Abstract
One of the most significant challenges facing investigators, laboratory animal veterinarians, and IACUCs, is how to balance appropriate analgesic use, animal welfare, and analgesic impact on experimental results. This is particularly true for in vivo studies on immune system function and inflammatory disease. Often times the effects of analgesic drugs on a particular immune function or model are incomplete or don't exist. Further complicating the picture is evidence of the very tight integration and bidirectional functionality between the immune system and branches of the nervous system involved in nociception and pain. These relationships have advanced the concept of understanding pain as a protective neuroimmune function and recognizing pathologic pain as a neuroimmune disease. This review strives to summarize extant literature on the effects of pain and analgesia on immune system function and inflammation in the context of preclinical in vivo studies. The authors hope this work will help to guide selection of analgesics for preclinical studies of inflammatory disease and immune system function.
Abbreviations and acronyms: CB,Endocannabinoid receptor; CD,Crohn disease; CFA, Complete Freund adjuvant; CGRP,Calcitonin gene-related peptide; COX,Cyclooxygenase; CTL, Cytotoxic T-Lymphocytes; DAMP,Damage-associated molecular pattern molecules; DRG,Dorsal root ganglion; DSS, Dextran sodium sulphate; ECS,Endocannabinoid system; IBD, Inflammatory bowel disease; IFA,Incomplete Freund adjuvant; Las, Local anesthetics; PAMP,Pathogen-associated molecular pattern molecules; PGE2, Prostaglandin E2; P2Y, ATP purine receptor Y; P2X, ATP purine receptor X; TNBS, 2,4,6-Trinitrobenzene sulphonic acid; TRP, Transient receptor potential ion channels; TRPV, Transient Receptor Potential Vanilloid; TG,Trigeminal ganglion; UC,Ulcerative colitis
The immune system is comprised of 2 arms, innate and adaptive immunity, which function in concert to protect an organism from pathogens and toxins. The immune system also plays an integral role in the process of tissue repair after injury.58,127 The process by which the immune system responds to pathogens, toxins, and tissue injury and initiates tissue repair is known as inflammation. Inflammation and its association with pain have been recognized since it was first described by the Roman, Aulus Celsus.177 More recently, it has been elucidated that the immune and nervous systems interact to mediate and modulate central and peripheral nociceptive processes that influence acute and chronic pain.69,95,190,217 There is a dizzying array of cells, receptors, enzymes, cytokines, peptides, and neurotransmitters that constitute the inflammatory process and neuroimmune interactions related to pain. To further complicate the picture, drugs that are used to control pain, modulate immune function and the immune system can produce endogenous analgesics.129,200 The goal of this article is to improve the reader's understanding of the relationship between pain, analgesia, and immune function in the context of preclinical in vivo studies. We hope this article will serve as a guide for laboratory animal professionals, IACUCs, and investigators in the selection of appropriate analgesics for preclinical studies of inflammatory disease and immune system function.
Neuroimmune Interactions
With regards to pain and analgesia, it is critical to understand the complex interactions between the immune and nervous systems. It has been postulated that a well-regulated neuroimmune response to infection, noxious stimuli, and tissue injury represents a cohesive system for host defense and tissue healing.36 Thus, conceptual siloing of immune and nervous system responses to pain is no longer appropriate.
Neurogenic Inflammation
Multiple lines of evidence indicate that nociceptive neurons can initiate and modulate inflammation. Considering the speed at which they respond to any form of insult, (traumatic, thermal, chemical) and their broad tissue distribution, nociceptive neurons are uniquely poised to function as monitors and rapid initiators of a neuroimmune response.36 When triggered by noxious stimuli or alarmins (ATP, uric acid, hydroxynonenals) from damaged tissue, receptors primarily in the Transient Receptor Potential ion channels (TRP) and ATP Purine Receptor X (P2×) and Y (P2Y) family activate nociceptors which release neuropetides that initiate a response referred to as neurogenic inflammation.36,95 Specifically, tachykinins (substance P and neurokinin A) and calcitonin gene-related peptide (CGRP), released from nociceptive neurons, act on vascular endothelium and smooth muscle cells, causing vasodilation and increased endothelial permeability.16,116,143,174,179,185 Activated nociceptive neurons also release neuropeptides and cytokines which attract and activate innate and adaptive immune cells.65,85,86,104,187,219 Over a course of days, the inflammatory response recruits monocytes, which differentiate into macrophages. Over time, macrophages undergo phenotype changes from inflammatory/host defense (M1) to antiinflammatory/wound healing (M2) cells as the wound microenvironment changes.58,127 Thus, nociceptor activation can induce the factors that cause the classic signs of inflammation: rubor, tumor, calor, and dolor, and may contribute to wound healing.
Bidirectional Interactions
Interactions between the immune system and nervous system are bidirectional and not all nociceptor driven. Nociceptive neurons express and respond to a receptor profile similar to that of leukocytes. These include receptors for cytokines, eicosanoids (prostaglandins), Toll-like receptors, and ATP purine receptors P2× and P2Y.76,147,209,214 Leukocytes express TRP receptors, macrophages, express high levels of Transient Receptor Potential Vanilloid (TRPV2), and mast cells express TRPV1. To further complicate the picture, both leukocytes and nociceptors express µ, δ, and κ opioid receptors, and T-lymphocytes, granulocytes and monocytes-macrophages can release endogenous opioid peptides.129,133,154,200
The pattern of aforementioned receptor expression suggests that the immune system can modulate nociception, and the nervous system can modulate inflammation. Nociceptors can also be directly activated by infectious agents, damage-associated molecular pattern molecules (DAMPs) and pathogen-associated molecular pattern molecules (PAMPs) through Toll-like receptors.35,59,146,214 It has been well established that inflammatory mediators activate nociceptors (causing pain) and can initiate neural plasticity in nociceptive pathways. This results in peripheral and central nociceptor sensitization.15,43,199 Clinically, peripheral and central nociceptor sensitization manifests as allodynia, hyperesthesia, and hyperalgesia.208
Neuroimmune activation of nociceptive neurons occurs in the PNS and CNS. In response to injury or alarmin stimulation, leukocytes, endothelial cells, and neurons in the dorsal root ganglion (DRG), trigeminal ganglia (TG) and the dorsal horn of the spinal cord release eicosanoids, growth factors, kinins, and cytokines.120,121,173,223 This milieu of biochemicals binds to receptors on nociceptive neurons, which are coupled to TRPV receptors and ion channels, resulting in increased neuron activity and sensitization.110,111,164,199,206,208
Microglial Activation
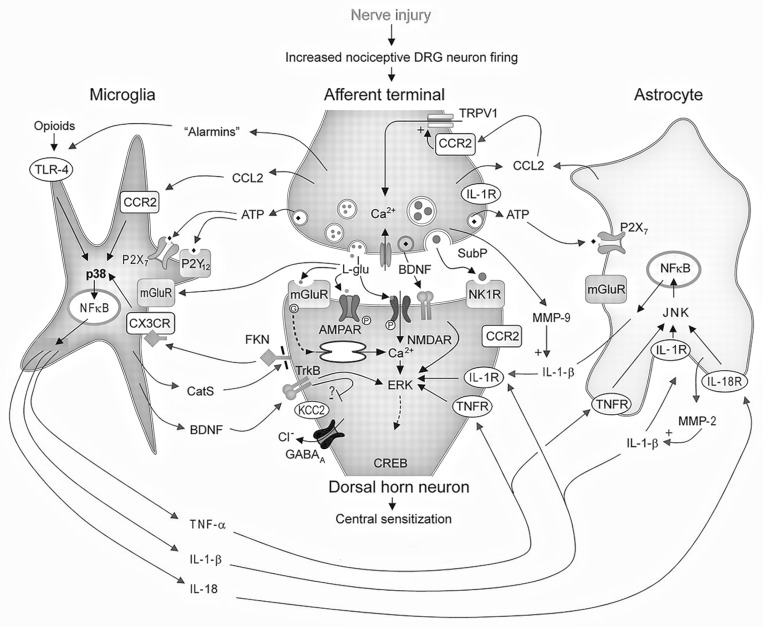
Activation and proliferation of microglial cells is thought to be a central feature of the neuroimmune interface both in physiologic and pathologic pain, and a primary element in the development of central sensitization and potentially, chronic pain (Figure 1).216 Microglia are the predominant cell type in the CNS, and reside at a critical interface; the synapse between 1st and 2nd order nociceptive neurons in the spinal dorsal horn. The activation of microglia represents a key feature of the neuroimmune interface, since activation can occur through neuronal, immune and pathogen mediated pathways.49,96,217 In addition, sex differences in glial activation have been reported, which may contribute to the established sexual dimorphism of pain.49,134
Figure 1.
Interactions between nociceptive neurons and microglial cells after neuronal damage or activation by alarmins are depicted. l-glutamate (l-glu), substance P (SubP), adenosine triphosphate (ATP), brain-derived neurotrophic factor (BDNF), cysteine-cysteine chemokine ligand CCL2 neurokinin-1 receptors (NK-1R), extracellular signal-regulated kinase (ERK), α-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid receptors (AMPAR), cyclic adenosine monophosphate response element binding protein (CREB). ATP purinergic receptors, (P2X7, P2Y12 and P2Y13R), mitogen-activated kinase (p38), c-jun-N terminal kinase (JNK), nuclear factor kappa B (NFκB), Interleukin-1β (IL1β) and its receptor, (IL-1R) tumor necrosis factor α (TNFα) and its receptor (TNFR), chloride (Cl−) transporter (KCC2), gamma aminobutyric acid A receptor (GABAA), chemokine ligand 2 (CCL2), chemokine receptor 2,3 (CCR2, CCR3), Cathepsin S (CatS), fractalkine (FKN, also termed CX3C-chemokine ligand 1), chemokine receptor 1 (CX3CR), Matrix metalloprotease 2, 9 (MMP2 MMP9), toll-like receptor 4 (TLR4).Reprinted by permission from Wolters Kluwer Health. Central neuron-glia interactions and neuropathic pain, Eduardo E. Benarroch 2010
Microglia are activated by ATP, CC-chemokine ligand 2 and 21, CX3CL1 (fracktalkine), and neuregulin 1, released from 1st order neurons during high threshold activation or injury.148,212 Of particular note is that the receptor for CX3CL1, CX3CR1, is only expressed on microglia and may represent a unique neuroimmune interface.212 Pathogens, DAMPs and PAMPs can directly activate microglial cells through binding to Toll-like receptors.72,184 Cytokines released from leukocytes both activate microglial cells and directly contribute to nociceptive hyperactivity. When activated, microglia release proinflammatory cytokines, reactive oxygen species, brain-derived neurotrophic factor, and integrins. This biochemical barrage results in enhanced excitability in 2nd order neurons, increased release of substance P, glutamate and excitatory amino acids from primary afferent neurons, astrocyte activation, inhibition of inhibitory interneurons and recruitment of T-cells.216
Endocannabinoid System
The Endocannabinoid system (ECS), an endogenous “on-demand” messaging system comprised of lipophilic ligands, their receptors, and synthetic proteins, represents another neuroimmune interface.167 The ECS has widespread and varied physiologic functions throughout the body including neuroimmune modulatory effects. The 2 principle endocannabinoid receptors (CB) are CB1 and CB2. CB1 receptors are found primarily in presynaptic neurons and are abundant in peripheral and central nociceptive pathways.1,82,83,102 CB2 receptors are expressed at lower levels in neurons and principally reside in peripheral tissues and leukocytes, including microglia.162 While CB1 expression appears constitutive in the CNS, CB2 is highly induced by inflammation and tissue injury.20,21,40,41,125 However, it remains unclear if the increase in CB2 is due to increased expression in resident leukocytes or due to infiltration from CB2 expressing monocytes. In response to high levels of activity, the primary endocannabinoid ligands 2-arachidonoylglycerol and arachidonoylethanolamide are synthesized from membrane phospholipids in postsynaptic neurons.4,45,222 Glial cell production of endocannabinoids has also been demonstrated in vitro, and is postulated to occur during neuronal injury.32,52 Ligand binding to CB1r results in antinociception by activation of descending antinociceptive pathways and inhibition of nociceptive neurotransmission and supraspinal processing.1,82,102,139,153 Cannabinoids exert broad antiinflammatory effects on peripheral leukocytes and glial cells, including reduced proinflammatory cytokine release, increased antiinflammatory cytokine release, decreased cell migration and activation, and inducing apoptosis.30,167
Effect of Pain on Immune System Function
Clearly, the nervous and immune systems are inexorably linked. However, separating the effect of pain resulting from tissue injury and the direct effect of tissue damage on immune function can be problematic. In addition, the effects of chronic pain on immune system function are significantly different than the effects of acute pain, and often involves a chronic inflammatory stimulus. Experimental procedures that employ noxious stimuli which do not (or should not) cause tissue damage have been shown to suppress selective immune function. For example, foot shock has been shown to suppress NK cell activity and mitogen induced cell proliferation. 172,183,193 Suppression of antigen stimulated IgG production and a reduced in vitro proliferative response to alloantigens (as assessed by mixed lymphocyte reaction) has been demonstrated in a tail-shock model.63,112 These studies suggest the possibility that pain or aversion (stress) induce the release of immunosuppressive hormones that modulate immune function in these models.
Surgery
Surgical procedures have well documented and marked effects on immune system function in humans, including increased susceptibility to infection, delayed wound healing, and enhanced tumor growth and spread of metastatic cancer.44,77,135 Similar data exists in animal models. Reduced NK and B-cell and T- cell activity and enhanced tumor growth have been demonstrated in rat and mouse surgical model.7,19,159,168,186,204,205 Impairment of macrophage function, including reduced phagocytosis of pathogens, microbicidal activity and H2O2 release and seemingly paradoxical increased TNFα release has been shown after surgical procedures in rodents.92,142,149,189 Macrophage dysfunction shows a phasic response over time in surgical models and decreased antigen presentation can last for a week.142 T-cell dysfunction characterized, by decreased production of IL2, IFNγ, and loss of T cell receptor – ζ occurs after laparotomy in mice, and may be due to T-cell suppression by myeloid CD11b+/Gr-1+ cells that infiltrate the spleen after surgery.132 Serum levels of the proinflammatory cytokines IL6 and IL1β transiently increase after laparotomy, and the potent angiogenic cytokine Vascular Endothelial Growth Factor, implicated in enhanced tumor growth, increases significantly around 6 to 12 d after surgery.168 Seven days after surgical trauma and hemorrhage, there is a shift in splenic T-helper cytokine profiles from Th1 (decreased IFNγ, IL2) to Th2 (increased IL4, IL5, IL6, and IL10) in mouse.130 Shifts in Th1 to Th2 phenotypes are associated with increased susceptibility to viral, bacterial and helminth infections and the development of sepsis.87,105 In addition, the effects of the surgical trauma and hemorrhage protocol on immune function are more pronounced in 18 to 20 mo old animals compared with 6 to 8 wk old.98
The mechanisms underlying immunosuppression in surgery and trauma models are complex and involve, pain, activation of the Hypothalamic-Pituitary-Axis, sympathetic nervous system activation, tissue trauma, and the effects of anesthesia and analgesia.81,105,107 Despite evidence that analgesics can inhibit immune function, a significant body of research suggests that robust pain management in humans reduces surgically related immunosuppression.3,9,17,18,97,192,225, 227 Although not as extensive as the human literature, similar findings have been made in rodents, which suggest that surgical pain management improves immune function and reduces tumor spread.14,74,165,166,192
Analgesic Modulation of Immune Function.
