Abstract
Purpose
To determine if an eye drop containing omega-3 fatty acids (Refresh Optive MEGA-3®, Allergan plc, Dublin, Ireland) increases the lipid layer thickness (LLT) of the tear film versus a non-emollient eye drop (Refresh Optive, Allergan plc).
Methods
Patients (≥30 years) with baseline LLT ≤75 nm completed the Current Symptoms Survey (CSS – a visual analog survey of dry eye symptoms), and LLT was measured pre- and post-instillation (15 and 60 mins) of their randomly assigned treatment. After washout, patients were tested with the other treatment. Primary endpoint: change in LLT from baseline. Secondary endpoint: CSS results.
Results
Of 21 patients enrolled, 19 completed the study. With the omega-3–containing eye drop, the mean (standard deviation) LLT increase from baseline at 15 mins was statistically significant in the overall field (8.8 [11.5] nm; P<0.001), and in each individual zone (superior, central, and inferior). At 1 hr, the LLT change from baseline was statistically significant overall (4.4 [9.7] nm; P<0.02) and in the inferior and central zones. With the aqueous eye drop, LLT change from baseline was only significant at 15 mins in the inferior field. The CSS analysis revealed a ≥8.68-unit decrease in mean average dryness score from baseline at 15 and 60 mins post-instillation of the lipid-based treatment (P≤0.03).
Conclusion
The eye drop containing omega-3 fatty acids increased LLT at 15 mins, maintaining it at 1 hr post-instillation. Dryness symptoms also improved and maintained improved levels 1 hr after instillation, indicating that the product may benefit symptomatic patients with evaporative dry eye.
Keywords: dry eye, artificial tears, ophthalmic solution, omega-3 fatty acids, survey, tear lipid layer
Introduction
Dry-eye-related symptoms are reported by 5% to 33% of the general population and by 49.5% of computer users.1 The symptoms and diagnosis of dry eye have become a large concern with the increase in the prevalence of dry eye disease. The largest portion of dry eye is attributed to evaporative dry eye, in which the lipid layer of the tears becomes thin and/or unstable, ultimately leading to tear break-up.2 Lipid deficiencies in the tears can be a result of a decrease in the quantity of functional meibomian glands, or a reduction in the amount of meibum produced by these glands.3 The eye blink is responsible for mechanically compressing these glands to express meibum into the tear film to form the lipid layer.4 The severity of dry eye often determines the treatment prescribed by clinicians, and artificial tears are very commonly used as an early treatment plan for these patients.5
Expression of meibomian glands and distribution of meibum depend upon the eye blink. Situations in which the eye blink rate is decreased could therefore lead to a reduction or abnormal distribution of meibum. For example, studies conducted to identify the likely causes of eye discomfort with computer use found that the most likely factor is the reduction in blink rate, from 15.5 blinks per minute when in primary gaze to six blinks per minute when using a handheld screen.6 Such a decrease in blink rate would be expected to reduce the expression of lipids from the meibomian glands, thereby reducing the stability of the tear film overall.
Patients with meibomian gland deficiency and those who have dry eye symptoms secondary to low blink rate may benefit from maintaining an enhanced and more stable lipid layer to keep the tear film protected from evaporation. Studies have found that eye drops containing emollients can help to increase the lipid layer thickness (LLT) of the tear film in the short term.7,8
Omega-3 fatty acids can be found in the normal tear film, with profiles of these lipids correlating with dry eye severity.9 While previous studies have found conflicting results as to whether dietary supplementation with omega-3 fatty acids can aid in the treatment of dry eye,10,11 no studies to date have been done to determine if an eye drop containing omega-3 fatty acids can thicken the lipid layer of the tears. The purpose of this study was to compare the increase in LLT before and after instillation of a preservative-free omega-3–containing emollient artificial tear (Refresh Optive MEGA-3®, Allergan plc, Dublin, Ireland) with that of a preservative-free non-emollient artificial tear (Refresh Optive®, Allergan plc), using a specialized noninvasive instrument (stroboscopic video color microscope [SVCM]).
Methods
Study Design
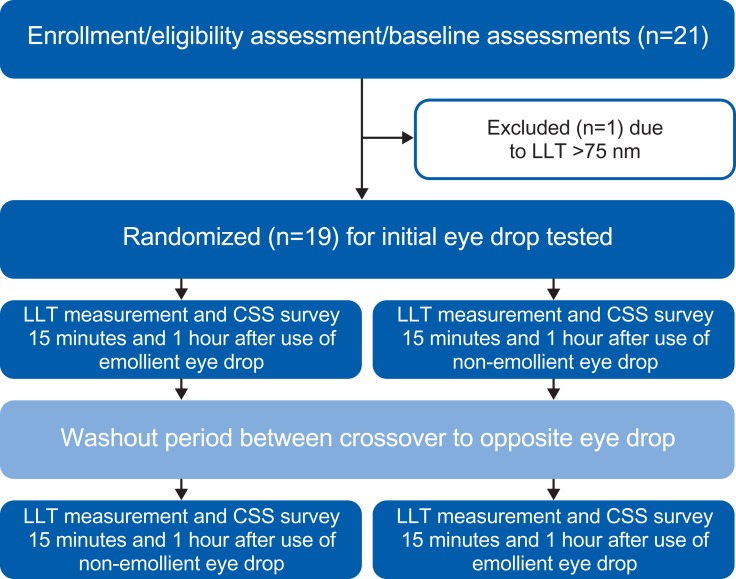
This randomized, double-masked, crossover study (ClinicalTrials.gov identifier: NCT03380624) was completed under the approval of the institutional review board at The Ohio State University and adhered to the tenets of the Declaration of Helsinki. All patients provided written consent before participation in the study. A graphical overview of the study is presented in Figure 1.
