Abstract
The neuroactive steroid 3α-5α-tetrahydroprogesterone (allopregnanolone), a metabolite of progesterone, is a positive allosteric modulator of GABAA receptors, and low levels have been implicated in the etiology of mood disorders. However, it is not known whether metabolism of progesterone to allopregnanolone varies across the menstrual cycle or is low after menopause. We hypothesized that the allopregnanolone/progesterone ratio would decrease from the follicular to luteal phase. We also hypothesized that postmenopausal women would have lower levels of progesterone and allopregnanolone but similar allopregnanolone/progesterone ratios as premenopausal women in the follicular phase. Serum fasting allopregnanolone and progesterone levels were measured by gas chromatography-mass spectrometry in ten premenopausal women at the follicular, mid-cycle, and luteal phases of the menstrual cycle and in twenty-four postmenopausal women. Although allopregnanolone and progesterone levels increased from the follicular to luteal phase, the allopregnanolone/progesterone ratio decreased 8-fold [0.33 ± 0.08 (follicular) vs 0.16 ± 0.09 (mid-cycle) vs 0.04 ± 0.007 (luteal), p=0.0003]. Mean allopregnanolone and progesterone levels were lower in postmenopausal than premenopausal women at all menstrual cycle phases (p<0.01). The mean allopregnanolone/progesterone ratio was similar in postmenopausal and premenopausal women in the follicular phase (0.39 ± 0.08 vs 0.33 ± 0.08, p=0.94) but was significantly lower at mid-cycle and in the luteal phase than in postmenopausal women (p<0.01). In conclusion, the serum allopregnanolone/progesterone ratio decreases 8-fold from the follicular to luteal phase and is lower at mid-cycle and the luteal phase than in postmenopausal women. Whether these data have implications for luteal phase and other mood disorders merits further study.
Keywords: neuroactive steroids, allopregnanolone, menstrual cycle, menopause
1. Introduction
Neuroactive steroids are metabolites of sex steroids that modulate neurotransmission. The best studied neuroactive steroid is the progesterone metabolite 3α-5α-tetrahydroprogesterone, also known as allopregnanolone, which is made both in the brain and peripherally by the adrenal glands, ovaries, and testes. Progesterone is converted to allopregnanolone in a two-step process by the enzymes 5α-reductase, which converts progesterone to 5α-dihydroprogesterone (5α-DHP), and 3α-hydroxysteroid-dehydrogenase (3α-HSD), which converts 5α-DHP to allopregnanolone (King, 2013). In the brain, allopregnanolone acts as a positive allosteric modulator at GABAA receptors with 10 times the potency of benzodiazepines (King, 2013; Majewska et al., 1986; Morrow et al., 1987). Moreover, there are limited clinical data linking relative allopregnanolone deficiency with mood disorders. Low serum and cerebrospinal fluid (CSF) levels of allopregnanolone have been observed in patients with mood disorders such as depression, anxiety, and post-traumatic stress disorder (Eser et al., 2006; Rasmusson et al., 2006; Uzunova et al., 1998), and several studies have demonstrated an inverse relationship between allopregnanolone levels and severity of depressive symptoms (Dichtel et al., 2018; Girdler et al., 2001; Wang et al., 1996). In addition, a recently published placebo-controlled trial demonstrating efficacy of an oral positive allosteric modulator of GABAA receptors (SAGE-217) administered for 14 days to patients with major depressive disorder supports a potential link (Gunduz-Bruce et al., 2019).
While cyclical variations of estrogen and progesterone are implicated in the etiology of luteal phase disorders such as premenstrual syndrome (PMS), premenstrual dysphoric disorder (PMDD), and catamenial epilepsy, it has been postulated that allopregnanolone may also play a role. Most studies measuring allopregnanolone in premenopausal women across the menstrual cycle and all studies of postmenopausal women have been subject to limitations of immunoassay cross-reactivity (Bixo et al., 1997; Genazzani et al., 1998; Girdler et al., 2001; Monteleone et al., 2000; Nyberg et al., 2005; Wang et al., 1996). Furthermore, whether conversion of progesterone to allopregnanolone decreases across the menstrual cycle or after menopause is unknown. It is now possible to measure neuroactive steroid levels by gas chromatography-mass spectrometry (GC/MS) after separation by high pressure liquid chromatography (HPLC), which can accurately distinguish allopregnanolone from precursor steroids, including progesterone, as well as structurally similar isomers with markedly different neuroactive effects, thus overcoming limitations of immunoassays (Cheney et al., 1995a). Two recent studies using GC/MS demonstrated a rise in allopregnanolone across the menstrual cycle (Martinez et al., 2016; Pineles et al., 2018). However, progesterone was not simultaneously measured at all menstrual cycle phases, so it is unknown whether the ratio of allopregnanolone to progesterone varies during the menstrual cycle. Given the potential protective function of allopregnanolone in depression as well as the deleterious mood effects demonstrated in some studies of exogenous progesterone administration (Andreen et al., 2003; Klatzkin et al., 2006; Natale et al., 2001), an understanding of the relationship between progesterone and allopregnanolone across the cycle may provide insight into the pathogenesis of mood symptoms associated with the luteal phase.
