Abstract
Oxalate crystal-induced renal inflammation is associated with progressive kidney failure due to activation of the NLRP3/CASP-1 inflammasome. It has been suggested previously that purinergic P2X7 receptor signaling is critical for crystal-induced inflammasome activation and renal injury. Therefore, we investigated the role of the P2X7 receptor in response to crystal-induced cytokine release, inflammation, and kidney failure using in vitro and in vivo models. Dendritic cells and macrophages derived from murine bone marrow and human peripheral blood mononucleated cells stimulated with calcium-oxalate crystals, monosodium urate crystals, or ATP lead to the robust release of interleukin-1beta (IL-1ß). Treatment with the P2X7 inhibitor A740003 or the depletion of ATP by apyrase selectively abrogated ATP-induced, but not oxalate and urate crystal-induced IL-1ß release. In line with this finding, dendritic cells derived from bone marrow (BMDCs) from P2X7−/− mice released reduced amounts of IL-1ß following stimulation with ATP, while oxalate and urate crystal-induced IL-1ß release was unaffected. In sharp contrast, BMDCs from Casp1−/− mice exhibited reduced IL-1ß release following either of the three stimulants. In addition, P2X7−/− mice demonstrated similar degrees of crystal deposition, tubular damage and inflammation when compared with WT mice. In line with these findings, increases in plasma creatinine were no different between WT and P2X7−/− mice. In contrast to previous reports, our results indicate that P2X7 receptor is not required for crystal-induced CKD and it is unlikely to be a suitable therapeutic target for crystal-induced progressive kidney disease.
Subject terms: End-stage renal disease, Inflammasome
Introduction
The kidney is highly predisposed to crystalopathies, since one of its main functions is to filter, secrete and concentrate substances via urine formation. Oxalic acid, which is not significantly metabolized by mammals1,2, is mainly excreted by the kidney1–3. Oxalate, its ionized form, can form highly insoluble complexes with calcium2. When oxalate homeostasis is disturbed, either by endogenous over-production (primary hyperoxaluria)1,4, excessive exogenous provision (secondary hyperoxaluria)1,2,4, or renal dysfunction, oxalate accumulates in the body, may harm the kidneys and lead to end-stage renal disease4. Inflammasomes are large multiprotein complexes activated in response to pathogen- and damage-associated molecular patterns (PAMPs, DAMPs)5,6. Various inflammasomes have been identified, but by far the best studied is the Nacht Domain-, Leucin-Rich Repeat-, and PYD containing Protein 3 (NLRP3, Nalp3, Crypopyrin) inflammasome, mainly expressed in myeloid cells such as dendritic cells and macrophages. Several crystalline materials, among them oxalate7,8, urate9,10, cholesterol11, silica12–14, alum15 and hydroxyapatite16, have been shown to be capable of activating the inflammasome machinery. Oxalate crystals have been demonstrated to stimulate IL-1ß in dendritic cells and Nlrp3−/− mice are protected from oxalate nephropathy7,8. Moreover, it is well established that the purinergic receptor P2X7 and ATP are involved in inflammation and immunity. P2X7 is expressed by virtually all cells of innate immunity and mediates NLRP3 inflammasome activation, resulting in IL-1ß release4,10,12,17. Moreover, it has been demonstrated that (1) silica12,18 and uric acid10,17 activate the inflammasome via P2X7 signaling, (2) treatment with a P2X7 inhibitor reduces IL-1ß release10,12,18, (3) oxalate crystal-induced ATP release contributes to kidney inflammation8 and (4) several studies have suggested that P2X7 may be a suitable pharmacological target in various renal diseases, such as diabetic nephropathy19,20, glomerulonephritis21, hypertension20, kidney injury induced by metabolic syndrome22 and ischemic acute kidney injury23. Hence, the present study is directed at defining the role of P2X7 receptor in crystal-induced inflammation and kidney disease.
Results
Oxalate, urate crystals, and ATP induce IL-1ß release in vitro
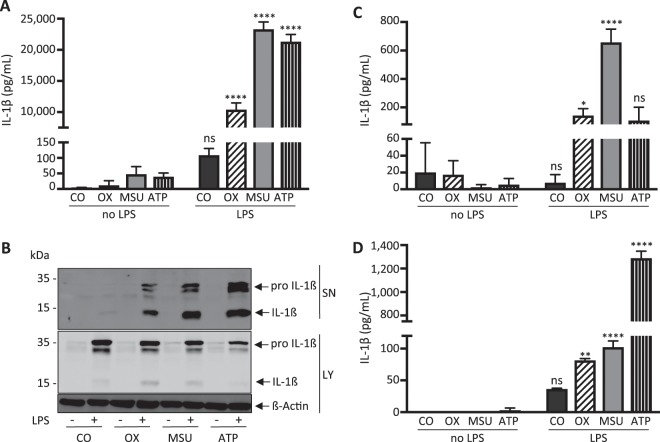
In a first series of experiments we investigated IL-1ß cytokine release from BMDCs in response to calcium oxalate crystals, monosodium urate crystals and ATP in vitro. As shown in Fig. 1A,B, all three stimuli activated murine BMDCs to synthesize (lysate) and release (supernatant) IL-1β following priming with LPS as previously reported8,10,24. To examine whether our findings are specific to BMDCs or can be elicited in different mononuclear cells, we further investigated BMDMs. As demonstrated in Fig. 1C, BMDMs released IL-1ß in response to crystals and ATP similar to BMDCs. In order to demonstrate that our findings are not limited to murine mononuclear cells, we next prepared human PBMCs and demonstrated the release of IL-1ß in response to all three stimuli as shown in Fig. 1D.
Figure 1.
