SUMMARY
The current concepts for development of autoreactive B cells in SLE focus on extrinsic stimuli and factors that provoke B cells into tolerance loss. Traditionally, major tolerance loss pathways are thought to be regulated by factors outside the B cell including autoantigen engagement of the B cell receptor (BCR) with simultaneous type I interferon (IFN) produced by dendritic cells, especially plasmacytoid dendritic cells (pDCs). Later, in autoreactive follicles, B cells encounter T follicular helper cells (Tfh) that produce interleukin (IL)-21, IL-4 and pathogenic cytokines, IL-17 and IFN gamma (IFNɣ). This review discusses these mechanisms and also highlights recent advances pointing to the peripheral transitional B cell stage as a major juncture where transient autocrine IFNβ expression by developing B cells imprints a heightened susceptibility to external factors favoring differentiation into autoantibody producing plasmablasts. Recent studies highlight transitional B cell heterogeneity as a determinant of intrinsic resistance or susceptibility to tolerance loss through the shaping of B cell responsiveness to cytokines and other environment factors.
Keywords: SLE, lupus, BXD2, autoantibodies, autoimmunity, IFNβ, type I IFNs, lymphotoxin beta, IL-17, IL-21, regulator of G-protein signaling, transitional stage 1 B cells, marginal zone-precursor B cells, germinal center B cells, plasmacytoid dendritic cells, marginal zone macrophages, follicular T-helper cells
“Look like the innocent flower, but be the serpent under it.”
William Shakespeare ~
1. INTRODUCTION
Autoreactive B cell development in SLE is often considered in terms of the extrinsic stimuli and factors that drive B cells into autoantibody production. This review focuses on the novel concept that autoreactive B cells are initially programmed at the transitional stage for heightened susceptibility for development into autoantibody secreting plasmablasts (Figure 1). During peripheral B cell development in the spleen, transitional B cells expressing elevated levels of endogenous intracellular interferon-β (IFNβ) exhibit enhanced survival and activation through the B cell receptor (BCR), a feature associated with more active SLE, particularly in African American (AA) patients1. Following passage through the transitional stage, a second major defect is the failure of B cells to maintain the integrity of tolerogenic splenic marginal zone macrophages (MZM), which normally function to remove apoptotic cell debris in a non-inflammatory fashion through upregulation of indoleamine-pyrrole 2,3-dioxygenase (IDO)2. This failure is amplified by the type I IFN-driven migratory properties of marginal zone-precursor (MZ-P) B cells as they lose their tethering to sphingosine-1-phosphate (S1P) and drift across the follicular exclusion barrier3–8. Here, they act as potent antigen-carrying, costimulatory cells expressing inflammatory cytokine IL-6 and low levels of the anti-inflammatory cytokine IL-109. In addition, Ag transporting MZ-P B cells express high levels of membrane lymphotoxin β (mLTβ) that can potently stimulate follicular dendritic cells (FDCs) to initiate germinal center (GC) formation5,6,10. A third B cell defect occurs during the T-dependent GC or extra GC stage of development in which B cells respond not only to IL-4 and IL-21, but also upregulate IL-17 receptor A (IL-17RA) and type II interferon-γ receptor (IFNɣR). Simulation by IL-17 on IL-17RA+ B cells induces up-regulation of regulator of G-protein signaling (RGS)13 and RGS16, leading to prolonged retention in the GC, upregulation of activation-induced cytidine deaminase (AICDA), and migratory behavior that can prolong the interaction between follicular T-helper cells (Tfh) and GC B cells11–15. Alternatively, upregulation of IFNɣR in the presence of IFNɣ producing Tfh (Tfh-IL-IFNɣ) can lead to T-bet+CD11c+CXCR5−IgD−CD27− double negative 2 (DN2) autoreactive B cells16–20. This review will highlight the high degree to which autoreactive B cells both directly and indirectly self-regulate their development through autocrine cytokine signaling during development and promoting the disintegration of critical tolerance barriers. This creates an environment ripe for pathogenic T cells to drive differentiation of B cells into pathogenic autoAb production.
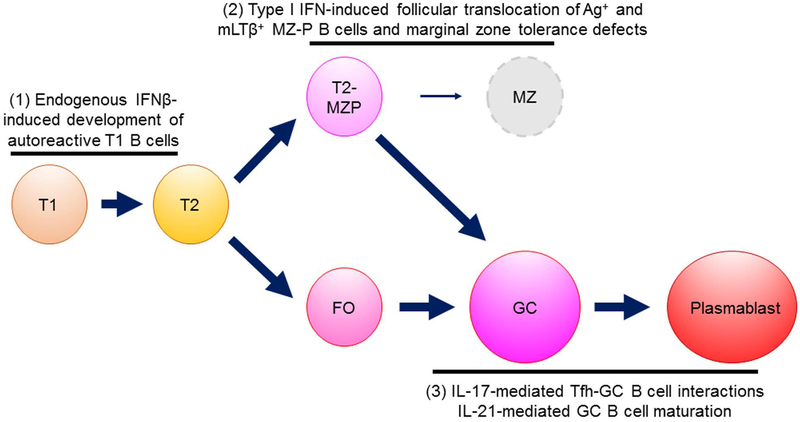
Figure 1.
Overview of three key mechanisms for development of autoreactive B cells. (1) At the early T1 transitional stage, endogenous IFNβ promotes activation and survival of B cells through enhancing responses to TLR signaling, including TLR7. (2) At the T2/MZ-P stage, B cells are susceptible to type I IFN induced follicular translocation. This event disrupts the tolerogenic MZM barrier which depends on mLTβ+ B cells which further enhances defective apoptotic cell clearance and increased type 1 interferon in the MZ. The translocated B cells can serve as excellent antigen-presenting cells with high levels of CD86 and class II MHC, and in the context of mLTβ can strongly stimulate FDCs to initiate a spontaneous autoreactive GC development. (3) During the GC or extra GC T-dependent phase of response, Tfh-IL21 initiates the T-B interaction and survival of GC B cells, but subsequent stimulation of B cells by Tfh-IL17 or Tfh-IFNɣ promotes development of either highly pathogenic and prolonged T-B interactions due to increased RGS13 and RGS16 or IFNɣ-induced upregulation of T-bet leading to development of the autoreactive DN2 B cell (T-bet+CD11c+IgDloCXCR5lo). We hypothesize that not all B cells are equally as susceptible to each of these pathogenic mechanisms; Rather, early events such as endogenous IFNβ and ensuing changes in chromatin organization influence individualized developmental trajectories.
2. PRE-GERMINAL CENTER STAGE B-CELL AUTOREGULATION
2.1. Control of the Pre-Immune Repertoire
B cell development in the bone marrow occurs in a progressive series of events leading to the assembly, expression, and signaling of the B cell antigen receptor (BCR). During early B cell genesis, a cascade of microenvironmental and stromal-derived signals including IL-7 induce the rearrangement of the immunoglobulin (Ig) locus and surface expression of a pre-B cell receptor comprised of an IgM heavy-chain with a surrogate light-chain, which does not efficiently bind antigen21–23. B cells then activate the light-chain locus and express a bonafide light-chain gene that enables production of a mature BCR capable of binding antigen. Tonic signaling through the BCR at this stage promotes positive selection, while high-affinity immunoglobulin binding to self-antigen triggers apoptotic cell death, contributing to the elimination of highly self-reactive and polyreactive B cells24,25. Autoreactive B cells can be rescued for survival through induced alteration of the structure and specificity of their antigen receptors, resulting in a less self-reactive immunoglobulin receptor, a process known as receptor editing26,27. B cells that are not clonally deleted leave the bone marrow as transitional B cells and migrate to the peripheral lymphoid organs. Studies by Nussenzweig and co-workers28 have shown that approximately 75% and 60% of bone marrow emigrant B cells are HEP-2 autoreactive or polyreactive, respectively, but this is reduced to approximately 40% and 7% as B cells transition from the immature new emigrant stage to the mature naïve stage within lymphoid organs.
In addition to clonal deletion and receptor editing, developing B cells that encounter low avidity self-antigen also may enter into an anergic state. Early studies using the HEL-anti-HEL mouse system by Goodnow and colleagues demonstrated that strong BCR crosslinking by membrane-bound HEL resulted in clonal deletion at the early transitional of peripheral B cell development29,30. In contrast, lower affinity and sustained signaling through the BCR by soluble HEL resulted in induction of B cell unresponsiveness or anergy, as defined by unresponsiveness to antigen stimulation29,30. Anergic B cells exhibit impaired signaling, proliferation and differentiation to antibody-secreting cells after antigen receptor stimulation either during immunization with exogenous HEL or in response to the innate Toll-like receptor (TLR) agonists CpG-containing DNA and lipopolysaccharide31,32. Mechanistically, Cambier and colleagues have shown that anergic B cells exhibit impaired Ca2+ mobilization and impaired phosphorylation events downstream of BCR crosslinking in part by recruitment of phosphatases to the BCR apparatus which counteracts strong BCR signaling33. Adoptive transfer of anergic B cells with subsequent induction of gene expression further showed that the inhibitory signaling pathways involved a tyrosine phosphatase SHP-1 and inositol phosphatase SHIP-1, which are both required to maintain anergy34. Activation events that disrupt anergy leads to rapid cell activation, proliferation and short-lived plasma cells that reside in extra-follicular foci34. Previous studies by Goodnow and co-workers showed similar anergy loss in mice lacking Lyn, SHP-1 and CD22. The mechanistic basis was found to be phosphorylation of CD22 by Lyn, which then recruits SHP-1 to the CD22/BCR complex35. More recently Dorner and Lipsky have proposed that the anti‐B cell therapy Epratuzumab binds CD22, interfering with CD22 function and BCR signaling.
In addition to direct modulation of BCR structure and function, other extrinsic and intrinsic factors can affect activation, survival and development of autoreactive B cells. The transitional T1 stage in the periphery has been identified as a major tolerance checkpoint where autoreactive B cells are highly susceptible to clonal deletion. Following bone marrow selection, the earliest B cell stage to enter the circulation in humans is defined as CD10+CD24HiCD38HiCD27Neg 37. The most immature peripheral T1 stage is distinguished by high levels of surface IgM, but low surface IgD38–41. In mice, T1 B cells exhibit a similar phenotype and are commonly classified as CD93+IgMHiIgDLoCD23Lo. These immature T1 B cells from the BM percolate into the spleen marginal sinuses and red pulp where they must migrate along chemokine and integrin-mediated signals for positive selection into the T-cell-rich periarteriolar lymphoid sheath (PALS) of the white pulp42,43. During this process, BCR-mediated negative selection serves to remove self-reactive B cells that encounter strong BCR stimulation44, and the remaining T1 B cells acquire IgD and CD23 expression and become T2 B cells, which lose sensitivity to negative selection and become responsive T-cell help43,45. T1 B cell negative selection is consistent with their inability to induce c-Myc, cyclin D2, BclxL and A1 following BCR stimulation46,47. Positive selection also occurs at the T1 stage, as suggested by the phenotypes of mice deficient in various components involved in BCR signal transduction. Syk-deficient mice, for example, exhibit a developmental block at the T1 stage48. In general, weak BCR signaling is thought to favor positive selection, whereas strong BCR signaling encountered by autoreactive clones leads to negative selection.
2.2. T1 B cell regulation by autocrine/paracrine IFNβ
Autocrine/paracrine-acting regulatory cytokine production in B cells is often associated with a transitional B cell phenotype49–51. Consistent with this, the transitional B cell stage has been associated with outcomes in various immunological settings, including allograft survival, tumor suppression responses and progression of autoimmune responses51–54 and thus appears to be a critically important developmental checkpoint that determines the fate and function of peripheral B cells. In both SLE and RA patients, the transitional B cell checkpoint is characterized by a failure to exclude new autoreactive BM emigrant B cells from the naïve pool41,55,56, underscoring the importance of identifying intrinsic and extrinsic factors that modulate T1 B cell survival in SLE. Among the potential mediators of T1 B cell survival, BAFF signaling has been a major subject of studies and clinical trials due to its critical role in B cell survival. However, the earliest IgMhiIgDlo T1 B cell stage manifests low BAFF responsiveness, and BCR- and BAFF-mediated signals are not well integrated at this stage57,58. belimumab treatment in human SLE did not affect T1 B cell frequencies or numbers, but led to dramatic reductions in T2, T3 and mature B cell subtypes59 consistent with studies in mice in which T1 B cells are largely unaffected by BAFF manipulation60,61. It remains unclear whether BAFF promotes T1 progression to the T2 stage in vivo. However, several lines of evidence clearly pin-point the T2 stage as the stage where productive BAFF signaling begins. At the T2 B cell stage, BAFF-R expression is dramatically upregulated and associated with preferential responses to BAFF in vivo61,62. Spleen T1 B cells in contrast express low levels of BAFF-R and are not expanded in BAFF transgenic mice nor in mice administered exogenous BAFF63. Together, available data suggest BAFF is not the main survival factor for immature T1 B cells arriving the spleen.
We recently showed that transitional T1 B cells possess a propensity for type I IFN production and responses, including a primary role for high-affinity IFNβ. In contrast to BAFF-R, IFNAR1 is highly expressed on both T1 and T2 B cells and is down-regulated on mature B cells61. Similarly, the highest levels of type I IFN transcripts, including Ifna4, Ifna7 and Ifnb, are observed in T1 B cells with subsequent down-regulation in mature MZ, FO and GC subsets, suggesting that transitional B cells are poised for responses to autocrine and/or paracrine type I IFN signaling. Single cell gene expression analysis of type I IFN and IFN response genes (IRGs) along with developmental markers Cd93 and Cd23 revealed distinct gene expression patterns in T1 B cells suggesting a model where autocrine IFNβ signaling leads to an ordered unfolding expression of genes, including Tlr7, other type I IFNs and IRGs during the T1 to T2 progression61. This is reminiscent of the priming role that IFNβ can elicit in other cell types, whereby low levels of IFNβ leads to the accumulation of antiviral factors enabling rapid amplification of subsequent antiviral responses64. Indeed, T1 B cells isolated from Ifnb+/+:Ifnb−/− BM chimeras revealed impaired expression of Tlr7, Tlr3, Ifna1, Ifna7 and Pkr in Ifnb−/− T1 B cells, and specific neutralization of IFNβ impaired TLR7 responses in transitional B cells61. This action of type I IFNs at the transitional B cell stage is notable, in that it provides a link between the reduced stringency of counter-selection at this early stage58 with later development of autoAbs specific for DNA, RNA and associated nuclear autoAg65,66. Within the same environment, IFNβ deficient B cells were less able to survive and develop into GC B cells, compared to IFNβ competent B cells (Figure 2).
