Heritable forms of epidermolysis bullosa (EB), a heterogeneous group of skin fragility disorders, manifest with blistering and erosions with high degree of morbidity (1–3). The phenotypic spectrum is highly variable, and in the most severe cases the patients die within the early postnatal period or within a few months of life due to complications of fragile skin, such as infections, sepsis, dehydration, and metabolic alterations. Some of these cases are also associated with extracutaneous manifestations in the syndromic forms of EB, affecting the lungs, kidneys, and heart (4). There is no effective treatment for EB. In PNAS, Jacków et al. (5) report on a CRISPR/Cas9-mediated therapy approach for a form of EB.
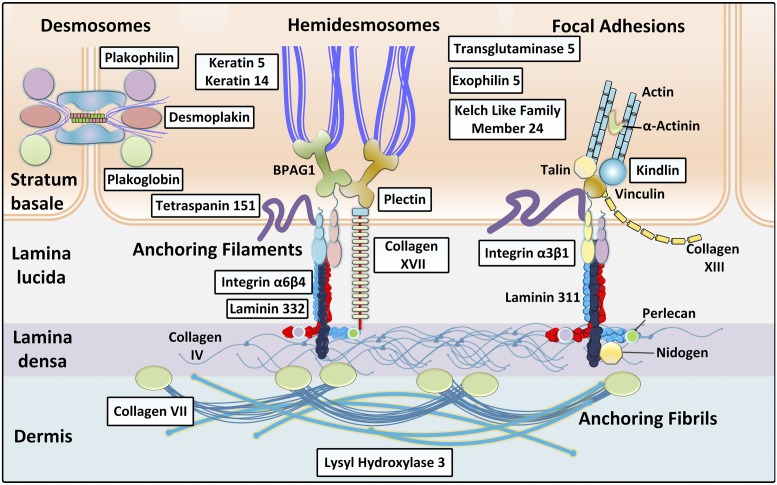
Critical for understanding the molecular basis of EB is the recognition of adhesion complexes consisting of interacting proteins, which form a network responsible for keratinocyte cell–cell adhesion and stable association of the epidermis and the underlying dermis (Fig. 1). Currently, there are 21 distinct genes that have been demonstrated to harbor mutations in different forms of EB (6, 7). The topographic location of the mutant genes within the epidermis and the cutaneous basement membrane zone, the types and combinations of the mutations, and their consequences at the mRNA and protein levels collectively explain the tremendous phenotypic variability in this group of disorders.
Fig. 1.
Schematic representation of the cutaneous basement membrane zone depicting attachment complexes critical for stable cell–cell contacts and association of epidermis to the underlying dermis. The boxed proteins, which are encoded by 21 distinct genes, are mutated in different forms of EB, the prototype of heritable blistering skin disorders. Adapted from ref. 4, with permission from Elsevier.
One of the most severe forms of EB is the recessive dystrophic EB (RDEB), in which the most devastating cases manifest with extensive fragility with blistering and denudation of the skin resulting in extensive scarring and often associated with rapidly metastasizing cutaneous squamous cell carcinomas with high degree of mortality at a relatively early age (8–10). RDEB is also associated with extracutaneous findings, including scarring of the gastrointestinal track, particularly the esophagus, and blistering and erosions of the cornea (8). RDEB is caused by biallelic mutations in the COL7A1 gene, encoding type VII collagen, the major if not the exclusive protein component of the anchoring fibrils (11). The COL7A1 loss-of-function mutations are frequently nonsense mutations or small insertions or deletions leading to frameshift, but a number of missense mutations have also been reported (12, 13). In addition, there is a dominantly inherited form of dystrophic EB frequently associated with glycine substitution mutations, which interfere with the type VII collagen assembly into anchoring fibrils through dominant-negative mode of action (14).
