Abstract
Background
Few studies have investigated the combination of pregnancy complications that predict risk for cardiovascular disease (CVD) death and how risk changes with age. This report presents a comprehensive investigation of the relation of the occurrence of multiple pregnancy complications to CVD death over 5 decades in a large pregnancy cohort.
Methods and Results
We examined pregnancy events (1959-1967) and CVD death through 2011 in 14,062 women from the Child Health and Development Studies. CVD death was determined by linkage to California Vital Statistics and National Death Index. Women were a median age of 26 years at enrollment and 66 years in 2011. Pre-existing hypertension (Hazard Ratio, (HR)=3.5; 95% Confidence Interval (CI)=2.4,5.1); glycosuria (HR=4.2; CI=1.3,13.1); late-onset pre-eclampsia (after week 34, HR=2.0; CI=1.2,3.5); and hemoglobin decline over the 2nd and 3rd trimesters (HR=1.7; CI=1.2,2.7) predicted CVD death. Delivery of a small-for-gestation or preterm infant and early-onset pre-eclampsia (by week 34) significantly predicted premature CVD death (p<0.05 for age dependence). Preterm birth combined with hemorrhage, gestational hypertension, or pre-existing hypertension identified women with a 4 to 7-fold increased risk of CVD death. Pre-eclampsia in combination with pre-existing hypertension conferred a significant nearly 6-fold risk compared to a 4-fold risk for pre-existing hypertension alone.
Conclusions
We observed combinations of pregnancy complications that predict high risk of death and two new risk markers, glycosuria and hemoglobin decline. Obstetricians serve as primary care physicians for many young women and can readily use these complications to identify high-risk women to implement early prevention.
Keywords: cardiovascular disease risk factors, mortality, cohort study, pregnancy, pre-eclampsia, hypertension, high blood pressure, preterm delivery, small for gestational age, glycosuria, hemoglobin
Journal Subject Codes: [8] Epidemiology, [122] Secondary prevention, [121] Primary prevention
INTRODUCTION
The demands of pregnancy require an extensive and coordinated maternal response involving multiple systems and substantial cardiovascular adaptation. Pregnancy is characterized as a temporary state of metabolic syndrome accompanied by increased insulin resistance and hyperlipidemia.1 The cardiovascular system is also challenged as marked by the elevation of coagulation factors and by a doubling of blood volume.2 And despite the documented up-regulation of white blood cells and other inflammatory markers such as C-reactive protein and interleukin-6, the immune system must strike a cooperative balance that allows placental implantation and facilitates fetal growth while maintaining protection for the mother. Pregnancy complications such as pre-eclampsia, gestational diabetes, gestational hypertension and delivery of a preterm or intra-uterine growth retarded infant provide signals about the mother’s cardiovascular coping mechanisms to the challenges of pregnancy.
Mounting evidence supports the link between pregnancy response and cardiovascular disease years later3-26 sustaining the notion that pregnancy offers opportunities for identifying women at risk early in their lives when it may be possible to alter their risk trajectory.1,15,16,27,28
Long-term cohort studies have provided evidence that either hypertension during pregnancy or the occurrence of pre-eclampsia (hypertension in the presence of proteinuria) predict a woman’s risk for cardiovascular disease.4,5,8,9,14,19,22,26,29,30 Other pregnancy complications have also been investigated as CVD risk factors, including gestational diabetes,4,29 preterm delivery4,7,10 and delivery of a low birthweight or intra-uterine growth retarded infant.4,6,18,24 However, few studies, including our own, have investigated the combination of pregnancy complications that confer high risk, and whether they predict CVD early in life or later. This report addresses the gap in the literature by presenting a more comprehensive investigation of the relation of the co-occurrence of multiple pregnancy complications to CVD death risk over 5 decades in a large pregnancy cohort enrolled in the 1960’s. Our goal was to refine the risk spectrum in order to identify very-high-risk target groups for more intensive and early intervention, and to examine the effect of elevated levels of glycosuria and changes in hemoglobin during pregnancy on CVD risk.
METHODS
Study Design.
The Child Health and Development Studies (CHDS) was initiated in 1959 to investigate the prenatal determinants of pregnancy outcomes and child health and development. The target population included all members of the Kaiser Foundation Health Plan residing in the East Bay of the San Francisco Bay Area. Virtually all pregnant women (over 98%) receiving prenatal care from the Kaiser Health Plan at its clinics in Alameda County, California were recruited to the study. In all, 15,528 women and their 19,044 live-born children were enrolled during the 7-year recruitment period from 1959 until 1966, with deliveries extending into 1967.31 The CHDS cohort incorporates multiple race/ethnic groups and is socioeconomically broadly based. Notably, the CHDS has always had a large representation of African-Americans (22%) due to the demographic shift that occurred during the “Great Migration” from the South to points North and West, including Oakland California.
Pre-existing maternal conditions and incident pregnancy complications (6 months prior to and during pregnancy), clinical measures (e.g. prenatal and obstetric), and labor and delivery data were abstracted from medical records. Demographic information, and health-related behavior were collected from in-person interviews for all pregnancies, generally early in the first trimester. Due to the uniform access to care, women received regular prenatal workups including assessment of weight, blood pressure, urine albumin and glucose, and hemoglobin and hematocrit during their pregnancies. Blood pressures were taken by clinic nurses using a standardized protocol.32
The institutional review board of the Public Health Institute approved the study protocol and informed consent was obtained from all participants.
Cohort Surveillance.
Surveillance has continued for 5 decades by linkage to: 1) the California Department of Motor Vehicles, for a history of residence to identify the population at risk for morbidity and mortality, 2) the California Department of Vital Statistics, for identifying deaths and cause of death,9,33 and 3) the California Cancer Registry, for identifying cancer diagnoses.34-37
CHDS mothers are regularly matched to these sources using all of their known names (e.g. married and maiden) and addresses. The accumulation of a name and address history for each cohort member protects against establishing false matches and failing to identify true matches. Surveillance efforts routinely identify over 90% of CHDS mothers. Life table analyses estimating expected numbers of breast (unpublished) and testicular cancer cases34 show close comparability to observed numbers. Linkages to the National Death Index to evaluate the completeness of mortality surveillance have yielded very few missed cases. These quality control efforts affirm that our surveillance provides reliable and comprehensive coverage.
Outcome.
