Abstract
Background
Advanced interatrial block (aIAB) on the surface electrocardiogram (ECG), defined as a P‐wave duration ≥120 milliseconds with biphasic (±) morphology in inferior leads, is frequently associated with atrial fibrillation (AF). The aim of this study was to determine whether preoperative aIAB could predict new‐onset AF in patients with severe congestive heart failure (CHF) requiring cardiac resynchronization therapy (CRT).
Methods
Retrospective analysis of consecutive patients with CHF and no prior history of AF undergoing CRT for standard indications. A baseline 12‐lead ECG was obtained prior to device implantation and analyzed for the presence of aIAB. ECGs were scanned at 300 DPI and maximized 8×. Semiautomatic calipers were used to determine P‐wave onset and offset. The primary outcome was the occurrence of AF identified through analyses of intracardiac electrograms on routine device follow‐up.
Results
Ninety‐seven patients were included (74.2% male, left atrial diameter 45.5 ± 7.8 mm, 63% ischemic). Mean P‐wave duration was 138.5 ± 18.5 milliseconds and 37 patients (38%) presented aIAB at baseline. Over a mean follow‐up of 32 ± 18 months, AF was detected in 29 patients (30%) and the incidence was greater in patients with aIAB compared to those without it (62% vs 28%; P < 0.003). aIAB remained a significant predictor of AF occurrence after multivariate analysis (OR 4.1; 95% CI, 1.6–10.7; P < 0.003).
Conclusion
The presence of aIAB is an independent predictor of new‐onset AF in patients with severe CHF undergoing CRT.
Keywords: interatrial block, cardiac resynchronization, atrial fibrillation, heart failure
Atrial fibrillation (AF) and congestive heart failure (CHF) often coexist and share common pathophysiologic mechanisms.1 CHF predisposes to AF by increased filling pressures, atrial stretch, and dysregulation of intracellular calcium. On the other side, AF contributes to progression of CHF by several mechanisms, including loss of atrial systole, rapid ventricular rate with shorter diastolic filling time, and tachycardia‐induced cardiomyopathy. Furthermore, AF is associated with worse clinical outcomes in patients with CHF2, 3 and identifying predictors of AF in this population may help developing strategies to prevent further complications.
Interatrial block (IAB), a delay of conduction over the Bachmann bundle, is manifested on the 12‐lead electrocardiogram (ECG) by a P‐wave duration ≥120 milliseconds either with biphasic [±] morphology in the inferior leads (advanced IAB, aIAB) or without it (partial IAB, pIAB).4 The presence of aIAB is frequently associated with atrial tachyarrhythmias and has been found to be a predictor of AF in different clinical scenarios.5, 6, 7 The aim of this study was to determine whether the presence of aIAB is a predictor of new onset AF in patients with advanced CHF undergoing cardiac resynchronization therapy (CRT).
METHODS
Patients
We conducted a retrospective analysis of all patients with CHF undergoing CRT at Kingston General Hospital over a 60‐month period. Inclusion criteria were: (i) CHF with indication for CRT on the basis of current guidelines; (ii) successful implantation of a triple‐chamber device with leads positioned at the right atrium, right ventricle (apical or septal position), and a tributary branch of the coronary sinus; (iii) sinus rhythm at the time of the procedure; and (iv) no prior history of AF. Only de novo implantations were considered and patients were excluded if they had less than 6 month's follow‐up at our center.
Electronic charts were reviewed for demographics, comorbid conditions (diabetes mellitus, hypertension, and obstructive sleep apnea), etiology of cardiomyopathy, and echocardiographic parameters, including left atrial (LA) diameter and left ventricular ejection fraction (LVEF).
ECG Analysis
A standard 12‐lead ECG (filter 150 Hz, 25 mm/s, 10 mm/mV) was obtained in all patients prior to the device implantation. The ECGs were scanned at 300 DPI and blindly analyzed for the presence of IAB. The images were amplified 8× and the P‐wave duration was measured using semiautomatic calipers (Iconico, New York, NY, USA). This method of P‐wave measurement has been previously described and validated.8, 9 The onset of the P‐wave was identified as the point of initial upward or downward deflection from the baseline and the P‐wave offset as the returning point of the waveform to the baseline.
pIAB was defined as P‐wave duration ≥120 milliseconds without biphasic morphology (±) in the inferior leads, and aIAB was defined as a P‐wave ≥120 milliseconds and biphasic morphology (±) in the inferior leads (Fig. 1).5 The ECG analyses was performed by two blinded independent observers (F.S.A., A.E.).
