Abstract
We studied the dynamics of melatonin suppression and changes in cortisol levels in humans in response to light exposure at night using high-frequency blood sampling. Twenty-one young healthy participants were randomized to receive either intermittent bright (~9,500 lux) light (IBL), continuous bright light (CBL) or continuous dim (~1 lux) light (VDL) for 6.5 h during the biological night (n = 7 per condition). Melatonin suppression occurred rapidly within the first 5 min and continued until the end of each IBL stimuli (t1/2 = ~13 min). Melatonin recovery occurred more slowly between IBL stimuli (half-maximal recovery rate of ~46 min). Mean melatonin suppression (~40%) and recovery (~50%) were similar across IBL stimuli. Suppression dynamics under CBL were also rapid (t1/2 = ~18 min), with no recovery until the light exposure ended. There was a significant linear increase of cortisol levels between the start and end of each IBL stimulus. Under CBL conditions cortisol showed trimodal changes with an initial linear activating phase, followed by an exponential inhibitory phase, and a final exponential recovery phase. These results show that light exposure at night affects circadian driven hormones differently and that outcomes are influenced by the duration and pattern of light exposure.
Subject terms: Circadian mechanisms, Neurophysiology
Introduction
Melatonin secretion is acutely suppressed by light exposure at night1,2. While the intensity3,4, duration5 and spectral sensitivity4,6–8 of this response is well characterized in humans, the precise temporal dynamics of this response has not been studied in detail. Findings from studies of several animal models suggest that this response is rapid (t1/2 ~2–10 min)9–14, which is consistent with the rapid attenuation of enzymatic activity in the melatonin biosynthetic pathway10,15. The onset of melatonin suppression and the onset of melatonin recovery begin within ~5–15 min of the start and end of the light pulse, respectively. Recovery to baseline levels can be up to several hours in both humans and rats11,16,17. The suppression and recovery intervals in rats are accurate because high-frequency (every 15 min) microdialysis based sampling was used to measure melatonin in the extracellular space of the pineal gland11. In humans, however, the recovery-interval estimation of several hours may be less accurate since low-frequency sampling (hourly) was used after the light pulse ended17.
Unlike birds and reptiles18–20, the mammalian pineal gland is not directly light sensitive. Instead, a multisynaptic retinal-hypothalamic-pineal pathway carries photic signals from the eye to the suprachiasmatic nucleus (SCN), and to the pineal gland via the paraventricular nucleus of the hypothalamus, intermediolateral nucleus of the spinal cord and the superior cervical ganglion21. SCN lesion or transection of the multisynaptic pathway abolishes light-induced suppression of melatonin22,23. Another multisynaptic pathway has been identified leading from the SCN to the adrenal cortex24. Similar to melatonin, the circadian rhythm of glucocorticoids is also regulated by the SCN25,26. Light exposure in mice increases corticosterone levels via this sympathetic pathway without activating the hypothalamic-pituitary-adrenal axis27. The effects of light exposure on cortisol levels in humans are less clear, however. When humans were kept on a 3-h day, with 1 h of sleep in the dark and 2 h of wakefulness in the light in each 3-h interval, mean cortisol secretion was highest during the first hour of waking following the sleep interval28. Although this elevation in cortisol may have been the removal of sleep-induced suppression of cortisol29; nonetheless, the sleep to wake transition also corresponded with transitions from darkness to light, suggesting a stimulatory role of light exposure on human cortisol secretion. An increase in cortisol was reported during the first 15 min of the light exposure (2,000–4,500 lux) in the morning, although no change in cortisol in response to light exposure was observed during the evening30. Scheer et al.31, reported an increase in cortisol during the morning, within one hour after wake, but no change during an evening exposure using the same intensity (800 lux) and duration (1 hour) of light exposure. Bright light (5,500–10,000 lux) has been reported to induce a sustained decrease in cortisol during the descending32,33 and during the ascending phase of the cortisol rhythm32. Moreover, no change in cortisol levels have been reported in response to light exposure in other studies e.g., during a four-hour evening light exposure (5,000 lux)34, during a three-hour early night or late night exposure (5,000 lux)35, during a one-hour evening exposure (350 lux)36, or during a three-hour nighttime light exposure (600 lux)37. Therefore, the change in cortisol in response to light exposure appears to depend on the timing, the intensity, and possibly the duration of the light stimulus. Moreover, high-frequency sampling to assess the temporal dynamics of changes in cortisol secretion in response to light was not used in any of the studies noted above. Therefore, the aim of the current study was to investigate the precise temporal dynamics of changes in melatonin and cortisol levels induced by nocturnal bright light exposure.
Methods
The general methodology has been reported in detail elsewhere38. We provide here the methods specific to the current analysis.
Participants and pre-study conditions
Twenty-one healthy participants [24.6 ± 5.1 (SD) years old, 15 males, 6 females] were studied. The participants had no medical, psychiatric, or sleep disorders as determined by history, physical examination, electrocardiogram, blood and urine biochemical screening tests, and psychological screening questionnaires (Minnesota Multiphasic Personality Inventory and Beck Depression Inventory). A staff psychologist interviewed participants and those with a history of or a current psychiatric pathology were excluded. Participants reported that they were not taking any medication and were instructed to abstain from the use of alcohol, nicotine, recreational drugs, and foods or beverages containing caffeine for three weeks prior to the study. All participants were drug free at the time of study as verified by a comprehensive toxic analysis conducted upon admission to the laboratory. All experimental procedures were carried out in accordance with the principles of the Declaration of Helsinki, and the protocol was approved by the Human Research Committee at the Brigham and Women’s Hospital. Prior to beginning the protocol, all participants gave informed, written consent.
Study protocol
Participants were required to maintain a regular 8 h:16 h sleep:wake schedule at home for at least 3 weeks prior to admission to the laboratory. In order to ensure compliance with this protocol, participants were required to call into a date/time-stamped answering machine just prior to going to bed and immediately upon awakening, and the times were compared to sleep-wake logs on the day of admission. In addition, wrist activity and light exposure were monitored for 1 week immediately prior to admission to the laboratory (Actiwatch-L, Mini Mitter, Sunriver, OR, USA) and were used to verify the stability of their sleep-wake cycle during that last week and throughout the entire impatient protocol.
Upon admission to the study on experimental day 1, participants were maintained in an environment free from external time cues, including clocks, radios, television, visitors, and sunlight. Participants were continuously monitored by staff members specifically trained to avoid communicating time of day or the nature of the experimental conditions to the participants. Participants were adapted to the laboratory with three baseline days (Days 1–3), during which time they continued to sleep and wake at their habitual times (Fig. 1A). In order to assess their endogenous circadian phase before the light stimulus and to center the light stimulus during the following scheduled episodes of wakefulness, participants underwent a 26.2-h initial constant routine (CR1; procedure described below) from day 4 to day 5. The duration of the constant routine was designed so that the center of the 6.5-h light exposure session was 5.8 h before habitual wake time. On day 5, participants were randomly assigned to one of the three light exposure conditions (described below).
Figure 1.