Calcium oxalate, monosodium urate and ATP induce IL-1β release in LPS-primed murine BMDCs, BMDMs and human PBMCs in vitro. (A,B) Murine bone marrow-derived dendritic cells (BMDCs), (C) murine bone marrow-derived macrophages and (D) human peripheral blood mononuclear cells were primed with LPS (100 ng/ml) for 3 hours before either calcium oxalate crystals (100 µg/ml) (OX), monosodium urate crystals (300 µg/ml) (MSU) or ATP (5 mM) or no further stimulus as control (CO) were added for an additional 6 hours. Culture supernatants (SN) were collected and concentrations of IL-1ß were measured using ELISA. (A,C,D) In addition, supernatants and whole cell lysates (LY) from stimulated murine BMDCs were analyzed by western blotting. (B) All three stimuli induced IL-1ß release in all three cell types. Data are presented as mean ± SD of a representative experiment of a total of nine each performed with triplicate biological samples. Statistical analysis was performed using two-way ANOVA and post hoc analysis. ****P < 0.0001; **P < 0.01; *P < 0.05; ns, not significant compared with control treatment (without LPS). To improve the clarity of the presented western blots, blots are displayed in a cropped version. Full-length gels are presented in Supplementary Fig. 1.
Crystal-induced IL-1ß secretion is independent of P2X7 in vitro
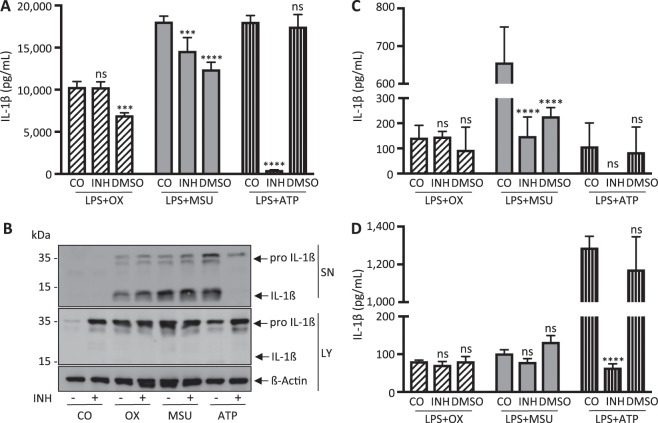
To investigate a potential role of P2X7 receptor in crystal-induced NLRP3-Casp1-inflammasome activation and cytokine release, we pretreated BMDCs, BMDMs and PBMCs with the selective P2X7 receptor antagonist A740003. As shown in Fig. 2A–D, pharmacological inhibition of P2X7 selectively abrogated ATP-induced IL-1ß release into the supernatant (Fig. 2B). In sharp contrast, crystal-induced IL-1ß release remained unaffected. Of note, DMSO applied as a vehicle control resulted in reduced IL-1ß release. This observation is consistent with previous reports that have demonstrated that DMSO downregulates and restrains NLRP3 activation through blockage of mitochondrial ROS generation25,26.
Figure 2.
Pharmacological inhibition of P2X7 does not affect crystal-induced IL-1β release in vitro. The P2X7 inhibitor A740003 (100 µM) (INH) dissolved in DMSO was applied to LPS-primed (A,B) murine bone marrow-derived dendritic cells, (C) murine bone marrow-derived macrophages and (D) human peripheral blood mononuclear cells. The inhibitor was added to the cells 15 minutes before stimulation with ATP or crystals for an additional 6 hours. A740003 did not affect oxalate (100 µg/ml) nor urate (300 µg/ml) crystal induced IL-1ß release. In contrast, ATP-induced IL-1ß release was completely abrogated in all cell types. IL-1ß release was analyzed using ELISA (A,C,D) for cell-free supernatants and western blotting (B) for whole cell lysates and supernatants from murine BMDCs. Note that DMSO affected IL-1ß release! Data are presented as mean ± SD of a representative experiment of a total of four each performed with triplicate biological samples. Statistical analysis was performed using two-way ANOVA and post hoc analysis. ****P < 0.0001; ***P < 0.001; ns, not significant compared with control treatment. To improve the clarity of the presented western blots, blots are displayed in a cropped version. Full-length gels are presented in Supplementary Fig. 2.
In a next series of experiments, we examined BMDCs from P2X7 deficient mice. As shown in Fig. 3A, crystal-induced IL-1ß release remained unaffected in BMDCs from P2X7−/− mice, whereas ATP-induced IL-1ß was completely abrogated. In sharp contrast, BMDCs from Casp1−/− mice exhibited significantly (P < 0.05) reduced IL-1ß release following either of the three stimulants, supporting the hypothesis of a defective final common pathway for both crystals and ATP in Casp1−/− mice.
Figure 3.
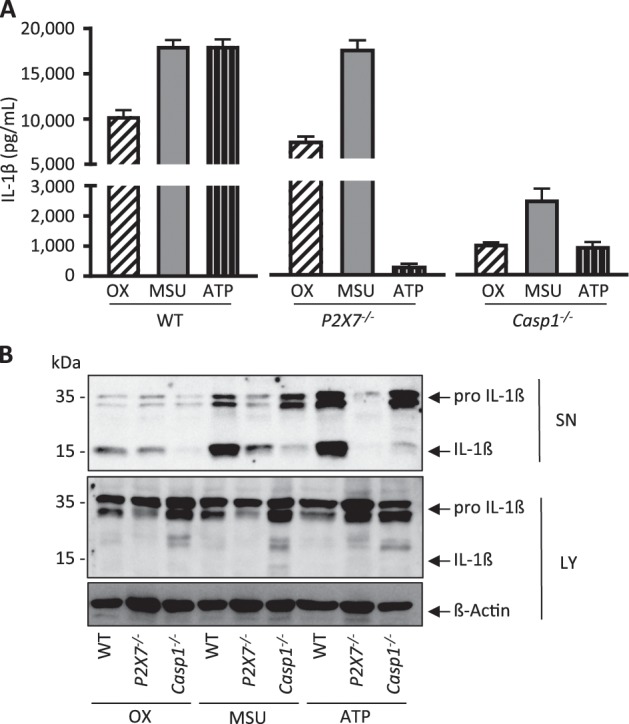
IL-1β secretion is caspase-1 dependent but does not require P2X7 signaling in vitro. Murine bone marrow-derived dendritic cells (BMDCs) from WT, P2X7−/− and Casp1−/− mice were treated with calcium oxalate crystals (100 µg/ml), monosodium urate crystals (300 µg/ml) or ATP (5 mM). (A,B) BMDCs from P2X7−/− mice showed completely abrogated ATP-induced IL-1β release, yet crystal-induced cytokine release remained unaffected. In contrast, BMDCs from Casp1−/− mice showed reduced IL-1β release for all three stimuli. IL-1ß release was analyzed using (A) ELISA and (B) western blotting. Data are presented as mean ± SD of a representative experiment of a total of three each performed with triplicate biological samples. To improve the clarity of the presented western blots, blots are displayed in a cropped version. Full-length gels are presented in Supplementary Fig. 3.