Opioids.
Opioids are some of the most common and potent analgesics used in laboratory animal medicine and in vivo research. Considerable effort has gone into elucidating the effects of opioids on immune function, however, the exact mechanism by which opioids modulate immune function has not been clearly elucidated. Postulated mechanisms for opioid modulation of immune function include alterations in the Hypothalamic-Pituitary-Axis, mu-opioid receptor activation, drug binding to nonopioid receptors on leukocytes, modulation of autonomic tone, drug structure, or a combination of effects.5,25 In general, opioids can be classified as drugs with mild to moderate effects on immune function (buprenorphine, hydromorphone, oxycodone, tramadol, hydrocodone, oxycodone) or marked effects on immune function (codeine, methadone, morphine, fentanyl, remifentanil).5 Because the effects of opioids on immune function vary by drug and species, this discussion will examine the immune modulatory profile of each drug individually.
Buprenorphine.
Arguably, buprenorphine is the most commonly used opioid in laboratory animal medicine. It appears that buprenorphine has the least effect on immune function, compared to other opiods, although not inert in this respect. When used as an analgesic in the guinea pig Sereny test (0.05 mg/kg BID for the duration of the test) buprenorphine had no effect on Shigella antigen induced or vaccine induced antibody responses or severity ratings.73 When infused to healthy dogs for 24 h, buprenorphine (1.7 µg/kg/h) had no effect on leukocyte stimulated cytokine production, apoptosis, neutrophil phagocytosis, or oxidative burst. Similar effects were noted for morphine.151
Pain induced by immunization with complete Freund adjuvant (CFA) and incomplete Freund adjuvant (IFA) in mice was reduced by buprenorphine (0.1 mg/kg BID X 72 h) and did not impair vaccine induced IgG titers.108 Infusion of buprenorphine in mouse for up to 7 d at 300 µg/day had no effect on NK cell activity and splenocyte lymphoproliferation, γ interferon release or IL2 production.140 In the mouse intracranial lymphocytic choriomeningitis virus model, infusion of buprenorphine (0.15 mg/kg/d) reduced pain scores and had no effect on the numbers of splenic CD8+,CD4+, NK1.1, and CD19+ cells or cytotoxic T-cell responses to viral epitopes.155 CNS Infiltration of leukocytes and virus-specific cytotoxic T cells in response to infection was also not affected.155 Administration of buprenorphine to mice at 2 mg/kg SID for 7 d had no effect on IgG and IgM titers in responses to sheep red blood cells, and increased the number of antibody producing cells.60 In the same study, using a contact hypersensitivity model, a process dependent on Th-1 lymphocytes and macrophage function, buprenorphine and oxycodone were shown to suppress reactions during the induction and effector phase.60 Nitric oxide release from macrophages was suppressed, and no significant effects on cytokine release from either unstimulated or LPS stimulated macrophages was noted.60 Although not reported as statistically significant, macrophage surface markers were also reduced by buprenorphine treatment.60
Buprenorphine can have strain and species dependent effects. In Lewis rat, buprenorphine reduced NK cell activity and suppressed mitogen stimulated proliferation and γ-interferon release from splenic lymphocytes in a dose-dependent fashion.33 Suppression of immune function was noted after single doses of buprenorphine either 0.1 and 1.0 mg/kg, although not at 0.01 mg/kg. The immunosupressive effects of buprenorphine were inhibited by administration of naltrexone, suggesting mu-receptor modulation of immune function in this study.33 Conversely, in Fischer rats, 2 doses of buprenorphine (0.1 mg/kg) given 5 h apart, were shown to preserve NK cell function in a surgical model64 and 0.66 nmol injected once into the midbrain had no effect on splenic NK cell, T cell, and macrophage function.68
The advent of sustained release formulations of buprenorphine invites questions as to the potential effects of such preparations on immune function. Evidence is emerging that sustained release buprenorphine has a different immunomodulatory fingerprint and may be less immunomodulatory than buprenorphine HCl.6,78
Morphine and Fentanyl.
Morphine and fentanyl have well documented immunosuppressant effects in humans. Owing to their infrequent use as analgesics, the effects of morphine and fentanyl on immune function in laboratory animals is not as well established. It is clear; however, that morphine and fentanyl have different immunomodulatory profiles, despite their antinociceptive action being primarily through mu receptor binding. In the mouse, fentanyl infusion (12.5 mg/h) over 7 d resulted in significant depression of NK cell activity, lymphoproliferation and IL2 and IFNγ release at day 1 and 3 of treatment.140 At day 7, immunotolerance appeared to develop, and no significant changes in the aforementioned dependent measures were noted.140 Several studies in mouse have documented the suppressive effects of morphine and fentanyl on macrophage dependent humoral responses, stimulation of reactive oxygen intermediate production, and the alteration of immune responses in a contact hypersensitivity model.60,61 Morphine and fentanyl inhibit LPS induced TNFα release after single doses.146 Repeated treatment every 8 h induces immunotolerance to morphine and sensitization to fentanyl after 6 to 8 doses.150 Single doses of morphine (0.1 to 10 mg/kg) had antiinflammatory effects in a murine incision model.38 However the relevance of all these findings to clinical analgesia is questionable.
Tramadol.
Although not commonly used, tramadol appears to have antinociceptive effects in rodents and dog.122,152,182,198,230 Tramadol is considered a drug with minimal immunosuppressive activity11,122,182,198,230 although it can have profound antiinflammatory action and in some models be an immunostimulant.23,181,230
Local Anesthetics
Local anesthetics (LAs) are extremely effective and are important drugs for pain prevention and management protocols. All LAs work through the same basic mechanism, by inhibiting voltage gated sodium channels in nociceptive neurons, blocking depolarization and thus, neurotransmission. Thus, LAs would be expected to exert an antiinflammatory effect by preventing the release of proinflammatory molecules that occurs when nociceptive neurons depolarize. Because a component of the pathophysiology of inflammatory pain is upregulation of sodium channels in nociceptive neurons, in this context, LAs inhibition of nociceptive neuron depolarization should prevent peripheral and central sensitization induced by inflammatory mediators.8 Most studies on LAs use lidocaine as the prototypical drug and occasionally bupivacaine, and assume comparable effects across all LAs. Leukocytes, excepting neutrophils, express voltage gated sodium channels, some of which may be important in microglia and macrophage function.42 The extent to which the direct inhibition of Na channels on leukocytes, interactions with other receptors such as G-protein-coupled receptors, and the indirect inhibition of inflammatory mediator release contributes to the immunomodulatory effects of LAs is not known. Another noteworthy phenomenon is that the antiinflammatory effects of LAs in vitro require supra-clinical drug concentrations and that in vivo effects occur at clinically relevant doses. LAs have been shown to modulate PMNs, macrophages, and cytokine release in a variety of models.82 PMN and macrophage functions (including chemotaxis, adherence, production of toxic oxygen species, phagocytosis, and cytokine release) are inhibited by LAs.10,12,13,70,84,191 Lidocaine has been shown to inhibit cell proliferation, cytokine production, and mitogen-activated protein kinase activation in T cells and upregulate regulatory T-cells that promote an antiinflammatory t-cell phenotype.84,94,101,118 One study in mouse showed that release of antiinflammatory cytokine IL10 may be enhanced by lidocaine.211 Questions remain as to how long the immunomodulatory effects of LAs persist after drug administration is complete. To date, there do not appear to be any studies that have addressed this question.
Nonsteriodal Antiinflammatory Drug (NSAID).
NSAID are arguably the most commonly used class of analgesic drugs in veterinary medicine and their use is prevalent in laboratory animal medicine. All NSAID work through the same primary mechanism; the inhibition of prostaglandin synthesis by inhibition of Cyclooxygenase (COX) isoenzymes 1 and or 2. NSAID anti-inflammatory and toxic effects, mediated by inhibition of prostaglandin synthesis, is exceptionally well documented in both human and veterinary literature. However, the analgesic effects of NSAID do not seem to rely on how selective an NSAID is for COX1 or 2. Recently, a host of prostaglandin and COX independent anti-inflammatory and analgesic effects have been proposed for NSAID. These effects vary by drug, but include antioxidant activity, inhibition of Nuclear Factor-κ B, inhibition of 5-lipoxygenase, prostaglandin receptor antagonism, anti-bradykinin actions and inhibition of fatty acid amide hydrolase, cytokine release, cell adhesion, and metabolism of arachidonic acid. 27,46,48,56,79,89,93,115,157,188 Since virtually every cell in the body constitutively expresses COX1, and COX2 can be markedly induced by inflammatory mediators, inhibition of COX has been ascribed to anti-inflammatory action in a staggering number of human and animal models. In addition, a wide range of behavioral actions have been associated with NSAID inhibition of COX.34,119,128,160 Data have been compiled on NSAID classified by chemical structure, COX selectivity, and putative mechanism of action. Any data on COX inhibitors must be carefully evaluated, since COX selectivity is almost always based on in vitro determinations using human cells, varies depending on the type of assay employed, and may not translate from human to animal cells or from one species to another.47,114,115,117,176 Thus the impact of any given NSAID on immune function in a particular animal species cannot be accurately extrapolated from other NSAID or human data.
Although macrophages and neutrophils are thought to be the principle target leukocytes for NSAID actions, T cells and NK cells may also be impacted by NSAID. In neurodegenerative diseases with an inflammatory component, such as Alzheimer, the immune function of neurons, microglia, astrocytes, and endothelial cells can all be altered by NSAID.123 In T cells, NSAID inhibition of COX1 interferes with T cell receptor dependent activation of p38 MAP-kinase, which blocks upregulation of COX2.163 Both isoforms of COX and their metabolites play a significant role in the differentiation of CD4+ T cells to Th1, Th2, and Th17 phenotypes. In general, COX and their eicosanoid products suppress Th1 differentiation, and augment Th2 and Th17 phenotypes and function.117 In this fashion, NSAIDs may profoundly alter immune function, impacting a wide variety of models and processes that depend on CD4+ T-cell differentiation.
The effects of NSAID on immune function varies by compound and species. The following section will discuss the effect of the most commonly used NSAID drugs (carprofen, ketoprofen, meloxicam), on immune indices.
Meloxicam.
In a mouse vaccination study using complete CFA, meloxicam was shown to reduce CFA associated pain without altering primary or secondary antibody responses.108 In 2 separate mouse models of infectious disease, meloxicam was shown to markedly reduced release of PGE2, TNFα, IFNγ, IL4, IL10 and increase IL2 release from splenocytes.144,145 Normalization of lymphoproliferation, and reduced parasitemia and mortality were noted in response to meloxicam in the T. cruzi study.145 Meloxicam has also been shown to inhibit Nuclear Factor-κ B activation in LPS stimulated mouse macrophages.89 Conversely, in a rabbit model of antigen induced arthritis, meloxicam was shown to decrease PGE2, leukocyte infiltration and release of IL8 and had no effect on monocyte chemotactic peptide-1.124 Meloxicam had no effect on LPS stimulated serum IL6 release and augmented TNF release in Guinea pig.180 To date, no data has been published on immune modulation by the sustained release formulation of meloxicam.
Carprofen.
Similar to results for meloxicam in mouse,105 carprofen had no significant effect on CFA enhanced polyclonal antibody production in rabbit.62 Carprofen reduced inflammatory cell infiltrates, thrombus weight, vein wall thickness, and serum IL6 in a mouse model of venous thrombosis.80 TNFα activity was reduced by carprofen in a rat subcutaneous pouch model of inflammation109 In a mouse model of traumatic brain injury, carprofen was shown to be neuroprotective and reduced brain levels of IL6 and IL1.208
Ketoprofen.
Although several studies on the effects of ketoprofen on immune endpoints in rat have been reported, the use of this drug in rat is likely contraindicated due to its potential for gastrointestinal toxicity194 and availability of other, less toxic options. In mice, ketoprofen has been shown to have profound effects on clinical endpoints, reducing cytokine release, and suppressing lymphocyte proportions of Th1 and Th17 cells in a collagen-induced arthritis model.37 In several mouse models, ketoprofen has been shown to increase TNFα levels which appears to be an effect of the S-isomer of the drug.66,67,141,158 In pig, ketoprofen can inhibit LPS stimulated cytokine release in vitro, although not in vivo, despite inhibiting PGE2 under both conditions.224
Model Specific Effects of Analgesia
Rodents are commonly used for studies of immunology, inflammation, and infectious disease. A partial list includes vaccine development, antibody production, inflammation induced with CFA or carrageenan, and models of inflammatory bowel disease and arthritis. The majority, if not all, of these studies are completed in rodents without analgesics despite being associated with significant levels of pain. A limited number of infectious disease models have assessed the effects of analgesia on immune endpoints and disease severity or mortality. The following section will discuss the effects of analgesia on immune function and in specific models.
Vaccines and Monoclonal Antibody Production.
