Abstract
Background
The currently used scheme for the classification of infarct location and extent in anterior myocardial infarction (MI) is intuitive rather than being evidence‐based, and recent evidence suggests that it may be misleading both in anatomic and prognostic sense.
Material and Methods
Consecutive patients with the diagnosis of anterior MI were enrolled. All electrocardiograms (ECG) were first classified according to established scheme and then reassessed using newer criteria for angiographic site of occlusion. The site of left anterior descending (LAD) occlusion was determined using multiple angiographic views. Clinic, echocardiographic and angiographic outcomes were compared.
Results
A total of 379 anterior MI cases were enrolled, final study population consisted of 267 patients. The established scheme did not predict infarct size or adverse outcomes. Location of the myocardium subtended by the occluded coronary network did not match with the anatomic location as ECG classification implies. Many high‐risk patients with proximal LAD were classified as “anteroseptal”, whereas the majority of the patients labeled as “extensive anterior MI” had in fact distal occlusions. On the other hand, expert interpretation was fairly accurate in predicting adverse outcomes and the site of angiographic involvement.
Conclusion
Classifying patients according to the established scheme neither gives prognostic information nor accurately localizes infarction. It should be regarded as obsolete and its use should be abandoned. Instead, the extent of infarction can be inferred from newer criteria provided by the angiographic correlation studies.
Highlights
The currently used scheme for the classification of infarct location and extent in anterior myocardial infarction (MI) is intuitive rather than being evidence‐based.
Recent evidence suggests that it may be misleading in both anatomic and prognostic sense.
In this study our results showed that the location of the myocardium subtended by the occluded coronary network did not match with the anatomic location as ECG classification implies. Many high‐risk patients with proximal LAD were classified as “anteroseptal”, whereas the majority of the patients labeled as “extensive anterior MI” had in fact distal occlusions. It also did not predict infarct size or adverse outcomes.
However, expert interpretation was fairly accurate in predicting adverse outcomes and the site of angiographic involvement.
Classifying patients according to the established scheme neither gives prognostic information nor accurately localizes infarction. This is a very important and clinically relevant result that points the critical need for a change in nearly a century‐old framework.
The established schema for anterior MI classification should be regarded as obsolete and its use should be abandoned. Instead, the extent of infarction can be inferred from newer criteria provided by angiographic correlation studies.
1. INTRODUCTION
Timely reperfusion is a life‐saving therapeutic target in patients with acute anterior myocardial infarction (MI), especially in patients with an extensive area‐at‐risk. The electrocardiogram (ECG) is the most commonly used and readily available diagnostic tool providing an opportunity to describe location and extent of infarction. However, currently used scheme for the classification of infarct location and extent (Table 1) is intuitive rather than being evidence‐based and the recent studies showed that the number or location of chest leads displaying ST‐segment elevation alone does not predict the extent of potentially damaged myocardium in anterior MI (Fiol et al., 2009). Furthermore, angiographic correlation studies hint that this scheme may be misleading in prediction of the site of left anterior descending (LAD) artery occlusion and estimation of infarct size (Arbane & Goy, 2000; Bayés de Luna, 2012; Fiol et al., 2009, 2004; Taglieri et al., 2014). This may be at least partially responsible for the fact that the failure to identify patients with a large area‐at‐risk is disappointingly common and results in lower quality care in the emergency room (Masoudi et al., 2006). In this study, we investigated whether established ECG scheme is able to correctly classify infarct localization and extent, and/or infer prognostic information. We also sought if expert interpretation using information from newer angiographic correlation studies provides a better anatomic and prognostic information.
Table 1.
The established scheme for classification of anterior wall myocardial infarctiona
| V1–V2 | Septal |
| V1–V4 | Anteroseptal |
| V3–V4 | Anteroapical (or mid‐anterior) |
| V3–V6 | Anterolateral |
| V5–V6 | Lateral |
| V1–V6 | Extensive anterior |
Additionally, ST‐segment elevation in lead I and aVL is generally labeled as “high‐lateral” myocardial infarction in this scheme.
2. MATERIAL AND METHODS
The study was undertaken at Dr. Siyami Ersek Thoracic and Cardiovascular Surgery Training and Research Hospital, Istanbul, which has a large local transfer network with around 1,500 STEMI patients per year referred for primary percutaneous coronary intervention. Institutional review board approval was obtained; the study was judged to be exempt from formal evaluation because it involved only analysis of existing records.
All patients from May 2017 to January 2018, who were admitted with the diagnosis of acute anterior ST‐segment elevation MI and underwent coronary angiography revealing acute occlusion of left anterior descending artery or its branches with Thrombolysis in Myocardial Infarction 0/1 flow, were enrolled. Patients with prior history of coronary artery bypass grafting, significant collaterals to the distal LAD territory (as defined below), left bundle branch block or any other secondary repolarization abnormality were excluded. Baseline characteristics were obtained via chart review and GRACE risk score (Fox et al., 2006) at admission was calculated retrospectively. Troponin I Abbott c4100i (Abbott Diagnostics, Chicago, IL, USA) was used as the troponin assay.