Crystal-induced IL-1ß secretion is independent of purinergic signalling in vitro
Since it has also been suggested that other P2X receptors such as P2X4 could play a role in inflammasome activation and IL-1ß cytokine release24, we next examined the effect of depleting ATP on crystal-induced cytokine release. As shown in Fig. 4, BMDCs treated with apyrase, an enzyme catalyzing ATP hydrolysis, completely abrogated ATP-induced IL-1ß release. In contrast, crystal-induced IL-1ß release remained unaffected, arguing against a significant involvement of purinergic signaling in crystal-induced IL-1ß release.
Figure 4.
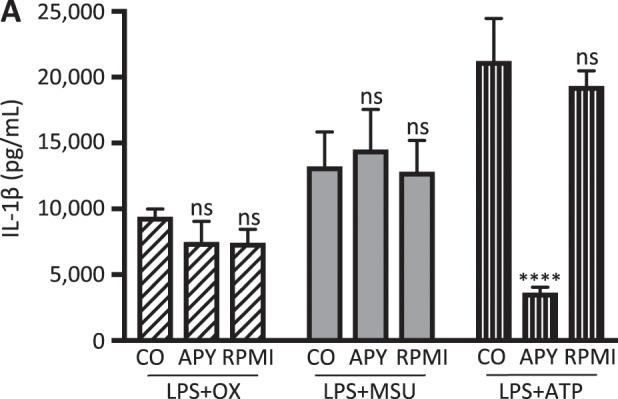
Pharmacological hydrolysis of ATP abrogates ATP-induced IL-1β secretion but does not affect crystal-induced IL-1ß secretion in vitro. Murine bone marrow-derived dendritic cells were treated with apyrase (10 U/ml) (APY) 15 minutes before adding either oxalate or urate crystals or ATP to the cells to induce hydrolysis of ATP. Crystal induced IL-1ß release remained unaffected whereas ATP-induced IL-1ß release was abrogated. Data are presented as mean ± SD of a representative experiment of a total of three each performed with triplicate biological samples. Statistical analysis was performed using two-way ANOVA and post hoc analysis. ****P < 0.0001; ns, not significant compared with control treatment.
P2X7−/− mice are not protected from crystal-induced renal failure in vivo
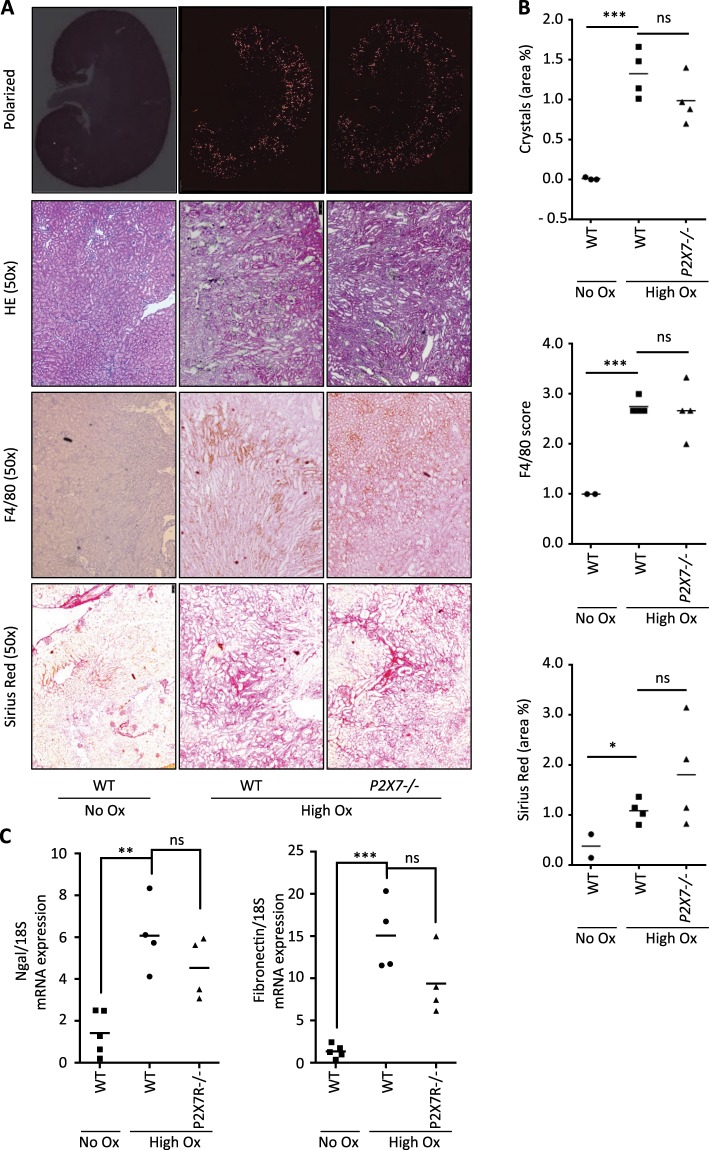
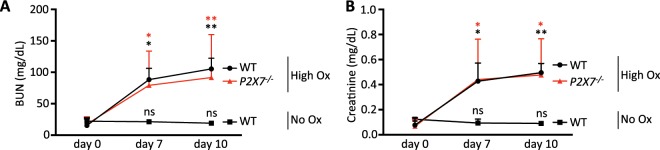
It has previously been suggested that ATP released from necrotic tubular cells may trigger NLRP3 inflammasome activation in vivo8. Moreover, treatment with apyrase has been shown to mitigate oxalate crystal-induced kidney injury8. Therefore, in a next series of experiments we examined the potential role of P2X7 receptor in crystal-induced progressive kidney injury in vivo. Crystal-induced renal failure was induced by feeding mice a high soluble oxalate diet for 10 days as previously described7. Control animals were fed an oxalate-free diet. As shown in Fig. 5A, renal histology of wild type and P2X7−/− mice fed a high soluble oxalate diet demonstrated profound crystal deposition, and analysis of hematoxylin and eosin (HE) staining revealed a high degree of tubular damage, as indicated by dilatation and rupture of tubules (Fig. 5A). This finding was also reflected in elevated mRNA expression of the renal injury biomarker, neutrophil gelatinase-associated lipocalin (NGAL)27, and the fibrosis marker, fibronectin (Fig. 5C). In addition, severe inflammation and fibrosis was noted as measured by infiltration of inflammatory cells positive for F4/80 and Sirius red staining (Fig. 5A). There was no difference in severity of crystal deposition, inflammation and fibrosis between WT and P2X7−/− mice with oxalate nephropathy (Fig. 5B). We next examined the renal function of P2X7−/− compared to wild type mice. As shown in Fig. 6, baseline blood urea nitrogen (BUN) and creatinine were determined on a control diet (oxalate-free diet). Subsequently, the diet was switched to a high soluble oxalate diet and progression of renal failure was monitored longitudinally at 7 and 10 days. Wild type and P2X7−/− mice demonstrated similar degrees of progressive renal failure over the following 10-day period as indicated by rising plasma BUN and creatinine compared to mice receiving a control diet suggesting that P2X7 receptor signaling is not involved in oxalate-crystal induced progressive renal failure. To exclude that our finding is due to a different strain of wild type mice used as compared with P2X7−/− mice, we repeated our experiments with wild-type littermates from our P2X7−/− strain. As shown in Supplementary Figs. 5 and 6, P2X7−/− mice and their wild type littermates receiving a soluble oxalate diet again demonstrate progressive kidney failure when compared with mice receiving a control diet (oxalate-free diet). mRNA expression of the renal injury biomarker, neutrophil gelatinase-associated lipocalin (NGAL)27, and the fibrosis marker, fibronectin (Supplementary Fig. 6) again demonstrated no difference between WT littermates and P2X7−/− mice, arguing against a strain effect to account for our findings.
