointly corrected for multiple testing by calculating the Benjamini–Hochberg false discovery rate (FDR) (Benjamini, 1995). Differentially expressed genes between groups were defined as those with a raw p-value of <0.001, an adjusted p-value (FDR) of <0.01, and an absolute fold-change value of >1.5. Gene expression changes were further visualized by Principal Component Analyses (PCA) using R. The pelleted chow group was selected as the referent group; upregulated genes are more highly expressed in the brains of powdered-chow exposed embryos compared to pelleted-chow exposed embryos, downregulated genes are more lowly expressed in the brains of powdered-chow exposed embryos compared to those exposed to pelleted-chow.
Functional analysis was performed on the differentially expressed genes using Ingenuity Pathway Analysis (IPA) (Qiagen, Redwood, CA, United States). The working file for the IPA analysis may be found in Supplementary File 1. Statistical significance within IPA was defined as p < 0.05, and activation state was predicted based on Z-scores ≥ 2 (activated) or ≤−2 (inhibited), in accordance with recommended thresholds (Kramer et al., 2014; Ingenuity Systems, 2019a, b [accessed April 12, 2019]). Only pathways including three or more differentially expressed genes were considered. We also performed whole transcriptome analysis of functional gene set regulation using Gene Set Enrichment Analysis (GSEA) (Subramanian et al., 2005), with a developmentally focused annotation (the Developmental Functional Annotation at Tufts or DFLAT) (Wick et al., 2014; Edlow et al., 2015). Gene sets with an FDR q < 0.05 were considered significantly dysregulated.
Offspring Biometry and Neurobehavioral Analyses
Neonatal Biometry and Developmental Milestone Assessment
For neonatal evaluations, 62 offspring exposed to pelleted chow in utero and during lactation and 31 offspring exposed to the maternal powdered chow diet were evaluated, with 1–2 offspring per sex per litter evaluated to avoid litter effects (n = 17 pelleted chow litters, n = 9 powdered chow litters). There were 15 females and 16 males in the powdered chow group, and 38 females and 24 males in the pelleted chow group. Biometry was performed on neonates from both diet groups daily from postnatal day 3 (P3) through P21 (weights), or P3-P15 (length measurements, only performed until eye opening due to limited accuracy secondary to pup movement after eye opening), to establish growth trajectories. Neonatal behavior was evaluated daily from P3 to either P15 when eye opening occurred (if this would confound the behavioral test) or P21 (weaning). Behavioral assessments started on P3 to avoid maternal stress and subsequent pup neglect or cannibalism that could impact neonatal survival and behavior. The amount of time (latency) needed to complete each test was recorded and analyzed. The neurobehavioral test protocols have been described in detail in previous publications (Hill et al., 2008; Guedj et al., 2015; Goodliffe et al., 2016). All behavioral experiments were conducted in the light phase, between 08:00 and 13:00. Test apparati were thoroughly cleaned with Sani-Cloth Plus wipes or 70% ethanol spray between mice, to minimize olfactory cues from previous trials. Mice were acclimated in the testing room in their home cages for at least 1 h prior to evaluation. For neonatal behavioral testing, pups were placed with nesting material in a bowl heated to 37°C. An investigator with extensive experience in neonatal developmental milestones performed all neonatal biometry and behavioral testing (Guedj).
A modified Fox scale (Hill et al., 2008) was used to evaluate developmental milestones in wild-type offspring exposed to powdered versus pelleted chow in utero and during lactation. This validated battery of behavioral tests evaluates body righting mechanisms, coordination and strength (surface righting and negative geotaxis), strength and coordination (cliff aversion and forelimb grasp), sensory system maturation (auditory startle, ear twitch, and eye opening), labyrinthine reflex (air righting), and the developmental transition from rotatory locomotion behavior to straight-line walking, reflecting the rostrocaudal development of limb coordination (open field) (Fox, 1965; Hill et al., 2008; Guedj et al., 2015, 2016; Goodliffe et al., 2016). The day of achievement of a developmental milestone was defined as the day at which the pup performed the task successfully for 2 days in a row. The time to achieve a developmental milestone (latency) and the presence or absence of a reflex was evaluated by a single investigator as stated above.
Adult Neurobehavioral Studies
Adult behavioral testing was performed on a subset of male offspring at 3 months of age, including 16 offspring exposed to pelleted chow in utero and during lactation, and 14 offspring exposed to maternal powdered chow diet. The open field test was used to evaluate locomotor activity and exploratory behavior, contextual fear conditioning was used to evaluate hippocampal learning and memory, and Rotarod test was used to evaluate motor coordination. Testing paradigms have been detailed in prior publications (Aziz et al., 2018; Guedj et al., 2018).
As previously described, open field testing utilized a 60-min trial paradigm in a 40 cm × 40 cm × 40 cm opaque plastic box, with animal tracking performed by the Ethovision 10.5 system (Noldus, Leesburg, VA, United States). The fear conditioning test was performed in a sound-attenuating cubicle with exhaust fan and stainless-steel grid floor (Med Associates, Fairfax, VT, United States), with a 5-min day one training session involving two mild foot shocks (0.5 mA for 2 s) administered at 180 and 240 s. On day two (the testing session), the mice were placed into the identical conditioning chamber for 5 min with no foot shocks, and mice were monitored for freezing (fear) behavior. Reduced freezing on day 2 was evaluated as a measure of hippocampal learning/memory deficit (failure to remember receiving a shock in the same environment on the day prior). Data were analyzed using the Freeze View software (Med Associates, Fairfax, VT, United States). Motor coordination was investigated using the rotarod test (Med Associates, Fairfax, VT, United States) using a 16 RPM, 24 RPM, and 32 RPM fixed speed protocol with two 120 s trials at each speed, separated by a 15 min inter-trial interval, as described previously (Aziz et al., 2018; Guedj et al., 2018). The latency to fall was recorded in seconds and analyzed for each mouse.
Statistical analyses: behavior
The Kolmogorov–Smirnov normality test and the Fisher variance equality test were performed on behavioral and biometric data to determine appropriate subsequent statistical tests. Significant differences between groups in biometry and in latency to developmental milestone achievement were evaluated via Mann–Whitney tests for single measures, or Wilcoxon signed-rank tests for repeated measures. Significant differences between groups were defined as p < 0.05 and p-values were corrected for multiple comparisons. Two-way ANOVA (maternal diet × offspring sex) was used to evaluate for the presence of significant interactions between maternal pregnancy diet and offspring sex on achievement of neonatal milestones.
Genotyping
Genotype and sex of embryos and offspring were determined via multiplex PCR amplification of DNA extracted from tail snips (embryos) or ear punches (neonates). Cite-F/Cite R primers targeting the neomycin cassette (present only in Ts1Cje mice) were used to determine Ts1Cje or wild-type status. Sry-F/Sry-R primers directed against the Sry gene (present only in males) was used to determine fetal/neonatal sex. For each reaction, Fez-F/Fez-R primers were used as endogenous controls. DNA extraction and purification methods, PCR conditions, and primer information and amplicon sizes have been described in detail in previous publications (Guedj et al., 2015, 2018; Ferres et al., 2016). Only wild-type embryos and offspring were evaluated in these experiments.
Results
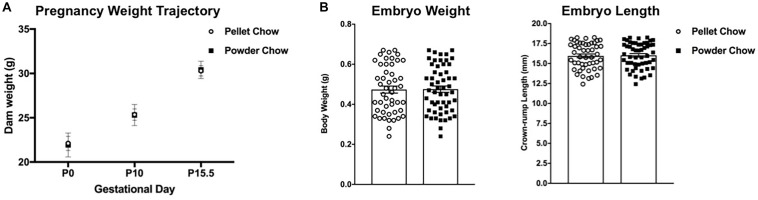
Pelleted Versus Powdered Chow Does Not Significantly Impact Dam Weight Gain During Pregnancy Nor Embryo Size
There were no significant differences between maternal diet groups with respect to dam weight trajectories in pregnancy (Figure 2A). Maternal pelleted versus powdered chow diet did not have a significant impact on embryo weights or crown rump lengths at E15.5 (Figure 2B).
FIGURE 2.
Dam weight trajectories in pregnancy, embryo weights and crown-rump lengths at embryonic day 15.5 (e15.5). There are no differences between pelleted and powdered chow for (A): dam weight gain in pregnancy at pregnancy day 0 (P0, day of mating), P10 and P15 or (B): embryonic (e15.5) weight (g) and crown-rump length (mm). N = 14 powdered chow and 14 pelleted chow litters for maternal weight gain and embryo size analyses.
Embryonic Day 15.5 Brain Gene Expression Profile Is Significantly Impacted by Maternal Pregnancy Diet Composition
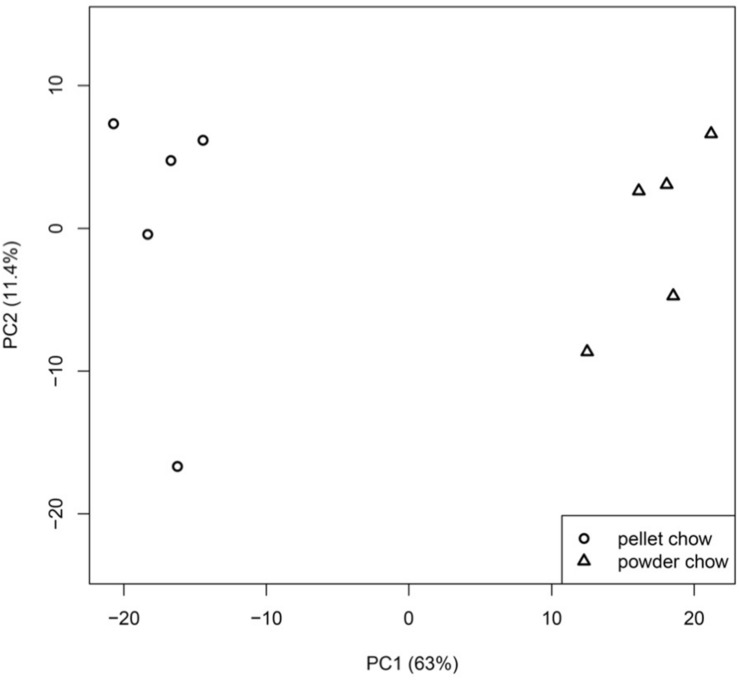
There were 1647 differentially-expressed genes (DEGs) in the embryonic brain exposed to maternal powdered chow diet compared to maternal pelleted chow diet. Principal component analysis demonstrated strong clustering of fetal brain gene expression by maternal pregnancy diet, with PC1 (indicating maternal pregnancy diet) accounting for 63% of the variation in fetal brain gene expression (Figure 3). Supplementary File 2 contains the list of significant DEGs including the Entrez Gene ID, gene name, chromosome location, fold change value, raw p- and BH-p values for expression levels between powdered chow-exposed embryonic brain compared to pelleted chow-exposed embryonic brain. Two-way ANOVA model (maternal diet × fetal sex) found no significant interaction effects between maternal diet and fetal sex on fetal brain gene expression, and no overlap between the genes affected by diet and those affected by sex.
FIGURE 3.
Principal component analysis (PCA) of embryonic day 15.5 brain gene expression profiles. Fetal brain gene expression clusters strongly by maternal pregnancy diet, with principal component or PC 1 (maternal pregnancy diet), accounting for 63% of the variation in fetal brain gene expression.
Pathways Analyses
Ingenuity pathways analysis
Pathway analyses performed in IPA suggested significant potential biological impact of the dysregulated brain gene expression due to different maternal diet composition. The canonical pathway Mitotic Roles of Polo-Like Kinase was significantly upregulated in the brains of powdered-chow exposed embryos (Z-score 2.4, key in cell cycle regulation). Other canonical pathways that were significantly affected with an adjusted p-value of <0.05 but for which a definite up- or down-regulation could not be determined based on the pattern of gene expression included: (1) Protein Ubiquitination Pathway, (2) Oxidative Phosphorylation Pathway, (3) Ataxia Telangiectasia Mutated Protein (ATM) Pathway (a canonical pathway key in regulation of the cell cycle and response to cellular stress and injury), and (4) Sirtuin Signaling Pathway (canonical pathway key in regulating metabolism and energy homeostasis by controlling lipid and glucose metabolism, ketone body synthesis, urea cycle and insulin secretion). Significantly dysregulated Canonical Pathways and their constituent differentially expressed genes in the embryonic brain exposed to powdered versus pelleted chow in utero are depicted in Table 2.
TABLE 2.
Significantly dysregulated canonical pathways in powdered chow-exposed fetal brain.
