Abstract
Elevated blood pressure (BP) has been proposed as a possible pathophysiological mechanism linking exposure to ambient air pollution and the increased risk of cardiovascular mortality and morbidity. In this study, we investigated the hourly relationship between ambient air pollutants and BP. BP measurements were extracted from the electronic health record database of the Seoul National University Bundang Hospital from February 2015 to June 2017. A total of 98,577 individual BP measurements were matched to the hourly levels of air pollutants. A generalized additive model was constructed for hour lags of 0–8 of air pollutants adjusting for age, sex, meteorological variables, and time trend. Systolic BP was shown to be significantly lower at 2–4 hours and 3–5 hours after increased levels of SO2 and CO, respectively (0.24 mmHg and 0.26 mmHg for an interquartile range, respectively). In contrast, O3 and NO2 were associated with significantly increased systolic BP at 3–5 lag hours and at 0–2 lag hours, respectively. BP elevation in association with O3 and NO2 was shown to be significantly greater in hypertensive patients than normotensive subjects. Our findings suggest that short-term exposure to air pollution may be associated with elevated BP.
Subject terms: Environmental impact, Renovascular hypertension
Introduction
Elevated blood pressure (BP) is the top leading risk factor for morbidity and mortality in both men and women1. Every 20 mmHg increase in systolic BP (or 10 mmHg increase in diastolic BP) has been shown to be associated with more than a two-fold increase in cardiovascular mortality2,3. It is currently estimated that over 30% of adults have hypertension (defined as systolic and/or diastolic BP ≥140/90 mmHg) worldwide4. BP is variable and can be affected by various environmental factors. Behavioral factors, including high dietary salt intake, obesity, physical inactivity, and alcohol abuse contribute to elevated BP5–7. Effects of environmental factors such as temporal trends, ambient temperature, and noise are also well known8. Elevated short-term BP variability and pathologic diurnal patterns have been shown to be associated with poor prognosis9,10.
Ambient air pollution is a major cause of death and disease. The World Health Organization (WHO) estimates that 7.6% of total global deaths are attributable to ambient air pollution11. The Global Burden of Disease study ranked ambient air pollution as sixth among other risk factors in terms of attributable disability-adjusted life-year12. Most of the disease burden associated with ambient fine particulate matter (PM) (PM with an aerodynamic diameter of <2.5 μm, PM2.5) is attributed to cardiovascular disease including ischemic heart disease and cerebrovascular disease. The estimated burden is particularly high in Asian countries such as China and India.
Recent studies have suggested that air pollution leads to elevated BP, and this may be an important mechanism defining the link between ambient air pollution and cardiovascular mortality and morbidity13,14. Studies have demonstrated a rapid increase in diastolic BP after controlled exposure to PM2.5 and ozone (O3)15,16. Observational studies and meta-analyses have also indicated that short-term exposure to air pollution, including PM2.5, increases BP and the frequency of emergency visits for hypertension17–19. In addition, long-term exposure to PM2.5 and nitrogen dioxide (NO2) has been associated with higher BP and development of hypertension19,20.
Most previous short-term effect studies utilizing a population-based database had limitations of assessing binary outcomes such as emergency visits for hypertension rather than absolute BP values18,21,22. In addition, because of constraints in time resolution, analyses were mostly limited to day lag models but reports including hour lag models have been scarce. Several studies that assessed hourly changes in BP comprised small sample sizes, which made it difficult to fully appreciate the association with sufficient statistical power15,16,23. Therefore, this study sought to investigate the short-term relationship between ambient air pollution and BP using electronic health record data from patients visiting a tertiary center in Korea. We hypothesized that air pollution contributes to pathological short-term BP changes, which may, in turn, lead to adverse cardiovascular outcomes. Large volumes of electronic health record data combined with a high temporal resolution enabled the examination of short-term effects of air pollution on BP on an hourly basis.
Results
Study subject profiles, air pollution levels, and meteorological data
A total of 98,577 subjects with available BP measurements were analyzed in this study. As shown in Table 1, mean age was 55.5 years and 50.2% were male. Among the study subjects, 31.7% had hypertension, 2.2% diabetes, and 10.4% dyslipidemia. The mean systolic and diastolic BP was 122.8 ± 16.3 mmHg and 74.1 ± 11.1 mmHg, respectively. Mean PR was 79.3 ± 13.4 per minute. Most measurements (97.8%) were obtained between 9 a.m. to 6 p.m. (Supplementary Fig. 1). Subjects with hypertension were older and more frequently associated with male sex and comorbidities. Calcium channel blockers were the most commonly used antihypertensive medication.
Table 1.