No published studies using GC/MS have investigated changes in allopregnanolone levels with menopausal status. Studies using immunoassays have demonstrated no difference in serum allopregnanolone levels between postmenopausal women and premenopausal women in the follicular phase (Genazzani et al., 1998), and higher postmortem brain concentrations of allopregnanolone in premenopausal women in the luteal phase compared to postmenopausal women (Bixo et al., 1997).
In the current study, we measured serum levels of allopregnanolone and progesterone by GC/MS at each phase of the menstrual cycle in 10 premenopausal women and at one time point in 24 postmenopausal women, all of whom were non-depressed. Given the high prevalence of luteal phase mood disorders and premenstrual symptoms among reproductive-age women (Direkvand-Moghadam A, 2014), we hypothesized that in the setting of a considerable increase in progesterone levels from the follicular to luteal phase, conversion to allopregnanolone would not parallel the increase in its precursor; therefore the ratio of allopregnanolone to progesterone would decrease from the follicular to the luteal phase. Additionally, we hypothesized that postmenopausal women would have-similar allopregnanolone to progesterone ratios as premenopausal women in the follicular phase in the context of low progesterone levels. A better understanding of the changes in neuroactive steroid levels and their precursors across the menstrual cycle and in menopause may identify therapeutic targets for luteal phase disorders and mood disorders.
2. Materials and Methods
2.1. Subjects
This study was approved by the Partners Healthcare Institutional Review Board and complied with Health Insurance Portability and Accountability Act guidelines. Written consent was obtained from all participants prior to study procedures. For all subjects, serum allopregnanolone and progesterone were measured at 0800 hours after an overnight fast. To assess the variability of allopregnanolone across the menstrual cycle, we studied 10 healthy premenopausal women [PRE], each at three times points in a single menstrual cycle: follicular phase (d 1-7), mid-cycle phase (d 13-16), and luteal phase (d 20-23). In order to be included in the study, women must have had a history of regular menstrual cycles since menarche. Ovulatory function and menstrual phase were confirmed by serum luteal progesterone >5 ng/mL as measured by immunoassay (Kratz and Lewandrowski, 1998). An immunoassay was used to confirm ovulation because there is no established cutoff for ovulation by GC/MS. The 10 premenopausal women were also compared at each menstrual cycle phase to 24 healthy postmenopausal women [POST]. Exclusion criteria for all subjects included patient-reported history of current or lifetime psychiatric disorder (including affective disorders, such as major depressive disorder or PMDD, and anxiety disorders), current cigarette smoking, the use of psychotropic medications, and the use of estrogens or progestins. Clinical characteristics of the premenopausal subjects have been reported as part of larger groups in parent studies (Miller et al., 2004; Miller et al., 2001; Sesmilo et al., 2001), but neither allopregnanolone levels nor progesterone levels by GC/MS have been reported for any subject in any parent study or subset analysis.
2.2. Hormone Analysis
Extraction, derivatization, and GC/MS analyses of allopregnanolone and progesterone were performed as previously described (Cheney et al., 1995b; Pibiri et al., 2008; Pinna et al., 2000) in the laboratory of investigator Graziano Pinna. Samples were stored at −80°C and run in one batch. After the samples were extracted with ethyl acetate and lyophilized, the steroids of interest were purified and separated by HPLC. Tritiated neuroactive steroids (American Radiolabeled Chemicals, St. Louis, MO, USA) were added to monitor the HPLC retention profile (Cheney et al., 1995b), while deuterated internal standards consisting of 1 pmol of deuterium-labeled neuroactive steroid (CDN Isotopes, Pointe-Claire, QC, and Steraloids, Newport, RI, USA) were used to allow quantification of the compound of interest and correct for procedural losses. Each steroid of interest was then derivatized for GC/MS (Pibiri et al., 2008; Pinna et al., 2000); mass spectrometry analysis was performed in the standard electron impact mode for both allopregnanolone and progesterone measurements. The quantity of each neuroactive steroid of interest was calculated by dividing the area under the peak of the neuroactive steroid in the sample by the area under the peak of the deuterated internal standard. The detection limit for allopregnanolone with this method was approximately 5 fmol. To convert allopregnanolone from pg/mL to nmol/L, multiply by 0.003185. To convert progesterone from pg/mL to nmol/L, multiply by 0.00318. To convert progesterone from ng/mL to nmol/L, multiply by 3.18.