Figure 1.
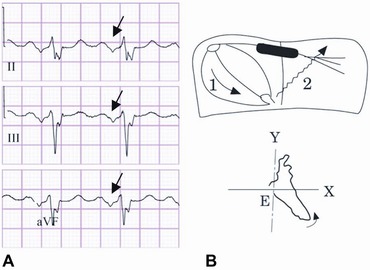
(A) Typical ECG of aIAB with P‐wave duration >120 milliseconds and biphasic morphology in inferior leads (see arrow). (B) Diagram of atrial conduction showing block of the electrical impulse in the upper and middle part of the interatrial septum and retrograde LA activation via muscular connections in the vicinity of coronary sinus (modified from Yamada et al.15).
Follow‐up
After implantation patients were followed in the device clinic at 3 months and every 6 months thereafter. The primary outcome was development of new onset AF identified through analyses of the intracardiac electrograms. AF was defined as an episode of switch mode or atrial high rate lasting at least 30 seconds with electrograms compatible with fast and disorganized atrial rhythm.
Statistical Analyses
Data were expressed as means and standard deviations for continuous variables, and frequencies and percentages for categorical variables. Following univariate comparisons (independent samples t‐tests and chi‐square tests), a multivariate logistic regression analysis was performed to identity predictors of AF. Variables were selected for inclusion into the multivariate model if they had a significance of P < 0.15 on univariate testing. Receiver operating curve (ROC) analysis was used to assess the adequacy of the final model, first by assessing the area under the curve (AUC) for the individual predictors alone, then assessing the AUC for the full multivariate model. P values < 0.05 were considered statistically significant.
RESULTS
A total of 390 patients received a de novo CRT implantation during the aforementioned period. Ninety‐seven patients were included based on the inclusion and exclusion criteria. Baseline characteristics are summarized in Table 1. Mean age was 67 ± 9.6 years; 73.5% were male, 65% had ischemic cardiomyopathy, and mean LA diameter was 45.5 ± 7.8 mm. Medications can be seen in Table 1.
Table 1.
Baseline Clinical Characteristics
| Clinical Variable | |
|---|---|
| Age (years) | 67 ± 9.6 |
| Male gender | 72 (74.2) |
| Hypertension | 39 (40.2) |
| Diabetes mellitus | 31 (32.0) |
| Obstructive sleep apnea | 12 (12.4) |
| Etiology of cardiomyopathy | |
| Ischemic | 61 (63) |
| Nonischemic | 35 (36) |
| Combined ischemic and nonischemic | 1 (1.0) |
| Mean LA size | 45.5 ± 7.8 |
| Mean LVEF | 23.8 ± 6.4 |
| Mean P‐wave duration (milliseconds) | 138.5 ± 18.5 |
| Advanced interatrial block | 37 (38) |
| Medical treatment | |
| Beta‐blocker | 92 (94.8) |
| ACE‐I/ARB | 90 (92.7) |
| Amiodarone | 7 (7.2) |
| Calcium channel blocker | 2 (2.1) |
| Digoxin | 15 (15.5) |
Values represent mean ± SD, number is parentheses represent percentage; LA = left atrium; LVEF = left ventricle ejection fraction; ACE‐I = angiotensin converting enzyme inhibitor; ARB = angiotensin receptor blocker.
Mean P‐wave duration was 138.5 ± 18.5 ms and IAB was detected in 75 patients (77%): 35 partial (36%) and 37 advanced (38%).
After a mean follow‐up of 32 ± 18 months, 29 patients (30%) developed AF. Table 2 presents the clinical characteristics of patients who developed AF versus those who remained in sinus rhythm. The patients who developed AF were older (70 ± 7.3 years vs 65.75 ± 10.2 years; P = 0.02), had more prolonged P‐waves (145.6 ± 21.3 milliseconds vs 135.5 ± 16 milliseconds; P 0.03) and had higher prevalence of aIAB (62% vs 28%; P 0.003). There was no significant difference between groups regarding gender, DM, HTN, OSA, etiology of cardiomyopathy, mean LA diameter, mean LVEF, and use of medications including beta‐blockers and Amiodarone.
Table 2.