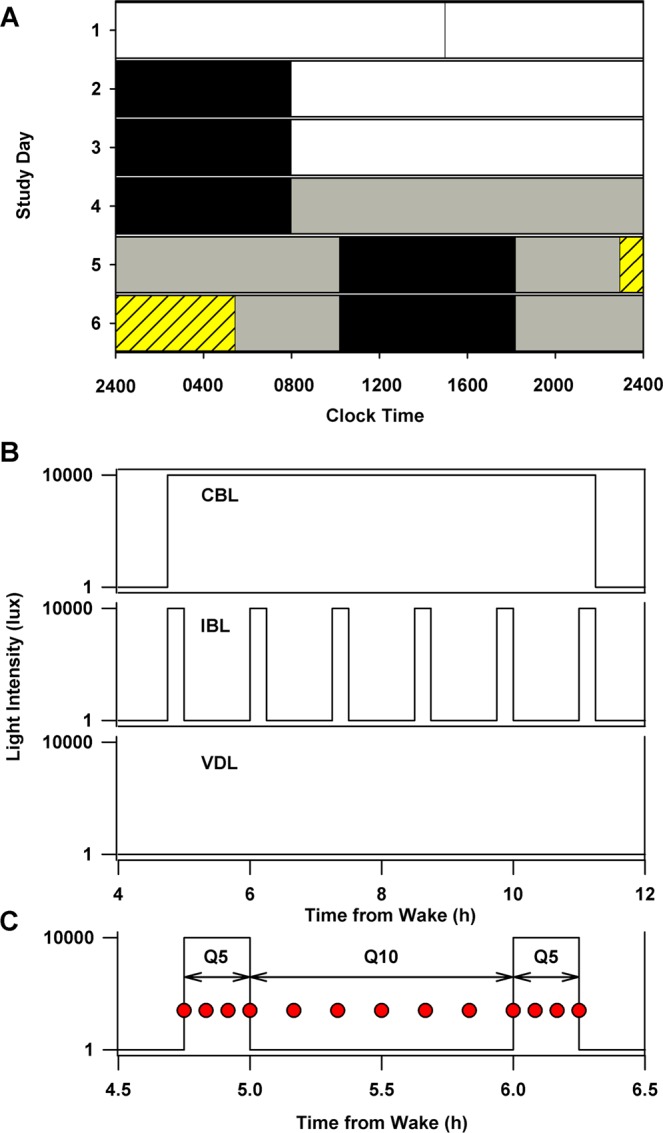
First 6 days of a 10-day experimental protocol and light exposure conditions. A representative study raster for an individual with 2400 h habitual bedtime and 0800 h habitual wake time (A). Scheduled sleep episodes (8 hours in darkness) are illustrated as black bars. During the baseline days (days 1–3), participants were exposed to ~90 lux during wakefulness (16 hours; white bars). For the remainder of the study, except during the light exposure session, participants were exposed to ~1.5 lux during wakefulness (grey bars). A ~ 26.2-h constant routine was scheduled on days 4–5. The light exposure session (yellow hashed bar) was scheduled on days 5–6, and consisted of 6.5 hours of exposure centered 5.8 hours before habitual wake time. Following the light exposure day, participants underwent a 64-h CR and were discharged after a ~22-h recovery sleep episode (days 7–10; not shown). Light exposure conditions (B): participants were exposed either to 6.5 hours of continuous bright light (CBL; ~9,500 lux), intermittent bright light [IBL; six 15-minute bright light (~9,500 lux) stimuli separated by 60 minutes of very dim light (<1 lux)] or very dim light (VDL; < 1 lux). High-frequency blood sampling used to assess melatonin and cortisol dynamics under the different light conditions, every 5 min (Q5) during the 15 min light exposure stimulus or every 10 min (Q10) during the 60 min recovery interval (C). The sampling frequency and patterns were the same under all three light exposure patterns.
During wake episodes, participants were free to move about the suite as desired, except that they were asked not to lie down, nap, or exercise. Participants’ compliance with the protocol was monitored by means of closed-circuit cameras and frequent interaction with technicians. The experimental suites were equipped with ceiling-mounted 4100 K fluorescent lamps [Phillips, Eindhoven, The Netherlands; additional details on light measurements and light spectrum reported in39]. A computer system automatically turned the lighting to the required pre-set intensity at the scheduled times. Maximum light intensities, as measured in the horizontal plane at a height of ~1.83 m using an IL1400 photometer (International Light, Newburyport, MA), were ~190 lux (~48 µW/cm2) during the waking hours of the three baseline days [~90 lux (~23 µW/cm2) measured in the vertical plane at a height of ~1.37 m]; < 8 lux [~1.5 lux ( < 0.4 µW/cm2 to ~0.1 µW/cm2) measured in the vertical plane at a height of ~1.37 m] during the constant routines and the day of light exposure; and depending on the experimental condition (described below), < 1 lux [~0.5 lux (0.1 µW/cm2) measured in the vertical plane at a height of ~1.37 m] or ~9,500 lux (~8 × 106 µW/cm2 in the direction of gaze at a wall-mounted target) during the light exposure session. Sleep episodes were conducted in darkness.
Constant routine procedure
A constant routine (CR) procedure40 was used in the current analysis to assess baseline levels of melatonin and cortisol under dim light conditions on the day prior to light exposure. The CR consisted of a regimen of enforced wakefulness in a semi-recumbent posture in constant dim illumination of ~1.5 lux. Participants were required to maintain a very low level of physical activity and were not permitted to change their posture throughout the constant routine. This posture was also maintained for urine samples and bowel movements. Nutritional intake was divided into hourly isocaloric snacks to maintain an equal caloric intake across the circadian cycle. Caloric requirements were calculated with use of the Wilmore nomogram41 to determine the basal metabolic rate, and adjusted upward by a 10% activity factor to account for increased energy expenditure during sleep deprivation of the constant routine42. A staff member monitored the participant to help maintain wakefulness and to ensure compliance with the posture and activity level requirements.
Light exposure conditions
Participants were randomly assigned to one of the three light exposure conditions (Fig. 1B). The three conditions were: continuous bright light (BL) of ~9,500 lux (~2,900 µW/cm2), defined as 100% bright light; intermittent bright light (IBL) consisting of six 15-minute bright light stimuli of ~9,500 lux [corresponding to ~6700 melanopic lux43] separated by 60 minutes of very dim light [<1 lux (~0.1 µW/cm2)], defined as 23% bright light; and continuous very dim light (VDL) of <1 lux, defined as 0% bright light. In all three conditions, the 30 min prior to and the 30 min following the light exposure session were conducted in <1 lux. Seven participants were randomized to each condition. Participants were seated in a chair from 2 h before until 2 h after the end of the light exposure session. A technician was present at all times during the light exposure session to ensure compliance with the protocol. During the light exposure sessions, participants were instructed to fix their gaze on a target mounted on the wall of their suite for 5 of every 10 min and then allowed free gaze for 5 of every 10 min to ensure consistency of light exposure between participants. This alternating fixed and free gaze episodes continued during the entire duration of the light exposure sessions. Light intensity measurements were taken in the direction of gaze every 5 min to ensure that participants were exposed to the targeted light intensity for the appropriate duration. Clear polycarbonate lenses filtered 99.9% of the light in the UV range from the light source. In addition, all participants wore clear goggles (model #S379; luminous transmittance 90%, UV absorption >99%, Uvex Safety, Smithfield, RI, USA) during the exposure sessions to bright light for additional protection against UV exposure.
Melatonin and cortisol assays
Blood samples were collected every 5 to 10 min during the light exposure session (Fig. 1C), through an indwelling intravenous catheter that was inserted into a forearm vein on day 2 of the study. A solution of heparinized saline (0.45% sodium chloride, 10 units of heparin/ml) was infused at a slow rate (20 ml/h, 200 IU heparin/h) between blood samples. We provided participants with ferrous gluconate (324 mg) pills to be taken at breakfast and dinner: (1) for a minimum of one week prior to participation in the inpatient research protocol; (2) during the inpatient portion of the protocol; and (3) for three weeks after completion of the protocol. Participants’ hemoglobin levels were tested every 1–2 day(s) to ensure appropriate levels (>11.0 for men, >10.3 for women). Blood samples were collected in ethylenediaminetetraacetate (EDTA)-K2 tubes, rapidly centrifuged at 4 °C, and the plasma was stored at or below −25 °C until assayed. Samples were assayed for melatonin using radioimmunoassay techniques (Diagnostech/Pharmasan, Osceola, WI, USA). The assay sensitivity was 2.5 pg/ml. The average intra-assay coefficients of variation (CVs) were 6.4% below 50 pmol/L, and 4.9% above. Plasma cortisol levels were determined by chemiluminescent assay (Beckman Coulter, Chaska, MN, USA); sensitivity, 0.4 mg/dL; intra- and inter-assay coefficients of variation, 6.4% and 7.9%, respectively.
Data analysis
Data are presented as mean ± SD unless stated otherwise. Melatonin and cortisol time course data were analyzed using both between-condition [data compared between individuals exposed to light exposure (IBL and CBL) and dim light conditions] and within-participant by condition analysis [data compared between light exposure (IBL and CBL) and dim light conditions under CR 24 h earlier] to ensure analytic robustness. The within-participant by condition analysis accounts for intra-individual differences in circadian phase, which may have affected hormone levels. In contrast, the between-condition analysis does not account for intra-individual differences.
