Abstract
STUDY QUESTION
Can kisspeptin treatment induce gonadotrophin responses and ovulation in preclinical models and anovulatory women with polycystic ovary syndrome (PCOS)?
SUMMARY ANSWER
Kisspeptin administration in some anovulatory preclinical models and women with PCOS can stimulate reproductive hormone secretion and ovulation, albeit with incomplete efficacy.
WHAT IS KNOWN ALREADY
PCOS is a prevalent, heterogeneous endocrine disorder, characterized by ovulatory dysfunction, hyperandrogenism and deregulated gonadotrophin secretion, in need of improved therapeutic options. Kisspeptins (encoded by Kiss1) are master regulators of the reproductive axis, acting mainly at GnRH neurons, with kisspeptins being an essential drive for gonadotrophin-driven ovarian follicular maturation and ovulation. Altered Kiss1 expression has been found in rodent models of PCOS, although the eventual pathophysiological role of kisspeptins in PCOS remains unknown.
STUDY DESIGN, SIZE, DURATION
Gonadotrophin and ovarian/ovulatory responses to kisspeptin-54 (KP-54) were evaluated in three preclinical models of PCOS, generated by androgen exposures at different developmental windows, and a pilot exploratory cohort of anovulatory women with PCOS.
PARTICIPANTS/MATERIALS, SETTING, METHODS
Three models of PCOS were generated by exposure of female rats to androgens at different periods of development: PNA (prenatal androgenization; N = 20), NeNA (neonatal androgenization; N = 20) and PWA (post-weaning androgenization; N = 20). At adulthood (postnatal day 100), rats were subjected to daily treatments with a bolus of KP-54 (100 μg/kg, s.c.) or vehicle for 11 days (N = 10 per model and treatment). On Days 1, 4, 7 and 11, LH and FSH responses were assessed at different time-points within 4 h after KP-54 injection, while ovarian responses, in terms of follicular maturation and ovulation, were measured at the end of the treatment. In addition, hormonal (gonadotrophin, estrogen and inhibin B) and ovulatory responses to repeated KP-54 administration, at doses of 6.4–12.8 nmol/kg, s.c. bd for 21 days, were evaluated in a pilot cohort of anovulatory women (N = 12) diagnosed with PCOS, according to the Rotterdam criteria.
MAIN RESULTS AND THE ROLE OF CHANCE
Deregulated reproductive indices were detected in all PCOS models: PNA, NeNA and PWA. Yet, anovulation was observed only in NeNA and PWA rats. However, while anovulatory NeNA rats displayed significant LH and FSH responses to KP-54 (P < 0.05), which rescued ovulation, PWA rats showed blunted LH secretion after repeated KP-54 injection and failed to ovulate. In women with PCOS, KP-54 resulted in a small rise in LH (P < 0.05), with an equivalent elevation in serum estradiol levels (P < 0.05). Two women showed growth of a dominant follicle with subsequent ovulation, one woman displayed follicle growth but not ovulation and desensitization was observed in another patient. No follicular response was detected in the other women.
LIMITATIONS, REASONS FOR CAUTION
While three different preclinical PCOS models were used in order to capture the heterogeneity of clinical presentations of the syndrome, it must be noted that rat models recapitulate many but not all the features of this condition. Additionally, our pilot study was intended as proof of principle, and the number of participants is low, but the convergent findings in preclinical and clinical studies reinforce the validity of our conclusions.
WIDER IMPLICATIONS OF THE FINDINGS
Our first-in-rodent and -human studies demonstrate that KP-54 administration in anovulatory preclinical models and women with PCOS can stimulate reproductive hormone secretion and ovulation, albeit with incomplete efficacy. As our rat models likely reflect the diversity of PCOS phenotypes, our results argue for the need of personalized management of anovulatory dysfunction in women with PCOS, some of whom may benefit from kisspeptin-based treatments.
STUDY FUNDING/COMPETING INTEREST(S)
This work was supported by research agreements between Ferring Research Institute and the Universities of Cordoba and Edinburgh. K.S. was supported by the Wellcome Trust Scottish Translational Medicine and Therapeutics Initiative (STMTI). Some of this work was undertaken in the MRC Centre for Reproductive Health which is funded by the MRC Centre grant MR/N022556/1. M.T.-S. is a member of CIBER Fisiopatología de la Obesidad y Nutrición, which is an initiative of Instituto de Salud Carlos III. Dr Mannaerts is an employee of Ferring International PharmaScience Center (Copenhagen, Denmark), and Drs Qi, van Duin and Kohout are employees of the Ferring Research Institute (San Diego, USA). Dr Anderson and Dr Tena-Sempere were recipients of a grant support from the Ferring Research Institute, and Dr Anderson has undertaken consultancy work and received speaker fees outside this study from Merck, IBSA, Roche Diagnostics, NeRRe Therapeutics and Sojournix Inc. Dr Skorupskaite was supported by the Wellcome Trust through the Scottish Translational Medicine and Therapeutics Initiative 102419/Z/13/A. The other authors have no competing interest.
Keywords: kisspeptin, polycystic ovary syndrome, gonadotrophins, ovulation, ovarian stimulation, preclinical models
Introduction
Polycystic ovary syndrome (PCOS) is the most common endocrinopathy in women of reproductive age (Sirmans and Pate 2013), with a prevalence of 8 to 13% (Franks 1995; Fauser et al. 2012; Teede et al. 2018b), which may rise to 20% depending on the population and diagnostic criteria (Sirmans and Pate 2013). The three cardinal manifestations of PCOS are ovulatory dysfunction, with irregularity or absence of menstrual cyclicity and oligo/anovulation; clinical and/or biochemical hyperandrogenism; and appearance of polycystic ovaries, with a diagnosis being made if two out of the three criteria are met (Rotterdam 2004; Teede et al. 2018b). In addition, sub/infertility is commonly observed (Gorry et al. 2006). Patients with PCOS frequently also suffer neuroendocrine and metabolic abnormalities, which impact on the clinical phenotype. These include derangements in gonadotrophin secretion with LH hyperpulsatility, likely reflecting perturbations in GnRH neuronal activity (Rebar et al. 1976; Sullivan and Moenter 2004; Blank et al. 2006; Goodarzi et al. 2011). In addition, up to 50% of women with PCOS fulfill the criteria for metabolic syndrome (Balen et al. 1995; Gambineri et al. 2002; Dunaif 1997; Sir-Petermann et al. 2009) and they have a markedly higher risk of developing type 2 diabetes (Glintborg 2016).
Accordingly, PCOS has considerable heterogeneity in medical presentation (Ehrmann, Barnes, and Rosenfield 1995; Goodarzi et al. 2011; Glintborg 2016), resulting in variable clinical phenotypes. This likely reflects a diverse and possibly multifactorial pathophysiology, in which genetic—as revealed by recent genome-wide association studies (GWAS)—and environmental factors are known to contribute, but whose underpinnings and relative importance remain unclear in most cases (Goodarzi et al. 2011; Goodarzi et al. 2012; Walters et al. 2012; Hayes et al. 2015; McAllister et al. 2015). The relevance of hyperandrogenism is documented by genetic, clinical and experimental studies that have documented the pivotal pathogenic role of androgen excess in the generation and perpetuation of the syndrome (Abbott et al. 2005; Goodarzi et al. 2011; Walters et al. 2012), as epitomized by the contribution of genetic variants of DENN domain-containing 1 (DENND1A), which is expressed in androgen-producing theca cells of the ovary and whose overexpression causes hyperandrogenism and a PCOS phenotype (McAllister et al. 2014; McAllister et al. 2015). Furthermore, sheep and non-human primate models of prenatal exposure to androgens display striking similarities with the clinical manifestations of women with PCOS (Abbott et al. 2005; Padmanabhan and Veiga-Lopez 2013), suggesting a developmental origin of the disease, based on the early mal-programming of key neuroendocrine (and possibly metabolic) pathways caused by high androgen levels. Likewise, the daughters of PCOS mothers, exposed to hyperandrogenism in utero, are prone to develop reproductive and metabolic alterations similar to PCOS (Sir-Petermann et al. 2009).
Studies on the impact of androgen exposure in various rodent species have also allowed recapitulation of some of the cardinal manifestations of PCOS (Walters et al. 2012; Caldwell et al. 2014), with variable or incomplete replication of the reproductive and metabolic features, depending on the developmental window of exposure and the androgen used (Walters et al. 2012; Caldwell et al. 2014). While no single rodent model of androgenization fully recapitulates the repertoire of alterations seen in women with PCOS (Walters et al. 2012; Caldwell et al. 2014), analysis of a combination of models might theoretically allow better targeting of specific clinical phenotypes (e.g. lean versus obese PCOS, PCOS with or without biochemical hyperandrogenism), thus permitting a more selective and potentially translational modeling of the different facets of this complex condition. They also overcome some of the limitations of use of more complex and ethically sensitive models, such as non-human primates (Walters et al. 2012).
Recent preclinical data suggest that the mechanisms whereby androgen excess contributes to the different components of PCOS are, to a large extent, mediated at central (neuronal) levels (Caldwell et al. 2017). These findings clearly illustrate the importance of neuroendocrine deregulation in the pathogenesis of PCOS, a contention that is further supported by recent GWAS (Hayes et al. 2015). These central components of the disease are thought to converge on GnRH neurons (Moore and Campbell 2017); however, these neurons seem to be devoid of androgen receptors (Herbison 2016), suggesting a role for intermediate pathways. Among the afferent signals to GnRH neurons, kisspeptins, the products of the Kiss1 gene, have recently emerged as master regulators of GnRH neurosecretion (Pinilla et al. 2012; Skorupskaite et al. 2014; Clarkson et al. 2017). Kiss1 neurons are crucial in mediating the feedback effects of sex steroids (Garcia-Galiano et al. 2012), which are deregulated in PCOS (Blank et al. 2006; Goodarzi et al. 2011; Moore and Campbell 2017), and they are a target of the organizing actions of gonadal steroids during key developmental periods (Garcia-Galiano et al. 2012), which are also sensitive to the disrupting actions of excessive androgen levels in various PCOS models (Kauffman et al. 2007). In keeping with this, as yet fragmentary data suggest that alterations in Kiss1 neurons, associated with pre- or postnatal exposures to androgens, may be relevant in generating PCOS-like manifestations in various rodent models (Brown et al. 2012; Yan et al. 2014; Marcondes et al. 2017; Osuka et al. 2017). However, disparate changes in hypothalamic Kiss1 expression and/or kisspeptin content have been reported depending on the model, and the actual pathophysiological relevance of developmental disruption of Kiss1 pathways remains unknown.
Due to these uncertainties in its etiology and pathophysiology, the treatment options for PCOS remain mostly symptomatic. Women seeking conception may require gonadotrophin stimulation, if treatment with first-line oral ovulation–induction agents fails (Teede et al. 2018b), but this carries a risk of poly-follicular development and multiple pregnancy, and even ovarian hyperstimulation syndrome (OHSS) (Wu et al. 2017). These features demonstrate the need for safer and more efficient protocols of ovarian stimulation. As kisspeptins are potent elicitors of the release of endogenous GnRH and thereby promote a more physiological gonadotrophin stimulus to the ovary, kisspeptin-based protocols have been recently proposed as a safer option for promoting oocyte maturation and ovulation (Jayasena et al. 2014; Abbara et al. 2015). In this context, we report herein the first proof-of-principle studies addressing the efficiency of a novel kisspeptin-based protocol of gonadotrophin and ovarian stimulation, in a series of preclinical models and a pilot cohort of anovulatory women with PCOS.
