Abstract
Introduction
Eculizumab has transformed outcomes for patients with atypical hemolytic uremic syndrome (aHUS). Its efficacy and safety profile was well characterized in the clinical trial program. The long-term safety profile was not previously assessed or compared against nontreated patients in an observational registry setting.
Methods
The Global aHUS Registry recruits patients with clinical diagnoses of aHUS. This analysis includes baseline characteristics and targeted safety events from adult and pediatric patients who were “ever treated” versus “never treated” with eculizumab in the first 5 years of the registry, through January 26, 2017.
Results
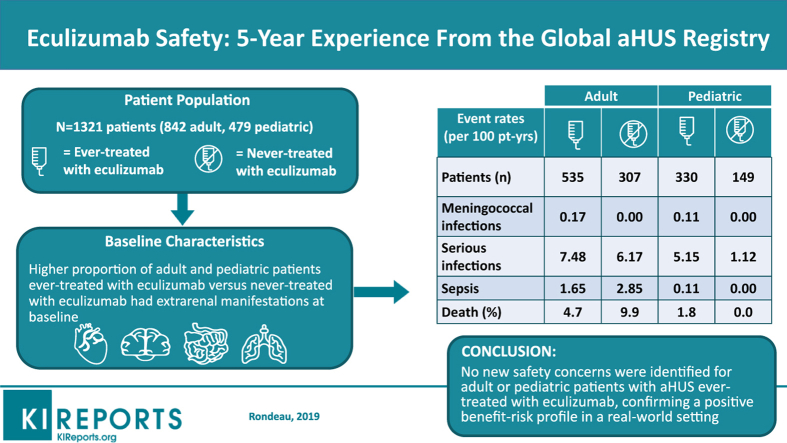
Overall, 1321 patients (adult, n = 842; pediatric, n = 479; ever treated, n = 865; never treated, n = 456) were enrolled. A higher proportion of ever-treated versus never-treated adult and pediatric patients had renal, cardiovascular, pulmonary, central nervous system, gastrointestinal symptoms, and hepatic impairment. No differences in safety event rates between ever-treated and never-treated patients were observed, except serious infections in pediatric patients (5.15 versus 1.12 events/100 patient-years for ever- and never-treated patients, respectively). Deaths were more frequent in adult (4.7% and 9.9% of ever- and never-treated patients) compared with pediatric patients (1.8% of ever-treated patients; no deaths in never-treated patients).Three meningococcal infections were reported in ever-treated patients; 1 infection led to a fatal outcome.
Conclusion
In this large observational dataset covering 5 years of registry enrollment, no new safety concerns were identified for adult or pediatric eculizumab-treated patients with aHUS, confirming a positive benefit−risk profile in a real-world setting.
Keywords: atypical hemolytic uremic syndrome, complement, thrombotic microangiopathy, safety
Graphical abstract
aHUS is a rare form of thrombotic microangiopathy (TMA) characterized by thrombocytopenia, hemolytic anemia, acute kidney injury, and/or other end-organ damage that occurs as a result of the dysregulation of the alternative complement pathway.1 In this clinical setting, uncontrolled activation of the alternative complement system results in inflammation, endothelial injury, platelet activation and aggregation, leukocyte recruitment, and procoagulative signaling.2, 3, 4 Without treatment, aHUS may progress to end-stage renal disease at onset in up to 46% of patients, and mortality may occur in up to 7% after 1 year.5 New-onset and later manifestations of TMA are also commonly associated with extrarenal organ damage.5, 6, 7, 8
Eculizumab (Soliris; Alexion Pharmaceuticals, Inc., Boston, MA) is a monoclonal antibody that inhibits cleavage of C5 to C5a and C5b-9 and prevents terminal complement activation.9, 10, 11 It is the only medicinal agent approved in 49 countries worldwide, including by the US Food and Drug Administration9 and European Medicines Agency,10 for the treatment of aHUS. Previous studies in the aHUS clinical program have demonstrated the efficacy and safety of eculizumab in both adult and pediatric patients diagnosed with aHUS.4, 12, 13, 14 However, the long-term safety profile of eculizumab has not been characterized or compared against nontreated patients in a large patient population in a real-world setting.
For rare diseases such as aHUS, a global registry with multiple study sites worldwide provides an opportunity to gather a large patient dataset and to gain further insight into clinical characterization, genetic correlations, and optimal therapeutic management.15 Here, an analysis of the eculizumab safety profile was performed using the Global aHUS Registry database. Specifically, baseline characteristics and targeted adverse events from adult and pediatric patients with aHUS who were “ever treated” versus “never treated” with eculizumab are reported.
Methods
The Global aHUS Registry15 (US National Institutes of Health, www.ClinicalTrials.gov Identifier NCT01522183) is an observational, noninterventional, multicenter, global study initiated in April 2012 to retrospectively and prospectively collect data on the natural history of aHUS in all eligible patients, as well as to evaluate long-term safety and effectiveness of eculizumab. The registry was designed to (i) record the natural history of the disease, regardless of disease management; and (ii) fulfill postmarketing regulatory requirements by providing long-term follow-up on the aHUS indication for eculizumab. The protocol was approved by an institutional review board or independent ethics committee at each participating center. All patients or their parents/guardians provided written informed consent prior to study entry.
Methodology for enrollment has been reported previously.15 In brief, clinicians were encouraged to enroll patients with a preexisting clinical diagnosis of aHUS (diagnosis of aHUS was not performed as part of the registry protocol).