Time-series melatonin and cortisol data were analyzed using two-way repeated measures with random intercepts mixed model analysis of variance using the restricted maximum likelihood estimation method (REML); fixed effects were set as light-exposure condition and duration of exposure. Simple main effects analyses were used to determine the effect of one factor separately for each level of the other factor. If a significant main or interaction effect was observed, then pairwise comparisons followed using 2-sided t-tests44.
To estimate the effects of each light stimulus on melatonin and cortisol levels, the percentage change from the start to the end of each light stimulus were determined. Similarly, to estimate recovery in melatonin and cortisol levels during the dim-light interval between light stimuli, the percentage change from the end of a light stimulus to the start of the next light stimulus were determined. To adjust for changes in hormone levels with circadian phase, pre- and post-light stimulus values were normalized to melatonin levels by expressing them as a ratio of the levels under dim light conditions 24 h earlier during CR, for each individual. The individual dim-light adjusted percentage-change values were subjected to outlier detection using 2-sided Rosner’s Extreme Studentized Deviate test for multiple outliers with a p-threshold of 0.0144. Three out of 42 data points were detected as outliers in the recovery dataset (2 points in the first stimulus and 1 point in the last stimulus) and none in the suppression dataset. Outliers were removed from further analysis. The individual dim-light adjusted values were then averaged across individuals and analyzed with one-way (stimulus number) repeated measures generalized linear models.
Additionally, accounting for the pulsatile secretion dynamics of cortisol and melatonin, we explored the linear change (increasing or decreasing) in melatonin and cortisol levels in response to bright light by linear regression of three consecutive points (concentrations at t = 5, 10 and 15 min) corresponding to 5 min after lights onset to the end of the light stimulus in the IBL condition, across all 6 stimuli. A positive slope corresponded to an increasing change in the levels between the start and end of the light stimulus, whereas a negative slope corresponded to a decreasing change. The number of positive and negative slopes during each stimulus was compared statistically using binomial probability tests.
To assess melatonin dynamics during and between each light stimulus, data were first expressed as a percentage of the fitted peak under CR for each individual and then averaged across individuals, for each light stimulus separately. This time series data were fitted with exponential-decay regression models [f(x) = a * e(−b * x)] during the light stimulus and 4-parameter logistic regression models [f(x) = ((a − b)/(1 + (x/c)d)) + b] during recovery (dim light between stimuli).
To assess the dynamics in melatonin and cortisol during continuous light exposure, data were first expressed as a percentage of the fitted peak under CR for each individual and then averaged across individuals. Melatonin time course data were fitted with a 3-parameter exponential-decay regression model [f(x) = (a − b) * e(−c * x) + b]. Cortisol time course data appeared to be triphasic and therefore a piece-wise fit was used with a linear model [f(x) = b + ax] for the first ~100 min, a 3-parameter exponential-decay model [f(x) = (a − b) * e(−c * x) + b] for the next ~100 min, and a 3-parameter exponential growth to maximum regression model [f(x) = a + b * (1-e(−c * x))] for remainder of the 6.5 hour light exposure. To compare the dynamics of decreasing melatonin levels following the start of continuous bright light exposure to the decrease in melatonin levels following the cessation of melatonin synthesis (SynOff)45,46, the t1/2 from the exponential decay phase under both conditions were compared using a F-test. The rate of decrease in melatonin levels following SynOff was calculated by applying a 3-parameter exponential-decay regression to the group mean melatonin profile derived under dim-light conditions during CR in the 24 h prior to the bright light exposure. SynOff was defined as previously described45 and could be calculated in 5 out of the 6 individuals who were exposed to continuous bright light.
Results
Melatonin and Cortisol Secretion Profiles
Melatonin suppression under the IBL and CBL conditions has been partially reported previously5,45,47. Using a between-condition analysis [data compared between individuals exposed to light exposure (IBL and CBL) and dim light conditions], we found that the melatonin levels were significantly different between dim light and continuous bright light (CBL) or intermittent bright light conditions (p < 0.01). As expected, melatonin levels increased across time under dim light conditions, and were suppressed throughout the entire duration of the CBL exposure (Fig. 2A) and by IBL stimuli (Fig. 2C) as revealed by a significant interaction between exposure duration and condition (p < 0.001). Simple main effects analyses of this interaction by t-tests showed that, compared to dim light, melatonin levels were lower under CBL at all times starting ~30 min after the onset of the bright light exposure. Additionally, melatonin levels during three of the six IBL stimuli were significantly lower as compared to the dim light condition (Fig. 2C). Moreover, examining the levels of melatonin at the start and end of the light stimulus showed that melatonin levels were significantly decreased at the end of the stimulus compared to the start of the stimulus during the last five IBL stimuli (Fig. 2C).
Figure 2.
Circulating melatonin and cortisol profiles under different lighting conditions. Melatonin and cortisol profiles under very dim light (VDL) and under constant routine (CR) conditions are shown in gray. Profiles under continuous bright light (CBL) exposure for melatonin (A) and cortisol (B) and intermittent bright light (IBL) exposure for melatonin (C) and cortisol (D) using between-conditions analysis and continuous bright light (CBL) exposure for melatonin (E) and cortisol (F) and intermittent bright light (IBL) exposure for melatonin (G) and cortisol (H) using within-participant by condition analysis. Light exposure patterns are shown as dotted lines. Significant differences in melatonin and cortisol levels between VDL or CR and CBL (A and B) or IBL (C and D) at specific exposure times are shown as ▲. Data shown are group mean (±SEM). Significant differences between pre- and post-light exposure levels of melatonin and cortisol levels under IBL condition is shown as *.
Similar to melatonin, cortisol levels were significantly different between dim light and CBL exposure. Cortisol levels were significantly lower under CBL as compared to the dim light condition during the latter half of the light exposure, which corresponded to the rising phase of the cortisol profile under dim light (Fig. 2B). In contrast, cortisol levels were not significantly reduced during IBL stimuli (Fig. 2D). Neither comparing cortisol levels during each light-stimulus interval in IBL and dim-light conditions, nor comparing cortisol levels at the start and end of each light stimulus showed significant differences (Fig. 2D).
Within-participant by condition analysis [data compared between light exposure (IBL and CBL) and dim light conditions under CR 24 h earlier] showed results mostly similar to the between-conditions analysis. One difference was that the melatonin levels were significantly lower during the last five IBL stimuli compared to the levels under the dim light condition using the within-participants by condition analysis (Fig. 2G), whereas only three pulses were different using the between-condition analysis (Fig. 2C). For cortisol, the only difference between the two types of analyses was that as compared to cortisol levels under dim light conditions, there was a significant increase in cortisol levels under CBL condition beginning about 1 h after the onset of bright light exposure and continuing for ~40 min during approximately the first quarter of the light exposure corresponding to the quiescent phase of the cortisol rhythm (Fig. 2F).
Stimulus-Wise Change in Melatonin and Cortisol under Intermittent Bright Light Exposure
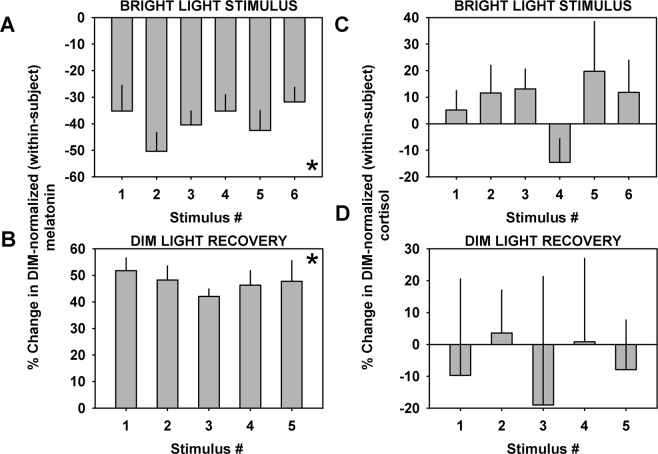
Melatonin levels were on average suppressed by ~40% (Fig. 3A) during each intermittent light stimulus, and recovered by ~50% (~1.5 times increase) between the end of one stimulus and the start of another (Fig. 3B) in the IBL condition. Melatonin levels significantly changed from zero during all of the IBL stimuli (p < 0.001) and during recovery (p < 0.0001). There were no statistically significant differences in the level of suppression (p = 0.50) and recovery (p = 0.85) (one-way GLM) between each of the stimuli after adjusting for the physiological increase in melatonin levels associated with changing circadian phase. There was no significant change in average cortisol levels during each intermittent light stimulus (Fig. 3C), or during recovery (Fig. 3D).
