Abstract
Unhealthy diets are a leading cause of death and disability globally. The WHO recommends Member States implement front-of-pack (FOP) nutrition labels to guide consumers towards healthier food choices, as part of comprehensive strategies to prevent diet-related non-communicable diseases. Interest in FOP nutrition labelling is increasing, but there is limited guidance for policymakers developing regulations necessary for effective implementation. A rapidly evolving evidence base, limited regulatory capacity and possibility of legal challenge by affected food industry stakeholders can create ‘regulatory chill’, whereby governments are dissuaded from progressive public health policymaking. We use a framework for analysing public health law and available best-practice guidance to evaluate key components of 31 FOP nutrition labelling regulations endorsed by governments up to June 2019. Analysis of regulatory form shows recent rapid uptake of label formats that are easier for consumers to understand and increasing use of mandatory legislation. However, policymakers must decide much more than whether to apply ‘stars’, ‘traffic lights’ or ‘stop signs’. The substance of effective regulation must contain strategic regulatory objectives, clear specifications for displaying the label on pack, a valid scoring mechanism and a justified scope for including foods. While there are limited data on current practice, good governance of FOP nutrition labelling regulation also requires transparency and accountability in processes of label development, implementation, evaluation and enforcement to promote continuous improvement and withstand undue commercial interference. Whether developing new FOP nutrition labels or reforming existing ones, our findings support policymakers to design and implement best-practice, evidence-informed regulation.
Keywords: food policy, public health nutrition, non-communicable diseases, food labelling, regulation
Summary box.
Simple, graphical front-of-pack (FOP) nutrition labels are part of the package of policies recommended by the WHO to address the growing global burden of diet-related non-communicable disease.
Food industry resistance to progressive FOP nutrition labels threatens to stifle policy innovation, creating need to support policymakers to design and implement best-practice regulation.
A framework for analysing public health law applied to 31 existing FOP nutrition labelling regulations endorsed by governments highlights strengths of current practice and areas where it could be further improved.
While public health attention has focused on label format (eg, stars, traffic lights, stop signs), regulators must also consider the legal framework used (ie, voluntary or mandatory), which foods are within scope, how foods will be scored and how labels should be displayed on pack, as well as incorporating procedural mechanisms to promote transparency and accountability at all stages of the policy cycle.
FOP nutrition labels implemented through evidence-informed, strategically designed regulation are less likely to be successfully contested in national and international courts and more likely to achieve public health impact.
Introduction
Unhealthy diets are a leading cause of death and disability globally.1 Unprecedented availability, accessibility and affordability of processed and prepackaged foods is a key driver of obesity and diet-related non-communicable diseases (NCDs) including high blood pressure, cardiovascular disease, type-2 diabetes and some cancers.2 3 As part of a comprehensive policy response to promoting healthier diets and preventing NCDs, the WHO recommends that governments implement front-of-pack (FOP) nutrition labels.4–11 These simple, often graphical labels provide at-a-glance information on nutritional quality on the primary display panel of foods and beverages (hereafter ‘foods’) to complement detailed nutrient declarations on back-of-pack. A growing body of evidence suggests such labels may aid consumer understanding of nutritional quality, encourage selection and purchase of healthier foods, and promote reformulation by industry.12–15
Existing authoritative guidance supports government action but provides limited detail on the appropriate content of national FOP nutrition labelling regulation.11 16 17 Recent policy developments at a national level include a shift away from ‘softer’ positive signposts that highlight healthier options within category such as Sweden’s green ‘Keyhole’,18 towards formats such as Chile’s ‘stop-sign’ warnings19 which signal product unhealthfulness and discourage consumption, thereby potentially decreasing sales.
Resistance to these newer FOP nutrition labels can be seen in legal challenges launched or threatened under domestic and international law, including at the World Trade Organization (WTO).20–22 The potential impact of different national requirements on food trade has also prompted the international food standards agency, the Codex Alimentarius Commission (Codex), to commence negotiating further guidance on FOP nutrition labelling.23 These developments illustrate the multisectoral nature of food governance and competing authorities at play: nutrition labelling lies within the remit of the health sector but requires balancing with the interests of stakeholders in agriculture, industry and trade.16 21 24
A significant body of health research compares the efficacy of different FOP nutrition label formats (eg, stars, traffic lights, stop signs),14 25 but there has been less systematic examination of other essential features of regulation. Questions faced by policymakers include which foods FOP nutrition labels should apply to, how foods should be scored and which stakeholders should be involved in developing, evaluating and enforcing FOP nutrition labelling.
A rapidly evolving public health evidence base, limited regulatory capacity and the possibility of costly legal challenge driven by affected food industry stakeholders creates the risk of ‘regulatory chill’, whereby governments are dissuaded from progressive public health policymaking.26 To avert this risk and accelerate continued policy innovation, our aim was to provide policymakers with support to prepare evidence-informed, strategically designed and well-governed FOP nutrition labelling regulations.
We analysed current practice using a framework developed by Reeve and Magnusson for analysing and improving the performance of public health regulation.27 Building on public health and regulatory studies literature, the framework allows evaluation of multiple ‘regulatory domains’: considering the form of regulation used, its substantive terms and conditions, and the application of good governance in its development, administration and enforcement. We adapted this framework to the specific context of FOP nutrition labelling regulation by reference to recent best-practice guidance from the WHO and World Cancer Research Fund (table 1).11 16 17
Table 1.
A framework for analysing and improving the performance of FOP nutrition labelling regulation
| Component | Recommendation | Application to FOP nutrition labelling |
| Domain One: Regulatory form | ||
| Regulatory framework | The regulatory framework is appropriate to the jurisdiction’s legal context.27 | Governments should consider mandatory legal frameworks to overcome suboptimal voluntary uptake.16 17 Existing laws (eg, constitutions) may determine whether mandatory implementation is legally possible.16 |
| FOP nutrition label format selection | The FOP nutrition label format selected supports the regulatory objective(s), that is, can be understood and used by consumers to inform healthier choices. | The FOP nutrition label format should be interpretive, that is, use words, colours and/or symbols to make judgments11 16 17 and be understandable to all population subgroups.11 16 29 Formats indicating unhealthfulness appear more effective in guiding consumers to nutritionally favourable products.17 |
| Domain Two: Regulatory substance | ||
| Regulatory Objective(s) | Clear, measurable objectives by which success of regulation can be assessed.27 | The aim of FOP nutrition labelling is to inform and guide consumers towards healthier food choices; a secondary aim is to stimulate healthier formulation by the food industry.11 16 17 |
| Operative terms and conditions | Key terms and conditions are clearly defined; regulatory rules are sufficiently expansive to achieve the regulatory objectives.27 | Operative terms include: display specifications that promote visibility and salience; nutrients and food components included that link to health evidence; valid scoring criteria and reference amount; justified scope (products included and excluded).11 16 17 |
| Policy coherence | Regulation is framed within comprehensive policies to promote healthier diets.4–7 | FOP nutrition labelling should be aligned with and enhance operation of other national public health and nutrition policies, food regulations and relevant WHO and Codex guidance.11 |
| Domain Three: Regulatory governance | ||
| Drafting regulatory rules and scheme design | Transparency and accountability mechanisms are incorporated into regulatory regimes from their inception, including when developing substantive regulatory rules.27 | Government retains ultimate responsibility and authority for setting regulatory objectives and scope; developing and validating scoring criteria with independent expert input; setting a review framework; leading a stakeholder engagement process for development of a trusted system, including use of multistakeholder committees or public consultation to develop label format, content and supporting documents (eg, style guides). Information should be transparent and easily accessible (eg, submissions to public consultation released, public meetings and minutes of committees).11 There should be appropriate safeguards for managing conflicts of interest.16 |
| Administration | Administration by a government or independent body which monitors and enforces compliance, publicly disseminates information on performance to facilitate external scrutiny.27 | Administration granted to an independent statutory authority (eg, food standards agency), government body (eg, ministry of health) or multistakeholder group with appropriate safeguards for managing conflicts of interest. The administrative body must be provided with requisite authority and resource to conduct monitoring and enforcement activities and to publicise performance outcomes.11 27 |
| Monitoring | Monitoring informs continuous improvement through collection of baseline data, setting of process and outcome indicators and timeframes for achievement, ongoing data collection.27 | Baseline and follow-up data collected on: uptake and label compliance by industry (eg, using a database with photographs of labels and/or licensing scheme), consumer understanding and use, product purchases, population dietary intakes, nutrient composition of foods and reformulation.11 16 |
| Evaluation | Structured, regular review ensures regulation meeting its objectives. A review framework set during development includes baseline data and, performance indicators and timeframes to evaluate effectiveness.27 | Government-led and/or carried out by independent body or research group (eg, auditor, consultant) with authority to assess achievement of the regulatory objectives using a transparent framework and sufficient data to assess whether performance indicators met in the specified timeframes.11 16 17 |
| Enforcement | A wide range of enforcement options are available, including incentives to encourage and reward high levels of compliance, ‘soft’ enforcement measures such as persuasion and more punitive measures for instances of serious or persistent non-compliance. Publication of decisions enhances transparency, allows development of ‘precedent’ for users.27 | Enforcement may be supported by premarket approval, for example, licensing; or auditing of products in the market. The administrative body possesses range of sanctions, including positive and negative publicity, written requests for action, withdrawal of right to use (positive) labels, fines or legal action under new or existing law. A complaints handling mechanism may allow all stakeholders to raise issues with regulation or instances of non-compliance.27 |
FOP, front-of-pack.
