Abstract
Despite the currently available pharmacotherapies, today, thirty percent of worldwide deaths are due to cardiovascular diseases (CVDs), whose primary cause is atherosclerosis, an inflammatory disorder characterized by the buildup of lipid deposits on the inside of arteries. Multiple cellular signaling pathways have been shown to be involved in the processes underlying atherosclerosis, and evidence has been accumulating for the crucial role of Notch receptors in regulating the functions of the diverse cell types involved in atherosclerosis onset and progression. Several classes of nutraceuticals have potential benefits for the prevention and treatment of atherosclerosis and CVDs, some of which could in part be due to their ability to modulate the Notch pathway. In this review, we summarize the current state of knowledge on the role of Notch in vascular health and its modulation by nutraceuticals for the prevention of atherosclerosis and/or treatment of related CVDs.
1. Introduction
“Prevention is better than cure” is the proverb that better describes the impact of eating habits in prophylaxis of many pathologies, including cardiovascular disease (CVD), one of the leading causes of death in industrialized countries [1]. The causes of CVDs are multifactorial and some of these, such as modifiable lifestyle (i.e., tobacco smoking and physical activity) and especially dietary habits, play a major role in this context. In fact, it has been widely demonstrated that a healthy diet, with balanced intake of vegetables, fruits, olive oil, and whole grains, reduces the risk of developing CVDs due to atherosclerosis. Therefore, in the last few years, novel nutritional strategies have been implemented, including dietary modifications and consumption of innovative functional foods and dietary supplements which fall in the category of nutraceutical products. Coined in the end of the ‘90s by Dr. Stephen De Felice, the neologism “nutraceutic” is a combination of the terms “nutrition” and “pharmaceutic” and indicates a discipline that studies the substances that are “a food or part of a food” which can provide “medical or health benefits, including the prevention and treatment of disease” [2]. More recently, nutraceuticals have been defined as “a substance that is cultivated/produced/extracted or synthesized under optimal and reproducible conditions and, when administered orally to normal subjects or patients, would provide the nutrient(s) required for bringing altered body structure and function back to normal, thus improving the health and well-being of the patients” [3]. Consistently, preclinical and clinical studies conducted in the last 20 years have shown an effect of nutraceuticals on prevention and progression of CVDs, specifically on atherosclerosis. Nevertheless, there are studies that did not confirm the protective effect of nutraceuticals, and to shed further light on the action of these compounds, different doses and modality of administration, taking also under consideration the presence/absence of other medications, are being investigated, together with studies of pharmacodynamics and pharmacokinetics. These contrasting results could be also due to the limited number of pathways so far investigated. An example in this context is provided by probiotics, whose supplementation efficacy has given contrasting results. A recently published study shows that probiotic supplements promote endothelial functions in humans with coronary artery disease (CAD) without altering traditional cardiovascular risk factors or the microbiota population but only their transcriptional activity, as indicated by their plasma metabolites profile [4]. Thus, by only looking at the different bacteria population of the microbiota, these effects of probiotics would have been missed. Additionally, by increasing the number of biomarkers of vascular function modified by nutraceuticals, the identification of “super responders” could be achieved, paving the way for “personalized nutraceutical treatments.”
The Notch pathway, originally studied for its role in promoting the survival of cancer cells, is emerging as a key player in the maintenance of the vascular wall health and in the regulation of inflammation [5, 6]. This pathway responds to agents, such as inflammatory cytokines and lipopolysaccharides, whose effects are contrasted by nutraceuticals. The aim of this review is to describe existing studies on the regulation of Notch by nutraceuticals, in order to determine whether the analysis of components of this pathway could represent novel clinical surrogates providing useful information when trying to assess the effect of traditional or emerging nutraceuticals.
2. The Steps of Atherosclerosis
Atherosclerosis is a complex multifactorial and multistep chronic inflammatory condition characterized by the progressive accumulation of lipid-loaded fibrous plaques within the artery wall. This pathology, arising from a cumulative damage of the vessel wall, can culminate in atherosclerotic plaque rupture and subsequent thrombus formation [7], leading to its most common clinical manifestations, namely, myocardial infarction (MI) or stroke [8]. The first stage of atherosclerosis development takes place in the endothelium, the intima layer of artery walls. The vascular endothelium is a selective semipermeable continuous monolayer of endothelial cells (ECs) that plays a major role in controlling the vascular tone and vascular wall thickness by synthesizing a plethora of autocrine and paracrine substances, such as nitric oxide (NO), prostacyclin, histamine, prostacyclin, angiotensin II (Ang II), heparin, tissue plasminogen activator (t-PA), and plasminogen activator inhibitor-1 (PAI-I), which affect vascular smooth muscle cell (VSMC) proliferation, leukocyte migration, platelet aggregation, and adhesion [9, 10]. Numerous systemic and hemodynamic risk factors contribute to the onset of endothelial dysfunction (defined as reduced levels of NO, increased endothelium permeability, caused by EC apoptosis and reduction of junctions between ECs, and increased expression of adhesion molecules) which is the first step toward plaque formation [11]. Endothelium discontinuity favors the entry of cholesterol-containing low-density lipoprotein (LDL) particles in the intima of large arteries [10], an event mediated by interaction between LDL apolipoprotein B100 (ApoB100) and matrix proteoglycans. LDL retention predisposes them to oxidative modification [12], and the resulting oxidized LDLs (oxLDL), by binding to a specific receptor, lectin-like oxLDL receptor-1 (LOX1), induce the expression of vascular cell adhesion molecule 1 (VCAM-1) and intercellular adhesion molecule 1 (ICAM-1) by ECs [13, 14]. These adhesion molecules, along with chemotactic molecules, such as monocyte chemoattractant protein-1 (MCP-1) secreted by ECs and VSMCs, mediate the transmigration of monocytes in the subendothelium, where differentiate into macrophages. These later adopt a M1 proinflammatory phenotype and encode a wide range of scavenger receptors (SRs) (i.e., SR-A1 and CD36), useful for facilitating the endocytosis oxLDLs [15]. This oxLDL overload results in acquisition of the “foam cell” phenotype by macrophage and fatty streak formation.
Furthermore, mast cells, T cells, and other inflammation mediators penetrate lesions and, together with foam cells, contribute to the maladaptive proatherosclerotic inflammatory response. Foam cells secrete chemotactic growth factors and metallopeptidases which, through degradation of the extracellular matrix (ECM), support proliferation and migration of VSMCs and leukocytes into the intima [16]. In the intima, the VSMCs produce interstitial collagen and elastin and form a fibrous cap that covers the plaque. This cap overlies the macrophage-derived foam cells, some of which die by apoptosis and release lipids that accumulate extracellularly. This process leads to the formation of a lipid-rich pool called the “necrotic core” of the plaque [7]. The stability of the plaques is influenced by the balance between cap and necrotic core. Stable plaques are characterized by the presence of a small core rich in lipids covered by a thick fibrous cap rich in collagen. Instead, unstable plaques have a thin fibrous cap over a large fatty core [17] and are prone to rupture which leads to the exposure of tissue factors to blood flow and the activation of coagulation cascade with consequent thrombus formation [7, 18]. An intraluminal thrombus in the coronary arteries may decrease blood flow causing ischemic cardiomyopathy: a complete vascular occlusion will cause MI.
3. Nutraceutical Supplementation for Atherosclerosis Prevention
Nutraceuticals are classified into (i) dietary supplements: products intended to supplement the diet, which contain one or more dietary ingredients with well-known nutritional functions (i.e., vitamins, minerals, herbs, or herbal active compounds) and (ii) functional foods: consumed as part of a normal diet, they consist in whole foods, along with “fortified, enriched, or enhanced” foods supplemented with known biologically active compounds that, in addition to macro- and micronutrients, provide physiological benefits and are intended for reducing the risk of developing chronic diseases [19]. Some examples of functional foods are garlic, apples, or soybean as remedies for treatment or prevention of a number of diseases, but also vitamin D-fortified milk, useful for counteracting osteoporosis [20]. Lastly, functional food category includes yogurts for their content in probiotics [21], live microorganisms with positive impact on the host through a beneficial action on the intestinal tract, and prebiotics, organic substances, found in several vegetables and fruits, which selectively enhance the activity of some groups of bacteria [22].
This traditional definition of nutraceutical classes intended that both nutraceutical ingredients and functional foods derived preferentially from food products. However, the hodiern nutraceutical includes also non-food-derived active metabolites, which are safe and useful as novel sources for modern nutraceuticals and functional foods, such as medicinal plant-derived compounds [23], marine bioactive compounds [22], and amino acids derived from bacteria fermentation [24].
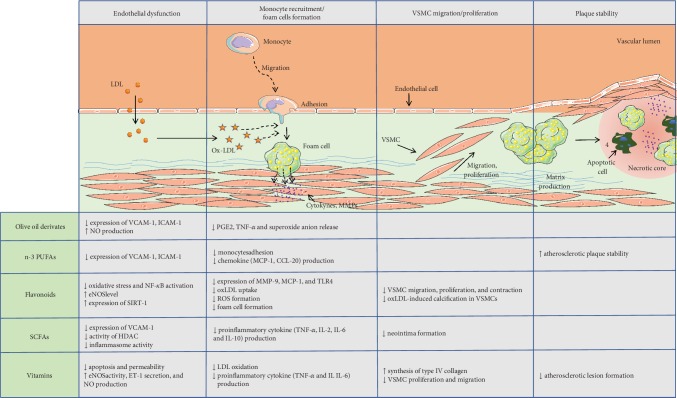
Several classes of nutraceuticals have been shown to have potential benefits in the treatment of atherosclerosis (reviewed in [25]) (Figure 1) and CVDs, and the ones for which strong evidence exists for atherosclerosis protection are described below and summarized in Table 1.
Figure 1.
Beneficial effects of major nutraceuticals on atherogenesis key steps. Highlights of the main findings of in vitro and in vivo studies which have investigated the mechanisms underlying the benefits of the major nutraceuticals (olive oil derivates, n-3 PUFAs, flavonoids, SCFAs, and vitamins) at different stages of atherosclerosis development, including endothelial dysfunction, monocyte recruitment, foam cell formation, VSMC migration and proliferation, and plaque stability.
Table 1.
Summary of cardiovascular benefits of major nutraceuticals in human studies.
| Nutraceutical | Study name | Study type | Number of participants/studies analyzed | Duration | Intervention | Summary of findings | References |
|---|---|---|---|---|---|---|---|
| Olive oil | NUTRAOLEUM | Clinical trial | 58 | 5 months | 30 mL/d of three virgin olive oils (VOOs): (1) a VOO (124 ppm of phenolic compounds and 86 ppm of triterpenes), (2) an optimized VOO (OVOO) (490 ppm of phenolic compounds and 86 ppm of triterpenes), and (3) a functional olive oil (FOO) high in phenolic compounds (487 ppm) and enriched with triterpenes (389 ppm) | Improved plasma HDL levels Decreased the level of systemic ET-1 |
[35] |
| VOHF | Clinical trial | 33 | 3 weeks | VOO (80 mg·kg−1), FVOO (500 mg·kg−1), and FVOO enriched with phenolic compounds from thyme FVOOT (500 mg·kg−1; 1 : 1) | Enhanced HDL content Increased endogenous antioxidant enzymes Reduced DNA oxidation level Increased fecal microbial metabolic activity Ameliorated endothelial function |
[36, 37] | |
|
| |||||||
| n-3 PUFAs | DART | Clinical trial | 2.033 | 6 months | Advised to eat about 300 g/week of oily fish or fish oil supplements giving an equivalent amount of n-3 PUFAs | 29% reduction in all-cause mortality | [311] |
| GISSI-Prevenzione | Clinical trial | 11.324 | 3.5 years | Supplements of n-3 PUFA (1 g/d), vitamin E (300 mg/d), both, or none | 20% reduction for total deaths 30% reduction for cardiovascular death 45% reduction for sudden deaths |
[52] | |
| JELIS | Clinical trial | 18.645 | 5 years | EPA (1800 mg/d) with statin or statin | 19% reduction in major coronary events | [51] | |
| Meta-analysis | 7.951 | Reduced overall mortality and sudden death Reduced mortality due to MI |
[2] | ||||
| Meta-analysis | 77.917 | No significant associations with CHD events and death No significant associations with nonfatal MI |
[56] | ||||
| Omega-FMD | Clinical trial | 74 | 3 months | Supplements of n-3 PUFA (2 g/d) or placebo | No improvement of endothelial function indices | [57] | |
| ASCEND | Clinical trial | 15,480 | 7.4 years | Supplements of n-3 PUFA (1 g/d) or placebo | No reduction in the rates of nonfatal serious adverse events | [58] | |
| REDUCE-IT | Clinical trial | 19.212 | 4.9 years | Supplements of icosapent ethyl (4 g/d) or placebo | 25% reduction in primary composite cardiovascular endpoint 26% reduction in secondary composite cardiovascular endpoint |
[60] | |
|
| |||||||
| Flavonoids | Zutphen Elderly Study | Prospective cohort study | 805 | 5 years | Reduced risk of CHD mortality Reduced incidence of MI |
[99] | |
| Rotterdam Study | Prospective cohort study | 4807 | 5.6 years | reduced incidence of MI | [100] | ||
| The Caerphilly study | Prospective cohort study | 1900 | 14 years | No change in incidence of ischemic heart disease | [101] | ||
| The Health Professionals Study | Prospective cohort study | 45589 | 2 years | No association between tea consumption and CVD | [102] | ||
| FLAVO | Clinical trial | 37 | 4 weeks | (-)-epicatechin (100 mg/d), quercetin-3-glucoside(160 mg/d), or placebo | Only (-)-epicatechin improved endothelial function and reduced inflammation | [103] | |
| SCFAs | Umbrella meta-analysis | 31 (meta-analysis) | 7-24% reduction in CHD and stroke 17-28% reduction in overall morbidity and mortality |
[108] | |||
| Meta-analysis | 752,848 | 12.4 years | 23% reduction in CVD mortality | [109] | |||
|
| |||||||
| Vitamins | ASAP | Clinical trial | 520 | 3 years | d-Alpha-tocopherol (182 mg/d), slow-release vitamin C (500 mg/d), both, or placebo | Delayed progression of atherosclerosis | [153, 154] |
| Women's Health Study | Clinical trial | 39.876 | 10.1 years | Natural-source vitamin E (600 IU) on alternate days | Reduced cardiovascular mortality in healthy women | [155] | |
| MRC/BHF | Clinical trial | 20.536 | 5 years | Vitamin supplementation (vitamin E, 600 mg/d; vitamin C, 250 mg/d; β-carotene, 20 mg/d) | No significant reduction in the incidence of cardiovascular events and CVD-related mortality | [156] | |
| GISSI-Prevenzione | Clinical trial | 11.324 | 3.5 years | Supplements of n-3 PUFA (1 g/d), vitamin E (300 mg/d), both, or none | [52] | ||
| VEAPS | Clinical trial | 353 | 3 years | DL alpha-tocopherol (400 IU/d) or placebo | [157] | ||
| HOPE | Clinical trial | 9.541 | 4.5 years | Natural-source vitamin E (400 IU/d) or placebo | [158] | ||
| SU.VI.MAX | Clinical trial | 1.162 | 7.2 ± 0.3 years | Combination of antioxidants (vitamin C, 120 mg/d; vitamin E, 30 mg/d; beta carotene, 6 mg/d; selenium, 100 μg/d; zinc 20 mg/d) or placebo | [159] | ||
| Meta-analysis | 51 (trials) | No significant reduction in mortality and cardiovascular risk | [160] | ||||
| Meta-analysis | 15.871 | No significant reduction in mortality and cardiovascular risk | [161] | ||||
| CARET | Clinical trial | 18.314 | 6 years | Beta-carotene (30 mg/d) and vitamin A (25000 IU/d) or placebo | 26% increase of CVD-related mortality | [162] | |
| Meta-analysis | Meta-analysis | 2,000,000 | No prevention of heart attacks, strokes, or cardiovascular death | [164] | |||
| Reduced risk of CHD incidence | |||||||
3.1. Olive Oil Derivates
Nutritional intake of olive oil, a key component of Mediterranean diet, has been associated with the prevention of CVDs, thanks to its content in monounsaturated fatty acids [26]. The 75% of fatty acids in olive oil is represented by monounsaturated oleic acid which counteracts endothelial dysfunction and reduces the tumor necrosis factor-alpha- (TNF-α-) induced apoptosis in VSMCs [27]. Olive oil is also a source of diverse phenolic compounds, such as hydroxytyrosol, oleocanthal, oleuropein, lignans, and pinoresinol, and numerous experimental, clinical, and epidemiological investigations support the beneficial properties of these olive derivates in CVDs [28, 29]. For instance, as for oleic acid, hydroxytyrosol reduces the expression of cell surface adhesion molecules (VCAM-1, ICAM-1, and E-selectin) in human umbilical vein endothelial cell line (HUVEC)[30]. Furthermore, hydroxytyrosol, as well as its derivates, inhibits the release of superoxide anions, prostaglandin E2 (PGE2), and TNF-α and the expression of cyclooxygenase 2 (COX2) in human monocytes [31]. Similarly, peracetylated hydroxytyrosol, a hydroxytyrosol derivate, attenuates lipopolysaccharide- (LPS-) induced proinflammatory cytokine production in murine peritoneal macrophages [32]. Interestingly, a diet rich in polyphenol powder, obtained from olive mill wastewater, enhanced antioxidant mechanisms and reduced oxidative stress-induced damage in chickens [33]. Moreover, in vitro treatment with hydroxytyrosol, or polyphenol extracts, from extra virgin olive oil, protects against the endothelial dysfunction induced by hyperglycemia and free fatty acids through modulation of NO production and endothelin-1 (ET-1) expression [34]. These observations have been confirmed by the NUTRAOLEUM study, a randomized double-blind controlled trial, which supported the hypothesis of beneficial effects of virgin olive oils on biomarkers of endothelial dysfunction in healthy adults. This study demonstrated that administration of olive oil enriched in phenols, especially in hydroxytyrosol, other than improving plasma high-density lipoprotein (HDL) levels, ameliorates the systemic ET-1 levels [35]. These data mirror those obtained in two other clinical trials which tested the effect of polyphenol-enriched olive oils on HDL- and endothelial-related markers in hypercholesterolemic subjects. In these studies, these functional olive oils promoted cardioprotective effects, as indicated by increased levels of fat-soluble antioxidants and antioxidant enzymes, improved HDL subclass distribution, reduced the level of DNA oxidation, and ameliorated endothelial function [36, 37]. Very recently, it has been also demonstrated that hydroxytyrosol blunts endothelial dysfunction by ameliorating mitochondrial function and reducing mitochondrial oxidative stress [38], suggesting a potential mitochondria-targeting antioxidant activity of hydroxytyrosol in the inflamed endothelium.
3.2. N-3 Polyunsaturated Fatty Acids
Polyunsaturated fatty acids (PUFAs) are fatty acids that contain two or more bonds in their carbon chains. Depending on the position of the carbon-carbon double bond closest to the omega (methyl) end of the molecule, PUFAs can be classified in omega-6 and omega-3 series of fatty acids which, other than being vital constituents of cell membranes, constitute the substrates for synthesis of eicosanoids (i.e., prostaglandins, prostacyclins, thromboxanes, and leukotrienes), mediators of inflammatory response and regulators of blood pressure and coagulation [39]. In the end of the 80's, an examination of the composition of Eskimos' diet revealed an association between the low mortality rate due to cardiovascular events in this ethnic group and their diet rich in PUFAs derived from sea fish [40]. This observation prompted the researchers to investigate the biological functions of these compounds and their role in human health and pathology prevention. During the past 20 years, the research on the role of omega-3 PUFAs in CVD has flourished, with the majority of scientific research being focused on eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA), both displaying beneficial cardiovascular effects. In fact, behind the possible cardioprotective effect of these omega-3 fatty acids, other than the reduction of triglyceride (TG) levels [39], additional modes of actions have been proposed, which include hypotensive effect [41], thrombosis reduction [42], and also decrease in malignant arrhythmias [43]. Moreover, numerous in vitro and in vivo investigations have shown that omega-3 PUFAs are able to modulate diverse key steps involved in atherosclerotic plaque formation (reviewed in [44]). Specifically, omega-3 PUFAs exert potent anti-inflammatory properties in polarized macrophages [45, 46] and are able to inhibit the expression of adhesion molecules by ECs [39, 47], thus decreasing leukocyte infiltration into the vascular wall [48]. In ApoE−/− mice, omega-3 PUFA treatments significantly attenuated the development and destabilization of atherosclerotic plaques [49]. In diabetic mice, supplementation with ω-3 fatty acids extracted from microalgae decreased the percentage of T lymphocyte CD4+-producing proinflammatory cytokines [45].
Several clinical trials have been conducted to assess the cardiovascular benefits of EPA and DHA treatments (i.e., diet and reinfarction trial (DART) [50], Japan EPA Liquid Intervention Study (JELIS) [51], and Gruppo Italiano per lo Studio della Sopravvivenza nell'infarto (GISSI) [52]). In 2002, a meta-analysis of eleven randomized clinical trials revealed that ω-3 PUFA supplementation reduced overall mortality, mortality due to myocardial infarction, and sudden death in patients with coronary heart disease (CHD) [53]. In the same year, the American Heart Association (AHA) recommended doses of total EPA and DHA between 2 and 4 g/day, to achieve a TG level reduction by 25-30% in normal and hyperlipidemic individuals [54]. After the publication of these guidelines, a large number of observational studies on omega-3 PUFA intake and CHD risk were conducted. In 2017, in an updated scientific advisory of AHA, the authors confirmed omega-3 fish oil supplements, in consultation with a physician, as a secondary—and not primary—prevention of sudden cardiac death in patients with prevalent CHD and in patients with heart failure [55]. In contrast, a recent meta-analysis of 10 trials involving 77917 individuals did not provide support for the AHA recommendations in individuals with a history of CHD for the prevention of fatal CHD, nonfatal MI, or any other vascular events [56]. Consistently, in a recent clinical trial, three months of treatment with PUFAs at a dose of 2 g/die did not improve endothelial function in patients with type 2 diabetes and high cardiovascular risk [57]. Similarly, in the ASCEND study, after a follow-up of 7.4 years, patients with diabetes and no evidence of cardiovascular disease, who received a daily supplement of ω-3 fatty acids, did not show a significantly lower incidence of serious vascular events than those who received placebo [58]. Despite the evidence from these last randomized trials that omega-3 has little or no effect on cardiovascular outcomes, clinical guidelines still recommend the use of omega-3 fatty acid supplements for the secondary prevention of CHD [55] and consider the beneficial effect of consuming fish and seafood, for their content in omega-3 PUFAs [59]. To date omega-3 PUFA effects on cardiovascular endpoints remain still unclear and might vary based on different types/doses of dietary omega-3 intakes and the presence of other medications (i.e., statins) in the clinical trials performed so far. Consistently, the recent results of the REDUCE-IT trial, which involved 19212 patients with elevated triglyceride levels at risk for ischemic events, showed that treatment with 4 g/die of EPA, a dose twice and 4-times higher compared to the dose tested in the Omega-FMD study [57] and ASCEND study [58], respectively, resulted in decreased risk of primary and secondary composite cardiovascular end points (i.e., cardiovascular death, nonfatal myocardial infarction, nonfatal stroke, coronary revascularization, or unstable angina) of 25 and 26%, respectively [60]. Several ongoing large randomized clinical trials (i.e., EVAPORATE [61], VITamin D and OmegA-3 TriaL, VITAL [62], STatin Residual Risk Reduction With EpaNova in HiGh CV Risk PatienTs With Hypertriglyceridemia, and STRENGTH (ClinicalTrials.gov Identifier NCT02104817)) will shed more light on the possible associations between omega-3 supplementation and reduction of risk of major cardiovascular events.
