Abstract
Lentiviral vectors are increasingly utilized in cell and gene therapy applications because they efficiently transduce target cells such as hematopoietic stem cells and T cells. Large-scale production of current Good Manufacturing Practices-grade lentiviral vectors is limited because of the adherent, serum-dependent nature of HEK293T cells used in the manufacturing process. To optimize large-scale clinical-grade lentiviral vector production, we developed an improved production scheme by adapting HEK293T cells to grow in suspension using commercially available and chemically defined serum-free media. Lentiviral vectors with titers equivalent to those of HEK293T cells were produced from SJ293TS cells using optimized transfection conditions that reduced the required amount of plasmid DNA by 50%. Furthermore, purification of SJ293TS-derived lentiviral vectors at 1 L yielded a recovery of 55% ± 14% (n = 138) of transducing units in the starting material, more than a 2-fold increase over historical yields from adherent HEK293T serum-dependent lentiviral vector preparations. SJ293TS cells were stable to produce lentiviral vectors over 4 months of continuous culture. SJ293TS-derived lentiviral vectors efficiently transduced primary hematopoietic stem cells and T cells from healthy donors. Overall, our SJ293TS cell line enables high-titer vector production in serum-free conditions while reducing the amount of input DNA required, resulting in a highly efficient manufacturing option.
Keywords: lentiviral, gene and cell therapy, HIV, scalable, lentivirus, production, immunotherapy, vector, pseudotype, suspension
Introduction
HIV-1-derived lentiviral vectors (LVs) are efficient gene transfer vehicles used in both basic and clinical research settings. The ability of LVs to efficiently shuttle DNA into mammalian cells enables researchers to explore the function of various genes of interest.1, 2, 3, 4, 5 Clinically, LVs are used ex vivo to deliver therapeutic genes or expression cassettes to primary target cells, such as hematopoietic stem cells (HSCs), to treat genetic disorders or infectious diseases.6, 7, 8, 9 LVs are well suited for use in ex vivo gene therapy because they can deliver a relatively large payload (>9 kb, including intron-containing genomes), have the ability to transduce dividing and non-dividing cells, and stably integrate into the genome of a target cell to provide lifelong correction to that cell and its progeny.10, 11, 12, 13 Further, the integration profile of LVs suggests that they are safer than gamma-retroviral vectors, and there have been no product-related malignant transformations reported to date with LVs used in clinical trials.14, 15, 16 The promise of LV cell and gene therapy has led to the initialization of nearly 200 clinical trials, with notable successes in the treatment of hemoglobinopathies, primary immunodeficiencies, and leukemias.15,17, 18, 19, 20, 21, 22, 23, 24, 25 In fact, LV-based cell and gene therapies have already been approved by the US Food and Drug Administration, Kymriah, and by the European Medicines Agency, Zynteglo. Furthermore, cancer immunotherapy, which currently includes the use of LV to modify T cell function, is an area of intense research. Thus, additional LV-based cellular therapies are likely to be approved in the future. However, the manufacturing of current Good Manufacturing Practices (cGMP)-grade LVs has been a challenge for early-phase clinical trials and is even more difficult for commercialization.26, 27, 28 Thus, advances in industrial-scale vector manufacturing are required to meet current and future clinical needs for lentiviral-based cell and gene therapies.
Research into the production and design of LVs for gene therapy has been ongoing for over 20 years, and although incremental advancements have been made, the current methodologies need optimization.29, 30, 31, 32 Today, standard LVs are made using a third generation production system that relies on the transient transfection of HEK293T cells.33 This transient transfection method can generate high-titer LVs free of replication-competent lentivirus. However, because of the adherent and serum-dependent nature of the HEK293T cell line, cGMP LV production is limited due to the need to use multi-stack plastic tissue culture vessels, for example, 10-stack Cell Factory systems, which increases the amount of handling, personnel and consumables costs, and production time. Furthermore, the use of animal-derived serum to maintain the HEK293T cell line increases the risk for contamination by adventitious viruses. In addition, obtaining large enough lots of serum can often be challenging and costly to acquire.
To address these challenges of industrial-scale cGMP-LV manufacturing, researchers have reported alternative means to produce LVs.27,34 Many strategies use HEK293-derived cells that are adapted to grow in suspension with serum-free media (SFM), fixed-bed bioreactors, and stable producer cell lines.35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49, 50, 51, 52 However, these novel production schemes have been problematic for large-scale cGMP production for several reasons, including multiple handling steps, reduced titers, a limited production scale, continued use of tissue culture flasks, and reliance on animal-derived serum. To address these concerns, we adapted a HEK293T cell line to grow in suspension using a commercially available SFM. This newly derived cell line, SJ293TS, maintains the growth characteristics and LV productivity of the parental HEK293T cell line. We further improved the system’s fitness for cGMP-manufacture of LVs by simplifying the cell manipulation steps in the manufacturing protocol and introduced an antibiotic-free (AF) selection marker on all transfected plasmids. We show that this new system produces equivalent amounts of vesicular stomatitis virus envelope glycoprotein (VSV-G)-pseudotyped LVs as are produced by conventional methods using adherent, serum-dependent HEK293T cells. Moreover, the SJ293TS system produces at least 10-fold higher LV titers than that provided by other suspension-adapted HEK293 cells, specifically Viral Production Cells and Expi293F cells. Importantly, we achieve a more than 2-fold increase in the recovery of LV transducing units (TUs) after downstream purification compared with historical data from HEK293T producer cells.53, 54, 55 Finally, we demonstrate that LVs produced from SJ293TS cells can efficiently transduce human cells including T cells and CD34+ cells.
Results
Deriving the SJ293TS Cell Line
In order to convert the adherent HEK293T cell line to grow in suspension, we employed a commercially available SFM, Freestyle 293 Expression media (FS293). The cells were adapted by replacing the standard growth media, DMEM plus 10% fetal bovine serum (FBS) (D10), with FS293 supplemented with 10% FBS and seeded onto both non-tissue culture-treated and tissue culture-treated plates. Gradually, we reduced the amount of serum in the media at 1-week time intervals, and cells were passaged three times per week. After 4 weeks, the cells were growing solely in the presence of FS293. Cells that were successfully adapted directly using non-tissue culture-treated plates appeared to be less aggregated than those grown on tissue culture-treated plates; however, both approaches were ultimately successful in adapting HEK293T cells to grow in suspension without serum. The final adapted cell line, designated as SJ293TS, was then cultured in a shaking incubator to make a master cell bank. The cells were thawed, and their growth rate was determined by seeding the cells at 5 × 105 cells/mL followed by counting every 24 h for 2 days (Figure S1). SJ293TS cells doubled every 23.4 ± 2.7 h and reached a cell density of up to 3 × 106 cells/mL without compromising cell viability. For regular maintenance of the SJ293TS cell line, cultures were kept between 3 × 105 and 3 × 106 cells/mL and passaged every 2–3 days.
Optimization of Transient Transfection Method
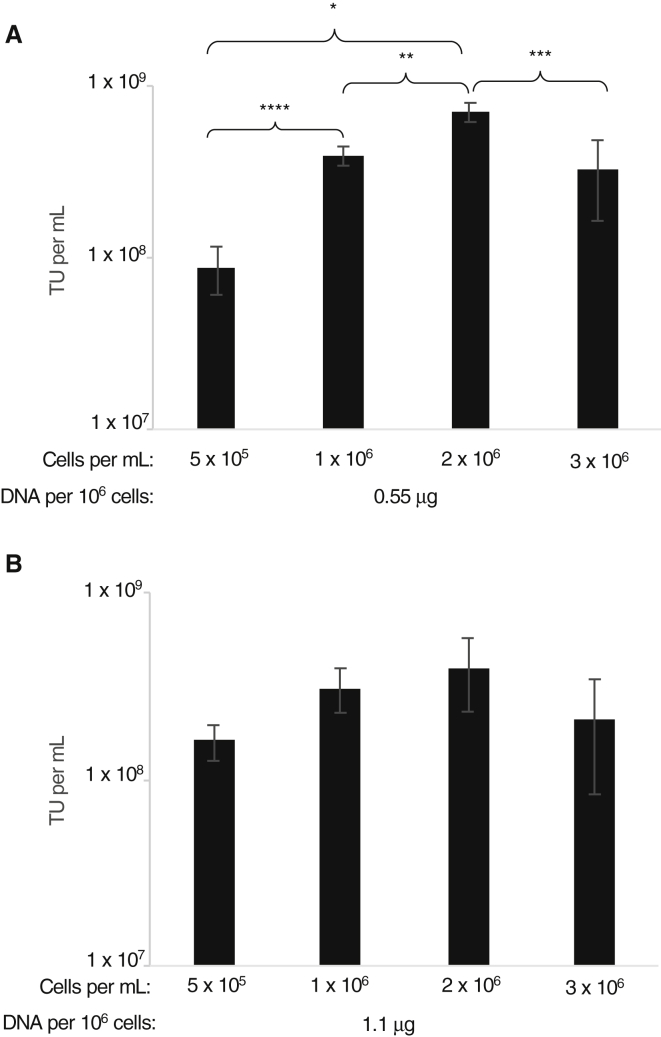
We assessed vector production using SJ293TS cells via our previously established transient transfection method.42,56 Initially, we aimed to optimize transfection conditions using a simple GFP-expressing LV (vector CL40-MND-GFP) in 20-mL cultures in 125-mL shaker flasks for quick and easy readout. First, we tested the effect of cell density at the time of transfection. Based on our previous experience with adherent HEK293T cells as a starting point, where cell density is approximately 1 × 106 cells/mL, we transfected SJ293TS cells at various densities ranging from 0.5 to 3 × 106 cells/mL at two different concentrations of plasmid DNA, 0.55 and 1.1 μg DNA per 106 cells. Twenty-four hours post transfection, the cells were pelleted by centrifugation and suspended in 20 mL of fresh media. Supernatants were collected 48 h post transfection and titered on HOS cells. Although LV production did not differ significantly among cells transfected at 1.1 μg DNA per 106 cells, LV output using SJ293TS cells transfected with 0.55 μg DNA per 106 cells was significantly impacted by seeding density, with the 2 × 106 cells/mL density achieving the highest titers (Figure 1). Using a cell density fixed at 2 × 106 cells/mL, we found that 0.55 μg DNA/mL was optimal for achieving the highest titer yields (Figure S2).
