Abstract
Background:
The study was carried out as part of the European Network for Patient Safety (EUNetPas) project in 2008-2010.
Objective:
To investigate facilitators and barriers in implementation process of selected medication safety practices across hospitals within European Union countries.
Methods:
This was an implementation study of seven selected medication safety practices in 55 volunteering hospitals of 11 European Union (EU) member states. The selected practices were two different versions of medicine bed dispensation; safety vest; discharge medication list for patients; medication reconciliation at patient discharge; medication reconciliation at patient admission and patient discharge, and sleep card. The participating hospitals submitted an evaluation report describing the implementation process of a chosen practice in their organisation. The reports were analysed with inductive content analysis to identify general and practice-specific facilitators and barriers to the practice implementation.
Results:
Altogether 75 evaluation reports were submitted from 55 hospitals in 11 EU member states. Implementation of the medication safety practices was challenging and more time consuming than expected. The major reported challenge was to change the work process because of the new practice. General facilitators for successful implementation were existence of safety culture, national guidelines and projects, expert support, sufficient resources, electronic patient records, interdisciplinary cooperation and clinical pharmacy services supporting the practice implementation.
Conclusions:
The key for the successful implementation of a medication safety practice is to select the right practice for the right problem, in the right setting and with sufficient resources in an organization with a safety culture.
Keywords: Patient Safety, Safety Management, Hospitals, Pharmacy Service Hospital, Medication Reconciliation, Patient Discharge, Patient Admission, Electronic Health Records, Delivery of Health Care, Health Plan Implementation, Surveys and Questionnaires, European Union, Europe
INTRODUCTION
Medication errors are one of the most common risks for patient safety.1,2 Medication errors are defined as any preventable event that may cause or lead to inappropriate medication use or patient harm while the medication is under the control of a healthcare professional, patient, or consumer.3 Medication safety interventions are among the most widely used patient safety interventions in health care.4 However, there is limited evidence of implementing or transferring interventions to different settings.4-7 This raises the need for research on medication safety interventions to understand settings, circumstances and factors for their successful implementation.5-8
European Union (EU) countries are responsible for promotion of their national patient safety strategies and actions.9 However, there is an increasing pressure for EU level coordination of patient safety and quality of care because of patients’ rights in cross-border care.10 Consequently, several recommendations on patient safety have been established at European level over time, covering also safe medication use in the member states.11,12 Medication safety has played a role in EU patient safety initiatives, such as the European Network for Patient Safety (EUNetPas, 2008-2010) and the European Union Network for Patient Safety and Quality of Care (PasQ, 2012-2016).13,14 Joint initiatives have facilitated shared learning from experiences of individual member states; it has been suggested that countries described as “recent adaptors” and “slow starters” could utilize the quality improvement strategies of “well established” EU countries.10
EUNetPas was the first patient safety program in the EU involving all 27 Member States and stakeholders.13 One of the core activities of EUNetPas focused on reducing medication errors in European hospitals by identifying safety practices and selecting some of the known practices to be implemented to hospitals in other member states. The aim of this study was to evaluate implementation process of selected safe medication practices within the EUNetPas project.
METHODS
Selection of medication safety practices
Applied from the definition of patient safety practices, medication safety practices (MSP) refer to interventions, strategies, or approaches intended to prevent or mitigate unintended consequences of the delivery of medication use and to improve medication safety.15 MSPs may include clinical interventions, organizational and behavioral interventions, and various combinations of these.
The EUNetPas medication safety project was divided into two phases: 1) Collection of real-life practices intended to improve medication safety in hospitals in various EU countries and selection of MSPs which could be implemented to other hospitals and countries; and 2) Investigating how well selected practices can be implemented across hospitals within EU countries in a given time frame. The present study focused primarily on phase 2 assessing facilitators and barriers for implementation of the practices.
The selection process of medication safety practices was carried out by the expert group responsible for the medication safety project (WP4) within EUNetPas. The expert group invited Member States and European stakeholders to take part in collecting MSPs applied in their hospitals. The expert group disseminated a call for proposals through national contact persons. These contact persons used their national networks to collect the proposals and sent them to WP4 expert group that made the selection. The selection criteria were that the practices must 1) take into account systems approach in the medication management process in the hospital (including prescribing, communication, medicine administration), 2) include actors’ (e.g., physicians, nurses, pharmacists and patients) involvement, and 3) be transferable to other hospitals. The selected practices were expected to be implemented in the given time frame (9 months) and to be easy and inexpensive to implement.
