Significance
Autoimmune inflammatory diseases of the retina represent a major source of vision loss worldwide. Single-cell RNA sequencing has been used as a comprehensive and unbiased approach to investigate cell types and gene expression patterns in the retinas of a mouse model of spontaneous, chronic, and progressive autoimmune uveoretinitis. This work defines the dominant immune effector cell types involved, reveals the development of tertiary lymphoid structures within the diseased retina, characterizes the conversion of monocytes to a macrophage/microglia state or a dendritic cell state, shows that essentially all resident retinal cell types respond to interferon gamma as part of the disease process, and suggests that Muller glia may act as antigen-presenting cells.
Keywords: Aire knockout, mouse model, ocular immunology, single-cell RNAseq, autoimmune uveitis
Abstract
Autoimmune uveoretinitis is a significant cause of visual loss, and mouse models offer unique opportunities to study its disease mechanisms. Aire−/− mice fail to express self-antigens in the thymus, exhibit reduced central tolerance, and develop a spontaneous, chronic, and progressive uveoretinitis. Using single-cell RNA sequencing (scRNA-seq), we characterized wild-type and Aire−/− retinas to define, in a comprehensive and unbiased manner, the cell populations and gene expression patterns associated with disease. Based on scRNA-seq, immunostaining, and in situ hybridization, we infer that 1) the dominant effector response in Aire−/− retinas is Th1-driven, 2) a subset of monocytes convert to either a macrophage/microglia state or a dendritic cell state, 3) the development of tertiary lymphoid structures constitutes part of the Aire−/− retinal phenotype, 4) all major resident retinal cell types respond to interferon gamma (IFNG) by changing their patterns of gene expression, and 5) Muller glia up-regulate specific genes in response to IFN gamma and may act as antigen-presenting cells.
Noninfectious posterior uveitis, an autoimmune and/or autoinflammatory disease of the pars plana of the ciliary body, vitreous, choroid, and retina, affects ∼0.01% of the population (1) and is estimated to be the fourth leading cause of severe vision loss in the industrialized world (2). When the disease encompasses the retina, it is often associated with autoreactivity to retinal antigens such as photoreceptor Arrestin and Interphotoreceptor Retinol Binding Protein (IRBP), and this is referred to as uveoretinitis (3).
Mouse models of autoimmune uveoretinitis offer unique opportunities to study the mechanisms of disease pathogenesis. The most widely used and best-characterized mouse model is referred to as experimental autoimmune uveitis/uveoretinitis (EAU) (4, 5). In the original EAU model, uveoretinitis is initiated by immunizing with a retinal antigen—typically IRBP or a peptide derived from IRBP—that has been emulsified in complete Freund’s adjuvant (CFA). One limitation of this EAU model is the use of mineral oil supplemented with heat-killed mycobacteria, the active ingredient in CFA, which causes nonphysiological stimulation of the innate immune response. Inflammation is further enhanced by injection of pertussis toxin, which is required for efficient disease production in most rodent strains. These treatments may alter the adaptive immune response, which is considered to be the central driver of uveoretinitis. In contrast, adjuvant-free models of uveitis rely on genetic backgrounds that increase the number of auto-reactive T cells and have a more protracted clinical course relative to the original EAU model (6).
Loss of central tolerance to retinal antigens has been shown to mediate the development of spontaneous uveoretinitis (7). Many retinal antigens, including IRBP, are expressed in the thymus under the control of the Autoimmune Regulator (AIRE) transcription factor (8), and the susceptibility of mouse strains to EAU has been shown to correlate inversely with the amount of IRBP expressed in the thymus (9). Aire knockout (Aire−/−) mice develop a spontaneous and chronic-progressive uveoretinitis as part of a multiorgan autoimmune phenotype, and, therefore, Aire−/− mice represent an adjuvant-free model of uveoretinitis secondary to a loss of central tolerance to retinal antigens (10–12). The Aire−/− mouse phenotype resembles autoimmune polyendocrinopathy-candidiasis-ectodermal dystrophy (13, 14), a human condition caused by loss-of-function mutations in the human AIRE gene. At present, the Aire−/− uveoretinitis model is relatively unexplored in terms of the immune cell types involved, the dominant effector response, and the response of resident retinal cells.
Droplet-based single-cell RNA sequencing (scRNA-seq) has emerged as a powerful and unbiased method for characterizing cell types in complex tissues in both normal and disease contexts (15, 16). Thus far, scRNA-seq has not yet been used to characterize uveoretinitis. To define the full range of cell types and cellular responses in a mouse model of uveoretinitis, we have characterized the neural retinas of Aire−/− mice on a C57BL/6J background using scRNA-seq, with additional analyses using immunostaining and in situ hybridization (ISH).
Results
scRNA-seq Reveals a Diverse Immune Cell Infiltrate in Uveoretinitis.
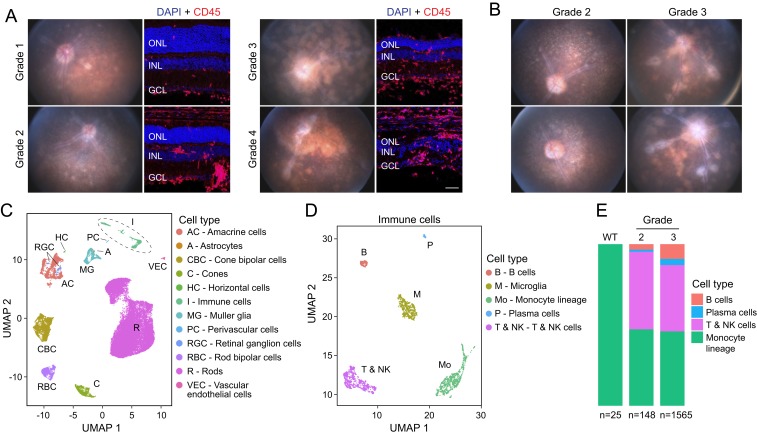
To characterize uveoretinitis in Aire−/− mice on a C57BL/6J background, we performed retinal imaging on mice between 5 and 25 wk of age and graded disease severity according to the published EAU clinical grading scale (SI Appendix, Fig. S1A) (17). We used mice between 10 and 16 wk of age for scRNA-seq and mice between 8 and 25 wk of age for immunohistochemistry. As previously described (6), Aire−/− mice develop a chronic-progressive spontaneous uveoretinitis, as seen in the fundus images and fluorescein angiograms obtained from one Aire−/− mouse at 5 time points over 20 wk (SI Appendix, Fig. S1B). Fig. 1A shows representative images of retina cross-sections with their corresponding fundus images for each clinical grade. In general, there was progressive retinal thinning and an increase in the number of infiltrating CD45-positive leukocytes with higher disease grade, consistent with previous descriptions of Aire−/− eyes on a B10.RIII background (6). In a minority of mice, the uveoretinitis was largely limited to one eye (SI Appendix, Fig. S1C).
Fig. 1.