To apply the framework, we created a database of existing regulations. Existing FOP nutrition labels were identified by a search of existing global policy databases and reports.16 17 28–30 We included government-led or officially supported (hereafter ‘government endorsed’) initiatives identified as FOP nutrition labelling by at least one of these sources. We adopted a broad definition of regulation to capture both voluntary FOP nutrition labels, for example, those implemented through sets of government-issued rules that industry voluntarily apply, and mandatory systems, which impose binding requirements on industry through government legislation. Inclusion of voluntary schemes acknowledges that this kind of ‘self-regulation’ is often relied on in lieu of more traditional government legislation and that public interest in their effectiveness thereby warrants their subjection to the same scrutiny. We refer to both mandatory and voluntary labelling schemes as ‘regulation’ throughout.
For each FOP nutrition label, we collated publicly available information on each component of regulation identified in the framework. Information from the primary global policy databases and reports was supplemented by targeted searches of peer-reviewed and grey literature, for example, government websites. Results are summarised in table format, and the database provided in full as online supplementary appendix 1.
bmjgh-2019-001882supp001.pdf (218.1KB, pdf)
For the purposes of this analysis paper, we present findings by regulatory domain to highlight strengths of existing practice and make recommendations for where and how FOP nutrition labelling regulation could be improved.
Regulatory form
Our search of current practice identified 31 unique FOP nutrition labels, each governed by their own regulatory rules. Some jurisdictions had more than one FOP nutrition label (eg, Israel had both red warnings for specific nutrients, and a green endorsement logo), and some labels operated in more than one jurisdiction (eg, Australia and New Zealand’s Health Star Ratings (HSR)).
In total, 32 governments had endorsed some form of FOP nutrition label (figure 1). Most (23/32) of these had done so since 2010 (online supplementary appendix 1). In 2019, countries including Argentina, Brazil, Canada, Fiji, Germany, Guatemala, India, Portugal and South Africa were reportedly considering FOP nutrition labelling,16 17 highlighting the need to disseminate regulatory best-practice.
Figure 1.
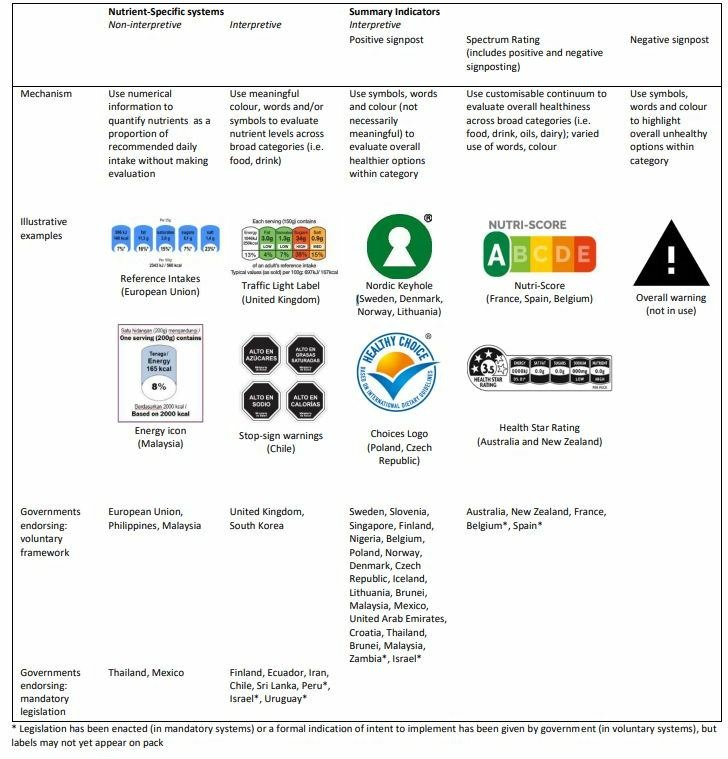
FOP nutrition labels by regulatory form (label format and legal framework). FOP, front-of-pack.
Regulatory framework
When designing regulation, an initial consideration for policymakers is selection of an appropriate legal framework (table 1).16 Mandatory FOP nutrition labels are specifically recommended by the WHO High Level Commission on Ending Childhood Obesity6 7 and provide an important way to overcome suboptimal uptake of voluntary labels.17 Despite sometimes being raised as a barrier to mandatory labels, international trade agreements governed by the WTO do allow governments to implement mandatory measures, provided regulation is crafted carefully to maintain coherence with international trade obligations.21 In each jurisdiction, policymakers must determine whether any existing law restricts implementation of a mandatory label. For example, European Union (EU) legislation may constrain EU member countries from applying mandatory national legislation.31 Where a mandatory label is not legally possible, a voluntary system can be pursued.16
While the majority of labelling schemes in current practice remained voluntary, 10 countries now have mandatory FOP nutrition labels (figure 1). Eight of these were adopted since 2014, including regulations in Ecuador,32 Iran,33 Sri Lanka,34 Mexico,35 Chile,36 Peru,37 Israel38 and Uruguay.39
Label format
For FOP nutrition labelling to promote healthier diets, policymakers must select a label format that consumers can understand and use (table 1). A significant body of public health evidence suggests formats with interpretive elements that show judgement or recommendation are most useful.11 13 14 As outlined in figure 1, these include nutrient-specific interpretive labels and multiple types of summary indicator formats.