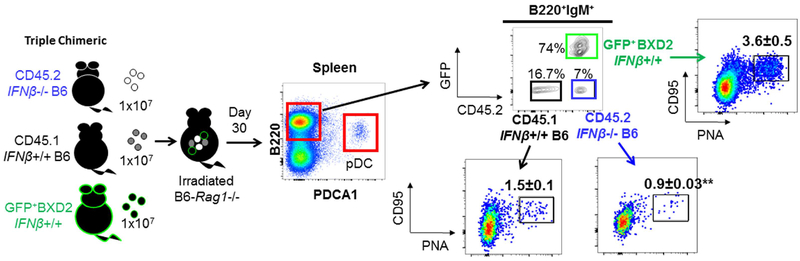
Figure 2.
Lupus susceptible B cells exhibit a higher potency to become GC B cells within the same environment. BM-chimeric mice were generated by reconstitution of CD45.2 Rag1‒⁄‒ mice with equal numbers of BM derived from CD45.2 B6-Ifnb‒⁄‒ mice, CD45.1 Ifnb+/+ (WT) B6 mice and GFP+ BXD2 mice. Recipient mice were sacrificed at day 30 after BM transfer. FACS analysis of pDCs, gated as PDCA1+B220lo cells or PNA+CD95+ GC B cells revealed that despite the identical developmental environment, B cells derived from BM of lupus prone BXD2 mice and those derived from Ifnb+/+ (WT) B6 mice exhibited a higher survival advantage and higher potency to develop into GC B cells vs. CD45.2 B6-Ifnb‒⁄‒ mice.
Examination of type I IFN production in circulating B cell subsets from SLE patients revealed expression of multiple type I IFNs, including IFNβ, beginning with the transitional B cell stage1. Interestingly, single cell gene expression studies in circulating SLE transitional B cells revealed distinct subpopulations, including type I IFN producers, IFN responders, and mixed IFN producer/responder clusters1. At the protein level, increased B cell IFNβ levels were positively associated with renal disease, anti-DNA and anti-Sm autoantibodies and African American race1. The transcription factor ARID3a which regulates accessibility of the IgH enhancer region was recently associated with increased expression of IFNα in transitional and naïve B cells from SLE patients67. Other potential candidates include components of the IFNβ enhanceosome, such as IRF7 and other trans-acting regulatory factors, which have been previously implicated in SLE68. A challenge for future studies will be identification of genetic and non-genetic factors promoting aberrant endogenous type I IFN production by SLE B cells.
2.3. Pathogenic effects of pDCs
pDCs are often considered the principal source of type I IFNs in vivo; however it is becoming clear that production of type I IFN, especially the rapidly induced high-affinity IFNβ, is accomplished a variety of cell types and cannot be mainly attributed to pDCs69. Our studies and others similarly suggest that pathological type I IFN production in SLE is not limited to pDCs1,67,70–72. pDCs have long been prime suspects owing to a large body of literature confirming their high capacity for type I IFN production following viral infection or stimulation with viral nucleic acid mimics73. Indeed, injection of Poly(I:C) into BXD2 mice induces pDCs to produce >10 fold more IFN transcripts compared to other cell types. However, BXD2 mice develop type I IFN-dependent autoantibodies targeting DNA and nuclear Ag in the absence of exogenous pDC stimulation, further suggesting additional complexity in type I IFN dysregulation in SLE. Interestingly in humans, pDC in vitro depletion studies have shown that while pDCs are responsible for the majority IFNα produced by healthy PBMCs, they account only for 57% of IFNα produced by SLE PBMCs70, further supporting that pathological IFNs in autoimmune disease derive from additional cell types. Such complexity is also implicated by observations that type I IFN signaling is not concordant across immune cell populations of an individual patient and tends to vary among patients74.
The cellular sources of type I IFN production depend on many factors, including the nature and multiplicity of the stimulus, temporal factors, as well as genetic factors69,71. In human studies, pDCs clearly have a significant presence in cutaneous lupus75 and are increased in tissues in later stages of SLE where immune complexes are available for deposition and stimulation76,77. Autonomous IFNβ production by T1 B cells developing within the MZ microenvironment may prime surviving autoreactive clones for subsequent responses to other sources of IFNα and nucleic acid sensing64, thus “imprinting” heightened antiviral-like responses onto the B cell compartment. Further interrogation of mechanisms associated with T1 B cell development and cytokine production, including single-cell analyses of molecular features and BCR usage will be necessary to obtain a better understanding of this critical subset.
2.4. The IFNβ-TLR7-IFNα loop of autoreactive B-cell development
Priming by IFNβ is especially important in the regulation of TLR7 responses61,78. Intracellular nucleic acid sensors are important in the pathogenesis of SLE, and TLR7 in particular is associated with the development of antibodies targeting RNA associated antigens in SLE79,80. Similarly, in autoimmune BXSB and TLR7 transgenic mice, overexpression of TLR7 causes severe lupus and antibodies to RNA-containing complexes81,82. TLR7 stimulation of B cells from wild type C57BL/6J is known to induce upregulation of type I IFNs including IFNβ, which has a pro-inflammatory role to promote TLR7 responses78. Our analyses of Ifnb−/− B cells extended this initial finding and further showed that B cell endogenous IFNβ and autocrine signaling is required for Tlr7 and Ifna gene induction in T1 B cells61. Experiments in chimeric mice revealed that B cell endogenous IFNβ exhibited an intrinsic survival advantage for B cells, where although IFNβ+/+ and IFNβ−/− B cells localized to the same regions of the spleen, IFNβ+/+ B cells survived to the mature and GC B cell stage in greater numbers (Figure 2)51. Functionally, genetic deficiency of IFNβ resulted in impaired transitional B cell upregulation of CD69 and CD86 in response to TLR7 stimulation in vitro, suggestive of an autocrine/paracrine feedback loop in transitional B cells61. Similar mechanisms of transitional B cell autocrine regulation have been observed in human transitional B cells, where transitional B cell produced IL10 resulted in CD86 down-regulation which functionally reduced T cell responses. Future studies will be necessary to determine if SLE non-responders to IFNAR blockade treatment is due to an intrinsic survival advantage that results from endogenous IFNβ83.
2.5. IFNα vs. IFNβ
There are 13 distinct type I IFN species in mice and humans, all of which bind the same heterodimeric receptor composed of IFNAR1 and IFNAR2, as distinct from receptors for type II and type III interferons84. SLE is highly associated with a type I IFN stimulated gene (ISG) signature85, but little is understood regarding the specific species of IFN subtypes underlying the signature. Recently gene expression analyses identified the potential roles of multiple interferons in SLE, including prominent expression of IFNβ-specific signatures86. The majority of type I IFN studies in SLE have focused predominantly on the effects of IFNα as the main driver of lupus pathogenesis. This focus on IFNα is partially due to differences in the relative abundance of the type I IFN subtypes, where some IFNα subtypes are more readily detectable in circulation vs. IFNβ87–89. Consistent with this, the induction of IFN-response genes PRKR, IFIT1, IFI44, MX1 in WISH cells by SLE plasma was inhibited >90% by anti-IFNα antibody, but not by anti-IFNβ or anti-IFNγ antibodies suggesting that IFNα is the dominant IFN in many SLE plasma samples90. In addition, a positive correlation between serum IFNα and various disease parameters has also been established91. However, these studies have been limited by an inability to detect IFN species less abundant in circulation including IFNβ. The relative lack of detectable IFNβ in serum is consistent with its much higher affinity for cell-surface bound IFNAR1 and IFNAR2, as well as a lower absolute abundance92. Functionally, the higher affinity of IFNβ for IFNAR1 and IFNAR2 increases the efficiency of ternary complex formation and stability, especially at low receptor surface concentrations92,93. Indeed, the regulation of IFNAR1 and IFNAR2 expression levels is a major mechanism controlling the potency of cell responsiveness to IFNβ94. Several studies have demonstrated that IFNβ induces endocytosis and degradation of IFNAR1 to regulate the cell signaling92,95,96. Down-regulation of IFNAR1 appears to occur rapidly in a tunable fashion, while IFNAR2 expression displays a slower basal turnover97. Additionally, both IFNAR2 and IFNAR1 can interact with IFNβ leading to signal transduction and changes in gene expression (IFNβ/IFNAR1 and IFNβ/IFNAR2)98, a property that may explain differences in the response to IFNβ treatment94.
2.6. What drives transitional B cell IFNβ production?
The driving mechanisms behind increased type I IFN production in the absence of detectable chronic viral infection is a long-standing and critical question in SLE, as precision targeting of these mechanisms will likely be prerequisite for therapeutic success (Figure 3A). As with most features of SLE, elucidation of these mechanisms is challenging due the complex contributions of genetic and environmental factors as well as inter-patient heterogeneity. Some evidence suggests that the aberrant transitional B cell type I IFN production observed in SLE may have earlier origins incipient during development in an inflammatory BM niche. IFNβ has been shown to promote autoreactive IgM expression by developing B cells in the BM by altering stringency of negative selection65,66. More recently, elevated IFNβ expression in bone marrow–derived mesenchymal stem cells from SLE patients was associated with increased production of reactive oxygen species and expression of mitochondrial antiviral signaling protein (MAVS), suggesting cell intrinsic mitochondrial dysfunction and type I IFN signaling in the BM hematopoietic microenvironment99. Increased oxidative stress and decreased glutathione (GSH) levels are frequently associated with SLE100,101 and are implicated as a trigger for spontaneous MAVS oligomerization and type I IFN production by circulating B cells, T cells and monocytes from SLE patients102. These mechanisms are associated with increased disease activity, as both oxidative stress and increased plasma levels of IFNβ have been associated with impending disease pre-flare103,104.
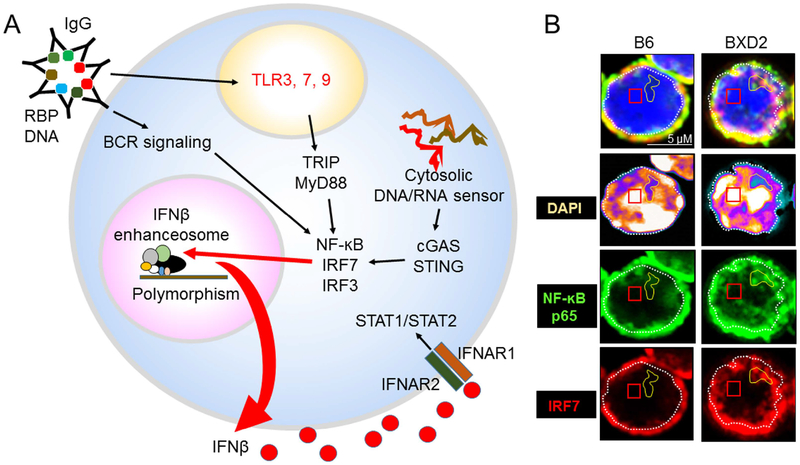
Figure 3.
Increased IFNβ enhanceosome components in lupus B cells. A. Uncleared ACs or AC-autoantibody immune complexes with DNA- or RNA-containing proteins signals BCR and the TLR3, 7, 9 endosome in B cells. Both IRF3 and IRF7 are critical for type I IFN induction and amplification following TLR signaling. Alternatively, defects in clearance of endogenous DNA/RNA could activate cytosolic sensors through the cGAS/STING pathway. This all leads to increased NF-kB, IRF7 and IRF3 which are important co-factors for the IFNβ enhanceosome. The IFNβ promoter contains four positive regulatory domains (PRD I-IV), which are occupied by overlapping transcription factor complexes115. IRF3 and IRF7 bind PRDI and III; the AP-1 heterodimer of ATF-2 and c-Jun binds PRDIV; and the NF-κB p50-RelA heterodimer binds PRDII115. Polymorphisms in IFNβ enhanceosome genes and aberrant induction of nucleic acid sensing pathways may contribute to elevated IFNβ expression, which leads to production of other IFN subtypes and amplification of nucleic acid sensing responses. B. Increased expression of IFNβ enhanceosome components in lupus B cells. Super-Resolution Structured Illumination Microscopy (SIM) imaging analysis showing the colocalization of NF-ĸB p65 and IRF7 in DAPIdim open chromatin region in B cells derived from lupus prone BXD2 mice but not normal B6 mice. As SLE B cells express endogenous IFNβ, these results suggest there is increased stimulation of the IFNβ enhanceosome.
A second and complimentary model holds that selection pressures due to exposure to pathogens endemic to Africa may account for increased type I IFN production and responses in the AA population. Although studies are limited, there are reports that SLE incidence is lower in Africa compared to North America and genetic studies have demonstrated the impact of genetic admixture on SLE risk105–108. Other population-level studies have shown that genetic variants across populations play a major role in shaping an individual’s immune response109, including variability in cytokine responses to various stimuli110–112. Extensive studies into the genetic associations in SLE identified that aberrant regulation of innate and adaptive immunity including increased type I IFN is a genetically conferred risk factor for development of SLE113,114. As B cell IFNβ was significantly increased in AA patients, this further suggests that polymorphisms in the IFNβ enhanceosome genes or other upstream genes may predispose some patients to dysregulated IFNβ production1. Future studies will be needed to determine if increased B cell endogenous IFNβ in African American patients can be a useful predictor of predisposition to development of SLE.
The IFNβ promoter contains four positive regulatory domains (PRD I-IV), which are occupied by overlapping transcription factor complexes115. IRF3 and IRF7 bind PRDI and III; the AP-1 heterodimer of ATF-2 and c-Jun binds PRDIV; and the NF-κB p50-RelA heterodimer binds PRDII115. Both IRF3 and IRF7 are critical for type I IFN induction and amplification following viral infection and activation of cytosolic nucleic acid sensing pathway (Figure 3A)116. As such, genetic variation in such loci regulating cytokine release could affect expression of type I IFNs and IFN signature genes as commonly observed in SLE. Indeed, the IRF7 locus has been identified as a trans-response expression quantitative trait locus (trans-reQTL) that regulates expression of multiple type I IFN genes following influenza expression117, and several studies have indeed identified variations in the IRF7 gene that confer elevated activation of IRF-7 and predisposes to the development of SLE68,118. Recent population level studies found marked differences between transcriptional immune responses in individuals of African vs. European ancestry109,119 and showed that genetic variants including a regulatory hotspot in the IFNB1 gene itself explain a substantial proportion of these population differences, concluding that adaptation and a genetic admixture shaped the immune responses of human populations109. Increased colocalization of NF-ĸB p65 and IRF7 has been identified in the nucleus of B cells derived from BXD2 mice (Figure 3B). Future studies will be needed to further delineate the multiple independent interacting loci and molecular mechanisms that account for aberrant type I IFN production and tolerance loss in SLE.