Considering the clinical severity of RDEB and the fact that there is currently no specific or effective treatment beyond protection from trauma and extensive bandaging as well as prevention of infections, several studies have recently focused on developing new therapies for this devastating, currently intractable disorder (15–18). In PNAS, Jacków et al. (5) report extensive studies employing CRISPR/Cas9-based correction of COL7A1 mutations in cells from 2 patients with RDEB. One of the patients had a homozygous frameshift mutation in exon 19 (c.2470insG), while the second one was a compound heterozygous for frameshift mutations in exons 19 and 32 (c.2470insG/c.3948insT). As the first step of their project, the investigators generated patient-specific inducible pluripotent stem cells (iPSCs). These cells were then subjected to clustered regularly interspaced short palindromic repeats (CRISPR)-associated nuclease/Cas9 (CRISPR/Cas9) homology-directed repair (HDR) of the pathogenic mutations. The researchers demonstrated that the CRISPR/Cas9-mediated HDR can introduce a corrected homologous sequence in the targeted gene and restore the normal gene sequence, which was shown by direct sequencing of the PCR products generated around the targeted region. The results revealed that ∼10% of the clones had undergone biallelic correction, and 40% of them monoallelic correction of the COL7A1 mutation in exon 19. Sequencing also revealed that the areas at the target sequence were free of off-target mutations. Next, the gene-corrected iPSCs were differentiated into either keratinocytes, which were shown to become functionally mature in ∼60 d, or fibroblasts, which after 31 d assumed characteristic morphology and expressed markers of mesodermal and fibroblastic differentiation, including type I and type III collagen as well as fibroblast-associated CD surface markers in a pattern similar to that of normal human fibroblasts. Importantly, the gene-edited fibroblasts derived from RDEB patients’ iPSCs synthesized and secreted type VII collagen, which assumed its characteristic stable triple-helical conformation. Finally, the investigators built 3-dimensional skin equivalents from gene-corrected keratinocytes and fibroblasts, which were then grafted onto immunocompromised mice. Analysis at 2 mo postgrafting demonstrated robust expression of type VII collagen in the xenografts in a pattern resembling that in wild-type mouse skin, and importantly, transmission electron microscopy revealed the formation of anchoring fibrils. Thus, this work attests to the feasibility of a CRISPR/Cas9-mediated gene correction to develop autologous cell therapies for RDEB in the form of skin grafts for skin replacement or for local treatment of nonhealing wounds.
With the eventual goal of clinical translation of this approach, there are regulatory concerns that need to be addressed as treatment development moves forward. Although it is not possible to anticipate all of the potential regulatory concerns relevant to gene-editing–based therapeutics and iPSCs, the major issues at the present time include safety, efficacy, and quality control. Toward these aims, the work describes several improvements and approaches that represent important steps toward clinical translation. Such improvements include a highly efficient method for gene correction assisted by high-fidelity Cas9 (SpyFicas9) nuclease, which was shown to have few if any detectable genome-wide off-target effects with a chemically modified synthetic guide RNA and single-strand DNA as a repair donor template for efficient gene editing (19). Another innovative feature of this work was the adoption of cell culture conditions devoid of animal products and, instead, using xeno-free, chemically defined culture media. The protocol developed by the investigators resulted in significantly higher reprogramming efficiency than has been reported previously, associated with enhanced safety by eliminating the undefined animal-derived components when developing cell-containing products for treatment of human diseases.
With the realization of the potential of CRISPR/Cas9-mediated genomic correction for human diseases, this technology has been tested preclinically in a number of disease models, including cells, both fibroblasts and keratinocytes, derived from patients with RDEB (19–24). These studies demonstrated HDR using different technical editing strategies and selection methods, including restoration of the reading frame of COL7A1 in cells carrying a frameshift mutation. In addition, an adenine-based editor ABE was shown to introduce a correct single-nucleotide substitution in primary fibroblasts and iPSCs with high efficiency without exogenous donor DNA template (20). The ability by Jacków et al. (5) to construct fully autologous skin equivalents made from gene-corrected keratinocytes and fibroblasts differentiated
In PNAS, Jacków et al. report extensive studies employing CRISPR/Cas9-based correction of COL7A1 mutations in cells from 2 patients with RDEB.
from iPSCs minimizes the risk of immune rejection, thus contributing to the maintenance of functional extracellular matrix and providing long-lasting survival of the graft. Collectively, the work by Jacków et al. significantly advances potential translation of these approaches toward clinical reality, providing an efficient and safe method for clinical application.