Materials and Methods
Preclinical studies
Animals and drugs
An initial pilot experiment, testing the effects of repeated kisspeptin administration in adult, cyclic female rats, was conducted at the Ferring Research Institute. To this end, Wistar rats, obtained from Charles River Laboratories (Wilmington, MA, USA), were housed three per cage, with a 12-h light/dark cycle, under controlled conditions of temperature (22 ± 2°C), and access to food and water ad libitum. This experiment was approved by the Institutional Animal Care and Use Committee (IACUC) of the Ferring Research Institute and conducted under the supervision of the Institutional Office that oversees its animal program.
For pharmacological experiments testing the effects of repeated kisspeptin administration in PCOS models, Wistar rats bred in the Animal facilities of the University of Córdoba were used. Pregnant dams were obtained by mating young cyclic females with adult males of proven fertility. The animals were housed under constant conditions of light (12-h light/dark cycles) and temperature (22 ± 2°C). The day the litters were born was considered Day 1 of age (postnatal day 1; PND1). Animals were weaned at PND21 and were provided with free access to tap water and fed ad libitum with a standard soy-free diet. The corresponding local and regional Ethical Committees (University of Cordoba and Junta de Andalusia) approved the experiments and animal protocols included in this study; all experiments were conducted in accordance with European Union normative for the use and care of experimental animals (EU Directive 2010/63/UE, September 2010).
The androgenic compounds, dihydrotestosterone (DHT) and testosterone propionate (T), were purchased from Sigma Chemical Co. (St. Louis, MO, USA). Kisspeptin-54 (KP-54) was custom-synthesized under GMP (good manufacturing practice) standards and kindly provided by Ferring Pharmaceuticals.
The different experimental groups (see next section) were generated using similar procedures of animal handling in the context of the large-scale studies addressing the impact of kisspeptin treatment on three different rat models of PCOS. In order to provide a complete characterization of the three PCOS phenotypes, the animals were periodically monitored postnatally up to adulthood (PND100), using noninvasive (external signs) and/or minimally invasive (blood markers after tail or venipuncture sampling) parameters. Thereafter, the experimental groups were subjected to protocols of daily injections of KP-54, as described in detail below. For logistic reasons, different cohorts of animals were successively generated by in-house breeding. Yet, in all series of studies, the animals were housed and handled under strictly similar conditions, thus allowing the rigorous contemporary analysis and later integration of all the data.
Experimental design: preclinical PCOS models
For preclinical analyses, we generated three different rat PCOS models, based on exposure to distinct androgens (DHT or T) at various developmental windows, as described in detail elsewhere (Pinilla et al. 2002; Sullivan and Moenter 2004; van Houten et al. 2012; Walters et al. 2012; Caldwell et al. 2014), and thoroughly validated and phenotypically characterized in our laboratory; the three models used are schematically depicted in Supplementary Fig. S1. First, a model of prenatal androgenization (PNA) was produced based on previously published protocols in mice (Sullivan and Moenter 2004; Moore et al. 2015), which were adapted and validated by us in pregnant rats, as defined in Supplementary Table SI. Based on these pilot studies, a protocol defined by daily s.c. injections of timed pregnant rats with a fixed dose of DHT (3 mg/day), between gestational days 16 and 19, was used; the female offspring (N = 20) of androgenized mothers was analyzed as the PNA model of PCOS. Dams injected with vehicle (olive oil) served to produce the control (non-androgenized) group. Second, a model of neonatal androgenization (NeNA) was produced by direct s.c. injection of female rats on PND1 with a single 100-μL bolus of 1.25 mg of T (N = 20). Selection of this PCOS model was based on previous studies from our lab (Pinilla et al. 2002), and initial phenotypic characterization of its major reproductive and metabolic features, as illustrated in Supplementary Fig. S2. PND1 females injected with olive oil were used as controls. Finally, a model of persistent postnatal androgenization was generated, following previous literature in mice (van Houten et al. 2012), by implanting female rats at the day of weaning (PND21) with silastic capsules filled with DHT (2.5 mg/capsule; inner diameter, 1.98 mm; outer diameter, 3.18 mm; Dow Corning, Seneffe, Belgium). This dose of DHT was selected after initial validation experiments, summarized in Supplementary Fig. S3, using a 3-fold higher dose of DHT (7.5 mg/capsule), which caused persistently elevated DHT but massively suppressed basal and kisspeptin-stimulated LH levels. Rats were followed for 90 days after DHT implants (N = 20), as the model of post-weaning androgenization (PWA).
Somatic, reproductive and metabolic indices were evaluated in the above groups, including body weight (BW) gain during the course of the study; vaginal opening (VO), as consensus external marker of puberty in female rats; estrous cyclicity in adulthood; basal serum LH and FSH levels; basal insulin and glucose levels; and body fat composition (Fat)—the latter was conducted by quantitative magnetic resonance, using the EchoMRI™ 700 analyzer (Houston, TX, USA software v.2.0), using validated protocols at our laboratory (Velasco et al. 2019). Based on previous normative data on the timing of puberty in the rat, VO was monitored daily between PND26 and 38 postpartum. In addition, estrous cyclicity was assessed following routine procedures in our lab (Gaytan et al. 2005; Pineda et al. 2010), by cytological analysis of vaginal smears, taken daily for 32 consecutive days (equivalent to eight complete cycles), from PND60 onwards. For operational reasons, vaginal cytology was only conducted in rats/experimental groups showing VO. In order to limit invasiveness and potential interference prior to pharmacological stimulation, basal LH, FSH, glucose and insulin levels, as well as body composition, were monitored at single-point determinations in adult animals (between PND80 and 100) before initiation of kisspeptin treatments, as schematically depicted in Supplementary Fig. S1. Blood sampling for hormonal monitoring was conducted between 9:00 and 10:00 a.m.; for animals displaying estrous cyclicity, sampling was carried out in diestrus 1.
Protocol of kisspeptin stimulation
Gonadotrophin and ovarian responses to a standard protocol of repeated KP-54 stimulation were monitored in the three PCOS models: PNA, NeNA and PWA. Prior to those studies, a pilot experiment involving daily injections of KP-54 or vehicle to control, cyclic female rats (N = 7) was conducted for reference and dose-validation purposes. In this initial experiment, duration of treatment was set to 5 days and the dose of KP-54 was 100 μg/kg BW (equivalent to 17 nmol/kg); blood samples were drawn each day, before and at 60, 120 and 240 min after KP-54 injection. The dose of KP-54 selected was in keeping with our previous studies addressing the gonadotropic effects of systemic administration of kisspeptin-10 and -54 (Navarro et al. 2005a; Navarro et al. 2005b; Tovar et al. 2006) and supported by detailed pharmacokinetic (PK) studies. Based on the results of this pilot experiment, PND100 rats of the three PCOS groups received daily s.c. injections of 100 μg/kg of KP-54 (N = 10 per PCOS model) or 0.9% saline (N = 10 per model) as vehicle, with daily injections being applied at 9:00–10:00 a.m. Considering the length of the rat ovarian cycle and the main objectives of the study (namely, to analyze gonadotropic and ovarian responses in different PCOS models, including rescue of ovulation), pharmacological treatments in the three models were applied for a period of 11 days (up to PND110). On Days 1 (first day of treatments), 4, 7 and 11, blood samples (250 μL) were collected at basal and 30, 60, 120 and 240 min following KP-54 or vehicle injections. Serum was separated by centrifugation and stored at −20°C until measurement of LH and FSH. Basal gonadotrophin levels were measured in serum samples taken 1 h before KP-54 injection. In addition, ovarian and uterus tissues were collected upon decapitation of the animals after completion of KP-54 injections for 11 days.
Ovarian tissue processing and morphometric analyses of follicles and ovulation
The ovaries were fixed in Bouin solution for at least 24 h, processed for paraffin embedding, serially sectioned (10 μm thick) and stained with hematoxylin and eosin. Small follicles, measuring less than 200 μm in diameter, were divided into resting follicles, corresponding to primordial follicles and early primary follicles (measuring up to 30 μm in diameter, and non-growing oocyte), primary follicles (with a layer of cuboidal granulosa cells and growing oocyte) and secondary follicles (with two or more layers of granulosa cells). The number of small follicles per ovary was obtained by using a systematic random sampling procedure. For this, every 10th section was scored and the number of resting and primary follicles containing the oocyte nucleus, as well as the number of secondary follicles containing the oocyte nucleolus, was recorded. As only 1 out of 10 sections was scored, the final number of follicles per ovary was obtained by multiplying by 10.
Counting of follicles measuring >200 μm was performed by scoring all sections, and counting the follicles only when the oocyte nucleolus was present in the section. Follicles were considered as atretic when displaying apoptotic granulosa cells and/or oocyte degeneration, in line with standard histological procedures (Osman 1985). In detail, antral follicles were cataloged as atretic when showing pyknosis (i.e. chromatin condensation in the nucleus) in granulosa cells: more than three cells with pyknotic nuclei per follicle section at early atretic stages, or numerous pyknotic cells through the whole granulosa layer, together with morphological alterations of the oocyte, at more advanced stages (Osman 1985). Previous studies in rats have reported that even early atretic follicles, displaying only few pyknotic nuclei but otherwise normal granulosa cells, show a low number of proliferating cells throughout the granulosa layer, thus indicating arrested follicle growth (Gaytan et al. 1996).
For semi-quantitative assessment of follicular maturation and ovulation in the different treatment groups, a scoring method, adapted from our recently published protocol for dating of puberty in rodents, was used. Histological analyses of the ovaries were conducted after completion of the 11-day treatment with KP-54 in the three PCOS models (PNA, NeNA and PWA). Depending on initial morphometric data and optimally fixed tissue availability, ovaries from 5–10 independent animals were studied per model. Equivalent analyses were conducted in control, cycling female rats. Scoring was based on the identification of the most advanced healthy antral follicle class (classes F1 to F5), combined with dating of the corpora lutea of the current cycle, in order to monitor whether ovulation has occurred during the treatment period and the time elapsed since the last ovulation. Counting of corpora lutea was conducted in serial sections of one ovary per animal. In cycling animals, including PNA rats, only the youngest generation of corpora lutea, corresponding to the current cycle, was counted. For further details, see Gaytan et al. (2017).
Hormonal and metabolic measurements
LH and FSH levels were measured using RIA kits from the National Hormone Peptide Program (Torrance, CA, USA), with reference preparations LH-RP-3 and FSH-RP-2, and intra- and inter-assay coefficients of variation below 8 and 10%, respectively. Leptin and insulin levels were measured using RIA kits from Linco Research; sensitivity was 0.5 (leptin) and 0.1 (insulin) ng/mL.
Glucose and insulin tolerance tests
Glucose and insulin tolerance tests (GTT and ITT) were applied to adult female rats of the PNA, NeNA and PWA models after a 12-h period of overnight fasting. To limit the potential interference on the effects of kisspeptin administration on ovarian parameters, GTT and ITT were conducted prior to the initiation of the pharmacological treatments, as depicted in Supplementary Fig. S1. Basal glucose levels were recorded 1 h before glucose overload. A bolus of glucose (1 g/kg BW) or insulin (1 U/kg BW) was applied by i.p. injection and glucose concentrations measured at 20 and 60 min. Glucose levels were assayed using a glucometer (Accu-Chek; Roche Diagnostics, Barcelona, Spain).
Presentation of data and statistics
Hormonal determinations were conducted in duplicate, with a total number of 7–10 samples per time-point and group, depending on final availability of serum samples. When appropriate, in addition to individual time-point measurements, integrated LH and FSH secretory responses were calculated as the area under the curve (AUC), following the trapezoidal rule. Hormonal data are presented as mean ± SEM. Results were analyzed using Student t test or ANOVA followed by Student–Newman–Keuls multiple-range tests (Prism GraphPad 5.0 software; GraphPad Software Inc., La Jolla, CA, USA). Significance level was set at P ≤ 0.05, and different letters or asterisks indicate statistical significance.
