Abstract
Short chain chlorinated paraffins (SCCPs) are a group of complex emerging persistent organic pollutants. In this study, the uptake, translocation, and transformation of four constitutionally defined SCCP isomers were studied using whole pumpkin (Cucurbita maxima × C. moschata) and soybean (Glycine max L. Merrill) seedlings via hydroponic exposure. Results showed that the daughter SCCPs were C10Cl5–8 and C11–13Cl5–6. The metabolic transformation of all tested isomers included dechlorination and chlorine rearrangement. In addition, carbon chain decomposition products were found for isomers with trichlorinated carbon atoms (CCl3-groups) in both pumpkin and soybean seedlings. This study provides the first evidence of carbon chain decomposition of SCCPs in whole plants, and it suggests new metabolism pathways of SCCPs in the environment. The influence of carbon chain length and degree of chlorination of SCCPs on their fate and behavior within different plant species were also investigated. Bioaccumulation of SCCPs in pumpkin and soybean increased with increasing carbon chain length and degree of chlorination. In comparison, soybean translocated and degraded parent SCCPs faster and to a greater extent than pumpkin, but pumpkin accumulated parent SCCPs to a greater extent than soybean. After 10 days exposure, less than 4% of the initial mass of exposed chemicals remained in solution of exposure groups. The parent chemicals accumulated in roots ranging from 23.6% to 59.9% for pumpkin and 1.98% to 54.5% for soybean and in stems ranging from 0.7% to 3.81% for pumpkin and 0.50% to 2.54% for soybean. These results give new perspectives on the transport, transformation, and fate of SCCPs in the environment.
Graphical Abstract
1. INTRODUCTION
Short chain chlorinated paraffins (SCCPs) are highly complex technical mixtures containing a great number of isomers with carbon chains ranging from C10 to C13. They are used as cutting fluids, flame retardants, plasticizers, and additives.1 Due to their wide distribution in the environment,2 high toxicity, and bioaccumulation in aquatic organisms,3 SCCPs were listed as restricted chemicals in the European water framework directive of 20003,4 and in metal and leather industries in the European Union in 2002.2,5 They were classified as persistent toxic substances by UNEP in 2003,6 priority toxic substances in the United States in 2009, and as persistent organic pollutants (POPs) by the Stockholm Convention in 2017.7 As a consequence, the production and usage of SCCPs will decrease in the near future,8 and medium chain chlorinated paraffins (MCCPs, C14–17) and long chain chlorinated paraffins (LCCPs, C18–30) are available alternatives of SCCPs.
Reports about SCCPs in the environment have increased along with the development of better cleanup procedures and new instrumental techniques over the past five years.4 The distributions of SCCPs in farmlands, marine sediments, mammals, sewage sludge, and aquatic ecosystem affected by sewage treatment plants have been studied.9–15 SCCPs in sediments from the Liaohe River Basin and Pearl River Delta and in the marine food web from Liaodong Bay in China were reported.16–18 Most studies to date have reported SCCPs environmental occurrence, distributions, and concentrations. Only a few reports have focused on the fates of SCCPs in various environmental biota.19,20
Environmental processes in plants affect the global cycling and environmental risk evaluation for these organic contaminants.21 Several POPs are known to be uptaken by plants. Uptake and translocation of polychlorinated biphenyls (PCBs) in crops, such as beans (Phaseolus vulgaris), cabbages (Brassica oleracea var. capotata L.), carrots (Daucus carota L.) and turnips (Barssica rapa L.), have been reported.22–24 Poplar plants were not only able to take up PCBs25 but also to hydroxylate 3,3′,4,4′-tetrachlorobiphenyl (PCB 77)26 and 4-monochlorobiphenyl (PCB 3).27 Maize (Zea may L.) can take up and metabolize PCBs and polybrominated diphenyl ethers (PBDEs) in hydroponic exposure experiments.28,29 Other POPs, such as 2,2-bis(chlorophenyl)1,1,1-trichloroethane (DDT) and chlordane, are known to bioaccumulate and translocate within vascular plants and crops.30,31 Nevertheless, there is limited knowledge on the uptake, translocation, and metabolism of SCCPs within whole plants.
We reported the uptake, bidirectional translocation, phytovolatilization, dechlorination, and chlorine rearrangement of 1,2,5,5,6,9,10-heptachlorodecane in pumpkin in our previous work.32 Results showed that leaves of pumpkins in blank controls were able to take up phytovolatilized daughter chlorodecanes from the air phase, which were subsequently translocated downward to the roots. For pumpkins that were exposed hydroponically to 1,2,5,5,6,9,10-heptachlorodecane, the uptake by roots and upward translocation from the roots to the shoots were dominant. Pumpkin and soybean were chosen as model plants for investigation due to their role as food plants and their high capacity for translocation, bioaccumulation, and biotransformation.31,33–37 Hydroponic exposures were chosen to eliminate the soil and rhizospheric interactions with SCCPs and to focus on biotransformations within plant tissues. Thus, to further study the universality and the time courses of these environmental processes of SCCPs in different plants, chloro-substituted decane, undecane, and tridecane with defined chlorine positions were hydroponically exposed to whole plants of pumpkin and soybean for 10 days. The effects of the carbon chain length and the number of chlorine atoms of SCCPs on their fate and metabolism were the special focus of this study.
2. MATERIALS AND METHODS
2.1. Chemicals and Reagents
All the individual SCCP standards were purchased from Chiron, Norway and Dr. Ehrensforfer GmbH, Germany. The standards included 1,1,1,3,8,10,10,10-octachlorodecane (1,1,1,3,8,10,10,10-OctaCD), 1,1,1,3,10,11-hexachloroundecane (1,1,1,3,10,11-HexCU), 1,1,1,3,12,13-hexachlorotridecane (1,1,1,3,12,13-HexCT), 1,2,5,6,9-pentachlorodecane (1,2,5,6,9-PentaCD), 1,2,5,6,9,10-hexachlorodecane (1,2,5,6,9,10-HexCD), hexachlorodecane (1,2,4,5,9,10-HexCD), 1,2,4,5,6,9,10-heptachlorodecane (1,2,4,5,6,9,10-HepCD), 1,2,5,5,6,9,10-heptachlorodecane (1,2,5,5,6,9,10-HepCD), and 1,1,1,3,8,9-hexachlorononane (1,1,1,3,8,9-HexCN). Their concentrations and purities are listed in Table S1. The concentrations of chlorodecane standard solutions obtained from Ehrenstorfer GmbH have been adjusted according to the purity of the compounds as indicated. The injection internal standard, ε-hexachlorocyclohexane (ε-HCH, 10 mg∙L−1 in cyclohexane, 99.9%), and CP reference standards of SCCP mixtures (C10–13 with chlorine contents of 51.5, 55.5, and 63.0%) and MCCP mixtures (C14–17 with 42.0%, 52.0%, and 57.0% chlorine content) were purchased from Ehrenstorfer GmbH (Augsburg, Germany). 13C10-trans-Chlordane (100 mg∙L−1 in n-nonane, 99.9%) was obtained from Cambridge Isotope Laboratories (Andover, USA) and used as the surrogate standard. Other details are described in Text S1.