Patients were not required to have a history of eculizumab therapy or a specific identified genetic or autoimmune complement abnormality. Patients with evidence of Shiga toxin-producing Escherichia coli infection and those with ADAMTS13 activity ≤5% (if performed) were excluded, consistent with previous clinical trials of eculizumab in aHUS.12
Medical records were used to collect disease history information, which was then maintained in a Web-based secure electronic data collection system. The following information was included in reports gathered during enrollment and every 6 months thereafter: patient demographics, medical and disease history, symptomatology, appropriate laboratory results, clinical and patient-reported outcomes, and safety of eculizumab.
Patients in the ever-treated group received ≥1 dose of eculizumab prior to, during, or after enrollment; patients who received eculizumab must have been vaccinated for meningococcal disease at least 2 weeks prior to receiving the first dose of eculizumab.9, 10 Patients in the never-treated group had no history of eculizumab use at any time. Reasons for not receiving eculizumab were not known, as therapy was determined by the treating physician. For ever-treated patients, baseline was defined as the date of eculizumab initiation, whereas for never-treated patients, baseline was defined as the date of registry enrollment. Targeted safety events were reported at baseline and follow-up visits with a focus on meningococcal infections, serious infections, sepsis, malignancy, hepatic impairment, infusion reactions, and death. Neither sepsis nor renal symptoms were defined specifically for adverse event reporting purposes; however, these events were reported when identified by the clinician. Serious infections were not necessarily classified as serious adverse events according to clinical trial regulations, and did not necessarily result in death, life-threatening adverse event, inpatient hospitalization or prolongation of existing hospitalization, persistent or significant incapacity or substantial disruption of the ability to conduct normal life functions, or a congenital anomaly/birth defect. Serious infections included, but were not limited to, Aspergillus infections and infections due to encapsulated bacteria such as Neisseria meningitidis, Neisseria gonorrhoeae, Streptococcus pneumoniae, and Haemophilus influenzae. Although considered a serious infection, meningococcal infection was analyzed separately because of its significance with regard to the mechanism of action of eculizumab.
All statistical analyses were performed using SAS statistical software (version 9.2; SAS Institute, Cary, NC).15 The data cutoff for the analysis was January 26, 2017. Event rates were calculated as the number of safety events that were reported since baseline divided by the cumulative duration of follow-up for all patients (expressed in patient-years).
Results
Patients
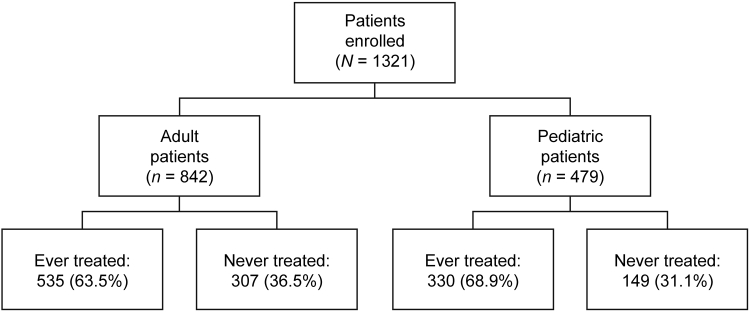
A total of 1321 patients from the registry were included in the analysis by the data cutoff date. Upon stratification, there were 842 (63.7%) adult and 479 (36.3%) pediatric patients (Figure 1). Of the adult patients, 535 (63.5%) were ever treated and 307 (36.5%) were never treated with eculizumab. There were 330 (68.9%) and 149 (31.1%) pediatric patients in the ever-treated and never-treated groups, respectively. The mean (SD) duration of eculizumab treatment (whether started before or after enrollment in the registry) was 2.17 (1.56) years for adult and 2.72 (1.74) for pediatric ever-treated patients, respectively.
Figure 1.
Patient population. Patient enrollment by age at baselinea and treatment with eculizumab. aPediatric patients were <18 years of age at baseline.
Median (range) age at baseline was 38.1 years (range, 18–80) and 41.9 years (range, 18–88) in ever-treated and never-treated adult patients, respectively, and was 5.9 years (range, 0–17.9) and 9.8 years (range, 0–17.9) in ever-treated and never-treated pediatric patients, respectively (Table 1). The median interval between diagnosis and eculizumab initiation (baseline) in ever-treated adult patients was 0.04 years (range, −0.08 to 36.9) and 0.04 years in ever-treated pediatric patients (range, −0.074 to 16.991), with median time from diagnosis to enrollment of 0.6 years (range, 0−37) in adult patients and 0.9 years (range, 0−20) in pediatric patients. In the never-treated group, the median time from diagnosis to enrollment was 2.2 years (range, 0−51) in adult patients and 4.3 years (range, 0−17) in pediatric patients. Fifty-one percent of never-treated and 28% of ever-treated patients were diagnosed with aHUS before establishment of the Global aHUS Registry in 2012. Approximately 11% of adult patients and 15% of pediatric patients had a family history of aHUS. The proportions of patients receiving dialysis (regardless of whether dialysis was acute [≤3 months] or chronic [>3 months]) and plasma exchange/plasma infusion prior to or at baseline did not differ between the 2 patient groups. However, other baseline characteristics suggest that ever-treated patients, particularly pediatric patients, had more severe disease at baseline than never-treated patients, as evidenced by a higher proportion of renal, cardiovascular, pulmonary, central nervous system, and gastrointestinal symptoms and hepatic impairment prior to or at baseline compared with patients who were never treated with eculizumab (Table 1).
