Pacific Coast tick fever is a febrile illness associated with the bite of Dermacentor occidentalis and results from an infection due to the intracellular pathogen Rickettsia 364D (also known by the proposed name “Rickettsia philipii”). Current molecular methods for the detection of this pathogen rely on the amplification of a conserved spotted fever group rickettsial gene (ompA) followed by DNA sequencing of the amplicon to identify the species.
KEYWORDS: Rickettsia 364D, Rickettsia philipii, Pacific Coast tick fever, real-time PCR, Rickettsia, TaqMan
ABSTRACT
Pacific Coast tick fever is a febrile illness associated with the bite of Dermacentor occidentalis and results from an infection due to the intracellular pathogen Rickettsia 364D (also known by the proposed name “Rickettsia philipii”). Current molecular methods for the detection of this pathogen rely on the amplification of a conserved spotted fever group rickettsial gene (ompA) followed by DNA sequencing of the amplicon to identify the species. This work describes the development of a Rickettsia 364D-specific TaqMan assay to simplify and accelerate the detection and identification processes. The assay demonstrated a sensitivity of 1 genomic copy per 4-μl sample and is highly specific for Rickettsia 364D. The utility of this assay for ecological and diagnostic samples was evaluated using banked specimens collected in a single-blind manner and yielded a clinical sensitivity and specificity of 100%. In conclusion, we describe the development and evaluation of a novel TaqMan real-time PCR assay for the detection and identification of Rickettsia 364D suitable for ecological and diagnostic applications.
INTRODUCTION
The original strain of Rickettsia 364D (strain 364-D) was isolated from Dermacentor occidentalis (the Pacific Coast tick) collected from southern California in 1966 (1). It was not until 2008 that this agent was confirmed to be a human pathogen (2). Fourteen cases of Rickettsia 364D infection, now called Pacific Coast tick fever (PCTF), have been identified in California (2, 3). Rickettsia 364D is most closely genetically related to Rickettsia rickettsii, the causative agent of Rocky Mountain spotted fever (RMSF). Mouse serotyping classification schemes recognize the type strain (strain 364-D) as a unique serotype (1, 4), and the name “Rickettsia philipii” has been proposed (5).
Pacific Coast ticks are found throughout much of California, with a range that extends from parts of Washington and southwestern Oregon to northern Baja California, Mexico. All three life stages of this mammophilic tick bite humans. Dermacentor occidentalis is a potential vector of several pathogens of human and veterinary importance, including Francisella tularensis, Colorado tick fever virus, Anaplasma bovis, R. rickettsii, and Rickettsia 364D (2, 6, 7). The estimated prevalence of Rickettsia 364D among D. occidentalis in California is approximately 2% to 8% (3, 5, 8, 9). Additional studies are required to better identify cases of PCTF and to understand the ecological factors involved in the maintenance of this pathogen in nature. Current molecular assays to detect Rickettsia 364D use conventional PCR amplification of the ompA gene, followed by DNA sequencing of the amplicon (2, 3, 5, 10). However, D. occidentalis ticks are more commonly infected with Rickettsia species other than Rickettsia 364D (5, 8), so this represents an inefficient method to screen large numbers of ticks for the pathogen. From a clinical perspective, evaluation of specimens using conventional PCR with sequence analysis can delay diagnostic confirmation of PCTF. The aim of this work was to develop a highly sensitive and specific assay that provides rapid and accurate diagnosis of PCTF in patients and facilitates large-scale entomological surveys to better define the occurrence and distribution of Rickettsia 364D in nature.
MATERIALS AND METHODS
Primer and probe design.
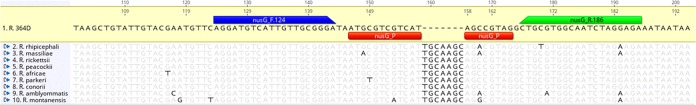
During a previous investigation to examine intergenic regions as a method to differentiate isolates of R. rickettsii, a 7-bp deletion in the intergenic region between nusG and rplK was identified in Rickettsia 364D that did not occur in any of the prototypical isolates of R. rickettsii (4). A comparison of this locus to nine other related spotted fever group (SFG) Rickettsia genomes (Rickettsia rhipicephali, Rickettsia massiliae, R. rickettsii, Rickettsia peacockii, Rickettsia africae, Rickettsia parkeri, Rickettsia conorii, Rickettsia amblyommatis, and Rickettsia montanensis) revealed that this deletion is unique to Rickettsia 364D. Geneious v10.2.2 (Biomatters Ltd.) was used to design primers nusG_F.124 (5′-AGG ATG TCA TTG TTG CGG GA-3′) and nusG_R.186 (5′-TCT CCT AGA TTG CCA CGC AG-3′), which produced a 62-bp amplicon spanning the deletion region. Probe nusG_P_FAM (6-carboxyfluorescein [FAM]-5′-ATG CGT CGT CAT AGC CGT AG-3′-BHQ1) was designed to overlap the deletion site (Fig. 1). All oligonucleotides were synthesized by the Biotechnology Core Facility Branch at the Centers for Disease Control and Prevention.