2.2. Hydroponic Exposure
Pumpkin (Cucurbita maxima × C. moschata) and soybean (Glycine max L. Merrill) seeds were obtained from Taigu Yinong Seed CO. Ltd. (Shanxi, China) and the Chinese Academy of Agricultural Sciences (Beijing, China), respectively. The seeds were pregerminated on filter paper and drenched with deionized water at 30 °C in darkness for 3–4 days. After germination, the seedlings were transplanted to sterilized perlite beds for 7–10 days of cultivation and then grown in the sterile deionized water without perlite for 2 days before exposure.
Healthy soybean seedlings (shoot height of 9–10 cm) and pumpkin seedlings (shoot height of 5–6 cm) were selected for the hydroponic exposure. Brown glass bottles (50 mL) were used as hydroponic reactors, which contained 40 mL of autoclaved deionized water (120 °C for 20 min) and four seedlings. Suitable amounts of individual SCCP standards in acetone were added into the reactor to make a solution containing four parent SCCP congeners. The initial exposure concentrations were 99.7, 202, 199, and 199 ng/mL for 1,2,5,6,9,10-HexCD, 1,1,1,3,8,10,10,10-OctaCD, 1,1,1,3,10,11-HexCU, and 1,1,1,3,12,13-HexCT, respectively, within the environmental levels reported for SCCPs. Higher concentrations were used for the latter three congeners owing to their relatively low instrumental responses in ECNI-MS.38 Though the exposure concentrations were set higher than the modeled water solubility of the parent chemicals (Table S2) to guarantee good instrumental responses, the uptake, translocation, and transformation of SCCPs by plants were not affected. Pumpkin and soybean plants grew well at those concentrations during the exposure. Bottles were wrapped with aluminum foil to support the plants, provide dark conditions for root growth, and suppress possible photolysis of SCCPs. As shown in Figure S1, planted blank controls were planted with four seedlings (no exposure chemicals were added to the solution), and the same volume of acetone (120 μL) was used to control for cross contamination. Unplanted chemical controls were set up at the same time. Glass rods were inserted into unplanted chemical controls to simulate the status of exposure groups and the volatilization process. Thus, the unplanted chemical controls were not sealed (they were not airtight). Exposure reactors, planted blank controls, and unplanted controls were all placed in the same exposure chamber. All the bottles and glass rods were autoclaved to avoid contamination by microorganisms. All procedures above to prepare the treated and control reactors were conducted in a laminar flow hood. Pumpkins and soybeans were separately exposed to SCCPs to determine the behaviors of SCCPs in different plant species.
The exposure experiments and plant cultivation (before exposure) were conducted in two separate chambers. To minimize the influences of potential laboratory background contaminations on the exposure experiments and to avoid the effects of exposed SCCPs on the lab air, the exposure chamber was sealed airtight and utilized internal air circulation. The wall of the exposure chamber was cleaned using ethanol-soaked Kimwipes and air-dried, and then the air in the chamber was filtered by polyurethane foams (PUFs, Tish Environmental Inc.) before and after exposure. The exposure and cultivation chambers were all set at 25 °C under 16 h light/8 h dark. Light was provided by fluorescent lighting with an intensity of 250 μmol m−2 s−1. During exposure, water loss caused by transpiration (10–18 mL/day for soybeans and 8–13 mL/day for pumpkins) was replaced by adding autoclaved deionized water using sterile syringes.
2.3. Sample Preparation
The treated and planted blank pumpkin plants were harvested and sampled on exposure day 1, 2, 4, 6, and 10. At each sampling time, there were three parallel planted blank and five parallel treated pumpkin reactors. Treated (5 parallel reactors) and planted blank (3 parallel reactors) soybean plants were only sampled on the 10th day to compare the behaviors of SCCPs in different plants at the end of the exposure period. Unplanted chemical controls (3 parallels) were also sampled at the end of the exposure. The roots, stems, and shoots of pumpkin and soybean plants were sampled as shown in Figure S1, respectively. The roots were rinsed with sterile deionized water and then dried by Kimwipes (Kimberly-Clark, Roswell, GA, USA). The rinse water was combined with the cultivation solution for analysis. All plant tissue samples were stored at −80 °C overnight, freeze-dried, ground, and stored at −20 °C until analysis. Extraction and cleanup procedures are described in details in Text S2.
2.4. Instrumental Qualitative and Quantitative Analysis
The analysis of chlorinated praraffins (CPs) was carried out on an Agilent 7890A gas chromatograph (GC) coupled with a 7000B triple quadrupole mass spectrometer (MS) in the electron capture negative ionization (ECNI) mode (Agilent, Palo Alto, CA) (details described in Text S3). The mass resolution and mass accuracy of the instrument are 1000 and ±0.3 Da, respectively. To evaluate the influences of potential laboratory background contamination, SCCPs and even MCCPs were all analyzed. The target chlorinated paraffins (CPs) and their SIM ions are shown in Table S3. Qualitative analysis of CPs was based on previous work.12 The most and the second most abundant isotope ions of [M – Cl]− for CPs were used for the quantification and identification in selected ion monitoring (SIM) mode, respectively. In addition, CPs were also identified by comparing the retention time and peak shape and by correcting the isotope ratio between samples and standards.
There are detection interferences between some of the SCCP congeners and even between SCCPs and MCCPs.39,40 For the target SCCP congeners, the two most abundant chloride-loss ions [M – Cl]− isotopes of SCCPs containing 7–9 Cl atoms and the most abundant chloride-adduct ions [M + Cl]− isotopes of congeners containing 4–6 Cl atoms overlapped with those congeners containing two more carbon atoms and one less chlorine atom and the second most abundant chloride-loss [M – Cl]− isotopes of congeners containing the same number of carbons and two more chlorines, respectively.39 So the determination of C10H15Cl7 can be interfered by C10H17Cl5 and C12H20Cl6; C10H14Cl8 can be interfered by C10H16Cl6 and C12H19Cl7 (no C12H19Cl7 congeners were detected in this work). The determination of daughter SCCP congeners, C10Cl5–7 and C11–13Cl5–6, was not affected by MCCPs when using LRMS in the ECNI mode except that C10H14Cl8 can be interfered by the presence of C15H26Cl6.39 However, no C15H26Cl6 were detected in this work. Currently, accurate quantification of SCCPs is impossible because of the limitation of commercially available individual congeners.41 Beaume et al.42 reported that the quantification of C10-chloroparaffins using the individual standards with monochlorine substituted carbon atoms has led to reproducible and reliable results. Thus, daughter congeners, C10–13Cl5, C10–13Cl6, C10Cl7, and C10Cl8, were quantitatively analyzed by 1,2,5,6,9-PentaCD, 1,2,5,6,9,10-HexCD, 1,2,5,5,6,9,10-HepCD, and 1,1,1,3,8,10,10,10-OctaCD, respectively, in this study. For the response factors of SCCPs in ECNI-MS, the position of chlorine within the molecule played an important role.41,42 Individual standards with three chlorine atoms at the terminal carbon atom (CCl3-group), e.g., 1,1,1,3,8,10,10,10-OctaCD, which has two CCl3-groups, have an unexpected relatively low response factor. Thus, concentration of daughter congeners without CCl3-groups would be biased when using individual standards with CCl3-groups (e.g., 1,1,1,3,8,10,10,10-OctaCD) as quantification standards and vice versa.
