Abstract
Aims
Fragmented QRS has emerged as a novel electrocardiographic parameter associated with adverse clinical events in various diseases. The aim of this study was to investigate the association of fQRS with in‐hospital and long‐term cardiovascular events in patients with ST‐segment elevation myocardial infarction (STEMI) and non‐ST segment elevation myocardial infarction (NSTEMI).
Methods and Results
We searched PubMed, Embase, Web of Science, and Cochrane Library up to October 2015 for eligible studies. We selected studies with fQRS defined with 12‐lead ECG during the index hospitalization of STEMI/NSTEMI. Primary outcomes were in‐hospital and long‐term cardiovascular events. In‐hospital mortality was significantly higher in fQRS (+) group (99/733; 13.5%) compared to fQRS (–) group (47/1293; 3.6%) (OR 4.03 95% CI 1.81–8.94; P = 0.0006). Long‐term mortality rate was higher in fQRS (+) group (89/473; 18.8%) compared to fQRS (–) group (54/1009; 5.3%) (OR 3.93 95% CI 1.92–8.05; P = 0.0002). In addition the frequency of long‐term MACE was higher in fQRS (+) group (46.9%) compared to fQRS (–) group (14.6%) (OR 5.13 95% CI 2.77–9.51; P < 0.00001)
Conclusion
Presence of fQRS on admission ECG was found to be predictor of mortality, MACE, deterioration of LV function, and presence of multivessel disease in patients with STEMI and NSTEMI.
Keywords: fragmented QRS, myocardial infarction, coronary artery disease
Acute myocardial infarction (AMI) is a leading and an important cause of morbidity and mortality worldwide and 12‐lead electrocardiography (ECG) still plays a key role in the diagnosis and management of patients. Various ECG parameters have been evaluated to predict prognosis in AMI. Fragmented QRS (fQRS) was defined as various RSR’ patterns with or without Q waves on ECG. Fragmented QRS complexes are novel ECG signals which are associated with varied conduction abnormalities and the delay of periinfarct conductions due to myocardial scarring or necrosis.1, 2
Correlation and prognostic importance of presence of fQRS on ECG have been shown in various cardiovascular diseases such as cardiomyopathy, coronary slow flow, left ventricular noncompaction, and Brugada syndrome.3, 4, 5, 6, 7 In addition, various studies have evaluated the prognostic importance of fQRS in patients with acute coronary syndrome (ACS), ST‐segment elevation (STEMI), or non‐ST segment elevation myocardial infarction (NSTEMI).8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18 Some recent papers reviewed the clinical importance of fQRS in patients with cardiovascular diseases.19, 20, 21 However, the literature regarding the definition and the prognostic value of fQRS in patients with AMI is heterogeneous and the findings are not easy to interpret. Thus, in this meta‐analysis we recruited studies of STEMI and NSTEMI and investigated the association of fQRS with short‐/long‐term mortality and major adverse cardiovascular events (MACE) in patients with AMI.
METHODS
Literature Search
We aimed to identify all published data relating the presence of fQRS to cardiovascular end points in patients with AMI. The electronic databases PubMed, MEDLINE, Embase, and Cochrane Library were searched to find primary references and reviews. Search terms included: fragmented QRS, QRS fragmentation, fQRS, coronary artery disease, CAD, myocardial infarction, MI, mortality, morbidity, survival, and prognosis. These terms were combined with the search algorithm, for example, “fragmented QRS and myocardial infarction.” The search was restricted to adults (>18 years of age) in English language peer‐reviewed journals from 1960 to October 2015. Abstracts of the articles published by the American College of Cardiology, the American Heart Association, the European Society of Cardiology, were also searched. Reviews and reference lists of retrieved articles were hand searched for potentially relevant publication not previously identified in the database search. The retrieved studies were examined to eliminate potential duplicates or overlapping data. Our analysis is based on the guidelines of the Meta‐analysis of Observational Studies in the Epidemiology Group.22
Study Selection
Studies recruiting patients with STEMI and/or NSTEMI were included. Diagnosis of an acute STEMI was made by the presence of new or presumed new ST‐segment elevation at the J point in >2 contiguous leads of >0.2 mV in leads V1, V2, or V3, and >0.1 mV in other leads. Marked ST depression, which was maximal in leads V1 through V3, without ST segment elevation in other leads, was designated as posterior wall MI and included in the STEMI group.23
Acute NSTEMI was defined by the detection of increases and/or decreases in cardiac biomarkers (troponin I), with >1 value above the 99th percentile of the upper reference limit, together with evidence of myocardial ischemia, which included typical symptoms of myocardial ischemia, electrocardiographic changes indicative of new ischemia (new ST‐T changes) or the development of pathologic Q waves on ECG, or imaging evidence (nuclear imaging, echocardiography, or left ventriculography) of a new loss of viable myocardium or new regional wall motion abnormality. Studies recruiting patients with stable CAD or patients with unstable angina pectoris (normal cardiac biomarker levels obtained 6–8 hours after presentation) were excluded. In addition, studies only including patients with ischemic or nonischemic cardiomyopathy, implantable cardioverter defibrillator (ICD), hypertrophic cardiomyopathy, congenital heart disease, Brugada syndrome, long QT syndrome, and Chagas’ disease were excluded.
