Abstract
Noradrenergic locus coeruleus (LC) neuron loss is a significant feature of mild cognitive impairment and Alzheimer’s disease (AD). The LC is the primary source of norepinephrine in the forebrain, where it modulates attention and memory in vulnerable cognitive regions such as prefrontal cortex (PFC) and hippocampus. Furthermore, LC-mediated norepinephrine signaling is thought to play a role in blood-brain barrier (BBB) maintenance and neurovascular coupling, suggesting that LC degeneration may impact the high comorbidity of cerebrovascular disease and AD. However, the extent to which LC projection system degeneration influences vascular pathology is not fully understood. To address this question in vivo, we stereotactically lesioned LC projection neurons innervating the PFC of six-month-old Tg344–19 AD rats using the noradrenergic immunotoxin, dopamine-β-hydroxylase IgG-saporin (DBH-sap), or an untargeted control IgG-saporin (IgG-sap). DBH-sap-lesioned animals performed significantly worse than IgG-sap animals on the Barnes maze task in measures of both spatial and working memory. DBH-sap-lesioned rats also displayed increased amyloid and inflammation pathology compared to IgG-sap controls. However, we also discovered prominent parenchymal albumin extravasation with DBH-sap lesions indicative of BBB breakdown. Moreover, microvessel wall-to-lumen ratios were increased in the PFC of DBH-sap compared to IgG-sap rats, suggesting that LC deafferentation results in vascular remodeling. Finally, we noted an early emergence of amyloid angiopathy in the DBH-sap-lesioned Tg344–19 AD rats. Taken together, these data indicate that LC projection system degeneration is a nexus lesion that compromises both vascular and neuronal function in cognitive brain areas during the prodromal stages of AD.
Keywords: Alzheimer’s disease, blood-brain barrier, cerebral amyloid angiopathy, locus coeruleus, vascular remodeling
INTRODUCTION
Alzheimer’s disease (AD) is believed to have an extensive preclinical stage since older people with a clinical diagnosis of no cognitive impairment or mild cognitive impairment (MCI) consistently reveal pathological signatures similar to those with frank AD [1–3]. Moreover, the majority of MCI and AD cases also present with cerebrovascular pathology such as microinfarctions, microbleeds, and white matter lesions [4–9], and vascular comorbidities are significant risk factors for cognitive decline [10–14]. Vascular lesions impair the structure and function of the neurovascular unit, which alters cerebral blood flow regulation, disrupts blood-brain barrier (BBB) function, and reduces the brain’s repair potential, supporting the notion that vascular pathology potentiates AD by reducing the threshold for cognitive impairment and accelerating the pace of dementia [15]. Hence, the identification of neurovascular pathologic events during the preclinical and prodromal stages of AD may provide a unified framework for understanding vascular contributions to AD and improving therapeutic target identification within a disease modifying window.
In this regard, we recently demonstrated that the locus coeruleus (LC) displays a ~30–35% loss of noradrenergic neurons during the transition from no cognitive impairment to amnestic MCI, and that decreases in LC neuron number were significantly associated with poorer performance on neuropsycho-logical tests of episodic memory, semantic memory, working memory, perceptual speed, and visuospatial ability [16]. Taken together with previous reports that LC neurons are among the first to display neurofibrillary tangle pathology [17, 18] and that LC neuron loss correlates with Braak stage and clinical status [19, 20], our observations support the concept that LC projection system degeneration is a prominent feature of prodromal AD that contributes to cognitive impairment [21]. However, the mechanisms by which the loss of forebrain norepinephrine (NE) contributes to AD are unresolved. LC-NE signaling modulates attention, memory, executive function, and arousal [22], and in vitro and in vivo studies show that NE exerts a wide array of neuroprotective and neurotrophic effects that reduce inflammation, oxidative stress, and plaque and tangle pathology [23–28]. On the other hand, the LC also provides central regulation of the cerebral vascular system [29–33], yet the extent to which LC degeneration impacts cerebrovascular pathology during AD progression has received virtually no attention. To address this knowledge gap, we administered a dopamine-β-hydroxylase IgG-saporin (DBH-sap) immunotoxin [34] into the prefrontal cortex (PFC) of Tg344–19 AD rats [35], which effectively mimicked LC-NE deafferentation of a major LC projection zone [36]. DBH-sap and control IgG-saporin (IgG-sap)-treated rats were compared for cognitive performance, AD-like pathology including parenchymal and vascular amyloidosis, and cerebrovascular pathology including markers for BBB function and vascular remodeling. As reported below, our results suggest that prodromal degeneration of LC projection neurons promotes forebrain vascular pathology, which may contribute to the onset of cognitive impairment during the progression of AD.
MATERIALS AND METHODS
Animals and timeline
We used male and female Tg344–19 AD rats on the Fischer 344 background, overexpressing both human amyloid-β precursor protein (AβPP) bearing the AβPP KM670/671NL Swedish mutation (APP-swe) and human presenilin-1 (PS1) bearing the exon 9 deletion mutation (PS1ΔE9) under the mouse prion protein gene promoter [35]. Tg344–19 AD rats manifest an age-dependent cerebral deposition of amyloid-β (Aβ) plaque-like pathology that precedes tauopathy, gliosis, apoptotic loss of neurons in the cerebral cortex and hippocampus, and cognitive disturbances [35, 37, 38]. Original breeding colonies were provided by the Rat Resource Center (St. Louis, MO; funded by NIH grant P40 OD011062). Rats were bred by backcrossing hemizygous transgene positive animals to wild type Fischer 344 littermates. All animals were pair-housed in 12 h:12 h reverse light-dark cycle conditions and ad libitum access to chow and water. The experimental timeline was as follows: 1) at six months of age, animals were administered IgG-sap or DBH-sap, 2) six weeks later, animals were behaviorally tested, and 3) following behavioral testing, the animals were sacrificed for postmortem studies. All procedures were conducted in accordance with guidelines set by the Institutional Animal Care and Use Committee of Michigan State University.
Stereotactic surgeries
A total of 27 age matched transgenic rats were used for this study, as follows: DBH-sap, male (n = 7); DBH-sap, female (n = 6); IgG-sap, male (n = 8); IgG-sap, female (n = 6). Male and female rats were randomized using a randomization table, anesthetized with equithesin (~3 ml/kg, i.p.), and placed in a stereotaxic frame. DBH-sap or control IgG-sap (Advanced Targeting Systems, San Diego, CA, 2.5 μ g/injection) were administered to the PFC bilaterally at coordinates (relative to bregma; from dura) AP + 1.2, ±2.0, DV −3.0 using a Hamilton syringe (26 s/2” needle; Hamilton, Reno, NV) [39]. The needle was lowered to the site and immunotoxin injection began immediately at a rate of 0.5 μl/min and remained in place after the injection for an additional 5 min before being slowly retracted. Following surgeries, rats were administered buprenorphine (1.2 mg/kg), placed on a heating pad, and returned to a clean home cage once they started to ambulate.
Barnes maze
Rats were evaluated behaviorally at six weeks after stereotactic surgeries. The investigator was blinded to rat treatment group by a randomization table prior to testing and by coding the videos for all testing outcomes. All experimental sessions were recorded by a video camera placed above the apparatus and analyzed with video-tracking AnyMaze software (Stoelting, Wood Dale, IL). The software detected the center of the animal body and recorded distance moved and time active based on color differences between animal coat color and testing apparatus recorded by the camera. The short-protocol Barnes Maze for spatial and working memory function [40] was adapted from Attar and colleagues [41]. Briefly, during the habituation session, the animals were slowly pulled to the escape hole in a clear cylinder and then given 120 s to freely enter the escape hole. Afterwards, the animals were submitted to a set of two daily training sessions, with at least two trials. The first training session was performed 24 h after the habituation session. All trials lasted 120 s or until the animals reached the escape box. However, if the rats did not reach the target hole, the experimenter gently guided the animal towards it at the end of the trial using a clear cylinder. After reaching the escape box, animals remained inside for at least 60 s before being returned to their home cages. The escape box was always located in the same place during training. Animals were tested in groups of four, in order that all trials averaged 20 min between each trial per animal. Retrieval of spatial learning was evaluated in the probe session, which was conducted after the rest day, 48 h after the last training day. The procedure was similar to the training trials, but the escape box was removed and rats were evaluated for 120 s. At the beginning of each session, the animals were placed in an opaque container at the center of the maze. The container was then pulled up, and the animal was released to explore the maze. Parameters analyzed in these experiments included time spent in target quadrant and latency to target hole entry (measures of spatial learning and memory) and incorrect revisits to holes already investigated (a measure of working or procedural memory). Incorrect revisits were defined as searching the same hole twice within a trial when the revisit occurred after the inspection of other holes [42].
Open field test
Locomotor activity was evaluated using a standard open field test [43]. The open field apparatus (Stoelting) consisted of an open topped, 2 times 2 acrylic black box measuring 40.6 × 40.6 × 38 cm. The box placed at table height and all experimental sessions were recorded by a video camera placed above the apparatus and analyzed with the video-tracking software. All movements were automatically recorded and time mobile and distance traveled were plotted and the data were used to measure locomotor activity. On the day of testing, rats in their home cages were brought into the experimental room. Rats remained in the experimental room for 30 min, after which each rat was placed into the center of the observation box and recording began immediately. Movement was recorded in 5-min bins for 30 min.
Elevated plus maze
The open field test can also be used to measure anxiety [44]. However, to ensure the DBH-sap lesion did not produce any anxiolytic or anxiogenic effects that might impact behavioral outcomes [45], we also tested the rats on the elevated plus maze [44]. The maze consisted of four arms arranged in a plus shape, elevated 50 cm off the floor, with two arms opened as extend platforms away from the maze (open arms) and the other two arms as platforms with walls (closed arms). Rats were put in the center of the platform facing the same open arm and were allowed to explore the maze for 5 min. Time spent in open compared to closed arms was analyzed as a measure of relative fear/anxiety.
Tissue preparation
Twenty-four hours after completing the final behavior trials, rats were deeply anesthetized (pentobarbital, 60 mg/kg, i.p.) and perfused intracardially with 0.9% saline containing 10,000 USP/L heparin. Rat brains were immediately removed and hemisected in the sagittal plane at midline using a brain block. One hemisphere was post-fixed in 4% paraformaldehyde (Electron Microscopy Sciences, Hatfield, PA) in 0.1 M phosphate buffer (pH 7.2) for 24–48 h and processed for immunohistochemistry, whereas the other hemisphere was flash frozen and processed for biochemical analyses.
