Abstract
Background:
Nonadherence to smoking cessation medication is a frequent problem. Identifying pre-quit predictors of nonadherence may help explain nonadherence and suggest tailored interventions to address it.
Aims:
Identify and characterize subgroups of smokers based on adherence to nicotine replacement therapy (NRT).
Method:
Secondary classification tree analyses of data from a 2-arm randomized controlled trial of Recommended Usual Care (R-UC, n=315) versus Abstinence-Optimized Treatment (A-OT, n=308) were conducted. R-UC comprised 8 weeks of nicotine patch plus brief counseling whereas A-OT comprised 3 weeks of pre-quit mini-lozenges, 26 weeks of nicotine patch plus mini-lozenges, 11 counseling contacts, and 7-11 automated reminders to use medication. Analyses identified subgroups of smokers highly adherent to nicotine patch use in both treatment conditions, and identified subgroups of A-OT participants highly adherent to mini-lozenges.
Results:
Varied facets of nicotine dependence predicted adherence across treatment conditions 4 weeks post-quit and between 4- and 16-weeks post-quit in A-OT, with greater baseline dependence and greater smoking trigger exposure and reactivity predicting greater medication use. Greater quitting motivation and confidence, and believing that stop smoking medication was safe and easy to use were associated with greater adherence.
Conclusion:
Adherence was especially high in those who were more dependent and more exposed to smoking triggers. Quitting motivation and confidence predicted greater adherence, while negative beliefs about medication safety and acceptability predicted worse adherence. Results suggest that adherent use of medication may reflect a rational appraisal of the likelihood that one will need medication and will benefit from it.
Keywords: Classification tree, adherence, nicotine replacement therapy, nicotine dependence, smoking cessation
1. INTRODUCTION
Nonadherence to smoking cessation medication is common amongst people trying to quit smoking (Mooney, et al., 2005; Shiffman, et al., 2008). In population studies and clinical trials, smokers typically take less than half of prescribed doses or discontinue medication prematurely (Burns and Levinson, 2008; Schlam et al., 2018). Poor adherence to smoking cessation medication is associated with failure to quit (Raupach, et al., 2014; Shiffman et al., 2008). Multiple adherence-focused interventions have been developed, but have modest effects on long-term abstinence rates (e.g., Graham et al., 2017; Hollands, et al., 2019; Schlam et al., 2016; Tseng et al., 2017; cf. Nieuwlaat et al., 2014). Nonadherence is an important limiter of smoking cessation treatment impact that has not been adequately treated.
An excellent recent literature review of 50 studies (Pacek et al., 2018) identified several correlates consistently associated with nonadherence to smoking cessation pharmacotherapy, including male sex, youth, racial minority status, low education, socioeconomic disadvantage; low quitting experience, motivation, and confidence (e.g., Shadel, et al., 2016); negative beliefs about medication (e.g., Voci, et al., 2016; Wiggers et al., 2006); exposure to others smoking (e.g., Cropsey et al., 2017; Grandi et al., 2016); and side effects. This review reported that greater nicotine dependence and stress were generally related to lower adherence (e.g., Balmford, et al., 2011), although there were some findings of positive relations with adherence (e.g., Hood et al., 2013; Okuyemi, et al., 2010; Voci, et al., 2016).
Despite the many studies reviewed by Pacek et al., (2018), important questions remain. Prior research defies easy integration due to inconsistencies across studies in the nature of the study designs, types of interventions, and types and breadth of predictors analyzed. The current study attempts to fill knowledge gaps by using somewhat novel and powerful methods. The present secondary analysis of adherence data in a randomized controlled trial (RCT) of a tobacco abstinence-optimized treatment (A-OT) versus recommended usual care (R-UC) adds to the literature on predictors of nicotine replacement therapy (NRT) adherence via several methodological features. First, analyses focused on a broad range of predictors (Pacek et al., 2018). Second, we report on adherence to the same agent (nicotine patches) in two different, randomly assigned treatments (A-OT vs. R-UC), which enables examination of adherence in intensive care that actively promoted adherence and in the less intensive R-UC. Third, we used efficient machine learning classification tree analyses to screen varied predictors, and their combinations, for associations with adherence. Previous research typically used regression-based analytic approaches (e.g., Cropsey et al., 2017; Vaz et al., 2016) that may fail to identify complex interactions amongst factors associated with adherence. Fourth, we examined correlates of adherence at different times (4 and 16 weeks following a target quit day (TQD)) in the A-OT that involved extended (26-week) treatment, to explore the extent to which adherence correlates vary over time. The present study relies on self-reported adherence data, as the parent trial was conducted in a pragmatic manner in primary care settings; burdensome and invasive assessment was minimized in keeping with a pragmatic research approach (Thorpe et al., 2009). The goal of this study is to identify reliable predictors, or combinations of predictors, that can differentiate subgroups of smokers with high versus low self-reported NRT adherence, using variables assessed prior to the quit attempt in a randomized RCT.
In the parent RCT (Piper et al., 2018), adults recruited in primary care were randomized to receive either a low-intensity R-UC intervention (including nicotine patch, n=315), or a more intensive, A-OT (n=308) comprising components found to be particularly effective in earlier factorial experiments (Piper et al., 2016; Schlam et al., 2016). Analyzing relations of pre-cessation predictors with adherence in the context of this RCT allows us to examine the extent to which of these relations are robust across the independent samples randomized to different conditions, and across different treatment contexts. Pre-cessation variables were selected as predictors rather than variables assessed during NRT use (e.g., lapsing, withdrawal symptoms) for three reasons: 1) to enhance causal inference via clear temporal precedence; 2) to have greater potential for clinical adoption by not relying on costly repeated assessments; and 3) to identify factors that could be used to prevent nonadherence rather than react to it. The current study aimed to identify reliable predictors of nonadherence relevant to both low and high intensity treatments and to identify risk subgroups who may benefit from future interventions to promote adherence.