3. BREAKDOWN OF THE FOLLICULAR EXCLUSION BARRIER
3.1. Follicular migration of autoreactive B cells
Regulation of B cell migration into follicles, termed “follicular exclusion,” represents a second level of pre-GC B cell tolerance120, as B cell access to follicles is associated with entry into the long-lived repertoire of re-circulating B cells. Developmentally, B cell potential for follicular migration is concurrent with progression beyond the T1 stage45. B cells that survive T1 negative selection progress to the T2 stage characterized by CD93, IgM and CD23 co-expression and regulatory properties. T2 cells expressing a high level of CD21 (CD21hi) and can further differentiate into MZ or FO B cells53. The MZ-P stage, an intermediate subset between T2 and MZ B cells, is marked by heightened responsiveness to BCR signals, T cell help, cytokines and signals promoting entry into the B cell follicle5,45. Phenotypically, MZ-P B cells are distinguished by expression of both the MZ B cell marker, CD21 (complement receptor 2), and the follicular B cell marker, CD23 (high-affinity IgE receptor) and can be further defined as CD93negCD1dhiIgMhi CD21hiCD23hi.
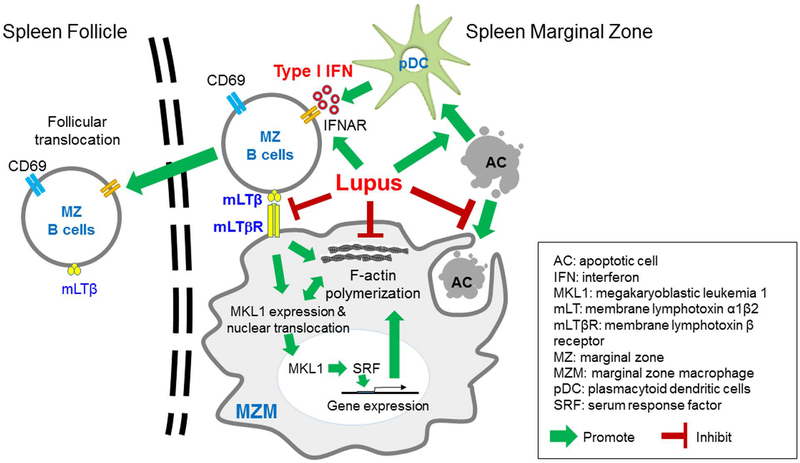
In the spleens of BXD2 mice, MZ-Ps are notable for their presence within the follicle and their extensive migratory properties5 (Figure 4). Normally, MZ B cell localization is under the influence of local levels of the red blood cell product sphingosine 1-phosphate (S1P) which stabilizes S1P receptor (S1P1)-positive MZ B cells in the spleen MZ region3. Cyster and colleagues found that type I IFN can rapidly down regulate the S1P1 leading to follicular inward migration of MZ B cells121. In the context of autoimmunity, we have shown that two key cell types in the MZ play counteracting roles to regulate MZ and MZ-P B cell localization and tolerance to autoantigens9,122: These are the plasmacytoid dendritic cell (pDC) and the marginal zone macrophage (MZM), as further discussed below.
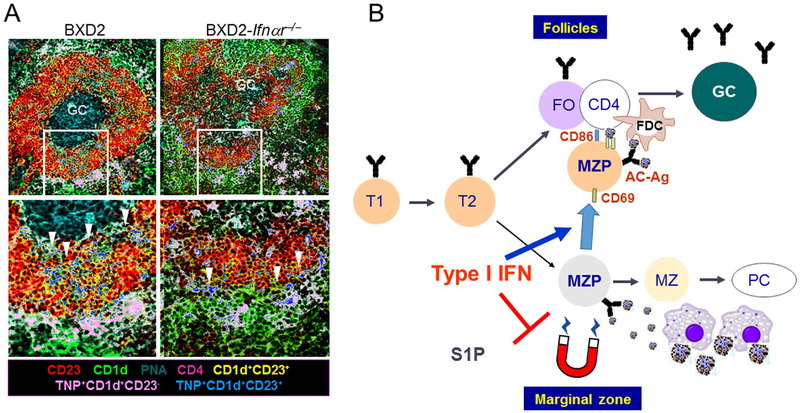
Figure 4.
A. Type I IFN-stimulated follicular translocation of antigen-delivery MZ-P B cells in lupus. In BXD2 mice, there was a significantly larger population of MZ-P B cells in the spleens of autoimmune BXD2 mice compared with B6 mice and IFNAR deficient BXD2 mice. In a TNP-ficoll injection experiment, TNP+ MZ-P B cells (blue) were aggregated inside the follicle and were in proximity with a GC. In contrast, TNP+ MZ-P B cells in BXD2-Ifnar−/− mice were found to reside predominantly in the periphery and had a lower ability to transport TNP into the GCs (adapted from Wang et al, J Immunol. 2010 Jan 1;184(1):442–51)5. B. Model where type I IFN driven follicular translocation of MZ-P B cells promotes apoptotic cell autoantigen transport into the GC region and ultimately B-cell tolerance loss. Generation of type I IFNs, generated by pDCs in the MZ, induced the expression of CD69 and suppressed the sphingosine-1-phosphate-induced chemotactic response, thereby promoting FO-oriented Ag transport by MZ-P B cells.
3.2. Breakdown of the follicular exclusion barrier by pDCs
Seminal work on follicular exclusion of autoreactive B cells was carried out by Cyster et al. in a paper which showed that HEL autoAg-engaged B cells were excluded from entering B cell follicles and instead remained at the T-cell–B-cell border120. The relevance of defective follicular exclusion in SLE patients was shown by Sanz and colleagues in a study showing that 9G4+ B cells successfully participated in GC reactions and expanded within the IgG memory and plasma cell compartments123. Our studies in lupus-prone BXD2 mice which develop spontaneous autoreactive spleen GCs revealed a breakdown in follicular exclusion of MZ-P B cells in the spleen9,122. The formation of aggregations of MZ-P B cells in the follicle is associated with the presence of type I IFN producing pDCs in the MZ, providing a connection between defective follicular exclusion of B cells and pDC-derived type I IFN in the regulation of the localization of B cells at the MZ-P stage.
Plasmacytoid dendritic cells are present in the splenic T cell area and in the red pulp124 of mice and humans where they are poised for abundant type I IFN production125. Apoptotic cell debris can readily interact with and can be taken up by pDCs to stimulate TLR3, 4, 7 and 9, in the endosome resulting in migration toward the MZ and local production of high levels of type I IFN124. In autoimmune BXD2 mice, pDCs are clustered in the MZ and outside the FO and peak in frequency at about 3 months of age, coinciding with the induction of anti-Ro and anti-SSA and slightly preceding induction of anti-dsDNA IgG by several weeks6,126. CpG-induced type I IFN production by pDCs also peaked in 3-mo-old mice, a trend which correlated with increased frequencies of MZ-P B cells5,6,14. Genetic disruption of type I IFN signaling using Ifnar−/− mice led to fewer PNA+ GCs, fewer autoAbs and reduced frequencies of MZ-P B cells. Mechanistically, type I IFN has multiple stimulatory effects on B cells, including rapid upregulation of CD69 which down-regulates S1P1 expression, leading to inward migration of MZ B cells121. In an autoimmune setting, freshly isolated MZ-P B cells exhibited the highest surface expression of CD69 and decreased levels of S1P transcripts5 suggesting MZ-P involvement in the follicular directed flux. Consistent with this, CD1dhiCD23hi B cells from IFNAR intact mice exhibit the highest expression of CD86, the highest capacity for T cell costimulation and are situated adjacent to the GC and opposite from the CD4+ T cell area, suggesting localization near the GC light zone5,6.
Compounding the strong effect of type I IFN on B cell migration, MZ-Ps in BXD2 mice also serve as potent antigen-presenting cells which can carry apoptotic cell debris into the follicle in a “Trojan horse” fashion6. MZ-Ps express high levels of class II MHC which can present autoantigens, as well as high levels of mLTβ which can activate FDCs to promote a strong GC response6,122. Consistent with this, histological analyses revealed accumulation of TNP-Ficoll carried primarily by MZ-P B cells in proximity to the GCs in BXD2 mice (Figure 4). Although MZ B cells could trap TNP-Ficoll, the majority of TNP+ MZ B cells were located in the MZ and not adjacent to the developing GC6. This pattern of Ag-loaded MZ-P localization was significantly reduced in BXD2-Ifnar−/− mice as evidenced by retention to the FO–MZ border (Figure 4). Together, these results suggest a mechanistic model in which the production of type I IFNs by pDCs in the marginal periphery of B cell FOs can upregulate CD69 expression on MZ-P B cells to induce intra-follicular aggregations that may facilitate GC induction. As described later in Section 4, inside the GC, other cytokines, including IL-17A promote autoAb formation through upregulating the expression of regulators of G protein signaling (RGS) in B cells, desensitizing responses to CXCL12 and CXCL13. This fosters a prolonged stable interaction of B and T cells in the GC, high levels of activation-induced cytidine deaminase (AICDA) and the development of pathogenic autoantibody-producing B cells11. These studies illustrate how multiple, sometimes antagonizing cytokines can co-exist in SLE by being produced and active at different sites during a GC B cell development.
AutoAbs in SLE are known to occur years before disease onset, suggesting a gradual pathogenic process that occurs over a long period127,128. The expansion of transitional and MZ-P B cells and their dysregulation by type I IFN appear to be early events that occur prior to disease onset and, when combined with later immune defects, eventually culminates in clinical disease. It remains unclear whether pDC-produced type I IFN is a major SLE initiator and at what stages of disease its pathological effects occur. Stimulation of pDCs with apoptotic cells induces type I IFN production in a FcγRIIa (CD32)-dependent mechanism pDCs129. This and the fact that pDCs lack a specific Ag receptor, unlike B cells, make pDCs unlikely primary sensors of nuclear antigens130. As there is a constant outpouring of B cells from the BM, type I IFN stimulated MZ-Ps have the potential to provide a continuous supply of autoAg to the GCs, which may ultimately support the generation of autoantibody-producing plasma cells.
3.2. Breakdown of the follicular exclusion barrier to autoantigens by MZM disruption
The MZ barrier receives large amounts of blood from the general circulation and thus constitutes a major site of Ag capture and regulation of immunity to circulating Ag. Although MZMs were first described in the 1980s, their Ag capture function was not fully appreciated until the identification of specific scavenger receptors131. Their ability to clear blood-borne antigen and AC-Ag is a complex process requiring recognition by scavenger receptors including macrophage receptor with collagenous structure (MARCO) and specific intracellular adhesion molecule-grabbing non-integrin receptor 1 (SIGN-R1), which send signals to the cytoskeletal apparatus to promote proper engulfment and vesicular trafficking to phagolysosomes as well as induction of tolerogenic signals132,133. Defective serum-dependent, Rho-mediated mechanosensing cytoskeletal reorganization has been identified in macrophages obtained from 6 strains of lupus mice prior to disease onset134.
Multiple cell types are resident in the MZ where they engage in interactive activities critical for immune surveillance and tolerance135. In addition to MZ-Bs, MZMs are also prominent in the spleen MZ where they have a specialized role to interact with and regulate responses to circulating apoptotic cell material4. MZ-B cells and MZMs form a close network and sustain each other through several mechanisms, including LT-β signaling whereby membrane LTβ (mLTβ) expressing MZBs signal to LTβR+ MZMs, promoting proper localization and function of MZMs7,122,136,137. MZMs in turn produce BAFF138 which is necessary for maintenance of mature B cells139. This reciprocal survival signaling in MZ-B and MZMs is central to self-tolerance, as MZMs are specialized for disposal of apoptotic cells and immune complexes in a non-inflammatory fashion involving the expression of TGF-β1 and IL-102,140. T2/MZ-P B cells can also contribute to local IL-10 production (sometimes referred to as B10 B cells) and can produce other tolerogenic factors including TGF-β1141,142. Together, MZ-Bs and MZMs form a “follicular exclusion” barrier in which autoimmune complexes cannot obtain access to the follicle, which may account for the use of extra-follicular pathways for generation of some short-lived autoreactive B cells. These mechanisms are of interest in SLE, as they provide a link between extra-follicular and intra-follicular B cell response to apoptotic cell debris.
3.3. Breakdown of the MZM follicular exclusion barrier in SLE
Systemic lupus erythematosus (SLE) and mouse models of lupus both exhibit central features of increased circulating apoptotic cell autoantigens (AC-Ags) and, in most cases, elevated type I IFN signaling9,143,144 (Figure 5). The most accepted model for development of SLE is that the presence of uncleared ACs or AC-autoantibody immune complexes with DNA- or RNA-containing proteins signals the production of type I IFNs by pDCs. This in turn drives T cell activation and B cell differentiation into autoantibody-producing B cells145–149. Although it is well accepted that increased circulating ACs can be due to reduced clearance of ACs in SLE, the underlying mechanism for the clearance defect is not completely understood9,150–152. Marginal zone macrophages (MZMs) surrounding the splenic follicles have been reported to promote efficient clearance of ACs accompanied by the induction of tolerance to AC-Ags2,140,153,154. Because of their advantageous anatomic location of MZMs, they act as the final follicular AC-Ag exclusion barrier9.
Figure 5.
Type I IFN indirectly induces MZM tolerance loss through follicular translocation of mLTβ expressing MZ B cells. SLE is characterized by increased type I IFN and circulating apoptotic cell-derived autoantigens (AC-Ags), both of which drive autoantibody production by B cells. We have found that in murine SLE models, type I IFN increased follicular translocation of MZ B cells in the spleen, which disrupted the interaction between these B cells and MZMs. The interaction between MZ B cells and MZMs was shown to activate the MKL1–mediated mechanosensing pathway, which was essential for MZMs to phagocytize ACs and thereby prevent follicular entry of AC-Ags. Moreover, these defects were also present in spleens from patients with SLE. The results of this study suggest that strategies to maintain this mechanosensing pathway may block follicular entry of AC-Ags and prevent the development of autoantibodies against these antigens (adapted from Li et al, J Clin Invest. 2015 Jul 1;125(7):2877–90)122.
Understanding the sequence of events that leads to breakdown of the follicular exclusion barrier provides insights into the chain of events that can lead to follicular translocation of B cells and autoantigens in SLE. One of the most fundamental observations in SLE is the increased circulation of apoptotic cell debris2,140,143,152. Related to this is the well-established interaction of such apoptotic cell debris with disposal mechanisms including uptake by DCs including plasmacytoid dendritic cells (pDCs), and with B cells through their BCR antigen receptor20,61,78,80,81,155–157. In SLE, such clearance mechanisms can lead to localization of apoptotic cell debris in the endosome and induction of type l interferons via stimulation through the endosomal TLRs including TLR7 and TLR9156–160. Secondary events of this process are two-fold. First, there is increased production of type l interferon, which is well established as being associated with SLE113,145,146,161–163. Second, there is the opportunity for autoantigens to be presented by class II MHC and up-regulating of CD865,6 leading to T cell tolerance loss164–167. As described above, type I IFNs can signal through the type I interferon receptor which is expressed on virtually all cells, leading to enhanced pro-inflammatory activity by the cell. Thus, the simplest explanation for breakdown of the follicular exclusion barrier by AC debris is that type I interferon acts directly on B cells to result in follicular translocation, as described above, and on MZMs that take up apoptotic cell debris to further reinforce a proinflammatory activity upon uptake of this debris.
