Abstract
Appetite change is a defining feature of major depressive disorder (MDD), yet little neuroscientific evidence exists to explain why some individuals experience increased appetite when they become depressed while others experience decreased appetite. Previous research suggests depression-related appetite changes can be indicative of underlying neural and inflammatory differences among MDD subtypes. The present study explores the relationship between systemic inflammation and brain circuitry supporting food hedonics for individuals with MDD. Sixty-four participants (31 current, unmedicated MDD and 33 healthy controls [HC]) provided blood samples for analysis of an inflammatory marker, C-reactive protein (CRP), and completed a functional magnetic resonance imaging (fMRI) scan in which they rated the perceived pleasantness of various food stimuli. Random-effects multivariate modeling was used to explore group differences in the relationship between CRP and the coupling between brain activity and inferred food pleasantness (i.e., strength of the relationship between activity and pleasantness ratings). Results revealed that for MDD with increased appetite, higher CRP in blood related to greater coupling between orbitofrontal cortex and anterior insula activity and inferred food pleasantness. Compared to HC, all MDD exhibited a stronger positive association between CRP and coupling between activity in striatum and inferred food pleasantness. These findings suggest that for individuals with MDD, systemic low-grade inflammation is associated with differences in reward and interoceptive-related neural circuitry when making hedonic inferences about food stimuli. In sum, altered immunologic states may affect appetite and inferences about food reward in individuals with MDD and provide evidence for physiological subtypes of MDD.
Keywords: fMRI, major depressive disorder, inflammation, C-reactive protein, appetite
1. Introduction
Major depressive disorder (MDD) is one of the most commonly diagnosed mental illnesses in the United States (The National Institute of Mental Health, 2014), with a lifetime prevalence of approximately 16 percent (Kessler et al., 2003). MDD is also one of the most disabling mental illnesses worldwide (Vos et al., 2012) and is associated with an increased risk for numerous physical health disorders, including cardiovascular disease (CVD) and obesity (Chapman et al., 2005). These concomitant illnesses (e.g., CVD and obesity) are often associated with the somatic symptoms of MDD (e.g., altered sleep and increased fatigue) in ways that are bidirectional and mutually-reinforcing (Luppino et al., 2010; Milaneschi et al., 2019). The marked changes in appetite and weight experienced by individuals with depression may be particularly salient in this relationship. Approximately one-half of individuals with MDD report a decrease in appetite (MDD-A−), while one-third of individuals with MDD demonstrate increased appetite (MDD-A+; Maxwell & Cole, 2009). These appetite change profiles tend to remain stable for individuals over recurrent depressive episodes (Stunkard et al., 1990).
Recent evidence has highlighted systemic inflammation as a potential biological substrate relating depression to its adverse long-term physical health outcomes, including CVD and obesity (Berg & Scherer, 2005; Bhattacharya et al., 2016; Dantzer et al., 2008; Milaneschi et al., 2019; Miller & Raison, 2016; Miller et al., 2002; Monteiro & Azevedo, 2010; Raison et al., 2006). Specifically, inflammation is theorized to be a precursor to the development of depressive symptoms for some individuals (Miller & Raison, 2016; Miller et al., 2002; Moieni et al., 2015; Shelton & Miller, 2011). However, systemic inflammation may not be present for all individuals with depression (Chamberlain et al., 2018; Lamers et al., 2013; Lamers et al., 2016; Lamers et al., 2018; Simmons et al., 2018). As has been noted previously (Simmons et al., 2018), the complex crosstalk between immune and metabolic signaling pathways may help account for the observation that increased appetite in depression is associated with elevated peripheral markers of inflammation. Compared to MDD-A−, MDD-A+ have been shown to have higher levels of C-reactive protein (CRP; Lamers et al., 2013; Lamers et al., 2018; Simmons et al., 2018), an immune system signaling protein produced by hepatocytes as an acute response to inflammation, infection, or tissue damage (Pepys & Hirschfield, 2003). Thus, the appetite change profiles experienced by most individuals with MDD may reflect an underlying immunologic pathophysiology. What remains unclear, however, is the specific intervening neurobiological processes that turn systemic inflammation into increased appetite and eating.
Prior work provides some evidence for links between systemic inflammation and the insula, striatum, and orbitofrontal cortex (OFC), which play important roles in homeostatic food significance and reward value (Simmons et al., 2016; Simmons et al., 2018). The insula is also implicated in interoception, or awareness of visceral sensations such as signals of hunger and thirst (Craig, 2002; Khalsa et al., 2009; Simmons et al., 2013). First, studies that elicit acute systemic inflammatory responses by administering endotoxins to non-depressed adults have reported altered activity throughout the insula, striatum, and OFC (Eisenberger et al., 2010; Harrison et al., 2015; Kullmann et al., 2013). Additionally, a meta-analysis has identified insular and striatal regions as primary structures implicated in systemic inflammation in depression (Byrne et al., 2016). Further, while passively viewing food pictures, MDD-A+ exhibit greater hemodynamic response in the insula, striatum, and OFC when compared to MDD-A− or healthy comparison participants (HC), while MDD-A− exhibit reduced activation in the insula as compared to MDD-A+ and HC (Simmons et al., 2016). Moreover, Simmons and colleagues (2018) reported that in MDD-A+, higher levels of the pro-inflammatory cytokine interleukin 6 (IL-6) were associated with increased insular response while passively viewing food pictures, although only at a statistical threshold uncorrected for multiple comparisons.
In summary, (1) depression with increased appetite is associated with systemic low-grade inflammation; (2) acute systemic inflammation is associated with altered activity in the insula, striatum and OFC; (3) the insula, striatum, and OFC are important structures in the perception and valuation of food stimuli; and (4) MDD-A+ exhibit greater activity in the insula, striatum, and OFC while passively viewing food pictures than MDD-A−. Together these earlier findings provide evidence linking systemic inflammation to the appetite change profiles and eating behaviors experienced by many individuals with MDD, but important questions remain unanswered. Passive food picture viewing is not synonymous with active hedonic valuations of food (i.e., one’s idiosyncratic interpretation of how pleasant a food appears), as assessing participants during active inferences of food pleasantness allows for a more precise examination of food-related neurocircuitry than passive food picture viewing. These hedonic food valuations are meaningful as they are central to everyday decisions about what, when, and how much to eat. Additionally, there is no extant evidence that these hedonic judgements are related to systemic inflammatory signals in individuals with MDD experiencing appetite change. What is missing is a test of brain activation in regions implicated in both pro-inflammatory states and food information processing (i.e., insula, striatum, and OFC) during hedonic food valuation, and how this might relate to levels of systemic inflammation in individuals with MDD. Such a study is critical to the development of a detailed account of the pathophysiology underlying depression, as it may inform more targeted treatments for MDD and the associated negative physical health outcomes. The present study aimed to address this gap in the literature.