Immunohistochemistry
Post-fixed brain hemispheres were transferred to 15% sucrose in 0.1 M phosphate buffer until saturated, then 30% sucrose in 0.1 M phosphate buffer until saturated. Brains were frozen on dry ice and sectioned at a 40 μm thickness in 1:12 series in the coronal plane using a freezing-sliding microtome (American Optical, Buffalo, NY). Serial sections were processed for immunohistochemistry (IHC) using the free-floating method [16, 46, 47]. All antibodies and dilutions used in these experiments are listed in Table 1. Control experiments including primary antibody deletions were performed for each antibody.
Table 1.
Antibodies used in the present study
| Antibody | Source | Dilution (application) |
|---|---|---|
| DBH rabbit polyelonal antiserum | Immunostar #22806 | 1:4000 (IHC) |
| albumin rabbit polyelonal antiserum | ProteinTech #16475-l-AP | 1:500 (IHC) 1:10,000 (WB) |
| smooth muscle actin (ACTA2) rabbit polyelonal antiserum | ProteinTech #23081-1-AP | 1:1000 (IHC) |
| MOAB-2 (Aβ 1–4) mouse IgG2b monoclonal antibody | Gift from Dr. Nicholas Kanaan, Michigan State University | 1:4000 (IHC) |
| GFAP rabbit polyelonal antiserum | Abeam #ab7260 | 1:10,000 (IHC) 1:20,000 (WB) |
| SMI71 mouse IgM monoclonal antibody | Biolegend #836804 | 1:1000 (IHC) |
| AT8 (tau phospho-serine 202, phospho-threonine 205) mouse IgGl mouse monoclonal antibody | Thermo Fisher #MN1020 | 1:100–1:5000 (IHC) |
| CP-13 (tau phospho-serine 202) mouse IgG2b monoclonal antibody | Gift from Dr. Peter Davies, Northwell Feinstein Institute | 1:100–1:5000 (IHC) |
Brightfield and fluorescence IHC
Tissue sections (1:12 series/experiment) were rinsed in Tris-buffered saline (TBS; pH 7.4) and nonfluorescence sections were quenched in 0.3% H2O2 for 1 h at room temperature. Tissue was then permeabilized with TBS+0.5% Triton X-100 (TBS-TX) and blocked in TBS-TX/10% normal goat serum for 1 h, followed by overnight incubation in primary antibody in TBS-TX/1% goat serum at 4°C under constant agitation. Following primary antibody incubation, sections were rinsed with TBS-TX and incubated in biotinylated goat anti-rabbit or anti-mouse IgG (1:500 dilution for both secondary antisera; Vector Labs, Burlingame, CA) or fluorescent goat anti-rabbit (1:500; Alexa Fluor 488; Thermo Scientific/Molecular Probes, Eugene, OR) or goat anti-mouse (1:500; Alexa Fluor 594) IgG for 2 h at room temperature, followed by TBS-TX washes. Brightfield IHC was further processed with the Vector Labs ABC detection kit for 1 h at room temperature and antibody labeling was visualized by exposure to 0.5 mg/ml 3,3’ diaminobenzidine (DAB) and 0.03% H2O2 in TBS or to DAB+2.5 mg/mL nickel ammonium sulfate and 0.03% H2O2 in TBS. Sections were mounted on subbed slides, dehydrated via ascending ethanol washes, cleared with xylenes, and cover-slipped with Cytoseal (ThermoFisher, Waltham, MA). Images were taken on a Nikon Eclipse 90i microscope with a Nikon DS-Ri1 camera.
LI-COR infrared IHC and imaging
Serial tissue sections (1:12) were rinsed in TBS-TX and blocked in TBS-TX/10% normal goat serum for 1 h. Sections were then incubated in primary antisera (Table 1) overnight at 4°C under constant agi tation. Following primary incubation, sections were rinsed with TBS-TX then incubated with LI-COR near-infrared secondary antibodies for 2 h at room temperature in the dark. Antibodies used were IRDye 800 conjugated goat anti-rabbit (1:500; LI-COR Biosciences, Lincoln, NE) and IRDye 680 conjugated goat anti-mouse IgG (1:500). Sections were then rinsed in TBS, mounted on subbed slides, dehydrated via ascending ethanol washes, cleared with xylenes, and cover-slipped with Cytoseal. Slides were left to dry for 48 h in the dark at room temperature. Slides were then scanned on a LI-COR Odyssey imaging station to determine DBH, Aβ, and albumin signal intensity (Table 1). For DBH signal intensity, boundaries were drawn around the entire PFC using the LI-COR Image Studio 3.1 software to obtain an average signal strength. Tracings began with sections at+5 mm from bregma and were terminated at bregma (~8–10 sections/animal) [39]. Reported integrated intensity measurements of DBH expression rostrocaudally across the sections were collected using the 800 nm channel and were normalized to background levels obtained from a 100-pixel sampling area in the basal ganglia of each brain. Data were represented as the mean cortical DBH integrated intensity measurement per brain. DBH staining intensity data showed that DBH signal loss was also found in cortical regions beyond PFC (Fig. 3), most likely due to the highly bifurcated morphology of corticopetal LC axons [48]. Therefore, to measure Aβ signal intensity, boundaries were drawn around the entire cortex and hippocampus to obtain an averaged signal strength normalized to area. For albumin signal intensity, boundaries were drawn around the entire cortex. Reported integrated intensity measurements of albumin or Aβ expression rostrocaudally across the sections were collected using the 800 nm channel and were normalized to background levels obtained in the intermediate reticulate nucleus (caudal to the LC) of each brain. Investigators were blinded to rat treatment group during all procedures. For CAA analysis, the same tissue sections and boundaries used to quantify Aβ load in each animal were analyzed in a blinded manner for MOAB-2-positive vascular profiles. Profiles were counted for 100% of the area using a Nikon Eclipse 90i microscope outfitted with a near-infrared filter cube (Chroma Tech, Bellows Falls, VT).
Fig. 3.
Cortical and hippocampal amyloidosis is accelerated in DBH-sap-lesioned Tg344–19 AD rats. Representative images showing increased MOAB-2 immunostaining in both the cortex and hippocampus of (A) IgG-sap- and (B) DBH-sap-lesioned animals. Modest vascular MOAB2 deposition is observed in large vessels the DBH-sap lesioned cortex (arrows and panel B inset). Optical densitometry measurements revealed (C) a~30 increase in cortical amyloid (p = 0.0008) and (D) a~20% increase in hippocampal amyloid (p = 0.015) at 6 weeks following DBH-sap lesions compared to IgG-sap. Dual-label fluorescence microscopy with MOAB-2 (red) and the vascular marker SMI71 (green) revealed additional evidence for emerging CAA following DBH-sap (F) compared to IgG-sap (E) lesions. G) Brightfield microscopy in MOAB-2 immunostained tissue revealed additional evidence for emergent CAA in the vicinity of amyloid plaques in the PFC of DBH-sap-treated animals. H) A semi-quantitative counting procedure of MOAB-2 labeled tissue sections showed a ten-fold increase in CAA-like vascular profiles in DBH-sap compared to IgG-sap rats. *p < 0.05; ***p < 0.001; ****p < 0.0001 via Student’s t test. Panels A and B scale bars = 1000 μm. Panel G scale bar = 50 μm.
Western blot analyses
The flash frozen hemisphere of the brain (stored at −80°C) was placed on a modified cold plate (Teca, Chicago, IL) at −18°C for 1 h in a Leica CM3050 S cryostat chamber before being microdissected with a small cortical tissue punch (1.5 mm diameter) at the level of the PFC rostral to the injection site. Frozen dissected structures were placed in pre-chilled microcentrifuge tubes and stored at −80°C until analysis.
Samples for western blot analyses were homogenized at 4°C for 2 h using the RIPA Lysis Buffer System (Santa Cruz, Dallas, TX). Total protein concentration was determined by the Pierce BCA Protein Assay (ThermoFisher). The western blot protocol was performed as previously described [49, 50]. Protein lysates (20 μg/sample) were electrophoresed in duplicate using SDS-PAGE Criterion gels (BIO-RAD, Hercules, CA) and transferred to Immobilon-FL membranes (Millipore, Bedford, MA). Membranes were incubated in primary antisera to albumin (as a measure of BBB leakage) or glial fibrillary acidic protein (GFAP; as an index of astrogliosis/inflammation) overnight. A monoclonal antibody to glyceraldehyde 3-phosphate dehydrogenase (GAPDH) was co-incubated with the albumin and GFAP antisera as a loading control (see Table 1). Blots were rinsed and then incubated using the LI-COR IRDye800-conjugated goat anti-rabbit and IRDye680-conjugated goat anti-mouse IgG (1:20,000) secondary antibodies. All antibody dilutions were made in TBS/2% nonfat dairy milk. Multiplexed signal intensities were imaged with both 700 and 800 nm channels in a single scan with a resolution of 169 μm using the LI-COR Odyssey image system. Albumin and GFAP immunoreactive signals were normalized to GAPDH for quantitative analysis using LI-COR Image Studio software.
Vessel wall-to-lumen measurements
Wall-to-lumen ratios (WLR) of PFC parenchymal arterioles were measured based on previous methods as an index of cerebral vessel remodeling, where increased wall: lumen measurements indicate stenosis, increased myogenic tone, and reduced vasoreactivity [51, 52]. Briefly, slides of α-actin 2 (smooth muscle actin)-labeled tissue (see Table 1) were randomized, the rater was blinded, and the first six α-actin 2-immunopositive PFC parenchymal arterioles coursing perpendicular to the visual plane as identified using Meander Scan (MBF Bioscience, Williston, VT) were measured. Since the majority of arterioles sampled were smaller than 50 μm in diameter and larger arterioles were not equally found in-plane between the two groups of rats, intergroup comparison of arteriole profile was based only on arterioles with an external diameter < 50 μm. Measurements were made on a Nikon Eclipse 90i microscope with a Nikon DS-Ri1 camera using Nikon Elements AR analysis software. WLR was calculated as follows [53]:
Sample size and power
A total of 27 age matched transgenic rats were used for this study, as follows: DBH-sap, male (n = 7); DBH-sap, female (n = 6); IgG-sap, male (n = 8); IgG-sap, female (n = 6). Power analyses using results from preliminary behavioral experiments indicated an n = 5/group would have 90% power to detect 1.25 standard deviations between DBH and IgG-sap-lesioned animals. The prospective experimental design required that DBH-sap-lesioned animals exhibit > 30% DBH signal loss compared to mean IgG-sap signal to be included in the study. No sex differences were observed in the animals in behavior or pathology (data not shown), consistent with previous observations in Tg344–19 rats [35, 38], so both sexes were evaluated together as DBH-sap or IgG-sap groups.
Statistics
Data analysis and graph creation were performed using GraphPad Prism software (GraphPad, version 7; La Jolla, CA). All data sets were verified for normality using D’Agostino and Pearson omnibus testing. To compare DBH-sap versus IgG-sap rats, Student’s t-tests were used. The level of statistical significance was set at p < 0.05 (two-tailed). Linear regression was performed to assess the relationships among the cognitive and pathological outcome variables.