The expert group received 63 MSPs from 16 Member States via national contact persons and their networks during 2008. Of these practices, the expert group selected the following seven ones for the implementation exercise: medicine bed dispensation (two versions); safety vest; discharge medication list for patients; medication reconciliation at discharge; medication reconciliation at admission and discharge; and sleep card.16 The selected practices are described in Online appendix 1 as they were presented for the participating organizations in the EUNetPas project.
Participating hospitals and carrying out the implementation
Hospitals for implementing one or more of the seven practices were recruited with the help of the expert group members and partners in 11 Member States that volunteered to participate (Austria, Belgium, Denmark, Finland, France, Greece, Ireland, Italy, Lithuania, the Netherlands and Portugal). The hospitals were able to independently choose the practice(s) for implementation. However, the hospitals had to commit to implementation of the selected practices and to evaluate the implementation process without any financial support from the EUNetPas. A nine-month time frame, starting from April 2009, was given for the practice implementation and submission of the evaluation report. Every hospital had a contact person who was responsible for introducing the practice in the hospital and sending the evaluation report to the expert group. The hospitals were provided with the description of the selected practices (Online appendix 1) together with a standard evaluation form. They were, however, able to independently plan the way and scope of implementation to adopt the practice into their medication management processes. There was no standardized implementation process introduced for the hospitals.
Evaluation form
The material for this study was based on the written evaluation reports from the hospitals that participated in the implementation of the selected practices. The reports was requested to be written in English. The reports were collected by EUNetPas expert group and delivered to the main researcher (CLL) for data analysis. The evaluation form (Online appendix 2) consisted of 19 open-ended questions which varied slightly depending on the practice.16 The core topics covered in the evaluation form were: hospital’s baseline situation in medication safety before the implementation of the practice; description of the implementation process; assessment of the implementation experience, and outcomes of implementation on medication safety. If the hospital implemented more than one practice, they reported separately the implementation process of each of them.
Data analysis
All evaluation reports were analysed by using inductive content analysis.17 The analysis was carried out by the main researcher and the analysis strategy was decided with other researchers prior starting the analysis and discussed regularly during the analysis process. MS Word software was applied in the analysis.
The success of the implementation process at nine months was rated by the main researcher basing on the narrative description in each report and estimate of the success by the reporter on the evaluation report (rating: failed, on-going or succeeded).
The facilitators and barriers for implementation of the practices were identified by categorizing themes arising from the data. In the context of this study, the barriers refer to healthcare structures and approaches, such as lack of resources or safety culture, which may prevent safety practice implementation locally.18 The facilitators enable and contribute to successful implementation of the practices. The facilitators and barriers identified from narratives to open questions in evaluation reports were compiled to three separate analysis: 1) identifying all facilitators (i.e., general facilitators, not depending on the practice), 2) identifying all barriers (i.e., general barriers, not depending on the practice) and 3) identifying facilitators and barriers that were practice-specific. As all facilitators and barriers were identified and listed, they were clustered to themes. Practice-specific facilitators and barriers were collected under each practice, but they were not clustered. Also, actors (i.e., healthcare providers) involved in the implementation process were identified as part of the content analysis.
RESULTS
At the initiation stage of the implementation project, the participating hospitals (n=79) from 11 EU countries committed to implement a total of 113 practices, but 75 evaluation reports were returned from 55 hospitals in 11 EU member states (Table 1). According to the returned reports, 59 % (n=67) of the planned practice implementations (n=113) were actually started in the hospitals and reported to the EUNetPas. Eight of the reports were returned unfilled as the implementation had not been started as planned. Of those hospitals that started the implementation, 78 % (n=52) reported that they were able to implement the practice as described or as modified. The implementation was rated as successful in these cases. The implementation was reported as partly successful (some units or professionals involved in implementation had adopted the practice) or the implementation was still on-going in 11 (16 %) of the cases at the end of the given time frame. Implementation was rated failed (n=4, 6 %) when the practice was not implemented at all although implementation efforts were made.