Figure 1.
Study design.
Abbreviations: LLT, lipid layer thickness; CSS, Current Symptoms Survey (a visual analog scale of eye dryness symptoms).
Eligibility Criteria
Patients were eligible for study enrollment if they were at least 30 years of age, in good general health (defined as no changes in medical conditions in the last month), and had a tear LLT measurement ≤75 nm at baseline. This requirement was consistent with previous studies of dry eye in which changes in LLT were evaluated.8,12 Excluded were patients taking ocular prescription medications or using artificial tears within 14 days of the screening visit, those with punctal plugs, current eye infections, past corneal eye surgery, or infectious diseases, as well as pregnant or lactating women. Contact lens wearers could only participate in the study if they refrained from lens wear for 2 days before the baseline visit and throughout the study until after the final visit. All potential participants were instructed not to use eye drops or eye makeup or to rub their eyes on a study visit day.
Study Materials
The study consisted of a baseline visit followed by two eye-drop visits, Visit 1 (immediately after the baseline visit) and Visit 2 (≥2 days after Visit 1), during which a single drop of an emulsified nano-lipid artificial tear containing omega-3 fatty acids/flaxseed oil or control nonemulsified artificial tear was administered.
Outcome Measures and Assessments
The primary outcome measures were changes in LLT, assessed by the SVCM, and ocular symptoms, evaluated by the Current Symptoms Survey (CSS).
The SVCM, developed by King-Smith at The Ohio State University,13 was used to measure the LLT over a 6-mm diameter circular field centered on the central cornea. The stroboscopic white light source and high-performance color camera (1400 × 1100 pixels) recorded approximately 22 images per second, with a flash duration of 0.04 msec. Software rated the quality of the measurement to ensure that only images of sufficient quality were analyzed, and then each pixel in the image was converted into an LLT measurement in nanometers. The 6-mm diameter field was also separated into three sections so that the LLT values could be assessed superiorly, centrally, and inferiorly, as well as over the entire/overall field. The inferior field is similar to that used in previous studies.7,8 Assessment of the central and superior areas of the cornea is appropriate as the tear film moves on the ocular surface during a blink.13 For this study, 900 frames were collected over 40 seconds for each measurement session. Previous studies of LLT have been shown to be consistent in patients over the course of the day without diurnal fluctuation, allowing baseline LLT measurement to serve as a stable comparison to measurements taken after instillation of an eye drop or other treatment of dry eye.14
The CSS is a visual analog survey in which participants are asked to indicate the level of a symptom present by marking a vertical line at a position along a 100-mm horizontal line.15,16 The rightward direction of the line is associated with more severe symptoms. The symptoms graded with the CSS include burning/stinging, grittiness/foreign body sensation, dryness, blurry/fluctuating vision, and overall ocular pain/discomfort.
At the baseline visit, the CSS was completed by each patient and an initial LLT measurement was then collected for each eye using the SVCM. Patients positioned their chin and forehead into a typical biomicroscopic chinrest, and the SVCM light was centered and focused on the lipid layer of the tears. Patients were asked to remain as still as possible and to look at the center of the lighted circle. The examiner began the video recording and instructed the patient to blink every 4 seconds (to simulate a natural blink pattern17) during the 40-second recording. After the data were saved, the process was repeated for the fellow eye. Patients with an LLT of 75 nm or less were eligible to continue with Visit 1 immediately following the baseline visit and were randomized to receive either the omega-3–containing or aqueous eye drops. Patients had the appropriate eye drop instilled in both eyes, with careful attention made to avoid touching or manipulating the eyelids so as to minimize meibum expression. Both eye drops were instilled using single-use vials, which appeared identical in order to mask the patient. The examiner had knowledge of the eye drop required for instillation, but the data files were saved without indication of the eye drop utilized in order to keep the analysis masked. Fifteen and 60 mins after the eye drop instillation, patients again completed the CSS and underwent LLT measurements, so that Visit-1 data were obtained before eye drop instillation, as well as 15 mins and 1 hr after.
Patients were scheduled to return at least 2 days after the first visit, as described in previous studies of LLT,7 and were reminded not to wear contact lenses or to use any eye drops. During Visit 2, patients repeated a baseline LLT measurement and CSS survey. The appropriate randomized eye drop was then instilled in each eye, and the patient completed the CSS and underwent LLT measurements 15 mins and 1 hr after artificial tear administration, as was done during Visit 1.