Characterization and scRNA-seq analysis of Aire−/− retinas. (A) Aire−/− retina fundus photographs (Left) and immunostaining of the corresponding cross-sections (Right) showing leukocytes (CD45; red) and nuclei (DAPI; blue). Representative examples of grade 1 to grade 4 uveoretinitis are shown. In this and other retina cross-sections, the following abbreviations are used: ONL, outer nuclear layer; INL, inner nuclear layer; GCL, ganglion cell layer. (Scale bar, 50 μm.) (B) Fundus photographs of 4 Aire−/− retinas used for scRNA-seq. (C) UMAP plot showing different cell-type clusters in a merged dataset from duplicate samples of Aire−/− retinas (shown in B) and age- and sex-matched WT control retinas. (D) UMAP plot of immune cells in the merged dataset. (E) Stacked bar plots showing the proportions of nonmicroglial immune cell types in scRNA-seq datasets from Aire−/− and WT retinas. The numbers of immune cells in each dataset are indicated below.
Using a droplet-based scRNA-seq platform (10× Genomics), we characterized 64,196 dissociated retinal cells from 8 samples (one neural retina per sample): 2 grade 2 Aire−/− mice (16,884 cells), 2 grade 3 Aire−/− mice (12,640 cells), and their wild-type (WT) littermate controls (34,672 cells) (Fig. 1B). The mean number of genes detected was 928 per cell (median: 584; interquartile range: 383 to 1,289). To control for sex effects, only female mice were studied. By principal component analysis, the samples were clearly segregated by disease grade (SI Appendix, Fig. S1D). On a Uniform Manifold Approximation and Projection (UMAP) (18) plot, the single-cell transcriptomes segregated predominantly by cell type (Fig. 1C), based on known markers for the 12 major retinal cell types (SI Appendix, Table S1), rather than by disease grade or batch (SI Appendix, Fig. S1E). (In this UMAP plot, microglia constitute a subset of the cell clusters labeled “Immune cells.”) A total of 1,266 presumed multiplets were excluded from subsequent analyses.
In Aire−/− retinas, scRNA-seq revealed multiple immune cell types. When visualized on a separate UMAP plot, these cells formed distinct clusters representing microglia, cells of monocyte lineage, T and Natural Killer (NK) cells, B cells, and plasma cells (Fig. 1D and SI Appendix, Table S2). In WT retinas, a small number of monocyte-derived cells that are transcriptionally distinct from microglia were present (Fig. 1 E, Left), but B cells, T and NK cells, and plasma cells were not detected. The absence or near-absence of lymphoid cells in WT retinas may be a feature of ocular immune privilege (5). In Aire−/− retinas, there were many more nonresident immune cells, and there was a preponderance of T and NK cells and monocyte-derived cells, accompanied by a smaller number of B cells and plasma cells (Fig. 1 E, Right). The proportion of B cells and plasma cells increased with disease severity [χ2 = 37.855, degree of freedom (df) = 6, P value = 1.199 × 10−6].
We note the lack of significant expression of Aire transcripts in resident retinal cells in the WT mouse retina, implying that the phenotype associated with Aire loss of function reflects Aire expression in nonretinal cells, presumably medullary thymic epithelial cells (8, 11, 12). In a previous study, transfer of thymi from Aire−/− mice into Aire-sufficient athymic mice was sufficient to induce autoantibodies to eye antigens (8).
Analysis of T Cell Diversity Reveals Th1 Cells as the Main Effector T Cells.
Since experimental uveoretinitis has been characterized as a T cell-driven disease (5), we analyzed the cluster of cells that express Cd3e or Klra1 transcripts or both—representing T cells and NK cells—by first embedding these cells on a separate UMAP plot (Fig. 2A). By classifying cells according to known markers (Fig. 2B and SI Appendix, Table S2), we identified the following 6 classes: Th1 cells (T-bet+, Ifng+, Cxcr6+, Cd4+, Cd8a−, Klra1−), Cd8a+ T cells (Cd8a+, Cd4-, Klra1−), T follicular helper (Tfh) cells (Bcl6+, Cxcr5+, Cd4+, Cd8a−), regulatory T (Treg) cells (Foxp3+, Cd4+, Il10+), NK cells (Klra1+, Cd3e−, Cd4−, Cd8a−), and NK T cells (Klra1+, Cd3e+, Cd8a+). We also found a population of apparently undifferentiated Cd4+ T cells that were enriched for S1pr1 but did not express any of the classical effector T cell markers (S1pr1+, Cd3+, Cd4+, Cd8a−). Only 3 of 714 T and NK cells in Aire−/− retinas were classified as Th17 cells, as defined by IL17a expression (Fig. 2 A and B). These presumed Th17 cells do not form a distinct cluster on the UMAP plot, but this could reflect a combination of the small number of cells and/or variation in their gene expression profiles.
Fig. 2.
Analysis of T cell diversity in Aire−/− retinas. (A) UMAP plot of Cd3-expressing cells from Aire−/− retinas showing different T cell and NK cell subtypes. (B) Heatmap showing, for each T and NK cell subtype (horizontal axis), the scaled mean unique molecular identifiers (UMIs) of transcripts for known cell-type markers (vertical axis). (C) Immunostaining of cross-sections of WT vs. Aire−/− retinas showing T-BET (green), IFNG (red), CD4 (magenta), and nuclei (DAPI; blue). T-BET+ IFNG+ CD4+ Th1 cells are indicated by arrows. In this and other figures, the regions in the square Insets are enlarged below. (D) UMAP replotting of Th1 cells from the UMAP plot in A enclosed in dashed lines showing 2 distinct clusters of Th1 cells: a Cd40-ligand (Cd40Lg)–positive cluster and an IL10-positive cluster (SI Appendix, Fig. S2B). (E) Immunostaining of cross-sections of WT vs. Aire−/− retinas showing T-BET (green), IL10 (red), CD4 (magenta), and nuclei (DAPI; blue). T-BET+ IL10+ CD4+ Tfh cells are indicated by arrows. (F) Heatmap showing 40 genes that are differentially expressed between the 2 Th1 clusters. (G, Left) Bar plots showing total UMIs for Tgfb1, Tgfb2, and Tgfb3. (G, Right) Heatmap showing mean expression per cell of Tgfb1, Tgfb2, and Tgfb3 for each cell type. (H) Retinal cross-sections showing fluorescent ISH for Tgfb2 (orange) and immunostaining for COL4A (green) and CD45 (magenta); nuclei are marked by DAPI (blue). (Scale bars in C, E, and H, 50 μm.)
The classification of the different T cell populations revealed Th1 cells as the predominant class of T helper cells in Aire−/− retinas. Direct quantification of Th1 and Th17 markers in Cd4+ T cells revealed a predominance of Th1 markers in both grade 2 and grade 3 disease (SI Appendix, Fig. S2A). Immunostaining of Aire−/− retinas for T-BET, interferon gamma (IFNG), and CD4 verified the presence of Th1 cells, which express all of these markers (Fig. 2C). The predominance of Th1 cells as effector T cells in the Aire−/− retina is consistent with previous reports that the main effector response in nonocular Aire−/− tissues is Th1 dominant (19). Closer examination of the scRNA-seq data derived from the Th1 population revealed 2 distinct clusters—one that is IL10+ (Interleukin-10+) and another that is Cd40lg+ (Cd40 ligand+) (Fig. 2D). These clusters appear to represent the previously described activated (Cd40lg+) and self-regulatory (Il10+) states, respectively (20). These 2 states were correlated with the differential expression of multiple genes (Fig. 2F) that may underlie the dynamics of Th1 cell self-regulation. Notably, both of these Th1 subpopulations express the IFN gamma (Ifng) gene and the Th1 markers Cxcr6 and Tbx21, the latter coding for T-BET (SI Appendix, Fig. S2B). The IL10-expressing Th1 cells do not express Foxp3, indicating that they are not regulatory T cells (SI Appendix, Fig. S2B). Fig. 2E shows accumulation of IL10 in a subset of T-BET+ and CD4+ T cells in the Aire−/− retina.
