Abstract
The use of immunomodulatory and immunosuppressive therapies in multiple sclerosis (MS) has allowed practitioners to regulate MS disease activity, with the caveat that these potent medications may render patients susceptible to opportunistic infections. The approval of fingolimod presented the first oral option for relapsing MS. Since 2015, postmarketing safety data have documented several published cases of cryptococcal meningitis and disseminated cryptococcosis associated with fingolimod use. However, surveillance mechanisms for opportunistic infections and management of active demyelinating disease with ongoing infection have not been adequately addressed. We present a case of isolated pulmonary cryptococcosis with the use of fingolimod to highlight the hurdles in balancing efficacious disease-modifying therapies for MS while treating an opportunistic infection associated with that therapy.
Keywords: Cryptococcus, Fingolimod, Multidisciplinary, Multiple sclerosis (MS), Opportunistic infection
Fingolimod was approved for the treatment of relapsing multiple sclerosis (MS) in 2010 and marketed as the first effective disease-modifying oral alternative to injection therapy.1 It is a sphingosine 1-phosphate receptor modulator, promoting receptor internalization and impairing egress of peripheral T and B cells from secondary lymphoid tissue into blood, thereby reducing access to the central nervous system (CNS). Receptor modulation leads to a redistribution of T cells to lymph nodes, with some studies suggesting neuroprotective effects.2 Although peripheral lymphopenia was observed in on-drug study participants versus the placebo group, increased incidence of infection was not reported in participants enrolled in the initial multisite randomized phase 3 controlled trials.2,3 However, postmarketing surveillance suggests that the prolonged use of fingolimod may present a greater risk of several different opportunistic infections, including cryptococcosis.4,5 The incidence of these pathogens could call into question the effectiveness of cellular immunity after fingolimod exposure, although earlier studies suggested that only T-cell trafficking was affected.6 Cryptococcus can be acquired through inhalation of spores and requires intact cellular immunity to prevent infection and dissemination. T-cell–mediated immunity in particular is critical in granuloma formation and containment of this pathogen.7 Guidelines on balancing immunomodulatory therapy to treat relapsing MS with treatment of such opportunistic infections have not yet been established.
Case Description
A 45-year-old man with relapsing-remitting MS diagnosed in 2001 presented to our institution in 2017 for enlarging pulmonary lymph nodes of unclear etiology. His social history was significant for work on an army base where he was exposed to dust and pigeon droppings on vehicles that he repaired. The lymph node enlargement was noted at an outside hospital 5 months earlier by serial computed tomography (CT) of the chest, originally performed due to worsening odynophagia. His MS history included initial right optic neuritis at age 28 years, with loss of peripheral vision followed by incomplete resolution after corticosteroids were given at an outside hospital. One year later he had another neurologic attack with bilateral upper extremity paresthesia. The diagnosis of MS was made based on history and examination, with supporting imaging characteristics and cerebrospinal fluid (CSF) analysis. He was initially started on interferon beta-1a, but this was discontinued after an unremitting flu-like syndrome. He was switched to interferon beta-1b, which he took for 4 years, but then he self-discontinued his medication and was lost to follow-up for several years before he began using fingolimod, prescribed by an outside neurologist.
