Abstract
The avian pallium is organised into clusters of neurons and does not have layered structures such as those seen in the mammalian neocortex. The evolutionary relationship between sub-regions of avian pallium and layers of mammalian neocortex remains unclear. One hypothesis, based on the similarities in neural connections of the motor output neurons that project to sub-pallial targets, proposed the cell-type homology between brainstem projection neurons in neocortex layers 5 or 6 (L5/6) and those in the avian arcopallium. Recent studies have suggested that gene expression patterns are associated with neural connection patterns, which supports the cell-type homology hypothesis. However, a limited number of genes were used in these studies. Here, we showed that chick orthologues of mammalian L5/6-specific genes, nuclear receptor subfamily 4 group A member 2 and connective tissue growth factor, were strongly expressed in the arcopallium. However, other chick orthologues of L5/6-specific genes were primarily expressed in regions other than the arcopallium. Our results do not fully support the cell-type homology hypothesis. This suggests that the cell types of brainstem projection neurons are not conserved between the avian arcopallium and the mammalian neocortex L5/6. Our findings may help understand the evolution of pallium between birds and mammals.
Subject terms: Neuroscience, Evolution
Introduction
The organisation of the avian telencephalic pallium differs considerably from that of the mammalian neocortex, which forms the neural basis of cognitive abilities in mammals1,2. Whilst large areas of the mammalian cortex exhibit a six-layered structure, large parts of the avian pallium are not laminated, but are instead organised into clusters of neurons (nuclei)2. The large masses of neurons make up the dorsal ventricular ridge (DVR), which is a highly elaborate pallial structure. The DVR consists of the mesopallium, nidopallium, and arcopallium. The difference in organisation has raised questions with regard to the evolutionary relationship between sub-regions of avian pallium and layers of mammalian neocortex3,4.
Although the overall organisation of the avian DVR and mammalian neocortex are different, the fundamental neural connections in sensory input and motor output pathways are common to mammals and birds5,6. For example, in mammals, layer 5 (L5) projection neurons of the motor cortex extend to the brainstem and spinal cord, and layer 6 (L6) projection neurons primarily project to the thalamus7. In the bird DVR, neurons in the anterior, dorsal, and intermediate parts of the arcopallium also project to the brainstem and rostral spinal cord8–11. This evidence indicates that the neural connections in the cell populations of the bird arcopallium and mammalian L5/6 similarly project to sub-pallial targets, such as brainstem and premotor areas (motor output). It has been proposed that brainstem projection neurons in the avian arcopallium are homologous to brainstem projection neurons in the neocortex L5/6 (cell-type homology hypothesis)5,6,12. Concomitantly, a study has shown that six chick orthologues of neocortex L5/6 markers were strongly expressed in the arcopallium, which is the motor output region of the bird DVR13. This suggests that gene expression patterns reflect the neural connection patterns of motor output projections to sub-pallial targets between the DVR and neocortex. Whilst their results therefore support the cell-type homology hypothesis, a limited number of genes was used. A recent largescale transcriptomic analysis revealed a mostly divergent pattern in pallial compartments between chicken and mouse14,15, suggesting the possibility that the expression of chick orthologues of L5/6 genes do not fully support the cell-type homology hypothesis.
In this study, we examined the expression of more chick orthologues of L5/6 genes and tested whether their expression patterns supported the cell-type homology hypothesis. If cell types are conserved between the brainstem projection neurons in the mammalian L5/6 and the avian arcopallium, most of the chick orthologues of L5/6-specific genes should be selectively expressed in the arcopallium. We used chick orthologues of mammalian neocortical L5/6 markers and performed in situ hybridisation in the chick telencephalon. We found nuclear receptor subfamily 4 group A member 2 (NR4A2) and connective tissue growth factor (CTGF) expression in the arcopallium which reflected the neural connection patterns between avian DVR and mammalian neocortex. However, the gene expression patterns of the other four did not. Their major expression regions were outside of the arcopallium, which shows that the expression patterns of the four genes did not reflect the neural connections in terms of motor output. Our results on the expression patterns of chick orthologues of L5/6 genes do not fully support the cell-type homology hypothesis, which suggests that the cell types of brainstem projection neurons are not conserved between the avian arcopallium and the neocortex L5/6.