All ECGs were reviewed by a senior cardiologist (E.A.), who was blinded to the angiographic and clinical outcomes. For multiple ECGs on the same patient, the earliest ECG with maximum ST‐segment deviation was used. ST‐segment elevation was measured at the J point and approximated to closest 0.5 mm. The chest leads with at least 1 mm ST‐segment elevation were used for classification according to the established MI localization scheme (Table 1). The reviewer also sought to predict the site of LAD occlusion using 12‐lead information, published criteria (Arbane & Goy, 2000; Bayés de Luna, 2012; Fiol et al., 2009, 2004; Taglieri et al., 2014) and subjective impression when published criteria were conflicting or inconclusive. Namely, Fiol's algorithm (Fiol et al., 2009) (ST‐segment depression in aVF + lead III ≥ 2.5 for prediction of occlusion proximal to D1, ST‐segment elevation in V1 + aVR ‐ V6 ≥ 0 for prediction of occlusion proximal to S1, isoelectric or elevated ST‐segments in inferior leads for prediction of distal occlusion), right bundle branch block or ST‐segment elevation in V1 ≥ 2.5 mm for prediction of occlusion proximal to S1, ST‐segment elevation or pathologic Q waves in aVL for prediction of occlusion proximal to DI were used (Arbane & Goy, 2000).
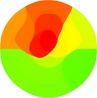
Echocardiographic wall motion score index was calculated using a 17‐segment model of the ventricle and a scoring system as follows: 1, normokinesia; 2, hypokinesia; 3, akinesia; 4, dyskinesia. For each ECG subclass, for three subclasses with respect to the prediction of the site of occlusion (proximal, mid, distal) and for three subclasses for angiographic involvement site (proximal, mid, distal), mean values of the wall motion score for each segment were separately calculated and color‐coded bullseye displays were constructed.
Cineangiograms were reviewed by two interventional cardiologists (E.B. and Ö.Y.), who were blinded to the electrocardiographic and clinical outcomes. Any disagreement was resolved by a third cardiologists’ opinion. The relation of the site of occlusion of the LAD artery to the origin of its major first diagonal (D1) and septal (S1) branch was determined using multiple angiographic views. Any stenosis >70% (>50% for left main coronary artery) affecting non‐infarct related arteries was defined as significant for the purpose of detecting multivessel disease. Since electrocardiographic studies used S1 and D1 for prediction of LAD occlusion site and defined LAD segments according to these branches rather than established angiographic segmentation scheme, any occlusion proximal to both D1 and S1 was defined as proximal, both distal to D1 and S1 as distal, and proximal to one of D1 and S1, but distal to the other as mid segment occlusion in our study. Collateral blood supply to the distal LAD territory was assessed by visual analysis and Rentrop grade ≥2 collaterals to the territory at risk were deemed as a significant collateral supply (Rentrop, Cohen, Blanke, & Phillips, 1985).
All measurements were presented as mean and standard deviation. Baseline characteristics were summarized using standard descriptive statistics. Comparisons of relevant parameters according to ECG group or expert prediction of angiographic occlusion site were performed by chi‐square or Kruskal–Wallis H test as appropriate. Trends across groups were assessed by Jonckheere–Terpstra test. A Spearman's rank order correlation was run to assess the relationship between expert prediction and the real angiographic site of occlusion. All statistical analyses were performed with SPSS (version 24.0; SPSS Inc., Chicago, IL, USA).
3. RESULTS
A total of 379 anterior MI cases were enrolled during study period. One‐hundred and twelve patients were excluded because one of several reasons including left bundle branch block (n = 19), secondary ST‐T abnormalities, such as left ventricular hypertrophy, preexcitation syndrome (n = 24), subacute ECG changes (n = 41), history of coronary artery bypass grafting (n = 21), significant collaterals to the infarct territory (n = 7). Therefore, final patient population consisted of 267 patients. Baseline clinical characteristics were summarized in Table 2, along with a comparison of baseline characteristics and clinical outcomes according to the site of LAD occlusion.
Table 2.