Figure 3.
Change in melatonin and cortisol levels during the light stimulus and recovery interval for each individual bright light stimulus. Change in melatonin during the light stimulus (A) and recovery intervals (B) and in cortisol during the light stimulus (C) and recovery intervals (D) observed with each 15-min stimulus during the intermittent light exposure pattern. Data shown are group mean (±SEM). Melatonin levels were significantly suppressed and recovered between pre- and post-light and post- and pre-light stimulus, respectively *. Cortisol did not show significant changes associated with any of the six pulses.
Additionally, the inhibitory effects of light stimuli on melatonin was confirmed by that 95.3% (40 out of 42) of the intermittent bright light stimuli resulted in an acute linear decrease of melatonin levels during the light pulse (binomial probability test; p < 0.0001, significant association). In contrast, cortisol concentrations increased linearly during the light pulses for 71.4% of the bright light stimuli (30 out of 42; binomial probability test; p < 0.005, significant association). As a control, melatonin and cortisol increased or decreased linearly at random at the same times during the CR 24 h earlier under dim light (54.8% increases and 45.2% decreases for melatonin, p = 0.64; 47.6% increases and 52.4% decreases for cortisol, p = 0.88, no association).
Melatonin Suppression and Recovery Dynamics to Intermittent Bright Light Exposure
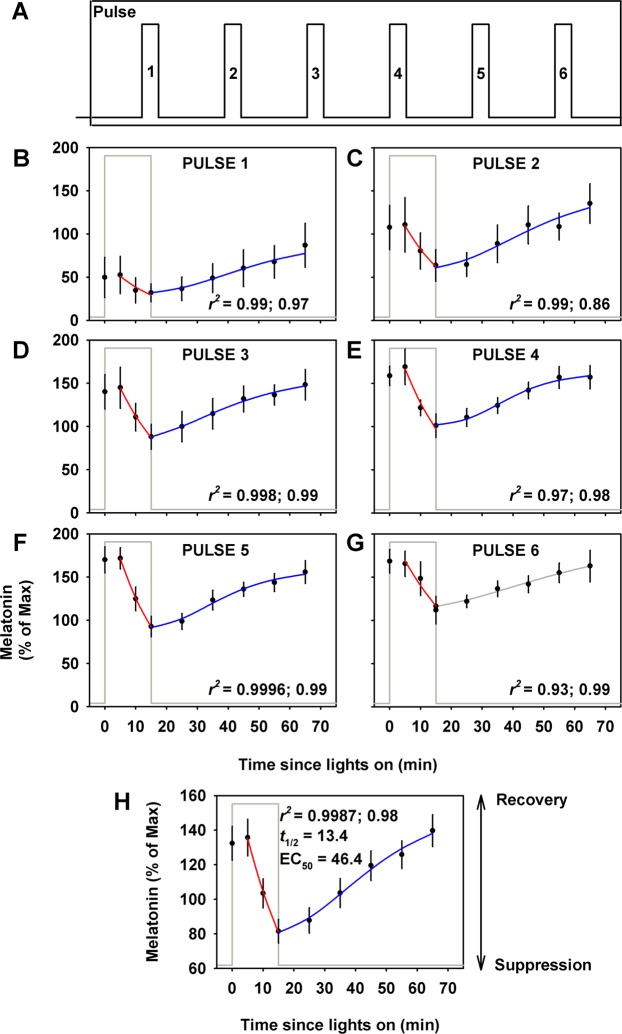
Group mean profiles were generated for each of the six stimuli individually (Fig. 4B–G) and across all six stimuli (Fig. 4H) using Q5 min sampling for melatonin during the 15-min light stimulus and Q10 min sampling during the 60-min recovery interval. Besides the first stimulus, all remaining five stimuli during the suppression interval were fit significantly using an exponential-decay regression model. The mean half-life (t1/2) across six stimuli was 13.5 ± 2.9 min. The t1/2 derived from the group mean profile across all six stimuli was 13.4 ± 1.3 min (Fig. 4H).
Figure 4.
Melatonin suppression and recovery dynamics. Linear and non-linear regression models were applied to group mean (±SEM) melatonin levels under intermittent light exposure. Stimulus pattern under the intermittent condition is shown for reference (A). Suppression regression-line is shown in red and recovery regression-line is shown in blue for each individual stimulus (B–G) and the group mean profile across all six stimuli (H). The recovery time-course for the last stimulus was under dim light after the 6.5 h exposure interval ended (gray line) and is shown for illustrative purposes and was not included in the recovery time-course profile analysis in (H). Adjusted r2 values from the regression analyses are shown for the suppression followed by the recovery interval; t1/2: half-life of suppression; EC50: half-maximal recovery. Data shown are group mean (±SEM).
Besides the second recovery interval, all remaining five recovery intervals were fit significantly using the logistic regression model. The mean EC50 across all five recovery intervals was 46.4 ± 11.3 min. The EC50 calculated from the recovery phase of the group mean profile was 46.4 ± 28.2 min (Fig. 4H).
Melatonin and Cortisol Responses to Continuous Bright Light Exposure
Under continuous bright light exposure there was a sustained suppression of melatonin across the entire light exposure duration (Fig. 5A). This response was best fit by an exponential decay regression model, applied to the group mean profile, which showed a half-life of 18.2 ± 1.4 min. This rate was significantly faster than the rate of melatonin decrease following estimated SynOff (56.2 ± 9.6 min, p < 0.0001). In contrast, cortisol levels under continuous bright light exposure showed trimodal dynamics. In the first ~100 min cortisol levels increased linearly (3.7% increase per minute of exposure) followed by an exponential decrease (t1/2 = 34.0 ± 26.3 min) over the next ~100 min and a final exponential recovery (t1/2 = 21.4 ± 14.5 min) phase for the remainder of the light stimulus (Fig. 5B).
Figure 5.
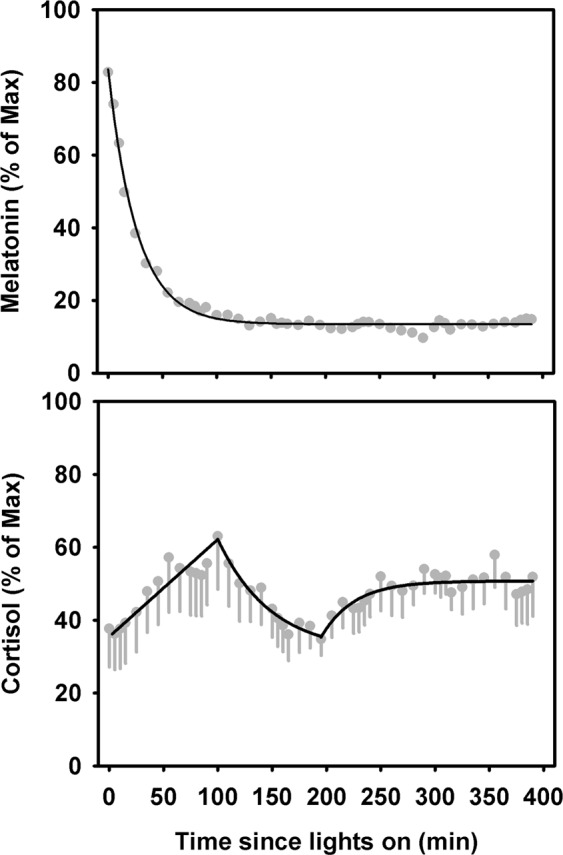
Change in melatonin and cortisol in response to continuous bright light exposure. Change in melatonin (A) and cortisol (B) as a function of duration of exposure under the CBL condition. Data shown are group mean (±SEM).
