Abstract
The use of allografts has become a vital option for orthopaedic surgeons in the treatment of a variety of musculoskeletal lesions, ranging from osteochondral defects in the glenohumeral joint to meniscal deficiency in the young athlete. Nevertheless, barriers to treating a patient with an allograft-based procedure may arise from concerns over disease transmission, the navigation of tissue banks that supply allografts, the process of obtaining insurance approval, or optimal storage methods. This review serves to support orthopaedic surgeons in the incorporation of allografts into their practice by quelling these potential concerns. Fresh osteochondral allografts, fresh-frozen meniscal allografts, soft tissue allografts, and off-the-shelf cartilage products are the focus of this review amid broad overviews of allograft safety and tissue bank practices in the United States.
Keywords: allografts, shoulder, general, knee, articular cartilage, tissue banks
Orthopaedic surgeons have incorporated allografts into their surgical practice for more than a century. Specifically, the first fresh osteochondral allograft (OCA) transplantation, referred to as “joint allotransplantation” at the time, was first documented to be performed by Lexer in 1908.48 The procedure became increasingly popular for the treatment of tumors and osteoarthritic lesions during the latter half of the 20th century, as larger case series on OCA transplantation began to appear in the literature in the 1960s and 1970s.38 The increasing use of allografts in orthopaedic surgery, however, pertains not strictly to those intended to treat osteochondral lesions; the United States saw a jump from 7525 tendon allografts distributed by tissue banks in 1993 to 750,000 tendon allografts distributed in 1999.44 As these trends of allograft utilization continue, the modern-day orthopaedic surgeon’s ability to obtain, store, and apply allografts from tissue banks has seen a proportional uptick of importance.
From the surgeon’s perspective, the benefits of having allografts at one’s disposal are clear. Among countless other examples, 1 common instance of their utility is the use of bone–patellar tendon–bone (BTB) allograft as well as soft tissue allograft (eg, semitendinosus) in revision anterior cruciate ligament (ACL) reconstruction, particularly in cases in which an autograft was used to treat the initial ACL rupture. Another example is the application of OCA transplantation to address a focal articular cartilage defect in the knee. This is in contrast to the osteochondral autograft equivalent, which may raise concerns over donor site morbidity.12,19
The use of an allograft requires extensive thought and planning. Surgeons and their patients must feel confident that the possibility of an infection or an immunogenic reaction poses only a minimal risk. The clinical practice itself, moreover, benefits greatly from familiarity with tissue banks and the operations under which they provide grafts. Additionally, to provide the best possible care for their patients, surgeons must have a comprehensive awareness of the grafts that are available, the proper way to handle and store those grafts, and the relevant outcomes described in the literature.
The purpose of this review was to provide orthopaedic surgeons with detailed information about the use of allografts with a focus on variety, safety, procurement, and storage, while briefly highlighting the outcomes associated with procedures involving allografts and the tissue banks that provide them to surgeons in the United States. Fresh OCAs, fresh-frozen meniscal allografts, frozen soft tissue (ligament and tendon) allografts, and off-the-shelf cartilage products will be discussed.
Allograft Safety
Sterilization
Federal law mandates that tissue banks adhere to donation and preparation standards that evaluate allograft tissues for infectious diseases. This involves 2 critical phases of evaluation: donor screening and tissue processing. Donor screening ranges from a thorough review of the donor’s medical records to an interview of an individual who knew the donor personally in order to flag any infectious disease risk factors, such as intravenous drug use. Tissue processing often involves culturing for bacteria and fungi, as well as a sterilization process that must meet US Food & Drug Administration (FDA)–regulated criteria. The overarching requirement of any approach to this process is to meet the FDA’s sterilization standards while preserving the mechanical and biological properties of the allograft.41 The American Association of Tissue Banks (AATB), a nongovernmental, nonprofit organization that serves as the main advising body in the United States for donor tissue handling practices, provides tissue banks with recommendations for validating the efficacy of their sterilization techniques. Such recommendations include targeted culturing for at least 1 organism within each of the following classifications: Gram-negative bacilli, Gram-positive bacilli, Gram-positive cocci, yeast, anaerobes, and mold.3 With respect to viruses, blood is screened for hepatitis B surface antigen, total antibody to hepatitis B core antigen, antibodies to hepatitis C virus, antibodies to human T-lymphotropic virus, and syphilis. Finally, antibodies to human immunodeficiency virus (HIV) are checked and nucleic acid testing for HIV is carried out.29
It is worth noting that the Center for Biologics Evaluation and Research (CBER) is the entity within the FDA that regulates the use of allografts that are discussed in this review. The CBER requires that companies that procure and distribute allografts register their grafts while providing guidance for good tissue practices. Further details are available at https://www.fda.gov/vaccines-blood-biologics/tissue-tissue-products.41
Despite the criteria enforced by the FDA and the recommendations passed down by the AATB, a substantial degree of variation surrounding the methodology of allograft sterilization still exists. The remainder of this section will primarily discuss the sterilization techniques applied to ACL allografts as a vehicle for highlighting this variation in methodology. Fresh OCAs are not typically subjected to irradiation or chemical sterilization and are preserved by unique methods; therefore, the safety of their use is discussed separately.
Gamma irradiation, the most commonly used sterilization technique, therefore lending itself to comparatively robust clinical data, involves ionization by photon emission.30 The potential detrimental effect of gamma irradiation on the biomechanical properties of allografts is a highly studied and rather controversial topic. A systematic review by Lansdown et al37 that included 18 studies evaluating the consequences of irradiation on allograft tissues used for ACL reconstruction described the detrimental biomechanical effects of moderate-dose irradiation on allografts but highlighted the mixed findings regarding the effects of low-dose (<2 mrad) irradiation. Specifically, 1 study included in their review13 reported a reduction in graft stiffness of 20% after 1 to 1.2 mrad irradiation and a reduction in load to failure (LTF), generally defined as the maximum load placed on a graft causing it to be displaced at a prespecified rate, of 20% after 2 mrad irradiation. Still, other studies37 that they included found minimal effects on LTF and stiffness, among other biomechanical measures, using comparable levels of irradiation. For example, Bhatia et al6 compared 1.2 mrad-irradiated semitendinosus allografts, nonirradiated allografts, and autografts in a rabbit model. Their study stated that there was no difference in maximum load or stiffness 8 weeks after ACL reconstruction. It would be remiss to fail to mention that Lansdown et al37 did note a dose-dependent relationship on LTF when higher doses of gamma irradiation (2-4 mrad) were used. Regarding what is known clinically, a systematic review49 that included 21 studies and 1453 patients compared the outcomes of primary ACL reconstruction using allografts that were sterilized using low-dose (<2.5 mrad) gamma irradiation and nonirradiated allografts. The authors found that the patients who received irradiated allografts had lower Lysholm scores, inferior stability outcomes on multiple stability measures, including Lachman, pivot-shift, and KT-1000/2000 arthrometer tests, and an increased propensity to undergo revision surgery.