To this end, we measured CRP levels in unmedicated MDD-A+ and MDD-A− as well as HC. Here, we focus on CRP, as this inflammatory marker has been reliably shown to differ between MDD-A+ and MDD-A− samples (Lamers et al., 2013; Lamers et al., 2018; Simmons et al., 2018). We asked all participants to complete a task requiring active judgments about the hedonic value of food stimuli while undergoing functional magnetic resonance imaging (fMRI). We examined whether CRP concentration in blood differentially related to the coupling between brain activity and inferred food pleasantness across groups (see Section 2.3 for more information on “coupling” analyses). More specifically, we hypothesized that there would be a significant interaction effect between CRP concentration and group on coupling between activity in the OFC, striatum, and insula and food pleasantness ratings. We expected that MDD-A+ would have a significantly stronger positive relationship between CRP concentration and this coupling (i.e., higher CRP related to stronger coupling between brain activity and pleasantness ratings) than MDD-A− and HC. Such a finding would suggest that elevated peripheral inflammation previously observed in MDD-A+ samples may influence how these individuals interpret the hedonic value of food.
2. Methods
2.1. Participants
The protocol for the present study was approved by Western IRB. All participants provided written informed consent to participate, in accordance with the Declaration of Helsinki. A total of 64 participants aged 18–49 years completed the protocol for the current study (female n = 45; mean age = 32.3, SD = 8.9). These participants are a subset of the sample described in studies by Simmons and colleagues (2016; 2018) reporting upon appetite change in MDD. Participants from that larger sample (N = 96) were not included in this subset if they were missing CRP data (n = 13), did not complete the Food Pleasantness fMRI Task (which was not reported in the previous projects; n = 7), or had poor quality imaging data (e.g., due to excessive motion; n = 12). No participants or their data were removed from analyses for having outlier status on variables of interest. Specifically, two participants (one HC and one MDD-A+) had lower CRP values than the rest of their respective groups (based on being [1.5*interquartile range] lower than the 25th percentile) but were not removed from the sample. Rather, in an effort to maintain power, we utilized robust statistical methods to account for the influence of these outliers.
Participant groups were established based on diagnostic status and symptomatology. HC participants (n = 33) did not meet criteria for a current or past Axis I psychiatric disorder on the Structured Clinical Interview for DSM-IV Axis I Disorders (SCID-IV; First et al., 1996), nor did they have any first-degree relatives who met criteria for those disorders. MDD participants (n = 31) were required to meet DSM-IV-TR criteria for a current major depressive episode involving depression-related appetite change. Twenty-five of the MDD participants had experienced recurrent depressive episodes, and the MDD-A+ and MDD-A− groups did not show significant differences in single versus recurrent episode diagnoses (X2 = 0.07, p = 0.79). Appetite change was evaluated with the appetite questions in the mood disorders module of the SCID-IV as well as in an additional interview with a psychiatrist. All MDD participants reported a weight change of at least five pounds in the same direction as their appetite change in the current depressive episode (i.e., MDD-A− with weight loss and MDD-A+ with weight gain). If participants did not meet the weight change threshold, research staff contacted a close relative or significant other to corroborate the participant’s reported appetite change profile. These appetite and weight changes were used to assign each participant to either the MDD-A+ (n = 14) or MDD-A− (n = 17) group. Because the researchers were involved in recruitment, screening, and diagnosis, they were unable to be blinded to participants’ diagnostic status. Volunteers were excluded from the study for use of psychotropic medications within three weeks of the fMRI scan (six weeks for Fluoxetine) and for current use of corticosteroid or anti-inflammatory medication. Additional exclusion criteria included previous diagnosis of major neurological or medical disorder, substance abuse, traumatic brain injury, current pregnancy or having a primary language other than English.
2.2. Research Design
The study involved two sessions - a screening visit and a neuroimaging visit. During the screening, all participants provided demographic information and were assessed for current psychiatric disorders using the SCID-IV. Participants fasted for 12 hours prior to the second visit. At 8:00 am on the morning of the visit, they were given a breakfast standardized across participants for micro- and macro-nutrients. After completing the meal, all participants completed the clinician-administered Hamilton Depression Rating Scale (HAM-D) to assess current depression severity (Hamilton, 1960). Participants also completed the Hamilton Anxiety Rating Scale (HAM-A; Hamilton, 1959), as well as the Snaith-Hamilton Pleasure Scale (SHAPS) to evaluate anhedonia, or lack of pleasure (Snaith et al., 1995). Both the HAM-D and the SHAPS were modified in order to remove items related to food, drink, appetite, and weight (i.e., two items from the HAM-D and three items from the SHAPS) to avoid a bias in scoring that would otherwise give MDD-A− higher depression and anhedonia scores.
After completing these measures, participants were situated in the scanner at a standardized time (i.e., 12:00 pm). Immediately prior to the fMRI scan, blood was drawn from each participant using BD Vacutainer ethylenediaminetetraacetic acid (EDTA) blood collection tubes. The whole blood samples were centrifuged for 10 minutes after collection. Then, plasma was removed and stored at −80 degrees Celsius until analysis. Plasma CRP was measured with a high sensitivity enzyme-linked immunosorbent assay (ELISA) kit obtained from R&D Systems (Minneapolis, USA). Assays were performed in duplicate and quality was assessed by determining coefficients of variation (%CV). The intra-assay %CV for the samples was 8.4
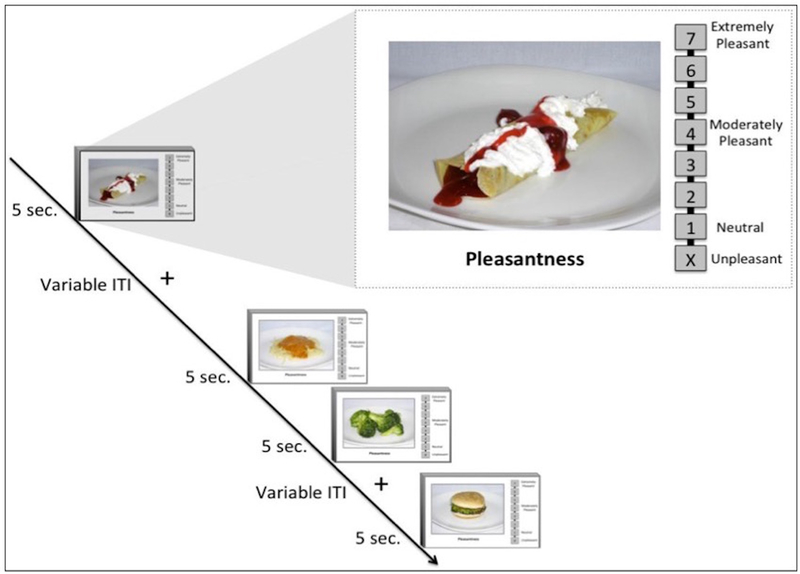
After the blood draw was complete, participants began the fMRI scan. During the Food Pleasantness Task (Simmons et al., 2014), participants viewed pictures of food and rated how pleasurable it would be to eat the food item in the present moment (Figure 1). Therefore, this task elicits brain activation related to participants’ real-time inferences of food pleasantness, which allows for a more personalized measurement of activity in food-related neurocircuitry than standard food picture tasks that do not incorporate subjects’ idiosyncratic food ratings. Participants completed four runs of the task, with each run consisting of 36 food pictures (144 total) presented for 5-second periods, separated by variable duration interstimulus intervals. During picture presentation, participants provided ratings using an MR-compatible scroll wheel on a Likert-type scale that ranged from 1 to 7, with 1 indicating neutral and 7 implying extreme pleasure. An additional response option of “X” represented unpleasantness, and participants rated an average of 24 food pictures (SD = 23) as unpleasant. Trials rated as unpleasant were not included in the present analyses. Food used for the stimuli were diverse and included a variety of food types (i.e., high/low fat and high/low sugar). As described by Simmons and colleagues (2014), the food stimuli were validated by a group of participants who rated the perceived fat and sugar content of each food.