Table 1. Participation of the 11 European Union member states in the implementation process of seven selected medication safety practices in the EUNetPas project.16.
| Practice | Hospitals that planned to participate in the implementation process (n) | Countries involved in the implementation and submitting the evaluation report (n of reports provided for countries submitting ≥1 reports) | Hospitals that started the implementation process n (%) | Implemen-tation faileda n (%) | Implementation partly succeeded or on-goinga n (%) | Implementation succeededa n (%) |
|---|---|---|---|---|---|---|
| Bed dispensation (A) | 10 | Portugal (3), Austria, Ireland | 5 (50) | 0 | 0 | 5 (100) |
| Bed dispensation (B) | 10 | Greece (3), Ireland (2), Italy (2), Lithuania | 8 (80) | 0 | 1 (13) | 7 (87) |
| Safety vest | 28 | Ireland (6), Finland (4), Portugal (4), Italy (2), Lithuania (2), France | 16 (57) | 3 (19) | 4 (25) | 9 (56) |
| Medication reconciliation at admission and discharge | 17 | Portugal (5), France (3), Ireland (2), Belgium, Italy | 12 (71) | 1 (8) | 2 (17) | 9 (75) |
| Discharge medication list for patients | 21 | Portugal (4), Ireland (2), Italy (2), Finland, France | 8 (38) | 0 | 1 (13) | 7 (87) |
| Medication reconciliation at discharge | 23 | Denmark (5), Portugal (4), Italy (3), The Netherlands (2), Austria, Ireland, Lithuania | 16 (70) | 0 | 3 (19) | 13 (81) |
| Sleep card | 4 | Ireland (2), Austria, Italy | 2 (50) | 0 | 0 | 2 (100) |
| Total | 113 | 75 (66%) | 67 | 4 (6) | 11 (16) | 52 (78) |
According to the evaluation reports submitted by the participating hospitals at the end of the nine-month implementation period
At least in a quarter (24%, n=16) of the cases the hospitals needed to modify the practice locally. Especially medication reconciliation practices needed local modification and they often ended up being more like discharge medication list practices. In the given implementation and evaluation time frame, it was not possible to assess whether the implementation was sustainable. Actors involved in practice implementation are described in Figure 1.
Figure 1. The actors involved in medication safety practice implementation process in hospitals participating in the study (n=67).

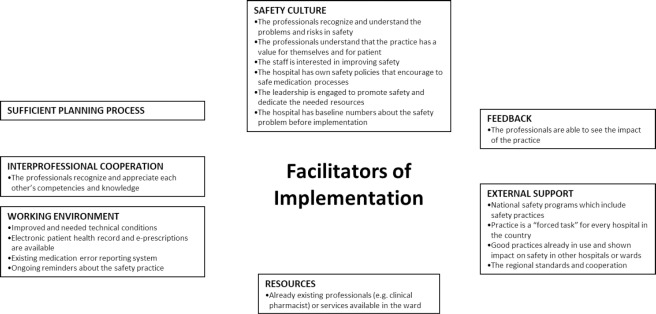
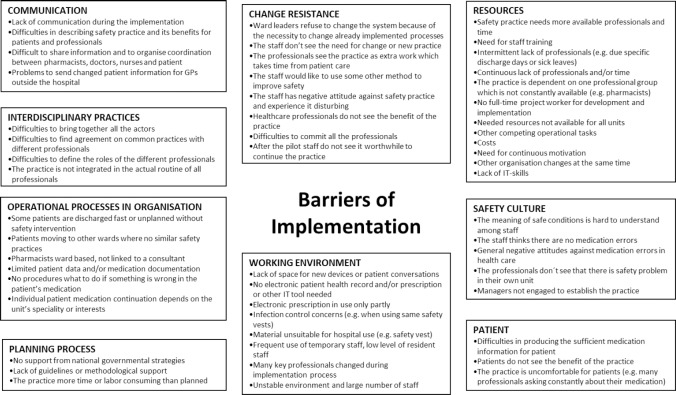
Most of the participating hospitals described problems they encountered during the practice implementation, but many facilitators were also identified (Figures 2 and 3). Safety culture and national patient safety programs were seen to be effective general facilitators for the implementation (Figure 2). The practice was more likely to be implemented if the planning was done with due care, health care professionals valued interprofessional cooperation, working environment and tools (e.g., information technology) enabled practice implementation, and the workers got updated feedback about the use of the practice.
Figure 2. General facilitators reported by hospitals (n=67) in implementing new medication safety practices.
Figure 3. General barriers reported by hospitals (n=67) in implementing new medication safety practices.
