Abstract
The marked augment of drug-resistance to traditional antibiotics underlines the crying need for novel replaceable antibacterials. Research advances have revealed the considerable sterilization potential of two-dimension graphene-based nanomaterials. Subsequently, two-dimensional nanomaterials beyond graphene (2D NBG) as novel antibacterials have also demonstrated their power for disinfection due to their unique physicochemical properties and good biocompatibility. Therefore, the exploration of antibacterial mechanisms of 2D NBG is vital to manipulate antibacterials for future applications. Herein, we summarize the recent research progress of 2D NBG-based antibacterial agents, starting with a detailed introduction of the relevant antibacterial mechanisms, including direct contact destruction, oxidative stress, photo-induced antibacterial, control drug/metallic ions releasing, and the multi-mode synergistic antibacterial. Then, the effect of the physicochemical properties of 2D NBG on their antibacterial activities is also discussed. Additionally, a summary of the different kinds of 2D NBG is given, such as transition-metal dichalcogenides/oxides, metal-based compounds, nitride-based nanomaterials, black phosphorus, transition metal carbides, and nitrides. Finally, we rationally analyze the current challenges and new perspectives for future study of more effective antibacterial agents. This review not only can help researchers grasp the current status of 2D NBG antibacterials, but also may catalyze breakthroughs in this fast-growing field.
Keywords: bacterial resistance, antibacterials, 2D NBG, antibacterial mechanisms, physicochemical properties
1. Introduction
The diseases caused by bacterial infections continue to be a major growing global health issue. According to statistics, pathogenic microorganisms including bacteria, fungi, viruses, protozoa, prions, and rickettsia caused millions of deaths all over the world annually 1-3. Subsequently, the advent of antibiotics has effectively inhibited bacterial infections and saved countless lives, which are attributed to their natural properties and abilities to selectively kill bacteria by disrupting cellular processes including protein synthesis, deoxyribonucleic acid (DNA) replication/repair, and cell-wall/membrane formation 4-7. However, the overuse or misuse of traditional antibiotics has resulted in the emergence and rapid increase of multi-drug resistance “superbugs” day after day, consequently leading to extremely severe side effects 8-10. It is estimated that if antibiotics resistance continues to emerge, antibiotic-resistant infections will cause over ten million deaths annually and considerable global economy loss 11-13. In addition, the formation of biofilm is another major obstacle that affects the bactericidal efficiency of traditional antibiotics 14-17. Therefore, it is an urgent demand to search for low toxic antibiotics or develop new classes of alternative antibacterial to alleviate or address the looming crisis of bacterial resistance 18-21.
Recently, sparked by the rapid development of nanoscience and technology, substantial efforts have been aimed to develop versatile nanomaterials as novel antibacterial against various bacterial pathogens based on their extraordinary physicochemical characteristics 22-25. Compared to traditional antibiotics, nanomaterials are less likely to trigger bacterial resistance owing to their good membrane permeability, benign biocompatibility and potential for multiple antibacterial actions 26-28. In addition, 2D nanomaterials with large surface area and easy surface functionalization enable intimate interactions with bacteria membranes, which help in enhancing the antibacterial effect. Moreover, the 2D nanomaterials based antibacterial agents can be used at a low dose than traditional antibiotics, hence overcoming the problem of resistance and diminishing other undesirable side effects to some extent 29-31. Significant progress has been achieved on the development of nanotechnology-based antibacterial nanoagents (graphene 32, noble metals 33, organic polymers 34, etc.) for combating multidrug resistance in bacteria through physical contact damage 35, oxidative stress 36, photo-induced antibacterial (such as photothermal therapy (PTT) 37, photocatalytic therapy (PTC) 38, and photodynamic therapy (PDT) 39), controlled drug/metallic ions releasing 40, and multi-mode synergistic antibacterial 41. Among various antibacterial nanoagents, 2D graphene and its derivatives have exhibited attractive applications in biomedicine (tumor diagnosis and therapy, neurological disorders treatment, and antibacterial) over the past decade 42-47, because of their intriguing biochemical properties, such as easy preparation and functionalization, high specific surface area, and good biocompatibility 48, 49. Moreover, the investigations of graphene-based nanomaterials also facilitated the exploration of new two-dimensional nanomaterials beyond graphene (2D NBG) 50. Fortunately, in recent years, 2D NBG has displayed attractive applications in the fields of catalysis, optical/electronic devices, and biomedicine, which could be attributed to favorable graphene-like physicochemical characteristics, such as large specific surface-area, ultrathin 2D nature, striking light-to-heat capability, extraordinary photocatalytic features, facile surface modification, and relatively benign biocompatibility 51-53. Particularly, recent advances reveal that 2D NBG has a robust antibacterial effect 54. Moreover, these graphene-like nanomaterials-oriented antibacterials presented different types of antibacterial mechanisms 55. To date, the family of 2D NBG nanomaterials have been enriched by many members, which include 2D layered transition metal dichalcogenides (TMDCs), transition metal oxides (TMDOs), graphitic carbon nitride (g-C3N4), black phosphorus (BP), layered double hydroxides (LDHs), transition metal carbides and nitrides (MXenes), and so on 52. Currently, broad researches have been pursued on antibacterial applications of 2D NBG, such as direct interaction mechanism between 2D NBG and bacteria 56. However, a comprehensive review with antibacterial progress, size/shape/phase structure and surface modification-oriented antibacterial advantages, future perspectives and challenges aiming specifically at 2D NBG for antibacterial is still rare. Therefore, it is obligatory to present a systematically summary of the recent research progress of 2D NBG in antibacterial field.
Herein, we provide a critical overview on the recent advances of 2D NBG-based antibacterial agents, specifically focusing on their dominant antibacterial mechanisms (schematic illustration in Figure 1). We first outline the underlying antibacterial mechanisms of 2D NBG and their derivatives as antibacterials based on the existing research studies. Then, a brief discussion of the relationship between the physicochemical characteristics of 2D NBG and antibacterial efficiency is presented. Furthermore, we enumerate some representative 2D NBG and their relevant antibacterial action. Finally, we provide our perspectives about the major obstacles in clinical translation of 2D NBG. We hope that this review can help researchers to understand the properties and antibacterial mechanisms of 2D NBG for antibacterial and draw public attention to develop novel efficient antibacterial strategies using the 2D NBG in the future.
Figure 1.
The existing antibacterial mechanisms and kinds of 2D NBG. Physical contact damage: Reproduced with permission from 58, copyright 2018 American Chemical Society. Oxidative stress: Reproduced with permission from 83, copyright 2016 American Chemical Society. Photo-induced generate heat or ROS for antibacterial. Controlled drug/metallic ions release: Reproduced with permission from 134, copyright 2018 Nature Publishing Group. Multi-mode Synergistic antibacterial: Reproduced with permission from 143, copyright 2016 American Chemical Society.
2. Antibacterial Mechanisms and Physicochemical Characteristics Related Influencing Factors of 2D NBG
To the best of our knowledge, graphene-based nanomaterials with outstanding antibacterial activities based on their extraordinary characteristics, and predominant antibacterial mechanisms have been systematically reviewed by Luo's group in 2016 57.
However, a thorough understanding of the underlying antibacterial mechanisms of 2D NBG remains in its infancy. Therefore, it is essential to summarize the existing antibacterial mechanisms of 2D NBG to manipulate antibacterial nanomaterials for future biomedical applications. According to recent achievements, we summarize that current antibacterial mechanisms of 2D NBG mainly include physical contact destruction, oxidative stress, photo-induced antibacterial, controlled drug/metallic ions release, and multi-mode synergistic antibacterial effects 54, 56. In the following section, we will comprehensively clarify each antibacterial mechanism of 2D NBG and offer some hints to improve antibacterial effects.
2.1 Physical Contact Destruction
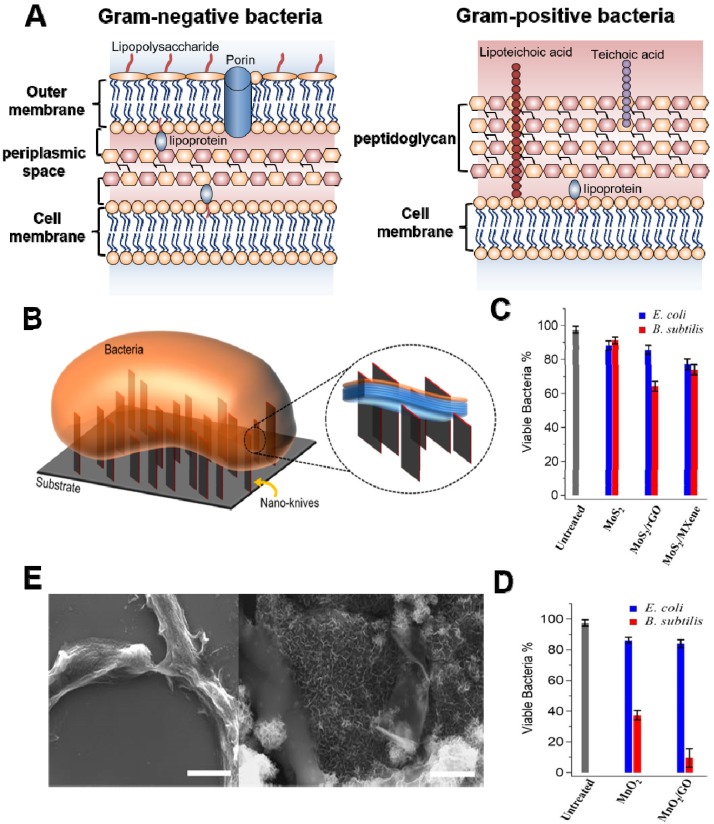
Several accumulated studies have demonstrated that the cell wall or membrane is an indispensable component in all bacteria, which is accountable for maintaining cell morphology, regulating osmotic pressure, and preventing infection 54. It has been verified that Gram- and Gram+ bacteria have different cell membrane structure (Figure 2A) 58-60. The former possesses twain distinct lipoprotein membranes: a lipopolysaccharide coated inner cell membrane and outer membrane, which is separated by a thin layer of peptidoglycan in the periplasmic space 59. Conversely, Gram+ bacteria are composed of single lipid bilayer cell membrane and multilayer thick peptidoglycan mesh outside of the cell membrane 60. It has been confirmed that sharp edges of nanosheets (also regarded as “nanoknives”) were probably compromising bacterial membrane integrity upon physical contact, which could lead to the leakage of intracellular components, such as ribonucleic acid (RNA), DNA, phospholipids, and proteins, etc. 61. This mechanism was initially proposed for graphene-based nanomaterials 35, 62. In recent years, various 2D NBG such as MoS2, WS2, MoO3, RuO2, MnO2, and Bi2Se3 have been used for physical contact disinfection because their sharp edges could damage cell wall or outer membrane 52. For instance, Alimohammadi et al. reported that MnO2 and MoS2 nanosheets with blade-like shape show remarkable antibacterial effects towards B. subtilis and E. coli (Figure 2B) 58. Intriguingly, they studied the antibacterial activities of randomly oriented and vertically aligned MoS2/MnO2 nanosheets on graphene oxide or Ti3C2 MXene nanosheets, and observed that vertically aligned MnO2 nanosheets exhibit higher antibacterial activity than randomly oriented nanosheets, which is ascribed to the sharp edges of vertically aligned MnO2 nanosheets drastic damaging the integrity of bacterial cell membrane (Figure 2C, D). Meanwhile, scanning electron microscope (SEM) images of the B. subtilis also verified that the sharp edge of MnO2 nanosheets can distinctly destroy bacterial morphology and ultimately kill bacteria, which also proved that 2D antibacterials probably mainly damage the peptidoglycan mesh of bacteria (Figure 2E). Similarly, Krishnamoorthy et al. revealed that the plate-like morphology of the as-prepared MoO3 functioned as “nanoknives” could kill bacteria through physical puncture of the outer bacterial wall 63, 64. Furthermore, Liu et al. found that WS2 nanosheets cling more bacterial and show a robust antibacterial activity 65. From the SEM and transmission electron microscope (TEM) images, they proposed that WS2 nanosheets with sharp edges could apparently damage the structural integrity of the bacterial membrane, which is induced by the direct contact of bacteria and WS2 nanosheets. Besides, Jiang et al. developed a novel Bi2Se3 nanodiscs through solvent thermal reaction for selectively treating Gram+ bacterial infections 66. They found that Bi2Se3 nanodiscs mainly damage bacterial wall/membrane by binding with lipoteichoic acid of Gram+ bacteria. It is worth noting that theoretical simulation results revealed that the physical contact destruction action of graphene nanosheets mainly depends on their sizes 67-70. However, it is not clear whether the size or other physicochemical properties of 2D NBG will affect their antibacterial activity. Therefore, physical contact destruction as one of the most common antibacterial mechanisms should be further studied. And the combination of theoretical simulation with experiment to deeply explore the effect of nanomaterial physicochemical properties on antibacterial activity is also recommended.
Figure 2.
(A) General membrane ultrastructure of Gram- and Gram+ bacterial species. (B) Schematic representation of direct physical interaction of the bacterial surface with sharp edges of vertically aligned nanosheets onto a substrate. Antibacterial activities of (C) MoS2 and (D) MnO2 nanosheets against E. coli and B. subtilis. (E) SEM images of the B. subtilis bacteria treated with MnO2 and MnO2/GO nanomaterials for 3 h in dark. Reproduced with permission from 58, copyright 2018 American Chemical Society.
2.2 Oxidative Stress Antibacterial
Oxidative stress can impede bacterial metabolism and continuously damage essential cellular functions, causing bacteria inactivation, and eventually killing bacteria 71, 72. Therefore, the generation of oxidative stress is one of the widely accepted antibacterial methods. In general, 2D nanomaterials-mediated oxidative stress mainly includes reactive oxygen species (ROS)-dependent and ROS-independent oxidative stress.
2.2.1 ROS-Dependent Oxidative Stress
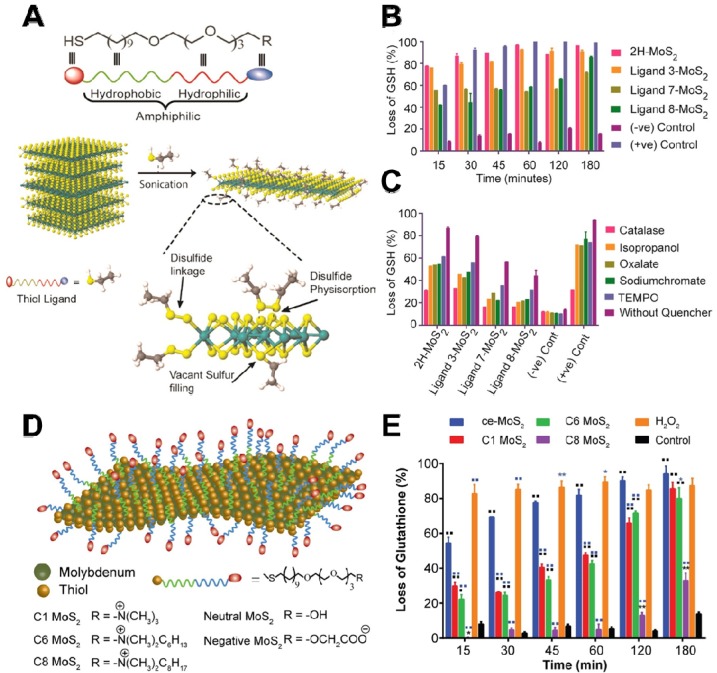
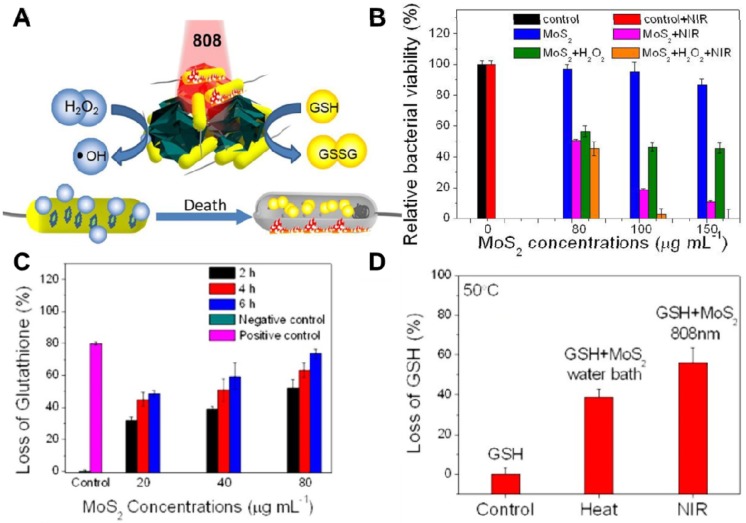
ROS-dependent oxidative stress is triggered by excessive accumulation of intracellular ROS, such as hydrogen peroxide (H2O2), hydroxyl radicals (•OH), superoxide anions (•O2-), and singlet molecular oxygen (1O2) under external/internal stimulus factors. Previous studies have demonstrated that graphene and its ramifications can generate ROS 8. In general, an excessive amount of ROS not only causes direct damage to the bacterial structure by breaking DNA single strand but also leads to hyperoxidation of intracellular components. Recently, 2D NBG has been found to exert antibacterial performances through the production of ROS under light-dependent or light-independent stimulus factors. Several cumulative studies have documented that 2D MoS2 nanosheets with supramolecular selfassembly property could selectively produce ROS in a target cell rather than control cells, which is beneficial for their further biomedical application 73. To verify the ROS-dependent oxidative stress, Karunakaran et al. used different thiol surfactant molecules to exfoliate and functionalize 2H-MoS2 (H stands for hexagonal) nanosheets for enhancing the antibacterial effect (Figure 3A) 74. By monitoring ROS levels using Ellman's assay, they found that 2H-MoS2 nanosheets with suitable conduction band could incur more loss of glutathione (GSH) when compared to 1T-MoS2 (T is trigonal) nanosheets (Figure 3B). Subsequently, they used different scavengers to ascertain the ROS type and found that H2O2 is the dominant ROS (Figure 3C). Moreover, they used fluorescent probe 2',7'-dichlorofluorescin diacetate (DCFDA) to estimate the amount of intracellular ROS, and the results revealed that positively charged 2H-MoS2 nanosheets can effectively attach to the bacterial surface and generate more ROS. In contrast, the negatively charged 2H-MoS2 nanosheets with non-interactive property produced less ROS in bacteria. Therefore, due to the different semiconducting property of 2H and 1T phase, when compared to 1T-MoS2, the ROS-dependent oxidative stress is more obvious in 2H-MoS2 nanosheets. Furthermore, the higher antibacterial capacity of exfoliated 1T-phase TMDCs (including MoS2, WS2, MoSe2) nanosheets is also attributed to the production of ROS 75, 76. In addition, Xiong et al. demonstrated that BP nanosheets can also strikingly kill E. coli and B. subtilis through ROS-dependent oxidative stress and membrane damage mechanism 77. These studies not only partially verified the generation of ROS in bacteria, but also proved that ROS-dependent oxidative stress is an important antibacterial process.