Electron beam (E-beam) irradiation differs from gamma irradiation in that the ionization is caused by the acceleration of mass- and charge-specific electrons.30 Like gamma irradiation, this technique has been scrutinized and its effects on the reliability of allografts have been discussed in the literature, although there exists a paucity of data related to clinical outcomes. Much like in the case of gamma irradiation, in their aforementioned review Lansdown et al37 described detrimental biomechanical effects in ACL allografts when E-beam doses exceeded 2.5 mrad. Interestingly, fractionation of E-beam irradiation, a method in which the irradiation is delivered in a series of smaller doses rather than 1 stronger dose, resulted in LTF values that were 21% to 89% of nonirradiated allografts but stiffness values that were similar. Comparatively, E-beam irradiation appears to be superior to gamma irradiation, which produced LTF values that were 81% to 94% of E-beam irradiation values and stiffness values that were 82% to 88% of E-beam irradiation values.37
Chemical sterilization is an approach that differs markedly from irradiation, with several techniques falling under this relatively broad category, including treatment with peracetic acid and the supercritical CO2 technique. Again, clinical analysis and comparison of these sterilization methods are rather absent in the literature, but biomechanical data are available.
Studies reporting on allograft sterilization with peracetic acid have drawn mixed conclusions, but the results are generally discouraging. At least 1 study performed on sheep has reported compromised stiffness and LTF values for ACL allografts treated with peracetic acid compared with those of nonsterilized allograft and autograft controls.37,55 Still, 1 study performed on ACL reconstruction in rabbits demonstrated a 48% increase in LTF 12 weeks postoperatively, adding uncertainty to the effect of peracetic acid sterilization.16
In the supercritical CO2 technique, allografts are sealed in packaging and placed into a CO2-filled chamber. The pressure and temperature of the chamber are then increased such that the gas condenses into a sterilizing solvent.5 One biomechanical study that compared unprocessed, gamma-irradiated (2-2.8 mrad), and supercritical CO2-treated anterior or posterior tibialis allografts did not find a statistically significant difference in failure stress or LTF.5 Bui et al9 applied a sheep model to examine the effects of supercritical CO2 treatment in meniscal allografts, contrasting the technique with gamma irradiation. They reported that both techniques yielded increased stiffness compared with unprocessed controls and suggested that the increase was milder in allografts in the supercritical CO2 group, although the latter finding was not statistically significant.
Preservation
As in the case of sterilization, allograft preservation may be achieved by way of more than 1 method, and the method that least diminishes allograft integrity remains a conversation that is ongoing in the literature. Fresh-freezing, freeze-drying, and cryopreservation are the methods discussed in this section, primarily involving allografts used in ACL reconstruction. While the data on clinical outcomes are sparse, substantial biomechanical data are available and presented herein.
Fresh-frozen allografts represent the simplest preservation method that tissue banks use commonly. Upon harvest, the graft is frozen for 2 to 4 weeks, during which time the serologic studies are obtained. After that phase, the graft is thawed and soaked in an antibiotic solution for 1 hour before being frozen to –80°C. The graft can then be stored for 3 to 5 years.32 Giannini et al23 examined the biomechanical effects of using the fresh-frozen preservation technique for posterior tibial tendon allografts, describing a decrease of 18.2% in ultimate load compared with fresh allograft controls and an increase in stiffness of 17.3%.
In freeze-drying, also known as lyophilization, the tissue is harvested and then frozen until the results of serologic testing are returned. The tissue is then soaked in an antibiotic solution, refrozen, and lyophilized such that the moisture content falls below 5%, allowing the graft to be stored at room temperature for 3 to 5 years.32 Freeze-dried BTB allografts sterilized with E-beam irradiation were reported by Gut et al26 to demonstrate a 50% reduction in LTF compared with donor-matched controls irradiated with the same dose. The same study found statistically nonsignificant but noteworthy decreases in elongation values for the freeze-dried allografts.26
The cryopreservation technique allows for up to a 10-year shelf life. It involves cellular water extraction and the use of cryoprotective media, such as dimethyl sulfoxide or glycerol, to aid in controlled freezing of the allograft. The graft undergoes long-term storage in liquid nitrogen at –196° C. It is worth noting that the cryoprotective solutions are associated with a diminished intravascular immune response by the host and heightened angiogenesis.32 Of additional importance is the distinction that cryopreservation may allow for cell viability, while fresh-frozen and freeze-dried grafts do not.58 Suhodolčan et al58 compared the biomechanical properties of cryopreserved BTB allografts using glycerol as a cryoprotective agent with fresh-frozen allografts. Their study suggested that, over a 9-month preservation period, the cryopreservation of allografts using glycerol as a cryoprotectant yields improved values of strain during cyclic measurements, ultimate stress, and ultimate stiffness at failure.58
Tissue Banks