2.3. Statistical Analysis
JMP Pro Statistical Database Software (version 14; SAS Institute, Cary, NC, USA) was used for statistical analyses. Two outlier values, defined as greater than 3 standard deviations from the mean, were excluded. All variables were log-transformed. Clinical characteristics and mean hormone levels between premenopausal women at each phase of the menstrual cycle and postmenopausal women were compared using analysis of variance. Trends in hormone levels across the follicular, mid-cycle, and luteal phases were determined using a repeated measures analysis. Data are reported as mean ± standard error of the mean unless otherwise noted. Statistical significance was defined as a two-tailed p-value <0.05.
3. Results
3.1. Clinical characteristics
By design, the mean age of postmenopausal women was higher than that of premenopausal women [61 ± 7 (SD) (range 52-73) vs 34 ± 7 (SD) (range 23-44) years. p<0.0001]. There was no difference in mean BMI between the groups [27.3 ± 4.9 (SD) (range 20.4-36.6) (POST) vs 25.8 ± 3.9 (SD) (range 19.9-31.4) kg/m2 (PRE), p=0.44]. Average time since onset of menopause for the postmenopausal women was 8.5 ± 5.4 (SD) years. All subjects were non-smokers.
3.2. Allopregnanolone and progesterone across the menstrual cycle
Allopregnanolone levels increased from the follicular to the luteal phase [27.3 ± 4.4 (follicular) vs 64.2 ± 21.3 (mid-cycle) vs 165.3 ± 37.1 (luteal) pg/mL, p=0.02; Figure 1a]. Progesterone levels also rose across the menstrual cycle [143 ± 44 (follicular) vs 1254 ± 515 (mid-cycle) vs 4694 ± 646 (luteal) pg/mL, p<0.0001; Figure 1b]. The allopregnanolone/progesterone ratio decreased 8-fold from the follicular to the luteal phase [0.33 ± 0.08 (follicular) vs 0.16 ± 0.09 (mid-cycle) vs 0.04 ± 0.007 (luteal), p=0.0003; Figure 1c].
Figure 1.
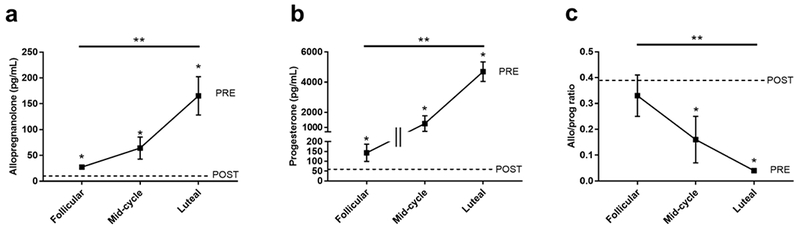
Both allopregnanolone (a) and progesterone (b) increased across the menstrual cycle. Mean levels of allopregnanolone (a) and progesterone (b) were lower in postmenopausal women compared to premenopausal women at all menstrual cycle phases. The allopregnanolone/progesterone ratio (c) decreased across the menstrual cycle and was lower than postmenopausal levels at the mid-cycle and in the luteal phase. *indicates p<0.05 for premenopausal women vs postmenopausal women. **indicates p<0.05 for hormone measurements across the entire menstrual cycle. Error bars indicate SEM. PRE = premenopausal women, POST = postmenopausal women.
3.3. Allopregnanolone and progesterone in postmenopausal women
Mean allopregnanolone levels were lower in postmenopausal women compared to premenopausal women at all menstrual cycle phases [12.7 ± 1.4 (POST) vs 27.3 ± 4.4 (follicular) pg/mL, p=0.0002; 12.7 ± 1.4 (POST) vs 64.2 ± 21.3 (mid-cycle) pg/mL, p<0.0001; 12.7 ± 1.4 (POST) vs 165.3 ± 37.1 (luteal) pg/mL, p<0.0001; Figure 1a]. Similarly, mean progesterone levels were lower in postmenopausal women compared to premenopausal women across the menstrual cycle [57 ± 12 (POST) vs 143 ± 44 (follicular) pg/mL, p=0.006; 57 ± 12 (POST) vs 1254 ± 515 (mid-cycle) pg/mL, p<0.0001; 57 ± 12 (POST) vs 4694 ± 646 (luteal) pg/mL, p<0.0001; Figure 1b]. The mean allopregnanolone/progesterone ratio was similar between postmenopausal women and premenopausal women in the follicular phase [0.39 ± 0.08 (POST) vs 0.33 ± 0.08 (follicular), p=0.94; Figure 1c] but was significantly higher in postmenopausal women compared to premenopausal women in the mid-cycle phase [0.39 ± 0.08 (POST) vs 0.16 ± 0.09 (mid-cycle), p=0.004; Figure 1c] and the luteal phase [0.39 ± 0.08 (POST) vs 0.04 ± 0.007 (luteal), p<0.0001; Figure 1c].