Table 1.
Patient demographics and baselinea clinical characteristics
| Variable |
Adult patients (n = 842) |
Pediatric patientsb(n = 479) |
||
|---|---|---|---|---|
| Ever treated with eculizumab (n = 535) | Never treated with eculizumab (n = 307) | Ever treated with eculizumab (n = 330) | Never treated with eculizumab (n = 149) | |
| Age at baseline, median (range), yr | 38.1 (18−80) | 41.9 (18−88) | 5.9 (0−17.9) | 9.8 (0−17.9) |
| Female, n (%) | 347 (64.9) | 178 (58.0) | 140 (42.4) | 71 (47.7) |
| Any family history of aHUS, n (%) | 62 (11.6) | 34 (11.1) | 51 (15.5) | 23 (15.4) |
| Time from diagnosis to enrollment, median (range), yr | 0.6 (0−37) (n = 521) | 2.2 (0−51) (n = 298) | 0.9 (0−20) (n = 328) | 4.3 (0−17) (n = 149) |
| FACIT-Fatiguec score at baseline, median | 30.0 (n = 59) | 38.0 (n = 192) | 33.0 (n = 12) | 46.5 (n = 100) |
| Kidney transplant,d n (%) | 102 (19.1) | 82 (26.7) | 21 (6.4) | 24 (16.1) |
| Dialysis,d n (%) | 308 (57.6) | 186 (60.6) | 166 (50.3) | 65 (43.6) |
| Acute dialysis (≤3 mo)e | 124 (40.3) | 66 (35.5) | 109 (65.7) | 39 (60.0) |
| Chronic dialysis (>3 mo)e | 138 (44.8) | 83 (44.6) | 45 (27.1) | 23 (35.4) |
| PE/PI,d n (%) | 341 (63.7) | 188 (61.2) | 170 (51.5) | 74 (49.7) |
| Symptoms and impairments,d n (%) | ||||
| Thrombosis | 81 (15.1) | 54 (17.6) | 27 (8.2) | 4 (2.7) |
| Malignant hypertensionf | 18 (3.4) | 12 (3.9) | 6 (1.8) | 0 |
| Renal symptoms | 416 (77.8) | 88 (28.7) | 269 (81.5) | 20 (13.4) |
| Hepatic impairment | 7 (1.3) | 8 (2.6) | 5 (1.5) | 1 (0.7) |
| Cardiovascular symptoms | 144 (26.9) | 35 (11.4) | 101 (30.6) | 4 (2.7) |
| Pulmonary symptoms | 84 (15.7) | 29 (9.4) | 46 (13.9) | 0 |
| Central nervous system symptoms | 142 (26.5) | 34 (11.1) | 82 (24.8) | 2 (1.3) |
| Gastrointestinal symptoms | 173 (32.3) | 40 (13.0) | 130 (39.4) | 5 (3.4) |
aHUS, atypical hemolytic uremic syndrome; FACIT, Functional Assessment of Chronic Illness Therapy; PE/PI, plasma exchange/plasma infusion.
Baseline was defined as the date of eculizumab initiation for ever-treated patients and the date of enrollment for never-treated patients.
Pediatric patients were <18 yr of age at baseline.
The FACIT-Fatigue is a 13-item scale evaluating the intensity of fatigue and impact on daily life using a 5-point Likert-type scale.35, 36 The score ranges from 0 (maximum fatigue) to 52 (no fatigue).35 An increase in score of ≥3 on this instrument is considered clinically important.37
Medical history and/or prior to baseline.
For several patients, the end date of dialysis was missing, so categorization as acute or chronic was not possible.
Malignant hypertension was reported by the physician, but specific diagnostic criteria were not given.
Follow-up
In ever-treated adult patients, mean (SD) length of follow-up after the first infusion of eculizumab was 2.32 (1.60) and 1.35 (0.92) years, respectively, among those who initiated treatment before and after registry enrollment. In ever-treated pediatric patients, length of follow-up was 2.83 (1.77) and 1.72 (0.89) years, respectively.
During the follow-up period from baseline to data cutoff, 108 (20.4%) adult ever-treated and 21 (7.7%) never-treated patients had dialysis, 123 (23.3%) and 21 (7.7%) had transfusions, and 43 (8.1%) and 13 (4.8%) received a kidney transplant. Findings were similar for pediatric ever-treated compared with never-treated patients with respect to dialysis (44 [13.4%] vs. 1 [0.7%]), transfusions (80 [24.4%) vs. 3 [2.2%]), and kidney transplants (23 [7.0%] vs. 1 [0.7%]).
Targeted Safety Events
Meningococcal infections occurred in 2 adult patients (0.17 per 100 patient-years; 95% confidence interval [CI] = 0.02−0.63) and 1 pediatric patient (0.11 per 100 patient-years; 95% CI = 0.00−0.62) ever treated with eculizumab (Table 2). Details of the meningococcal infections are reported in Table 3. The meningococcal infection resolved without sequelae in 2 patients, whereas a fulminant meningococcemia led to a fatal outcome in 1 adult patient.
Table 2.