FIG 1.
Primer and probe design. The TaqMan probe is designed to overlap a 7-bp gap in Rickettsia 364D compared with other closely related Rickettsia species. Blue, forward primer; green, reverse primer; red, FAM-labeled probe.
Assay optimization.
DNA was extracted from Vero E6 cells infected with Rickettsia 364D (strain 364-D) using a KingFisher mL magnetic particle purification system and a KingFisher cell and tissue DNA kit (Thermo Fisher Scientific, Waltham, MA) and eluted in 150 μl elution buffer. The PCRs were run on a CFX96 Touch real-time PCR detection system (Bio-Rad, Hercules, CA), and each reaction consisted of 400 nM (each) each primer, 200 nM probe, 10 μl QuantiTect probe master mix (Qiagen, Valencia, CA), 4.4 μl sterile PCR-grade water, and 4 μl template DNA in a final reaction volume of 20 μl. To determine the optimum annealing temperature of the assay, a gradient real-time PCR was performed using annealing temperatures from 55 to 65°C based on the estimated melting temperatures of the primers and probe.
Sensitivity and specificity testing.
A control plasmid was constructed using an Invitrogen TOPO TA Cloning kit for sequencing (Thermo Fisher Scientific, Waltham, MA) and an amplicon from strain 364-D. To confirm the sequence of the clone, the insertion site was sequenced in both directions using primers T7 Promoter and M13 Reverse. Sequencing reads were assembled using Geneious v10.2 (Biomatters Ltd., Auckland, New Zealand). The plasmid concentration was determined using a NanoDrop ND-1000 spectrophotometer (Thermo Fisher Scientific). The limit of detection of the assay was assessed using a 10-fold dilution series of the control plasmid, with concentrations ranging from 1,000,000 copies/4 μl to 1 copy/4 μl. To test the specificity of the assay, 92 individual DNA samples comprising 10 additional isolates of Rickettsia 364D (5), 27 isolates of R. rickettsii, 26 isolates of 24 other Rickettsia species, 10 strains of closely related bacteria and Escherichia coli, 9 tick species, and 8 cell lines were used (Table 1). All evaluations included a nontemplate control (sterile, PCR-grade water) and a positive control comprising the control plasmid.
TABLE 1.
Reference DNAs used to test assay specificity
| DNA sample |
|---|
| Rickettsia 364D 364-DT |
| Rickettsia 364D Cache Creek Ridge |
| Rickettsia 364D Crystal Cove |
| Rickettsia 364D El Moro Canyon |
| Rickettsia 364D Highland Creek |
| Rickettsia 364D Lake |
| Rickettsia 364D Mt. Konocti |
| Rickettsia 364D Orange |
| Rickettsia 364D Pierce Canyon |
| Rickettsia 364D Pine Mountain |
| Rickettsia 364D Wright Peak |
| Rickettsia rickettsii 78-RL |
| Rickettsia rickettsii AZ-3 |
| Rickettsia rickettsii Bitterroot |
| Rickettsia rickettsii Brazil |
| Rickettsia rickettsii BSF Bell |
| Rickettsia rickettsii BSF Rab1 |
| Rickettsia rickettsii BSF-Si7 |
| Rickettsia rickettsii Colombia |
| Rickettsia rickettsii Costa Rica |
| Rickettsia rickettsii Domino |
| Rickettsia rickettsii Gila |
| Rickettsia rickettsii Hauke |
| Rickettsia rickettsii Hino |
| Rickettsia rickettsii HLP-2 |
| Rickettsia rickettsii Iowa |
| Rickettsia rickettsii Lost Horse Canyon |
| Rickettsia rickettsii Morgan |
| Rickettsia rickettsii Panama1 |
| Rickettsia rickettsii PriceT |
| Rickettsia rickettsii Ripley |
| Rickettsia rickettsii RSMG |
| Rickettsia rickettsii Salt River |
| Rickettsia rickettsii São Paulo |
| Rickettsia rickettsii Sawtooth |
| Rickettsia rickettsii Sheila SmithT |