Results
Selection of the mammalian neocortical layer-specific marker genes
We selected six chick orthologues that have been shown to be mammalian neocortical L5/6-specific or -selective markers16–20, i.e. NR4A2, CTGF, neurofilament heavy polypeptide (NEFH), thymocyte selection-associated high mobility group box (TOX), chicken ovalbumin upstream promoter transcription factor interacting protein 2 (CTIP2) and forkhead box protein P2 (FOXP2). NR4A2 and CTGF expressed in neocortical L6 specifically, NEFH and TOX expressed in L5 specifically, and Ctip2 expressed in both L5 and L6, and Foxp2 was expressed in the L6 selectively in mice neocortex16–20 (Table 1). The six orthologues exhibited the following sequence similarities between chicks and mice: NR4A2: protein 94.6% and DNA 85.3%; CTGF: protein 92.6% and DNA 84.3%; NEFH: protein 62.6% and DNA 66.9%; TOX: protein 89.6% and DNA 82.1%; CTIP2: protein 84.4% and DNA 76.2%; and FOXP2: protein 93.8% and DNA 87.5%. Then, we confirmed their expression pattern by referring to the data in the Allen Mouse Brain Atlas21–23 (http://www.brain-map.org, Table 1). When we selected genes from Allen Brain Atlas, we did not focus on the function of genes. Regardless of the functions, we selected genes expressed in deep layers of neocortex selectively, with less expression in other parts of pallium, especially in the pallial amygdala. We performed in situ hybridisation and analysed the expression pattern of the chick orthologues in the chick brains.
Table 1.
Overview of markers of cortical layers 5 and 6 in this study.
| Accession number | Gene symbol | Gene name | Expression layer in cortex | References | Experiment number at Allen Brain Atlas |
|---|---|---|---|---|---|
| CR522946 | NR4A2 | Nuclear receptor subfamily 4, group A, member 2 | Layer 6 (Subplate) | Watakabe, et al.18,19, Molyneaux, et al.16 | 732 |
| NM_204274 | CTGF | Connective tissue growth factor | Layer 6 (Subplate) | Heuer, et al.17 | 1183 |
| XM_415310 | NEFH | Neurofilament, heavy polypeptide | Layer 5 | Molyneaux, et al.16 | 74512048 |
| XM_015282673 | TOX | Thymocyte selection-associated high mobility group box | Layer 5 | Artegiani, et al.20 | 71670691 |
| XM_003641410 | CTIP2 | Chicken ovalbumin upstream promoter transcription factor interacting protein 2 | Layer 5 and 6 | Molyneaux, et al.16 | 74990505 |
| NM_001318413 | FOXP2 | Forkhead box protein P2 | Layer 6 | Molyneaux, et al.16 | 72079884 |
NR4A2 expression in the telencephalon of chicks
We performed in situ hybridisation using NR4A2 as the mammalian neocortical layer 6b-specific marker in post-hatched day-1 (P1) naive chick brains. Strong signals were detected in the hyperpallium (Fig. 1a–f,A12.6-A7.8) and arcopallium (Fig. 1d–f,A8.8-A7.8). In addition, signals were detected in the mesopallium (Fig. 1a–d,A12.6-A8.8). Previous studies have detected NR4A2 expression in the hyperpallium and mesopallium in embryonic chicks24,25.
Figure 1.
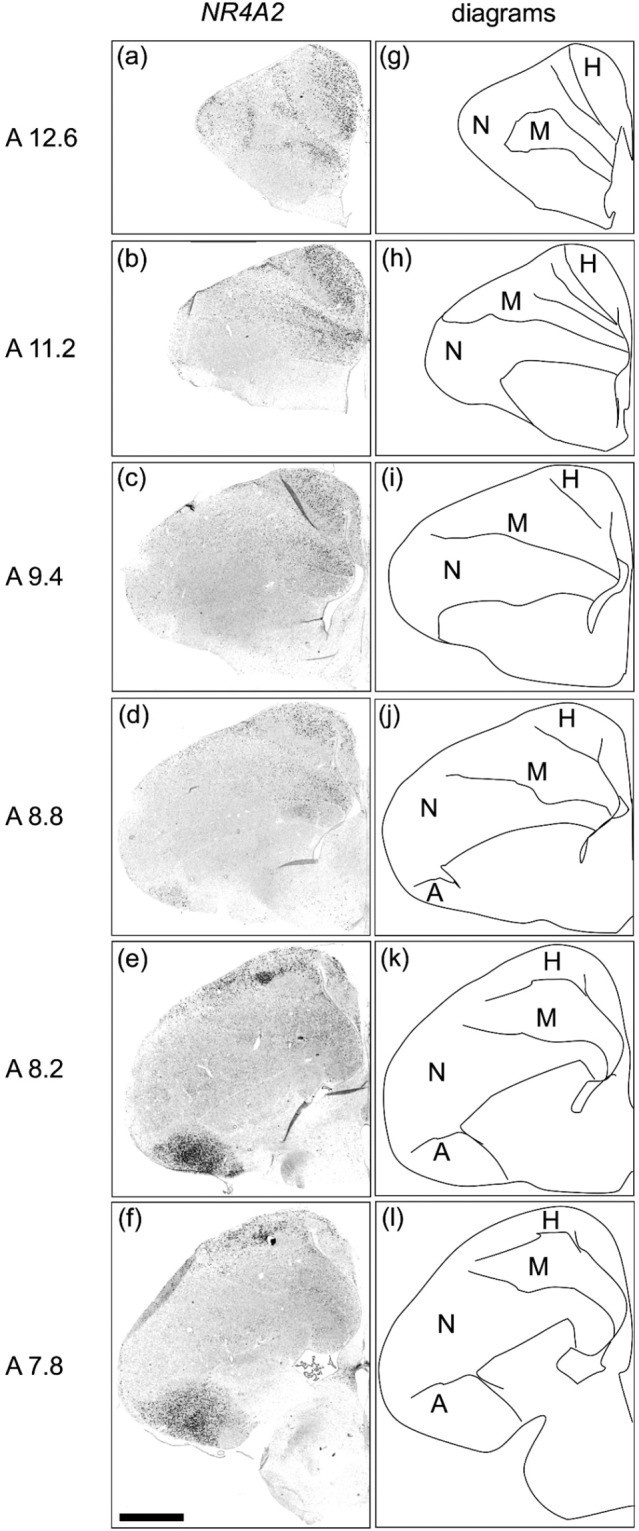
In situ hybridisation of NR4A2 in P1 chick brains. DIG-labelled RNA antisense (a–f) NR4A2 probe was used for in situ hybridisation in P1 chick brain coronal sections. For NR4A2, sections of two chicks were analysed, and representative images of chick brain sections are shown. (g–l) Diagrams of coronal sections are shown on the right panels. The levels of the sections (A12.6 to A7.8) correspond to those of the chick atlas by Kuenzel and Masson50. A, arcopallium; H, hyperpallium; M, mesopallium; N, nidopallium. Scale bar = 2.5 mm.