Of 31 current FOP nutrition labels, 26 used interpretive elements. Fourteen positive signposts used subjective words (eg, good, healthier), symbols (eg, ticks, hearts) and colour to signal relative healthfulness within category. However, included elements such as the Keyhole symbol did not necessarily hold intuitive meaning (figure 1). While the marketing value of positive signposts makes them appealing to industry, a summary of recent evidence suggests they encourage consumers to overestimate healthfulness and may also facilitate price premiums on healthier options that policymakers may find undesirable for low socioeconomic consumers.17
By contrast, the past 5 years has brought rapid innovation in labels that evaluate product unhealthfulness, which appear more effective in supporting consumers to choose nutritionally favourable products.17 These include 10 nutrient-specific formats which used descriptive words (eg, low, medium, high); meaningful colour (red, yellow, green, black) and salient symbols (eg, stop signs, traffic lights) to illustrate specific nutrient content (figure 1). Recent regulation adopted in Uruguay elected to use ‘excess’ rather than ‘high in’ for specific nutrients,39 reflecting research in that context that ‘high in’ was prone to more positive connotations.40
In a similar manner, Australasia’s HSR41 and France’s NutriScore42 (now also endorsed by Belgium and Spain) indicated both healthfulness and unhealthfulness but did so on a customisable continuum to summarise nutritional quality overall. Nutri-Score used colour to reinforce its evaluation (ie, A was dark green to signify healthiest)42 whereas HSR allowed manufacturers to display the logo in any colour.41 No jurisdiction had yet extended this focus on evaluating unhealthfulness to providing only a summary negative signpost (figure 1).
The remaining five labels (figure 1) were non-interpretive, that is, presented monochrome numerical information. While preferred by industry, consumers cannot understand and use such information,11 13 14 particularly those with lower nutrition literacy, education or members of minority ethnic groups.11 For FOP nutrition labelling to effectively guide consumers towards healthier choices, policymakers should avoid non-interpretive label formats.
Regulatory substance
Effective implementation of FOP nutrition labelling also requires policymakers to ensure sufficient attention to Domain Two, the substantive terms and content of regulation. In this domain of regulatory substance, loopholes and gaps in current practice provide insight for strengthening future regulation.
Regulatory objectives
The objectives of regulation provide the basis by which policy ‘success’ will be judged, both in routine evaluation and in the event of legal challenge.16 21 Strategic regulatory objectives should reflect measurable pathways of effect, for example, changes in consumer understanding, behaviour and/or food composition (table 1) demonstrable by short-term evaluation. While improved food labelling may contribute to broader goals, for example, prevention of NCDs or longer-term changes in obesity prevalence, difficulty in producing short-term evidence of these outcomes makes them less suited as explicit objectives of regulation.16 21
Current practice summarised in table 2 aligns with WHO Guidance on the objectives of FOP nutrition labelling.11 Where identified, all regulations specified a measurable consumer-focused objective that is, informing and/or helping them make healthier food choices. Uruguay’s law was even more strategic; its objective was to allow consumers to ‘identify foods with excessive amounts of specific nutrients’,39 a threshold foreseeably easier to demonstrate. Seventeen interpretive labelling regulations also contained explicit reference to the additional benefit of incentivising industry reformulation (table 2).
Table 2.
Substantive content of FOP nutrition labelling regulation
| Label, Jurisdiction (Year Endorsed) | Regulatory objectives | Display specifications | Nutrients/Food components included | Nutrient scoring mechanism | Reference amount used | Criteria validated | Scope | Specified exclusions | Policy coherence |
|
Nordic Keyhole
Sweden (1989); Denmark And Norway (2009), Lithuania (2013) |
Inform consumers, incentivise reformulation | Exact colour, clearance around label, separation from other claims | Sodium, saturated fat, total fat, total sugar, fibre, wholegrain, artificial sweeteners | Thresholds, multiple category | Per 100 g/100 mL, per serve, % energy | Construct validity; peer-reviewed | Packaged foods and drinks, unpackaged fresh foods, restaurant meals | Non-basic foods, for example, snacks | Education campaign, integration into school curricula |
|
High Salt Warning Label
Finland (1993) |
Inform consumers, incentivise reformulation | Main panel not specified | Sodium | Thresholds, multiple category | Per 100 g | Limited categories of high salt foods, (including unpackaged) | N/A | Education campaign, part of major heart health programme with public institution partners/media | |
|
Protective Food Logo
Slovenia (1993) |
Inform consumers | Sodium, saturated fat, total fat, added sugar, fibre, energy | Thresholds, multiple category | Per 100 g/100 mL | Packaged foods and drinks, restaurant meals | ||||
|
Healthier Choice Symbol
Singapore (1998) |
Inform consumers | Size, clearance around label, separation from other claims, written government endorsement | Sodium, saturated fat, total fat, total sugar, fibre, calcium, artificial sweeteners | Thresholds, multiple category | Per 100 g/100 mL, per serve | Predictive validity | Packaged foods and drinks, unpackaged fresh foods, restaurant meals | Education campaign, can be used in advertising with approval | |
|
Heart Symbol
Finland (2000) |
Inform consumers, incentivise reformulation |
Sodium, saturated fat, unsaturated fat, added sugar, fibre, energy | Thresholds, multiple category | Per 100 g/100 mL | Construct validity | Packaged foods and drinks, restaurant meals | Education campaign | ||
|
Heart Tick
Nigeria (2005) |
Inform consumers | Sodium, total fat, trans fat, added sugar, fibre | Thresholds, multiple category | Packaged foods and drinks | |||||
|
Traffic Light System
UK (2006) |
Inform consumers, Incentivise reformulation |
Legibility, colour, contrast and delineation | Sodium, saturated fat, total fat, total sugar, energy | Thresholds, foods and drinks | Per 100 g/100 mL, per serve | Packaged foods and drinks | Foods exempt from mandatory nutrition labelling in EU law | ||
|
Choices Programme
Poland (2008), Czech Republic (2011) |
Inform consumers | Colour (different colours used for basic and non-basic foods) | Sodium, saturated fat, trans fat, added sugar, fibre, energy, artificial sweeteners | Thresholds, multiple category | Per 100 g/100 mL, per serve, % energy | Multiple construct/ predictive validity peer-review | Packaged foods and drinks, unpackaged fresh foods | ||
|
Guideline Daily Amount
Thailand (2011) |
Written direction: ‘should consume in small amounts and exercise for better health’ | Sodium, total fat, total sugar, energy | N/A | Per serve | N/A | Limited categories | N/A | Education campaign (consumer facing and food manufacturers) | |
|
Traffic Light Labels
South Korea (2011) |
Inform consumers | Sodium, saturated fat, total fat, total sugar, caffeine | Per serve | Limited categories of children’s snack | N/A | Education campaign, part of regulated school zones restricting sales and advertising of unhealthy products | |||
|
Reference Intakes
European Union (2011) |
Inform consumers | Size, legibility | Sodium, saturated fat, total fat, total sugar, energy | N/A | Per serve | N/A | Packaged foods and drinks | ||
|
Guideline Daily Amount
Malaysia (2012) |
Inform consumers | Size, monochrome colours | Energy | N/A | Per serve | N/A | Packaged foods and drinks | Special purpose foods, infant formula | Education campaign |
|
Guideline Daily Amount
Philippines (2013) |
Inform consumers, incentivise reformulation |
Size, contrast, placement (lower right hand portion) | Energy | N/A | Per serve | N/A | Packaged foods and drinks | Education campaign (industry led) | |
|
Traffic Light Labels
Ecuador (2014) |
Inform consumers | Size, not compulsory principal panel | Sodium, saturated fat, total fat | Per 100 g/100 mL | Packaged foods and drinks | ‘Traditional’ (unprocessed) foods, for example, fruit, meat, milk; foods without nutritive value | Education campaign | ||
|
Guideline Daily Amount
Mexico (2014) |
Inform consumers | Size | Sodium, saturated fat, total fat, total sugar, energy | N/A | Per serve | N/A | Packaged foods and drinks | Herbs and spices, vinegar, foods with less than 1% daily amounts or bulk sale products | |