Software was utilized to process the SVCM video files into LLT measurements. Although this process is objective, data files remained masked, such that the investigator was unaware of the eye drop used for each data file until after each file was processed.
Statistical Analyses
Previous studies of LLT changes tested patients 15 mins after the instillation of eye drops.8,12 As this study was the first to test LLT changes following the use of an omega-3 fatty acid, and the first known study to measure tear LLT 1 hr after instillation of an eye drop, the sample size selected was for pilot data collection.
One eye of each patient was selected for analysis using a randomization table. A custom computer program was used to process each recording into LLT data for each of the 900 frames collected. The program filtered out frames during blinks and averaged the LLT data points over the entire field. Average LLT thicknesses were also calculated for the superior, central, and inferior zones. Each of the zones is one-third of the 6-mm diameter overall field (2 mm in height). The LLT of the inferior zone covers the area tested in previous studies of the lipid layer.7,8,18 The changes in LLT at the 15 min and 1 hr time points were compared to baseline with a paired t-test. Scores from the CSS were similarly compared to baseline scores with a paired t-test.
Results
Of 21 patients enrolled, 19 (13 females and six males) completed the study visits. The patients ranged in age from 34 to 63 years, with an average age (standard deviation [SD]) of 46.47 (8.74) years. Two patients who were screened were not eligible to complete the study.
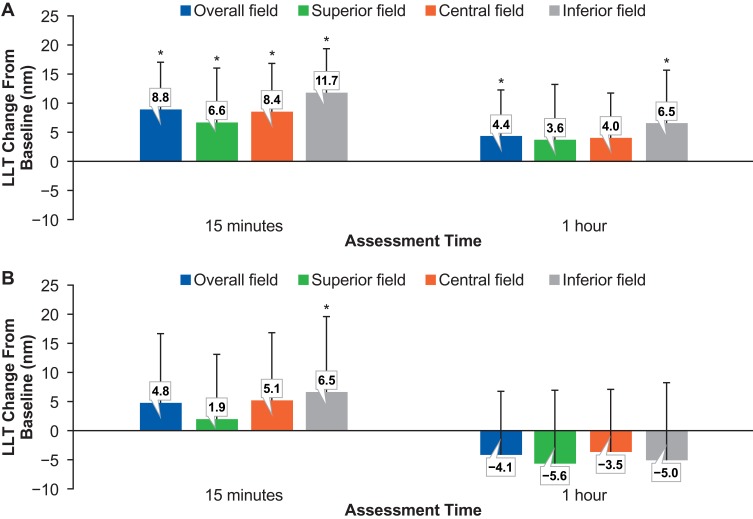
The mean increase in LLT from baseline at 15 mins post-instillation of the omega-3–containing eye drop was statistically significant in the overall field with an increase of 8.8 nm (P=0.0002). When evaluated separately, the mean change in LLT was statistically significant in each segment, with increases of 6.6 nm in the superior (P=0.006), 8.4 nm in the central (P=0.0003), and 11.7 nm in the inferior (P<0.0001) fields (Figure 2). When measured at 1 hr post-instillation, the change from baseline with the omega-3–containing eye drops was associated with a statistically significant increase of 4.4 nm for the overall field (P=0.02), 6.5 nm for the inferior zone (P=0.04), and 4.0 nm for the central zone (P=0.007). By comparison, the non–omega-3 eye drop showed a statistically significant increase in average LLT only in the inferior field (P=0.04) and only at the 15 mins measurement. Table 1 gives all LLT changes found in the study. Further analysis compared the change in average LLT after using the omega-3 eye drop to the change in average LLT after using the non–omega-3 eye drop. Specifically, a paired t-test of the values revealed that the change in LLT 1 hr post-instillation was statistically different when comparing the two eye drops in the overall field and in all zones measured (P<0.02), favoring the omega-3 eye drop. This difference in the LLT change was not significant at 15 mins post–eye drop instillation.
Figure 2.
Mean LLT change from baseline at 15 mins and 1 hr post-instillation of a single drop of the omega-3–containing artificial tear (A) or non-emollient (omega-3-free) artificial tear (B). The overall field (6 mm in diameter) was separated into three sections so that the mean LLT values could also be assessed superiorly, centrally, and inferiorly. *P<0.05 versus baseline. Error bars indicate standard deviations.
Abbreviation: LLT, lipid layer thickness.
Table 1.
Changes in Tear LLT After Instillation of Study Eye Drops
| Mean LLT Change from Baseline with Omega-3–Containing Eye Drop | Mean LLT Change from Baseline with Non–Omega-3-Containing Eye Drop | |
|---|---|---|
| Whole field | ||
| 15 mins | 8.8 nma | 4.8 nm |
| 1 hr | 4.4 nma | –4.1 nm |
| Superior field | ||
| 15 mins | 6.6 nma | 1.9 nm |
| 1 hr | 3.6 nm | –5.6 nm |
| Central field | ||
| 15 mins | 8.4 nma | 5.1 nm |
| 1 hr | 4.0 nm | –3.5 nm |
| Inferior field | ||
| 15 mins | 11.7 nma | 6.5 nma |
| 1 hr | 6.5 nma | –5.0 nm |
Notes: aStatistically significant change in lipid layer when compared with baseline (P<0.05).