Figure 5.
P2X7−/− mice demonstrate similar amounts of renal crystal deposition and inflammation in vivo. WT and P2X7−/− mice were placed on a high soluble oxalate diet to induce renal failure. A separate group of WT mice received a control diet. (A,B) Whole kidney scans show no significant difference in crystal deposition, tubular damage, inflammation and renal fibrosis between WT and P2X7−/− mice on high oxalate diet. (C) mRNA levels of renal injury and fibrosis markers Ngal and fibronectin showing no significant difference between WT and P2X7−/− mice on high oxalate diet. Data are presented as mean. n = 3–4 animals. Statistical analysis for the histopathological evaluation (B) was performed using unpaired t-test. Statistical analysis for mRNA expression (C) was performed using one-way ANOVA. ***P < 0.001; **P < 0.01; ns, not significant compared with mice on control diet (0% calcium/0% oxalate).
Figure 6.
P2X7−/− mice are not protected from oxalate-induced renal failure in vivo. (A,B) Renal function was assessed measuring plasma creatinine and blood urea nitrogen (BUN) from retro-orbital blood samples. P2X7−/− mice showed no protection from oxalate nephropathy in vivo. Statistical analysis was performed using two-way ANOVA. **P < 0.01; *P < 0.05; ns, not significant compared with mice on control diet (0% calcium/0% oxalate).
Discussion
Purinergic receptors have received major attention as drug targets in renal disease because of their role in glomerular, tubular, and vascular cell damage19,20,23. However, several P2X7 receptor antagonists have completed phase 2 clinical trials and these compounds have failed to deliver the expected benefit and interest in P2X7 receptor has declined28. Since CKD is a ‘catch-all’ term and syndrome representing a mix of various distinct disease etiologies, it is necessary to define the underlying pathophysiology and select appropriate patient cohorts to test any novel therapeutic pharmacological approach. To date, the involvement of P2X7 receptor in crystal-induced IL-1ß release and inflammasome activation is not clear and indeed rather contradictory. Previous reports have demonstrated that uric acid17 or silica crystals lead to ATP and IL-1ß release in BMDMs29. Similarly, crystal-induced lung injury was reduced in P2X7−/− mice as compared with WT mice30. In vitro studies using specific pharmacological inhibitors demonstrated that the P2X7 receptor participates in crystal-induced IL-1ß release, reactive oxygen production and particle phagocytosis18,30. However, several groups of investigators have failed to confirm a role for P2X7 receptor in crystal-induced inflammasome activation and IL-1 release using BMDCs from P2X7−/− mice13,16,31,32. Specifically, membrane permeation by crystalline materials was not dependent on P2X7 receptors and was suggested to be secondary to phagocytosis, because it was strongly inhibited by cytochalasin B and latrunculin32. Our findings using crystals such as monosodium urate and oxalate are in line with the latter observation.
The contrasting findings may be explained by different methodological approaches of preparing cell types, priming of cells or use of different sizes of crystals. Of interest, P2X7 inhibitors such as A740003 are dissolved in DMSO, which is a potent inflammasome inhibitor25 that may have been overlooked if this vehicle was not examined separately. Moreover, our data suggest that even in the setting of a complex interaction of tubular cell damage and inflammatory cell activation in vivo, P2X7 receptor signaling is dispensable. Using a model of acute oxalate crystal-induced kidney injury, it has been suggested that depletion of ATP by apyrase8 may prevent kidney failure. However, our findings suggest that this is unlikely to be mediated by the P2X7 receptor, because we observed no protection of crystal-induced renal inflammation or organ failure in P2X7−/− mice when compared with WT mice. Nevertheless, we cannot exclude the in vivo participation of other purinergic signaling pathways.
