Abstract
Phthalates have a history of reproductive toxicity in animal models and associations with adverse reproductive outcomes in women. Human exposure to dibutyl phthalate (DBP) occurs via consumer products (7–10 μg/kg/day) and medications (1–233 μg/kg/day). Most DBP toxicity studies have focused on high supraphysiological exposure levels; thus, very little is known about exposures occurring at environmentally relevant levels. CD-1 female mice (80 days old) were treated with tocopherol-stripped corn oil (vehicle control) or DBP dissolved in oil at environmentally relevant (10 and 100 μg/kg/day) or higher (1000 μg/kg/day) levels for 30 days to evaluate effects on DNA damage response (DDR) pathway genes and folliculogenesis. DBP exposure caused dose-dependent effects on folliculogenesis and gene expression. Specifically, animals exposed to the high dose of DBP had more atretic follicles in their ovaries, while in those treated with environmentally relevant doses, follicle numbers were no different from vehicle-treated controls. DBP exposure significantly reduced the expression of DDR genes including those involved in homologous recombination (Atm, Brca1, Mre11a, Rad50), mismatch repair (Msh3, Msh6), and nucleotide excision repair (Xpc, Pcna) in a dose-specific manner. Interestingly, staining for the DNA damage marker, γH2AX, was similar between treatments. DBP exposure did not result in differential DNA methylation in the Brca1 promoter but significantly reduced transcript levels for the maintenance DNA methyltransferase, Dnmt1, in the ovary. Collectively, these findings show that oral exposure to environmentally relevant levels of DBP for 30 days does not significantly impact folliculogenesis in adult mice but leads to aberrant ovarian expression of DDR genes.
Keywords: atresia, cell cycle, endocrine disruptors, environmental contaminants and toxicants, follicle, follicle development, follicle maturation, gene expression, ovary, rodents, toxicology, phthalate
Summary Sentence Exposure to human relevant doses of dibutyl phthalate results in significant disruption of DNA damage repair gene expression in the mouse ovary.
Introduction
In 2010, it was estimated that 48.5 million couples worldwide and 6.7 million women in the United States were unable to achieve pregnancy or carry a pregnancy to term [1]. Phthalates are a family of endocrine-disrupting chemicals (EDCs) with a history of reproductive and developmental toxicities in animal models (reviewed in [2, 3]). Phthalate exposure in women has been confirmed by detection of their metabolites in urine and follicular fluid [4]. In women, increased urinary phthalate burden has been associated with various adverse reproductive outcomes, which include early menopause [5], decreased hormone levels [6], early pregnancy loss [7, 8], decreased oocyte yield during IVF and low rate of clinical pregnancies and live births following assisted reproduction [9, 10], and low antral follicle counts [11]. Despite this valuable information, the mechanisms involved in these associations are still not understood.
Dibutyl phthalate (DBP) is an EDC commonly used in and able to leach from consumer products such as personal care products and plastic-coated medications. Based on urinary burden data, it is estimated that greater than 95% of the general population is exposed to DBP and that women of reproductive age tend to have higher burden than men [12]. Daily intake estimates for DBP in humans have been reported to range between 7 and 10 μg/kg/day in the general population [13, 14] and 1–233 μg/kg/day in individuals taking oral medications coated with DBP-containing plastic [15, 16]. The reproductive and developmental toxicities of DBP have been reported previously by an expert panel [14]; however, most studies used high doses that do not compare to human exposure estimates. Thus, there is still a significant gap in our knowledge about the effects of DBP exposure at environmentally relevant levels. In studies that include classical supraphysiological and environmentally relevant exposure levels, we have identified the process of folliculogenesis as a potential target for DBP action in the ovary. Specifically, we have shown that DBP exposure results in inhibition of antral follicle growth in vitro [17, 18] and in decreased antral follicle numbers in juvenile mice treated in vivo [19]. These studies have also identified pathways with important roles in the regulation of cell cycle, cell cycle arrest, and apoptosis as altered in response to DBP exposures in isolated antral follicles [17, 18] and whole ovary [19]. Specifically, in in vitro exposed antral follicles, DBP has been shown to inhibit growth, trigger follicle atresia, and disrupt cell cycle and apoptosis gene expression in a manner consistent with activation of cell cycle arrest followed by apoptosis [17, 18].
DNA damage can be lethal to the cell; thus, its detection within a cell results in the activation of a series of molecular events known as the DNA damage response (DDR; reviewed in [20]). Through various checkpoints during the cell cycle, DDR can halt the cell cycle progression, repair the DNA, and, if necessary, trigger apoptosis to prevent transmission of mutations due to DNA lesions. DDR is possible through the specialized functions of sensor proteins that recognize DNA damage, transducer proteins responsible for recruiting downstream effector proteins, and effector proteins that regulate cell cycle arrest, transcription and DNA repair, and apoptosis (reviewed in [21]). To date, six DDR mechanisms have been described including base excision repair, mismatch repair (MMR), nucleotide excision repair (NER), translesion DNA synthesis, nonhomologous end joining, and homologous recombination (HR; reviewed in [20, 21]). Deficiencies in the expression and activation of factors in these pathways render the cell defenseless in the face of DNA damage.
In the ovary, losing the ability to respond to DNA damage could lead to follicle loss and early menopause [22]. In fact, the ability of agents used during radio- and chemotherapy to cause ovarian DNA damage, trigger ovarian DDR responses, and cause follicle loss has been documented previously (reviewed in [23]). Most recently, the ability of metabolites of cyclophosphamide and the endocrine disruptor, bisphenol A, have been shown to cause ovarian follicle loss via modulation of the DDR signaling pathway in the ovary [24, 25]. Unfortunately, no studies have investigated whether exposures to phthalates interfere with DDR in the ovary.
As a first step toward eliminating this gap in our knowledge, the objective of this study was to test whether a subacute exposure to DBP resulted in (1) disrupted expression of DDR signaling pathway members; (2) increased accumulation of a DNA damage marker; and (3) alterations in follicle numbers. To accomplish this goal, we exposed adult mice to environmentally relevant levels of DBP for 30 consecutive days and used whole ovary samples to (1) measure differential expression of DDR-related genes; (2) assess accumulation of γH2AX foci in ovarian sections; and (3) determine alterations in ovarian follicle populations.
Materials and methods
All investigations using experimental animals were conducted in accordance with the Society for the Study of Reproduction’s specific guidelines and standards.
Chemicals
Dibutyl phthalate (DBP, CAS # 84-74-2; 99.6% purity) was obtained from Sigma-Aldrich (St. Louis, MO), and tocopherol-stripped corn oil (vehicle for DBP, CAS# 8001-30-7) was obtained from MP Biomedicals (Solon, OH). Antibody Research Resource Identifier (RRID) information is included in Supplementary Table 1.
Animals
Female CD-1 mice (age: 60 days old, n = 32) were obtained from Charles River Laboratories (Charles River, CA) and housed four per cage in single-use BPA and phthalate-free Econo-Cage Disposable Rodent Caging (Lab Products Inc., Seaford, DE, USA) at the University of Arizona Central Animal Facility with food and water provided ad libitum. Temperature was maintained at 22 ± 1 °C, and animals were subjected to 12 L:12D cycles. After arrival to our animal facility, mice acclimated to the facilities for at least 24 h before being handled. Estrous cycles and weight gain were monitored by daily vaginal smear readings and weighing for 3 weeks prior to the start of oral dosing. No differences in weight gain were observed during this predosing period. At the end of dosing, mice were euthanized by decapitation under isoflurane anesthesia. Major organs were excised and trimmed prior to weighing. All experiments and methods involving animals were approved by the University of Arizona Institutional Animal Care and Use Committee and conformed to the Guide for the Care and Use of Experimental Animals [26].
Dibutyl phthalate dosing
At the end of the 3-week predosing cyclicity assessment, normally cycling mice (n = 30; ~80 days old) were randomly assigned to receive tocopherol-stripped corn oil (vehicle, n = 7) or DBP dissolved in vehicle at 10 or 100 μg/kg/day (n = 8 each). An additional group of mice (n = 7) was treated with DBP at a higher dose for comparison (10-fold higher; 1000 μg/kg/day). Doses were selected to approximate estimates of daily exposure reported in humans via oral ingestion (7–10 μg/kg/day; [14]) and medication exposure (1–233 μg/kg/day; [16]). Animals were weighed, smeared, and dosed daily for 30 consecutive days. As described previously [19], all doses were administered orally by pipetting the dosing solution into the mouth past the incisors and into the cheek pouch.
