Abstract
Background
Exercise training is commonly recommended for individuals with fibromyalgia. This review is one of a series of reviews about exercise training for fibromyalgia that will replace the review titled "Exercise for treating fibromyalgia syndrome", which was first published in 2002.
Objectives
To evaluate the benefits and harms of mixed exercise training protocols that include two or more types of exercise (aerobic, resistance, flexibility) for adults with fibromyalgia against control (treatment as usual, wait list control), non exercise (e.g. biofeedback), or other exercise (e.g. mixed versus flexibility) interventions. Specific comparisons involving mixed exercise versus other exercises (e.g. resistance, aquatic, aerobic, flexibility, and whole body vibration exercises) were not assessed.
Search methods
We searched the Cochrane Library, MEDLINE, Embase, the Cumulative Index to Nursing and Allied Health Literature (CINAHL), Thesis and Dissertations Abstracts, the Allied and Complementary Medicine Database (AMED), the Physiotherapy Evidence Databese (PEDro), Current Controlled Trials (to 2013), WHO ICTRP, and ClinicalTrials.gov up to December 2017, unrestricted by language, to identify all potentially relevant trials.
Selection criteria
We included randomised controlled trials (RCTs) in adults with a diagnosis of fibromyalgia that compared mixed exercise interventions with other or no exercise interventions. Major outcomes were health‐related quality of life (HRQL), pain, stiffness, fatigue, physical function, withdrawals, and adverse events.
Data collection and analysis
Two review authors independently selected trials for inclusion, extracted data, and assessed risk of bias and the quality of evidence for major outcomes using the GRADE approach.
Main results
We included 29 RCTs (2088 participants; 98% female; average age 51 years) that compared mixed exercise interventions (including at least two of the following: aerobic or cardiorespiratory, resistance or muscle strengthening exercise, and flexibility exercise) versus control (e.g. wait list), non‐exercise (e.g. biofeedback), and other exercise interventions. Design flaws across studies led to selection, performance, detection, and selective reporting biases. We prioritised the findings of mixed exercise compared to control and present them fully here.
Twenty‐one trials (1253 participants) provided moderate‐quality evidence for all major outcomes but stiffness (low quality). With the exception of withdrawals and adverse events, major outcome measures were self‐reported and expressed on a 0 to 100 scale (lower values are best, negative mean differences (MDs) indicate improvement; we used a clinically important difference between groups of 15% relative difference). Results for mixed exercise versus control show that mean HRQL was 56 and 49 in the control and exercise groups, respectively (13 studies; 610 participants) with absolute improvement of 7% (3% better to 11% better) and relative improvement of 12% (6% better to 18% better). Mean pain was 58.6 and 53 in the control and exercise groups, respectively (15 studies; 832 participants) with absolute improvement of 5% (1% better to 9% better) and relative improvement of 9% (3% better to 15% better). Mean fatigue was 72 and 59 points in the control and exercise groups, respectively (1 study; 493 participants) with absolute improvement of 13% (8% better to 18% better) and relative improvement of 18% (11% better to 24% better). Mean stiffness was 68 and 61 in the control and exercise groups, respectively (5 studies; 261 participants) with absolute improvement of 7% (1% better to 12% better) and relative improvement of 9% (1% better to 17% better). Mean physical function was 49 and 38 in the control and exercise groups, respectively (9 studies; 477 participants) with absolute improvement of 11% (7% better to 15% better) and relative improvement of 22% (14% better to 30% better). Pooled analysis resulted in a moderate‐quality risk ratio for all‐cause withdrawals with similar rates across groups (11 per 100 and 12 per 100 in the control and intervention groups, respectively) (19 studies; 1065 participants; risk ratio (RR) 1.02, 95% confidence interval (CI) 0.69 to 1.51) with an absolute change of 1% (3% fewer to 5% more) and a relative change of 11% (28% fewer to 47% more). Across all 21 studies, no injuries or other adverse events were reported; however some participants experienced increased fibromyalgia symptoms (pain, soreness, or tiredness) during or after exercise. However due to low event rates, we are uncertain of the precise risks with exercise. Mixed exercise may improve HRQL and physical function and may decrease pain and fatigue; all‐cause withdrawal was similar across groups, and mixed exercises may slightly reduce stiffness. For fatigue, physical function, HRQL, and stiffness, we cannot rule in or out a clinically relevant change, as the confidence intervals include both clinically important and unimportant effects.
We found very low‐quality evidence on long‐term effects. In eight trials, HRQL, fatigue, and physical function improvement persisted at 6 to 52 or more weeks post intervention but improvements in stiffness and pain did not persist. Withdrawals and adverse events were not measured.
It is uncertain whether mixed versus other non‐exercise or other exercise interventions improve HRQL and physical function or decrease symptoms because the quality of evidence was very low. The interventions were heterogeneous, and results were often based on small single studies. Adverse events with these interventions were not measured, and thus uncertainty surrounds the risk of adverse events.
Authors' conclusions
Compared to control, moderate‐quality evidence indicates that mixed exercise probably improves HRQL, physical function, and fatigue, but this improvement may be small and clinically unimportant for some participants; physical function shows improvement in all participants. Withdrawal was similar across groups. Low‐quality evidence suggests that mixed exercise may slightly improve stiffness. Very low‐quality evidence indicates that we are 'uncertain' whether the long‐term effects of mixed exercise are maintained for all outcomes; all‐cause withdrawals and adverse events were not measured. Compared to other exercise or non‐exercise interventions, we are uncertain about the effects of mixed exercise because we found only very low‐quality evidence obtained from small, very heterogeneous trials. Although mixed exercise appears to be well tolerated (similar withdrawal rates across groups), evidence on adverse events is scarce, so we are uncertain about its safety. We downgraded the evidence from these trials due to imprecision (small trials), selection bias (e.g. allocation), blinding of participants and care providers or outcome assessors, and selective reporting.
Plain language summary
Mixed exercise programmes for adults with fibromyalgia
What is fibromyalgia and what is mixed exercise?
Fibromyalgia is a condition causing chronic pain and soreness throughout the body. People with this condition often feel depressed, tired, and stiff, and have difficulty sleeping. Mixed exercise is defined as regular sessions of two or more types of exercise including aerobic (walking or cycling), strengthening (lifting weights or pulling against resistance bands), or flexibility (stretching) exercise.
Study characteristics
Reviewers searched for studies until December 2017, and found 29 studies (2088 people) conducted in 12 different countries. The average age of study participants was 51 years, and 98% were female. The average exercise programme was 14 weeks long with three sessions of 50 to 60 minutes per week. All exercise programmes were fully or partially supervised. Reviewers were most interested in comparing mixed exercise groups to control groups (19 studies; 1065 people). People in control groups either received no treatment or continued their usual care.
Key results – mixed exercise vs control
Each outcome below is measured on a scale that goes from 0 to 100, where lower scores are better.
Health‐related quality of life (HRQL)
After 5 to 26 weeks, people who exercised were 7% better (3% better to 11% better) or improved by 7 points on a 100 point scale.
People who exercised rated their HRQL at 49 points.
People in the control group rated their HRQL at 56 points.
Pain
After 5 to 26 weeks, people who exercised had 5% less pain (1% better to 9% better) or improved by 5 points on a 100 point scale.
People who exercised rated their pain at 53 points.
People in the control group rated their pain at 58.6 points.
Tiredness
After 14 to 24 weeks, people who exercised were 13% less tired (8% better to 18% better) or improved by 13 points on a 100 point scale
People who exercised rated their tiredness at 59 points.
People in the control group rated their tiredness at 72 points.
Stiffness
After 16 weeks, people who exercised were 7% less stiff (1% better 1 to 12% better) or improved by 7 points on a 100 point scale.
People who exercised rated their stiffness at 61 points.
People in the control group rated their stiffness at 68 points.
Ability to do daily activities (physical function)
After 8 to 24 weeks, people who exercised were 11% better (7% to 15%) or improved by 11 points on a 100 point scale.
People who exercised rated their physical function at 38 points.
People in the control group rated their physical function at 49 points.
Harms ‐ Some participants experienced increased pain, soreness, or tiredness during or after exercise. Studies reported no injuries or other harms. However, reporting of harms was missing or incomplete in many studies. We are uncertain whether risk is increased with exercise.
Leaving the study early – 11% of control participants left the study early compared with 12% of exercisers.
Long‐term effects ‐ Analysis of long‐term effects of HRQL showed maintenance of mixed exercise effects at 6 to 12 weeks and at 13 to 26 weeks but not at 27 to 52 weeks. Very low‐quality evidence suggests that it is uncertain whether mixed exercises improve HRQL in the long term. Withdrawals and adverse events were not measured.
Other ‐ Reviewers found no evidence that the benefits and harms of mixed exercise were any different from education programmes, cognitive‐behavioural training, biofeedback, medication, or other types of exercise.
Conclusions and quality of evidence
Mixed exercise may improve HRQL and the ability to do daily activities, may decrease pain and tiredness, and may be acceptable to individuals with fibromyalgia. Low‐quality evidence suggests that mixed exercise may slightly improve stiffness. When compared to other exercise or non‐exercise interventions, we are uncertain about the effects of mixed exercise. Although mixed exercise appears to be well tolerated (similar numbers of people leaving the study across groups), evidence on harms was scarce, so we are uncertain about its safety. Reviewers considered the quality of evidence to be low to moderate because of small numbers of people in the studies, some issues involving study design, and the low quality of results.
Summary of findings
Summary of findings for the main comparison. MX exercise training compared to control for fibromyalgia.
| MX exercise training compared to control for fibromyalgia | ||||||
|
Patient or population: individuals with fibromyalgia
Settings: supervised group exercise with or without additional unsupervised home‐based exercise
Intervention: mixed exercise training with or without additional patient education
Comparison: control (no treatment or continued usual care) Outcome: measured at the end of the intervention | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No. of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | MX exercise training | |||||
| HRQL FIQ Total. Scale from 0 to 100; high scores indicate worse quality of life Median length of interventions: 12 weeks | Mean HRQL in control group was 56 | Mean HRQL in intervention groups was 6.95 lower (10.51 lower to 3.38 lower) | 610 (13 studies) | ⊕⊕⊕⊝ Moderatea,b | Includes both clinically important and unimportant improvement with exercisec: absolute difference 7% (95% CI 3% to 11%) improvement Relative change 12% (95% CI 18% to 6%)d; NNTBe |
|
| Pain FIQ Pain, VAS, and SF‐36 Bodily Pain. Scale from 0 to 100; high scores indicate worse pain Median length of interventions: 12 weeks | Mean pain at in control group was 58.6 | Mean pain in intervention groups was 5.2 lower (8.85 lower to 1.48 lower) | 832 (15 studies) | ⊕⊕⊕⊝ Moderatea | Clinically unimportant improvement with exercisec: absolute difference 5% (95% CI 1% to 9%) improvement Relative change 9% (95% CI 15% to 3%)d; NNTBe |
|
| Fatigue FIQ Fatigue, VAS, and SF‐36 vitality. Scale from 0 to 100; high scores indicate worse fatigue Median length of interventions: 16 weeks | Mean fatigue at baseline in control groups was 72.3 | Mean fatigue in intervention groups was 12.93 lower (17.79 lower to 8.07 lower) | 493 (11 studies) | ⊕⊕⊕⊝ Moderatea | Includes both clinically important and unimportant improvement with exercisec: absolute difference 13% (95% CI 8% to 18%) improvement Relative change 18% (95% CI 24% to 11%)d; NNTBe |
|
| Stiffness FIQ Stiffness and VAS. Scale from: 0 to 100; high scores indicate worse stiffness Median length of interventions: 12 weeks | Mean stiffness at baseline in control groups was 67.6 | Mean stiffness in intervention groups was 6.51 lower (12.28 lower to 0.74 lower) | 261 (5 studies) | ⊕⊕⊝⊝ Lowa,f | Includes both clinically important and unimportant improvement with exercisec: absolute difference 7% (95% CI 1% to 12%) improvement Relative change 9% (95% CI 17% to 1%)d; NNTBe |
|
| Physical function FIQ Physical Function, SF‐36 Physical Function, AIMS, and HAQ. Scale converted to 0 to 100; high scores indicate worse physical function Median length of interventions: 12 weeks | Mean physical function in control group was 49.2 | Mean physical function in intervention groups was 10.99 lower (14.8 lower to 7.18 lower) | 477 (9 studies) | ⊕⊕⊕⊝ Moderatea | Includes both clinically important and unimportant improvement with exercisec: absolute difference 11% (95% CI 7% to 15%) improvement Relative change 22% (95% CI 30% to 14%)d; NNTBe |
|
| All‐cause withdrawal All‐cause withdrawals from studies Median length of interventions: 16 weeks | Study population |
RR 1.02 (0.69 to 1.51) |
1065 (19 studies) | ⊕⊕⊕⊝ Moderatea | Absolute difference 1% more withdrawals with exercise (3% fewer to 5% more) Relative change 11% more (28% less to 47% more); NNTBe |
|
| 11 per 100 | 12 per 100 (8 to 16) | |||||
| Adverse events ‐ increase in symptoms, injuries, or serious adverse events | Not all studies measured or reported events in the control groups | Incompletely reported across studies | No reliable estimate | ⊕⊝⊝⊝ Very lowa,f,g | In 8 of the 21 studies, some participants experienced increased symptoms (pain, soreness, or tiredness) during or after exercise. Reporting of adverse events was missing or incomplete in many studies, and we could not calculate reliable estimates | |
| *The basis for the assumed risk is the mean of the controls at baseline. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). AIMS: The Arthritis Impact Measurement Scales; CI: confidence interval; FIQ: Fibromyalgia Impact Questionnaire; HAQ: Health Assessment Questionnaire; HRQL: health‐related quality of life; MD: mean difference; MX: mixed; NNTB: number needed to benefit; RR: risk ratio; SD: standard deviation; SF‐36: Short Form‐36; VAS: visual analogue scale. | ||||||
| GRADE Working Group grades of evidence. High quality: further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: we are very uncertain about the estimate. | ||||||
aPossible sources of risk of bias include lack of allocation concealment, lack of blinding of participants and care providers, and uncertainty regarding selective reporting. bModerate heterogeneity ‐ issue explored (i.e. using peek and poke technique for I² and tau², investigated studies in which data errors were suspected, subgroups, etc.) and not downgraded for heterogeneity. cWe assumed a minimal clinically important between‐group difference (MCID) of 15 points on the 100‐point continuous pain scale (15% absolute difference for pain) and a relative difference of 15% on all other functional scales (HRQL, fatigue, stiffness, function).
dWe calculated the relative change as the MD divided by the pooled baseline mean of control groups and used the control group baseline SD from van Eijk‐Hustings 2013 (HRQL 55.4 on FIQ Total score 0 to 100; Pain 55 on FIQ Pain VAS score 0 to 100; Fatigue 74 on FIQ Fatigue score 0 to 100; Stiffness 68 on FIQ Stiffness score 0 to 100; Physical Function 34 on FIQ Physical Function score 0 to 100) in these calculations.
eNNTB was not calculated, as none of the outcomes showed a clinically important between‐group difference. fImprecision: fewer than 400 participants in the studies.
gIndirectness, adverse events reported inconsistently and unsystematically, either post hoc for one of the comparisons or extrapolated from dropouts.
Background
Description of the condition
Fibromyalgia is a chronic centralised pain disorder marked by widespread muscular tenderness (Clauw 2014). Most people with fibromyalgia experience concurrent gastrointestinal (e.g. abdominal pain, irritable bowel syndrome) and somatosensory symptoms (e.g. hyperalgesia, allodynia, paraesthesias), in addition to fatigue and disturbances in sleep, memory, mood, and cognition (Burckhardt 2005; Clauw 2014; Mease 2005). The myriad of symptoms significantly affects quality of life and results in both physical and psychosocial disability with far reaching implications for individuals’ families, employment opportunities, and independence (Burckhardt 1993; Burckhardt 2005; Mease 2005). Moreover, people with fibromyalgia are often intolerant of physical activity and tend to have a sedentary lifestyle that increases the risk of additional morbidity (Park 2007; Raftery 2009).
The American College of Rheumatology (ACR) published the first diagnostic criteria in 1990 (Wolfe 1990). When this method was used, fibromyalgia was diagnosed when a person experienced widespread pain (above and below the waist bilaterally) for longer than three months and tenderness at at least 11 of 18 specific tender points on physical exam. Because of ongoing concerns with the 1990 criteria, the ACR published an alternative method of diagnosis that was symptom based and eliminated the need for the specific tender point exam but required the examiner to identify areas of pain (Wolfe 2010). The 2010 criteria were further modified to require only self‐report of symptoms through the Fibromyalgia Survey Questionnaire (Wolfe 2011). This questionnaire includes a measure of widespread pain (using a body map, patients identify which of the 19 points are painful); a symptom severity scale containing items related to fatigue, cognition, sleep disturbances, and somatic complaints; and additional questions about the duration of symptoms (three months) and other possible diagnoses (Wolfe 2011). Questions are scored to determine whether a person qualifies with a "case definition" of fibromyalgia. This tool has been found to classify 88% of cases that meet the ACR 1990 criteria (Wolfe 2010). Although the measures focussing on tender point counts have been widely applied in clinical and research settings, the modified ACR 2010 method allows for greater classification of men with fibromyalgia (because men tend to have fewer tender points, yet suffer from many other fibromyalgia‐associated symptoms) (Jones 2015;Walitt 2015), and this method accurately conceptualises the core symptoms of fibromyalgia as a continuum of pain centralisation (Wolfe 2009; Wolfe 2010; Wolfe 2011).
The prevalence of fibromyalgia in Canada, the United States (US), France, Germany, Italy, Portugal, and Spain has been reported to range from 1.1% in Canada (McNalley 2006) to 6.6% in Italy (Branco 2010), with global mean prevalence of 2.7% (Queiroz 2013). Historically, women with this condition greatly outnumber men (Branco 2010; McNalley 2006; Wolfe 1995). Similar to other rheumatological conditions, the prevalence of fibromyalgia in China is substantially lower than in Western countries at about 0.05% (Zeng 2008). Use of the new ACR criteria has yielded similar and higher prevalence rates and a female‐to‐male ratio more consistently approaching 2:1 (Vincent 2013). A study recently conducted in Minnesota, in the US, determined that the prevalence of fibromyalgia was 6.4% in the general adult population (n = 830) according to ACR 2010 criteria (with no statistical difference in prevalence between males and females; Vincent 2013). Another recent study conducted in Scotland (n = 1604) reported prevalence of 5.4% with ACR 2010 criteria versus 1.7% with ACR 1990 criteria (Jones 2015). The female‐to‐male ratio was 2.3:1 for ACR 2010 classified individuals compared to 13.7:1 for ACR 1990 classified patients. However, the National Health Interview Survey used the ACR 2010 criteria with a large sample (n = 8446) and found that the prevalence of fibromyalgia in North American adults was lower at 1.75%, with women affected approximately two times more often than men (Walitt 2015).
To date, no definitive aetiology or pathophysiology has been identified for fibromyalgia. However, current evidence supports the model of central amplification of pain perception that is both developed and maintained by a variety of factors influencing neurotransmitter and neurohormonal dysregulation (Bennett 1999; Clauw 2011; Desmeules 2003). Based on this theory, treatment and management of fibromyalgia require multiple modalities and an integrative multi‐disciplinary approach that includes pharmacological and other therapies (e.g. exercise, cognitive therapy, relaxation, education; Burckhardt 2005; Carville 2008).
Description of the intervention
Exercise is a type of physical activity that consists of "planned, structured, and repetitive bodily movement done to improve and/or maintain one or more components of physical fitness" and health (ACSM 2013). This review defines mixed exercise training programmes (hereafter mixed exercise) as those that include substantial components of at least two of the following types of exercise: (1) aerobic or cardiorespiratory exercise, (2) resistance or muscle strengthening exercise, and (3) flexibility exercise (exclusive of all exercises in the warm‐up and cool‐down; see Appendix 1). Aerobic exercise primarily affects the cardiovascular and respiratory systems, resulting in increased ability to extract oxygen from the lungs and deliver oxygen to the tissues, allowing an individual to perform more work at a given submaximal level (ACSM 2013). Functional capacity can also be enhanced by resistance training, which alters neuromuscular strength, endurance, or power, depending on the specific exercise prescription. Flexibility exercises affect function by ensuring that soft tissues around the joints allow for full range of motion (Pollock 1998).
To be considered for inclusion in this review, we required that the intervention consists of at least two of the three major types of exercise (aerobic, resistance, flexibility) (i.e. aerobic and resistance; aerobic and flexibility; resistance and flexibility; or aerobic, resistance, and flexibility). Each type of exercise had to contribute as a significant part of the exercise intervention. Other types of exercise, such as co‐ordination, balance, and relaxation (involving voluntary muscle contractions), could also contribute to the intervention. Because education on self‐management is frequently provided with exercise, we included interventions that combined mixed exercise with self‐management programmes (when exercise made up less than 50% of the full intervention). We excluded interventions that combined mixed exercise with other non‐exercise interventions, for example, massage.
How the intervention might work
Regularly engaging in exercise training is important for reducing risks associated with numerous chronic diseases and for maintaining or improving physical fitness and functional independence (ACSM 2013;Garber 2011). However, people with fibromyalgia often associate exacerbations of symptoms with exercise and routinely exhibit low levels of cardiovascular fitness (Turk 2002), as well as low levels of muscular fitness (Bennett 1989; Bennett 1998), which increase their risk for additional morbidity (Park 2007; Raftery 2009).
Aerobic and resistance exercise programmes have been shown to lower blood pressure, improve blood lipid and other coronary profiles, enhance insulin sensitivity, and contribute to weight management in the general population (Garber 2011). In addition to direct effects of exercise training on the cardiovascular and respiratory systems, aerobic exercise alters brain chemistry (Barclay 2014; Klaperski 2014; Lopresti 2013; Moylan 2013; Puetz 2006), which can improve mood and reduce fatigue, stress, anxiety, and depression (Klaperski 2014; Moylan 2013; Puetz 2006). Aerobic exercise stimulates the hypothalamus to release increased levels of neurotransmitters including endorphins (Barclay 2014; Lopresti 2013; Scheef 2012), which can lower levels of perceived pain and improve sleep quality (Scheef 2012; Yang 2012). Although the specific effects of aerobic exercise in people with fibromyalgia have not been definitively determined, studies have demonstrated improved HRQL (Kayo 2011; Sanudo 2010b), reduced pain (Sanudo 2010b;,Sencan 2004), lessened fatigue (Kayo 2011), and enhanced physical function (Kayo 2011;Sanudo 2010b).
People with fibromyalgia often present with generalised decreased muscle strength and endurance, along with high levels of muscle fatigue (Kingsley 2009). Due to general deconditioning and lack of physical activity, joint range of motion may be limited (Dierick 2011; Goes 2015). It has been postulated that people with fibromyalgia may have an exaggerated response to muscle microtrauma. Microtrauma is a normal, expected outcome that is associated with novel or strenuous exercise. This could lead to unusually high levels of localised pain in response to relatively low levels of exercise, as well as more widespread pain through disordered central processing (Jones 2002). Resistance training, which focusses on improving muscle strength, endurance, and power capabilities, may result in greater tolerance and more success with daily activities requiring a large, prolonged, or fast muscular effort (e.g. lifting tasks, climbing tasks, maintenance of postural control). For people with fibromyalgia, resistance training may increase tolerance of muscle microtrauma, repair, and adaptation that occurs with exercise, thus reducing pain responses. In addition to improved muscle strength and pain tolerance, a recent meta‐analysis found reduced muscle tenderness and improved HRQL and physical function in response to resistance training (Busch 2013). Flexibility exercises can increase functional range of motion and can contribute to improved postural stability and balance (Garber 2011).
Mixed exercise training might offer unique advantages beyond those derived from interventions employing only one type of exercise. For carry‐over into daily life and optimal societal functioning, individuals benefit from adaptive effects associated with multiple forms of exercise (aerobic, resistance, and flexibility) that offer the potential for training cardiorespiratory, vascular, and neuromusculoskeletal systems. However, to reach the recommended weekly frequency and duration for each type of exercise (Garber 2011), individuals must be highly dedicated and must devote a significant amount of time to exercise. For this reason, exercise professionals may compromise and prescribe lower dosages of each type of exercise to keep the overall programme manageable. However, then people with fibromyalgia may not achieve the physiological changes typically associated with recommended training levels. Some combinations of exercise have been shown to result in better outcomes compared to those achieved when programmes focus on only one form of exercise. For example, a recent systematic review demonstrated that, in people with type 2 diabetes, combined aerobic and resistance training resulted in improved glucose control and blood lipids beyond those achieved with aerobic or resistance training conducted in isolation (Schwingshackl 2014). Similarly, combined aerobic and resistance training programmes have been shown to result in superior weight and fat loss and improvements in cardiorespiratory fitness among overweight and obese people compared to either programme conducted on its own (Ho 2012). Although these effects are relevant and important for addressing risk factors and common comorbidities in people with fibromyalgia (e.g. obesity, low cardiorespiratory fitness, type 2 diabetes), it is not known whether mixed exercise programmes have a compounded effect on signs and symptoms related to fibromyalgia. It is possible that combined aerobic and resistance training programmes may have an additive effect on reducing pain through the release of neurotransmitters centrally and via local muscular adaptations that improve exercise tolerance and allow participants to reach greater intensities of aerobic exercise for longer periods of time.
Why it is important to do this review
Incorporating exercise into one's daily routine is not a small endeavour. It is the responsibility of clinicians and researchers to identify for individuals with fibromyalgia both the effects they can expect of exercise training in terms of fibromyalgia symptoms and the most efficacious methods of achieving those effects. This review aims to explore the effectiveness of various combinations of types and training volumes of mixed exercise for improvement of fibromyalgia symptoms and physical function. This review also examined what outcomes are most impacted by mixed exercises, types of mixed interventions that have been tested, and the relative effects of these interventions.
Objectives
To evaluate the benefits and harms of mixed exercise interventions (interventions that include two or more forms of exercise) in adults with fibromyalgia
To assess the following specific comparisons
Mixed versus control conditions (e.g. wait list, treatment as usual, pharmaceutical treatment only, delayed treatment, education about fibromyalgia and lifestyle activities, daily activities not including physical activity)
Mixed versus non‐exercise interventions (e.g. biofeedback, relaxation, cognitive‐behavioural therapy)
Mixed versus other exercise interventions (e.g. remedial exercise, flexibility and posture)
Methods
Criteria for considering studies for this review
Types of studies
We included trials described as randomised, even if methods of generating the random sequence were unclear or unreported, or if the method of allocating participants was likely to be quasi‐random (i.e. by alternation, date of birth, or similar pseudo‐randomised method). Studies using a cross‐over design and cluster randomised controlled trials (RCTs) were not included.
Types of participants
We included studies that examined adults with fibromyalgia (18 years of age and older). We selected studies that used published criteria for diagnosis (or classification) of fibromyalgia. Diagnosis could be based on ACR 1990 criteria ‐ the preliminary diagnostic tool (Wolfe 1990), ACR 2010 criteria (Wolfe 2010), or a follow‐up survey questionnaire (Wolfe 2011). Although we noted some differences between the published fibromyalgia diagnostic (or classification) criteria, for the purposes of this review, we considered all to be acceptable and comparable. We set no restriction on the number of participants included in the trials.
Types of interventions
We examined trials that studied mixed exercise training interventions, which have been defined in detail under Description of the intervention (also see Appendix 1), regardless of frequency, duration, or intensity. We excluded studies providing such exercise interventions as Pilates, yoga, Tai Chi, manual therapy, and those focussed on a single region of the body. We also excluded studies with more than 50% of the time spent in aquatic exercise. Aquatic exercise studies are included in the systematic review on aquatic exercise training for fibromyalgia (Bidonde 2014a).
Comparators
Acceptable comparators included (1) controls (e.g. wait list, usual care, no intervention), (2) other exercise‐only interventions, and (3) non‐exercise interventions (e.g. relaxation, cognitive‐behavioural therapy, biofeedback, medication).
Comparators not included in this review are strength‐only training, aquatic exercise training, vibration‐only exercise, and flexibility‐only training. These were included in the reviews on resistance exercise training (Busch 2013), aquatic exercise training (Bidonde 2014a), whole body vibration exercise training (Bidonde 2017a), and flexibility exercise training (Kim SY 2019).
Types of outcome measures
We designated seven outcomes as major outcomes: HRQL, pain intensity, fatigue, stiffness, physical function, number of participants who withdrew or dropped out, and adverse events; and three as minor outcomes: submaximal cardiorespiratory function, muscle strength, and number of participants with greater than 30% improvement in pain. In selecting these outcomes, we considered the consensus statement regarding the core set of outcome measures for clinical trials in fibromyalgia provided by Choy 2009, along with the anticipated effects of mixed exercise training on physical fitness. We extracted data for selected outcomes at any time points measured; however, we included baseline, post‐treatment, and follow‐up (or long‐term) time points in this review. Each included study was required to report measurement of one or more outcomes at these time periods. Five outcomes were assessed using self‐report measures: HRQL, pain intensity, fatigue, stiffness, and physical function. Two outcomes were assessed using assessor‐reported measures: cardiorespiratory submaximal and muscle strength. Two outcomes were measured using counts: number of participants who withdrew from the study, and number of participants with a reduction in pain intensity greater than 30%.
Major outcomes
When an included study used more than one instrument to measure a particular outcome, we applied the following preferred hierarchy to choose the outcome for analysis.
Health‐related quality of life (HRQL) ‐ this outcome consists of multi‐dimensional indices used to measure general health status or HRQL, or both (Choy 2009). When included studies used more than one instrument to measure HRQL, we preferentially extracted data from the Fibromyalgia Impact Questionnaire (FIQ total; Burckhardt 1991), followed by the Short Form Questionnaire (the Short Form (SF)‐36 total or the SF‐12 total; Busija 2011; Ware 1993), then the EuroQol‐5D (standardised instrument used to measure HRQL; Wolfe 1997).
Pain intensity ‐ for the purpose of this review, we focussed on one aspect of the pain experience – pain intensity. When a single study reported more than one measure of pain intensity, we preferentially extracted measures of average pain intensity (as opposed to worst, least, or current pain) assessed by visual analogue scale (VAS; Ferreira‐Valente 2011), FIQ Pain, FIQ‐translated, and the McGill Pain VAS, followed by the Numerical Pain Rating Scale. When studies did not report uni‐dimensional measures of pain intensity, we extracted composite measures that include pain intensity and interference (SF‐36 or Rand 36 Bodily Pain Scale; Ware 1993), or pain intensity and suffering from pain (Multi‐dimensional Pain Inventory ‐ Pain Severity Scale).
Fatigue ‐ fatigue is recognised by individuals with fibromyalgia and clinicians alike as an important symptom (Choy 2009). Fatigue can be measured in a global manner, as when an individual rates fatigue on a single‐item scale or uses a multi‐dimensional tool that breaks the experience of fatigue down into two or more dimensions, such as general fatigue, physical fatigue, mental fatigue, reduced motivation, reduced activity, and degree of interference with activities of daily living (Boomershine 2012). We accepted both uni‐dimensional and multi‐dimensional measures for this outcome. When included studies used more than one instrument to measure fatigue, we preferentially extracted the fatigue VAS (FIQ/FIQ‐Translated Fatigue, or single‐item fatigue VAS), followed by the SF‐36 or Rand 36 Vitality subscale, the Chalder Fatigue Scale (total), the Fatigue Severity Scale (FSS), and the Multi‐dimensional Fatigue Inventory.
Stiffness ‐ in focus groups conducted by Arnold 2008, individuals with fibromyalgia "... remarked that their muscles were constantly tense. Participants alternately described feeling as if their muscles were ‘lead jelly’ or ‘lead Jell‐O', and this resulted in a general inability to move with ease and a feeling of stiffness". We used a common measure of stiffness encountered in this literature ‐ the FIQ stiffness subscale.
Physical function ‐ this outcome focusses on the basic actions and complex activities considered "essential for maintaining independence, and those considered discretionary that are not required for independent living, but may have an impact on quality of life" (Painter 1999). Given that cardiorespiratory fitness, neuromuscular attributes (e.g. muscular strength, endurance, power), and muscle and joint flexibility are important determinants of physical function, this outcome is highly relevant as an outcome of exercise interventions. When more than one measure of physical function was available within a study, we preferentially extracted data for the FIQ physical impairment scale (Burckhardt 1991), followed by the Health Assessment Questionnaire disability scale (HAQ), the SF‐36 or Rand 36 Physical Function Scale; the Sickness Impact Profile – Physical Disability (Bergner 1981), and the Multi‐dimensional Pain Inventory Household Chores Scale (Huskisson 1976; Huskisson 1983).
Adverse events ‐ we extracted the proportion of participants who experienced adverse events during the intervention (e.g. injuries, exacerbations of pain, other fibromyalgia symptoms). If this information was not available, we described the nature of the adverse events in a narrative report.
Withdrawals ‐ we recorded the proportion or number of participants who withdrew or dropped out of the study for any reason.
Minor outcomes
We present here a rationale and preferential listing of minor outcomes. We designated as minor outcomes two fitness variables that potentially could improve with mixed exercise training.
Submaximal cardiorespiratory function (CR submax) ‐ there are two major categories of submaximal tests: predictive and performance tests. Predictive tests are submaximal tests that are used to predict maximal aerobic capacity (Noonan 2000). Performance tests involve measuring responses to standardised physical activities that are typically encountered in everyday life. In this review, we preferentially extracted data from work completed at a specified exercise heart rate (e.g. Physical Working Capacity (PWC)170 test), followed by distance walked in six minutes (meters), the two‐minute walk test (meters), walking time for a set distance (seconds), the anaerobic threshold test, and timed walking distance (e.g. Quarter Mile Walk Test).
Muscle strength ‐ muscle strength is a measure of the ability of a muscle to generate force. It is commonly expressed as maximal voluntary contraction (MVC) during isometric testing; one‐repetition maximum (1RM) during dynamic isotonic testing (Howley 2001); and/or peak torque during isokinetic or isometric testing. When more than one measure of strength is reported, we preferentially extracted dynamic test results over isometric tests results, lower limb over upper limb tests, and extensor muscle strength over flexor muscle strength.
Improvement in pain greater than 30% ‐ a 30% reduction is considered a benchmark for a moderately important change in pain intensity, and consensus groups such as Initiative on Methods, Measurement, and Pain Assessment in Clinical Trials (IMMPACT) recommend this measure for interpreting clinical trial efficacy (Dworkin 2008). When available, we extracted data on the number of participants who met this criterion for intervention efficacy.
Search methods for identification of studies
Note: this is an update of the Busch 2002, and Busch 2007 reviews. Current search strategies differ from the strategies used in previous versions of this review (for previous search strategies, see Table 2).
1. Search strategy used for Busch 2002 FMS and exercise (first edition).
| Process | Particulars |
| Databases used | MEDLINE (1966‐12/2000), CINAHL (1982‐12/2000), HealthSTAR (1990‐12/2000), Sports Discus (1975‐12/2000), Embase (1974‐12/2000), Cochrane Controlled Trials Register (2000, Issue 4) |
| Adjunctive search methods | Reference lists from identified articles, meta‐analyses, and reviews of all types of treatment for FMS were reviewed independently by 2 review authors and all promising references were scrutinised. We searched without language restriction and translated all non‐English studies that were initially identified as possibly meeting the inclusion criteria |
| Search strategy used for MEDLINE | Search strategy on SilverPlatter v3.0 for Windows |
| 1 "Fibromyalgia"/ all subheadings 2 fibromyalgia 3 fibrositis 4 fibromyalgia or fibrositis 5 #1 or #4 6 explode "Exertion"/ all subheadings 7 "Physical‐Fitness"/ all subheadings 8 explode "Physical‐Therapy"/ all subheadings 9 "Exercise‐Test"/ all subheadings 10 "Exercise‐Tolerance"/ all subheadings 11 explode "Sports"/ all subheadings 12 "Pliability"/ all subheadings 13 #6 or #7 or #8 or #9 or #10 or #11 or #12 14 exertion* 15 exercis* 16 physical 17 motion 18 fitness 19 therapy 20 therapies 21 (physical or motion) near (fitness or therapy or therapies) 22 physical 23 endurance 24 physical near endurance 25 manipulation* 26 skating 27 running 28 jogging 29 swimming 30 bicycling 31 cycling 32 walking 33 rowing 34 weight 35 training 36 muscle 37 strengthening 38 skating or running or jogging or swimming or bicycling or cycling or walking or rowing or weight training or muscle strengthening 39 #13 or #14 or #15 or #21 or #24 or #25 or #38 40 #5 and #39 41 explode "Research‐Design"/ all subheadings 42 explode "Clinical‐Trials"/ all subheadings 43 #41 or #42 44 #40 and #43 45 PT = "CLINICAL‐TRIAL" 46 #40 and (PT = "CLINICAL‐TRIAL") 47 #44 or #46 |
The team Information Specialist conducted a comprehensive search of nine databases for physical activity interventions for adults with fibromyalgia. We screened the citations found in the electronic and manual searches and then classified them by type of exercise training. This comprehensive search yielded physical activity intervention studies that included a subset of mixed exercise training interventions.
Electronic searches
We searched the following databases from database inception to December 2017, using methods outlined in Chapter 6 of the Cochrane Handbook for Systematic Reviews of Interventions (Lefebvre 2011). We used an RCT filter for the Embase database and applied no language restrictions. Full search strategies for each database are found in the appendices, as indicated in this list.
Medline (OVID), Medline–In Process, MEDLINE 1946 to December 2017 (Appendix 2).
Embase (OVID), Embase Classic+Embase 1947 to December 2017 (Appendix 3).
-
Cochrane Library (Wiley) to December 2017 (http://www.thecochranelibrary.com/view/0/index.html) (Appendix 4).
Cochrane Database of Systematic Reviews (Cochrane Reviews).
Database of Abstracts of Reviews of Effects (DARE).
Cochrane Central Register of Controlled Trials (CENTRAL).
Health Technology Assessment Database (HTA).
NHS Economic Evaluation Database (EED).
Cumulative Index to Nursing and Allied Health Literature (CINAHL) (EBSCO) 1982 to December 2017 (Appendix 5).
Physiotherapy Evidence Database (PEDro) (www.pedro.org.au/) to December 2017 (Appendix 6).
Dissertation Abstracts (ProQuest) to December 2017 (Appendix 7).
Current Controlled Trials accessed to October 25, 2013 (Appendix 8).
ClinicalTrials.gov to December 2017 (Appendix 8).
World Health Organization (WHO) International Clinical Trials Registry Platform (www.who.int/ictrp/) to December 2017 (Appendix 9).
Allied and Complementary Medicine (AMED) (OVID) 1985 to December 2017 (Appendix 10).
Searching other resources
Two review authors independently reviewed reference lists from key journals, identified articles, meta‐analyses, and reviews; scrutinised all promising or potential references; and added appropriate titles to the search output.
Data collection and analysis
Review authors
Review authors were members of the Cochrane Musculoskeletal Group ‐ Exercise for Fibromyalgia Team (for a complete list, see Acknowledgements). The authors of this review were trained in data extraction using a standardised orientation programme. Review authors worked independently and in pairs with at least one physical therapist in each pair to extract data. The team met regularly to discuss progress, to clarify procedures, to make decisions regarding inclusion or exclusion and classification of outcome variables, and to work collaboratively in the production of this review.
Selection of studies
Two review authors used a set of predetermined criteria to independently examine the titles and abstracts of studies generated from searches (see Appendix 11). We used Covidence software to assist with independent screening of literature as of December 2017. We retrieved full‐text publications for all titles and abstracts and translated all non‐English reports. We examined the full‐text reports to determine if the study met the selection criteria. We resolved disagreements between the two review authors and questions regarding interpretation of inclusion criteria in discussion with partners, unless the pair agreed to take the issue to the team. For this review update, we reassessed whether each study from the previous review met the inclusion criteria. In keeping with Rosenthal's recommendations (Rosenthal 1995), we linked and presented as one all publications (including published protocols and trial registry records) referring to the same primary study (what we called 'companions') but presenting follow‐up data in consequent publications.
Data extraction and management
We used electronic data extraction forms developed and refined in our previous reviews to facilitate independent data extraction and consensus (Busch 2008). Pairs of review authors independently extracted the data. We resolved disagreements by consensus or by consultation with a third person if necessary. Two review authors transferred data into Review Manager software (RevMan 2014). We double‐checked that data were entered correctly by comparing data presented in the software with those provided in the study reports. We noted in the Characteristics of included studies table whether outcome data were not reported in a useable way, instances when data were obtained directly from RCT authors, and times when data were transformed or estimated from a graph. If both unadjusted and adjusted values for the same outcome were reported, we extracted the adjusted values. If the data were analysed based on an intention‐to‐treat (ITT) sample and another sample (e.g. per‐protocol, as‐treated), we extracted ITT data. For this updated review, we reassessed studies included in the previous review due to changes in methods (e.g. risk of bias) (Busch 2002;Busch 2007; Busch 2008).
We extracted the following data from the included studies.
Methods: study design, total duration of study, details of any 'run‐in' period, number of study centres and locations, study setting, and date of study.
Participants: N, mean age, age range, gender, disease duration, diagnostic criteria, inclusion and exclusion criteria.
-
Interventions, comparisons, concomitant treatments based on:
for all interventions with an exercise component: type of exercise, frequency, intensity, mode, duration, progression (if any), and congruence with American College of Sports Medicine (ACSM) guidelines on the quantity and quality of exercise for developing and maintaining cardiorespiratory and musculoskeletal fitness in apparently health adults (Garber 2011) (Appendix 12); and
for the intervention non‐exercise components; frequency, duration, and main characteristics.
-
Outcomes: major and minor outcomes as indicated above based on:
means, medians, standard deviations, or confidence intervals for tests at baseline and post‐intervention and follow‐up assessment(s) for continuous outcomes (HRQL, physical function, pain intensity, fatigue, stiffness, muscle strength, and CR submax);
if post‐test data were not available, means and standard deviations of change scores;
numerical or narrative information per group describing adverse events (e.g. injuries, exacerbations);
number of participants with improvement in pain greater than 30%; and
number of dropouts and reasons for each intervention.
Methodological quality of the trial as outlined below in the Risk of bias in included studies section.
Notes: country, language, author contact ‐ funding for trial, protocol identifier, and notable declarations of interest of trial authors.
Analysis of exercise interventions
We used the FITT‐VP framework (frequency, intensity, time, type, volume, pattern, progression) (ACSM 2013, pages 178‐188) to extract information about each component of the exercise interventions. We recorded exercise intensity as both published percentages of maximal heart rate (HRmax) or heart rate reserve (HRR) and the corresponding ACSM descriptors (ACSM 2013, page 165). For clarity, we have chosen to use type to differentiate among aerobic, resistance, and flexibility exercises, and mode to describe the actual exercise within each type of exercise. For example, for the aerobic exercise type, modes could be walking, cycling, or swimming. For resistance‐type exercise, modes could be lifting weights or using a resistance machine like the Nautilus. For flexibility, the mode could be stretching, range of motion, or hold relax. We have also used the word duration instead of time to refer to the length of exercise sessions. We have included information about pattern and progression (if any) under the categories of frequency, intensity, and time.
We evaluated whether exercise interventions achieved congruence with ACSM guidelines for improving or maintaining cardiorespiratory, neuromuscular fitness by comparing the programmes versus current ACSM guidelines for apparently healthy individuals (see Table 3) (Garber 2011).
2. Exercise intensity.
| Intensity | % VO2 reserve or % HRR | % HRmax | % VO2 max | RPE (6 to 20 scale) |
| Very light | < 30 | < 57 | < 37 | < 9 |
| Light | 30 to 39 | 57 to 63 | 37 to 45 | 9 to 11 |
| Moderate | 40 to 59 | 64 to 76 | 46 to 63 | 12 to 13 |
| Vigorous (hard) | 60 to 89 | 77 to 95 | 64 to 90 | 14 to 17 |
| Near maximal to maximal | ≥ 90 | ≥ 96 | ≥ 90 | ≥ 18 |
Garber 2011, ACSM 2013 (page 165).
HRmax: maximal heart rate; HRR: heart rate reserve; RPE: rating of perceived exertion; VO2: oxygen uptake.
Assessment of risk of bias in included studies
We followed the procedures recommended in the Cochrane Handbook for Systematic Reviews of Interventions to assess bias. Two review authors independently evaluated the risk of bias in each included study using a customised form based on the Cochrane 'Risk of bias' tool (Higgins 2011a). This tool addresses six specific domains: selection, performance, detection, reporting, attrition, and other biases. For other sources of bias, we considered things such as baseline inequities despite randomisation, adherence, or within‐study inequities in the duration of interventions.
We rated each domain as being at low, high, or unclear risk of bias. We assigned the criterion 'unclear risk' when absence or ambiguity of the information blocked assessors' ability to determine the potential for bias. In such cases, we revised the assessments if study authors responded to our requests for more information. We resolved disagreements between review authors on classifying risk of bias through discussion at consensus meetings. If we could not reach agreement, we referred the issue to the review team for a decision.
We divided the detection bias domain into blinding of subjective and assessor‐reported outcomes. For subjective outcome assessment (i.e. self‐report outcomes), we reported detection bias as low risk if participants were blind to treatment allocation. When studies did not include any assessor‐reported or subjective test, we rated detection bias related to assessor blinding as low risk and added an explanation (the current risk of bias tool does not allow us to rate this as not applicable or to leave the criterion blank). For example, we rated the criterion as low risk and added, "Not applicable; no assessor‐related tests were applied to measure cardiorespiratory submaximal function or muscle strength."
We synthesised risk of bias assessments by generating 'Risk of bias' summary figures using Review Manager 5 (RevMan 2014).
Measures of treatment effect
For continuous data, we used group post‐test means and standard deviations to calculate effect sizes. We expressed effect sizes preferentially in the form of mean differences (MDs) and 95% confidence intervals (95% CIs). When different scales were used to measure the same outcome, we calculated standardised mean differences (SMDs) with corresponding 95% CIs instead. We back‐translated SMDs to a typical scale (e.g. 0 to 10 for pain) by multiplying the SMD by a typical among‐person standard deviation (e.g. the standard deviation of the control group at baseline from the most representative trial). We analysed dichotomous data as risk ratios (RRs; difference in adherence after the intervention minus difference before the intervention) and 95% confidence intervals. This is a relative effect rather than an absolute effect; the effect size reflects baseline performance as well as change in performance, and it is not bound between ‐100% and +100%. We used RevMan 2014 software to generate forest plots to display the results. When evaluating long‐term effects, we grouped data for all post‐intervention follow‐up assessments into four intervals: 6 to 12 weeks, 13 to 26 weeks, 27 to 52 weeks, and longer than 52 weeks post intervention.
In the comments column of the Table 1, we provided the absolute percent difference and the number needed to treat for an additional beneficial outcome (NNTB), or the number needed to treat for an additional harmful outcome (NNTH). We provided the NNTB or the NNTH only when the outcome showed a clinically important between‐group difference. We calculated the NNTB for continuous measures using the Wells calculator (available at the CMSG Editorial office; http://musculoskeletal.cochrane.org/). For dichotomous outcomes, such as dropouts, we calculated the NNTH from the control group event rate and the relative risk using the Visual Rx NNT calculator.
In accordance with the Philadelphia Panel, we assumed a minimal clinically important between‐group difference (MCID) of 15 points on a 100‐point continuous pain scale (or an absolute difference of 15%) and a relative difference of 15% on all functional scales as clinically relevant. We used the MCID in calculating the NNTB for continuous outcomes. For dichotomous outcomes, we calculated the absolute risk difference using the risk difference statistic in RevMan 2014 with the result expressed as a percentage. We calculated the relative percent change for dichotomous data as Risk ratio ‐ 1, and expressed this as a percentage. For continuous outcomes, we calculated the absolute benefit as improvement in the intervention group minus improvement in the control group, in the original units and expressed as a percentage. We calculated the relative change as the MD divided by the pooled baseline mean of the control groups according to the standards of the Cochrane Musculoskeletal Group (http://musculoskeletal.cochrane.org/).
Unit of analysis issues
Although many randomised trials have only two parallel arms (i.e. groups), some have three or four parallel arms; thus a single randomised trial can yield several relevant comparisons. This review examined any relevant comparison that allowed evaluation of the effects of mixed exercise training interventions on people with fibromyalgia. For example, a three‐arm trial comparing mixed versus drug treatment versus sham could appear in two separate analyses: mixed versus sham; and mixed versus drug treatment. If a control group was used as a comparator twice in the same analysis, the sample size of the control group was halved. In the event that two arms of the same trial were included in a comparison, we planned to aggregate and present the data as one.
Dealing with missing data
When numerical data were missing, we contacted the study author to request the additional data required for analysis. We used open‐ended questions to obtain the information needed to assess risk of bias or treatment effect. We have noted correspondence with authors in the 'Notes' section of the Characteristics of included studies. We were unable to get a response from authors of the following studies: Alentorn‐Geli 2008; Garcia‐Martinez 2011; Genc 2002; Rooks 2007; van Santen 2002a; and van Santen 2002b. When numerical data were available only in graphic form, we used Engauge version 5.1 to extrapolate means and standard deviations by digitalising data points on the graphs (Mitchell 2012).
For dichotomous outcomes (e.g. number of withdrawals), we calculated the withdrawal rate by using the number of participants randomised in the group as the denominator. For continuous outcomes (e.g. post‐test pain score), we calculated the MD or the SMD based on the number of individuals analysed at that time point. When the number of individuals analysed was not presented for each time point, we used the number of individuals randomised to each group at baseline. When means were not reported, medians were accepted. When post‐test standard deviations were unavailable, we used standard deviations of the pre‐test scores as estimates. When variance was expressed using statistics other than standard deviation (e.g. standard error, confidence interval, P value), we computed standard deviations according to the methods recommended in Chapter 7 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011 Ch7). When missing standard deviations could not be derived via the methods described above, we imputed them from other studies in the meta‐analysis.
Assessment of heterogeneity
We assessed statistical heterogeneity through visual inspection of the forest plot to assess for obvious differences in results between studies, and using the I² and chi² statistical tests. As recommended in the Cochrane Handbook for Systematic Reviews of Interventions (Deeks 2017 Ch9), we followed the interpretation of an I² value from 0% to 40% as 'might not be important'; from 30% to 60% as representing 'moderate’ heterogeneity; from 50% to 90% as representing 'substantial' heterogeneity; and from 75% to 100% as representing 'considerable' heterogeneity. Because I² has overlapping categories (i.e. 0% to 40%, 30% to 60%) or "ambiguous" zones, when we found moderate to substantial statistical heterogeneity (i.e. I² between 50% and 60%), we explored it thoroughly. In addition, we assessed clinical and methodological diversity in terms of participants, interventions, outcomes, and study characteristics to determine whether a meta‐analysis was appropriate.
When removing a trial from the analysis, we recalculated both heterogeneity and effect size. Given that values between 50% and 60% fall in an 'ambiguous' zone, if we could find no apparent causes of heterogeneity, we kept the trial in the analysis and documented our decision. We interpreted the Chi² test with P ≤ 0.10 as indicating evidence of statistical heterogeneity.
Assessment of reporting biases
Between studies reporting biases: we produced funnel plots to investigate publication reporting bias when a large enough sample of studies (i.e. more than 10 studies) was available or was included in the meta‐analysis for the mixed versus control comparison (Sterne 2017).
Within studies reporting biases: when a published or trial registry record/protocol was available, we compared the number and order of outcomes in the study protocol versus outcomes in the published report. We screened the Clinical Trial Registers at the International Clinical Trials Registry Platform of the World Health Organization (http://apps.who.int/trialssearch) and at ClinicalTrials.gov (http://clinicaltrials.gov) for the RCT registry records of articles published after 2005. We documented the trial number or the availability of a published protocol in the 'Risk of bias' table (Risk of bias in included studies).
Data synthesis
When two or more studies reported the same outcome and interventions were deemed homogeneous enough, we pooled the data (meta‐analysis) using RevMan (RevMan 2014). Before pooling data, we ensured that the directionality of the data permitted pooling; we arithmetically reversed selected scales as needed so higher values consistently had the same meaning. We ensured that scaling factors were consistent to permit calculation of MD (e.g. 10‐cm scales were expressed in mm to match other 100‐mm scales). We presented results grouped by common comparator, for example, mixed versus control, mixed versus no exercise, etc. We included all studies for adverse events and for withdrawals. We included studies in the meta‐analyses regardless of risk of bias rating. We used the random‐effects model for all meta‐analyses (Sterne 2017).
Meta‐regression
If a large number of trials were available (at least 10 per variable), we planned to conduct a meta‐regression to explore variation in results based on the exercise characteristics of included studies (Deeks 2017 Ch9). In other words, we aimed to estimate the treatment effect by controlling for differences across studies and determining which study level co‐variate accounted for the heterogeneity. We planned to use a random‐effects model and SPSS statistical software for analysis (Berkery 1995; Berlin 1994; Berlin 2002; Thompson 2002). We did not identify enough trials to conduct a meta‐regression for this review.
GRADE and 'Summary of findings' tables
We used the GRADE approach to assess the quality of evidence related to each of the major outcomes at the end of intervention (Schünemann 2017 ch12). We used GRADEpro 2011 software to import data from Review Manager and create a 'Summary of findings' table for the major outcomes for the mixed exercise training versus control comparison. In Table 1, we integrated analysis of the quality of evidence and the magnitude of effect of the interventions.
For assessments of the overall quality of evidence for each outcome that included pooled data, we downgraded the evidence from 'high quality' by one level for serious (or by two levels for very serious) study limitations (risk of bias), indirectness of evidence, inconsistency, imprecision of effect estimates, or potential publication bias.
Subgroup analysis and investigation of heterogeneity
We planned to explore the relative effects of age and exercise volume (frequency × duration × intensity) on the impact of mixed exercise for pain intensity and HRQL and the primary comparison. We planned subgroups for age to be younger (45 years or younger) and older (over 45 years). Age 45 was proposed as a cut‐off based on changes in hormone levels and lifestyle (physical activity participation) that occur with aging (Shephard 1998). Subgroups for exercise volume were based upon ACSM criteria (meets ACSM criteria/does not meet ACSM criteria) according to ACSM 2013. We also planned to explore the effects of combining/adding an education component to the mixed exercise intervention.
We planned to use the formal test for subgroup interactions in RevMan 2014, and to use caution in interpreting subgroup analyses, as advised in Section 9.6 of the Cochrane Handbook for Systematic Reviews of Interventions (Deeks 2017 Ch9). We also aimed to compare the magnitude of effects between subgroups by assessing overlap of the confidence intervals of the summary estimated. Non‐overlap of confidence intervals could indicate statistical significance.
Sensitivity analysis
We explored the impact of including studies with high or unclear risk of selection, detection, and attrition biases in the meta‐analyses using sensitivity analyses. We restricted sensitivity analyses to two major outcomes (HRQOL and pain intensity) and the primary comparison (mixed exercise interventions vs control (usual care, no intervention, placebo or sham exercise, or minimal intervention)).
Results
Description of studies
See Characteristics of included studies; Characteristics of excluded studies; Characteristics of studies awaiting classification; and Characteristics of ongoing studies.
Results of the search
The search resulted in a total of 6533 journal and trial registry records. After 2771 duplicates were removed, 3762 records remained to be screened. We excluded 3477 records on citation and abstract screening. We assessed 285 full‐text articles, published study protocols, theses, and trial registry records for eligibility and excluded 91 full‐text articles, three theses, and five trial registry records. Twenty‐nine published studies (29 articles, one companion article, and three companion trial registry records) and five ongoing studies (two published protocols, three trial registry records, and two companion trial registry records) met the inclusion criteria for this review (see Figure 1, Characteristics of included studies, and Characteristics of ongoing studies). An additional 13 articles and two trial registry records representing 12 unique studies are awaiting classification (Characteristics of studies awaiting classification).
1.
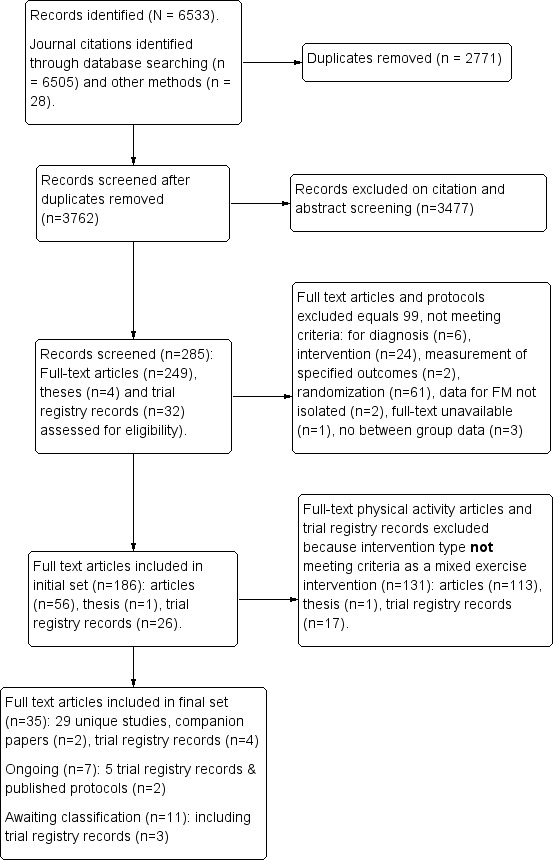
Study flow diagram. (Note: the term 'protocol' refers to both published study protocols and trial registry records; the term 'companion' refers to either a protocol or an additional publication for the same study.)
Included studies
We included 29 unique research studies for analysis. Jones published two papers on the same RCT ‐ one in 2007 and one in 2008; we will refer to this work as Jones 2007. van Eijk also published two papers on the same RCT ‐ one in 2013 and a follow up study in 2015, which we will refer to as van Eijk‐Hustings 2013. Of the study protocols that met our criteria, four described included studies (Alentorn‐Geli 2008; Baptista 2012; Giannotti 2014; van Eijk‐Hustings 2013), and the remaining studies were not yet completed; we therefore classified them as ongoing (da Silva 2015Gusi N; Mendonça Araújo F; Montañez‐Aguilera J; Ruiz Ruiz J) (see Characteristics of ongoing studies).
Studies were published between 1994 and 2015; 27 were written in English and two were translated from Turkish (Genc 2002; Yuruk 2008). Studies were conducted in 12 different countries (Spain 7; Netherlands 4; US 4; Italy 3; Turkey 3; Canada 2; Brazil 1; Finland 1; India 1; Norway 1; Sweden 1; and United Kingdom 1). We contacted 21 study authors using open‐ended questions and received 15 answers (see "Notes" section in Characteristics of included studies table). We have summarised in Table 4 the inclusion and exclusion criteria for the 29 trials considered in this review. Genc 2002 did not list any exclusion criteria.
3. Inclusion and exclusion criteria (all included trials).
| Inclusion criteria | Exclusion criteria |
|
|
ACSM: American College of Sports Medicine; SNRI: serotonin‐norepinephrine reuptake inhibitor.