Descriptive statistics on study subjects and blood pressure measurements.
| Values | Total (n = 98,577) | Hypertension (n = 31,267) | Normotension (n = 67,310) |
|---|---|---|---|
| Age, years | 55.5 ± 14.9 | 61.8 ± 14.1 | 52.6 ± 14.4 |
| Male sex, n (%) | 49,452 (50.2) | 16,866 (53.9) | 32,586 (48.4) |
| Body mass index, kg/m2* | 23.83 ± 0.49 | 25.16 ± 0.41 | 23.83 ± 0.49 |
| Blood pressure measurements | |||
| Systolic blood pressure, mmHg | 122.8 ± 16.3 | 127.1 ± 17.8 | 120.8 ± 15.1 |
| Diastolic blood pressure, mmHg | 74.1 ± 11.1 | 75.6 ± 11.8 | 73.4 ± 10.6 |
| Pulse rate, per minute | 79.3 ± 13.4 | 78.3 ± 13.8 | 79.8 ± 13.2 |
| Comorbidities | |||
| Diabetes mellitus, n (%) | 7553 (7.7) | 5732 (18.3) | 1821 (2.7) |
| Dyslipidemia, n (%) | 10,288 (10.4) | 6584 (21.1) | 3704 (5.5) |
| Medication, n (%) | |||
| Antihypertensive agents | |||
| ACEI/ARB | 13,582 (13.8) | 13,582 (43.4) | — |
| Calcium channel blockers | 17,505 (17.8) | 17,505 (56.0) | — |
| β-blockers | 17,090 (17.3) | 17,090 (54.7) | — |
| Diuretics | 7565 (7.7) | 7565 (24.2) | — |
| Statin | 17,813 (18.1) | 13,632 (43.6) | 4181 (6.2) |
Data are reported as numbers and percentages for categorical variables and mean ± standard deviation (SD) for continuous ones. *Body mass index was only available in 59,215 subjects (61.1%).
ACEI denotes angiotensin-converting-enzyme inhibitor; ARB, angiotensin II receptor blockers.
Table 2 summarizes the meteorological and air pollution data obtained during the study period. The mean concentrations of PM2.5, O3, and NO2 were 23 μg/m3, 17 ppb, and 29 ppb, respectively. The mean temperature was 13.4 °C. Supplementary Table 1 shows the correlation matrix between air pollutants, temperature, and relative humidity during the study period. PM2.5, PM10, SO2, CO showed moderate relationships with each other. NO2 and O3 showed more dramatic diurnal variations: O3 level was highest during daytime when NO2 concentration was lowest (Supplementary Fig. 2).
Table 2.
Descriptive statistics on environmental variables during the study period.
| Values | Total |
|---|---|
| Air pollutants, median (interquartile range) | |
| PM2.5, μg/m3 | 23 (18) |
| PM10, μg/m3 | 44 (33) |
| SO2, ppb | 4 (2) |
| CO, ppm | 0.5 (0.2) |
| O3, ppb | 17 (26) |
| NO2, ppb | 29 (24) |
| Meteorological data, mean (SD) | |
| Temperature, °C | 13.4 (18.2) |
| Atmospheric pressure, hPa | 1017.0 (13.3) |
| Humidity, % | 66 (37) |
Medians (IQR) or mean (standard deviation) are presented.
PM2.5 denotes fine particulate matter (<2.5 µm); PM10, fine particulate matter (<10 µm); SO2, Sulfur dioxide; CO, carbon monoxide; O3, ozone; NO2, Nitrogen dioxide.
Short-term relationship between air pollutants and blood pressure
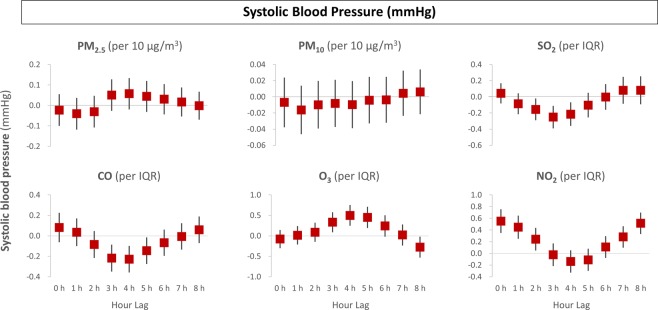
Different pollutants showed different effects with varying time lags. Systolic BP had no significant association with particulate matter of PM2.5 or PM10 from 0 to 8 hours before BP measurements. Nonetheless, gaseous pollutants showed significant lag effects with systolic BP (Fig. 1). SO2 and CO significantly reduced systolic BP at 2–4 lag hours and at 3–5 lag hours, respectively, while O3 and NO2 were associated with elevated systolic BP at 3–5 lag hours and at 0–2 lag hours, respectively.