RESULTS
DBH-sap lesioned animals exhibit spatial and working memory deficits
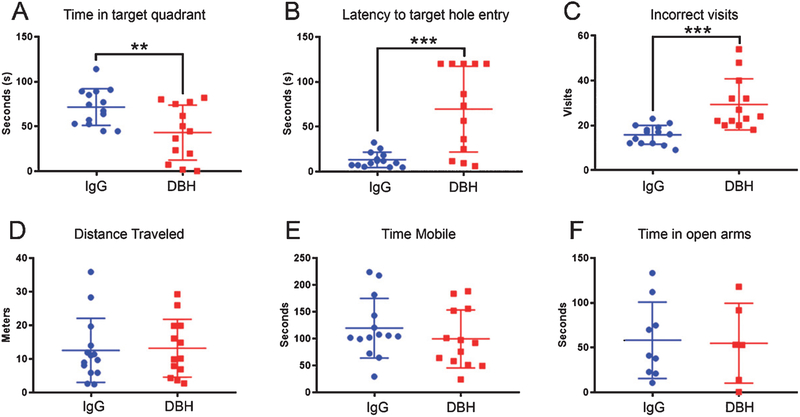
DBH-sap and IgG-sap-treated Tg344–19 AD rats were evaluated on the Barnes Maze at six weeks after surgery [40, 41]. Animals with the DBH-sap lesion spent significantly less time in the target quadrant during the probe trial (Fig. 1A; p = 0.0083) and were significantly slower to find the target hole (Fig. 1B; p = 0.0002) than IgG-sap rats. Both of these measures indicate a deficit in spatial memory. Interestingly, the DBH-sap animals also showed a greater number of revisits to holes that they had already investigated during the probe trial (Fig. 1 C; p = 0.0003) suggesting working memory deficits [54]. By contrast, there were no significant differences between DBH-sap or IgG-sap-lesioned animals during the open field test, which included measurements of distance traveled (Fig. 1D; p = 0.85) and time mobile (Fig. 1E; p = 0.35). Although DBH-sap lesions were administered in PFC, LC axons projecting into the telencephalon through the dorsal tegmental bundle are highly bifurcated, with lateral branches providing an immense, reciprocal innervation of the amygdala [55, 56]. To ensure that there were no amygdala-related anxiogenic or anxiolytic effects of the lesions that might impact cognitive or motor behavior, we evaluated the animals using the elevated plus maze [57]. There were no differences in the time spent in the open arms during this task (Fig. 1F; p = 0.8).
Fig. 1.
DBH-sap-lesioned Tg344–19 AD rats are impaired in measures of spatial and working memory at six weeks after surgery. Animals were evaluated on the Barnes Maze six weeks after stereotactic PFC administration of DBH IgG- or control IgG-saporin. Data are displayed as scatter plots with mean ± SD. A) DBH-sap-lesioned animals spent significantly less time in the target quadrant during the probe trial (p = 0.0083), suggesting LC-mediated deficits in spatial memory. B) DBH-sap animals were also significantly slower to find the target hole during probe trial (p = 0.0002), which is another measure of spatial memory. C) DBH-sap-lesioned rats made more revisits to holes already investigated (p = 0.0003), suggesting an LC-mediated deficit in working memory compared to IgG-sap rats. By contrast, open field testing showed no significant differences between DBH-sap or IgG-sap-lesioned animals in (D) distance traveled (p = 0.85) or (E) time mobile (p = 0.35), indicating no locomotor or anxiogenic effects of the lesion. F) Elevated plus maze testing as an additional measure of fear or anxiety-related behaviors revealed no differences in the time spent in the open arms (p = 0.8). **p < 0.01; ***p < 0.001 via Student’s t test.
The DBH-sap lesion results in noradrenergic deafferentation of PFC
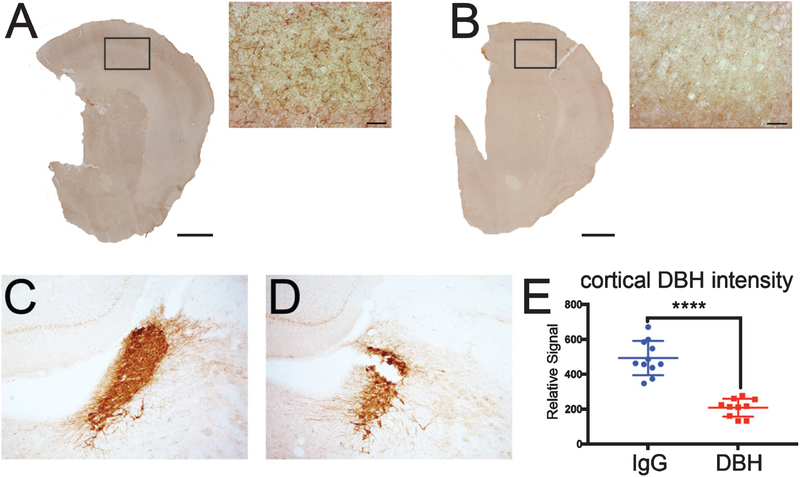
DBH-sap has been previously validated as a NE-specific immunotoxin in intraventricular surgeries [34, 58, 59]. DBH IHC revealed a striking loss of fibers within PFC, as well as in adjacent cortical and subcortical regions, in DBH-sap (Fig. 2B) compared to IgG-sap-lesioned animals (Fig. 2A). LC fiber loss beyond the PFC injection site likely reflected degeneration of LC axon bifurcations [45, 48]. Optical densitometry showed a 50% reduction in DBH-immunopositive profiles in the PFC of DBH-sap compared to IgG-sap animals (Fig. 2E; p < 0.0001). Notably, IHC of brainstem tissue from the same animals revealed a loss of DBH-immunoreactive profiles in the rostral LC, suggesting a concomitant loss of forebrain-projecting LC noradrenergic neurons in DBH-sap (Fig. 2D) compared to IgG-sap-lesioned animals (Fig. 2 C).
Fig. 2.
Evidence for LC system degeneration following the DBH-sap lesion in Tg344–19 AD rats. Representative DBH immunostaining in control IgG-sap lesioned cortex (A) and DBH-sap lesioned cortex (B). Note the loss of noradrenergic fibers following DBH-sap lesions. DBH-sap lesions also resulted in a loss of DBH-immunoreactive neuron density within the LC (D) compared to IgG-sap lesions (C). E) Quantitative optical densitometry measurements revealed a~60% loss of cortical DBH immunoreactivity in DBH-sap compared to IgGsap-lesioned rats. Data are displayed as a scatter plot with mean ± SD. ****p < 0.0001 via Student’s t test. Panels A and B scale bars: large images = 1000 μm; inset images = 50 μm.
DBH-sap lesioned Tg344 rats exhibit increased amyloid pathology and microgliosis in the cortex and hippocampus
We investigated the extent to which DBH-sap lesions result in increased amyloid pathology in the PFC target lesion field, which would replicate the effects of intraperitoneal injections of the neurotoxin N-(2-chloroethyl)-N-ethyl-2-bromobenzylamine (DSP-4) on exacerbating amyloidosis in transgenic mouse models of AD [26, 60–63]. We performed IHC using the MOAB-2 antibody, which recognizes human Aβ residues 1–4 but not AβPP [64]. DBH-sap-lesioned Tg34419 AD rats exhibited increased numbers and size of MOAB-2-labeled plaques in the cortex and hippocampus (Fig. 3B) compared to IgG-sap animals (Fig. 3A). Quantitative analysis of MOAB-2 label density revealed a significant ~30% increase in amyloid burden in the cortex (Fig. 3 C; p < 0.001) and a significant ~20% increase in amyloid pathology in the hippocampus (Fig. 3D; p < 0.05) of DBH-sap compared to IgG-sap-lesioned animals at six weeks post-surgery. Strikingly, we also found evidence that DBH-sap lesions resulted in the emergence of amyloid deposition within PFC microvessels (Fig. 3B, F,G) suggesting the onset of cerebral amyloid angiopathy (CAA) months before it is typically observed in these animals at 16 months of age [35]. There was a significant increase in the number of MOAB-2-labeled, CAA-like vascular profiles in the cortex and hippocampus of DBH-sap-treated (9.9 ± 2.8 [mean ± SD]) compared to IgG-sap-treated (0.8±1.3) animals (Fig. 3 H). Finally, using the tau antibodies AT8 and CP-13, which are directed against phosphoepitopes associated with neurofibrillary tangles in AD [65, 66] (Table 1), we did not detect any immunohistochemical evidence for tau pathology in these animals (data not shown).
Evidence for BBB disruption in DBH-sap-lesioned Tg344–19 AD rats
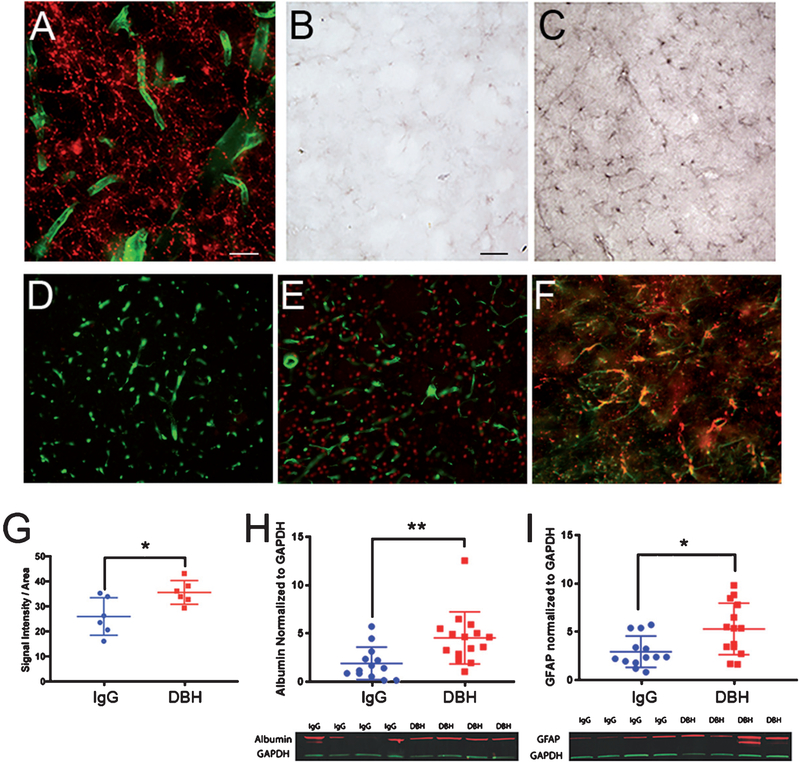
Dual-label immunofluorescence studies showed a dense network of DBH-positive fibers apposed to parenchymal microvessels labeled with SMI71[67], which recognizes a vascular/BBB epitope (Fig. 4A). To investigate the impact of the DBH-sap lesion on BBB integrity, we analyzed albumin immunoreactivity in PFC tissue of DBH-sap or IgG-sap-lesioned Tg344–19 AD rats. A striking increase in albumin staining was observed in the DBH-sap animals (Fig. 4 C, E) compared to IgG-sap animals (Fig. 4B, D), suggesting BBB breakdown and leakage and/or increased microvascular permeability upon LC deafferentation. This observation was confirmed using optical densitometry of albumin immunoreactivity in adjacent fixed tissue sections (Fig. 4 G; p = 0.0242) and quantitative western blotting of frozen tissue from the opposite hemisphere of the same animals (Fig. 4 H; p = 0.0052). Interestingly, further dual-label immunohistochemical investigations revealed a substantial colocalization of albumin with the reactive astrocytic marker GFAP in the DBH-sap-lesioned animals (Fig. 4F). Quantitative immunoblotting of frozen cortical tissue containing PFC revealed a 50% increase in GFAP protein in DBH-sap compared to IgG-sap-lesioned animals (Fig. 4I; p = 0.011). Taken together, these data show an astroglial immune response following LC deaf-ferentation, similar to that observed in other LC lesion paradigms [26]; however, this response may be due at least in part due to cerebrovascular damage and BBB leakage.