Th1 cells are sensitive to transforming growth factor-beta (TGF-β), which has been shown to promote either an effector state or a self-regulatory state, depending on the context (21). Promoting a self-regulatory state associated with induction of Il10 appears to be the predominant response for mature Th1 cells, as seen, for example, in a mouse model of experimental autoimmune encephalitis (22). Our scRNA-seq data show that Tgfb2 is the principal TGF-β family member expressed in both WT and Aire−/− retinas and that it is expressed by multiple retinal cell types, predominantly in the inner nuclear layer (INL) (Fig. 2G), which was confirmed by fluorescent ISH (Fig. 2H). There was no difference in the mean abundance of Tgfb2 transcripts between WT and Aire−/− retinas (SI Appendix, Fig. S2 C and D). Tgfb1 transcripts were present at a lower level and were detected principally in infiltrating immune cells, especially in cells of the monocyte lineage.
Tfh Cells and the Formation of Tertiary Lymphoid Structures.
The cluster of Bcl6+, Cxcr5+, Cd4+, and Cd8a− cells observed with scRNA-seq of Aire−/− retinas match the profile of Tfh cells (23, 24). This raises the possibility that tertiary lymphoid structures (TLSs) could have formed within Aire−/− retinas, a phenomenon that has been reported in the R161H mouse line, a mouse model of spontaneous uveoretinitis that is driven by transgenic expression of a T cell receptor (TCR) that recognizes IRBP (25).
By immunostaining of Aire−/− retinas, we observed aggregates of CD4+ T cells, CD8+ T cells, and CD19+ B cells (Fig. 3A). There appeared to be a predominance of CD4+ T cells over CD8+ T cells, as reflected in the scRNA-seq data, where 61.3% (χ2 = 8.1667, df = 1, P value = 4.27 × 10−3) and 76.3% (χ2 = 255.12, df = 1, P value < 2.2 × 10−16) of all T cells detected in grade 2 and grade 3 disease, respectively, were Cd4+ T cells (SI Appendix, Fig. S2E). To assess whether these cell aggregates might resemble TLSs, we assessed markers indicative of TLSs. This analysis revealed the close proximity of BCL6+;CD4+ presumptive Tfh cells and CD19+ B cells in these cell aggregates (Fig. 3B). We also detected the classical germinal center marker Peripheral Lymph Node Addressin (PNAd) in close proximity to aggregates of CD19+ B cells and CD4+ T cells (Fig. 3C). PNAd is a glycoprotein that promotes the homing of T and B cells to TLSs and is a marker for high endothelial venules (HEVs), which are postcapillary venous structures important for lymphocyte homing and trafficking (26). We found PNAd+ blood vessels in close proximity to the cell aggregates (Fig. 3C). The aggregates also exhibited close apposition of CCR7+;CD4+ T cells and CCR7+ antigen-presenting cells (APCs), characterized by the expression of the major histocompatibility complex (MHC) class II proteins and visualized by immunostaining with an antibody directed against I-A and I-E MHC class II proteins (Fig. 3D). CCR7 has been shown to be important for organizing the T cell zone in lymph nodes and is necessary for the formation of TLSs (27). Finally, the detection of phosphorylated ZAP70 in CD4+ T cells and the presence of the costimulatory molecule CD80 indicate that TCR activation was occurring in these presumed TLSs (Fig. 3E). Consistent with CD4+ T cell activation and signaling, the TLSs also contained CD19+, SDC1+ plasmablasts, and CD19− and SDC1+ plasma cells (Fig. 3F).
Fig. 3.
Presence of tertiary lymphoid structures in Aire−/− retinas. (A–D) Immunostaining of cross-sections of WT vs. Aire−/− retinas. (A) CD8 (green), CD19 (red), CD4 (magenta), and nuclei (DAPI; blue). (B) BCL6 (green), CD19 (red), CD4 (magenta), and nuclei (DAPI; blue). BCL6+CD4+ cells are indicated by arrows. (C) PNAd (green), CD19 (red), CD4 (magenta), and nuclei (DAPI; blue). A PNAd+ vessel resembling an HEV is indicated by the arrows. (D) MHC class II (I-A and I-E; green), CCR7 (red), CD4 (magenta), and nuclei (DAPI; blue). (E) CD80 (green), pZAP70 (red), CD4 (magenta), and nuclei (DAPI; blue). (F) SDC1 (green), CD19 (red), CD4 (magenta), and nuclei (DAPI; blue). CD19− SDC1+ plasma cells are indicated by arrows; a CD19+ SDC1+ plasmablast is indicated by the arrowheads. (Scale bars in A–F, 50 μm.)
Monocyte Transitional States: Microglia-Like Macrophages and Dendritic Cells.
As shown in Fig. 1 C and D, retinal microglia and cells of the monocyte lineage form transcriptionally distinct clusters when visualized on a UMAP plot. Based on recent scRNA-seq studies of microglia and macrophages in the brain and retina (28–30), we were able to further resolve the cells of the monocyte lineage into monocytes, monocyte-derived macrophages (mo-MΦs), monocyte-derived dendritic cells (mo-DCs), replicating monocytes, perivascular macrophages, and plasmacytoid dendritic cells (Fig. 4A). Each of these populations of cells expressed distinct markers (SI Appendix, Fig. S3A). Two populations of cells, termed macrophage-committed monocytes (MΦ-committed Mo) and dendritic cell-committed monocytes (DC-committed Mo), expressed macrophage and dendritic markers, respectively, in addition to monocyte markers (SI Appendix, Fig. S3B).
Fig. 4.
Divergent fates of monocyte lineage cells in Aire−/− retinas. (A) UMAP plot from Aire−/− retinas showing different subtypes of monocyte lineage cells. (B) Cell trajectory of monocyte lineage cells showing a bifurcation into Mo-MΦs and Mo-DCs. (C) Heatmap showing branch expression analysis modeling of the branch point between Mo-MΦs (Left) and Mo-DCs (Right). (D) Expression of Cd14, Mrc1, P2ry12, Smad7, and Zbtb46 along the cell trajectory shown in B. (E) Immunostaining of cross-sections of WT vs. Aire−/− retinas showing P2RY12 (green), CD14 (red), IBA1 (magenta), and nuclei (DAPI; blue). A P2RY12+ CD14+ cell is indicated by the arrows. (F) Immunostaining as in E for P2RY12 (green), MRC1 (red), IBA1 (magenta), and nuclei (DAPI; blue). Most IBA1+ P2RY12+ cells are MRC1− (indicated by arrowheads) with a minority being MRC1+ (indicated by arrows). (G) Barplots showing mean log10 normalized expression per cell for Cd68, Aif1, and Lgals. *P value < 0.05 and **P value < 0.01. (H) Immunostaining as in E for CD68 (green), IBA1 (red), Galectin 3 (magenta), and nuclei (DAPI; blue). (Scale bars in E, F, and H, 50 μm.)