3.3. Flavonoids
Flavonoids, antioxidants present in fruit and vegetables, represent the most abundant polyphenolic constituents in foods of plant origin [63]. It has been estimated that the daily dietary intake of flavonoids is at least 1 gram per person and thus it is higher than that of all other known dietary antioxidants (e.g., vitamins C and E intake from food are estimated less than 100 mg/day) [64, 65]. Flavonoids present a 15-carbon (C6-C3-C6) skeleton, which generally consists of two phenyl rings and a heterocyclic ring. Approximately 8000 flavonoids have been identified based on C3 structure variations and degree of oxidation, and these include flavonols, flavones, isoflavones, flavanones, flavan-3-ols, and anthocyanidins [66]. In the context of atherosclerosis, flavonoids exert various beneficial effects, such as improvement of endothelial function and reduced oxidative stress and inflammation, and also antiplatelet, antihypertensive, and vasodilatory actions, inhibition of cholesterol synthesis, alteration of HDL function, and increased insulin sensitivity (reviewed in [67–69]). Flavanols are the subclass of flavonoids [70, 71] which have attracted most of the attention in the cardiovascular research community, since numerous epidemiological and mechanistic studies have supported the role of flavanols, particularly catechins, contained in cocoa and green tea, in counteracting endothelial dysfunction and atherosclerosis development [63, 72–76]. The mechanisms underlying the cardiovascular protective effects of catechins may include the inhibition of endothelial cell apoptosis by decreasing oxidative stress and ameliorating mitochondrial injury [77] and attenuation of proliferation of VSMCs by regulating anti-inflammatory and antioxidative enzyme heme oxygenase-1 (HO-1) [78]. In macrophage cell lines, catechins also mediate suppression of expression of metalloproteinase-9 (MMP-9), monocyte chemoattractant protein-1 MCP-1, and Toll-like receptor 4 (TLR4), which have a major role in atherosclerosis [79]. Moreover, in macrophages, epigallocatechin-3-gallate (EGCG) suppresses oxLDL uptake and foam cell formation [80]. In ApoE−/− mice, catechin consumption has been associated with reduced susceptibility of LDL to oxidation and aggregation and thus to the reduced atherosclerotic lesion area [81]. Recently, in vivo findings show that catechins reduce circulating LDL cholesterol and protect HUVECs against oxidative injury and decrease arterial vasoconstriction through reduction of H2O2 activity and eNOS level restoration [82, 83].
Similarly to flavanols, flavonols, and primarily quercetin, reduce atherosclerosis lesion area in vivo by affecting the oxidative stress status [84, 85]. The mechanism underlying this protection, which resembles those of flavanols, may involve attenuation of endothelial dysfunction [86], induction of HO-1 [87] and sirtuin-1 (SIRT-1) [88], and reduction of NF-κB activity [89]. It has been also suggested that quercetin may also inhibit VSMC migration, proliferation, and contraction [90, 91] and oxLDL-induced calcification in VSMCs, by targeting the ROS/TLR4 signaling pathway [92]. Recently, it has been also demonstrated that quercetin inhibits ox-LDL-induced ROS formation in mouse peritoneal macrophages by limiting the activation of NADPH-oxidase, which in turn may lead to the observed attenuation of high-fat diet-induced atherosclerosis in ApoE−/− mice [93, 94]. Additionally, quercetin reduces ROS levels in aortas of hypertensive rats by interfering with MMP-2 activity, thus limiting vascular remodeling [95]. Similarly, quercetin metabolites, like quercetin-3-glucuronide, generally found in onions, broccoli, and apples, have been shown to exhibit antioxidant, anti-inflammatory, and also antihyperglycemic properties both in vitro and in vivo [96–98].
The Zutphen Elderly Study was the first investigation in 805 men (aged 65-84 years, followed up for 5 years) which assessed the inverse correlation between flavonoid intake from tea, with a high content in quercetin, and the mortality from CHD, together with the incidence of MI [99]. Such correlation was confirmed by the Rotterdam Study, in which it was found that increased intake of tea and flavonoids may contribute to the primary prevention of ischemic heart disease [100]. Conversely, the protective effect of consumption of quercetin-rich tea, against ischemic heart disease, was not confirmed in other studies, such as the Caerphilly Study of Welsh men [101] and the Health Professionals Follow-Up Study [102]. Recently, the FLAVO study, a randomized, double-blind, placebo-controlled crossover trial performed in 37 (pre)hypertensive men and women (40-80 years), compared the cardioprotective effects of quercetin and epicatechin supplementation, both exerting similar in vitro and in vivo atheroprotective functions. The study has shown that, unlike quercetin-3-glucoside, supplementation of pure epicatechin improves endothelial function and reduces inflammation, thus reducing CVD risk factors [103].
Together, these data suggest that flavonoids, especially quercetin and catechins, might exert cardiovascular health benefits but larger trials are needed to draw conclusive evidence for the cardiovascular protective effects of these natural antioxidants, in the presence and absence of commonly used therapies for CVD.
3.4. Short-Chain Fatty Acids
In 1954, Walker and Ardvidsson [104] and Higginson and Pepler [105] were the first to suggest that low plasma lipid levels and the absence of atherosclerosis in primitive African populations were mostly due to their fiber-rich diet. In the 70's, this hypothesis was formalized by Burkitt et al. [106] and Trowell [107], proposing that the interaction among the dietary components can disturb the course of atherosclerosis onset, pointing out at the pivotal role played by fibers in modulating the serum lipid level. Since then, diverse epidemiological studies have reported an inverse relationship between fiber consumption and cardiovascular risk and that the beneficial effects of fibers would primarily reside in an improvement of the serum lipid profile (reduced total serum and LDL cholesterol levels) and reduction in blood pressure and systemic inflammation. In a recent umbrella review of all published meta-analyses (from January 1, 1980 to January 31, 2017), it emerged that dietary fibers can reduce chances of developing CHD and stroke between 7% to 24% and the overall morbidity and mortality caused by cardiovascular disease from 17% to 28% [108]. These observations mirror those described in another systematic meta-analysis review, performed in 2014, which investigated the association of fiber consumption and all-cause and cause-specific mortality in 42 cohorts (from 25 studies). This analysis showed a 23% reduction of the mortality rate for CVDs and a concomitant 17% decrease for cancer-related death and a 23% reduction for all-cause mortalities [109]. Based on these scientific evidences, the Food and Drug Administration (FDA) strongly recommends consumption of fiber-rich food for health promotion and disease prevention, suggesting that an adequate intake of dietary fibers should be at least 25 grams/day for a 2000-calorie diet (https://www.accessdata.fda.gov/scripts/InteractiveNutritionFactsLabel/factsheets/Dietary_Fiber.pdf).
The beneficial effects on lowering total serum cholesterol are attributed to three major mechanisms: (i) prevention of bile salt reabsorption from the small intestine which leads to increased fecal excretion of bile acids, (ii) reduced glycemic index and insulin resistance, which can result in the inhibition of hydroxymethylglutaryl coenzyme A (HMG-CoA) reductase activity and hepatic cholesterol synthesis [110, 111], and (iii) production, from anaerobic bacterial fermentation of undigestible fibers, of short chain fatty acids (SCFAs), mainly acetate, propionate, and butyrate [112]. SCFAs inhibit the production of proinflammatory cytokines and the recruitment of immune cells to ECs, mechanisms mediated by binding to the free fatty acid (FFA) receptor types 2 and 3 and G-protein-coupled receptor 109A (GPR109A) and by inhibiting intracellular activity of histone deacetylases (HDACs), enzymes which can regulate multiple molecular processes involved in atherogenesis [97, 113–115]. Recently, it has emerged that SCFAs inhibit LPS- and TNF-α-induced endothelial expression of VCAM-1, through activation of GPR41/43, which are SCFA receptors expressed in ECs, and inhibition of HDAC activity [116, 117]. The anti-inflammatory activity of SCFAs has been also evaluated in vivo: in a partially ligated carotid artery (PLCA) mouse model of atherosclerosis, it has been found that butyrate repressed endothelial Nlrp3 (Nlr family pyrin domain-containing 3) inflammasome activation in ECs, via redox signaling pathways, and prevented arterial neointima formation. On the contrary, acetate and propionate exerted minimal inhibitory effects on inflammasome activation and even seem to augment the arterial neointima formation in the PLCA model [117]. The antiatherosclerotic activity of SCFAs was also confirmed by a recent study by Chen and collaborators showing that SCFAs from pectin fermentation are able to inhibit intestinal cholesterol absorption, to decrease serum total and low-density lipoprotein cholesterol and to protect ApoE−/− against diet-induced atherosclerosis [118]. Also, in this case, the authors showed that the beneficial effects of SCFAs are mediated only by butyrate through a mechanism which involves increased expression, in the small intestinal mucosa, of ATP-binding cassette (ABC) transporters G5 (Abcg5) and G8 (Abcg8), which limit intestinal absorption and facilitate biliary secretion of cholesterol [118, 119].
3.5. Vitamins
An adequate intake of vitamins, from food or dietary supplements, is commonly considered as indispensable for maintenance of good health, and thus, since their discovery in the early 1900s, vitamins have been considered the most promising nutraceuticals for the prevention of diverse pathologies, including atherosclerosis-derived CVDs. In this context, vitamins C and E have received the most attention since, according to the “oxidation hypothesis” of atherosclerosis onset [120, 121], antioxidant organic compounds, like these two vitamins, could represent the first line of defense against LDL oxidation, the first step in the formation of atherosclerotic plaques. Moreover, during the last two decades, other antiatherogenic mechanisms of action have been ascribed to vitamins C and E (reviewed in [25, 122, 123]), such as stimulation of endothelial cell proliferation, thanks to their ability to increase the synthesis of type IV collagen, reduction of apoptosis induced by high glucose conditions, LPS or TNF-α [122, 123], enhancement of eNOS activity by stabilization of the eNOS cofactor tetrahydrobiopterin (BH4) [124], and tightening of the permeability barrier of ECs [124]. Similarly, for vitamin E, the antiatherosclerotic action has been found, which is independent from its antioxidant properties, such as reduced cholesterol synthesis by inhibiting HMG-CoA reductase, increased eNOS activity, reduced NF-κB-dependent synthesis of ICAM-1 and VCAM-1, reduced platelet aggregation, and inhibition of VSMC proliferation [123, 125].
These in vitro findings were confirmed by studies in animal models of atherosclerosis (as reviewed in [126]). For instance, vitamin E, in an age-dependent manner, is able to inhibit atherosclerosis in ApoE−/− mice by decreasing serum oxLDL and vasculature mRNA expression of genes involved in cholesterol transportation [127]. Similarly, α-tocopheryl phosphate (a natural form of vitamin E) limits atherosclerosis lesions in hypercholesterolemic rabbits [128] and in ApoE−/− mice by limiting aortic superoxide formation and by reducing circulating plasma levels of proinflammatory markers [129], Similarly, vitamin E deficiency, caused by disruption of the α-tocopherol transfer protein gene, increased the severity of atherosclerotic lesions [130].
Likewise, vitamin C limits endothelial dysfunction in animal models of atherosclerosis: long-term supplementation (26/28 weeks) of vitamin C restored eNOS activity in the aorta of ApoE−/− mice [131], whereas chronic hypoascorbemia has been associated to an elevated lipoprotein(a) (Lp(a)) accumulation in the vasculature and increased atherosclerotic lesion development in gulonolactone-oxidase-deficient (Gulo−/−)/Lp(a)+ mice, a model lacking of endogenous ascorbate synthesis and expressing human Lp(a) [132].
In vivo studies have investigated the antiatherosclerotic effect of a combined vitamin C and vitamin E supplementation in mouse models of this pathology: vitamin C/vitamin E cocktail inhibited the development of fatty streak lesions in the LDLr−/− mice [133] and limited aortic Vegf and Vegfr-2 expression in ApoE−/− mice [134] compared to nontreated littermates. In a very recent study, in atherogenic diet-fed (scavenger receptor class B type 1) SR-B1 KO/ApoER61h/h mice, a murine model of dyslipidemia, progressive atherosclerosis, CHD and premature ischemic death, combined with administration of vitamins C and E reduced serum total cholesterol and triglyceride levels, improved HDL antioxidant function, and lowered serum TNF-α levels [135].
Other than vitamins C and E, vitamins A and D show potential antiatherosclerotic properties (as reviewed by [136]). The wide range of vitamin D beneficial functions includes reduction of endothelial dysfunction and VSMC proliferation and migration, as well as downregulation of the atherosclerosis-related inflammatory and immune processes [136]. Less is known about vitamin A (retinol) in this context: retinoic acid (RA) metabolites, derivatives of vitamin A, limit VSMC growth, differentiation, and proliferation [137] and prevent high-fat diet- (HFD-) induced atherogenesis in ApoE−/− mice via the upregulation of aforementioned transporters ABC-A1 and ABC-G1 [138]. Peculiarly, in the same animal model, vitamin A deficiency stimulates atherogenesis, prevented by β-carotene supplementation [139]. All-trans retinoic acid (ATRA), a derivative of vitamin A, has been recently shown to reduce the plaque size in a rabbit model of HFD-induced atherosclerosis by inhibiting platelet aggregation, by decreasing caveolin-1 expression and ET-1 secretion and by enhancing eNOS activity and NO formation [140–143].
Despite the promising in vitro and in vivo findings supporting the antiatherosclerotic properties of vitamins and their metabolites, at the clinical level, the results obtained have been contradictory: albeit the vast majority of the literature have correlated a low level of the above described vitamins with early atherosclerosis onset, major risk of a CVD events, and heart failure in human [144–152], it is undeniable that the various clinical trials, performed so far, lack consistency. For instance, a few studies have evidenced that supplementation of vitamins C and E, alone or in combination, may delay the progression of atherosclerosis [153, 154] and reduce cardiovascular mortality in healthy women [155], whereas many other trials, like the MRC/BHF Heart Protection Study Heart Protection Study Collaborative Group [156], GISSI-Prevenzione [52], VEAPS [157], HOPE [158], and Supplementation en Vitamines et Mineraux Antioxydants (SU.VI.MAX) [159], reported that these vitamins do not produce any significant difference in the incidence of cardiovascular events and CVD-related mortality. Contextually, in 2011, a systematic review of 51 trials showed no significant reduction in mortality and cardiovascular risk associated with vitamin D supplementation [160], having conclusions mirroring those obtained from vitamin C-centered meta-analysis performed in 2016 [161]. It is expected that new insights will arise from the ongoing randomized, double-blind, placebo-controlled VITAL trial, which will evaluate the long-term effects of high-dose vitamin D supplementation on CVD events in 25874 U.S. adults [62].
Only a few trials have been performed to assess the effects of vitamin A on CVDs. In 1996, the beta-carotene and retinol efficacy trial (CARET) tested the effect of beta carotene and vitamin A supplementation on the incidence of lung cancer and cardiovascular death in 18314 high-risk participants, specifically smokers and workers exposed to asbestos [162]. This trial was stopped because participants randomly assigned to vitamin supplementation, compared to the placebo group, exhibited a 28% increase in incidence of lung cancer and a 17% increase of overall mortality rate and a higher rate (26%) of cardiovascular disease mortality, which decreased during the 6-year follow-up after the vitamin integration was stopped [162, 163].
A recent meta-analysis of 18 studies, which involved a total of 2 million participants, concluded that taking multivitamins does not prevent heart attacks, strokes, or cardiovascular death, even though it seems to be associated with a lower risk of CHD incidence [164]. In the absence of studies showing their efficacy in primary prevention of CVDs, the AHA does not recommend vitamin supplementation for healthy subjects, and similarly, the U.S. Preventive Services Task Force (USPSTF) states that the current evidence is insufficient (I) to assess the balance of benefits and harms of the use of multivitamins for the prevention of cardiovascular disease or cancer (I statement). The USPSTF also discourages (D) the use of β-carotene or vitamin E supplements for the prevention of cardiovascular disease or cancer (D recommendation) [165].
3.6. Other Emerging Antiatherogenic Nutraceuticals
3.6.1. Berberine
Berberine (BBR), a quaternary ammonium salt from the protoberberine group of isoquinoline alkaloids (5,6-dihydrodibenzoquinolizinium derivative) found in Berberis species plants (Berberidaceae), exhibits many different types of biological activities, including its effectiveness in lipid disorders and hyperglycemia [166]. The poor intestinal absorption and bioavailability of BBR are the main drawback when orally administered even though its metabolites maintain higher concentration in plasma, behaving like the pharmacologically active forms of BBR; its main metabolite berberrubine (M1) tautomerizes to a highly conjugated, electroneutral quinoid structure [167] reaching a high plasma concentration as a consequence of a more efficient intestinal absorption [167].
Several preclinical studies as well as clinical trials suggest a beneficial role of BBR in endothelial dysfunction and dyslipidemia [166]; in ECs, BBR attenuates LDL oxidation induced by ROS and reduces apoptosis modulation, chromosome condensation, cytochrome c release, and caspase-3 activation. It has also been reported that BBR reversed NOX4-derived ROS production in HUVECs, at least in part due to the regulation of adenosine monophosphate-activated protein kinase (AMPK) activation. In both cultured endothelial cells and blood vessels isolated from rat aorta, BBR enhanced eNOS and promoted a glutathione peroxidase (GSH-Px) and superoxide dismutase (SOD) hyperactivation in the liver of mice, attenuating H2O2-induced ROS [168]. BBR elevates LDL receptor (LDLR) expression in human liver cells through inhibition of proprotein convertase subtilisin/kexin type 9 (PCSK9) transcription, an enzyme that posttranscriptionally upregulates LDLR [169]. In rat liver, a combination of BBR with simvastatin increased the LDLR gene expression to a level significantly higher in comparison to monotherapies [166]. In human macrophage-derived foam cells treated with oxLDL, BBR inhibits the expression of LOX-1 as well as the oxLDL uptake by macrophages and reduces foam cell formation in a dose-dependent manner by activating the AMPK-SIRT1-PPARγ pathway [170]. We recently reported that BBR prevents the oxLDL- and TNF-α-induced LOX1 expression and oxidative stress in HUVECs, key events leading to NOX, MAPK/Erk1/2, and NF-κB activation linked to endothelial dysfunction [171], and consistently, Abidi and colleagues had previously shown, both in vitro and in vivo, that BBR reduces VCAM-1 expression induced by LPS [172]. We recently observed that M1 inhibited intracellular xanthine oxidase activity, one of the major sources of ROS in vasculature, and reduced the expression ICAM-1 [173]. The lipid-lowering activity of BBR, alone or in association with other nutraceuticals, has been clearly confirmed in a relatively large number of randomized clinical trials which support the safety of a short-term use of this nutraceutical, especially when used at a lipid-lowering dose [166].
3.6.2. Carotenoids
Carotenoids represent a group of pigments widely distributed in nature. They contribute to the red, orange, and yellow colors found in many flowers, fruits, and vegetables, where they act as photoprotectors and as attractant for insects and animals for pollination and seed dispersal. Since animals and humans are unable to synthesize carotenoids de novo, carotenoids are essential nutrients and important health beneficial compounds [174, 175]. Carotenoid consumption improves the metabolic profile, decreasing the incidence of diabetes, lowers LDL levels, and improves blood pressure by ameliorating the bioavailability of NO [175]. Apart from the well-established function of carotenoids as provitamin A, some carotenoids, such as lycopene and astaxanthin, are strong antioxidants and have a protective function in reducing the risk of both cancer and cardiovascular diseases [175, 176]. Xanthophylls, such as lutein and zeaxanthin, are essential components of the macular pigments in the eyes and offer protection against macular degeneration, the leading cause of age-related blindness [175, 177, 178]. Lutein exhibits strong antioxidant properties in vitro and in vivo [178], and low serum levels of lutein have been associated both with increased values of common carotid intimal medial thickness (CCA-IMT) and myocardial infarction [179]. An 8-year follow-up study, performed on 43.738 men with no history of cardiovascular disease or diabetes, showed a significant inverse correlation between lutein intake and risk for ischemic stroke [179]. A case control study found that the risk for MI was inversely correlated with adipose tissue lutein content and inversely proportional to dietary lutein intake [178].
Due to their antioxidant activity, lycopene, lutein, zeaxanthin, and astaxanthin are able to attenuate the atherosclerotic process. Lycopene, a fat-soluble carotenoid without provitamin A activity, is the pigment responsible for the distinctive red color in tomatoes and watermelon, and it is a powerful antioxidant and free radical quencher [180]. High plasma lycopene levels have been associated with reduction in aortic stiffness in patients with metabolic syndrome [181]. Conversely, low plasma levels of lycopene were associated with increased risk of atherosclerotic lesions and with an increased risk of acute coronary events or stroke [179]. Similarly, in a case control study performed in patients suffering from heart failure (NYHA class II-III), the left ventricular ejection fraction was significantly and positively correlated with plasma lycopene levels: NYHA class II patients showed significantly higher levels of lycopene than class III patients [179]. High lycopene consumption has been associated with a decreased risk of CVD, including atherosclerosis, myocardial infarction, and stroke. In a study performed on healthy male volunteers, lycopene supplementation improved the endothelial function, together with a significant decrease in serum levels of CRP, ICAM-1, and VCAM-1 and an improvement in the atherosclerotic risk factors (lipid profile and systolic blood pressure level) [181]. A meta-analysis using a random effects model of all studies between 1955 and September 2010 investigating the effect of lycopene on blood lipids or blood pressure for a minimum duration of 2 weeks suggests that lycopene taken in doses ≥ 25 mg daily is effective in reducing LDL cholesterol by about 10% which is comparable to the effect of low doses of statins in patients with slightly elevated cholesterol levels [180].