Lack of time for practice implementation was the most commonly mentioned barrier which was reported in 34% (n=23) of the evaluation forms and was related to all seven practices. The relatively short nine-month time allocated for practice implementation in the study appeared as a major barrier especially for the medication reconciliation practice. Evaluation phase of the practice was still on-going in many hospitals when the project ended.
The barriers were often associated with the lack of resources and non-compliance of the unit’s staff for MSP implementation (Figure 3). Especially physicians, as a professional group, were seen to be unwilling to develop shared practices and commit to them. Many hospitals reported problems in implementing the practice in the existing processes of the unit or a lack of tools supporting implementation (e.g., no electronic patient records available). Lack of safety culture and operational processes that did not promote implementation (e.g., managers’ engagement) were mentioned in several reports. The practice was also seen as uncomfortable for patients in some cases (e.g., their medication history asked several times and by many professionals during medication reconciliation process). There was also a need for more external expert support in planning the implementation process. In the early stages of the project, many hospitals were planning to implement more than one medication safety practice. However, other concurrent projects were often barriers for actual implementation of multiple practices.
Specific facilitators and barriers for each practice are described in Table 2. The barriers and facilitators for two types of bed dispensation and medication reconciliation practices were identified as similar. Only two reports concerned implementation of the sleep card which did not yield enough information about barriers and facilitators.
Table 2. Practice-specific facilitators and barriers of medication safety practices (n=67) implementation reported by the hospitals participating in the study (n=55).
| Practice | Barriers | Facilitators |
|---|---|---|
| Safety vest (n=16)* | • Difficulties to commit the staff to use of the vest. • Negative attitudes against wearing the vest. •Wearing safety vest may be experienced as disturbing. • Nurses like to be available for questions and they think the vest will prevent it. • The staff would like to use other methods to make medication dispensing more peaceful. • The vest does not stop the primary cause of interruptions (e.g. phone calls). • The meaning of peace at work is hard to understand for the staff. • Vest material unsuitable for hospital use. • Infection control concerns when sharing the vests between many healthcare professionals. • The costs of the vests more than expected. • Some relatives of patients are embarrassed to ask help from a nurse wearing the vest even if they have no choice. • Some relatives see the vest as an “alert” and the nurse wearing the vest is interrupted more often than nurses without it. |
• Ongoing reminders to use the vest. |
| Medication reconciliation (n=28)* | • No electronic patient records are available or part of the prescriptions are handwritten. • Electronic patient records do not support medication reconciliation procedure or the actors (e.g. nurse, pharmacist, doctor) have insufficient skills to use the electronic records. • Incomplete patient records and medication documentation (e.g. previous medications). • Individual patient medication continuation depends on the specialty or interests of the unit. • No procedure what to do if there is something wrong in patient’s medication. • Difficult to share medication information and organize coordination of medication reconciliation procedure between pharmacists, doctors, nurses and patients. • Time-consuming procedure and/or it takes more time than anticipated. • The doctors and patients are dependent of the presence of pharmacist at the unit and there are limited number of pharmacists in the organization. • Discharges are sometimes fast or unplanned, and may take place when pharmacist is not present at the unit. Patient will leave or medicines are sent to patient’s home before pharmacist has a chance to check the medication. • Difficulties in presenting the reconciliation process to patient in a simple way. • The reconciliation process (at admission and discharge) is not completed if the patient is moving to next ward where there is no possibility to pharmacist’s intervention. • Lack of available space for patient medication overview discussions. • Workload not evenly spread throughout the week. • Difficulties to commit doctors for the practice and to describe medication reconciliation benefits for patients and healthcare professionals. • Patient feels uncomfortable when a large number of professionals are asking them about their medications. • Pharmacy technicians’ skills and role unexploited. • Problems to send discharge letters to GPs in relevant time. • Lack of guidelines to medication reconciliation. |
• Electronic patient records which support medication reconciliation process are in use. • Other pharmacist services already available in unit and/or medication reconciliation is part of pharmacist’s normal visit to wards. • The presence of pharmacist is appreciated by doctors. • There is regional standards and cooperation for medication reconciliation. |
| Medication list (n=8)* | • No electronic patient record system available. • Difficulties to bring together all the actors (e.g. nurses, pharmacists and doctors) and to find agreement on the practice procedure with doctors. • After procedure agreement all the actors do not involve in the process (especially doctors). • Doctors and patients are dependent of the presence of pharmacist at the unit. • Difficulties in presenting the medication list to patient in an understandable way. |
• Already existing clinical pharmacy services in the unit. • Already existing medication reconciliation process in the unit. • The presence of pharmacist appreciated by doctors. |
| Bed dispensing (n=13)* | • "Staff - bottleneck" (e.g. number of nursing staff at the time of sick leaves). • Lack of space for medication carts at the wards. • The financial costs of the carts. • Non - acceptance by nurses to change the traditional style to dispense medicines. |
• Electronic prescription and patient records in use (if computers available for carts). |
*n=hospitals involved in implementation
A medication process that already included a safety practice, was in some hospitals identified as a good basis for the implementation of new safety practices (e.g., when medication list was implemented in an already existing medication reconciliation process). Implementation of a safety practice in all wards of a hospital simultaneously encouraged the implementation of the practice more on those wards having acute safety problems (e.g., safety vest in wards with medication distribution room vs. wards without the room).