Before presentation at our institution, the patient developed progressive dry cough and pain while swallowing, with no new neurologic symptoms. A chest CT revealed multiple pulmonary nodules (Figure 1). Several months later, repeated chest CT showed growth of the largest nodule, triggering an evaluation for malignancy. After a failed bronchoscopy attempt, the patient opted for further investigation at our institution. He was first seen by infectious disease specialists at our institution 3 years after fingolimod initiation and was admitted to the hospital, during which time he underwent a bronchoscopy with bronchoalveolar lavage and distal airway evaluation. During hospitalization, he was afebrile, with normal hepatic and kidney function and a normal white blood cell count of 47,000/mm3, with a low absolute lymphocyte count of 680/μL (reference range, 800–3300/μL). Bronchoalveolar lavage cell count, bacterial culture, viral smears, and fungal and acid-fast bacilli analysis and culture were performed. Serum cryptococcus and aspergillus antigens, along with histoplasmosis, blastomyces antibodies, and urine histoplasma antigen were evaluated. His test results were positive for the cryptococcus antigen in serum (1:80 titer) with rare Cryptococcus neoformans in fungal culture from bronchoalveolar lavage. Results of acid-fast bacilli, bacterial, and viral testing were negative. Histoplasmosis antibodies in serum were positive, with a 1:8 titer and negative urine histoplasma antigen. The CSF studies were unrevealing, with no nucleated cells, a glucose level of 55 mg/dL, and a mildly elevated protein level of 66 mg/dL. The CSF fungal culture was negative for Cryptococcus neoformans or Cryptococcus gattii antigen.
Figure 1.
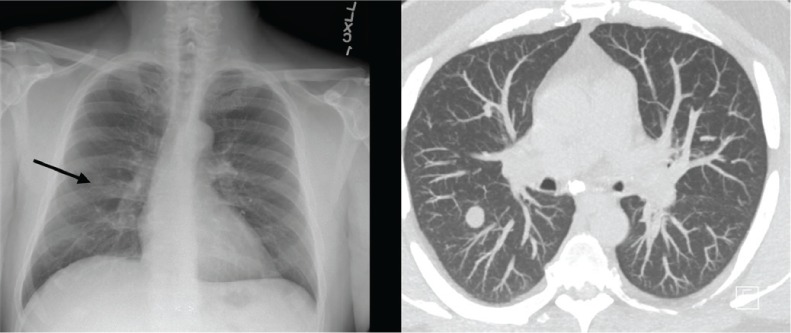
Chest radiograph and chest computed tomographic scan showing multiple pulmonary nodules
Fingolimod therapy was discontinued. Given the lack of evidence of cryptococcal fungus in the CNS, the patient was administered oral fluconazole 800 mg daily, which was decreased to 400 mg daily after 2 weeks. At that point, the entire evaluation had been performed by practitioners outside of neurology. The patient was then referred to the John L. Trotter MS Center (St. Louis, MO) to verify the MS diagnosis and to determine whether continued treatment with a disease-modifying therapy (DMT) was needed.
A complete neurologic history was obtained, confirming multiple previous demyelinating events. Neurologic examination was notable for residual right internuclear ophthalmoplegia and central scotoma, without weakness, sensory deficits, or ataxia. Brain magnetic resonance imaging (MRI) before fingolimod discontinuation demonstrated multiple ovoid, periventricular, and cortical/juxtacortical lesions, including at least one new lesion (Figure 2 and Figure S1 (83.7KB, pdf) , which is published in the online version of this article at ijmsc.org).
Figure 2.

Brain magnetic resonance image at diagnosis of pulmonary cryptococcosis
T2-weighted/fluid-attenuated inversion recovery periventricular hyperintensities typical of demyelination.
The patient's history and neurologic examination and brain MRI findings were compatible with the diagnosis of MS, and treatment with an MS DMT was deemed to be appropriate. In the setting of an active fungal infection, we opted to use an MS medication with no suppressive effects on the immune system and no known risks of opportunistic infections that still decreased MS relapse rates.8,9 Given his previous adverse effects with the beta-interferons, glatiramer acetate was selected. Other oral therapies, although preferred by the patient, all had risk of immunosuppression, posing potential risk for a person with an active infection.10
At 3-month follow-up on glatiramer acetate, he reported sensory symptoms, thought to be a clinical relapse. An MRI showed new nonenhancing MS lesions in the cerebrum and brainstem (Figure 3 and Figure S2 (83.7KB, pdf) ). Concurrent chest CT showed a decrease in pulmonary nodules despite ongoing cough and sputum production. Priority was given to optimizing MS therapy as the pulmonary cryptococcus seemed to be resolving. Taking into consideration his breakthrough demyelinating disease, the need for higher-efficacy DMT with a different mechanism of action, yet still not strongly immunosuppressive, was prioritized. Dimethyl fumarate therapy was initiated. Three months after starting dimethyl fumarate the patient reported being at his neurologic baseline. At 6 months, he had no new symptoms, and neurologic examination findings were improved; the only findings were old findings of right eye decreased vision with afferent pupillary defect and left upgoing toe. The MRIs of the neuroaxis showed no new lesions. Chest CT showed mild improvement. The patient continues to take dimethyl fumarate.