Discussion
In the current study we used high-frequency sampling under continuous and intermittent light exposure patterns to characterize the dynamics in melatonin and cortisol changes in response to nocturnal light exposure. Our results show that melatonin and cortisol secretion are differentially affected by nighttime light exposure. The change in melatonin levels is rapid, sustained and consistent across the continuous and intermittent light exposure patterns. The change in cortisol, however, is complex and appears to change based on the duration of light exposure. There is a linear increase in cortisol levels in the first ~15 minutes of light exposure, which is sustained for up to ~1.7 h of the continuous light exposure. Following that initial increase, cortisol levels decrease exponentially for an additional ~1.7 h, and finally stabilize during the final ~3 h of the continuous light exposure.
Since the changes in melatonin levels were sustained and consistent across light exposure patterns, we further assessed the dynamics of this response. Under the intermittent exposure pattern, melatonin levels reached half maximal suppression in ~13 min after the start of the 15-min light stimulus and recovered to half-maximal levels in ~29 min after the end of the light stimulus. Melatonin suppression and recovery was similar for all stimuli, after accounting for endogenous changes in the levels of melatonin caused by changes in circadian phase.
Under the continuous exposure pattern, melatonin levels reached half maximal suppression in ~18 min after the start of the 6.5-h light stimulus and did not recover until after the end of the light stimulus. Although the t1/2 of suppression was 5 minutes longer under the continuous exposure pattern compared to the intermittent pattern, this is likely due to the timing of the onset of the light exposure relative to endogenous phase. Indeed, the continuous light exposure that started at a similar phase as the first pulse of the intermittent exposure pattern, had a suppression t1/2 of ~20 min, similar to the suppression t1/2 of ~18 min under the continuous exposure pattern. The sustained suppression of melatonin under continuous light demonstrates a sustained signal reaching the SCN and pineal. These results are in agreement with previous reports showing sustained melatonin suppression in response to polychromatic white light exposure for up to 12 h3,48. The sustained response is most likely driven by intrinsically photosensitive retinal ganglion cells (ipRGCs) as non-visual responses to light, including melatonin suppression, are mediated primarily by ipRGCs4,49. Interestingly, the rate of melatonin suppression was significantly faster under both intermittent (t1/2 of ~13 min) and continuous (t1/2 of ~18 min) light exposure as compared to the rate of decrease in melatonin levels following the cessation of melatonin synthesis estimated as SynOff (t1/2 of ~56 min)45. There are several possible explanations for this observation. The first possibility is that melatonin synthesis and/or secretion may not stop abruptly at SynOff, but rather decrease over some time, whereas synthesis and/or secretion is abruptly halted in response to bright light. This would imply that the half-life of melatonin is closer to 15 minutes than 1 hour. However, melatonin elimination half-life following intravenous administration of melatonin in humans has been estimated to be ~40 minutes. Thus, the much shorter half-life that we have observed during exposure to bright light suggests a second possibility, namely that light exposure hastens the elimination/catabolism of melatonin by an as-yet unknown mechanism. Future research will be needed to confirm the results of this exploratory analysis with appropriate measures, controls and statistical power.
Contrary to the rapid and consistent response of melatonin to continuous and intermittent light exposure, cortisol dynamics differed between continuous and intermittent light exposure. The within-participant by condition analysis revealed a significant acute increase in cortisol levels in response to continuous light exposure, followed by a slow recovery and suppression toward the end of the continuous light exposure. During intermittent bright light exposure, there was also a significant cortisol elevation as assessed by linear regression analysis of the cortisol levels during the 15-min stimuli, but no systematic changes between each light pulse. Averaging cortisol levels across individuals obscures these effects, but this might be due to the pulsatile nature of cortisol secretion50.
The switch from increasing to decreasing cortisol levels may be explained by the dual-drive mechanism proposed for the daily cortisol rhythm, which includes time-of-day sensitive activating and inhibitory drives51. Moreover, two different mechanisms have been proposed for maintaining cortisol rhythms, including a direct neural pathway originating from the SCN to the adrenal gland24 and molecular clocks in the adrenal cells that gate steroid synthesis and cortisol secretion52. Light exposure signals alter the clock gene expression patterns in the adrenal gland and are dependent on the direct sympathetic innervations of the adrenal from the SCN via the splanchic nerve27,53,54. Therefore, it is likely that the signaling dynamics change in either or both of these pathways with duration of exposure and time of day. An alternative explanation for this multimodal effect of light on cortisol secretion could be related to the negative feedback exerted by cortisol on its tropic hormones, CRH and ACTH. The initial activation of cortisol secretion occurring at the beginning of the light exposure could induce a subsequent decreased secretion, by altering cortisol pulsatility, in reducing either pulse amplitude or frequency. This mechanism has been described in response to a large range of cortisol-inducing stimuli, including exercise55, food intake56, ACTH1-24 administration57, and temperature increases58. A similar mechanism could be involved in response to light.
We cannot exclude that the initial increase in cortisol levels following the initiation of light exposure is secondary to a stress response, as we did not evaluate markers of stress during light exposure (e.g., psychological stress with psychometric scales, or physiological stress with ACTH or heart rate). While changes in heart rate have been reported in some studies31,34,59, but not in others30, the changes are only observed during the early morning but not at other times of the day34. Importantly, the change in heart rate is not associated with a change in vagal tone59, a reliable marker of stress response, suggesting that the change in heart rate in not secondary to a stress response. Furthermore, studies in rodent models suggest that the rise in corticosterone with light exposure is not associated with a corresponding change in ACTH27,54, which lends additional support to the changes in glucocorticoids observed in our study and others’ is likely not a stress response. Additional studies are required to investigate the mechanisms, however.
Aberrant light exposure is common in modern society with exposure to less light during the daytime and more at night60. Millions of people use their personal electronic devices before bed61 and millions more are engaged in shift work with frequently changing sleep-wake and light-dark cycles. While initial research suggested that very bright light was required to induce physiologic effects in humans, relatively more recent work shows that half-maximal response of 10,000 lux light is achieved with ~100 lux light including melatonin suppression, circadian phase resetting and the alerting responses3,62. Regular indoor intensity room light exposure prior to bed suppresses melatonin onset and shortens melatonin duration in humans63,64, including the light exposure from personal electronic devices65–67. Moreover, short duration stimuli are more effective than long duration stimuli, on a per minute basis (e.g., each minute in a single 12 min stimulus resets the pacemaker 8 times more than per minute of a 4 h stimulus5). Recently, millisecond flashes delivered over one hour has also been shown to induce significant phase shifts68,69. Our work shows that the suppressive effects of light exposure on melatonin occur rapidly whereas recovery is a relatively slower process. There are robust and complex changes in cortisol, suggesting an activation of the hypothalamic-pituitary-adrenal axis and modulation of the feedback mechanism in this pathway. Overall, the findings show that the influence of light on circadian driven endocrine physiology is dependent upon exposure patterns (continuous versus intermittent light exposure), which underscores the need to consider inter-individual differences in these responses instead of relying solely on group responses. The health consequences of the effects of intermittent light exposure, or even a single light stimulus of short duration, particularly during the biological night, are unknown, but they cannot be excluded, and deserve further investigations under highly controlled protocols.
Acknowledgements
We thank Dr. Melissa St. Hilaire, the research staff, and research participants at the Division of Sleep Medicine, BWH; the technical, dietary, nursing and medical staff at the Center for Clinical Investigation at the Brigham and Women’s Hospital. This work was supported by NAG 5-3952 from the National Aeronautics and Space Agency, and by General Clinical Research Center grant GCRC-M01- RR02635 from the National Center for Research Resources. SAR, SWL and CAC were supported in part by the National Space Biomedical Research Institute through NASA NCC 9-58. SAR was supported by NIH/NHLBI T32-HL007901. KPW was supported in part by NIH/NHLBI HL109706. CG was supported in part by ANR 12-TECS-0013-01, and by EU ENG62-REG3-APP035.
