Abstract
Two new octanorlanostane-type triterpenes, euphraticanoids A and B (1 and 2), two new trinorsesquiterpenoids, euphraticanoids C and D (3 and 4), and eight known triterpenoids (5, 6, 8–13) along with one steroid (7) were isolated from Populus euphratica resins. The structures of these new compounds, including their absolute configurations, were characterized by spectrocsopic, chemical, and computational methods. Biological evaluation revealed that compounds 4, 7–9, 12, and 13 display neuroprotective activities in H2O2-induced HT-22 cells with 4, 8, and 9 occurring in a concentration-dependent manner and 7, 12, and 13 reaching the maximum effects at 20 μM. Meanwhile, the neuroprotective properties of all isolates were accessed using glutamate-induced SH-SY5Y cells and disclosed that compounds 3, 4, 8, and 9 could dose-dependently protect neural cell injury in a concentration range of 10–40 μM. Finally, a brief structure–activity relationship was briefly discussed.
Keywords: Populus euphratica, plant resins, sesquiterpenoids, triterpenoids, neuroprotection
1. Introduction
Populus euphratica, a plant of the family Salicaceae, spreads over the world in places such as China, Russia, Mongolia, India, and Iran. In China, the tree is mainly distributed in the west of China with abiotic surroundings exemplified as desert or saline and alkaline lands [1]. The resins secreted by the tree, known by the elegant name “the tears of poplar,” have been used to treat tuberculous adenitis, throat, and duodenal ulcer swelling in China [2]. Previous studies revealed the presence of salicin derivatives, volatile oils, and phenolics in the resins of P. euphratica [3,4]. In recent years, we have become interested in chemical investigations of medicinal plant resins. As a result, an increasing number of structurally novel terpenoids have been characterized [5,6,7]. In a continuous study on medicinal resins, the title material was investigated, resulting in the isolation of diterpenoids with cytotoxic and potent wound-healing promotion properties [8,9,10]. The current work is an in-depth investigation on P. euphratica resins, which led to the characterization of 12 terpenoids and one steroid with euphraticanoid compounds A–D (1–4) being new ones (Figure 1). To get an insight into the biological profiling of these secondary metabolites, neuroprotective properties of all the isolates were evaluated in either H2O2 or glutamate-induced neural or human neuroblastoma cells. In this paper, we describe the isolation, structure characterization, and neuroprotective activities of all the isolated compounds.
Figure 1.
The structures of compounds 1–13.
2. Results and Discussion
2.1. Structure Elucidation of the Compounds
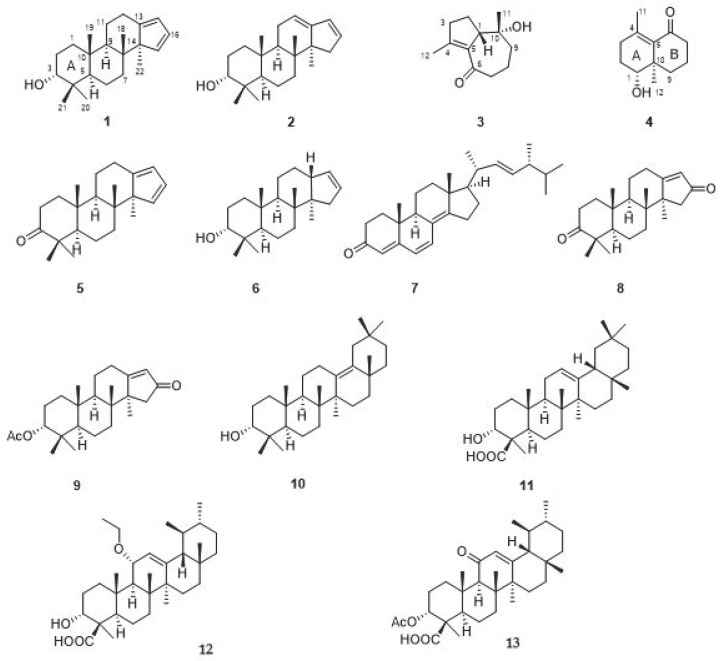
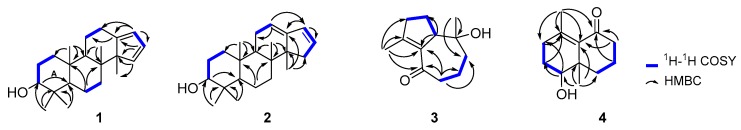
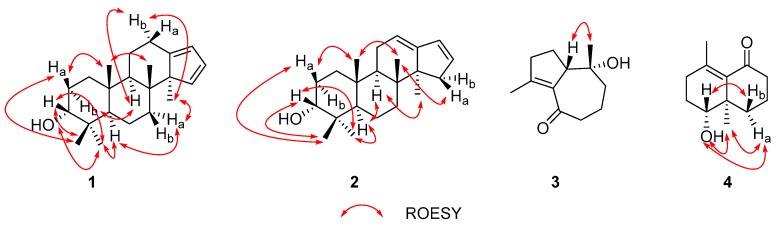
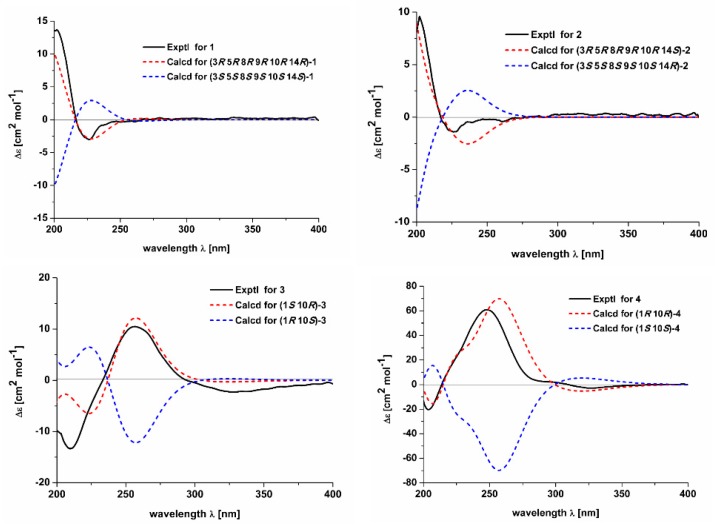
Compound 1 was obtained as a white powder with a positive optical rotation ([α]D25 +2.04; in MeOH). Its molecular formula was deduced as C22H34O on the basis of its HRESIMS (high resolution electrospray ionization mass spectroscopy), 13C NMR, and DEPT spectra. The 1H NMR spectrum of 1 shows signals for five methyl singlets (δH 1.05, 0.98, 0.84, 0.84, and 0.64). The 13C NMR and DEPT spectra (Table 1) indicate 22 carbons ascribed to five methyl, six methylene, six methine with three sp2 ones, and five non-protonated carbons (four aliphatic and one olefinic). A comparison NMR data of 1 with those of commiphorane G2 [11] revealed their resemblance except for a 13C NMR chemical shift difference occurring at C-1, C-2, C-3, and C-4. The 1H-1H COSY spectrum (Figure 2) shows correlations of H-1/H-2/H-3 (δH 3.42), in combination with the chemical shift of C-3 (δC 76.3), suggesting the chemical shift alterations in ring A (Figure 2) between 1 and commiphorane G2 might result from the configuration at C-3. To confirm this conclusion, a ROESY experiment (Figure 3) was utilized, and the ROESY cross peaks of Ha-2/H3-19, H3-21, H3-19/H3-18 indicate these protons are adjacent in space and assigned at β-orientation. Further, cross peaks of H-5/Ha-7, H3-20; H3-22/Ha-7, Hb-12, Hb-12/H-9, and H-9/Hb-2 suggest that these protons are at α-oriented. Finally, we examined the relative configuration of C-3 using the nuclear Overhauser effect (NOE) irradiation. A NOE enhancement was observed between 3-OH and H3-20 (Figure S14), evidently indicating the α-orientation of 3-OH in 1 contrary to that of commiphorane G2. Further NMR data comparison of ring A of 1 with the counterpart of epimansumbinol (6) [12] which bears a 3α-OH found their accordance, also securing the above conclusion. Thus, the planar structure and the relative configuration of 1 was deduced as shown in Figure 1. To clarify the absolute configuration of 1, electronic circular dichroism (ECD) calculation was carried out at B3LYP/6-311 + G(d) l level. The results show that the calculated ECD spectrum of (3R,5R,8R,9R,10R,14R)-1 (Figure 4) agrees well with the experimental one, indicating the absolute configuration of 1 is 3R,5R,8R,9R,10R,14R. In this way, the structure of 1 was identified and named as euphraticanoid A.