Together, our current findings suggest that while NLRP3 deficiency or its pharmacological inhibition prevents renal inflammation and failure7,8,33, P2X7 receptor stimulation is not required for oxalate crystal-induced kidney injury. Therefore, clinical studies examining P2X7 antagonists should not include crystal nephropathies, since this may obscure a potential benefit of these compounds in certain subsets of renal disease.
Methods
In vitro studies
Murine bone marrow-derived dendritic cells and macrophages
Bone marrow-derived dendritic cells (BMDCs) were isolated as previously described34 from either C57BL/6N, P2X7−/− and Casp1−/− mice. In brief, bone marrow cells were isolated from murine femur and tibia and differentiated at 37 °C in RPMI 1640 medium (Thermo Fisher Scientific, Waltham, Massachusetts, USA) supplemented with 10% heat inactivated fetal bovine serum (FBS) (FBS 10270, Gibco®, Thermo Fisher Scientific, Waltham, Massachusetts, USA), 1% penicilline-streptomycine (10,000U/10,000 μg ml−1) (Biochrom, Berlin, Germany) and 50 mM 2-Mercaptoethanol (Sigma Aldrich, St. Louis, Missouri, USA) and added recombinant murine granulocyte/monocyte-culture stimulating factor (GM-CSF) (PeproTech, Rocky Hill, New Jersey, USA) at a density of 2.5 × 106 cells 10 ml−1 for 8 days. Fresh medium and GM-CSF were added on day 3 and 6 after isolation. Cells were harvested on day 8 and viable cells were determined using 0.4% trypan blue solution (Thermo Fisher Scientific, Waltham, Massachusetts, USA). For experiments, cells were seeded into multi-well tissue culture plates at a density of 1 × 106 cells ml−1. For bone marrow-derived macrophages (BMDMs), bone marrow cells were obtained from murine femur and tibia and cultured in RPMI 1640 medium supplemented with 20% FBS, 30% conditioned media from L929 cells (containing macrophage-colony stimulating factor), 25 mM HEPES (Carl Roth, Karlsruhe, Germany) and 1% penicillin/streptomycin at 37 °C as described previously35,36. Cells were differentiated for 7 days and fresh media was added on days 4 and 6 of culturing. BMDMs were seeded for stimulation in 24-well tissue culture plates at a density of 200,000 cells ml−1.
Human peripheral blood mononuclear cells
Peripheral blood mononuclear cells (PBMCs) were isolated from healthy voluntary donors as described previously. Briefly, 15 ml of Lymphoflot (BioRad, Hercules, California, USA) were added to 20 ml of EDTA-blood and centrifuged 25 minutes at 2,000 rpm (Heraus Megafuge 40 R, Thermo Fisher Scientific, Waltham, Massachusetts, USA) and 20 °C without breaks and without acceleration. PBMCs were obtained in 10 ml PBS (Biochrom, Berlin, Germany) and washed three times. Washed cells were taken up in RPMI 1640 medium supplemented with 10% FBS, 1% penicilline-streptomycine and 50 mM 2-Mercaptoethanol. Cells were seeded for stimulation at a density of 2 × 106 cells ml−1 in 24-well tissue plates.
Cell culturing
Cells were stimulated with 100 ng ml−1 LPS for 3 hours (Ultra pure lipopolysaccharide from E. coli 0111:B4 strain, InvivoGen, San Diego, California, USA), followed by addition of a second stimulus for supplemental 6 hours. The second stimulus consisted either of calcium oxalate (100 μg ml−1 from a 1 mg ml−1 stock stored at 4 °C) (Sigma Aldrich, St. Louis, Montana, USA), ATP (5 mM from a prepared 100 mM stock solution stored at −20 °C) (InvivoGen, San Diego, California, USA) or monosodium urate crystals (300 μg ml−1 from 5 mg ml−1 stock solution stored at −20 °C) (InvivoGen, San Diego, California, USA). The selective P2X7 inhibitors A740003 or apyrase (both from Sigma Aldrich, St. Louis, Montana, USA) were applied 15 minutes prior to the addition of the second stimulus. A740003 was dissolved in pure DMSO and cells were treated with 100 μM A74000310 or the equivalent amount of DMSO alone (0.5%). Apyrase was dissolved in RPMI and cells were treated with 10U apyrase ml−1 or the corresponding amount of RPMI only. At the end of every series supernatants were collected and RIPA buffer (Sigma Aldrich, St. Louis, Montana, USA) containing complete EDTA-free protease inhibitor (Roche, Mannheim, Germany) was added to each well to gain whole cell lysates.
Western blot
Cells lysated with RIPA buffer containing protease inhibitor as described above were sonicated using a Bioruptor Plus (Diagenode, Philadelphia, Pennsyvania, USA). Protein concentration was determined using the standard Lowry method using the Bio-Rad DC™ Protein Assay (Bio-Rad, Hercules, California, USA). Cell-free supernatants (500 μl) were concentrated with ultra filters (Vivaspin 500, Membrane 5,000 MWCO PES, Sartorius Stedim Biotech, Göttingen, Germany). 20 µl of concentrated supernatants or 20 μg of cell lysates were subjected to 12% SDS-PAGE and immunoblotted with IL-1β antibodies 1:1000 (Sigma Aldrich, St. Louis, Missouri, USA). Peroxidase-conjugated monoclonal antibody against ß-actin (Sigma Aldrich, St. Louis, Missouri, USA) was used as a loading control.
Cytokine enzyme-linked immunosorbant assay (ELISA)
Secreted levels of IL-1ß were measured from supernatant using BD OptEIATM ELISA Set (BD Biosciences, Franklin Lakes, New Jersey, USA) according to manufacturer’s instructions.
