Abstract
Background
Hypertension entails atrial remodeling that affect P‐wave (PW) duration on electrocardiogram (ECG). PW indices (e.g., variance, dispersion, and terminal force) are associated with a higher risk for atrial fibrillation (AF), but their calculation requires multiple measurements of PW duration, limiting their use in clinical practice. We evaluated whether PW duration in specific ECG leads may identify patients with increased susceptibility to AF in a population of hypertensive patients.
Methods
In a case–control study, AF and control subjects were matched for age, sex, and left atrial (LA) dimensions. PW duration was measured from digitally stored ECGs. Logistic regression was used to assess the association of PW duration and indices with AF.
Results
We enrolled 44 hypertensive AF patients (16 paroxysmal and 28 persistent) and 44 hypertensive controls. AF and control subjects were matched for sex (males, n = 27), age (67 ± 8 years), LA diameter (40 ± 5 mm), and were comparable for left ventricular mass (45 ± 11 g/m2.7 vs 48 ± 12 g/m2.7, P = 0.19), ejection fraction (58 ± 7% in both groups), and prevalence of mild valvular heart disease (7% vs 5%; P = 0.64). PW duration in lead aVR was significantly higher in AF patients as compared with controls (115 ± 18 ms vs 101 ± 14 ms; P < 0.0001) and was the best independent predictor of AF in multivariable logistic regression (PW ≥ 100 ms: RR = 3.7; 95% CI: 1.3–10.3; P = 0.02).
Conclusions
Simple measurement of PW duration in lead aVR allows effective identification of AF patients in a population of hypertensives. Confirmation of this finding in a larger population would provide a simple and effective risk marker of AF in hypertensive patients.
Keywords: atrial fibrillation, P wave, hypertension, aVR lead
Atrial fibrillation (AF) is the most common arrhythmia in clinical practice and the number of patients affected is increasing, along with ageing of the population.1 Arterial hypertension appears to be responsible for AF more than any other risk factor.2 Indeed, animal studies demonstrated that long standing hypertension induces atrial structural and electrical remodeling (i.e., shortening of refractory periods, slowing of conduction velocity, and ectopic firings) that increase the susceptibility for AF.3, 4 Of interest, atrial remodeling results in slowing of atrial conduction that can be easily detected as P‐wave (PW) prolongation on surface electrocardiogram (ECG).5
PW indices such as duration, variance, dispersion, and terminal force are considered a reliable noninvasive marker of atrial conduction.6 Indeed, PW prolongation has been reported as a predictor of AF after bypass surgery and is associated with a higher risk of progression from paroxysmal to persistent AF.7, 8 Moreover, PW dispersion (PWD) (i.e., the difference between the maximum and the minimum PW duration on a standard 12‐lead ECG) and PW variance (i.e., the square of the standard deviation [SD)] of all PW durations) have been shown to identify patients with a higher risk of AF among subjects with clinical risk factors and structural heart disease. 6, 9, 10 However, PW‐derived indices require multiple measurements of PW duration, limiting their use in clinical practice.
We sought to evaluate whether simple measurement of PW duration in specific ECG leads may identify patients with increased susceptibility to AF in a population of hypertensives.
METHODS
Study Population
Case (AF) and control (no‐AF) hypertensive patients were consecutively recruited among those admitted to the adult inpatient or outpatient services of the Hypertension Unit at St. Andrea Hospital in Rome, Italy. The case group included patients with hypertension and at least two episodes of AF documented with standard or Holter ECG. Exclusion criteria were age <18 or >85 years, hyperthyroidism, electrolyte imbalance, severe chronic kidney dysfunction (eGFR < 30 mL/min by the Cockroft–Gault formula), left ventricular (LV), ejection fraction (EF) <40%, and severe valvular heart disease. Patients with implanted pacemakers were also excluded.
Patients with history of systemic arterial hypertension referred to the same Institution but without history of AF served as control group. Hypertension was defined as values ≥140 mmHg for systolic blood pressure and/or ≥90 mmHg for diastolic blood pressure. 11 A 1:1 matching ratio was predefined. Matching criteria included gender, age (difference <5 years), and left atrial dimensions (difference ± 3 mm). A greedy matching algorithm was implemented so to select control patient closely matching case patient within patients’ pool.
