Abstract
Background
Cardiac restitution is the ability of the heart to recover from one beat to the next. Ventricular arrhythmia vulnerability can occur when the heart does not properly adjust to sudden changes in rate or in hemodynamics leading to excessive temporal and/or spatial heterogeneity in conduction or repolarization. Restitution has historically been used to study, by invasive means, the dynamics of the relationship between action potential duration (APD) and diastolic interval (DI) in sedated subjects using various pacing protocols. Even though the analogous measures of APD and DI can be obtained using the surface ECG to acquire the respective QT and TQ intervals for ECG restitution, this methodology has not been widely adopted for a number of reasons.
Methods
Recent development of more advanced software algorithms enables ECG intervals to be measured accurately, on a continuous beat‐to‐beat basis, in an automated manner, and under highly dynamic conditions (i.e., ambulatory or exercise) providing information beyond that available in the typical resting state.
Results
Current breakthroughs in ECG technology will allow ECG restitution measures to become a practical approach for providing quantitative measures of the risks for ventricular arrhythmias as well as cardiac stress in general.
Conclusions
In addition to a review of the underlying principles and caveats of ECG restitution, a new approach toward an advancement of more integrated restitution biomarkers is proposed.
Keywords: arrhythmia, beat‐to‐beat, cardiac stress, QT prolongation, restitution, ECG biomarker
1. Background
Assessment of the risk for arrhythmia is a challenge that physicians, regulators, and developers of new medications all must face. To date, the primary biomarker in use for evaluation of cardiac safety in pharmaceutical trials is the corrected QT interval (QTc; International Conference on Harmonisation, ICH E14, 2005), even though it is well known that this measure is problematic including being associated with instances of false negatives and false positives (Garnett et al., 2012; Sager et al., 2014). The traditional methodology for use of ECG data in clinical studies is collection of tracings at designated time points when the subject is resting in a supine position. This is intended to stabilize the heart rate, hemodynamics, and autonomic state. To reduce the natural beat‐to‐beat variability even further, some methods reject heart beats outside of narrow limits and/or correct for QT hysteresis (Malik, Hnatkova, Kowalski, Keirns, & van Gelderen, 2012) based on changes in heart rate over time. It is precisely under these stable conditions that there is the least chance of detecting abnormal findings for any arrhythmia biomarker, particularly QTc, thus minimizing the potential utility for quantitative assessment of cardiac safety.
1.1. Physiology
Cardiac restitution is the ability of the heart to recover from one beat to the next. At its simplest, it is the relationship of how long the heart is in contraction compared to how much rest it has between beats to permit recovery. This, of course, varies at different heart rates. To help visualize restitution, if a normal healthy person were asked to do one push‐up every minute and rest between them, they could sustain that activity for a substantial period of time. However, if the same person were asked to do one push‐up every other second with only a second rest between efforts, they would soon tire. Further, you can imagine adding weights onto the back of that person during the push‐ups to make the task even more difficult. This would be analogous to the cardiovascular restitution effort of the heart contracting against the afterload of hypertension. Restitution can measure the integrated mechanoelectrical impacts on the heart and, using a multi‐lead system, can also quantify spatial and temporal differences. In simple terms, the harder the heart has to work in less time, the greater the stress and chance of failure (i.e., tachyarrhythmia). Most importantly, this is least likely to be evident under conditions of stable heart rate and rest.
Restitution has been studied for several decades by both direct and indirect measures in vivo (Riccio, Koller, & Gilmour, 1999; Taggart et al., 2003), on the isolated heart tissue (Bass, 1975; Boyett & Jewell, 1980; Pastore, Laurita, & Rosenbaum, 2006), and through computational models (Cherry & Fenton, 2004; Clayton & Taggart, 2005; Zeng, Laurita, Rosenbaum, & Rudy, 1995). The most common method of analysis examines the duration of action potential (APD) versus the previous diastolic interval (DI). The APD, in effect the duration of the cardiac working phase, is dependent on the previous resting phase between each cardiac cycle, during which recovery is a function of the cardiac ion channels and ion exchange pump kinetics (Chudin, Goldhaber, Garfinkel, Weiss, & Kogan, 1999; Delmar, Ibarra, Davidenko, Lorente, & Jalife, 1991). Nolasco and Dahlen (1968) first showed in frog ventricular muscle that when the slope of the APD/DI restitution curve exceeded a value of 1, periodic beating indicative of cardiac stability transforms into a pattern called “alternans” in which the action potential varies from beat‐to‐beat during steady pacing. A vast literature base of theoretical papers have shown that this value of 1 is critical for transition from stability to instability as illustrated by Koller, Riccio, and Gilmour (1998) in Figure 1. Action potential duration (APD) undergoes expanding alternations at faster heart rates as the slope becomes >1. This is the basis for the clinically useful T‐wave alternans test (Verrier & Nearing, 1994) at a standard controlled exercise heart rate of 110 bpm. As the oscillations in repolarization increase with the APD/DI ratio >1, complex forms of conduction block become apparent, leading to ventricular arrhythmia (Karma & Gilmour, 2007).
Figure 1.
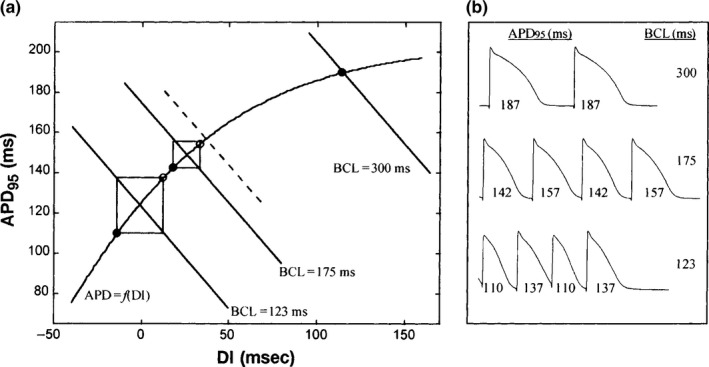
(a) Iteration of the dynamic restitution function APD = f(DI) from isolated canine Purkinje fibers reported by Koller et al. (1998). The slope of the restitution function is <1 to the right of the dashed line and >1 to the left of the dashed line. Solid −45° lines correspond to BCL 300, 175, and 123 ms, respectively, where BCL = APD + DI. (b) Action potential recording corresponding to data shown in A for BCL = 300, 175, and 123 ms
1.2. Early ECG restitution studies
In 1947, Taran and Szilagyi (1947) studied the ratio of the QT interval (analogous to APD) in relationship to the preceding TQ interval (resting phase of the heart or analogous to DI) in children with rheumatic carditis. They found that this ratio averaged 1.46 at rest and markedly increased in parallel with the severity of the disease. Another study in patients with carditis of nonrheumatic etiology by Gittleman, Thorner, and Griffith (1951) showed that the QT/TQ ratio increased above 1.0 in 74.5% of their own cases and 78.6% in cases observed in the literature at the time.