Table 1.
1H (600 MHz) and 13C (150 MHz) NMR data of 1 and 2 in CDCl3.
| 1 | 2 | ||||
|---|---|---|---|---|---|
| no. | δ H | δ C | no. | δ H | δ C |
| 1 | Ha: 1.50 (m) | 34.2, CH2 | 1 | Ha: 1.38 (m) | 33.2, CH2 |
| Hb: 1.40 (m) | Hb: 1.33 (overlap) | ||||
| 2 | Ha: 1.97 (m) | 25.6, CH2 | 2 | Ha: 1.96 (m) | 25.3, CH2 |
| Hb: 1.57 (m) | Hb: 1.56 (m) | ||||
| 3 | 3.42 (t-like, 2.63) | 76.3, CH | 3 | 3.42 (t-like, 2.8) | 76.3, CH |
| 4 | 37.8, C | 4 | 37.6, C | ||
| 5 | 1.36 (m) | 49.7, CH | 5 | 1.33 (overlap) | 49.7, CH |
| 6 | Ha: 1.48 (m) | 18.4, CH2 | 6 | Ha: 1.48 (m) | 18.3, CH2 |
| Hb: 1.38 (m) | Hb: 1.44 (m) | ||||
| 7 | Ha: 1.84 (m) | 37.3, CH2 | 7 | Ha: 1.62 (m) | 34.5, CH2 |
| Hb: 1.51 (overlap) | Hb: 1.31 (m) | ||||
| 8 | 41.4, C | 8 | 37.6, C | ||
| 9 | 1.51 (overlap) | 51.2, CH | 9 | 1.83 (dd, 10.6, 7.1) | 48.2, CH |
| 10 | 37.9, C | 10 | 37.5, C | ||
| 11 | Ha: 1.70 (m) | 23.1, CH2 | 11 | Ha: 2.13 (m) | 23.6, CH2 |
| Hb: 1.34 (m) | Hb: 1.93 (m) | ||||
| 12 | Ha: 2.61 (ddd, 13.6, 4.7, 1.7) | 26.4, CH2 | 12 | 5.40 (t, 3.7) | 114.7, CH |
| Hb: 2.16 (tdd, 13.6, 5.2, 1.7) | |||||
| 13 | 156.7, C | 13 | 151.9, C | ||
| 14 | 61.4, C | 14 | 49.4, C | ||
| 15 | 6.16 (d, 5.4) | 142.7, CH | 15 | Ha: 2.59 (brd,17.4) | 41.4, CH2 |
| Hb: 1.85 (brdt,17.4, 1.8) | |||||
| 16 | 6.21 (dd, 5.4, 1.7) | 129.6, CH | 16 | 5.83 (dt, 5.7, 2.6) | 133.5, CH |
| 17 | 5.79 (q-like, 1.7) | 120.2, CH | 17 | 6.04 (dt, 5.7, 1.8) | 131.3, CH |
| 18 | 0.64 (s) | 15.5, CH3 | 18 | 0.71 (s) | 17.6, CH3 |
| 19 | 0.84 (s) | 16.3, CH3 | 19 | 0.93 (s) | 15.2, CH3 |
| 20 | 0.98 (s) | 28.5, CH3 | 20 | 0.97 (s) | 28.5, CH3 |
| 21 | 0.84 (s) | 22.4, CH3 | 21 | 0.86 (s) | 22.3, CH3 |
| 22 | 1.05 (s) | 17.3, CH3 | 22 | 1.08 (s) | 24.9, CH3 |
Figure 2.
Key 1H−1H COSY and HMBC correlations for 1–4.
Figure 3.
Key ROESY correlations for 1–4.
Figure 4.
The calculated and experimental ECD spectra of 1–4.
Compound 2 was isolated as a white powder with a positive optical rotation ([α]D25 +7.27, in MeOH). The molecular formula of 2 was assigned as C22H34O aided with its HRESIMS, 13C NMR, and DEPT spectra. The 1H NMR spectrum of 2 exhibits five methyl (δH 1.08, 0.97, 0.93, 0.86, and 0.71). The 13C NMR and DEPT spectra (Table 1) display 22 carbons classified into five methyl, six methylene, six methine (three sp2), and five non-protonated carbons. These NMR signals resemble those of 1, indicating they are analogs. Compound 2 differs from 1 only in the position of two double bonds. The Δ12(13) and Δ16(17) double bonds in 2 rather than the Δ13(17) and Δ15(16) ones in 1 were observed to be supported by the 1H-1H COSY correlations of H-11/H-12 (δH 5.40) and H-15 (δH 2.59, 1.85)/H-16/H-17, and HMBC correlations of H-12/C-17 and H3-22/C-15. Therefore, the planar stucture of 2 was assigned. The relative stereochemistry of 2 was identical to that of 1 by inspection of their ROESY data (Figure 3). For the relative configuration at C-3, ROESY correlations of H-3/H3-20, H3-21 are observed (Figure 2), which indicated that H-3 is an equatorial position. Thus, concluding from the molecular model study, there are two possibilities: H-3β orientation/chair conformation of ring A and H-3α orientation/boat conformation of ring A, while the former is a more stable configuration, thus we deduced that the relative configuration of H-3 is β-orientation. In addition, the chemical shift of C-3 in 2 is in accordance with that of such types of structures wherein 3α-OH is around 3 ppm upshifted relative to 3β-OH, further securing the configuration at C-3 [11,12,13]. To clarify the absolute configuration of 2, ECD calculations were performed. It was found that the calculated (3R,5R,8R,9R,10R,14S)-2 matches well with that experimental curve, demonstrating the absolute configuration of 2 is 3R,5R,8R,9R,10R,14S (Figure 4), which confirmed the deduction of the molecular model study. Collectively, the structure of 2 was finally deduced and named as euphraticanoid B.