Tissue collection
At the end of dosing, both ovaries from each mouse were excised and processed for subsequent analyses. Specifically, one ovary from each mouse was cut in half and placed in either formalin or Bouin’s fixative for subsequent immunofluorescent or hematoxylin and eosin (H&E) staining, respectively. The contralateral ovary was also cut in half and snap frozen in liquid nitrogen and stored at −80 °C for subsequent RNA/DNA and protein extraction, respectively. Tissues were collected as animals reached the stage of diestrus after 30–32 days of dosing. Only tissues from mice in diestrus at euthanasia were used for subsequent analyses to maintain stage of the estrous cycle constant.
Estrous cyclicity
The estrous cycles of each animal were monitored for 3 weeks by vaginal cytology prior to being added to the study. Vaginal smears were collected and interpreted using criteria previously described with minor modifications [19, 27, 28]. Briefly, vaginal washings were performed by restraining animals and carefully pipetting 20 μL of sterile-filtered phosphate-buffered saline (PBS) into the vaginal opening. Washings were placed in 96-well plates and observed unstained under an inverted microscope. A second individual evaluated digital images of a subset of the smears (~10–20% of all washings). All smears, fresh and digital, were evaluated without knowledge of treatment by experienced staff. The cyclicity pattern of an animal was considered abnormal if the animal showed at least one of the following: no cycles (no change in stage), extended estrus (>4 days), or extended diestrus (>6 days) during the prestudy period. Two animals were identified as having abnormal cycles based on these criteria and were, thus, excluded from the study.
Classification and enumeration of ovarian follicles and corpora lutea
Ovarian samples were prepared for classification and enumeration of ovarian follicles and corpora lutea (CL) as previously described [19, 28]. Briefly, one-half ovary per animal was fixed in Bouin’s solution (2 h), transferred to 70% ethanol, and embedded in paraffin. Paraffin-embedded ovarian fragments were serially sectioned at 5 μm thickness, mounted on glass slides, and processed for H&E staining. Oocyte containing follicles and CL were counted on every 20th section without knowledge of treatment. Follicles were classified as atretic using previously published criteria [29], which included having at least one of the following: (1) follicular cell pyknosis, (2) granulosa/theca cell layer disorganization, (3) oocyte fragmentation, and (4) theca cell hypertrophy. Healthy follicles were further classified by stage using criteria previously published [19, 28]. Specifically, follicles were classified as primordial if they consisted of a single oocyte surrounded by a single layer of squamous granulosa cells, primary if the oocyte was surrounded by a single layer comprised of ≥50% cuboidal granulosa cells, secondary if the oocyte was surrounded by two or more layers of cuboidal granulosa cells and a theca layer, and antral if the oocyte was surrounded by multiple layers of cuboidal granulosa cells, theca cells, and contained an antrum. Follicle counts are presented as percentages from total counted follicles per animal to account for differences in ovarian fragment size due to the halving of each ovary at the time of collection. Follicle percentages were used when making statistical comparisons between treatment groups. CL counts were divided by the number of sections counted.
RNA extraction and cDNA synthesis
Total RNA and DNA were extracted from individual ovarian samples (n = 4–5 mice per treatment) using AllPrep DNA/RNA Mini kits (Qiagen, Valencia, CA) with a DNAse treatment (Qiagen; 15 min) step to eliminate potential genomic DNA contamination from the RNA extracts. RNA concentration was determined at 260 nm using a Synergy H1m microplate reader equipped with a Take3 micro-volume plate (Biotek, Winooski, VT). RNA samples (1 μg) were reverse transcribed using an iScript cDNA synthesis kit (Bio-Rad, Hercules, CA). Each cDNA sample was diluted 1:5 with nuclease-free water prior to analysis.
Standard real-time PCR
All standard qPCR reactions were done in triplicate and contained 1 μL of cDNA, 1 μL of gene-specific primers (500 nM; Integrated DNA Technologies, Coralville, IA), 3 μL of nuclease-free water, and 5 μL of SsoFast EvaGreen Supermix (Bio-Rad) for a final volume of 10 μL per reaction. Primers for Dnmt1, as well as the reference genes, β-actin (Actb), glyceraldehyde 3-phosphate dehydrogenase (Gapdh), and TATA-box binding protein (Tbp) were purchased premade as PrimePCR qPCR primer assays from Bio-Rad (Dnmt1:qMmuCED0044266) or from Integrated DNA Technologies (Actb: Mm.PT.58.33540333, Gapdh: Mm.PT.39a.1, Tbp: Mm.PT.58.10867035).
qPCR arrays
Pathway-specific gene expression was determined using Bio-Rad PrimePCR arrays (Mouse Brca1 and Brca2 in DNA Repair, Cat # 10029171). Specifically, qPCR reactions were set up in 96-well qPCR plates preloaded with lyophilized pathway-specific primers. Each reaction consisted of 1 μL of cDNA, 9 μL of nuclease-free water, and 10 μL of SsoFast EvaGreen Supermix for a final volume of 20 μL. Each array included 24 genes of interest, 3 reference genes, and 5 assay controls (genomic DNA, PCR control, RT control, and two RNA quality controls).
Real-time PCR data analysis
All qPCR experiments (standard and array formats) were completed using a CFX Connect Real-time System (Bio-Rad) using manufacturer-recommended qPCR programs. Expression data were generated using the Gene Study function of Bio-Rad’s CFX Manager Software, which utilizes the ΔΔCt mathematical model for relative quantification of real-time PCR data. Reported data consist of mean relative mRNA expression ratios from four to five separate ovarian samples per treatment.
Immunostaining for phosphorylated H2AX
One-half ovary per animal was fixed in formalin (2 h), transferred to 70% ethanol, and embedded in paraffin. Paraffin-embedded ovarian samples were serially sectioned at 5 μm thickness, mounted on glass slides, and processed for immunostaining. After air drying, the sections were deparaffinized in xylene, followed by rehydration through 100–70% ethanol. Antigen was unmasked by immersing tissue sections in preheated 1X eBioscience IHC Antigen Retrieval Solution (Invitrogen, Madison, WI) and steaming for 20 min prior to cooling in hot solution for 30 min at room temperature with the container lid open. After washing with ddH2O, sections were blocked in 1% BSA/1X PBS for 1 h at room temperature prior to incubation with rabbit antigamma H2AX (phosphor S139; Cat# ab26350) polyclonal IgG (1:250 in 1% BSA/0.3% Triton X-100/1X PBS, Abcam, Cambridge, MA) overnight at 4 °C. The antibody control sections (negative controls) were incubated in antibody dilution solution only. After antibody incubation, tissue sections were subjected to three PBS washes and one ddH2O wash followed by dehydration through 70–100% ethanol. Sections were then mounted with VECTASHIELD mounting medium with DAPI (Vector Laboratories, Burlingame, CA). Photographs with 200× magnification were taken using a Leica CTR 550 fluorescent microscope with camera. Individual sections of each imaged ovary were subjected to quantification of p-H2AX staining using the opensource Python-based program, Foci Counter 1.0 (http://focicounter.sourceforge.net/index.html). In total, 9–12 sections per ovary were quantified and the total p-H2AX brightness of each section calculated by dividing the foci brightness over the total brightness of the section. Additionally, images were manually analyzed by quantifying the number of positive small follicles, antral follicles, and CL and dividing each number by the total number observed of each to generate a percentage. An ovarian feature was considered positive for p-H2AX when at least one p-H2AX foci was observed.
Protein extraction
Total protein was extracted from one-half ovary per mouse (n = 3 mice per treatment). Ovary samples were homogenized in 200 μL T-PER reagent (lysis buffer, Thermo Scientific, Rockford, IL) containing protease inhibitors (cOmplete Mini Protease Inhibitor Cocktail, Roche, Brandford, CT) using a variable-speed Tissue Tearor homogenizer. As recommended by manufacturer, ovary lysates were subjected to three freeze/thaw cycles (10 min at −80 °C then thawed on ice) followed by centrifugation (10,000× g for 5 min). The supernatant was collected, and protein concentration determined using a Pierce BCA Protein Assay kit (Thermo Scientific) following manufacturer instructions.
SDS-PAGE and Western blotting
Protein samples (10 μg) were denatured at 95 °C for 5 min in 1X Laemmli Sample Buffer (Bio-Rad) and loaded on a Bio-Rad mini-protein TGX stain-free gel using 1X Tris/Glycine/SDS (Bio-Rad) as the running buffer. The protein samples, including molecular weight markers (Precision Plus protein Dual Color Standards, Bio-Rad), were separated at 70 V for 30 min followed by 100 V for 45 min. The stain-free gel was then UV activated for 1 min and imaged in a Bio-Rad ChemiDoc XRS+ Imaging System equipped with Image Lab software. The intensity of total lane protein in each sample was determined, recorded, and later used as a loading control for normalization of the blot.