Abbreviation: LLT, lipid layer thickness.
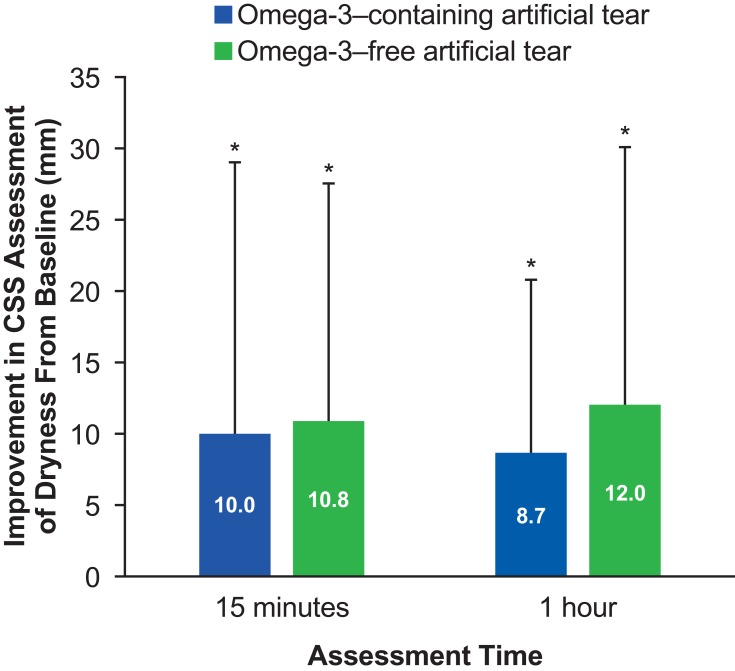
Scores from the CSS analysis showed a statistically significant decrease in the average dryness score from baseline (14.1 [22.2]) to 15 mins post-instillation of the omega-3–containing eye drop (4.1 [6.2]; P=0.03), as measured on a visual analog scale of 0–100. Similarly, the average CSS score was 5.4 (16.5) at 1 hr post–lipid eye drop instillation, and this was also a significant decrease from baseline (P=0.006) (Figure 3). The average dryness score change from baseline (16.0 [21.7]) to 15 mins post-instillation of the aqueous eye drop (5.2 [10.2]) and to 1 hr post-instillation (4.0 [6.7]) was also statistically significant (P=0.01 and P=0.01, respectively). Comparison of the changes in CSS average dryness score between the two eye drops at each time point was not statistically significant at 15 mins (P=0.8) or at 1 hr (P=0.2).
Figure 3.
Improvement in mean ocular dryness score from baseline to 15 mins and 1 hr post-instillation of a single drop of the omega-3–containing artificial tear or a non-emollient (omega-3-free) artificial tear. Assessments are based on the CSS, a 100-mm VAS of various dry eye symptoms. Changes from baseline were statistically significant at both time points with either product. *P≤0.03 versus baseline. There were no statistically significant differences between eye drops at either time points (P≥0.277, 2-sided t-test). Error bars indicate standard deviations.
Abbreviations: CSS, Current Symptoms Survey (a VAS of eye dryness symptoms); VAS, visual analog scale.
Of note, no adverse events were reported for any of the study participants enrolled in the study.
Discussion
Objective results of this study showed a measurable and statistically significant mean increase in all zones of the tear lipid layer, including the superior portion, which has not been studied in previous investigations of lipid-containing eye drops.7,8,18 No previous studies have measured an increase in tear LLT an hour after eye drop instillation, showing that this omega-3–containing eye drop makes changes that persist longer than previously recorded. In addition, previous studies of the lipid layer have only measured the LLT over the lower portion of the ocular surface, where the lipid layer is thickest and pulled upward by the blink.19 Tear analyses by Braun et al have shown that the lipid layer of the superior tear film is thinnest, creating ripples as it is replenished during a blink in normal blink patterns.13
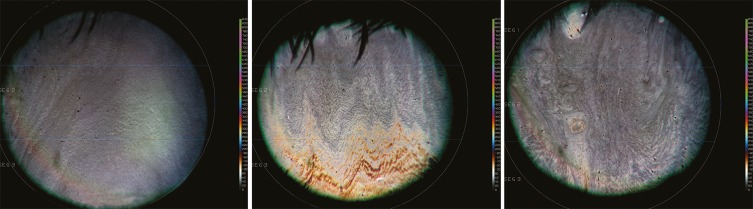
The inter-blink LLT measurements obtained in this study showed increased colors (ie, increased LLT thickness) that were more prominent inferiorly. They also revealed that even the central and the typically thinned superior zone of the tear lipid layer increased in thickness with use of an omega-3–containing eye drop in the short term (Figure 4). These inter-blink measurements are of particular interest with the increase in computer screen use, which has been associated with greatly decreased blink rate.20–22 Maintaining LLT between blinks in all zones likely improves the stability of the overall tear film, which in turn helps to prevent evaporation between blinks.