Figure 1.
Transfection Optimization of SJ293TS Cells to Produce Lentiviral Vectors
(A and B) To optimize transfection conditions, we seeded SJ293TS cells at increasing cell densities and transfected with either (A) 0.55 or (B) 1.1 μg of total plasmid DNA per 106 cells by using a simple reporter lentiviral vector. *p = 0.0002, **p = 0.0167, ***p = 0.0053, ****p = 0.0193. No significant differences were detected among conditions tested in (B). n = 3. Data are expressed as the mean ± the standard deviation.
Next, we examined the timing for optimal polyplex formation between polyethylenimine (PEI) and plasmid DNA under the conditions described in Materials and Methods. A single master transfection mixture was made and incubated for up to 30 min. At various time points, an aliquot was taken from the transfection master mixture and used to transfect SJ293TS cells in a 20-mL culture. Although no significant differences were observed, peak vector production occurred when the incubation continued for 5 min, whereas longer incubation periods tended to decrease titer (Figure S3). Thus, all subsequent transfections of SJ293TS cells were performed after a 5-min incubation of PEI and plasmid DNA.
Comparison of LV Production by SJ293TS Cells with Other HEK293-Based Cell Lines
To evaluate LV production in the suspension-adapted SJ293TS cell line, we compared 1-L productions of SJ293TS cells in 5-L shaker flasks against the parent HEK293T cells in 10-stack Cell Factory systems. For this comparison, three different clinically relevant LVs were produced: two containing shRNAmiR against human BCL11A with fluorophores (vectors SJL118 and SJL121) and the other with an expression cassette for a second generation anti-CD19 chimeric antigen receptor (vector αCD19-CAR). Media were replaced 24 h post transfection as described above, and vector was harvested 24 h later. HEK293T titers were 1.8-, 1.4-, and 2-fold higher than SJ293TS cells (Table 1).
Table 1.
Titers of Either HEK293T or SJ293TS Cell-Derived Lentiviral Vectors Produced at 1-L Scale
| Producer Cell Line in Culture Vessel | Vector | TUs/mLa |
|---|---|---|
| HEK293T cells in Cell Factory | SJL118 | 1.5 × 108 |
| SJ293TS cells in 5-L shaker flask | SJL118 | 8.2 × 107 |
| HEK293T cells in Cell Factory | SJL121 | 5.3 × 108 |
| SJ293TS cells in 5-L shaker flask | SJL121 | 3.7 × 108 |
| HEK293T cells in Cell Factory Prep 1 | αCD19-CAR | 2.00 × 108 |
| HEK293T cells in Cell Factory Prep 2 | αCD19-CAR | 2.00 × 108 |
| SJ293TS cells in 5-L shaker flask | αCD19-CAR | 1.00 × 108 |
One liter total volume, unprocessed supernatant titer.
There are few commercially available suspension HEK293 cell lines. We compared the SJ293TS cell line against two different HEK293-based suspension cell lines commercially available, namely, Expi293F cells and Viral Production Cells. All three cell lines were transfected, at the 20-mL scale, to produce CL40-MND-GFP. A master polyplex was formed, and an equal amount was distributed to each flask. In addition to GFP-LV, a more complex LV production, V5m3-Sardinia expressing the human γ-globin gene, was compared in a similar setting. The SJ293TS cell line produced 10- to 25-fold more TUs than either Viral Production Cells or Expi293F cells, regardless of vector. Statistical significance was reached only with the GFP-expressing vector, with p values of 0.0252 and 0.0239 in comparison with Viral Production Cells and Expi293F cells, respectively (Figure 2).
Figure 2.
Comparison of SJ293TS, Viral Production Cells, and Expi293F Cells as Lentiviral Vector Producer Cell Lines
Production of either pCL40-MND-GFP (black bars) or pCL30-V5m3-Sardinia (gray bars) from SJ293TS, Viral Production Cells (VPCs), or Expi293F cells at the 20-mL scale. *p = 0.0252, **p = 0.0239. n = 3. Data are expressed as the mean ± the standard deviation.
Improvements to SJ293TS LV Production Method
In order to improve the productivity and suitability of the SJ293TS cell line for cGMP manufacturing, we made modifications to the LV production process. Because there is growing concern using antibiotics for plasmid manufacturing, we looked for an alternative. An AF selection strategy, RNA-OUT, has been developed in which an approximately 150-bp cassette expresses a small antisense RNA against a counter-selectable marker expressed by the specialized bacterial host cell.57 We replaced the β-lactamase expression cassette on our helper and transfer plasmids with RNA-OUT, which has the added benefit of reducing the size of the plasmid backbone. We also reduced the posttransfection cell culture handling by diluting transfected cells with an equal volume of fresh media rather than performing a full media exchange.
In the context of these changes, we also improved vector production by optimizing plasmid ratios used to transiently transfect SJ293TS cells. We found that a ratio of 14:4:2:0.25 of transfer vector:HIV-1 gagpol:Env:HIV-1 rev, compared with our previously used standard plasmid ratio of 12:6:2:0.25, led to a 1.7-fold increase in the titer of a pre-clinical LV (vector SJL644), which expresses a second generation anti-CD123 chimeric antigen receptor (p > 0.05) and a reduction in the amount of HIV-1 p24 present in harvested supernatants (p > 0.05) (Figures S4A and S4B). Interestingly, by using an estimate of 104 physical particles per picogram of HIV-1 p24, the physical particles per TU decreased significantly from an average of 319 to 170 (p = 0.0071) (Figure S4B). Thus, the new plasmid ratio was implemented for all further SJ293TS vector productions.