The effect of exercise on the QT/TQ ratio was studied by Yu, Bruce, Lovejoy, and Pearson in 1950 in normal healthy subjects and patients with cardiopulmonary disease. Normal subjects at rest had a mean ratio of 0.94 ± 0.13 that increased to 1.33 ± 0.2 during walking on a treadmill at 1.7–2.6 mph for up to 10 min. Recovery to baseline occurred within minutes. Conversely, patients with hypertensive cardiovascular disease (HCVD), congenital heart disease (CHD), or pulmonary disease (PD) had baseline values of 1.16 ± 0.2, 1.44 ± 0.29, and 1.08 ± 0.15, respectively. During exercise, the values increase to 1.67 ± 0.24 in HCVD, 1.72 ± 0.42 in CHD, and 1.34 ± 0.26 in PD. Recovery was substantially delayed. In this same study, in the seven patients in whom precordial pain was noted during exercise, the QT/TQ ratios ranged from 1.56 to 2.31 with an average of 1.94.
As continuous digital ECG recording and automated software for analysis of beat‐to‐beat data progressed at the turn of this century, particularly in the preclinical domain, investigators began using more sophisticated measures in conscious ambulatory animals (Fossa, Depasquale, Raunig, Avery, & Leishman, 2002). A new method of analysis for beat‐to‐beat assessment of the dynamic QT interval was introduced (Fossa et al., 2005). This method eliminated the erroneous QT correction factors and utilized the continuous recordings to compare QT intervals obtained during all normal physiological states (Garnett et al., 2012). All or any heart beat during a treatment period of interest could be compared to all beats with similar RR intervals extracted from a 24‐hr baseline recording. This methodology was soon adapted to assess ECG restitution of beat‐to‐beat QT and TQ intervals from the same continuous ECG recording in animal and human studies (Fossa, Wisialowski, & Crimin, 2006; Fossa & Zhou, 2010; Fossa et al., 2007).
2. Current Usage
2.1. Temporal heterogeneity analysis
In 2008, beat‐to‐beat ECG restitution was applied for clinical use in several pharmaceutical development studies (Fossa & Zhou, 2010; Garnett et al., 2012). One of those studies reported was a Thorough QT (TQT) study with the alpha‐2A adrenergic receptor agonist guanfacine (Intuniv; Fossa, Zhou, Robinson, Purkayastha, & Martin, 2014) which has been used as an antihypertensive based on its centrally mediated action to reduce heart rate and blood pressure. This alteration of the sympathetic‐vagal balance was felt to lead to false negatives QTc determinations at low heart rates and false positives at higher heart rates or during hysteresis (i.e., reflex tachycardia) because of effects on the dynamicity of the QT‐RR and QT‐TQ interval relationship (Fossa & Zhou, 2012; Fossa et al., 2011; Garnett et al., 2012). The FDA requested a retrospective analysis of the study data using the beat‐to‐beat methods because of “variation in autonomic tone in response to drug‐induced heart rate and possibly blood pressure changes” (FOI, http://www.accessdata.fda.gov/drugsatfda_docs/nda/2009/022037_intuniv_toc.cfm) .
Dynamic beat‐to‐beat ECG restitution findings showed consistently significant improvements in parameters of cardiac stabilization at all time points after both therapeutic and supratherapeutic dosing of guanfacine. Those endpoints were a reduction in median QT/TQ ratio (QTTQ50); a reduction in the upper 98th percentile of QT/TQ ratios (QTTQ98) that represent the most extremely stressed beats; a reduction in the total percentage of beats with a QT/TQ ratio >1, indicating less overall time spend on the steep phase of the restitution curve; increases in median TQ interval and values in the lowest 5 percentile of TQ intervals, indicative of more rest time per beat. At peak guanfacine levels, improvements of between 20% and 30% were dose‐related despite mean placebo‐adjusted QTc prolongation up to 20.7 ms. The positive control, moxifloxacin, showed significant impairment of these parameters indicative of increased cardiac instability. Intuniv® was approved with no adverse QTc labeling. Five years after the study and NDA approval, a comprehensive review of the published data and adverse events (AEs) reported to the Freedom of Information FDA‐AERS database provided no evidence of, or individual case reports pointing to an association of guanfacine with Torsades de Pointes (TdP). A postmarketing review of the clinical data from the guanfacine‐Intuniv program indicated no evidence of pro‐arrhythmic effects.
2.2. Spatial heterogeneity analysis
Within the last few years, ECG restitution indexes were introduced by Nicolson et al. (2012, 2014) for risk stratification in patients with ischemic cardiomyopathy. Two endpoints Regional Restitution Instability Index (R2I2) and Peak ECG Restitution Slope (PERS) were used for prospective prediction of sudden cardiac death (SCD) or ventricular arrhythmia (VA‐ventricular tachycardia or fibrillation >30 s or terminated by implantable cardioverter defibrillator [ICD] shock). Prior to receiving an ICD, patients (n = 62) had ejection fractions <35% and evidence of nonsustained ventricular tachycardia on a 24‐hr Holter. To generate restitution curves, ICDs were programmed to perform electrical stimulation in a set protocol with surface leads recording the resulting ECG. The R2I2 was derived from the QTpeak (onset of QRS to peak of T wave) and TPq (previous beat T‐wave peak to onset of QRS) at each surface lead. The difference between the mean gradient and the restitution curve gradient from each 40‐ms stimulation segment was calculated. R2I2 is defined as the standard deviation (SD) of these values from each lead as a measure of spatial heterogeneity. The PERS was derived from an average of all leads and is primarily a temporal measure of restitution. The relative risk of SCD or VA in patients with high R2I2 values was 6.5 times than that of low‐risk R2I2 patients. In patients experiencing SCD or VA, the relative risk for patients with PERS values ≥1.21 was 4.9 times that of patients with PERS values <1.21. In patients with both positive R2I2 values ≥1.03 and PERS values ≥1.21, the relative risk of VA/SCD was 21.6 times that of patients who were negative for both.
Another ECG restitution measure of spatial heterogeneity examines the relationship between Tpeak to Tend (Tpe) and the RR intervals at different steady‐state conditions (Ramirez, Minchole, Bolea, Laguna, & Pueyo, 2013). Using a single principal component lead to emphasize the T wave, Delta‐Restitution (DRest) was used to discriminate between groups of patients with and without SCD. Dispersion of heterogeneity, signified by increases in DRest, was proposed to stratify heart failure patients with ventricular arrhythmia that could lead to SCD. This same DRest measure was used to compare findings to QTc in an analysis of healthy patients who had received oral sotalol and three subjects who had TdP after intravenous sotalol challenge (Minchole, Bueno‐Orovio, Laguna, Pueyo, & Rodriguez, 2015). Patients with TdP showed increased DRest and provided the best discrimination from healthy subjects, including the use of QTcF.
2.3. Other uses
Analysis of restitution using ECG beat‐to‐beat QT‐TQ interval interaction has been used as a surrogate for the systolic–diastolic interaction in diabetic cardiac autonomic neuropathy (CAN) progression (Imam, Karmaka, Jelinek, Palaniswami, & Khandoker, 2015). This group of patients has previously been reported to have a 53% 5‐year mortality rate due to cardiovascular complications (Ewing, Martyn, Young, & Clarke, 1985). They found that the QT/TQ ratio gradually increases with severity of CAN progression along with a decrease in variability. However, another measure that normalizes the TQ interval as a measure of diastolic dysfunction was proposed as the TQ/RR interval. Since the progression of the disease appears to be more related to the mechanical inability of the ventricles to relax, this measure was more sensitive for determining the progression of CAN. Both measures performed better than time‐domain heart rate variability analysis.
