Abstract
A history of child abuse (CA) is associated with morbidity and mortality in adulthood, and one proposed mechanism is dysregulation of the hypothalamic-pituitary-adrenal (HPA) axis. Therefore, we evaluated whether a history of physical and sexual CA was associated with daily rhythms of HPA hormones (cortisol and dehydroepiandrosterone (DHEA)) among postmenopausal women (mean age: 60.6 years). In 2013, 233 participants from the Nurses’ Health Study II provided up to 5-timed saliva samples over the course of a day: immediately upon awakening, 45 minutes, 4 hours, and 10 hours after waking, and prior to going to sleep. Among these 233 participants, 217 provided ≥4 timed saliva samples. Assessment of physical and sexual CA history occurred in 2001 using the Revised Conflict Tactics Scale. Cumulative CA history was derived by combining reports of physical and sexual abuse prior to age 18. Piecewise linear mixed models compared diurnal rhythms of cortisol and DHEA between participants with none-to-moderate CA (n=104, reference group) versus high-to-severe CA (n=113). Models adjusted for characteristics at each saliva collection, health status, sleep quality, medications, and hormone use. Compared to those with none-to-moderate CA, women with high-to-severe CA had different diurnal rhythms in the early and evening hours, including blunted (less steep) early declines in DHEA (% difference (%D)= 10.7, 95% CI 4.3, 17.5), and steeper evening declines in both cortisol and DHEA (cortisol %D = −2.5, 95% CI −4.8, −0.1, and DHEA %D= −3.9, 95% CI −6.0, −1.8). In conclusion, high-to-severe abuse history prior to age 18 was more strongly associated with differences in DHEA rather than cortisol, suggesting that early life abuse may be related to dysregulation of stress-response mechanisms later in life.
Keywords: Child abuse, Cortisol, DHEA, Postmenopausal, Cortisol to DHEA ratio
INTRODUCTION
A history of child abuse (CA) is associated with some of the leading causes of death and disability in adulthood (1–3). Although abuses during childhood can include emotional abuse and neglect, physical and sexual abuse prior to age 18 can be particularly severe and are associated with a wide range of adverse mental and physical health outcomes in adulthood (4–9), with negative long-term health outcomes more common in women as compared to men (10–12). Abuse during childhood has been shown to result in biological and behavioral sequelae, including changes to neuroendocrine structures and function (13). An important mechanism linking CA to outcomes in adulthood is the dysregulation of allostatic systems designed to adapt and respond to acute stressors, whereby repeated adverse experiences lead to increased allostatic load (14, 15). One such system is the hypothalamic-pituitary-adrenal (HPA) axis, a major stress-related allostatic system with two main adrenal hormones, cortisol and its antagonist dehydroepiandrosterone (DHEA).
Pulsating cortisol rhythms are preserved with physiological ageing, although higher nocturnal levels have been observed in older individuals (16, 17). Cortisol levels naturally rise in the morning prior to awakening, decline throughout the day, and are at their lowest in the evening. Awakening bursts of secretory activity enhance the immune system (18, 19), declines throughout the day allow for synaptic turnover that facilitate learning (20, 21), and the total area under the curve (AUC) is a summary measure that reflects the magnitude of this response throughout the day (22). These “on and off” fluctuations are important for coordinating daily activities and normal stress-response, and the loss of such adaptions in the form of flattened rhythms have been observed with exposure to chronic stressors (23, 24). DHEA diurnal rhythms are characterized by elevated post-awakening peaks followed by decreases throughout the day, and in contrast to cortisol exhibit an age-related decline (16, 25). Further, their ratio (cortisol:DHEA) can help shed light on the “catabolic/anabolic balance” (26, 27) and may be important in understanding stress-reactivity (28). Since both cortisol and DHEA are secreted in response to adrenocorticotrophin (ACTH), changes in one in relation to the other may provide a read out of adrenocortical secretory mechanisms, where one hormone may become up-or down-regulated in response to chronic stress over time. Higher ratios due to higher cortisol levels have been observed with chronic stress, including childhood maltreatment (29), and lower ratios due to higher DHEA have been associated with greater resilience in some (30), but not all (31), studies.
Changes in cortisol and DHEA diurnal rhythms have been reported in relation to CA history in adolescent and young adult populations (32–34). Notably, the impacts of CA on dysregulated adrenal hormone secretion may be cumulative over time. For example, a history of CA was more strongly associated with blunted cortisol secretion among older (aged 36–61) compared to younger adults (aged 18–35) (35). Evidence of HPA axis dysregulation, including a flattened diurnal rhythm, or overall higher daily levels, may provide a window into later developing disease risk (14, 24). Flattened salivary cortisol rhythms are associated with coronary calcification (36), and HPA axis dysregulation may be an important pathway linking a history of CA with subclinical cardiovascular disease and inflammation in older women (8, 37). Further, DHEA levels are also associated with cardiovascular health in postmenopausal women (38). Demonstrating the potential for long-term health effects, such dysregulations are also associated with incident fractures (22), memory loss (23), cardiovascular disease (24, 25), and estrogen-receptor positive breast cancers (26) in older women. Notably, adverse childhood experiences have been directly associated with skeletal fractures, mental health disorders, ischemic heart disease, cancer, and chronic lung and liver diseases (1).
Few studies have examined the potential link between CA history and HPA axis dysregulation in older populations, particularly in older women when the adrenal gland becomes a major androgenic source. Thus, we investigated the association between CA history and diurnal rhythms of cortisol, DHEA, and their ratio (cortisol:DHEA) in postmenopausal women.
METHODS
Study Population
The Nurses’ Health Study II (NHSII) is an ongoing longitudinal study of U.S. female registered nurses established in 1989. At baseline, 116,429 women returned a health and lifestyle questionnaire, and biennial questionnaires are mailed to update information on diseases diagnoses and exposures. The Mind Body Study (MBS) is a sub-study among NHSII participants (age range 45–69 years) that aims to characterize psychosocial stress and its associations with biomarkers by collecting additional biospecimens to measure HPA axis markers as well as a detailed questionnaire of validated, stress-related scales, and is described in detail elsewhere (39). Briefly, women who previously provided a blood sample were invited via email to participate (N=688), oversampling women who reported physical or sexual CA history on a questionnaire mailed in 2001 (4, 5) in order to increase the distribution of stress-related phenotypes (40). Respondents who returned the 2001 questionnaire were similar to non-respondents in age, race, BMI, hypertension, diabetes, depression, and medication use. MBS participants signed informed consent and completed an online psychosocial stress questionnaire and provided 5 timed saliva samples over the course of one day in 2013 (N=233). In general, women who completed the MBS were similar to the overall NHS2 cohort, although were more likely to take psychotropic medications and, by design, were more likely to experience CA (39). Study protocols for NHSII and MBS were approved by the institutional review board of the Brigham’s and Women’s Hospital.
Timed saliva samples
MBS participants were mailed a kit containing collection instructions, equipment, and return packaging. Participants were instructed to collect saliva at standard collection times: immediately upon awakening (before getting out of bed), 45 minutes after waking, 4 hours after waking, 10 hours after waking, and just prior to bed. This technique provides information about diurnal cortisol secretion rhythms which allows for the detailed calculation of multiple aspects of the diurnal rhythm while capturing peak and nadir levels (41), and is not a measure of stress reactivity (for which data are not available in the current sample). Collection details are further described by Huang and colleagues (39). MBS participants remained in bed prior to the first saliva collection (40), and at each collection were instructed to fill out a log indicating the time, whether they brushed their teeth, ate, drank, exercised, and whether they felt happy, excited, content, worried, anxious, or fearful at that time. Samples were refrigerated upon collection. Participants were instructed to ship samples with a cold pack to the laboratory using overnight mail on the following day, which were aliquoted and stored in the vapor phase of liquid nitrogen freezers. Assays for cortisol and DHEA were conducted at the Laboratory for Biological Health Psychology (Dr. Nicholas Rohleder) at Brandeis University using a competitive chemiluminescence immunoassay (CLIA-approved) with high sensitivity. All samples were measured in duplicate, and samples with CVs > 10% were repeated. CVs for blinded QC pools were < 6%. Cortisol and DHEA in saliva are stable with delayed freezing of up to 4 days (42).