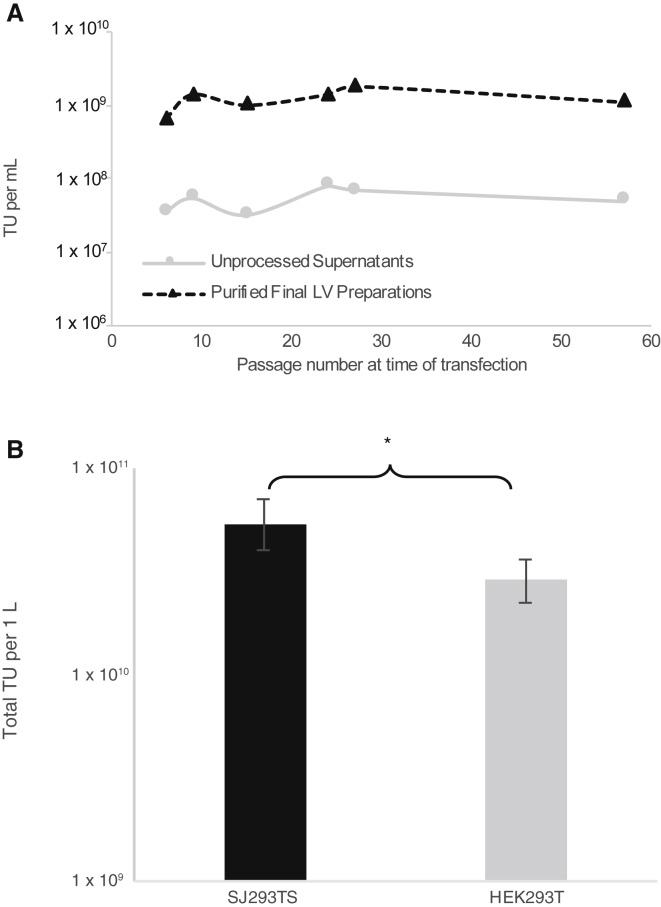
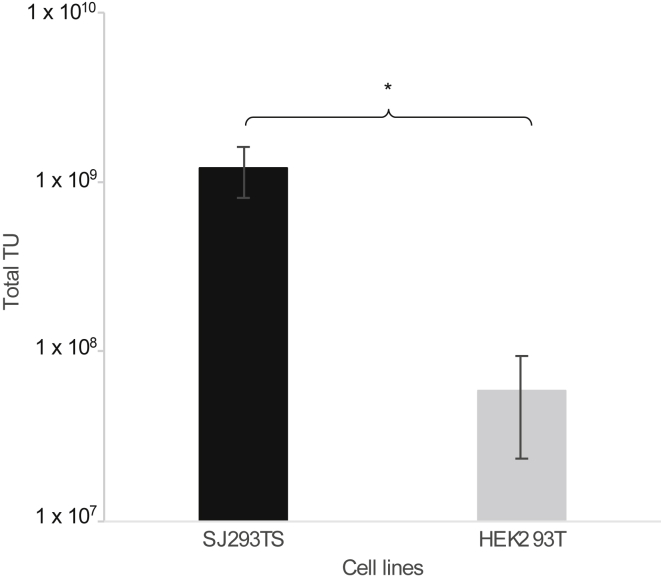
The robustness and stability of our new manufacturing process was evaluated by continuously passaging SJ293TS cells in culture for over 4 months and performing periodic 1-L LV production using another pre-clinical vector that also expresses a second generation anti-CD123 chimeric antigen receptor (vector SJL643). To keep the total harvested volume at 1 L, 500 mL of SJ293TS cells was transfected at a cell density of 2 × 106 cells/mL in a 5-L shaker flask. Titers from both unprocessed and purified material were highly reproducible, with respective averages of 5.3 ± 1.9 × 107 and 1.2 ± 0.4 × 109 TUs/mL (Figure 3A). When the same vector was produced by using HEK293T cells in 10-stack Cell Factory systems using ampicillin-resistant plasmids, the titers obtained from SJ293TS cells were 1.8-fold higher, which was statistically significant, p = 0.02554 (Figure 3B). Also, transfection of HEK293T cells consumed 2-fold more plasmid DNA than did SJ293TS cell transfection (1,012.5 versus 506.25 μg, respectively), and due to several media exchanges, the use of 10-stack Cell Factory systems used 3-fold more media than did SJ293TS cells in 5-L shaker flasks.
Figure 3.
Consistent High-Titer Production of Clinically Relevant Lentiviral Vectors by SJ293TS Cells over Time and Comparison with HEK293T Cell-Derived Titers at 1-L Scale
(A) Titers from unprocessed supernatants (solid gray line with circles) and purified final lentiviral vector preparations (black dashed line with triangles) from periodic production of VSV-G-pseudotyped SJL643 from SJ293TS cells continuously passaged over 4 months. (B) Total lentiviral vector transducing units (TUs) obtained from 1 L of transfected HEK293T cells or SJ293TS cells. *p = 0.02554. n = 3 for HEK293T cells and n = 6 for SJ293TS cells. Data are expressed as the mean ± the standard deviation.
Further characterization of a subset of these vector preparations indicated that the amounts of HIV-1 p24 in unprocessed supernatants and purified final LV preparations were between 0.65 ± 0.35 and 16.95 ± 10.1 μg of HIV-1 p24 per mL, respectively (Table S1). The average number of physical particles per TU was not significantly different after downstream processing (p > 0.05).
Downstream Processing of LVs Derived from SJ293TS Cells
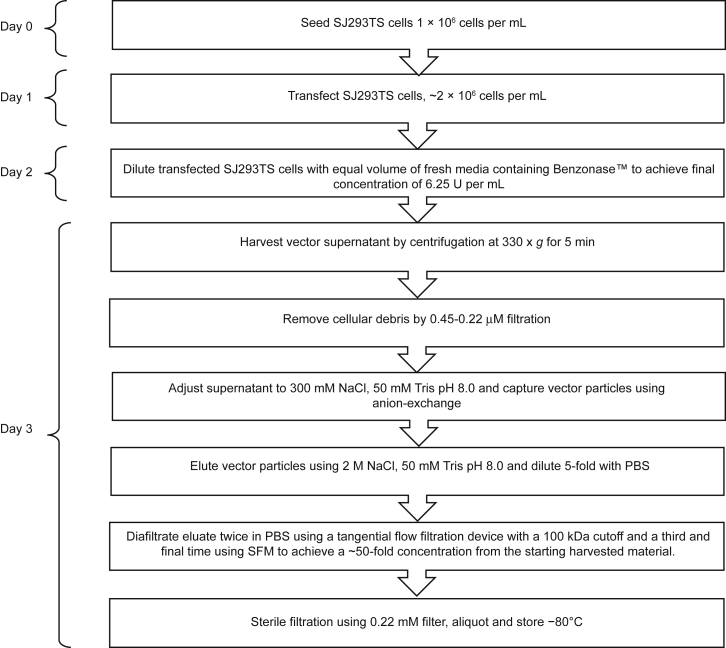
During vector production, Benzonase, an RNA-DNA endonuclease, was added to producer cells 24 h post transfection to reduce plasmid and genomic DNA levels in vector supernatants. Upon harvest, 1-L LV preparations derived from SJ293TS cells were purified using scale-down cGMP-like processes. In brief, after clarification, LV harvests were subjected to anion-exchange chromatography, tangential flow filtration and 0.22 μM filtration to reduce contaminating host cell protein and DNA, increase the concentration of LV TUs per milliliter, and ensure sterility of the final product (Figure 4). We applied these processing methods to 1-L research-grade LV preparations (n = 138) at our institutional core facility. After 0.22 μM filtration, the average overall recovery rate was 55% ± 14% of the total TUs present in the starting material. This represents an approximately 2-fold increase over previously published yields from adherent HEK293T serum-dependent cGMP-grade LV preparations, which ranged from 16% to 33% depending on the study.53,54
Figure 4.
SJ293TS Lentiviral Vector Production Diagram
Diagram outlining daily steps for the production and purification of lentiviral vectors from SJ293TS cells.
Four different 1-L LV preparations (before and after processing) were subjected to digital droplet PCR (ddPCR) analyses to detect contaminating plasmids as measured by the amplification of the RNA-OUT selection marker common to all transfected plasmids. Although plasmid DNAs were detected in all samples tested, the number of plasmid copies in the supernatant at harvest was reduced by 3–4 logs from the calculated plasmid input (at the time of transfection) (p ≤ 0.001) and did not change significantly after purification (p > 0.05) (Figure S5). These data suggest that treatment of the vector harvest with a relatively low number of units of Benzonase during vector production significantly reduces contaminating plasmid content in the harvest and purified material.58 As a consequence of this reduction, residual vector plasmid DNA is unlikely to confound vector titration. To test this, we performed ddPCR on genomic DNA from HOS cells transduced with either unconcentrated or concentrated LV preparations. No plasmid signal was detected by ddPCR in genomic DNA from cells harvested on either day 4 or day 7 (data not shown). Furthermore, calculated titers from these samples were comparable, with vector copy numbers (VCNs) ranging from 0.259 to 3.81. Interestingly, day 4 titers were only 9% ± 11% higher than day 7 titers (Table 2); thus, to accelerate LV titer determination, we performed ddPCR on HOS cells 4 days post transduction.
Table 2.
HOS Cell Vector Copy Number at Days 4 and 7 Posttransduction
| Lentiviral Vector Sample | Day 4 VCN | Day 7 VCN |
|---|---|---|
| Prep 1 unconcentrated | 2.62 | 2.32 |
| Prep 1 finala | 2.09 | 1.83 |
| Prep 2 unconcentrated | 1.63 | 1.42 |
| Prep 2 finala | 2.12 | 1.84 |
| Prep 3 unconcentrated | 2.48 | 2.31 |
| Prep 3 finala | 3.81 | 3.54 |
| Prep 4 unconcentrated | 0.259 | 0.206 |
| Prep 4 finala | 0.954 | 0.809 |
| Prep 5 unconcentrated | 1.32 | 1.30 |
| Prep 5 finalb | 1.83 | 1.73 |
| Prep 6 unconcentrated | 1.83 | 1.55 |
| Prep 6 finalb | 10.7 | 12.5 |
Fifty-fold concentration post ion-exchange, ultra-filtration, and sterile filtration.
Thirty-fold concentration by ultra-centrifugation.