Results from linkage to the California Vital Status files were used to append year of death and underlying cause. Since follow-up of the cohort spanned over 50 years, codes for the underlying cause of death from several ICD revisions were used to define cardiovascular disease (CVD) death, including 420.1 for ICD-7; 410 and 412 for ICD-8; 410, 411, 414 and 429 for ICD-9, and I21, I24 and I25 for ICD-10.9,33
Pregnancy complications.
Due to the scope and breadth of the CHDS archive, multiple pregnancy complications were available for investigation including: pre-eclampsia, pre-existing hypertension, gestational hypertension, glycosuria, hemoglobin change during pregnancy, hemorrhage, and delivery of a preterm or small for gestational age (SGA) baby. These variables were coded as 1 for observed versus 0 for not observed for each CHDS pregnancy.
Proteinuria was defined as a reading of ≥1 (0.5% or 300 mg/24 hours) on a urine dipstick. Blood pressure was measured as systolic over diastolic pressure in millimeters of mercury. Pre-eclampsia was defined as any of the following occurring after 20 weeks of gestation:38,39 two or more blood pressure readings of ≥140/90 mm Hg and proteinuria; or, two or more systolic readings ≥140 mm Hg and proteinuria; or, two or more diastolic readings of ≥90 mm Hg and proteinuria. Early onset pre-eclampsia was defined as occurring by the 34th completed week of pregnancy and late onset was defined as occurring after the 34th completed week.9 Preexisting hypertension was defined as one or more blood pressure reading(s) of ≥140/90 mm Hg before 20 weeks of pregnancy or physician-coded hypertension from the medical record. Gestational hypertension was defined as one or more blood pressure reading(s) of ≥140/90 mm Hg that developed after the 20th week of pregnancy and not accompanied by proteinuria. Preterm delivery was defined as delivery before the 37th completed week of gestation. Small for gestational age (SGA) was defined as below the 10th percentile of the standardized (z-score) sex-specific birthweight-for-gestation distribution for all CHDS births. Sex-specific birthweight-for-gestation z-scores were calculated by subtracting the individual birthweight from the mean for each gestational week and dividing the difference by the standard deviation of the mean. Glycosuria was defined as one or more readings of ≥2 (13.9 mmol/L or 250 mg/100 mL) on a urine dipstick.4 This measure was used as a surrogate indicator for gestational diabetes since prenatal care standards during CHDS pregnancies did not include the routine oral glucose tolerance screening procedures that are now common practice for gestational diabetes. Hemoglobin change over the gestation was calculated as the difference between 3rd trimester and 2nd trimester hemoglobin adjusted for the days between the measures ([3rd trimester hemoglobin – 2nd trimester hemoglobin]/[gestational days between measures]). Hemoglobin change ranged from positive to negative values, with negative values representing women who had declines in hemoglobin over the 2nd and 3rd trimesters. Women who had the greatest absolute decline in hemoglobin, corresponding to the lowest decile of the change score, were coded as ‘1’ on a measure of hemoglobin decline, while all other women were coded as ‘0’. Hemorrhage was defined as bleeding late in pregnancy from a normally situated uterus; or, during labor and delivery. Irregular menstruation was used as a surrogate to examine CVD risk associated with polycystic ovarian syndrome (PCOS).33 It was defined as self-report or physician report of irregular menstrual cycles, self or physician report of long cycles (>35 days); or physician coded oligomenorrhea, anovulatory cycles, or irregular menses.
Covariates.
Covariates were derived from information given during pregnancy interview and from medical records. Race was categorized as Caucasian, African-American, Hispanic, Asian, or other. Dichotomous variables were created to represent African Americans, Hispanics, Asians and others, relative to Caucasians. Age was based on reported age at the observed pregnancy. Parity reflects number of previous liveborn births prior to the observed pregnancy. Cigarette smoking identifies women who smoked during pregnancy and was based on report at observed pregnancy. Overweight was defined as having a body mass index (BMI) of 25 kg/m2 or more at the observed pregnancy. BMI was calculated from weight (kg) divided by height (m) squared, measured or reported at interview or first prenatal visit. Weight was adjusted to compensate for variation in the timing of measurement by regressing weight on gestational age using the locally weighted scatterplot smoothing technique.40 Adjusted weight was then imputed as the fitted mean weight at day 104 of gestation (median value for day of interview) plus the residual from the regression procedure. This adjustment removes differences in weight due to variation in the timing of the measurement during pregnancy. Measured weight at interview was used in preference over self-reported pre-pregnancy weight since the latter was only collected after 20% of interviews had already been completed. Adjustment using measured weight avoids sample attrition, preserves statistical power and produced results similar to those for adjustment using pre-pregnancy weight (data not shown). Pregnancy weight gain was calculated using measured weight abstracted during prenatal care records over the course of pregnancy. This variable was not an independent predictor nor did it confound the associations of focus.
Statistical Methods.
Hazard ratios were calculated using competing risks Cox proportional hazards models to estimate associations for CVD death and account for the possible influence of risk from all other causes of death. Age at death or follow-up was used as the censoring variable for these models. It was calculated using mother’s age at observed pregnancy and her survival time over the follow-up period. Survival time was calculated by subtracting the year of death or censorship (based on vital status and residence history) from the year of delivery of the observed pregnancy.
Covariate-adjusted models were constructed using covariates that were either independently significant and/or confounders that altered the magnitude of the associations for pregnancy complications by 10% or more after mutual adjustment. Separate models were used to estimate each association to avoid potential collinearity among pregnancy complications. Risks for co-occurring complications were estimated using a model that included mutually exclusive dummy variables to represent combinations of the paired events. For example, to estimate the risk of gestational hypertension and preterm delivery, three dummy variables were included to represent the following non-overlapping scenarios: 1) gestational hypertension alone, 2) preterm delivery alone, and 3) co-occurring gestational hypertension and preterm delivery.
The proportional hazards assumption was tested by including an interaction term for each pregnancy complication and age at follow-up. Contrasts for significant interaction terms were run at 5-year age intervals over the follow-up period to identify the age when risk becomes statistically null and to estimate subgroup effects. All pregnancy complications were tested for interaction with race, coded as African American versus all other, and race-specific associations were examined in models stratified by race. All analyses were performed using SAS 9.3.
Analysis sample.