Figure 1.
Time-lag effects of air pollution on systolic blood pressure. The x-axis represents hour lags, while the y-axis indicates adjusted effects on systolic blood pressure.
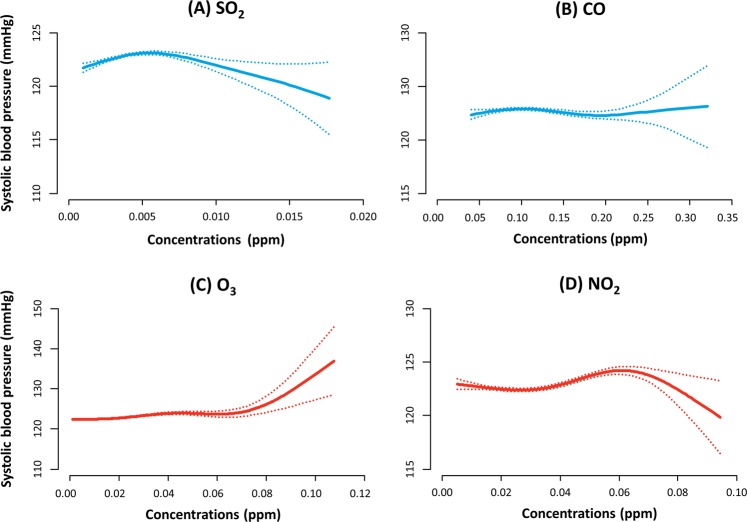
Figure 2 shows the nonlinear relationship of running means of SO2 (3–5 lag hours), CO (2–4 lag hours), O3 (3–5 lag hours) and NO2 (0–2 lag hours) with systolic BP. An IQR increase of O3 (26 ppb) was associated with an increase in systolic BP by 0.89 ± 0.10 mmHg at 3–5 lag hours when unadjusted and by 0.55 ± 0.14 mmHg when adjusted. An IQR increase of NO2 (24 ppb) was associated with an increase in systolic BP by 0.65 ± 0.09 mmHg at 0–2 lag hours when unadjusted, and by 0.42 ± 0.12 mmHg after multivariable adjustment. In the meantime, SO2 and CO were shown to be associated with increased systolic BP: −0.24 ± 0.08 mmHg at 2–4 lag hours by an IQR of SO2 (2 ppb) and −0.26 ± 0.07 mmHg at 3–5 hour lags by an IQR of CO (0.2 ppm)
Figure 2.
Spline curves showing the non-linear relationship of (A) SO2, (B) CO, (C) O3, and (D) NO2 with systolic blood pressure. The running mean of lag hours 3–5 was used for SO2 and O3, and that of lag hour 2–4 for CO and lag hours 0–2 for NO2.
Time lag effects of air pollutants on diastolic BP were generally consistent with those of systolic BP (Supplementary Fig. 3). SO2 and CO significantly reduced diastolic BP, while O3 and NO2 were associated with elevated diastolic BP. None of the pollutants showed significant associations with PR (Supplementary Fig. 4).
Subgroup analysis
Effect estimates of air pollutants on BP were analyzed stratified by the presence of hypertension (Fig. 3). The relationship of O3 and NO2 with systolic BP showed significant interaction with hypertension. BP-elevating effects of O3 and NO2 were significantly greater in patients with hypertension than in normotensive subjects. Although there was a significant interaction, the impact of PM10 was not significant in either of the subgroups. PM2.5 showed a similar trend without statistical significance. No significant interaction was present for SO2 and CO levels. No significant interactions were observed between BP and BMI across air pollutants (Supplementary Fig. 5).
Figure 3.
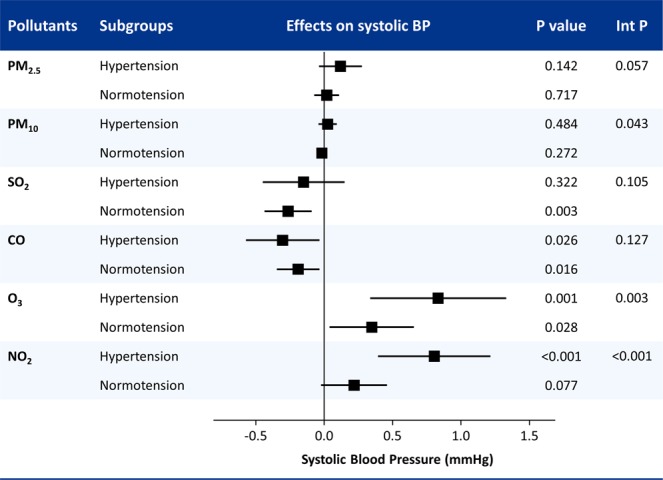
Subgroup analysis for the associations between air pollutants and systolic blood pressure (BP) stratified by the presence of hypertension. Int P, interaction P values; PM2.5, fine particulate matter with an aerodynamic diameter of <2.5 µm; PM10; CO, carbon monoxide; SO2, sulfur dioxide; NO2, nitrogen dioxide; O3, ozone.