In vivo studies
Animal studies
All experiments were performed on male age- and gender-matched 8–12 week old mice. C57BL/6 N mice (wild type control animals) were purchased from Charles River Laboratories (Sulzfeld, Germany). P2X7−/− (B6-P2rx7tm1Ipch) were a gift from GlaxoSmithKline and have been described in detail elsewhere37. The absence of mRNA transcript was confirmed using qPCR as shown in Supplementary Fig. 4. Casp1−/− (B6-Casp1tm2.1Flv)38 were kindly provided by Till Strowig (Helmholtz Centre for Infection Research, Braunschweig, Germany). The mice were housed in groups of four with a 12-hour dark/light cycle with unlimited access to food and water. Mouse synthetic diets were obtained from Ssniff (Ssniff-Spezialdiäten GmbH, Soest, Germany). The high soluble oxalate diet was manufactured by adding 50 mmol sodium oxalate kg−1 to a virtually calcium- and oxalate free diet as previously described39. All mice were fed with a calcium- and oxalate free diet three days prior to switching to the high-oxalate diet. All experimental protocols were approved by the Committee on Animal Health and Care of the Government of Unterfranken (Permit Number: 55.2-2532.1-40/14) and conform to international guidelines on the ethical use of animals.
Assessment of renal function
Kidney function was monitored by determination of blood urea nitrogen (BUN) and plasma creatinine. Retro-orbital blood samples were collected at indicated time points as previously described7. Plasma BUN and creatinine levels were measured using a Cobas Integra 800 auto-analyzer (Roche, Germany).
Histopathological evaluation
Kidney sections from C57BL/6N and P2X7−/− mice were fixed in zinc (in TRIS-based buffer) over night, embedded in paraffin, and stained with hematoxylin and eosin (HE). Whole kidney sections were scanned with polarization microscopy using a Leica microscope (Leica DM 6000B, Wetzlar, Germany). Oxalate crystal deposition was quantified using ImageJ software (National Institutes of Health, Bethesda, Maryland, USA). By setting an intensity threshold crystals were separated from background tissue. Total pixels above this threshold are expressed as a percentage of total kidney surface area as previously described7. Tubulointerstitial fibrosis was detected by Sirius Red staining. Kidney sections were stained with 0.1% Sirius Red in saturated picric acid for 1 hour, followed by dehydration with 100% ethanol and finally washed in xylene. Sirius red positive areas were detected in whole kidney scans using ImageJ software as previously described40 and are presented as percentage area per kidney scan.
Immunostaining
2 μm sections of murine kidneys fixed in 4% paraformaldehyde were used for immunostaining as previously described7. Briefly, an avidin-biotin immunoperoxidase method was used (ABC-Kit, Vector laboratories, Burlingame, CA, USA) in combination with ImmPACT DAB as substrate (Vector laboratories, Burlingame, CA, USA) and monoclonal rat anti mouse F4/80 (1:500, BioRad, Hercules, California, USA) antibodies directed against macrophages/monocytes. Peroxidase positive areas (dark staining) were quantified in whole kidney scans by three different observers in blinded fashion using a five-point scoring system as following: 1, none; 2, <25%; 3, 25%-50%; 4, 51%-75%; 5, >75%.
Real-time reverse transcription-polymerase chain reaction (RT-PCR)
Total RNA was isolated from frozen kidney tissue using PureLink RNA Mini Kit (Ambion life technologies, California, USA) following manufacturer’s instructions, adding treatment with DNase (Qiagen, Venlo, Netherlands). Frozen tissue was homogenized in 600 μl RNA lysis buffer containing 1% tris(2-carboxyethyl)phosphine (Marchery-Nagel, Düren, Germany) using a T25 basic ULTRA-TURRAX® dispersing device (IKA-Werke GmbH & CO. KG, Staufen, Germany). RNA quantity was assessed spectrophotometrically using the Nanodrop 2000 (Thermo Fisher Scientific, Waltham, Massachusetts, USA). 100 ng of RNA were transcribed into cDNA. All reagents for cDNA preparation including RevertAid Reverse Transcriptase, reaction buffer, RiboLock RNase inhibitor, random hexamer primer and dNTP mix were obtained from Thermo Fisher Scientific (Waltham, Massachusetts, USA). Real-time PCR on cDNA was performed using a StepOne PlusTM Real Time-PCR system (Applied Biosystems, Waltham, Massachusetts, USA) using SYBR Green Master Mix (Thermo Fisher Scientific, Waltham, Massachusetts, USA). All primers were obtained from Sigma Aldrich (St. Louis, Missouri, USA). Primer sequences used are listed in Table 1. mRNA expression values of all genes were normalized to 18S rRNA as a housekeeping gene. For ease of comparison, expression of each gene in wild type animals was set to 1 and values are given relative to their respective control.
Table 1.
Primer sequences used to determine mRNA expression levels.
| Target | Primer sequence (5′-3′) |
|---|---|
| 18S |
Forward: TTGATTAAGTCCCTGCCCTTTGT Reverse: CGATCCGAGGGCCTCACTA |
| Fibronectin |
Forward: GTGTAGCACAACTTCCAATTACGAA Reverse: GGAATTTCCGCCTCGAGTCT |
| NGAL |
Forward: GGCAGCTTTACGATGTACAGCAC Reverse: TCTGATCCAGTAGCGACAGCC |
| P2X7 |
Forward: CTGGTTTTCGGCACTGGA Reverse: CCAAAGTAGGACAGGGTGGA |
Statistical analysis
Statistical analysis was performed using unpaired t-test, one- or two-way ANOVA and post-hoc analysis as indicated using GraphPad Prism Version 7.00 (GraphPad Software, La Jolla, CA, USA) assuming normal distribution of the values and equality of variances. For the comparison of two independent groups unpaired t-test was used. One- and two-way ANOVA were used to compare more than two groups and to assess more than one dependent variable, respectively.