Most mothers, 74%, had only a single observed study pregnancy during the CHDS data collection (1959-1967). To avoid dependent observations for the 26% who had more than one observed CHDS pregnancy, we randomly selected only one pregnancy per woman for analysis. Pregnancies missing parity or terminating in multiple births, abortion, and fetal death before 20 weeks’ gestation were excluded from analysis because these pregnancies were too short to observe most of the predictors of interest in this study. Since gestations longer than 20 weeks were required to define and observe most complications, pregnancies ending before 20 weeks were excluded because we were either unable to classify pregnancy complications for them or were concerned about misclassifying them. In addition, 96 mothers with heart disease at baseline were excluded. This resulted in an analysis sample that included 14,062 women (Supplemental Figure 1) who were racially diverse (67% Caucasian, 23% African-American, 3% Hispanic, 4% Asian, 3% Other) and largely multiparous (66%), with a median age of 26 years at first study pregnancy and a median follow-up age of 66 years, ranging up to 95 years. Supplemental Table 1 provides baseline characteristics for mothers in the analysis sample and demonstrates the comparability of analysis sample mothers with all CHDS mothers. We observed 368 CVD deaths with a median age at death of 66 years. Information on hemoglobin was available for only a subset of the analysis sample, N=7,317. Sensitivity analyses for all but one complication could be examined in the analysis sample compared to the reduced sample with available hemoglobin data. Associations were comparable in both samples. Glycosuria could not be examined in the smaller sample due to low prevalence.
RESULTS
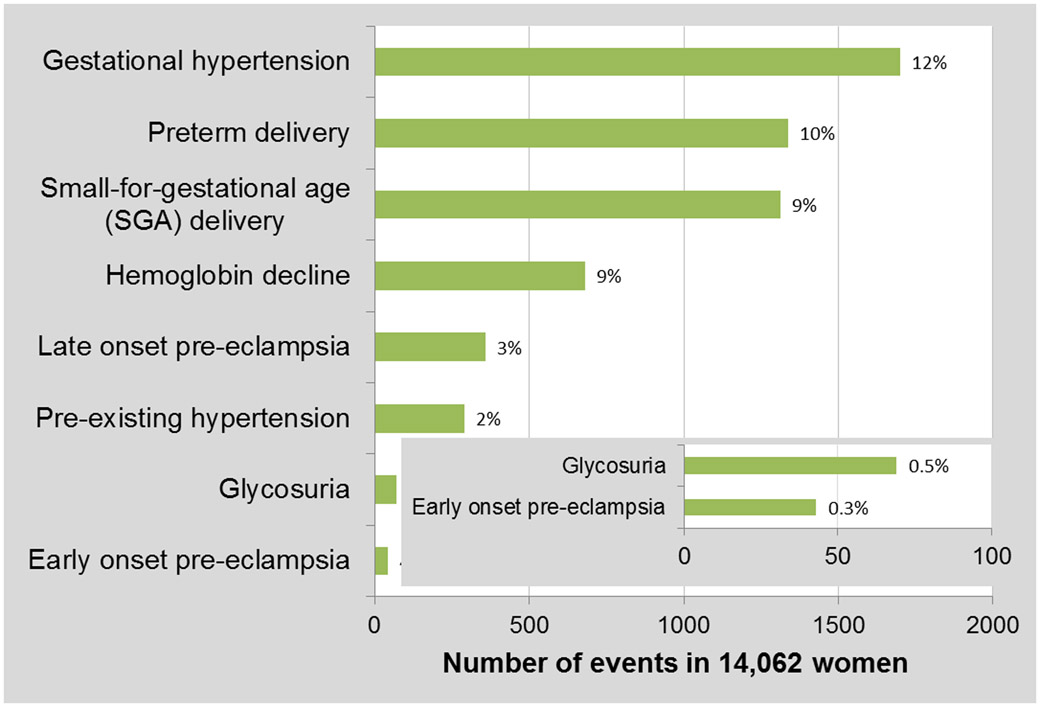
Gestational hypertension was the most commonly observed pregnancy complication, followed by preterm and SGA delivery, then hemoglobin decline, late-onset pre-eclampsia and pre-existing hypertension (Figure 1). Glycosuria and early-onset pre-eclampsia were least common and each occurred at a much lower frequency than other events. Supplemental Table 2 provides the number of CVD deaths, person-years of observation and age-adjusted rates for each pregnancy complication.
Figure 1.
Frequency and percent of pregnancy complications.
We found significant interactions between age at follow-up and early-onset pre-eclampsia (p<0.05), between age at follow-up and preterm delivery (p<0.01) and between age at follow-up and SGA delivery (p=0.01). All terms were negative indicating declining CVD risk with age, however the magnitude of the interaction term for early-onset pre-eclampsia (β=−0.07, p-value<0.01) was nearly double that for preterm delivery (β=−0.04, p-value<0.01) and SGA delivery (β=−0.04, p-value=0.01).
We examined interactions between race (dichotomized as African America versus all other) and all pregnancy complications. Only the interaction between race and gestational hypertension was significant (p=0.04). The risk of CVD estimated for gestational hypertension among African Americans was 1.7 (95% CI=1.1, 2.7) compared to 0.92 (95% CI=0.6, 1.4) among non-African Americans. Thus gestational hypertension was a significant marker of CVD risk only for African Americans.
Table 1 provides modeled CVD associations for each pregnancy complication and the effects of adjustment. For complications with significant age dependence, early-onset pre-eclampsia, preterm delivery and SGA delivery, CVD associations are estimated at age 60 years. This age was chosen because it reflected the latest age in the follow-up period when all three complications were still significant predictors. The first column shows unadjusted associations. The second column gives associations adjusted for age, race, parity, BMI and cigarette smoking at the observed pregnancy. Estimated CVD associations for pregnancy complications are significant and persistent even after adjustment for potential confounders.
Table 1.