Participants
This review included 2088 participants, of whom 2028 (98%) were female. Nearly 70% of the studies in this review involved only females. The duration of disease or symptoms since diagnosis ranged from 4 to 19.4 years; 12 studies did not report this information, and one study reported that most participants were at one to 10 years since their diagnosis. The average age of participants was 51 years (study means ranged from 43.2 to 59 years, range of ages across all studies was 27.5 to 62.3); one study did not report the age of participants (Sanudo 2012). All participants had a diagnosis of fibromyalgia ‐ most according to ACR 1990 criteria (Wolfe 1990), one based on ACR 2010 criteria (Giannotti 2014; Wolfe 2010), one based on Yunus' guidelines (Buckelew 1998; Yunus 1981), and one ‐ Verstappen 1997 ‐ based on Wolfe's earlier guideline (Wolfe 1988).
Outcomes
Outcomes and outcome measures (number of studies using the tool) used in the mixed exercise versus control comparison are stated below (for detailed information on remaining comparisons, see Characteristics of included studies ‐ 'outcomes').
HRQL: FIQ Total (14), The Arthritis Impact Measurement Scales (Dutch‐AIMS) (1).
Pain: FIQ pain (6), VAS (6), SF‐36 bodily pain (3), Fibromyalgia Actitivity Score (FAS) pain (1).
Fatigue: FIQ fatigue (4), VAS (2), SF‐36 vitality (4), FSS (1).
Stiffness: FIQ stiffness (4), VAS (1).
Physical function: FIQ impairment (3), SF‐36 physical function (4), AIMS physical function (1), HAQ (1), Sickness Impact Profile (SIP) physical function (1).
Cardiorespiratory: six‐minute walk test (4).
Muscle strength: maximum voluntary contraction of knee extensors (Newtons) (1), right grip strength (Newtons) (1), concentric knee extension (Newtons) (1), static arm pull (kg) (1).
Number of participants with ≥ 30% reduction in pain (0).
Design
All studies were randomised clinical trials with a one to three parallel‐group study design. Seven studies had three arms (Alentorn‐Geli 2008; Burckhardt 1994; Clarke‐Jenssen 2014; Sanudo 2010b; Sanudo 2013; van Eijk‐Hustings 2013; van Santen 2002a), and three had four arms (Buckelew 1998; Jones 2007; Rooks 2007). The remaining studies (n = 19) had two arms. The arms included mixed exercise only compared to control, mixed exercise plus education compared to control, and mixed exercise only compared to another form of exercise or intervention. There were 506 participants in control conditions who did not change their treatments over the study period. Information on arms included in the analyses can be found in the Characteristics of included studies table.
Interventions
Among the full sample of studies (n = 29), average length of treatment was 13 weeks (median 12 weeks, range 3 to 26 weeks). A detailed description of the exercise interventions, including each of frequency, intervention, time, and type and mode (FITT) parameters, is presented in the Characteristics of included studies table and in Table 5 and Table 6.
4. Detailed description of exercise interventions ‐ mixed exercise versus control.
| Author, year | Full exercise programmea | Type | Mode, intensity, timeb | Congruence with ACSM guidelines |
| Mixed exercise only vs control | ||||
| Alentorn‐Geli 2008 | MX (AE+FX+Relax) + Placebo whole body vibration · Supervised sessions 2/week for 6 weeks · Total duration of each exercise session (excluding relax): ˜ 60' |
AE | Primarily level ground walking with games, dance, moderate to vigorous intensity (65% to 85% HRmax) × 30' | No2 |
| RT | None | n/a | ||
| FX | 5 whole body static stretches involving lower and upper extremities, neck and back, 5 reps held for 30" to 'stop point' with 30" rests, for 25' | Y | ||
| Other | Relax, Placebo whole body vibration | n/a | ||
| Baptista 2012 | MX (Belly dance)c · Supervised sessions 2/week for 16 weeks · Home programme 2/week for 12 weeks (week 4 to 16) · Total duration of exercise sessions: 60' |
AE + RT + FX | Supervised sessions: Belly dance for 45', intensity unspecified Home programme: Belly dance > 30', intensity unspecified 5 whole body static stretches involving lower and upper extremities, neck, and back; 5 reps held for 30" to 'stop point' with 30" rests, for 25' |
No1 |
| Other | None | ‐‐‐ | ||
| Buckelew 1998 | MX (AE+RT+FX+Posture + Biomechanics + Instruction in use of hot and cold and massage) Active phase: · Supervised sessions 1/week for 6 weeks · Active phase: home programme 2×/week for 6 weeks |
AE (Active phase) | Walking, light to moderate intensity (60% to 70% HRmax) × unspecified part of 1 to 3 hours total | No1 |
| RT (Active phase) | Unspecified | No1 | ||
| FX (Active phase) | Unspecified beyond "active range of motion" | No1 | ||
| Other (Active phase) | Instruction in posture and biomechanics, hot and cold modalities, and massage | No1 | ||
|
Maintenance phase: · 1 support meeting/mo for 104 weeks · Maintenance phase: home programme unspecified frequency for 104 weeks · Total duration of exercise sessions: unspecified |
AE (Maintenance phase) | Unspecified for all types of exercise | No1 | |
| Other | Support meeting | n/a | ||
| Da Costa 2005 | MX (AQ AE+Land AE+RT+FX) ‐ Phase 1: home programme frequency; participant selected for 12 weeks ‐ Phase 1: supervised meetings at weeks 0, 1, 3, 9 ‐ Total duration of exercise sessions: dependent on individual prescription and exercise intensity |
AE | Individually prescribed programme. Participant‐selected mode including walking, swimming, dancing, aqua fitness, light to moderate intensity (60% to 70% HRmax) progressed to moderate to vigorous intensity (75% to 85% HRmax) for participant‐selected time between 60 and 120 min/week | No1 |
| RT | Varied with individual prescription, 3/week. May have included callisthenics with max reps, free weight exercise at 12 to 15 RM for upper and lower limbs and trunk | No1 | ||
| FX | Varied with individual prescription: 3 reps of static stretches with 15 to 30" holds for upper and lower limbs, intensity unspecified | No1 | ||
| Other | None | n/a | ||
| Etnier 2009 | MX (AE+RT+FX) ‐ Supervised 3/week for 18 weeks ‐ Total duration of exercise sessions: 60' |
AE | Walking, moderate to vigorous intensity (55% to 65% HRR) × unspecified time | No1 |
| RT | 8 isotonic and isometric exercises for unspecified muscle group at 'light' intensity | No1 | ||
| FX | Unspecified FX | No1 | ||
| Other | None | n/a | ||
| Garcia‐Martinez 2011 | MX (AE+RT+FX) · Supervised sessions 3/week for 12 weeks · Total duration of each exercise session: 60' |
AE | Unspecified mode, light to moderate intensity (60% to 70% HRmax) progressed to moderate to vigorous intensity (75% to 85% HRmax) for 20' | No2 |
| RT | Unspecified, RT+FX=20' | n/a | ||
| FX | Unspecified, RT+FX=20' | Y | ||
| Other | None | n/a | ||
| Jones 2007 | MX (AE+RT+FX+Bal+Relax) · Supervised sessions 3/week for 26 weeks · Total duration of each exercise session: 60' |
AE | Low‐impact floor aerobics, light intensity (40% to 50% HRmax) or RPE 10 to 12 on 0 to 20 scaled. Duration for warm‐up + AE = 30' | No2 |
| RT | Isotonic exercises for all major muscle groups using elastic bands and free weights, unspecified intensity for 10' | No1 | ||
| FX | Unspecified static and non‐ballistic stretches for all major muscle groups for 5', reps/sets, intensity unspecified for 5' | No2 | ||
| Other | Balance and relaxation for 15' | n/a | ||
| Sanudo 2010b | MX (AE+RT+FX) · Supervised sessions 2/week for 24 weeks · Total duration of each exercise session: 45' to 60' |
AE | Walking, jogging, moderate intensity (65% to 70% HRmax) for 10 to 15' | No2 |
| RT | Unspecified isotonic exercises with free weights for 8 muscle groups, 1 set of 8 to 10 reps with 1 to 3 kg for 15 to 20' | Y | ||
| FX | Static stretches for 8 to 9 muscle groups of upper, lower limbs and trunk, 1 set of 3 reps with 30" holds, intensity unspecified, for unspecified timee | Y | ||
| Other | ‐‐‐ | n/a | ||
| Sanudo 2011 | MX (AE+RT+FX) · Supervised sessions + home program 2/week x 24 weeks · Total duration of each exercise session: 45 to 55' |
AE | Walking with arm movements, jogging, moderate intensity (65% to 70% HRmax) for 10 to 15' | No2 |
| RT | Isotonic exercises with free weights for 8 muscle groups of upper and lower limbs and trunk, initially light intensity, progressed to participant tolerated loads for 15 to 20' | No1 | ||
| FX | Static stretches for 8 to 9 exercise stations, 1 set of 3 reps with 30" holds for 10' intensity unspecified, for 10'e | Y | ||
| Other | ‐‐ | n/a | ||
| Sanudo 2012 | MX (AE+RT+FX) · Phase 1: unsupervised sessions 2/week × 26 weeks · Total duration of exercise session: 45' to 60' |
AE | Walking with arm movements, jogging, moderate intensity (65% to 70% HRmax) for unspecified time | No2 |
| RT | Isotonic exercises with free weights for 8 muscle groups, 1 set of 8 to 10 reps with 1 to 3 kg for 15 to 20' | Y | ||
| FX | Static stretches for 8 to 9 muscle groups of upper, lower limbs and trunk, 1 set of 3 reps with 30" holds, intensity unspecified, for 10' e | Y | ||
| Other | ‐‐ | n/a | ||
| Sanudo 2013 | MX (AE+RT+FX) · Supervised sessions 2/week for 8 weeks · Total duration of exercise session: 45' to 60' |
AE | Walking, moderate intensity (65% to 70% HRmax) for 10 to 15' | No2 |
| RT | Isotonic exercises with free weights for 8 muscle groups of trunk, upper, lower limbs, 1 set of 8 to 10 reps with 1 to 3 kg for 15 to 20' | No1 | ||
| FX | Static stretches for 8 to 9 muscle groups of upper, lower limbs and trunk, 1 set of 3 reps with 30" holds, intensity unspecified, for 10' e | Y | ||
| Other | ‐‐ | n/a | ||
| Valkeinen 2008 | MX (RT+AE) · Supervised RT sessions, partially supervised AE sessions, 3/week (approximately 1.5/week for each AE and RT) for 21 weeks · Total duration of exercise sessions 30' to 90' depending on exercise type |
AE | Cycle ergometry, walking, low to vigorous intensity (below to above anaerobic threshold) for 30 to 60' | Y |
| RT | Isotonic exercise on unspecified equipment for leg extensors + other main muscle groups, 2 to 4 sets at 15 to 20 RM progressed to 2 to 6 sets at 5 to 8 RM for 60' to 90' | No2 | ||
| FX | ‐‐ | n/a | ||
| Other | ‐‐ | |||
| van Eijk‐Hustings 2013 | MX (AE+RT) · Phase 1: supervised sessions 2/week for 12 weeks · Phase 1: HP 1/week for 12 weeks · Total duration of exercise session: 60' |
AE | Supervised AE: exercises on floor of gym with and without steps at low to moderate intensity (55% to 64% HRmax) for 30' Unsupervised AE: unspecified | No1 |
| RT | Supervised RT: unspecified isotonic exercises using weights for major muscle groups for 15'. Unsupervised RT: unspecified | No1 | ||
| FX | ‐‐ | n/a | ||
| Other | ‐‐ | n/a | ||
| van Santen 2002a | MX (AE+FX+RT) · Supervised sessions 2/week × 24 weeks · Unsupervised sessions 1/week × 24 weeks · Total duration of each exercise session: 60' |
AE | Unspecified mode at participant‐selected intensity, AE+FX+Balance=30' | No1 |
| RT | Unspecified isometric exercises at participant‐selected intensity for 10' | No1 | ||
| FX | Unspecified stretches at participant‐selected intensity, AE+FX+ Balance=30' | no1 | ||
| Other | Balance for an unspecified portion of 30' | n/a | ||
| Verstappen 1997 | MX (AE+RT+FX+Co‐ordination) · Supervised session 2/week for 26 weeks · Home programme 1 to 2/week for 26 weeks · Total duration of each exercise session: 50' |
AE | Cycle ergometry or treadmill running at participant‐selected intensity, AE+RT+FX+Co‐ordination=30' | No2 |
| RT | Isotonic exercise using Nautilus equipment for upper limbs, lower limbs, and abdomen, at participant‐selected intensity, AE+RT+FX+Co‐ordination=30' | No1 | ||
| FX | Unspecified stretches, AE+RT+FX+Co‐ordination=30' | No1 | ||
| Other | Co‐ordination exercises, AE+RT+FX+Co‐ordination=30' | n/a | ||
| Mixed Exercise + Education versus Control | ||||
| Burckhardt 1994 | MX (AQ and Land AE+FX) ‐ Phase 1 ‐‐ 1 supervised and 2 unsupervised sessions per week for 6 weeks ‐ Supervised group session: duration 60' ‐ Unsupervised session duration unknown ‐ Phase 2 ‐‐ 6 weeks unsupervised exercise, 1 follow‐up session to discuss and modify exercise |
AE | Unspecified pool exercise, walking, swimming, or cycling, intensity not specified | No1 |
| RT | None | n/a | ||
| FX | Unspecified stretching and range of motion | No1 | ||
| Other | none | n/a | ||
| Clarke‐Jenssen 2014 (2 intervention arms with identical exercise protocols) | MX (AQ/Land: AE+RT+FX+RX)+ED+Group discussion and Resting, in Warm Climate · Active phase: supervised sessions 5/week for 4 weeks (Land AE 5/week, AQ and RT alternating, each 2 or 3/week, Relax 2/week, Rest 5/week) · Total duration of each exercise session 115' |
AE | Land: Walking at low to moderate intensity (slightly out of breath) for 45'. AQ: 'emphasis on aerobic exercise' (page 678), low to moderate intensity (slightly out of breath) for 45' | No1 |
| RT | Unspecified details, "emphasis on body awareness, balance and strengthening exercises", with RT at moderate intensity for 45' | No1 | ||
| FX | Unspecified mode of stretches for all main muscle groups, reps, sets and intensity unspecified, for 15' | No1 | ||
| Other | Relax using hold relax technique for 45' education, small group discussion, rest for 60' | n/a | ||
| Giannotti 2014 | MX (AE+RT+FX+Ther ex) + ED · Phase 1: supervised sessions 2/week for 10 weeks · Phase 1: home programme 3+/week for 10 weeks · Total duration of each exercise session 60' |
AE | Cycle ergometry at vigorous intensity (70% functional capacity) for 10' in sessions 10 to 20 | No2 |
| RT | Strengthening exercises, no equipment used, for spine and lower limbs, 1 set of 10 reps at unspecified intensity for 10' in sessions 8 to 20 | No2 | ||
| FX | Stretches for spine, upper and lower limbs, 2 reps held 50 to 60"/stretch, intensity unspecified. FX+Ther ex=25' | Y | ||
| Other | Ther ex. FX+Ther ex=25'. Education sessions 1 to 7 about FM and management plus correction of ex performance for 10' in sessions 8 to 20 | n/a | ||
| Hunt 2000 | MX (AE+RT+FX)+ED · Supervised sessions: 1/week for 5 weeks · Home programme: 7/week for 5 weeks · Total duration of each exercise session: unspecified |
AE | Cycle ergometry or stepping at moderate intensity (RPE 3 to 4/10) for 15' | No2 |
| RT | 8 lower body and core callisthenics without weights and isometric exercises performed for 2'/exercise | No1 | ||
| FX | 12 static stretches for trunk, upper and lower limbs, 5 reps held for 5", intensity unspecified, for unspecified time | Y | ||
| Other | Education regarding advice on sleep, relax, pain managementt | n/a | ||
| Paolucci 2015 | MX (AE+RT+agility, balance, postural exercises+ED) · Phase 1: supervised sessions 2/week for 5 weeks · Phase 1: home programme unspecified times/week · Total duration of each exercise session: 60' |
AE | Walking + stair steps at light intensity (60% HRmax) for 20' | No2 |
| RT | Callisthenics for upper + lower limbs and trunk 3 sets × 10 reps for unspecified time | No1 | ||
| FX | Static stretches for upper and lower limbs and trunk, 3 reps of 30 to 60" intensity unspecified for unspecified time | No1 | ||
| Other | Agility, balance, posture, breathing exercises, education | n/a | ||
| Salaffi 2015 | MX (AE+RT+FX)+ED · Supervised sessions 2/week for 12 weeks · Total duration of each exercise session: unspecified |
AE | Participant‐preferred mode at light to moderate intensity (60% to 70% HRmax) progressed to moderate to vigorous intensity (75% to 85% HRmax) for 60 to 120'/week | No2 |
| RT | Prescribed on individual basis. Isotonic exercises with weights for upper and lower limbs, 1 set × 10 reps with 1 to 3 kg for upper limbs, 3 to 5 kg for lower limbs, progression encouraged, 1 kg/week for unspecified time | No1 | ||
| FX | Stretches prescribed on basis of individual need; no further details provided | Y | ||
| Other | Education | n/a | ||
ACSM: American College of Sport Medicine; AE:aerobic; AQ: aquatic exercise; AQ AE: aquatic aerobic; Bal: balance; ED: education; FM: fibromyalgia; FX: flexibility; HRmax: heart rate maximum; HRR: heart rate response; Land: land exercise; Land AE: land aerobic; min/week: minutes per week; MX: mixed exercise; n/a: not applicable; RM: repetition maximum; RPE: rating of perceived rating scale; RT: resistance; Relax: relaxation; reps: repetitions; Ther ex: therapeutic exercise; Y: yes.
aTotal duration of each exercise session includes warm‐up+cool‐down=all AE, RT, FX.
b AE intensity is usually expressed as a descriptor (such as moderate) followed by the physiological equivalent (such as % HRmax). RT intensity is usually expressed as the numbers of repetitions and sets at a specific RM.
c Reviewers classified this belly dance intervention to be a combination of AE+RT+FX based on the physiological demands of this form of exercise.
d Authors use two conflicting descriptors of AE intensity: 40% to 50% HRmax = low intensity, and RPE of 10 to 12/20 = moderate intensity.
eWhen no details about any component of the FX were available to reviewers, we entered "Unspecified" without listing all unspecified components in this table.
1 = not enough information to evaluate congruence with ACSM guidelines.
2 = frequency, duration, and/or intensity did not meet ACSM guidelines.
5. Detailed description of exercise interventions ‐ mixed exercise vs other interventions.
| Author, year, intervention | Full exercise programmea | Type | Mode, intensity, time b,c | Congruence with ACSM guidelines |
| MX only vs ˜EX | ||||
| Alentorn‐Geli 2008 | MX (AE+FX+Relax)+Placebo whole body vibration ‐ Supervised sessions 2/week for 6 weeks ‐ Total duration of exercise sessions (excluding relax): ˜ 60' |
AE | Primarily level ground walking with games, dance, moderate to vigorous intensity (65% to 85% HRmax) for 30' | No2 |
| RT | None | n/a | ||
| FX | 5 whole body static stretches involving lower and upper extremities, neck, and back, 5 reps held for 30" to 'stop point' with 30" rests, for 25' | Y | ||
| Other | Relax, Placebo whole body vibration | n/a | ||
| Buckelew 1998 | MX (AE+RT+FX+Posture+Biomechanics+ Instruction in use of hot and cold and massage) Active phase: · Supervised sessions 1/week for 6 weeks · Active phase: home programme 2×/week for 6 weeks |
AE (Active phase) | Walking, light to moderate intensity (60% to 70% HRmax) × unspecified part of 1 to 3 hour total | No1 |
| RT (Active phase) | Unspecified | No1 | ||
| FX (Active phase) | Unspecified beyond "active range of motion" | No1 | ||
| Other (Active phase) | Instruction in posture and biomechanics, hot and cold modalities, and massage | n/a | ||
| Active phase: Home Program | Unspecified for all types of exercise | No1 | ||
|
Maintenance phase: · 1 support meeting/mo for 104 weeks. Maintenance phase: home programme unspecified frequency for 104 weeks · Total duration of exercise sessions: unspecified |
Home programme | Unspecified for all types of exercise | No1 | |
| Other | Support meeting | n/a | ||
| (a) MX only vs Ed, SMT, or CBT | ||||
| Alentorn‐Geli 2008 | MX (AE+FX+Relax)+Placebo whole body vibration · Supervised sessions 2/week for 6 weeks · Total duration of exercise sessions (excluding relax): ˜ 60' |
AE | Primarily level ground walking with games, dance, moderate to vigorous intensity (65 to 85% HRmax) for 30' | No2 |
| RT | None | n/a | ||
| FX | 5 whole body static stretches involving lower and upper extremities, neck, and back, 5 reps held for 30" to 'stop point' with 30" rests, for 25' | Y | ||
| Other | Relax, Placebo whole body vibration | n/a | ||
| Rooks 2007b | MX (AE+FX) · Supervised sessions 2/week for 16 weeks · Home programme 1/week for 16 weeks · Total duration of each exercise session: 60' |
AE | Treadmill walking at participant‐determined moderate effort, progressed from 5' to 45' | No2 |
| RT | ‐‐ | n/a | ||
| FX | Unspecified stretches for primary body movements unspecified reps, sets, intensity × unspecified time | No1 | ||
| Other | ‐‐ | ‐‐ | ||
| MX (RT+AE+FX) · Supervised sessions 2/week for 16 weeks · Home programme 1/week for 16 weeks · Total duration of each exercise session: 60' |
AE | Treadmill walking at participant‐determined moderate effort, progressed from 5' to 20' | No1 | |
| RT | Isotonic exercises using machines and hand weights for upper and lower limbs and trunk, 1 set 'easy' progressed to 2 sets of 10 to 12 at unspecified RM for 25' | No1 | ||
| FX | Unspecified stretches for primary body movements unspecified reps, sets, intensity × unspecified time | No1 | ||
| Other | ‐‐ | ‐‐ | ||
| Rivera Redondo 2004 | AQ+Land MX (AE+FX+ST) · Active: supervised sessions 5/week for 8 weeks · Follow‐up: home programme "daily" for 52 weeks · Total duration of each exercise session: 45' |
AE | Conflicting and unclear information provided from publication plus author communications results in an unclear understanding of AE. AE intensity was light to vigorous (50% to 80% HRmax) and included AQ exercise (details not specified) and cycle ergometry on land | No1 |
| RT | Isotonic for upper limbs and trunk. Conflicting and unclear information provided from publication plus author communications results in an unclear understanding of RT | No1 | ||
| FX | Conflicting and unclear information provided from publication plus author communications results in an unclear understanding of FX | No1 | ||
| Other | ‐‐ | ‐‐ | ||
| Martin 1996 | MX (AE+FX+RT) · Supervised sessions 3/week for 6 weeks · Total duration of each exercise session: 60' |
AE | Walking at light to vigorous intensity (60% to 80% HRmax) for 20' | No1 |
| RT | Isotonic exercises for upper, lower limbs and trunk at unspecified intensity for 20' | No1 | ||
| FX | Unspecified stretches upper, lower limbs and trunk at unspecified sets, reps, intensity for 20' | No1 | ||
| Other | ‐‐ | ‐‐ | ||
| Buckelew 1998 | MX (AE+RT+FX+Posture+Biomechanics +Instruction in use of hot and cold and massage) Active phase: · Supervised sessions 1/week for 6 weeks · Active phase: home programme 2×/week for 6 weeks |
AE (Active phase) | Walking, light to moderate intensity (60% to 70% HRmax) × unspecified part of 1 to 3 hour total | No1 |
| RT (Active phase) | Unspecified | No1 | ||
| FX (Active phase) | Unspecified beyond "active range of motion" | No1 | ||
| Other (Active phase) | Instruction in posture and biomechanics, hot and cold modalities, and massage | No1 | ||
| Home programme (Active phase | Unspecified for all types of exercise | No1 | ||
|
Maintenance phase: · 1 support meeting/mo for 104 weeks · Maintenance phase: home programme unspecified frequency for 104 weeks · Total duration of exercise sessions: unspecified |
Home programme | Unspecified for all types of exercise | No1 | |
| Other | Support meeting | n/a | ||
| Joshi 2009 | MX (RT,FX+ relax) · Home programme of (RT+FX 2×/week, Relax 4×/week) for 26 weeks · Supervised session 1/mo for 26 weeks · Total duration of exercise sessions: at least 20' (at least 10', twice daily) |
AE | ‐‐ | n/a |
| RT | Isotonic or isometric exercises against gravity, body weights or using light weights, unspecified intensity for 3 to 4', twice daily for shoulder/shoulder girdle, trunk, and limb extensors, 1 set of 10 reps primarily at unspecified intensity for part of each session of 10' or more, twice daily | No1 | ||
| FX | Static stretches for neck, shoulders, shoulder girdles; other details unspecified for 2' to 3', twice daily | No1 | ||
| Other | Relax for 2 to 3' twice daily | n/a | ||
| Jones 2007 | MX (AE+RT+FX+Bal+Relax) · Supervised sessions 3/week for 26 weeks · Total duration of each exercise session: 60' |
AE | Low‐impact floor aerobics, light intensity (40% to 50% HRmax) or RPE 10 to 12 on 0 to 20 scaled.Duration for warm‐up + AE = 30' | No2 |
| RT | Isotonic exercises for all major muscle groups using elastic bands and free weights, unspecified intensity for 10' | No1 | ||
| FX | Unspecified static and non‐ballistic stretches for all major muscle groups, unspecified set reps and intensity, for 5' | No2 | ||
| Other | Balance and relaxation for 15' | n/a | ||
| MX only vs ˜MX Ex | ||||
| (a) MX only vs AE only | ||||
| Sanudo 2010b | MX (AE+RT+FX) · Supervised sessions 2/week for 24 weeks · Total duration of each exercise session: 45' to 60' |
AE | Walking, jogging, moderate intensity (65% to 70% HRmax) for 10 to 15' | No2 |
| RT | Unspecified isotonic exercises with free weights for 8 muscle groups, 1 set of 8 to 10 reps with 1 to 3 kg for 15 to 20' | Y | ||
| FX | Static stretches for 8 to 9 muscle groups of upper, lower limbs and trunk, 1 set of 3 reps with 30" holds, intensity unspecified for unspecified time | Y | ||
| Other | ||||
| van Santen 2002b | MX (AE+RT+FX) · Supervised sessions 2/week for 20 weeks · Unsupervised sessions 1/week for 20 weeks · Total duration of each exercise session: 60' |
AE | Unspecified mode at participant‐selected intensity alternating with balance and flexibility exercises for 30' | No2 |
| RT | Isometric exercises, unspecified muscle groups and intensity for 10' | No1 | ||
| FX | Unspecified 'general' flexibility exercises during AE (AE+balance+FX=30') | No1 | ||
| Other | Balance exercises during AE | n/a | ||
| (b) MX only vs Other Ex | ||||
| Demir‐Gocmen 2013 | MX (FX+Balance‐Co‐ordination) · Supervised sessions 3/week for 12 weeks · Total duration of each exercise session: 60' |
AE | ‐‐ | n/a |
| RT | ‐‐ | n/a | ||
| FX | Unspecified stretches and muscle groups, 1 set of 10 reps per exercise at intensity as tolerated for 15' | Y | ||
| Other | Balance‐co‐ordination on 1 and 2 feet, without and with a partner for 25' | n/a | ||
| Genc 2002 | MX (RT+FX+Posture) · Unsupervised sessions 3/week for 3 weeks · Total duration of each exercise session: unspecified |
AE | ‐‐ | n/a |
| RT | Unspecified mode and intensity for cervical, thoracic, lumbar muscle RT for unspecified time | No1 | ||
| FX | Unspecified flexibility exercises | No1 | ||
| Other | Moist heat and postural awareness education | n/a | ||
| Yuruk 2008 | MX (RT+FX) · Home programme 3/week for 8 weeks · Phone calls 1/week for 8 weeks · Total duration of each exercise session: 30' |
AE | ‐‐ | n/a |
| RT | Isometric exercises for neck, isotonic for shoulder girdle and shoulders, unspecified intensity, RT+FX=20' | No1 | ||
| FX | Unspecified mode for neck, upper back, shoulders at unspecified sets, reps, intensity, RT+FX=20' | No1 | ||
| Other | Posture exercises | n/a | ||
| (a) MX only (1) vs MX only (2) | ||||
| Rooks 2007 | MX (AE+FX) · Supervised sessions 2/week for 16 weeks · Home programme 1/week for 16 weeks · Total duration of each exercise session: 60' |
AE | Treadmill walking at participant‐determined moderate effort, progressed from 5' to 45' | No2 |
| RT | ‐‐ | n/a | ||
| FX | Unspecified stretches for primary body movements unspecified reps, sets, and intensity for unspecified time | No1 | ||
| Other | ‐‐ | |||
| MX (RT+AE+FX) · Supervised sessions 2/week for 16 weeks · Home programme 1/week for 16 weeks · Total duration of each exercise session: 60' |
AE | Treadmill walking at participant‐determined moderate effort, progressed from 5' to 20' | No1 | |
| RT | Isotonic exercises using machines and hand weights for upper and lower limbs and trunk, 1 set 'easy' progressed to 2 sets of 10 to 12 at unspecified RM for 25' | No1 | ||
| FX | Unspecified stretches for primary body movements unspecified reps, sets, and intensity for unspecified time | No1 | ||
| Other | ||||
| Yuruk 2008 | MX (RT+FX) · Home programme 3/week for 8 weeks · Phone calls 1/week for 8 weeks · Total duration of each exercise session: 30' |
AE | ‐‐ | n/a |
| RT | Isometric exercises for neck, isotonic for shoulder girdle and shoulders, unspecified intensity, RT+FX=20' | No1 | ||
| FX | Unspecified mode for neck, upper back, shoulders at unspecified sets, reps, intensity, RT+FX=20' | No1 | ||
| Other | Posture exercises | n/a | ||
| Giannotti 2014 | MX (AE+RT+FX+Ther ex)+ED · Phase 1: supervised sessions 2/week for 10 weeks · Phase 1: home programme 3+/week for 10 weeks · Total duration of each exercise session 60' |
AE | Cycle ergometry at vigorous intensity (70% functional capacity) for 10' in sessions 10 to 20 | No2 |
| RT | Strengthening exercises, no equipment used, for spine and lower limbs, 1 set of 10 reps at unspecified intensity for 10' in sessions 8 to 20 | No2 | ||
| FX | Stretches for spine, upper and lower limbs, 2 reps held 50 to 60"/stretch, intensity unspecified. FX+Therapeutic ex=25' | Y | ||
| Other | Therapeutic ex. FX+Therapeutic ex=25'. Education sessions 1 to 7 about FM and management plus correction of ex performance for 10' in sessions 8 to 20 | n/a | ||
| Hunt 2000 | MX (AE+ST+FX)+ED · Supervised sessions: 1/week for 5 weeks · Home programme: 7/week for 5 weeks · Total duration of each exercise session: unspecified |
AE | Cycle ergometry or stepping at moderate intensity (RPE 3 to 4/10) for 15' | No2 |
| RT | 8 lower body and core callisthenics without weights and isometric exercises performed for 2'/exercise | No1 | ||
| FX | 12 static stretches for trunk, upper and lower limbs, 5 reps held for 5", intensity unspecified, for unspecified time | Y | ||
| Other | Education regarding advice on sleep, RX, pain management) | n/a | ||
| Paolucci 2015 | MX (AE+RT+ agility, balance, postural exercises)+ ED) · Phase 1: supervised sessions 2/week for 5 weeks · Phase 1: home programme unspecified times/week · Total duration of each exercise session: 60' |
AE | Walking + stair steps at light intensity (60% HRmax) for 20' | No2 |
| RT | Callisthenics for upper + lower limbs and trunk 3 sets × 10 reps for unspecified time | No1 | ||
| FX | Static stretches for upper and lower limbs and trunk, 3 reps of 30 to 60" intensity unspecified for unspecified time | No1 | ||
| Other | Agility, balance, posture, breathing exercises, education | n/a | ||
| Salaffi 2015 | MX (AE+RT+FX)+ED · Supervised sessions 2/week for 12 weeks · Total duration of each exercise session: unspecified |
AE | Participant‐preferred mode at light to moderate intensity (60% to 70% HRmax) progressed to moderate to vigorous intensity (75% to 85% HRmax) for 60 to 120'/week | No2 |
| RT | Prescribed on individual basis. Isotonic exercises with weights for upper and lower limbs, 1 set × 10 reps with 1 to 3 kg for upper limbs, 3 to 5 kg for lower limbs, progression encouraged, 1 kg/week for unspecified time | No1 | ||
| FX | Stretches prescribed on basis of individual need; no further details provided | Y | ||
| Other | Education | n/a | ||
ACSM: American College of Sport Medicine; AE: aerobic; AQ: aquatic exercise; Bal: balance; ED: education; Ex: exercise; FX: flexibility; HRmax: heart rate maximum; Land: land exercise; MX: mixed exercise; n/a: not applicable; reps: repetitions; Relax: relaxation; RM: repetition maximum; RPE: rating of perceived rating scale; RT: resistance; Therap ex: therapeutic exercise; Y: yes.
a Total duration of each exercise session includes warm‐up+cool‐down= all AE, RT, FX.
bAE intensity is usually expressed as a descriptor (such as moderate) followed by the physiological equivalent (such as % HRmax). RT intensity is usually expressed as the numbers of reps and sets at a specific RM.
c When no details about any component of the FX were available to reviewers, we entered "Unspecified" without listing all unspecified components in this table.
d Authors use two conflicting descriptors of AE intensity: 40% to 50% HRmax = low intensity, and RPE of 10 to 12/20 = moderate intensity.
Y = yes.
1not enough information to evaluate congruence with ACSM guidelines.
2frequency, duration, and/or intensity did not meet ACSM guidelines.
ACSM congruence
Studies that met ACSM criteria for development and maintenance of fitness in apparently healthy adults in terms of intensity, frequency, and duration were as follows (Garber 2011).
Aerobic: one study (Valkeinen 2008).
Resistance (strength): two studies (Sanudo 2010b; Sanudo 2012).
Flexibility: nine studies (Alentorn‐Geli 2008; Garcia‐Martinez 2011; Giannotti 2014; Hunt 2000; Salaffi 2015; Sanudo 2010b; Sanudo 2011; Sanudo 2012; Sanudo 2013).
Most of the programmes that did not meet the guidelines had actually failed to provide enough information about their interventions for review authors to judge. Specific to aerobic training, other studies fell short of the 150 minutes per week of moderate‐intensity exercise on five or more days per week that is recommended. Two or three days per week of supervised participation in exercise studies is commonly found in exercise studies. To achieve congruence with ACSM guidelines, moderate exercise must be performed for at least 150 minutes on five or more days per week. This means that a home and/or unsupervised component performed on two or more days per week is essential to meet the guidelines. Although some researchers stated that participants were encouraged to perform exercise at home, information about such home programmes was insufficient to determine whether these criteria had been achieved.
Mixed exercise vs control
There were 21 studies comparing mixed exercise versus a control programme. Of 21 studies included in the main comparison (mixed exercise vs control), average length of treatment was 14 weeks (median 12 weeks, range 4 to 26 weeks), and eight studies had one or more post‐intervention follow‐ups: two studies from 6 to 12 weeks (Buckelew 1998; Paolucci 2015), four studies from 13 to 26 weeks (Baptista 2012; Da Costa 2005; Giannotti 2014; Sanudo 2012), four studies from 27 to 52 weeks (Buckelew 1998; Clarke‐Jenssen 2014; Da Costa 2005; van Eijk‐Hustings 2013), and one study for longer than 52 weeks post intervention (Buckelew 1998). Six studies had an education component as part of the intervention (Burckhardt 1994; Clarke‐Jenssen 2014; Giannotti 2014; Hunt 2000; Paolucci 2015; Salaffi 2015). One study compared three groups: an exercise intervention carried out in a cold climate, an identical exercise carried out in a warm climate, and a control (the two exercise groups were aggregated and presented as one) (Clarke‐Jenssen 2014). All studies included supervised group sessions (median 2 per week) and home exercise programmes. Specifics of the home session(s) were left to the participants.
Frequency ‐ the number of sessions per week varied between 1 and 7 (mean 3.1). Most studies included regular supervised sessions (median 2 per week); however Da Costa 2005 and Hunt 2000 were primarily home exercise programmes that included supervised sessions to enhance participant exercise performance.
Intensity ‐ intensity for aerobic exercise ranged from 40% to 50% HRmax in Jones 2007 up to 85% HRmax in Alentorn‐Geli 2008. For resistance exercise, intensity generally was not noted other than selected by participants.
Time (duration) ‐ fifteen studies required participants to do sessions of 45 to 60 minutes of mixed exercise; exercise sessions were 115 minutes in Clarke‐Jenssen 2014, 30 to 90 minutes in Valkeinen 2008, and 60 to 180 minutes in Buckelew 1998. In three studies, the length of exercise sessions was unspecified or unclear.
Type ‐ sixteen programmes used a combination of aerobic, resistance, and flexibility exercise; two combined aerobic and resistance exercise (Valkeinen 2008; van Eijk‐Hustings 2013); two combined aerobic and flexibility exercise (Alentorn‐Geli 2008; Burckhardt 1994); and one used belly dance (Baptista 2012), which was classified as a combination of the three types of exercise. Five included other forms of exercise such as agility or co‐ordination or balance or therapeutic exercises (Giannotti 2014; Jones 2007; Paolucci 2015; van Santen 2002a; Verstappen 1997), and three included relaxation (Alentorn‐Geli 2008; Clarke‐Jenssen 2014; Jones 2007). Most studies included a warm‐up and a warm‐down. Three exercise interventions were carried out in part in water (Burckhardt 1994; Clarke‐Jenssen 2014; Da Costa 2005). Two interventions were primarily home exercise programmes (Da Costa 2005; Hunt 2000).
Mode ‐ the mode of aerobic exercise varied from walking to jogging, occasionally with upper body movement, with some studies using a stationary bike or treadmill. Of the 19 studies that included resistance training, isotonic and isometric types of muscle strengthening were used. Seven used free weights (n = 6) or specialised equipment (Nautilus, n = 1). Two studies combined free weights with callisthenics (Da Costa 2005), or with elastic bands (Jones 2007). One study used isotonic exercise with unspecified equipment (Valkeinen 2008), and one used isotonic exercise with unspecified equipment plus isometric exercise (Etnier 2009). One study used isometric strengthening exercises only (van Santen 2002a), one used callisthenics only (Paolucci 2015), and one combined callisthenics with isometric exercise (Hunt 2000). One study used belly dance (that we estimate included isometric and isotonic muscle modes of muscle strengthening) (Baptista 2012). Two studies did not specify the resistance training mode used in the interventions (Buckelew 1998; Clarke‐Jenssen 2014). Two studies did not specify the exercise mode for any type of exercise used in the interventions (Garcia‐Martinez 2011;Giannotti 2014). Details regarding the exercise interventions are provided in Table 5.
The control group received usual care or treatment as usual, delayed treatment.
Mixed exercise only vs other exercise
We found a series of studies comparing mixed exercise only versus other types of exercise programmes. Rooks 2007 compared mixed exercise to mixed exercise. Mixed exercise was compared to aerobic exercise (Sanudo 2010b; van Santen 2002b), to remedial exercise, to relaxation and mobilisations (Genc 2002), to a home programme of flexibility training (Demir‐Gocmen 2013), and to resistance training (Yuruk 2008). Details regarding the interventions are provided in Characteristics of included studies and in Table 5.
Mixed exercise vs other intervention
We found a series of studies comparing mixed exercise to a variety of other interventions. One study compared mixed exercise plus education to education only (Burckhardt 1994). Another study compared mixed exercise to relaxation (Martin 1996), and a third study compared mixed exercise to cognitive‐behavioural training (Rivera Redondo 2004). Two studies compared mixed exercise to biofeedback (Buckelew 1998; van Santen 2002a), and two others compared mixed exercise to medications (amitriptyline – Joshi 2009; pyridostigmine – Jones 2007). One study compared mixed exercise to a fibromyalgia self‐help programme (Rooks 2007). Details regarding the interventions are provided in Characteristics of included studies and in Table 5.
Excluded studies
We excluded 3477 records on citation and abstract screening, as they did not meet the inclusion criteria for this review (see Figure 1). We examined 285 full‐text articles and excluded 91 full‐text articles, three theses, and five trial registry records. We excluded full‐text articles and trial registry records because they did not meet the selection criteria related to the following: not an RCT/randomisation (n = 61), diagnosis (n = 6), intervention (n = 24), outcomes not measured (n = 2), no between‐group data (n = 3), full‐text unavailable (n = 1), and data for fibromyalgia not isolated (n = 2). The remaining 186 full‐text articles and trial registry records represented RCTs examining the effects of physical activity interventions for fibromyalgia. A further 131 articles were screened out because (1) the physical activity intervention did not meet the inclusion criteria for this review intervention, or (2) the study was reviewed or was designated to be reviewed in another Cochrane Review in this series (see Figure 1, Table 7, and Excluded studies).
6. Physical activity studies ruled out.
| RCT | Numbers of Groups and Interventions | Review |
| Altan 2004 | 2 groups: AQ‐MX (AE+FX+Relax), Bal | AQ |
| Altan 2009 | 2 groups: MX (RT+FX) [Pilates], Relax+FX | FX |
| Amanollahi 2013 | 3 groups: FX, Friction massage | FX |
| Arcos‐Carmona 2011 | 2 groups: AQ+Land MX (AE+Relax in land), Control (placebo magnet therapy) | AQ |
| Assis 2006 | 2 groups: AE, AQ‐AE | AQ |
| Astin 2003 | 2 groups: Mindfulness Meditation, | CAMS |
| Baptista 2012 | 2 groups: MX (AE‐FX ‐ Belly Dance), Wait list control | MX |
| Bircan 2008 | 2 groups: AE, RT | RT |
| Bjersing 2012 | 2 groups: Nordic walking, AE | AE |
| Bojner 2006 | 2 groups: Dance/Movement, Control | Dance |
| Bressan 2008 | 2 groups: FX, AE | FX |
| Calandre 2009 | 2 groups: FX, AiChi | AQ |
| Carson 2010, Carson 2012 | 2 groups: COMP (Yoga, med'n, breathing ex, ED), Wait list control | CAMS |
| Castel 2013 | 2 groups: Comp [(Land+AQ MX (AE+ST+FX+Co‐ord))+CBT], Conventional pharmacological | CAMS |
| Cedraschi 2004 | 2 groups: Comp (AQ+Land AE, Relax, ED), Control | Comp |
| De Andrade 2008 | 2 groups: AQ‐(AE), AQ‐(AE) SPA | AQ |
| de Araujo 2013 | 2 groups: AE, RT | RT |
| de Melo Vitorino 2006 | 2 groups: AQ‐MX (RT+AE+Relax), MX (AE+Relax) | AQ |
| Evcik 2008 | 2 groups: AQ ‐MX (FX+AE+Relax), MX (AE‐ST‐FX‐Relax) | AQ |
| Field 2003 | 2 groups: COMP (Self‐Massage+FX), Relax | FX |
| Fontaine 2007 | 2 groups: LPA (likely mostly aerobic), ED | AE |
| Fontaine 2010, Fontaine 2011 | 2 groups: LPA (likely mostly aerobic), ED (Fibro education‐non‐ex group) | AE |
| Gavi 2014 | 2 groups: FX, RT | FX |
| Genc 2015 | 2 groups: FX, AE | FX |
| Gomes da Silva 2008 | 2 groups: AQ (AE+FX), TENS | AQ |
| Gusi 2010, Olivares 2011, Adsuar 2012 | 2 groups: WBV, Control TAU | WBV |
| Gusi 2006, Tomas‐Carus 2007a_8214, Tomas‐Carus 2007b_8215, Tomas‐Carus 2007c_8212 | 2 groups: AQ‐MX (AE+RT), Control | AQ |
| Hakkinen 2001, Hakkinen 2002 | 3 groups: RT (Fibromyalgia),RT (Healthy), Control (Fibromyalgia) | RT |
| Hammond 2006 | 2 groups: COMP [ED+SMP+MX(AE+Tai Chi 15'+ST+FX)], Relax | COMP |
| Hecker 2011 | 2 groups: AQ MX (FX, AE, ROM), MX (FX, AE, ROM) | AQ |
| Hooten 2012 | 2 groups: COMP [MX(RT+FX)+pain prg], COMP [MX(AE+FX)+pain prg] | COMP |
| Ide 2008 | 2 groups: AQ‐COMP (AE+Relax), Control (Supervised ˜PA Recreational Activities) | AQ |
| Isomeri 1993 | 3 groups: AE, RT+ meds, AE + meds | AE |
| Jentoft 2001 | 2 groups: AQ‐(AE+RT+FX), MX (AE+RT+FX) | AQ |
| Jones 2002 | 2 groups: ST, FX | RT |
| Jones 2012 | 2 groups: TaiChi, ED | CAMS |
| Kayo 2011 | 3 groups: AE, RT, Control | RT/AE |
| Keel 1998 | 2 groups: Comp (MX (AE+FX)+ED, Relax, group discussion), Relax (they called Control) | COMP |
| King 2002 | 4 groups: AE (AQ +/or Land), ED, Comp AE (AQ +/or Land)+ED, Control | AE |
| Larsson 2015 | 2 groups: RT, Relax | RT |
| Lemstra 2005 | 2 groups: Comp (MX (AE+FX+RT)+ED+SM+ SMP+Massage), Control | COMP |
| Liu 2012 | 2 groups: Qi Gong, Sham Qi Gong | CAMS |
| Lopez‐Rodriguez 2012 | 2 groups: AQ‐AE+Dance, FX | FX |
| Lynch 2012 | 2 groups: Qi Gong, Wait list control | CAMS |
| Mannerkorpi 2000 | 2 groups: AQ‐MX (AE+FX), ED | AQ |
| Mannerkorpi 2009 | 2 groups: COMP AQ MX (FX, AE, Co‐ord)+ED, ED | AQ |
| Mannerkorpi 2010 | 2 groups: AE (moderate intensity), AE (low intensity) | AE |
| Martin 2014 | 2 groups: Comp MX (AE+RT+FX)+CBT+ED, Control | COMP |
| Martin‐Nogueras 2012 | 2 groups: Comp MX (RT+FX+Relax)+PT with modalities, Control | COMP |
| Matsutani 2007 | 2 groups: COMP (ED+Laser+FX) COMP (ED+FX) | COMP |
| Matsutani 2012 | 2 groups: AE, FX | FX |
| McCain 1988 | 2 groups: AE, FX | FX |
| Mengshoel 1992, Mengshoel 1993 | 2 groups: AE‐Dance, Control | AE |
| Munguia‐Izquierdo 2007; Munguia‐Izquierdo 2008 | 2 groups: AQ‐MX (ST+AE), Control (FM), Control (Healthy) | AQ |
| Nichols 1994 | 2 groups: AE, Control | AE |
| Palekar 2014 | 3 groups: Pilates, Yoga | Other |
| Ramsay 2000 | 2 groups: AE, AE (CV) | AE |
| Richards 2002 | 2 groups: AE, Comp Relax+FX | FX |
| Sanudo Corrales 2010c | 2 groups: AE, Control | AE |
| Sanudo 2010c 8410 | 2 groups: AE, Control | AE |
| Sanudo 2015 | 2 groups: AE, Control | AE |
| Schachter 2003 | 3 groups: AE‐long bout, AE‐short bout, Control (TAU) | AE |
| Schmidt 2011 | 3 groups: Comp (Meditation+Yoga), Comp (Relax+FX), Control (Wait list) | CAMS |
| Sencan 2004 | 3 groups: AE, Meds, Control | AE |
| Tomas Carus 2008, Tomas Carus 2007d_8216, Gusi 2008 | 2 groups: AQ ‐ MX (RT+AE), Control | AQ |
| Valencia 2009 | 2 groups: COMP [Relax+MX(AE+FX)], FX (Meziere method) | FX |
| Valim 2003 | 2 groups: AE, FX | FX |
| Valkeinen 2004, Valkeinen 2005 | 3 groups: ST Fibromyalgia, ST Healthy, Control (Fibromyalgia) | RT |
| van Koulil 2010 | 2 groups: Comp CBT1+AQ/Land (AE+RT+FX+Hydro), Comp CBT2+AQ/Land (AE+RT+FX+Hydro) | COMP |
| Wang 2010 | 2 groups: Tai Chi, Comp (FX+ED) | CAMS |
| Wigers 1996 | 3 groups: AE, SMT, Control (TAU) | AE |
AE: aerobic exercise:, AE‐FX: aerobic flexibility; AQ: aquatic exercise; AQ‐AE: aquatic aerobic exercise; AQ‐MX: aquatic mixed exercise; CAMS: complementary and alternative; CBT: cognitive behavioural therapy; COMP: composite intervention/review; Co‐ord: coordination; CV: cardiovascular; ED: education; ex: exercise; FM: fibromyalgia; FX: Flexibility; Hydro: hydrotherapy; Land: exercise performed in land; LPA: leisure physical activity; Med'n: meditation; meds: medications; MX: mixed exercise; PA: physical activity; PT: physical therapy; Relax: relaxation; ROM: range of motion; RT: resistance exercise; SMP/T: Self‐management program/treatment; TAU: treatment as usual; TENS: transcutaneous electrical nerve stimulation; WBV: whole body vibration;
Risk of bias in included studies
The most frequently identified biases across studies were inadequate blinding, selective reporting, and allocation concealment. Results of the 'Risk of bias' assessment for the 29 studies are provided in the Risk of bias in included studies table and in Figure 2 and Figure 3. The 'Risk of bias' assessments were based on primary article data and published or registered protocols when available, and were supplemented by responses from authors.
2.
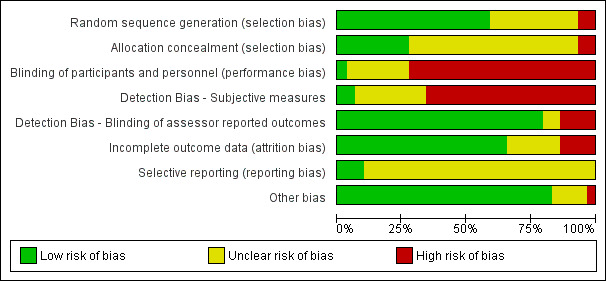
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.
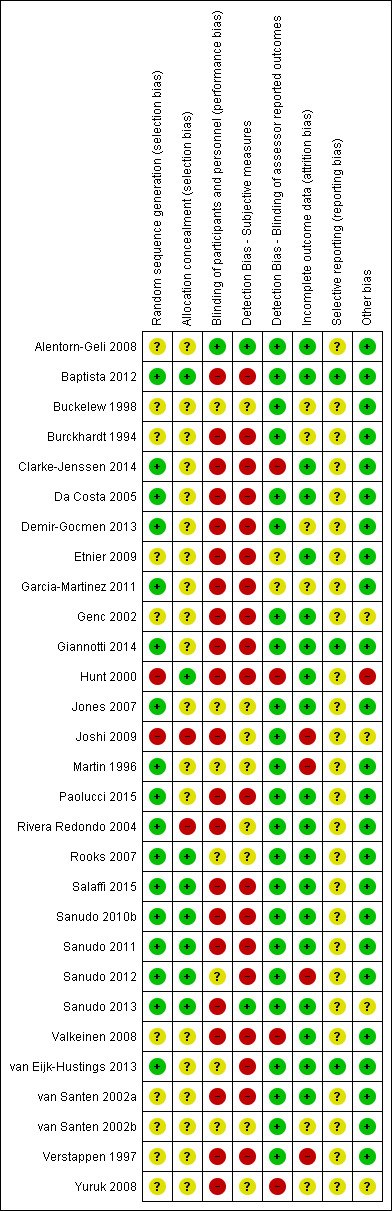
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Mixed versus control
Of the 21 studies comparing mixed exercise to control, 13 used an acceptable method of random sequence generation (computer‐generated sequence, coin toss, drawing of cards or lots), and we rated them as low risk. For seven studies, we rated random sequence generation as unclear risk. We rated only one study as high risk (Hunt 2000).
With regards to allocation methods, of the 21 studies included in this comparison, eight utilised acceptable methods such as central allocation using telephone, web‐based, or pharmacy‐controlled randomisation; or sequentially numbered opaque, sealed envelopes. We rated them as low risk. For 12 studies, we rated risk of bias as unclear, as they did not present sufficient information to allow definitive judgement. One study did not use acceptable allocation methods, we rated it as high risk (Paolucci 2015).
Mixed versus other exercise or non‐exercise interventions
Overall, we rated only five studies as low risk for both sequence generation and allocation methods. Among the eight studies in this comparison group, we rated four as low risk as they utilised an acceptable method of random sequence generation. Three studies were unclear in their method of random sequence generation (Genc 2002; van Santen 2002b; Yuruk 2008), and Joshi 2009 was the only study rated as high risk. For allocation concealment in this comparison, we rated one study as low risk (Rooks 2007), five studies as unclear risk, and two studies as high risk (Joshi 2009; Rivera Redondo 2004).
Blinding
We divided the blinding domain into blinding of participants and personnel (performance bias) and blinding of outcome assessors (subjective and assessor‐reported outcomes) (detection bias). For exercise studies, blinding of participants and care providers from treatment allocation is very rare.
Perfomance bias
Mixed versus control
Among the 21 studies included in this comparison, we rated blinding of participants and personnel as low risk in one study (Alentorn‐Geli 2008), unclear risk in four studies (Buckelew 1998; Jones 2007; Sanudo 2012; van Eijk‐Hustings 2013), and high risk in 16 studies.
Mixed versus other interventions
Of the eight studies comparing mixed versus other interventions, we rated three as unclear risk (Martin 1996; Rooks 2007; van Santen 2002b), and we rated the remaining five studies as high risk.
Detection bias – subjective outcome
Mixed versus control
Of the 21 studies in this comparison, we deemed two studies to have low risk (Alentorn‐Geli 2008; Sanudo 2013) (Sanudo did not have a subjective outcome, but RevMan software does not present this option). We considered 17 studies to have high risk of bias, and two studies to have unclear risk (Buckelew 1998; Jones 2007).
Mixed versus other exercise or non‐exercise interventions
Among the eight studies in this comparison group, we rated six as unclear risk and two as high risk (Demir‐Gocmen 2013; Genc 2002).
Detection bias – assessor‐reported outcome
Mixed versus control
With regards to blinding of assessor‐reported outcomes, of the 21 studies included in this comparison, ten studies used assessor‐reported tests (e.g. cardiorespiratory submaximal function, muscle strength measurement). Seven were rated as low risk (i.e. outcome assessor was blinded to group assignment). Risk of detection bias was high in three studies (i.e. assessor was not blinded) (Clarke‐Jenssen 2014; Hunt 2000; Valkeinen 2008), and risk was unclear in two studies (Etnier 2009; Garcia‐Martinez 2011).
The remaining nine studies did not use an assessor‐reported test and were classified as 'low risk' (i.e. not applicable for detection bias). (Note: number is 19 because one study ‐ Clarke‐Jenssen 2014 ‐ is used twice.)
Mixed versus other exercise or non‐exercise interventions
Among the eight studies in this comparison, we rated one study as low risk (Rooks 2007), one as high risk (Yuruk 2008), and six as low risk ('not applicable') for detection bias.
Incomplete outcome data
Mixed versus control
Sixteen of the 21 studies included in this comparison reported complete outcome data and were rated as low risk. Seven studies analysed data using ITT analysis. Missing outcome data were balanced in numbers across intervention groups, with similar reasons for missing data across groups in Jones 2007 and Salaffi 2015. Missing outcome data were balanced in numbers across intervention groups, and reasons for missing outcome data were unlikely to be related to true outcomes in Alentorn‐Geli 2008,Giannotti 2014,Paolucci 2015, and Valkeinen 2008. There were no missing data at post‐test in Clarke‐Jenssen 2014 and Hunt 2000. We rated three studies that did not present sufficient information to allow definitive judgement as unclear risk (Buckelew 1998; Burckhardt 1994; Garcia‐Martinez 2011). Sanudo 2012 and Verstappen 1997 had incomplete outcome data and were rated as high risk. (Note: Clarke‐Jenssen 2014 is used twice.)
Mixed versus other exercise or non‐exercise interventions
Among the eight studies in this comparison, we rated three studies as low risk. Data were analysed using ITT analysis (Rooks 2007); missing outcome data were balanced in numbers across intervention groups in Rivera Redondo 2004 and Genc 2002. Three studies were rated as unclear risk (Demir‐Gocmen 2013; van Santen 2002b; Yuruk 2008). Joshi 2009 and Martin 1996 had incomplete outcome data and were rated as high risk.
Selective reporting
Among the 21 studies included in the main comparison, we found study protocols for three of the included studies (Baptista 2012; Giannotti 2014; van Eijk‐Hustings 2013). After comparing the protocol with the study, we rated these studies as low risk for selective reporting bias. We classified the remaining 18 studies as unclear risk. We rated all eight studies comparing mixed versus exercise or other interventions as unclear risk.
Between‐studies reporting bias: reported under Effects of interventions.
Other potential sources of bias
Overall, we rated risk due to other sources of bias as low (approximately 80%; Figure 2) in the 29 studies. We rated four studies as unclear risk because information was insufficient to assess whether an important risk of bias existed (Genc 2002; Joshi 2009; Sanudo 2013; Yuruk 2008). We did not find information on baseline inequities despite randomisation, and we did not find within‐study inequities in duration of the intervention. Poor adherence is also a potential source of bias in exercise studies.
Effects of interventions
See: Table 1
We have presented the effects of interventions per comparison (mixed vs control, mixed vs non‐exercise, and mixed vs other) and by outcome (major and minor), followed by long‐term effects, minimal clinically important differences, heterogeneity, and subgroup and sensitivity analyses. For five major outcomes, negative numbers mean improvement. We converted all scores to a common scale of 0 to 100, with higher scores corresponding to poorer health. Specific outcome measures and tools utilised by primary study authors are recorded in the Characteristics of included studies table.
Mixed exercise versus control
Major outcomes
HRQL (self‐reported, FIQ total, scale 0 to 100, higher scores corresponding to poorer health)
We meta‐analysed 13 of 15 studies that evaluated HRQL (median duration 12 weeks, range 5 to 26 weeks). Nine studies had mixed exercise only interventions, and four studies had mixed exercise plus education interventions. Two studies included an aquatic component (Burckhardt 1994; Da Costa 2005).
Due to statistical heterogeneity (I² = 67%) and clinical and methodological heterogeneity, we excluded two studies from the meta‐analysis: van Santen 2002a because unlike all the other studies, it used the Sickness Impact Profile as an outcome measure, and Baptista 2012 because unlike the other studies, it provided a belly dance intervention. When these two studies were eliminated, heterogeneity remained in the ambiguous zone (I² = 51%). Although removal of Etnier 2009 would have further lowered statistical heterogeneity (I² = 29%), we could find no rationale based on review of clinical features of the study to eliminate it from the meta‐analysis. All 13 studies included in the meta‐analysis used the FIQ total as the outcome measure. Pooled mean post‐test scores for HRQL were 56 and 49 in the control and exercise groups, respectively. The mean improved by 6.95 FIQ units in the intervention group (mean difference (MD) ‐6.95, 95% confidence interval (CI) ‐10.51 to ‐3.38; 13 studies; 610 participants; Analysis 1.1; absolute difference 7%, 95% CI 3% to 11%; relative difference 12%, 95% CI 6% to 18%). Moderate‐quality evidence shows that mixed exercise probably improves HRQL for individuals with fibromyalgia.