Figure 3.
(A) Scheme for the exfoliation of 2H-MoS2 with amphophilic ligand. (B) Abiotic oxidative stress estimation by Ellman's assay with 0.4 mM glutathione. (C) Estimation of the type of ROS species in the presence of different ROS scavengers. Reproduced with permission from 74, copyright 2018 American Chemical Society. (D) Schematic representation of functionalized ce-MoS2 with thiol ligands of varied charge and hydrophobicity. (E) Abiotic glutathione oxidation assay for quantification of oxidative stress generated. Reproduced with permission from 83, copyright 2016 American Chemical Society.
2.2.2 ROS-Independent Oxidative Stress
Although the above-mentioned reviews underline the crucial role of ROS-dependent oxidative stress, several studies have revealed that ROS-independent oxidative stress also possesses a favorable bactericidal effect. In detail, ROS-independent oxidative stress mainly damages or oxidizes cellular structure and components (such as lipids, proteins, and DNA) by nanomaterials-induced direct oxidation rather than ROS production 78, 79. GSH, a ubiquitous antioxidant in vivo, not only can serve as an intracellular redox state indicator but also can indicate the oxidative stress antibacterial effect owing to its easy oxidation into glutathione disulfide (GSSG) 80. In 2008, Alvarez's group firstly proposed this antibacterial mechanism and they observed that the antibacterial ability of fullerene (nC60) does not rely on light and oxygen, which are the two conditions essential for ROS generation 81. Similarly, Yang et al. used a chemically exfoliated (ce) method to synthesize ce-MoS2 nanosheets and studied their antibacterial activity 82. They found that ce-MoS2 nanosheets could dramatically reduce the viability of E. coli in a short time, and show concentration- and time-dependent GSH oxidation capacity. Therefore, the antibacterial activity of ce-MoS2 nanosheets is attributed to ROS-independent oxidative stress. In addition, Mrinmoy's group revealed that the surface charge and hydrophobicity of ce-MoS2 nanosheets can be altered by modifying with different thiol ligands (Figure 3D), and such functionalized ce-MoS2 nanosheets can be endowed with remarkable antibacterial effect by ROS-independent oxidative stress and depolarization of the bacterial membrane 83. Intriguingly, ce-MoS2 nanosheets showed higher GSH oxidizability than functionalized MoS2 nanosheets (Figure 3E), which is responsible for the ROS-independent oxidative stress generated by ce-MoS2 nanosheets. What's more, they also found that the surface functionalization can affect antibacterial pathway, and non-functionalized MoS2 nanosheets against bacteria mainly through ROS-induced oxidative stress, while functionalized MoS2 nanosheets kill bacterial through both ROS-independent oxidative stress and bacterial membrane depolarization. Although vast research has substantiated the striking germicidal ability of graphene oxide (GO) and MoS2 nanosheets, the relevant study of 2D nanosheet-coated films for biomedical devices is still rare. Based on this, Kim et al. prepared a GO-MoS2 nanocomposite film with outstanding antibacterial effects 84. They found that GO-MoS2 nanofilms have higher ROS-independent oxidation capacity, and its antibacterial effect is mostly achieved through ROS-independent oxidative stress. Furthermore, Navale et al. also reported that the reduced graphene oxide-tungsten disulphide (rGO-WS2) nanosheets had a significant bacterial inhibitory effect compared to pure WS2 or rGO. They observed that rGO-WS2 composites have the highest oxidation capacity towards GSH, and proposed that the GSH membrane mechanism stress may originate from the direct contact with nanosheets, and ROS-independent oxidation stress was the major antibacterial mechanism 85.
2.3 Photo-Induced Antibacterial Effects
It is well-known that light-induced antibacterial mainly utilized photoconversion of excited photoactive fluorophores in photosensitizer, photothermal agents and other semiconductor nanomaterials into heat or ROS for PTT, PCT or PDT 37, 38, 86, 87. Therefore, in recent years, these photo-induced antibacterial methods have been considered as a promising antibacterial mechanism due to their peculiar merits such as noninvasiveness, targeted selective treatment, and minimized side effects.
2.3.1 Photothermal Antibacterial
Photothermal antibacterial is an important antibacterial approach, which refers to the efficient generation of heat by nanomaterials under the irradiation of light at a proper power density to kill bacteria 37. Near-infrared (NIR) photothermal nanoagents are preferred for antibacterial due to their deep biological tissue penetration capability and minimal damage to healthy tissues. In recent years, researchers have demonstrated that various 2D NBG display remarkable photothermal antibacterial ability owing to their strong photothermal conversion efficiencies, such as MoS2 88, metal-based nanomaterials 89, Sb2Se3 90, LDH-based compounds 91, and BP nanosheets 92. Among them, MoS2 nanosheets have attracted considerable attention as typical photothermal antibacterial agents due to their high NIR photothermal conversion efficiency 93, 94. Based on it, Zhang et al. prepared versatile chitosan functionalized magnetic MoS2 as bacterial cross-linking and photothermal agents for in vitro photothermal sterilization (Figure 4A), and in vivo focal infection treatment (Figure 4B) 95. The nano-agent could cause rapid aggregation and bacteria arrest in both S. aureus and E. coli, thereby enhancing the photothermal antibacterial effect. Under NIR irradiation, the temperature of the nano-agent solution (100 μg mL-1) could reach around 45 oC within 10 min, and the bacteria survival rate decreased over 90% (Figure 4C). Besides, this nano-agent nanoplatform could also effectively convert NIR light into localized heat for treating in vivo focal infection. More recently, Ma et al. developed a core-shell gold nanorod@layered double hydroxide (GNR@LDH) nanomaterial for enlarging PTT antibacterial and tumor therapy 91. They found that the thermal energy conversion of GNR@LDH can be significantly enhanced after 808 nm laser irradiation, which is mainly due to the production of electron deficiency on gold surface. As shown above, the burgeoning photothermal nanomaterials with strong NIR light absorbance/convert capacity revealed that the generated heat can not only kill drug-resistant bacteria but also inhibit the formation of biofilm. Therefore, 2D NBG based PTT is deemed as a safe and efficient strategy to combat bacterial infections.
Figure 4.
(A) In vitro conjugating and photothermal killing of bacteria, (B) and in vivo photothermal treatment of a focal infection using chitosan functionalized magnetic MoS2. (C) Antibacterial activity of the chitosan functionalized magnetic MoS2 nanocomposites with or without NIR laser irradiation for S. aureus and E. coli. Reproduced with permission from 95, copyright 2016 Royal Society of Chemistry. (D) Schematic that shows the PPMS/BPS inactivating bacteria through generating 1O2 in the presence and absence of light, and the stability of BPS can be improved by PPMS. (E) Antibacterial performance in vitro. Reproduced with permission from 122, copyright 2018 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim.
2.3.2 Photocatalytic/Photodynamic Antibacterial
It is well-known that the photo-induced production of ROS could cause distinct oxidative stress to bacteria. Generally, the photo-mediated ROS generation mainly includes two ways: photocatalytic and photodynamic process. Photocatalytic antibacterial refers to light-induced bacteria inactivation, which depends on the efficient generation of highly oxidative ROS by the separation of holes (h+) and electrons (e-) in the valence band (VB) and conduction band (CB) under light irradiation 96. For many 2D NBG, they can generate photo-induced ROS to attack bacteria under ultra violet (UV) or visible light illumination. Normally, the photocatalytic disinfection process mainly includes three steps: (i) 2D NBG with large surface area can bind to bacterial surface, which provide more active sites for catalytic reactions; (ii) the formation of a photo-induced charge-separated state; (iii) the interactions of activated charge-separated centers and neighboring oxygen and water molecules can produce ROS 97. Bismuth oxybromide (BiOBr) nanosheets, as a major 2D layered semiconductor, have attracted tremendous attention owing to its outstanding physicochemical prosperities and excellent visible light photocatalytic activity 98. To further efficiently harvest solar energy, Wong's group fabricated bismuth oxybromide (BiOBr) nanosheets and doped with boron (B), where they further found that B-BiOBr nanosheets could effectively enhance photocatalytic bacteria inactivation through photogenerated h+ mediated mechanism 99. Firstly, light motivated BiOBr to generate e- in its CB, and leave h+ in its VB. Then, B as a good e- acceptor could promote an extra e- from VB to CB and cause abundant h+ gathering in VB, which could enhance the e-/h+ pair separation efficiency of BiOBr. Finally, the amount of h+ with higher oxidative ability could directly oxidize bacteria, leading to photocatalytic bacteria inactivation. They also found that B-BiOBr nanosheets show apparent photocatalytic disinfection activity towards E. coli, and the increased content of B dopant could dramatically enhance the antibacterial properties. Moreover, Wu et al. demonstrated that self-doped Bi5+ or Bi2O4 decorated BiOBr nanosheets display a much higher photocatalytic inactivation activity than pure BiOBr nanosheets 100, 101. Recently, g-C3N4 has been a rising star in the field of photocatalyst disinfection as it could harvest substantial visible light for achieving highly efficient photocatalytic performance 102-104. For example, Huang et al. for the first time revealed that g-C3N4 nanosheets exhibited excellent photocatalytic bactericidal effects toward E. coli under visible light irradiation, without any bacterial regrowth 105. Several cumulative studies have documented that exfoliating the bulk g-C3N4 into g-C3N4 nanosheets could improve the photocatalytic performance. To this end, Zhao et al. fabricated single layer g-C3N4 nanosheets by thermal etching and ultrasonic exfoliation. They found that a total of 2 × 107 CFU mL-1 of E. coli could be destroyed completely within 4 h after incubated with single layer g-C3N4 nanosheets under visible light irradiation, which is much higher than bulk g-C3N4 nanosheets 106. Besides, some other 2D NBG, such as MoS2, TiO2, Bi2WO6, ZnO, as well as metal-free nanomaterials, also exhibited outstanding photocatalytic antibacterial activity 97, 104, 105, 107-109. To further improve the photocatalytic antibacterial activity, the integration of various photocatalysts or co-catalysts, such as Ag-ZnO/g-C3N4 110, g-C3N4 NS/RGO/CA 111, V2O5/BiVO4 112, graphene/g-C3N4 113, TiO2/g-C3N4 114, TiO2-Bi2WO6 108, and Bi2MoO6/g-C3N4 115, has been widely investigated as an effective modification strategy. Although photocatalytic disinfection represents one of the most promising disinfection mechanisms, important issues such as catalyst immobilization, recycling methods, photocatalytic reactor design as well as optimization of disinfection parameters of 2D NBG need to be addressed for future practical applications.
In addition to above photocatalytic-induced bacteria elimination, PDT as another light-induced ROS generating modality for minimally invasive antibacterial has also been reported 86. It mainly employs appropriate excitation light and oxygen molecules as exogenous stimuli to selectively activate photosensitizers at the targeted region, producing various cytotoxic ROS like 1O2 and •OH to effectively damage the bacterial membrane, nucleic acids, proteins, and even DNA. Additionally, PDT can also eliminate biofilms by degrading their excretive products 116. Notably, PDT has several advantages over other conventional antibacterial strategies owing to its noninvasive property, massive loading capability, and fast healing process. PDT can be carried out through two paths: type I and type II. Under light irradiation, photosensitizers migrate from ground state to single excited state, and then reach to triple excited state 117, 118. For type I, the triple excited-state photosensitizers directly react with the biological substrates to generate •OH or •O2- through electron transfer, and this process not only break the structural integrity of the bacterial membranes but also enhance ionic permeability of bacteria cell membrane 119. For type II, triple excited-state photosensitizers could directly transfer energy with the surrounding 3O2 to produce cytotoxic 1O2, and the 1O2 can effectively kill bacteria by causing oxidative damages to unsaturated lipids, enzymes, DNA, peptides, and other cellular components 120. Currently, various 2D nanomaterials such as MoS2 nanosheets, BP nanosheets, fullerenes, graphene, ZnO, and TiO2, show inherent photosensitivity, and they have been widely explored for PDT through generating ROS upon light irradiation 121. Recently, Tan et al. designed a novel PDT antibacterial system based on the integration of BP nanosheets and poly (4-pyridonemethylstyrene) (PPMS), achieving the storage and release of 1O2 for rapid disinfection and infection prevention (Figure 4D) 122. Intriguingly, BP nanosheets were used as a photosensitizer to generate 1O2 under light irradiation. Simultaneously, the PPMS were composited with the BP nanosheets to store singlet 1O2, and the 1O2 was released by thermal decomposition. They found that this system showed an excellent PDT antibacterial effect with or without light irradiation (Figure 4E). Therefore, 2D-based nano-photosensitizers as light-triggered bactericides show great potential for PDT antibacterial.
2.4 Controlled Drug/Metallic Ions Release for Antibacterial
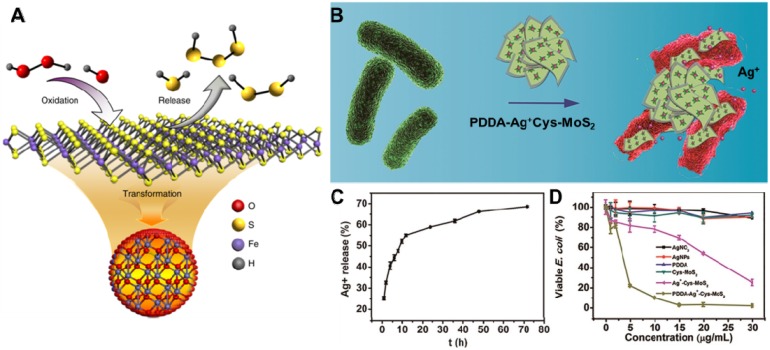
The ability of controllable release drug/metallic ions is widely accepted as an important antibacterial process of 2D NBG 123. Due to their large surface area, 2D NBG can act as nanocarriers with controlled release of antibacterial agents under particular stimuli 124. Currently, the release of antibacterial agents mainly include either converting unstable organosulfur compounds into stable inorganic sulfur compounds 125 or loading traditional antibiotics (cefazolin 126, tetracycline hydrochloride 127, and curcumin etc. 128), noble metallic ions (such as Ag ions 129-131) and molecular donor (NO 132, H2S donor 133) into 2D NBG surface. Ultimately, released drugs or metallic ions on the surface of 2D NBG can inactivate the bacteria through interfering essential cellular components such as cell membrane integrity, respiration, and adenosine triphosphate (ATP) production. Recently, Gao's group proposed a novel nano-conversion strategy for enhancing antibacterial activity through converting natural organosulfur compounds into nano-iron sulfides (FeS), where the enhanced antibacterial effect benefited from cysteine-nFeS (Cys-nFeS) with enzyme-like activity could effectively increase the release of bactericidal polysulfanes (Figure 5A) 134. The antibacterial results also showed that Cys-nFeS not only display a broad antibacterial activity against Pseudomonas aeruginosa (P. aeruginosa), E. coli, and S. aureus, but also could effectively inhibit the formation of biofilms and accelerate infected-wound healing. Moreover, it is well-known that Ag ions can combine with proteins, nucleic acid, and enzymes in microorganisms, causing a serious deformation of the bacteria membrane to ultimately kill bacteria 135. To this end, Cao et al. constructed an efficient and benign antibacterial depot (PDDA-Ag+-Cys-MoS2) with Cys-modified MoS2 loaded with minimum Ag ions and coated with cationic polyelectrolyte (Figure 5B) 136. This system displayed remarkable antibacterial activity toward both E. coli and S. aureus compared with an equivalent amount of silver nitrate, due to its enhancing accessibility of released Ag ions to the bacterial wall (Figure 5C, D). In addition, antibiotic-loaded MoS2 nanosheets and MoS2‑modified curcumin nanostructures could effectively release antibacterial agents against multidrug-resistant bacteria 127, 128. To enhance the antibacterial activity of this disinfection process, high concentrations of 2D NBG nanocarriers are necessary. However, due to the toxicity of metallic ions, it is important to regulate the concentration of metal ions in an acceptable range. Therefore, the effective removal of metal ions and 2D NBG from in vivo also requires a deeper investigation in the future.
Figure 5.
(A) Scheme of polysulfane release from nFeS. Reproduced with permission from 134, copyright 2018 Nature Publishing Group. (B) The PDDA-Ag+-Cys-MoS2 depot for antibacterial applications. (C) Cumulative silver ion release profiles from PDDA-Ag+-Cys-MoS2 samples. (D) Viability analyses of E. coli. Reproduced with permission from 136, copyright 2017 American Chemical Society.
2.5 Synergistic Antibacterial
In the previous sections, we have detailed introduced the typical antibacterial mechanisms of 2D NBG based on their intrinsic antibacterial properties. However, the complexity, diversity, and multidrug resistance of bacteria seriously impede the development potential of 2D NBG for disinfection. The antibacterial mechanisms mentioned above also suffer from their respective shortcomings, which limit their further antibacterial application. For example, the mechanism of physical contact destruction could only kill the bacteria attached on the surface of 2D NBG, and the long-term antibacterial efficiency could significantly decrease with the degradation of nanomaterials. For the oxidative stress antibacterial mechanism, the antibacterial effects mainly depend on the specific surface area, conductivity, and size of the 2D NBG. Although light-induced antibacterial mechanisms were deemed as one of the generally accepted efficient antibacterial strategies, the targeted killing bacteria ability, the hypoxia infected areas, and the photocatalytic efficiency are still prominent factors limited their antibacterial performances. In addition, for the release of metallic ions or drugs, the overuse may also bring serious toxicity to normal tissues. Thus, current study trend has gradually converted from the concentration on mono-mode antibacterial to 2D NBG-mediated multi-mode synergistic antibacterial for enhanced antibacterial effect. Currently, 2D NBG was often employed as nanocarriers to deliver other antibacterial agents (such as drugs, metallic ions, gas donor, and antibacterial polymers) into infected areas, which attain striking synergistic antibacterial effects that are stronger than that of any antibacterial strategy alone. Here, we summarize several common 2D NBG-mediated synergistic antibacterial methods: (i) synergy of PTT with PDT 137-139; (ii) synergy of PTT with metallic ions release (Ag, Au, etc.); (iii) synergy of chemotherapy with PTT 140; (iv) synergy of PTT with oxidative stress 79; (v) synergy of PTT with catalytic 139, 141-144; (vi) and tri-modal synergistic antibacterial 145, 146. At the same time, the synergetic antibacterial mechanism and application are detailedly discussed.
