Abstract
Background:
Athletic training rooms have a high prevalence of bacteria, including multidrug-resistant organisms, increasing the risk for both local and systematic infections in athletes. There are limited data outlining formal protocols or standardized programs to reduce bacterial and viral burden in training rooms as a means of decreasing infection rate at the collegiate and high school levels.
Hypothesis:
Adaptation of a hygiene protocol would lead to a reduction in bacterial and viral pathogen counts in athletic training rooms.
Study Design:
Cohort study.
Level of Evidence:
Level 3.
Methods:
Two high school and 2 collegiate athletic training rooms were studied over the course of the 2017-2018 academic year. A 3-phase protocol, including introduction of disinfectant products followed by student-athlete and athletic trainer education, was implemented at the 4 schools. Multiple surfaces in the athletic training rooms were swabbed at 4 time points throughout the investigation. Bacterial and viral burden from swabs were analyzed for overall bacterial aerobic plate count (APC), bacterial adenosine triphosphate activity, influenza viral load, and multidrug-resistant organisms such as methicillin-resistant Staphylococcus aureus (MRSA) and vancomycin-resistant enterococcus (VRE).
Results:
Overall bacterial load, as measured by APC, was reduced by 94.7% (95% CI, 72.6-99.0; P = 0.003) over the course of the investigation after protocol implementation. MRSA and VRE were found on 24% of surfaces prior to intervention and were reduced to 0% by the end of the study. Influenza was initially detected on 25% of surfaces, with no detection after intervention. No cases of athletic training room–acquired infections were reported during the study period.
Conclusion:
A uniform infection control protocol was effective in reducing bacterial and viral burden, including multidrug-resistant organisms, when implemented in the athletic training rooms of 2 high schools and 2 colleges.
Clinical Relevance:
A standardized infection control protocol can be utilized in athletic training rooms to reduce bacterial and viral burden.
Keywords: infection, athletic training room, bacteria, MRSA
While musculoskeletal injuries represent the most commonly reported health risks during athletic participation, more recent attention has been paid to the high risk of infection among athletes. The athletic training room represents a shared environment involving close contact among athletes and, in the presence of poor hygiene and contamination, can predispose athletes to infection. Several studies and surveys have documented the presence of high bacterial burden in both high school and collegiate training room facilities.6,14,19 Furthermore, high rates of multidrug-resistant organisms, such as vancomycin-resistant enterococcus (VRE) and methicillin-resistant Staphylococcus aureus (MRSA), have been documented to exist within training rooms.7,9 While MRSA is highly publicized because of its propensity to cause localized soft tissue infections, MRSA is also a potential source of bacteremia, pneumonia, and urinary tract infections.16,18 Such infections have been associated with significant morbidity, with up to 70% of patients acquiring MRSA infections requiring hospitalization and intravenous antibiotics.18
The prevention, containment, and treatment of MRSA infections continues to be challenging in collegiate and high school athletes, especially contact sport athletes. Of 21 infection outbreaks in competitive athletes over a 5-year period, 1 in 3 were caused by MRSA.4 A recent survey reported the incidence of MRSA infections as 26.8 per 10,000 athletes in 2015-2016 and 20.3 per 10,000 athletes in 2016-2017.1 Infection incidence was highest in contact sport athletes, such as wrestlers and football players, with incidence rates of 248 per 10,000 and 71 per 10,000, respectively.1 Additional studies have confirmed higher rates of nasal carriers of methicillin-sensitive Staphylococcus aureus and MRSA in contact sport athletes when compared with the general population,7 with the athletic training room serving as a likely transmission source. Montgomery et al13 sampled 10 athletic training rooms in secondary schools in rural communities and reported that 46% of surfaces tested positive for MRSA. Additionally, the National Wrestling Coaches Association found high bio-burdens of bacteria, particularly skin and respiratory bacteria, on collegiate wrestling mats.22 After implementation of a standardized cleaning protocol, the authors reported a 76% reduction in bacterial load using residual disinfectants in comparison with nonresidual cleaners.22 Given the high frequency of MRSA exposure in collegiate and high school athletes, infection control and decontamination protocols warrant further investigation to examine the extent to which implementation decreases the risk of illness.1,10
Professional athletic leagues maintain high standards for hygiene within athletic facilities based on national and international guidelines. Standardized protocols have been outlined by the Centers for Disease Control and Prevention (CDC)2,3 and the World Health Organization (WHO)20,21 to establish best practices for hand hygiene and infection control. The National Football League uses the model outlined by the Duke Infection Control Outreach Network (DICON) to apply CDC and WHO infection control principles throughout the league.11 The National Collegiate Athletic Association publishes a sports medicine handbook to outline CDC guidelines for hygiene and wound care in athletic training rooms.15 In contrast, it is difficult to replicate and implement standardized protocols at the high school level because of limited resources and trained personnel.