Child abuse
An adaptation of the Revised Conflict Tactics Scale (43) was used to ask participants about their physical and sexual abuse experiences during childhood (up to and including age 17). Physical abuse was assessed separately for young childhood (up to age 11) and adolescence (ages 11–17) by asking participants to report the frequency with which they experienced abuse by a parent, step-parent or adult guardian. Physical abuse categories of none, mild (being pushed, grabbed, or shoved at any frequency or being kicked, bitten, or punched once or hit with something once), moderate (being hit with something more than once or physically attacked once), and severe (being kicked, bitten, or punched or physically attacked more than once or ever choked or burned) were assessed (5). Responses were then categorized as either: no physical abuse, mild or moderate child and/or adolescent physical abuse, severe child or adolescent physical abuse (not both), and severe child and adolescent physical abuse. Sexual abuse was assessed separately for young childhood (up to age 11) and as an adolescent (ages 11–17) by asking participants to report the frequency with which a parent, step-parent or adult guardian touched them in a sexual way when they did not want to; or forced (or attempted to force) them into any sexual activity. Responses were then categorized as either: never, touched at least once, forced sex at least once as child or as teen (not both), and forced sex at least once as child and teen. Participants who reported having ever been touched or forced into sex were categorized as sexually abused. Cumulative physical and sexual child abuse (CA) experiences were then grouped into six mutually exclusive categories based on severity, with higher values indicating more severe cumulative and physical abuse. For example, (1) indicated no physical or sexual abuse, and (6) indicated severe physical abuse and forced sex in both childhood and adolescence (Appendix). Cumulative physical and sexual abuse categories of none-to-moderate (1–2) and high-to-severe (3–6) were used in analyses to increase power. Cumulative physical and sexual abuse categories of none-to-moderate (1–2), high (3–4), severe (5–6) were also explored. Reports of severe physical abuse and forced sex were restricted to those with high-to-severe CA (APPENDIX Table 1). Participants with both physical and sexual CA were more likely to have been diagnosed with clinical depression and be on anti-depressants.
Covariates
The baseline NHSII questionnaire asked about birth date and height. Information on multiple adult factors that may act as precision variables (i.e., explain variance in the outcome, but are not associated with CA) or potential mediators were collected at the time of sample collection or on biennial questions near the time of collection. Biennial questionnaires provided information on: weight, lifetime duration of night shiftwork, smoking, hypertension, type II diabetes, sleep apnea, menopausal status, surgical ovary removal, and history of physician-diagnosed depression, as well as alcohol and caffeine consumption, which were collected using a validated semi-quantitative food frequency questionnaire (44, 45). In 2008, a modified version of the brief trauma questionnaire (BTQ) was used to ascertain women’s experiences of 16 traumatic events, including abuse prior to age 18, and derived from the Brief Trauma Interview (46). Participants were then asked to recall their worst trauma and age of first occurrence. PTSD symptoms were evaluated as a potential precision variable using the 7-item Brief Screening Scale for DSM-IV PTSD based on the worst trauma, irrespective of whether the worst trauma was abuse during childhood. A cutoff of 4 or more symptoms was then used as the basis of categorization for suggestive PTSD symptoms, which maximized sensitivity and specificity (0.85 and 0.84, respectively) when compared to the Clinician-Administered PTSD Scale (47).
Within 1-month of the saliva collection participants provided information on medication use and psychosocial factors, and designed to overlap the time period of the saliva collection. We considered medications that may be related to cortisol or DHEA levels in saliva (oral steroids, DHEA medication, testosterone use, hormone therapy) or with CA (antidepressants, beta-blockers, minor tranquilizers). Sleep patterns, which have been related to the salivary stress hormones in the past month (48), were assessed using the Pittsburgh Sleep Quality Index (PSQI) (49). Insomnia with short sleep duration was defined as trouble falling asleep, trouble maintaining sleep, or early awakening, with ≤6 hours of sleep (50). Global PSQI scores greater than 5 have >0.8 sensitivity and specificity to distinguish clinically relevant sleep disturbances. Psychometric properties for psychosocial MBS questionnaires are described in detail elsewhere (39). Symptoms of anxiety in the past two weeks were assessed using the 7-item Generalized Anxiety Disorder (GAD-7) Scale (Cronbach’s alpha=0.89 in MBS (40)), and a score >5 was used as the basis for probable anxiety
Statistical analyses
Analyses excluded participants with missing information on CA history (N=8), missing two or more timed saliva collections (N=6), missing information on menopausal status (N=1), or extreme outliers at three or more timed saliva collections (>3 standard deviations (SD) from the mean; N=1). Our final sample size consisted of 217 participants, 214 provided all five timed saliva samples (40). Collection times were referenced to time at awakening. Cortisol, DHEA, and their ratio (cortisol to DHEA) were log-transformed due to right skewness (51, 52). The latter was log-transformed after calculating the ratio of cortisol to DHEA. Log-transformations resulted in approximately normal distributions. In visual depictions of diurnal rhythms of cortisol, DHEA and their ratio, the collection times of 45 minutes after awakening, 4 hours after awakening, and 16 hours after awakening were each used as points of inflection for the diurnal rhythm, similar to prior studies (48, 53, 54).
To evaluate differences in the diurnal components comparing participants with high-severe versus none-moderate CA (reference group), we fit linear mixed models with piecewise linear spline functions at each of the designated inflection points and cross-product interaction terms between the indicator variable for CA with each of the three spline functions. Components of diurnal rhythms were evaluated separately for cortisol, DHEA and their ratio, and included: 1) waking level, 2) awakening response (the change from waking level to 45 minutes after awakening), 3) early decline (the change from 45 minutes after awakening to 4 hours after awakening), 4) late decline (the change from 4 hours after awakening to 16 hours after awakening), 5) late night rise (change from 16 hours after awakening to bedtime), 6) the area under the curve (AUC) from awakening to 16 hours after awakening, and 7) the overall slope (the change from awakening to bedtime). Models were fit separately for log-transformed cortisol, log-transformed DHEA, and log-transformed ratio of cortisol:DHEA. Based on estimates from the mixed model, percent differences were calculated by exponentiating the corresponding coefficient or the linear combination of coefficients and subtracting one. To assess relationships with CA severity more finely, we repeated models with the following categories: none-moderate (reference, score 1–2), high (score 3–4), and severe (score 5–6). In secondary analyses, we evaluated the joint associations of CA and PTSD symptoms, respectively, using the following categories: 1) none-moderate and <4 PTSD symptoms (n=97), 2) none-moderate and ≥4 PTSD symptoms (n=16), 3) high-severe and <4 PTSD symptoms (n=70), and 4) high-severe and ≥4 PTSD symptoms (n=34).
