Abstract
MicroRNAs are post-transcriptional regulators of gene expression, crucial for neuronal differentiation, survival, and activity. Age-related dysregulation of microRNA biogenesis increases neuronal vulnerability to cellular stress and may contribute to the development and progression of neurodegenerative diseases. All major neurodegenerative disorders are also associated with oxidative stress, which is widely recognized as a potential target for protective therapies. Albeit often considered separately, microRNA networks and oxidative stress are inextricably entwined in neurodegenerative processes. Oxidative stress affects expression levels of multiple microRNAs and, conversely, microRNAs regulate many genes involved in an oxidative stress response. Both oxidative stress and microRNA regulatory networks also influence other processes linked to neurodegeneration, such as mitochondrial dysfunction, deregulation of proteostasis, and increased neuroinflammation, which ultimately lead to neuronal death. Modulating the levels of a relatively small number of microRNAs may therefore alleviate pathological oxidative damage and have neuroprotective activity. Here, we review the role of individual microRNAs in oxidative stress and related pathways in four neurodegenerative conditions: Alzheimer’s (AD), Parkinson’s (PD), Huntington’s (HD) disease, and amyotrophic lateral sclerosis (ALS). We also discuss the problems associated with the use of oversimplified cellular models and highlight perspectives of studying microRNA regulation and oxidative stress in human stem cell-derived neurons.
Keywords: microRNA, oxidative stress, ROS, translation regulation, neurodegeneration, Alzheimer’s disease, Parkinson’s disease, Huntington’s disease, ALS
1. Introduction
Neurodegenerative diseases, such as Alzheimer’s (AD), Parkinson’s (PD), Huntington’s (HD) disease, and Amyotrophic Lateral Sclerosis (ALS), are devastating and currently incurable conditions causing severe cognitive and/or motor impairments predominantly in aged people [1,2]. The incidence of age-related neurodegeneration is expected to increase due to aging population and increased life expectancy in the developed countries. Alzheimer’s disease (AD) and other dementias are estimated to affect up to 50 million people worldwide [3]. Another 10 million patients are suffering from Parkinson’s disease (PD), which occurs in ≈2% of people over 70 years of age [4]. To develop curative therapies for neurodegenerative diseases, it is crucial to elucidate molecular mechanisms regulating neuron survival and degeneration.
Oxidative stress has been implicated in predisposing neurons to death either directly or indirectly as a consequence of mitochondrial dysfunction, pathological protein aggregation, specific neurotransmitter (dopamine) metabolism, inflammation, or deregulation of antioxidant pathways [5,6,7,8,9,10]. The brain is particularly susceptible to oxidative stress due to high oxygen consumption (reflecting high ATP demand) and the reliance on mitochondrial activity, intracellular calcium, and a relatively weak endogenous antioxidant defense, among other reasons [11,12]. Reactive oxygen species (ROS) cause oxidative damage to proteins, lipids, and nucleic acids, compromising critical cellular functions and activating cell death pathways [13]. Oxidative stress and oxidative damage are commonly observed in different neurodegenerative diseases and, therefore, therapies aiming to reduce cellular ROS levels may offer neuroprotective treatments for multiple neurodegenerative conditions. However, attempts to treat neurodegenerative diseases with antioxidant drugs have mostly been unsuccessful, in part, due to insufficient blood–brain barrier penetration, short treatment duration, or incorrect timing of therapy application [13,14,15]. Alternative therapeutic interventions may aim to counteract oxidative damage by stimulating endogenous neuronal antioxidant defense pathways [16]. In this review, we explore the concept of targeting specific microRNAs regulating or regulated by these pathways as a strategy to protect neurons in neurodegenerative diseases.
MicroRNAs are short regulatory RNA molecules which affect translation and stability of their mRNA targets by guiding RNA-induced silencing complex (RISC) predominantly to 3′ untranslated region (UTR) [17,18]. MicroRNAs are predicted to regulate the activity of about a half of all protein coding genes, reducing fluctuations in protein expression [19,20].
MicroRNAs are expressed as precursor hairpins which undergo sequential processing in the nucleus and cytoplasm by specific protein complexes containing ribonucleases Drosha and Dicer; mature functional microRNAs are then loaded to Argonaute family protein Ago2, a central component of the RISC complex [18]. MicroRNAs are critical for neuronal functions both during development and in the adult brain [21,22]. Loss of mature microRNA functions by genetic deletion of Dicer or Ago2 is embryonic lethal [23,24], whereas deletion of Dicer during embryogenesis severely impairs neuronal development [21,25,26,27,28,29]. Inducible deletion of Dicer in postnatal Purkinje cells and in adult forebrain and dopamine neurons causes their progressive loss and severe behavioral phenotypes [30,31,32,33,34]. While some neuronal populations survive Dicer deletion, their functions are clearly affected [35,36,37,38]. Similarly, loss of Ago2 in adult neurons is dispensable for their survival, but it nevertheless affects neuronal functions resulting in a behavioral phenotype [39]. MicroRNAs have been implicated in modulation of neuronal signaling by regulating neuronal excitability, dendritogenesis, local translation in dendritic spines, and neurotransmitter release [21,40,41,42]. Age and disease-related downregulation of the microRNA biogenesis pathway in adult neurons can lead to changes in their survival, functions, and connectivity. Inhibition of Dicer activity and resulting changes in microRNA expression levels have been observed in aging and in neurological and neurodegenerative diseases [30,42,43,44,45,46,47,48,49,50]. Deregulation of microRNA biogenesis is causing cellular stress and, vice versa, increased stress causes deregulation of microRNA biogenesis, creating a vicious cycle leading to eventual cell death [51,52,53]. In line with this hypothesis, stimulation of microRNA biogenesis is neuroprotective in mouse models of ALS and PD [30,52,54].
A small number of microRNAs can regulate hundreds of transcripts and may enable a crosstalk between different cellular pathways [55,56]. For example, several microRNAs can be targeting many genes involved in antioxidant defense pathways [53]. Modulating the levels of a relatively small number of microRNAs which regulate the oxidative stress response in neurons may therefore alleviate pathological oxidative damage and have neuroprotective activity. However, it is not trivial to identify such microRNAs and their target genes in aged and degenerating neuronal populations. Below, we review the current literature addressing the interplay between oxidative stress and microRNAs in major neurodegenerative diseases.
2. Alzheimer’s Disease
Dementia is estimated to affect more than 50 million people worldwide with the prognosis of doubling in the next 20 years [57]. AD, which is an irreversible neurodegenerative disorder affecting both cognition and emotional behavior of affected persons (usually at the age of 65 and older) [58], is considered to be the cause of 50–75% of all dementia cases with no effective treatment to stop or slow down the disease [59,60].
Despite intensive studies, the real causes of AD development are still not clear. Extracellular accumulation of amyloid-β (Aβ) peptides and hyperphosphorylation of the microtubule-associated Tau protein are the main hallmarks of AD development at the molecular and cellular level leading to the accumulation of senile plagues and neurofibrillary tangles, respectively. Mutations in PSEN1, PSEN2, APP genes, variants of APOE gene, and posttranscriptional modifications of AD-associated proteins can also contribute to the development of this neurodegenerative disease. Taken together, these changes result in synaptic loss, neuronal cell death, and cognitive impairment reviewed in [61,62].
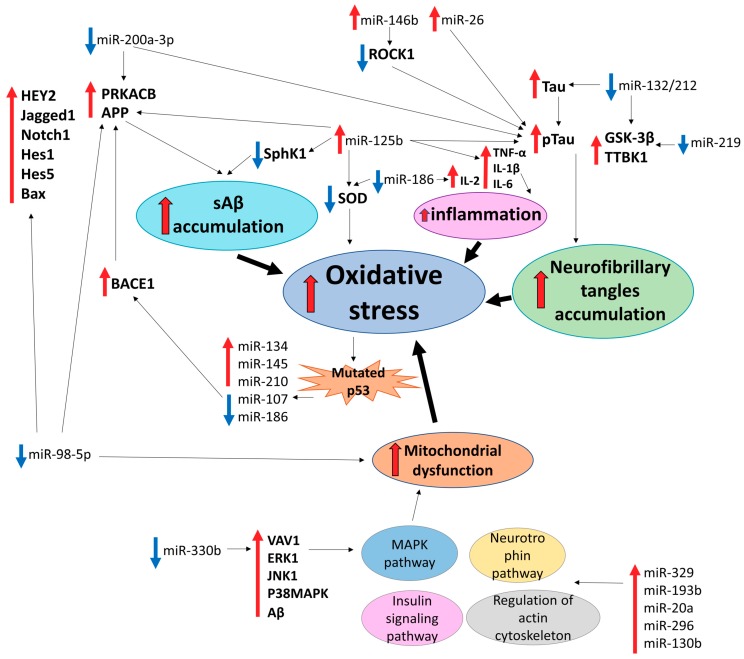
According to numerous studies, microRNA contribute to the development of AD regulating accumulation of Aβ peptides and Tau phosphorylation [63,64,65,66,67,68]. However, accumulation of insoluble protein aggregates is not the only, and, possibly, not the main pathological process driving AD progression. Oxidative stress is of particular importance for AD development as it causes chronic inflammation at the early stages of neurodegeneration, which leads to mitochondrial dysfunction, oxidative damage of nucleic acids, changes in genes expression, and abnormal modifications of lipids and proteins [69]. Oxidative stress causes both up- and downregulation of different microRNAs and, conversely, many microRNAs can regulate oxidative stress response [70] (Figure 1).
Figure 1.
MicroRNAs implicated in oxidative stress-related cellular pathways in Alzheimer’s disease.
Li et al. demonstrated that soluble Aβ peptides (sAβ) known to generate ROS [71] reliably induced expression of miR-134, miR-145 and miR-210. In the same study, expression of miR-107 was markedly reduced, supporting a bilateral effect of sAβ-induced ROS on microRNA expression [72]. Decreased levels of miR-107 is associated with early stages of AD progression. This microRNA directly targets BACE1 mRNA encoding β-secretase enzyme that processes APP to Aβ peptides [73]. In AD patients with the APOE4 genotype, decreased levels of miR-107 have been demonstrated along with the increased production of Aβ peptides. Accumulation of Aβ induced oxidative stress in APOE4 leads to the deregulation of the TP53 gene. In addition to its role in cancer, p53 protein (encoded by a TP53 gene) can be involved in cell death in AD patients with upregulation at the early stages of the disease and downregulation during neurodegeneration [74]. Previously, p53 mutations that may be associated with oxidative stress were observed in AD patients and AD animal models [75,76]. Since miR-107 is downregulated in cell lines with mutated p53 [77], p53 mutations and accumulation of Aβ may result in the decrease of miR-107 levels in AD patients. Moreover, 8-oxo-2′deoxyguanosine RNA modifications caused by oxidative stress can serve as an additional factor of decreasing miR-107 levels [78]. Levels of another microRNA, miR-186, are decreased through aging. This microRNA targets 3′UTR of BACE1 and is implicated in the mitigation of the oxidative stress effects in AD pathogenesis [79].
Another study revealed that the upregulation of miR-342-5p is important for neurogenesis and neuroprotection in an AD mouse model. Downregulation of Ankylin G, a direct target of miR-342-5p, results in AD axonopathy [80]. Liang et al. showed a decrease of miR-153 expression following sAβ treatment of M17 human neuroblastoma cells in combination with H2O2. APP and APLP2, an APP homologue, are confirmed as direct targets of miR-153, providing additional evidence of microRNA-based regulation of the essential stage of AD progression and the role of oxidative stress in this process [81].
Phosphorylation of Tau protein followed by the accumulation of neurofibrillary tangles is affected by the formation of ROS. Numerous studies confirmed the role of oxidative stress on Tau acetylation and subsequent phosphorylation by GSK-3 kinase or other pathways [82,83,84]. Several microRNAs also contribute to the regulation of Tau phosphorylation. MiR-200a-3p targets BACE1 and PRKACB (catalytic subunit of PKA), reducing Aβ accumulation and Tau hyperphosphorylation, respectively [85]. Li et al. identified overexpressed miR-219 in brains of AD patients. In the SH-SY5Y cell line, miR-219 downregulated Tau phosphorylation by targeting TTBK1 and GSK-3β [86]. GSK-3β alongside with Rbfox1, EP300, and Calpain 2 are directly targeted by miR-132/212, which are among the most downregulated microRNAs in AD [87]. Moreover, Tau mRNA is directly targeted by miR-132/212 [88]. In contrast to the abovementioned cases, overexpression of miR-146b in the AD brain induced abnormal Tau phosphorylation by targeting ROCK1 kinase [89]. Absalon et al. demonstrated a neuroprotective effect of sequence-specific inhibition of miR-26 in primary cortical neurons treated with H2O2. miR-26 is known to be upregulated in AD patients and contributes to Tau hyperphosphorylation and Aβ accumulation [90].
Screening of AD-associated microRNAs in H2O2-treated primary hippocampal neurons and a senescent mouse model demonstrated strong upregulation of miR-329, miR-193b, miR-20a, miR-296, and miR-130b. Expression of miR-329 played a critical role in the activity-dependent dendritic outgrowth of hippocampal neurons, whereas miR-130b expressed in the hippocampus was related to chronic stress-induced depression. miR-20a targeted neuronal differentiation markers BCL2, MEF2D and MAP3K12 (ZPK/MUK/DLK), suggesting its key role in the regulation of gene expression during brain development. According to KEGG analysis, upregulated microRNAs participated in the cellular processes closely connected to the occurrence and development of AD, in particular, neurotrophin signaling pathway, MAPK pathway, insulin signaling pathway, and regulation of actin cytoskeleton. This can indicate the importance of abovementioned microRNAs for the development of AD. [91]. miR-330a has also been reported to contribute to alleviation of oxidative stress and mitochondria dysfunction in AD by targeting mRNAs of VAV1, ERK1, JNK1, P38MAPK, and Aβ, which are all upregulated in AD mice, indicating the involvement of the MAPK pathway in AD [92].
The Notch pathway is among important cellular processes that can be associated with oxidative stress [93,94,95]. The Notch-HEY2 pathway in the hippocampal neurons of AD mice was activated with the downregulation of miR-98-5p compared to normal hippocampal neurons. APP correlated with levels of miR-98-5p, alongside HEY2, Jagged1, Notch1, Hes1, Hes5, and Bax genes of the Notch pathway, indicating the inhibitory effect of miR-98-5p on these genes and, thus, AD progression. Furthermore, miR-98-5p promoted the growth of hippocampal neurons, inhibited neuronal apoptosis, and improved oxidative stress and mitochondrial dysfunction of AD mice, whereas HEY2 was reported to have opposite effects. These results contradict previous data about promotion of Aβ production by miR-98-5p and upregulation of this microRNA in AD mouse models. Further studies would possibly clarify a more precise role of miR-98 in AD [96].