Figure 1.
An overview of the Food Pleasantness Rating Task. Current analyses focused only on pleasantness ratings of food rather than unpleasant (source: Simmons et al., 2014).
Note. Reprinted by permission from Springer Nature: Springer, Brain Structure and Function (The ventral pallidum and orbitofrontal cortex support food pleasantness inferences, Simmons et al.) Copyright (2014).
2.3. fMRI Data Collection and Preprocessing
A 3-Tesla, whole-body MRI scanner with a 32-channel phased array head coil was used to collect the imaging data. A single-shot, gradient-recalled echo-planar imaging (EPI) sequence was used to identify blood oxygenation level-dependent (BOLD) contrasts. Forty-four axial slices were collected per volume, and 248 volumes were collected in each run of the Food Pleasantness Task (TR = 2500 ms, TE = 22 ms). The volume of each voxel was approximately 1.72×1.72×2.90 mm3. Each participant’s EPI images were aligned to an anatomical scan, which was acquired with a Tl-weighted magnetization-prepared rapid gradient-echo (MPRAGE) sequence.
These fMRI data were preprocessed for each individual participant using the Analysis of Functional Neuroimaging (AFNI) software package (Cox, 1996). The first four volumes of each EPI time course were removed to isolate a steady-state fMRI signal, and a slice timing correction was then applied to the remaining volumes of each EPI scan. In one combined step, the EPI time courses were then corrected for motion, aligned to the anatomical scan, warped to Talairach space, and resampled to a 1.75×1.75×1.75 mm3 voxel size. Finally, data were smoothed using a 6-mm full-width, half-maximum Gaussian kernel and normalized to the percent signal change from each voxel’s mean signal.
Each participant’s fMRI data were visually inspected and quality checked for excessive motion and alignment. Individual TRs with motion parameters greater than 0.3 (i.e., roughly 0.3mm motion relative to the previous TR) or with outlier parameters greater than 0.05 (i.e., more than 5% of voxels in TR are outliers) were censored during single-subject regression analyses. Additionally, any participant with greater than 20% of time points censored due to excessive motion was excluded from group analyses. Data were analyzed on a single-subject level using multiple linear regression. Included in the regression model were covariates of non-interest that accounted for motion correction and the mean, linear, quadratic, and cubic signal trends for each run of the task. The food picture stimuli were modeled by convolving a canonical hemodynamic function with a box car function with 5-second width, beginning at the onset of each trial period. The amplitude of the hemodynamic response function for each picture stimulus was modulated according to the participant’s pleasantness rating for the depicted food (i.e., amplitude modulation regression; AM). This AM regression analysis provided beta values that reflect the relationship between the BOLD response during hedonic evaluation of a food stimulus and the participant’s pleasantness rating of that food while seeing it in the scanner. More specifically, the AM beta coefficients reflect the magnitude of the BOLD signal change per unit-change in pleasantness ratings. The practical interpretation of these betas is that they represent the coupling between brain activity and pleasantness ratings. As a result, a positive beta value (i.e., stronger coupling) signifies that a participant exhibited greater brain activity when they rated a food as more pleasant (and vice versa). The more strongly coupled this relationship, the greater the entrainment between food ratings and BOLD activation. Examining this coupling is meaningful because it allows us to assay a participant’s brain activation in relation to their subjective, idiosyncratic food preferences and therefore provides a more precise examination of food-related neurocircuitry than passive food picture viewing. See the work of Simmons and colleagues (2014) for more detail on the AM analysis method for the Food Pleasantness Task.
2.4. Data Analytic Plan
Before conducting any analyses, normality of the distribution of CRP concentration for each group was evaluated using Shapiro-Wilks tests. Because distributions were non-normal, CRP values were log-transformed. Group fMRI analysis was conducted using voxel-wise, random-effects multivariate modeling (AFNI’s robust 3dMVM) to assess the group by CRP interaction on coupling between BOLD activation and pleasantness ratings. Robust 3dMVM utilizes MM-estimation, which has both a high breakdown point and high efficiency, indicating it performs well in robust regression (Yu & Yao, 2017). For this analysis, the log-transformed CRP concentration and group (i.e., MDD-A+, MDD-A−, HC) were included as predictors, and the beta coefficients (i.e., coupling between BOLD activation and pleasantness ratings) from each participant’s AM regression constituted the dependent variable. The main effects of CRP and group were analyzed. However, because we hypothesized that MDD-A+ would exhibit an increased relationship between CRP and coupling between brain activity and pleasantness ratings, the primary effect of interest was the interaction between group and CRP. Because previous literature suggested that the OFC, striatum, and insula play a role in food hedonics, and are all regions where activity has been associated with peripheral inflammation, we focused our analyses involved small volume corrections within a priori anatomical masks of these regions. All masks used for small-volume correction were based on anatomical definitions used in previously published studies. The OFC mask was created using AFNI’s TT_N27 template brain volume. The striatum mask contained masks of the caudate, putamen, and ventral striatum, which were also used in the previous study involving the Food Pleasantness Task (Simmons et al., 2014). Each of these striatum masks was defined using the procedures described by Mawlawi and colleagues (2001). Insula masks were created using ‘DKD_Desai_PM,’ a maximum probability atlas available in AFNI (Destrieux et al., 2010). For more information on the anatomical definitions of the masks used, see Supplemental Materials (Section 1.0).
To identify specific relationships underlying the interaction effect between group and CRP concentration on the coupling between BOLD signal change and pleasantness ratings, follow-up robust regression analyses were conducted in R for each significant cluster. These post-hoc regression analyses included group as a between-subject variable and were completed using R’s ‘lmrob’ function (R Core Team, 2017). These post-hoc regressions allowed us to examine the hypothesized group differences in the slope of the relationship between CRP concentration and the coupling between BOLD signal change and pleasantness ratings. This allows for the examination of whether CRP differentially influences the relationship between subjective pleasantness ratings and BOLD signal change in response to food stimuli. Additional exploratory post-hoc regressions examined the unique relationship between CRP concentration and the coupling between BOLD signal change and pleasantness ratings for each group (see Supplemental Table 6 for these results).
Given evidence that traditional methods of fMRI multiple comparison corrections do not adequately control false-positive inferences (Eklund et al., 2015), we employed the spatial autocorrelation function (acf) option for AFNI programs 3dFWHMx (estimating intrinsic smoothness based on residuals) and 3dClustSim (estimating probability of false positives), yielding a more stringent cluster threshold to address concerns about cluster thresholding methods and false positive rates (Eklund et al., 2016). A cluster-size multiple comparisons correction threshold of p < 0.05 was used for all analyses. Results were considered significant at conservative voxel-wise thresholds of p < 0.0005 for whole-brain voxel-wise results (a minimum cluster size of 595 mm3; presented in Supplemental Materials Section 2.0) and p < 0.005 for small volume correction analyses within the pre-specified regions of interest. Minimum cluster thresholds were 86 mm3 for OFC and 160 mm3 for insula. As striatum was comprised of the putamen, caudate, and ventral striatum, the cluster threshold for these masks were 80, 64, and 48 mm3 respectively.