However, adding to the complexity of this process, we also observed that uptake of apoptotic cell debris by MZMs not only changes the phenotype of these macrophages, but also results in dissipation of the MZMs. To investigate if MZM dissipation is a direct effect of type I interferon, Rag1−/− BXD2 mice were reconstituted with bone marrow from GFP−BXD2-IFNαR−/− and GFP+BXD2-IFNαR+/+ mice122. Four months after reconstitution, both MZ B cells and MZMs were repopulated with cells that did and did not express the IFNAR. As previously reported, there was enhanced follicular translocation of IFNAR+ MZ B cells, and only IFNAR- B cells remained in the MZ. However, both IFNAR+ and IFNAR- MZMs populated the MZ at equivalently, indicating that type I interferon does not directly impact MZM numbers or structure. Building upon previous observations that membrane lymphotoxin-β (mLTβ) expression on MZ B cells sustained MZMs7,122,136,137, we demonstrated that abrogated mLTβ expression on B cells usng LTβflox/flox CD19-Cre-B6 mice led to loss of MZMs122.
As one of the major functions of MZMs is to efficiently take up apoptotic cell debris in a non-inflammatory fashion, a potential link between LTβ receptor and molecules associated with this function were investigated. Using high-throughput transcriptomics, we investigated how signaling through LTβ receptor on MZMs might affect their function. Since in vitro studies could result in artifacts due to disruption of the MZM from its environment during cell preparation, studies were carried out in vivo using BXD2 mice that were treated with either control PBS or LTβR-Fc to block LTβR signaling. The results indicated that in the absence of LTβ receptor, there is a defect in the megakaryoblastic leukemia 1 (MKL1) mechanosensing pathway which resulted in failure of LTβR deficient MZMs to retain their efficient phagocytosis clearance function and anti-inflammatory function. MKL1 functions with serum response factor (SRF) as an important transcription factor regulating cytoskeletal gene expression in macrophages168.This important role of LTβR signaling to maintain MZMs was observed in BXD2 mice9,11,14,15,61,126,169–172 and B6.SLE1.SLE2.SLE3 (B6.TC mice)8. There was also a decreased expression of MKL1 and loss of MARCO+CD11c+MZ-B cells by histological section of spleens of SLE patients.
The LTβR-MKL1 mechanosensing pathway regulates endocytic uptake, actin polymerization and RhoA, which is required for proper cytoskeletal activities that regulate both uptake of apoptotic cells and the organization of intracellular compartments to efficiently process the apoptotic cell debris after it enters the MZM173,174. Future investigations into molecular mechanisms of proper uptake of apoptotic cells and their non-inflammatory digestion within a phagocytic cell may shed further insights into this as a common pathogenic mechanism in SLE and may explain such defects in other tissues. For example, defects in apoptotic cell debris uptake and elimination are a major pathogenic mechanism in mesangial proliferative glomerulonephritis which involves mesangial cells175 and also, membranous glomerulonephritis which involves defects in apoptotic cell disposal by the podocytes lining the outside of basement membranes in the capillary loop of the glomerulus176,177. Importantly, such apoptotic cell uptake defects may be related to several under-explored mechanisms as described in this section, and studies should extend beyond effects of proinflammatory cytokines (such as type I interferon, interferon-γ, IL-6 or IL-17)178, to include intracellular defects in the organization of membrane contact molecules and within internal organelles associated with proper apoptotic cell debris.
4. TOLERANCE LOSS IN THE GC
4.1. STIMULATION OF RESTING B CELLS WITH AUTOANTIGENS AND CO-STIMULATORY MOLECULES
Despite the existence of BM and transitional B cell stage negative selection and anergy induction mechanisms, mature autoreactive B cells do find their way into GC reactions, as inferred from the high levels of mutations observed in the IgVH genes of circulating plasma cells (PCs) in SLE179. For activated of naive autoreactive B cells escaping central tolerance, the nature of secondary signals during B cell antigen presentation to T cells is a major gate-keeper of GC initiation. GC formation begins with antigen recognition and acquisition by resting B cells180, followed by B cell migration to the T:B border181. Here, B cells receive co-stimulatory signals from CD4+ T cells182,183. This interaction triggers full activation, proliferation, the initiation of differentiation182, as well as a coalescence in close association with a network of stromal cells known as follicular dendritic cells (FDCs)184,185. B cells compete for limited antigen in an affinity-dependent manner, such that B cells with higher-affinity BCRs can gradually outcompete lower-affinity B cells184,185. After autoAg stimulation, co-stimulatory molecules, including B cell expressed inducible T cell co-stimulator (ICOS) ligand, B7 molecules (CD80 and CD86), and CD40 provide a positive co-stimulatory signal to T cells through ICOS, CD28 and CD40L, respectively182. An additional crucial level of control is achieved by the expression of inhibitory receptors that can transduce negative signals. B cell PD-L1 provides a negative signal through interaction with T cell PD-L, which is linked to an immuno-tyrosine inhibitory motif (ITIM) and an immuno-tyrosine domain switching motif (ITSM)186. This inhibitory motif can dampen signaling through TCR-CD3-ICOS family and dominates during a low-affinity CD86 class II MHC and TCR interaction.
CTLA-4 is another well-studied inhibitory receptor which binds both CD80 and CD86 and effectively opposes B cell interaction CD28182,187. An important role of CD86 to promote SLE is suggested by increased expression of CD86 on circulating B cells in SLE subjects188 and its association with renal disease51. Inhibition of the CD28-CD86 interaction in BXD2 mice through administration of an adenovirus expressing CTLA4-Ig resulted in a long-term normalization of AICDA expression in the B cells, suppression of both autoAb producing B cells and IgG autoAbs189. Similarly, knockout of CD86 prevented the co-stimulatory activity of MZ-P B cells, even though they retained their ability to translocate into the FO6. As CD86-CD28 engagement induces both AICDA and signals for SHM and CSR within autoreactive B cells, high levels of CD86 has the potential to fully potentiate follicular naïve (or transitional/MZ-P B cells) to stimulate TFHs. Moreover, these signals can potentially overcome the inhibitory effects of PD-L1, even in the context of low affinity binding of class II MHC plus antigen to TCR on the T cell190–192.
4.2. STIMULATION OF AUTOREACTIVE B CELLS BY TFH
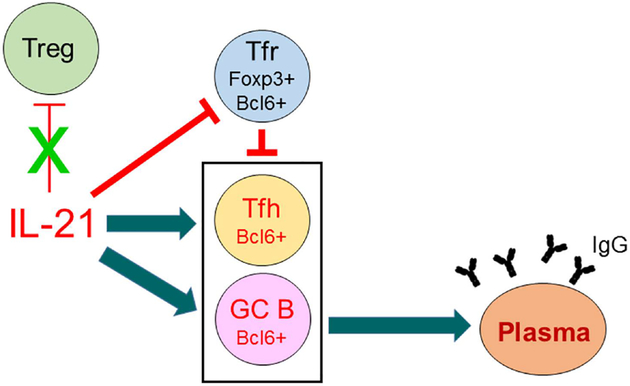
GC-dependent B cell responses are initiated similar to GC-independent B cell responses in which there is an initial contact of an antigen activated B cell at the T/B border193. The T-follicular helper cell that initiates this response does so using interactions to initially promote survival with an interaction with the GC B cell. Whether this GC reaction is normal or pathogenic is determined by the cytokines produced by the Tfh and the cytokine receptors expressed by the GC B cell194–196. After successful interaction with T cells, the B cell has a potential to encounter a spectrum of T-follicular helper cells each of which possess a different transcription factor that drives a different program and different cytokines that can promote B cell development. The canonical T-follicular helper cell is one in which STAT3 signaling, either through IL-6 or IL-21, can upregulate Bcl6, the canonical transcription factor for Tfhs (Figure 6)197–202. We have also identified that IL-21 selectively inhibits Foxp3+Bcl6+CXCR5+ICOS+ follicular regulatory T cell (Tfr) commitment without influencing total Treg cells (Figure 6). Bcl6 turns on a program of Tfh genes that can promote B cell development and at the same time, turns off the Tfh’s ability to develop into other Tfhs. However, this suppression has been shown to be incomplete and also, subject to a synchronous development. Weinstein and co-workers have shown that Tfhs exhibit synchronous development from Bcl6-IL21 producing Tfh to a IL-4 producing Tfh196. IL-4 producing Tfhs have not been shown to be especially pathogenic in SLE but may play a role in enhancing AICDA in B cells. In contrast to these normal GC encounters, Tfhs that express IL-17 or IFNɣ (Tfh-IL-17, Tfh-IFNɣ) (with the underlying T cell program transcription factors being RORɣt and T-bet) then interact with the corresponding receptors, IL-17R or IFNɣR on B cells to induce development of autoantibody producing B cells11,15,171,194,203. The underlying mechanism for production of such autoantibodies is by definition due to a loss of tolerance at some step before or at this GC stage of development.
Figure 6.
IL-21 breaks GC B-cell tolerance through the stimulation of Tfh and GC B cells and the inhibition of Tfr CD4 T cells. In BXD2 mice, IL-21 regulates GC formation by preferential expansion of Tfh cells, relative to Tfr cells. These CXCR5+ICOS+ subpopulations of CD4 T cells are located in the GC as we previously described171,172. In BXD2-Il21−/− mice, the frequency of Foxp3+ Tfr cells was increased, whereas there was a significant decrease in the frequency and the number of Foxp3− Tfh cells, resulting in an increase in the ratio of Foxp3+/Foxp3− CXCR5+ICOS+CD4 T cells. There was no change in the frequency and the ratio of conventional Treg to total CD4 T cells. Together, these results suggest that IL-21 regulates GC development through an effect that is limited to the relatively small subpopulations of Tfh and Tfr cells, which underscores the important role of these two small subpopulations in regulating GCs.
4.3. IL-17, RGS13, AND PROLONGED RETENTION IN THE GC
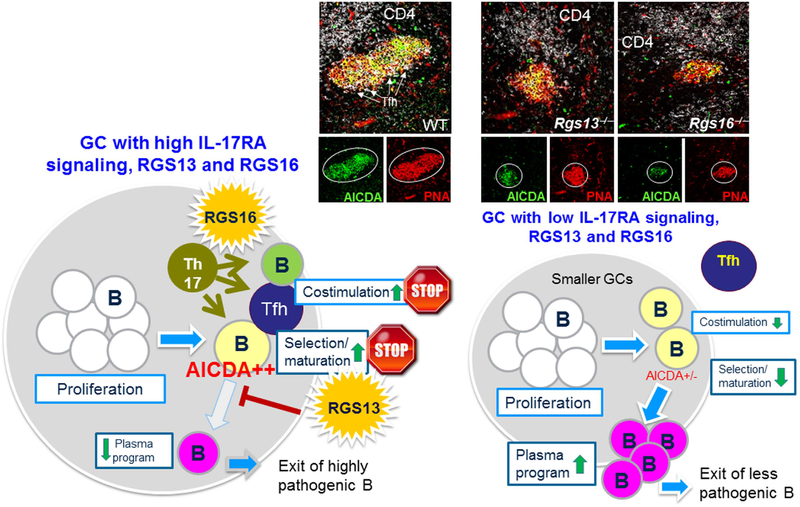
In SLE, pathogenic Tfhs include those that produce interferon-γ (Tfh-IFNγ) or IL-17 (Tfh-IL-17). In 2008, we first showed in BXD2 mice that GC T cells expressed high levels of IL-17 and that GC B cells expressed high levels of IL-17RA11. IL-17 did not have a direct proliferative or survival effect on B cells but could stabilize the T-B interaction by upregulation of RGS13 and RGS16 which desensitized their migratory response to a CXCL-13 chemokine gradient from the follicular dendritic cell (Figure 7)12,204. IL‐17 effectively induces expression of regulator of G protein signaling 13 (RGS13), a GTPase accelerator (GTPase‐activating protein) for Gα subunits that can control the magnitude and duration of chemotactic responses to CXCL12 and CXCL1313,205. The resulting stabilization of T-B interaction is similar to stabilizations that have previously been described by CTLA4 which suppresses T cell migratory behavior or PD-L1186,206. However, instead of inhibiting migration signaling outside the GC, these interactions now inhibit migration of B and T cells inside the GC14. We showed that these interactions lock B and T cells into a state of persistent or prolonged interaction. RGS13 expression in BXD2 mice occurred exclusively in GC B cells, and its expression was stimulated by IL‐17, but not IL‐21. Importantly, IL-17 induction of RGS13 enabled retention and close contact of GC B cells and CD4 T cells, which was necessary for AICDA up‐regulation11. Although BXD2‐Rgs13−/− mice exhibited equal numbers of GC B cells surrounding CD4 T cells, their levels of AICDA expression were significantly attenuated. We subsequently showed that IL-17 can directly activate through the IL-17 on B cells through the ACT1/Traf6 pathway and directly activate the p50/p65 NF-kB pathway15. This activation pathway has turned out to be a central mechanism for regulating B cell activation responses through multiple signaling pathways. B cells lacking IL-17RA upregulate the chromatin transcriptional repressor, p50/p50 homodimers which can block NF-ĸB signaling through multiple molecules including IRF7, IgG, CD40, and others207. These results show the broad-spectrum importance of IL-17RA signaling toward autoreactive B cell development and support that such IL-17 signaling is an important contributor to development of autoantibody producing B cells. In the Tfh cells, we have further identified that IL-17 is an extrinsic stop signal that it acts through the induction of RGS16 on post-differentiated IL-17RA(+) Tfh to enable interaction with responder B cells in the LZ niche. These data suggest a novel concept that Tfh differentiation and its stabilization in the LZ are two separate checkpoints and that IL-21 and IL-17 act at each checkpoint to enable pathogenic GC development (Figure 7).
Figure 7.
Prolonged Tfh and GC B cell interactions through the effects of IL-17-mediated upregulation of RGS13 and RGS16. Chemotactic responses play a key role in orchestrating the cell-cell interactions in the GCs. This process involves active shuttling of the antigen-carrying B cells between the marginal zone and the GCs. We have shown that within the GCs of autoimmune BXD2 mice, IL-17A upregulates the expression of regulators of G protein signaling (RGS) in B cells to desensitize the G protein-coupled receptor (GPCR) signaling pathway of CXCL12 and CXCL13 chemokines. Consistent with this, IL-17 also stimulated the expression of RGS16 in Tfh to promote Tfh migration arrest at the GC light zone to facilitate Tfh and GC B cell interactions. This promotes a prolonged stable interaction of B and T cells in the GC that induces high levels of activation-induced cytidine deaminase (AICDA) thereby enabling development of pathogenic autoantibody-producing B cells.