Since monocytes, MΦ-committed Mo, mo-MΦs, DC-committed Mo, and mo-DCs formed a continuum on a UMAP plot from Aire−/− retinas (Fig. 4A), we constructed a cell trajectory map for these cell types. Starting with Ccr2+ monocytes, this trajectory bifurcates into mo-MΦs (branch 1) and mo-DCs (branch 2) (Fig. 4B). The cell trajectory analysis shows that, as monocytes progress toward MΦs, they reduce expression of the monocyte markers Anxa8, Ly6c2, and Ccr2, while increasing expression of macrophage markers, including Trem2 and Hexb (Fig. 4C). Interestingly, the mo-MΦs also increase expression of classical microglial markers such as Tmem119 and P2ry12. This observation is consistent with the findings of a previous study indicating that bone-marrow–derived monocytes can migrate into both the normal and the injured retina to assume a microglia-like ramified morphology with expression of microglial markers (31). Similarly, as monocytes progress toward mo-DCs, they increase expression of DC markers, including Clec9a and Zbtb46 (Fig. 4C). Fig. 4D illustrates, on a cell-by-cell basis, the changing patterns of expression for 5 markers: Cd14, which is enriched in monocytes, MΦ-committed Mo, and mo-MΦs; Mrc1, which codes for CD206 and is expressed in monocytes and MΦ-committed Mo; P2ry12, which is enriched in mo-MΦs (and microglia); Smad7, which is a marker of TGF-β signaling and is up-regulated as monocytes convert to mo-MΦs and to mo-DCs; and Zbtb46, a marker of dendritic cells, which is enriched in mo-DCs.
A recent study has shown that, in mouse models of photoreceptor degeneration, resident microglia migrate to the subretinal space and adhere to the apical retinal pigment epithelium (RPE), whereas infiltrating monocytes and mo-MΦs remain in the neural retina (29). In a similar fashion, immunostaining of Aire−/− retinas showed P2RY12+;CD14+ mo-MΦs and IBA1+;MRC1+ MΦ-committed-Mo predominantly in the neural retina, with only occasional P2RY12+;CD14+ cells in the subretinal space (Fig. 4 E and F). Interestingly, colonization of the microglial niche by mo-MΦs has been shown to be TGF-β dependent in the brain and retina (32, 33).
In previous studies, it has been difficult to distinguish infiltrating mo-MΦs from resident microglia because the 2 cell types exhibit similar patterns of gene expression and undergo similar gene expression changes in response to disease (34). Therefore, we examined gene expression patterns and disease-associated changes in those patterns in mo-MΦs and resident microglia in Aire−/− retinas. Some genes, such as Hexb and Trem2, are expressed at similar levels in both mo-MΦs and resident microglia (SI Appendix, Fig. S4A). Other genes are more specific to one or the other population. In particular, we examined Galectin-3 (Lgals3) and Cd68, as these 2 genes are enriched and up-regulated in subretinal microglia in a light damage model (29). In Aire−/− retinas, Lgals3 was up-regulated in both mo-MΦs and resident microglia (P value < 0.01 for both), but Cd68 was unchanged (Fig. 4G). Interestingly, we observed increased immunostaining for both Galectin-3 and CD68 in IBAI+ cells in Aire−/− retinas (Fig. 4H), suggesting that CD68 may be regulated posttranscriptionally. Both Galectin-3 and CD68 were detected in IBAI+ cells in the neural retina and the subretinal space, in contrast to their predominant subretinal localization in a light damage model (29). Transcripts coding for IBA1 (Aif1), a commonly used marker for microglia and macrophages, were only modestly up-regulated (P value < 0.05 for microglia and P value < 0.01 for mo-MΦs) (Fig. 4G), whereas IBA1 immunostaining was substantially increased in Aire−/− retinas in both mo-MΦs and microglia (Fig. 4H). Lgals3 and Aif1 are representative of the 145 genes that are up-regulated in both mo-MΦs and microglia in Aire−/− retinas. Thirty of these genes are shown in SI Appendix, Fig. S4B.
Taken together, these findings show that, although mo-MΦs and microglia express similar markers and undergo similar gene expression changes in Aire−/− retinas, they still maintain distinct transcriptional profiles.
Widespread Responses to IFN Gamma Among Resident Retinal Cells.
We next examined the gene expression changes in resident retinal cell types in Aire−/− retinas. Cross-sample normalization was first performed via mean-scaling of the raw transcript copies per cell in each cell type independently. Using Monocle2 (35, 36), we generated a negative binomial regression model that included parameters for genotype, cell type, sequencing depth, and batch effects. This permitted the calculation of a z-scored genotype regression coefficient for each gene and the identification of differentially expressed genes on a per-cell type basis. To identify gene sets with differential representation in WT vs. Aire−/− datasets, genes were ranked based on the z-scored genotype regression coefficient for each gene in each cell type, and this ranking was used for a preranked Gene Set Enrichment Analysis (GSEA) (37). This preranked GSEA was performed with the Hallmark gene sets curated by the Molecular Signatures DataBase (MSigDB) (38) to identify gene sets that were enriched in Aire−/− vs. WT retinas in both grade 2 and grade 3 disease.
To examine gene expression changes that may underlie a common disease process across multiple cell types, we examined MSigDB Hallmark gene sets that were significantly enriched (q-value < 0.05) in 3 or more cell types and found multiple enriched gene sets in both grade 2 and grade 3 disease (Fig. 5 A and B). Notably, there were more enriched gene sets in grade 3 disease compared to grade 2 disease, which is consistent with the increased disease severity. Of these genes sets, the IFN-responsive gene sets appeared to be the most significantly enriched across all resident retinal cell types. As only IFN gamma, but not -alpha or -beta, transcripts were detected in the Aire−/− retina dataset, we presume that these gene expression changes were driven by IFN gamma, consistent with the predominant Th1 response. By examining the regression coefficients of IFN gamma stimulated genes as defined in the Hallmark Interferon Gamma Response gene set, we identified genes that were differentially expressed across multiple cell types, as well as those that were differentially expressed in specific cell types (Fig. 5 C and D).
Fig. 5.
IFN gamma response in resident cells in Aire−/− retinas. (A and B) Statistically significant MSigDB Hallmark pathways in 3 or more cell types in GSEA reveal pathways enriched in Aire−/− over WT retinas across multiple retinal cell types in grade 2 and grade 3 disease. *P value < 0.05. (C and D) Heatmaps showing GSEA for the IFN gamma response gene set in grade 2 (A) and grade 3 (B) Aire−/− retinas. (Left) Single column showing normalized enrichment score (NES) for each cell type. (Right) Heatmap showing normalized regression coefficients for each cell type (rows represent cell types; columns represent genes). (E) Immunostaining of cross-sections of WT vs. Aire−/− retinas showing IRF1 (green), CD45 (red), COL4A1 (magenta), and nuclei (DAPI; blue). (F) Immunostaining as in E for PSMB9 (green), CD45 (red), COL4A1 (magenta), and nuclei (DAPI; blue). (Scale bars in E and F, 50 μm.)