Associations of pregnancy complications with CVD death
| Unadjusted |
Adjusted for Covariates* |
|||||
|---|---|---|---|---|---|---|
| HR† |
95% CI‡ |
HR |
95% CI |
|||
| Associated | Lower |
Upper |
Lower |
Upper |
||
| Early-onset pre-eclampsia§ | 6.7 | 2.74 | 16.20 | 3.6 | 1.04 | 12.19 |
| Pre-existing hypertension | 4.6 | 3.32 | 6.41 | 3.5 | 2.35 | 5.07 |
| Glycosuria | 4.3 | 1.62 | 11.61 | 4.2 | 1.33 | 13.10 |
| Late-onset pre-eclampsia | 2.5 | 1.36 | 3.87 | 2.0 | 1.18 | 3.46 |
| Preterm delivery§ | 2.5 | 1.82 | 3.47 | 2.1 | 1.40 | 3.01 |
| Hemoglobin decline∥ | 1.8 | 1.19 | 2.80 | 1.7 | 1.12 | 2.70 |
| SGA delivery§# | 1.8 | 1.24 | 2.55 | 1.6 | 1.02 | 2.42 |
| Gestational hypertension** | ||||||
| African American | 1.7 | 1.10 | 2.65 | 1.8 | 1.09 | 2.82 |
| Non-African American | 0.9 | 0.63 | 1.36 | 1.0 | 0.68 | 1.52 |
| Not Associated | ||||||
| Hemorrhage | 1.4 | 0.91 | 2.30 | 1.4 | 0.85 | 2.42 |
| Irregular menses/PCOS*** | 1.0 | 0.76 | 1.41 | 1.1 | 0.80 | 1.51 |
Covariates include age, race, parity, BMI and cigarette smoking status at observed pregnancy. Age is represented continuously. Parity is represented using two dummy variables for primiparas and multiparas with 3 or more prior live births versus else. BMI is dichotomized as >25kg/m2 versus else. Race is represented using two dummy variables for African-Americans and Asians versus else.
HR=Hazard Ratio estimated using competing risks Cox proportional hazards models where two outcomes, CVD death and all other death are modeled. Associations for CVD death are tabled here.
95% CI=95% Confidence Interval (Lower limit, Upper limit).
Due to interactions between early-onset pre-eclampsia and age at follow-up (p<0.05), between preterm delivery and age at follow-up (p<0.01) and between SGA delivery and age at follow-up (p=0.01) results are tabled for the CVD association estimated at age 60 years. This age was chosen because it reflected the latest age in the follow-up period when all three complications were still significant predictors.
SGA=Small-for-gestational-age.
Information on hemoglobin was available for only a subset of the sample, N=7,317.
Interaction for race by gestational hypertension was significant, p=0.04.
Irregular menses is a proxy for Polycystic Ovarian Syndrome (PCOS). We previously reported an association between irregular menses and coronary heart disease, a more restrictive subset of CVD.33
Although maternal education (less than high school degree, (HR=1.6; 95% CI=1.2, 2.2)) and occupation (as a laborer, (HR=1.5; 95% CI=1.1, 2.2)) were independently associated with increased CVD risk these socio-economic indicators did not explain associations between CVD and pregnancy complications.
Hemorrhage occurred in just over 3% and irregular menses in 13% of mothers. Neither was associated with CVD death, although we previously reported an association between irregular menses and coronary heart disease, a more restrictive subset of CVD.33 There was evidence that hemorrhage accompanied by preterm delivery in the 35th-36th weeks of gestation was associated with a significantly increased CVD risk (HR=3.9; 95% CI=1.6, 9.6). And although CVD risk for preterm delivery alone was elevated both before (HR=1.8; CI=1.2, 2.8) and after 35 weeks (HR=1.6; CI=1.0, 2.5), there was no evidence of increased risk for the co-occurrence of hemorrhage with preterm delivery before 35 weeks.
Overall, 64% of mothers had no complications (9,059), 31% had a single event (4,293) and 5% had two or more (710). Thus, most pregnancy complications occurred as single events unaccompanied by any other (Figure 2). Eighty percent of women with gestational hypertension, the most common complication, had no other event. All other complications occurred as single events at least half the time, except early-onset pre-eclampsia which occurred most frequently in combination with others (67% of occurrences). The type and frequency of combinations of pregnancy complications observed among CHDS mothers are presented in Figure 2.
Figure 2.
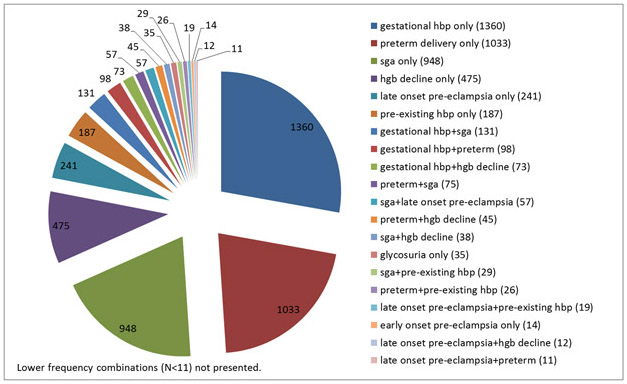
Type and frequency of observed combinations of pregnancy complications.
The CVD risk associated with the most common combinations of pregnancy complications is shown in Table 2. These combinations were associated with a statistically significant almost 3-fold or greater risk of CVD. In particular combinations that included pre-existing hypertension and another pregnancy complication were associated with a 4 to 7-fold increase in risk. Pre-eclampsia in combination with SGA delivery also showed evidence of higher risk.
Table 2.
Associations of paired pregnancy complications with CVD risk
| 95% CI* |
|||
|---|---|---|---|
| Combinations of pregnancy complications† |
HR‡ |
Lower |
Upper |
| SGA§ + preterm delivery | 2.6 | 1.06 | 6.20 |
| Gestational hypertension + hemoglobin decline | 2.8 | 1.15 | 6.92 |
| Pre-eclampsia∥ + SGA# | 3.7 | 1.12 | 12.10 |
| Preterm delivery (weeks 35-36) + hemorrhage | 3.9 | 1.63 | 9.56 |
| SGA + pre-existing hypertension | 4.8 | 1.78 | 12.91 |
| Gestational hypertension + preterm delivery# | 5.0 | 2.64 | 9.60 |
| Pre-eclampsia∥ + pre-existing hypertension | 5.6 | 2.09 | 15.18 |
| Preterm delivery + pre-existing hypertension | 7.1 | 3.49 | 14.55 |
CI=95% Confidence Interval (Lower limit, Upper limit)
Risk was estimated for each combination of pregnancy complications separately using a competing risks proportional hazards model. In order to avoid collinearity between paired pregnancy events, mutually exclusive dummy variables were included in each model. Using the first combination, SGA + preterm delivery as an example, three dummy variables were created to represent the following non-overlapping scenarios: 1) SGA only and not preterm delivery, 2) preterm delivery only and not SGA, and 3) SGA plus preterm delivery combined. The association for the co-occurrence of both events is tabled here. Models were adjusted for age, race and parity at study pregnancy. Age is represented continuously. Parity is represented using two dummy variables for primiparas and multiparas with 3 or more prior live births versus else.
HR=Hazard Ratio
SGA=small-for-gestational-age.