Author contributions
Study design: C.A.C., C.G., K.P.W. Data acquisition: C.G., K.P.W. Data analysis/interpretation: S.A.R., S.W.L., C.G., K.P.W., C.A.C. Manuscript drafting, critical revision, and approval of the final article: S.A.R., S.W.L., C.G., K.P.W., C.A.C.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Competing interests
Competing interests are reported for the last two years. C.G. has had the following commercial interests related to lighting: consulting contracts with Dayvia, Lucibel; unrestricted equipment gifts from Dayvia, HeLight. C.G. is listed as an inventor on 2 patents filed by Inserm Transfert on a wearable health and lifestyle device, and on a lighting apparatus with optimum stimulation of non-visual functions. S.A.R. holds patents for prevention of circadian rhythm disruption by using optical filters and improving sleep performance in subjects exposed to light at night; S.A.R. owns equity in Melcort Inc.; S.A.R. is a co-investigator on studies sponsored by Biological Illuminations, LLC; Vanda Pharmaceuticals Inc., Seoul Semiconductor Co. LTD. S.A.R. has provided paid consulting services to Sultan & Knight Limited t/a Circadia, Bambu Vault LLC. S.A.R. has received honoraria as an invited speaker and travel funds from Starry Skies Lake Superior, University of Minnesota Medical School, PennWell Corp., Seoul Semiconductor Co. LTD. K.P.W. reports funding from the National Institutes of Health, Office of Naval Research, The PAC-212 conference, Philips Inc., CurAegis Technologies (formerly Torvec Inc); Consulting fees from or served as a paid member of scientific advisory boards for NIH, CurAegis Technologies; Speaker honorarium fees from American College of Chest Physicians, The Obesity Society, and Obesity Medicine Association. S.W.L. reports receiving consulting fees from Atlanta Falcons, Atlanta Hawks, Carbon Limiting Technologies Ltd on behalf of PhotoStar LED, Perceptive Advisors, PlanLED, Serrado Capital, Slingshot Insights, and has ongoing consulting contracts with Akili Interactive, Consumer Sleep Solutions, Delos Living LLC, Environmental Light Sciences LLC, Focal Point LLC, Headwaters Inc., Hintsa Performance AG, Light Cognitive, Mental Workout, OpTerra Energy Services Inc., Pegasus Capital Advisors LP, Wyle Integrated Science and Engineering; has received unrestricted equipment gifts from Biological Illuminations LLC; Bionetics Corporation; and F. Lux Software LLC; royalties from Oxford University Press; honoraria plus travel, accommodation or meals for invited seminars, conference presentations or teaching from Estee Lauder, Informa Exhibitions, and Teague; travel, accommodation and/or meals only (no honoraria) for invited seminars, conference presentations or teaching from Hintsa Performance AG, Lightfair and USGBC; ongoing investigator-initiated research grants from Biological Illumination LLC and F. Lux Software LLC; has completed service agreements with Rio Tinto Iron Ore and Vanda Pharmaceuticals Inc.; and has completed three sponsor-initiated clinical research contracts with Vanda Pharmaceuticals Inc. Dr. Lockley also holds a process patent for the use of short-wavelength light for resetting the human circadian pacemaker and improving alertness and performance which is assigned to the Brigham and Women’s Hospital per Hospital policy. Dr. Lockley has also served as a paid expert witness in two cases related to sleep, circadian rhythms, work hours and/or light. Dr Lockley also serves as a Program Leader in the Cooperative Research Centre for Alertness, Safety and Productivity. C.A.C. reports grants from Cephalon Inc., Jazz Pharmaceuticals Plc., Inc., National Football League Charities, Optum, Philips Respironics, Inc., Regeneron Pharmaceuticals, ResMed Foundation, San Francisco Bar Pilots, Sanofi S.A., Sanofi-Aventis, Inc, Schneider Inc., Sepracor, Inc, Mary Ann & Stanley Snider via Combined Jewish Philanthropies, Sysco, Takeda Pharmaceuticals, Teva Pharmaceuticals Industries, Ltd., and Wake Up Narcolepsy; and personal fees from Bose Corporation, Boston Celtics, Boston Red Sox, Cephalon, Inc., Columbia River Bar Pilots, Ganésco Inc., Institute of Digital Media and Child Development, Klarman Family Foundation, Samsung Electronics, Quest Diagnostics, Inc., Teva Pharma Australia, Vanda Pharmaceuticals, Washington State Board of Pilotage Commissioners, Zurich Insurance Company, Ltd. In addition, CAC holds a number of process patents in the field of sleep/circadian rhythms (e.g., photic resetting of the human circadian pacemaker) and holds an equity interest in Vanda Pharmaceuticals, Inc. Since 1985, CAC has also served as an expert on various legal and technical cases related to sleep and/or circadian rhythms, including those involving the following commercial entities: Casper Sleep Inc., Comair/Delta Airlines, Complete General Construction Company, FedEx, Greyhound, HG Energy LLC, Purdue Pharma, LP, South Carolina Central Railroad Co., Steel Warehouse Inc., Stric-Lan Companies LLC, Texas Premier Resource LLC, and United Parcel Service (UPS). CAC receives royalties from the New England Journal of Medicine; McGraw Hill; Houghton Mifflin Harcourt/Penguin; and Philips Respironics, Inc. for the Actiwatch-2 and Actiwatch-Spectrum devices. Interests for S.A.R., S.W.L. and C.A.C. were reviewed and managed by Brigham and Women’s Hospital and Partners HealthCare in accordance with their conflict of interest policies.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Wurtman RJ, Axelrod J, Phillips LS. Melatonin synthesis in the pineal gland: control by light. Science. 1963;142:1071–1073. doi: 10.1126/science.142.3595.1071. [DOI] [PubMed] [Google Scholar]
- 2.Lewy AJ, Wehr TA, Goodwin FK, Newsome DA, Markey SP. Light suppresses melatonin secretion in humans. Science. 1980;210:1267–1269. doi: 10.1126/science.7434030. [DOI] [PubMed] [Google Scholar]
- 3.Zeitzer JM, Dijk DJ, Kronauer RE, Brown EN, Czeisler CA. Sensitivity of the human circadian pacemaker to nocturnal light: Melatonin phase resetting and suppression. J Physiol. 2000;526:695–702. doi: 10.1111/j.1469-7793.2000.00695.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Prayag AS, Najjar RP, Gronfier C. Melatonin suppression is exquisitely sensitive to light and primarily driven by melanopsin in humans. J Pineal Res. 2019;66:e12562. doi: 10.1111/jpi.12562. [DOI] [PubMed] [Google Scholar]
- 5.Chang AM, et al. Human responses to bright light of different durations. J Physiol. 2012;590:3103–3112. doi: 10.1113/jphysiol.2011.226555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brainard GC, et al. Action spectrum for melatonin regulation in humans: Evidence for a novel circadian photoreceptor. J Neurosci. 2001;21(16):6405–6412. doi: 10.1523/JNEUROSCI.21-16-06405.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Thapan K, Arendt J, Skene DJ. An action spectrum for melatonin suppression: Evidence for a novel non-rod, non-cone photoreceptor system in humans. J Physiol. 2001;535:261–267. doi: 10.1111/j.1469-7793.2001.t01-1-00261.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Najjar RP, et al. Aging of non-visual spectral sensitivity to light in humans: compensatory mechanisms? PLoS One. 2014;9:e85837. doi: 10.1371/journal.pone.0085837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brainard GC, Richardson BA, Petterborg LJ, Reiter RJ. The effect of different light intensities on pineal melatonin content. Brain Res. 1982;233:75–81. doi: 10.1016/0006-8993(82)90931-3. [DOI] [PubMed] [Google Scholar]