Compound 3 was obtained as yellow oils with negative optical rotation ([α]D25 −10.94 in MeOH). The molecular formula of 3 was deduced as C12H18O2 derived from its HRESIMS, 13C NMR, and DEPT spectra. The 1H NMR spectrum of 3 (Table 2) gives signals for two methyl singlets. The 13C NMR and DEPT spectra (Table 2) contain 12 carbon signals classified into two methyl, five methylene, one methine, one oxygenated tertiary carbon, two sp2 quaternary carbons, and one keto-carbonyl carbon. The structure architecture of 3 was mainly assembled with the aid of 2D NMR experiments. The 1H-1H COSY spectrum shows spin systems consisting of H-13/H-2/H-3 and H-7/H-8/H-9. The HMBC correlations of H3-12/C-3, C-4, C-5 and H-1, H-2/C-5 led to conclude the presence of five-membered ring. Additional HMBC correlations of H-7, H-8/C-6 (δC 202.1), H-8, H-9/C-10 (δC 75.1), H-7/C-5, and H-9/C-1, in combination with the chemical shifts of C-5, C-6, and C-10 allowed to deduce a seven-membered ring as shown (Figure 2). Finally, the HMBC correlations of H3-11/C-1, C-9, C-10 and the above mentioned H3-12/C-3, C-4, C-5 clarify the positions of two methyl groups. In this way, the planar structure of 3 was identified. The relative configuration of 3 was assigned by ROESY data (Figure 3), which gives a correlation of H-1/H3-11, indicating that these protons are adjacent to each other. Finally, the absolute configuration of 3 was established by ECD calculations. It was found that the calculated ECD of (1S,10R)-3 (Figure 4) is in accordance with that of experimental one, eventually clarifying the absolute configuration of 3 to be 1S,10R, with a trivial name euphraticanoid C.
Table 2.
1H (600 MHz) and 13C (150 MHz) NMR data of 3 and 4 in CDCl3.
| 3 | 4 | ||||
|---|---|---|---|---|---|
| no. | δ H | δ C | no. | δ H | δ C |
| 1 | 3.18 (m) | 56.7, CH | 1 | 3.61 (dd, 11.4, 4.6) | 76.6, CH |
| 2 | Ha: 1.99 (m) | 23.7, CH2 | 2 | 1.72 (m) | 26.7, CH2 |
| Hb: 1.93 (m) | |||||
| 3 | Ha: 2.45 (m) | 39.0, CH2 | 3 | 2.19 (m) | 32.4, CH2 |
| Hb: 2.36 (m) | |||||
| 4 | 158.3, C | 4 | 139.0, C | ||
| 5 | 135.3, C | 5 | 138.2, C | ||
| 6 | 202.1, C | 6 | 205.8, C | ||
| 7 | 2.46 (m) | 45.2, CH2 | 7 | Ha: 2.49 (m) | 43.2, CH2 |
| Hb: 2.29 (m) | |||||
| 8 | Ha: 1.86 (m) | 21.4, CH2 | 8 | Ha: 1.94 (m) | 20.8, CH2 |
| Hb:1.49 (m) | Hb: 1.90 (m) | ||||
| 9 | Ha: 1.95 (m) | 46.8, CH2 | 9 | Ha: 2.09 (ddd, 13.2, 5.2, 3.3) | 36.7, CH2 |
| Hb: 1.72(m) | Hb: 1.53 (ddd, 13.2, 11.1, 6.4) | ||||
| 10 | 75.1, C | 10 | 42.7, C | ||
| 11 | 0.99 (s) | 20.8, CH3 | 11 | 1.74 (s) | 21.2, CH3 |
| 12 | 2.07 (brs) | 17.2, CH3 | 12 | 0.81 (s) | 18.8, CH3 |
| 10 -OH | 4.83 (s) a | 1 -OH | 4.67 (d, 4.8) a | ||
a In DMSO−d6.
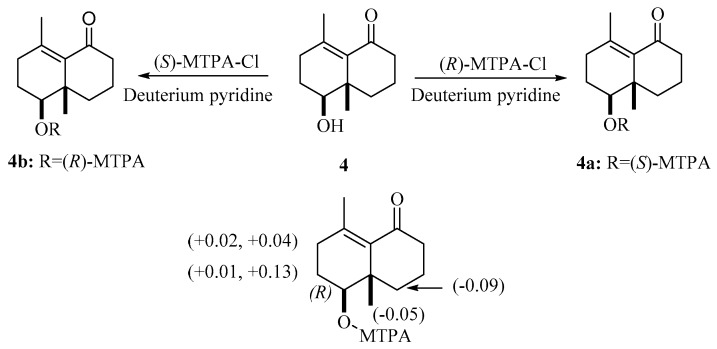
Compound 4, obtained as a yellow oil with positive optical rotation ([α]D25 +83.91 in MeOH), was found to have a molecular formula of C12H18O2 by analysis of its HRESIMS, 13C NMR, and DEPT spectra. The 1H NMR spectrum of 4 (Table 2) displays two methyl singlets. The 13C NMR and DEPT spectra (Table 2) contain 12 carbon signals ascribed to two methyl, five methylene, one methine, two sp2 and one sp3 quaternary carbons, and one keto-carbonyl carbon. In the manner of 3, the structure of 4 was mainly constructed with the assistance of 2D NMR data. The 1H-1H COSY spectrum of 4 shows correlations of H-1/H-2/H-3 and H-7/H-8/H-9. Starting from two spin systems, the HMBC correlations of H-3/C-4, C-5, H3-11/C-3, C-4, C-5, H-1, H-2/C-10, and H-1/C-5, in consideration of the chemical shift of C-1 suggest the presence of a six-membered ring (A) and the substituted groups thereof. In addition, HMBC correlations of H-8, H-9/C-10, H-9/C-5, H-7, H-8/C-6, H-7/C-5, and H3-12/C-1, C-9, C-10, in combination with the chemical shift of C-6 (δC 205.8), imply the presence of another six-membered ring (B) which fuses with ring A via the formation of C-5–C-10 (Figure 1). Collectively, the planar structure of 4 was assigned. There are two chiral centers in the molecule. ROESY correlations of H3-12, 1-OH (in DMSO-d6)/H-9a, H-1/H-9b indicate the spacial relationship of one OH group and 10-CH3 (Figure 3). To clarify the absolute configuration of 4, ECD calculations were utilized, which shows that the calculated ECD curve of (1R,10R)-4 matches well with the calculated one, evidently indicating the absolute configuration are 1R,10R. Meanwhile, the absolute configuration of 4 was confirmed by Mosher’s method [14]. In brief, treatment of 4 with (R)- or (S)-a-methoxy-atrifluoromethyl phenylacetic acyl chloride (MTPA-Cl) in deuterated pyridine was carried out to acquire the (S)-MTPA ester (4a) and (R)-MTPA ester (4b) (Figure 5), respectively. Further analysis of the 1H NMR signals of 4a and 4b indicates a 10R configuration judged from the ΔδH values of 4a and 4b. As a result, the absolute configuration of 4 was finally confirmed with a trivial name of euphraticanoid D.