Baseline characteristicsa
| All (N = 267) | Proximal LAD occlusion (n = 93) | Occlusion between D1 and S1 (n = 69) | Occlusion Distal to S1 and D1 (n = 93) | P‐valueb (p for trend) | |
|---|---|---|---|---|---|
| Demographic parameters | |||||
| Age, years | 59 ± 12 | 60 ± 13 | 58 ± 12 | 59 ± 13 | 0.724 |
| Male | 201 (75) | 69 (74) | 54 (78) | 69 (73) | 0.829 |
| White | 267 (100) | 93 (100) | 69 (100) | 93 (100) | 1.000 |
| Hypertension | 118 (44) | 42 (45) | 25 (36) | 47 (50) | 0.230 |
| Diabetes | 73 (27) | 28 (30) | 18 (26) | 26 (28) | 0.868 |
| Smoker | 137 (51) | 48 (52) | 37 (54) | 44 (46) | 0.613 |
| Prior MI | 30 (11) | 13 (14) | 4 (6) | 12 (13) | 0.379 |
| Prior PCI | 30 (11) | 13 (14) | 5 (7) | 11 (12) | 0.585 |
| Clinical parameters | |||||
| Heart rate, bpm | 86 ± 20 | 86 ± 20 | 85 ± 22 | 87 ± 20 | 0.628 |
| SBP, mmHg | 137± 32 | 119 ± 33 | 138 ± 22 | 151 ± 30 | <0.001 |
| Hgb, g/dl | 13.8 ± 1.8 | 13.7 ± 1.7 | 14.1 ± 1.9 | 13.6 ± 1.9 | 0.287 |
| Creatinine, mg/dl | 0.9 ± 0.4 | 0.9 ± 0.3 | 0.9 ± 0.6 | 0.9 ± 0.2 | 0.667 |
| Peak troponin, ng/ml | 34 ± 19 | 37 ± 18 | 35 ± 20 | 31 ± 19 | 0.062 (0.022) |
| LVEF, % | 42 ± 10 | 39 ± 10 | 40 ± 10 | 43 ± 10 | 0.167 (0.067) |
| WMSI |
|

|

|

|
|
| 1.4 ± 0.4 | 1.5 ± 0.4 | 1.4 ± 0.4 | 1.4 ± 0.4 | 0.174 (0.060) | |
| Time to ECG, minutes | 97 ± 118 | 80 ± 84 | 88 ± 100 | 120 ± 152 | 0.265 |
| Parameters related to mortality and morbidity | |||||
| Killip class ≥2 | 27 (10.1%) | 12 (13.1%) | 10 (14.5%) | 5 (5.3%) | 0.099 (0.059) |
| GRACE risk score | 149 ± 34 | 157 ± 37 | 150 ± 32 | 142 ± 30 | 0.024 (0.006) |
| Intubation | 33 (12.4%) | 17 (18.3%) | 8 (11.6%) | 8 (8.6%) | 0.134 |
| CPR | 31 (11.6%) | 16 (17.2%) | 8 (11.6%) | 7 (7.5%) | 0.128 |
| In‐hospital mortality | 26 (9.7%) | 15 (16.1%) | 6 (8.7%) | 5 (5.4%) | 0.047 |
| Angiographic involvement | |||||
| LMCA | 8 (3%) | 6 (6%) | 0 (0%) | 2 (2%) | 0.330 |
| LAD | 267 (100%) | 93 (100%) | 69 (100%) | 93 (100%) | 1.000 |
| RCA | 74 (28%) | 22 (24%) | 24 (35%) | 22 (23%) | 0.292 |
| Cx | 69 (25%) | 24 (26%) | 16 (23%) | 23 (24%) | 0.904 |
Bpm, beats per minute; Cx, circumflex artery; Hgb, hemoglobin; GRACE, global registry of acute coronary events study; LAD, left anterior descending artery; LMCA, left main coronary artery; LVEF, left ventricular ejection fraction; MI, myocardial infarction; PCI, percutaneous coronary intervention; RCA, right coronary artery; SBP, systolic blood pressure; WMSI, wall motion score index.
Values are presented as mean (SD) or number (percentage) as appropriate.
p‐value for inter‐group comparison.
Of 267 patients, 43 (16.1%) were classified as “septal,” 49 (18.4%) as “anteroseptal,” 37 (13.7%) as “anteroapical,” 13 (4.9%) as “anterolateral,” 1 (0.4%) as “lateral,” 124 (46.4%) as “extensive anterior” MI according to leads displaying ST‐segment elevation on ECG. One patient with lateral MI was included in anterolateral group. No significant difference was observed in any of the baseline characteristics across the groups. When clinical parameters and adverse outcome measures, such as Killip class on admission, the need for endotracheal intubation or cardiopulmonary resuscitation (CPR) during hospital stay, in‐hospital mortality; or measures of infarct size, such as peak troponin level, ejection fraction, wall motion score index, were compared among groups, there was no significant difference in any of these parameters (Table 3). Also, there were no significant differences in individual segmental wall motion scores across the groups.
Table 3.