Only the studies that used 12‐lead ECG during the index hospitalization of MI for the definition of fQRS were included. The description of Das for definition of fQRS was searched for in the articles and other definitions such as “QRS distortion” were omitted.1 Das has defined fQRS as; the presence of an additional R wave (R′) or notching in the nadir of the S wave, or the presence of 1R′ (fragmentation) in two contiguous leads, corresponding to a major coronary artery territory where the QRS duration <120 ms. One study was excluded because the ECGs were obtained 2 months after AMI.8
Persistent QRS fragmentation was defined as presence of QRS fragmentation throughout the hospital stay including the discharge ECG, or the last ECG performed in case of death. Transient QRS fragmentation was defined as presence of fragmentation in at least one ECG but not in all ECGs recorded during the hospitalization or follow‐up period. Studies using methods other than 12‐lead ECG such as vectorcardiography, magnetocardiography, and signal‐averaged ECG were also excluded.
Study End Points
The primary outcome of interest was the occurrence of a first fatal event during the study period. Major adverse cardiovascular events (MACE) were defined as recurrent myocardial infarction, target vessel revascularization (percutaneous or surgical) or death from these events.
Quality Assessment
The risk of study bias was evaluated with Quality in Prognosis Studies (QUIPS) tool which includes six domains: participation, attrition, prognostic factor measurement, outcome measurement, study confounding, statistical analysis and reporting.24 Publication bias was evaluated by generating a funnel plot of the logarithm of effect size against the standard error for each trial.
Statistical Analysis
The significance between two groups was estimated by odds ratio (OR) and weighted mean difference (WMD) with a 2‐tailed 95% confidence intervals (CI). A fixed‐effect model was used for homogenous studies, whereas a random‐effect model was used for heterogeneous studies. Statistic I 2 was used to describe the percentage of total across‐studies variation due to study‐to‐study heterogeneity. Subgroup analyses were performed to explore and control potential confounders. A two‐sided P value <0.05 was considered statistically significant. Statistical analysis was performed by using Review Manager 5.0 (The Cochrane Collaboration, Oxford, United Kingdom).
RESULTS
Search Results
The study selection process is illustrated in Figure 1. In total, 204 studies (excluding duplicates) were identified by our literature search. After the exclusion of nonrelevant studies, case reports and reviews by title and abstracts, 41 studies including patients with acute MI were retrieved for further consideration. In 27 of these studies, data on morbidity/mortality were not reported. Three studies were excluded as morbidity/mortality data regarding subgroup analysis of STEMI/NSTEMI were not reported. Finally, 10 studies were included in our systematic review.9, 10, 11, 12, 13, 14, 15, 16, 17, 18
Figure 1.
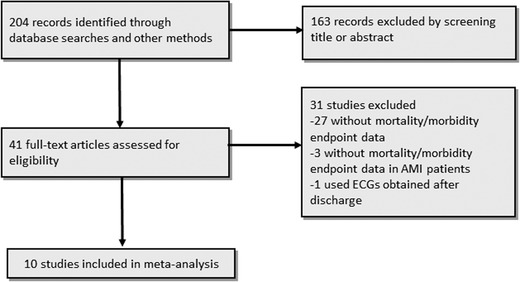
Flow‐diagram for inclusion of studies in the meta‐analysis.