Figure 5.
ΔδH values of the Mosher esters 4a and 4b.
The nine known compounds were identified as populeuphroid L (5) [7], epimansumbinol (6) [12], ergosta-4,6,8(15),22-tetraen-3-one (7) [15], mansumbin-13(17)-en-3,16-dione (8) [16], 3α-acetoxy-mansumbin-13(17)-en-16-one (9) [17], 3-epi-δ-amyrin (10) [18], α-boswellic acid (11) [19], 11α-ethoxy-β-boswellic acid (12) [20], acetyl-11-keto-β-boswellic acid (13) [21] by a comparison of their spectroscopic data with those reported in the literature. Although compound 12 is a known one, it might be an artefact produced during extraction procedure.
2.2. Biological Evaluation
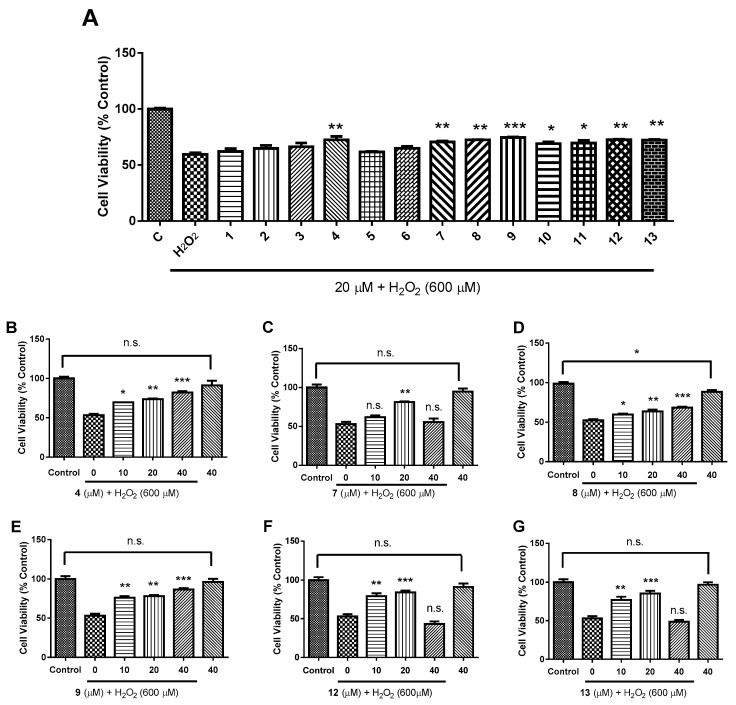
Neuroexcitotoxicity and oxidative stress have been implicated as playing a dominant role in neurodegenerative disorders such as Alzheimer’s disease (AD), ischemic stroke, as well as Parkinson’s disease (PD) [22,23,24,25,26]. In this study, all the compounds isolated from P. euphratica resin were applied to detect neuroprotective bioactivities against glutamate-induced excitotoxicity in SH-SY5Y cells and to examine their antioxidative effects against H2O2 in HT-22 cells. In primary screening, HT-22 cells were pretreated with 20 μM of different compounds, following by 600 μM H2O2 stimulation for 24 h. Our results show that eight out of thirteen compounds could significantly prevent H2O2-induced oxidative stress with compounds 4, 7–9, 12, and 13 are more potent (Figure 6A). Therefore, compounds 4, 7–9, 12, and 13 were submitted to a dose-dependent response experiment. The results show that 4, 8, and 9 could dose-dependently protect neural cells from H2O2-induced oxidative stress injury (Figure 6B,D,E), and 7, 12, and 13 possess neuroprotective property against oxidative stress at lower concentrations (10 μM and 20 μM) (Figure 6C,F,G). Of note, neuroprotective effects of 7, 12, and 13 reach the maximum at 20 μM and decline at 40 μM. Last but not the least, all the compounds except 8 show no cytotoxicity toward HT-22 cells even at 40 μM (Figure 6D). Although 8 is cytotoxicity against HT-22 cells at 40 μM, it appears that the cytotoxic effect might be negligible. Interestingly, compounds 1, 2, 5, 6, 8, and 9 are all octanortriterpenoids. However, the fact that 8 and 9 are active and the other analogs are inactive indicate the importance of α, β-unsaturated ketone to keep the activity. In addition, it was observed that compounds 12 and 13 are active, while in contrast 10 and 11 are inactive, implying that ursane-type rather than oleanane-type triterpenoids might contribute to neuroprotection. Boldly, it was found that compounds 4, 7–9, and 13 all bear a common α, β-unsaturated ketone, whether such a functional group is essential for keeping the activity needs further exploration.
Figure 6.
Neuroprotective activities of compounds 1−13 against H2O2-mediated oxidative stress in HT-22 cells. Cell viability was determined by CCK-8 assay. (A): Primary screening at 20 μM; (B–G): Dose-dependent curve of 4, 7–9, 12, and 13. Control was PBS-treated cells. “40” in bar charts means that cells were only treated by 40 μM compound. n = 3, all data in bar charts represent means ± SEM. The symbol n.s. means no significance, * p < 0.05, ** p < 0.01, *** p < 0.001, one-way ANOVA with post-hoc comparison Turkey.