Parameters related to infarct size, extent, mortality and morbidity according to electrocardiographic localizationa
| Septal (STE V1–V2) (n = 43) | Anteroseptal (STE V1–V4) (n = 49) | Anteroapical (STE V2–V4) (n = 37) | Anterolateral (STE V2–V6) (n = 14) | Extensive anterior (STE V1–V6) (n = 124) | p‐value | |
|---|---|---|---|---|---|---|
| Parameters Related to Infarct Size and Extent | ||||||
| Peak troponin, ng/ml | 29 ± 20 | 36 ± 20 | 34 ± 18 | 31 ± 18 | 36 ± 19 | 0.142 |
| LVEF, % | 44 ± 11 | 41 ± 10 | 45 ± 9 | 42 ± 14 | 40 ± 10 | 0.108 |
| WMSI |

|

|

|

|

|

|
| 1.3 ± 0.4 | 1.4 ± 0.4 | 1.4 ± 0.4 | 1.5 ± 0.4 | 1.4 ± 0.4 | 0.354 | |
| Parameters related to mortality and morbidity | ||||||
| Killip Class ≥2 | 3 (7.2%) | 7 (14.2%) | 3 (8.1%) | 1 (7.0%) | 13 (10.4%) | 0.708 |
| GRACE risk score | 150 ± 40 | 152 ± 38 | 146 ± 32 | 158 ± 33 | 146 ± 30 | 0.730 |
| Intubation | 4 (9.5%) | 9 (18.4%) | 1 (2.7%) | 3 (21.4%) | 16 (13%) | 0.185 |
| CPR | 4 (9.5%) | 10 (20.4%) | 1 (2.7%) | 3 (21.4%) | 13 (10.6%) | 0.087 |
| In‐hospital mortality | 4 (9.5%) | 8 (16.3%) | 1 (2.7%) | 2 (15.4%) | 11 (8.9%) | 0.299 |
| Angiographic correlations | ||||||
| Proximal | 18 (48.6%) | 26 (54.2%) | 9 (25.7%) | 4 (28.6%) | 36 (29.5%) | 0.011 |
| Between S1 and D1 | 5 (13.5%) | 4 (8.3%) | 6 (17.1%) | 2 (14.3%) | 16 (13.1%) | |
| Between D1 and S1 | 4 (10.8%) | 11 (22.9%) | 4 (11.4%) | 0 (0%) | 18 (14.8%) | |
| Distal | 10 (27.0%) | 7 (14.6%) | 16 (45.7%) | 8 (57.1%) | 52 (42.6%) | |
| Proximal to S1 | 22 (59.5%) | 37 (77.1%) | 13 (37.1%) | 4 (28.6%) | 54 (44.3%) | <0.001 |
| Proximal to D1 | 22 (59.5%) | 30 (62.5%) | 15 (42.9%) | 6 (42.9%) | 52 (42.6%) | 0.097 |
CPR, cardiopulmonary resuscitation; D1, first major diagonal artery; GRACE, global registry of acute coronary events study; LVEF, left ventricular ejection fraction; S1, first major septal artery; STE, ST‐segment elevation; WMSI, wall motion score index.
Values are presented as mean (SD) or number (percentage) as appropriate.
After exclusion of 11 patients with isolated diagonal branch occlusion, the distribution of the site of LAD occlusion across groups was significantly heterogeneous. However, location of the myocardium subtended by the occluded coronary network does not seem to match with the anatomic location as the ECG classification implies. For example, angiographically proximal occlusions (supposedly extensive anterior MI) were more frequent in “septal” and “anteroseptal” groups rather than “extensive anterior” group. When these two groups were separately compared, proximal occlusion was significantly more frequent in “anteroseptal” group than “extensive anterior” group (54.1% vs. 29.5%, p < 0.001). Conversely, angiographically distal occlusions (supposedly anteroapical MI) were more common in “anteroapical,” “anterolateral” and “extensive anterior” groups. Of note, isolated diagonal branch occlusions were classified in “septal” group due to isolated ST‐segment elevation in V2 (in addition to ST‐segment elevation in lead I, aVL and concomitant ST‐segment depression in inferior leads; “South African flag sign”) (Durant & Singh, 2015; Sclarovsky et al., 1994).
On the other hand, expert interpretation classified 93 (34.8%) ECGs as proximal LAD occlusion (supposedly “extensive anterior” involvement), 33 (12.4%) as occlusion proximal to S1 (supposedly “anteroseptal” involvement), 37 (13.9%) as occlusion proximal to D1 (supposedly “anterolateral” involvement), 93 (34.8%) as distal LAD occlusion (supposedly “anteroapical” involvement), 11 (4.1%) as isolated diagonal occlusion (supposedly “lateral” or “high‐lateral” involvement). When occlusions between S1 and D1 were grouped together as mid‐LAD occlusion and isolated diagonal occlusions were excluded, expert interpretation accurately predicted adverse outcomes and angiographic site of occlusion in a graded fashion (Table 4). When angiographic occlusion site was similarly stratified as three groups, there was a strong correlation between expert prediction and the real angiographic site of occlusion (rs = 0.580; p < 0.001).