DISCUSSION
The current study indicates that the implementation of MSPs into the daily practice of hospitals is challenging and requires often local adaptation of the procedures. As previous research is scarce, our study provides unique information on general and practice-specific facilitators and barriers of MSP implementation in European hospitals. Our study describes the implementation process rather than the success or impact of implementation.6
Our study indicates that implementation of MSP is not only challenging, but also time consuming, especially when the practice requires changing the existing work processes. It should be noted that there are multiple actors in hospitals, such as professionals and care units which have a central role in the implementation process and should be identified in early stages of the implementation. Most importantly, hospitals need to be provided with enough support and guidance to assist in the implementation and evaluation process of new MSPs, like noted also previously.10 According to our findings, national guidelines and safety projects seem to serve as good facilitators for practice implementation in hospitals. Previous evidence indicate that national healthcare quality campaigns even influence on those hospitals that do not directly participate in the campaigns.19
As learnt from this EUNetPas project, implementing MSPs across hospitals and countries by only introducing general descriptions of the given practices may not be the most efficient way of successful practice implementation. Instead, the hospitals need to participate more actively in identifying their own priority medication safety problems in order to improve safety of their medication processes. Following this, the hospitals would need to identify the best practice for a particular safety problem, e.g., safety vest does not eliminate the primary cause for interruptions in dispensing if phone is still ringing. Previous studies have found that the lack of effectiveness may merely be a reflection of an implementation failure than actual ineffectiveness of the practice.20 It is also suggested that quality improvement strategies and practices are not equally effective in different settings.10
This study, among other studies, indicated that when considering the implementation of a MSP in hospitals, the primary focus should be on safety culture.21-23 In supporting system-based organizational culture, the healthcare professionals are more likely to understand the medication safety risks at their own hospital, commit themselves to MSP implementation and give value to the implementation, as identified in our study. In addition to safety culture, the role of hospital leadership was identified as central. Indeed, the leadership, resources and commitment to quality and safety have been identified as key enabling factors in patient safety improvement work.2,21,22,24 Our findings also indicated that active interprofessional cooperation is essential for the successful implementation of practices.
Our study demonstrated that there are general facilitators and barriers for MSP implementation, but each MSP has also practice-specific factors influencing the implementation. An interesting notion was that practices presumed as easy and inexpensive to implement still failed to implement. This was especially the case with the safety vest; the challenges with this particular practice have been also noticed in previous studies.25 According to our findings, electronic patient records facilitate implementation especially in the case of the bed dispensation, medication list and medication reconciliation practices. If technical devices, such as carts in bed dispensation, are implemented, the organization must be prepared for the additional space it requires. Moreover, the process where the device is implemented is also likely to change. Medication reconciliation might be the practice that has been studied most after EUNetPas project.14,26,27 In this study some hospitals planned to implement medication reconciliation but ended to implement “only” medication list. This may be considered as partly unsuccessful implementation but noticing recent discussion, correct medication list is a key for successful implementation of medication reconciliation.28
Already established clinical pharmacy services was one key facilitator in the implementation of medication reconciliation and medication list practices. This was due to the hospitals’ possibility to integrate some parts of the new medication safety practices as part of pharmacist’s daily work. In countries where pharmacists were not involved in the interdisciplinary team or did not work on the wards, it was more likely that nurses and doctors took the lead. Although pharmacists would be an ideal part of the medication safety practice implementation team, our study indicated that their work resources were often limited, hindering their participation in the implementation activities. The EUNetPas project was done almost 10 years ago but the challenges in pharmacists’ resources still exist European wide.