| Rickettsia rickettsii Tarheel |
| Rickettsia rickettsii Taiaçu |
| Rickettsia amblyommatis WB-8-2T |
| Rickettsia africae Z9-HuT |
| Rickettsia akari KaplanT |
| Rickettsia asemboensis NMRCiiT |
| Rickettsia asiatica IO-1T |
| Rickettsia australis Cutlack |
| Rickettsia bellii 369-CT |
| Rickettsia canadensis McKiel 24 |
| Rickettsia conorii subsp. indica |
| Rickettsia conorii Malish 7T |
| Rickettsia felis Marseille-URRFXCal2T |
| Rickettsia honei TT118 |
| Rickettsia massiliae Mtu1T |
| Rickettsia montanensis OSU 85-930 |
| Rickettsia monteiroi IntervalesT |
| Rickettsia parkeri Portsmouth |
| Rickettsia peacockii Rustic |
| Rickettsia rhipicephali Burgdorfer3-7-Female 6T |
| Rickettsia rhipicephali Ect |
| Rickettsia sibirica 246T |
| Rickettsia slovaca BT |
| Rickettsia vini BreclavT |
| Rickettsia typhi WilmingtonT |
| Rickettsia sp. strain SF143 |
| Rickettsia sp. strain SF145 |
| Rickettsia sp. Tillamook str. 23 |
| Anaplasma phagocytophilum USG3 |
| Ehrlichia canis OklahomaT |
| Ehrlichia chaffeensis ArkansasT |
| Ehrlichia muris eauclairensis WisconsinT |
| Ehrilichia muris muris AS145T |
| Orientia tsutsugamushi KarpT |
| Bartonella elizabethae |
| Bartonella henselae Houston-1T |
| Bartonella quintana |
| Bartonella vinsonii Berkofii |
| Amblyomma americanum |
| Amblyomma maculatum |
| Dermacentor andersoni |
| Dermacentor occidentalis |
| Dermacentor variabilis |
| Haemaphysalis leporispalustris |
| Ixodes pacificus |
| Ixodes scapularis |
| Rhipicephalus sanguineus |
| DH82 (canine macrophage cells) |
| EA.hy926 (human endothelial cells) |
| Escherichia coli |
| Human lung endothelium cells (HULECs) |
| Human dermal endothelium cells (HMECs) |
| Human aorta cells |
| ISE6 (Ixodes scapularis tick cells) |
| Vero E6 (African green monkey epithelial cells) |
| XTC-2 (African clawed frog cells) |
To assess the clinical sensitivity of the assay, we evaluated sterile collection swabs containing whole blood spiked with live Rickettsia 364D bacteria to reproduce the most commonly used specimen collection technique for PCR (i.e., eschar swab). An aliquot of whole blood, obtained from an anonymous human donor from the Tennessee Blood Services was initially tested by the TaqMan assay to confirm that the sample was negative for Rickettsia 364D. Dilutions of cultured Rickettsia 364D (strain Lake) were inoculated into 100-μl aliquots of blood to achieve final concentrations of 1 × 106 to 1 organism/4 μl (10-fold dilution series), assuming a 100% recovery rate from the DNA extraction procedure. Each aliquot was then absorbed onto a Fisherbrand bacteriology culture collection and transport system swab (Thermo Fisher Scientific) and allowed to dry overnight. DNA was extracted from the swabs using a DNeasy blood and tissue kit (Qiagen, Valencia, CA) as follows. The dried swabs were placed into vials containing 400 μl phosphate-buffered saline (PBS), 180 μl buffer ATL, and 20 μl proteinase K and incubated at 56°C for 3 hours with intermittent vortexing. The swabs were then removed from the vials, 400 μl buffer AL was added, and the samples were incubated at 70°C for 10 minutes. Four hundred microliters of 100% ethanol was then added to the samples, and the samples were put into spin columns and extracted following the manufacturer’s directions, with the samples eluted in 200 μl buffer AE. The samples were tested in duplicate along with an extraction blank (whole blood alone), a no-template control, and a plasmid positive control.