In summary, recent progress in development of treatments for EB gives hope to the patients, their parents, and their caregivers for future improvement in quality of life. CRISPR/Cas9-mediated gene targeting is one of the approaches that shows clear promise for potential breakthroughs, and the study by Jacków et al. has developed a systematic pathway leading from mutation correction in iPSCs to formation of functional skin equivalents that can be applied to patients suffering from RDEB. Theoretically, similar correction approaches could be applicable to other forms of EB with mutations in different genes. While the preclinical approaches developed here look promising, the translation of these findings to clinical treatment still faces critical questions. What size skin grafts can be transplanted to the patients and at what age? How long do the grafts remain functional in terms of skin stability and expression of the corrected protein? While the grafting procedures are largely targeting eroded areas of skin, how can we develop techniques for prevention of blistering and erosions by strengthening the dermal–epidermal junction of the normal-appearing skin in these patients? As the grafting targeted to the skin provides correction only of cutaneous findings, how will the treatment of extracutaneous manifestations be accomplished? Are there immunologic consequences to the application of grafts to the skin of patients, who in some cases have been completely devoid of the corresponding protein? Could complementary cell-, protein-, and gene-based correction approaches in combination improve the efficacy of the treatment? Answers to some of these questions will become available once early clinical trials have been initiated, but there is optimism in the field in light of recent grafting experiments with gene-corrected cells that demonstrated improvement in the functionality of the skin and overall quality of life of patients with EB (25–27).
Acknowledgments
I thank Carol Kelly for assistance with manuscript preparation and Amir Saeidian for Fig. 1 modifications. The author’s original work was supported by the NIH and DEBRA International.
Footnotes
The author declares no competing interest.
See companion article on page 26846.
References
- 1.Fine J. D., et al. , Inherited epidermolysis bullosa: Updated recommendations on diagnosis and classification. J. Am. Acad. Dermatol. 70, 1103–1126 (2014). [DOI] [PubMed] [Google Scholar]
- 2.Uitto J., Has C., Vahidnezhad H., Youssefian L., Bruckner-Tuderman L., Molecular pathology of the basement membrane zone in heritable blistering diseases: The paradigm of epidermolysis bullosa. Matrix Biol. 57-58, 76–85 (2017). [DOI] [PubMed] [Google Scholar]
- 3.Has C., et al. , Clinical practice guidelines for laboratory diagnosis of epidermolysis bullosa. Br. J. Dermatol. 10.1111/bjd.18128 (15 May 2019). [DOI] [PMC free article] [PubMed]
- 4.Vahidnezhad H., Youssefian L., Saeidian A. H., Uitto J., Phenotypic spectrum of epidermolysis bullosa: The paradigm of syndromic versus non-syndromic skin fragility disorders. J. Invest. Dermatol. 139, 522–527 (2019). [DOI] [PubMed] [Google Scholar]
- 5.Jacków J., et al. , CRISPR/Cas9-based targeted genome editing for correction of recessive dystrophic epidermolysis bullosa using iPS cells. Proc. Natl. Acad. Sci. U.S.A. 116, 26846–26852 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Uitto J., Bruckner-Tuderman L., McGrath J. A., Riedl R., Robinson C., EB2017—progress in epidermolysis bullosa research toward treatment and cure. J. Invest. Dermatol. 138, 1010–1016 (2018). [DOI] [PubMed] [Google Scholar]
- 7.Has C., Nyström A., Saeidian A. H., Bruckner-Tuderman L., Uitto J., Epidermolysis bullosa: Molecular pathology of connective tissue components in the cutaneous basement membrane zone. Matrix Biol. 71-72, 313–329 (2018). [DOI] [PubMed] [Google Scholar]
- 8.Fine J. D., Mellerio J. E., Extracutaneous manifestations and complications of inherited epidermolysis bullosa: Part II. Other organs. J. Am. Acad. Dermatol. 61, 387–402, quiz 403–404 (2009). [DOI] [PubMed] [Google Scholar]