Clinical studies
Patients
Otherwise healthy women aged 18–42 years and with BMI 18–32 kg/m2 with PCOS were recruited. PCOS was diagnosed based on the Rotterdam criteria (Rotterdam 2004), with oligomenorrhea/amenorrhea for at least 1 year and at least one of hyperandrogenism or hyperandrogenemia (above laboratory normal of total testosterone i.e. >1.9 nmol/L, measured by mass spectrometry) or free androgen index (>6.5), or polycystic appearance of ovaries (as defined by Rotterdam criteria), in the absence of other causes. No hormonal treatment had been taken for at least 2 months. The study had ethical committee approval (South East Scotland Research Ethics Committee, ref 15/SS/0029), and all women gave informed consent.
Protocols of kisspeptin stimulation
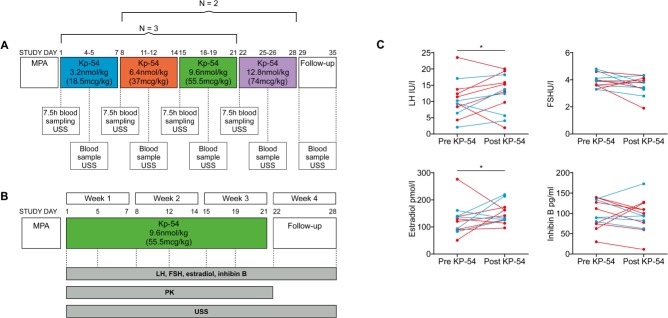
Medroxyprogesterone acetate (10 mg bd for 7 days, Pfizer, Tadworth, UK) was administered to induce a withdrawal bleed. Women were administered GMP grade KP-54 (Bachem GmbH, Weil am Rhein, Germany) s.c. twice daily for 21 days, starting on Days 2–4 of menses, if transvaginal ultrasound examination on that day showed no follicles of ≥10 mm diameter.
Two treatment regimens were used. For the first five women, the initial dose was 3.2 nmol/kg s.c. twice daily, administered for 7 days, with twice weekly monitoring. Blood sampling via a peripheral cannula was performed for 30 min before and for 7 h after the first s.c. injection of KP-54 to determine the extent and duration of gonadotrophin secretion after KP-54 administration, and to obtain samples for PK evaluation. KP-54 was administered at 0 min into the lower anterior abdominal wall, and blood sampled for serum LH at −30-, −15- and 0-min intervals before administration of KP-54, and at 10-, 20-, 30-, 60-, 90- and 120-min intervals followed by hourly sampling over the 7-h period after administration of KP-54. If there was no evidence of a response (defined as the development of a follicle ≥12 mm) on Day 8, the dose was increased to 6.4 nmol/kg s.c, bd. This dose was administered for a further 7 days and increased to 9.6 nmol/kg s.c. bd for a third period of 7 days if there was no evidence of a response. The dose of 9.6 nmol/kg s.c. bd had no effect on follicular development in the first three subjects, so for the subsequent two subjects the dose escalation regimen started at 6.4 nmol/kg s.c. bd, increasing to 9.6 nmol/kg s.c. bd and to 12.8 nmol/kg s.c. bd at 7-day intervals with assessments as described. If at the end of any 7-day treatment period there was evidence of a response, women were reassessed (ultrasound and blood sampling for reproductive hormones) twice weekly until the outcome of that follicle was apparent i.e. either atresia or ovulation had occurred.
A second group of seven women were treated with 9.6 nmol/kg s.c. twice daily for 21 days. Study Day 1 followed progestagen-induced menses as before: women attended for ultrasound, single-point blood sampling for reproductive hormones, followed by administration of the first dose of KP-54. Thereafter, women were reviewed twice weekly, with ultrasound examination and venesection for reproductive hormones. If a response was detected, monitoring continued until the outcome of that follicle development was apparent with a further assessment of 7 days after disappearance of any dominant follicle (i.e. presumed ovulation) with repeat ultrasound and hormone assessment. Women were followed up by telephone to ascertain the date of subsequent menses.
Hormonal analyses
Serum samples were stored at −20°C until analysis. LH and FSH were measured by in-house ELISA using monoclonal antibodies (Medix Biochemica, Espoo, Finland), and inhibin B was assayed also by ELISA (Beckman Coulter High Wycombe, UK). Inter-assay and intra-assay coefficients of variation were <5% for the LH, FSH and inhibin B assays. Estradiol and progesterone were determined by ELISA (Demeditec Diagnostics GmbH, Kiel, Germany). Inter-assay and intra-assay coefficients of variation are 10.5 and 8% for the estradiol assay, and 7.5 and 6.5% for the progesterone assay, respectively.
PK analyses
For PK analysis sample collection, blood samples were collected into lithium-heparin tubes containing protease inhibitor cocktail (Sigma P8340) on ice. Samples were immediately centrifuged, and plasma stored at −20°C or lower until shipped to Ferring Research Institute (San Diego) for analysis. Bioanalysis included compound extraction followed by standard lipid chromatography–mass spectrometry methods to determine Kisspeptin-54 concentration in each samples. Analyte concentration was calculated from internal standard-normalized peak areas and calibration curves. The dynamic range of the assays was 0.25–500 ng/mL.
Statistical analyses
Hormone concentrations were analyzed by repeated-measures ANOVA after log transformation (Prism v7 for Mac, GraphPad Software, San Diego, CA, USA). Significance level was set at P ≤ 0.05.
Results
Preclinical studies
Preclinical studies included the use of three models of androgenization of female rats, targeting different developmental windows of androgen excess, as adapted from previous literature (Pinilla et al. 2002; Sullivan and Moenter 2004; van Houten et al. 2012; Walters et al. 2012; Caldwell et al. 2014). These models were PNA (for prenatal androgenization); NeNA (for neonatal androgenization); and PWA (for post-weaning androgenization). A combination of models was investigated as a means to replicate at least some of the pathophysiological substrate (i.e. androgen excess) and different phenotypes of PCOS. These models are described in detail in the Methods section and schematically depicted in Supplementary Fig. S1. Notably, selection of these models was based on thorough validation experiments in our laboratory, which are summarized in Supplementary Figs S2 and S3, and Supplementary Table SI.
Basic reproductive and metabolic features in preclinical models of PCOS
Phenotypic analyses of major reproductive indices of the three PCOS models revealed that PNA rats displayed perturbed VO, denoting pubertal onset, with variable prevalence. Thus, while all controls had VO by PND36, 22 out of 44 PNA rats failed to complete VO (50%). Moreover, the age of vaginal canalization in the subgroup of PNA rats with VO was partially delayed (control: 34.0 ± 0.14 PND versus PNA: 36.4 ± 0.18 PND; P < 0.01). In contrast, none of the NeNA rats showed VO, in line with previous literature (Pinilla et al. 2002), whereas in the PWA group, since DHT administration started post-weaning, all the animals showed complete VO. Monitoring of estrous cyclicity by means of vaginal smears in adulthood revealed that PNA rats with VO had grossly preserved cyclicity, with regular succession of estrous cycle phases at adult age, while PWA rats were completely acyclic at adulthood, with vaginal cellularity indicating persistent diestrus. Vaginal smears were not accessible in NeNA rats, as they did not show canalization of the vagina at any age. Anyhow, evidence for acyclicity and anovulation in this model was obtained by ovarian morphometric analyses (see below).
PNA rats remained lean as adults and did not show significant alterations in basic reproductive parameters (including LH levels, and ovarian and uterus weights (OW, UW)), except for a decrease in basal FSH levels (Fig. 1, upper panels). In contrast, NeNA rats were moderately heavier than controls and displayed a significant suppression of basal LH concentrations, as well as OW and UW, without changes in basal FSH levels (Fig. 1, middle panels). In addition, the absence of corpora lutea denoted lack of ovarian cyclicity and anovulation. Similarly, PWA rats showed increased terminal BW and markedly suppressed LH levels, together with lower OW and UW, as well as FSH concentrations (Fig. 1, lower panels). No corpus luteum was detected in ovarian sections of PWA rats, as reflection of their anovulatory state. Thus, there seems to be a gradual impact of developmental androgenization on the above reproductive indices, with the following scheme: PNA << NeNA < PWA.
Figure 1.
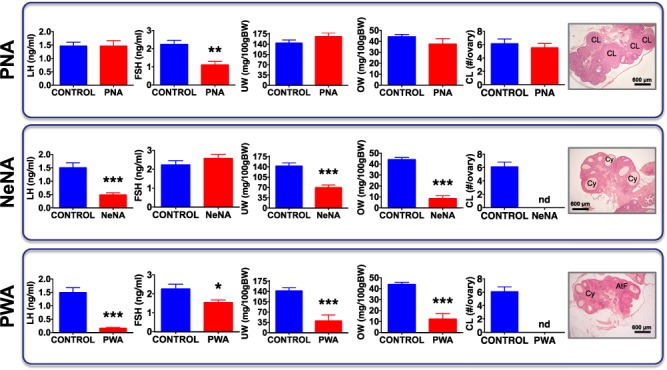
Baseline reproductive features of the three preclinical rat models of PCOS. Major reproductive features are presented from the three preclinical models of polycystic ovary syndrome (PCOS) (PNA: prenatal androgenization, NeNA: neonatal androgenization and PWA: post-weaning androgenization) at adulthood, prior to pharmacological intervention. Bar graphs represent data on serum LH and FSH levels, uterus weight (UW), ovarian weight (OW) and number of fresh corpora lutea (i.e. of the current cycle) in a representative subset of randomly selected animals (N = 10/group) of the three models. Data from the corresponding non-androgenized control animals are also shown. In addition, representative histological images of ovarian sections from the three PCOS models are presented. Note that, like control cycling females, PNA rats showed several generations of corpora lutea, as sign of ovulation. In contrast, corpora lutea were absent in NeNA and PWA rats, which presented atretic follicles that at advanced stages appeared as follicular cysts. Each histogram represents the mean ± SEM. Data were analyzed by Student t tests (*P < 0.05, **P < 0.01, ***P < 0.001 versus corresponding control groups). CL: corpus luteum; Cy: cyst-like structures; AtF: atretic follicle. Scale bar: 600 μm.
Further phenotypic characterization of the PCOS models included morphometric analyses at the ovarian level, with a particular focus on small follicles. No significant changes in the total number of small follicles (<200 μm in diameter), neither in the abundance of resting, primary or secondary follicles, were detected in PNA rats (Supplementary Fig. S4, upper panels). In clear contrast, both NeNA and PWA rats displayed an increase in the total number of small follicles, with more primordial and primary follicles but fewer secondary follicles (Supplementary Fig. S4, mid and lower panels). In addition, the ovaries of adult PNA rats presented large preovulatory follicles, as well as corpora lutea, indicating spontaneous ovulation. Conversely, progression to the preovulatory stage was arrested in the ovaries of adult NeNA and PWA rats, which did not show corpora lutea, indicating lack of spontaneous ovulation. In fact, NeNA and PWA rats presented numerous atretic follicles that, at advanced stages, adopted a cyst-like appearance (Fig. 1). This phenomenon was not detected in PNA rats.
Metabolic analyses revealed that, in keeping with final BW data, PNA rats showed a postnatal BW gain profile similar to that of controls. However, they showed a modest but significant increase in body fat content in adulthood, together with perturbed glucose tolerance and partial insulin resistance, as revealed by GTT and ITT (Fig. 2, upper panels). NeNA rats became heavier than control females from PND90 onwards, with a significant increase in body fat and altered GTT and ITT responses (Fig. 2, mid panels). PWA rats were also heavier than controls, with the increase in total BW already detectable at PND50 and a marked increase in body fat content, together with significantly perturbed GTT and ITT responses (Fig. 2, lower panels). We thus observed a gradual impact of the different treatments, in this case on the metabolic profile, with the same scheme: PNA << NeNA < PWA.