2.5. Quality Assurance and Quality Control (QA/QC)
The glassware was kept at 450 °C for 6 h and rinsed with dichloromethane three times before use. Laboratory backgrounds were controlled and detected by plants grown in a cultivation chamber that was free from parent individual SCCPs exposure (before exposure). The results are shown in Table S4. All the results on the SCCPs in this work subtracted the laboratory background concentrations. The details for subtraction are shown in Text S4 of the Supporting Information. Linear calibration curves were plotted by the ratios between peak areas of standards and injection internal standard against the concentrations of the compounds. The concentration ranges and the correlation coefficients (r2) of calibration curves were 1.0–7.0 ng/μL and 0.998 for 1,2,5,6,9-PentaCD, 0.5–5 ng/μL and 0.992 for 1,2,5,6,9,10-HexCD, 0.4–4 ng/μL and 0.995 for 1,2,5,5,6,9,10-HepCD, and 2–9 ng/μL and 0.996 for 1,1,1,3,8,10,10,10-OctaCD, respectively. Method detection limits (MDLs) for parent individual SCCP standards were 0.25–0.41 ng/mL for water samples and 15.8–213 ng/g dry weight for plant samples (Table S2). Spiked recoveries of 1,2,5,6,9,10-HexCD, 1,1,1,3,8,10,10,10-OctaCD, 1,1,1,3,10,11-HexCU, and 1,1,1,3,12,13-HexCT were in the ranges of 87.6–117%, 58.1–110%, 64.8–101%, and 67.6–117%, respectively. The recoveries of surrogate standards in shoots, stems, roots, and solution were in the ranges of 71.2–87.8%, 70.6–81.7%, 85.6–108%, and 74.7–104%, respectively. All results were corrected by surrogate recoveries.
3. RESULTS AND DISCUSSION
3.1. The Time Courses of Parent SCCPs in Hydroponic Solutions
In unplanted chemical controls, the total recoveries of parent SCCPs were 92.7 ± 5.4% for 1,2,5,6,9,10-HexCD, 96.7 ± 5.8% for 1,1,1,3,10,11-HexCU, 103 ± 16% for 1,1,1,3,8,10,10,10-OctaCD, and 109 ± 15% for 1,1,1,3,12,13-HexCT after 10 days exposure (Table 1). The recoveries indicated that no significant volatilization and transformation unrelated to plants occurred in the exposure systems during the 10-day experiments. No parent SCCPs were found in solution of the planted blank controls during 10 days exposure.
Table 1.
Mass Balance and Distributions of Parent SCCPs (% of Mass Applied) in Exposure Reactors and Unplanted Chemical Controls on Day 10a
| reactors | compounds | solutions (%) | roots (%) | stems (%) | shoots (%) | total SCCPs recovered (%)e |
|---|---|---|---|---|---|---|
| unplanted chemical controls | 1,2,5,6,9,10-HexCD | 92.7 ± 5.4b | c | c | c | 92.7 ± 5.4 |
| 1,1,1,3,8,10,10,10-OctaCD | 103 ± 16 | c | c | c | 103 ± 16 | |
| 1,1,1,3,10,11-HexCU | 96.7 ± 5.8 | c | c | c | 96.7 ± 5.8 | |
| 1,1,1,3,12,13-HexCT | 109 ± 15 | c | c | c | 109 ± 15 | |
| pumpkin exposure groups | 1,2,5,6,9,10-HexCD | 1.28 ± 0.34 | 23.6 ± 6.6 | 0.70 ± 0.46 | ndd | 25.6 ± 6.7 |
| 1,1,1,3,8,10,10,10-OctaCD | 3.13 ± 0.44 | 59.9 ± 12.3 | 3.81 ± 2.22 | ndd | 66.9 ± 13.7 | |
| 1,1,1,3,10,11-HexCU | 1.16 ± 0.18 | 33.4 ± 11.6 | 0.84 ± 0.64 | ndd | 35.4 ± 12.1 | |
| 1,1,1,3,12,13-HexCT | 3.53 ± 1.50 | 40.5 ± 15.6 | 1.31 ± 0.80 | ndd | 45.3 ± 16.6 | |
| soybean exposure groups | 1,2,5,6,9,10-HexCD | 5.35 ± 1.80 | 1.98 ± 0.65 | 0.53 ± 0.31 | ndd | 7.90 ± 1.90 |
| 1,1,1,3,8,10,10,10-OctaCD | 3.75 ± 3.55 | 54.5 ± 5.17 | 2.54 ± 2.04 | ndd | 60.8 ± 8.4 | |
| 1,1,1,3,10,11-HexCU | 0.39 ± 0.16 | 2.29 ± 0.70 | 0.50 ± 0.30 | ndd | 3.20 ± 0.90 | |
| 1,1,1,3,12,13-HexCT | 0.79 ± 0.55 | 5.25 ± 0.90 | 0.81 ± 0.65 | ndd | 6.90 ± 1.50 |
The recovered parent SCCPs in different compartments expressed as the mass percentage of initial exposure chemicals. The initial mass was 4000 ng for 1,2,5,6,9,10-HexCD, 8098 ng for 1,1,1,3,8,10,10,10-OctaCD, 7973 ng for 1,1,1,3,10,11-HexCU, and 7980 ng for 1,1,1,3,12,13-HexCT.
Mean value ± standard deviation, n = 5 for exposure groups, n = 3 for unplanted chemical controls.
No sample for detection.
Nondetectable, nd.
The summation of different compartment.
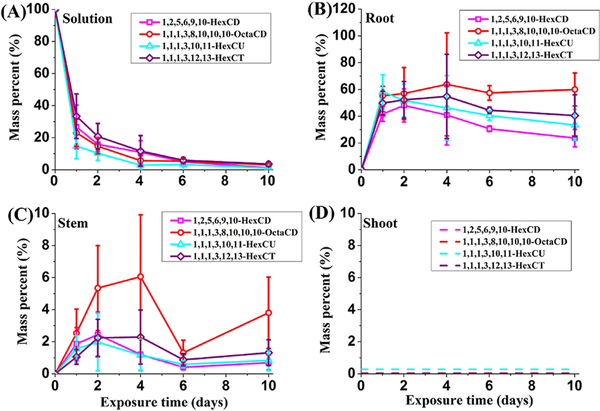
In the pumpkin exposure groups, the mass percentages of parent SCCPs remaining in hydroponic solutions fell sharply during the first 4 days and then decreased slowly (Figure 1A). In the end, less than 4% of the initial mass of 1,2,5,6,9,10-HexCD, 1,1,1,3,8,10,10,10-OctaCD, 1,1,1,3,10,11-HexCU, and 1,1,1,3,12,13-HexCT remained in solution. Since there was little volatilization and chemical transformation in the system according to the results of unplanted chemical controls, the removal of parent SCCPs from solutions was mainly caused by the adsorption and uptake by plant roots.32 Levels of different parent SCCPs in aqueous solutions suggested that the removal efficiencies of different parent SCCP congeners by pumpkin roots from hydroponic solution were similar.