The AATB accredits over 120 tissue banks in the United States and seeks to promote ethical procurement and treatment of tissue.2 The most prominent domestic tissue banks are JRF Ortho, MTF Biologics, RTI Surgical, and LifeNet Health. Every tissue bank is required to be registered with the FDA’s Establishment Registration and Listing for Human Cells, Tissues, and Cellular and Tissue-Based Products.61 Grafts are most commonly received via organ procurement organizations, but tissue banks also source from hospital morgues, operating rooms, coroners, and modernized funeral homes.29 Although variation exists from tissue bank to tissue bank, allografts typically cannot be returned,7 even though storage and preparation of the graft type is taken into account when the need for a return arises.4 Exceptions to this rule tend to be granted because of damage incurred during shipping and handling or a discrepancy between the order placed and the product received.46 The standard timetable for reporting an unsatisfactory graft is 30 days.46
Fresh OCA Tissue
Fresh OCA transplantation, which is the transplantation of a size-matched, allogenic core of viable hyaline cartilage atop subchondral bone, is used to treat chondral and osteochondral lesions primarily of the knee, as well as lesions in the ankle, hip, and shoulder.24,56
Fresh OCA tissues are ideally harvested from healthy donors aged 14 to 50 years within 24 hours of death.24,62 Optimal candidates for graft harvest have established healthy gross articular cartilage,24,62 and this tissue is aseptically harvested to reduce the risk of contamination during procurement. Before implantation, the graft is screened for infectious organisms by targeted culturing and historian interviews as described in the section titled Allograft Safety. 64 Correct matching between donor and recipient and subsequent minimization of immunological response is of concern whenever a foreign tissue is transferred to a new host. Unique to OCAs, host immune response to chondrocytes is negated by the extracellular matrix of hyaline cartilage, which creates immune-privileged tissue by masking chondrocytes.56 Still, the subchondral bone and marrow of grafts remain a concern for host rejection.56 At the time of surgery, a pulsed lavage is used to cleanse the graft of marrow elements, which serves to minimize the host immune response.45 This is especially critical in larger bone grafts (>10 cm2 ), which tend to elicit more potent and systemic immune response than smaller grafts.56
It is critical to maintain chondrocyte viability during the storage and transportation of OCAs. Sustainability of cellular metabolism and matrix is achieved through storage of grafts in a cultured medium, oftentimes with the addition of fetal bovine serum, which has been shown to preserve function and viability of chondrocytes.8 Chondrocyte viability is roughly 28 days, with 14 days of testing resulting in a 14-day window to use grafts once they have been cleared.64 During this 14-day window, grafts are stored at either 4°C, which is associated with lower risk of infection but also lower chondrocyte viability, or 37°C, which is associated with higher risk of infection but also higher chondrocyte viability.64
Despite some degree of variation from company to company, tissue banks maintain a relatively simple and uniform process of fresh OCA order submission. Once the decision-making process has concluded and the surgeon is prepared to order the graft from a tissue bank, the surgeon must provide the tissue bank with a form denoting the desired graft (eg, a distal tibia or a femoral condyle), along with laterality and the procedure to be performed, oftentimes in addition to the patient’s sex and basic biometric data. This form, along with the radiographs discussed later that allow for size matching, may be submitted electronically using a website account or via paper mail.4,34 It is important to note, nevertheless, that the surgeons and their staff may need to work with the patient to obtain prior authorization from the patient’s insurance provider before placing the allograft order. Given that the fresh OCA transplantation is a relatively new procedure compared with, for example, a total knee arthroplasty (TKA), insurance companies often require that their own medical team review the patient’s records before the procedure is deemed medically necessary. For this review to take place, it is generally necessary that any relevant previous imaging, consultation notes, or surgical notes are electronically forwarded to the insurance company, along with a completed cover sheet where appropriate International Classification of Diseases–10 and Current Procedural Terminology codes are denoted. The cover sheet is often a preformed template provided by the insurance company. Once the insurance company provides a written statement agreeing that the procedure is indeed medically necessary and covered by the patient’s plan, the surgeon should unimpededly move forward with the allograft ordering process.
Fresh Femoral Condyle, Trochlea, and Patella OCAs
Fresh distal femur (Figure 1), patellar (Figure 2), and trochlear (Figure 3) OCAs are ordered from tissue banks. In order for the tissue bank to select and distribute a size-matched allograft, the surgeon must provide an anteroposterior (AP) radiograph that includes a sizing marker, allowing the distributor to calculate the image’s magnification.56 A lateral view radiograph of the index knee at the level of the tibia, directly below and parallel to the joint line, should also be obtained and provided to the tissue bank.24 Magnetic resonance imaging (MRI) provides the surgeons with a second option, although studies have shown that MRI has the potential to underestimate the size of the lesion.56 Visualization of subchondral involvement and/or edema in the lesion can be gained through cartilage-specific MRI, whereas bone scintigraphy can be used to evaluate the compartment overload, particularly in patients who have undergone prior meniscectomy.56
Figure 1.
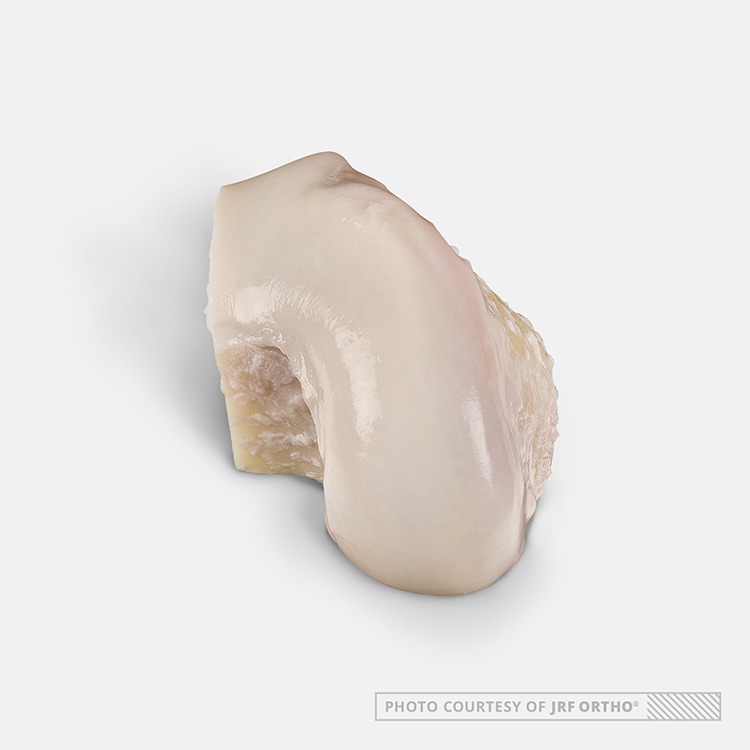
A fresh lateral femoral hemicondylar osteochondral allograft. Image courtesy of JRF Ortho.
Figure 2.

A fresh patellar osteochondral allograft. Image courtesy of JRF Ortho.
Figure 3.

A fresh femoral trochlear osteochondral allograft. Image courtesy of JRF Ortho.