Ketocarotenoid astaxanthin is the main carotenoid present in aquatic animals (salmon, trout, red seabream, shrimp, lobster, and fish eggs), contributing to the pinkish-red color of their flesh, and also in some birds (flamingoes and quails in particular) [179]. Astaxanthin exhibits a free radical-quenching potency that is, on an equimolar basis, double than the potency of β-carotene [178], about 100-fold greater than the antioxidant potency of α-tocopherol [178], and approximately 6000 times the potency of ascorbic acid [178]. Astaxanthin demonstrated to exert beneficial effects on the heart, both by reducing inflammation and by modifying blood levels of LDL-C and HDL-C; moreover, it reduces macrophage infiltration and apoptosis in vascular lesions, thus improving plaque stability by increasing adiponectin [179]. Astaxanthin inhibits also the production of oxLDL [178] and their uptake by activated intravascular macrophages [178] and inhibits the release of atherogenic ROS, NO, and proinflammatory cytokines [178]. 8 weeks of a dietary supplementation with 2 mg of astaxanthin daily by a group of healthy postmenopausal women produced a significantly greater increase in total plasma antioxidant machinery than what was elicited by placebo and a significantly greater decrease in the plasma concentration of thiobarbituric acid-reactive substances (the mixed reaction products of nonenzymatic oxidative lipid peroxidation) [178]. Dietary astaxanthin also contributes to healthy blood flow through the vasculature by promoting aortic and coronary artery vasodilation and increases the flexibility of red blood cell membranes (with an acceleration of red blood cell flow through the blood vessels) [178].
3.6.3. Red Yeast Rice
Red yeast rice (RYR) is a Chinese herbal supplement produced by fermenting white rice with the yeast, Monascus purpureus, used to flavour, color, and preserve foods and as a traditional medicine for many years. RYR contains a variety of monacolins, which inhibit HMG-CoA reductase, the rate-limiting step in cholesterol synthesis. Approximately 90% of the total monacolin content of RYR consists of monacolin K, chemically identical to lovastatin, and its hydroxy acid form, monacolin KA [182]. Other active ingredients with the potential to lower cholesterol in commercially available RYR products include plant sterols (β-sitosterol, campesterol, and stigmasterol), isoflavones, and monounsaturated fatty acids [183]. The first prospective, double-blind, placebo-controlled study evaluating RYR in an American population was conducted by Heber et al. in 1999 in eighty-three healthy adults with untreated hyperlipidemia that followed the AHA cardioprotective diet (less than 10% of calories from saturated fat and less than 300 mg from cholesterol per day). They were randomly assigned to receive 2.4 g per day of RYR or placebo, for 12 weeks. Compared to baseline, LDL-C levels decreased by 22% in the RYR-treated group [184]. Other clinical trials have found that a relatively small dose of RYR (equivalent to a daily lovastatin dose of 5 to 7 mg) is as effective as 20 to 40 mg of pure lovastatin in lowering cholesterol [185]. Becker et al. compared the efficacy of an alternative treatment composed by RYR, fish oil, and therapeutic lifestyle changes with simvastatin 40 mg per day in 74 primary prevention patients with known or newly diagnosed hypercholesterolemia [186]. Depending on baseline LDL-C, patients took 1200 mg of RYR (10 mg lovastatin) or 1800 mg (15 mg lovastatin) twice a day, for 12 weeks. At the end of the study, both groups had a similar reduction in LDL-C and no significant differences were found between the two groups. Interestingly, participants in the RYR-fish oil and life style change group lost more weight during the study (−4.7 ± 2.4 kg vs −0.3 ± 2.2 kg) and had a significant reduction in triglycerides compared with the simvastatin group. Li et al. published a large meta-analysis, which examined the effectiveness and safety of RYR as an alternative approach for treating dyslipidemia. Thirteen randomized, placebo-controlled trials were included (from 1999 to 2013) with treatment duration of 4 weeks to 6 months and no serious side effects were reported. Overall, RYR significantly lowered total and LDL-C levels (P < 0.001) compared with placebo and this effect did not appear to be related to the dose, duration of therapy, or geographic location [187]. In another small observational study, 25 dyslipidemic patients with a history of intolerance to lipid-lowering medications were treated with RYR for more than 4 weeks. In accordance with other studies, RYR significantly lowered LDL-C by 21% in this clinical population during the period of treatment [188]. The China coronary secondary prevention study (CCSPS) so far is the only randomized, double-blinded, placebo-controlled, multicentered study demonstrating that monacolin K reduces cardiovascular risk [189]. This trial recruited 4870 Chinese patients with a history of MI and moderate hypercholesterolemia. Patients were randomized to receive twice-daily treatment of a capsule of Xuezhikang (XZK), containing 2.5 to 3.2 mg of monacolin K equivalent to a total daily lovastatin dose of 10 to 12.8 mg or placebo. After 4.5 years, XZK was associated with a highly significant reduction in frequency of coronary events (10.4% in the placebo vs 5.7% in the XZK group) and a relative risk reduction of 45% [189]. Treatment with XZK also significantly decreased total mortality by 33%, cardiovascular deaths by 30%, and the need for coronary revascularization by 33%. Total cholesterol and LDL-C levels decreased by 13 and 20%, respectively, compared to baseline. Adverse effects were similar in both groups, and the XZK appeared to be well tolerated. A substudy of elderly hypertensive patients in the same CCSPS cohort found that monacolin K was effective in lowering the rates of coronary events and death from CHD compared with placebo [190].
Several clinical trials have shown RYR to be safe, effective, and well tolerated both alone or in combination with other nutraceuticals; however, the studies are small and of short duration [191]. Even if RYR is perceived as a “natural” product providing fewer side effects, it should be taken into account that monacolin K is identical to lovastatin and therefore may present an increased risk of muscular and other side effects especially in patients with a history of SAM. Myopathy, hepatoxicity, and rhabdomyolysis have all been reported in patients taking RYR, as one would expect from any statin therapy [192]. For this reason, RYR should be taken under the guidance of a physician who will closely monitor its efficacy, safety, and tolerability [193]. In the USA, RYR has been used as an alternative to statin therapy in treating patients with mild to moderate hypercholesterolemia, especially among patients who might be intolerant to standard therapy due to statin-associated myalgia (SAM). For this reason, the FDA has prohibited the sale of all RYR products containing monacolin K, because it is considered an unapproved drug; however, many RYR supplements are still on the market.
3.6.4. Allicin
Garlic (Allium sativum) has been used as a spice, food, and medicine for over 5000 years and is one of the earliest documented herbs utilized for the maintenance of health (as a diuretic and for the immune system and gastrointestinal health) and for treatment of disease, including circulatory disorders and infections [194]. Functional sulfur-containing components described in garlic include alliin, allicin, diallyl sulfide, diallyl disulfide, diallyl trisulfide, ajoene, and S-allylcysteine. Allicin is a thiosulfinate and in nature is produced after damage of the plant tissue by an enzymatic reaction [195].
Aged garlic extract in cell culture prevented endothelial cell dysfunctions caused by oxidative stress by increasing cellular concentrations of thiol antioxidants, such as cysteine and glutathione (GSH) [196]. A correlation between low red blood cell glutathione (GSH), which plays important roles in cellular redox status and signaling, and increased plasma homocysteine (HCy) has been linked to an increased incidence of hypertension [197]; in an animal model of hyperhomocysteinemia, induced by a severely folate-depleted diet in rats, aged garlic extract decreased plasma HCy concentrations by 30% [198]. The potential effect of garlic on HCy levels has been reported in a small clinical trial of atherosclerosis patients randomized to aged garlic extract (P = 0.08) [199]. Garlic has been also shown to have blood pressure- (BP-) lowering properties in hypertensive patients [200]: allicin, decomposes rapidly to its degradation products which results in the release of hydrogen sulfide (H2S) [201], a potent gaseous signaling molecule which lowers blood pressure (BP) by the relaxation of smooth muscle cells surrounding the blood vessel [194]. The H2S-dependent BP-reducing effect is thought to be primarily mediated through sulfhydration of ATP-sensitive potassium (KATP) channels, which in turn leads to voltage-sensitive channel opening and relaxation of vascular smooth muscle cells [202]. However, other potassium channels may also be affected by H2S and additional mechanisms have been suggested in determining the opening/closing of K+ channels, including a possible cooperation between H2S and NO [202]. In CVD models, the administration of H2S prevents myocardial injury and dysfunction [203] and aged garlic extract was shown to normalize NO output from endothelial cells by preventing the decline of BH4 levels [194]. In addition, garlic, due to the high content of polysulfides, may help in providing the nutrients needed for maintaining optimum redox balances for several eNOS-dependent signaling pathways important in vascular relaxation [194]. Additionally, allicin is able to suppress cholesterol biosynthesis [204–206] and platelet aggregation. So far, five trials have demonstrated a strong effect of garlic on inhibition of platelet aggregation, whereas one trial reported no effect [207].
3.6.5. Curcuminoids
Curcuminoids, extracted from the rhizomes of Curcuma longa, are naturally occurring polyphenols used for centuries in indigenous medicine to treat various diseases, such as common colds, arthritis, diarrhea, and upper respiratory disorders. The curcuminoids best characterized are curcumin, demethoxycurcumin (DMC), and bisdemethoxycurcumin (BDMC), all belonging to the diarylheptanoid family [208]. A lot of evidence suggests that curcuminoids have a diverse range of molecular targets; curcumin, the main component of curcuma extract, has several biological and pharmacological properties including anti-inflammatory, antioxidant, antithrombotic, antiatherosclerotic, anticonvulsant, and anticancer properties and cardio- and neuroprotective activities (reviewed in [209]).
Curcuminoid treatment improved glycemic factors, hepatic function, and serum cortisol levels in subjects with overweight and impaired fasting glucose in a randomized double-blind placebo-controlled trial involving 80 overweight subjects [210]. Among curcuminoids, curcumin is the best characterized and studied; its antioxidant and anti-inflammatory properties are therefore considered a multifunction phytochemical that can interact with multiple molecular targets, modulating cell growth, inflammation, and apoptosis signaling pathways (reviewed in [211]). Firstly studied for its beneficial properties in the gastrointestinal tract, it has been observed that curcumin has a beneficial role in chronic conditions such as intestinal dysmotility disorders and in the prevention and maintenance of remission of intestinal bowel disease (IBD), requiring long-time treatment [212]. Moreover, curcumin seems to show protective properties from metabolic syndrome, decreasing insulin resistance, obesity, hypertriglyceridemia, and hypertension and preventive properties from complications. It has been evidenced that curcumin possesses hypolipidemic effects, which together with its antioxidant and anti-inflammatory activities can contribute to reducing the incidence of atherosclerosis [213]. The remarkable antioxidant capacity of curcumin reduces lipid peroxidation and the generation of oxLDL and, consequently, reduces the inflammatory response and the progression of atherosclerosis [213].
Recently, it has been observed that curcumin can inhibit hypoxia-inducible factor 1α (HIF-1α) thus repressing the total cholesterol and lipid level in macrophage under hypoxic condition [214]. The benefit of curcumin in patients at risk of atherosclerosis has also been described; after 6 months of curcumin dietary supplementation, patients with type 2 diabetes had lower pulse wave velocity which improved the metabolic profile [215]. Furthermore, the use of curcumin for 8 weeks improved flow-mediated dilatation in 32 postmenopausal women [216]. A major limitation to using curcuminoids as a nutraceutical is its poor bioavailability, owing to inadequate absorption in the gut and as it is rapidly broken down and quickly excreted from the body. Several strategies are being pursued in an attempt to increase their bioavailability, including the use of liposomal curcumin, nanoparticles, and a curcumin phospholipid complex.
4. Notch Signaling Modulation by a Nutraceutical Approach
The Notch signaling is a highly conserved short-intercellular communication system deeply investigated for the possible role as a novel therapeutic target in cancer [217], which is becoming more and more recognized as a key player in the maintenance of vascular homeostasis. In the next paragraphs, we will discuss the basics of this pathway, the role played by its dysregulation in atherosclerosis and what is currently known about the effects of nutraceuticals on Notch.
4.1. The Basics of Notch Signaling
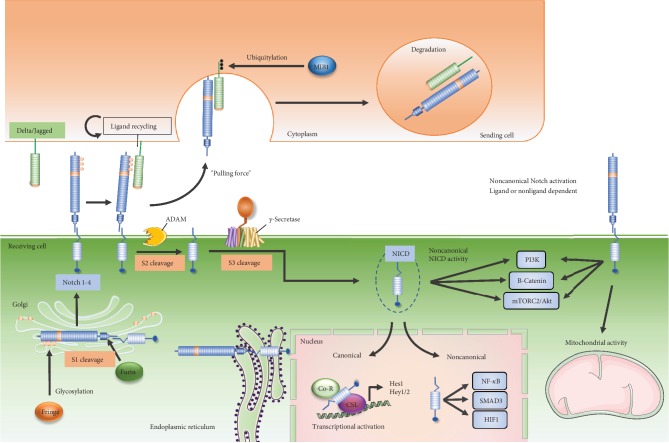
In mammals, there are four highly homologous receptors (Notch-1-4) and five ligands belonging to Delta-like (Dll-1, 3, and 4) or Jagged (Jagged-1 and 2) families. Notch receptors are synthesized as single-chain precursors and are cleaved by Furin (S1 cleavage) into an extracellular domain (NECD, rich in epidermal growth factor- (EGF-) like repeats) and a transmembrane subunit in the Golgi apparatus, generating the functional heterodimeric receptor, linked by Ca2+-dependent noncovalent bonds. Here, the EGF-like domains can be modified by the adding of O-fucose glycans by the Glycosil transferase Fringe [218], thus determining which ligands can be bound by the Notch receptors. Similarly, the ligands are also single-pass type I transmembrane proteins and present an extracellular domain formed by EGF-like repeats [219]. The canonical activation of Notch signaling arises from the interaction between the Notch receptors and their ligands on adjacent cells (Figure 2).
Figure 2.
Canonical and noncanonical Notch signaling pathway. In the canonical Notch pathway, precursor of Notch receptors undergoes Furin-mediated cleavage (S1) in the Golgi apparatus, which is necessary to form the functional heterodimeric receptor. Upon Notch glycosylation by the Fringe family of glycosyltransferases, the Notch receptor translocates to the plasma membrane, where it interacts with a Delta/Jagged ligand, present on the surface of an adjacent cell. Notch signaling is activated when the ligand, bound to the receptor, is ubiquitylated by MIB1, an event that generates the mechanical force necessary for exposing the second cleavage site of Notch receptors. This event leads ADAM to perform the second cleavage (S2). The third cleavage (S3), by the γ-secretase complex, promotes the release of the intracellular domain of the receptor (NICD). NICD translocates into the nucleus where it promotes the transcription of canonical Notch target genes, such as Hey1 and 2 and HES1. The noncanonical Notch signaling pathway may be γ-secretase dependent or independent. This later may also occur either in the presence or in the absence of its ligand. Noncanonical Notch signaling is also independent of CSL, and it is mediated by the interaction with PI3K, mTORC2, AKT, Wnt, NF-κB, YY1, or HIF-1α pathways at either the cytoplasmic and/or nuclear levels.
Accordingly to the “pulling force” theory, the Notch signaling is activated when the E3 ubiquitin-protein ligase (MIB1) modifies the Notch ligands, when bound to NECD, allowing the ligand endocytosis and generating the mechanical force necessary for exposing the second cleavage site of Notch receptors, thus driving the successive proteolytic cleavages mediated by A disintegrin and metalloprotease (ADAM) surface protease (S2 cleavage) [220]. A second intramembranous cut by γ-secretase (S3 cleavage) mediates the release of the Notch intracellular domain (NICD), the active form of the receptor. NICD translocates into the nucleus where it binds the transcription factor CSL (CBF-1, suppressor of Hairless and Lag-1) also known as RBP-Jκ (recombinant signal-binding protein 1 for Jκ) transcription factor, thus promoting the transcription of Notch target genes. The most characterized direct Notch target genes are the negative regulator of transcription and belong to the Hairy and Enhancer of Split (HES) and Hairy and Enhancer of Split with YRPW motif (HEY) gene families [221] although several other targets have been described [222]. Under pathological conditions, like cancer and activation of the immune system, Notch signaling can also act in a RPB-Jκ-independent manner (“noncanonical” fashion) signaling [223]. In the noncanonical pathway, NICD interacts with other transcription factors, such as SMAD3 (small mother against decapentaplegic 3), YY1 (Yin Yang 1), HIF-1α, and NF-κB (nuclear factor kappa-light-chain-enhancer of activated B cells), into the nucleus, whereas, in the cytoplasm, this signaling may occur via the uncleaved Notch receptor, still bound to membrane, or via the NICD, through interaction with PI3K (phosphoinositide 3-kinase)/Akt/Wnt/β-catenin pathways [224]. Recently, it has been also reported that Notch, by interacting with PTEN-induced kinase 1 (PINK1), can activate the mTORC2/Akt pathway, thus influencing the mitochondrial function and cell survival [225]. Posttranslational modifications (methylation, hydroxylation, acetylation, ubiquitylation, and phosphorylation) and the interplay with other signaling pathways, such as NF-κB, estrogen receptor- (ER-) alpha, G protein-coupled ER (GPER), ErbB2, and vascular endothelium growth factor receptors (VEGFRs) [226–231], increase the complexity of this signaling pathways which, in a cell type- and context-dependent manner, can influence a plethora of biological processes.
4.2. Notch in the Endothelium
A large number of in vitro and in vivo studies have convincingly established the critical role of Notch signaling during development of the vascular system in which Notch is indispensable for a correct arteriovenous specification [232] and its malfunctioning has been correlated to vascular abnormalities, not compatible with life. Briefly, Notch-1 homozygous mutant and Notch-1/Notch-4 double homozygous mutant embryos displayed severe defects in angiogenic vascular remodeling [5, 233, 234]. Similarly, expression of active Notch-4 in vasculature leads to an anomalous vessel structure and embryonic lethality at embryonic day 10 (E10) [235]. Furthermore, the vascular defects of Notch transcriptional regulators (RBP-Jκ or Mib1) on homozygous mutant mice embryos are similar to defects developed by mutant embryos for Notch receptors and died prior to E11.5 [236, 237]. Likewise, Hey1 or Hey2 and Dll-4 or Jagged-1 mutant mice exhibit defects in their vasculature during embryogenesis and died from massive hemorrhage [238–241].
Given the pivotal role played by Notch signaling in vascular embryogenesis, it is not surprising that Notch is also essential in maintaining the homeostasis of the adult vasculature. In ECs, all Notch receptors, Dll-1 and 4 and Jagged-1 and 2 ligands, are expressed [242] and it is well-known that Notch, by intricate crosstalk with VEGF-A, controls arterial angiogenesis, also under proinflammatory conditions [243], by affecting the balance between tip cells and stalk cell [244].
During the last decade, Notch has been under the spotlight of the atherosclerosis research field since, except for a few studies [116, 245–247], numerous studies demonstrated that Notch may counteract endothelial dysfunction and atherosclerotic plaque development. Quillard et al. provided the first in vitro evidence that proinflammatory conditions (i.e., TNF-α) dysregulate Notch signaling leading to increased levels of ICAM-1 and VCAM-1 and to NF-κB-mediated apoptosis [248, 249]. These data were corroborated by Briot et al. showing that in human aortic ECs, siRNA-mediated reduction of Notch-1 is sufficient to increase the expression of inflammatory markers and adhesion molecules and that treatment of ECs with oxidized lipids and proinflammatory cytokines (TNF-α and interleukin-1-beta (IL1β)) decreased Notch-1 expression [250]. In agreement with this study, we have recently demonstrated that 17β-estradiol is able to limit TNF-α-induced apoptosis in ECs by activating Notch-1 [226]. In support of the protective role of Notch in the endothelium, Wang and collaborators reported that in bone marrow ECs, RBP-Jκ inhibits miR-155/NF-κB axis activation [251], data being reinforced by the Nus et al. study which showed that RBP-Jκ heterozygous inactivation results in aortic valve calcification under dyslipidemic condition [252]. Similarly, in human-induced pluripotent stem cell- (iPSC-) derived ECs, Notch-1 haploinsufficiency interferes with EC response to shear stress, causing the unlock of proosteogenic and inflammatory networks [253]. The interplay between Notch and shear stress is an emerging relevant topic in the context of vascular biology [6]. Diverse Notch signaling components in the endothelium respond to shear stress [254], and Notch-1 is essential for preserving EC tight junctions and their normal transcriptional/epigenetic response to shear stress [253, 255]. Polacheck et al. suggested that Notch-1 may be activated by shear stress through a Dll-4-dependent mechanism, triggering a noncanonical Notch pathway [256]. Schober et al. showed instead that in aorta regions exposed to turbulent flow, thus prone to plaque formation, disturbed shear stress may lead to expression of the Notch inhibitor Delta-like 1 homolog (Dlk1), through the downregulation of miR126-5p, leading to reduced expression of HES5, a Notch-1 target gene essential for restoring the dyslipidemia-injured endothelium [257]. Noteworthy, we have recently demonstrated that heart rate reduction by ivabradine treatment induces an atheroprotective gene profile and HES5 expression in the aortic arch endothelium of apolipoprotein E-deficient (ApoE−/−) mice, which was linked to maintenance of endothelial integrity and reduction in the plaque area in their aortic root [258].
4.3. Notch in Vascular Smooth Muscle Cells
The correct morphology and functionality of VSMCs are also indispensable for guaranteeing the stability and function of adult vasculature, and in the first stages of atherosclerosis, VSMCs, switching from a contractile/quiescent to a secretory/inflammatory/migratory state, play a role in plaque formation. Many studies suggest that Notch is necessary for maintaining VSMCs in a contractile/quiescent phenotype. In rat VSMCs, the IL-1β-induced secretory/migrating phenotype is blunted by Notch-3 overexpression and enhanced by treatment with DAPT, a γ-secretase inhibitor [259] but, contrary to this finding, DAPT also seems to prevent SMC migration and proliferation induced by AngII [260].
We recently demonstrated that cholesterol-induced VSMC transdifferentiation is associated with reduced levels of Jag1 and Hey2 and increased levels of Dll-4 mRNAs [261]. In human aortic VSMCs, Notch-3 also promotes transcription of prosurvival genes, which resulted to be significantly decreased in the aortas of Notch-3−/− mice [262]. Similarly, Notch-3 knockdown by RNA interference caused VSMC to have higher proliferation, migration, and apoptosis rates, with a concomitant abnormal morphology configuration [263]. Consistently, Ragot et al. showed that the loss of the Notch-3-RBP-Jκ pathway in VSMCs led to cardiac vasculature alterations in response to AngII-induced hypertension, thus to the development of cardiac hypertrophy [264]. Reduced expression of Notch-1 and Dll-4 has been also found in the aortic wall of patients with abdominal aortic aneurysm, which was correlated to decreased VSMC content in the vessels [265]. Chen and collaborators found that Notch-1 repression is required for miR-34a-mediated VSMC proliferation and migration [266]. Redmond et al. also reported that Notch-1 activation may guide the neointimal formation and VSMC proliferation in the carotid artery ligation mouse model, phenomenon prevented by Notch-1 siRNA injected following carotid ligation [267]. In conclusion, based on the existing data, which seem to indicate an opposite effect of Notch-1 and Notch-3 in controlling VSMC activity and phenotype, the role played by Notch in this context still needs to be clarified.