Figure 2.
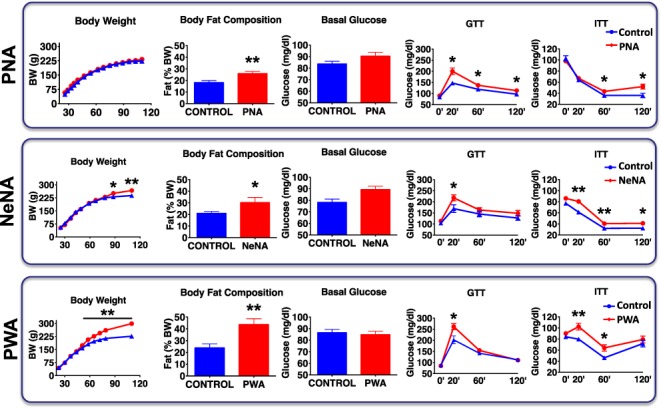
Baseline metabolic features of the three preclinical rat models of PCOS. Major metabolic indices are presented in a representative subset of randomly selected animals (N = 10/group) of the three preclinical models of PCOS (PNA, NeNA and PWA) at adulthood, prior to pharmacological intervention. Graphs correspond to data of body weight (BW) evolution, body fat composition (% Fat), basal glucose levels, glucose tolerance tests (GTT) and insulin tolerance tests (ITT). Data from the corresponding non-androgenized control animals are also show. Data are presented as the mean ± SEM. Data were analyzed by Student t tests or ANOVA followed by Student–Newman–Keuls tests (*P < 0.05, **P < 0.01 versus corresponding control groups).
Gonadotrophin responses to KP-54 stimulation in preclinical models of PCOS
The effects of daily boluses of kisspeptin-54 (KP-54; 100 μg/kg BW) on gonadotrophin secretory profiles in the above PCOS models were studied at different time intervals, over a total period of 11 days of administration. These pharmacological experiments were based on a pilot study addressing the effects of daily injections of KP-54 in control, cyclic female rats, for a period of 5 days. The results from this pilot study, summarized in Supplementary Fig. S5, unambiguously demonstrated robust LH and FSH responses to such KP-54 stimulation, with variable desensitization over the 5-day period. For operational reasons, related to the inherent variability associated with spontaneous cyclicity, and the main focus of the study being the pharmacological experiments in PCOS models, in these experiments, gonadotrophin responses were monitored only in a reference group of control cyclic rats after a single bolus of KP-54 (1-D).
In PNA rats, LH responses to acute administration of KP-54 (on Day 1 of the treatment; 1-D) were grossly similar to those of control rats, with a massive secretory response that peaked between 60 (PNA) and 120 (control) min, and decreased to basal levels thereafter. Repeated administration of daily boluses of KP-54 resulted in significant LH responses that were detected at Day 4 (4-D), Day 7 (7-D) and Day 11 (11-D) after the initiation of KP-54 treatment, although the LH secretory response was progressively smaller (Fig. 3, upper panels). In NeNA rats, acute LH secretory responses to KP-54 were largely preserved, although the peak values at 120 min were partially attenuated versus controls. As with PNA rats, repeated injections of KP-54 resulted in a significant LH response at all time-points but with partial desensitization of LH responses over the study period (Fig. 3, mid panels). Finally, PWA rats showed significant, but markedly blunted, LH responses to KP-54 injection on 1-D, and a complete desensitization of LH responses after repeated administration of KP-54 was observed, with no detectable response on 4-D, 7-D and 11-D of treatment (Fig. 3, lower panels).
Figure 3.
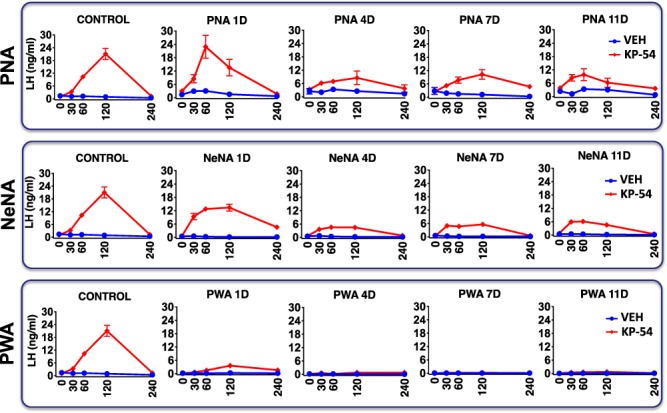
LH responses to KP-54 in the three preclinical rat models of PCOS. Profiles of serum LH responses to kisspeptin-54 (KP-54) administration are presented from the three preclinical models of PCOS (PNA, NeNA and PWA) at adulthood. The animals were injected for 11 days with a daily bolus of KP-54 (100 μg/kg; N = 10 per PCOS model) or 0.9% saline (N = 10 per model), and serial blood sampling was conducted on Days 1, 4, 7 and 11 of treatment, just before (0) or at the indicated time intervals after s.c. KP-54 injection. Androgenized animals from each PCOS model, injected with vehicle, were run in parallel for comparative purposes. In addition, the profiles of LH responses to a single bolus of KP-54 or vehicle in control (non-androgenized) female rats are also shown in the left panels. Data are presented as the mean ± SEM. PNA 1D: postnatal day 1.
The profiles of FSH responses to repeated KP-54 administration were also markedly different across the PCOS models, and from LH profiles. In control female rats, a single bolus of KP-54 evoked a moderate FSH response, with an overall increase of 2-fold over basal levels. In PNA rats, acute FSH responses to KP-54 on 1-D were fully preserved, and even moderately increased, with persistently elevated FSH levels over the 240-min period following KP-54 administration. However, complete desensitization of the effect on FSH release took place upon repeated injections of KP-54, so that no increase in circulating FSH was detectable on 4-D, 7-D or 11-D after initiation of KP-54 treatment (Fig. 4, upper panels). In clear contrast, in NeNA and PWA rats, FSH secretory responses to KP-54 injections were massively elevated on 1-D, with >5-fold increase in peak secretory levels at 120 min. Moreover, blunted but significant increases in FSH concentrations were detected after KP-54 injections in both models of postnatal androgenization, on 4-D, 7-D and 11-D after beginning of KP-54 treatments (Fig. 4, mid and lower panels).
Figure 4.
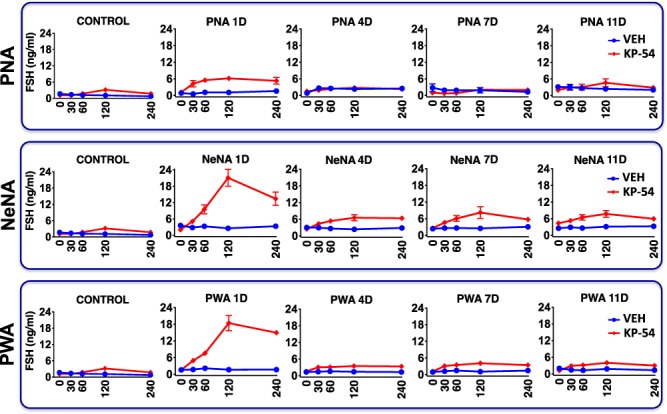
FSH responses to KP-54 in the three preclinical rat models of PCOS. Profiles of serum FSH responses to KP-54 administration are presented from the three preclinical models of PCOS (PNA, NeNA and PWA) at adulthood. The animals were injected for 11 days with a daily bolus of KP-54 (100 μg/kg; N = 10 per PCOS model) or 0.9% saline (N = 10 per model), and serial blood sampling was conducted on Days 1, 4, 7 and 11 of treatment, just before (0) or at the indicated time intervals after s.c. KP-54 injection. Androgenized animals from each PCOS model, injected with vehicle, were run in parallel for comparative purposes. In addition, the profiles of LH responses to a single bolus of KP-54 or vehicle in control (non-androgenized) female rats are also shown in the left panels. Data are presented as the mean ± SEM.
In addition to time-course profiles, net (integral) LH and FSH responses to KP-54 were calculated for each PCOS model as AUC during the 240-min following KP-54 injection. To ease comparison of net LH and FSH responses to KP-54 administration across the three PCOS models used, AUC data are presented in a single figure (Fig. 5). Robust AUC LH responses to KP-54 were detected in PNA rats on 1-D, with a rather moderate decay in the magnitude of responses over the duration of the treatment. In NeNA rats, acute (1-D) net LH responses to KP-54 were similar to those of the PNA model, but were significantly smaller thereafter, on 4-D, 7-D and 11-D. Finally, in PWA rats, acute AUC LH responses to KP-54 were already significantly attenuated on 1-D, while they were completely absent at later day-points during KP-54 treatment. In the case of FSH, net acute increases were detected on 1-D following bolus KP-54 injection in all the models; these responses were exaggerated in NeNA and PWA rats. In addition, AUC FSH responses were significantly higher in NeNA than in PNA and PWA rats at later time-points (4-D, 7-D and 11-D) of the KP-54 treatment.
Figure 5.
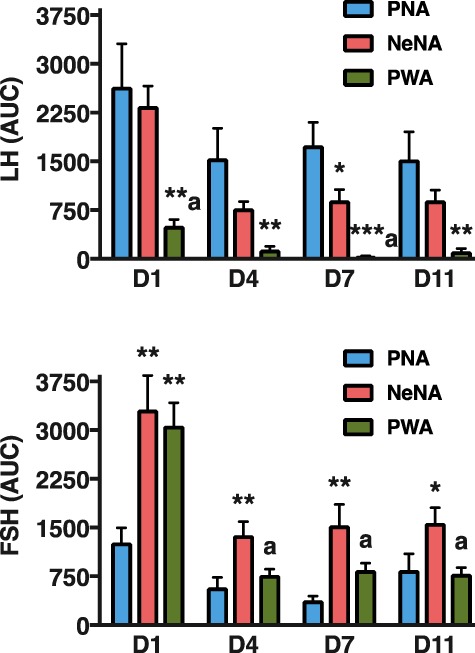
Integral gonadotrophin responses to KP-54 in the three preclinical rat models of PCOS. Integral LH (upper panel) and FSH (lower panel) responses to KP-54 administration are presented from the three preclinical models of PCOS (PNA, NeNA and PWA; N = 10 per model) at adulthood. Integral responses were calculated as AUC over the 240-min period following KP-54 administration, for each indicated day of treatment. Data in these graphs are the AUC values of the hormonal profiles presented in Figs 3 and 4. Data are presented as the mean ± SEM. Data were analyzed by ANOVA followed by Student–Newman–Keuls tests (**P < 0.01 versus corresponding PNA values; aP < 0.01 versus corresponding NeNA values).
Ovarian responses to KP-54 stimulation in preclinical models of PCOS
Detailed morphometric analyses were conducted in the three PCOS models at the end of KP-54 treatments (11-D), to monitor ovarian responses to repeated kisspeptin stimulation. In addition, OW and UWs were also recorded. In keeping with initial phenotypic data, OW and UW were fully preserved in PNA rats treated with vehicle; in fact, a moderate increase in UW was observed in this model. Repeated treatment with KP-54 significantly increased OW, without altering UW, in PNA rats. In contrast, OW and UW were decreased in both NeNA and PWA rats. While no responses to KP-54 in terms of gross OW and UW were detected in the PWA model, NeNA rats showed a significant increase in OW at the end of the 11-D treatment with daily boluses of KP-54 (Fig. 6-I).
Figure 6.