Clinical Characteristics of Patients with and without Atrial Fibrillation at Follow‐Up
| Atrial Fibrillation | No Atrial Fibrillation | ||
|---|---|---|---|
| Clinical Variable | (n = 29) | (n = 68) | P Value |
| Age (years) | 70 ± 7.3 | 65.75 ± 10.2 | 0.02 |
| Gender | 0.31 | ||
| Male | 24(82.2) | 48(70.6) | |
| Female | 5 (17.2) | 20 (29.4) | |
| Hypertension | 10 (34.5) | 29 (42.6) | 0.50 |
| Diabetes mellitus | 8 (27.6) | 23 (33.8) | 0.63 |
| Obstructive sleep apnea | 1 (3.4) | 11 (16.2) | 0.10 |
| Ischemic cardiomyopathy | 22 (75.9) | 39 (57.4) | 0.11 |
| Mean LA size | 47.4 ± 7.8 | 44.7 ± 7.7 | 0.18 |
| Mean LVEF | 24.8 ± 6.7 | 23.3 ± 6.3 | 0.3 |
| Mean P‐wave duration (milliseconds) | 145.6 ± 21.3 | 135.5 ± 16.3 | 0.03 |
| Advanced interatrial block | 18 (62) | 19 (28) | 0.003 |
| Medications | |||
| Beta‐blockers | 27 (93) | 61 (89.7) | 0.5 |
| Amiodarone | 2 (6.9) | 6 (8.9) | 0.5 |
Values represent mean ± SD, number is parentheses represent percentage; LA = left atrium; LVEF = left ventricle ejection fraction.
Variables entered into the logistic regression (on the basis of a significance of P < 0.15 in univariate testing) included age, OSA, etiology of cardiomyopathy, P‐wave duration, and aIAB. There was a strong association between P‐wave duration and aIAB (P = 0.003 on independent samples t‐testing) but in the multivariable model for AF, aIAB had the stronger association and P‐wave duration was eliminated. Table 3 summarizes the multivariate analysis in which aIAB was a statistically significant predictor of AF and age fell just short of significance. Mean P‐wave duration was the last to be eliminated from the model, at P = 0.128. ROC analysis indicated that both components of the final model classified the group significantly better than chance alone. The AUC for age was 0.632 (95% CI, 0.519–0.745, P = 0.043) and the AUC for aIAB was 0.671 (95% CI, 0.550–0.791, P = 0.008). The combined model had an AUC of 0.720 (95% CI, 0.604–0.836, P = 0.001), suggesting that these two factors in combination classified the patients better than chance alone, and that the two variables together were superior to either one alone.
Table 3.
Predictors of Atrial Fibrillation on Multivariate Analyses
| Clinical Variable | Odds Ratio | 95% Confidence Interval | P Value |
|---|---|---|---|
| Age (years) | 1.05 | 1.00–1.11 | 0.053 |
| Advanced IAB | 4.13 | 1.60–10.70 | 0.003 |
Area under the curve for the final model was 0.720 (95% CI, 0.604–0.836), P = 0.001.
DISCUSSION
AF affects 15–30% of patients with CHF10 and the prevalence increases with progression of NYHA class.11 In addition, patients with CHF and AF have an increased cardiovascular mortality compared to patients in sinus rhythm.2, 3, 12 Although the association between both conditions is well documented, the clinical factors that predict the occurrence of AF in this subgroup of patients have not been yet completely elucidated.
The main finding of this study is that aIAB predicted the development of new‐onset AF in patients with severe CHF undergoing CRT. We found no significant differences in gender, LA diameter, LVEF, or prevalence of hypertension between patients with and without AF during the follow‐up, which is compatible with previous studies13, 14, 15 and suggests that classic predictors of AF in the general population might not predict AF in the subset of patients with CHF. Pozzoli et al.,13 in a prospective study of 344 patients with CHF and sinus rhythm, found that 8% of them developed AF over a period of 19 ± 12 months, but its occurrence could not be predicted by any of the baseline clinical, electrocardiographic or echocardiographic variables. Similar results were reported by Crijns et al.,14 who described an incidence of 9% after 3.4 years in patients with moderate‐to‐severe CHF who had sinus rhythm at baseline; these patients were older (70 years vs 66 years, P < 0.007) and had slightly lower blood pressure and plasma norepinephrine levels (P < 0.05), but were otherwise similar. The incidence of AF in our study was significantly higher than in the mentioned series, which is probably explained by the more severe disease in the patients in our cohort. In addition, the presence of an implantable cardiac device provides continuous electrocardiographic monitoring, allowing detection of subclinical and self‐limited AF episodes.