- 10.Brainard GC, Richardson BA, King TS, Matthews SA, Reiter RJ. The suppression of pineal melatonin content and N -acetyltransferase activity by different light irradiances in the Syrian hamster: A dose-response relationship. Endocrinology. 1983;113:293–296. doi: 10.1210/endo-113-1-293. [DOI] [PubMed] [Google Scholar]
- 11.Kanematsu N, Honma S, Katsuno Y, Honma KI. Immediate response to light of rat pineal melatonin rhythm: analysis by in vivo microdialysis. Am J Physiol. 1994;266:R1849–R1855. doi: 10.1152/ajpregu.1994.266.6.R1849. [DOI] [PubMed] [Google Scholar]
- 12.Klein DC, Weller JL. Rapid light-induced decrease in pineal serotonin N-acetyltransferase activity. Science. 1972;177:532–533. doi: 10.1126/science.177.4048.532. [DOI] [PubMed] [Google Scholar]
- 13.Rollag MD, Panke ES, Trakulrungsi W, Trakulrungsi C, Reiter RJ. Quantification of daily melatonin synthesis in the hamster pineal gland. Endocrinology. 1980;106:231–236. doi: 10.1210/endo-106-1-231. [DOI] [PubMed] [Google Scholar]
- 14.Illnerova H, Vanecek J. Response of rat pineal serotonin N-acetyltransferase to one min light pulse at different night times. Brain Res. 1979;167:431–434. doi: 10.1016/0006-8993(79)90841-2. [DOI] [PubMed] [Google Scholar]
- 15.Illnerova H, Vanecek J, Krecek J, Wetterberg L, Saaf J. Effect of one minute exposure to light at night on rat pineal serotonin N-acetyltransferase and melatonin. J Neurochem. 1979;32:673–675. doi: 10.1111/j.1471-4159.1979.tb00407.x. [DOI] [PubMed] [Google Scholar]
- 16.Honma S, Kanematsu N, Katsuno Y, Honma K. Light suppression of nocturnal pineal and plasma melatonin in rats depends on wavelength and time of day. Neurosci Lett. 1992;147:201–204. doi: 10.1016/0304-3940(92)90595-X. [DOI] [PubMed] [Google Scholar]
- 17.McIntyre IM, Norman TR, Burrows GD, Armstrong SM. Human melatonin response to light at different times of the night. Psychoneuroendocrinology. 1989;14:187–193. doi: 10.1016/0306-4530(89)90016-4. [DOI] [PubMed] [Google Scholar]
- 18.Tosini G, Bertolucci C, Foà A. The circadian system of reptiles: A multioscillatory and multiphotoreceptive system. Physiol Behav. 2001;72:461–471. doi: 10.1016/S0031-9384(00)00423-6. [DOI] [PubMed] [Google Scholar]
- 19.Vigh B, Vigh-Teichmann I, Rohlich P, Aros B. Immunoreactive opsin in the pineal organ of reptiles and birds. Z Mikrosk Anat Forsch. 1982;96:113–129. [PubMed] [Google Scholar]
- 20.Hamm HE, Takahashi JS, Menaker M. Light-induced decrease of serotonin N-acetyltransferase activity and melatonin in the chicken pineal gland and retina. Brain Res. 1983;266:287–293. doi: 10.1016/0006-8993(83)90660-1. [DOI] [PubMed] [Google Scholar]
- 21.Moore RY. Neural control of the pineal gland. Behav Brain Res. 1996;73:125–130. doi: 10.1016/0166-4328(96)00083-6. [DOI] [PubMed] [Google Scholar]
- 22.Klein DC, Moore RY. Pineal N-acetyltransferase and hydroxyindole-O-methyltransferase: Control by the retinohypothalamic tract and the suprachiasmatic nucleus. Brain Res. 1979;174:245–262. doi: 10.1016/0006-8993(79)90848-5. [DOI] [PubMed] [Google Scholar]
- 23.Moore RY, Klein DC. Visual pathways and the central neural control of a circadian rhythm in pineal serotonin N-acetyltransferase activity. Brain Res. 1974;71:17–33. doi: 10.1016/0006-8993(74)90188-7. [DOI] [PubMed] [Google Scholar]
- 24.Buijs RM, et al. Anatomical and functional demonstration of a multisynaptic suprachiasmatic nucleus adrenal (cortex) pathway. Eur J Neurosci. 1999;11:1535–1544. doi: 10.1046/j.1460-9568.1999.00575.x. [DOI] [PubMed] [Google Scholar]
- 25.Kalsbeek A, van, d. V, Buijs RM. Decrease of endogenous vasopressin release necessary for expression of the circadian rise in plasma corticosterone: a reverse microdialysis study. J Neuroendocrinol. 1996;8:299–307. doi: 10.1046/j.1365-2826.1996.04597.x. [DOI] [PubMed] [Google Scholar]
- 26.Czeisler CA, Klerman EB. Circadian and sleep-dependent regulation of hormone release in humans. Recent Prog Horm Res. 1999;54:97–132. [PubMed] [Google Scholar]
- 27.Ishida A, et al. Light activates the adrenal gland: timing of gene expression and glucocorticoid release. Cell Metab. 2005;2:297–307. doi: 10.1016/j.cmet.2005.09.009. [DOI] [PubMed] [Google Scholar]
- 28.Weitzman ED, et al. Effects of a prolonged 3-hour sleep-wake cycle on sleep stages, plasma cortisol, growth hormone, and body temperature in man. J Clin Endocrinol.Metab. 1974;38:1018–1030. doi: 10.1210/jcem-38-6-1018. [DOI] [PubMed] [Google Scholar]
- 29.Weitzman ED, Zimmerman JC, Czeisler CA, Ronda J. Cortisol secretion is inhibited during sleep in normal man. J Clin Endocrinol Metab. 1983;56:352–358. doi: 10.1210/jcem-56-2-352. [DOI] [PubMed] [Google Scholar]
- 30.Leproult R, Colecchia EF, L’Hermite-Balériaux M, Van Cauter E. Transition from dim to bright light in the morning induces an immediate elevation of cortisol levels. J Clin Endocrinol Metab. 2001;86:151–157. doi: 10.1210/jcem.86.1.7102. [DOI] [PubMed] [Google Scholar]
- 31.Scheer FAJL, Buijs RM. Light affects morning salivary cortisol in humans. J Clin Endocrinol Metab. 1999;84:3395–3398. doi: 10.1210/jcem.84.9.6102. [DOI] [PubMed] [Google Scholar]
- 32.Jung CM, et al. Acute effects of bright light exposure on cortisol levels. J Biol Rhythms. 2010;25:208–216. doi: 10.1177/0748730410368413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kostoglou-Athanassiou I, Treacher DF, Wheeler MJ, Forsling ML. Bright light exposure and pituitary hormone secretion. Clin Endocrinol. 1998;48:73–79. doi: 10.1046/j.1365-2265.1998.00355.x. [DOI] [PubMed] [Google Scholar]
- 34.Rüger M, Gordijn MC, Beersma DG, de Vries B, Daan S. Time-of-day-dependent effects of bright light exposure on human psychophysiology: comparison of daytime and nighttime exposure. Am J Physiol Regul Integr Comp Physiol. 2006;290:R1413–R1420. doi: 10.1152/ajpregu.00121.2005. [DOI] [PubMed] [Google Scholar]
- 35.Leproult R, Van Reeth O, Byrne MM, Sturis J, Van Cauter E. Sleepiness, performance, and neuroendocrine function during sleep deprivation: Effects of exposure to bright light or exercise. J Biol Rhythms. 1997;12:245–258. doi: 10.1177/074873049701200306. [DOI] [PubMed] [Google Scholar]
- 36.Thalen BE, Morkrid L, Kjellman BF, Wetterberg L. Cortisol in light treatment of seasonal and non-seasonal depression: relationship between melatonin and cortisol. Acta Psychiatr Scand. 1997;96:385–394. doi: 10.1111/j.1600-0447.1997.tb09934.x. [DOI] [PubMed] [Google Scholar]
- 37.McIntyre IM, Norman TR, Burrows GD, Armstrong SM. Melatonin, cortisol and prolactin response to acute nocturnal light exposure in healthy volunteers. Psychoneuroendocrinology. 1992;17(2/3):243–248. doi: 10.1016/0306-4530(92)90063-D. [DOI] [PubMed] [Google Scholar]
- 38.Gronfier C, Wright KP, Jr., Kronauer RE, Jewett ME, Czeisler CA. Efficacy of a single sequence of intermittent bright light pulses for delaying circadian phase in humans. Am J Physiol Endocrinol Metab. 2004;287:E174–E181. doi: 10.1152/ajpendo.00385.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gronfier C, Wright KP, Jr., Kronauer RE, Czeisler CA. Entrainment of the human circadian pacemaker to longer-than-24-h days. Proc Natl Acad Sci USA. 2007;104:9081–9086. doi: 10.1073/pnas.0702835104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Duffy JF, Dijk DJ. Getting through to circadian oscillators: Why use constant routines? J Biol Rhythms. 2002;17:4–13. doi: 10.1177/074873002129002294. [DOI] [PubMed] [Google Scholar]
- 41.Wilmore, D. W. The metabolic management of the critically ill. (Plenum Press, 1977).