Despite the known potential for infection in the athletic training room, there remains a lack of knowledge among athletes, parents, and athletic trainers about best practices to limit the spread of infection. A survey of collegiate and high school athletic trainers found that that while the majority of athletic trainers were aware of the risks of MRSA, 35% of athletic trainers reported performing inadequate hand hygiene while being unaware of proper disinfectant solutions.8 As such, while guidelines for infection management exist, many student-athletes and athletic trainers at the collegiate and high school levels need further training and a better understanding of effective infection control protocols.
The purpose of this investigation was to examine the outcomes of a year-long quality improvement study aimed at reducing bacterial and viral burden in athletic training rooms at the collegiate and high school levels by creating an infection control protocol consisting of hand hygiene solutions, surface disinfectants, athletic trainer education, and student-athlete teaching.
Methods
Study Design
The institutional review board reviewed the protocol and designated the investigation a quality improvement study prior to study initiation. There were 2 suburban high schools and 2 suburban colleges selected for inclusion in the investigation. These schools were representative of the surrounding community and agreed to participate after discussion and approval from athletic trainers and athletic directors.
Bacterial swabs of high-touch surfaces were obtained at 4 separate time points during the academic year. Baseline samples were taken at the start of the school year in September 2017 (time 0). Subsequent samples were obtained in November 2017 (time 1), February 2018 (time 2), and May 2018 (time 3) to correspond with infection control interventions. Sampled surfaces included water bottle lids, water cooler nozzles, training room benches, front door handles, and drawer/cabinet handles. The number of surfaces was proportional to the size of the athletic training room, ranging from 24 to 28 samples at each facility, and varied in total quantity from visit to visit because of availability (eg, if no water bottles were clean and ready to use by athletes, no samples were obtained).
An infection control program was formulated based on CDC and DICON guidelines.2,3,12,20,21 Key components included utilization of disinfectant products with rapid, broad-spectrum antimicrobial efficacy for skin and surfaces, teaching athletic trainers principles of infection control and proper use, and educating student-athletes on hygiene measures. An alcohol-based hand sanitizer (PURELL Foam Handwash; GOJO Industries Inc) was selected along with an antimicrobial spray for hard surfaces (PURELL Surface Spray; GOJO Industries Inc). Educational components involved distribution of electronic and paper educational tools, presented to the athletic trainers, coaches, athletes, and parents at each of the 4 schools. Informative posters were placed around the training rooms and locker rooms to reinforce concepts of proper hand hygiene and infection awareness. Each athletic training room was equipped with written guidelines, and daily checklists were provided to athletic trainers to ensure compliance.