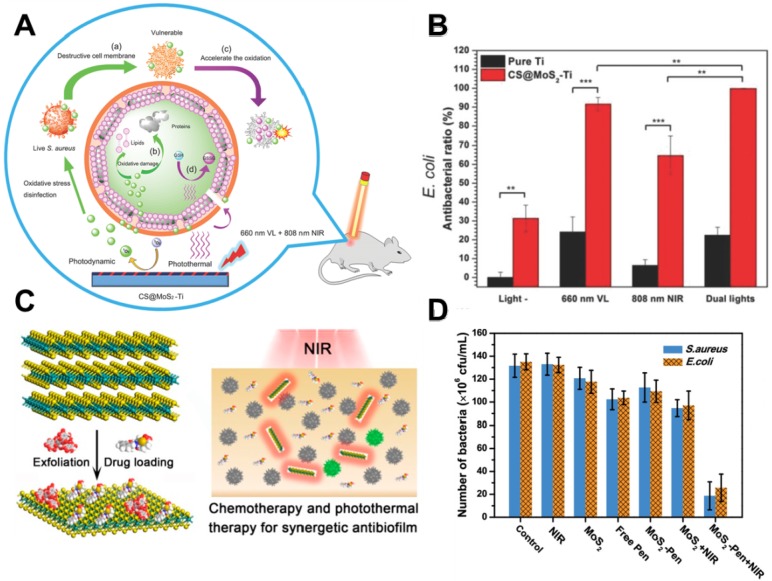
In recent years, PTT antibacterial has been widely accepted by researchers. The generation of heat by PTT can not only directly destroy the bacteria but also accelerate blood flow so as to effectively improve the intracellular generation of ROS for enhanced PDT. To this end, Wu's group synthesized a chitosan-assisted MoS2 (CS@MoS2) hybrid with a coating on the surface of Ti material, which showed rapid in situ antibacterial effect 147. They demonstrated that the synergistic effects of PDT and PTT actions of CS@ MoS2-Ti under dual lights irradiation (660 nm visible light and 808 nm NIR) could significantly enhance the antibacterial effect towards E. coli and S. aureus (Figure 6A, B). Besides, Wu's group prepared hybrid nanosheets based on g-C3N4-Zn2+@graphene oxide for rapid sterilization and accelerated wound healing under dual light irradiation 139. They found that the hybrid nanosheets could achieve an antibacterial ratio over 99.1% within a short time due to the synergistic effects of PDT and PTT. In addition, Wang's group used polydopamine (PDA), MoS2 nanosheets and silver nanoparticles to form MoS2@PDA-Ag nanosheets (MPA) as a novel dual antibacterial nanoagent 148. On the one hand, under NIR irradiation, the MPA nanosheets could generate heat, and directly damage the bacterial biofilm and the bacterial membrane. On the other hand, PTT also accelerates Ag ions release, enhancing the antibacterial efficacy of Ag ions. Therefore, the therapy strategy of combining PTT and metallic ions release is helpful for addressing the lower single antibacterial effect. In addition, Yuan et al. fabricated a functional MoS2/PDA-arginine-glycine-aspartic acid (RGD) to inhibit bacterial infection in situ and improve osseointegration 79. They found that NIR-induced hyperthermia of MoS2/PDA-RGD samples could efficiently increase GSH oxidation. Besides, the intrinsic ROS-independent oxidative stress of MoS2 nanosheets could also destroy the integrity of the bacterial membrane, finally significantly killing bacteria. After NIR irradiation, the antibacterial efficiency of MoS2/PDA-RGD reached 96.6% for S. aureus and 97.8% for E. coli, which is ascribed to the synergism between PTT and oxidative stress. Some antibiotic drugs, such as penicillin (pen), curcumin and lysozyme can be controllably released from 2D NBG surface. Based on which, Zhang et al. constructed an antibacterial nanomaterial through a polyphenol-assisted exfoliation strategy to exfoliate MoS2 nanosheets and loading antibiotic drugs pen onto MoS2 nanosheets surface (Figure 6C), which exhibited robust antibacterial effect via a synergetic of NIR-driven PTT and Pen-induced chemotherapy (Figure 6D) 140. Furthermore, our previous study demonstrated that polyethylene glycol functionalized MoS2 nanoflowers (PEG-MoS2 NFs) with peroxidase-like catalytic activity could be used for treating in vivo wound infection, which could convert low dosage clinical H2O2 into more toxic •OH. Together with the NIR photothermal ability of MoS2, it could realize synergetic PTT/catalytic antibacterial (Figure 7A, B) 143. Intriguingly, PEG-MoS2 NFs exhibited a time-dependent and hyperthermia-enhanced oxidation behavior, and after incubation with PEG-MoS2 NFs (80 μg mL-1) for 6 h the statistical loss of GSH can reach to 73.4% (Figure 7C). Furthermore, compared to the water bath group at 50 °C, the hyperthermia induced by PEG-MoS2 NFs showed a much higher GSH oxidation level (Figure 7D). Recently, Qu's groups firstly developed a nanozyme with abundant defects that have enhancing bacterial capturing abilities and efficient enzyme catalysis antibacterial effects 144. Briefly, the nanozyme with rough surface not only can efficiently trap bacteria, but also the defect-rich edges exhibit higher intrinsic peroxidase-like activity than pristine structures, which generate amounts of toxic •OH around the bacteria and exhibit considerable bacterial inhibition effect. Aside from the above mentioned dual-mode synergistic antibacterial, Yin et al. designed a tri-modal synergistic antibacterial agent (MoO3-x-Ag) for cleaning the bacterial contaminated water environment 145. They found that the as-designed MoO3-x-Ag killed pathogenic bacteria mainly through (i) photothermal effect of MoO3-x nanosheets upon NIR irradiation; (ii) NIR light excitation of MoO3-x-Ag trigger the release of Ag ions; and (iii) photocatalytic reaction. Moreover, Roy et al. prepared chitosan exfoliated MoS2 nanosheets (CS-MoS2) and investigated their intrinsic antibacterial mechanism 146. They found that the CS-MoS2 nanosheets could kill bacteria through a synergistic effect of ROS-dependent oxidative stress, physical contact caused membrane damage and metabolic inactivation.
Figure 6.
(A) Schematic illustration of CS@MoS2 hybrid coating constructed on the surface of Ti material for synergistic photodynamic and photothermal antibacterial action under the dual lights irradiation. (B) The antibacterial activity E. coli and S. aureus of pure Ti and CS@MoS2-Ti under the conditions of darkness (light) and after exposing to 660 nm visible light; 808 nm NIR; dual lights for 10 min. Reproduced with permission from 137, copyright 2018 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim. (C) The Pen-loaded MoS2 nanosheets exhibit outstanding antibiofilm activity via synergetic chemotherapy and photothermal therapy. (D) Number of S. aureus and E. coil in the presence of different antibacterial agents: control, NIR light, MoS2 nanosheets, free Pen, MoS2-Pen nanosheets, MoS2 nanosheets with NIR light, and MoS2-Pen nanosheets with NIR light. Reproduced with permission from 140, copyright 2018 American Chemical Society.
Figure 7.
(A) Schematic illustration of PEG-MoS2 for peroxidase catalyst and photothermal synergistic antibacterial. (B) Relative bacterial viability of Ampr E. coli incubated with different concentrations PEG-MoS2 NFs with or without H2O2 (100 μM) under 808 nm laser irradiation. (C) Loss of GSH plot after incubation with PEG-MoS2 at different time intervals. (D) Loss of GSH plots heated by water bath and NIR 808 nm irradiation, respectively, for 20 min at 50°C. Reproduced with permission from 143, copyright 2018 American Chemical Society.
2.6 Effect of Physicochemical Properties on the Antibacterial of 2D NBG
It is well-known that the antibacterial effect of 2D nanomaterials can be significantly influenced by their intrinsic physicochemical properties, such as size, shape, number of layers, phase structure, and surface functionalization. Firstly, the size of 2D nanomaterials is a key factor in governing the antibacterial effect because size can strongly influence the dispersibility and adsorption ability, which are crucial to the interactions between 2D nanomaterials and bacteria. For instance, a relatively smaller size of Ag nanoparticles can increase the interaction with bacteria and exhibit higher antibacterial activity 149-151. Then, the shapes of 2D nanomaterials are also found to largely influence the germicidal ability.
It was revealed that the shapes of 2D nanomaterials would affect its interaction strength with the bacterial membrane, which greatly influences electron-transfer-based oxidative stress antibacterial activities. For example, Ananth et al. prepared spherical and sheet-like ruthenium oxide (RuO2) nanomaterials, and their shape-dependent anti-bacterial activities were carried out. Results showed that RuO2 nanosheets displayed higher antibacterial activity compared with RuO2 nanospheres 152. Moreover, the numbers of 2D NBG layers also influence antibacterial performance. Normally, thinner nanomaterials can easily damage the bacterial cell wall, and causing outstanding antibacterial effect. For instance, Sun et al. used N,N'-dimethylpropyleneurea as a new exfoliating solvent to effectively produce higher crystalline and free defects of BP nanosheets, and found that exfoliated BP nanosheets exhibited thickness-dependent photothermal antibacterial effects 153. In addition, the phase structure of layered TMDCs nanomaterial also affects their antibacterial activity. In general, due to the differences in coordination geometry between metal and chalcogen atoms, TMDCs mainly include the 1T phase and 2H phase 154. The 1T phase structure of TMDCs with metallic character can be used in cell destruction using NIR PTT effect 88, while the 2H phase structure with semiconducting and photoluminescence property can be utilized in visible light-induced water disinfection 97. According to research findings, the antibacterial capacity of exfoliated 1T-MoS2 nanosheets is higher than the annealed exfoliated 2H-MoS2 nanosheets, which is attributed to the higher electron conductivity in 1T-MoS2 nanosheets 75. Finally, the surface functionalization also affects the antibacterial performance of 2D NBG through changing their surface charge 155. For instance, 2D nanomaterials modified with positively charged groups could efficiently attach to the negatively charged bacterial membrane, which presents higher antibacterial activities 74.
3. 2D NBG Antibacterial Nanomaterials
In the past few years, there have been large amounts of research that concentrate on 2D NBG and their bactericidal applications. Taking inspiration from the excellent antibacterial performances of graphene, the potential antibacterial applications of 2D NBG, such as transition-metal dichalcogenides/oxides (TMDC/Os), metal-based nanocompounds, C3N4, BP, MXenes and other burgeoning 2D NBG materials have also been researched. Here, a summary of the recent progress of the 2D NBG antibacterial applications is in Table 1, and the detailed introduction is given below.
Table 1.
A summary of 2D NBG for antibacterial application
| Category | Nanomaterials | Morphology, size & surface modification | Type of Bacteria | Antibacterial Mechanism & effect | Refs. |
|---|---|---|---|---|---|
| TMDC/Os | CS@MoS2 | Monolayer NSs, Chitosan modified | E. coli, S. aureus | PDT & PTT cause the disruption of membrane integrity & leakage of cytoplasmic components; >99% | 41, 137, 156 |
| Magnetic MoS2 | NSs, Chitosan functionalized | E. coli., S. aureus | Enhance the conjugation of bacterial & PTT; >90% | 95 | |
| MoS2-Pen | Monolayer NSs, 38.6± 1.3 nm, Pen-loaded | E. coli., S. aureus | chemotherapy & PTT synergetic effect; kill the majority of bacterial | 140 | |
| PEG-MoS2 | 334 nm NFs, PEG modified | E. coli, B. subtilis | Peroxidase-like catalysis and PTT synergetic antibacterial; up to 97% | 143 | |
| MoS2-BNN6 | Single-layer NSs, 50~80 nm, α-cyclodextrin modified | Ampr E. coli, E. faecalis | PTT & NO-enhanced free radical generation synergetic antibacterial, highly effective bacterial inactivation (>97.2%) | 157 | |
| pyramid MoS2@Ag | Triangles, 5 to 10 μm | E. coli | Photocatalytic generate ROS; more than 99.99% | 158 | |
| MoSe2 | 100 nm NSs, without modified | E. coli, B. subtilis | Peroxidase-like activity could catalyze H2O2 into •OH for antibacterial; ~100% | 159 | |
| MoO3 | plate-like structures | E. coli, S. aureus, E. faecalis, B. subtilis | Disruption of the bacterial cell wall; Effective | 63 | |
| MoO3-x-Ag | Around 300 nm NSs, without modified | E. coli, S. aureus | PTT, Ag+ release & photocatalytic synergic effect; 99.2% of E. coli and 97.0% of S. aureus are killed | 145 | |
| WS2 | 2~3 layers 200 nm NSs | E. coli, S. aureus | ROS generating & damage the structural integrity of bacterial membrane | 65 | |
| WX2-ssDNA | 65~650 nm NSs | E. coli | ROS-independent oxidative stress; around 82.3% | 78 | |
| PDMS/WS2 | Single-layer less than 1 µm nanoflowers | E. coli | ROS generating; more than 99.99% | 160 | |
| WS2 | Monolayer NSs, Ag decorated | E. coli | Ag enhance the photocatalytic efficiency; up to 96% | 161 | |
| Metal-based | Pd@Ag | hexagonal plate-like shapes, 85 nm | E. coli, S. aureus | the synergic effect of PTT & NIR-triggered Ag+ release; almost 100% bacteria | 162 |
| Ag/CS@MnO2-Ti | Chitosan functionalized | E. coli, S. aureus | PTT & Ag ions release synergic effect; up to 99.00% | 163 | |
| TiO2 | Sizes controllable NSs, Amine modified | E. coli | Photocatalytic antibacterialactivity; around 89% | 164 | |
| MoS2-TiO2 | Sheet-like morphology | E. coli, S. aureus | Effective | 165 | |
| Nitride-based | g-C3N4 | Plane structural, etching with HCl | E. coli | Photocatalytic & self-cleaning; reach 100% | 166 |
| g-C3N4-Au | irregular nanosheets, about 200 nm, 1-pyrenebutanoic acid modified | E. coli | light-triggered ROS generation; Effective | 167 | |
| Bi2MoO6/g-C3N4 | Plane structural | E. coli | PCT antibacterial; significantly effect | 115 | |
| Ag@g-C3N4 | Ultrathin NSs, 20~40 nm | E. coli, S. aureus & P. aeruginosa | photocatalytic generated ROS; Effective | 168, 169 | |
| Ag/PDA/g-C3N4 | PDA modified | E. coli | Photocatalytic & Ag NPs; 99.2% of E. coli can be killed within 2 h | 170 | |
| AgVO3 QDs/g-C3N4 | Sheet-like morphology | S. aureus, Salmonella | Photocatalytic generated ROS; killing 96.4% of bacterial within 10 min | 171 | |
| BP-based | BP | 215.84 ± 82.09 nm NSs | E. coli, B. subtilis | ROS-dependent oxidative stress & membrane damage; up to 91.65% and 99.69% for E. coli and B. subtilis | 77 |
| BP@silk fibroin | About 200 nm layer NSs, silk fibroin modified | E. coli, B. subtilis | NIR-mediated PTT bactericidal; eliminate most of the bacteria | 92 | |
| PPMS/BPS | 588 nm NSs, with modified | E. coli, S. aureus | PDT; 99.3% against E. coli and 99.2% against S. aureus | 122 | |
| AuNPs/BP | Ultrathin NSs | E. coli | Catalytic synergistic Au; up to 94.7% | 172 | |
| MXenes | Ti3C2Tx | Multilayer transparent flakes, about 400 nm | E. coli, S. aureus | Physical contact lead to membrane damage; > 98% | 173 |
| MoS2/MXene | About 350 nm NSs | E. coli, S. aureus | Sharp edges lead to membrane damage; >90% | 58 | |
| Ti3C2Tx MXene | Single-layer NSs, few hundred nanometers | E. coli, S. aureus | Inhibit the bacterial growth and efficiently hinder the biofilm formation; > 99% growth inhibition | 174 | |
| Others | B-BiOBr | Square-like NSs | E. coli | Photocatalytic generated ROS; Effective | 99 |
| lyso@ZnMgAl-LDH | Flower-like morphology; Lyso modified | E. coli, S. aureus | LDH enhanced lysozyme antibacterial; fewest bacterial colonies | 175 | |
| Ag-LDH | Multilayers platelet-like, 300~500 nm NSs | E. coli, B. subtilis | Kill the planktonic bacteria and biofilm inhibition; killing almost 100% of bacteria | 150 | |
| Sliver/h-BN | Double layer NSs | Chlorophenols arthrobacter | Remarkable antibacterial activity | 176 | |
| In2Se3 | Multilayer NSs, about 300 nm | E. coli | PTT antibacterial; the bacterial inactivation percentage is 98% | 177 | |
| RuO2 | Spherical/sheet like structure, PEG modified | V. anguillarum, E. tarda, S. iniae & S. parauberis | Shape dependent direct contact or oxidative stress; Effective | 152 |
Abbreviations: BNN6: N,N'-di-sec-butyl-N,N'-dinitroso-1,4-phenylenediamine; PEG: polyethylene glycol; TEG: tetraethylene glycol; Pseudomonas aeruginosa: P. aeruginosa; PPMS: poly (4-pyridonemethylstyrene); PDDA: polyelectrolyte poly(diallyldimethylammonium chloride); ssDNA: single-stranded DNA; PDMS: polydimethylsiloxane; PDA: polydopamine; LDH: layered double hydroxide.
3.1 Transition-Metal Dichalcogenides/Oxides Antibacterial Nanomaterials
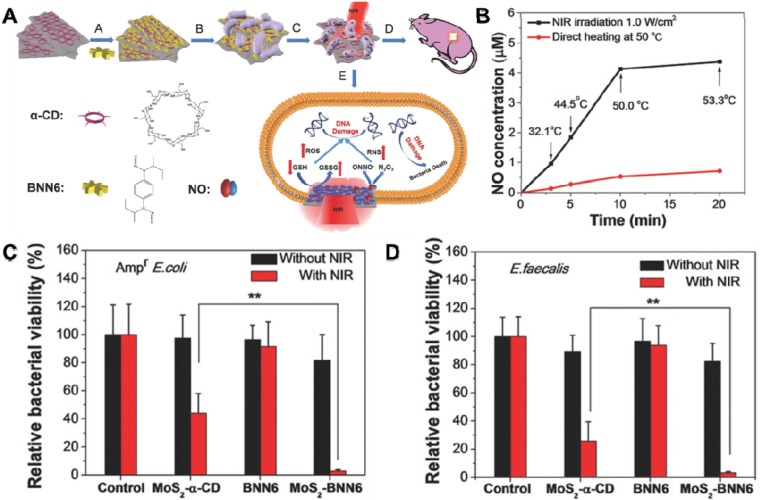
In the last few decades, 2D TMDC/Os with general chemical formula of ABX (A: transition-metal, B: chalcogen), have been studied extensively in nanotechnology field owing to their unique graphene-like properties, which are composed of a “sandwich” structure of “B-A-B” or “B-A-O” through weak Van der Waals forces 178-180. Prototypical TMDC/Os, MoS2, MoO2, MoSe2, WO3-x, and WS2 are the most extensively explored ones 181, 182. For example, MoS2 nanosheets have exhibited great potential in the biomedical field due to their unique properties correlated with their 2D ultrathin atomic layer structure and high surface area 183. Specifically, owing to its layer-dependent bandgap, MoS2 nanosheets with very broad photodetection ability can be used as a candidate for photothermal/photocatalytic antibacterial 183, 184. Taking advantage of this feature, our groups fabricated a biocompatible 808 nm laser-mediated NO-releasing MoS2-BNN6 nanovehicles for low-cost, rapid, and effective antibacterial (Figure 8A) 157. We demonstrated that MoS2 nanosheets with high photothermal conversion efficiency and hyperthermia could control NO delivery and release upon 808 nm laser irradiation (Figure 8B). The synergistic effect of PTT and NO release lead to outstanding germicidal ability toward Ampr E. coli (Figure 8C) and E. faecalis (Figure 8D). Moreover, comet assay results proved that the MoS2-BNN6 nanovehicles presented with PTT/NO synergetic antibacterial activities could cause great DNA damage. The in vivo wound healing in mice demonstrated the practical applicability of this antibacterial strategy. Moreover, Liu et al. fabricated few-layered vertically aligned MoS2 (FLV-MoS2) films with the ability to harvest the whole spectrum of visible light for effective photocatalytic water disinfection 97. Due to the increased bandgap of MoS2, from 1.3 eV (bulk material) to 1.55 eV (FLV-MoS2), FLV-MoS2 could generate considerable ROS for bacteria inactivation in water under visible light irradiation. They also found that the additional deposition of Cu or Au onto FLV-MoS2 films could assist electron-hole pair separation and catalyze ROS production reactions, which allowed FLV-MoS2 to realize a rapid inactivation of >99.999% bacteria within only 20 min. Similarly, Cheng et al. constructed a pyramid-type MoS2 (pyramid MoS2) on transparent glass using the chemical vapor deposition method for highly effective water disinfection under visible light irradiation 158. They also found that the pyramid MoS2 has a smaller bandgap, which could harvest a wide spectrum of sunlight and generate more ROS for killing bacteria.