Fig. 4.
Cortical intraparenchymal albumin levels are increased in DBH-sap-lesioned Tg344–19 AD rats with evidence for albumin uptake by astrocytes. A) Dual-label fluorescence microscopy with DBH (red) and SMI71 (green) reveal dense apposition of LC noradrenergic fibers with parenchymal microvessels. B, C) Brightfield photomicrographs of PFC albumin immunoreactivity in IgG-sap- (B) or DBH-sap-lesioned rats (C) indicated an increase in parenchymal albumin deposition in with DBH-sap treatments. D, E) Dual-label fluorescence IHC with albumin (red) and SMI71 (green) further demonstrate prominent albumin extravasation within PFC in DBH-sap (E) compared to IgG-sap (D) animals. F) Dual-label fluorescence IHC with albumin (red) and GFAP (green) demonstrate albumin uptake by astrocytes within the PFC of DBH-sap-lesioned rats. G) Semi-quantitative analysis using LICOR infrared tissue immunoreactivity revealed a~40% increase in cortical albumin load in DBH-sap lesioned animals (p = 0.024) H, I) Quantitative western blot analyses of cortical tissue demonstrated a significant~60% increase in albumin levels (p = 0.005) and a significant~50% increase in GFAP levels (p = 0.011). Data are displayed as scatter plots with mean ± SD. Representative western blots are shown beneath the scatterplots. *p < 0.05; **p < 0.01 via Student’s t test. Panel A scale bar = 100 μm. Panel B scale bar = 50 μm (applies to panels C-F).
Evidence for small vessel remodeling in DBH-sap-lesioned Tg344–19 AD rats
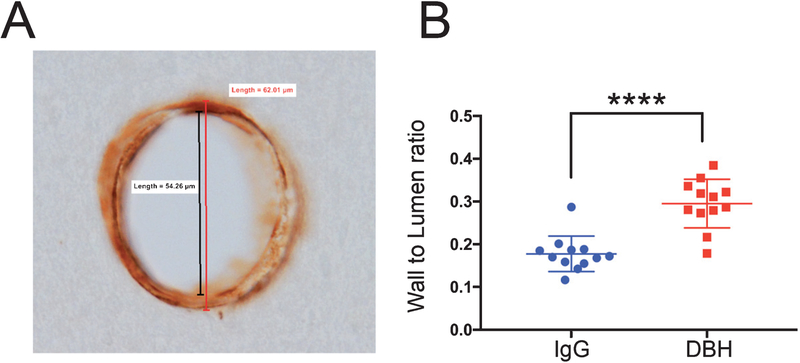
The wall-to-lumen ratio is an important parameter in vascular medicine as it indicates the extent of vascular wall stenosis and vessel remodeling [52, 68,69]. PFC parenchymal arterioles that were perpendicular to the plane of view were evaluated for this ratio in DBH- and IgG-sap-lesioned animals (Fig. 5A). Quantitative analysis revealed that arterioles in DBH-sap-lesioned animals displayed an ~45% increase in wall-to-lumen ratio compared to IgG-sap animals (Fig. 5B; p < 0.0001) indicating a compensatory remodeling of the vessel structure with presumably pathological stenosis as a result of the loss of LC-NE signaling.
Fig. 5.
Wall-to-lumen ratio of parenchymal arterioles is increased in DBH-sap lesioned Tg344–19 rats. A) Representative brightfield photomicrograph of an arteriole labeled with smooth muscle actin (ACTA2). Outside wall diameter measurement is compared to luminal diameter to produce the wall-to-lumen ratio value. B) Quantitative analysis revealed a significant ~75% increase vascular wall-to-lumen ratio in the cortex of DBH-sap- compared to IgG-sap-lesioned animals. Data are displayed as a scatter plot with mean ± SD. ****p < 0.0001 via Student’s t test.
Cortical albumin load and vascular wall-to-lumen ratios predict cognitive impairment in Tg344–19 AD rats
Regression analysis was performed to test for relationships among the cognitive and pathological outcome variables measured six weeks after the IgG-sap or DBH-sap lesions. Notably, lower DBH-immunoreactive fiber intensity in the cortex predicted increased amyloid load (p = 0.02, r2 = 0.43) but not GFAP levels as measured by western blotting (p = 0.1, r2 = 0.24). By contrast, reductions in DBH signal predicted all measures of vascular pathology, including albumin load (p = 0.0004, r2 = 0.73), albumin protein levels as measured by western blotting (p = 0.004, r2 = 0.58), and wall-to-lumen ratio (p = 0.002 r2 = 0.62). Predictors for the three Barnes maze cognitive outcomes are shown in Table 2. With respect to spatial memory tasks, the time animals spent in the target quadrant was related to DBH fiber intensity, albumin load, albumin levels, and wall-tolumen ratio, whereas the latency to find the target hole was influenced only by albumin load, albumin levels, and wall-to-lumen ratio. By contrast, lower DBH fiber intensity, higher albumin levels, and increased wall-to-lumen ratio predicted a greater number of incorrect revisits made by the rats, suggesting that these factors influenced working memory performance. Notably, amyloid load and GFAP levels did not reveal significant associations with cognition on any of the Barnes maze tasks.
Table 2.
Pathologic predictors of cognitive impairment in IgG-sap and DBH-sap-lesioned Tg344–19 AD rats
| Pathologic index | Time in target quadrant | Latency to target hole | Incorrect visits |
|---|---|---|---|
| DBH fiber intensity | p = 0.02, r2 = 0.43 | p = 0.08, r2 = 0.28 | p = 0.03, r2 = 0.4 |
| amyloid load | p = 0.28, r2 = 0.11 | p = 0.4, r2 = 0.07 | p = 0.08, r2 = 0.27 |
| GFAP (western) | p = 0.06, r2 = 0.31 | p = 0.26, r2 = 0.12 | p = 0.35,r2 = 0.09 |
| albumin load | p = 0.04, r2 = 0.35 | p = 0.005, r2 = 0.57 | p = 0.13, r2 = 0.2 |
| albumin (western) | p = 0.04, r2 = 0.35 | p = 0.05, r2 = 0.34 | p = 0.0008, r2 = 0.7 |
| wall-to-lumen ratio | p = 0.002, r2 = 0.62 | p = 0.02, r2 = 0.44 | p = 0.03, r2 = 0.4 |
DISCUSSION
In the early 1970 s, several groups noted DBH-containing fibers in close association with parenchymal arterioles and capillaries in monkey and rat forebrain (Fig. 4A), which persisted following superior cervical ganglionectomy [70, 71]. Subsequent studies using electrophysiological and pharmacologic stimulation of the LC demonstrated central regulation of cerebral blood flow and vascular permeability by this nucleus [29, 31]. More recently, it was reported that LC stimulation in rats increased cerebral perfusion to stimulate cortical neuronal activity [33]. In light of these data, we hypothesized that LC degeneration negatively impacts cerebrovascular function in target fields and hastens the progression of AD. However, to our knowledge, the extent to which this phenomenon occurs has not been examined experimentally. In the present study, we investigated the effects of lesioning the noradrenergic LC forebrain projection system on vascular pathology in AD by injecting a central noradrenergic immunotoxin into the PFC of six-month-old Tg344–19 AD rats. Transgenic and genome-edited rats may offer some advantages over mice for lesion studies, including more defined motor and cognitive behaviors, greater synaptic complexity, genetics and pharmacokinetics more similar to humans, and a larger brain and body size [38, 72]. The DBH-sap lesion resulted not only in LC fiber loss but also in LC neurodegeneration compared to the IgG-sap group. DBH-sap-mediated degeneration of LC forebrain input resulted in deficits in working and spatial memory, increased parenchymal and vascular amyloidosis, astroglial activation, as well as evidence for BBB breakdown and leakage and vascular remodeling consistent with decreased vasoreactivity. Therefore, this in vivo paradigm is well-suited as a model of preclinical coeruleo-forebrain disconnection and its role as a nexus lesion in promoting both neural and vascular dysfunction during the onset of AD.
Behavioral testing of DBH-sap and IgG-saplesioned animals at six weeks post-surgery revealed impairment in working and spatial memory on the Barnes maze (Fig. 1) as a consequence of the sustained loss of LC innervation of PFC and hippocampus (Fig. 2). These effects were reminiscent of previous studies showing that LC lesions with the neurotoxin DSP-4 [73] disrupts spatial memory in transgenic mouse models of AD [26, 60–63] and that LC stimulation or pharmacological increases in NE levels enhance cognitive performance [38, 74,75]. Although we specifically targeted PFC with the immunotoxin, the highly bifurcated nature of long, poorly myelinated LC axons likely led to deafferentation of adjacent cortical structures and hippocampus, as well [48]. Cognitive function depends greatly on LC-NE system integrity [22, 76–81]. In this regard, both β1 [82] and β2 [83] receptor signaling are critically required for spatial learning in animal models. Moreover, LC innervation of the cortical mantle modulates PFC activity in the context of attentional function [22, 79] and selective impairment in NE transmission within the PFC leads to disrupted working memory [84, 85]. In a similar vein, we demonstrate that DBH-sap lesioned animals displayed a higher number of incorrect visits on the Barnes maze, which has been proposed to represent deficits in prefrontal-mediated working memory and attentional function in rodents [42]. This result is reminiscent of findings by Janitzky and colleagues, who reported deficits in the attentional set-shifting paradigm following optogenetic silencing of the LC in mice [86]. Taken together with recent clincial pathologic studies in well-characterized autopsy cohorts [16, 19, 20], these results support the prevailing concept that LC degeneration contributes to cognitive impairment in AD.
