Abstract
When a malaria parasite invades a host erythrocyte, it pushes itself in and invaginates a portion of the host membrane, thereby sealing itself inside and establishing itself in the resulting vacuole. The parasitophorous vacuolar membrane (PVM) that surrounds the parasite is modified by the parasite using its secretory organelles. To survive within this enveloping membrane, the organism must take in nutrients, secrete wastes, export proteins into the host cell and eventually egress. Here, we review current understanding of the unique solutions Plasmodium has evolved to these challenges and discuss the remaining questions.
Keywords: Plasmodium, parasitophorous vacuole
Parasitophorous Vacuolar Membrane of Malaria Parasites
While it is not yet one hundred years after the flurry of activity by the earliest electron microscopists that defined the concepts and paradigms of cellular membrane topology, their hypotheses have moved well beyond theories – they are generally established now as the law that governs protein and vesicle trafficking within cells. And given how different the cytoplasm and extracellular fluid are, it stands to reason that the separate surfaces that make up the membrane bilayer should also be very different; indeed, the plasma membrane has asymmetrical composition of inner and outer leaflets. Measured by area, the membrane that surrounds the related parasite Toxoplasma tachyzoite as it pushes itself into a host cell prior to pinching off is nearly completely derived from host cell membrane[1]. Likewise, the lipids of the membrane enveloping the malaria parasite are derived, at least partially, from the erythrocyte membrane[2]. Thus it is appealing to imagine that the malaria parasite, Plasmodium, also simply invaginates a portion of the host membrane, sealing itself inside and establishing a parasitophorous vacuole (PV) that retains the inverted asymmetry of all endosomal membranes (Figure 1). But however appealing, it cannot be this simple. For one thing, the parasitophorous vacuolar membrane (PVM, see Glossary) that surrounds the malaria parasite is unique in its paucity of host protein -- most integral membrane proteins may be actively excluded and lipids may be added during its formation[3,4]. For another, PVM topology seems backwards: whereas most host cellular vacuoles would be bound by a membrane transporting proteins (synthesized in the host cellular cytoplasm) into the vacuolar lumen, the PVM transports proteins (synthesized in the parasite cytoplasm) out of the vacuole and into the host cell cytoplasm, a feature also seen in many intracellular bacterial vacuoles[5,6]. But unlike bacterial vacuoles in nucleated cells, malaria parasites do not have to face destruction by the host cell and instead focus on obtaining nutrients and evading extracellular immunity. Structure and function have been adapted by Plasmodium to fit its particular needs.
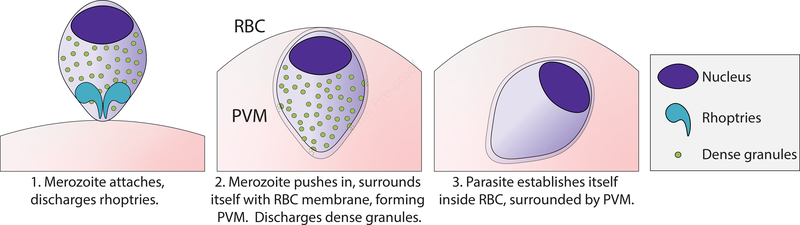
Figure 1. Formation of the PVM.
An infectious merozoite attaches to an erythrocyte, discharging rhoptries to facilitate invasion and add content to the recipient membrane. The merozoite pushes its way in, surrounding itself with RBC membrane and forming the PVM. It discharges dense granules to contribute to the PVM. The parasite then established itself inside the erythrocyte, surrounded by the PVM.
Biogenesis and Structure
During invasion, the parasite modifies the PVM by secreting components of the rhoptry organelles[7,8]; once inside, discharge of dense granules contributes to formation of the PVM as well[9–11]. Undoubtably, lipid alterations occur with invasion [7,12,13] but how and when these alterations occur, whether the lipid changes are symmetric or asymmetric, and how lipid composition changes throughout the life cycle of the parasite are unknown. In fact, there is not even consensus on the lipid composition of the PVM. Clearly, the mechanisms of lipid homeostasis in this organism are poorly understood.
PVM/PPM Relationship
The PVM is closely apposed to the parasite plasma membrane (PPM) and recent evidence suggests attachment points between the two[14]. Specifically, soluble proteins in the parasitophorous vacuole are excluded from portions of the vacuolar space. Also, knockdown of the single-pass membrane protein EXP1 causes increased separation of PVM from the PPM[15]. The abundant single-pass early transcribed membrane proteins (ETRAMPS) could participate in formation of attachment points between the two membranes as well[16]. Further, blockade of protein export across the PVM causes blebbing of sections of PVM into the erythrocyte cytoplasm, away from the PPM but tethered at foci where the two membranes remain together[17,18]. A similar phenotype has been observed in liver-stage parasites[19].
Tubovesicular Network
In contrast to the blebbing described above, normal extensions of PVM into the erythrocytic space do occur, called the tubovesicular network (TVN)[20]. This network is speculated to provide membrane for the formation of Maurer’s clefts[21,22], a Golgi-like structure out in the host erythrocyte that is responsible for trafficking exported parasite effector proteins to the erythrocyte surface and perhaps vesicular destinations as well. The extra surface area provided by the TVN may also allow the remarkable gross shape fluctuations in early-stage parasites that facilitate growth and ameboid activity within the erythrocyte[23,24].