Most recently, restitution has been used in a rural setting as a readily available way of assessing arrhythmia liability in epileptic patients (Al‐Nimer, Al‐Mahdawi, & Abdullah, 2017). In a study comparing 71 healthy subjects versus 64 newly diagnosed epileptic patients, QT nomograms did not disclose significant findings while restitution steepness showed that epileptic patients were vulnerable. Abnormal ECG findings were observed in epileptics of <18 and >50 years of age compared with healthy subjects.
3. Restitution Obstacles: Translation of Preclinical APD Restitution to Human ECG Restitution
3.1. Restitution slope
The concept of using restitution for prediction of human arrhythmia liability has been fraught with controversy (Franz, 2003). The vast majority of APD versus DI data indicates that a steep curve in this relationship is associated with the onset of ventricular arrhythmia (Garfinkel et al., 2000; Kurz, Ren, & Franz, 1994) but there are experiments, particularly during ischemia, that show otherwise (Cherry & Fenton, 2004; Kurz et al., 1994; Taggart et al., 1986). There are several reasons for the disparity, but these have been widely ignored or at least underappreciated leading to further confounding data. This confusion surrounding restitution seems partially responsible for the slow adoption of methodologies related to its use.
In early studies by Bass (1975) and more precisely by Franz, Schaefer, Schottler, Seed, and Noble (1983), the restitution relationship was shown to be triphasic, not monotonic or single exponential. When the human restitution APD/DI relationship is measured in short increments <20 ms, the following are observed: a steep initial phase at very fast heart rates, a temporary downward turn at around a DI of approximately 100 ms creating a “hump,” then a final asymptotic phase that approaches a flat plateau at a DI of 500–600 ms (Figure 2) are observed. The triphasic nature of the curve shifts leftward during faster steady‐state heart rates but is dependent on the previous DI or immediate beat history (Franz, Swerdlow, Liem, & Schaefer, 1988). This is as expected in an in vivo heart that is never in a hemodynamic steady‐state with preload and afterload pressures differing beat‐by‐beat. Consequently, restitution of contractility is also affected (Burkhoff, Yue, Franz, Hunter, & Sagawa, 1984) all of which ultimately affect the multitude of ion channels that have different rate dependency kinetics.
Figure 2.
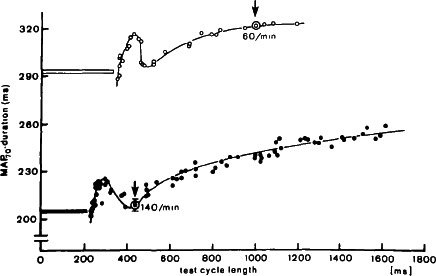
Electrical restitution curves at two different pacing rates. The circled data points (marked by arrows) show steady‐state responses (mean ± 1 SD) at indicated rate. The horizontal bar before each curve indicates the duration of membrane depolarization above 70% as part of the test cycle length. From Franz et al. (1983)
3.2. Experimental protocols
Another major factor contributing to the disparity in restitution results is protocol dependency. Almost every protocol which employs pacing will produce a different result because of, to name a few: cycle length used, DI with subsequent stimulus jump after steady‐state pacing (S1S2), the number and frequency of pulses, the type of tissue used, cardiac memory, and physiological environmental conditions such as anesthetics. Therefore, unless a standardized methodology is adopted, it is difficult to compare, at least quantitatively, any results between protocols.
3.3. Translational practicalities
Most would agree that as experimental models proceed from cellular to isolated tissue, to whole organ, to in vivo with species differences, the level of complexity increases. Restitution can be assessed by many models but the intact human heart is infinitely more complex with differences in spatial and temporal heterogeneities, varying autonomic, hemodynamic and neurohormonal influences, structural/pathophysiological anomalies, and muscle artifact from chest leads. Many believe that the human ECG recording is not suitable for translation of restitution principles. However, examining human ECG restitution data is surprisingly similar to preclinical findings despite all the mentioned complexities.
In 1999, Gilmour et al. (1997) showed in patients with acquired QT prolongation, as was found for APD restitution, the QT interval shows rate and time dependence attributable to the DI or previous TQ and QT‐TQ history, not the immediately preceding RR interval. It was also observed, over the time course of minutes, that an increase in the QT/TQ ratio, analogous to restitution slope, precipitated arrhythmias while a decrease in the QT/TQ ratio during of 5–16 s durational episode of TdP was sufficient to terminate the arrhythmia (Figure 3) despite the QT interval not reaching a steady state. This would seem to emphasize the importance of measuring the TQ interval.
Figure 3.
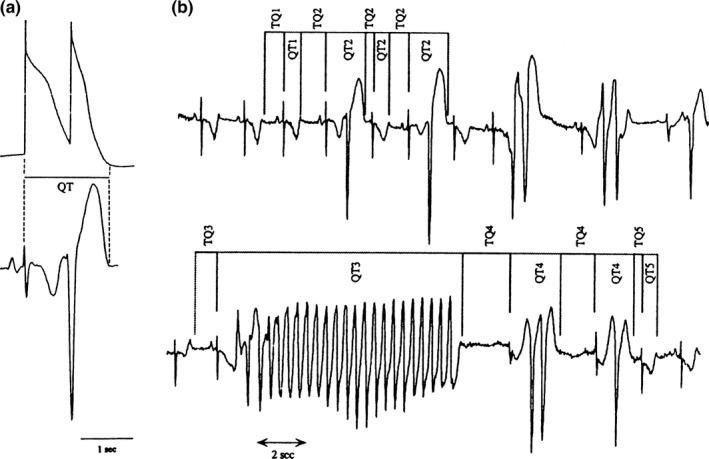
Relation between QT and TQ intervals during episodes of ventricular arrhythmias in patients with acquired prolongation of ventricular repolarization. (a) Hypothetic correspondence between a cellular action potential and early after depolarization‐induced triggered response (upper record) and the QT interval of the scalar ECG (lower record). (b) Example of QT and TQ intervals measured during an episode of ventricular arrhythmias: 1 = intervals during sinus rhythm preceding initiation of arrhythmias; 2 = intervals associated with first three complexes during episodes of ventricular arrhythmias; 3 = intervals associated with Torsade de pointes; 4 = intervals associated with complexes that followed Torsade de pointes before the end of the episode; and 5 = intervals associated with sinus complexes immediately after termination of the episode. From Gilmour et al. (1997)
A study looking at ECG restitution prior to TdP induced by sotalol was reported (Fossa et al., 2007). Healthy volunteers given oral doses of sotalol as high as 320 mg had QTTQ50 ratios well below 1 when all beats over a 24‐hr period were examined. Only 25% of the beats over 24‐hr had a QT/TQ ratio >1 with an upper 98% bound of 1.52. A similar analysis was conducted from a coronary artery disease patient who at baseline had 98% of beats with a QT/TQ ratio >1 and a QTTQ98 of 1.9. After sotalol challenge, within 2 hr of the TdP episode, the upper bounds of the QTTQ98 ratio was 2.3 with several R on T beats having QT/TQ ratios >5 immediately prior to arrhythmia.