4. Discussion
This preliminary study is the first to characterize the allopregnanolone to progesterone ratio, as a proxy for metabolism of progesterone to allopregnanolone, across the menstrual cycle and in non-depressed post-menopausal women using serum GC/MS measurements for both allopregnanolone and progesterone. As we hypothesized and now show, the ratio of allopregnanolone to progesterone decreases across the menstrual cycle; our data show that this decrease is 8-fold from the follicular to the luteal phase despite the increase in both progesterone and allopregnanolone across the cycle. Pineles et al. recently demonstrated by GC/MS that the ratio of ALLO (which includes allopregnanolone and its stereoisomer pregnanolone) to 5α-DHP – the immediate allopregnanolone precursor – increases from the follicular to the luteal phase (Pineles et al., 2018), which may be due to increased gene expression of 3α-HSD by estradiol (Mitev et al., 2003; Pineles et al., 2018). Despite these findings suggesting increased activity of 3α-HSD during the luteal phase, we show that levels of allopregnanolone do not increase proportionally to progesterone levels. We hypothesize that 5α-reductase may be saturated in the luteal phase from high progesterone levels, as it has been shown that 5α-reduction is the rate-limiting step in conversion of progesterone to allopregnanolone (Cai et al., 2018; Do Rego et al., 2009). Alternatively, it is possible that more progesterone is metabolized to pregnanolone through increased activity of 5α-reductase or 3α-HSD. Preclinical studies suggest that modulation of GABAA transmission by allopregnanolone plays a role in the pathogenesis of mood disorders. There are as yet few clinical data supporting a link between allopregnanolone and mood disorders, although, of note, a recently published placebo-controlled trial of an oral positive allosteric modulator of GABAA receptors (SAGE-217) demonstrated efficacy in major depressive disorder when administered for 14 days (Gunduz-Bruce et al., 2019). Our finding that the allopregnanolone/progesterone ratio decreases in the luteal phase suggests that relative allopregnanolone deficiency may be implicated in premenstrual syndrome or luteal phase mood disorders, although the cause of PMDD is likely multifactorial with hormonal, genetic, and psychiatric contributors. More studies investigating the role of allopregnanolone in the pathogenesis of mood disorders are needed.
Studies in women with PMDD have found that serum allopregnanolone levels and/or the allopregnanolone/progesterone ratio at the luteal phase are low (Monteleone et al., 2000; Nyberg et al., 2005; Rapkin et al., 1997), high (Girdler et al., 2001), or no different compared to healthy women (Martinez et al., 2016; Schmidt et al., 1994; Timby et al., 2016; Wang et al., 2004; Wang et al., 1996), and one suggested that changes in allopregnanolone levels rather than low absolute levels contribute to premenstrual symptoms (Martinez et al., 2016). However, none measured both allopregnanolone and progesterone levels by GC/MS across the menstrual cycle. As progesterone levels are variable in premenopausal women, and the capacity to convert progesterone to allopregnanolone via 5α-reduction may also vary, we hypothesize that the decrease in the allopregnanolone to progesterone ratio from the follicular to the luteal phase may be more pronounced in women with PMDD. Our findings thus add to the limited studies measuring allopregnanolone and progesterone by GC/MS and lay the foundation for future work investigating the relationship between neuroactive steroid levels and mood symptoms in women with luteal phase disorders such as PMDD.
Our finding that allopregnanolone, when measured by GC/MS, increases across the menstrual cycle is consistent with prior studies using GC/MS (Havlikova et al., 2006; Martinez et al., 2016; Pineles et al., 2018) and immunoassays (Girdler et al., 2001; Monteleone et al., 2000; Nyberg et al., 2005; Wang et al., 1996) and is in line with the known increase in levels of the allopregnanolone precursor, progesterone, produced after ovulation by the corpus luteum during the luteal phase of the menstrual cycle. Ottander et al. showed in an in vitro study that the corpus luteum also makes allopregnanolone directly, which may account for some of the rise in allopregnanolone in the luteal phase (Ottander et al., 2005). Of note, the progesterone levels we measured are lower than those measured by immunoassay and can be explained by the greater specificity of GC/MS assays.
Additionally, we found that allopregnanolone and progesterone levels are lower in postmenopausal women than premenopausal women at all phases of the menstrual cycle. A study by Ke et al. similarly demonstrated lower serum allopregnanolone levels by LC/MS-MS in postmenopausal women than premenopausal women but did not control for timing of the menstrual cycle (Ke et al., 2017). In contrast, Genazzani et al. found similar serum allopregnanolone levels by immunoassay in postmenopausal women and premenopausal women during the follicular phase despite lower progesterone levels in the postmenopausal women (Genazzani et al., 1998); however, they did not report or control for BMI, which may be relevant given prior work from our group showing lower levels of allopregnanolone in overweight/obese women than lean women (Dichtel et al., 2018). No prior study has compared serum allopregnanolone and progesterone levels by GC/MS in postmenopausal women and premenopausal women across the menstrual cycle.