Domains
Careful observation of the PVM reveals a heterogeneous lateral distribution of PVM components, alternatively described as beaded, patchy, or piebald[14,23,25], with limited protein flow between foci as assessed by photobleaching experiments[26]. The integral membrane protein EXP2 by live fluorescence microscopy is proximal to a soluble parasitophorous vacuole marker (signal peptide-tagged mRuby3), while the PPM lipid transporter PfNCR1 (Niemann-Pick type C-related protein 1)[27] anticorrelates and may correspond to attachment domains with lipid flow between PVM and PPM[14].
Protein Targeting
While it has been established that a signal sequence is sufficient to get a reporter into the PV lumen[26], PVM targeting may be less straightforward. A study on the gametocyte protein Pfs16 suggested that its N-terminal signal sequence plus a C-terminal motif containing a hydrophobic stretch was enough to target a GFP reporter to the PVM, and possesses a motif also found in other PVM proteins such as Exp1 and Exp2 [28]. The protein PfAK2 was shown to contain an N-terminal sequence sufficient for targeting a GFP reporter to the PVM, likely anchored by myristate and palmitate moieties, and this was presented as an alternate targeting sequence [29]. Still more work remains to be done to understand PVM targeting.
Function
Any consideration of PVM function must start with an inevitable comparison to a related apicomplexan parasite Babesia, whose PVM disappears soon after formation[30,31]. The PVM is not an obligate structure of every intracellular apicomplexan organism; fundamentally intracellular parasites can thrive without a continuous macromolecular barrier to host cytosol[31,32]. But unless the malaria parasite PVM is vestigial, i.e. a remnant of the invasion process, its continuing presence in all malaria species indicates a selective advantage in the cytoplasm of erythrocytes, and its existence mandates that proteins evolve to modify the PVM in order to carry out essential functions for the replication of viable daughter cells: the PVM must facilitate intake of nutrients, excretion of wastes and export of protein effectors for manipulation of the environment outside the parasite (Figure 2, Key Figure). Ultimately, the PVM creates a physical barrier for the daughter cells to overcome when it is time to leave. The parasite needs to destroy the PVM in order to egress.
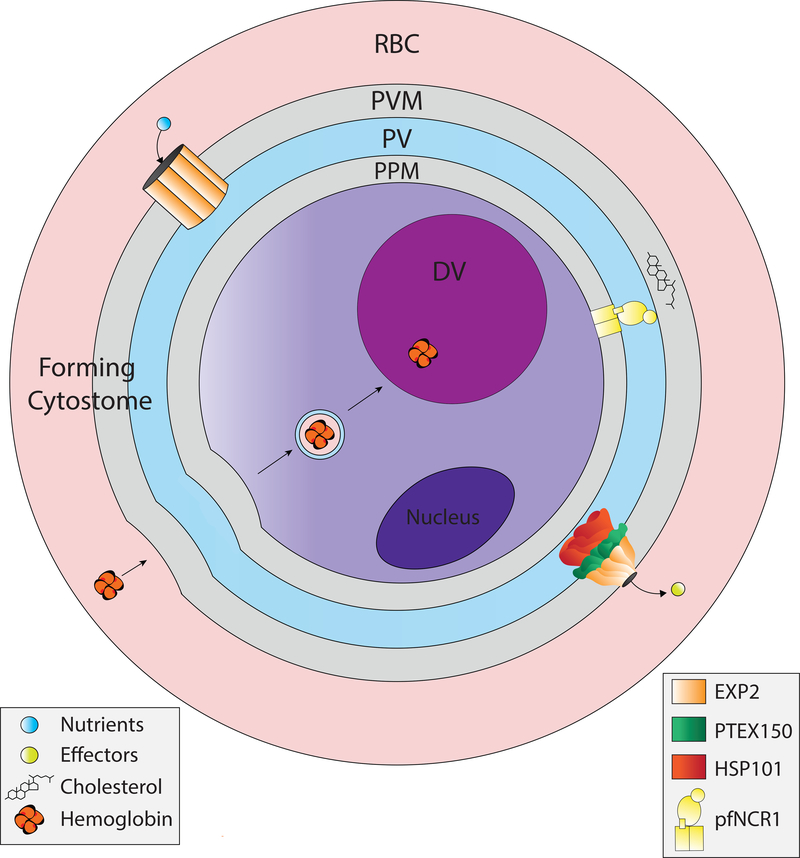
Figure 2, Key Figure. Functions of the PVM.
The PVM has a translocon complex called PTEX to export effector proteins into the host cell. There is a nutrient channel formed by EXP2 to import nutrients. It likely excretes wastes as well. EXP2 is depicted in the two separate macromolecular complexes, detailed in Figure 3. There is a forming cytostome that spans the PVM and PPM to ingest hemoglobin. There is a lipid transporter, PfNCR1, that contributes to membrane lipid homeostasis, likely by keeping cholesterol out of the plasma membrane. DV, digestive vacuole.