The ECG restitution curve was described in 2006 (Manriquez, Zhang, Medigue, Papelier, & Sorine, 2006). Using handgrip isometric exercise to generate variation in the QT interval versus the preceding TQ interval, the restitution curves were derived over a wide range of TQ intervals (250–750 ms). In parallel, the data were entered into a computational model for electrical activity of cardiac membranes. The action potential model was simulated using a train of impulses with frequency equal to the heart rate frequency. Despite the macroscopic differences, the curves for APD and ECG restitution were very similar.
The translation of beat‐to‐beat data from dog to monkey to man is excellent. Table 1 shows a summary of baseline values for the QTTQ50 ratio compared with the previously described clinical data. Baseline values from FDA/regulatory submitted toxicology and clinical studies have also been included but not previously reported. It is clear from the author's experience that despite differences in autonomic balance, health status, physiology of the QT interval, environmental conditions for measurement, etc., causing the absolute values to vary between species, the QTTQ50 ratio is rather constant with rested values being below 1 and incremental increases in stress producing values above 1 to a maximum of generally around 2. Also shown in Table 1 is the QTTQ98 ratio or top 2% of beats that reflects the most extreme values throughout the collection period. The upper bounds of these beats are also consistent between species.
Table 1.
Comparison of baseline ECG restitution findings for QT/TQ interval ratios in healthy humans, dogs and monkeys and humans during autonomic challenge or disease states
| Subject | Measurement period | Average study values | References | |
|---|---|---|---|---|
| QT/TQ 50 | QT/TQ 98 | |||
| Healthy humans | 10 s at supine resting baseline | 0.73–0.76 | 1.14 | Fossa et al. (2014), Gross, (1952) |
| 2‐hr supine resting baseline | 0.63 | 1.11 | Fossa and Zhou (2010) | |
| 2 hr at sleep | 0.65–0.70 | 1.11–1.23 | Fossa et al. (2007), Fossa and Zhou (2010) | |
| 24‐hr baselines | 0.70–0.85 | 1.09–1.52 | Fossa and Zhou (2010), Fossa et al. (2007, 2014)a | |
| Baseline:10‐min exercise (walking) | 0.94:1.33 | NA | Yu et al. (1950) | |
| Bike exercise (maximum burst) | 2.13 | 3.25 | Fossa and Zhou (2010) | |
| Supine to standing | 1.09 | 1.80 | Fossa and Zhou (2010) | |
| Phenylephrine bolus challenge during supine rest | 0.63 | 0.92 | Fossa and Zhou (2010) | |
| Isoproterenol bolus during rest | 1.79 | 2.63 | Fossa and Zhou (2010) | |
| Rheumatic carditis | 10‐s baseline | 1.46 | NA | Taran and Szilagyi (1947) |
| Hypertension | Baseline:10‐min walk | 1.16:1.67 | NA | Taran and Szilagyi (1947) |
| Congenital heart disease | Baseline:10‐min walk | 1.44:1.72 | NA | Taran and Szilagyi (1947) |
| Pulmonary disease | Baseline:10‐min walk | 1.08:1.34 | NA | Taran and Szilagyi (1947) |
| Monkey | 24‐hr ambulatory baseline | 1.01 | 1.85 | b |
| Canine | 24‐hr ambulatory baseline | 0.41–0.69 | 1.20–1.60 | b |
Regulatory TQT studies submitted to FDA.
Regulatory toxicology studies submitted to FDA.
3.4. Practical implementation
Only recently have software systems capable of analyzing high‐resolution beat‐to‐beat ECG intervals and other morphological changes in the waveforms been available. Such software needs to be fully automated with minimal need to manually edit data particularly in periods of greater heart rate changes or in‐band noise (i.e., somatic muscle interference). Some software systems are now capable of this during resting supine conditions but require much more validation and oversight for ambulatory data. This impacts the utility and commercial costs which have hindered their use. With the advent of newer algorithms systems that can process beat‐to‐beat data without need for as much manual effort, the utility has become more evident and their use is expected to be more widespread.
4. Advancing ECG Restitution
4.1. Addressing protocol dependencies
With the controversies outlined in mind, ECG restitution can be improved to address many of the issues that would seem to deter its use. In fact, restitution under normal sinus rhythm, whether ambulatory or nocturnal, is ideal because it eliminates the utilization of pacing protocol bias. All data from a continuous 24‐hr Holter collected under “unstressed or treatment free” conditions can be used as a baseline to encompass the spectrum of physiologic states that influence restitution, including activity, meals, and sleep. Any sinus arrhythmia (i.e., respiratory) and normal beat‐to‐beat variability that are often removed in TQT studies remain in beat‐to‐beat studies. Consequently, the impact of hysteresis/cardiac memory, magnitude, and heterogeneity are included within the restitution data, all necessary and critical components to assessing arrhythmia liability properly.
4.2. Addressing restitution slope
The triphasic or hump nature of the restitution relationship requires attention so that errors in interpretation related to slope do not occur. Using continuously collected normal sinus rhythm data, when the QT and previous TQ intervals of successive beats are plotted during heart rate acceleration, as described by Franz et al. (1983), the hump is readily apparent (Figure 2) because RR intervals differ by <20 ms in continuum and are not protocol dependent. Normal healthy volunteers given isoproterenol to accelerate the heart with concomitant DECREASES in blood pressure show a smaller hump in the restitution curve (top panels of Figure 4) than the same subject given epinephrine which produces concomitant INCREASES in blood pressures at the same heart rates (bottom panels of Figure 4) (Fossa et al., 2005; Magnano, Holleran, Ramakrishnan, Reiffel, & Bloomfield, 2004). The larger hump is presumably due to the increased contractile force required of the heart per beat to eject blood during increases in afterload (i.e., push‐up example), thus causing the QT interval to become transiently prolonged, due to the inward Ca+2 current (Magnano et al., 2004). Epinephrine also shows larger temporal heterogeneity compared with isoproterenol as apparent by the wider scatter of beats upon heart rate acceleration. Depending on the heart rate range in which the slope of the restitution is measured it can be flat, steep, or even negative. Therefore, for this reason in 2005, the impact of determining restitution as the beat‐to‐beat QT/TQ versus RR interval relationship was explored. Analysis of the continuous ECG data in this manner removed the hump and allowed all the data to be represented in a unidirectional manner with only positive changes in slope. A review of all subsequent publications by the author in humans and animals using this methodology shows that this relationship is extremely reproducible within a subject and can discern a number of physiological states to be further described below (Fossa & Zhou, 2010; Fossa et al., 2005, 2006, 2007, 2011, 2014).
Figure 4.