Discussion
The present study evaluated the short-term relationship of ambient air pollution and BP. BP was shown to be elevated within several hours after exposure to increased levels of O3 and NO2, and to be depressed after exposure to SO2 and CO. PM showed no significant short-term effects on BP. The increase in systolic BP related with short-term exposure to O3 and NO2 was significantly greater in hypertensive than in normotensive subjects.
Air pollution contributes to cardiovascular mortality and morbidity24–27. Proposed pathways include increased systemic inflammation, oxidative stress, endothelial dysfunction, atherogenesis, altered autonomic control, arrhythmogenesis, and an increase in BP13,14. Studies linking air pollution and arterial BP have shown conflicting results. Several epidemiologic studies have suggested acute increases in systemic BP with higher daily PM levels (effect magnitudes ranging from 1- to 4-mmHg increase in systolic and diastolic BP per 10 µg/m3 elevation in PM)28–31. However, other studies failed to show any significant associations32–34. Several distinctive features make the present study different from the previous ones. First, the time frame considered was hours after exposure, which is much shorter than previous short-term studies that were mostly based on day lag model analysis. Second, while previous studies have provided strong evidence for adverse cardiovascular effects of PM, the present study showed that extremely short-term effects on BP were greater with gaseous pollutants.
Ozone causes oxidative stress. Studies have shown there is a short-term increase in cardiovascular mortality in response to O3 exposure35,36. A recent quasi-experimental study also showed that short-term O3 exposure leads to an increase in BP in healthy adults37. A possible mechanism linking elevated BP and O3 exposure is increased serotonin-induced vasoconstriction and decreased acetylcholine-induced vasodilation38. NO2 is produced from the combustion of fossil fuel in industrial factories or automobiles. Previous studies have yielded conflicting results regarding the short-term effects of NO2 on BP19,39. This inconsistency between previous studies might be partly explained by the difference in study methodology such as exposure time and study sample size, as well as population susceptibility and regional differences.
The present study showed negative relationships of CO and SO2 with systolic BP. CO is the product of incomplete combustion of carbon. Vascular effects of CO have been reported to resemble those of nitric oxide (NO). Experimental studies have suggested endogenously produced CO contributes to vasorelaxation in the cerebrovascular circulation40. Penney and his collaborators have performed thorough animal studies to find that the response to CO consists of systemic arterial hypotension and reduced systemic vascular resistance41,42. A human study also showed decreased peripheral resistance after acute exposure to CO43.
The burning of fossil fuels by power plants and other industrial facilities accounts for the largest source of SO2 in the atmosphere. Experimental studies demonstrated acute exposure to SO2 and SO2 derivatives (SOx) had a dose- and time-response relationship with a decrease in BP in rat models44. Vasodilatation is suggested as the key mechanism that SO2 inhibits Ca2+ entry through both potential-dependent calcium channels and receptor-operating calcium channels, and intracellular Ca2+ release45.
In the present study, we found no significant short-term relationship between PM and BP. Evidence supporting longer-term effects of PM air pollutants on hypertension is more convincing. Studies have linked short- and long-term exposure to PM and the risk of hypertension19,46. In addition, a randomized trial showed an intervention to reduce indoor air pollution resulted in improved BP control47. PM causes systemic inflammatory/oxidative response and autonomic dysfunction, which may in turn leads to elevated BP13. Therefore, long lags (than within several hours) may be required to observe meaningful response in BP.
This study has several limitations that originate from the study design. First, the changes in systolic BP was no greater than 1 mmHg per IQR, which confers little clinical significance. Second, this study was observational and provides little insight into the pathophysiology. Although different pollutants showed significant associations at different time points in this study, future studies are required to understand the differential effects. Third, concentrations measured in a nearby monitoring station were used as a proxy for an individual’s exposure to pollutants, which is a potential source of bias. However, measuring an individual’s exposure to air pollutants requires special efforts including portable devices, which is difficult to achieve for large-scale studies such as the present study. This study was one of the largest to measure absolute BP levels and analyze short-term effects of air pollution. Fourth, a further limitation is the representativeness of the study sample. The present study participants were limited to patients of a tertiary medical center. Fifth, BP was not measured in a controlled condition. Recent studies have suggested the importance of ‘white-coat effects’, which means measured BP values may vary widely according to the measurement methods48. Sixth, this study evaluated between-subject differences in BP only. Because the number of repeated measurements varied according to the subjects, we were not able to analyze within-subject BP changes. Finally, while this study aimed at evaluating very short-term relationship, we could not assess day lag effects with this study design.