Two major decision points for proliferating GC B cells is whether and when their progeny undergo (1) Ig class-switching and (2) the switch to Blimp1 and differentiation into plasma cells. The reduced AICDA expression and lower titers of autoantibody production in RSG13‐deficient mice suggest a role for RGS13 in this fate decision choice. Indeed, while RGS13 deficiency was associated with a significant reduction in the frequency of GC B cells, there was a significant increase in CD138+B220+ plasmablasts14. High‐power microscopy further confirmed higher numbers of IgMbright plasmablasts located in bridging channels and outside of the marginal zone in the BXD2‐Rgs13−/− mice compared to the BXD2 mice. This increase in plasmablast numbers was associated with changes in gene expression in sorted GC B cells, including increased PC program genes Blimp1, IRF‐4, and XBP‐1 and significantly lower expression GC program genes Bach2, Pax5, and AICDA, but not Bcl614. Functionally, immunization of wild‐type BXD2 and BXD2‐Rgs13−/− with NP21‐CGG, a T cell–dependent antigen revealed that, from week 2 to week 4, the development of anti‐NP7 (high-affinity) and anti‐NP33 (all-affinity) IgG1 and IgG2b antibodies were significantly reduced in BXD2‐Rgs13−/− mice, consistent with a reduction in GC‐mediated SHM in the BXD2-Rgs13−/− B cells. Taken together, IL-17–induced RGS13 favors the GC program and delays the transition of GC B cells to PC, leading to increased CSR, SHM, and production of pathogenic autoantibodies in a model of “delay‐driven diversity” (Figure 7)208,209. In this model, antibody affinity maturation processes, including SHM and CSR, can be controlled at the point at which GC B cells initiate the transition to PC differentiation208,209. This model provides a framework for the understanding of IL-17–mediated promotion of GC formation and autoantibody production in autoimmune disease. Factors such as IL-17 that can directly regulate the expression of RGS13 have the ability to influence the size of GCs, as well as temporal differences in expression of key GC-PC differentiation genes and the extent of affinity maturation and CSR in the GC which in turn can affect disease severity. Future studies will be needed to determine if regulation of B cell migration can be safely use to prevent autoimmune disease.
4.4. TYPE II IFN AND AUTOREACTIVE B CELLS IN SLE
A second pathogenic cytokine in the GC B cell development is IFNɣ produced by Tfh-IFNɣ. Such Tfhs express the transcription factor, T-bet and drive the development of T-bet+ B cells16,18–20,210,211. This B cell phenotype is similar to that previously found age-associated B cell (ABC) and overlap with memory populations17,18. As described above, this can occur outside the GC to produce short-lived responses or inside the GC to produce higher affinity and more pathogenic B cells and lead potentially to a longer-lived memory B cell. These interactions are best characterized using viral GC responses which promote a strong Tfh-IFNɣ response consistent with the Th1 response to a virus. These interactions produce IFNɣ and promote development of high-affinity B cells to undergo SHM and class switching to produce neutralizing antibody to the virus. In the context of autoimmunity, B cells require additional helper factors including TLR7, strong IgG immunoglobulin crosslinking, IL-21 and other factors, as described above.
In SLE, it has been long-recognized that there is an increased population of circulating activated B cells that have been characterized by a CD19hiCD21−CD38loIgMlo CD23− phenotype212. There was also a lower expression of CD25 (IL-2Ralpha) in both naïve and memory B cells in SLE patients, suggesting defects in development of immunomodulatory CD25+ B cells213. One of the fundamental B cell defects in SLE is the inability to maintain naïve B cells in a resting state, either due to an incomplete tolerance or anergy induction during transitional B cell development or exposure to stimulatory T cell cytokines or an inflammatory environment (potentially BAFF from macrophages). Especially important for SLE is the exposure of resting naïve B cells to type II IFNɣ17. This results in upregulation of the Th1 transcription factor, T-bet, and down regulation of the BACH2 suppressor. BACH2 is one of the key transcription factors leading to a program preventing development of antibody-secreting B cells, whereas reprogramming by IFNɣ in the context of TLR7 stimulation can upregulate T-bet and reprogram the B cells to become activated naïve which can then, in the presence of IL-21 and further stimulation, upregulate Blimp1 and lead to antibody-secreting cells16,214. This development pathway is not dependent on a GC but is dependent upon T cell cytokines. As both IL-17 and IFNɣ can promote development of autoreactive B cells, further trials with an anti-interleukin-12/23 (IL-12/23) monoclonal antibody such as Ustekinumab (Stelara), which is blocks the p40 common chain215,216 will be important217,218. Such therapies might inhibit development of T cells that produce IL-17 or IFNɣ, and be effective to prevent the T-dependent phase of pathogenic B cell development in SLE.
5. CONCLUSIONS
A focus of this review has been that pathogenic autoAb development in SLE is not merely an outcome of mis-directed B cell responses in which the driving Ags are self-derived; rather pathogenic autoAbs are the outcome of a gradual dissolution in tolerance checkpoints and barriers realized in large part by the B cells themselves. Thus, B cells are not viewed as bystanders “caught up” or recruited into responses as a consequence of extrinsic factors such as exposure to excess autoantigens, type I IFN production, innate environmental factors such as BAFF, and T cell-derived factors such as IL-21, IL-17 or IFNɣ. Moreover, the rapidly advancing frontier of single-cell technologies and our own single-cell studies suggest that B cells at the early transitional stage are endowed with a unique spectrum of gene expression destined to follow certain trajectories1,61,219. We propose that these endogenous properties such as IFNβ expression are maintained to guide subsequent B cell responses at the MZ-P stage and beyond in which responses to apoptotic cell debris, type I IFNs, and mLTβ are integrated in a balancing act to either maintain a tolerogenic MZM barrier or initiate to a cascade of follicular translocation, stimulation of FDCs and initiation of an early GC response. The development of autoreactive GCs thus possesses a component of spontaneity, as their development is in a sense continuous with prior defects, rather than the result of a strong immunization, such as by an infectious agent. Unresolved questions are whether the initial propensity of early T1 stage B cells to produce IFNβ is due to polymorphisms in transcription factor components of the IFNβ enhanceosome or is secondary to stimulation through cytosolic and/or endosomal DNA/RNA sensors. The finding that IFNβ production is enhanced in AAs compared to EAs suggest a genetic contribution, but does not exclude other possibilities.
Another complexity in SLE autoreactive B cell development is the opposing effects that immunologic factors have depending on their localization (e.g. outside or inside the follicle). For example, outside the follicle, B cell expression of mLTβ has a protective role to maintain a tolerogenic MZM barrier. Yet, these same mLTβ+ B cells, when translocated into the follicle (through the action of type I IFNs to induce CD69), can stimulate FDCs to initiate an active GC response10,122. BAFF is another factor with dual roles. Produced by MZMs, it maintains MZ B cells and is necessary for MZ barrier integrity122,220. However, when present and signaling at the T2 or the GC stage of development, BAFF can promote survival and tolerance loss of autoreactive B cells221–224.
Similarly, regulation of chemotaxis can either enhance or inhibit immune responses. Interruption of a chemokine signaling is not always beneficial. In the case of T and B cells in which contact is established by chemokine gradients, IL-17 can function to induce chemokine unresponsiveness, effectively stabilizing and prolonging T:B interactions. Thus, turning off motion or chemotaxis can accentuate development of an immune response and autoreactive B cells. Fingolimod represents a practical example of this principle; interruption of S1P1 responses to high levels of extra-follicular S1P may prevent egress of immune cells from a follicle, and at the same time, promote compartmentalization and an intense immune response within the follicle. Thus, termination of fingolimod treatment may exacerbate multiple sclerosis in certain patients225,226, presumably due to the release of follicular inflammatory cells into the circulation or local tissues.
Another unresolved question is whether the transitional B-cell checkpoint can influence B and T-cell interactions at the GC stage. While transitional B cell properties can influence intrinsic responsiveness to TLRs and T cell polarization51,61, it is less clear whether transitional stage events imprint specific patterns of cytokine receptor expression. The regulation of cytokine receptor expression is important, as the pathogenic cytokines produced by Tfh-IL-21, Tfh-IL-17 or Tfh-IFNɣ cells are insufficient for adaptive immunity and require the expression of specific receptors in order to drive GC B cell responses. The molecular basis for when and how specific profiles of cytokine receptor expression are acquired is poorly defined for B cells, but ongoing advances in single-cell technologies are providing the tools and approaches to answer these questions. Such studies will be needed to probe relationships between developmental transitions (i.e. T1-T2-mature B), chromatin states, gene expression, and specific pathogenic phenotypes.
ACKNOWLEDGEMENTS
We thank past and present members of the Mountz lab for helpful discussions. J.D.M. was supported by grants from VA Merit Review grant (I01BX004049), NIH grants R01-AI-071110, R01 AI134023, and Lupus Research Alliance Distinguished Innovator Award. H.C.H. was supported by the Lupus Research Alliance Novel Research Award. J.A.H. was supported by the 2T32AI007051–39 Immunology T32 Training Grant and the LFA Finzi Summer Fellowship. Flow cytometry analysis and high resolution imaging analysis were supported by the and the P30-AR-048311 and the P30-AI-027767.
Footnotes
CONFLICT OF INTEREST
The authors have no conflict of interest to declare.
References
- 1.Hamilton JA, Wu Q, Yang P, et al. Cutting Edge: Intracellular IFN-beta and Distinct Type I IFN Expression Patterns in Circulating Systemic Lupus Erythematosus B Cells. J Immunol. 2018;201(8):2203–2208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McGaha TL, Chen Y, Ravishankar B, van Rooijen N, Karlsson MC. Marginal zone macrophages suppress innate and adaptive immunity to apoptotic cells in the spleen. Blood. 2011;117(20):5403–5412. [DOI] [PubMed] [Google Scholar]
- 3.Cinamon G, Matloubian M, Lesneski MJ, et al. Sphingosine 1-phosphate receptor 1 promotes B cell localization in the splenic marginal zone. Nat Immunol. 2004;5(7):713–720. [DOI] [PubMed] [Google Scholar]
- 4.Kraal G Cells in the marginal zone of the spleen. Int Rev Cytol. 1992;132:31–74. [DOI] [PubMed] [Google Scholar]
- 5.Wang JH, Li J, Wu Q, et al. Marginal zone precursor B cells as cellular agents for type I IFN-promoted antigen transport in autoimmunity. J Immunol. 2010;184(1):442–451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang JH, Wu Q, Yang P, et al. Type I interferon-dependent CD86(high) marginal zone precursor B cells are potent T cell costimulators in mice. Arthritis Rheum. 2011;63(4):1054–1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.You Y, Myers RC, Freeberg L, et al. Marginal zone B cells regulate antigen capture by marginal zone macrophages. J Immunol. 2011;186(4):2172–2181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhou Z, Niu H, Zheng YY, Morel L. Autoreactive marginal zone B cells enter the follicles and interact with CD4+ T cells in lupus-prone mice. BMC immunology. 2011;12:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li H, Wu Q, Li J, et al. Cutting Edge: defective follicular exclusion of apoptotic antigens due to marginal zone macrophage defects in autoimmune BXD2 mice. J Immunol. 2013;190(9):4465–4469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Myers RC, King RG, Carter RH, Justement LB. Lymphotoxin alpha1beta2 expression on B cells is required for follicular dendritic cell activation during the germinal center response. European journal of immunology. 2013;43(2):348–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hsu HC, Yang P, Wang J, et al. Interleukin 17-producing T helper cells and interleukin 17 orchestrate autoreactive germinal center development in autoimmune BXD2 mice. Nat Immunol. 2008;9(2):166–175. [DOI] [PubMed] [Google Scholar]
- 12.Moratz C, Harrison K, Kehrl JH. Regulation of chemokine-induced lymphocyte migration by RGS proteins. Methods in enzymology. 2004;389:15–32. [DOI] [PubMed] [Google Scholar]
- 13.Shi GX, Harrison K, Wilson GL, Moratz C, Kehrl JH. RGS13 regulates germinal center B lymphocytes responsiveness to CXC chemokine ligand (CXCL)12 and CXCL13. J Immunol. 2002;169(5):2507–2515. [DOI] [PubMed] [Google Scholar]
- 14.Wang JH, New JS, Xie S, et al. Extension of the germinal center stage of B cell development promotes autoantibodies in BXD2 mice. Arthritis Rheum. 2013;65(10):2703–2712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xie S, Li J, Wang JH, et al. IL-17 activates the canonical NF-kappaB signaling pathway in autoimmune B cells of BXD2 mice to upregulate the expression of regulators of G-protein signaling 16. J Immunol. 2010;184(5):2289–2296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jenks SA, Cashman KS, Zumaquero E, et al. Distinct Effector B Cells Induced by Unregulated Toll-like Receptor 7 Contribute to Pathogenic Responses in Systemic Lupus Erythematosus. Immunity. 2018;49(4):725–739 e726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Peng SL, Szabo SJ, Glimcher LH. T-bet regulates IgG class switching and pathogenic autoantibody production. Proc Natl Acad Sci U S A. 2002;99(8):5545–5550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rubtsova K, Rubtsov AV, Thurman JM, Mennona JM, Kappler JW, Marrack P. B cells expressing the transcription factor T-bet drive lupus-like autoimmunity. J Clin Invest. 2017;127(4):1392–1404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang S, Wang J, Kumar V, et al. IL-21 drives expansion and plasma cell differentiation of autoreactive CD11c(hi)T-bet(+) B cells in SLE. Nat Commun. 2018;9(1):1758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zumaquero E, Stone SL, Scharer CD, et al. IFNgamma induces epigenetic programming of human T-bet(hi) B cells and promotesTLR7/8 and IL-21 induced differentiation. Elife. 2019;8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sakaguchi N, Melchers F. Lambda 5, a new light-chain-related locus selectively expressed in pre-B lymphocytes. Nature. 1986;324(6097):579–582. [DOI] [PubMed] [Google Scholar]