Figure 8.
(A) Schematic illustration of MoS2-BNN6 as NIR laser-mediated NO release nanovehicle for synergistic eliminating bacteria. (B) Effects of direct heating and 808 nm laser irradiation on NO release from MoS2-BNN6. The corresponding bacterial viabilities of (C) Ampr E. coli, D) E. faecalis treated with PBS, MoS2-α-CD, BNN6, and MoS2-BNN6 without or with 808 nm laser irradiation (1.0 W cm-2, 10 min). Reproduced with permission from 157, copyright 2018 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim.
Aside from the photo-induced germicidal ability, the peroxidase-like activity of MoS2/MoSe2 nanosheets has also been verified. Our group constructed functionalized nano-MoS2 with peroxidase catalytic and NIR PTT for synergetic antibacterial 143. Besides, the imbalance of bacterial flora may increase resistance, which demands the development of strain-selective bactericidal strategies. To this end, Qu's groups designed an intelligent photo-responsive Gram- selective antibacterial system citraconic anhydride-modified MoS2 185. This nanosystem possesses charge-selective antibacterial potential because the surface charge could be modulated by altering light irradiation time, and the simultaneous lower pH value would activate the peroxidase-like activity of MoS2 nanozymes as well as increase antibacterial effects. In addition, Huang et al. used carboxyl-modified silk fibroin as the exfoliating agent to high-yield synthesize thin-layer MoSe2 nanosheets, and revealed that the superior peroxidase-like activity of MoSe2 nanosheets could effectively resist bacterial infections and promote wound healing 159. Furthermore, the antibacterial activity of WX2 nanosheets has also been valued in recent years 186. Bang et al. designed a facile and effective exfoliation technique to fabricate WX2 (X=S or Se) nanosheets by using single-stranded DNA (ssDNA) of high ssDNA molecular weight, and investigated the antibacterial activity of the as-prepared WX2. They found that WSe2-ssDNA nanosheets had remarkable antibacterial activity due to their strong GSH oxidation capacity, which suggested the ROS-independent oxidative stress antibacterial properties of WSe2-ssDNA nanosheets 65, 85.
3.2 Metal-Based 2D Antibacterial Nanomaterials
It has been demonstrated that metallic nanoparticles, including Ag, Au 187, Ti, Cu 188, Zn 189, Mg 190-192 and Ni 192, exhibit potential antibacterial activity. However, compared to 2D nanomaterials, these metallic nanoparticles show compromised bactericidal efficiency due to its small specific surface area and fewer surface-active sites. Therefore, the strategy to overcome the shortcomings and obtain high-performance antibacterial effect of metal alone is either to decorate 2D nanosheets with metallic nanoparticles or prepare 2D metallic nanosheets 193-195. Towards this direction, Mo et al. prepared Pd@Ag nanosheets through the reduction of Ag ions with formaldehyde on the surface of Pd nanosheets. It could realize a synergetic antibacterial effect of PTT and Ag ions released under the irradiation of NIR light (Figure 9A) 162. The amounts of released Ag from Pd@Ag nanosheets were monitored by inductively coupled plasma mass spectrometry (ICP-MS), and results showed that NIR irradiation could significantly increase silver release compared to non-irradiated controls (Figure 9B). Interestingly, the bacteria viability indicated that NIR laser or Pd@Ag nanosheets alone did not kill bacteria effectively, while Pd@Ag nanosheets upon NIR irradiation could realize efficient bacteria-killing effect as almost 100% bacteria was killed after the synergistic treatment for 10 min (Figure 9C, D). Therefore, Pd@Ag nanosheets with outstanding photothermal conversion effect could not only generate heat to kill bacteria, but also destroy the bacterial membrane with released Ag ions under the irradiation of NIR light. Recently, 2D nanosized metallic oxides, such as TiO2 196, CuO 188, and ZnO 197, 198, have shown excellent antibacterial performance and drawn widespread attention due to their favorable photocatalytic activities, benign biosafety, strong oxidizing power, and rich surface-active sites. Among them, ultrathin TiO2 nanosheets are confirmed as the most outstanding antibacterial, which is attributed to their numerous advantages, such as low-cost, low-toxicity, good stability, as well as robust oxidizing capability. In general, TiO2 nanosheets have three phase structure: brookite, rutile and anatase. Among them, anatase TiO2 nanosheets have been widely applied as a photocatalyst 196. To explore the in-depth capacity of TiO2, Arnab et al. prepared MoS2-TiO2 nanocomposites with significant antibacterial ability 165. In addition, Ma et al. fabricated ultrathin Fe3O4-TiO2 nanosheets (Fe3O4-TNS) using lamellar reverse micelles and solvothermal method for efficient antibacterial application 199. On the one hand, Fe3O4-TNS could effectively capture photo-induced electron and accelerate the separation of electron-hole pairs. On the other hand, Fe3O4-TNS showed superior antibacterial activity to E. coli under the irradiation of solar light. Moreover, Wang et al. designed a bifunctional layered Cu2S nanoflowers/biopolymer-incorporated electrospun membrane for tumor therapy and as a source of Cu ions for wound healing 89. Despite the tremendous antibacterial potential of metal-based nanomaterial shown from these studies, it is important to investigate their cytotoxicity and immune response effect on human cells. Therefore, metal-based antibacterial nanomaterials deserve further research regarding their in vivo biological effect before clinical sterilization application.
Figure 9.
(A) Schematic illustration of the Pd@Ag nanosheets for synergetic treatment of bacteria. (B) Silver released from 5.9 and 9.5 ppm of Pd@Ag nanosheets with and without light irradiation. Histograms of (C) E. coli and (D) S. aureus viability treated with Pd@Ag nanosheets under NIR irradiation. Reproduced with permission from 162, copyright 2015 The Royal Society of Chemistry.
3.3 Nitride-Based Antibacterial Nanomaterials
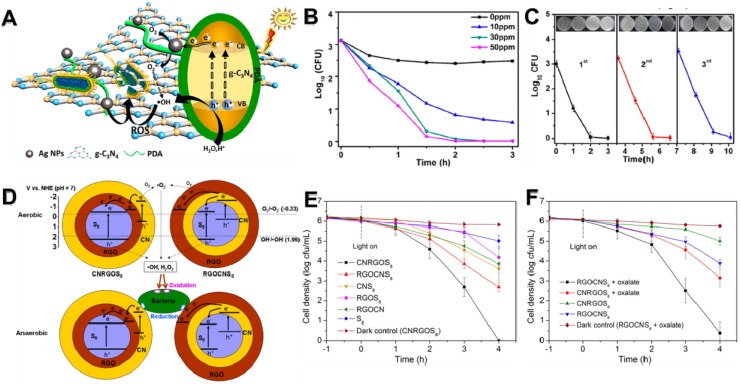
C3N4 was first synthesized by Berzelius in the 1830s, subsequently Liebig named it “melon” 111. Recently, g-C3N4 nanosheets, a burgeoning nitride-based polymeric semiconductor material with laminar structure and narrow bandgap, have attracted giant attention on the antibacterial field owing to their highly efficient photocatalytic performance, large surface area, good chemical stability and adjustable electron band structure 200. Wu et al. once synthesized an Ag/polydopamine/g-C3N4 bio-photocatalyst antibacterial agent using in situ reduction method (Figure 10A) 170. On the one hand, the photocatalytic activity of Ag/polydopamine/g-C3N4 was improved through Ag NPs and polydopamine under the irradiation of visible light, followed by the generation of abundant ROS (particularly •OH). On the other hand, light could accelerate the release of Ag ions. Finally, the Ag/polydopamine/g-C3N4 displayed excellent antibacterial activity by a synergistic effect between Ag ions and PCT of g-C3N4 nanosheets (Figure 10B). Simultaneously, this bio-photocatalyst has commendable biocompatibility and outstanding stable antibacterial activity (Figure 10C). To further advance the use of g-C3N4 as photocatalysts, Wang et al. firstly designed a novel metal-free heterojunction through coating cyclooctasulfur (α-S8) with rGO and g-C3N4 nanosheets for photocatalytic disinfection under visible light 113. They found that the photocatalytic bacteria inactivation mechanism of this nano-heterojunction mainly through photogenerated ROS in an aerobic environment or through electron transfer induced oxidative stress under anaerobic conditions (Figure 10D). Intriguingly, the results showed that a S8 (core)/rGO (inner shell)/g-C3N4 (outer shell) (CNRGOS8) arrangement exhibited higher antibacterial effect under aerobic conditions (Figure 10E), whereas a S8 (core)/g-C3N4 (inner shell)/rGO (outer shell) (RGOCNS8) arrangement showed excellent antibacterial ability under anaerobic conditions (Figure 10F). In addition, Li and co-workers fabricated g-C3N4 nanosheets functionalized hydrophilic composite membranes, and found that the antibacterial ability largely increased with the modification of g-C3N4 nanosheets 166. Furthermore, Wang et al. reported that vanadate quantum dots-inset g-C3N4 (vanadate QDs/g-C3N4) nanosheets showed faster photocatalytic disinfection with abundant ROS compared to bare g-C3N4 nanosheets 171. Moreover, to reduce the toxicity risk of heavy metal species, Chen's groups created a novel all-organic self-assembled semiconductor photocatalytic nanomaterial C3N4/PDINH heterostructure for photocatalytic antibacterial by recrystallization of PDINH (perylene-3,4,9,10-tetracarboxylic diimide) on the surface of C3N4 nanosheets in situ 201. Intriguingly, they found that the absorption spectrum of heterostructure were greatly extended from UV to NIR, enhancing the photocatalytic effect to generate more ROS for better bactericidal, while revealing low-toxicity to healthy tissue cells. Simultaneously, the C3N4/PDINH heterostructure could quickly cure the in vivo infected area, and promote wound regeneration. Taken together, although C3N4-based photocatalyst has reported in various biomedical fields, its photocatalytic efficiency is still relatively low to meet the requirements of practical applications. Therefore, enhancing the catalytic efficiency of C3N4 nanosheets in the future is highly expected.
Figure 10.
(A) Bactericidal mechanism for Ag/PDA/g-C3N4 bio-photocatalyst. (B) Bactericidal time curve profiles of E. coli treated with different concentrations of Ag/PDA/g-C3N4 (1:2) within 3 h. (C) Three-cycle run experiments of photocatalytic disinfection of 30 ppm Ag/PDA/g-C3N4 (1:2) under visible-light irradiation. Reproduced with permission from 170, copyright 2018 American Chemical Society. (D) Schematic illustration of the VLD photocatalytic bacterial inactivation mechanisms of CNRGOS8/RGOCNS8 in aerobic condition, and CNRGOS8/RGOCNS8 in anaerobic condition. Photocatalytic inactivation efficiency against E. coli K-12 without (E) and with (F) 0.5 mmol/L sodium oxalate in the presence of the samples (100 mg/L) in anaerobic condition under visible light irradiation. Reproduced with permission from 113, copyright 2018 American Chemical Society.
3.4 Black Phosphorus-Based Antibacterial Nanomaterials
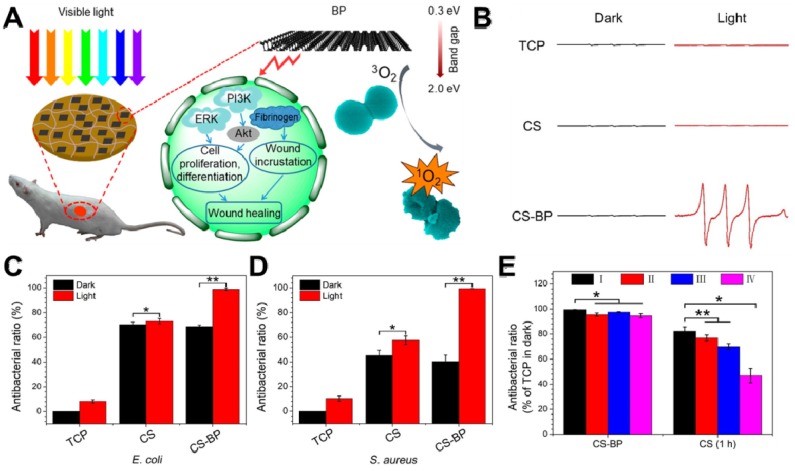
BP, a rising star of 2D nanomaterials family, has attracted great attention since 2014 202, 203. Recently, 2D BP nanosheets have attracted widespread attention due to their fascinating thermal/optical/electrical performance, and the weak Van der Waals forces allow its easy exfoliation into ultrathin 2D nanosheets 204, 205. Owing to their metal-free semiconductor features and thickness-dependent band gap, BP nanosheets possess a very broad light absorption across entire visible regions 206. Therefore, 2D layered BP nanosheets can be used to produce 1O2 under visible light, which can be applied to PDT 207. Towards this direction, Mao et al. fabricated a BP-based hybrid hydrogel (CS-BP) therapeutic system for repeatable PDT disinfection and promote wound healing (Figure 11A) 39. They used electron spin resonance (ESR) spectra to trap 1O2, and found that only CS-BP hydrogel could rapidly generate 1O2 under visible light irradiation (Figure 11B). Meanwhile, under light irradiation with simulated sunlight, CS hydrogel showed moderate bactericidal efficacy while CS-BP could kill most bacteria (E. coli and S. aureus) within 10 min (Figure 11C, D). Moreover, the CS-BP hydrogel still maintain favorable reusable antibacterial efficacy even after four repeatedly challenge with high concentrations of S. aureus (Figure 11E). In addition, Huang et al. prepared thin-layer BP@ silk fibroin nanosheets with subtle solution-processability using silk fibroin as an exfoliating agent, and revealed that BP@ silk fibroin nanosheets not only could be fabricated into various formats but also show excellent PTT disinfection and wound repair ability 92. Based on the above attractive findings, BP-based nanomaterials hold great promise for material science, biomedicine, and biotechnology in the future.
Figure 11.
(A) Sterilization under visible light irradiation and the process of stimulating skin cell behaviors that can promote the regenerative activities of the skin cells and actively participate in skin regeneration to accelerate bacteria-accompanied wound healing using BP-based hydrogel. (B) Electron spin resonance spectra. The corresponding abilities of the samples (TCP, the CS hydrogel, and the CS-BP hydrogel) to kill E. coli (C) and S. aureus (D); (E) Corresponding abilities of the samples to kill S. aureus; the CS-BP hydrogel and CS hydrogel were repeatedly challenged with S. aureus under simulated sunlight for 10 min and in the darkness for 1 h, respectively, repeatedly up to four times. Reproduced with permission from 39, copyright 2018 American Chemical Society.
3.5 MXenes Antibacterial Nanomaterials
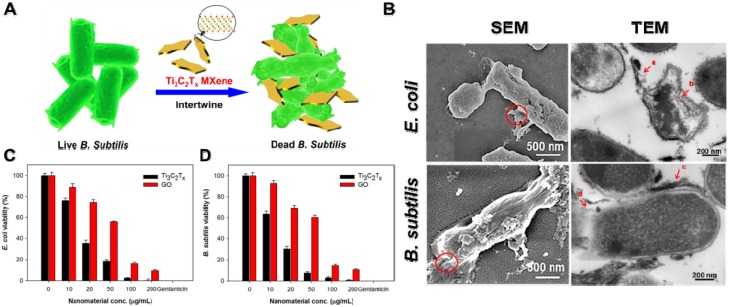
MXenes are a very new member of 2D transition metal carbides/nitrides with a general formula of Mn+1AnTx (M stands for transition metal, A is carbon or nitrogen, and T is surface-terminating functional groups, such as -OH, -O, or -F) 25, 208. So far, approximate 70 kinds of MXenes have been reported, among which Ti3C2Tx is the most representative MXenes material 209. Recently, studies demonstrated that Ti3C2Tx MXenes nanosheets display outstanding antibacterial performances owing to their ultrathin lamellar morphology and unique physiochemical properties 209-211. For instance, Rasool et al. synthesized Ti3C2Tx MXenes colloidal suspension using ultrasonication in argon (Ar) gas and first demonstrated their outstanding antibacterial behavior towards E. coli and B. subtilis (Figure 12A) 173, 212. In comparison to GO, the Ti3C2Tx MXenes nanosheets displayed distinct dose-dependent bactericidal efficacy, and up to 98% of bacterial were killed after incubated with 200 μg/mL Ti3C2Tx for 4 h (Figure 12C, D). According to the SEM and TEM images, they proposed that the antibacterial mechanism of Ti3C2Tx MXenes nanosheets were ascribed to the synergism of sharp edge induced membrane damage and electron transfer rendered oxidative stress (Figure 12B). Then, they further fabricated Ti3C2Tx MXenes nanosheets coated with polyvinylidene fluoride (PVDF) membranes by vacuum-assisted filtration method and further studied their antibacterial ability towards E. coli and B. subtilis 174. Compared to fresh Ti3C2Tx/PVDF membranes, the aged Ti3C2Tx/PVDF membranes were more active in enhancing the overall antibacterial properties owing to the synergistic effect between Ti3C2Tx nanosheets and the formation of anatase TiO2 nanocrystals with sharp edges. Moreover, Shamsabadi et al. reported that colloidal Ti3C2Tx MXenes nanosheets showed both size- and exposure time-dependent antibacterial properties. They found that the direct interactions between sharp edges of Ti3C2Tx MXenes nanosheets and bacteria membrane cause serious damage to the bacterial membrane 212. Taking into consideration that MXenes' chemical behavior depends on the kind of transition metals (such as Mo, Nb, V) and surface-terminating functional groups, different physicochemical properties are closely relevant to antibacterial effects. Therefore, fine regulated ultrathin MXenes nanosheets will open a wide door for multiple and extensive application in antibacterial coatings and biomedical applications.
Figure 12.