4.4. Notch in Macrophages
During atherosclerotic plaque formation, intraintima macrophages respond to extracellular and intracellular signals which regulate their phenotypes, resulting in high levels of heterogeneity and plasticity among macrophage subtypes [268]. The “classical” model of macrophage activation indicates that the predominant phenotypes are characterized by a proinflammatory M1 and alternative M2 profiles [268]. Many studies in vitro clearly indicate that the Dll-4/Notch-1 axis is involved in promoting a M1 phenotype in macrophages [269–272]. Outtz et al. demonstrated that macrophages from Notch-1+/- mice displayed decreased LPS/IFNγ-mediated induction of proinflammatory IL-6, IL-12, and TNF-α compared with wild-type mice [273]. These data have been confirmed by Xu and collaborators that showed in macrophages isolated from Notch-1−/− mice a decreased basal and LPS-induced NF-κB and HIF-1α activation, indicative that induction of the M1 pathway is dependent on Notch signaling [274]. Defects in NF-κB p50 nuclear localization were observed in DAPT-treated macrophages and in RBP-Jκ-deficent macrophages, indicative of crosstalk between Notch and NF-κB pathways [275]. Lastly, DAPT treatment during MI diminished the number of macrophages in the infarcted area and significantly increased the M2 macrophage polarization [276], data resembling those obtained by Singla et al. that confirmed reduction of the proinflammatory M1 phenotype following monocyte treatment with DAPT or Notch-1 siRNA [277].
Pabois et al., by using an EC/monocyte coculture system, demonstrated that endothelial Dll-4 induces M1 polarization [278], and consistently, Koga et al. showed that Dll-4 antibody administration resulted in reduced vein graft lesion development in LDLr−/− mice and concomitantly decreased macrophage accumulation and expression of proinflammatory M1 genes [279]. Recently, the Pagie et al. study showed that Dll-4, other than promoting LPS/IFNγ-mediated M1 polarization, interferes with IL-4-mediated M2 macrophage polarization [280]. In elucidating the mechanism underlying the Notch signaling-mediated M1/M2 polarization, Lin et al. suggested that activation of Notch signaling might downregulate, through HES family corepressors, the signal regulatory protein α (SIRPα), a M2 phenotype inducer, which conversely resulted to be upregulated in RBP-Jκ-deficient bone marrow-derived macrophages [281]. Consistently, bone marrow-derived macrophages from RBP-Jκ−/− mice displayed, under LPS/IFNγ stimulation, a M2 phenotype (reduced expression of TNF-α, IL-6, and inducible-nitric oxide synthase (iNOS)), which can be reversed by transfection with miR-148a-3p mimic, a Notch-1-target miRNA which promotes M1 polarization [282]. Similarly, Miranda and collaborators, in an attempt to identify the link between the macrophage subtype and the resistance to insulin in HFD-induced obesity mouse models, found that miR-30, targeting Dll-4, is associated with obesity-induced inflammation and proinflammatory cytokine production in adipose tissue macrophages isolated from visceral fat of obese mice [283]. Specifically, they demonstrated that miR-30 inhibition is sufficient to promote the Dll-4/Notch-1 axis and proinflammatory cytokine (TNF-α and CCL2) production, this later is blunted by using anti-Dll-4 antibody. Conversely, lentiviral overexpression of miR-30 in RAW264.7 cells resulted in reduced M1 polarization and TNF-α/CCL2 production [283]. Taken together, with the previously published studies [269], these studies indicate that Dll-4/Notch-1 assume in macrophage a central function in determining the balance of M1/M2 subpopulations; thus, it could represent a valid anti-inflammatory target for limiting excessive activation of proinflammatory programs during atherosclerosis onset.
4.5. Nutraceuticals Acting through the Notch Pathway
Several authors reported that natural extracts can be used in cancer prevention; few of them focused the studies on Notch signaling, obtaining interesting results. EGCG is able to inhibit Notch signaling in BALB/c nude mice, previously injected with cancer stem cells (CSC), inhibiting tumor formation [284]. Honokiol, a phenolic compound isolated from the bark of Magnolia officinalis Rehder (Magnoliaceae), is able to counteract the growth of melanospheres formed by CSC isolated from two melanoma cell lines downregulating Notch-2 receptor, HES1, and cyclin D1 expression [285]. Withaferin-A (WA), a bioactive compound derived from Withania somnifera, inhibits Notch-1 signaling and cell proliferation in three colon cancer cell lines [286]. Two bioactive compounds (tricin and p-coumaric acid) present in leaf extract of Sasa quelpaertensis Nakai (Poaceae) are able to inhibit the growth of CSCs deriving from different colon cancer cell lines through Dll-1 and Notch-1 inhibition and downregulation of biomarkers related to tumor vascularization (VEGF and HIF-1α) [287]. Curcumin inhibits the activation of Notch-1 and the expression of Jagged-1 as well as HES1 in esophageal cancer cell lines [288]. It has also been shown that curcumin inhibits the expression of Notch-1-specific microRNAs including miR-21and miR-34a and upregulates the cancer suppressor let-7a miRNA. Moreover, Notch downstream genes are overexpressed in pancreatic cancer and curcumin induces apoptosis through reduction of the Notch-1 signaling pathway and downregulation of cyclin D1 and Bcl-xL. Curcumin downregulated the expression of Notch-1 leading to increased apoptosis and cell cycle arrest in hepatic and oral cancer cells, activating NF-κB and its target genes (Bcl-2, cyclin D1, VEGF, and MMP-9) [289]. Curcumin inhibits the DNA-binding ability of NICD in prostate cancer cells [290] and decreases also CSC markers in lymphoma/leukemia cells, at least in part through inhibiting their self-renewal [291]. Resveratrol, a natural phenol present in grape skin with known anticancer effects [292], is able to suppress proliferation and to induce a p53-mediated apoptosis in acute lymphoblastic leukemia cell via inhibition of the Notch signaling pathway [293]. These studies suggest that curcumin treatment is an attractive new strategy for several types of cancers at least in part thanks to its capability to downregulate Notch-1 signaling.
In human epidermal growth factor receptor 2- (HER-2-) positive breast cancer cells, characterized by HER2 gene amplification [294], all-trans retinoic acid (ATRA) inhibited γ-secretase and Notch-1 processing, involved in cell migration and proliferation [295]. Oroxylin A, a natural compound extracted from the root of Scutellaria baicalensis, inhibited the hypoxia-induced invasion and migration of ERα-positive breast cancer cells by suppressing the Notch processing [296]. Alpinetin can suppress the proliferation and invasiveness of gastric stem cells (GSCs) and induce apoptosis through Notch inhibition in glioma stem cells [297]. Cowanin has a strong inhibitory activity of the Notch signaling target proteins, HES1 and HES5, affecting the activity of the γ-secretase complex on several cancer cells [298].
Still, little is known about the modulation of Notch signaling by natural bioactive compounds and the consequent control of many features that characterize endothelial dysfunction. EGCG prevents the oxLDL decrease of Jagged-1 and Notch pathway-related proteins (MATH1, HES1, and HES5) and inhibits apoptosis in vitro in human vascular ECs and in ApoE−/− mice [276]. Furthermore, EGCG inhibits macrophage accumulation and inflammation response in the skin wounds of streptozotocin- (STZ-) induced DM mice at least in part through Notch signaling modulation [299]. DHA, an omega-3 fatty acid, increases Notch-3 and HES1 transcription and enhances γ-secretase complex activity, thus reducing fibrinolytic/MMP activity in transdifferentiated VSMCs toward a migrative/proliferative phenotype [300]. Norisoboldine, an alkaloid compound isolated from Radix Linderae, suppresses synovial angiogenesis, thanks to its inhibition of VEGF-induced endothelial cell migration via a cAMP-PKA-NF-κB/Notch-1 signaling pathway [301]. Both in rat aortic subintimal macrophages and in in vitro-differentiated macrophage cell diosgenin, a phytosteroid sapogenin extracted from the tubers of Dioscorea wild yam suppresses the nuclear translocation of NICD [302]. Resveratrol can attenuate neointimal VSMC hyperplasia in rats, following balloon injury, through a mechanism that involves inhibition of the Notch signaling [303]. Activation of Notch signaling reduces myocardial ischemia reperfusion injury (MI/RI), by activating key components of survival pathways, namely, PI3K-Akt, NOS, and mitochondrial K+-ATP (mitoKATP) channels [231]; BBR, polydatin, and 2,3,5,4′-tetrahydroxystilbene-2-O-β-D-glucoside upregulate Notch-1/HES1 signaling attenuating myocardial apoptosis both in cultured cardiomyocytes and in rats subjected to MI/RI [304–306].
Nutraceuticals have been studied also in the context of age-related diseases [307] and few studies reported an involvement of Notch in the effect of natural extract treatment on Alzheimer disease. Dihydroergocristine (DHEC), a component of ergoloid mesylates approved by the FDA for the treatment of hypertension and dementia, suppresses the production of Aβ peptides by inhibiting the γ-secretase complex in neurons [308]. Also, (20S)-Rg3, a triterpene natural compound known as ginsenoside and one compound (SPI-014) isolated from Actaea racemosa reduced Aβ peptide levels in neurons in vitro and in a mouse model of Alzheimer disease, at least in part decreasing the association of presenilin 1 (PS1) fragments with catalytic components of the γ-secretase complex localized in lipid rafts [309].
Indeed, the modulation of Notch signaling in several age-related diseases is an emerging approach in clinical practice and the correct use of plants could help to decrease the incidence as well as the healthcare costs of these pathologies.
5. Conclusions
The increased understanding of how diet affects disease, together with high healthcare costs and the aging population, has generated interest in food as a tool for disease prevention and health enhancement. There is growing evidence that components of food may play an integral role in the link between food and health; thus, a diet, mainly based on plant and plant-derived nutraceuticals, can contribute to reduce health care costs, while supporting economic development in rural communities.
Preclinical investigations have consistently shown that bioactive compounds, present in plants and certain foods, inhibit those biological processes linked to atherosclerosis onset. In the clinical settings, while a large number of studies have shown a close association between an imbalanced diet, with low consumption of fruits, vegetables, fibers, vitamins, and fish, and the increase risk of incidence of CVDs, other studies have failed to show results in primary, or secondary, prevention of atherosclerosis-related coronary artery disease, stroke, and heart failure trough consumption of nutraceuticals. This suggests that there is still much left to be understood about the stability of these compounds in vivo, the best route and dose of administration, and differences in response related to gender and age. Moreover, more investigations are needed to fully understand how nutraceuticals' effect varies in the presence of those medications commonly used by the subjects involved in this type of studies. This gap could be filled by a wider characterization of the molecular pathways regulated by these compounds. The Notch signaling is a well-known master regulator of embryogenesis and postnatal maintenance of self-renewing tissues, and its detrimental role when trying to achieve cancer cell apoptosis is unquestioned. To date, γ-secretase inhibitors (GSIs) are the most studied Notch-inhibiting agents which increase response to chemotherapy [310]. Furthermore, the modulation of Notch signaling by nutraceuticals to interfere with cancer progression (Table 2) is a research field that has been steadily growing in the last ten years.
Table 2.
List of nutraceuticals acting through Notch signaling modulation.
| Nutraceutical | Disease | Major findings | Role of Notch | References |
|---|---|---|---|---|
| Epigallocatechin-3-gallate (EGCG) | Cardiovascular | EGCG inhibits macrophage accumulation and inflammation response in the skin wounds of STZ-induced diabetes mellitus | EGCG reduces expression of Notch-1 and 2 in wound tissues of diabetic mice | [299] |
| In RAW 264.7, EGCG limits LPS-mediated release of proinflammatory IL-1β | EGCG reduces expression of Notch-1 and 2 and of Notch target gene HES1. EGCG binds Notch-1 and limits its activity | |||
| In HUVECs, EGCG induces expression of iNOS and eNOS and inhibits oxLDL-mediated apoptosis | EGCG restores the expression of Jagged-1 and of target proteins (MATH1, HES1, and HES5) | [276] | ||
| Jagged-1 is the key effector of EGCG-protective effect against oxLDL-induced endothelial dysfunction | ||||
| EGCG attenuates the HFD-induced accumulation of atherosclerotic plaque in ApoE-deficient mice | EGCG protects ApoE-KO mice from atherosclerosis through the Jagged-1/Notch-1 pathway | |||
| Cancer | EGCG inhibits the self-renewal capacity of head and neck squamous carcinoma (HNSC) cancer stem cells (CSCs) by suppressing their sphere forming capacity and attenuates the expression of stem cell markers. EGCG augments cisplatin-mediated chemosensitivity | EGCG decreases HNSC CSC traits by inhibiting the Notch-1 pathway | [225] | |
| Norisoboldine | Cardiovascular | Norisoboldine suppresses VEGF-induced HUVEC migration | Norisoboldine induces VEGF-mediated migration through activation of Notch-1 | [301] |
| Docosahexaenoic acid (DHA) | Cardiovascular | DHA significantly decreases VSMC migration/proliferation induced by IL-1β as well as fibrinolytic/MMP activity | DHA increases Notch-3 expression and HES1 transcription and enhances γ-secretase complex activity | [300] |
| Diosgenin | Cardiovascular | Diosgenin reduces the HFD-induced atherogenesis in rat aorta | Diosgenin prevents nuclear translocation of NICD in aorta and in differentiated macrophage cells | [302] |
| Berberine (BBR) | Cardiovascular | BBR significantly improves cardiac function recovery and decreases myocardial apoptosis, infarct size, serum creatine kinase, and lactate dehydrogenase levels in rats following myocardial IRI | Both in vitro and in vivo, BBR upregulates NICD translocation and HES1 expression | [304] |
| In H9C2, BBR attenuates simulated IRI-induced myocardial apoptosis | In vitro, antiapoptotic effect of BBR is blocked by Notch-1 or HES1 siRNA | |||
| Polydatin | Cardiovascular | Following myocardial IRI, polydatin preserves cardiac function, ameliorates myocardial oxidative/nitrative stress damage, and reduces myocardial infarct size in STZ-induced diabetic rats | Polydatin exerts cardioprotection against diabetic myocardial IRI by activating myocardial Notch-1/HES1 signaling. DAPT blunts the beneficial effects of polydatin | [305] |
| 2,3,5,4′-Tetrahydroxystilbene-2-O-β-D-glucoside (TSG) | Cardiovascular | TSG significantly improves cardiac function and suppresses IRI-induced myocardial apoptosis | Both in vitro and in vivo, TSG upregulates NICD and HES1 expression | [306] |
| In H9C2, TSG pretreatment dose-dependently decreases simulated IRI-induced apoptosis | In vitro, antiapoptotic effect of TSG is blocked by DAPT | |||
| Honokiol | Cancer | Honokiol inhibits the growth of melanospheres formed by CSC | In vitro, Honokiol downregulates Notch-2, HES1, and cyclin D1 expression | [285] |
| Withaferin-A | Cancer | In three colon cancer cell lines, Withaferin-A mediates c-Jun-NH(2)-kinase-mediated apoptosis | Withaferin-A inhibits Notch-1 signaling | [286] |
| Tricin and p-coumaric acid | Cancer | Tricin and p-coumaric acid inhibits the growth of CSCs and VEGF and HIF1α expression | Tricin and p-coumaric acid inhibits Dll-1 and Notch-1 expression | [287] |
| Curcumin | Cancer | Curcumin inhibits hepatocellular cancer cell (HCC) proliferation | Curcumin decreases NICD expression in HCC | [289] |
| Curcumin treatment results in a 40% decrease in tumor growth in a nude mouse xenograft model | ||||
| Curcumin inhibits proliferation and colony formation in esophageal cancer cell lines and upregulates expression of let-7a miRNA | Curcumin reduces Notch-1 activation and expression of Jagged-1 and HES1 | [288] | ||
| Curcumin reduces expression of Notch-1-specific microRNAs (miR-21 and miR-34a) and upregulates tumor suppressor let-7a miRNA | ||||
| Curcumin decreases markers associated with CSCs in Burkitt lymphoma and acute myeloid leukemia cells | Curcumin reduces expression of Notch-1 and cyclin D1 | [93] | ||
| Resveratrol | Cancer | Resveratrol increases apoptosis and suppresses proliferation in MOLT-4 acute lymphoblastic leukemia cells | Resveratrol reduces NICD levels in a dose-dependent manner and inhibits the expression of HES1 | [293] |
| Cardiovascular | Resveratrol inhibits phenotypic switching of neointimal VSMCs after balloon injury in rats | Resveratrol decreases Notch-1, Jagged-1, Hey1, and Hey2 mRNA in balloon-injured arteries at 7 days | [303] | |
| All-trans retinoic acid (ATRA) | Cancer | ATRA exerts a strong antimigratory action in the HER2-positive SKBR3 cell line | ATRA inhibits Notch-1 pathway | [295] |
| Oroxylin A | Cancer | Oroxylin A inhibits the hypoxia-induced invasion and migration of ERα-positive breast cancer cells | Oroxylin A inhibits NICD translocation into the nucleus | [296] |
| Alpinetin | Cancer | Alpinetin suppresses the proliferation and invasiveness of glioma stem cells (GSCs) and induces their apoptosis | Alpinetin reduces Notch-1 activity. Notch reactivation, by using recombinant Jagged-1, rescues the effect of alpinetin on GSCs | [297] |
| Cowanin | Cancer | Cowanin shows potent cytotoxicity against human leukemic HPB-ALL cells | Cowanin degrades nicastrin, a component of γ-secretase, thus hampering Notch-1 activation | [298] |
As discussed in this review, the implications of Notch in atherogenesis are wide ranging, from protection against endothelial dysfunction to the modulation of VSMCs and macrophage phenotypes, but a detailed analysis of the signaling upstream and downstream of Notch, in each cell context, has not been performed yet. Additionally, due to the distinct roles played in the above cited cell type (anti-inflammatory in endothelial cells vs proinflammatory in macrophages), targeting Notch in the context of atherosclerosis could result to be particularly challenging. There is a limited number of studies showing that some classes of nutraceutical compounds exert antiatherosclerotic activity, at least partially, through modulation of Notch signaling (Table 2). Given the important role of Notch in atherosclerosis, it could be of interest to investigate the effect on vascular Notch of widely investigated and used nutraceutical compounds, in order to gain a better understanding of their biological effects. Additionally, high-throughput analyses could be applied to investigate the effects on Notch of novel, still uncharacterized plant compounds. Based on the established role of Notch as anticancer therapy and on the emerging role of this pathway in atherosclerosis, it is not implausible to imagine a combination of specific tissue-targeted nutraceutical Notch modulators as a novel therapeutic strategy in this context.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
Authors' Contributions
Cristiana Caliceti and Paola Rizzo contributed equally to this work.