As hypothesized, we found that the allopregnanolone/progesterone ratio is similar between premenopausal women in the follicular phase and postmenopausal women selected for lack of depression. We also found that as the allopregnanolone/progesterone ratio declined at mid-cycle and in the luteal phase, it became lower than that of postmenopausal women. Of note, estimation of the mid-cycle phase was not precise because we did not document the timing of ovulation, which can vary among individuals. However, we did confirm luteal phase (d 20-23) using the established cut-off (serum progesterone >5 ng/mL by immunoassay) (Leiva et al., 2015).
The main limitations of this study include its small sample size, estimation of the mid-cycle time point in the premenopausal sample, and measurement of serum rather than central levels of allopregnanolone, although many studies have shown an excellent correlation between serum and central levels of allopregnanolone (Bixo et al., 1997; Kancheva et al., 2011; Kancheva et al., 2010). Additionally, we relied on self-report of psychiatric disturbances and did not assess alcohol intake or potential use of illicit drugs. Acute intoxication has been shown to raise allopregnanolone levels (Torres and Ortega, 2003, 2004); as no subjects appeared acutely intoxicated at the time of laboratory assessment, it is unlikely that alcohol or illicit drug use systemically impacted the results of the study. Standardized sample collection and the assay procedures were strengths of this study. Nevertheless, it is not possible to compare the raw neuroactive steroid levels from this study to those reported in the literature or to use these data toward establishment of normal ranges across the menstrual cycle or for menopause. Absolute steroid levels may vary between GC/MS assay batches due to the effects of storage time on the internal standards used or due to small variations in the extraction or derivatization processes. Use of serum vs plasma or variations in blood collection and processing procedures also may affect steroid levels. Studies are thus needed to determine the best uniform blood collection, processing, and GC/MS analytic procedures for measurement of these neuroactive steroids before clinically useful normal ranges can be established.
In conclusion, our study showed an 8-fold decrease in the ratio of allopregnanolone to progesterone from the follicular to luteal phase of the menstrual cycle despite an increase in allopregnanolone and progesterone across the menstrual cycle. Moreover, compared to postmenopausal women, the allopregnanolone/progesterone ratio was similar in premenopausal women in the follicular phase, but significantly lower at mid-cycle and in the luteal phase. Additional larger studies using standardized specific and sensitive mass spectrometric methodologies and careful control for effects of ambient stress are needed to establish normative levels of these neuroactive steroids across the menstrual cycle and after menopause in healthy women, as well as in women with disorders in which synthesis of allopregnanolone is dysregulated. Further studies are also needed to advance our knowledge of the complex relationship between cortisol and allopregnanolone activity in the brain, as the normal negative feedback of allopregnanolone on the hypothalamic-pituitary-adrenal axis may be disrupted in states of stress and depression (Mody and Maguire, 2011; Rasmusson et al., 2001). A better understanding of the role of centrally acting steroids including allopregnanolone in the pathophysiology of luteal phase mood disorders such as PMDD could give rise to potential new targeted therapies for these conditions.
Highlights.
The allopregnanolone/progesterone ratio decreases 8-fold across the menstrual cycle
Allopregnanolone and progesterone levels are very low in menopause
The ratio is lower in the luteal phase than even in postmenopausal women
These data may have implications for luteal phase mood disorders
Acknowledgments
Funding: This work was supported by the National Institutes of Health (Grant Numbers T32 DK007028, K24 HL092902, K23 DK113220, K23 AT0080434, R34 MH099315, and R34 MH099310) and the Harvard Catalyst/The Harvard Clinical and Translational Science Center (Grant Number 1UL1TR001102 and Grant Number M01-RR-01066, from the National Center for Research Resources).
Declaration of interest: Dr. Mischoulon has received research support from Nordic Naturals. He has provided unpaid consulting for Pharmavite LLC and Gnosis USA, Inc. He has received honoraria for speaking from the Massachusetts General Hospital Psychiatry Academy, Blackmores, and PeerPoint Medical Education Institute, LLC. He has received royalties from Lippincott Williams & Wilkins for published book “Natural Medications for Psychiatric Disorders: Considering the Alternatives.” Within the past three years, Dr. Rasmusson has served as a paid scientific consultant to Resilience Therapeutics and Cohen Veterans Bioscience. A list of Dr. Fava's lifetime disclosures can be found online: http://mghcme.org/faculty/faculty-detail/maurizio_fava. Dr. Miller is the recipient of an investigator-initiated research grant from Amgen. She also receives study drug at no cost and support for assays from Marinus Pharmaceuticals. The other authors declare no conflicts of interest.