1.1. Analysis.
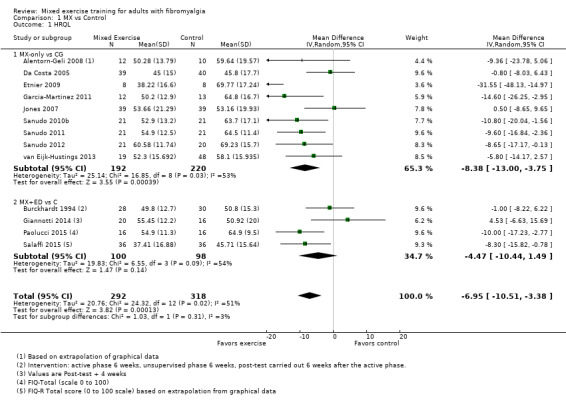
Comparison 1 MX vs Control, Outcome 1 HRQL.
Seven studies provided information on long‐term effects. Analysis of long‐term effects of HRQL showed maintenance of mixed exercise effects at 6 to 12 weeks (MD ‐10.5, 95% CI ‐17.48 to ‐3.52; 1 study; 32 participants) and at 13 to 26 weeks (MD ‐8.44, 95% CI ‐15.22 to ‐1.66; 4 studies; 224 participants) but not at 27 to 52 weeks (MD ‐5.29, 95% CI ‐11.42 to 0.84; 2 studies; 146 participants; Analysis 2.1). Very low‐quality evidence suggests that it is uncertain whether mixed exercises improve HRQL in the long term (see Table 8).
2.1. Analysis.
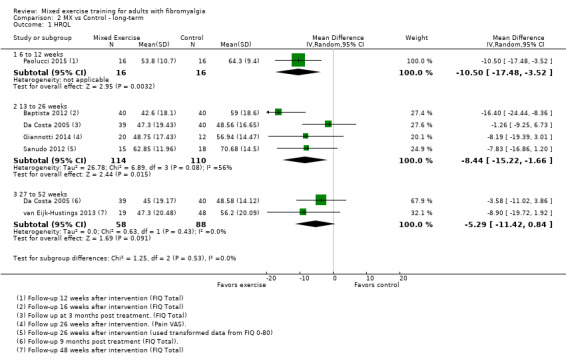
Comparison 2 MX vs Control ‐ long‐term, Outcome 1 HRQL.
7. Quality of evidence ‐ GRADE assessment for long‐term effects of MX vs Control.
| Quality assessment | № of participants | Quality | Importance | |||||||
| № of studies | Study design | Risk of bias | Inconsistency | Indirectness | Imprecision | Other considerations | Mixed exercise | Control | ||
| HRQL ‐ 6 to 12 weeks | ||||||||||
| 1 | randomised trials | very seriousa | not serious | not serious | seriousb | Single study | 16 | 16 | ⊕⊝⊝⊝ very low | CRITICAL |
| HRQL ‐ 13 to 26 weeks | ||||||||||
| 4 | randomised trials | very seriousc | seriousd | not serious | seriousb | I²: 56% | 114 | 110 | ⊕⊝⊝⊝ very low | CRITICAL |
| HRQL ‐ 27 to 52 weeks | ||||||||||
| 2 | randomised trials | seriousa | not serious | not serious | seriousb | 58 | 88 | ⊕⊝⊝⊝ very low | CRITICAL | |
| Pain ‐ 6 to 12 weeks | ||||||||||
| 1 | randomised trials | very seriouse | not serious | not serious | seriousb | Single study | 26 | 27 | ⊕⊝⊝⊝ very low | CRITICAL |
| Pain ‐ 13 to 26 weeks | ||||||||||
| 2 | randomised trials | very seriouse | not serious | not serious | seriousb | 59 | 52 | ⊕⊝⊝⊝ very low | CRITICAL | |
| Pain ‐ 27 to 52 weeks | ||||||||||
| 5 | randomised trials | very seriousc | seriousd | not serious | seriousb | I²: 84% | 209 | 199 | ⊕⊝⊝⊝ very low ‐ | CRITICAL |
| Pain ‐ > 52 weeks | ||||||||||
| 1 | randomised trials | very seriouse | not serious | not serious | seriousb | Single study | 26 | 27 | ⊕⊝⊝⊝ very low | CRITICAL |
| Fatigue ‐ 13 to 26 weeks | ||||||||||
| 2 | randomised trials | seriousf | not serious | not serious | seriousb | 60 | 52 | ⊕⊝⊝⊝ very low | IMPORTANT | |
| Fatigue ‐ 27 to 52 weeks | ||||||||||
| 1 | randomised trials | seriousg | not serious | not serious | very seriousb | Single study | 19 | 48 | ⊕⊝⊝⊝ very low ‐ | IMPORTANT |
| Stiffness ‐ 13 to 26 weeks | ||||||||||
| 1 | randomised trials | seriousf | not serious | not serious | very seriousb | Single study | 20 | 12 | ⊕⊝⊝⊝ very low | IMPORTANT |
| Stiffness ‐ 27 to 52 weeks | ||||||||||
| 1 | randomised trials | seriousf | not serious | not serious | very seriousb | Single study | 19 | 48 | ⊕⊝⊝⊝ very low | IMPORTANT |
| Physical Function ‐ 6 to 12 weeks | ||||||||||
| 1 | randomised trials | very seriouse | not serious | not serious | seriousb | Single study | 26 | 27 | ⊕⊝⊝⊝ very low | IMPORTANT |
| Physical Function ‐ 13 to 26 weeks | ||||||||||
| 3 | randomised trials | seriousf | seriousd | not serious | very seriousb | I²: 63% | 79 | 100 | ⊕⊝⊝⊝ very low | IMPORTANT |
| Physical Function ‐ 27 to 52 weeks | ||||||||||
| 1 | randomised trials | very seriouse | not serious | not serious | seriousb | Single study | 26 | 27 | ⊕⊝⊝⊝ very low | IMPORTANT |
| Physical Function ‐ > 52 weeks | ||||||||||
| 1 | randomised trials | very seriouse | not serious | not serious | seriousb | Single study | 26 | 27 | ⊕⊝⊝⊝ very low | IMPORTANT |
| All cause withdrawal and Adverse events not reported | ||||||||||
HRQL: health‐related quality of life.
aHigh or unclear risk of bias related to selection, performance, and selective reporting.
bSmall sample size and/or wide confidence interval.
cHigh or unclear risk of bias related to selection, performance, detection, and incomplete outcome reporting.
dModerate or substantial heterogeneity (I² = 30% to 60% may represent moderate heterogeneity or 50% to 90% may represent substantial heterogeneity).
eUnclear risk of bias related to selection (randomisation and allocation), detection, attrition, and reporting.
fUnclear or high risk of bias related to selection, performance, and detection.
gUnclear or high risk of bias related to selection, performance, detection, and reporting.
A funnel plot was generated and was somewhat asymmetrical (Figure 4), suggesting the possibility of publication bias.
4.
Funnel plot of comparison: 1 MX vs Control ‐ outcome: 1.1 HRQL.
Lack of evidence of an effect was found in the subgroup analysis for mixed exercise only versus mixed exercise plus education (Chi² = 1.03, P = 0.31).
Pain intensity (self‐reported, 0 to 100 scale, higher scores corresponding to greater pain)
We meta‐analysed 15 studies (832 participants, median duration 12 weeks, range 6 to 26 weeks). Ten studies provided mixed exercise only interventions (487 participants), and four studies provided mixed exercise plus education interventions (345 participants). Two studies included an aquatic component (Burckhardt 1994; Da Costa 2005).
Pooled mean post‐test scores were 58.6 and 53 in the control and exercise groups, respectively. Mean pain intensity at post‐test was 5.17 units less in the mixed exercise groups than in the control groups (MD ‐5.17, 95% CI ‐8.85 to ‐1.48; 15 studies; Analysis 1.4; absolute difference 5%, 95% CI 1% to 9%; relative difference 8.9%, 95% CI 3% to 14.8%). Moderate‐quality evidence indicates that mixed exercise probably decreases pain for individuals with fibromyalgia.
1.4. Analysis.
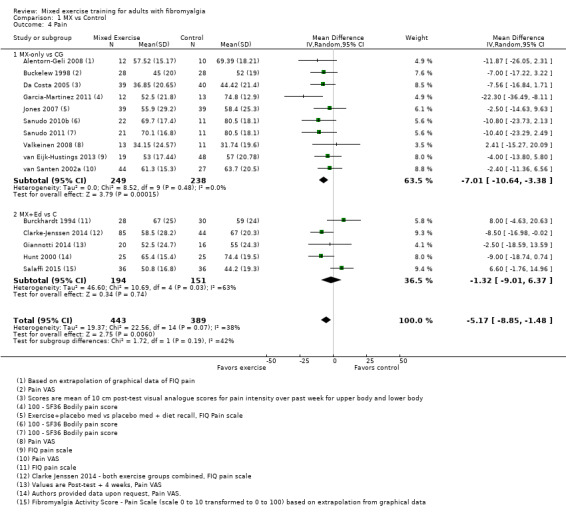
Comparison 1 MX vs Control, Outcome 4 Pain.
Analysis of long‐term effects on pain showed that effects of mixed exercise protocols were not maintained at 6 to 12 weeks (MD ‐5.00, 95% CI ‐15.50 to 5.50; 1 study; 53 participants), at 13 to 26 weeks (MD ‐4.80, 95% CI‐14.25 to 4.65; 2 studies; 111 participants), at 27 to 52 weeks (MD ‐8.33, 95% CI ‐19.03 to 2.36; 5 studies; 408 participants), and at more than 2 years (MD ‐5.00, 95% CI‐14.16 to 4.16; 1 study; 53 participants; Analysis 2.2). It is uncertain whether mixed exercise reduces pain because the quality of evidence is very low (see Table 8).
2.2. Analysis.
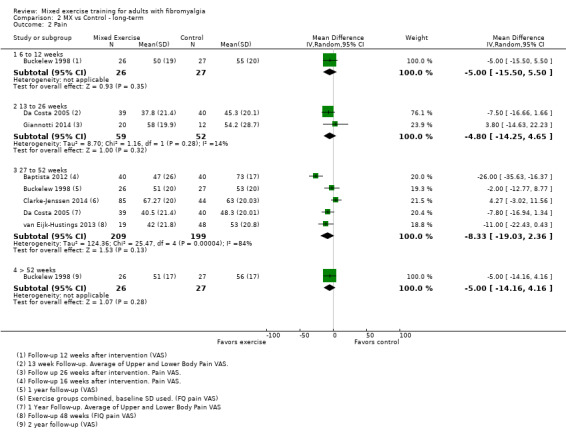
Comparison 2 MX vs Control ‐ long‐term, Outcome 2 Pain.
Due to statistical heterogeneity (I² = 72%), we explored clinical heterogeneity and excluded one study from the meta‐analysis: we excluded Baptista 2012 because unlike the other studies, it provided a belly dance intervention. This exclusion brought statistical heterogeneity to acceptable limits (I² = 37%).
On visual inspection, the funnel plot was asymmetrical, indicating the possibility of publication bias (Figure 5).
5.
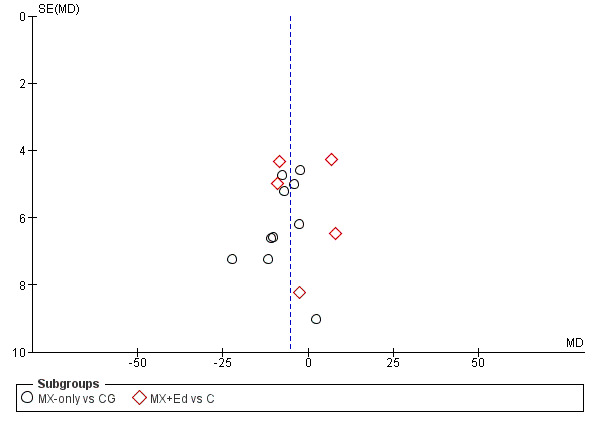
Funnel plot of comparison: 1 MX vs Control, outcome: 1.4 Pain.
We found no evidence of an effect in the subgroup analysis for mixed exercise versus mixed exercise plus education (Chi² = 1.32, P = 0.25).
Fatigue (self‐reported, 0 to 100 scale, higher scores corresponding to greater fatigue)
We meta‐analysed 11 studies (493 participants, median duration 16 weeks, range 6 to 26 weeks). Pooled mean post‐test scores were 72 and 59 in the control and exercise groups, respectively. Mean fatigue improved by 12.9 units more in the mixed exercise groups than in the control groups (MD ‐12.93, 95% CI ‐17.79 to ‐8.07; 11 studies; 493 participants; Analysis 1.7; absolute difference 13%, 95% CI 8% to 18%; relative change ‐17.7%, 95% CI ‐24.4% to ‐11.1%). Mixed exercise probably reduces fatigue (moderate‐quality evidence).
1.7. Analysis.
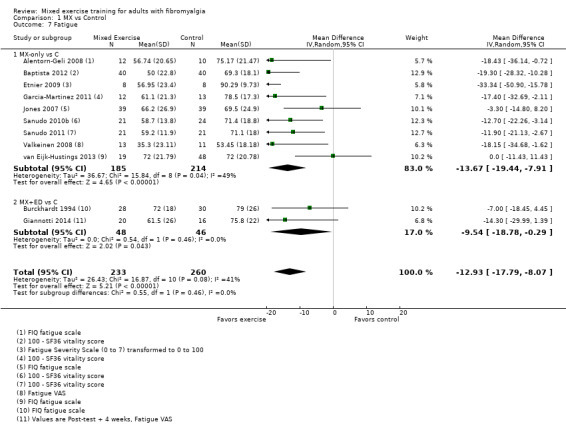
Comparison 1 MX vs Control, Outcome 7 Fatigue.
Analysis of long‐term effects on fatigue shows that effects were not maintained for mixed exercise interventions at 13 to 26 weeks (MD ‐6.48, 95% CI‐16.25 to 3.29; 2 studies; 112 participants) but an effect is seen at 27 to 52 weeks (MD ‐15.00, 95% CI ‐29.07 to ‐0.93; 1 study; 67 participants; Analysis 2.3). It is uncertain whether mixed exercises reduce fatigue in the long term because the quality of this evidence is very low (see Table 8).
2.3. Analysis.
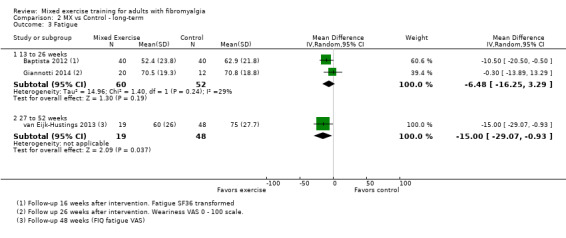
Comparison 2 MX vs Control ‐ long‐term, Outcome 3 Fatigue.
Due to statistical heterogeneity (I² = 70%), we explored clinical heterogeneity and excluded two studies from the meta‐analysis: Hunt 2000 because of inconsistencies in the data and high risk of selection, performance, and reporting bias. On evaluation, we could not identify any obvious clinical issues to explain the statistical heterogeneity in van Santen 2002a, but perhaps comparison of the difference in sample size of the two groups may have led to violated assumptions (i.e. heteroscedascity between groups). When these two studies were eliminated, heterogeneity was within acceptable limits (I² = 41%).
We found no evidence of an effect in the subgroup analysis for mixed exercise only versus mixed exercise plus education (Chi² = 0.55, df = 1, P = 0.46).
Stiffness (self‐reported, 0 to 100 mm FIQ scale, higher scores corresponding to greater stiffness)
We meta‐analysed five studies (261 participants, median duration 12 weeks, range 6 to 26 weeks) and noted no statistical heterogeneity (I² = 0%); we included all five studies in the meta‐analysis.
Pooled mean post‐test scores were 68 and 61 in the control and exercise groups, respectively. Mean stiffness improved by 6.5 units more in in the mixed exercise groups than in the control groups (MD ‐6.51, 95% CI ‐12.28 to ‐0.74; 5 studies; 261 participants; Analysis 1.8; absolute change difference 7%, 95% CI 1% to 12%; relative change 8.9%, 95% CI 0.74% to 16.8%). Based on these results, mixed exercise may slightly reduce stiffness (low‐quality evidence). Analysis of long‐term effects on stiffness shows the effect of mixed exercise interventions was not maintained at 13 to 26 weeks (MD 6.80, 95% CI ‐9.39 to 22.99; 1 study; 32 participants) nor at 27 to 52 weeks (MD ‐14.00, 95% CI ‐29.80 to 1.80; 1 study; 67 participants; Analysis 2.4). It is uncertain wether mixed exercise reduces stiffness because the quality of this evidence is very low (see Table 8).
1.8. Analysis.
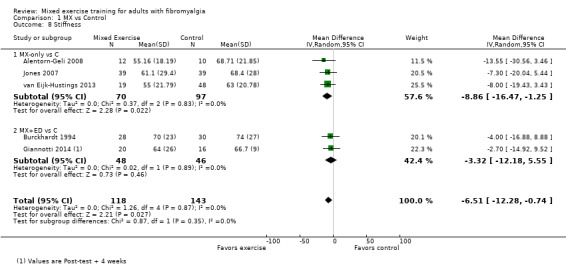
Comparison 1 MX vs Control, Outcome 8 Stiffness.
2.4. Analysis.
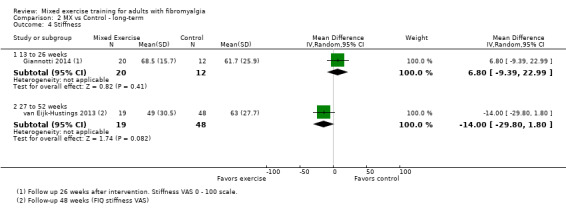
Comparison 2 MX vs Control ‐ long‐term, Outcome 4 Stiffness.
We did not identify enough studies for this outcome to evaluate publication bias.
Lack of evidence of an effect was found in the subgroup analysis for mixed exercise only versus mixed exercise plus education (Chi² = 0.87, df = 1, P = 0.35).
Physical function (self‐reported, 0 to 100 FIQ impairment scale, higher scores corresponding to greater limitation)
We meta‐analysed nine studies (477 participants, median duration 12, range 10 to 24 weeks). Seven studies had mixed exercise only interventions (141 participants), and three studies had mixed exercise plus education interventions (82 participants).
Pooled mean post‐test scores were 49 and 38 in the control and exercise groups, respectively. Mean physical function improved by 10.99 units more in the mixed exercise groups than in the control groups (MD ‐10.99, 95% CI ‐14.80 to ‐7.18; 9 studies; 477 participants; Analysis 1.9; absolute difference 11%, 95% CI 7% to 15%; relative change 22%, 95% CI ‐29.8 to ‐14.4). Thus, mixed exercises probably improve physical function (moderate‐quality evidence).
1.9. Analysis.
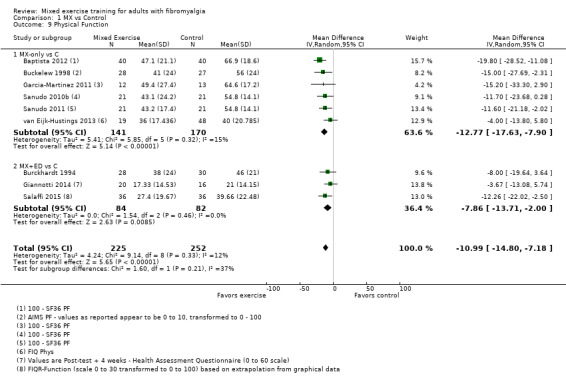
Comparison 1 MX vs Control, Outcome 9 Physical Function.
Analysis of long‐term effects showed that effects of mixed exercise interventions on physical function were maintained at 6 to 12 weeks (MD ‐18.00, 95% CI ‐31.74 to ‐4.26; 1 study; 53 participants), at 27 to 52 weeks (MD ‐20.00, 95% CI ‐31.85 to ‐8.15; 1 study; 53 participants), and longer than 52 weeks (MD ‐21.00, 95% CI ‐33.41 to ‐8.59; 1 study; 53 participants) but not at 13 to 26 weeks (MD ‐8.13, 95% CI ‐18.24 to 1.97; 3 studies; 179 participants; Analysis 2.5). It is uncertain whether mixed exercise improves physical function over the long term because the quality of this evidence is very low (see Table 8).
2.5. Analysis.
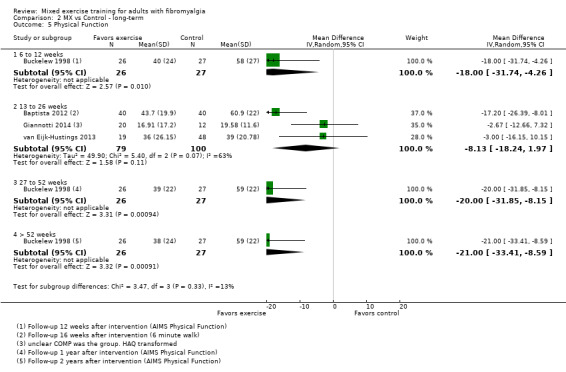
Comparison 2 MX vs Control ‐ long‐term, Outcome 5 Physical Function.
During meta‐analysis, substantial statistical heterogeneity (I² = 59%) was noted, arising chiefly from two studies (Valkeinen 2008; van Santen 2002a). We explored possible sources of clinical heterogeneity, and although there were some minor clinical differences between these two studies and the others, the most notable issue was very high variability in the data (standard deviations (SDs) exceeded mean scores in the control groups). Results for the self‐report instrument (SIP physical function) in Valkeinen 2008 seem to contradict findings in the assessor‐reported tests of physical fitness, suggesting inconsistency in the data. Effects as measured by van Santen 2002a may have been masked by pre‐test differences. When the two studies were eliminated, statistical heterogeneity decreased dramatically (I² = 12%).
There was lack of evidence of an effect in the subgroup analysis for mixed exercise only versus mixed exercise plus education (Chi² = 1.60, P = 0.21).
All‐cause withdrawal
Two studies did not clearly quantify withdrawals and were excluded from the meta‐analysis (Da Costa 2005;van Eijk‐Hustings 2013). We meta‐analysed withdrawal rates from the remaining 19 studies (1065 participants, median duration 16 weeks). Rates for the mixed exercise only training groups (n1/N1) versus the control group (n2/N2) were 0/12 versus 2/11 (Alentorn‐Geli 2008); 2/40 versus 3/40 (Baptista 2012); 2/30 versus 5/35 (Buckelew 1998); 0/8 versus 0/8 (Etnier 2009); 2/14 versus 1/14 (Garcia‐Martinez 2011); 8/47 versus 15/54 (Jones 2007); 4/21 versus 1/21 (Sanudo 2010b); 3/21 versus 1/21 (Sanudo 2011); 3/21 versus 1/20 (Sanudo 2012); 1/15 versus 5/16 (Sanudo 2013a); 2/15 versus 0/11 (Valkeinen 2008); 3/50 versus 1/29 (van Santen 2002a); and 13/58 versus 2/29 (Verstappen 1997). Rates for mixed exercise plus education groups versus control groups were 5/33 versus 5/35 (Burckhardt 1994); 10/88 versus 3/44 (Clarke‐Jenssen 2014); 1/21 versus 4/20 (Giannotti 2014); 0/25 versus 0/25 (Hunt 2000); 3/19 versus 2/18 (Paolucci 2015); and 2/38 versus 2/38 (Salaffi 2015). Reasons for participants to withdraw from studies have been footnoted in the meta‐analysis (Analysis 1.10).
1.10. Analysis.
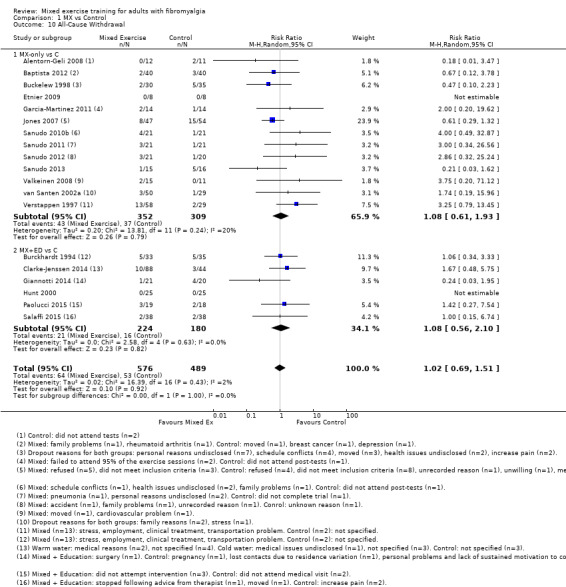
Comparison 1 MX vs Control, Outcome 10 All‐Cause Withdrawal.
There was no evidence of substantial heterogeneity among the 19 studies (I² = 20%). The pooled all‐cause withdrawal rate in the exercise groups was 64/576 as compared to 53/489 in the control groups (risk ratio (RR) 1.02, 95% CI 0.69 to 1.51; 19 studies; 1065 participants; absolute change 1% more withdrawals with exercise, 95% CI ‐3% to 5%; relative change 11%, 95% CI ‐28% to 47%; Analysis 1.10). In the subgroup analysis, withdrawal rates for mixed exercise only training groups and for mixed exercise plus education groups versus control groups were 43/352 versus 37/309 (RR 1.08, 95% CI 0.61 to 1.93; 13 studies; 661 participants; Analysis 1.10) and 21/224 versus 16/180 (RR 1.08, 95% CI 0.56 to 2.10; 5 studies; 404 participants), respectively. There were no subgroup differences in all‐cause withdrawals between mixed exercise with and without education groups (Chi² = 0.09, df = 1, P = 0.76; Analysis 1.10). Thus, mixed exercise probably leads to slightly less withdrawal (moderate‐quality evidence).
Adverse events
Reporting of adverse events (injuries, exacerbations, or other) was inconsistent in the 21 studies. Some study authors did not specify whether illness, exacerbations, or adverse events were experienced by participants (Hunt 2000; Paolucci 2015; Salaffi 2015). Two study authors stated that participants had none of these concerns (Giannotti 2014; Valkeinen 2008). We were unable to pool the data due to studies reporting variability and inconsistencies for this outcome.
Following is a summary of the data related to adverse events.
Injuries: five studies indicated there were no injuries (Alentorn‐Geli 2008; Sanudo 2010b; Sanudo 2013; Valkeinen 2008; van Eijk‐Hustings 2013); the remainder did not report on injuries.
Exacerbation of fibromyalgia symptoms: six study authors reported on the presence of exacerbations in the exercise group in narrative form (Alentorn‐Geli 2008; Etnier 2009; van Eijk‐Hustings 2013; Clarke‐Jenssen 2014), without specifying the group (Buckelew 1998), and without mentioning the control group (Salaffi 2015). For example, Etnier 2009 mentioned that flare‐up of symptoms limited participants' progression but did not provide details. Clarke‐Jenssen 2014, in describing participant absences from treatment sessions, stated, "the main reason for absence was temporarily increased pain." Salaffi 2015 did not specify exacerbations per group nor in the control group. The other study authors were not specific or did not report exacerbations.
Other adverse events:Verstappen 1997 reported that 7 of 45 (15%) individuals in the mixed exercise group experienced intolerable pain during or after exercise (compared to none in the control group; n = 27), and van Santen 2002a stated that "some individuals" in the mixed exercise group (unspecified number of individuals) had substantial post‐exercise pain. Otherwise, no adverse effects specific to mixed exercise were reported. Therefore, events were insufficient for pooling of these data.
Minor outcomes
Cardiorespiratory submax (assessor‐reported test, six‐minute walk test, units were meters, higher numbers mean improvement)
All five studies used the six‐minute walk test (median duration 18.5 weeks, range 12 to 26 weeks) (Burckhardt 1994; Clarke‐Jenssen 2014; Giannotti 2014; Sanudo 2010b; Sanudo 2012). Pooled mean post‐test scores were 477 meters and 536 meters in the control and exercise groups, respectively. Mean post‐test cardiorespiratory submax was 52.8 meters more in the mixed exercise groups than in the control groups (MD 52.77, 95% CI 34.11 to 71.43; 5 studies; 306 participants; Analysis 1.11; relative change 12.4%, 95% CI 8% to 17%).
1.11. Analysis.
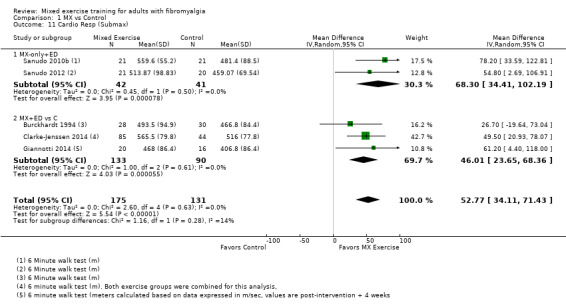
Comparison 1 MX vs Control, Outcome 11 Cardio Resp (Submax).
Because of the diversity of measures used and the differing directionality of the scales, two studies were excluded from the meta‐analysis (Etnier 2009, which used the Quarter Mile Walk Test, and Verstappen 1997, which measured heart rate at fixed workload during a cycle ergometer test). Due to statistical heterogeneity (I² = 57%), we explored clinical heterogeneity and excluded another study from the meta‐analysis: Baptista 2012 because unlike the other studies, this study provided a belly dance intervention. This exclusion brought statistical heterogeneity to acceptable limits (I² = 4%).
Of the three studies that we excluded from the meta‐analysis, Etnier 2009 found no significant difference in time to walk a quarter of a mile (MD 21.00 seconds, 95% CI ‐56.93 to 98.93; P > 0.05), and Baptista 2012 and Verstappen 1997 found significant improvements in cardiovascular submax in the exercise groups. Verstappen 1997 found an average of eight fewer heartbeats per minute to exercise at a fixed workload (MD ‐8.00, 95% CI ‐15.29 to ‐0.71; P < 0.05), and Baptista 2012 found an increase of 99.2 meters walked in six minutes (MD 99.20, 95% CI 66.09 to 132.31; P < 0.05).
Analysis of long‐term effects showed maintenance of statistically significant effects of mixed exercise intervention on distance walked in six minutes at 13 to 26 weeks (MD 61.71, 95% CI 15.37 to 108.05; 3 studies; Analysis 2.6).
2.6. Analysis.
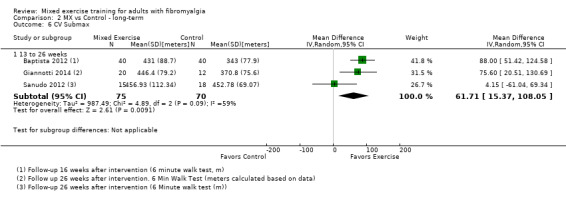
Comparison 2 MX vs Control ‐ long‐term, Outcome 6 CV Submax.
Strength (observational test, variety of measures, higher scores mean greater muscle strength)
Four studies that compared mixed exercise interventions with control interventions (163 participants, median duration 22.5 weeks, range 12 to 26 weeks) measured muscle strength as an outcome. Instruments used were MVC Quads (peak of three tries; Garcia‐Martinez 2011), grip strength (dynamometer; Sanudo 2010b), concentric leg extension (Valkeinen 2008), and static arm pull (Verstappen 1997).
Because of the diversity of strength measures used, we deemed it inappropriate to meta‐analyse the data. Two studies found no statistically significant differences (Sanudo 2010b; Valkeinen 2008), Verstappen 1997 found significant results favouring the control group, and Garcia‐Martinez 2011 found statistically significant results favouring the mixed exercise group (Analysis 1.12).
1.12. Analysis.
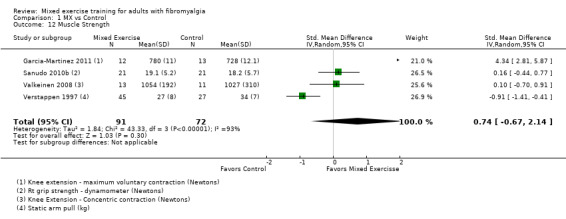
Comparison 1 MX vs Control, Outcome 12 Muscle Strength.
Improvement in pain greater than 30%
No studies measured the number of participants experiencing percentage of improvement in pain.
Subgroup analysis
Subgroup analysis of the relative effects of age (45 years or younger and over 45 years) was not carried out due to proximity of the means to the set cut‐off of 45 years. This proximity would prevent us from seeing meaningful differences.
Subgroup analyses related to the interventions meeting ACSM criteria was not carried out due to heterogeneity among the studies.
The results of subgroup analysis undertaken to explore the effects of combining an education component with a mixed exercise intervention have been reported above.
Sensitivity analysis
Sensitivity analysis was carried out to determine the impact of risk of bias related to selection bias and attrition bias on two outcomes ‐ HRQL and pain intensity (Analysis 1.2).
1.2. Analysis.
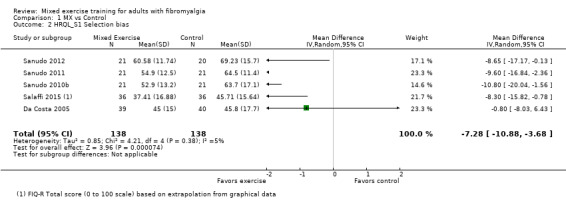
Comparison 1 MX vs Control, Outcome 2 HRQL_S1 Selection bias.
HRQL
Elimination of studies with high or unclear risk of allocation bias from the meta‐analysis left five studies. Results showed minimal impact on the magnitude, direction, and significance of the difference between mixed exercise and control for HRQL based on selection bias (see Analysis 1.2; Table 9). Sensitivity analysis was also carried out to determine the impact of risk of attrition bias. Eliminating studies with high or unclear attrition bias from the meta‐analysis left 10 of the 13 studies. Minimal impact on the magnitude, direction, and significance of results was observed (see Analysis 1.3; Table 9).
8. Sensitivity analyses: mixed exercise vs control.
| Outcome |
Low risk of selection bias MD [95% CI LL, UL], number of studies (participants), I² |
Low risk of attrition bias MD [95% CI LL, UL], number of studies (participants), I² |
All studies MD [95% CI LL, UL], number of studies (participants), I² |
| HRQL | ‐7.28 [‐10.88, ‐3.68], 5 studies (276), I² = 5% | ‐6.97 [‐11.26, ‐2.68], 10 studies (596), I² = 55% | ‐6.95 [‐10.51, ‐3.38], 13 studies (610), I² = 51% |
| Pain | ‐4.75 [‐13.76, 4.27], 4 studies (216), I² = 65% | ‐4.74 [‐8.09, ‐1.38], 12 studies (693), I² = 13% | ‐5.17 [‐8.85, ‐1.48], 15 studies (832), I² = 38% |
CI: confidence interval; LL: lower limb; MD: mean difference; UL: upper limb.
1.3. Analysis.
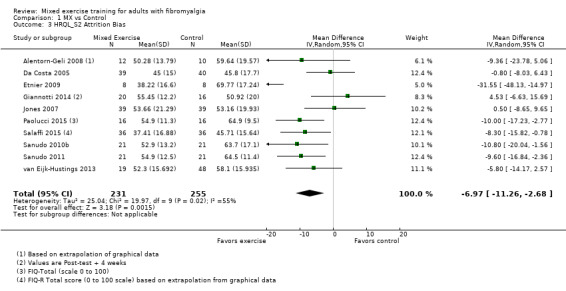
Comparison 1 MX vs Control, Outcome 3 HRQL_S2 Attrition Bias.
Pain intensity
Elimination of studies with high or unclear risk of selection bias left four studies (Da Costa 2005; Salaffi 2015; Sanudo 2010b; Sanudo 2011;Analysis 1.5). Minimal impact was observed in the magnitude and direction of effect size (see Analysis 1.5; Table 9), but the effect was no longer statistically significant. Sensitivity analysis was carried out to determine the impact of attrition bias. Three of the 13 studies had high or unclear risk of attrition bias and were eliminated from the sensitivity analysis. Minimal impact of pain on the magnitude, direction, and significance of effect size was observed (see Analysis 1.6; Table 9).
1.5. Analysis.
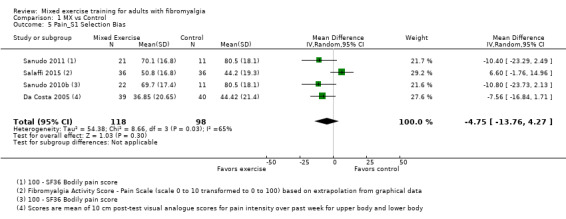
Comparison 1 MX vs Control, Outcome 5 Pain_S1 Selection Bias.
1.6. Analysis.
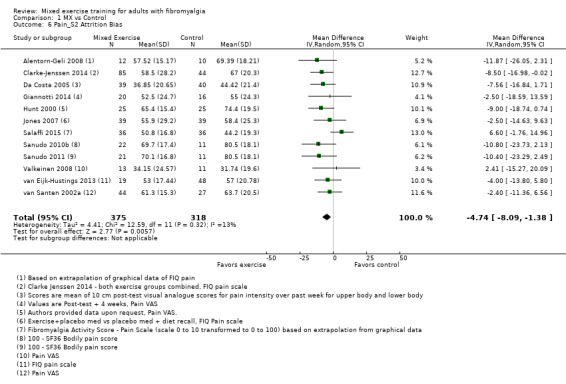
Comparison 1 MX vs Control, Outcome 6 Pain_S2 Attrition Bias.
Although detection bias is a definite possibility, sensitivity analyses could not be carried out because we found too few studies to contrast.
Mixed exercise versus non‐exercise intervention
Results of analyses of mixed exercise versus non‐exercise interventions are summarised below and in Table 10.
9. Outcomes of comparisons for mixed exercise versus other or non‐exercise interventions.
| Major outcomes | Minor outcomes | ||||||
| Comparator | HRQL (MD, scale 0 to 100)a, b | Pain Intensity (MD, scale 0 to 100)a, b | Fatigue (MD, scale 0 to 100)a, b | Stiffness (MD, scale 0 to 100)a, b | Physical function (MD, scale 0 to 100)a, b | Cardiovascular submax (MD, 6‐minute walk test, meters)a, c | Strength (MD)a, c |
| Non‐exercise comparators | |||||||
| Self‐help programme | ‐0.77 [‐8.36, 6.81], 2 studies, n = 153 |
‐2.25 [‐15.55, 11.06], 2 studies, n = 153 |
‐1.14 [‐11.13, 8.85], 2 studies, n = 152 |
‐3.68 [‐12.71, 5.36], 2 studies, n = 155 |
‐5.24 [‐12.88, 2.39], 2 studies, n = 153 |
‐‐‐ | ‐‐‐ |
| Cogniive‐behavioural training | ‐3.50 [‐12.24, 5.24], 1 study, n = 40 |
‐4.00 [‐19.84, 11.84], 1 study, n = 40 |
‐7.00 [‐22.67, 8.67], 1 study, n = 40 |
4.00 [‐13.98, 21.98], 1 study, n = 40 |
2.20 [‐9.39, 13.79], 1 study, n = 41 |
‐‐‐ | ‐‐‐ |
| Relaxation | ‐4.51 [‐13.08, 4.07], 1 study, n = 38 |
‐‐‐ | ‐‐‐ | ‐‐‐ | ‐‐‐ | ‐‐‐ | ‐‐‐ |
| Biofeedback | 0.80 [‐2.97, 4.57], 1 study, n = 82 |
‐2.35 [‐9.59, 4.88], 2 studies, n = 135 |
7.00 [‐0.16, 14.16], 1 study, n = 82 |
‐‐‐ | ‐0.56 [‐4.58, 3.46], 2 study, n = 136 |
‐‐‐ | ‐‐‐ |
| Medication | 0.72 [‐5.67, 7.11], 1 study, n = 231 |
3. 00 [‐9.79, 15.79], 1 study, n = 75 |
‐6.10 [‐18.81, 6.61], 1 study, n = 75 |
0.50 [‐12.61, 13.61], 1 study, n = 75 |
‐‐‐ | ‐‐‐ | ‐‐‐ |
| Mixed exercise vs other exercise comparators | |||||||
| AE | 0.80 [‐8.64, 10.24], 1 study, n = 43 |
4.61 [‐3.16, 12.38], 2 studies, n = 73 |
‐3.70 [‐13.10, 5.70], 1 study, n = 43 |
‐‐‐ | 1.76 [‐9.54, 13.05], 2 studies, n = 73 |
21.60 [‐20.98, 64.18], 1 study, n = 43 |
1.30 [‐1.53, 4.13], 1 study, n = 43 |
| Remedial exercise | 3.59 [‐1.89, 9.07], 1 study, n = 32 |
‐‐‐ | ‐‐‐ | ‐‐‐ | ‐‐‐ | ‐‐‐ | ‐‐‐ |
| Home programme (flexibility) | ‐6.82 [‐22.12, 8.48], 1 study, n = 43 |
‐4.60 [‐18.03, 8.83], 1 study, n = 43 |
‐‐‐ | ‐‐‐ | ‐‐‐ | ‐‐‐ | ‐‐‐ |
| (AE+FX) vs (RT+AE+FX) | 1.90 [‐4.68, 8.48], 1 study, n = 70 |
‐4.00 [‐14.61, 6.61], 1 study, n = 70 |
0.00 [‐11.03, 11.03], 1 study, n = 70 |
3.00 [‐9.19, 15.19], 1 study, n = 70 |
‐2.10 [‐11.45, 7.25], 1 study, n = 70 |
‐19.00 [‐52.29, 14.29], 1 study, n = 70 |
‐‐‐ |
| (Callisthenics+AE+FX) vs (RT+FX+posture) | ‐2.20 [‐11.81, 7.41], 1 study, n = 27 |
‐13.00 [‐26.29, 0.29], 1 study, n = 27 |
‐9.00 [‐25.65, 7.65], 1 study, n = 27 |
‐11.00 [‐28.16, 6.16], 1 study, n = 27 |
10.00 [‐0.30, 20.30], 1 study, n = 27 |
‐‐‐ | ‐‐‐ |
AE: aerobic exercise; FX: flexibility exercise; HRQL: health related quality of life; MD: mean difference; RT: resistance exercise
aValues are MD [95% CI lower limit, 95% CI upper limit].
bPositive values for MD indicate that the comparator was more effective than the mixed exercise; negative values for MD indicate that mixed exercise was more effective than the comparator.
cPositive values for MD indicate that mixed exercise was more effective than the comparator; negative values for MD indicate that comparator was more effective than the mixed exercise.
Mixed exercise versus self‐help programmes
One study compared two mixed exercise interventions to a self‐help programme in a total of 97 participants (Rooks 2007). We found evidence of no effect on HRQL (MD ‐4.81, 95% CI ‐11.41 to 1.79), pain intensity (MD ‐8.93, 95% CI ‐18.77 to 0.92), fatigue (MD ‐6.00, 95% CI ‐14.54 to 2.54), stiffness (MD ‐8.52, 95% CI ‐18.87 to 1.83), and physical function (standardised mean difference (SMD) ‐0.40, 95% CI ‐0.84 to 0.05). Participants in Rooks 2007 had no exacerbations or serious adverse events in response to mixed exercise. Rates for all‐cause withdrawal in the mixed exercise training group versus the self‐help programme were 16/51 versus 23/50 (Rooks 2007). It is uncertain whether mixed exercise improves HRQL and physical function, or reduces pain, fatigue, stiffness, or withdrawals, because the quality of this evidence was very low (see Table 11).
10. Quality of evidence ‐ GRADE assessment for mixed exercise vs non‐exercise.
| Quality assessment | № of individuals | Quality | Importance | |||||||
| № of studies | Study design | Risk of bias | Inconsistency | Indirectness | Imprecision | Other considerations | Mixed exercise | Other non‐Ex | ||
| HRQL: MX vs self‐help programme | ||||||||||
| 1 | randomised trials | seriousa | not serious | not serious | very seriousb | Single study | 70 | 27 | ⊕⊝⊝⊝ very low | CRITICAL |
| HRQL: MX+ED vs ED | ||||||||||
| 1 | randomised trials | very seriousc | not serious | not serious | seriousb | Single study | 28 | 28 | ⊕⊝⊝⊝ very low | CRITICAL |
| HRQL: MX vs relaxation | ||||||||||
| 1 | randomised trials | very seriousc | not serious | not serious | seriousb | Single study | 18 | 20 | ⊕⊝⊝⊝ very low | CRITICAL |
| HRQL: MX vs biofeedback | ||||||||||
| 1 | randomised trials | very seriousd | not serious | not serious | seriousb | Single study | 44 | 38 | ⊕⊝⊝⊝ very low | CRITICAL |
| HRQL: MX vs medication | ||||||||||
| 2 | randomised trials | very seriouse | not serious | not serious | seriousb | 113 | 118 | ⊕⊝⊝⊝ very low | CRITICAL | |
| HRQL: MX vs cogniive‐behavioural training | ||||||||||
| 1 | randomised trials | very seriousd | not serious | not serious | seriousb | Single study | 19 | 21 | ⊕⊝⊝⊝ very low | CRITICAL |
| Pain intensity: MX vs self‐help programme | ||||||||||
| 1 | randomised trials | seriousa | not serious | not serious | very seriousb | Single study | 70 | 27 | ⊕⊝⊝⊝ very low | CRITICAL |
| Pain intensity: MX vs cognitive‐behavioural therapy | ||||||||||
| 1 | randomised trials | very seriousd | not serious | not serious | very seriousb | Single study | 19 | 21 | ⊕⊝⊝⊝ very low | CRITICAL |
| Pain intensity: MX+ED vs ED | ||||||||||
| 1 | randomised trials | very seriousc | not serious | not serious | seriousb | Single study | 28 | 28 | ⊕⊝⊝⊝ very low | CRITICAL |
| Pain intensity: MX vs biofeedback | ||||||||||
| 2 | randomised trials | very seriousc | not serious | not serious | seriousb | 70 | 65 | ⊕⊝⊝⊝ very low | CRITICAL | |
| Pain intensity: MX vs medication | ||||||||||
| 1 | randomised trials | seriousa | not serious | not serious | very seriousb | Single study | 39 | 36 | ⊕⊝⊝⊝ very low | CRITICAL |
| Fatigue: MX vs self‐help programme | ||||||||||
| 1 | randomised trials | seriousa | not serious | not serious | very seriousb | Single study | 70 | 26 | ⊕⊝⊝⊝ very low | IMPORTANT |
| Fatigue: MX vs cognitive‐behavioural therapy | ||||||||||
| 1 | randomised trials | seriousa | not serious | not serious | very seriousb | Single study | 19 | 21 | ⊕⊝⊝⊝ very low | IMPORTANT |
| Fatigue: MX+ED vs ED | ||||||||||
| 1 | randomised trials | very seriousc | not serious | not serious | seriousb | Single study | 28 | 28 | ⊕⊝⊝⊝ very low | IMPORTANT |
| Fatigue: MX vs biofeedback | ||||||||||
| 1 | randomised trials | seriousd | not serious | not serious | very seriousb | Single study | 44 | 38 | ⊕⊝⊝⊝ very low | IMPORTANT |
| Fatigue: MX vs med | ||||||||||
| 1 | randomised trials | seriousa | not serious | not serious | very seriousb | Single study | 39 | 36 | ⊕⊝⊝⊝ very low | IMPORTANT |
| Stiffness: MX vs self‐help programme | ||||||||||
| 1 | randomised trials | seriousa | not serious | not serious | very seriousb | Single study | 73 | 26 | ⊕⊝⊝⊝ very low | IMPORTANT |
| Stiffness: MX vs cognitive‐behavioural therapy | ||||||||||
| 1 | randomised trials | very seriousd | not serious | not serious | seriousb | Single study | 19 | 21 | ⊕⊝⊝⊝ very low | IMPORTANT |
| Stiffness: MX+ED vs ED | ||||||||||
| 1 | randomised trials | very seriousc | not serious | not serious | seriousb | Single study | 28 | 28 | ⊕⊝⊝⊝ very low | IMPORTANT |
| Stiffness: MX vs Medication | ||||||||||
| 1 | randomised trials | seriousf | not serious | not serious | very seriousb | Single study | 39 | 36 | ⊕⊝⊝⊝ very low | IMPORTANT |
| Physical function: MX vs self‐help programme | ||||||||||
| 1 | randomised trials | seriousa | not serious | not serious | very seriousb | Single study | 70 | 27 | ⊕⊝⊝⊝ very low | IMPORTANT |
| Physical function: MX vs cognitive‐behavioural training | ||||||||||
| 1 | randomised trials | very seriousd | not serious | not serious | seriousb | Single study | 19 | 22 | ⊕⊝⊝⊝ very low | IMPORTANT |
| Physical function: MX+ED vs ED | ||||||||||
| 1 | randomised trials | very seriousc | not serious | not serious | seriousb | Single study | 28 | 28 | ⊕⊝⊝⊝ very low | IMPORTANT |
| Physical function: MX vs biofeedback | ||||||||||
| 2 | randomised trials | very seriouse | not serious | not serious | seriousb | 72 | 64 | ⊕⊝⊝⊝ very low | IMPORTANT | |
| All‐cause withdrawal ‐ MX only vs ED, SMP, CBT | ||||||||||
| 6 | randomised trials | very seriousc | not serious | seriousg | seriousb | 37/213 (17.4%) | 38/211 (18.0%) | ⊕⊝⊝⊝ very low | IMPORTANT | |
| All‐cause withdrawal ‐ MX only vs biofeedback | ||||||||||
| 2 | randomised trials | very seriousd | not serious | not serious | very seriousb | 5/78 (6.4%) | 9/70 (12.9%) | ⊕⊝⊝⊝ very low | IMPORTANT | |
| All‐cause withdrawal ‐ MX only vs medication | ||||||||||
| 2 | randomised trials | very seriousc | serioush | not serious | seriousb | 22/135 (16.3%) | 22/140 (15.7%) | ⊕⊝⊝⊝ very low | IMPORTANT | |
CBT: cognitive‐behavioural therapy; ED: education; HRQL: health‐related quality of life; MX: mixed exercise; SH/MT: self‐help‐management programme.
aUnclear risk of performance, detection, and reporting bias.
bSmall sample size and/or wide confidence interval.
cUnclear or high risk of bias related to selection, performance, detection, attrition, and reporting.
dUnclear or high risk of bias related to selection, performance, detection, and reporting.
eUnclear or high risk of selection, performance, detection, attrition, reporting, and other types of biases.
fUnclear risk of selection, detection, and reporting biases.
gDifferences in comparison.
hHigh heterogeneity (I² = 87%).
Mixed exercise versus cognitive‐behavioural therapy
One study compared mixed exercise to cognitive‐behavioural therapy in a total of 97 participants (Rivera Redondo 2004). We found evidence of no differences in effect between groups in HRQL (MD ‐3.50, 95% CI ‐12.24 to 5.24), pain intensity (MD ‐4.00, 95% CI ‐19.84 to 11.84), fatigue (MD ‐7.00, 95% CI ‐22.67 to 8.67), stiffness (MD 4.00, 95% CI ‐13.98 to 21.98) and physical function (SMD 0.11, 95% CI ‐0.50 to 0.73). There was no mention of adverse events in Rivera Redondo 2004. Rates for all‐cause withdrawal in the mixed exercise training group versus the cognitive‐behavioural training group were 4/19 versus 2/21. It is uncertain whether mixed exercises improve HRQL and physical function, or reduce pain, fatigue, stiffness, or withdrawals, because the quality of this evidence was very low (see Table 11).
Mixed exercise plus education versus education
One study compared mixed exercise and education to education alone in a total of 56 participants (Burckhardt 1994). We found evidence of no effect on HRQL (MD 6.10, 95% CI ‐1.73 to 13.93), pain intensity (MD 11.00, 95% CI ‐2.63 to 24.63), fatigue (MD 10.00, 95% CI ‐3.71 to 23.71), stiffness (MD 5.00, 95% CI ‐8.71 to 18.71), and physical function (SMD ‐0.04, 95% CI ‐0.57 to 0.48). Rates of all‐cause withdrawal in the mixed exercise training group versus the education group were 5/33 versus 3/31 (Burckhardt 1994). Burckhardt 1994 did not report on adverse events. It is uncertain wether mixed exercise improves HRQL and physical function, or reduces pain, fatigue, stiffness, or withdrawals, because the quality of this evidence was very low (see Table 11).
Mixed exercise versus relaxation
One study compared mixed exercise to relaxation in a total of 38 participants (Martin 1996). We found lack of evidence of an effect on HRQL (MD ‐4.51, 95% CI ‐13.08 to 4.07). The mixed exercise group in Martin 1996 "complained of increased muscle pain and stiffness when they started exercise" (page 1052), but it was noted that participants were able to "undertake an exercise program that includes strength training without adverse events" (page 1053). Rates of all‐cause withdrawal in the mixed exercise training group versus the relaxation group were 12/30 versus 10/30. It is uncertain whether mixed exercise improves HRQL or reduces withdrawals because the quality of this evidence was very low (see Table 11).
Mixed exercise versus biofeedback
Two studies compared mixed exercise to biofeedback (Buckelew 1998; van Santen 2002a). We found lack of evidence of an effect (due to imprecision) for HRQL (MD 0.80, 95% CI ‐2.97 to 4.57; 1 study; 82 participants), pain (MD ‐2.35, 95% CI ‐9.59 to 4.88; 135 participants; 2 studies), fatigue (MD 7.00, 95% CI ‐0.16 to 14.16; 82 participants; 1 study), or physical function (SMD ‐0.08, 95% CI ‐0.41, to 0.26; 136 participants; 2 studies). Two participants dropped out due to increased pain, but their assigned group(s) were not specified (Buckelew 1998). An unspecified number of participants in the mixed exercise group in van Santen 2002a complained of substantial post‐exercise pain, and two individuals in the biofeedback/relaxation group dropped out because biofeedback was stressful for them. Rates of all‐cause withdrawal in the mixed exercise training groups versus the biofeedback groups were 5/78 versus 9/70. It is uncertain whether mixed exercise improves HRQL and physical function, or reduces pain, fatigue, or withdrawals, because the quality of this evidence was very low (see Table 11).
Mixed exercise versus medication
Two studies compared mixed exercise to medication (amitriptyline ‐ Joshi 2009; pyridostigmine ‐ Jones 2007). We found lack of evidence of an effect on HRQL (MD 0.72, 95% CI ‐5.67 to 7.11; 231 participants; 2 studies), pain (MD 3.00, 95% CI ‐9.79 to 15.79; 75 participants; 1 study), fatigue (MD ‐6.10, 95% CI ‐18.81 to 6.61; 75 participants; 1 study), or stiffness (MD 0.50, 95% CI ‐12.61 to 13.61; 75 participants; 1 study). Joshi 2009 provided no information on adverse events. Participants taking pyridostigmine (combined with mixed exercise or diet monitoring) reported greater numbers of adverse events compared to those given placebo medication (combined with mixed exercise or diet monitoring; Jones 2007). The percentage of participants (placebo vs pyridostigmine) reporting various side effects were as follows: abdominal complaints (40% vs 62%), nausea/vomiting (22% vs 29%), headache (93% vs 85%), hot flash/flush (15% vs 26%), diarrhoea (43% vs 77%), muscle cramps (2% vs 25%), and fatigue (17% vs 20%). It is uncertain whether mixed exercise improves HRL, or reduces pain, fatigue, or stiffness, because the quality of this evidence was very low (see Table 11).
Improvement in pain greater than 30%
No studies measured this outcome.
Mixed exercise versus other exercise
Results of analyses of mixed exercise versus other exercise interventions are summarised below and in Table 10.
Mixed exercise versus aerobic exercise only
Two studies compared mixed exercise to aerobic exercise (Sanudo 2010b; van Santen 2002b). We found lack of evidence of an effect between groups (Analysis 4.1) in terms of HRQL (MD 0.80, 95% CI ‐8.64 to 10.24; 1 study; 43 participants), pain intensity (MD 4.61, 95% CI ‐3.16 to 12.38; 2 studies; 73 participants), fatigue (MD ‐3.70, 95% CI ‐13.10 to 5.70; 1 study; 43 participants), physical function (see footnote added to the forest plot) (SMD 0.06, 95% CI ‐0.40 to 0.52; 2 studies; 73 participants), CR submax (MD 21.60, 95% CI ‐20.98 to 64.18; 1 study; 43 participants), and strength (MD 1.30 Newtons grip strength, 95% CI ‐1.53 to 4.13; 1 study; 43 participants). Rates of all‐cause withdrawal for the mixed exercise training groups versus the aerobic exercise groups were 4/36 versus 4/10. In Sanudo 2010b, one individual in the aerobic exercise group was unable to exercise after an injury was sustained; however study authors did not specify whether this injury occurred in response to testing or training, or whether it was unrelated to the programme. There was no mention of any adverse events occurring in the mixed exercise group in this study. In van Santen 2002b, participants in the aerobic exercise programme "stated that they felt completely "broken‐down" for more than 24 hours after the training sessions and that they had hardly recovered before the next training session was due. It took about a month after the study started before all participants cycled on the desired high level of intensity." van Santen 2002b also noted that almost all participants in both the aerobic and mixed exercise groups "judged their fitness training as too time consuming, painful and stressful." It is uncertain whether mixed exercise improves HRQL and physical function, or decreases pain, fatigue, and withdrawals, because the quality of evidence was very low (Table 12).
4.1. Analysis.
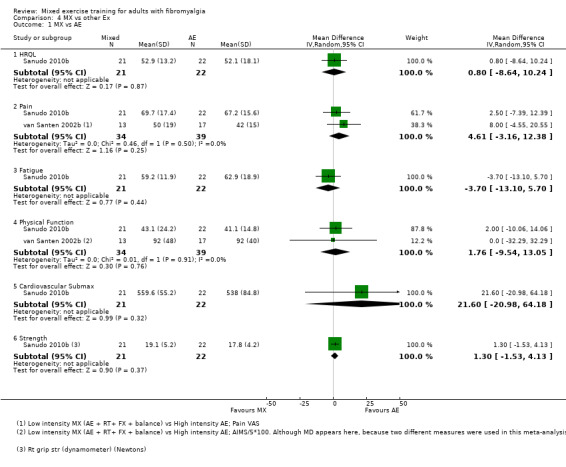
Comparison 4 MX vs other Ex, Outcome 1 MX vs AE.