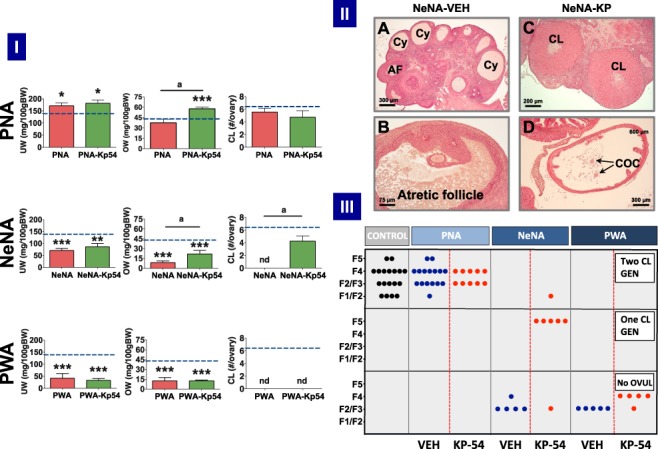
Ovarian responses to KP-54 in the three preclinical rat models of PCOS. In panel I, UW and OW, as well as the number of fresh CL per ovary, after 11-day treatment with KP-54 or vehicle are shown from the three preclinical models of PCOS (PNA, NeNA and PWA); dotted lines indicate reference OW, UW and CL in control (non-androgenized) adult female rats. Data are presented as mean ± SEM and were analyzed by ANOVA followed by Student–Newman–Keuls multiple range tests (*P < 0.05, **P < 0.01, ***P < 0.001 versus control rats; aP < 0.01 versus PNA rats treated with Vehicle). In panel II, representative images of ovarian histology of adult NeNA rats treated as adults with vehicle (A, B) or KP-54 (C, D). The presence of CL, Cy, atrophic follicles (AF) and cumulus oocyte complexes (COCs) is indicated. Note that while NeNA rats treated with vehicle failed to show CL (as index of anovulation), KP-54 administration to NeNA rats rescued ovulation, as denoted by the appearance of CL and COC. Scale bars are as follows: A (300 μm); B (75 μm); C (200 μm); and D (300 μm). In panel III, scoring of follicular maturation and ovulatory status in PNA, NeNA and PWA rats, following treatment as adults with either vehicle (blue dots) or KP-54 (red dots), is presented. Note that each dot represents the score of individual ovaries. Depending on initial morphometric data and optimally fixed tissue availability, ovaries from 5–10 independent animals were studied per model. Control, non-androgenized rats are included (black dots) for reference purposes. Analyses were conducted in ovarian samples obtained after completion of the 11-day period of treatment with KP-54 or vehicle. Scoring of follicular maturation was based on the identification of the most advanced stage of large growing follicles, divided into these classes: F1 (275–350 μm), F2 (351–400 μm), F3 (401–450 μm), F4 (451–575 μm) and F5 (>575 μm). The presence of one or two generations (GEN) of corpora lutea denoted the occurrence of one or two ovulatory cycles.
An ovarian maturation score, depicted in Fig. 6-III, and based on the identification of the most advanced stage for corpora lutea and/or large growing follicles, was applied to quantify the follicular/ovulatory impact of KP-54 treatment in the three PCOS models. Large growing follicles were divided into five classes: F1 (275–350 μm), F2 (351–400 μm), F3 (401–450 μm), F4 (451–575 μm) and F5 (>575 μm) following previously used classifications (Osman 1985; Gaytan et al. 2017). Random analysis of cyclic female rats, used as controls, revealed a scattered distribution of individuals, all having two identifiable generations of corpora lutea (as index of regular ovulation), together with growing follicles that, depending on the phase of the estrous cycle, were at different stages of maturation, ranging from F1/F2 follicles in some individuals to F5 (preovulatory) in others. All PNA rats treated with vehicle showed a similar profile, with identifiable corpora lutea and large growing follicles at various stages of maturation, depending on the individual. While our score does not allow discrimination of subtle irregularities in cyclicity, these are clear signs of persistent ovulatory function in PNA rats, as confirmed also by the preserved number of total corpora lutea per ovary (Fig. 6-I). Treatment of PNA rats with KP-54 for 11 days did not grossly alter this phenotype, as signs of ovulation were present at the end of the treatment and the number of fresh corpora lutea per ovary was maintained. In fact, some evidence for synchronization of follicular maturation and ovulation was observed, as all PNA rats treated with KP-54 for 11 days showed a narrower range of maturation of growing follicles (mainly at F3/F4 as most advanced stage) than vehicle-treated PNA animals and controls.
In sharp contrast, NeNA and PWA rats treated with vehicle were clearly anovulatory, with no corpora lutea in any animal examined (Fig. 6-I). However, responses to KP-54 were markedly different between these two models of early androgenization. In NeNA rats, repeated kisspeptin stimulation was sufficient to elicit ovulation in a large majority of individuals (six out of seven of the series analyzed), as revealed by the presence of one or even two generations of corpora lutea, and the complete normalization of the mean number of corpora lutea per ovary (Fig. 6-I). These responses are further illustrated in Fig. 6-II. Thus, while NeNA rats treated with vehicle displayed clear cyst-like structures (panel A) and atretic antral follicles (panels A and B) as the most advanced stage of follicular growth, without corpora lutea, NeNA rats treated with KP-54 for 11 days did show morphologically discernible corpora lutea (panel C), and cumulus–oocyte complexes in the oviduct (panel D), as clear signs of successful ovulation.
Conversely, none of the PWA rats analyzed reached an ovulatory stage after repeated KP-54 stimulation, although a subtle progression towards a most advance stage of follicular maturation was detected. While PWA rats treated with vehicle consistently showed that the most advanced follicles were at stages F2/F3, the majority of PWA rats treated with KP-54 showed F4 follicles (Fig. 6-III). Detailed histological analyses provided further insight into the differential features of ovarian response to KP-54 in this anovulatory model. Thus, although in PWA rats treated with KP-54 no single animal ovulated (hence, no fresh corpus luteum was detected; Fig. 6-I), positive responses to kisspeptin treatment were evidenced by a significant reduction in the percentage of atretic follicles, both small (diameter ≥200 μm) and large (≥350 μm), as well as an increase in the total number of large (>350 μm) healthy follicles (P = 0.06) (Supplementary Fig. S6).
Clinical studies
Clinical studies consisted of two arms. First, a dose-exploration study was performed, with dose escalation at weekly intervals, to evaluate PKs and LH responses to KP-54 (administered by s.c. injection twice daily) in women with PCOS. The doses chosen were similar to those used in previous clinical studies in women (Dhillo et al. 2007; Jayasena et al. 2009; Jayasena et al. 2014), although it should be noted that KP-54 had not been previously administered to women with PCOS for induction of ovulation. In addition, a protocol of 21-day administration of KP-54 twice daily was applied to a pilot cohort of women with PCOS, who were followed for LH and ovarian/ovulatory responses. These protocols are described in detail in the Methods section and schematically depicted in Fig. 7. A summary of the major clinical characteristics at baseline of the PCOS patients enrolled in this study, all of whom had anovulation and polycystic ovaries on ultrasound, is presented in Table I. Of note, five of the patients enrolled had signs of hyperandrogenism/hyperandrogenemia (i.e. phenotype A), while seven did not (i.e. phenotype D).
Figure 7.
Schematic of protocol, and gonadotrophin and ovarian hormone responses to KP-54 in 12 individual women with PCOS. In the left panel, a schematic showing the timing of KP-54 administration in the (A) dose-exploration arm and (B) constant dose arm of the study. In both cases, KP-54 was started after a progestogen-induced withdrawal menses and administered s.c. bd for 21 days. Group sizes were N = 5 in (A; split into N = 3 and 2, in two escalation protocols, as depicted) and N = 7 in (B). In (A), multiple blood samples were taken over periods of 7.5 h, as indicated for pharmacokinetic (PK) analysis, and in both, blood samples and transvaginal ultrasound were performed twice weekly as indicated. In the right panels, serum hormone concentrations before and after repeated administration of KP-54 at 3.2, 6.4, 9.6 and 12.8 nmol/kg, and 7 days following the last dose. KP-54 was administered s.c. twice daily for 21 days; Day 1 samples were taken immediately before first dose. Pre- (day-1) and post-KP (7-day after completion of treatments) hormonal levels are shown. Significant overall rises in LH and estradiol (E2) levels during the period of KP-54 administration were detected (Student t tests), with no changes in FSH or inhibin B levels. Blue symbols indicate those patients treated with increasing doses of KP-54, and red symbols denote those treated with a fixed dose of 9.6 nmol/kg twice daily throughout. MPA: medroxyprogesterone acetate, 10 mg bd for 7 days to induce a withdrawal bleed. USS: ultrasound scan.
Table I.
Baseline characteristics of the women with polycystic ovary syndrome enrolled in the clinical studies.
| Subject | Age (years) |
BMI (kg/m2) |
Months since last menses |
LH (IU/L) | FSH (IU/L) | Estradiol (pmol/L) |
Testosterone (nmol/L) |
FAI | Inhibin B (pg/mL) |
PCOS phenotype |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 32 | 22 | 7 | 10.3 | 4.1 | 139 | 2.5 | 3.0 | 135.3 | A |
| 2 | 24 | 22 | 6 | 9.3 | 4.8 | 140 | 1.5 | 3.2 | 89.4 | D |
| 3 | 32 | 31 | 13 | 2.2 | 3.6 | 84 | 0.6 | 2.7 | 90.7 | D |
| 4 | 34 | 25 | 18 | 17.1 | 3.3 | 161 | 3.2 | 4.8 | 137 | A |
| 5 | 30 | 29 | 12 | 6.5 | 4.7 | 94 | 0.7 | 1.2 | 80.7 | D |
| 6 | 28 | 33 | 5 | 8.5 | 3.9 | 93 | 1.8 | 4.5 | 75.4 | D |
| 7 | 23 | 25 | 3 | 13.8 | 4.0 | 138 | 1.2 | 1.4 | 62.5 | D |
| 8 | 29 | 33 | 3 | 9.5 | 3.6 | 53 | 1.1 | 5.0 | 30.6 | D |
| 9 | 32 | 26 | 24 | 4.3 | 3.3 | 91 | 0.6 | 0.8 | 77.5 | D |
| 10 | 25 | 17 | 24 | 11.5 | 4.6 | 130 | 2.5 | 3.0 | 127.6 | A |
| 11 | 25 | 22 | 6 | 12.4 | 3.6 | 121 | 1.9 | 3.2 | 111.8 | A |
| 12 | 34 | 25 | 22 | 23.5 | 3.9 | 276 | 3.2 | 4.8 | 140.3 | A |
| Mean ± SEM | 29 ± 1.2 | 26 ± 1.7 | 12 ± 2.6 | 10.7 ± 1.6 | 3.7 ± 0.1 | 124.0 ± 16.2 | 1.7 ± 0.3 | 3.1 ± 0.4 | 88.0 ± 15.3 |
All women had polycystic ovaries at ultrasound. Polycystic ovary syndrome (PCOS) phenotype A = oligo/amenorrhea, clinical or biochemical hyperandrogenism and polycystic ovaries; phenotype D = oligo/amenorrhea and polycystic ovaries. Reference range for testosterone, 0.3–1.9 nmol/l, and for FAI (Free Androgen Index), <5.2.
PK analyses
PK analysis showed a clear dose relationship, with peak plasma concentrations of KP-54 seen at 30–90 min, depending on dose. KP-54 administration resulted in plasma concentrations of 2 to 12 nmol/L kisspeptin, 120 min after injection of 3.2–12.8-nmol/kg doses. PK modeling of simulation of a 12.8 nmol/kg s.c. twice daily dosing showed C-max ~10 nM, 1-h post injection, with C-trough ~0.1 nM 12-h post injection (Supplementary Fig. S7A).