| Pathway and constituent genes | Gene name | Entrez gene ID (mouse) | Expr log ratio | Expr false discovery rate (q-value) | Location | Type(s) |
| Mitotic Polo-Like Kinase Pathway p = 7.01 × 10^−7 23 of 61 molecules in pathway dysregulated, Z-score 2.50 (upregulated) | ||||||
| ANAPC4 | Anaphase promoting complex subunit 4 | 52206 | 0.603 | 1.60E–04 | Nucleus | Enzyme |
| ANAPC5 | Anaphase promoting complex subunit 5 | 59008 | 0.817 | 3.53E–06 | Nucleus | Other |
| ANAPC13 | Anaphase promoting complex subunit 13 | 69010 | 1.682 | 7.13E–06 | Nucleus | Other |
| CDC7 | Cell division cycle 7 | 12545 | 0.929 | 4.89E–05 | Nucleus | Kinase |
| CDC16 | Cell division cycle 16 | 69957 | 0.739 | 6.44E–05 | Nucleus | Other |
| CDC26 | Cell division cycle 26 | 66440 | 0.784 | 6.28E–05 | Nucleus | Other |
| CDC25C | Cell division cycle 25C | 12532 | 0.916 | 9.78E–04 | Nucleus | Phosphatase |
| FBXO5 | F-box protein 5 | 67141 | 0.911 | 1.06E–04 | Nucleus | Enzyme |
| KIF11 | Kinesin family member 11 | 16551 | 0.953 | 1.21E–03 | Nucleus | Other |
| KIF23 | Kinesin family member 23 | 71819 | 0.641 | 1.94E–03 | Cytoplasm | Other |
| PLK2 | Polo like kinase 2 | 20620 | 0.751 | 2.12E–04 | Nucleus | Kinase |
| PLK3 | Polo like kinase 3 | 12795 | –0.649 | 1.33E–03 | Nucleus | Kinase |
| PPP2R2A | Protein phosphatase 2 regulatory subunit B alpha | 71978 | 0.64 | 4.54E–05 | Cytoplasm | Phosphatase |
| PPP2R2C | Protein phosphatase 2 regulatory subunit B gamma | 269643 | –0.606 | 7.46E–05 | Nucleus | Phosphatase |
| PPP2R3A | Protein phosphatase 2 regulatory subunit B alpha | 235542 | 0.589 | 2.94E–05 | Nucleus | Phosphatase |
| PPP2R5B | Protein phosphatase 2 regulatory subunit B beta | 225849 | –0.63 | 2.40E–05 | Cytoplasm | Phosphatase |
| PPP2R5E | Protein phosphatase 2 regulatory subunit B epsilon | 26932 | 0.684 | 1.05E–04 | Cytoplasm | Phosphatase |
| PRC1 | Protein regulator of cytokinesis 1 | 233406 | 1.286 | 6.32E–04 | Nucleus | Other |
| PTTG1 | Pituitary tumor-transforming 1 | 30939 | –0.645 | 3.78E–03 | Nucleus | Transcription regulator |
| SLK | STE20 like kinase | 20874 | 0.642 | 5.30E–05 | Nucleus | Kinase |
| SMC3 | Structural maintenance of chromosomes 3 | 13006 | 1.111 | 2.99E–05 | Nucleus | Other |
| SMC1A | Structural maintenance of chromosomes 1A | 24061 | 0.905 | 5.41E–05 | Nucleus | Transporter |
| STAG2 | Stromal antigen 2 | 20843 | 0.838 | 1.03E–04 | Nucleus | Transcription regulator |
| Protein Ubiquitination Pathway p = 7.9 × 10^−4, 46 of 249 molecules dysregulated, Z-score NA | ||||||
| ANAPC4 | Anaphase promoting complex subunit 4 | 52206 | 0.603 | 1.60E–04 | Nucleus | Enzyme |
| ANAPC5 | Anaphase promoting complex subunit 5 | 59008 | 0.817 | 3.53E–06 | Nucleus | Other |
| B2M | Beta-2-microglobulin | 12010 | –1.193 | 4.03E–05 | Plasma Membrane | Transmembrane receptor |
| BAP1 | BRCA1 associated protein 1 | 104416 | –0.799 | 3.53E–06 | Nucleus | Peptidase |
| BIRC2 | Baculoviral IAP repeat containing 2 | 11797 | 0.764 | 6.38E–04 | Cytoplasm | Enzyme |
| CUL1 | Cullin 1 | 26965 | 0.768 | 8.98E–05 | Nucleus | Enzyme |
| DNAJC1 | DnaJ heat shock protein family (Hsp40) member C1 | 13418 | 0.877 | 2.00E–05 | Cytoplasm | Transcription regulator |
| DNAJC2 | DnaJ heat shock protein family (Hsp40) member C2 | 22791 | 0.925 | 3.10E–05 | Nucleus | Transcription regulator |
| DNAJC3 | DnaJ heat shock protein family (Hsp40) member C3 | 100037258 | 0.7 | 8.26E–05 | Cytoplasm | Other |
| DNAJC7 | DnaJ heat shock protein family (Hsp40) member C7 | 56354 | 0.692 | 3.40E–05 | Cytoplasm | Other |
| DNAJC8 | DnaJ heat shock protein family (Hsp40) member C8 | 68598 | 0.903 | 1.09E–03 | Nucleus | Other |
| DNAJC9 | DnaJ heat shock protein family (Hsp40) member C9 | 108671 | 0.772 | 2.55E–04 | Nucleus | Other |
| DNAJC10 | DnaJ heat shock protein family (Hsp40) member C10 | 66861 | 0.81 | 4.67E–05 | Cytoplasm | Enzyme |
| DNAJC14 | DnaJ heat shock protein family (Hsp40) member C14 | 74330 | –0.965 | 1.52E–05 | Cytoplasm | Other |
| DNAJC19 | DnaJ heat shock protein family (Hsp40) member C19 | 100503724 | 0.667 | 1.26E–03 | Cytoplasm | Other |
| DNAJC21 | DnaJ heat shock protein family (Hsp40) member C21 | 78244 | 1.18 | 2.94E–05 | Other | Other |
| DNAJC30 | DnaJ heat shock protein family (Hsp40) member C30 | 66114 | –0.81 | 6.44E–04 | Cytoplasm | Other |
| ELOC | elongin C | 67923 | –0.674 | 2.48E–04 | Nucleus | Transcription regulator |
| FBXW7 | F-box and WD repeat domain containing 7 | 50754 | 0.637 | 2.65E–05 | Nucleus | Enzyme |
| HSPA4L | Heat shock protein family A (Hsp70) member 4 like | 18415 | 0.794 | 7.77E–05 | Cytoplasm | Other |
| MDM2 | MDM2 proto-oncogene | 17246 | 0.628 | 1.08E–04 | Nucleus | Transcription regulator |
| PSMA2 | Proteasome subunit alpha 2 | 19166 | 0.789 | 2.18E–05 | Cytoplasm | Peptidase |
| PSMA4 | Proteasome subunit alpha 4 | 26441 | 1.429 | 1.36E–05 | Cytoplasm | Peptidase |
| PSMA7 | Proteasome subunit alpha 7 | 26444 | 2.048 | 4.96E–06 | Cytoplasm | Peptidase |
| PSMB1 | Proteasome subunit beta 1 | 19170 | 0.828 | 2.49E–05 | Cytoplasm | Peptidase |
| PSMB2 | Proteasome subunit beta 2 | 26445 | –0.729 | 1.78E–04 | Cytoplasm | Peptidase |
| PSMB3 | Proteasome subunit beta 3 | 26446 | –1.71 | 1.15E–06 | Cytoplasm | Peptidase |
| PSMB4 | Proteasome subunit beta 4 | 19172 | –0.756 | 2.91E–03 | Cytoplasm | Peptidase |
| PSMB6 | Proteasome subunit beta 6 | 19175 | 1.201 | 5.89E–05 | Nucleus | Peptidase |
| PSMC1 | Proteasome 26S subunit, ATPase 1 | 19179 | 0.732 | 1.05E–04 | Nucleus | Peptidase |
| PSMC2 | Proteasome 26S subunit, ATPase 2 | 19181 | 0.945 | 5.53E–05 | Nucleus | Peptidase |
| PSMC5 | Proteasome 26S subunit, ATPase 5 | 19184 | 0.636 | 1.38E–03 | Nucleus | Transcription regulator |
| PSMC6 | Proteasome 26S subunit, ATPase 6 | 67089 | 0.646 | 4.40E–04 | Nucleus | Peptidase |
| PSMD7 | Proteasome 26S subunit, non-ATPase 7 | 17463 | 0.589 | 6.59E–04 | Cytoplasm | Other |
| PSMD11 | Proteasome 26S subunit, non-ATPase 11 | 69077 | 1.09 | 1.99E–05 | Cytoplasm | Other |
| PSMD12 | Proteasome 26S subunit, non-ATPase 12 | 66997 | 0.636 | 3.26E–04 | Cytoplasm | Other |
| PSME1 | Proteasome activator subunit 1 | 19186 | 0.89 | 3.19E–05 | Cytoplasm | Other |
| PSME2 | Proteasome activator subunit 2 | 19188 | –0.639 | 3.67E–04 | Cytoplasm | Peptidase |
| SUGT1 | SGT1 homolog, MIS12 kinetochore complex assembly cochaperone | 67955 | 0.662 | 6.36E–04 | Nucleus | Other |
| UBE2L3 | Ubiquitin conjugating enzyme E2 L3 | 22195 | –0.726 | 2.08E–05 | Nucleus | Enzyme |
| UBE2V1 | ubiquitin conjugating enzyme E2 V1 | 66589 | 1.141 | 4.21E–04 | Nucleus | Transcription regulator |
| UBE2V2 | Ubiquitin conjugating enzyme E2 V2 | 70620 | –0.929 | 7.41E–05 | Cytoplasm | Enzyme |
| UBE2Z | Ubiquitin conjugating enzyme E2 Z | 268470 | –0.627 | 2.56E–05 | Nucleus | Enzyme |
| UCHL5 | Ubiquitin C-terminal hydrolase L5 | 56207 | 0.752 | 1.28E–04 | Cytoplasm | Peptidase |
| USP25 | Ubiquitin specific peptidase 25 | 30940 | 0.769 | 3.59E–05 | Cytoplasm | Peptidase |
| USP47 | Ubiquitin specific peptidase 47 | 74996 | 0.82 | 3.64E–05 | Cytoplasm | Peptidase |
| Oxidative Phosphorylation Pathway p-value 0.01, 21 of 92 molecules dysregulated, Z-score 0.22 | ||||||
| ATP5F1C | ATP synthase F1 subunit gamma | 11949 | 0.775 | 1.50E–03 | Cytoplasm | Transporter |
| ATP5F1D | ATP synthase F1 subunit delta | 66043 | –1.576 | 4.29E–05 | Cytoplasm | Transporter |
| COX11 | Cytochrome c oxidase copper chaperone COX11 | 69802 | –0.958 | 3.03E–05 | Cytoplasm | Enzyme |
| COX17 | Cytochrome c oxidase copper chaperone COX17 | 12856 | 1.965 | 2.95E–06 | Cytoplasm | Enzyme |
| COX6A1 | Cytochrome c oxidase subunit 6A1 | 12861 | 0.804 | 3.30E–05 | Cytoplasm | Enzyme |
| Cox6c | Cytochrome c oxidase subunit 6C | 12864 | –0.6 | 2.13E–04 | Cytoplasm | Enzyme |
| COX7A2L | Cytochrome c oxidase subunit 7A2 like | 20463 | 2.175 | 1.32E–06 | Cytoplasm | Enzyme |
| COX8A | Cytochrome c oxidase subunit 8A | 12868 | –0.819 | 5.82E–05 | Cytoplasm | Enzyme |
| CYB5A | Cytochrome b5 type A | 109672 | –0.586 | 3.74E–04 | Cytoplasm | Enzyme |
| CYC1 | Cytochrome c1 | 66445 | –1.226 | 1.10E–04 | Cytoplasm | Enzyme |
| NDUFA1 | NADH:ubiquinone oxidoreductase subunit A1 | 54405 | 1.593 | 2.91E–05 | Cytoplasm | Enzyme |
| NDUFA4 | NDUFA4, mitochondrial complex associated | 17992 | 1.402 | 8.33E–06 | Cytoplasm | Enzyme |
| NDUFA6 | NADH:ubiquinone oxidoreductase subunit A6 | 67130 | 0.774 | 1.86E–04 | Cytoplasm | Enzyme |
| NDUFB2 | NADH:ubiquinone oxidoreductase subunit B2 | 68198 | 0.751 | 5.90E–04 | Cytoplasm | Enzyme |
| NDUFB6 | NADH:ubiquinone oxidoreductase subunit B6 | 230075 | –0.899 | 3.84E–03 | Cytoplasm | Enzyme |
| NDUFB9 | NADH:ubiquinone oxidoreductase subunit B9 | 66218 | 1.305 | 3.93E–06 | Cytoplasm | Enzyme |
| NDUFB11 | NADH:ubiquinone oxidoreductase subunit B11 | 104130 | –0.749 | 2.27E–04 | Cytoplasm | Enzyme |
| NDUFS4 | NADH:ubiquinone oxidoreductase subunit S4 | 17993 | –0.801 | 8.94E–05 | Cytoplasm | Enzyme |
| NDUFS6 | NADH:ubiquinone oxidoreductase subunit S6 | 407785 | 0.609 | 1.67E–03 | Cytoplasm | Enzyme |
| NDUFS7 | NADH:ubiquinone oxidoreductase core subunit S7 | 75406 | –1.177 | 1.01E–05 | Cytoplasm | Enzyme |
| UQCRFS1 | Ubiquinol-cytochrome c reductase, Rieske iron-sulfur polypeptide 1 | 66694 | 0.704 | 2.81E–05 | Cytoplasm | Enzyme |
| Ataxia Telangiectasia Mutated Protein Pathway p-value 0.02, 20 of 89 molecules dysregulated, Z-score 0.24 | ||||||
| CDC25C | Cell division cycle 25C | 12532 | 0.916 | 9.78E–04 | Nucleus | Phosphatase |
| CDK2 | Cyclin dependent kinase 2 | 12566 | 0.678 | 6.59E–04 | Nucleus | Kinase |
| GADD45A | Growth arrest and DNA damage inducible alpha | 13197 | 0.682 | 6.13E–05 | Nucleus | Other |
| GADD45G | Growth arrest and DNA damage inducible gamma | 23882 | 0.827 | 3.22E–04 | Nucleus | Other |
| MDM2 | MDM2 proto-oncogene | 17246 | 0.628 | 1.08E–04 | Nucleus | Transcription regulator |
| PPM1D | Protein phosphatase, Mg2 + Mn2 + dependent 1D | 53892 | –0.588 | 5.01E–05 | Cytoplasm | Phosphatase |
| PPP2R2A | Protein phosphatase 2 regulatory subunit Balpha | 71978 | 0.64 | 4.54E–05 | Cytoplasm | Phosphatase |
| PPP2R2C | Protein phosphatase 2 regulatory subunit Bgamma | 269643 | –0.606 | 7.46E–05 | Nucleus | Phosphatase |
| PPP2R3A | Protein phosphatase 2 regulatory subunit B”alpha | 235542 | 0.589 | 2.94E–05 | Nucleus | Phosphatase |
| PPP2R5B | Protein phosphatase 2 regulatory subunit B’beta | 225849 | –0.63 | 2.40E–05 | Cytoplasm | Phosphatase |
| PPP2R5E | Protein phosphatase 2 regulatory subunit B’epsilon | 26932 | 0.684 | 1.05E–04 | Cytoplasm | Phosphatase |
| RAD50 | RAD50 double strand break repair protein | 19360 | 0.626 | 6.95E–04 | Nucleus | Enzyme |
| RAD51 | RAD51 recombinase | 19361 | 0.72 | 9.83E–04 | Nucleus | Enzyme |
| RNF8 | Ring finger protein 8 | 58230 | 0.66 | 7.22E–05 | Nucleus | Enzyme |
| SMC2 | Structural maintenance of chromosomes 2 | 14211 | 0.644 | 6.72E–04 | Nucleus | Transporter |
| SMC3 | Structural maintenance of chromosomes 3 | 13006 | 1.111 | 2.99E–05 | Nucleus | Other |
| SMC1A | Structural maintenance of chromosomes 1A | 24061 | 0.905 | 5.41E–05 | Nucleus | Transporter |
| SUV39H1 | Suppressor of variegation 3-9 homolog 1 | 20937 | –0.598 | 2.77E–05 | Nucleus | Enzyme |
| TLK1 | Tousled like kinase 1 | 228012 | 0.803 | 2.21E–04 | Nucleus | Kinase |
| TLK2 | Tousled like kinase 2 | 24086 | 0.767 | 1.88E–04 | Cytoplasm | Kinase |
| Sirtuin Signaling Pathway p-value 0.04, 40 of 251 molecules dysregulated, Z = −0.76 | ||||||
| ATG13 | Autophagy related 13 | 51897 | –0.765 | 7.34E–05 | Cytoplasm | Other |
| ATP5F1C | ATP synthase F1 subunit gamma | 11949 | 0.775 | 1.50E–03 | Cytoplasm | Transporter |
| ATP5F1D | ATP synthase F1 subunit delta | 66043 | –1.576 | 4.29E–05 | Cytoplasm | Transporter |
| BPGM | Bisphosphoglycerate mutase | 12183 | 1.425 | 2.29E–05 | Extracellular Space | Phosphatase |
| CYC1 | Cytochrome c1 | 66445 | –1.226 | 1.10E–04 | Cytoplasm | Enzyme |
| GABARAPL1 | GABA type A receptor associated protein like 1 | 57436 | –0.756 | 7.58E–05 | Cytoplasm | Other |
| GABPA | GA binding protein transcription factor subunit alpha | 14390 | 0.632 | 8.98E–04 | Nucleus | Transcription regulator |
| GADD45A | Growth arrest and DNA damage inducible alpha | 13197 | 0.682 | 6.13E–05 | Nucleus | Other |
| GADD45G | Growth arrest and DNA damage inducible gamma | 23882 | 0.827 | 3.22E–04 | Nucleus | Other |
| H1FX | H1 histone family member X | 243529 | –0.828 | 3.19E–05 | Nucleus | Other |
| HIST1H1C | Histone cluster 1 H1 family member c | 50708 | –0.686 | 1.03E–03 | Nucleus | Other |
| MAPK3 | Mitogen-activated protein kinase 3 | 26417 | –0.752 | 1.89E–04 | Cytoplasm | Kinase |
| MAPK15 | Mitogen-activated protein kinase 15 | 332110 | –0.699 | 8.98E–04 | Cytoplasm | Kinase |
| NDUFA1 | NADH:ubiquinone oxidoreductase subunit A1 | 54405 | 1.593 | 2.91E–05 | Cytoplasm | Enzyme |
| NDUFA4 | NDUFA4, mitochondrial complex associated | 17992 | 1.402 | 8.33E–06 | Cytoplasm | Enzyme |
| NDUFA6 | NADH:ubiquinone oxidoreductase subunit A6 | 67130 | 0.774 | 1.86E–04 | Cytoplasm | Enzyme |
| NDUFB2 | NADH:ubiquinone oxidoreductase subunit B2 | 68198 | 0.751 | 5.90E–04 | Cytoplasm | Enzyme |
| NDUFB6 | NADH:ubiquinone oxidoreductase subunit B6 | 230075 | –0.899 | 3.84E–03 | Cytoplasm | Enzyme |
| NDUFB9 | NADH:ubiquinone oxidoreductase subunit B9 | 66218 | 1.305 | 3.93E–06 | Cytoplasm | Enzyme |
| NDUFB11 | NADH:ubiquinone oxidoreductase subunit B11 | 104130 | –0.749 | 2.27E–04 | Cytoplasm | Enzyme |
| NDUFS4 | NADH:ubiquinone oxidoreductase subunit S4 | 17993 | –0.801 | 8.94E–05 | Cytoplasm | Enzyme |
| NDUFS6 | NADH:ubiquinone oxidoreductase subunit S6 | 407785 | 0.609 | 1.67E–03 | Cytoplasm | Enzyme |
| NDUFS7 | NADH:ubiquinone oxidoreductase core subunit S7 | 75406 | –1.177 | 1.01E–05 | Cytoplasm | Enzyme |
| POLR1B | RNA polymerase I subunit B | 20017 | –0.696 | 2.49E–04 | Nucleus | Enzyme |
| POLR1D | RNA polymerase I and III subunit D | 20018 | 0.738 | 1.55E–04 | Nucleus | Enzyme |
| POLR2F | RNA polymerase II subunit F | 69833 | –1.462 | 7.63E–06 | Nucleus | Enzyme |
| PPIF | Peptidylprolyl isomerase F | 105675 | –0.614 | 2.65E–04 | Cytoplasm | Enzyme |
| SIRT3 | Sirtuin 3 | 64384 | –0.596 | 1.30E–04 | Cytoplasm | Enzyme |
| SIRT6 | Sirtuin 6 | 50721 | –1.22 | 8.33E–06 | Nucleus | Enzyme |
| SLC25A4 | Solute carrier family 25 member 4 | 11739 | 0.819 | 3.35E–05 | Cytoplasm | Transporter |
| SMARCA5 | SWISNF related, matrix associated, actin dependent regulator of chromatin, subfamily a, member 5 | 93762 | 1.405 | 1.36E–04 | Nucleus | Transcription regulator |
| SOD2 | Superoxide dismutase 2 | 20656 | –0.601 | 6.71E–05 | Cytoplasm | Enzyme |
| SUV39H1 | Suppressor of variegation 3-9 homolog 1 | 20937 | –0.598 | 2.77E–05 | Nucleus | Enzyme |
| TIMM44 | Translocase of inner mitochondrial membrane 44 | 21856 | 0.69 | 2.08E–05 | Cytoplasm | Transporter |
| TIMM17A | Translocase of inner mitochondrial membrane 17A | 21854 | 0.604 | 8.33E–06 | Cytoplasm | Transporter |
| TOMM6 | Translocase of outer mitochondrial membrane 6 | 66119 | –0.93 | 2.99E–05 | Cytoplasm | Other |
| TOMM70 | Translocase of outer mitochondrial membrane 70 | 28185 | 0.896 | 4.80E–05 | Cytoplasm | Transporter |
| TUBA4A | Tubulin alpha 4a | 22145 | –0.708 | 2.79E–03 | Cytoplasm | Other |
| UQCRFS1 | Ubiquinol-cytochrome c reductase, Rieske iron-sulfur polypeptide 1 | 66694 | 0.704 | 2.81E–05 | Cytoplasm | Enzyme |
| ZIC2 | Zic family member 2 | 22772 | –0.645 | 1.52E–04 | Nucleus | Transcription regulator |
Significant activation and inhibition of several transcriptional factors and other regulatory elements of interest was predicted by the Upstream Regulator analysis within IPA (bias-corrected Z-score of ≥2.0 or ≤−2.0 predict significant activation or inhibition of a specific upstream regulator as described in Section “Fetal Brain Gene Expression Studies” (Ingenuity Systems, 2019a, b [accessed April 12, 2019]). Significantly activated upstream regulators in fetal brains exposed to powdered chow in utero included transcriptional regulators and G-protein coupled receptors implicated in negative regulation of apoptosis, regulation of the cell cycle, synaptic plasticity, brain immune and inflammatory response, sensory nervous system development, and circadian rhythm regulation. Significantly inhibited upstream regulators included transcriptional regulators, growth factors, peptidases and cytokines implicated in cognition and learning, mitochondrial apoptosis, maintenance of vascular integrity, and vasoprotection and neuroprotection in the setting of hypoxic stimuli. Significantly activated or inhibited upstream regulators and their downstream differentially expressed genes in the embryonic brain exposed to powdered versus pelleted chow in utero are depicted in Table 3.