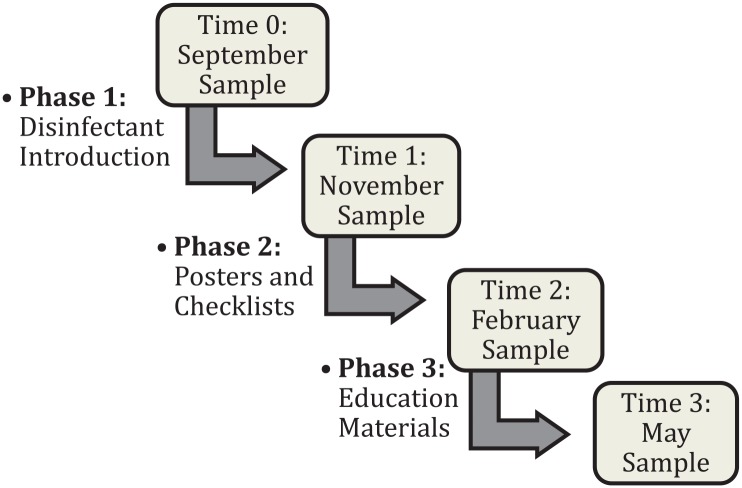
The infection control program was implemented in 3 phases throughout the year to track changes in bacterial and viral load. Phase 1 (between time 0 and time 1) involved installation of products at the point of care in athletic training rooms. Phase 2 (between time 1 and time 2) involved the initiation of educational interventions with the placement of posters and checklists. Posters featuring athletes following CDC protocols were designed by the research team and placed throughout the training room and locker room (see Appendix 1, available in the online version of this article). Checklists were provided in each training room for reference by the athletic trainer, reminding him or her to use surface and hand disinfectants daily. Phase 3 (between time 2 and time 3) involved targeted educational materials distribution. Athletic trainers distributed informational slides to each coach, which were then shared with the athletes. Additional educational emails/handouts were given to parents and athletes. Figure 1 outlines the study design. Athletic trainers were required to record and report any incidence of infection noted during the study period.
Figure 1.
Overview of study design. The above sampling periods are noted in the boxes, with each intervention phase initiated between samples.
Specimen Collection and Analysis
Sampling occurred on weekdays at peak times of athlete presence in the training rooms, generally between 3:00 pm and 7:00 pm. After swab collection, specimens were transported to an approved laboratory and maintained under refrigeration until testing for total and specific microorganisms. Overall cleanliness was quantified by aerobic plate count (APC), while adenosine triphosphate (ATP) assays using CHARM (novaLUM II ATP Detection System; Charm Sciences) and Hygiena (SystemSURE Plus) systems were performed for microbial testing. Samples were also assessed for presence or absence of MRSA, VRE, Enterococcus, and Staphylococcus subspecies. Additional surface samples for influenza were obtained in November 2017 (time 1) and February 2018 (time 2). Appendix 2 (available online) contains the microbiology and assay details.
Statistical Analysis
R (version 3.4.3) and Minitab (version 18) software packages were both used for statistical analysis. Linear models were fit using the lme4 package in R. Individual value, residual, and normal probability plots were used to assess model assumptions and check for outliers. All statistically significant results were reported, with significance set at P < 0.05. To determine percentage reduction and statistical significance over time for ATP and APC results, mixed-effects linear regression models were used. Models included fixed effects for each surface tested and whether the sample came from a high school or a college, with random effects for facility and the date the sample was taken.
To determine the percentage reduction and statistical significance following each study phase, additional models were created with an added fixed effect for whether the sample was taken before or after each intervention. The data were then split by high schools and colleges to determine percentage reduction and statistical significance within each of those categories separately. Models using these split data sets were structured like the previously described mixed-effects regression models, but without the fixed effect for whether the sample was taken at a high school or college. Comparisons between time periods for microbiological species tested for presence or absence were completed using Bayesian analysis. Analysis was performed using the average of beta posteriors for each school and a noninformative beta prior.