After imaging, the gel was briefly rinsed in ddH2O and equilibrated for 15 min in transfer buffer (1X Tris/Glycine, Bio-Rad) followed by transfer to a nitrocellulose membrane at 100 V for 1 h at 4 °C. Following transfer, the membrane was blocked in blocking buffer (5% nonfat dry milk in 1X TTBS buffer, Bio-Rad) for 1 h and then incubated overnight at 4 °C with anti-BRCA1 (C-20) rabbit IgG solution (1:200 in blocking buffer, Cat # sc-642, Santa Cruz Biotechnology, Dallas, TX) to detect the expression of BRCA1 protein. After three 10 min washes in 1X TTBS, the membrane was incubated with goat antirabbit IgG-HRP solution (1:5,000 in blocking buffer, Cat # sc-2004, Santa Cruz Biotechnology) for 1 h at room temperature. The membrane was then washed with TTBS and detected using a SuperSignal West Femto Maximum Sensitivity Substrate kit (Thermo Scientific). Imaging of the chemiluminescent blot was performed using a Bio-Rad ChemiDoc XRS+ Imaging System equipped with Image Lab software following manufacturer instructions. The molecular weight and the intensity of each band were determined by the Image Lab software. The total protein intensity detected in the stain-free gel was used for normalization of the blot, and the BRCA1 protein expression in DBP-treated samples relative to vehicle control samples was calculated.
Quantitative analysis of DNA methylation
Analysis of DNA methylation was performed by the University of Arizona Genetics Core (http://uagc.arl.arizona.edu) using EpiTYPER assays on a Sequenom’s MassARRAY system as previously described [30, 31]. Based on previous studies evaluating methylation in the Brca1 promoter region, we selected the region spanning −700 to 299 as the region of interest in this study [32]. Genomic DNA extracted from whole ovaries using AllPrep DNA/RNA Mini kits (Qiagen) was subjected to bisulfite treatment using the EpiTect Bisulfite kit (Qiagen). The promoter region of interest was amplified using primers specific to quantitative mass spectrometric analysis using EpiTYPER technology (Sequenom, San Diego, CA). Amplified DNA was transcribed in vitro, cleaved using RNAse A, and subjected to Matrix-Assisted Laser Desorption/Ionization Time-of-Flight Mass Spectrometry (MALDI-TOF MS) analysis. DNA methylation standards (0–100%) were used to control for PCR amplification bias.
Statistical analysis
All non-qPCR data were analyzed using SPSS Statistics 25 software (IBM Chicago, IL). As described in the “Real-time PCR Data Analysis” section, all qPCR data were analyzed using Bio-Rad’s CFX Manager software. Prior to statistical analysis, non-qPCR data were subjected to normality and homogeneity of variance tests to best determine appropriate statistical tests. For data meeting the necessary assumptions, comparisons between treatment groups were done by ANOVA followed by Dunnett’s post hoc test with vehicle set as control. Data not meeting the assumptions for ANOVA were subjected to Kruskal-Wallis nonparametric test followed by Mann-Whitney’s post hoc tests as applicable. For all comparisons, statistical significance was assigned at p ≤ 0.05.
Results
Effect of DBP dosing on body and organ weights
Exposure to DBP did not cause overt toxicity as demonstrated by normal behavior, physical appearance, and no changes in the normalized weights of major organs (Supplementary Table 2). Mice treated with DBP at 100 and 1000 μg/kg/day experienced increased weight gain during the dosing period (Supplementary Figure 1A), but the p-values obtained for these comparisons did not reach statistical significance (ANOVA p = 0.1).
Effects of DBP dosing on estrous cyclicity
To determine whether repeated daily dosing with DBP for 30 days disrupts estrous cyclicity in adult mice, we compared the percentage of study time that each animal spent on each stage of the mouse estrous cycle (proestrus, estrus, and diestrus) between treatments. There were no differences in the percentage of time spent in proestrus, estrus, and diestrus among all treatment groups during the predosing and dosing periods (Supplementary Figure 1B).
Effect of DBP exposure on DNA repair gene expression
We used a commercially available mouse-specific DNA damage and repair qPCR array to determine whether DBP exposure caused differential expression of key DDR genes in the ovary. A complete list of the genes tested including their expression data and p-values is included in Supplementary Tables 3–4. Using this approach, we identified 11 genes whose expression in the ovary was significantly altered by DBP exposure. Among these genes, we noticed that the gene expression data produced three dose-specific groups of genes. Using this information, we grouped the genes into those that were sensitive to (1) all exposure levels, (2) middle and highest levels, and (3) highest dose only. Two genes, Brca1 and Brca2, were sensitive to DBP treatment irrespective of the exposure level (Figure 1A). Additional genes were sensitive to DBP exposure at the middle and highest doses including Atm, Ddb2, Msh3, Msh6, and Rad50 (Figure 1B). Finally, four genes were only altered when mice were exposed to the highest dose of DBP used in this study and included Mre11a, Pcna, Trp53b1, and Xpc (Figure 1B).
Figure 1.
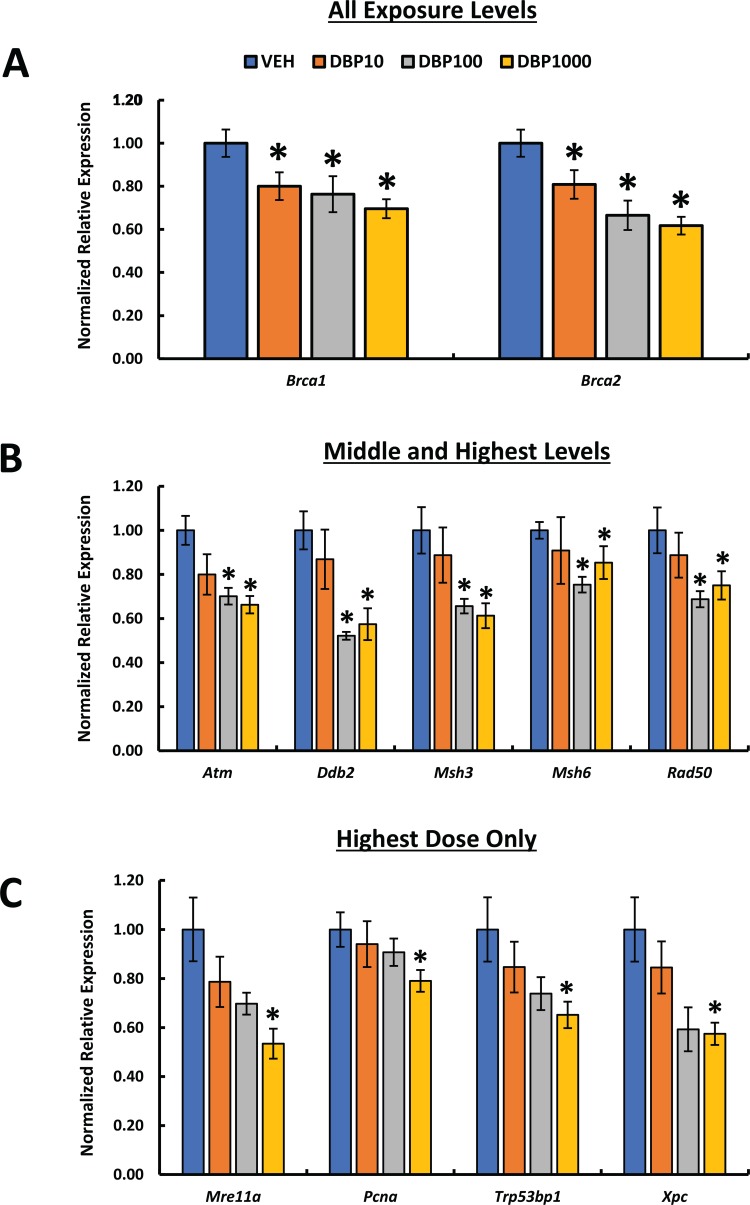
Effect of oral DBP exposure on ovarian DDR gene expression. Adult cycling female CD-1 mice were treated daily with vehicle or DBP and their ovaries processed for qPCR as described in Materials and Methods. Gene expression data were normalized to housekeeping gene expression and presented as mean normalized relative expression ± SEM. Expression data are grouped based on dose-specific effects into changes in response to all exposure levels (A), middle and highest doses (B), and highest dose only (C). Asterisks (*) indicate statistical differences versus vehicle (p ≤ 0.05; n = 5 mice/treatment).