Targeted safety events from baselinea to last follow-up
| Variable |
Adult patients (n = 842) |
Pediatric patientsb(n = 479) |
||
|---|---|---|---|---|
| Ever treated with eculizumab (n = 535) | Never treated with eculizumab (n = 307) | Ever treated with eculizumab (n = 330) | Never treated with eculizumab (n = 149) | |
| Patients with evaluable data, n | 529 | 272 | 328 | 136 |
| Meningococcal infections | ||||
| Patients with event (n) | 2 | 0 | 1 | 0 |
| Events (n) | 2 | 0 | 1 | 0 |
| Events/100 patient-yrc | 0.17 | 0.00 | 0.11 | 0 |
| 95% CI | 0.02−0.43 | 0.00−0.71 | 0.00−0.62 | 0.00−1.12 |
| Serious infection | ||||
| Patients with event (n) | 46 | 14 | 32 | 2 |
| Events (n) | 86 | 26 | 46 | 3 |
| Events/100 patient-yrc | 7.48 | 6.17 | 5.15 | 1.12 |
| 95% CI | 5.98−9.24 | 4.03−9.04 | 3.77−6.87 | 0.23−3.28 |
| Sepsis | ||||
| Patients with event (n) | 14 | 8 | 1 | 0 |
| Events (n) | 19 | 12 | 1 | 0 |
| Events/100 patient-yrc | 1.65 | 2.85 | 0.11 | 0.00 |
| 95% CI | 0.99−2.58 | 1.47−4.98 | 0.00−0.62 | 0.00−1.12 |
| Malignancy | ||||
| Patients with event (n) | 3 | 5 | 1 | 0 |
| Events (n) | 4 | 6 | 1 | 0 |
| Events/100 patient-yrc | 0.35 | 1.42 | 0.11 | 0.00 |
| 95% CI | 0.09−0.89 | 0.52−3.10 | 0.00−0.62 | 0.00−1.12 |
| Hepatic impairment | ||||
| Patients with event (n) | 6 | 1 | 2 | 0 |
| Events (n) | 7 | 1 | 2 | 0 |
| Events/100 patient-yrc | 0.61 | 0.24 | 0.22 | 0.00 |
| 95% CI | 0.24−1.25 | 0.01−1.32 | 0.03−0.81 | 0.00−1.12 |
| Infusion reaction | ||||
| Patients with event (n) | 4 | 0 | 5 | 0 |
| Events (n) | 6 | 0 | 7 | 0 |
| Events/100 patient-yrc | 0.52 | 0.00 | 0.78 | 0.00 |
| 95% CI | 0.19−1.14 | 0.00−0.71 | 0.32−1.62 | 0.00−1.12 |
| Deaths, n (%) | 25 (4.7) | 27 (9.9) | 6 (1.8) | 0 |
CI, confidence interval.
Baseline was defined as the date of eculizumab initiation for ever-treated patients and the date of enrollment for never-treated patients.
Pediatric patients were <18 yr of age at baseline.
Rates were calculated as (the number of events since baseline divided by the sum [for all patients] of the duration from baseline to the last follow-up date [expressed in patient-yr]).
Table 3.
Meningococcal infections
| Patient | Age group | Description |
|---|---|---|
| 1 | Pediatrica |
|
| 2 | Adultb |
|
| 3 | Adultc |
|
Similar rates of sepsis, malignancy, and hepatic impairment were reported from baseline to the date of last follow-up among patients who were ever treated and never treated with eculizumab (Table 2). Four infusion reactions were reported in adult patients and 5 in pediatric patients. A higher proportion of pediatric patients in the ever-treated group reported serious infections (32 patients with 46 events; 5.15 events per 100 patient-years; 95% CI = 3.77−6.87) compared with the never-treated group (2 patients with 3 events; 1.12 events per 100 patient-years; 95% CI = 0.23−3.28). Serious infections during eculizumab treatment in pediatric patients included 17 unspecified, 12 viral, 3 infections in central lines/catheters, 2 pneumonia, 2 pyelonephritis, and 1 each of influenza, Clostridium difficile, otitis media, tonsillitis, and upper respiratory tract infection. Eight pediatric ever-treated patients had infections while off eculizumab treatment. Eighteen (56%) and 7 (22%) pediatric ever-treated patients with serious infections required dialysis or had renal transplants, respectively. Infections reported in never-treated patients were pyrexia and viral infection (n = 1) and possible line infection (n = 1). Both patients had received dialysis and had renal transplants. Serious infections were also reported more frequently in ever-treated compared with never-treated adult patients (7.48 [95% CI = 5.98−9.24] versus 6.17 [95% CI = 4.03−9.04] per 100 patient-years).
Although death was reported more frequently in never-treated (9.9%) compared with ever-treated (4.7%) adult patients (Table 2), causes of death in both subgroups included sepsis/infections, cardiovascular disease, and cancer (Table 4 and Supplementary Tables S1 and S2). In ever-treated adult patients, 1 patient died from fulminant meningococcemia and 1 patient died from a ruptured cerebral aneurysm related to Aspergillus infection (Table 4). In all, 12 of the 25 (48.0%) ever-treated adult patients were on eculizumab at the time of death. Six of 25 deaths (24.0%) were reported in adult patients who had discontinued eculizumab for >2 months, and the remaining 7 had discontinued eculizumab <2 months.
Table 4.