2. METHODS
2.1. Participants
Participants were recruited from primary care clinics in two Wisconsin health systems. To be eligible, participants needed to be: willing to quit smoking in the next 30 days, at least 18 years old, smoking at least 5 cigarettes per day for the previous 6 months, literate in English, planning to remain in the area for at least 12 months, and reachable by telephone. Exclusion criteria included: current use of bupropion or other smoking medication; history of stroke, heart attack, transient ischemic attack, or an abnormal electrocardiogram in the past 4 weeks; diagnosis of psychosis; or pregnancy or unwillingness to use an approved method of birth control during treatment. Eligible patients provided written informed consent at a study visit at their primary care clinic. Study procedures were approved by an Institutional Review Board.
2.2. Treatments
Participants were randomized to either R-UC or A-OT, unblinded, in a 1:1 ratio, blocked by sex. Consistent with the Public Health Service (PHS) Clinical Guideline Treating Tobacco Use and Dependence (Fiore et al., 2008), R-UC comprised (1) 8 weeks of nicotine patches for use starting on the TQD; (2) one 10-minute, in-person counseling session; (3) faxed referral to the Wisconsin Tobacco Quit Line (WTQL) offering one proactive counseling call with a trained quit coach and unlimited opportunities for the participant to call in for support; and (4) instructions for installing the QUITNOW application, a free smoking cessation mobile app from the WTQL. R-UC participants were instructed to start using patches on the TQD and received a full 8-week supply of patches at a visit one week pre-TQD.
A-OT participants received (1) nicotine mini-lozenges for use in the 3 weeks pre-TQD; (2) 26 weeks of post-quit combination nicotine patches and nicotine mini-lozenges; (3) three 20-minute, in-person cessation counseling sessions focused on providing support and help coping with withdrawal symptoms and smoking triggers; (4) eight 15-minute phone counseling sessions to provide support for smoking cessation and problem solving; and (5) 7-11 automated calls to promote medication adherence (7 calls for those who achieved 7-day abstinence by 8 weeks post-TQD, 11 calls for those smoking 8 weeks post-TQD). A-OT participants were asked to use 9-20 mini-lozenges daily (1 every 1-2 hours) starting 3 weeks pre-quit and tapering off in weeks 25-26 post TQD, and were instructed to wear one patch daily starting on the TQD. Mini-lozenges were dispensed 3 weeks pre-quit, and patches and more mini-lozenges were dispensed one week pre-quit and after follow-ups 8 and 16 weeks post-TQD (if still using medication). A-OT offered more skill-based, supportive counseling to encourage medication adherence and boost cessation success than did R-UC.
2.3. Measures
2.3.1. Predictors assessed at baseline
Baseline predictors of adherence are presented with descriptive statistics in Table1 (demographics and smoking history) and Supplementary Tables 1 (other categorical predictors) and 2 (continuous predictors).
Table 1.
Sample characteristics, overall and by treatment condition
| Total sample (N=623) | Recommended usual care (n=315) | Abstinence-Optimized treatment (n=308) | ||
|---|---|---|---|---|
| Variable | Level | n (%) | ||
| Gender | Female | 357 (57.3) | 184 (58.4) | 173 (56.2) |
| Male | 266 (42.7) | 131 (41.6) | 135 (43.8) | |
| Race | White | 431 (69.3) | 211 (67.0) | 220 (71.7) |
| Member of a racial minority | 191 (30.7) | 104 (33.3) | 87 (28.3) | |
| Marital status | Married | 225 (36.5) | 112 (35.6) | 113 (37.4) |
| unmarried | 392 (63.5) | 203 (64.4) | 189 (62.6) | |
| Education | Less than college | 346 (55.6) | 188 (59.9) | 158 (51.3) |
| Some college or more | 276 (44.4) | 126 (40.1) | 150 (48.7) | |
| Household income | Less than $24,999 per year | 298 (53.1) | 147 (51.2) | 151 (55.1) |
| $25,000 or more per year | 263 (46.9) | 140 (48.8) | 123 (44.9) | |
| Mean (SD) | ||||
| Age in years | 49.7 (12.7) | 49.4 (12.9) | 50.0 (12.5) | |
| Age at first cigarette | 14.7 (3.8) | 14.8 (3.8) | 14.7 (3.9) | |
| Cigarettes per day | 16.8 (9.4) | 17.1 (9.8) | 16.5 (9.0) | |
| FTCD score | 4.8 (2.2) | 4.9 (2.2) | 4.8 (2.2) | |
| Number of past quit attempts | 4.1 (6.0) | 3.8 (4.5) | 4.4 (7.3) | |
Note: There were no statistically significant differences on any variables between treatment conditions. FTCD: Fagerström Test of Cigarette Dependence.
Tobacco dependence was measured by the 6-item Fagerström Test of Cigarette Dependence (FTCD, Fagerström, 2012; Heatherton, et al., 1991, Cronbach’s α=0.58 in this sample) and the 37-item Brief Wisconsin Inventory of Smoking Dependence Motives (Brief WISDM, Smith et al., 2010) with 11 subscales: Affiliative Attachment (α=0.85), Affective Enhancement (α=0.82), Automaticity (α=0.86), Loss of Control (α=0.79), Cognitive Enhancement (α=0.87), Craving (α=0.82), Cue Exposure/Associative Processes (α=0.63), Social and Environmental Goads (α=0.91), Taste and Sensory Properties (α=0.86), Tolerance (α=0.75), and Weight Control (α=0.82).