|
Nutrio-Sello
Mexico (2014) |
Inform consumers, incentivise reformulation |
Size, colour | Sodium, saturated fat, total sugar, energy | Per Serve | Packaged foods and drinks | ||||
|
Health Star Rating System
Australia (2014), New Zealand (2014) |
Inform consumers, incentivise reformulation | Five design variants permitted, colour not specified provided sufficient contrast | Sodium, saturated fat, total sugar, protein, fibre, fruit vegetable nut legume, energy | Overall algorithm, operating in six broad categories | Per 100 g/100 mL | Construct validity; peer-reviewed | Packaged foods and drinks | Foods without nutrient declaration, infant formula, formulated supplementary sports foods, foods for special dietary uses, alcohol | Education campaign, aims to align with dietary guidelines, nutrition claims legislation, nutrient reference values, some link to public procurement |
|
Stop Sign Labels
Chile (2015) |
Inform consumers, incentivise reformulation |
Font, size, removal of proximal marketing tools (eg, cartoons), small packs can display on side, written government endorsement | sodium, saturated fat, total sugar, energy | Thresholds, foods and drinks | Per 100 g/100 mL | Packaged foods and drinks | Products with no added sugars, sodium or saturated fat, bulk foods, infant formula, baby food unless has added sugar, foods for special uses, supplements, some sports foods, table-top sugar substitutes | Education campaign Implementing law integrates with advertising restrictions, school food procurement | |
|
Traffic Light Labels
Iran (2015) |
Inform consumers, incentivise reformulation |
Sodium, total fat, trans fat, total sugar, energy | Thresholds, foods and drinks | Per 100 g/100 mL | Packaged foods and drinks | ||||
|
Weqaya Logo
United Arab Emirates (2015) |
Inform consumers, incentivise reformulation |
Sodium, saturated fat, total fat, trans fat, total sugar, fibre, wholegrain, artificial sweeteners | Thresholds, multiple category | Per 100 g/100 mL, Per Serve | Packaged foods and drinks, restaurant meals | Deep fried foods | Link to hospital retail procurement, food outlet menu labelling, part of broader Weqaya NCD reduction programme | ||
|
Healthy Living Guarantee
Croatia (2015) |
Inform consumers, incentivise reformulation | Written government endorsement | Sodium, saturated fat, total fat, fibre, wholegrain, added aromas and preservatives | Thresholds, multiple category | Per 100 g/100 mL | Packaged foods and drinks, unpackaged fresh foods | One of five components of Healthy Living National Programme | ||
|
Traffic Light Labels
Sri Lanka (2016 drinks, 2019 food) |
Inform consumers, incentivise reformulation | Size, colour, multiple languages, main panel | Sodium, total fat, total sugar | Thresholds, foods and drinks | Per 100 g/100 mL | Packaged foods and drinks | Primary agricultural products, spices, foods for special dietary uses, bulk packs, infant formula | Education campaign, part of National Multisectoral Action Plan on NCDs | |
|
Healthier Choices Logo
Thailand (2016) |
Inform consumers | Size, separation from other statements, contrast | Sodium, saturated fat, total fat, total sugar, fibre, calcium, protein | Hybrid: overall algorithm broad categories and thresholds, multiple categories | Per 100 g/100 mL | Limited categories of food and drink | Infant formula, special purpose foods, supplements, alcohol, medical foods, water | Education campaign | |
|
Red Warning Labels
Israel (2017) |
Principal panel (small pack exception), ‘conspicuous manner’, size, colour | Sodium, saturated fat, total sugar | Thresholds, foods and drinks | Per 100 g/100 mL | Packaged foods and drinks | Water, tea, coffee, yeast, infant formula, alcohol, special purpose foods | Part of national development strategy | ||
|
Green Signpost
Israel (2017) |
Inform consumers | Sodium, saturated fat, total sugar, fibre | Thresholds, multiple categories | Not yet outlined | Products with artificial sweeteners; products that carry red warning labels | Part of national development strategy | |||
|
Stop Sign Labels
Peru (2017) |
Inform consumers, incentivise reformulation |
Size, front panel, placement (top right corner), written government endorsement and directions ‘avoid excessive consumption’ | Sodium, saturated fat, trans fat, total sugar | Thresholds, foods and drinks | Per 100 g/100 mL | Packaged foods and drinks | Stop signs must also appear in advertisements, linked to school food procurement | ||
|
Nutri-Score
France (2017), Spain (2018), Belgium (2019) |
Inform consumers, incentivise reformulation |
Colour, size, placement (lower left hand corner) | Sodium, saturated fat, total sugar, protein, fibre, fruit vegetable nut legume, energy | Overall algorithm, operating in three broad categories | Per 100 g/100 mL | Multiple construct and predictive, peer-reviewed | Packaged foods and drinks | Infant formula, aromatic herbs, teas, coffees, yeasts, alcohol | Part of National Nutrition Health Programme, use will be mandatory in advertisements |
|
Healthier Choice Logo
Malaysia (2017) |
Inform consumers, incentivise reformulation |
Max and min size, colour, separation from brand name, paired with energy icon | Sodium, saturated fat, trans fat, added sugar, fibre, wholegrain, calcium | Thresholds, multiple category | Per 100 g/100 mL, per serve, % wholegrain | Packaged foods and drinks | Education campaign, integrated into school curriculum, part of National Action Plan for Nutrition | ||
|
Healthier Choice Symbol
Brunei (2017) |
Inform consumers | Sodium, saturated fat, total fat, trans fat, total sugar, added sugar, fibre, wholegrain, energy, cholesterol | Thresholds, multiple category | Per 100 g/100 mL, % energy, % composition | Packaged foods and drinks | Education campaign | |||
|
Stop Sign Labels
Uruguay (2018) |
Inform consumers, incentivise reformulation |
Size, main panel, cannot be covered by other elements, placement ‘preferably at top’ | Sodium, saturated fat, total fat, total sugar (NB fat not counted if from nuts and seeds, sugar does not count if lactose or naturally present in fruit and vegetables) | Thresholds, foods and drinks | Per 100 g/100 mLrecent rapid uptake of label | Packaged foods and drinks | Foods with no added sugars, fats and sodium; foods for medicinal purposes, supplements, infant formula and baby food, sweeteners | Linked to school curricula, linked to public procurement | |
|
Good Food Logo
Zambia (2018) |
Anticipated to cover nutrients relevant to NCDs and micronutrient deficiencies | Education campaign |
Notes: Blank cells indicate no relevant information was identified; N/A indicates metric not applicable for example, non-interpretive labels do not employ nutrient criteria.
FOP, front-of-pack; NCD, non-communicable disease.
Specifications for displaying FOP nutrition labelling on pack
Regulation can specify requirements to enhance visibility and salience of the FOP nutrition label on pack. Examination of current practice identified at least some display specifications in 22 FOP nutrition labelling regulations. Specifications included size, contrast, colour and/or font (table 2). Where display was not explicitly required on the front or principal panel, it sometimes occurred elsewhere (eg, Ecuador),16 weakening visibility.
Only four regulations directed uniform placement, for example, top right (table 2). More common were minimum space requirements around the FOP nutrition label; the Nordic Keyhole also specified separation from competing claims (eg high in protein).18 Chile’s law required removal of marketing tools on pack (eg cartoon characters), for foods carrying a stop sign label.19 Six regulations incorporated written government endorsement (eg, Ministry of Health); two provided directions for use (eg avoid excessive consumption)(table 2). These progressive examples, along with lessons from refined display specifications in settings such as tobacco control,43 suggest potential for future regulatory innovation.
Included nutrients and/or food components
As part of a comprehensive approach to NCDs, nutrients and/or food components included in regulation should relate to evidence of diet-related risk.1 Current WHO guidance also suggests they be focused on nutrients already required to be declared on back-of-pack.11
Information on current practice was identified in the regulations of 30 labels (table 2). Twenty-eight included one or more nutrients associated with increased NCD risk: sodium (28); saturated fat (22), total sugar (21), trans fat (8), total fat (8) and added sugar (6).