Causes of death
| Cause, n (%) |
Adult patients (n = 842) |
Pediatric patientsa(n = 479) |
||
|---|---|---|---|---|
| Ever treated with eculizumab (n = 535) | Never treated with eculizumab (n = 307) | Ever treated with eculizumab (n = 330) | Never treated with eculizumab (n = 149) | |
| Patients with evaluable data, n | 529 | 272 | 328 | 136 |
| Total deaths | 25 (4.7) | 27 (9.9) | 6 (1.8) | 0 |
| Deaths by cause | ||||
| Infection | 8 (1.5)b | 7 (2.6) | 2 (0.6) | 0 |
| Cancer | 6 (1.1)c | 5 (1.8)d | 1 (0.3) | 0 |
| Unknown/other | 5 (0.9) | 1 (0.4) | 1 (0.3) | 0 |
| Cardiovascular event | 4 (0.8) | 5 (1.8) | 0 | 0 |
| aHUS | 1 (0.2)e | 6 (2.2) | 1 (0.3) | 0 |
| Gastrointestinal event | 1 (0.2) | 2 (0.7) | 1 (0.3) | 0 |
| Medication-related event | 0 | 1 (0.4)f | 0 | 0 |
aHUS, atypical hemolytic uremic syndrome; TMA, thrombotic microangiopathy.
Pediatric patients were <18 yr of age at baseline.
Included 1 patient who died as a result of fulminant meningococcemia and 1 patient who died of a ruptured cerebral aneurysm related to Aspergillus infection.
Two patients had acute myeloid leukemia. One patient each had pancreatic cancer, cervical cancer, glioblastoma, and hepatocellular carcinoma with malignant neoplasm.
One patient each had anal cancer, breast carcinoma, leukemia, liver metastases, and prostate cancer.
Patient was on chronic dialysis, received 1 dose of eculizumab subsequent to a TMA manifestation, and died the next day with serious, severe acute respiratory failure and acute cardiac failure.
Patient died of respiratory failure secondary to lung injury associated with bleomycin.
In the pediatric population, death was reported in 6 (1.8%) ever-treated patients (Table 2). Causes of death are presented in Tables 4 and 5. At the time of death, 4 of 6 pediatric ever-treated patients (66.7%) were on eculizumab. No deaths were reported in never-treated pediatric patients (Table 2).
Table 5.
Deaths in pediatric patients ever treated with eculizumab
| Patient agea (yr) | Cause of death | Additional details |
|---|---|---|
| <1 | Infection |
|
| 1 | Infection |
|
| 2 | Unknown |
|
| 2 | Gastrointestinal event |
|
| 13 | Cancer |
|
| 17 | aHUS |
|
aHUS, atypical hemolytic uremic syndrome.
Patient age at enrollment in the registry.
Discussion
The Global aHUS Registry provides a substantial observational dataset with a large patient population and represents a longitudinal analysis of patients at different stages of disease and treatment status/duration, with varying degrees of severity and comorbidities. The results of this analysis including 5 years of enrollment in the registry did not identify new safety concerns in pediatric and adult patients who were treated with eculizumab. Overall, the safety profile demonstrated in the clinical trial program4, 12, 13, 14 was confirmed with this registry analysis in a larger patient population with longer follow-up.
In the current study, the most frequently reported safety events in all patients with aHUS, regardless of eculizumab treatment status, were serious infections and sepsis. Serious infections occurred at a higher rate in the ever-treated patient group in the pediatric population. However, it should be emphasized that “ever treated” included any patient who ever received a single dose of eculizumab; the analysis was not designed to determine timing of safety events with respect to eculizumab treatment status at the time of the event. Therefore, not all of these patients necessarily received long-term treatment or were on eculizumab at the time of infection. Previous clinical trials of eculizumab have included safety outcomes, including infection rates, in pediatric patients with aHUS. A 26-week clinical trial of eculizumab in patients with aHUS who were <18 years of age included few reports of serious infections, with the most common infections being of the upper respiratory tract and occurring in 32% of patients.14 A recent interim analysis of a long-term, observational clinical study enrolling adult and pediatric patients who completed a clinical trial of eculizumab in aHUS16 included safety outcomes, revealing low (<5%) rates of serious infections in patients, but did not include data subgroups by age or by treatment status (ever treated versus never treated with eculizumab). In the current study, a higher proportion of pediatric patients who were treated with eculizumab developed a serious infection following treatment versus those who were never treated (5.15 [95% CI = 3.77−6.87] versus 1.12 [95% CI = 0.23−3.28] per 100 patient-years); most were unspecified/viral in cause and resolved with appropriate management. It should be noted that higher proportions of ever-treated versus never-treated pediatric and adult patients required dialysis, transfusions, or had renal transplants, which are well-known confounding and independent predisposing factors for infections17, 18, 19 from baseline through data cutoff. In addition, both pediatric and adult ever-treated versus never-treated patients had more frequent renal and extrarenal symptoms and impairment, again indicating a more severe baseline disease.