- 22.Melchers F, Karasuyama H, Haasner D, et al. The surrogate light chain in B-cell development. Immunol Today. 1993;14(2):60–68. [DOI] [PubMed] [Google Scholar]
- 23.Nagasawa T Microenvironmental niches in the bone marrow required for B-cell development. Nat Rev Immunol. 2006;6(2):107–116. [DOI] [PubMed] [Google Scholar]
- 24.Sandel PC, Monroe JG. Negative selection of immature B cells by receptor editing or deletion is determined by site of antigen encounter. Immunity. 1999;10(3):289–299. [DOI] [PubMed] [Google Scholar]
- 25.Pelanda R, Torres RM. Central B-cell tolerance: where selection begins. Cold Spring Harb Perspect Biol. 2012;4(4):a007146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tiegs SL, Russell DM, Nemazee D. Receptor editing in self-reactive bone marrow B cells. J Exp Med. 1993;177(4):1009–1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gay D, Saunders T, Camper S, Weigert M. Receptor editing: an approach by autoreactive B cells to escape tolerance. J Exp Med. 1993;177(4):999–1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mietzner B, Tsuiji M, Scheid J, et al. Autoreactive IgG memory antibodies in patients with systemic lupus erythematosus arise from nonreactive and polyreactive precursors. Proc Natl Acad Sci U S A. 2008;105(28):9727–9732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Goodnow CC, Crosbie J, Adelstein S, et al. Altered immunoglobulin expression and functional silencing of self-reactive B lymphocytes in transgenic mice. Nature. 1988;334(6184):676–682. [DOI] [PubMed] [Google Scholar]
- 30.Cambier JC, Gauld SB, Merrell KT, Vilen BJ. B-cell anergy: from transgenic models to naturally occurring anergic B cells? Nat Rev Immunol. 2007;7(8):633–643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rui L, Healy JI, Blasioli J, Goodnow CC. ERK signaling is a molecular switch integrating opposing inputs from B cell receptor and T cell cytokines to control TLR4-driven plasma cell differentiation. J Immunol. 2006;177(8):5337–5346. [DOI] [PubMed] [Google Scholar]
- 32.Rui L, Vinuesa CG, Blasioli J, Goodnow CC. Resistance to CpG DNA-induced autoimmunity through tolerogenic B cell antigen receptor ERK signaling. Nat Immunol. 2003;4(6):594–600. [DOI] [PubMed] [Google Scholar]
- 33.Vilen BJ, Famiglietti SJ, Carbone AM, Kay BK, Cambier JC. B cell antigen receptor desensitization: disruption of receptor coupling to tyrosine kinase activation. J Immunol. 1997;159(1):231–243. [PMC free article] [PubMed] [Google Scholar]
- 34.Getahun A, Beavers NA, Larson SR, Shlomchik MJ, Cambier JC. Continuous inhibitory signaling by both SHP-1 and SHIP-1 pathways is required to maintain unresponsiveness of anergic B cells. J Exp Med. 2016;213(5):751–769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cornall RJ, Cyster JG, Hibbs ML, et al. Polygenic autoimmune traits: Lyn, CD22, and SHP-1 are limiting elements of a biochemical pathway regulating BCR signaling and selection. Immunity. 1998;8(4):497–508. [DOI] [PubMed] [Google Scholar]
- 36.Dorner T, Shock A, Goldenberg DM, Lipsky PE. The mechanistic impact of CD22 engagement with epratuzumab on B cell function: Implications for the treatment of systemic lupus erythematosus. Autoimmun Rev. 2015;14(12):1079–1086. [DOI] [PubMed] [Google Scholar]
- 37.Kaminski DA, Wei C, Qian Y, Rosenberg AF, Sanz I. Advances in human B cell phenotypic profiling. Front Immunol. 2012;3:302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Agrawal S, Smith SA, Tangye SG, Sewell WA. Transitional B cell subsets in human bone marrow. Clin Exp Immunol. 2013;174(1):53–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Uckun FM. Regulation of human B-cell ontogeny. Blood. 1990;76(10):1908–1923. [PubMed] [Google Scholar]
- 40.Sims GP, Ettinger R, Shirota Y, Yarboro CH, Illei GG, Lipsky PE. Identification and characterization of circulating human transitional B cells. Blood. 2005;105(11):4390–4398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yurasov S, Wardemann H, Hammersen J, et al. Defective B cell tolerance checkpoints in systemic lupus erythematosus. J Exp Med. 2005;201(5):703–711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mebius RE, Kraal G. Structure and function of the spleen. Nat Rev Immunol. 2005;5(8):606–616. [DOI] [PubMed] [Google Scholar]
- 43.Henderson RB, Grys K, Vehlow A, et al. A novel Rac-dependent checkpoint in B cell development controls entry into the splenic white pulp and cell survival. J Exp Med. 2010;207(4):837–853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Petro JB, Gerstein RM, Lowe J, Carter RS, Shinners N, Khan WN. Transitional type 1 and 2 B lymphocyte subsets are differentially responsive to antigen receptor signaling. J Biol Chem. 2002;277(50):48009–48019. [DOI] [PubMed] [Google Scholar]
- 45.Chung JB, Silverman M, Monroe JG. Transitional B cells: step by step towards immune competence. Trends Immunol. 2003;24(6):343–349. [DOI] [PubMed] [Google Scholar]
- 46.Su TT, Rawlings DJ. Transitional B lymphocyte subsets operate as distinct checkpoints in murine splenic B cell development. J Immunol. 2002;168(5):2101–2110. [DOI] [PubMed] [Google Scholar]
- 47.Andrews SF, Rawlings DJ. Transitional B cells exhibit a B cell receptor-specific nuclear defect in gene transcription. J Immunol. 2009;182(5):2868–2878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Turner M, Gulbranson-Judge A, Quinn ME, Walters AE, MacLennan IC, Tybulewicz VL. Syk tyrosine kinase is required for the positive selection of immature B cells into the recirculating B cell pool. J Exp Med. 1997;186(12):2013–2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cherukuri A, Salama AD, Carter CR, et al. Reduced human transitional B cell T1/T2 ratio is associated with subsequent deterioration in renal allograft function. Kidney Int. 2017;91(1):183–195. [DOI] [PubMed] [Google Scholar]
- 50.Blair PA, Chavez-Rueda KA, Evans JG, et al. Selective targeting of B cells with agonistic anti-CD40 is an efficacious strategy for the generation of induced regulatory T2-like B cells and for the suppression of lupus in MRL/lpr mice. J Immunol. 2009;182(6):3492–3502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nova-Lamperti E, Fanelli G, Becker PD, et al. IL-10-produced by human transitional B-cells down-regulates CD86 expression on B-cells leading to inhibition of CD4+T-cell responses. Sci Rep. 2016;6:20044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Newell KA, Asare A, Kirk AD, et al. Identification of a B cell signature associated with renal transplant tolerance in humans. J Clin Invest. 2010;120(6):1836–1847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Evans JG, Chavez-Rueda KA, Eddaoudi A, et al. Novel suppressive function of transitional 2 B cells in experimental arthritis. J Immunol. 2007;178(12):7868–7878. [DOI] [PubMed] [Google Scholar]
- 54.Ganti SN, Albershardt TC, Iritani BM, Ruddell A. Regulatory B cells preferentially accumulate in tumor-draining lymph nodes and promote tumor growth. Sci Rep. 2015;5:12255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Blair PA, Norena LY, Flores-Borja F, et al. CD19(+)CD24(hi)CD38(hi) B cells exhibit regulatory capacity in healthy individuals but are functionally impaired in systemic Lupus Erythematosus patients. Immunity. 2010;32(1):129–140. [DOI] [PubMed] [Google Scholar]
- 56.Samuels J, Ng YS, Coupillaud C, Paget D, Meffre E. Impaired early B cell tolerance in patients with rheumatoid arthritis. J Exp Med. 2005;201(10):1659–1667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Scholz JL, Oropallo MA, Sindhava V, Goenka R, Cancro MP. The role of B lymphocyte stimulator in B cell biology: implications for the treatment of lupus. Lupus. 2013;22(4):350–360. [DOI] [PubMed] [Google Scholar]
- 58.Hoek KL, Carlesso G, Clark ES, Khan WN. Absence of mature peripheral B cell populations in mice with concomitant defects in B cell receptor and BAFF-R signaling. J Immunol. 2009;183(9):5630–5643. [DOI] [PubMed] [Google Scholar]
- 59.Huang W, Quach TD, Dascalu C, et al. Belimumab promotes negative selection of activated autoreactive B cells in systemic lupus erythematosus patients. JCI Insight. 2018;3(17). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Schiemann B, Gommerman JL, Vora K, et al. An essential role for BAFF in the normal development of B cells through a BCMA-independent pathway. Science. 2001;293(5537):2111–2114. [DOI] [PubMed] [Google Scholar]
- 61.Hamilton JA, Wu Q, Yang P, et al. Cutting Edge: Endogenous IFN-beta Regulates Survival and Development of Transitional B Cells. J Immunol. 2017;199(8):2618–2623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Meyer-Bahlburg A, Andrews SF, Yu KO, Porcelli SA, Rawlings DJ. Characterization of a late transitional B cell population highly sensitive to BAFF-mediated homeostatic proliferation. J Exp Med. 2008;205(1):155–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hsu BL, Harless SM, Lindsley RC, Hilbert DM, Cancro MP. Cutting edge: BLyS enables survival of transitional and mature B cells through distinct mediators. J Immunol. 2002;168(12):5993–5996. [DOI] [PubMed] [Google Scholar]
- 64.Taniguchi T, Takaoka A. A weak signal for strong responses: interferon-alpha/beta revisited. Nat Rev Mol Cell Biol. 2001;2(5):378–386. [DOI] [PubMed] [Google Scholar]
- 65.Vasconcellos R, Braun D, Coutinho A, Demengeot J. Type I IFN sets the stringency of B cell repertoire selection in the bone marrow. Int Immunol. 1999;11(2):279–288. [DOI] [PubMed] [Google Scholar]
- 66.Demengeot J, Vasconcellos R, Modigliani Y, Grandien A, Coutinho A. B lymphocyte sensitivity to IgM receptor ligation is independent of maturation stage and locally determined by macrophage-derived IFN-beta. Int Immunol. 1997;9(11):1677–1685. [DOI] [PubMed] [Google Scholar]
- 67.Ward JM, Ratliff ML, Dozmorov MG, et al. Human effector B lymphocytes express ARID3a and secrete interferon alpha. J Autoimmun. 2016;75:130–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Fu Q, Zhao J, Qian X, et al. Association of a functional IRF7 variant with systemic lupus erythematosus. Arthritis Rheum. 2011;63(3):749–754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ali S, Mann-Nuttel R, Schulze A, Richter L, Alferink J, Scheu S. Sources of Type I Interferons in Infectious Immunity: Plasmacytoid Dendritic Cells Not Always in the Driver’s Seat. Front Immunol. 2019;10:778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Blanco P, Palucka AK, Gill M, Pascual V, Banchereau J. Induction of dendritic cell differentiation by IFN-alpha in systemic lupus erythematosus. Science. 2001;294(5546):1540–1543. [DOI] [PubMed] [Google Scholar]
- 71.Rodero MP, Decalf J, Bondet V, et al. Detection of interferon alpha protein reveals differential levels and cellular sources in disease. J Exp Med. 2017;214(5):1547–1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Akkaya M, Akkaya B, Miozzo P, et al. B Cells Produce Type 1 IFNs in Response to the TLR9 Agonist CpG-A Conjugated to Cationic Lipids. J Immunol. 2017;199(3):931–940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Colonna M, Trinchieri G, Liu YJ. Plasmacytoid dendritic cells in immunity. Nat Immunol. 2004;5(12):1219–1226. [DOI] [PubMed] [Google Scholar]
- 74.Sharma S, Jin Z, Rosenzweig E, Rao S, Ko K, Niewold TB. Widely divergent transcriptional patterns between SLE patients of different ancestral backgrounds in sorted immune cell populations. J Autoimmun. 2015;60:51–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Vermi W, Lonardi S, Morassi M, et al. Cutaneous distribution of plasmacytoid dendritic cells in lupus erythematosus. Selective tropism at the site of epithelial apoptotic damage. Immunobiology. 2009;214(9–10):877–886. [DOI] [PubMed] [Google Scholar]
- 76.Fiore N, Castellano G, Blasi A, et al. Immature myeloid and plasmacytoid dendritic cells infiltrate renal tubulointerstitium in patients with lupus nephritis. Molecular immunology. 2008;45(1):259–265. [DOI] [PubMed] [Google Scholar]
- 77.Tucci M, Quatraro C, Lombardi L, Pellegrino C, Dammacco F, Silvestris F. Glomerular accumulation of plasmacytoid dendritic cells in active lupus nephritis: role of interleukin-18. Arthritis Rheum. 2008;58(1):251–262. [DOI] [PubMed] [Google Scholar]
- 78.Green NM, Laws A, Kiefer K, et al. Murine B cell response to TLR7 ligands depends on an IFN-beta feedback loop. J Immunol. 2009;183(3):1569–1576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Theofilopoulos AN, Kono DH, Beutler B, Baccala R. Intracellular nucleic acid sensors and autoimmunity. J Interferon Cytokine Res. 2011;31(12):867–886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Chauhan SK, Singh VV, Rai R, Rai M, Rai G. Distinct autoantibody profiles in systemic lupus erythematosus patients are selectively associated with TLR7 and TLR9 upregulation. Journal of clinical immunology. 2013;33(5):954–964. [DOI] [PubMed] [Google Scholar]
- 81.Celhar T, Magalhaes R, Fairhurst AM. TLR7 and TLR9 in SLE: when sensing self goes wrong. Immunologic research. 2012;53(1–3):58–77. [DOI] [PubMed] [Google Scholar]
- 82.Deane JA, Pisitkun P, Barrett RS, et al. Control of toll-like receptor 7 expression is essential to restrict autoimmunity and dendritic cell proliferation. Immunity. 2007;27(5):801–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Furie R, Khamashta M, Merrill JT, et al. Anifrolumab, an Anti-Interferon-alpha Receptor Monoclonal Antibody, in Moderate-to-Severe Systemic Lupus Erythematosus. Arthritis Rheumatol. 2017;69(2):376–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.de Weerd NA, Samarajiwa SA, Hertzog PJ. Type I interferon receptors: biochemistry and biological functions. J Biol Chem. 2007;282(28):20053–20057. [DOI] [PubMed] [Google Scholar]
- 85.Crow MK. Type I interferon in the pathogenesis of lupus. J Immunol. 2014;192(12):5459–5468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Catalina MD, Bachali P, Geraci NS, Grammer AC, Lipsky PE. Gene expression analysis delineates the potential roles of multiple interferons in systemic lupus erythematosus. Commun Biol. 2019;2:140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Hooks JJ, Jordan GW, Cupps T, Moutsopoulos HM, Fauci AS, Notkins AL. Multiple interferons in the circulation of patients with systemic lupus erythematosus and vasculitis. Arthritis Rheum. 1982;25(4):396–400. [DOI] [PubMed] [Google Scholar]
- 88.Wong D, Kea B, Pesich R, et al. Interferon and biologic signatures in dermatomyositis skin: specificity and heterogeneity across diseases. PloS one. 2012;7(1):e29161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Crow MK. Autoimmunity: Interferon alpha or beta: which is the culprit in autoimmune disease? Nature reviews Rheumatology. 2016;12(8):439–440. [DOI] [PubMed] [Google Scholar]