CTGF expression in the telencephalon of chicks
Strong signals were detected in the mesopallium (Fig. 2a–f,A13.8-A7.4) and arcopallium (Fig. 2d–f,A8.8-A7.4). In addition, signals were detected in the hyperpallium (Fig. 2a–e,A13.8-A8.0) and parahippocampal area (APH) (Fig. 2f,A7.4). To the best of our knowledge, this is first time that CTGF expression in the arcopallium has been reported. CTGF expression in the hyperpallium and mesopallium has been described by Wang et al.24.
Figure 2.
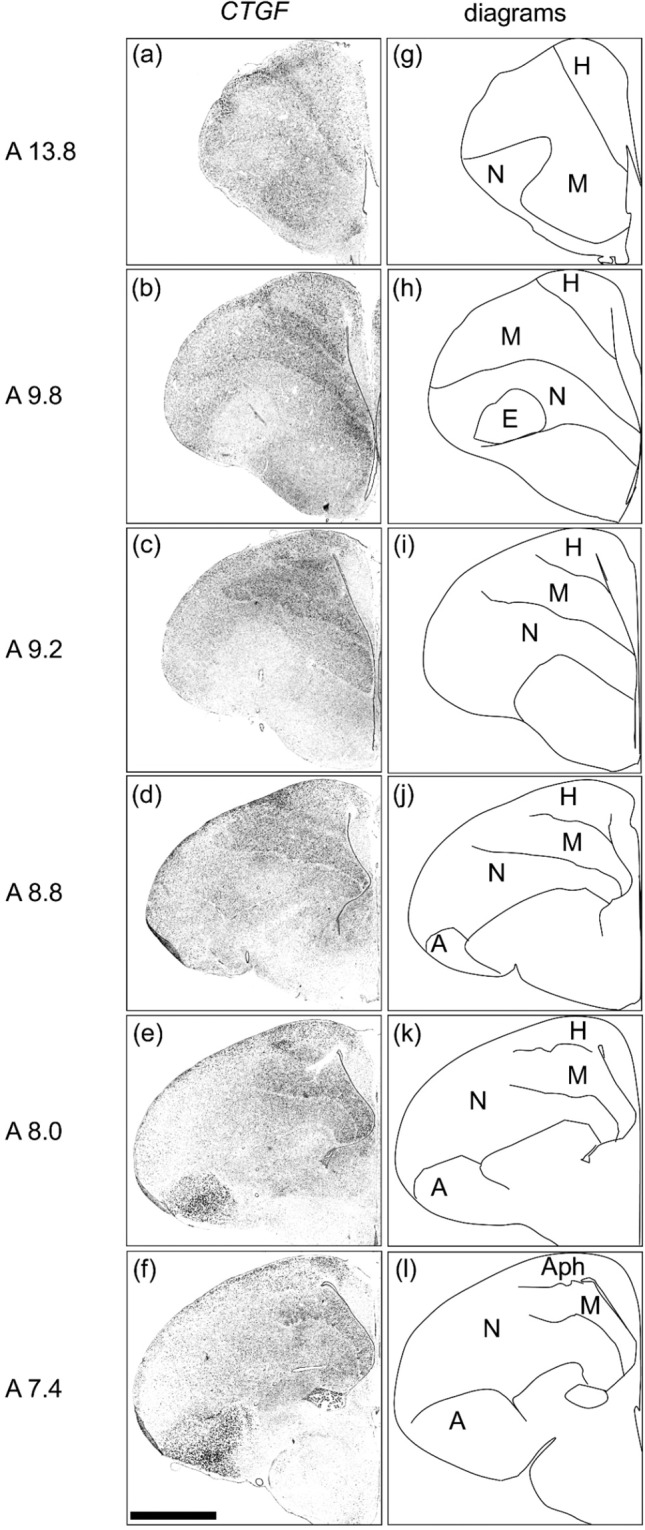
In situ hybridisation of CTGF in P1 chick brains. DIG-labelled RNA antisense (a–f) CTGF probe was used for in situ hybridisation in P1 chick brain coronal sections. For CTGF, sections of three chicks were analysed and representative images of two chick brain sections are shown. (g–l) Diagrams of coronal sections are shown on the right panels. The levels of the sections (A13.8 to A7.4) correspond to those of the chick atlas by Kuenzel and Masson50. A, arcopallium; Aph, area parahippocampalis; E, entopallium; H, hyperpallium; M, mesopallium; N, nidopallium. Scale bar = 2.5 mm.