3. Results
3.1. Participant Characteristics
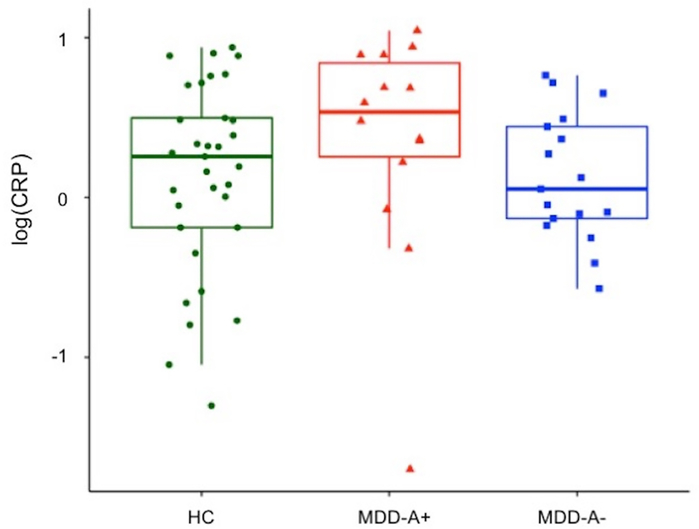
The MDD-A+, MDD-A−, and HC groups did not differ significantly in age, body mass index (BMI), or gender composition. The three groups exhibited significant differences in average food pleasantness ratings (p < 0.01). Follow-up pairwise comparisons revealed that MDD-A− rated food stimuli as significantly less pleasant than MDD-A+ (p < 0.01, Bonferroni-corrected) and HC (p < 0.04, Bonferroni-corrected). Pleasantness ratings of food stimuli made by MDD-A+ did not differ significantly from those made by HC (p = 0.68, Bonferroni-corrected). No significant differences in CRP concentration between the three groups were observed (p = 0.15; Figure 2). Exploratory analysis using a Wilcoxon non-parametric test (i.e., Wilcoxon rank-sum test; ‘wilcox.test’ in R) revealed a trend toward higher CRP in the MDD-A+ group (W= 166.5, p = 0.06). This trend is in line with the report by Simmons and colleagues (2018) which previously demonstrated that MDD-A+ had significantly higher CRP levels than MDD-A− using a larger sample of which the present sample is a subset. Importantly, the two MDD groups of the present sample did not exhibit significant differences on measures of depression (HAM-D), anxiety (HAM-A), or anhedonia (SHAPS; see Table 1). Because group differences in anhedonia were not observed, appetite change rather than anhedonia appears to more broadly account for MDD-A− participants’ significantly lower food pleasantness ratings.
Figure 2.
CRP concentration distribution by group.
Note. CRP = C-reactive protein. HC = healthy control. MDD-A+ = MDD with increased appetite. MDD-A− = MDD with decreased appetite.
Table 1.
Participant demographics and characteristics.
| MDD-A+ (n = 14) | MDD-A− (n = 17) | HC (n = 33) | Statistics | ||||
|---|---|---|---|---|---|---|---|
| Male (%) | Male (%) | Male (%) | X2 | df | p | ||
| Gender | 2 (14.29) | 8 (47.06) | 9 (27.27) | 4.14 | 2 | 0.13a | |
| Mean (SD) | Mean (SD) | Mean (SD) | F | t | df | p | |
| Age | 34.36 (9.71) | 32.12 (8.94) | 31.48 (8.65) | 0.51 | - | 2 | 0.60b |
| Body Mass Index (kg/m2) | 30.31 (5.04) | 27.25 (5.14) | 28.65 (5.15) | 1.38 | - | 2 | 0.26b |
| Average Food Pleasantness Rating | 4.32 (0.92) | 3.23 (0.91) | 3.96 (0.96) | 5.72 | - | 2 | 0.01b |
| CRP (mg/L) | 4.26 (3.43) | 1.95 (1.76) | 2.63 (2.60) | - | - | - | - |
| log(CRP) | 0.36 (0.71) | 0.12 (0.40) | 0.14 (0.59) | - | 3.81 | 2 | 0.15c |
| Modified Hamilton Depression Rating Scale | 18.85 (5.53) | 17.00 (5.55) | 1.67 (2.12) | - | 0.93 | 27.90 | 0.36d |
| Hamilton Anxiety Rating Scale | 19.00 (6.30) | 18.41 (8.03) | 1.94 (2.44) | - | 0.23 | 28.95 | 0.82d |
| Modified Snaith-Hamilton Pleasure Scale | 22.64 (4.83) | 26.00 (7.09) | 15.33 (5.14) | - | −1.56 | 28.12 | 0.13d |
Note. MDD-A+ = major depressive disorder with appetite increase. MDD-A− = major depressive disorder with appetite decrease. HC = healthy comparison subjects. CRP = C-reactive protein. Appetite and weight related items were removed from the Hamilton Depression Rating Scale. All food and drink related items were removed from the Snaith-Hamilton Pleasure Scale.
Chi-squared test
One-way ANOVA test
One-way Kruskal-Wallis test
Two Sample t-test between MDD groups.
3.2. Neuroimaging Results
Ratings of food pleasantness were related to activation within a network of regions implicated in valuation and reward, including medial prefrontal cortex, insula, OFC, and caudate (see Supplemental Table 1). While the current study focused on CRP by group interactions, whole-brain main effects of group (i.e., observed within regions including posterior cingulate cortex, parietal cortex, and fusiform gyrus) and CRP (i.e., observed within regions including culmen, parietal cortex, and fusiform gyrus) are described in Supplemental Materials (Supplemental Tables 2 and 3). Additionally, the present study focused on results in a priori anatomical regions; however, the whole brain results for the CRP by group interaction are included in Supplemental Materials as other brain regions may also be implicated in this relationship (Supplemental Table 4).