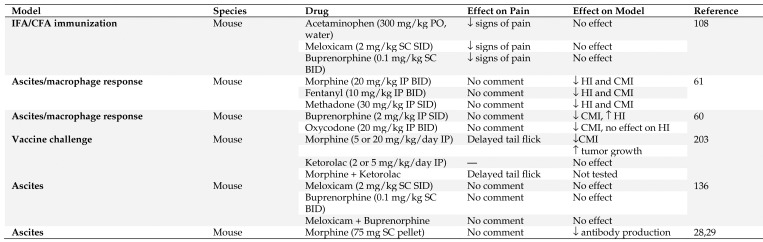
The administration of vaccines is not generally associated with pain; however, the administration of infectious agents or neoplastic cells that the vaccines are targeting may be associated with significant pain. This is especially true with the recent focus on the use of vaccines and immunotherapies to treat various cancers. Unfortunately, very few studies have attempted to look at the effects of analgesics on vaccine efficacy (see Figure 2). Kolstad and colleagues demonstrated that acetaminophen, meloxicam, and buprenorphine decreased signs of pain in male C57BL/6J mice, but did not decrease the antibody response to immunization with antigen in either CFA or IFA.108 However, in conflict with this, Filipczak and colleagues showed that the timing of administration and the type of opioid administered affects the cell- and humoral- mediate immune response in CBA mice, with oxycodone having the weakest immunomodulatory properties in mice.60,61 Another group, who recognized that analgesics are never withheld from cancer patients, specifically studied the effects of physiologically relevant doses of analgesics on an antitumor vaccine. This study found that morphine administered alone suppressed the antitumor effect of the antigen-specific DNA vaccine, but when coadministered with ketorolac, analgesia was provided to female C57BL/6 mice without compromising the antigen-specific immunity and antitumor effect of the naked DNA vaccine.203
Figure 2.
Summary of vaccine and antibody production models in which analgesic effects were evaluated. HI – humeral immunity, CMI – cell mediated immunity, SID – once a day, BID – twice a day, PO – by mouth, SC – subcutaneous, IP - intraperitoneal
While vaccines may not be painful, monoclonal antibody production can be associated with significant amounts of pain and distress.170,178 In vivo growth of hybridoma cells, resulting in accumulation of ascites fluid, has been reported to be a source of pain and distress, as has the injection of adjuvants and antibodies used to induce ascites.202 The effects of morphine on antibody production has been evaluated in a number of studies, and results suggest that it may suppress antibody production in a strain, but not sex dependent manner.28 More specifically, morphine consistently suppressed the primary antibody response in C3HeB/FeJ, C3H/HeJ, and C57BL/6 but not CxBk/ByJ or Balb/cByJ mice.28 In addition, C57BL/6J bgJ/bgJ mice, which tend to be less sensitive than other strains to analgesic effects of morphine, were shown to have a decreased capacity to respond to antigenic challenge when implanted with morphine pellets.29
In contrast to morphine, clinically relevant doses of meloxicam, buprenorphine, or a combination of both, did not affect antibody production in male BALB/c mice injected with pristane followed by hybridoma cells for antibody production, compared with saline controls.136
Due to the variety of immunomodulatory effects seen in vaccine and antibody production studies, caution should be used with any analgesic agent. Partial µ-agonists (for example buprenorphine) or combinations of NSAID and partial µ-agonist can likely be used, but pilot studies may be necessary to identify any potential confounding effects of drug administration.
Inflammation models.
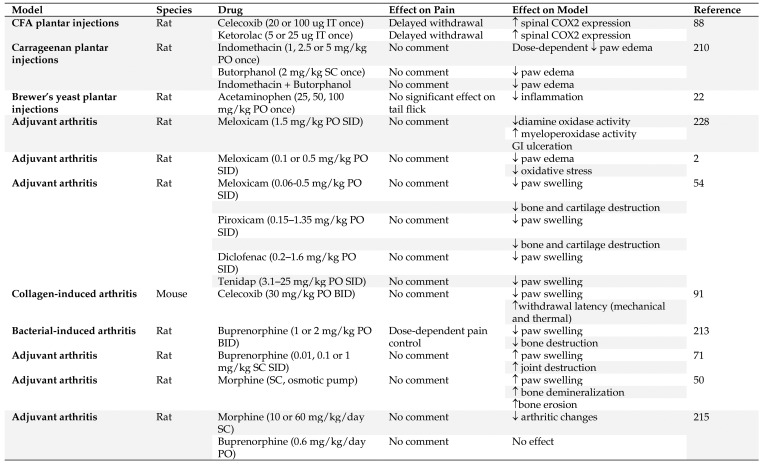
Inflammation and associated pain is a primary component of many disease and injury conditions. Inflammatory pain can result from thermal, chemical, or mechanical injuries via nociceptors in the neural system. Mice and rats are used in a variety of different inflammation models that mimic the human condition, most commonly without any analgesia despite the knowledge that these conditions are associated with significant pain in humans. Figure 3 summarizes the effects that analgesics have been reported to have in models of inflammation.
Figure 3.
Summary of inflammation models in which analgesic effects were evaluated. IT – intrathecal, SID – once a day, BID – twice a day, PO – by mouth, SC – subcutaneous, IP - intraperitoneal
Complete Freund Adjuvant and Carrageenan.
An inflammatory state can be created by injecting chemical agents, such as CFA or carrageenan. Plantar intradermal injections of CFA have been used to study the effects of COX isoenzymes and is also a good model for studying novel analgesics for rheumatoid arthritis.152 Both ketorolac and celecoxib, administered intrathecally, transiently increased expression of inducible COX2 in the spinal cord of male Sprague–Dawley rats with adjuvant induced inflammation and relieved thermal hyperalgesia through blockade of COX.88 In CFA-induced unilateral paw inflammation in a rodent model, µ and κ agonists decrease the severity of inflammation.201 Similarly, carrageenan injection induces granuloma formation which has been used to evaluate general antiinflammatory agents. Butorphanol decreased paw inflammation following carrageenan injections, with or without concurrent administration of indomethacin in Sprague–Dawley rats,210 and acetaminophen reduced inflammatory hyperalgesia without affecting inflammation and central hyperalgesia in male Sprague–Dawley rats.22 It appears that both NSAID and opioids can have strong inflammation-modulating effects in these models and that their use is best avoided to avoid confounding analysis of the inflammatory response.
Rheumatoid Arthritis.
Rheumatoid arthritis (RA) is a painful, chronic, autoimmune disease. Rodent models of rheumatoid arthritis are similarly painful, and significant refinement of these models to improve rodent welfare is necessary. NSAIDs are the mainstay therapy for pain relief in human RA patients, and opioids are rarely used. Although NSAID may provide appropriate analgesia for rodent subjects in models of RA, they can also markedly confound experimental results, by significantly modulating the inflammatory response and decreasing disease severity.2,54,75,91,228 Opioids have shown variable effects on model endpoints that depend on the animal stock or strain used, type of opioid administered route of administration, and method of arthritis induction.50,71,213,215 A full discussion of the various effects of both NSAID and opioid analgesic agents can be found in the review by Peterson and colleagues.171 Because of the mixed response to conventional analgesics, pilot studies should be performed to evaluate the confounding effects of any analgesic and nonpharmacological measures are strongly recommended to enhance animal comfort and welfare.
Inflammatory Bowel Disease (IBD).
IBD is a complex inflammatory disease that is generally considered to include both ulcerative colitis (UC) and Crohn disease (CD). Inflammatory lesions are generally limited to the large intestines and rectum in UC, but can occur in any part of the gastrointestinal tract in CD.175 Regardless of the type of IBD, the condition is generally associated with significant abdominal pain, and requires management with an analgesic regimen in humans. Current work in mice shows that activation of the polymodal ion channel TRPV1 is also associated with chronic abdominal pain in the dextran sodium sulphate model (DSS) of ulcerative colitis.110 Unfortunately, translational rodent models frequently ignore the pain component of the disease process and analgesics are not commonly provided.
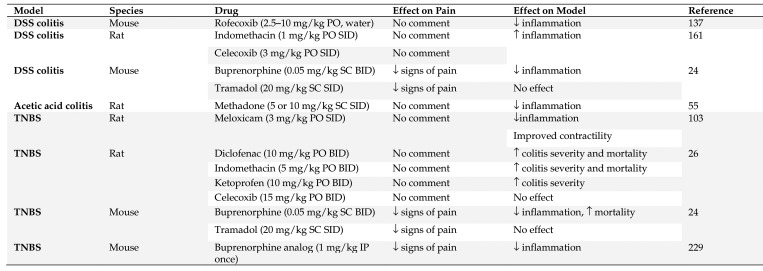
Many different methods are commonly employed to induce experimental inflammatory bowel disease. These are associated with acute and chronic intestinal inflammation and they all recapitulate different aspects of IBD.53,175,221,222 Pain is an essential feature of IBD and optimal treatment in animals can aid the translation to human medicine, where the challenge of intestinal pain is frequently met with opioids.24,31 This is because IBD is characterized by periods of remission and reactivation, and NSAID consumption is considered a primary cause of disease reactivation.57,106 Figure 4 summarizes the effects that analgesics have been reported to have in models of IBD.
Figure 4.
Summary of IBD models in which analgesic effects were evaluated. SID – once a day, BID – twice a day, PO – by mouth, SC – subcutaneous, IP - intraperitoneal
In human medicine, it is not uncommon to also use nontraditional analgesic agents to manage the visceral pain associated with IBD.31,197 This includes: antidepressants, peppermint oil (antispasmodic), 5-HT3 receptor antagonists, nonabsorbed antibiotics (such as, rifaximin), secretogogues, H1-receptor antagonists, Neurokinin-2 receptor antagonists, and GABAergic agents.31,197 These agents remain largely untested in animals, but may provide alternative means of analgesia for the pain associated with experimental models of both UC and CD.
Ulcerative Colitis.
Dextran sodium sulphate (DSS) causes a progressive chemical injury to the intestinal epithelium, resulting in exposure of the lamina propria and submucosal compartment to luminal antigens and enteric bacteria, thereby triggering inflammation.100 The effectiveness of DSS-induced UC depends on several factors, including dosage (typically 1% to 5% DSS), duration (acute or chronic), manufacturer or batch of DSS, strain of animals (C3H/HeJ and BALB/c mice strains are more susceptible), sex of animals (male mice are more susceptible), and microbiota of animals (for example germ free compared with SPF).24,51,100,126,169 Several NSAID and opioids have been evaluated in both mice and rats in the DSS model for their effects on the inflammatory process. Rofecoxib decreased inflammation in male BABL/c mice,137 whereas indomethacin and celecoxib both worsened the severity of inflammation in both sexes of Wistar rats.161,195 Interestingly, although celecoxib administration exacerbated inflammation it protected from ulceration.195 Buprenorphine was generally antiinflammatory in both BALB/c and CD1 mice, whereas tramadol did not affect inflammation, based on scoring of gut histology. Both treatment regimens appeared to provide adequate analgesia, and the authors recommend tramadol for future studies in either strain of mice.24
Oxazolone causes a superficial inflammatory acute colitis that is limited to the distal colon.100, 126, 226 Animals demonstrate weight loss, diarrhea, ulcers, and loss of epithelial cells in the large intestines. Although rodents are anesthetized for intrarectal administration of Oxazolone, to the authors’ knowledge, there have been no studies on the effects of analgesics, nor has analgesic use been documented in this model.100,126,226
Acetic acid administration causes a chemical injury to the mucosal epithelium that induces a transient phenotype mimicking UC.53,126,131 The injury is characterized by ulceration of the distal colon and crypt abnormalities that begin to heal within days in mice and a few weeks in rats.53,126,131 Few studies have evaluated the effects of analgesics in this model. In one study, specifically looking at the gastroprotectant effects of opioids, methadone improved macroscopic and microscopic disease scores of colitis in male Wistar rats previously treated with acetic acid.55
Crohn Disease.
In the 2,4,6-Trinitrobenzene sulphonic acid (TNBS) model of Crohn disease, TNBS disrupts the epithelial layer of the colon and exposes the underlying lamina propria to bacterial components that lead to a severe transmural infiltrative colitis.100 Colitis is associated with diarrhea, rectal prolapse, and weight loss. Several NSAID and opioids have been evaluated in both mice and rats in the TNBS model for their effects on the inflammatory process. Administration of rofecoxib reduced the colonic damage and inflammation in Wistar rats.138 Administration of meloxicam to male Sprague–Dawley rats restored colonic contractility and decreased colonic inflammation.103 Diclofenac, indomethacin, and ketoprofen all exacerbated colitis in male Wistar rats, but celecoxib had no significant effect.26 In BALB/c mice, tramadol administration did not affect inflammation, but buprenorphine was antiinflammatory.24 BU08070, a buprenorphine analog, produced a concentration-dependent decrease in inflammation and visceral pain-induced behaviors in male BALB/c mice.229
To keep murine models of UC and CD consistent with human treatments, opioids are generally recommended as therapeutics to decrease model associated discomfort and improve animal welfare. However, pilot studies are warranted to evaluate for potential confounding effects of opioid or NSAID analgesia in the specific model type and species. Based on the collective body of literature described herein, tramadol should be considered for IBD studies due to its clinical efficacy for relieving visceral pain and its lack of modulatory effects on inflammation.
Infectious Disease Models.