Results
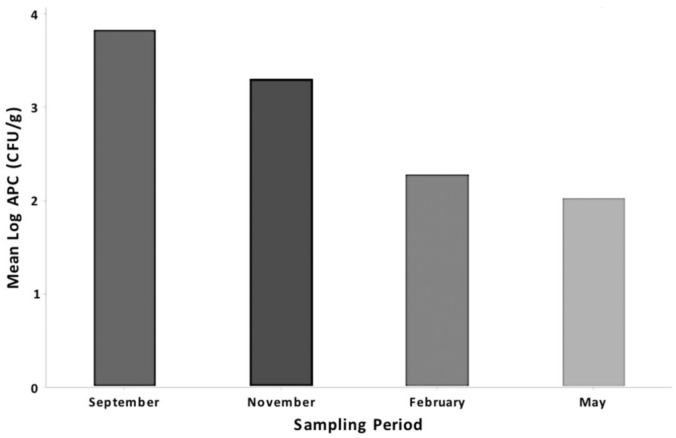
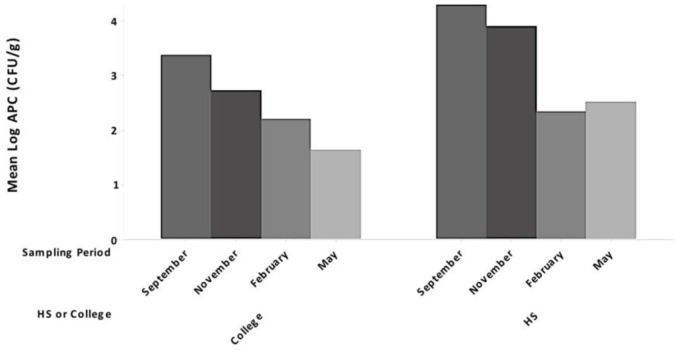
There were no reported infections in student-athletes throughout the study period. A steady decline in APCs was observed at all 4 test locations after implementation of phase 1 in November 2017 to the conclusion of the investigation in May 2018 (Table 1). After implementation and completion of the full quality improvement program, no VRE- or MRSA-positive samples were detected. A steady decline in mean logAPC (in colony-forming units per gram) values for both colleges and high schools over the course of the sampling period was appreciated (Figures 2 and 3). No samples tested positive for Escherichia coli during the investigation, while few samples were positive for coliforms.
Table 1.
Bacterial results summary
| School | Measure | Mean Results (All Surfaces) | |||
|---|---|---|---|---|---|
| September 2017 | November 2017 | February 2018 | May 2018 | ||
| College A | Micro (CFU APC) log mean counts | 2.127 | 2.393 | 1.512 | 1.271 |
| No. of MRSA + VRE hits | 2/12 (16.7%) | 2/12 (16.7%) | 1/8 (12.5%) | 0/8 (0%) | |
| College B | Micro (CFU APC) log mean counts | 4.467 | 3.058 | 2.635 | 1.987 |
| No. of MRSA + VRE hits | 3/14 (21.4%) | 1/13 (7.7%) | 1/13 (7.7%) | 0/8 (0%) | |
| High school A | Micro (CFU APC) log mean counts | 3.836 | 4.263 | 2.232 | 2.457 |
| No. of MRSA + VRE hits | 4/12 (33.3%) | 6/11 (54.5%) | 1/13 (7.7%) | 0/5 (0%) | |
| High school B | Micro (CFU APC) log mean counts | 4.780 | 3.597 | 2.457 | 2.573 |
| No. of MRSA + VRE hits | 3/12 (25%) | 3/12 (25%) | 1/12 (8.3%) | 0/7 (0%) | |
APC, aerobic plate count; CFU, colony-forming unit; MRSA, methicillin-resistant Staphylococcus aureus; VRE, vancomycin-resistant enterococcus.
Figure 2.
Mean logAPC (in CFU/g) values for all schools for each sampling period, pooled across all surface types. APC, aerobic plate count; CFU, colony-forming unit.
Figure 3.
Mean logAPC (in CFU/g) values for all colleges and high schools separately for each sampling period, pooled across all surface types. APC, aerobic plate count; CFU, colony-forming unit; HS, high school.
Percentage reductions from pre- to postintervention (time 0 to aggregate of time 1 through time 3) are shown in Table 2 for APC and ATP measures. Bacterial load, as measured by APC, was reduced by 94.7% (95% CI, 72.6%-99.0%; P = 0.003) from time 0 to the end of the study. When measured using the Hygiena ATP meter, there was a statistically significant reduction in bacterial burden across all schools and surfaces by 60.2% (95% CI, 0.92%-84.0%; P = 0.048). When analyzing high schools and colleges separately, there was a statistically significant reduction in APC measurements from pre- to postintervention of 96.3% for high schools (95% CI, 79.9%-99.3%; P < 0.001) and 92.2% for colleges (95% CI, 33.3%-99.1%; P = 0.029). There was no significant difference for ATP testing when comparing pre- to postintervention in high schools or colleges.
