Abstract
Cholecystokinin (CCK), through the CCK-2 receptor, exerts complex effects on anxiety. While CCK agonists are panicogenic, CCK-2 antagonists fail to alleviate human anxiety. Preclinical studies with CCK-2 antagonists are also inconsistent because their anxiolytic effects largely depend on the behavioral paradigm and antecedent stress. The controversy might be accounted by the neuromodulatory role for CCK in anxiety which is ill-defined. If this is its actual role, blocking CCK-2 will have carry-over effects on the anxiety baseline over time. To test this hypothesis, the consequences of acute administration of the CCK-2 antagonist Ly225.910 (0.1 mg Kg−1) was evaluated in the temporal expression of aversion toward exploration-conflicting tasks. Ly225.910 effects were evaluated in rats exposed to the elevated plus-maze (EPM) twice, an approach-avoidance anxiety-like test. While LY225.910-treated rats had less anxiety than vehicle-treated rats, the difference was reversed during the EPM retest 24 hours later without drug. Moreover, Ly225.910 effects in stress-induced cognitive impairment was measured giving the novel-object discrimination (NOD) test to rats not habituated to the exploration apparatus to elicit neophobia. After a first encounter with objects (“old”), Ly225.910-treated rats did not recognize the “novel” object introduced 6 hours later. Ly225.910-exposed rats did not discriminate the new location of the “novel object” when it was repositioned in the arena 24 hours later. Ly225.910-treated rats also failed to explore objects. In line with its neuromodulatory role, aversive carry-over effects of Ly225.910 suggest that CCK-2 activation by endogenous CCK, rather than triggering anxiety, may return the anxiety state to its normal level.
Keywords: Cholecystokinin, Receptor, Cholecystokinin B, Performance Anxiety, Behavior Rating Scale
1. INTRODUCTION
Cholecystokinin is a member of a sulfated peptide family, whose small CCK-8 and CCK-4 forms are potent neurotransmitters (Dockray, 1980). Two CCK receptor types, CCK-1 and CCK-2, are expressed at relatively low and high levels respectively in the brain (Innis and Snyder, 1980). Meanwhile the CCK-2 binds sulfated and non-sulfated forms of CCK-8, as well as CCK-4, the CCK1 is highly specific for the sulfated octapeptide of CCK. The CCK-2 (formerly CCK-B) type has traditionally been linked with the expression of anxiety-like behaviors in rodents (Singh et al., 1991; Van Megen et al., 1996; Chen et al., 2006), and the neurobiology of panic attacks in humans (Bradwejn et al., 1990; reviewed in Zwanzger et al., 2012). Although an array of potent, selective CCK-2 receptor antagonists have been developed, no clinical trials so far have demonstrated their effectiveness in alleviating anxiety and panic attacks in humans (Kramer et al., 1995; Rodgers et al., 1995; Harro, 2006). Reconciling this body of evidence may profit from a fuller understanding of the precise role of CCK in anxiety.
The CCK system is recruited only under conditions of high frequency neuronal firing (Rotzinger and Vaccarino, 2003). Based on this finding, a recent refinement of the hypothesis suggests that endogenous CCK may “modulate” specific affective states related to anxiety, rather than trigger anxiety itself (Adamec et al., 1997; Becker et al., 2001; Hebb et al., 2003; Panksepp et al., 2004; Kellner et al., 2000; Eser et al., 2008). For example, activation of CCK mediates the anticipatory states before the development of anxiety in rats (Becker et al., 2001; Panksepp et al., 2004; Panksepp et al., 2007) and humans (Philipp et al., 1992; Aluoja et al., 1997; Brawman-Mintzer et al., 1997; Benedetti et al., 2006). Even of greater importance, the presumed “neuromodulatory role” for CCK in anxiety still remains ill-defined. If that is the actual role of endogenous CCK, the blockade of its main receptor in the brain (CCK-2R, for instance, avoidance-approach conflicting situations may condition subsequent emotional responses when repeatedly exposed to the same situation.
To this purpose, the effects of acute administration of Ly225.910, a potent and selective CCK-2 receptor antagonist, was first evaluated based on the performance of the elevated-plus-maze (EPM) test during the test (anxiety state) and retest (anxiety sensitization) sessions (Bertoglio and Carobrez, 2000). To measure whether the development of anxiety causes impairments in memory consolidation, rats were forced to explore and recognize novel objects according to the recognition paradigm (Ennaceur and Delacour, 1988) without habituation to the exploration apparatus to elicit neophobic responses. In both behavioral paradigms, pharmacological effects of Ly225.910 were determined acutely while its carry-over effects determined once the antagonism were cleared.
2. METHODS
2.1. Animals
Male Sprague–Dawley rats from Charles River (Wilmington, MA, USA), weighing 250–300 g at the beginning of the experiment, were housed in groups of three per cage on a 12-h light/dark cycle (lights on at 7 a.m.), with access to food and water ad libitum. Behavioral testing was conducted during the light hours in a different room from where the rats had been housed. In all experiments, behavior was videotaped for later analysis by an observer blind to the conditions. Animals were treated in accordance with the National Institutes of Health guide for the care and use of Laboratory animals (NIH Publications No. 8023, revised 1978) and in accordance with the University of Michigan Committee on the Use and Care of Animals.