Figure 4.
LLT images taken using the stroboscopic video color microscope. The image on the left was taken at baseline. The center and right-side images were taken 15 mins and 1 hr post-instillation of a single drop of the omega-3–containing artificial tear, respectively. A color scale to the right of each image indicates the LLT thickness represented by different colors of the LLT images, with more vibrant colors corresponding to greater LLT.
Abbreviation: LLT, lipid layer thickness.
In addition to the objective measurements, patients completed a survey to determine if changes in symptoms corresponded with the changes in LLT. The CSS was given to all patients before each LLT measurement in order to monitor the patients’ symptoms, while also measuring the objective findings from the SVCM. Of the five categories of symptoms surveyed at baseline on the CSS, only dryness showed an average score above 10 mm in this study population, which is consistent with a population of patients who do not require prescription medication for dry eye. Since this CSS-based dryness category was the only one with a relatively high starting value, the fact that it was the only category to show improvement was not unexpected. The increase in LLT coupled with the decrease in the dryness symptom CSS value suggests that the omega-3–containing artificial tear may provide better relief and tear film stability at 60 mins than the control eye drop.
Artificial tears have long been used for treating ocular discomfort, with differing compositions targeting a variety of underlying causes of dry eye. The improvement in symptoms of dryness with the omega-3-containing eye drop was not statistically significant different from the improvement observed with the aqueous eye drop. An artificial tear that can alleviate dry eye symptoms immediately, while also improving the thickness of the tear lipid layer to provide stability, can provide relief to symptoms regardless of the etiology of the dry eye. The improvement in dry eye symptoms, as well as the increase in LLT observed after use of the omega-3–containing eye drop, indicate that this eye drop is able to provide both of these features, making it attractive to patients with meibomian gland deficiency who may have a deficient lipid layer, as well as patients with an aqueous tear deficiency or temporary dry eye symptoms.
The fact that the lipid component of this eye drop also includes flaxseed oil may provide additional benefit to the relief of symptoms and increase stability of the LLT. There are currently very few published preclinical and clinical studies evaluating the effects of topical artificial tears containing flaxseed oil (or its main omega-3 fatty acid, alpha-linolenic acid) in dry eye. A preclinical study has reported potent anti-inflammatory properties of alpha-linolenic acid on human corneal cells in vitro.23 In a mouse model of dry eye, topical treatment with alpha-linolenic acid significantly decreased signs of dry eye and inflammation at both the cellular and molecular levels.24 Similarly, in another study, the addition of flaxseed oil to hyaluronic acid–based eye drops reduced corneal epithelial barrier disruption and decreased levels of ocular surface proinflammatory markers, compared with hyaluronic acid therapy alone.25 A study of dogs with keratoconjunctivitis sicca found that topical application of a periophthalmic fatty acid cream coupled with hyaluronate eye drops resulted in statistically significant improvements in mean Shirmer’s Tear Test results after 8 weeks of use, with similar improvements in hyperemia and ocular discharge.26 Similar benefits, relating to a reduction in tear proinflammatory cytokines with a topical long-chain omega-3 fatty acid preparation, have recently been documented in individuals with contact lens discomfort and mild ocular surface inflammation. These authors raised the possibility of topical omega-3 fatty acids acting through a local ocular pathway to impart anti-inflammatory effects.27 In a multicenter, double-masked, randomized, 3-month study conducted in the United States and Australia, patients with signs and symptoms of dry eye (baseline Ocular Surface Disease Index [OSDI] score 18–65) who administered one to two drops of an artificial tear containing carboxymethylcellulose, glycerin, compatible solutes, trehalose, and emulsified castor and flaxseed oil in each eye at least twice daily exhibited greater reduction from baseline in the combined corneal and conjunctival staining score at all follow-up time points (≤90 days), compared with patients who instilled the control eye drop (containing the same ingredients except trehalose and flaxseed oil; P<0.03). Patients with more severe dry eye disease also had a greater reduction in corneal staining alone at all follow-up time points (P≤0.0338).28 These previous studies revealed additional benefits as a result of the use of an omega-3 fatty acid-containing eye drop formulation. It is not yet known whether these results are related to the increase in LLT found in this study, or if additional anti-inflammatory properties exist with these fatty acids when used on the ocular surface.