The study protocol was approved by the local Ethic Committee, and all participants gave written informed consent.
Echocardiography
All participants underwent Doppler echocardiographic examination using an Acuson Sequoia C512 (Siemens Medical Solution, Mountain View, CA, USA) with a multifrequency transducer (2.5–4 MHz); images were performed using standardized acquisition methods. LV dimensions were measured at end‐diastole (recognized as the peak of the R wave of the ECG) and end‐systole, just below the mitral leaflets through the standard left parasternal window. LV EF was calculated according to Simpson method. Left atrial size was calculated as the anteroposterior diameter and measured as the distance from the leading edge of the posterior aortic wall to the leading edge of the posterior left atrial wall at end‐systole. LV mass was calculated and normalized by height2.7.12 LV hypertrophy was defined according to standard criteria.13
ECG Analysis
All study patients had to be in sinus rhythm on the day of examination. Antiarrhythmic drugs were suspended at least 2 days before ECG collection.
A 12‐lead surface ECG was obtained for all patients in the supine position using a Mortara Eli 350 ECG machine (Milwaukee, WI, USA). The 12‐lead ECG was recorded at a paper speed of 25 mm/s and 1 mV/cm standardization. All electrocardiograms were scanned at 600 dpi and PW parameters measured on magnified views on a high‐resolution computer screen by two investigators blinded to patient clinical information. Each investigator assessed all available ECG traces and final PW durations are given as the average values of the two measurements. PW interobserver coefficient of variation (SD of differences between two observations divided by the mean value and expressed as percentage) was found to be 2.65%. Vertical calipers were used to identify the beginning and end of the PWs. In order to exclude from the analysis PWs with ambiguous onset or offset, one PW per lead was measured for each subject based on technical quality. The onset of the PW was defined as the point of the first visible upward departure of the trace from the bottom of the baseline for the positive waves and as the point of first downward departure from the top of the baseline for negative waves. The return to the baseline of the bottom of the trace in positive waves and of the top of the trace in negative waves was considered the end of the PW. ECGs with measurable PWs in <9 out of 12 ECG leads were excluded from the study.
The following indices were derived from each ECG:
The maximum PW duration in any measurable leads (P maximum);
PWD, defined as the difference between the maximum PW duration and the minimum PW duration (PWD = Pmax – Pmin);
PW variance, defined as the square root of the SD of PW durations; and
PW‐terminal force in precordial lead V1. The force was obtained as the algebric product of the duration and amplitude in the negative terminal portion of the PW.
Statistical Analysis
Continuous variables are presented as mean ± SD of the mean. Categorical variables are presented as absolute counts and percentage. Groups were compared using Student's t‐test or Wilcoxon rank sum test for continuous and chi‐square or Fisher's exact test for categorical variables, as appropriate.
Correlation among variables was assessed by the Pearson method. Univariate logistic regression analysis was performed to explore the association between AF status and clinical as well as ECG variables (including PW measurements). A multivariable logistic regression analysis was performed to correct for relevant demographic and clinical characteristics.
Receiver operating characteristic (ROC) curves were constructed showing threshold values of PW variables that defined patients with AF. Sensitivity and specificity of different cutoff levels were calculated. The validity of the model was measured by means of the area under ROC curve. All tests were two‐sided, and a P value of less than 0.05 was considered statistically significant. All data analyses were performed with SPSS software package (version 21.0, SPSS, Inc., Chicago, IL, USA).
RESULTS
A total of 44 hypertensive patients with nonfamilial AF (16 paroxysmal and 28 persistent) and 44 matched hypertensive controls were recruited. Clinical and echocardiographic characteristics of patients with and without AF are reported in Table 1. AF and control patients were well matched with regard to age, gender and left atrial dimensions. Also, there were no significant differences regarding the prevalence of diabetes, hypercholesterolemia, pre‐existing mild LV dysfunction, and most relevant echocardiographic characteristics (Tables 1 and 2). As expected, AF patients were more commonly on anti‐arrhythmic drugs (Table 1). Two control patients with coronary artery disease and paroxysmal supraventricular tachycardia were on amiodarone.