TABLE 3.
Significantly dysregulated upstream regulators and constituent downstream genes in powdered chow-exposed fetal brain.
| Upstream regulator | Entrez gene name | Putative function | Molecule type | Predicted activation state | Bias-corrected Z-score | Target molecules in dataset |
| EP400 | E1A binding protein p400 | Cell cycle regulation; chromatin organization; histone H2A acetylation; histone H4 acetylation | other | Activated | 2.063 | CDCA3, CENPF, FBXO5, INCENP, MCM3, SUV39H2 (direction of regulation predicts EP400 activation in 6/6) |
| ISL1 | ISL LIM homeobox 1 | Encodes a member of the LIM/homeodomain family of transcription factors. The encoded protein may play an important role in regulating insulin gene expression. | Transcription regulator | Activated | 2.009 | CRABP1, NPY, NXPH4, OLIG1 (direction of expression predicts ISL1 activation in 4/4) |
| PTGER2 | Prostaglandin E receptor 2 | Encodes a receptor for prostaglandin E2, a metabolite of arachidonic acid. Within the brain, PGE2 receptors are involved in the regulation of synaptic activity and plasticity, in brain maturation, and are key mediators of the brain’s response to inflammation. This gene has been implicated in negative regulation of the apoptotic process | G-protein coupled receptor | Activated | 3.623 | ARFGEF1, CENPE, CENPF, CEP55, CKAP2L, ECT2, KIF11, KIF15, KIF20A, KIF2C, MELK, MKI67, NUF2, PRC1, STIL, TPX2, TTK (direction of expression predicts PTGER2 activation in 17/17) |
| POU4F1 | POU class 4 homeobox 1 | Encodes a member of the POU-IV class of neural transcription factors. Implicated in sensory nervous system development, axonogenesis, and negative regulation of central nervous system apoptosis | Transcription regulator | Activated | 2.013 | CHRNA3, CRABP1, NECAB2, NPY, NXPH4, OLIG1, RTN4RL2, SSTR4 (direction of expression predicts activation of POU4F1 in 4/8) |
| CLOCK | Clock circadian regulator | Encodes a protein that is a key regulator of circadian rhythms. Polymorphisms in this gene are associated with obesity and metabolic syndrome in certain populations. | Transcription regulator | Activated | 2.248 | CADM2, CLIP1, CTGF, GCC2, GTF2E1, ITM2C, LYPD6, OPN3, OSTM1, PCGF5, PIP4K2C, QSER1, THUMPD1, TIMP4 (direction of expression predicts CLOCK activation in 11/14 genes) |
| BDNF | Brain derived neurotrophic factor | Encodes a member of the nerve growth factor family of proteins. Inhibition of expression is associated with cognitive deficits and neurogenerative disorders such as Alzheimer’s, Parkinson’s, and Huntington’s disease. | Growth factor | Inhibited | –2.781 | ALDH7A1, ANXA5, Cdkn1c, DNAJC21, DRD2, EGR1, HSD17B4, HSPA4L mir-10, mir-154, NCDN, NPY, PCDH8, RGS4, SCCPDH, SFR1, TMED2, Tmsb4x, VAMP2, VGF (direction of expression predicts BDNF inhibition in 12/20) |
| IL33 | Interleukin 33 | Encodes a cytokine that binds to the IL1RL1/ST2 receptor. Encoded protein is involved in the maturation of Th2 cells and the activation of mast cells, basophils, eosinophils and natural killer cells. Gene has been implicated in microglial activation and the brain’s innate immune response. | cytokine | Inhibited | –3.102 | ACAT1, ARRB1, CCL3L3, ENO2, HACD3, NFKBIB, PRPF4B, RAI14, RAMP3, RASGRP1 (direction of expression predicts IL33 inhibition in 8/12) |
| BNIP3L | BCL2 interacting protein 3 like | Encodes a protein that belongs to the pro-apoptotic subfamily within the Bcl-2 family of proteins. The encoded protein directly targets mitochondria and causes apoptotic changes, including loss of membrane potential and the release of cytochrome c. | Other | Inhibited | –2.94 | CCND3, CENPE, CENPF, CKAP2, CST3, GADD45A, GPSM2, KIF11, NT5C3A, NUF2, PRIM1, RAD54L, TOP2A (direction of expression predicts inhibition in 12/13) |
| KLF3 | Kruppel like factor 3 | Transcription factor that is a key regulator of adipogenesis and B cell development. KLF3 serves as a key regulator of neuronal development, and dysregulation of regulators in the KLF famiuly has been linked to has been linked to various neurological disorders. KLFs may play a key role in brain vasoprotection and neuroprotection in response to ischemic or hypoxic stimuli. | Transcription regulator | Inhibited | –2.241 | AGGF1, ANXA5, CEP63, CHCHD10, CSNK1G3, EEF1AKMT1, EPRS, HNRNPH1, HYPK, IGF2BP3 (direction of expression predicts inhibition in 25/35) |
| F2 | Coagulation Factor II; Thrombin | Coagulation factor II is proteolytically cleaved to form thrombin in the first step of the coagulation cascade which ultimately results in the stemming of blood loss. F2 also plays a role in maintaining vascular integrity during development and postnatal life. | Peptidase | Inhibited | –2.821 | B4GALT1, BIRC2, CTGF, DOK5, EGR1, F3, PPIF, RAC1, RHOJ, SLC2A6 (direction of expression predicts inhibition in 10/12) |
| C5 | Complement 5 | Encodes a component of the complement system, part of the innate immune system that plays an important role in inflammation, host homeostasis, and host defense against pathogens | Cytokine | Inhibited | –2.101 | CCL3L3, EFNB2, EGR1, F3, PLK3 (direction of expression predicts inhibition in 4/5) |
Within the Downstream Effect Analysis, 56 Molecular, Cellular, and Physiological System Development Functions met criteria for significant dysregulation in the brains of powdered-chow exposed embryos compared to pelleted (p-value < 0.05 and involving three or more genes in the dataset). Many pathways related to cell cycle regulation were significantly dysregulated, including pathways related to the G1/S Phase, G2 Phase, G2/M transition, Mitosis, Interphase, formation of mitotic spindle, segregation of chromosomes, centrosome duplication, and cytokinesis. The function “segregation of chromosomes” within the Cell Cycle category was predicted to be significantly increased (activation Z-score 2.2, p = 0.003). Many terms related to DNA damage response were also significantly dysregulated. Other themes that emerged from the IPA Downstream Effects pathways analyses included dysregulation of pathways related to cell death (predicted to be decreased, activation Z-score -3.53, p < 0.001) and neuronal survival; to brain innate immune response (pathways related to microglia, macrophage phagocytic activity, and natural killer cell production); to synaptic transmission and plasticity (transmission in the hippocampal region was highlighted twice); to dopaminergic neuron firing and morphology; to amino acid transport (predicted to be decreased, activation Z-score −2.9, p = 0.03); and to fatty acid transport and lipid storage. All significantly dysregulated pathways and biofunctions identified by IPA in embryonic brains exposed to powdered compared to pelleted chow in utero are described in Supplementary File 3.
Gene set enrichment analysis with a development-specific annotation (GSEA/DFLAT)
Two hundred sixty-one gene sets were significantly dysregulated (FDR q < 0.05) in the fetal brain exposed to powdered compared to pelleted chow in utero (215 sets upregulated and 46 downregulated). A complete list of significantly dysregulated gene sets may be found in Supplementary File 4. GSEA/DFLAT identified many of the same dysregulated biological processes as IPA, with cell cycle dysregulation, DNA damage response, brain immune function, amino acid transport, and neurotransmitter regulation again figuring prominently.
Cell cycle regulation was the most disrupted biofunction in powdered-chow exposed brains, with more than half of the upregulated gene sets relating to cell cycle function and regulation. Representative dysregulated gene sets related to the cell cycle include chromatin assembly and disassembly, spindle formation, mitotic spindle assembly checkpoint, centromere complex assembly, regulation of chromosome segregation (and many similar gene sets), cell cycle regulation by ubiquitin-protein ligase, regulation of the metaphase to anaphase transition, M/G1 transition, sister chromatid cohesion, G2/M transition, and many others. DNA damage response and DNA processing were the second most affected biofunctions based on the number of dysregulated gene sets (double-stranded break repair, signal transduction in response to DNA damage, DNA integrity checkpoint regulation, K63-linked polyubiquitin binding, telomere maintenance, telomere organization, recombinational repair, replication fork, double-strand break repair via homologous recombination, DNA packaging and other similar), followed by RNA processing (mRNA splicing via spliceosome/multiple spliceosome-related gene sets, RNA splicing via transesterification reactions, mRNA transport and localization, ribosomal RNA metabolism and processing, mRNA export from nucleus and other similar). Immune function (both humoral and innate) was another area in which many gene sets were upregulated, including production of molecular mediators of immune response, immunoglobulin production and diversification of immune receptors, immunoglobulin-mediated immune response, somatic recombination of immunoglobulin gene segments, B cell activation involved in immune response and other similar. Multiple gene sets related to protein synthesis and function were dysregulated (primarily downregulated) including ribosome biogenesis, translational initiation and termination, downregulation of amino acid transport/amino acid transmembrane transporter activity, glutamine amino acid metabolic process, and multiple sets related to carboxylic and organic acid transporter activity. Downregulation of gene sets related to neuronal apoptosis and cell death was also noted, as were gene sets related to neurotransmitter transport, neurotransmitter regulation, and neurotransmitter receptor activity. Regulation of cAMP biosynthesis and metabolism, and adenylate cyclase activity were also downregulated in the powdered-chow exposed brains, as were gene sets related to membrane lipid metabolism, endoplasmic reticulum function, and potassium and calcium ion transport.
Maternal Dietary Composition Has a Significant Impact on Neonatal Biometry and Behavior
Neonatal Biometry
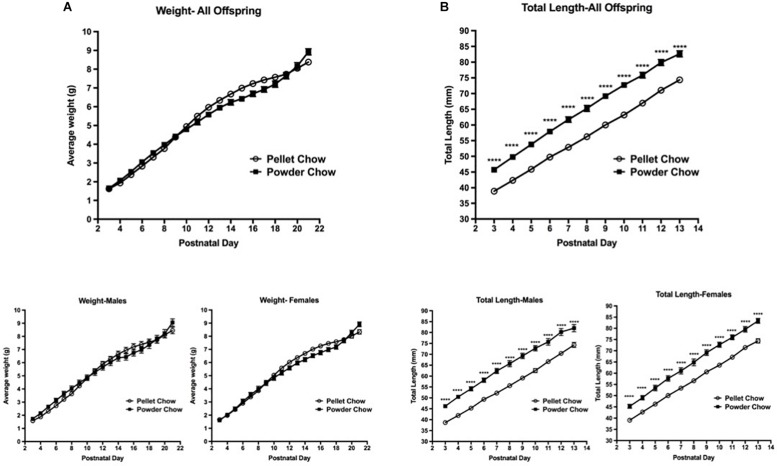
Biometric analyses were performed on 31 powder-chow exposed and 62 pelleted-chow exposed neonates. These included 15 females and 16 males in the powdered chow group and 38 females and 24 males in the pelleted chow group. There was no significant difference between the two maternal diet groups with respect to offspring weight trajectory, which was true when male and female offspring were grouped and when they were examined in a sex-stratified fashion (Figure 4A). The powdered chow-exposed offspring were significantly longer at every postnatal day than pelleted chow-exposed counterparts, which was true both for grouped and sex-stratified analyses (Figure 4B).
FIGURE 4.
Neonatal offspring weight and length trajectory. There were no significant differences in offspring weight (A) between maternal diet groups, both when sexes were grouped (above) and in sex-stratified analyses (below). Powdered chow-exposed offspring were significantly longer at every postnatal day than pelleted chow-exposed counterparts (B), both when sexes were grouped (above) and in sex-stratified analyses (below). N = 31 powder and 62 pelleted chow-exposed neonates. ****p < 0.0001.