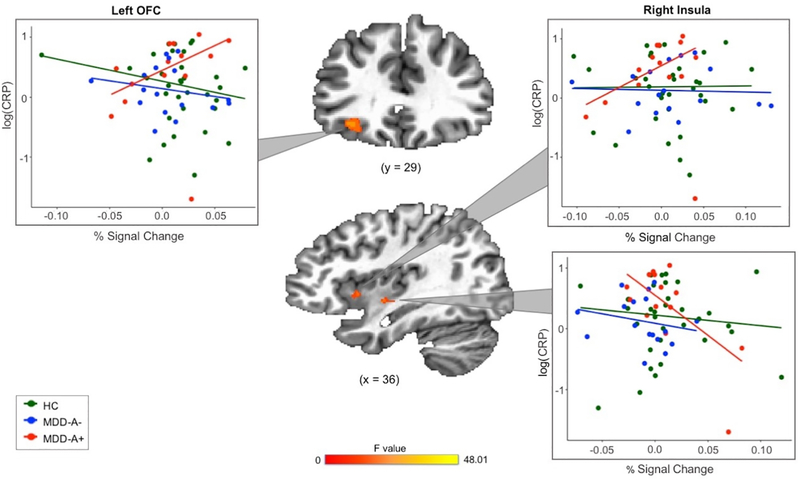
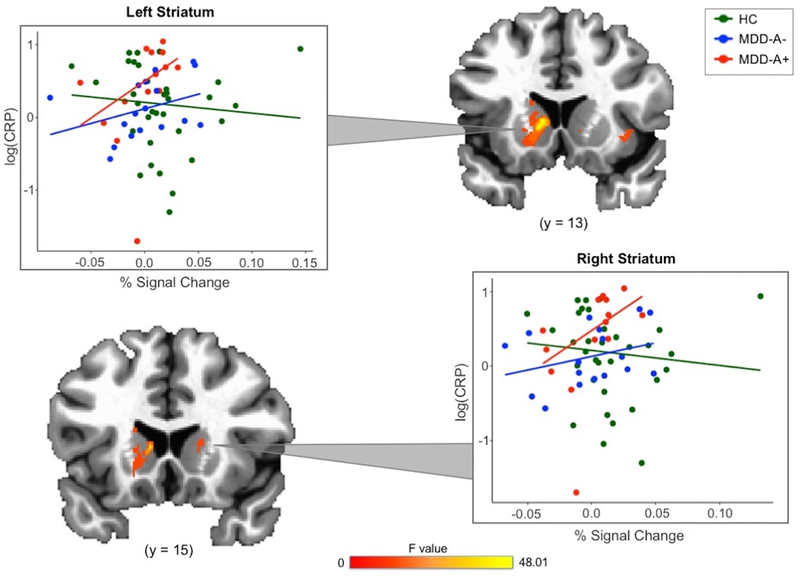
The results of the robust 3dMVM revealed clusters in left OFC, right insula, and bilateral striatum that demonstrated a significant interaction effect between group and CRP (voxel-wise p < 0.005; see Table 2 and Figures 3 and 4), indicating that the coupling between participants’ BOLD signal change and pleasantness ratings of food stimuli is influenced by the interaction between their group status and CRP concentration. Similar results remained when controlling for gender (Supplemental Table 5).
Table 2.
ROI analysis of Group*CRP interaction effects on the coupling between BOLD activation and pleasantness ratings of food stimuli (p < 0.005).
| Regions within Cluster | L/R | Vol (mm3) | x | y | z | BA |
|---|---|---|---|---|---|---|
| Caudate, Putamen, Ventral Striatum | L | 2058.0 | −7.9 | 14.8 | 7.4 | - |
| OFC | L | 262.6 | −32.4 | 28.8 | −4.9 | 11, 47 |
| Anterior/Mid-Insula | R | 198.3 | 42.9 | −6.2 | −1.4 | 13 |
| Posterior Mid-Insula | R | 182.2 | 35.9 | 9.5 | 0.4 | 13 |
| Putamen | R | 80.4 | 20.1 | 6.0 | 3.9 | - |
| Caudate | R | 75.0 | 14.9 | 14.8 | 7.4 | - |
| Caudate | R | 75.0 | 18.4 | −8.0 | 21.4 | - |
| Ventral Striatum | R | 69.7 | 11.4 | 9.5 | 2.1 | - |
Note. L = left hemisphere. R = right hemisphere. Vol = Volume in cubic millimeters. BA = Brodmann Area. Talairach coordinates reflect the area of peak activation in the cluster. All data included in this table was collected from the ‘whereami’ function in AFNI.
Figure 3.
Robust regression analyses revealed a more positive relationship between CRP and coupling between food peasantness ratings and BOLD activation in OFC and anterior insula clusters for MDD-A+. In mid/posterior insula, MDD-A+ demonstrated a more negative relationship between CRP and coupling between peasantness ratings and BOLD activation.
Note. HC = healthy control. MDD-A+ = MDD with increased appetite. MDD-A− = MDD with decreased appetite.
Figure 4.
Robust regression analyses revealed a more positive relationship between CRP and coupling between food pleasantness ratings and striatal BOLD activation for all MDD.
Note. HC = healthy control. MDD-A+ = MDD with increased appetite. MDD-A− = MDD with decreased appetite. Data for the right striatum represent individual subject averages from four separate clusters of activation.
Follow-up (i.e., post-hoc) robust regression analyses were performed to identify the specific relationships underlying the group x CRP interactions. In the left OFC cluster, CRP was related to stronger coupling between BOLD signal change and pleasantness ratings for MDD-A+ compared to both MDD-A− (b = −0.07, t = −4.58, p < 0.001) and HC (b = −0.07, t = −5.79, p < 0.001), with no significant differences observed between MDD-A− and HC (b = −0.004, t = −0.30, p = 0.76; Figure 3). In short, compared to participants from the other two groups, MDD-A+ participants exhibited a greater increase in left OFC activity per unit change in their food pleasantness ratings.
The regression models involving insula activation revealed contrasting results. In the right anterior/mid-insula, greater CRP was related to stronger coupling between BOLD signal change and pleasantness ratings in MDD-A+ compared to MDD-A− (b = −0.05, t = −2.14, p < 0.05) and HC (b = −0.07, t = −4.16, p < 0.001). There were no significant differences between MDD-A− and HC (b = 0.02, t = 0.79, p = 0.43). In the right posterior mid-insula, higher CRP was related to weaker coupling between BOLD signal change and pleasantness ratings in MDD-A+ than HC (b = −0.04, t = −3.64, p < 0.001). MDD-A− did not differ significantly from MDD-A+ (b = 0.01, t = 1.16, p = 0.25) or HC (b = −0.02, t = −1.90, p = 0.06; Figure 3).
Compared to HC, both MDD-A+ and MDD-A− higher CRP was related to stronger coupling between BOLD signal change and pleasantness ratings in right (MDD-A+: b = 0.03, t = 3.20, p < 0.01; MDD-A−: b = 0.05, t = 2.91, p < 0.01) and left (MDD-A+: b = 0.03, t = 2.68, p < 0.01; MDD-A−: b = 0.05, t = 4.45, p < 0.001) striatum clusters. Statistically significant differences between MDD-A+ and MDD-A− were not observed for right (b = 0.02, t = 1.15; p = 0.26) or left striatum clusters (b = 0.03, t = 1.86; p = 0.07; Figure 4). However, the left striatum appears to have a trending difference between the MDD groups.
4. Discussion
The present study examined potential neural mechanisms underlying the relationship between systemic inflammation, indexed here by CRP concentration in plasma, and depression. There were two key findings. First, in participants with depression-related appetite increase, higher inflammation was associated with a stronger coupling between inferred food pleasantness and activity in both the OFC and anterior/mid-insula as well as a weaker coupling in posterior insula. Second, in MDD participants regardless of the direction of appetite change profile, higher inflammation was associated with a stronger coupling between inferred food pleasantness and activity in the striatum. These findings suggest that CRP may differentially moderate the relationship between food pleasantness ratings and BOLD activation across MDD appetite change profiles and specific brain regions involved in food hedonics. Therefore, peripheral inflammation may play a role in the behavioral and neural processes underlying why some individuals with MDD experience increased appetite while others experience decreased appetite.