Table 2.
Percentage reduction pooled across schools as noted and all surfaces
| % Reduction | 95% CI | P | |
|---|---|---|---|
| All schools | |||
| Charm ATP | 36.3 | −39.7%, 75.6% | 0.321 |
| Hygiena ATP | 60.2 | 0.92%, 84.0% | 0.048 a |
| APC | 94.7 | 72.6%, 99.0% | 0.003 a |
| High schools only | |||
| Charm ATP | 51.4 | −58.9%, 90.3% | 0.305 |
| Hygiena ATP | 63.3 | −63.1%, 95.0% | 0.255 |
| APC | 96.3 | 79.9%, 99.3% | <0.001 a |
| Colleges only | |||
| Charm ATP | 17.0 | −77.2%, 84.3% | 0.774 |
| Hygiena ATP | 62.3 | −20.7%, 88.8% | 0.090 |
| APC | 92.2 | 33.3%, 99.1% | 0.029 a |
APC, aerobic plate count; ATP, adenosine triphosphate.
Statistically significant reduction.
Influenza was detected on 25% of the surfaces initially with ≥195 viral particles on each contaminated site, which included front door handles (college A, 195 viral particles; high school A, 218 viral particles), drawer handles (high school A, 293 viral particles), water bottle lids (college A, 462 viral particles), and water cooler nozzles (college A, 222 viral particles). Influenza was not detected during the February sampling after implementation of program education.
Discussion
As a shared environment, athletic training rooms act as a source for the spread of infection. Although DICON has served as a blueprint for infection control in the National Football League, there are limitations on the implementation of such programs at the high school and collegiate levels because of limited resources and personnel. To decrease bacterial and viral burden within the training room, this investigation sought to provide practical, feasible resources aimed at educating athletic trainers and student-athletes to minimize infection risk and transmission.
Phase 1 of our study involved the introduction of hand hygiene and surface disinfectant solutions into the athletic training room. This resulted in a modest, albeit nonsignificant, reduction in overall bacterial burden, with a slight increase in the amount of MRSA and VRE detected in the training rooms. Anecdotally, it was observed that while resources were now available, many student-athletes were not consistently utilizing them. The authors suspect that athletic trainers and athletes were not adequately educated on principles of infection control in the training room, similar to the findings reported by Kahanov et al,8 in which 35% of athletes were noncompliant with hand hygiene while many athletic trainers were unaware of how to properly disinfect surfaces for MRSA.
The next 2 phases of this investigation focused on education to address this gap in understanding and compliance with the use of disinfectants. Phase 2 involved the addition of posters and checklists in the training rooms to raise awareness by focusing on hygiene as a crucial element to ensure athlete health. Phase 3 involved direct education of the athletic trainers, coaches, and student-athletes. After these phases, there was a significant improvement in bacterial and viral burdens within the training rooms, indicative of the effectiveness of education in decreasing bio-burden and the potential risk of infection.
Schools had a cumulative MRSA rate of 24% (12/50 surfaces) prior to intervention, lower than the 46% rate of surface MRSA infections in the 10 training rooms sampled by Montgomery et al.13 After implementation of the final phase of our study, both MRSA and VRE were no longer detected in any of the 4 training rooms. These findings are consistent with those reported by Oller et al,14 who reported the presence of MRSA on 31% of surfaces in a single Division II collegiate locker room and weight room, followed by complete elimination after implementation of an infectious control protocol. The protocol employed by Oller et al involved education of custodial staff and student-athletes, focusing on proper use of disinfectants by the custodial staff.14 Instead, our protocol empowered the student-athletes to use hand and surface disinfectants after each training room encounter.