Nutrient Acquisition and Waste Excretion
The clearest problem with retaining a continuous spheroidal bilayer as a retaining wall is that it is a barrier to the flux of nutrients and waste materials. From the bloodstream the parasite requires pantothenate for CoA synthesis, glucose for glycolytic metabolism, isoleucine for protein synthesis (most amino acids are obtained from hemoglobin but there is no isoleucine in human hemoglobin), a purine and certain lipids[33–35]. Pathways of lipid acquisition in the infected RBC are poorly defined but for most hydrophilic small molecules, entry into the infected erythrocyte is via parasite-derived new permeation pathways[36], especially PSAC[37,38], a channel whose components are exported by the parasite out to the erythrocyte membrane[39–41]. Glucose, in contrast, appears to predominantly enter via the host glucose transporter Glut 1[42]. These molecules must then pass through the PVM to get to specific transporters at the PPM. At the PVM, there is a channel established by the parasite protein EXP2[17] that allows nutrients smaller than 1.4 kDa to pass through[43,44]. Molecular assignment of the nutrient channel activity to EXP2 was based on observation of altered electrical gating properties in EXP2 mutants lacking a C-terminal charged region[17]. Knockdown of this channel protein is lethal to the parasite[17]. A similar channel exists in T. gondii[45]. In addition to facilitating nutrient import, this channel is thought to allow export of wastes, especially the glycolytic product lactate, as well as excess amino acids, ATP and glutathione (ATP and glutathione may be exported to maintain host cell homeostasis and oxidative balance)[46–48].
Hemoglobin Degradation
Hemoglobin consumption is also required for parasite maturation, as alluded to above, both to acquire small molecules (such as amino acids and heme) and to maintain osmotic homeostasis[47,49–51]. Hemoglobin is brought in by means of an endocytic apparatus called the cytostome that spans the PVM and PPM membranes[52], effecting delivery to the acidic digestive vacuole where proteolysis, heme sequestration and amino acid/peptide transport to the parasite cytoplasm take place. Formation of the cytostome is poorly understood, but could take place at the membrane domains where the PVM and PPM are closely apposed and likely contiguous[14].
Effector Protein Export
The constraint of a PVM necessitates that the parasite translocate protein effectors across this membrane into the erythrocyte. Exported proteins contribute to the aforementioned PSAC nutrient channel at the infected erythrocyte surface. Others form knob structures under the erythrocyte surface to cluster variant surface antigens called PfEMPs that it sends out to mediate adherence to the vascular endothelium (thereby avoiding the spleen). Still others form microvesicles, modify erythrocyte cytoskeleton rigidity or manipulate vascular tone[53,54]. The translocon required for export (PTEX) was first identified as a PVM complex with a putative pore protein (EXP2) and a AAA+-ATPase that could potentially unfold translocon cargo (HSP101)[10,55]. HSP101 was recently shown to have unfoldase activity[56]. The core complex also contains an adaptor protein, PTEX150. Knockdown experiments have demonstrated the essential roles of all three components in effector export[57,58]. Establishment of the PSAC nutrient channel is impaired by translocon disruption[58]. Accessory translocon components (PTEX88 and TRX2) are non-essential in cultured parasites but could play roles in export of particular effectors[59,60]. The translocon-associated protein RON3 has recently been shown to be essential for glucose acquisition and protein export[61], and further work is required to determine the mechanism of RON3 action and the nature of its interaction with PTEX. How the translocon determines which proteins get exported and which remain in the PV or its membrane remains unknown. There is information important for export at the N-terminus of mature effector proteins, but no clear motif or recognition mechanism has been identified.
The cryoEM structure of the malaria parasite translocon[62] shows a twisted hexamer of HSP101 docked into the funnel-shaped EXP2 heptameric channel, attached via a heptameric PTEX150 adapter (Figure 3). Two conformations of the translocon were seen, with a compressed or extended HSP101 multimer interacting with offset, unraveled single chains of cargo protein inside the translocon, suggesting a processive threading or compaction mechanism of translocation. It is interesting that Plasmodium EXP2 appears to have a dual role in nutrient uptake and in protein export[14]. These appear to be different functions, perhaps involving different multimeric complexes[17]. The T. gondii EXP2 ortholog Gra17 has only the nutrient channel function[45]. Both parasites possess HSP101s, but it seems that Plasmodium has evolved the adaptor protein PTEX150 to recruit EXP2 and HSP101 to allow protein translocation. Recent work shows that the protein translocation function of the translocon can be competent when nutrient import channel activity is insufficient[63].
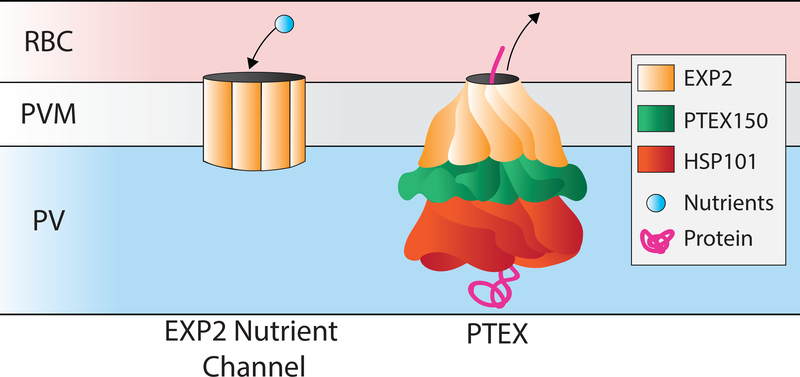
Figure 3. Dual functions of EXP2.