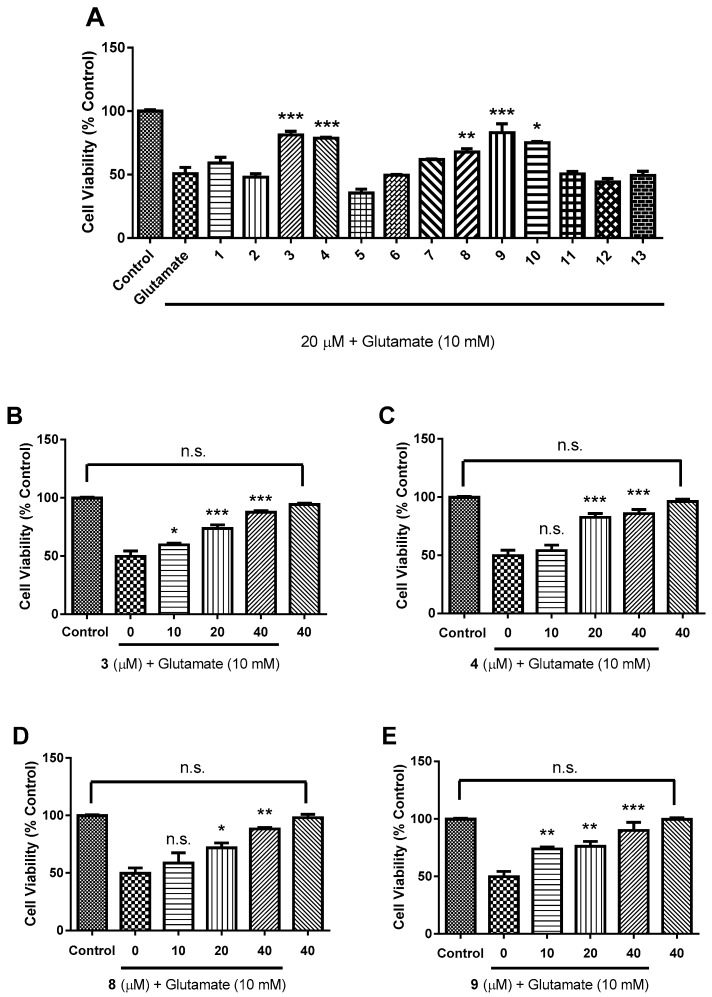
The neuroprotective properties of all the compounds were also observed in glutamate-induced SH-SY5Y cells. Cells were pretreated with 20 μM of different compounds followed by 10 mM glutamate stimulation for 24 h. The results show that 3, 4, 8–10 could significantly prevent glutamate-induced excitotoxicity (Figure 7A). Thereafter, 3, 4, 8, and 9 were assessed for a dose-dependent response and revealed that the neuro protection of 4, 8, and 9 against glutamate-induced excitotoxicity is dose-dependent (Figure 7B−E). Despite that octanortriterpenoids 8 and 9 are different types of compounds from 3 and 4, their neuroprotective activities against glutamate-induced excitotoxicity might imply that the presence of an α,β-unsaturated ketone is pivotal for keeping neuroprotection.
Figure 7.
Neuroprotective activities of compounds 1–13 against glutamate-induced excitotoxicity in SH-SY5Y cells. Cell viability was determined by CCK-8 assay. (A): Primary screening at 20 μM; (B–E): the neuroprotection of 3, 4, 8 and 9 was dose-dependent. Control was PBS-treated cells. “40” in bar charts means that cells were only treated by 40 μM compound. n = 3, all data in bar charts represent means ± SEM. The symbol n.s. means no significance, * p < 0.05, ** p < 0.01, *** p < 0.001, one-way ANOVA with post-hoc comparison Turkey.
3. Experimental Section
3.1. General Procedures
UV spectra were obtained on a Shimadzu UV-2600 spectrometer (Shimadzu Corporation, Tokyo, Japan). CD spectra were measured on a Chirascan instrument (Agilent Technologies, Santa Clara, CA, USA). NMR spectra were recorded on a Bruker AV-600 spectrometer (Bruker, Karlsruhe, Germany) with TMS as an internal standard. HRESIMS of 1–4 was collected by a Shimazu LC-20AD AB SCIEX triple TOF 5600+ MS spectrometer (Shimadzu Corporation, Tokyo, Japan). Column chromatography was undertaken on silica gel (200–300 mesh, Qingdao Marine Chemical Inc., Qingdao, China), MCI gel CHP 20P (75–150 μm, Mitsubishi Chemical Industries, Tokyo, Japan), RP-18 (40–60 µm; Daiso Co., Tokyo, Japan), and Sephadex LH-20 (Amersham Pharmacia, Uppsala, Sweden). Optical rotations were measured on a Bellingham + Stanley ADP 440 + digital polarimeter (Bellingham & Stanley, Kent, UK). Semi-preparative or analytic HPLC was carried out using an Agilent 1200 liquid chromatograph (Agilent Technologies, Santa Clara, CA, USA). The column used was a YMC-Pack ODS-A 250 × 9.4 mm, i.d., 5 µm, or a Phenomenex Kinetex (250 × 10 mm, i.d., 5 μm).
3.2. Plant Material
The resins of P. euphratica were collected by Ming-Yang Zong from Bayin, Xinjiang Autonomous Region, in November 2011. A voucher specimen (CHYX0573) identified by Prof. Bin Qiu at Yunnan University of Traditional Chinese Medicine is deposited at School of Pharmaceutical Sciences, Shenzhen University, China.