Figure 1.
Mass percentage of parent SCCPs spiked to solution as measured in the solutions (A), roots (B), stems (C), and shoots (D) when pumpkin plants were exposed to SCCPs over time. No parent SCCPs were detected in the shoots (lower than the method detection limits (MDLs) as the dashed lines show).
3.2. Effects of Carbon Chain Length and Degree of Chlorination on the Behavior of Parent SCCPs in Pumpkins
In pumpkin plants exposed to SCCPs, uptake and accumulation of various parent SCCPs were investigated over time (shown as mass percentage in Figure 1B, C, and D and consistent with Figure S2 shown as concentrations). The mass percentage of parent chemicals associated with roots and stems increased first and then decreased somewhat during the 10-day exposure time. In roots, 1,1,1,3,8,10,10,10-OctaCD, 1,1,1,3,12,13-HexCT, 1,1,1,3,10,11-HexCU, and 1,2,5,6,9,10-HexCD reached the highest amount on day 4, 4, 1, and 2, respectively. Namely, 1,2,5,6,9,10-HexCD and 1,1,1,3,10,11-HexCU reached the highest mass accumulation in roots faster than 1,1,1,3,12,13-HexCT and 1,1,1,3,8,10,10,10-OctaCD. The percentages of parent chemicals associated with roots consistently showed the order of 1,1,1,3,8,10,10,10-OctaCD > 1,1,1,3,12,13-HexCT > 1,1,1,3,10,11-HexCU > 1,2,5,6,9,10-HexCD after the second day. At the end of the exposure, 59.9%, 40.5%, 33.4%, and 23.6% of the initial dose of parent 1,1,1,3,8,10,10,10-OctaCD, 1,1,1,3,12,13-HexCT, 1,1,1,3,10,11-HexCU, and 1,2,5,6,9,10-HexCD was accumulated by pumpkin roots. In stems, the parent chemicals were lower in both mass percentages and concentrations than roots and apparently originated from the upward translocation from roots. The time for reaching the highest accumulation and the mass accumulation order of parent SCCPs in stem were similar to those of roots, respectively (it is likely that the last data point at 10 days for 1,1,1,3,8,10,10,10-OctaCD in stem is in error–it was too large and possessed a high standard deviation). These findings indicate that the levels and the time spent to reach the highest accumulation for parent SCCPs in pumpkin plants increased with the increasing degree of chlorination when SCCPs had the same carbon chain length, and they also increased with the increasing carbon chain length when SCCPs had the same number of chlorine atoms.
No parent SCCPs were found in pumpkin shoots of the exposure groups or any of the tissue samples of planted blank controls during the exposure period. It was consistent with our previous work,32 in which very little of 1,2,5,5,6,9,10-heptachlorodecane (less than 1% of initial mass) was detected in pumpkin shoots after 10-day exposure. Accordingly, the total recoveries of parent SCCPs in the exposure pumpkin reactors, including parent SCCPs in solutions, roots, stems, and shoots, were in the range from 25.6 ± 6.7% to 66.9 ± 13.7% at end of the exposure (day 10). The huge amounts of unrecovered parent SCCPs were attributed to both biotransformation by plants and phytovolatilization.32
3.3. Discovery of Carbon Chain Decomposition Products
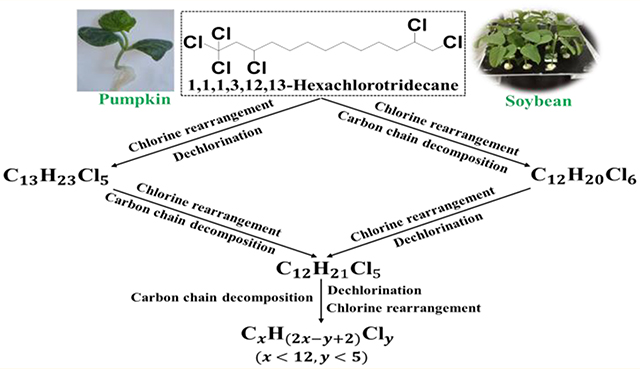
Transformation of parent SCCPs was analyzed for all pumpkin samples during the 10-day exposure. The daughter SCCPs, including C10H17Cl5, C10H16Cl6 (except 1,2,5,6,9,10-HexCD), C10H15Cl7, C10H14Cl8 (except 1,1,1,3,8,10,10,10-OctaCD), C11H19Cl5, C11H18Cl6 (except 1,1,1,3,10,11-HexCU), C12H21Cl5, C12H20Cl6, C13H23Cl5, and C13H22Cl6 (except 1,1,1,3,12,13-HexCT), were found in all pumpkin tissues of the exposure groups. Compared with the chlorine atoms of parent SCCPs, the dechlorination and chlorine rearrangement products were all identified by ECNI.43 The selected parent SCCPs contained 10, 11, and 13 carbon atoms, respectively. However, the daughter chemicals included SCCPs containing 12 carbon atoms. The carbon chain decomposition was also reported in studies on the thermal degradation of CP52 and CP70, polychlorododecane with 59 and 70% chlorine contents, and other commercial CPs.44–46 The products detected in this study verified that the carbon chain of parent chlorotridecane was decomposed (shortened) under the mediation of pumpkin plants and formed chlorododecanes. Because the exposure chemicals were a mixture of two chlorodecanes, one chloroundecane and one chlorotridecane, it was difficult to positively identify the carbon chain decomposition products of parent chlorodecanes and chloroundecane. However, it was reasoned that the carbon chains of the parent chloroundecane and chlorodecanes and even the daughter dodecanes might also be decomposed in the exposure systems since these SCCPs have similar chemical structures and properties.
The impurities of exposure standards were determined to ensure the authenticity of the experimental results. In parent 1,1,1,3,8,10,10,10-OctaCD standard, SCCP congeners, C10H16Cl6 (1.5%), C10H15Cl7 (0.7%), and C10H14Cl8 (1.1%),were detected as impurities. C11H18Cl6 (4.9%) and C13H22Cl6 (8.2%) were detected as impurities in 1,1,1,3,10,11-HexaCU and 1,1,1,3,12,13-HexaCT, respectively. No target CPs were found as impurities in 1,2,5,6,9,10-HexCD standard. Thus, none of the SCCPs containing 12 carbon atoms were detected as impurities. The mass percentages of SCCP congeners C10Cl6–8, C11Cl6, and C13Cl6 (as the impurities in parent SCCP standards) in unplanted controls were in the same amounts as that in standards after 10 days exposure. Our results indicated that those SCCP congeners in unplanted controls came from the impurities of the corresponding parent chemicals, further confirming that no transformation occurred in the unplanted controls. These results verified that carbon chain decomposition from chlorotridecane to chlorododecanes definitely occurred under the mediation of pumpkins. Impurities in parent SCCP standards might also be involved in the metabolism within pumpkins; however, their contributions should be negligible due to their initial low concentrations.