EXP2 forms a nutrient channel, and separately forms the PTEX translocation channel in association with Hsp101 and PTEX 150. EXP2 is depicted as a homomultimer for the nutrient channel, though tertiary and quaternary structural details are unknown and there may be other components of this complex that are undefined. For PTEX, EXP2 is shown as a twisted, funnel-shaped multimeric channel in association with other core components PTEX150 and HSP101, based on the cryo-EM structure[62].
Egress from the Host Cell
A final challenge for the PVM-enveloped malaria parasite is that at the end of its growth and replication, it must get out of the PVM (and host erythrocyte) to invade new red blood cells. To do so, about 10 minutes before egress[64], the parasite initiates the cGMP/Ca++-triggered secretion of subtilisin-like protease 1 (SUB1) into the PV[65–67] (Figure 4). There, SUB1 activates egress effectors called SERAs (a family of cysteine proteases) and MSPs (a family of merozoite surface proteins involved in invasion as well as egress) [67–70]. SUB1 knockout prevents PV swelling, rupture and dissolution[67]. MSPs and SERAs are thought to play later roles in exit from the host cell, so how SUB1 mediates PVM destruction is unclear. Knock down or chemical inhibition of the aspartic protease plasmepsin X prevents maturation of SUB1 and phenocopies SUB1 knockout, placing this enzyme at the top of the proteolytic cascade (Fig 4)[71,72]. Plasmepsin X inhibition is a promising avenue for antimalarial chemotherapy.
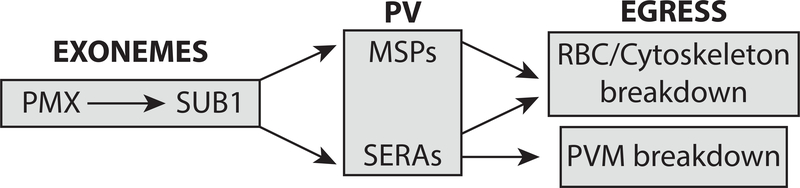
Figure 4. Proteolytic egress cascade.
Plasmepsin X (PMX) is the maturase for subtilisin-like protease 1 (SUB1), which activates cysteine proteases called SERAs and merozoite surface proteins (MSPs), leading to PVM, RBC membrane and cytoskeleton breakdown. The specific roles of these later effectors in the egress process are largely unknown.
Unanswered Questions
PVM Replenishment
Although we are starting to learn about this important membrane surrounding the intraerythrocytic malaria parasite, much remains unknown. The Unanswered Questions section begins with a paradox – while PVM depletion is expected after invasion due to the need for significant membrane area to form Maurer’s clefts and to transit hemoglobin to the digestive vacuole, in fact the PVM grows during the first day of intraerythrocytic development. Thus, we do not understand how this membrane is replenished; there must be mechanisms of membrane recycling and rebuilding. Various lipid components are synthesized, scavenged or a combination of the two[73,74]. Details of lipid acquisition for a number of lipid species is still sketchy. Lipid gradients between membranes are maintained[75], so there must be homeostatic mechanisms that are largely undiscovered. PfNCR1 is a lipid transporter that appears to contribute to membrane lipid maintenance[76] from its topologically bewildering position in the PPM. Its substrate is most likely cholesterol but this hypothesis has not been formally demonstrated. The return journey for a lipid molecule from the digestive vacuole back to the PVM, while seemingly essential, remains to be elucidated.
PVM Formation
The contributions of merozoite organelle secretion (rhoptries, dense granules) in formation of the PVM has been studied extensively in Toxoplasma[77], but the surface has barely been scratched in Plasmodium, in part due to the smaller size of the host cell, the parasite, and its granules.
PVM Structure
We do not understand the structure of the domains of the PVM or how the attachment points to the PPM are formed. The abundant ETRAMP proteins and the related protein EXP1 are likely to be structurally important[78], but more work needs to be done.
Hemoglobin Ingestion
The ingestion of hemoglobin through a cytostome that spans the PVM and PPM is understood only at a rudimentary morphological level[79]. Molecular components and mechanisms remain to be discovered.
Specificity of Protein Export
As alluded to previously, the puzzle of the specificity of protein export across the PVM is yet to be solved[80], as well as the roles for most translocated protein effectors. More generally, the presumed plasma membrane exocytosis of translocon substrates by fusion of Golgi-derived cargo vesicles is not understood, nor are the mechanisms by which newly synthesized transmembrane proteins, which should be retained by the PPM, get to a translocon that is in the wrong membrane – the PVM.