Despite being affected, microRNAs themselves can trigger oxidative stress in neurons promoting neurodegeneration. miR-125b is known as an important factor of AD progression, promoting APP, BACE1, and Tau overexpression and hyperphosphorylation [97]. In mouse neuroblastoma Neuro2a APPSwe/Δ9 cells, overexpression of miR-125b enhanced oxidative stress by decreasing levels of superoxide dismutase (SOD) together with the stimulation of apoptosis. Additionally, this microRNA stimulates overexpression of TNF-α, IL-1β, and IL-6 inflammatory cytokines, further supporting the connection between inflammation and oxidative stress in degeneration of neurons. Moreover, miR-125b significantly decreased expression of SphK1, which improves memory, learning, and suppresses formation of Aβ peptides [98]. The biological activities of IL2, another inflammatory cytokine, correlate with the JAK/STAT pathway involved in AD development by inducing astrocyte reactivity. A recent study by Wu et al. demonstrated the role of miR-186, a tumor suppressor microRNA, in the downregulation of IL2. Rat brains with decreased expression of miR-186 had been characterized by the elevated levels of IL2, JAK/STAT, Bax, and Cleaved-caspase 3 genes and ROS, whereas BCL2 and SOD activity were downregulated [99].
3. Parkinson’s Disease
PD is a common progressive neurodegenerative disorder. PD is primarily characterized by degeneration of dopamine neurons in the substantia nigra pars compacta (SNpc) and their projections to the corpus striatum. Dopamine neuron loss leads to manifestation of PD motor symptoms, such as bradykinesia, resting tremor, postural instability, and rigidity [6]. Additionally, PD patients exhibit a broad range of non-motor symptoms, such as depression, sleep disorders, and dementia. Many of them precede the appearance of motor symptoms and worsen with progression of PD [100].
PD is an age-related disorder, affecting approximately 1% of the population over 60 years old and this number reaches 4-5% in the population over 85-years old. Despite many years of research, mechanisms underlying the pathology of PD are still not well understood. Several genetic mutations associated with PD have been identified and account for at least 5–10% of PD cases; however, in most of the cases, the etiology of PD is unknown [101].
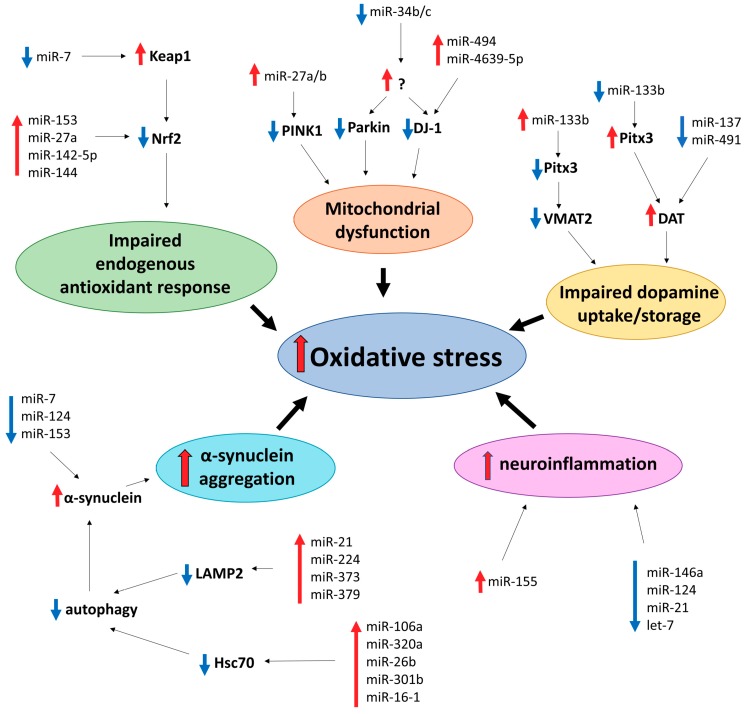
Many different mechanisms have been proposed to drive neuronal death in PD, including oxidative stress. Major sources of oxidative stress in dopamine neurons include dopamine metabolism, mitochondrial dysfunction, impairment of the endogenous antioxidant system, aggregation of the α-synuclein protein, and neuroinflammation (Figure 2) [102].
Figure 2.
MicroRNAs implicated in oxidative stress-related cellular pathways in Parkinson’s disease.
Selective vulnerability of dopamine neurons suggests a role of dopamine itself in pathogenesis of PD. Normally, dopamine that is newly synthesized or uptaken from the synaptic cleft is removed from the cytosol and stored in synaptic vesicles by vesicular monoamine transporter 2 (VMAT2). Excess of cytosolic dopamine readily oxidizes and forms ROS [6]. MiR-133b indirectly inhibits expression of VMAT2 via downregulation of Pitx3 [103,104]. Therefore, its upregulation may contribute to PD pathology, since dopamine neurons with reduced VMAT2 expression showed increased sensitivity to dopamine-mediated toxicity [105]. Additionally, increased dopamine transporter (DAT)-mediated dopamine uptake may result in oxidative damage and neuronal degeneration [106]. Interestingly, miR-133b can also alter expression of DAT via the same route as VMAT2 [103]. Therefore, decreased levels of miR-133b may result in elevated levels of DAT, contributing to oxidative stress. This suggestion is particularly interesting in the light of findings that miR-133b is downregulated in the midbrain of PD patients [26]. MiR-137 and miR-491 negatively regulate DAT expression and uptake of dopamine by DAT in vitro [107], and decreased expression of these microRNAs may also implicate them in oxidative stress in PD.
Dysfunctional mitochondria is one of the main sources of ROS. Several mutations in genes encoding proteins PINK1, Parkin, and DJ-1 can affect mitochondrial function, increase oxidative stress, and cause autosomal recessive PD in humans [6]. PINK1, together with Parkin, are mitochondrial quality control regulators: they induce disposal of dysfunctional mitochondria reviewed in [108]. PINK1 exhibits a neuroprotective effect in dopamine neurons by inhibiting ROS production [109], while PINK1 knockout in human and mouse dopamine neurons causes increased ROS generation [110]. MiR-27a and miR-27b suppress expression of PINK1 [111], which potentially can induce oxidative stress. Additionally, miR-27a may be implicated in downregulation of mitochondrial complex I subunit NDUFS4 and, together with miR-155, mitochondrial complex V subunit ATP5G3 [112].
DJ-1 is a multifunctional protein and, amongst various roles, it is a regulator of mitochondrial activity and an important player in mediating the oxidative stress response [113,114]. In addition to its role in familial cases of PD, damaged by irreversible oxidation DJ-1 was also reported in the brains of sporadic PD patients [115]. Increased levels of miR-494 downregulate DJ-1 levels and increase cell vulnerability to oxidative stress both in vitro and in vivo [116]. Upregulation of mir-4639-5p, also targeting DJ-1 expression, increases oxidative stress and causes cell death in SH-SY5Y cells, a frequently used dopamine neuron-like model, and its increased expression was reported in PD patients [117]. In addition, miR-34b and miR-34c are downregulated in PD patients (particularly in the SNpc), and their depletion was correlated with mitochondrial dysfunction, increased oxidative stress, and a moderate decrease of SH-SY5Y cell viability. Decreased expression of miR-34b/c was coupled with downregulated expression of Parkin and DJ-1, although mechanism of their action is unclear [118].
The Nrf2-antioxidant response element (ARE) pathway is an endogenous antioxidant system, shown to be downregulated in neurodegenerative diseases. Nrf2 is regulated by Keap1, which facilitates its degradation. Oxidative stress induces translocation of Nrf2 to the nucleus, activating expression of genes, which encode proteins involved in the oxidative stress response, such as SOD1 and GSH (for more details, see [119,120]). miR-7 is capable of repressing Keap1 [121]; what is particularly interesting in the light of this report is that miR-7 is downregulated in the SNpc of PD patients, and its downregulation results in a loss of dopamine neurons in vivo [122]. MiR-153, miR-27a, miR-142-5p, and miR-144 can directly downregulate Nrf2 expression in SH-SY5Y cells [123], potentially contributing to an impaired oxidative stress response.
Histopathologically, PD is characterized by formation of inclusions in neuronal soma (Lewy bodies) or processes (Lewy neurites) with the protein α-synuclein as a major component [124]. Mutations in encoding α-synuclein gene, SNCA, and its duplication and triplication were reported to cause familial cases of PD [125]. α-synuclein is capable of inducing oxidative stress and increased levels of ROS, although the exact mechanism is still unclear [126,127,128,129,130]. Multiple microRNAs were reported to control α-synuclein expression, including miR-7, miR-214, miR-153, and miR-34b/c, and their downregulation may contribute to α-synuclein-mediated neurotoxicity in PD [131,132,133,134]. α-synuclein aggregation can also be mediated through its impaired removal by chaperon-mediated autophagy. For example, miR-21, miR-224, miR-373, and miR-379 were demonstrated to downregulate LAMP2 expression, and miR-26b, miR-106a, miR-301b, miR-320a, and miR-16-1 were shown to suppress expression of Hsc70 [135,136,137]. Upregulation of some of these microRNAs were detected in PD patients [120]. MicroRNA regulation of α-synuclein expression has recently been systematically reviewed elsewhere [138]. Altogether, the literature describes multiple mechanisms for microRNAs to contribute to α-synuclein accumulation, which consequently could lead to oxidative stress.
Neuroinflammation, mediated by microglia and to a lesser extent by astrocytes and oligodendrocytes, was shown to play an important role in PD pathophysiology. Particularly, activated microglia can produce numerous cytotoxic substances, including superoxide, and therefore contribute to oxidative stress in the brain (for more details, see [139,140]). Some microRNAs were reported to be implicated in neuroinflammation, such as miR-155 (pro-inflammatory), and miR-146a and miR-124 (anti-inflammatory) [124]. Interestingly, miR-155 was found to be upregulated in an α-synuclein in vivo model of PD and was proposed to mediate α-synuclein-induced inflammation [141,142]. Additionally, increased levels of miR-155 were reported in PD patients. In the same study, downregulation of miR-146a was also demonstrated [143]. MiR-124 attenuates microglia activation and improves survival of dopamine neurons in the MPTP model of PD [144]. PD-associated proteins, including Parkin, DJ-1, and α-synuclein, can induce neuroinflammation by activating microglia [145].
4. Amyotrophic Lateral Sclerosis
Amyotrophic lateral sclerosis (ALS) is a neurodegenerative disease characterized by a loss of upper and lower motor neurons in the brain and spinal cord [146], which leads to loss of voluntary control over muscles and subsequent muscle atrophy. Patients gradually experience worsening symptoms of muscle weakness, problems with speaking, chewing and swallowing, and eventually breathing difficulties most often leading to death due to respiratory failure. About one out of 300–500 humans is affected by ALS, with the incidence being higher in men. The risk increases with age and survival is estimated at 3-4 years after onset. ALS presents either in a sporadic or a familial form. There are many genes associated with the familial form and a few mutations which are known to be the cause, the most common ones being on RNA binding protein FUS (FUS), TAR DNA-binding protein 43 (TARDBP), chromosome 9 open reading frame 72 (C9orf72) and Cu2+/Zn2+ superoxide dismutase (SOD1) [147]. Notably, TARDBP and FUS are involved in RNA biology, including microRNA processing [148]. Non-genetic factors are also implicated in ALS. For instance, environmental insults can cause oxidative stress through the release of free radicals, mainly ROS and reactive nitrogen species, which may lead to epigenetic modifications and changes in gene expression relevant for ALS [149].
Supporting evidence for the role of oxidative stress in ALS was demonstrated by a recent meta-analysis which showed that malondialdehyde, 8-hydroxyguanosine, and Advanced Oxidation Protein Product were significantly elevated in the peripheral blood of ALS patients when compared to controls, as opposed to levels of antioxidant glutathione and uric acid which were downregulated [150]. Other oxidative stress markers such as Cu, SOD, glutathione peroxidase, Co-Q10, and transferrin did not have a link to ALS.
The progressive loss of motor neurons happens relatively fast compared to other neurodegenerative diseases and causes a wide variety of clinical symptoms related to motor deficits, making early diagnosis of ALS challenging. Thus, there is an active search for biomarkers of the disease and microRNAs could represent one option as their expression signatures have been studied in patients. Many studies have identified differential expression of small RNAs, including microRNAs, in the muscle, cerebrospinal fluid, motor neuron progenitors, and blood as well as in post mortem tissue samples (spinal cord, brain stem, and the brain) of both sporadic and familial ALS patients compared to healthy controls [151,152,153,154,155]. Besides being valuable as biomarkers, many microRNAs are also studied from a therapeutic point of view as regulating them may provide an option to treat ALS. For example, using an AAV-mediated artificial microRNA targeting SOD1, which is involved in reducing ROS and one of the causal genes of ALS, has shown efficient silencing of the gene in macaques [156].
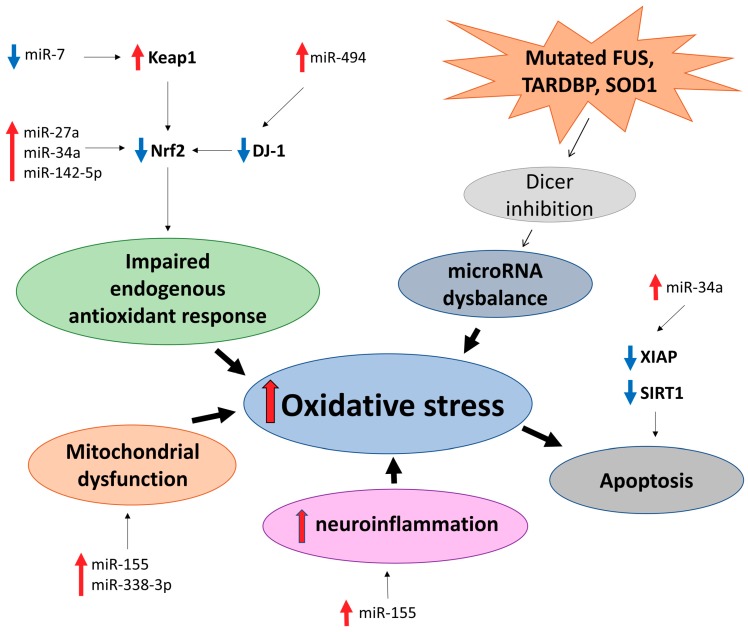
A number of differentially expressed miRNAs in ALS patients versus controls regulate genes involved in oxidative stress, e.g., reducing or counteracting ROS/reactive nitrogen species and may be useful as biomarkers and/or therapeutics. For example, miR-27a, miR-34a, miR-155, miR-142-5p, and miR-338-3p have been studied as biomarkers and potential therapeutic targets in relation to ALS and are involved in oxidative stress directly or indirectly [153,155,157,158] (Figure 3).
Figure 3.