Early and late-onset pre-eclampsia were combined to maximize power for investigating the risk of pre-eclampsia co-occurring with other pregnancy events.
Co-occurring events involving pre-eclampsia, and preterm and SGA delivery were tested for age-dependence. Only the co-occurrence of gestational hypertension with preterm delivery and of pre-eclampsia with SGA were significantly age-dependent (p=0.0119 and p=0.0018 respectively). The hazard ratios for these paired events are estimated at age 60 for co-occurring gestational hypertension with preterm delivery, and at age 55 for co-occurring pre-eclampsia with SGA delivery.
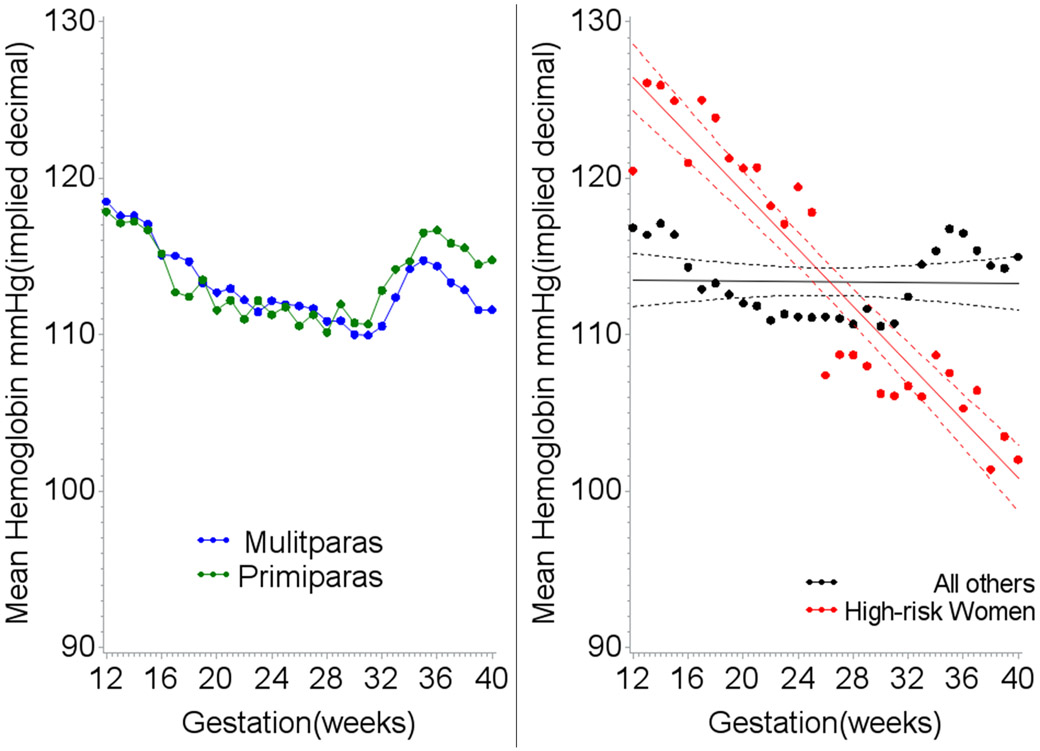
Women with the greatest hemoglobin decline during pregnancy experienced nearly a 2-fold increased risk of CVD (Table 1). Patterns of hemoglobin by trimester for these high risk women were clearly distinct from other women (Figures 3a and 3b). Women at higher risk had on average both higher levels of hemoglobin early in the second trimester and lower levels in the third trimester. Hemoglobin decline remained a significant predictor of CVD death after adjustment for early high hemoglobin levels (Table 3, Model #3).
Figure 3.
A. Mean hemoglobin levels for all women by week of gestation and parity. B. Mean hemoglobin levels for high risk women (greatest rate of hemoglobin decline) compared to all others by week of gestation.
Table 3.
CVD Associations for hemoglobin decline, high early hemoglobin and the effect of co-adjustment
| 95% CI* |
|||
|---|---|---|---|
| Model† |
HR‡ |
Lower |
Upper |
| Model #1 | |||
| Hemoglobin decline§ only | 1.8 | 1.14 | 2.71 |
| Model #2 | |||
| High early hemoglobin∥only | 1.4 | 1.00 | 1.90 |
| Model #3 | |||
| Hemoglobin decline | 1.6 | 1.04 | 2.54 |
| High early hemoglobin | 1.3 | 0.89 | 1.84 |
95% CI=95% Confidence Interval (Lower limit, Upper limit).
All models were adjusted for age, race and parity. Age is represented continuously. Parity is represented using two dummy variables for primiparas and multiparas with 3 or more prior live births versus else. Race is represented using two dummy variables for African-Americans and Asians versus else.
HR=Hazard Ratio.
Hemoglobin decline is defined as the lowest decile of hemoglobin change between the 2nd and 3rd trimesters.
High early hemoglobin is defined as the highest quartile based on the distribution of all first measures in the 2nd trimester.
DISCUSSION
We examined a constellation of pregnancy complications including pre-eclampsia, pre-existing and gestational hypertension, SGA and pre-term delivery; and, glycosuria and hemoglobin decline during pregnancy. Over a third of CHDS mothers experienced one or more of these events during their pregnancy and the risk of having any one of these complications ranged from a 1.6-fold to a 4-fold increased risk of subsequent CVD death.
In the absence of other complications, pre-eclampsia, and in particular early-onset pre-eclampsia, pre-existing hypertension and the presence of glycosuria were the strongest predictors of CVD death. Pre-term and SGA delivery and hemoglobin decline in the absence of other complications were more modest, but significant predictors of CVD death. These findings lend substantive and corroborative evidence to previous reports3-8,10,12,14-20,22,24,26,29 and are consistent with the concept that an extreme response to the challenges of pregnancy expose or initiate an underlying vulnerability to CVD.
Pregnant women undergo dramatic changes in cardiovascular function including increased blood volume and cardiac output.41 They develop hyperlidemia,2,41 experience blood pressure change (decline then increase),2,32,41 accumulate visceral fat42 and become insulin resistant.43 Thus adaptations in normal pregnancy mimic components of metabolic syndrome.44 Pro-inflammatory responses occur over the course of pregnancy, both at the site of implantation, and in the periphery.45 Many have posited that this multi-system assault acts as a “stress test” for identifying women who are at higher risk of cardiovascular disease later in life.1,27,46 Our findings support this hypothesis.