Eight items from the Wisconsin Beliefs Assessment on Smoking and Cessation (WI-BASC, Schlam et al., 2018; Schlam et al., 2016) and one novel item assessed agreement that stop-smoking medicine is: less important than willpower, not needed if smoking urges are gone, not needed if smoking again, not needed for longer than a couple of weeks, not needed in the recommended amounts, ineffective, addictive, dangerous, or hard to use as instructed. Items were rated on a 7-point Likert scale ranged from 1= “Strongly disagree” to 7= “Strongly agree” and were analyzed individually.
Withdrawal symptoms and affect were assessed with items from the Wisconsin Smoking Withdrawal Scale (WSWS, Welsch et al., 1999) and the Positive and Negative Affect Schedule (PANAS, Watson, et al., 1988). Two items assessed stress-elicited distress and craving to smoke: “Think about the most stressful thing that happened today. How upsetting was it?” and “How much did you want to smoke when this happened?” and two items assessed quitting motivation and confidence: “How motivated are you to quit smoking?” and “How confident are you that you can quit smoking successfully?” rated on a scale from 1= “Not at all” to 7= “Extremely”. Self-reported history of psychiatric disorder and binge drinking (National Institute of Alcohol Abuse and Alcoholism, 2004) were also assessed.
2.3.2. Adherence outcomes
Past-week patch use was assessed via timeline follow-back (Sobell, et al., 1988) during phone interviews 4 weeks post-TQD (for both R-UC and A-OT) and 16 weeks post-TQD (for A-OT). Mini-lozenge use in the past week was assessed at 4 and 16 weeks post-TQD in A-OT. Based on non-normal distributions of adherence, daily patch use was coded as binary (0=used patches 6 or fewer days in the past week and 1=used patches every day for the past week) and mean daily mini-lozenge use was coded as an ordered categorical variable (0=used 0 mini-lozenges per day in the past week, 1=used a mean of 0.1-3.9 mini-lozenges per day in the past week, and 2=used a mean of 4 or more mini-lozenges per day in the past week).
2.4. Data Analysis Plan
The Generalized, Unbiased, Interaction Detection and Estimation (GUIDE; (Loh, 2002, 2009) www.stat.wisc.edu/~loh/quide.html) classification tree modeling program was used to identify predictors of patch use (in R-UC and A-OT) and mini-lozenge use in A-OT, and to distinguish subgroups of smokers based on medication use. Primary analyses were conducted separately in the R-UC and A-OT conditions. Given the differences in treatment conditions in terms of medication intensity (patch alone vs. patch and mini-lozenge), duration (8 vs. 26 weeks), and counseling intensity, duration, and focus on medication adherence (all greater in A-OT than R-UC), we anticipated that predictor-adherence relations might vary across conditions and did not want to obscure this in pooled analyses. We examined interactions between treatment condition and predictors of patch use at 4 weeks post-TQD to see if treatment context significantly moderated predictor-adherence relations during the period of common patch availability. Because GUIDE makes no underlying distributional assumptions about predictors or outcomes and effectively handles non-linear outcomes by means of partitioning (Loh, 2002), we modeled adherence outcomes as categorical. Analyses treating mini-lozenge use as continuous yielded similar results (not shown). Both continuous (e.g., age) and categorical predictors (e.g., sex) were included without transformation because GUIDE identifies optimal cut-points for each continuous variable.
GUIDE is a nonparametric, algorithm-based method that uses binary recursive partitioning cuts to construct a hierarchical decision tree based on a defined outcome. In each partitioning step, GUIDE uses chi-square tests to evaluate associations between each predictor and outcome, and selects the most important predictor variables (tree splitters) to subdivide the sample into groups, with each group as homogeneous on the outcome as possible. Through a successive partitioning process, GUIDE identifies the unique combinations of predictor variables and cut-points that most accurately capture subgroups that differ maximally on the outcome; these are partitioned graphically as a decision tree. The GUIDE approach was selected given its advantages over traditional logistic regression. Logistic regression typically determines the average associations between predictors and an outcome across a sample, without exhaustively searching all possible interactions among predictors (which is not feasible with numerous predictors). In contrast, GUIDE examines interactions amongst all predictor combinations and aids interpretation by identifying subgroups of individuals that are homogeneous in adherence via tree diagrams. Logistic regression tests interactions across an entire sample of individuals. GUIDE, however, initially splits individuals based on the best predictors and the resulting subgroups are then recursively and separately split so that the most informative predictors are identified for each subgroup. This accords with the notion that very different factors may predict outcomes (e.g., adherence) in one subgroup versus another. In addition, GUIDE offers several advantages over other decision tree modeling programs (e.g., CART; Breiman, et al, 1984) by producing unbiased splits, treating missing predictor data as informative, and identifying predictor variables that exceed a threshold (1.0) for importance across possible tree models (Loh, 2014). Importance score ratios greater than 1.0 indicate that a variable is important in predicting the outcome across trees, as determined across all relations within the predictor variable space (both as a sole variable and across all interactions). Importance scores do not indicate the direction of association between a predictor and outcome, however. To verify the direction of the association in the absence of a classification tree, bivariate logistic regression analyses were conducted for each predictor with an importance score above 1.0 for a particular adherence outcome. These logistic regression analyses were conducted to explore relations identified as important by GUIDE; they were not used to test the significance of relations between predictors and adherence outcomes.