Concluding Remarks
Given that Babesia escapes from its PVM, as discussed above, the mystery remains as to why intraerythrocytic malaria parasites go to the trouble of maintaining a PVM, given the attendant challenges of access to the host cell. In the related apicomplexan parasite Toxoplasma gondii[81,82] and in liver-stage Plasmodium[83], the PVM protects the parasite from the host cell, which is trying to kill it via GTPase-mediated attack, apoptosis and/or autophagy. Protein components of the PVM in these cases are mostly different from those of intraerythrocytic Plasmodium[78]. The parasite does devote a significant portion of its genome to establishing the Maurer’s clefts[84] that allow protein sorting in the host cell to get virulence determinants to the surface. Perhaps the PVM is needed mainly to extend into the host cell and provide membrane for Maurer’s cleft formation, though the PVM is maintained even after this organelle is formed. In certain respects, the maintenance of the PVM creates a compartment, an organelle out of the parasite that looks like a cytoplasmic inclusion to the host cell, and the parasite decorates its new house to its advantage. Alternatively, it could be that the parasite is protecting itself from something in the erythrocyte such as a protein, an RNA, an osmotic force. There is much that remains to be elucidated about PVM structure and function (see Outstanding Questions). We look forward to learning more about this fascinating outer covering of the intraerythrocytic malaria parasite that is itself an inner liner to the host erythrocyte.
OUTSTANDING QUESTIONS.
How is the PVM replenished upon depletion from formation of Maurer’s clefts and cytostomes?
How does the malaria parasite alter the nascent PVM?
How do PVM domains and attachment foci form?
How does hemoglobin ingestion work?
How is protein export specified? What do all these exported effectors do?
Why does the parasite maintain a PVM?
Highlights.
Recent work suggests that the PVM is not a homogeneous membrane, but rather has domains that are specialized for transport and for lipid exchange between membranes
The PVM is tethered to the PPM at distinct attachment points
A translocon complex called PTEX exports protein effectors into the host erythrocyte
The structure of this complex has been solved by cryoEM and appears to function by a threading/compaction mechanism
The translocon pore protein, EXP2, has a separate function as a nutrient channel across the PVM
Egress from the host cell requires a proteolytic cascade centered on SUB1, which is required for breaking down the PVM as well as rupture of the RBC
ACKNOWLEDGEMENT
Illustrations by Abigail Kimball in association with InPrint at Washington University in St Louis. This work is funded in part by the Division of Intramural Research of the NICHD.
GLOSSARY
- Cytostome
a mouth-like structure that spans the PVM and PPM and effects ingestion of hemoglobin by the parasite
- Digestive Vacuole
the lysosome-like compartment where hemoglobin is degraded and heme is sequestered as hemozoin
- Egress
the process of parasite escape from the host cell after replication and packaging of daughter cells (merozoites)
- EXP2
a PVM protein that forms the channel component in PTEX for protein export and also forms a channel for nutrient import
- Maurer’s clefts
a membranous structure elaborated by the parasite in the cytoplasm of the host erythrocyte, involved in targeting exported effectors to locations in the host cell including the erythrocyte surface
- Parasite plasma membrane (PPM)
the membrane that bounds the parasite
- Parasitophorous vacuolar membrane (PVM)
the membrane that envelops the parasite as it enters the host erythrocyte
- Plasmepsin X
an aspartic protease that processes and activates SUB1; its blockade prevents egress and positions it as an attractive drug target
- PSAC
a parasite-genetically encoded channel at the host cell surface that facilitates nutrient uptake
- PTEX
the translocon complex at the PVM that facilitates effector protein export
- Subtilisin-like protease 1 (SUB1)
a proteolytic enzyme central to the process of egress
- Tubovesicular network (TVN)
the membrane network that extends from the PVM and is thought to provide membrane for the Maurer’s clefts
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Suss-Toby E et al. (1996) Toxoplasma invasion: the parasitophorous vacuole is formed from host cell plasma membrane and pinches off via a fission pore. Proc. Natl. Acad. Sci 93, 8413–8418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ward GE et al. (1993) The origin of parasitophorous vacuole membrane lipids in malaria-infected erythrocytes. J. Cell Sci 106, 237–248 [DOI] [PubMed] [Google Scholar]
- 3.Murphy SC et al. (2006) Lipid rafts and malaria parasite infection of erythrocytes (Review). Mol. Membr. Biol 23, 81–88 [DOI] [PubMed] [Google Scholar]
- 4.Lauer S et al. (2000) Vacuolar uptake of host components, and a role for cholesterol and sphingomyelin in malarial infection. EMBO J. 19, 3556–3564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cornejo E et al. (2017) How to rewire the host cell: A home improvement guide for intracellular bacteria. J. Cell Biol DOI: 10.1083/jcb.201701095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kwon DH and Song HK A structural view of xenophagy, a battle between host and microbes., Molecules and Cells. (2018) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lingelbach K and Joiner KA (1998) The parasitophorous vacuole membrane surrounding Plasmodium and Toxoplasma: An unusual compartment in infected cells. J. Cell Sci 111, 1467–1475 [DOI] [PubMed] [Google Scholar]