11. Quality of evidence ‐ GRADE assessment for mixed exercise vs other exercise.
| Quality assessment | № of individuals | Quality | Importance | ||||||||
| № of studies | Study design | Risk of bias | Inconsistency | Indirectness | Imprecision | Other considerations | Two exercise types | Other exercise | |||
| MX vs AE ‐ HRQL | |||||||||||
| 1 | randomised trials | very seriousa | not serious | not serious | seriousb | Single study | 21 | 22 | ⊕⊝⊝⊝ very low | CRITICAL | |
| MX vs AE ‐ Pain | |||||||||||
| 2 | randomised trials | very seriousc | not serious | not serious | seriousb | 34 | 39 | ⊕⊝⊝⊝ very low | CRITICAL | ||
| MX vs AE ‐ Fatigue | |||||||||||
| 1 | randomised trials | very seriousa | not serious | not serious | seriousb | Single study | 21 | 22 | ⊕⊝⊝⊝ very low | IMPORTANT | |
| MX vs AE ‐ Physical function | |||||||||||
| 2 | randomised trials | very seriousc | not serious | not serious | seriousb | 34 | 39 | ⊕⊝⊝⊝ very low | IMPORTANT | ||
| MX vs remedial exercise ‐ HRQL | |||||||||||
| 1 | randomised trials | very seriousd | not serious | not serious | seriousb | Single study | 16 | 16 | ⊕⊝⊝⊝ very low | CRITICAL | |
| MX vs HPrg (FX) ‐ HRQL | |||||||||||
| 1 | randomised trials | very seriousd | not serious | not serious | seriousb | Single study | 23 | 20 | ⊕⊝⊝⊝ very low | CRITICAL | |
| MX vs HPrg (FX) ‐ Pain | |||||||||||
| 1 | randomised trials | very seriousd | not serious | not serious | seriousb | Single study | 23 | 20 | ⊕⊝⊝⊝ very low | CRITICAL | |
| MX (AE+FX) vs MX (RE+AE+FX) ‐ HRQL | |||||||||||
| 1 | randomised trials | seriouse | not serious | not serious | seriousb | Single study | 35 | 35 | ⊕⊝⊝⊝ very low | CRITICAL | |
| MX (AE+FX) vs MX (RE+AE+FX) ‐ Pain | |||||||||||
| 1 | randomised trials | seriouse | not serious | not serious | seriousb | Single study | 35 | 35 | ⊕⊝⊝⊝ very low | CRITICAL | |
| MX (AE+FX) vs MX (RE+AE+FX) ‐ Fatigue | |||||||||||
| 1 | randomised trials | seriouse | not serious | not serious | seriousb | Single study | 35 | 35 | ⊕⊝⊝⊝ very low | IMPORTANT | |
| MX (AE+FX) vs MX (RE+AE+FX) ‐ Stiffness | |||||||||||
| 1 | randomised trials | seriouse | not serious | not serious | seriousb | Single study | 35 | 35 | ⊕⊝⊝⊝ very low | IMPORTANT | |
| MX (AE+FX) vs MX (RE+AE+FX) ‐ Physical function | |||||||||||
| 1 | randomised trials | seriouse | not serious | not serious | seriousb | Single study | 35 | 35 | ⊕⊝⊝⊝ very low | IMPORTANT | |
| MX (Callisthenics+AE+FX) vs MX (RE+FX+Posture) ‐ HRQL | |||||||||||
| 1 | randomised trials | very seriousf | not serious | not serious | seriousb | Single study | 14 | 13 | ⊕⊝⊝⊝ very low | CRITICAL | |
| MX (Callisthenics+AE+FX) vs MX (RE+FX+Posture) ‐ Pain | |||||||||||
| 1 | randomised trials | very seriousf | not serious | not serious | seriousb | Single study | 14 | 13 | ⊕⊝⊝⊝ very low | CRITICAL | |
| MX (Callisthenics+AE+FX) vs MX (RE+FX+Posture) ‐ Fatigue | |||||||||||
| 1 | randomised trials | very seriousf | not serious | not serious | seriousb | Single study | 14 | 13 | ⊕⊝⊝⊝ very low | IMPROTANT | |
| MX (Callisthenics+AE+FX) vs MX (RE+FX+Posture) ‐ Stiffness | |||||||||||
| 1 | randomised trials | very seriousf | not serious | not serious | seriousb | Single study | 14 | 13 | ⊕⊝⊝⊝ very low | IMPORTANT | |
| MX (Callisthenics+AE+FX) vs MX (RE+FX+Posture) ‐ Physical function | |||||||||||
| 1 | randomised trials | very seriousf | not serious | not serious | seriousb | Single study | 14 | 13 | ⊕⊝⊝⊝ very low | IMPORTANT | |
| All‐cause withdrawal | |||||||||||
| 6 | randomised trials | very seriousg | not serious | serioush | seriousb | 22/142 (15.5%) | 25/145 (17.2%) | ⊕⊝⊝⊝ very low | IMPORTANT | ||
AE: aerobic; CI: confidence interval; FX: flexibility; HRQL: health‐related quality of life; MD: mean difference; MX: mixed exercise; RE: remedial exercise.
aHigh risk of performance (blinding of participant or personnel) and detection bias, and unclear risk of reporting bias.
bSmall sample size (fewer than 300) and wide confidence intervals.
cHigh (performance and detection) and unclear risk of bias (selection, reporting, attrition) issues.
dUnclear risk of selection, reporting, and other, and high risk of performance and detection bias.
eUnclear risk of performance, selection, and reporting biases.
fUnclear risk of selection, detection, attrition, reporting, and other biases. High risk of performance and detection (subjective outcome measures) biases.
gUnclear or high risk of performance and detection bias; unclear selection, attrition, and reporting biases.
hInterventions and comparators and heterogeneity.
Mixed exercise versus remedial exercise, relaxation, and mobilisations
One study compared mixed exercise to remedial exercise, relaxation, and mobilisations (Genc 2002; 32 participants). We found lack of evidence of an effect between groups in the only outcome reported (i.e. HRQL, MD 3.59, 95% CI ‐1.89 to 9.07; Analysis 4.2). Rates of all‐cause withdrawal for the mixed exercise training group versus the remedial exercise, relaxation, and mobilisation group were 0/15 versus 0/15. Adverse events were not reported. It is uncertain whether mixed exercise improves HRQL or reduces withdrawal because the quality of this evidence was very low (see Table 12).
4.2. Analysis.
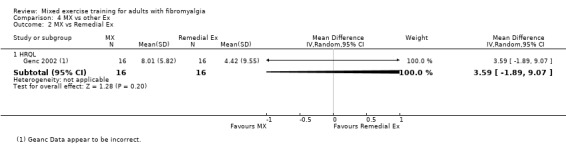
Comparison 4 MX vs other Ex, Outcome 2 MX vs Remedial Ex.
Mixed exercise versus flexibility home programme
One study compared mixed exercise to a flexibility home programme (Demir‐Gocmen 2013; 43 participants). We found lack of evidence of an effect between groups in HRQL (MD ‐6.82, 95% CI ‐22.12 to 8.48) or pain intensity (MD ‐4.60, 95% CI ‐18.03, 8.83; Analysis 4.3). Rates of all‐cause withdrawal for the mixed exercise training group versus the flexibility exercise group were 2/25 versus 5/25. No adverse events were related to either exercise programme. It is uncertain whether mixed exercise improves HRQL or reduces pain and all‐cause withdrawal because the quality of this evidence was very low (see Table 12).
4.3. Analysis.
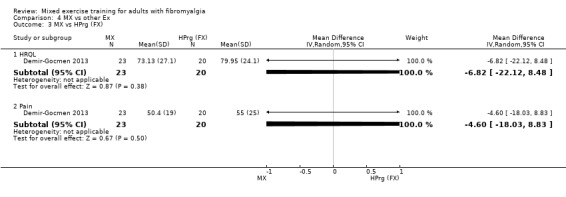
Comparison 4 MX vs other Ex, Outcome 3 MX vs HPrg (FX).
Mixed exercise (aerobic + flexibility) versus mixed exercise (resistance + aerobic + flexibility)
One study compared one mixed exercise intervention (two components) versus mixed exercise (three components) (Rooks 2007; 70 participants). We found lack of evidence of an effect between groups in HRQL (MD 1.90, 95% CI ‐4.68 to 8.48), pain intensity (MD ‐4.00, 95% CI ‐14.61 to 6.61), fatigue (MD 0.00, 95% CI ‐11.03 to 11.03), stiffness (MD 3.00, 95% CI ‐9.19 to 15.19), physical function (MD ‐2.10, 95% CI ‐11.45 to 7.25), or CR submax (MD ‐19.00, 95% CI ‐52.29 to 14.29; Analysis 4.4). Rates of all‐cause withdrawal for the mixed with two components versus the mixed with three components groups were 16/51 versus 16/51. Participants reported no serious adverse events in response to these mixed exercise programmes. It is uncertain whether mixed exercise (aerobic + flexibility) improves HRQL and physical function, or reduces pain, fatigue, stiffness, or all‐cause withdrawal, because the quality of this evidence was very low (see Table 12).
4.4. Analysis.
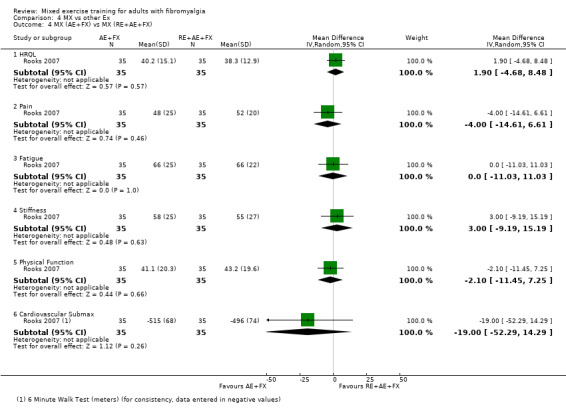
Comparison 4 MX vs other Ex, Outcome 4 MX (AE+FX) vs MX (RE+AE+FX).
Mixed exercise (callisthenics + aerobic + flexibility) versus mixed exercise (resistance + flexibility + posture exercise)
One study compared mixed exercise with callisthenics + aerobic exercise + flexibility exercise versus mixed exercise with resistance exercise + flexibility + posture (Yuruk 2008; 27 participants). We found lack of evidence of an effect between groups in HRQL (MD ‐2.20, 95% CI ‐11.81 to 7.41), pain intensity (MD ‐13.00, 95% CI ‐26.29 to 0.29), fatigue (MD ‐9.00, 95% CI ‐25.65 to 7.65), stiffness (MD ‐11.00, 95% CI ‐28.16 to 6.16), or physical function (MD 10.00, 95% CI ‐0.30 to 20.30; Analysis 4.5). Rates of all‐cause withdrawal for the mixed exercise with callisthenics + aerobic + flexibility group versus the mixed exercise with resistance + flexibility + posture group were 0/14 versus 0/13. No adverse events were related to either exercise programme. In summary, it is uncertain whether mixed exercise (callisthenics + aerobic + flexibility) improves HRQL and physical function, or reduces pain, fatigue, stiffness, and all‐cause withdrawal, because the quality of this evidence was very low (see Table 12).
4.5. Analysis.
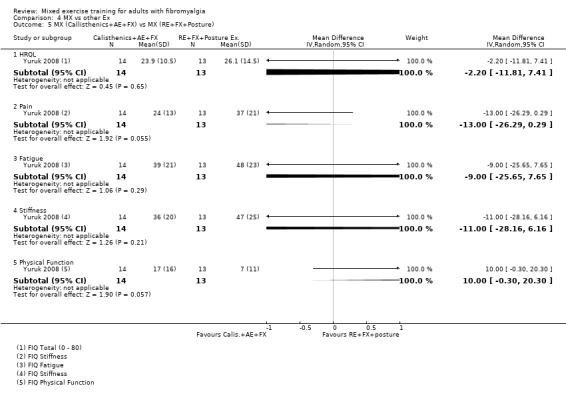
Comparison 4 MX vs other Ex, Outcome 5 MX (Callisthenics+AE+FX) vs MX (RE+FX+Posture).
Improvement in pain greater than 30%
No studies measured this outcome.
Discussion
Summary of main results
Meta‐analyses of mixed exercise interventions versus controls (21 studies, five to 26 weeks in length) provided low‐ to moderate‐quality evidence of beneficial effects on all major and minor outcomes post exercise. Only three outcomes reached the threshold for clinical relevance (health‐related quality of life (HRQL), fatigue, and physical function), but the confidence intervals included both clinically unimportant (< 15%) and clinically relevant (> 15%) improvements; improvements with exercise in the remaining outcomes were small and therefore were not deemed clinically relevant. Statistically significant effects on HRQL and cardiorespiratory (CR) submax but not on pain, fatigue, stiffness, or physical function were maintained for up to 26 weeks. In eight of the 21 studies, some participants experienced increased fibromyalgia symptoms (pain, soreness, or tiredness) during or after exercise. Across all 21 studies, no injuries or other serious adverse events were reported; however, in many studies, reporting of adverse events was missing or incomplete. There were no differences in all‐cause withdrawal rates nor in subgroup analyses comparing mixed exercise interventions and the mixed exercise plus education intervention for any outcomes. Sensitivity analysis showed no substantial impact of selection or attrition bias on HRQL or pain in the comparison of mixed exercise interventions versus controls.
There were no statistically significant differences in any of the outcomes for comparisons of mixed exercise interventions versus a variety of non‐exercise interventions including self‐help and education programmes, cognitive‐behavioural training, biofeedback, and medications. Nor were there any differences in the head‐to‐head comparisons of mixed exercise interventions versus any other exercise type – aerobic exercise, remedial exercise, and flexibility. Further, when different mixed exercise interventions made up of differing components were compared, no differences in outcome variables were found. Because so few studies were available for each of the comparisons in this category, when possible, meta‐analyses involved two studies at the most, and the overall quality of this evidence was very poor due to problems with risk of bias and imprecision.
Overall completeness and applicability of evidence
There were several gaps in reporting across the 29 studies, and we contacted several trial authors to obtain more information. Notable areas of inadequate reporting were study methods (allocation concealment, measuring and reporting adverse events, adherence, key features of the intervention). Nevertheless, the body of literature in the mixed versus control comparison is sufficiently large to address our objective related to the benefits and harms of mixed exercise. Given the large number of randomised controlled trials (RCTs) included in this comparison and the stability in effect size observed, we believe it is unlikely that missing or new trials would substantially alter the estimated median effect of mixed exercise for fibromyalgia.
Most of the studies included only females (nearly 70%). Thus our conclusions are limited to female participants.
The number of studies in this review is not sufficiently large to compare exercise to other interventions (pharmaceutical or non‐pharmaceutical interventions), nor is the literature sufficiently explicit or large enough to answer clinical questions about the ideal routine or combination of exercises for patients with fibromyalgia.
No studies used the outcome recommended by IMMPACT (Initiative on Methods, Measurement, and Pain Assessment in Clinical Trials) ‐ the proportion of participants who experienced greater than 30% improvement. Symptoms remain the main focus of clinical trials, with a small number addressing physical fitness.
Quality of the evidence
This review included 29 studies covering a wide range of mixed exercise interventions. The evidence presented in this review comes from trials published in academic journals and trial registries, and from trial authors. Using the GRADE system for major outcomes, we found evidence of low to moderate quality for benefits in HRQL, pain, fatigue, stiffness, and all‐cause withdrawal, with mixed intervention training versus control at the end of treatment. We downgraded the evidence because there are limitations related to imprecision (e.g. total sample size smaller than 400 participants) and to risk of bias, such as lack of allocation concealment, lack of blinding of participants and care providers, and uncertainty regarding selective reporting.
We rated the quality of evidence as very low for long‐term benefits of mixed interventions for all outcomes owing to limitations in the risk of bias (selection, performance, selective reporting, and incomplete outcome reporting), moderate to severe heterogeneity, and imprecision (see Table 8). In comparisons of mixed interventions versus non‐exercise or other exercise interventions, the quality of the evidence was very low. We downgraded the quality of the evidence because of limitations in the risk of bias assessment including lack of allocation concealment, lack of blinding of participants and care providers, and uncertainty regarding selective reporting, as well as very low numbers of trial participants, wide confidence intervals, and high heterogeneity (see Table 11 and Table 12).
Risk of bias assessment highlighted concerns regarding insufficient information on allocation concealment, blinding of participants and care providers, and detection bias related to self‐report instruments. Although lack of allocation concealment can result in overestimation of effect (Odgaard‐Jenssen 2011), the importance of this criterion in the trials included in this review was shown by the sensitivity analysis to have no effect. One limitation of exercise studies is that blinding is difficult. Non‐blinded participants who are aware of their intervention may differ from blinded participants in how they report outcomes or in the quality of the participant‐instructor‐assessor relationship, inducing dissimilar rates of effect. This body of evidence relies heavily on subjective self‐reported outcome measures. Several studies were small and probably were underpowered.
Other issues to consider when interpreting these results include the following: (1) some studies assessed large numbers of outcomes, increasing the probability of finding statistically significant differences for outcomes by chance; (2) the diversity of psychometric and other outcome measures used made interpretation of statistically pooled outcome data difficult; and (3) important clinical heterogeneity was present among the studies and this remains a major challenge. We noted a trend over time towards improved reporting in the RCTs with clear improvement after 2010, which coincides with the implementation of CONSORT.
Potential biases in the review process
Limitations inherent in the primary literature include incomplete description of exercise protocols, inadequate documentation of adherence to exercise prescriptions, and inconsistent reporting of adverse events. In secondary comparisons, there were few studies for each comparison to assess publication bias through assessment of asymmetry. Despite efforts to reduce the impact of publication bias in the review, the possibility remains that some studies (with positive or negative findings) may not have been identified by the search. Analysis of a funnel plot that appeared asymmetrical (Figure 4; Figure 5) indicated a relationship between treatment effect estimates and study size (small‐study effects). However, we did not test the funnel plots, and some authors have argued that visual interpretation of funnel plots is too subjective to be useful. Funnel plot asymmetry can be due to heterogeneity, reporting bias, or chance and may also be an artefact of the statistics chosen to be plotted. We performed many meta‐analyses; therefore some of the findings may have resulted from chance. Many pooled results were statistically and clinically heterogeneous, mainly because of the small number of included studies and the breadth of interventions and participant characteristics reported. Because of this, these results must be interpreted with caution. Contacting authors for additional information may have improved the accuracy of the information reported in most cases but also may have introduced a 'response bias' into the risk of bias assessment.
In our review process, we did attempt to control for biases through the following processes.
We applied no language restrictions to our search.
We updated searches every six months and utilised multiple databases.
We complemented our database literature searches with handsearching.
We contacted primary authors for clarification and for additional information where indicated, although responses were not always obtained. We asked our questions in an open‐ended fashion so as to avoid leading questions or answers.
We searched clinical trial registries (i.e. clinicaltrials.gov and the WHO International Clinical Trials Registry Platform) to identify unpublished trials and to increase our chances of detecting selective reporting of outcomes. Publication bias may lead to overestimation of a treatment effect by up to 12% (Moher 1999).
Our multi‐disciplinary team had a range of expertise in library science, systematic reviewing and methods, critical appraisal, clinical rheumatology, exercise physiology, physiotherapy, kinesiology, and knowledge translation.
We used a standardised procedure for selection and inclusion of studies in the review, and review authors were trained in data extraction through a standardised process.
Two members of our multi‐disciplinary team provided the perspective of consumers (i.e. one team member had fibromyalgia, and a second team member had another rheumatic disease).
We used intention‐to‐treat data preferentially.
Agreements and disagreements with other studies or reviews
Several reviews have examined fibromyalgia and exercise, but none have focussed exclusively on the effectiveness of mixed training interventions. We have chosen to comment on reviews in which interventions investigated were similar to ours (Brosseau 2008; Cerrillo‐Urbina 2015; Garcia‐Hermoso 2015; Häuser 2010). It is difficult to directly compare findings across these reviews because each one has defined mixed exercise differently, or has classified exercise that consists of more than one mode as aerobic exercise, as was the case in Brosseau 2008.
The effect of physical exercise on the symptoms of fibromyalgia in post‐menopausal women was explored through the Cerrillo‐Urbina 2015 systematic review and meta‐analysis. This review focussed on one main outcome: global well‐being as measured by the total Fibromyalgia Impact Questionnaire (FIQ). As in our review, review authors considered other measures of symptoms (e.g. pain, fatigue, stiffness). Unlike in our review, review authors used the Physiotherapy Evidence Databese (PEDro) Scale to evaluate methodological quality. Nineteen studies are included in the Cerrillo‐Urbina 2015 review, with four studies examining combined exercise programmes (Garcia‐Martinez 2011; Rooks 2007; Sanudo 2010b; Sanudo 2011). In this context, combined exercise referred to programmes that included all aerobic, resistance, and flexibility training. In contrast, our definition of mixed exercise required the inclusion of two or more of these different exercise modes. Review authors pooled data from three of the studies that combined exercise and did not find a statistically significant effect on global well‐being (d = ‐0.63, 95% confidence interval (CI) ‐0.99 to 0.27).
Three of the four studies comprising the review authors’ combined exercise group for meta‐analysis were also included in our review (Rooks 2007; Garcia‐Martinez 2011; Sanudo 2011). Self‐esteem was also noted to improve with combined exercise in the Cerrillo‐Urbina 2015 review. Pain, fatigue, sleep, stiffness, and anxiety improved with all types of exercise studied. Four additional studies in Cerrillo‐Urbina 2015 overlap with the studies in our review (Alentorn‐Geli 2008; Burckhardt 1994; Da Costa 2005; Sanudo 2010b); however, these studies were categorised by Cerrillo‐Urbina 2015 as providing aerobic or aquatic exercise, rather than combined exercise. Rates of exercise adherence were reported to be high, with the exception of one study (not included in our review), which reported a dropout rate of 38% (Meyer 2000). Similar to our review, no serious adverse effects were reported with the different modes of exercise. One study not included in our review reported increased pain among some participants upon initiation of exercise (Mannerkorpi 2000).
The Garcia‐Hermoso 2015 systematic review examined the efficacy and structure of exercise programmes for people with fibromyalgia. These review authors focussed on one main outcome: functional aerobic capacity as measured by the six‐minute walk test. Thirteen studies were included (12 RCTs), and, as in Cerrillo‐Urbina 2015, the PEDro scale was used to evaluate study quality. Seventy‐five per cent of the included studies met at least 50% of the PEDro criteria. The included studies were grouped into five categories (strengthening, aerobic, mixed, aquatic, and multi‐disciplinary, which consisted of pool‐ and land‐based programmes), with exercise programmes combining aerobic, strength, and flexibility training considered as mixed. Only two studies met the criterion of providing mixed exercise (Sanudo 2010b; Sanudo 2012). The review authors reported that functional aerobic capacity did not increase with mixed exercise, but they noted that they were able to calculate effect size based only on the Sanudo 2012 study. This review meta‐analysed five studies comparing mixed exercise interventions with controls using the six‐minute walk test to evaluate changes in aerobic fitness (Burckhardt 1994; Clarke‐Jenssen 2014; Giannotti 2014; Sanudo 2010b; Sanudo 2012). The mean change was 52.8 meters (95% CI 34.11 to 71.43) in favour of mixed exercise.
Garcia‐Hermoso 2015 reported high exercise adherence (more than 80%) and a low dropout rate (less than 19%) for the two mixed exercise studies in this review. In our review, we meta‐analysed withdrawal rates from a total of 19 studies (1065 participants) and determined that there was lack of evidence of an effect on withdrawal rates between exercise and control participants. The pooled all‐cause withdrawal rate in the exercise groups was 64/576 as compared to 53/489 in the control groups (risk difference (RD) 0.01, 95% CI ‐0.03 to 0.05). Garcia‐Hermoso 2015 also concluded that many studies did not give sufficient detail on exercise intensity nor on adverse events.
Häuser 2010 provided a review and meta‐analysis that compared fibromyalgia outcomes between aerobic exercise and control conditions. The analysis also involved a comparison of different types of aerobic exercise (land‐based, water‐based, and mixed). Here, mixed exercise was defined as "a combination of [aerobic exercise] with stretching and/or muscle strength, the length of [aerobic exercise] should exceed the time with the other types of exercise" (Häuser 2010). In total, the review included 28 RCTs, with seven of these contributing to a subgroup analysis comparing the three types of aerobic exercise. Subgroup analyses were limited by the small number of studies that provided sufficient detail about the exercise programme provided to categorise interventions into one of the three types of aerobic exercise. Eight studies from our review were also included in Häuser 2010 (Alentorn‐Geli 2008;Da Costa 2005;Etnier 2009;Jones 2007;Martin 1996;Rooks 2007;Valkeinen 2008;van Santen 2002a). In Häuser 2010, the exercise subgroup analysis for pain at the end of treatment found evidence of no effect (effect size ‐0.03, 95% CI ‐0.45 to 0.39). In the 15 studies where we performed meta‐analysis to determine the effect of mixed exercise on pain, there was an absolute difference of 5% (95% CI 1% to 9%) in favour of the exercise participants. Häuser 2010 concluded that combining aerobic exercise with stretching or strengthening exercises was not superior to providing aerobic exercise alone.
The Ottawa Panel was created to develop guidelines on aerobic exercise for adults with fibromyalgia (Brosseau 2008). This group completed a literature review on the effects of aerobic exercise on pain, quality of life, endurance, and psychological well‐being, among others. Their review included RCTs and studies of other designs, such as cohort and case‐control studies, whereas our review was restricted to RCTs. Due to heterogeneity across studies in Brosseau 2008, the data could not be pooled for meta‐analysis. In contrast, we were able to complete pooled analyses. Where we used the Cochrane ‘Risk of bias’ tool to evaluate study quality, the Ottawa Panel used the 5‐point Jadad Scale (Jadad 1996). Finally, one important distinction between the two reviews was the classification of exercise mode. The Panel’s definition of aerobic exercise allowed for the inclusion of resistance, relaxation, and flexibility exercises – what we have defined as mixed exercise (i.e. two or more types of exercise in the main component of the exercise session). As a result, four of the 16 studies included in the Brosseau 2008 overlap with our review (Da Costa 2005;van Santen 2002a; van Santen 2002b, Verstappen 1997). The Ottawa Panel concluded that there was evidence supporting the role of aerobic exercise in fibromyalgia management, with the greatest improvements seen in pain relief and HRQL. Similarly, our results showed that mixed exercise has small, statistically significant effects on outcomes, including pain and HRQL.
Authors' conclusions
Implications for practice.
Mixed exercise interventions may be effective for individuals with fibromyalgia. Yet, the evidence showed small to moderate effects with considerable variation in the way interventions were designed and delivered. Mixed interventions can be made of a mix of components, which may interact in synergistic or opposing ways, or may be interdependent. It is important for practitioners to understand that this review did not investigate the diversity of interactions among the components. We are unable to draw conclusions on which component, or what combination of exercise components, is more effective.
Mixed exercise interventions that include multiple forms of exercise (e.g. aerobic, resistance, and flexibility) as well as non‐exercise components (e.g. education) have the potential to influence cardiorespiratory, vascular, and neuromusculoskeletal physiology, along with psychological and behavioural factors. We found no additional effect by including an educational component. However, some reviews have found that printed educational materials have a small (but potentially important) effect (Farmer 2008; Grimshaw 2004).
Based upon the significant improvement in CR submax (distance walked in six minutes), it appears that participants in the mixed groups obtained a benefit in physical fitness. Although the evidence for this secondary outcome is rated as poor, this is encouraging.
To facilitate the applicability of results for practitioners, we have provided rich descriptions of settings, implementation details, supervision, intensity, frequency, mode, and, to a degree, context.
Given the multitude of settings and the precise exercise regimens making up the mixed exercise interventions covered in this review, studies within each category are still too few to allow conclusions on specific intervention characteristics (e.g. type of mixed combination, duration of intervention, supervision) that may impact effectiveness.
Future systematic reviews may attempt to evaluate these programmes using techniques that account for these complexities and may evaluate the mechanisms of action and the influence of different settings, contexts, and populations.
Most of the included studies were carried out in developed countries. Critical contextual factors for consideration include the following: individual(s) delivering the intervention, intervention scheduling, communication regarding the intervention, understanding and uptake by the participant, space, resources, materials and equipment, and intervention supervision and monitoring of care. The availability, accessibility, and affordability of any or all of these factors may positively or negatively affect the implementation and sustainability of any mixed exercise intervention.
Implications for research.
Several implications for further research arose from this review. We have used the EPICOT approach to describe these implications (Brown 2006).
Evidence
There were 29 trials meeting our PICO and inclusion criteria; this is clear evidence that this type of intervention is popular and well accepted by individuals with fibromyalgia. As well, the growth in this body of evidence suggests that researchers believe this may be a more effective type of intervention. Investigators need to design better quality trials, with more rigorous methods of allocation concealment and specifications for blinding of participants and professionals involved in the trial. Creation of and agreement on a consistent terminology across studies will be favourable; to date a broad range and considerable variation have been seen in these studies. Evidence on adverse events is dissimilar and often is not reported among studies. This is critical for individuals and practitioners, and new studies should ensure that this information is included.
Population
The participants included were mainly women. It is necessary to clarify the effects of mixed exercise training on males with fibromyalgia
Researchers investigating exercise interventions are encouraged to describe physical fitness levels and physical activity participation of individuals recruited to these studies; baseline values are important for understanding effects of the intervention, follow‐up results, and overall dose response to exercise.
Population mainly consisted of middle‐aged Caucasian women living in developed countries, which make results difficult to generalise to other populations and settings. At the same time, this brings awareness of the need for studies coming from other parts of the world; future research is encouraged to investigate participants of different ages, ethnicities, and countries.
We were unable to perform a subgroup analysis based on age; researchers are encouraged to provide subgroup analysis (e.g. 45 and over or under 45) in their RCTs.
Intervention
Researchers need to provide more and better described information with respect to:
exercise frequency, intensity, time (duration), type (and mode), and progression, to more precisely identify exercise volume and to determine if the prescribed exercise protocols meet current American College of Sports Medicine (ACSM) guidelines.
Supervision, adherence to supervised and unsupervised sessions, and improved descriptions on adherence will allow systematic review authors to identify and compare characteristics that may help to explain the effects of mixed interventions. Accurate measurement of unsupervised physical activity would benefit future research studies. Unsupervised components of exercise interventions (e.g. home programmes) can add a significant amount of total activity to a prescribed programme and thus can potentially affect the impact of a prescribed programme. Although supervised components of the exercise interventions were more comprehensively described, researchers should strive to quantify the volume of both supervised and unsupervised physical activities.
The results of this review reflect the high variability of mixed exercise types and exercise dosages (frequency, intensity, time (duration), outcomes of interest, and follow‐up from none (majority of studies) to 52 weeks).
Ideally, trials should follow the CONSORT guidelines (Schultz 2010). Prescribed mixed exercise interventions and control conditions need to be described in sufficient detail, so researchers can replicate or utilise these interventions. Adequate recording of the types and exact dosage of mixed exercise interventions, based on standard, accepted recommendations (e.g. ACSM), would benefit translating evidence into practice and data pooling. An international standardisation of a 'core outcome measure set' for people with fibromyalgia is needed to improve reporting of outcome effects and to assist in the systematic review process.
Outcome measures related to symptoms were often compatible and we were able to meta‐analyse the data. Outcome measures for physical functioning components (e.g. CR submax, strength) remain heterogeneous, not allowing pooling of the information; research in this area should focus on reaching agreement to support pooling of the information and advancement of knowledge of this particular topic.
Long‐term benefits of exercise interventions are unclear due to relative lack of follow‐up, limited length of follow‐up, or limited follow‐up phase information; future research is encouraged in this area. Determining the long‐term clinical effectiveness and cost‐effectiveness of mixed exercise interventions and how best to ensure that short‐term beneficial effects are maintained over time are important lines of enquiry.
With respect to further research, trials need to better identify how to best support people with fibromyalgia engaging in mixed exercise interventions, which people with fibromyalgia would benefit most from which mixed exercise in general and from which combinations in particular, and which modes of exercise delivery and support would lead to better adherence and improved outcomes.
Comparators
This review included comparisons of mixed training versus non‐exercise interventions (self‐help and education programmes, cognitive‐behavioural training, relaxation, biofeedback, and medications) or other exercise interventions (aerobic exercise, resistance exercise, flexibility exercise), as well as head‐to‐head comparisons of two mixed exercise protocols. We found an insufficient number of studies to adequately evaluate these comparisons.
Although we included 29 studies in this Cochrane Review and we have established the effectiveness of physical activity for individuals with fibromyalgia when compared to controls, we are still unable to know or respond to which intervention is better than the other due to lack of head‐to‐head comparisons. We do not have enough studies to be able to meta‐analyse data in (other than control) comparisons.
Outcomes
Improved reporting of the occurrence of adverse events (injuries, exacerbations of fibromyalgia, and other associated adverse effects) is needed.
Assessment of adherence to the prescribed frequency, duration, and intensity of exercise should be an integral part of all RCTs studying the effects of exercise interventions. Further research is needed to elucidate a dose‐response relationship. Formal follow‐up periods are needed to assess the stability of responses. In addition, further work to validate a set of outcome measures for fibromyalgia research, such as has been initiated by OMERACT, is needed to allow comparisons across studies and elucidation of the more effective interventions. Determination of the minimum clinically important difference and responsiveness of the core measures is also needed.
Timestamp
The need for an update of this review should be considered in three to five years. The utility of future updates of this review will depend on the availability of new, well‐designed (and well‐reported) trials and our ability to recognise, abstract, and analyse important explanatory factors related to mixed interventions for individuals with fibromyalgia.
What's new
| Date | Event | Description |
|---|---|---|
| 13 December 2018 | New citation required and conclusions have changed | Review updated, new trials included |
History
Review first published: Issue 5, 2019
| Date | Event | Description |
|---|---|---|
| 14 June 2008 | New search has been performed | "Exercise for treating fibromyalgia syndrome" review updated and restructured. This review has been split into several reviews, each focusing on a particular type of exercise training or physical activity. This review addresses mixed exercise training. The others are:
|
| 17 August 2007 | New citation required and conclusions have changed | Substantive amendments made. See published notes for details |
Notes
This review is a major update of previous reviews completed in 2002 and 2007.
Acknowledgements
We would like to acknowledge the following.
-
Exercise for Fibromyalgia Cochrane Review Team.
The Review team contributed to various tasks on reviews of exercise and fibromyalgia (screening of citations, abstracts, and full‐text articles for inclusion/exclusion and related consensus activities, data extraction and related consensus activities, regular attendance at monthly meetings, contributing to discussion regarding relevant concepts, interpretation and discussion of results, translation of non‐English articles, and knowledge translation).
Currently the Review Team is led by Angela Busch and is made up of 14 members, including two consumers (Janet Gunderson, Anne Lyddiatt), one librarian/information specialist (Catherine Boden), and nine reviewers (kinesiologists ‐ Julia Bidonde, Suelen Goés, Heather Foulds; physical therapists ‐ Tom Overend, Candice Schachter, Sandra Webber, Kristen Musselman, Susan Tupper, Ina Van Der Spuy, Vanina Dal Bello Haas, Soo Kim).
Former members of the team include the following: information specialist Tamara Rader, physiotherapists Rachel Richards and Laurel Schafer, nutritionist Adrienne Danyliw, consumer Mary Brachaniec, and research assistant Christopher Ross.
We would also like to acknowledge the following.
Louise Falzon for help with the literature search (2002, 2007).
Tanis Kershaw, Joelle Harris, and Saf Malleck for administrative support for the Review Team.
We thank the following authors for their responses: Baptista 2012; Buckelew 1998; Burckhardt 1994; Da Costa 2005; Demir‐Gocmen 2013; Etnier 2009; Giannotti 2014; Hunt 2000; Rivera Redondo 2004; Salaffi 2015; Sanudo 2010b; van Eijk‐Hustings 2013; Yuruk 2008.
Appendices
Appendix 1. Glossary of terms
| Term | Meaning |
| Aerobic (cardiorespiratory) exercise training | Aerobic exercise training primarily affects the circulatory system and the respiratory system. Following aerobic exercise training, the heart pumps out more blood per beat and there are more capillaries available to transfer this blood to the working muscles and to the lungs. In addition, the lungs become more efficient in moving air in and out and in transferring oxygen into the blood and removing carbon dioxide. As a result of these improvements in heart and lung function, people have an increased total work capacity, and they can do a higher rate of work at a given submaximal level (ACSM 2013) |
| Resistance (muscular fitness) training | Resistance training can take several forms, producing more strength, more power, or more endurance in the muscles. The effects of resistance training are seen in the muscles and in their neuromuscular effectors (Ferguson 2014; ACSM 2014 9th Guidelines) |
| Cardiorespiratory fitness | The ability of the cardiovascular and respiratory systems to supply oxygen to muscles during sustained physical activity |
| Cognitive‐behavioural therapy | A form of therapy in which the goal is to diminish symptoms by correcting distorted thinking based on negative self‐perceptions and expectations |
| Companion study | A companion study is a second report of a study’s results focussing on different outcomes than the original study |
| Complex intervention | An intervention comprising multiple components that interact to produce change. Complexity may also relate to the difficulty of behaviours targeted by interventions, the number of organisational levels targeted, or the range of outcomes |
| Concomitant | Existing or concurring with something else |
| Detraining | Losing the physical and health effects gained during exercise training by stopping exercise |
| Exercise | Physical activity that is planned, structured, and repetitive, and [that] has as a final or intermediate objective of improvement or maintenance of physical fitness (Garber 2011) |
| Exercise training | Programme that is designed to meet individual health and physical fitness goals; a single exercise session should include a warm‐up, stretching, conditioning, and cool‐down components. A programme may include an improvement phase during which the work during exercise is gradually progressed (increased) as well as maintenance phases. The rate of progression depends on the individual's health status and exercise tolerance |
| Exercise volume | The total amount of exercise performed, usually expressed per day and per week. ACSM guidelines are based on evidence that certain amounts or volumes of regular exercise produce various physical and health benefits. Exercise volume is used in creating exercise prescriptions that can improve physical fitness and in evaluating whether training programmes have met the guidelines (ACSM 2013; Garber 2011) |
| FITT‐VP principle | A widely accepted approach to classifying and prescribing exercise advocated by the American College of Sports Medicine. The acronym stands for frequency, intensity, type (i.e. mode), time (duration of exercise sessions), volume, and pattern/progression. This classification system can be applied to exercise that can be used to improve or maintain cardiorespiratory (aerobic), muscular, and/or neuromotor fitness |
| Flexibility | The passive or active range of motion at a joint |
| Heart rate reserve (HRR) | Heart rate reserve (HRR) is the difference between resting heart rate (HRrest) and maximum heart rate (HRmax). Heart rate reserve can be used when determining exercise heart rates. Percentage of HRR can be used to describe and categorise intensity of aerobic exercise. |
| Hormones | Any of various internally secreted compounds, such as insulin or thyroxine, formed in endocrine glands that affect the functions of specifically receptive organs or tissues when transported to them by body fluids |
| Inflammatory | Pathology of or caused by inflammation; (inflammation) biological response of body tissues to harmful stimuli like irritants, damaged cells, or pathogens |
| Maximal aerobic performance | Maximum rate of oxygen consumption by the body as measured during incremental exercise |
| Maximum heart rate (HRmax) | The highest number of beats per minute the heart can reach during maximum physical exertion. It is unique to each individual and depends on hereditary factors and age. Maximal heart rate is used when determining exercise heart rates. Percentage of HRmax can be used to describe and categorise intensity of aerobic exercise. HRmax is commonly estimated (predicted HRmax; see below). (Ferguson 2014; ACSM 2014, page 168) rather than measured |
| Exercise‐induced muscle microtrauma | Trauma caused to muscle cells by physical activity |
| Min x d−1 | Minutes per day |
| Muscle strength | The amount of force a muscle can generate |
| Neuromotor exercise | “Neuromotor exercise training involved motor skills such as balance, coordination, gait, and agility” (Ferguson 2014; ACSM 2014 page 189) |
| Neurotransmitters | Any of several chemical substances, such as epinephrine or acetylcholine, that transmit nerve impulses across a synapse to a postsynaptic element (nerve, muscle, or gland) |
| Non‐pharmacological | Treatment that does not include medication |
| OMERACT | OMERACT (Outcome Measures in Rheumatology) is an independent initiative of international health professionals interested in outcome measures in rheumatology. Over the last 20 years, OMERACT has served a critical role in the development and validation of clinical and radiographic outcome measures in rheumatoid arthritis, osteoarthritis, psoriatic arthritis, fibromyalgia, and other rheumatic diseases (www.omeract.org). OMERACT is linked to the Cochrane Collaboration Musculoskeletal Review Group where the outcomes endorsed by OMERACT are recommended for use in Cochrane Systematic Reviews |
| Pathophysiology | The physiology of abnormal or diseased organisms or their parts |
| Pattern | Pattern refers to number of exercise sessions per day and length of rests between sets of exercise (Garber 2011) |
| Perceived exertion | Amount of effort that is perceived by someone during physical activity, usually rated on scales of 6 to 20 or 1 to 10 |
| Physical activity | Any bodily movement produced by skeletal muscles that results in energy expenditure above resting (basal) levels. Physical activity broadly encompasses exercise, sports, and physical activities done as part of daily living, occupation, leisure, and active transportation (Garber 2011) |
| Physical fitness | The ability to carry out daily tasks with vigour and alertness, without undue fatigue and with ample energy to enjoy [leisure] pursuits and to meet unforeseen emergencies. Physical fitness is operationalised as "[a set of] measurable health and skill‐related attributes" |
| Physical function | The capacity of an individual to carry out the physical activities of daily living. Physical function reflects motor function and control, physical fitness, and habitual physical activity and is an independent predictor of functional independence, disability, and morbidity |
| Physiology | The branch of biology dealing with the functions and activities of living organisms and their parts, including all physical and chemical processes |
| Predicted maximum heart rate (HRmax‐p) | Predicted HRmax‐p is an estimate of maximum heart rate (HRmax) using an equation without the need for an individual to perform a maximal stress test (Ferguson 2014 ACSM 2014, page 168). Percentage of predicted HRmax can be used to describe and categorise intensity of aerobic exercise |
| ;Prevalence | Rate of occurrence of a condition, usually expressed on a per year basis |
| Protocol | Study protocols provide a description of the proposed methods for a randomised controlled trial. In this systematic review, the term refers specifically to a published paper describing and delineating the methods planned by researchers for the conduct of an RCT (published RCT study protocol), and also to the methodological details made public through registration of the clinical trial in a trial registry database (trial registry record) |
| Skewness | Not every distribution of data is symmetrical ‐ sets of data that are not symmetrical are said to be asymmetrical. The measure of how asymmetrical a distribution can be is called skewness |
| Sleep disturbance | A score derived from a questionnaire that measures sleep quantity and quality. The Medical Outcomes Survey Sleep Scale measures 6 dimensions of sleep (initiation, staying asleep, quantity, adequacy, drowsiness, shortness of breath, snoring) |
| Somatic comorbidities | Conditions of the body related to a disease |
| Symptoms | Patients' perceptions of an 'abnormal' physical, emotional, or cognitive state |
| Tenderness | Pain evoked by tactile pressure on the skin surface |
| Hyperalgesia, allodynia, paraesthesias | Hyperalgesia ('increased pain'), "an increased response to a stimulus which is normally painful"; allodynia ('other pain'), "pain due to a stimulus which does not normally provoke pain". Thus, allodynia involves a change in quality of sensation, whether touch or heat or cold, for example, paraesthesia ('beyond feeling') is "an abnormal sensation, whether spontaneous or evoked" that is not unpleasant |
| Tender points | A set of specific points on the body surface where pain is registered during testing for fibromyalgia |
| Trial register | A trial register is a searchable database of records of registered trials. "Trial registration [is] the publication of an internationally‐agreed set of information about the design, conduct, and administration of clinical trials" (WHO; http://www.who.int/ictrp/en/). Some trial registers also contain a results database in which researchers can report results of their primary and secondary outcome measures. Also referred to as trial registry |
| Neurohormones | Hormones that stimulate neural mechanisms or are released when activated by neural stimuli |
| Dysregulation | impairment of a physiological regulatory mechanism |
| Exacerbation | Worsening of signs and symptoms |
| Neuromuskuloskeletal | Including components of the nervous system (e.g. peripheral nerves and the brain), the muscular system (muscles and tendons), and the skeletal system (bones) |
| Efficacy | The extent to which an intervention is beneficial under ideal circumstances (when other factors can be controlled, e.g. during research studies) |
Appendix 2. MEDLINE (OVID) search strategy
Fibromyalgia/
fibromyalgi$.tw.
fibrositis.tw.
or/1‐3
exp Exercise/
Physical Exertion/
Physical Fitness/
exp Physical Endurance/
exp Sports/
Pliability/
exertion$.tw.
exercis$.tw.
sport$.tw.
((physical or motion) adj5 (fitness or therapy or therapies)).tw.
(physical$ adj2 endur$).mp. [mp=title, abstract, original title, name of substance word, subject heading word, keyword heading word, protocol supplementary concept word, rare disease supplementary concept word, unique identifier]
manipulat$.tw.
(skate$ or skating).tw.
jog$.tw.
swim$.tw.
bicycl$.tw.
(cycle$ or cycling).tw.
walk$.tw.
(row or rows or rowing).tw.
weight train$.tw.
muscle strength$.tw.
exp Yoga/
yoga.tw.
exp Tai Ji/
tai chi.tw.
ai chi.tw.
exp Vibration/
vibration.tw.
pilates.tw.
or/5‐33
4 and 34
Appendix 3. Embase (OVID) search strategy
FIBROMYALGIA/
fibromyalgi$.tw.
fibrositis.tw.
or/1‐3
exp exercise/
fitness/
exercise tolerance/
exp sport/
pliability/
exertion$.tw.
exercis$.tw.
sport$.tw.
((physical or motion) adj5 (fitness or therapy or therapies)).tw.
(physical$ adj2 endur$).tw.
manipulat$.tw.
(skate$ or skating).tw.
jog$.tw.
swim$.tw.
bicycl$.tw.
(cycle$ or cycling).tw.
walk$.tw.
(row or rows or rowing).tw.
weight train$.tw.
muscle strength$.tw.
or/5‐24
4 and 25
(random$ or placebo$).ti,ab.
((single$ or double$ or triple$ or treble$) and (blind$ or mask$)).ti,ab.
controlled clinical trial$.ti,ab.
RETRACTED ARTICLE/
or/27‐30
(animal$ not human$).sh,hw.
31 not 32
26 and 33
Appendix 4. Cochrane Library (Wiley) search strategy
#1 MeSH descriptor: [Exercise] explode all trees
#2 MeSH descriptor: [Exercise Therapy] explode all trees
#3 MeSH descriptor: [Physical Therapy Modalities] explode all trees
#4 exercise:ti,ab
#5 MeSH descriptor: [Physical Fitness] explode all trees
#6 MeSH descriptor: [Exercise Tolerance] explode all trees
#7 MeSH descriptor: [Sports] explode all trees
#8 MeSH descriptor: [Pliability] explode all trees
#9 MeSH descriptor: [Physical Exertion] explode all trees
#10 MeSH descriptor: [Motion] explode all trees
#11 MeSH descriptor: [Physical Endurance] explode all trees
#12 swim:ti,ab
#13 skate:ti,ab
#14 jog:ti,ab
#15 bike:ti,ab
#16 cycle:ti,ab
#17 walk:ti,ab
#18 row:ti,ab
#19 weight train:ti,ab
#20 muscle strength:ti,ab
#21 #1 or #2 or #3 or #4 or #5 or #6 or #7 or #8 or #9 or #10 or #11 or #12 or #13 or #14 or #15 or #16 or #17 or #18 or #19 or #20
#22 MeSH descriptor: [Fibromyalgia] explode all trees
#23 fibromyalgia:ti,ab
#24 #22 or #23
#25 #21 and #24
Appendix 5. CINAHL (Ebscohost) search strategy
S01 (MH "Fibromyalgia")
S02 TI fibromyalgia or AB fibromyalgia
S03 TI fibrositis or AB fibrositis
S04 (MH "Exercise+")
S05 (MH "Exertion+")
S06 (MH "Physical Fitness")
S07 (MH "Exercise Test+")
S08 (MH "Sports+")
S09 (MH "Pliability")
S10 (MH "Physical Endurance+")
S11 TI exertion* or AB exertion*
S12 TI exercis* or AB exercis*
S13 TI sport* or AB sport*
S14 TI physical N5 fitness or TI physical N5 therapy or TI physical N5 therapies or AB physical N5 fitness or AB physical N5 therapy or AB physical N5 therapies
S15 TI motion N5 fitness or TI motion N5 therapy or TI motion N5 therapies or AB motion N5 fitness or AB motion N5 therapy or AB motion N5 therapies
S16 TI physical* N2 endur* or AB physical* N2 endur*
S17 ( skate* or skating ) or AB ( skate* or skating )
S18 TI jog* or AB jog*
S19 TI swim* or AB swim*
S20 TI bicycl* or AB bicycl*
S21 TI ( (cycle* or cycling) ) or AB ( (cycle* or cycling) )
S22 TI walk* or AB walk*
S23 TI (row or rows or rowing ) or AB ( row or rows or rowing )
S24 TI weight train* or AB weight train*
S25 TI muscle strength* or AB muscle strength*
S26 TI manipulat* or AB manipulat*
S27 MH "Yoga") OR (MH "Yoga Pose")
S28 TX yoga
S29 TX tai chi
S30 (MM "Tai Chi")
S31 TX tai ji
S32 TX pilates
S33 (MH "Pilates") OR "pilates"
S34 (MH "Vibration")
S35 TX vibration
S36 S1 OR S2 OR S3
S37 S4 OR S5 OR S6 OR S7 OR S8 OR S9 OR S10 OR S11 OR S12 OR S13 OR S14 OR S15 OR S16 OR S17 OR S18 OR S19 OR S20 OR S21 OR S22 OR S23 OR S24 OR S25 OR S26 OR S27 OR S28 OR S29 OR S30 OR S31 OR S32 OR S33 OR S34 OR S35
S38 S36 AND S37
Appendix 6. PEDro Physiotherapy Evidence Database (http://www.pedro.org.au/) search strategy
fibromyalg* AND fitness training
fibromyalg* AND strength training
fibrositis
Appendix 7. Dissertation Abstracts (ProQuest) search strategy
Terms searched fibromyalg* or fibrositis (in citation or abstract)
Appendix 8. Current Controlled Trials & ClinicalTrials.gov (http://clinicaltrials.gov) search strategy
Terms searched fibromyalg* or fibrositis
Appendix 9. WHO International Clinical Trials Registry Platform (http://www.who.int/ictrp/en/) search strategy
Terms searched fibromyalg* or fibrositis in Condition
Appendix 10. AMED (OVID) Allied and Complementary Medicine search strategy
OVID AMED (Allied and Complementary Medicine) <1985 to December 2015>
Fibromyalgia/
fibromyalgi$.tw.
fibrositis.tw.
or/1‐3
exp exercise/
physical fitness/
exp physical endurance/
exp sports/
Pliability/
exertion$.tw.
exercis$.tw.
sport$.tw.
((physical or motion) adj5 (fitness or therapy or therapies)).tw.
(physical$ adj2 endur$).tw.
manipulat$.tw.
(skate$ or skating).tw.
jog$.tw.
swim$.tw.
bicycl$.tw.
(cycle$ or cycling).tw.
walk$.tw.
(row or rows or rowing).tw.
weight train$.tw.
muscle strength$.tw.
exp pilates/
exp yoga/
Tai chi/
tai ji.tw.
yoga.tw.
(hatha or kundalini or ashtanga or bikram).tw.
pilates.tw.
exp exercise therapy/
or/5‐32
4 and 33
Appendix 11. Screening criteria
Level One screen
Based on the title and abstract of the report:
Does the study deal exclusively with fibromyalgia? No – exclude, Yes or uncertain ‐ go to step two
Does it include exercise? No – exclude, Yes or uncertain – go to step two
Does the study deal exclusively with adults? No – exclude, Yes or uncertain – go to step two
Is it an RCT? No – exclude, Yes or uncertain – go to step two
Level Two screen
Based on the full text of the report or protocol:
Does the study deal exclusively with fibromyalgia? No – exclude, Yes ‐ go to step three, Uncertain – add to list of questions for author and proceed to step three
Is the diagnosis of fibromyalgia based on published criteria? No – exclude, Yes ‐ include, Uncertain ‐ contact author and proceed to step three
Does the study deal exclusively with adults? No – exclude, Yes ‐ go onto step three, Uncertain ‐ contact author and proceed to step three
Is it an RCT (the study uses terms such as "random", "randomized", "RCT", or "randomization" to describe the study design or assignment of subjects to groups)? No – exclude, Yes ‐ go onto step three, Uncertain ‐ add to list of questions for author and proceed to step three,
Does it include at least one physical activity or exercise intervention? No – exclude, Yes – go onto step three, Uncertain ‐ add to list of questions for author and proceed to step three
Are between‐group data provided for the outcomes? No (the study does not contain only fibromyalgia, or results are reported such that effects on fibromyalgia cannot be isolated) – exclude, Yes – include the study, Uncertain about one or more of steps 1 to 3 – reserve judgement until authors are contacted.
Level three screen (Classification of interventions in the included studies)
-
Classification of design
Number of interventions
-
Types of comparisons
Head‐to‐head comparison?
Exercise to control?
etc.