In the analyses presented, cases with missing adherence outcome data were omitted. Because data may not be missing at random (e.g., if subjects with missing data were especially likely to be nonadherent), we conducted sensitivity analyses under a range of assumptions about missing adherence data. We ran three imputed models for every outcome, assuming three levels of nonadherence for the missing cases (e.g., with 50%, 70%, 90% of missing responses coded as nonadherent). Missing values in each outcome were imputed based on predicted probabilities derived from logistic regression models and random draws from a Bernoulli distribution. Results of sensitivity analyses for each adherence outcome were essentially the same as those in which missing cases were omitted, and thus are not reported. Missing data for predictors were treated as missing, as GUIDE treats missing predictors as informative, categorical values.
3. RESULTS
Table 1 presents demographic and smoking history data for the 623 adult smokers randomized in this RCT. Of the 315 R-UC participants, 76.5% (n=241) reported on patch use at 4 weeks post-TQD. Of 308 A-OT participants, 61.7% (n=190) and 63.0% (n=194) completed adherence assessments 4 and 16 weeks post-TQD, respectively. Approximately 49.8% of R-UC participants and 71.1% of A-OT participants assessed reported using patches every day in the previous 7 days at 4 weeks (χ2 (N=431) =19.9, p<.001). In A-OT, 69.1% of participants assessed reported using at least 4 mini-lozenges per day in the past week at 4 weeks post-TQD. At 16 weeks, 52.6% of assessed A-OT participants reported daily patch use and 43.8% reported using at least 4 mini-lozenges per day in the past week.
3.1. Patch use at 4 weeks.
In R-UC, quitting motivation was the only splitting variable in the GUIDE classification tree. Participants high in baseline motivation to quit (n=152) were more likely to use patches daily (n=87, 57.2%) than the less motivated (n=89), of whom 33 (37.1%) used patches daily. WISDM Taste and Sensory Properties scores and stress-elicited craving had high importance scores and were both associated with higher probabilities of daily patch use (Table 2). In A-OT, no baseline predictors were associated with daily patch use at 4 weeks and no classification tree model was identified. In the pooled R-UC and A-OT sample, an interaction between treatment condition and baseline stress-elicited craving was detected. Follow-up logistic regression analyses revealed that stress-elicited craving was more weakly related with daily patch use in A-OT than in R-UC (AOR=0.86, 95% CI=0.72-1.03).
Table 2.
Ranking the predictors with importance scores greater than 1.0 in models of daily patch use (in R-UC) and mean daily mini-lozenge use at 4 weeks (in A-OT)
| Outcome | Predictor | Importance score | Odds Ratio (95% CI) |
|---|---|---|---|
| Daily patch use at 4 weeks in R-UC | WISDM Taste and Sensory Properties | 1.54 | 1.10 (0.96-1.27) |
| Quitting motivation | 1.17 | 1.32 (1.00-1.75) | |
| Stress-elicited craving | 1.09 | 1.08 (0.97-1.21) | |
| Mean daily lozenge use at 4 weeks in A-OT | WISDM Cognitive Enhancement | 2.33 | 1.20 (0.96-1.52) |
| WISDM Tolerance | 1.45 | 1.04 (1.04-1.69) | |
| Age at first cigarette | 1.19 | 1.11 (0.98-1.26) | |
| Bans on smoking at workplace | 1.05 | 3.07 (1.15-8.18) | |
| Number of smokers in the household | 1.01 | 1.81 (0.93-3.54) |
Odds ratios in logistic regression predicting adherence (values <1.0 indicate higher values on the predictor are associated with lower log odds of adherence; values >1.0 indicate that high scores on the predictor are associated with increased log odds of adherence); 95% CI: 95% confidence interval. R-UC = Recommended usual care. A-OT = Abstinence-Optimized treatment. WISDM = Wisconsin Inventory of Smoking Dependence Motives.
3.2. Mini-lozenge use at 4 weeks.
Although no classification tree model was identified, importance scores revealed that greater WISDM Cognitive Enhancement and Tolerance scores, older age at first cigarette, having a smoking ban at work, and having more smokers in the household were all positively related to mean daily mini-lozenge use in A-OT at 4 weeks (Table 2).
3.3. Patch use at 16 weeks.
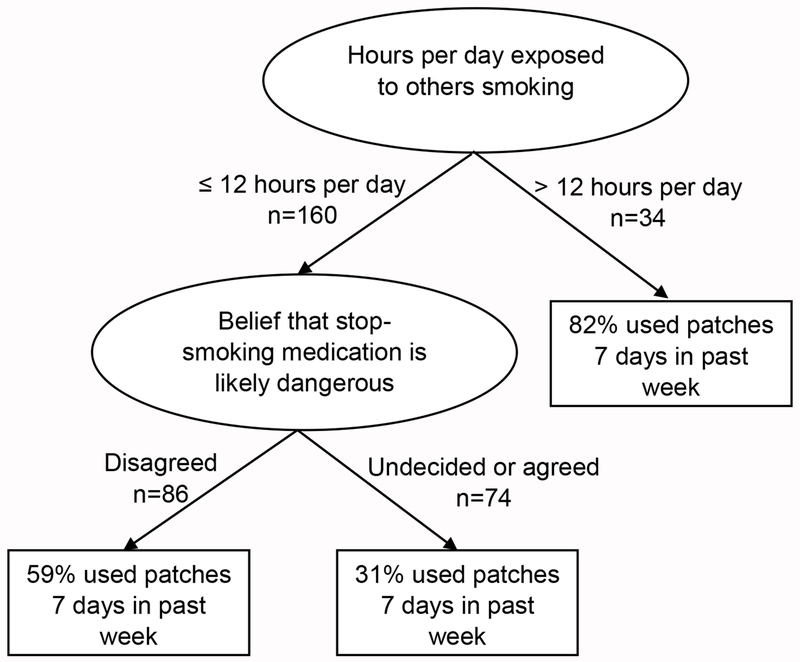
In A-OT, hours of exposure to others smoking was the first splitting variable in the classification tree of daily patch use at 16 weeks, and belief that cessation medication is dangerous was the second splitting variable (Figure 1). Participants who reported at baseline that they were exposed to others smoking >12 hours/day (n=34) showed the highest rate of daily patch use (n=28, 82.4%). Among participants exposed to others smoking ≤12 hours/day (n=160), those not viewing medication as dangerous (n=86) were more likely to use the patch every day (n=51, 59.3%) versus others (n=74, of whom only 23, 31.1% used patches daily). Importance scores and logistic regression analyses revealed that not viewing cessation medication as dangerous, greater WISDM Taste and Sensory Properties scores, more hours of exposure to others smoking, and greater quitting confidence predicted greater likelihood of daily patch use at 16 weeks in A-OT (Table 3).