References
- 1.Andreen L, Bixo M, Nyberg S, Sundstrom-Poromaa I, Backstrom T, 2003. Progesterone effects during sequential hormone replacement therapy. Eur J Endocrinol 148, 571–577. [DOI] [PubMed] [Google Scholar]
- 2.Bixo M, Andersson A, Winblad B, Purdy RH, Backstrom T, 1997. Progesterone, 5alpha-pregnane-3,20-dione and 3alpha-hydroxy-5alpha-pregnane-20-one in specific regions of the human female brain in different endocrine states. Brain Res 764, 173–178. [DOI] [PubMed] [Google Scholar]
- 3.Cai H, Zhou X, Dougherty GG, Reddy RD, Haas GL, Montrose DM, Keshavan M, Yao JK, 2018. Pregnenolone-progesterone-allopregnanolone pathway as a potential therapeutic target in first-episode antipsychotic-naive patients with schizophrenia. Psychoneuroendocrinology 90, 43–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cheney DL, Uzunov D, Costa E, Guidotti A, 1995a. Gas chromatographic-mass fragmentographic quantitation of 3 alpha-hydroxy-5 alpha-pregnan-20-one (allopregnanolone) and its precursors in blood and brain of adrenalectomized and castrated rats. J Neurosci 15, 4641–4650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cheney DL, Uzunov D, Guidotti A, 1995b. Pregnenolone sulfate antagonizes dizocilpine amnesia: role for allopregnanolone. Neuroreport. 6, 1697–1700. [DOI] [PubMed] [Google Scholar]
- 6.Dichtel LE, Lawson EA, Schorr M, Meenaghan E, Paskal ML, Eddy KT, Pinna G, Nelson M, Rasmusson AM, Klibanski A, Miller KK, 2018. Neuroactive steroids and affective symptoms in women across the weight spectrum. Neuropsychopharmacology 43, 1436–1444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Direkvand-Moghadam A, S. K, Delpisheh A, Kaikhavandi S, 2014. Epidemiology of premenstrual syndrome (PMS)-a systematic review and meta-analysis study. J Clin Diagn Res 8, 106–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Do Rego JL, Seong JY, Burel D, Leprince J, Luu-The V, Tsutsui K, Tonon MC, Pelletier G, Vaudry H, 2009. Neurosteroid biosynthesis: enzymatic pathways and neuroendocrine regulation by neurotransmitters and neuropeptides. Front Neuroendocrinol 30, 259–301. [DOI] [PubMed] [Google Scholar]
- 9.Eser D, Romeo E, Baghai TC, di Michele F, Schule C, Pasini A, Zwanzger P, Padberg F, Rupprecht R, 2006. Neuroactive steroids as modulators of depression and anxiety. Neuroscience 138, 1041–1048. [DOI] [PubMed] [Google Scholar]
- 10.Genazzani AR, Petraglia F, Bernardi F, Casarosa E, Salvestroni C, Tonetti A, Nappi RE, Luisi S, Palumbo M, Purdy RH, Luisi M, 1998. Circulating levels of allopregnanolone in humans: gender, age, and endocrine influences. J Clin Endocrinol Metab 83, 2099–2103. [DOI] [PubMed] [Google Scholar]
- 11.Girdler SS, Straneva PA, Light KC, Pedersen CA, Morrow AL, 2001. Allopregnanolone levels and reactivity to mental stress in premenstrual dysphoric disorder. Biol Psychiatry 49, 788–797. [DOI] [PubMed] [Google Scholar]
- 12.Gunduz-Bruce H, Silber C, Kaul I, Rothschild AJ, Riesenberg R, Sankoh AJ, Li H, Lasser R, Zorumski CF, Rubinow DR, Paul SM, Jonas J, Doherty JJ, Kanes SJ, 2019. Trial of SAGE-217 in patients with major depressive disorder. N Engl J Med 381, 903–911. [DOI] [PubMed] [Google Scholar]
- 13.Havlikova H, Hill M, Kancheva L, Vrbikova J, Pouzar V, Cerny I, Kancheva R, Starka L, 2006. Serum profiles of free and conjugated neuroactive pregnanolone isomers in nonpregnant women of fertile age. J Clin Endocrinol Metab 91, 3092–3099. [DOI] [PubMed] [Google Scholar]
- 14.Kancheva R, Hill M, Novak Z, Chrastina J, Kancheva L, Starka L, 2011. Neuroactive steroids in periphery and cerebrospinal fluid. Neuroscience 191, 22–27. [DOI] [PubMed] [Google Scholar]
- 15.Kancheva R, Hill M, Novak Z, Chrastina J, Velikova M, Kancheva L, Riha I, Starka L, 2010. Peripheral neuroactive steroids may be as good as the steroids in the cerebrospinal fluid for the diagnostics of CNS disturbances. J Steroid Biochem Mol Biol 119, 35–44. [DOI] [PubMed] [Google Scholar]