We also found different expression patterns of NR4A2 and CTGF in the arcopallium (Fig. 3,A7.6 and A7.0). NR4A2 was expressed almost ubiquitously in the lateral, ventral, and intermediate arcopallium, while CTGF was highly expressed in the medial and ventral arcopallium, but not in the lateral arcopallium. Neither NR4A2 nor CTGF was expressed in the dorsal arcopallium.
Figure 3.
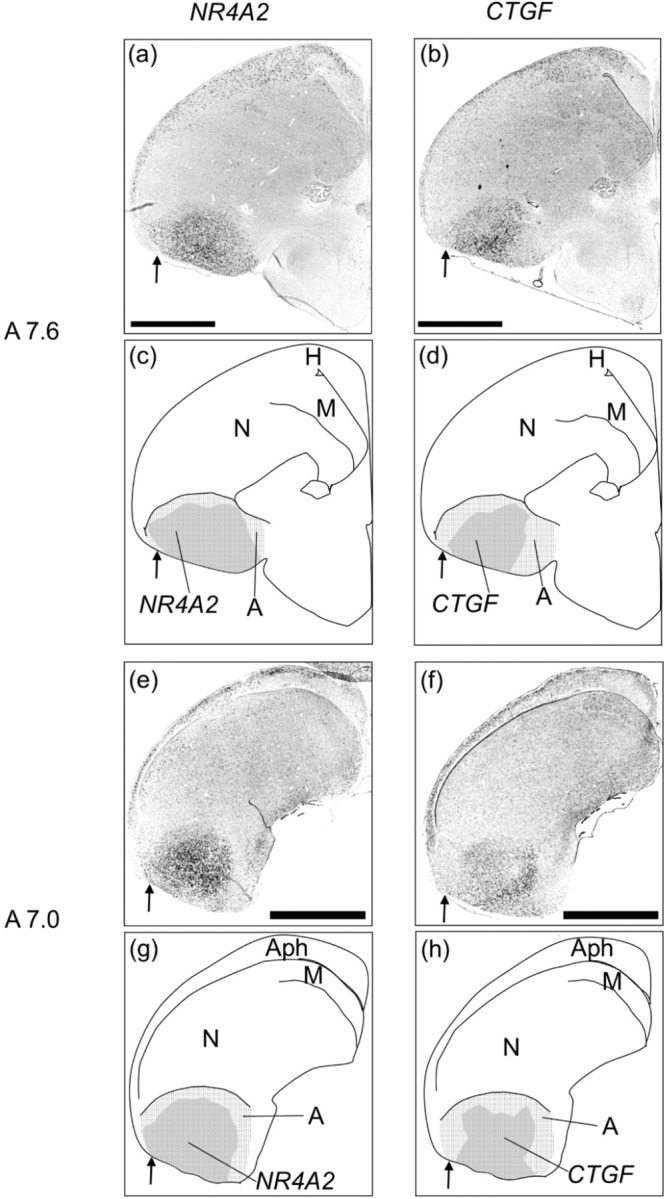
In situ hybridisation of NR4A2 and CTGF in P1 chick brains using neighboring sections. In situ hybridisation using DIG-labeled RNA antisense NR4A2 (a,e) and CTGF (b,f) probes with P1 chick brain coronal sections were shown, respectively. Panels (c), (d), (g), and (h) indicated the diagrams of the panels of the (a), (b), (e), and (f), respectively. The shaded regions, (c,g) for NR4A2 and (d,h) for CTGF, indicated the regions of signal detected in the arcopallium (dot pattern regions), respectively. Arrows indicated the area of the lateral arcopallium (a–h). The levels of the sections (A7.6 to A7.0) correspond to those of the chick atlas by Kuenzel and Masson50. A, arcopallium; Aph, area parahippocampalis; H, hyperpallium; M, mesopallium; N, nidopallium. Scale bar = 2.5 mm.