An additional 61 DNA extracts, comprising 30 D. occidentalis ticks (Table 2) and 31 clinical specimens (Table 3), which were previously tested at the California Department of Public Health Viral and Rickettsial Disease Laboratory (VRDL), were similarly evaluated using the same amplification parameters on an Applied Biosystems (AB) 7500 fast real-time PCR system (Thermo Fisher Scientific). Clinical specimens originated from 12 confirmed cases of PCTF, 2 confirmed cases of murine typhus, 1 confirmed case of RMSF, and 10 samples submitted for varicella-zoster virus screening. All specimens were tested using a two-assay algorithm that consisted of an initial screening using either a pan-Rickettsia real-time assay or an SFG Rickettsia real-time assay (11, 12). Species identification was then accomplished using a nested PCR assay and DNA sequencing of a segment of the ompA gene (2, 13, 14). An assay targeting the human RNAse P gene (15) was used to evaluate clinical specimens for the presence of substances that may interfere with the Rickettsia 364D PCR assay.
TABLE 2.
Dermacentor occidentalis samples used to test assay specificitya
| Specimen no. | Pan-Rickettsia real-time PCR result | Previous species identification | 364D real-time PCR result |
|---|---|---|---|
| 1 | Detected | Rickettsia 364D | Detected |
| 2 | Detected | Rickettsia 364D | Detected |
| 3 | Detected | Rickettsia 364D | Detected |
| 4 | Detected | Rickettsia 364D | Detected |
| 5 | Detected | Rickettsia 364D | Detected |
| 6 | Detected | Rickettsia 364D | Detected |
| 7 | Detected | Rickettsia 364D | Detected |
| 8 | Detected | Rickettsia 364D | Detected |
| 9 | Detected | Rickettsia 364D | Detected |
| 10 | Detected | Rickettsia 364D | Detected |
| 11 | Detected | R. rhipicephali | Not detected |
| 12 | Detected | R. rhipicephali | Not detected |
| 13 | Detected | R. rhipicephali | Not detected |
| 14 | Detected | R. rhipicephali | Not detected |
| 15 | Detected | R. rhipicephali | Not detected |
| 16 | Detected | R. rhipicephali | Not detected |
| 17 | Detected | R. rhipicephali | Not detected |
| 18 | Detected | R. rhipicephali | Not detected |
| 19 | Detected | R. rhipicephali | Not detected |
| 20 | Detected | R. rhipicephali | Not detected |
| 21 | Detected | R. bellii | Not detected |
| 22 | Detected | R. bellii | Not detected |
| 23 | Detected | R. bellii | Not detected |
| 24 | Not detected | Not done | Not detected |
| 25 | Not detected | Not done | Not detected |
| 26 | Not detected | Not done | Not detected |
| 27 | Not detected | Not done | Not detected |
| 28 | Not detected | Not done | Not detected |
| 29 | Not detected | Not done | Not detected |
| 30 | Not detected | Not done | Not detected |
Shading indicates samples positive for Rickettsia 364D.
TABLE 3.
Human DNA samples used to test assay specificitya
| Sample no. | Specimen type | Diagnosis | Rickettsial real-time PCR result | Previous species identification | 364D real-time PCR result |
|---|---|---|---|---|---|
| 1 | Lesion swab/scab | Rickettsia 364D | SYBR SFGR detected | Rickettsia 364D | Detected |
| 2 | Lesion swab | Rickettsia 364D | SYBR SFGR detected | Rickettsia 364D | Detected |
| 3 | Eschar | Rickettsia 364D | SYBR SFGR detected | Rickettsia 364D | Detected |
| 4 | Eschar | Rickettsia 364D | SYBR SFGR detected | Rickettsia 364D | Detected |
| 5 | Eschar | Rickettsia 364D | SYBR SFGR detected | Unable to determine | Detected |
| 6 | Lesion swab | Rickettsia 364D | PanR detected | Rickettsia 364D | Detected |
| 7 | Eschar | Rickettsia 364D | PanR detected | Rickettsia 364D | Detected |
| 8 | Eschar | Rickettsia 364D | PanR detected | N/A | Detected |
| 9 | Lesion swab | Varicella-zoster virus | Not done | N/A | Not detected |