MicroRNAs implicated in oxidative stress-related cellular pathways in Amyotrophic Lateral Sclerosis (AMS).
miR-34a regulates an X-linked inhibitor of apoptosis (XIAP) that is linked to oxidative stress-induced senescence and Sirtuin 1 (SIRT1), which is protective against oxidative stress-induced apoptosis [154,159]. Of interest, SIRT1 is downregulated in PD [160]. Moreover, ALS patient-derived cell lines have a reduction of miR-34a, which is rescued by treatment with enoxacin, a small-molecule drug stimulating microRNA biogenesis [154]. Thus, enoxacin and other microRNA biogenesis stimulating drugs can potentially be used as ALS therapy [52].
The Nrf2-ARE pathway regulates many genes involved in redox reactions and has been linked to ALS [161]. It is regulated by several microRNAs, directly by e.g., aforementioned miR-27a and miR-34a and indirectly by e.g., miR-7 and miR-494, which regulate Nrf2 modulating proteins [116,121,123,162]. Furthermore, inhibiting miR-142-5p reduces oxidative stress via upregulation of the Nrf2-ARE signaling pathway, and it is downregulated in the CSF of sporadic ALS patients [155,163].
MiR-155 has been shown to be upregulated in both sporadic and familial ALS patients, and inhibiting it in the brains of SOD1G93A mice increases both survival and disease duration [157]. Additionally, miR-338-3p regulates certain subunits of mitochondrial OXPHOS complexes [164] and is also implicated in ALS in human patients and mouse models [158,165]. A broader microRNA dysregulation has also been observed in human ALS patient motor neurons and overexpression of ALS-causing genes FUS, TARDBP, and SOD1 seem to inhibit pre-miRNA processing by Dicer. Enhancing Dicer with enoxacin improves neuromuscular function in two separate ALS mouse models [52]. Therefore, a treatment strategy not only taking into account oxidative stress, but also microRNA dysregulation could prove to be useful for ALS patients. However, this and the relationship of microRNAs and oxidative stress should be studied much more carefully before engagement of clinical trials.
5. Huntington’s Disease
HD is a relatively rare hereditary disorder with the highest prevalence in the white Caucasian population (about 1:10,000 to 1:20,000) reviewed in [166]. HD is caused by abnormal expansion of a repeated trinucleotide (CAG) sequence in the huntingtin (HTT) gene, translated to a long polyglutamine stretch in mutant huntingtin (mHTT) protein or, via repeat associated non-ATG (RAN) translation, to homopolymeric proteins prone to aggregation (for detailed review, see [166,167,168]). Longer CAG repeats correlate with an earlier age of onset of disease symptoms, which include severe motor (chorea, bradykinesia, and dystonia), cognitive (executive function, memory, attention and visuospatial functions) and psychiatric (anxiety, aggression, apathy and depression) disturbances, combined with sleep and circadian disorders, weight loss, skeletal muscle wasting, testicular atrophy, and peripheral immune system alterations [166,168]. The majority of these symptoms are caused by degeneration of striatal GABAergic medium spiny neurons and the cortical neurons projecting to them, accompanied by astrogliosis and microglia activation. Glutamate excitotoxicity, caused by reduced astrocyte glutamate uptake, further exacerbates neurodegeneration. Progressive atrophy of the striatum and cerebral cortex leads to patient death at 15–20 years from the disease onset [166,168].
On a molecular level, HD is characterized by the presence of nuclear inclusions and cytoplasmic aggregates containing mHTT and RAN translation proteins, transcription dysregulation (including large changes in microRNAs), inhibition of proteasome activity and autophagy, defects in synaptic neurotransmission, mitochondrial dysfunction, and oxidative stress [8,166,168,169,170]. A direct link between microRNA dysregulation in HD and oxidative stress has not been evidently described in the literature; however, both are highly relevant for HD as summarized below. Moreover, drawing from research on other neurodegenerative disorders (particularly ALS and PD), it seems plausible that global dysregulation of microRNAs in HD and oxidative stress might form a vicious cycle exacerbating each other and potentially worsening disease progression [51].
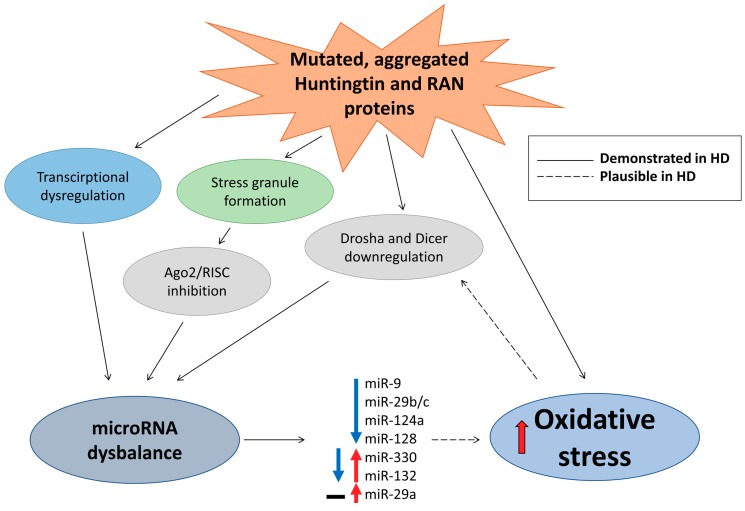
Dysregulation of transcription caused by interaction of mHTT with Repressor Element 1 Silencing Transcription Factor (REST) affected, among other targets, the expression of several REST-regulated microRNAs in mouse HD models and, importantly, in post mortem cortex samples of HD patients, where upregulation of miR-29a and miR-330 and downregulation of miR-132 was observed [171]. Similarly, analysis of cortical microRNA expression in the brains of patients at different HD stages identified progressive downregulation of miR-9, miR-9*, miR-29b, and miR-124a, whereas, in contrast to the study of Johnson et al. [171], no changes of miR-29a and significant upregulation of miR-132 at late disease stages were observed [172]. Interestingly, both wild-type and mHTT interact with Ago2 and localize to P bodies, suggesting that mHTT can affect Ago2 and, consequently, RISC complex activity in HD [173]. Importantly, recent results confirmed the effect of mHTT on Ago2, demonstrating that aggregation of mHTT, through autophagy impairment, can lead to Ago2 accumulation in a mouse HD model and HD patients and, consequently, to global dysregulation of microRNA levels and activity [174]. mHTT mRNA can also lead to generation of small CAG-repeated RNAs, whose generation and neurotoxic activity depend on Dicer and Ago2, potentially affecting microRNA biogenesis [175]. Thus, both transcription and processing of microRNAs appear to be dysregulated in HD. Indeed, analysis of HD mouse models identified common downregulation of miR-22, miR-29c, miR-128, miR-132, miR-138, miR-218, miR-222, miR-344, and miR-674*, as well as reduced levels of Drosha and Dicer mRNA [176]. In line with these results, microRNA sequencing and differential expression analysis demonstrated deregulation of multiple microRNAs in the frontal cortex and striatum of HD patients [177]. Moreover, because microRNA silencing machinery may be impeded in HD due to Ago2 translocation to stress granules [173,174], observed changes in specific microRNAs should be interpreted with caution as they might not reflect a functional outcome on target mRNA regulation (Figure 4).
Figure 4.
MicroRNAs implicated in oxidative stress-related cellular pathways in Huntington’s Disease.
The unequivocal cause for HD is the CAG expanse in the HTT gene and a higher number of CAG repeats leads to an earlier manifestation of the disease. However, large variations in age of disease onset among individuals with moderate (<55) CAG repeat numbers, together with variations in disease progression, strongly imply genetic and environmental modifiers of the disease which could exacerbate detrimental effects of mHTT explaining observed variability [178,179]. Oxidative stress or, conversely, capacity of antioxidant defense systems seem highly plausible as modifiers of HD [8,180]. Markers of oxidative stress rise with transition from the asymptomatic to symptomatic phase in HD patients [181], and oxidative stress is widely described as the main contributor to cell death in HD [8]. While there are no studies specifically addressing the link between microRNAs and oxidative stress in HD, some of the above-mentioned microRNAs, such as miR-9, miR-29, miR-124, and miR-128, changed in HD models and patients, have also been predicted to target genes involved in the oxidative stress response [53]. Similarly, general dysregulation of the microRNA network observed in HD will affect neuronal susceptibility to stress, including oxidative stress [182,183], which putatively could affect pace of disease progression. Conversely, it is tempting to speculate that strategies based on boosting microRNAs processing machinery could slow down demise of neurons in HD similarly to what we and others have shown in models of ALS [52] and PD [30].
Overall, while both oxidative stress and microRNA dysregulation are established features in HD, their interaction remains largely unexplored, yet an intriguing and promising topic for further studies.
6. Common and Unique MicroRNAs Affecting Oxidative Stress in Neurodegenerative Diseases
As discussed above, neurodegenerative diseases share many similarities, including mitochondrial dysfunction, formation, and spread of insoluble protein inclusions and, as reviewed here, oxidative stress and deregulation of microRNA networks. Among multiple microRNAs associated with neurodegenerative diseases, we have focused on those implicated in the oxidative stress response (Figure 1, Figure 2, Figure 3 and Figure 4 and Table 1). For many microRNAs, association with oxidative stress was not reported in the original publication, which frequently only demonstrated the change in its level in a selected neurodegenerative condition. In such cases, we consulted other studies, like [53], to identify if a particular microRNA can be involved in the oxidative stress response. Comparison of microRNAs associated with each of the four neurodegenerative diseases reviewed here identified only a small set of common microRNAs affecting the oxidative stress response in different neurodegenerative conditions (Table 1), and no single common oxidative stress-implicated microRNA was reported to be associated with three or four diseases. However, compared to AD and PD, relatively few studies have addressed changes in microRNA levels in ALS and HD, and, therefore, it is reasonable to expect that many more microRNAs associated with these diseases are awaiting their discovery. Nevertheless, many microRNAs are common at least between two neurodegenerative conditions (Table 1); among them are miR-34, miR-124, miR-132, miR-26, mir-7 which are highly expressed in the brain [184,185] and regulate multiple oxidative stress-related pathways (Figure 1, Figure 2, Figure 3 and Figure 4). Such microRNAs are particularly attractive as potential therapeutic targets for the treatment of neurodegeneration. However, the unique microRNAs also deserve attention, as they may be reflecting fundamental differences in the development and progression of particular neurodegenerative disease and serve as specific biomarkers, facilitating and accelerating disease diagnosis.
Table 1.
MicroRNAs associated with neurodegenerative diseases (AD, PD, ALS, and HD) and implicated in regulation of oxidative stress and related cellular pathways. Bolded are microRNAs associated with more than one neurodegenerative disease.
| Disease | Associated microRNAs Involved in Oxidative Stress Regulation | References |
|---|---|---|
| Alzheimer’s disease | miR-107 | [72,73,78] |
| miR-125b | [97] | |
| miR-130b | [91] | |
| miR-132/212 | [87,88] | |
| miR-134 | [72] | |
| miR-145 | [72] | |
| miR-146b | [89] | |
| miR-153 | [81] | |
| miR-186 | [79,99] | |
| miR-193b | [91] | |
| miR-200a-3p | [85] | |
| miR-20a | [91] | |
| miR-210 | [72] | |
| miR-219 | [86] | |
| miR-26 | [90] | |
| miR-296 | [91] | |
| miR-329 | [91] | |
| miR-330a | [92] | |
| miR-342-5p | [80] | |
| miR-98-5p | [96] | |
| Parkinson’s disease | miR-106a | [135] |
| miR-124 | [124,144] | |
| miR-133b | [103] | |
| miR-137 | [106] | |
| miR-142-5p | [123] | |
| miR-144 | [123] | |
| miR-146a | [124] | |
| miR-153 | [123,133] | |
| miR-155 | [124,141] | |
| miR-16-1 | [137] | |
| miR-214 | [132] | |
| miR-224 | [135] | |
| miR-26b | [135] | |
| miR-27a/b | [110,111,123] | |
| miR-301b | [135] | |
| miR-320 | [136] | |
| miR-34b/c | [118,134] | |
| miR-373 | [135] | |
| miR-379 | [135] | |
| mir-4639-5p | [117] | |
| miR-491 | [106] | |
| miR-494 | [116] | |
| miR-7 | [121,122,131,133] | |
| ALS | miR-142-5p | [155,163] |
| miR-155 | [157] | |
| miR-27a | [158,165] | |
| miR-338-3p | [162] | |
| miR-34a | [116,154] | |
| Huntington’s disease | miR-124a | [172] |
| miR-128 | [176] | |
| miR-132 | [171,172,176] | |
| miR-29a/b/c | [171,172,176] | |
| miR-330 | [171] | |
| miR-9 | [172] |
7. Challenges and Perspectives
The above reviewed results clearly demonstrate the intrinsic link between oxidative stress and microRNAs in ageing and disease. However, there are many questions, experimental details, and technical difficulties that need to be solved to bring microRNA-based therapies to clinical use. We undoubtedly have learned a lot about microRNAs and oxidative stress from experiments in cultured cells and extrapolating results from cancer research, but we should exercise caution in translating the findings obtained in cell culture to human neurons. Despite continuous improvement of computational algorithms, prediction and validation of microRNA-mRNA regulation remains challenging [186,187]. Many reported results on microRNA-mRNA regulation are obtained using luciferase reporter assays and transient transfection of microRNA mimics, which are known to cause unspecific general effects on the microRNA biogenesis pathway [188]. The use of proper controls (scrambled microRNAs and reporters with mutated putative binding sites) in such studies is, therefore, absolutely crucial for their validity. Additional caution in interpretation of microRNA overexpression studies should be taken since achieved and functionally effective overexpression levels might be orders of magnitude higher than normally observed.
MicroRNA expression profiles in neurons and glia in vivo are cell type-specific and different from cultured immortalized cells, as are 3′UTR isoforms [189,190], and, moreover, they change with age and the stage of the disease. Furthermore, expression patterns of microRNAs and their putative targets are distinct in different neuronal populations [191]. Thus, ideally, we should address regulation of endogenous mRNA by endogenous microRNAs, for example, by utilizing target protectors introduced to post-mitotic neurons at the lowest possible concentrations, using proper controls [192,193]. Development of new genetic methods, such as CRISPR/Cas9-mediated gene knockout [194,195,196], greatly facilitated loss-of-function genetic studies, enabling relatively easy deletion of both individual microRNAs and whole microRNA families in cultured cells and in vivo [197,198,199,200]. Both knockout and base editing using CRISPR/Cas9 [201,202,203,204] can be further utilized to selectively mutate or create microRNA binding site(s) on 3′UTR of a particular gene, allowing for precisely addressing the consequences of modulation of individual microRNA-mRNA binding. Identified neuroprotective microRNAs can be introduced to the brain using gene therapy vectors, similar to the ones used in clinical trials for neurotrophic factor expression in neurodegenerative disorders [205].