Because of its mechanism of action, the use of eculizumab is associated with increased risk of meningococcal infections.9, 10, 20 In this registry, revaccination occurs at the discretion of the individual treating physician. Of the 842 ever-treated patients with evaluable safety data in this analysis, 3 patients (0.4%) developed meningococcal infections. Of the 3 patients, 2 did not receive prophylactic antibiotics, and 1 patient received penicillin prophylaxis but was infected with a penicillin-resistant meningococcal strain. Despite the severity of the meningococcal infections, 2 of the 3 patients recovered from the infection without sequelae after receiving appropriate antibiotic therapy and did not require interruption of their eculizumab therapy. One adult patient, who did not receive prophylactic antibiotics, developed fulminant meningococcemia with Waterhouse-Friderichsen syndrome presentation and died on the same day. The N. meningitidis strain (ST-12758) identified by polymerase chain reaction was not covered by the vaccination received by the patient.21 In past aHUS clinical trials,4, 12, 13, 14 in which vaccination was required for enrollment, meningococcal infections were reported in 2 adult patients (2% overall). Fakhouri et al.4 reported 1 case of meningococcal meningitis with unknown serogroup that led to discontinuation of therapy, and another case of meningococcal sepsis with B serogroup. In both cases, the patients were vaccinated against meningococcal serogroups A, C, W, and Y, but were not on prophylactic antibiotics.4 Recent findings from a 10-year pharmacovigilance analysis of eculizumab included meningococcal infection rate of 0.25 per 100 patient-years overall, including 0.24 per 100 patient-years in patients with paroxysmal nocturnal hemoglobinuria and 0.29 per 100 patient-years in patients with aHUS.22
According to the Centers for Disease Control and Prevention (CDC), vaccinations against serogroups A, B, C, W, and Y may reduce the risk of meningococcal infection, but may not provide complete protection.20, 23, 24 The CDC recommends that adult eculizumab-treated patients receive a 2-dose series of MenACWY, with revaccination every 5 years.25 As rates of vaccination against these serogroups increase, serogroup B has become a common cause of invasive meningococcal disease in developed nations.26 It is possible that increased use of the serogroup B vaccine,27, 28 which is still not available worldwide, will help prevent disease caused by that particular serogroup, although more research is needed. The European Medicines Agency recommends prophylactic antibiotic therapy in patients who initiate eculizumab <2 weeks after receiving meningococcal vaccine,10 whereas the US Food and Drug Administration does not make recommendations regarding prophylaxis.9 Some countries (e.g., France) require or recommend antibioprophylaxis throughout the duration of eculizumab therapy.29 Although case reports have demonstrated the effectiveness of prophylactic antibiotics in reducing the risk of developing meningococcal infection,30, 31 use and dosing in patients who have already been vaccinated has not been standardized.29
Prescribing information for use of eculizumab also cautions regarding the risk of Aspergillus infections during treatment.9, 10 In the current analysis, 1 adult ever-treated patient with a history of liver and kidney transplant was noted to have recurrent bacterial and Aspergillus infections; subsequently, the patient suffered a ruptured cerebral aneurysm and died 1.5 months after discontinuing therapy. Aspergillus infections have been reported previously in rare instances32, 33 in the setting of aHUS and eculizumab treatment. Thus, treating physicians should have clinical awareness of this additional potential complication, particularly with regard to immunosuppressed patients.
Few hepatic impairment events were reported in adult or pediatric patients in this safety analysis, and none of them led to treatment discontinuation. Hepatic impairment, typically asymptomatic and transient, has rarely been reported in patients with aHUS who have been treated with eculizumab.34 In the aHUS clinical trial program, analyses of liver enzymes in pediatric14 and adult4 patients treated with eculizumab determined that more patients had elevated levels before initiation of therapy compared with after eculizumab initiation. Most of these patients had normalization of liver enzymes over 26 weeks of treatment with eculizumab.
Serious infusion reaction rates were low in adult (6 events in 4 patients; 0.52 per 100 patient-years) and pediatric patients (7 events in 5 patients; 0.78 per 100 patient-years) ever treated with eculizumab. Overall, these findings are consistent with those observed in the clinical trial program over up to 2 years of exposure.4, 12, 13, 14
In the current analysis, instances of death were highest in the never-treated adult patients (9.9%), followed by ever-treated adults (4.7%) and ever-treated pediatric patients (1.8%). This disparity may be attributed, at least in part, to greater disease progression in the adult population, particularly in the never-treated patients. Deaths occurred primarily as a result of underlying or coexisting diseases. As determined in the clinical trial program, deaths related to eculizumab therapy are rare and limited to meningococcal infections.4, 12, 13, 14
In conclusion, this analysis of the Global aHUS Registry after 5 years of enrollment demonstrated no new safety concerns in either adult or pediatric patients with aHUS treated with eculizumab. Furthermore, the results of our analysis confirm the safety profile of eculizumab previously established in the clinical trial setting and, therefore, a positive benefit−risk profile in a real-world setting.
Disclosure
ER has received consulting fees and travel support from Alexion Pharmaceuticals, Inc. SRC has received research funding from Alexion Pharmaceuticals, Inc. IA-D is an employee and stockholder of Alexion Pharmaceuticals, Inc. BM is an employee and stockholder of Alexion Pharmaceuticals, Inc. NJAW has received speaker fees from Alexion Pharmaceuticals, Inc., was the national coordinator for the aHUS Registry for Alexion Pharmaceuticals, Inc., and was a former Honorary Chair in Paediatric Nephrology at the Royal Manchester Children’s Hospital during the time the research was conducted. DL serves as Israel’s national coordinator for the Alexion Pharmaceuticals, Inc.–sponsored aHUS Registry.
Acknowledgments
Medical writing and editorial support were funded by Alexion Pharmaceuticals, Inc. The sponsor and investigators thank the patients and their families for their participation in and support for this clinical study. The authors would like to acknowledge Peloton Advantage, LLC, an OPEN Health Company, which provided editorial and medical writing support by Kristen W. Quinn, PhD, and Envision Pharma Inc, which provided medical editing support by Kersten Reich, MPH, and Linda V. Wychowski, PhD, with funding from Alexion Pharmaceuticals, Inc. ClinicalTrials.gov identifier: NCT01522183.