3.3. Extraction and Isolation
The dried resins of the title plant (50 kg) was soaked with 95% EtOH (300 L × 3 × 24 h) to afford a crude extract, which was suspended in water and partitioned with EtOAc to afford an EtOAc soluble extract (12 kg). This extract was cut into eight fractions (Fr.1–Fr.8) by using a silica gel column with petroleum ether–acetone (50:1, 35:1, 20:1, 15:1, 10:1, 7:1, 3:1,1:1) as solvents. Fr.1 (640 g) was separated via MCI gel CHP 20P eluted with aqueous MeOH (50%–100%) to provide nine portions (Fr.1.1–Fr.1.9). Among them, Fr.1.5 (136 g) was subjected to a RP-18 column eluted with aqueous MeOH (70%–100%) to provide nine portions (Fr.1.5.1–Fr.1.5.9). Fr.1.5.5 (38.2 g) was further separated via a silica gel column washed with petroleum ether–CH2Cl2 (30:1, 20:1, 15:1, 13:1, 10:1, 8:1, 4:1, 2:1, 1:1, 0:100) to provide six portions (Fr.1.5.5.1–Fr.1.5.5.6). Fr.1.5.5.4 (5.731 g) was further separated via vacuum liquid chromatography and washed with petroleum ether–EtOAc (30:1) to provide six portions (Fr.1.5.5.4.1–Fr.1.5.5.4.6). Of them, Fr.1.5.5.4.4 (373 mg) were subjected to preparative TLC (petroleum ether-CH2Cl2 (2:1) to give Fr.1.5.5.4.4.1–Fr.1.5.5.4.4.6. Fr.A.5.5.4.4.3 (9 mg) was submitted to Sephadex LH-20 (MeOH) to afford a portion (8 mg), which was further purified by semi-preparative HPLC with aqueous MeOH (94%) to afford compound 6 (2.64 mg, tR = 29.605 min; flow rate: 3 mL/min). Fr.A.5.5.4.4.4 (13 mg) was submitted to Sephadex LH-20 (MeOH) to give a portion (10 mg), which was purified by semi-preparative HPLC (aqueous MeCN, 40%) to give two portions. 1 (1.58 mg, tR = 13.575 min; flow rate: 3 mL/min) and 2 (0.88 mg mg, tR = 14.728 min; flow rate: 3 mL/min) was obtained from Fr.A.5.5.4.4.4.1 (2.50 mg, tR = 28.328 min, flow rate: 3 mL/min) by HPLC separation (aqueous MeOH, 94%). Fr.1.5.5.5 (1.609 g) was further separated via a silica gel column washed with petroleum ether–EtOAc (50:1,25:1,15:1,10:1) to provide seven portions (Fr.1. 5.5.5.1–Fr.1. 5.5.5.7). Fr.1.5.5.5.6 (128 mg) were subjected to preparative TLC (petroleum ether -CH2Cl2, 2:1) to give Fr.1. 5.5.5.6.1–Fr.1. 5.5.5.6.4. Fr.1.5.8 (721.0 mg) was purified by Sephadex LH-20(MeOH) to afford two parts (Fr.1.5.8.1 and Fr.1.5.8.2). Of which, Fr.1.5.8.2 (240.0 mg) was further divided into six parts (Fr.1.5.8.2.1–Fr.1.5.8.2.6) by a vacuum liquid chromatography eluted with petroleum ether–EtOAC (100:1–1:1). Fr.1.5.8.2.4 (56.9 mg) was separated by semi-preparative HPLC with aqueous MeOH (97%) to afford 7 (8.9 mg, tR = 35.6 min, flow rate: 3 mL/min). Fr.1.6 (36 g) was divided into eight parts (Fr.1.6.1–Fr.1.6.8) by using a silica gel column eluted with petroleum ether–acetone (250:1–1:1). Fr.1.6.4 (4.6 g) was submitted to Sephadex LH-20 (MeOH) to yield four fractions (Fr.A.6.4.1–Fr.A.6.4.4). Fr.1.6.4.3 (2.2 g) was subjected to a RP-18 column eluted with aqueous MeOH (65%–100%) to yield five fractions (Fr.1.6.4.3.1–Fr.1.6.4.3.5). 5 (29.4 mg, tR = 18.9 min, flow rate: 3 mL/min) was obtained from Fr.1.6.4.3.4 (500.0 mg) by a silica gel column eluted with petroleum ether–acetone (300:1–1:1) and HPLC separation (aqueous MeCN, 82%). Fr.1.7 (60 g) was subjected to a RP-18 column eluted with aqueous MeOH (60%–100%) to yield thirteen fractions (Fr.1.7.1–Fr.1.7.13). Fr.1.7.3 (332 mg) was further separated via Sephadex LH-20 (MeOH) and semi-preparative HPLC with aqueous MeCN (87%) to afford 8 (26.4 mg, tR = 12.3 min, flow rate: 3 mL/min). Fr.1.7.4 (193.0 mg) was passed through Sephadex LH-20 (MeOH) to yield two fractions (Fr.1.7.4.1 and Fr.1.7.4.2). Fr.1.7.4.1 (103 mg) was further purified by semi-preparative HPLC with aqueous MeCN (75%) to afford 9 (6.4 mg, tR = 18.8 min; flow rate: 3 mL/min). Fr.1.7.13 (33.2 g) was divided into six fractions (Fr.1.7.13.1–Fr.1.7.13.6) by using silica gel chromatography (petroleum ether–acetone, 40:1–1:1). Compound 10 (3.0 g) was purified from Fr.1.7.13.1 (14.0 g) by using gradient silica gel chromatography (petroleum ether–CHCl3, 10:1–1:1). Fr. 5 (370 g) was separated via MCI gel CHP 20P eluted with aqueous MeOH (40%–100%) to provide seven portions (Fr.5.1–Fr.5.7). Fr.5.1 (2.354 g) was passed through Sephadex LH-20 (MeOH) to yield two fractions (Fr.5.1.1 and Fr.5.1.2). Fr.5.1.1 (1.036 g) was separated via RP-18 eluted with aqueous MeOH (45%–100%) to provide six portions (Fr.5.1.1.1–Fr.5.1.1.6). Fr.5.1.1.2 (158 mg) was purified by semi-preparative HPLC (aqueous MeCN, 24%) to yield 3 (61.08 mg, tR = 21 min; flow rate: 3 mL/min) and three fractions (Fr.5.1.1.2.1–Fr.5.1.1.2.3). Fr.5.1.1.2.3 (21.0 mg) was further purified by semi-preparative HPLC to afford 4 (9.05 mg, tR = 24.047 min, flow rate: 3 mL/min) by HPLC separation (aqueous MeOH, 60%). Compounds 11 (9.95 mg, tR = 40.028 min; flow rate: 3 mL/min), 12 (3.16 mg, tR = 36.118 min; flow rate: 3 mL/min) and 13 (6.65mg, tR = 19.938 min; flow rate: 3 mL/min) were afforded from the portion of Fr.5.6 (50.3 mg) by semi-preparative HPLC (aqueous MeOH, 90%).
3.4. Compound Characterization Data
Compound 1: White powder. [α]20D +2.0 (c 0.10, MeOH); CD (MeOH), ∆ε202 +13.70, ∆ε226 −2.99, ∆ε280 +0.30; UV (MeOH) λmax (log ε) 256 (0.18) nm; HRESIMS: m/z 315.2676 [M + H]+ (calcd. for C22H35O, 315.2682); 1H and 13C NMR data, see Table 1.
Compound 2: White powder. [α]20D +7.3 (c 0.06, MeOH); CD (MeOH), ∆ε202 +9.59, ∆ε226 −1.40; UV (MeOH) λmax (log ε) 238 (0.28) nm; HRESIMS: m/z 315.2672 [M + H]+ (calcd. for C22H35O, 315.2682); 1H and 13C NMR data, see Table 1
Compound 3: Yellow syrup. [α]20D −10.9 (c 0.06, MeOH); CD (MeOH), ∆ε201 −8.59, ∆ε211 −13.47, ∆ε256 +10.56, ∆ε328 −2.31; UV (MeOH) λmax (log ε) 257 (1.08) nm; HRESIMS: m/z 195.1371 [M + H]+ (calcd. for C12H19O2, 195.1380); 1H and 13C NMR data, see Table 2.
Compound 4: Yellow syrup. [α]20D +83.9 (c 0.09, MeOH); CD (MeOH), ∆ε204 −20.38, ∆ε248 +60.83, ∆ε326 −2.87; UV (MeOH) λmax (log ε) 248 (1.14) nm; HRESIMS: m/z 195.1373 [M + H]+ (calcd. for C12H19O2, 195.1380); 1H and 13C NMR data, see Table 2.