Generally, fresh OCA transplantation in the knee yields promising results. In 1 study, midterm outcome analysis of OCA transplantation with or without concomitant procedures revealed an 87% graft survival rate at 5 years with a 37% reoperation rate, which was primarily arthroscopic debridement.21 A systematic review by Assenmacher et al4 that included 291 patients across 5 studies with OCA transplantation in the knee found a failure rate of 25% at an average follow-up of 12.3 years. They also identified patients younger than 35 years, male sex, and transplanted grafts smaller than 2 cm2 as predictors of more favorable outcomes, as well as the tendency for grafts transplanted to the patellofemoral compartment to be less successful.4 Further, the literature on return to play (RTP) after OCA transplantation reads favorably. One study examined 13 high-level, high school, intercollegiate, and professional athletes (15 knees) who underwent femoral condyle OCA transplantation with an average follow-up of 5.9 years. The study reported that 7 athletes (54%) returned to competitive sports at an average of 7.9 months. An adjusted RTP rate of 77% was calculated when graduation was taken into account as the chief reason for not returning to the preinjury level of competitive sport.42 With respect to revision OCA transplantation, although the outcomes appear to be inferior to those of primary OCA transplantation, the current literature suggests it is a viable treatment option for patients in whom primary OCA transplantation had failed.31 One study that was carried out on 33 patients (33 knees) who had undergone revision OCA transplantation, with an average follow-up of 10 years, found a graft survivorship of 79% at 5 years and 61% at 10 years. The mean time to failure was 5.5 years, and 95% of the patients in the study expressed satisfaction with the procedure.31
Fresh Distal Tibial and Humeral Head OCAs
The glenohumeral joint dislocates more frequently than any other joint in the body.27 Patients younger than 20 years, generally the age group most susceptible to such dislocations, demonstrate a 95% rate of recurrence.14,27 Combining this information with Nakagawa et al’s 47 report that 60% of patients with traumatic anterior shoulder instability had bipolar lesions (glenoid and humeral head), the value of a procedure that can restore bony integrity to the glenohumeral joint is clear. Humeral head and distal tibial allografts (Figure 4) provide orthopaedic surgeons with 2 options for accomplishing this restoration.
Figure 4.

A fresh distal tibial osteochondral allograft. Image courtesy of JRF Ortho.
Given the therapeutic utility of the viable chondrocytes on their articular surfaces, both humeral head and distal tibial allografts are kept fresh by commercial tissue banks and therefore are ordered by a process similar to that of fresh OCAs intended for use in the knee. The humeral head and distal tibial allograft ordering process also involves size matching, although computed tomography (CT) imaging is recommended for this category of allografts.22,52,57,63 To be used within 28 days of harvesting and optimally stored at 4°C, the surgeon should open the fresh allograft at the time of surgery and place it in a sterile, room-temperature solution of saline.52,63 For distal tibial allografts, the most lateral aspect of the graft is harvested.22
Riff et al53 reported on midterm outcomes of fresh humeral head OCA transplantation in 18 patients. With an average follow-up of 66.5 months, they described 7 failures (39%), including 4 conversions to total shoulder arthroplasty (22%). Eleven patients (61%) expressed satisfaction and stated they would undergo the procedure again, while postoperative radiographic analysis at an average of 14.8 months revealed that 90% of grafts were incorporated.53 A cohort of 27 male patients who underwent fresh distal tibial allograft reconstruction of the glenoid underwent postoperative clinical and radiographic evaluation at an average of 45 and 17 months, respectively. Investigators found excellent clinical results with respect to patient-reported outcomes and joint stability, as well as an 89% healing rate as seen on CT, with a 3% average allograft lysis rate.51
Fresh-Frozen Meniscal Allografts
When nonoperative treatments for patients with meniscal tears have been exhausted, surgical options may include partial meniscectomy, subtotal meniscectomy, and meniscal repair. However, in a young, meniscus-deficient patient whose symptoms affect the activities of daily living and/or recreation, meniscal allograft transplantation (MAT) is a viable joint-preserving option (Figure 5).15
Figure 5.

A fresh-frozen lateral meniscal allograft. Image courtesy of JRF Ortho.
Fresh-frozen meniscal allografts are obtained from tissue banks in a fashion similar to the process by which fresh OCAs are obtained, as detailed above. Meniscal defect dimensions are quantified using AP and lateral radiographs with size-matched magnification markers, and an appropriate graft can be selected and distributed by tissue banks using this imaging.29,50 In contrast to OCAs, however, fresh-frozen meniscal allografts can be stored indefinitely at –80°C and, before usage, thawed in a water bath at a temperature of 37°C before being removed from its package and placed in a sterile saline solution.17 These allografts are typically sourced from donors younger than 45 years and must be harvested and stored within 24 hours of death.10,20
Summarizing the literature on outcomes after MAT has been historically challenging because of the variety of techniques used to carry out the procedure, the propensity for concomitant procedures to be performed, and the predominance of level 4 studies on the subject.20 Still, outcomes remain enouraging. One study followed 172 patients who underwent MAT for a mean follow-up of 59 months (minimum of 48 months), defining failure as the need for a revision MAT or TKA, and recorded just 8 failures (4.7%). Although a 32% reoperation rate was reported, the reoperations were predominantly debridement or hardware removal (44/64; 69%). At the final follow-up, the allograft survival rate was 88% among those who had a subsequent procedure and 98% among those who did not require a second operation.43 Additionally, RTP outcomes were explored for 13 high school or higher-level athletes who underwent MAT, with findings that 10 of the athletes (77%) returned to their previous level of activity at a mean of 16.5 months. Of the cohort, 70% returned to their desired level of play and 93% of athletes gave marks of 8 of 10 or greater on satisfaction measures.11 Data on revision MAT, clinical data especially, are limited. Nevertheless, 7 of 8 patients (88%) who underwent revision MAT in a study with a mean follow-up of 3.8 years reported that they were mostly or completely satisfied and would undergo the surgery again. The authors reported 1 failure, which was a patient who progressed to TKA 34 months following the revision MAT.65
Soft Tissue Allografts for Ligament Reconstruction
Soft tissue allografts, namely those used for ligament reconstruction, provide orthopaedic surgeons with an excellent option when a patient lacks autologous tissue.54 Tissue banks commercially distribute soft tissue allografts, including fascia lata, tibialis anterior, tibialis posterior, semitendinosus, gracilis, Achilles tendon (Figure 6), and patellar tendon (Figure 7), with the last 2 representing the most commonly utilized.54 The ordering process for soft ligament allografts mirrors that of fresh OCAs with the exception of including radiographs for size matching.4,34 For simplicity, this section focuses on information pertaining to allografts used in ACL reconstruction.