Body Righting, Strength and Coordination
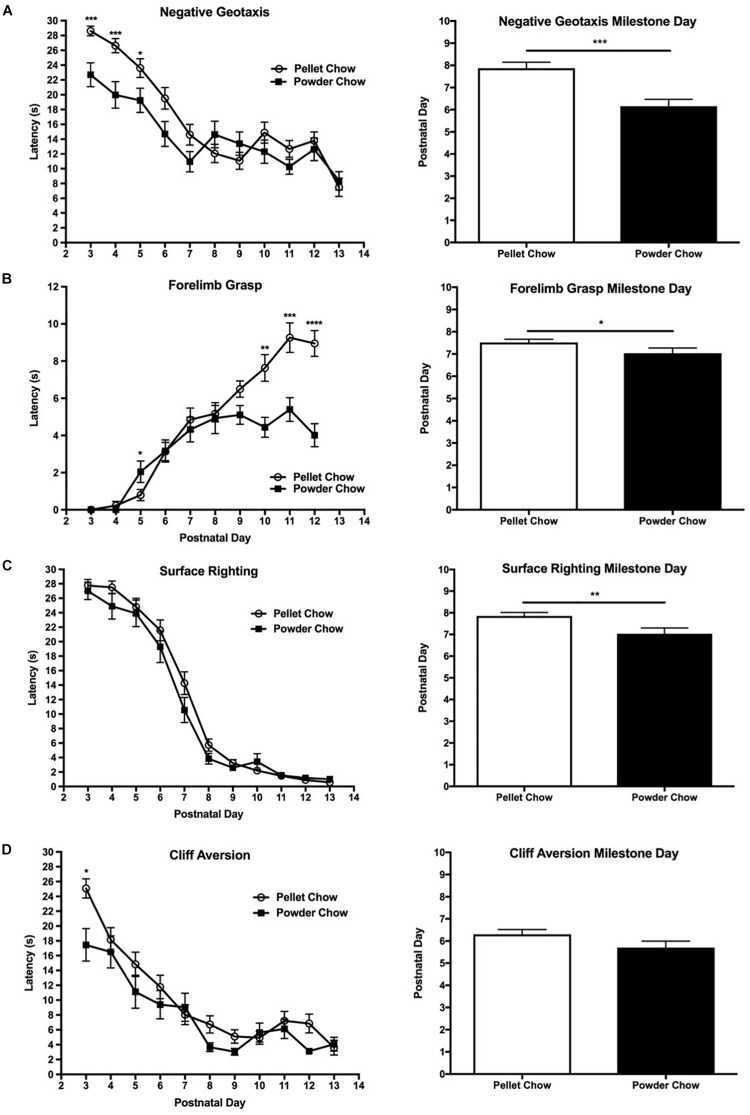
Powdered-chow exposed neonates achieved the body righting, strength, and coordination-related milestones significantly faster than the pelleted-chow exposed neonates. The negative geotaxis task was used to evaluate body righting mechanisms, strength, and coordination. The powdered-chow neonates had a significantly shorter daily latency on the negative geotaxis task and achieved that milestone an average of 1.7 days sooner than the pelleted-chow neonates (p = 0.0002, Figure 5A). Two-way ANOVA demonstrated no significant interaction terms between maternal diet and offspring sex, and no significant main effects of offspring sex on achievement of the negative geotaxis milestone [F(1,88) = 0.65 p = 0.42 for interaction term, F(1,88) = 0.14, p = 0.71 for offspring sex].
FIGURE 5.
Neonatal performance on tests of coordination, strength and body righting. Powdered chow-exposed neonates achieved coordination, strength, and body-righting milestones significantly earlier than pelleted-chow exposed neonates. Graphs on the left depict neonatal latency (mean ± SEM) to complete the behavioral task by postnatal day. Graphs on the right depict differences between neonates from the two maternal diet groups in the day of developmental milestone achievement (defined as the day at which the pup performed the task successfully for 2 days in a row). (A) Negative geotaxis test; (B) Forelimb grasp test; (C) Surface righting test; (D) Cliff aversion test. N = 31 powder and N = 62 pellet-chow exposed neonates. ∗p < 0.05; ∗∗p < 0.01, ∗∗∗p < 0.001.
The forelimb grasp task was used to evaluate strength. Powdered chow-exposed offspring had significantly shorter daily latency on the forelimb grasp task, in addition to a faster overall acquisition of the forelimb grasp milestone (approximately 0.5 days earlier, p = 0.01, Figure 5B). Two-way ANOVA demonstrated no significant interaction terms between maternal diet and offspring sex, and no significant main effects of offspring sex on achievement of the forelimb grasp milestone [F(1,88) = 0.87, p = 0.35 for interaction term, F(1,88) = 0.04, p = 0.85 for offspring sex].
Surface righting task was used to evaluate body righting mechanisms, strength and coordination. The powdered-chow neonates did not have a significantly shorter latency on the surface righting task on any specific day, but achieved the surface righting milestone significantly earlier than the pelleted chow neonates (PND 7 versus 8, p = 0.007, Figure 5C). Two-way ANOVA demonstrated no significant interaction terms between maternal diet and offspring sex, and no significant main effects of offspring sex on achievement of the surface righting milestone [F(1,88) = 0.65, p = 0.42 for interaction term, F(1,88) = 1.31, p = 0.26 for offspring sex].
The cliff aversion test was used to evaluate strength and coordination. Powdered chow-exposed neonates achieved the cliff aversion milestone a mean of 0.6 days earlier than pelleted chow-exposed, but this finding did not achieve statistical significance (p = 0.11, Figure 5D). Two-way ANOVA demonstrated no significant interaction terms between maternal diet and offspring sex, and no significant main effects of offspring sex on achievement of the cliff aversion milestone [F(1,88) = 0.03, p = 0.87 for interaction term, F(1,88) = 1.18, p = 0.28 for offspring sex].
Transition From Rotatory/Pivoting Locomotion Behavior to Straight-Line Walking
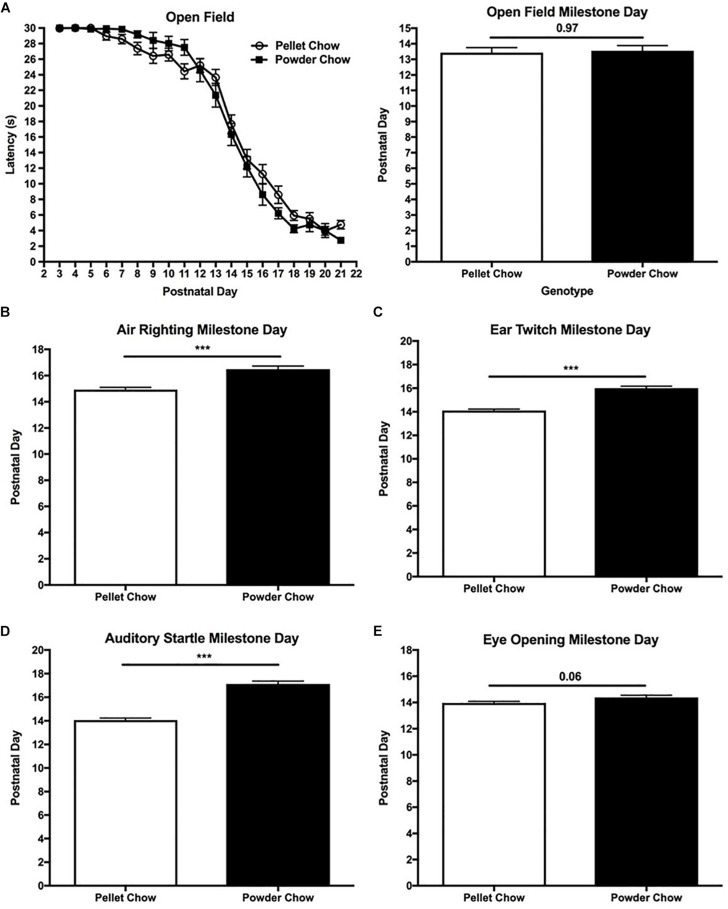
There were no significant differences between powdered-chow and pelleted-chow neonates with respect to daily time spent ambulating in a rotatory fashion and latency to achieve the extinction of rotatory behavior on open field testing (∼day 13 for both groups, Figure 6A). Two-way ANOVA demonstrated no significant interaction terms between maternal diet and offspring sex, and no significant main effects of offspring sex on extinction of rotatory behavior [F(1,88) = 0.43, p = 0.51 for interaction term, F(1,88) = 0.15, p = 0.69 for offspring sex].
FIGURE 6.
Neonatal extinction of rotatory behavior and acquisition of sensory maturation reflexes. Powdered chow-exposed neonates were significantly delayed in their achievement of sensory maturation reflexes including air righting (B), ear twitch (C), and auditory startle (D), compared to pelleted chow-exposed neonates. Eye opening (E) also trended toward delay in the powdered chow-exposed neonates. There were no significant differences in neonatal extinction of rotatory behavior between maternal diet groups (evaluated by open field testing, A). N = 31 powder and N = 62 pellet-chow exposed neonates. ∗∗∗p < 0.001.
Sensory Maturation
Unlike the body strength and coordination tasks, powdered chow-exposed neonates were significantly delayed in achievement of the sensory maturation reflexes compared to their pelleted chow counterparts. Powdered chow exposed neonates were delayed by 1.5 days in achieving the air righting reflex (evaluates the labyrinthine reflex initiated by the vestibular system, in addition to body righting and coordination) compared to pelleted chow-exposed neonates (p < 0.0001, Figure 6B). Two-way ANOVA demonstrated no significant interaction terms between maternal diet and offspring sex, and no significant main effects of offspring sex on achievement of the air righting milestone [F(1,88) = 1.45, p = 0.23 for interaction term, F(1,88) = 0.71, p = 0.40 for offspring sex]. Powdered chow offspring were 2 days delayed in achieving the ear twitch (tactile reflex, p < 0.0001), 3 days delayed in auditory startle (auditory reflex, p < 0.0001), and 0.4 days delayed in eye opening (p = 0.06), compared to their pelleted chow-exposed counterparts. Similar to the other neonatal milestones, two-way ANOVA demonstrated no significant interactions between maternal diet and offspring sex and no significant main effects of offspring sex on attainment of any sensory maturation reflexes [ear twitch F(1,88) = 0.21, p = 0.65 for interaction term, F(1,88) = 1.78, p = 0.19 for offspring sex; auditory startle F(1,88) = 1.86, p = 0.18 for interaction term, F(1,88) = 0.02, p = 0.88 for offspring sex; eye opening F(1,88) = 0.35, p = 0.55 for interaction term, F(1,88) = 1.48, p = 0.23 for offspring sex]. Figures 6C–E depicts the day of milestone achievement for powdered versus pelleted chow-exposed neonates for the aforementioned sensory maturation reflexes.
Maternal Dietary Composition in Pregnancy and Lactation Has a Significant Impact on Adult Locomotor Activity and Hippocampal Learning
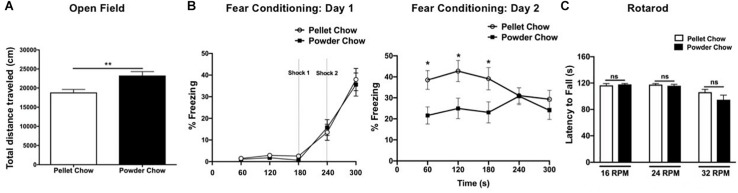
Powdered chow-exposed adult offspring traveled a significantly greater distance in the open field arena compared to pellet chow-exposed adults, consistent with hyperactivity (23,296 ± 1019 cm versus 18,853 ± 779.1 cm, p = 0.001, Figure 7A). Powdered chow-exposed adults demonstrated significantly reduced freezing on day 2 of fear conditioning at 60, 120, and 180 s, consistent with a hippocampal learning/memory deficit (p = 0.01, p = 0.017, and p = 0.039, respectively, Figure 7B). There were no significant differences between powdered chow-exposed and pellet chow-exposed adults on the rotarod test at any speed (Figure 7C).
FIGURE 7.
Adult offspring locomotor behavior, hippocampal learning, and motor coordination. Powdered chow-exposed adults demonstrated hyperactivity (significantly greater distance traveled in the open field test, A) and hippocampal learning/memory deficits (significantly reduced freezing on day 2 of contextual fear conditioning, B). There were no differences between groups in adult motor coordination (Rotarod testing, C). N = 14 powder and N = 16 pellet-chow exposed adults, except for open field where N = 13 powder and 16 pellet-chow exposed adults. ∗p < 0.05; ∗∗p < 0.01.
Discussion
In this study, we demonstrated that maternal diet in pregnancy has a significant impact on fetal brain gene expression signatures and neonatal behavior, even in the absence of overt maternal over- or undernutrition. Specifically, we found that a maternal diet relatively enriched for carbohydrates and n-3 long-chain polyunsaturated fatty acids, and relatively deficient in micronutrients, antioxidants, macroelements, and the amino acids alanine, alargine, and glycine, was associated with differential expression of over 1600 fetal brain genes, significantly increased neonatal length, earlier achievement of strength and coordination-associated milestones, and delayed neonatal sensory maturation. Importantly, these changes occurred in the absence of any differences in maternal weight gain in pregnancy or fetal weight/length changes at embryonic day 15.5, and in the absence of any neonatal weight differences, suggesting that the differences in fetal brain gene expression and neonatal behavior were not attributable to overt nutrient excess or deficiency. We also found neurobehavioral changes persisting into adult life in the offspring, with maternal powdered chow diet in pregnancy and lactation associated with hyperactivity of on open field testing, and hippocampal learning deficits on contextual fear conditioning. Both diets were commercially available “chow” diets, highlighting the critical importance of selection of appropriate control diets for those examining the impact of maternal dietary or other environmental manipulations on fetal brain development and offspring behavior.
Impact of Dietary Micro- and Macronutrients on Brain and Organismal Development
The powdered-chow diet was relatively deficient in manganese, iodine, iron, zinc, and copper, which were 9.5-fold, 71-, 5. 4-, 2-, and 2.5-fold higher in pelleted chow, respectively. These micronutrients have all been shown to influence neurodevelopment in both human and animal model studies (Beard and Connor, 2003; Armony-Sivan et al., 2004; Hirzel et al., 2006; Corniola et al., 2008; Gao et al., 2009; Adamo and Oteiza, 2010; Gogia and Sachdev, 2012; Chung et al., 2015; Claus Henn et al., 2017). Maternal supplementation of these micronutrients has been demonstrated to improve neonatal and pregnancy outcomes, including incidence of congenital anomalies, low birth weight, low IQ or developmental delay, preterm birth, preeclampsia, and preterm premature rupture of membranes (Keats et al., 2019; Mousa et al., 2019).
It is important to note the limitations of available data on the impact of particular micronutrients on fetal and offspring brain development, particularly data from human cohorts. Key limitations include: (1) Many of the associations between maternal micronutrient deficiency and offspring neurodevelopmental outcomes are based on small observational studies that utilize maternal dietary report/recall (therefore are subject to recall bias); (2) Deficiency of a particular micronutrient is likely to co-occur with other nutrient deficiencies and potentially other conditions which may also impact fetal and offspring brain development, such as low socioeconomic status/food insecurity, poor maternal health in general, and prematurity (raising the potential for confounding). Thus, data linking any single micronutrient deficiency to adverse neurodevelopmental outcomes, particularly in observational human cohorts, should be interpreted with caution.