Early-onset pre-eclampsia was not only one of the strongest predictors of CVD death but also showed evidence of age-dependence. The impact of early-onset pre-eclampsia appeared relatively swiftly, associated with very high CVD mortality by age 60. Other very-high risk groups identified by multiple complications were: pre-existing hypertension combined with preterm or SGA delivery; pre-existing hypertension combined with pre-eclampsia; gestational hypertension combined with preterm delivery; preterm delivery accompanied by hemorrhage; and pre-eclampsia combined with SGA delivery. Although this last combination may be a consequence of the severity of the pre-eclampsia, the combination of pre-eclampsia complicated by IUGR or preterm delivery has been observed by others to enhance CVD risk beyond that reported for pre-eclampsia alone.12,47
We observed a new marker of CVD risk – hemoglobin decline between the 2nd and 3rd trimesters of pregnancy. In general, CHDS mothers showed a steady decline in hemoglobin beginning in early pregnancy through the 32nd week of gestation. Between weeks 32 and 36 hemoglobin increased, then declined again until the 38th week, after which it stabilized until delivery at levels just slightly below those observed in early pregnancy (Figure 3a). These are established pregnancy hemoglobin patterns and are due to the interplay between changes in blood plasma and red cell volume response during fetal growth and development.2 Early high hemoglobin is a marker of poor fetal growth, placental infarction and higher blood viscosity,48 and was marginally associated with higher CVD death in CHDS mothers. Greater hemoglobin decline was also a significant risk factor for maternal CVD and may indicate a failure to produce red cells in sufficient volume to “catch up” to plasma volume increases, or could indicate a defect that results in late increases in blood volume. Thus, hemoglobin decline may be an early indicator of the lack of an adaptive response that ultimately results in increased CVD in these women. In contrast to our results for maternal CVD death, Stephansson and colleagues reported that hemoglobin decline was not an independent risk factor for stillbirth after accounting for higher earlier hemoglobin,48 suggesting multiple pathways for the etiology of stillbirth and the etiology of maternal CVD.
Figure 3a also shows that hemoglobin patterns were similar for both primiparas and mutliparas, although levels in multiparas didn’t peak as high by the 36th week and remained lower thereafter. This difference – higher levels for primiparas – was also observed for blood pressure change during pregnancy in the CHDS (Supplemental Figure 2) and supports the theory that pregnancy response is altered after first pregnancy.
Our study has clear limitations. Covariate information measured at the observed pregnancy is used to represent lifetime exposure status. Completed parity and pregnancy complications that occurred in subsequent pregnancies are not captured. To the extent these exposures occur or change (e.g. smoking and weight) subsequent to study observation and over the life-span, baseline measures may miss-estimate the reported associations and not truly adjust out the contribution of covariates to risk of CVD. Previously, we reported CVD associations separately for primiparous and multiparous pregnancies9 and found comparable results in both groups suggesting that adjustment for completed parity would have little impact on results. Despite these limitations baseline characteristics predict risk; and, the associations observed for both pregnancy complications and covariates showed the expected direction and magnitude, lending reassurance about the observed effects.
Glycosuria was used as a surrogate measure of gestational diabetes since oral glucose tolerance testing was not standard practice when CHDS mothers were pregnant. This measure likely captures more severe, uncontrolled gestational diabetes which is consistent with both the strength of the association we observed for CVD (>4-fold increase in risk) and with the lower prevalence of gestational diabetes observed in our cohort (0.5% compared to 2-5%49). We would expect lower prevalence of gestational diabetes among CHDS mothers because they are a “healthy” population with low levels of obesity (<5%) and low levels of pregnancy weight gain (median pregnancy weight gain was just under 20 lbs.) Thus, our study cannot evaluate the effect of gestational diabetes mellitus, but does estimate the effect of a commonly available clinical marker, glycosuria in pregnancy.
Despite the sizeable nature of the CHDS cohort, some combinations of pregnancy events were observed in small numbers and were therefore less reliable for estimating CVD risk as evidenced by broad confidence intervals. Still, findings support the conclusion that pregnancies complicated by multiple events identify women who are at heightened risk of subsequent CVD. As for any observational study we cannot rule out confounding of reported associations by unmeasured variables. While, the CHDS population is diverse (Supplemental Table 1), we cannot be certain that these findings apply to all women. As for all observational studies, we cannot we rule out bias or uncontrolled confounding as a contribution to results. The outcome, CVD death rather than morbidity, poses both advantages and disadvantages. It is well-defined and likely captures the most severe disease, but prohibits the examination of the intermediary disease course.
Study strengths include a sizeable sample and prospective observation of pregnancy events. The CHDS is one of few long-term prospective studies able to examine the effect of multiple pregnancy complications on subsequent maternal cardiovascular disease mortality, having captured clinical information on pregnancy events 50 years ago when mothers were in their childbearing years and who are now well into the age of disease risk. Associations were based on clinical measures and interview data collected during pregnancy and are not subject to recall bias years later at the time of the outcome. Associations we observed for CVD are not observed for cancer death or all other non-CVD death (data not shown) supporting our hypothesis that pregnancy responses are related specifically to CVD pathology.
That combinations of risk factors carry higher risk than each individually (compare Table 1 and Table 2), suggests multiple disease pathways to CVD, or the possibility these combinations capture different aspects of a common etiology. This supports the hypothesis that pregnancy complications share pathophysiology, already programmed prior to the challenge of pregnancy, that ultimately lead to CVD.50 Our findings corroborate those of prior researchers showing associations between pregnancy complications and increased CVD risk and add to the literature by the consideration of multiple indicators of risk during pregnancy, including glycosuria and hemoglobin change. The underlying pathways that link pregnancy complications to CVD might provide clues about new early pregnancy biomarkers (e.g. markers of inflammatory and endocrine response) that identify women at risk for adverse pregnancy outcomes. Further research is required to investigate this possibility.
In summary, we identified hemoglobin decline in pregnancy as a new predictor of CVD death and found particularly high risk for some pregnancy complications. The presence of pre-existing hypertension coupled with pre-eclampsia, preterm birth, or SGA birth, strongly predict CVD death. Furthermore, we found that early-onset pre-eclampsia predicts death before age 60. Physicians should provide early, prompt surveillance and intervention for women with these high-risk pregnancy complications.