- 16.Ke Y, Gonthier R, Labrie F, 2017. A sensitive and accurate LC-MS/MS assay with the derivatization of 1-Amino-4-methylpiperazine applied to serum allopregnanolone, pregnenolone and androsterone in pre- and postmenopausal women. Steroids 118, 25–31. [DOI] [PubMed] [Google Scholar]
- 17.King SR, 2013. Neurosteroids and the Nervous System. Springer-Verlag New York, New York. [Google Scholar]
- 18.Klatzkin RR, Morrow AL, Light KC, Pedersen CA, Girdler SS, 2006. Associations of histories of depression and PMDD diagnosis with allopregnanolone concentrations following the oral administration of micronized progesterone. Psychoneuroendocrinology 31, 1208–1219. [DOI] [PubMed] [Google Scholar]
- 19.Kratz A, Lewandrowski KB, 1998. Case records of the Massachusetts General Hospital. Weekly clinicopathological exercises. Normal reference laboratory values. N Engl J Med 339, 1063–1072. [DOI] [PubMed] [Google Scholar]
- 20.Leiva R, Bouchard T, Boehringer H, Abulla S, Ecochard R, 2015. Random serum progesterone threshold to confirm ovulation. Steroids 101, 125–129. [DOI] [PubMed] [Google Scholar]
- 21.Majewska MD, Harrison NL, Schwartz RD, Barker JL, Paul SM, 1986. Steroid hormone metabolites are barbiturate-like modulators of the GABA receptor. Science 232, 1004–1007. [DOI] [PubMed] [Google Scholar]
- 22.Martinez PE, Rubinow DR, Nieman LK, Koziol DE, Morrow AL, Schiller CE, Cintron D, Thompson KD, Khine KK, Schmidt PJ, 2016. 5alpha-reductase inhibition prevents the luteal phase increase in plasma allopregnanolone levels and mitigates symptoms in women with premenstrual dysphoric disorder. Neuropsychopharmacology 41, 1093–1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Miller KK, Rosner W, Lee H, Hier J, Sesmilo G, Schoenfeld D, Neubauer G, Klibanski A, 2004. Measurement of free testosterone in normal women and women with androgen deficiency: comparison of methods. J Clin Endocrinol Metab 89, 525–533. [DOI] [PubMed] [Google Scholar]
- 24.Miller KK, Sesmilo G, Schiller A, Schoenfeld D, Burton S, Klibanski A, 2001. Androgen deficiency in women with hypopituitarism. J Clin Endocrinol Metab 86, 561–567. [DOI] [PubMed] [Google Scholar]
- 25.Mitev YA, Darwish M, Wolf SS, Holsboer F, Almeida OF, Patchev VK, 2003. Gender differences in the regulation of 3 alpha-hydroxysteroid dehydrogenase in rat brain and sensitivity to neurosteroid-mediated stress protection. Neuroscience 120, 541–549. [DOI] [PubMed] [Google Scholar]
- 26.Mody I, Maguire J, 2011. The reciprocal regulation of stress hormones and GABA(A) receptors. Front Cell Neurosci 6, 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Monteleone P, Luisi S, Tonetti A, Bernardi F, Genazzani AD, Luisi M, Petraglia F, Genazzani AR, 2000. Allopregnanolone concentrations and premenstrual syndrome. Eur J Endocrinol 142, 269–273. [DOI] [PubMed] [Google Scholar]
- 28.Morrow AL, Suzdak PD, Paul SM, 1987. Steroid hormone metabolites potentiate GABA receptor-mediated chloride ion flux with nanomolar potency. Eur J Pharmacol 142, 483–485. [DOI] [PubMed] [Google Scholar]
- 29.Natale V, Albertazzi P, Zini M, Di Micco R, 2001. Exploration of cyclical changes in memory and mood in postmenopausal women taking sequential combined oestrogen and progestogen preparations. Bjog 108, 286–290. [DOI] [PubMed] [Google Scholar]
- 30.Nyberg S, Andersson A, Zingmark E, Wahlstrom G, Backstrom T, Sundstrom-Poromaa I, 2005. The effect of a low dose of alcohol on allopregnanolone serum concentrations across the menstrual cycle in women with severe premenstrual syndrome and controls. Psychoneuroendocrinology 30, 892–901. [DOI] [PubMed] [Google Scholar]
- 31.Ottander U, Poromaa IS, Bjurulf E, Skytt A, Backstrom T, Olofsson JI, 2005. Allopregnanolone and pregnanolone are produced by the human corpus luteum. Mol Cell Endocrinol 239, 37–44. [DOI] [PubMed] [Google Scholar]