In summary, this study suggests that air pollution affects BP within hours after exposure. Gaseous pollutants such as O3 and NO2 increased systolic and diastolic BP in the short term. Patients with hypertension were shown to be more vulnerable to the BP-elevating effects than normotensive subjects. Short-term effects of PM were not significant, while SO2 and CO were associated with a reduction in BP within hours.
Methods
Study database and study participants
A retrospective analysis of single-center electronic health record data was performed. The study individuals were extracted from the Seoul National University Bundang Hospital clinical data warehouse. The center is a tertiary referral hospital in Korea and is equipped with complete electronic medical records reaching stage 7 criteria according to the Electronic Medical Record Adoption Model of the Healthcare Information and Management System Society. This study conformed to the principles of the Helsinki declaration of 1975 (revised version 2013). The study protocol was approved by the institutional review board of Seoul National University Bundang Hospital (No. B-1709-420-003), which waived the need for written informed consent from the subjects due to the retrospective nature of the study.
All consecutive subjects aged 18 years or older who were ambulatory and visited outpatient clinics with available BP measurements from February 2015 to June 2017 were enrolled. Subjects who visited the emergency department were excluded. The levels of systolic and diastolic BP, and pulse rate (PR) as well as the exact time of measurements (date, hour, minutes, and seconds) were extracted. Information on demographics, comorbidities, and medication history was collected from the data warehouse. Hypertension was defined as International Classification of Diseases, 10th Revision (ICD-10) code I10‒I15 and/or taking antihypertensive medications; diabetes as ICD-10 code (E11‒14) and/or glucose-lowering agents; and dyslipidemia as ICD-10 code (E78) and/or the use of lipid-lowering therapy.
Blood pressure measurement
BP was measured using automated oscillometric devices, including HBP-9020 (Omron Healthcare Co., Ltd., Japan), TM-2655 (Kensei Industry Co., Ltd., Japan), and Easy-X 800 R (Jawon Medical Co. Ltd, Republic of Korea), which were equipped with an elbow detection sensor and movable cuff to provide accurate measurements. Upon visiting the outpatient department, patients were instructed to measure their own sitting BP after resting for over 5 minutes. Two or more measurements were generally recommended, but not mandatory. Measured values were automatically matched to the patients’ identification code and recorded in the electronic health record database.
When a subject performed multiple measurements of BP within an hour, the mean of the last two values was chosen. Two measurements more than an hour apart were considered separate measurements. If a subject made multiple visits during the study period, the first value was chosen because only between-subject effects and not within-subject effects were considered in this study. The primary study endpoint was systolic BP, while secondary endpoints included diastolic BP and PR. The rationale for systolic BP as the primary endpoint is the recent evidence showing that systolic BP has greater effect on adverse outcomes than diastolic BP 3,49.
Air pollution and meteorological data
Hourly average concentrations of PM2.5, PM with an aerodynamic diameter of <10 μm (PM10), carbon monoxide (CO), sulfur dioxide (SO2), NO2, and O3 were obtained from the nearest monitoring station of the air quality regulatory monitoring network. The station (Unjung-Dong station) is located 5.9 km from the hospital and is installed in a residential area with a purpose to monitor urban air quality. This station collects air samples from 10‒20 meters above the ground level and indicates air quality within 70 km. PM2.5 and PM10 were measured by the β-ray absorption method, CO by the non-dispersive infrared method, SO2 by pulse ultraviolet fluorescence, NO2 by chemiluminescence, and O3 by ultraviolet photometry. Meteorological data, including temperature (°C), relative humidity (%), and air pressure (hPa) were obtained from the nearest station 15.1 km from the meteorological observation network run by the Korean Meteorological Administration.
Statistical analyses
Each BP and PR measurement was matched to the hourly levels of air pollutants and meteorological variables by linking the date and the time of measurements. Hourly BP or PR, hourly air pollution, smoothed meteorology, and an hourly time trend were put into generalized additive model with a Gaussian link function. Adjusted variables included study subjects’ demographic factors (age and sex), meteorological variables (temperature, atmospheric pressure, and humidity), time variables (year, month, day of the week, and hourly time trend). Natural splines were used for smoothing independent variables. Three degrees of freedom were chosen for temperature, atmospheric pressure, and humidity, while 8 for an hourly time trend based on explorations using 1 to 10 degrees of freedom. To investigate delayed effects of air pollution on BP or PR, we estimated the effects of air pollution on the concurrent hour through 8 hours prior to BP or PR. Eight-hour lag was chosen as the maximum lag because outpatients rarely stay more than 8 hours (working hour: 9 a.m.‒5 p.m.). A nonlinear relationship between air pollution levels and systolic BP was illustrated with the smooth function using penalized splines. Subgroup analysis was performed for the presence of hypertension and body mass index (BMI) (<25 kg/m2 and ≥25 kg/m2). Running means of lag hours during which the association was significant were used as exposure (lag hour 3 to 4 for PM2.5, 2 to 4 for SO2, 3 to 5 for CO, 3 to 5 for O3, and 0 to 2 for NO2). Descriptive statistics on study population were reported as means ± standard deviation (SD) for continuous variables and as numbers (percentages) for categorical variables. Effect estimates of BP or PR were presented for interquartile increments, as the difference between the 25th and 75th percentiles, for the concentration of each air pollutant.