Figure 1.
GUIDE classification tree predicting daily patch use at 16 weeks in A-OT
Table 3.
Ranking the predictors with importance scores greater than 1.0 in models of daily patch use and mean daily lozenge use at 16 weeks in A-OT
| Outcome | Predictor | Importance score | Odds Ratio (95% CI) |
|---|---|---|---|
| Daily patch use at 16 weeks in A-OT | Belief medication is likely dangerous | 2.22 | 0.73 (0.60-0.88) |
| WISDM Taste and Sensory Properties | 1.84 | 1.21 (1.02-1.43) | |
| Hours exposure to others smoking | 1.67 | 1.31 (1.06-1.62) | |
| Quitting confidence | 1.15 | 1.12 (0.91-1.39) | |
| Mean daily lozenge use at 16 weeks in A-OT | WISDM Primary Dependence | 1.51 | 1.44 (1.10-1.88) |
| WISDM Secondary Dependence | 1.41 | 1.31 (0.98-1.75) | |
| WISDM Automaticity | 1.15 | 1.23 (1.01-1.50) | |
| WISDM Taste and Sensory Properties | 1.08 | 1.12 (0.92-1.36) | |
| WISDM Tolerance | 1.06 | 1.29 (1.06-1.57) | |
| WSWS sleep disturbance | 1.03 | 0.93 (0.80-1.08) |
Odds ratios in logistic regression predicting adherence (values <1.0 indicate higher values on the predictor are associated with lower log odds of abstinence; values >1.0 indicate that high scores on the predictor are associated with increased log odds of adherence); 95% CI: 95% confidence interval. A-OT = Abstinence-Optimized treatment. WISDM = Wisconsin Inventory of Smoking Dependence Motives; WSWS = Wisconsin Smoking Withdrawal Scale. WISDM Primary Dependence Motives scale = mean of Automaticity, Loss of Control, Craving, and Tolerance. WISDM Secondary Dependence Motives scale = mean of Affiliative Attachment, Cognitive Enhancement, Cue Exposure/Associative Processes, Affective Enhancement, Social/Environmental Goads, Taste and sensory properties, and Weight Control.
3.4. Mini-lozenge use at 16 weeks.
Believing that stop-smoking medication is hard to use as instructed was the primary splitting variable predicting lower mean daily mini-lozenge use at 16 weeks in A-OT. Those who disagreed that stop-smoking medicine is hard to use (n=142) were more likely to use at least 4 mini-lozenges per day (n=68, 47.9%) than those who endorsed this baseline belief (n=52), of whom only 17 (32.7%) used at least 4 mini-lozenges per day. Importance scores identified WISDM Primary, Secondary, Automaticity, Taste and Sensory Properties, and Tolerance scores, and WSWS sleep disturbance at baseline (Table 3) as important predictors. Higher levels of dependence on these subscales were associated with more daily mini-lozenge use, whereas sleep disturbance was associated with less use.
4. DISCUSSION
In this secondary analysis of data from a smoking cessation RCT, diverse pre-cessation individual difference and environmental exposure variables were meaningfully related to NRT use. Although the forms of medication (nicotine patch vs. mini-lozenge) and adjuvant treatments (e.g., counseling intensity, adherence reminders) varied across analyses, similar variables emerged as predictors of adherence across analyses.
Nicotine dependence facets predicted greater use of NRT, in contrast to most previous research showing lower adherence among more nicotine-dependent smokers (Balmford et al., 2011; Hollands et al., 2013; Pacek et al., 2018). However, the Pacek et al. (2018) review noted that previous findings on this relation have been somewhat mixed. In the current study, higher WISDM Taste and Sensory Properties scores predicted greater use of both the patch and the mini-lozenge in A-OT. Higher scores on at least one facet of primary dependence motives (Tolerance, Automaticity) and at least one facet of secondary dependence motives (e.g., Cognitive Enhancement, Taste and Sensory Properties) predicted greater use of mini-lozenges at both follow-ups. These positive relations suggest that NRT adherence may be driven by perceived dependence. Individuals may be rational actors titrating NRT to their perceived needs. These relations also could reflect an indirect effect of dependence: i.e., the most dependent may benefit most from NRT and this benefit may enhance adherence.