Role of the Funding Source
This analysis was funded by Alexion Pharmaceuticals, Inc., Boston, MA. Alexion Pharmaceuticals, Inc., was responsible for the collection, management, and analysis of information contained in the Global aHUS Registry. Alexion Pharmaceuticals, Inc., contributed to data interpretation, preparation, review, and approval of the manuscript for submission. All authors had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Data Sharing
Qualified academic investigators may request participant-level, de-identified clinical data and supporting documents (statistical analysis plan and protocol) pertaining to this study. Further details regarding data availability, instructions for requesting information, and our data disclosure policy will be available on the Alexion.com website (http://alexion.com/research-development).
Footnotes
Tables S1. Deaths in adult ever-treated patients.
Table S2. Deaths in adult never-treated patients.
Supplementary Material
References
- 1.Fakhouri F., Zuber J., Fremeaux-Bacchi V. Haemolytic uraemic syndrome. Lancet. 2017;390:681–696. doi: 10.1016/S0140-6736(17)30062-4. [DOI] [PubMed] [Google Scholar]
- 2.Noris M., Remuzzi G. Atypical hemolytic-uremic syndrome. N Engl J Med. 2009;361:1676–1687. doi: 10.1056/NEJMra0902814. [DOI] [PubMed] [Google Scholar]
- 3.Wong E.K., Kavanagh D. Anticomplement C5 therapy with eculizumab for the treatment of paroxysmal nocturnal hemoglobinuria and atypical hemolytic uremic syndrome. Transl Res. 2015;165:306–320. doi: 10.1016/j.trsl.2014.10.010. [DOI] [PubMed] [Google Scholar]
- 4.Fakhouri F., Hourmant M., Campistol J.M. Terminal complement inhibitor eculizumab in adult patients with atypical hemolytic uremic syndrome: a single-arm, open-label trial. Am J Kidney Dis. 2016;68:84–93. doi: 10.1053/j.ajkd.2015.12.034. [DOI] [PubMed] [Google Scholar]
- 5.Fremeaux-Bacchi V., Fakhouri F., Garnier A. Genetics and outcome of atypical hemolytic uremic syndrome: a nationwide French series comparing children and adults. Clin J Am Soc Nephrol. 2013;8:554–562. doi: 10.2215/CJN.04760512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Noris M., Caprioli J., Bresin E. Relative role of genetic complement abnormalities in sporadic and familial aHUS and their impact on clinical phenotype. Clin J Am Soc Nephrol. 2010;5:1844–1859. doi: 10.2215/CJN.02210310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hofer J., Giner T., Jozsi M. Complement factor H-antibody-associated hemolytic uremic syndrome: pathogenesis, clinical presentation, and treatment. Semin Thromb Hemost. 2014;40:431–443. doi: 10.1055/s-0034-1375297. [DOI] [PubMed] [Google Scholar]
- 8.Schaefer F., Ardissino G., Ariceta G. Clinical and genetic predictors of atypical hemolytic uremic syndrome phenotype and outcome. Kidney Int. 2018;94:408–418. doi: 10.1016/j.kint.2018.02.029. [DOI] [PubMed] [Google Scholar]
- 9.US Food and Drug Administration . Alexion Pharmaceuticals; Boston, MA: 2018. Soliris (eculizumab) [prescribing information] [Google Scholar]
- 10.European Medicines Agency . Alexion Europe SAS; Paris, France: 2018. Soliris (eculizumab) [summary of product characteristics] [Google Scholar]
- 11.Kaplan B.S., Ruebner R.L., Spinale J.M. Current treatment of atypical hemolytic uremic syndrome. Intractable Rare Dis Res. 2014;3:34–45. doi: 10.5582/irdr.2014.01001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Legendre C.M., Licht C., Muus P. Terminal complement inhibitor eculizumab in atypical hemolytic-uremic syndrome. N Engl J Med. 2013;368:2169–2181. doi: 10.1056/NEJMoa1208981. [DOI] [PubMed] [Google Scholar]
- 13.Licht C., Greenbaum L.A., Muus P. Efficacy and safety of eculizumab in atypical hemolytic uremic syndrome from 2-year extensions of phase 2 studies. Kidney Int. 2015;87:1061–1073. doi: 10.1038/ki.2014.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Greenbaum L.A., Fila M., Ardissino G. Eculizumab is a safe and effective treatment in pediatric patients with atypical hemolytic uremic syndrome. Kidney Int. 2016;89:701–711. doi: 10.1016/j.kint.2015.11.026. [DOI] [PubMed] [Google Scholar]
- 15.Licht C., Ardissino G., Ariceta G. The global aHUS Registry: methodology and initial patient characteristics. BMC Nephrol. 2015;16:207–214. doi: 10.1186/s12882-015-0195-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Menne J., Delmas Y., Fakhouri F. Eculizumab prevents thrombotic microangiopathy in patients with atypical haemolytic uraemic syndrome in a long-term observational study. Clin Kidney J. 2018;12:196–205. doi: 10.1093/ckj/sfy035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Karkar A. Infection control guidelines in hemodialysis facilities. Kidney Res Clin Pract. 2018;37:1–3. doi: 10.23876/j.krcp.2018.37.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ainley L.I., Hewitt P.E. Haematology patients and the risk of transfusion transmitted infection. Br J Haematol. 2018;180:473–483. doi: 10.1111/bjh.15030. [DOI] [PubMed] [Google Scholar]