Most extant studies on the effects of analgesics on immune function and disease in infectious disease models have used NSAID to explore the role of COX and prostaglandins in disease pathogenesis.113,144,145,156,196,218 The one notable exception is a study on the effect of buprenorphine in a mouse model of intracranial lymphocytic choriomeningitis virus (LCMV) infection.155 Intracranial LCMV in mouse is used to model CTL-mediated meningitis, and produces characteristic fatal meningitis 6 to 8 d post infection, which may be associated with significant pain and distress.99 Mice intracranially infected with LCMV and treated with buprenorphine (0.05 mg/kg s.c) followed by osmotic pump delivery (0.15 mg/kg/day) for 1 wk, had markedly reduced pain scores and no clinical signs of pain.155 Buprenorphine treatment had no effect on LCMV-induced CTL responses or LCMV induced brain infiltration by lymphocytes and virus specific CTLs.155
Conclusion
The balance between appropriate analgesic use for animal welfare, and analgesic impact on experimental results continues to present significant challenges to the research community.171 Furthermore, relatively little is currently known about the role of gender in the interaction between analgesics and immune function. However, gender has a major influence on both the prevalence and severity of pain and sex related differences in neuroimmune interactions (in particular glial cell function) appears to underpin this phenomenon.49,96,134 Thus in light of NIH directives, better understanding of gender-related differences in the effects of pain and analgesia on neuroimmune function in preclinical studies is critically important. In human medicine, archaic concepts such as “pain medication may mask clinical signs” and “nobody ever died from pain” have been refuted by years of research and clinical experience. It would be unethical and malpractice to withhold analgesics from human patients experiencing pain from cancer, autoimmune disease, infection or the innumerable other diseases which cause pain. In this context, the possibility should be considered that in some instances the translatability of animal models may be improved if analgesics are administered, not withheld, and used in a manner that more closely matches human treatment.39,90 What is clear from this review is that many questions remain regarding the impact of analgesics on immune function and that there is no one drug that represents the “Magic Bullet” analgesic for all models. In many cases, the literature is incomplete, or does not exist, necessitating empirical choices or pilot studies to evaluate or optimize the use of analgesics for in vivo studies of immunology and inflammation. Responsibility for appropriate analgesic drug use in the absence of published data lies with the investigator, and is shared with laboratory animal veterinarians and IACUC members. Our hope is that research and development of new analgesic drugs and regimens will progress and help improve our ability to appropriately manage pain and minimally impact experimental results
References
- 1.Agarwal N, Pacher P, Tegeder I, Amaya F, Constantin CE, Brenner GJ, Rubino T, Michalski CW, Marsicano G, Monory K, Mackie K, Marian C, Batkai S, Parolaro D, Fischer MJ, Reeh P, Kunos G, Kress M, Lutz B, Woolf CJ, Kuner R. 2007. Cannabinoids mediate analgesia largely via peripheral type 1 cannabinoid receptors in nociceptors. Nat Neurosci 10:870–879. 10.1038/nn1916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Agha AM, El-Khatib AS, Al-Zuhair H. 1999. Modulation of oxidant status by meloxicam in experimentally induced arthritis. Pharmacol Res 40:385–392. 10.1006/phrs.1999.0522. [DOI] [PubMed] [Google Scholar]
- 3.Ahlers O, Nachtigall I, Lenze J, Goldmann A, Schulte E, Höhne C, Fritz G, Keh D. 2008. Intraoperative thoracic epidural anaesthesia attenuates stress-induced immunosuppression in patients undergoing major abdominal surgery. Br J Anaesth 101:781–787. 10.1093/bja/aen287. [DOI] [PubMed] [Google Scholar]
- 4.Ahn K, McKinney MK, Cravatt BF. 2008. Enzymatic pathways that regulate endocannabinoid signaling in the nervous system. Chem Rev 108:1687–1707. 10.1021/cr0782067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Al-Hashimi M, Scott SWM, Thompson JP, Lambert DG. 2013. Opioids and immune modulation: more questions than answers. Br J Anaesth 111:80–88. 10.1093/bja/aet153. [DOI] [PubMed] [Google Scholar]
- 6.Allen A, Kendall LV. 2018. Measuring immune system perturbations associated with the use of buprenorphine in laboratory mice. Abstracts presented at the AALAS 69th National Meeting, Baltimore, Maryland, October 28–01 November 2018. J Am Assoc Lab Anim Sci 57:609. [Google Scholar]
- 7.Allendorf JD, Bessler M, Horvath KD, Marvin MR, Laird DA, Whelan RL. 1999. Increased tumor establishment and growth after open vs laparoscopic surgery in mice may be related to differences in postoperative T-cell function. Surg Endosc 13:233–235. 10.1007/s004649900952. [DOI] [PubMed] [Google Scholar]
- 8.Amir R, Argoff CE, Bennett GJ, Cummins TR, Durieux ME, Gerner P, Gold MS, Porreca F, Strichartz GR. 2006. The role of sodium channels in chronic inflammatory and neuropathic pain. J Pain 7:S1–S29. 10.1016/j.jpain.2006.01.444. [DOI] [PubMed] [Google Scholar]
- 9.Amodeo G, Bugada D, Franchi S, Moschetti G, Grimaldi S, Panerai A, Allegri M, Sacerdote P. 2018. Immune function after major surgical interventions: the effect of postoperative pain treatment. J Pain Res 11:1297–1305. 10.2147/JPR.S158230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ando T, Ogawa J, Fujiwara H, Yokotachi S, Maeda K, Kohiruimaki M, Ohtsuka H, Watanabe D. 2009. Effect of lidocaine hydrochloride on the function of bovine peripheral leukocytes. J Vet Med Sci 71:387–390. 10.1292/jvms.71.387. [DOI] [PubMed] [Google Scholar]
- 11.Axiak-Bechtel SM, Tsuruta K, Amorim J, Donaldson R, Lino G, Honaker A, Monibi F, Dodam J, DeClue A. 2015. Effects of tramadol and o-desmethyltramadol on canine innate immune system function. Vet Anaesth Analg 42:260–268. 10.1111/vaa.12201. [DOI] [PubMed] [Google Scholar]
- 12.Azuma Y, Ohura K. 2004. Immunological modulation by lidocaine-epinephrine and prilocaine-felypressin on the functions related to natural immunity in neutrophils and macrophages. Curr Drug Targets Immune Endocr Metabol Disord 4:29–36. 10.2174/1568008043339974. [DOI] [PubMed] [Google Scholar]
- 13.Azuma Y, Shinohara M, Wang PL, Suese Y, Yasuda H, Ohura K. 2000. Comparison of inhibitory effects of local anesthetics on immune functions of neutrophils. Int J Immunopharmacol 22:789–796. 10.1016/S0192-0561(00)00040-0. [DOI] [PubMed] [Google Scholar]
- 14.Bar-Yosef S, Melamed R, Page GG, Shakhar G, Shakhar K, Ben-Eliyahu S. 2001. Attenuation of the tumor-promoting effect of surgery by spinal blockade in rats. Anesthesiology 94:1066–1073. 10.1097/00000542-200106000-00022. [DOI] [PubMed] [Google Scholar]
- 15.Baral P, Udit S, Chiu IM. 2019. Pain and immunity: implications for host defence. Nat Rev Immunol 19:433–447. 10.1038/s41577-019-0147-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Beggs S, Liu XJ, Kwan C, Salter MW. 2010. Peripheral nerve injury and TRPV1-expressing primary afferent C-fibers cause opening of the blood-brain barrier. Mol Pain 6:1–12 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2984489/pdf/1744-8069-6-74.pdf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Beilin B, Bessler H, Mayburd E, Smirnov G, Dekel A, Yardeni I, Shavit Y. 2003. Effects of preemptive analgesia on pain and cytokine production in the postoperative period. Anesthesiology 98:151–155. 10.1097/00000542-200301000-00024. [DOI] [PubMed] [Google Scholar]
- 18.Beilin B, Shavit Y, Trabekin E, Mordashev B, Mayburd E, Zeidel A, Bessler H. 2003. The effects of postoperative pain management on immune response to surgery. Anesth Analg 97:822–827. 10.1213/01.ANE.0000078586.82810.3B. [DOI] [PubMed] [Google Scholar]
- 19.Ben-Eliyahu S, Page GG, Yirmiya R, Shakhar G. 1999. Evidence that stress and surgical interventions promote tumor development by suppressing natural killer cell activity. Int J Cancer 80:880–888. . [DOI] [PubMed] [Google Scholar]
- 20.Benito C, Kim WK, Chavarria I, Hillard CJ, Mackie K, Tolon RM, Williams K, Romero J. 2005. A glial endogenous cannabinoid system is upregulated in the brains of macaques with simian immunodeficiency virus-induced encephalitis. J Neurosci 25:2530–2536. 10.1523/JNEUROSCI.3923-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Benito C, Tolón RM, Pazos MR, Núñez E, Castillo AI, Romero J. 2008. Cannabinoid CB2 receptors in human brain inflammation. Br J Pharmacol 153:277–285. 10.1038/sj.bjp.0707505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bianchi M, Panerai AE. 1996. The dose-related effects of paracetamol on hyperalgesia and nociception in the rat. Br J Pharmacol 117:130–132. 10.1111/j.1476-5381.1996.tb15164.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bianchi M, Rossoni G, Sacerdote P, Panerai AE. 1999. Effects of tramadol on experimental inflammation. Fundam Clin Pharmacol 13:220–225. 10.1111/j.1472-8206.1999.tb00342.x. [DOI] [PubMed] [Google Scholar]
- 24.Blennerhassett MG, Lourenssen SR, Parlow LRG, Ghasemlou N, Winterborn AN. 2017. Analgesia and mouse strain influence neuromuscular plasticity in inflamed intestine. Neurogastroenterol Motil 29:1–12. 10.1111/nmo.13097. [DOI] [PubMed] [Google Scholar]
- 25.Börner C, Lanciotti S, Koch T, Hollt V, Kraus J. 2013. µ opioid receptor agonist-selective regulation of interleukin-4 in T lymphocytes. J Neuroimmunol 263:35–42. 10.1016/j.jneuroim.2013.07.012. [DOI] [PubMed] [Google Scholar]
- 26.Breganó JW, Barbosa DS, El Kadri MZ, Rodrigues MA, Cecchini R, Dichi I. 2014. Comparison of selective and non selective cyclo-oxygenase 2 inhibitors in experimental colitis exacerbation: role of leukotriene B4 and superoxide dismutase. Arq Gastroenterol 51:226–234. 10.1590/S0004-28032014000300012. [DOI] [PubMed] [Google Scholar]
- 27.Bryant CE, Farnfield BA, Janicke HJ. 2003. Evaluation of the ability of carprofen and flunixin meglumine to inhibit activation of nuclear factor k B. Am J Vet Res 64:211–215. 10.2460/ajvr.2003.64.211. [DOI] [PubMed] [Google Scholar]
- 28.Bussiere JL, Adler MW, Rogers TJ, Eisenstein TK. 1992. Differential effects of morphine and naltrexone on the antibody response in various mouse strains. Immunopharmacol Immunotoxicol 14:657–673. 10.3109/08923979209005416. [DOI] [PubMed] [Google Scholar]
- 29.Bussiere JL, Adler MW, Rogers TJ, Eisenstein TK. 1993. Effects of in vivo morphine treatment on antibody responses in C57BL/6 bgJ/bgJ (beige) mice. Life Sci 52:PL43–PL48. 10.1016/0024-3205(93)90157-X. [DOI] [PubMed] [Google Scholar]
- 30.Cabral GA, Griffin-Thomas L. 2009. Emerging role of the cannabinoid receptor CB2 in immune regulation: therapeutic prospects for neuroinflammation. Expert Rev Mol Med 11:1–31. 10.1017/S1462399409000957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Camilleri M, Boeckxstaens G. 2017. Dietary and pharmacological treatment of abdominal pain in IBS. Gut 66:966–974. 10.1136/gutjnl-2016-313425. [DOI] [PubMed] [Google Scholar]
- 32.Carrier EJ, Kearn CS, Barkmeier AJ, Breese NM, Yang W, Nithipatikom K, Pfister SL, Campbell WB, Hillard CJ. 2004. Cultured rat microglial cells synthesize the endocannabinoid 2-arachidonylglycerol, which increases proliferation via a CB2 receptor-dependent mechanism. Mol Pharmacol 65:999–1007. 10.1124/mol.65.4.999. [DOI] [PubMed] [Google Scholar]
- 33.Carrigan KA, Saurer TB, Ijames SG, Lysle DT. 2004. Buprenorphine produces naltrexone reversible alterations of immune status. Int Immunopharmacol 4:419–428. 10.1016/j.intimp.2004.01.011. [DOI] [PubMed] [Google Scholar]
- 34.Chen Q, Luo Y, Kuang S, Yang Y, Tian X, Ma J, Mai S, Xue L, Yang J. 2017. Cyclooxygenase-2 signalling pathway in the cortex is involved in the pathophysiological mechanisms in the rat model of depression. Sci Rep 7:1–12. 10.1038/s41598-017-00609-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chiu IM, Heesters BA, Ghasemlou N, Von Hehn CA, Zhao F, Tran J, Wainger B, Strominger A, Muralidharan S, Horswill AR, Bubeck Wardenburg J, Hwang SW, Carroll MC, Woolf CJ. 2013. Bacteria activate sensory neurons that modulate pain and inflammation. Nature 501:52–57. 10.1038/nature12479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chiu IM, von Hehn CA, Woolf CJ. 2012. Neurogenic inflammation and the peripheral nervous system in host defense and immunopathology. Nat Neurosci 15:1063–1067. 10.1038/nn.3144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Choi JK, Kim SW, Kim DS, Lee JY, Lee S, Oh HM, Ha YS, Yoo J, Park PH, Shin TY, Kwon TK, Rho MC, Kim SH. 2016. Oleanolic acid acetate inhibits rheumatoid arthritis by modulating T cell immune responses and matrix-degrading enzymes. Toxicol Appl Pharmacol 290:1–9. 10.1016/j.taap.2015.11.005. [DOI] [PubMed] [Google Scholar]