Figure 3.
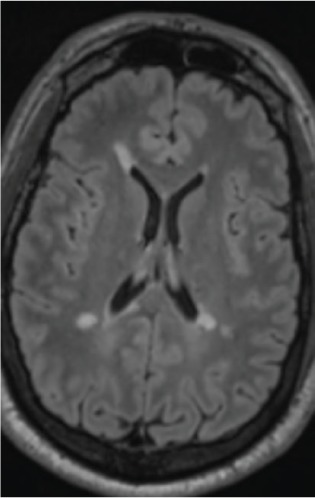
Brain magnetic resonance image 3 months after discontinuing fingolimod use, initiating glatiramer acetate therapy, and reporting clinical relapse in interim
New T2-weighted/fluid-attenuated inversion recovery nonenhancing periventricular lesion.
Discussion
Disease-modifying agents are critical for effective management of relapsing MS and for reducing long-term disability in most patients.11–13 However, some agents come with a risk of opportunistic, and at times life-threatening, infections. As DMTs become fixtures in the management of MS, and patients continue taking them for longer durations, managing the consequences of immune system alteration while controlling disease activity has become an additional challenge for neurologists.
To date, several cases of cryptococcal infection have been reported in patients using fingolimod (Table 1).1,6,14–19 These cases included CNS cryptococcosis as well as other sites of dissemination, but none described isolated pulmonary involvement. Due to the emergence of these earlier cases, the prescribing information for fingolimod was changed (May 2018) to include its association with cryptococcal infection, with higher risk after 2 years of therapy. Perhaps the present patient was also more susceptible to cryptococcosis due to his exposure to dust and pigeon droppings at his place of work. Based on characteristics of the published cases, clinicians should also have a low threshold for evaluation of cryptococcosis infection in those aged 45 years or older. Duration of therapy, immune system senescence, lymphopenia, or some combination of these factors may all contribute to the development of this fungal disease.11 We are limited by the range of data collected on each case in the literature, and, therefore, it is challenging to identify which factors are most consequential to the development of cryptococcosis with fingolimod use. Given its rarity in those treated with fingolimod, the presence of cryptococcosis may not be immediately considered. This brings up the possible role of surveillance in identifying opportunistic infections. In certain cases, cryptococcosis may mimic MS exacerbations, delaying antifungal therapy or unnecessarily advancing DMT and perpetuating immunosuppression.4 Because cryptococcosis is a pulmonary-related infection, performing a baseline chest radiograph on fingolimod initiation or after age 40 years, checking CSF cryptococcal antigen if an atypical lesion is seen on systemic or brain imaging, and measuring CD4 levels are some measures to be considered. Performing screening mechanisms that can identify this fungal infection in the preliminary stages may allow for prompt treatment of the infection and minimize the time off DMT.
Table 1.