A two-sided p-value < 0.05 was considered statistically significant. All statistical analyses were performed using R programming version 3.2.4 (http://www.R-project.org; The R Foundation for Statistical Computing, Vienna, Austria).
Supplementary information
Acknowledgements
We thank Ms. Moon Ju Kim for performing data collection and statistical analyses. This work was supported by the Basic Science Research Program through the National Research Foundation of Korea [Grant Number 2019R1C1C1006611].
Author contributions
Concept of study: Y.-J.C., S.-H. Kim, S.-H. Kang, S.-Y.K. and O.-J.K. Study design: Y.-J.C., S.-H. Kang, and S.-H. Kim. Statistical analysis: Y.-J.C., S.-H. Kim, S.-H. Kang, S.-Y.K. and O.-J.K. Interpretation of results: Y.-J.C., S.-H. Kang, C.-H.Y., H.-Y.L., T.-J.Y., I.-H.C. and C.-H.K. Drafting the paper: Y.-J.C. and S.-H. Kang. All the authors contributed critical review of the manuscript.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
is available for this paper at 10.1038/s41598-019-56413-y.
References
- 1.Collaborators GBDRF. Global, regional, and national comparative risk assessment of 84 behavioural, environmental and occupational, and metabolic risks or clusters of risks for 195 countries and territories, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2018;392:1923–1994. doi: 10.1016/S0140-6736(18)32225-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lewington S, et al. Age-specific relevance of usual blood pressure to vascular mortality: a meta-analysis of individual data for one million adults in 61 prospective studies. Lancet. 2002;360:1903–1913. doi: 10.1016/s0140-6736(02)11911-8. [DOI] [PubMed] [Google Scholar]
- 3.Choi YJ, et al. Reconsidering the cut-off diastolic blood pressure for predicting cardiovascular events: a nationwide population-based study from Korea. Eur Heart J. 2019;40:724–731. doi: 10.1093/eurheartj/ehy801. [DOI] [PubMed] [Google Scholar]
- 4.Mills KT, et al. Global Disparities of Hypertension Prevalence and Control: A Systematic Analysis of Population-Based Studies From 90 Countries. Circulation. 2016;134:441–450. doi: 10.1161/CIRCULATIONAHA.115.018912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Forman JP, Stampfer MJ, Curhan GC. Diet and lifestyle risk factors associated with incident hypertension in women. JAMA. 2009;302:401–411. doi: 10.1001/jama.2009.1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Whelton PK, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Hypertension. 2018;71:e13–e115. doi: 10.1161/HYP.0000000000000065. [DOI] [PubMed] [Google Scholar]
- 7.Williams B, et al. 2018 ESC/ESH Guidelines for the management of arterial hypertension. Eur Heart J. 2018;39:3021–3104. doi: 10.1093/eurheartj/ehy339. [DOI] [PubMed] [Google Scholar]
- 8.Brook RD, Weder AB, Rajagopalan S. “Environmental hypertensionology” the effects of environmental factors on blood pressure in clinical practice and research. J Clin Hypertens (Greenwich) 2011;13:836–842. doi: 10.1111/j.1751-7176.2011.00543.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mancia G. Short- and long-term blood pressure variability: present and future. Hypertension. 2012;60:512–517. doi: 10.1161/HYPERTENSIONAHA.112.194340. [DOI] [PubMed] [Google Scholar]
- 10.Kario K, et al. Morning surge in blood pressure as a predictor of silent and clinical cerebrovascular disease in elderly hypertensives: a prospective study. Circulation. 2003;107:1401–1406. doi: 10.1161/01.cir.0000056521.67546.aa. [DOI] [PubMed] [Google Scholar]
- 11.World Health Organization, Air pollution, https://www.who.int/airpollution/en/ (2019).