Multivariable models adjusted for factors that could influence diurnal rhythms, including categorical time-varying saliva collection characteristics (brushed teeth, ate, drank, exercised, felt happy, excited, content, and felt worried, anxious, or fearful), age (continuous), PTSD (≥4 symptoms, yes/no), body mass index (continuous), night shiftwork (continuous), hypertension (yes/no), type II diabetes (yes/no), caffeine intake (continuous), alcohol consumption (continuous), current smoking status (yes/no), sleep apnea (yes/no), poor sleep (yes/no), history of physician-diagnosed depression (yes/no), probable anxiety (GAD>5, yes/no), medication use (antidepressants, beta-blockers, minor tranquilizers, oral steroids, and hormone use, yes/no for all), and surgical ovary removal (yes/no). We considered four models with different levels of statistical adjustment (see footnote Table 2). Since delayed sleep times result in a more marked cortisol rise (55), we conducted sensitivity analyses excluding participants who went to sleep one half hour later than usual (6.2% of participants with none-moderate CA and 11.5% of participants with high-severe CA). We also conducted sensitivity analyses excluding participants taking DHEA medications (4.4% of participants with none-moderate CA and 1.0% of participants with high-severe CA). All analyses were conducted in SAS 9.4 (Cary, NC).
Table 2:
Percent differences (%D) and 95% confidence intervals (CIs) of diurnal biomarker components comparing participants with a cumulative physical and sexual child abuse (CA) history of high-severe (N=104) to none-moderate (N=113, reference group).
| N=217 | Model 1 | Model 2 | Model 3 | Model 4 | ||||
|---|---|---|---|---|---|---|---|---|
| %D | 95% CI | %D | 95% CI | %D | 95% CI | %D | 95% CI | |
| Cortisol | ||||||||
| Waking level a | 1.6 | −15.7, 22.5 | −1.4 | −18.4, 19.1 | −5.0 | −21.6, 15.1 | −7.7 | −23.8, 11.8 |
| Awakening response b | 3.0 | −22.1, 36.0 | 3.5 | −21.7, 36.8 | 3.6 | −22.0, 37.7 | 3.7 | −22.0, 37.8 |
| Early decline c | 4.1 | −2.4, 10.9 | 4.2 | −2.3, 11.0 | 5.7 | −1.0, 12.8 | 5.7 | −1.0, 12.8 |
| Late decline d | −2.5 | −4.8, −0.2 | −2.6 | −4.8, −0.2 | −2.5 | −4.8, −0.1 | −2.5 | −4.8, −0.1 |
| Night rise e | 17.6 | −13.6, 60.1 | 17.4 | −13.8, 59.9 | 22.2 | −10.4, 66.6 | 22.1 | −10.5, 66.5 |
| AUC f | 0.5 | −1.8, 2.9 | 0.1 | −2.3, 2.5 | 0.2 | −2.3, 2.7 | −0.2 | −2.7, 2.2 |
| Overall slope g | −0.6 | −2.3, 1.0 | −0.7 | −2.3, 1.0 | −0.3 | −2.0, 1.4 | −0.3 | −2.0, 1.4 |
| Dehydroepiandrosterone (DHEA) | ||||||||
| Waking level | 12.7 | −13.9, 47.5 | 8.8 | −16.7, 42.1 | 12.1 | −15.7, 49.2 | 12.8 | −15.3, 50.3 |
| Awakening response | −11.3 | −31.4, 14.7 | −11.2 | −31.1, 14.3 | −10.6 | −31.1, 16.0 | −10.6 | −31.1, 16.0 |
| Early decline | 10.2 | 3.9, 16.8 | 10.1 | 3.9, 16.6 | 10.6 | 4.2, 17.5 | 10.7 | 4.3, 17.5 |
| Late decline | −3.7 | −5.8, −1.6 | −3.8 | −5.8, −1.7 | −3.9 | −6.0, −1.8 | −3.9 | −6.0, −1.8 |
| Night rise | 25.5 | −5.6, 66.8 | 30.0 | −1.8, 72.1 | 33.1 | 0.0, 77.2 | 32.7 | −0.3, 76.6 |
| AUC | 2.1 | −1.9, 6.1 | 1.4 | −2.6, 5.5 | 2.1 | −2.2, 6.4 | 2.2 | −2.1, 6.5 |
| Overall slope | −0.5 | −2.1, 1.0 | −0.6 | −2.1, 1.0 | −0.6 | −2.1, 1.0 | −0.6 | −2.1, 1.0 |
| Cortisol to DHEA ratio | ||||||||
| Waking level | −10.0 | −30.6, 16.8 | −9.4 | −30.0, 17.2 | −15.5 | −35.3, 10.4 | −17.6 | −37.2, 8.1 |
| Awakening response | 16.3 | −11.1, 52.2 | 16.6 | −10.7, 52.1 | 16.1 | −11.2, 51.7 | 16.2 | −11.1, 51.9 |
| Early decline | −5.5 | −11.1, 0.5 | −5.4 | −10.9, 0.5 | −4.5 | −10.2, 1.5 | −4.5 | −10.2, 1.5 |
| Late decline | 1.2 | −1.0, 3.3 | 1.2 | −1.0, 3.3 | 1.4 | −0.7, 3.7 | 1.4 | −0.7, 3.7 |
| Night rise | −5.0 | −28.0, 25.5 | −7.5 | −29.9, 25.0 | −8.5 | −30.7, 20.8 | −8.4 | −30.6, 21.0 |
| AUC | −1.6 | −5.7, 2.5 | −1.4 | −5.5, 2.7 | −2.0 | −6.2, 2.3 | −2.4 | −6.7, 2.0 |
| Overall slope | −0.1 | −1.5, 1.2 | −0.1 | −1.5, 1.2 | 0.2 | −1.2, 1.6 | 0.2 | −1.2, 1.6 |
Values in bold are statistically significant at the alpha 0.05 level
biomarker level at awakening
change in biomarker from awakening to 45 minutes after awakening
change in biomarker from 45 minutes after awakening to 4 hours after awakening
change in biomarker from 4 hours after awakening to 16 hours after awakening
change in biomarker from 16 hours after awakening to bedtime
area under the curve, total biomarker levels from wake time to 16 hours after awakening
change in biomarker from awakening to bedtime
Model 1=unadjusted; Model 2=Age, four or more PTSD symptoms, saliva collection characteristics (ate, drank, brushed, exercised, happy, or worried); Model 3=Model 2 + history of shiftwork, BMI, hypertension, type 2 diabetes, sleep apnea, smoking, caffeine, alcohol intake, insomnia, and poor sleep; Model 4= Model 3 + history of physician diagnosed depression, anxiety, beta-blocker use, minor tranquilizer use, oral steroid use, DHEA medication, surgical ovary removal, and current hormone use
RESULTS
Participants were predominately Non-Hispanic White (>95%) and the mean age was 60.6 (SD=4.0; Table 1). Nearly half of all study participants had a history of high-severe cumulative physical and sexual CA (N=104, 47.9%). Compared to women with a history of none-moderate CA, those with a history of high-severe CA were more likely to have a history of chronic diseases (hypertension, diabetes, depression), use antidepressants and minor tranquilizers, and have increased levels of psychosocial distress (anxiety, PTSD symptomology, insomnia symptoms). Individuals with high-severe CA were also more likely to report abuse during childhood as their worst lifetime trauma (40.3% vs. 7.6%) and younger age at worst trauma (irrespective of whether that trauma was abuse during childhood) (ages 25.5 vs. 30.2). CA group differences were statistically significant at the alpha 0.05 level for PTSD symptomology, child abuse as worst trauma, age at worst trauma, and minor tranquilizer use. Participants with a history of severe CA (N=26) had the worst health and sleep (Supplemental Table 1).