| 10 | Skin scraping | Varicella-zoster virus | Not done | N/A | Not detected |
| 11 | Lesion swab | Varicella-zoster virus | Not done | N/A | Not detected |
| 12 | Lesion swab | Varicella-zoster virus | Not done | N/A | Not detected |
| 13 | Lesion swab | Varicella-zoster virus | Not done | N/A | Not detected |
| 14 | Lesion swab | Varicella-zoster virus | Not done | N/A | Not detected |
| 15 | Lesion swab | Varicella-zoster virus | Not done | N/A | Not detected |
| 16 | Lesion swab | Varicella-zoster virus | Not done | N/A | Not detected |
| 17 | Lesion swab | Varicella-zoster virus | Not done | N/A | Not detected |
| 18 | Lesion swab | Varicella-zoster virus | Not done | N/A | Not detected |
| 19 | Serum | Rickettsia 364D | Not done | N/A | Not detected |
| 20 | Serum | Rickettsia 364D | Not done | N/A | Not detected |
| 21 | Serum | Rickettsia 364D | Not done | N/A | Not detected |
| 22 | Serum | Rickettsia 364D | Not done | N/A | Not detected |
| 23 | Serum | Rickettsia 364D | Not done | N/A | Not detected |
| 24 | Serum | Rickettsia 364D | Not done | N/A | Not detected |
| 25 | Serum | Rickettsia 364D | Not done | N/A | Not detected |
| 26 | Serum | Rickettsia 364D | Not done | N/A | Not detected |
| 27 | Serum | Rickettsia 364D | Not done | N/A | Not detected |
| 28 | Serum | Rickettsia 364D | Not done | N/A | Not detected |
| 29 | Plasma | R. typhi (PCR) | PanR detected | R. typhi | Not detected |
| 30 | Serum | R. typhi (PCR) | PanR detected | R. typhi | Not detected |
| 31 | Whole blood | R. rickettsii (PCR confirmed at CDC) | PanR detected | Unable to determine | Not detected |
Shading indicates samples positive for Rickettsia 364D. N/A, not applicable; SFGR, spotted fever group Rickettsia; PanR, pan-Rickettsia real-time PCR.
RESULTS
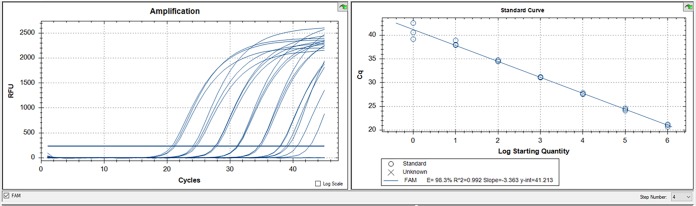
The positive-control plasmid was used to determine the optimal reaction conditions. Based on Cq values, the optimal annealing temperature was determined to be 61°C. The limit of detection was determined to be 1 copy per 4 μl, with an R2 value of 0.992 and an amplification efficiency of 98.3% (Fig. 2).
FIG 2.
Evaluation of Rickettsia 364D-specific TaqMan assay sensitivity and efficiency. Standard curve produced using serial 10-fold dilutions of the control plasmid (1 × 106 copies/4 μl to 1 copy/4 μl). All samples were run in triplicate. The sensitivity of the assay was found to be 1 copy/4 μl, the R2 value was 0.992, and the PCR efficiency was 98.3%.
To assess the specificity and accuracy of the real-time PCR assay, a total of 153 positive- and negative-control DNA samples were evaluated (Tables 1 and 2). The assay correctly detected all 21 Rickettsia 364D-positive samples comprising 11 unique isolates and 10 Rickettsia 364D-positive specimens of D. occidentalis. None of the 53 other rickettsial isolates (25 distinct species) and none of the 13 D. occidentalis ticks infected with either R. rhipicephali (n = 10) or Rickettsia bellii (n = 3) were detected. In a similar manner, the assay did not detect DNA from any of the 10 species in closely related genera, including Ehrlichia, Anaplasma, Orientia, and Bartonella; 9 species of hard ticks endemic to the United States; 8 uninfected D. occidentalis; 8 cell lines of human, animal, or tick origin; or E. coli.
To assess the clinical sensitivity of the assay, human whole blood was spiked with known concentrations of Rickettsia 364D and absorbed onto specimen collection swabs. The DNA was extracted, and the samples screened for the detection of rickettsial DNA. The clinical sensitivity was found to be between 100 and 1,000 genomic copies per 4 μl, with an R2 value of 0.992 and an amplification efficiency of 110.1% (results not shown).