References
- 1.Reiner Z., Catapano A. L., De Backer G., et al. ESC/EAS Guidelines for the management of dyslipidaemias: the task force for the management of dyslipidaemias of the European Society of Cardiology (ESC) and the European Atherosclerosis society (EAS) European Heart Journal. 2011;32(14):1769–1818. doi: 10.1093/eurheartj/ehr158. [DOI] [PubMed] [Google Scholar]
- 2.Biesalski H. K. Nutraceuticals: the link between nutrition and medicine. Journal of Toxicology: Cutaneous and Ocular Toxicology. 2002;21(1-2):9–30. doi: 10.1081/cus-120004324. [DOI] [Google Scholar]
- 3.Gupta R. C. Nutraceuticals: Efficacy, Safety and Toxicity. Elsevier Science; 2016. [Google Scholar]
- 4.Mobin M., Tisha M. S., Sudhi T., et al. Lactobacillus plantarum 299v supplementation improves vascular endothelial function and reduces inflammatory biomarkers in men with stable coronary artery disease. Circulation Research. 2018;123(9):1091–1102. doi: 10.1161/circresaha.118.313565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rizzo P., Miele L., Ferrari R. The Notch pathway: a crossroad between the life and death of the endothelium. European Heart Journal. 2013;34(32):2504–2509. doi: 10.1093/eurheartj/ehs141. [DOI] [PubMed] [Google Scholar]
- 6.Vieceli Dalla Sega F., Aquila G., Fortini F., et al. Context-dependent function of ROS in the vascular endothelium: the role of the Notch pathway and shear stress. BioFactors. 2017;43(4):475–485. doi: 10.1002/biof.1359. [DOI] [PubMed] [Google Scholar]
- 7.Badimon L., Vilahur G. Thrombosis formation on atherosclerotic lesions and plaque rupture. Journal of Internal Medicine. 2014;276(6):618–632. doi: 10.1111/joim.12296. [DOI] [PubMed] [Google Scholar]
- 8.Libby P., Ridker P. M., Hansson G. K. Progress and challenges in translating the biology of atherosclerosis. Nature. 2011;473(7347):317–325. doi: 10.1038/nature10146. [DOI] [PubMed] [Google Scholar]
- 9.Deanfield J. E., Halcox J. P., Rabelink T. J. Endothelial function and dysfunction: testing and clinical relevance. Circulation. 2007;115(10):1285–1295. doi: 10.1161/CIRCULATIONAHA.106.652859. [DOI] [PubMed] [Google Scholar]
- 10.Mehta D., Malik A. B. Signaling mechanisms regulating endothelial permeability. Physiological Reviews. 2006;86(1):279–367. doi: 10.1152/physrev.00012.2005. [DOI] [PubMed] [Google Scholar]
- 11.Widmer R. J., Lerman A. Endothelial dysfunction and cardiovascular disease. Global Cardiology Science & Practice. 2014;2014(3):43–308. doi: 10.5339/gcsp.2014.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Williams K. J., Tabas I. The response-to-retention hypothesis of early atherogenesis. Arteriosclerosis, Thrombosis, and Vascular Biology. 1995;15(5):551–561. doi: 10.1161/01.ATV.15.5.551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cushing S. D., Berliner J. A., Valente A. J., et al. Minimally modified low density lipoprotein induces monocyte chemotactic protein 1 in human endothelial cells and smooth muscle cells. Proceedings of the National Academy of Sciences of the United States of America. 1990;87(13):5134–5138. doi: 10.1073/pnas.87.13.5134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McMurray H. F., Parthasarathy S., Steinberg D. Oxidatively modified low density lipoprotein is a chemoattractant for human T lymphocytes. The Journal of Clinical Investigation. 1993;92(2):1004–1008. doi: 10.1172/JCI116605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ley K., Miller Y. I., Hedrick C. C. Monocyte and macrophage dynamics during atherogenesis. Arteriosclerosis, Thrombosis, and Vascular Biology. 2011;31(7):1506–1516. doi: 10.1161/ATVBAHA.110.221127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jennings L. K. Mechanisms of platelet activation: need for new strategies to protect against platelet-mediated atherothrombosis. Thrombosis and Haemostasis. 2009;102(8):248–257. doi: 10.1160/TH09-03-0192. [DOI] [PubMed] [Google Scholar]
- 17.Lendon C. L., Davies M. J., Born G. V., Richardson P. D. Atherosclerotic plaque caps are locally weakened when macrophages density is increased. Atherosclerosis. 1991;87(1):87–90. doi: 10.1016/0021-9150(91)90235-U. [DOI] [PubMed] [Google Scholar]
- 18.Aikawa M., Libby P. The vulnerable atherosclerotic plaque: pathogenesis and therapeutic approach. Cardiovascular Pathology. 2004;13(3):125–138. doi: 10.1016/S1054-8807(04)00004-3. [DOI] [PubMed] [Google Scholar]
- 19.Hasler C. M. Functional foods: benefits, concerns and challenges-a position paper from the american council on science and health. The Journal of Nutrition. 2002;132(12):3772–3781. doi: 10.1093/jn/132.12.3772. [DOI] [PubMed] [Google Scholar]
- 20.Ariganjoye R. Pediatric hypovitaminosis D: molecular perspectives and clinical implications. Global Pediatric Health. 2017;4 doi: 10.1177/2333794X16685504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lin D. C. Probiotics as functional foods. Nutrition in Clinical Practice. 2003;18(6):497–506. doi: 10.1177/0115426503018006497. [DOI] [PubMed] [Google Scholar]
- 22.Al-Sheraji S. H., Ismail A., Manap M. Y., Mustafa S., Yusof R. M., Hassan F. A. Prebiotics as functional foods: a review. Journal of Functional Foods. 2013;5(4):1542–1553. doi: 10.1016/j.jff.2013.08.009. [DOI] [Google Scholar]
- 23.Sedighi M., Bahmani M., Asgary S., Beyranvand F., Rafieian-Kopaei M. A review of plant-based compounds and medicinal plants effective on atherosclerosis. Journal of Research in Medical Sciences : The Official Journal of Isfahan University of Medical Sciences. 2017;22(1):p. 30. doi: 10.4103/1735-1995.202151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gul K., Singh A. K., Jabeen R. Nutraceuticals and functional foods: the foods for the future world. Critical Reviews in Food Science and Nutrition. 2016;56(16):2617–2627. doi: 10.1080/10408398.2014.903384. [DOI] [PubMed] [Google Scholar]
- 25.Moss J. W., Ramji D. P. Nutraceutical therapies for atherosclerosis. Nature Reviews Cardiology. 2016;13(9):513–532. doi: 10.1038/nrcardio.2016.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Estruch R., Ros E., Salas-Salvadó J., et al. Primary prevention of cardiovascular disease with a Mediterranean diet. The New England Journal of Medicine. 2013;368(14):1279–1290. doi: 10.1056/NEJMoa1200303. [DOI] [PubMed] [Google Scholar]
- 27.Perdomo L., Beneit N., Otero Y. F., et al. Protective role of oleic acid against cardiovascular insulin resistance and in the early and late cellular atherosclerotic process. Cardiovascular Diabetology. 2015;14(1):p. 75. doi: 10.1186/s12933-015-0237-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Barbaro B., Toietta G., Maggio R., et al. Effects of the olive-derived polyphenol oleuropein on human health. International Journal of Molecular Sciences. 2014;15(10):18508–18524. doi: 10.3390/ijms151018508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Reboredo-Rodriguez P., Varela-Lopez A., Forbes-Hernandez T. Y., et al. Phenolic compounds isolated from olive oil as nutraceutical tools for the prevention and management of cancer and cardiovascular diseases. International Journal of Molecular Sciences. 2018;19(8) doi: 10.3390/ijms19082305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dell'Agli M., Fagnani R., Mitro N., et al. Minor components of olive oil modulate proatherogenic adhesion molecules involved in endothelial activation. Journal of Agricultural and Food Chemistry. 2006;54(9):3259–3264. doi: 10.1021/jf0529161. [DOI] [PubMed] [Google Scholar]
- 31.Rosignoli P., Fuccelli R., Fabiani R., Servili M., Morozzi G. Effect of olive oil phenols on the production of inflammatory mediators in freshly isolated human monocytes. The Journal of Nutritional Biochemistry. 2013;24(8):1513–1519. doi: 10.1016/j.jnutbio.2012.12.011. [DOI] [PubMed] [Google Scholar]
- 32.Montoya T., Aparicio-Soto M., Castejon M. L., et al. Peracetylated hydroxytyrosol, a new hydroxytyrosol derivate, attenuates LPS-induced inflammatory response in murine peritoneal macrophages via regulation of non-canonical inflammasome, Nrf2/HO1 and JAK/STAT signaling pathways. The Journal of Nutritional Biochemistry. 2018;57:110–120. doi: 10.1016/j.jnutbio.2018.03.014. [DOI] [PubMed] [Google Scholar]
- 33.Papadopoulou A., Petrotos K., Stagos D., et al. Enhancement of antioxidant mechanisms and reduction of oxidative stress in chickens after the administration of drinking water enriched with polyphenolic powder from olive mill waste waters. Oxidative Medicine and Cellular Longevity. 2017;2017:10. doi: 10.1155/2017/8273160.8273160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Storniolo C. E., Rosello-Catafau J., Pinto X., Mitjavila M. T., Moreno J. J. Polyphenol fraction of extra virgin olive oil protects against endothelial dysfunction induced by high glucose and free fatty acids through modulation of nitric oxide and endothelin-1. Redox Biology. 2014;2:971–977. doi: 10.1016/j.redox.2014.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sanchez-Rodriguez E., Lima-Cabello E., Biel-Glesson S., et al. Effects of virgin olive oils differing in their bioactive compound contents on metabolic syndrome and endothelial functional risk biomarkers in healthy adults: a randomized double-blind controlled trial. Nutrients. 2018;10(5):p. 626. doi: 10.3390/nu10050626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Farras M., Fernandez-Castillejo S., Rubio L., et al. Phenol-enriched olive oils improve HDL antioxidant content in hypercholesterolemic subjects. A randomized, double-blind, cross-over, controlled trial. The Journal of Nutritional Biochemistry. 2018;51:99–104. doi: 10.1016/j.jnutbio.2017.09.010. [DOI] [PubMed] [Google Scholar]
- 37.Pedret A., Fernandez-Castillejo S., Valls R. M., et al. Cardiovascular benefits of phenol-enriched virgin olive oils: new insights from the virgin olive oil and HDL functionality (VOHF) study. Molecular Nutrition & Food Research. 2018;62(16, article e1800456) doi: 10.1002/mnfr.201800456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Calabriso N., Gnoni A., Stanca E., et al. Hydroxytyrosol ameliorates endothelial function under inflammatory conditions by preventing mitochondrial dysfunction. Oxidative Medicine and Cellular Longevity. 2018;2018:14. doi: 10.1155/2018/9086947.9086947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wiktorowska-Owczarek A., Berezinska M., Nowak J. Z. PUFAs: structures, metabolism and functions. Advances in Clinical and Experimental Medicine. 2015;24(6):931–941. doi: 10.17219/acem/31243. [DOI] [PubMed] [Google Scholar]
- 40.Bang H. O., Dyerberg J., Sinclair H. M. The composition of the Eskimo food in north western Greenland. The American Journal of Clinical Nutrition. 1980;33(12):2657–2661. doi: 10.1093/ajcn/33.12.2657. [DOI] [PubMed] [Google Scholar]
- 41.Howe P. R. Dietary fats and hypertension focus on fish oil. Annals of the New York Academy of Sciences. 1997;827:339–352. doi: 10.1111/j.1749-6632.1997.tb51846.x. [DOI] [PubMed] [Google Scholar]
- 42.Harris W. S. Omega-3 fatty acids, thrombosis and vascular disease. International Congress Series. 2004;1262:380–383. doi: 10.1016/j.ics.2003.12.097. [DOI] [Google Scholar]
- 43.Leaf A., Kang J. X., Xiao Y. F., Billman G. E. Clinical prevention of sudden cardiac death by n-3 polyunsaturated fatty acids and mechanism of prevention of arrhythmias by n-3 fish oils. Circulation. 2003;107(21):2646–2652. doi: 10.1161/01.CIR.0000069566.78305.33. [DOI] [PubMed] [Google Scholar]
- 44.Dessi M., Noce A., Bertucci P., et al. Atherosclerosis, dyslipidemia, and inflammation: the significant role of polyunsaturated fatty acids. ISRN Inflammation. 2013;2013:13. doi: 10.1155/2013/191823.191823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gutierrez-Pliego L. E., Martinez-Carrillo B. E., Resendiz-Albor A. A., et al. Effect of supplementation with n-3 fatty acids extracted from microalgae on inflammation biomarkers from two different strains of mice. Journal of Lipids. 2018;2018:10. doi: 10.1155/2018/4765358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Poland M., Ten Klooster J. P., Wang Z., et al. Docosahexaenoyl serotonin, an endogenously formed n-3 fatty acid-serotonin conjugate has anti-inflammatory properties by attenuating IL-23-IL-17 signaling in macrophages. Biochimica et Biophysica Acta (BBA) - Molecular and Cell Biology of Lipids. 2016;1861(12, Part A):2020–2028. doi: 10.1016/j.bbalip.2016.09.012. [DOI] [PubMed] [Google Scholar]
- 47.Yamagata K. Docosahexaenoic acid regulates vascular endothelial cell function and prevents cardiovascular disease. Lipids in Health and Disease. 2017;16(1):p. 118. doi: 10.1186/s12944-017-0514-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Baker E. J., Yusof M. H., Yaqoob P., Miles E. A., Calder P. C. Omega-3 fatty acids and leukocyte-endothelium adhesion: novel anti-atherosclerotic actions. Molecular Aspects of Medicine. 2018;64:169–181. doi: 10.1016/j.mam.2018.08.002. [DOI] [PubMed] [Google Scholar]
- 49.Takashima A., Fukuda D., Tanaka K., et al. Combination of n-3 polyunsaturated fatty acids reduces atherogenesis in apolipoprotein E-deficient mice by inhibiting macrophage activation. Atherosclerosis. 2016;254:142–150. doi: 10.1016/j.atherosclerosis.2016.10.002. [DOI] [PubMed] [Google Scholar]
- 50.Ness A. R., Hughes J., Elwood P. C., Whitley E., Smith G. D., Burr M. L. The long-term effect of dietary advice in men with coronary disease: follow-up of the diet and reinfarction trial (DART) European Journal of Clinical Nutrition. 2002;56(6):512–518. doi: 10.1038/sj.ejcn.1601342. [DOI] [PubMed] [Google Scholar]
- 51.Yokoyama M., Origasa H., Matsuzaki M., et al. Effects of eicosapentaenoic acid on major coronary events in hypercholesterolaemic patients (JELIS): a randomised open-label, blinded endpoint analysis. The Lancet. 2007;369(9567):1090–1098. doi: 10.1016/S0140-6736(07)60527-3. [DOI] [PubMed] [Google Scholar]
- 52.Marchioli R., Schweiger C., Tavazzi L., Valagussa F. Efficacy of n‐3 polyunsaturated fatty acids after myocardial infarction: results of GISSI‐prevenzione trial. Lipids. 2001;36(S1):S119–S126. doi: 10.1007/s11745-001-0694-8. [DOI] [PubMed] [Google Scholar]
- 53.Bucher H. C., Hengstler P., Schindler C., Meier G. N-3 polyunsaturated fatty acids in coronary heart disease: a meta-analysis of randomized controlled trials. The American Journal of Medicine. 2002;112(4):298–304. doi: 10.1016/S0002-9343(01)01114-7. [DOI] [PubMed] [Google Scholar]
- 54.Kris-Etherton P. M., Harris W. S., Appel L. J., for the Nutrition Committee Fish consumption, fish oil, omega-3 fatty acids, and cardiovascular disease. Circulation. 2002;106(21):2747–2757. doi: 10.1161/01.CIR.0000038493.65177.94. [DOI] [PubMed] [Google Scholar]
- 55.Siscovick D. S., Barringer T. A., Fretts A. M., et al. Omega-3 polyunsaturated fatty acid (fish oil) supplementation and the prevention of clinical cardiovascular disease: a science advisory from the American Heart Association. Circulation. 2017;135(15):e867–e884. doi: 10.1161/CIR.0000000000000482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Aung T., Halsey J., Kromhout D., et al. Associations of omega-3 fatty acid supplement use with cardiovascular disease risks: meta-analysis of 10 trials involving 77 917 individuals. JAMA Cardiology. 2018;3(3):225–234. doi: 10.1001/jamacardio.2017.5205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Siniarski A., Haberka M., Mostowik M., et al. Treatment with omega-3 polyunsaturated fatty acids does not improve endothelial function in patients with type 2 diabetes and very high cardiovascular risk: a randomized, double-blind, placebo-controlled study (omega-FMD) Atherosclerosis. 2018;271:148–155. doi: 10.1016/j.atherosclerosis.2018.02.030. [DOI] [PubMed] [Google Scholar]
- 58.The ASCEND Study Collaborative Group. Effects of n-3 fatty acid supplements in diabetes mellitus. The New England Journal of Medicine. 2018;379(16):1540–1550. doi: 10.1056/nejmoa1804989. [DOI] [PubMed] [Google Scholar]
- 59.Piepoli M. F., Hoes A. W., Agewall S., et al. 2016 European Guidelines on cardiovascular disease prevention in clinical practice: The Sixth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (constituted by representatives of 10 societies and by invited experts) developed with the special contribution of the European Association for Cardiovascular Prevention & Rehabilitation (EACPR) European Heart Journal. 2016;37(29):2315–2381. doi: 10.1093/eurheartj/ehw106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bhatt D. L., Steg P. G., Miller M., et al. Cardiovascular risk reduction with icosapent ethyl for hypertriglyceridemia. The New England Journal of Medicine. 2019;380(1):11–22. doi: 10.1056/nejmoa1812792. [DOI] [PubMed] [Google Scholar]
- 61.Budoff M., Brent Muhlestein J., Le V. T., May H. T., Roy S., Nelson J. R. Effect of VASCEPA (icosapent ethyl) on progression of coronary atherosclerosis in patients with elevated triglycerides (200-499 mg/dL) on statin therapy: rationale and design of the EVAPORATE study. Clinical Cardiology. 2018;41(1):13–19. doi: 10.1002/clc.22856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bassuk S. S., Manson J. E., Lee I. M., et al. Baseline characteristics of participants in the VITamin D and OmegA-3 TriaL (VITAL) Contemporary Clinical Trials. 2016;47:235–243. doi: 10.1016/j.cct.2015.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Grassi D., Desideri G., Ferri C. Flavonoids: antioxidants against atherosclerosis. Nutrients. 2010;2(8):889–902. doi: 10.3390/nu2080889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Scalbert A., Manach C., Morand C., Remesy C., Jimenez L. Dietary polyphenols and the prevention of diseases. Critical Reviews in Food Science and Nutrition. 2005;45(4):287–306. doi: 10.1080/1040869059096. [DOI] [PubMed] [Google Scholar]
- 65.Scalbert A., Williamson G. Dietary intake and bioavailability of polyphenols. The Journal of Nutrition. 2000;130(8):2073S–2085S. doi: 10.1093/jn/130.8.2073S. [DOI] [PubMed] [Google Scholar]
- 66.Kumar S., Pandey A. K. Chemistry and biological activities of flavonoids: an overview. ScientificWorldJournal. 2013;2013, article 162750:16. doi: 10.1155/2013/162750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bieganska-Hensoldt S., Rosolowska-Huszcz D. Polyphenols in preventing endothelial dysfunction. Postȩpy Higieny i Medycyny Doświadczalnej (Online) 2017;71:227–235. doi: 10.5604/01.3001.0010.3808. [DOI] [PubMed] [Google Scholar]
- 68.Millar C. L., Duclos Q., Blesso C. N. Effects of dietary flavonoids on reverse cholesterol transport, HDL metabolism, and HDL function. Advances in Nutrition. 2017;8(2):226–239. doi: 10.3945/an.116.014050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Siasos G., Tousoulis D., Tsigkou V., et al. Flavonoids in atherosclerosis: an overview of their mechanisms of action. Current Medicinal Chemistry. 2013;20(21):2641–2660. doi: 10.2174/0929867311320210003. [DOI] [PubMed] [Google Scholar]
- 70.Panche A. N., Diwan A. D., Chandra S. R. Flavonoids: an overview. Journal of Nutritional Science. 2016;5, article e47 doi: 10.1017/jns.2016.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Pourcel L., Routaboul J. M., Cheynier V., Lepiniec L., Debeaujon I. Flavonoid oxidation in plants: from biochemical properties to physiological functions. Trends in Plant Science. 2007;12(1):29–36. doi: 10.1016/j.tplants.2006.11.006. [DOI] [PubMed] [Google Scholar]
- 72.Davinelli S., Corbi G., Zarrelli A., et al. Short-term supplementation with flavanol-rich cocoa improves lipid profile, antioxidant status and positively influences the AA/EPA ratio in healthy subjects. The Journal of Nutritional Biochemistry. 2018;61:33–39. doi: 10.1016/j.jnutbio.2018.07.011. [DOI] [PubMed] [Google Scholar]
- 73.Esser D., Geleijnse J. M., Matualatupauw J. C., et al. Pure flavonoid epicatechin and whole genome gene expression profiles in circulating immune cells in adults with elevated blood pressure: a randomised double-blind, placebo-controlled, crossover trial. PLoS One. 2018;13(4, article e0194229) doi: 10.1371/journal.pone.0194229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Grassi D., Aggio A., Onori L., et al. Tea, flavonoids, and nitric oxide-mediated vascular reactivity. The Journal of Nutrition. 2008;138(8):1554S–1560S. doi: 10.1093/jn/138.8.1554S. [DOI] [PubMed] [Google Scholar]
- 75.Grassi D., Desideri G., Croce G., Tiberti S., Aggio A., Ferri C. Flavonoids, vascular function and cardiovascular protection. Current Pharmaceutical Design. 2009;15(10):1072–1084. doi: 10.2174/138161209787846982. [DOI] [PubMed] [Google Scholar]
- 76.Grassi D., Desideri G., Necozione S., et al. Cocoa consumption dose-dependently improves flow-mediated dilation and arterial stiffness decreasing blood pressure in healthy individuals. Journal of Hypertension. 2015;33(2):294–303. doi: 10.1097/HJH.0000000000000412. [DOI] [PubMed] [Google Scholar]
- 77.Zhou X., Liang L., Zhao Y., Zhang H. Epigallocatechin-3-gallate ameliorates angiotensin II-induced oxidative stress and apoptosis in human umbilical vein endothelial cells through the activation of Nrf2/caspase-3 signaling. Journal of Vascular Research. 2017;54(5):299–308. doi: 10.1159/000479873. [DOI] [PubMed] [Google Scholar]
- 78.Liu P. L., Liu J. T., Kuo H. F., Chong I. W., Hsieh C. C. Epigallocatechin gallate attenuates proliferation and oxidative stress in human vascular smooth muscle cells induced by interleukin-1β via heme oxygenase-1. Mediators of Inflammation. 2014;2014:8. doi: 10.1155/2014/523684.523684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Li Y. F., Wang H., Fan Y., et al. Epigallocatechin-3-gallate inhibits matrix metalloproteinase-9 and monocyte chemotactic protein-1 expression through the 67-κDa laminin receptor and the TLR4/MAPK/NF-κB signalling pathway in lipopolysaccharide-induced macrophages. Cellular Physiology and Biochemistry. 2017;43(3):926–936. doi: 10.1159/000481643. [DOI] [PubMed] [Google Scholar]