Table 1:
Characteristics of Mind Body Study participants in 2013 by cumulative physical and sexual child abuse (CA) history.
| Characteristics | Cumulative physical and sexual CA history | ||
|---|---|---|---|
| All | None-Moderate | High-Severe | |
| N (%) | 217 (100.0) | 113 (52.1) | 104 (47.9) |
| Mean age in years (SD) | 60.6 (4.0) | 61.0 (3.9) | 60.1 (4.4) |
| Mean body mass index (kg/m2) | 26.6 (6.1) | 26.1 (6.1) | 27.1 (6.0) |
| History of type 2 diabetes % | 4.6 | 2.7 | 6.7 |
| History of hypertension % | 34.6 | 27.4 | 42.3 |
| Surgical ovary removal % | 28.8 | 24.1 | 34.0 |
| Current smokers % | 3.2 | 1.8 | 4.8 |
| Alcohol drinking, gram/day (SD) | 7.6 (11.1) | 7.4 (9.7) | 7.9 (12.4) |
| Caffeine intake (mg/day) | 178 (145) | 163 (130) | 195 (158) |
| Total caloric intake (kcal/day) | 1773 (554) | 1813 (569) | 1729 (535) |
| Recent shiftwork in the past 4 years | 6.9 | 5.3 | 8.9 |
| History of shiftwork % | |||
| None | 23.0 | 29.2 | 16.4 |
| ≤5 years | 52.5 | 49.6 | 55.8 |
| >5 years | 24.4 | 21.2 | 27.9 |
| Mental health and trauma | |||
| History of physician-diagnosed depression % | 32.3 | 23.9 | 41.4 |
| Probable anxiety (GAD score ≥5) | 19.9 | 14.4 | 26.0 |
| PTSD symptoms ≥4 % | 23.0 | 14.2 | 32.7 |
| Mean age of worst traumatic event (SD)* | 29.6 (13.7) | 30.2 (13.0) | 25.5 (16.4) |
| Child abuse worst trauma % | 18.4 | 3.5 | 34.6 |
| Sleep | |||
| Awake ≥18 hours % | 4.6 | 4.4 | 4.8 |
| Bedtime 0.5hr later than usual % | 8.8 | 6.2 | 11.5 |
| History of sleep apnea % | 6.1 | 4.5 | 7.8 |
| History of insomnia % | 19.8 | 14.2 | 26.0 |
| Poor sleep (PSQI score >5) % | 61.7 | 51.8 | 72.7 |
| Medication use | |||
| History of antidepressant use % | 24.9 | 17.7 | 32.7 |
| History of minor tranquilizer use % | 8.3 | 3.5 | 13.5 |
| Beta-blocker use % | 13.8 | 9.7 | 18.3 |
| DHEA medication use % | 2.8 | 4.4 | 1.0 |
| Oral steroid use % | 4.2 | 4.4 | 3.9 |
| Testosterone therapy use % | 0.9 | 1.8 | 0.0 |
Abbreviations: GAD-7= Generalized Anxiety Disorder 7-item scale; PSQI= Pittsburgh sleep quality index, PTSD= post-traumatic stress disorder
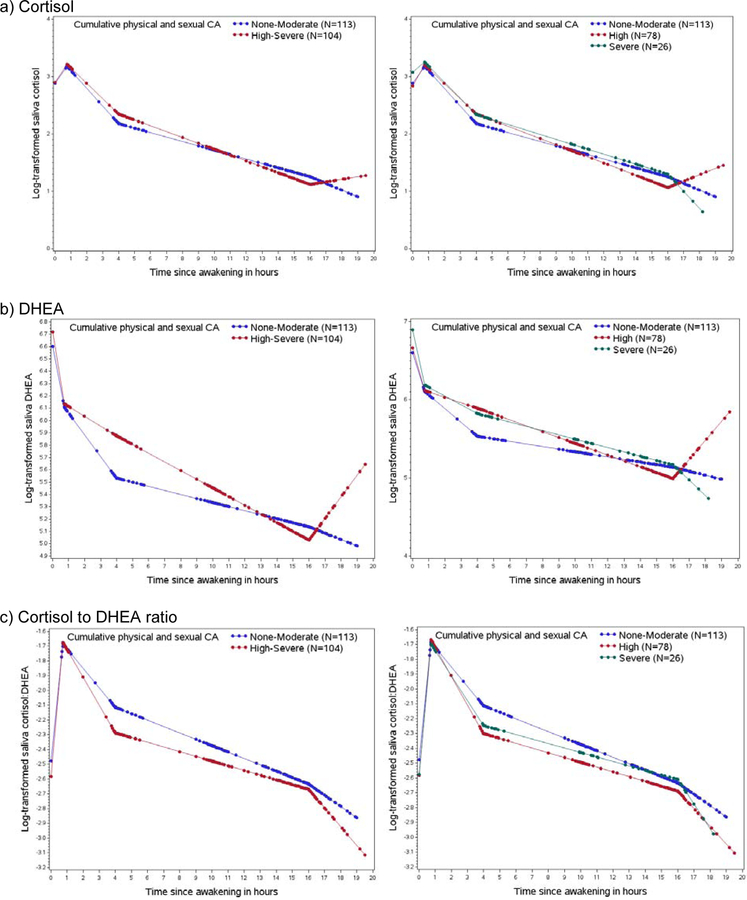
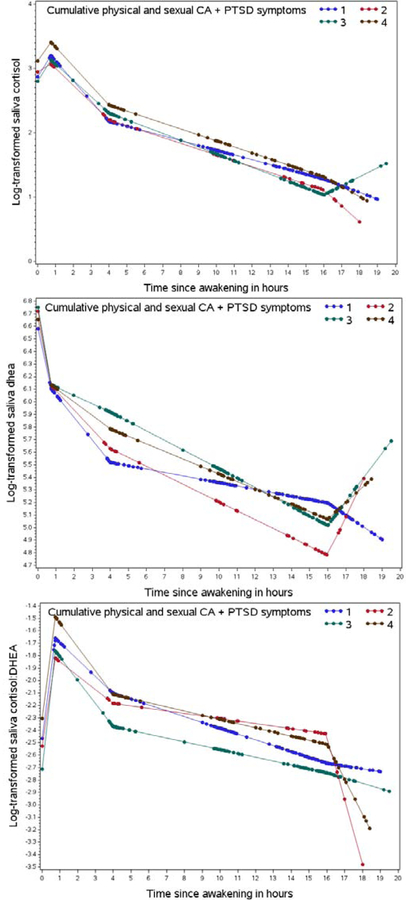
Participants with a history of high-severe CA had higher DHEA levels throughout the course of the day and lower cortisol to DHEA ratios, while cortisol levels appeared similar for most measures (Figure 1). In fully adjusted analyses, participants with a history of high-severe versus none-moderate CA had blunted (less steep) early declines in DHEA (%D= 10.7, 95% CI 4.3, 17.5), steeper evening declines in DHEA (%D= −3.9, 95% CI −6.0, −1.8), and steeper evening declines in cortisol (% difference (%D)= −2.5, 95% CI −4.8, −0.1) (Table 2). Although not statistically significant in the fully adjusted model, there was a suggestion of a 32.7% higher night rise in DHEA (95%CI: −0.3, 76.6) and 22.1% higher night rise in cortisol (95%CI: −10.5, 66.5) among those with high-severe CA history versus non-moderate. No statistically significant differences were observed for the cortisol:DHEA ratio. The results were similar, when separating high and severe CA history in comparison to none-moderate (Figure 1). In secondary analyses of the association between comorbid CA/PTSD and diurnal rhythms, blunted early declines in DHEA were observed among participants with a history of high-severe versus non-moderate CA, irrespective of PTSD symptomology (Figure 2). In sensitivity analyses excluding participants who stayed up more than 0.5 hour later than usual on the night of saliva collection, results were similar, except we observed a significantly higher night rise in DHEA for those with high-severe CA history (%D= 46.5, 95% CI 0.9, 112.6) compared to none-moderate CA (Supplemental Table 2). Findings did not differ when DHEA medication users were excluded (data not shown).