- 9.Cho R. J., et al. , APOBEC mutation drives early-onset squamous cell carcinomas in recessive dystrophic epidermolysis bullosa. Sci. Transl. Med. 10, eaas9668 (2018). [DOI] [PubMed] [Google Scholar]
- 10.Castelo B., et al. , Epidemiology and natural history of cutaneous squamous cell carcinoma in recessive dystrophic epidermolysis bullosa patients: 20 years’ experience of a reference centre in Spain. Clin. Transl. Oncol. 21, 1573–1577 (2019). [DOI] [PubMed] [Google Scholar]
- 11.Chung H. J., Uitto J., Type VII collagen: The anchoring fibril protein at fault in dystrophic epidermolysis bullosa. Dermatol. Clin. 28, 93–105 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Varki R., Sadowski S., Uitto J., Pfendner E., Epidermolysis bullosa. II. Type VII collagen mutations and phenotype-genotype correlations in the dystrophic subtypes. J. Med. Genet. 44, 181–192 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vahidnezhad H., et al. , Dystrophic epidermolysis bullosa: COL7A1 mutation landscape in a multi-ethnic cohort of 152 extended families with high degree of customary consanguineous marriages. J. Invest. Dermatol. 137, 660–669 (2017). [DOI] [PubMed] [Google Scholar]
- 14.Uitto J., In this issue: Glycine substitution mutations in the COL7A1 gene: Implications for inheritance of dystrophic epidermolysis bullosa—dominant vs. recessive. Acta Derm. Venereol. 91, 259–261 (2011). [DOI] [PubMed] [Google Scholar]
- 15.Woodley D. T., Chen M., Recessive dystrophic epidermolysis bullosa: Advances in the laboratory leading to new therapies. J. Invest. Dermatol. 135, 1705–1707 (2015). [DOI] [PubMed] [Google Scholar]
- 16.Prodinger C., Reichelt J., Bauer J. W., Laimer M., Epidermolysis bullosa: Advances in research and treatment. Exp. Dermatol. 28, 1176–1189 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Marinkovich M. P., Tang J. Y., Gene therapy for epidermolysis bullosa. J. Invest. Dermatol. 139, 1221–1226 (2019). [DOI] [PubMed] [Google Scholar]
- 18.Dourado Alcorte M., Sogayar M. C., Demasi M. A., Patent landscape of molecular and cellular targeted therapies for recessive dystrophic epidermolysis bullosa. Expert Opin. Ther. Pat. 29, 327–337 (2019). [DOI] [PubMed] [Google Scholar]
- 19.Bonafont J., et al. , Clinically relevant correction of recessive dystrophic epidermolysis bullosa by dual sgRNA CRISPR/Cas9-mediated gene editing. Mol. Ther. 27, 986–998 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Osborn M. J., et al. , Base editor correction of COL7A1 in recessive dystrophic epidermolysis bullosa patient-derived fibroblasts and iPSCs. J. Invest. Dermatol., 10.1016/j.jid.2019.07.701 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Takashima S., et al. , Efficient gene reframing therapy for recessive dystrophic epidermolysis bullosa with CRISPR/Cas9. J. Invest. Dermatol. 139, 1711–1721.e4 (2019). [DOI] [PubMed] [Google Scholar]
- 22.Hainzl S., et al. , COL7A1 editing via CRISPR/Cas9 in recessive dystrophic epidermolysis bullosa. Mol. Ther. 25, 2573–2584 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Webber B. R., et al. , CRISPR/Cas9-based genetic correction for recessive dystrophic epidermolysis bullosa. NPJ Regen. Med., 10.1038/npjregenmed.2016.14 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Izmiryan A., et al. , Ex vivo COL7A1 correction for recessive dystrophic epidermolysis bullosa using CRISPR/Cas9 and homology-directed repair. Mol. Ther. Nucleic Acids 12, 554–567 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hirsch T., et al. , Regeneration of the entire human epidermis using transgenic stem cells. Nature 551, 327–332 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bauer J. W., et al. , Closure of a large chronic wound through transplantation of gene-corrected epidermal stem cells. J. Invest. Dermatol. 137, 778–781 (2017). [DOI] [PubMed] [Google Scholar]
- 27.Eichstadt S., et al. , Phase 1/2a clinical trial of gene-corrected autologous cell therapy for recessive dystrophic epidermolysis bullosa. JCI Insight 4, 130554 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]