Figure 6.

An Achilles tendon allograft. Image courtesy of JRF Ortho.
Figure 7.

A preshaped bone–patellar tendon–bone allograft. Image courtesy of JRF Ortho.
Literature surrounding the use of allograft tissue for ACL reconstruction recommends that the performing surgeon obtain weightbearing radiographs of the knee. Although this is not necessary preoperatively for size-matching purposes, as mentioned earlier, such radiographs will provide the surgeon with additional information that may predict graft failure. For example, routine weightbearing radiographs may demonstrate compartment narrowing caused by chondral injury, and hip-knee-ankle radiographs may identify malalignment.36 As in the case of fresh OCAs, MRIs may also prove valuable in the identification of cartilage, meniscal, or additional ligament pathology, all of which could affect the surgeon’s decision making.36
Soft tissue allografts should be harvested within 24 hours of the donor’s death. Tissue banks then store the grafts at –80°C, and portions of the soft tissue and the attached bone undergo aerobic and anaerobic testing for the presence of pathogens. After this screening process, which also includes the previously mentioned review of medical records among other checkpoints, the graft remains viable for surgical application for 3 to 5 years.54 Distribution from the tissue bank involves temperature-controlled conditions by way of dry ice. Thawing the soft tissue allograft takes roughly 1 hour if it arrives to the location of surgery at a temperature of –20°C, and once the graft is thawed, the surgeon can begin to manipulate it according to preference. To expedite the thawing process, the surgeon may elect to place the graft in a sterile saline solution warmed to 37°C for 30 minutes.54
Several studies have investigated the relationship between a soft tissue donor’s characteristic background and the quality of the donor’s harvested graft. Biomechanical analyses have largely revealed that no difference in tensile strength, stiffness, or displacement exists between soft tissue allografts harvested from young donors and their elder counterparts.25,28 However, a weak yet statistically significant negative correlation between donor age and graft modulus has been described in the literature.28 With respect to the bony portion of BTB allografts, bone mineral density tends to be higher in grafts harvested from males compared with females. Again, this metric does not vary by donor age.28
In the context of primary ACL reconstruction, the rates of re-rupture have been reported to be significantly higher in the application of allografts compared with autografts, with literature generally recommending the use of autografts when the option presents.35,40 With respect to revision ACL reconstruction, the literature reads similarly; autografts tend to yield better outcomes than allografts. However, tendon allografts are indicated in revision ACL reconstruction in certain circumstances, which include older age and prior use of an autograft.39
Off-the-Shelf Cartilage Products
Off-the-shelf OCAs and cartilage matrix products have become popular in the past decade as an alternative to fresh OCAs and osteochondral autografts. These products can be stored in the surgical facility on the shelf and can be customized to the patient’s defect. Commercially available products in the United States include Cartiform (Osiris), ProChondrix (AlloSource), Chondrofix (Zimmer Biomet), BioCartilage (Arthrex Inc), and DeNovo NT Natural Tissue Graft (Zimmer Biomet). In addition, precut fresh OCA cores are available in a variety of sizes (JRF).
Cartiform is a cryopreserved, viable OCA that contains factors that promote cartilage healing, including extracellular matrix, viable chondrocytes, and chondrogenic proteins. It is used in combination with microfracture of a lesion base as a single-stage procedure and features a porous structure that enhances flexibility of the graft, promotes the preservation of native chondrocytes, and facilitates mesenchymal stem cell migration to the Cartiform graft after marrow stimulation. The flexibility of the graft allows it to conform to the contours of any joint surface and to be cut into any shape or a smaller size. Fixation is typically accomplished with fibrin glue and sutures or suture anchors. Cartiform remains viable for 24 months stored at −80°C and is available in 4 sizes (diameter): 10, 20, 12 × 19, and 20 × 25 mm. Clinical outcomes are not yet available.
ProChondrix, a cellular, 3-dimensional fresh OCA with viable chondrocytes, extracellular matrix, and growth factors, is another off-the-shelf solution and may be used with microfracture to treat chondral or osteochondral lesions. Fibrin glue fixation is recommended, with or without additional fixation techniques. The shelf life for ProChondrix is 35 days when stored at 4°C and it is available in 5 sizes (diameter): 9, 11, 13, 15, 17, and 20 mm. The depth of the graft can be intraoperatively tailored to match the depth of the defect being treated. Clinical outcomes are not yet available.
Chondrofix is another off-the-shelf OCA and was introduced in 2012 for single-stage treatment of full-thickness articular cartilage lesions. The decellularized bone-cartilage construct is cylindrical, precut to a depth of 10 mm, and is available in diameters of 7, 9, 11, and 15 mm. The shelf life for Chondrofix is 24 months when stored at 40°C or less and it should never be frozen. In 2016, Farr et al18 reported discouraging preliminary data on patients treated with Chondrofix. A 72% failure rate was noted among the 32 knees treated in the series at a mean follow-up of 1.29 years. The average defect size of the cohort was 2.9 ± 2.0 cm2 with a median of 2 allografts implanted per knee.
An allogenic, dehydrated, micronized cartilage scaffold, BioCartilage forms a paste when mixed with platelet-rich plasma or bone marrow aspirate concentrate and is used as a viable, 1-stage augment to microfracture procedures. BioCartilage is rich in type II collagen as well as various growth factors, and it provides mesenchymal stem cells from the subchondral bone with access to the microfractured lesion. This product is associated with a distinct scaffold delivery system and is secured with fibrin glue. The shelf life for BioCartilage is 5 years at room temperature. Human clinical outcome data are not yet available.1
Similar to BioCartilage, DeNovo NT Graft is a particulated juvenile cartilage implant used as a single-stage surgical treatment for cartilage defects. DeNovo differs from BioCartilage, however, in that it is not intended to augment microfracture procedures. The product is applied directly to the cartilage defect and secured with fibrin glue. From the time of procurement, the shelf life is 49 days in storage medium between 19°C and 26°C.59,60 Although they are relatively short term and limited, preliminary results for DeNovo are promising. A total of 13 patients (15 knees) with patellar cartilage defects evaluated at a mean of 28.8 months after treatment with DeNovo showed a mean defect surface area coverage of 89%, including 12 knees (80%) that had 90% fill or greater. Improvements in various patient-reported outcome surveys, including the Knee injury and Osteoarthritis Outcome Score, International Knee Documentation Committee, Tegner, and the visual analog pain scale, were noted as well.60
Finally, fresh OCA core (JRF Ortho) is another off-the-shelf, single-stage surgical option for the treatment of focal, full-thickness osteochondral lesions <20 mm in diameter (Figure 8). These precut cores are composed of hyaline cartilage, including viable chondrocytes, atop bone and can be ordered in diameters of either 10 or 16 mm, each at an intraoperatively adjustable length of 12 mm. Fresh OCA cores can be kept at 1°C to 10°C in a hospital or surgery center refrigerator for up to 35 days.33 Clinical outcomes are not yet available.