Table 1.
Baseline Characteristics
| Variable | AF Group (n = 44) | Control Group (n = 44) | P Value |
|---|---|---|---|
| Demographic | |||
| Male sex, n (%) | 27 (61%) | 27 (61%) | Matched |
| Age (years) | 67.2 ± 8 | 67.2 ± 8 | Matched |
| Clinical | |||
| Diabetes, n (%) | 8 (18%) | 11 (25%) | 0.43 |
| Hypercholesterolemia, n (%) | 24 (54%) | 27 (61%) | 0.51 |
| BMI | 27.9 ± 4 | 27.6 ± 4 | 0.71 |
| Systolic BP (mmHg) | 124 ± 16 | 121 ± 16 | 0.42 |
| Diastolic BP (mmHg) | 73 ± 10 | 71 ± 8 | 0.37 |
| LV dysfunction, n (%) | 10 (23%) | 9 (20%) | 0.79 |
| Drug therapy | |||
| Beta‐blockers, n (%) | 17 (39%) | 25 (57%) | 0.09 |
| ACE‐inhibitors or ARB, n (%) | 29 (66%) | 36 (82%) | |
| Anti‐arrhythmic drugs, n (%) | 18 (41%) | 2 (4%) | |
| Flecainide | 2 (5%) | 0 | |
| Propafenone | 3 (7%) | 0 | 0.09 |
| Amiodarone | 11 (25%) | 2 (4%) | <0.001 |
| Dronedarone | 1 (2%) | 0 | |
| Sotalol | 1 (2%) | 0 | |
Table 2.
Echocardiographic Characteristics
| Variable | AF Group (n = 44) | Control Group (n = 44) | P Value |
|---|---|---|---|
| LVEDD (mm) | 50 ± 6 | 50 ± 5 | 0.82 |
| LVESD (mm) | 34 ± 6 | 34 ± 6 | 0.72 |
| LV EF (%) | 58 ± 7 | 58 ± 7 | 0.58 |
| LV mass (g, 2.7) | 45 ± 11 | 48 ± 12 | 0.19 |
| Left atrium diameter (mm) | 40 ± 5 | 40 ± 5 | Matched |
| Valvular heart disease, n (%) | 3 (7%) | 2 (5%) | 0.64 |
PW duration in leads I, aVR, aVF, aVL, V1, V2, V4, and V6 was significantly longer in cases as compared to controls. Among derived parameters, only PW max was significantly higher in AF patients (Table 3). Treatment with amiodarone did not affect PW duration in any ECG leads or PW‐derived indexes (P value ranging from 0.37 to 0.96 for all comparisons).
Table 3.
PW Duration and Derived Indices in AF versus Control Patients with Hazard Ratios and 95% Confidence Intervals
| Univariate Analysis | Multivariate Analysis | ||||||
|---|---|---|---|---|---|---|---|
| AF Group | Control | Hazard | Hazard | ||||
| (n = 44) | Group (n = 44) | P Value | Ratio (95% CI) | P Value | Ratio (95% CI) | P Value | |
| P‐wave duration (ms) | |||||||