Study Quality
The methodological quality of the included studies was generally good, without high risk of bias (Table 1). In six studies with long‐term follow‐up, only Lorgis et al. reported data regarding loss to follow‐up.9, 10, 11, 12, 16, 17 Six studies reported in‐hospital adverse events, thus it may be assumed that no cases were lost to follow‐up.13, 14, 15, 16, 17, 18 In addition, it was not clear how three studies accounted for potential confounders, raising the small possibility of result distortion.13, 15, 18 The funnel plot did not suggest evidence of publication bias (Fig. 2).
Table 1.
Quality in Prognosis Studies Analysis of Internal Validity
| Prognostic | Statistical | |||||
|---|---|---|---|---|---|---|
| Study | Study | Factor | Outcome | Study | Analysis | |
| Participation | Attrition | Measuring | Measuring | Confounding | and Reporting | |
| Arı 2012 | Low | Medium | Low | Low | Low | Low |
| Guo 10 | Low | Medium | Low | Medium | Low | Low |
| Lorgis 11 | Low | Low | Low | Low | Low | Low |
| Yıldırım 2013 | Low | Low | Low | Low | Medium | Medium |
| Bekler 12 | Low | Medium | Low | Low | Low | Low |
| Akgul 2014 | Low | Medium | Low | Low | Low | Low |
| Sheng 13 | Low | Low | Medium | Low | Medium | Medium |
| Stavileci 14 | Low | Low | Low | Low | Low | Low |
| Tanrıverdi 18 | Low | Low | Low | Medium | Medium | Medium |
| Bozbeyoğlu 17 | Low | Low | Low | Low | Low | Low |
Figure 2.
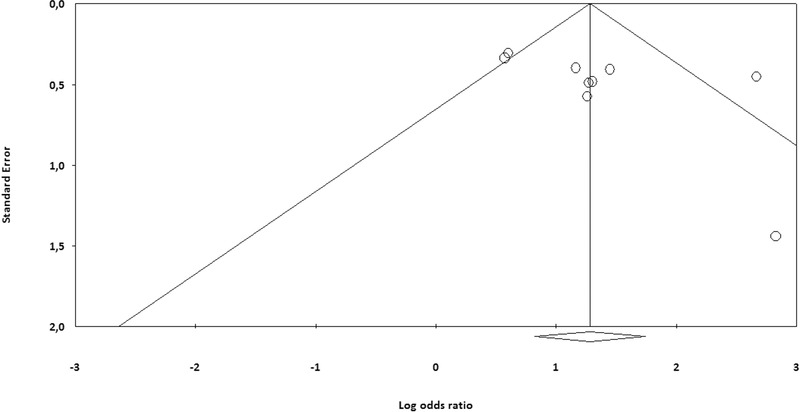
Funnel plot for the included studies.
Baseline Characteristics of Included Studies
The baseline characteristics of ten included studies are shown in Table 2. The study size differed from 85 to 433 subjects and in total 2766 cases were included in this meta‐analysis, 1064 of them were assigned in the fQRS (+) group (38.5%) and 1702 of them were assigned in the fQRS (–) group (61.5%). Four studies were designed as prospective and others were retrospective trials.9, 15, 16, 17 Male patients were predominantly enrolled in these studies. The mean age of cases ranged from 54 to 71 years in fQRS (+) groups and 55 to 62 years in the fQRS (–) groups. Five studies included patients only with STEMI (n = 1398)9, 14, 15, 16, 18 and three studies included patients only with NSTEMI (n = 761).10, 12, 17 Two studies included patients with both STEMI and NSTEMI (n = 607).11, 13 In total, 1626 patients (58.7%) had STEMI and 1440 patients had NSTEMI (41.3%).
Table 2.