In contrast to previous studies (e.g., Cropsey et al., 2017; Grandi et al., 2016), greater environmental exposure and reactivity to smoking triggers predicted greater NRT adherence. Craving cigarettes in response to environmental stress predicted patch use in R-UC, and to a lesser degree, in A-OT. These findings add to a mixed literature on relations between stress and cessation therapy adherence. While some studies identify stress as predictive of nonadherence (Voci, et al., 2016), others show stress-related increases in NRT use (Okuyemi, et al., 2010). Unlike earlier studies that found that exposure to others smoking predicted worse adherence (Cropsey et al., 2017; Grandi et al., 2016; Pacek et al., 2018), we found mini-lozenge use was greater among those living with smokers at 4 weeks, and hours of exposure to others smoking was the first splitter in the classification tree of greater patch use at 16 weeks. Perhaps people who perceive many smoking triggers are more adherent as a strategy for reducing this risk. Also quitting motivation and confidence were predictive of greater medication use, as in past studies (Pacek et al., 2018; Shadel et al., 2016). Those with greater motivation used more patches in R-UC and those with more confidence in quitting used more patches 16 weeks into A-OT. Thus, while much attention has been focused on the fact that smokers’ misconceptions about medication may undermine adherence (Cummings et al., 2004; Mooney, et al., 2006), many smokers appear to act as rational actors; they use more medication if they really want to quit and if they believe they need it due to high dependence and exposure to smoking triggers.
Endorsement of specific beliefs regarding NRT dangerousness and difficulty of use predicted lower use of patches and mini-lozenges, respectively, at 16 weeks in A-OT. Prior research has also found that beliefs about treatment predict adherence (Pacek et al., 2018). Modifying such beliefs to improve adherence is difficult, as shown by recent studies of interventions focused on medication beliefs (Hollands et al., 2019; Schlam et al., 2016; Smith et al., 2013). Meta-analysis of adherence interventions suggests that practical interventions may be more effective than trying to change beliefs (Hollands et al., 2019). The use of automated phone reminders to use NRT in A-OT may be such a practical intervention contributing to the higher self-reported rates of patches in A-OT vs. R-UC, although the multiple intervention components used in the A-OT treatment make that explanation difficult to evaluate.
Collectively, these results suggest a simple clinical decision model to predict adherence based on baseline individual differences. This approach may identify individuals at particularly high risk for nonadherence that may benefit from targeted interventions to promote medication use. If the hypothesis that individuals who perceive greater need for medication (due to high levels of self-reported dependence, stress reactivity, and exposure to smoking triggers) use more smoking medication is corroborated, then interventions that increase perceived need may also enhance adherence. One reason for inconsistency in relations between these results and the literature on adherence correlates may be that some measures may capture a risk factor (e.g., nicotine dependence) whereas others capture awareness of the significance of the risk factor (e.g., awareness of the tendency to lose control over smoking). If the rational actor hypothesis is correct, then it is the awareness of personal risk (rather than risk per se) that may drive adherence. Providing feedback about levels of dependence, stress reactivity, or environmental risk may motivate adherence.
Limitations of this study include the possibility that the particular pattern of results may reflect psychometric rather than construct-related differences among measures (i.e., the most reliable rather than the most valid measures emerged as predictors of adherence). Also, this study relied upon self-reported adherence, which is prone to presentation and recall bias but is more practical and affordable in large clinical trials (Hollands et al., 2019; Lam and Fresco, 2015). Objective and more rigorous measures of NRT adherence were not feasible due to concerns about burden and possible assessment reactivity in this pragmatic trial. As Pacek et al. (2018) have noted, it is important to improve the rigor of adherence assessments in the future (e.g., through electronic monitoring of use as in Schlam et al., 2016). Text messaging reminders and mobile assessments may be useful in enhancing the accuracy of self-reported adherence in the future (Dowshen, et al., 2013; Ershad Sarabi, et al., 2016). In addition, missing adherence data may have affected results, although sensitivity analyses across a range of missing data values yielded similar results.
5. Conclusions
Classification tree models identified nicotine dependence, environmental exposure to smoking triggers, motivational variables, and medication beliefs as important predictors of nicotine patch and mini-lozenge use across two different intensities of smoking cessation treatment for adult smokers motivated to quit. Results suggest that for many smokers the adherent use of medication reflects a rational appraisal of the likelihood that they will need medication and will benefit from it. This information may be useful in developing targeted intervention strategies to promote adherence, and thereby enhance cessation success.
Supplementary Material
Highlights.
Classification trees identified smokers high vs. low in adherence to medication.
Nicotine dependence, quitting motivation, and smoking triggers predicted adherence.
Negative beliefs about nicotine medication predicted nonadherence.
Acknowledgements
We would like to thank the participants in this research, co-investigators on the P01 Center Grant that funded this research, and the staff and students at the University of Wisconsin Center for Tobacco Research and Intervention, particularly Christopher Todd Hayes-Birchler and Wendy Theobald, for their contributions to this research.
Role of funding sources
This work was supported by a grant 5P01CA180945-05 from the National Cancer Institute (Michael C. Fiore and Timothy B. Baker, Principal Investigators).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of interest
No conflict declared.