| I | 104 ± 19 | 93 ± 17 | 0.01 | 1.03 (1.00–1.06) | 0.01 | 1.03 (1.00–1.06) | 0.02 |
| II | 113 ± 23 | 109 ± 17 | 0.35 | 1.01 (0.98–1.03) | 0.35 | ||
| III | 101 ± 18 | 95 ± 17 | 0.17 | 1.02 (0.99–1.04) | 0.17 | ||
| aVR | 115 ± 18 | 101 ± 14 | <0.0001 | 1.05 (1.02–1.09) | 0.001 | 1.05 (1.02–1.10) | 0.001 |
| aVR > 100 ms; n (%) | 20 (53%) | 32 (80%) | 0.01 | 3.6 (1.32–9.81) | 0.01 | 3.75 (1.35–10.39) | 0.01 |
| aVL | 95 ± 25 | 81 ± 19 | 0.02 | 1.02 (1.00–1.05) | 0.02 | 1.03 (1.00–1.06) | 0.02 |
| aVF | 110 ± 22 | 101 ± 16 | 0.03 | 1.02 (1.00–1.05) | 0.04 | 1.02 (0.99–1.05) | 0.06 |
| V1 | 102 ± 19 | 92 ± 23 | 0.02 | 1.02 (1.00–1.04) | 0.03 | 1.03 (1.00–1.05) | 0.01 |
| V2 | 98 ± 21 | 86 ± 21 | 0.01 | 1.03 (1.00–1.05) | 0.02 | 1.03 (1.00–1.05) | 0.02 |
| V3 | 103 ± 18 | 98 ± 19 | 0.17 | 1.02 (0.99–1.04) | 0.17 | ||
| V4 | 110 ± 20 | 100 ± 21 | 0.03 | 1.02 (1.00–1.04) | 0.03 | 1.02 (1.00–1.04) | 0.04 |
| V5 | 108 ± 23 | 102 ± 18 | 0.18 | 1.01 (0.99–1.03) | 0.18 | ||
| V6 | 110 ± 22 | 101 ± 15 | 0.03 | 1.02 (1.00–1.05) | 0.04 | 1.03 (1.00–1.05) | 0.02 |
| Derived indices | |||||||
| Min P wave duration. (ms) | 79 ± 15 | 72 ± 19 | 0.09 | 1.02 (0.99–1.04) | 0.10 | ||
| Max P wave duration. (ms) | 129 ± 18 | 117 ± 14 | 0.001 | 1.04 (1.01–1.08) | 0.003 | 1.05 (1.01–1.08) | 0.002 |
| P wave variance (ms) | 299 ± 233 | 230 ± 192 | 0.13 | 1.00 (0.99–1.00) | 0.14 | ||
| P wave dispersion (ms) | 50 ± 20 | 45 ± 20 | 0.22 | 1.01 (0.99–1.03) | 0.22 | ||
| P wave terminal force (ms) | 3.6 ± 4.3 | 3.2 ± 3.1 | 0.61 | 1.03 (0.91–1.15) | 0.61 | ||
In ROC analysis, PW duration in lead aVR showed the greatest AUC (0.79; P = 0.002) (Figure 1). Of note, PW duration in aVR and PWD were well correlated in AF patients (r = 0.52; P < 0.001) but not in control subjects (r = 0.18; P = 0.27) (Figure 2).
Figure 1.
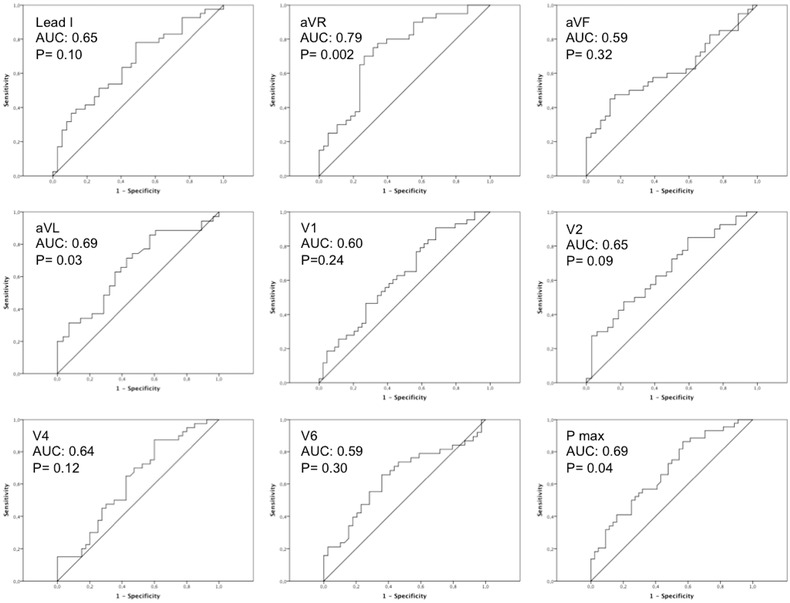
Receiver operating characteristic curves displaying the relationship between sensitivity and 1‐specificity across all possible threshold values of PW duration in individual leads that define patients with AF. P‐wave duration in lead aVR displays the largest AUC (0.79; P = 0.002).