NEFH expression in the telencephalon of chicks
Strong signals were detected in the basorostralis (Fig. 4a,A12.6,b,A11.8), entopallium (Fig. 4c,A11.4,d,A9.6), and globus pallidus (Fig. 4d,A9.6,e,A8.8). Relatively weak signals were also detected in the hyperpallium and the entire DVR, including the arcopallium (Fig. 4a–f,A12.6-A7.4). The regions in which we detected strong signals appeared to correspond to a part of the intercalated nidopallium, which Jarvis et al. proposed to be a distinctive and continuous formation of the entopallium, basorostralis, and Field L26. Strong NEFH expression was restricted to the basorostralis and entopallium, but was not found in the Field L.
Figure 4.
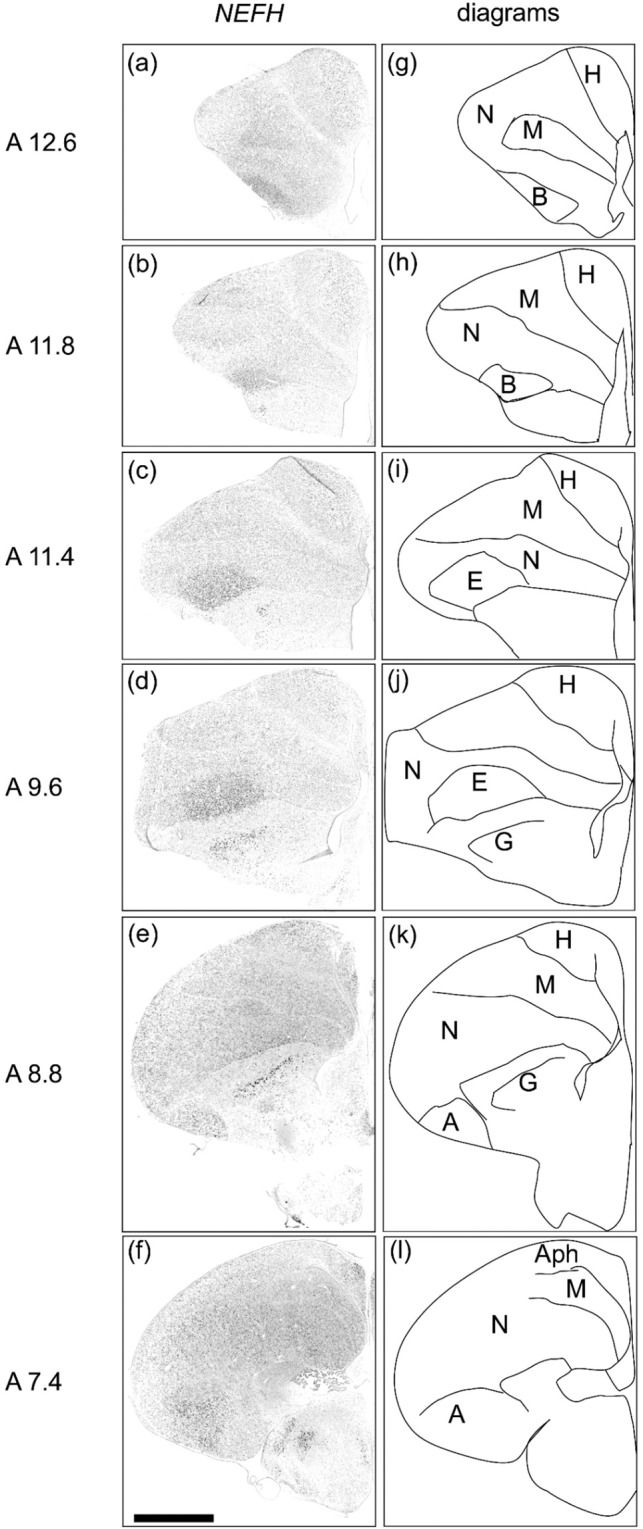
In situ hybridisation of NEFH in P1 chick brains. DIG-labelled RNA antisense (a–f) NEFH probe was used for in situ hybridisation in P1 chick brain coronal sections. For NEFH, sections of four chicks were analysed and representative images of three chick brain sections are shown. (g–l) Diagrams of coronal sections are shown on the right panels. The levels of the sections (A12.6 to A7.4) correspond to those of the chick atlas by Kuenzel and Masson50. A, arcopallium; Aph, area parahippocampalis; B, basorostralis; E, entopallium; G, globus pallidus; H, hyperpallium; M, mesopallium; N, nidopallium. Scale bar = 2.5 mm.
TOX expression in the telencephalon of chick
Strong signals were detected in the mesopallium (Fig. 5a–f,A13.8-A7.6). In addition, relatively weak signals were detected in the hyperpallium (Fig. 5a–f,A13.8-A7.6) and arcopallium (Fig. 5d–f,A8.8-A7.6).
Figure 5.