(A) Schematic illustration of the interaction between Ti3C2Tx and bacteria. (B) SEM and TEM images of E. coli and B. subtilis cells treated with Ti3C2Tx MXenes. Cell viability measurements of C) E. coli and D) B. subtilis treated with an aqueous suspension of Ti3C2Tx MXenes. Reproduced with permission from 173, copyright 2016 American Chemical Society.
3.6 Others 2D NBG Antibacterial Nanomaterials
In addition to the above-mentioned 2D NBG antibacterial nanomaterials, some other new members of 2D nanomaterials, such as layered double hydroxides (LDHs) 213-216, laponite (Lap) 217, 218, hexagonal boron nitride (BN) 176, III2-VI3 compounds (In2Se3, Bi2Se3, Sb2Se3) 90, 177, 219, 220, and RuO2 have also been deemed as promising antibacterial agents. For example, Wang et al. prepared various morphology of lysozyme modified LDHs through tuning the ratio of cations, and found that bloom flower structure of LDHs not only could load more lysozyme but also adhere to more bacteria, which show excellent antibacterial activity and wound healing ability 175. Moreover, Ghadiri et al. fabricated a Laponite/mafenide/alginate (Lap/Maf/Alg) film with remarkable antibacterial effects and good wound healing applications 221. Furthermore, Zhu et al. synthesized large quantities of In2Se3 nanosheets using a solvent exfoliation method. Their results revealed that the In2Se3 nanosheets possess excellent photothermal performance, which causes significant antibacterial effect 177. Similarly, Miao et al. synthesized new atomically thin antimony Sb2Se3 nanosheets using a liquid exfoliation method, which could kill bacteria through physical contact destruction and short-time hyperthermia sterilization under laser irradiation 90. Therefore, various 2D antibacterial materials with excellent bacteria inhibition efficacy should gain more researchers' attention.
4. Conclusions and Future Perspectives
In recent years, the increasing of bacteria multidrug resistance to traditional antibiotics has greatly hampered the development of antibacterial applications. Fortunately, various emerging 2D NBG, which show promising potential and good opportunities in addressing antibacterial issues, have demonstrated their excellent ability to kill drug-resistant bacteria. In this review, we systematically summarize the research progress of versatile 2D NBG from their antibacterial mechanisms to materials classification, and the effect of unique physiochemical properties on their antibacterial applications. Similar to graphene, 2D NBG also has features such as high specific surface area, better stability, and good biosafety, which make them one of the most advanced, attractive, and promising antibacterial nano-agents. Currently, the existing antibacterial mechanisms of 2D NBG mainly include physical contact destruction, oxidative stress, photo-induced (photothermal, photocatalytic and photodynamic) heat/ROS production to damage cellular components, controlled drugs or metallic ions releasing, and multi-mode synergistic antibacterial. Among them, synergistic antibacterial is the most effective bactericidal method, and photo-induced antibacterial, especially photocatalytic antibacterial, has attracted far-ranging attention in recent years. Furthermore, we also discuss the effect of the physiochemical characteristic of 2D NBG on antibacterial effects. In addition, we have divided the 2D NBG into the following categories: TMDC/Os, Metal-based, Nitride-based, BP, MXenes and other 2D NBG antibacterial nanoagents. Although extensive research demonstrated that 2D NBG has excellent antibacterial activities, studies regarding the clinical translation of the 2D NBG are still rare. Therefore, in order to realize more practical application, it requires us to focus on several important issues in the future:
I) The systematical evaluation of the biosafety of 2D NBG is all-important before being translated into clinical practice. Currently, 2D NBG has been exhibited tremendous potential in antibacterial, water disinfection, wound healing, and other biomedical filed. However, biosafety consideration is a prerequisite for the clinical antibacterial translation of these 2D nanomaterials. Although some reports have been conducted to investigate the dosage, incubation time, and surface modification-related biosafety of 2D NBG, such research is still insufficient and remains controversial for the biosafety evaluation of 2D NBG 222. Most recent studies have demonstrated that 2D NBG presents good biocompatibility and low toxic effects after suitable functionalization, however, their long-term biological effects in vivo still unexplored 223, 224. Firstly, although metal or metallic ions possess apparent antibacterial properties, studies of exploring and reducing their in vivo long-term toxicity are needed. Secondly, to accelerate the metabolism of 2D NBG, we can improve their biodegradability through surface functionalization or design ultrasmall size of 2D NBG to make them more easily cleared out of the body 225. Thirdly, developing 2D NBG with targeting antibacterial ability could not only specifically target bacteria to improve antibacterial efficiency but also reduce their toxicity and side effects to normal tissues. Finally, the mechanism for clearance pathways and other biological effects of 2D nanomaterials are still not clear. Thus, putting all these unresolved biosafety issues in the first place for deep investigation is essential, which is beneficial for their future preclinical or clinical antibacterial application.
II) The deeper exploration of the interactions between 2D NBG-bacteria interfaces and related antibacterial mechanisms. To date, lots of antibacterial mechanisms of 2D NBG have been widely recognized, but most of the mechanism studies are far from comprehensive. Thus, it is essential to deeply understand the antibacterial mechanisms and explore the interactions between nanomaterials and intracellular components of microorganisms.
III) The optimization of the physicochemical properties of 2D NBG nanomaterials for enhancing antibacterial effects. As mentioned above, size, shape, phase structure, number of layers and surface functional modification, all of them affect antibacterial activities. For example, different size of MoS2 nanosheets possesses different drugs load capacity, leading to different antibacterial activities. Moreover, the shape-dependent antibacterial effects of VS2 nanosheets were also revealed. Therefore, the systematic study on unique physicochemical properties relevant to the antibacterial effect should be carried out to gain the satisfying antibacterial efficiency of 2D NBG for better preclinical use.
IV) The exploration of the optimal modality of synergistic antibacterial. In this review, we have summarized various combined modalities for synergistic bactericidal. However, no reliable research has compared these synergistic therapeutic strategies together, causing some studies to randomly combine several treatment strategies to improve antibacterial efficacy. In addition, most of the current in vivo researches mainly focus on superficial wound healing, the infection in the deep site of tissue is still rarely studied. Furthermore, the ways of drug delivery also play an important role in improving the synergistic antibacterial effects. Therefore, to obtain the optimal synergistic modality of synergistic antibacterial, it is needed to compare existing synergistic antibacterial effect under the same experimental conditions or propose new synergy mechanisms of 2D NBG.
V) The promotion of the clinical translation of 2D NBG fungicide. Currently, various 2D NBG antibacterial nanoagents were studied by researchers, but too few clinic antibacterial agents were developed. Therefore, their clinic translation process still remains to be a giant challenge. Therefore, tremendous efforts are needed to facilitate the clinical translation of 2D NBG.
VI) The optimization of antibacterial effects of 2D NBG by computational simulation. Currently, with the explosive development of science and technology, computers can do exactly what humans cannot do through simulations. For example, Fan's group using coarse-grained molecular dynamics simulations studied the physical and mechanical interaction between lipid liposomes and hydrophobic nanosheets 226. They found that the size and the orientation of 2D nanomaterials affect the interaction between cells and nanomaterials, which is a good supplement of the experiment. Based on it, we can further study the unresolved antibacterial mechanisms by the combination of computational simulation and theoretical calculation.
Taken together, we believe that this review regarding 2D NBG antibacterials can not only deepen researchers' interest in the antibacterial field, but also provide a new insight in exploring the unknown bactericidal mechanism. We hope that more research can be carried out to develop new types of safe 2D NBG antibacterials for future clinical transformation.
Acknowledgments
This work was supported by the National Basic Research Program of China (2016YFA2021600 and 2018YFA0208900), the National Natural Science Foundation of China (51822207, 51772292, 31571015, 11621505, and 51772293), Chinese Academy of Sciences Youth Innovation Promotion Association (2013007), and CAS Key Research Program of Frontier Sciences (QYZDJ-SSW-SLH022).
Abbreviations
- 2D NBG
two-dimensional nanomaterials beyond graphene
- ATP
adenosine triphosphate
- Ar
argon
- B. subtilis
Bacillus subtilis
- BP
black phosphorus
- B
boron
- BNN6
N,N'-di-sec-butyl-N,N'-dinitroso-1,4-phenylenediamine
- CFU
colony-forming unit
- CB
conduction band
- Cys
cysteine
- CS
chitosan
- CNRGOS8
S8(core)/rGO(inner shell)/g-C3N4(outer shell)
- DNA
deoxyribonucleic acid
- DCFDA
2',7'-dichlorofluorescin diacetate
- E. coli
Escherichia coli
- e-
electrons
- FLV-MoS2
few-layered vertically aligned MoS2
- Fe3O4-TNS
Fe3O4-TiO2 nanosheets
- GSH
glutathione
- GSSG
glutathione disulfide
- Gram-
Gram-negative
- Gram+
Gram-positive
- GNR@LDH
gold nanorod@layered double hydroxide
- g-C3N4
graphitic carbon nitride
- H2O2
hydrogen peroxide
- h+
holes
- h-BN
hexagonal boron nitride
- ICP-MS
inductively coupled plasma mass spectrometry
- LDHs
layered double hydroxides
- LAP
laponite
- lyso
lysozyme
- MRSA
methicillin-resistant S. aureus
- MXenes
transition metal carbides
- NIR
near-infrared
- NSs
nanosheets
- •OH
hydroxyl radicals
- •O2-
superoxide anions
- 1O2
singlet molecular oxygen
- PTT
photothermal therapy
- PCT
photocatalytic therapy
- PDT
photodynamic therapy
- PDDA
poly diallyldimethylammonium
- PPMS
poly (4-pyridonemethylstyrene)
- P. aeruginosa
Pseudomonas aeruginosa
- PDA
polydopamine
- Pen
penicillin
- PEG
polyethylene glycol
- PPMS
poly (4-pyridonemethylstyrene)
- PDDA
polyelectrolyte poly(diallyldimethylammonium chloride)
- PDMS
polydimethylsiloxane
- PDINH
(perylene-3,4,9,10-tetracarboxylic diimide)
- PVDF
polyvinylidene fluoride
- QDs
quantum dots
- RNA
ribonucleic acid
- ROS
reactive oxygen species
- rGO-WS2
reduced graphene oxide-tungsten disulphide
- RGOCNS8
S8(core)/g-C3N4(inner shell)/rGO(outer shell)
- SEM
scanning electron microscope
- ssDNA
single-stranded DNA
- α-S8
cyclooctasulfur
- TEM
transmission electron microscope
- TEG
tetraethylene glycol
- TMDCs
transition metal dichalcogenides
- TMDOs
transition metal oxides
- TEG
tetraethylene glycol
- UV
ultra violet
- VB
valence band
References
- 1.Medzhitov R. Recognition of microorganisms and activation of the immune response. Nature. 2007;449:819–26. doi: 10.1038/nature06246. [DOI] [PubMed] [Google Scholar]
- 2.Parvez MK, Parveen S. Evolution and Emergence of Pathogenic Viruses: Past, Present, and Future. Intervirology. 2017;60:1–7. doi: 10.1159/000478729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Martens EC, Neumann M, Desai MS. Interactions of commensal and pathogenic microorganisms with the intestinal mucosal barrier. Nat Rev Microbiol. 2018;16:457–70. doi: 10.1038/s41579-018-0036-x. [DOI] [PubMed] [Google Scholar]
- 4.Ling LL, Schneider T, Peoples AJ, Spoering AL, Engels I, Conlon BP. et al. A new antibiotic kills pathogens without detectable resistance. Nature. 2015;517:455–9. doi: 10.1038/nature14098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kohanski MA, Dwyer DJ, Hayete B, Lawrence CA, Collins JJ. A common mechanism of cellular deathinduced by bactericidal antibiotics. Cell. 2007;130:797–810. doi: 10.1016/j.cell.2007.06.049. [DOI] [PubMed] [Google Scholar]
- 6.Ter Kuile BH, Hoeksema M. Antibiotic Killing through Incomplete DNA Repair. Trends Microbiol. 2018;26:2–4. doi: 10.1016/j.tim.2017.11.006. [DOI] [PubMed] [Google Scholar]
- 7.Kohanski MA, Dwyer DJ, Collins JJ. How antibiotics kill bacteria: from targets to networks. Nat Rev Microbiol. 2010;8:423–35. doi: 10.1038/nrmicro2333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Anand A, Unnikrishnan B, Wei S, Chou C, Zhang L, Huang C. Graphene oxide and carbon dots as broad-spectrum antimicrobial agents-a minireview. Nanoscale Horiz. 2019;4:117–37. doi: 10.1039/c8nh00174j. [DOI] [PubMed] [Google Scholar]
- 9.He X, Xiong LH, Zhao Z, Wang Z, Luo L, Lam JWY. et al. AIE-based theranostic systems for detection and killing of pathogens. Theranostics. 2019;9:3223–48. doi: 10.7150/thno.31844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pope CF, Sullivan DM, McHugh TD, Gillespie SH. A practical guide to measuring mutation rates in antibiotic resistance. Antimicrob Agents Chemother. 2008;52:1209–14. doi: 10.1128/AAC.01152-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Andersson DI. Improving predictions of the risk of resistance development against new and old antibiotics. Clin Microbiol Infect. 2015;21:894–8. doi: 10.1016/j.cmi.2015.05.012. [DOI] [PubMed] [Google Scholar]
- 12.Ragheb MN, Thomason MK, Hsu C, Nugent P, Gage J, Samadpour AN. et al. Inhibiting the Evolution of Antibiotic Resistance. Mol Cell. 2019;73:157–65. doi: 10.1016/j.molcel.2018.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Syal K, Mo M, Yu H, Iriya R, Jing W, Guodong S. et al. Current and emerging techniques for antibiotic susceptibility tests. Theranostics. 2017;7:1795–805. doi: 10.7150/thno.19217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen Z, Wang Z, Ren J, Qu X. Enzyme mimicry for combating bacteria and biofilms. Acc Chem Res. 2018;51:789–99. doi: 10.1021/acs.accounts.8b00011. [DOI] [PubMed] [Google Scholar]
- 15.Qiao Z, Yao Y, Song S, Yin M, Luo J. Silver nanoparticles with pH induced surface charge switchable properties for antibacterial and antibiofilm applications. J Mater Chem B. 2019;7:830–40. doi: 10.1039/c8tb02917b. [DOI] [PubMed] [Google Scholar]
- 16.Dai X, Zhao Y, Yu Y, Chen X, Wei X, Zhang X. et al. All-in-one NIR-activated nanoplatforms for enhanced bacterial biofilm eradication. Nanoscale. 2018;10:18520–30. doi: 10.1039/c8nr04748k. [DOI] [PubMed] [Google Scholar]
- 17.Mendoza RA, Hsieh J-C, Galiano RD. The Impact of Biofilm Formation on Wound Healing. Wound Healing-Current Perspectives. IntechOpen; 2019. pp. 235–250. [Google Scholar]
- 18.Albada B, Metzler-Nolte N. Highly potent antibacterial organometallic peptide conjugates. Acc Chem Res. 2017;50:2510–8. doi: 10.1021/acs.accounts.7b00282. [DOI] [PubMed] [Google Scholar]
- 19.Wang C, Wang M, Xu T, Zhang X, Lin C, Gao W. et al. Engineering Bioactive Self-Healing Antibacterial Exosomes Hydrogel for Promoting Chronic Diabetic Wound Healing and Complete Skin Regeneration. Theranostics. 2019;9:65–76. doi: 10.7150/thno.29766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dizaj SM, Lotfipour F, Barzegar-Jalali M, Zarrintan MH, Adibkia K. Antimicrobial activity of the metals and metal oxide nanoparticles. Mater Sci Eng C. 2014;44:278–84. doi: 10.1016/j.msec.2014.08.031. [DOI] [PubMed] [Google Scholar]
- 21.Cattoir V, Felden B. Future Antibacterial Strategies: From Basic Concepts to Clinical Challenges. J Infect Dis. 2019;220:350–60. doi: 10.1093/infdis/jiz134. [DOI] [PubMed] [Google Scholar]
- 22.Miller KP, Wang L, Benicewicz BC, Decho AW. Inorganic nanoparticles engineered to attack bacteria. Chem Soc Rev. 2015;44:7787–807. doi: 10.1039/c5cs00041f. [DOI] [PubMed] [Google Scholar]
- 23.Chen H, Gu Z, An H, Chen C, Chen J, Cui R. et al. Precise nanomedicine for intelligent therapy of cancer. Sci China: Chem. 2018;61:1503–52. [Google Scholar]