- 8.Hiller NL et al. (2003) Identification of a Stomatin Orthologue in Vacuoles Induced in Human Erythrocytes by Malaria Parasites. J. Biol. Chem 278, 48413–48421 [DOI] [PubMed] [Google Scholar]
- 9.Culvenor JG et al. (1991) Plasmodium falciparum ring-infected erythrocyte surface antigen is released from merozoite dense granules after erythrocyte invasion. Infect. Immun 59, 1183–1187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bullen HE et al. (2012) Biosynthesis, Localization, and Macromolecular Arrangement of the Plasmodium falciparum Translocon of Exported Proteins (PTEX). J. Biol. Chem 287, 7871–7884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bannister L. et al. (2000) A Brief Illustrated Guide to the Ultrastructure of Plasmodium falciparum Asexual Blood Stages. Parasitol. Today 16, 427–433 [DOI] [PubMed] [Google Scholar]
- 12.Dluzewski AR et al. (1995) Origins of the parasitophorous vacuole membrane of the malaria parasite: Surface area of the parasitized red cell. Eur. J. Cell Biol 68, 446–449 [PubMed] [Google Scholar]
- 13.Dluzewski AR et al. (1992) Origins of the parasitophorous vacuole membrane of the malaria parasite, Plasmodium falciparum, in human red blood cells. J. Cell Sci 102, 527–532 [DOI] [PubMed] [Google Scholar]
- 14.Garten M et al. Membrane contact sites mediate lipid exchange at the Plasmodium-red blood cell interface. In process of submission to Biorxiv. [Google Scholar]
- 15.Nessel T et al. (2019) EXP1 is required for organization of the intraerythrocytic malaria parasite vacuole. BioRxiv DOI: 10.1101/752634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Spielmann T et al. (2006) Organization of ETRAMPs and EXP-1 at the parasite-host cell interface of malaria parasites. Mol. Microbiol 59, 779–794 [DOI] [PubMed] [Google Scholar]
- 17.Garten M et al. (2018) EXP2 is a nutrient-permeable channel in the vacuolar membrane of Plasmodium and is essential for protein export via PTEX. Nature Microbiol. 3, 1090–1098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Charnaud SC et al. (2018) Spatial organization of protein export in malaria parasite blood stages. Traffic 19, 605–623 [DOI] [PubMed] [Google Scholar]
- 19.Kalanon M et al. (2016) The Plasmodium translocon of exported proteins component EXP2 is critical for establishing a patent malaria infection in mice. Cell. Microbiol DOI: 10.1111/cmi.12520 [DOI] [PubMed] [Google Scholar]
- 20.Elmendorf HG (1994) Plasmodium falciparum exports the Golgi marker sphingomyelin synthase into a tubovesicular network in the cytoplasm of mature erythrocytes. J. Cell Biol 124, 449–462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Spycher C et al. (2006) Genesis of and Trafficking to the Maurer’s Clefts of Plasmodium falciparum-Infected Erythrocytes. Mol. Cell. Biol 26, 4074–4085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wickert H (2003) Evidence for trafficking of PfEMP1 to the surface of -infected erythrocytes via a complex membrane network. Eur. J. Cell Biol 82, 271–284 [DOI] [PubMed] [Google Scholar]
- 23.Wickham ME (2001) Trafficking and assembly of the cytoadherence complex in Plasmodium falciparum-infected human erythrocytes. EMBO J. 20, 5636–5649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Grüring C et al. (2011) Development and host cell modifications of Plasmodium falciparum blood stages in four dimensions. Nat. Commun DOI: 10.1038/ncomms1169 [DOI] [PubMed] [Google Scholar]
- 25.Riglar DT et al. (2013) Spatial association with PTEX complexes defines regions for effector export into Plasmodium falciparum-infected erythrocytes. Nat. Commun 4, 1415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Adisa A et al. (2003) The Signal Sequence of Exported Protein-1 Directs the Green Fluorescent Protein to the Parasitophorous Vacuole of Transfected Malaria Parasites. J. Biol. Chem 278, 6532–6542 [DOI] [PubMed] [Google Scholar]
- 27.Istvan ES et al. (2019) Plasmodium niemann-pick type C1- related protein is a druggable target required for parasite membrane homeostasis. Elife 8, e40529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Eksi S and Williamson KC (2011) Protein Targeting to the Parasitophorous Vacuole Membrane of Plasmodium falciparum. Eukaryot. Cell 10, 744–752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Thavayogarajah T et al. (2015) Alternative Protein Secretion in the Malaria Parasite Plasmodium falciparum. PLoS One 10, e0125191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rudzinska M et al. (1976) An electron microscopic study of Babesia microti invading erythrocytes. Cell Tissue Res. 169, 323–334 [DOI] [PubMed] [Google Scholar]
- 31.Repnik U et al. (2015) The apicomplexan parasite B abesia divergens internalizes band 3, glycophorin A and spectrin during invasion of human red blood cells. Cell. Microbiol 17, 1052–1068 [DOI] [PubMed] [Google Scholar]