- 38.Clark JD, Shi X, Li X, Qiao Y, Liang D, Angst MS, Yeomans DC. 2007. Morphine reduces local cytokine expression and neutrophil infiltration after incision. Mol Pain 3:1–12. 10.1186/1744-8069-3-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Clutton RE. 2018. A review of factors affecting analgesic selection in large animals undergoing translational research. Vet J 236:12–22. 10.1016/j.tvjl.2018.04.006. [DOI] [PubMed] [Google Scholar]
- 40.Concannon RM, Okine BN, Finn DP, Dowd E. 2015. Differential upregulation of the cannabinoid CB2 receptor in neurotoxic and inflammation-driven rat models of Parkinson's disease. Exp Neurol 269:133–141. 10.1016/j.expneurol.2015.04.007. [DOI] [PubMed] [Google Scholar]
- 41.Concannon RM, Okine BN, Finn DP, Dowd E. 2016. Upregulation of the cannabinoid CB2 receptor in environmental and viral inflammation-driven rat models of Parkinson's disease. Exp Neurol 283:204–212. 10.1016/j.expneurol.2016.06.014. [DOI] [PubMed] [Google Scholar]
- 42.Craner MJ, Damarjian TG, Liu S, Hains BC, Lo AC, Black JA, Newcombe J, Cuzner ML, Waxman SG. 2005. Sodium channels contribute to microglia/macrophage activation and function in EAE and MS. Glia 49:220–229. 10.1002/glia.20112. [DOI] [PubMed] [Google Scholar]
- 43.D'Mello R, Dickenson AH. 2008. Spinal cord mechanisms of pain. Br J Anaesth 101:8–16. 10.1093/bja/aen088. [DOI] [PubMed] [Google Scholar]
- 44.Dąbrowska AM, Słotwiński R. 2014. The immune response to surgery and infection. Cent Eur J Immunol 39:532–537. 10.5114/ceji.2014.47741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Di S, Boudaba C, Popescu IR, Weng FJ, Harris C, Marcheselli VL, Bazan NG, Tasker JG. 2005. Activity-dependent release and actions of endocannabinoids in the rat hypothalamic supraoptic nucleus. J Physiol 569:751–760. 10.1113/jphysiol.2005.097477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Díaz-González F, Gonzalez-Alvaro I, Campanero MR, Mollinedo F, del Pozo MA, Munoz C, Pivel JP, Sanchez-Madrid F. 1995. Prevention of in vitro neutrophil-endothelial attachment through shedding of L-selectin by nonsteroidal antiinflammatory drugs. J Clin Invest 95:1756–1765. 10.1172/JCI117853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Díaz-González F, Sánchez-Madrid F. 2015. NSAIDs: learning new tricks from old drugs. Eur J Immunol 45:679–686. 10.1002/eji.201445222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dik B, Coskun D, Bahcivan E, Er A. 2019. Doxycycline and meloxicam can treat neuroinflammation by increasing activity of antioxidant enzymes in rat brain. Pak J Pharm Sci 32:391–396. [PubMed] [Google Scholar]
- 49.Dodds KN, Beckett EA, Evans SF, Grace PM, Watkins LR, Hutchinson MR. 2016. Glial contributions to visceral pain: implications for disease etiology and the female predominance of persistent pain. Transl Psychiatry 6:1–13. 10.1038/tp.2016.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Earl JR, Claxson AW, Blake DR, Morris CJ. 1994. Proinflammatory effects of morphine in the rat adjuvant arthritis model. Int J Tissue React 16:163–170. [PubMed] [Google Scholar]
- 51.Eichele DD, Kharbanda KK. 2017. Dextran sodium sulfate colitis murine model: An indispensable tool for advancing our understanding of inflammatory bowel diseases pathogenesis. World J Gastroenterol 23:6016–6029. 10.3748/wjg.v23.i33.6016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Eljaschewitsch E, Witting A, Mawrin C, Lee T, Schmidt PM, Wolf S, Hoertnagl H, Raine CS, Schneider-Stock R, Nitsch R, Ullrich O. 2006. The endocannabinoid anandamide protects neurons during CNS inflammation by induction of MKP-1 in microglial cells. Neuron 49:67–79. 10.1016/j.neuron.2005.11.027. [DOI] [PubMed] [Google Scholar]
- 53.Elson CO, Sartor RB, Tennyson GS, Riddell RH. 1995. Experimental models of inflammatory bowel disease. Gastroenterology 109:1344–1367. 10.1016/0016-5085(95)90599-5. [DOI] [PubMed] [Google Scholar]
- 54.Engelhardt G, Homma D, Schnitzler C. 1995. Meloxicam: a potent inhibitor of adjuvant arthritis in the Lewis rat. Inflamm Res 44:548–555. 10.1007/BF01757360. [DOI] [PubMed] [Google Scholar]
- 55.Fakhraei N, Javadian N, Rahimian R, Nili F, Rahimi N, Hashemizadeh S, Dehpour AR. 2018. Involvement of central opioid receptors in protective effects of methadone on experimental colitis in rats. Inflammopharmacology 26:1399–1413. 10.1007/s10787-018-0538-1. [DOI] [PubMed] [Google Scholar]
- 56.Favia AD, Habrant D, Scarpelli R, Migliore M, Albani C, Bertozzi SM, Dionisi M, Tarozzo G, Piomelli D, Cavalli A, De Vivo M. 2012. Identification and characterization of carprofen as a multitarget fatty acid amide hydrolase/cyclooxygenase inhibitor. J Med Chem 55:8807–8826. 10.1021/jm3011146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Feagins LA, Cryer BL. 2010. Do nonsteroidal antiinflammatory drugs cause exacerbations of inflammatory bowel disease? Dig Dis Sci 55:226–232. 10.1007/s10620-009-1042-7. [DOI] [PubMed] [Google Scholar]
- 58.Ferrante CJ, Leibovich SJ. 2012. Regulation of macrophage polarization and wound healing. Adv Wound Care (New Rochelle) 1:10–16. 10.1089/wound.2011.0307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ferraz CC, Henry MA, Hargreaves KM, Diogenes A. 2011. Lipopolysaccharide from Porphyromonas gingivalis sensitizes capsaicin-sensitive nociceptors. J Endod 37:45–48. 10.1016/j.joen.2007.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Filipczak-Bryniarska I, Nazimek K, Nowak B, Kozlowski M, Wąsik M, Bryniarski K. 2018. In contrast to morphine, buprenorphine enhances macrophage-induced humoral immunity and, as oxycodone, slightly suppresses the effector phase of cell-mediated immune response in mice. Int Immunopharmacol 54:344–353. 10.1016/j.intimp.2017.11.039. [DOI] [PubMed] [Google Scholar]
- 61.Filipczak-Bryniarska I, Nowak B, Sikora E, Nazimek K, Woroń J, Wordliczek J, Bryniarski K. 2012. The influence of opioids on the humoral and cell-mediated immune responses in mice. The role of macrophages. Pharmacol Rep 64:1200–1215. 10.1016/S1734-1140(12)70916-7. [DOI] [PubMed] [Google Scholar]
- 62.Fishback JE, Stronsky SM, Green CA, Bean KD, Froude JW. 2016. Antibody production in rabbits administered Freund's complete adjuvant and carprofen concurrently. Lab Anim (NY) 45:63–66. 10.1038/laban.937. Erratum: Antibody production in rabbits administered Freund's complete adjuvant and carpofen concurrently. Lab Anim NY. 2016. [DOI] [PubMed] [Google Scholar]
- 63.Fleshner M, Bellgrau D, Watkins LR, Laudenslager ML, Maier SF. 1995. Stress-induced reduction in the rat mixed lymphocyte reaction is due to macrophages and not to changes in T cell phenotypes. J Neuroimmunol 56:45–52. 10.1016/0165-5728(94)00132-8. [DOI] [PubMed] [Google Scholar]
- 64.Franchi S, Panerai AE, Sacerdote P. 2007. Buprenorphine ameliorates the effect of surgery on hypothalamus-pituitary-adrenal axis, natural killer cell activity and metastatic colonization in rats in comparison with morphine or fentanyl treatment. Brain Behav Immun 21:767–774. 10.1016/j.bbi.2007.01.001. [DOI] [PubMed] [Google Scholar]
- 65.Fujino K, Takami Y, de la Fuente SG, Ludwig KA, Mantyh CR. 2004. Inhibition of the vanilloid receptor subtype-1 attenuates TNBS-colitis. J Gastrointest Surg 8:842–847, discussion 847–848. 10.1016/j.gassur.2004.07.011. [DOI] [PubMed] [Google Scholar]
- 66.Ghezzi P, Melillo G, Meazza C, Sacco S, Pellegrini L, Asti C, Porzio S, Marullo A, Sabbatini V, Caselli G, Bertini R. 1998. Differential contribution of R and S isomers in ketoprofen anti-inflammatory activity: role of cytokine modulation. J Pharmacol Exp Ther 287:969–974. [PubMed] [Google Scholar]
- 67.Ghezzi P, Sacco S, Agnello D, Marullo A, Caselli G, Bertini R. 2000. Lps induces IL6 in the brain and in serum largely through TNF production. Cytokine 12:1205–1210. 10.1006/cyto.2000.0697. [DOI] [PubMed] [Google Scholar]
- 68.Gomez-Flores R, Weber RJ. 2000. Differential effects of buprenorphine and morphine on immune and neuroendocrine functions following acute administration in the rat mesencephalon periaqueductal gray. Immunopharmacology 48:145–156. 10.1016/S0162-3109(00)00198-3. [DOI] [PubMed] [Google Scholar]
- 69.Grace PM, Hutchinson MR, Maier SF, Watkins LR. 2014. Pathological pain and the neuroimmune interface. Nat Rev Immunol 14:217–231. 10.1038/nri3621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Gray A, Marrero-Berrios I, Weinberg J, Manchikalapati D, SchianodiCola J, Schloss RS, Yarmush J. 2016. The effect of local anesthetic on proinflammatory macrophage modulation by mesenchymal stromal cells. Int Immunopharmacol 33:48–54. 10.1016/j.intimp.2016.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hall TJ, Jagher B, Schaeublin M, Wiesenberg I. 1996. The analgesic drug buprenorphine inhibits osteoclastic bone resorption in vitro, but is proinflammatory in rat adjuvant arthritis. Inflamm Res 45:299–302. 10.1007/BF02280995. [DOI] [PubMed] [Google Scholar]
- 72.Hanke ML, Kielian T. 2011. Toll-like receptors in health and disease in the brain: mechanisms and therapeutic potential. Clin Sci (Lond) 121:367–387. 10.1042/CS20110164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hanson CE, Ruble GR, Essiet I, Hartman AB. 2001. Effects of buprenorphine on immunogenicity and protective efficacy in the guinea pig keratoconjunctivitis model (Sereny test). Comp Med 51:224–229. [PubMed] [Google Scholar]
- 74.Hasegawa A, Iwasaka H, Hagiwara S, Hasegawa R, Kudo K, Kusaka J, Asai N, Noguchi T. 2011. Remifentanil and glucose suppress inflammation in a rat model of surgical stress. Surg Today 41:1617–1621. 10.1007/s00595-010-4457-z. [DOI] [PubMed] [Google Scholar]
- 75.Hawkins P, Armstrong R, Boden T, Garside P, Knight K, Lilley E, Seed M, Wilkinson M, Williams RO. 2015. Applying refinement to the use of mice and rats in rheumatoid arthritis research. Inflammopharmacology 23:131–150. 10.1007/s10787-015-0241-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Helley MP, Abate W, Jackson SK, Bennett JH, Thompson SW. 2015. The expression of Toll-like receptor 4, 7 and co-receptors in neurochemical sub-populations of rat trigeminal ganglion sensory neurons. Neuroscience 310:686–698. 10.1016/j.neuroscience.2015.09.069. [DOI] [PubMed] [Google Scholar]
- 77.Hensler T, Hecker H, Heeg K, Heidecke CD, Bartels H, Barthlen W, Wagner H, Siewert JR, Holzmann B. 1997. Distinct mechanisms of immunosuppression as a consequence of major surgery. Infect Immun 65:2283–2291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Herndon NL, Bandyopadhyay S, Hod EA, Prestia KA. 2016. Sustained-release buprenorphine improves postsurgical clinical condition but does not alter survival or cytokine levels in a murine model of polymicrobial sepsis. Comp Med 66:455–462. [PMC free article] [PubMed] [Google Scholar]
- 79.Herrera-García A, Domínguez-Luis M, Arce-Franco M, López-Fernández J, Feria M, Barreiro O, Sánchez-Madrid F, Díaz-González F. 2013. In vivo modulation of the inflammatory response by nonsteroidal antiinflammatory drug-related compounds that trigger L-selectin shedding. Eur J Immunol 43:55–64. 10.1002/eji.201242805. [DOI] [PubMed] [Google Scholar]
- 80.Hish GA, Jr, Diaz JA, Hawley AE, Myers DD, Jr, Lester PA. 2014. Effects of analgesic use on inflammation and hematology in a murine model of venous thrombosis. J Am Assoc Lab Anim Sci 53:485–493. [PMC free article] [PubMed] [Google Scholar]
- 81.Hogan BV, Peter MB, Shenoy HG, Horgan K, Hughes TA. 2011. Surgery induced immunosuppression. Surgeon 9:38–43. 10.1016/j.surge.2010.07.011 [DOI] [PubMed] [Google Scholar]
- 82.Hohmann AG. 2002. Spinal and peripheral mechanisms of cannabinoid antinociception: behavioral, neurophysiological and neuroanatomical perspectives. Chem Phys Lipids 121:173–190. 10.1016/S0009-3084(02)00154-8. [DOI] [PubMed] [Google Scholar]
- 83.Hohmann AG, Herkenham M. 1999. Localization of central cannabinoid CB1 receptor messenger RNA in neuronal subpopulations of rat dorsal root ganglia: a double-label in situ hybridization study. Neuroscience 90:923–931. 10.1016/S0306-4522(98)00524-7. [DOI] [PubMed] [Google Scholar]
- 84.Hollmann MW, Durieux ME. 2000. Local anesthetics and the inflammatory response: a new therapeutic indication? Anesthesiology 93:858–875. 10.1097/00000542-200009000-00038. [DOI] [PubMed] [Google Scholar]