Summary of Study Characteristics
| Male | fQRS (+) | QRS | Follow | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Age | Gender | ST/NS | ECG | ratio, | width (ms) | LV EF, % | ‐up, | Death,n | MACE, | |||
| Name | n | Type | fQRS +/– | fQRS +/– | TEMI | Time | n (%) | fQRS +/− | fQRS +/– | Months | fQRS +/– | n fQRS +/– |
| Arı 2012 | 85 | Prosp | 54/55 | 29/41 | 85/0 | 48 | 34 (40) | 73/68 | 39/43 | 6.6 ± 2.3 | NR | 10/3 |
| Guo 10 | 179 | Retro | 62/60 | 66/47 | 0/179 | 48 | 106 (59) | NR | 57.2/57.1 | 10.2 | 18/4 | 46/22 |
| Lorgis 11 | 307 | Retro | 71/62 | 103/113 | 129/178 | 36 | 145 (47) | 80/80 | 55/50 | 28.2 | 25/17 | 72/20 |
| Yıldırım 2013 | 355 | Prosp | 59/55 | 175/90 | 355/0 | 48 | 217 (61) | NR | 42/50 | In‐hospital | 14/0 | 55/9 |
| Bekler 12 | 149 | Retro | 64/59 | 38/74 | 0/149 | Adm | 46 (31) | NR | 46/50 | 18 | 12/9 | 33/20 |
| Akgul 2014 | 414 | Prosp | 60/53 | 59/269 | 414/0 | 48 | 91 (22) | 94/88 | 44/49 | 12 | 22/7 | 37/39 |
| Sheng 13 | 300 | Retro | 68 (total) | 204 (total) | 99/201 | 48 | 169 (56) | NR | NR | In‐hospital | 40/19 | NR |
| Stavileci 14 | 296 | Retro | 61/61 | 70/156 | 296/0 | Adm | 80 (27) | NR | 37/44 | In‐hospital | 16/12 | NR |
| Tanrıverdi 18 | 248 | Retro | 65/62 | 69/120 | 248/0 | 48 | 91 (37) | 108/102 | 35/47 | In‐hospital | 13/7 | NR |
| Bozbeyoğlu 17 | 433 | Prosp | 63/62 | 59/231 | 0/433 | Adm | 85 (20) | 103/95 | 45/50 | In‐hospital,12 months | 12/17 | NR |
ADM = admission; ECG = electrocardiography; LV EF = left ventricular ejection fraction; MACE = major adverse cardiovascular events; NSTEMI = non‐ST segment myocardial infarction; NR = not reported; Prosp = prospective; Retro = retrospective; STEMI = ST segment myocardial infarction.
QRS Fragmentation
All of the studies have defined QRS fragmentation using ECGs obtained during acute MI and mostly within 48 hours of hospitalization (Table 2). The rate of QRS fragmentation ranged between 20% and 61% and was 38.5 % in total. In the STEMI population, fQRS was detected in 568 of 1527 patients (37.2%) and in NSTEMI population, fQRS was detected in 327 of 939 patients (34.8%) which was not statistically different (P = 0.41). Five studies reported QRS duration which was not different between fQRS (+) and (−) groups.9, 11, 16, 17, 18
Five studies reported the persistence of QRS fragmentation during the hospitalization or follow‐up period.10, 11, 13, 14, 18 Two studies included subjects with transient QRS fragmentation in the control group10, 14 and two studies included cases with transient QRS fragmentation in the fQRS (+) group.13, 18 Lorgis et al. reported the rate of adverse events separately in patients with transient and persistent QRS fragmentation subgroups.11The rate of transient fQRS in the study populations ranged between 12.5% and 54%.11, 13, 18 The rates of permanent QRS fragmentation ranged between 3% and 74%.10, 11, 13, 14, 18 When, only patients with STEMI were considered, the rate of fQRS persistence was reported as 27% and 66%.14, 18 Sheng et al. reported the average onset time of fQRS as 2.9 days but at the end of 7 days only 5% of the fQRS (+) cases had persistent fQRS.13
Left Ventricular Ejection Fraction
All of the studies except for Sheng et al. reported left ventricular EF in study groups. In six studies, left ventricular EF was lower in fQRS (+) (ranged between 35% and 45%) compared to fQRS (–) group (ranged between 43% and 50%).9, 14, 15, 16, 17, 18 Whereas, three studies reported an insignificant difference of left ventricular EF between the groups.10, 11, 12 Seven studies reporting left ventricular EF in mean ± standard deviation were analyzed with the random effects approach. The overall effect showed that patients who had fQRS on admission ECG had a significantly lower LVEF than patients without fQRS (WMD −6.01, 95% CI [−9.08, −2.94], P < 0.00001) (Fig. 3)
Figure 3.