Upon postmortem evaluation, we observed increased Aβ deposition and astroglial activation in the cortex and hippocampus of our lesioned animals (Figs. 3 and 4), similar to previous findings [26, 60–63]. However, it is notable that amyloid load in Tg344–19 AD rats lesioned at six months of age resembled the degree of amyloid accrual observed in these animals at ~16 months of age but without experimental LC degeneration [35]. Furthermore, we also noted the emergence of CAA in parenchymal vessels in the LC-lesioned animals, which was virtually absent at this age in unlesioned rats and has not been previously noted prior to 16 months of age (Fig. 3) [35]. The mechanism for this accelerated pace of vascular amyloid deposition is unclear, but may be related to impaired amyloid clearance if NE depletion results in vascular hypertrophy and increased myogenic tone, as suggested by the observed DBH-sap-induced increases in arteriole wall-to-lumen ratio (Fig. 5, see below). Alternatively, recent computational data suggest that vascular smooth muscle cells provide the motive force for intramural periarterial drainage (IPAD) of brain solutes including Aβ [87, 88]. Given data that VSMCs express adrenergic receptors [89], LC deafferentation during AD may impair the motive forces for IPAD, thus contributing to reduced Aβ clearance and increased CAA.
Tg344–19 AD rats reportedly display age-dependent endogenous hyperphosphorylated tau and argyrophilic tangles in the hippocampus and cortex by 16 months [35, 38] and even an early accrual of tau pathology within the LC at 6 months [38]. When examining the brains of our control or lesioned animals for tau pathology (Table 1) at 6–8 months, including a detailed analysis of pontine tissue, we were unable to detect an immunopositive signal above background denoting the presence of tau pathological epitopes. This may be due to methodological differences (e.g., free-floating versus paraffin-embedded tissue [38]) or even age-related phenotypic differences between our colony and others. Nonetheless, future studies will re-examine the accrual of tau pathologic epitopes following DBH- or IgG-sap lesions in older cohorts and different tissue preparation protocols.
While these observations of impaired cognition and exacerbated amyloid-related pathology were consistent with previous in vivo studies [26, 60–63], the primary goal of the present study was to evaluate the extent to which LC deafferentation impacts cerebrovascular function in target fields. In this regard, we noted prominent extravasation of the blood protein albumin within the PFC of DBH-sap-lesioned animals. As all rats were equally saline-perfused at sacrifice, parenchymal albumin deposition is likely the result of impaired BBB integrity specific to the DBH-sap lesion. Perivascular accumulation of serum albumin, plasma proteins, and immunoglobulins have been detected in microvascular segments associated with senile plaques and CAA in AD brains [90,91], while increased cerebrospinal fluid/serum ratio of albumin observed in AD patients has been long used as a proxy for BBB disruption [92–94]. From a translational perspective, these results suggest that LC degeneration during the preclinical and prodromal stages of AD [16, 19, 20] contributes to the BBB pathology observed during AD [95–97], driving disease pathophysiology by increasing BBB permeability and allowing blood-derived molecules and microbes to enter the brain. This insult may in turn trigger multiple neurodegenerative pathways such as inflammation and oxidative stress, as well as impairing neural connectivity via reduced perfusion and dysregulated neurovascular coupling [98, 99]. In support of this hypothesis, Kalinin and colleagues noted a loss of forebrain tight junction protein expression following DSP-4 lesions in wild type rats [100]. Furthermore, Rorabaugh and colleagues found that designer receptor exclusively activated by designer drugs (DREADD)-induced LC activation restored normal reversal learning to aged Tg344-AD rats, indicating that enhancing LC tone can improve cognition, even in the presence of AD pathology [38]. We posit that this may be the result of increased cerebrovascular perfusion from LC activation [33], though this would need to be tested directly. Future studies will determine the extent to which cerebral blood flow and hemodynamic responsiveness along forebrain neural circuits are impaired following LC projection system deafferentation.
Interestingly, we also demonstrated an overlap between reactive astroglial profiles and albumin suggesting that a proportion of astrogliosis in AD and LC-lesioned animal models involves uptake of albumin and other blood-derived proteins in response to BBB breakdown [101]. As astrocytic end feet serve as a first line of defense against the infiltration of potentially neurotoxic blood products from the cerebrovasculature [102] and astrocytes are recruited to wall off damaged areas and restore BBB integrity [101, 103], this has been traditionally viewed as a neuroprotective response [104]. However, studies from the stroke and epilepsy fields have demonstrated that albumin uptake by astrocytes reduces astrocytic expression of inward-rectifying potassium channels, which may compromise their ability to buffer extracellular K+ [105, 106] and may form a mechanistic basis for network hyperexcitability [107]. Astrocytic uptake of albumin also reduces aquaporin 4 expression, which likely impacts vascular permeability [108]. Furthermore, albumin has been shown to induce astrocytic release of the pro-inflammatory ligands interleukin-1β [109] and monocyte chemotactic protein-1 [110]. Hence, astrocyte activation and uptake of albumin in response to BBB breakdown may ultimately compromise neuronal function and protection within the neurovascular unit where LC innervation has been lost.
Perhaps even more strikingly, we found that DBH-sap-lesioned animals display increased cerebrovascular wall-to-lumen ratios in PFC target fields compared to IgG-sap rats. This measure is widely viewed as indicative of vascular remodeling and hypertrophy, increased myogenic tone, and blunted vasoreactivity [52, 68, 69, 111]. Vascular hypertrophy is of particular physiologic importance since it has been is hypothesized to be the result of a remodeling process [53, 112–115] that limits blood flow during maximal vasodilatation and increases vascular responsiveness to constrictor stimuli [53]. Future physiological studies will identify specific changes in tone, constriction, and dilation in LC-denervated parenchymal arterioles [116]. Notably, Joo and colleagues detected dystrophic mural cell morphology and altered hypercapnic responses indictive of vascular remodeling in Tg344–19 AD rats compared to nontransgenic littermates at 9 months of age, suggesting that amyloid accrual itself has the potential to compromise blood flow [117]. The present findings complement and expand upon this observation to show that a loss of LC-NE input to blood vessels likely further exacerbates blood flow to areas of higher order cognition [32, 33, 45, 118]. Moreover, our demonstration that markers of vascular dysfunction arising from DBH-sap lesions, but not parenchymal amyloid load or astrogliosis (Table 2), predicts cognitive outcomes in these animals underscore the importance of LC-mediated vascular tone in regulating cognition in health and disease.
In summary, we provide translational evidence that degeneration of the vulnerable LC noradrenergic projection system impairs forebrain cerebrovascular function during the preclinical and prodromal stages of AD [16–20], thus providing an additional mechanism for the therapeutic benefit of targeting NE-mediated neuronal activity [119–121]. A key caveat to this interpretation is that LC neurons also release other neurochemical transmitters along with NE such as galanin, neuropeptide Y, dopamine, and even brain-derived neurotrophic factor, albeit at lower levels [122–125]. Future directions will use this animal model to test the extent to which NE replacement therapies such as the NE precursor L-threo-3,4-dihydroxyphenylserine (L-DOPS) [126] or genetic approaches to manipulate the levels of NE or non-NE LC transmitters ameliorate forebrain vascular pathology induced by LC projection system degeneration. Moreover, this model is well-suited to determine the extent to which other neurochemical projection systems regulating neurovascular function, such as the cholinergic nucleus basalis or the serotonergic raphe [127, 128], might undergo compensatory cerebrovascular innervation patterns in response to LC deafferentation. Finally, this model can also be used to explore potential neurotoxic mechanisms of BBB leakage of proteins such as albumin or fibrinogen [99, 129–131] or the preclinical value of using blood proteins such as platelet-derived growth factor receptor-β as a diagnostic and prognostic biomarker of disease progression [132]. Altogether, the present findings point to the continuing need to consider noradrenergic system pathophysiology as a key and early component associated with the progression of AD and posit that strategies aimed at LC neuroprotection or NE replacement remain viable therapeutic options.
ACKNOWLEDGMENTS
This study was supported by NIH grants AG060731, AG014449, AG053760, and AG042146; the Saint Mary’s Foundation; and Miles for Memories of Battle Creek, MI, USA.
Footnotes
Authors’ disclosures available online (https://www.j-alz.com/manuscript-disclosures/19-0090r1).