Effect of DBP exposure on γ-H2AX staining
Double-stranded breaks (DSBs) resulting from a DNA-damaging insult lead to the phosphorylation of histone H2A variant H2AX, thus generating H2AX that has been phosphorylated at Ser 139 (p-H2AX). Detection of this molecule has been used to examine DNA damage as a sensitive marker of this process. We tested whether the presence of p-H2AX foci in the ovaries of DBP-treated mice differed from that in vehicle controls (Figures 2–4). Immunofluorescent staining of ovarian sections for p-H2AX showed punctate staining that was rare in follicles, except for the theca cells of some large follicles, but abundant in the interstitium and CL of each treatment group. Among all ovarian structures, CL and interstitium had the greatest frequency of pH2AX staining in all treatment groups, and this pattern was not affected by DBP exposure (Figure 2 and Supplementary Table 5). Similarly, the level of p-H2AX staining in the theca of some large follicles of DBP-treated mice did not differ from that observed in vehicle controls (Figure 3 and Supplementary Table 5). To determine the overall level of p-H2AX in the ovarian sections, we marked p-H2AX foci in each image per ovary and measured their brightness related to the whole ovarian section. There was no statistically significant difference in p-H2AX brightness between the treatments. Interestingly, a small trend for decreased p-H2AX brightness was observed in the ovaries of mice treated with DBP at 10 μg/kg/day (Figure 4). Finally, we reviewed our qPCR array data for the expression of H2afx transcript and observed that it was not affected by any of the DBP treatments (Supplementary Table 3).
Figure 2.
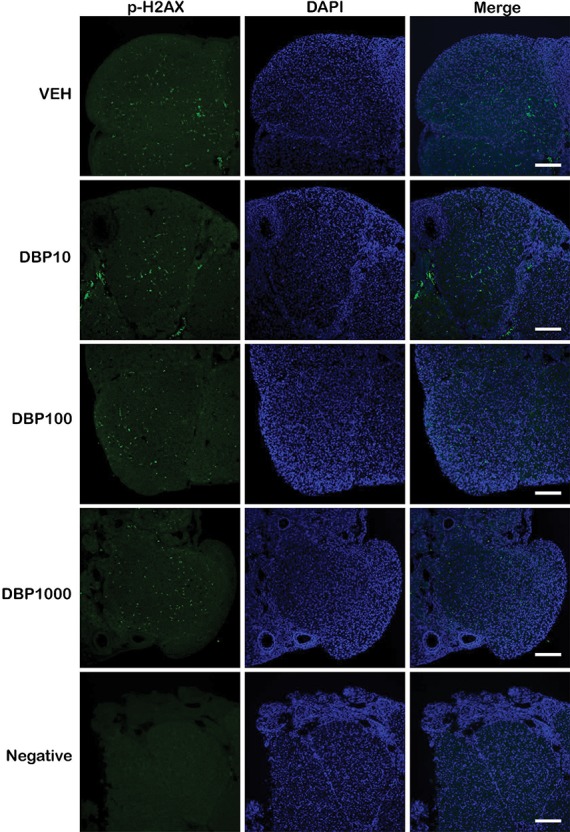
Effect of oral DBP exposure on corpus luteum p-H2AX. Adult cycling female CD-1 mice were treated daily with vehicle or DBP and their ovaries processed for immunofluorescence as described in Materials and Methods. Representative images (from n = 3 mice per treatment) show p-H2AX (green), DAPI (blue), or merged staining in CL of mice treated with vehicle control (VEH), 10 μg/kg/day (DBP10), 100 μg/kg/day (DBP100), 1000 μg/kg/day (DBP1000), or a no primary antibody negative control (negative). Images were taken at 200× magnification. Scale bar represents 100 μm.
Figure 4.
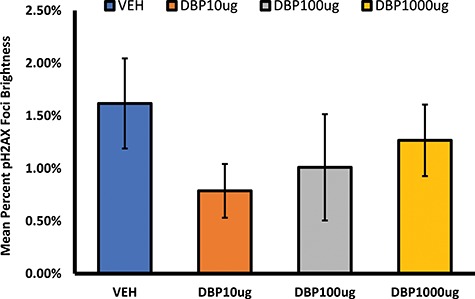
Effect of oral DBP exposure on overall p-H2AX staining. Adult cycling female CD-1 mice were treated daily with vehicle or DBP and their ovaries processed for immunofluorescent staining and image analysis as described in Materials and Methods. Percent brightness from p-H2AX foci data are presented as mean ± SEM.
Figure 3.
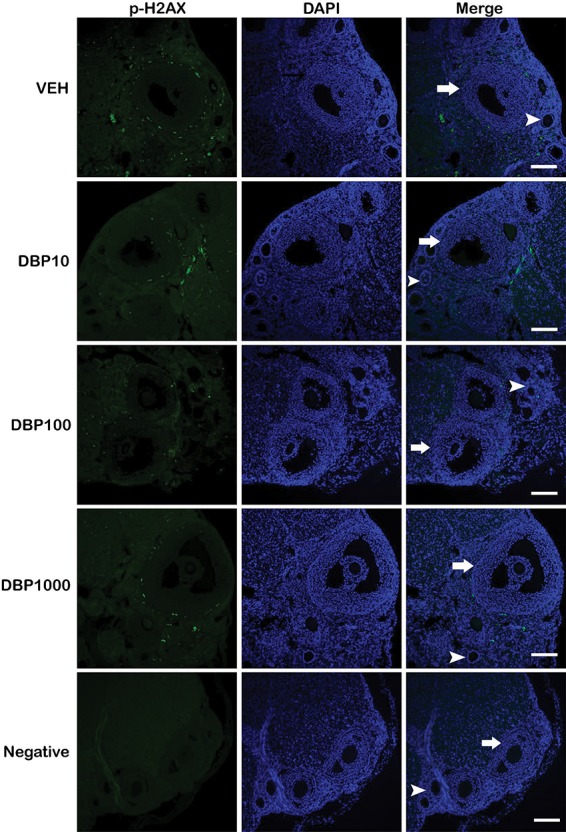
Effect of oral DBP exposure on ovarian follicle p-H2AX. Adult cycling female CD-1 mice were treated daily with vehicle or DBP and their ovaries processed for immunofluorescence as described in Materials and Methods. Representative images (from n = 3 mice per treatment) show p-H2AX (green), DAPI (blue), or merged staining in ovarian follicles of mice treated with vehicle control (VEH), 10 μg/kg/day (DBP10), 100 μg/kg/day (DBP100), 1000 μg/kg/day (DBP1000), or a no primary antibody negative control (negative). Images were taken at 200× magnification. Scale bar represents 100 μm. Arrows show large antral follicles, and arrowheads point at small follicles.
Effect of DBP exposure on ovarian BRCA1 protein
Based on its sensitivity to DBP exposure in this study and its importance in fertility and ovarian aging [33, 34], we became interested in further understanding the consequences of DBP-induced down-regulation of the Brca1 gene in the mouse ovary. Therefore, ovary protein samples were subjected to western blotting to determine the effect of DBP exposure on levels of BRCA1 protein in the ovary. In agreement with our gene expression data, we observed that the ovaries of mice treated with DBP for 30 days had significantly lower BRCA1 protein levels than vehicle-treated controls (Figure 5).
Figure 5.
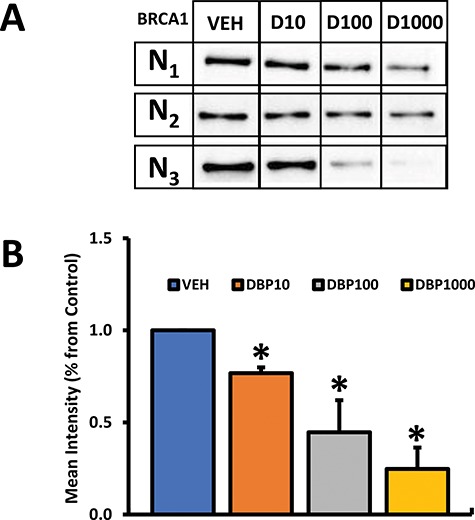
Effect of oral DBP exposure on ovarian BRCA1 protein levels. Adult cycling female CD-1 mice were treated daily with vehicle or DBP and their ovaries processed for western blotting as described in Materials and Methods. Panel A shows resulting blots with BRCA1 staining (N1–N3 indicate individual ovarian protein samples for each treatment group), while panel B shows resulting densitometry data obtained by averaging the intensity of individual bands in panel A. Protein expression data are presented as mean normalized relative expression ± SEM (panel B). Data were compared between treatments using Kruskal-Wallis nonparametric followed by Mann-Whitney post hoc test. Asterisks (*) indicate statistical differences versus vehicle (p ≤ 0.05; n = 3 mice/treatment).