Suitability of SJ293TS-Derived LV for Clinical Gene and Cell Therapy
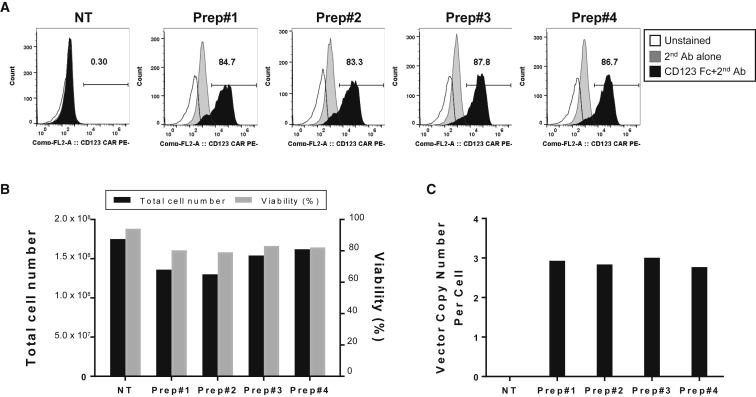
During our process development, we have produced clinically relevant LV, and we wanted to test how these vectors transduced target cell populations, mainly human T cells or CD34+ HSCs. LV produced from the SJ293TS cell line efficiently transduced healthy donor-derived human T cells (Figure 5). Four distinct vector preparations containing two different second generation anti-CD123-CAR vectors, pSJL605 and pSJL643, were used to transduce primary human T cells at a MOI of 25. Seven days post transduction, all four T cell populations expressed high levels of anti-CD123 CAR on the surface and maintained levels of expansion and viability comparable with those of mock-transduced cells (Figures 5A and 5B). Of note, the average VCNs per cell were remarkably consistent between the four different LV batches, demonstrating the reproducibility of our production method (Figure 5C).
Figure 5.
Efficient Transduction of Healthy Human T Cells by SJ293TS-Derived Lentiviral Vector
CD4+ and CD8+ human T cells from a single healthy donor were transduced with four separate lentiviral vector preparations derived from SJ293TS cells consisting of two different anti-CD123-CAR lentiviral vectors at a MOI of 25. (A–C) Seven days post transduction, the CD123 CAR expression was determined by flow cytometry analysis (A); the total number of cells, viability (B), and vector copy numbers (C) were also measured.
Next, we tested transduction efficiency on human CD34+ cells known for resistance to VSV-G-pseudotyped LVs. Similar to primary T cells, LV derived from SJ293TS cells efficiently transduced peripheral blood mobilized human CD34+ cells harvested from different healthy donors. To confirm that efficient transduction does not result from one particular batch of LV, we produced multiple vector preparations of two different LVs, each expressing a small hairpin RNA along with a fluorophore from either the human hemoglobin locus control region DNA hypersensitivity sites HS2 and HS3 and with the β-globin promoter (vector SJL425) or the human glycophorin A promoter with a BCL11A enhancer (vector SJL319). CD34+ cells were transduced at a MOI of 50 and subsequently plated into MethoCult in order to assess VCN from hematopoietic lineage cells. Twelve days later, MethoCult colonies were pooled together, and the average VCN was determined via ddPCR. SJL425 achieved VCNs of 0.89 and 2.71 on two different donors; two separate preparations of SJL319 exhibited VCNs of 0.76 and 1.05 on a third donor. The data are limited, but the nearly 3-fold VCN difference observed in MethoCult cultures using the SJL425 vector preparation likely reflects differences in donor permissivity.
Baboon Endogenous Retroviral R-less Glycoprotein
An alternative envelope glycoprotein, the baboon endogenous retrovirus (BaEV), has been shown to greatly improve human B cell transduction otherwise resistant to VSV-G-pseudotyped LV.59 Although promising, limitation of this envelope protein results from low titer partially due to syncytia formation during LV production in adherent HEK293T cells. We reasoned that vector production in suspension cells might alleviate syncytia formation and could lead to increased titers. To test whether SJ293TS cells might improve LV production pseudotyped with baboon endogenous retroviral R-less (BaEV-R-less), we compared titers of vectors produced by HEK293T and SJ293TS cells at similar scales using a GFP-expressing LV (vector CL40-MND-GFP). SJ293TS and HEK293T cells were transfected in culture volumes of 20 and 10 mL, respectively. However, because SJ293TS cells are transfected with half the concentration of DNA as HEK293T cells are, the total amount of DNA transfected into each cell line was identical. Thus, using the same amount of transfected DNA, SJ293TS cells provided 20-fold more TU than did HEK293T cells (Figure 6). Furthermore, three different BaEV-R-less-pseudotyped LVs were produced from SJ293TS cells at the 1-L scale and concentrated 50-fold by using the downstream processing described above. Depending on the vector, titers ranged from 0.2 to 2 × 108 TUs/mL with an average overall yield of 33% ± 2.9% from the starting supernatant. These data demonstrate that SJ293TS can be used to produce BaEV-R-less-pseudotyped LVs at titers higher than those of adherent HEK293T cells and can be purified using ion-exchange and tangential flow filtration.
Figure 6.
Total BaEV-R-less-Pseudotyped Lentiviral Vector Transducing Units Produced by SJ293TS and HEK293T Cells
SJ293TS cells produce 20-fold more TUs than HEK293T cells do when an equal amount of transfected DNA is used. *p = 0.0054. n = 4. Data are expressed as the mean ± the standard deviation.
Discussion
The use of LVs as a therapeutic modality is showing promise in many early clinical studies and has already achieved commercial approval for some lentiviral-based therapies.6,7,17, 18, 19, 20, 21, 22, 23, 24, 25, 26,60 Because most lentiviral gene and cellular therapies are currently in phase I/II clinical trials, demands for cGMP-grade LVs are achievable.24 However, with the steady rise in the number of LV-based clinical trials and the expectation that more LV-based therapies will progress to late-stage clinical studies involving larger patient accruals, the production of cGMP-grade LVs may become a bottleneck for developing treatments. Indeed, most recent delay in commercialization partly stems from an unreliable vector supply chain.
The development of stable packaging and producer cell lines has been employed to mitigate some of the limitations of current LV production processes.39, 40, 41, 42, 43,45, 46, 47, 48, 49, 50, 51, 52,61, 62, 63 A common feature of these new systems is the reliance on adherent, serum-dependent cells, which may not be amenable to commercial-scale cGMP production. Further, although producer cell line stability and titers have been improved by tightly regulating the levels of cytotoxic components, these systems inherently introduce a level of complexity in producer cell maintenance and manipulation that could contaminate the final product. Finally, the development of stable LV producer cell lines requires a significant up-front investment in time to introduce the necessary elements and fully assess the ideal clonal cell line. Overall, these limitations are reflected by the relatively few clinical trials that utilize stable cell lines for their LV supply (ClinicalTrials.org: NCT03573700, NCT01512888, and NCT01306019).20,25
The most commonly used method for cGMP LV manufacturing is transient transfection of HEK293T cells to generate high-titer supernatants. This process has undergone extensive development over 20 years, and the flexibility of the system enables it to readily incorporate changes in vector design. cGMP manufacture of clinical-grade LVs is typically performed by using multi-stack trays due to the adherent and serum-dependent nature of the HEK293T cell line. This process is laborious, costly, and thus not conducive to scale-up. Instead, manufacturers often resort to scaling-out, which increases the number of production vessels and, consequently, the difficulty of vector production. To avoid these restrictions, HEK293 cells grown in suspension have been used instead of HEK293T cells to produce LVs with titers typically lower than those achieved with HEK293T cells.35,36,39,41 Valkama et al.44 reported using HEK293T cells in a fixed-bed bioreactor to produce LVs; however, the described method still required the initial expansion of cells on plastic multi-trays before seeding the bioreactor. In 2011, Lesch et al.37 produced LVs by transducing HEK293T cells with baculoviruses; however, this protocol included FBS and sodium butyrate during the production steps, which may complicate its integration into cGMP clinical-grade LV production. For a thorough review of various LV production systems, please see Merten et al.27
We describe a robust, cGMP-compatible LV production system using SJ293TS cells. By adapting HEK293T cells to grow in suspension using SFM, we avoid the drawbacks of using animal-derived products, such as serum, and the limits associated with the high cost and increased personnel handling time associated with tissue-culture-treated plasticware. Our production system relies on the well-defined, widely adapted, and highly productive HEK293T transient transfection method facilitating its use in cGMP-grade LV manufacturing. Further, because cGMP-grade reagents are a major expense in the production of clinical-grade LVs, our SJ293TS production system not only reduces overall consumables and media costs, including plasmid DNA, but provides for a nearly 2-fold increase in titer (Figure 3B) and post purification yields compared with previous historical data using adherent, serum-dependent HEK293T cells. The SJ293TS cell line is highly stable and can be continuously cultured for up to 4 months with no loss in the ability to produce high-titer LVs. These cells also produced high-titer alternative-envelope pseudotyped LVs such as the BaEV-R-less glycoprotein. Although SJ293TS cells have many advantages, as described above, over adherent, serum-dependent HEK293T cells to produce LV, one potential disadvantage would be the requirement for current ongoing development programs to invest time in adopting a new LV manufacturing protocol. However, we believe that the benefits of this new LV production scheme outweigh any potential drawbacks.