Table 4.
Parameters related to infarct size, extent, mortality and morbidity according to expert interpretationa
| Proximal to D1 and S1 (n = 67) | Between S1 and D1 (n = 83) | Distal to S1 and D1 (n = 104) | P‐value (P for trend) | |
|---|---|---|---|---|
| Parameters related to infarct size and extent | ||||
| Peak troponin, ng/ml | 38 ± 18 | 35 ± 19 | 33 ± 19 | 0.343 (0.147) |
| LVEF, % | 42 ± 10 | 39 ± 10 | 42 ± 9 | 0.241 (0.772) |
| WMSI |

|

|

|

|
| 1.4 ± 0.3 | 1.4 ± 0.4 | 1.4 ± 0.4 | 0.499 (0.978) | |
| Parameters related to mortality and morbidity | ||||
| Killip class ≥2 | 10 (15.4%) | 10 (12.3%) | 7 (6.8%) | 0.149 (0.050) |
| GRACE risk score | 154 ± 39 | 150 ± 35 | 147 ± 30 | 0.730 (0.419) |
| Intubation | 14 (20.9%) | 10 (12.0%) | 8 (7.7%) | 0.039 |
| CPR | 14 (20.9%) | 10 (12.0%) | 6 (5.8%) | 0.011 |
| In‐hospital mortality | 13 (19.4%) | 8 (9.6%) | 4 (3.8%) | 0.004 |
| Angiographic correlations | ||||
| Proximal | 50 (75.8%) | 26 (31.7%) | 16 (15.7%) | <0.001 |
| Between S1 and D1 | 3 (4.5%) | 24 (29.3%) | 4 (3.9%) | |
| Between D1 and S1 | 7 (10.6%) | 19 (23.2%) | 10 (9.8%) | |
| Distal | 6 (9.1%) | 13 (15.9%) | 72 (70.6%) | |
| Proximal to S1 | 57 (86.4%) | 45 (54.9%) | 26 (25.5%) | <0.001 |
| Proximal to D1 | 53 (80.3%) | 49 (59.8%) | 20 (19.6%) | <0.001 |
CPR, cardiopulmonary resuscitation; D1, first major diagonal artery; GRACE, global registry of acute coronary events study; LVEF, left ventricular ejection fraction; S1, first major septal artery; WMSI, wall motion score index.
Values are presented as mean (SD) or number (percentage) as appropriate.
4. DISCUSSION
The prediction of infarct size and localization is more than an academic exercise, as it can influence the selection of acute reperfusion strategy, help in risk stratification, lead to better referral decisions, promote a vigilant search for associated complications. Although ECG can help clinicians in differentiating a proximal LAD occlusion that will ultimately result in extensive anterior MI from a more limited an anteroapical MI caused by distal LAD occlusion, the failure to identify the former is common and results in lower quality care and worse outcomes (Engelen et al., 1999; Karha et al., 2003). In the earlier days of clinical electrocardiography, the presence of abnormal Q‐waves in specific lead groups was related to several anatomic locations on the basis of correlations with the postmortem anatomic gold standard (Table 1) (Myers, Klein, & Hiratzka, 1949; Roberts & Gardin, 1978). Despite later studies did not reproduce these results (Savage, Wagner, Ideker, Podolsky, & Hackel, 1977; Sullivan, Vlodaver, Tuna, Long, & Edwards, 1978) and a new terminology based on cardiac magnetic resonance imaging has been proposed (Bayés de Luna et al., 2006), the older terminology not only persisted but also replicated itself into ST‐segment terminology after the Q/non‐Q to ST‐/non‐ST‐MI paradigm shift. Unfortunately, many educational sources and textbooks still reiterate this older scheme and may contribute to this inappropriate risk stratification (Mirvis & Goldberger, 2015; Prutkin, 2018; Thomas & Brady, 2018).
To our knowledge, our study, for the first time, aimed to show that the established scheme for anterior MI classification (Table 1) misguides clinicians in predicting infarct location and extent and their prognostic implications. This classification scheme systematically classifies high‐risk patients to a lower risk stratum, while patients with limited infarctions consistently classified in high‐risk stratum. Actually, the scheme seems to distribute patients across groups so unselectively that all information about infarct size and prognostication becomes blurred. Furthermore, our data also show no correlation between the site of LAD occlusion and the location of MI as ECG class implies. For example, our results indicated that the majority of the patients labeled as “septal‐anteroseptal” group had in fact “extensive anterior MI,” whereas the majority of the patients labeled as “extensive anterior MI” did not have proximal LAD occlusion. Patients labeled as having “septal” infarctions actually had diagonal branch occlusion. Lastly, the established scheme did not provide any information on infarct size in terms of peak troponin or ejection fraction, despite angiographic stratification showed such a trend.