Fewer regulations incorporated food components associated with decreased NCD risk: fibre (15), whole grain (5) and ‘fruit, vegetable, nut and legume’ content (2). This may reflect caution in highlighting packaged sources of these components over whole food counterparts.11
Other components included in current regulations were energy (14), artificial sweeteners (4), calcium (3), protein (3), cholesterol (1), unsaturated fat (1), caffeine (1) and added preservatives (1). Zambia indicated it would address NCDs and micronutrient deficiency,44 though nutrient criteria were not identified. To mitigate the risk of potential challenge, policymakers should ensure all inclusions are informed by health evidence.
Nutrient profiling criteria or scoring system
Nutrient profiling is the science of classifying or ranking foods according to their nutritional composition for reasons related to preventing disease and promoting health.17 For FOP nutrition labelling to successfully guide consumers towards healthier choices, regulation must specify a valid set of nutrient profiling criteria, that is, ones that appropriately evaluate nutritional quality.11 16 17
Nutrient profiling criteria were identified for 22/26 interpretive labels. Positive signposts used nutrient thresholds that varied by category; some had >60 categories (table 2). Nutrient-specific interpretive labels also used nutrient thresholds but across broader categories of ‘foods’ and ‘drinks’ to determine eligibility for a label (eg, stop sign) or its colour coding (eg, red, orange, green). The two overall spectrum ratings used algorithms to score overall nutritional quality, for example, HSR combined points from up to seven food components to generate an overall rating of 0.5–5.0 in one of six categories.41
To produce meaningful scores, regulation must specify a reference amount of the product to be used. Most regulations (20/31) applied criteria per 100 g/100 mL (table 2), consistent with WHO recommendation that this is most useful to facilitate standardised comparisons between products. ‘Per serving’ was used always or sometimes as a basis for nutrient profiling in 12 regulations, with potential to cause consumer confusion.11
Validation of nutrient profiling criteria is important to assess whether they correctly classify product healthfulness. However, some form of validity testing was identified for only six labels (table 2). Five criteria had been assessed against other measures of healthiness (eg, dietary guidelines); only three had been subject to stronger predictive testing of links between scoring criteria and health outcomes.45 Our results support existing calls to improve the science and selection of nutrient profiling criteria to ensure FOP nutrition labelling promotes genuinely healthier choices.11 45
Scope of products included in regulation
WHO guidance suggests regulations apply across most packaged foods to facilitate product comparisons and improve familiarity with the FOP nutrition label.11 Most current regulations (26/31) aligned with this guidance, applying broadly to packaged foods and drinks that carried a nutrient declaration. Some regulations applied beyond packaged food: six applied to unpackaged foods (eg, fruit, vegetables, fresh meat and seafood); five applied to meals served in restaurants or other catering services (table 2).
WHO guidance also suggests FOP nutrition labelling may not be appropriate for some foods.11 While exclusions were often not clearly specified in current regulation, no FOP nutrition labelling was allowed on infant formula; other frequent exclusions were foods for special dietary or medical uses, supplements and alcoholic beverages (online supplementary appendix 1). Foods not displaying nutrient declarations (eg, herbs, tea, small packs) were commonly exempted (but not necessarily excluded) (table 2).
Some exclusions varied by label format: for example, Chile and Uruguay excluded products with no added sugars, sodium or saturated fat, meaning intrinsic sources of these nutrients did not carry stop-sign warnings.19 39 Conversely, the positive Keyhole was not permitted on snacks.18
Findings suggest some flexibility for policymakers to determine national scope, provided decisions are justified by health evidence and apply equally to local and imported products.21
Policy coherence
Recognising no single action will be sufficient to address the global burden of unhealthy diets; policymakers should support and frame FOP nutrition labelling as part of a comprehensive suite of measures to promote healthier diets.16 21 To maximise public health impact, the substance of regulation should be aligned with existing measures, for example, food-based dietary guidelines (table 1).11
We identified 16 FOP nutrition labels that were supported by education campaigns to promote label use and/or broader nutrition messages, but only six that explicitly identified FOP nutrition labelling as part of broader nutrition, NCD or development plans (table 2). There is potential for innovative regulation to extend utility of FOP nutrition labelling beyond the point of food purchase: six regulations integrated labelling with other interventions, including Chile who stopped foods carrying labels from being sold in schools or marketed to children,19 and France’s agreement to make Nutri-Score mandatory in all food advertisements from 2021.42
Regulatory governance
In addition to form and substance, good governance of the processes by which regulation is developed, implemented, evaluated and enforced is critical to consumer confidence in FOP nutrition labelling (table 1).27 A summary of data identified on current practice is provided in table 3. The limited nature of available data suggests need for greater attention from policymakers and/or increased transparency in activities undertaken.
Table 3.
Governance of FOP nutrition labelling regulation
| Label, Jurisdiction (Year endorsed) | Initiated by | Stakeholder engagement | Nutrient criteria developed by | Administration | Monitoring | Government-supported evaluation/Review | Review of nutrient criteria | Enforcement strategies |
|
Nordic Keyhole
Sweden (1989); Denmark And Norway (2009), Lithuania (2013) |
Government, Swedish Food Standards Agency with reference to Nordic Nutrition Recommendations | Government (varies by jurisdiction) | Consumer use, Uptake (industry self-report), compliance, reformulation | Government led evaluation, timeline and framework varies by jurisdiction | Yes, criteria reviewed five times since 1989 | Varies by jurisdiction, includes publicity (Denmark), legal remedies under trademark and food law (Sweden) | ||
|
High Salt Warning Label
Finland (1993) |
Government, multisectoral engagement | Government (Ministry of Social Affairs and Health) | Reported, detail not available | Broader cardiovascular initiative evaluated, reformulation reported | Thresholds lowered in 2009, extended to unpackaged equivalents 2016 | Audits | ||
|
Protective Food Logo
Slovenia (1993) |
Civil society (Slovenian Heart Foundation) | Independent experts from Society of Cardiovascular Health | Civil society (Slovenian Heart Foundation) | |||||
|
Healthier Choice Symbol
Singapore (1998) |
Government | Government, developed with industry to promote feasibility | Government (Health Promotion Board) | Consumer use, register of products using | Government led evaluation resulted in revisions in 2015 | Yes, criteria updated on rolling basis | Licensing, audits, publicity, legal remedies under trademark, removal of approval to use | |
|
Heart Symbol
Finland (2000) |
Civil society (Finnish Heart Association and Finnish Diabetic Association) | Food industry and consumer groups consulted, endorsed by Ministry of Social Affairs and Health | Independent experts, input from government Food Safety Authority and reference to national dietary guidelines | Civil society | Consumer use, uptake | Regular update by independent expert group | Audits | |
|
Heart Tick
Nigeria (2005) |
Civil society (Nigerian Heart Foundation) | Civil society, approved by National Agency for Food and Drug Administration | Civil society, later joined Choices Programme | Now subject to Choices Programme updates | Licensing by Nigerian Heart Foundation | |||
|
Traffic Light System
UK (2006) |
Government | Public consultation | Government | Government (Food Standards Agency) | Uptake (industry self-reported) | |||
|
Choices Programme
Poland (2008), Czech Republic (2011) |
Industry | Multistakeholder collaboration | Independent expert group separate of industry collaborators for this process | Multistakeholder committee | Varies by jurisdiction, Consumer use, reformulation | Reviewed every 3–4 years by independent expert committee | Licensing | |
|
Guideline Daily Amount
Thailand (2011) |