Figure 2.
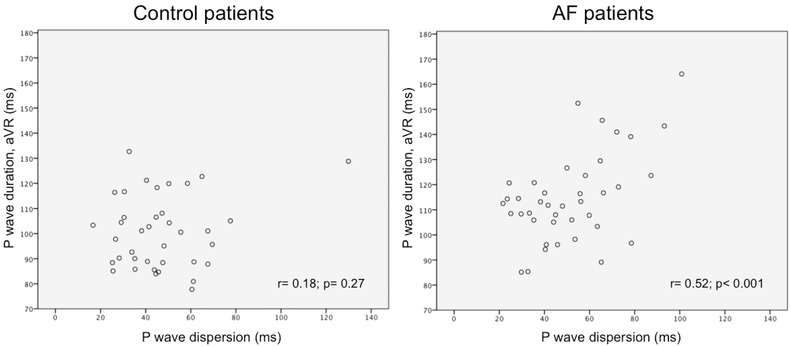
Correlation between P wave (PW) duration in lead aVR and PW dispersion in the study population. PW duration in aVR and PPW dispersion are well correlated in AF (r = 0.52; P < 0.001) but not in control patients (r = 0.18; P = 0.27).
PW duration in leads I, aVR, aVL, V1, V2, V4, V6, and maximum PW duration were associated with AF in univariate logistic regression analysis and retained statistical significance even after correction for LV mass and valvular heart disease (Table 3). When considered as a dichotomous variable (< vs ≥ 100 ms), PW duration in aVR was strongly and independently associated with AF (RR: 3.7; 95% CI: 1.3–10.3; P = 0.01) (Table 3).
PW duration in lead aVR was comparable among patients with paroxysmal and persistent AF (115 ± 18 ms vs 114 ± 18 ms; P = 0.88).
DISCUSSION
Our study does provide evidence that PW duration in lead aVR > 100 ms in patients with systemic arterial hypertension is strongly associated with susceptibility to AF even after adjusting for major cardiovascular disease and risk factors.
Hypertension is the most common coexisting cardiovascular disease in patients who have AF.14 A large body of data suggests that cardiovascular adaptive changes to the chronically elevated afterload play a major role in increasing susceptibility to AF.14, 15, 16 Accordingly, epidemiology of AF closely resembles that of systemic arterial hypertension.17 Despite progresses in pharmacological and nonpharmacological treatment of AF, long‐term results are suboptimal in terms of recurrence rates and thromboembolic complications. Indeed, identification of hypertensive patients at higher risk for AF would be critical to develop tailored therapies. In this view, an optimal screening tool to stratify patients at increased risk for AF should be feasible, inexpensive, and noninvasive to be of any clinical utility.
ECG is of routine use in patients with hypertension. Indeed, in view of its large accessibility, high reproducibility, simple interpretation, and cost‐effectiveness, the most recent sets of European guidelines11 stated that standard ECG must be performed to each and every hypertensive patients during screening and follow‐up to evaluate the presence of signs of LV hypertrophy and cardiac arrhythmias.
Our study investigated whether a simplified analysis of the PW may identify hypertensive patients at high risk for AF using a case–control approach. The heterogeneity of structural and electrophysiological properties of atrial myocardium is thought to play a major role in the occurrence and maintenance of AF because of the increased likelihood of focal firing, shortened refractoriness and delayed conduction.18 On the other hand, PW abnormalities as detected from standard 12‐lead ECG have been thought to reflect left atrial enlargement and altered conduction.19, 20
Several PW duration and morphology indexes (e.g., dispersion, variance, and terminal force), have been described as predictors of AF or transition from paroxysmal to persistent AF.8, 10, 21, 22, 23, 24 However, the need for multiple PW measurements and complex analysis limits their use in clinical practice. In our study, we found that PW duration in lead aVR in standard 12‐lead ECG is a simple and easily applicable method to identify AF patients among hypertensive patients. PW variance and dispersion were also higher in AF as compared to control patients. However, unlike previous studies, this difference did not reach statistical significance. Strict matching for age, sex, LA dimensions and correction for LV mass and valvular heart disease may explain the differences between this and previous reports.