- 42.Jung CM, et al. Energy expenditure during sleep, sleep deprivation and sleep following sleep deprivation in adult humans. J Physiol. 2011;589:235–244. doi: 10.1113/jphysiol.2010.197517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lucas RJ, et al. Measuring and using light in the melanopsin age. Trends Neurosci. 2014;37:1–9. doi: 10.1016/j.tins.2013.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rahman SA, et al. Diurnal spectral sensitivity of the acute alerting effects of light. Sleep. 2014;37:271–281. doi: 10.5665/sleep.3396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.St Hilaire MA, Gronfier C, Zeitzer JM, Klerman EB. A physiologically-based mathematical model of melatonin including ocular light suppression and interactions with the circadian pacemaker. J Pineal Res. 2007;43:294–304. doi: 10.1111/j.1600-079X.2007.00477.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Brown EN, Choe Y, Shanahan TL, Czeisler CA. A mathematical model of diurnal variations in human plasma melatonin levels. Am J Physiol. 1997;272:E506–E516. doi: 10.1152/ajpcell.1997.272.3.C937. [DOI] [PubMed] [Google Scholar]
- 47.Rahman SA, et al. Functional decoupling of melatonin suppression and circadian phase resetting in humans. J Physiol. 2018;596:2147–2157. doi: 10.1113/JP275501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wright KP, Jr., Myers BL, Plenzler SC, Drake CL, Badia P. Acute effects of bright light and caffeine on nighttime melatonin and temperature levels in women taking and not taking oral contraceptives. Brain Res. 2000;873:310–317. doi: 10.1016/S0006-8993(00)02557-9. [DOI] [PubMed] [Google Scholar]
- 49.Gooley JJ, et al. Spectral responses of the human circadian system depend on the irradiance and duration of exposure to light. Sci Transl Med. 2010;2:31ra33. doi: 10.1126/scitranslmed.3000741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gronfier C, et al. Pulsatile cortisol secretion and EEG delta waves are controlled by two independent but synchronized generators. Am J Physiol. 1998;275:E94–E100. doi: 10.1152/ajpendo.1998.275.1.E94. [DOI] [PubMed] [Google Scholar]
- 51.Kalsbeek A, van Heerikhuize JJ, Wortel J, Buijs RM. A diurnal rhythm of stimulatory input to the hypothalamo-pituitary-adrenal system as revealed by timed intrahypothalamic administration of the vasopressin V1 antagonist. J Neurosci. 1996;16:5555–5565. doi: 10.1523/JNEUROSCI.16-17-05555.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Oster H, et al. The circadian rhythm of glucocorticoids is regulated by a gating mechanism residing in the adrenal cortical clock. Cell Metab. 2006;4:163–173. doi: 10.1016/j.cmet.2006.07.002. [DOI] [PubMed] [Google Scholar]
- 53.Kiessling S, Sollars PJ, Pickard GE. Light stimulates the mouse adrenal through a retinohypothalamic pathway independent of an effect on the clock in the suprachiasmatic nucleus. PLoS One. 2014;9:e92959. doi: 10.1371/journal.pone.0092959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rahman SA, Kollara A, Brown TJ, Casper RF. Selectively filtering short wavelengths attenuates the disruptive effects of nocturnal light on endocrine and molecular circadian phase markers in rats. Endocrinology. 2008;149:6125–6135. doi: 10.1210/en.2007-1742. [DOI] [PubMed] [Google Scholar]
- 55.Brandenberger G, Follenius M. Influence of timing and intensity of muscular exercise on temporal patterns of plasma cortisol levels. J Clin Endocrinol.Metab. 1975;40:845–849. doi: 10.1210/jcem-40-5-845. [DOI] [PubMed] [Google Scholar]
- 56.Follenius M, Brandenberger G, Hietter B. Diurnal cortisol peaks and their relationships to meals. J Clin Endocrinol Metab. 1982;55:757–761. doi: 10.1210/jcem-55-4-757. [DOI] [PubMed] [Google Scholar]
- 57.Brandenberger G, Follenius M, Muzet A. Interactions between spontaneous and provoked cortisol secretory episodes in man. J Clin Endocrinol Metab. 1984;59:406–411. doi: 10.1210/jcem-59-3-406. [DOI] [PubMed] [Google Scholar]
- 58.Follenius M, Brandenberger G, Oyono S, Candas V. Cortisol as a sensitive index of heat-intolerance. Physiol Behav. 1982;29:509–513. doi: 10.1016/0031-9384(82)90274-8. [DOI] [PubMed] [Google Scholar]
- 59.Scheer FAJL, van Doornen LJP, Buijs RM. Light and diurnal cycle affect human heart rate: possible role for the circadian pacemaker. J Biol Rhythms. 1999;14:202–212. doi: 10.1177/074873099129000614. [DOI] [PubMed] [Google Scholar]
- 60.Wright KP, Jr., et al. Entrainment of the human circadian clock to the natural light-dark cycle. Curr Biol. 2013;23:1554–1558. doi: 10.1016/j.cub.2013.06.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.National Sleep Foundation. Annual Sleep in America Poll Exploring Connections with Communications Technology Use and Sleep. (National Sleep Foundation, Washington, DC, 2011).
- 62.Cajochen C, Zeitzer JM, Czeisler CA, Dijk DJ. Dose-response relationship for light intensity and ocular and electroencephalographic correlates of human alertness. Behav Brain Res. 2000;115:75–83. doi: 10.1016/S0166-4328(00)00236-9. [DOI] [PubMed] [Google Scholar]
- 63.Gooley JJ, et al. Exposure to Room Light before Bedtime Suppresses Melatonin Onset and Shortens Melatonin Duration in Humans. J Clin Endocrinol Metab. 2011;96:E463–E472. doi: 10.1210/jc.2010-2098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Chellappa SL, et al. Non-visual effects of light on melatonin, alertness and cognitive performance: can blue-enriched light keep us alert? PLoS One. 2011;6:e16429. doi: 10.1371/journal.pone.0016429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.West KE, et al. Blue light from light-emitting diodes elicits a dose-dependent suppression of melatonin in humans. J Appl Physiol. 2011;110:619–626. doi: 10.1152/japplphysiol.01413.2009. [DOI] [PubMed] [Google Scholar]
- 66.Cajochen C, et al. Evening exposure to a light-emitting diodes (LED)-backlit computer screen affects circadian physiology and cognitive performance. J Appl Physiol. 2011;110:1432–1438. doi: 10.1152/japplphysiol.00165.2011. [DOI] [PubMed] [Google Scholar]
- 67.van der Lely S, et al. Blue Blocker Glasses as a Countermeasure for Alerting Effects of Evening Light-Emitting Diode Screen Exposure in Male Teenagers. J Adolesc Health. 2014 doi: 10.1016/j.jadohealth.2014.08.002. [DOI] [PubMed] [Google Scholar]
- 68.Zeitzer JM, Ruby NF, Fisicaro RA, Heller HC. Response of the human circadian system to millisecond flashes of light. PLoS One. 2011;6:e22078. doi: 10.1371/journal.pone.0022078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zeitzer JM, Fisicaro RA, Ruby NF, Heller HC. Millisecond flashes of light phase delay the human circadian clock during sleep. J Biol Rhythms. 2014;29:370–376. doi: 10.1177/0748730414546532. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.