- 90.Hua J, Kirou K, Lee C, Crow MK. Functional assay of type I interferon in systemic lupus erythematosus plasma and association with anti-RNA binding protein autoantibodies. Arthritis Rheum. 2006;54(6):1906–1916. [DOI] [PubMed] [Google Scholar]
- 91.Bengtsson AA, Sturfelt G, Truedsson L, et al. Activation of type I interferon system in systemic lupus erythematosus correlates with disease activity but not with antiretroviral antibodies. Lupus. 2000;9(9):664–671. [DOI] [PubMed] [Google Scholar]
- 92.Schreiber G, Piehler J. The molecular basis for functional plasticity in type I interferon signaling. Trends Immunol. 2015;36(3):139–149. [DOI] [PubMed] [Google Scholar]
- 93.Lamken P, Lata S, Gavutis M, Piehler J. Ligand-induced assembling of the type I interferon receptor on supported lipid bilayers. Journal of molecular biology. 2004;341(1):303–318. [DOI] [PubMed] [Google Scholar]
- 94.Hurtado-Guerrero I, Pinto-Medel MJ, Urbaneja P, et al. Activation of the JAK-STAT Signaling Pathway after In Vitro Stimulation with IFNss in Multiple Sclerosis Patients According to the Therapeutic Response to IFNss. PloS one. 2017;12(1):e0170031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Ivashkiv LB, Donlin LT. Regulation of type I interferon responses. Nat Rev Immunol. 2014;14(1):36–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Zheng H, Qian J, Varghese B, Baker DP, Fuchs S. Ligand-stimulated downregulation of the alpha interferon receptor: role of protein kinase D2. Molecular and cellular biology. 2011;31(4):710–720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Marijanovic Z, Ragimbeau J, van der Heyden J, Uze G, Pellegrini S. Comparable potency of IFNalpha2 and IFNbeta on immediate JAK/STAT activation but differential down-regulation of IFNAR2. The Biochemical journal. 2007;407(1):141–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.de Weerd NA, Vivian JP, Nguyen TK, et al. Structural basis of a unique interferon-beta signaling axis mediated via the receptor IFNAR1. Nat Immunol. 2013;14(9):901–907. [DOI] [PubMed] [Google Scholar]
- 99.Gao L, Bird AK, Meednu N, et al. Bone Marrow-Derived Mesenchymal Stem Cells From Patients With Systemic Lupus Erythematosus Have a Senescence-Associated Secretory Phenotype Mediated by a Mitochondrial Antiviral Signaling Protein-Interferon-beta Feedback Loop. Arthritis Rheumatol. 2017;69(8):1623–1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Wu T, Xie C, Han J, et al. Metabolic disturbances associated with systemic lupus erythematosus. PloS one. 2012;7(6):e37210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Morel L Immunometabolism in systemic lupus erythematosus. Nature reviews Rheumatology. 2017;13(5):280–290. [DOI] [PubMed] [Google Scholar]
- 102.Buskiewicz IA, Montgomery T, Yasewicz EC, et al. Reactive oxygen species induce virus-independent MAVS oligomerization in systemic lupus erythematosus. Sci Signal. 2016;9(456):ra115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Perl A Oxidative stress in the pathology and treatment of systemic lupus erythematosus. Nature reviews Rheumatology. 2013;9(11):674–686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Munroe ME, Vista ES, Merrill JT, Guthridge JM, Roberts VC, James JA. Pathways of impending disease flare in African-American systemic lupus erythematosus patients. J Autoimmun. 2017;78:70–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Niewold TB. Advances in lupus genetics. Curr Opin Rheumatol. 2015;27(5):440–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Molineros JE, Maiti AK, Sun C, et al. Admixture mapping in lupus identifies multiple functional variants within IFIH1 associated with apoptosis, inflammation, and autoantibody production. PLoS Genet. 2013;9(2):e1003222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Molineros JE, Kim-Howard X, Deshmukh H, Jacob CO, Harley JB, Nath SK. Admixture in Hispanic Americans: its impact on ITGAM association and implications for admixture mapping in SLE. Genes Immun. 2009;10(5):539–545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Rees F, Doherty M, Grainge MJ, Lanyon P, Zhang W. The worldwide incidence and prevalence of systemic lupus erythematosus: a systematic review of epidemiological studies. Rheumatology (Oxford). 2017;56(11):1945–1961. [DOI] [PubMed] [Google Scholar]
- 109.Quach H, Rotival M, Pothlichet J, et al. Genetic Adaptation and Neandertal Admixture Shaped the Immune System of Human Populations. Cell. 2016;167(3):643–656 e617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Li Y, Oosting M, Smeekens SP, et al. A Functional Genomics Approach to Understand Variation in Cytokine Production in Humans. Cell. 2016;167(4):1099–1110 e1014. [DOI] [PubMed] [Google Scholar]
- 111.Li Y, Oosting M, Deelen P, et al. Inter-individual variability and genetic influences on cytokine responses to bacteria and fungi. Nat Med. 2016;22(8):952–960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Ter Horst R, Jaeger M, Smeekens SP, et al. Host and Environmental Factors Influencing Individual Human Cytokine Responses. Cell. 2016;167(4):1111–1124 e1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Niewold TB, Hua J, Lehman TJ, Harley JB, Crow MK. High serum IFN-alpha activity is a heritable risk factor for systemic lupus erythematosus. Genes Immun. 2007;8(6):492–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Bentham J, Morris DL, Graham DSC, et al. Genetic association analyses implicate aberrant regulation of innate and adaptive immunity genes in the pathogenesis of systemic lupus erythematosus. Nat Genet. 2015;47(12):1457–1464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Gough DJ, Messina NL, Clarke CJ, Johnstone RW, Levy DE. Constitutive type I interferon modulates homeostatic balance through tonic signaling. Immunity. 2012;36(2):166–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Sato M, Suemori H, Hata N, et al. Distinct and essential roles of transcription factors IRF-3 and IRF-7 in response to viruses for IFN-alpha/beta gene induction. Immunity. 2000;13(4):539–548. [DOI] [PubMed] [Google Scholar]
- 117.Lee MN, Ye C, Villani AC, et al. Common genetic variants modulate pathogen-sensing responses in human dendritic cells. Science. 2014;343(6175):1246980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Salloum R, Franek BS, Kariuki SN, et al. Genetic variation at the IRF7/PHRF1 locus is associated with autoantibody profile and serum interferon-alpha activity in lupus patients. Arthritis Rheum. 2010;62(2):553–561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Nedelec Y, Sanz J, Baharian G, et al. Genetic Ancestry and Natural Selection Drive Population Differences in Immune Responses to Pathogens. Cell. 2016;167(3):657–669 e621. [DOI] [PubMed] [Google Scholar]
- 120.Cyster JG, Goodnow CC. Antigen-induced exclusion from follicles and anergy are separate and complementary processes that influence peripheral B cell fate. Immunity. 1995;3(6):691–701. [DOI] [PubMed] [Google Scholar]
- 121.Shiow LR, Rosen DB, Brdickova N, et al. CD69 acts downstream of interferon-alpha/beta to inhibit S1P1 and lymphocyte egress from lymphoid organs. Nature. 2006;440(7083):540–544. [DOI] [PubMed] [Google Scholar]
- 122.Li H, Fu YX, Wu Q, et al. Interferon-induced mechanosensing defects impede apoptotic cell clearance in lupus. J Clin Invest. 2015;125(7):2877–2890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Cappione A 3rd, Anolik JH, Pugh-Bernard A, et al. Germinal center exclusion of autoreactive B cells is defective in human systemic lupus erythematosus. J Clin Invest. 2005;115(11):3205–3216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Asselin-Paturel C, Brizard G, Pin JJ, Briere F, Trinchieri G. Mouse strain differences in plasmacytoid dendritic cell frequency and function revealed by a novel monoclonal antibody. J Immunol. 2003;171(12):6466–6477. [DOI] [PubMed] [Google Scholar]
- 125.Asselin-Paturel C, Trinchieri G. Production of type I interferons: plasmacytoid dendritic cells and beyond. J Exp Med. 2005;202(4):461–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Hsu HC, Zhou T, Kim H, et al. Production of a novel class of polyreactive pathogenic autoantibodies in BXD2 mice causes glomerulonephritis and arthritis. Arthritis Rheum. 2006;54(1):343–355. [DOI] [PubMed] [Google Scholar]
- 127.Eriksson C, Kokkonen H, Johansson M, Hallmans G, Wadell G, Rantapaa-Dahlqvist S. Autoantibodies predate the onset of systemic lupus erythematosus in northern Sweden. Arthritis Res Ther. 2011;13(1):R30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Arbuckle MR, McClain MT, Rubertone MV, et al. Development of autoantibodies before the clinical onset of systemic lupus erythematosus. N Engl J Med. 2003;349(16):1526–1533. [DOI] [PubMed] [Google Scholar]
- 129.Bave U, Magnusson M, Eloranta ML, Perers A, Alm GV, Ronnblom L. Fc gamma RIIa is expressed on natural IFN-alpha-producing cells (plasmacytoid dendritic cells) and is required for the IFN-alpha production induced by apoptotic cells combined with lupus IgG. J Immunol. 2003;171(6):3296–3302. [DOI] [PubMed] [Google Scholar]
- 130.Christensen SR, Shlomchik MJ. Regulation of lupus-related autoantibody production and clinical disease by Toll-like receptors. Semin Immunol. 2007;19(1):11–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.van Vliet E, Melis M, van Ewijk W. Marginal zone macrophages in the mouse spleen identified by a monoclonal antibody. Anatomical correlation with a B cell subpopulation. J Histochem Cytochem. 1985;33(1):40–44. [DOI] [PubMed] [Google Scholar]
- 132.Allen LA, Aderem A. Molecular definition of distinct cytoskeletal structures involved in complement- and Fc receptor-mediated phagocytosis in macrophages. J Exp Med. 1996;184(2):627–637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.May RC, Machesky LM. Phagocytosis and the actin cytoskeleton. Journal of cell science. 2001;114(Pt 6):1061–1077. [DOI] [PubMed] [Google Scholar]
- 134.Fan H, Patel VA, Longacre A, Levine JS. Abnormal regulation of the cytoskeletal regulator Rho typifies macrophages of the major murine models of spontaneous autoimmunity. Journal of leukocyte biology. 2006;79(1):155–165. [DOI] [PubMed] [Google Scholar]
- 135.Cerutti A, Cols M, Puga I. Marginal zone B cells: virtues of innate-like antibody-producing lymphocytes. Nat Rev Immunol. 2013;13(2):118–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Koni PA, Sacca R, Lawton P, Browning JL, Ruddle NH, Flavell RA. Distinct roles in lymphoid organogenesis for lymphotoxins alpha and beta revealed in lymphotoxin beta-deficient mice. Immunity. 1997;6(4):491–500. [DOI] [PubMed] [Google Scholar]
- 137.Alimzhanov MB, Kuprash DV, Kosco-Vilbois MH, et al. Abnormal development of secondary lymphoid tissues in lymphotoxin beta-deficient mice. Proc Natl Acad Sci U S A. 1997;94(17):9302–9307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Craxton A, Magaletti D, Ryan EJ, Clark EA. Macrophage- and dendritic cell--dependent regulation of human B-cell proliferation requires the TNF family ligand BAFF. Blood. 2003;101(11):4464–4471. [DOI] [PubMed] [Google Scholar]
- 139.Schneider P, Takatsuka H, Wilson A, et al. Maturation of marginal zone and follicular B cells requires B cell activating factor of the tumor necrosis factor family and is independent of B cell maturation antigen. J Exp Med. 2001;194(11):1691–1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Ravishankar B, Liu H, Shinde R, et al. Tolerance to apoptotic cells is regulated by indoleamine 2,3-dioxygenase. Proc Natl Acad Sci U S A. 2012;109(10):3909–3914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Lenert P, Brummel R, Field EH, Ashman RF. TLR-9 activation of marginal zone B cells in lupus mice regulates immunity through increased IL-10 production. Journal of clinical immunology. 2005;25(1):29–40. [DOI] [PubMed] [Google Scholar]
- 142.Miles K, Heaney J, Sibinska Z, et al. A tolerogenic role for Toll-like receptor 9 is revealed by B-cell interaction with DNA complexes expressed on apoptotic cells. Proc Natl Acad Sci U S A. 2012;109(3):887–892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Elkon KB, Silverman GJ. Naturally occurring autoantibodies to apoptotic cells. Advances in experimental medicine and biology. 2012;750:14–26. [DOI] [PubMed] [Google Scholar]
- 144.Rowland SL, Riggs JM, Gilfillan S, et al. Early, transient depletion of plasmacytoid dendritic cells ameliorates autoimmunity in a lupus model. J Exp Med. 2014;211(10):1977–1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Baechler EC, Batliwalla FM, Karypis G, et al. Interferon-inducible gene expression signature in peripheral blood cells of patients with severe lupus. Proc Natl Acad Sci U S A. 2003;100(5):2610–2615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Bennett L, Palucka AK, Arce E, et al. Interferon and granulopoiesis signatures in systemic lupus erythematosus blood. J Exp Med. 2003;197(6):711–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Jego G, Palucka AK, Blanck JP, Chalouni C, Pascual V, Banchereau J. Plasmacytoid dendritic cells induce plasma cell differentiation through type I interferon and interleukin 6. Immunity. 2003;19(2):225–234. [DOI] [PubMed] [Google Scholar]
- 148.Kiefer K, Oropallo MA, Cancro MP, Marshak-Rothstein A. Role of type I interferons in the activation of autoreactive B cells. Immunology and cell biology. 2012;90(5):498–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Obermoser G, Pascual V. The interferon-alpha signature of systemic lupus erythematosus. Lupus. 2010;19(9):1012–1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Casciola-Rosen LA, Anhalt G, Rosen A. Autoantigens targeted in systemic lupus erythematosus are clustered in two populations of surface structures on apoptotic keratinocytes. J Exp Med. 1994;179(4):1317–1330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Fransen JH, Hilbrands LB, Ruben J, et al. Mouse dendritic cells matured by ingestion of apoptotic blebs induce T cells to produce interleukin-17. Arthritis Rheum. 2009;60(8):2304–2313. [DOI] [PubMed] [Google Scholar]