Translation of the results from animal to human settings has long been an issue in neurodegeneration research, with many neuroprotective treatments successfully working in rodent and even primate models, but not in human patients, failing at the stage of double-blinded randomized clinical trials [206]. Neither genetic nor toxin-based rodent models fully recapitulate features of neurodegenerative diseases. While many AD and PD models focus on protein aggregation, other factors contributing to neurodegeneration clearly exist. Mouse and human midbrain progenitors and dopamine neurons have distinct RNA expression profiles and species-specific differences, for example, the presence of neuromelanin and differences in dopamine oxidation [10,207]. Genetic mutations, which lead to early onset familial PD in humans, do not recapitulate the disease when introduced to rodents [208,209,210]. The lack of appropriate neurodegenerative disease models greatly impairs studies of the disease-related microRNAs. While the majority of microRNAs are conserved between rodents and humans, a number of primate- and human-specific microRNAs have been identified [211,212]. Furthermore, existing data demonstrate that some genes may exhibit human-specific regulation by microRNAs [213,214]. These questions have been partly addressed by the analysis of microRNA–mRNA interactions in neurons derived from patients at different stages of disease progression; however, obtaining high quality RNA in sufficient amounts from specific neuronal populations in post mortem brain samples is technically very challenging. Development of more sensitive methods, such as single cell microRNA–mRNA co-sequencing [215] would greatly improve the analysis of patient samples. Studies of post mortem tissue samples are also limited in that they only provide a snapshot of microRNAs changed at a particular disease stage, whereas longitudinal studies would have been much more informative.
Fortunately, current advances in differentiation of patient-derived induced pluripotent stem cells towards specific neuronal populations have finally allowed studying neurodegeneration and, particularly, microRNA alterations, in human disease models [216,217]. However, the protocols for human stem cell reprogramming and differentiation are still challenging, and the obtained neurons have embryonic or early postnatal phenotype, rather than adult neurons affected by neurodegeneration in patients. Culturing cells in artificial in vitro environments can affect their mRNA and microRNA expression patterns and oxidative stress levels (for a review of the current state of the field and challenges, see [216]). We are still lacking the methods to reliably detect and monitor levels of oxidative damage in live cells [218]. Development of such experimental techniques and models would also enable longitudinal studies to address the question on whether oxidative stress is a cause or consequence of other processes affecting neuronal survival, such as mitochondrial dysfunction, protein aggregation, or microRNA biogenesis disruption.
Focusing exclusively on neurons will not be sufficient to understand neurodegeneration—astrocytes, oligodendrocytes, and microglia are important players which may also be involved in modulating oxidative stress effects, for example, by regulating neuroinflammation. Therefore, to uncover molecular mechanisms behind human neurodegenerative diseases, we need to study human models representing and recapitulating the interaction between several neural cell types. Three-dimensional human brain organoids offer a great hope for neurodegeneration modeling [219], though it remains to be seen whether such organoids could be developed to the stage mature enough to model properties of the aged or even the adult brain. In this respect, one very promising direction would be to establish humanized animal models based on transplantation of human neural cell precursors to the rodent brain. A similar strategy has already been successfully implemented to obtain humanized mice with brains chimeric for human glia [220]. For example, it has recently been shown that, after transplantation to the rat midbrain, a proportion of human embryonic stem cell-derived neuron precursors will differentiate to nigral dopamine neurons, integrate into appropriate neuronal circuits, and regrow axons to innervate their natural targets [221,222,223]. It is therefore possible in principle to obtain rodents containing human glia, microglia, and neurons correctly differentiated and integrated into host neuronal circuits and use these humanized animals to model degeneration of human neurons in a human-specific cell environment. Clearly, more work is needed to overcome technical and ethical hurdles; however, recent progress in the development of molecular tools and cellular models gives a strong hope that successful treatments to cure neurodegenerative diseases may finally be available.
Acknowledgments
We thank Katrina Albert for careful proofreading and critical comments on the manuscript.
Abbreviations
| Aβ | Amyloid-β |
| AD | Alzheimer’s disease |
| ALS | Amyotrophic Lateral Sclerosis |
| APP | Amyloid precursor protein |
| ARE | Antioxidant response element |
| ATP | Adenosine triphosphate |
| BACE1 | Beta-secretase 1 |
| CRISPR | Clustered regularly interspaced short palindromic repeats |
| DAT | Dopamine transporter |
| GSH | Glutathione |
| HD | Huntington’s disease |
| mHTT | mutant Huntingtin |
| PD | Parkinson’s disease |
| PSEN | Presenilin |
| RAN | Repeat associated non-ATG |
| REST | Repressor Element 1 Silencing Transcription Factor |
| RISC | RNA-induced silencing complex |
| ROS | Reactive oxygen species |
| sAβ | soluble amyloid-β |
| SNpc | Substantia nigra pars compacta |
| SOD | Superoxide dismutase |
| VMAT2 | Vesicular monoamine transporter 2 |
| UTR | Untranslated region |
Author Contributions
Conceptualization: J.K., D.G., I.P., P.C. and A.D.; writing—original draft preparation, J.K., D.G., I.P., P.C. and A.D.; writing—review and editing, J.K., D.G., I.P., P.C. and A.D.; supervision, A.D.; funding acquisition, D.G., I.P. and A.D.
Funding
This research was funded by the Academy of Finland grants #293392, #319195, Instrumentarium Science Foundation, the Doctoral Program for Drug Research, and the Finnish National Agency for Education (EDUFI) and statutory funds of the Maj Institute of Pharmacology, PAS, Poland. Open access funding provided by the University of Helsinki.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Erkkinen M.G., Kim M.O., Geschwind M.D. Clinical Neurology and Epidemiology of the Major Neurodegenerative Diseases. Cold Spring Harb Perspect. Biol. 2018;10 doi: 10.1101/cshperspect.a033118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brown R.H., Al-Chalabi A. Amyotrophic Lateral Sclerosis. N. Engl. J. Med. 2017;377:162–172. doi: 10.1056/NEJMra1603471. [DOI] [PubMed] [Google Scholar]
- 3.Collaborators G.B.D.N. Global, regional, and national burden of neurological disorders, 1990-2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2019;18:459–480. doi: 10.1016/S1474-4422(18)30499-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pringsheim T., Jette N., Frolkis A., Steeves T.D. The prevalence of Parkinson’s disease: A systematic review and meta-analysis. Mov. Disord. 2014;29:1583–1590. doi: 10.1002/mds.25945. [DOI] [PubMed] [Google Scholar]
- 5.Lin M.T., Beal M.F. Mitochondrial dysfunction and oxidative stress in neurodegenerative diseases. Nature. 2006;443:787–795. doi: 10.1038/nature05292. [DOI] [PubMed] [Google Scholar]
- 6.Blesa J., Trigo-Damas I., Quiroga-Varela A., Jackson-Lewis V.R. Oxidative stress and Parkinson’s disease. Front. Neuroanat. 2015;9:91. doi: 10.3389/fnana.2015.00091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tonnies E., Trushina E. Oxidative Stress, Synaptic Dysfunction, and Alzheimer’s Disease. J. Alzheimers Dis. 2017;57:1105–1121. doi: 10.3233/JAD-161088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kumar A., Ratan R.R. Oxidative stress and Huntington’s disease: The good, the bad, and the ugly. J. Huntingtons Dis. 2016;5:217–237. doi: 10.3233/JHD-160205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Niedzielska E., Smaga I., Gawlik M., Moniczewski A., Stankowicz P., Pera J., Filip M. Oxidative stress in neurodegenerative diseases. Mol. Neurobiol. 2016;53:4094–4125. doi: 10.1007/s12035-015-9337-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Burbulla L.F., Song P., Mazzulli J.R., Zampese E., Wong Y.C., Jeon S., Santos D.P., Blanz J., Obermaier C.D., Strojny C., et al. Dopamine oxidation mediates mitochondrial and lysosomal dysfunction in Parkinson’s disease. Science. 2017;357:1255–1261. doi: 10.1126/science.aam9080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cobley J.N., Fiorello M.L., Bailey D.M. 13 reasons why the brain is susceptible to oxidative stress. Redox Biol. 2018;15:490–503. doi: 10.1016/j.redox.2018.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pacelli C., Giguere N., Bourque M.J., Levesque M., Slack R.S., Trudeau L.E. Elevated mitochondrial bioenergetics and axonal arborization size are key contributors to the vulnerability of dopamine neurons. Curr. Biol. 2015;25:2349–2360. doi: 10.1016/j.cub.2015.07.050. [DOI] [PubMed] [Google Scholar]
- 13.Liu Z., Zhou T., Ziegler A.C., Dimitrion P., Zuo L. Oxidative stress in neurodegenerative diseases: From molecular mechanisms to clinical applications. Oxid. Med. Cell Longev. 2017;2017:2525967. doi: 10.1155/2017/2525967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim G.H., Kim J.E., Rhie S.J., Yoon S. The role of oxidative stress in neurodegenerative diseases. Exp. Neurobiol. 2015;24:325–340. doi: 10.5607/en.2015.24.4.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Murphy M.P. Antioxidants as therapies: Can we improve on nature? Free Radic. Biol. Med. 2014;66:20–23. doi: 10.1016/j.freeradbiomed.2013.04.010. [DOI] [PubMed] [Google Scholar]
- 16.Vasconcelos A.R., Dos Santos N.B., Scavone C., Munhoz C.D. Nrf2/ARE pathway modulation by dietary energy regulation in neurological disorders. Front. Pharmacol. 2019;10:33. doi: 10.3389/fphar.2019.00033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bartel D.P. MicroRNAs: Genomics, biogenesis, mechanism, and function. Cell. 2004;116:281–297. doi: 10.1016/S0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- 18.Bartel D.P. Metazoan MicroRNAs. Cell. 2018;173:20–51. doi: 10.1016/j.cell.2018.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Krol J., Loedige I., Filipowicz W. The widespread regulation of microRNA biogenesis, function and decay. Nat. Rev. Genet. 2010;11:597–610. doi: 10.1038/nrg2843. [DOI] [PubMed] [Google Scholar]
- 20.Schmiedel J.M., Klemm S.L., Zheng Y., Sahay A., Bluthgen N., Marks D.S., van Oudenaarden A. Gene expression. MicroRNA control of protein expression noise. Science. 2015;348:128–132. doi: 10.1126/science.aaa1738. [DOI] [PubMed] [Google Scholar]
- 21.Rajman M., Schratt G. MicroRNAs in neural development: From master regulators to fine-tuners. Development. 2017;144:2310–2322. doi: 10.1242/dev.144337. [DOI] [PubMed] [Google Scholar]
- 22.Im H.I., Kenny P.J. MicroRNAs in neuronal function and dysfunction. Trends Neurosci. 2012;35:325–334. doi: 10.1016/j.tins.2012.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bernstein E., Kim S.Y., Carmell M.A., Murchison E.P., Alcorn H., Li M.Z., Mills A.A., Elledge S.J., Anderson K.V., Hannon G.J. Dicer is essential for mouse development. Nat. Genet. 2003;35:215–217. doi: 10.1038/ng1253. [DOI] [PubMed] [Google Scholar]
- 24.Morita S., Horii T., Kimura M., Goto Y., Ochiya T., Hatada I. One Argonaute family member, Eif2c2 (Ago2), is essential for development and appears not to be involved in DNA methylation. Genomics. 2007;89:687–696. doi: 10.1016/j.ygeno.2007.01.004. [DOI] [PubMed] [Google Scholar]
- 25.Kawase-Koga Y., Otaegi G., Sun T. Different timings of Dicer deletion affect neurogenesis and gliogenesis in the developing mouse central nervous system. Dev. Dyn. 2009;238:2800–2812. doi: 10.1002/dvdy.22109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kim J., Inoue K., Ishii J., Vanti W.B., Voronov S.V., Murchison E., Hannon G., Abeliovich A. A MicroRNA feedback circuit in midbrain dopamine neurons. Science. 2007;317:1220–1224. doi: 10.1126/science.1140481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Huang T., Liu Y., Huang M., Zhao X., Cheng L. Wnt1-cre-mediated conditional loss of Dicer results in malformation of the midbrain and cerebellum and failure of neural crest and dopaminergic differentiation in mice. J. Mol. Cell Biol. 2010;2:152–163. doi: 10.1093/jmcb/mjq008. [DOI] [PubMed] [Google Scholar]