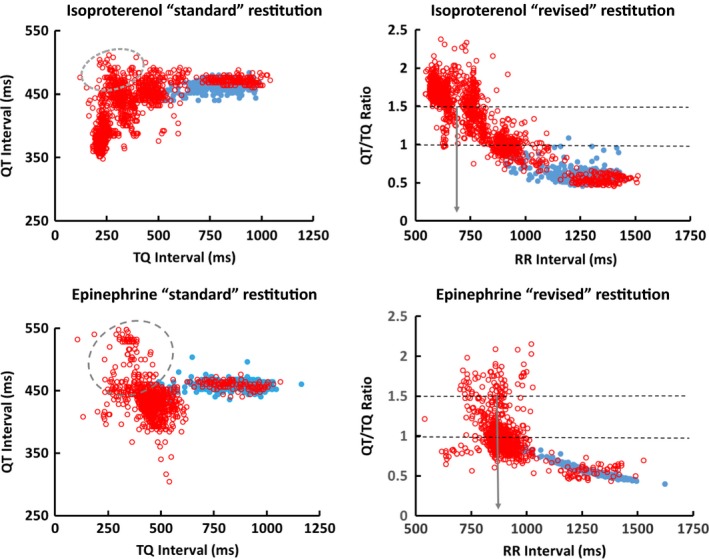
ECG restitution of isoproterenol (top panels) and epinephrine (bottom panels) infusions plotted as the “standard” beat‐to‐beat QT versus TQ interval and “revised” QT/TQ versus RR interval relationships from the same subject. Resting beats from 10‐min baseline (solid blue dots) and 20‐min infusion with either isoproterenol or epinephrine (hollow red circles). Dashed circle area of plots shows hump in restitution relationship during heart rate acceleration. The gray arrows indicate median RR interval at a QT/TQ ratio of 1.5. Note how QT/TQ ratio shifts to right (larger RR interval) with epinephrine versus isoproterenol presumably due to increase workload from increased blood pressure
The QT/TQ versus RR interval relationship in healthy normal subjects is similar in most respects to the normal QT versus TQ interval relationship in that QT/TQ ratio values <1 are produced on a flat portion of the curve at high RR intervals (low heart rates) and QT/TQ ratio values over 1 occur at shorter RR intervals (higher heart rates). Since the relationship is unidirectional, one can use the curvature to determine more precisely at what heart rate the work exceeds rest (i.e., ratios > 1). Thus, knowing at what RR interval the QT/TQ ratio crosses a value of 1 or some other defined set point is very useful. For example, low heart rates are an effect of physical conditioning. As athletes train, they maintain a lower heart rate for longer periods of time and maintain a lower QT/TQ ratio versus RR interval relationship. Contrary to this, unhealthy individuals will show higher QT/TQ ratios at comparable or slower heart rates.
4.3. Integrating temporal and spatial heterogeneity information
The curvature of the QT/TQ versus RR interval relationship is also valuable to assess. We know that as QT/TQ interval ratios increase more quickly at lower heart rates, the risks for arrhythmia increase dramatically. This curvature is largely defined by cardiac memory during heart rate accelerations; the more memory, the tighter the curvature. Figure 5 shows a conceptualized view of the QT/TQ ratio relationship as it would move upward and rightward. This would reflect stress and arrhythmia risk to the heart. To clarify the term “stress” this is not implied to mean any specific electrical, hemodynamic, or contractile mechanism but used more as a term of the integrated impact of all possible mechanisms on the general cardiac fitness; much like how the term is used in a treadmill “stress test.” These directional changes reflect qualitative differences in how the heart is reacting to increased integrated stresses or adaptation. Two examples of this can be observed in Figures 4 and 6. In Figure 4, one can see the rightward shift in the right panels which display the actual beat curvature of the data. Epinephrine, which increases blood pressure with vasoconstriction, produces more rapid increase in QT/TQ interval ratio at lower heart rates compared with isoproterenol, a drug that vasodilates and lowers blood pressure while increasing heart rate. In Figure 6, a similar response is obtained during impaired repolarization induced with dofetilide (a selective hERG potassium channel blocker) in the absence of significant heart rate change. This latter point can be observed in Figure 6 by noting almost no movement in the center of the highest density of beats (reddish area) with regard to RR interval despite the curvature shifting up and to the right. However, to utilize these changes in risk assessment, we need quantify the density of beats that make up these relationships.
Figure 5.
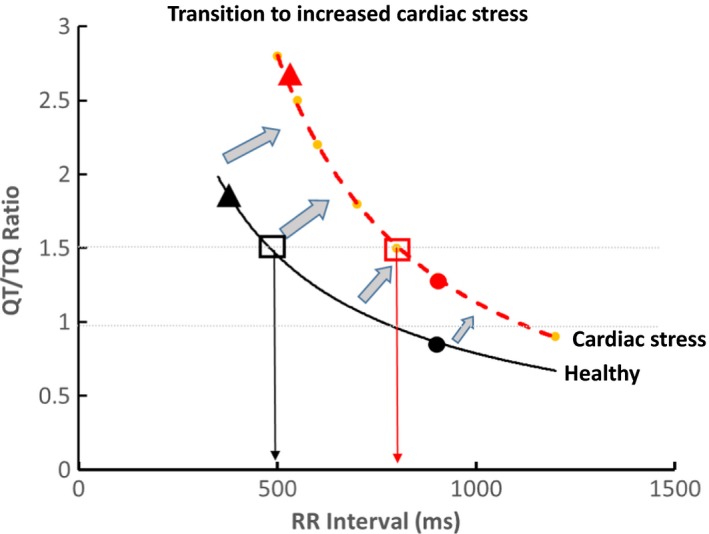
ECG restitution curve conceptualized transition from normal to cardiac stress. In healthy individual, the median QT/TQ (QTTQ50) ratio (filled circles) is under a value of 1 with the top 2% of beats (QTTQ98 as filled triangles) under a value of 2. Cardiac stress (red dashed line) causes upward and rightward shift so that increases in median QTTQ50 and/or QTTQ98 occur at lower heart rates (high RR intervals)
Figure 6.
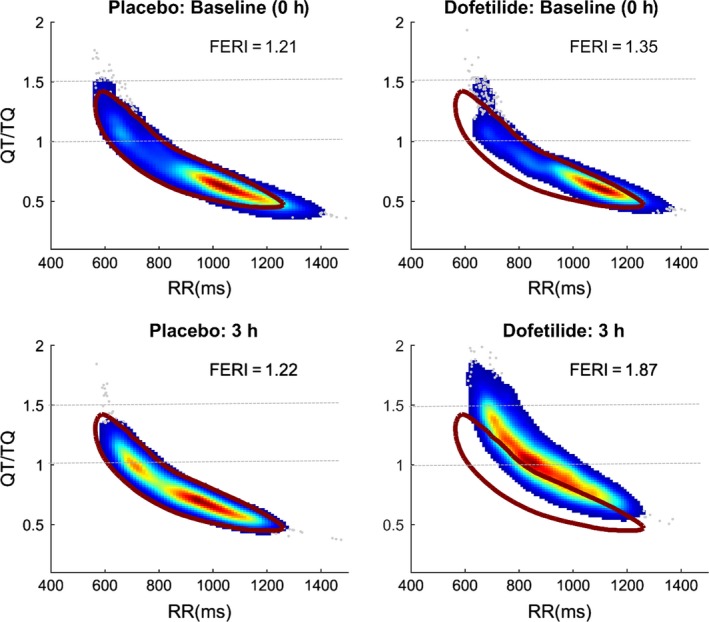
ECG restitution of dofetilide in the same subject compared with responses before and after placebo treatment. Data were obtained from Holters that were collected from a previously reported study (Johannesen et al., 2014) and archived in the University of Rochester Telemetry and Holter ECG Warehouse (Telemetry and Holter ECG Warehouse, University of Rochester Medical Center, 2017). QT/TQ versus RR interval values for each beat determined for a 1‐hr predose period followed by time‐matched data of the same duration ending at 3 hr after dosing are displayed. The density of beats is evident with the gradient colors of least (blue) to highest (red). Brown circle area represents the 95% confidence bounds for all 24‐hr baseline data obtained for that individual subject prior to study. Dofetilide causes FERI to increase during a notable shift in both height and curvature to the right of baseline data
The magnitude and heterogeneity of the changes allow us to quantify the risk. In the past, the median QT/TQ ratio (QTTQ50) of any particular time period has been used to estimate the center density of the data (shown in Figure 5 as the solid circles) with the upper 98th percentile of the QT/TQ intervals (QTTQ98, shown as solid triangles) to estimate the value for the 2% of extreme outliers that may pose the highest risk. To understand how the curve is moving (right or left, up or down) yet to standardize the measure for use across different datasets, all beats from the curve with a QT/TQ ratio of 1.5 are extracted from the dataset and the median RR interval value of those beats at which they occur is determined (RRQTTQ1.5). An automated program for analysis of these extracted beats can be created as is currently done for dynamic beat‐to‐beat analysis (Fossa & Zhou, 2010).