-
Control group
Classify type of control
-
Exercise
Enter the type of exercise interventions used in the study
Complete the naming of the intervention groups
Appendix 12. 2011 ACSM position stand: guidance for prescribing exercise
The following recommendations are from Garber 2011
Recommendations for cardiorespiratory fitness
Moderate‐intensity cardiorespiratory exercise training for ≥ 30 minutes/d on ≥ 5 days per week for a total of ≥ 150 minutes per week, vigorous‐intensity cardiorespiratory exercise training for ≥ 20 minutes/d on ≥ 3 days per week (≥75 minutes/week), or a combination of moderate‐ and vigorous‐intensity exercise to achieve a total energy expenditure of ≥ 500 to 1000 MET min/week
Recommendations for muscular fitness
On two to three days per week, adults should also perform resistance exercises for each of the major muscle groups, and neuromotor exercise involving balance, agility, and co‐ordination
Two to four sets of resistance exercise per muscle group is recommended but even a single set of exercise may significantly improve muscle strength and size
Rest interval between sets if more than one set is performed: two to three minutes
Resistance equivalent of 60% to 80% of one repetition max (1RM) effort. For novices, 60% to 70% of 1RM is recommended; for experienced exercisers, ≥ 80% may be appropriate
The selected resistance should permit the completion of 8 to 12 repetitions per set or the number needed to induce muscle fatigue but not exhaustion
For people who wish to focus on improving muscular endurance, a lower intensity (< 50% of 1RM) can be used with 15 to 25 repetitions in no more than two sets
Recommendations for flexibility
A series of flexibility exercises for each major muscle‐tendon group with a total of 60 seconds per exercise on ≥ 2 days per week is recommended. A series of exercises targeting the major muscle‐tendon units of the shoulder girdle, chest, neck, trunk, lower back, hips, posterior and anterior legs, and ankles is recommended. For most individuals, this routine can be completed within 10 minutes
Stretches should be held for 1 to 30 seconds at the point of tightness or slight discomfort. Older persons may realise greater improvements in range of motion with longer stretching durations (30 to 60 seconds). A 20% to 75% maximum contraction held for three to six seconds followed by a 10‐ to 30‐second assisted stretch is recommended for PNF techniques
Repeating each flexibility exercise two to four times is effective
Data and analyses
Comparison 1. MX vs Control.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 HRQL | 13 | 610 | Mean Difference (IV, Random, 95% CI) | ‐6.95 [‐10.51, ‐3.38] |
| 1.1 MX‐only vs CG | 9 | 412 | Mean Difference (IV, Random, 95% CI) | ‐8.38 [‐13.00, ‐3.75] |
| 1.2 MX+ED vs C | 4 | 198 | Mean Difference (IV, Random, 95% CI) | ‐4.47 [‐10.44, 1.49] |
| 2 HRQL_S1 Selection bias | 5 | 276 | Mean Difference (IV, Random, 95% CI) | ‐7.28 [‐10.88, ‐3.68] |
| 3 HRQL_S2 Attrition Bias | 10 | 486 | Mean Difference (IV, Random, 95% CI) | ‐6.97 [‐11.26, ‐2.68] |
| 4 Pain | 15 | 832 | Mean Difference (IV, Random, 95% CI) | ‐5.17 [‐8.85, ‐1.48] |
| 4.1 MX‐only vs CG | 10 | 487 | Mean Difference (IV, Random, 95% CI) | ‐7.01 [‐10.64, ‐3.38] |
| 4.2 MX+Ed vs C | 5 | 345 | Mean Difference (IV, Random, 95% CI) | ‐1.32 [‐9.01, 6.37] |
| 5 Pain_S1 Selection Bias | 4 | 216 | Mean Difference (IV, Random, 95% CI) | ‐4.75 [‐13.76, 4.27] |
| 6 Pain_S2 Attrition Bias | 12 | 693 | Mean Difference (IV, Random, 95% CI) | ‐4.74 [‐8.09, ‐1.38] |
| 7 Fatigue | 11 | 493 | Mean Difference (IV, Random, 95% CI) | ‐12.93 [‐17.79, ‐8.07] |
| 7.1 MX‐only vs C | 9 | 399 | Mean Difference (IV, Random, 95% CI) | ‐13.67 [‐19.44, ‐7.91] |
| 7.2 MX+ED vs C | 2 | 94 | Mean Difference (IV, Random, 95% CI) | ‐9.54 [‐18.78, ‐0.29] |
| 8 Stiffness | 5 | 261 | Mean Difference (IV, Random, 95% CI) | ‐6.51 [‐12.28, ‐0.74] |
| 8.1 MX‐only vs C | 3 | 167 | Mean Difference (IV, Random, 95% CI) | ‐8.86 [‐16.47, ‐1.25] |
| 8.2 MX+ED vs C | 2 | 94 | Mean Difference (IV, Random, 95% CI) | ‐3.32 [‐12.18, 5.55] |
| 9 Physical Function | 9 | 477 | Mean Difference (IV, Random, 95% CI) | ‐10.99 [‐14.80, ‐7.18] |
| 9.1 MX‐only vs C | 6 | 311 | Mean Difference (IV, Random, 95% CI) | ‐12.77 [‐17.63, ‐7.90] |
| 9.2 MX+ED vs C | 3 | 166 | Mean Difference (IV, Random, 95% CI) | ‐7.86 [‐13.71, ‐2.00] |
| 10 All‐Cause Withdrawal | 19 | 1065 | Risk Ratio (M‐H, Random, 95% CI) | 1.02 [0.69, 1.51] |
| 10.1 MX‐only vs C | 13 | 661 | Risk Ratio (M‐H, Random, 95% CI) | 1.08 [0.61, 1.93] |
| 10.2 MX+ED vs C | 6 | 404 | Risk Ratio (M‐H, Random, 95% CI) | 1.08 [0.56, 2.10] |
| 11 Cardio Resp (Submax) | 5 | 306 | Mean Difference (IV, Random, 95% CI) | 52.77 [34.11, 71.43] |
| 11.1 MX‐only+ED | 2 | 83 | Mean Difference (IV, Random, 95% CI) | 68.30 [34.41, 102.19] |
| 11.2 MX+ED vs C | 3 | 223 | Mean Difference (IV, Random, 95% CI) | 46.01 [23.65, 68.36] |
| 12 Muscle Strength | 4 | 163 | Std. Mean Difference (IV, Random, 95% CI) | 0.74 [‐0.67, 2.14] |
Comparison 2. MX vs Control ‐ long‐term.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 HRQL | 6 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.1 6 to 12 weeks | 1 | 32 | Mean Difference (IV, Random, 95% CI) | ‐10.5 [‐17.48, ‐3.52] |
| 1.2 13 to 26 weeks | 4 | 224 | Mean Difference (IV, Random, 95% CI) | ‐8.44 [‐15.22, ‐1.66] |
| 1.3 27 to 52 weeks | 2 | 146 | Mean Difference (IV, Random, 95% CI) | ‐5.29 [‐11.42, 0.84] |
| 2 Pain | 6 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 2.1 6 to 12 weeks | 1 | 53 | Mean Difference (IV, Random, 95% CI) | ‐5.0 [‐15.50, 5.50] |
| 2.2 13 to 26 weeks | 2 | 111 | Mean Difference (IV, Random, 95% CI) | ‐4.80 [‐14.25, 4.65] |
| 2.3 27 to 52 weeks | 5 | 408 | Mean Difference (IV, Random, 95% CI) | ‐8.33 [‐19.03, 2.36] |
| 2.4 > 52 weeks | 1 | 53 | Mean Difference (IV, Random, 95% CI) | ‐5.0 [‐14.16, 4.16] |
| 3 Fatigue | 3 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 3.1 13 to 26 weeks | 2 | 112 | Mean Difference (IV, Random, 95% CI) | ‐6.48 [‐16.25, 3.29] |
| 3.2 27 to 52 weeks | 1 | 67 | Mean Difference (IV, Random, 95% CI) | ‐15.00 [‐29.07, ‐0.93] |
| 4 Stiffness | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 4.1 13 to 26 weeks | 1 | 32 | Mean Difference (IV, Random, 95% CI) | 6.80 [‐9.39, 22.99] |
| 4.2 27 to 52 weeks | 1 | 67 | Mean Difference (IV, Random, 95% CI) | ‐14.0 [‐29.80, 1.80] |
| 5 Physical Function | 4 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 5.1 6 to 12 weeks | 1 | 53 | Mean Difference (IV, Random, 95% CI) | ‐18.0 [‐31.74, ‐4.26] |
| 5.2 13 to 26 weeks | 3 | 179 | Mean Difference (IV, Random, 95% CI) | ‐8.13 [‐18.24, 1.97] |
| 5.3 27 to 52 weeks | 1 | 53 | Mean Difference (IV, Random, 95% CI) | ‐20.0 [‐31.85, ‐8.15] |
| 5.4 > 52 weeks | 1 | 53 | Mean Difference (IV, Random, 95% CI) | ‐21.0 [‐33.41, ‐8.59] |
| 6 CV Submax | 3 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 6.1 13 to 26 weeks | 3 | 145 | Mean Difference (IV, Random, 95% CI) | 61.71 [15.37, 108.05] |
Comparison 3. MX vs other non‐Ex.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 HRQL | 7 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.1 HRQL: MX vs Self‐Help Programme | 1 | 97 | Mean Difference (IV, Random, 95% CI) | ‐4.81 [‐11.41, 1.79] |
| 1.2 HRQL: MX+ED vs ED | 1 | 56 | Mean Difference (IV, Random, 95% CI) | 6.10 [‐1.73, 13.93] |
| 1.3 HRQL: MX vs Relaxation | 1 | 38 | Mean Difference (IV, Random, 95% CI) | ‐4.51 [‐13.08, 4.07] |
| 1.4 HRQL: MX vs Biofeedback | 1 | 82 | Mean Difference (IV, Random, 95% CI) | 0.80 [‐2.97, 4.57] |
| 1.5 HRQL: MX vs Med | 2 | 231 | Mean Difference (IV, Random, 95% CI) | 0.72 [‐5.67, 7.11] |
| 1.6 HRQL: MX vs Cogniive‐Behavioural Training | 1 | 40 | Mean Difference (IV, Random, 95% CI) | ‐3.5 [‐12.24, 5.24] |
| 2 Pain Intensity | 6 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 2.1 Pain: MX vs Self‐Help Programme | 1 | 97 | Mean Difference (IV, Random, 95% CI) | ‐8.93 [‐18.77, 0.92] |
| 2.2 Pain: MX vs Cognitive‐Behavioural Therapy | 1 | 40 | Mean Difference (IV, Random, 95% CI) | ‐4.0 [‐19.84, 11.84] |
| 2.3 Pain: MX+ED vs Ed | 1 | 56 | Mean Difference (IV, Random, 95% CI) | 11.0 [‐2.63, 24.63] |
| 2.4 Pain: MX vs Biofeedback | 2 | 135 | Mean Difference (IV, Random, 95% CI) | ‐2.35 [‐9.59, 4.88] |
| 2.5 Pain: MX vs Medication | 1 | 75 | Mean Difference (IV, Random, 95% CI) | 3.00 [‐9.79, 15.79] |
| 3 Fatigue | 5 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 3.1 Fatigue: MX vs Self‐Help Programme | 1 | 96 | Mean Difference (IV, Random, 95% CI) | ‐4.00 [‐14.54, 2.54] |
| 3.2 Fatigue: MX vs Cognitive‐Behavioural Therapy | 1 | 40 | Mean Difference (IV, Random, 95% CI) | ‐7.0 [‐22.67, 8.67] |
| 3.3 Fatigue: MX+ED vs Ed | 1 | 56 | Mean Difference (IV, Random, 95% CI) | 10.0 [‐3.71, 23.71] |
| 3.4 Fatigue: MX vs Biofeedback | 1 | 82 | Mean Difference (IV, Random, 95% CI) | 7.0 [‐0.16, 14.16] |
| 3.5 Fatigue: MX vs Med | 1 | 75 | Mean Difference (IV, Random, 95% CI) | ‐6.10 [‐18.81, 6.61] |
| 4 Stiffness | 4 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 4.1 Stiffness: MX vs Self‐Help Programme | 1 | 99 | Mean Difference (IV, Random, 95% CI) | ‐8.52 [‐18.87, 1.83] |
| 4.2 Stiffness: MX vs Cognitive‐Behavioural Therapy | 1 | 40 | Mean Difference (IV, Random, 95% CI) | 4.0 [‐13.98, 21.98] |
| 4.3 Stiffness: MX+ED vs Ed | 1 | 56 | Mean Difference (IV, Random, 95% CI) | 5.0 [‐8.71, 18.71] |
| 4.4 Stiffness: MX vs Med | 1 | 75 | Mean Difference (IV, Random, 95% CI) | 0.5 [‐12.61, 13.61] |
| 5 Physical Function | 5 | Std. Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 5.1 PF: MX vs Self‐Help Programme | 1 | 97 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.40 [‐0.84, 0.05] |
| 5.2 PF: MX vs Cognitive‐Behavioural Training | 1 | 41 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.11 [‐0.50, 0.73] |
| 5.3 PF: MX+ED vs Ed | 1 | 56 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.04 [‐0.57, 0.48] |
| 5.4 PF: MX vs Biofeedback | 2 | 136 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.08 [‐0.41, 0.26] |
| 6 All‐Cause Withdrawal | 8 | 847 | Risk Difference (M‐H, Random, 95% CI) | ‐0.00 [‐0.05, 0.04] |
| 6.1 MX only vs ED, SMT, CBT | 6 | 424 | Risk Difference (M‐H, Random, 95% CI) | 0.00 [‐0.03, 0.03] |
| 6.2 MX only vs Biofeedback | 2 | 148 | Risk Difference (M‐H, Random, 95% CI) | ‐0.06 [‐0.16, 0.04] |
| 6.3 MX only vs Meds | 2 | 275 | Risk Difference (M‐H, Random, 95% CI) | ‐0.02 [‐0.27, 0.24] |
3.1. Analysis.
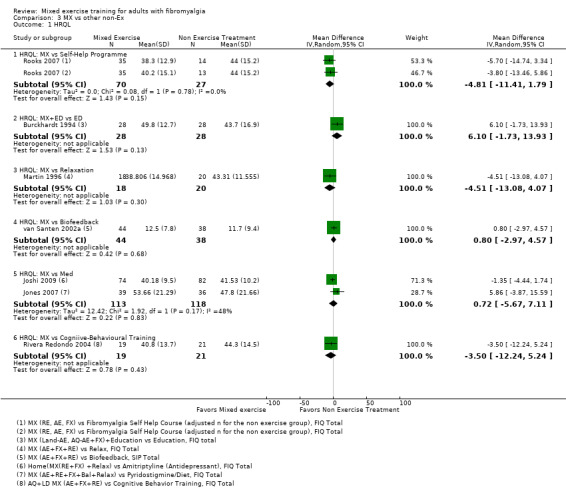
Comparison 3 MX vs other non‐Ex, Outcome 1 HRQL.
3.2. Analysis.
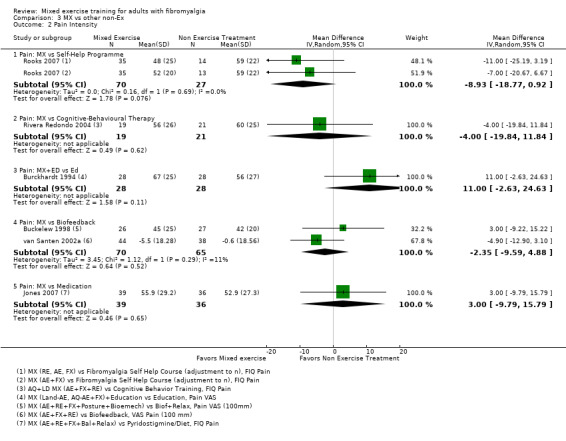
Comparison 3 MX vs other non‐Ex, Outcome 2 Pain Intensity.
3.3. Analysis.
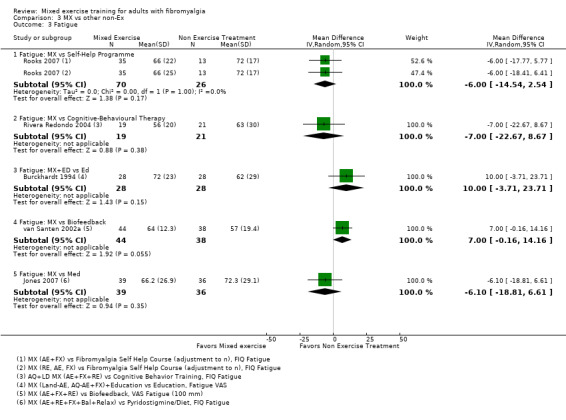
Comparison 3 MX vs other non‐Ex, Outcome 3 Fatigue.
3.4. Analysis.
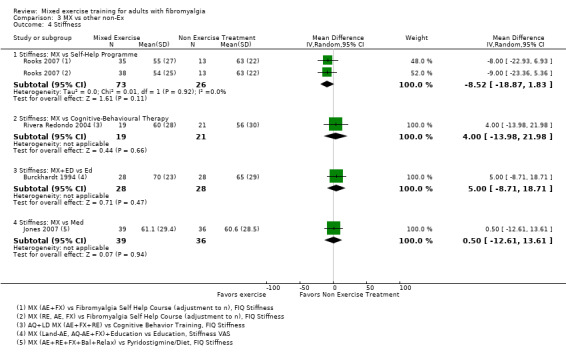
Comparison 3 MX vs other non‐Ex, Outcome 4 Stiffness.
3.5. Analysis.
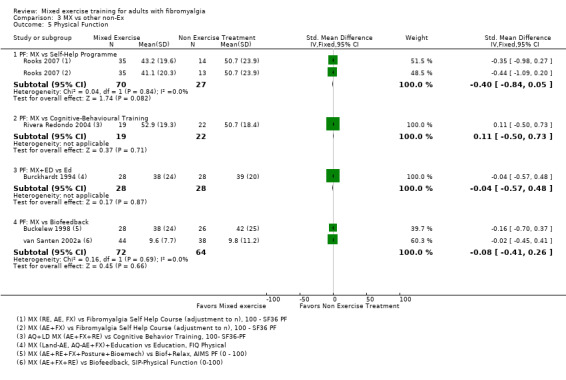
Comparison 3 MX vs other non‐Ex, Outcome 5 Physical Function.
3.6. Analysis.
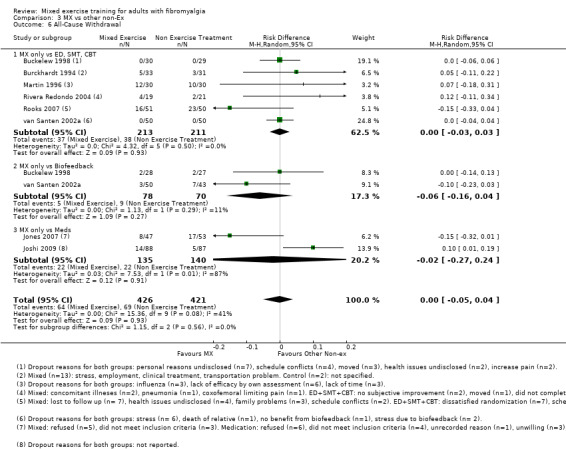
Comparison 3 MX vs other non‐Ex, Outcome 6 All‐Cause Withdrawal.
Comparison 4. MX vs other Ex.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 MX vs AE | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.1 HRQL | 1 | 43 | Mean Difference (IV, Random, 95% CI) | 0.80 [‐8.64, 10.24] |
| 1.2 Pain | 2 | 73 | Mean Difference (IV, Random, 95% CI) | 4.61 [‐3.16, 12.38] |
| 1.3 Fatigue | 1 | 43 | Mean Difference (IV, Random, 95% CI) | ‐3.70 [‐13.10, 5.70] |
| 1.4 Physical Function | 2 | 73 | Mean Difference (IV, Random, 95% CI) | 1.76 [‐9.54, 13.05] |
| 1.5 Cardiovascular Submax | 1 | 43 | Mean Difference (IV, Random, 95% CI) | 21.60 [‐20.98, 64.18] |
| 1.6 Strength | 1 | 43 | Mean Difference (IV, Random, 95% CI) | 1.30 [‐1.53, 4.13] |
| 2 MX vs Remedial Ex | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 2.1 HRQL | 1 | 32 | Mean Difference (IV, Random, 95% CI) | 3.59 [‐1.89, 9.07] |
| 3 MX vs HPrg (FX) | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 3.1 HRQL | 1 | 43 | Mean Difference (IV, Random, 95% CI) | ‐6.82 [‐22.12, 8.48] |
| 3.2 Pain | 1 | 43 | Mean Difference (IV, Random, 95% CI) | ‐4.60 [‐18.03, 8.83] |
| 4 MX (AE+FX) vs MX (RE+AE+FX) | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 4.1 HRQL | 1 | 70 | Mean Difference (IV, Random, 95% CI) | 1.90 [‐4.68, 8.48] |
| 4.2 Pain | 1 | 70 | Mean Difference (IV, Random, 95% CI) | ‐4.0 [‐14.61, 6.61] |
| 4.3 Fatigue | 1 | 70 | Mean Difference (IV, Random, 95% CI) | 0.0 [‐11.03, 11.03] |
| 4.4 Stiffness | 1 | 70 | Mean Difference (IV, Random, 95% CI) | 3.0 [‐9.19, 15.19] |
| 4.5 Physical Function | 1 | 70 | Mean Difference (IV, Random, 95% CI) | ‐2.10 [‐11.45, 7.25] |
| 4.6 Cardiovascular Submax | 1 | 70 | Mean Difference (IV, Random, 95% CI) | ‐19.0 [‐52.29, 14.29] |
| 5 MX (Callisthenics+AE+FX) vs MX (RE+FX+Posture) | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 5.1 HRQL | 1 | 27 | Mean Difference (IV, Random, 95% CI) | ‐2.20 [‐11.81, 7.41] |
| 5.2 Pain | 1 | 27 | Mean Difference (IV, Random, 95% CI) | ‐13.00 [‐26.29, 0.29] |
| 5.3 Fatigue | 1 | 27 | Mean Difference (IV, Random, 95% CI) | ‐9.0 [‐25.65, 7.65] |
| 5.4 Stiffness | 1 | 27 | Mean Difference (IV, Random, 95% CI) | ‐11.0 [‐28.16, 6.16] |
| 5.5 Physical Function | 1 | 27 | Mean Difference (IV, Random, 95% CI) | 10.0 [‐0.30, 20.30] |
| 6 All‐Cause Withdrawal | 6 | 287 | Risk Difference (M‐H, Random, 95% CI) | ‐0.01 [‐0.07, 0.05] |
| 6.1 MX vs AE‐only | 2 | 76 | Risk Difference (M‐H, Random, 95% CI) | 0.00 [‐0.10, 0.10] |
| 6.2 MX vs Remedial Ex | 1 | 32 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [‐0.11, 0.11] |
| 6.3 MX vs HomePrg (FX) | 1 | 50 | Risk Difference (M‐H, Random, 95% CI) | ‐0.12 [‐0.31, 0.07] |
| 6.4 MX (AE+FX) vs MX (RE+AE+FX) | 1 | 102 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [‐0.18, 0.18] |
| 6.5 MX (Callisthenics+AE+FX) vs MX (RE+FX+Posture) | 1 | 27 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [‐0.13, 0.13] |
4.6. Analysis.
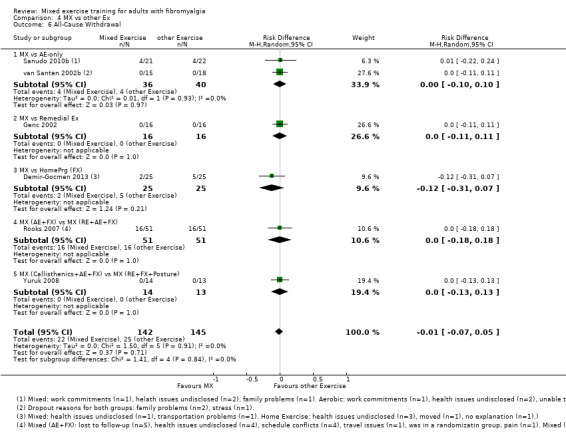
Comparison 4 MX vs other Ex, Outcome 6 All‐Cause Withdrawal.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Alentorn‐Geli 2008.
| Methods | 3 groups: (a) mixed exercise (AE+FX+Relax)+Vib, (b) mixed exercise (AE+FX+Relax)+placebo Vib, (c) control (medication as usual) Length: 6 weeks. Follow‐up: none Study design: randomised clinical trial with parallel groups |
|
| Participants | Female:Male: 33:0 Age, years: (a) 55.2 (SE 3.4), (b) 53.7 (SE 2.7), (c) 59.3 (SE 2.3) Duration of Illness, years: 9.8 (SE 0.8) to 10.5 (SE 0.8) Inclusion: women, diagnosis of FM (ACR 1990) for at least 3 years Exclusion: any orthopaedic limitation or cardiovascular, pulmonary, or metabolic disease that would preclude exercise |
|
| Interventions | (a)Mixed exercise, relaxation, vibration (n = 11): total duration (over 12 sessions) of aerobic exercise, stretching, and relaxation was 9 hours, 6 hours, and 4 hours, respectively: (1) exercise protocol ‐ Frequency: 2/week; Duration: 90 min (WU 15 min, AE 30 min, FX 25 min, Relax 20 min), Intensity: AE moderate to vigorous intensity (65% to 85% HRmax); FX to stop point; Mode: AE: primarily level ground walking with games dance; FX: 5 × 5 whole body stretches, 30 s hold, 30 s relax, involving hamstrings, calves, Achilles tendons, shoulders, arms, gluteals, cervical spine, low back, upper back, chest, hip adductors, (2) vibration exercise ‐ Frequency: 2/week; Duration: 4.5 min sessions 1 min and 2 min, 18 min sessions 3 to 12; Intensity: body weight resistance; Mode: six 30 s lower extremity exercises (static and dynamic), vibratory stimulus: vibration frequency 30 Hz with 2 mm amplitude; (3) relaxation exercise ‐ Mode: diaphragmatic respiration, progressive muscular relaxation, contraction – relaxation, imagery techniques, pharmacological care as usual* (b) Mixed exercise, relaxation, placebo vibration (n = 12): (1) exercise protocol ‐ as per group (a), (2) placebo vibration ‐ as per group (a) but the apparatus did not produce vibrations, (3) relaxation exercise ‐ as per group (a) (c)Control (n = 10): pharmacological care as usual |
|
| Outcomes | Health‐related quality of life (FIQ Total), pain (FIQ), fatigue (FIQ), stiffness (FIQ), depression (FIQ) Measurements: 0 and 6 weeks |
|
| Adherence | Group (a) attendance = 93%, group (b) attendance = 92% | |
| Congruence of EX protocol with ACSM criteria for aerobic, strength or flexibility | Groups (a) and (b): AE exercise did not meet ACSM criteria based on frequency; FX: met the criteria | |
| Notes | Country: Spain Language: English Author contact: study author contacted by email; no response Data extraction: point estimates and variability estimates extrapolated from graphs Trial registry record or protocol available: none related to this study Conflict of interest: none reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to permit judgement: "Women were randomized into three treatment groups" (page 976) |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgment of risk |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Although authors do not specify blinding of participants, reviewers considered authors' steps to prevent knowledge of the group intervention as low risk In addition to implementing a "sham" intervention (vibratory apparatus was turned on yet did not produce vibration) (page 977), steps were taken to reduce contact between intervention groups. In addition, "The administration and analysis of the questionnaires were performed by an investigator who was blind to the treatment group" (page 977). Individuals not aware of placebo effect: "We informed both EVG and EG that they would receive a perceptible and imperceptible vibratory stimulus, respectively, thus maintaining the potential of a placebo effect consistent in both groups" (page 977) |
| Detection Bias ‐ Subjective measures All outcomes | Low risk | All outcomes (HRQL, pain intensity, fatigue, stiffness) were self‐reported |
| Detection Bias ‐ Blinding of assessor reported outcomes All outcomes | Low risk | Not applicable; no assessor‐reported tests were applied to measure cardiorespiratory submaximal function or muscle strength |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Attrition rates: (a) = 1/12 (8%), (b) = 0/12 (0%), (c) = 2/12 (17%). Attrition was attributed to "a no‐show on testing day"; ITT analysis not utilised; unlikely that attrition affected the results |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement of risk |
| Other bias | Low risk | Study appears to be free of other sources of bias |
Baptista 2012.
| Methods | 2 groups: (a) mixed exercise (dance = AE+RT), (b) control Length: 16 weeks. Follow‐up: 16 weeks Study design: randomised clinical trial with parallel groups |
|
| Participants | Female:Male: 80:0 Age, years (SD): (a) 49.5 (11.0), (b) 49.1 (11.5) Duration of Illness: not specified Inclusion: women between ages 18 and 65 years, diagnosis of FM (ACR1990), no change in treatment over 4 weeks before study entry, provided informed consent Exclusion: other rheumatic diseases, painful joint diseases, uncontrolled cardiopulmonary diseases, diseases of the lower limbs, uncontrolled diabetes |
|
| Interventions | (a)Mixed exercise (dance) (n = 40) Frequency: supervised group programme 2/week plus home programme 2/week from week 4 to 16; Duration: supervised programme 60 min (WU 5 min, dance 45 min, cool‐down 10 min), home programme at least 30'; Intensity: not specified; Mode: belly dance (classified by reviewers to be a mixed programme including AE+ST) (b)Control (n = 40): wait‐list. The control group did not receive any intervention |
|
| Outcomes | Health‐related quality of life (FIQ Total), self‐reported physical function (SF‐36), mental health (SF‐36), pain (VAS, SF‐36), fatigue (SF‐36), depression (BDI), Anxiety (State‐Trait Anxiety Inventory, Parts 1 and 2), cardiorespiratory function submax (6MWT); other: social and emotional health, self‐image Measurements: 0, 16, and 32 weeks |
|
| Adherence | Attendance was used to gauge adherence to supervised sessions during the intervention (median attendance = 26.4 of 32 sessions). Home programme performance was not evaluated | |
| Congruence of EX protocol with ACSM criteria for aerobic, strength or flexibility | Insufficient information to permit judgement | |
| Notes | Country: Brazil Language: English Author contact: email (2 September 2013) from author provided SD for ages, details of the exercise protocol, median attendance Trial registry record or protocol available: NCT00961805; clinicaltrials.gov Funding source: CAPES scholarship Conflict of interest: none reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer random number generator used |
| Allocation concealment (selection bias) | Low risk | Sequentially numbered, opaque, sealed envelopes used |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Email response: participants were not blinded |
| Detection Bias ‐ Subjective measures All outcomes | High risk | Self‐report instruments were used to measure health‐related quality of life, pain, fatigue, and physical function |
| Detection Bias ‐ Blinding of assessor reported outcomes All outcomes | Low risk | Submaximal cardiorespiratory function test was performed by a physiotherapist trained in administering the tests who was blinded to group assignment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | ITT analysis utilised. In cases in which treatment was interrupted, participants were asked to come in and undergo the evaluations; if participants did not attend, the method of adjusting for missing data was the "last observation carried forward" technique" |
| Selective reporting (reporting bias) | Low risk | Based on the ClinicalTrials.gov study protocol, all outcomes were accounted for in the final report |
| Other bias | Low risk | Study appears to be free of other sources of bias |
Buckelew 1998.
| Methods | 4 groups: (a) biofeedback/relaxation training, (b) MX (AE+RT+FX + posture + biomechanics + instruction in use of hot and cold modalities and massage), (c) biofeedback/relaxation + MX (AE+RT+FX + posture + biomechanics + instruction in use of hot and cold modalities and massage), (d) education/attention control Length: Phase 1 (active) 6 weeks, Phase 2 (maintenance) 104 weeks. Follow‐up: none Study design: randomised clinical trial with parallel groups |
|
| Participants | Female:Male: 108:11 Age, years (SD): (a) 44.1 (9.6), (b) 45.6 (9.4), (c) 41.9 (8.1), (d) 44.3 (11.2) Duration of Illness, years (SD): (a) 11.6 (10.0), (b) 11.6 (8.9), (c) 12.9 (9.3), (d) 10.0 (9.0) Inclusion: diagnosis of FM (Yunus 1981) Exclusion: organic brain syndrome, psychotic disorder, unstable or uncontrolled medical condition, major communicative disorder, rheumatoid arthritis, widespread osteoarthritis, subjective pain of less than 4 on a 10‐point scale, current participation in regular aerobic exercise, biofeedback training within the past year |
|
| Interventions | (a)Biofeedback/relaxation (n = 29). Phase 1: Frequency: individual supervised sessions ‐ 1/week, home programme – 2+/week; Duration: 1.5 to 3 hours; Mode: cognitive and muscular relaxation strategies and education regarding application of same to ADL. Phase 2: Frequency: group meetings ‐ 1/month; Duration: 60 min; Mode: home programme as Phase 1 (b) Exercise protocol (n = 30).Phase 1: Frequency/Duration: individual supervised sessions ‐ 1 to 3 hours 1/week, home programme 2+/week; Intensity: AE light to moderate (60% to 70% HRmax); FX unspecified, RT unspecified; Mode: AE walking, FX active ROM, RT unspecified. Phase 2: Frequency: group meeting ‐ 1/month, home programme unspecified; Duration: 60 min; Mode: participant group meetings for maintenance, home programme unspecified frequency (c) Biofeedback + Exercise (n = 30). Phases 1 and 2: biofeedback/relaxation same as (a), exercise protocol same as (b) (d)Education/attention control (n = 30). Phase 1: Frequency: 1/week (unknown if group or individual); Duration: 1.5 to 3 hours; Mode: educational information regarding diagnosis and treatment of FM and general health topics. Phase 2: Frequency: 1/month; Duration: 60 min; Mode: participant group meetings for maintenance |
|
| Outcomes | Pain (VAS), tenderness (TP count; myalgia score, dolorimeter), depression (CES‐D), mental health (Global Severity Index from the Symptom Checklist 90‐Revised), self‐efficacy (Arthritis Self‐Efficacy Scale), sleep (0 to 12 sleep score); other: disease severity (physician rating), physical activity (AIMS), pain behaviour (video analysis) Measurements: 0 and 6 weeks (Phase 1); 13, 52, and 104 weeks (Phase 2) |
|
| Adherence | Not specified | |
| Congruence of EX protocol with ACSM criteria for aerobic, strength or flexibility | Insufficient information to permit judgement | |
| Notes | Country: United States Language: English Author contact: email from author (2005) provided means and standard deviations for Tables 3 and 4 Trial registry record or protocol available: none Funding source: National Institute of Arthritis and Musculoskeletal and Skin Diseases, National Institute on Disability and Rehabilitation Research Conflict of interest: none reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Authors did not specify blinding of participants and personnel, but took steps to prevent knowledge of the groups’ intervention. "Subjects not informed of specific details about each of the 4 groups." However, this information is not adequate to make a judgement on the risk of performance bias |
| Detection Bias ‐ Subjective measures All outcomes | Unclear risk | Self‐report instruments were used to measure pain intensity and physical function. Comparator (education) likely minimised risk; however, participant blinding not reported, hence unclear risk |
| Detection Bias ‐ Blinding of assessor reported outcomes All outcomes | Low risk | Not applicable; no assessor‐reported tests were applied to the control group to measure cardiorespiratory submaximal function or muscle strength |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | ITT analysis not utilised Attrition at 6 weeks: (a) 2/29 (7%), (b) 2/30 (7%), (c) 4/30 (13%), (d) 2/30 (7%) Pooled reasons for dropouts reported: 7 = personal undisclosed, 4 = schedule conflict with work, 3 = moved, 2 = health issues undisclosed, 2 = increased pain) Attrition at 2‐year follow‐up: (a) 4/29 (14%), (b) 4/30 (13%), (c) 7/30 (23%), (d) 3/30 (10%) |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement of risk |
| Other bias | Low risk | Study appears to be free of other sources of risk of bias |
Burckhardt 1994.
| Methods | 3 groups: (a) mixed exercise and education, (b) education, (c) wait list control Length: Phase 1 (active) 6 weeks, Phase 2 (home programme) 6 weeks. Follow‐up 1: Follow‐up 2: 16 to 24 weeks Study design: randomised clinical trial with parallel groups |
|
| Participants | Female:Male: 86:0 Age, years (SD): pooled: 46.5 (8.3) Duration of symptoms, years (SD): pooled 7.5 (5.5) Inclusion: diagnosis of FM (ACR 1990), normal lab results (haemoglobin, free thyroxine, ESR, antinuclear antibodies, rheumatoid factor, creatinine phosphokinase), understand Swedish Exclusion: any other rheumatic disease |
|
| Interventions | (a)Exercise and Education (n = 28). Phase 1: Frequency: 1/week group exercise session × 6 weeks, encouraged to exercise on own at home during the week, Duration: supervised group session ‐ 1 hour, duration of unsupervised home exercise unknown; Intensity: unspecified, Mode: not clearly defined. Unspecified stretches and ROM each session, 2 of the 6 sessions were in the pool Education as described in (b) plus individual time to develop a training programme of walking, swimming, or cycling Phase 2: encouraged to continue to exercise on own,Frequency/Duration/Intensity: unknown; Mode: unknown (b)Education only (n = 28).Phase 1. Frequency: 1/week × 6 weeks, Duration: 1.5 hours, Mode: group self‐management classes ‐ information on the disease, the role of stress, coping, problem‐solving, assertiveness training, relaxation, and the importance of physical conditioning. Phase 2: home programme unspecified (c) Wait list control (n = 30) |
|
| Outcomes | Health‐related quality of life (FIQ Total), physical function (FIQ), pain (FIQ), fatigue (FIQ), stiffness (FIQ), cardiorespiratory submax (6MWT), anxiety (FIQ), depression (VAS, 10 cm), sleep disturbance (FIQ rested), flexibility (sit‐and‐reach), muscle endurance (sit‐to‐stand, # reps/min), tenderness (TP count); other: interference with work, feel bad, coping Measurements: 0, 12, 24, and 48 weeks |
|
| Adherence | No monitoring of duration or intensity Adherence criteria: excluded only if attended 1 to 2 of the classes Adherence: no information on how often those included attended |
|
| Congruence of EX protocol with ACSM criteria for aerobic, strength or flexibility | Insufficient information to permit judgement | |
| Notes | Country: Sweden Language: English Author contact: email from author (2008) provided means and standard deviations for Table 2 Trial registry record or protocol available: none Funding source: Riksförbundet mot Reumatism and the Ragnar och Lisa Stenbergs Fund Conflict of interest: none reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Participants were randomly assigned to 1 of 3 groups by the principal investigator after determining subject eligibility and pretesting (reviewing laboratory tests and TP results). No information about specific randomisation protocol |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | (b) Participants received education before (c) began, so intergroup communication could not take place; unlikely that participants and personnel delivering intervention were blinded |
| Detection Bias ‐ Subjective measures All outcomes | High risk | Self‐report instruments were used to measure health‐related quality of life, pain intensity, fatigue, stiffness, and physical function |
| Detection Bias ‐ Blinding of assessor reported outcomes All outcomes | Low risk | A trained PT, blinded to group, tested cardiorespiratory submaximal test |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | ITT analysis not utilised. Analysis based on completers (87% of participants) |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement of risk |
| Other bias | Low risk | Study appears to be free of other sources of risk of bias |
Clarke‐Jenssen 2014.
| Methods | 3 groups: (a) MX warm climate, (b) MX cold climate, (c) control Length: 4 weeks. Follow‐up: 3 months, 12 months Study design: randomised clinical trial with parallel groups |
|
| Participants | Female:Male = 119:10 Age, years (SD) pooled: 45 (9) Duration of Illness, years (SD): 14 (10) Inclusion: FM (ACR 1990), age between 18 and 60 years, independent in activities of daily living, capable of participating in a light exercise group on land and in warm water, understanding written and oral Norwegian Exclusion: serious physical or psychiatric diagnosis, alcohol or drug abuse, being pregnant or breast‐feeding, receiving more than 50% disability pension |
|
| Interventions | (a) Warm climate MX (AQ/LD: AE+RT+FX+RX)+ED+ Group discussion and Resting (n = 42): Frequncy: 5/week; Duration: 115 min (WU ns, AE 45 min, FX 15 min, RT 2 to 3/week 45 min; Relax 45 min, CD ns), Intensity: low to moderate (no values); Mode: AE = daily walking on land 45 min and AQ component 2 to 3/week 45 min; FX: 15 min after the walking/all main muscle groups; RELAX 2/week 45 min – hold relax technique. Non‐exercise protocol: Patient education 1/week (update on pain, fibromyalgia, self‐efficacy, and physical activity); small group discussions 1/week; resting daily 1 hour × 2 (b)Cold climate MX (AQ/LD: AE+RT+FX+RX)+ED+ Group discussion and Resting (n = 43): Frequency: 5/week; Duration: 115 min (WU ns, AE 45 min, FX 15 min, RT 2 to 3/week 45 min; Relax 20 min, CD ns), Intensity: low to moderate (no values); Mode: AE = walking on land 45 min and AQ component 2 to 3/week 45 min; FX: 15 min after the walking/all main muscle groups; RELAX 2/week 45 min – hold relax technique. Non‐exercise protocol: Patient education 1/week (update on pain, fibromyalgia, self‐efficacy, and physical activity); small group discussions 1/week; resting daily 1 hour × 2 (c)Control: treatment as usual (n = 44) |
|
| Outcomes | Health‐related quality of life (FIQ Total, SF‐36), pain (FIQ, VAS, Pain Mannequin ‐ McGill Pain Questionnaire), tenderness (TP count), depression (Hospital Anxiety and Depression), anxiety (Hospital Anxiety and Depression), self‐efficacy (Arthritis Self‐Efficacy Scale), strength (grip strength), cardiovascular submax (6MWT) Measurements: 0, 4, 12, and 52 weeks |
|
| Adherence | Group (a) attendance = 95%, Group (b) attendance = 91% | |
| Congruence of EX protocol with ACSM criteria for aerobic, strength or flexibility | Groups (a) and (b): not enough information to permit judgement | |
| Notes | Country: Norway Language: English Author contact: none Trial registry record or protocol available: none Funding source: Section for Climate Therapy, Oslo University Hospital, Rikshopitalet, Norwegian Fibromyalgia Association Conflict of interest: none reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A random numbers table was used; participants were stratified according to age and gender. Participants were randomised after inclusion but before baseline data were collected |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not reported; unlikely that participants and personnel delivering intervention were blinded |
| Detection Bias ‐ Subjective measures All outcomes | High risk | A self‐report instrument was used to measure pain intensity |
| Detection Bias ‐ Blinding of assessor reported outcomes All outcomes | High risk | Participants spent 4 weeks in a sunny climate that inevitably resulted in a tan; participants' groups were revealed to the assessor; assessors were not blinded to groups |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No attrition |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement of risk |
| Other bias | Low risk | Study appears to be free of other sources of bias |
Da Costa 2005.
| Methods | 2 groups: (a) mixed (AQ AE, Land AE+FX+RT), (b) control Length: 12 weeks. Follow‐up: 13 weeks, 39 weeks Study design: randomised clinical trial with parallel groups |
|
| Participants | Female:Male: 79:0 Age, years (SD): (a) 49.2 (8.7), (b) 52.3 (10.8) Duration of Illness, years (SD):(a) 10.5 (8.4), (b) 11.2 (7.6) Inclusion: women with diagnosis of FM (ACR 1990) Exclusion: concomitant disease that precludes participation in exercise, contraindication to exercise identified by the examining physician, change in medication in previous 2 weeks, regular participation in moderate‐intensity exercise (> 30 min 3/week) |
|
| Interventions | (a)Exercise protocol (n = 39) – individually prescribed home programme, primarily land‐based with initial 90 min prescription and supervised instruction and 3x30 min follow‐up sessions; warm‐up, cool‐down details unspecified; Frequency: AE: unspecified beyond participant selection within prescribed duration of 60 to 120 min/week, RT: 3 week × 12 weeks; FX unspecified. Duration: 1.5 to 3 hours; instructed to practice 2 additional times/week. Intensity: AE: light to moderate (60% to 70% HRmax) progressed to moderate to vigorous (75% to 85% HRmax); RT: max reps for callisthenics, 12 to 15 RM for free weight; FX light stretches held 15 to 30 s × 3 reps Mode: AE: participant selected, included walking, swimming, dancing, or aqua fitness. FX: static stretches, upper and lower extremity. RT: isotonic ex included callisthenics free weights and body weight, for upper and lower extremity, trunk (b)Control (n = 40) ‐ treatment as usual |
|
| Outcomes | Health‐related quality of life (FIQ Total), pain (10‐cm VAS multi‐site mean for upper body and lower body), mental health (Global Severity Index from the Symptom Checklist 90‐Revised: total score) Measurements: 0, 12, 24, and 48 weeks |
|
| Adherence | Participant logs were used to record frequency, duration, intensity (using HR monitors), type of exercise, FM symptoms Average weekly adherence rate for AE and FX from participant logs = ratio of sessions reported to sessions prescribed: AE adherence: 67.4% (SD 34.2%), FX adherence: 65.9% (SD 33.8%), RT adherence: unspecified |
|
| Congruence of EX protocol with ACSM criteria for aerobic, strength or flexibility | AE: does not meet criteria for healthy adults, but meets criteria for extremely deconditioned individuals; RT: meets criteria; FX: meets criteria | |
| Notes | Country: Canada Language: English Author contact: additional information about exercise programme (mode, targeted muscle groups, sets, reps, timing for ST and FX prescribed and performed) was provided, June 2005 Trial registry record or protocol available: none Funding source: the Arthritis Society (#TAS99/0134) Conflict of interest: none reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation list used |
| Allocation concealment (selection bias) | Unclear risk | The project co‐ordinator was responsible for enrolling participants and was blinded to the allocation sequence. At the point of group assignment, the project co‐ordinator was provided with the participant's group assignment by one of the investigators (DD), who had no contact with the student participants. Participants were then informed of their group allocation by the project co‐ordinator |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | The exercise physiologist was responsible for (a) and the project co‐ordinator for (b). (a) participants met individually 4 times with an exercise physiologist only. The project co‐ordinator interacted with (b) group through "contact" (unspecified) to review the questionnaire battery; unlikely that participants and personnel delivering intervention were blinded |
| Detection Bias ‐ Subjective measures All outcomes | High risk | Self‐report instruments were used to measure health‐related quality of life and pain intensity |
| Detection Bias ‐ Blinding of assessor reported outcomes All outcomes | Low risk | Not applicable; no assessor‐reported tests were applied to measure cardiorespiratory submaximal function or muscle strength |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | ITT analysis utilised. Missing data imputed using last value carried forward method |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement of risk |
| Other bias | Low risk | Study appears to be free of other sources of bias |
Demir‐Gocmen 2013.
| Methods | 2 groups: (a) supervised group MX (FX + balance‐co‐ordination), (b) unsupervised individual home FX Length: 12 weeks. Follow‐up: 12 weeks Study design: randomised clinical trial with parallel groups |
|
| Participants | Female:Male: 50:0 Age, years (SD): (a) 44.7 (5.3), (b) 44.4 (5.2) Duration of Illness, years: not specified Inclusion: women between ages 20 and 50 with diagnosis of FM (ACR 1990) Exclusion: inflammatory rheumatic disease, severe musculoskeletal deformities and mechanical problems limiting capacity for exercise, unstable hypertension, severe cardiac and respiratory problems, post menopause, diabetes, hypoglycaemia, vitamin D deficiency, hypothyroidism, hyperthyroidism, osteoporosis, vertigo, hearing and visual problems, joint prosthesis or implants that would contraindicate exercise, neurological disease |
|
| Interventions | (a)Exercise protocol 1 ‐ Supervised Group MX (FX + balance‐co‐ordination) (n = 25).Frequency: Supervised, 3/week; Duration: Total 60 min (WU 10 min, CD 10 min, FX 15 min, Balance‐co‐ordination 25 min); Intensity: FX as tolerated, 10 reps/exercise; Mode: FX ‐ Unspecified. Balance‐co‐ordination ‐ balancing on 1 and 2 feet, tandem exercises, standing with a partner, bending, squatting, lateral and backward movements, skipping, scissoring, rolling, and twisting (b) Exercise protocol 2 ‐ Unsupervised Individual Home FX (n = 25).Frequency: Unsupervised home programme 3/week; Duration: Total 60 min (WU 10 min, CD 10 min, FX 40 min); Intensity: as tolerated, 10 reps/exercise; Mode: Unspecified |
|
| Outcomes | Health‐related quality of life (FIQ Total), pain (VAS), tenderness (TP count), depression (BDI), co‐ordination (Four Square Step Test), balance (Timed Up and Go Test, Berg Balance Scale, Activity Specific Balance Confidence Scale, balance measures on TeknoBody PK stabilometry balance platform) Measurements: 0, 12, and 24 weeks |
|
| Adherence | Group (a) attendance was used to represent adherence. Group (b) participant‐completed exercise charts; participants received 2/week telephone calls regarding adherence | |
| Congruence of EX protocol with ACSM criteria for aerobic, strength or flexibility | Groups (a) and (b): FX: congruent with ACSM guidelines | |
| Notes | Country: Turkey Language: English Author contact: email response (3 June 2013) from author; details of study methods, exercise protocols, information about injuries, exacerbations, adverse effects, and adherence provided Trial registry record or protocol available: none Funding source: none reported Conflict of interest: none reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Email response: "the randomization was done using randomization table" |
| Allocation concealment (selection bias) | Unclear risk | Not reported in publication or email response |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not reported; unlikely that participants and personnel delivering intervention were blinded |
| Detection Bias ‐ Subjective measures All outcomes | High risk | Self‐report instruments were used to measure health‐related quality of life and pain intensity |
| Detection Bias ‐ Blinding of assessor reported outcomes All outcomes | Low risk | Not applicable; no assessor reported tests were applied to measure cardiorespiratory submaximal function or muscle strength |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Dropouts: (a) 2/25 (8%), (b) 5/25 (20%) Email response: "ITT was not used since no drop‐out was due to any side effect of the study" |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement of risk |
| Other bias | Low risk | Baseline data indicate imbalance on one outcome measure; the outcome was not a major outcome or an important subject attribute |
Etnier 2009.
| Methods | 2 groups: (a) supervised group mixed exercise, (b) control Length: 18 weeks. Follow‐up: none Study design: randomised clinical trial with parallel groups |
|
| Participants | Female:Male: 16:0 Age, years (SD) years: pooled: 54.7 (9.3) Duration of Illness: most participants reported having symptoms as teenagers and receiving a medical diagnosis within the last 1 to 10 years Inclusion: diagnosis of FM (ACR 1990), over 18 years of age, participating in exercise ≤ 1 day/week, meeting ACSM criteria for safe conduct of exercise, willing to participate in control or exercise group Exclusion: none stated |
|
| Interventions | (a)Exercise ‐ MX (AE+ST+FX) (n = 8),Frequency: 3/week; Duration: 60 min; Intensity: AE moderate to vigorous intensity (55% to 65% HRmax); RT unspecified, Mode: AE walking, RT 8 stations of light resistance exercise (unspecified) and static bridging, FX unspecified (b)Control (n = 8) – no exercise, delayed treatment |
|
| Outcomes | Health‐related quality of life (FIQ Total), fatigue (Fatigue Severity Scale), depression (CES‐Depression Scale), submax cardiorespiratory (Quarter Mile Walk Test); other: cognitive function (Rey Auditory Verbal Learning Test, Wisconsin Card Sorting, Pincus Cognitive Symptoms Inventory, Paced Addition Serial Attention Task, Stroop Interference Task) Measurements: 0 and 18 weeks |
|
| Adherence | Group (a) mean percentage of sessions attended = 65% | |
| Congruence of EX protocol with ACSM criteria for aerobic, strength or flexibility | Group (a) AE did not meet ACSM criteria ‐ exercise performed for 15 min only. RT and FX not enough information to determine | |
| Notes | Country: United States of America Language: English Author contact: Email received from authors 26 January 2011 provided pre‐test scores for all outcomes Trial registry record or protocol available: none Funding source: the University of North Carolina Greensboro Office of Research and Public/Private Sector Partnerships Conflict of interest: none reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | The second author conducted and supervised the exercise sessions. Blinding not reported; unlikely that participants and personnel delivering intervention were blinded |
| Detection Bias ‐ Subjective measures All outcomes | High risk | Self‐report instruments were used to measure pain intensity and fatigue |
| Detection Bias ‐ Blinding of assessor reported outcomes All outcomes | Unclear risk | Blinding not reported for the test of submaximal cardiorespiratory function |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Dropouts after baseline testing but before randomisation: 6/22 (27.7%) |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement of risk |
| Other bias | Low risk | Study appears to be free of other sources of risk of bias |
Garcia‐Martinez 2011.
| Methods | 2 groups: (a) MX (AE+RT+FX), (b) control Length: 12 weeks. Follow‐up: none Study design: randomised clinical trial with parallel groups |
|
| Participants | Female:Male: 28:0 Age, years (SD): (a) 58.6 (7.8), (b) 59.3 (4.8) Duration of Illness, years (SD): (a) 9.9 (3.8), (b) 10.6 (4.1) Inclusion: women with diagnosis of FM (ACR1990) Exclusion: serious cardiovascular, pulmonary, endocrine, neurological, or renal disease; inflammatory rheumatic disease; participation in a physical therapy or exercise programme in the last 6 months |
|
| Interventions | (a) Exercise protocol (n = 14) ‐ Frequency: 3/week supervised; Duration: 60 min (WU (AE) 10 min, AE 20 min, RT+FX 20 min, CD 10 min), Intensity: AE light to moderate intensity (60% to 70% HRmax) progressed to moderate to vigorous intensity (as high as 75% to 85% HRmax) depending on participants' adaptation; RT+FX: not specified; Mode: AE = not specified; RT+FX: not specified. (b) Control (n = 14) – daily activities, which did not include any physical exercise similar to those in the programme |
|
| Outcomes | Health‐related quality of life (FIQ total, SF‐36), self‐reported physical function (SF‐36 Physical Function summary, SF‐36 Physical Function, SF‐36 Role Physical), pain (SF‐36), fatigue (SF‐36), muscle strength (MVC knee extension), mental health (SF‐36 Mental Health summary, SF‐36 Mental Health), muscle endurance (maximum reps of concentric knee extension), flexibility (forward reach in long sitting); other: self‐esteem (Rosenberg Self‐Esteem Scale), self‐concept (Erdmann Auto‐Concept Scales: general self, personal self, optimism/pessimism, physical activity motivation, social self, inhibition, global), SF‐36 role emotional, SF‐36 social function Measurements: 0 and 12 weeks |
|
| Adherence | Attendance was tracked. Participants were excluded from analysis if they attended less than 95% of exercise sessions | |
| Congruence of EX protocol with ACSM criteria for aerobic, strength or flexibility | AE: does not meet criteria for healthy adults. The frequency of exercise in this intervention was 3 times per week. The protocol included 20 min of aerobic work that began at 60% to 70% HRmax and progressed gradually to "as high as 75‐85% HRmax depending on the subjects’ adaptations". RT: not enough information to permit judgement; FX: not enough information to permit judgement | |
| Notes | Country: Spain Language: English Author contact: author emailed twice, no response from author Trial registry record or protocol available: none Funding source: none reported Conflict of interest: none reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random numbers table used |
| Allocation concealment (selection bias) | Unclear risk | Not specified |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not reported; unlikely that participants or personnel delivering the exercise were blinded |
| Detection Bias ‐ Subjective measures All outcomes | High risk | Self‐report instruments were used to measure health‐related quality of life, pain, fatigue, and physical function |
| Detection Bias ‐ Blinding of assessor reported outcomes All outcomes | Unclear risk | Not reported whether or not assessor for muscle strength test was blinded |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | ITT analysis not utilised (a): 2/14 (14%) failed to complete 95% of exercise sessions; reasons for poor adherence not specified (b): 1/14 (7%) failed to attend measurements; reasons not specified Reason for missing outcome data likely related to true outcome, with imbalance for missing data across intervention groups |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement of risk |
| Other bias | Low risk | Study appears to be free of other sources of risk of bias |
Genc 2002.
| Methods | 2 groups: (a) mixed exercise (FX+RT+ posture), (b) remedial exercise, relaxation, self‐mobilisation Length: 3 weeks. Follow‐up: none Study design: randomised clinical trial with parallel groups |
|
| Participants | Female:Male: 32:0 Age, years (SD): (a) 27.9 (SD 5.4), (b) 27.5 (SD 5.6) Duration of illness: unknown Inclusion: women with diagnosis of FM (ACR1990) Exclusion: not reported |
|
| Interventions | (a)Mixed exercise ‐ MX (FX+RT+posture, n = 16) ‐ Frequency: 3/week; Duration: unknown; Intensity: unknown; Mode: flexibility and strengthening for thoracic, cervical, and lumbar muscles plus moist heat and posture awareness education (b) Exercise ‐ remedial exercise, relaxation, self‐mobilisation (n = 16) ‐ Frequency: 3/week; Duration: unknown; Intensity: unknown; Mode: isometric relaxation for upper parts of trapezius, supraspinatus, and levator scapula muscles. Remedial exercises for cervical, thoracic, and lumbar regions, and active mobilisations plus moist heat and posture awareness education |
|
| Outcomes | Health‐related quality of life (FIQ Total), flexibility (spinal ROM: forward reach in long sitting, extension, right and left lateral flexion; cervical spine ROM: flexion, extension, right and left lateral flexion and rotation) Measurements at: baseline and 3 weeks following initiation of treatment |
|
| Adherence | Not reported | |
| Congruence of EX protocol with ACSM criteria for aerobic, strength or flexibility | Not enough information to permit judgement | |
| Notes | Country: Turkey Language: Turkish [English translation of methods was obtained by reviewers. Reviewers were not able to obtain a complete translation, thus gaps in CIS table information have been indicated by ‘Unknown’] Author contact: we were unable to contact the authors Trial registry record or protocol available: none Funding source: none reported Conflict of interest: none reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Translator stated that trial was described as randomised, but method of randomisation not specified |
| Allocation concealment (selection bias) | Unclear risk | No information provided by translator |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No information provided by translator on blinding of participants; unlikely that participants and personnel delivering intervention were blinded |
| Detection Bias ‐ Subjective measures All outcomes | High risk | A self‐report instrument was used to measure health‐related quality of life |
| Detection Bias ‐ Blinding of assessor reported outcomes All outcomes | Low risk | Not applicable; no assessor‐reported tests were applied to measure cardiorespiratory submaximal function or muscle strength |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | As per translator and Table 2 of the paper, n = 16 for both groups, unchanged from pre to post |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement of risk |
| Other bias | Unclear risk | No information provided by translator |
Giannotti 2014.
| Methods | 2 groups: (a) composite [Ed+MX (AE+RT+FX+therapeutic ex)], (b) control Length: 10 weeks; Follow‐up: 36 weeks Study design: randomised clinical trial |
|
| Participants | Female:male: 39:2 Age, years (SD): (a) 51.3 (6.3), (b) 52.8 (10.7) Duration of illness, years (SD): composite: (a) 7.6 (8.8); (b) 7.1 (5.2) Inclusion: diagnosis of FM (ACR 2010 criteria), ages between 35 and 65 years, BMI between 18 and 35 kg/m² Exclusion: diabetes; other rheumatic diseases including severe osteoarthritis and osteoporosis; severe musculoskeletal alterations; use of assistive devices to perform daily activities; orthopaedic surgery such as spine or hip/knee in previous year; patients who attended physical therapy and rehab treatments or had modified their usual FM pharmacological therapy in the 3 months before enrolment in the study |
|
| Interventions | (a)MX (AE+RT+FX+Ther Ex) + Education (n = 21) ‐ Overall Frequency: 2/week. Education: sessions #1‐7; MX ‐ Frequency: FX+Ther Ex ‐ 2/week; RT: in sessions #8 to 20, AE ‐ in sessions #15 to 20. Duration: total 60 min, FX + Ther Ex 25 min, 2 reps held for 50 to 60 s per stretch, AE 10 min, RT 10 min; Intensity: FX not specified, RT 1 set of 10 reps not progressed, AE vigorous (70% max functional capacity); Mode: FX static for spine, upper and lower limbs, RT not specified beyond no equipment used, for spine and lower limbs, AE cycle ergometry (additional information provided by author). Home programme ‐ Frequency: 3+/week during intervention and follow‐up (b)Control ‐ treatment as usual (n = 20) |
|
| Outcomes | Multi‐dimensional function (FIQ Total, FM assessment status), pain (VAS), fatigue (Fatigue Severity Scale, VAS), sleep disorders (VAS), stiffness (VAS), tenderness (TP count), physical function (HAQ), submax cardiorespiratory function (6MWT), flexibility (spinal flexion, extension, left and right inclination, left and right rotation); other: BMI, thoracic kyphosis, lumbar lordosis Measurements taken at 0, 12 (post intervention), and 36 weeks (follow‐up) |
|
| Adherence | Group (a) attendance was used to gauge adherence. For home programme: participant diaries describing #session performed/week | |
| Congruence of EX protocol with ACSM criteria for aerobic, strength or flexibility | RT: no. Intensity not adequate, no progression (additional information from author). AE: no, duration too low. FX yes | |
| Notes | Country: Italy Language: English Author contacted: yes. Information provided on 23 February 2015 Trial registry record or protocol available: DRKS00005071 http://drks‐neu.uniklinik‐freiburg.de/drks_web/navigate.do?navigationId=trial.HTML&TRIAL_ID=DRKS00005071 Funding source/declaration of interest: none |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random number table was used |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement of risk |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Email response: study participants were not informed about the specific study hypotheses; unlikely that participants and personnel delivering the intervention were blinded |
| Detection Bias ‐ Subjective measures All outcomes | High risk | Self‐report instruments were used to measure health‐related quality of life, pain intensity, fatigue, stiffness, and physical function |
| Detection Bias ‐ Blinding of assessor reported outcomes All outcomes | Low risk | Email response from authors: outcome assessors for cardiorespiratory function test were blinded to group |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Reasons for missing outcome data were unlikely to be related to true outcome |
| Selective reporting (reporting bias) | Low risk | Study protocol is available; all of the study’s pre‐specified primary outcomes have been reported in the pre‐specified way |
| Other bias | Low risk | Study appears to be free of other sources of bias |
Hunt 2000.
| Methods | 2 groups: (a) mixed exercise (AE+FX+RT) + self‐management programme, (b) control Length: 5 weeks. Follow‐up: none Study design: randomised clinical trial with parallel groups |
|
| Participants | Female:Male: 42:8 Age, years (SD): (a) 44.6 (8.6), (b) 46.9 (7.5) Duration of Illness, years (SD): (a) 4.1 (4.7), (b) 4.6 (4) Inclusion: all patients entered into the study had been given a diagnosis of primary fibromyalgia following assessment by a consultant rheumatologist in clinic (ACR 1990), referred for PT from rheumatology clinic Exclusion: blood test excluded rheumatological disease (test not specified) |
|
| Interventions | (a)MX (AE+RT+agility, balance, postural exercises) + ED (n = 25): AE Frequency: 1/week in class, daily at home; AE Duration: 15 min; AE Intensity: 3 to 4/10 (moderate), "cycle or step in short bursts until out of breath" (email), "patients gradually increase pace and intensity within their level of perceived exertion"; AE Mode: stationary cycling and stepping (step‐ups); RT Frequency: 1/week in class, daily at home; RT Duration: 2 min each of 8 endurance exercises; RT Intensity: not specified, but "encouraged to progress their programme", increase gradually; RT Mode: 8 lower body and core callisthenic exercises with no weights (e.g. bridging, curl‐ups, hip abduction in side lying, straight leg raise, hip adduction in side lying, isometric abdominal, hip, and knee flexion, trunk twist in crook‐lying); FX Frequency: 1/week in class, daily at home; FX Duration; each stretch held 5 s, 5 reps each; FX Intensity: not specified; FX Mode: 12 stretches for neck, shoulders, chest, gastrocnemius, hamstrings; Education: planning, pacing, goal setting, advice on sleep management, relaxation techniques, pain management (b)Control (n = 25) – not reported |
|
| Outcomes | Pain (VAS 10 cm), fatigue (VAS 10 cm), sleep disturbance (6‐point ordinal scale), submax cardiorespiratory (cycling ergometer test ‐ RPE, Borg CR‐10); other: helplessness (Rheumatology Attitude Index) Measurements: 0, 5 weeks; a subset of participants returned at week 6 for semi‐structured interview |
|
| Adherence | No attrition for Groups (a) and (b) | |
| Congruence of EX protocol with ACSM criteria for aerobic, strength or flexibility | AE: frequency, duration, and/or intensity did not meet ACSM guidelines; RT: not enough information to evaluate congruence with ACSM guidelines; FX: yes, likely 10 min/d | |
| Notes | Country: United Kingdom Language: English Author contact: email response (29 March 2011) from author; details regarding study design and numerical data for pain and fatigue provided Trial registry record or protocol available: none Funding source: North West Regional Health Authority Conflict of interest: none reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Email response: "number tables were not used. Referrals were sent to PT dept and numbered and dated on arrival. Alternate patients were allocated to the treatment group" |
| Allocation concealment (selection bias) | Low risk | Email response: "all allocation was carried out by an independent individual, clerical support staff, who simply allocated patients to Group A or B with no knowledge of which was the treatment group" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Email response: "participants and care study personnel knew group identity of the patients" |
| Detection Bias ‐ Subjective measures All outcomes | High risk | Self‐report instruments were used to measure pain intensity and fatigue |
| Detection Bias ‐ Blinding of assessor reported outcomes All outcomes | High risk | Email response from author: "observers measuring outcomes were not blinded to group assignment" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No dropouts |
| Selective reporting (reporting bias) | Unclear risk | Author provided point estimates for pain VAS and fatigue VAS when requested. Insufficient information to permit judgement of risk |
| Other bias | High risk | Baseline differences between groups |
Jones 2007.