Effect of DBP exposure on BRCA1 promoter methylation
Because both mRNA and protein were decreased, we became interested in understanding how DBP exposure could affect Brca1 expression in the ovary of treated mice. To do so, we subjected DNA samples to DNA methylation analysis using EpiTYPER assays. The EpiTYPER assay allows for detection and quantitative analysis of DNA methylation using base-specific cleavage and MALDI-TOF MS. Base-specific cleavage generated 28 cleavage products of which 12 were considered to represent single CpGs (potential methylation sites) and were analyzed. When considered together, overall methylation in all 12 cleavage products was not significantly changed by DBP exposure (Figure 6); however, the fragment labeled CpG6 showed a nonstatistically significant (p > 0.05) trend for increased methylation (Figure 6A).
Figure 6.
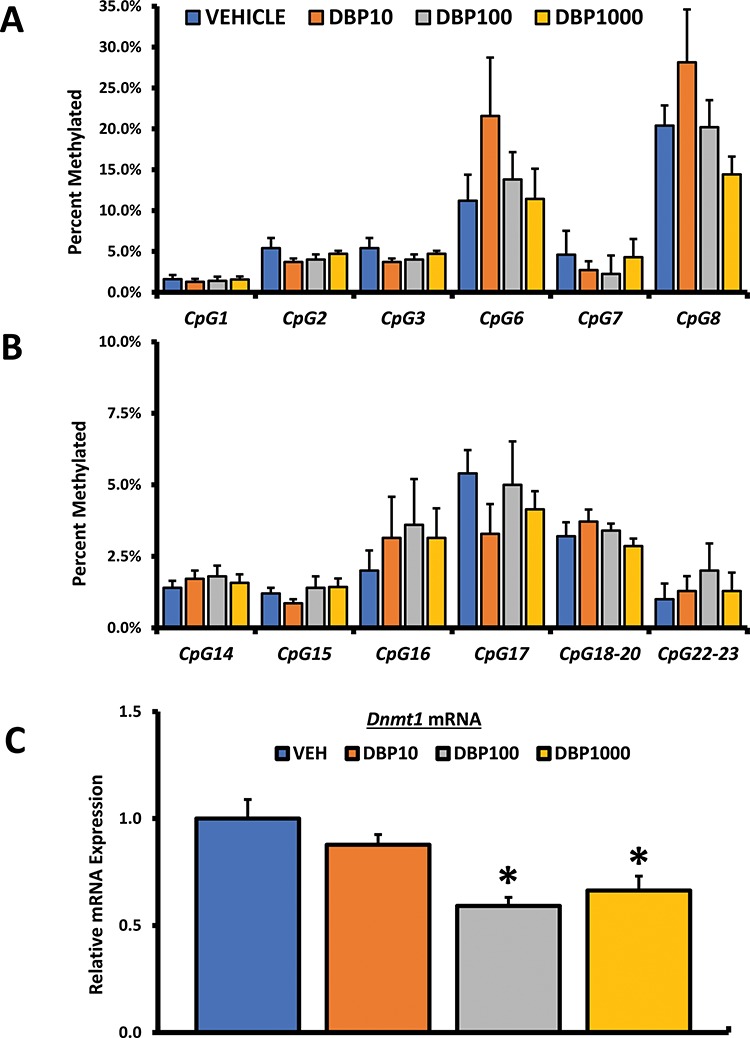
Effect of oral DBP exposure on Brca1 promoter methylation. Adult cycling female CD-1 mice were treated daily with vehicle or DBP and their ovaries processed for DNA methylation or qPCR analysis as described in Materials and Methods. Methylation data are presented as mean percent methylated ± SEM. Panel A shows methylation data for the cleavage sites CpG1 to 3 and CpG6 to 8, while panel B shows data for sites CpG14 to 18–20 and CpG22–23. Panel C shows mean relative expression for the DNA methyltransferase Dnmt1. Data were compared between treatments using Kruskal-Wallis nonparametric followed by Mann-Whitney post hoc test with significance set at p ≤ 0.05 (n = 5–7 mice per treatment).
Effect of DBP exposure on ovarian DNA methyltransferase expression
Although increased methylation in fragment CpG6 did not achieve statistical significance, it sparked our interest in investigating whether DBP exposure affects the expression DNA methyltransferases in the ovary. We focused on testing the effect of DBP on mRNA levels of Dnmt1, an abundant methyltransferase involved in the maintenance of DNA methylation in mammals. Real-time PCR analysis of Dnmt1 revealed that the ovaries from mice treated with DBP at 100 and 1000 μg/kg/day expressed significantly lower levels of this enzyme (Figure 6C).
Effect of DBP exposure on ovarian follicle numbers
Acute exposure (10 days) to DBP caused differences in the number of antral follicles in a previous study using juvenile mice (35 days old at dosing; [19]); thus, we were interested in expanding our understanding of the effects of DBP on follicular survival and dynamics in our cohort of adult mice (dosing started at ~80 days old) subjected to a longer exposure (30 days). First, we tested whether DBP exposure resulted in alterations in the balance between healthy and atretic follicles in the ovary. The percentage of healthy and atretic follicles in the ovaries of mice treated with environmentally relevant levels of DBP was not different from that observed in control mouse ovaries. However, exposure to the classical high dose of DBP (1000 mg/kg/day) resulted in a statistically significant increase in atretic follicles in the ovary of treated mice (Figure 7A). A corresponding decrease in healthy follicles was also observed in the ovaries of these mice. Healthy follicles were further classified into different developmental stages to test whether DBP exposure affected follicular dynamics. No differences in the percentage of follicles at the secondary (Figure 7B) and antral stages (Figure 7C) of development were observed between controls and DBP-treated ovaries. A slight alteration in the balance between primordial and primary follicles was observed in the ovaries of mice treated with the general population exposure level of DBP (10 μg/kg/day), but this difference in follicle percentages was not statistically significant (Figure 7B). Finally, the number of CL present in the ovaries of treated mice did not differ between vehicle and DBP treatment groups (data not shown).
Figure 7.
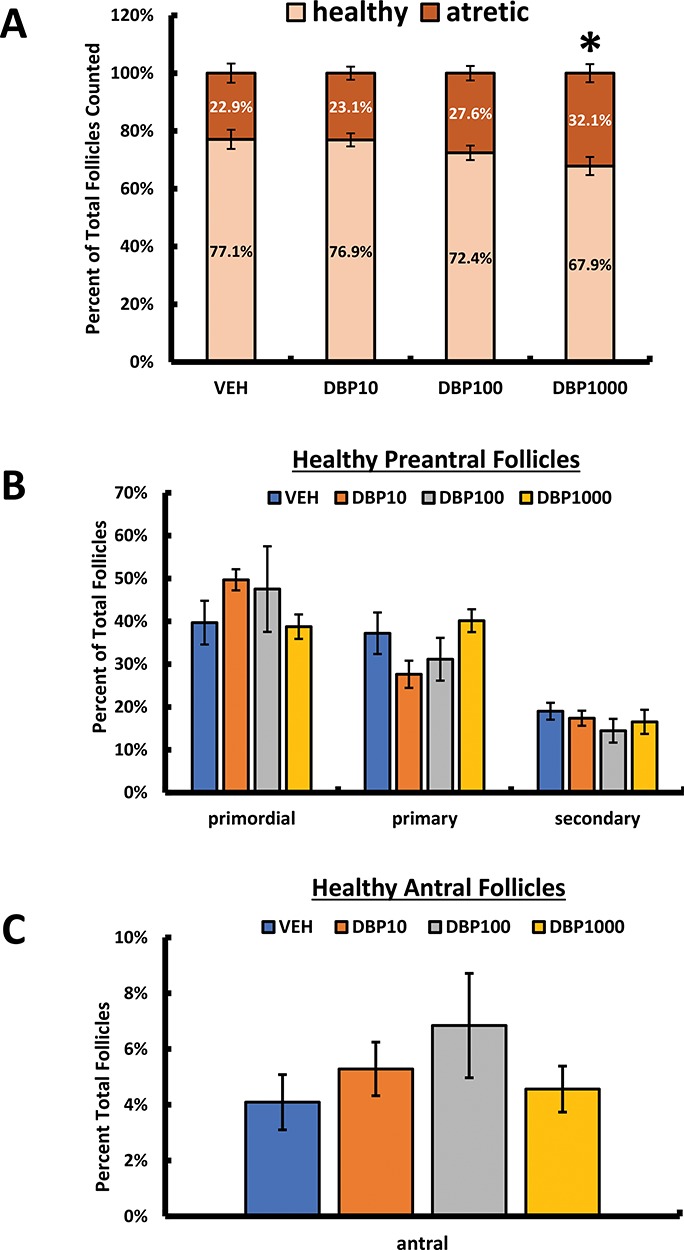
Effect of oral DBP exposure on ovarian follicle numbers. Adult cycling female CD-1 mice were treated daily with vehicle or DBP and their ovaries processed for H&E staining and follicle counting as described in Materials and Methods. Data are presented as the mean percent of total follicles counted ± SEM. Panel A shows data for healthy and atretic follicles, while panel B shows data for health preantral and panel C healthy antral follicles. Data were compared between treatments using Kruskal-Wallis nonparametric followed by Mann-Whitney post hoc test with significance set at p ≤ 0.05. Asterisk (*) indicates statistical differences versus vehicle (p ≤ 0.05; n = 5–7 mice/treatment).