PW prolongation is generally accepted as the most reliable noninvasive marker of atrial conduction and it has been associated with a history AF.21, 25 However, several studies failed to demonstrate significant PW prolongation in patients with AF,26, 27 and shorter PW duration has been associated with lone AF, 28 suggesting that locally delayed intra‐ and interatrial conduction rather than global conduction slowing may occur. Accordingly, Josephson et al.29 first suggested that biphasic PWs in the right precordial leads may represent an interatrial conduction defect not necessarily associated to left atrial enlargement.
Interatrial conduction time is associated with increased incidence of AF in patients without history of AF.30 The Bachmann's bundle is the most common interatrial conductive route, and is therefore involved in interatrial conduction impairment.31, 32 Moreover, as assessed with noncontact mapping,33 the depolarization breakthrough to the left atrium may also involve the posterior–inferior region of the atrial septum in up to one‐third of patients. Indeed, Becker et al. identified extensive fibro‐fatty replacement of Buchmann's bundle in patients with history of AF.34 Fibrosis may, therefore, slow the conduction over the Bachmann's bundle and the other interatrial pathways, thus resulting in focal rather than global slowing of atrial conduction and entailing PW enlargement in selected ECG leads. Although the augmented limb leads were developed to derive more localized information than the bipolar leads, lead aVR has been largely ignored in clinical practice. Indeed, the purpose of lead aVR was to obtain specific information from the right upper side of the heart.35 In this view, PW prolongation in aVR may identify an early intra‐ and interatrial conduction defect that is associated with AF in hypertensive patients.
Study Limitations
First, the small number of patients and the case–control design of the study are the major limitations of the study so that we can not exclude the presence of unexpected bias. Our findings will serve as the basis to conduct a large, prospective, and epidemiologic study. Second, the long elimination half‐life of amiodarone did not allow a complete wash‐out of the drug. As amiodarone has been reported both to shorten PWD 36 and to have a neutral effect on PW measurements,37 the relevance of this limitation is undefined at present. However, treatment with amiodarone affect neither PW duration nor PW‐derived indexes in our study population.
CONCLUSIONS
We here report that simple measurement of PW duration in lead aVR allows effective identification of AF patients in a population of hypertensive patients independently from cardiovascular risk factors and LA dimensions. Confirmation of this finding in a larger population would provide a simple and effective risk marker of AF susceptibility in hypertensive patients.
REFERENCES
- 1. Go AS, Hylek EM, Phillips KA, et al. Prevalence of diagnosed atrial fibrillation in adults: National implications for rhythm management and stroke prevention: The AnTicoagulation and Risk Factors in Atrial Fibrillation (ATRIA) Study. J Am Med Assoc 2001;285:2370–2375. [DOI] [PubMed] [Google Scholar]
- 2. Andrade J, Khairy P, Dobrev D, et al. The clinical profile and pathophysiology of atrial fibrillation: Relationships among clinical features, epidemiology, and mechanisms. Circ Res 2014;114:1453–1468. [DOI] [PubMed] [Google Scholar]
- 3. Choisy SC, Arberry LA, Hancox JC, et al. Increased susceptibility to atrial tachyarrhythmia in spontaneously hypertensive rat hearts. Hypertension 2007;49:498–505. [DOI] [PubMed] [Google Scholar]
- 4. Nattel S, Burstein B, Dobrev D. Atrial remodeling and atrial fibrillation: Mechanisms and implications. Circ Arrhythm Electrophysiol 2008;1:62–73. [DOI] [PubMed] [Google Scholar]