Finally, our findings showed that the patient involvement may be crucial for the compliance to MSP. Our study also supports previous findings that if many new practices were implemented at the same time, there is a high possibility that the resources were not sufficient for implementing all of them.24
The expert group of WP4 decided to use inventory method instead of purely evidence-base for selecting medication safety practices because systems-based patient and medication safety work was still in its infancy phase in the late 2000s globally and in Europe. Evidence on effectiveness of various practices intended to reduce medication errors in hospitals was scarce at that time. It is very likely that today the selection of medication safety practices would be more evidence-based. However, most of the practices included in the EUNetPas exercise are still valid and widely in use in European hospitals. According to our understanding, at least medication reconciliation, medication list and safety vest would be chosen again if the exercise would be repeated today. The focus of bedside dispensing would now be more likely in new automated technologies. What has remarkably changed within the last ten years is the implementation of electronic patient record systems, electronic prescribing and medication risk management systems in many countries, although these systems are not yet optimally working and supporting seamless and safe patient care.
The approach of this study is still, after 10 years of EUNetPas, valid and unique. There are few published studies that have shared experiences of processes for implementing medication safety practices from one hospital to another, even across countries. The major barrier in the implementation of the new medication safety practices were found to be in changing work processes because of the implementation of the new practices. The successful practice implementation was best facilitated by existence of safety culture, national guidelines and projects, expert support, sufficient resources, electronic patient records, interprofessional cooperation and clinical pharmacy services. These are fundamental issues that still today play a role in safety of health systems in EU and globally, influencing also medication safety.2,6,7,29
Study limitations
The majority of the study participants were not from native English speaking countries, while the free-text evaluation reports needed to be written in English, affecting the quality and contents of the reports. Especially challenging was to estimate the actual phase of the implementation process. Hospitals were not contacted if there was some unclear or missing information but the researchers avoided interpretations in these cases.
Only a few reports were received from hospitals that did not start the implementation process, causing a loss of information about barriers effecting the starting of the implementation. In addition to these hospitals, there was a high number of hospitals that did not send a report, most likely because they did not start the implementation. It would have been valuable to contact them and find out the reasons and barriers hindering their participation. The barriers of planning and implementing the practices were clearly more common in the returned evaluation reports in comparison to the facilitators. However, several key facilitators were identified, giving essential information for hospitals planning to implement the described safety practices or improving their existing ones.
The MSPs were seldom implemented completely according to the EUNetPas practice examples (Online appendix 1) as the practices needed to be adapted to units, working cultures and processes. This could have influenced the experienced facilitators and barriers. As this was retrospective document analysis focusing on identifying general and practice-specific facilitators and barriers for MSP implementation, the real-life conditions and environments affecting the implementation of each practice were not investigated. This may be seen influencing to external validity of the study.6 Furthermore, it was not possible to detect the sustainability of the practices from evaluation reports.7 Consequently, future research should evaluate the long-term effects and successes of the MSPs implemented.
CONCLUSIONS
Implementing MSPs in an international short-term project is challenging, especially when changes in work processes are required. Even simple and inexpensive practices will not be successfully implemented if the professionals or patients involved do not understand the need and benefits of the new practice. The key for a successful implementation of a MSP is to select the right practice for the right problem, in the right setting and with sufficient resources. The successful implementation requires a presence of safety culture, including committed leadership, and interdisciplinary cooperation. External support and involving a pharmacist may facilitate implementation of some MSPs. Every MSPs in this study had also practice-specific facilitators and barriers that should be considered in the implementation process. When planning future European-wide patient safety projects as EUNetPas was, focus should be on evidence-based MSPs with clear implementation strategy.
ACKNOWLEDGEMENTS
We would like to thank the following organizations and countries for their valuable contribution to the study: EUNetPas WP4 expert group, European Hospital and Healthcare Federation (HOPE); involved partners from European Federation of Nurses, European Association of Hospital Pharmacists, Pharmacist Group of the European Union, and the EU member states participating the study (Austria, Belgium, Denmark, Finland, France, Greece, Ireland, Lithuania, Portugal, the Netherlands and Italy).
Footnotes
CONFLICT OF INTEREST
None.