| Methods | 4 groups: (a) pyridostigmine + mixed exercise (AE+RT+FX+Balance+Relax), (b) pyridostigmine + diet monitoring, (c) placebo pyridostigmine + mixed exercise (AE+RT+FX+Balance+Relax), (d) placebo pyridostigmine + diet monitoring Length: 26 weeks. Follow‐up: none Study design: randomised clinical trial with parallel groups |
|
| Participants | Female:male: pooled: 160:5, (a) 40:0, (b) 39:0, (c) and (d) not specified Age, years (SD): pooled: 49.5 (8.1), (a) 49.1 (9.0), (b) 49.3 (7.9), (c) 49.6 (7.7), (d) 49.8 (7.9) Duration of illness, years (SD): (a) 15.0 (10.5), (b) 14.8 (9.7), (c) 16.9 (11.9), (d) 14.9 (10.6) Inclusion: adults ages 18 to 65, diagnosis of FM (ACR 1990), medically capable to participate in exercise programme Exclusion: other rheumatic disorder; current or past history of cardiovascular, pulmonary, neurological, endocrine, or renal disease that would preclude involvement in treadmill testing to VO2 max or alter the GH/IGF‐1 axis; use of the following medications: pyridostigmine, high‐dose beta‐blockers, systemic steroids; currently exercising more than 30 min per week; Beck Depression Scale score (modified for FM) ≥ 29; BMI > 45 kg/m²; pregnant or nursing women; planned surgery during study period; ongoing, unresolved disability litigation |
|
| Interventions |
(a) Pyridostigmine + supervised group mixed exercise (AE+RT+FX+Balance+Relax) (n = 40) ‐ Frequency: 3/week; Duration: 60 min (30 min WU and AE, 10 min RT, 5 min FX, 5 min Balance, 10 min Relax); Intensity: AE classification both as light intensity (40% to 50% HRmax) and light to moderate intensity (10 to 12 on 0 to 20 scale on Borg's rating of perceived exertion); RT: intensity not specified; Mode: AE low‐impact, floor aerobics, RT dynamic exercise using elastic bands and free weights for all major muscle groups, FX static and non‐ballistic stretching of all major muscle groups, balance static and dynamic standing on foam and balance boards, Relax guided imagery with breathing awareness (b) Pyridostigmine + diet monitoring (diet monitoring by registered nurse) (n = 36) ‐ Frequency: 1 phone call/week, 1 visit/month; Duration: visit duration 2 hours (c) Placebo pyridostigmine + supervised group mixed exercise (AE+RT+FX+Balance+Relax) (n = 39) ‐ Frequency: 3/week; Duration: 60 min (30 min WU and AE, 10 min RT, 5 min FX, 5 min Balance, 10 min Relax); Intensity: AE at 40% to 50% HRmax or 10 to 12 out of 20 on Borg's rating of perceived exertion (light intensity); RT: intensity not specified; Mode: AE low‐impact, floor aerobics, RT dynamic exercise using elastic bands and free weights for all major muscle groups, FX static and non‐ballistic stretching of all major muscle groups, Balance static and dynamic standing on foam and balance boards, Relax guided imagery with breathing awareness (d) Placebo pyridostigmine + diet monitoring – diet monitoring by registered nurse (n = 39) ‐ Frequency: 1 phone call/week, 1 visit/month; Duration: visit duration 2 hours |
|
| Outcomes | Health‐related quality of life (FIQ Total, Quality of Life Scale), pain (FIQ), fatigue (FIQ), depression (BDI‐R, FIQ), sleep (FIQ), stiffness (FIQ), tenderness (TP count, Total Myalgic score), anxiety (FIQ), cardiorespiratory max (Treadmill Test Balke Protocol modified for FM‐VO2 max, time), muscle endurance (# sit‐to‐stand in 30 s), flexibility (forward reach in long sitting, overhead external rotation to behind back internal rotation), balance (Flamingo stand); other: side effects of med/placebo (count), % body fat (skin fold test, bioelectrical impedance), hormone levels Measurements: 0 and 26 weeks |
|
| Adherence | (a) and (c): analysis included only participants who attended > 50% of sessions (b) and (d): not specified |
|
| Congruence of EX protocol with ACSM criteria for aerobic, strength or flexibility | (a) and (c): AE: met criteria specified for moderately to highly deconditioned individuals; RT: not enough information to determine; FX: no (5 min duration/session too short) | |
| Notes | Country: United States of America Language: English. Author contact: n/a Trial registry record or protocol available: none Funding source: National Institute of Nursing Research grant 5R01‐NR‐8150‐4, General Clinical Research Center grant M01‐RR‐000334, medications provided by Valeant Pharmaceuticals, exercise equipment provided by TheraBand Conflict of interest: none reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random assignment study team statistician, who had no contact with participants. Participants were randomised via stratified block (age in 5‐year blocks, BMI in 3‐point blocks, and sex) |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not enough information for clear judging. The PYD arm was double‐blinded but the EX vs attention control could not be double‐blinded. This instructor was not responsible for collecting outcomes |
| Detection Bias ‐ Subjective measures All outcomes | Unclear risk | Self‐report measures used for health‐related quality of life, pain intensity, fatigue, and stiffness, but placebo used as comparator. Not enough information to judge if participants were aware of study hypothesis and group assignment |
| Detection Bias ‐ Blinding of assessor reported outcomes All outcomes | Low risk | Not applicable; no assessor‐reported tests were applied to measure cardiorespiratory submaximal function or muscle strength |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | ITT analysis not utilised (a) 3/43 (7%) ‐ all for medical reasons unrelated to intervention (b) 6/42 (14%) ‐ 3 were unwilling, 1 had relocated, and 2 dropped for medical reasons (c) 0/39 (0%) (d) 2/41 (5%) ‐ 1 was unwilling, 1 dropped out for medical reasons; medical reasons were not well described |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement of risk |
| Other bias | Low risk | Study appears to be free of other sources of risk of bias |
Joshi 2009.
| Methods | 2 groups: (a) mixed exercise (RT+FX+relaxation), (b) amitriptyline Length: 26 weeks. Follow‐up: none Study design: randomised clinical trial with parallel groups |
|
| Participants | Female:Male = 166:9 Age, years (SD): (a) 38.8 (9.8), (b) 38 (8.7) Duration of Illness, months (SD): (a) 16.4 (15.9), (b) 19.4 (9.0) Inclusion: 18 to 60 years of age, muscular pain at least 12 weeks' duration, diagnosis of FM (ACR 1990) Exclusion: pregnancy or lactation, history of trauma, fracture, fever, malignancy, chronic renal or hepatic disorders, alcohol abuse, cerebrovascular or neurological abnormality |
|
| Interventions | (a) Mixed exercise (RT+FX+relaxation; n = 88) ‐Frequency: unsupervised home programme of RT and FX 2/d × 2 days/week and relaxation 2/d × 4 days/week. Supervised 1/month; Duration: RT and FX at least 10 min, relaxation 4 to 6 min; Intensity: not specified; Mode: RT isotonic or isometric exercise against resistance of gravity, body weight, light weight for shoulder/shoulder girdle, trunk and limb extensors; FX static stretches for neck, shoulder/shoulder girdle (b)Amitriptyline (n = 87) ‐ received open‐label amitriptyline 25 mg once daily at bedtime. Dose was increased to 50 mg if no benefit was seen |
|
| Outcomes | Health‐related quality of life (FIQ Total) Measurements: 0 week, 26 weeks |
|
| Adherence | (a) 78% adherence (b) 75% adherence |
|
| Congruence of EX protocol with ACSM criteria for aerobic, strength or flexibility | (a) RT and FX did not meet ACSM criteria | |
| Notes | Country: India Language: English Author contact: none Trial registry record or protocol available: none Funding source: none reported Conflict of interest: none reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Open‐label alternate patient treatment allocation strategy |
| Allocation concealment (selection bias) | High risk | See above |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Unlikely that participants and personnel delivering intervention were blinded |
| Detection Bias ‐ Subjective measures All outcomes | Unclear risk | A self‐report instrument was used to measure health‐related quality of life but comparator (amitriptyline) likely minimised risk |
| Detection Bias ‐ Blinding of assessor reported outcomes All outcomes | Low risk | Not applicable; no assessor‐reported tests were applied to measure cardiorespiratory submaximal function or muscle strength |
| Incomplete outcome data (attrition bias) All outcomes | High risk | "As treated" analysis utilised with substantial departure of the intervention received from that assigned at randomisation; dropouts: (a) 14/88 (15.9%), (b) 5/87 (5.7%); reasons for missing outcome data likely to be related to true outcome |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement of risk |
| Other bias | Unclear risk | Insufficient information to permit judgement of risk |
Martin 1996.
| Methods | 2 groups: (a) mixed exercise (AE+RT+FX), (b) relaxation Length: 6 weeks. Follow‐up: none Study design: randomised clinical trial with parallel groups |
|
| Participants | Female:Male: study entry: not reported, analysis: 37:1 Age, years (SD): (a) 43.9 (9.7), (b) 45.7 (9.9) Duration of Illness, years (SD): (a) 8.9 (6.8), (b) 10.4 (7.5) Inclusion: diagnosis of FM (ACR 1990), willingness to undertake the exercise programme Exclusion: cardiovascular, pulmonary, neurological, or renal disease that precluded participation in exercise; medication that could significantly affect normal physiological response to exercise |
|
| Interventions | (a) Mixed exercise (AE+RT+FX; n = 30) – Frequency: 3/week; Duration: 60 min (AE 20 min, RT 20 min, FX 20 min); Intensity: AE light to vigorous intensity (60% to 80% maxHR), RTand FX not specified; Mode: AE walking, RT isotonic exercises for upper/lower/trunk muscles, FX mode not specified for upper/lower/trunk muscles (b) Relaxation (n = 30) ‐ Frequency: 3/week;Duration: 60 min; Mode: visualisation, yoga, and autogenic relaxation |
|
| Outcomes | Health‐related quality of life (FIQ Total, Illness Intrusive Questionnaire); self‐efficacy (Arthritis Self‐Efficacy Scale), tenderness (TP count, total myalgic score), pain (VAS), cardiorespiratory max (modified Balke treadmill protocol, time to volitional fatigue), muscle strength (isokinetic peak torque at 90, 180, and 240 degrees/s for knee extensors and flexors, shoulder internal and external rotators), muscle endurance (isokinetic fatigue curve, details unspecified), flexibility (forward reach in long sitting, shoulder forward flexion) Measurements: 0 and 6 weeks |
|
| Adherence | Not specified | |
| Congruence of EX protocol with ACSM criteria for aerobic, strength or flexibility | (a) AE ‐ did not meet ACSM guidelines. RT and FX not enough information to permit judgement | |
| Notes | Country: Canada Language: English Author contact: none Trial registry record or protocol available: none Funding source: Canadian Fitness and Lifestyle Research Institute Conflict of interest: none reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Table of random numbers used |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Group (a) and (b) participants did not have contact; personnel blinding not reported; unlikely that personnel delivering intervention were blinded |
| Detection Bias ‐ Subjective measures All outcomes | Unclear risk | A self‐report instrument was used to measure health‐related quality of life and pain intensity but comparator (relaxation) likely minimised risk |
| Detection Bias ‐ Blinding of assessor reported outcomes All outcomes | Low risk | Not applicable; no assessor‐reported tests were applied to measure cardiorespiratory submaximal function or muscle strength |
| Incomplete outcome data (attrition bias) All outcomes | High risk | High dropout rates; ITT analysis not utilised |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement of risk |
| Other bias | Low risk | Study appears to be free of other sources of bias |
Paolucci 2015.
| Methods | 2 groups: (a) mixed exercise + education [MX (AE, agility, balance, posture, RT, FX)+ED], (b) control (AAU) Length: 5 weeks. Follow‐up: 17 weeks Study design: randomised clinical trial with parallel groups |
|
| Participants | Female:Male: study entry: not reported, analysis: 32:0 Age, years (SD): (a) 50.1 (8.9), (b) 48.1 (10.4) Duration of illness: not reported Inclusion: diagnosis of FM (ACR 1990); baseline FIQ score > 50; absence of other severe somatic or psychiatric/neurological disorders and other diseases that prevent physical loading, as severe scoliosis or kyphoscoliosis, previous spine surgery, vertebral fracture, sciatic pain, neoplasia; not currently attending another type of physical therapy; stable pharmacological treatment for 3 months before the study Exclusion: using antidepressants such as SNRI |
|
| Interventions | (a) Mixed exercise + Education (MX (AE, Agility, balance, posture, RT, FX)+ED; n = 16) ‐ Frequency: 2/week × 5 weeks of supervised group exercise, followed by 2/week × 12 weeks of home programme, unsupervised exercise; Duration: total 60 min, AE 20 min; Intensity: AE 60% HRmax (age‐220); Mode: low‐impact AE ‐ fast walking in a circle alternating with periods of up and down the stairs‐3 stair steps × 10 min, RT ‐ 4 strengthening exercises for hip and trunk extensors in supine and prone lying and on hands and knees (3 sets of 10 reps each exercise), agility training and balance exercises, postural exercises for the back and proprioceptive exercises for the trunk in supine position, diaphragmatic breathing; FX ‐ static stretching (shoulder/upper body, hamstrings, quadriceps, gluteus maximus/hip, gastrocnemius/soleus, lower back/abdomen, inner thigh/groin) exercises for 30 s to 60 s, repeated 3 times, diaphragmatic breathing exercises and relaxation. ED ‐ brief educational intervention performed by a physiatrist: symptoms of FM, importance of correct motor habits. An instructional booklet describing and illustrating the exercises was given to patients for use during the home programme (b) Control (n = 16) ‐ continue with normal activities. A clinical diary was utilised | |
| Outcomes | Health‐related quality of life (FIQ Total, Illness Perception Questionnaire); other: Minnesota Multiphasic Personality Inventory Profile Measurements: 0, 5, and 17 weeks |
|
| Adherence | Monitoring methods: none stated; adherence criteria: none stated; adherence: (a) 84% attended all sessions | |
| Congruence of EX protocol with ACSM criteria for aerobic, strength or flexibility | AE: did not meet ACSM criteria; RT: not enough information to permit judgement | |
| Notes | Country: Italy Language: English Author contact: none Trial registry record or protocol available: none Funding source: none reported Conflict of interest: none reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation utilised |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement of risk |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants were fully informed about the study procedures; personnel blinding not reported; unlikely that personnel delivering interventions were blinded |
| Detection Bias ‐ Subjective measures All outcomes | High risk | A self‐report instrument was used to measure health‐related quality of life |
| Detection Bias ‐ Blinding of assessor reported outcomes All outcomes | Low risk | Not applicable; no assessor‐reported tests were applied to measure cardiorespiratory submaximal function or muscle strength |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | "During the five week treatment period, 3 subjects of TG were dropped from the protocol because they did not attempt therapy sessions. Two patients of CG were removed at the T1, due to their absence at the medical visit. Therefore, data of 16 subjects of TG and 16 subjects of CG were analysed in this study" Reasons for missing outcome data unlikely to be related to true outcome |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement of risk |
| Other bias | Low risk | Study appears to be free of other sources of bias |
Rivera Redondo 2004.
| Methods | 2 groups: (a) mixed exercise (AQ (unspecified exercises) + land (AE+RT+FX)), (b) cognitive‐behavioural therapy Length: 8 weeks. Follow‐up: 26 weeks and 52 weeks Study design: randomised clinical trial with parallel groups |
|
| Participants | Female:Male: 40:0 Age, years (SD): pooled: 52.5 (8.8) Duration of Illness: not reported Inclusion: females with diagnosis of FM (ACR 1990) Exclusion: serious concomitant disease |
|
| Interventions | (a) Mixed exercise – MX (AQ (unspecified exercises) + Land (AE+RT+FX) (n = 19) ‐ Frequency: supervised 5/week (AQ 1/week, AE+RT 2/week, FX 2/week);Duration: 45 min; Intensity: light to vigorous (50% to 80% HRmax); Mode: AQ unspecified exercise in warm pool, AE cycle ergometry, RT isotonic exercises for upper limbs, unspecified exercises for trunk, FX static stretching for upper limb/lower limb/trunk muscles. Follow‐up: participants were instructed to maintain daily physical exercises at home. Note ‐ caution is needed here because conflicting and unclear information was provided in the published research report and in the author's response to our inquiry regarding the exercise intervention (b) Cognitive‐behavioural therapy (n = 21) ‐ 1/week for 2.5 hours focussing on managing chronic pain and increasing self‐efficacy |
|
| Outcomes | Health‐related quality of life (FIQ Total, SF‐36 general health, FIQ Feel Good), self‐reported physical function (FIQ, SF‐36 physical functioning, SF‐36 physical role), self‐efficacy (CPSS physical function, CPSS pain management, CPSS symptoms); Chronic Pain Coping Inventory (asking for assistance, guarding, resting, relaxation, task persistence, exercise, social support, self statements); mental health (SF‐36 mental health, social functioning, role emotional), pain (FIQ Pain, SF‐36 bodily pain, 5‐point Likert scale), fatigue (FIQ Fatigue, SF‐36 vitality), sleep disturbance (FIQ Sleep); stiffness (FIQ Stiffness), tenderness (TP count), depression (Beck Depression Index, FIQ Depression), anxiety (Beck Anxiety Inventory, FIQ Anxiety), cardiorespiratory function max (cycle ergometry VO2 max), muscle endurance (for shoulder abduction, knee extension, trunk flexion/extension) composite scores of ROM + pain + endurance; flexibility (ROM spine, upper limbs, lower limbs) Measurements at: baseline (0 weeks), post intervention (8 weeks), first follow‐up (26 weeks), second follow‐up (52 weeks) |
|
| Adherence | Attendance: (a) 84%, (b) 72% | |
| Congruence of EX protocol with ACSM criteria for aerobic, strength or flexibility | (a): AE, RT, and FX: not enough information to permit judgement | |
| Notes | Country: Spain Language: English Author contact: email response (November 2005) from author; information about study design, age of participants, exercise intervention, exercise protocol clarification provided Trial registry record or protocol available: none Funding source: none reported Conflict of interest: none reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Author communication ‐ "patients were allocated to study groups by means of a random numbers table generated by the SPSS program" |
| Allocation concealment (selection bias) | High risk | Author communication ‐ "allocation of patients was not concealed" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participant and personnel blinding not specified; unlikely that participant and personnel delivering the intervention were blinded |
| Detection Bias ‐ Subjective measures All outcomes | Unclear risk | Self‐report instruments were used to measure health‐related quality of life, pain, fatigue, stiffness, and physical function but active comparator used |
| Detection Bias ‐ Blinding of assessor reported outcomes All outcomes | Low risk | Not applicable; no assessor‐reported tests were applied to measure cardiorespiratory submaximal function or muscle strength |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Missing outcome data balanced in numbers across intervention groups, with similar reasons for missing data in both groups |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement of risk |
| Other bias | Low risk | Study appears to be free of other sources of bias |
Rooks 2007.
| Methods | 4 groups: (a) mixed exercise (AE+FX), (b) mixed exercise (RT+AE+FX), (c) RT + Fibromyalgia Self‐Help Course, (d) control (Fibromyalgia Self‐Help Course) Length: 16 weeks. Follow‐up: 26 weeks Study design: randomised clinical trial with parallel groups |
|
| Participants | Female:Male: pooled: 207:0 Age, years (SD): (a) 48 (11), (b) 50 (11), (c) 50 (11), (d) 50 (12) Duration of Illness, years (SD): (a) 5 (4), (b) 6 (4), (c) 6 (5), (d) 6 (6) Inclusion: women 18 to 75 years of age, diagnosis of FM (ACR1990) Exclusion: medical conditions that limited participants’ ability to perform the exercise protocol or for whom moderate‐level exercise was contraindicated |
|
| Interventions | (a) Mixed exercise (AE+FX; n = 51) ‐ Frequency: 2/week supervised, 1/week home programme; Duration: 60 min (WU 5 min AE 5 min initially progressed to 45 min; FX not specified); Intensity: AE participant self‐determined moderate effort; FX not specified; Mode: AE treadmill walking; FX: primary body movements (b)Mixed exercise (AE+RT+FX; n = 51) ‐ Frequency: 2/week supervised, 1/week home programme; Duration: 60 min (WU 5 min, AE 5 min initially progressed to 20 min, RT 25 min, FX not specified); Intensity: AE not specified, RT 1 set of 6 repetitions at 'easy' intensity (resistance level the participant could perform with proper technique) progressing to 2 sets of 10 to 12 repetitions (unspecified RM), participant determined progress through increased number of repetitions; FX not specified; Mode: AE treadmill walking, RT isotonic exercises using machines and handheld weights for LE and UE and trunk, FX primary body movements (c)RT + Fibromyalgia Self‐Help Course (n = 55) ‐ RT as described in (b), Fibromyalgia Self‐Help Course as described in (d) (d)Fibromyalgia Self‐Help Course (n = 50) – 7‐session programme providing information about FM and self‐management skills. Series of 5‐ to 15‐minute lectures with facilitated group discussion and supplementary readings for a total of 120 minutes, conducted every 2 weeks |
|
| Outcomes | Health‐related quality of life (FIQ Total, SF‐36 general health); self‐reported physical function (SF‐36 physical function, SF‐36 role physical); mental health (SF‐36 mental health subscale, SF‐36 social function, SF‐36 role emotional); self‐efficacy (Adapted Arthritis Self‐Efficacy Scale, pain and other symptom subscales); pain (FIQ Pain, SF‐36 bodily pain); fatigue (FIQ Daily Fatigue, FIQ Morning Fatigue, SF‐36 vitality); depression (FIQ Depression, Beck Depression Inventory total); anxiety (FIQ Anxiety); stiffness (FIQ Stiffness); cardiorespiratory function submax (6‐minute walk test distance, walking speed, resting heart rate, post‐test exercise heart rate); muscle strength (chest press, leg press) Measurements at: baseline (16 weeks), follow‐up (26 weeks) |
|
| Adherence | Attendance: (a) 73%, (b) 78%, (c) 78%, (d) 77% | |
| Congruence of EX protocol with ACSM criteria for aerobic, strength or flexibility | AE = frequency, duration, and/or intensity did not meet ACSM guidelines; FX = not enough information to permit judgement | |
| Notes | Country: USA Language: English Author contact: emailed author July 17, 2011; no response Trial registry record or protocol available: none Funding source Arthritis Foundation Investigator Award, National Institutes of Health Grants (K23 AR48305, RO3 AR047398, K24 AR02123, P60 AR47782, RR01032) Conflict of interest: none reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Generated randomisation utilised; stratified randomisation by level of functional status, using the FIQ (score < 40 or ≥ 40) |
| Allocation concealment (selection bias) | Low risk | Opaque envelopes, sealed, numbered sequentially, and stored in a locked cabinet utilised |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported; unlikely that participants or personnel providing the intervention were blinded |
| Detection Bias ‐ Subjective measures All outcomes | Unclear risk | Self‐report instruments were used to measure health‐related quality of life, pain intensity, fatigue, stiffness, and physical function, but intervention compared to self‐help intervention |
| Detection Bias ‐ Blinding of assessor reported outcomes All outcomes | Low risk | Assessors who measured cardiorespiratory submaximal function were blinded to group |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | ITT analysis utilised |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement of risk |
| Other bias | Low risk | Study appears to be free of other sources of bias |
Salaffi 2015.
| Methods | 2 groups: (a) MX (AE+RT)+ED), (b) control Length of Intervention: 12 weeks. Follow‐up: none Study design: randomised clinical trial, parallel groups |
|
| Participants | Female:Male = 65:7 Age, years (SD): pooled: 49, (a) 48.3 (11.3), (b) 49.6 (12.3) Duration of illness, years (SD): (a)10.1 (9.6), (b) 8.5 (8.8) (from time of diagnosis): mean time from pain onset 9.3 (range 1 to 20) Inclusion: aged 18 to 65 years, diagnosis of FM (ACR); average numerical rating scale (NRS) pain score ≥ 4; on stable doses of FM medications for ≥ 4 weeks; willing to limit the introduction of new FM medications Exclusion: cardiovascular disease; moderate to severe chronic lung disease; uncontrolled hypertension; uncontrolled thyroid disorders; orthopaedic or musculoskeletal conditions prohibiting moderate to intense exercise; active suicidal ideation; planned elective surgery during the study period; inflammatory rheumatic conditions (i.e. rheumatoid arthritis, systemic lupus erythematosus, and other connective tissue disease); schizophrenia or other psychoses; participation in moderate or vigorous exercise for ≥ 3 days a week |
|
| Interventions | (a) Multi‐component MX (AE+RT+FX)+ED) (n = 18): outpatient programme consisting ofFrequency: 2/week + home programme recommended; Duration: AE: 60 to 120 min/week, RT: unspecified, FX: unspecified I: AE 60% to 85% HRmax (began at 60% to 70% of maxHR and gradually increased to 75% to 85%) (light to moderate intensity) FX: initial loads were 1 to 3 kg for upper limbs and 3 to 5 kg for lower limbs, and participants were encouraged to increase the load by 1 kg/week over the course of the 12 weeks; Mode: stretching and strength exercises were prescribed on the basis of individual needs, with 1 set of 10 repetitions completed at individually specified loads. All sessions were supervised by 2 physiotherapists uninvolved in clinical assessments ED/Non‐exercise: 45 min of educational activities with a physician and physiotherapist covering topics related to characteristics of FM, such as its nature and usual course, treatment options, appropriate organisation of daily activities, and physician/patient relationships. Participants were given a basis for understanding and applying self‐control techniques, along with an opportunity to discuss the difficulties of everyday life and to share possible solutions Pharmacological treatment arranged during the recruitment phase was not modified and included tricyclic antidepressants (amitriptyline, maximum dose 75 mg/24 h), an anti‐inflammatory drug (ibuprofen, maximum dose 1800 mg/d), an analgesic (paracetamol, maximum dose 3 g/24 h), and a central opioid analgesic (tramadol, maximum dose 400 mg/24 h). Participants were asked to not change medications during the study period (b)Control (n = 18): medications as usual |
|
| Outcomes | Pain (FAS), fatigue (FAS), sleep (FAS), function (FIQR); other: FM overall impact (FIQR), FM symptoms (FIQR), FIQR Total Score, FAS Total Score Measurements: 0 and 12 weeks |
|
| Adherence | (a) attendance 97.9%. | |
| Congruence of EX protocol with ACSM criteria for aerobic, strength or flexibility | AE = frequency, duration, and/or intensity did not meet ACSM guidelines; RT = not enough information to evaluate congruence with ACSM guidelines | |
| Notes | Country: Italy Language: English Author contact: email 7 July 2015 provided answers on the exercise protocol Trial registry record or protocol available: none Funding source: none reported Conflict of interest: none reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation utilised |
| Allocation concealment (selection bias) | Low risk | Computer‐generated randomisation list prepared by biostatisticians uninvolved in the clinical conduct of the trial; list kept at a purpose‐designed control centre, which allocated assigned treatment when telephoned by clinical investigators, who were blinded to the allocation sequence |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not reported; unlikely that participants or personnel delivering the intervention were blinded |
| Detection Bias ‐ Subjective measures All outcomes | High risk | Self‐report instruments were used to measure health‐related quality of life, pain, and physical function |
| Detection Bias ‐ Blinding of assessor reported outcomes All outcomes | Low risk | Not applicable; no assessor‐reported tests were applied to measure cardiorespiratory submaximal function or muscle strength |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | "Seventy‐two of the 76 randomised patients completed the three‐month study. Among these patients, one patient stopped the physiotherapy, according advices of therapists, two participants in the control group explicitly cited an increase in pain as the reason for dropping out, and one patient moved to another region. These four patients were not included in the subsequent analysis" |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement of risk |
| Other bias | Low risk | Study appears to be free of other sources of bias |
Sanudo 2010b.
| Methods | 3 groups: (a) mixed exercise (AE+RT+FX), (b) aerobic exercise, (c) control Length: 24 weeks. Follow‐up: none Study design: randomised clinical trial with parallel groups |
|
| Participants | Female:Male: 64:0 Age, years (SD): (a) 55.9 (1.7), (b) 55.9 (1.6), (c) 56.6 (1.9) Duration of illness: not reported Inclusion: women with diagnosis of FM (ACR 1990) Exclusion: presence of inflammatory rheumatic disease and severe psychiatric illness, or respiratory or cardiovascular disease that prevents physical exertion; receiving psychological or physical therapy |
|
| Interventions | (a) Mixed exercise (AE+RT+FX; n = 21) ‐ Frequency: 2/week; Duration: 45‐60 min; Intensity: AE moderate intensity (65% to 70% HRmax); Mode: RT: 1 set of 8 to 10 reps for 8 different muscle groups with 1 to 3 kg load, FX: 1 set of 3 reps for 8 or 9 different static stretches (upper limb, lower limb, and trunk) held 30 s; AE: continuous movement with arm movements and jogging (b) Aerobic exercise (n = 22) ‐ Frequency: 2/week; Duration: 45 to 60 min (WU 10 min – slow walks, CD 10 min – slow walks, easy movements and relaxation training); Intensity: AE light to moderate steady state (60% to 65% HRmax) and vigorous intensity interval training (75% to 80% HRmax); Mode: WU: slow walks, easy movements of progressive intensity. AE – steady state: walking with arm movements and jogging, AE ‐ interval training: aerobic dance and jogging, CD: slow walks, easy movements, and relaxation training (c)Control (n = 21) ‐ usual medical treatment for FM, normal daily activities that did not include structured exercise |
|
| Outcomes | Health‐related quality of life (FIQ Total, SF‐36 overall, SF‐36 GH), physical function (SF‐36 PF, SF‐36 RP); mental health (SF‐36 MH), pain (SF‐36 BP), fatigue (SF‐36, VT), depression (FIQ VAS, BDI), stiffness (FIQ VAS), cardiorespiratory submax (6‐minute walk test distance), muscle strength (grip strength), flexibility (goniometer measure of hip and shoulder ROM) Measurements: 0 and 24 weeks |
|
| Adherence | Attendance: (a) 86%, (b) 89% | |
| Congruence of EX protocol with ACSM criteria for aerobic, strength or flexibility | (a) and (b) AE did not meet ACSM criteria (a) FX and RT met ACSM criteria |
|
| Notes | Country: Spain Language: English Author contact: Email response received 5 April 2012; confirmed no overlap in participants with Sanudo 2011 Trial registry record or protocol available: none Funding source: University of Seville Conflict of interest: none reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A computer‐generated random number sequence was used |
| Allocation concealment (selection bias) | Low risk | Randomisation completed by an individual not involved in recruitment or assessment of patients; randomisation list kept at a separate location in a locked filing cabinet |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not reported; unlikely that participants or personnel delivering the intervention were blinded |
| Detection Bias ‐ Subjective measures All outcomes | High risk | Self‐report instruments were used to measure health‐related quality of life, pain, fatigue, and physical function |
| Detection Bias ‐ Blinding of assessor reported outcomes All outcomes | Low risk | Assessor‐reported tests for cardiorespiratory submaximal function and muscle strength were carried out by an assessor blinded to group assignment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | ITT analysis utilised |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement of risk |
| Other bias | Low risk | Study appears to be free of other sources of bias |
Sanudo 2011.
| Methods | 2 groups: (a) mixed exercise (AE+RT+FX), (b) control Length: 24 weeks. Follow‐up: none Study design: randomised clinical trial with parallel groups |
|
| Participants | Female:Male: 42:0 Age, years (SD): (a) 55.5 (7.1), (b) 56.2 (8.5) Duration of illness: not reported Inclusion: women aged 18 to 65 years, diagnosis of FM (ACR 1990) Exclusion: any significant concomitant medical illness, such as inflammatory rheumatic disease or respiratory or cardiovascular disease that would prevent physical exercise; severe psychiatric illness; attended physical therapy or psychological therapy in the previous 3 months |
|
| Interventions | (a) Mixed exercise (AE+RT+FX) (n = 21) ‐ Frequency: 2/week; Duration: 45 to 55 min (WU 10 min – multi‐joint movements, AE: 10 to 15 min RT: 15 to 20 min, CD 10 min – flexibility); Intensity: AE moderate intensity (65% to 70% HRmax), RT initially light then progressed to participant‐tolerated loads, Mode: AE walking with arm movements and jogging, RT: isotonic concentric and eccentric, free weights for 8 muscles groups (upper limb, lower limb, and trunk); FX Static stretches for 8 or 9 exercise stations, 1 set of 3 reps with 30 s hold for 10 min (b) Control (n = 21) ‐ participants continued their normal daily activities with no structured exercise and continued with their current medication and use of 'rescue' analgesic as normal |
|
| Outcomes | Health‐related quality of life (FIQ Total, SF‐36), physical function (SF‐36), pain (SF‐36), fatigue (SF‐36), stiffness (FIQ VAS), depression (BDI), mental health (SF‐36), sleep (FIQ) Measurements: 0 and 24 weeks |
|
| Adherence | Attendance; (a) mean %: 85% | |
| Congruence of EX protocol with ACSM criteria for aerobic, strength or flexibility | (a) AE: frequency, duration, and/or intensity did not meet ACSM guidelines; RT ‐ not enough information to determine; FX met ACSM criteria | |
| Notes | Country: Spain Language: English Author contact: none Trial registry record or protocol available: none Funding source: none reported Conflict of interest: none reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random number table used |
| Allocation concealment (selection bias) | Low risk | Randomisation unknown until participant accepted or declined to participate; randomisation sequence not disclosed to the researcher responsible for day‐to‐day running of the trial until participants completed baseline assessments |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not reported; unlikely that participants or personnel delivering the intervention were blinded |
| Detection Bias ‐ Subjective measures All outcomes | High risk | Self‐report instruments were used to measure health‐related quality of life, pain, fatigue, and physical function |
| Detection Bias ‐ Blinding of assessor reported outcomes All outcomes | Low risk | Not applicable; no assessor‐reported tests were applied to measure cardiorespiratory submaximal function or muscle strength |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | ITT analysis utilised, last observation carried forward method Attrition: (a) 3/21 (14%) ‐ due to concomitant illness (pneumonia; n = 1) and for personal reasons (n = 2); (b) 1/21 (5%) ‐ reason unknown |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement of risk |
| Other bias | Low risk | Study appears to be free of other sources of bias |
Sanudo 2012.
| Methods | 2 groups: (a) mixed exercise: MX (AE+RT+FX), (b) control Length: 26 weeks. Follow‐up: 26 weeks Study design: randomised clinical trial with parallel groups |
|
| Participants | Female:Male: 41:0 Age: not reported Duration of Illness: not reported Inclusion: women with FM (ACR 1990) Exclusion: concomitant conditions such as inflammatory rheumatic disease, respiratory or cardiovascular disease, and severe psychiatric illness |
|
| Interventions | (a) MX (AE+RT+FX; n = 21)Frequency: 2/week; Duration: 45 to 60 min (WU 10 min – slow walking, gentle movements of progressive intensity, CD 10 min ‐ flexibility); Intensity: AE moderate intensity (65% to 70% HRmax), RT: loads 1 to 3 kg for different exercises; Mode: AE: continues walking with arm movements and jogging, RT 1 set 8 to 10 reps, 8 different muscle groups (deltoids, biceps, neck, hips, back and chest muscles), FX 1 set of 3 reps for 8 or 9 different exercises, maintaining stretch for 30 seconds (deltoids, biceps, neck, hips, back and chest muscles) (b) Control (n = 20): usual medical treatment, continued daily activities not including exercise |
|
| Outcomes | Health‐related quality of life (FIQ Total, Spanish version SF‐36), depression (BDI), cardiorespiratory submax (6MWT) Measurements: 0, 26, and 52 weeks |
|
| Adherence | (a) Participants were taught how to monitor their heart rate and adjust their activity to maintain the correct exercise intensity. Although target intensities were planned, participants were informed that they could return to a lower level of intensity as needed | |
| Congruence of EX protocol with ACSM criteria for aerobic, strength or flexibility | (a) AE did not meet ACSM criteria; RT and FX met ACSM criteria | |
| Notes | Country: Spain Language: English Author contact: Email response from authors 24 June 2013; provided information on study methods, intervention particulars, adherence, and outcomes Trial registry record or protocol available: none Funding source: University of Seville Conflict of interest: none reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Author response: "computer‐generated random number table" used for randomisation |
| Allocation concealment (selection bias) | Low risk | Author response: "Yes, central allocation" |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Author response: "participants were blind to the intervention (but no description provided)" Blinding of personnel not reported; author did not specifically answer this question in email response; unlikely that participants or personnel delivering the intervention were blinded |
| Detection Bias ‐ Subjective measures All outcomes | High risk | A self‐report instrument was used to measure health‐related quality of life |
| Detection Bias ‐ Blinding of assessor reported outcomes All outcomes | Low risk | Author response: "participants were asked not to discuss their care during the assessment with the assessor" |
| Incomplete outcome data (attrition bias) All outcomes | High risk | High dropout rates; ITT analysis not utilised ‐ "unequal numbers of participants withdrew from EG vs CG. Intially 21 in EG and 20 in CG but by end of 156 weeks there were 13 in EG and 12 in CG" |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement of risk |
| Other bias | Low risk | Study appears to be free of other sources of bias |
Sanudo 2013.
| Methods | 3 groups: (a) mixed exercise (AE+ST+FX) + Vib, (b) mixed exercise (AE+ST+FX), (c) control Length: 8 weeks. Follow‐up: none Study design: randomised controlled trial, 3 parallel groups |
|
| Participants | Female:Male: 46:0 Age, years (SD):(a) 57.15 (6.8), (b) 62.3 (9.8), (c) 55.6 (7.9) Duration of illness: not reported Inclusion: participants in a local FM support group (Seville, Spain) and in physician practices, diagnosis of FM (ACR 1990) Exclusion: no previous experience with vibratory training; 1 or more possible contraindications (acute hernia; thrombosis; diabetes; epilepsy; metabolic or neuromuscular disease; osteoporosis; osteoarthritis; orthopaedic injury and prosthesis); respiratory or cardiovascular disease that prevents physical exertion; taking drugs that could interfere with balance control; receiving psychological or physical therapy (to avoid possible interaction) |
|
| Interventions |
(a) Mixed exercise (AE+ST+FX) + Vib (n = 15): Mixed exercise ‐ Frequency: 2×/week, community‐based group exercises with supervision; Duration: 45 to 60 min (WU 10 min, AE 10 to 15 min, RT 15 to 20 min (1 set of 8 to 10 reps for 8 different muscle groups; load 1 to 3 kg), FX 10 min (FX (1 set of 3 reps for 8 or 9 different exercises, maintained for 30 s)); Intensity: AE moderate intensity (65% to 70% HRmax); Mode: AE ‐ walking mode not specified, RT and FX focussed on main areas of pain (deltoids, biceps, neck, hips, back, and chest). Vibration ‐ Frequency: 3×/week progressive training (2‐leg stance with knees @120 degrees of knee flexion), 30 Hz, peak‐to‐peak displacement 4 mm (71.1 m/s‐2˜7.2 g); Duration: weeks 1 to 2: 6 sets of 30 s, 45 s recovery between sets with participants standing with both feet on platform), and 4 sets of 30 s single leg (right and left immediately following each other), weeks 3 and 4: 7 sets 30 s/45 s rest (bilateral squat) and 5 sets of 30 s/45 s rest (unilateral squat). Weeks 5 and 6: 8 sets of 30 s/45 s rest (bilateral squat) and 6 sets of 30 s/45 s rest (unilateral squat). Weeks 7 and 8: 9 sets of 30 s, 45 s rest (bilateral squat), and 7 sets of 30 s, 45 s rest (unilateral squat) (b) Mixed exercise (AE+ST+FX; n = 15) ‐ Frequency: 2×/week, community‐based group exercise with supervision; Duration: 45 to 60 min (WU 10 min, AE 10 to 15 min, RT 15 to 20 min (1 set of 8 to 10 reps for 8 different muscle groups; load 1 to 3 kg), FX 10 min (FX (1 set of 3 reps for 8 or 9 different exercises, maintained for 30 s)); Intensity: AE moderate intensity (65% to 70% HRmax); Mode: AE ‐ walking mode not specified, RT and FX focussed on main areas of pain (deltoids, biceps, neck, hips, back, and chest) (c) Control (n = 16) ‐ usual care; no additional information provided |
|
| Outcomes | Power (# of reps of ½ squats in 1 min), balance(Biodex F1C Stability System: overall stability index open eyes, overall stability index closed eyes) Measurements: 0 and 8 weeks |
|
| Adherence | Not reported | |
| Congruence of EX protocol with ACSM criteria for aerobic, strength or flexibility | (a) and (b) AE did not meet ACSM criteria; RT not enough information to permit judgement; FX met ACSM criteria | |
| Notes | Country: Spain Language: English Author contact: email received 17 March 2014; provided clarification regarding sample size (only 5 participants in (c) dropped out of study), 11 participants were assessed at post‐test, assessor blinding Trial registry record or protocol available: none Funding source: none reported Conflict of interest: none reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random number sequence utilised |
| Allocation concealment (selection bias) | Low risk | Randomisation sequence was not disclosed to the researcher responsible for day‐to‐day running of the trial until participants had completed their baseline assessments |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants were blinded to group assignment before baseline measurements, after which all participants were informed of group assignment; blinding of personnel not reported; unlikely that participants or personnel delivering the intervention were blinded |
| Detection Bias ‐ Subjective measures All outcomes | Low risk | Not applicable; no self‐report measures were used |
| Detection Bias ‐ Blinding of assessor reported outcomes All outcomes | Low risk | Not applicable; none of the designated assessor‐reported tests were applied to measure cardiorespiratory submaximal function or muscle strength |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | ITT analysis utilised |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement of risk |
| Other bias | Unclear risk | Study appears to be free of other sources of bias |
Valkeinen 2008.
| Methods | 2 groups: (a) mixed exercise (AE+RT), (b) control Length: 21 weeks. Follow‐up: none Study design: randomised clinical trial with parallel groups |
|
| Participants | Female:Male = 26:0 Age, years (SD): (a) 59 (3), (b) 58 (3) Duration of illness: not reported. Inclusion: women aged over 50 years, diagnosis of FM (ACR 1990) Exclusion: severe cardiovascular disease, diabetes, severe osteoarthritis of large joints, thyroid disorders, other diseases that might confound study results; participation in regular aerobic and strength training; predictable difficulties in attending training sessions |
|
| Interventions |
(a) Mixed exercise (AE+RT; n = 15) ‐ Frequency: 3/week, alternately 2/week AE and 1/week RT and vice versa, averaging 1.5/week AE and 1.5/week RT, AE partially supervised, RT supervised; Duration: WU+CD unspecified, AE 30 to 60 min, RT 60 to 90 min; Intensity: AE low to vigorous intensity (from 'under aerobic threshold to over anaerobic threshold', page 1662), RT: 2 to 4 sets at 15 to 20 RM progressed to 2 to 6 sets at 5 to 8 RM, light to vigorous; Mode: AE = cycle ergometry, walking; RT = isotonic concentric using unspecified equipment (b): Controls (n = 11) ‐ activity as usual |
|
| Outcomes | Self‐reported physical function (HAQ total), patient‐rated global (VAS 100 cm), pain (VAS 100 cm), fatigue (VAS 100 cm), sleep quality (VAS 100 cm), cardiorespiratory function max (cycle ergometry VO2peak test: measuring peak/max VO2, blood lactate, heart rate, workload, and work time), strength (bilateral leg extension 1 RM, maximal isometric force for leg extension, elbow flexion, grip strength, trunk extension, and trunk flexion), walking speed for 10 min; time to climb 10 stairs without handrails Measurements: ‐2, 0, and 21 weeks |
|
| Adherence | Not reported | |
| Congruence of EX protocol with ACSM criteria for aerobic, strength or flexibility | (a) AE: met ACSM criteria for healthy adults; RT did not meet ACSM criteria | |
| Notes | Country: Finland Language: English Author contact: none Trial registry record or protocol available: none Funding source: Ministry of Education of Finland and Peurunka‐Medical Rehabilitation Foundation, Laukaa, Finland Conflict of interest: none reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to permit judgement of risk |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not reported; unlikely that participants or personnel delivering the intervention were blinded |
| Detection Bias ‐ Subjective measures All outcomes | High risk | Self‐report instruments were used to measure pain intensity, fatigue, and physical function |
| Detection Bias ‐ Blinding of assessor reported outcomes All outcomes | High risk | First author supervised strength and walking measurements and knew group assignment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | ITT analysis not utilised Attrition: (a) 2/15 (13%) ‐ 1 moved away, 1 had cardiovascular symptoms "unrelated to the present training"; (b) 0/11 (0%) |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement of risk |
| Other bias | Low risk | Study appears to be free of other sources of bias |
van Eijk‐Hustings 2013.
| Methods | 3 groups: (a) multi‐disciplinary (composite), (b) mixed (AE+RT), (c) control Length: 12 weeks. Follow‐up: 52 weeks Study design: randomised clinical trial with parallel groups |
|
| Participants | Female:Male: 129:7 Age, years (SD): (a) 41.6 (8.8), (b) 43.9 (7.6), (c) 42.9 (11) Duration of illness, years (SD): (a) 7.1 (6.8), (b) 6.2 (7.0), (c) 7.1 (6.4) Inclusion: recent (> 3 months) diagnosis of fibromyalgia (ACR 1990), literate, ages between 18 and 65 years, being seen in outpatient rheumatology clinics at 3 medical centres (Southern Netherlands), agreed to participate in the study Exclusion: pregnancy, involvement in litigation concerning work disability procedures, use of other non‐pharmacological treatment (psychological or physical treatment), alcohol or drug abuse, use of walking device |
|
| Interventions |
(a) Multi‐disciplinary (n analysed = 67). Multi‐disciplinary intervention aimed at optimising daily functioning, coping with pain and disability (sociotherapy, physiotherapy (MX (AE+RT+Relaxation)+Therapeutical exercise), psychotherapy, creative arts therapy) ‐ Frequency: 12‐week course, 3 half‐days/week (sociotherapy: 2×/week, physiotherapy: 2×/week, psychotherapy: 1×/week, creative arts therapy: 1×/week). Follow‐up consisted of 5 meetings over 9 months. Plus, a maximum of 7 individual therapy sessions; Duration: 2 therapy sessions per day of 90 minutes; Follow‐up: not specified; Intensity: not specified; Mode: AE = not specified. RT = strength training on arms and legs; free weights and callisthenics exercise (additional information from author) (b) Mixed exercise (n analysed = 19). Supervised land intervention: aerobic exercise and resistance training ‐ Frequency: 2×/week, home programme 1×/week; Duration: 60 min (WU: 10 min, AE: 30 min and RT: 15 min, CD: 5 min); home programme duration not specified; Intensity: AE: low to moderate intensity ‐ 55% to 64% of predicated maxHR; RT: not specified; Mode: AE: exercises on the floor of the gym, with or without help of steps (additional information from authors); RT: strength major muscle groups, type of RT not specified beyond weights. Home programme not specified beyond participants received a digital video for home exercises and were asked to perform home exercises 1×/week. Home exercises were not monitored (c) Control ‐ usual care (n = 48). Minimum = individualised education about FMS and lifestyle advice by a rheumatologist or a specialised rheumatology nurse within 1 or 2 consultations; could have also included diversity of other treatments such as physiotherapy or social support from the rheumatology nurse |
|
| Outcomes | Health‐related quality of life (FIQ Total, Societal Value for Health ‐ EQ‐5D, Overall Impression of Health ‐ EQ‐5D), self‐reported physical function (FIQ Physical Function, contractual hours of paid work per week, hours unpaid tasks and chores per week, hours leisure and social activities per week), pain (FIQ Pain VAS), fatigue (FIQ Fatigue VAS), stiffness (FIQ Stiffness VAS), depression (FIQ Depression VAS), sleep (FIQ Unrefreshed Sleep VAS), anxiety (FIQ Anxiety VAS); other: (hours sick leave per week, FIQ Days Feel Good, FIQ Days Not Missed Work, FIQ Job Ability, healthcare utilisation (# of contacts with GPs, medical specialists, physiotherapists, paramedical professionals)) | |
| Adherence | (a) 7 participants did not attend > 70% of scheduled sessions; (b) of those who started the intervention, only 8 participants attended > 70% of scheduled sessions | |
| Congruence of EX protocol with ACSM criteria for aerobic, strength or flexibility | Not enough information to permit judgement | |
| Notes | Country: Netherlands Language: English Author contacted: response received 24 November 2014; information on exercise protocol, study design, and adverse effects Trial registry record or protocol available: ISRCTN32542621 Funding source/declaration of interest: Maastricht University Medical Centre; Care Renewal Grants of medical insurance companies in the region |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random numbers utilised |
| Allocation concealment (selection bias) | Unclear risk | Opaque, sealed envelopes, following the order of consent to participate, utilised |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | (a) and (b) participants not informed about alternative treatment conditions; (c) participants were not informed about any intervention; unlikely personnel delivering the intervention were blinded |
| Detection Bias ‐ Subjective measures All outcomes | High risk | Self‐report instruments were used to measure health‐related quality of life, pain intensity, fatigue, stiffness, and physical function |
| Detection Bias ‐ Blinding of assessor reported outcomes All outcomes | Low risk | Not applicable; no assessor‐reported tests were applied to measure cardiorespiratory submaximal function or muscle strength |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | ITT analysis utilised |
| Selective reporting (reporting bias) | Low risk | Study protocol is available and all of the study’s pre‐specified primary outcomes (including adverse effects) have been reported in the pre‐specified way |
| Other bias | Low risk | Study appears to be free of other sources of bias |
van Santen 2002a.
| Methods | 3 groups: (a) mixed exercise (AE+RT+FX + Balance), (b) biofeedback + relax, (c) control Length: 24 weeks. Follow‐up: none Study design: randomised clinical trial with parallel groups |
|
| Participants | Female:Male: pooled: 129:0 Age, years: (a) 46.2 (range 26 to 59), (b) 44.4 (range 26 to 60), (c) 42.8 (range 26 to 59) Duration of illness, years: (a) 9.7 (range 1 to 37), (b) 10.1 (range 1 to 38), (c) 15.4 (range 3 to 40) Inclusion: diagnosis of FM (ACR 1990), female, aged 18 to 60 years, living within 30 km of the 2 research sites Exclusion: presence of comorbidity (ischaemic heart disease, arrhythmia, exercise‐induced asthma, unsettled disability compensation disputes, incapacitating psychological distress), localised myalgia |
|
| Interventions | (a) Mixed exercise (AE+RT+FX+Balance; n = 50) ‐ Frequency: 3/week (2 sessions supervised, 1 session unsupervised); Duration: 60 min (WU 10 min, AE+FX+Balance 30 min, RT 10 min, CD 10); Intensity: participant selected for AE, RT, and FX; Mode: RT isometric contractions, unspecified for AE and FX (b)Biofeedback + Relax (n = 50) – Frequency: 2/d (2/week supervised for first 8 weeks); Duration: 30 min. Intensity: not applicable; Mode: progressive relaxation technique (biofeedback added at supervised sessions) (c) Control (n = 29) ‐ usual care |
|
| Outcomes | Health‐related quality of life (AIMS Total, SIP Total), self‐reported physical function (SIP physical), patient‐rated global (5‐point Likert scale), mental health (SCL‐90R total, SIP psychological), pain intensity (VAS), tenderness (TP count, Total myalgic score), fatigue (VAS); cardiorespiratory max (max cycle ergometer test – maximum workload, RPE) Measurements: 0 and 24 weeks |
|
| Adherence | Attendance: (a) 37 of 47 completers attended > 67%, (b) 38 of 43 completers attended > 67% | |
| Congruence of EX protocol with ACSM criteria for aerobic, strength or flexibility | (a) Not enough information to permit judgement for AE, RT, and FX | |
| Notes | Country: Netherlands Language: English Author contact: contact attempted; no response Trial registry record or protocol available: none Funding source: Dutch Arthritis Society Conflict of interest: none reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not reported; unlikely that participants or personnel delivering the intervention were blinded |
| Detection Bias ‐ Subjective measures All outcomes | High risk | Self‐report instruments were used to measure health‐related quality of life, pain intensity, fatigue, and physical function |
| Detection Bias ‐ Blinding of assessor reported outcomes All outcomes | Low risk | Not applicable; no assessor‐reported tests were applied to measure cardiorespiratory submaximal function or muscle strength |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | ITT analysis utilised |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement of risk |
| Other bias | Low risk | Study appears to be free of other sources of bias |
van Santen 2002b.
| Methods | 2 groups: (a) high‐intensity AE, (b) low‐intensity MX (AE+RT+FX + Balance) Length: 20 weeks. Follow‐up: none Study design: randomised clinical trial, parallel groups |
|
| Participants | Female:Male: 33:0 Age, years: (a) 39 (range 20 to 54), (b) 45 years (range 25 to 58) Duration of illness, years: (a) 9 (range 2 to 27), (b) 12 (range 1 to 36) Inclusion: women ages 18 to 60 years, diagnosis of FM (ACR 1990), living within 30 km radius of research location Exclusion: ischaemic heart disease, arrhythmia, exercise‐induced asthma, unsettled disability compensation disputes, incapacitating psychological distress |
|
| Interventions |
(a) Exercise protocol (n = 18) ‐ Frequency: 3/week supervised; Duration: 60 min (WU 10 to 15 min, AE 45 min); Intensity: AE moderate or higher intensity (at least 70% HRmax); Mode: WU ball games and lower extremity stretching exercises, AE cycle ergometry (b) Exercise protocol (n = 15) ‐ Frequency: 2/week supervised and 1/week independent; Duration: 60 min (WU 10 min, AE 30 min, RT 10 min, CD 10 min); Intensity: patient directed; Mode: WU aerobic exercises and postural muscle stretching, AE+FX+balance ‐ unspecified exercises, RT isometric muscle strengthening; CD aerobic, stretching, and relaxation exercises |
|
| Outcomes | Multi‐dimensional function (AIMS social activities, AIMS health perception), self‐reported physical function (AIMS mobility, AIMS dexterity, AIMS ADL, AIMS physical activity), mental health (SCL‐90 global severity of psychological distress, phobic anxiety, somatisation, obsession/compulsion, interpersonal sensitivity, hostility, psychoticism) patient‐rated global (VAS), pain (AIMS pain), fatigue (VAS), sleep (SCL‐90 sleep), tenderness (TP count, Total myalgic score), depression (AIMS depression, SCL‐90 depression), anxiety (AIMS anxiety, SCL‐90 anxiety), cardiorespiratory function max/peak (cycle ergometry peak workload, peak RPE); other: AIMS social role Measurements: 0 and 20 weeks |
|
| Adherence | Not specified; (a) and (b) ‐ about 50% of participants were not able to fully comply with the training sessions | |
| Congruence of EX protocol with ACSM criteria for aerobic, strength or flexibility | (a) and (b) AE did not meet ACSM guidelines; RT and FX not enough information tp permit judgement | |
| Notes | Country: the Netherlands Language: English Author contact: via email 19 June 2011; response not received Trial registry record or protocol available: none Funding source: Dutch Arthritis Association Conflict of interest: none reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to permit judgement of risk |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement of risk |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported; (a) and (b) exercise conducted in different facilities with different instructors, so groups were unlikely to come into contact with other group members or other instructors; unlikely that participants or personnel delivering the intervention were blinded to the intervention |
| Detection Bias ‐ Subjective measures All outcomes | Unclear risk | Self‐report instruments were used to measure pain and physical function but likely minimised risk (both arms received treatment) |
| Detection Bias ‐ Blinding of assessor reported outcomes All outcomes | Low risk | Not applicable; no assessor‐reported tests were applied to measure cardiorespiratory submaximal function or muscle strength |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | ITT analysis utilised, but data not presented. Data for completers only (> 67% participation) presented |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement of risk |
| Other bias | Low risk | Study appears to be free of other sources of bias |
Verstappen 1997.
| Methods | 2 groups: (a) mixed exercise (AE+RT+FX), (b) control Length: 26 weeks. Follow‐up: none Study design: randomised clinical trial with parallel groups |
|
| Participants | Female:Male: 72:0 Age, years (SD): (a) 46.6 (8.3), (b) 42.8 (8.4) Duration of illness: not reported Inclusion: diagnosis of FM (Wolfe 1988), female, ages 18 to 60 years, registered with outpatient clinic within 2 years of study commencement Exclusion: ischaemic heart disease, cardiac arrhythmia, exercise‐induced bronchospasm, psychiatric disorder, currently involved in health insurance procedures |
|
| Interventions | (a) Mixed exercise (AE+RT+FX; n = 45) ‐ Frequency: 2/week plus home programme 1/week; Duration: 50 min (WU 10 min, AE+RT+FX 30 min, CD 10 min); Intensity: participant selected for AE and RT, FX unspecified; Mode: AE cycle ergometry or treadmill running, RT concentric and eccentric strengthening with Nautilus equipment for upper body, abdomen, and legs, FX not specified (b) Control (n = 27) not specified |
|
| Outcomes | Health‐related quality of life (patient‐rated, 5‐point ordinal scale), pain intensity (VAS); tenderness (TP count), cardiorespiratory max (max cycle ergometer – peak workload, peak HR, peak RPE), cardiorespiratory submax (max cycle ergometer test – HR at 50 W, RPE at 50 W), co‐ordination (time to perform 15 ball bounces, time to perform 25 hand plate taps), balance (displacing centre of gravity while standing on 1 leg), flexibility (sit and reach in long sitting), power (vertical jump, time to perform 15 sit‐ups), muscle endurance (isometric quads endurance), strength (grip strength); other: feeling of fitness (VAS), housekeeping (VAS), BMI, % body fat Measurements: 0 and 26 weeks |
|
| Adherence | (a) Not reported | |
| Congruence of EX protocol with ACSM criteria for aerobic, strength or flexibility | (a) AE did not meet ACSM criteria for healthy adults but met ACSM criteria for extremely deconditioned; RT and FX not enough information to permit judgement | |
| Notes | Country: the Netherlands Language: English Author contact: none Trial registry record or protocol available: none Funding source: Nationaal Rheumafonds Conflict of interest: none reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to permit judgement of risk |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not reported; unlikely that participants or personnel delivering the intervention were blinded |
| Detection Bias ‐ Subjective measures All outcomes | High risk | Self‐report instruments were used to measure quality of life and pain intensity |
| Detection Bias ‐ Blinding of assessor reported outcomes All outcomes | Low risk | Assessor‐reported tests for cardiorespiratory submaximal function and muscle strength were carried out by an assessor blinded to group assignment |
| Incomplete outcome data (attrition bias) All outcomes | High risk | ITT analysis not utilised (b) originally recruited 29 participants, data for 27 participants reported for post‐test; reasons for dropouts not reported. Error in Table 1 |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement of risk |
| Other bias | Low risk | Study appears to be free of other sources of bias |
Yuruk 2008.
| Methods | 2 groups: (a) mixed exercise (RT+FX), (b) RT Length: 8 weeks. Follow‐up: none Study design: randomised clinical trial with parallel groups |
|
| Participants | Female:Male: 27:0 Age, years (SD): (a) 47.9 (10.9), (b) 47.5 (8.8) Duration of illness: not reported Inclusion: diagnosis of FM (ACR 1990) Exclusion: coronary artery disease, hypertension that limited participation in physical exercise, diabetes mellitus, systemic orthopaedic and neurological problems, regular use of pain killers or antidepressants, participation in regular exercise for the last 3 months or participation in physical therapy in the last 6 months |
|
| Interventions | (a) Mixed exercise (RT+FX; n = 13) ‐ Frequency: 3/week home programme with weekly phone calls to participants; Duration: 30 min (WU 5 min, RT+FX 20 min, CD 5 min); Intensity: not specified; Mode: RT ‐ isometric exercises for neck, isotonic exercises for shoulder girdle and shoulders; FX ‐ stretching for neck, upper back, shoulders; postural exercises unspecified (b)RT (n = 14) ‐ Frequency: 3/week supervised; Duration: 20 to 30 min (WU 5 min, CD 5 min); Intensity: body weight vs gravity (callisthenics), progressed by number of repetitions, based on eliciting HR elevation no greater than 55% HRmax; Mode: callisthenics for upper and lower limbs, chest |
|
| Outcomes | Health‐related quality of life (FIQ Total, FIQ Feel Good, SF‐36 general health), self‐reported physical function (FIQ Physical Function, SF‐36 physical functioning, SF‐36 physical role); mental health (SF‐36 mental health, SF‐36 social functioning, SF‐36 role emotional); pain (FIQ Pain, SF‐36 bodily pain), fatigue (FIQ Fatigue, SF‐36 vitality), stiffness (FIQ Stiffness), tenderness (TP count), depression (FIQ Depression), anxiety (FIQ Anxiety), strength (grip strength ‐ dynamometer), flexibility (forward reach in long sitting); other: FIQ Days Worked Measurements: 0 and 8 weeks |
|
| Adherence | Not reported | |
| Congruence of EX protocol with ACSM criteria for aerobic, strength or flexibility |
(a) RT and FX ‐ not enough information to evaluate congruence with ACSM guidelines (b) RT ‐ not enough information to evaluate congruence with ACSM guidelines |
|
| Notes | Country: Turkey Language: Turkish Author contact: email response received 3 May 2013. Information included interventions, adverse effects, outcomes Trial registry record or protocol available: none Conflict of interest: none reported Funding source: none reported Other: methods and results translated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Email question: "How was randomization and allocation conducted in this study? Were participants blinded to the hypothesis?" Author response: "We conducted a simple randomization procedure (flipping a coin was use to assign the participant within each group)" |
| Allocation concealment (selection bias) | Unclear risk | See above; specific response to email question not provided |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Author response: "Yes, participants were blinded to the hypothesis"; "this study was a master thesis. All evaluations and interventions were done with same physiotherapist. We didn’t have blinding procedure" |
| Detection Bias ‐ Subjective measures All outcomes | Unclear risk | Self‐report instruments were used to measure health‐related quality of life, pain intensity, fatigue, stiffness, and physical function, but comparator (RE) likely minimised risk |
| Detection Bias ‐ Blinding of assessor reported outcomes All outcomes | High risk | Author response: "all evaluations and interventions were done with same physiotherapist. We didn’t have blinding procedure" |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Author response: "this study was my master thesis and we didn't report all outcome data. This study was a part of my study. Other measurements included subjective pain at rest (VAS) and cardiorespiratory endurance (measured with maximal oxygen consumption test in treadmill). We wanted to publish another research report including VO2max but we didn't" |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement of risk |
| Other bias | Unclear risk | Email question: "do any of the authors declare any conflicts of interest relate to the study?" Author response: "not available" Translation conducted Insufficient information to permit judgement of risk |
6MWT: six‐minute walk test; ACR: American College of Rheumatology; ACSM: American College of Sports Medicine; ADL: activities of daily living; AE: aerobic exercise; AIMS: Arthritis Impact Measurement Scales; AQ: aquatic; BDI: Beck Depression Inventory; BMI: body mass index; BP: bodily pain; CD: cool‐down; CES‐D: Centre for Epidemiological Studies‐Depression; CIS: characteristics of included studies; CPSS: Chronic Pain Self‐Efficacy Scale; ED: education; ex: exercise; EQ‐5D: standardised assessment of health‐related quality of life; ESR: erythrocyte sedimentation rate; FIQ: Fibromyalgia Impact Questionnaire; FM: fibromyalgia; FX: flexibility exercise; GH: general health; HAQ: Health Assessment Questionnaire; HR: heart rate; HRmax: heart rate maximum; HRQL: health‐related quality of life; IGF‐1: insulin‐like growth factor‐1; ITT: intention‐to‐treat; LD: land; max: maximum; LE: lower extremity; MH: mental health; min: minute; MVC: maximum voluntary contraction; MX: mixed; ns: not stated; PF: physical function; PT: physical therapy; Relax: relaxation; reps: repetitions; RF: Role functional; RM: repetition maximum; ROM: range of motion; RPE: rate of perceived exertion; RT: resistance training; SCL‐90R: Symptom Checklist 90‐Revised; SD: standard deviation; SE: standard error; SF: Short Form; SNRI: serotonin‐norepinephrine reuptake inhibitor; ST: strength; Ther Ex: therapeutic exercise; TP: tender point; UE: upper extremity; VAS: visual analogue scale; Vib: vibration; VO2: oxygen uptake; VT: vitality; WU: warm‐up.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Ahlgren 2001 | Diagnosis ‐ trapezius myalgia |
| Astin 2003 | Did not meet exercise criteria (QiGong) |
| Bailey 1999 | Not randomised (1‐group design) |
| Bakker 1995 | Between‐group analysis not conducted |
| Dawson 2003 | One group before‐after design |
| Gandhi 2000 | Not randomised ‐ 3‐group design: (1) non‐exercising control (n = 12), (2) hospital‐based exercise (n = 10), (3) home‐based videotaped exercise programme (n = 10) |
| Geel 2002 | Not randomised |
| Guarino 2001 | Diagnosis ‐ Gulf War syndrome |
| Han 1998 | Not randomised (geographic control) |
| Karper 2001 | Not randomised (programme evaluation) |
| Kendall 2000 | Did not meet exercise criteria (body awareness) |
| Kingsley 2005 | Diagnosis of FM made by physician or rheumatologist, but when contacted, authors did not verify the use of published criteria (e.g. ACR 1990 classification) |
| Mason 1998 | Not randomised (participants enrolled in multi‐modal treatment compared to those who were unable to participate due to insurance reasons) |
| Meiworm 2000 | Not randomised (participants self‐selected their groups) |
| Mobily 2001 | Case study |
| Nielen 2000 | Not randomised (cross‐sectional case control study of fitness) |
| Norregaard 1997 | Physical activity that did not meet criteria for mixed exercise |
| Offenbacher 2000 | Non‐experimental ‐ narrative review |
| Oncel 1994 | Insufficient description of exercise (1 group received "medical therapy and exercise"; no further information about the exercise intervention given) |
| Peters 2002 | Diagnosis ‐ persistent unexplained symptoms |
| Pfeiffer 2003 | One‐group before‐after design |
| Piso 2001 | Not randomised ‐ our translator reported: "the authors wrote only how they recruited nine of the patients. They wrote nothing about if and how the patients were allocated to the two groups" We were unsuccessful on several attempts to contact the authors for clarification |
| Rooks 2002 | One‐group design |
| Salek 2005 | Not an RCT |
| Thieme 2003 | Did not meet exercise criteria (passive PT with light movement in water ‐ active exercise was too small a component, was not described or quantified sufficiently) |
| Tiidus 1997 | One‐group repeated measures design |
| Vlaeyen 1996 | Insufficient description of the mode of exercise "Each session ended with a physical exercise such as swimming or bicycling, excluding systematic physical or fitness training" |
| Worrel 2001 | One‐group design |
FM: fibromyalgia; PT: physical therapy; RCT: randomised controlled trial.