3.4. Time Courses of Daughter SCCPs in Pumpkin Seedlings
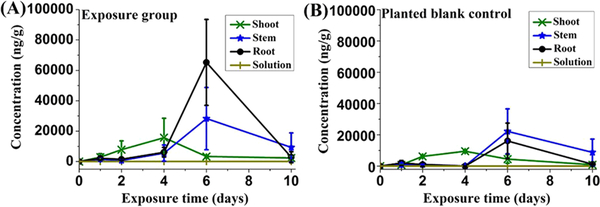
Temporal variations showed that the concentrations of different groups of SCCP congeners (Table S5, Figure S3A–D) and the total daughter SCCPs (Figure 2A) in all tissues of exposed pumpkins increased and then decreased. The highest total concentrations in shoots, stems, and roots were found at day 4, 6, and 6, respectively. The temporal changes of daughter SCCPs in pumpkins were an amalgamation of several different processes. This could be illustrated by the example of shoots. The origins of daughter SCCPs in shoots included upward translocation from roots and stems, transformation from parent SCCPs within the shoots, and reabsorption of the phytovolatilized daughter SCCPs from the air phase. Elimination processes from shoots included further transformation, downward translocation, and phytovolatilization.32 The trend of total daughter SCCPs in shoots suggested that elimination processes became predominant after 4 days exposure.
Figure 2.
Total concentrations of daughter SCCPs in solutions, roots, stems, and shoots for exposure group (A) and planted blank control (B) in pumpkin plants over time. No daughter SCCPs were found in the solutions of the exposure group or the planted blank control.
In planted blank pumpkin controls, daughter SCCPs were also detected in shoots, stems, and roots (Figure 2B and Figure S3E–H, Table S6). This result illustrates that phytovolatilized SCCPs from the exposed pumpkins were absorbed by shoots and then translocated downward to the roots of planted blank controls. The highest amounts in the shoots, stems, and roots were found on day 4, 6, and 6, respectively, which was consistent with that of exposure pumpkin groups. The accumulation of SCCPs in the plant tissues of planted blank pumpkin controls illustrated that considerable mass of phytovolatilized SCCPs transferred through the air and was followed by the usual dynamics within plant compartments of downward translocation and further transformation.
3.5. Parent SCCPs and Their Metabolism in Different Plant Species
To study the universality of the uptake, translocation, and transformation of SCCPs in food plants and the difference of those behaviors in different plant species, another model plant, soybean, was also introduced in this exposure experiment. The soybean plant has been reported to take up and translocate organic compounds such as chlorobenzenes and chlorobiphenyl.37,47 Fates of parent and daughter SCCPs in soybean plants after a 10-day exposure were investigated and compared with the results of pumpkins at day 10. The mass percentages of parent SCCPs (accounting for their initial dose) in different compartments of exposure soybean reactors are shown in Table 1 and Figure S4A. Similar to pumpkin, no parent chemicals were found in the exposed soybean shoots, and none were detected in the whole plants of the planted blank soybean controls. The mass percentages of parent SCCPs associated with roots and stems of exposed soybeans also displayed an order of 1,1,1,3,8,10,10,10-OctaCD > 1,1,1,3,12,13-HexCT > 1,1,1,3,10,11-HexCU > 1,2,5,6,9,10-HexCD.
Concentration ratios of parent chemicals accumulated in stem and in root (S/R) were used to evaluate the upward translocation ability of SCCPs. In exposed soybeans and pumpkins, S/R values were 0.45 ± 0.14 and 0.05 ± 0.03 for 1,2,5,6,9,10-HexCD, 0.08 ± 0.05 and 0.12 ± 0.07 for 1,1,1,3,8,10,10,10-OctaCD, 0.36 ± 0.06 and 0.05 ± 0.03 for 1,1,1,3,10,11-HexCU, and 0.26 ± 0.13 and 0.06 ± 0.03 for 1,1,3,12,13-HexCT, respectively. Namely, S/R values for 1,2,5,6,9,10-HexCD (p < 0.01), 1,1,1,3,10,11-HexCU (p < 0.01), and 1,1,3,12,13-HexCT (p < 0.05) in exposure soybeans were significantly higher than the corresponding S/R values in exposure pumpkins. The S/R value for 1,1,1,3,8,10,10,10-OctaCD in soybean was lower than that in pumpkin; however, there was no significant difference (p > 0.05). It indicated that the extent of upward translocation of parent SCCPs in soybean was greater than that in pumpkin.
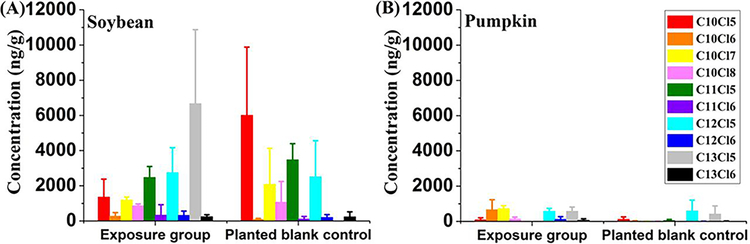
The parent congeners showed that the ratios of 1,2,5,6,9,10-HexCD:1,1,1,3,8,10,10,10-OctaCD:1,1,1,3,10,11-HexCU:1,1,1,3,12,13-HexCT were 1:5:1:2 for both pumpkin and soybean stems, 1:28:1:3 for soybean roots, and 1:3:1:2 for pumpkin roots, respectively. Experiments clearly revealed that the congener containing 8 chlorine and 10 carbon atoms was accumulated significantly more than congeners containing 6 chlorine and 10–13 carbon atoms, and this phenomenon was even more obvious in soybeans than pumpkins.
The amounts of 1,1,1,3,12,13-HexCT, 1,2,5,6,9,10-HexCD, and 1,1,1,3,10,11-HexCU accumulated by the roots of exposed soybeans were all significantly lower than those of exposed pumpkins (1,1,1,3,12,13-HexCT, 1,2,5,6,9,10-HexCD, and 1,1,1,3,10,11-HexCU, p < 0.01) (with the exception of 1,1,1,3,8,10,10,10-OctaCD which showed similar accumulation levels by roots in both plant species, p > 0.05). In the stems, levels of parent SCCPs in exposed soybeans were also lower than those in the exposed pumpkins, although there was no significant difference (p > 0.05). As shown in Table 1, the total recoveries of parent chemicals in the soybean exposed plants were far lower than those of the pumpkin exposure group for 1,1,1,3,12,13-HexCT, 1,2,5,6,9,10-HexCD, and 1,1,1,3,10,11-HexCU (p < 0.01). These results suggest that the accumulation of parent SCCPs in soybean was much lower than that in pumpkin. Unplanted chemical controls showed that no volatilization or transformation of parent SCCPs (unrelated to plants) occurred in these reactors. Similar to the findings in our previous study,32 the unrecovered parent chemicals were assumed to be transformed and phytovolatilized. Significantly greater mass percentages of SCCPs were biotransformed and phytovolatilized by soybean than pumpkin seedlings.