- 24.Jiang X, Wang C, Fitch S, Yang F. Targeting Tumor Hypoxia Using Nanoparticle-engineered CXCR4-overexpressing Adipose-derived Stem Cells. Theranostics. 2018;8:1350–60. doi: 10.7150/thno.22736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu Z, Lin H, Zhao M, Dai C, Zhang S, Peng W. et al. 2D Superparamagnetic Tantalum Carbide Composite MXenes for Efficient Breast-Cancer Theranostics. Theranostics. 2018;8:1648–64. doi: 10.7150/thno.23369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li Y, Liu X, Tan L, Cui Z, Jing D, Yang X. et al. Eradicating Multidrug-Resistant Bacteria Rapidly Using a Multi-Functional g-C3N4@Bi2S3 Nanorod Heterojunction with or without Antibiotics. Adv Funct Mater. 2019;29:1900946. [Google Scholar]
- 27.Wang Y, Jin Y, Chen W, Wang J, Chen H, Sun L. et al. Construction of nanomaterials with targeting phototherapy properties to inhibit resistant bacteria and biofilm infections. Chem Eng J. 2019;358:74–90. [Google Scholar]
- 28.Chitgupi U, Qin Y, Lovell JF. Targeted Nanomaterials for Phototherapy. Nanotheranostics. 2017;1:38–58. doi: 10.7150/ntno.17694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang LS, Gupta A, Rotello VM. Nanomaterials for the treatment of bacterial biofilms. ACS Infect Dis. 2016;2:3–4. doi: 10.1021/acsinfecdis.5b00116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Beyth N, Houri-Haddad Y, Domb A, Khan W, Hazan R. Alternative Antimicrobial Approach: Nano-Antimicrobial Materials. Evid-Based Complement & Alternat Med. 2015;2015:1–16. doi: 10.1155/2015/246012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hemeg HA. Nanomaterials for alternative antibacterial therapy. Int J Nanomedicine. 2017;12:8211–25. doi: 10.2147/IJN.S132163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gu Z, Zhu S, Yan L, Zhao F, Zhao Y. Graphene-based smart platforms for combined cancer therapy. Adv Mater. 2019;31:1800662. doi: 10.1002/adma.201800662. [DOI] [PubMed] [Google Scholar]
- 33.Li X, Robinson SM, Gupta A, Saha K, Jiang Z, Moyano DF. et al. Functional gold nanoparticles as potent antimicrobial agents against multi-drug-resistant bacteria. ACS Nano. 2014;8:10682–6. doi: 10.1021/nn5042625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sun Z, Li Y, Guan X, Chen L, Jing X, Xie Z. Rational design and synthesis of covalent organic polymers with hollow structure and excellent antibacterial efficacy. RSC Adv. 2014;4:40269–72. [Google Scholar]
- 35.Mangadlao JD, Santos CM, Felipe MJL, de Leon ACC, Rodrigues DF, Advincula RC. On the antibacterial mechanism of graphene oxide (GO) Langmuir-Blodgett films. Chem Commun. 2015;51:2886–9. doi: 10.1039/c4cc07836e. [DOI] [PubMed] [Google Scholar]
- 36.Liu Y, Zhao Y, Sun B, Chen C. Understanding the toxicity of carbon nanotubes. Acc Chem Res. 2013;46:702–13. doi: 10.1021/ar300028m. [DOI] [PubMed] [Google Scholar]
- 37.Xu J, Yao K, Xu Z. Nanomaterials with a photothermal effect for antibacterial activities: an overview. Nanoscale. 2019;11:8680–91. doi: 10.1039/c9nr01833f. [DOI] [PubMed] [Google Scholar]
- 38.Wang W, Li G, Xia D, An T, Zhao H, Wong PK. Photocatalytic nanomaterials for solar-driven bacterial inactivation: recent progress and challenges. Environ Sci Nano. 2017;4:782–99. [Google Scholar]
- 39.Mao C, Xiang Y, Liu X, Cui Z, Yang X, Li Z. et al. Repeatable photodynamic therapy with triggered signaling pathways of fibroblast cell proliferation and differentiation to promote bacteria-accompanied wound healing. ACS Nano. 2018;12:1747–59. doi: 10.1021/acsnano.7b08500. [DOI] [PubMed] [Google Scholar]
- 40.Yang X, Yang J, Wang L, Ran B, Jia Y, Zhang L. et al. Pharmaceutical intermediate-modified gold nanoparticles: against multidrug-resistant bacteria and wound-healing application via an electrospun scaffold. ACS Nano. 2017;11:5737–45. doi: 10.1021/acsnano.7b01240. [DOI] [PubMed] [Google Scholar]
- 41.Zhang W, Mou Z, Wang Y, Chen Y, Yang E, Guo F. et al. Molybdenum disulfide nanosheets loaded with chitosan and silver nanoparticles effective antifungal activities: in vitro and in vivo. Mater Sci Eng, C. 2019;97:486–97. doi: 10.1016/j.msec.2018.12.052. [DOI] [PubMed] [Google Scholar]
- 42.Xin Q, Shah H, Nawaz A, Xie W, Akram MZ, Batool A. et al. Antibacterial carbon-based nanomaterials. Adv Mater. 2018;0:1804838. doi: 10.1002/adma.201804838. [DOI] [PubMed] [Google Scholar]
- 43.Yin F, Hu K, Chen Y, Yu M, Wang D, Wang Q. et al. SiRNA Delivery with PEGylated Graphene Oxide Nanosheets for Combined Photothermal and Genetherapy for Pancreatic Cancer. Theranostics. 2017;7:1133–48. doi: 10.7150/thno.17841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Han H, Qiu Y, Shi Y, Wen W, He X, Dong L. et al. Glypican-3-targeted precision diagnosis of hepatocellular carcinoma on clinical sections with a supramolecular 2D imaging probe. Theranostics. 2018;8:3268–74. doi: 10.7150/thno.24711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lokerse WJ, Bolkestein M, Ten Hagen TL, de Jong M, Eggermont AM, Grull H. et al. Investigation of Particle Accumulation, Chemosensitivity and Thermosensitivity for Effective Solid Tumor Therapy Using Thermosensitive Liposomes and Hyperthermia. Theranostics. 2016;6:1717–31. doi: 10.7150/thno.14960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jacobson O, Weiss ID, Szajek LP, Niu G, Ma Y, Kiesewetter DO. et al. PET imaging of CXCR4 using copper-64 labeled peptide antagonist. Theranostics. 2011;1:251–62. doi: 10.7150/thno/v01p0251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ji H, Sun H, Qu X. Antibacterial applications of graphene-based nanomaterials-Recent achievements and challenges. Adv Drug Delivery Rev. 2016;105:176–89. doi: 10.1016/j.addr.2016.04.009. [DOI] [PubMed] [Google Scholar]
- 48.Jia Q, Yang F, Huang W, Zhang Y, Bao B, Li K. et al. Low Levels of Sox2 are required for Melanoma Tumor-Repopulating Cell Dormancy. Theranostics. 2019;9:424–35. doi: 10.7150/thno.29698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.You R, Cao YS, Huang PY, Chen L, Yang Q, Liu YP. et al. The Changing Therapeutic Role of Chemo-radiotherapy for Loco-regionally Advanced Nasopharyngeal Carcinoma from Two/Three-Dimensional Radiotherapy to Intensity-Modulated Radiotherapy: A Network Meta-Analysis. Theranostics. 2017;7:4825–35. doi: 10.7150/thno.21815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Karahan HE, Wiraja C, Xu C, Wei J, Wang Y, Wang L. et al. Graphene materials in antimicrobial nanomedicine: current status and future perspectives. Adv Healthc Mater. 2018;7:1701406. doi: 10.1002/adhm.201701406. [DOI] [PubMed] [Google Scholar]
- 51.Bhirde AA, Liu G, Jin A, Iglesias-Bartolome R, Sousa AA, Leapman RD. et al. Combining portable Raman probes with nanotubes for theranostic applications. Theranostics. 2011;1:310–21. doi: 10.7150/thno/v01p0310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kurapati R, Kostarelos K, Prato M, Bianco A. Biomedical uses for 2D materials beyond graphene: current advances and challenges ahead. Adv Mater. 2016;28:6052–74. doi: 10.1002/adma.201506306. [DOI] [PubMed] [Google Scholar]
- 53.Epah J, Palfi K, Dienst FL, Malacarne PF, Bremer R, Salamon M. et al. 3D Imaging and Quantitative Analysis of Vascular Networks: A Comparison of Ultramicroscopy and Micro-Computed Tomography. Theranostics. 2018;8:2117–33. doi: 10.7150/thno.22610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sun W, Wu FG. Two-dimensional materials for antimicrobial applications: graphene materials and beyond. Chem Asian J. 2018;13:3378–410. doi: 10.1002/asia.201800851. [DOI] [PubMed] [Google Scholar]
- 55.Han W, Wu Z, Li Y, Wang Y. Graphene family nanomaterials (GFNs)-promising materials for antimicrobial coating and film: A review. Chem Eng J. 2019;358:1022–37. [Google Scholar]
- 56.Miao H, Teng Z, Wang C, Chong H, Wang G. Recent progress in two-dimensional antimicrobial nanomaterials. Chem Asian J. 2019;25:929–44. doi: 10.1002/chem.201801983. [DOI] [PubMed] [Google Scholar]
- 57.Zou X, Zhang L, Wang Z, Luo Y. Mechanisms of the antimicrobial activities of graphene materials. J Am Chem Soc. 2016;138:2064–77. doi: 10.1021/jacs.5b11411. [DOI] [PubMed] [Google Scholar]
- 58.Alimohammadi F, Gh MS, Attanayake NH, Thenuwara AC, Gogotsi Y, Anasori B. et al. Antimicrobial properties of 2D MnO2 and MoS2 nanomaterials vertically aligned on graphene materials and Ti3C2 MXene. Langmuir. 2018;34:7192–200. doi: 10.1021/acs.langmuir.8b00262. [DOI] [PubMed] [Google Scholar]
- 59.Schwechheimer C, Kuehn MJ. Outer-membrane vesicles from Gram-negative bacteria: biogenesis and functions. Nat Rev Microbiol. 2015;13:605–19. doi: 10.1038/nrmicro3525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Brown L, Wolf JM, Prados-Rosales R, Casadevall A. Through the wall: extracellular vesicles in Gram-positive bacteria, mycobacteria and fungi. Nat Rev Microbiol. 2015;13:620–30. doi: 10.1038/nrmicro3480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lu X, Feng X, Werber JR, Chu C, Zucker I, Kim J-H. et al. Enhanced antibacterial activity through the controlled alignment of graphene oxide nanosheets. Proc Natl Acad Sci U S A. 2017;114:E9793–E801. doi: 10.1073/pnas.1710996114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hu W, Peng C, Luo W, Lv M, Li X, Li D. et al. Graphene-based antibacterial paper. ACS Nano. 2010;4:4317–23. doi: 10.1021/nn101097v. [DOI] [PubMed] [Google Scholar]
- 63.Krishnamoorthy K, Veerapandian M, Yun K, Kim SJ. New function of molybdenum trioxide nanoplates: Toxicity towards pathogenic bacteria through membrane stress. Colloids Surf, B. 2013;112:521–4. doi: 10.1016/j.colsurfb.2013.08.026. [DOI] [PubMed] [Google Scholar]
- 64.Desai N, Mali S, Kondalkar V, Mane R, Hong C, Bhosale P. Chemically grown MoO3 nanorods for antibacterial activity study. J Nanomed Nanotechnol. 2015;6:1000338. [Google Scholar]
- 65.Liu X, Duan G, Li W, Zhou Z, Zhou R. Membrane destruction-mediated antibacterial activity of tungsten disulfide (WS2) RSC Adv. 2017;7:37873–81. [Google Scholar]
- 66.Ouyang J, Wen M, Chen W, Tan Y, Liu Z, Xu Q. et al. Multifunctional two dimensional Bi2Se3 nanodiscs for combined antibacterial and anti-inflammatory therapy for bacterial infections. Chem Commun. 2019;55:4877–80. doi: 10.1039/c9cc01129c. [DOI] [PubMed] [Google Scholar]
- 67.Dallavalle M, Calvaresi M, Bottoni A, Melle Franco M, Zerbetto F. Graphene can wreak havoc with cell membranes. ACS Appl Mater Interfaces. 2015;7:4406–14. doi: 10.1021/am508938u. [DOI] [PubMed] [Google Scholar]
- 68.Tu Y, Lv M, Xiu P, Huynh T, Zhang M, Castelli M. et al. Destructive extraction of phospholipids from Escherichia coli membranes by graphene nanosheets. Nat Nanotechnol. 2013;8:594–601. doi: 10.1038/nnano.2013.125. [DOI] [PubMed] [Google Scholar]
- 69.Li Y, Yuan H, von dem Bussche A, Creighton M, Hurt RH, Kane AB. et al. Graphene microsheets enter cells through spontaneous membrane penetration at edge asperities and corner sites. Proceedings of the National Academy of Sciences. 2013;110:12295. doi: 10.1073/pnas.1222276110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Chen P, Yan L-T. Physical principles of graphene cellular interactions: computational and theoretical accounts. J Mater Chem B. 2017;5:4290–306. doi: 10.1039/c6tb03310e. [DOI] [PubMed] [Google Scholar]
- 71.Zheng H, Ma R, Gao M, Tian X, Li Y, Zeng L. et al. Antibacterial applications of graphene oxides: structure-activity relationships, molecular initiating events and biosafety. Sci Bull. 2018;63:133–42. doi: 10.1016/j.scib.2017.12.012. [DOI] [PubMed] [Google Scholar]
- 72.Li YF, Ouyang SH, Tu LF, Wang X, Yuan WL, Wang GE. et al. Caffeine Protects Skin from Oxidative Stress-Induced Senescence through the Activation of Autophagy. Theranostics. 2018;8:5713–30. doi: 10.7150/thno.28778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ji DK, Zhang Y, Zang Y, Li J, Chen GR, He XP. et al. Targeted intracellular production of reactive oxygen species by a 2D molybdenum disulfide glycosheet. Adv Mater. 2016;28:9356–63. doi: 10.1002/adma.201602748. [DOI] [PubMed] [Google Scholar]
- 74.Karunakaran S, Pandit S, Basu B, De M. Simultaneous exfoliation and functionalization of 2H-MoS2 by thiolated surfactants: applications in enhanced antibacterial activity. J Am Chem Soc. 2018;140:12634–44. doi: 10.1021/jacs.8b08994. [DOI] [PubMed] [Google Scholar]
- 75.Fan J, Li Y, Nguyen HN, Yao Y, Rodrigues DF. Toxicity of exfoliated-MoS2 and annealed exfoliated-MoS2 towards planktonic cells, biofilms, and mammalian cells in the presence of electron donor. Environ Sci Nano. 2015;2:370–9. [Google Scholar]
- 76.Kim TI, Kim J, Park IJ, Cho KO, Choi SY. Chemically exfoliated 1T-phase transition metal dichalcogenide nanosheets for transparent antibacterial applications. 2D Mater; 2019. p. 6. 025025. [Google Scholar]
- 77.Xiong Z, Zhang X, Zhang S, Lei L, Ma W, Li D. et al. Bacterial toxicity of exfoliated black phosphorus nanosheets. Ecotoxicol Environ Saf. 2018;161:507–14. doi: 10.1016/j.ecoenv.2018.06.008. [DOI] [PubMed] [Google Scholar]
- 78.Bang GS, Cho S, Son N, Shim GW, Cho BK, Choi SY. DNA-assisted exfoliation of tungsten dichalcogenides and their antibacterial effect. ACS Appl Mater Interfaces. 2016;8:1943–50. doi: 10.1021/acsami.5b10136. [DOI] [PubMed] [Google Scholar]
- 79.Yuan Z, Tao B, He Y, Liu J, Lin C, Shen X. et al. Biocompatible MoS2/PDA-RGD coating on titanium implant with antibacterial property via intrinsic ROS-independent oxidative stress and NIR irradiation. Biomaterials. 2019;217:119290. doi: 10.1016/j.biomaterials.2019.119290. [DOI] [PubMed] [Google Scholar]
- 80.Perreault F, de Faria AF, Nejati S, Elimelech M. Antimicrobial properties of graphene oxide nanosheets: why size matters. ACS Nano. 2015;9:7226–36. doi: 10.1021/acsnano.5b02067. [DOI] [PubMed] [Google Scholar]
- 81.Lyon DY, Brunet L, Hinkal GW, Wiesner MR, Alvarez PJJ. Antibacterial activity of fullerene water suspensions (nC60) is not due to ROS-mediated damage. Nano Lett. 2008;8:1539–43. doi: 10.1021/nl0726398. [DOI] [PubMed] [Google Scholar]
- 82.Yang X, Li J, Liang T, Ma C, Zhang Y, Chen H. et al. Antibacterial activity of two-dimensional MoS2 sheets. Nanoscale. 2014;6:10126–33. doi: 10.1039/c4nr01965b. [DOI] [PubMed] [Google Scholar]
- 83.Pandit S, Karunakaran S, Boda SK, Basu B, De M. High antibacterial activity of functionalized chemically exfoliated MoS2. ACS Appl Mater Interfaces. 2016;8:31567–73. doi: 10.1021/acsami.6b10916. [DOI] [PubMed] [Google Scholar]
- 84.Kim TI, Kwon B, Yoon J, Park IJ, Bang GS, Park Y. et al. Antibacterial activities of graphene oxide-molybdenum disulfide nanocomposite films. ACS Appl Mater Interfaces. 2017;9:7908–17. doi: 10.1021/acsami.6b12464. [DOI] [PubMed] [Google Scholar]
- 85.Navale GR, Rout CS, Gohil KN, Dharne MS, Late DJ, Shinde SS. Oxidative and membrane stress-mediated antibacterial activity of WS2 and rGO-WS2 nanosheets. RSC Adv. 2015;5:74726–33. [Google Scholar]
- 86.Tavares A, Carvalho CMB, Faustino MA, Neves MGPMS, Tomé JPC, Tomé AC. et al. Antimicrobial photodynamic therapy: study of bacterial recovery viability and potential development of resistance after treatment. Marine Drugs. 2010;8:91–105. doi: 10.3390/md8010091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Wegener M, Hansen MJ, Driessen AJM, Szymanski W, Feringa BL. Photocontrol of antibacterial activity: shifting from UV to red light activation. J Am Chem Soc. 2017;139:17979–86. doi: 10.1021/jacs.7b09281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Chou SS, Kaehr B, Kim J, Foley BM, De M, Hopkins PE. et al. Chemically exfoliated MoS2 as near-infrared photothermal agents. Angew Chem Int Ed. 2013;52:4160–4. doi: 10.1002/anie.201209229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Wang X, Lv F, Li T, Han Y, Yi Z, Liu M. et al. Electrospun micropatterned nanocomposites incorporated with Cu2S nanoflowers for skin tumor therapy and wound healing. ACS Nano. 2017;11:11337–49. doi: 10.1021/acsnano.7b05858. [DOI] [PubMed] [Google Scholar]
- 90.Miao Z, Fan L, Xie X, Ma Y, Xue J, He T. et al. Liquid exfoliation of atomically thin antimony selenide as an efficient two-dimensional antibacterial nanoagent. ACS Appl Mater Interfaces. 2019;11:26664–73. doi: 10.1021/acsami.9b08320. [DOI] [PubMed] [Google Scholar]