- 5. Lau YF, Yiu KH, Siu CW, et al. Hypertension and atrial fibrillation: Epidemiology, pathophysiology and therapeutic implications. J Hum Hypertens 2012;26:563–569. [DOI] [PubMed] [Google Scholar]
- 6. Magnani JW, Williamson MA, Ellinor PT, et al. P wave indices: Current status and future directions in epidemiology, clinical, and research applications. Circ Arrhythm Electrophysiol 2009;2:72–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Steinberg JS, Zelenkofske S, Wong SC, et al. Value of the P‐wave signal‐averaged ECG for predicting atrial fibrillation after cardiac surgery. Circulation 1993;88:2618–2622. [DOI] [PubMed] [Google Scholar]
- 8. Abe Y, Fukunami M, Yamada T, et al. Prediction of transition to chronic atrial fibrillation in patients with paroxysmal atrial fibrillation by signal‐averaged electrocardiography: A prospective study. Circulation 1997;96:2612–2616. [DOI] [PubMed] [Google Scholar]
- 9. Koide Y, Yotsukura M, H Ando, et al. Usefulness of P‐wave dispersion in standard twelve‐lead electrocardiography to predict transition from paroxysmal to persistent atrial fibrillation. Am J Cardiol 2008;102:573–577. [DOI] [PubMed] [Google Scholar]
- 10. Andrikopoulos GK, Dilaveris PE, Richter DJ, et al. Increased variance of P wave duration on the electrocardiogram distinguishes patients with idiopathic paroxysmal atrial fibrillation. Pacing Clin Electrophysiol 2000;23:1127–1132. [DOI] [PubMed] [Google Scholar]
- 11. Mancia G, Fagard R, Narkiewicz K, et al. 2013 Practice guidelines for the management of arterial hypertension of the European Society of Hypertension (ESH) and the European Society of Cardiology (ESC): ESH/ESC Task Force for the Management of Arterial Hypertension. J Hypertens 2013;31:1925–1938. [DOI] [PubMed] [Google Scholar]
- 12. de Simone G, Daniels SR, Devereux RB, et al. Left ventricular mass and body size in normotensive children and adults: Assessment of allometric relations and impact of overweight. J Am Coll Cardiol 1992;20:1251–1260. [DOI] [PubMed] [Google Scholar]
- 13. Verdecchia P, Carini G, Circo A, et al. Left ventricular mass and cardiovascular morbidity in essential hypertension: The MAVI study. J Am Coll Cardiol 2001;38:1829–1835. [DOI] [PubMed] [Google Scholar]
- 14. Nabauer M, Gerth A, Limbourg T, et al. The Registry of the German Competence Network on Atrial Fibrillation: Patient characteristics and initial management. Europace 2009;11:423–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Tsang TS, Gersh BJ, Appleton CP, et al. Left ventricular diastolic dysfunction as a predictor of the first diagnosed nonvalvular atrial fibrillation in 840 elderly men and women. J Am Coll Cardiol 2002;40:1636–1644. [DOI] [PubMed] [Google Scholar]
- 16. Schotten U, Verheule S, Kirchhof P, et al. Pathophysiological mechanisms of atrial fibrillation: A translational appraisal. Physiol Rev 2011;91:265–325. [DOI] [PubMed] [Google Scholar]
- 17. Benjamin EJ, Levy D, Vaziri SM, et al. Independent risk factors for atrial fibrillation in a population‐based cohort. The Framingham Heart Study. J Am Med Assoc 1994;271:840–844. [PubMed] [Google Scholar]
- 18. Heijman J, Voigt N, Nattel S, et al. Cellular and molecular electrophysiology of atrial fibrillation initiation, maintenance, and progression. Circ Res 2014;114:1483–1499. [DOI] [PubMed] [Google Scholar]
- 19. Platonov PG. Atrial conduction and atrial fibrillation: What can we learn from surface ECG? Cardiol J 2008;15:402–407. [PubMed] [Google Scholar]
- 20. Bayes de Luna A, Platonov P, Cosio FG, et al. Interatrial blocks. A separate entity from left atrial enlargement: A consensus report. J Electrocardiol 2012;45:445–451. [DOI] [PubMed] [Google Scholar]