References
- Balmford J, Borland R, Hammond D, & Cummings KM (2011). Adherence to and reasons for premature discontinuation from stop-smoking medications: data from the ITC Four-Country Survey. Nicotine Tob Res, 13(2), 94–102. doi: 10.1093/ntr/ntq215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breiman L, Friedman JH, Olshen RA, & Stone CJ (1984). Classification and regression trees. Belmont, CA: Chapman & Hall/CRC Press. [Google Scholar]
- Burns EK, & Levinson AH (2008). Discontinuation of nicotine replacement therapy among smoking-cessation attempters. Am J Prev Med, 34(3), 212–215. [DOI] [PubMed] [Google Scholar]
- Cropsey KL, Clark CB, Stevens EN, Schiavon S, Lahti AC, & Hendricks PS (2017). Predictors of medication adherence and smoking cessation among smokers under community corrections supervision. Addict Behav, 65, 111–117. doi: 10.1016/j.addbeh.2016.10.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummings KM, Hyland A, Giovino GA, Hastrup JL, Bauer JE, & Bansal MA(2004). Are smokers adequately informed about the health risks of smoking and medicinal nicotine? Nicotine Tob Res, 6(Suppl 3), S333–S340. [DOI] [PubMed] [Google Scholar]
- Dowshen N, Kuhns LM, Gray C, Lee S, Garofalo R (2013) Feasibility of interactive text message response (ITR) as a novel, real-time measure of adherence to antiretroviral therapy for HIV+ youth. AIDS Behav, 17(6), 2237–2243. doi: 10.1007/s10461-013-046406 [DOI] [PubMed] [Google Scholar]
- Ershad Sarabi R, Sadoughi F, Jamshidi Orak R, & Bahaadinbeigy K (2016). The Effectiveness of Mobile Phone Text Messaging in Improving Medication Adherence for Patients with Chronic Diseases: A Systematic Review. Iranian Red Crescent medical journal, 18(5), e25183. doi: 10.5812/ircmj.25183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fagerström K (2012). Determinants of tobacco use and renaming the FTND to the Fagerström Test for Cigarette Dependence. Nicotine Tob Res, 14(1), 75–78. doi: 10.1093/ntr/ntr137 [DOI] [PubMed] [Google Scholar]
- Fiore MC, Jaen CR, Baker TB, Bailey WC, Benowitz N, Curry SJ, … Wewers ME (2008). Treating tobacco use and dependence: 2008 update. Rockville, MD: U.S. Department of Health and Human Services, U.S. Public Health Service. [Google Scholar]
- Graham AL, Papandonatos GD, Cha S, Erar B, Amato MS, Cobb NK, … Abrams DB (2017). Improving adherence to smoking cessation treatment: intervention effects in a web-based randomized trial. Nicotine Tob Res, 19(3), 324–332. doi: 10.1093/ntr/ntw282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grandi SM, Eisenberg MJ, Joseph L, O’Loughlin J, Paradis G, & Filion KB (2016). Cessation treatment adherence and smoking abstinence in patients after acute myocardial infarction. Am Heart J, 173, 35–40. doi: 10.1016/j.ahj.2015.12.003 [DOI] [PubMed] [Google Scholar]
- Heatherton TF, Kozlowski LT, Frecker RC, & Fagerstrom KO (1991). The Fagerstrom Test for Nicotine Dependence: a revision of the Fagerstrom Tolerance Questionnaire. Br J Addict, 86(9), 1119–1127. [DOI] [PubMed] [Google Scholar]
- Hollands GJ, Sutton S, McDermott MS, Marteau TM, & Aveyard P (2013). Adherence to and consumption of nicotine replacement therapy and the relationship with abstinence within a smoking cessation trial in primary care. Nicotine Tob Res, 15(9), 1537–1544. doi: 10.1093/ntr/ntt010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollands GJ, Naughton F, Farley A, Lindson N, & Aveyard P (2019). Interventions to increase adherence to medications for tobacco dependence. Cochrane Database of Systematic Reviews, Issue 8 Art. No.: CD009164. DOI 10.1002/14651858.CD009164.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hood NE, Ferketich AK, Paskett ED, & Wewers ME (2013). Treatment adherence in a lay health adviser intervention to treat tobacco dependence. Health education research, 28(1), 72–82. doi: 10.1093/her/cys081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam WY, & Fresco P (2015). Medication Adherence Measures: An Overview. BioMed research international, 217047. doi: 10.1155/2015/217047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loh W-Y (2002). Regression trees with unbiased variable selection and interaction detection. Statistics Sinica, 12, 361–386. [Google Scholar]
- Loh W-Y (2009). Improving the precision of classification trees. Ann Appl Stat, 31710–1737. [Google Scholar]
- Loh WY (2014). Fifty years of classification and regression trees. Int Stat Rev, 34, 329–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mooney M, Babb D, Jensen J, & Hatsukami D (2005). Interventions to increase use of nicotine gum: a randomized, controlled, single-blind trial. Nicotine Tob Res, 7(4), 565–579. doi: 10.1080/14622200500185637 [DOI] [PubMed] [Google Scholar]
- Mooney ME, Leventhal AM, & Hatsukami DK (2006). Attitudes and knowledge about nicotine and nicotine replacement therapy. Nicotine Tob Res, 8(3), 435–46. [DOI] [PubMed] [Google Scholar]
- National Institute of Alcohol Abuse and Alcoholism. (2004). NIAAA Council approves definition of binge drinking3. Retrieved from http://pubs.niaaa.nih.gov/publications/Newsletter/winter2004/Newsletter_Number3.pdf