Currently reported cases of cryptococcal infections in patients with MS who used fingolimod
| Case report | Age at onset of infection, y | Sex | MS durationa at time of diagnosis, y | Fingolimod exposure | Compatible clinical features | CD4 count, / μL | Lymphocyte count, /μL |
|---|---|---|---|---|---|---|---|
| Present case | 45 | M | 16 | 3 y | Pain and difficulty with swallowing | — | 680 |
| Anene-Maidoh et al,17 2018 | 61 | F | 10 | 4 y 10 mo | Severe headaches, confusion, worsening gait, and seizures | 69 (23%) | 321 |
| Pham et al,18 2017 | 61 | F | 10 | 3 y | Neck pain, headache, nausea, vertigo, and weight loss | — | 120 |
| Seto et al,14 2016 | 63 | M | 30 | 2 y | Cutaneous nodular lesion of his jaw, lethargy, progressive cognitive impairment, and low-grade fever | 145 | 300 |
| Ward et al,16 2016 | 67 | F | — | 4 yb | Dysarthria, diplopia, vomiting, and confusion | — | 2390 (60–800 while on fingolimod) |
| Grebenciucova et al,6 2016 | 62 | M | — | 3 y | Headache, dizziness, confusion | — | 336 |
| Forrestel et al,15 2016 | 62 | F | — | 3 y | Tender forehead nodule | 56 | 650 |
| Achtnichts et al,1 2015 | 40 | M | 3 | 2 y | Throbbing bilateral, retro-orbital, and temporal headache, photophobia, and lethargy | 56 | 400 |
| Huang19 2015 | 50 | M | — | 3 y 3 mo | Open left cheek lesion, headache, neck pain | — | 500 |
Abbreviation: MS, multiple sclerosis.
aMS duration indicates time since MS diagnosis when cryptococcal infection was diagnosed.
bSymptoms occurred 6 to 8 weeks after fingolimod discontinuation.
Herein we reported a method to manage active demyelinating disease with an ongoing opportunistic infection while highlighting the challenges we faced. Determining which DMT should replace fingolimod, identifying appropriate follow-up and repeated imaging periods, and selecting another DMT when an MS relapse was identified are potential areas for further studies. Throughout his course, the present patient's therapy was prioritized based on the current primary health concern—whether it be infectious or MS activity.20 A multidisciplinary approach involving specialists in neurology and infectious disease may become more important as increasing numbers of patients with MS continue taking immune-altering therapies for prolonged periods and unusual or opportunistic infections arise. In the case of cryptococcal infections, consultation with infectious disease specialists has been shown to dramatically decrease mortality.21
On retrospective evaluation of the present patient, transition from one therapy to another was based on ongoing concerns of CNS involvement of fungal disease versus MS relapse. It is likely that 3 months of glatiramer acetate therapy was not enough time to allow for effective therapeutic coverage. Nonetheless, new symptoms during that time instigated a radiologic investigation and switch of DMT. An MRI at 6 months may have been a more appropriate time to start surveillance for breakthrough disease; however, with the ongoing concern for fungal disease spreading to the CNS, earlier imaging was obtained. When choosing therapy after glatiramer acetate use, we considered the need to use an effective DMT yet still maintain enough immune integrity to continue to resolve the cryptococcal disease. Both teriflunomide and dimethyl fumarate were discussed; however, with the lack of head-to-head trials, direct comparison of treatments is not possible. Either may have been a reasonable choice.11,12
Due to the recent approvals and relatively short periods of continuous treatment with immune system–altering therapies in patients with MS, current experience may not reflect the full extent of infections that may be encountered on longer-term exposure to new DMTs. Therefore, it is increasingly important that neurologists maintain an awareness of the potential for DMT-related infections and begin to develop evidence-based surveillance strategies and management guidelines. The unique features of this case do not allow generalization for management but rather represent challenges that may need to be overcome for improved patient care. Future studies should identify risk factors, including whether a threshold level of lymphocytes or lymphocyte subsets (eg, CD4 T cells or other T-cell subsets) correlate with the development of cryptococcosis in patients taking fingolimod.11 Remaining aware of specific infections associated with specific DMTs, and vigilance for symptoms that would be atypical for MS, is recommended to improve patient outcomes by early identification of opportunistic infections. Management of opportunistic disease will generally require a multidisciplinary approach to optimize outcomes for infection and MS.