REFERENCES
- [1].Albert MS, Dekosky ST, Dickson D, Dubois B, Feldman HH, Fox NC, Gamst A, Holtzman DM, Jagust WJ, Petersen RC, Snyder PJ, Carrillo MC, Thies B, Phelps CH (2011) The diagnosis of mild cognitive impairment due to Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement 7, 270–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Markesbery WR, Schmitt FA, Kryscio RJ, Davis DG, Smith CD, Wekstein DR (2006) Neuropathologic substrate of mild cognitive impairment. Arch Neurol 63, 38–46. [DOI] [PubMed] [Google Scholar]
- [3].Mufson EJ, Binder LI, Counts SE, DeKosky ST, Toledo-Morrell L, Ginsberg SD, Ikonomovic MD, Perez SE, Scheff SW (2012) Mild cognitive impairment: Pathology and mechanisms. Acta Neuropathol 123, 13–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Jellinger KA, Attems J (2007) Neuropathological evaluation of mixed dementia. J Neurol Sci 257, 80–87. [DOI] [PubMed] [Google Scholar]
- [5].Arvanitakis Z, Capuano AW, Leurgans SE, Bennett DA, Schneider JA (2016) Relation of cerebral vessel disease to Alzheimer’s disease dementia and cognitive function in elderly people: A cross-sectional study. Lancet Neurol 15, 934–943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Attems J, Jellinger KA (2014) The overlap between vascular disease and Alzheimer’s disease–lessons from pathology. BMC Med 12, 206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Bailey TL, Rivara CB, Rocher AB, Hof PR (2004) The nature and effects of cortical microvascular pathology in aging and Alzheimer’s disease. Neurol Res 26, 573–578. [DOI] [PubMed] [Google Scholar]
- [8].Schneider JA, Arvanitakis Z, Leurgans SE, Bennett DA (2009) The neuropathology of probable Alzheimer disease and mild cognitive impairment. Ann Neurol 66, 200–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Smallwood A, Oulhaj A, Joachim C, Christie S, Sloan C, Smith AD, Esiri M (2012) Cerebral subcortical small vessel disease and its relation to cognition in elderly subjects: A pathological study in the Oxford Project to Investigate Memory and Ageing (OPTIMA) cohort. Neuropathol Appl Neurobiol 38, 337–343. [DOI] [PubMed] [Google Scholar]
- [10].Gorelick PB, Scuteri A, Black SE, Decarli C, Greenberg SM, Iadecola C, Launer LJ, Laurent S, Lopez OL, Nyenhuis D, Petersen RC, Schneider JA, Tzourio C, Arnett DK, Bennett DA, Chui HC, Higashida RT, Lindquist R, Nilsson PM, Roman GC, Sellke FW, Seshadri S, American Heart Association Stroke Council, Council on Epidemiology and Prevention, Council on Cardiovascular Nursing, Council on Cardiovascular Radiology and Intervention, and Council on Cardiovascular Surgery and Anesthesia (2011) Vascular contributions to cognitive impairment and dementia: A statement for healthcare professionals from the american heart association/american stroke association. Stroke 42, 2672–2713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Haring B, Wu C, Coker LH, Seth A, Snetselaar L, Manson JE, Rossouw JE, Wassertheil-Smoller S (2016) Hypertension, dietary sodium, and cognitive decline: Results from the Women’s Health Initiative Memory Study. Am J Hypertens 29, 202–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].McDonald C, Pearce MS, Kerr SR, Newton JL (2017) Blood pressure variability and cognitive decline in older people: A 5-year longitudinal study. J Hypertens 35, 140–147. [DOI] [PubMed] [Google Scholar]
- [13].Rajan KB, Arvanitakis Z, Lynch EB, McAninch EA, Wilson RS, Weuve J, Barnes LL, Bianco AC, Evans DA (2016) Cognitive decline following incident and preexisting diabetes mellitus in a population sample. Neurology 87, 1681–1687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Reitz C, Tang MX, Manly J, Mayeux R, Luchsinger JA (2007) Hypertension and the risk of mild cognitive impairment. Arch Neurol 64, 1734–1740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Iadecola C (2010) The overlap between neurodegenerative and vascular factors in the pathogenesis of dementia. Acta Neuropathol 120, 287–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Kelly SC, He B, Perez SE, Ginsberg SD, Mufson EJ, Counts SE (2017) Locus coeruleus cellular and molecular pathology during the progression of Alzheimer’s disease. Acta Neuropathol Commun 5, 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Braak H, Del Tredici K (2011) The pathological process underlying Alzheimer’s disease in individuals under thirty. Acta Neuropathol 121, 171–181. [DOI] [PubMed] [Google Scholar]
- [18].Grudzien A, Shaw P, Weintraub S, Bigio E, Mash DC, Mesulam MM (2007) Locus coeruleus neurofibrillary degeneration in aging, mild cognitive impairment and early Alzheimer’s disease. Neurobiol Aging 28, 327–335. [DOI] [PubMed] [Google Scholar]
- [19].Arendt T, Bruckner MK, Morawski M, Jager C, Gertz HJ (2015) Early neurone loss in Alzheimer’s disease: Cortical or subcortical? Acta Neuropathol Commun 3, 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Theofilas P, Ehrenberg AJ, Dunlop S, Di Lorenzo Alho AT, Nguy A, Leite REP, Rodriguez RD, Mejia MB, Suemoto CK, Ferretti-Rebustini REL, Polichiso L, Nascimento CF, Seeley WW, Nitrini R, Pasqualucci CA, Jacob Filho W, Rueb U, Neuhaus J, Heinsen H, Grinberg LT (2017) Locus coeruleus volume and cell population changes during Alzheimer’s disease progression: A stereological study in human postmortem brains with potential implication for early-stage biomarker discovery. Alzheimers Dement 13, 236–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Simic G, Stanic G, Mladinov M, Jovanov-Milosevic N, Kostovic I, Hof PR (2009) Does Alzheimer’s disease begin in the brainstem? Neuropathol Appl Neurobiol 35, 532–554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Aston-Jones G, Cohen JD (2005) An integrative theory of locus coeruleus-norepinephrine function: Adaptive gain and optimal performance. Annu Rev Neurosci 28, 403–450. [DOI] [PubMed] [Google Scholar]
- [23].Chalermpalanupap T, Schroeder JP, Rorabaugh JM, Liles LC, Lah JJ, Levey AI, Weinshenker D (2018) Locus coeruleus ablation exacerbates cognitive deficits, neuropathology, and lethality in P301S tau transgenic mice. J Neurosci 38, 74–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Counts SE, Mufson EJ (2010) Noradrenaline activation of neurotrophic pathways protects against neuronal amyloid toxicity. J Neurochem 113, 649–660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Feinstein DL, Kalinin S, Braun D (2016) Causes, consequences, and cures for neuroinflammation mediated via the locus coeruleus: Noradrenergic signaling system. J Neurochem 139, 154–178. [DOI] [PubMed] [Google Scholar]
- [26].Heneka MT, Galea E, Gavriluyk V, Dumitrescu-Ozimek L, Daeschner J, O’Banion MK, Weinberg G, Klockgether T, Feinstein DL (2002) Noradrenergic depletion potentiates beta -amyloid-induced cortical inflammation: Implications for Alzheimer’s disease. J Neurosci 22, 2034–2042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Liu X, Ye K, Weinshenker D (2015) Norepinephrine protects against amyloid-beta toxicity via TrkB. J Alzheimers Dis 44, 251–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Madrigal JL, Kalinin S, Richardson JC, Feinstein DL (2007) Neuroprotective actions of noradrenaline: Effects on glutathione synthesis and activation of peroxisome proliferator activated receptor delta. J Neurochem 103, 2092–2101. [DOI] [PubMed] [Google Scholar]
- [29].Hartman BK, Swanson LW, Raichle ME, Preskorn SH, Clark HB (1980) Central adrenergic regulation of cerebral microvascaulr permeability and blood flow: Anatomic and physiologic evidence. In The Cerebral Microvasculature, Eisenberg HM, ed. Plenum Press, New York, pp. 113–126. [Google Scholar]
- [30].Preskorn SH, Hartman BK, Raichle ME, Swanson LW, Clark HB (1980) Central adrenergic regulation of cerebral microvascular permeability and blood flow: Pharamcologic evidence. In The Cerebral Microvasculature, Eisenberg HM, ed. Plenum Press, New York, pp. 127–138. [DOI] [PubMed] [Google Scholar]
- [31].Raichle ME, Hartman BK, Eichling JO, Sharpe LG (1975) Central noradrenergic regulation of cerebral blood flow and vascular permeability. Proc Natl Acad Sci U S A 72, 3726–3730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Bekar LK, Wei HS, Nedergaard M (2012) The locus coeruleus-norepinephrine network optimizes coupling of cerebral blood volume with oxygen demand. J Cereb Blood Flow Metab 32, 2135–2145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Toussay X, Basu K, Lacoste B, Hamel E (2013) Locus coeruleus stimulation recruits a broad cortical neuronal network and increases cortical perfusion. J Neurosci 33, 3390–3401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Wrenn CC, Picklo MJ, Lappi DA, Robertson D, Wiley RG (1996) Central noradrenergic lesioning using anti-DBH-saporin: Anatomical findings. Brain Res 740, 175–184. [DOI] [PubMed] [Google Scholar]
- [35].Cohen RM, Rezai-Zadeh K, Weitz TM, Rentsendorj A, Gate D, Spivak I, Bholat Y, Vasilevko V, Glabe CG, Breunig JJ, Rakic P, Davtyan H, Agadjanyan MG, Kepe V, Barrio JR, Bannykh S, Szekely CA, Pechnick RN, Town T (2013) A transgenic Alzheimer rat with plaques, tau pathology, behavioral impairment, oligomeric abeta, and frank neuronal loss. J Neurosci 33, 6245–6256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Agster KL, Mejias-Aponte CA, Clark BD, Waterhouse BD (2013) Evidence for a regional specificity in the density and distribution of noradrenergic varicosities in rat cortex. J Comp Neurol 521, 2195–2207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Pentkowski NS, Berkowitz LE, Thompson SM, Drake EN, Olguin CR, Clark BJ (2018) Anxiety-like behavior as an early endophenotype in the TgF344-AD rat model of Alzheimer’s disease. Neurobiol Aging 61, 169–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Rorabaugh JM, Chalermpalanupap T, Botz-Zapp CA, Fu VM, Lembeck NA, Cohen RM, Weinshenker D (2017) Chemogenetic locus coeruleus activation restores reversal learning in a rat model of Alzheimer’s disease. Brain 140, 3023–3038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Paxinos G, Watson C (2013) The Rat Brain in Stereotaxic Coordinates, Academic Press, Cambridge, MA. [Google Scholar]
- [40].Barnes CA (1979) Memory deficits associated with senescence: A neurophysiological and behavioral study in the rat. J Comp Physiol Psychol 93, 74–104. [DOI] [PubMed] [Google Scholar]
- [41].Attar A, Liu T, Chan WT, Hayes J, Nejad M, Lei K, Bitan G (2013) A shortened Barnes maze protocol reveals memory deficits at 4-months of age in the triple-transgenic mouse model of Alzheimer’s disease. PLoS One 8, e80355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Rosenfeld CS, Ferguson SA (2014) Barnes maze testing strategies with small and large rodent models. J Vis Exp, e51194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Walsh RN, Cummins RA (1976) The Open-Field Test: A critical review. Psychol Bull 83, 482–504. [PubMed] [Google Scholar]