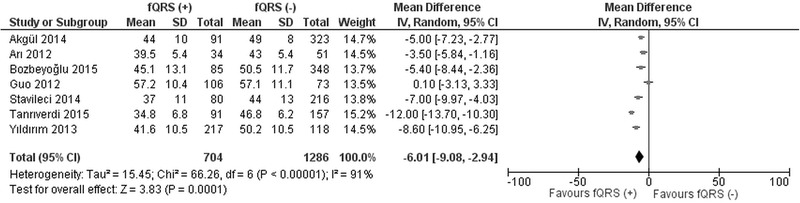
Forest plot for left ventricular ejection fraction between fQRS (+) and fQRS (–) groups. The relative size of the data markers indicates the weight of the sample size from each study.
Coronary Angiography
In six studies the frequency of 3‐vessel disease was reported.10, 12, 14, 16, 17, 18 In four studies, the frequency of 3‐vessel disease was higher in fQRS (+) group (ranged between 42% and 61%) compared to fQRS (–) group (ranged between 15% and 33%). In total, the frequency of 3‐vessel disease was 46.4% in fQRS (+) group and was 24.2% in fQRS (–) group (P < 0.01).Whereas, two studies reported an insignificant difference between groups regarding multivessel involvement. 12, 17 Most of the patients with STEMI were treated with primary percutaneous coronary intervention (77.6%).
Comparison of Clinical Outcome
Six studies reported in‐hospital mortality13, 14, 15, 16, 17, 18 and six studies reported long‐term adverse events.9, 10, 11, 12, 16, 17 Most of the studies defined MACE as mortality, reinfarction, or repeat target vessel revascularization whereas Sheng et al. and Stavileci et al. defined MACE as arrhythmic complications during hospitalization. Arı et al. did not report mortality rates but reported the outcome as MACE only.
Regarding in‐hospital events, mortality was significantly higher in fQRS (+) group (99/733; 13.5%) compared to fQRS (–) group (47/1293; 3.6%) (OR 4.03; 95% CI, 1.81–8.94; P = 0.0006) (Fig. 4). When only STEMI studies were included in the analysis, mortality was higher in fQRS (+) group (57/479; 11.9%) compared to fQRS (–) group (21/814; 2.6%) (OR 6.01; 95% CI, 2.37–15.23; P = 0.0002).14, 15, 16, 18
Figure 4.
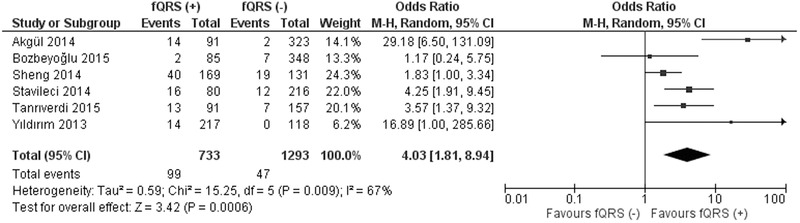
Forest plot for in‐hospital mortality between fQRS (+) and fQRS (–) groups. The relative size of the data markers indicates the weight of the sample size from each study.
Five studies reported mortality rates during a follow up period ranging between 6.6 and 28.2 months.10, 11, 12, 16, 17 The mortality rate was higher in fQRS (+) group (89/473; 18.8%) compared to fQRS (–) group (54/1009; 5.3%) (OR 3.93; 95% CI, 1.92–8.05; P = 0.0002) (Fig. 5). In addition the frequency of MACE was higher in fQRS (+) group (46.9%) compared to fQRS (–) group (14.6%) (OR 5.13; 95% CI, 2.77–9.51; P < 0.00001) (Fig. 6). When only 3 NSTEMI studies are included in the analysis, mortality rate was higher in fQRS (+) group (17.7%) compared to fQRS (–) group (5.7%) (OR 3.42; 95% CI, 2.01–5.82; P < 0.00001).10, 12, 17
Figure 5.
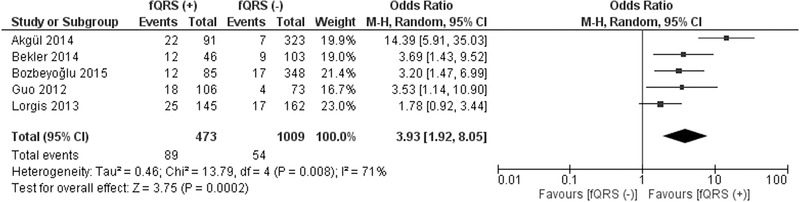
Forest plot for long‐term mortality between fQRS (+) and fQRS (–) groups. The relative size of the data markers indicates the weight of the sample size from each study.