- 85.Horváth A, Tékus V, Bencze N, Szentes N, Scheich B, Bölcskei K, Szőke É, Mócsai A, Tóth-Sarudy É, Mátyus P, Pintér E, Helyes Z. 2018. Analgesic effects of the novel semicarbazide-sensitive amine oxidase inhibitor SZV 1287 in mouse pain models with neuropathic mechanisms: Involvement of transient receptor potential vanilloid 1 and ankyrin 1 receptors. Pharmacol Res 131:231–243. 10.1016/j.phrs.2018.02.006. [DOI] [PubMed] [Google Scholar]
- 86.Horváth G, Kemény Á, Barthó L, Molnár P, Deli J, Szente L, Bozo T, Pál S, Sándor K, Szőke E, Szolcsanyi J, Helyes Z. 2015. Effects of some natural carotenoids on TRPA1- and TRPV1-induced neurogenic inflammatory processes in vivo in the mouse skin. J Mol Neurosci 56:113–121. 10.1007/s12031-014-0472-7. [DOI] [PubMed] [Google Scholar]
- 87.Hsing CH, Wang JJ. 2015. Clinical implication of perioperative inflammatory cytokine alteration. Acta Anaesthesiol Taiwan 53:23–28. 10.1016/j.aat.2015.03.002. [DOI] [PubMed] [Google Scholar]
- 88.Hsueh SF, Lu CY, Chao CS, Tan PH, Huang YW, Hsieh SW, Hsiao HT, Chung NC, Lin SH, Huang PL, Lyu PC, Yang LC. 2004. Nonsteroidal antiinflammatory drugs increase expression of inducible COX2 isoform of cyclooxygenase in spinal cord of rats with adjuvant induced inflammation. Brain Res Mol Brain Res 125:113–119. 10.1016/j.molbrainres.2004.03.016. [DOI] [PubMed] [Google Scholar]
- 89.Hu YF, Guo Y, Cheng GF. 2001. [[Inhibitory effects of indomethacin and meloxicam on NF-kappa B in mouse peritoneal macrophages]] Yao Xue Xue Bao 36:161–164. [Article in Chinese]. [PubMed] [Google Scholar]
- 90.Hummel M, Whiteside GT. 2017. Measuring and realizing the translational significance of preclinical in vivo studies of painful osteoarthritis. Osteoarthritis Cartilage 25:376–384. 10.1016/j.joca.2016.08.007. [DOI] [PubMed] [Google Scholar]
- 91.Inglis JJ, Notley CA, Essex D, Wilson AW, Feldmann M, Anand P, Williams R. 2007. Collagen-induced arthritis as a model of hyperalgesia: functional and cellular analysis of the analgesic actions of tumor necrosis factor blockade. Arthritis Rheum 56:4015–4023. 10.1002/art.23063. [DOI] [PubMed] [Google Scholar]
- 92.Iwanaka T, Arkovitz MS, Arya G, Ziegler MM. 1997. Evaluation of operative stress and peritoneal macrophage function in minimally invasive operations. J Am Coll Surg 184:357–363. [PubMed] [Google Scholar]
- 93.Jarrar YB, Jarrar Q, Abed A, Abu-Shalhoob M. 2019. Effects of nonsteroidal anti-inflammatory drugs on the expression of arachidonic acid-metabolizing Cyp450 genes in mouse hearts, kidneys and livers. Prostaglandins Other Lipid Mediat 141:14–21. 10.1016/j.prostaglandins.2019.02.003. [DOI] [PubMed] [Google Scholar]
- 94.Jeon YT, Na H, Ryu H, Chung Y. 2015. Modulation of dendritic cell activation and subsequent Th1 cell polarization by lidocaine. PLoS One 10:1–17. 10.1371/journal.pone.0139845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Ji RR, Chamessian A, Zhang YQ. 2016. Pain regulation by nonneuronal cells and inflammation. Science 354:572–577. 10.1126/science.aaf8924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Ji RR, Nackley A, Huh Y, Terrando N, Maixner W. 2018. Neuroinflammation and central sensitization in chronic and widespread pain. Anesthesiology 129:343–366. 10.1097/ALN.0000000000002130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Joshi GP, Ogunnaike BO. 2005. Consequences of inadequate postoperative pain relief and chronic persistent postoperative pain. Anesthesiol Clin North America 23:21–36. 10.1016/j.atc.2004.11.013. [DOI] [PubMed] [Google Scholar]
- 98.Kang SC, Matsutani T, Choudhry MA, Schwacha MG, Rue LW, Bland KI, Chaudry IH. 2004. Are the immune responses different in middle-aged and young mice following bone fracture, tissue trauma and hemorrhage? Cytokine 26:223–230. 10.1016/j.cyto.2004.03.005. [DOI] [PubMed] [Google Scholar]
- 99.Kang SS, McGavern DB. 2008. Lymphocytic choriomeningitis infection of the central nervous system. Front Biosci 13:4529–4543. 10.2741/3021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Kawada M, Arihiro A, Mizoguchi E. 2007. Insights from advances in research of chemically induced experimental models of human inflammatory bowel disease. World J Gastroenterol 13:5581–5593. 10.3748/wjg.v13.i42.5581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Kawasaki T, Kawasaki C, Sata T, Chaudry IH. 2011. Lidocaine suppresses mouse Peyer's Patch T cell functions and induces bacterial translocation. Surgery 149:106–113. 10.1016/j.surg.2010.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Kelly S, Donaldson LF. 2008. Peripheral cannabinoid CB1 receptors inhibit evoked responses of nociceptive neurones in vivo. Eur J Pharmacol 586:160–163. 10.1016/j.ejphar.2008.03.003. [DOI] [PubMed] [Google Scholar]
- 103.Khan I, Oriowo MA. 2006. Mechanism underlying the reversal of contractility dysfunction in experimental colitis by cyclooxygenase-2 inhibition. Inflammopharmacology 14:28–35. 10.1007/s10787-006-1507-7. [DOI] [PubMed] [Google Scholar]
- 104.Kihara N, de la Fuente SG, Fujino K, Takahashi T, Pappas TN, Mantyh CR. 2003. Vanilloid receptor-1 containing primary sensory neurones mediate dextran sulphate sodium induced colitis in rats. Gut 52:713–719. 10.1136/gut.52.5.713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Kimura F, Shimizu H, Yoshidome H, Ohtsuka M, Miyazaki M. 2010. Immunosuppression following surgical and traumatic injury. Surg Today 40:793–808. 10.1007/s00595-010-4323-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Klein A, Eliakim R. 2010. Non steroidal antiinflammatory drugs and inflammatory bowel disease. Pharmaceuticals (Basel) 3:1084–1092. 10.3390/ph3041084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Koksoy S, Sahin Z, Karsli B. 2013. Comparison of the effects of desflurane and bupivacaine on Th1 and Th2 responses. Clin Lab 59:1215–1220. 10.7754/Clin.Lab.2013.120413. [DOI] [PubMed] [Google Scholar]
- 108.Kolstad AM, Rodriguiz RM, Kim CJ, Hale LP. 2012. Effect of pain management on immunization efficacy in mice. J Am Assoc Lab Anim Sci 51:448–457. [PMC free article] [PubMed] [Google Scholar]
- 109.Kotiw M, Morgan M, Taylor SM, Shiels IA. 2010. Detection of anti-TNFα activity in canine hyperimmune serum using a TNFα inhibition assay. Vet Clin Pathol 39:46–52. 10.1111/j.1939-165X.2009.00166.x. [DOI] [PubMed] [Google Scholar]
- 110.Lapointe TK, Basso L, Iftinca MC, Flynn R, Chapman K, Dietrich G, Vergnolle N, Altier C. 2015. TRPV1 sensitization mediates postinflammatory visceral pain following acute colitis. Am J Physiol Gastrointest Liver Physiol 309:G87–G99. 10.1152/ajpgi.00421.2014. [DOI] [PubMed] [Google Scholar]
- 111.Latremoliere A, Woolf CJ. 2009. Central sensitization: a generator of pain hypersensitivity by central neural plasticity. J Pain 10:895–926. 10.1016/j.jpain.2009.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Laudenslager ML, Fleshner M, Hofstadter P, Held PE, Simons L, Maier SF. 1988. Suppression of specific antibody production by inescapable shock: stability under varying conditions. Brain Behav Immun 2:92–101. 10.1016/0889-1591(88)90010-4. [DOI] [PubMed] [Google Scholar]
- 113.Lee YJ, Chuang YC. 2010. Ibuprofen augments proinflammatory cytokine release in a mouse model of Vibrio vulnificus infection. Microbiol Immunol 54:542–550. 10.1111/j.1348-0421.2010.00249.x. [DOI] [PubMed] [Google Scholar]
- 114.Lees P, Giraudel J, Landoni MF, Toutain PL. 2004. PK-PD integration and PK-PD modelling of nonsteroidal antiinflammatory drugs: principles and applications in veterinary pharmacology. J Vet Pharmacol Ther 27:491–502. 10.1111/j.1365-2885.2004.00618.x. [DOI] [PubMed] [Google Scholar]
- 115.Lees P, Landoni MF, Giraudel J, Toutain PL. 2004. Pharmacodynamics and pharmacokinetics of nonsteroidal antiinflammatory drugs in species of veterinary interest. J Vet Pharmacol Ther 27:479–490. 10.1111/j.1365-2885.2004.00617.x. [DOI] [PubMed] [Google Scholar]
- 116.Li D, Ren Y, Xu X, Zou X, Fang L, Lin Q. 2008. Sensitization of primary afferent nociceptors induced by intradermal capsaicin involves the peripheral release of calcitonin gene-related Peptide driven by dorsal root reflexes. J Pain 9:1155–1168. 10.1016/j.jpain.2008.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Li H, Edin ML, Gruzdev A, Cheng J, Bradbury JA, Graves JP, DeGraff LM, Zeldin DC. 2013. Regulation of T helper cell subsets by cyclooxygenases and their metabolites. Prostaglandins Other Lipid Mediat 104-105:74–83. 10.1016/j.prostaglandins.2012.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Li H, Li C, Zhang H, Zhang L, Cheng R, Li M, Guo Y, Zhang Z, Lu Z, Zhuang Y, Yan M, Gu Y, Feng X, Liang J, Yu X, Wang H, Yao Z. 2016. Effects of lidocaine on regulatory T cells in atopic dermatitis. J Allergy Clin Immunol 137:613–617.e5. 10.1016/j.jaci.2015.07.039. [DOI] [PubMed] [Google Scholar]
- 119.Li H, Luo Y, Xu Y, Yang L, Hu C, Chen Q, Yang Y, Ma J, Zhang J, Xia H, Li Y, Yang J. 2018. Meloxicam improves cognitive impairment of diabetic rats through COX2-PGE2-EPs-cAMP/pPKA Pathway. Mol Pharm 15:4121–4131. 10.1021/acs.molpharmaceut.8b00532. [DOI] [PubMed] [Google Scholar]
- 120.Lima GK, Zolini GP, Mansur DS, Freire Lima BH, Wischhoff U, Astigarraga RG, Dias MF, das Graças Almeida Silva M, Béla SR, do Valle Antonelli LR, Arantes RM, Gazzinelli RT, Báfica A, Kroon EG, Campos MA. 2010. Toll-like receptor (TLR) 2 and TLR9 expressed in trigeminal ganglia are critical to viral control during herpes simplex virus 1 infection. Am J Pathol 177:2433–2445. 10.2353/ajpath.2010.100121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Lin JJ, Du Y, Cai WK, Kuang R, Chang T, Zhang Z, Yang YX, Sun C, Li ZY, Kuang F. 2015. Toll-like receptor 4 signaling in neurons of trigeminal ganglion contributes to nociception induced by acute pulpitis in rats. Sci Rep 5:1–14. 10.1038/srep12549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Liu YM, Zhu SM, Wang KR, Feng ZY, Chen QL. 2008. Effect of tramadol on immune responses and nociceptive thresholds in a rat model of incisional pain. J Zhejiang Univ Sci B 9:895–902. 10.1631/jzus.B0820039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Lleo A, Galea E, Sastre M. 2007. Molecular targets of non-steroidal anti-inflammatory drugs in neurodegenerative diseases. Cell Mol Life Sci 64:1403–1418. 10.1007/s00018-007-6516-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.López-Armada MJ, Sanchez-Pernaute O, Largo R, Diez-Ortego I, Palacios I, Egido J, Herrero-Beaumont G. 2002. Modulation of cell recruitment by anti-inflammatory agents in antigen-induced arthritis. Ann Rheum Dis 61:1027–1030. 10.1136/ard.61.11.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.López A, Aparicio N, Pazos MR, Grande MT, Barreda-Manso MA, Benito-Cuesta I, Vazquez C, Amores M, Ruiz-Perez G, Garcia-Garcia E, Beatka M, Tolon RM, Dittel BN, Hillard CJ, Romero J. 2018. Cannabinoid CB2 receptors in the mouse brain: relevance for Alzheimer's disease. J Neuroinflammation 15:1–11. 10.1186/s12974-018-1174-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Low D, Nguyen DD, Mizoguchi E. 2013. Animal models of ulcerative colitis and their application in drug research. Drug Des Devel Ther 7:1341–1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Lucas T, Waisman A, Ranjan R, Roes J, Krieg T, Muller W, Roers A, Eming SA. 2010. Differential roles of macrophages in diverse phases of skin repair. J Immunol 184:3964–3977. 10.4049/jimmunol.0903356. [DOI] [PubMed] [Google Scholar]
- 128.Luo Y, Kuang S, Li H, Ran D, Yang J. 2017. cAMP/PKA-CREB-BDNF signaling pathway in hippocampus mediates cyclooxygenase 2-induced learning/memory deficits of rats subjected to chronic unpredictable mild stress. Oncotarget 8:35558–35572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Machelska H, Stein C. 2000. Pain control by immune-derived opioids. Clin Exp Pharmacol Physiol 27:533–536. 10.1046/j.1440-1681.2000.03287.x. [DOI] [PubMed] [Google Scholar]
- 130.Mack VE, McCarter MD, Naama HA, Calvano SE, Daly JM. 1996. Dominance of T-helper 2–type cytokines after severe injury. Arch Surg 131:1303–1308. 10.1001/archsurg.1996.01430240057007. [DOI] [PubMed] [Google Scholar]
- 131.MacPherson BR, Pfeiffer CJ. 1978. Experimental production of diffuse colitis in rats. Digestion 17:135–150. 10.1159/000198104. [DOI] [PubMed] [Google Scholar]
- 132.Makarenkova VP, Bansal V, Matta BM, Perez LA, Ochoa JB. 2006. CD11b+/Gr-1+ myeloid suppressor cells cause t cell dysfunction after traumatic stress. J Immunol 176:2085–2094. 10.4049/jimmunol.176.4.2085. [DOI] [PubMed] [Google Scholar]
- 133.Makman MH. 1994. Morphine receptors in immunocytes and neurons. Adv Neuroimmunol 4:69–82. 10.1016/S0960-5428(05)80002-6. [DOI] [PubMed] [Google Scholar]