We expected that compared to MDD-A− and HC, higher CRP in MDD-A+ would be associated with stronger coupling between food pleasantness ratings and activity in reward and interoceptive regions. This result was observed within left OFC, a region commonly associated with decision making and behavioral drives including food cravings (Elliott et al., 2000; Lowe et al., 2009). The same area of the OFC was also one of two regions identified in the original publication with the Food Pleasantness Task, further highlighting the role of the OFC in on-line hedonic inferences of food stimuli (Simmons et al., 2014). As described by Simmons and colleagues (2018), it is possible that systemic inflammation in some individuals with depression may lead to disrupted metabolic signaling through the hypothalamus and a subsequent blunting of homeostatic satiety signals that inform reward valuation. This model would indeed account for the present study’s observation that the MDD-A+ participants exhibited both heightened inflammation and altered coupling between activity in the OFC and food reward inferences. Other models, however, could also apply. For example, elevated inflammatory cytokines and resulting changes in the relative availability of kynurenine pathway metabolites may alter neural activity more directly, particularly in OFC as well as in general reward circuitry (Felger et al., 2015).
Two significant clusters were observed within the insula that exhibited contrasting effects in the MDD-A+ group. This may reflect the functional diversity of specific regions of the insular cortex (Kurth et al., 2010; Simmons et al., 2013) as well as the impact of peripheral inflammation across the insular cortex (Harrison et al., 2015). In the anterior insula, a region involved in emotional processing and attending to salient stimuli (Kurth et al., 2010; Simmons et al., 2013), higher CRP was related to stronger coupling between activation and hedonic ratings for MDD-A+ compared to MDD-A− and HC. In the mid- to posterior insula cluster, higher CRP was related to weaker coupling between activation and hedonic ratings for MDD-A+ compared to HC. The mid and posterior regions of the insula are primarily implicated in interoceptive awareness of the body’s homeostatic state (Simmons et al., 2013). In combining these two findings from the insula, our results suggest that when MDD-A+ are in a pro-inflammatory state, and they may be unable to effectively integrate interoceptive/homeostatic signals (e.g., hunger versus satiety) into their valuation of salient food stimuli.
In bilateral striatum, both MDD groups exhibited a stronger positive relationship between CRP and the coupling between BOLD activation and hedonic ratings than was observed in HC participants. This is consistent with the aforementioned evidence that inflammation may particularly affect the activity of reward circuitry in individuals with MDD (Felger et al., 2015; Felger & Treadway, 2017; Miller & Raison, 2016), with greater inflammation associated with altered hedonic inferences of food, irrespective of appetite change directionality. Our results suggest that increased inflammation in MDD is linked to tighter coupling between striatum activity and hedonic inferences about food. However, striatum activity alone certainly does not drive differential appetite responses in MDD. Rather, OFC and insula activation in the presence of inflammation may further modulate responses to food cues, thereby contributing to whether an individual with MDD ultimately exhibits an increased or decreased appetite phenotype. Specifically, inflammation may be related to unique OFC and insula responses to interpreting the pleasantness of food cues in MDD-A+. This may reveal a process by which inflammation leads MDD-A+ to be more sensitive to food-related sensory information but to be unable to properly recognize interoceptive signals of hunger and satiety. This may then result in MDD-A+ having greater motivation to consume foods.
The present study included a well-characterized sample of unmedicated MDD participants, with groups that were similar in age and BMI. The study used a variety of data collection methods including self-report, behavioral, immunologic, and neuroimaging approaches. Analyses were conducted using robust methods and were conservatively corrected for multiple comparisons. The Food Pleasantness Task used for neuroimaging analyses has demonstrated replicable effects across populations of healthy participants (Simmons et al., 2014). Analysis of the Food Pleasantness Task accounted for participants’ idiosyncratic food preferences through AM regression. Nevertheless, it is important to acknowledge that the sample size of participants with depression was relatively small which limited the power of our analyses. Moreover, the small samples and gender distribution across groups limited our ability to study sex differences. It is notable, however, that the gender distribution of the present sample is representative of the greater incidence of MDD in females than males (Essau et al., 2010). Next, our hypotheses were based on prior findings (from our group and others) of increased inflammation being associated with MDD-A+. However, some previous studies have also found elevated cytokine levels in patient subtypes which may involve appetite loss (Maes et al. 1993; Huang & Lee, 2007; Bai et al., 2015). Although our current findings suggest that inflammation may play a unique role in the neurocircuitry underlying food hedonics in MDD-A+, further research is needed to parse apart the relationship between cytokine levels and appetite change profiles in MDD. Additionally, we did not explore differences in results for MDD participants based on onset of depression. However, we expect that results would not differ much depending on disease chronicity due to the finding that appetite change profiles generally remain stable over recurrent depressive episodes (Stunkard et al., 1990). Furthermore, our examination of the relationship between peripheral inflammation and appetite change profiles was only conducted within the scope of major depressive disorder. Therefore, our results cannot be applied to other clinical populations with inflammation related to appetite and weight changes (e.g., cancer cachexia, anorexia nervosa, etc.). The focus of this manuscript was specifically on the role played by inflammation, as indexed by CRP, in appetite change profiles of MDD. Nevertheless, it is important to recognize that other non-inflammatory factors likely also influence the neurobiology underlying these profiles, including numerous metabolic and endocrine signaling molecules (e.g., leptin, ghrelin, cortisol, etc.). For instance, previous findings from our group suggest that increased peripheral inflammation may lead to leptin insensitivity, which impacts the regulation of appetite (i.e., through the hypothalamus; Simmons et al., 2018). Similarly, ghrelin has been shown to have unique relationships with hypothalamic BOLD activation in response to food pictures across MDD-A+ and MDD-A− groups (Cerit et al., 2019). Finally, the data were collected cross-sectionally, so interpretations of causation between inflammation, depression, appetite changes, and neural activation patterns should not be made.
Recognizing the limitations of the present study, future research should explore the relationship between inflammation and depression-related appetite change profiles in a larger, more representative sample. The current findings, while studied in relation to hedonic inferences of food, may also suggest relationships between depression and other aspects of appetite regulation (e.g., motivational salience and homeostatic signaling). Further investigations concerning these various aspects of appetite regulation could be a fruitful endeavor for future depression research. Exploring the underlying mechanisms between inflammation and food-related anhedonia in MDD may be particularly useful, as our results suggest that MDD-A− find foods to be significantly less pleasant than MDD-A+. Additionally, studying the onset and development of inflammation in MDD could provide a longitudinal perspective of how inflammation relates to behavioral outcomes such as appetite change or interoception. To expand on the present findings involving CRP, future studies may wish to explore specific inflammatory/cytokine signaling pathways in depression with appetite change (e.g., using IL-1 or IL-6). Because previous findings suggest that IL-6 concentration may differ between MDD-A+ and MDD-A− (Lamers et al., 2013; Rudolph et al., 2014; Simmons et al., 2018), we replicated the analyses presented herein to include IL-6 rather than CRP concentration (see Supplemental Materials Section 3.0). These analyses are not included in the main text because they were likely impacted by reduced power, as only 45 of the 64 participants had usable IL-6 assays. However, the results suggest that similar to CRP, IL-6 may also differentially relate to the coupling between food pleasantness ratings and BOLD activation across groups. This is to be expected, as CRP production is stimulated by IL-6. Lastly, intervention studies would be useful in determining whether these inflammatory, interoceptive, and appetite disturbances relate to one’s ability to respond to treatment and whether they may normalize with symptom remission.