In addition to the quantification of bacterial burden, this investigation represents the first study to our knowledge tracking influenza burden in athletic training rooms. Pope and Koenig17 outlined the risk that influenza poses in the athletic training room, but no sampling or interventions were performed. Meanwhile, the detrimental respiratory effects of influenza, along with other respiratory tract infections, have been well documented.12 It is important to note that this portion of the study in which influenza samples were obtained (November and February) occurred across phase 2, after the initiation of student-athlete education, which was felt to be optimal as schools had both disinfectant products distributed and some educational material implemented. There were limitations, however, given the high degree of variability inherent to the influenza virus,5 even during the winter season. Additional data points between November and February would have been beneficial to better trend the changes in influenza burden; however, resource constraints limited our sample size and sampling frequency.
Conclusion
The implementation of a standardized infectious control protocol revolving around student-athlete and athletic trainer education effectively eliminated multidrug-resistant bacteria and influenza while significantly lowering overall bacterial and viral burden in high school and college athletic training rooms. Future investigations tracking pathogen incidence and transmission in additional schools are warranted to further evaluate the efficacy of this protocol and its effects on infection incidence and outcomes at other institutions in different geographic areas.
Supplemental Material
Supplemental material, 34686_Appendix_1 for Infection Risk Reduction Program on Pathogens in High School and Collegiate Athletic Training Rooms by Mark W. LaBelle, Derrick M. Knapik, James W. Arbogast, Steve Zhou, Lisa Bowersock, Albert Parker and James E. Voos in Sports Health: A Multidisciplinary Approach
Supplemental Material
Supplemental material, 34686_Appendix_2 for Infection Risk Reduction Program on Pathogens in High School and Collegiate Athletic Training Rooms by Mark W. LaBelle, Derrick M. Knapik, James W. Arbogast, Steve Zhou, Lisa Bowersock, Albert Parker and James E. Voos in Sports Health: A Multidisciplinary Approach
Acknowledgments
The authors recognize Stephanie Bock and Chelsea Conley for their help in sample collection, and John N. Rapko, PhD, for his help in data handling and analysis of the ATP meter science. We also acknowledge ATL (Advanced Testing Labs) and Phil Geis, PhD, for execution of all microbiology testing and in writing the microbiology methods appendix.
Footnotes
The following authors declared potential conflicts of interest: J.W.A. is a scientist with GOJO Industries, Inc; L.B. and A.P. received payment and fees from GOJO Industries, Inc; and J.E.V. is a consultant from Arthrex and received royalties from Stryker, Arthrex, and Linvatec. This research was supported by GOJO Industries.
References
- 1. Braun T, Kahanov L. Community-associated methicillin-resistant Staphylococcus aureus infection rates and management among student-athletes. Med Sci Sports Exerc. 2018;50(9):1802-1809. [DOI] [PubMed] [Google Scholar]
- 2. Centers for Disease Control and Prevention. Environmental infection control guidelines. https://www.cdc.gov/infectioncontrol/guidelines/environmental/index.html. Accessed August 9, 2018.