- 134.Mapplebeck JC, Beggs S, Salter MW. 2016. Sex differences in pain: a tale of 2 immune cells. Pain 157 Suppl 1:S2–S6. 10.1097/j.pain.0000000000000389. [DOI] [PubMed] [Google Scholar]
- 135.Marik PE, Flemmer M. 2012. The immune response to surgery and trauma: Implications for treatment. J Trauma Acute Care Surg 73:801–808. 10.1097/TA.0b013e318265cf87. [DOI] [PubMed] [Google Scholar]
- 136.Marko ST, Little SF, Benton CG, Kelly R, Field AE, Laufer RS. 2014. Effect of analgesics on monoclonal antibody ascites production in mice administered upon recognition of pain. ISRN Immunology 2014:1–8. 10.1155/2014/350796 [DOI] [Google Scholar]
- 137.Martín AR, Villegas I, Alarcón de la Lastra C. 2005. The COX2 inhibitor, rofecoxib, ameliorates dextran sulphate sodium induced colitis in mice. Inflamm Res 54:145–151. 10.1007/s00011-004-1337-2. [DOI] [PubMed] [Google Scholar]
- 138.Martín AR, Villegas I, La Casa C, Alarcón de la Lastra C. 2003. The cyclo-oxygenase-2 inhibitor, rofecoxib, attenuates mucosal damage due to colitis induced by trinitrobenzene sulphonic acid in rats. Eur J Pharmacol 481:281–291. 10.1016/j.ejphar.2003.09.033. [DOI] [PubMed] [Google Scholar]
- 139.Martin WJ, Patrick SL, Coffin PO, Tsou K, Walker JM. 1995. An examination of the central sites of action of cannabinoid-induced antinociception in the rat. Life Sci 56:2103–2109. 10.1016/0024-3205(95)00195-C. [DOI] [PubMed] [Google Scholar]
- 140.Martucci C, Panerai AE, Sacerdote P. 2004. Chronic fentanyl or buprenorphine infusion in the mouse: similar analgesic profile but different effects on immune responses. Pain 110:385–392. 10.1016/j.pain.2004.04.020. [DOI] [PubMed] [Google Scholar]
- 141.Mascagni P, Sabbatini V, Biordi L, Martinotti S, Allegretti M, Marullo A, Caselli G, Bertini R. 2000. R- and S-isomers of nonsteroidal anti-inflammatory drugs differentially regulate cytokine production. Eur Cytokine Netw 11:185–192. [PubMed] [Google Scholar]
- 142.McCarter MD, Mack VE, Daly JM, Naama HA, Calvano SE. 1998. Trauma-induced alterations in macrophage function. Surgery 123:96–101. 10.1016/S0039-6060(98)70234-X. [DOI] [PubMed] [Google Scholar]
- 143.Meseguer V, Alpizar YA, Luis E, Tajada S, Denlinger B, Fajardo O, Manenschijn JA, Fernandez-Pena C, Talavera A, Kichko T, Navia B, Sanchez A, Senaris R, Reeh P, Perez-Garcia MT, Lopez-Lopez JR, Voets T, Belmonte C, Talavera K, Viana F. 2014. TRPA1 channels mediate acute neurogenic inflammation and pain produced by bacterial endotoxins. Nat Commun 5:1–14. 10.1038/ncomms4125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Michelin MA, Figueiredo F, Cunha FQ. 2002. Involvement of prostaglandins in the immunosuppression occurring during experimental infection by Paracoccidioides brasiliensis. Exp Parasitol 102:170–177. 10.1016/S0014-4894(03)00053-5. [DOI] [PubMed] [Google Scholar]
- 145.Michelin MA, Silva JS, Cunha FQ. 2005. Inducible cyclooxygenase released prostaglandin mediates immunosuppression in acute phase of experimental Trypanosoma cruzi infection. Exp Parasitol 111:71–79. 10.1016/j.exppara.2005.05.001. [DOI] [PubMed] [Google Scholar]
- 146.Miller RE, Belmadani A, Ishihara S, Tran PB, Ren D, Miller RJ, Malfait AM. 2015. Damage-associated molecular patterns generated in osteoarthritis directly excite murine nociceptive neurons through Toll-like receptor 4. Arthritis Rheumatol 67:2933–2943. 10.1002/art.39291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Miller RJ, Jung H, Bhangoo SK, White FA. 2009. Cytokine and chemokine regulation of sensory neuron function. Handb Exp Pharmacol 194:417–449. 10.1007/978-3-540-79090-7_12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Milligan ED, Zapata V, Chacur M, Schoeniger D, Biedenkapp J, O'Connor KA, Verge GM, Chapman G, Green P, Foster AC, Naeve GS, Maier SF, Watkins LR. 2004. Evidence that exogenous and endogenous fractalkine can induce spinal nociceptive facilitation in rats. Eur J Neurosci 20:2294–2302. 10.1111/j.1460-9568.2004.03709.x. [DOI] [PubMed] [Google Scholar]
- 149.Moehrlen U, Schwoebel F, Reichmann E, Stauffer U, Gitzelmann CA, Hamacher J. 2005. Early peritoneal macrophage function after laparoscopic surgery compared with laparotomy in a mouse mode. Surg Endosc 19:958–963. 10.1007/s00464-004-2118-2. [DOI] [PubMed] [Google Scholar]
- 150.Molina-Martínez LM, Gonzalez-Espinosa C, Cruz SL. 2014. Dissociation of immunosuppressive and nociceptive effects of fentanyl, but not morphine, after repeated administration in mice: fentanyl-induced sensitization to LPS. Brain Behav Immun 42:60–64. 10.1016/j.bbi.2014.06.011. [DOI] [PubMed] [Google Scholar]
- 151.Monibi FA, Dodam JR, Axiak-Bechtel SM, Amorim J, Zhang Y, Tsuruta K, Mann FA, DeClue AE. 2015. Morphine and buprenorphine do not alter leukocyte cytokine production capacity, early apoptosis, or neutrophil phagocytic function in healthy dogs. Res Vet Sci 99:70–76. 10.1016/j.rvsc.2015.01.010. [DOI] [PubMed] [Google Scholar]
- 152.Montilla-García Á, Tejada M, Perazzoli G, Entrena JM, Portillo-Salido E, Fernández-Segura E, Cañizares FJ, Cobos EJ. 2017. Grip strength in mice with joint inflammation: A rheumatology function test sensitive to pain and analgesia. Neuropharmacology 125:231–242. 10.1016/j.neuropharm.2017.07.029. [DOI] [PubMed] [Google Scholar]
- 153.Morisset V, Urban L. 2001. Cannabinoid-induced presynaptic inhibition of glutamatergic EPSCs in substantia gelatinosa neurons of the rat spinal cord. J Neurophysiol 86:40–48. 10.1152/jn.2001.86.1.40. [DOI] [PubMed] [Google Scholar]
- 154.Mousa SA, Machelska H, Schafer M, Stein C. 2002. Immunohistochemical localization of endomorphin-1 and endomorphin-2 in immune cells and spinal cord in a model of inflammatory pain. J Neuroimmunol 126:5–15. 10.1016/S0165-5728(02)00049-8. [DOI] [PubMed] [Google Scholar]
- 155.Mundt S, Groettrup M, Basler M. 2015. Analgesia in mice with experimental meningitis reduces pain without altering immune parameters. ALTEX 32:183–189. [DOI] [PubMed] [Google Scholar]
- 156.Muñoz-Miralles J, Trindade BC, Castro-Córdova P, Bergin IL, Kirk LA, Gil F, Aronoff DM, Paredes-Sabja D. 2018. Indomethacin increases severity of Clostridium difficile infection in mouse model. Future Microbiol 13:1271–1281. 10.2217/fmb-2017-0311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Nagahisa A, Okumura T. 2017. Pharmacology of grapiprant, a novel EP4 antagonist: receptor binding, efficacy in a rodent postoperative pain model, and a dose estimation for controlling pain in dogs. J Vet Pharmacol Ther 40:285–292. 10.1111/jvp.12349. [DOI] [PubMed] [Google Scholar]
- 158.Nakamura K, Radhakrishnan K, Wong YM, Rockson SG. 2009. Anti-inflammatory pharmacotherapy with ketoprofen ameliorates experimental lymphatic vascular insufficiency in mice. PLoS One 4:1–7. 10.1371/journal.pone.0008380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Nelson CJ, Lysle DT. 1998. Severity, time, and β-adrenergic receptor involvement in surgery-induced immune alterations. J Surg Res 80:115–122. 10.1006/jsre.1998.5429. [DOI] [PubMed] [Google Scholar]
- 160.Nemeth CL, Miller AH, Tansey MG, Neigh GN. 2016. Inflammatory mechanisms contribute to microembolism-induced anxiety-like and depressive-like behaviors. Behav Brain Res 303:160–167. 10.1016/j.bbr.2016.01.057. [DOI] [PubMed] [Google Scholar]
- 161.Okayama M, Hayashi S, Aoi Y, Nishio H, Kato S, Takeuchi K. 2007. Aggravation by selective COX1 and COX2 inhibitors of dextran sulfate sodium (DSS)-induced colon lesions in rats. Dig Dis Sci 52:2095–2103. 10.1007/s10620-006-9597-z. [DOI] [PubMed] [Google Scholar]
- 162.Onaivi ES, Ishiguro H, Gong JP, Patel S, Perchuk A, Meozzi PA, Myers L, Mora Z, Tagliaferro P, Gardner E, Brusco A, Akinshola BE, Liu QR, Hope B, Iwasaki S, Arinami T, Teasenfitz L, Uhl GR. 2006. Discovery of the presence and functional expression of cannabinoid CB2 receptors in brain. Ann N Y Acad Sci 1074:514–536. 10.1196/annals.1369.052. [DOI] [PubMed] [Google Scholar]
- 163.Paccani SR, Boncristiano M, Ulivieri C, D'Elios MM, Del Prete G, Baldari CT. 2002. Nonsteroidal antiinflammatory drugs suppress T-cell activation by inhibiting p38 MAPK induction. J Biol Chem 277:1509–1513. 10.1074/jbc.M110676200. [DOI] [PubMed] [Google Scholar]
- 164.Pace MC, Passavanti MB, De Nardis L, Bosco F, Sansone P, Pota V, Barbarisi M, Palagiano A, Iannotti FA, Panza E, Aurilio C. 2018. Nociceptor plasticity: a closer look. J Cell Physiol 233:2824–2838. 10.1002/jcp.25993. [DOI] [PubMed] [Google Scholar]
- 165.Page GG, Ben-Eliyahu S, Yirmiya R, Liebeskind JC. 1993. Morphine attenuates surgery-induced enhancement of metastatic colonization in rats. Pain 54:21–28. 10.1016/0304-3959(93)90095-7. [DOI] [PubMed] [Google Scholar]
- 166.Page GG, Blakely WP, Ben-Eliyahu S. 2001. Evidence that postoperative pain is a mediator of the tumor-promoting effects of surgery in rats. Pain 90:191–199. 10.1016/S0304-3959(00)00403-6. [DOI] [PubMed] [Google Scholar]
- 167.Pandey R, Mousawy K, Nagarkatti M, Nagarkatti P. 2009. Endocannabinoids and immune regulation. Pharmacol Res 60:85–92. 10.1016/j.phrs.2009.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Pera M, Nelson H, Rajkumar SV, Young-Fadok TM, Burgart LJ. 2003. Influence of postoperative acute-phase response on angiogenesis and tumor growth: open vs. laparoscopic-assisted surgery in mice. J Gastrointest Surg 7:783–790. 10.1016/S1091-255X(03)00111-2. [DOI] [PubMed] [Google Scholar]
- 169.Perše M, Cerar A. 2012. Dextran sodium sulphate colitis mouse model: traps and tricks. J Biomed Biotechnol 2012:1–13. 10.1155/2012/718617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Peterson NC. 2000. Behavioral, clinical, and physiologic analysis of mice used for ascites monoclonal antibody production. Comp Med 50:516–526. [PubMed] [Google Scholar]
- 171.Peterson NC, Nunamaker EA, Turner PV. 2017. To treat or not to treat: the effects of pain on experimental parameters. Comp Med 67:469–482. [PMC free article] [PubMed] [Google Scholar]
- 172.Pezzone MA, Dohanics J, Rabin BS. 1994. Effects of footshock stress upon spleen and peripheral blood lymphocyte mitogenic responses in rats with lesions of the paraventricular nuclei. J Neuroimmunol 53:39–46. 10.1016/0165-5728(94)90062-0. [DOI] [PubMed] [Google Scholar]
- 173.Qi J, Buzas K, Fan H, Cohen JI, Wang K, Mont E, Klinman D, Oppenheim JJ, Howard OM. 2011. Painful pathways induced by TLR stimulation of dorsal root ganglion neurons. J Immunol 186:6417–6426. 10.4049/jimmunol.1001241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Quallo T, Gentry C, Bevan S, Broad LM, Mogg AJ. 2015. Activation of transient receptor potential ankyrin 1 induces CGRP release from spinal cord synaptosomes. Pharmacol Res Perspect 3:1–10. 10.1002/prp2.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Randhawa PK, Singh K, Singh N, Jaggi AS. 2014. A review on chemical-induced inflammatory bowel disease models in rodents. Korean J Physiol Pharmacol 18:279–288. 10.4196/kjpp.2014.18.4.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Rao P, Knaus EE. 2008. Evolution of nonsteroidal anti-inflammatory drugs (NSAIDs): cyclooxygenase (COX) inhibition and beyond. J Pharm Pharm Sci 11:81s–110s. 10.18433/J3T886. [DOI] [PubMed] [Google Scholar]
- 177.Rather LJ. 1971. Disturbance of function (functio laesa): the legendary 5th cardinal sign of inflammation, added by Galen to the 4 cardinal signs of Celsus. Bull N Y Acad Med 47:303–322. [PMC free article] [PubMed] [Google Scholar]
- 178.National Research Council (US) Committee on Methods of Producing Monoclonal Antibodies. 1999. Monoclonal antibody production. Washington (DC): National Academies Press. [PubMed] [Google Scholar]
- 179.Rigoni M, Trevisani M, Gazzieri D, Nadaletto R, Tognetto M, Creminon C, Davis JB, Campi B, Amadesi S, Geppetti P, Harrison S. 2003. Neurogenic responses mediated by vanilloid receptor-1 (TRPV1) are blocked by the high affinity antagonist, iodo-resiniferatoxin. Br J Pharmacol 138:977–985. 10.1038/sj.bjp.0705110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Roth J, Hübschle T, Pehl U, Ross G, Gerstberger R. 2002. Influence of systemic treatment with cyclooxygenase inhibitors on lipopolysaccharide-induced fever and circulating levels of cytokines and cortisol in guinea-pigs. Pflugers Arch 443:411–417. 10.1007/s004240100718. [DOI] [PubMed] [Google Scholar]
- 181.Sacerdote P, Bianchi M, Gaspani L, Panerai AE. 1999. Effects of tramadol and its enantiomers on Concanavalin-A induced-proliferation and NK activity of mouse splenocytes: involvement of serotonin. Int J Immunopharmacol 21:727–734. 10.1016/S0192-0561(99)00048-X. [DOI] [PubMed] [Google Scholar]
- 182.Sacerdote P, Bianchi M, Manfredi B, Panerai AE. 1997. Effects of tramadol on immune responses and nociceptive thresholds in mice. Pain 72:325–330. 10.1016/S0304-3959(97)00055-9. [DOI] [PubMed] [Google Scholar]