Maternal manganese deficiency and manganese excess have both been associated with worse performance on psychomotor and mental development indexes in human cohorts (Chung et al., 2015; Claus Henn et al., 2017). Zinc deficiency during brain development is associated with adverse neurodevelopmental outcomes in humans and in animal models, with deficits including motor delay and impairments, impaired task attention, engagement, and social behavior (Hirzel et al., 2006; Gogia and Sachdev, 2012). In rodent models, zinc deficiency is associated with cell cycle arrest, mediated in part through dysregulation of the ERK1/2, p53, and NF-kappa B pathways, and zinc deficiency during development is associated with both impaired neuronal precursor cell proliferation and induction of apoptosis (Corniola et al., 2008; Gao et al., 2009; Adamo and Oteiza, 2010). Iodine was the most discrepant micronutrient among the two experimental diets, and has been demonstrated to have a significant impact on neurodevelopment, with effects likely mediated by thyroid hormone deficiency (Skeaff, 2011). Iodine is required for neuronal growth, synapse formation, and myelination during brain development (Prado and Dewey, 2014). Iron was also significantly different between the two diets (relatively deficient in powdered chow), and has been shown to be critical to early life brain development. Iron deficiency has been associated with deficits in learning and memory in children, as well as alterations in neuron energy metabolism, dopamine signaling, and dendrite complexity in animal models (Beard and Connor, 2003; Georgieff, 2007; Prado and Dewey, 2014; Bastian et al., 2016). Particularly relevant to the deficits observed on neonatal behavioral testing in the powdered chow-exposed neonates, iron deficiency has been associated with abnormal neonatal reflexes and impaired auditory processing and auditory cortex development in human cohorts (Armony-Sivan et al., 2004; Siddappa et al., 2004).
The powdered chow was also relatively deficient in all vitamins, with vitamins A, B1, B2, Niacin, B6, pantothenic acid, B12, biotin, and folate all two- to four-fold higher in pelleted chow and Vitamin E 1000-fold higher in pelleted chow. Vitamins A, E, B6, B12 and folic acid have all been demonstrated to impact brain development, with deficiency during key developmental windows associated in human cohorts with adverse neurodevelopmental outcomes including cognitive and motor deficits, as well as autism spectrum disorder (Dias et al., 2013; Wachs et al., 2014; Altamimi, 2018; Mousa et al., 2019). Vitamin E, which was 1000-fold lower in the powdered chow diet, is known to serve as an antioxidant, with fetal neuroprotective effects demonstrated primarily inrat and hamster models in the setting of maternal toxic exposures and/or oxidative stress (Erdemli et al., 2016; Sampayo-Reyes et al., 2017; Sakamoto et al., 2018; Zhang Y. et al., 2018). Supraphysiologic/supra-nutritional maternal vitamin E consumption has been demonstrated in rodent models to induce enduring changes in hippocampal synaptic plasticity of offspring, with increased synaptic density/reduced synaptic pruning observed in the adult offspring hippocampus, and associated cognitive/learning deficits (Betti et al., 2011; Salucci et al., 2014). Thus, both Vitamin E deficiency and excess appear to have deleterious impact on the developing fetal brain.
In addition to being deficient in the antioxidants Vitamin A and E, the powdered chow lacked the isoflavones daidzein and genistein. Studies in rodent models have demonstrated an important effect of dietary soy-derived phytoestrogens on learning and memory, and in mediating anti-inflammatory and neuroprotective effects on the brain (Wang et al., 2013). Both genistein and daidzein have been demonstrated to cross the blood-brain barrier, and exert antioxidant and neuroprotective effects by several mechanisms including mediation of programed cell death and reduction of RAGE-related NF-kB activation and neurovascular production of pro-inflammatory cytokines (Zeng et al., 2004; Xi et al., 2013; Zhang Q. et al., 2018). Both isoflavones have also been demonstrated to improve cognitive function. One putative mechanism for this is upregulation of brain-derived neurotrophic factors (Pan et al., 1999; Lund et al., 2001). Daidzein has been demonstrated in rodent models to exert neuroprotective and cell-proliferative effects in the context of stroke, and in the setting of high-fat diet-induced apoptosis and gliosis (Subedi et al., 2017).
While the powdered chow was deficient for micronutrients and antioxidants, it was relatively enriched for omega-3 long-chain polyunsaturated fatty acids (in particular the n-3 LC-PUFA linolenic acid), which have been demonstrated to influence neurodevelopment. The powdered chow contained 1.6-fold higher n-3 LC-PUFAs than pelleted chow, and had a more favorable n-6/n-3 long-chain PUFA ratio than the pelleted chow (Abedi and Sahari, 2014). LC-PUFAs are fatty acids with at least 18–20 carbons, whose omega-6 (n-6) or omega-3 (n-3) designations depend on the position of the first double bond relative to the methyl end group of the fatty acid (Venegas-Caleron et al., 2010). LC-PUFAs are important in neurotransmitter synthesis and release, immune system regulation, the clotting cascade, phospholipid membrane structure in the brain and retina, and cholesterol metabolism (Li and Hu, 2009; Abedi and Sahari, 2014). N-3 LC-PUFAs are highly concentrated in the mammalian retina and brain, and play a key role in normal visual and brain function due to their involvement in neurotransmitter biosynthesis, signal transduction, and monoamine neurotransmitter receptor binding and activity (Li and Hu, 2009). The powdered chow also had a higher content of carbohydrates when compared with the pelleted chow. There is a dearth of information in the literature about the impact of dietary carbohydrates on neurodevelopment, although overall maternal dietary quality, one measure of which includes total caloric consumption, has been demonstrated to be associated with neurodevelopmental outcomes (Malin et al., 2018). The slight increase in calories in the powdered chow (3.7 kcal/g versus 3.1 kcal/g in the pelleted chow), which is primarily attributable to the increased carbohydrate content, could impact overall organismal development, including of the developing brain.
Pathways Analysis of Fetal Brain Transcriptome Data in Context
Key themes in the functional analyses of differentially expressed fetal brain genes included dysregulation of the cell cycle, of DNA damage response, and of apoptosis. Significant upregulation of the canonical pathway Mitotic Roles of Polo-Like Kinase (key in cell cycle regulation), and significant dysregulation of multiple downstream pathways implicated in cell cycle regulation, was noted in the powdered chow-exposed fetal brain. The polo-like kinases are a highly conserved family of proteins identified in yeast, Xenopus, C. elegans and mammals, playing a key regulatory role for entry into and exit from mitosis, centrosome separation and maturation, and promoting the onset of cytokinesis, among other functions (Glover et al., 1998; Donaldson et al., 2001). Polo-like kinases have been implicated in neurogenesis and synaptic plasticity (Kauselmann et al., 1999; Sakai et al., 2012; Genin et al., 2014), suggesting that activation of polo-like kinases might reflect increased demand for new neural progenitor cells or new neuronal connections. Whether the significant upregulation of this canonical pathway means that there is increased flux through the cell cycle in the brains of powdered chow-exposed embryos, and if there is increased flux, whether this reflects increased neurogenesis, increased cell death, or both, is beyond the scope of these experiments to determine. These results point to the need for additional experiments investigating the impact of micronutrient deficiency on cellular proliferation and death in the developing brain, specifically as these processes relate to increased flux through the cell cycle mediated by polo-like kinases.
DNA damage response was another key dysregulated biological process in the functional analyses, and might be related to the significant dysregulation of cell-cycle related biological functions and canonical pathways. There are numerous examples of micronutrient deficiencies associated with DNA damage (Ames, 1999). In particular, iron is an essential cofactor for proteins which regulate DNA replication, repair, and cell cycle progression (Zhang, 2014; Jung et al., 2019). One such protein for which iron is an essential cofactor is the enzyme GADD45, which was noted to be significantly dysregulated in the powdered chow-exposed embryonic brains, and is involved in cell cycle arrest after DNA damage (Gao et al., 1999; Steegmann-Olmedillas, 2011). Dietary zinc supplementation has also been linked with reduced DNA strand breaks in human subjects; proteins involved in DNA repair, antioxidant, and immune functions were restored after dietary zinc was increased (Song et al., 2009; Zyba et al., 2017).
Negative regulation of apoptosis in the powdered chow-exposed fetal brain was another key theme in the functional analyses, including activation of both upstream regulators that downregulate apoptosis, and downstream pathways that negatively regulate apoptosis. This finding is consistent with gene expression patterns suggesting reduced apoptosis in the human fetal brain exposed to maternal obesity, which may itself be a state of relative micronutrient deficiency (Edlow et al., 2014). Multiple micronutrients that are deficient in the powdered chow diet have been linked to increased apoptosis in animal models, including iodine, manganese, zinc, and copper (Beard and Connor, 2003; Armony-Sivan et al., 2004; Vanlandingham et al., 2005; Georgieff, 2007; Tamm et al., 2008; Gao et al., 2009; Cui et al., 2018). Zinc deficiency, in particular, is strongly associated with apoptosis and abnormal neural progenitor regulation in the developing brain (Corniola et al., 2008; Gao et al., 2009; Adamo and Oteiza, 2010). Thus, downregulation of apoptosis-related pathways in the powdered chow-exposed fetal brain could be compensatory. Determining whether apoptosis is up- or down-regulated in the powdered chow-exposed/relatively micronutrient-deficient brain, and whether the downregulation of apoptosis seen on pathways analysis is compensatory or causative are important directions for future investigation.
Other key themes in both the IPA and GSEA/DFLAT analyses included decreased amino acid transport in the powdered chow-exposed fetal brain; dysregulated synaptic transmission and plasticity; brain innate immune response (including pathways related to microglia/macrophage phagocytic activity, as well as killer cell activity); and dopaminergic signaling. The changes in amino acid transport pathways could reflect the powdered chow’s relative deficiency of multiple amino acids, especially glycine, alanine and arginine. Several components deficient in the powdered chow diet have been demonstrated to play an important role in synaptic plasticity, in particular zinc and iron (Beard and Connor, 2003; Jorgenson et al., 2005; Georgieff, 2007). LC-PUFAs, which are relatively enriched in the powdered chow diet, have also been demonstrated to play an important role in synaptogenesis, learning and memory, and synaptic plasticity (Georgieff and Innis, 2005; Georgieff, 2007; Crupi et al., 2013). Deficiency of iron, zinc, and copper, and reduced antioxidant capacity conferred by relative deficiency of antioxidants (including the specific isoflavones daidzein and genistein), have been associated with altered microglial function and brain innate immune response (Cunningham-Rundles et al., 2009; Ibrahim et al., 2010; Chinta et al., 2013). Iron and copper deficiency (both relatively deficient in the powdered-chow exposed diet) have been demonstrated to impact dopamine synthesis, metabolism and release in the striatum (Prohaska and Brokate, 2001; Beard and Connor, 2003; Georgieff, 2007).
Contextualizing Neonatal Biometric Parameters and Behavioral Testing
The multiple differences between the two diets preclude attribution of neonatal growth changes or performance on any particular behavioral test to relative excess or deficiency of a particular dietary component. One possible interpretation of the neonatal length and behavioral differences between diet groups, however, is that the increased length and improved attainment of strength-and coordination-related tasks in the powdered chow-exposed pups may be attributable to aspects of the diet that were relatively enriched in powdered chow, while the delay in sensory milestone achievement might be attributable to relative deficiencies in the powdered chow diet. Thus, the relative enrichment of powdered chow for the n-3 LC-PUFA linolenic acid and carbohydrates could be a driver for increased length and improved strength and coordination in the pups, while the relative deficiency in macroelements, micronutrients and antioxidants could be a driver of the delays in sensory maturation noted.
Although our study design does not permit the explicit testing of these hypotheses, the existing literature suggests there is biologic plausibility for both omega-3 LC-PUFAs and carbohydrate enrichment increasing body length, and for omega-3 LC-PUFA enrichment improving strength and coordination. Prior studies have found an association between omega-3:omega-6 maternal dietary ratios and neonatal length in mice (Santillan et al., 2010). Maternal diet correlates with neonatal length in humans, as well (Rodriguez-Bernal et al., 2010; Hjertholm et al., 2018). Associations have also been noted between maternal dietary carbohydrate content and offspring body size, although weight and/or fat mass is more frequently reported to be significantly increased in the setting of increased carbohydrate consumption, compared to length (Renault et al., 2015; Crume et al., 2016).
LC-PUFAs are highly transferred between mother and neonate in breast milk and play a key role in synaptogenesis, synaptic plasticity, and myelination (Georgieff, 2007; Crupi et al., 2013). The majority of animal studies have demonstrated omega-3 fatty acid supplementation is associated with improvement in cognitive tasks, visual acuity, neurogenesis, and other brain-development-related endpoints (Lauritzen et al., 2016). Studies evaluating the benefit of maternal omega-3 fatty acid supplementation on cognition in humans, however, have been mixed. Inconsistent with our results demonstrating sensory maturation deficits in powdered chow-exposed offspring, a study in Turkish infants demonstrated that DHA-enriched formula was associated with more rapid acquisition of brainstem auditory-evoked potentials (Unay et al., 2004). Consistent with our results demonstrating improved coordination in powdered-chow exposed offspring, a small randomized trial found that omega-3 fatty acid supplementation was associated with improved hand-eye coordination in 2.5-year-old children (Dunstan et al., 2008). However, two comprehensive reviews, including one of nine randomized controlled studies, found no consistent sustained benefit of omega-3 supplementation for infant cognition or visual development (Lo et al., 2012; Prado and Dewey, 2014). Overall, studies evaluating the benefit of maternal omega-3 fatty acid supplementation on offspring developmental outcomes have demonstrated the most benefit in preterm infants (SanGiovanni et al., 2000; O’Connor et al., 2001; Makrides et al., 2010).
With respect to strength, omega-3 LC-PUFAs have been demonstrated to improve muscle contractility in rats (Patten et al., 2002), and in humans when omega-3 fatty acid supplementation was combined with an exercise and resistance-training regimen (Rodacki et al., 2012). This may be mediated by enhanced sensitivity of the muscle to acetylcholine, a neurotransmitter that stimulates muscle contraction (Jeromson et al., 2015). Finally, it is possible that the increased body length itself had a favorable impact on strength and coordination-related neonatal behavioral tasks; this association has not been previously reported, and may be an interesting direction for future study.
There is also biologic plausibility for micronutrient and antioxidant deficiency impacting sensory maturation. In a guinea pig model, mild maternal iron deficiency during pregnancy and lactation was associated with abnormal auditory function in postnatal day 24 offspring (Jougleux et al., 2011). Maternal iodine deficiency, even if mild, has also been associated with disorders of auditory processing in human offspring (Azizi et al., 1995; Hynes et al., 2013), and in rodents, early postnatal iodine deficiency and hypothyroidism resulted in decreased dendritic branching in the visual and auditory cortex (Dussault and Ruel, 1987). Maternal and neonatal zinc deficiency has also been associated in multiple studies with abnormal development of brain regions that play critical roles in processing sensory information (Hagmeyer et al., 2014), and with neurosensory disorders in children (Prasad, 1996). Although maternal and perinatal zinc deficiency has more classically been associated with deficits in offspring learning and memory and social behavior (Golub et al., 1995; Hagmeyer et al., 2014), the brain areas rich in zinc-containing glutamatergic neurons (cerebral cortex and limbic structures) also play a key role in sensory processing (Hagmeyer et al., 2014). With respect to ways in which relative antioxidant deficiency may contribute to deficits in sensory maturiation, early life oxidative stress has been demonstrated to impair the development of parvalbumin-expressing fast-spiking interneurons, leading to deficits in sensory processing (Cabungcal et al., 2013).
Contextualizing Adult Offspring Neurobehavioral Testing
With respect to the finding of hippocampal learning deficits in powdered-chow exposed adult offspring, multiple human studies have demonstrated relative micronutrient deficiency negatively impacts offspring cognition, with micronutrient supplementation of pregnant women and their infants/children associated with improved child cognition (standardized reading and math scores and information processing measures) (Prado and Dewey, 2014). However, these data are limited by concomitant increased caloric intake and/or improved protein intake during the period of supplementation for most studies, and lack of standardization of micronutrient supplementation content. While nearly all the micronutrients and antioxidants relatively deficient in the powdered-chow diet have been linked to hippocampal development and learning/memory, the strongest data in human and animal model studies are for iron and iodine deficiency in early development resulting in abnormal hippocampal development and cognitive and learning deficits that endure into adult life (Dussault and Ruel, 1987; Jorgenson et al., 2003, 2005; Lozoff et al., 2006; Beard, 2007; de Escobar et al., 2007; Walker et al., 2007; Fretham et al., 2011; Prado and Dewey, 2014).