Systemically, the ratio of omega-3 to omega-6 fatty acids has been described as a determinant of the body’s inflammatory status.29 Supporting a role for omega-3 fatty acids in tear film dysfunction, an elevated ratio of omega-6 to omega-3 tear lipids was reported in patients with dry eye (proportionately to the degree of tear film dysfunction and corneal staining).9 If omega-3 fatty acids are essential fatty acids that can only be obtained through dietary sources, it may not be surprising that the effects of dietary or supplements of omega-3 fatty acid on dry eye have also been evaluated. An observational study of 39,876 female health professionals has previously shown that a lower intake of dietary omega-3 fatty acids was associated with an increased risk of dry eye; an odds ratio (95% confidence interval) of 2.51 (1.13, 5.58) was indeed reported for a diet with an omega-6 to omega-3 ratio ≥15:1 (common with a typical American diet), compared with <4:1 (theoretically ideal diet; P=0.01).30 Since then, several other studies evaluating the effects of omega-3 fatty acids on dry eye have been reported, but whether oral supplements can improve signs and/or treat/relieve symptoms of dry eye remains controversial, as evidenced by recent publications.11 This apparent lack of consistency could be due to differences in the source and type of omega-3 fatty acids used in those studies and/or the parameters assessed. One randomized, double-masked, placebo-controlled study showed that a supplement containing omega-3 fatty acids from krill oil (mostly available as phospholipids) was more effective in reducing levels of interleukin-17 (a proinflammatory cytokine) than those from fish oil (mostly available as triacylglycerides); although both supplements statistically significantly reduced tear osmolarity at 90 days, compared with baseline, a statistically significant decrease in OSDI score was only observed in the group receiving the krill oil supplement.31 In a randomized, double-masked, placebo-controlled study in which patients with dry eye received supplements of fish and flaxseed oil or placebo, 70% and 37% of patients were asymptomatic at day 90, respectively, compared with baseline. Although the omega-3 fatty acid supplements had no statistically significant effect on the meibum lipid composition or aqueous tear evaporation rate, they did increase tear production and tear volume, based on Schirmer testing and fluorimetry.32 In a study of postmenopausal women with moderate to severe keratoconjunctivitis sicca, neither an oral supplement of omega-3 fatty acids (including alpha-linolenic [196 mg], eicosapentaenoic [126 mg], docosahexaenoic [99 mg], and docosapentaenoic [39 mg] acids) nor placebo had any effects on tear production, tear break-up time, or corneal/conjunctival staining at 6 months, compared with baseline. However, patients who received the omega-3 supplement had improved OSDI (SD) scores (21 [4]) and a lower asymmetry index (0.37 [0.03]), compared with patients who received placebo (OSDI, 34 [5]; 0.51 [0.03]; P=0.005). In addition, expression of the proinflammatory markers HLA-DR and CD11c at 6 months was decreased by 36% (9%) and 34% (7%) in the supplement group, compared with the placebo group (P=0.001).33 The above findings are clinically relevant, especially when considering that dry eye disease was the only ocular disease associated with an increased incidence between 2008 and 2012.34
Conclusions
Although aqueous-based artificial tears remain a common regimen of dry eye management, formulations for the treatment of evaporative dry eye not only improve symptoms of dryness, but also reveal increases in tear LLT up to 1 hr after use. In this study, using an eye drop containing omega-3 fatty acids increased the tear lipid layer, with thicker LLT observed at 15 mins post–eye drop instillation. Portions of the tear film even continued to show improved LLT an hour after use of the eye drop, without any report of adverse events. As symptoms of dryness were also improved in patients using these eye drops, these findings suggest that there are potential benefits to patients with dry eye and those with a lifestyle that may decrease blinking and –consequently− lipid secretion into the tears.
Acknowledgment
The authors would like to acknowledge Casey Ramirez-Cortes and Erin Ross for their work in compiling and analyzing the data for this study.
Data Sharing Statement
Data reported in this manuscript are available within the article. Study-level data including the study protocol are available. To request access to the data, the researcher must sign a data use agreement. All proposals should be directed to Fogt.78@osu.edu for up to 36 months following article publication.
Disclosure
This study was sponsored by Allergan plc, Dublin, Ireland, and conducted by the Innovation in Vision and Eye Care Research Group (iVERG) at The Ohio State University College of Optometry. Financial arrangements of the authors with companies whose products may be related to the present report are listed below, as declared by the authors.
JS. Fogt has received research grants from Alcon, Allergan, Contamac, Innovega, Johnson & Johnson, Nevakar, and Paragon Vision Sciences, and consulting honoraria from Alcon and Valeant Pharmaceuticals. JT. Barr has received research grants from Alcon, Allergan, Contamac, Innovega, and Paragon Vision Sciences, consulting honoraria from Alcon, Contamac, Innovega, Lentechs, Proteris, Shire, and Valeant Pharmaceuticals, and grants from The Ohio State University. E. King-Smith has received research funding from Allergan. H. Liu is an employee of Allergan. N. Fogt reports grant from Allergan, during the conduct of the study.
Editorial assistance was provided to the authors by Evidence Scientific Solutions Inc, Philadelphia, PA, and was funded by Allergan plc, Dublin, Ireland. Neither honoraria nor payments were made for authorship. The authors report no other conflicts of interest in this work.