- 80.Chen S. J., Kao Y. H., Jing L., et al. Epigallocatechin-3-gallate reduces scavenger receptor A expression and foam cell formation in human macrophages. Journal of Agricultural and Food Chemistry. 2017;65(15):3141–3150. doi: 10.1021/acs.jafc.6b05832. [DOI] [PubMed] [Google Scholar]
- 81.Hayek T., Fuhrman B., Vaya J., et al. Reduced progression of atherosclerosis in apolipoprotein E–deficient mice following consumption of red wine, or its polyphenols quercetin or catechin, is associated with reduced susceptibility of LDL to oxidation and aggregation. Arteriosclerosis, Thrombosis, and Vascular Biology. 1997;17(11):2744–2752. doi: 10.1161/01.ATV.17.11.2744. [DOI] [PubMed] [Google Scholar]
- 82.Anandh Babu P. V., Sabitha K. E., Shyamaladevi C. S. Green tea extract impedes dyslipidaemia and development of cardiac dysfunction in streptozotocin-diabetic rats. Clinical and Experimental Pharmacology and Physiology. 2006;33(12):1184–1189. doi: 10.1111/j.1440-1681.2006.04509.x. [DOI] [PubMed] [Google Scholar]
- 83.Chang H. H., Chien C. Y., Chen K. H., Huang S. C., Chien C. T. Catechins blunt the effects of oxLDL and its primary metabolite phosphatidylcholine hydroperoxide on endothelial dysfunction through inhibition of oxidative stress and restoration of eNOS in rats. Kidney & Blood Pressure Research. 2017;42(5):919–932. doi: 10.1159/000485082. [DOI] [PubMed] [Google Scholar]
- 84.Bhaskar S., Kumar K. S., Krishnan K., Antony H. Quercetin alleviates hypercholesterolemic diet induced inflammation during progression and regression of atherosclerosis in rabbits. Nutrition. 2013;29(1):219–229. doi: 10.1016/j.nut.2012.01.019. [DOI] [PubMed] [Google Scholar]
- 85.Phie J., Krishna S. M., Moxon J. V., Omer S. M., Kinobe R., Golledge J. Flavonols reduce aortic atherosclerosis lesion area in apolipoprotein E deficient mice: a systematic review and meta-analysis. PLoS One. 2017;12(7, article e0181832) doi: 10.1371/journal.pone.0181832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Lodi F., Jimenez R., Moreno L., et al. Glucuronidated and sulfated metabolites of the flavonoid quercetin prevent endothelial dysfunction but lack direct vasorelaxant effects in rat aorta. Atherosclerosis. 2009;204(1):34–39. doi: 10.1016/j.atherosclerosis.2008.08.007. [DOI] [PubMed] [Google Scholar]
- 87.Shen Y., Ward N. C., Hodgson J. M., et al. Dietary quercetin attenuates oxidant-induced endothelial dysfunction and atherosclerosis in apolipoprotein E knockout mice fed a high-fat diet: a critical role for heme oxygenase-1. Free Radical Biology and Medicine. 2013;65:908–915. doi: 10.1016/j.freeradbiomed.2013.08.185. [DOI] [PubMed] [Google Scholar]
- 88.Hung C. H., Chan S. H., Chu P. M., Tsai K. L. Quercetin is a potent anti-atherosclerotic compound by activation of SIRT1 signaling under oxLDL stimulation. Molecular Nutrition & Food Research. 2015;59(10):1905–1917. doi: 10.1002/mnfr.201500144. [DOI] [PubMed] [Google Scholar]
- 89.Indra M. R., Karyono S., Ratnawati R., Malik S. G. Quercetin suppresses inflammation by reducing ERK1/2 phosphorylation and NF kappa B activation in leptin-induced human umbilical vein endothelial cells (HUVECs) BMC Research Notes. 2013;6(1):p. 275. doi: 10.1186/1756-0500-6-275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Ishizawa K., Yoshizumi M., Kawai Y., et al. Pharmacology in health food: metabolism of quercetin in vivo and its protective effect against arteriosclerosis. Journal of Pharmacological Sciences. 2011;115(4):466–470. doi: 10.1254/jphs.10R38FM. [DOI] [PubMed] [Google Scholar]
- 91.Kim S. G., Kim J. R., Choi H. C. Quercetin-induced AMP-activated protein kinase activation attenuates vasoconstriction through LKB1-AMPK signaling pathway. Journal of Medicinal Food. 2018;21(2):146–153. doi: 10.1089/jmf.2017.4052. [DOI] [PubMed] [Google Scholar]
- 92.Liang Q., Chen Y., Li C., Lu L. Quercetin attenuates Ox-LDL-induced calcification in vascular smooth muscle cells by regulating ROS-TLR4 signaling pathway. Nan Fang Yi Ke Da Xue Xue Bao. 2018;38(8):980–985. doi: 10.3969/j.issn.1673-4254.2018.08.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Li S., Cao H., Shen D., Jia Q., Chen C., Xing S. L. Quercetin protects against ox-LDL-induced injury via regulation of ABCAl, LXR-α and PCSK9 in RAW264.7 macrophages. Molecular Medicine Reports. 2018;18(1):799–806. doi: 10.3892/mmr.2018.9048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Xiao L., Liu L., Guo X., et al. Quercetin attenuates high fat diet-induced atherosclerosis in apolipoprotein E knockout mice: a critical role of NADPH oxidase. Food and Chemical Toxicology. 2017;105:22–33. doi: 10.1016/j.fct.2017.03.048. [DOI] [PubMed] [Google Scholar]
- 95.Pereira S. C., Parente J. M., Belo V. A., et al. Quercetin decreases the activity of matrix metalloproteinase-2 and ameliorates vascular remodeling in renovascular hypertension. Atherosclerosis. 2018;270:146–153. doi: 10.1016/j.atherosclerosis.2018.01.031. [DOI] [PubMed] [Google Scholar]
- 96.Derlindati E., Dall'Asta M., Ardigo D., et al. Quercetin-3-O-glucuronide affects the gene expression profile of M1 and M2a human macrophages exhibiting anti-inflammatory effects. Food & Function. 2012;3(11):1144–1152. doi: 10.1039/c2fo30127j. [DOI] [PubMed] [Google Scholar]
- 97.Liu Y., Cao Y., Fang S., et al. Antidiabetic effect of Cyclocarya paliurus leaves depends on the contents of Antihyperglycemic flavonoids and antihyperlipidemic triterpenoids. Molecules. 2018;23(5, article 1042) doi: 10.3390/molecules23051042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Ohara K., Wakabayashi H., Taniguchi Y., Shindo K., Yajima H., Yoshida A. Quercetin-3-O-glucuronide induces ABCA1 expression by LXRα activation in murine macrophages. Biochemical and Biophysical Research Communications. 2013;441(4):929–934. doi: 10.1016/j.bbrc.2013.10.168. [DOI] [PubMed] [Google Scholar]
- 99.Hertog M. G., Feskens E. J., Hollman P. C., Katan M. B., Kromhout D. Dietary antioxidant flavonoids and risk of coronary heart disease: the Zutphen Elderly Study. The Lancet. 1993;342(8878):1007–1011. doi: 10.1016/0140-6736(93)92876-U. [DOI] [PubMed] [Google Scholar]
- 100.Geleijnse J. M., Launer L. J., Van der Kuip D. A., Hofman A., Witteman J. C. Inverse association of tea and flavonoid intakes with incident myocardial infarction: the Rotterdam Study. The American Journal of Clinical Nutrition. 2002;75(5):880–886. doi: 10.1093/ajcn/75.5.880. [DOI] [PubMed] [Google Scholar]
- 101.Hertog M. G., Sweetnam P. M., Fehily A. M., Elwood P. C., Kromhout D. Antioxidant flavonols and ischemic heart disease in a Welsh population of men: the Caerphilly Study. The American Journal of Clinical Nutrition. 1997;65(5):1489–1494. doi: 10.1093/ajcn/65.5.1489. [DOI] [PubMed] [Google Scholar]
- 102.Grobbee D. E., Rimm E. B., Giovannucci E., Colditz G., Stampfer M., Willett W. Coffee, caffeine, and cardiovascular disease in men. The New England Journal of Medicine. 1990;323(15):1026–1032. doi: 10.1056/nejm199010113231504. [DOI] [PubMed] [Google Scholar]
- 103.Dower J. I., Geleijnse J. M., Gijsbers L., Zock P. L., Kromhout D., Hollman P. C. Effects of the pure flavonoids epicatechin and quercetin on vascular function and cardiometabolic health: a randomized, double-blind, placebo-controlled, crossover trial. The American Journal of Clinical Nutrition. 2015;101(5):914–921. doi: 10.3945/ajcn.114.098590. [DOI] [PubMed] [Google Scholar]
- 104.Walker A. R., Arvidsson U. B. Fat intake, serum cholesterol concentration, and atherosclerosis in the South African Bantu. I. Low fat intake and the age trend of serum cholesterol concentration in the South African Bantu. Journal of Clinical Investigation. 1954;33(10):1358–1365. doi: 10.1172/JCI103012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Higginson J., Pepler W. J. Fat intake, serum cholesterol concentration, and atherosclerosis in the South African Bantu. Part II. Atherosclerosis and coronary artery disease. Journal of Clinical Investigation. 1954;33(10):1366–1371. doi: 10.1172/JCI103013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Burkitt D. P., Walker A. R., Painter N. S. Effect of dietary fibre on stools and transit-times, and its role in the causation of disease. The Lancet. 1972;300(7792):1408–1411. doi: 10.1016/S0140-6736(72)92974-1. [DOI] [PubMed] [Google Scholar]
- 107.Trowell H. Dietary fibre, ischaemic heart disease and diabetes mellitus. The Proceedings of the Nutrition Society. 1973;32(3):151–157. doi: 10.1079/PNS19730033. [DOI] [PubMed] [Google Scholar]
- 108.McRae M. P. Dietary fiber is beneficial for the prevention of cardiovascular disease: an umbrella review of meta-analyses. Journal of Chiropractic Medicine. 2017;16(4):289–299. doi: 10.1016/j.jcm.2017.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Liu L., Wang S., Liu J. Fiber consumption and all-cause, cardiovascular, and cancer mortalities: a systematic review and meta-analysis of cohort studies. Molecular Nutrition & Food Research. 2015;59(1):139–146. doi: 10.1002/mnfr.201400449. [DOI] [PubMed] [Google Scholar]
- 110.Juhel C., Tosini F., Steib M., et al. Cholesterol-lowering effect of non-viscous soluble dietary fiber Nutriose6 in moderately hypercholesterolemic hamsters. Indian Journal of Experimental Biology. 2011;49(3):219–228. [PubMed] [Google Scholar]
- 111.Lundin E. A., Zhang J. X., Lairon D., et al. Effects of meal frequency and high-fibre rye-bread diet on glucose and lipid metabolism and ileal excretion of energy and sterols in ileostomy subjects. European Journal of Clinical Nutrition. 2004;58(10):1410–1419. doi: 10.1038/sj.ejcn.1601985. [DOI] [PubMed] [Google Scholar]
- 112.Weickert M. O., Pfeiffer A. F. H. Impact of dietary fiber consumption on insulin resistance and the prevention of type 2 diabetes. The Journal of Nutrition. 2018;148(1):7–12. doi: 10.1093/jn/nxx008. [DOI] [PubMed] [Google Scholar]
- 113.Ohira H., Tsutsui W., Fujioka Y. Are short chain fatty acids in gut microbiota defensive players for inflammation and atherosclerosis? Journal of Atherosclerosis and Thrombosis. 2017;24(7):660–672. doi: 10.5551/jat.RV17006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Pons D., de Vries F. R., van den Elsen P. J., Heijmans B. T., Quax P. H., Jukema J. W. Epigenetic histone acetylation modifiers in vascular remodelling: new targets for therapy in cardiovascular disease. European Heart Journal. 2009;30(3):266–277. doi: 10.1093/eurheartj/ehn603. [DOI] [PubMed] [Google Scholar]
- 115.Zheng X.-X., Zhou T., Wang X.-A., Tong X.-H., Ding J.-W. Histone deacetylases and atherosclerosis. Atherosclerosis. 2015;240(2):355–366. doi: 10.1016/j.atherosclerosis.2014.12.048. [DOI] [PubMed] [Google Scholar]
- 116.Poulsen L. C., Edelmann R. J., Kruger S., et al. Inhibition of endothelial NOTCH1 signaling attenuates inflammation by reducing cytokine-mediated histone acetylation at inflammatory enhancers. Arteriosclerosis, Thrombosis, and Vascular Biology. 2018;38(4):854–869. doi: 10.1161/ATVBAHA.117.310388. [DOI] [PubMed] [Google Scholar]
- 117.Yuan X., Wang L., Bhat O. M., Lohner H., Li P. L. Differential effects of short chain fatty acids on endothelial Nlrp3 inflammasome activation and neointima formation: antioxidant action of butyrate. Redox Biology. 2018;16:21–31. doi: 10.1016/j.redox.2018.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Chen Y., Xu C., Huang R., Song J., Li D., Xia M. Butyrate from pectin fermentation inhibits intestinal cholesterol absorption and attenuates atherosclerosis in apolipoprotein E-deficient mice. The Journal of Nutritional Biochemistry. 2018;56:175–182. doi: 10.1016/j.jnutbio.2018.02.011. [DOI] [PubMed] [Google Scholar]
- 119.Yu X. H., Qian K., Jiang N., Zheng X. L., Cayabyab F. S., Tang C. K. ABCG5/ABCG8 in cholesterol excretion and atherosclerosis. Clinica Chimica Acta. 2014;428:82–88. doi: 10.1016/j.cca.2013.11.010. [DOI] [PubMed] [Google Scholar]
- 120.Jessup W., Kritharides L., Stocker R. Lipid oxidation in atherogenesis: an overview. Biochemical Society Transactions. 2004;32(1):134–138. doi: 10.1042/bst0320134. [DOI] [PubMed] [Google Scholar]
- 121.Steinberg D., Parthasarathy S., Carew T. E., Khoo J. C., Witztum J. L. Beyond cholesterol. The New England Journal of Medicine. 1989;320(14):915–924. doi: 10.1056/NEJM198904063201407. [DOI] [PubMed] [Google Scholar]
- 122.May J. M., Harrison F. E. Role of vitamin C in the function of the vascular endothelium. Antioxidants & Redox Signaling. 2013;19(17):2068–2083. doi: 10.1089/ars.2013.5205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Rashidi B., Hoseini Z., Sahebkar A., Mirzaei H. Anti-atherosclerotic effects of vitamins D and E in suppression of atherogenesis. Journal of Cellular Physiology. 2017;232(11):2968–2976. doi: 10.1002/jcp.25738. [DOI] [PubMed] [Google Scholar]
- 124.Ulker E., Parker W. H., Raj A., Qu Z. C., May J. M. Ascorbic acid prevents VEGF-induced increases in endothelial barrier permeability. Molecular and Cellular Biochemistry. 2016;412(1-2):73–79. doi: 10.1007/s11010-015-2609-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Siti H. N., Kamisah Y., Kamsiah J. The role of oxidative stress, antioxidants and vascular inflammation in cardiovascular disease (a review) Vascular Pharmacology. 2015;71:40–56. doi: 10.1016/j.vph.2015.03.005. [DOI] [PubMed] [Google Scholar]
- 126.Ozkanlar S., Akcay F. Antioxidant vitamins in atherosclerosis--animal experiments and clinical studies. Advances in Clinical and Experimental Medicine. 2012;21(1):115–123. [PubMed] [Google Scholar]
- 127.Tang F., Lu M., Zhang S., et al. Vitamin E conditionally inhibits atherosclerosis in ApoE knockout mice by anti-oxidation and regulation of vasculature gene expressions. Lipids. 2014;49(12):1215–1223. doi: 10.1007/s11745-014-3962-z. [DOI] [PubMed] [Google Scholar]
- 128.Libinaki R., Tesanovic S., Heal A., et al. Effect of tocopheryl phosphate on key biomarkers of inflammation: implication in the reduction of atherosclerosis progression in a hypercholesterolaemic rabbit model. Clinical and Experimental Pharmacology & Physiology. 2010;37(5-6):587–592. doi: 10.1111/j.1440-1681.2010.05356.x. [DOI] [PubMed] [Google Scholar]
- 129.Libinaki R., Vinh A., Tesanovic-Klajic S., Widdop R., Gaspari T. The effect of tocopheryl phosphates (TPM) on the development of atherosclerosis in apolipoprotein-E deficient mice. Clinical and Experimental Pharmacology & Physiology. 2017;44(Supplement 1):107–116. doi: 10.1111/1440-1681.12821. [DOI] [PubMed] [Google Scholar]
- 130.Terasawa Y., Ladha Z., Leonard S. W., et al. Increased atherosclerosis in hyperlipidemic mice deficient in α-tocopherol transfer protein and vitamin E. Proceedings of the National Academy of Sciences of the United States of America. 2000;97(25):13830–13834. doi: 10.1073/pnas.240462697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.d'Uscio L. V., Milstien S., Richardson D., Smith L., Katusic Z. S. Long-term vitamin C treatment increases vascular tetrahydrobiopterin levels and nitric oxide synthase activity. Circulation Research. 2003;92(1):88–95. doi: 10.1161/01.RES.0000049166.33035.62. [DOI] [PubMed] [Google Scholar]
- 132.Cha J., Niedzwiecki A., Rath M. Hypoascorbemia induces atherosclerosis and vascular deposition of lipoprotein(a) in transgenic mice. American Journal of Cardiovascular Disease. 2015;5(1):53–62. [PMC free article] [PubMed] [Google Scholar]
- 133.Crawford R. S., Kirk E. A., Rosenfeld M. E., LeBoeuf R. C., Chait A. Dietary antioxidants inhibit development of fatty streak lesions in the LDL receptor–deficient mouse. Arteriosclerosis, Thrombosis, and Vascular Biology. 1998;18(9):1506–1513. doi: 10.1161/01.ATV.18.9.1506. [DOI] [PubMed] [Google Scholar]
- 134.Nespereira B., Perez-Ilzarbe M., Fernandez P., Fuentes A. M., Paramo J. A., Rodriguez J. A. Vitamins C and E downregulate vascular VEGF and VEGFR-2 expression in apolipoprotein-E-deficient mice. Atherosclerosis. 2003;171(1):67–73. doi: 10.1016/j.atherosclerosis.2003.08.009. [DOI] [PubMed] [Google Scholar]
- 135.Contreras-Duarte S., Chen P., Andia M., et al. Attenuation of atherogenic apo B-48-dependent hyperlipidemia and high density lipoprotein remodeling induced by vitamin C and E combination and their beneficial effect on lethal ischemic heart disease in mice. Biological Research. 2018;51(1):p. 34. doi: 10.1186/s40659-018-0183-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Menezes A. R., Lamb M. C., Lavie C. J., DiNicolantonio J. J. Vitamin D and atherosclerosis. Current Opinion in Cardiology. 2014;29(6):571–577. doi: 10.1097/HCO.0000000000000108. [DOI] [PubMed] [Google Scholar]
- 137.Wakino S., Kintscher U., Kim S., et al. Retinoids inhibit proliferation of human coronary smooth muscle cells by modulating cell cycle regulators. Arteriosclerosis, Thrombosis, and Vascular Biology. 2001;21(5):746–751. doi: 10.1161/01.ATV.21.5.746. [DOI] [PubMed] [Google Scholar]
- 138.Zhou W., Lin J., Chen H., Wang J., Liu Y., Xia M. Retinoic acid induces macrophage cholesterol efflux and inhibits atherosclerotic plaque formation in ApoE-deficient mice. The British Journal of Nutrition. 2015;114(4):509–518. doi: 10.1017/S0007114515002159. [DOI] [PubMed] [Google Scholar]
- 139.Relevy N. Z., Harats D., Harari A., et al. Vitamin A-deficient diet accelerated atherogenesis in apolipoprotein E−/− mice and dietary β-carotene prevents this consequence. BioMed Research International. 2015;2015:9. doi: 10.1155/2015/758723.758723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Tao L., Nie Y., Wang G., et al. All‑trans retinoic acid reduces endothelin‑1 expression and increases endothelial nitric oxide synthase phosphorylation in rabbits with atherosclerosis. Molecular Medicine Reports. 2018;17(2):2619–2625. doi: 10.3892/mmr.2017.8156. [DOI] [PubMed] [Google Scholar]
- 141.Wu Y., Wang X., Zhou Q., et al. ATRA improves endothelial dysfunction in atherosclerotic rabbits by decreasing CAV‑1 expression and enhancing eNOS activity. Molecular Medicine Reports. 2018;17(5):6796–6802. doi: 10.3892/mmr.2018.8647. [DOI] [PubMed] [Google Scholar]
- 142.Zarei L., Bahrami M., Farhad N., Froushani S. M. A., Abbasi A. All-trans retinoic acid effectively reduces atheroma plaque size in a rabbit model of high-fat-induced atherosclerosis. Advances in Clinical and Experimental Medicine. 2018;27(12):1631–1636. doi: 10.17219/acem/74552. [DOI] [PubMed] [Google Scholar]
- 143.Zhou B., Pan Y., Hu Z., et al. All-trans-retinoic acid ameliorated high fat diet-induced atherosclerosis in rabbits by inhibiting platelet activation and inflammation. Journal of Biomedicine & Biotechnology. 2012;2012:9. doi: 10.1155/2012/259693.259693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Alkhatatbeh M. J., Abdul-Razzak K. K., Khasawneh L. Q., Saadeh N. A. High prevalence of vitamin D deficiency and correlation of serum vitamin D with cardiovascular risk in patients with metabolic syndrome. Metabolic Syndrome and Related Disorders. 2017;15(5):213–219. doi: 10.1089/met.2017.0003. [DOI] [PubMed] [Google Scholar]
- 145.Anderson J. L., May H. T., Horne B. D., et al. Relation of vitamin D deficiency to cardiovascular risk factors, disease status, and incident events in a general healthcare population. The American Journal of Cardiology. 2010;106(7):963–968. doi: 10.1016/j.amjcard.2010.05.027. [DOI] [PubMed] [Google Scholar]
- 146.Khaw K. T., Bingham S., Welch A., et al. Relation between plasma ascorbic acid and mortality in men and women in EPIC-Norfolk prospective study: a prospective population study. The Lancet. 2001;357(9257):657–663. doi: 10.1016/S0140-6736(00)04128-3. [DOI] [PubMed] [Google Scholar]
- 147.Kushi L. H., Folsom A. R., Prineas R. J., Mink P. J., Wu Y., Bostick R. M. Dietary antioxidant vitamins and death from coronary heart disease in postmenopausal women. The New England Journal of Medicine. 1996;334(18):1156–1162. doi: 10.1056/NEJM199605023341803. [DOI] [PubMed] [Google Scholar]
- 148.Martins D., Wolf M., Pan D., et al. Prevalence of cardiovascular risk factors and the serum levels of 25-hydroxyvitamin D in the United States: data from the Third National Health and Nutrition Examination Survey. Archives of Internal Medicine. 2007;167(11):1159–1165. doi: 10.1001/archinte.167.11.1159. [DOI] [PubMed] [Google Scholar]
- 149.Matilainen T., Vartiainen E., Puska P., et al. Plasma ascorbic acid concentrations in the Republic of Karelia, Russia and in North Karelia, Finland. European Journal of Clinical Nutrition. 1996;50(2):115–120. [PubMed] [Google Scholar]
- 150.Pfister R., Sharp S. J., Luben R., Wareham N. J., Khaw K. T. Plasma vitamin C predicts incident heart failure in men and women in European prospective investigation into cancer and nutrition-Norfolk prospective study. American Heart Journal. 2011;162(2):246–253. doi: 10.1016/j.ahj.2011.05.007. [DOI] [PubMed] [Google Scholar]
- 151.Rimm E. B., Stampfer M. J., Ascherio A., Giovannucci E., Colditz G. A., Willett W. C. Vitamin E consumption and the risk of coronary heart disease in men. The New England Journal of Medicine. 1993;328(20):1450–1456. doi: 10.1056/NEJM199305203282004. [DOI] [PubMed] [Google Scholar]
- 152.Stampfer M. J., Hennekens C. H., Manson J. E., Colditz G. A., Rosner B., Willett W. C. Vitamin E consumption and the risk of coronary disease in women. The New England Journal of Medicine. 1993;328(20):1444–1449. doi: 10.1056/NEJM199305203282003. [DOI] [PubMed] [Google Scholar]
- 153.Salonen J. T., Nyyssonen K., Salonen R., et al. Antioxidant Supplementation in Atherosclerosis Prevention (ASAP) study: a randomized trial of the effect of vitamins E and C on 3-year progression of carotid atherosclerosis. Journal of Internal Medicine. 2000;248(5):377–386. doi: 10.1046/j.1365-2796.2000.00752.x. [DOI] [PubMed] [Google Scholar]