One example of a gene that was significantly up-regulated across all retinal cell types is the transcription factor Irf1, a major target of IFN gamma signaling (SI Appendix, Fig. S5A). Additionally, expression of Psmb9, an IRF1 target gene, was increased in all retinal cell types (SI Appendix, Fig. S5B). PSMB9 is a subunit of the immunoproteasome, which is responsible for the processing of peptides for display by class I MHC proteins. Consistent with scRNA-seq data, immunostaining for IRF1 and PSMB9 showed widespread up-regulation in Aire−/− retinas (Fig. 5 E and F). These observations imply that resident retinal cells exhibit enhanced antigen presentation in Aire−/− retinas, thereby further stimulating the autoimmune process.
The Muller Glial Response to IFN Gamma.
Although all resident retinal cell types appear to be responsive to IFN gamma, the transcript heatmaps in Fig. 5 C and D show the greatest responses in Muller glia. Consistent with this transcriptome analysis, immunostaining of Aire−/− retinas showed that IRF1 was most significantly up-regulated in Muller glia (marked by nuclear SOX9 immunostaining in the INL) (Fig. 6A and SI Appendix, S5 A and E).
Fig. 6.
Activation of IFN gamma target genes in Muller glia in Aire−/− retinas. (A) Immunostaining of cross-sections of WT vs. Aire−/− retinas showing SOX9 (green), IRF1 (red), CD45 (magenta), and nuclei (DAPI; blue). SOX9 marks RPE nuclei (near the top of the image), Muller glia nuclei in the INL, and astrocyte nuclei in the GCL. (B) Heatmap showing, by cell type, IFN gamma target genes that are preferentially up-regulated in Muller glia in Aire−/− vs. WT retinas. Individual cells are arrayed across the horizontal axis, and genes are shown along the vertical axis. Up-regulation of genes indicated by arrows was validated by fluorescent ISH in C. (C) Retinal cross-sections as in A showing fluorescent ISH of C4b transcripts (orange) and immunofluorescence of CD45 (magenta); nuclei marked by DAPI (blue). (D) Retinal cross-sections as in A showing fluorescent ISH of Cd274 transcripts (green) and immunofluorescent staining of CD45 (magenta); nuclei are marked by DAPI (blue). (E) Heatmap as in B showing, by cell type, MHC class II genes that are significantly up-regulated in Aire−/− vs. WT retinas. MHC class II genes include the I-A and I-E alloantigens that can be detected by the anti-MHC II I-A/I-E antibody (arrows). (F) Retinal cross-sections as in A showing MHC II (I-A/I-E) (green), SOX9 (red), CD4 (magenta), and nuclei (DAPI; blue). A CD4+ T cell (indicated by arrowheads) is seen associating with an MHC class II+ process of a SOX9+ Muller glial cell (nucleus indicated by arrows). In addition, SOX9− cells with intense MHC class II staining (indicated by dashed arrows) scattered across the retina are mostly likely microglia, Mo-MΦs, or Mo-DCs. (Scale bars in A, C, D, and F, 50 μm.)
Using a specificity score of ≥0.4 (Monocle2) as a threshold for Muller glia, we identified IFN gamma target genes that were up-regulated and enriched in Muller glia in Aire−/− retinas. Examples are shown in Fig. 6B (see also SI Appendix, Fig. S5 C and D). The increase in expression of known IFN gamma target genes C4b and Cd274 was confirmed by fluorescent ISH (Fig. 6 C and D). C4b is a complement factor that promotes inflammation, as deletion of C4b is protective in EAU (39). Cd274 codes for PD-L1, a checkpoint regulator that dampens the adaptive immune response and has been implicated in suppressing EAU (40). In addition, the up-regulation of Vcam1 in Muller glia is consistent with an earlier study on EAU, which showed the up-regulation of VCAM1 in Muller glial radial processes by immunostaining (41). Vcam1 codes for Vascular Cell Adhesion Molecule 1, a protein involved in leukocyte migration into sites of inflammation.
Interestingly, multiple MHC class II genes are preferentially up-regulated in Muller glia in Aire−/− retinas (Fig. 6E). These genes are typically expressed in APCs including macrophages and dendritic cells. By immunostaining, MHC class II proteins were found to be enriched in Muller glial cell bodies (marked by arrow indicating nuclear SOX9 in Fig. 6F) and in their radial processes (Fig. 6F). Some of these MHC class II+ radial processes appeared to be in direct apposition to CD4+ T cells (arrowhead in Fig. 6F), suggesting that Muller glia may be functioning as APCs in Aire−/− retinas.
Discussion
This paper describes the application of scRNA-seq to characterize spontaneous uveoretinitis in Aire−/− mice. Based on the abundances of different immune cell types, the patterns of gene expression in immune and retinal cells, and the results of a parallel analysis by immunostaining and ISH, we infer that 1) the dominant effector response in Aire−/− retinas is Th1-driven, 2) the development of tertiary lymphoid structures constitutes an integral part of the Aire−/− retinal phenotype, 3) a subset of monocytes convert to either a macrophage/microglia state or a dendritic cell state, 4) all major classes of resident retinal cells respond to IFN gamma as evidenced by changes in their patterns of gene expression, and 5) Muller glia up-regulate specific genes in response to IFN gamma and may act as APCs. This study adds to a growing body of evidence showing that ocular inflammation is context-specific in terms of the molecular mechanisms and immune cell types involved (5).
The dominant effector response in uveitis is known to differ among animal models. In the classical EAU model, the use of killed mycobacteria in CFA promotes a Th17-dominant response that requires IL-17 for both the induction and the progression of uveitis (42). In contrast, in the R161H transgenic mouse line, which develops spontaneous uveitis through the expression of an IRBP-specific TCR transgene, Th1 cells appear to play a central role based on the large IFN gamma response (43). The present work supports a similarly central role for Th1 cells in Aire−/− uveoretinitis based on the abundance of Th1 cells, the presence of IFN gamma transcripts, and an IFN gamma-response gene expression signature in both immune and resident retinal cell types. These findings are consistent with previous work showing that many nonocular tissue pathologies in Aire−/− mice are Th1 dependent (19).
By immunostaining of Aire−/− retinas, we observed aggregates of T follicular helper cells, B cells, plasmablasts, plasma cells, and APCs that resemble TLSs. TLSs can be defined as unencapsulated, but structurally and functionally organized, aggregates of lymphoid cells that form in tissues other than primary lymphoid organs (i.e., thymus and bone marrow) or secondary lymphoid organs (i.e., lymph nodes, spleen, and Peyer’s patches) in the context of chronic inflammation (26, 44, 45). We use the term TLSs, instead of tertiary lymphoid organs (TLOs) because we have demonstrated some but not all of the features of TLOs (44). TLO features include 1) anatomically distinct yet adjacent B and T cell compartments, 2) the presence of PNAd-positive HEVs in the T cell compartment, and 3) the presence of germinal center reactions as evidenced by the presence of plasmablasts and plasma cells. In contrast to the diffuse inflammatory cellular infiltrates seen in the EAU model, the spatial organization of the retinal TLSs studied here more closely resembles that of lymphoid tissues. Similar TLSs have been described in the R161H transgenic mouse line (25), another model of spontaneous uveitis (described in the preceding paragraph). In R161H mice, retinas with TLSs initially have lower clinical and histological disease scores as well as slower loss of visual function (as measured by the electroretinogram) compared to retinas that lack TLSs, but the presence of well-developed, late-stage TLSs with abundant plasma cells was associated with increased visual loss and disease progression, presumably due to autoantibody production (25). Similarly, the presence of plasma cells in TLSs in Aire−/− retinas suggests that autoantibodies may also contribute to disease progression in Aire−/− uveoretinitis. There is evidence that autoantibodies play a pathogenic role in the EAU model (46) and in human uveitis (47, 48).