- 32.Shaw MK Cell invasion by Theileria sporozoites., Trends in Parasitology. (2003) [DOI] [PubMed] [Google Scholar]
- 33.Liu J et al. (2006) Plasmodium falciparum ensures its amino acid supply with multiple acquisition pathways and redundant proteolytic enzyme systems. Proc. Natl. Acad. Sci. U. S. A 103, 8840–8845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Divo AA et al. (1985) Nutritional Requirements of Plasmodium falciparum in Culture. I. Exogenously Supplied Dialyzable Components Necessary for Continuous Growth. J. Protozool 32, 59–64 [DOI] [PubMed] [Google Scholar]
- 35.Asahi H (2009) Plasmodium falciparum: Chemically defined medium for continuous intraerythrocytic growth using lipids and recombinant albumin. Exp. Parasitol 121, 22–28 [DOI] [PubMed] [Google Scholar]
- 36.Kutner S et al. (1983) Permselectivity changes in malaria (Plasmodium falciparum) infected human red blood cell membranes. J. Cell. Physiol 114, 245–251 [DOI] [PubMed] [Google Scholar]
- 37.Nguitragool W et al. (2011) Malaria Parasite clag3 Genes Determine Channel-Mediated Nutrient Uptake by Infected Red Blood Cells. Cell 145, 665–677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Desai SA et al. (2000) A voltage-dependent channel involved in nutrient uptake by red blood cells infected with the malaria parasite. Nature 406, 1001–1005 [DOI] [PubMed] [Google Scholar]
- 39.Counihan NA et al. (2017) Plasmodium falciparum parasites deploy RhopH2 into the host erythrocyte to obtain nutrients, grow and replicate. Elife 6, e23217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sherling ES et al. (2017) The Plasmodium falciparum rhoptry protein RhopH3 plays essential roles in host cell invasion and nutrient uptake. Elife 6, e23239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ito D et al. (2017) An essential dual-function complex mediates erythrocyte invasion and channel-mediated nutrient uptake in malaria parasites. Elife 6, e23485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kirk K and Saliba K (2007) Targeting Nutrient Uptake Mechanisms in Plasmodium. Curr. Drug Targets 8, 75–88 [DOI] [PubMed] [Google Scholar]
- 43.Desai SA and Rosenberg RL (1997) Pore size of the malaria parasite’s nutrient channel. Proc. Natl. Acad. Sci 94, 2045–2049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Desai SA et al. (1993) A nutrient-permeable channel on the intraerythrocytic malaria parasite. Nature 362, 643–646 [DOI] [PubMed] [Google Scholar]
- 45.Gold DA et al. (2015) The Toxoplasma Dense Granule Proteins GRA17 and GRA23 Mediate the Movement of Small Molecules between the Host and the Parasitophorous Vacuole. Cell Host Microbe 17, 642–652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Atamna H and Ginsburg H (1997) The Malaria Parasite Supplies Glutathione to its Host Cell - Investigation of Glutathione Transport and Metabolism in Human Erythrocytes Infected with Plasmodium Falciparum. Eur. J. Biochem 250, 670–679 [DOI] [PubMed] [Google Scholar]
- 47.Krugliak M et al. (2002) Intraerythrocytic Plasmodium falciparum utilizes only a fraction of the amino acids derived from the digestion of host cell cytosol for the biosynthesis of its proteins. Mol. Biochem. Parasitol 119, 249–256 [DOI] [PubMed] [Google Scholar]
- 48.Kanaani J and Ginsburg H (1989) Metabolic interconnection between the human malarial parasite Plasmodium falciparum and its host erythrocyte. Regulation of ATP levels by means of an adenylate translocator and adenylate kinase. J. Biol. Chem 264, 3194. [PubMed] [Google Scholar]
- 49.Sigala PA and Goldberg DE (2014) The Peculiarities and Paradoxes of Plasmodium Heme Metabolism. Annu. Rev. Microbiol 68, 259–278 [DOI] [PubMed] [Google Scholar]
- 50.Francis SE et al. (1997) Hemoglobin metabolism in the malaria parasite Plasmodium falciparum. Annu. Rev. Microbiol 51, 97–123 [DOI] [PubMed] [Google Scholar]
- 51.Lew VL (2003) Excess hemoglobin digestion and the osmotic stability of Plasmodium falciparum-infected red blood cells. Blood 101, 4189–4194 [DOI] [PubMed] [Google Scholar]
- 52.Bakar NA et al. (2010) Digestive-vacuole genesis and endocytic processes in the early intraerythrocytic stages of Plasmodium falciparum. J. Cell Sci 123, 441–450 [DOI] [PubMed] [Google Scholar]
- 53.Matthews KM et al. (2019) Illuminating how malaria parasites export proteins into host erythrocytes. Cell. Microbiol 21, e13009. [DOI] [PubMed] [Google Scholar]
- 54.Spillman NJ et al. (2015) Protein Export into Malaria Parasite–Infected Erythrocytes: Mechanisms and Functional Consequences. Annu. Rev. Biochem 84, 813–841 [DOI] [PubMed] [Google Scholar]
- 55.de Koning-Ward TF et al. (2009) A newly discovered protein export machine in malaria parasites. Nature 459, 945–949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Matthews KM et al. (2019) Uncoupling the threading and unfoldase actions of plasmodium HSP101 reveals differences in export between soluble and insoluble proteins. MBio DOI: 10.1128/mBio.01106-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Elsworth B et al. (2014) PTEX is an essential nexus for protein export in malaria parasites. Nature 511, 587–591 [DOI] [PubMed] [Google Scholar]