A critical consideration for LV for clinical use is the ability to transduce clinically relevant target cells. Our data demonstrate that LVs derived from SJ293TS cells are capable of efficiently transducing both primary human CD34+ cells from granulocyte-colony-stimulating factor mobilized peripheral blood of volunteers and healthy donor-derived peripheral T cells. Thus, our data suggest that the SJ293TS-production system represents a significant advancement in the production of LVs because it is stable, eliminates the need for animal-derived reagents, and is amenable to true scale-up manufacturing while increasing vector yields post purification. Future work will be focused on demonstrating this production scheme at the 5-L scale by using a stirred-tank bioreactor.
We have begun to establish a master cell bank derived from SJ293TS for future LV manufacturing at Children’s GMP.
Materials and Methods
Cell Lines
HEK293T and HOS cells were obtained from the American Type Culture Collection (ATCC CRL-11268 and CLR-1543, respectively) and maintained in DMEM (Corning, Corning, NY, USA) supplemented with 10% FBS (Seradigm, Radnor, PA, USA) and 2 mM l-alanyl-l-glutamine (Corning) (D10) at 37°C with 5% CO2. Expi293F cells and Viral Production Cells were purchased and maintained in Expi293 Expression and LV-MAX production media, respectively (Thermo Fisher Scientific, Waltham, MA, USA). SJ293TS cells were maintained in Freestyle 293 Expression media (Thermo Fisher Scientific) and, along with Expi293F cells and Viral Production Cells, were cultured at 37°C with 8% CO2 and shaking at 125 rpm.
Plasmids
First, the plasmid, pCL20-MSCV-GFP, was retrofitted to RNA-OUT, replacing the 858-bp β-lactamase open reading frame with a 138-bp shRNA expression cassette for sucrose selection, by Nature Technology (Lincoln, NE, USA).57 The resulting AF selection plasmid is named as pCL20-MSCV-GFP-AF2. The 2,988-bp NheI-SalI fragment from plasmids, pCAG-kGP1-1R, pCAG4-RTR2, and pCAG-VSVG, was removed and replaced with the 2,165-bp NheI-SalI fragment containing the RNA-OUT cassette from pCL20c-MSCV-GFP-AF2 to make pCAG-kGP1-1R-AF, pCAG4-RTR2-AF, and pCAG-VSVG-AF, respectively. The 2,753-bp BsaAI-NheI fragment from both pCAG4-RTR2 and pCAG4-RTR2-AF was replaced with a 720-bp synthetic DNA fragment consisting of the CMV promoter and the HIV-1 rev cDNA to make pCMV-Rev and pCMV-Rev-AF, respectively. The BaEV-R-less glycoprotein was synthesized as described in Girard-Gagnepain et al.64 and cloned into pCAG4-Ampho by using DraIII and PstI to make pCAG4-BaEV-R-less. For pCAG4-BaEV-R-less-AF, a 2,512-bp HinDIII-SalI fragment in pCAG4-BaEV-R-less was replaced with a 1,693-bp HinDIII-SalI fragment from pCAG4-VSVG-AF. Sequence files for all plasmids used in this study are available upon request.
LV Production
Production of LV from adherent HEK293T cells was performed as described in Throm et al.,42 except that pCAG-kGP1-1R (gagpol) and pCAG-VSVG (VSV-G) were substituted for pCAGG-HIVgpco (codon-optimized gagpol) and pHDM-G (VSV-G), respectively.56 In brief, HEK293T cells were seeded to achieve ∼80% confluency at the time of transfection, typically 5 × 106 cells per 10-cm plate. The day of transfection, the media were exchanged. Transfection of cells in 10-cm plates was performed as follows: 12 μg of transfer vector, 6 μg of pCAG-kGP1-1R, 2 μg of pCAG-VSVG, and either 2 μg of pCAG4-RTR2 (rev/tat) or 0.25 μg of pCMV-Rev (rev). When SJ293TS cells were used to produce LV in 20-mL cultures, cells were seeded at 1 × 106 cells/mL. The next day, cells were transfected with the specified LV transfer plasmid and the helper plasmids as follows: 14 μg of transfer vector, 4 μg of pCAG-kGP1-1R, 2 μg of pCAG-VSVG, and either 2 μg pCAG4-RTR2 or 0.25 μg pCMV-Rev. pCAG4-RTR2, which expresses HIV-1 rev and tat, was used to produce LVs as part of our core institutional services and in Figures 1, S2, and S3, Table 1, and pCL30-V5m3-Sardinia in Figure 2, whereas pCMV-Rev was used to produce LVs in all other data presented. Substitution of pCMV-Rev for pCAG4-RTR2 slightly reduced the total amount of plasmid DNA in the transfection of SJ293TS cells from 0.55 μg per 1 × 106 cells to 0.50625 μg per 1 × 106 cells. All transfections were performed using PEIpro (Polyplus Transfection, Strasbourg, France). Transfection reagents were scaled up proportionally depending on the scale of vector production. Before mixing, plasmid DNA was diluted in PBS (Corning) to 40 μg/mL, and PEIpro was diluted to 80 μg/mL in PBS. The transfection mixture was then incubated for approximately 5 min before being added directly to the cell culture. Twenty-four hours post transfection, the media were replaced or, in some instances, transfected SJ293TS cells were diluted with an equal volume of fresh media. At the same time, Benzonase was added to the cell culture to achieve a final concentration of 6.25 U/mL. Vector supernatants were harvested 48 h post transfection and clarified by centrifugation to remove cells, filtration through 0.45- and 0.22-μm filters, and either subjected to further purification or stored at −80°C before titration. Production of 1-L LV preparations by adherent HEK293T cells was made using 10-stack Cell Factory systems (Thermo Fisher Scientific). RNA-OUT-containing helper plasmids were always used together, and the plasmid pCAG-VSVG-AF was substituted with pCAG-BaEV-R-less-AF to produce BaEV-R-less pseudotyped LVs. For Expi293F cells and Viral Production Cells, vector was harvested at the optimal 72 h post transfection.
For downstream processing, LV containing supernatant was adjusted to 300 mM NaCl with 50 mM Tris (pH 8.0) before being loaded onto a 5-mL Mustang Q XT5 ion-exchange capsule (Pall Life Sciences, Port Washington, NY, USA) per the manufacturer’s instructions using an Akta Avant chromatography system (GE Healthcare Bio-Sciences, Pittsburgh, PA, USA). The column was then washed with 10 column volumes of 300 mM NaCl with 50 mM Tris (pH 8.0), and LV particles were eluted from the column by using 2 M NaCl, 50 mM Tris (pH 8.0). The eluate was diluted 5-fold with PBS and diafiltrated twice in PBS using a Vivaflow 50 with a 100,000 molecular weight cutoff (Sartorius AG, Göttingen, Germany) according to the manufacturer’s instructions before a final diafiltration was performed in X-VIVO 10 or X-VIVO 15 media (Lonza) to achieve a final concentration of about 50-fold from the starting material. The vector was then 0.22 μM filtered, aliquoted, and stored at −80°C before being titrated.
HIV-1 p24 ELISA
HIV-1 p24 determination was performed using RETROtek HIV-1 p24 Antigen ELSIA kit (ZeptoMetrix Corporation, Buffalo, NY, USA) according to the manufacturer’s instructions.
LV Copy Number Determination and Plasmid Detection
To determine viral titers, we seeded HOS cells at a density of 1 × 105 cells/well of a six-well plate at least 2 h before transduction. Vector aliquots were thawed at room temperature, serially diluted, and added to HOS cells in the presence of Polybrene (5–8 μg/mL; MilliporeSigma, Burlington, MA, USA) in a total volume of 1 mL. The next day, the media were exchanged. For fluorescent-reporter vectors, transduced HOS cells were analyzed 3–4 days post transduction by flow cytometry. For vectors titered by PCR, transduced HOS cells were cultured until day 4 or 7, when genomic DNA was isolated by using the Quick-DNA Miniprep kit (Zymo Research, Irvine, CA, USA). To determine the VCN, genomic DNA was digested with MspI and used as a template in PCR by using a ddPCR instrument (QX200; Bio-Rad, Carlsbad, CA, USA). The following primer-probe sets were used to amplify the HIV psi sequence and the endogenous control gene, RPP30, 5′-ACTTGAAAGCGAAAGGGAAAC-3′, 5′-CACCCATCTCTCTCCTTCTAGCC-3′ and probe 5′-56-FAM-AGCTCTCTC-ZEN-GACGCAGGACTCGGC-3IABkFQ-3′ and 5′-GCGGCTGTCTCCACAAGT-3′, 5′-GATTTGGACCTGCGAGCG-3′ and probe 5′-5HEX-CTGACCTGA-ZEN-AGGCTCT-3IABkFQ-3′, respectively. Vector titers and copy number were determined by calculating the number of copies of HIV psi to every two copies of RPP30, multiplied by the number of cells transduced, and if necessary, multiplied by the dilution factor.