- 28.Davis T.H., Cuellar T.L., Koch S.M., Barker A.J., Harfe B.D., McManus M.T., Ullian E.M. Conditional loss of Dicer disrupts cellular and tissue morphogenesis in the cortex and hippocampus. J. Neurosci. 2008;28:4322–4330. doi: 10.1523/JNEUROSCI.4815-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.De Pietri Tonelli D., Pulvers J.N., Haffner C., Murchison E.P., Hannon G.J., Huttner W.B. miRNAs are essential for survival and differentiation of newborn neurons but not for expansion of neural progenitors during early neurogenesis in the mouse embryonic neocortex. Development. 2008;135:3911–3921. doi: 10.1242/dev.025080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chmielarz P., Konovalova J., Najam S.S., Alter H., Piepponen T.P., Erfle H., Sonntag K.C., Schutz G., Vinnikov I.A., Domanskyi A. Dicer and microRNAs protect adult dopamine neurons. Cell Death Dis. 2017;8:e2813. doi: 10.1038/cddis.2017.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kaneko H., Dridi S., Tarallo V., Gelfand B.D., Fowler B.J., Cho W.G., Kleinman M.E., Ponicsan S.L., Hauswirth W.W., Chiodo V.A., et al. DICER1 deficit induces Alu RNA toxicity in age-related macular degeneration. Nature. 2011;471:325–330. doi: 10.1038/nature09830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pang X., Hogan E.M., Casserly A., Gao G., Gardner P.D., Tapper A.R. Dicer expression is essential for adult midbrain dopaminergic neuron maintenance and survival. Mol. Cell Neurosci. 2014;58:22–28. doi: 10.1016/j.mcn.2013.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hebert S.S., Papadopoulou A.S., Smith P., Galas M.C., Planel E., Silahtaroglu A.N., Sergeant N., Buee L., De Strooper B. Genetic ablation of Dicer in adult forebrain neurons results in abnormal tau hyperphosphorylation and neurodegeneration. Hum. Mol. Genet. 2010;19:3959–3969. doi: 10.1093/hmg/ddq311. [DOI] [PubMed] [Google Scholar]
- 34.Schaefer A., O’Carroll D., Tan C.L., Hillman D., Sugimori M., Llinas R., Greengard P. Cerebellar neurodegeneration in the absence of microRNAs. J. Exp. Med. 2007;204:1553–1558. doi: 10.1084/jem.20070823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Konopka W., Kiryk A., Novak M., Herwerth M., Parkitna J.R., Wawrzyniak M., Kowarsch A., Michaluk P., Dzwonek J., Arnsperger T., et al. MicroRNA loss enhances learning and memory in mice. J. Neurosci. 2010;30:14835–14842. doi: 10.1523/JNEUROSCI.3030-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vinnikov I.A., Hajdukiewicz K., Reymann J., Beneke J., Czajkowski R., Roth L.C., Novak M., Roller A., Dörner N., Starkuviene V., et al. Hypothalamic miR-103 protects from hyperphagic obesity in mice. J. Neurosci. 2014;34:10659–10674. doi: 10.1523/JNEUROSCI.4251-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cuellar T.L., Davis T.H., Nelson P.T., Loeb G.B., Harfe B.D., Ullian E., McManus M.T. Dicer loss in striatal neurons produces behavioral and neuroanatomical phenotypes in the absence of neurodegeneration. Proc. Natl. Acad. Sci. USA. 2008;105:5614–5619. doi: 10.1073/pnas.0801689105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mang G.M., Pradervand S., Du N.H., Arpat A.B., Preitner F., Wigger L., Gatfield D., Franken P. A neuron-specific deletion of the microRNA-processing enzyme DICER induces severe but transient obesity in mice. PLoS ONE. 2015;10:e0116760. doi: 10.1371/journal.pone.0116760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schaefer A., Im H.I., Veno M.T., Fowler C.D., Min A., Intrator A., Kjems J., Kenny P.J., O’Carroll D., Greengard P. Argonaute 2 in dopamine 2 receptor-expressing neurons regulates cocaine addiction. J. Exp. Med. 2010;207:1843–1851. doi: 10.1084/jem.20100451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schratt G. microRNAs at the synapse. Nat. Rev. Neurosci. 2009;10:842–849. doi: 10.1038/nrn2763. [DOI] [PubMed] [Google Scholar]
- 41.Antoniou A., Khudayberdiev S., Idziak A., Bicker S., Jacob R., Schratt G. The dynamic recruitment of TRBP to neuronal membranes mediates dendritogenesis during development. EMBO Rep. 2018;19 doi: 10.15252/embr.201744853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Thomas K.T., Gross C., Bassell G.J. microRNAs Sculpt Neuronal Communication in a Tight Balance That Is Lost in Neurological Disease. Front. Mol. Neurosci. 2018;11:455. doi: 10.3389/fnmol.2018.00455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dimmeler S., Nicotera P. MicroRNAs in age-related diseases. EMBO Mol. Med. 2013;5:180–190. doi: 10.1002/emmm.201201986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Eacker S.M., Dawson T.M., Dawson V.L. Understanding microRNAs in neurodegeneration. Nat. Rev. Neurosci. 2009;10:837–841. doi: 10.1038/nrn2726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Eacker S.M., Dawson T.M., Dawson V.L. The interplay of microRNA and neuronal activity in health and disease. Front. Cell Neurosci. 2013;7:136. doi: 10.3389/fncel.2013.00136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Heman-Ackah S.M., Hallegger M., Rao M.S., Wood M.J. RISC in PD: The impact of microRNAs in Parkinson’s disease cellular and molecular pathogenesis. Front. Mol. Neurosci. 2013;6:40. doi: 10.3389/fnmol.2013.00040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mouradian M.M. MicroRNAs in Parkinson’s disease. Neurobiol. Dis. 2012;46:279–284. doi: 10.1016/j.nbd.2011.12.046. [DOI] [PubMed] [Google Scholar]
- 48.Sonntag K.C. MicroRNAs and deregulated gene expression networks in neurodegeneration. Brain Res. 2010;1338:48–57. doi: 10.1016/j.brainres.2010.03.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kurzynska-Kokorniak A., Koralewska N., Pokornowska M., Urbanowicz A., Tworak A., Mickiewicz A., Figlerowicz M. The many faces of Dicer: The complexity of the mechanisms regulating Dicer gene expression and enzyme activities. Nucleic Acids Res. 2015;43:4365–4380. doi: 10.1093/nar/gkv328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Leggio L., Vivarelli S., L’Episcopo F., Tirolo C., Caniglia S., Testa N., Marchetti B., Iraci N. microRNAs in Parkinson’s disease: From pathogenesis to novel diagnostic and therapeutic approaches. Int. J. Mol. Sci. 2017;18:2698. doi: 10.3390/ijms18122698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Emde A., Hornstein E. miRNAs at the interface of cellular stress and disease. EMBO J. 2014;33:1428–1437. doi: 10.15252/embj.201488142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Emde A., Eitan C., Liou L.L., Libby R.T., Rivkin N., Magen I., Reichenstein I., Oppenheim H., Eilam R., Silvestroni A., et al. Dysregulated miRNA biogenesis downstream of cellular stress and ALS-causing mutations: A new mechanism for ALS. EMBO J. 2015;34:2633–2651. doi: 10.15252/embj.201490493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Engedal N., Zerovnik E., Rudov A., Galli F., Olivieri F., Procopio A.D., Rippo M.R., Monsurro V., Betti M., Albertini M.C. From oxidative stress damage to pathways, networks, and autophagy via MicroRNAs. Oxid. Med. Cell Longev. 2018;2018:4968321. doi: 10.1155/2018/4968321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Vinnikov I.A., Domanskyi A. Can we treat neurodegenerative diseases by preventing an age-related decline in microRNA expression? Neural Regen. Res. 2017;12:1602–1604. doi: 10.4103/1673-5374.217328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Salmena L., Poliseno L., Tay Y., Kats L., Pandolfi P.P. A ceRNA hypothesis: The Rosetta Stone of a hidden RNA language? Cell. 2011;146:353–358. doi: 10.1016/j.cell.2011.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sumazin P., Yang X., Chiu H.S., Chung W.J., Iyer A., Llobet-Navas D., Rajbhandari P., Bansal M., Guarnieri P., Silva J., et al. An extensive microRNA-mediated network of RNA-RNA interactions regulates established oncogenic pathways in glioblastoma. Cell. 2011;147:370–381. doi: 10.1016/j.cell.2011.09.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Prince M., Ali G.-C., Guerchet M., Prina A.M., Albanese E., Wu Y.-T. Recent global trends in the prevalence and incidence of dementia, and survival with dementia. Alzheimer’s Res. Ther. 2016;8:23. doi: 10.1186/s13195-016-0188-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tramutola A., Lanzillotta C., Perluigi M., Butterfield D.A. Oxidative stress, protein modification and Alzheimer disease. Brain Res. Bull. 2017;133:88–96. doi: 10.1016/j.brainresbull.2016.06.005. [DOI] [PubMed] [Google Scholar]
- 59.Van Cauwenberghe C., Van Broeckhoven C., Sleegers K. The genetic landscape of Alzheimer disease: Clinical implications and perspectives. Genetics Med. 2015;18:421. doi: 10.1038/gim.2015.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Qiu C., De Ronchi D., Fratiglioni L. The epidemiology of the dementias: An update. Curr. Opin. Psychiatry. 2007;20:380–385. doi: 10.1097/YCO.0b013e32816ebc7b. [DOI] [PubMed] [Google Scholar]
- 61.Prasad K.N. Oxidative stress and pro-inflammatory cytokines may act as one of the signals for regulating microRNAs expression in Alzheimer’s disease. Mech. Ageing Dev. 2017;162:63–71. doi: 10.1016/j.mad.2016.12.003. [DOI] [PubMed] [Google Scholar]
- 62.Lanoiselée H.-M., Nicolas G., Wallon D., Rovelet-Lecrux A., Lacour M., Rousseau S., Richard A.-C., Pasquier F., Rollin-Sillaire A., Martinaud O., et al. APP, PSEN1, and PSEN2 mutations in early-onset Alzheimer disease: A genetic screening study of familial and sporadic cases. PLoS Med. 2017;14:e1002270. doi: 10.1371/journal.pmed.1002270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Patel N., Hoang D., Miller N., Ansaloni S., Huang Q., Rogers J.T., Lee J.C., Saunders A.J. MicroRNAs can regulate human APP levels. Mol. Neurodegener. 2008;3:10. doi: 10.1186/1750-1326-3-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hébert S.S., Horré K., Nicolaï L., Bergmans B., Papadopoulou A.S., Delacourte A., De Strooper B. MicroRNA regulation of Alzheimer’s Amyloid precursor protein expression. Neurobiol. Dis. 2009;33:422–428. doi: 10.1016/j.nbd.2008.11.009. [DOI] [PubMed] [Google Scholar]
- 65.Liu C.G., Wang J.L., Li L., Xue L.X., Zhang Y.Q., Wang P.C. MicroRNA-135a and -200b, potential Biomarkers for Alzheimer׳s disease, regulate β secretase and amyloid precursor protein. Brain Res. 2014;1583:55–64. doi: 10.1016/j.brainres.2014.04.026. [DOI] [PubMed] [Google Scholar]
- 66.Kang Q., Xiang Y., Li D., Liang J., Zhang X., Zhou F., Qiao M., Nie Y., He Y., Cheng J., et al. MiR-124-3p attenuates hyperphosphorylation of Tau protein-induced apoptosis via caveolin-1-PI3K/Akt/GSK3β pathway in N2a/APP695swe cells. Oncotarget. 2017;8:24314–24326. doi: 10.18632/oncotarget.15149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Long J.M., Ray B., Lahiri D.K. MicroRNA-153 physiologically inhibits expression of amyloid-β precursor protein in cultured human fetal brain cells and is dysregulated in a subset of Alzheimer disease patients. J. Biol. Chem. 2012;287:31298–31310. doi: 10.1074/jbc.M112.366336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wang X., Tan L., Lu Y., Peng J., Zhu Y., Zhang Y., Sun Z. MicroRNA-138 promotes tau phosphorylation by targeting retinoic acid receptor alpha. FEBS Lett. 2015;589:726–729. doi: 10.1016/j.febslet.2015.02.001. [DOI] [PubMed] [Google Scholar]
- 69.Prasad K.N. Simultaneous activation of Nrf2 and elevation of antioxidant compounds for reducing oxidative stress and chronic inflammation in human Alzheimer’s disease. Mech. Ageing Dev. 2016;153:41–47. doi: 10.1016/j.mad.2016.01.002. [DOI] [PubMed] [Google Scholar]
- 70.Amakiri N., Kubosumi A., Tran J., Reddy P.H. Amyloid beta and microRNAs in Alzheimer’s disease. Front. Neurosci. 2019;13 doi: 10.3389/fnins.2019.00430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Varadarajan S., Yatin S., Aksenova M., Butterfield D.A. Review: Alzheimer’s amyloid β-peptide-associated free radical oxidative stress and neurotoxicity. J. Struct. Biol. 2000;130:184–208. doi: 10.1006/jsbi.2000.4274. [DOI] [PubMed] [Google Scholar]
- 72.Li J.J., Dolios G., Wang R., Liao F.-F. Soluble beta-amyloid peptides, but not insoluble fibrils, have specific effect on neuronal icroRNA expression. PLoS ONE. 2014;9:e90770. doi: 10.1371/journal.pone.0090770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wang W.-X., Rajeev B.W., Stromberg A.J., Ren N., Tang G., Huang Q., Rigoutsos I., Nelson P.T. The expression of microRNA miR-107 decreases early in Alzheimer’s disease and may accelerate disease progression through regulation of β-site amyloid precursor protein-cleaving enzyme 1. J. Neurosci. 2008;28:1213–1223. doi: 10.1523/JNEUROSCI.5065-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Monte S., Wands J. Molecular indices of oxidative stress and mitochondrial dysfunction occur early and often progress with severity of Alzheimer’s disease. J. Alzheimer’s Dis. 2006;9:167–181. doi: 10.3233/JAD-2006-9209. [DOI] [PubMed] [Google Scholar]
- 75.Dorszewska J., Oczkowska A., Suwalska M., Rozycka A., Florczak-Wyspianska J., Dezor M., Lianeri M., Jagodzinski P.P., Kowalczyk M.J., Prendecki M., et al. Mutations in the exon 7 of Trp53 gene and the level of p53 protein in double transgenic mouse model of Alzheimer’s disease. Folia Neuropathol. 2014;52:30–40. doi: 10.5114/fn.2014.41742. [DOI] [PubMed] [Google Scholar]
- 76.Dorszewska J., Oczkowska A., Suwalska M., Rozycka A., Florczak J., Dezor M., Lianeri M., Jagodzinski P., Kowalczyk M., Prendecki M., et al. Mutations of TP53 gene and oxidative stress in Alzheimer’s disease patients. Adv. Alzheimer’s Dis. 2014;3:24–32. doi: 10.4236/aad.2014.31004. [DOI] [Google Scholar]
- 77.Chen L., Zhang R., Li P., Liu Y., Qin K., Fa Z.Q., Liu Y.J., Ke Y.Q., Jiang X.D. P53-induced microRNA-107 inhibits proliferation of glioma cells and down-regulates the expression of CDK6 and Notch-2. Neurosci. Lett. 2013;534:327–332. doi: 10.1016/j.neulet.2012.11.047. [DOI] [PubMed] [Google Scholar]
- 78.Prendecki M., Florczak-Wyspianska J., Kowalska M., Ilkowski J., Grzelak T., Bialas K., Kozubski W., Dorszewska J. APOE genetic variants and apoE, miR-107 and miR-650 levels in Alzheimer’s disease. Folia Neuropathol. 2019;57:106–116. doi: 10.5114/fn.2019.84828. [DOI] [PubMed] [Google Scholar]