Government | Legislative process | N/A | Government (Food and Drug Administration) | Consumer use, uptake | Government, positive results published | N/A | |
|
Traffic Light Labels
South Korea (2011) |
Government | Legislative process | Government, Minister delegated authority to set thresholds | Government (Ministry for Food and Drug Safety) | Legal remedies under implementing act, including fines | |||
|
Reference Intakes
European Union (2011) |
Government | Legislative process, public consultation, | N/A | N/A | ||||
|
Guideline Daily Amount
Malaysia (2012) |
Industry | Was initiative of International Food and Beverage Alliance, endorsed by government | N/A | Government (Ministry of Health) | ||||
|
Guideline Daily Amount
Philippines (2013) |
Government | N/A | Government (Food and Drug Administration) | Licensing | ||||
|
Traffic Light Labels
Ecuador (2014) |
Government | Legislative process | Government, adapted from UK traffic lights but adapted to Ecuadorian intake | Government (Ministry of Public Health) | Consumer use | |||
|
Guideline Daily Amount
Mexico (2014) |
Government | Legislative process, lack of transparency in consultation noted as concern by civil society | N/A | Government (Mexican Federal Commission of Sanitary Risk Prevention) | N/A | |||
|
Nutrio-Sello
Mexico (2014) |
Government | Developed by International Food and Beverage Alliance, not publicly available | Government (Mexican Federal Commission of Sanitary Risk Prevention) | Licensing | ||||
|
Health Star Rating System
Australia (2014), New Zealand (2014) |
Government | Multistakeholder collaboration with industry, public health, consumer involvement; public consultation | Multistakeholder committee, including industry, public health, consumer groups | Multistakeholder committee, has authority to manage complaints | Framework available: includes consumer use, uptake, reformulation, compliance. Two year monitoring report and data fed into 5 year review. | Formal 5 year review led by government-appointed independent consultant, public consultation | Yes, focus of formal 5 year review | Audits, written requests for amendments, complaint handling mechanism, listing on HSR website |
|
Stop Sign Labels
Chile (2015) |
Government, multisectoral engagement | Legislative process, public consultation, strategic media engagement | Government, independent experts, tested against data from US Department of Agriculture Database | Government (Ministry of Health) | Consumer use, reformulation, uptake, compliance | Formal evaluation in cooperation with academics, includes changes in product purchases and dietary intakes | Progressively lowering thresholds built into legislation | Audits prioritising school foods |
|
Traffic Light Labels
Iran (2015) |
Government | Legislative process, consultation with technical committee including food industry and nutrition representatives | Government (Food and Drug Administration) | Uptake | Written requests for amendments, withhold marketing permission | |||
|
Weqaya Logo
United Arab Emirates (2015) |
Government | Government led public consultation | Government, thresholds derived from Nordic Keyhole | Government (Health Authority, Food Control Authority, Quality and Conformity Council) | Licensing, audits | |||
|
Healthy Living Guarantee
Croatia (2015) |
Government | Government | Government (Croatian Institute of Public Health) | Government evaluation as part of broader Healthy Living strategy | Licensing | |||
|
Traffic Light Labels
Sri Lanka (2016 drinks, 2019 food) |
Government | Legislative process, public consultation | Independent experts, drawing on other country experiences | Government (Ministry of Health) | Reformulation, sales | General sanctions available under Food Act | ||
|
Healthier Choices Logo
Thailand (2016) |
Government | Multistakeholder, developed with agreement of industry, academics and public sector | Adapted from Choices Programme criteria developed by independent experts | Government (owned by Thai Food and Drug Administration, use managed by Nutrition Promotion Foundation) | Uptake | Licensing, unauthorised use can face jail sentences and fines | ||
|
Red Warning Labels
Israel (2017) |
Government | Legislative process, multistakeholder committee with open meetings and publicly available recordings, public consultation | Government, independent experts | Government | Planned, no detail available | Planned, stated to include sales data | Progressively lowering thresholds built into legislation | |
|
Green Signpost
Israel (2017) |
Government | Multistakeholder committee with open meetings and publicly available recordings, public consultation | Government, independent experts, adapted from Nordic Keyhole | Government | ||||
|
Stop Sign Labels
Peru (2017) |
Government | Legislative process | Government | Progressively lowering thresholds built into legislation | Fines | |||
|
Nutri-Score
France (2017), Spain (2018), Belgium (2019) |
Government | Formal process directed by government under Health Law, public consultation, | Government, French High Council of Public Health commissioned to affirm | Government (Santé Publique France) | Consumer Use, Uptake (industry must register use on government website), Reformulation | Government-supported, conducted after 3 years, conducted by Observatory of Nutritional Food Quality. | Planned to review as part of evaluation framework | Written requests for action, suspending or revoking right to use logo, legal action to protect trademark misuse |
|
Healthier Choice Logo
Malaysia (2017) |
Government | Multistakeholder collaboration, input from Choices Programme | Adapted from Choices Programme developed by independent expert group | Government (Ministry of Health) | Uptake (list of products available on government website) | Criteria reviewed ‘from time to time’ | Licensing, audits, withdrawal of approval, other legal remedies | |
|
Healthier Choice Symbol
Brunei (2017) |
Government | Government, adapted from Singapore’s Healthier Choice Logo and WHO criteria | Government (Ministry of Health) | Uptake, surveillance of labels in supermarkets | Licensing, written requests for amendment | |||
|
Stop Sign Labels
Uruguay (2018) |
Government, multisectoral engagement | Legislative process, international expert input, public consultation | Government, modifying the Pan American Health Organisation Nutrient Profile Model | Government (Ministry of Public Health) | Stated but detail not available | Legal remedies under Law of Public Health | ||
|
Good Food Logo
Zambia (2018) |
Multistakeholder | Multistakeholder with experts from government, nutritionists, academics, scientists with input from private sector | Government |
Notes: Blank cells indicate no relevant information was identified; N/A indicates metric not applicable for example, non-interpretive labels do not employ nutrient criteria.
FOP, front-of-pack.
Drafting regulatory rules and scheme design
WHO recommends that governments initiate FOP nutrition labelling and retain specific responsibility for setting regulatory objectives, scope and nutrient scoring criteria (table 1).11 Government-led development is perceived as more credible by consumers.17
All mandatory and most voluntary current regulations were initiated by governments, typically through health ministries or statutory food authorities. Five governments noted early engagement across sectors (eg, trade, agriculture, education)(table 3), which is recommended to promote whole-of-government coordination.16 21 Three voluntary regulations were initiated by civil society, two by industry and one was multistakeholder.
During policy development, government-led stakeholder engagement can facilitate due process and assist development of feasible and acceptable regulation.11 At the same time, there is a need to ensure that public health policymaking is conducted with safeguards to appropriately manage conflicts of interest.46
We identified at least some detail on stakeholder engagement for 19 FOP nutrition labels. Mandatory regulations followed standard procedures for legislative development. In jurisdictions with functioning rule of law, these typically embed principles of good governance such as due process and public consultation. While useful in promoting accountability, these procedures are not necessarily failsafe: in Mexico, lack of transparency during public consultation raised concern that industries were more heavily consulted than academics or civil society organisations.47 Mexico subsequently adopted a non-interpretive label (figure 1). Voluntary regulations were also frequently preceded by public consultation, but in limited cases also elevated industry to collaborator or member of committees developing the substance of regulation (table 3). Beyond a right to be heard through consultation, these collaborative arrangements create additional need for safeguards to ensure health objectives are not undermined by commercial interests.