Supplementary information
Acknowledgements
We would like to thank Susanne Rößler, Michaela Arend, Margot Rehm und Astrid Ebenau-Eggers for expert technical assistance. This study was supported by grants to FK of the Deutsche Forschungsgemeinschaft (DFG, project KN 1148/2-1 and KN 1148/4-1, Projektnummer 394046635 – SFB 1365), the Oxalosis and Hyperoxaluria Foundation, and TRENAL, a thematic network grant of the Deutscher Akademischer Austauschdienst. K-U.E. reports personal fees from Akeba, Amgen, Bayer, Fresenius, Genzyme, Shire and Vifor. FWKT is supported by Ken and Mary Minton Chair of Medicine and the NIHR Biomedical Research Centre based at Imperial College Healthcare NHS Trust and Imperial College London. FWKT has received research project grants from AstraZeneca Limited, Baxter Biosciences, Boehringer Ingelheim, and MedImmune. He has consultancy agreements with Rigel Pharmaceuticals, Novartis and Baxter Biosciences. F.K. reports personal fees from Allena, Oxthera, Fresenius and Sanofi. H.L. is a recipient of a TRENAL, IZKF Friedrich-Alexander-Universität Erlangen-Nürnberg scholarship and kidney stars award from the American Society of Nephrology. This study was performed in fulfillment of her requirements for obtaining the degree “Dr. med.”.
Author contributions
H.L., F.K. and M.R. wrote the main manuscript text. H.L. prepared Figures 1–6. H.L., M.R., R.J.U., K.M., A.C.N., L.M.T., K-U.E., F.W.K.T. and F.K. reviewed the manuscript.
Data availability
The datasets generated and analyzed during the current study are available from the corresponding author at reasonable request.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
is available for this paper at 10.1038/s41598-019-56560-2.
References
- 1.Glew RH, et al. Nephropathy in dietary hyperoxaluria: A potentially preventable acute or chronic kidney disease. World J. Nephrol. 2014;3:122–142. doi: 10.5527/wjn.v3.i4.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Williams HE. Oxalic acid and the hyperoxaluric syndromes. Kidney Int. 1978;13:410–417. doi: 10.1038/ki.1978.59. [DOI] [PubMed] [Google Scholar]
- 3.Elder TD, Wyngaarden JB. The biosynthesis and turnover of oxalate in normal and hyperoxaluric subjects. J. Clin. Invest. 1960;39:1337–1344. doi: 10.1172/JCI104151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ermer T, Eckardt KU, Aronson PS, Knauf F. Oxalate, inflammasome, and progression of kidney disease. Curr. Opin. Nephrol. Hypertens. 2016;25:363–371. doi: 10.1097/MNH.0000000000000229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lorenz G, Darisipudi MN, Anders HJ. Canonical and non-canonical effects of the NLRP3 inflammasome in kidney inflammation and fibrosis. Nephrol. Dial. Transplant. 2014;29:41–48. doi: 10.1093/ndt/gft332. [DOI] [PubMed] [Google Scholar]
- 6.Schroder K, Tschopp J. The inflammasomes. Cell. 2010;140:821–832. doi: 10.1016/j.cell.2010.01.040. [DOI] [PubMed] [Google Scholar]
- 7.Knauf F, et al. NALP3-mediated inflammation is a principal cause of progressive renal failure in oxalate nephropathy. Kidney Int. 2013;84:895–901. doi: 10.1038/ki.2013.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mulay SR, et al. Calcium oxalate crystals induce renal inflammation by NLRP3-mediated IL-1beta secretion. J. Clin. Invest. 2013;123:236–246. doi: 10.1172/JCI63679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Martinon F, Petrilli V, Mayor A, Tardivel A, Tschopp J. Gout-associated uric acid crystals activate the NALP3 inflammasome. Nature. 2006;440:237–241. doi: 10.1038/nature04516. [DOI] [PubMed] [Google Scholar]
- 10.Gicquel T, et al. IL-1beta production is dependent on the activation of purinergic receptors and NLRP3 pathway in human macrophages. FASEB J. 2015;29:4162–4173. doi: 10.1096/fj.14-267393. [DOI] [PubMed] [Google Scholar]
- 11.Duewell P, et al. NLRP3 inflammasomes are required for atherogenesis and activated by cholesterol crystals. Nature. 2010;464:1357–1361. doi: 10.1038/nature08938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nakanishi K, Tsukimoto M, Tanuma S, Takeda K, Kojima S. Silica nanoparticles activate purinergic signaling via P2X7 receptor in dendritic cells, leading to production of pro-inflammatory cytokines. Toxicol. In Vitro. 2016;35:202–211. doi: 10.1016/j.tiv.2016.06.003. [DOI] [PubMed] [Google Scholar]
- 13.Cassel SL, et al. The Nalp3 inflammasome is essential for the development of silicosis. Proc. Natl. Acad. Sci. USA. 2008;105:9035–9040. doi: 10.1073/pnas.0803933105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dostert C, et al. Innate immune activation through Nalp3 inflammasome sensing of asbestos and silica. Science. 2008;320:674–677. doi: 10.1126/science.1156995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Eisenbarth SC, Colegio OR, O’Connor W, Sutterwala FS, Flavell RA. Crucial role for the Nalp3 inflammasome in the immunostimulatory properties of aluminium adjuvants. Nature. 2008;453:1122–1126. doi: 10.1038/nature06939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jin C, et al. NLRP3 inflammasome plays a critical role in the pathogenesis of hydroxyapatite-associated arthropathy. Proc. Natl. Acad. Sci. USA. 2011;108:14867–14872. doi: 10.1073/pnas.1111101108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Eleftheriadis T, et al. Uric acid induces caspase-1 activation, IL-1beta secretion and P2X7 receptor dependent proliferation in primary human lymphocytes. Hippokratia. 2013;17:141–145. [PMC free article] [PubMed] [Google Scholar]
- 18.Kojima S, et al. Purinergic signaling via P2X7 receptor mediates IL-1beta production in Kupffer cells exposed to silica nanoparticle. Toxicology. 2014;321:13–20. doi: 10.1016/j.tox.2014.03.008. [DOI] [PubMed] [Google Scholar]