Discussion
We exposed adult CD-1 mice to environmentally relevant doses of DBP, a phthalate ester commonly detected in women of reproductive age, for 30 consecutive days and determined whether exposure resulted in disrupted DDR signaling factor expression and alternations in folliculogenesis. Our novel findings demonstrate that oral exposure to DBP results in dose-specific disruptions on the expression of genes involved in HR, MMR, and NER in the ovary. DBP exposure did not cause increased p-H2AX foci relative to control in this study, thus suggesting that exposure to this phthalate does not result in direct DNA damage. Based on the critical role of these pathways, our findings raise questions about whether phthalate exposures could lead to increased ovarian sensitivity to future DNA-damaging insults.
Cellular exposure to a DNA-damaging insult results in the formation of DSBs which is quickly followed by the formation of p-H2AX foci. Formation of p-H2AX foci has been shown to occur prior to ovarian follicle loss [24], thus understanding whether phthalate exposure results in p-H2AX accumulation in the ovary is important. No studies have measured p-H2AX in the ovaries of phthalate-treated mice, but the effects of another phthalate, di-2-ethylhexyl phthalate (DEHP), on p-H2AX have been previously investigated [35]. In that study, DEHP exposure resulted in increased p-H2AX and comet tail moment, both accepted indicators of DNA damage. Here, we measured whether exposure to DBP results in accumulation of such foci in the ovary of orally treated mice. Compared to ovaries from vehicle-treated controls, the ovaries from DBP-treated mice did not show increased presence of p-H2AX foci, thus suggesting that DBP exposure does not result in increased formation of DSBs. These findings suggest that the mechanism of action of DBP in the ovary does not involve direct DNA damage. It is important to note that although these findings provide evidence against the formation of DSBs in response to DBP exposure, they do not rule out the possibility that other forms of DNA damage that do not increase p-H2AX (e.g. nucleotide excision and cross-linking, [36]) could be involved. Our gene expression results suggest that DBP exposure could also affect the integrity of repair pathways associated with nucleotide excision and nucleotide misincorporation; thus, the effects of DBP on these other mechanisms should be investigated.
Upon detection of a DNA DSB, the trimeric protein complex formed by MRE11-RAD50-NBS1 (MRN complex) recruits and activates the ataxia telangiectasia mutated (ATM) kinase. ATM activation leads to the phosphorylation of several effectors including BRCA1, CHK2, and TRP53, which mediate ATM effects on DNA repair, cell cycle arrest, and apoptosis (reviewed in [20]). In this study, DBP exposure led to decreased expression of Mre11a, Rad50, Atm, Brca1, Brca2, and Trp53b1 mRNA. These observations suggest that cells in the ovaries of DBP-treated mice will have difficulty with sensing DSBs, activating ATM-dependent DDR, and transducing signals to downstream pathways (cell cycle arrest and apoptosis). Though no other studies have reported effects of phthalates on Mre11a and Rad50, our findings are consistent with the scarce data available on the interactions of phthalates with Atm expression. Specifically, Atm expression has been shown to be decreased in the fetal rat testes following in utero exposure to DBP in two separate studies (supplementary data accompanying [37, 38]). In young ICR mice, DEHP treatment (0–1600 mg/kg/day) via gavage for 6 weeks resulted in a significant decrease in ovarian expression of various genes including Atm [39]. Finally, a meta-analysis of breast cancer and uterine leiomyoma studies utilized pathway enrichment analysis to identify genes associated with urinary phthalate metabolites levels. The ATM signaling pathway was enriched among the 59 genes associated with DEHP metabolites in that meta-analysis [40]. Collectively, these studies provide evidence that phthalates may interact with ATM-mediated DDR in reproductive tissues.
The MMR pathway is key to the repair of nucleotide misincorporation, base–base mismatches, and insertion/deletion mispairs, which can lead to altered DNA structure and defects during meiotic recombination (reviewed in [41]). The heterotrimeric complexes known as MutS (MSH2, MSH3, MSH6) and MutL (MLH1, MLH3, PMS1, PMS2) homologs as well as proliferating cell nuclear antigen (PCNA), replication protein A (RPA), and exonucleases are involved in this pathway. Our results show that following DBP exposure, the ovaries of treated mice have significantly lower transcript levels of Msh3 and Msh6 and a trend for lower Msh2 levels. Furthermore, exposure to the highest dose of DBP used in this study also significantly reduced transcript levels for Pcna in the ovary. Our findings are consistent with supplementary data supporting two microarray studies evaluating fetal testicular gene expression, which reported decreased expression of Msh2, Msh6, and Pcna after in utero exposure to DBP [37, 38]. Furthermore, immunoreactivity for PCNA was significantly decreased in fetal testes of rats exposed in utero (gestational days 6–19) to di-n-hexyl phthalate and dicyclohexyl phthalate (0–500 mg/kg/day; [42]). Based on the role of MMR in oocytes (reviewed in [23]), it is critical to test the possibility that phthalate exposure could interfere with the ability of oocytes to respond to DNA-damaging events under physiological (DNA replication and recombination) and exogenous (DNA damaging exposures) conditions in the future.
Nucleotide excision repair (NER) is important as an excision mechanism for bulky, helix-distorting lesions [43]. During NER, damage is recognized thus triggering the removal of the short single-stranded DNA segment containing the lesion and activating the synthesis of a new strand from its undamaged complementary sequence. Xpc encodes a key DNA damage recognition protein involved in the global genome repair branch of NER. Humans and mice deficient in Xpc function are extremely sensitive to DNA damage and prone to tumor formation [44, 45]. Xpc null mice have an increased propensity to slowly accumulate mutations in response to induction of oxidative stress [46]. Our findings show that the ovaries from DBP-treated mice expressed significantly less Xpc transcript when compared to controls. Because phthalates have been shown to induce oxidative stress in ovarian follicles [47, 48], it is possible that down-regulation of Xpc in DBP-treated ovaries could result in increased susceptibility to the DNA-damaging effects of reactive oxygen species.
In this study, oral exposure to DBP resulted in significantly decreased transcript and protein levels for Brca1 in the ovary without affecting DNA methylation of its promoter. Although most commonly associated with carcinogenesis in the breast and ovary, Brca1 has also been reported to have an important role in reproduction. Specifically, women bearing Brca1 mutations have been shown to ovulate less oocytes, undergo early menopause, and have lower ovarian reserve as suggested by lower serum anti-mullerian hormone levels when compared to nonmutation carriers [33, 34]. In mice, BRCA1 deficiency leads to decreased oocyte yields in response to ovarian stimulation, small litter sizes, reduced follicular endowment, and increased accumulation of DSBs [34]. Interestingly, similar findings have been reported in human studies evaluating associations between phthalates and adverse reproductive health outcomes. Specifically, phthalates have been associated with early menopause, decreased oocyte yield, and reduced ovarian reserve in women [5, 9, 11]. In mice, daily oral exposures to DEHP [49, 50] and DBP [19] have been shown to result in alterations in folliculogenesis in juvenile mice. In vitro, various phthalates have been shown to target ovarian follicles individually [17, 18, 51] and in mixtures [52]. In this study, we used hemiovaries to classify and enumerate ovarian follicles in the ovaries of vehicle- and DBP-treated mice and observed a significant decrease in the percentage of healthy follicles in the ovaries of adult mice treated with DBP (1000 μg/kg/day). Although follicle dynamics were not significantly different between controls and DBP-treated mice, the trends for increased primordial and decreased primary in the low-dose DBP group are intriguing and a potential hint that disruption in folliculogenesis could occur in a chronic exposure scenario. It is important to note that the use of follicle percentages in this study could have prevented us from observing changes in total follicle number; thus, future studies specifically aimed at characterizing the effects of DBP on folliculogenesis are still needed. Nonetheless, taken together, these findings highlight a potential relationship between phthalates, the DDR machinery, and ovarian health.