The particular need to maintain the scientific integrity of committees developing nutrient profiling criteria is recognised in WHO recommendations that this process run parallel but independent and siloed from broader stakeholder engagement.11 Where detail was identified for current regulations, only 4/22 governments engaged industry in nutrient profiling development (table 3). They include Australia and New Zealand’s HSR system, where a government-commissioned independent review recently recommended industry be distanced from future nutrient profiling updates.41 The need for nutrient profiling to remain free from commercial interests can be seen elsewhere in the industry-led Choices Programme, which created a separate ‘independent scientific committee’ specifically for this task.48
Unlike the Framework Convention on Tobacco Control’s Article 5.3 in a tobacco control context,49 there is currently no binding international law requiring government to exclude the food industry from health policy development. Available guidance includes a proposed approach for prevention and management of conflicts of interest in national nutrition policymaking discussed by the WHO Executive Board in 2018.46 In Canada, where FOP nutrition labelling regulations are currently being developed, government has introduced new standards for transparency of stakeholder communications for healthy eating initiatives. In addition to publishing responses to formal consultations, all meetings and correspondence with stakeholders including industry are published on the government’s website. Any documents provided during meetings are also accessible to the public upon request.50 Divergence in current practice more broadly highlights the need for further guidance for policymakers on appropriate engagement with the food industry in the governance of FOP nutrition labelling.23
Administration
To promote accountability, labelling regulations should be administered by government or an independent body with requisite authority to monitor and enforce regulation (table 1). Evaluation of current practice suggested governments administered 25/31 current labelling schemes. Three were administered by civil society organisations, which were independent but may lack authority or resource to enforce regulation (table 3). HSR and Choices used multistakeholder committees, again creating need for conflict of interest safeguards. In the context of administrative committees, appropriate measures could include conducting meetings publicly with publicly available minutes, and stakeholders recusing themselves from decisions where a conflict exists (eg, where an industry representative is required to assess the compliance of one of their members with regulation).
Monitoring and evaluation
Structured, regular review ensures regulation is meeting its objectives and supports continuous improvement. Best-practice requires policymakers to set a framework during development that includes baseline data, performance indicators and appropriate timeframes (table 1).
The absence of systematic monitoring and evaluation with publicly available outcomes was a key limitation of current practice in our evaluation. Government-supported monitoring was reported for just over half of regulations; only one-third of regulations had been subject to some form of evaluation. There was suboptimal monitoring of outcomes necessary to demonstrate achievement of regulatory objectives: 10 labels monitored consumer understanding and use, 7 monitored reformulation. Only 11 monitored industry uptake, necessary to judge impact and compliance with regulation (table 3).
Some newer regulations showed elements of stronger practice. HSR had a government-appointed independent reviewer with publicly available Terms of Reference.41 Nutri-Score was implemented with publicly funded evaluation using a variety of data sources.42 Governments of Chile and Israel proposed evaluation with independent academics;36 Israel also planned to obtain sales data (online supplementary appendix 1).
FOP nutrition label performance was also subject of ad hoc independent research. For example, the HSR website noted 45 peer-reviewed papers between 2014 and 2019.41 While contributing valuable insight, this work does not obviate the need for systematic government-supported evaluation.
Specific evaluation of nutrient profiling criteria is necessary to ensure regulation evolves with nutrition science.11 Only one-third of current regulations had relevant mechanisms to trigger this process. Legislation in Chile, Israel and Peru incorporated progressively lowering nutrient thresholds to incentivise continuing industry reformulation; Finland had also updated salt warning legislation. Five positive signposts reviewed criteria at prespecified intervals; Keyhole criteria had been updated five times.18 HSR’s algorithm was a focus of its 5-year review.41
Enforcement
Effective enforcement of regulation promotes compliance with regulation (table 1). Sanctions or rewards were identified in only around half of FOP nutrition label regulations but provided insight into the range of options available (table 3). Ten labels licensed products prior to market sometimes with associated cost (online supplementary appendix 1), though utility of this feature may be practically limited to positive signposts. Other incentives for voluntary uptake included listing participating companies on government websites.
Once on shelves, at least seven regulations audited products for compliance. Sanctions for non-compliance varied widely. Positive signposts could withdraw approval for use. Four labels used written requests to manufacturers for amendment (table 3). Non-compliance with Ecuador’s label could result in cancellation of advertising permits.32 In South Korea, Sri Lanka and Uruguay, general sanctions under relevant Food Acts applied, for example, fines and potentially jail sentences (online supplementary appendix 1). Even in voluntary systems, legal action was possible, for example, to protect misuse of trademarked logos. HSR was the only system identified that had its own complaints handling mechanism.41
For all systems, lack of identified information on whether sanctions are being used suggests potential to strengthen enforcement action and/or improve transparency of activity.
Conclusion
Development and implementation of FOP nutrition labelling regulation requires much more than the selection of ‘stars’, ‘traffic lights’ or ‘stop signs.’ While growing government endorsement of more effective label formats and use of mandatory legal frameworks holds public health promise, policymakers must still ensure the substance of regulation is strategically drafted to support policy objectives. They must also apply principles of good governance in regulatory processes to protect health policymaking from undue commercial interference, support continuous improvement and retain the trust of consumers as end users.
This paper applies a comprehensive public health law framework to demonstrate how factors often considered outside the realm of public health such as regulatory drafting and good governance can support or undermine health outcomes. This study benefited from its global remit but was limited by reliance on publicly available information, particularly on governance processes. Whether developing new FOP nutrition labels or reforming existing ones, these findings provide support for policymakers to design and implement best-practice, evidence-informed regulation.
Footnotes
Handling editor: Seye Abimbola
Twitter: @alikjones, @@ClionaNiMhurchu
Correction notice: This article has been corrected since it published Online.
Contributors: AJ, BN and AMT designed the study. AJ conducted the research and wrote the first and subsequent drafts. BN, BR, CNM and AMT provided input on the first and subsequent drafts.
Funding: AJ is funded by an Australian Government Research Training Stipend.
Disclaimer: The HSRAG did not have any role in the study design, data collection and analysis, decision to publish or preparation of the manuscript.
Competing interests: CNM is a member of the New Zealand Health Star Rating Advisory Group (HSRAG).
Patient consent for publication: Not required.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement: All data relevant to the study are included in the article or uploaded as supplementary information.
References
- 1. Afshin A, Sur PJ, Fay KA, et al. Health effects of dietary risks in 195 countries, 1990-2017: a systematic analysis for the global burden of disease study 2017. Lancet 2019;393:1958–72. 10.1016/S0140-6736(19)30041-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Stuckler D, McKee M, Ebrahim S, et al. Manufacturing epidemics: the role of global producers in increased consumption of unhealthy commodities including processed foods, alcohol, and tobacco. PLoS Med 2012;9:e1001235 10.1371/journal.pmed.1001235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hall KD, Ayuketah A, Brychta R, et al. Ultra-processed diets cause excess calorie intake and weight gain: an inpatient randomized controlled trial of AD libitum food intake. Cell Metab 2019;30:67–77. 10.1016/j.cmet.2019.05.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. World Health Organization Global action plan for the prevention and control of noncommunicable diseases 2013-2020 2013.
- 5. World Health Organization 'Best buys' and other recommended interventions for the prevention and control of noncommunicable diseases, updated (2017) appendix III of the global action plan for the prevention and control of non-communicable diseases 2013-2020. Geneva, Switzerland, 2017. [Google Scholar]
- 6. World Health Organization Final report of the Commission on ending childhood obesity. Geneva, 2016. [Google Scholar]
- 7. World Health Organization Implementation plan of the who Commission on ending childhood obesity. Geneva, Switzerland: WHO, 2017. [Google Scholar]
- 8. United Nations Food and Agriculture Organization and World Health Organization Second International Conference on nutrition outcome document: framework for action ICN2 2014/3. in: World Health organization and food and agriculture organization. Rome, 2014. [Google Scholar]
- 9. World Health Organization Technical meeting on nutrition labelling for promoting healthy diets Lisbon, Portugal, 2015. Available: https://www.who.int/nutrition/events/2015_meeting_nutrition_labelling_diet_9to11dec/en/ [Accessed 8 January 2019].