Figure 6.
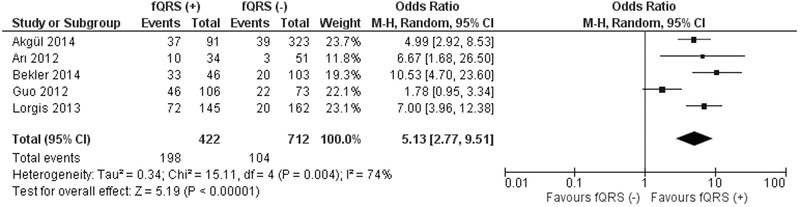
Forest plot for long‐term major adverse cardiovascular events between fQRS (+) and fQRS (–) groups. The relative size of the data markers indicates the weight of the sample size from each study.
When 2 studies which required persistence of fQRS(+) throughout the hospitalization period were excluded,10, 14 presence of fQRS was still found to be correlated with higher risk of mortality in the remaining study population (OR 3.59; 95% CI, 1.99–6.47; P < 0.00001), in STEMI subgroup (OR 8.08; 95% CI, 2.65–24.65; P < 0.00001),15, 16, 18 and in NSTEMI subgroup (OR 3.20; 95% CI, 1.46–6.99; P < 0.00001).12, 17
DISCUSSION
This meta‐analysis included 10 studies involving 2766 patients. The main findings of the current meta‐analysis are (1) the frequency of fQRS on ECGs obtained within 24 hours of hospital admission is not different between STEMI and NSTEMI groups (2) presence of fQRS is correlated with higher rate of multivessel disease on CAG and lower LVEF on echocardiography (3) presence of fQRS is associated with higher risk of in‐hospital and long‐term mortality and adverse cardiovascular events in patients with AMI.
This is the first meta‐analysis focused on STEMI and NSTEMI. Two prior meta‐analyses included patients with stable/unstable CAD, cardiomyopathy and ICD implantation.19, 20 AMI is the most severe form of CAD which is associated with high risk of left ventricular failure, arrhythmias and mortality compared to stable patients. Fragmentation of QRS has been shown to be correlated with myocardial scar, left ventricular dysfunction and arrhythmias in patients with various cardiovascular diseases.2, 19 In fact, AMI is the initial event in most of patients that lead to heart failure, arrhythmias and ICD implantation. Determination of QRS fragmentation during the acute phase of AMI (especially within 48 hours) is feasible and as we have shown in this meta‐analysis, presence of fQRS may have clinical value in establishing patients with higher risk for short and long term adverse cardiac events.
In this analysis, we have found that presence of fQRS is correlated with short‐ and long‐term adverse cardiac event regardless of the AMI type. In addition, a higher portion of patients with fQRS had multivessel disease which may result in incomplete revascularization. We have found that patients with fQRS (+) have lower LVEF compared to fQRS (–) patients. This finding is concordant with previous reports which found lower LVEF, and larger left ventricular systolic and diastolic dimensions and volumes in patients with fQRS and CAD.21, 25 As we included only AMI patients, we can assume that appearance of QRS fragmentation even in the early phase of AMI may indicate larger infarct size and worse LV systolic function. This correlation may partially explain the worse prognosis in patients with fQRS (+).
The prognostic value of QRS fragmentation during the acute phase of ACS was first proposed by Das et al.26 In that study, they have reported that fQRS had higher sensitivity for diagnosis of STEMI or NSTEMI compared to Q waves, T‐wave inversion or ST‐segment depression. In addition, they have found higher long‐term mortality rates in fQRS(+) group compared to fQRS (–) group. This study was not included in this meta‐analysis because the mortality and morbidity rates were not reported in the AMI subgroup. In another study, Pietrasik et al. reported incidence of QRS fragmentation as 53% in AMI patients after 2 months.8 They found that presence of QRS fragmentation and/or Q waves was not associated with long term adverse events. Interestingly, they found that patients with resolved Q waves and persistent fQRS had the highest risk for long‐term adverse events.