On the other hand, the rising trend of electrocardiographic prediction of angiographic occlusion site is fairly well evidence‐based (Arbane & Goy, 2000; Bayés de Luna, 2012; Engelen et al., 1999; Fiol et al., 2009, 2004; Sasaki, Yotsukura, Sakata, Yoshino, & Ishikawa, 2001; Taglieri et al., 2014). In these studies, the direction and the magnitude of the ST vector seemed to convey information about involved myocardial segments rather than chest leads directly overlying involved myocardial tissue. For example, a distal LAD occlusion or an occlusion proximal to D1 generally causes ST‐vector to be directed anterolaterally, which results in ST‐segment elevation in leads V1 through V6. In this situation, ECG is labeled as “extensive anterior” MI by the older scheme, but instead, the occluded coronary network deprives apical or anterolateral region of blood supply. On the contrary, proximal LAD occlusion frequently displays ST‐segment elevation in leads V1 to V4 and ST‐segment depression in V5 and V6, due to a ST vector directed to dominant basal segments, which is incorrectly classified as “anteroseptal” MI in the older scheme (Allencherril et al., 2018a, 2018b; Bandeali et al., 2012; Bayés de Luna, 2012; Huang, Tran, Jneid, Wilson, & Birnbaum, 2011). However, a complex interaction with reciprocal changes and vessel anatomy also influences the leads showing ST‐segment elevation in addition to the site of occlusion. For example, while ST‐segment elevation in I and aVL and ST depression in the inferior leads are specific signs of LAD occlusion before the first diagonal branch, their sensitivity is low, as proximal occlusion of a long LAD often does not cause ST‐segment deviation in the limb leads. Therefore, the deadliest cases of proximal occlusion of a wrapping LAD could be misinterpreted as small infarcts caused by distal occlusion of a short LAD (Sasaki et al., 2001).
Our results show that expert interpretation of the ECG using the published criteria can accurately predict the site of LAD occlusion in a sizable portion of the patients. In the current study, in accordance with the previous studies (Arbane & Goy, 2000; Bayés de Luna, 2012; Engelen et al., 1999; Fiol et al., 2009, 2004; Taglieri et al., 2014) angiographic and electrocardiographic site of occlusion were reasonably correlated, although there is still room for improvement. For example, expert interpretation correctly classified high‐risk patients in terms of adverse outcomes, but infarct size as assessed by peak troponin, ejection fraction, and wall motion scores did not show a significant difference across groups. One can notice that the graded decrease in infarct extent according to actual angiographic LAD occlusion site presented in Table 2 could not be reproduced in Table 4 by the predicted occlusion site according to expert interpretation. This may be due to the fact that the accuracy of expert prediction was moderate at best and the criteria used in this study still incorrectly classified approximately one‐fourth of the patients. Nevertheless, results presented here show that expert interpretation of the ECG is better than the established scheme and, most importantly, it can predict adverse outcomes and provides a better risk stratification. This is a very critical and clinically relevant result that points the critical need for a change in nearly a century‐old framework.
Our study has several limitations. This is a retrospective chart review study, with all known limitations associated with bias. Because of retrospective nature of the study, standard lead placement could not be confirmed. Expert interpretation of ECG is hard to standardize and use of different criteria may result in different conclusions. Echocardiographic wall motion scores may have influenced by the ECG diagnosis as the echocardiographers were not blinded. Cohort size may be small for subclassifications. Another imaging test with higher diagnostic accuracy such as cardiac magnetic resonance might be of greater value for the assessment of infarct localization. Due to variations in coronary anatomy, the definitions of the site of coronary occlusion may differ among interventionalists. Angiographic involvement site may not always be associated with a standard infarction location. Angiographic variables, such as the length of the LAD and the concomitant size of size branches, can also influence the amplitude and location of ST‐segment elevation; but these variables were not specifically taken into account in our study.
In conclusion, classifying patients according to the established scheme, which is predominantly based on chest leads, neither gives prognostic information nor accurately localizes infarction. It should be regarded as obsolete and its use should be abandoned. Rather, extent of infarction should be classified according to newer angiographic occlusion prediction site studies. However, further studies with imaging correlations are needed to develop more accurate algorithms.
CONFLICT OF INTEREST
No authors have any conflicts of interest to report.