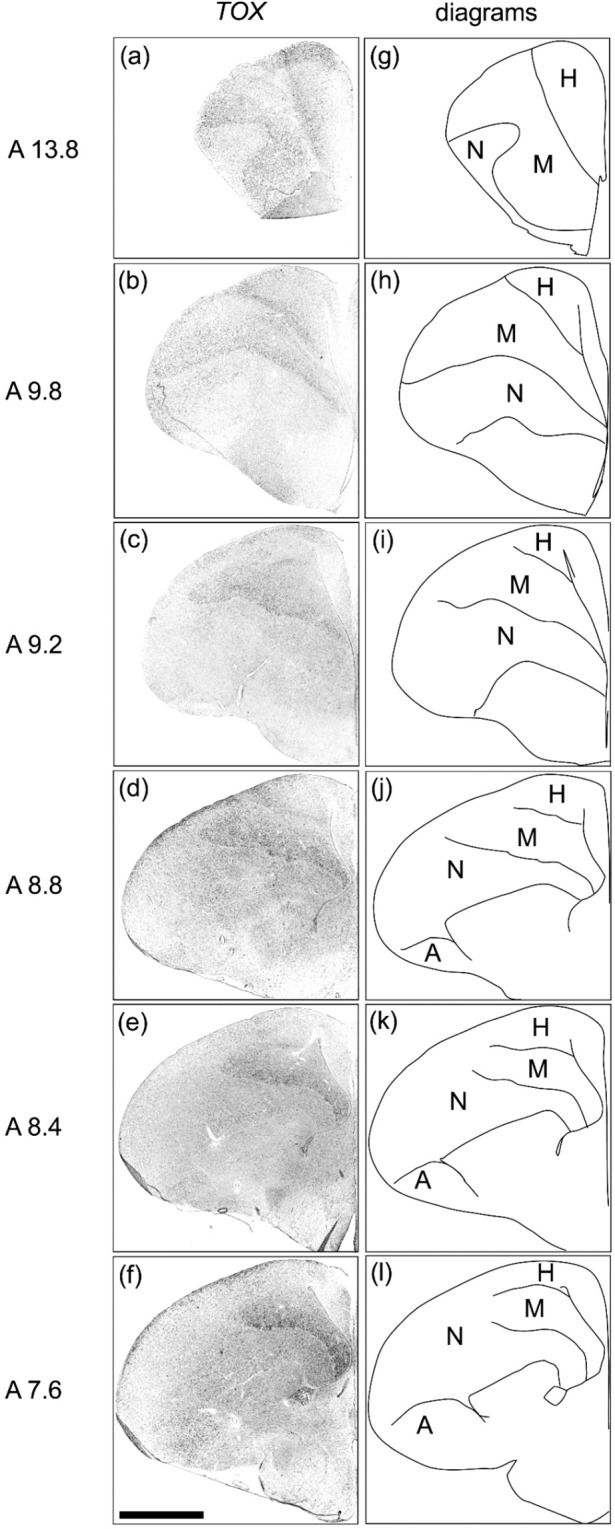
In situ hybridisation of TOX in P1 chick brains. DIG-labelled RNA antisense. (a–f) TOX probe was used for in situ hybridisation in P1 chick brain coronal sections. For TOX, sections of three chicks were analysed and representative images of two chick brain sections are shown. (g–l) Diagrams of coronal sections are shown on the right panels. The levels of the sections (A13.8 to A7.6) correspond to those of the chick atlas by Kuenzel and Masson50. A, arcopallium; H, hyperpallium; M, mesopallium; N, nidopallium. Scale bar = 2.5 mm.
CTIP2 and FOXP2 expressions in the telencephalon of chick
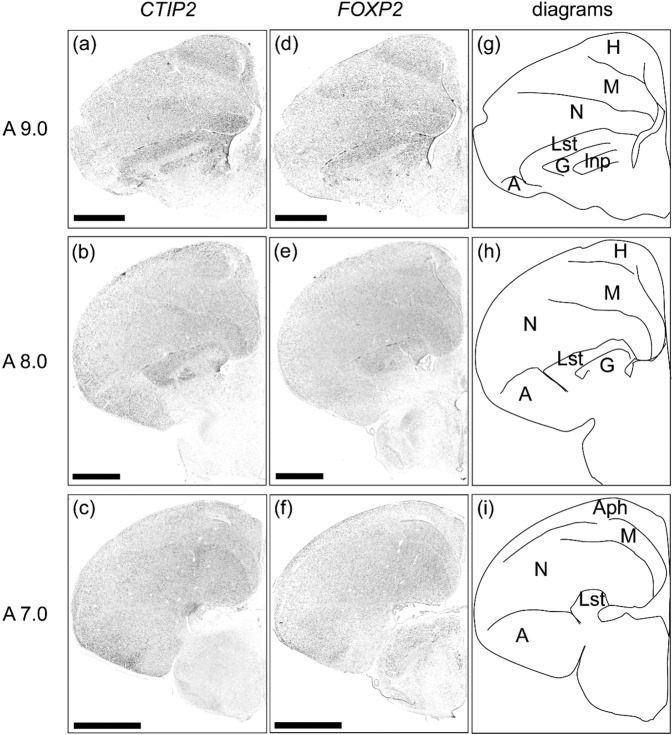
We further examined expression patterns of the chick orthologue of mammalian neocortical deep layer markers, CTIP2 and FOXP2, in the chick telencephalon. As for CTIP2, strong signals were detected in the lateral striatum (LSt), intrapeduncular nucleus (INP), and nidopallium (Fig. 6a,A9.0), and LSt and nidopallium (Fig. 6b,c,A8.0. A7.0). In addition, weak signals were detected in the hyperpallium and the entire DVR (Fig. 6a–c,A9.0-A7.0). As for FOXP2, a strong signal was detected in the LSt (Fig. 6d,e,A9.0,A8.0). Weak signals were also detected in the hyperpallium and the entire DVR (Fig. 6d–f).