Figure 8.

A fresh osteochondral allograft core. Image courtesy of JRF Ortho.
Conclusion
One certainty in the continuously evolving field of orthopaedic surgery is the importance of having allografts as an option for the treatment of a variety of musculoskeletal lesions. The purpose of this review was to equip orthopaedic surgeons with the know-how required to treat their patients with these allografts. Potential impediments were addressed through the discussion of safety, tissue banks, the role of insurance coverage, approaches to graft storage, associated outcomes, and more. The information herein presented should grant orthopaedic surgeons a heightened confidence in incorporating allograft-based procedures into their practices.
Footnotes
One or more of the authors has declared the following potential conflict of interest or source of funding: B.J.C. has received research support from Aesculap/B. Braun, Arthrex, National Institutes of Health (NIAMS & NICHD), and Regentis; consulting fees from Anica Therapeutics, Arthrex, Bioventus, Geistlich Pharma, Genzyme, Pacira, Regentis, Smith & Nephew, Vericel, and Zimmer; royalties from Arthrex, DJO, Elsevier, and Operative Techniques in Sports Medicine; educational support from Arthrex and Pacira; financial/material support from Athletico and JRF Ortho; nonconsulting fees from Carticept Medical and LifeNet Health; stock/stock options in Ossio and Regentis; and hospitality payments from Aesculap/B.Braun, DePuy, and GE Healthcare. R.M.F. has received institutional support from Arthrex and Smith & Nephew; research support from Arthrex; and royalties from Elsevier; and is a consultant for Arthrex, JRF, and AlloSource. AOSSM checks author disclosures against the Open Payments Database (OPD). AOSSM has not conducted an independent investigation on the OPD and disclaims any liability or responsibility relating thereto.
References
- 1. Abrams G, Mall N, Fortier L, Roller B, Cole B. BioCartilage: Background and operative technique. Oper Tech Sports Med. 2013;21(2):116–124. [Google Scholar]
- 2. American Association of Tissue Banks. About us: https://www.aatb.org/?q=about-us. Accessed January 17, 2019.
- 3. American Association of Tissue Banks. Microbiological Process Validation & Surveillance Program. http://www.aatb.org/standards/guidance-documents. Published 2016. Accessed December 31, 2018.
- 4. Assenmacher AT, Pareek A, Reardon PJ, Macalena JA, Stuart MJ, Krych AJ. Long-term outcomes after osteochondral allograft: a systematic review at long-term follow-up of 12.3 years. Arthroscopy. 2016;32(10):2160–2168. [DOI] [PubMed] [Google Scholar]
- 5. Baldini T, Caperton K, Hawkins M, McCarty E. Effect of a novel sterilization method on biomechanical properties of soft tissue allografts. Knee Surg Sports Traumatol Arthrosc. 2016;24(12):3971–3975. [DOI] [PubMed] [Google Scholar]
- 6. Bhatia S, Bell R, Frank RM, et al. Bony incorporation of soft tissue anterior cruciate ligament grafts in an animal model: autograft versus allograft with low-dose gamma irradiation. Am J Sports Med. 2012;40(8):1789–1798. [DOI] [PubMed] [Google Scholar]
- 7. Bone Bank Allografts. Bone Bank Allografts Product Catalog. http://www.bonebank.com. Published 2017. Accessed October 12, 2017.
- 8. Bugbee WD, Pallante-Kichura AL, Görtz S, Amiel D, Sah R. Osteochondral allograft transplantation in cartilage repair: graft storage paradigm, translational models, and clinical applications. J Orthop Res. 2016;34(1):31–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bui D, Lovric V, Oliver R, Bertollo N, Broe D, Walsh WR. Meniscal allograft sterilisation: effect on biomechanical and histological properties. Cell Tissue Bank. 2015;16(3):467–475. [DOI] [PubMed] [Google Scholar]
- 10. Bursac P, York A, Kuznia P, Brown LM, Arnoczky SP. Influence of donor age on the biomechanical and biochemical properties of human meniscal allografts. Am J Sports Med. 2009;37(5):884–889. [DOI] [PubMed] [Google Scholar]
- 11. Chalmers PN, Karas V, Sherman SL, Cole BJ. Return to high-level sport after meniscal allograft transplantation. Arthroscopy. 2013;29(3):539–544. [DOI] [PubMed] [Google Scholar]
- 12. Christian DR, Oliver-Welsh L, Yanke AB, Cole BJ. Staging and practical issues in complex cases. In: Farr J, Gomoll A, eds. Cartilage Restoration: Practical Clinical Applications. 2nd ed. Basel, Switzerland: Springer International; 2018. [Google Scholar]
- 13. Curran AR, Adams DJ, Gill JL, Steiner ME, Scheller AD. The biomechanical effects of low-dose irradiation on bone-patellar tendon-bone allografts. Am J Sports Med. 2004;32(5):1131–1135. [DOI] [PubMed] [Google Scholar]
- 14. Cutts S, Prempeh M, Drew S. Anterior shoulder dislocation. Ann R Coll Surg Engl. 2009;91(1):2–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Cvetanovich GL, Yanke AB, McCormick F, Bach BR, Cole BJ. Trends in meniscal allograft transplantation in the United States, 2007 to 2011. Arthroscopy. 2015;31(6):1123–1127. [DOI] [PubMed] [Google Scholar]
- 16. Dong S, Huangfu X, Xie G, et al. Decellularized versus fresh-frozen allografts in anterior cruciate ligament reconstruction: An in vitro study in a rabbit model. Am J Sports Med. 2015;43(8):1924–1934. [DOI] [PubMed] [Google Scholar]
- 17. Fabbriciani C, Lucania L, Milano G, Schiavone Panni A, Evangelisti M. Meniscal allografts: cryopreservation vs deep-frozen technique. An experimental study in goats. Knee Surg Sports Traumatol Arthrosc. 1997;5(2):124–134. [DOI] [PubMed] [Google Scholar]
- 18. Farr J, Gracitelli GC, Shah N, Chang EY, Gomoll AH. High failure rate of a decellularized osteochondral allograft for the treatment of cartilage lesions. Am J Sports Med. 2016;44(8):2015–2022. [DOI] [PubMed] [Google Scholar]
- 19. Fox JA, Pierce M, Bojchuk J, Hayden J, Bush-Joseph CA, Bach BR. Revision anterior cruciate ligament reconstruction with nonirradiated fresh-frozen patellar tendon allograft. Arthroscopy. 2004;20(8):787–794. [DOI] [PubMed] [Google Scholar]