- 19.Kreft E, Kowalski R, Jankowski M, Szczepanska-Konkel M. Renal vasculature reactivity to agonist of P2X7 receptor is increased in streptozotocin-induced diabetes. Pharmacol. Rep. 2016;68:71–74. doi: 10.1016/j.pharep.2015.06.140. [DOI] [PubMed] [Google Scholar]
- 20.Vonend O, et al. Glomerular expression of the ATP-sensitive P2X receptor in diabetic and hypertensive rat models. Kidney Int. 2004;66:157–166. doi: 10.1111/j.1523-1755.2004.00717.x. [DOI] [PubMed] [Google Scholar]
- 21.Taylor SR, et al. P2X7 deficiency attenuates renal injury in experimental glomerulonephritis. J. Am. Soc. Nephrol. 2009;20:1275–1281. doi: 10.1681/ASN.2008060559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Solini A, et al. The purinergic 2X7 receptor participates in renal inflammation and injury induced by high-fat diet: possible role of NLRP3 inflammasome activation. J. Pathol. 2013;231:342–353. doi: 10.1002/path.4237. [DOI] [PubMed] [Google Scholar]
- 23.Yan Y, et al. P2X7 receptor inhibition protects against ischemic acute kidney injury in mice. Am. J. Physiol. Cell Physiol. 2015;308:C463–472. doi: 10.1152/ajpcell.00245.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sakaki H, et al. P2X4 receptor regulates P2X7 receptor-dependent IL-1beta and IL-18 release in mouse bone marrow-derived dendritic cells. Biochem. Biophys. Res. Commun. 2013;432:406–411. doi: 10.1016/j.bbrc.2013.01.135. [DOI] [PubMed] [Google Scholar]
- 25.Coll RC, Robertson A, Butler M, Cooper M, O’Neill LA. The cytokine release inhibitory drug CRID3 targets ASC oligomerisation in the NLRP3 and AIM2 inflammasomes. PLoS One. 2011;6:e29539. doi: 10.1371/journal.pone.0029539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.He Y, et al. 3,4-methylenedioxy-beta-nitrostyrene inhibits NLRP3 inflammasome activation by blocking assembly of the inflammasome. J. Biol. Chem. 2014;289:1142–1150. doi: 10.1074/jbc.M113.515080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mishra J, et al. Neutrophil gelatinase-associated lipocalin: a novel early urinary biomarker for cisplatin nephrotoxicity. Am. J. Nephrol. 2004;24:307–315. doi: 10.1159/000078452. [DOI] [PubMed] [Google Scholar]
- 28.Menzies RI, Tam FW, Unwin RJ, Bailey MA. Purinergic signaling in kidney disease. Kidney Int. 2017;91:315–323. doi: 10.1016/j.kint.2016.08.029. [DOI] [PubMed] [Google Scholar]
- 29.Riteau N, et al. ATP release and purinergic signaling: a common pathway for particle-mediated inflammasome activation. Cell Death Dis. 2012;3:e403. doi: 10.1038/cddis.2012.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Moncao-Ribeiro LC, et al. P2X7 receptor modulates inflammatory and functional pulmonary changes induced by silica. PLoS One. 2014;9:e110185. doi: 10.1371/journal.pone.0110185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Iyer SS, et al. Necrotic cells trigger a sterile inflammatory response through the Nlrp3 inflammasome. Proc. Natl. Acad. Sci. USA. 2009;106:20388–20393. doi: 10.1073/pnas.0908698106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Munoz-Planillo R, et al. K(+) efflux is the common trigger of NLRP3 inflammasome activation by bacterial toxins and particulate matter. Immunity. 2013;38:1142–1153. doi: 10.1016/j.immuni.2013.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ludwig-Portugall I, et al. An NLRP3-specific inflammasome inhibitor attenuates crystal-induced kidney fibrosis in mice. Kidney Int. 2016;90:525–539. doi: 10.1016/j.kint.2016.03.035. [DOI] [PubMed] [Google Scholar]
- 34.Lutz MB, et al. An advanced culture method for generating large quantities of highly pure dendritic cells from mouse bone marrow. J. Immunol. Methods. 1999;223:77–92. doi: 10.1016/S0022-1759(98)00204-X. [DOI] [PubMed] [Google Scholar]
- 35.Maier A, et al. Hypoxia-inducible protein 2 Hig2/Hilpda mediates neutral lipid accumulation in macrophages and contributes to atherosclerosis in apolipoprotein E-deficient mice. FASEB J. 2017;31:4971–4984. doi: 10.1096/fj.201700235R. [DOI] [PubMed] [Google Scholar]
- 36.Schleicher U, Bogdan C. Generation, culture and flow-cytometric characterization of primary mouse macrophages. Methods Mol. Biol. 2009;531:203–224. doi: 10.1007/978-1-59745-396-7_14. [DOI] [PubMed] [Google Scholar]
- 37.Chessell IP, et al. Disruption of the P2X7 purinoceptor gene abolishes chronic inflammatory and neuropathic pain. Pain. 2005;114:386–396. doi: 10.1016/j.pain.2005.01.002. [DOI] [PubMed] [Google Scholar]
- 38.Case CL, et al. Caspase-11 stimulates rapid flagellin-independent pyroptosis in response to Legionella pneumophila. Proc. Natl. Acad. Sci. USA. 2013;110:1851–1856. doi: 10.1073/pnas.1211521110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mulay Shrikant R., Eberhard Jonathan N., Pfann Victoria, Marschner Julian A., Darisipudi Murthy N., Daniel Christoph, Romoli Simone, Desai Jyaysi, Grigorescu Melissa, Kumar Santhosh V., Rathkolb Birgit, Wolf Eckhard, Hrabě de Angelis Martin, Bäuerle Tobias, Dietel Barbara, Wagner Carsten A., Amann Kerstin, Eckardt Kai-Uwe, Aronson Peter S., Anders Hans Joachim, Knauf Felix. Oxalate-induced chronic kidney disease with its uremic and cardiovascular complications in C57BL/6 mice. American Journal of Physiology-Renal Physiology. 2016;310(8):F785–F795. doi: 10.1152/ajprenal.00488.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Street Jonathan M., Souza Ana Carolina P., Alvarez-Prats Alejandro, Horino Taro, Hu Xuzhen, Yuen Peter S. T., Star Robert A. Automated quantification of renal fibrosis with Sirius Red and polarization contrast microscopy. Physiological Reports. 2014;2(7):e12088. doi: 10.14814/phy2.12088. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated and analyzed during the current study are available from the corresponding author at reasonable request.