- Nieuwlaat R, Wilczynski N, Navarro T, Hobson N, Jeffery R, Keepanasseril A, … Haynes RB (2014). Interventions for enhancing medication adherence. Cochrane Database Syst Rev(11), CD000011. doi: 10.1002/14651858.CD000011.pub4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okuyemi KS, Zheng H, Guo H, & Ahluwalia JS (2010). Predictors of adherence to nicotine gum and counseling among African-American light smokers. J Gen Intern Med, 25(9), 969–976. doi: 10.1007/s11606-010-1386-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacek LR, McClernon FJ, & Bosworth HB (2018). Adherence to pharmacological smoking cessation interventions: a literature review and synthesis of correlates and barriers. Nicotine Tob Res, 20(10), 1163–1172. doi: 10.1093/ntr/ntx210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piper, Cook JW, Schlam TR, Jorenby DE, Smith SS, Collins LM, … Baker TB (2018). A randomized controlled trial of an optimized smoking treatment delivered in primary care. Ann Behav Med, 52(10):854–864. doi: 10.1093/abm/kax059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piper, Fiore MC, Smith SS, Fraser D, Bolt DM, Collins LM, … Baker TB (2016). Identifying effective intervention components for smoking cessation: a factorial screening experiment. Addiction, 111(1), 129–141. doi: 10.1111/add.13162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raupach T, Brown J, Herbec A, Brose L, & West R (2014). A systematic review of studies assessing the association between adherence to smoking cessation medication and treatment success. Addiction, 109(1), 35–43. doi: 10.1111/add.12319 [DOI] [PubMed] [Google Scholar]
- Schlam, Cook JW, Baker TB, Hayes-Birchler T, Bolt DM, Smith SS, … Piper ME (2018). Can we increase smokers’ adherence to nicotine replacement therapy and does this help them quit? Psychopharmacology (Berl), 235(7), 2065–2075. doi: 10.1007/s00213-018-4903-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlam, Fiore MC, Smith SS, Fraser D, Bolt DM, Collins LM, … Baker TB (2016). Comparative effectiveness of intervention components for producing long-term abstinence from smoking: a factorial screening experiment. Addiction, 111(1), 142–155. doi: 10.1111/add.13153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shadel WG, Galvan FH, & Tucker JS (2016). Developing a nicotine patch adherence intervention for HIV-positive Latino smokers. Addict Behav, 59, 52–57. doi: 10.1016/j.addbeh.2016.03.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiffman S, Sweeney CT, Ferguson SG, Sembower MA, & Gitchell JG (2008). Relationship between adherence to daily nicotine patch use and treatment efficacy: secondary analysis of a 10-week randomized, double-blind, placebo-controlled clinical trial simulating over-the-counter use in adult smokers. Clin Ther, 30(10), 1852–1858. doi: 10.1016/j.clinthera.2008.09.016 [DOI] [PubMed] [Google Scholar]
- Smith SS, Keller PA, Kobinsky KH, Baker TB, Fraser DL, Bush T, … Fiore MC (2013). Enhancing tobacco quitline effectiveness: identifying a superior pharmacotherapy adjuvant. Nicotine Tob Res, 15(3), 718–728. doi: 10.1093/ntr/nts186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SS, Piper ME, Bolt DM, Fiore MC, Wetter DW, Cinciripini PM, & Baker TB (2010). Development of the Brief Wisconsin Inventory of Smoking Dependence Motives. Nicotine Tob Res, 12(5), 489–499. doi: 10.1093/ntr/ntq032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobell LC, Sobell MB, Leo GI, & Cancilla A (1988). Reliability of a timeline method: Assessing normal drinkers’ reports of recent drinking and a comparative evaluation across several populations. Br J Addict, 83(4), 393–402. [DOI] [PubMed] [Google Scholar]
- Thorpe KE, Zwarenstein M, Oxman AD, Treweek S, Furberg CD, Altman DG, … Chalkidou K (2009). A pragmatic-explanatory continuum indicator summary (PRECIS): a tool to help trial designers. J Clin Epidemiol, 62 (5), 464–475. doi: 10.1016/j.jclinepi.2008.12.011. [DOI] [PubMed] [Google Scholar]
- Tseng TY, Krebs P, Schoenthaler A, Wong S, Sherman S, Gonzalez M, … Shelley D (2017). Combining text messaging and telephone counseling to increase varenicline adherence and smoking abstinence among cigarette smokers living with HIV: a randomized controlled study. AIDS Behav, 21(7), 1964–1974. doi: 10.1007/s10461-016-1538-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaz LR, Aveyard P, Cooper S, Leonardi-Bee J, Coleman T, & Team S. T. (2016). The association between treatment adherence to nicotine patches and smoking cessation in pregnancy: a secondary analysis of a randomized controlled trial. Nicotine Tob Res, 18(10), 1952–1959. doi: 10.1093/ntr/ntw080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voci SC, Zawertailo LA, Hussain S, & Selby PL (2016). Association between adherence to free nicotine replacement therapy and successful quitting. Addict Behav, 61, 25–31. doi: 10.1016/j.addbeh.2016.05.012 [DOI] [PubMed] [Google Scholar]
- Watson D, Clark LA, & Tellegen A (1988). Development and validation of brief measures of positive and negative affect: The PANAS scales. J Pers Soc Psychol, 54(6), 1063–1070. doi: 10.1037/0022-3514.54.6.1063 [DOI] [PubMed] [Google Scholar]
- Welsch SK, Smith SS, Wetter DW, Jorenby DE, Fiore MC, & Baker TB (1999). Development and validation of the Wisconsin Smoking Withdrawal Scale. Exp Clin Psychopharmacol, 7(4), 354–361. doi: 10.1037/1064-1297.7.4.354 [DOI] [PubMed] [Google Scholar]
- Wiggers LC, Smets EM, Oort FJ, Storm-Versloot MN, Vermeulen H, van Loenen LB, … Legemate DA (2006). Adherence to nicotine replacement patch therapy in cardiovascular patients. Int J Behav Med, 13(1), 79–88. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.