Retinal microglia, which arise from the yolk sac, are difficult to distinguish from mo-MΦs as the 2 cell populations share many of the same markers and both microglia and mo-MΦs up-regulate many of the same genes and can occupy new tissue niches in response to disease. A previous study that utilized irradiated mice injected with bone marrow (BM) cells marked by enhanced green fluorescent protein showed that BM-derived monocytes can populate the normal retina and assume microglial-like morphology with expression of classical microglial markers (31). The migration of BM-derived cells was enhanced in a model of N-methyl-N-nitrosourea–induced retinal injury, and the BM-derived microglial-like cells were restricted to the juxtapapillary and peripheral regions of the retina (31). More recently, fate-mapping studies in mouse models of photoreceptor injury and degeneration have shown that resident microglia migrate to the subretinal space where they adhere to the apical RPE, while mo-MΦs occupy the vacated microglial niche in the neuroretina and do not migrate to the subretinal space (29, 34). By immunostaining of Aire−/− retinas, we observed CD14- and P2ry12-positive cells (i.e., mo-MΦs) mainly in the neural retina, although some were found in the subretinal space adhering to the apical RPE. The differences across retinal disease models imply that the tissue niches occupied by microglia and mo-MΦs can vary depending on the disease context.
Our pseudotime analysis expands on this dynamic picture by implying that many monocytes transition from a state characterized by markers such as Ccr2 and Ly6c2 to a mo-MΦ state characterized by markers such as Hexb and Trem2. The rapid down-regulation of Ccr2 in monocytes infiltrating the retina has also been described in the context of photoreceptor degeneration (49). Mo-MΦs are difficult to distinguish from resident retinal microglia as they also express conventional microglial markers such as P2ry12 and Tmem119, a finding that is consistent with recent scRNA-seq studies of fluorescence-activated cell sorting-purified microglia and mo-MΦs isolated from murine retina and brain (28–30, 50). The down-regulation of genes such as Ccr2 and the up-regulation of genes such as Tmem119 may account for the previously observed heterogeneity in gene expression of monocyte-derived cells at early time points after these cells have infiltrated the retina in the context of light-induced photoreceptor degeneration (29, 30). The pseudotime analysis also implies that a distinct subset of monocytes transitions to a dendritic cell state characterized by markers such as Clec9a and Zbtb46. Interestingly, both the mo-MΦ and mo-DC states were associated with an up-regulation of Smad7, a marker of TGF-β signaling.
The scRNA-seq and ISH analyses reveal TGF-β2 expression in the INL in Aire−/− retinas. TGF-β2 has potent immunosuppressive properties (21, 51) and is thought to contribute to ocular immune privilege (5). In the context of Aire−/− uveoretinitis, TGF-β2 may mitigate against excessive immune-mediated destruction through its effects on Th1 cells and microglia/mo-MΦs. Consistent with this idea, the induction of IL10 expression in Th1 cells has been shown to be TGF-β dependent (22), and the Th1 cells in the Aire−/− retina exhibit distinct Cd40lg+ and IL10+ states that most likely represent activated and self-regulatory states, respectively (20). Also consistent with this idea, genetic ablation of Tgfbr2 in spinal cord mo-MΦs (32) and in retinal microglia (33) resulted in down-regulation of classical microglial markers such as Tmem119 and conversion to a more proinflammatory phenotype.
The IFN gamma response gene expression signature that we observe in all major classes of resident retinal cells is characterized by widespread IRF1 up-regulation and up-regulation of putative IFN gamma target genes across retinal cell types. The role of IFN gamma in uveoretinitis appears to be multifaceted. Retinal production of IFN gamma, either by expression of a transgene (52) or by infusing an IRBP-specific Th1-like T cell line that produces large amounts of IFN gamma (53), appears to be uveitogenic. Moreover, disease induction in an EAU model based on injection of antigen-exposed dendritic cells requires host production of IFN gamma (54). However, removing IFN gamma, either by systemic administration of a neutralizing antibody (55) or by genetic deletion (56), exacerbated uveoretinitis in EAU instead of conferring a protective effect. These differences in the effects of IFN gamma in the context of uveoretinitis have been attributed to differences in the stage of the disease at which IFN gamma exposure occurs, with early exposure to IFN gamma eliciting an antiinflammatory effect and late exposure eliciting a proinflammatory effect (57, 58).
One intriguing instance of a cell type-specific up-regulation of putative IFN gamma target genes is the increased production of MHC class II messenger RNAs and proteins in Muller glia, with evidence of direct contact between Muller glia processes and CD4+ T cells. Muller glia are the major glial component of the retina, and their processes densely ramify throughout the entirety of the neural retina between the nerve fiber layer and the outer limiting membrane. Thus, any immune cell infiltrating the retina will come into contact with Muller glia processes. Under normal conditions, Muller glia are thought to contribute to ocular immune privilege by inhibiting the proliferation and activation of lymphocytes through a direct-contact mechanism, as demonstrated in cultured Muller glia (59). The addition of IFN gamma in vitro has been shown to induce production of MHC class II proteins in cultured Muller glia (60) and enable them to act as APCs in a MHC class II-dependent fashion to stimulate the proliferation of cocultured T cells (61). Consistent with these in vitro studies, our findings suggest that Muller glia may act as APCs in vivo in the context of Aire−/− uveoretinitis, although the relative contributions of Muller glia compared to more conventional APCs in the uveoretinitis disease process remain to be determined.
In sum, the present study demonstrates the utility of scRNA-seq as an unbiased tool for investigating immune-mediated pathogenesis in the retina, including a systematic assessment of the cell types in the inflammatory milieu and the response of the resident retinal cells to disease progression. This work suggests that scRNA-seq could be used to identify biomarkers and therapeutic targets in ocular inflammatory disorders.
Materials and Methods
All animal experiments were approved by and conducted in accordance with the regulations of the Institutional Animal Care and Use Committee at Johns Hopkins University School of Medicine (protocol MO16M367). Mouse husbandry, scRNA-seq, in situ hybridization, immunostaining, and computational methods are described in SI Appendix. scRNAseq data were submitted to the National Center for Biotechnology Information Gene Expression Omnibus database under accession numbers GSE132229 and GSM3854512–3854519. The annotated datasets can be viewed at https://jacobheng.shinyapps.io/uveoretinitis/ and http://loom.gofflab.org. Supplementary code for processing and visualizing the scRNA-seq data can be found in an R package, cellwrangler, available at https://github.com/jacobheng/cellwrangler.