PRACTICE POINTS
A case of isolated pulmonary cryptococcosis in a patient with MS taking fingolimod is presented. By working with an infectious disease team, the need to treat the MS disease process was balanced with the need to treat the fungal infection.
Guidelines for surveillance for opportunistic infections are increasingly needed as more patients are treated with disease-modifying therapies for longer durations and at older ages.
Guidelines for the concurrent treatment of MS disease activity and infections, especially opportunistic infections, are needed. A multidisciplinary approach is recommended.
Supplementary Material
Acknowledgments
We thank Dr. David Clifford, Melba and Forest Seay Professor of Clinical Neuropharmacology in Neurology, Washington University in St. Louis, who provided support, insight, and expertise on this challenging case.
Financial Disclosures
Dr. Samudralwar reports receiving honoraria from Sanofi Genzyme (consultant) and Biogen (speakers' bureau). Dr. Cross reports receiving honoraria as a consultant for Biogen, Celgene, EMD Serono, Genentech, Genzyme, Novartis, and TG Therapeutics. Dr. Spec declares no conflicts of interest.
Funding/Support
Dr. Cross was supported in part by the Manny and Rosalyn Rosenthal–Dr. John L. Trotter MS Center Chair in Neuroimmunology of the Barnes-Jewish Hospital Foundation.
Prior Presentation
Aspects of this study were presented in abstract form at the Annual Meeting of the Consortium of Multiple Sclerosis Centers (CMSC); May 30–June 2, 2018; Nashville, TN, USA.
References
- 1.Achtnichts L, Obreja O, Conen A, Fux CA, Nedeltchev K. Cryptococcal meningoencephalitis in a patient with multiple sclerosis treated with fingolimod. JAMA Neurol. 2015;72:1203–1205. doi: 10.1001/jamaneurol.2015.1746. [DOI] [PubMed] [Google Scholar]
- 2.Cohen JA, Barkhof F, Comi G et al. Oral fingolimod or intramuscular interferon for relapsing multiple sclerosis. N Engl J Med. 2010;362:402–415. doi: 10.1056/NEJMoa0907839. [DOI] [PubMed] [Google Scholar]
- 3.Kappos L, Radue E-W, O'Connor P et al. A placebo-controlled trial of oral fingolimod in relapsing multiple sclerosis. N Engl J Med. 2010;362:387–401. doi: 10.1056/NEJMoa0909494. [DOI] [PubMed] [Google Scholar]
- 4.Arvin AM, Wolinsky JS, Kappos L et al. Varicella-zoster virus infections in patients treated with fingolimod: risk assessment and consensus recommendations for management. JAMA Neurol. 2015;72:31–39. doi: 10.1001/jamaneurol.2014.3065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Berger JR, Cree BA, Greenberg B et al. Progressive multifocal leukoencephalopathy after fingolimod treatment. Neurology. 2018;90:e1815–e1821. doi: 10.1212/WNL.0000000000005529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Grebenciucova E, Reder AT, Bernard JT. Immunologic mechanisms of fingolimod and the role of immunosenescence in the risk of cryptococcal infection: a case report and review of literature. Mult Scler Relat Disord. 2016;9:158–162. doi: 10.1016/j.msard.2016.07.015. [DOI] [PubMed] [Google Scholar]
- 7.Spec A, Powderly WG. Cryptococcal meningitis in AIDS. Handb Clin Neurol. 2018;152:139–150. doi: 10.1016/B978-0-444-63849-6.00011-6. [DOI] [PubMed] [Google Scholar]
- 8.Wijnands JMA, Zhu F, Kingwell E et al. Disease-modifying drugs for multiple sclerosis and infection risk: a cohort study. J Neurol Neurosurg Psychiatry. 2018;89:1050–1056. doi: 10.1136/jnnp-2017-317493. [DOI] [PubMed] [Google Scholar]