- 79.Kim J., Yoon H., Chung D.E., Brown J.L., Belmonte K.C., Kim J. miR-186 is decreased in aged brain and suppresses BACE1 expression. J. Neurochem. 2016;137:436–445. doi: 10.1111/jnc.13507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Sun X., Wu Y., Gu M., Zhang Y. MiR-342-5p decreases ankyrin G levels in Alzheimer’s disease transgenic mouse models. Cell Rep. 2014;6:264–270. doi: 10.1016/j.celrep.2013.12.028. [DOI] [PubMed] [Google Scholar]
- 81.Liang C., Zhu H., Xu Y., Huang L., Ma C., Deng W., Liu Y., Qin C. MicroRNA-153 negatively regulates the expression of amyloid precursor protein and amyloid precursor-like protein 2. Brain Res. 2012;1455:103–113. doi: 10.1016/j.brainres.2011.10.051. [DOI] [PubMed] [Google Scholar]
- 82.Guo X.D., Sun G.L., Zhou T.T., Wang Y.Y., Xu X., Shi X.F., Zhu Z.Y., Rukachaisirikul V., Hu L.H., Shen X. LX2343 alleviates cognitive impairments in AD model rats by inhibiting oxidative stress-induced neuronal apoptosis and tauopathy. Acta Pharmacol. Sin. 2017;38:1104–1119. doi: 10.1038/aps.2016.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Salminen A., Kaarniranta K., Kauppinen A. Crosstalk between oxidative stress and SIRT1: Impact on the aging process. Int. J. Mol. Sci. 2013;14:3834–3859. doi: 10.3390/ijms14023834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Dias-Santagata D., Fulga T.A., Duttaroy A., Feany M.B. Oxidative stress mediates tau-induced neurodegeneration in Drosophila. J. Clin. Investig. 2007;117:236–245. doi: 10.1172/JCI28769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Wang L., Liu J., Wang Q., Jiang H., Zeng L., Li Z., Liu R. MicroRNA-200a-3p mediates neuroprotection in Alzheimer-related deficits and attenuates amyloid-beta overproduction and tau hyperphosphorylation via coregulating BACE1 and PRKACB. Front. Pharmacol. 2019;10 doi: 10.3389/fphar.2019.00806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Li J., Chen W., Yi Y., Tong Q. miR-219-5p inhibits tau phosphorylation by targeting TTBK1 and GSK-3β in Alzheimer’s disease. J. Cell. Biochem. 2019;120:9936–9946. doi: 10.1002/jcb.28276. [DOI] [PubMed] [Google Scholar]
- 87.El Fatimy R., Li S., Chen Z., Mushannen T., Gongala S., Wei Z., Balu D.T., Rabinovsky R., Cantlon A., Elkhal A., et al. MicroRNA-132 provides neuroprotection for tauopathies via multiple signaling pathways. Acta Neuropathol. 2018;136:537–555. doi: 10.1007/s00401-018-1880-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Smith P.Y., Hernandez-Rapp J., Jolivette F., Lecours C., Bisht K., Goupil C., Dorval V., Parsi S., Morin F., Planel E., et al. MiR-132/212 deficiency impairs tau metabolism and promotes pathological aggregation in vivo. Hum. Mol. Gen. 2015;24:6721–6735. doi: 10.1093/hmg/ddv377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Wang G., Huang Y., Wang L.-L., Zhang Y.-F., Xu J., Zhou Y., Lourenco G.F., Zhang B., Wang Y., Ren R.-J., et al. MicroRNA-146a suppresses ROCK1 allowing hyperphosphorylation of tau in Alzheimer’s disease. Sci. Rep. 2016;6:26697. doi: 10.1038/srep26697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Absalon S., Kochanek D.M., Raghavan V., Krichevsky A.M. MiR-26b, upregulated in Alzheimer’s disease, activates cell cycle entry, tau-phosphorylation, and apoptosis in postmitotic neurons. J. Neurosci. 2013;33:14645–14659. doi: 10.1523/JNEUROSCI.1327-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Zhang R., Zhang Q., Niu J., Lu K., Xie B., Cui D., Xu S. Screening of microRNAs associated with Alzheimer’s disease using oxidative stress cell model and different strains of senescence accelerated mice. J. Neurol. Sci. 2014;338:57–64. doi: 10.1016/j.jns.2013.12.017. [DOI] [PubMed] [Google Scholar]
- 92.Zhou Y., Wang Z.-F., Li W., Hong H., Chen J., Tian Y., Liu Z.-Y. Protective effects of microRNA-330 on amyloid β-protein production, oxidative stress, and mitochondrial dysfunction in Alzheimer’s disease by targeting VAV1 via the MAPK signaling pathway. J. Cell. Biochem. 2018;119:5437–5448. doi: 10.1002/jcb.26700. [DOI] [PubMed] [Google Scholar]
- 93.Gonzalez-Foruria I., Santulli P., Chouzenoux S., Carmona F., Chapron C., Batteux F. Dysregulation of the ADAM17/Notch signalling pathways in endometriosis: From oxidative stress to fibrosis. Mol. Hum. Reprod. 2017;23:488–499. doi: 10.1093/molehr/gax028. [DOI] [PubMed] [Google Scholar]
- 94.Jiao W.E., Wei J.F., Kong Y.G., Xu Y., Tao Z.Z., Chen S.M. Notch signaling promotes development of allergic rhinitis by suppressing Foxp3 expression and treg cell differentiation. Int. Arch. Allergy Immunol. 2019;178:33–44. doi: 10.1159/000493328. [DOI] [PubMed] [Google Scholar]
- 95.Zhu P., Yang M., He H., Kuang Z., Liang M., Lin A., Liang S., Wen Q., Cheng Z., Sun C. Curcumin attenuates hypoxia/reoxygenationinduced cardiomyocyte injury by downregulating Notch signaling. Mol. Med. Rep. 2019;20:1541–1550. doi: 10.3892/mmr.2019.10371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Chen F.-Z., Zhao Y., Chen H.-Z. MicroRNA-98 reduces amyloid β-protein production and improves oxidative stress and mitochondrial dysfunction through the Notch signaling pathway via HEY2 in Alzheimer’s disease mice. Int. J. Mol. Med. 2019;43:91–102. doi: 10.3892/ijmm.2018.3957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Zhang L., Dong H., Si Y., Wu N., Cao H., Mei B., Meng B. miR-125b promotes tau phosphorylation by targeting the neural cell adhesion molecule in neuropathological progression. Neurobiol. Aging. 2019;73:41–49. doi: 10.1016/j.neurobiolaging.2018.09.011. [DOI] [PubMed] [Google Scholar]
- 98.Jin Y., Tu Q., Liu M. MicroRNA125b regulates Alzheimer’s disease through SphK1 regulation. Mol. Med. Rep. 2018;18:2373–2380. doi: 10.3892/mmr.2018.9156. [DOI] [PubMed] [Google Scholar]
- 99.Wu D.-M., Wen X., Wang Y.-J., Han X.-R., Wang S., Shen M., Fan S.-H., Zhuang J., Zhang Z.-F., Shan Q., et al. Effect of microRNA-186 on oxidative stress injury of neuron by targeting interleukin 2 through the janus kinase-signal transducer and activator of transcription pathway in a rat model of Alzheime’s disease. J. Cell. Physiol. 2018;233:9488–9502. doi: 10.1002/jcp.26843. [DOI] [PubMed] [Google Scholar]
- 100.Jellinger K.A. Neuropathobiology of non-motor symptoms in Parkinson disease. J. Neural Transm. 2015;122:1429–1440. doi: 10.1007/s00702-015-1405-5. [DOI] [PubMed] [Google Scholar]
- 101.Tysnes O.B., Storstein A. Epidemiology of Parkinson’s disease. J. Neural Transm. 2017;124:901–905. doi: 10.1007/s00702-017-1686-y. [DOI] [PubMed] [Google Scholar]
- 102.Wei Z., Li X., Li X., Liu Q., Cheng Y. Oxidative stress in Parkinson’s disease: A systematic review and meta-analysis. Front. Mol. Neurosci. 2018;11:236. doi: 10.3389/fnmol.2018.00236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Hwang D.Y., Hong S., Jeong J.W., Choi S., Kim H., Kim J., Kim K.S. Vesicular monoamine transporter 2 and dopamine transporter are molecular targets of Pitx3 in the ventral midbrain dopamine neurons. J. Neurochem. 2009;111:1202–1212. doi: 10.1111/j.1471-4159.2009.06404.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Li Y., Li C., Chen Z., He J., Tao Z., Yin Z.Q. A microRNA, mir133b, suppresses melanopsin expression mediated by failure dopaminergic amacrine cells in RCS rats. Cell Signal. 2012;24:685–698. doi: 10.1016/j.cellsig.2011.10.017. [DOI] [PubMed] [Google Scholar]
- 105.Caudle W.M., Richardson J.R., Wang M.Z., Taylor T.N., Guillot T.S., McCormack A.L., Colebrooke R.E., Di Monte D.A., Emson P.C., Miller G.W. Reduced vesicular storage of dopamine causes progressive nigrostriatal neurodegeneration. J. Neurosci. 2007;27:8138–8148. doi: 10.1523/JNEUROSCI.0319-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Masoud S.T., Vecchio L.M., Bergeron Y., Hossain M.M., Nguyen L.T., Bermejo M.K., Kile B., Sotnikova T.D., Siesser W.B., Gainetdinov R.R., et al. Increased expression of the dopamine transporter leads to loss of dopamine neurons, oxidative stress and l-DOPA reversible motor deficits. Neurobiol. Dis. 2015;74:66–75. doi: 10.1016/j.nbd.2014.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Jia X., Wang F., Han Y., Geng X., Li M., Shi Y., Lu L., Chen Y. MiR-137 and miR-491 negatively regulate dopamine transporter expression and function in neural cells. Neurosci. Bull. 2016;32:512–522. doi: 10.1007/s12264-016-0061-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Barodia S.K., Creed R.B., Goldberg M.S. Parkin and PINK1 functions in oxidative stress and neurodegeneration. Brain Res. Bull. 2017;133:51–59. doi: 10.1016/j.brainresbull.2016.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Wang H.L., Chou A.H., Wu A.S., Chen S.Y., Weng Y.H., Kao Y.C., Yeh T.H., Chu P.J., Lu C.S. PARK6 PINK1 mutants are defective in maintaining mitochondrial membrane potential and inhibiting ROS formation of substantia nigra dopaminergic neurons. Biochim. Biophys. Acta. 2011;1812:674–684. doi: 10.1016/j.bbadis.2011.03.007. [DOI] [PubMed] [Google Scholar]
- 110.Wood-Kaczmar A., Gandhi S., Yao Z., Abramov A.Y., Miljan E.A., Keen G., Stanyer L., Hargreaves I., Klupsch K., Deas E., et al. PINK1 is necessary for long term survival and mitochondrial function in human dopaminergic neurons. PLoS ONE. 2008;3:e2455. doi: 10.1371/annotation/ba489c2a-5cf2-481c-aff7-d2c8c4ecdcfa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Kim J., Fiesel F.C., Belmonte K.C., Hudec R., Wang W.X., Kim C., Nelson P.T., Springer W., Kim J. miR-27a and miR-27b regulate autophagic clearance of damaged mitochondria by targeting PTEN-induced putative kinase 1 (PINK1) Mol. Neurodegener. 2016;11:55. doi: 10.1186/s13024-016-0121-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Prajapati P., Sripada L., Singh K., Bhatelia K., Singh R., Singh R. TNF-alpha regulates miRNA targeting mitochondrial complex-I and induces cell death in dopaminergic cells. Biochim. Biophys. Acta. 2015;1852:451–461. doi: 10.1016/j.bbadis.2014.11.019. [DOI] [PubMed] [Google Scholar]
- 113.Hayashi T., Ishimori C., Takahashi-Niki K., Taira T., Kim Y.C., Maita H., Maita C., Ariga H., Iguchi-Ariga S.M. DJ-1 binds to mitochondrial complex I and maintains its activity. Biochem. Biophys. Res. Commun. 2009;390:667–672. doi: 10.1016/j.bbrc.2009.10.025. [DOI] [PubMed] [Google Scholar]
- 114.Ariga H., Takahashi-Niki K., Kato I., Maita H., Niki T., Iguchi-Ariga S.M. Neuroprotective function of DJ-1 in Parkinson’s disease. Oxid. Med. Cell Longev. 2013;2013:683920. doi: 10.1155/2013/683920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Choi J., Sullards M.C., Olzmann J.A., Rees H.D., Weintraub S.T., Bostwick D.E., Gearing M., Levey A.I., Chin L.S., Li L. Oxidative damage of DJ-1 is linked to sporadic Parkinson and Alzheimer diseases. J. Biol. Chem. 2006;281:10816–10824. doi: 10.1074/jbc.M509079200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Xiong R., Wang Z., Zhao Z., Li H., Chen W., Zhang B., Wang L., Wu L., Li W., Ding J., et al. MicroRNA-494 reduces DJ-1 expression and exacerbates neurodegeneration. Neurobiol. Aging. 2014;35:705–714. doi: 10.1016/j.neurobiolaging.2013.09.027. [DOI] [PubMed] [Google Scholar]
- 117.Chen Y., Gao C., Sun Q., Pan H., Huang P., Ding J., Chen S. MicroRNA-4639 is a regulator of DJ-1 expression and a potential early diagnostic marker for Parkinson’s disease. Front. Aging Neurosci. 2017;9:232. doi: 10.3389/fnagi.2017.00232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Minones-Moyano E., Porta S., Escaramis G., Rabionet R., Iraola S., Kagerbauer B., Espinosa-Parrilla Y., Ferrer I., Estivill X., Marti E. MicroRNA profiling of Parkinson’s disease brains identifies early downregulation of miR-34b/c which modulate mitochondrial function. Hum. Mol. Genet. 2011;20:3067–3078. doi: 10.1093/hmg/ddr210. [DOI] [PubMed] [Google Scholar]
- 119.Ramsey C.P., Glass C.A., Montgomery M.B., Lindl K.A., Ritson G.P., Chia L.A., Hamilton R.L., Chu C.T., Jordan-Sciutto K.L. Expression of Nrf2 in neurodegenerative diseases. J. Neuropathol. Exp. Neurol. 2007;66:75–85. doi: 10.1097/nen.0b013e31802d6da9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Xie Y., Chen Y. MicroRNAs: Emerging targets regulating oxidative stress in the models of Parkinson’s disease. Front. Neurosci. 2016;10:298. doi: 10.3389/fnins.2016.00298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Kabaria S., Choi D.C., Chaudhuri A.D., Jain M.R., Li H., Junn E. MicroRNA-7 activates Nrf2 pathway by targeting Keap1 expression. Free Radic. Biol. Med. 2015;89:548–556. doi: 10.1016/j.freeradbiomed.2015.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.McMillan K.J., Murray T.K., Bengoa-Vergniory N., Cordero-Llana O., Cooper J., Buckley A., Wade-Martins R., Uney J.B., O’Neill M.J., Wong L.F., et al. Loss of MicroRNA-7 regulation leads to alpha-synuclein accumulation and dopaminergic neuronal loss in vivo. Mol. Ther. 2017;25:2404–2414. doi: 10.1016/j.ymthe.2017.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Narasimhan M., Patel D., Vedpathak D., Rathinam M., Henderson G., Mahimainathan L. Identification of novel microRNAs in post-transcriptional control of Nrf2 expression and redox homeostasis in neuronal, SH-SY5Y cells. PLoS ONE. 2012;7:e51111. doi: 10.1371/journal.pone.0051111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Slota J.A., Booth S.A. MicroRNAs in neuroinflammation: Implications in disease pathogenesis, biomarker discovery and therapeutic applications. Noncoding RNA. 2019;5:35. doi: 10.3390/ncrna5020035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Farrer M.J. Genetics of Parkinson disease: Paradigm shifts and future prospects. Nat. Rev. Genet. 2006;7:306–318. doi: 10.1038/nrg1831. [DOI] [PubMed] [Google Scholar]