2.2. Elevated Plus-Maze (EPM) Test
The EPM test derives from the innate aversion of rodents to open spaces, whereby animals that spend less time exploring open arms are thought to behave more anxious (reviewed in Carobrez and Bertoglio, 2005). The apparatus was constructed of black-painted Plexiglas with four arms (45 cm long and 12 cm wide) arranged in a cross and elevated 70 cm from the floor. Two opposite arms were enclosed by 40-cm-high walls. The other two arms were open, with rims at the edge to prevent rats from falling off. A source of dim light (70 lux) was placed above the center of the maze. Each rat was placed in the central square of the maze facing one of the closed arms and was allowed to freely explore the maze for 5 min. After the completion of the first trial (Test) rats were returned to their home cages and remained there until the performance of the second trial (Retest) that was conducted 24h later. Recorded behavioral parameters included the percent of time spent in open arms and the percent of entries made into open arms. An entry into any arm was defined as four paws placed on it with forward motion. The maze was thoroughly cleaned with 70% ethanol between each test session.
2.3. Novel-object discrimination (NOD) test
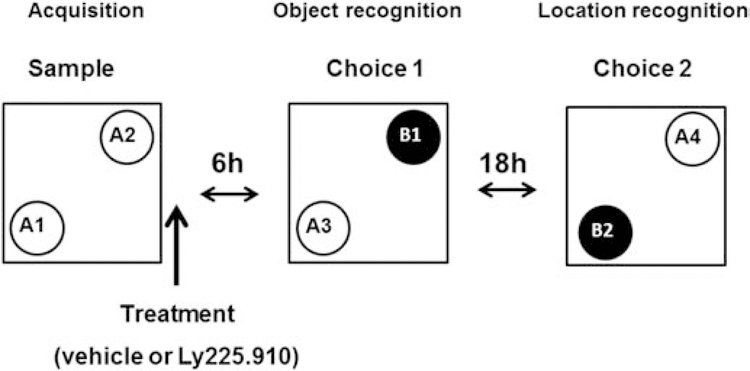
The method was originally described by Ennaceur and Delacour (1988). The test consisted of acquisition (sample) and recognition (choice) phases, but in contrast to the traditional method, rats were not previously habituated to the exploration arena to promote neophobic responses to small objects (Besheer and Bevins, 2000). There was an additional choice phase where the previously introduced novel object was shifted to a new position to regain novelty. The NOD arena consisted of a squared Open Field (OF) test (Belzung and Griebel, 2001) apparatus made of white Plexiglas® (base dimensions 1 m2; height 40 cm). The OF arena and the experimental room were illuminated with a bright white light (~75 lux at floor level). The objects to be discriminated were (A) a cane (7 cm base x 13 cm high) and (B) a Lego® block (8 cm base x 15 cm high). Objects were arranged diagonally in opposite corners 25 cm from the edges (Fig.1). These objects were not known to have any ethological significance for the rats and they had never been associated with reinforcement.
Fig.1.
NOD test series layout. The test consisted of 5 min object acquisition, 6 h latency, 5 min object recognition, 18 h latency, and 5 min object location. Note that the empty circles represent two identical copies of the same “old” object that becomes the familiar object in Choice 1 when a new (“novel”) object (black circles) is introduced in the arena. The “novel object” switched positions with the “old object” to regain novelty in the new location.
Rats were exposed to a sample phase and two choice phases distributed over 24 h (Fig.1). For the sample (familiarization) phase, rats were placed in a corner and exposed to two identical copies of the same sample (“old”) object (A1 and A2) for a limited period of time (5 min). Rats received IP injections (1ml Kg-1) of either vehicle or LY225.910 immediately after the completion of the sample phase and were returned to their home cages. After a memory retention interval of 6 hours, rats were put back into the open arena (Choice 1-object recognition) in which one of the “old” objects were substituted by an exact copy (A3) and the other by a new (“novel”) object (B1) to assess memory retention. Rats were re-exposed 18 hours later (24h after the sample phase) to copies of the “novel object” (B2) and “old object” (A4) occupying switched positions in the same diagonal of the OF to regain novelty in the new location (Choice 2-location recognition). Positions of the objects were counter-balanced between rats to avoid bias. All objects and OF were thoroughly wiped with 70% alcohol.
“Exploration of an object” was defined as directing the nose to the object at a distance of less than 2 cm and /or touching it with the nose. Turning around or sitting on the object was not considered exploration. The time spent exploring each of the objects was used as the basic measure to determine object recognition. A discrimination index (D) for each rat was reckoned during the choice phases. D-index was defined as the ratio of the amount of time exploring the “novel object” (N) minus the time exploring the old object (O) over the total time exploring both objects according to the formula D= (N−O) / (N+O). A D-value above 0 indicates “novel object” discrimination, and equal to 0 (or below 0) no object discrimination. Animals that failed exploration of objects were excluded from the NOD analysis.
2.4. Drug administration
The selective CCK-2 antagonist Ly225.910, which is a potent CCK2 receptor antagonist (IC50 = 9.3 nM for inhibition of 125I-labeled CCK-8 sulfate binding at mouse brain membranes) (Yu et al., 1991; Suman-Chauhan et al., 1996); was purchased from Tocris (Ellisville, Missouri). It was prepared fresh at 0.1 mg per ml in 0.9% NaCl plus DMSO (5%) and administered at 1 ml/Kg intraperitoneal (IP) at the dose of 0.1 mg/Kg, which is reported to be effective in ameliorating heightened states of anxiety as measured in the EPM test (Farook et al., 2004). It was used just one dose of antagonist given the excellent selectivity of Ly225.910 for CCK-2 (IC50 = 1.4 nM) over CCK-1 (IC50 >10,000 nM) receptors (Yu et al. 1991). Two independent cohorts of rats were used for each behavioral test. In the EPM cohort, 25 rats received Ly225.910 while the other 25 rats were given vehicle 30 min before the first EPM test. Rats were divided into two groups afterwards: half of the sample (12 vehicle-treated rats and 13 Ly225.910-treated rats) received saline injections 30 min before the EPM retest, whereas the rest (13 vehicle-treated rats and 12 Ly225.910-treated rats) remained unstressed. A total of 32 rats were used in the NOD test series, half of them received Ly225.910 and the other half received vehicle immediately after the completion of the sample (object familiarization) phase.