Figure 1:
Diurnal rhythms by cumulative physical and sexual child abuse (CA) history. Dots represent predicted values from mixed models at the actual time of the saliva collection. Legends are provided within each figure.
Figure 2:
Diurnal rhythms of cumulative physical and sexual child abuse (CA) history and PTSD symptoms. Dots represent predicted values from mixed models at the actual time of the saliva collection. Legend: (1) blue represents none-moderate CA and <4 PTSD symptoms (n=97), (2) red represents non-moderate CA and ≥4 PTSD symptoms (n=16), (3) green represents high-severe CA and <4 PTSD symptoms (n=70), and (4) brown represents high-severe CA and ≥ 4 PTSD (n=34).
DISCUSSION
In this sample of postmenopausal women, we observed that a history of high-severe cumulative physical and sexual CA was associated with steeper evening declines in cortisol and DHEA, and blunted early declines in DHEA compared to those with none-moderate CA. Interestingly, there was also a much higher increase in the night rise for cortisol and DHEA levels among women with high-severe CA. Results for CA history were similar among those with and without PTSD symptomology, although sample sizes within each group were limited. In addition, no clear differences in cortisol:DHEA ratios were observed, although visual depictions of the diurnal rhythm suggested lower cortisol:DHEA ratios over the course of the day among individuals with a history of high-severe CA.
Although few studies have directly evaluated the relationship between CA history and DHEA, dysregulated DHEA has been associated with a history of abuse during childhood. For example, among a clinical sample of 33 PTSD patients in their mid-30’s (26 women and 7 men), sexual and/or physical abuse prior to the age of 12 was associated with higher mean DHEA levels collected intravenously over the course of two hours at 15-minute intervals (56). Further, elevated hair levels of DHEA, but not cortisol, were observed among new mothers with a history of child maltreatment (mean age 32.5) (57). Overall, these results are similar to what we observed among older postmenopausal women, with blunted declines (flattened diurnal rhythm) in DHEA and increased night rises, suggesting that a history of child adversity may be associated with higher overall androgenic exposure over the lifecourse. While this adaptation may offset the effect of cortisol, high androgen levels have been associated with increased risk of a number of chronic diseases in women (58–62). Further work is necessary to understand the impact of alterations of DHEA diurnal rhythms linking CA history and subsequent health and disease risk, particularly in postmenopausal women who experience reduced DHEA secretion with aging.
We observed steeper late declines with cortisol, which is not consistent with a study among postmenopausal women with no hormone use, showing no association of sexual abuse history in childhood or adulthood with cortisol secretion patterns (63). Furthermore, flattened daytime rhythms of cortisol have been documented among adults exposed to chronic and prolonged stress in childhood (33, 64). Despite our findings of steeper rather than blunted cortisol declines, differences comparing CA categories were small (Figure 1, %D=2.5), and no differences were observed for AUC, an estimation for total diurnal cortisol output. Consistent with prior work finding elevated nocturnal adrenal levels with physiological ageing in men and women (16, 17, 65), we observed suggestively higher nocturnal cortisol levels of cortisol. Higher nocturnal cortisol and DHEA secreted in response to adrenocorticotrophin (ACTH) may be indicative of upstream dysregulations during expected nadirs for those exposed to chronic stress in early life. In older women, it appears that diurnal cortisol rhythms may not be substantially influenced by history of childhood CA, although increasing evidence suggests that childhood CA alters cortisol levels in response to acute stressors, such as the Trier social stress test (66–69).
Prior studies have suggested that comorbid distress, such as PTSD, may exacerbate the impact of history of CA on later cortisol and DHEA levels (34, 56). A recent meta-analysis details the literature describing DHEA and PTSD (70), with at least two studies investigating childhood physical and sexual abuse as the main trauma type (34, 71), and all participants were women. For example, when comparing women (mean age of 31–34) with a history of sexual CA and PTSD versus neither, the 24-hour AUC was lower for cortisol and higher for DHEA, although not statistically significant (34). Contrasting results were observed among female adolescents with PTSD due to a single sexual trauma, who had lower plasma DHEAS levels than healthy girls exposed to neither (72). In our study we generally observed similar results for women who experienced high-severe CA regardless of PTSD status. No differences in cerebrospinal fluid DHEA levels were observed comparing premenopausal women with PTSD related to a history of childhood physical abuse versus non-trauma-exposed controls (71). Several factors may play into the different results across the studies, the most notable of which is limited power to assess CA history and PTSD simultaneously due to small subgroup sample sizes. Other possible explanations include differences in the hormones measured (e.g., DHEAS has a longer half-life than DHEA resulting in less diurnal variation) and different hormonal milieu of adolescent, premenopausal and postmenopausal women. Larger studies are needed that simultaneously collect cortisol and DHEA while assessing PTSD symptomology and other comorbid forms of distress along with history of CA among a larger number of participants, including men.
Observations of suggestively lower salivary cortisol:DHEA ratios due to higher DHEA levels in relation to CA, may indicate an up-regulation of DHEA relative to cortisol in our sample of postmenopausal women. Recently, Kimonis and colleagues reported higher salivary cortisol:DHEA ratios, due to elevated cortisol levels, was associated with childhood maltreatment (including neglect and physical, sexual, and emotional abuse) among incarcerated male juvenile offenders (29). Differences across studies are possibly due to differences in study design, particularly participant ages, abuse categorizations, and saliva collection procedures. In a recent review of the literature reporting on cortisol:DHEA ratios and psychopathology (73), age is highlighted as an important factor when evaluating this ratio in light of age-related changes in DHEA. Long-term modifications to adrenocortical mechanisms in response to chronic stress, such as abuse during childhood, may differ over time and with proximity to the events in question. Further, differences in mediating coping mechanisms and resources (economic, social support, etc.) across the life course may influence observed associations between child abuse history and cortisol:DHEA ratios. Given our select sample of postmenopausal women, the observed upregulation of DHEA in relation to cortisol may be partly a function of these women being highly resilient high-functioning survivors of moderate-severe child abuse.
Our study has some limitations. First, we did not account for multiple testing when we examined the association between CA history and the individual diurnal components. Nevertheless, the consistency of associations with respect to DHEA reduces the possibility of false positive findings. Second, we collected saliva samples over the course of only one day, which may lead to misclassification as cortisol has day-to-day variation. We addressed this, in part, by evaluating the association between CA history and the overall slope from awakening to bedtime, which has less day-to-day variation (74). Third, evaluation of diurnal rhythms across categories of comorbid CA and PTSD were limited by sample size. Fourth, abuse from non-parents and guardians may have been missed. We also did not assess other forms of child abuse (e.g. emotional abuse and neglect) and we did not have information on duration of abuse (months vs. years) or therapy-seeking. Abuse recollection also occurred many years after childhood. Given the MBS study design of oversampling women with CA (39), few women had no physical or sexual CA, limiting power to use this as a reference group. While this could lead to some exposure misclassification, and thus attenuate our results, studies have largely observed health-related associations with severe or repeated forms of physical and sexual CA (4–9), justifying our decision to focus our evaluation on high to severe CA categories. Lastly, our findings may not be generalizable to populations where prevalence of CA and access to certain resources (e.g. antidepressants, social support) differ. For example, if nurses in our study with a history of severe child abuse were more likely to be high-functioning compared to severe child abuse survivors in the general population, differences in cortisol may have been harder to detect. Residual confounding by socioeconomic status may also have impacted results.