- 126.Hsu L.J., Sagara Y., Arroyo A., Rockenstein E., Sisk A., Mallory M., Wong J., Takenouchi T., Hashimoto M., Masliah E. alpha-synuclein promotes mitochondrial deficit and oxidative stress. Am. J. Pathol. 2000;157:401–410. doi: 10.1016/S0002-9440(10)64553-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Martin L.J., Pan Y., Price A.C., Sterling W., Copeland N.G., Jenkins N.A., Price D.L., Lee M.K. Parkinson’s disease alpha-synuclein transgenic mice develop neuronal mitochondrial degeneration and cell death. J. Neurosci. 2006;26:41–50. doi: 10.1523/JNEUROSCI.4308-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Perfeito R., Ribeiro M., Rego A.C. Alpha-synuclein-induced oxidative stress correlates with altered superoxide dismutase and glutathione synthesis in human neuroblastoma SH-SY5Y cells. Arch. Toxicol. 2017;91:1245–1259. doi: 10.1007/s00204-016-1788-6. [DOI] [PubMed] [Google Scholar]
- 129.Deas E., Cremades N., Angelova P.R., Ludtmann M.H., Yao Z., Chen S., Horrocks M.H., Banushi B., Little D., Devine M.J., et al. Alpha-synuclein oligomers interact with metal ions to induce oxidative stress and neuronal death in Parkinson’s disease. Antioxid. Redox Signal. 2016;24:376–391. doi: 10.1089/ars.2015.6343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Di Maio R., Barrett P.J., Hoffman E.K., Barrett C.W., Zharikov A., Borah A., Hu X., McCoy J., Chu C.T., Burton E.A., et al. alpha-Synuclein binds to TOM20 and inhibits mitochondrial protein import in Parkinson’s disease. Sci. Transl. Med. 2016;8:342ra378. doi: 10.1126/scitranslmed.aaf3634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Junn E., Lee K.W., Jeong B.S., Chan T.W., Im J.Y., Mouradian M.M. Repression of alpha-synuclein expression and toxicity by microRNA-7. Proc. Natl. Acad. Sci. USA. 2009;106:13052–13057. doi: 10.1073/pnas.0906277106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Wang Z.H., Zhang J.L., Duan Y.L., Zhang Q.S., Li G.F., Zheng D.L. MicroRNA-214 participates in the neuroprotective effect of Resveratrol via inhibiting alpha-synuclein expression in MPTP-induced Parkinson’s disease mouse. Biomed. Pharmacother. 2015;74:252–256. doi: 10.1016/j.biopha.2015.08.025. [DOI] [PubMed] [Google Scholar]
- 133.Doxakis E. Post-transcriptional regulation of alpha-synuclein expression by mir-7 and mir-153. J. Biol. Chem. 2010;285:12726–12734. doi: 10.1074/jbc.M109.086827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Kabaria S., Choi D.C., Chaudhuri A.D., Mouradian M.M., Junn E. Inhibition of miR-34b and miR-34c enhances alpha-synuclein expression in Parkinson’s disease. FEBS Lett. 2015;589:319–325. doi: 10.1016/j.febslet.2014.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Alvarez-Erviti L., Seow Y., Schapira A.H., Rodriguez-Oroz M.C., Obeso J.A., Cooper J.M. Influence of microRNA deregulation on chaperone-mediated autophagy and alpha-synuclein pathology in Parkinson’s disease. Cell Death Dis. 2013;4:e545. doi: 10.1038/cddis.2013.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Li G., Yang H., Zhu D., Huang H., Liu G., Lun P. Targeted suppression of chaperone-mediated autophagy by miR-320a promotes alpha-synuclein aggregation. Int. J. Mol. Sci. 2014;15:15845–15857. doi: 10.3390/ijms150915845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Zhang Z., Cheng Y. miR-16-1 promotes the aberrant alpha-synuclein accumulation in parkinson disease via targeting heat shock protein 70. Sci. World J. 2014;2014:938348. doi: 10.1155/2014/938348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Recasens A., Perier C., Sue C.M. Role of microRNAs in the Regulation of alpha-Synuclein Expression: A Systematic Review. Front. Mol. Neurosci. 2016;9:128. doi: 10.3389/fnmol.2016.00128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Dias V., Junn E., Mouradian M.M. The role of oxidative stress in Parkinson’s disease. J. Parkinsons Dis. 2013;3:461–491. doi: 10.3233/JPD-130230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Block M.L., Zecca L., Hong J.S. Microglia-mediated neurotoxicity: Uncovering the molecular mechanisms. Nat. Rev. Neurosci. 2007;8:57–69. doi: 10.1038/nrn2038. [DOI] [PubMed] [Google Scholar]
- 141.Thome A.D., Harms A.S., Volpicelli-Daley L.A., Standaert D.G. microRNA-155 regulates alpha-synuclein-induced inflammatory responses in models of Parkinson disease. J. Neurosci. 2016;36:2383–2390. doi: 10.1523/JNEUROSCI.3900-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Zhou Y., Lu M., Du R.H., Qiao C., Jiang C.Y., Zhang K.Z., Ding J.H., Hu G. MicroRNA-7 targets Nod-like receptor protein 3 inflammasome to modulate neuroinflammation in the pathogenesis of Parkinson’s disease. Mol. Neurodegener. 2016;16:11–28. doi: 10.1186/s13024-016-0094-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Caggiu E., Paulus K., Mameli G., Arru G., Sechi G.P., Sechi L.A. Differential expression of miRNA 155 and miRNA 146a in Parkinson’s disease patients. eNeurologicalSci. 2018;13:1–4. doi: 10.1016/j.ensci.2018.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Yao L., Ye Y., Mao H., Lu F., He X., Lu G., Zhang S. MicroRNA-124 regulates the expression of MEKK3 in the inflammatory pathogenesis of Parkinson’s disease. J. Neuroinflamm. 2018;15:13. doi: 10.1186/s12974-018-1053-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Wilhelmus M.M., Nijland P.G., Drukarch B., de Vries H.E., van Horssen J. Involvement and interplay of Parkin, PINK1, and DJ1 in neurodegenerative and neuroinflammatory disorders. Free Radic. Biol. Med. 2012;53:983–992. doi: 10.1016/j.freeradbiomed.2012.05.040. [DOI] [PubMed] [Google Scholar]
- 146.Van Es M.A., Hardiman O., Chio A., Al-Chalabi A., Pasterkamp R.J., Veldink J.H., van den Berg L.H. Amyotrophic lateral sclerosis. Lancet. 2017;390:2084–2098. doi: 10.1016/S0140-6736(17)31287-4. [DOI] [PubMed] [Google Scholar]
- 147.Volk A.E., Weishaupt J.H., Andersen P.M., Ludolph A.C., Kubisch C. Current knowledge and recent insights into the genetic basis of amyotrophic lateral sclerosis. Med. Genet. 2018;30:252–258. doi: 10.1007/s11825-018-0185-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Guerrero E.N., Wang H., Mitra J., Hegde P.M., Stowell S.E., Liachko N.F., Kraemer B.C., Garruto R.M., Rao K.S., Hegde M.L. TDP-43/FUS in motor neuron disease: Complexity and challenges. Prog. Neurobiol. 2016;145–146:78–97. doi: 10.1016/j.pneurobio.2016.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Belzil V.V., Katzman R.B., Petrucelli L. ALS and FTD: An epigenetic perspective. Acta Neuropathol. 2016;132:487–502. doi: 10.1007/s00401-016-1587-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Wang Z., Bai Z., Qin X., Cheng Y. Aberrations in oxidative stress markers in amyotrophic lateral sclerosis: A systematic review and meta-analysis. Oxid. Med. Cell Longev. 2019;2019:1712323. doi: 10.1155/2019/1712323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Joilin G., Leigh P.N., Newbury S.F., Hafezparast M. An overview of MicroRNAs as biomarkers of ALS. Front. Neurol. 2019;10:186. doi: 10.3389/fneur.2019.00186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Kovanda A., Leonardis L., Zidar J., Koritnik B., Dolenc-Groselj L., Ristic Kovacic S., Curk T., Rogelj B. Differential expression of microRNAs and other small RNAs in muscle tissue of patients with ALS and healthy age-matched controls. Sci. Rep. 2018;8:5609. doi: 10.1038/s41598-018-23139-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Ricci C., Marzocchi C., Battistini S. MicroRNAs as biomarkers in amyotrophic lateral sclerosis. Cells. 2018;7:219. doi: 10.3390/cells7110219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Rizzuti M., Filosa G., Melzi V., Calandriello L., Dioni L., Bollati V., Bresolin N., Comi G.P., Barabino S., Nizzardo M., et al. MicroRNA expression analysis identifies a subset of downregulated miRNAs in ALS motor neuron progenitors. Sci. Rep. 2018;8:10105. doi: 10.1038/s41598-018-28366-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Waller R., Wyles M., Heath P.R., Kazoka M., Wollff H., Shaw P.J., Kirby J. Small RNA sequencing of sporadic amyotrophic lateral sclerosis cerebrospinal fluid reveals differentially expressed miRNAs related to neural and glial activity. Front. Neurosci. 2017;11:731. doi: 10.3389/fnins.2017.00731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Borel F., Gernoux G., Sun H., Stock R., Blackwood M., Brown R.H., Jr., Mueller C. Safe and effective superoxide dismutase 1 silencing using artificial microRNA in macaques. Sci. Transl. Med. 2018;10 doi: 10.1126/scitranslmed.aau6414. [DOI] [PubMed] [Google Scholar]
- 157.Koval E.D., Shaner C., Zhang P., du Maine X., Fischer K., Tay J., Chau B.N., Wu G.F., Miller T.M. Method for widespread microRNA-155 inhibition prolongs survival in ALS-model mice. Hum. Mol. Genet. 2013;22:4127–4135. doi: 10.1093/hmg/ddt261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Li C., Wei Q., Gu X., Chen Y., Chen X., Cao B., Ou R., Shang H. Decreased glycogenolysis by miR-338-3p promotes regional glycogen accumulation within the spinal cord of amyotrophic lateral sclerosis mice. Front. Mol. Neurosci. 2019;12:114. doi: 10.3389/fnmol.2019.00114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Yamakuchi M., Ferlito M., Lowenstein C.J. miR-34a repression of SIRT1 regulates apoptosis. Proc. Natl. Acad. Sci. USA. 2008;105:13421–13426. doi: 10.1073/pnas.0801613105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Singh P., Hanson P.S., Morris C.M. SIRT1 ameliorates oxidative stress induced neural cell death and is down-regulated in Parkinson’s disease. BMC Neurosci. 2017;18:46. doi: 10.1186/s12868-017-0364-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Paladino S., Conte A., Caggiano R., Pierantoni G.M., Faraonio R. Nrf2 pathway in age-related neurological disorders: Insights into MicroRNAs. Cell Physiol. Biochem. 2018;47:1951–1976. doi: 10.1159/000491465. [DOI] [PubMed] [Google Scholar]
- 162.Ba Q., Cui C., Wen L., Feng S., Zhou J., Yang K. Schisandrin B shows neuroprotective effect in 6-OHDA-induced Parkinson’s disease via inhibiting the negative modulation of miR-34a on Nrf2 pathway. Biomed. Pharmacother. 2015;75:165–172. doi: 10.1016/j.biopha.2015.07.034. [DOI] [PubMed] [Google Scholar]
- 163.Wang N., Zhang L., Lu Y., Zhang M., Zhang Z., Wang K., Lv J. Down-regulation of microRNA-142-5p attenuates oxygen-glucose deprivation and reoxygenation-induced neuron injury through up-regulating Nrf2/ARE signaling pathway. Biomed. Pharmacother. 2017;89:1187–1195. doi: 10.1016/j.biopha.2017.03.011. [DOI] [PubMed] [Google Scholar]
- 164.Aschrafi A., Kar A.N., Natera-Naranjo O., MacGibeny M.A., Gioio A.E., Kaplan B.B. MicroRNA-338 regulates the axonal expression of multiple nuclear-encoded mitochondrial mRNAs encoding subunits of the oxidative phosphorylation machinery. Cell Mol. Life Sci. 2012;69:4017–4027. doi: 10.1007/s00018-012-1064-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Shioya M., Obayashi S., Tabunoki H., Arima K., Saito Y., Ishida T., Satoh J. Aberrant microRNA expression in the brains of neurodegenerative diseases: miR-29a decreased in Alzheimer disease brains targets neurone navigator 3. Neuropathol. Appl. Neurobiol. 2010;36:320–330. doi: 10.1111/j.1365-2990.2010.01076.x. [DOI] [PubMed] [Google Scholar]
- 166.McColgan P., Tabrizi S.J. Huntington’s disease: A clinical review. Eur. J. Neurol. 2018;25:24–34. doi: 10.1111/ene.13413. [DOI] [PubMed] [Google Scholar]
- 167.Banez-Coronel M., Ayhan F., Tarabochia A.D., Zu T., Perez B.A., Tusi S.K., Pletnikova O., Borchelt D.R., Ross C.A., Margolis R.L., et al. RAN translation in huntington disease. Neuron. 2015;88:667–677. doi: 10.1016/j.neuron.2015.10.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Ross C.A., Tabrizi S.J. Huntington’s disease: From molecular pathogenesis to clinical treatment. Lancet Neurol. 2011;10:83–98. doi: 10.1016/S1474-4422(10)70245-3. [DOI] [PubMed] [Google Scholar]
- 169.Moumne L., Betuing S., Caboche J. Multiple aspects of gene dysregulation in Huntington’s disease. Front. Neurol. 2013;4:127. doi: 10.3389/fneur.2013.00127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Zheng J., Winderickx J., Franssens V., Liu B. A Mitochondria-associated oxidative stress perspective on Huntington’s disease. Front. Mol. Neurosci. 2018;11:329. doi: 10.3389/fnmol.2018.00329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Johnson R., Zuccato C., Belyaev N.D., Guest D.J., Cattaneo E., Buckley N.J. A microRNA-based gene dysregulation pathway in Huntington’s disease. Neurobiol. Dis. 2008;29:438–445. doi: 10.1016/j.nbd.2007.11.001. [DOI] [PubMed] [Google Scholar]
- 172.Packer A.N., Xing Y., Harper S.Q., Jones L., Davidson B.L. The bifunctional microRNA miR-9/miR-9* regulates REST and CoREST and is downregulated in Huntington’s disease. J. Neurosci. 2008;28:14341–14346. doi: 10.1523/JNEUROSCI.2390-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Savas J.N., Makusky A., Ottosen S., Baillat D., Then F., Krainc D., Shiekhattar R., Markey S.P., Tanese N. Huntington’s disease protein contributes to RNA-mediated gene silencing through association with Argonaute and P bodies. Proc. Natl. Acad. Sci. USA. 2008;105:10820–10825. doi: 10.1073/pnas.0800658105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Pircs K., Petri R., Madsen S., Brattas P.L., Vuono R., Ottosson D.R., St-Amour I., Hersbach B.A., Matusiak-Bruckner M., Lundh S.H., et al. Huntingtin aggregation impairs autophagy, leading to argonaute-2 accumulation and global MicroRNA dysregulation. Cell Rep. 2018;24:1397–1406. doi: 10.1016/j.celrep.2018.07.017. [DOI] [PubMed] [Google Scholar]