With respect to the finding of hyperactivity/increased locomotion in powdered-chow exposed offspring, human studies evaluating the impact of vitamin and micronutrient deficiencies on offspring attention deficit hyperactivity disorder (ADHD) risk have reported mixed results (Li et al., 2019). For example, maternal multivitamin and folate intake were associated with a lower risk of ADHD diagnosis and medication use in the Danish National Birth Cohort, but not in a New Zealand birth cohort (Virk et al., 2018; D’Souza et al., 2019). Deficiencies in B vitamins and Vitamin D have also been linked to increased ADHD risk in children (Morales et al., 2015; Altun et al., 2018; Fasihpour et al., 2019; Kotsi et al., 2019), although some studies have failed to find these associations (Gustafsson et al., 2015). Inconsistent with our finding of hyperactivity in the powder-chow exposed offspring, recent human and rodent studies have reported that increased cord blood n-6/n-3 fatty acid ratio or relative maternal deficiency of n-3 fatty acids is associated with increased risk for ADHD in offspring (Fedorova and Salem, 2006; Sakayori et al., 2016; Lopez-Vicente et al., 2019), A recent systematic meta-analysis concluded that maternal omega-3 fatty acid intake has not been consistently associated with offspring ADHD risk, however (Li et al., 2019). Poor maternal dietary quality, particularly diets high in sugar, fat and processed foods (which may in some cases be a marker for micronutrient deficiency), has been more consistently linked to ADHD risk in offspring (Rijlaarsdam et al., 2017; Galera et al., 2018). Animal model studies have demonstrated more consistent associations between micronutrient deficiency during development and hyperactivity of offspring, with relative deficiency of B vitamins and iron most strongly implicated (Lalonde et al., 2008; LeBlanc et al., 2009; Fiset et al., 2015).
Strengths, Limitations and Future Directions
As one of the first studies to directly examine the impact of maternal micronutrient and antioxidant deficiency on fetal brain development and offspring behavior in the absence of overt maternal over- or undernutrition, this study begins to address a significant knowledge gap. Pathway analysis of differentially expressed fetal brain genes provided unique insights into biological processes that could be impacted by the relative excess and deficient dietary components in the powdered chow. We hope that the gene expression changes and dysregulated pathways highlighted here can act as a starting point for future studies designed to examine the impact of specific combinations of maternal dietary components on fetal neurodevelopment and offspring behavior. The use of multiple pathways analysis tools, including a tool with annotation specific to fetal development (DFLAT), provides the most comprehensive picture of dysregulated biological processes (Edlow et al., 2015). The use of a comprehensive and validated battery of neonatal behavioral assessments performed by a single experienced investigator is also a strength. The multiple dietary components that differ between the study diets is a limitation, as it does not permit us to attribute specific gene expression changes or putative biological functional changes to particular macro- or micronutrient components, only to demonstrate that “chow” diet composition has a profound impact on the developing fetal brain and enduring consequences for offspring behavior. However, as a recent publication notes, studies that examine the impact of deficiency or enrichment for a single micro- or macronutrient have limited “real world” applicability, and to maximize translatability, studies should focus on the neurodevelopmental impact of combinations of nutrients, given that nutrients are actually ingested in combination (Malin et al., 2018). Because offspring were weaned to the same diet they were exposed to in utero and during lactation, we cannot determine whether the adult behavioral differences were attributable solely to the intrauterine and lactational environment, the postnatal diet exposure, or a combination of the two. Although the study was not designed to interrogate the contribution of paternal diet, a strength of the study design is that male sires were exposed to the same diet as the dams, and male sires were kept on a powdered or pelleted chow diet throughout the duration of the study, with no crossover of male sires between maternal diet groups.
The relatively small number of microarrays per maternal diet group could be viewed as a potential limitation, but five biological replicates per group is sufficient in microarray studies to detect differences in global gene expression (Allison et al., 2006; Tarca et al., 2006). While this number of microarrays is sufficient to interrogate the impact of maternal diet on fetal brain gene expression, the study was not specifically designed or powered to evaluate sex differences in fetal brain gene expression in the setting of this maternal exposure. Therefore, the lack of a significant sex-diet interaction on two-way ANOVA modeling of the differentially expressed brain genes should be interpreted with caution. One piece of evidence in favor of no actual fetal sex effect is the additional lack of a significant effect of offspring sex in the neonatal behavioral analyses. The large number of offspring per sex in the neonatal behavioral experiments (15–37/sex/maternal diet group) suggests that in the context of this particular maternal dietary exposure, fetal and offspring sex truly may not be a significant modifier. Additional studies designed and powered specifically to look at the impact of fetal sex in the setting of these maternal dietary exposures are necessary to definitively exclude fetal sex as an effect modifier. Other future directions include specific enrichment or depletion of maternal diet for only one or a small combination of micro- or macronutrients to explicitly test some of the hypotheses put forward here regarding the impact of LC-PUFA and carbohydrate enrichment on neonatal length, strength and coordination, and the impact of relative micronutrient and antioxidant deficiency on neonatal sensory maturation. In addition, specific evaluation of the mother’s milk for particular dietary components would be a useful adjunct to future studies.
Summary
In this study, we demonstrate the important role of maternal diet composition in fetal brain development and offspring behavior, even in the absence of maternal over- or undernutrition. We show that commercially available chow diets are not interchangeable, and the content of maternal chow diet significantly impacts embryonic brain gene expression, neonatal behavior, and adult behavior in mice. These findings underscore the importance of selecting a maternal chow diet matched for macro- and micronutrients when investigating fetal and offspring brain development and behavior.
Data Availability Statement
The datasets generated for this study can be found in the GEO, accession number GSE133525.
Ethics Statement
The animal study was reviewed and approved by the Tufts Medical Center IACUC.
Author Contributions
AE, FG, and DB contributed to conceiving and designing the study. AE, FG, and JP performed the statistical analysis of behavioral and microarray data. FG performed the neonatal behavioral and microarray experiments. AE performed in silico microarray experiments and wrote the first draft of the manuscript. DS wrote sections of the manuscript. All authors contributed to the manuscript revision, and read and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Funding. This study was funded by the National Institutes of Child Health and Human Development (NICHD R01HD058880 to DB; 2K12HD000849 to AE via the Reproductive Scientist Development Program; and NICHD R01HD100022-01 to AE), and a sponsored research grant from Verinata Health, Inc., an Illumina company (to DB). This work was also supported in part by the National Human Genome Research Institute’s Intramural Research Program, National Institutes of Health, Bethesda, MD, United States through grant Z1A HG200399-04 (Investigator: Diana Bianchi). The funders had no role in the study design, interpretation of results, or writing of the manuscript. Funding for open access publication fees is provided by the Massachusetts General Hospital Vincent Memorial Fund.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnins.2019.01335/full#supplementary-material
References
- Abedi E., Sahari M. A. (2014). Long-chain polyunsaturated fatty acid sources and evaluation of their nutritional and functional properties. Food Sci. Nutr. 2 443–463. 10.1002/fsn3.121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adamo A. M., Oteiza P. I. (2010). Zinc deficiency and neurodevelopment: the case of neurons. Biofactors 36 117–124. 10.1002/biof.91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allison D. B., Cui X., Page G. P., Sabripour M. (2006). Microarray data analysis: from disarray to consolidation and consensus. Nat. Rev. Genet. 7 55–65. 10.1038/nrg1749 [DOI] [PubMed] [Google Scholar]
- Altamimi M. (2018). Could autism be associated with nutritional status in the palestinian population? The outcomes of the palestinian micronutrient survey. Nutr. Metab. Insights. 11:1178638818773078. 10.1177/1178638818773078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altun H., Sahin N., Belge Kurutas E., Gungor O. (2018). Homocysteine, pyridoxine, folate and vitamin B12 levels in children with attention deficit hyperactivity disorder. Psychiatr. Danub. 30 310–316. 10.24869/psyd.2018.310 [DOI] [PubMed] [Google Scholar]
- Ames B. N. (1999). Micronutrient deficiencies. A major cause of DNA damage. Ann. N. Y. Acad. Sci. 889 87–106. 10.1111/j.1749-6632.1999.tb08727.x [DOI] [PubMed] [Google Scholar]
- Antonow-Schlorke I., Schwab M., Cox L. A., Li C., Stuchlik K., Witte O. W., et al. (2011). Vulnerability of the fetal primate brain to moderate reduction in maternal global nutrient availability. Proc. Natl. Acad. Sci. U.S.A. 108 3011–3016. 10.1073/pnas.1009838108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armony-Sivan R., Eidelman A. I., Lanir A., Sredni D., Yehuda S. (2004). Iron status and neurobehavioral development of premature infants. J. Perinatol. 24 757–762. 10.1038/sj.jp.7211178 [DOI] [PubMed] [Google Scholar]
- Aziz N. M., Guedj F., Pennings J. L. A., Olmos-Serrano J. L., Siegel A., Haydar T. F., et al. (2018). Lifespan analysis of brain development, gene expression and behavioral phenotypes in the Ts1Cje, Ts65Dn and Dp(16)1/Yey mouse models of down syndrome. Dis. Model Mech. 11:dmm031013. 10.1242/dmm.031013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azizi F., Kalani H., Kimiagar M., Ghazi A., Sarshar A., Nafarabadi M., et al. (1995). Physical, neuromotor and intellectual impairment in non-cretinous schoolchildren with iodine deficiency. Int. J. Vitam. Nutr. Res. 65 199–205. [PubMed] [Google Scholar]
- Bang O. Y., Hong H. S., Kim D. H., Kim H., Boo J. H., Huh K., et al. (2004). Neuroprotective effect of genistein against beta amyloid-induced neurotoxicity. Neurobiol. Dis. 16 21–28. 10.1016/j.nbd.2003.12.017 [DOI] [PubMed] [Google Scholar]
- Bastian T. W., von Hohenberg W. C., Mickelson D. J., Lanier L. M., Georgieff M. K. (2016). Iron deficiency impairs developing hippocampal neuron gene expression, energy metabolism, and dendrite complexity. Dev. Neurosci. 38 264–276. 10.1159/000448514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beard J. (2007). Recent evidence from human and animal studies regarding iron status and infant development. J. Nutr 137 524S–530S. 10.1093/jn/137.2.524s [DOI] [PubMed] [Google Scholar]
- Beard J. L., Connor J. R. (2003). Iron status and neural functioning. Annu. Rev. Nutr. 23 41–58. [DOI] [PubMed] [Google Scholar]
- Benjamini Y. H. Y. (1995). Controlling the false discovery rate: a practical and powerful approach to multiple testing. J. Roy. Statist. Soc. Ser. B 57 289–300. 10.1111/j.2517-6161.1995.tb02031.x [DOI] [Google Scholar]
- Betti M., Ambrogini P., Minelli A., Floridi A., Lattanzi D., Ciuffoli S., et al. (2011). Maternal dietary loads of alpha-tocopherol depress protein kinase C signaling and synaptic plasticity in rat postnatal developing hippocampus and promote permanent deficits in adult offspring. J. Nutr. Biochem. 22 60–70. 10.1016/j.jnutbio.2009.11.014 [DOI] [PubMed] [Google Scholar]
- Brion M. J., Zeegers M., Jaddoe V., Verhulst F., Tiemeier H., Lawlor D. A., et al. (2011). Intrauterine effects of maternal prepregnancy overweight on child cognition and behavior in 2 cohorts. Pediatrics 127 e202–e211. 10.1542/peds.2010-0651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabungcal J. H., Steullet P., Kraftsik R., Cuenod M., Do K. Q. (2013). Early-life insults impair parvalbumin interneurons via oxidative stress: reversal by N-acetylcysteine. Biol. Psychiatry 73 574–582. 10.1016/j.biopsych.2012.09.020 [DOI] [PubMed] [Google Scholar]
- Chinta S. J., Ganesan A., Reis-Rodrigues P., Lithgow G. J., Andersen J. K. (2013). Anti-inflammatory role of the isoflavone diadzein in lipopolysaccharide-stimulated microglia: implications for Parkinson’s disease. Neurotox. Res. 23 145–153. 10.1007/s12640-012-9328-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung S. E., Cheong H. K., Ha E. H., Kim B. N., Ha M., Kim Y., et al. (2015). Maternal blood manganese and early neurodevelopment: the mothers and children’s environmental health (MOCEH) study. Environ. Health Perspect. 123 717–722. 10.1289/ehp.1307865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claus Henn B., Bellinger D. C., Hopkins M. R., Coull B. A., Ettinger A. S., Jim R., et al. (2017). Maternal and cord blood manganese concentrations and early childhood neurodevelopment among residents near a mining-impacted superfund site. Environ. Health Perspect. 125:067020. 10.1289/EHP925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corniola R. S., Tassabehji N. M., Hare J., Sharma G., Levenson C. W. (2008). Zinc deficiency impairs neuronal precursor cell proliferation and induces apoptosis via p53-mediated mechanisms. Brain Res. 1237 52–61. 10.1016/j.brainres.2008.08.040 [DOI] [PubMed] [Google Scholar]
- Crume T. L., Brinton J. T., Shapiro A., Kaar J., Glueck D. H., Siega-Riz A. M., et al. (2016). Maternal dietary intake during pregnancy and offspring body composition: the healthy start study. Am. J. Obstet. Gynecol. 215 609 e1–609.e8. 10.1016/j.ajog.2016.06.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crupi R., Marino A., Cuzzocrea S. (2013). n-3 fatty acids: role in neurogenesis and neuroplasticity. Curr. Med. Chem. 20 2953–2963. 10.2174/09298673113209990140 [DOI] [PubMed] [Google Scholar]
- Cui Y., Zhang Z., Zhang B., Zhao L., Hou C., Zeng Q., et al. (2018). Excessive apoptosis and disordered autophagy flux contribute to the neurotoxicity induced by high iodine in Sprague-Dawley rat. Toxicol. Lett. 297 24–33. 10.1016/j.toxlet.2018.08.020 [DOI] [PubMed] [Google Scholar]
- Cunningham-Rundles S., Lin H., Ho-Lin D., Dnistrian A., Cassileth B. R., Perlman J. M. (2009). Role of nutrients in the development of neonatal immune response. Nutr. Rev. 67(Suppl. 2), S152–S163. 10.1111/j.1753-4887.2009.00236.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darnton-Hill I., Mkparu U. C. (2015). Micronutrients in pregnancy in low- and middle-income countries. Nutrients 7 1744–1768. 10.3390/nu7031744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Escobar G. M., Obregon M. J., del Rey F. E. (2007). Iodine deficiency and brain development in the first half of pregnancy. Public Health Nutr. 10 1554–1570. 10.1017/S1368980007360928 [DOI] [PubMed] [Google Scholar]