- [44].Carola V, D’Olimpio F, Brunamonti E, Mangia F, Renzi P (2002) Evaluation of the elevated plus-maze and open-field tests for the assessment of anxiety-related behaviour in inbred mice. Behav Brain Res 134, 49–57. [DOI] [PubMed] [Google Scholar]
- [45].Szabadi E (2013) Functional neuroanatomy of the central noradrenergic system. J Psychopharmacol 27, 659–693. [DOI] [PubMed] [Google Scholar]
- [46].Counts SE, Nadeem M, Lad SP, Wuu J, Mufson EJ (2006) Differential expression of synaptic proteins in the frontal and temporal cortex of elderly subjects with mild cognitive impairment. J Neuropathol Exp Neurol 65, 592–601. [DOI] [PubMed] [Google Scholar]
- [47].Counts SE, Perez SE, He B, Mufson EJ (2014) Intravenous immunoglobulin reduces tau pathology and preserves neuroplastic gene expression in the 3xTg mouse model of Alzheimer’s disease. Curr Alzheimer Res 11, 655–663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Counts SE, Mufson EJ (2012) Locus coeruleus In The human nervous system, Mai JK, Paxinos G, eds. Academic, London. [Google Scholar]
- [49].Polinski NK, Manfredsson FP, Benskey MJ, Fischer DL, Kemp CJ, Steece-Collier K, Sandoval IM, Paumier KL, Sortwell CE (2016) Impact of age and vector construct on striatal and nigral transgene expression. Mol Ther Methods Clin Dev 3, 16082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Counts SE, Lah JJ, Levey AI (2001) The regulation of presenilin-1 by nerve growth factor. J Neurochem 76, 679–689. [DOI] [PubMed] [Google Scholar]
- [51].Baumbach GL, Hajdu MA (1993) Mechanics and composition of cerebral arterioles in renal and spontaneously hypertensive rats. Hypertension 21, 816–826. [DOI] [PubMed] [Google Scholar]
- [52].Dorrance AM, Rupp NC, Nogueira EF (2006) Miner-alocorticoid receptor activation causes cerebral vessel remodeling and exacerbates the damage caused by cerebral ischemia. Hypertension 47, 590–595. [DOI] [PubMed] [Google Scholar]
- [53].Hart MN, Heistad DD, Brody MJ (1980) Effect of chronic hypertension and sympathetic denervation on wall/lumen ratio of cerebral vessels. Hypertension 2, 419–423. [DOI] [PubMed] [Google Scholar]
- [54].Kesby JP, Kim JJ, Scadeng M, Woods G, Kado DM, Olefsky JM, Jeste DV, Achim CL, Semenova S (2015) Spatial cognition in adult and aged mice exposed to high-fat diet. PLoS One 10, e0140034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].McCall JG, Siuda ER, Bhatti DL, Lawson LA, McElligott ZA, Stuber GD, Bruchas MR (2017) Locus coeruleus to basolateral amygdala noradrenergic projections promote anxiety-like behavior. Elife 6, e18247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Price JL, Amaral DG (1981) An autoradiographic study of the projections of the central nucleus of the monkey amygdala. J Neurosci 1, 1242–1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Walf AA, Frye CA (2007) The use of the elevated plus maze as an assay of anxiety-related behavior in rodents. Nat Protoc 2, 322–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Ostock CY, Lindenbach D, Goldenberg AA, Kampton E, Bishop C (2014) Effects of noradrenergic denervation by anti-DBH-saporin on behavioral responsivity to L-DOPA in the hemi-parkinsonian rat. Behav Brain Res 270, 75–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Patrone LGA, Biancardi V, Marques DA, Bicego KC, Gargaglioni LH (2018) Brainstem catecholaminergic neurones and breathing control during postnatal development in male and female rats. J Physiol 596, 3299–3325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Hammerschmidt T, Kummer MP, Terwel D, Martinez A, Gorji A, Pape HC, Rommelfanger KS, Schroeder JP, Stoll M, Schultze J, Weinshenker D, Heneka MT (2013) Selective loss of noradrenaline exacerbates early cognitive dysfunction and synaptic deficits in APP/PS1 mice. Biol Psychiatry 73, 454–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Heneka MT, Ramanathan M, Jacobs AH, Dumitrescu-Ozimek L, Bilkei-Gorzo A, Debeir T, Sastre M, Galldiks N, Zimmer A, Hoehn M, Heiss WD, Klockgether T, Staufenbiel M (2006) Locus ceruleus degeneration promotes Alzheimer pathogenesis in amyloid precursor protein 23 transgenic mice. J Neurosci 26, 1343–1354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Jardanhazi-Kurutz D, Kummer MP, Terwel D, Vogel K, Dyrks T, Thiele A, Heneka MT (2010) Induced LC degeneration in APP/PS1 transgenic mice accelerates early cerebral amyloidosis and cognitive deficits. Neurochem Int 57, 375–382. [DOI] [PubMed] [Google Scholar]
- [63].Kalinin S, Polak PE, Lin SX, Sakharkar AJ, Pandey SC, Feinstein DL (2012) The noradrenaline precursor L-DOPS reduces pathology in a mouse model of Alzheimer’s disease. Neurobiol Aging 33, 1651–1663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Youmans KL, Tai LM, Kanekiyo T, Stine WB Jr., Michon SC, Nwabuisi-Heath E, Manelli AM, Fu Y, Riordan S, Eimer WA, Binder L, Bu G, Yu C, Hartley DM, LaDu MJ (2012) Intraneuronal Abeta detection in 5xFAD mice by a new Abeta-specific antibody. Mol Neurodegener 7, 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Duff K, Knight H, Refolo LM, Sanders S, Yu X, Picciano M, Malester B, Hutton M, Adamson J, Goedert M, Burki K, Davies P (2000) Characterization of pathology in transgenic mice over-expressing human genomic and cDNA tau transgenes. Neurobiol Dis 7, 87–98. [DOI] [PubMed] [Google Scholar]
- [66].Goedert M, Jakes R, Vanmechelen E (1995) Monoclonal antibody AT8 recognises tau protein phosphorylated at both serine 202 and threonine 205. Neurosci Lett 189, 167–169. [DOI] [PubMed] [Google Scholar]
- [67].Sternberger NH, Sternberger LA (1987) Blood-brain barrier protein recognized by monoclonal antibody. Proc Natl Acad Sci U S A 84, 8169–8173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Amaral SL, Zorn TM, Michelini LC (2000) Exercise training normalizes wall-to-lumen ratio of the gracilis muscle arterioles and reduces pressure in spontaneously hypertensive rats. J Hypertens 18, 1563–1572. [DOI] [PubMed] [Google Scholar]
- [69].Harazny JM, Ritt M, Baleanu D, Ott C, Heckmann J, Schlaich MP, Michelson G, Schmieder RE (2007) Increased wall:Lumen ratio of retinal arterioles in male patients with a history of a cerebrovascular event. Hypertension 50, 623–629. [DOI] [PubMed] [Google Scholar]
- [70].Edvinsson L, Lindvall M, Nielsen KC, Owman C (1973) Are brain vessels innervated also by central (non-sympathetic) adrenergic neurones? Brain Res 63, 496–499. [DOI] [PubMed] [Google Scholar]
- [71].Hartman BK, Zide D, Udenfriend S (1972) The use of dopamine -hydroxylase as a marker for the central noradrenergic nervous system in rat brain. Proc Natl Acad Sci U S A 69, 2722–2726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Ellenbroek B, Youn J (2016) Rodent models in neuro-science research: Is it a rat race? Dis Model Mech 9, 1079–1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Szot P, Miguelez C, White SS, Franklin A, Sikkema C, Wilkinson CW, Ugedo L, Raskind MA (2010) A comprehensive analysis of the effect of DSP4 on the locus coeruleus noradrenergic system in the rat. Neuroscience 166, 279–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Hansen N, Manahan-Vaughan D (2015) Locus coeruleus stimulation facilitates long-term depression in the dentate gyrus that requires activation of beta-adrenergic receptors. Cereb Cortex 25, 1889–1896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Totah NK, Logothetis NK, Eschenko O (2015) Atomoxetine accelerates attentional set shifting without affecting learning rate in the rat. Psychopharmacology (Berl) 232, 3697–3707. [DOI] [PubMed] [Google Scholar]
- [76].Arnsten AF, Goldman-Rakic PS (1984) Selective prefrontal cortical projections to the region of the locus coeruleus and raphe nuclei in the rhesus monkey. Brain Res 306, 9–18. [DOI] [PubMed] [Google Scholar]
- [77].Arnsten AF, Goldman-Rakic PS (1985) Alpha 2-adrenergic mechanisms in prefrontal cortex associated with cognitive decline in aged nonhuman primates. Science 230, 1273–1276. [DOI] [PubMed] [Google Scholar]
- [78].Aston-Jones G, Bloom FE (1981) Activity of norepinephrine-containing locus coeruleus neurons in behaving rats anticipates fluctuations in the sleep-waking cycle. J Neurosci 1, 876–886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Berridge CW, Waterhouse BD (2003) The locus coeruleusnoradrenergic system: Modulation of behavioral state and state-dependent cognitive processes. Brain Res Brain Res Rev 42, 33–84. [DOI] [PubMed] [Google Scholar]
- [80].Gibbs ME, Hutchinson DS, Summers RJ (2010) Noradrenaline release in the locus coeruleus modulates memory formation and consolidation; roles for alpha- and beta-adrenergic receptors. Neuroscience 170, 1209–1222. [DOI] [PubMed] [Google Scholar]
- [81].Sara SJ (2009) The locus coeruleus and noradrenergic modulation of cognition. Nat Rev Neurosci 10, 211–223. [DOI] [PubMed] [Google Scholar]
- [82].Ardestani PM, Evans AK, Yi B, Nguyen T, Coutellier L, Shamloo M (2017) Modulation of neuroinflammation and pathology in the 5XFAD mouse model of Alzheimer’s disease using a biased and selective beta-1 adrenergic receptor partial agonist. Neuropharmacology 116, 371–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Hagena H, Hansen N, Manahan-Vaughan D (2016) beta-Adrenergic control of hippocampal function: Subserving the choreography of synaptic information storage and memory. Cereb Cortex 26, 1349–1364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Arnsten AF, Jin LE (2014) Molecular influences on working memory circuits in dorsolateral prefrontal cortex. Prog Mol Biol Transl Sci 122, 211–231. [DOI] [PubMed] [Google Scholar]
- [85].Ramos BP, Arnsten AF (2007) Adrenergic pharmacology and cognition: Focus on the prefrontal cortex. Pharmacol Ther 113, 523–536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Janitzky K, Lippert MT, Engelhorn A, Tegtmeier J, Goldschmidt J, Heinze HJ, Ohl FW (2015) Optogenetic silencing of locus coeruleus activity in mice impairs cognitive flexibility in an attentional set-shifting task. Front Behav Neurosci 9, 286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Aldea R, Weller RO, Wilcock DM, Carare RO, Richardson G (2019) Cerebrovascular smooth muscle cells as the drivers of intramural periarterial drainage of the brain. Front Aging Neurosci 11, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Carare RO, Bernardes-Silva M, Newman TA, Page AM, Nicoll JA, Perry VH, Weller RO (2008) Solutes, but not cells, drain from the brain parenchyma along basement membranes of capillaries and arteries: Significance for cerebral amyloid angiopathy and neuroimmunology. Neuropathol Appl Neurobiol 34, 131–144. [DOI] [PubMed] [Google Scholar]