- 58.Beck JR et al. (2014) PTEX component HSP101 mediates export of diverse malaria effectors into host erythrocytes. Nature 511, 592–595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Matz JM et al. (2015) In Vivo Function of PTEX88 in Malaria Parasite Sequestration and Virulence. Eukaryot. Cell 14, 528–534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Matthews K et al. (2013) The Plasmodium translocon of exported proteins (PTEX) component thioredoxin-2 is important for maintaining normal blood-stage growth. Mol. Microbiol 89, 1167–1186 [DOI] [PubMed] [Google Scholar]
- 61.Low LM et al. (2019) Deletion of Plasmodium falciparum Protein RON3 Affects the Functional Translocation of Exported Proteins and Glucose Uptake. MBio 10, 01460–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ho C-M et al. (2018) Malaria parasite translocon structure and mechanism of effector export. Nature 561, 70–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Mesén-Ramírez P et al. (2019) EXP1 is critical for nutrient uptake across the parasitophorous vacuole membrane of malaria parasites. PLOS Biol. DOI: 10.1371/journal.pbio.3000473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Glushakova S et al. (2018) Rounding precedes rupture and breakdown of vacuolar membranes minutes before malaria parasite egress from erythrocytes. Cell. Microbiol 20, e12868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Collins CR et al. (2013) Malaria Parasite cGMP-dependent Protein Kinase Regulates Blood Stage Merozoite Secretory Organelle Discharge and Egress. PLoS Pathog. 9, e1003344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Yeoh S et al. (2007) Subcellular Discharge of a Serine Protease Mediates Release of Invasive Malaria Parasites from Host Erythrocytes. Cell 131, 1072–1083 [DOI] [PubMed] [Google Scholar]
- 67.Thomas JA et al. (2018) A protease cascade regulates release of the human malaria parasite Plasmodium falciparum from host red blood cells. Nat. Microbiol 3, 447–455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ruecker A et al. (2012) Proteolytic Activation of the Essential Parasitophorous Vacuole Cysteine Protease SERA6 Accompanies Malaria Parasite Egress from Its Host Erythrocyte. J. Biol. Chem 287, 37949–37963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Child MA et al. (2010) Regulated maturation of malaria merozoite surface protein-1 is essential for parasite growth. Mol. Microbiol 78, 187–202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Das S et al. (2015) Processing of Plasmodium falciparum Merozoite Surface Protein MSP1 Activates a Spectrin-Binding Function Enabling Parasite Egress from RBCs. Cell Host Microbe 18, 433–444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Nasamu AS et al. (2017) Plasmepsins IX and X are essential and druggable mediators of malaria parasite egress and invasion. Science (80-. ). 358, 518–522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Pino P et al. (2017) A multistage antimalarial targets the plasmepsins IX and X essential for invasion and egress. Science (80-. ). 358, 522–528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ben Mamoun C et al. (2009) Targeting the lipid metabolic pathways for the treatment of malaria. Drug Dev. Res 71, 44–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Gulati S et al. (2015) Profiling the Essential Nature of Lipid Metabolism in Asexual Blood and Gametocyte Stages of Plasmodium falciparum. Cell Host Microbe 18, 371–381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Tokumasu F et al. (2014) Inward cholesterol gradient of the membrane system in P. falciparum-infected erythrocytes involves a dilution effect from parasite-produced lipids. Biol. Open 3, 529–541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Istvan ES et al. (2019) Plasmodium Niemann-Pick type C1-related protein is a druggable target required for parasite membrane homeostasis. Elife 8, e40529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Mercier C et al. (2005) Dense granules: Are they key organelles to help understand the parasitophorous vacuole of all apicomplexa parasites? Int. J. Parasitol 35, 829–849 [DOI] [PubMed] [Google Scholar]
- 78.Spielmann T et al. (2012) Molecular make-up of the Plasmodium parasitophorous vacuolar membrane. Int. J. Med. Microbiol 302, 179–186 [DOI] [PubMed] [Google Scholar]
- 79.Elsworth B et al. (2019) Elucidating Host Cell Uptake by Malaria Parasites. Trends Parasitol. 35, 333–335 [DOI] [PubMed] [Google Scholar]
- 80.Goldberg DE and Cowman AF (2010) Moving in and renovating: exporting proteins from Plasmodium into host erythrocytes. Nat. Rev. Microbiol 8, 617–621 [DOI] [PubMed] [Google Scholar]
- 81.Hakimi M-A et al. (2017) Toxoplasma Effectors Targeting Host Signaling and Transcription. Clin. Microbiol. Rev 30, 615–645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Saeij JP and Frickel E-M (2017) Exposing Toxoplasma gondii hiding inside the vacuole: a role for GBPs, autophagy and host cell death. Curr. Opin. Microbiol 40, 72–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Agop-Nersesian C et al. (2018) Host cell cytosolic immune response during Plasmodium liver stage development. FEMS Microbiol. Rev 42, 324–334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Maier AG et al. (2008) Exported Proteins Required for Virulence and Rigidity of Plasmodium falciparum-Infected Human Erythrocytes. Cell 134, 48–61 [DOI] [PMC free article] [PubMed] [Google Scholar]