2.5. Statistics
Statistical analyses were carried out using SPSS®16 for Windows. Differences in the exploration of the EPM were evaluated using repeated measures two-way repeated ANOVA with the interaction of Treatment (Ly225.910 vs. vehicle) and Injection (saline injection vs. no injection) as between-subjects factor, and Trial (test vs. retest) as within-subject factor. Paired t-test were used to compare EPM performance across trials. Independent-samples t-test were conducted when any factor or interaction reached statistical significance. Additionally, a t-test a priori analysis was made to check treatment effects during the first EPM trial. NOD data were analyzed by unpaired t-test (comparing vehicle-treated with Ly225.910-treated rats) and by one-sample t-test comparing performance with chance levels (test value 0.0). The alpha value was set at p < 0.05.
3. RESULTS
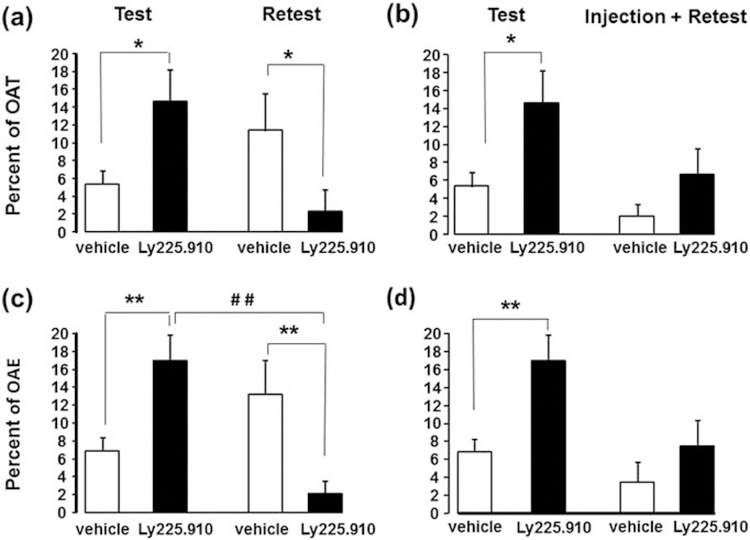
Figure 1 displays the behavior of Ly225.910-treated rats compared to vehicle-treated rats in response to the two-trial (test-retest) EPM. A priori comparison conducted on the EPM test showed that Ly225.910-treated rats showed a greater percentage of both open-arm time (t (50) −2.39, p = 0.021; Fig.1a,b) and of open-arm entries (t (50) −3.20, p = 0.002; Fig.2c,d) than vehicle-treated counterparts as expected by the assumed anxiolytic effects of acute CCK-2 antagonism. The two-way repeated ANOVA revealed a significant within-subjects treatment effect in both the percent of open-arm time (F(1,46) = 5.43, p = 0.024) and in the percent of open-arm entries (F(1,46) = 7.48, p = 0.009). Only Ly225.910-treated rats consistently reduced open-arm exploration across trials (percent of open-arm time: t (23) 2.21, p = 0.038, Fig.2a; percent of open-arm entries: t (23) 2.63, p = 0.015, Fig.2c). As to between-groups differences, there was only a significant Treatment x Injection interaction for the percent of open-arm entries (F(1,46) = 4.96, p = 0.031; Fig.2c and Fig.2d). When facing the EPM retest, vehicle-treated rats entered the open-arm more than their Ly225.910-treated counterparts, but only when rats did not receive saline injections upon the second EPM session (t (20) 3.37, p = 0.005, Fig.2c). Stress (injections) upon EPM retest eliminated these differences (Fig. 1b and Fig. 1d).
Fig.2.
Carry-over effects of Ly225.910 (0.1mg Kg−1) on the two-trial elevated plus-maze performance. Anxiety-like indices are the following: (a, b) Percent of time in open arms (OAT) and (c, d). Percent of entries into open arms (OAE). Results are displayed as mean ± SEM. * p < 0.05, ** p < 0.01, compared with vehicle-treated counterpart, # # p < compared to the same group on test (two-way (Treatment x Injection) repeated ANOVA followed by t-test analysis, N = 12–25).
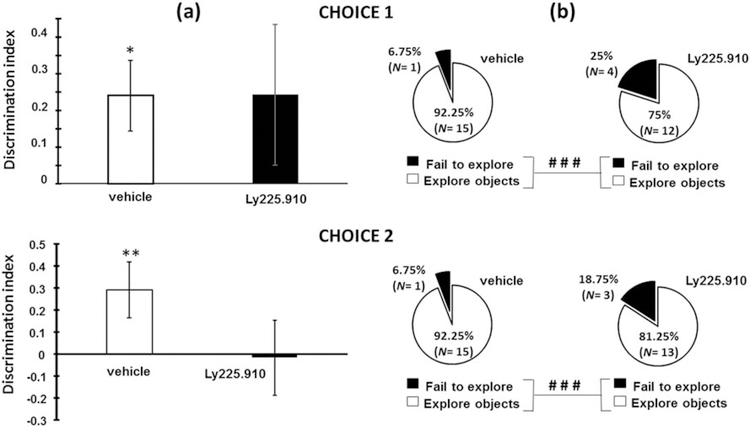
To evaluate whether aversive carry-over effects of acute Ly225.910 could impair cognitive function (attention and memory), rats were evaluated for object-recognition under neophobic conditions since rats were not previously habituated to the exploration apparatus. The NOD test is based on the spontaneous tendency of rodents to spend more time exploring a new (“novel”) object when it is paired with a familiar (“old”) one (Fig.1). The choice to explore a novel object requires judgment of the previous occurrence of objects made on the basis of their relative familiarity (recognition). Discrimination ratios (Fig.3a) did not differ between groups either in Choice 1 (object recognition, t (25) 0.88, p = 0.931) or in Choice 2 (location recognition, t (26) 1.831, p = 0.079). However, while vehicle-treated showed significant object discrimination (Choice 1: t (14) 2.572, p = 0.022) and location discrimination (Choice 2: t (14) 3.006, p = 0.009), Ly229.910-treated rats could discriminate nothing (Choice 1: t (11) 1.314, p = 0.216; Choice 2: t (12) −0.101, p = 0.921). Interestingly, the proportion of animals that failed to explore the objects (Fig.3b) in the Ly225.910-treated group was significantly greater as compared to the vehicle-treated group (Choice 1: p < 0.001; p < 0.001 Fisheŕs Exact Test), perhaps due to the higher anxiety levels.