Figure 6.
In situ hybridisation of CTIP2 and FOXP2 in P1 chick brains. DIG-labelled RNA antisense (a–c) CTIP2 and (d–f) FOXP2 probes for in situ hybridisation in P1 chick brain coronal sections. For CTIP2 and FOXP2, sections of four chicks were analysed, and representative images of three chick brain sections are shown. (g–i) Diagrams of coronal sections are shown on the right panels. The levels of the sections (A9.0 to A7.0) correspond to those of the chick atlas by Kuenzel and Masson50. A, arcopallium; Aph, area parahippocampalis; G, globus pallidus; H, hyperpallium; Ins, intrapeduncular nucleus; Lst, lateral striatum; M, mesopallium; N, nidopallium. Scale bar = 2.5 mm.
Discussion
One feature shared by the avian arcopallium and mammalian neocortex L5/6 consists of the neural connections of motor output neurons projecting to sub-pallial targets5–12. In this study, we found that chick orthologous genes of mammalian neocortical L5/6 markers, NR4A2 and CTGF, were strongly expressed in a neuronal population of the arcopallium (Fig. 7). Thus, the expression of NR4A2 and CTGF orthologues in the arcopallium could reflect the neural connections in terms of motor output projection between birds and mammals. In contrast, we also found that chick orthologous genes of mammalian neocortical L5/6 markers, TOX and NEFH, were strongly expressed in regions other than the arcopallium (Fig. 7), which suggests that the expression pattern of these two genes does not reflect the neural connections in terms of motor output. In addition, CTIP2 was strongly expressed in regions other than the arcopallium and FOXP2 was expressed in the entire pallium (Fig. 7). The expression of chick orthologues of L5/6 genes did not always support the cell-type homology hypothesis, which suggests that the cell types of brainstem projection neurons between the avian arcopallium and the neocortex L5/6 were not conserved. The genes we used are also expressed in other part of pallium in mammals. However, those genes are basically not expressed together outside of neocortical layers. Neocortical deep layers are the only pallial regions in which all six genes we studied are expressed together (Table S1). Therefore, if the cell-type of brainstem projection neurons in arcopallium and those in deep layer L5/6 are homologous, all six chick orthologues for deep layer markers should be expressed in the arcopallium. Contrary to this assumption, our results showed that two of them were majorly expressed in the arcopallium, while the other genes were not. Thus, not all expression patterns of chick orthologues of L5/6 genes support the cell-type homology hypothesis.
Figure 7.
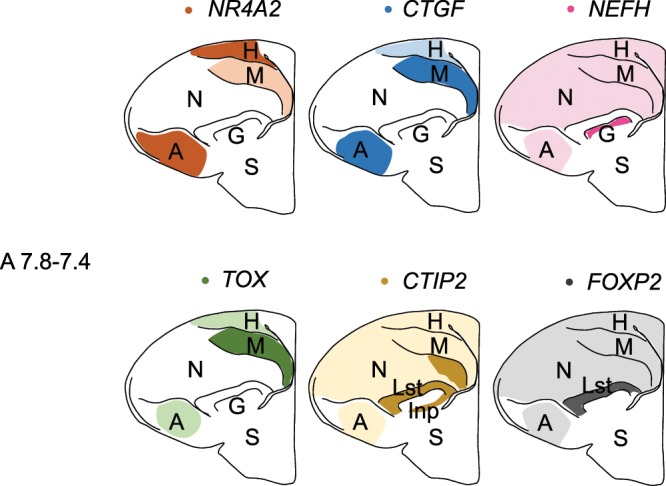
Schematic summary of the expression patterns of the 6 chick orthologues for mammalian L5/6-specific genes (NR4A2, CTGF, NEFH, TOX, CTIP2, FOXP2) in P1 chicks. The representative expression patterns at the levels of sections around A7.8-A7.4 are shown by coloured areas (orange, NR4A2; blue, CTGF; magenta, NEFH; green, TOX; yellow, CTIP2; grey, FOXP2). The darker colours indicate a higher level of gene expression. The levels of the sections correspond to those of the chick atlas by Kuenzel and Masson50. A, arcopallium; B, basorostralis; E, entopallium; G, globus pallidus; H, hyperpallium; Inp, intrapeduncular nucleus; Lst, lateral striatum; M, mesopallium; N, nidopallium; S, striatum.