- 154.Salonen R. M., Nyyssonen K., Kaikkonen J., et al. Six-year effect of combined vitamin C and E supplementation on atherosclerotic progression: the Antioxidant Supplementation in Atherosclerosis Prevention (ASAP) study. Circulation. 2003;107(7):947–953. doi: 10.1161/01.CIR.0000050626.25057.51. [DOI] [PubMed] [Google Scholar]
- 155.Lee I. M., Cook N. R., Gaziano J. M., et al. Vitamin E in the primary prevention of cardiovascular disease and cancer: the Women’s Health Study: a randomized controlled trial. JAMA. 2005;294(1):56–65. doi: 10.1001/jama.294.1.56. [DOI] [PubMed] [Google Scholar]
- 156.Heart Protection Study Collaborative Group. MRC/BHF Heart Protection Study of antioxidant vitamin supplementation in 20 536 high-risk individuals: a randomised placebo-controlled trial. The Lancet. 2002;360(9326):23–33. doi: 10.1016/S0140-6736(02)09328-5. [DOI] [PubMed] [Google Scholar]
- 157.Hodis H. N., Mack W. J., LaBree L., et al. Alpha-tocopherol supplementation in healthy individuals reduces low-density lipoprotein oxidation but not atherosclerosis: the Vitamin E Atherosclerosis Prevention Study (VEAPS) Circulation. 2002;106(12):1453–1459. doi: 10.1161/01.CIR.0000029092.99946.08. [DOI] [PubMed] [Google Scholar]
- 158.Heart Outcomes Prevention Evaluation Study. Vitamin E supplementation and cardiovascular events in high-risk patients. The New England Journal of Medicine. 2000;342(3):154–160. doi: 10.1056/NEJM200001203420302. [DOI] [PubMed] [Google Scholar]
- 159.Zureik M., Galan P., Bertrais S., et al. Effects of long-term daily low-dose supplementation with antioxidant vitamins and minerals on structure and function of large arteries. Arteriosclerosis, Thrombosis, and Vascular Biology. 2004;24(8):1485–1491. doi: 10.1161/01.ATV.0000136648.62973.c8. [DOI] [PubMed] [Google Scholar]
- 160.Elamin M. B., Abu Elnour N. O., Elamin K. B., et al. Vitamin D and cardiovascular outcomes: a systematic review and meta-analysis. The Journal of Clinical Endocrinology and Metabolism. 2011;96(7):1931–1942. doi: 10.1210/jc.2011-0398. [DOI] [PubMed] [Google Scholar]
- 161.Moser M. A., Chun O. K. Vitamin C and heart health: A review based on findings from epidemiologic studies. International Journal of Molecular Sciences. 2016;17(8) doi: 10.3390/ijms17081328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Omenn G. S., Goodman G. E., Thornquist M. D., et al. Effects of a combination of beta carotene and vitamin A on lung cancer and cardiovascular disease. The New England Journal of Medicine. 1996;334(18):1150–1155. doi: 10.1056/NEJM199605023341802. [DOI] [PubMed] [Google Scholar]
- 163.Goodman G. E., Thornquist M. D., Balmes J., et al. The beta-carotene and retinol efficacy trial: incidence of lung cancer and cardiovascular disease mortality during 6-year follow-up after stopping β-carotene and retinol supplements. Journal of the National Cancer Institute. 2004;96(23):1743–1750. doi: 10.1093/jnci/djh320. [DOI] [PubMed] [Google Scholar]
- 164.Kim J., Choi J., Kwon S. Y., et al. Association of multivitamin and mineral supplementation and risk of cardiovascular disease: a systematic review and meta-analysis. Circulation: Cardiovascular Quality and Outcomes. 2018;11(7) doi: 10.1161/circoutcomes.117.004224. [DOI] [PubMed] [Google Scholar]
- 165.Moyer V. A., on behalf of the US Preventive Services Task Force Vitamin, mineral, and multivitamin supplements for the primary prevention of cardiovascular disease and cancer: U.S. Preventive services Task Force recommendation statement. Annals of Internal Medicine. 2014;160(8):558–564. doi: 10.7326/M14-0198. [DOI] [PubMed] [Google Scholar]
- 166.Caliceti C., Franco P., Spinozzi S., Roda A., Cicero A. F. Berberine: new insights from pharmacological aspects to clinical evidences in the management of metabolic disorders. Current Medicinal Chemistry. 2016;23(14):1460–1476. doi: 10.2174/0929867323666160411143314. [DOI] [PubMed] [Google Scholar]
- 167.Spinozzi S., Colliva C., Camborata C., et al. Berberine and its metabolites: relationship between physicochemical properties and plasma levels after administration to human subjects. Journal of Natural Products. 2014;77(4):766–772. doi: 10.1021/np400607k. [DOI] [PubMed] [Google Scholar]
- 168.Caliceti C., Rizzo P., Cicero A. F. Potential benefits of berberine in the management of perimenopausal syndrome. Oxidative Medicine and Cellular Longevity. 2015;2015:9. doi: 10.1155/2015/723093.723093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Dong B., Li H., Singh A. B., Cao A., Liu J. Inhibition of PCSK9 transcription by berberine involves down-regulation of hepatic HNF1α protein expression through the ubiquitin-proteasome degradation pathway. Journal of Biological Chemistry. 2015;290(7):4047–4058. doi: 10.1074/jbc.M114.597229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Abd El-Wahab A. E., Ghareeb D. A., Sarhan E. E., Abu-Serie M. M., El Demellawy M. A. In vitro biological assessment of Berberis vulgaris and its active constituent, berberine: antioxidants, anti-acetylcholinesterase, anti-diabetic and anticancer effects. BMC Complementary and Alternative Medicine. 2013;13(1):p. 218. doi: 10.1186/1472-6882-13-218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Caliceti C., Rizzo P., Ferrari R., et al. Novel role of the nutraceutical bioactive compound berberine in lectin-like OxLDL receptor 1-mediated endothelial dysfunction in comparison to lovastatin. Nutrition, Metabolism, and Cardiovascular Diseases. 2017;27(6):552–563. doi: 10.1016/j.numecd.2017.04.002. [DOI] [PubMed] [Google Scholar]
- 172.Abidi P., Zhou Y., Jiang J. D., Liu J. Extracellular signal-regulated kinase–dependent stabilization of hepatic low-density lipoprotein receptor mRNA by herbal medicine berberine. Arteriosclerosis, Thrombosis, and Vascular Biology. 2005;25(10):2170–2176. doi: 10.1161/01.ATV.0000181761.16341.2b. [DOI] [PubMed] [Google Scholar]
- 173.Porru E., Franco P., Calabria D., et al. Combined analytical approaches to define biodistribution and biological activity of semi-synthetic berberrubine, the active metabolite of natural berberine. Analytical and Bioanalytical Chemistry. 2018;410(15):3533–3545. doi: 10.1007/s00216-018-0884-2. [DOI] [PubMed] [Google Scholar]
- 174.Fraser P. D., Bramley P. M. The biosynthesis and nutritional uses of carotenoids. Progress in Lipid Research. 2004;43(3):228–265. doi: 10.1016/j.plipres.2003.10.002. [DOI] [PubMed] [Google Scholar]
- 175.Lu S., Li L. Carotenoid metabolism: biosynthesis, regulation, and beyond. Journal of Integrative Plant Biology. 2008;50(7):778–785. doi: 10.1111/j.1744-7909.2008.00708.x. [DOI] [PubMed] [Google Scholar]
- 176.Hadley C. W., Miller E. C., Schwartz S. J., Clinton S. K. Tomatoes, lycopene, and prostate cancer: progress and promise. Experimental Biology and Medicine. 2002;227(10):869–880. doi: 10.1177/153537020222701006. [DOI] [PubMed] [Google Scholar]
- 177.Krinsky N. I., Landrum J. T., Bone R. A. Biologic mechanisms of the protective role of lutein and zeaxanthin in the eye. Annual Review of Nutrition. 2003;23(1):171–201. doi: 10.1146/annurev.nutr.23.011702.073307. [DOI] [PubMed] [Google Scholar]
- 178.Riccioni G., Speranza L., Pesce M., Cusenza S., D'Orazio N., Glade M. J. Novel phytonutrient contributors to antioxidant protection against cardiovascular disease. Nutrition. 2012;28(6):605–610. doi: 10.1016/j.nut.2011.11.028. [DOI] [PubMed] [Google Scholar]
- 179.Ciccone M. M., Cortese F., Gesualdo M., et al. Dietary intake of carotenoids and their antioxidant and anti-inflammatory effects in cardiovascular care. Mediators of Inflammation. 2013;2013:11. doi: 10.1155/2013/782137.782137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Ried K., Fakler P. Protective effect of lycopene on serum cholesterol and blood pressure: meta-analyses of intervention trials. Maturitas. 2011;68(4):299–310. doi: 10.1016/j.maturitas.2010.11.018. [DOI] [PubMed] [Google Scholar]
- 181.Wolak T., Paran E. Can carotenoids attenuate vascular aging? Vascular Pharmacology. 2013;59(3-4):63–66. doi: 10.1016/j.vph.2013.07.006. [DOI] [PubMed] [Google Scholar]
- 182.Ma J., Li Y., Ye Q., et al. Constituents of red yeast rice, a traditional Chinese food and medicine. Journal of Agricultural and Food Chemistry. 2000;48(11):5220–5225. doi: 10.1021/jf000338c. [DOI] [PubMed] [Google Scholar]
- 183.Musselman M. E., Pettit R. S., Derenski K. L. A review and update of red yeast rice. Journal of Evidence-Based Complementary & Alternative Medicine. 2012;17(1):33–39. doi: 10.1177/2156587211429703. [DOI] [Google Scholar]
- 184.Heber D., Yip I., Ashley J. M., Elashoff D. A., Elashoff R. M., Go V. L. Cholesterol-lowering effects of a proprietary Chinese red-yeast-rice dietary supplement. The American Journal of Clinical Nutrition. 1999;69(2):231–236. doi: 10.1093/ajcn/69.2.231. [DOI] [PubMed] [Google Scholar]
- 185.Lin C. C., Li T. C., Lai M. M. Efficacy and safety of Monascus purpureus Went rice in subjects with hyperlipidemia. European Journal of Endocrinology. 2005;153(5):679–686. doi: 10.1530/eje.1.02012. [DOI] [PubMed] [Google Scholar]
- 186.Becker D. J., Gordon R. Y., Morris P. B., et al. Simvastatin vs therapeutic lifestyle changes and supplements: randomized primary prevention trial. Mayo Clinic Proceedings. 2008;83(7):758–764. doi: 10.4065/83.7.758. [DOI] [PubMed] [Google Scholar]
- 187.Fortini C., Cesselli D., Beltrami A. P., et al. Alteration of Notch signaling and functionality of adipose tissue derived mesenchymal stem cells in heart failure. International Journal of Cardiology. 2014;174(1):119–126. doi: 10.1016/j.ijcard.2014.03.173. [DOI] [PubMed] [Google Scholar]
- 188.Venero C. V., Venero J. V., Wortham D. C., Thompson P. D. Lipid-lowering efficacy of red yeast rice in a population intolerant to statins. The American Journal of Cardiology. 2010;105(5):664–666. doi: 10.1016/j.amjcard.2009.10.045. [DOI] [PubMed] [Google Scholar]
- 189.Lu Z., Kou W., Du B., et al. Effect of Xuezhikang, an extract from red yeast Chinese rice, on coronary events in a Chinese population with previous myocardial infarction. The American Journal of Cardiology. 2008;101(12):1689–1693. doi: 10.1016/j.amjcard.2008.02.056. [DOI] [PubMed] [Google Scholar]
- 190.Sirtori C. R., Galli C., Anderson J. W., Arnoldi A. Nutritional and nutraceutical approaches to dyslipidemia and atherosclerosis prevention: focus on dietary proteins. Atherosclerosis. 2009;203(1):8–17. doi: 10.1016/j.atherosclerosis.2008.06.019. [DOI] [PubMed] [Google Scholar]
- 191.Banach M., Patti A. M., Giglio R. V., et al. The role of nutraceuticals in statin intolerant patients. Journal of the American College of Cardiology. 2018;72(1):96–118. doi: 10.1016/j.jacc.2018.04.040. [DOI] [PubMed] [Google Scholar]
- 192.Burke F. M. Red yeast rice for the treatment of dyslipidemia. Current Atherosclerosis Reports. 2015;17(4):p. 495. doi: 10.1007/s11883-015-0495-8. [DOI] [PubMed] [Google Scholar]
- 193.Childress L., Gay A., Zargar A., Ito M. K. Review of red yeast rice content and current Food and Drug Administration oversight. Journal of Clinical Lipidology. 2013;7(2):117–122. doi: 10.1016/j.jacl.2012.09.003. [DOI] [PubMed] [Google Scholar]
- 194.Borlinghaus J., Albrecht F., Gruhlke M. C., Nwachukwu I. D., Slusarenko A. J. Allicin: chemistry and biological properties. Molecules. 2014;19(8):12591–12618. doi: 10.3390/molecules190812591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Ried K., Fakler P. Potential of garlic (Allium sativum) in lowering high blood pressure: mechanisms of action and clinical relevance. Integrated Blood Pressure Control. 2014;7:71–82. doi: 10.2147/IBPC.S51434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Weiss N., Papatheodorou L., Morihara N., Hilge R., Ide N. Aged garlic extract restores nitric oxide bioavailability in cultured human endothelial cells even under conditions of homocysteine elevation. Journal of Ethnopharmacology. 2013;145(1):162–167. doi: 10.1016/j.jep.2012.10.045. [DOI] [PubMed] [Google Scholar]
- 197.Muda P., Kampus P., Zilmer M., et al. Homocysteine and red blood cell glutathione as indices for middle-aged untreated essential hypertension patients. Journal of Hypertension. 2003;21(12):2329–2333. doi: 10.1097/00004872-200312000-00022. [DOI] [PubMed] [Google Scholar]
- 198.Yeh Y. Y., Yeh S. M. Homocysteine-lowering action is another potential cardiovascular protective factor of aged garlic extract. The Journal of Nutrition. 2006;136(3):745S–749S. doi: 10.1093/jn/136.3.745S. [DOI] [PubMed] [Google Scholar]
- 199.Budoff M. J., Takasu J., Flores F. R., et al. Inhibiting progression of coronary calcification using aged garlic extract in patients receiving statin therapy: a preliminary study. Preventive Medicine. 2004;39(5):985–991. doi: 10.1016/j.ypmed.2004.04.012. [DOI] [PubMed] [Google Scholar]
- 200.Ried K., Frank O. R., Stocks N. P. Aged garlic extract lowers blood pressure in patients with treated but uncontrolled hypertension: a randomised controlled trial. Maturitas. 2010;67(2):144–150. doi: 10.1016/j.maturitas.2010.06.001. [DOI] [PubMed] [Google Scholar]
- 201.Benavides G. A., Squadrito G. L., Mills R. W., et al. Hydrogen sulfide mediates the vasoactivity of garlic. Proceedings of the National Academy of Sciences of the United States of America. 2007;104(46):17977–17982. doi: 10.1073/pnas.0705710104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Jiang B., Tang G., Cao K., Wu L., Wang R. Molecular mechanism for H2S-induced activation of KATP channels. Antioxidants & Redox Signaling. 2010;12(10):1167–1178. doi: 10.1089/ars.2009.2894. [DOI] [PubMed] [Google Scholar]
- 203.Bradley J. M., Organ C. L., Lefer D. J. Garlic-derived organic polysulfides and myocardial protection. The Journal of Nutrition. 2016;146(2):403S–409S. doi: 10.3945/jn.114.208066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Focke M., Feld A., Lichtenthaler K. Allicin, a naturally occurring antibiotic from garlic, specifically inhibits acetyl-CoA synthetase. FEBS Letters. 1990;261(1):106–108. doi: 10.1016/0014-5793(90)80647-2. [DOI] [PubMed] [Google Scholar]
- 205.Gebhardt R., Beck H., Wagner K. G. Inhibition of cholesterol biosynthesis by allicin and ajoene in rat hepatocytes and HepG2 cells. Biochimica et Biophysica Acta. 1994;1213(1):57–62. doi: 10.1016/0005-2760(94)90222-4. [DOI] [PubMed] [Google Scholar]
- 206.Gupta N., Porter T. D. Garlic and garlic-derived compounds inhibit human squalene monooxygenase. The Journal of Nutrition. 2001;131(6):1662–1667. doi: 10.1093/jn/131.6.1662. [DOI] [PubMed] [Google Scholar]
- 207.Yun H. M., Ban J. O., Park K. R., et al. Potential therapeutic effects of functionally active compounds isolated from garlic. Pharmacology & Therapeutics. 2014;142(2):183–195. doi: 10.1016/j.pharmthera.2013.12.005. [DOI] [PubMed] [Google Scholar]
- 208.Strimpakos A. S., Sharma R. A. Curcumin: preventive and therapeutic properties in laboratory studies and clinical trials. Antioxidants & Redox Signaling. 2008;10(3):511–546. doi: 10.1089/ars.2007.1769. [DOI] [PubMed] [Google Scholar]
- 209.Li C., Miao X., Li F., et al. Curcuminoids: implication for inflammation and oxidative stress in cardiovascular diseases. Phytotherapy Research. 2019 doi: 10.1002/ptr.6324. [DOI] [PubMed] [Google Scholar]
- 210.Cicero A. F. G., Sahebkar A., Fogacci F., Bove M., Giovannini M., Borghi C. Effects of phytosomal curcumin on anthropometric parameters, insulin resistance, cortisolemia and non-alcoholic fatty liver disease indices: a double-blind, placebo-controlled clinical trial. European Journal of Nutrition. 2019 doi: 10.1007/s00394-019-01916-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Li H., Sureda A., Devkota H. P., et al. Curcumin, the golden spice in treating cardiovascular diseases. Biotechnology Advances. 2019 doi: 10.1016/j.biotechadv.2019.01.010. In press. [DOI] [PubMed] [Google Scholar]
- 212.Micucci M., Aldini R., Cevenini M., et al. Curcuma longa L. as a therapeutic agent in intestinal motility disorders. 2: safety profile in mouse. PLoS One. 2013;8(11, article e80925) doi: 10.1371/journal.pone.0080925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Panahi Y., Ahmadi Y., Teymouri M., Johnston T. P., Sahebkar A. Curcumin as a potential candidate for treating hyperlipidemia: a review of cellular and metabolic mechanisms. Journal of Cellular Physiology. 2018;233(1):141–152. doi: 10.1002/jcp.25756. [DOI] [PubMed] [Google Scholar]
- 214.Ouyang S., Yao Y. H., Zhang Z. M., Liu J. S., Xiang H. Curcumin inhibits hypoxia inducible factor-1α-induced inflammation and apoptosis in macrophages through an ERK dependent pathway. European Review for Medical and Pharmacological Sciences. 2019;23(4):1816–1825. doi: 10.26355/eurrev_201902_17145. [DOI] [PubMed] [Google Scholar]
- 215.Chuengsamarn S., Rattanamongkolgul S., Phonrat B., Tungtrongchitr R., Jirawatnotai S. Reduction of atherogenic risk in patients with type 2 diabetes by curcuminoid extract: a randomized controlled trial. The Journal of Nutritional Biochemistry. 2014;25(2):144–150. doi: 10.1016/j.jnutbio.2013.09.013. [DOI] [PubMed] [Google Scholar]
- 216.Akazawa N., Choi Y., Miyaki A., et al. Curcumin ingestion and exercise training improve vascular endothelial function in postmenopausal women. Nutrition Research. 2012;32(10):795–799. doi: 10.1016/j.nutres.2012.09.002. [DOI] [PubMed] [Google Scholar]
- 217.Takebe N., Miele L., Harris P. J., et al. Targeting Notch, Hedgehog, and Wnt pathways in cancer stem cells: clinical update. Nature Reviews Clinical Oncology. 2015;12(8):445–464. doi: 10.1038/nrclinonc.2015.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Moloney D. J., Panin V. M., Johnston S. H., et al. Fringe is a glycosyltransferase that modifies Notch. Nature. 2000;406(6794):369–375. doi: 10.1038/35019000. [DOI] [PubMed] [Google Scholar]
- 219.Suckling R. J., Korona B., Whiteman P., et al. Structural and functional dissection of the interplay between lipid and Notch binding by human Notch ligands. The EMBO Journal. 2017;36(15):2204–2215. doi: 10.15252/embj.201796632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Musse A. A., Meloty-Kapella L., Weinmaster G. Notch ligand endocytosis: mechanistic basis of signaling activity. Seminars in Cell & Developmental Biology. 2012;23(4):429–436. doi: 10.1016/j.semcdb.2012.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Kopan R. Notch signaling. Cold Spring Harbor Perspectives in Biology. 2012;4(10) doi: 10.1101/cshperspect.a011213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Borggrefe T., Oswald F. The Notch signaling pathway: transcriptional regulation at Notch target genes. Cellular and Molecular Life Sciences. 2009;66(10):1631–1646. doi: 10.1007/s00018-009-8668-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Ayaz F., Osborne B. A. Non-canonical Notch signaling in cancer and immunity. Frontiers in Oncology. 2014;4:p. 345. doi: 10.3389/fonc.2014.00345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Andersen P., Uosaki H., Shenje L. T., Kwon C. Non-canonical Notch signaling: emerging role and mechanism. Trends in Cell Biology. 2012;22(5):257–265. doi: 10.1016/j.tcb.2012.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Lee K. S., Wu Z., Song Y., et al. Roles of PINK1, mTORC2, and mitochondria in preserving brain tumor-forming stem cells in a noncanonical Notch signaling pathway. Genes & Development. 2013;27(24):2642–2647. doi: 10.1101/gad.225169.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Fortini F., Vieceli Dalla Sega F., Caliceti C., et al. Estrogen receptor β–dependent Notch1 activation protects vascular endothelium against tumor necrosis factor α (TNFα)-induced apoptosis. Journal of Biological Chemistry. 2017;292(44):18178–18191. doi: 10.1074/jbc.M117.790121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Li J. L., Harris A. L. Crosstalk of VEGF and Notch pathways in tumour angiogenesis: therapeutic implications. Frontiers in Bioscience. 2009;14:3094–3110. doi: 10.2741/3438. [DOI] [PubMed] [Google Scholar]
- 228.Lou Y. L., Guo F., Liu F., et al. miR-210 activates notch signaling pathway in angiogenesis induced by cerebral ischemia. Molecular and Cellular Biochemistry. 2012;370(1-2):45–51. doi: 10.1007/s11010-012-1396-6. [DOI] [PubMed] [Google Scholar]
- 229.Osipo C., Golde T. E., Osborne B. A., Miele L. A. Off the beaten pathway: the complex cross talk between Notch and NF-κB. Laboratory Investigation. 2008;88(1):11–17. doi: 10.1038/labinvest.3700700. [DOI] [PubMed] [Google Scholar]
- 230.Osipo C., Patel P., Rizzo P., et al. ErbB-2 inhibition activates Notch-1 and sensitizes breast cancer cells to a γ-secretase inhibitor. Oncogene. 2008;27(37):5019–5032. doi: 10.1038/onc.2008.149. [DOI] [PubMed] [Google Scholar]
- 231.Rocca C., Femmino S., Aquila G., et al. Notch1 mediates preconditioning protection induced by GPER in normotensive and hypertensive female rat hearts. Frontiers in Physiology. 2018;9:p. 521. doi: 10.3389/fphys.2018.00521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Lehoux S., Jones E. A. Shear stress, arterial identity and atherosclerosis. Thrombosis and Haemostasis. 2016;115(03):467–473. doi: 10.1160/TH15-10-0791. [DOI] [PubMed] [Google Scholar]
- 233.Krebs L. T., Xue Y., Norton C. R., et al. Notch signaling is essential for vascular morphogenesis in mice. Genes & Development. 2000;14(11):1343–1352. [PMC free article] [PubMed] [Google Scholar]