Observed differences in DHEA rather than cortisol may in part be due to vast physiological changes in androgen production in postmenopausal women, including DHEA and its sulfate (DHEAS) (75–77). Age-related declines in DHEA are commonly reported in men and women (16), and such changes are positively correlated with changes in other circulating hormones (76), which we did not adjust for in analyses. Recent studies also show that such declines across the menopausal transition, and after, are not consistent across racial-ethnic groups (75, 78, 79). Our study population is predominately Non-Hispanic White (>95%). Further, day-to-day levels of salivary DHEA are more robust compared to cortisol (25), and may also partly explain why observed associations with CA history were stronger with DHEA. Thus, observed associations with cortisol:DHEA ratios may have also been impacted. Differences in salivary flow rates of cortisol and DHEA have also been reported (80), and were not taken into account in these analyses.
Notable strengths of our study include the use of five timed saliva samples to characterize the diurnal rhythms of cortisol and DHEA simultaneously, allowing us to examine individual aspects that comprise the diurnal rhythm in addition to AUC and overall slope. Further, our sample was embedded in a well-phenotyped prospective cohort with highly reliable self-reports of health and lifestyle, and our statistical modeling accounted for a number of potential confounding and intermediate factors.
CONCLUSION
Abuse history during childhood was associated with differences in DHEA diurnal rhythms, potentially leading to higher androgenic exposures; however, no clear differences were observed with cortisol rhythms. Increasing data support the notion that childhood adversity can lead to alterations in HPA axis activity that last throughout the life course, even into postmenopause, and may be a mechanism by which CA influences chronic disease development later in life. To better understand the underlying mechanisms and health consequences of abuse during childhood, further studies are needed in larger diverse sample sizes of men and women that collect neuroendocrine biomarkers (resting and reactive) and assess psychological distress, coping, and functionality, among others. Ultimately, such findings will help identify buffers and resilience factors, improve the health of survivors of abuse during childhood, and help advocate for its prevention.
Supplementary Material
HIGHLIGHTS.
Diurnal profiles differed in postmenopausal women with a history of severe early abuse
Specifically, diurnal DHEA rhythm differed with effects most evident in the evening.
Early abuse was more consistently associated with altered DHEA than cortisol.
Child abuse may impact later health via dysregulation of the HPA axis hormones.
ACKNOWLEDGMENTS
The authors assume full responsibility for analyses and interpretation of these data. The authors would also like to thank the Nurses’ Health study participants for their invaluable contributions, without which this research would not have been possible.
FUNDING
This work was supported by the National Institute of Health (grant number UM1 CA176726, R01 CA163451) and Department of Defense award, W81XWH-13–1-0493. T.H. is a recipient of the American Heart Association Postdoctoral Fellowship (Founders Affiliate) Award (16POST27480007). O.R.O was supported by funding from the National Institute of Health Training Grant in Psychiatric Epidemiology (T32-MH-017119).
Footnotes
CONFLICTS OF INTEREST
None.
DISCLOSURE SUMMARY
The authors have nothing to disclose.
References
- 1.Felitti VJ, Anda RF, Nordenberg D, Williamson DF, Spitz AM, Edwards V, et al. Relationship of childhood abuse and household dysfunction to many of the leading causes of death in adults. The Adverse Childhood Experiences (ACE) Study. Am J Prev Med. 1998;14(4):245–58. [DOI] [PubMed] [Google Scholar]
- 2.Kessler RC, McLaughlin KA, Green JG, Gruber MJ, Sampson NA, Zaslavsky AM, et al. Childhood adversities and adult psychopathology in the WHO World Mental Health Surveys. Br J Psychiatry. 2010;197(5):378–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gilbert LK, Breiding MJ, Merrick MT, Thompson WW, Ford DC, Dhingra SS, et al. Childhood adversity and adult chronic disease: an update from ten states and the District of Columbia, 2010. Am J Prev Med. 2015;48(3):345–9. [DOI] [PubMed] [Google Scholar]
- 4.Rich-Edwards JW, Mason S, Rexrode K, Spiegelman D, Hibert E, Kawachi I, et al. Physical and sexual abuse in childhood as predictors of early-onset cardiovascular events in women. Circulation. 2012;126(8):920–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rich-Edwards JW, Spiegelman D, Lividoti Hibert EN, Jun HJ, Todd TJ, Kawachi I, et al. Abuse in childhood and adolescence as a predictor of type 2 diabetes in adult women. Am J Prev Med. 2010;39(6):529–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Molnar BE, Buka SL, Kessler RC. Child sexual abuse and subsequent psychopathology: results from the National Comorbidity Survey. Am J Public Health. 2001;91(5):753–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Springer KW, Sheridan J, Kuo D, Carnes M. Long-term physical and mental health consequences of childhood physical abuse: results from a large population-based sample of men and women. Child Abuse Negl. 2007;31(5):517–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Thurston RC, Chang Y, Derby CA, Bromberger JT, Harlow SD, Janssen I, et al. Abuse and subclinical cardiovascular disease among midlife women: the study of women’s health across the nation. Stroke. 2014;45(8):2246–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bertone-Johnson ER, Whitcomb BW, Missmer SA, Karlson EW, Rich-Edwards JW. Inflammation and early-life abuse in women. Am J Prev Med. 2012;43(6):611–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Thompson MP, Kingree JB, Desai S. Gender differences in long-term health consequences of physical abuse of children: data from a nationally representative survey. Am J Public Health. 2004;94(4):599–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.MacMillan HL, Fleming JE, Trocme N, Boyle MH, Wong M, Racine YA, et al. Prevalence of child physical and sexual abuse in the community. Results from the Ontario Health Supplement. JAMA. 1997;278(2):131–5. [PubMed] [Google Scholar]
- 12.Briere J, Elliott DM. Prevalence and psychological sequelae of self-reported childhood physical and sexual abuse in a general population sample of men and women. Child Abuse Negl. 2003;27(10):1205–22. [DOI] [PubMed] [Google Scholar]
- 13.Frodl T, O’Keane V. How does the brain deal with cumulative stress? A review with focus on developmental stress, HPA axis function and hippocampal structure in humans. Neurobiol Dis. 2013;52:24–37. [DOI] [PubMed] [Google Scholar]
- 14.McEwen BS. Stress, adaptation, and disease. Allostasis and allostatic load. Ann N Y Acad Sci. 1998;840:33–44. [DOI] [PubMed] [Google Scholar]
- 15.Juster RP, McEwen BS, Lupien SJ. Allostatic load biomarkers of chronic stress and impact on health and cognition. Neurosci Biobehav Rev. 2010;35(1):2–16. [DOI] [PubMed] [Google Scholar]
- 16.Ferrari E, Cravello L, Muzzoni B, Casarotti D, Paltro M, Solerte SB, et al. Age-related changes of the hypothalamic-pituitary-adrenal axis: pathophysiological correlates. Eur J Endocrinol. 2001;144(4):319–29. [DOI] [PubMed] [Google Scholar]