References
- 1.Sheppard AL, Wolffsohn JS. Digital eye strain: prevalence, measurement and amelioration. BMJ Open Ophthalmol. 2018;3(1):e000146. doi: 10.1136/bmjophth-2018-000146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.King-Smith PE. The evaporation barrier of the tear film lipid layer. Invest Ophthalmol Vis Sci. 2016;57(3):959. doi: 10.1167/iovs.16-19161 [DOI] [PubMed] [Google Scholar]
- 3.Nelson JD, Shimazaki J, Benitez-del-Castillo JM, et al. The international workshop on meibomian gland dysfunction: report of the definition and classification subcommittee. Invest Ophthalmol Vis Sci. 2011;52(4):1930–1937. doi: 10.1167/iovs.10-6997b [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Knop E, Knop N, Millar T, Obata H, Sullivan DA. The international workshop on meibomian gland dysfunction: report of the subcommittee on anatomy, physiology, and pathophysiology of the meibomian gland. Invest Ophthalmol Vis Sci. 2011;52(4):1938–1978. doi: 10.1167/iovs.10-6997c [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Moshirfar M, Pierson K, Hanamaikai K, Santiago-Caban L, Muthappan V, Passi SF. Artificial tears potpourri: a literature review. Clin Ophthalmol. 2014;8:1419–1433. doi: 10.2147/OPTH.S65263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Argiles M, Cardona G, Perez-Cabre E, Rodriguez M. Blink rate and incomplete blinks in six different controlled hard-copy and electronic reading conditions. Invest Ophthalmol Vis Sci. 2015;56(11):6679–6685. doi: 10.1167/iovs.15-16967 [DOI] [PubMed] [Google Scholar]
- 7.Fogt JS, Kowalski MJ, King-Smith PE, et al. Tear lipid layer thickness with eye drops in meibomian gland dysfunction. Clin Ophthalmol. 2016;10:2237–2243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Korb DR, Scaffidi RC, Greiner JV, et al. The effect of two novel lubricant eye drops on tear film lipid layer thickness in subjects with dry eye symptoms. Optom Vis Sci. 2005;82(7):594–601. doi: 10.1097/01.opx.0000171818.01353.8c [DOI] [PubMed] [Google Scholar]
- 9.Walter SD, Gronert K, McClellan AL, Levitt RC, Sarantopoulos KD, Galor A. ω-3 tear film lipids correlate with clinical measures of dry eye. Invest Ophthalmol Vis Sci. 2016;57(6):2472–2478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dry Eye Assessment and Management Study Research Group, Asbell PA, Maguire MG, et al. n-3 fatty acid supplementation for the treatment of dry eye disease. N Engl J Med. 2018;378(18):1681–1690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Giannaccare G, Pellegrini M, Sebastiani S, et al. Efficacy of omega-3 fatty acid supplementation for treatment of dry eye disease: a meta-analysis of randomized clinical trials. Cornea. 2019. doi: 10.1097/ICO.0000000000001884 [DOI] [PubMed] [Google Scholar]
- 12.JS KM F, King-Smith PE, Barr J. Interferometric measurements of the tear lipid layer thickness following instillation of lipid-containing eye drops and nonlipid-containing eye drops. Invest Ophthalmol Vis Sci. 2016;57. doi: 10.1167/iovs.16-19420 [DOI] [Google Scholar]
- 13.Braun RJ, King-Smith PE, Begley CG, Li L, Gewecke NR. Dynamics and function of the tear film in relation to the blink cycle. Prog Retin Eye Res. 2015;45:132–164. doi: 10.1016/j.preteyeres.2014.11.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.JS KM F, Fogt N, King-Smith PE, Barr J. Evaluation of lipid layer thickness of the tears over the course of a day. Invest Ophthalmol Vis Sci. 2017;58. doi: 10.1167/iovs.16-20610 [DOI] [Google Scholar]
- 15.Schaumberg DA, Gulati A, Mathers WD, et al. Development and validation of a short global dry eye symptom index. Ocul Surf. 2007;5(1):50–57. doi: 10.1016/S1542-0124(12)70053-8 [DOI] [PubMed] [Google Scholar]
- 16.Amparo F, Schaumberg DA, Dana R. Comparison of two questionnaires for dry eye symptom assessment: the ocular surface disease index and the symptom assessment in dry eye. Ophthalmology. 2015;122(7):1498–1503. doi: 10.1016/j.ophtha.2015.02.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ishak B, Thye J, Mohd Ali B, Mohidin N. Blinking characteristics and corneal staining in different soft lens materials. World Acad Sci Eng Technol. 2012;6(12):1662–1665. [Google Scholar]