Supplementary Material
Acknowledgments
We thank David Mohr (Johns Hopkins Medical Institutions Genetic Research Core Facility) for his assistance with sequencing the scRNA-seq libraries; Melissa Olson and Kakali Sarkar for their assistance with preparing some of the scRNA-seq libraries; Peter Campochiaro for sharing his fundus imaging equipment and expertise; Daniel Saban, Jeff Mumm, Mark Soloski, Debebe Theodros, Yanshu Wang, and Chen Yu for helpful advice; and Peter Dimitrion, Mark Sabbagh, and Mark Soloski for helpful comments on the manuscript. This work was supported by the Jerome L. Greene Foundation (J.S.H and J.N.); the Thomas J. Kelly and Mary L. Kelly Young Scholar Award (to J.S.H); the Howard Hughes Medical Institute (J.W. and J.N.); the Johns Hopkins University Catalyst and Synergy Awards (L.A.G.); and the Chan-Zuckerberg Initiative Donor Advised Fund Grant 2018-183445 (to G.L.S.-O. and L.A.G.).
Footnotes
The authors declare no competing interest.
Data deposition: scRNA-seq data reported in this paper have been deposited in the National Center for Biotechnology Information Gene Expression Omnibus database (accession nos. GSE132229 and GSM3854512–GSM3854519). The annotated datasets can be viewed at https://jacobheng.shinyapps.io/uveoretinitis/ and http://loom.gofflab.org. Supplementary code for processing and visualizing the scRNA-seq data can be found in an R package, cellwrangler, available at https://github.com/jacobheng/cellwrangler.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.1915571116/-/DCSupplemental.
References
- 1.Thorne J. E., et al. , Prevalence of noninfectious uveitis in the United States: A claims-based analysis. JAMA Ophthalmol. 134, 1237–1245 (2016). [DOI] [PubMed] [Google Scholar]
- 2.Suttorp-Schulten M. S., Rothova A., The possible impact of uveitis in blindness: A literature survey. Br. J. Ophthalmol. 80, 844–848 (1996). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.de Smet M. D., et al. , Cellular immune responses of patients with uveitis to retinal antigens and their fragments. Am. J. Ophthalmol. 110, 135–142 (1990). [DOI] [PubMed] [Google Scholar]
- 4.Caspi R. R., A look at autoimmunity and inflammation in the eye. J. Clin. Invest. 120, 3073–3083 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Perez V. L., Caspi R. R., Immune mechanisms in inflammatory and degenerative eye disease. Trends Immunol. 36, 354–363 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen J., et al. , Comparative analysis of induced vs. spontaneous models of autoimmune uveitis targeting the interphotoreceptor retinoid binding protein. PLoS One 8, e72161 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lambe T., et al. , Limited peripheral T cell anergy predisposes to retinal autoimmunity. J. Immunol. 178, 4276–4283 (2007). [DOI] [PubMed] [Google Scholar]
- 8.Anderson M. S., et al. , Projection of an immunological self shadow within the thymus by the aire protein. Science 298, 1395–1401 (2002). [DOI] [PubMed] [Google Scholar]
- 9.Egwuagu C. E., Charukamnoetkanok P., Gery I., Thymic expression of autoantigens correlates with resistance to autoimmune disease. J. Immunol. 159, 3109–3112 (1997). [PubMed] [Google Scholar]
- 10.Jiang W., Anderson M. S., Bronson R., Mathis D., Benoist C., Modifier loci condition autoimmunity provoked by Aire deficiency. J. Exp. Med. 202, 805–815 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.DeVoss J., et al. , Spontaneous autoimmunity prevented by thymic expression of a single self-antigen. J. Exp. Med. 203, 2727–2735 (2006). Correction in: J. Exp. Med. 204, 203 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fujikado N., et al. , Aire inhibits the generation of a perinatal population of interleukin-17A-producing γδ T cells to promote immunologic tolerance. Immunity 45, 999–1012 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bourgault S., et al. , Retinal degeneration in autoimmune polyglandular syndrome type 1: A case series. Br. J. Ophthalmol. 99, 1536–1542 (2015). [DOI] [PubMed] [Google Scholar]
- 14.Couturier A., Brézin A. P., Ocular manifestations of autoimmune polyendocrinopathy syndrome type 1. Curr. Opin. Ophthalmol. 27, 505–513 (2016). [DOI] [PubMed] [Google Scholar]
- 15.Heng J. S., et al. , Hypoxia tolerance in the Norrin-deficient retina and the chronically hypoxic brain studied at single-cell resolution. Proc. Natl. Acad. Sci. U.S.A. 116, 9103–9114 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Papalexi E., Satija R., Single-cell RNA sequencing to explore immune cell heterogeneity. Nat. Rev. Immunol. 18, 35–45 (2018). [DOI] [PubMed] [Google Scholar]
- 17.Agarwal R. K., Silver P. B., Caspi R. R., Rodent models of experimental autoimmune uveitis. Methods Mol. Biol. 900, 443–469 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Becht E., et al. , Dimensionality reduction for visualizing single-cell data using UMAP. Nat. Biotechnol. 37, 38–44 (2018). [DOI] [PubMed] [Google Scholar]
- 19.Devoss J. J., et al. , Effector mechanisms of the autoimmune syndrome in the murine model of autoimmune polyglandular syndrome type 1. J. Immunol. 181, 4072–4079 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Murugaiyan G., Agrawal R., Mishra G. C., Mitra D., Saha B., Functional dichotomy in CD40 reciprocally regulates effector T cell functions. J. Immunol. 177, 6642–6649 (2006). [DOI] [PubMed] [Google Scholar]
- 21.Sanjabi S., Oh S. A., Li M. O., Regulation of the immune response by TGF-β: From conception to autoimmunity and infection. Cold Spring Harb. Perspect. Biol. 9, a022236 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Huss D. J., et al. , TGF-beta enhances effector Th1 cell activation but promotes self-regulation via IL-10. J. Immunol. 184, 5628–5636 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hutloff A., T follicular helper-like cells in inflamed non-lymphoid tissues. Front. Immunol. 9, 1707 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rao D. A., T cells that help B cells in chronically inflamed tissues. Front. Immunol. 9, 1924 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kielczewski J. L., Horai R., Jittayasothorn Y., Chan C.-C., Caspi R. R., Tertiary lymphoid tissue forms in retinas of mice with spontaneous autoimmune uveitis and has consequences on visual function. J. Immunol. 196, 1013–1025 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pipi E., et al. , Tertiary lymphoid structures: Autoimmunity goes local. Front. Immunol. 9, 1952 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wengner A. M., et al. , CXCR5- and CCR7-dependent lymphoid neogenesis in a murine model of chronic antigen-induced arthritis. Arthritis Rheum. 56, 3271–3283 (2007). [DOI] [PubMed] [Google Scholar]
- 28.Jordão M. J. C., et al. , Single-cell profiling identifies myeloid cell subsets with distinct fates during neuroinflammation. Science 363, eaat7554 (2019). [DOI] [PubMed] [Google Scholar]