- 17.Van Cauter E, Leproult R, Kupfer DJ. Effects of gender and age on the levels and circadian rhythmicity of plasma cortisol. J Clin Endocrinol Metab. 1996;81(7):2468–73. [DOI] [PubMed] [Google Scholar]
- 18.Dhabhar FS, McEwen BS. Acute stress enhances while chronic stress suppresses cell-mediated immunity in vivo: a potential role for leukocyte trafficking. Brain Behav Immun. 1997;11(4):286–306. [DOI] [PubMed] [Google Scholar]
- 19.Dhabhar FS, Miller AH, Stein M, McEwen BS, Spencer RL. Diurnal and acute stress-induced changes in distribution of peripheral blood leukocyte subpopulations. Brain Behav Immun. 1994;8(1):66–79. [DOI] [PubMed] [Google Scholar]
- 20.Liston C, Cichon JM, Jeanneteau F, Jia Z, Chao MV, Gan WB. Circadian glucocorticoid oscillations promote learning-dependent synapse formation and maintenance. Nat Neurosci. 2013;16(6):698–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liston C, Gan WB. Glucocorticoids are critical regulators of dendritic spine development and plasticity in vivo. Proc Natl Acad Sci U S A. 2011;108(38):16074–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fekedulegn DB, Andrew ME, Burchfiel CM, Violanti JM, Hartley TA, Charles LE, et al. Area under the curve and other summary indicators of repeated waking cortisol measurements. Psychosom Med. 2007;69(7):651–9. [DOI] [PubMed] [Google Scholar]
- 23.McEwen BS, Karatsoreos IN. Sleep Deprivation and Circadian Disruption: Stress, Allostasis, and Allostatic Load. Sleep Med Clin. 2015;10(1):1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McEwen BS. What Is the Confusion With Cortisol? Chronic Stress. 2019;3:1–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hucklebridge F, Hussain T, Evans P, Clow A. The diurnal patterns of the adrenal steroids cortisol and dehydroepiandrosterone (DHEA) in relation to awakening. Psychoneuroendocrinology. 2005;30(1):51–7. [DOI] [PubMed] [Google Scholar]
- 26.Mocking RJ, Pellikaan CM, Lok A, Assies J, Ruhe HG, Koeter MW, et al. DHEAS and cortisol/DHEAS-ratio in recurrent depression: State, or trait predicting 10-year recurrence? Psychoneuroendocrinology. 2015;59:91–101. [DOI] [PubMed] [Google Scholar]
- 27.Jin RO, Mason S, Mellon SH, Epel ES, Reus VI, Mahan L, et al. Cortisol/DHEA ratio and hippocampal volume: A pilot study in major depression and healthy controls. Psychoneuroendocrinology. 2016;72:139–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Russo SJ, Murrough JW, Han MH, Charney DS, Nestler EJ. Neurobiology of resilience. Nat Neurosci. 2012;15(11):1475–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kimonis ER, Fleming GE, Wilbur RR, Groer MW, Granger DA. Dehydroepiandrosterone (DHEA) and its ratio to cortisol moderate associations between maltreatment and psychopathology in male juvenile offenders. Psychoneuroendocrinology. 2019;101:263–71. [DOI] [PubMed] [Google Scholar]
- 30.Yehuda R, Brand SR, Golier JA, Yang RK. Clinical correlates of DHEA associated with post-traumatic stress disorder. Acta Psychiatr Scand. 2006;114(3):187–93. [DOI] [PubMed] [Google Scholar]
- 31.Kimonis ER, Fleming GE, Wilbur RR, Groer MW, Granger DA. Dehydroepiandrosterone (DHEA) and its ratio to cortisol moderate associations between maltreatment and psychopathology in male juvenile offenders. Psychoneuroendocrinology. 2018;101:263–71. [DOI] [PubMed] [Google Scholar]
- 32.Heim C, Newport DJ, Heit S, Graham YP, Wilcox M, Bonsall R, et al. Pituitary-adrenal and autonomic responses to stress in women after sexual and physical abuse in childhood. JAMA. 2000;284(5):592–7. [DOI] [PubMed] [Google Scholar]
- 33.Heim C, Ehlert U, Hellhammer DH. The potential role of hypocortisolism in the pathophysiology of stress-related bodily disorders. Psychoneuroendocrinology. 2000;25(1):1–35. [DOI] [PubMed] [Google Scholar]
- 34.Bremner D, Vermetten E, Kelley ME. Cortisol, dehydroepiandrosterone, and estradiol measured over 24 hours in women with childhood sexual abuse-related posttraumatic stress disorder. J Nerv Ment Dis. 2007;195(11):919–27. [DOI] [PubMed] [Google Scholar]
- 35.Carpenter LL, Tyrka AR, Ross NS, Khoury L, Anderson GM, Price LH. Effect of childhood emotional abuse and age on cortisol responsivity in adulthood. Biol Psychiatry. 2009;66(1):69–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Matthews K, Schwartz J, Cohen S, Seeman T. Diurnal cortisol decline is related to coronary calcification: CARDIA study. Psychosom Med. 2006;68(5):657–61. [DOI] [PubMed] [Google Scholar]
- 37.Matthews KA, Chang YF, Thurston RC, Bromberger JT. Child abuse is related to inflammation in mid-life women: role of obesity. Brain Behav Immun. 2014;36:29–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hirokawa K, Ohira T, Nagayoshi M, Kajiura M, Imano H, Kitamura A, et al. Dehydroepiandrosterone-sulfate is associated with cardiovascular reactivity to stress in women. Psychoneuroendocrinology. 2016;69:116–22. [DOI] [PubMed] [Google Scholar]
- 39.Huang T, Trudel-Fitzgerald C, Poole EM, Sawyer S, Kubzansky LD, Hankinson SE, et al. The Mind-Body Study: study design and reproducibility and interrelationships of psychosocial factors in the Nurses’ Health Study II. Cancer Causes Control. 2019;30(7):779–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Huang T, Trudel-Fitzgerald C, Poole EM, Sawyer S, Kubzansky LD, Hankinson SE, et al. The Mind Body Study: Study design and reproducibility and interrelationships of psychosocial factors in the Nurses’ Health Study II (ACCEPTED). Cancer Causes & Control. [DOI] [PMC free article] [PubMed]
- 41.Vining RF, McGinley RA, Maksvytis JJ, Ho KY. Salivary cortisol: a better measure of adrenal cortical function than serum cortisol. Ann Clin Biochem. 1983;20 (Pt 6):329–35. [DOI] [PubMed] [Google Scholar]
- 42.Inder WJ, Dimeski G, Russell A. Measurement of salivary cortisol in 2012-laboratory techniques and clinical indications. Clin Endocrinol (Oxf). 2012;77(5):645–51. [DOI] [PubMed] [Google Scholar]
- 43.MA S, RJ G. Physical Violence in American Families: Risk Factors and Adaptations to Violence in 8,145 Families. New Brunswick: Transaction Publishers; 1990. [Google Scholar]
- 44.Willett WC, Sampson L, Stampfer MJ, Rosner B, Bain C, Witschi J, et al. Reproducibility and validity of a semiquantitative food frequency questionnaire. Am J Epidemiol. 1985;122(1):51–65. [DOI] [PubMed] [Google Scholar]
- 45.Spitzer RL, Kroenke K, Williams JB, Lowe B. A brief measure for assessing generalized anxiety disorder: the GAD-7. Arch Intern Med. 2006;166(10):1092–7. [DOI] [PubMed] [Google Scholar]
- 46.Schnurr PP, Lunney CA, Sengupta A, Spiro A 3rd. A longitudinal study of retirement in older male veterans. J Consult Clin Psychol. 2005;73(3):561–6. [DOI] [PubMed] [Google Scholar]
- 47.Kimerling R, Ouimette P, Prins A, Nisco P, Lawler C, Cronkite R, et al. Brief report: Utility of a short screening scale for DSM-IV PTSD in primary care. J Gen Intern Med. 2006;21(1):65–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Huang T, Poole EM, Vetter C, Rexrode KM, Kubzansky LD, Schernhammer E, et al. Habitual sleep quality and diurnal rhythms of salivary cortisol and dehydroepiandrosterone in postmenopausal women. Psychoneuroendocrinology. 2017;84:172–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Buysse DJ, Reynolds CF 3rd, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28(2):193–213. [DOI] [PubMed] [Google Scholar]