Finally, mice treated orally with DBP at 100 and 1000 μg/kg/day for 30 days showed a trend for increased body weight gain during dosing compared to vehicle-treated controls. Although it was not statistically significant in our study, this observation is consistent with findings from epidemiological studies showing associations between phthalate burden, body mass index, and waist circumference [53], faster prospective weight gain [54], metabolic syndrome [55], central obesity [56], total and trunk fat mass, and subcutaneous adipose tissue [57]. Additional studies have proposed that phthalates are obesogenic based on their ability to interact with peroxisome proliferator-activated receptors [58]. Recent studies have demonstrated a relationship between obesity status and ability to activate the DDR pathway. Specifically, work by Ganesan et al. [24, 25] demonstrated that obesity status disrupted DDR activation in 18-week-old agouti mice exposed to phosphoramide mustard and dimethylbenz[a]anthracene. Although the trend for weight gain observed in the present study is small (~6% more than control) versus the agouti mouse (obese starting at ~8 weeks of age), these results highlight the importance of teasing out the influence of metabolic disorders when evaluating outcomes of EDC toxicity in animal studies.
In conclusion, here we provide evidence that environmentally relevant exposures to the phthalate DBP lead to decreased expression of key DDR factors including BRCA1 and key players in the HR, MMR, and NER pathways. These findings highlight the need to further understand the effects of low-dose exposures to EDCs such as phthalates and determine whether DDR gene expression changes induced by these exposures subsequently lead to a state of increased sensitivity to DNA damage, risk of carcinogenesis, and exacerbated ovarian disease.
Supplementary Material
Acknowledgments
The authors wish to acknowledge Nivedita Sen, Lindsay Rasmussen, and Jahaira Vera for technical help during tissue collections and Doug Cromey (Southwest Health Sciences Center Cellular Imaging Facility Core) for assistance with image processing. The technical advice and performance of the DNA methylation analyses by the University of Arizona Genetics Core (http://uagc.arl.arizona.edu) are also appreciated.
Conflict of Interest
The authors have declared that no conflict of interest exists.
Conference Presentation: This work was presented in part at the 51st Annual Meeting of The Society for the Study of Reproduction, 10–13 July 2018, New Orleans, LA, USA.
REFERENCES
- 1. Mascarenhas M, Flaxman S, Boerma T, Vanderpoel S, Stevens G. National, regional, and global trends in infertility prevalence since 1990: a systematic analysis of 277 health surveys. PLOS Med 2012; 9:e1001356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kay V, Chambers C, Foster W. Reproductive and developmental effects of phthalate diesters in females. Crit Rev Toxicol 2013; 43:200–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hannon P, Flaws J. The effects of phthalates on the ovary. Front Endocrinol 2015; 6:1–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Du Y, Fang Y, Wang Y, Zeng Q, Guo N, Zhao H, Li Y. Follicular fluid and urinary concentrations of phthalate metabolites among infertile women and associations with in vitro fertilization parameters. Reprod Toxicol 2016; 61:142–150. [DOI] [PubMed] [Google Scholar]
- 5. Grindler N, Allsworth J, Macones G, Kannan K, Roehl K, Cooper A. Persistent organic pollutants and early menopause in U.S. women. PLoS One 2015; 10:e0116057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Meeker J, Ferguson K. Urinary phthalate metabolites are associated with decreased serum testosterone in men, women, and children from NHANES 2011–2012. J Clin Endocrinol Metab 2014; 99:4346–4352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Peng F, Ji W, Zhu F, Peng D, Yang M, Liu R, Pu Y, Yin L. A study on phthalate metabolites, bisphenol A and nonylphenol in the urine of Chinese women with unexplained recurrent spontaneous abortion. Environ Res 2016; 150:622–628. [DOI] [PubMed] [Google Scholar]
- 8. Toft G, Jonsson B, Lindh C, Jense T, Hjollund N, Vested A, Bonde J. Association between pregnancy loss and urinary phthalate levels around the time of conception. Environ Health Perspect 2012; 120:458–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hauser R, Gaskins A, Souter I, Smith K, Dodge L, Ehrlich S, Meeker J, Calafat A, Williams P. Urinary phthalate metabolite concentrations and reproductive outcomes among women undergoing in vitro fertilization: results from the EARTH study. Environ Health Perspect 2016; 124:831–839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Messerlian C, Wylie B, Mínguez-Alarcón L, Williams P, Ford J, Souter I, Calafat A, Hauser R, Study Team EARTH. Urinary concentrations of phthalate metabolites and pregnancy loss among women conceiving with medically assisted reproduction. Epidemiology 2016; 27:879–888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Messerlian C, Souter I, Gaskins A, Williams P, Ford J, Chiu Y, Calafat A, Hauser R, Study Team EARTH. Urinary phthalate metabolites and ovarian reserve among women seeking infertility care. Hum Reprod 2016; 31:75–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Centers for Disease Control and Prevention. Third National Report on Human Exposure to Environmental Chemicals. NCEH Pub. No. 05-0570 2005.
- 13. International Programme on Chemical Safety (IPCS). Di-n-butyl phthalate. 1997.
- 14. Kavlock R, Boekelheide K, Chapin R, Cunningham M, Faustman E, Foster P, Golub M, Henderson R, Hinberg I, Little R, Seed J, Shea K et al. . NTP Center for the Evaluation of risks to human reproduction: phthalates expert panel report on the reproductive and developmental toxicity of di-n-butyl phthalate. Reprod Toxicol 2002; 16:489–527. [DOI] [PubMed] [Google Scholar]
- 15. Hernandez-Diaz S, Su YC, Mitchell AA, Kelley KE, Calafat AM, Hauser R. Medications as a potential source of exposure to phthalates among women of childbearing age. Reprod Toxicol 2013; 37:1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hernandez-Diaz S, Mitchell AA, Kelley KE, Calafat AM, Hauser R. Medications as a potential source of exposure to phthalates in the U.S. population. Environ Health Perspect 2009; 117:185–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Craig Z, Hannon P, Wang W, Ziv-Gal A, Flaws J. Di-n-butyl phthalate disrupts the expression of genes involved in cell cycle and apoptotic pathways in mouse ovarian antral follicles. Biol Reprod 2013; 88:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Rasmussen L, Sen N, Vera J, Liu X, Craig Z. Effects of in vitro exposure to dibutyl phthalate, mono-butyl phthalate, and acetyl tributyl citrate on ovarian antral follicle growth and viability. Biol Reprod 2017; 96:1105–1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Sen N, Liu X, Craig Z. Short term exposure to di-n-butyl phthalate (DBP) disrupts ovarian function in young CD-1 mice. Reprod Toxicol 2015; 53:15–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ciccia A, Elledge S. The DNA damage response: making it safe to play with knives. Mol Cell 2010; 40:179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Sancar A, Lindsey-Boltz L, Unsal-Kacmaz K, Linn S. Molecular mechanisms of mammalian DNA repair and the DNA damage checkpoints. Annu Rev Biochem 2004; 73:39–85. [DOI] [PubMed] [Google Scholar]
- 22. Collins J, Jones K. DNA damage responses in mammalian oocytes. Reproduction 2016; 152:R15–R22. [DOI] [PubMed] [Google Scholar]
- 23. Stringer J, Winship A, Liew S, Hutt K. The capacity of oocytes for DNA repair. Cell Mol Life Sci 2018; 75:2777–2792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ganesan S, Keating A. Bisphenol A-induced ovotoxicity involves DNA damage induction to which the ovary mounts a protective response indicated by increased expression of proteins involved in DNA repair and xenobiotic biotransformation. Toxicol Sci 2016; 152:169–180. [DOI] [PubMed] [Google Scholar]
- 25. Ganesan S, Keating A. The ovarian DNA damage repair response is induced prior to phosphoramide mustard-induced follicle depletion, and ataxia telangiectasia mutated inhibition prevents PM-induced follicle depletion. Toxicol Appl Pharmacol 2016; 292:65–74. [DOI] [PubMed] [Google Scholar]