- 29.O’Koren E. G., et al. , Microglial function is distinct in different anatomical locations during retinal homeostasis and degeneration. Immunity 50, 723–737.e7 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ronning K. E., Karlen S. J., Miller E. B., Burns M. E., Molecular profiling of resident and infiltrating mononuclear phagocytes during rapid adult retinal degeneration using single-cell RNA sequencing. Sci. Rep. 9, 4858 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kaneko H., Nishiguchi K. M., Nakamura M., Kachi S., Terasaki H., Characteristics of bone marrow-derived microglia in the normal and injured retina. Invest. Ophthalmol. Vis. Sci. 49, 4162–4168 (2008). [DOI] [PubMed] [Google Scholar]
- 32.Lund H., et al. , Fatal demyelinating disease is induced by monocyte-derived macrophages in the absence of TGF-β signaling. Nat. Immunol. 19, 1–7 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ma W., et al. , Absence of TGFβ signaling in retinal microglia induces retinal degeneration and exacerbates choroidal neovascularization. eLife 8, e42049 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.O’Koren E. G., Mathew R., Saban D. R., Fate mapping reveals that microglia and recruited monocyte-derived macrophages are definitively distinguishable by phenotype in the retina. Sci. Rep. 6, 20636 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Trapnell C., et al. , The dynamics and regulators of cell fate decisions are revealed by pseudotemporal ordering of single cells. Nat. Biotechnol. 32, 381–386 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Qiu X., et al. , Single-cell mRNA quantification and differential analysis with Census. Nat. Methods 14, 309–315 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Subramanian A., et al. , Gene set enrichment analysis: A knowledge-based approach for interpreting genome-wide expression profiles. Proc. Natl. Acad. Sci. U.S.A. 102, 15545–15550 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liberzon A., et al. , The Molecular Signatures Database (MSigDB) hallmark gene set collection. Cell Syst. 1, 417–425 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhang L., Bell B. A., Li Y., Caspi R. R., Lin F., Complement component C4 regulates the development of experimental autoimmune uveitis through a T cell-intrinsic mechanism. Front. Immunol. 8, 1116 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lee D. J., Taylor A. W., Recovery from experimental autoimmune uveitis promotes induction of antiuveitic inducible Tregs. J. Leukoc. Biol. 97, 1101–1109 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Makhoul M., et al. , Characterization of retinal expression of vascular cell adhesion molecule (VCAM-1) during experimental autoimmune uveitis. Exp. Eye Res. 101, 27–35 (2012). [DOI] [PubMed] [Google Scholar]
- 42.Luger D., et al. , Either a Th17 or a Th1 effector response can drive autoimmunity: Conditions of disease induction affect dominant effector category. J. Exp. Med. 205, 799–810 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Horai R., et al. , Spontaneous ocular autoimmunity in mice expressing a transgenic T cell receptor specific to retina: A tool to dissect mechanisms of uveitis. Curr. Mol. Med. 15, 511–516 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Neyt K., Perros F., GeurtsvanKessel C. H., Hammad H., Lambrecht B. N., Tertiary lymphoid organs in infection and autoimmunity. Trends Immunol. 33, 297–305 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Colbeck E. J., Ager A., Gallimore A., Jones G. W., Tertiary lymphoid structures in cancer: Drivers of antitumor immunity, immunosuppression, or bystander sentinels in disease? Front. Immunol. 8, 1830 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pennesi G., et al. , A humanized model of experimental autoimmune uveitis in HLA class II transgenic mice. J. Clin. Invest. 111, 1171–1180 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Heiligenhaus A., Miserocchi E., Heinz C., Gerloni V., Kotaniemi K., Treatment of severe uveitis associated with juvenile idiopathic arthritis with anti-CD20 monoclonal antibody (rituximab). Rheumatology (Oxford) 50, 1390–1394 (2011). [DOI] [PubMed] [Google Scholar]
- 48.Jiménez-Alonso J., et al. , CD5+ B cells and uveitis. Ann. Rheum. Dis. 61, 854–855 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sennlaub F., et al. , CCR2(+) monocytes infiltrate atrophic lesions in age-related macular disease and mediate photoreceptor degeneration in experimental subretinal inflammation in Cx3cr1 deficient mice. EMBO Mol. Med. 5, 1775–1793 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hammond T. R., et al. , Single-cell RNA sequencing of microglia throughout the mouse lifespan and in the injured brain reveals complex cell-state changes. Immunity 50, 253–271.e6 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Flavell R. A., Sanjabi S., Wrzesinski S. H., Licona-Limón P., The polarization of immune cells in the tumour environment by TGFbeta. Nat. Rev. Immunol. 10, 554–567 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Geiger K., et al. , Transgenic mice expressing IFN-gamma in the retina develop inflammation of the eye and photoreceptor loss. Invest. Ophthalmol. Vis. Sci. 35, 2667–2681 (1994). [PubMed] [Google Scholar]
- 53.Xu H., Rizzo L. V., Silver P. B., Caspi R. R., Uveitogenicity is associated with a Th1-like lymphokine profile: Cytokine-dependent modulation of early and committed effector T cells in experimental autoimmune uveitis. Cell. Immunol. 178, 69–78 (1997). [DOI] [PubMed] [Google Scholar]
- 54.Tang J., et al. , Autoimmune uveitis elicited with antigen-pulsed dendritic cells has a distinct clinical signature and is driven by unique effector mechanisms: Initial encounter with autoantigen defines disease phenotype. J. Immunol. 178, 5578–5587 (2007). [DOI] [PubMed] [Google Scholar]
- 55.Caspi R. R., et al. , Endogenous systemic IFN-gamma has a protective role against ocular autoimmunity in mice. J. Immunol. 152, 890–899 (1994). [PubMed] [Google Scholar]
- 56.Jones L. S., et al. , IFN-gamma-deficient mice develop experimental autoimmune uveitis in the context of a deviant effector response. J. Immunol. 158, 5997–6005 (1997). [PubMed] [Google Scholar]
- 57.Tarrant T. K., et al. , Interleukin 12 protects from a T helper type 1-mediated autoimmune disease, experimental autoimmune uveitis, through a mechanism involving interferon γ, nitric oxide, and apoptosis. J. Exp. Med. 189, 219–230 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Grajewski R. S., et al. , Activation of invariant NKT cells ameliorates experimental ocular autoimmunity by a mechanism involving innate IFN-γ production and dampening of the adaptive Th1 and Th17 responses. J. Immunol. 181, 4791–4797 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Caspi R. R., Roberge F. G., Nussenblatt R. B., Organ-resident, nonlymphoid cells suppress proliferation of autoimmune T-helper lymphocytes. Science 237, 1029–1032 (1987). [DOI] [PubMed] [Google Scholar]
- 60.Mano T., Tokuda N., Puro D. G., Interferon-gamma induces the expression of major histocompatibility antigens by human retinal glial cells. Exp. Eye Res. 53, 603–607 (1991). [DOI] [PubMed] [Google Scholar]
- 61.Roberge F. G., Caspi R. R., Nussenblatt R. B., Glial retinal Müller cells produce IL-1 activity and have a dual effect on autoimmune T helper lymphocytes. Antigen presentation manifested after removal of suppressive activity. J. Immunol. 140, 2193–2196 (1988). [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.