Transfected RNA-OUT plasmids were detected via ddPCR using the following primer-probe set: 5′- GACGCTCAGTGGAACGAAA-3′, 5′-AACAAGATGTGCGAACTCGATA-3′, and 5′-56-FAM-ACACGACTC-ZEN-TCTTTACCAATTCTACCACC-3IABkFQ-3′.
hCD34+ Cell Transduction and Colony Forming Unit Assay
The St. Jude Human Applications Laboratory or Key Biologics (Memphis, TN, USA) purified CD34+ cells from granulocyte-colony-stimulating factor mobilized peripheral blood of healthy volunteers. CD34+ cells were cultured in X-VIVO 10 (Lonza, Walkersville, MD, USA) with 100 ng/mL of stem cell factor (SCF), Fms-related tyrosine kinase 3 ligand (FLT3-ligand), and thrombopoietin (TPO) (CellGenix, Freiburg, Germany), 50 U/mL of penicillin/streptomycin (Corning), and 2 mM l-alanyl-l-glutamine (Corning). Cells were primed overnight in media at a density of 1 × 106 cells/mL and seeded at a density of 2 × 106 cells/mL at the time of transduction with vector in the presence of 8 μg/mL protamine sulfate (St. Jude Children’s Research Hospital pharmacy), 6.25 U/mL of Benzonase (MilliporeSigma), 1% recombinant human serum albumin (Grifols Biologics, Los Angeles, CA, USA), and 10 μM prostaglandin E2 (Cayman Chemical, Ann Arbor, MI, USA). Twenty-four hours post transduction, cells were seeded in MethoCult H4434 Classic (STEMCELL Technologies, Vancouver, BC, Canada), and colonies were pooled on post transduction day 12 for analysis by ddPCR.
T Cell Transduction
Healthy donor-derived human peripheral blood apheresis cells were purchased from Key Biologics. The cells were labeled with anti-CD4/CD8 microbeads, and CD4+/CD8+ T cells were purified by using the CliniMACS system (Miltenyi Biotec, Bergisch Gladbach, Germany). CD4+ and CD8+ T cells from peripheral blood mononuclear cells were suspended in X-VIVO 15 media with 5% human AB serum (Valley Biomedicals, Winchester, VA, UA) and 10 ng/mL of recombinant human IL-7 and IL-15 (Miltenyi Biotec) (X-VIVO 15–5% HSA/IL-7/IL-15, hereafter). Then, cells were activated with T Cell TransAct (Miltenyi Biotec) according to the manufacturer’s protocol in six-well plates (Thermo Fisher Scientific) at 37°C with 5% CO2 for 24 h. The activated T cells were washed and suspended in X-VIVO 15–5% HSA/IL-7/IL-15 followed by transduction with four different batches of anti-CD123 chimeric antigen receptor-expressing vectors at a MOI of 25 in a total volume of 1 mL. After 22 h of transduction, the cells were transferred to a G-Rex plate (Wilson Wolf, St. Paul, MN, USA) and incubated in 30 mL of X-VIVO 15–5% HSA/IL-7/IL-15. CD123-specific chimeric antigen receptor-expressing T cell expression, viability, and expansion were determined 5 days post transduction.
Flow Cytometry
A CytoFlex (Beckman Coulter, Brea, CA, USA) instrument was used to acquire immunofluorescence data. FlowJo v.10 (FlowJo, Ashland, OR, USA) was used for data analysis and graphic representation. Genetically modified anti-CD123 chimeric antigen receptor-expressing T cells were detected by using a recombinant human IL-3RA/CD123 Fc chimera (Abcam, Cambridge, UK) and phycoerythrin (PE)-conjugated goat anti-human Fc-IgG (immunoglobulin G; Southern Biotech, Birmingham, AL, USA). We used 7-aminoactinomycin D (7-AAD) (TONBO Biosciences, San Diego, CA, USA) to distinguish live cells from dead cells. Cells were collected and washed twice with PBS (Corning) before CD123 Fc Chimera was added. The cells were incubated for 30 min at 4°C in the dark, washed twice, and incubated with PE-conjugated goat anti-human Fc-IgG in PBS with 2% FBS (fluorescence-activated cell sorting [FACS] buffer) for 30 min at 4°C in the dark. The cells were washed twice and then suspended with FACS buffer containing 7-AAD before analysis.
Statistical Analyses
Statistical analysis was performed using R version 3.3.3 (https://www.r-project.org/). A t test or a one-way ANOVA followed by Tukey’s post hoc analysis was used for analyses. Significance was defined as a p value <0.05.
Author Contributions
M.B., J.K.R., C.-C.W., B.F., F.F., B.H.Y., S.D., M.M.W., Y.-I.K., and J.M. developed methods, performed experiments, and analyzed data. J.K.R. conducted all statistical analyses. S.Z., M.M.W., B.R., and R.E.T. conceived experiments and interpreted data. R.E.T. wrote the manuscript. All authors read and approved the final manuscript.
Conflicts of Interest
The authors declare no competing interests.
Acknowledgments
This study was supported with funding from the National Heart, Lung, and Blood Institute (grant PO1 HL-53749), the Assisi Foundation of Memphis, and the American Lebanese Syrian Associated Charities.
Footnotes
Supplemental Information can be found online at https://doi.org/10.1016/j.omtm.2019.11.011.
Supplemental Information
References
- 1.Zheng Y., Yu F., Wu Y., Si L., Xu H., Zhang C., Xia Q., Xiao S., Wang Q., He Q. Broadening the versatility of lentiviral vectors as a tool in nucleic acid research via genetic code expansion. Nucleic Acids Res. 2015;43:e73. doi: 10.1093/nar/gkv202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ranzani M., Annunziato S., Calabria A., Brasca S., Benedicenti F., Gallina P., Naldini L., Montini E. Lentiviral vector-based insertional mutagenesis identifies genes involved in the resistance to targeted anticancer therapies. Mol. Ther. 2014;22:2056–2068. doi: 10.1038/mt.2014.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ranzani M., Cesana D., Bartholomae C.C., Sanvito F., Pala M., Benedicenti F., Gallina P., Sergi L.S., Merella S., Bulfone A. Lentiviral vector-based insertional mutagenesis identifies genes associated with liver cancer. Nat. Methods. 2013;10:155–161. doi: 10.1038/nmeth.2331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mak A.B., Moffat J. A versatile lentiviral expression system to identify mammalian protein-protein interactions. Methods. 2012;57:409–416. doi: 10.1016/j.ymeth.2012.06.005. [DOI] [PubMed] [Google Scholar]
- 5.Moffat J., Grueneberg D.A., Yang X., Kim S.Y., Kloepfer A.M., Hinkle G., Piqani B., Eisenhaure T.M., Luo B., Grenier J.K. A lentiviral RNAi library for human and mouse genes applied to an arrayed viral high-content screen. Cell. 2006;124:1283–1298. doi: 10.1016/j.cell.2006.01.040. [DOI] [PubMed] [Google Scholar]
- 6.Naldini L. Genetic engineering of hematopoiesis: current stage of clinical translation and future perspectives. EMBO Mol. Med. 2019;11:e9958. doi: 10.15252/emmm.201809958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kumar S.R., Markusic D.M., Biswas M., High K.A., Herzog R.W. Clinical development of gene therapy: results and lessons from recent successes. Mol. Ther. Methods Clin. Dev. 2016;3:16034. doi: 10.1038/mtm.2016.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Levine B.L., Miskin J., Wonnacott K., Keir C. Global Manufacturing of CAR T Cell Therapy. Mol. Ther. Methods Clin. Dev. 2016;4:92–101. doi: 10.1016/j.omtm.2016.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hu B., Tai A., Wang P. Immunization delivered by lentiviral vectors for cancer and infectious diseases. Immunol. Rev. 2011;239:45–61. doi: 10.1111/j.1600-065X.2010.00967.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Naldini L., Blömer U., Gallay P., Ory D., Mulligan R., Gage F.H., Verma I.M., Trono D. In vivo gene delivery and stable transduction of nondividing cells by a lentiviral vector. Science. 1996;272:263–267. doi: 10.1126/science.272.5259.263. [DOI] [PubMed] [Google Scholar]
- 11.Kumar M., Keller B., Makalou N., Sutton R.E. Systematic determination of the packaging limit of lentiviral vectors. Hum. Gene Ther. 2001;12:1893–1905. doi: 10.1089/104303401753153947. [DOI] [PubMed] [Google Scholar]