The role of interatrial conduction disorders in the genesis of atrial arrhythmias was first described by Bayés de Luna several decades ago and this association is particularly strong for aIAB.16 This electrocardiographic pattern, characterized by a negative terminal component in the P wave of inferior leads, results from block of the electrical impulse at the Bachmann bundle zone, with retrograde activation of the LA via muscular connections in the vicinity of the coronary sinus.17, 18 The delayed and heterogeneous activation of the LA results in dispersion of atrial refractory periods, interatrial dyssynchrony and impaired LA mechanical function,19, 20 all of which favor the development of AF. Thus, IAB (partial or advanced) has been found to be associated with new‐onset AF in patients from a general hospital population,21, recurrence of AF postelectrical or pharmacological cardioversion,22, 23, AF recurrence after pulmonary vein isolation,24 and progression of paroxysmal AF to the persistent or permanent forms of the disease.25, 26
In patients with CHF, the association of P‐wave abnormalities with development of AF was first described by Yamada et al.15 using signal averaged ECG (SAECG). They reported abnormal P‐wave duration by SAECG in 38% of patients at baseline and higher incidence of paroxysmal AF in subjects with rather than without abnormal P‐wave duration during the follow‐up (32% vs 2%; P = 0.0002). Similarly, Holmqvist et al.27, 28 have analyzed unfiltered P wave on SAECG and have shown similar results in heart failure population.
Using conventional 12‐lead ECG in patients with Chagas’ cardiomyopathy and implantable cardioverter defibrillators (ICDs), we found that IAB was present in 18.8% of patients and was strongly associated with newly diagnosed AF and inappropriate therapies by the ICD.29 This study corroborates these findings in a different HF population composed mainly of patients with ischemic and idiopathic cardiomyopathy. It is interesting that although P‐wave duration alone was significantly higher in patients that developed AF; it fell out of significance in the multivariate analysis. This finding is in agreement with the studies done by Holmqvist et al.,27, 28 suggesting that the activation sequence of the LA is probably more important for the genesis of atrial arrhythmias than the total atrial activation time. This can explain why P‐wave duration by itself has showed some inconsistence to predict AF in other studies.13, 14
Holmqvist et al.27 have also shown that abnormal P‐wave morphology patterns were an independent risk predictor for development of AF. Although the technique of assessing IAB by analyzing unfiltered P wave on SAECG has been previously validated30; it is not very widespread and is unavailable in many centers. It was also interesting to note that the reported prevalence of the pattern of P‐wave morphology consistent with aIAB in studies by Holmqvist et al.27, 28 was much lower than in our study (0.2% and 0.1% respectively). This could be explained on the basis of the difference in the methodology used for assessing IAB, as well as on a disparity on age and NYHA class. In our study, patients were much older (67 ± 9.6 years vs. 63 ± 10 years or 64 ± 11 years in Holmqvist study, depending on the functional class). Regarding functional class, in our study almost all were in NYHA III‐IV at the time of implantation whereas functional class was much lower in Holmqvist. Finally, the disparity in the methodology used to measure the P‐wave and the dissimilar definitions of aIAB may account for a significant difference on the prevalence of aIAB between studies.
A question that arises is whether CRT may have a favorable effect on interatrial conduction abnormalities. There is evidence that biventricular pacing is associated with favorable reverse remodeling effect on the LA, manifested by decrease in LA volume that correlates closely with reduction in LV dimensions.30, 31, 32, 33 The effects of CRT on interatrial conduction or P‐wave duration have not been specifically assessed; however, the risk of atrial tachyarrhythmias decreases post‐CRT,31, 32, 33 which suggests that the structural atrial remodeling entails some degree of electrical remodeling.
STUDY LIMITATIONS
First, the retrospective nature of this study could introduce bias in the analysis. Second, the sample size is small and these findings need to be verified in larger cohorts of patients. Finally, although patients with prior documented AF were excluded, it is impossible to be sure that some of them had not had undocumented asymptomatic episodes.
CONCLUSION
aIAB is frequent in patients with CHF requiring CRT and is a strong independent predictor of new‐onset AF. We believe that this could be relevant for clinical management because many of these episodes are asymptomatic and these patients may benefit from a closer follow‐up by device interrogation and maybe earlier consideration of anticoagulant therapy in case of severe LV dysfunction.