Gonadotrophin and ovarian responses after repeated administration of KP-54 in anovulatory women with PCOS
During a period of 7 h after administration of four different dosages of KP-54, no clear response or dose response was observed in LH levels (Supplementary Fig. S7B). Dose escalation in five women did not show clear-cut LH responses in individual women over the 21-day period of administration, nor did it reveal evidence of a dose–response relationship. Gonadotrophin and ovarian responses to a fixed dose (9.6 nmol/kg, twice daily) of KP-54 for 3 weeks were further evaluated in a pilot cohort of seven anovulatory women with PCOS. As women in both of the two arms were treated for 21 days, all doses were combined for analysis (N = 12). This integral analysis revealed that repeated administration of KP-54 resulted in a small but significant rise in LH (pre KP-54 10.8 ± 1.8 versus post KP-54 13.4 ± 1.6 IU/L, P = 0.038, Fig. 7), with an equivalent elevation in serum estradiol levels across 21 days of KP-54 administration (pre KP-54113 ± 9.7 versus post KP-54148.6 ± 11.9 pmol/L, P = 0.029, Fig. 7). In contrast, there was no significant change in FSH (pre KP-54 3.9 ± 0.2 versus post KP-54 3.5 ± 0.2 IU/L) or inhibin B levels (pre KP-54 92.6 ± 9.9 versus post KP-54 93.3 ± 12.7 pg/mL; Fig. 7).
Our analyses revealed variability in the individual patterns of response: LH responses were detected in five out of seven women with PCOS treated with the fixed dose of 9.6 nmol/kg twice daily, and in two of them growth of a dominant follicle with subsequent ovulation occurred (Subjects 5 and 7 in Table I). In both cases, while emergence of a growing follicle occurred during KP-54 administration, ongoing follicle growth and subsequent ovulation continued after the 21 days of administration. Of these two women, one had been amenorrhoeic for the previous 12 months, while the other had reported only two to three menses per year. An example of an ovulatory response is shown in Fig. 8A and B. There was a small but consistent increase in the size of the dominant follicle during 3 weeks of KP-54 treatment, with a more marked rise immediately thereafter and a coincident increase in serum estradiol and endometrial thickness culminating in an LH surge, disappearance of the dominant follicle and a subsequent serum progesterone level of 25.4 nmol/l detected thereafter.
Figure 8.
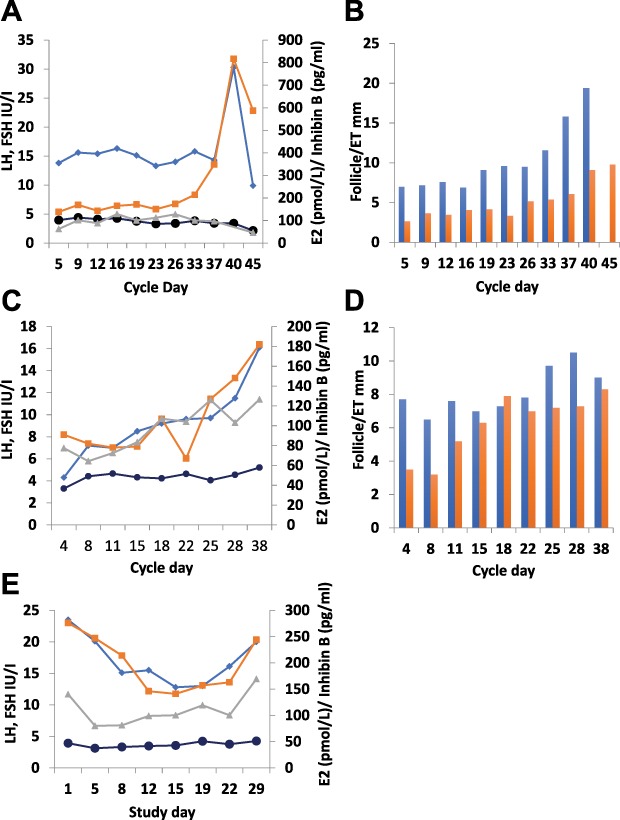
Individual gonadotrophin and ovarian hormone responses to KP-54 in women with PCOS. Serum hormones and ultrasound-measured diameter of the leading follicle and endometrial thickness (ET) in individual women (panels A/B, C/D and E display data from each of the three women) administered 9.6 nmol/kg s.c. bd for 21 days to illustrate the variation in response. Blue line: LH; black line, FSH; orange line, E2; grey line, inhibin B. Blue columns, follicle diameter; orange columns, ET. In the woman whose results are shown in panels A/B, there was a small but consistent increase in the size of the dominant follicle during 3 weeks of KP-54 treatment, with a more marked rise immediately thereafter, with a coincident increase in serum E2 and ET. This culminated in an LH surge, disappearance of the dominant follicle and a subsequent serum progesterone level of 25.4 nmol/L detected thereafter, indicating ovulation. In panels C/D, a rise in LH with a limited ovarian response is illustrated, with a rise in E2 during the third week of treatment and a transient increase in diameter of the leading follicle to >10 mm. Panel E shows evidence of desensitization, with declines in LH and E2, accompanied by some light vaginal bleeding.
A rise in LH with a limited ovarian response is illustrated in Fig. 8C and D. There was a progressive rise in LH during KP-54 administration, with a rise in estradiol during the third week of treatment. An increase in diameter of the leading follicle to >10 mm was detected, but this was not sustained.
Of note, evidence of desensitization was observed in one woman (Fig. 8E). In this patient, LH fell during treatment, though with a recovery towards pretreatment levels during the third week of treatment. Estradiol also fell, and this was accompanied by some light vaginal bleeding. FSH and inhibin B showed very similar patterns of change with an immediate fall followed by a small but sustained increase during the rest of the treatment period; no change in leading follicle diameter was detected. This woman had a markedly elevated baseline LH (23.5 IU/L), with pretreatment testosterone levels of 3.2 nmol/L.
Discussion
PCOS is a highly prevalent endocrinopathy, but our understanding of the pathophysiology and, thus, its clinical management suffer from serious gaps, with the need for substantial research efforts being highlighted by a recent international, evidence-based guideline (Teede et al. 2018a, 2018b). Despite its health burden to a large proportion of the female population at reproductive age, the therapeutic options for PCOS remain symptomatic, of moderate efficacy when conception is desired, and associated with potentially life-threatening complications, such as OHSS. In this context, novel pharmacological strategies based on the use of kisspeptin analogs might provide improved opportunities for effective ovulation induction. Several reasons support this rationale: kisspeptin stimulation is likely to achieve a more physiological gonadotropic stimulus than exogenous gonadotrophin priming, thus being less prone to result in multiple follicle development and OHSS (Abbara et al. 2017), and as described in men (Chan et al. 2011), kisspeptins may help to reset or synchronize the GnRH pulse generator, thus helping to mitigate potential dysregulation of the GnRH/gonadotrophin axis predicted in women with PCOS (Goodarzi et al. 2011; Blank et al. 2006). Additionally, kisspeptins have been suggested to operate directly at the ovary (Castellano et al. 2006; Gaytan et al. 2009; Owens et al. 2018), but the relevance of this is unclear. Rational testing of such a pharmacological option should consider the heterogeneity of the syndrome, which may lead to variability in responses in terms of gonadotrophin secretion and rescue of ovulation.
Our results, using a combination of preclinical models of PCOS, based on androgenization of female rats at different developmental windows—intended to recapitulate the heterogeneity of the human disease—and a pilot cohort of anovulatory women with PCOS, demonstrate the capacity of administration of KP-54 to evoke gonadotrophin responses and ovulation in PCOS conditions, albeit with variable efficacy. This is well illustrated by our data in rat models of androgenization, defined by diverse metabolic and reproductive features, in which responses to KP-54 partially diverged. Thus, while both NeNA and PWA rats were anovulatory, robust LH and FSH responses, together with rescue of ovulation, were only observed in neonatally androgenized rats. In contrast, PWA rats, with persistently elevated androgen levels, displayed blunted LH (but exaggerated FSH) responses that promoted trophic follicular responses at the ovarian level but failed to induce ovulation. Hence, it might be argued that in PCOS conditions linked to persistent hyperandrogenism, the efficiency of KP-54 to elicit sustained LH and ovulatory responses might be compromised, while kisspeptin stimulation might be more able to induce ovulation in anovulatory women with PCOS linked to early androgenization—without marked elevation of circulating androgens. While the clinical data are insufficient to allow clear identification of likely phenotypes in which kisspeptins might be effective, neither of the two women who ovulated had elevated serum testosterone levels. In PNA rats, which were less severely affected by gestational androgen exposure, with a lean and ovulatory phenotype, KP-54 administration only induced some synchronization of ovulation, which nonetheless might be of interest to increase fecundity. These observations are of relevance, considering recent reports on neuroendocrine alterations in other mouse models of PCOS, generated by prenatal hormonal manipulation and androgen excess (Moore et al. 2015; Tata et al. 2018). Interestingly, similar protocols of administration of KP-54 to cyclic, ovulatory rats revealed that repeated kisspeptin stimulation evoked a paradoxical suppression of ovulatory function in a subset of control animals (Supplementary Fig. S5); a response that was not observed in PNA rats. This suggests, although this is yet to be investigated, that women with PCOS who are ovulatory but with cycle irregularities might also benefit from kisspeptin treatments to increase reproductive efficiency.
An analogous diversity of responses was detected in our first-in-woman, pilot study. Repeated administration of KP-54 to a group of anovulatory women with PCOS revealed modest, albeit significant, integral LH and estradiol responses to kisspeptin stimulation in a majority of individuals, with successful ovulation induction in two out of seven patients. While the limited number of women included in this study prevents more general conclusions, the parallels with our preclinical data reinforce the potential value of kisspeptin stimulation in the management of anovulatory PCOS. The magnitude of LH responses in women was much more modest than in the preclinical models, even if compared with PWA rats, which displayed the lowest LH secretion after KP-54 administration. Differences in terms of effective dose and protocols of KP-54 dosage and blood sampling might partially explain such differences.
Interestingly, pharmacological tests in our various rat models of androgenization revealed also notable divergences in the profiles of LH versus FSH responses to kisspeptin stimulation. Hence, while PNA rats showed robust LH, but modest FSH responses, similar to cyclic control animals, our models of postnatal androgenization (NeNA and PWA) displayed exaggerated FSH responses, which are likely to have resulted in the observed trophic effects on ovarian follicles. Only NeNA rats had concurrent LH and FSH secretory responses to KP-54. The fact that this was the only model in which kisspeptin treatment effectively rescued ovulation strongly suggests that efficiency of kisspeptins to induce ovulation in PCOS conditions is bound to their capacity to elicit robust LH and FSH responses.
In spite of this evidence, it is also intriguing that effective ovulatory rescue in NeNA rats occurred even despite some degree of desensitization of the gonadotropic responses to KP-54. Furthermore, follicular atresia was diminished, and the total number of large healthy follicles was increased in PWA rats at the end of the period of KP-54 administration, in spite of complete elimination of LH responses and severe attenuation of FSH responses to KP-54. While the contribution of the cumulative effect of (even submaximal) gonadotrophin stimulation to this phenomenon cannot be discarded, these data are also compatible with a direct positive effect of kisspeptins at the ovarian level. Local expression of kisspeptins and the Kiss1 receptor has been documented in rodent and primate species (Castellano et al. 2006; Gaytan et al. 2009; Owens et al. 2018), and fragmentary data have suggested putative local actions of kisspeptins (Owens et al. 2018), whose nature and physiological relevance are yet to be substantiated.