Metabolism of SCCPs in soybean was further studied. Similar to the verification of daughter SCCPs in the pumpkin experiment, carbon chain decomposition products as well as the dechlorination and chlorine rearrangement products of SCCPs were also found in soybean seedlings of the exposure group and planted blank controls (Figure 3A, Table S5, S6). The total concentrations of daughter SCCPs in shoots, stems, and roots of soybean (Figure 3 and Figure S4B) were significantly higher than those of pumpkin (shoots, stems, and roots, p < 0.01), providing the direct evidence for the greater transformation ability in soybean for these selected SCCP parent chemicals. Significantly higher concentrations of daughter SCCPs were observed in planted blank soybeans than planted blank pumpkins (p < 0.01) (Figure 3). According to the results above, soybean exhibited greater translocation, biotransformation, and phytovolatilization abilities than pumpkin, meaning that more daughter SCCPs were released into the air, and the air concentrations of metabolites were higher in soybean experiments than that in pumpkin experiments. In addition, lipid content of shoots was higher in soybean than in pumpkin.48,49 This might be the main reason resulting in higher concentrations of SCCPs in planted blank soybean controls.
Figure 3.
Concentrations of daughter SCCPs in exposure groups and planted blank controls of soybean (A) and pumpkin (B) seedlings after 10 days exposure.
3.6. Environmental Implication
This study provides the first evidence for carbon chain decomposition of SCCPs mediated by plants using the hydroponic exposure experiment with an environmental concentration. Though three out of four parent SCCPs congeners are less common in the technical mixture, showing higher chlorination on the terminal carbon atom (1,1,1- and 10,10,10-chlorinated positions), the carbon chain decomposition mediated by plants was confirmed by those commercially available individual standards. The higher bioavailability of parent compounds in the hydroponic cultivation system than that in a real soil-plant system facilitates the results’ observation. Further dechlorination or production of other transformation products that have not been analyzed in these exposure systems, and the possible transformation of SCCPs in the air phase after phytovolatilizaiton,32 still requires further study.
The results of this work provide new insight into possible pathways of transport and transformation of SCCPs and even MCCPs in the environment. Dechlorination and carbon chain decomposition of MCCPs probably also occurs in the environment, likely affecting ecological and food chain transfers and could lead to significant environmental problems because of the similar chemical structure and physicochemical properties between MCCPs and SCCPs.
The degradation of CPs depends on the degree of chlorination.50 The mass of exposed chemicals with lower degree of chlorination, 1,2,5,6,9,10-HexCD (61.0% Cl), 1,1,1,3,10,11-HexCU (58.7% Cl), and 1,1,1,3,12,13-HexCT (54.5% Cl), remaining in plants was less than that of 1,1,1,3,8,10,10,10-OctaCD (67.9% Cl) after 10 days exposure. Madeley et al. reported that SCCPs with a degree of chlorination less than 60% appeared to be rapidly and completely degraded.51 Half-lives of C12–CP and C16–CP in sediment increased with the increasing degree of chlorination.1 CPs with lower degrees of chlorination are likely less persistent, similar to that of PCBs (the lower degree of chlorination, the faster the metabolism).52 Plants have developed complex detoxification mechanisms to transform the parent chemical to nonphytotoxic metabolites and to prevent harmful effects from the exposure to these compounds.53 The metabolites are more soluble and then less toxic during the three phase process known as the green liver model (activation, conjugation, and sequestration).54 The water solubility and vapor pressure of CPs increase with decreasing degree of chlorination. Geng et al. reported that SCCPs with a longer carbon chain and higher chlorine content were prone to producing higher cytotoxicity to human hepatoma HepG2 cell.55 Our study shows that pumpkin and soybean seedlings are likely detoxifying the SCCPs via dechlorination and carbon chain decomposition.
Supplementary Material
ACKNOWLEDGMENTS
This work was jointly supported by the National Key Research and Development Project (2018YFC1800702), the National Natural Science Foundation of China (21677158, 21621064), and the Chinese Academy of Science (XDB14010400). J.L.S. was supported by the Iowa Superfund Research Program (ISRP), National Institute of Environmental Health Science (nIEHS), Grant Number P42ES013661, and by the 1000- Talents Program of the Chinese Academy of Sciences.
Footnotes
The authors declare no competing financial interest.
ASSOCIATED CONTENT
Supporting Information
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acs.est.9b01215.
Details of chemicals and reagents and information on individual SCCP congeners (Text S1, Table S1), extraction and cleanup, instrumentation, target CPs and MDLs (Texts S2 and S3, Tables S3 and S2), plant background (Text S4, Table S4), schematic of experiments (Figure S1), SCCPs in exposure groups and planted blank controls (Tables S5 and S6, Figures S2–S4) (PDF)
REFERENCES
- (1).Feo ML; Eljarrat E; Barcelo D Occurrence, fate and analysis of polychlorinated n-alkanes in the environment. TrAC, Trends Anal. Chem 2009, 28 (6), 778–791. [Google Scholar]
- (2).Bayen S; Obbard JP; Thomas GO Chlorinated paraffins: A review of analysis and environmental occurrence. Environ. Int 2006, 32 (7), 915–929. [DOI] [PubMed] [Google Scholar]
- (3).Pellizzato F; Ricci M; Held A; Emons H Analysis of short-chain chlorinated paraffins: a discussion paper. J. Environ. Monit 2007, 9 (9), 924–930. [DOI] [PubMed] [Google Scholar]
- (4).Van Mourik LM; Leonards PE; Gaus C; de Boer J Recent developments in capabilities for analysing chlorinated paraffins in environmental matrices: A Review. Chemosphere 2015, 136, 259–272. [DOI] [PubMed] [Google Scholar]
- (5).European. Community, Directive 2002/45/EC of The European Parliament and of the Council of amending for the twentieth time Council Directive 76/769/ EEC relating to restrictions on the marketing and use of certain dangerous substances and preparations (short_chain chlorinated paraffins). Office Journal of European Communities; Office for Official Publications of the European Communities: Luxembourg, 2002. [Google Scholar]
- (6).UNEP, Regionally based assessment of persistent organic substances; 2003.