- 183.Sacerdote P, Manfredi B, Bianchi M, Panerai AE. 1994. Intermittent but not continuous inescapable footshock stress affects immune responses and immunocyte beta-endorphin concentrations in the rat. Brain Behav Immun 8:251–260. 10.1006/brbi.1994.1023. [DOI] [PubMed] [Google Scholar]
- 184.Saito O, Svensson CI, Buczynski MW, Wegner K, Hua XY, Codeluppi S, Schaloske RH, Deems RA, Dennis EA, Yaksh TL. 2010. Spinal glial TLR4-mediated nociception and production of prostaglandin E(2) and TNF. Br J Pharmacol 160:1754–1764. 10.1111/j.1476-5381.2010.00811.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Samsam M, Coveñas R, Csillik B, Ahangari R, Yajeya J, Riquelme R, Narváez JA, Tramu G. 2001. Depletion of substance P, neurokinin A and calcitonin gene-related peptide from the contralateral and ipsilateral caudal trigeminal nucleus following unilateral electrical stimulation of the trigeminal ganglion; a possible neurophysiological and neuroanatomical link to generalized head pain. J Chem Neuroanat 21:161–169. 10.1016/S0891-0618(01)00088-6. [DOI] [PubMed] [Google Scholar]
- 186.Sandoval BA, Robinson AV, Sulaiman TT, Shenk RR, Stellato TA. 1996. Open versus laparoscopic surgery: a comparison of natural antitumoral cellular immunity in a small animal model. Am Surg 62:625–630, discussion 630–621. [PubMed] [Google Scholar]
- 187.Sano T, Utsumi D, Amagase K, Matsumoto K, Tominaga M, Higuchi K, Takeuchi T, Kato S. 2017. Lafutidine, a histamine H2 receptor antagonist with mucosal protective properties, attenuates 5-fluorouracil-induced intestinal mucositis in mice through activation of extrinsic primary afferent neurons. J Physiol Pharmacol 68:79–90. [PubMed] [Google Scholar]
- 188.Schlosburg JE, Kinsey SG, Lichtman AH. 2009. Targeting fatty acid amide hydrolase (FAAH) to treat pain and inflammation. AAPS J 11:39–44. 10.1208/s12248-008-9075-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Schmelzer TM, Heath JJ, Hope WW, Mostafari A, Novitsky YW, Heniford BT. 2008. The effect of preoperative corticosteroids on peritoneal macrophage function after laparoscopic and open abdominal surgery in a rat model. Am J Surg 196:920–924, discussion 924–925. 10.1016/j.amjsurg.2008.07.023. [DOI] [PubMed] [Google Scholar]
- 190.Scholz J, Woolf CJ. 2007. The neuropathic pain triad: neurons, immune cells and glia. Nat Neurosci 10:1361–1368. 10.1038/nn1992. [DOI] [PubMed] [Google Scholar]
- 191.Serra MF, Anjos-Valotta EA, Olsen PC, Couto GC, Jurgilas PB, Cotias AC, Pao CR, Ferreira TP, Arantes AC, Pires AL, Cordeiro RS, Silva PM, Martins MA. 2012. Nebulized lidocaine prevents airway inflammation, peribronchial fibrosis, and mucus production in a murine model of asthma. Anesthesiology 117:580–591. 10.1097/ALN.0b013e31826687d5. [DOI] [PubMed] [Google Scholar]
- 192.Shavit Y, Fridel K, Beilin B. 2006. Postoperative pain management and proinflammatory cytokines: animal and human studies. J Neuroimmune Pharmacol 1:443–451. 10.1007/s11481-006-9043-1. [DOI] [PubMed] [Google Scholar]
- 193.Shavit Y, Martin FC, Yirmiya R, Ben-Eliyahu S, Terman GW, Weiner H, Gale RP, Liebeskind JC. 1987. Effects of a single administration of morphine or footshock stress on natural killer cell cytotoxicity. Brain Behav Immun 1:318–328. 10.1016/0889-1591(87)90034-1. [DOI] [PubMed] [Google Scholar]
- 194.Shientag LJ, Wheeler SM, Garlick DS, Maranda LS. 2012. A therapeutic dose of ketoprofen causes acute gastrointestinal bleeding, erosions, and ulcers in rats. J Am Assoc Lab Anim Sci 51:832–841. [PMC free article] [PubMed] [Google Scholar]
- 195.Singh VP, Patil CS, Jain NK, Kulkarni SK. 2004. Aggravation of inflammatory bowel disease by cyclooxygenase-2 inhibitors in rats. Pharmacology 72:77–84. 10.1159/000079135. [DOI] [PubMed] [Google Scholar]
- 196.Smith RL, Kajiyama G, Schurman DJ. 1997. Staphylococcal septic arthritis: antibiotic and nonsteroidal antiinflammatory drug treatment in a rabbit model. J Orthop Res 15:919–926. 10.1002/jor.1100150619. [DOI] [PubMed] [Google Scholar]
- 197.Srinath AI, Walter C, Newara MC, Szigethy EM. 2012. Pain management in patients with inflammatory bowel disease: insights for the clinician. Therap Adv Gastroenterol 5:339–357. 10.1177/1756283X12446158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Stachtari CC, Thomareis ON, Tsaousi GG, Karakoulas KA, Chatzimanoli FI, Chatzopoulos SA, Vasilakos DG. 2016. Interaction of a cannabinoid-2 agonist with Tramadol on nociceptive thresholds and immune responses in a rat model of incisional pain. Am J Ther 23:e1484–e1492. 10.1097/MJT.0000000000000131. [DOI] [PubMed] [Google Scholar]
- 199.Stein C, Clark JD, Oh U, Vasko MR, Wilcox GL, Overland AC, Vanderah TW, Spencer RH. 2009. Peripheral mechanisms of pain and analgesia. Brain Res Rev 60:90–113. 10.1016/j.brainresrev.2008.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Stein C, Hassan AH, Przewlocki R, Gramsch C, Peter K, Herz A. 1990. Opioids from immunocytes interact with receptors on sensory nerves to inhibit nociception in inflammation. Proc Natl Acad Sci USA 87:5935–5939. 10.1073/pnas.87.15.5935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Stein C, Küchler S. 2013. Targeting inflammation and wound healing by opioids. Trends Pharmacol Sci 34:303–312. 10.1016/j.tips.2013.03.006. [DOI] [PubMed] [Google Scholar]
- 202.Stills HF., Jr 2005. Adjuvants and antibody production: dispelling the myths associated with Freund's complete and other adjuvants. ILAR J 46:280–293. 10.1093/ilar.46.3.280. [DOI] [PubMed] [Google Scholar]
- 203.Sun WZ, Chang MC, Hsiao PN, Chen CA, Hsu YT, Hsieh CY, Cheng WF. 2010. Morphine-sparing effect by COX1 inhibitor sustains analgesic function without compromising antigen-specific immunity and antitumor effect of naked DNA vaccine. Int J Immunopathol Pharmacol 23:91–104. 10.1177/039463201002300109. [DOI] [PubMed] [Google Scholar]
- 204.Tai LH, de Souza CT, Belanger S, Ly L, Alkayyal AA, Zhang J, Rintoul JL, Ananth AA, Lam T, Breitbach CJ, Falls TJ, Kirn DH, Bell JC, Makrigiannis AP, Auer RA. 2013. Preventing postoperative metastatic disease by inhibiting surgery-induced dysfunction in natural killer cells. Cancer Res 73:97–107. 10.1158/0008-5472.CAN-12-1993. [DOI] [PubMed] [Google Scholar]
- 205.Tai LH, Tanese de Souza C, Sahi S, Zhang J, Alkayyal AA, Ananth AA, Auer RA. 2014. A mouse tumor model of surgical stress to explore the mechanisms of postoperative immunosuppression and evaluate novel perioperative immunotherapies. J Vis Exp 85:1–7. 10.3791/51253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Tajti J, Vecsei L. 2009. [[The mechanism of peripheral and central sensitization in migraine. A literature review]] Neuropsychopharmacol Hung 11:15–21.[Article in Hungarian]. [PubMed] [Google Scholar]
- 207.Thau-Zuchman O, Shohami E, Alexandrovich AG, Trembovler V, Leker RR. 2012. The antiinflammatory drug carprofen improves long-term outcome and induces gliogenesis after traumatic brain injury. J Neurotrauma 29:375–384. 10.1089/neu.2010.1673. [DOI] [PubMed] [Google Scholar]
- 208.Treede RD. 2016. Gain control mechanisms in the nociceptive system. Pain 157:1199–1204. 10.1097/j.pain.0000000000000499. [DOI] [PubMed] [Google Scholar]
- 209.Üceyler N, Schäfers M, Sommer C. 2009. Mode of action of cytokines on nociceptive neurons. Exp Brain Res 196:67–78. 10.1007/s00221-009-1755-z. [DOI] [PubMed] [Google Scholar]
- 210.Vachon P, Moreau JP. 2002. Butorphanol decreases edema following carrageenan-induced paw inflammation in rats. Contemp Top Lab Anim Sci 41:15–17. [PubMed] [Google Scholar]
- 211.Van Der Wal S, Vaneker M, Steegers M, Van Berkum B, Kox M, Van Der Laak J, Van Der Hoeven J, Vissers K, Scheffer GJ. 2015. Lidocaine increases the antiinflammatory cytokine IL10 following mechanical ventilation in healthy mice. Acta Anaesthesiol Scand 59:47–55. 10.1111/aas.12417. [DOI] [PubMed] [Google Scholar]
- 212.Verge GM, Milligan ED, Maier SF, Watkins LR, Naeve GS, Foster AC. 2004. Fractalkine (CX3CL1) and fractalkine receptor (CX3CR1) distribution in spinal cord and dorsal root ganglia under basal and neuropathic pain conditions. Eur J Neurosci 20:1150–1160. 10.1111/j.1460-9568.2004.03593.x. [DOI] [PubMed] [Google Scholar]
- 213.Volker D, Bate M, Gentle R, Garg M. 2000. Oral buprenorphine is antiinflammatory and modulates the pathogenesis of streptococcal cell wall polymer-induced arthritis in the Lew/SSN rat. Lab Anim 34:423–429. 10.1258/002367700780387732. [DOI] [PubMed] [Google Scholar]
- 214.Wadachi R, Hargreaves KM. 2006. Trigeminal nociceptors express TLR-4 and CD14: a mechanism for pain due to infection. J Dent Res 85:49–53. 10.1177/154405910608500108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Walker JS, Chandler AK, Wilson JL, Binder W, Day RO. 1996. Effect of mu-opioids morphine and buprenorphine on the development of adjuvant arthritis in rats. Inflamm Res 45:557–563. 10.1007/BF02342227. [DOI] [PubMed] [Google Scholar]
- 216.Watkins LR, Maier SF. 2002. Beyond neurons: evidence that immune and glial cells contribute to pathological pain states. Physiol Rev 82:981–1011. 10.1152/physrev.00011.2002. [DOI] [PubMed] [Google Scholar]
- 217.Watkins LR, Maier SF. 2005. Immune regulation of central nervous system functions: from sickness responses to pathological pain. J Intern Med 257:139–155. 10.1111/j.1365-2796.2004.01443.x. [DOI] [PubMed] [Google Scholar]
- 218.Weng TC, Chen CC, Toh HS, Tang HJ. 2011. Ibuprofen worsens Streptococcus pyogenes soft tissue infections in mice. J Microbiol Immunol Infect 44:418–423. 10.1016/j.jmii.2011.04.012. [DOI] [PubMed] [Google Scholar]
- 219.White FA, Bhangoo SK, Miller RJ. 2005. Chemokines: integrators of pain and inflammation. Nat Rev Drug Discov 4:834–844. 10.1038/nrd1852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Wilson RI, Nicoll RA. 2001. Endogenous cannabinoids mediate retrograde signalling at hippocampal synapses. Nature 410:588–592. 10.1038/35069076. [DOI] [PubMed] [Google Scholar]
- 221.Wirtz S, Neufert C, Weigmann B, Neurath MF. 2007. Chemically induced mouse models of intestinal inflammation. Nat Protoc 2:541–546. 10.1038/nprot.2007.41. [DOI] [PubMed] [Google Scholar]
- 222.Wirtz S, Popp V, Kindermann M, Gerlach K, Weigmann B, Fichtner-Feigl S, Neurath MF. 2017. Chemically induced mouse models of acute and chronic intestinal inflammation. Nat Protoc 12:1295–1309. 10.1038/nprot.2017.044. [DOI] [PubMed] [Google Scholar]
- 223.Wu FX, Bian JJ, Miao XR, Huang SD, Xu XW, Gong DJ, Sun YM, Lu ZJ, Yu WF. 2010. Intrathecal siRNA against Toll-like receptor 4 reduces nociception in a rat model of neuropathic pain. Int J Med Sci 7:251–259. 10.7150/ijms.7.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Wyns H, Meyer E, Plessers E, Watteyn A, van Bergen T, Schauvliege S, De Baere S, Devreese M, De Backer P, Croubels S. 2015. Modulation by gamithromycin and ketoprofen of in vitro and in vivo porcine lipopolysaccharide-induced inflammation. Vet Immunol Immunopathol 168:211–222. 10.1016/j.vetimm.2015.09.014. [DOI] [PubMed] [Google Scholar]
- 225.Yang C, Chang H, Zhang T, Liang C, Li E. 2015. Pre-emptive epidural analgesia improves post-operative pain and immune function in patients undergoing thoracotomy. ANZ J Surg 85:472–477. 10.1111/ans.12746. [DOI] [PubMed] [Google Scholar]
- 226.Yang J, Zhao J, Nakaguchi T, Gregersen H. 2009. Biomechanical changes in oxazolone-induced colitis in BALB/C mice. J Biomech 42:811–817. 10.1016/j.jbiomech.2009.01.028. [DOI] [PubMed] [Google Scholar]
- 227.Yardeni IZ, Beilin B, Mayburd E, Levinson Y, Bessler H. 2009. The effect of perioperative intravenous lidocaine on postoperative pain and immune function. Anesth Analg 109:1464–1469. 10.1213/ANE.0b013e3181bab1bd. [DOI] [PubMed] [Google Scholar]
- 228.Zheng YQ, Wei W, Shen YX, Dai M, Liu LH. 2004. Oral and nasal administration of chicken type II collagen suppresses adjuvant arthritis in rats with intestinal lesions induced by meloxicam. World J Gastroenterol 10:3165–3170. 10.3748/wjg.v10.i21.3165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Zielińska M, Ben Haddou T, Cami-Kobeci G, Sałaga M, Jarmuż A, Padysz M, Kordek R, Spetea M, Husbands SM, Fichna J. 2015. Anti-inflammatory effect of dual nociceptin and opioid receptor agonist, BU08070, in experimental colitis in mice. Eur J Pharmacol 765:582–590. 10.1016/j.ejphar.2015.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Zou WY, Guo QL, Cai J, Wang E, Yang HW, Xu DM, Wang YC. 2008. [[Effect of intrathecal pumping tramadol on the immune function in rats with formalin pain]] Zhong Nan Da Xue Xue Bao Yi Xue Ban 33:404–409.[Article in Chinese]. [PubMed] [Google Scholar]