- 91.Ma K, Li Y, Wang Z, Chen Y, Zhang X, Chen C. et al. A core-shell gold nanorod@layered double hydroxide nanomaterial with high efficient photothermal conversion and its application in antibacterial and tumor therapy. ACS Appl Mater Interfaces. 2019;11:29630–40. doi: 10.1021/acsami.9b10373. [DOI] [PubMed] [Google Scholar]
- 92.Huang X, Wei J, Zhang M, Zhang X, Yin X, Lu C. et al. Water-based black phosphorus hybrid nanosheets as a moldable platform for wound healing applications. ACS Appl Mater Interfaces. 2018;10:35495–502. doi: 10.1021/acsami.8b12523. [DOI] [PubMed] [Google Scholar]
- 93.Yin W, Yan L, Yu J, Tian G, Zhou L, Zheng X. et al. High-throughput synthesis of single-layer MoS2 nanosheets as a near-infrared photothermal-triggered drug delivery for effective cancer therapy. ACS Nano. 2014;8:6922–33. doi: 10.1021/nn501647j. [DOI] [PubMed] [Google Scholar]
- 94.Yu J, Yin W, Zheng X, Tian G, Zhang X, Bao T. et al. Smart MoS2/Fe3O4 nanotheranostic for magnetically targeted photothermal therapy guided by magnetic resonance/photoacoustic imaging. Theranostics. 2015;5:931–45. doi: 10.7150/thno.11802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Zhang W, Shi S, Wang Y, Yu S, Zhu W, Zhang X. et al. Versatile molybdenum disulfide based antibacterial composites for in vitro enhanced sterilization and in vivo focal infection therapy. Nanoscale. 2016;8:11642–8. doi: 10.1039/c6nr01243d. [DOI] [PubMed] [Google Scholar]
- 96.Liu Y, Zeng X, Hu X, Hua J, Zhang X. Two-dimensional nanomaterials for photocatalytic water disinfection: recent progress and future challenges. J Chem Technol Biotechnol. 2019;94:22–37. [Google Scholar]
- 97.Liu C, Kong D, Hsu PC, Yuan H, Lee HW, Liu Y. et al. Rapid water disinfection using vertically aligned MoS2 nanofilms and visible light. Nat Nanotechnol. 2016;11:1098–105. doi: 10.1038/nnano.2016.138. [DOI] [PubMed] [Google Scholar]
- 98.Wu D, Wang B, Wang W, An T, Li G, Ng TW. et al. Visible-light-driven BiOBr nanosheets for highly facet-dependent photocatalytic inactivation of Escherichia coli. J Mater Chem A. 2015;3:15148–55. [Google Scholar]
- 99.Wu D, Yue S, Wang W, An T, Li G, Yip HY. et al. Boron doped BiOBr nanosheets with enhanced photocatalytic inactivation of Escherichia coli. Appl Catal B. 2016;192:35–45. [Google Scholar]
- 100.Wu D, Yue S, Wang W, An T, Li G, Ye L. et al. Influence of photoinduced Bi-related self-doping on the photocatalytic activity of BiOBr nanosheets. Appl Surf Sci. 2017;391:516–24. [Google Scholar]
- 101.Wu D, Ye L, Yue S, Wang B, Wang W, Yip HY. et al. Alkali-induced in situ fabrication of Bi2O4-decorated BiOBr nanosheets with excellent photocatalytic performance. J Phys Chem C. 2016;120:7715–27. [Google Scholar]
- 102.Murugesan P, Moses JA, Anandharamakrishnan C. Photocatalytic disinfection efficiency of 2D structure graphitic carbon nitride-based nanocomposites: a review. J Mater Sci. 2019;54:12206–35. [Google Scholar]
- 103.Cao S, Low J, Yu J, Jaroniec M. Polymeric Photocatalysts Based on Graphitic Carbon Nitride. Adv Mater. 2015;27:2150–76. doi: 10.1002/adma.201500033. [DOI] [PubMed] [Google Scholar]
- 104.Adhikari SP, Awasthi GP, Lee J, Park CH, Kim CS. Synthesis, characterization, organic compound degradation activity and antimicrobial performance of g-C3N4 sheets customized with metal nanoparticles-decorated TiO2 nanofibers. RSC Adv. 2016;6:55079–91. [Google Scholar]
- 105.Huang J, Ho W, Wang X. Metal-free disinfection effects induced by graphitic carbon nitride polymers under visible light illumination. Chem Commun. 2014;50:4338–40. doi: 10.1039/c3cc48374f. [DOI] [PubMed] [Google Scholar]
- 106.Zhao H, Yu H, Quan X, Chen S, Zhang Y, Zhao H. et al. Fabrication of atomic single layer graphitic-C3N4 and its high performance of photocatalytic disinfection under visible light irradiation. Appl Catal B. 2014;152-153:46–50. [Google Scholar]
- 107.Liu B, Han X, Wang Y, Fan X, Wang Z, Zhang J. et al. Synthesis of g-C3N4/BiOI/BiOBr heterostructures for efficient visiblelight-induced photocatalytic and antibacterial activity. J Mater Sci: Mater Electron. 2018;29:14300–10. [Google Scholar]
- 108.Jia Y, Zhan S, Ma S, Zhou Q. Fabrication of TiO2-Bi2WO6 binanosheet for enhanced solar photocatalytic disinfection of E. coli: insights on the mechanism. ACS Appl Mater Interfaces. 2016;8:6841–51. doi: 10.1021/acsami.6b00004. [DOI] [PubMed] [Google Scholar]
- 109.Tian X, Sun Y, Fan S, Boudreau MD, Chen C, Ge C. et al. Photogenerated Charge Carriers in Molybdenum Disulfide Quantum Dots with Enhanced Antibacterial Activity. ACS Appl Mater Interfaces. 2019;11:4858–66. doi: 10.1021/acsami.8b19958. [DOI] [PubMed] [Google Scholar]
- 110.Adhikari SP, Pant HR, Kim JH, Kim HJ, Park CH, Kim CS. One pot synthesis and characterization of Ag-ZnO/g-C3N4 photocatalyst with improved photoactivity and antibacterial properties. Colloids Surf, A Physicochem Eng Asp. 2015;482:477–84. [Google Scholar]
- 111.Zhao H, Chen S, Quan X, Yu H, Zhao H. Integration of microfiltration and visible-light-driven photocatalysis on g-C3N4 nanosheet/reduced graphene oxide membrane for enhanced water treatment. Appl Catal B. 2016;194:134–40. [Google Scholar]
- 112.Wang Y, Long Y, Zhang D. Novel bifunctional V2O5/BiVO4 nanocomposite materials with enhanced antibacterial activity. J Taiwan Inst Chem Eng. 2016;68:387–95. [Google Scholar]
- 113.Wang W, Yu JC, Xia D, Wong PK, Li Y. Graphene and g-C3N4 nanosheets cowrapped elemental α-Sulfur as a novel metal-free heterojunction photocatalyst for bacterial inactivation under visible-light. Environ Sci Technol. 2013;47:8724–32. doi: 10.1021/es4013504. [DOI] [PubMed] [Google Scholar]
- 114.Xu J, Li Y, Zhou X, Li Y, Gao Z, Song Y. et al. Graphitic C3N4-sensitized TiO2 nanotube layers: a visible-light activated efficient metal-free antimicrobial platform. Chemistry. 2016;22:3947–51. doi: 10.1002/chem.201505173. [DOI] [PubMed] [Google Scholar]
- 115.Li J, Yin Y, Liu E, Ma Y, Wan J, Fana J. et al. In situ growing Bi2MoO6 on g-C3N4 nanosheets with enhanced photocatalytic hydrogen evolution and disinfection of bacteria under visible light irradiation. J Hazard Mater. 2017;321:183–92. doi: 10.1016/j.jhazmat.2016.09.008. [DOI] [PubMed] [Google Scholar]
- 116.De Melo WC, Avci P, de Oliveira MN, Gupta A, Vecchio D, Sadasivam M. et al. Photodynamic inactivation of biofilm: taking a lightly colored approach to stubborn infection. Expert Rev Anti Infect Ther. 2013;11:669–93. doi: 10.1586/14787210.2013.811861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Abrahamse H, Hamblin MR. New photosensitizers for photodynamic therapy. Biochem J. 2016;473:347–64. doi: 10.1042/BJ20150942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Chilakamarthi U, Giribabu L. Photodynamic therapy: past, present and future. Chem Rec. 2017;17:775–802. doi: 10.1002/tcr.201600121. [DOI] [PubMed] [Google Scholar]
- 119.Fan W, Huang P, Chen X. Overcoming the Achilles' heel of photodynamic therapy. Chem Soc Rev. 2016;45:6488–519. doi: 10.1039/c6cs00616g. [DOI] [PubMed] [Google Scholar]
- 120.Biel MA. Photodynamic therapy of bacterial and fungal biofilm infections. Totowa, NJ: Humana Press; 2010. pp. 175–194. [DOI] [PubMed] [Google Scholar]
- 121.Jia Q, Song Q, Li P, Huang W. Rejuvenated Photodynamic Therapy for Bacterial Infections. Adv Healthc Mater. 2019;8:e1900608. doi: 10.1002/adhm.201900608. [DOI] [PubMed] [Google Scholar]
- 122.Tan L, Li J, Liu X, Cui Z, Yang X, Yeung KWK. et al. In situ disinfection through photoinspired radical oxygen species storage and thermal-triggered release from black phosphorous with strengthened chemical stability. Small. 2018;14:1703197. doi: 10.1002/smll.201703197. [DOI] [PubMed] [Google Scholar]
- 123.Richter AP, Brown JS, Bharti B, Wang A, Gangwal S, Houck K. et al. An environmentally benign antimicrobial nanoparticle based on a silver-infused lignin core. Nat Nanotechnol. 2015;10:817–23. doi: 10.1038/nnano.2015.141. [DOI] [PubMed] [Google Scholar]
- 124.Zhang Q, Sun C, Zhao Y, Zhou S, Hu X, Chen P. Low Ag-doped titanium dioxide nanosheet films with outstanding antimicrobial property. Environ Sci Technol. 2010;44:8270–5. doi: 10.1021/es1019383. [DOI] [PubMed] [Google Scholar]
- 125.Ali SR, Pandit S, De M. 2D-MoS2-based β-Lactamase inhibitor for combination therapy against drug-resistant bacteria. ACS Appl Bio Mater. 2018;1:967–74. doi: 10.1021/acsabm.8b00105. [DOI] [PubMed] [Google Scholar]
- 126.Ryu SJ, Jung H, Oh JM, Lee JK, Choy JH. Layered double hydroxide as novel antibacterial drug delivery system. J Phys Chem Solids. 2010;71:685–8. [Google Scholar]
- 127.Zhang X, Zhang W, Liu L, Yang M, Huang L, Chen K. et al. Antibiotic-loaded MoS2 nanosheets to combat bacterial resistance via biofilm inhibition. Nanotechnology. 2017;28:225101–12. doi: 10.1088/1361-6528/aa6c9b. [DOI] [PubMed] [Google Scholar]
- 128.Singh AK, Mishra H, Firdaus Z, Yadav S, Aditi P, Nandy N. et al. MoS2-modified curcumin nanostructures: the novel theranostic hybrid having potent antibacterial and antibiofilm activities against multidrug-resistant hypervirulent klebsiella pneumoniae. Chem Res Toxicol. 2019;8:1599–618. doi: 10.1021/acs.chemrestox.9b00135. [DOI] [PubMed] [Google Scholar]
- 129.Feng Q, Wu J, Chen G, Cui F, Kim TN, Kim JO. A mechanistic study of the antibacterial effect of silver ions on Escherichia coli and Staphylococcus aureus. J Biomed Mater Res. 2000;52:662–8. doi: 10.1002/1097-4636(20001215)52:4<662::aid-jbm10>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 130.Sondi I, Salopek-Sondi B. Silver nanoparticles as antimicrobial agent: a case study on E. coli as a model for Gram-negative bacteria. J Colloid Interface Sci. 2004;275:177–82. doi: 10.1016/j.jcis.2004.02.012. [DOI] [PubMed] [Google Scholar]
- 131.Chernousova S, Epple M. Silver as antibacterial agent: ion, nanoparticle, and metal. Angew Chem Int Ed. 2013;52:1636–53. doi: 10.1002/anie.201205923. [DOI] [PubMed] [Google Scholar]
- 132.Yu S, Li G, Liu R, Ma D, Xue W. Dendritic Fe3O4@Poly(dopamine)@PAMAM nanocomposite as controllable NO-releasing material: a synergistic photothermal and NO antibacterial study. Adv Funct Mater. 2018; 1707. 440-54. [Google Scholar]
- 133.Wu J, Li Y, He C, Kang J, Ye J, Xiao Z. et al. Novel H2S releasing nanofibrous coating for In Vivo dermal wound regeneration. ACS Appl Mater Interfaces. 2016;8:27474–81. doi: 10.1021/acsami.6b06466. [DOI] [PubMed] [Google Scholar]
- 134.Xu Z, Qiu Z, Liu Q, Huang Y, Li D, Shen X. et al. Converting organosulfur compounds to inorganic polysulfides against resistant bacterial infections. Nat Commun. 2018;9:3713–26. doi: 10.1038/s41467-018-06164-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Xu Y, Li S, Yue X, Lu W. Review of silver nanoparticles (AgNPs)-cellulose antibacterial composites. Bioresources. 2018;13:2150–70. [Google Scholar]
- 136.Cao F, Ju E, Zhang Y, Wang Z, Liu C, Li W. et al. An efficient and benign antimicrobial depot based on silver-infused MoS2. ACS Nano. 2017;11:4651–9. doi: 10.1021/acsnano.7b00343. [DOI] [PubMed] [Google Scholar]
- 137.Zhou F, Mei L, Lei T, Duo C, Jin Y, Yang L. et al. Electrophoretic deposited stable chitosan@MoS2 coating with rapid in situ bacteria-killing ability under dual-light irradiation. Small. 2018;14:1704347. doi: 10.1002/smll.201704347. [DOI] [PubMed] [Google Scholar]
- 138.Zhang X, Zhang C, Yang Y, Zhang H, Huang X, Hang R. et al. Light-assisted rapid sterilization by a hydrogel incorporated with Ag3PO4/MoS2 composites for efficient wound disinfection. Chem Eng J. 2019;374:596–604. [Google Scholar]
- 139.Li Y, Liu X, Tan L, Cui Z, Yang X, Zheng Y. et al. Rapid Sterilization and Accelerated Wound Healing Using Zn2+ and Graphene Oxide Modified g-C3N4 under Dual Light Irradiation. Adv Funct Mater. 2018;28:1800299. [Google Scholar]
- 140.Zhang C, Hu D, Xu J, Ma M, Xing H, Yao K. et al. Polyphenol-assisted exfoliation of transition metal dichalcogenides into nanosheets as photothermal nanocarriers for enhanced antibiofilm activity. ACS Nano. 2018;12:12347–56. doi: 10.1021/acsnano.8b06321. [DOI] [PubMed] [Google Scholar]
- 141.Lin Y, Han D, Li Y, Tan L, Liu X, Cui Z. et al. Ag2S@WS2 Heterostructure for Rapid Bacteria-Killing Using Near-Infrared Light. ACS Sustainable Chem Eng. 2019;7:14982–90. [Google Scholar]
- 142.Li B, Tan L, Liu X, Li Z, Cui Z, Liang Y. et al. Superimposed surface plasma resonance effect enhanced the near-infrared photocatalytic activity of Au@Bi2WO6 coating for rapid bacterial killing. J Hazard Mater. 2019;380:120818. doi: 10.1016/j.jhazmat.2019.120818. [DOI] [PubMed] [Google Scholar]
- 143.Yin W, Yu J, Lv F, Yan L, Zheng L, Gu Z. et al. Functionalized nano-MoS2 with peroxidase catalytic and near-infrared photothermal activities for safe and synergetic wound antibacterial applications. ACS Nano. 2016;10:11000–11. doi: 10.1021/acsnano.6b05810. [DOI] [PubMed] [Google Scholar]
- 144.Cao F, Zhang L, Wang H, You Y, Wang Y, Gao N, Defect-rich adhesive nanozymes as efficient “antibiotics” for enhanced bacterial inhibition. Angew Chem Int Ed; 2019. DOI:10.1002/anie.201908289. [DOI] [PubMed] [Google Scholar]
- 145.Yin Q, Tan L, Lang Q, Ke X, Bai L, Guo K. et al. Plasmonic molybdenum oxide nanosheets supported silver nanocubes for enhanced near-infrared antibacterial activity: Synergism of photothermal effect, silver release and photocatalytic reactions. Appl Catal B. 2018;224:671–80. [Google Scholar]
- 146.Roy S, Mondal A, Yadav V, Sarkar A, Banerjee R, Sanpui P. et al. Mechanistic insight into the antibacterial activity of chitosan exfoliated MoS2 nanosheets: membrane damage, metabolic inactivation, and oxidative stress. ACS Appl Bio Mater. 2019;2:2738–55. doi: 10.1021/acsabm.9b00124. [DOI] [PubMed] [Google Scholar]
- 147.Li M, Li L, Su K, Liu X, Zhang T, Liang Y, Highly effective and noninvasive near-infrared eradication of a Staphylococcus aureus biofilm on implants by a photoresponsive coating within 20 min. Adv Sci. 2019; 1900. 599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Yuwen L, Sun Y, Tan G, Xiu W, Zhang Y, Weng L. et al. MoS2@polydopamine-Ag nanosheets with enhanced antibacterial activity for effective treatment of Staphylococcus aureus biofilms and wound infection. Nanoscale. 2018;10:16711–20. doi: 10.1039/c8nr04111c. [DOI] [PubMed] [Google Scholar]
- 149.Lok C, Ho C, Chen R, He Q, Yu W, Sun H. et al. Silver nanoparticles: partial oxidation and antibacterial activities. J Biol Inorg Chem. 2007;12:527–34. doi: 10.1007/s00775-007-0208-z. [DOI] [PubMed] [Google Scholar]
- 150.Chen C, Gunawan P, Lou X, Xu R. Silver nanoparticles deposited layered double hydroxide nanoporous coatings with excellent antimicrobial activities. Adv Funct Mater. 2012;22:780–7. [Google Scholar]
- 151.Tang S, Zheng J. Antibacterial activity of silver nanoparticles: structural effects. Adv Healthc Mater. 2018;7:1701503. doi: 10.1002/adhm.201701503. [DOI] [PubMed] [Google Scholar]
- 152.Ananth A, Dharaneedharan S, Gandhi MS, Heo MS, Mok YS. Novel RuO2 nanosheets-Facile synthesis, characterization and application. Chem Eng J. 2013;223:729–36. [Google Scholar]
- 153.Sun Z, Zhang Y, Yu H, Yan C, Liu Y, Hong S. et al. New solvent-stabilized few-layer black phosphorus for antibacterial applications. Nanoscale. 2018;10:12543–53. doi: 10.1039/c8nr03513j. [DOI] [PubMed] [Google Scholar]
- 154.Kan M, Wang J, Li X, Zhang S, Li Y, Kawazoe Y. et al. Structures and phase transition of a MoS2 monolayer. J Phys Chem C. 2014;118:1515–22. [Google Scholar]
- 155.Ke Y, Liu C, Zhang X, Xiao M, Wu G. Surface Modification of Polyhydroxyalkanoates toward Enhancing Cell Compatibility and Antibacterial Activity. Macromol Mater Eng. 2017;302:1700258. [Google Scholar]
- 156.Cao W, Yue L, Wang Z. High antibacterial activity of chitosan-molybdenum disulfide nanocomposite. Carbohydr Polym. 2019;215:226–34. doi: 10.1016/j.carbpol.2019.03.085. [DOI] [PubMed] [Google Scholar]
- 157.Gao Q, Zhang X, Yin W, Ma D, Xie C, Zheng L. et al. Functionalized MoS2 nanovehicle with near-infrared laser-mediated nitric oxide release and photothermal activities for advanced bacteria-infected wound therapy. Small. 2018;0:1802290–305. doi: 10.1002/smll.201802290. [DOI] [PubMed] [Google Scholar]
- 158.Cheng P, Zhou Q, Hu X, Su S, Wang X, Jin M. et al. Transparent glass with growth of pyramid-type MoS2 for highly efficient water disinfection under visible light irradiation. ACS Appl Mater Interfaces. 2018;10:23444–50. doi: 10.1021/acsami.8b06656. [DOI] [PubMed] [Google Scholar]