- 234.Limbourg F. P., Takeshita K., Radtke F., Bronson R. T., Chin M. T., Liao J. K. Essential role of endothelial Notch1 in angiogenesis. Circulation. 2005;111(14):1826–1832. doi: 10.1161/01.CIR.0000160870.93058.DD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Uyttendaele H., Ho J., Rossant J., Kitajewski J. Vascular patterning defects associated with expression of activated Notch4 in embryonic endothelium. Proceedings of the National Academy of Sciences of the United States of America. 2001;98(10):5643–5648. doi: 10.1073/pnas.091584598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Koo B. K., Lim H. S., Song R., et al. Mind bomb 1 is essential for generating functional Notch ligands to activate Notch. Development. 2005;132(15):3459–3470. doi: 10.1242/dev.01922. [DOI] [PubMed] [Google Scholar]
- 237.Krebs L. T., Shutter J. R., Tanigaki K., Honjo T., Stark K. L., Gridley T. Haploinsufficient lethality and formation of arteriovenous malformations in Notch pathway mutants. Genes & Development. 2004;18(20):2469–2473. doi: 10.1101/gad.1239204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Fischer A., Schumacher N., Maier M., Sendtner M., Gessler M. The Notch target genes Hey1 and Hey2 are required for embryonic vascular development. Genes & Development. 2004;18(8):901–911. doi: 10.1101/gad.291004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Gale N. W., Dominguez M. G., Noguera I., et al. Haploinsufficiency of delta-like 4 ligand results in embryonic lethality due to major defects in arterial and vascular development. Proceedings of the National Academy of Sciences of the United States of America. 2004;101(45):15949–15954. doi: 10.1073/pnas.0407290101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Trindade A., Kumar S. R., Scehnet J. S., et al. Overexpression of delta-like 4 induces arterialization and attenuates vessel formation in developing mouse embryos. Blood. 2008;112(5):1720–1729. doi: 10.1182/blood-2007-09-112748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Xue Y., Gao X., Lindsell C. E., et al. Embryonic lethality and vascular defects in mice lacking the Notch ligand Jagged1. Human Molecular Genetics. 1999;8(5):723–730. doi: 10.1093/hmg/8.5.723. [DOI] [PubMed] [Google Scholar]
- 242.Lindner V., Booth C., Prudovsky I., Small D., Maciag T., Liaw L. Members of the Jagged/Notch gene families are expressed in injured arteries and regulate cell phenotype via alterations in cell matrix and cell-cell interaction. The American Journal of Pathology. 2001;159(3):875–883. doi: 10.1016/S0002-9440(10)61763-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Caliceti C., Aquila G., Pannella M., et al. 17β-estradiol enhances signalling mediated by VEGF-A-delta-like ligand 4-notch1 axis in human endothelial cells. PLoS One. 2013;8(8, article e71440) doi: 10.1371/journal.pone.0071440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Jakobsson L., Bentley K., Gerhardt H. VEGFRs and Notch: a dynamic collaboration in vascular patterning. Biochemical Society Transactions. 2009;37(6):1233–1236. doi: 10.1042/BST0371233. [DOI] [PubMed] [Google Scholar]
- 245.Liu Z. J., Tan Y., Beecham G. W., et al. Notch activation induces endothelial cell senescence and pro-inflammatory response: implication of Notch signaling in atherosclerosis. Atherosclerosis. 2012;225(2):296–303. doi: 10.1016/j.atherosclerosis.2012.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Nus M., Martinez-Poveda B., MacGrogan D., et al. Endothelial Jag1-RBPJ signalling promotes inflammatory leucocyte recruitment and atherosclerosis. Cardiovascular Research. 2016;112(2):568–580. doi: 10.1093/cvr/cvw193. [DOI] [PubMed] [Google Scholar]
- 247.Verginelli F., Adesso L., Limon I., et al. Activation of an endothelial Notch1-Jagged1 circuit induces VCAM1 expression, an effect amplified by interleukin-1β. Oncotarget. 2015;6(41):43216–43229. doi: 10.18632/oncotarget.6456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Quillard T., Devalliere J., Chatelais M., et al. Notch2 signaling sensitizes endothelial cells to apoptosis by negatively regulating the key protective molecule survivin. PLoS One. 2009;4(12, article e8244) doi: 10.1371/journal.pone.0008244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Quillard T., Devalliere J., Coupel S., Charreau B. Inflammation dysregulates Notch signaling in endothelial cells: implication of Notch2 and Notch4 to endothelial dysfunction. Biochemical Pharmacology. 2010;80(12):2032–2041. doi: 10.1016/j.bcp.2010.07.010. [DOI] [PubMed] [Google Scholar]
- 250.Briot A., Civelek M., Seki A., et al. Endothelial NOTCH1 is suppressed by circulating lipids and antagonizes inflammation during atherosclerosis. The Journal of Experimental Medicine. 2015;212(12):2147–2163. doi: 10.1084/jem.20150603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Wang L., Zhang H., Rodriguez S., et al. Notch-dependent repression of miR-155 in the bone marrow niche regulates hematopoiesis in an NF-κB-dependent manner. Cell Stem Cell. 2014;15(1):51–65. doi: 10.1016/j.stem.2014.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Nus M., MacGrogan D., Martinez-Poveda B., et al. Diet-induced aortic valve disease in mice haploinsufficient for the Notch pathway effector RBPJK/CSL. Arteriosclerosis, Thrombosis, and Vascular Biology. 2011;31(7):1580–1588. doi: 10.1161/ATVBAHA.111.227561. [DOI] [PubMed] [Google Scholar]
- 253.Theodoris C. V., Li M., White M. P., et al. Human disease modeling reveals integrated transcriptional and epigenetic mechanisms of NOTCH1 haploinsufficiency. Cell. 2015;160(6):1072–1086. doi: 10.1016/j.cell.2015.02.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Masumura T., Yamamoto K., Shimizu N., Obi S., Ando J. Shear stress increases expression of the arterial endothelial marker ephrinB2 in murine ES cells via the VEGF-Notch signaling pathways. Arteriosclerosis, Thrombosis, and Vascular Biology. 2009;29(12):2125–2131. doi: 10.1161/ATVBAHA.109.193185. [DOI] [PubMed] [Google Scholar]
- 255.Mack J. J., Mosqueiro T. S., Archer B. J., et al. NOTCH1 is a mechanosensor in adult arteries. Nature Communications. 2017;8(1):p. 1620. doi: 10.1038/s41467-017-01741-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Polacheck W. J., Kutys M. L., Yang J., et al. A non-canonical Notch complex regulates adherens junctions and vascular barrier function. Nature. 2017;552(7684):258–262. doi: 10.1038/nature24998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Schober A., Nazari-Jahantigh M., Wei Y., et al. MicroRNA-126-5p promotes endothelial proliferation and limits atherosclerosis by suppressing Dlk1. Nature Medicine. 2014;20(4):368–376. doi: 10.1038/nm.3487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Aquila G., Morelli M. B., Vieceli Dalla Sega F., et al. Heart rate reduction with ivabradine in the early phase of atherosclerosis is protective in the endothelium of ApoE-deficient mice. Journal of Physiology and Pharmacology. 2018;69(1):35–52. doi: 10.26402/jpp.2018.1.04. [DOI] [PubMed] [Google Scholar]
- 259.Clement N., Gueguen M., Glorian M., et al. Notch3 and IL-1β exert opposing effects on a vascular smooth muscle cell inflammatory pathway in which NF-κB drives crosstalk. Journal of Cell Science. 2007;120(19):3352–3361. doi: 10.1242/jcs.007872. [DOI] [PubMed] [Google Scholar]
- 260.Wu J. R., Yeh J. L., Liou S. F., Dai Z. K., Wu B. N., Hsu J. H. Gamma-secretase inhibitor prevents proliferation and migration of ductus arteriosus smooth muscle cells through the Notch3-HES1/2/5 pathway. International Journal of Biological Sciences. 2016;12(9):1063–1073. doi: 10.7150/ijbs.16430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Aquila G., Fortini C., Pannuti A., et al. Distinct gene expression profiles associated with Notch ligands Delta-like 4 and Jagged1 in plaque material from peripheral artery disease patients: a pilot study. Journal of Translational Medicine. 2017;15(1):p. 98. doi: 10.1186/s12967-017-1199-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Baeten J. T., Lilly B. Differential regulation of NOTCH2 and NOTCH3 contribute to their unique functions in vascular smooth muscle cells. The Journal of Biological Chemistry. 2015;290(26):16226–16237. doi: 10.1074/jbc.M115.655548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Liu N., Li Y., Chen H., Wei W., An Y., Zhu G. RNA interference-mediated NOTCH3 knockdown induces phenotype switching of vascular smooth muscle cells in vitro. International Journal of Clinical and Experimental Medicine. 2015;8(8):12674–12684. [PMC free article] [PubMed] [Google Scholar]
- 264.Ragot H., Monfort A., Baudet M., et al. Loss of Notch3 signaling in vascular smooth muscle cells promotes severe heart failure upon hypertension. Hypertension. 2016;68(2):392–400. doi: 10.1161/HYPERTENSIONAHA.116.07694. [DOI] [PubMed] [Google Scholar]
- 265.Doyle A. J., Redmond E. M., Gillespie D. L., et al. Differential expression of Hedgehog/Notch and transforming growth factor-β in human abdominal aortic aneurysms. Journal of Vascular Surgery. 2015;62(2):464–470. doi: 10.1016/j.jvs.2014.02.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Chen Q., Yang F., Guo M., et al. miRNA-34a reduces neointima formation through inhibiting smooth muscle cell proliferation and migration. Journal of Molecular and Cellular Cardiology. 2015;89, Part A:75–86. doi: 10.1016/j.yjmcc.2015.10.017. [DOI] [PubMed] [Google Scholar]
- 267.Redmond E. M., Liu W., Hamm K., Hatch E., Cahill P. A., Morrow D. Perivascular delivery of Notch 1 siRNA inhibits injury-induced arterial remodeling. PLoS One. 2014;9(1, article e84122) doi: 10.1371/journal.pone.0084122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Bobryshev Y. V., Ivanova E. A., Chistiakov D. A., Nikiforov N. G., Orekhov A. N. Macrophages and their role in atherosclerosis: pathophysiology and transcriptome analysis. BioMed Research International. 2016;2016:13. doi: 10.1155/2016/9582430.9582430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Fukuda D., Aikawa E., Swirski F. K., et al. Notch ligand delta-like 4 blockade attenuates atherosclerosis and metabolic disorders. Proceedings of the National Academy of Sciences of the United States of America. 2012;109(27):E1868–E1877. doi: 10.1073/pnas.1116889109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Fung E., Tang S. M., Canner J. P., et al. Delta-like 4 induces notch signaling in macrophages: implications for inflammation. Circulation. 2007;115(23):2948–2956. doi: 10.1161/CIRCULATIONAHA.106.675462. [DOI] [PubMed] [Google Scholar]
- 271.Monsalve E., Perez M. A., Rubio A., et al. Notch-1 up-regulation and signaling following macrophage activation modulates gene expression patterns known to affect antigen-presenting capacity and cytotoxic activity. The Journal of Immunology. 2006;176(9):5362–5373. doi: 10.4049/jimmunol.176.9.5362. [DOI] [PubMed] [Google Scholar]
- 272.Monsalve E., Ruiz-Garcia A., Baladron V., et al. Notch1 upregulates LPS-induced macrophage activation by increasing NF-κB activity. European Journal of Immunology. 2009;39(9):2556–2570. doi: 10.1002/eji.200838722. [DOI] [PubMed] [Google Scholar]
- 273.Outtz H. H., Wu J. K., Wang X., Kitajewski J. Notch1 deficiency results in decreased inflammation during wound healing and regulates vascular endothelial growth factor receptor-1 and inflammatory cytokine expression in macrophages. The Journal of Immunology. 2010;185(7):4363–4373. doi: 10.4049/jimmunol.1000720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Xu J., Chi F., Guo T., et al. NOTCH reprograms mitochondrial metabolism for proinflammatory macrophage activation. The Journal of Clinical Investigation. 2015;125(4):1579–1590. doi: 10.1172/JCI76468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Wongchana W., Kongkavitoon P., Tangtanatakul P., et al. Notch signaling regulates the responses of lipopolysaccharide-stimulated macrophages in the presence of immune complexes. PLoS One. 2018;13(6, article e0198609) doi: 10.1371/journal.pone.0198609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Yin J., Huang F., Yi Y., Yin L., Peng D. EGCG attenuates atherosclerosis through the Jagged-1/Notch pathway. International Journal of Molecular Medicine. 2016;37(2):398–406. doi: 10.3892/ijmm.2015.2422. [DOI] [PubMed] [Google Scholar]
- 277.Singla D. K., Wang J., Singla R. Primary human monocytes differentiate into M2 macrophages and involve Notch-1 pathway. Canadian Journal of Physiology and Pharmacology. 2017;95(3):288–294. doi: 10.1139/cjpp-2016-0319. [DOI] [PubMed] [Google Scholar]
- 278.Pabois A., Pagie S., Gerard N., et al. Notch signaling mediates crosstalk between endothelial cells and macrophages via Dll4 and IL6 in cardiac microvascular inflammation. Biochemical Pharmacology. 2016;104:95–107. doi: 10.1016/j.bcp.2016.01.016. [DOI] [PubMed] [Google Scholar]
- 279.Koga J. I., Nakano T., Dahlman J. E., et al. Macrophage Notch Ligand Delta-like 4 promotes vein graft lesion development: implications for the treatment of vein graft failure. Arteriosclerosis, Thrombosis, and Vascular Biology. 2015;35(11):2343–2353. doi: 10.1161/ATVBAHA.115.305516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Pagie S., Gerard N., Charreau B. Notch signaling triggered via the ligand DLL4 impedes M2 macrophage differentiation and promotes their apoptosis. Cell Communication and Signaling: CCS. 2018;16(1):p. 4. doi: 10.1186/s12964-017-0214-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Lin Y., Zhao J. L., Zheng Q. J., et al. Notch signaling modulates macrophage polarization and phagocytosis through direct suppression of signal regulatory protein α expression. Frontiers in Immunology. 2018;9:p. 1744. doi: 10.3389/fimmu.2018.01744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Huang F., Zhao J. L., Wang L., et al. miR-148a-3p mediates notch signaling to promote the differentiation and M1 activation of macrophages. Frontiers in Immunology. 2017;8, article 1327 doi: 10.3389/fimmu.2017.01327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Miranda K., Yang X., Bam M., Murphy E. A., Nagarkatti P. S., Nagarkatti M. MicroRNA-30 modulates metabolic inflammation by regulating Notch signaling in adipose tissue macrophages. International Journal of Obesity. 2018;42(6):1140–1150. doi: 10.1038/s41366-018-0114-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Lee S. H., Nam H. J., Kang H. J., Kwon H. W., Lim Y. C. Epigallocatechin-3-gallate attenuates head and neck cancer stem cell traits through suppression of Notch pathway. European Journal of Cancer. 2013;49(15):3210–3218. doi: 10.1016/j.ejca.2013.06.025. [DOI] [PubMed] [Google Scholar]
- 285.Kaushik G., Venugopal A., Ramamoorthy P., et al. Honokiol inhibits melanoma stem cells by targeting notch signaling. Molecular Carcinogenesis. 2015;54(12):1710–1721. doi: 10.1002/mc.22242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Koduru S., Kumar R., Srinivasan S., Evers M. B., Damodaran C. Notch-1 inhibition by Withaferin-A: a therapeutic target against colon carcinogenesis. Molecular Cancer Therapeutics. 2010;9(1):202–210. doi: 10.1158/1535-7163.MCT-09-0771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Min S. J., Lim J. Y., Kim H. R., Kim S. J., Kim Y. Sasa quelpaertensis leaf extract inhibits colon cancer by regulating cancer cell stemness in vitro and in vivo. International Journal of Molecular Sciences. 2015;16(12):9976–9997. doi: 10.3390/ijms16059976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Subramaniam D., Ponnurangam S., Ramamoorthy P., et al. Curcumin induces cell death in esophageal cancer cells through modulating Notch signaling. PLoS One. 2012;7(2, article e30590) doi: 10.1371/journal.pone.0030590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Ning L., Wentworth L., Chen H., Weber S. M. Down-regulation of Notch1 signaling inhibits tumor growth in human hepatocellular carcinoma. American Journal of Translational Research. 2009;1(4):358–366. [PMC free article] [PubMed] [Google Scholar]
- 290.Chen J., Wang F. L., Chen W. D. Modulation of apoptosis-related cell signalling pathways by curcumin as a strategy to inhibit tumor progression. Molecular Biology Reports. 2014;41(7):4583–4594. doi: 10.1007/s11033-014-3329-9. [DOI] [PubMed] [Google Scholar]
- 291.Li Y., Domina A., Lim G., Chang T., Zhang T. Evaluation of curcumin, a natural product in turmeric, on Burkitt lymphoma and acute myeloid leukemia cancer stem cell markers. Future Oncology. 2018;14(23):2353–2360. doi: 10.2217/fon-2018-0202. [DOI] [PubMed] [Google Scholar]
- 292.Kumar A., Rimando A. M., Levenson A. S. Resveratrol and pterostilbene as a microRNA-mediated chemopreventive and therapeutic strategy in prostate cancer. Annals of the New York Academy of Sciences. 2017;1403(1):15–26. doi: 10.1111/nyas.13372. [DOI] [PubMed] [Google Scholar]
- 293.Cecchinato V., Chiaramonte R., Nizzardo M., et al. Resveratrol-induced apoptosis in human T-cell acute lymphoblastic leukaemia MOLT-4 cells. Biochemical Pharmacology. 2007;74(11):1568–1574. doi: 10.1016/j.bcp.2007.08.001. [DOI] [PubMed] [Google Scholar]
- 294.Starczynski J., Atkey N., Connelly Y., et al. HER2 gene amplification in breast cancer: a rogues' gallery of challenging diagnostic cases: UKNEQAS interpretation guidelines and research recommendations. American Journal of Clinical Pathology. 2012;137(4):595–605. doi: 10.1309/AJCPATBZ2JFN1QQC. [DOI] [PubMed] [Google Scholar]
- 295.Zanetti A., Affatato R., Centritto F., et al. All-trans-retinoic acid modulates the plasticity and inhibits the motility of breast cancer cells: role of notch1 and transforming growth factor (TGFβ) Journal of Biological Chemistry. 2015;290(29):17690–17709. doi: 10.1074/jbc.M115.638510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Cheng Y., Zhao K., Li G., et al. Oroxylin A inhibits hypoxia-induced invasion and migration of MCF-7 cells by suppressing the Notch pathway. Anti-Cancer Drugs. 2014;25(7):778–789. doi: 10.1097/CAD.0000000000000103. [DOI] [PubMed] [Google Scholar]
- 297.Wang J., Yan Z., Liu X., Che S., Wang C., Yao W. Alpinetin targets glioma stem cells by suppressing Notch pathway. Tumour Biology. 2016;37(7):9243–9248. doi: 10.1007/s13277-016-4827-2. [DOI] [PubMed] [Google Scholar]
- 298.Arai M. A., Akamine R., Tsuchiya A., et al. The Notch inhibitor cowanin accelerates nicastrin degradation. Scientific Reports. 2018;8(1):p. 5376. doi: 10.1038/s41598-018-23698-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299.Huang Y. W., Zhu Q. Q., Yang X. Y., et al. Wound healing can be improved by (−)-epigallocatechin gallate through targeting Notch in streptozotocin-induced diabetic mice. The FASEB Journal. 2018;33(1):953–964. doi: 10.1096/fj.201800337r. [DOI] [PubMed] [Google Scholar]
- 300.Delbosc S., Glorian M., Le Port A. S., Bereziat G., Andreani M., Limon I. The benefit of docosahexanoic acid on the migration of vascular smooth muscle cells is partially dependent on Notch regulation of MMP-2/-9. The American Journal of Pathology. 2008;172(5):1430–1440. doi: 10.2353/ajpath.2008.070951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Lu Q., Tong B., Luo Y., et al. Norisoboldine suppresses VEGF-induced endothelial cell migration via the cAMP-PKA-NF-κB/Notch1 pathway. PLoS One. 2013;8(12, article e81220) doi: 10.1371/journal.pone.0081220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302.Binesh A., Devaraj S. N., Devaraj H. Inhibition of nuclear translocation of notch intracellular domain (NICD) by diosgenin prevented atherosclerotic disease progression. Biochimie. 2018;148:63–71. doi: 10.1016/j.biochi.2018.02.011. [DOI] [PubMed] [Google Scholar]
- 303.Zhang J., Chen J., Xu C., et al. Resveratrol inhibits phenotypic switching of neointimal vascular smooth muscle cells after balloon injury through blockade of Notch pathway. Journal of Cardiovascular Pharmacology. 2014;63(3):233–239. doi: 10.1097/FJC.0000000000000040. [DOI] [PubMed] [Google Scholar]
- 304.Yu L., Li F., Zhao G., et al. Protective effect of berberine against myocardial ischemia reperfusion injury: role of Notch1/Hes1-PTEN/Akt signaling. Apoptosis. 2015;20(6):796–810. doi: 10.1007/s10495-015-1122-4. [DOI] [PubMed] [Google Scholar]
- 305.Yu L., Li Z., Dong X., et al. Polydatin protects diabetic heart against ischemia-reperfusion injury via Notch1/Hes1-mediated activation of Pten/Akt signaling. Oxidative Medicine and Cellular Longevity. 2018;2018:18. doi: 10.1155/2018/2750695.2750695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306.Zhang M., Yu L. M., Zhao H., et al. 2,3,5,4′-Tetrahydroxystilbene-2-O-β-D-glucoside protects murine hearts against ischemia/reperfusion injury by activating Notch1/Hes1 signaling and attenuating endoplasmic reticulum stress. Acta Pharmacologica Sinica. 2017;38(3):317–330. doi: 10.1038/aps.2016.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307.Sikora E., Scapagnini G., Barbagallo M. Curcumin, inflammation, ageing and age-related diseases. Immunity & Ageing. 2010;7(1):p. 1. doi: 10.1186/1742-4933-7-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308.Lei X., Yu J., Niu Q., Liu J., Fraering P. C., Wu F. The FDA-approved natural product dihydroergocristine reduces the production of the Alzheimer’s disease amyloid-β peptides. Scientific Reports. 2015;5(1, article 16541) doi: 10.1038/srep16541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309.Kang M. S., Baek S. H., Chun Y. S., et al. Modulation of lipid kinase PI4KIIα activity and lipid raft association of presenilin 1 underlies γ-secretase inhibition by ginsenoside (20S)-Rg3. The Journal of Biological Chemistry. 2013;288(29):20868–20882. doi: 10.1074/jbc.M112.445734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310.Yuan X., Wu H., Xu H., et al. Notch signaling: an emerging therapeutic target for cancer treatment. Cancer Letters. 2015;369(1):20–27. doi: 10.1016/j.canlet.2015.07.048. [DOI] [PubMed] [Google Scholar]
- 311.Burr M. L., Fehily A. M., Gilbert J. F., et al. Effects of changes in fat, fish, and fibre intakes on death and myocardial reinfarction: diet and reinfarction trial (DART) Lancet. 1989;2(8666):757–761. doi: 10.1016/s0140-6736(89)90828-3. [DOI] [PubMed] [Google Scholar]