- Dias F. M., Silva D. M., Doyle F. C., Ribeiro A. M. (2013). The connection between maternal thiamine shortcoming and offspring cognitive damage and poverty perpetuation in underprivileged communities across the world. Med. Hypotheses 80 13–16. 10.1016/j.mehy.2012.09.011 [DOI] [PubMed] [Google Scholar]
- Donaldson M. M., Tavares A. A., Hagan I. M., Nigg E. A., Glover D. M. (2001). The mitotic roles of polo-like kinase. J. Cell Sci. 114(Pt 13), 2357–2358. [DOI] [PubMed] [Google Scholar]
- D’Souza S., Waldie K. E., Peterson E. R., Underwood L., Morton S. M. B. (2019). Antenatal and postnatal determinants of behavioural difficulties in early childhood: evidence from growing up in New Zealand. Child Psychiatry Hum. Dev. 50 45–60. 10.1007/s10578-018-0816-6 [DOI] [PubMed] [Google Scholar]
- Dunstan J. A., Simmer K., Dixon G., Prescott S. L. (2008). Cognitive assessment of children at age 2(1/2) years after maternal fish oil supplementation in pregnancy: a randomised controlled trial. Arch. Dis. Child. Fetal. Neonatal. Ed. 93 F45–F50. [DOI] [PubMed] [Google Scholar]
- Dussault J. H., Ruel J. (1987). Thyroid hormones and brain development. Annu. Rev. Physiol. 49 321–334. [DOI] [PubMed] [Google Scholar]
- Edlow A. G. (2017). Maternal obesity and neurodevelopmental and psychiatric disorders in offspring. Prenat. Diagn. 37 95–110. 10.1002/pd.4932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edlow A. G., Guedj F., Pennings J. L., Sverdlov D., Neri C., Bianchi D. W. (2016a). Males are from Mars, and females are from Venus: sex-specific fetal brain gene expression signatures in a mouse model of maternal diet-induced obesity. Am. J. Obstet. Gynecol. 214 623 e1–1623 e10. 10.1016/j.ajog.2016.02.054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edlow A. G., Hui L., Wick H. C., Fried I., Bianchi D. W. (2016b). Assessing the fetal effects of maternal obesity via transcriptomic analysis of cord blood: a prospective case-control study. BJOG 123 180–189. 10.1111/1471-0528.13795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edlow A. G., Slonim D. K., Wick H. C., Hui L., Bianchi D. W. (2015). The pathway not taken: understanding omics data in the perinatal context. Am. J. Obstet. Gynecol. 213:59 e1-57e172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edlow A. G., Vora N. L., Hui L., Wick H. C., Cowan J. M., Bianchi D. W. (2014). Maternal obesity affects fetal neurodevelopmental and metabolic gene expression: a pilot study. PLoS One 9:e88661. 10.1371/journal.pone.0088661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erdemli M. E., Turkoz Y., Altinoz E., Elibol E., Dogan Z. (2016). Investigation of the effects of acrylamide applied during pregnancy on fetal brain development in rats and protective role of the vitamin E. Hum. Exp. Toxicol. 35 1337–1344. 10.1177/0960327116632049 [DOI] [PubMed] [Google Scholar]
- Fasihpour B., Moayeri H., Shariat M., Keihanidoust Z., Effatpanah M., Khedmat L. (2019). Vitamin D deficiency in school-age Iranian children with attention-deficit/hyperactivity disorder (ADHD) symptoms: a critical comparison with healthy controls. Child Neuropsychol. 13 1–15. 10.1080/09297049.2019.1665638 [DOI] [PubMed] [Google Scholar]
- Fedorova I., Salem N., Jr. (2006). Omega-3 fatty acids and rodent behavior. Prostaglandins Leukot Essent. Fatty Acids 75 271–289. 10.1016/j.plefa.2006.07.006 [DOI] [PubMed] [Google Scholar]
- Ferres M. A., Bianchi D. W., Siegel A. E., Bronson R. T., Huggins G. S., Guedj F. (2016). Perinatal natural history of the Ts1Cje mouse model of down syndrome: growth restriction, early mortality, heart defects, and delayed development. PLoS One 11:e0168009. 10.1371/journal.pone.0168009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiset C., Rioux F. M., Surette M. E., Fiset S. (2015). Prenatal iron deficiency in guinea pigs increases locomotor activity but does not influence learning and memory. PLoS One 10:e0133168. 10.1371/journal.pone.0133168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox W. M. (1965). Reflex-ontogeny and behavioural development of the mouse. Anim. Behav. 13 234–241. [DOI] [PubMed] [Google Scholar]
- Fretham S. J., Carlson E. S., Georgieff M. K. (2011). The role of iron in learning and memory. Adv. Nutr. 2 112–121. 10.3945/an.110.000190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galera C., Heude B., Forhan A., Bernard J. Y., Peyre H., Van der Waerden J., et al. (2018). Prenatal diet and children’s trajectories of hyperactivity-inattention and conduct problems from 3 to 8 years: the EDEN mother-child cohort. J. Child Psychol. Psychiatry 59 1003–1011. 10.1111/jcpp.12898 [DOI] [PubMed] [Google Scholar]
- Gao H. L., Zheng W., Xin N., Chi Z. H., Wang Z. Y., Chen J., et al. (2009). Zinc deficiency reduces neurogenesis accompanied by neuronal apoptosis through caspase-dependent and -independent signaling pathways. Neurotox. Res. 16 416–425. 10.1007/s12640-009-9072-7 [DOI] [PubMed] [Google Scholar]
- Gao J., Lovejoy D., Richardson D. R. (1999). Effect of iron chelators with potent anti-proliferative activity on the expression of molecules involved in cell cycle progression and growth. Redox Rep. 4 311–312. 10.1179/135100099101534990 [DOI] [PubMed] [Google Scholar]
- Genin E. C., Caron N., Vandenbosch R., Nguyen L., Malgrange B. (2014). Concise review: forkhead pathway in the control of adult neurogenesis. Stem Cells 32 1398–1407. 10.1002/stem.1673 [DOI] [PubMed] [Google Scholar]
- Georgieff M. K. (2007). Nutrition and the developing brain: nutrient priorities and measurement. Am. J. Clin. Nutr. 85 614S–620S. [DOI] [PubMed] [Google Scholar]
- Georgieff M. K., Innis S. M. (2005). Controversial nutrients that potentially affect preterm neurodevelopment: essential fatty acids and iron. Pediatr. Res. 57(5 Pt 2), 99R–103R. 10.1203/01.pdr.0000160542.69840.0f [DOI] [PubMed] [Google Scholar]
- Gerster H. (1998). Can adults adequately convert alpha-linolenic acid (18:3n-3) to eicosapentaenoic acid (20:5n-3) and docosahexaenoic acid (22:6n-3)? Int. J. Vitam. Nutr. Res. 68 159–173. [PubMed] [Google Scholar]
- Glover D. M., Hagan I. M., Tavares A. A. (1998). Polo-like kinases: a team that plays throughout mitosis. Genes Dev. 12 3777–3787. 10.1101/gad.12.24.3777 [DOI] [PubMed] [Google Scholar]
- Gogia S., Sachdev H. S. (2012). Zinc supplementation for mental and motor development in children. Cochrane Database. Syst. Rev. 12:CD007991. 10.1002/14651858.CD007991.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golub M. S., Keen C. L., Gershwin M. E., Hendrickx A. G. (1995). Developmental zinc deficiency and behavior. J. Nutr. 125(8 Suppl.), 2263S–2271S. 10.1093/jn/125.suppl_8.2263S [DOI] [PubMed] [Google Scholar]
- Goodliffe J. W., Olmos-Serrano J. L., Aziz N. M., Pennings J. L., Guedj F., Bianchi D. W., et al. (2016). Absence of prenatal forebrain defects in the Dp(16)1Yey/+ mouse model of down syndrome. J. Neurosci. 36 2926–2944. 10.1523/JNEUROSCI.2513-15.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guedj F., Pennings J. L., Ferres M. A., Graham L. C., Wick H. C., Miczek K. A., et al. (2015). The fetal brain transcriptome and neonatal behavioral phenotype in the Ts1Cje mouse model of Down syndrome. Am. J. Med. Genet. A 167A 1993–2008. 10.1002/ajmg.a.37156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guedj F., Pennings J. L., Massingham L. J., Wick H. C., Siegel A. E., Tantravahi U., et al. (2016). An integrated human/murine transcriptome and pathway approach to identify prenatal treatments for down syndrome. Sci. Rep. 6:32353. 10.1038/srep32353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guedj F., Pennings J. L. A., Siegel A. E., Alsebaa F., Massingham L. J., Tantravahi U., et al. (2018). Apigenin as a candidate prenatal treatment for trisomy 21: effects in human amniocytes and the Ts1Cje mouse model. bioRxiv. [preprint]. 10.1101/495283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gustafsson P., Rylander L., Lindh C. H., Jonsson B. A., Ode A., Olofsson P., et al. (2015). Vitamin D status at birth and future risk of attention Deficit/Hyperactivity Disorder (ADHD). PLoS One 10:e0140164. 10.1371/journal.pone.0140164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagmeyer S., Haderspeck J. C., Grabrucker A. M. (2014). Behavioral impairments in animal models for zinc deficiency. Front. Behav. Neurosci. 8:443. 10.3389/fnbeh.2014.00443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill J. M., Lim M. A., Stone M. M. (2008). “Developmental milestones in the newborn mouse,” in Neuropeptide Techniques, Vol. 39 eds Gozes I., Totowa N. J. (Totowa, NJ:: Humana Press; ), 131–149. 10.1007/978-1-60327-099-1_10 [DOI] [Google Scholar]
- Hirzel K., Muller U., Latal A. T., Hulsmann S., Grudzinska J., Seeliger M. W., et al. (2006). Hyperekplexia phenotype of glycine receptor alpha1 subunit mutant mice identifies Zn(2+) as an essential endogenous modulator of glycinergic neurotransmission. Neuron 52 679–690. 10.1016/j.neuron.2006.09.035 [DOI] [PubMed] [Google Scholar]
- Hjertholm K. G., Iversen P. O., Holmboe-Ottesen G., Mdala I., Munthali A., Maleta K., et al. (2018). Maternal dietary intake during pregnancy and its association to birth size in rural Malawi: a cross-sectional study. Matern. Child Nutr. 14 10.1111/mcn.12433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hynes K. L., Otahal P., Hay I., Burgess J. R. (2013). Mild iodine deficiency during pregnancy is associated with reduced educational outcomes in the offspring: 9-year follow-up of the gestational iodine cohort. J. Clin. Endocrinol. Metab. 98 1954–1962. 10.1210/jc.2012-4249 [DOI] [PubMed] [Google Scholar]
- Ibrahim A. S., El-Shishtawy M. M., Pena A., Jr., Liou G. I. (2010). Genistein attenuates retinal inflammation associated with diabetes by targeting of microglial activation. Mol. Vis. 16 2033–2042. [PMC free article] [PubMed] [Google Scholar]
- Ingenuity Systems, (2019a). Calculating and Interpreting the p-values for Functions, Pathways and Lists in IPA. Redwood City, CA: Ingenuity Systems. [Google Scholar]
- Ingenuity Systems. (2019b). Ingenuity Upstream Regulator Analysis in IPA. Redwood City, CA: Ingenuity Systems. [Google Scholar]
- Jeromson S., Gallagher I. J., Galloway S. D., Hamilton D. L. (2015). Omega-3 fatty acids and skeletal muscle health. Mar. Drugs 13 6977–7004. 10.3390/md13116977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson K. L., Tao K., Stroh H., Kallenbach L., Peter I., Richey L., et al. (2010). Increased fetal cell trafficking in murine lung following complete pregnancy loss from exposure to lipopolysaccharide. Fertil 93 1718–1721e1712. 10.1016/j.fertnstert.2009.08.042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones A. D., Zhao G., Jiang Y. P., Zhou M., Xu G., Kaciroti N., et al. (2016). Maternal obesity during pregnancy is negatively associated with maternal and neonatal iron status. Eur. J. Clin. Nutr. 70 918–924. 10.1038/ejcn.2015.229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jorgenson L. A., Sun M., O’Connor M., Georgieff M. K. (2005). Fetal iron deficiency disrupts the maturation of synaptic function and efficacy in area CA1 of the developing rat hippocampus. Hippocampus 15 1094–1102. 10.1002/hipo.20128 [DOI] [PubMed] [Google Scholar]
- Jorgenson L. A., Wobken J. D., Georgieff M. K. (2003). Perinatal iron deficiency alters apical dendritic growth in hippocampal CA1 pyramidal neurons. Dev. Neurosci. 25 412–420. 10.1159/000075667 [DOI] [PubMed] [Google Scholar]
- Jougleux J. L., Rioux F. M., Church M. W., Fiset S., Surette M. E. (2011). Mild maternal iron deficiency anemia during pregnancy and lactation in guinea pigs causes abnormal auditory function in the offspring. J. Nutr. 141 1390–1395. 10.3945/jn.110.135715 [DOI] [PubMed] [Google Scholar]
- Jung M., Mertens C., Tomat E., Brune B. (2019). Iron as a central player and promising target in cancer progression. Int. J. Mol. Sci. 20 E273. 10.3390/ijms20020273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang S. S., Kurti A., Fair D. A., Fryer J. D. (2014). Dietary intervention rescues maternal obesity induced behavior deficits and neuroinflammation in offspring. J. Neuroinflammation 11:156. 10.1186/s12974-014-0156-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kauselmann G., Weiler M., Wulff P., Jessberger S., Konietzko U., Scafidi J., et al. (1999). The polo-like protein kinases Fnk and Snk associate with a Ca(2+)- and integrin-binding protein and are regulated dynamically with synaptic plasticity. EMBO J. 18 5528–5539. 10.1093/emboj/18.20.5528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keats E. C., Haider B. A., Tam E., Bhutta Z. A. (2019). Multiple-micronutrient supplementation for women during pregnancy. Cochrane Database. Syst. Rev. 3 CD004905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimmons J. E., Blanck H. M., Tohill B. C., Zhang J., Khan L. K. (2006). Associations between body mass index and the prevalence of low micronutrient levels among US adults. MedGenMed 8:59. [PMC free article] [PubMed] [Google Scholar]
- Kotsi E., Kotsi E., Perrea D. N. (2019). Vitamin D levels in children and adolescents with attention-deficit hyperactivity disorder (ADHD): a meta-analysis. Atten. Defic. Hyperact. Disord. 11 221–232. 10.1007/s12402-018-0276-7 [DOI] [PubMed] [Google Scholar]
- Krakowiak P., Walker C. K., Bremer A. A., Baker A. S., Ozonoff S., Hansen R. L., et al. (2012). Maternal metabolic conditions and risk for autism and other neurodevelopmental disorders. Pediatrics 129 e1121–e1128. 10.1542/peds.2011-2583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer A., Green J., Pollard J., Jr., Tugendreich S. (2014). Causal analysis approaches in ingenuity pathway analysis. Bioinformatics 30 523–530. 10.1093/bioinformatics/btt703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lalonde R., Barraud H., Ravey J., Gueant J. L., Bronowicki J. P., Strazielle C. (2008). Effects of a B-vitamin-deficient diet on exploratory activity, motor coordination, and spatial learning in young adult Balb/c mice. Brain Res. 1188 122–131. 10.1016/j.brainres.2007.10.068 [DOI] [PubMed] [Google Scholar]
- Laraia B. A., Bodnar L. M., Siega-Riz A. M. (2007). Pregravid body mass index is negatively associated with diet quality during pregnancy. Public Health Nutr. 10 920–926. 10.1017/s1368980007657991 [DOI] [PubMed] [Google Scholar]
- Lauritzen L., Brambilla P., Mazzocchi A., Harslof L. B., Ciappolino V., Agostoni C. (2016). DHA effects in brain development and function. Nutrients 8:E6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeBlanc C. P., Fiset S., Surette M. E., Turgeon O., Brien H., Rioux F. M. (2009). Maternal iron deficiency alters essential fatty acid and eicosanoid metabolism and increases locomotion in adult guinea pig offspring. J. Nutr. 139 1653–1659. 10.3945/jn.109.106013 [DOI] [PubMed] [Google Scholar]
- Li D., Hu X. (2009). Fish and its multiple human health effects in times of threat to sustainability and affordability: are there alternatives? Asia. Pac. J. Clin. Nutr. 18 553–563. [PubMed] [Google Scholar]