4.4. The need for simplicity
The FDA biomarker qualification team has expressed a strong interest in having a single integrated measure to assess restitution for evaluation in drug studies (personal communication Teleconference Minutes, FDA meeting with Biomarker Qualification Process Team, 2015. Attendees: Amur, Colatsky, Dang, Noone, Rogers, Sanyal, Stockbridge, Strauss, Wang, Zhang). As multiple measurement parameters are possible, as previously described with the guanfacine study (Fossa et al., 2014), it is not known at this time how to weigh the importance of each parameter for final risk assessment. In the guanfacine study, all measures showed improvements so it was clear restitution was unequivocally improved but that may not be the case in other situations. Some measures, like TQ interval may be more sensitive in situations of ischemia, whereas QT interval or QTTQ98 ratio may be more sensitive in cases of impaired repolarization as in the dofetilide example when heart rate is not significantly affected.
5. A New Restitution Index Proposal
A new, simplified, integrated measure of restitution is proposed by this author, namely “FERI” or ECG Restitution Index to avoid confusion with other restitution indexes that may follow. Although at this time, FERI may appear speculative due to the lack of cited publications of its use in datasets with known outcomes or positive controls, there is currently an ongoing effort for its use with the FDA Telemetry Holter ECG Warehouse database (Telemetry and Holter ECG Warehouse, University of Rochester Medical Center, 2017) expected to be published soon. It is the intent of this proposal to describe the rationale for that work and allow others to build upon its use if deemed suitable when those data are available for review.
where the RR interval is in units of seconds.
The advantage of this index would be that it would be translatable across all continuous beat‐to‐beat ECG studies where an adequate heart range was acquired. Heart rate variation is necessary to achieve at least QTTQ98 intervals of approximately 1.5 to assure an accurate measure of stress for evaluation of healthy subjects as well as patients. In addition, a 24‐hr Holter may not be required to fully assess the subject if heart rates of approximately 110 bpm can be achieved over a shorter period of time once reference values are established. This also would potentially allow for comparison to other tests, such as T‐wave alternans data, for further quantification.
5.1. Rationale of FERI formula
To standardize the assessment of the magnitude and heterogeneity of the beat‐to‐beat QT/TQ ratios across heart rates, the index equation is broken into several components.
5.2. Temporal heterogeneity
The magnitude and heterogeneity are determined by the addition of the QTTQ50 and QTTQ98 values in a set of beats with a defined time window (i.e., time point) with the sum divided by 2. This will produce a single QT/TQ ratio value that incorporates the range of values represented by the median and the upper bounds of the QT/TQ ratios (QTTQh). For example, if the median QTTQh is increasing, it reflects not only the increase in the median but also the temporal heterogeneity between the median and upper bound which may be more indicative of arrhythmia risk. If QTTQh declines, the opposite is true. It should be noted that a specific QTTQh value for the mean of the QTTQ50 and QTTQ98 was desired instead of a simple delta (i.e., QTTQ98–QTTQ50), so that the magnitude of the final value is not lost.
5.3. Movement of curvature
The second part of the index adjust the QT/TQ versus RR interval relationship so that if the curve occurs at lower heart rates (larger RR interval value in seconds), more risk is assigned to the QTTQh. This quantifies how the curve is moving (right or left, up or down) as well as standardizes the measure for use across different datasets. A value of 1.5 was chosen instead of 1 because it has been shown in previous experience by this author (Fossa et al., 2007) and by Nicolson et al. (2014) that in normal and high‐risk patients the peak ECG restitution asymptote occurs after the maximum curvature between QT/TQ ratios of 1 and 1.5. All beats from the dataset with a QT/TQ ratio of 1.5 are extracted from the dataset and the median RR interval value of those beats at which they occur is determined. That RR interval value (expressed in seconds not milliseconds to cancel out units) is multiplied times the QTTQh. Figure 6 shows a final computation of FERI applied to a single subject's data before and after treatment with either placebo or dofetilide (Johannesen et al., 2014). The baseline and time‐matched placebo values are all consistently between 1.24 and 1.28 whereas in the period (1‐hr duration of beats) near the 3 hr C max of dofetilide, the FERI value increases to 1.89.
5.4. Spatial heterogeneity
Additional assessment of spatial heterogeneity can be obtained by multiplying the resulting FERI times the SD or other measures of variability between leads at any particular set point or the confidence width of the data obtained via a single lead. The more comprehensive measure of FERI would be designated under a different acronym based on the methodology used so as not to confuse established values for each index. For example FERI12 could be the average of FERI times the standard deviation of FERI from each of 12 chest leads.
6. Conclusions
The application of restitution principles to continuous ECG data appears to be a very promising methodology for assessing arrhythmia vulnerability and as an integrated measure of general cardiac stress. The ability to quantify dynamic parameters of the heart as changes in autonomic state occur is a major advancement beyond general stratification to allow potentially real‐risk prognosis. Many of the approaches toward ECG restitution have focused on highly controlled situations such as acquisition of data during a precise pacing protocols. Although the data are powerful, their utility has been quite restrictive. Combining the best attributes of temporal and spatial heterogeneity components of restitution reflected in the continuous ECG obtained during normal activity is the best hope toward developing a truly integrated measure of cardiac safety. ECG restitution indexes have been proposed which may be widely used in regulatory studies and perhaps in even small clinical environments once their predictive abilities are fully established. As advances in software algorithms allow accurate, automated assessment of ambulatory ECG intervals and other morphological parameters, the ease, utility, and commercial viability will become apparent.
Acknowledgments
The author thanks Dr. Daniel Goodman for his insightful comments in the writing of this manuscript and VivaQuant LLC (St. Paul, MN) for analysis of data and generation of graphics for Figure 6.