- 10. World Health Organization Joint FAO/WHO workshop on Front-of-Pack nutrition labelling Prince. Canada: Edward Island, 2013. https://www.who.int/nutrition/events/2013_FAO_WHO_workshop_frontofpack_nutritionlabelling/en/ [Google Scholar]
- 11. World Health Organization Guiding principles and framework manual for front-of-pack labelling for promoting healthy diet. Geneva, Switzerland, 2019. [Google Scholar]
- 12. Shangguan S, Afshin A, Shulkin M, et al. A meta-analysis of food labeling effects on consumer diet behaviors and industry practices. Am J Prev Med 2019;56:300–14. 10.1016/j.amepre.2018.09.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Neal B, Crino M, Dunford E, et al. Effects of different types of Front-of-Pack labelling information on the healthiness of food purchases—A randomised controlled trial. Nutrients 2017;9:1284 10.3390/nu9121284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Cecchini M, Warin L. Impact of food labelling systems on food choices and eating behaviours: a systematic review and meta-analysis of randomized studies. Obes Rev 2016;17:201–10. 10.1111/obr.12364 [DOI] [PubMed] [Google Scholar]
- 15. Vyth EL, Steenhuis IH, Roodenburg AJ, et al. Front-of-pack nutrition label stimulates healthier product development: a quantitative analysis. Int J Behav Nutr Phys Act 2010;7 10.1186/1479-5868-7-65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. World Cancer Research Fund International Building momentum: lessons on implementing a robust front-of-pack food label. London, UK, 2019. [Google Scholar]
- 17. Kelly B, Jewell J. What is the evidence on the policy specifications, development processes and effectiveness of existing front-of-pack food labelling policies in the who European region?. health evidence network (hen) synthesis report 61. Copenhagen: WHO Regional Office for Europe, 2018. [PubMed] [Google Scholar]
- 18. National Food Agency Sweden The keyhole, 2019. Available: https://www.livsmedelsverket.se/en/food-and-content/labelling/nyckelhalet [Accessed 6 June 2019].
- 19. Ministerio de Salud Chile Ley 20.606 Sobre La Composición de Los Alimentos Y SU Publicidad 2012.
- 20. Aguayo, Ecclefield, Martínez Pepsico subsidiary sues Treasury for food labeling law Marca sur 2017;14. [Google Scholar]
- 21. Thow AM, Jones A, Hawkes C, et al. Nutrition labelling is a trade policy issue: lessons from an analysis of specific trade concerns at the world trade organization. Health Promot Int 2017;17:daw109–daw09. 10.1093/heapro/daw109 [DOI] [PubMed] [Google Scholar]
- 22. Ahmed A, Richtel M, Jacobs A. In NAFTA talks, us tries to limit junk food warning labels. New York Times 2018;20. [Google Scholar]
- 23. Codex Alimentarius Commission Agenda Item 6 for Codex Committee on Food Labelling 13-17 May 2019 - Proposed Draft Guidelines on Front-of-Pack Nutrition Labelling 2019.
- 24. Thow AM, Jones A, Schneider CH, et al. Global governance of front-of-pack nutrition labelling: a qualitative analysis. Nutrients 2019;11:268 10.3390/nu11020268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Egnell M, Talati Z, Hercberg S, et al. Objective understanding of Front-of-Package nutrition labels: an international comparative experimental study across 12 countries. Nutrients 2018;10:1542 10.3390/nu10101542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. World Health Organization Regional Office for Europe (WHO EURO) Key considerations for the use of law to prevent noncommunicable diseases in the who European region: report of an intensive legal training and capacity-building workshop on law and noncommunicable diseases, 2016. [Google Scholar]
- 27. Reeve B, Magnusson R. Regulation of food advertising to children in six jurisdictions: a framework for analyzing and improving the performance of regulatory instruments. Ariz J Int't Comp Law 2018;35. [Google Scholar]
- 28. World Health Organization Global database on the implementation of nutrition action (GINA), 2019. Available: https://extranet.who.int/nutrition/gina/en [Accessed 10 April 2019].
- 29. World Cancer Research Fund International WCRF International Food Policy Framework for Healthy Diets: NOURISHING. Available: http://www.wcrf.org/policy_public_affairs/nourishing_framework/index.php
- 30. World Health Organization Global nutrition policy review 2016-2017. Geneva, 2018. [Google Scholar]
- 31. European Commission Regulation (EU) NO 1169/2011 of the European Parliament and of the Council on the provision of food information to consumers. OJ 2011;54. [Google Scholar]
- 32. Government of Ecuador Reglamento Sanitario Sustitutivo de Etiquetado de Alimentos Procesados para El Consumo Humano. No 2014;318. [Google Scholar]
- 33. Zargaraan A, Dinarvand R, Hosseini H. Nutritional traffic light labeling and taxation on unhealthy food products in Iran: health policies to prevent non-communicable diseases. Iran Red Crescent Med J 2017;19:e57874. [Google Scholar]
- 34. Gazette of the Socialist Republic of Sri Lanka Food (colour coding for sugar, salt and fat) regulations 2019.
- 35. Diario Oficial de la Federación México Modificación de la Norma Oficial mexicana NOM-051-SCFI/SSA1-2010, Especificaciones generales de etiquetado para alimentos Y bebidas no alcohólicas preenvasados, 2014. [Google Scholar]
- 36. Food and Agriculture Organization of the United Nations,, Organization PAH . Approval of a new food act in Chile: process summary. Santiago, 2017. [Google Scholar]
- 37. Congress of Peru Law 30021 – law for the promotion of healthy eating for children and adolescents, 2013. [Google Scholar]
- 38. Government of Israel Regulations for the Protection of Public Health (Food)(Nutritional Labeling, 2017. [Google Scholar]
- 39. Government of Uruguay Executive decree No. 272/2018 on the labelling of packaged foods, 2018. [Google Scholar]
- 40. Cabrera M, Machín L, Arrúa A, et al. Nutrition warnings as front-of-pack labels: influence of design features on healthfulness perception and attentional capture. Public health nutrition 2017;20:3360–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Department of Health The health StAR rating system (website): Australian government. Available: http://healthstarrating.gov.au/internet/healthstarrating/publishing.nsf/content/home [Accessed 27 July 2017].
- 42. Santé Publique France Nutri-Score. Available: https://www.santepubliquefrance.fr/determinants-de-sante/nutrition-et-activite-physique/articles/nutri-score [Accessed 6 June 2019].
- 43. Hammond DJW, ON: Tobacco Labelling Resource Centre 2009 In: Tobacco labelling & packaging toolkit: a guide to FCTC article 11, 2009. [Google Scholar]
- 44. Scaling Up Nutrition (SUN) Network The good food Logo: sun business network, 2018. Available: http://scalingupnutrition.org/wp-content/uploads/2018/06/The-Good-Food-Logo.pdf [Accessed 20 June 2019].
- 45. Townsend MS. Where is the science? what will it take to show that nutrient profiling systems work? Am J Clin Nutr 2010;91:1109S–15. 10.3945/ajcn.2010.28450F [DOI] [PubMed] [Google Scholar]
- 46. World Health Organization Safeguarding against possible conflicts of interest in nutrition programmes draft approach for the prevention and management of conflicts of interest in the policy development and implementation of nutrition programmes at country level. executive board paper EB142/23, 2017. [Google Scholar]
- 47. UK Health Forum Public health and the food and drinks industry: the governance and ethics of interaction. lessons from research, policy and practice. London: UKHF, 2018. [Google Scholar]
- 48. Choices Programme The Choices Programme [Website], 2019. Available: https://www.choicesprogramme.org/ [Accessed 10 July 2019].
- 49. Conference of the Parties to the Framework Convention on Tobacco Control Guidelines for implementation of article 5.3 of the who framework convention on tobacco control, 2008. [Google Scholar]
- 50. Health Canada Transparency of stakeholder communications for healthy eating initiatives, 2016. Available: https://www.canada.ca/en/services/health/campaigns/vision-healthy-canada/healthy-eating/transparency-stakeholder-communications-healthy-eating-initiatives.html [Accessed 10 August 2019].
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjgh-2019-001882supp001.pdf (218.1KB, pdf)