An important confounding factor in evaluation of QRS fragmentation in CAD patients is the timing of the ECG and persistence of QRS fragmentation throughout the study period. The studies are heterogeneous and persistence of ECG findings were not reported in all studies. In this meta‐analysis, five studies reported data regarding duration of QRS fragmentation10, 11, 13, 14, 18 In subgroup analysis, we have found that documentation of QRS fragmentation within 48 hours of hospitalization is adequate and is correlated with worse outcome in STEMI and NSTEMI patients. The data regarding the rate of persistence of QRS fragmentation after AMI is controversial, thus, to our opinion use of the ECGs obtained within 48 hours of hospitalization is adequate for risk stratification of patients.
The exact mechanism of fQRS on ECG has not been fully elucidated in the literature but most of the studies have concluded the main causative mechanism is cardiac fibrosis and scarring. Presence of QRS fragmentation is accepted as a sensitive marker of myocardial scar after AMI.2 Myocardial damage causes heterogeneity of myocardial segments and a conduction delay around the infarction zone or scar accounts for the reason for fragmentation in QRS. Even if arrhythmic complications were not reported in detail in this analysis, sudden cardiac death is a major cause of death in patients with CAD and MI. Thus, presence of fQRS may be correlated with arrhythmic complications and SCD that leads to higher mortality risk.
Study Limitations
Several potential limitations of present meta‐analysis should be taken into account. First, there is publication bias on considering only published studies. In addition, language was restricted to English. About half of the studies were retrospective which warrants more large scale randomized controlled trials. The duration of follow‐up was not same for all studies. In addition, it is hard to evaluate the additive prognostic value of QRS fragmentation besides reduced LVEF in patients with AMI.
CONCLUSION
Presence of fQRS on admission ECG was a predictor of mortality, MACE, deterioration of LV function and presence of multivessel disease in patients with STEMI and NSTEMI. Further evaluation of clinical use of QRS fragmentation in patients with AMI are needed to establish the risk of arrhythmic complications and mortality in patients with fQRS.
Ann Noninvasive Electrocardiol 2016;21(6):604–612
REFERENCES
- 1. Das MK, Khan B, Jacob S, et al. Significance of a fragmented QRS complex versus a Q wave in patients with coronary artery disease. Circulation. 2006;113:2495–2501. [DOI] [PubMed] [Google Scholar]
- 2. Das MK, Suradi H, Maskoun W, et al. Fragmented wide QRS on a 12‐lead ECG: A sign of myocardial scar and poor prognosis. Circ Arrhythm Electrophysiol 2008;1:258–268. [DOI] [PubMed] [Google Scholar]
- 3. Das MK, Maskoun W, Shen C, et al. Fragmented QRS on twelve‐lead electrocardiogram predicts arrhythmic events in patients with ischemic and nonischemic cardiomyopathy. Heart Rhythm 2010;7:74–80. [DOI] [PubMed] [Google Scholar]
- 4. Das MK, Saha C, El Masry H, et al. Fragmented QRS on a 12‐lead ECG: a predictor of mortality and cardiac events in patients with coronary artery disease. Heart Rhythm 2007;4:1385–1392. [DOI] [PubMed] [Google Scholar]
- 5. Yilmaz H, Gungor B, Kemaloglu T, et al. The presence of fragmented QRS on 12‐lead ECG in patients with coronary slow flow. Kardiol Pol 2014;72:14–19. [DOI] [PubMed] [Google Scholar]
- 6. Ning XH, Tang M, Chen KP, et al. The prognostic significance of fragmented QRS in patients with left ventricular noncompaction cardiomyopathy. Can J Cardiol 2012;28:508–514. [DOI] [PubMed] [Google Scholar]
- 7. Morita H, Kusano KF, Miura D, et al. Fragmented QRS as a marker of conduction abnormality and a predictor of prognosis of Brugada syndrome. Circulation 2008;118:1697–1704. [DOI] [PubMed] [Google Scholar]
- 8. Pietrasik G, Goldenberg I, Zdzienicka J, et al. Prognostic significance of fragmented QRS complex for predicting the risk of recurrent cardiac events in patients with Q‐wave myocardial infarction. Am J Cardiol 2007;100:583–586. [DOI] [PubMed] [Google Scholar]