- 12.Lewis P.F., Emerman M. Passage through mitosis is required for oncoretroviruses but not for the human immunodeficiency virus. J. Virol. 1994;68:510–516. doi: 10.1128/jvi.68.1.510-516.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ramezani A., Hawley R.G. Overview of the HIV-1 Lentiviral Vector System. Curr. Protoc. Mol. Biol. 2002;Chapter 16:Unit 16.21. doi: 10.1002/0471142727.mb1621s60. [DOI] [PubMed] [Google Scholar]
- 14.Modlich U., Navarro S., Zychlinski D., Maetzig T., Knoess S., Brugman M.H., Schambach A., Charrier S., Galy A., Thrasher A.J. Insertional transformation of hematopoietic cells by self-inactivating lentiviral and gammaretroviral vectors. Mol. Ther. 2009;17:1919–1928. doi: 10.1038/mt.2009.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McGarrity G.J., Hoyah G., Winemiller A., Andre K., Stein D., Blick G., Greenberg R.N., Kinder C., Zolopa A., Binder-Scholl G. Patient monitoring and follow-up in lentiviral clinical trials. J. Gene Med. 2013;15:78–82. doi: 10.1002/jgm.2691. [DOI] [PubMed] [Google Scholar]
- 16.Cesani M., Plati T., Lorioli L., Benedicenti F., Redaelli D., Dionisio F., Biasco L., Montini E., Naldini L., Biffi A. Shedding of clinical-grade lentiviral vectors is not detected in a gene therapy setting. Gene Ther. 2015;22:496–502. doi: 10.1038/gt.2015.10. [DOI] [PubMed] [Google Scholar]
- 17.Maude S.L., Frey N., Shaw P.A., Aplenc R., Barrett D.M., Bunin N.J., Chew A., Gonzalez V.E., Zheng Z., Lacey S.F. Chimeric antigen receptor T cells for sustained remissions in leukemia. N. Engl. J. Med. 2014;371:1507–1517. doi: 10.1056/NEJMoa1407222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Porter D.L., Hwang W.T., Frey N.V., Lacey S.F., Shaw P.A., Loren A.W., Bagg A., Marcucci K.T., Shen A., Gonzalez V. Chimeric antigen receptor T cells persist and induce sustained remissions in relapsed refractory chronic lymphocytic leukemia. Sci. Transl. Med. 2015;7:303ra139. doi: 10.1126/scitranslmed.aac5415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cavazzana-Calvo M., Payen E., Negre O., Wang G., Hehir K., Fusil F., Down J., Denaro M., Brady T., Westerman K. Transfusion independence and HMGA2 activation after gene therapy of human β-thalassaemia. Nature. 2010;467:318–322. doi: 10.1038/nature09328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.De Ravin S.S., Wu X., Moir S., Anaya-O’Brien S., Kwatemaa N., Littel P., Theobald N., Choi U., Su L., Marquesen M. Lentiviral hematopoietic stem cell gene therapy for X-linked severe combined immunodeficiency. Sci. Transl. Med. 2016;8:335ra57. doi: 10.1126/scitranslmed.aad8856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Biffi A., Montini E., Lorioli L., Cesani M., Fumagalli F., Plati T., Baldoli C., Martino S., Calabria A., Canale S. Lentiviral hematopoietic stem cell gene therapy benefits metachromatic leukodystrophy. Science. 2013;341:1233158. doi: 10.1126/science.1233158. [DOI] [PubMed] [Google Scholar]
- 22.Eichler F., Duncan C., Musolino P.L., Orchard P.J., De Oliveira S., Thrasher A.J., Armant M., Dansereau C., Lund T.C., Miller W.P. Hematopoietic Stem-Cell Gene Therapy for Cerebral Adrenoleukodystrophy. N. Engl. J. Med. 2017;377:1630–1638. doi: 10.1056/NEJMoa1700554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Aiuti A., Biasco L., Scaramuzza S., Ferrua F., Cicalese M.P., Baricordi C., Dionisio F., Calabria A., Giannelli S., Castiello M.C. Lentiviral hematopoietic stem cell gene therapy in patients with Wiskott-Aldrich syndrome. Science. 2013;341:1233151. doi: 10.1126/science.1233151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ginn S.L., Amaya A.K., Alexander I.E., Edelstein M., Abedi M.R. Gene therapy clinical trials worldwide to 2017: An update. J. Gene Med. 2018;20:e3015. doi: 10.1002/jgm.3015. [DOI] [PubMed] [Google Scholar]
- 25.Mamcarz E., Zhou S., Lockey T., Abdelsamed H., Cross S.J., Kang G., Ma Z., Condori J., Dowdy J., Triplett B. Lentiviral Gene Therapy Combined with Low-Dose Busulfan in Infants with SCID-X1. N. Engl. J. Med. 2019;380:1525–1534. doi: 10.1056/NEJMoa1815408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Milone M.C., O’Doherty U. Clinical use of lentiviral vectors. Leukemia. 2018;32:1529–1541. doi: 10.1038/s41375-018-0106-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Merten O.W., Hebben M., Bovolenta C. Production of lentiviral vectors. Mol. Ther. Methods Clin. Dev. 2016;3:16017. doi: 10.1038/mtm.2016.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McCarron A., Donnelley M., McIntyre C., Parsons D. Challenges of up-scaling lentivirus production and processing. J. Biotechnol. 2016;240:23–30. doi: 10.1016/j.jbiotec.2016.10.016. [DOI] [PubMed] [Google Scholar]
- 29.Zufferey R., Nagy D., Mandel R.J., Naldini L., Trono D. Multiply attenuated lentiviral vector achieves efficient gene delivery in vivo. Nat. Biotechnol. 1997;15:871–875. doi: 10.1038/nbt0997-871. [DOI] [PubMed] [Google Scholar]
- 30.Kim V.N., Mitrophanous K., Kingsman S.M., Kingsman A.J. Minimal requirement for a lentivirus vector based on human immunodeficiency virus type 1. J. Virol. 1998;72:811–816. doi: 10.1128/jvi.72.1.811-816.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Reiser J., Harmison G., Kluepfel-Stahl S., Brady R.O., Karlsson S., Schubert M. Transduction of nondividing cells using pseudotyped defective high-titer HIV type 1 particles. Proc. Natl. Acad. Sci. USA. 1996;93:15266–15271. doi: 10.1073/pnas.93.26.15266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kuroda H., Kutner R.H., Bazan N.G., Reiser J. Simplified lentivirus vector production in protein-free media using polyethylenimine-mediated transfection. J. Virol. Methods. 2009;157:113–121. doi: 10.1016/j.jviromet.2008.11.021. [DOI] [PubMed] [Google Scholar]
- 33.Dull T., Zufferey R., Kelly M., Mandel R.J., Nguyen M., Trono D., Naldini L. A third-generation lentivirus vector with a conditional packaging system. J. Virol. 1998;72:8463–8471. doi: 10.1128/jvi.72.11.8463-8471.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Segura M.M., Mangion M., Gaillet B., Garnier A. New developments in lentiviral vector design, production and purification. Expert Opin. Biol. Ther. 2013;13:987–1011. doi: 10.1517/14712598.2013.779249. [DOI] [PubMed] [Google Scholar]
- 35.Segura M.M., Garnier A., Durocher Y., Coelho H., Kamen A. Production of lentiviral vectors by large-scale transient transfection of suspension cultures and affinity chromatography purification. Biotechnol. Bioeng. 2007;98:789–799. doi: 10.1002/bit.21467. [DOI] [PubMed] [Google Scholar]
- 36.Ansorge S., Lanthier S., Transfiguracion J., Durocher Y., Henry O., Kamen A. Development of a scalable process for high-yield lentiviral vector production by transient transfection of HEK293 suspension cultures. J. Gene Med. 2009;11:868–876. doi: 10.1002/jgm.1370. [DOI] [PubMed] [Google Scholar]
- 37.Lesch H.P., Laitinen A., Peixoto C., Vicente T., Makkonen K.E., Laitinen L., Pikkarainen J.T., Samaranayake H., Alves P.M., Carrondo M.J. Production and purification of lentiviral vectors generated in 293T suspension cells with baculoviral vectors. Gene Ther. 2011;18:531–538. doi: 10.1038/gt.2010.162. [DOI] [PubMed] [Google Scholar]