FUNDING
This work was supported by Helsinki University Pharmacy and Finnish Cultural Foundation.
Contributor Information
Carita Linden-Lahti, MSc. Helsinki University Hospital, HUS Pharmacy; & Division of Pharmacology and Pharmacotherapy, Faculty of Pharmacy, University of Helsinki.Finland carita.linden@helsinki.fi.
Anna-Riia HOLMSTRÖM, PhD. Helsinki University Hospital, HUS Pharmacy; & Division of Pharmacology and Pharmacotherapy, Faculty of Pharmacy, University of Helsinki.Finland..
Pirjo Pennanen, MD. City of Vantaa, Preventive Healthcare. Vantaa (Finland)..
Marja Airaksinen, PhD. Division of Pharmacology and Pharmacotherapy, Faculty of Pharmacy, University of Helsinki.Finland..
References
- 1.Kohn KT, Corrigan JM, Donaldson MS, editors. Institute of Medicine. To Err Is Human:Building a Safer Health System. Washington, DC: National Academy Press; 2000. [Google Scholar]
- 2.World Health Organization (WHO) [accessed May 31, 2019];Medication without harm:WHO´s Third Global Patient Safety Challenge. http://www.who.int/patientsafety/medication-safety/en .
- 3.National Coordinating Council for Medication Error Reporting and Prevention (NCCMERP) [accessed Nov 15, 2017];About medication errors:What is a medication error? http://www.nccmerp.org/about-medication-errors .
- 4.Dückers M, Faber M, Cruijsberg J, Grol R, Schoonhoven L, Wensing M. Safety and risk management interventions in hospitals - a systematic review of the literature. Med Care Res Rev. 2009;66(6 Suppl):90S–119S. doi: 10.1177/1077558709345870. [DOI] [PubMed] [Google Scholar]
- 5.Foy R, Eccles M, Grimshaw J. Why does primary care need more implementation research? Fam Pract. 2001;18(4):353–355. doi: 10.1093/fampra/18.4.353. [DOI] [PubMed] [Google Scholar]
- 6.Bauer MS, Damschroder L, Hagedorn H, Smith J, Kilbourne AM. An introduction to implementation science for the non-specialist. BMC Psychol. 2015;3:32. doi: 10.1186/s40359-015-0089-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rapport F, Clay-Williams R, Churruca K, Shih P, Hogden A, Braithwaite J. The struggle of translating science into action:Foundational concepts of implementation science. J Eval Clin Pract. 2018;24(1):117–126. doi: 10.1111/jep.12741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ovretveit J. Understanding the conditions for improvement:research to discover which context influences affect improvement success. BMJ Qual Saf. 2011;20(Suppl 1):i18–i23. doi: 10.1136/bmjqs.2010.045955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.The Council of the European Union. Council conclusions on patient safety and quality of care, including the prevention and control of healthcare-associated infections and antimicrobial resistance (2014/C 438/05) Official Journal of the European Union. 2014;C438:7–11. [Google Scholar]
- 10.Groene O, Klazinga N, Walshe K, Cucic C, Shaw CD, Suñol R. Learning from MARQuIS:future direction of quality and safety in hospital care in the European Union. Qual Saf Health Care. 2009;18(Suppl I):i69–i74. doi: 10.1136/qshc.2008.029447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Council of Europe. [accessed May 31, 2019];Creation of a better medication safety culture in Europe:Building up safe medication practices. Expert Group on Safe Medication Practices. 2006 https://www.edqm.eu/medias/fichiers/Report_2006.pdf .
- 12.European Medicines Agency (EMA) [accessed May 31, 2019];Medication errors. https://www.ema.europa.eu/en/human-regulatory/post-authorisation/pharmacovigilance/medication-errors/recommendations-medication-errors .
- 13. [accessed Aug 15, 2016];European Network for Patient Safety (EUNetPas):Project homepages. http://90plan.ovh.net/~extranetn/index.php?option=com_content&task=view&id=1&Itemid=2 .
- 14.The European Union Network for Patient Safety and Quality of Care (PaSQ) [accessed Nov 15, 2017];Project homepages. http://www.pasq.eu/Project/Project.aspx .