- (7).UNEP, United Nations Environment Programme, Risk Management Evaluation on Short Chain Chlorinated Paraffins UNEP/POPS/POPRC.12/11/Add; Stockholm Convention on Persistent Organic Pollutants, Geneva, 2016; p 3. [Google Scholar]
- (8).Gluge J; Schinkel L; Hungerbuhler K; Cariou R; Bogdal C Environmental Risks of Medium-Chain Chlorinated Paraffins (MCCPs): A Review. Environ. Sci. Technol 2018, 52 (12), 6743–6760. [DOI] [PubMed] [Google Scholar]
- (9).Zeng LX; Chen R; Zhao ZS; Wang T; Gao Y; Li A; Wang YW; Jiang GB; Sun LG Spatial Distributions and Deposition Chronology of Short Chain Chlorinated Paraffins in Marine Sediments across the Chinese Bohai and Yellow Seas. Environ. Sci. Technol 2013, 47 (20), 11449–11456. [DOI] [PubMed] [Google Scholar]
- (10).Zeng LX; Lam JCW; Wang YW; Jiang GB; Lam PKS Temporal Trends and Pattern Changes of Short- and Medium- Chain Chlorinated Paraffins in Marine Mammals from the South China Sea over the Past Decade. Environ. Sci. Technol 2015, 49 (19), 11348–11355. [DOI] [PubMed] [Google Scholar]
- (11).Zeng LX; Li HJ; Wang T; Gao Y; Xiao K; Du YG; Wang YW; Jiang GB Behavior, Fate, and Mass Loading of Short Chain Chlorinated Paraffins in an Advanced Municipal Sewage Treatment Plant. Environ. Sci. Technol 2013, 47 (2), 732–740. [DOI] [PubMed] [Google Scholar]
- (12).Zeng LX; Wang T; Han WY; Yuan B; Liu Q; Wang YW; Jiang GB Spatial and Vertical Distribution of Short Chain Chlorinated Paraffins in Soils from Wastewater Irrigated Farmlands. Environ. Sci. Technol 2011, 45 (6), 2100–2106. [DOI] [PubMed] [Google Scholar]
- (13).Zeng LX; Wang T; Ruan T; Liu Q; Wang YW; Jiang GB Levels and distribution patterns of short chain chlorinated paraffins in sewage sludge of wastewater treatment plants in China. Environ. Pollut 2012, 160, 88–94. [DOI] [PubMed] [Google Scholar]
- (14).Zeng LX; Wang T; Wang P; Liu Q; Han SL; Yuan B; Zhu NL; Wang YW; Jiang GB Distribution and Trophic Transfer of Short-Chain Chlorinated Paraffins in an Aquatic Ecosystem Receiving Effluents from a Sewage Treatment Plant. Environ. Sci. Technol 2011, 45 (13), 5529–5535. [DOI] [PubMed] [Google Scholar]
- (15).Zeng LX; Zhao ZS; Li HJ; Wang T; Liu Q; Xiao K; Du YG; Wang YW; Jiang GB Distribution of Short Chain Chlorinated Paraffins in Marine Sediments of the East China Sea: Influencing Factors, Transport and Implications. Environ. Sci. Technol 2012, 46 (18), 9898–9906. [DOI] [PubMed] [Google Scholar]
- (16).Ma XD; Zhang HJ; Wang Z; Yao ZW; Chen JW; Chen JP Bioaccumulation and trophic transfer of short chain chlorinated paraffins in a marine food web from Liaodong Bay, North China. Environ. Sci. Technol 2014, 48 (10), 5964–5971. [DOI] [PubMed] [Google Scholar]
- (17).Gao Y; Zhang HJ; Su F; Tian YZ; Chen JP Environmental Occurrence and Distribution of Short Chain Chlorinated Paraffins in Sediments and Soils from the Liaohe River Basin, P. R. China. Environ. Sci. Technol. 2012, 46 (7), 3771–3778. [DOI] [PubMed] [Google Scholar]
- (18).Chen MY; Luo XJ; Zhang XL; He MJ; Chen SJ; Mai BX Chlorinated paraffins in sediments from the Pearl River Delta, South China: spatial and temporal distributions and implication for processes. Environ. Sci. Technol 2011, 45 (23), 9936–9943. [DOI] [PubMed] [Google Scholar]
- (19).Krogseth IS; Breivik K; Arnot JA; Wania F; Borgen AR; Schlabach M Evaluating the environmental fate of short-chain chlorinated paraffins (SCCPs) in the Nordic environment using a dynamic multimedia model. Environ. Sci-Proc. Imp 2013, 15 (12), 2240–2251. [DOI] [PubMed] [Google Scholar]
- (20).Wei GL; Liang XL; Li DQ; Zhuo MN; Zhang SY; Huang QX; Liao YS; Xie ZY; Guo TL; Yuan ZJ Occurrence, fate and ecological risk of chlorinated paraffins in Asia: A review. Environ. Int 2016, 92–93, 373–387. [DOI] [PubMed] [Google Scholar]
- (21).Collins C; Fryer M; Grosso A Plant uptake of non ionic organic chemicals. Environ. Sci. Technol 2006, 40 (1), 45–52. [DOI] [PubMed] [Google Scholar]
- (22).Sawhney BL; Hankin L Plant Contamination by PCBs from Amended Soils. J. Food Prot 1984, 47 (3), 232–236. [DOI] [PubMed] [Google Scholar]
- (23).Iwata Y; Gunther FA Translocation of the polychlorinated biphenyl Aroclor 1254 from soil into carrots under field conditions. Arch. Environ. Contam. Toxicol 1976, 4 (1), 44–59. [DOI] [PubMed] [Google Scholar]
- (24).Webber RM; Anderson JL; Jhon MS Hydrodynamic studies of adsorbed diblock copolymers in porous membranes. Macromolecules 1990, 23 (4), 1026–1034. [Google Scholar]
- (25).Liu JY; Schnoor JL Uptake and translocation of lesser-chlorinated polychlorinated biphenyls (PCBs) in whole hybrid poplar plants after hydroponic exposure. Chemosphere 2008, 73 (10), 1608–1616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (26).Zhai GS; Lehmler H-J; Schnoor JL Identification of hydroxylated metabolites of 3,3′,4,4′-tetrachlorobiphenyl and metabolic pathway in whole poplar plants. Chemosphere 2010, 81 (4), 523–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (27).Zhai GS; Lehmler HJ; Schnoor JL Hydroxylated metabolites of 4-monochlorobiphenyl and its metabolic pathway in whole poplar plants. Environ. Sci. Technol 2010, 44 (10), 3901–3907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (28).Wang S; Zhang SZ; Huang HL; Zhao MM; Lv JT Uptake, translocation and metabolism of polybrominated diphenyl ethers (PBDEs) and polychlorinated biphenyls (PCBs) in maize (Zea mays L.). Chemosphere 2011, 85 (3), 379–385. [DOI] [PubMed] [Google Scholar]
- (29).Wang S; Zhang SZ; Huang HL; Lu AX; Ping H Debrominated, hydroxylated and methoxylated metabolism in maize (Zea mays L.) exposed to lesser polybrominated diphenyl ethers (PBDEs). Chemosphere 2012, 89 (11), 1295–1301. [DOI] [PubMed] [Google Scholar]
- (30).Mattina MJI; Iannucci-Berger W; Dykas L Chlordane uptake and its translocation in food crops. J. Agric. Food Chem 2000, 48 (5), 1909–1915. [DOI] [PubMed] [Google Scholar]