- 9. Ari H, Cetinkaya S, Ari S, et al. The prognostic significance of a fragmented QRS complex after primary percutaneous coronary intervention. Heart Vessels 2012;27:20–28. [DOI] [PubMed] [Google Scholar]
- 10. Guo R, Zhang J, Li Y, et al. Prognostic significance of fragmented QRS in patients with non‐ST elevation myocardial infarction: Results of a 1‐year, single‐center follow‐up. Herz 2012;37:789–795. [DOI] [PubMed] [Google Scholar]
- 11. Lorgis L, Jourda F, Hachet O, et al. Prognostic value of fragmented QRS on a 12‐lead ECG in patients with acute myocardial infarction. Heart Lung 2013;42:326–331. [DOI] [PubMed] [Google Scholar]
- 12. Bekler A, Gazi E, Erbag G, et al. [Relationship between presence of fragmented QRS on 12‐lead electrocardiogram on admission and long‐term mortality in patients with non‐ST elevated myocardial infarction]. Turk Kardiyol Dern Ars 2014;42:726–732. [DOI] [PubMed] [Google Scholar]
- 13. Sheng QH, Hsu CC, Li JP, et al. Correlation between fragmented QRS and the short‐term prognosis of patients with acute myocardial infarction. J Zhejiang Univ Sci B 2014;15(1):67–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Stavileci B, Cimci M, Ikitimur B, et al. Significance and usefulness of narrow fragmented QRS complex on 12‐lead electrocardiogram in acute ST‐segment elevation myocardial infarction for prediction of early mortality and morbidity. Ann Noninvasive Electrocardiol 2014;19:338–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Yildirim E, Karacimen D, Ozcan KS, et al. The relationship between fragmentation on electrocardiography and in‐hospital prognosis of patients with acute myocardial infarction. Med Sci Monit 2014;20:913–919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Akgul O, Uyarel H, Pusuroglu H, et al. Predictive value of a fragmented QRS complex in patients undergoing primary angioplasty for ST elevation myocardial infarction. Ann Noninvasive Electrocardiol 2015;20:263–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Bozbeyoglu E, Yildirimturk O, Yazici S, et al. Fragmented QRS on admission electrocardiography predicts long‐term mortality in patients with Non‐ST‐segment elevation myocardial infarction. Ann Noninvasive Electrocardiol 2015. Sep 22 [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Tanriverdi Z, Dursun H, Simsek MA, et al. The predictive value of fragmented QRS and QRS distortion for high‐risk patients with STEMI and for the reperfusion success. Ann Noninvasive Electrocardiol 2015;20:578–585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Rosengarten JA, Scott PA, Morgan JM. Fragmented QRS for the prediction of sudden cardiac death: A meta‐analysis. Europace 2015;17:969–977. [DOI] [PubMed] [Google Scholar]
- 20. Xu Y, Qiu Z, Xu Y, et al. The role of fQRS in coronary artery disease. A meta‐analysis of observational studies. Herz 2015;40 (Suppl):8–15. [DOI] [PubMed] [Google Scholar]
- 21. Gong B, Li Z. Total mortality, major adverse cardiac events, and echocardiographic‐derived cardiac parameters with fragmented QRS complex. Ann Noninvasive Electrocardiol 2015. Nov 2 [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Stroup DF, Berlin JA, Morton SC, et al. Meta‐analysis of observational studies in epidemiology: a proposal for reporting. Meta‐analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA 2000;283(15):2008–2012. [DOI] [PubMed] [Google Scholar]
- 23. Thygesen K, Alpert JS, Jaffe AS, et al. Third universal definition of myocardial infarction. J Am Coll Cardiol 2012;60(16):1581–1598. [DOI] [PubMed] [Google Scholar]
- 24. Hayden JA, van der Windt DA, Cartwright JL, et al. Assessing bias in studies of prognostic factors. Ann Intern Med 2013;158(4):280–286. [DOI] [PubMed] [Google Scholar]
- 25. Uslu N, Gul M, Cakmak HA, et al. The assessment of relationship between fragmented QRS complex and left ventricular wall motion score index in patients with ST elevation myocardial infarction who underwent primary percutaneous coronary intervention. Ann Noninvasive Electrocardiol 2015;20:148–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Das MK, Michael MA, Suradi H, et al. Usefulness of fragmented QRS on a 12‐lead electrocardiogram in acute coronary syndrome for predicting mortality. Am J Cardiol 2009;104:1631–1637. [DOI] [PubMed] [Google Scholar]