- 19.Zuber J., Le Q.M., Morris H. Targeted strategies in the prevention and management of atypical HUS recurrence after kidney transplantation. Transplant Rev. (Orlando) 2013;27:117–125. doi: 10.1016/j.trre.2013.07.003. [DOI] [PubMed] [Google Scholar]
- 20.McNamara L.A., Topaz N., Wang X. High risk for invasive meningococcal disease among patients receiving eculizumab (Soliris) despite receipt of meningococcal vaccine. MMWR Morb Mortal Wkly Rep. 2017;66:734–737. doi: 10.15585/mmwr.mm6627e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Platonov A.E., Mironov K.O., Matosova S.V. Genetic characteristics of the isolate of Neisseria meningitidis induced a lethal meningococcus infection in a patient with atypical hemolytic-ruemic syndrome receiving the therapy of ’eculizumab‘. Mol Diagn. 2017;1:225–227. [Google Scholar]
- 22.Socié G., Caby-Tosi M.P., Marantz J.L. Eculizumab in paroxysmal nocturnal haemoglobinuria and atypical haemolytic uraemic syndrome: 10-year pharmacovigilance analysis. Br J Haematol. 2019;185:297–310. doi: 10.1111/bjh.15790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Drug Safety and Risk Management Advisory Committee . Alexion Pharmaceuticals, Inc; Cheshire, CT: 2014. Briefing Document for Soliris® (eculizumab) [Google Scholar]
- 24.Gackler A, Kaulfuss M, Rohn H, et al. Failure of first meningococcal vaccination in patients with atypical haemolytic uraemic syndrome treated with eculizumab [e-pub ahead of print]. Nephrol Dial Transplant. 10.1093/ndt/gfy225. Accessed June 15, 2019. [DOI] [PubMed]
- 25.Centers for Disease Control and Prevention . Centers for Disease Control and Prevention; Atlanta, GA: 2019. Immunization schedules. [Google Scholar]
- 26.European Centre for Disease Prevention and Control . European Centre for Disease Prevention and Control; Stockholm, Sweden: 2017. Invasive meningococcal disease. Annual Epidemiological Report for 2015. [Google Scholar]
- 27.European Medicines Agency . Novartis; Siena, Italy: 2013. Bexsero (meningococcal group B vaccine) [European public assessment report] [Google Scholar]
- 28.Trumenba [package insert] Wyeth Pharmaceuticals Inc, A subsidiary of Pfizer Inc; Philadelphia, PA: 2018. [Google Scholar]
- 29.Loirat C., Fakhouri F., Ariceta G. An international consensus approach to the management of atypical hemolytic uremic syndrome in children. Pediatr Nephrol. 2016;31:15–39. doi: 10.1007/s00467-015-3076-8. [DOI] [PubMed] [Google Scholar]
- 30.Mussoni M.P., Veneziano F.A., Boetti L. Innovative therapeutic approach: sequential treatment with plasma exchange and eculizumab in a pregnant woman affected by atypical hemolytic-uremic syndrome. Transfus Apher Sci. 2014;51:134–136. doi: 10.1016/j.transci.2014.08.027. [DOI] [PubMed] [Google Scholar]
- 31.Ohta T., Urayama K., Tada Y. Eculizumab in the treatment of atypical hemolytic uremic syndrome in an infant leads to cessation of peritoneal dialysis and improvement of severe hypertension. Pediatr Nephrol. 2015;30:603–608. doi: 10.1007/s00467-014-2975-4. [DOI] [PubMed] [Google Scholar]
- 32.de Andrade L.G.M., Contti M.M., Nga H.S. Long-term outcomes of the atypical hemolytic uremic syndrome after kidney transplantation treated with eculizumab as first choice. PLoS One. 2017;12 doi: 10.1371/journal.pone.0188155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vellanki V.S., Bargman J.M. Aspergillus niger peritonitis in a peritoneal dialysis patient treated with eculizumab. Ren Fail. 2014;36:631–633. doi: 10.3109/0886022X.2014.882712. [DOI] [PubMed] [Google Scholar]
- 34.Hayes W., Tschumi S., Ling S.C. Eculizumab hepatotoxicity in pediatric aHUS. Pediatr Nephrol. 2015;30:775–781. doi: 10.1007/s00467-014-2990-5. [DOI] [PubMed] [Google Scholar]
- 35.Yellen S.B., Cella D.F., Webster K. Measuring fatigue and other anemia-related symptoms with the Functional Assessment of Cancer Therapy (FACT) measurement system. J Pain Symptom Manage. 1997;13:63–74. doi: 10.1016/s0885-3924(96)00274-6. [DOI] [PubMed] [Google Scholar]
- 36.Cella D., Lai J.S., Chang C.H. Fatigue in cancer patients compared with fatigue in the general United States population. Cancer. 2002;94:528–538. doi: 10.1002/cncr.10245. [DOI] [PubMed] [Google Scholar]
- 37.Cella D., Eton D.T., Lai J.S. Combining anchor and distribution-based methods to derive minimal clinically important differences on the Functional Assessment of Cancer Therapy (FACT) anemia and fatigue scales. J Pain Symptom Manage. 2002;24:547–561. doi: 10.1016/s0885-3924(02)00529-8. [DOI] [PubMed] [Google Scholar]
- 38.Parikh S.R., Lucidarme J., Bingham C. Meningococcal B vaccine failure with a penicillin-resistant strain in a young adult on long-term eculizumab. Pediatrics. 2017;140 doi: 10.1542/peds.2016-2452. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.