Fig.3.
Effects of Ly225.910 (0.1mg Kg−1) on the object-recognition (NOD) task. The figure portrays (a) the discrimination index in the two 5-min choice (1-object recognition and 2-location recognition) sessions and (b) the proportion of animals that failed to explore objects. The data are in mean ± SEM; * p < 0.05; ** p < 0.01 compared to no discrimination (One-sample t-test, N=11–15); # # # p < 0.001 compared to Ly225.910-treated group (Fisheŕs Exact Test, N=16).
4. DISCUSSION
There is now a substantial literature indicating that a single prior un-drugged exposure to the EPM usually results in increased open arm avoidance on subsequent trials, perhaps indicating increased anxiety. By example, the anxiolytic efficacy of benzodiazepines is either markedly reduced or completely abolished by prior un-drugged test experience (Bourin, 2019). Rats exposed to the treatment with the CCK-2 receptor antagonist Ly225.910 are more prone to develop anticipatory anxiety to oncoming approach-avoidance conflicts. Anxiolytic-like effects rendered by Ly225.910 in the EPM test shifted to heightened anxiety during the retest. This finding is in agreement with the fact that stress-induced enhancement of fear conditioning activates the amygdala cholecystokinin system in a rat model of post-traumatic stress disorder (Feng et al., 2014). In addition, Ly225.910-exposed rats neither recognized a novel object nor explored objects because of the interference of anxiety in cognitive function. Carry-over effects of Ly225.910 suggest that one of the neuromodulatory roles for endogenous CCK may consist of the long-term reestablishment of anxiety baseline after experiencing incidental (unconditioned) stress.
The efficacy of CCK-2 receptor antagonists as anxiolytic in preclinical models has been questioned (Griebel et al., 1997) since it may inhibit the interference of prior stress rather than ameliorate the present anxiety state (Kõks et al., 2000; Wang et al. 2011). The rationale of the present study was to reverse this cause-effect relationship, that is, the carry-over effects of a single anxiolytic dose of Ly225.920 in the baseline of anxiety. The EPM task is a robust anxiety-like test based on the rat’s innate avoidance of aversive novel environments which induces a shift in the type of anxiety (i.e., anxiety sensitization) on retest (Bertoglio and Carobrez, 2000) likely due to aversive learning (File, 1993). Rats treated with a single dose of Ly225.910 explored open arms more intensively than their vehicle counterparts which gives support to the contention that blocking the CCK-2 receptor may have anxiolytic-like effects (Farook et al., 2004; Rezayat et al., 2005; Wang et al., 2005) in the EPM test (Wilson et al., 1998; Li et al., 2013). Aversion towards open arms is influenced by single or multiple pre-exposures to the maze (File, 1993; Treit et al., 1993; Bertoglio and Carobrez, 2000). Indeed, stressful stimuli present upon initial exposure to the EPM render an elevation of plasma corticosterone on retest (Albrechet-Souza et al., 2007), which means that rodents may learn about the potentially dangerous areas of the maze (Bertoglio & Carobrez, 2004). Interestingly, only rats exposed to the Ly225.910 treatment showed a significant shift in emotional states from an unconditioned (EPM test) to a learned (EPM retest) aversive response toward the open arms (Bertoglio and Carobrez, 2000) upon retest.
It could be argued that Ly225.910 effects across trials were the result of either floor effects in the vehicle-treated group or of the lack of anxiolytic effects in the Ly225.910-treated group once the CCK-2R antagonist was cleared. However it was not the case as rats exposed to Ly225.910 showed evidence of greater open arms avoidance than vehicle-treated rats when both groups remained undisturbed (no saline injections) upon retest (Fig.1a,c). In the EPM retest, open arms avoidance of undisturbed Ly225.910-treated rats was similar to that caused by prior stress (saline injections) in vehicle-treated rats (Hogg, 1996). CCK-2R antagonists tend to augment the anxiogenic effects of NMDA in the rat EPM test (Vasar et al., 1993). Accordingly, carry-over Ly225.910 effects across EPM trials may denote trans-synaptic activations of excitatory amino acids (Hökfelt et al., 2002) long after drug has cleared.