- 159.Huang X, Wei J, Liu T, Zhang X, Bai S, Yang H. Silk fibroin-assisted exfoliation and functionalization of transition metal dichalcogenide nanosheets for antibacterial wound dressings. Nanoscale. 2017;9:17193–8. doi: 10.1039/c7nr06807g. [DOI] [PubMed] [Google Scholar]
- 160.Masimukkub S, Hub YC, Linc ZH, Chanc SW, Chouc TM, Wu JM. High efficient degradation of dye molecules by PDMS embedded abundant single-layer tungsten disulfide and their antibacterial performance. Nano Energy. 2018;46:338–46. [Google Scholar]
- 161.Kumar P, Kataria S, Roy S, Jaiswal A, Balakrishnan V. Photocatalytic water disinfection of CVD grown WS2 monolayer decorated with Ag nanoparticles. ChemistrySelect. 2018;3:7648–55. [Google Scholar]
- 162.Mo S, Chen X, Chen M, He C, Lu Y, Zheng N. Two-dimensional antibacterial Pd@Ag nanosheets with a synergetic effect of plasmonic heating and Ag+ release. J Mater Chem B. 2015;3:6255–60. doi: 10.1039/c5tb00830a. [DOI] [PubMed] [Google Scholar]
- 163.Wang X, Su K, Tan L, Liu X, Cui Z, Jing D. et al. Rapid and highly effective noninvasive disinfection by hybrid Ag/CS@MnO2 nanosheets using near-infrared light. ACS Appl Mater Interfaces. 2019;11:15014–27. doi: 10.1021/acsami.8b22136. [DOI] [PubMed] [Google Scholar]
- 164.Kaushik R, Vineeth Daniel P, Mondal P, Halder A. Transformation of 2-D TiO2 to mesoporous hollow 3-D TiO2 spheres-comparative studies on morphology-dependent photocatalytic and anti-bacterial activity. Microporous Mesoporous Mater. 2019;285:32–42. [Google Scholar]
- 165.Pal A, Jana TK, Roy T, Pradhan A, Maiti R, M. Choudhury S, et al. MoS2-TiO2 nanocomposite with excellent adsorption performance and high antibacterial activity. ChemistrySelect. 2018;3:81–90. [Google Scholar]
- 166.Li R, Ren Y, Zhao P, Wang J, Liu J, Zhang Y. Graphitic carbon nitride (g-C3N4) nanosheets functionalized composite membrane with self-cleaning and antibacterial performance. J Hazard Mater. 2019;365:606–14. doi: 10.1016/j.jhazmat.2018.11.033. [DOI] [PubMed] [Google Scholar]
- 167.Dai J, Song J, Qiu Y, Wei J, Hong Z, Li L. et al. Gold nanoparticle-decorated g-C3N4 nanosheets for controlled generation of reactive oxygen species upon 670 nm laser illumination. ACS Appl Mater Interfaces. 2019;11:10589–96. doi: 10.1021/acsami.9b01307. [DOI] [PubMed] [Google Scholar]
- 168.Liu C, Wang L, Xu H, Wang S, Gao S, Ji X. et al. “One pot” green synthesis and the antibacterial activity of g-C3N4/Ag nanocomposites. Mater Lett. 2016;164:567–70. [Google Scholar]
- 169.Khan ME, Han TH, Khan MM, Karim MR, Cho MH. Environmentally sustainable fabrication of Ag@g-C3N4 nanostructures and their multifunctional efficacy as antibacterial agents and photocatalysts. ACS Appl Nano Mater. 2018;1:2912–22. [Google Scholar]
- 170.Wu Y, Zhou Y, Xu H, Liu Q, Li Y, Zhang L. et al. Highly active, superstable, and biocompatible Ag/polydopamine/g-C3N4 bactericidal photocatalyst: synthesis, characterization, and mechanism. ACS Sustainable Chem Eng. 2018;6:14082–94. [Google Scholar]
- 171.Wang R, Kong X, Zhang W, Zhu W, Huang L, Wang J. et al. Mechanism insight into rapid photocatalytic disinfection of salmonella based on vanadate QDs-interspersed g-C3N4 heterostructures. Appl Catal B. 2018;225:228–37. [Google Scholar]
- 172.Wu Q, Liang M, Zhang S, Liu X, Wang F. Development of functional black phosphorus nanosheets with remarkable catalytic and antibacterial performance. Nanoscale. 2018;10:10428–35. doi: 10.1039/c8nr01715h. [DOI] [PubMed] [Google Scholar]
- 173.Rasool K, Helal M, Ali A, Ren CE, Gogotsi Y, Mahmoud KA. Antibacterial activity of Ti3C2Tx MXene. ACS Nano. 2016;10:3674–84. doi: 10.1021/acsnano.6b00181. [DOI] [PubMed] [Google Scholar]
- 174.Rasool K, Mahmoud KA, Johnson DJ, Helal M, Berdiyorov GR, Gogotsi Y. Efficient antibacterial membrane based on two-dimensional Ti3C2Tx (MXene) nanosheets. Sci Rep. 2017;7:1598–609. doi: 10.1038/s41598-017-01714-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Wang Z, Yu H, Ma K, Chen Y, Zhang X, Wang T. et al. Flower-like surface of three-metal-component layered double hydroxide composites for improved antibacterial activity of lysozyme. Bioconjugate Chem. 2018;29:2090–9. doi: 10.1021/acs.bioconjchem.8b00305. [DOI] [PubMed] [Google Scholar]
- 176.Gao G, Mathkar A, Martins EP, Galvão DS, Gao D, Autreto PAdS. et al. Designing nanoscaled hybrids from atomic layered boron nitride with silver nanoparticle deposition. J Mater Chem A. 2014;2:3148–54. [Google Scholar]
- 177.Zhu C, Shen H, Liu H, Lv X, Li Z, Yuan Q. Solution-processable two-dimensional In2Se3 nanosheets as efficient photothermal agents for elimination of bacteria. Chemistry. 2018;24:19060–5. doi: 10.1002/chem.201804360. [DOI] [PubMed] [Google Scholar]
- 178.Li X, Shan J, Zhang W, Su S, Yuwen L, Wang L. Recent advances in synthesis and biomedical applications of two-dimensional transition metal dichalcogenide nanosheets. Small. 2017;13:1602660–88. doi: 10.1002/smll.201602660. [DOI] [PubMed] [Google Scholar]
- 179.Kalantar zadeh K, Ou JZ, Daeneke T, Strano MS, Pumera M, Gras SL. Two-dimensional transition metal dichalcogenides in biosystems. Adv Funct Mater. 2015;25:5086–99. [Google Scholar]
- 180.Zhang B, Zou S, Cai R, Li M, He Z. Highly-efficient photocatalytic disinfection of Escherichia coli under visible light using carbon supported Vanadium Tetrasulfide nanocomposites. Appl Catal B. 2018;224:383–93. [Google Scholar]
- 181.Yong Y, Cheng X, Bao T, Zu M, Yan L, Yin W. et al. Tungsten sulfide quantum dots as multifunctional nanotheranostics for in vivo dual-modal image-guided photothermal/radiotherapy synergistic therapy. ACS Nano. 2015;9:12451–63. doi: 10.1021/acsnano.5b05825. [DOI] [PubMed] [Google Scholar]
- 182.Dong X, Yin W, Zhang X, Zhu S, He X, Yu J. et al. Intelligent MoS2 nanotheranostic for targeted and enzyme-/pH-/NIR-responsive drug delivery to overcome cancer chemotherapy resistance guided by PET imaging. ACS Appl Mater Interfaces. 2018;10:4271–84. doi: 10.1021/acsami.7b17506. [DOI] [PubMed] [Google Scholar]
- 183.Yang L, Wang J, Yang S, Lu Q, Li P, Li N. Rod-shape MSN@MoS2 Nanoplatform for FL/MSOT/CT Imaging-Guided Photothermal and Photodynamic Therapy. Theranostics. 2019;9:3992–4005. doi: 10.7150/thno.32715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Wu N, Yu Y, Li T, Ji X, Jiang L, Zong J. et al. Investigating the influence of MoS2 nanosheets on E. coli from metabolomics level. PLoS One. 2016;11:e0167245. doi: 10.1371/journal.pone.0167245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Niu J, Sun Y, Wang F, Zhao C, Ren J, Qu X. Photomodulated nanozyme used for a gram-selective antimicrobial. Chem Mater. 2018;30:7027–33. [Google Scholar]
- 186.Fakhri A, Gupta VK, Rabizadeh H, Agarwal S, Sadeghi N, Tahami S. Preparation and characterization of WS2 decorated and immobilized on chitosan and polycaprolactone as biodegradable polymers nanofibers: Photocatalysis study and antibiotic-conjugated for antibacterial evaluation. Int J Biol Macromol. 2018;120:1789–93. doi: 10.1016/j.ijbiomac.2018.09.207. [DOI] [PubMed] [Google Scholar]
- 187.Li J, Cha R, Zhao X, Guo H, Luo H, Wang M. et al. Gold nanoparticles cure bacterial infection with benefit to intestinal microflora. ACS Nano. 2019;13:5002–14. doi: 10.1021/acsnano.9b01002. [DOI] [PubMed] [Google Scholar]
- 188.Shahmiri M, Ibrahim N, Shayesteh F, Asim N, Motallebi N. Preparation of PVP-coated copper oxide nanosheets as antibacterial and antifungal agents. J Mater Res. 2013;28:3109–18. [Google Scholar]
- 189.Raghupathi KR, Koodali RT, Manna AC. Size-dependent bacterial growth inhibition and mechanism of antibacterial activity of zinc oxide nanoparticles. Langmuir. 2011;27:4020–8. doi: 10.1021/la104825u. [DOI] [PubMed] [Google Scholar]
- 190.Li Y, Liu G, Zhai Z, Liu L, Li H, Yang K. et al. Antibacterial properties of magnesium in vitro and in an in vivo model of implant-associated methicillin-resistant Staphylococcus aureus infection. Antimicrob Agents Chemother. 2014;58:7586–91. doi: 10.1128/AAC.03936-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Robinson DA, Griffith RW, Shechtman D, Evans RB, Conzemius MG. In vitro antibacterial properties of magnesium metal against Escherichia coli, Pseudomonas aeruginosa and Staphylococcus aureus. Acta Biomater. 2010;6:1869–77. doi: 10.1016/j.actbio.2009.10.007. [DOI] [PubMed] [Google Scholar]
- 192.Behin J, Shahryarifar A, Kazemian H. Ultrasound-assisted synthesis of Cu and Cu/Ni nanoparticles on NaP zeolite support as antibacterial agents. Chem Eng Technol. 2016;39:2389–403. [Google Scholar]
- 193.Chondath SK, Poolakkandy RR, Kottayintavida R, Thekkangil A, Gopalan NK, Vasu ST. et al. Water-Chloroform Interface Assisted Microstructure Tuning of Polypyrrole-Silver Sheets. ACS Appl Mater Interfaces. 2019;11:1723–31. doi: 10.1021/acsami.8b18943. [DOI] [PubMed] [Google Scholar]
- 194.Song X, Hu J, Zeng H. Two-dimensional semiconductors: recent progress and future perspectives. J Mater Chem C. 2013;1:2952–69. [Google Scholar]
- 195.Wang G, Xing Z, Zeng X, Feng C, McCarthy DT, Deletic A. et al. Ultrathin titanium oxide nanosheets film with memory bactericidal activity. Nanoscale. 2016;8:18050–6. doi: 10.1039/c6nr06313f. [DOI] [PubMed] [Google Scholar]
- 196.Wang B, Leung MKH, Lu X, Chen S. Synthesis and photocatalytic activity of boron and fluorine codoped TiO2 nanosheets with reactive facets. Appl Energy. 2013;112:1190–7. [Google Scholar]
- 197.Zanni E, Chandraiahgari RC, De Bellis G, Montereali RM, Armiento G, Ballirano P. et al. Zinc oxide nanorods-decorated graphene nanoplatelets: a promising antimicrobial agent against the cariogenic bacterium streptococcus mutans. Nanomaterials. 2016;6:179–93. doi: 10.3390/nano6100179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.He W, Kim HK, Wamer WG, Melka D, Callahan JH, Yin J. Photogenerated charge carriers and reactive oxygen species in ZnO/Au hybrid nanostructures with enhanced photocatalytic and antibacterial activity. J Am Chem Soc. 2014;136:750–7. doi: 10.1021/ja410800y. [DOI] [PubMed] [Google Scholar]
- 199.Ma S, Zhan S, Jia Y, Zhou Q. Superior antibacterial activity of Fe3O4-TiO2 nanosheets under solar light. ACS Appl Mater Interfaces. 2015;7:21875–83. doi: 10.1021/acsami.5b06264. [DOI] [PubMed] [Google Scholar]
- 200.Ong WJ, Tan LL, Ng YH, Yong ST, Chai SP. Graphitic carbon nitride (g-C3N4)-based photocatalysts for artificial photosynthesis and environmental remediation: are we a step closer to achieving sustainability? Chem Rev. 2016;116:7159–329. doi: 10.1021/acs.chemrev.6b00075. [DOI] [PubMed] [Google Scholar]
- 201.Wang L, Zhang X, Yu X, Gao F, Shen Z, Zhang X. et al. An all-organic semiconductor C3N4/PDINH heterostructure with advanced antibacterial photocatalytic therapy activity. Adv Mater. 2019;0:1901965. doi: 10.1002/adma.201901965. [DOI] [PubMed] [Google Scholar]
- 202.Li Z, Wu L, Wang H, Zhou W, Liu H, Cui H. et al. Synergistic antibacterial activity of black phosphorus nanosheets modified with titanium aminobenzenesulfanato complexes. ACS Appl Nano Mater. 2019;2:1202–9. [Google Scholar]
- 203.Lei W, Liu G, Zhang J, Liu M. Black phosphorus nanostructures: recent advances in hybridization, doping and functionalization. Chem Soc Rev. 2017;46:3492–509. doi: 10.1039/c7cs00021a. [DOI] [PubMed] [Google Scholar]
- 204.Luo M, Fan T, Zhou Y, Zhang H, Mei L. 2D black phosphorus-based biomedical applications. Adv Funct Mater. 2019;29:1808306. [Google Scholar]
- 205.Choi JR, Yong KW, Choi JY, Nilghaz A, Lin Y, Xu J. et al. Black Phosphorus and its Biomedical Applications. Theranostics. 2018;8:1005–26. doi: 10.7150/thno.22573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Ge X, Xia Z, Guo S. Recent advances on black phosphorus for biomedicine and biosensing. Adv Funct Mater. 2019;29:1900318. [Google Scholar]
- 207.Ouyang J, Liu R, Chen W, Liu Z, Xu Q, Zeng K. et al. A black phosphorus based synergistic antibacterial platform against drug resistant bacteria. J Mater Chem B. 2018;6:6302–10. doi: 10.1039/c8tb01669k. [DOI] [PubMed] [Google Scholar]
- 208.Liu G, Shen J, Ji Y, Liu Q, Liu G, Yang J. et al. Two-dimensional Ti2CTx MXene membranes with integrated and ordered nanochannels for efficient solvent dehydration. J Mater Chem A. 2019;7:12095–104. [Google Scholar]
- 209.Anasori B, Lukatskaya MR, Gogotsi Y. 2D metal carbides and nitrides (MXenes) for energy storage. Nat Rev Mater. 2017;2:16098. [Google Scholar]
- 210.Lin H, Chen Y, Shi J. Insights into 2D MXenes for versatile biomedical applications: current advances and challenges ahead. Adv Sci. 2018;5:1800518. doi: 10.1002/advs.201800518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Han X, Jing X, Yang D, Lin H, Wang Z, Ran H. et al. Therapeutic mesopore construction on 2D Nb2C MXenes for targeted and enhanced chemo-photothermal cancer therapy in NIR-II biowindow. Theranostics. 2018;8:4491–508. doi: 10.7150/thno.26291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Arabi Shamsabadi A, Sharifian Gh M, Anasori B, Soroush M. Antimicrobial mode-of-action of colloidal Ti3C2Tx MXene nanosheets. ACS Sustainable Chem Eng. 2018;6:16586–96. [Google Scholar]
- 213.Mishra G, Dash B, Pandey S, Mohanty PP. Antibacterial actions of silver nanoparticles incorporated Zn-Al layered double hydroxide and its spinel. J Environ Chem Eng. 2013;1:1124–30. [Google Scholar]
- 214.Yang Q, Chang Y, Zhao H. Preparation and antibacterial activity of lysozyme and layered double hydroxide nanocomposites. Water Res. 2013;47:6712–8. doi: 10.1016/j.watres.2013.09.002. [DOI] [PubMed] [Google Scholar]
- 215.Wang Q, O'Hare D. Recent advances in the synthesis and application of layered double hydroxide (LDH) nanosheets. Chem Rev. 2012;112:4124–55. doi: 10.1021/cr200434v. [DOI] [PubMed] [Google Scholar]
- 216.Mishra G, Dash B, Pandey S. Layered double hydroxides: A brief review from fundamentals to application as evolving biomaterials. Appl Clay Sci. 2018;153:172–86. [Google Scholar]
- 217.Leon Morales CF, Leis AP, Strathmann M, Flemming HC. Interactions between laponite and microbial biofilms in porous media: implications for colloid transport and biofilm stability. Water Res. 2004;38:3614–26. doi: 10.1016/j.watres.2004.05.009. [DOI] [PubMed] [Google Scholar]
- 218.Das SS, Neelam, Hussain K, Singh S, Hussain A, Faruk A. et al. Laponite-based nanomaterials for biomedical applications: a review. Curr Pharm Des. 2019;25:424–43. doi: 10.2174/1381612825666190402165845. [DOI] [PubMed] [Google Scholar]
- 219.Ding W, Zhu J, Wang Z, Gao Y, Xiao D, Gu Y. et al. Prediction of intrinsic two-dimensional ferroelectrics in In2Se3 and other III2-VI3 van der Waals materials. Nat Commun. 2017;8:14956–64. doi: 10.1038/ncomms14956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Gorle G, Bathinapatla A, Chen Y, Ling Y. Near infrared light activatable PEI-wrapped bismuth selenide nanocomposites for photothermal/photodynamic therapy induced bacterial inactivation and dye degradation. RSC Adv. 2018;8:19827–34. doi: 10.1039/c8ra02183j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Ghadiri M, Chrzanowski W, Rohanizadeh R. Antibiotic eluting clay mineral (Laponite(R)) for wound healing application: an in vitro study. J Mater Sci Mater Med. 2014;25:2513–26. doi: 10.1007/s10856-014-5272-7. [DOI] [PubMed] [Google Scholar]
- 222.Zhao F, Zhao Y, Liu Y, Chang X, Chen C, Zhao Y. Cellular uptake, intracellular trafficking, and cytotoxicity of nanomaterials. Small. 2011;7:1322–37. doi: 10.1002/smll.201100001. [DOI] [PubMed] [Google Scholar]
- 223.Mei L, Zhang X, Yin W, Dong X, Guo Z, Fu W. et al. Translocation, biotransformation-related degradation, and toxicity assessment of polyvinylpyrrolidone-modified 2H-phase nano-MoS2. Nanoscale. 2019;11:4767–80. doi: 10.1039/c8nr10319d. [DOI] [PubMed] [Google Scholar]
- 224.Hussain SM, Stolle LKB, Schrand AM, Murdock RC, Yu KO, Mattie DM. et al. Toxicity evaluation for safe use of nanomaterials: recent achievements and technical challenges. Adv Mater. 2009;21:1549–59. [Google Scholar]
- 225.Feng Q, Liu Y, Huang J, Chen K, Huang J, Xiao K. Uptake, distribution, clearance, and toxicity of iron oxide nanoparticles with different sizes and coatings. Sci Rep. 2018;8:2082–94. doi: 10.1038/s41598-018-19628-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Li Z, Zhang Y, Ma J, Meng Q, Fan J. Modeling Interactions between Liposomes and Hydrophobic Nanosheets. Small. 2019;15:1804992. doi: 10.1002/smll.201804992. [DOI] [PubMed] [Google Scholar]