- 12.Cohen AJ, et al. Estimates and 25-year trends of the global burden of disease attributable to ambient air pollution: an analysis of data from the Global Burden of Diseases Study 2015. Lancet. 2017;389:1907–1918. doi: 10.1016/S0140-6736(17)30505-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brook RD, et al. Particulate matter air pollution and cardiovascular disease: An update to the scientific statement from the American Heart Association. Circulation. 2010;121:2331–2378. doi: 10.1161/CIR.0b013e3181dbece1. [DOI] [PubMed] [Google Scholar]
- 14.Newby DE, et al. Expert position paper on air pollution and cardiovascular disease. Eur Heart J. 2015;36:83–93b. doi: 10.1093/eurheartj/ehu458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Urch B, et al. Acute blood pressure responses in healthy adults during controlled air pollution exposures. Environ Health Perspect. 2005;113:1052–1055. doi: 10.1289/ehp.7785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brook RD, et al. Inhalation of fine particulate air pollution and ozone causes acute arterial vasoconstriction in healthy adults. Circulation. 2002;105:1534–1536. doi: 10.1161/01.cir.0000013838.94747.64. [DOI] [PubMed] [Google Scholar]
- 17.Dvonch JT, et al. Acute effects of ambient particulate matter on blood pressure: differential effects across urban communities. Hypertension. 2009;53:853–859. doi: 10.1161/HYPERTENSIONAHA.108.123877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Szyszkowicz M, Rowe BH, Brook RD. Even low levels of ambient air pollutants are associated with increased emergency department visits for hypertension. Can J Cardiol. 2012;28:360–366. doi: 10.1016/j.cjca.2011.06.011. [DOI] [PubMed] [Google Scholar]
- 19.Cai Y, et al. Associations of Short-Term and Long-Term Exposure to Ambient Air Pollutants With Hypertension: A Systematic Review and Meta-Analysis. Hypertension. 2016;68:62–70. doi: 10.1161/HYPERTENSIONAHA.116.07218. [DOI] [PubMed] [Google Scholar]
- 20.Chan SH, et al. Long-Term Air Pollution Exposure and Blood Pressure in the Sister Study. Environ Health Perspect. 2015;123:951–958. doi: 10.1289/ehp.1408125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Guo Y, et al. Gaseous air pollution and emergency hospital visits for hypertension in Beijing, China: a time-stratified case-crossover study. Environ Health. 2010;9:57. doi: 10.1186/1476-069X-9-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brook RD, Kousha T. Air Pollution and Emergency Department Visits for Hypertension in Edmonton and Calgary, Canada: A Case-Crossover Study. Am J Hypertens. 2015;28:1121–1126. doi: 10.1093/ajh/hpu302. [DOI] [PubMed] [Google Scholar]
- 23.Delfino RJ, et al. Traffic-related air pollution and blood pressure in elderly subjects with coronary artery disease. Epidemiology. 2010;21:396–404. doi: 10.1097/EDE.0b013e3181d5e19b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shah AS, et al. Global association of air pollution and heart failure: a systematic review and meta-analysis. Lancet. 2013;382:1039–1048. doi: 10.1016/S0140-6736(13)60898-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kang SH, et al. Ambient air pollution and out-of-hospital cardiac arrest. Int J Cardiol. 2016;203:1086–1092. doi: 10.1016/j.ijcard.2015.11.100. [DOI] [PubMed] [Google Scholar]
- 26.Kim, H. et al. Cardiovascular Effects of Long-Term Exposure to Air Pollution: A Population-Based Study With 900 845 Person-Years of Follow-up. J Am Heart Assoc6, 10.1161/JAHA.117.007170 (2017). [DOI] [PMC free article] [PubMed]
- 27.Di Q, et al. Air Pollution and Mortality in the Medicare Population. N Engl J Med. 2017;376:2513–2522. doi: 10.1056/NEJMoa1702747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chuang KJ, Chan CC, Shiao GM, Su TC. Associations between submicrometer particles exposures and blood pressure and heart rate in patients with lung function impairments. J Occup Environ Med. 2005;47:1093–1098. doi: 10.1097/01.jom.0000181749.03652.f9. [DOI] [PubMed] [Google Scholar]
- 29.Ibald-Mulli A, Stieber J, Wichmann HE, Koenig W, Peters A. Effects of air pollution on blood pressure: a population-based approach. Am J Public Health. 2001;91:571–577. doi: 10.2105/ajph.91.4.571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zanobetti A, et al. Ambient pollution and blood pressure in cardiac rehabilitation patients. Circulation. 2004;110:2184–2189. doi: 10.1161/01.CIR.0000143831.33243.D8. [DOI] [PubMed] [Google Scholar]