Growing evidence suggests a metabolic dimension of kisspeptins, which might participate in the control of BW, glucose homeostasis and insulin secretion (Wolfe and Hussain 2018; Velasco et al. 2019), parameters that are commonly altered in PCOS patients. However, conflicting results have been presented to date on the actual pathophysiological roles of kisspeptins in the control of metabolic homeostasis; e.g. while studies in mouse models suggested a role of liver-born kisspeptins in the pathogenesis of impaired insulin secretion and glucose intolerance (Song et al. 2014), more recent data in humans revealed a positive effect of kisspeptin administration on basal and glucose-stimulated insulin secretion (Izzi-Engbeaya et al. 2018). Likewise, administration of kisspeptin-10 has been reported to heighten glucose-stimulated insulin release in rhesus monkeys (Wahab et al. 2011). Moreover, pharmacological studies in humans have not found any detrimental effects of kisspeptins on key metabolic traits, such as blood glucose, BW or BMI (Jayasena et al. 2009). Thus, although not covered by our current experimental design, the analysis of kisspeptin effects on the metabolic features of preclinical models and women with PCOS warrants specific investigation.
Recent preclinical data have not only substantiated the importance of disturbed androgen signaling in the pathogenesis of PCOS but also highlighted the relevant contribution of its central (brain) actions in the generation of the syndrome. Accordingly, central transducers of deregulated androgen actions on GnRH neurons might theoretically play a major pathogenic role and might constitute novel targets for therapy. Our combined pharmacological studies in various preclinical models and women with PCOS strongly suggest that kisspeptins may fulfill these criteria, as these analyses document that, albeit with variable effectiveness, stimulation with KP-54 was able to evoke detectable gonadotrophin responses and, in a subset of cases, effectively rescued ovulation. Indeed, manipulation of the activity of another hypothalamic neuropeptide, neurokinin B, whose action is closely linked to that of kisspeptin in the control of GnRH secretion, has been shown to affect pulsatile section of LH and testosterone levels in women with PCOS (George et al. 2016). All in all, our findings provide the basis for the design of novel, kisspeptin-based treatments for the management of some anovulatory forms of PCOS.
Authors’ roles
A.R.R. had a leading role in the design and conduction of preclinical studies, the analysis and presentation of data and the preparation and revision of the manuscript; K.S. had a relevant role in the design, conduction and analysis of clinical studies, as well as in the preparation and revision of the manuscript; F.G. was responsible for the conduction of histological analyses of ovarian samples in preclinical studies and had a very relevant role in the analysis and discussion of the whole dataset, as well as in manuscript revision; E.T. and C.P-L. actively participated in the conduction of preclinical (animal) studies, as well as in data collection and hormonal and metabolic analyses; B.M.M. actively participated in the design and analysis of data of the clinical of studies; S.Q. contributed to the design of the clinical studies and participated in the analysis of data, with a major role in pharmacokinetic analyses; S.L. and M.M.-L. actively participated in the conduction of preclinical (animal) studies, as well as in data collection and hormonal & metabolic analyses; C.L.-R. was actively involved in the generation of the various animal models, as well as in tissue sampling and hormonal analyses; M.S.A., M.A.S.-G., M.J.V. and L.P. had a substantial contribution to the implementation of preclinical studies in various PCOS models and actively participated in hormonal and metabolic analyses, as well as in the evaluation of data; M.v.D. and T.A.K. had a very active role in the design of preclinical and clinical studies, substantially participated in the discussion and analysis of data and were actively involved in the preparation of the first draft of the manuscript and its final revision; R.A.A. coordinated the clinical studies and the analysis of the data derived from them, with a very substantial role in manuscript preparation and revision; M.T.-S. was responsible for the design and coordination of the study, with a leading role in preclinical experiments. M.T.-S. was responsible for the revision and analysis of data and had a leading role in manuscript preparation and its revision. R.A.A. and M.T.-S. were responsible for writing the first draft of the paper, which was revised and approved by A.R.R., M.v.D, T.A.K. and the rest of the authors. All the authors take full responsibility for the work.
Funding
Ferring Research Institute; Universities of Cordoba and Edinburgh; Wellcome Trust Scottish Translational Medicine and Therapeutics Initiative (STMTI) (to K.S.); MRC Centre (MR/N022556/1).
Conflict of interest
M.T.-S. is a member of CIBER Fisiopatología de la Obesidad y Nutrición, which is an initiative of Instituto de Salud Carlos III. B.M.M. is an employee of Ferring International PharmaScience Center (Copenhagen, Denmark), and S.Q., M.v.D. and T.A.H. are employees of Ferring Research Institute (San Diego, USA). R.A.A. and M.T.-S. were recipients of a grant support from the Ferring Research Institute, which partially funded this study. R.A.A. has undertaken consultancy work and received speaker fees outside this study from Merck, IBSA, Roche Diagnostics, NeRRe Therapeutics and Sojournix Inc. The other authors have no conflict of interest to disclose.
Supplementary Material
References
- Abbara A, Clarke S, Islam R, Prague JK, Comninos AN, Narayanaswamy S, Papadopoulou D, Roberts R, Izzi-Engbeaya C, Ratnasabapathy R et al. A second dose of kisspeptin-54 improves oocyte maturation in women at high risk of ovarian hyperstimulation syndrome: a phase 2 randomized controlled trial. Hum Reprod 2017;32:1915–1924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abbara A, Jayasena CN, Christopoulos G, Narayanaswamy S, Izzi-Engbeaya C, Nijher GM, Comninos AN, Peters D, Buckley A, Ratnasabapathy R et al. Efficacy of kisspeptin-54 to trigger oocyte maturation in women at high risk of ovarian hyperstimulation syndrome (OHSS) during in vitro fertilization (IVF) therapy. J Clin Endocrinol Metab 2015;100:3322–3331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abbott DH, Barnett DK, Bruns CM, Dumesic DA. Androgen excess fetal programming of female reproduction: a developmental aetiology for polycystic ovary syndrome? Hum Reprod Update 2005;11:357–374. [DOI] [PubMed] [Google Scholar]
- Balen AH, Conway GS, Kaltsas G, Techatrasak K, Manning PJ, West C, Jacobs HS. Polycystic ovary syndrome: the spectrum of the disorder in 1741 patients. Hum Reprod 1995;10:2107–2111. [DOI] [PubMed] [Google Scholar]
- Blank SK, McCartney CR, Marshall JC. The origins and sequelae of abnormal neuroendocrine function in polycystic ovary syndrome. Hum Reprod Update 2006;12:351–361. [DOI] [PubMed] [Google Scholar]
- Brown RE, Wilkinson DA, Imran SA, Caraty A, Wilkinson M. Hypothalamic kiss1 mRNA and kisspeptin immunoreactivity are reduced in a rat model of polycystic ovary syndrome (PCOS). Brain Res 2012;1467:1–9. [DOI] [PubMed] [Google Scholar]
- Caldwell AS, Middleton LJ, Jimenez M, Desai R, McMahon AC, Allan CM, Handelsman DJ, Walters KA. Characterization of reproductive, metabolic, and endocrine features of polycystic ovary syndrome in female hyperandrogenic mouse models. Endocrinology 2014;155:3146–3159. [DOI] [PubMed] [Google Scholar]
- Caldwell ASL, Edwards MC, Desai R, Jimenez M, Gilchrist RB, Handelsman DJ, Walters KA. Neuroendocrine androgen action is a key extraovarian mediator in the development of polycystic ovary syndrome. Proc Natl Acad Sci U S A 2017;114:E3334–E3343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellano JM, Gaytan M, Roa J, Vigo E, Navarro VM, Bellido C, Dieguez C, Aguilar E, Sanchez-Criado JE, Pellicer A et al. Expression of KiSS-1 in rat ovary: putative local regulator of ovulation? Endocrinology 2006;147:4852–4862. [DOI] [PubMed] [Google Scholar]
- Chan YM, Butler JP, Pinnell NE, Pralong FP, Crowley WF Jr, Ren C, Chan KK, Seminara SB. Kisspeptin resets the hypothalamic GnRH clock in men. J Clin Endocrinol Metab 2011;96:E908–E915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarkson J, Han SY, Piet R, McLennan T, Kane GM, Ng J, Porteous RW, Kim JS, Colledge WH, Iremonger KJ et al. Definition of the hypothalamic GnRH pulse generator in mice. Proc Natl Acad Sci U S A 2017;114:E10216–E10223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhillo WS, Chaudhri OB, Thompson EL, Murphy KG, Patterson M, Ramachandran R, Nijher GK, Amber V, Kokkinos A, Donaldson M et al. Kisspeptin-54 stimulates gonadotropin release most potently during the preovulatory phase of the menstrual cycle in women. J Clin Endocrinol Metab 2007;92:3958–3966. [DOI] [PubMed] [Google Scholar]
- Dunaif A. Insulin resistance and the polycystic ovary syndrome: mechanism and implications for pathogenesis. Endocr Rev 1997;18:774–800. [DOI] [PubMed] [Google Scholar]
- Ehrmann DA, Barnes RB, Rosenfield RL. Polycystic ovary syndrome as a form of functional ovarian hyperandrogenism due to dysregulation of androgen secretion. Endocr Rev 1995;16:322–353. [DOI] [PubMed] [Google Scholar]
- Fauser BC, Tarlatzis BC, Rebar RW, Legro RS, Balen AH, Lobo R, Carmina E, Chang J, Yildiz BO, Laven JS et al. Consensus on women’s health aspects of polycystic ovary syndrome (PCOS): the Amsterdam ESHRE/ASRM-sponsored 3rd PCOS Consensus Workshop Group. Fertil Steril; 97 2012;e25:28–38. [DOI] [PubMed] [Google Scholar]
- Franks S. Polycystic ovary syndrome. N Engl J Med 1995;333:853–861. [DOI] [PubMed] [Google Scholar]
- Gambineri A, Pelusi C, Vicennati V, Pagotto U, Pasquali R. Obesity and the polycystic ovary syndrome. Int J Obes Relat Metab Disord 2002;26:883–896. [DOI] [PubMed] [Google Scholar]
- Garcia-Galiano D, Pinilla L, Tena-Sempere M. Sex steroids and the control of the Kiss1 system: developmental roles and major regulatory actions. J Neuroendocrinol 2012;24:22–33. [DOI] [PubMed] [Google Scholar]
- Gaytan F, Gaytan M, Castellano JM, Romero M, Roa J, Aparicio B, Garrido N, Sanchez-Criado JE, Millar RP, Pellicer A et al. KiSS-1 in the mammalian ovary: distribution of kisspeptin in human and marmoset and alterations in KiSS-1 mRNA levels in a rat model of ovulatory dysfunction. Am J Physiol Endocrinol Metab 2009;296:E520–E531. [DOI] [PubMed] [Google Scholar]
- Gaytan F, Morales C, Bellido C, Aguilar E, Sanchez-Criado JE. Proliferative activity in the different ovarian compartments in cycling rats estimated by the 5-bromodeoxyuridine technique. Biol Reprod 1996;54:1356–1365. [DOI] [PubMed] [Google Scholar]