- 9.Wolinsky JS, Borresen TE, Dietrich DW et al. GLACIER: an open-label, randomized, multicenter study to assess the safety and tolerability of glatiramer acetate 40 mg three-times weekly versus 20 mg daily in patients with relapsing-remitting multiple sclerosis. Mult Scler Relat Disord. 2015;4:370–376. doi: 10.1016/j.msard.2015.06.005. [DOI] [PubMed] [Google Scholar]
- 10.Vargas DL, Tyor WR. Update on disease-modifying therapies for multiple sclerosis. J Investig Med. 2017;65:883–891. doi: 10.1136/jim-2016-000339. [DOI] [PubMed] [Google Scholar]
- 11.Boster A, Nicholas J, Wu N et al. Comparative effectiveness research of disease-modifying therapies for the management of multiple sclerosis: analysis of a large health insurance claims database. Neurol Ther. 2017;6:91–102. doi: 10.1007/s40120-017-0064-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fox RJ, Chan A, Zhang A et al. Comparative effectiveness using a matching-adjusted indirect comparison between delayed-release dimethyl fumarate and fingolimod for the treatment of multiple sclerosis. Curr Med Res Opin. 2017;33:175–183. doi: 10.1080/03007995.2016.1248380. [DOI] [PubMed] [Google Scholar]
- 13.Rae-Grant A, Day GS, Marrie RA et al. Practice guideline recommendations summary: disease-modifying therapies for adults with multiple sclerosis: report of the Guideline Development, Dissemination, and Implementation Subcommittee of the American Academy of Neurology. Neurology. 2018;90:777–788. doi: 10.1212/WNL.0000000000005347. [DOI] [PubMed] [Google Scholar]
- 14.Seto H, Nishimura M, Minamiji K et al. Disseminated cryptococcosis in a 63-year-old patient with multiple sclerosis treated with fingolimod. Intern Med. 2016;55:3383–3386. doi: 10.2169/internalmedicine.55.7255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Forrestel AK, Modi BG, Longworth S, Wilck MB, Micheletti RG. Primary cutaneous cryptococcus in a patient with multiple sclerosis treated with fingolimod. JAMA Neurol. 2016;73:355–356. doi: 10.1001/jamaneurol.2015.4259. [DOI] [PubMed] [Google Scholar]
- 16.Ward MD, Jones DE, Goldman MD. Cryptococcal meningitis after fingolimod discontinuation in a patient with multiple sclerosis. Mult Scler Relat Disord. 2016;9:47–49. doi: 10.1016/j.msard.2016.06.007. [DOI] [PubMed] [Google Scholar]
- 17.Anene-Maidoh TI, Paschall RM, Scott Graham R. Refractory cryptococcal meningoencephalitis in a patient with multiple sclerosis treated with fingolimod: a case report. Interdisciplinary Neurosurgery. 2018;12:8–9. [Google Scholar]
- 18.Pham C, Bennett I, Jithoo R. Cryptococcal meningitis causing obstructive hydrocephalus in a patient on fingolimod. BMJ Case Rep. 2017;2017 doi: 10.1136/bcr-2017-220026. bcr-2017-220026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huang D. Disseminated cryptococcosis in a patient with multiple sclerosis treated with fingolimod. Neurology. 2015;85:1001–1003. doi: 10.1212/WNL.0000000000001929. [DOI] [PubMed] [Google Scholar]
- 20.Members of the MS in the 21st Century Steering Group. Rieckmann P, Centonze D, Elovaara I et al. Unmet needs, burden of treatment, and patient engagement in multiple sclerosis: a combined perspective from the MS in the 21st Century Steering Group. Mult Scler Relat Disord. 2018;19:153–160. doi: 10.1016/j.msard.2017.11.013. [DOI] [PubMed] [Google Scholar]
- 21.Spec A, Olsen MA, Raval K, Powderly WG. Impact of infectious diseases consultation on mortality of cryptococcal infection in patients without HIV. Clin Infect Dis. 2017;64:558–564. doi: 10.1093/cid/ciw786. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