- Li M., Fallin M. D., Riley A., Landa R., Walker S. O., Silverstein M., et al. (2016). The association of maternal obesity and diabetes with autism and other developmental disabilities. Pediatrics 137:e20152206. 10.1542/peds.2015-2206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M., Francis E., Hinkle S. N., Ajjarapu A. S., Zhang C. (2019). Preconception and prenatal nutrition and neurodevelopmental disorders: a systematic review and meta-analysis. Nutrients 11:E1628. 10.3390/nu11071628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo A., Sienna J., Mamak E., Djokanovic N., Westall C., Koren G. (2012). The effects of maternal supplementation of polyunsaturated Fatty acids on visual, neurobehavioural, and developmental outcomes of the child: a systematic review of the randomized trials. Obstet. Gynecol. Int. 2012:591531. 10.1155/2012/591531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Vicente M., Ribas Fito N., Vilor-Tejedor N., Garcia-Esteban R., Fernandez-Barres S., Dadvand P., et al. (2019). Prenatal omega-6:omega-3 ratio and attention deficit and hyperactivity disorder symptoms. J. Pediatr. 209:e204. 10.1016/j.jpeds.2019.02.022 [DOI] [PubMed] [Google Scholar]
- Lozoff B., Beard J., Connor J., Barbara F., Georgieff M., Schallert T. (2006). Long-lasting neural and behavioral effects of iron deficiency in infancy. Nutr. Rev. 64(5 Pt 2), S34–S43. discussion S72-91., [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lund T. D., West T. W., Tian L. Y., Bu L. H., Simmons D. L., Setchell K. D., et al. (2001). Visual spatial memory is enhanced in female rats (but inhibited in males) by dietary soy phytoestrogens. BMC Neurosci. 2:20. 10.1186/1471-2202-2-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makrides M., Gibson R. A., McPhee A. J., Yelland L., Quinlivan J., Ryan P., et al. (2010). Effect of DHA supplementation during pregnancy on maternal depression and neurodevelopment of young children: a randomized controlled trial. JAMA 304 1675–1683. 10.1001/jama.2010.1507 [DOI] [PubMed] [Google Scholar]
- Malin A. J., Busgang S. A., Cantoral A. J., Svensson K., Orjuela M. A., Pantic I., et al. (2018). Quality of prenatal and childhood diet predicts neurodevelopmental outcomes among children in mexico city. Nutrients 10:E1093. 10.3390/nu10081093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morales E., Julvez J., Torrent M., Ballester F., Rodriguez-Bernal C. L., Andiarena A., et al. (2015). Vitamin D in pregnancy and attention deficit hyperactivity disorder-like symptoms in childhood. Epidemiology 26 458–465. 10.1097/EDE.0000000000000292 [DOI] [PubMed] [Google Scholar]
- Mousa A., Naqash A., Lim S. (2019). Macronutrient and micronutrient intake during pregnancy: an overview of recent evidence. Nutrients 11:E443. 10.3390/nu11020443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Connor D. L., Hall R., Adamkin D., Auestad N., Castillo M., Connor W. E., et al. (2001). Growth and development in preterm infants fed long-chain polyunsaturated fatty acids: a prospective, randomized controlled trial. Pediatrics 108 359–371. [DOI] [PubMed] [Google Scholar]
- Pan Y., Anthony M., Clarkson T. B. (1999). Effect of estradiol and soy phytoestrogens on choline acetyltransferase and nerve growth factor mRNAs in the frontal cortex and hippocampus of female rats. Proc. Soc. Exp. Biol. Med. 221 118–125. 10.3181/00379727-221-44393 [DOI] [PubMed] [Google Scholar]
- Patten G. S., Abeywardena M. Y., McMurchie E. J., Jahangiri A. (2002). Dietary fish oil increases acetylcholine- and eicosanoid-induced contractility of isolated rat ileum. J. Nutr. 132 2506–2513. 10.1093/jn/132.9.2506 [DOI] [PubMed] [Google Scholar]
- Pinhas-Hamiel O., Newfield R. S., Koren I., Agmon A., Lilos P., Phillip M. (2003). Greater prevalence of iron deficiency in overweight and obese children and adolescents. Int. J. Obes. Relat. Metab. Disord. 27 416–418. 10.1038/sj.ijo.0802224 [DOI] [PubMed] [Google Scholar]
- Prado E. L., Dewey K. G. (2014). Nutrition and brain development in early life. Nutr. Rev. 72 267–284. 10.1111/nure.12102 [DOI] [PubMed] [Google Scholar]
- Prasad A. S. (1996). Zinc deficiency in women, infants and children. J. Am. Coll. Nutr. 15 113–120. 10.1080/07315724.1996.10718575 [DOI] [PubMed] [Google Scholar]
- Prohaska J. R., Brokate B. (2001). Dietary copper deficiency alters protein levels of rat dopamine beta-monooxygenase and tyrosine monooxygenase. Exp. Biol. Med. 226 199–207. 10.1177/153537020122600307 [DOI] [PubMed] [Google Scholar]
- Renault K. M., Carlsen E. M., Norgaard K., Nilas L., Pryds O., Secher N. J., et al. (2015). Intake of carbohydrates during pregnancy in obese women is associated with fat mass in the newborn offspring. Am. J. Clin. Nutr. 102 1475–1481. 10.3945/ajcn.115.110551 [DOI] [PubMed] [Google Scholar]
- Rijlaarsdam J., Cecil C. A., Walton E., Mesirow M. S., Relton C. L., Gaunt T. R., et al. (2017). Prenatal unhealthy diet, insulin-like growth factor 2 gene (IGF2) methylation, and attention deficit hyperactivity disorder symptoms in youth with early-onset conduct problems. J. Child Psychol. Psychiatry 58 19–27. 10.1111/jcpp.12589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodacki C. L., Rodacki A. L., Pereira G., Naliwaiko K., Coelho I., Pequito D., et al. (2012). Fish-oil supplementation enhances the effects of strength training in elderly women. Am. J. Clin. Nutr. 95 428–436. 10.3945/ajcn.111.021915 [DOI] [PubMed] [Google Scholar]
- Rodriguez-Bernal C. L., Rebagliato M., Iniguez C., Vioque J., Navarrete-Munoz E. M., Murcia M., et al. (2010). Diet quality in early pregnancy and its effects on fetal growth outcomes: the infancia y medio ambiente (childhood and environment) mother and child cohort study in spain. Am. J. Clin. Nutr. 91 1659–1666. 10.3945/ajcn.2009.28866 [DOI] [PubMed] [Google Scholar]
- Sakai D., Dixon J., Dixon M. J., Trainor P. A. (2012). Mammalian neurogenesis requires Treacle-Plk1 for precise control of spindle orientation, mitotic progression, and maintenance of neural progenitor cells. PLoS Genet. 8:e1002566. 10.1371/journal.pgen.1002566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakamoto M., Chan H. M., Domingo J. L., Koriyama C., Murata K. (2018). Placental transfer and levels of mercury, selenium, vitamin E, and docosahexaenoic acid in maternal and umbilical cord blood. Environ. Int. 111 309–315. 10.1016/j.envint.2017.11.001 [DOI] [PubMed] [Google Scholar]
- Sakayori N., Tokuda H., Yoshizaki K., Kawashima H., Innis S. M., Shibata H., et al. (2016). Maternal Nutritional Imbalance between linoleic acid and alpha-linolenic acid increases offspring’s anxious behavior with a sex-dependent manner in mice. Tohoku. J. Exp. Med. 240 31–37. 10.1620/tjem.240.31 [DOI] [PubMed] [Google Scholar]
- Salucci S., Ambrogini P., Lattanzi D., Betti M., Gobbi P., Galati C., et al. (2014). Maternal dietary loads of alpha-tocopherol increase synapse density and glial synaptic coverage in the hippocampus of adult offspring. Eur. J. Histochem. 58:2355. 10.4081/ejh.2014.2355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sampayo-Reyes A., Tamez-Guerra R. S., Bermudez de Leon M., Vargas-Villarreal J., Lozano-Garza H. G., Rodriguez-Padilla C., et al. (2017). Tocopherol and selenite modulate the transplacental effects induced by sodium arsenite in hamsters. Reprod Toxicol. 74 204–211. 10.1016/j.reprotox.2017.10.003 [DOI] [PubMed] [Google Scholar]
- SanGiovanni J. P., Parra-Cabrera S., Colditz G. A., Berkey C. S., Dwyer J. T. (2000). Meta-analysis of dietary essential fatty acids and long-chain polyunsaturated fatty acids as they relate to visual resolution acuity in healthy preterm infants. Pediatrics 105 1292–1298. 10.1542/peds.105.6.1292 [DOI] [PubMed] [Google Scholar]
- Santillan M. E., Vincenti L. M., Martini A. C., de Cuneo M. F., Ruiz R. D., Mangeaud A., et al. (2010). Developmental and neurobehavioral effects of perinatal exposure to diets with different omega-6:omega-3 ratios in mice. Nutrition 26 423–431. 10.1016/j.nut.2009.06.005 [DOI] [PubMed] [Google Scholar]
- Scholing J. M., Olthof M. R., Jonker F. A., Vrijkotte T. G. (2018). Association between pre-pregnancy weight status and maternal micronutrient status in early pregnancy. Public Health Nutr. 21 2046–2055. 10.1017/S1368980018000459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siddappa A. M., Georgieff M. K., Wewerka S., Worwa C., Nelson C. A., Deregnier R. A. (2004). Iron deficiency alters auditory recognition memory in newborn infants of diabetic mothers. Pediatr. Res. 55 1034–1041. 10.1203/01.pdr.0000127021.38207.62 [DOI] [PubMed] [Google Scholar]
- Skeaff S. A. (2011). Iodine deficiency in pregnancy: the effect on neurodevelopment in the child. Nutrients 3 265–273. 10.3390/nu3020265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song Y., Chung C. S., Bruno R. S., Traber M. G., Brown K. H., King J. C., et al. (2009). Dietary zinc restriction and repletion affects DNA integrity in healthy men. Am. J. Clin. Nutr. 90 321–328. 10.3945/ajcn.2008.27300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steegmann-Olmedillas J. L. (2011). The role of iron in tumour cell proliferation. Clin. Transl. Oncol. 13 71–76. 10.1007/s12094-011-0621-1 [DOI] [PubMed] [Google Scholar]
- Subedi L., Ji E., Shin D., Jin J., Yeo J. H., Kim S. Y. (2017). Equol, a dietary daidzein gut metabolite attenuates microglial activation and potentiates neuroprotection in vitro. Nutrients 9:E207. 10.3390/nu9030207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subramanian A., Tamayo P., Mootha V. K., Mukherjee S., Ebert B. L., Gillette M. A., et al. (2005). Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc. Natl. Acad. Sci. U.S.A. 102 15545–15550. 10.1073/pnas.0506580102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamm C., Sabri F., Ceccatelli S. (2008). Mitochondrial-mediated apoptosis in neural stem cells exposed to manganese. Toxicol. Sci. 101 310–320. 10.1093/toxsci/kfm267 [DOI] [PubMed] [Google Scholar]
- Tarca A. L., Romero R., Draghici S. (2006). Analysis of microarray experiments of gene expression profiling. Am. J. Obstet. Gynecol. 195 373–388. 10.1016/j.ajog.2006.07.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theiler K. (1989). Theiler Staging Criteria for Mouse Embryo Development. Available at: https://www.emouseatlas.org/emap/ema/theiler_stages/downloads/theiler2.pdf (accessed April, 2018) [Google Scholar]
- Tozuka Y., Kumon M., Wada E., Onodera M., Mochizuki H., Wada K. (2010). Maternal obesity impairs hippocampal BDNF production and spatial learning performance in young mouse offspring. Neurochem. Int. 57 235–247. 10.1016/j.neuint.2010.05.015 [DOI] [PubMed] [Google Scholar]
- Tozuka Y., Wada E., Wada K. (2009). Diet-induced obesity in female mice leads to peroxidized lipid accumulations and impairment of hippocampal neurogenesis during the early life of their offspring. FASEB J. 23 1920–1934. 10.1096/fj.08-124784 [DOI] [PubMed] [Google Scholar]
- Unay B., Sarici S. U., Ulas U. H., Akin R., Alpay F., Gokcay E. (2004). Nutritional effects on auditory brainstem maturation in healthy term infants. Arch. Dis. Child. Fetal Neonatal Ed. 89 F177–F179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Burg J. W., Sen S., Chomitz V. R., Seidell J. C., Leviton A., Dammann O. (2016). The role of systemic inflammation linking maternal BMI to neurodevelopment in children. Pediatr. Res. 79 3–12. 10.1038/pr.2015.179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanlandingham J. W., Tassabehji N. M., Somers R. C., Levenson C. W. (2005). Expression profiling of p53-target genes in copper-mediated neuronal apoptosis. Neuromolecular Med. 7 311–324. 10.1385/nmm:7:4:311 [DOI] [PubMed] [Google Scholar]
- Veena S. R., Gale C. R., Krishnaveni G. V., Kehoe S. H., Srinivasan K., Fall C. H. (2016). Association between maternal nutritional status in pregnancy and offspring cognitive function during childhood and adolescence; a systematic review. BMC Pregnancy Childbirth 16:220. 10.1186/s12884-016-1011-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venegas-Caleron M., Sayanova O., Napier J. A. (2010). An alternative to fish oils: metabolic engineering of oil-seed crops to produce omega-3 long chain polyunsaturated fatty acids. Prog. Lipid. Res. 49 108–119. 10.1016/j.plipres.2009.10.001 [DOI] [PubMed] [Google Scholar]
- Virk J., Liew Z., Olsen J., Nohr E. A., Catov J. M., Ritz B. (2018). Pre-conceptual and prenatal supplementary folic acid and multivitamin intake, behavioral problems, and hyperkinetic disorders: a study based on the danish national birth cohort (DNBC). Nutr. Neurosci. 21 352–360. 10.1080/1028415X.2017.1290932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wachs T. D., Georgieff M., Cusick S., McEwen B. S. (2014). Issues in the timing of integrated early interventions: contributions from nutrition, neuroscience, and psychological research. Ann. N. Y. Acad. Sci. 1308 89–106. 10.1111/nyas.12314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker S. P., Wachs T. D., Gardner J. M., Lozoff B., Wasserman G. A., Pollitt E., et al. (2007). Child development: risk factors for adverse outcomes in developing countries. Lancet 369 145–157. 10.1016/s0140-6736(07)60076-2 [DOI] [PubMed] [Google Scholar]
- Wang Q., Ge X., Tian X., Zhang Y., Zhang J., Zhang P. (2013). Soy isoflavone: the multipurpose phytochemical (Review). Biomed Rep. 1 697–701. 10.3892/br.2013.129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- White C. L., Pistell P. J., Purpera M. N., Gupta S., Fernandez-Kim S. O., Hise T. L., et al. (2009). Effects of high fat diet on morris maze performance, oxidative stress, and inflammation in rats: contributions of maternal diet. Neurobiol. Dis. 35 3–13. 10.1016/j.nbd.2009.04.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wick H. C., Drabkin H., Ngu H., Sackman M., Fournier C., Haggett J., et al. (2014). DFLAT: functional annotation for human development. BMC Bioinformatics 15:45. 10.1186/1471-2105-15-45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winther G., Elfving B., Muller H. K., Lund S., Wegener G. (2018). Maternal high-fat diet programs offspring emotional behavior in adulthood. Neuroscience 388 87–101. 10.1016/j.neuroscience.2018.07.014 [DOI] [PubMed] [Google Scholar]
- Xi Y. D., Li X. Y., Ding J., Yu H. L., Ma W. W., Yuan L. H., et al. (2013). Soy isoflavone alleviates Abeta1-42-induced impairment of learning and memory ability through the regulation of RAGE/LRP-1 in neuronal and vascular tissue. Curr. Neurovasc. Res. 10 144–156. 10.2174/1567202611310020007 [DOI] [PubMed] [Google Scholar]
- Zeng H., Chen Q., Zhao B. (2004). Genistein ameliorates beta-amyloid peptide (25-35)-induced hippocampal neuronal apoptosis. Free Radic. Biol. Med. 36 180–188. 10.1016/j.freeradbiomed.2003.10.018 [DOI] [PubMed] [Google Scholar]
- Zhang C. (2014). Essential functions of iron-requiring proteins in DNA replication, repair and cell cycle control. Protein. Cell 5 750–760. 10.1007/s13238-014-0083-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q., Xie H., Chen D., Yu B., Huang Z., Zheng P., et al. (2018). Dietary daidzein supplementation during pregnancy facilitates fetal growth in rats. Mol. Nutr. Food Res. 62:e1800921. 10.1002/mnfr.201800921 [DOI] [PubMed] [Google Scholar]
- Zhang Y., Wang H., Li Y., Peng Y. (2018). A review of interventions against fetal alcohol spectrum disorder targeting oxidative stress. Int. J. Dev. Neurosci. 71 140–145. 10.1016/j.ijdevneu.2018.09.001 [DOI] [PubMed] [Google Scholar]
- Zyba S. J., Shenvi S. V., Killilea D. W., Holland T. C., Kim E., Moy A., et al. (2017). A moderate increase in dietary zinc reduces DNA strand breaks in leukocytes and alters plasma proteins without changing plasma zinc concentrations. Am. J. Clin. Nutr. 105 343–351. 10.3945/ajcn.116.135327 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated for this study can be found in the GEO, accession number GSE133525.