- 20. Frank RM, Cole BJ. Meniscus transplantation. Curr Rev Musculoskelet Med. 2015;8(4):443–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Frank RM, Lee S, Levy D, et al. Osteochondral allograft transplantation of the knee: analysis of failures at 5 years. Am J Sports Med. 2017;45(4):864–874. [DOI] [PubMed] [Google Scholar]
- 22. Frank RM, Romeo AA, Provencher MT. Glenoid reconstruction with distal tibia allograft for recurrent anterior shoulder instability. Orthopedics. 2017;40(1):e199–e205. [DOI] [PubMed] [Google Scholar]
- 23. Giannini S, Buda R, Di Caprio F, et al. Effects of freezing on the biomechanical and structural properties of human posterior tibial tendons. Int Orthop. 2008;32(2):145–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Gortz S, Bugbee WD. Fresh osteochondral allografts: graft processing and clinical applications. J Knee Surg. 2006;19(3):231–240. [DOI] [PubMed] [Google Scholar]
- 25. Greaves LL, Hecker AT, Brown CH. The effect of donor age and low-dose gamma irradiation on the initial biomechanical properties of human tibialis tendon allografts. Am J Sports Med. 2008;36(7):1358–1366. [DOI] [PubMed] [Google Scholar]
- 26. Gut G, Marowska J, Jastrzebska A, Olender E, Kamiński A. Structural mechanical properties of radiation-sterilized human bone-tendon-bone grafts preserved by different methods. Cell Tissue Bank. 2016;17(2):277–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Haber DB, Sanchez A, Sanchez G, Ferrari MB, Ferdousian S, Provencher MT. Bipolar bone loss of the shoulder joint due to recurrent instability: use of fresh osteochondral distal tibia and humeral head allografts. Arthrosc Tech. 2017;6(3):e893–e899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hampton DM, Lamb J, Klimkiewicz JJ. Effect of donor age on patellar tendon allograft ACL reconstruction. Orthopedics. 2012;35(8):e1173–e1176. [DOI] [PubMed] [Google Scholar]
- 29. Harner CD, Lo MY. Future of allografts in sports medicine. Clin Sports Med. 2009;28(2):327–340, ix. [DOI] [PubMed] [Google Scholar]
- 30. Hoburg A, Keshlaf S, Schmidt T, et al. High-dose electron beam sterilization of soft-tissue grafts maintains significantly improved biomechanical properties compared to standard gamma treatment. Cell Tissue Bank. 2015;16(2):219–226. [DOI] [PubMed] [Google Scholar]
- 31. Horton MT, Pulido PA, McCauley JC, Bugbee WD. Revision osteochondral allograft transplantations: do they work? Am J Sports Med. 2013;41(11):2507–2511. [DOI] [PubMed] [Google Scholar]
- 32. Hulet C, Sonnery-Cottet B, Stevenson C, et al. The use of allograft tendons in primary ACL reconstruction. Knee Surg Sports Traumatol Arthrosc. 2019;27(6):1754–1770. [DOI] [PubMed] [Google Scholar]
- 33. Jones KJ, Mosich GM, Williams RJ. Fresh precut osteochondral allograft core transplantation for the treatment of femoral cartilage defects. Arthrosc Tech. 2018;7(8):e791–e795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. JRF Ortho. JRF Ortho ordering process http://jrfortho.org/products/details/fresh-osteochondral-oca-cores. Accessed February 27, 2019. [Google Scholar]
- 35. Kraeutler MJ, Bravman JT, McCarty EC. Bone–patellar tendon–bone autograft versus allograft in outcomes of anterior cruciate ligament reconstruction: a meta-analysis of 5182 patients. Am J Sports Med. 2013;41(10):2439–2448. [DOI] [PubMed] [Google Scholar]
- 36. Kuhn MA, Ross G. Allografts in the treatment of anterior cruciate ligament injuries. Sports Med Arthrosc Rev. 2007;15(3):133–138. [DOI] [PubMed] [Google Scholar]
- 37. Lansdown DA, Riff AJ, Meadows M, Yanke AB, Bach BR. What factors influence the biomechanical properties of allograft tissue for ACL reconstruction? A systematic review. Clin Orthop Relat Res. 2017;475(10):2412–2426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Mankin HJ, Fogelson FS, Thrasher AZ, Jaffer F. Massive resection and allograft transplantation in the treatment of malignant bone tumors. N Engl J Med. 1976;294(23):1247–1255. [DOI] [PubMed] [Google Scholar]
- 39. MARS Group. Effect of graft choice on the outcome of revision anterior cruciate ligament reconstruction in the Multicenter ACL Revision Study (MARS) cohort. Am J Sports Med. 2014;42(10):2301–2310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Mascarenhas R, Erickson BJ, Sayegh ET, et al. Is there a higher failure rate of allografts compared with autografts in anterior cruciate ligament reconstruction: a systematic review of overlapping meta-analyses. Arthroscopy. 2015;31(2):364–372. [DOI] [PubMed] [Google Scholar]
- 41. McAllister DR, Joyce MJ, Mann BJ, Vangsness CT., Jr Allograft update: the current status of tissue regulation, procurement, processing, and sterilization. Am J Sports Med. 2007;35(12):2148–2158. [DOI] [PubMed] [Google Scholar]
- 42. McCarthy MA, Meyer MA, Weber AE, et al. Can competitive athletes return to high-level play after osteochondral allograft transplantation of the knee? Arthroscopy. 2017;33(9):1712–1717. [DOI] [PubMed] [Google Scholar]
- 43. McCormick F, Harris JD, Abrams GD, et al. Survival and reoperation rates after meniscal allograft transplantation: analysis of failures for 172 consecutive transplants at a minimum 2-year follow-up. Am J Sports Med. 2014;42(4):892–897. [DOI] [PubMed] [Google Scholar]
- 44. McDermott ID, Thomas NP, Poniatowski S, Warwick RM. Soft tissue allografts in the knee: a survey of UK usage and a report of a combined user/provider collaborative group. Knee. 2006;13(1):72–75. [DOI] [PubMed] [Google Scholar]
- 45. Mohr J, Germain M, Winters M, et al. Disinfection of human musculoskeletal allografts in tissue banking: a systematic review. Cell Tissue Bank. 2016;17(4):573–584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. MTF Biologics. Tissue & Product Catalog. www.mtfbiologics.org/our-products. Accessed October 12, 2017.