- Gaytan F, Morales C, Leon S, Heras V, Barroso A, Avendano MS, Vazquez MJ, Castellano JM, Roa J, Tena-Sempere M. Development and validation of a method for precise dating of female puberty in laboratory rodents: the puberty ovarian maturation score (Pub-Score). Sci Rep 2017;7:46381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaytan M, Sanchez MA, Morales C, Bellido C, Millan Y, Martin de Las Mulas J, Sanchez-Criado JE, Gaytan F. Cyclic changes of the ovarian surface epithelium in the rat. Reproduction 2005;129:311–321. [DOI] [PubMed] [Google Scholar]
- George JT, Kakkar R, Marshall J, Scott ML, Finkelman RD, Ho TW, Veldhuis J, Skorupskaite K, Anderson RA, McIntosh S et al. Neurokinin B receptor antagonism in women with polycystic ovary syndrome: a randomized, placebo-controlled trial. J Clin Endocrinol Metab 2016;101:4313–4321. [DOI] [PubMed] [Google Scholar]
- Glintborg D. Endocrine and metabolic characteristics in polycystic ovary syndrome. Dan Med J 2016;63: pii: B5232. [PubMed] [Google Scholar]
- Goodarzi MO, Dumesic DA, Chazenbalk G, Azziz R. Polycystic ovary syndrome: etiology, pathogenesis and diagnosis. Nat Rev Endocrinol 2011;7:219–231. [DOI] [PubMed] [Google Scholar]
- Goodarzi MO, Jones MR, Li X, Chua AK, Garcia OA, Chen YD, Krauss RM, Rotter JI, Ankener W, Legro RS et al. Replication of association of DENND1A and THADA variants with polycystic ovary syndrome in European cohorts. J Med Genet 2012;49:90–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorry A, White DM, Franks S. Infertility in polycystic ovary syndrome: focus on low-dose gonadotropin treatment. Endocrine 2006;30:27–33. [DOI] [PubMed] [Google Scholar]
- Hayes MG, Urbanek M, Ehrmann DA, Armstrong LL, Lee JY, Sisk R, Karaderi T, Barber TM, McCarthy MI, Franks S et al. Genome-wide association of polycystic ovary syndrome implicates alterations in gonadotropin secretion in European ancestry populations. Nat Commun 2015;6:7502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbison AE. Control of puberty onset and fertility by gonadotropin-releasing hormone neurons. Nat Rev Endocrinol 2016;12:452–466. [DOI] [PubMed] [Google Scholar]
- Izzi-Engbeaya C, Comninos AN, Clarke SA, Jomard A, Yang L, Jones S, Abbara A, Narayanaswamy S, Eng PC, Papadopoulou D et al. The effects of kisspeptin on beta-cell function, serum metabolites and appetite in humans. Diabetes Obes Metab 2018;20:2800–2810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jayasena CN, Abbara A, Comninos AN, Nijher GM, Christopoulos G, Narayanaswamy S, Izzi-Engbeaya C, Sridharan M, Mason AJ, Warwick J et al. Kisspeptin-54 triggers egg maturation in women undergoing in vitro fertilization. J Clin Invest 2014;124:3667–3677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jayasena CN, Nijher GM, Chaudhri OB, Murphy KG, Ranger A, Lim A, Patel D, Mehta A, Todd C, Ramachandran R et al. Subcutaneous injection of kisspeptin-54 acutely stimulates gonadotropin secretion in women with hypothalamic amenorrhea, but chronic administration causes tachyphylaxis. J Clin Endocrinol Metab 2009;94:4315–4323. [DOI] [PubMed] [Google Scholar]
- Kauffman AS, Gottsch ML, Roa J, Byquist AC, Crown A, Clifton DK, Hoffman GE, Steiner RA, Tena-Sempere M. Sexual differentiation of Kiss1 gene expression in the brain of the rat. Endocrinology 2007;148:1774–1783. [DOI] [PubMed] [Google Scholar]
- Marcondes RR, Carvalho KC, Giannocco G, Duarte DC, Garcia N, Soares-Junior JM, da Silva I, Maliqueo M, Baracat EC, Maciel GAR. Hypothalamic transcriptional expression of the kisspeptin system and sex steroid receptors differs among polycystic ovary syndrome rat models with different endocrine phenotypes. Clinics (Sao Paulo) 2017;72:510–514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAllister JM, Legro RS, Modi BP, Strauss JF 3rd.. Functional genomics of PCOS: from GWAS to molecular mechanisms. Trends Endocrinol Metab 2015;26:118–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAllister JM, Modi B, Miller BA, Biegler J, Bruggeman R, Legro RS, Strauss JF 3rd.. Overexpression of a DENND1A isoform produces a polycystic ovary syndrome theca phenotype. Proc Natl Acad Sci U S A 2014;111:E1519–E1527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore AM, Campbell RE. Polycystic ovary syndrome: understanding the role of the brain. Front Neuroendocrinol 2017;46:1–14. [DOI] [PubMed] [Google Scholar]
- Moore AM, Prescott M, Marshall CJ, Yip SH, Campbell RE. Enhancement of a robust arcuate GABAergic input to gonadotropin-releasing hormone neurons in a model of polycystic ovarian syndrome. Proc Natl Acad Sci U S A 2015;112:596–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navarro VM, Castellano JM, Fernandez-Fernandez R, Tovar S, Roa J, Mayen A, Barreiro ML, Casanueva FF, Aguilar E, Dieguez C et al. Effects of KiSS-1 peptide, the natural ligand of GPR54, on follicle-stimulating hormone secretion in the rat. Endocrinology 2005a;146:1689–1697. [DOI] [PubMed] [Google Scholar]
- Navarro VM, Castellano JM, Fernandez-Fernandez R, Tovar S, Roa J, Mayen A, Nogueiras R, Vazquez MJ, Barreiro ML, Magni P et al. Characterization of the potent luteinizing hormone-releasing activity of KiSS-1 peptide, the natural ligand of GPR54. Endocrinology 2005b;146:156–163. [DOI] [PubMed] [Google Scholar]
- Osman P. Rate and course of atresia during follicular development in the adult cyclic rat. J Reprod Fertil 1985;73:261–270. [DOI] [PubMed] [Google Scholar]
- Osuka S, Iwase A, Nakahara T, Kondo M, Saito A, Bayasula, Nakamura T, Takikawa S, Goto M, Kotani T et al. Kisspeptin in the hypothalamus of two rat models of polycystic ovary syndrome. Endocrinology 2017;158:367–377. [DOI] [PubMed] [Google Scholar]
- Owens LA, Abbara A, Lerner A, O'Floinn S, Christopoulos G, Khanjani S, Islam R, Hardy K, Hanyaloglu AC, Lavery SA et al. The direct and indirect effects of kisspeptin-54 on granulosa lutein cell function. Hum Reprod 2018;33:292–302. [DOI] [PubMed] [Google Scholar]
- Padmanabhan V, Veiga-Lopez A. Sheep models of polycystic ovary syndrome phenotype. Mol Cell Endocrinol 2013;373:8–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pineda R, Garcia-Galiano D, Roseweir A, Romero M, Sanchez-Garrido MA, Ruiz-Pino F, Morgan K, Pinilla L, Millar RP, Tena-Sempere M. Critical roles of kisspeptins in female puberty and preovulatory gonadotropin surges as revealed by a novel antagonist. Endocrinology 2010;151:722–730. [DOI] [PubMed] [Google Scholar]
- Pinilla L, Aguilar E, Dieguez C, Millar RP, Tena-Sempere M. Kisspeptins and reproduction: physiological roles and regulatory mechanisms. Physiol Rev 2012;92:1235–1316. [DOI] [PubMed] [Google Scholar]
- Pinilla L, Barreiro ML, Gonzalez LC, Tena-Sempere M, Aguilar E. Comparative effects of testosterone propionate, oestradiol benzoate, ICI 182,780, tamoxifen and raloxifene on hypothalamic differentiation in the female rat. J Endocrinol 2002;172:441–448. [DOI] [PubMed] [Google Scholar]
- Rebar R, Judd HL, Yen SS, Rakoff J, Vandenberg G, Naftolin F. Characterization of the inappropriate gonadotropin secretion in polycystic ovary syndrome. J Clin Invest 1976;57:1320–1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rotterdam EA-SPcwg Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome (PCOS). Hum Reprod 2004;19:41–47. [DOI] [PubMed] [Google Scholar]
- Sir-Petermann T, Codner E, Perez V, Echiburu B, Maliqueo M, Ladron de Guevara A, Preisler J, Crisosto N, Sanchez F, Cassorla F et al. Metabolic and reproductive features before and during puberty in daughters of women with polycystic ovary syndrome. J Clin Endocrinol Metab 2009;94:1923–1930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sirmans SM, Pate KA. Epidemiology, diagnosis, and management of polycystic ovary syndrome. Clin Epidemiol 2013;6:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skorupskaite K, George JT, Anderson RA. The kisspeptin-GnRH pathway in human reproductive health and disease. Hum Reprod Update 2014;20:485–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song WJ, Mondal P, Wolfe A, Alonso LC, Stamateris R, Ong BW, Lim OC, Yang KS, Radovick S, Novaira HJ et al. Glucagon regulates hepatic kisspeptin to impair insulin secretion. Cell Metab 2014;19:667–681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan SD, Moenter SM. Prenatal androgens alter GABAergic drive to gonadotropin-releasing hormone neurons: implications for a common fertility disorder. Proc Natl Acad Sci U S A 2004;101:7129–7134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tata B, Mimouni NEH, Barbotin AL, Malone SA, Loyens A, Pigny P, Dewailly D, Catteau-Jonard S, Sundstrom-Poromaa I, Piltonen TT et al. Elevated prenatal anti-Mullerian hormone reprograms the fetus and induces polycystic ovary syndrome in adulthood. Nat Med 2018;24:834–846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teede HJ, Misso ML, Costello MF, Dokras A, Laven J, Moran L, Piltonen T, Norman RJ, International PN . Recommendations from the international evidence-based guideline for the assessment and management of polycystic ovary syndrome. Fertil Steril 2018a;110:364–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teede HJ, Misso ML, Costello MF, Dokras A, Laven J, Moran L, Piltonen T, Norman RJ, International PN . Recommendations from the international evidence-based guideline for the assessment and management of polycystic ovary syndrome. Hum Reprod 2018b;33:1602–1618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tovar S, Vazquez MJ, Navarro VM, Fernandez-Fernandez R, Castellano JM, Vigo E, Roa J, Casanueva FF, Aguilar E, Pinilla L et al. Effects of single or repeated intravenous administration of kisspeptin upon dynamic LH secretion in conscious male rats. Endocrinology 2006;147:2696–2704. [DOI] [PubMed] [Google Scholar]
- van Houten EL, Kramer P, McLuskey A, Karels B, Themmen AP, Visser JA. Reproductive and metabolic phenotype of a mouse model of PCOS. Endocrinology 2012;153:2861–2869. [DOI] [PubMed] [Google Scholar]
- Velasco I, Leon S, Barroso A, Ruiz-Pino F, Heras V, Torres E, Leon M, Ruohonen ST, Garcia-Galiano D, Romero-Ruiz A et al. Gonadal hormone-dependent versus -independent effects of kisspeptin signaling in the control of body weight and metabolic homeostasis. Metabolism 2019;98:84–94. [DOI] [PubMed] [Google Scholar]
- Wahab F, Riaz T, Shahab M. Study on the effect of peripheral kisspeptin administration on basal and glucose-induced insulin secretion under fed and fasting conditions in the adult male rhesus monkey (Macaca mulatta). Horm Metab Res 2011;43:37–42. [DOI] [PubMed] [Google Scholar]
- Walters KA, Allan CM, Handelsman DJ. Rodent models for human polycystic ovary syndrome. Biol Reprod 2012;86:1–12. [DOI] [PubMed] [Google Scholar]
- Wolfe A, Hussain MA. The emerging role(s) for kisspeptin in metabolism in mammals. Front Endocrinol (Lausanne) 2018;9:184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu L, Sun Y, Wan J, Luan T, Cheng Q, Tan Y. A proteomic analysis identifies candidate early biomarkers to predict ovarian hyperstimulation syndrome in polycystic ovarian syndrome patients. Mol Med Rep 2017;16:272–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan X, Yuan C, Zhao N, Cui Y, Liu J. Prenatal androgen excess enhances stimulation of the GNRH pulse in pubertal female rats. J Endocrinol 2014;222:73–85. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.