The NOD paradigm was chosen to elucidate the interference of anxiety with cognitive functions (Vargas et al., 2015). Although the NOD test captures memory alterations, it will also produce mild stress (Moore et al, 2013; Aguilar et al., 2018) if the task is conducted under neophobic (no habituation) conditions (Besheer and Bevins, 2000). Exposure to Ly225.910 impaired object discrimination in rats, an effect that was not delay-dependent (King et al., 2004) as rats naïve to the antagonist were able to discriminate between objects even when the “novel object” was repositioned in Choice 2. Poor NOD performance of Ly225.910-exposed rats was likely to be due to their heightened anxiety because a significant number of these rats did not explore objects (Fig. 3b). Carry-over effects of acute Ly225.910 in NOD is in agreement with the role played by CCK in the cognitive processes related to fear control (Chen et al., 2006) and with previous reports of repeated exploration-conflict tasks (Ballaz et al., 2007). Anticipatory fear induced by the forced exposure to an unfamiliar setting (Belzung and Griebel, 2001) accounts for the aversive carry-over effects. Learning had been studied in this context in order to evaluate the influence of memory in test-retest. It has been shown that the use of amnesic agents such as scopolamine did not modify the test-retest observed when administering chlordiazepoxide in the elevated plus-maze (Calzavara et al. 2005). Atropine sulfate, a muscarinic cholinergic receptor antagonist known for its amnesic properties, did not significantly raise the number of punished crossings in retest mice in the four plate test (FPT) (Ripoll et al., 2005). In contrast, other studies concluded that test–retest implies an aversive learning in trial 1 that is transferred to trial 2 (Vargas et al., 2006).
Beyond its role similar to an excitatory neurotransmitter in anxiety (Vasar et al., 1993; Li et al., 2013), the tone of CCK activation is likely to contribute to emotional stability in humans (Verbanck et al., 1984; Harro et al., 1992). In rodents, elevated CCK expression in cortical areas relevant to emotionality and cognition mediates the anticipation to stress as it occurs (Becker et al., 2001; Panksepp et al., 2007). The fact that mean panic attack frequency in panic-disorder patients under chronic treatment with a potent CCK-2 antagonist was even greater than the placebo group (Kramer et al., 1995) gives support to the notion that some CCKergic tone is required for mood stability. In this vein, this research demonstrates that the down-regulation of the CCK-2 receptor is related to greater anxiety in the rat long term (Wunderlich et al., 2000). Although the use of another CCK-2 antagonist (e.g., CI-988) or a higher dose of Ly225.910 would have strengthened the study, despite the lack of data concerning to the half-life of Ly225.910, these limitations do not preclude that the adaptive role of CCK in anxiety (Ladurelle et al., 1995; Hökfelt et al., 2002) applies to this study. Interestingly, this study put the focus on the long-term consequences of the CCK-2R antagonism in anxiety which has been neglected and requires further research.
5. CONCLUSION
In summary, the present research shows that the activation of the CCK-2 receptor by endogenous CCK may normalize levels of anxiety long term (Bourin 1998). It also sheds some light on the so-called “neuromodulatory” role for CCK in anxiety.
Highlights.
Cholecystokinin (CCK) through the CCK-2 receptor play a complex role in anxiety
Acute CCK-2 receptor antagonist Ly225.910 is anxiolytic in the elevated plus-maze
Ly225.910 carry-over effects elicits aversion during the elevated plus-maze retest
Ly225.910 alters object recognition and reduces object exploration due to anxiety
CCK through CCK-2 receptor may return anxiety to baseline at long-term
ACKNOWLEDGEMENTS
We thank the Pritzker Neuropsychiatric Disorders Research Consortium Fund LLC (http://www.pritzkerneuropsych.org) for its support.
FUNDING SOURCE
This work was supported by NIDA P01 DA021633, The Office of Naval Research (ONR) Grants N00014-09-1-0598 and N00014-12-1-0366 to SJW and HA, as well as the Pritzker Neuropsychiatric Disorders Research Consortium Fund LLC (http://www.pritzkerneuropsych.org).
ABBREVIATIONS:
- EPM
Elevated Plus Maze
- NOD
Novel Object Discrimination
- CCK
cholecystokinin
- CCK-2R
cholecystokinin 2-receptor
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of interest: none.
REFERENCES
- Adamec RE, Shallow T, Budgell J, 1997. Blockade of CCK(B) but not CCK(A) receptors before and after the stress of predator exposure prevents lasting increases in anxiety-like behavior: implications for anxiety associated with posttraumatic stress disorder. Behav. Neurosci 111, 935–939. 10.1037/0735-7044.111.2.435 [DOI] [PubMed] [Google Scholar]