- 47. Nakagawa S, Ozaki R, Take Y, Iuchi R, Mae T. Relationship between glenoid defects and Hill-Sachs lesions in shoulders with traumatic anterior instability. Am J Sports Med. 2015;43(11):2763–2773. [DOI] [PubMed] [Google Scholar]
- 48. Nikolaou VS, Giannoudis PV. History of osteochondral allograft transplantation. Injury. 2017;48(7):1283–1286. [DOI] [PubMed] [Google Scholar]
- 49. Park SS, Dwyer T, Congiusta F, Whelan DB, Theodoropoulos J. Analysis of irradiation on the clinical effectiveness of allogenic tissue when used for primary anterior cruciate ligament reconstruction. Am J Sports Med. 2015;43(1):226–235. [DOI] [PubMed] [Google Scholar]
- 50. Pollard ME, Kang Q, Berg EE. Radiographic sizing for meniscal transplantation. Arthroscopy. 1995;11(6):684–687. [DOI] [PubMed] [Google Scholar]
- 51. Provencher MT, Frank RM, Golijanin P, et al. Distal tibia allograft glenoid reconstruction in recurrent anterior shoulder instability: clinical and radiographic outcomes. Arthroscopy. 2017;33(5):891–897. [DOI] [PubMed] [Google Scholar]
- 52. Provencher MT, Ghodadra N, LeClere L, Solomon DJ, Romeo AA. Anatomic osteochondral glenoid reconstruction for recurrent glenohumeral instability with glenoid deficiency using a distal tibia allograft. Arthroscopy. 2009;25(4):446–452. [DOI] [PubMed] [Google Scholar]
- 53. Riff AJ, Yanke AB, Shin JJ, Romeo AA, Cole BJ. Midterm results of osteochondral allograft transplantation to the humeral head. J Shoulder Elbow Surg. 2017;26(7):e207–e215. [DOI] [PubMed] [Google Scholar]
- 54. Robertson A, Nutton RW, Keating JF. Current trends in the use of tendon allografts in orthopaedic surgery. J Bone Joint Surg Br. 2006;88(8):988–992. [DOI] [PubMed] [Google Scholar]
- 55. Scheffler SU, Gonnermann J, Kamp J, Przybilla D, Pruss A. Remodeling of ACL allografts is inhibited by peracetic acid sterilization. Clin Orthop Relat Res. 2008;466(8):1810–1818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Sherman SL, Garrity J, Bauer K, Cook J, Stannard J, Bugbee W. Fresh osteochondral allograft transplantation for the knee: current concepts. J Am Acad Orthop Surg. 2014;22(2):121–133. [DOI] [PubMed] [Google Scholar]
- 57. Snir N, Wolfson TS, Hamula MJ, Gyftopoulos S, Meislin RJ. Arthroscopic anatomic humeral head reconstruction with osteochondral allograft transplantation for large Hill-Sachs lesions. Arthrosc Tech. 2013;2(3):e289–e293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Suhodolčan L, Brojan M, Kosel F, Drobnič M, Alibegović A, Brecelj J. Cryopreservation with glycerol improves the in vitro biomechanical characteristics of human patellar tendon allografts. Knee Surg Sports Traumatol Arthrosc. 2013;21(5):1218–1225. [DOI] [PubMed] [Google Scholar]
- 59. Tompkins M, Adkisson D, Bonner K. DeNovo NT allograft. Oper Tech Sports Med. 2013;21(2):82–89. [Google Scholar]
- 60. Tompkins M, Hamann JC, Diduch DR, et al. Preliminary results of a novel single-stage cartilage restoration technique: particulated juvenile articular cartilage allograft for chondral defects of the patella. Arthroscopy. 2013;29(10):1661–1670. [DOI] [PubMed] [Google Scholar]
- 61. United States Food & Drug Administration. U.S. Food & Drug Administration Tissue Establishment Registration. https://www.fda.gov/BiologicsBloodVaccines/GuidanceComplianceRegulatoryInformation/EstablishmentRegistration/TissueEstablishmentRegistration/default.htm. Accessed January 17, 2019.
- 62. Vangsness CT, Triffon MJ, Joyce MJ, Moore TM. Soft tissue for allograft reconstruction of the human knee: a survey of the American Association of Tissue Banks. Am J Sports Med. 1996;24(2):230–234. [DOI] [PubMed] [Google Scholar]
- 63. Wang KC, Waterman BR, Cotter EJ, Frank RM, Cole BJ. Fresh osteochondral allograft transplantation for focal chondral defect of the humerus associated with anchor arthropathy and failed SLAP repair. Arthrosc Tech. 2017;6(4):e1443–e1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Wydra FB, York PJ, Vidal AF. Allografts: osteochondral, shell, and paste. Clin Sports Med. 2017;36(3):509–523. [DOI] [PubMed] [Google Scholar]
- 65. Yanke AB, Chalmers PN, Frank RM, Friel NA, Karas V, Cole BJ. Clinical outcome of revision meniscal allograft transplantation: minimum 2-year follow-up. Arthroscopy. 2014;30(12):1602–1608. [DOI] [PubMed] [Google Scholar]