Current findings provide an enhanced neurobiological understanding of MDD and potential subtypes of this heterogenous disorder. Results suggest that MDD treatments targeting inflammation may result in normalization of food responses, particularly in individuals with increased appetite. These findings, along with further delineation of inflammatory or appetite subtypes, may ultimately lead to more targeted, personalized, and effective treatments for MDD, and the prevention of its concomitant adverse health outcomes such as obesity and cardiovascular disease.
Supplementary Material
Depression with increased appetite (MDD-A+) has been linked to systemic inflammation
We explored how food hedonics in MDD relate to systemic inflammation (plasma CRP)
Striatum activity in all participants with MDD positively correlated with CRP
OFC and anterior insula activity positively correlated with CRP in only MDD-A+
Food hedonics in MDD appetite change profiles differentially relate to inflammation
Acknowledgments
The authors would like to thank Katie Thompson, Joel Barcalow, Jennifer Dobson, and Casey Mullins for their assistance with subject assessment and recruitment. We also thank the University of Oklahoma Integrative Immunology Center for assistance with running immunoassays and Dr. Jennifer Stewart for providing helpful feedback on this manuscript.
Funding: This research was supported by the National Institute of Mental Health (K01MH096175) grant to WKS and NARSAD Young Investigator Award to WKS.
Declarations of interest: WKS and WCD are employees of Janssen Research & Development, LLC., of Johnson & Johnson, and hold equity in Johnson & Johnson. WKS and WCD are coinventors on a patent regarding appetite change in depression. RLA receives funding from the National Institute of Mental Health (K23MH108707). RLA and TKT receive funding from the National Institute of General Medical Sciences (P20GM121312). All other authors have no financial interests to disclose.
Footnotes
Publisher's Disclaimer: This is a PDF file of an article that has undergone enhancements after acceptance, such as the addition of a cover page and metadata, and formatting for readability, but it is not yet the definitive version of record. This version will undergo additional copyediting, typesetting and review before it is published in its final form, but we are providing this version to give early visibility of the article. Please note that, during the production process, errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bai YM, Su TP, Li CT, Tsai SJ, Chen MH, Tu PC, & Chiou WF (2015). Comparison of pro-inflammatory cytokines among patients with bipolar disorder and unipolar depression and normal controls. Bipolar disorders, 17(3), 269–277. [DOI] [PubMed] [Google Scholar]
- Berg AH, & Scherer PE (2005). Adipose tissue, inflammation, and cardiovascular disease. Circulation Research, 96(9), 939–949. [DOI] [PubMed] [Google Scholar]
- Bhattacharya A, Derecki NC, Lovenberg TW, & Drevets WC (2016). Role of neuro-immunological factors in the pathophysiology of mood disorders. Psychopharmacology, 233(9), 1623–1636. [DOI] [PubMed] [Google Scholar]
- Byrne ML, Whittle S, & Allen NB (2016). The role of brain structure and function in the association between inflammation and depressive symptoms: a systematic review. Psychosomatic Medicine, 78(4), 389–400. [DOI] [PubMed] [Google Scholar]
- Cent H, Christensen K, Moondra P, Klibanski A, Goldstein JM, & Holsen LM (2019). Divergent associations between ghrelin and neural responsivity to palatable food in hyperphagic and hypophagic depression. Journal of affective disorders, 242, 29–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chamberlain SR, Cavanagh J, de Boer P, Mondelli V, Jones DN, Drevets WC, … Pariante CM (2018). Treatment-resistant depression and peripheral C-reactive protein. The British Journal of Psychiatry, 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman DP, Perry GS, & Strine TW (2005). The Vital Link Between Chronic Disease and Depressive Disorders. Preventing Chronic Disease, 2(1). [PMC free article] [PubMed] [Google Scholar]
- Cox RW (1996). AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Computers and Biomedical Research, 29(3), 162–173. [DOI] [PubMed] [Google Scholar]
- Craig AD (2002). How do you feel? Interoception: the sense of the physiological condition of the body. Nature Reviews Neuroscience, 3(8), 655–656. [DOI] [PubMed] [Google Scholar]
- Dantzer R, O’Connor JC, Freund GG, Johnson RW, & Kelley KW (2008). From inflammation to sickness and depression: when the immune system subjugates the brain. Nature Reviews Neuroscience, 9(1), 46–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Destrieux C, Fischl B, Dale A, & Halgren E (2010). Automatic parcellation of human cortical gyri and sulci using standard anatomical nomenclature. NeuroImage, 53(1), 1–15. doi: 10.1016/j.neuroimage.2010.06.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberger NI, Berkman ET, Inagaki TK, Rameson LT, Mashal NM, & Irwin MR (2010). Inflammation-Induced Anhedonia: Endotoxin Reduces Ventral Striatum Responses to Reward. Biological Psychiatry, 68(8), 748–754. doi: 10.1016/j.biopsych.2010.06.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eklund A, Nichols T, & Knutsson H (2015). Can parametric statistical methods be trusted for fMRI based group studies? arXivpreprint arXiv:1511.01863. [Google Scholar]
- Eklund A, Nichols TE, & Knutsson H (2016). Cluster failure: why fMRI inferences for spatial extent have inflated false-positive rates. Proceedings of the National Academy of Sciences, 201602413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott R, Dolan RJ, & Frith CD (2000). Dissociable functions in the medial and lateral orbitofrontal cortex: evidence from human neuroimaging studies. Cerebral Cortex, 10(3), 308–317. [DOI] [PubMed] [Google Scholar]
- Essau CA, Lewinsohn PM, Seeley JR, & Sasagawa S (2010). Gender differences in the developmental course of depression. Journal of Affective Disorders, 127(1–3), 185–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felger JC, Li Z, Haroon E, Woolwine BJ, Jung MY, Hu X, & Miller AH (2015). Inflammation is associated with decreased functional connectivity within corticostriatal reward circuitry in depression. Molecular Psychiatry, 21, 1358–1365. doi: 10.1038/mp.2015.168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felger JC, & Treadway MT (2017). Inflammation Effects on Motivation and Motor Activity: Role of Dopamine. Neuropsychopharmacology, 42(1), 216–241. doi: 10.1038/npp.2016.143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M & Williams JB (1996). Structured Clinical Interview for the DSM-IV Axis I Disorders. [DOI] [PubMed] [Google Scholar]
- Hamilton M (1959). The assessment of anxiety states by rating. British Journal of Medical Psychology, 32(1), 50–55. [DOI] [PubMed] [Google Scholar]
- Hamilton M (1960). A rating scale for depression. Journal of Neurology, Neurosurgery, and Psychiatry, 23(1), 56–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison NA, Cooper E, Dowell NG, Keramida G, Voon V, Critchley HD, & Cercignani M (2015). Quantitative magnetization transfer imaging as a biomarker for effects of systemic inflammation on the brain. Biological Psychiatry, 78(1), 49–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang TL, & Lee CT (2007). T-helper 1/T-helper 2 cytokine imbalance and clinical phenotypes of acute-phase major depression. Psychiatry and Clinical Neurosciences, 61(4), 415–420. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Berglund P, Demler O, Jin R, Koretz D, Merikangas KR, … Wang PS (2003). The epidemiology of major depressive disorder: results from the National Comorbidity Survey Replication (NCS-R). JAMA, 289(23), 3095–3105. doi: 10.1001/jama.289.23.3095 [DOI] [PubMed] [Google Scholar]