- Aguilar BL, Malkova L, N¨Gouemo P, Forcelli PA, 2018. Geneticaly epilepsy-prone rats display anxiety-like behaviors and neuropsychiatric comorbidilities of epilepsy. Front. Neurol 9, 476. doi: 10.3389/fneur.2018.00476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albrechet-Souza L, Cristina de Carvalho M, Rodrigues Franci C, Brandão ML, 2007. Increases in plasma corticosterone and stretched-attend postures in rats naive and previously exposed to the elevated plus-maze are sensitive to the anxiolytic-like effects of midazolam. Horm. Behav 52, 267–273. doi: 10.1016/j.yhbeh.2007.05.002 [DOI] [PubMed] [Google Scholar]
- Aluoja A, Shlik J, Vasar V, Kingisepp PH, Jagomagi K, Vasar E, Bradwejn J, 1997. Emotional and cognitive factors connected with response to cholecystokinin tetrapeptide in healthy volunteers. Psychiatry Res 66, 59–67. 10.1016/S0165-1781(96)02948-4 [DOI] [PubMed] [Google Scholar]
- Ballaz SJ, Akil H, Watson SJ, 2007. The CCK-system mediates adaptation to novelty-induced stress in the rat: a pharmacological evidence. Neurosci. Lett 428, 27–32. doi: 10.1016/j.neulet.2007.09.035 [DOI] [PubMed] [Google Scholar]
- Becker C, Linthorst AC, Reul JM, 2001. Enhanced cortical extracellular levels of cholecystokinin-like material in a model of anticipation of social defeat in the rat. J. Neurosci 21, 262–269. 10.1523/JNEUROSCI.21-01-00262.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belzung C, Griebel G, 2001. Measuring normal and pathological anxiety-like behaviour in mice: a review. Behav. Brain Res 125, 141–149. 10.1016/S0166-4328(01)00291-1 [DOI] [PubMed] [Google Scholar]
- Benedetti F, Amanzio M, Vighetti S, Asteggiano G, 2006. The biochemical and neuroendocrine bases of the hyperalgesic nocebo effect. J. Neurosci 26, 12014–1222. doi: 10.1523/JNEUROSCI.2947-06.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertoglio LJ, Carobrez AP, 2004. Scopolamine given pre-Trial 1 prevents the one-trial tolerance phenomenon in the elevated plus-maze Trial 2. Behav. Pharmacol 15, 45–54. doi: 10.1097/00008877-200402000-00006 [DOI] [PubMed] [Google Scholar]
- Bertoglio LJ, Carobrez AP, 2000. Previous maze experience required to increase open arms avoidance in rats submitted to the elevated plus-maze model of anxiety. Behav. Brain Res 108, 197–203. 10.1016/S0166-4328(99)00148-5 [DOI] [PubMed] [Google Scholar]
- Besheer J, Bevins RA, 2000. The role of environmental familiarization in novel-object preference. Behav. Processes 50, 19–29. 10.1016/S0376-6357(00)00090-5 [DOI] [PubMed] [Google Scholar]
- Bourin M, 1998. Cholecystokinin as a target for neuropsychiatric drugs. Drug News Perspect 11, 342–349. doi: 10.1358/dnp.1998.11.6.657281 [DOI] [PubMed] [Google Scholar]
- Bourin M, 2019. The test retest model of anxiety: An appraisal of findings to explain benzodiazepine tolerance. Pharmacol. Biochem. Behav 178, 39–41. doi: 10.1016/j.pbb.2017.12.009 [DOI] [PubMed] [Google Scholar]
- Bourin M, Dailly E, 2004. Cholecystokinin and panic disorder. Acta Neuropsychiatr 16, 85–93. doi: 10.1111/j.1601-5215.2004.0076.x [DOI] [PubMed] [Google Scholar]
- Bradwejn J, Koszycki D, Meterissian G, 1990. Cholecystokinin-tetrapeptide induces panic attacks in patients with panic disorder. Can. J. Psychiatry, 35, 83–85. doi: 10.1177/070674379003500115 [DOI] [PubMed] [Google Scholar]
- Brawman-Mintzer O, Lydiard RB, Bradwejn J, Villarreal G, Knapp R, Emmanuel N, Ware MR, He Q, Ballenger JC, 1997. Effects of the cholecystokinin agonist pentagastrin in patients with generalized anxiety disorder. Am. J. Psychiatry 154, 700–702. doi: 10.1176/ajp.154.5.700 [DOI] [PubMed] [Google Scholar]
- Calzavara MB, Patti CL, Lopez GB, Abílio VC, Silva RH, Frussa-Filho R, 2005. Role of learning of open arm avoidance in the phenomenon of one-trial tolerance to the anxiolytic effect of chlordiazepoxide in mice. Life Sci 76, 2235–2246. doi: 10.1016/j.lfs.2004.10.040 [DOI] [PubMed] [Google Scholar]
- Carobrez AP, Bertoglio LJ, 2005. Ethological and temporal analyses of anxiety-like behavior: the elevated plus-maze model 20 years on. Neurosci. Biobehav. Rev 29, 1193–1205. doi: 10.1016/j.neubiorev.2005.04.017 [DOI] [PubMed] [Google Scholar]
- Chen Q, Nakajima A, Meacham C, Tang YP, 2006. Elevated cholecystokininergic tone constitutes an important molecular/neuronal mechanism for the expression of anxiety in the mouse. Proc. Natl. Acad. Sci. USA 103, 3881–3886. doi: 10.1073/pnas.0505407103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dockray R, 1980. Cholecystokinins in rat cerebral cortex: identification, purification and characterization by immunochemical methods. Brain Res 188, 155–165. doi: 10.1016/0006-8993(80)90564-8 [DOI] [PubMed] [Google Scholar]
- Ennaceur A, Delacour J, 1988. A new one-trial test for neurobiological studies of memory in rats. 1: Behavioral data. Behav. Brain Res 31, 47–59. 10.1016/0166-4328(88)90157-X [DOI] [PubMed] [Google Scholar]
- Eser D, Wenninger S, Baghai T, Schüle C, Rupretch R, 2008. Impact of state and trait anxiety on the panic response to CCK-4. J. Neural Transm 115, 917–920. doi: 10.1007/s00702-008-0047-2 [DOI] [PubMed] [Google Scholar]