Conflict of interest: None.
REFERENCES
- 1. Anter E, Jessup M, Callans DJ. Atrial fibrillation and heart failure: Treatment considerations for a dual epidemic. Circulation 2009;119:2516–2525. [DOI] [PubMed] [Google Scholar]
- 2. Swedberg K, Olsson LG, Charlesworth A, et al. Prognostic relevance of atrial fibrillation in patients with chronic heart failure on long‐term treatment with beta‐blockers: Results from COMET. Eur H Journal 2005;26:1303–1308. [DOI] [PubMed] [Google Scholar]
- 3. Dries DL, Exner DV, Gersh BJ, et al. Atrial fibrillation is associated with an increased risk for mortality and heart failure progression in patients with asymptomatic and symptomatic left ventricular systolic dysfunction: a retrospective analysis of the SOLVD trials. Studies of left ventricular dysfunction. J Am Coll Cardiol 1998;32:695–703. [DOI] [PubMed] [Google Scholar]
- 4. Bayés de Luna A, Platonov P, Cosio FG, et al. Interatrial blocks: A separate entity from left atrial enlargement: A consensus report. J Electrocardiol 2012;45:445–451. [DOI] [PubMed] [Google Scholar]
- 5. Conde D, Baranchuk A. Interatrial block as anatomical–electrical substrate for supraventricular arrhythmias: Bayes’ syndrome. Arch Mex Cardiol 2014;84:32–40. [DOI] [PubMed] [Google Scholar]
- 6. Enriquez A, Conde D, Hopman W, et al. Advanced interatrial block is associated with recurrence of atrial fibrillation post pharmacological cardioversion. Cardiovasc Ther 2014;32:52–56. [DOI] [PubMed] [Google Scholar]
- 7. De Luna AB, Guindo J, Vinolas X, et al. Third‐degree inter‐atrial block and supraventricular tachyarrhythmias. Europace 1999;1:43–46. [DOI] [PubMed] [Google Scholar]
- 8. Dilaveris P, Batchvarov V, Gialafos J, et al. Comparison of different methods for manual P wave duration measurement in 12‐lead electrocardiograms. PACE 1999;22:1532–1538. [DOI] [PubMed] [Google Scholar]
- 9. Andrikopoulos GK, Dilaveris PE, Richter DJ, et al. Increased variance of P wave duration on the electrocardiogram distinguishes patients with idiopathic paroxysmal atrial fibrillation. PACE 2000;23:1127–1132. [DOI] [PubMed] [Google Scholar]
- 10. Naccarelli GV, Hynes B, Wolbrette DL, et al. Atrial fibrillation in heart failure. J Cardiovasc Electrophysiol 2003;14:S281–S286. [DOI] [PubMed] [Google Scholar]
- 11. Maisel WH, Stevenson LW. Atrial fibrillation in heart failure: Epidemiology, pathophysiology, and rationale for therapy. Am J Cardiol 2003;91:2–8. [DOI] [PubMed] [Google Scholar]
- 12. Benjamin EJ, Wolf PA, D'Agostino RB, et al. Impact of atrial fibrillation on the risk of death the Framingham Heart Study. Circulation 1998;98:946–952. [DOI] [PubMed] [Google Scholar]
- 13. Pozzoli M, Cioffi G, Traversi E, et al. Predictors of primary atrial fibrillation and concomitant clinical and hemodynamic changes in patients with chronic heart failure: A prospective study in 344 patients with baseline sinus rhythm. J Am Coll Cardiol 1998;32:197–204. [DOI] [PubMed] [Google Scholar]
- 14. Crijns HJ, Tjeerdsma G, de Kam PJ, et al. Prognostic value of the presence and development of atrial fibrillation in patients with advanced chronic heart failure. Eur Heart J 2000;21:1238–1245. [DOI] [PubMed] [Google Scholar]
- 15. Yamada T, Fukunami M, Shimonagata T, et al. Prediction of paroxysmal atrial fibrillation in patients with congestive heart failure: A prospective study. J Am Coll Cardiol 2000;35:405–413. [DOI] [PubMed] [Google Scholar]
- 16. Bayes de Luna A, Cladellas M, Oter R, et al. Interatrial conduction block and retrograde activation of the left atrium and paroxysmal supraventricular tachyarrhythmia. Eur Heart J 1988;9:1112–1118. [DOI] [PubMed] [Google Scholar]
- 17. Puech P. L'activite´ Electrique Auriculaire Normale et Pathologuique. Paris, Masson, 1956, p. 206. [Google Scholar]