- [89].Piascik MT, Perez DM (2001) Alpha1-adrenergic receptors: New insights and directions. J Pharmacol Exp Ther 298, 403–410. [PubMed] [Google Scholar]
- [90].Wisniewski HM, Kozlowski PB (1982) Evidence for blood-brain barrier changes in senile dementia of the Alzheimer type (SDAT). Ann N Y Acad Sci 396, 119–129. [DOI] [PubMed] [Google Scholar]
- [91].Wisniewski HM, Vorbrodt AW, Wegiel J (1997) Amyloid angiopathy and blood-brain barrier changes in Alzheimer’s disease. Ann N Y Acad Sci 826, 161–172. [DOI] [PubMed] [Google Scholar]
- [92].Alafuzoff I, Adolfsson R, Bucht G, Winblad B (1983) Albumin and immunoglobulin in plasma and cerebrospinal fluid, and blood-cerebrospinal fluid barrier function in patients with dementia of Alzheimer type and multi-infarct dementia. J Neurol Sci 60, 465–472. [DOI] [PubMed] [Google Scholar]
- [93].Elovaara I, Icen A, Palo J, Erkinjuntti T (1985) CSF in Alzheimer’s disease. Studies on blood-brain barrier function and intrathecal protein synthesis. J Neurol Sci 70, 73–80. [DOI] [PubMed] [Google Scholar]
- [94].Skoog I, Wallin A, Fredman P, Hesse C, Aevarsson O, Karlsson I, Gottfries CG, Blennow K (1998) A population study on blood-brain barrier function in 85-year-olds: Relation to Alzheimer’s disease and vascular dementia. Neurology 50, 966–971. [DOI] [PubMed] [Google Scholar]
- [95].Montagne A, Zhao Z, Zlokovic BV (2017) Alzheimer’s disease: A matter of blood-brain barrier dysfunction? J Exp Med 214, 3151–3169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Zenaro E, Piacentino G, Constantin G (2017) The blood-brain barrier in Alzheimer’s disease. Neurobiol Dis 107, 41–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Zlokovic BV (2008) The blood-brain barrier in health and chronic neurodegenerative disorders. Neuron 57, 178–201. [DOI] [PubMed] [Google Scholar]
- [98].Iadecola C (2013) The pathobiology of vascular dementia. Neuron 80, 844–866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Sweeney MD, Sagare AP, Zlokovic BV (2018) Blood-brain barrier breakdown in Alzheimer disease and other neurodegenerative disorders. Nat Rev Neurol 14, 133–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Kalinin S, Feinstein DL, Xu HL, Huesa G, Pelligrino DA, Galea E (2006) Degeneration of noradrenergic fibres from the locus coeruleus causes tight-junction disorganisation in the rat brain. Eur J Neurosci 24, 3393–3400. [DOI] [PubMed] [Google Scholar]
- [101].Abbott NJ, Ronnback L, Hansson E (2006) Astrocyteendothelial interactions at the blood-brain barrier. Nat Rev Neurosci 7, 41–53. [DOI] [PubMed] [Google Scholar]
- [102].Minagar A, Shapshak P, Fujimura R, Ownby R, Heyes M, Eisdorfer C (2002) The role of macrophage/microglia and astrocytes in the pathogenesis of three neurologic disorders: HIV-associated dementia, Alzheimer disease, and multiple sclerosis. J Neurol Sci 202, 13–23. [DOI] [PubMed] [Google Scholar]
- [103].Bonkowski D, Katyshev V, Balabanov RD, Borisov A, Dore-Duffy P (2011) The CNS microvascular pericyte: Pericyte-astrocyte crosstalk in the regulation of tissue survival. Fluids Barriers CNS 8, 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Barreto G, White RE, Ouyang Y, Xu L, Giffard RG (2011) Astrocytes: Targets for neuroprotection in stroke. Cent Nerv Syst Agents Med Chem 11, 164–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Seiffert E, Dreier JP, Ivens S, Bechmann I, Tomkins O, Heinemann U, Friedman A (2004) Lasting blood-brain barrier disruption induces epileptic focus in the rat somatosensory cortex. J Neurosci 24, 7829–7836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Stewart TH, Eastman CL, Groblewski PA, Fender JS, Verley DR, Cook DG, D’Ambrosio R (2010) Chronic dysfunction of astrocytic inwardly rectifying K+channels specific to the neocortical epileptic focus after fluid percussion injury in the rat. J Neurophysiol 104, 3345–3360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Salar S, Lapilover E, Muller J, Hollnagel JO, Lippmann K, Friedman A, Heinemann U (2016) Synaptic plasticity in area CA1 of rat hippocampal slices following intraventricular application of albumin. Neurobiol Dis 91, 155–165. [DOI] [PubMed] [Google Scholar]
- [108].Kovacs R, Heinemann U, Steinhauser C (2012) Mechanisms underlying blood-brain barrier dysfunction in brain pathology and epileptogenesis: Role of astroglia. Epilepsia 53 Suppl 6, 53–59. [DOI] [PubMed] [Google Scholar]
- [109].Vezzani A, Baram TZ (2007) New roles for interleukin1 Beta in the mechanisms of epilepsy. Epilepsy Curr 7, 45–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [110].Calvo CF, Amigou E, Tence M, Yoshimura T, Glowinski J (2005) Albumin stimulates monocyte chemotactic protein-1 expression in rat embryonic mixed brain cells. J Neurosci Res 80, 707–714. [DOI] [PubMed] [Google Scholar]
- [111].Folkow B, Hallback M, Lundgren Y, Sivertsson R, Weiss L (1973) Importance of adaptive changes in vascular design for establishment of primary hypertension, studied in man and in spontaneously hypertensive rats. Circ Res 32, Suppl 1:2–16. [DOI] [PubMed] [Google Scholar]
- [112].Heagerty AM, Bund SJ, Aalkjaer C (1988) Effects of drug treatment on human resistance arteriole morphology in essential hypertension: Direct evidence for structural remodelling of resistance vessels. Lancet 2, 1209–1212. [DOI] [PubMed] [Google Scholar]
- [113].Jacobsen JC, Hornbech MS, Holstein-Rathlou NH (2011) Significance of microvascular remodelling for the vascular flow reserve in hypertension. Interface Focus 1, 117–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [114].Korsgaard N, Aalkjaer C, Heagerty AM, Izzard AS, Mulvany MJ (1993) Histology of subcutaneous small arteries from patients with essential hypertension. Hypertension 22, 523–526. [DOI] [PubMed] [Google Scholar]
- [115].Schiffrin EL, Deng LY, Larochelle P (1992) Blunted effects of endothelin upon small subcutaneous resistance arteries of mild essential hypertensive patients. J Hypertens 10, 437–444. [DOI] [PubMed] [Google Scholar]
- [116].Matin N, Fisher C, Jackson WF, Dorrance AM (2016) Bilateral common carotid artery stenosis in normotensive rats impairs endothelium-dependent dilation of parenchymal arterioles. Am J Physiol Heart Circ Physiol 310, H1321–1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [117].Joo IL, Lai AY, Bazzigaluppi P, Koletar MM, Dorr A, Brown ME, Thomason LA, Sled JG, McLaurin J, Stefanovic B (2017) Early neurovascular dysfunction in a transgenic rat model of Alzheimer’s disease. Sci Rep 7, 46427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [118].Cohen Z, Molinatti G, Hamel E (1997) Astroglial and vascular interactions of noradrenaline terminals in the rat cerebral cortex. J Cereb Blood Flow Metab 17, 894–904. [DOI] [PubMed] [Google Scholar]
- [119].Chalermpalanupap T, Kinkead B, Hu WT, Kummer MP, Hammerschmidt T, Heneka MT, Weinshenker D, Levey AI (2013) Targeting norepinephrine in mild cognitive impairment and Alzheimer’s disease. Alzheimers Res Ther 5, 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [120].Chamberlain SR, Robbins TW (2013) Noradrenergic modulation of cognition: Therapeutic implications. J Psychopharmacol 27, 694–718. [DOI] [PubMed] [Google Scholar]
- [121].Weinshenker D (2018) Long road to ruin: Noradrenergic dysfunction in neurodegenerative disease. Trends Neurosci 41, 211–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [122].Counts SE, Chen EY, Che S, Ikonomovic MD, Wuu J, Ginsberg SD, Dekosky ST, Mufson EJ (2006) Galanin fiber hypertrophy within the cholinergic nucleus basalis during the progression of Alzheimer’s disease. Dement Geriatr Cogn Disord 21, 205–214. [DOI] [PubMed] [Google Scholar]
- [123].Fawcett JP, Bamji SX, Causing CG, Aloyz R, Ase AR, Reader TA, McLean JH, Miller FD (1998) Functional evidence that BDNF is an anterograde neuronal trophic factor in the CNS. J Neurosci 18, 2808–2821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [124].Holets VR, Hokfelt T, Rokaeus A, Terenius L, Goldstein M (1988) Locus coeruleus neurons in the rat containing neuropeptide Y, tyrosine hydroxylase or galanin and their efferent projections to the spinal cord, cerebral cortex and hypothalamus. Neuroscience 24, 893–906. [DOI] [PubMed] [Google Scholar]
- [125].Kempadoo KA, Mosharov EV, Choi SJ, Sulzer D, Kandel ER (2016) Dopamine release from the locus coeruleus to the dorsal hippocampus promotes spatial learning and memory. Proc Natl Acad Sci U S A 113, 14835–14840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [126].Biaggioni I, Robertson D (1987) Endogenous restoration of noradrenaline by precursor therapy in dopamine-beta-hydroxylase deficiency. Lancet 2, 1170–1172. [DOI] [PubMed] [Google Scholar]
- [127].Hotta H, Masamoto K, Uchida S, Sekiguchi Y, Takuwa H, Kawaguchi H, Shigemoto K, Sudo R, Tanishita K, Ito H, Kanno I (2013) Layer-specific dilation of penetrating arteries induced by stimulation of the nucleus basalis of Meynert in the mouse frontal cortex. J Cereb Blood Flow Metab 33, 1440–1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [128].Lincoln J (1995) Innervation of cerebral arteries by nerves containing 5-hydroxytryptamine and noradrenaline. Pharmacol Ther 68, 473–501. [DOI] [PubMed] [Google Scholar]
- [129].Ahn HJ, Glickman JF, Poon KL, Zamolodchikov D, Jno-Charles OC, Norris EH, Strickland S (2014) A novel Abeta-fibrinogen interaction inhibitor rescues altered thrombosis and cognitive decline in Alzheimer’s disease mice. J Exp Med 211, 1049–1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [130].Cortes-Canteli M, Paul J, Norris EH, Bronstein R, Ahn HJ, Zamolodchikov D, Bhuvanendran S, Fenz KM, Strickland S (2010) Fibrinogen and beta-amyloid association alters thrombosis and fibrinolysis: A possible contributing factor to Alzheimer’s disease. Neuron 66, 695–709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [131].Petersen MA, Ryu JK, Akassoglou K (2018) Fibrinogen in neurological diseases: Mechanisms, imaging and therapeutics. Nat Rev Neurosci 19, 283–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [132].Nation DA, Sweeney MD, Montagne A, Sagare AP, D’Orazio LM, Pachicano M, Sepehrband F, Nelson AR, Buennagel DP, Harrington MG, Benzinger TLS, Fagan AM, Ringman JM, Schneider LS, Morris JC, Chui HC, Law M, Toga AW, Zlokovic BV (2019) Blood-brain barrier breakdown is an early biomarker of human cognitive dysfunction. Nat Med 25, 270–276. [DOI] [PMC free article] [PubMed] [Google Scholar]