Fossa AA. Beat‐to‐beat ECG restitution: A review and proposal for a new biomarker to assess cardiac stress and ventricular tachyarrhythmia vulnerability. Ann Noninvasive Electrocardiol. 2017;22:e12460 10.1111/anec.12460
References
- International Conference on Harmonisation. ICH Harmonized Tripartite Guideline E14 . (2005). Clinical evaluation of QT/QTc interval prolongation and proarrhythmic potential for non‐antiarrhythmic drugs. Guidance to industry. Federal Register, 70, 61134–61135. [PubMed] [Google Scholar]
- Al‐Nimer, M. S. , Al‐Mahdawi, S. A. , & Abdullah, N. M. (2017). Epileptic patients are at risk of cardiac arrhythmias: A novel approach using QT‐nomogram, tachogram and cardiac restitution plots. Journal of Neurosciences in Rural Practice, 8, 7–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bass, B. G. (1975). Restitution of the action potential in cat papillary muscle. American Journal of Physiology, 228, 1717–1724. [DOI] [PubMed] [Google Scholar]
- Boyett, M. R. , & Jewell, B. R. (1980). Analysis of the effects of changes in rate and rhythm upon electrical activity in the heart. Progress in Biophysics and Molecular Biology, 36, 1–52. [DOI] [PubMed] [Google Scholar]
- Burkhoff, D. , Yue, D. T. , Franz, M. R. , Hunter, W. C. , & Sagawa, K. (1984). Mechanical restitution of isolated perfused canine ventricles. Heart and Circulatory Physiology, 15, H8–H16. [DOI] [PubMed] [Google Scholar]
- Cherry, E. M. , & Fenton, F. H. (2004). Suppression of alternans and conduction blocks despite steep APD restitution: electrotonic, memory and conduction velocity restitution effects. The American Journal of Physiology – Heart and Circulatory Physiology, 286(6), H2332–H2341. [DOI] [PubMed] [Google Scholar]
- Chudin, E. , Goldhaber, J. , Garfinkel, A. , Weiss, J. , & Kogan, B. (1999). Intracellular Ca2+ dynamics and the stability of ventricular tachycardia. Biophysical Journal, 77, 2930–2941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clayton, R. H. , & Taggart, P. (2005). Regional differences in APD restitution can initiate wavebreak and re‐entry in cardiac tissue: A computational study. BioMedical Engineering OnLine, 4, 54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delmar, M. , Ibarra, J. , Davidenko, J. , Lorente, P. , & Jalife, J. (1991). Dynamics of the background outward current of single guinea pig ventricular myocytes. Ionic mechanisms of hysteresis in cardiac cells. Circulation Research, 69, 1316–1326. [DOI] [PubMed] [Google Scholar]
- Ewing, D. J. , Martyn, C. N. , Young, R. J. , & Clarke, B. F. (1985). The value of cardiovascular autonomic function tests: 10 years experience in diabetes. Diabetes Care, 8, 491–498. [DOI] [PubMed] [Google Scholar]
- Fossa, A. A. , Depasquale, M. J. , Raunig, D. L. , Avery, M. J. , & Leishman, D. J. (2002). The relationship of clinical QT prolongation to outcome in the conscious dog using a beat‐to‐beat QT‐RR interval assessment. Journal of Pharmacology and Experimental Therapeutics, 202, 828–833. [DOI] [PubMed] [Google Scholar]
- Fossa, A. A. , Langdon, G. , Couderc, J.‐P. , Zhou, M. , Darpo, B. , Wilson, F. , … Davis, J. D. (2011). The use of beat‐to‐beat electrocardiogram analysis to distinguish QT/QTc interval changes caused by moxifloxacin from those caused by vardenafil. Clinical Pharmacology and Therapeutics, 90, 449–454. [DOI] [PubMed] [Google Scholar]
- Fossa, A. A. , Wisialowski, T. , & Crimin, K. (2006). QT prolongation modifies dynamic restitution and hysteresis of the beat‐to‐beat QT‐TQ interval relationship during normal sinus rhythm under varying states of repolarization. Journal of Pharmacology and Experimental Therapeutics, 316, 498–506. [DOI] [PubMed] [Google Scholar]
- Fossa, A. A. , Wisialowski, T. , Crimin, K. , Wolfgang, E. , Couderc, J.‐P. , Hinterseer, M. , … Sarapa, N. (2007). Analyses of dynamic beat‐to‐beat QT‐TQ interval (ECG restitution) changes in humans under normal sinus rhythm and prior to an event of Torsades dePointes during QT prolongation caused by sotalol. Annals of Noninvasive Electrocardiology, 12, 338–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fossa, A. A. , Wisialowski, T. , Magnano, A. , Wolfgang, E. , Winslow, R. , Gorczyca, W. , … Raunig, D. L. (2005). Dynamic beat‐to‐beat modeling of the QT‐RR interval relationship: Analysis of QT prolongation during alterations of autonomic state versus human ether a‐go‐go‐related gene inhibition. Journal of Pharmacology and Experimental Therapeutics, 312, 1–11. [DOI] [PubMed] [Google Scholar]
- Fossa, A. A. , & Zhou, M. (2010). Assessing QT prolongation and electrocardiography restitution using a beat‐to‐beat method. Journal of Cardiology, 17, 230–243. [PubMed] [Google Scholar]
- Fossa, A. A. , & Zhou, M. (2012). Letter to the Editor in response to “Vardenafil‐associated QTc changes: Not merely a normal autonomic process”. Clinical Pharmacology and Therapeutics, 91, 580–581. [DOI] [PubMed] [Google Scholar]
- Fossa, A. A. , Zhou, M. , Robinson, A. , Purkayastha, J. , & Martin, P. (2014). Use of ECG restitution (beat‐to‐beat QT‐TQ interval analysis) to assess arrhythmogenic risk of QTc prolongation with guanfacine. Annals of Noninvasive Electrocardiology, 19, 582–594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franz, M. R. (2003). The electrical restitution curve revisited: Steep or flat slope—Which is better? Journal of Cardiovascular Electrophysiology, 14, S140–S147. [DOI] [PubMed] [Google Scholar]
- Franz, M. R. , Schaefer, J. , Schottler, M. , Seed, W. A. , & Noble, M. (1983). Electrical and mechanical restitution of the human heart at different rates of stimulation. Circulation Research, 53, 815–822. [DOI] [PubMed] [Google Scholar]
- Franz, M. R. , Swerdlow, C. D. , Liem, L. B. , & Schaefer, J. (1988). Cycle length dependence of human action potential during in vivo. Effects of single extrastimuli, sudden sustained rate acceleration and deceleration, and different steady‐state frequencies. Journal of Clinical Investigation, 82, 67–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garfinkel, A. , Kim, Y. H. , Voroshilovsky, O. , Qu, Z. , Kil, J. R. , Lee, M. H. , … Chen, P. S. (2000). Preventing ventricular fibrillation by flattening cardiac restitution. Proceedings of the National Academy of Sciences of the United States of America, 97, 6061–6066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garnett, C. E. , Zhu, H. , Malik, M. , Fossa, A. A. , Zhang, J. , Badalini, F. , … Rodriguez, I. (2012). Methodologies to characterize the QT/corrected QT interval in the presence of drug‐induced heart rate changes or other autonomic effects. American Heart Journal, 163, 912–930. [DOI] [PubMed] [Google Scholar]