- 15.Shekelle PG, Pronovost PJ, Wachter RM. Assessing the Evidence for Context-Sensitive Effectiveness and Safety of Patient Safety Practices - Developing Criteria. [accessed May 31, 2019];AHRQ Publication No. 11-0006-EF. 2010 https://archive.ahrq.gov/research/findings/final-reports/contextsensitive/context.pdf .
- 16.European Network for Patient Safety (EuNetPas) Good medication safety practices in Europe –Compendium I:Results of the implementation. EuNetPas. 2010 [Google Scholar]
- 17.Hsieh HF, Shannon SE. Three approaches to qualitative content analysis. Qual Health Res. 2005;15(9):1277–1288. doi: 10.1177/1049732305276687. [DOI] [PubMed] [Google Scholar]
- 18.Vrbnjak D, Denieffe S, O'Gorman C, Pajnkihar M. Barriers to reporting medication errors and near misses among nurses:A systematic review. Int J Nurs Stud. 2016;63:162–178. doi: 10.1016/j.ijnurstu.2016.08.019. [DOI] [PubMed] [Google Scholar]
- 19.Hansen LO, Herrin J, Nembhard IM, Busch S, Yuan CT, Krumholz HM, Bradley EH. National quality campaigns:who benefits? Qual Saf Health Care. 2010 Aug;19(4):275–278. doi: 10.1136/qshc.2009.036087. [DOI] [PubMed] [Google Scholar]
- 20.Graig P, Dieppe P, Macintyre S, Michie S, Nazareth I, Petticrew M. Developing and evaluating complex interventions:the new Medical Research Council guidance. BMJ. 2008;337:a1655. doi: 10.1136/bmj.a1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Halligan M, Zecevic A. Safety culture in healthcare:a review of concepts, dimensions, measures and progress. BMJ Qual Saf. 2011 Apr;20(4):338–343. doi: 10.1136/bmjqs.2010.040964. [DOI] [PubMed] [Google Scholar]
- 22.Taylor SL, Dy S, Foy R, Hempel S, McDonald KM, Ovretveit J, Pronovost PJ, Rubenstein LV, Wachter RM, Shekelle PG. What context features might be important determinants of the effectiveness of patient safety practice interventions? BMJ Qual Saf. 2011 Jul;20(7):611–617. doi: 10.1136/bmjqs.2010.049379. [DOI] [PubMed] [Google Scholar]
- 23.Holmström AR, Laaksonen R, Airaksinen M. How to make medication error reporting systems work –Factors associated with their successful development and implementation. Health Policy. 2015;119(8):1046–1054. doi: 10.1016/j.healthpol.2015.03.002. [DOI] [PubMed] [Google Scholar]
- 24.Burnett S, Benn J, Pinto A, Parand A, Iskander S, Vincent C. Organisational readiness:exploring the preconditions for success in organisation-wide patient safety improvement programmes. Qual Saf Health Care. 2010;19(4):313–317. doi: 10.1136/qshc.2008.030759. [DOI] [PubMed] [Google Scholar]
- 25.Westbrook JI, Li L, Hooper TD, Raban MZ, Middleton S, Lehnbom EC. Effectiveness of a “Do not interrupt“bundled intervention to reduce interruptions during medication administration:a cluster randomised controlled feasibility study. BMJ Qual Saf. 2017;26(9):734–742. doi: 10.1136/bmjqs-2016-006123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mekonnen AB, McLachlan AJ, Brien JA. Pharmacy-led medication reconciliation programmes at hospital transitions:a systematic review and meta-analysis. J Clin Pharm Ther. 2016;41(2):128–144. doi: 10.1111/jcpt.12364. [DOI] [PubMed] [Google Scholar]
- 27.Redmond P, Grimes TC, McDonnell R, Boland F, Hughes C, Fahey T. Impact of medication reconciliation for improving transitions of care. Cochrane Database Syst Rev. 2018;8:CD010791. doi: 10.1002/14651858.CD010791.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rose AJ, Fischer SH MD, Paasche-Orlow MK. Beyond Medication Reconciliation:The Correct Medication List. JAMA. 2017;317(20):2057–2058. doi: 10.1001/jama.2017.4628. [DOI] [PubMed] [Google Scholar]
- 29.The National Patient Safety Foundation. [accessed May 31, 2019];Free from Harm –Accelerating Patient Safety Improvement Fifteen Years after To Err Is Human. http://www.ihi.org/resources/Pages/Publications/Free-from-Harm-Accelerating-Patient-Safety-Improvement.aspx .