To assess the clinical accuracy and specificity of the assay, DNA extracts of 31 clinical specimens were also evaluated. These included specimens from 8 cases of previously diagnosed PCTF (skin biopsy specimens or swabs) and an additional 13 serum, plasma, or whole-blood specimens from 13 cases of suspected or confirmed PCTF, endemic typhus, or RMSF. Eleven of these samples were tested with the 364D assay and a pan Rickettsia or an SFG Rickettsia real-time assay (the 10 PCTF serum samples were not previously tested using the pan Rickettsia assays). Of these 11 samples, all were positive for a Rickettsia species, including the 8 eschar swabs and biopsy specimens from 8 cases of PCTF, 2 blood specimens from confirmed cases of murine typhus, and 1 of RMSF. Using the Rickettsia 364D assay, only the eight eschar swabs and biopsy samples tested positive. Six of these samples were previously shown to be positive for Rickettsia 364D by DNA sequencing (sample 8 did not undergo DNA sequencing). However, a seventh sample, which was known to be positive for unidentified rickettsial DNA, was from a patient in whom the 2-assay algorithm had confirmed PCTF using another lesion swab. The remaining three samples tested negative with the new assay and were also negative for Rickettsia 364D using the 2-assay algorithm. Although Rickettsia 364D DNA has never been detected in patient blood, 10 serum samples from confirmed PCTF patients were screened using the new assay. Additionally, 10 lesion swabs obtained for varicella-zoster virus testing were also tested. All 20 samples were negative using the Rickettsia 364D assay. The RNase P target was detected in all 30 clinical specimens tested. Overall, both the clinical accuracy and specificity of the assay were 100% compared with previous results.
DISCUSSION
This work describes the development and application of a highly sensitive and specific TaqMan real-time PCR assay for Rickettsia 364D that provides a simplified molecular method to more rapidly evaluate samples for ecological studies and provide more timely and accurate results for the diagnosis of persons suspected to have PCTF. As tested, the clinical sensitivity of the assay (100 to 1,000 genomic copies per 4 μl) did not match the limit of detection of the assay (1 copy per 4 μl) when tested using the control plasmid. However, the extraction procedure was not optimized for the recovery of DNA from collection swabs, and no extraction procedure is 100% effective at recovering the entire input DNA quantity. Therefore, it is unknown what the actual concentration of rickettsial DNA was in each of the eluted samples and how these concentrations relate to the input concentration. Nevertheless, the novel TaqMan assay showed complete clinical concordance with the two-assay algorithm currently used at the VRDL when evaluated against a panel of 31 archival specimens of blood, serum, plasma, eschar, and swab biopsy specimens. Current molecular methods for the detection of Rickettsia 364D rely on DNA sequencing of PCR amplicons (2, 3, 10); this new assay will facilitate the detection of Rickettsia 364D by providing a more rapid and cost-effective method than is currently available.
Much about the ecology of Rickettsia 364D remains unknown. To date, D. occidentalis is the only tick species in which Rickettsia 364D has been detected (5). Other human-biting tick species coexist with D. occidentalis in areas where Rickettsia 364D has been identified, and some of these species have been shown to be competent vectors of other, closely related rickettsial species. Additionally, more work needs to be conducted to determine which vertebrate hosts are associated with the transmission or maintenance of Rickettsia 364D in nature. A more thorough understanding of the natural history of Rickettsia 364D is needed to better define the public health significance posed by this pathogen. This novel assay provides a new tool to help answer these important ecological questions.