- Gilmour Jr, R. F. , Riccio, M. L. , Locati, E. H. , Maison‐Blanche, P. , Coumel, P. , & Schwartz, P. J. (1997). Time‐ and rate‐dependent alterations of the QT interval precede the onset of Torsade de Pointes in patients with acquired QT prolongation. American Journal of Cardiology, 30, 209–217. [DOI] [PubMed] [Google Scholar]
- Gittleman, I. W. , Thorner, M. C. , & Griffith, G. C. (1951). The Q‐T interval of the electrocardiogram in acute myocarditis in adults with autopsy correlation. American Heart Journal, 41, 78–90. [DOI] [PubMed] [Google Scholar]
- Gross, D. (1952). A single numerical correlation between the quotient QT/TQ and cardiac rate in healthy adults. American Journal of Physiology, 170, 121–125. [DOI] [PubMed] [Google Scholar]
- Imam, M. H. , Karmaka, C. K. , Jelinek, H. F. , Palaniswami, M. , & Khandoker, A. H. (2015). Analyzing systolic‐diastolic interval interaction characteristics in diabetic cardiac autonomic neuropathy progression. IEEE Journal of Translational Engineering in Health and Medicine, 3, 1900510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johannesen, L. , Vicente, J. , Mason, J. W. , Sanabria, C. , Waite‐Laborr, K. , Hong, M. , … Strauss, S. G. (2014). Differentiating drug‐induced multichannel block on the electrocardiogram: Randomized study of dofetilide, quinidine, ranolazine, and verapamil. Clinical Pharmacology and Therapeutics, 96, 549–558. [DOI] [PubMed] [Google Scholar]
- Karma, A. , & Gilmour Jr, R. F. (2007). Nonlinear dynamics of heart rhythm disorders. Physics Today, 60, 51–57. [Google Scholar]
- Koller, M. L. , Riccio, M. L. , & Gilmour Jr, R. F. (1998). Dynamic restitution of action potential duration during electrical alternans and ventricular fibrillation. American Journal of Physiology, 275, H1635–H1642. [DOI] [PubMed] [Google Scholar]
- Kurz, R. W. , Ren, X. L. , & Franz, M. R. (1994). Dispersion and delay of electrical restitution in the globally ischaemic heart. European Heart Journal, 15, 547–553. [DOI] [PubMed] [Google Scholar]
- Magnano, A. R. , Holleran, S. , Ramakrishnan, R. , Reiffel, J. A. , & Bloomfield, D. M. (2004). Autonomic modulation of the u wave during sympathomimetic stimulation and vagal inhibition in normal individuals. PACE, 27, 1484–1492. [DOI] [PubMed] [Google Scholar]
- Malik, M. , Hnatkova, K. , Kowalski, D. , Keirns, J. J. , & van Gelderen, E. M. (2012). Importance of subject‐specific QT/RR curvatures in the design of individual heart rate corrections of the QT interval. Journal of Electrocardiology, 45, 571–581. [DOI] [PubMed] [Google Scholar]
- Manriquez, A. I. , Zhang, Q. , Medigue, C. , Papelier, Y. , & Sorine, M. (2006). Electrocardiogram‐based restitution curve. Computers in Cardiology, 33, 493–496. [Google Scholar]
- Minchole, A. , Bueno‐Orovio, A. , Laguna, P. , Pueyo, E. , & Rodriguez, B. (2015). ECG‐based estimation of dispersion of APD restitution as a tool to stratify sotalol‐induced arrhythmic risk. Journal of Electrocardiology, 48, 867–873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicolson, W. B. , McCann, G. P. , Brown, P. D. , Sandilands, A. J. , Stafford, P. J. , Schlindwein, F. S. , … Ng, G. A. (2012). A novel surface electrocardiogram‐based marker of ventricular arrhythmia risk in patients with ischemic cardiomyopathy. Journal of the American Heart Association, 1(4), e001552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicolson, W. B. , McCann, G. P. , Smith, M. I. , Sandilands, A. J. , Stafford, P. J. , Schlindwein, F. S. , … Ng, G. A. (2014). Prospective evaluation of two novel ECG‐based restitution biomarkers for prediction of sudden cardiac death risk in ischaemic cardiomyopathy. Heart, 100, 1878–1885. [DOI] [PubMed] [Google Scholar]
- Nolasco, J. B. , & Dahlen, R. W. (1968). A graphic method for the study of alternation in cardiac action potentials. Journal of Applied Physiology, 25, 191–196. [DOI] [PubMed] [Google Scholar]
- Pastore, J. M. , Laurita, K. R. , & Rosenbaum, D. S. (2006). Importance of spatiotemporal heterogeneity of cellular restitution in mechanism of arrhythmogenic discordant alternans. Heart Rhythm: The Official Journal of the Heart Rhythm Society, 3, 711–719. [DOI] [PubMed] [Google Scholar]
- Ramirez, J. , Minchole, A. , Bolea, J. , Laguna, P. , & Pueyo, E. (2013). Prediction of sudden cardiac death in chronic heart failure patients by analysis of restitution dispersion. Computers in Cardiology, 40, 1–4. [Google Scholar]
- Riccio, M. L. , Koller, M. L. , & Gilmour Jr, R. F. (1999). Electrical restitution and spatiotemporal organization during ventricular fibrillation. Circulation Research, 84, 955–963. [DOI] [PubMed] [Google Scholar]
- Sager, P. T. , Gintant, G. , Turner, J. R. , Turner, R. J. , Pettit, S. , & Stockbridge, N. (2014). Rechanneling the cardiac proarrhythmia safety paradigm: A meeting report from the Cardiac Safety Research Consortium. American Heart Journal, 167, 292–300. [DOI] [PubMed] [Google Scholar]
- Taggart, P. , Sutton, P. , Chalabi, Z. , Boyett, M. R. , Simon, R. , Elliott, D. , & Gill, J. S. (2003). Effect of adrenergic stimulation on action potential restitution in humans. Circulation, 107, 285–289. [DOI] [PubMed] [Google Scholar]
- Taggart, P. , Sutton, P. , Runnalls, M. , O'Brian, W. , Donaldson, R. , Hayward, R. , … Treasure, T. (1986). Use of monophasic action potential recordings during routine coronary‐artery bypass surgery as an index of localized myocardial ischaemia. Lancet, 1, 1462–1465. [DOI] [PubMed] [Google Scholar]
- Taran, L. M. , & Szilagyi, N. (1947). The duration of the electrical extrasystole in acute rheumatic carditis in children. American Heart Journal, 33, 14. [DOI] [PubMed] [Google Scholar]
- Telemetry and Holter ECG Warehouse, University of Rochester Medical Center (2017). FDA2‐ trial of potassium, calcium and late sodium blockers, IDENTIFICATION: E‐OTH‐12‐0109‐021.
- Verrier, R. L. , & Nearing, B. D. (1994). Electrophysiologic basis for T wave alternans as an index of vulnerability to ventricular fibrillation. Journal of Cardiovascular Electrophysiology, 5, 445–461. [DOI] [PubMed] [Google Scholar]
- Yu, P. N. G. , Bruce, R. A. , Lovejoy, F. W. , & Pearson, R. (1950). Observations on the change of ventricular systole (QT interval) during exercise. Journal of Clinical Investigation, 29, 279–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng, J. , Laurita, K. R. , Rosenbaum, D. S. , & Rudy, Y. (1995). Two components of the delayed rectifier K+ current in ventricular myocytes of the guinea pig type: Theoretical formulation and their role in repolarization. Circulation Research, 77, 140–152. [DOI] [PubMed] [Google Scholar]