- Khalsa SS, Rudrauf D, Feinstein JS, & Tranel D (2009). The pathways of interoceptive awareness. Nature Neuroscience, 12(12), 1494–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kullmann JS, Grigoleit JS, Lichte P, Kobbe P, Rosenberger C, Banner C, … Elsenbruch S (2013). Neural response to emotional stimuli during experimental human endotoxemia. Human Brain Mapping, 34(9), 2217–2227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurth F, Zilles K, Fox PT, Laird AR, & Eickhoff SB (2010). A link between the systems: functional differentiation and integration within the human insula revealed by meta-analysis. Brain Structure and Function, 214(5–6), 519–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamers F, Bot M, Jansen R, Chan M, Cooper J, Bahn S, & Penninx B (2016). Serum proteomic profiles of depressive subtypes. Translational Psychiatry, 6(7), e851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamers F, Milaneschi Y, de Jonge P, Giltay E, & Penninx B (2018). Metabolic and inflammatory markers: associations with individual depressive symptoms. Psychological Medicine, 48(7), 1102–1110. [DOI] [PubMed] [Google Scholar]
- Lamers F, Vogelzangs N, Merikangas K, De Jonge P, Beekman A, & Penninx B (2013). Evidence for a differential role of HPA-axis function, inflammation and metabolic syndrome in melancholic versus atypical depression. Molecular Psychiatry, 18(6), 692–699. [DOI] [PubMed] [Google Scholar]
- Lowe MR, van Steenburgh J, Ochner C, & Coletta M (2009). Neural correlates of individual differences related to appetite. Physiology & Behavior, 97(5), 561–571. [DOI] [PubMed] [Google Scholar]
- Luppino FS, de Wit LM, Bouvy PF, Stijnen T, Cuijpers P, Penninx BW, & Zitman FG (2010). Overweight, obesity, and depression: a systematic review and meta-analysis of longitudinal studies. Archives of General Psychiatry, 67(3), 220–229. [DOI] [PubMed] [Google Scholar]
- Maes M, Scharpé S, Meltzer HY, Bosmans E, Suy E, Calabrese J, & Cosyns P (1993). Relationships between interleukin-6 activity, acute phase proteins, and function of the hypothalamic-pituitary-adrenal axis in severe depression. Psychiatry Research, 49(1), 11–27. [DOI] [PubMed] [Google Scholar]
- Mawlawi O, Martinez D, Slifstein M, Broft A, Chatterjee R, Hwang D-R, … Van Heertum R (2001). Imaging human mesolimbic dopamine transmission with positron emission tomography: I. Accuracy and precision of D2 receptor parameter measurements in ventral striatum. Journal of Cerebral Blood Flow & Metabolism, 21(9), 1034–1057. [DOI] [PubMed] [Google Scholar]
- Maxwell MA, & Cole DA (2009). Weight change and appetite disturbance as symptoms of adolescent depression: Toward an integrative biopsychosocial model. Clinical Psychology Review, 29(3), 260–273. [DOI] [PubMed] [Google Scholar]
- Milaneschi Y, Simmons WK, van Rossum EF, & Penninx BW (2019). Depression and obesity: evidence of shared biological mechanisms. Molecular Psychiatry, 24, 18–33. [DOI] [PubMed] [Google Scholar]
- Miller AH, & Raison CL (2016). The role of inflammation in depression: from evolutionary imperative to modern treatment target. Nature Reviews Immunology, 16(1), 22–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller GE, Stetler CA, Carney RM, Freedland KE, & Banks WA (2002). Clinical depression and inflammatory risk markers for coronary heart disease. The American Journal of Cardiology, 90(12), 1279–1283. [DOI] [PubMed] [Google Scholar]
- Moieni M, Irwin MR, Jevtic I, Olmstead R, Breen EC, & Eisenberger NI (2015). Sex Differences in Depressive and Socioemotional Responses to an Inflammatory Challenge: Implications for Sex Differences in Depression. Neuropsychopharmacology, 40(7), 1709–1716. doi: 10.1038/npp.2015.17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monteiro R, & Azevedo I (2010). Chronic inflammation in obesity and the metabolic syndrome. Mediators of Inflammation, 2010, 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pepys MB, & Hirschfield GM (2003). C-reactive protein: a critical update. The Journal of Clinical Investigation, 111(12), 1805–1812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Core Team. (2017). R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; 2017. [Google Scholar]
- Raison CL, Capuron L, & Miller AH (2006). Cytokines sing the blues: inflammation and the pathogenesis of depression. Trends in Immunology, 27(1), 24–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudolf S, Greggersen W, Kahl KG, Huppe M, & Schweiger U (2014). Elevated IL-6 levels in patients with atypical depression but not in patients with typical depression. Psychiatry Research, 217(1–2), 34–38. [DOI] [PubMed] [Google Scholar]
- Shelton RC, & Miller AH (2011). Inflammation in depression: is adiposity a cause? Dialogues in Clinical Neuroscience, 13(1), 41–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmons WK, Avery JA, Barcalow JC, Bodurka J, Drevets WC, & Bellgowan P (2013). Keeping the body in mind: insula functional organization and functional connectivity integrate interoceptive, exteroceptive, and emotional awareness. Human Brain Mapping, 34(11), 2944–2958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmons WK, Burrows K, Avery JA, Kerr KL, Bodurka J, Savage CR, & Drevets WC (2016). Depression-related increases and decreases in appetite: dissociable patterns of aberrant activity in reward and interoceptive neurocircuitry. American Journal of Psychiatry, 173(4), 418–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmons WK, Burrows K, Avery JA, Kerr KL, Taylor A, Bodurka J, … Drevets WC (2018). Appetite changes reveal depression subgroups with distinct endocrine, metabolic, and immune states. Molecular Psychiatry. doi: 10.1038/s41380-018-0093-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmons WK, Rapuano KM, Ingeholm JE, Avery J, Kallman S, Hall KD, & Martin A (2014). The ventral pallidum and orbitofrontal cortex support food pleasantness inferences. Brain Structure and Function, 219(2), 473–483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snaith R, Hamilton M, Morley S, Humayan A, Hargreaves D, & Trigwell P (1995). A scale for the assessment of hedonic tone the Snaith-Hamilton Pleasure Scale. The British Journal of Psychiatry, 167(1), 99–103. [DOI] [PubMed] [Google Scholar]
- Stunkard AJ, Fernstrom MH, Price RA, Frank E, & Kupfer DJ (1990). Direction of weight change in recurrent depression: consistency across episodes. Archives of General Psychiatry, 47(9), 857–860. [DOI] [PubMed] [Google Scholar]
- The National Institute of Mental Health. (2014). Major depression among adults. Retrieved from https://www.nimh.nih.gov/health/statistics/prevalence/major-depression-among-adults.shtml
- Vos T, Flaxman AD, Naghavi M, Lozano R, Michaud C, Ezzati M, … Aboyans V (2012). Years lived with disability (YLDs) for 1160 sequelae of 289 diseases and injuries 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. The Lancet, 380(9859), 2163–2196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu C, & Yao W (2017). Robust linear regression: A review and comparison. Communications in Statistics-Simulation and Computation, 46(8), 6261–6282. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.