Bozbeyoğlu E, Aslanger E, Yıldırımtürk Ö, et al. The established electrocardiographic classification of anterior wall myocardial infarction misguides clinicians in terms of infarct location, extent and prognosis. Ann Noninvasive Electrocardiol. 2019;24:e12628 10.1111/anec.12628
REFERENCES
- Allencherril, J. , Fakhri, Y. , Engblom, H. , Heiberg, E. , Carlsson, M. , Dubois‐Rande, J. L. , … Birnbaum, Y. (2018a). The significance of ST‐elevation in aVL in anterolateral myocardial infarction: An assessment by cardiac magnetic resonance imaging. Annals of Noninvasive Electrocardiology, 23, e12580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allencherril, J. , Fakhri, Y. , Engblom, H. , Heiberg, E. , Carlsson, M. , Dubois‐Rande, J. L. , … Birnbaum, Y. (2018b). Correlation of anteroseptal ST elevation with myocardial infarction territories through cardiovascular magnetic resonance imaging. Journal of Electrocardiology, 51, 563–568. [DOI] [PubMed] [Google Scholar]
- Arbane, M. , & Goy, J. J. (2000). Prediction of the site of total occlusion in the left anterior descending coronary artery using admission electrocardiogram in anterior wall acute myocardial infarction. American Journal of Cardiology, 85, 487–491. 10.1016/S0002-9149(99)00777-8 [DOI] [PubMed] [Google Scholar]
- Bandeali, S. J. , Stone, S. , Huang, H. D. , Kayani, W. T. , Wilson, J. M. , & Birnbaum, Y. (2012). Comparison of segmental wall motion abnormalities on echocardiography in patients with anteroseptal versus extensive anterior wall ST‐segment elevation myocardial infarction. Journal of Electrocardiology, 45, 551–555. 10.1016/j.jelectrocard.2012.07.001 [DOI] [PubMed] [Google Scholar]
- Bayés de Luna, A. (Ed.) (2012). Ischemia and necrosis In Clinical electrocardiography (4th ed., pp. 216–276). Chichester, UK: Wiley‐Blackwell Publishing. [Google Scholar]
- Bayés de Luna, A. , Wagner, G. , Birnbaum, Y. , Nikus, K. , Fiol, M. , Gorgels, A. , … Zereba, W. (2006). A new terminology for left ventricular walls and location of myocardial infarcts that present Q wave based on the standard of cardiac magnetic resonance imaging: A statement for healthcare professionals from a committee appointed by the International Society for Holter and Noninvasive Electrocardiography. Circulation, 114, 1755–1760. 10.1161/CIRCULATIONAHA.106.624924 [DOI] [PubMed] [Google Scholar]
- Durant, E. , & Singh, A. (2015). Acute first diagonal artery occlusion: A characteristic pattern of ST elevation in noncontiguous leads. American Journal of Emergency Medicine, 33, 1326.e3–1326.e5. 10.1016/j.ajem.2015.02.008 [DOI] [PubMed] [Google Scholar]
- Engelen, D. J. , Gorgels, A. P. , Cheriex, E. C. , De Muinck, E. D. , Ophuis, A. J. , Dassen, W. R. , … Wellens, H. J. (1999). Value of the electrocardiogram in localizing the occlusion site in the left anterior descending coronary artery in acute anterior myocardial infarction. Journal of the American College of Cardiology, 34, 389–395. 10.1016/S0735-1097(99)00197-7 [DOI] [PubMed] [Google Scholar]
- Fiol, M. , Carrillo, A. , Cygankiewicz, I. , Velasco, J. , Riera, M. , Bayés‐Genis, A. , … Bayés de Luna, A. (2009). A new electrocardiographic algorithm to locate the occlusion in left anterior descending coronary artery. Clinical Cardiology, 32, E1–6. 10.1002/clc.20347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiol, M. , Cygankiewicz, I. , Guindo, J. , Flotats, A. , Genis, A. B. , Carreras, F. , … Bayés de Luna, A. (2004). Evolving myocardial infarction with ST elevation: Ups and downs of ST in different leads identifies the culprit artery and location of the occlusion. Annals of Noninvasive Electrocardiology, 9, 180–186. 10.1111/j.1542-474X.2004.92538.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox, K. A. , Dabbous, O. H. , Goldberg, R. J. , Pieper, K. S. , Eagle, K. A. , Van de Werf, F. , … Granger, C. B. (2006). Prediction of risk of death and myocardial infarction in the six months after presentation with acute coronary syndrome: Prospective multinational observational study (GRACE). BMJ, 333, 1091 10.1136/bmj.38985.646481.55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang, H. D. , Tran, V. , Jneid, H. , Wilson, J. M. , & Birnbaum, Y. (2011). Comparison of angiographic findings in patients with acute anteroseptal versus anterior wall ST‐elevation myocardial infarction. American Journal of Cardiology, 107, 827–832. 10.1016/j.amjcard.2010.10.070 [DOI] [PubMed] [Google Scholar]