- Farook JM, Zhu YZ, Wang Q, Moochhala SM, Lee L, Wong PT, 2004. Analysis of strain difference in behavior to cholecystokinin (CCK) receptor mediated drugs in PVG hooded and Sprague-Dawley rats using elevated plus-maze test apparatus. Neurosci. Lett 358, 215–219. doi: 10.1016/j.neulet.2004.01.027 [DOI] [PubMed] [Google Scholar]
- Feng T, Yang S, Wen D, Sun Q, Li Y, Ma C, Cong B, 2014. Stress-induced enhancement of fear conditioning activates the amygdalar cholecystokinin system in a rat model of post-traumatic stress disorder. Neuroreport 25, 1085–1090. doi: 10.1097/WNR.0000000000000232 [DOI] [PubMed] [Google Scholar]
- File SE, 1993. The interplay of learning and anxiety in the elevated plus-maze. Behav. Brain Res 58, 199–202. 10.1016/0166-4328(93)90103-W [DOI] [PubMed] [Google Scholar]
- Griebel G, Perrault G, Sanger DJ, 1997. CCK receptor antagonists in animal models of anxiety: comparison between exploration tests, conflict procedures and a model based on defensive behaviours. Behav. Pharmacol 8, 549–560. [DOI] [PubMed] [Google Scholar]
- Harro J, 2006. CCK and NPY as anti-anxiety treatment targets: promises, pitfalls, and strategies. Aminoacids 31, 215–230. doi: 10.1007/s00726-006-0334-x [DOI] [PubMed] [Google Scholar]
- Harro J, Marcusson J, Oreland L, 1992. Alterations in brain cholecystokinin receptors in suicide victims. Eur. Neuropsychopharmacol 2, 57–63. 10.1016/0924-977X(92)90037-9 [DOI] [PubMed] [Google Scholar]
- Hebb AL, Zacharko RM, Bowie JP, Drolet G, 2003. Differential startle reactivity following central CCK-8S and systemic Boc CCK-4 administration in mice: antecedent stressor history and testing condition. Behav. Neurosci 117, 704–715. 10.1037/0735-7044.117.4.704 [DOI] [PubMed] [Google Scholar]
- Hökfelt T, Blacker D, Broberger C, Herrera-Marschitz M, Snyder G, Fisone G, Cortes R, Morino P, You ZB, Ogren SO, 2002. Some aspects on the anatomy and function of central cholecystokinin systems. Pharmacol. Toxicol 91, 382–386. 10.1034/j.1600-0773.2002.910617.x [DOI] [PubMed] [Google Scholar]
- Hogg S, 1996. A review of the validity and variability of the elevated plus-maze as an animal model of anxiety. Pharmacol. Biochem. Behav 54, 21–30. 10.1016/0091-3057(95)02126-4 [DOI] [PubMed] [Google Scholar]
- Innis RB, Snyder S, 1980. Distinct cholecystokinin receptors in brain and pancreas. Proc. Natl. Acad. Sci. USA 77, 6917–6921. doi: 10.1073/pnas.77.11.6917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kellner M, Wiedemann K, Yassouridis A, Levengood R, Guo LS, Holsboer F, Yehuda R, 2000. Behavioral and endocrine response to cholecystokinin tetrapeptide in patients with posttraumatic stress disorder. Biol. Psychiatry 47, 107–111. 10.1016/S0006-3223(99)00118-3 [DOI] [PubMed] [Google Scholar]
- King MV, Sleight AJ, Woolley ML, Topham IA, Marsden CA, Fone KC, 2004. 5-HT6 receptor antagonists reverse delay-dependent deficits in novel object discrimination by enhancing consolidation--an effect sensitive to NMDA receptor antagonism. Neuropharmacology 47, 195–204. doi: 10.1016/j.neuropharm.2004.03.012 [DOI] [PubMed] [Google Scholar]
- Kõks S, Männistö PT, Bourin M, Shlik J, Vasar V, Vasar E, 2000. Cholecystokinin-induced anxiety in rats: relevance of pre-experimental stress and seasonal variations. J. Psychiatry Neurosci 25, 33–42. [PMC free article] [PubMed] [Google Scholar]
- Kramer MS, Cutler NR, Ballenger JC, Patterson WM, Mendels J, Chenault A, Shrivastava R, Matsura-Wolfe D, Lines C, Reines S, 1995. A placebo-controlled trial of L-365,260, a CCKB antagonist, in panic disorder. Biol. Psychiatry 37, 462–466. doi: 10.1016/0006-3223(94)00190-E [DOI] [PubMed] [Google Scholar]
- Ladurelle N, Roques BP, Daugé V, 1995. The transfer of rats from a familiar to a novel environment prolongs the increase of extracellular dopamine efflux induced by CCK8 in the posterior nucleus accumbens. J. Neurosci 15, 3118–3127. 10.1523/JNEUROSCI.15-04-03118.1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Ohta H, Izumi H, Matsuda Y, Seki M, Toda T, Akiyama M, Matsushima Y, Goto Y, Kaga M, Inagaki M, 2013. Behavioral and cortical EEG evaluations confirm the roles of both CCKA and CCKB receptors in mouse CCK-induced anxiety. Behav. Brain Res 237, 325–332. doi: 10.1016/j.bbr.2012.09.051 [DOI] [PubMed] [Google Scholar]
- Moore S, Deshpande K, Stinnett GS, Seasholtz AF, Murphy GG, 2013. Conversion of short term memory to long-term memort in the novel object recognition paradigm. Neurobiol. Learn Mem 105, 174–185. doi: 10.1016/j.nlm.2013.06.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panksepp J, Burgdorf J, Beinfeld MC, Kroes RA, Moskal JR, 2004. Regional brain cholecystokinin changes as a function of friendly and aggressive social interactions in rats. Brain Res 1025, 75–84. doi: 10.1016/j.brainres.2004.07.076 [DOI] [PubMed] [Google Scholar]