- 38.Witting S.R., Li L.H., Jasti A., Allen C., Cornetta K., Brady J., Shivakumar R., Peshwa M.V. Efficient large volume lentiviral vector production using flow electroporation. Hum. Gene Ther. 2012;23:243–249. doi: 10.1089/hum.2011.088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sanber K.S., Knight S.B., Stephen S.L., Bailey R., Escors D., Minshull J., Santilli G., Thrasher A.J., Collins M.K., Takeuchi Y. Construction of stable packaging cell lines for clinical lentiviral vector production. Sci. Rep. 2015;5:9021. doi: 10.1038/srep09021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Broussau S., Jabbour N., Lachapelle G., Durocher Y., Tom R., Transfiguracion J., Gilbert R., Massie B. Inducible packaging cells for large-scale production of lentiviral vectors in serum-free suspension culture. Mol. Ther. 2008;16:500–507. doi: 10.1038/sj.mt.6300383. [DOI] [PubMed] [Google Scholar]
- 41.Manceur A.P., Kim H., Misic V., Andreev N., Dorion-Thibaudeau J., Lanthier S., Bernier A., Tremblay S., Gélinas A.M., Broussau S. Scalable Lentiviral Vector Production Using Stable HEK293SF Producer Cell Lines. Hum. Gene Ther. Methods. 2017;28:330–339. doi: 10.1089/hgtb.2017.086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Throm R.E., Ouma A.A., Zhou S., Chandrasekaran A., Lockey T., Greene M., De Ravin S.S., Moayeri M., Malech H.L., Sorrentino B.P., Gray J.T. Efficient construction of producer cell lines for a SIN lentiviral vector for SCID-X1 gene therapy by concatemeric array transfection. Blood. 2009;113:5104–5110. doi: 10.1182/blood-2008-11-191049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wielgosz M.M., Kim Y.S., Carney G.G., Zhan J., Reddivari M., Coop T., Heath R.J., Brown S.A., Nienhuis A.W. Generation of a lentiviral vector producer cell clone for human Wiskott-Aldrich syndrome gene therapy. Mol. Ther. Methods Clin. Dev. 2015;2:14063. doi: 10.1038/mtm.2014.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Valkama A.J., Leinonen H.M., Lipponen E.M., Turkki V., Malinen J., Heikura T., Ylä-Herttuala S., Lesch H.P. Optimization of lentiviral vector production for scale-up in fixed-bed bioreactor. Gene Ther. 2018;25:39–46. doi: 10.1038/gt.2017.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ikeda Y., Takeuchi Y., Martin F., Cosset F.L., Mitrophanous K., Collins M. Continuous high-titer HIV-1 vector production. Nat. Biotechnol. 2003;21:569–572. doi: 10.1038/nbt815. [DOI] [PubMed] [Google Scholar]
- 46.Cockrell A.S., Ma H., Fu K., McCown T.J., Kafri T. A trans-lentiviral packaging cell line for high-titer conditional self-inactivating HIV-1 vectors. Mol. Ther. 2006;14:276–284. doi: 10.1016/j.ymthe.2005.12.015. [DOI] [PubMed] [Google Scholar]
- 47.Xu K., Ma H., McCown T.J., Verma I.M., Kafri T. Generation of a stable cell line producing high-titer self-inactivating lentiviral vectors. Mol. Ther. 2001;3:97–104. doi: 10.1006/mthe.2000.0238. [DOI] [PubMed] [Google Scholar]
- 48.Marin V., Stornaiuolo A., Piovan C., Corna S., Bossi S., Pema M., Giuliani E., Scavullo C., Zucchelli E., Bordignon C. RD-MolPack technology for the constitutive production of self-inactivating lentiviral vectors pseudotyped with the nontoxic RD114-TR envelope. Mol. Ther. Methods Clin. Dev. 2016;3:16033. doi: 10.1038/mtm.2016.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Stornaiuolo A., Piovani B.M., Bossi S., Zucchelli E., Corna S., Salvatori F., Mavilio F., Bordignon C., Rizzardi G.P., Bovolenta C. RD2-MolPack-Chim3, a packaging cell line for stable production of lentiviral vectors for anti-HIV gene therapy. Hum. Gene Ther. Methods. 2013;24:228–240. doi: 10.1089/hgtb.2012.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Farson D., Witt R., McGuinness R., Dull T., Kelly M., Song J., Radeke R., Bukovsky A., Consiglio A., Naldini L. A new-generation stable inducible packaging cell line for lentiviral vectors. Hum. Gene Ther. 2001;12:981–997. doi: 10.1089/104303401750195935. [DOI] [PubMed] [Google Scholar]
- 51.Ni Y., Sun S., Oparaocha I., Humeau L., Davis B., Cohen R., Binder G., Chang Y.N., Slepushkin V., Dropulic B. Generation of a packaging cell line for prolonged large-scale production of high-titer HIV-1-based lentiviral vector. J. Gene Med. 2005;7:818–834. doi: 10.1002/jgm.726. [DOI] [PubMed] [Google Scholar]
- 52.Klages N., Zufferey R., Trono D. A stable system for the high-titer production of multiply attenuated lentiviral vectors. Mol. Ther. 2000;2:170–176. doi: 10.1006/mthe.2000.0103. [DOI] [PubMed] [Google Scholar]
- 53.Greene M.R., Lockey T., Mehta P.K., Kim Y.S., Eldridge P.W., Gray J.T., Sorrentino B.P. Transduction of human CD34+ repopulating cells with a self-inactivating lentiviral vector for SCID-X1 produced at clinical scale by a stable cell line. Hum. Gene Ther. Methods. 2012;23:297–308. doi: 10.1089/hgtb.2012.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Merten O.W., Charrier S., Laroudie N., Fauchille S., Dugué C., Jenny C., Audit M., Zanta-Boussif M.A., Chautard H., Radrizzani M. Large-scale manufacture and characterization of a lentiviral vector produced for clinical ex vivo gene therapy application. Hum. Gene Ther. 2011;22:343–356. doi: 10.1089/hum.2010.060. [DOI] [PubMed] [Google Scholar]
- 55.Bandeira V., Peixoto C., Rodrigues A.F., Cruz P.E., Alves P.M., Coroadinha A.S., Carrondo M.J. Downstream processing of lentiviral vectors: releasing bottlenecks. Hum. Gene Ther. Methods. 2012;23:255–263. doi: 10.1089/hgtb.2012.059. [DOI] [PubMed] [Google Scholar]
- 56.Hanawa H., Persons D.A., Nienhuis A.W. Mobilization and mechanism of transcription of integrated self-inactivating lentiviral vectors. J. Virol. 2005;79:8410–8421. doi: 10.1128/JVI.79.13.8410-8421.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Luke J., Carnes A.E., Hodgson C.P., Williams J.A. Improved antibiotic-free DNA vaccine vectors utilizing a novel RNA based plasmid selection system. Vaccine. 2009;27:6454–6459. doi: 10.1016/j.vaccine.2009.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ausubel L.J., Hall C., Sharma A., Shakeley R., Lopez P., Quezada V., Couture S., Laderman K., McMahon R., Huang P. Production of CGMP-Grade Lentiviral Vectors. Bioprocess Int. 2012;10:32–43. [PMC free article] [PubMed] [Google Scholar]
- 59.Levy C., Fusil F., Amirache F., Costa C., Girard-Gagnepain A., Negre D., Bernadin O., Garaulet G., Rodriguez A., Nair N. Baboon envelope pseudotyped lentiviral vectors efficiently transduce human B cells and allow active factor IX B cell secretion in vivo in NOD/SCIDγc-/- mice. J. Thromb. Haemost. 2016;14:2478–2492. doi: 10.1111/jth.13520. [DOI] [PubMed] [Google Scholar]
- 60.Bach P.B., Giralt S.A., Saltz L.B. FDA Approval of Tisagenlecleucel: Promise and Complexities of a $475 000 Cancer Drug. JAMA. 2017;318:1861–1862. doi: 10.1001/jama.2017.15218. [DOI] [PubMed] [Google Scholar]
- 61.Pacchia A.L., Adelson M.E., Kaul M., Ron Y., Dougherty J.P. An inducible packaging cell system for safe, efficient lentiviral vector production in the absence of HIV-1 accessory proteins. Virology. 2001;282:77–86. doi: 10.1006/viro.2000.0787. [DOI] [PubMed] [Google Scholar]
- 62.Sparacio S., Pfeiffer T., Schaal H., Bosch V. Generation of a flexible cell line with regulatable, high-level expression of HIV Gag/Pol particles capable of packaging HIV-derived vectors. Mol. Ther. 2001;3:602–612. doi: 10.1006/mthe.2001.0296. [DOI] [PubMed] [Google Scholar]
- 63.Kafri T., van Praag H., Ouyang L., Gage F.H., Verma I.M. A packaging cell line for lentivirus vectors. J. Virol. 1999;73:576–584. doi: 10.1128/jvi.73.1.576-584.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Girard-Gagnepain A., Amirache F., Costa C., Lévy C., Frecha C., Fusil F., Nègre D., Lavillette D., Cosset F.L., Verhoeyen E. Baboon envelope pseudotyped LVs outperform VSV-G-LVs for gene transfer into early-cytokine-stimulated and resting HSCs. Blood. 2014;124:1221–1231. doi: 10.1182/blood-2014-02-558163. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.