- Karha, J. , Murphy, S. A. , Kirtane, A. J. , de Lemos, J. A. , Aroesty, J. M. , Cannon, C. P. , … Gibson, C. M. (2003). Evaluation of the association of proximal coronary culprit artery lesion location with clinical outcomes in acute myocardial infarction. American Journal of Cardiology, 92, 913–918. 10.1016/S0002-9149(03)00969-X [DOI] [PubMed] [Google Scholar]
- Masoudi, F. A. , Magid, D. J. , Vinson, D. R. , Tricomi, A. J. , Lyons, E. E. , Crounse, L. , … Rumsfeld, J. S. (2006). Implications of the failure to identify high‐risk electrocardiogram findings for the quality of care of patients with acute myocardial infarction: Results of the Emergency Department Quality in Myocardial Infarction (EDQMI) study. Circulation, 114, 1565–1571. 10.1161/CIRCULATIONAHA.106.623652 [DOI] [PubMed] [Google Scholar]
- Mirvis, D. M. , & Goldberger, A. L. (2015). Electrocardiography In Mann D. L., Zipes D. P., Libby P., Bonow R. O., & Braunwald E. (Eds.), Braunwald’s heart disease: a textbook of cardiovascular medicine (10th ed., pp. 114–154). Philadelphia, PA: Elsevier. [Google Scholar]
- Myers, G. B. , Klein, H. A. , & Hiratzka, T. (1949). Correlation of electrocardiographic and pathologic findings in posterolateral infarction. American Heart Journal, 38, 837–862. [DOI] [PubMed] [Google Scholar]
- Prutkin, J. M. (2018)ECG tutorial: Myocardial ischemia and infarction In Goldberger A. L., & Saperia G. M. (Eds.), UpToDate. Waltham, MA: UpToDate Inc; Retrieved from https://www.uptodate.com/contents/ecg-tutorial-myocardial-ischemia-andinfarction?search=ecg%20tutorial%20myocardial%20infdarction&source=search_result&selectedTitle=1~150&usage_type=default&display_rank=1 [Google Scholar]
- Rentrop, K. P. , Cohen, M. , Blanke, H. , & Phillips, R. A. (1985). Change in collateral channel filling immediately after controlled coronary artery occlusion by an angioplasty balloon in human subjects. Journal of the American College of Cardiology, 5, 587–592. [DOI] [PubMed] [Google Scholar]
- Roberts, W. C. , & Gardin, J. M. (1978). Location of myocardial infarcts: A confusion of terms and definitions. American Journal of Cardiology, 42, 868–872. 10.1016/0002-9149(78)90110-8 [DOI] [PubMed] [Google Scholar]
- Sasaki, K. , Yotsukura, M. , Sakata, K. , Yoshino, H. , & Ishikawa, K. (2001). Relation of ST‐segment changes in inferior leads during anterior wall acute myocardial infarction to length and occlusion site of the left anterior descending coronary artery. American Journal of Cardiology, 87, 1340–1345. 10.1016/S0002-9149(01)01549-1 [DOI] [PubMed] [Google Scholar]
- Savage, R. M. , Wagner, G. S. , Ideker, R. E. , Podolsky, S. A. , & Hackel, D. B. (1977). Correlation of postmortem anatomic findings with electrocardiographic changes in patients with myocardial infarction. Circulation, 55, 279–285. [DOI] [PubMed] [Google Scholar]
- Sclarovsky, S. , Birnbaum, Y. , Solodky, A. , Zafrir, N. , Wurzel, M. , & Rechavia, E. (1994). Isolated mid‐anterior myocardial infarction: A special electrocardiographic sub‐type of acute myocardial infarction consisting of ST‐elevation in non‐consecutive leads and two different morphologic types of ST‐depression. International Journal of Cardiology, 46, 37–47. 10.1016/0167-5273(94)90115-5 [DOI] [PubMed] [Google Scholar]
- Sullivan, W. , Vlodaver, Z. , Tuna, N. , Long, L. , & Edwards, J. E. (1978). Correlation of electrocardiographic and pathologic findings in healed myocardial infarction. American Journal of Cardiology, 42, 724–732. 10.1016/0002-9149(78)90090-5 [DOI] [PubMed] [Google Scholar]
- Taglieri, N. , Saia, F. , Alessi, L. , Cinti, L. , Reggiani, M. L. , Lorenzini, M. , … Rapezzi, C. (2014). Diagnostic performance of standard electrocardiogram for prediction of infarct related artery and site of coronary occlusion in unselected STEMI patients undergoing primary percutaneous coronary intervention. European Heart Journal: Acute Cardiovascular Care, 3, 326–339. 10.1177/2048872614530665 [DOI] [PubMed] [Google Scholar]
- Thomas, J. J. , & Brady, W. J. (2018). Acute coronary syndromes In Walls R. M., Hockberger R. S., & Gausche‐Hill M. (Eds.), Rosen’s emergency medicine: concepts and clinical practice (9th ed., pp. 891–928). Philadelphia, PA: Elsevier. [Google Scholar]