3.5. ECD Calculation for Compounds 1–4
Conformation search using molecular mechanics calculations was performed in CONFLEX version 7.0 with MMFF force field with an energy window for acceptable conformers (ewindow) of 5 kcal/mol above the ground state, a maximum number of conformations per molecule (maxconfs) of 100, and an RMSD cutoff (rmsd) of 0.5Å. Then the predominant conformers were optimized at B3LYP/6-311+G(d) level in Gaussian 09 [27]. The optimized conformation geometries and thermodynamic parameters of all selected conformations were provided. The optimized conformers of 1–4 were used for the ECD calculation, which were performed with Gaussian 09 (B3LYP/6-311+G(d)). The solvent effects were taken into account by the polarizable-conductor calculation model (PCM, methanol as the solvent). Percentages for each conformation are shown in Tables S34–S37.
3.6. MTPA Esterification of 4
Compound 4 (1 mg) was dissolved in 600 μL of anhydrous deuteration pyridine, which was divided into two equal portions in NMR sample tube. To each portion was added 1.5 μL of either R-MTPA-Cl or S-MTPA-Cl, and then the mixtures were kept at room temperature for 2 h. Finally, the 1H NMR data were collected using the mixtures without purification.
3.7. Bioactivity Assay
Mouse hippocampus cell line (HT-22 cells) was purchased from iCell company in Shanghai, China, and human neuroblastoma cell line (SH-SY5Y) was purchased from Cellcook Company in Guangzhou, China. The experimental procedures of cell culture and treatments were performed as described previously [28]. Briefly, cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM, Gibco, Grand Island, NY, USA) with the addition of 10% fetal bovine serum (FBS, Gibco, Australia), 100 U/mL penicillin and 100 U/mL streptomycin (Gibco, Thornton, Australia) at 37 °C in a humidified atmosphere of 95% air and 5% CO2 incubator. SH-SY5Y cells were plated at a density of 2 × 104/well of a 96-well plate for 24 h. Thereafter, SH-SY5Y cells were pretreated with compounds (10, 20 and 40 μM) or PBS, and followed by a 24 h stimulation of either 10 mM glutamate (Sigma) or PBS (HyClone). HT-22 cells were pretreated with compounds (10, 20 and 40 μM) or PBS and followed by a 24 h stimulation of either 600 μM H2O2 (Sigma) or PBS. After all treatments, 10 μL/well of CCK-8 solution was added into each well and incubated for 1 h. The absorbance was determined at 450 nm by using a microplate reader (BioTek, Winooski, VT, USA).
All quantified biological data are expressed as means ± standard error of the mean (SEM) of n independent experiments. Statistical analyses were performed by one-way analysis of variance (ANOVA) following by a post-hoc multiple-comparison Tukey test whereby p < 0.05 (** p < 0.01, *** p < 0.001) was considered significant.
4. Conclusions
To conclude, the current study led to the characterization of two new nortriterpenoids (1 and 2), two new norsesquiterpenoids (3 and 4), and nine known compounds (5–13) from P. euphratica resins. Biological evaluation revealed that 4, 7–9, 12, and 13 are neuroprotective agents and the presence of α,β-unsaturated ketone in the structure might be crucial for keeping the activity. This study might shed light on further structure modification for developing new generation of neuroprotective drugs.
Acknowledgments
We are grateful to Ji-Gang Wang at Artemisinin Research Center of Institute of Chinese Materia Medica, China Academy of Chinese Medical Sciences and Shenzhen People’s Hospital for his critical reading for the manuscript.
Supplementary Materials
The following are available online. Figures S1–S14: NMR spectra of 1, Figure S15: HREIMS of 1, Figures S16–S23: NMR spectra of 2, Figure S24: HRESIMS of 2, Figures S25–S31: NMR spectra of 3, Figure S32: HREIMS of 3, Figures S33–S39: NMR spectra of 4, Figure S40: HRESIMS of 4, 5. Figures S41–S43: NMR spectra of 4a, 6. Figures S44–S46: NMR spectra of 4b, Figures S47–S50: The lowest energy conformers of 1–4. Table S1: Extracted heats and weighting factors of the optimized conformers of 1–4, Table S2: The Cartesian coordinates of the lowest energy conformers for 1–4.
Author Contributions
Y.-X.C. conceived, designed the experiments and wrote the paper, Y.D. carried out biological experiments. Y.-Y.L. and D.-L.H. performed chemical experiments. D.-P.Q. carried out ECD calculations. Y.-M.Y. gave advice for structure identification. All the authors read and approved the final manuscript.
Funding
This study was supported by the National Key Research and Development Program of China (2017YFA0503900) and National Science Fund for Distinguished Young Scholars (81525026).
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Sample Availability: Samples of the compounds 1‒13 are available from the authors.