- 3. Centers for Disease Control and Prevention. Guideline for hand hygiene in health-care settings: recommendations of the Healthcare Infection Control Practices Advisory Committee and the HIPAC/SHEA/APIC/IDSA Hand Hygiene Task Force. MMWR Recomm Rep. 2002;51(RR-16):1-45. [PubMed] [Google Scholar]
- 4. Collins JC, O’Connell B. Infectious disease outbreaks in competitive sports, 2005-2010. J Athl Train. 2012;47:516-518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Domenech de, Cellès M, Arduin H, Varon E, et al. Characterizing and comparing the seasonality of influenza-like illnesses and invasive pneumococcal diseases using seasonal waveforms. Am J Epidemiol. 2018;187:1029-1039. [DOI] [PubMed] [Google Scholar]
- 6. Hostetter KS, Lux M, Shelley K, Drummond JL, Laguna P. MRSA as a health concern in athletic facilities. J Environ Health. 2011;74:18-25. [PubMed] [Google Scholar]
- 7. Jiménez-Truque N, Saye EJ, Soper N, et al. Association between contact sports and colonization with Staphylococcus aureus in a prospective cohort of collegiate athletes. Sports Med. 2017;47:1011-1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kahanov L, Gilmore EJ, Eberman LE, Roberts J, Semerjian T, Baldwin L. Certified athletic trainers’ knowledge of methicillin-resistant Staphylococcus aureus and common disinfectants. J Athl Train. 2011;46:415-423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kahanov L, Kim YK, Eberman L, Dannelly K, Kaur H, Ramalinga A. Staphylococcus aureus and community-associated methicillin-resistant Staphylococcus aureus (CA-MRSA) in and around therapeutic whirlpools in college athletic training rooms. J Athl Train. 2015;50:432-437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Karanika S, Kinamon T, Grigoras C, Mylonakis E. Colonization with methicillin-resistant Staphylococcus aureus and risk for infection among asymptomatic athletes: a systematic review and metaanalysis. Clin Infect Dis. 2016;63:195-204. [DOI] [PubMed] [Google Scholar]
- 11. Kayes KS, Engemann JJ, Fulmer EM, Clark CC, Noga EM, Sexton DJ. Favorable impact of an infection control network on nosocomila infection rates in community hospitals. Infect Control Hosp Epidemiol. 2006;27:228-232. [DOI] [PubMed] [Google Scholar]
- 12. Lorenc TM, Kernan MT. Lower respiratory infections and potential complications in athletes. Curr Sports Med Rep. 2006;5:80-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Montgomery K, Ryan TJ, Krause A, Starkey C. Assessment of athletic health care facility surfaces for MRSA in the secondary school setting. J Environ Health. 2010;72(6):8-11. [PubMed] [Google Scholar]
- 14. Oller AR, Province L, Curless B. Staphylococcus aureus recovery from environmental and human locations in 2 collegiate athletic teams. J Athl Train. 2010;45:222-229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Parsons JT. 2014-2015 NCAA Sports Medicine Handbook. Indianapolis, IN: National Collegiate Athletic Association; 2014. [Google Scholar]
- 16. Pecci M, Comeau D, Chawla V. Skin conditions in the athlete. Am J Sports Med. 2009;37:406-418. [DOI] [PubMed] [Google Scholar]
- 17. Pope JS, Koenig SM. Pulmonary disorders in the training room. Clin Sports Med. 2005;24:541-564. [DOI] [PubMed] [Google Scholar]
- 18. Rihn JA, Michaels MG, Harner CD. Community-acquired methicillin-resistant Staphylococcus aureus: an emerging problem in the athletic population. Am J Sports Med. 2005;33:1924-1929. [DOI] [PubMed] [Google Scholar]
- 19. Turbeville SD, Cowan LD, Greenfield RA. Infectious disease outbreaks in competitive sports: a review of the literature. Am J Sports Med. 2006;34:1860-1865. [DOI] [PubMed] [Google Scholar]
- 20. World Health Organization. Hand hygiene tools and resources. http://www.who.int/infection-prevention/tools/hand-hygiene/en/. Accessed August 9, 2018.
- 21. World Health Organization. WHO guidelines on hand hygiene in health care: a summary. First Global Patient Safety Challenge: Clean Care is Safer Care. Geneva, Switzerland: World Health Organization; 2009:64. [PubMed] [Google Scholar]
- 22. Young LM, Motz VA, Markey ER, Young SC, Beaschler RE. Recommendations for best disinfectant practices to reduce the spread of infection via wrestling mats. J Athl Train. 2017;52:82-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, 34686_Appendix_1 for Infection Risk Reduction Program on Pathogens in High School and Collegiate Athletic Training Rooms by Mark W. LaBelle, Derrick M. Knapik, James W. Arbogast, Steve Zhou, Lisa Bowersock, Albert Parker and James E. Voos in Sports Health: A Multidisciplinary Approach
Supplemental material, 34686_Appendix_2 for Infection Risk Reduction Program on Pathogens in High School and Collegiate Athletic Training Rooms by Mark W. LaBelle, Derrick M. Knapik, James W. Arbogast, Steve Zhou, Lisa Bowersock, Albert Parker and James E. Voos in Sports Health: A Multidisciplinary Approach