Supplementary Material
Acknowledgments:
We thank the CHDS families for their participation in this study. We acknowledge the late Jacob Yerushalmy who had the foresight to design and implement the CHDS; the late Barbara van den Berg, the second Director of the CHDS, whose steadfast allegiance and tireless efforts were responsible for granting the CHDS longevity; Roberta Christianson for her prior work on blood pressure change during pregnancy and her insight about hemoglobin as an important clinical marker for heart disease; Morgana Mongraw-Chaffin for her prior collaboration on pre-eclampsia; Nickilou Krigbaum for her meticulous assistance with file preparation and Lauren Zimmermann for assistance with analysis and preparation of tables. The point of view and conclusions expressed in this paper are those of the authors and do not necessarily represent the official position or policies of the funding agency.
Funding Sources: This work was supported by funding from the Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health, Department of Health and Human Services Contract No. HHSN275201100020C.
Footnotes
Disclosures: None.
References:
- 1.Sattar N, Greer IA. Pregnancy complications and maternal cardiovascular risk: Opportunities for intervention and screening? Br Med J. 2002;325:157–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hytten FE, Leitch I. The physiology of human pregnancy. Oxford, England: Blackwell Scientific Publications; 1971. [Google Scholar]
- 3.Brown MC, Best KE, Pearce MS, Waugh J, Robson SC, Bell R. Cardiovascular disease risk in women with pre-eclampsia: Systematic review and meta-analysis. Eur J Epidemiol. 2013;28:1–19. [DOI] [PubMed] [Google Scholar]
- 4.Fraser A, Nelson SM, Macdonald-Wallis C, Cherry L, Butler E, Sattar N, Lawlor DA. Associations of pregnancy complications with calculated cardiovascular disease risk and cardiovascular risk factors in middle age: The avon longitudinal study of parents and children. Circulation. 2012;125:1367–1380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Skjaerven R, Wilcox AJ, Klungsøyr K, Irgens LM, Vikse BE, Vatten LJ, Lie RT. Cardiovascular mortality after pre-eclampsia in one child mothers: Prospective, population based cohort study. Br Med J. 2012;345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bukowski R, Davis KE, Wilson PW. Delivery of a small for gestational age infant and greater maternal risk of ischemic heart disease. PLoS ONE. 2012;7:e33047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hastie CE, Smith GC, MacKay DF, Pell JP. Maternal risk of ischaemic heart disease following elective and spontaneous pre-term delivery: Retrospective cohort study of 750 350 singleton pregnancies. Int J Epidemiol. 2011;40:914–919. [DOI] [PubMed] [Google Scholar]
- 8.Romundstad PR, Magnussen EB, Smith GD, Vatten LJ. Hypertension in pregnancy and later cardiovascular riskclinical perspective common antecedents? Circulation. 2010;122:579–584. [DOI] [PubMed] [Google Scholar]
- 9.Mongraw-Chaffin ML, Cirillo PM, Cohn BA. Preeclampsia and cardiovascular disease death: Prospective evidence from the child health and development studies cohort. Hypertension. 2010;56:166–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Catov JM, Wu CS, Olsen J, Sutton-Tyrrell K, Li J, Nohr EA. Early or recurrent preterm birth and maternal cardiovascular disease risk. Ann Epidemiol. 2010;20:604–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lykke JA, Langhoff-Roos J, Sibai BM, Funai EF, Triche EW, Paidas MJ. Hypertensive pregnancy disorders and subsequent cardiovascular morbidity and type 2 diabetes mellitus in the mother. Hypertension. 2009;53:944–951. [DOI] [PubMed] [Google Scholar]
- 12.McDonald SD, Malinowski A, Zhou Q, Yusuf S, Devereaux PJ. Cardiovascular sequelae of preeclampsia/eclampsia: A systematic review and meta-analyses. Am Heart J. 2008;156:918–930. [DOI] [PubMed] [Google Scholar]
- 13.Berends AL, de Groot CJ, Sijbrands EJ, Sie MP, Benneheij SH, Pal R, Heydanus R, Oostra BA, van Duijn CM, Steegers EA. Shared constitutional risks for maternal vascular-related pregnancy complications and future cardiovascular disease. Hypertension. 2008;51:1034–1041. [DOI] [PubMed] [Google Scholar]
- 14.Bellamy L, Casas J-P, Hingorani AD, Williams DJ. Pre-eclampsia and risk of cardiovascular disease and cancer in later life: Systematic review and meta-analysis. Br Med J. 2007;335: 974. Epub 2007 Nov 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Harskamp RE, Zeeman GG. Preeclampsia: At risk for remote cardiovascular disease. Am J Med Sci. 2007;334:291–295. [DOI] [PubMed] [Google Scholar]
- 16.Garovic VD, Hayman SR. Hypertension in pregnancy: An emerging risk factor for cardiovascular disease. Nat Clin Pract Nephrol. 2007;3:613–622. [DOI] [PubMed] [Google Scholar]
- 17.Kaaja RJ, Greer IA. Manifestations of chronic disease during pregnancy. JAMA. 2005;294:2751–2757. [DOI] [PubMed] [Google Scholar]
- 18.Smith GD, Sterne J, Tynelius P, Lawlor DA, Rasmussen F. Birth weight of offspring and subsequent cardiovascular mortality of the parents. Epidemiol. 2005;16:563–569. [DOI] [PubMed] [Google Scholar]
- 19.Funai EF, Friedlander Y, Paltiel O, Tiram E, Xue X, Deutsch L, Harlap S. Long-term mortality after preeclampsia. Epidemiol. 2005;16:206–215. [DOI] [PubMed] [Google Scholar]
- 20.Ray JG, Vermeulen MJ, Schull MJ, Redelmeier DA. Cardiovascular health after maternal placental syndromes (champs): Population-based retrospective cohort study. Lancet. 2005;366:1797–1803. [DOI] [PubMed] [Google Scholar]
- 21.Sattar N, Ramsay J, Crawford L, Cheyne H, Greer IA. Classic and novel risk factor parameters in women with a history of preeclampsia. Hypertension. 2003;42:39–42. [DOI] [PubMed] [Google Scholar]
- 22.Irgens HU, Reisaeter L, Irgens LM, Lie RT. Long term mortality of mothers and fathers after pre-eclampsia: Population based cohort study. Br Med J. 2001;323:1213–1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Smith GCS, Pell JP, Walsh D. Pregnancy complications and maternal risk of ischaemic heart disease: A retrospective cohort study of 129290 births. Lancet. 2001;357:2002–2006. [DOI] [PubMed] [Google Scholar]