- Panksepp J, Burgdorf J, Beinfeld MC, Kroes RA, Moskal JR, 2007. Brain regional neuropeptide changes resulting from social defeat. Behav. Neurosci 121, 1364–1371. doi: 10.1037/0735-7044.121.6.1364 [DOI] [PubMed] [Google Scholar]
- Philipp E, Wilckens T, Fries EC, Platte P, Pirke KM, 1992. Cholecystokinin, gastrin and stress hormone responses in marathon runners. Peptides 13, 125–128. 10.1016/0196-9781(92)90150-2 [DOI] [PubMed] [Google Scholar]
- Rezayat M, Roohbakhsh A, Zarrindast MR, Massoudi R, Djahanguiri B, 2005. Cholecystokinin and GABA interaction in the dorsal hippocampus of rats in the elevated plus-maze test of anxiety. Physiol. Behav 84, 775–782. doi: 10.1016/j.physbeh.2005.03.002 [DOI] [PubMed] [Google Scholar]
- Ripoll N, Nic Dhonnchadha BA, Sébille V, Bourin M, Hascoët M, 2005. The four-plates test-retest paradigm to discriminate anxiolytic effects. Psychopharmacology (Berl) 180, 73–83. doi: 10.1007/s00213-004-2130-1 [DOI] [PubMed] [Google Scholar]
- Rodgers RJ, Johnson NJ, 1995. Cholecystokinin and anxiety: promises and pitfalls. Crit. Rev. Neurobiol 9, 345–369. [PubMed] [Google Scholar]
- Rotzinger S, Vaccarino FJ, 2003. Cholecystokinin receptor subtypes: role in the modulation of anxiety-related and reward-related behaviours in animal models. J. Psychiatry Neurosci 28, 171–181. [PMC free article] [PubMed] [Google Scholar]
- Singh L, Lewis AS, Field MJ, Hughes J, Woodruff GN, 1991. Evidence for an involvement of the brain cholecystokinin B receptor in anxiety. Proc. Natl. Acad. Sci, USA 88, 1130–1133. doi: 10.1073/pnas.88.4.1130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suman-Chauhan N, Meecham KG, Webdale L, Hunter JC, Pritchard MC, Woodruff GN, Hill DR, 1996. The influence of guanyl nucleotide on agonist and antagonist affinity at guinea pig CCK-B/gastrin receptors: binding studies using [3H] PD140376. Regul. Pept 65, 37–43. 10.1016/0167-0115(96)00070-5 [DOI] [PubMed] [Google Scholar]
- Treit D, Menard J, Royan C, 1993. Anxiogenic stimuli in the elevated plus-maze. Pharmacol. Biochem. Behav 44, 463–469. 10.1016/0091-3057(93)90492-C [DOI] [PubMed] [Google Scholar]
- van Megen HJ, Westenberg HG, den Boer JA, Kahn RS, 1996. Cholecystokinin in anxiety. Eur. Neuropsychopharmacol 6, 263–280. 10.1016/S0924-977X(96)00038-7 [DOI] [PubMed] [Google Scholar]
- Vargas KM, Da Cunha C, Andreatini R, 2006. Amphetamine and pentylenetetrazole given post-trial 1 enhance one-trial tolerance to the anxiolytic effect of diazepam in the elevated plus-maze in mice. Prog. Neuropsychopharmacol. Biol. Psychiatry 30, 1394–1402. doi: 10.1016/j.pnpbp.2006.05.004 [DOI] [PubMed] [Google Scholar]
- Vargas-López V, Torres-Berrio A, González-Martínez L, Múnera A, Lamprea MR, 2015. Acute restrain stress and cortiscoterone transiently disrupts novelty preference in an object recognition task. Behav. Brain Res 291, 60–66. 10.1016/j.bbr.2015.05.006 [DOI] [PubMed] [Google Scholar]
- Vasar E, Harro J, Lang A, Soosaar A, Oöpik T, Kôks S, Sihver S, Volke V, 1993. Anti-exploratory effect of N-methyl-D-aspartate in elevated plus-maze. Involvement of NMDA and CCK receptors. Eur. Neuropsychopharmacol 3, 63–73. 10.1016/0924-977X(93)90296-X [DOI] [PubMed] [Google Scholar]
- Verbanck BM, Lotstra F, Gilles G, Linkowski P, Mendlewicz J, Vanderhaeghen JJ, 1984. Reduced cholecystokinin immunoreactivity in the cerebrospinal fluid of patients with psychiatric disorders. Life Sci, 34, 67–72. doi: 10.1016/0024-3205(84)90331-x [DOI] [PubMed] [Google Scholar]
- Wang H, Wong PT, Spiess J, Zhu YZ, 2005. Cholecystokinin-2 (CCK2) receptor-mediated anxiety-like behaviors in rats. Neurosci. Biobehav. Rev 29, 1361–1373. doi: 10.1016/j.neubiorev.2005.05.008 [DOI] [PubMed] [Google Scholar]
- Wang H, Spiess J, Wong PT, Zhu YZ, 2011. Blockade of CRF1 and CCK2 receptors attenuated the elevated anxiety-like behavior induced by immobilization stress. Pharmacol. Biochem. Behav 98, 362–368. doi: 10.1016/j.pbb.2011.01.022 [DOI] [PubMed] [Google Scholar]
- Wilson J, Watson WP, Little HJ, 1998. CCK(B) antagonists protect against anxiety-related behaviour produced by ethanol withdrawal, measured using the elevated plus maze. Psychopharmacology (Berl.) 137, 120–131. doi: 10.1007/s002130050601 [DOI] [PubMed] [Google Scholar]
- Wunderlich GR, Raymond R, DeSousa NJ, Nobrega JN, Vaccarino FJ, 2002. Decreased CCK(B) receptor binding in rat amygdala in animals demonstrating greater anxiety-like behavior. Psychopharmacology (Berl) 164, 193–199. doi: 10.1007/s00213-002-1181-4 [DOI] [PubMed] [Google Scholar]
- Yu MJ, Thrasher KJ, McCowan JR, Mason NR, Mendelsohn LG, 1991. Quinazolinone cholecystokinin-B receptor ligands. J. Med. Chem 34, 1505–1508. doi: 10.1021/jm00108a040 [DOI] [PubMed] [Google Scholar]
- Zwanzger P, Domschke K, Bradwejn J, 2012. Neuronal network of panic disorder: the role of the neuropeptide cholecystokinin. Depress. Anxiety 29, 762–774. doi: 10.1002/da.21919 [DOI] [PubMed] [Google Scholar]