- 26. Institute of Laboratory Animal Resources. Guide for the Care and Use of Laboratory Animals. Washington, DC: National Academies Press; 1996. [Google Scholar]
- 27. Goldman J, Murr A, Cooper R. The rodent estrous cycle: characterization of vaginal cytology and its utility in toxicological studies. Birth Defects Res B Dev Reprod Toxicol 2007; 80:84–97. [DOI] [PubMed] [Google Scholar]
- 28. Rasmussen L, Sen N, Liu X, Craig Z. Effects of oral exposure to the phthalate substitute acetyl tributyl citrate on female reproduction in mice. J Appl Toxicol 2017; 37:668–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Paulose T, Hannon P, Peretz J, Craig Z, Flaws J. Estrogen receptor alpha overexpressing mouse antral follicles are sensitive to atresia induced by methoxychlor and its metabolites. Reprod Toxicol 2012; 33:353–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ehrich M, Nelson M, Stanssens P, Zabeau M, Liloglou T, Xinarianos G, Cantor C, Field J, van den Boom D. Quantitative high-throughput analysis of DNA methylation patterns by base-specific cleavage and mass spectrometry. Proc Natl Acad Sci 2005; 102:15785–15790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ollikainen M, Smith K, Joo E, Ng H, Andronikos R, Novakovic B, Abdul Aziz N, Carlin J, Morley R, Saffery R, Craig J. DNA methylation analysis of multiple tissues from newborn twins reveals both genetic and intrauterine components to variation in the human neonatal epigenome. Hum Mol Genet 2010; 19:4176–4188. [DOI] [PubMed] [Google Scholar]
- 32. Li D, Chen N, Cao J, Sun W, Zhou Y, Li C, Wang X. BRCA1 as a nicotinamide adenine nucleotide (NAD)-dependent metabolic switch in ovarian cancer. Cell Cycle 2014; 13:2564–2571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Oktay K, Kim J, Barad D, Babayev S. Association of BRCA1 mutations with occult primary ovarian insufficiency: a possible explanation for the link between infertility and breast/ovarian cancer risks. J Clin Oncol 2010; 28:240–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Oktay K, Turan V, Titus S, Stobezki R, Liu L. BRCA mutations, DNA repair deficiency, and ovarian aging. Biol Reprod 2015; 93:67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kim S, Park G, Yoo Y, Jeong J, Nam K, Jee S, Lim K, Lee Y. Di-2-ethylhexylphthalate promotes thyroid cell proliferation and DNA damage through activating thyrotropin-receptor-mediated pathways in vitro and in vivo. Food Chem Toxicol 2019; 124:265–272. [DOI] [PubMed] [Google Scholar]
- 36. Revet I, Feeney L, Bruguera S, Wilson W, Dong T, Oh D, Dankort D, Cleaver J. Functional relevance of the histone gammaH2AX in the response to DNA damaging agents. Proc Natl Acad Sci 2011; 108:8663–8667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Gaido K, Hensley J, Liu D, Wallace D, Borghoff S, Johnson K, Hall S, Boekelheide K. Fetal mouse phthalate exposure shows that gonocyte multinucleation is not associated with decreased testicular testosterone. Toxicol Sci 2007; 97:491–503. [DOI] [PubMed] [Google Scholar]
- 38. Johnson KJ, Hensley JB, Kelso MD, Wallace DG, Gaido KW. Mapping gene expression changes in the fetal rat testis following acute dibutyl phthalate exposure defines a complex temporal cascade of responding cell types. Biol Reprod 2007; 77:978–989. [DOI] [PubMed] [Google Scholar]
- 39. Liu J, Wang W, Zhu J, Li Y, Luo L, Huang Y, Zhang W. Di(2-ethylhexyl) phthalate (DEHP) influences follicular development in mice between the weaning period and maturity by interfering with ovarian development factors and microRNAs. Environ Toxicol 2018; 33:535–544. [DOI] [PubMed] [Google Scholar]
- 40. Fu Z, Zhao F, Chen K, Xu J, Li P, Xia D, Wu Y. Association between urinary phthalate metabolites and risk of breast cancer and uterine leiomyoma. Reprod Toxicol 2017; 74:134–142. [DOI] [PubMed] [Google Scholar]
- 41. Jiricny J. The multifaceted mismatch-repair system. Nat Rev Mol Cell Biol 2006; 7:335–346. [DOI] [PubMed] [Google Scholar]
- 42. Ahbad M, Barlas N. Influence of in utero di-n-hexyl phthalate and dicyclohexyl phthalate on fetal testicular development in rats. Toxicol Lett 2015; 233:125–137. [DOI] [PubMed] [Google Scholar]
- 43. Lans H, Marteijn J, Schumacher B, Hoeijmakers J, Jansen G, Vermeulen W. Involvement of global genome repair, transcription coupled repair, and chromatin remodeling in UV DNA damage response changes during development. PLoS Genet 2010; 6:e1000941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Kraemer K, Lee M, Scotto J. DNA repair protects against cutaneous and internal neoplasia: evidence from xeroderma pigmentosum. Carcinogenesis 1984; 5:511–514. [DOI] [PubMed] [Google Scholar]
- 45. Kraemer K, Lee M, Scotto J. Xeroderma pigmentosum. Cutaneous, ocular, and neurologic abnormalities in 830 published cases. Arch Dermatol 1987; 123:241–250. [DOI] [PubMed] [Google Scholar]
- 46. Melis J, Kuiper R, Zwart E, Robinson J, Pennings J, van C, Luitjen M, van Steeg H. Slow accumulation of mutations in Xpc−/− mice upon induction of oxidative stress. DNA Repair 2013; 12:1081–1086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Wang W, Craig Z, Basavarajappa M, Gupta R, Flaws J. Di (2-ethylhexyl) phthalate inhibits growth of mouse antral follicles through an oxidative stress pathway. Toxicol Appl Pharmacol 2012; 258:288–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Wang W, Craig Z, Basavarajappa M, Hafner K, Flaws J. Mono (2-ethylhexyl) phthalate induces oxidative stress and inhibits growth of mouse ovarian antral follicles. Biol Reprod 2012; 87:152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Hannon PR, Niermann S, Flaws JA. Acute exposure to di(2-ethylhexyl) phthalate in adulthood causes adverse reproductive outcomes later in life and accelerates reproductive aging in female mice. Toxicol Sci 2016; 150:97–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Hannon P, Peretz J, Flaws J. Daily exposure to di(2-ethylhexyl) phthalate alters estrous cyclicity and accelerates primordial follicle recruitment potentially via dysregulation of the phosphatidylinositol 3-kinase signaling pathway in adult mice. Biol Reprod 2014; 90:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Gupta RK, Singh JM, Leslie TC, Meachum S, Flaws JA, Yao HH. Di-(2-ethylhexyl) phthalate and mono-(2-ethylhexyl) phthalate inhibit growth and reduce estradiol levels of antral follicles in vitro. Toxicol Appl Pharmacol 2010; 242:224–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Zhou C, Flaws J. Effects of an environmentally relevant phthalate mixture on cultured mouse Antral follicles. Toxicol Sci 2017; 156:217–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Yaghjyan L, Sites S, Ruan Y, Chang S. Associations of urinary phthalates with body mass index, waist circumference and serum lipids among females: National Health and Nutrition Examination Survey 1999-2004. Int J Obes 2015; 39:994–1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Song Y, Hauser R, Hu F, Franke A, Liu S, Sun Q. Urinary concentrations of bisphenol A and phthalate metabolites and weight change: a prospective investigation in US women. Int J Obes 2017; 38:1532–1537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. James-Todd T, Stahlhut R, Meeker J, Powell S, Hauser R, Huang T, Rich-Edwards J. Urinary phthalate metabolite concentrations and diabetes among women in the National Health and Nutrition Examination Survey (NHANES) 2001-2008. Environ Health Perspect 2012; 120:1307–1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Dong R, Zhou T, Chen J, Zhang M, Zhang H, Wu M, Li S, Zhang L, Chen B. Gender- and age-specific relationships between phthalate exposures and obesity in Shanghai adults. Arch Environ Contam Toxicol 2017; 73:431–441. [DOI] [PubMed] [Google Scholar]
- 57. Lind P, Roos V, Ronn M, Johansson L, Ahlstrom H, Kullberg J, Lind L. Serum concentrations of phthalate metabolites are related to abdominal fat distribution two years later in elderly women. Environ Health 2012; 11:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Pereira-Fernandes A, Demaegdt H, Vandermeiren K, Hectors T, Jorens P, Blust R, Vanparys C. Evaluation of a screening system for obesogenic compounds: screening of endocrine disrupting compounds and evaluation of the PPAR dependency of the effect. PLoS One 2013; 14:e77481. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