- 32.Pibiri F, Nelson M, Guidotti A, Costa E, Pinna G, 2008. Decreased corticolimbic allopregnanolone expression during social isolation enhances contextual fear: A model relevant for posttraumatic stress disorder. Proc Natl Acad Sci U S A. 105, 5567–5572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pineles SL, Nillni YI, Pinna G, Irvine J, Webb A, Arditte Hall KA, Hauger R, Miller MW, Resick PA, Orr SP, Rasmusson AM, 2018. PTSD in women is associated with a block in conversion of progesterone to the GABAergic neurosteroids allopregnanolone and pregnanolone measured in plasma. Psychoneuroendocrinology 93, 133–141. [DOI] [PubMed] [Google Scholar]
- 34.Pinna G, Uzunova V, Matsumoto K, Puia G, Mienville JM, Costa E, Guidotti A, 2000. Brain allopregnanolone regulates the potency of the GABA(A) receptor agonist muscimol. Neuropharmacology. 39, 440–448. [DOI] [PubMed] [Google Scholar]
- 35.Rapkin AJ, Morgan M, Goldman L, Brann DW, Simone D, Mahesh VB, 1997. Progesterone metabolite allopregnanolone in women with premenstrual syndrome. Obstet Gynecol 90, 709–714. [DOI] [PubMed] [Google Scholar]
- 36.Rasmusson AM, Lipschitz DS, Wang S, Hu S, Vojvoda D, Bremner JD, Southwick SM, Charney DS, 2001. Increased pituitary and adrenal reactivity in premenopausal women with posttraumatic stress disorder. Biol Psychiatry 50, 965–977. [DOI] [PubMed] [Google Scholar]
- 37.Rasmusson AM, Pinna G, Paliwal P, Weisman D, Gottschalk C, Charney D, Krystal J, Guidotti A, 2006. Decreased cerebrospinal fluid allopregnanolone levels in women with posttraumatic stress disorder. Biol Psychiatry 60, 704–713. [DOI] [PubMed] [Google Scholar]
- 38.Schmidt PJ, Purdy RH, Moore PH Jr., Paul SM, Rubinow DR, 1994. Circulating levels of anxiolytic steroids in the luteal phase in women with premenstrual syndrome and in control subjects. J Clin Endocrinol Metab 79, 1256–1260. [DOI] [PubMed] [Google Scholar]
- 39.Sesmilo G, Miller KK, Hayden D, Klibanski A, 2001. Inflammatory cardiovascular risk markers in women with hypopituitarism. J Clin Endocrinol Metab 86, 5774–5781. [DOI] [PubMed] [Google Scholar]
- 40.Timby E, Backstrom T, Nyberg S, Stenlund H, Wihlback AC, Bixo M, 2016. Women with premenstrual dysphoric disorder have altered sensitivity to allopregnanolone over the menstrual cycle compared to controls-a pilot study. Psychopharmacology (Berl) 233, 2109–2117. [DOI] [PubMed] [Google Scholar]
- 41.Torres JM, Ortega E, 2003. Alcohol intoxication increases allopregnanolone levels in female adolescent humans. Neuropsychopharmacology 28, 1207–1209. [DOI] [PubMed] [Google Scholar]
- 42.Torres JM, Ortega E, 2004. Alcohol intoxication increases allopregnanolone levels in male adolescent humans. Psychopharmacology (Berl) 172, 352–355. [DOI] [PubMed] [Google Scholar]
- 43.Uzunova V, Sheline Y, Davis JM, Rasmusson A, Uzunov DP, Costa E, Guidotti A, 1998. Increase in the cerebrospinal fluid content of neurosteroids in patients with unipolar major depression who are receiving fluoxetine or fluvoxamine. Proc Natl Acad Sci U S A 95, 3239– 3244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang C, Cunningham G, Dobs A, Iranmanesh A, Matsumoto AM, Snyder PJ, Weber T, Berman N, Hull L, Swerdloff RS, 2004. Long-term testosterone gel (AndroGel) treatment maintains beneficial effects on sexual function and mood, lean and fat mass, and bone mineral density in hypogonadal men. J Clin Endocrinol Metab 89, 2085–2098. [DOI] [PubMed] [Google Scholar]
- 45.Wang M, Seippel L, Purdy RH, Backstrom T, 1996. Relationship between symptom severity and steroid variation in women with premenstrual syndrome: study on serum pregnenolone, pregnenolone sulfate, 5 alpha-pregnane-3,20-dione and 3 alpha-hydroxy-5 alpha-pregnan-20-one. J Clin Endocrinol Metab 81, 1076–1082. [DOI] [PubMed] [Google Scholar]