Characteristics of studies awaiting assessment [ordered by study ID]
Amris K 2016.
| Methods | 2 groups: (a) ADAPT programme and (b) ACTIVE programme Length: 16 weeks Design: quasi‐randomised control trial with parallel group |
| Participants | Inclusion: women > 18 years of age who fulfilled the 1990 American College of Rheumatology (ACR) classification
criteria for fibromyalgia Exclusion: (a) severe physical impairment necessitating assistance in personal activities of daily living, (b) concurrent history of major psychiatric disorder not related to the pain disorder, (c) other medical conditions capable of causing patients symptoms (e.g. uncontrolled inflammatory/autoimmune disorder, uncontrolled endocrine disorder, malignancy), (d) not Danish‐speaking, (e) enrolment in any other clinical trial within the last 30 days |
| Interventions | (a) ADAPT programme included 16 2‐hour sessions and aimed to improve activities of daily living (ADL) ability by means of adaptation. Authors used compensatory and educational models as primary means to teach participants how to adapt more successfully. The sessions took place in a clinical ADL unit (i.e. a 2‐room flat used to observe and practice ADL task performance in a simulated, but naturalistic, home environment) (b) ACTIVE programme included 10 2‐hour sessions and aimed at improving ADL ability by means of graded physical activity. Education was the primary means to implement strategies to increase physical activity in everyday life. The programme was conducted in a clinical unit fitted for group discussions and performance of light exercises (e.g. resistance band exercises) |
| Outcomes | Activities of daily life ability (AMPS), health‐related quality of life (SF‐36), physical functioning (MOS and SF‐36 subscale), disease severity (FIQ), pain (FIQ Subscale) |
| Notes | Country: Denmark Awaiting response from author for confirmation of population diagnosis, randomisation, and details of the intervention (June 2018) |
Collado‐Mateo 2017.
| Methods | 2 groups: (a) VirtualEx‐FM, (b) control Length: 8 weeks Design: single‐blinded randomised controlled trial with parallel group |
| Participants | Inclusion: (1) women between 30 and 75 years of age, (2) with fibromyalgia diagnosed by a rheumatologist according to the criteria of the American College of Rheumatology, (3) able to communicate effectively with study staff, and (4) had read, understood, and signed the written informed consent form Exclusion: (1) pregnant, (2) changed their usual care therapies during the 8 weeks of treatment, (3) had contraindications for physical exercise |
| Interventions | (a) VirtualEx‐FM: participants exercised 2×/week (1 hour per session) using Kinect (Microsoft). Participants attended the local
FM association’s facilities and performed each session in groups of 3. Training was based on an exergame, the VirtualEx‐FM, which has been specifically designed by the research group to improve physical conditioning and the ability to perform activities of daily living of women with FM. This programme consists of 3 virtual environments developed to allow the patient to perform several motor training exercises. The VirtualEx‐FM focusses on postural control and co‐ordination of the upper and lower limbs, aerobic conditioning, strength, and mobility, while always keeping the quality of movement patterns in mind. The VirtualEx‐FM session has the following parts: warm‐up using a video in which an expert performs joint movements of the upper and lower limbs. Participants are encouraged to imitate these movements. The speed of these movements can be manually controlled at 0.5, 1, 1.5, and 2. The second part is an aerobic component performed by following dance steps marked by a professional kinesiologist and dance teacher. The third portion is postural control and co‐ordination, which are trained through a game, in which participants have to reach an apple that appears and disappears near them. The body part used by the participant to reach for the apple is indicated. Finally, walk training is developed using a circuit comprising a trail of footprints on a virtual floor. Participants must step on the virtual footprints and walk on the circuit. Amplitude and cadence are controlled by the technician. The interface allows selection of different types of steps: a normative step, on tiptoe, heel walking, raised knees, and raised heels (b) Control: continued their normative daily life |
| Outcomes | Impact of disease (FIQ and FIQ‐R), quality of life (EQ‐5D‐5L), attendance, pain (VAS and algometer), self‐reported number of falls, fear of falling (VAS), work absence (self‐reported), number of visits to the health system, perceived effort (Borg scale), body composition (bioelectrical impedance analysis (Tanita BC‐418)), depression (GDS), well‐being (SWLS), activities of daily living (FAB), dynamic balance (time up and go), lower limb strength (30‐step chair stand test and 10‐step stair climbing test with and without carrying a load), hand‐grip strength (grip‐strength dynamometer), aerobic endurance (six‐minute walk test), upper body strength (arm curl test), velocity (time needed to walk 20 metres will be recorded), balance (Biodex Balance System) |
| Notes | Country: Spain Awaiting final team decision on classification/new type of intervention |
Genc 2015.
| Methods | 2 groups: (a) home exercise (FX+ST), (b) home exercise (FX+ST+AE) Length: 6 weeks Design: randomised controlled trial with parallel design |
| Participants | Inclusion: female, admitted to the fibromyalgia outpatient clinic of the Physical Medicine and Rehabilitation Department of the institution, diagnosed by the 1990 American College of Rheumatology classification criteria Exclusion: individuals with an endocrine, metabolic, infectious, or neurological disease; cancer; connective tissue disorder; a cardiac, respiratory, or orthopaedic disease that might have hindered AE; hormonal dysfunction; pregnancy; menopause; or a cognitive function hampering assessments. Individuals receiving any treatment such as psychological or physical therapy for the last 3 months and individuals who were in need of medication for anxiety or depression during the study |
| Interventions | (a) Home exercises: flexibility and stretching (b) Home exercises: flexibility, stretching, and aerobic |
| Outcomes | Pain (VAS), tender point count, morning stiffness duration (minutes), fatigue, cardiovascular fitness (ergospirometric exercise tolerance test), functional disability (FIQ), and health‐related quality of life (SF‐36). Sleep quality (difficulty in falling asleep (the number of nights/week on which the patient experienced difficulty falling asleep), frequent awakening during sleep (0 = none, 1 = some of the nights, 2 = every night), and quality of sleep (0 = good, 1 = moderate, 2 = unrefreshing). Plasma ACTH, IGF‐1, and GH levels (immunoradiometric assays); fasting basal levels of GH, IGF‐1, adrenocorticotropic hormone (ACTH), and cortisol (blood samples) |
| Notes | Country: Turkey Awaiting final team decision on classification |
Kibar 2015.
| Methods | 2 groups: (a) balance and flexibility, (b) flexibility Length: 6 weeks Design: randomised controlled trial with parallel design |
| Participants | Inclusion: (a) age range, 18 to 65 years, (b) diagnosis of fibromyalgia by an experienced physiatrist based on the 2010 American College of Rheumatology diagnostic criteria Exclusion: vitamin B12, 25‐OH vitamin D, and folate deficiencies; diabetes mellitus; neurological diseases; rheumatoid diseases; eye and internal ear pathologies; advanced cardiovascular or lung pathologies; and uncontrolled hypertension or hypotension. In addition, those who underwent surgery, who had injuries in their lower extremities (knees, hips, ankles, feet), and who were admitted to a physical therapy and/or exercise programme for their pain within the last year |
| Interventions | (a) Balance and flexibility: (flexibility) participants engaged in 2 sessions of active static exercises and were informed of the necessity of exercising 5 days/week. Participants performed stretches in 8 large muscle groups (neck, back, lower back, biceps, triceps, gluteus, iliopsoas, quadriceps femoris, hamstring, gastrocsoleus) in three 60‐second static stretching repetitions. To the extent that patients were capable, they held the static stretches for 30 to 60 seconds. Ten minutes of walking in place was recommended as warm‐up for the stretching exercises. A physiotherapist supervised the entire programme; (balance) these exercises involved postures that gradually reduced the base of support (2‐legged stand, semi‐tandem stand, tandem stand, 1‐legged stand), dynamic movements that disturbed the centre of gravity (tandem walk, circle turns), exercises that stressed the postural muscle groups (heel or toe stands), and exercises that reduced sensory input (standing with eyes closed). Training was for 20 sessions over a 4‐week period (20 minutes for each session, 5 days/week). The group also received 5 minutes of static and 5 minutes of dynamic balance training with the KAT device 3 days/week. This device has a movable platform and a tilt sensor that is connected to a computer. Participants maintained their balance by tilting the platform in all directions without moving their feet (b) Flexibility: participants engaged in active static exercises. They performed stretches in 8 large muscle groups (neck, back, lower back, biceps, triceps, gluteus, iliopsoas, quadriceps femoris, hamstring, gastrocsoleus) in three 60‐second static stretching repetitions. To the extent that patients were capable, they held the static stretches for 30 to 60 seconds. Ten minutes of walking in place was recommended as warm‐up for the stretching exercises. A physiotherapist supervised the entire programme |
| Outcomes | Fall history (interview), functional balance (Berg Balance Scale), dynamic and static balances (KAT device), risk of fall (Hendrich II fall risk model), disease impact (FIQ), quality of life (Nottingham Health Profile), depression (Beck Depression Inventory) |
| Notes | Country: Trurkey Awaiting final team decision on classification |
Kurt 2016.
| Methods | 3 groups: (a) balneotherapy, (b) balneotherapy and aerobic exercise, (c) aerobic exercise Length: unclear. Follow‐up: 3 months Design: randomised controlled trial |
| Participants | Inclusion: female patients 18 to 65 years of age with diagnosis of fibromyalgia according to the American College of Rheumatology 2010 diagnostic criteria, stable on pharmacological treatment over the last 3 months Exclusion: patients who had cardiac, respiratory, gastrointestinal, renal, or hematological disorders and neurological or psychiatric disorders too severe to allow participation in balneotherapy or exercise programme. Pregnancy or cancer, having advanced osteoarthritis, joint malformation, spinal disorders, or trauma within the last 3 months; inflammatory rheumatic disorders, history of smoking, having had modifications related to fibromyalgia medications within the last 3 months or alcohol intake. Those who participated in a physical therapy programme within the last year were also excluded |
| Interventions | (a) and (b) Balneotherapy: 20‐minute balneotherapy program 5 days a week for a total of 15 sessions at 42 ± 1° C in Kırehir Terme oligometallic thermal water containing a total mineralisation content of 556 mg/L bicarbonate, 98.2 mg/L sulphur, 34.5 mg/L magnesium, 226 mg/L calcium, 232 mg/L chlorine, and 2.6 mg/L fluorine (b) and (c) Groups were administered an aerobic exercise programme 5 days a week for a total of 15 sessions, which initially started with 25 minutes and was extended to 35 minutes 1 week later in a gradual intensification pattern The exercise programme included muscle stretching, strengthening, and relaxation exercises with few repeats to increase heart rate by 60% to 70%, particularly involving muscles of the cervical, thoracic, and lumbar regions |
| Outcomes | Depression (Beck Depression Scale, FIQ Depression), anxiety (FIQ), sleep quality (Pittsburgh Sleep Quality Index), tenderness (18 points), functional status (FIQ), stiffness (FIQ), pain (FIQ), fatigue (FIQ) |
| Notes | Country: Turkey Awaiting author response on confirmation of length of intervention, details of the aerobic intervention, and protocol availability |
Mutlu 2013.
| Methods | 2 groups: (a) mixed exercise (AE+FX+Relax)+Vib, (b) mixed exercise (AE+FX+Relax)+placebo Vib, (c) control (medication as usual) Length: 6 weeks. Follow‐up: none Study design: randomised clinical trial with parallel groups |
| Participants | Inclusion: women, diagnosis of FM (ACR 1990) for at least 3 years Exclusion: any orthopaedic limitation; cardiovascular, pulmonary, or metabolic disease that would preclude exercise |
| Interventions | (a) Mixed exercise, relaxation, vibration: total duration (over 12 sessions) of aerobic exercise, stretching, and relaxation was 9 hours, 6 hours, and 4 hours, respectively: (1) exercise protocol ‐ Frequency: 2/week; Duration: 90 min (WU 15 min, AE 30 min, FX 25 min, relax 20 min), Intensity: AE moderate to vigorous intensity (65% to 85% HRmax); FX to stop point; Mode: AE: primarily level ground walking with games dance; FX: 5 × 5 whole body stretches, 30 s hold, 30 s relax, involving hamstrings, calves, Achilles tendons, shoulders, arms, gluteals, cervical spine, low back, upper back, chest, hip adductors, (2) vibration exercise ‐ Frequency: 2/week; Duration: 4.5 min sessions 1 min and 2 min, 18 min sessions 3 to 12; Intensity: body weight resistance; Mode: six 30 s lower extremity exercises (static and dynamic), vibratory stimulus: vibration frequency 30 Hz with 2 mm amplitude; (3) relaxation exercise ‐ Mode: diaphragmatic respiration, progressive muscular relaxation, contraction – relaxation, and imagery techniques; pharmacological care as usual* (b) Mixed exercise, relaxation, placebo vibration: (1) exercise protocol ‐ as per Group (a), (2) placebo vibration ‐ as per Group (a) but the apparatus did not produce vibrations, (3) relaxation exercise ‐ as per Group (a) (c) Control: pharmacological care as usual |
| Outcomes | Health‐related quality of life (FIQ Total), pain (FIQ), fatigue (FIQ), stiffness (FIQ), depression (FIQ) |
| Notes | Country: Turkey Awaiting final team decision on classification |
Paolucci 2016.
| Methods | 3 groups: (a) physical exercise, (b) perceptive rehabilitation, (c) control Length: 12 weeks Study design: randomised controlled trial with parallel assignment |
| Participants | Inclusion: (a) fibromyalgia diagnosed according to the criteria proposed by the American College of Rheumatology (ACR 1990 and 2010), (b) aged between 18 and 60 years, (c) visual analogue scale (VAS) for pain > 5 Exclusion: presence of concomitant autoimmune diseases, psychiatric disorders, other causes of chronic pain, other diseases that prevented physical loading, severe scoliosis or kyphoscoliosis, surgery of the spine, vertebral fractures, sciatic pain, tumours, enrolled in another type of physical therapy programme, myocardial infarction, lower extremity arterial disease, major neurological problems, diabetes, gastrointestinal disease, chronic respiratory disease, kidney disease, poor vision |
| Interventions | (a) Exercise: includes 10 one‐hour sessions, held 2×/week with 4 individuals (group). Different types of exercises such as low‐impact to moderate aerobic training (gradually starting from 50% of the Fc max to 70% to 80% of the Fc max); walking fast in a circle, alternating with periods of going up and down the stairs (3 steps for 10 minutes) for a total of 20 consecutive minutes; posture exercises for the back and proprioceptive exercises for the trunk in the supine position to improve axial stability, including diaphragmatic breathing. Heart rate was monitored through the use of a heart rate monitor, which allowed not to exceed the threshold. A brief educational session was done by a physiatrist (b) Perceptive rehabilitation: therapeutic system that is based on the interaction between the patient's back or painful area and a support surface, composed of small latex cones of various dimensions (height: 3 to 8 cm; base diameter: 2 to 4 cm) and elasticities. The inferior bases of these cones are applied to a rigid wood surface using elastic strips (c) Control: 1 one‐hour brief educational session with breathing, relaxation, and stretching exercises to perform at home. They were asked to continue with their regular lifestyle |
| Outcomes | Disease impact (FIQ), pain (fibromyalgia assessment status), activities of daily living (Health Assessment Questionnaire) |
| Notes | Country: Italy Awaiting final team decision on classification |
Ranque 2017.
| Methods | 2 groups: (a) adapted physical activity programme, (b) standard care Length: 24 weeks Design: feasibility study |
| Participants | Inclusion Exclusion |
| Interventions | (a) Adapted physical activity "Fibromyactiv" programme ‐ 2 hours 3×/week (b) Standard care |
| Outcomes | FIQ |
| Notes | Country: France Awaiting translation for final team decision on classification |
Salvat 2017.
| Methods | 2 groups: (a) control or (b) multi‐disciplinary treatment Design: retrospective analysis of a randomised, simple‐blinded, clinical trial |
| Participants | Inclusion: female, diagnosis according to diagnostic criteria of the American College of Rheumatology, age 18 to 60, 3 to 8 years of schooling Exclusion: coexistence of another severe chronic pain pathology (e.g. sciatica, complex regional pain syndrome), diagnosis of inflammatory rheumatic disease, physical inability to perform exercises, an open wound, a skin disease, under psychiatric and/or psychological treatment within the past 3 years, significant suicidal ideation, cognitive or sensorial deterioration, pending disability‐related legal resolution |
| Interventions | (a) Control: usual (pharmacological) care (b) Multi‐disciplinary group: received 24 physical and cognitive–behavioural therapy (CBT) sessions during 2 consecutive hours twice weekly in reduced groups of 8 patients (12 weeks). The physical therapy component involved 2 sessions per week including hydrokinesitherapy and strength training. The CBT programme included information about FM, theory of pain perception, cognitive restructuring skills training, CBT for primary insomnia, assertiveness training, goal setting, activity pacing and pleasant activity scheduling training, life values, and relapse prevention. All were given a pedometer (Yamax Digi‐walker SW‐200) to encourage activity and an audio CD to practice Schultz autogenic training at home |
| Outcomes | Impact of disease (FIQ); functional status (WONCA); submaximal aerobic capacity (6MWT); sleep quality (The Medical Outcome Study Sleep Scale to Sleep Index Problems); coping skills (Coping Strategies Questionnaire) |
| Notes | Country: Spain Awaiting team decision on classification (re: design) |
Sevimli 2015.
| Methods | 3 groups: (a) isometric strength stretching, (b) aerobic gymnastics, and (c) aquatic aerobic exercise program (AAEP) Length: 12 weeks Design: randomised, parallel design |
| Participants | Inclusion: diagnosis according to ACR definition, ages 18 to 50 Exclusion: postmenopausal, over 50 years of age, cardiovascular problems, Cushing syndrome, younger than 18 years |
| Interventions | (a) Isometric strength and stretching (n = 25): performed home‐based isometric strength and stretching exercise programme lasting 15 minutes per day (b) Aerobic gymnastics (n = 25): attended a gymnastic‐based aerobic exercise programme with group therapy 2 times per week (c) Aquatic exercise (n = 25): attended a pool‐based aquatic aerobic exercise programme with group therapy 2 times per week. Durations of the exercise programme was 40 minutes for AE program and AAEP in the first month, 45 minutes in the second month, and 50 minutes in the third month |
| Outcomes | Pain (VAS), health status (FIQ), aerobic submax (Six‐Minute Walk Test), quality of life (SF‐36), depression (Beck Depression Inventory) |
| Notes | Country: Turkey Awaiting final team decision on classification |
Toprak 2017.
| Methods | 2 groups: (a) exercise programme with connective tissue massage (CTM), and (b) exercise programme without CTM Length: 6 weeks Design: randomised controlled trial with parallel design |
| Participants | Inclusion: women diagnosed by a rheumatologist according to 1990 criteria of ACR, referred to the Physiotherapy and Rehabilitation clinic, 18 to 65 years of age Exclusion: neurological, infectious, endocrine, and other inflammatory rheumatic diseases; severe psychological disorders; any condition interfering with exercise (advanced cardiac respiratory or orthopaedic problems); malignancy; pregnancy; intervention including exercise programme or physical therapy in the last 6 months |
| Interventions | (a) Exercise programme: 2×/week led by a physical therapist × 60 min. It was composed of 10‐min warm‐up exercises, 40‐min aerobic and strengthening exercises, 10‐min cool‐down, stretching exercises including neck, trunk, and upper and lower limb muscles. Aerobic exercise consisted of 20 min of walking on a treadmill. Muscle strengthening exercises were performed with elastic resistive bands for 20 min, for strengthening deep neck muscles, deltoid, latissimus dorsi, serratus anterior, scapular retractor muscles, pectoralis major, shoulder external rotator muscles, erector spinae, abdominalis, gluteus, and quadriceps muscles. Exercises started with yellow or red Thera‐Bands (Hygenic Corporation, Akron, OH, USA) at mild or medium tension. When they performed 15 repetitions without serious pain or fatigue, participants progressed to the next colour resistance band in the sequence of green and blue. They had 10 repetitions with a holding period of 10 s each. CTM: 2×/week for a total of 12 sessions by the same experienced physical therapist. Patients were in a sitting position; CTM included the lumbosacral region and the lower thoracic, scapular, interscapular, and cervical regions. Each session lasted around 5 to 20 min (b) Same exercise programme as above without CTM |
| Outcomes | Pain (VAS), fatigue (VAS), sleep problem (VAS), health status (FIQ), quality of life (SF‐36) |
| Notes | Country: Turkey Awaiting final team decision on classification |
6MWT: six‐minute walking test; AAEP: aquatic aerobic exercise program; ACR: American College of Rheumatology; ACTH: adrenocorticotropic hormone; ADL: activities of daily living; AE: aerobic exercise; AMPS: Activities of daily life ability; CBT: cognitive‐behavioural therapy; CD: cool‐down; CTM: connective tissue massage; EQ‐5D‐5L: five‐level standardised assessment of health‐related quality of life; FAB: activities of daily living; FIQ: Fibromyalgia Impact Questionnaire; FM: fibromyalgia; FX: flexibility exercise; GDS: Geriatric Depression Scale; GH: growth hormone; HRmax: heart rate maximum; IGF‐1: insulin‐like growth factor‐1; KAT:kinesthetic ability trainer; MOS: Medical Outcome Study; Relax: relaxation; SF‐36: Short Form‐36; ST: strength; SWLS: Satisfaction With Life Scale; VAS: visual analogue scale; Vib: vibration; WONCA: World Organization of Family Doctors; WU: warm‐up.
Characteristics of ongoing studies [ordered by study ID]
da Silva 2015.
| Trial name or title | Effects of exercise training and photobiomodulation therapy (EXTRAPHOTO) on pain in women with fibromyalgia and temporomandibular disorder: study protocol for a randomised controlled trial |
| Methods | 4 groups: (a) phototherapy, (b) mixed exercise (AE+FX+facial exercise), (c) phototherapy + mixed exercise, (d) control (placebo phototherapy) Length: 10 weeks. Follow‐up: none Study design: randomised clinical trial with parallel groups |
| Participants | Inclusion: (1) women ≥ 35 years of age presenting with at least a 5‐year diagnosis of FM and TMD, optimised drug treatment; (2) · cognitive independence to respond to inquiries; (3) functionally independent regarding daily physical activity; (4) availability and ability to fully comply with the training process and phototherapy, no contraindication to the research procedures Exclusion: (1) prior regular and structured physical activity programme; (2) missing more than 3 times from treatment; (3) presence of psychiatric disorders; (4) missing teeth and/or use of dentures; (5) history of trauma to the face; (6) currently undergoing orthodontic intervention; (7) any contraindication to exercise or phototherapy; (8) suspicion of other conditions: osteoarthritis, bursitis, tendinitis, rheumatoid arthritis, palindromic rheumatism, polymyalgic rheumatic disease, hydroxyapatite crystal disease, systemic lupus erythematosus, dermatomyositis‐polymyositis complex, Lyme disease, hypothyroidism or hyperthyroidism, hyperparathyroidism; (9) previous history of hepatitis, Epstein‐Barr virus infection, and Sjögren, McArdle, Addison, Cushing, and paraneoplastic syndromes |
| Interventions | (a) PTO: Frequency: 2/week; Intensity: 39.3 Joules of total energy; Time: 300 s; Mode: irradiation applied to each active FM tender point and to temporomandibular joints (bilaterally) using Pain Away portable device (9‐diode cluster) (b) Mixed exercise: Frequency: 2/week, Time: 3 reps of 30 s followed by 30 s of rest to each muscle; Intensity: produce mild discomfort; Mode: active stretching to biceps, trapezius, latissimus dorsi, pectoralis, paraspinal, hamstrings, and quadriceps. AE: Duration: 30 minutes; Intensity: 75% of age‐predicted maximum heart rate (220 − age in years); Mode: walking on electronic motorised treadmill (LX‐150 Movement: www.movement.com.br/index.php?principal=1; Sao Paulo, Brazil) without inclination. Exercises for TMD: Time: 3 repetitions for every movement. Mode: Maximum oral opening will be required for the first exercise; the second exercise will be a tongue slippage on the palate; and the third exercise will be oral lateralisation to the right and left with contraction of the masseter muscle. This exercise will be conducted with the participant’s mouth filled with air for 3 s. Ultimately, circular fingertip motions will be applied with slight pressure on the temporomandibular joint and the masseter muscle (c) PTO + Mixed exercise (d) Control: placebo PTO |
| Outcomes | Pain intensity (visual analogue scale, McGill Pain Questionnaire), pain thresholds (digital algometer), FM symptoms (Fibromyalgia Impact Questionnaire), Quality of life (SF‐36), serotonin levels (enzyme‐linked immunosorbent assay of salivary samples) Measured at baseline and 10 weeks |
| Starting date | Start date: March 2013 End date: March 2015 |
| Contact information | Correspondence: fisioterapeutamariana@gmail.com Nove de Julho University, Rua Vergueiro, 235, Liberdade, São Paulo, SP, 01504‐000, Brazil |
| Notes | Recruitment status: unknown ClinicalTrials.gov identifier: NCT02279225 |
Gusi N.
| Trial name or title | Effectivity of virtual reality physical exercise programme in brain and motor aging in fibromyalgia |
| Methods | 2 groups: (a) VirtualEx‐FM programme, (b) control Length: 24 weeks Design: randomised controlled trial |
| Participants | Inclusion: (a) women, (b) between 30 and 75 years, (c) diagnosis of fibromyalgia by a rheumatologist, (d) able to communicate effectively with study staff, (e) can read and signed the written informed consent Exclusion: (a) pregnancy, (b) changes in usual care therapies during 8 weeks of treatment, (c) contraindications for physical exercise |
| Interventions | (a) VirtualEx‐FM programme: consists of 2 weekly 1‐hour sessions for 24 weeks. It is based on a Motion‐Controlled Video Game on Microsoft Xbox Kinect carried out indoors in a room at the local fibromyalgia association's building (b) Control: no details specified |
| Outcomes | Health‐related quality of life (EQ‐5D‐5L and Whoqol), disease impact (FIQ and FIQ‐R), cost‐effectiveness (EQ‐5D‐5L and WHOQOL), lower limb strength (30 s chair stand test, 10‐step stair climbing test), hand‐grip strength (grip‐strength dynamometer), aerobic endurance (Canadian Aerobic Fitness Test, 6‐min walking), upper body strength (Arm Curl Test), balance (Biodex Balance System), upper and lower body flexibility (chair sit‐and‐reach and back scratch), cognitive tasks (Functional Assessment of Biomechanics and a wireless electroencephalography system (Enobio, Neuroelectrics)), electrical activity and volumes (Enobio (Neuroelectrics, Cambridge, MA, USA), magnetic resonance imaging (MRI)), Cognitive impairment (Mini‐Mental State Examination and Stroop test), psychophysiological response (EEG register), pain‐related fear (Tampa Scale for Kinesiophobia), cortisol and melatonin levels (using saliva samples), pain (visual analogue scale, current pain today, algometer on tender points), depression (Geriatric Depression Scale), body composition (bioelectrical impedance analysis) and waist‐to‐hip ratio, perceived effort (Borg Scale). Drug treatment, cost‐effectiveness (number of visits to the healthcare system), self‐reported work absence (number of days participants missed work), fear of falling (VAS from 0 (no fear) to 100 (extreme fear) and using the FES‐I questionnaire), number of falls, volume of physical activity during free time (international physical activity questionnaire) Sociodemographics: gender, age, education level, profession, income level, religiosity, postal code, and familial situation. Other diseases, current treatment and therapies, years since diagnosis of FM, years since first symptoms. Sleep quality, latency, duration, efficiency, disturbances, use of sleep medication (Pittsburgh Sleep Quality Index), health habits (EUROPALIQ) |
| Starting date | Start date: November 2017 |
| Contact information | Correspondence: Dr. Narcis Gusi; ngusi@unex.es |
| Notes | Status: no longer recruiting ISRCTN65034180: https://doi.org/10.1186/ISRCTN65034180 |
Mendonça Araújo F.
| Trial name or title | Effect of interferential current combined with exercise in patients with fibromyalgia: randomised clinical trial |
| Methods | 2 groups: (a) mixed exercise (AE+FX+ST) plus application of interferential current, (b) mixed exercise (AE+FX+ST) plus application of interferential current placebo Length: not specified ‐ mention 24 sessions Study design: randomised clinical trial, parallel, double‐blind, with 2 arms |
| Participants | Inclusion: (1) fibromyalgia, (2) diagnosed according to criteria of the American College of Rheumatology, (3) female, (4) aged 18 to 60 years, (5) without physical therapy concomitant Exclusion: (1) concomitant rheumatological disease, (2) severe psychiatric disorders, (3) any contraindication to prevent the use of interferential current, such as allergies to electrodes, cardiac pacemaker, pregnancy, epilepsy, skin conditions, or deficient skin sensation in the areas of electrode placement |
| Interventions | (a) Mixed exercise (AE+FX+ST) plus application of interferential current; the exercise protocol consists of 15 minutes of stretching, 10 minutes of aerobic exercise, and 15 minutes of muscle strengthening. Concomitant with exercise, interferential current (IFC) will be applied in paravertebral region for 40 minutes. Four auto‐adhesive electrodes will be placed diagonally on the upper angle of the scapula region and lumbar spine, just above the iliac crests. Frequency of amplitude modulated at 100 Hz will be used. Pulse amplitude or intensity of stimulation will be maintained at a strong but comfortable level as reported by the participant. At 5‐minute intervals, the intensity level will be increased again (b) Mixed exercise (AE+FX+ST) plus application of interferential current placebo; each session consists of an exercise protocol, previously described, and application of placebo. For application of placebo, electrodes are applied in the paravertebral region, but the IFC device will work only in the first 40 seconds, then no current will be released to the patient |
| Outcomes | Pain (numerical scale of 11 points, pressure pain threshold, McGill pain questionnaire, temporal summation test and modulation condition of pain test), quality of life, sleep quality, muscle strength, and cutaneous sensitive threshold. Reduction of depression, anxiety, physical disability; pain‐related negative expectations; fear of movement; red areas marked in thermographic image; and number of cytokines. To assess these variables, the following will be used: Fibromyalgia Impact Questionnaire, Short Form 36 Health Survey, Pittsburgh Sleep Quality Index, dynamometer, Von Frey filaments, Beck Depression Inventory, State‐Trait Anxiety Inventory, Roland Morris Disability Questionnaire, sit‐to‐stand test, six‐minute walk test, Pain Catastrophizing Scale, Tampa Kinesiophobia Scale, infrared thermographic camera, and blood collection. For verification of expected outcomes, variation of at least 5% for each scale and questionnaire used before, during, and after the intervention will be considered |
| Starting date | Registration date: December 2016 |
| Contact information | Correspondence: Fernanda Mendonça Araújo; nanda.maraujo@hotmail.com Avenida Augusto Franco, n. 3553, bloco G, apto. 503 49047‐040, Aracaju Brazil |
| Notes | Recruitment status: completed ICTRP web portal main ID: RBR‐6dk3y3 |
Montañez‐Aguilera J.
| Trial name or title | Change in sleep quality of patients with fibromyalgia subjected to a protocol based on physical exercise and stretching |
| Methods | 2 groups: (a) aerobic exercise, (b) stretching + aerobics Length: 24 weeks Design: randomised controlled trial with parallel design |
| Participants | Inclusion: (a) 18 years of age or older; (b) diagnosis of fibromyalgia according to criteria established by the American College of Rheumatology, on the basis of its publication of 1990 or the current revision for 2010; (c) acceptance to volunteer and give oral consent Exclusion: (a) do not present any pathology for which physical exercise is contraindicated; (b) do not suffer another serious somatic illness or severe psychological disorder; no severe dementia (MMSE < 10); (c) not participating at the time of the study in any other physical or psychological intervention |
| Interventions | (a) Aerobic exercise protocol of moderate intensity, 3 sessions per week, about 12 minutes, pedaling on a static bike (b) Aerobic exercise protocol of moderate intensity, 3 sessions per week, about 12 minutes, pedaling on a static bike plus muscle stretching programme at the end of the aerobic exercise for the main muscle groups of the body |
| Outcomes | Sleep quality (Pittsburgh Sleep Quality Index, sleep scale), sleepiness (Epworth Sleepiness Scale), pain (VAS), disease impact (FIQ) |
| Starting date | Start date: August 2016 Completion date: March 2017 |
| Contact information | Correspondence: F. Javier Montañez‐Aguilera Moncada, Valencia, Spain, 46113 |
| Notes | Recruitment status: completed ClinicalTrials.gov identifier: NCT02876965; https://clinicaltrials.gov/ct2/show/NCT02876965 |
Ruiz Ruiz J.
| Trial name or title | Exercise in women with fibromyalgia Official title: Land‐ and water‐based exercise intervention in women with fibromyalgia: the Al‐Andalus physical activity randomised controlled trial |
| Methods | 3 groups: (a) water base exercises, (b) land base exercises, (c) control interventions Length: 24 weeks Design: randomised controlled trial |
| Participants | Inclusion: (a) 35 to 65 years; (b) meeting American College of Rheumatology criteria: widespread pain for longer than 3 months, and pain with 4 kg/cm of pressure reported for 11 or more of 18 tender points; c) no other severe somatic or psychiatric disorders, or other diseases that prevent physical loading (answer "no" to all questions on the Physical Activity Readiness Questionnaire‐PAR‐Q); (d) not engaged in regular physical activity > 20 minutes on > 3 days/week; (e) planning to stay in the same association during the study; (f) able to ambulate, with or without assistance; (g) able to communicate; (h) must be capable and willing to provide consent Exclusion: (a) acute or terminal illness; (b) myocardial infarction in the past 3 months; (c) not able to ambulate; (d) unstable cardiovascular disease or other medical condition; (e) upper or lower extremity fracture in the past 3 months; (f) severe dementia (MMSE < 10); (g) unwillingness to complete study requirements or to be randomised into control or training group; (h) presence of neuromuscular disease or drugs affecting neuromuscular function |
| Interventions | (a) Water‐based exercise intervention will consist of aerobic, muscular strength, and flexibility exercises in water (b) Land‐based exercise intervention will consist of aerobic, muscular strength, and flexibility exercises on land (c) No intervention: control group |
| Outcomes | Impact of disease (FIQ), tenderness (18 patients), pain (VAS, Pain Catastrophizing Scale), body composition (weight, height, BMI, skeletal body mass, total body water and fat free mass (bioelectrical impedance analysis)), functional capacity (Functional Senior Fitness Test Battery), fatigue (Multidimensional Fatigue Inventory), sleep quality (Pittsburgh Sleep Quality Index), health‐related quality of life (SF‐36), cognitive function (Mini Mental State Examination) |
| Starting date | Starting date: November 2011 Completion date: December 2014 |
| Contact information | J. Ruiz Ruiz, University of Granada |
| Notes | Recruitment status: completed ClinitalTrials.gov Identifier: NCT01490281; https://clinicaltrials.gov/ct2/show/NCT01490281 |
AE: aerobic exercise; BMI: body mass index; EEG: electroencephalography; EQ‐5D‐5L: five‐level standardised assessment of health‐related quality of life; EUROPALIQ: health habits questionnaire; FES: Falls Eficacy Scale; FIQ: Fibromyalgia Impact Questionnaire; FM: fibromyalgia; FX: flexibility training; IFC: interferential current; MMSE: Mini Mental State Examination; MRI: magnetic resonance imaging; SF‐36: Short Form‐36; ST: strength; TMD: temporomandibular disorder; VAS: visual analogue scale; WHOQOL: World Health Organization Quality of Life.
Differences between protocol and review
Given the growth in the literature, the original review has now been split into several reviews (i.e. resistance, aquatic, aerobic, flexibility, whole body vibration, and mixed exercises). There are several differences between the 2007 review and this update, including the following.
Team membership has increased and changed since the 2002 update. Some members of the team wrote a new (WBV) protocol to reflect and incorporate new advances in evidence synthesis methodology. Team members who helped with writing the protocol ‐ Busch 2013 ‐ differ from those preparing the original protocol ‐ Busch 2002.
In 2007, we used 11 items of the van Tulder (van Tulder 2003) methodological criteria that reflect internal validity to classify studies into high‐, moderate‐, and low‐quality studies. For data synthesis, greater weight was placed on moderate‐ to high‐quality studies comparing exercise‐only interventions to controls. In this review, we used the Cochrane risk of bias tool (Higgins 2017 Ch8_ROB).
Methodological differences between the 2007 review and this update include revisions suggested by the 2011 Cochrane Handbook for Systematic Reviews of Interventions, and by MECIR Standards 2015, in addition to revisions to the search terms and databases, incorporation of the standardised electronic screening programme, and use of data extraction sheets and training programmes for review authors.
Meta‐regression and intervention/education subgroup analyses were not planned in the Busch 2013 protocol but were incorporated in this review.
Contributions of authors
| Task | Review Author |
| Conceived the review and led the team | AJB, JB |
| Designed and reviewed the (WBV) protocol | AJB, KS, IVdS, ST, JB, TO, CB |
| Designed and implemented the search strategy | CB |
| Screened for inclusion and exclusion | CB, AJB, JB, (SK not an author in this review) |
| Extracted data and assessed risk of bias | AJB, CLS, VDHS, JB, KEM, SW, SMG, TO |
| Contributed expert opinion on exercise physiology and systematic review methodology | CLS, TO, AJB, JB, SW |
| Performed statistical analysis and GRADE assessment | AJB, JB |
| Prepared initial manuscripts drafts | AJB, JB, CLS, KEM, SW, SMG, TO, CB |
| Commented on and reviewed the manuscript final version | All authors and consumers |
| Contributed to plain language summary write‐up | Consumers (Janet Gunderson and Anne Lyddiatt) and CB, JB, AJB |
Sources of support
Internal sources
School of Rehabiliation Science, University of Saskatchewan, Canada.
College of Medicine, University of Saskatchewan, Canada.
External sources
No sources of support supplied
Declarations of interest
We confirm that any present or past affiliations or other involvement in any organisation or entity with an interest in this review which might lead me/us to have a real or perceived conflict of interest is listed below.
None known.
New
References
References to studies included in this review
Alentorn‐Geli 2008 {published data only}
- Alentorn‐Geli E, Padilla J, Moras G, Lazaro Haro C, Fernandez‐Sola J. Six weeks of whole‐body vibration exercise improves pain and fatigue in women with fibromyalgia. Journal of Alternative & Complementary Medicine 2008;14(8):975‐81. [DOI] [PubMed] [Google Scholar]
- Alentorn‐Geli, E. PROTOCOL: Effects of Whole‐Body Vibration Exercise on Serum IGF‐1 in Fibromyalgia. ClinicalTrials.gov, March 31, 2008; Vol. NCT00650715.
Baptista 2012 {published data only}
- Baptista AS. Effectiveness of Dance on Patients With Fibromyalgia (Study Protocol). ClinicalTrials.gov (http://clinicaltrials.gov/show/NCT00961805), August 2009.