- 175.Banez-Coronel M., Porta S., Kagerbauer B., Mateu-Huertas E., Pantano L., Ferrer I., Guzman M., Estivill X., Marti E. A pathogenic mechanism in Huntington’s disease involves small CAG-repeated RNAs with neurotoxic activity. PLoS Genet. 2012;8:e1002481. doi: 10.1371/journal.pgen.1002481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Lee S.T., Chu K., Im W.S., Yoon H.J., Im J.Y., Park J.E., Park K.H., Jung K.H., Lee S.K., Kim M., et al. Altered microRNA regulation in Huntington’s disease models. Exp. Neurol. 2011;227:172–179. doi: 10.1016/j.expneurol.2010.10.012. [DOI] [PubMed] [Google Scholar]
- 177.Marti E., Pantano L., Banez-Coronel M., Llorens F., Minones-Moyano E., Porta S., Sumoy L., Ferrer I., Estivill X. A myriad of miRNA variants in control and Huntington’s disease brain regions detected by massively parallel sequencing. Nucleic Acids Res. 2010;38:7219–7235. doi: 10.1093/nar/gkq575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Wexler N.S., Lorimer J., Porter J., Gomez F., Moskowitz C., Shackell E., Marder K., Penchaszadeh G., Roberts S.A., Gayan J., et al. Venezuelan kindreds reveal that genetic and environmental factors modulate Huntington’s disease age of onset. Proc. Natl. Acad. Sci. USA. 2004;101:3498–3503. doi: 10.1073/pnas.0308679101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Rosenblatt A., Liang K.Y., Zhou H., Abbott M.H., Gourley L.M., Margolis R.L., Brandt J., Ross C.A. The association of CAG repeat length with clinical progression in Huntington disease. Neurology. 2006;66:1016–1020. doi: 10.1212/01.wnl.0000204230.16619.d9. [DOI] [PubMed] [Google Scholar]
- 180.Johri A., Beal M.F. Antioxidants in Huntington’s disease. Biochim. Biophys. Acta. 2012;1822:664–674. doi: 10.1016/j.bbadis.2011.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Duran R., Barrero F.J., Morales B., Luna J.D., Ramirez M., Vives F. Oxidative stress and plasma aminopeptidase activity in Huntington’s disease. J. Neural. Transm. 2010;117:325–332. doi: 10.1007/s00702-009-0364-0. [DOI] [PubMed] [Google Scholar]
- 182.Olejniczak M., Kotowska-Zimmer A., Krzyzosiak W. Stress-induced changes in miRNA biogenesis and functioning. Cell Mol. Life Sci. 2018;75:177–191. doi: 10.1007/s00018-017-2591-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Asada S., Takahashi T., Isodono K., Adachi A., Imoto H., Ogata T., Ueyama T., Matsubara H., Oh H. Downregulation of Dicer expression by serum withdrawal sensitizes human endothelial cells to apoptosis. Am. J. Physiol. Heart Circ. Physiol. 2008;295:H2512–H2521. doi: 10.1152/ajpheart.00233.2008. [DOI] [PubMed] [Google Scholar]
- 184.Inukai S., de Lencastre A., Turner M., Slack F. Novel microRNAs differentially expressed during aging in the mouse brain. PLoS ONE. 2012;7:e40028. doi: 10.1371/journal.pone.0040028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Ludwig N., Leidinger P., Becker K., Backes C., Fehlmann T., Pallasch C., Rheinheimer S., Meder B., Stahler C., Meese E., et al. Distribution of miRNA expression across human tissues. Nucleic Acids Res. 2016;44:3865–3877. doi: 10.1093/nar/gkw116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Agarwal V., Bell G.W., Nam J.W., Bartel D.P. Predicting effective microRNA target sites in mammalian mRNAs. Elife. 2015;4 doi: 10.7554/eLife.05005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Witkos T.M., Koscianska E., Krzyzosiak W.J. Practical aspects of microRNA target prediction. Curr. Mol. Med. 2011;11:93–109. doi: 10.2174/156652411794859250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Jin H.Y., Gonzalez-Martin A., Miletic A.V., Lai M., Knight S., Sabouri-Ghomi M., Head S.R., Macauley M.S., Rickert R.C., Xiao C. Transfection of microRNA mimics should be used with caution. Front. Genet. 2015;6:340. doi: 10.3389/fgene.2015.00340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Jovicic A., Roshan R., Moisoi N., Pradervand S., Moser R., Pillai B., Luthi-Carter R. Comprehensive expression analyses of neural cell-type-specific miRNAs identify new determinants of the specification and maintenance of neuronal phenotypes. J. Neurosci. 2013;33:5127–5137. doi: 10.1523/JNEUROSCI.0600-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Nam J.W., Rissland O.S., Koppstein D., Abreu-Goodger C., Jan C.H., Agarwal V., Yildirim M.A., Rodriguez A., Bartel D.P. Global analyses of the effect of different cellular contexts on microRNA targeting. Mol. Cell. 2014;53:1031–1043. doi: 10.1016/j.molcel.2014.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.He M., Liu Y., Wang X., Zhang M.Q., Hannon G.J., Huang Z.J. Cell-type-based analysis of microRNA profiles in the mouse brain. Neuron. 2012;73:35–48. doi: 10.1016/j.neuron.2011.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Svoboda P. A toolbox for miRNA analysis. FEBS Lett. 2015;589:1694–1701. doi: 10.1016/j.febslet.2015.04.054. [DOI] [PubMed] [Google Scholar]
- 193.Knauss J.L., Bian S., Sun T. Plasmid-based target protectors allow specific blockade of miRNA silencing activity in mammalian developmental systems. Front. Cell Neurosci. 2013;7:163. doi: 10.3389/fncel.2013.00163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Ran F.A., Hsu P.D., Wright J., Agarwala V., Scott D.A., Zhang F. Genome engineering using the CRISPR-Cas9 system. Nat. Protoc. 2013;8:2281–2308. doi: 10.1038/nprot.2013.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Jinek M., Chylinski K., Fonfara I., Hauer M., Doudna J.A., Charpentier E. A programmable dual-RNA-guided DNA endonuclease in adaptive bacterial immunity. Science. 2012;337:816–821. doi: 10.1126/science.1225829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Gasiunas G., Barrangou R., Horvath P., Siksnys V. Cas9-crRNA ribonucleoprotein complex mediates specific DNA cleavage for adaptive immunity in bacteria. Proc. Natl. Acad. Sci. USA. 2012;109:E2579–E2586. doi: 10.1073/pnas.1208507109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Amin N.D., Bai G., Klug J.R., Bonanomi D., Pankratz M.T., Gifford W.D., Hinckley C.A., Sternfeld M.J., Driscoll S.P., Dominguez B., et al. Loss of motoneuron-specific microRNA-218 causes systemic neuromuscular failure. Science. 2015;350:1525–1529. doi: 10.1126/science.aad2509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Chang H., Yi B., Ma R., Zhang X., Zhao H., Xi Y. CRISPR/cas9, a novel genomic tool to knock down microRNA in vitro and in vivo. Sci. Rep. 2016;6:22312. doi: 10.1038/srep22312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Kurata J.S., Lin R.J. MicroRNA-focused CRISPR-Cas9 library screen reveals fitness-associated miRNAs. RNA. 2018;24:966–981. doi: 10.1261/rna.066282.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Back S., Necarsulmer J., Whitaker L.R., Coke L.M., Koivula P., Heathward E.J., Fortuno L.V., Zhang Y., Yeh C.G., Baldwin H.A., et al. Neuron-specific genome modification in the adult rat brain using CRISPR-Cas9 transgenic rats. Neuron. 2019;102:105–119. doi: 10.1016/j.neuron.2019.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Hess G.T., Tycko J., Yao D., Bassik M.C. Methods and applications of CRISPR-mediated base editing in eukaryotic genomes. Mol. Cell. 2017;68:26–43. doi: 10.1016/j.molcel.2017.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Zafra M.P., Schatoff E.M., Katti A., Foronda M., Breinig M., Schweitzer A.Y., Simon A., Han T., Goswami S., Montgomery E., et al. Optimized base editors enable efficient editing in cells, organoids and mice. Nat. Biotechnol. 2018;36:888–893. doi: 10.1038/nbt.4194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Hu J.H., Miller S.M., Geurts M.H., Tang W., Chen L., Sun N., Zeina C.M., Gao X., Rees H.A., Lin Z., et al. Evolved Cas9 variants with broad PAM compatibility and high DNA specificity. Nature. 2018;556:57–63. doi: 10.1038/nature26155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Anzalone A.V., Randolph P.B., Davis J.R., Sousa A.A., Koblan L.W., Levy J.M., Chen P.J., Wilson C., Newby G.A., Raguram A., et al. Search-and-replace genome editing without double-strand breaks or donor DNA. Nature. 2019 doi: 10.1038/s41586-019-1711-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Domanskyi A., Saarma M., Airavaara M. Prospects of neurotrophic factors for Parkinson’s disease: Comparison of protein and gene therapy. Hum. Gene Ther. 2015;26:550–559. doi: 10.1089/hum.2015.065. [DOI] [PubMed] [Google Scholar]
- 206.Espay A.J., Brundin P., Lang A.E. Precision medicine for disease modification in Parkinson disease. Nat. Rev. Neurol. 2017;13:119–126. doi: 10.1038/nrneurol.2016.196. [DOI] [PubMed] [Google Scholar]
- 207.La Manno G., Gyllborg D., Codeluppi S., Nishimura K., Salto C., Zeisel A., Borm L.E., Stott S.R.W., Toledo E.M., Villaescusa J.C., et al. Molecular diversity of midbrain development in mouse, human, and stem cells. Cell. 2016;167:566–580. doi: 10.1016/j.cell.2016.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Creed R.B., Goldberg M.S. New developments in genetic rat models of Parkinson’s disease. Mov. Disord. 2018;33:717–729. doi: 10.1002/mds.27296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Chesselet M.F., Richter F. Modelling of Parkinson’s disease in mice. Lancet Neurol. 2011;10:1108–1118. doi: 10.1016/S1474-4422(11)70227-7. [DOI] [PubMed] [Google Scholar]
- 210.Blesa J., Przedborski S. Parkinson’s disease: Animal models and dopaminergic cell vulnerability. Front. Neuroanat. 2014;8:155. doi: 10.3389/fnana.2014.00155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Berezikov E., Thuemmler F., van Laake L.W., Kondova I., Bontrop R., Cuppen E., Plasterk R.H. Diversity of microRNAs in human and chimpanzee brain. Nat. Genet. 2006;38:1375–1377. doi: 10.1038/ng1914. [DOI] [PubMed] [Google Scholar]
- 212.Londin E., Loher P., Telonis A.G., Quann K., Clark P., Jing Y., Hatzimichael E., Kirino Y., Honda S., Lally M., et al. Analysis of 13 cell types reveals evidence for the expression of numerous novel primate- and tissue-specific microRNAs. Proc. Natl. Acad. Sci. USA. 2015;112:E1106–E1115. doi: 10.1073/pnas.1420955112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.McLoughlin H.S., Wan J., Spengler R.M., Xing Y., Davidson B.L. Human-specific microRNA regulation of FOXO1: Implications for microRNA recognition element evolution. Hum. Mol. Genet. 2014;23:2593–2603. doi: 10.1093/hmg/ddt655. [DOI] [PubMed] [Google Scholar]
- 214.Franca G.S., Hinske L.C., Galante P.A., Vibranovski M.D. Unveiling the impact of the genomic architecture on the evolution of vertebrate microRNAs. Front. Genet. 2017;8:34. doi: 10.3389/fgene.2017.00034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Wang N., Zheng J., Chen Z., Liu Y., Dura B., Kwak M., Xavier-Ferrucio J., Lu Y.C., Zhang M., Roden C., et al. Single-cell microRNA-mRNA co-sequencing reveals non-genetic heterogeneity and mechanisms of microRNA regulation. Nat. Commun. 2019;10:95. doi: 10.1038/s41467-018-07981-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Engle S.J., Blaha L., Kleiman R.J. Best practices for translational disease modeling using human iPSC-derived neurons. Neuron. 2018;100:783–797. doi: 10.1016/j.neuron.2018.10.033. [DOI] [PubMed] [Google Scholar]
- 217.Tolosa E., Botta-Orfila T., Morato X., Calatayud C., Ferrer-Lorente R., Marti M.J., Fernandez M., Gaig C., Raya A., Consiglio A., et al. MicroRNA alterations in iPSC-derived dopaminergic neurons from Parkinson disease patients. Neurobiol. Aging. 2018;69:283–291. doi: 10.1016/j.neurobiolaging.2018.05.032. [DOI] [PubMed] [Google Scholar]
- 218.Katerji M., Filippova M., Duerksen-Hughes P. Approaches and methods to measure oxidative stress in clinical samples: Research applications in the cancer field. Oxid. Med. Cell Longev. 2019;2019:1279250. doi: 10.1155/2019/1279250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Grenier K., Kao J., Diamandis P. Three-dimensional modeling of human neurodegeneration: Brain organoids coming of age. Mol. Psychiatry. 2019 doi: 10.1038/s41380-019-0500-7. [DOI] [PubMed] [Google Scholar]
- 220.Windrem M.S., Schanz S.J., Morrow C., Munir J., Chandler-Militello D., Wang S., Goldman S.A. A competitive advantage by neonatally engrafted human glial progenitors yields mice whose brains are chimeric for human glia. J. Neurosci. 2014;34:16153–16161. doi: 10.1523/JNEUROSCI.1510-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Cardoso T., Adler A.F., Mattsson B., Hoban D.B., Nolbrant S., Wahlestedt J.N., Kirkeby A., Grealish S., Bjorklund A., Parmar M. Target-specific forebrain projections and appropriate synaptic inputs of hESC-derived dopamine neurons grafted to the midbrain of parkinsonian rats. J. Comp. Neurol. 2018;526:2133–2146. doi: 10.1002/cne.24500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Grealish S., Diguet E., Kirkeby A., Mattsson B., Heuer A., Bramoulle Y., Van Camp N., Perrier A.L., Hantraye P., Bjorklund A., et al. Human ESC-derived dopamine neurons show similar preclinical efficacy and potency to fetal neurons when grafted in a rat model of Parkinson’s disease. Cell Stem Cell. 2014;15:653–665. doi: 10.1016/j.stem.2014.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Adler A.F., Cardoso T., Nolbrant S., Mattsson B., Hoban D.B., Jarl U., Wahlestedt J.N., Grealish S., Bjorklund A., Parmar M. HESC-derived dopaminergic transplants integrate into basal ganglia circuitry in a preclinical model of Parkinson’s disease. Cell Rep. 2019;28:3462–3473. doi: 10.1016/j.celrep.2019.08.058. [DOI] [PMC free article] [PubMed] [Google Scholar]