- 24.Smith GD, Whitley E, Gissler M, Hemminki E. Birth dimensions of offspring, premature birth, and the mortality of mothers. Lancet. 2000;356:2066–2067. [DOI] [PubMed] [Google Scholar]
- 25.Chesley LC. Recognition of the long-term sequelae of eclampsia. Am J Obstet Gynecol. 2000;182:249–250. [DOI] [PubMed] [Google Scholar]
- 26.Hannaford P, Ferry S, Hirsch S. Cardiovascular sequelae of toxaemia of pregnancy. Heart. 1997;77:154–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rich-Edwards JW, McElrath TF, Karumanchi SA, Seely EW. Breathing life into the lifecourse approach: Pregnancy history and cardiovascular disease in women. Hypertension. 2010;56:331–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Magee LA, von Dadelszen P. Pre-eclampsia and increased cardiovascular risk. Br Med J. 2007;335:945–946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mannisto T, Mendola P, Vaarasmaki M, Jarvelin MR, Hartikainen AL, Pouta A, Suvanto E. Elevated blood pressure in pregnancy and subsequent chronic disease risk. Circulation. 2013;127:681–690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ray JG, Vermeulen MJ, Schull MJ, Redelmeier DA. Cardiovascular health after maternal placental syndromes (champs): Population-based retrospective cohort study. Lancet. 2005;366:1797–1803. [DOI] [PubMed] [Google Scholar]
- 31.van den Berg BJ, Christianson RE, Oechsli FW. The california child health and development studies of the school of public health, university of california at berkeley. Paediatr Perinat Epidemiol. 1988;2:265–282. [DOI] [PubMed] [Google Scholar]
- 32.Christianson RE. Studies on blood pressure during pregnancy 1. Influence of parity and age. Am J Obstet Gynecol. 1976;125:509–513. [DOI] [PubMed] [Google Scholar]
- 33.Wang ET, Cirillo PM, Vittinghoff E, Bibbins-Domingo K, Cohn BA, Cedars MI. Menstrual irregularity and cardiovascular mortality. J Clin Endocrinol. 2011;96:E114–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cohn BA, Cirillo PM, Christianson RE. Prenatal ddt exposure and testicular cancer: A nested case-control study. Arch Environ Occup Health. 2010;65:127–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cohn BA, Cirillo PM, Christianson RE, van den Berg BJ, Siiteri PK. Placental characteristics and reduced risk of maternal breast cancer. J Natl Cancer Inst. 2001;93:1133–1140. [DOI] [PubMed] [Google Scholar]
- 36.Cohn BA, Wolff MS, Cirillo PM, Sholtz RI, Christianson RE, van den Berg BJ, Siiteri PK. Timing of ddt exposure and breast cancer before age 50. Lancet. 2002;13:S197. [Google Scholar]
- 37.Whittemore AS, Cirillo PM, Feldman D, Cohn BA. Prostate specific antigen levels in young adulthood predict prostate cancer risk: Results from a cohort of black and white americans. J Urol. 2005;174:872–876; discussion 876. [DOI] [PubMed] [Google Scholar]
- 38.Brown MA, Lindheimer MD, de Swiet M, Van Assche A, Moutquin JM. The classification and diagnosis of the hypertensive disorders of pregnancy: Statement from the international society for the study of hypertension in pregnancy (isshp). Hypertens Pregnancy. 2001;20:IX–XIV. [DOI] [PubMed] [Google Scholar]
- 39.Roberts JM, Pearson G, Cutler J, Lindheimer M. Summary of the nhlbi working group on research on hypertension during pregnancy. Hypertension. 2003;41:437–445. [DOI] [PubMed] [Google Scholar]
- 40.Cleveland WS. Robust locally weighted regression and smoothing scatterplots. J Am Stat Assoc. 1979;74: 829–836. [Google Scholar]
- 41.Abduljalil K, Furness P, Johnson TN, Rostami-Hodjegan A, Soltani H. Anatomical, physiological and metabolic changes with gestational age during normal pregnancy: A database for parameters required in physiologically based pharmacokinetic modelling. Clin. Pharmacokinet. 2012;51:365–396. [DOI] [PubMed] [Google Scholar]
- 42.Kinoshita T, Itoh M. Longitudinal variance of fat mass deposition during pregnancy evaluated by ultrasonography: The ratio of visceral fat to subcutaneous fat in the abdomen. Gynecol Obstet Invest. 2006;61:115–118. [DOI] [PubMed] [Google Scholar]
- 43.Vejrazkova D, Vcelak J, Vankova M, Lukasova P, Bradnova O, Halkova T, Kancheva R, Bendlova B. Steroids and insulin resistance in pregnancy. J Steroid Biochem Mol Biol. 2014;139:122–9. doi: 10.1016/j.jsbmb.2012.11.007. Epub 2012 Nov 29. [DOI] [PubMed] [Google Scholar]
- 44.Koren O, Goodrich JK, Cullender TC, Spor A, Laitinen K, Backhed HK, Gonzalez A, Werner JJ, Angenent LT, Knight R, Backhed F, Isolauri E, Salminen S, Ley RE. Host remodeling of the gut microbiome and metabolic changes during pregnancy. Cell. 2012;150:470–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mor G, Cardenas I. The immune system in pregnancy: A unique complexity. Am J Reprod Immunol. 2010;63:425–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Williams D Pregnancy: A stress test for life. Curr Opin Obstet Gynecol. 2003;15:465–471. [DOI] [PubMed] [Google Scholar]
- 47.Rodie VA, Freeman DJ, Sattar N, Greer IA. Pre-eclampsia and cardiovascular disease: Metabolic syndrome of pregnancy? Atherosclerosis. 2004;175:189–202. [DOI] [PubMed] [Google Scholar]
- 48.Stephansson O, Dickman PW, Johansson A, Cnattingius S. Maternal hemoglobin concentration during pregnancy and risk of stillbirth. JAMA. 2000;284:2611–2617. [DOI] [PubMed] [Google Scholar]
- 49.Ferrara A, Hedderson MM, Quesenberry CP, Selby JV. Prevalence of gestational diabetes mellitus detected by the national diabetes data group or the carpenter and coustan plasma glucose thresholds. Diabetes Care. 2002;25:1625–1630. [DOI] [PubMed] [Google Scholar]
- 50.Intapad S, Alexander BT. Future cardiovascular risk: Interpreting the importance of increased blood pressure during pregnancy. Circulation. 2013;127:668–669. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.