- Baptista AS, Villela AL, Jones A, Natour J. Effectiveness of dance in patients with fibromyalgia: a randomized, single‐blind, controlled study. Clinical & Experimental Rheumatology 2012;30(6 Suppl 74):18‐23. [PubMed] [Google Scholar]
Buckelew 1998 {published data only}
- Buckelew SP, Conway R, Parker J, Deuser WE, Read J, Witty TE, et al. Biofeedback/relaxation training and exercise interventions for fibromyalgia: a prospective trial. Arthritis Care and Research 1998;11(3):196‐209. [DOI] [PubMed] [Google Scholar]
Burckhardt 1994 {published data only}
- Burckhardt CS, Mannerkorpi K, Hedenberg L, Bjelle A. A randomized, controlled clinical trial of education and physical training for women with fibromyalgia. Journal of Rheumatology 1994;21(4):714‐20. [PubMed] [Google Scholar]
Clarke‐Jenssen 2014 {published data only}
- Clarke‐Jenssen AC, Mengshoel AM, Strumse YS, Forseth KO. Effect of a fibromyalgia rehabilitation programme in warm versus cold climate: a randomized controlled study. Journal of Rehabilitation Medicine 2014;46(7):676‐83. [DOI] [PubMed] [Google Scholar]
Da Costa 2005 {published data only}
- Costa D, Abrahamowicz M, Lowensteyn I, Bernatsky S, Dritsa M, Fitzcharles MA, et al. A randomized clinical trial of an individualized home‐based exercise programme for women with fibromyalgia. Rheumatology 2005;44(11):1422‐7. [DOI] [PubMed] [Google Scholar]
Demir‐Gocmen 2013 {published data only}
- Demir‐Gocmen D, Altan L, Korkmaz N, Arabaci R. Effect of supervised exercise program including balance exercises on the balance status and clinical signs in patients with fibromyalgia. Rheumatology International 2013;33(3):743‐50. [DOI] [PubMed] [Google Scholar]
Etnier 2009 {published data only}
- Etnier JL, Karper WB, Gapin JI, Barella LA, Chang YK, Murphy KJ. Exercise, fibromyalgia, and fibrofog: a pilot study. Journal of Physical Activity & Health 2009;6(2):239‐46. [DOI] [PubMed] [Google Scholar]
Garcia‐Martinez 2011 {published data only}
- Garcia‐Martinez AM, Paz JA, Marquez S. Effects of an exercise programme on self‐esteem, self‐concept and quality of life in women with fibromyalgia: a randomized controlled trial. Rheumatol International 2011;32(7):1869‐76. [DOI] [PubMed] [Google Scholar]
Genc 2002 {published data only}
- Genc A, Sagiroglu E. Comparison of two different exercise programs in fibromyalgia treatment. [Turkish]. Fizyoterapi Rehabilitasyon 2002;13(2):90‐5. [Google Scholar]
Giannotti 2014 {published data only}
- Giannotti E, Koutsikos K, Pigatto M, Rampudda ME, Doria A, Masiero S. Medium‐/long‐term effects of a specific exercise protocol combined with patient education on spine mobility, chronic fatigue, pain, aerobic fitness and level of disability in fibromyalgia. BioMed Research International 2014;2014(Article ID 474029):9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masiero S. Medium‐long term effects of a specific exercise protocol combined with patient education on spine mobility, chronic fatigue, pain, aerobic fitness and level of disability in fibromyalgia: a case‐control study. http://drks‐neu.uniklinik‐freiburg.de/drks_web/navigate.do?navigationId=trial.HTML&TRIAL_ID=DRKS00005071, 2013/06/14. [DOI] [PMC free article] [PubMed]
Hunt 2000 {published data only}
- Hunt J, Bogg J. An evaluation of the impact of a FM self‐management programme on patient morbidity and coping. Advancing in Physiotherapy 2000;2(4):168‐75. [Google Scholar]
Jones 2007 {published data only}
- Jones KD, Burckhardt CS, Deodhar AA, Perrin NA, Hanson GC, Bennett RM. A six‐month randomized controlled trial of exercise and pyridostigmine in the treatment of fibromyalgia. Arthritis & Rheumatism 2008;58(2):612‐22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones KD, Deodhar AA, Burckhardt CS, Perrin NA, Hanson GC, Bennett RM. A combination of 6 months of treatment with pyridostigmine and triweekly exercise fails to improve insulin‐like growth factor‐I levels in fibromyalgia, despite improvement in the acute growth hormone response to exercise. Journal of Rheumatology 2007;34(5):1103‐11. [PubMed] [Google Scholar]
Joshi 2009 {published data only}
- Joshi MN, Joshi R, Jain AP. Effect of amitriptyline vs. physiotherapy in management of fibromyalgia syndrome: what predicts a clinical benefit?. Journal of Postgraduate Medicine 2009;55(3):185‐9. [DOI] [PubMed] [Google Scholar]
Martin 1996 {published data only}
- Martin L, Nutting A, MacIntosh BR, Edworthy SM, Butterwick D, Cook J. An exercise program in the treatment of fibromyalgia. Journal of Rheumatology 1996;23(6):1050‐3. [PubMed] [Google Scholar]
Paolucci 2015 {published data only}
- Paolucci T, Vetrano M, Zangrando F, Vulpiani MC, Grasso MR, Trifoglio D, et al. MMPI‐2 profiles and illness perception in fibromyalgia syndrome: the role of therapeutic exercise as adapted physical activity. Journal of Back and Musculoskeletal Rehabilitation 2015;28(1):101‐9. [DOI: ] [DOI] [PubMed] [Google Scholar]
Rivera Redondo 2004 {published data only}
- Rivera RJ, Moratalla JC, Valdepenas MF, Garcia VY, Oses PJJ, Ruiz ZJ, et al. Long‐term efficacy of therapy in patients with fibromyalgia: a physical exercise‐based program and a cognitive‐behavioral approach. Arthritis and Rheumatism 2004;51(2):184‐92. [DOI] [PubMed] [Google Scholar]
Rooks 2007 {published data only}
- Rooks DS, Gautam S, Romeling M, Cross ML, Stratigakis D, Evans B, et al. Group exercise, education, and combination self‐management in women with fibromyalgia: a randomized trial. Archives of Internal Medicine 2007;167(20):2192‐200. [DOI] [PubMed] [Google Scholar]
Salaffi 2015 {published data only}
- Salaffi F, Ciapetti A, Gasparini S, Atzeni F, Sarzi‐Puttini P, Baroni M. Web/Internet‐based telemonitoring of a randomized controlled trial evaluating the time‐integrated effects of a 24‐week multicomponent intervention on key health outcomes in patients with fibromyalgia. Clinical and Experimental Rheumatology 2015;33(1 Suppl 88):S93‐S101. [PubMed] [Google Scholar]
Sanudo 2010b {published data only}
- Sanudo B, Galiano D, Carrasco L, Blagojevic M, Hoyo M, Saxton J. Aerobic exercise versus combined exercise therapy in women with fibromyalgia syndrome: a randomized controlled trial. Archives of Physical and Medical Rehabilitation 2010;91(12):1838‐43. [DOI] [PubMed] [Google Scholar]
Sanudo 2011 {published data only}
Sanudo 2012 {published data only}
- Sanudo B, Carrasco L, Hoyo M, McVeigh JG. Effects of exercise training and detraining in patients with fibromyalgia syndrome: a 3‐yr longitudinal study. American Journal of Physical Medicine & Rehabilitation 2012;91(7):561‐73. [DOI] [PubMed] [Google Scholar]
Sanudo 2013 {published data only}
- Sanudo B, Carrasco L, Hoyo M, Oliva‐Pascual‐Vaca A, Rodriguez‐Blanco C. Changes in body balance and functional performance following whole‐body vibration training in patients with fibromyalgia syndrome: a randomized controlled trial. Journal of Rehabilitation Medicine 2013;45(7):678‐84. [DOI] [PubMed] [Google Scholar]
Valkeinen 2008 {published data only}
- Valkeinen H, Alen M, Hakkinen A, Hannonen P, Kukkonen‐Harjula K, Hakkinen K. Effects of concurrent strength and endurance training on physical fitness and symptoms in postmenopausal women with fibromyalgia: a randomized controlled trial. Archives of Physical Medicine and Rehabilitation 2008;89(9):1660‐6. [DOI] [PubMed] [Google Scholar]
van Eijk‐Hustings 2013 {published data only}
- Kroese MEAL. Fibromyalgia on the move. Randomised study on the effect and cost‐effectiveness of a multidisciplinary part‐time daycare intervention. BioMed Central 2013; Vol. ISRCTN32542621:http://www.isrctn.com/ISRCTN32542621. [DOI: 10.1186/ISRCTN32542621] [DOI]
- Eijk‐Hustings Y, Kroese M, Boonen A, Bessems‐Beks M, Landewe R. Predictors for health improvement in patients with fibromyalgia: a 2‐year follow‐up study. Clinical Rheumatology 2015;34:133‐41. [DOI: 10.1007/s10067-013-2371-7] [DOI] [PubMed] [Google Scholar]
- Eijk‐Hustings Y, Kroese M, Tan F, Boonen A, Bessems‐Beks M, Landewe R. Challenges in demonstrating the effectiveness of multidisciplinary treatment on quality of life, participation and health care utilisation in patients with fibromyalgia: a randomised controlled trial. Clinical Rheumatology 2013;32(2):199‐209. [DOI] [PubMed] [Google Scholar]
van Santen 2002a {published data only}
- vanSanten M, Bolwijn P, Verstappen F, Bakker C, Hidding A, Houben H, et al. A randomized clinical trial comparing fitness and biofeedback training versus basic treatment in patients with fibromyalgia. Journal of Rheumatology 2002;29(3):575‐81. [PubMed] [Google Scholar]
van Santen 2002b {published data only}
- vanSanten M, Bolwijn P, Landewe R, Verstappen F, Bakker C, Hidding A, et al. High or low intensity aerobic fitness training in fibromyalgia: does it matter?. Journal of Rheumatology 2002;29(3):582‐7. [PubMed] [Google Scholar]
Verstappen 1997 {published data only}
- Verstappen FTJ, Santen‐Hoeuftt HMS, Bolwijn PH, LS, Kuipers H. Effects of a group activity program for fibromyalgia patients on physical fitness and well being. Journal of Musculoskeletal Pain 1997;5(4):17‐28. [Google Scholar]
Yuruk 2008 {published data only}
- Yuruk OB, Gultekin Z. Comparison of two different exercise programs in women with fibromyalgia syndrome [Turkish]. Fizyoterapi Rehabilitasyon 2008;19(1):15‐23. [Google Scholar]
References to studies excluded from this review
Ahlgren 2001 {published data only}
- Ahlgren C, Waling K, Kadi F, Djupsjobacka M, Thornell L, Sundelin G. Effects on physical performance and pain from three dynamic training programs for women with work‐related trapezius myalgia. Journal of Rehabilitation Medicine 2001;33(4):162‐9. [PubMed] [Google Scholar]
Astin 2003 {published data only}
- Astin JA, Berman BM, Bausel B, Lee WL, Hochberg M, Forys KL. The efficacy of mindfulness meditation plus Qigong movement therapy in the treatment of fibromyalgia: a randomized controlled trial. Journal of Rheumatology 2003;30(10):2257‐62. [PubMed] [Google Scholar]
Bailey 1999 {published data only}
- Bailey A, Starr L, Alderson M, Moreland J. A comparative evaluation of a fibromyalgia rehabilitation program. Arthritis Care & Research 1999;12(5):336‐40. [DOI] [PubMed] [Google Scholar]
Bakker 1995 {published data only}
- Bakker C, Rutten M, Santen‐Hoeufft M, Bolwijn P, Doorslaer E, Linden S. Patient utilities in fibromyalgia and the association with other outcome measures. Journal of Rheumatology 1995;22:1536‐43. [PubMed] [Google Scholar]
Dawson 2003 {published data only}
- Dawson KA, Tiidus PM, Pierrynowski M, Crawford JP, Trotter J. Evaluation of a community‐based exercise program for diminishing symptoms of fibromyalgia. Physiotherapy Canada 2003;55:17‐22. [Google Scholar]
Gandhi 2000 {published data only}
- Gandhi N. Effect of an exercise program on quality of life of women with fibromyalgia. Effect of an Exercise Program on Quality of Life of Women With Fibromyalgia. Eugene, OR, USA: Microform Publications, University of Oregon, 2000. [Google Scholar]
Geel 2002 {published data only}
- Geel SE, Robergs RA. The effect of graded resistance exercise on fibromyalgia symptoms and muscle bioenergetics: a pilot study. Arthritis and Rheumatism 2002;47:82‐6. [DOI] [PubMed] [Google Scholar]
Guarino 2001 {published data only}
- Guarino P, Peduzzi P, Donta ST, et al. A multicenter two by two factorial trial of cognitive behavioral therapy and aerobic exercise for Gulf War veterans' illnesses: design of a Veterans Affairs Cooperative Study (CSP #470). Controlled Clinical Trials 2001;22:310‐32. [DOI] [PubMed] [Google Scholar]
Han 1998 {published data only}
- Han SS. Effects of a self‐help program including stretching exercise on symptom reduction in patients with fibromyalgia. Taehan Kanho 1998;37:78‐80. [PubMed] [Google Scholar]
Karper 2001 {published data only}
- Karper WB, Hopewell R, Hodge M. Exercise program effects on women with fibromyalgia syndrome. Clinical Nurse Specialist 2001;15:67‐75. [DOI] [PubMed] [Google Scholar]
Kendall 2000 {published data only}
- Kendall SA, Brolin MK, Soren B, Gerdle B, Henriksson KG. A pilot study of body awareness programs in the treatment of fibromyalgia syndrome. Arthritis Care and Research 2000;13:304‐11. [DOI] [PubMed] [Google Scholar]
Kingsley 2005 {published data only}
- Kingsley JD, Panton LB, Toole T, Sirithienthad P, Mathis R, McMillan V. The effects of a 12‐week strength‐training program on strength and functionality in women with fibromyalgia. Archives of Physical Medicine and Rehabilitation 2005;86(9):1713‐21. [DOI] [PubMed] [Google Scholar]
Mason 1998 {published data only}
- Mason LW, Goolkasian P, McCain GA. Evaluation of multimodal treatment program for fibromyalgia. Journal of Behavioral Medicine 1998;21(2):163‐78. [DOI] [PubMed] [Google Scholar]
Meiworm 2000 {published data only}
- Meiworm L, Jakob E, Walker UA, Peter HH, Keul J. Patients with fibromyalgia benefit from aerobic endurance exercise. Clinical Rheumatology 2000;19(4):253‐7. [DOI] [PubMed] [Google Scholar]
Mobily 2001 {published data only}
- Mobily KE, Verburg MD. Aquatic therapy in community‐based therapeutic recreation: pain management in a case of fibromyalgia. Therapeutic Recreation Journal 2001;35:57‐69. [Google Scholar]
Nielen 2000 {published data only}
- Nielens H, Boisset V, Masquelier E. Fitness and perceived exertion in patients with fibromyalgia syndrome. Clinical Journal of Pain 2000;16:209‐13. [DOI] [PubMed] [Google Scholar]
Norregaard 1997 {published data only}
- Norregaard J, Lykkegaard JJ, Mehlsen J, Danneskiold Samsoe B. Exercise training in treatment of fibromyalgia. Journal of Musculoskeletal Pain 1997;5(1):71‐9. [Google Scholar]
Offenbacher 2000 {published data only}
- Offenbacher M, Stucki G. Physical therapy in the treatment of fibromyalgia. Scandinavian Journal of Rheumatology 2000;Suppl 29:78. [DOI] [PubMed] [Google Scholar]
Oncel 1994 {published data only}
- Oncel A, Eskiyurt N, Leylabadi M. The results obtained by different therapeutic measures in the treatment of generalized fibromyalgia syndrome. Tip Fakultesi Mecmuasi 1994;57(4):45‐9. [Google Scholar]
Peters 2002 {published data only}
- Peters S, Stanley I, Rose M, Kaney S, Salmon P. A randomized controlled trial of group aerobic exercise in primary care patients with persistent, unexplained physical symptoms. Family Practice 2002;19:665‐74. [DOI] [PubMed] [Google Scholar]
Pfeiffer 2003 {published data only}
- Pfeiffer AT. Effects of a 1.5‐day multidisciplinary outpatient treatment program for fibromyalgia: a pilot study. American Journal of Physical Medicine & Rehabilitation 2003;82:186‐91. [DOI] [PubMed] [Google Scholar]
Piso 2001 {published data only}
- Piso U, Kuther G, Gutenbrunner C, Gehrke A. Analgesic effects of sauna in fibromyalgia. [German]. Physikalische Medizin Rehabilitationsmedizin Kurortmedizin 2001;11(3):94‐9. [Google Scholar]
Rooks 2002 {published data only}
- Rooks DS, Silverman CB, Kantrowitz FG. The effects of progressive strength training and aerobic exercise on muscle strength and cardiovascular fitness in women with fibromyalgia: a pilot study. Arthritis and Rheumatism 2002;47(1):22‐8. [DOI] [PubMed] [Google Scholar]
Salek 2005 {published data only}
- Salek AK, Khan MM, Ahmed SM, Rashid MI, Emran MA, Mamun MA. Effect of aerobic exercise on patients with primary fibromyalgia syndrome. Mymensingh Medical Journal 2005;14:141‐4. [PubMed] [Google Scholar]
Thieme 2003 {published data only}
- Thieme K, Gronica‐Ihle E, Flor H. Operant behavioral treatment of fibromyalgia: a controlled study. Arthritis Care & Research 2003;49:314‐20. [DOI] [PubMed] [Google Scholar]
Tiidus 1997 {published data only}
- Tiidus PM. Manual massage and recovery of muscle function following exercise: a literature review. Journal of Orthopeadics and Sports Physical Therapy 1997;25(2):107‐12. [DOI] [PubMed] [Google Scholar]
Vlaeyen 1996 {published data only}
- Vlaeyen JW, Teeken‐Gruben NJ, Goossens ME, Rutten‐van Molken MP, Pelt RA, Eek H, et al. Cognitive‐educational treatment of fibromyalgia: a randomized clinical trial. I. Clinical effects. Journal of Rheumatology 1996;23(7):1237‐45. [PubMed] [Google Scholar]
Worrel 2001 {published data only}
- Worrel LM, Krahn LE, Sletten CD, Pond GR. Treating fibromyalgia with a brief interdisciplinary program: initial outcomes and predictors of response. Mayo Clinical Proceedings 2001;76:384‐90. [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
Amris K 2016 {published and unpublished data}
- Amris K. Interdisciplinary rehabilitation of patients with chronic widespread pain (IMPROvE). ClinicalTrials.gov Recruitment status: completed. [NCT01352052]
- Bulow C, Amris K, Bandak E, Danneskiold‐Samsøe B, Ejlersen Wæhrens E. Improving activities of daily living ability in women with fibromyalgia: an exploratory, quasi‐randomized, phase‐two study, IMPROvE trial. Journal of Rehabilitative Medicine 2017;49:241‐50. [DOI: 10.2340/16501977-2198] [DOI] [PubMed] [Google Scholar]
Collado‐Mateo 2017 {published data only}
- Collado‐Mateo D, Dominguez‐Muñoz FJ, Adsuar JC, Garcia‐Gordillo MA, Gusi N. Effects of exergames on quality of life, pain, and disease effect in women with fibromyalgia: a randomized controlled trial. Archives of Physical Medicine and Rehabilitation 2017;98(9):1725‐31. [DOI] [PubMed] [Google Scholar]
- Collado‐Mateo D, Dominguez‐Muñoz FJ, Adsuar JC, Merellano‐Navarro E, Gusi N. Exergames for women with fibromyalgia: a randomised controlled trial to evaluate the effects on mobility skills, balance and fear of falling. PeerJ 2017;5:e3211. [DOI: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gusi N. Effects of a virtual reality‐based physical exercise on health‐related quality of life, pain, activities of daily living, physical fitness, and wellbeing in fibromyalgia patients. A randomized controlled trial. ACTRN12615000836538. [https://www.anzctr.org.au/Trial/Registration/TrialReview.aspx?id=368858]
Genc 2015 {published data only}
- Genc A, Tur BS, Aytur YK, Oztuna D, Erdogan MF. Does aerobic exercise affect the hypothalamic‐pituitary‐adrenal hormonal response in patients with fibromyalgia syndrome?. Journal of Physical Therapy Science 2015;27(7):2225‐31. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kibar 2015 {published data only}
Kurt 2016 {published data only}
- Kurt EE, Kocak FA, Erdem HR, Tuncay F, Kelez F. Which non‐pharmacological treatment is more effective on clinical parameters in patients with fibromyalgia: balneotherapy or aerobic exercise?. Archives of Rheumatology 2016;31(2):162‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mutlu 2013 {published data only}
- Mutlu B, Paker N, Bugdayci D, Tekdos D, Kesiktas N. Efficacy of supervised exercise combined with transcutaneous electrical nerve stimulation in women with fibromyalgia: a prospective controlled study. Rheumatology International 2013;33(3):649‐55. [DOI] [PubMed] [Google Scholar]
Paolucci 2016 {published data only}
- Paolucci T. A new rehabilitation tool in fibromyalgia. ClinicalTrial.gov June 2015. [NCT02472093]
- Paolucci T, Baldari C, Franco M, Didona D, Reis V, Vetrano M, et al. A new rehabilitation tool in fibromyalgia: the effects of perceptive rehabilitation on pain and function in a clinical randomized controlled trial. Evidence‐based Complementary and Alternative Medicine 2016;2016:7574589. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ranque 2017 {published data only}
- Ranque Garnier S, Zerdab A, Laurin J, Donnet A. "Fibromyactiv": single‐center, prospective, randomized pilot study. Adapted physical exercise efficacy on quality of life of patients with fibromyalgia. Douleurs 2017;18(2):87‐104. [Google Scholar]
Salvat 2017 {published data only}
- Salvat I, Zaldivar P, Monterde S, Montull S, Miralles I, Castel A. Functional status, physical activity level, and exercise regularity in patients with fibromyalgia after multidisciplinary treatment: retrospective analysis of a randomized controlled trial. Rheumatology International 2017;37(3):377‐87. [DOI] [PubMed] [Google Scholar]
Sevimli 2015 {published data only}
- Sevimli D, Kozanoglu E, Guzel R, Doganay A. The effects of aquatic, isometric strength‐stretching and aerobic exercise on physical and psychological parameters of female patients with fibromyalgia syndrome. Journal of Physical Therapy Science 2015;27(6):1781‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Toprak 2017 {published data only}
- Toprak CS, Anaforoglu KB, Yasa ME, Sahbaz PC, Un YN, Kucuksahin O, et al. A comparison of the effects of exercises plus connective tissue massage to exercises alone in women with fibromyalgia syndrome: a randomized controlled trial. Rheumatology International 2017;37(11):1799‐806. [DOI] [PubMed] [Google Scholar]
References to ongoing studies
da Silva 2015 {published data only}
- Silva MM. Protocol phototherapy treatment for fibromyalgia and temporomandibular joint dysfunction (FM and DTM). ClinicalTrials.gov 2014 (Recruitment status: unknown). [NCT02279225]
- Silva MM, Albertini R, Leal‐Junior EC, Tarso Camillo C, Silva JA Jr, Bussadori SK, et al. PROTOCOL. Effects of exercise training and photobiomodulation therapy (EXTRAPHOTO) on pain in women with fibromyalgia and temporomandibular disorder: study protocol for a randomized controlled trial. Trials 2015;16:252. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gusi N {unpublished data only}
- Gusi N. Effectivity of virtual reality physical exercise program in brain and motor aging in fibromyalgia. ISRCTN65034180 Recruitment status: completed. [https://doi.org/10.1186/ISRCTN65034180]
Mendonça Araújo F {unpublished data only}
- Mendonça Araújo F. Effect of interferential current combined with exercise in patients with fibromyalgia: randomized clinical trial. http://apps.who.int/trialsearch/Trial2.aspx?TrialID=RBR‐6dk3y3. Recruitment status: recruiting. [RBR‐6dk3y3]
Montañez‐Aguilera J {unpublished data only}
- Montañez‐Aguilera J. Change on sleep quality of patients with fibromyalgia subjected to a protocol based on physical exercise and stretching. ClinicalTrials.gov Identifier Recruitment status: completed. [NCT02876965]
Ruiz Ruiz J {unpublished data only}
- Carbonell‐Baeza A, Ruiz JR, Aparicio VA, Ortega FB, Munguia‐Izquierdo D, Alvarez‐Gallardo IC, et al. Land‐ and water‐based exercise intervention in women with fibromyalgia: the al‐Andalus physical activity randomised controlled trial. BMC Musculoskeletal Disorders 2012;13:18. [DOI: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz Ruiz J. Exercise in women with fibromyalgia. ClinicalTrials.gov Recruitment status: completed. [NCT01490281]
Additional references
ACSM 2013
- American College of Sports Medicine. ACSM's Guidelines for Exercise Testing and Prescription. 9th Edition. Baltimore, MD, USA: Lippincott Williams & Wilkins, 2013. [DOI] [PubMed] [Google Scholar]
Adsuar 2012
- Adsuar JC, Pozo‐Cruz B, Parraca JA, Olivares PR, Gusi N. Whole body vibration improves the single‐leg stance static balance in women with fibromyalgia: a randomized controlled trial. Journal of Sports Medicine & Physical Fitness 2012;52(1):85‐91. [PubMed] [Google Scholar]
Altan 2004
- Altan L, Bingol U, Aykac M, Koc Z, Yurtkuran M. Investigation of the effects of pool‐based exercise on fibromyalgia syndrome. Rheumatology International 2004;24(5):272‐7. [DOI] [PubMed] [Google Scholar]
Altan 2009
- Altan L, Korkmaz N, Bingol U, Gunay B. Effect of pilates training on people with fibromyalgia syndrome: a pilot study. Archives of Physical Medicine and Rehabilitation 2009;90(12):1983‐8. [DOI] [PubMed] [Google Scholar]
Amanollahi 2013
- Amanollahi A, Naghizadeh J, Khatibi A, Hollisaz MT, Shamseddini AR, Saburi A. Comparison of impacts of friction massage, stretching exercises and analgesics on pain relief in primary fibromyalgia syndrome: a randomized clinical trial. Tehran University Medical Journal 2013;70(10):616‐22. [Google Scholar]
Arcos‐Carmona 2011
- Arcos‐Carmona IM, Castro‐Sanchez AM, Mataran‐Penarrocha GA, Gutierrez‐Rubio AB, Ramos‐Gonzalez E, Moreno‐Lorenzo C. [Effects of aerobic exercise program and relaxation techniques on anxiety, quality of sleep, depression, and quality of life in patients with fibromyalgia: a randomized controlled trial]. Medina Clinica 2011;137(9):398‐401. [DOI] [PubMed] [Google Scholar]
Arnold 2008
- Arnold LM, Crofford LJ, Mease PJ, Burgess SM, Palmer SC, Abetz L, et al. Patient perspectives on the impact of fibromyalgia. Patient Education and Counseling 2008;73(1):114‐20. [DOI] [PMC free article] [PubMed] [Google Scholar]
Assis 2006
- Assis MR, Silva LE, Alves AM, Pessanha AP, Valim V, Feldman D, et al. A randomized controlled trial of deep water running: clinical effectiveness of aquatic exercise to treat fibromyalgia. Arthritis Care & Research 2006;55(1):57‐65. [DOI] [PubMed] [Google Scholar]
Barclay 2014
- Barclay T, Richards S, Schoffstall J, Magnuson C, McPhee C, Price J, et al. A pilot study on the effects of exercise on depression symptoms using levels of neurotransmitters and EEG as markers. European Journal of Psychology and Educational Studies 2014;1(1):30‐5. [Google Scholar]
Bennett 1989
- Bennett RM, Clark SR, Goldberg L, Nelson D, Bonafede RP, Porter J, et al. Aerobic fitness in patients with fibrositis. A controlled study of respiratory gas exchange and 133xenon clearance from exercising muscle. Arthritis & Rheumatism 1989;32(4):454‐60. [DOI] [PubMed] [Google Scholar]
Bennett 1998
- Bennett RM, Clark SC, Walczyk J. A randomized, double‐blind, placebo‐controlled study of growth hormone in the treatment of fibromyalgia. American Journal of Medicine 1998;104(3):227‐31. [DOI] [PubMed] [Google Scholar]
Bennett 1999
- Bennett RM. Emerging concepts in the neurobiology of chronic pain: evidence of abnormal sensory processing in fibromyalgia. Mayo Clinic Proceedings 1999;74(4):385‐98. [DOI] [PubMed] [Google Scholar]
Bergner 1981
- Bergner M, Bobbitt RA, Carter WB, Gilson BS. The Sickness Impact Profile: development and final revision of a health status measure. Medical Care 1981;19(8):787‐805. [DOI] [PubMed] [Google Scholar]
Berkery 1995
- Berkery CS, Hoaglin DC, Mosteller F, Colditz GA. A random‐effects regression model for meta‐analysis. Statistics in Medicine 1995;14(4):395‐411. [DOI] [PubMed] [Google Scholar]
Berlin 1994
Berlin 2002
- Berlin JA, Santanna J, Schmid CH, Szczech LA, Feldman HI. Individual patient versus group level data meta‐regressions for the investigation of subgroup effects: ecological bias rears its ugly head. Statistics in Medicine 2002;21(2):371‐87. [DOI] [PubMed] [Google Scholar]
Bircan 2008
- Bircan C, Karasel SA, Akgun B, El O, Alper S. Effects of muscle strengthening versus aerobic exercise program in FM. Rheumatology International 2008;28(6):527‐32. [DOI] [PubMed] [Google Scholar]
Bojner 2006
- Bojner Horwitz E, Kowalski J, Theorell T, Anderberg UM. Dance/movement therapy in fibromyalgia patients: changes in self‐figure drawings and their relation to verbal self‐rating scales. Arts in Psychotherapy 2006;33(1):11‐25. [Google Scholar]
Boomershine 2012
- Boomershine CS. A comprehensive evaluation of standardized assessment tools in the diagnosis of fibromyalgia and in the assessment of fibromyalgia severity. Pain Research and Treatment 2012;2012:653714. [DOI] [PMC free article] [PubMed] [Google Scholar]
Branco 2010
- Branco JC, Bannwarth B, Failde I, Abello Carbonell J, Blotman F, Spaeth M, et al. Prevalence of FM: a survey in five European countries. Seminars in Arthritis and Rheumitism 2010;39(6):448‐53. [DOI] [PubMed] [Google Scholar]
Bressan 2008
- Bressan LR, Matsutani LA, Assumpcao A, Marques AP, Cabral CMN. Effects of muscle stretching and physical conditioning as physical therapy treatment for patients with fibromyalgia [Portuguese]. Revista Brasileira de Fisioterapia / Brazilian Journal of Physical Therapy 2008;12(2):88‐93. [Google Scholar]
Brosseau 2008
- Brosseau L, Wells GA, Tugwell P, Egan M, Wilson KG, Dubouloz CJ, et al. Ottawa panel evidence‐based clinical practice guidelines for aerobic fitness exercises in the management of fibromyalgia: part 1. Physical Therapy 2008;88(7):857‐71. [DOI] [PubMed] [Google Scholar]
Brown 2006
- Brown P, Brunnhuber K, Chalkidou K, et al. How to formulate research recommendations. British Medical Journal 2006;333(7572):804‐6. [DOI: 10.1136/bmj.38987.492014.94] [DOI] [PMC free article] [PubMed] [Google Scholar]
Burckhardt 1991
- Burckhardt CS, Clark SR, Bennett RM. The Fibromyalgia Impact Questionnaire: development and validation. Journal of Rheumatology 1991;18(5):728‐33. [PubMed] [Google Scholar]
Burckhardt 1993
- Burckhardt CS, Archenholtz B, Bjelle A. Quality of life of women with systemic lupus erythematosus: a comparison with women with rheumatoid arthritis. Journal of Rheumatology 1993;20:977‐81. [PubMed] [Google Scholar]
Burckhardt 2005
- Buckhardt CS, Goldenberg D, Crofford L, Gerwin R, Gowens S, Jackson K, et al. Guideline for the Management of Fibromyalgia Syndrome Pain in Adults and Children. Clinical Practice Guideline. Vol. 4, Glenview, IL: American Pain Society, 2005. [Google Scholar]
Busija 2011
- Busija L, Pausenberger E, Haines TP, Haymes S, Buchbinder R, Osborne RH. Adult measures of general health and health‐related quality of life: Medical Outcomes Study Short Form 36‐Item (SF‐36) and Short Form 12‐Item (SF‐12) Health Surveys, Nottingham Health Profile (NHP), Sickness Impact Profile (SIP), Medical Outcomes Study Short Form 6D (SF‐6D), Health Utilities Index Mark 3 (HUI3), Quality of Well‐Being Scale (QWB), and Assessment of Quality of Life (AQoL). Arthritis Care and Research 2011;63 Suppl 11:S383‐412. [DOI] [PubMed] [Google Scholar]
Calandre 2009
- Calandre EP, Rodriguez‐Claro ML, Rico‐Villademoros F, Vilchez JS, Hidalgo J, Gado‐Rodriguez A. Effects of pool‐based exercise in fibromyalgia symptomatology and sleep quality: a prospective randomised comparison between stretching and Ai Chi. Clinical & Experimental Rheumatology 2009;27(5 Suppl 56):S21‐8. [PubMed] [Google Scholar]
Carson 2010
- Carson JW, Carson KM, Jones KD, Bennett RM, Wright CL, Mist SD. A pilot randomized controlled trial of the Yoga of Awareness program in the management of fibromyalgia. Pain 2010; Vol. 151, issue 2:530‐9. [DOI] [PMC free article] [PubMed]
Carson 2012
- Carson JW, Carson KM, Jones KD, Mist SD, Bennett RM. Follow‐up of yoga of awareness for fibromyalgia: results at 3 months and replication in the wait‐list group. Clinical Journal of Pain 2012;28(9):804‐13. [DOI] [PMC free article] [PubMed] [Google Scholar]
Carville 2008
- Carville SF, Choy EH. Systematic review of discriminating power of outcome measures used in clinical trials of fibromyalgia. Journal of Rheumatology 2008;35(11):2094‐105. [DOI] [PubMed] [Google Scholar]
Castel 2013
- Castel A, Fontova R, Montull S, Perinan R, Poveda MJ, Miralles I, et al. Efficacy of a multidisciplinary fibromyalgia treatment adapted for women with low educational levels: a randomized controlled trial [with consumer summary]. Arthritis Care & Research 2013;65(3):421‐31. [DOI] [PubMed] [Google Scholar]
Cedraschi 2004
- Cedraschi C, Desmeules J, Rapiti E, Baumgartner E, Cohen P, Finckh A, et al. Fibromyalgia: a randomised, controlled trial of a treatment programme based on self management. Annals of Rheumatic Diseases 2004;63(3):290‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Cerrillo‐Urbina 2015
- Cerrillo‐Urbina AJ, Garcia‐Hermoso A, Sánchez‐López M, Martínez‐Vizcaíno V. Effect of exercise programs on symptoms of fibromyalgia in peri‐menopausal age women: a systematic review and meta‐analysis of randomized controlled trials. Myopain 2015;23(1‐2):56‐70. [Google Scholar]
Choy 2009
- Choy EH, Mease PJ. Key symptom domains to be assessed in fibromyalgia (outcome measures in rheumatoid arthritis clinical trials). Rheumatic Disease Clinics of North America 2009;35(2):329‐37. [DOI] [PubMed] [Google Scholar]
Clauw 2011
- Clauw DJ, Arnold LM, McCarberg BH. The science of fibromyalgia. Mayo Clinical Proceedings 2011;86(9):907‐11. [DOI] [PMC free article] [PubMed] [Google Scholar]
Clauw 2014
- Clauw DJ. Fibromyalgia: a clinical review. JAMA 2014;311(15):1547‐55. [DOI] [PubMed] [Google Scholar]
Covidence [Computer program]
- Veritas Health Innovation. Covidence systematic review software. Melbourne, Australia: http://www.covidence.org, 2015.
De Andrade 2008
- Andrade SC, Carvalho RFPP, Soares AS, Abreu Freitas RP, Madeiros Guerra LM, Vilar MJ. Thalassotherapy for fibromyalgia: a randomized controlled trial comparing aquatic exercises in sea water and water pool. Rheumatology International 2008;29(2):147‐52. [DOI] [PubMed] [Google Scholar]
de Araujo 2013
- Araujo Farias D, Abrahao AA, Rossato M, Bezerra Ede S. Effects of two different training methods in women with fibromyalgia syndrome. Research in Sports Medicine 2013;21(3):280‐5. [DOI] [PubMed] [Google Scholar]
de Melo Vitorino 2006
- Melo Vitorino DF, Carvalho LBC, do Prado GF. Hydrotherapy and conventional physiotherapy improve total sleep time and quality of life of fibromyalgia patients: randomized clinical trial. Sleep Medicine 2006;7(3):293‐6. [DOI] [PubMed] [Google Scholar]
Deeks 2017 Ch9
- Deeks JJ, Higgins JPT, Altman DG (editors). Chapter 9. Analysing data and undertaking meta‐analyses. In: Higgins JPT, Churchill R, Chandler J, Cumpston MS (editors). Cochrane Handbook for Systematic Reviews of Interventions version 5.2.0 (updated June 2017). Cochrane, 2017. Available from www.training.cochrane.org/handbook. The Cochrane Collaboration.
Desmeules 2003
- Desmeules JA, Cedraschi C, Rapiti E, Baumgartner E, Finckh A, Cohen P, et al. Neurophysiologic evidence for a central sensitization in patients with fibromyalgia. Arthritis and Rheumatism 2003;48(5):1420‐9. [DOI] [PubMed] [Google Scholar]
Dierick 2011
- Dierick F, Detrembleur C, Trintignac G, Masquelier E. Nature of passive musculoarticular stiffness increase of ankle in female subjects with fibromyalgia syndrome. European Journal of Applied Physiology 2011;111(9):2163‐71. [DOI] [PubMed] [Google Scholar]
Dworkin 2008
- Dworkin RH, Turk DC, Wyrwich KW, Beaton D, Cleeland CS, Farrar JT, et al. Interpreting the clinical importance of treatment outcomes in chronic pain clinical trials: IMMPACT recommendations. Journal of Pain 2008;9(2):105‐21. [DOI] [PubMed] [Google Scholar]
Evcik 2008
- Evcik D, Yugit I, Pusak H, Kavuncu V. Effectiveness of aquatic therapy in the treatment of fibromyalgia syndrome: a randomized controlled open study. Rheumatology International 2008;28(9):885‐90. [DOI] [PubMed] [Google Scholar]
Ferguson 2014
- Ferguson B. ACSM's guidelines for exercise testing and prescription, 9th edition, 2014. Journal of the Canadian Chiropractic Association 2014;58(3):328. [Google Scholar]
Ferreira‐Valente 2011
- Ferreira‐Valente MA, Pais‐Ribeiro JL, Jensen MP. Validity of four pain intensity rating scales. Pain 2011;152(10):2399‐404. [DOI] [PubMed] [Google Scholar]
Field 2003
- Field T, Delage J, Hernandez RM. Movement and massage therapy reduce fibromyalgia pain. Journal of Bodywork and Movement Therapies 2003;7(1):49‐52. [Google Scholar]
Fontaine 2007
- Fontaine KR, Haas S. Effects of lifestyle physical activity on health status, pain, and function in adults with fibromyalgia syndrome. Journal of Musculoskeletal Pain 2007;15(1):3‐9. [Google Scholar]
Fontaine 2010
- Fontaine KR, Conn L, Clauw DJ. Effects of lifestyle physical activity on perceived symptoms and physical function in adults with fibromyalgia: results of a randomized trial. Arthritis Research and Therapy 2010;12(2):R55. [DOI] [PMC free article] [PubMed] [Google Scholar]
Fontaine 2011
- Fontaine KR, Conn L, Clauw DJ. Effects of lifestyle physical activity in adults with fibromyalgia: results at follow‐up. Journal of Clinical Rheumatology 2011; Vol. 17, issue 2:64‐8. [DOI] [PMC free article] [PubMed]
Garber 2011
- Garber CE, Blissmer B, Deschenes MR, Franklin BA, Lamonte MJ, Lee IM, et al. American College of Sports Medicine position stand. Quantity and quality of exercise for developing and maintaining cardiorespiratory, musculoskeletal, and neuromotor fitness in apparently healthy adults: guidance for prescribing exercise. Medical Science and Sports Exercise 2011;43(7):1334‐59. [DOI] [PubMed] [Google Scholar]
Garcia‐Hermoso 2015
- Garcia‐Hermoso A, Saavedra JM, Escalante Y. Effects of exercise on functional aerobic capacity in adults with fibromyalgia syndrome: a systematic review of randomized controlled trials. Journal of Back Musculoskeletal Rehabilitation 2015;28(4):609‐19. [DOI] [PubMed] [Google Scholar]
Goes 2015
- Goes SM, Leite N, Stefanello JM, Homann D, Lynn SK, Rodacki AL. Ankle dorsiflexion may play an important role in falls in women with fibromyalgia. Clinical Biomechanics (Bristol, Avon.) 2015;30(6):593‐8. [DOI] [PubMed] [Google Scholar]
GRADEpro 2011 [Computer program]
- GRADE Working Group. GRADEprofiler. Version 3.6.1. GRADE Working Group, 2011.
Gusi 2006
- Gusi N, Tomas‐Carus P, Hakkinen A, Hakkinen K, Ortega‐Alonso A. Exercise in waist‐high warm water decreases pain and improves health‐related quality of life and strength in the lower extremities in women with fibromyalgia. Arthritis Rheumatology 2006;55(1):66‐73. [DOI] [PubMed] [Google Scholar]
Gusi 2008
- Gusi N, Tomas‐Carus P. Cost‐utility of an 8‐month aquatic training for women with fibromyalgia: a randomized controlled trial. Arthritis Research & Therapy 2008;10(1):R24. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gusi 2010
- Gusi N, Parraca JA, Olivares PR, Leal A, Adsuar JC. Tilt vibratory exercise and the dynamic balance in fibromyalgia: a randomized controlled trial. Arthritis Care Research (Hoboken) 2010;62(8):1072‐8. [DOI] [PubMed] [Google Scholar]
Hakkinen 2002
- Hakkinen K, Pakarinen A, Hannonen P, Hakkinen A, Airaksinen O, Valkeinen H, et al. Effects of strength training on muscle strength, cross‐sectional area, maximal electromyographic activity, and serum hormones in premenopausal women with FM. Journal of Rheumatology 2002;29(6):1287‐95. [PubMed] [Google Scholar]
Hammond 2006
- Hammond A, Freeman K. Community patient education and exercise for people with fibromyalgia: a parallel group randomized controlled trial. Clinical Rehabilitation 2006;20(10):835‐46. [DOI] [PubMed] [Google Scholar]
Hecker 2011
- Hecker CD, Melo C, Tomazoni SS, Lopes‐Martins RAB, Leal Junior ECP. Analysis of effects of kinesiotherapy and hydrokinesiotherapy on the quality of patients with fibromyalgia ‐ a randomized clinical trial [Portuguese]. Fisioterapia em Movimento 2011;24(1):57‐64. [Google Scholar]
Higgins 2011 Ch7
- Higgins JPT, Deeks JJ. Chapter 7. Selecting studies and collecting data. In: Higgins JPT, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.training.cochrane.org/handbook.
Higgins 2017 Ch8_ROB
- Higgins JPT, Altman DG, Sterne JAC (editors). Chapter 8. Assessing risk of bias in included studies. In: Higgins JPT, Churchill R, Chandler J, Cumpston MS (editors), Cochrane Handbook for Systematic Reviews of Interventions version 5.2.0 (updated June 2017). Cochrane, 2017. Available from www.training.cochrane.org/handbook.
Ho 2012
- Ho SS, Dhaliwal SS, Hills AP, Pal S. The effect of 12 weeks of aerobic, resistance or combination exercise training on cardiovascular risk factors in the overweight and obese in a randomized trial. BMC Public Health 2012;12:704. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hooten 2012
- Hooten WM, Qu W, Townsend CO, Judd JW. Effects of strength vs aerobic exercise on pain severity in adults with fibromyalgia: a randomized equivalence trial. Pain 2012;153(4):915‐23. [DOI] [PubMed] [Google Scholar]
Howley 2001
- Howley ET. Type of activity: resistance, aerobic and leisure versus occupational physical activity. Medicine and Science in Sports Exercise 2001;33(6 Suppl):S364‐9. [DOI] [PubMed] [Google Scholar]
Huskisson 1976
- Huskisson EC, Jones J, Scott PJ. Application of visual‐analogue scales to the measurement of functional capacity. Rheumatology and Rehabilitation 1976;15(3):185‐7. [DOI] [PubMed] [Google Scholar]
Huskisson 1983
- Huskisson EC, Sturrock RD, Tugwell P. Measurement of patient outcome. British Journal of Rheumatology 1983;22:86‐9. [DOI] [PubMed] [Google Scholar]
Häuser 2010
- Hauser W, Klose P, Langhorst J, Moradi B, Steinbach M, Schiltenwolf M, et al. Efficacy of different types of aerobic exercise in fibromyalgia syndrome: a systematic review and meta‐analysis of randomised controlled trials. Arthritis Research & Therapy 2010;12(3):R79. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ide 2008
- Ide MR, Laurindo LMM, Rodrigues‐Junior AL, Tanaka C. Effect of aquatic respiratory exercise‐based program in patients with fibromyalgia. APLAR Journal of Rheumatology 2008;11(2):131‐40. [Google Scholar]
Isomeri 1993
- Isomeri R, Mikkelsson M, Latikka P, Kammonen K. Effects of amitriptyline and cardiovascular fitness training on pain in patients with primary fibromyalgia. Journal of Musculoskeletal Pain 1993;1:253‐60. [Google Scholar]
Jadad 1996
- Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJ, Gavaghan DJ, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary?. Controlled Clinical Trials 1996;17(1):1‐12. [DOI] [PubMed] [Google Scholar]
Jentoft 2001
- Jentoft ES, Kvalvik AG, Mengshoel AM. Effects of pool‐based and land‐based aerobic exercise on women with fibromyalgia/chronic widespread muscle pain. Arthritis and Rheumatism 2001;45(1):42‐7. [DOI] [PubMed] [Google Scholar]
Jones 2002
- Jones KD, Burckhardt CS, Clark SR, Bennett RM, Potempa KM. A randomized controlled trial of muscle strengthening versus flexibility training in FM. Journal of Rheumatology 2002;29(5):1041‐8. [PubMed] [Google Scholar]
Jones 2015
- Jones GT, Atzeni F, Beasley M, Fluss E, Sarzi‐Puttini P, MacFarlane GJ. The prevalence of fibromyalgia in the general population: a comparison of the American College of Rheumatology 1990, 2010, and modified 2010 classification criteria. Arthritis & Rheumatology 2015;67(2):568‐75. [DOI] [PubMed] [Google Scholar]
Kayo 2011
- Kayo AH, Peccin MS, Sanches CM, Trevisani VF. Effectiveness of physical activity in reducing pain in patients with FM: a blinded randomized clinical trial. Rheumatology International 2011;32(8):2285‐92. [DOI] [PubMed] [Google Scholar]
Keel 1998
- Keel PJ, Bodoky C, Gerhard U, Muller W. Comparison of integrated group therapy and group relaxation training for fibromyalgia. Clinical Journal of Pain 1998;14(3):232‐8. [DOI] [PubMed] [Google Scholar]
King 2002
- King SJ, Wessel J, Bhambhani Y, Sholter D, Maksymowych W. The effects of exercise and education, individually or combined, in women with fibromyalgia. Journal of Rheumatology 2002;29(12):2620‐7. [PubMed] [Google Scholar]
Kingsley 2009
- Kingsley JD, Panton LB, McMillan V, Figueroa A. Cardiovascular autonomic modulation after acute resistance exercise in women with fibromyalgia. Archives of Physical Medicine and Rehabilitation 2009;90(9):1628‐34. [DOI] [PubMed] [Google Scholar]
Klaperski 2014
- Klaperski S, Dawans B, Heinrichs M, Fuchs R. Effects of a 12‐week endurance training program on the physiological response to psychosocial stress in men: a randomized controlled trial. Journal of Behavioral Medicine 2014;37(6):1118‐33. [DOI] [PubMed] [Google Scholar]
Lefebvre 2011
- Lefebvre C, Manheimer E, Glanville J. Searching for studies. In: Higgins JPT, Green S editor(s). Cochrane Handbook for Systematic Reviews of Interventions (Version 5.1.0). The Cochrane Collaboration, 2011. [Google Scholar]
Lemstra 2005
- Lemstra M, Olszynski WP. The effectiveness of multidisciplinary rehabilitation in the treatment of fibromyalgia: a randomized controlled trial. Clinical Journal of Pain 2005;21(2):166‐74. [DOI] [PubMed] [Google Scholar]
Liu 2012
- Liu W, Zahner L, Cornell M, Le T, Ratner J, Wang Y, et al. Benefit of Qigong exercise in patients with fibromyalgia: a pilot study. International Journal of Neuroscience 2012;122(11):657‐64. [DOI] [PubMed] [Google Scholar]
Lopez‐Rodriguez 2012
- Lopez‐Rodriguez MM, Castro‐Sanchez AM, Fernandez‐Martinez M, Mataran‐Penarrocha GA, Rodriguez‐Ferrer ME. [Comparison between aquatic‐biodance and stretching for improving quality of life and pain in patients with fibromyalgia]. Atencion Primaria 2012;44(11):641‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lopresti 2013
- Lopresti AL, Hood SD, Drummond PD. A review of lifestyle factors that contribute to important pathways associated with major depression: diet, sleep and exercise. Journal of Affective Disorders 2013;148(1):12‐27. [DOI] [PubMed] [Google Scholar]
Lynch 2012
- Lynch M, Sawynok J, Hiew C, Marcon D. A randomized controlled trial of qigong for fibromyalgia. Arthritis Research and Therapy 2012;14(4):R178. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mannerkorpi 2000
- Mannerkorpi K, Nyberg B, Ahlmen M, Ekdahl C. Pool exercise combined with an education program for patients with fibromyalgia syndrome. A prospective, randomized study. Journal of Rheumatology 2000;27(10):2473‐81. [PubMed] [Google Scholar]
Mannerkorpi 2009
- Mannerkorpi K, Nordeman L, Ericsson A, Arndorw M. Pool exercise for patients with fibromyalgia or chronic widespread pain: a randomized controlled trial and subgroup analyses. Journal of Rehabilitation Medicine 2009;41(9):751‐60. [DOI] [PubMed] [Google Scholar]
Mannerkorpi 2010
- Mannerkorpi K, Nordeman L, Cider A, Jonsson G. Does moderate‐to‐high intensity Nordic walking improve functional capacity and pain in fibromyalgia? A prospective randomized controlled trial. Arthritis Research & Therapy 2010;12(5):R189. [DOI] [PMC free article] [PubMed] [Google Scholar]
Martin‐Nogueras 2012
- Martin‐Nogueras AM, Calvo‐Arenillas JI. Efficacy of physiotherapy treatment on pain and quality of life in patients with fibromyalgia [Spanish]. Rehabilitacion 2012;46(3):199‐206. [Google Scholar]
Matsutani 2007
- Matsutani LA, Marques AP, Ferreira EA, Assumpcao A, Lage LV, Casarotto RA, et al. Effectiveness of muscle stretching exercises with and without laser therapy at tender points for patients with fibromyalgia. Clinical & Experimental Rheumatology 2007;25(3):410‐5. [PubMed] [Google Scholar]
Matsutani 2012
- Matsutani LA, Assumpcao A, Marques AP. Stretching and aerobic exercises in the treatment of fibromyalgia: pilot study [Portuguese]. Fisioterapia em Movimento 2012;25(2):411‐8. [Google Scholar]
McCain 1988
- McCain GA, Bell DA, Mai FM, Halliday PD. A controlled study of the effects of a supervised cardiovascular fitness training program on the manifestations of primary fibromyalgia. Arthritis and Rheumatism 1988;31(9):1135‐41. [DOI] [PubMed] [Google Scholar]
McNalley 2006
- McNalley JD, Matheson DA, Bakowshy VS. The epidemiology of self‐reported fibromyalgia in Canada. Chronic Diseases in Canada 2006;27(1):9‐16. [PubMed] [Google Scholar]
Mease 2005
- Mease P. Fibromyalgia syndrome: review of clinical presentation, pathogenesis, outcome measures, and treatment. Journal of Rheumatology Supplement 2005;75:6‐21. [PUBMED: 16078356] [PubMed] [Google Scholar]
Mengshoel 1992
- Mengshoel AM, Komnaes HB, Forre O. The effects of 20 weeks of physical fitness training in female patients with fibromyalgia. Clinical & Experimental Rheumatology 1992;10(4):345‐9. [PubMed] [Google Scholar]
Mengshoel 1993
- Mengshoel AM, Forre O. Physical fitness training in patients with fibromyalgia. Musculoskeletal Pain 1993;1(4):267‐72. [Google Scholar]
Meyer 2000
- Meyer BB, Lemley KJ. Utilizing exercise to affect the symptomology of fibromyalgia: a pilot study. Medicine and Science in Sports and Exercise 2000;32(10):1691‐7. [DOI] [PubMed] [Google Scholar]
Mitchell 2012 [Computer program]
- Mitchell M. Engauge Digitizer ‐ Digitizing Software. Version 5.1. Online, 2012. [digitizer.sourceforge.net]
Moher 1999
- Moher D, Cook DJ, Eastwood S, Olkin I, Rennie D, Stroup DF. Improving the quality of reports of meta‐analyses of randomised controlled trials: the QUOROM statement. Quality of Reporting of Meta‐analyses. Lancet 1999;354(9193):1896‐900. [DOI] [PubMed] [Google Scholar]
Moylan 2013
- Moylan S, Eyre HA, Maes M, Baune BT, Jacka FN, Berk M. Exercising the worry away: how inflammation, oxidative and nitrogen stress mediates the beneficial effect of physical activity on anxiety disorder symptoms and behaviours. Neuroscience & Biobehavioral Reviews 2013;37(4):573‐84. [DOI] [PubMed] [Google Scholar]
Munguia‐Izquierdo 2007
- Munguia‐Izquierdo D, Legaz‐Arrese A. Exercise in warm water decreases pain and improves cognitive function in middle‐aged women with fibromyalgia. Clinical & Experimental Rheumatology 2007;25(6):823‐30. [PubMed] [Google Scholar]
Munguia‐Izquierdo 2008
- Munguia‐Izquierdo D, Legaz‐Arrese A. Assessment of the effects of aquatic therapy on global symptomatology in patients with fibromyalgia syndrome: a randomized controlled trial. Archives of Physical Medicine & Rehabilitation 2008;89(12):2250‐7. [DOI] [PubMed] [Google Scholar]
Nichols 1994
- Nichols DS, Glenn TM. Effects of aerobic exercise on pain perception, affect, and level of disability in individuals with fibromyalgia. Physical Therapy 1994;74:327‐32. [DOI] [PubMed] [Google Scholar]
Noonan 2000
- Noonan V, Dean E. Submaximal exercise testing: clinical application and interpretation. Physical Therapy 2000;80(8):782‐807. [PubMed] [Google Scholar]
Odgaard‐Jenssen 2011
- Odgaard‐Jenssen J, Vist GE, Timmer A, Knuz R, Akl EA, Shunemmann H, et al. Randomisation to protect against selection bias in healthcare trials. Cochrane Database of Systematic Reviews 2011, Issue 4. [DOI: 10.1002/14651858.MR000012.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Olivares 2011
- Olivares PR, Gusi N, Parraca JA, Adsuar JC, Pozo‐Cruz B. Tilting whole body vibration improves quality of life in women with fibromyalgia: a randomized controlled trial. Journal of Alternative and Complementary Medicine 2011;17(8):723‐8. [DOI] [PubMed] [Google Scholar]
Painter 1999
- Painter P, Stewart AL, Carey S. Physical functioning: definitions, measurement, and expectations. Advances in Renal Replacement Therapy 1999;6(2):110‐23. [DOI] [PubMed] [Google Scholar]
Park 2007
- Park J, Knudson S. Medically unexplained physical symptoms. Health Reports 2007;18(1):43‐7. [PubMed] [Google Scholar]
Philadelphia Panel
- Philadelphia Panel 2001. Philadelphia Panel evidence‐based clinical practice guidelines on selected rehabilitation interventions for knee pain. Physical Therapy 2001;81(10):1675‐700. [PubMed] [Google Scholar]
Pollock 1998
- Pollock ML, Gaesser GA, Butcher JD, Després Dishman RK, Franklin BA, Ewing GC. ACSM position stand: the recommended quantity and quality of exercise for developing and maintaining cardiorespiratory and muscular fitness and flexibility in healthy adults. Medicine and Science in Sports and Exercise 1990;30(6):975‐91. [DOI: 10.1097/00005768-199806000-00032] [DOI] [PubMed] [Google Scholar]
Puetz 2006
- Puetz TW. Physical activity and feelings of energy and fatigue: epidemiological evidence. Sports Medicine 2006;36(9):767‐80. [DOI] [PubMed] [Google Scholar]
Queiroz 2013
- Queiroz LP. Worldwide epidemiology of fibromyalgia. Current Pain and Headache Reports 2013;17(356):1‐6. [DOI] [PubMed] [Google Scholar]
Raftery 2009
- Raftery G, Bridges M, Heslop P, Walker DJ. Are fibromyalgia patients as inactive as they say they are?. Clinical Rheumatology 2009;28(6):711‐4. [DOI] [PubMed] [Google Scholar]
Ramsay 2000
- Ramsay C, Moreland J, Ho M, Joyce S, Walker S, Pullar T. An observer‐blinded comparison of supervised and unsupervised aerobic exercise regimens in fibromyalgia. Rheumatology 2000;39(5):501‐5. [DOI] [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- The NCC. Review Manager (RevMan). Version 5.3. Copenhaugen: The Cochrane Collaboration, 2014.
Richards 2002
- Richards SC, Scott DL. Prescribed exercise in people with fibromyalgia: parallel group randomised controlled trial. BMJ 2002;325(7357):185. [DOI] [PMC free article] [PubMed] [Google Scholar]
Rosenthal 1995
- Rosenthal R. Writing meta‐analytic reviews. Psychological Bulletin 1995;118:183‐92. [Google Scholar]
Schachter 2003
- Schachter CL, Busch AJ, Peloso P, Sheppard MS. The effects of short versus long bouts of aerobic exercise in sedentary women with fibromyalgia: a randomized controlled trial. Physical Therapy 2003;83(4):340‐58. [PubMed] [Google Scholar]
Scheef 2012
- Scheef L, Jankowski J, Daamen M, Weyer G, Klingenberg M, Renner J, et al. An fMRI study on the acute effects of exercise on pain processing in trained athletes. Pain 2012;153(8):1702‐14. [DOI] [PubMed] [Google Scholar]
Schmidt 2011
- Schmidt S, Grossman P, Schwarzer B, Jena S, Naumann J, Walach H. Treating fibromyalgia with mindfulness‐based stress reduction: results from a 3‐armed randomized controlled trial. Pain 2011;152(2):1838‐43. [DOI] [PubMed] [Google Scholar]
Schultz 2010
- Schulz KF, Altman DG, Moher D. CONSORT 2010 statement: updated guidelines for reporting parallel group randomised trials. BMJ 2010;340:c332. [DOI] [PMC free article] [PubMed] [Google Scholar]
Schwingshackl 2014
- Schwingshackl L, Missbach B, Dias S, Konig J, Hoffmann G. Impact of different training modalities on glycaemic control and blood lipids in patients with type 2 diabetes: a systematic review and network meta‐analysis. Diabetologia 2014;57(9):1789‐97. [DOI] [PubMed] [Google Scholar]
Schünemann 2017 ch12
- Schünemann HJ, Oxman AD, Vist GE, Higgins JPT, Deeks JJ, Glasziou P, et al. Chapter 12. Interpreting results and drawing conclusions. In: Higgins JPT, Churchill R, Chandler J, Cumpston MS (editors), Cochrane Handbook for Systematic Reviews of Interventions version 5.2.0 (updated June 2017). Cochrane, 2017. Available from www.training.cochrane.org/handbook.
Sencan 2004
- Sencan S, Ak S, Karan A, Muslumanoglu L, Ozcan E, Berker E. A study to compare the therapeutic efficacy of aerobic exercise and paroxetine in fibromyalgia syndrome. Journal of Back & Musculoskeletal Rehabilitation 2004;17(2):57‐61. [Google Scholar]
Sterne 2017
- Sterne JAC, Egger M, Moher D, Boutron I (editors). Chapter 10. Addressing reporting biases. In: Higgins JPT, Churchill R, Chandler J, Cumpston MS (editors). Cochrane Handbook for Systematic Reviews of Interventions version 5.2.0 (updated June 2017). Cochrane, 2017. Available from www.training.cochrane.org/handbook. [OTHER]
Thompson 2002
- Thompson SG, Higgins JPT. How should meta‐regression analyses be undertaken and interpreted. Statistics in Medicine 2002;21(11):1559‐73. [DOI] [PubMed] [Google Scholar]
Tomas‐Carus 2007a_8214
- Tomas‐Carus P, Hakkinen A, Gusi N, Leal A, Hakkinen K, Ortega‐Alonso A. Aquatic training and detraining on fitness and quality of life in fibromyalgia. Medicine & Science in Sports & Exercise 2007;39(7):1044‐50. [DOI] [PubMed] [Google Scholar]
Tomas‐Carus 2007b_8215
- Tomas‐Carus P, Raimundo A, Adsuar JC, Olivares P, Gusi N. Effects of aquatic training and subsequent detraining on the perception and intensity of pain and number [Spanish]. Apunts Medicina de l'Esport 2007;154:76‐81. [Google Scholar]
Tomas‐Carus 2007c_8212
- Tomas‐Carus P, Gusi N, Leal A, Garcia Y, Orega‐Alonso A. The fibromyalgia treatment with physical exercise in warm water reduces the impact of the disease on female patients' physical and mental health [Spanish]. Reumatologia Clinica 2007;3(1):33‐7. [DOI] [PubMed] [Google Scholar]
Turk 2002
- Turk DC. Suffering and dysfunction in fibromyalgia syndrome. Journal of Musculoskeletal Pain 2002;10(1‐2):85‐96. [Google Scholar]
Valencia 2009
- Valencia M, Alonso B, Alvarez MJ, Barrientos MJ, Ayan C, Sanchez VM. Effects of 2 physiotherapy programs on pain perception, muscular flexibility, and illness impact in women with fibromyalgia: a pilot study. Journal of Manipulative and Physiological Therapeutics 2009;32(1):84‐92. [DOI] [PubMed] [Google Scholar]
Valim 2003
- Valim V, Oliveira L, Suda A, Silva L, Assis M, Barros Neto T, et al. Aerobic fitness effects in fibromyalgia. Journal of Rheumatology 2003;30(5):1060‐9. [PubMed] [Google Scholar]
Valkeinen 2004
- Valkeinen H, Alen M, Hannonen P, Hakkinen A, Airaksinen O, Hakkinen K. Changes in knee extension and flexion force, EMG and functional capacity during strength training in older females with FM and healthy controls. Rheumatology 2004;43(2):225‐8. [DOI] [PubMed] [Google Scholar]
Valkeinen 2005
van Koulil 2010
- Koulil S, Lankveld W, Kraaimaat FW, Helmond T, Vedder A, Hoorn H, et al. Tailored cognitive‐behavioral therapy and exercise training for high‐risk patients with fibromyalgia. Arthritis Care and Research 2010;62(10):1377‐85. [DOI] [PubMed] [Google Scholar]
Vincent 2013
- Vincent A, Lahr BD, Wolfe F, Clauw DJ, Whipple MO, Oh TH, et al. Prevalence of fibromyalgia: a population‐based study in Olmsted County, Minnesota, utilizing the Rochester Epidemiology Project. Arthritis Care Research 2013;65(5):786‐92. [DOI] [PMC free article] [PubMed] [Google Scholar]
Visual Rx [Computer program]
- Dr. Christopher Cates EBM website. http://www.nntonline.net. Visual Rx. Version 3. Dr. Christopher Cates EBM website. http://www.nntonline.net, 2008.
Walitt 2015
- Walitt B, Nahin RL, Katz RS, Bergman MJ, Wolfe F. The prevalence and characteristics of fibromyalgia in the 2012 National Health Interview Survey. PLoS One 2015;10(9):e0138024. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wang 2010
- Wang C, Schmid CH, Rones R, Kalish R, Yinh J, Goldenberg DL, et al. A randomized trial of tai chi for fibromyalgia. New England Journal of Medicine 2010;363(8):743‐54. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ware 1993
- Ware JE, Snow KK, Kosinski M, Gandek B, New EMCHHI. SF‐36 Health Survey: Manual and Interpretation Guide. Boston, MA, USA: The Health Institute, New England Medical Center, 1993. [Google Scholar]
Wigers 1996
- Wigers SH, Stiles TC, Vogel PA. Effects of aerobic exercise versus stress management treatment in fibromyalgia. A 4.5 year prospective study. Scandinavian Journal of Rheumatology 1996;25(2):77‐86. [DOI] [PubMed] [Google Scholar]
Wolfe 1988
- Wolfe F. Fibrositis, fibromyalgia, and musculoskeletal disease: the current status of the fibrositis syndrome. Archives of Physical Medicine and Rehabilitation 1988;69:527‐31. [PubMed] [Google Scholar]
Wolfe 1990
- Wolfe F, Smythe HA, Yunus MB, Bennett RM, Bombardier C, Goldenberg DL, et al. The American College of Rheumatology 1990 criteria for the classification of fibromyalgia. Report of the Multicenter Criteria Committee. Arthritis & Rheumatism 1990;33(2):160‐72. [DOI] [PubMed] [Google Scholar]
Wolfe 1995
- Wolfe F, Ross K, Anderson J, Russell IJ, Hebert L. The prevalence and characteristics of fibromyalgia in the general population. Arthritis & Rheumatism 1995;38(1):19‐28. [DOI] [PubMed] [Google Scholar]
Wolfe 1997
- Wolfe F, Anderson J, Harkness D, Bennett RM, Caro XJ, Goldenberg DL, et al. Work and disability status of persons with fibromyalgia. Journal of Rheumatology 1997;24(6):1171‐8. [PubMed] [Google Scholar]
Wolfe 2009
- Wolfe F. Fibromyalgianess. Arthritis Rheumatism 2009;61(6):715‐6. [DOI] [PubMed] [Google Scholar]
Wolfe 2010
- Wolfe F, Clauw DJ, Fitzcharles MA, Goldenberg DL, Katz RS, Mease P, et al. The American College of Rheumatology preliminary diagnostic criteria for fibromyalgia and measurement of symptom severity. Arthritis Care and Research 2010;62(5):600‐10. [DOI] [PubMed] [Google Scholar]
Wolfe 2011
- Wolfe F, Clauw DJ, Fitzcharles MA, Goldenberg DL, Hauser W, Katz RS, et al. Fibromyalgia criteria and severity scales for clinical and epidemiological studies: a modification of the ACR Preliminary Diagnostic Criteria for Fibromyalgia. Journal of Rheumatology 2011;38(6):1113‐22. [PUBMED: 21285161] [DOI] [PubMed] [Google Scholar]
Yang 2012
- Yang PY, Ho KH, Chen HC, Chien MY. Exercise training improves sleep quality in middle‐aged and older adults with sleep problems: a systematic review. Journal of Physiotherapy 2012;58(3):157‐63. [DOI: 10.1016/S1836-9553(12)70106-6] [DOI] [PubMed] [Google Scholar]
Yunus 1981
- Yunus M, Masi AT, Calabro JJ, Miller KA, Feigenbaum SL. Primary fibromyalgia (fibrositis): clinical study of 50 patients with matched normal controls. Seminars in Arthritis and Rheumatism 1981;11:151‐71. [DOI] [PubMed] [Google Scholar]
Zeng 2008
- Zeng QY, Chen R, Darmawan J, Xiao ZY, Chen SB, Wigley R, et al. Rheumatic diseases in China. Arthritis Research & Therapy 2008;10(1):R17. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to other published versions of this review
Bidonde 2014a
- Bidonde J, Busch AJ, Webber SC, Schachter CL, Danyliw A, Overend TJ, et al. Aquatic exercise training for fibromyalgia. Cochrane Database of Systematic Reviews. Issue 10. Art. No.: CD011336. DOI: 10.1002/14651858.CD011336 2014. [DOI] [PMC free article] [PubMed]
Bidonde 2017
- Bidonde J, Busch AJ, Schachter CL, Overend TJ, Kim SY, Góes SM, et al. Aerobic exercise training for adults with fibromyalgia. Cochrane Database of Systematic Reviews 21;6:CD012700. DOI: 10.1002/14651858.CD012700 June 2017. [DOI] [PMC free article] [PubMed]
Bidonde 2017a
- Bidonde J, Busch AJ, Spuy I, Tupper S, Kim SY, Boden C. Whole body vibration exercise training for fibromyalgia. Cochrane Database of Systematic Reviews 26;9:CD011755. DOI: 10.1002/14651858.CD011755.pub2. Sep 2017. [DOI] [PMC free article] [PubMed]
Busch 2001
- Busch AJ, Schachter CL, Peloso PM. Fibromyalgia and exercise training: a systematic review of randomized clinical trials. Physical Therapy Review 2001;6:287‐306. [Google Scholar]
Busch 2002
Busch 2007
- Busch AJ, Barber KAR, Overend TJ, Peloso PMJ, Schachter CL. Exercise for treating fibromyalgia syndrome. Cochrane Database of Systematic Reviews 2007; Vol. 17, issue 4:CD003786. [DOI] [PMC free article] [PubMed]
Busch 2008
- Busch AJ, Schachter CL, Overend TJ, Peloso PM, Barber KA. Exercise for fibromyalgia: a systematic review. Journal of Rheumatology 2008;35(6):1130‐44. [PubMed] [Google Scholar]
Busch 2013
- Busch AJ, Webber SC, Richards RS, Bidonde J, Schachter CL, Schafer LA, et al. Resistance exercise training for fibromyalgia. Cochrane Database Systematic Review 20;(12):CD010884. DOI: 10.1002/14651858.CD010884 2013. [DOI] [PMC free article] [PubMed]
Kim SY 2019
- (forthcoming) Kim SY, Busch A, Boden C, Goez SM, Foulds HJA, Spuy I, et al. Flexibility exercise training for adults with fibromyalgia. Cochrane Database of Systematic Reviews. Forthcoming. [DOI] [PMC free article] [PubMed]