- 152.Peng Y, Martin DA, Kenkel J, Zhang K, Ogden CA, Elkon KB. Innate and adaptive immune response to apoptotic cells. J Autoimmun. 2007;29(4):303–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Ravishankar B, Shinde R, Liu H, et al. Marginal zone CD169+ macrophages coordinate apoptotic cell-driven cellular recruitment and tolerance. Proc Natl Acad Sci U S A. 2014;111(11):4215–4220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Miyake Y, Asano K, Kaise H, Uemura M, Nakayama M, Tanaka M. Critical role of macrophages in the marginal zone in the suppression of immune responses to apoptotic cell-associated antigens. J Clin Invest. 2007;117(8):2268–2278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Herlands RA, Christensen SR, Sweet RA, Hershberg U, Shlomchik MJ. T cell-independent and toll-like receptor-dependent antigen-driven activation of autoreactive B cells. Immunity. 2008;29(2):249–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Avalos AM, Busconi L, Marshak-Rothstein A. Regulation of autoreactive B cell responses to endogenous TLR ligands. Autoimmunity. 2010;43(1):76–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Walsh ER, Pisitkun P, Voynova E, et al. Dual signaling by innate and adaptive immune receptors is required for TLR7-induced B-cell-mediated autoimmunity. Proc Natl Acad Sci U S A. 2012;109(40):16276–16281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Suthers AN, Sarantopoulos S. TLR7/TLR9- and B Cell Receptor-Signaling Crosstalk: Promotion of Potentially Dangerous B Cells. Front Immunol. 2017;8:775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Desnues B, Macedo AB, Roussel-Queval A, et al. TLR8 on dendritic cells and TLR9 on B cells restrain TLR7-mediated spontaneous autoimmunity in C57BL/6 mice. Proc Natl Acad Sci U S A. 2014;111(4):1497–1502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Krieg AM, Vollmer J. Toll-like receptors 7, 8, and 9: linking innate immunity to autoimmunity. Immunol Rev. 2007;220:251–269. [DOI] [PubMed] [Google Scholar]
- 161.Theofilopoulos AN, Baccala R, Beutler B, Kono DH. Type I interferons (alpha/beta) in immunity and autoimmunity. Annu Rev Immunol. 2005;23:307–336. [DOI] [PubMed] [Google Scholar]
- 162.Kirou KA, Lee C, George S, et al. Coordinate overexpression of interferon-alpha-induced genes in systemic lupus erythematosus. Arthritis Rheum. 2004;50(12):3958–3967. [DOI] [PubMed] [Google Scholar]
- 163.Baechler EC, Gregersen PK, Behrens TW. The emerging role of interferon in human systemic lupus erythematosus. Curr Opin Immunol. 2004;16(6):801–807. [DOI] [PubMed] [Google Scholar]
- 164.Giles JR, Kashgarian M, Koni PA, Shlomchik MJ. B Cell-Specific MHC Class II Deletion Reveals Multiple Nonredundant Roles for B Cell Antigen Presentation in Murine Lupus. J Immunol. 2015;195(6):2571–2579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Sobel ES, Kakkanaiah VN, Kakkanaiah M, Cheek RL, Cohen PL, Eisenberg RA. T-B collaboration for autoantibody production in lpr mice is cognate and MHC-restricted. J Immunol. 1994;152(12):6011–6016. [PubMed] [Google Scholar]
- 166.Crow MK, DelGiudice-Asch G, Zehetbauer JB, et al. Autoantigen-specific T cell proliferation induced by the ribosomal P2 protein in patients with systemic lupus erythematosus. J Clin Invest. 1994;94(1):345–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Eisenberg R Mechanisms of systemic autoimmunity in murine models of SLE. Immunologic research. 1998;17(1–2):41–47. [DOI] [PubMed] [Google Scholar]
- 168.Sullivan AL, Benner C, Heinz S, et al. Serum response factor utilizes distinct promoter- and enhancer-based mechanisms to regulate cytoskeletal gene expression in macrophages. Molecular and cellular biology. 2011;31(4):861–875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Mountz JD, Yang P, Wu Q, et al. Genetic segregation of spontaneous erosive arthritis and generalized autoimmune disease in the BXD2 recombinant inbred strain of mice. Scandinavian journal of immunology. 2005;61(2):128–138. [DOI] [PubMed] [Google Scholar]
- 170.Hsu HC, Yang P, Wu Q, et al. Inhibition of the catalytic function of activation-induced cytidine deaminase promotes apoptosis of germinal center B cells in BXD2 mice. Arthritis Rheum. 2011;63(7):2038–2048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Ding Y, Li J, Wu Q, et al. IL-17RA is essential for optimal localization of follicular Th cells in the germinal center light zone to promote autoantibody-producing B cells. J Immunol. 2013;191(4):1614–1624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Ding Y, Li J, Yang P, et al. Interleukin-21 promotes germinal center reaction by skewing the follicular regulatory T cell to follicular helper T cell balance in autoimmune BXD2 mice. Arthritis Rheumatol. 2014;66(9):2601–2612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Smith EC, Teixeira AM, Chen RC, et al. Induction of megakaryocyte differentiation drives nuclear accumulation and transcriptional function of MKL1 via actin polymerization and RhoA activation. Blood. 2013;121(7):1094–1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Smith EC, Thon JN, Devine MT, et al. MKL1 and MKL2 play redundant and crucial roles in megakaryocyte maturation and platelet formation. Blood. 2012;120(11):2317–2329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Kwok SK, Tsokos GC. New insights into the role of renal resident cells in the pathogenesis of lupus nephritis. Korean J Intern Med. 2018;33(2):284–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Han TS, Schwartz MM, Lewis EJ. Association of glomerular podocytopathy and nephrotic proteinuria in mesangial lupus nephritis. Lupus. 2006;15(2):71–75. [DOI] [PubMed] [Google Scholar]
- 177.Sakhi H, Moktefi A, Bouachi K, et al. Podocyte Injury in Lupus Nephritis. J Clin Med. 2019;8(9). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Anders HJ, Lichtnekert J, Allam R. Interferon-alpha and -beta in kidney inflammation. Kidney Int. 2010;77(10):848–854. [DOI] [PubMed] [Google Scholar]
- 179.Jacobi AM, Odendahl M, Reiter K, et al. Correlation between circulating CD27high plasma cells and disease activity in patients with systemic lupus erythematosus. Arthritis Rheum. 2003;48(5):1332–1342. [DOI] [PubMed] [Google Scholar]
- 180.Cyster JG. B cell follicles and antigen encounters of the third kind. Nat Immunol. 2010;11(11):989–996. [DOI] [PubMed] [Google Scholar]
- 181.Schwickert TA, Victora GD, Fooksman DR, et al. A dynamic T cell-limited checkpoint regulates affinity-dependent B cell entry into the germinal center. J Exp Med. 2011;208(6):1243–1252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Frauwirth KA, Thompson CB. Activation and inhibition of lymphocytes by costimulation. J Clin Invest. 2002;109(3):295–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Garside P, Ingulli E, Merica RR, Johnson JG, Noelle RJ, Jenkins MK. Visualization of specific B and T lymphocyte interactions in the lymph node. Science. 1998;281(5373):96–99. [DOI] [PubMed] [Google Scholar]
- 184.Heesters BA, Myers RC, Carroll MC. Follicular dendritic cells: dynamic antigen libraries. Nat Rev Immunol. 2014;14(7):495–504. [DOI] [PubMed] [Google Scholar]
- 185.Mesin L, Ersching J, Victora GD. Germinal Center B Cell Dynamics. Immunity. 2016;45(3):471–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Riella LV, Paterson AM, Sharpe AH, Chandraker A. Role of the PD-1 pathway in the immune response. Am J Transplant. 2012;12(10):2575–2587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Qureshi OS, Zheng Y, Nakamura K, et al. Trans-endocytosis of CD80 and CD86: a molecular basis for the cell-extrinsic function of CTLA-4. Science. 2011;332(6029):600–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Folzenlogen D, Hofer MF, Leung DY, Freed JH, Newell MK. Analysis of CD80 and CD86 expression on peripheral blood B lymphocytes reveals increased expression of CD86 in lupus patients. Clin Immunol Immunopathol. 1997;83(3):199–204. [DOI] [PubMed] [Google Scholar]
- 189.Hsu HC, Wu Y, Yang P, et al. Overexpression of activation-induced cytidine deaminase in B cells is associated with production of highly pathogenic autoantibodies. J Immunol. 2007;178(8):5357–5365. [DOI] [PubMed] [Google Scholar]
- 190.Nishimura H, Honjo T. PD-1: an inhibitory immunoreceptor involved in peripheral tolerance. Trends Immunol. 2001;22(5):265–268. [DOI] [PubMed] [Google Scholar]
- 191.Neumann K, Ostmann A, Breda PC, et al. The co-inhibitory molecule PD-L1 contributes to regulatory T cell-mediated protection in murine crescentic glomerulonephritis. Sci Rep. 2019;9(1):2038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Stefanski AL, Wiedemann A, Reiter K, Hiepe F, Lino AC, Dorner T. Enhanced PD-1 and diminished PD-L1 upregulation capacity mark post-activated lupus B cells. Arthritis Rheumatol. 2019. [DOI] [PubMed] [Google Scholar]
- 193.Crotty S Follicular helper CD4 T cells (TFH). Annu Rev Immunol. 2011;29:621–663. [DOI] [PubMed] [Google Scholar]
- 194.Mountz JD, Hsu HC, Ballesteros-Tato A. Dysregulation of T Follicular Helper Cells in Lupus. J Immunol. 2019;202(6):1649–1658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Shulman Z, Gitlin AD, Weinstein JS, et al. Dynamic signaling by T follicular helper cells during germinal center B cell selection. Science. 2014;345(6200):1058–1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Weinstein JS, Herman EI, Lainez B, et al. TFH cells progressively differentiate to regulate the germinal center response. Nat Immunol. 2016;17(10):1197–1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Choi YS, Kageyama R, Eto D, et al. ICOS receptor instructs T follicular helper cell versus effector cell differentiation via induction of the transcriptional repressor Bcl6. Immunity. 2011;34(6):932–946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Choi YS, Yang JA, Crotty S. Dynamic regulation of Bcl6 in follicular helper CD4 T (Tfh) cells. Curr Opin Immunol. 2013;25(3):366–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Choi YS, Yang JA, Yusuf I, et al. Bcl6 expressing follicular helper CD4 T cells are fate committed early and have the capacity to form memory. J Immunol. 2013;190(8):4014–4026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Johnston RJ, Poholek AC, DiToro D, et al. Bcl6 and Blimp-1 are reciprocal and antagonistic regulators of T follicular helper cell differentiation. Science. 2009;325(5943):1006–1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Liu X, Nurieva RI, Dong C. Transcriptional regulation of follicular T-helper (Tfh) cells. Immunol Rev. 2013;252(1):139–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Poholek AC, Hansen K, Hernandez SG, et al. In vivo regulation of Bcl6 and T follicular helper cell development. J Immunol. 2010;185(1):313–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Tarlinton D IL-17 drives germinal center B cells? Nat Immunol. 2008;9(2):124–126. [DOI] [PubMed] [Google Scholar]
- 204.Estes JD, Thacker TC, Hampton DL, et al. Follicular dendritic cell regulation of CXCR4-mediated germinal center CD4 T cell migration. J Immunol. 2004;173(10):6169–6178. [DOI] [PubMed] [Google Scholar]
- 205.Johnson EN, Druey KM. Functional characterization of the G protein regulator RGS13. J Biol Chem. 2002;277(19):16768–16774. [DOI] [PubMed] [Google Scholar]
- 206.Cubas RA, Mudd JC, Savoye AL, et al. Inadequate T follicular cell help impairs B cell immunity during HIV infection. Nat Med. 2013;19(4):494–499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Guan H, Hou S, Ricciardi RP. DNA binding of repressor nuclear factor-kappaB p50/p50 depends on phosphorylation of Ser337 by the protein kinase A catalytic subunit. J Biol Chem. 2005;280(11):9957–9962. [DOI] [PubMed] [Google Scholar]
- 208.Muto A, Ochiai K, Kimura Y, et al. Bach2 represses plasma cell gene regulatory network in B cells to promote antibody class switch. The EMBO journal. 2010;29(23):4048–4061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Watanabe-Matsui M, Muto A, Matsui T, et al. Heme regulates B-cell differentiation, antibody class switch, and heme oxygenase-1 expression in B cells as a ligand of Bach2. Blood. 2011;117(20):5438–5448. [DOI] [PubMed] [Google Scholar]
- 210.Jackson SW, Jacobs HM, Arkatkar T, et al. B cell IFN-gamma receptor signaling promotes autoimmune germinal centers via cell-intrinsic induction of BCL-6. J Exp Med. 2016;213(5):733–750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Manni M, Ricker E, Pernis AB. Regulation of systemic autoimmunity and CD11c(+) Tbet(+) B cells by SWEF proteins. Cell Immunol. 2017;321:46–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Tipton CM, Fucile CF, Darce J, et al. Diversity, cellular origin and autoreactivity of antibody-secreting cell population expansions in acute systemic lupus erythematosus. Nat Immunol. 2015;16(7):755–765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Amu S, Tarkowski A, Dorner T, Bokarewa M, Brisslert M. The human immunomodulatory CD25+ B cell population belongs to the memory B cell pool. Scandinavian journal of immunology. 2007;66(1):77–86. [DOI] [PubMed] [Google Scholar]
- 214.Huang C, Geng H, Boss I, Wang L, Melnick A. Cooperative transcriptional repression by BCL6 and BACH2 in germinal center B-cell differentiation. Blood. 2014;123(7):1012–1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Oppmann B, Lesley R, Blom B, et al. Novel p19 protein engages IL-12p40 to form a cytokine, IL-23, with biological activities similar as well as distinct from IL-12. Immunity. 2000;13(5):715–725. [DOI] [PubMed] [Google Scholar]
- 216.Kastelein RA, Hunter CA, Cua DJ. Discovery and biology of IL-23 and IL-27: related but functionally distinct regulators of inflammation. Annu Rev Immunol. 2007;25:221–242. [DOI] [PubMed] [Google Scholar]
- 217.Costedoat-Chalumeau N, Houssiau FA. Ustekinumab: a promising new drug for SLE? Lancet. 2018;392(10155):1284–1286. [DOI] [PubMed] [Google Scholar]
- 218.van Vollenhoven RF, Hahn BH, Tsokos GC, et al. Efficacy and safety of ustekinumab, an IL-12 and IL-23 inhibitor, in patients with active systemic lupus erythematosus: results of a multicentre, double-blind, phase 2, randomised, controlled study. Lancet. 2018;392(10155):1330–1339. [DOI] [PubMed] [Google Scholar]
- 219.Bendall SC, Davis KL, Amir el AD, et al. Single-cell trajectory detection uncovers progression and regulatory coordination in human B cell development. Cell. 2014;157(3):714–725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Rauch M, Tussiwand R, Bosco N, Rolink AG. Crucial role for BAFF-BAFF-R signaling in the survival and maintenance of mature B cells. PloS one. 2009;4(5):e5456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Carrillo-Ballesteros FJ, Oregon-Romero E, Franco-Topete RA, et al. B-cell activating factor receptor expression is associated with germinal center B-cell maintenance. Exp Ther Med. 2019;17(3):2053–2060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Kalled SL. Impact of the BAFF/BR3 axis on B cell survival, germinal center maintenance and antibody production. Semin Immunol. 2006;18(5):290–296. [DOI] [PubMed] [Google Scholar]
- 223.Cancro MP, D’Cruz DP, Khamashta MA. The role of B lymphocyte stimulator (BLyS) in systemic lupus erythematosus. J Clin Invest. 2009;119(5):1066–1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Zhang J, Roschke V, Baker KP, et al. Cutting edge: a role for B lymphocyte stimulator in systemic lupus erythematosus. J Immunol. 2001;166(1):6–10. [DOI] [PubMed] [Google Scholar]
- 225.Sato K, Niino M, Kawashima A, Yamada M, Miyazaki Y, Fukazawa T. Disease Exacerbation after the Cessation of Fingolimod Treatment in Japanese Patients with Multiple Sclerosis. Intern Med. 2018;57(18):2647–2655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Czlonkowska A, Smolinski L, Litwin T. Severe disease exacerbations in patients with multiple sclerosis after discontinuing fingolimod. Neurol Neurochir Pol. 2017;51(2):156–162. [DOI] [PubMed] [Google Scholar]