- 18.Korb DR, Blackie CA, Finnemore VM, Douglass T. Effect of using a combination of lid wipes, eye drops, and omega-3 supplements on meibomian gland functionality in patients with lipid deficient/evaporative dry eye. Cornea. 2015;34(4):407–412. doi: 10.1097/ICO.0000000000000366 [DOI] [PubMed] [Google Scholar]
- 19.Korb DR. British Contact Lens Association. The Tear Film: Structure, Function, and Clinical Examination. Oxford: Butterworth-Heinemann; 2002. [Google Scholar]
- 20.Portello JK, Rosenfield M, Chu CA. Blink rate, incomplete blinks and computer vision syndrome. Optom Vis Sci. 2013;90(5):482–487. doi: 10.1097/OPX.0b013e31828f09a7 [DOI] [PubMed] [Google Scholar]
- 21.Tsubota K, Nakamori K. Dry eyes and video display terminals. N Engl J Med. 1993;328(8):584. doi: 10.1056/NEJM199302253280817 [DOI] [PubMed] [Google Scholar]
- 22.Tsubota K. Tear dynamics and dry eye. Prog Retin Eye Res. 1998;17(4):565–596. doi: 10.1016/S1350-9462(98)00004-4 [DOI] [PubMed] [Google Scholar]
- 23.Erdinest N, Shmueli O, Grossman Y, Ovadia H, Solomon A. Anti-inflammatory effects of alpha linolenic acid on human corneal epithelial cells. Invest Ophthalmol Vis Sci. 2012;53(8):4396–4406. doi: 10.1167/iovs.12-9724 [DOI] [PubMed] [Google Scholar]
- 24.Rashid S, Jin Y, Ecoiffier T, Barabino S, Schaumberg DA, Dana MR. Topical omega-3 and omega-6 fatty acids for treatment of dry eye. Arch Ophthalmol. 2008;126(2):219–225. doi: 10.1001/archophthalmol.2007.61 [DOI] [PubMed] [Google Scholar]
- 25.Li Z, Choi JH, Oh HJ, Park SH, Lee JB, Yoon KC. Effects of eye drops containing a mixture of omega-3 essential fatty acids and hyaluronic acid on the ocular surface in desiccating stress-induced murine dry eye. Current Eye Research. 2014;39(9):871–878. doi: 10.3109/02713683.2014.884595 [DOI] [PubMed] [Google Scholar]
- 26.Amalfitano C, Pasolini MP, Nieddu A, et al. The effect of periocular fatty acids and 0.15% hyaluronate eye drops application on keratoconjunctivitis sicca in dogs: an exploratory study. Top Companion Anim M. 2019;35:18–25. doi: 10.1053/j.tcam.2019.03.001 [DOI] [PubMed] [Google Scholar]
- 27.Downie LE, Gad A, Wong CY, et al. Modulating contact lens discomfort with anti-inflammatory approaches: a randomized controlled trial. Invest Ophthalmol Vis Sci. 2018;59(8):3755–3766. doi: 10.1167/iovs.18-24758 [DOI] [PubMed] [Google Scholar]
- 28.Hom MM, Berdy GJ, Downie LE, et al. Clinical evaluation of a novel lipid-containing lubricant eye drop with omega-3 oil and trehalose. Invest Ophthalmol Vis Sci. 2017;58(8):2671. doi: 10.1167/iovs.16-20610 [DOI] [Google Scholar]
- 29.Calder PC. N-3 polyunsaturated fatty acids and inflammation: from molecular biology to the clinic. Lipids. 2003;38(4):343–352. doi: 10.1007/s11745-003-1068-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Miljanović B, Trivedi KA, Dana MR, Gilbard JP, Buring JE, Schaumberg DA. Relation between dietary n-3 and n-6 fatty acids and clinically diagnosed dry eye syndrome in women. Am J Clin Nutr. 2005;82(4):887–893. doi: 10.1093/ajcn/82.4.887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Deinema LA, Vingrys AJ, Wong CY, Jackson DC, Chinnery HR, Downie LE. A randomized, double-masked, placebo-controlled clinical trial of two forms of omega-3 supplements for treating dry eye disease. Ophthalmology. 2017;124(1):43–52. doi: 10.1016/j.ophtha.2016.09.023 [DOI] [PubMed] [Google Scholar]
- 32.Wojtowicz JC, Butovich I, Uchiyama E, Aronowicz J, Agee S, McCulley JP. Pilot, prospective, randomized, double-masked, placebo-controlled clinical trial of an omega-3 supplement for dry eye. Cornea. 2011;30(3):308–314. doi: 10.1097/ICO.0b013e3181f22e03 [DOI] [PubMed] [Google Scholar]
- 33.Sheppard JD Jr., Singh R, McClellan AJ, et al. Long-term supplementation with n-6 and n-3 PUFAs improves moderate-to-severe keratoconjunctivitis sicca: a randomized double-blind clinical trial. Cornea. 2013;32(10):1297–1304. doi: 10.1097/ICO.0b013e318299549c [DOI] [PubMed] [Google Scholar]
- 34.Bradley JL, Özer Stillman I, Pivneva I, Guerin A, Evans AM, Dana R. Dry eye disease ranking among common reasons for seeking eye care in a large US claims database. Clin Ophthalmol (Auckland, NZ). 2019;13:225–232. doi: 10.2147/OPTH.S188314 [DOI] [PMC free article] [PubMed] [Google Scholar]