- (31).Lunney AI; Zeeb BA; Reimer KJ Uptake of weathered DDT in vascular plants Potential for phytoremediation. Environ. Sci. Technol 2004, 38 (22), 6147–6154. [DOI] [PubMed] [Google Scholar]
- (32).Li YL; Hou XW; Yu M; Zhou QF; Liu JY; Schnoor JL; Jiang GB Dechlorination and chlorine rearrangement of 1,2,5,5,6,9,10-heptachlorodecane mediated by the whole pumpkin seedlings. Environ. Pollut 2017, 224, 524–531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (33).Sun JT; Liu JY; Liu YW; Yu M; Jiang GB Reciprocal Transformation between Hydroxylated and Methoxylated Polybromi-nated Diphenyl Ethers in Young Whole Pumpkin Plants. Environ. Sci. Technol. Lett 2014, 1 (4), 236–241. [Google Scholar]
- (34).Yu M; Liu JY; Wang T; Sun JT; Liu RZ; Jiang GB Metabolites of 2,4,4′-tribrominated diphenyl ether (BDE-28) in pumpkin after in vivo and in vitro exposure. Environ. Sci. Technol 2013, 47 (23), 13494–13501. [DOI] [PubMed] [Google Scholar]
- (35).Sun JT; Liu JY; Yu M; Wang C; Sun YZ; Zhang AQ; Wang T; Lei Z; Jiang GB In vivo metabolism of 2,2′,4,4′-tetrabromodiphenyl ether (BDE-47) in young whole pumpkin plant. Environ. Sci. Technol 2013, 47 (8), 3701–3707. [DOI] [PubMed] [Google Scholar]
- (36).Zhang HN; Wen B; Hu XY; Wu YL; Pan Y; Huang HL; Liu L; Zhang SZ Uptake, Translocation, and Metabolism of 8:2 Fluorotelomer Alcohol in Soybean (Glycine max L. Merrill). Environ. Sci. Technol 2016, 50 (24), 13309–13317. [DOI] [PubMed] [Google Scholar]
- (37).Fries GF; Marrow GS Chlorobiphenyl movement from soil to soybean plants. J. Agric. Food Chem 1981, 29 (4), 757–759. [DOI] [PubMed] [Google Scholar]
- (38).Rusina TP; Korytar P; de Boer J Comparison of quantification methods for the analysis of polychlorinated alkanes using electron capture negative ionisation mass spectrometry. Int. J. Environ. Anal. Chem 2011, 91 (4), 319–332. [Google Scholar]
- (39).Reth M; Oehme M Limitations of low resolution mass spectrometry in the electron capture negative ionization mode for the analysis of short- and medium-chain chlorinated paraffins. Anal. Bioanal. Chem 2004, 378 (7), 1741–1747. [DOI] [PubMed] [Google Scholar]
- (40).Yuan B; Alsberg T; Bogdal C; MacLeod M; Berger U; Gao W; Wang YW; de Wit CA Deconvolution of Soft Ionization Mass Spectra of Chlorinated Paraffins To Resolve Congener Groups. Anal. Chem 2016, 88 (18), 8980–8988. [DOI] [PubMed] [Google Scholar]
- (41).Yuan B; Bogdal C; Berger U; MacLeod M; Gebbink WA; Alsberg T; de Wit CA Quantifying Short-Chain Chlorinated Paraffin Congener Groups. Environ. Sci. Technol 2017, 51 (18), 10633–10641. [DOI] [PubMed] [Google Scholar]
- (42).Beaume F; Coelhan M; Parlar H Determination of C10- chloroalkane residues in fish matrices by short column gas chromatography/electron capture negative ion low resolution mass spectrometry applying single pure and representative synthesised chlorodecanes as standards. Anal. Chim. Acta 2006, 565 (1), 89–96. [Google Scholar]
- (43).Schinkel L; Lehner S; Heeb NV; Lienemann P; McNeill K; Bogdal C Deconvolution of Mass Spectral Interferences of Chlorinated Alkanes and Their Thermal Degradation Products: Chlorinated Alkenes. Anal. Chem. 2017, 89 (11), 5923–5931. [DOI] [PubMed] [Google Scholar]
- (44).Bergman A; Hagman A; Jacobsson S; Jansson B; Ahlman M THERMAL-DEGRADATION OF POLYCHLORINATED ALKANES. Chemosphere 1984, 13 (2), 237–250. [Google Scholar]
- (45).Xin SZ; Gao W; Wang YW; Jiang GB Thermochemical emission and transformation of chlorinated paraffins in inert and oxidizing atmospheres. Chemosphere 2017, 185, 899–906. [DOI] [PubMed] [Google Scholar]
- (46).Xin SZ; Gao W; Wang YW; Jiang GB Identification of the Released and Transformed Products during the Thermal Decomposition of a Highly Chlorinated Paraffin. Environ. Sci. Technol 2018, 52 (17), 10153–10162. [DOI] [PubMed] [Google Scholar]
- (47).Tam DD; Shiu WY; Qiang K; Mackay D Uptake of chlorobenzenes by tissues of the soybean plant: Equilibria and kinetics. Environ. Toxicol. Chem. 1996, 15 (4), 489–494. [Google Scholar]
- (48).Wen B; Wu YL; Zhang HN; Liu Y; Hu XY; Huang HL; Zhang SZ The roles of protein and lipid in the accumulation and distribution of perfluorooctane sulfonate (PFOS) and perfluorooctanoate (PFOA) in plants grown in biosolids-amended soils. Environ. Pollut. 2016, 216, 682–688. [DOI] [PubMed] [Google Scholar]
- (49).Huang H; Zhang S; Christie P; Wang S; Xie M Behavior of decabromodiphenyl ether (BDE-209) in the soil-plant system: uptake, translocation, and metabolism in plants and dissipation in soil. Environ. Sci. Technol 2010, 44 (2), 663–667. [DOI] [PubMed] [Google Scholar]
- (50).De Boer J; El-Sayed Ali T; Fiedler H; Legler J; Muir DC; Nikiforov VA; Tomy GT; Tsunemi K Chlorinated Paraffins. In The Handbook of Environmental Chemistry, Chlorinated Paraffins;De Boer J, Ed.; Springer-Verlag Berlin: Berlin, 2010; Vol. 10, DOI: 10.1007/978-3-642-10761-0. [DOI] [Google Scholar]
- (51).Madeley JR; Birtley RDN Chlorinated Paraffins and the Environment 0.2. Aquatic and Avian Toxicology. Environ. Sci. Technol 1980, 14 (10), 1215–1221. [Google Scholar]
- (52).Grimm FA; Hu D; Kania-Korwel I; Lehmler HJ; Ludewig G; Hornbuckle KC; Duffel MW; Bergman A; Robertson LW Metabolism and metabolites of polychlorinated biphenyls. Crit. Rev. Toxicol 2015, 45 (3), 245–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (53).Schnoor JL; Licht LA; Mccutcheon SC; Wolfe NL; Carreira LH Phytoremediation of Organic and Nutrient Contaminants. Environ. Sci. Technol 1995, 29 (7), 318A–323A. [DOI] [PubMed] [Google Scholar]
- (54).Van Aken B; Correa PA; Schnoor JL Phytoremediation of Polychlorinated Biphenyls: New Trends and Promises. Environ. Sci. Technol 2010, 44 (8), 2767–2776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (55).Geng NB; Zhang HJ; Zhang BQ; Wu P; Wang FD; Yu ZK; Chen JP Effects of Short-Chain Chlorinated Paraffins Exposure on the Viability and Metabolism of Human Hepatoma HepG2 Cells. Environ. Sci. Technol 2015, 49 (5), 3076–3083. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.