ACKNOWLEDGMENTS
The findings and conclusions in this article are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
REFERENCES
- 1.Philip RN, Casper EA, Burgdorfer W, Gerloff RK, Hughes LE, Bell EJ. 1978. Serologic typing of rickettsiae of the spotted fever group by microimmunfluorescence. J Immunol 121:1961–1968. [PubMed] [Google Scholar]
- 2.Shapiro MR, Fritz CL, Tait K, Paddock CD, Nicholson WL, Abramowicz KF, Karpathy SE, Dasch GA, Sumner JW, Adem PV, Scott JJ, Padgett KA, Zaki SR, Eremeeva ME. 2010. Rickettsia 364D: a newly recognized cause of eschar-associated illness in California. Clin Infect Dis 50:541–548. doi: 10.1086/649926. [DOI] [PubMed] [Google Scholar]
- 3.Padgett KA, Bonilla D, Eremeeva ME, Glaser C, Lane RS, Porse CC, Castro MB, Messenger S, Espinosa A, Hacker J, Kjemtrup A, Ryan B, Scott JJ, Hu R, Yoshimizu MH, Dasch GA, Kramer V. 2016. The Eco-epidemiology of Pacific Coast tick fever in California. PLoS Negl Trop Dis 10:e0005020. doi: 10.1371/journal.pntd.0005020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Karpathy SE, Dasch GA, Eremeeva ME. 2007. Molecular typing of isolates of Rickettsia rickettsii by use of DNA sequencing of variable intergenic regions. J Clin Microbiol 45:2545–2553. doi: 10.1128/JCM.00367-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Paddock CD, Yoshimizu MH, Zambrano ML, Lane RS, Ryan BM, Espinosa A, Hacker JK, Karpathy SE, Padgett KA. 2018. Rickettsia species isolated from Dermacentor occidentalis (Acari: Ixodidae) from California. J Med Entomol 55:1555–1560. doi: 10.1093/jme/tjy100. [DOI] [PubMed] [Google Scholar]
- 6.Furman DP, Loomis EC. 1984. The ticks of California. Bulletin of the California Insect Survey 25 University of California Press, Berkeley, CA. [Google Scholar]
- 7.Lane RS, Anderson JR, Yaninek JS, Burgdorfer W. 1985. Diurnal host seeking of adult Pacific Coast ticks, Dermacentor occidentalis (Acari: Ixodidae), in relation to vegetational type, meterlolgical factors, and rickettsial infection rates in California, USA. J Med Entomol 22:558–571. doi: 10.1093/jmedent/22.5.558. [DOI] [PubMed] [Google Scholar]
- 8.Wikswo ME, Hu R, Dasch GA, Krueger L, Arugay A, Jones K, Hess B, Bennett S, Kramer V, Eremeeva ME. 2008. Detection and identification of spotted fever group rickettsiae in Dermacentor species from southern California. J Med Entomol 45:509–516. doi: 10.1093/jmedent/45.3.509. [DOI] [PubMed] [Google Scholar]
- 9.Billeter SA, Yoshimizu MH, Hu R. 2017. Species composition and temporal distribution of adult ticks and prevalence of Borrelia burgdorferi sensi lato and Rickettsia species in Orange Country. J Vector Ecol 42:189–192. doi: 10.1111/jvec.12255. [DOI] [PubMed] [Google Scholar]
- 10.Johnston SH, Glaser CA, Padgett K, Wadford DA, Espinosa A, Espinosa N, Eremeeva ME, Tait K, Hobson B, Shtivelman S, Hsieh C, Messenger S. 2013. Rickettsia spp. 364D causing a cluster of eschar-associated illness, California. Pediatr Infect Dis J 32:1036–1039. doi: 10.1097/INF.0b013e318296b24b. [DOI] [PubMed] [Google Scholar]
- 11.Eremeeva ME, Dasch GA, Silverman DJ. 2003. Evaluation of a PCR assay for quantitation of Rickettsia rickettsii and closely related spotted fever group rickettsiae. J Clin Microbiol 41:5466–5472. doi: 10.1128/jcm.41.12.5466-5472.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kato CY, Chung IH, Robinson LK, Austin AL, Dasch GA, Massung RF. 2013. Assessment of real-time PCR assay for detection of Rickettsia spp. and Rickettsia rickettsii in banked clinical samples. J Clin Microbiol 51:314–317. doi: 10.1128/JCM.01723-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Regnery RL, Spruill CL, Plikaytis BD. 1991. Genotypic identification of rickettsiae and estimation of intraspecies sequence divergence for portions of two rickettsial genes. J Bacteriol 173:1576–1589. doi: 10.1128/jb.173.5.1576-1589.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Roux V, Fournier PE, Raoult D. 1996. Differentiation of spotted fever group rickettsiae by sequencing and analysis of restriction fragment length polymorphism of PCR-amplified DNA of the gene encoding the protein rOmpA. J Clin Microbiol 34:2058–2065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Emery SL, Erdman DD, Bowen MD, Newton BD, Winchell JM, Meyer RF, Tong S, Cook BT, Holloway BP, McCaustland KA, Rota PA, Bankamp B, Lowe LE, Ksiazek TG, Bellini WJ, Anderson LJ. 2004. Real-time reverse transcription-polymerase chain reaction assay for SARS-associated coronavirus. Emerg Infect Dis 10:311–316. doi: 10.3201/eid1002.030759. [DOI] [PMC free article] [PubMed] [Google Scholar]