- 18. Cosío FG, Martín‐Peñato A, Pastor A, et al. Atrial activation mapping in sinus rhythm in the clinical electrophysiology laboratory: Observations during Bachmann's bundle block. J Cardiovasc Electrophysiol 2004;15:524–531. [DOI] [PubMed] [Google Scholar]
- 19. Daubert JC, Pavin D, Jauvert G, Mabo P. Intra‐ and interatrial conduction delay: Implications for cardiac pacing. Pacing Clin Electrophysiol 2004;27:507–525. [DOI] [PubMed] [Google Scholar]
- 20. Goyal SB, Spodick DH. Electromechanical dysfunction of the left atrium associated with interatrial block. Am Heart J 2001;142:823–827. [DOI] [PubMed] [Google Scholar]
- 21. Agarwal YK, Aronow WS, Levy JA, Spodick DH. Association of interatrial block with development of atrial fibrillation. Am J Cardiol 2003;91:882. [DOI] [PubMed] [Google Scholar]
- 22. Gonna H, Gallagher MM, Guo XH, et al. P‐wave abnormality predicts recurrence of atrial fibrillation after electrical cardioversion: A prospective study. Ann Noninvasive Electrocardiol 2014;19:57–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Enriquez A, Conde D, Hopman W, et al. Advanced interatrial block is associated with recurrence of atrial fibrillation post pharmacological cardioversion. Cardiovasc Ther 2014;32:52–56. [DOI] [PubMed] [Google Scholar]
- 24. Caldwell J, Koppikar S, Barake W, et al. Prolonged P‐wave duration is associated with atrial fibrillation recurrence after successful pulmonary vein isolation for paroxysmal atrial fibrillation. J Interv Card Electrophysiol 2014;39:131–138. [DOI] [PubMed] [Google Scholar]
- 25. Koide Y, Yotsukura M, Ando H, et al. Usefulness of P‐wave dispersion in standard twelve lead electrocardiography to predict transition from paroxysmal to persistent atrial fibrillation. Am J Cardiol 2008;102:573–577. [DOI] [PubMed] [Google Scholar]
- 26. Dixen U, Vang Larsen M, Ravn L, et al. Signal‐averaged P wave duration and the long‐term risk of permanent atrial fibrillation. Scan Cardiovasc J 2008;42:31–37. [DOI] [PubMed] [Google Scholar]
- 27. Holmqvist F, Platonov PG, McNitt S, et al. Abnormal P‐wave morphology is a predictor of atrial fibrillation development and cardiac death in MADIT II patients. Ann Noninvasive Electrocardiol 2010;15:63–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Holmqvist F, Platonov PG, Solomon S et al and MADIT CRT investigators. P‐wave morphology is associated with echocardiographic response to cardiac resynchronization therapy in MADIT‐CRT patients. Ann Noninvasive Electrocardiol 2013;18:510–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Enriquez A, Conde D, Femenia F, et al. Relation of interatrial block to new‐onset atrial fibrillation in patients with Chagas cardiomyopathy and implantable cardioverter‐defibrillators. Am J Cardiol 2014;113:1740–1743. [DOI] [PubMed] [Google Scholar]
- 30. Holmqvist F, Havmoller R, Platonov P, Carlson J. Signal averaged P wave analysis for delineation of interatrial conduction—Further validation of the method. BMC Cardiovasc Disord 2007;7:29–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kiès P, Leclercq C, Bleeker GB, et al. Cardiac resynchronisation therapy in chronic atrial fibrillation: Impact on left atrial size and reversal to sinus rhythm. Heart 2006;92:490–494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Fung JW, Yip GW, Zhang Q, et al. Improvement of left atrial function is associated with lower incidence of atrial fibrillation and mortality after cardiac resynchronization therapy. Heart Rhythm 2008;5:780–786. [DOI] [PubMed] [Google Scholar]
- 33. Brenyo A, Link MS, Barsheshet A, et al. Cardiac resynchronization therapy reduces left atrial volume and the risk of atrial tachyarrhythmias in MADIT‐CRT (Multicenter Automatic Defibrillator Implantation Trial with Cardiac Resynchronization Therapy). J Am Coll Cardiol 2011; 58:1682–1689. [DOI] [PubMed] [Google Scholar]