- 21. Magnani JW, Johnson VM, Sullivan LM, et al. P wave duration and risk of longitudinal atrial fibrillation in persons >/ = 60 years old (from the Framingham Heart Study). Am J Cardiol 2011;107:917–921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Dilaveris PE, Gialafos EJ, Chrissos D, et al. Detection of hypertensive patients at risk for paroxysmal atrial fibrillation during sinus rhythm by computer‐assisted P wave analysis. J Hypertens 1999;17:1463–1470. [DOI] [PubMed] [Google Scholar]
- 23. Tsao CW, Josephson ME, Hauser TH, et al. Accuracy of electrocardiographic criteria for atrial enlargement: Validation with cardiovascular magnetic resonance. J Cardiovasc Magn Reson 2008;10:7–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Blanche C, Tran N, Carballo D, et al. Usefulness of P‐wave signal averaging to predict atrial fibrillation recurrences after electrical cardioversion. Ann Noninvase Electrocardiol 2014;19:266–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Gonna H, Gallagher MM, Guo XH, et al. P‐wave abnormality predicts recurrence of atrial fibrillation after electrical cardioversion: A prospective study. Ann Noninvasive Electrocardiol 2014;19:57–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Nemirovsky D, Hutter R, Gomes JA. The electrical substrate of vagal atrial fibrillation as assessed by the signal‐averaged electrocardiogram of the P wave. Pacing Clin Electrophysiol 2008;31:308–313. [DOI] [PubMed] [Google Scholar]
- 27. Jurkko R, Vaananen H, Mantynen V, et al. High‐resolution signal‐averaged analysis of atrial electromagnetic characteristics in patients with paroxysmal lone atrial fibrillation. Ann Noninvasive Electrocardiol 2008;13:378–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Chang IC, Austin E, Krishnan B, et al. Shorter minimum P‐wave duration is associated with paroxysmal lone atrial fibrillation. J Electrocardiol 2014;47:106–112. [DOI] [PubMed] [Google Scholar]
- 29. Josephson ME, Kastor JA, Morganroth J. Electrocardiographic left atrial enlargement. Electrophysiologic, echocardiographic and hemodynamic correlates. Am J Cardiol 1977;39:967–971. [DOI] [PubMed] [Google Scholar]
- 30. Deftereos S, Kossyvakis C, Efremidis M, et al. Interatrial conduction time and incident atrial fibrillation: A prospective cohort study. Heart Rhythm 2014;11(7):1095–1101. [DOI] [PubMed] [Google Scholar]
- 31. Platonov PG. P‐wave morphology: Underlying mechanisms and clinical implications. Ann Noninvasive Electrocardiol 2012;17:161–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Holmqvist F, Husser D, Tapanainen JM, et al. Interatrial conduction can be accurately determined using standard 12‐lead electrocardiography: Validation of P‐wave morphology using electroanatomic mapping in man. Heart Rhythm 2008;5:413–418. [DOI] [PubMed] [Google Scholar]
- 33. Betts TR, Roberts PR, et al. High‐density mapping of left atrial endocardial activation during sinus rhythm and coronary sinus pacing in patients with paroxysmal atrial fibrillation. J Cardiovasc Electrophysiol 2004;15:1111–1117. [DOI] [PubMed] [Google Scholar]
- 34. Becker AE. How structurally normal are human atria in patients with atrial fibrillation? Heart Rhythm 2004;1:627–631. [DOI] [PubMed] [Google Scholar]
- 35. George A, Arumugham PS, Figueredo VM. aVR—The forgotten lead. Exp Clin Cardiol 2010;15:e36–e44. [PMC free article] [PubMed] [Google Scholar]
- 36. Boriani G, Diemberger I, Biffi M, et al. P wave dispersion and short‐term vs. late atrial fibrillation recurrences after cardioversion. Int J Cardiol 2005;101:355–361. [DOI] [PubMed] [Google Scholar]
- 37. Kalus JS, Kluger J, Caron MF, et al. An evaluation of postoperative P‐wave variables after cardiothoracic surgery. J Electrocardiol 2004;37:127–132. [DOI] [PubMed] [Google Scholar]