- 50.Vgontzas AN, Liao D, Pejovic S, Calhoun S, Karataraki M, Basta M, et al. Insomnia with short sleep duration and mortality: the Penn State cohort. Sleep. 2010;33(9):1159–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bland JM, Altman DG. Transformations, means, and confidence intervals. BMJ. 1996;312(7038):1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Feng C, Wang H, Lu N, Chen T, He H, Lu Y, et al. Log-transformation and its implications for data analysis. Shanghai Arch Psychiatry. 2014;26(2):105–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ryan R, Booth S, Spathis A, Mollart S, Clow A. Use of Salivary Diurnal Cortisol as an Outcome Measure in Randomised Controlled Trials: a Systematic Review. Ann Behav Med. 2016;50(2):210–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Adam EK, Kumari M. Assessing salivary cortisol in large-scale, epidemiological research. Psychoneuroendocrinology. 2009;34(10):1423–36. [DOI] [PubMed] [Google Scholar]
- 55.Weitzman ED, Zimmerman JC, Czeisler CA, Ronda J. Cortisol secretion is inhibited during sleep in normal man. J Clin Endocrinol Metab. 1983;56(2):352–8. [DOI] [PubMed] [Google Scholar]
- 56.Kellner M, Muhtz C, Peter F, Dunker S, Wiedemann K, Yassouridis A. Increased DHEA and DHEA-S plasma levels in patients with post-traumatic stress disorder and a history of childhood abuse. J Psychiatr Res. 2010;44(4):215–9. [DOI] [PubMed] [Google Scholar]
- 57.Schury K, Koenig AM, Isele D, Hulbert AL, Krause S, Umlauft M, et al. Alterations of hair cortisol and dehydroepiandrosterone in mother-infant-dyads with maternal childhood maltreatment. BMC Psychiatry. 2017;17(1):213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Greendale GA, Kritz-Silverstein D, Seeman T, Barrett-Connor E. Higher basal cortisol predicts verbal memory loss in postmenopausal women: Rancho Bernardo Study. J Am Geriatr Soc. 2000;48(12):1655–8. [DOI] [PubMed] [Google Scholar]
- 59.Greendale GA, Unger JB, Rowe JW, Seeman TE. The relation between cortisol excretion and fractures in healthy older people: results from the MacArthur studies-Mac. J Am Geriatr Soc. 1999;47(7):799–803. [DOI] [PubMed] [Google Scholar]
- 60.Johannes CB, Stellato RK, Feldman HA, Longcope C, McKinlay JB. Relation of dehydroepiandrosterone and dehydroepiandrosterone sulfate with cardiovascular disease risk factors in women: longitudinal results from the Massachusetts Women’s Health Study. J Clin Epidemiol. 1999;52(2):95–103. [DOI] [PubMed] [Google Scholar]
- 61.Shufelt C, Bretsky P, Almeida CM, Johnson BD, Shaw LJ, Azziz R, et al. DHEA-S levels and cardiovascular disease mortality in postmenopausal women: results from the National Institutes of Health--National Heart, Lung, and Blood Institute (NHLBI)-sponsored Women’s Ischemia Syndrome Evaluation (WISE). J Clin Endocrinol Metab. 2010;95(11):4985–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Tworoger SS, Missmer SA, Eliassen AH, Spiegelman D, Folkerd E, Dowsett M, et al. The association of plasma DHEA and DHEA sulfate with breast cancer risk in predominantly premenopausal women. Cancer Epidemiol Biomarkers Prev. 2006;15(5):967–71. [DOI] [PubMed] [Google Scholar]
- 63.Woods NF, Mitchell ES, Smith-Dijulio K. Cortisol levels during the menopausal transition and early postmenopause: observations from the Seattle Midlife Women’s Health Study. Menopause. 2009;16(4):708–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gunnar MR, Vazquez DM. Low cortisol and a flattening of expected daytime rhythm: potential indices of risk in human development. Dev Psychopathol. 2001;13(3):515–38. [DOI] [PubMed] [Google Scholar]
- 65.Kern W, Dodt C, Born J, Fehm HL. Changes in cortisol and growth hormone secretion during nocturnal sleep in the course of aging. J Gerontol A Biol Sci Med Sci. 1996;51(1):M3–9. [DOI] [PubMed] [Google Scholar]
- 66.Carpenter LL, Shattuck TT, Tyrka AR, Geracioti TD, Price LH. Effect of childhood physical abuse on cortisol stress response. Psychopharmacology (Berl). 2011;214(1):367–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.MacMillan HL, Georgiades K, Duku EK, Shea A, Steiner M, Niec A, et al. Cortisol response to stress in female youths exposed to childhood maltreatment: results of the youth mood project. Biol Psychiatry. 2009;66(1):62–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Cook EC, Chaplin TM, Sinha R, Tebes JK, Mayes LC. The stress response and adolescents’ adjustment: the impact of child maltreatment. J Youth Adolesc. 2012;41(8):1067–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Pierrehumbert B, Torrisi R, Glatz N, Dimitrova N, Heinrichs M, Halfon O. The influence of attachment on perceived stress and cortisol response to acute stress in women sexually abused in childhood or adolescence. Psychoneuroendocrinology. 2009;34(6):924–38. [DOI] [PubMed] [Google Scholar]
- 70.van Zuiden M, Haverkort SQ, Tan Z, Daams J, Lok A, Olff M. DHEA and DHEA-S levels in posttraumatic stress disorder: A meta-analytic review. Psychoneuroendocrinology. 2017;84:76–82. [DOI] [PubMed] [Google Scholar]
- 71.Rasmusson AM, Pinna G, Paliwal P, Weisman D, Gottschalk C, Charney D, et al. Decreased cerebrospinal fluid allopregnanolone levels in women with posttraumatic stress disorder. Biol Psychiatry. 2006;60(7):704–13. [DOI] [PubMed] [Google Scholar]
- 72.Usta MB, Tuncel OK, Akbas S, Aydin B, Say GN. Decreased dehydroepiandrosterone sulphate levels in adolescents with post-traumatic stress disorder after single sexual trauma. Nord J Psychiatry. 2016;70(2):116–20. [DOI] [PubMed] [Google Scholar]
- 73.Kamin HS, Kertes DA. Cortisol and DHEA in development and psychopathology. Horm Behav. 2017;89:69–85. [DOI] [PubMed] [Google Scholar]
- 74.Segerstrom SC, Boggero IA, Smith GT, Sephton SE. Variability and reliability of diurnal cortisol in younger and older adults: implications for design decisions. Psychoneuroendocrinology. 2014;49:299–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kim C, Harlow SD, Zheng H, McConnell DS, Randolph JF Jr. Changes in androstenedione, dehydroepiandrosterone, testosterone, estradiol, and estrone over the menopausal transition. Womens Midlife Health. 2017;3. [DOI] [PMC free article] [PubMed]
- 76.McConnell DS, Stanczyk FZ, Sowers MR, Randolph JF Jr., Lasley BL. Menopausal transition stage-specific changes in circulating adrenal androgens. Menopause. 2012;19(6):658–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Labrie F. All sex steroids are made intracellularly in peripheral tissues by the mechanisms of intracrinology after menopause. J Steroid Biochem Mol Biol. 2015;145:133–8. [DOI] [PubMed] [Google Scholar]
- 78.Lasley BL, Santoro N, Randolf JF, Gold EB, Crawford S, Weiss G, et al. The relationship of circulating dehydroepiandrosterone, testosterone, and estradiol to stages of the menopausal transition and ethnicity. J Clin Endocrinol Metab. 2002;87(8):3760–7. [DOI] [PubMed] [Google Scholar]
- 79.Crawford S, Santoro N, Laughlin GA, Sowers MF, McConnell D, Sutton-Tyrrell K, et al. Circulating dehydroepiandrosterone sulfate concentrations during the menopausal transition. J Clin Endocrinol Metab. 2009;94(8):2945–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Vining RF, McGinley RA, Symons RG. Hormones in saliva: mode of entry and consequent implications for clinical interpretation. Clin Chem. 1983;29(10):1752–6. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.