References
- 1.Gong X., Li Z., Zhang N., Wang J., Han J.H., Ren K., Zhang C.H., Li M.H. Anti-inflammatory activity and chemical composition of resin extracts from Populus diversifolia Schrenk. Mod. Chin. Med. 2018;12:1185–1189. [Google Scholar]
- 2.Wei W., Rena K., Yang X.W. New salicin derivatives from the leaves of Populus euphratica. Asian Nat. Prod. Res. 2015;17:491–496. doi: 10.1080/10286020.2015.1028920. [DOI] [PubMed] [Google Scholar]
- 3.Luo J.R., Jiang H.E., Zhao Y.X., Liu Y.Q., Qian J.F. Volatiles from the heartwood of Populus euphratica in ancient tomb. Nat. Prod. Res. Dev. 2007;19:819–821. [Google Scholar]
- 4.Luo J.R., Jiang H.E., Zhao Y.X., Liu Y.Q., Qian J.F. Components of the heartwood of Populus euphratica from an ancient tomb. Chem. Nat. Compd. 2008;44:6–9. doi: 10.1007/s10600-008-0003-2. [DOI] [Google Scholar]
- 5.Liu J.W., Liu Y., Yan Y.M., Yang J., Lu X.F., Cheng Y.X. Commiphoratones A and B, Two sesquiterpenedimers from Resina Commiphora. Org. Lett. 2018;20:2220–2223. doi: 10.1021/acs.orglett.8b00561. [DOI] [PubMed] [Google Scholar]
- 6.Dong L., Cheng L.Z., Yan Y.M., Wang S.M., Cheng Y.X. Commiphoranes A−D, carbon skeletal terpenoids rom Resina Commiphora. Org. Lett. 2017;19:286–289. doi: 10.1021/acs.orglett.6b03661. [DOI] [PubMed] [Google Scholar]
- 7.Zhu C.Z., Hu B.Y., Liu J.W., Cai Y., Chen X.C., Qin D.P., Cheng Y.X., Zhang Z.D. Anti-mycobacterium tuberculosis terpenoids from Resina Commiphora. Molecules. 2019;24:1475. doi: 10.3390/molecules24081475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu K.X., Zhu Y.X., Yan Y.M., Zeng Y., Jiao Y.B., Qin F.Y., Liu J.W., Zhang Y.Y., Cheng Y.X. Discovery of populusone, a skeletal stimulator of umbilical cord mesenchymal stem cells from Populus euphratica exudates. Org. Lett. 2019;21:1837–1840. doi: 10.1021/acs.orglett.9b00423. [DOI] [PubMed] [Google Scholar]
- 9.Cao Q., Wang S.X., Cheng Y.X. Abietane diterpenoids with potent cytotoxic activities from the resins of Populus euphratica. Nat. Prod. Commun. 2019 doi: 10.1177/1934578X19850029. [DOI] [Google Scholar]
- 10.Liu K.X., Qin D.P., Zhu Y.X., Wang S.X., Jiao Y.B., Ge P.L., Cheng Y.X. Populeuphrines A and B, two new cembrane diterpenoids from the resins of Populus euphratica. Nat. Prod. Res. 2019:1–9. doi: 10.1080/14786419.2019.1610753. [DOI] [PubMed] [Google Scholar]
- 11.Dong L., Luo Q., Cheng L.Z., Yan Y.M., Cheng Y.X., Wang S.M. New terpenoids from Resina Commiphora. Fitoterapia. 2017;117:147–153. doi: 10.1016/j.fitote.2017.01.013. [DOI] [PubMed] [Google Scholar]
- 12.Matsuda H., Morikawa T., Ando S., Oominami H., Murakami T., Kimura I., Yoshikawa M. Absolute stereostructures of polypodane- and octanordammarane-type triterpenes with nitric oxide production inhibitory activity from guggul-gum resins. Bioorgan. Med. Chem. 2004;12:3037–3046. doi: 10.1016/j.bmc.2004.03.020. [DOI] [PubMed] [Google Scholar]
- 13.Nakamura S., Iwami J., Matsuda H., Mizuno S., Yoshikawa M. Absolute stereostructures of inoterpenes A–F from sclerotia of Inonotus obliquus. Tetrahedron. 2009;65:2443–2450. doi: 10.1016/j.tet.2009.01.076. [DOI] [Google Scholar]
- 14.Ohtani I., Kusumi T., Kashman Y., Kakisawa H. High-field FT NMR application of Mosher’s method. The absolute configurations of marine terpenoids. J. Am. Chem. Soc. 1991;113:4092–4096. doi: 10.1021/ja00011a006. [DOI] [Google Scholar]
- 15.Lee W.Y., Park Y.K., Ahn J.K., Park S.Y., Lee H. Cytotoxic activity of ergosta-4,6,8(14),22-tetraen-3-one from the sclerotia of Polyporus umbellatus. Bull. Korean Chem. Soc. 2005;26:1464–1466. [Google Scholar]
- 16.Provan G.J., Gray A.I., Waterman P.G. Mansumbinane derivatives from stem bark of Commiphora kua. Phytochemistry. 1992;31:2065–2068. doi: 10.1016/0031-9422(92)80364-K. [DOI] [Google Scholar]
- 17.Wang Y.G., Ma Q.G., Tian J., Ren J., Wang A.G., Ji T.F., Wang J.B., Su Y.L. Hepatoprotective triterpenes from the gum resin of Boswellia carterii. Fitoterapia. 2016;109:266–273. doi: 10.1016/j.fitote.2015.12.018. [DOI] [PubMed] [Google Scholar]
- 18.He A., Wang M., Hao H., Zhuang D.C., Li G.H. Hepatoprotective triterpenes from Sedum sarmentosum. Phytochemistry. 1998;49:2607–2610. doi: 10.1016/S0031-9422(98)00434-8. [DOI] [Google Scholar]
- 19.Culioli G., Mathe C., Archier P., Vieillescazes C. A lupane triterpene from frankincense (Boswellia sp., Burseraceae) Phytochemistry. 2003;62:537–541. doi: 10.1016/S0031-9422(02)00538-1. [DOI] [PubMed] [Google Scholar]
- 20.Al-Harrasi A., Ali L., Rehman N.U., Hussain J., Hussain H., Al-Rawahi A., Rizvi T.S. 11 α-ethoxy-β-boswellic acid and nizwanone, a new boswellic acid derivative and a new triterpene, respectively, from Boswellia sacra. Chem. Biodivers. 2013;10:1501–1506. doi: 10.1002/cbdv.201200231. [DOI] [PubMed] [Google Scholar]
- 21.Li F.S., Yan D.L., Liu R.R., Xu K.P., Tan G.S. Chemical constituents of Boswellia carterii (Frankincense) Chin. J. Nat. Med. 2010;115:25–27. doi: 10.3724/SP.J.1009.2010.00025. [DOI] [Google Scholar]
- 22.Pradip K.K., Anuradha K., Shivika R., Supriya S., Santoshkumar T., Chandishwar N., Neetu T. Mechanism of oxidative stress and synapse dysfunction in the pathogenesis of Alzheimer’s disease: Understanding the therapeutics strategies. Mol. Neurobiol. 2016;53:648–661. doi: 10.1007/s12035-014-9053-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Erika B., George G. Oxidative stress differentially induces tau dissociation from neuronal microtubules in neurites of neurons cultured from different regions of the embryonic Gallus domesticus brain. J. Neurosci. Res. 2019 doi: 10.1002/jnr.24541. [DOI] [PubMed] [Google Scholar]
- 24.Zhao H.P., Han Z.P., Ji X.M., Luo Y.M. Epigenetic regulation of oxidative stress in ischemic stroke. Aging Dis. 2016;7:295–306. doi: 10.14336/AD.2015.1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vera D., Eunsung J., Mouradian M.M. The role of oxidative stress in Parkinson’s disease. J. Parkinsons. Dis. 2013;3:461–491. doi: 10.3233/JPD-130230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pamela J.S. Excitatory amino acid receptors, excitotoxicity, and the human nervous system. Curr. Opin. Neurol. Neurosurg. 1993;6:414–422. [PubMed] [Google Scholar]
- 27.Wu B., He S., Wu X.D., Wu D.K., Pan Y.J. Cadinane and eudesmane sesquiterpenoids from Chloranthas henryi. Helv. Chim. Acta. 2007;90:1586–1592. doi: 10.1002/hlca.200790166. [DOI] [Google Scholar]
- 28.Arezoo R., Hamid R.S., Azar H., Seyed H.M., Mohammad T.B. 3-Acetyl-11-keto-β-boswellic acid attenuated oxidative glutamate toxicity in neuron-like cell lines by apoptosis inhibition. J. Cell. Biochem. 2019 doi: 10.1002/jcb.29413. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.