- 31.Choi JH, et al. Seasonal variation of effect of air pollution on blood pressure. J Epidemiol Community Health. 2007;61:314–318. doi: 10.1136/jech.2006.049205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Harrabi I, Rondeau V, Dartigues JF, Tessier JF, Filleul L. Effects of particulate air pollution on systolic blood pressure: A population-based approach. Environ Res. 2006;101:89–93. doi: 10.1016/j.envres.2006.01.012. [DOI] [PubMed] [Google Scholar]
- 33.Ibald-Mulli A, et al. Effects of particulate air pollution on blood pressure and heart rate in subjects with cardiovascular disease: a multicenter approach. Environ Health Perspect. 2004;112:369–377. doi: 10.1289/ehp.6523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Madsen C, Nafstad P. Associations between environmental exposure and blood pressure among participants in the Oslo Health Study (HUBRO) Eur J Epidemiol. 2006;21:485–491. doi: 10.1007/s10654-006-9025-x. [DOI] [PubMed] [Google Scholar]
- 35.Jerrett M, et al. Long-term ozone exposure and mortality. N Engl J Med. 2009;360:1085–1095. doi: 10.1056/NEJMoa0803894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zanobetti A, Schwartz J. Mortality displacement in the association of ozone with mortality: an analysis of 48 cities in the United States. Am J Respir Crit Care Med. 2008;177:184–189. doi: 10.1164/rccm.200706-823OC. [DOI] [PubMed] [Google Scholar]
- 37.Day DB, et al. Association of Ozone Exposure With Cardiorespiratory Pathophysiologic Mechanisms in Healthy Adults. JAMA Intern Med. 2017;177:1344–1353. doi: 10.1001/jamainternmed.2017.2842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Paffett ML, et al. Ozone Inhalation Impairs Coronary Artery Dilation via Intracellular Oxidative Stress: Evidence for Serum-Borne Factors as Drivers of Systemic Toxicity. Toxicol Sci. 2015;146:244–253. doi: 10.1093/toxsci/kfv093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chang LT, et al. Short-term exposure to noise, fine particulate matter and nitrogen oxides on ambulatory blood pressure: A repeated-measure study. Environ Res. 2015;140:634–640. doi: 10.1016/j.envres.2015.06.004. [DOI] [PubMed] [Google Scholar]
- 40.Sutherland BA, Harrison JC, Nair SM, Sammut IA. Inhalation gases or gaseous mediators as neuroprotectants for cerebral ischaemia. Curr Drug Targets. 2013;14:56–73. doi: 10.2174/138945013804806433. [DOI] [PubMed] [Google Scholar]
- 41.Penney DG. Hemodynamic response to carbon monoxide. Environ Health Perspect. 1988;77:121–130. doi: 10.1289/ehp.8877121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kanten WE, Penney DG, Francisco K, Thill JE. Hemodynamic responses to acute carboxyhemoglobinemia in the rat. Am J Physiol. 1983;244:H320–327. doi: 10.1152/ajpheart.1983.244.3.H320. [DOI] [PubMed] [Google Scholar]
- 43.Heistad DD, Wheeler RC. Effect of carbon monoxide on reflex vasoconstriction in man. J Appl Physiol. 1972;32:7–11. doi: 10.1152/jappl.1972.32.1.7. [DOI] [PubMed] [Google Scholar]
- 44.Meng Z, Geng H, Bai J, Yan G. Blood pressure of rats lowered by sulfur dioxide and its derivatives. Inhal Toxicol. 2003;15:951–959. doi: 10.1080/08958370390215785. [DOI] [PubMed] [Google Scholar]
- 45.Meng Z, Zhang H. The vasodilator effect and its mechanism of sulfur dioxide-derivatives on isolated aortic rings of rats. Inhal Toxicol. 2007;19:979–986. doi: 10.1080/08958370701515175. [DOI] [PubMed] [Google Scholar]
- 46.Lin H, et al. Long-Term Effects of Ambient PM2.5 on Hypertension and Blood Pressure and Attributable Risk Among Older Chinese Adults. Hypertension. 2017;69:806–812. doi: 10.1161/HYPERTENSIONAHA.116.08839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.McCracken JP, Smith KR, Diaz A, Mittleman MA, Schwartz J. Chimney stove intervention to reduce long-term wood smoke exposure lowers blood pressure among Guatemalan women. Environ Health Perspect. 2007;115:996–1001. doi: 10.1289/ehp.9888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.O’Brien E, et al. Use and interpretation of ambulatory blood pressure monitoring: recommendations of the British Hypertension Society. Bmj-Brit Med J. 2000;320:1128–1134. doi: 10.1136/bmj.320.7242.1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Flint AC, et al. Effect of Systolic and Diastolic Blood Pressure on Cardiovascular Outcomes. N Engl J Med. 2019;381:243–251. doi: 10.1056/NEJMoa1803180. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.