In birds, the arcopallium has motor output neurons that project to sub-pallial targets and is considered to be involved in motor control27–29. For example, arcopallium-ablated chicks exhibit less approach behaviours to the imprinting object during filial imprinting30, which suggests that the arcopallium is involved in enhancing the subject’s motivation for approach behaviour and/or locomotor activity. We previously found that NR4A2 was upregulated, accompanying filial imprinting, using cDNA microarray and quantitative RT-PCR31. NR4A2 is a transcription factor activated by several signalling cascades32. It is possible that NR4A2 modulates arcopallium activity and increases the subject’s motivation for approach behaviour and/or locomotor activity during filial imprinting.
CTGF is a secreted protein which belongs to the Cyr61/CTGF/NOV (CCN) protein family. It binds to and modulates the activity of other growth factors, including insulin-like growth factor, transforming growth factor ß, and bone morphogenetic proteins33–35. One study found that CCNs in the nervous system are involved in neuroprecursor proliferation, neuronal survival, and differentiation in mice35. Recently, Khodosevich et al. found that changes in CTGF expression levels in the olfactory bulb led to modifications in local neuronal circuitry and olfactory behaviours36. It is possible that CTGF modulates the activity of efferent neurons in the arcopallium and increases the subject’s motivation for approach behaviours or locomotor activity during filial imprinting.
NEFH encodes one of the neurofilament triplet components. The level of neurofilament protein has been associated with the extent of neuronal cell myelination37, whereby NEFH-positive cells are a more heavily myelinated neuronal population. We found that the major expression regions for NEFH were the basorostralis and entopallium in the DVR and globus pallidus. The major expression regions for NEFH appeared to correspond to a part of the intercalated nidopallium, which supports previous work from Jarvis et al.26. Those authors found that the intercalated nidopallium receives sensory projections from the thalamus out of the pallium26, which suggests that NEFH is involved in roles in the cells that have input projection from the thalamus. It is likely that in chicks, NEFH-positive cells are a highly myelinated neuronal population within which projection neurons with long dendrites can be found.
TOX is a multifunctional transcription factor involved in corticogenesis via the promotion of neurite outgrowth and regulating the fate of newborn neurons in mouse embryos20,38. TOX is expressed in the thymus, liver, and brain, and has been studied on the role in lymphocyte development of mice39. We found that TOX was primarily expressed in the mesopallium in chicks (Fig. 7), which is not known to have the characteristic projections to sub-pallial targets40–42. We think that TOX is likely to be involved in roles such as neurite outgrowth in neurons within the telencephalon.
The arcopallium is a heterogeneous organisation that consists of a somato-motor region and a limbic region27–29. We are beginning to understand which subregions of the arcopallium are related to motor or limbic functions. Recently, one study that used an anterograde tracer found that the lateral arcopallium had characteristic projections to the hippocampus and septum, as well as wide areas of limbic nuclei in the hypothalamus and medial areas of the striatum11. These results suggest that the lateral arcopallium is involved in emotion-related behaviours. In addition, diverse projections to midbrain areas were found to derive from the medial arcopallium region40. The NR4A2 and CTGF expression patterns found in the present study indicate that the cell populations in the lateral arcopallium were NR4A2+/CTGF− and that those in the medial arcopallium were NR4A2+/CTGF+ (Fig. 3). Therefore, a combination of NR4A2 and CTGF expression could be used to precisely characterise cell populations in the subregions of the arcopallium in the chick brain.
Much like the mammalian L5/6, the hyperpallium also has motor output neurons projecting to the spinal cord or thalamus5,6,12. All six orthologues for L5/6-specific genes used in this study were expressed in neural populations of the hyperpallium of chicks (Fig. 7), which shows that the expression patterns reflected the neural connections patterns in terms of motor output projections to sub-pallial targets. Given that the avian dorsal pallium derivative region (hyperpallium) is considered to be homologous to the mammalian neocortex43,44, this is reasonable.
The mesopallium is not known to have the characteristic projections to sub- pallial targets. The major projections from the mesopallium are distributed within the telencephalon, and the mesopallium has strong reciprocal fibre connections with regions in the nidopallium40–42. We found that all six orthologous genes used in this study were expressed in the mesopallium (Fig. 7). This suggests that, in the mesopallium, these six genes were expressed in neurons projecting within the telencephalon rather than in neurons projecting to the spinal cord out of the telencephalon. In the case of NR4A2, mouse Nr4a2 has been reported to be expressed not only in the neocortex, but also in other parts of the pallium (the claustrum)25. Furthermore, one study reported that an early NR4A2-positive population within the chick mesopallium represents the lateropallial claustrum homologue in mouse embryos25. It is important to consider the gene expression patterns in other parts of the pallium, such as the claustrum, together with a hodological analysis of connections to compare the gene expression patterns in the mammalian cortex and in birds’ DVR.
Analysing homologies through developmental origins is useful to understand the evolution of brain complexities between mammals and birds. As mammalian neocortex derives from the dorsal pallium, while the DVR of birds derives from the lateroventral pallium43,44, the neocortex and DVR are not homologous in terms of developmental-based homology. In contrast, the mammalian brain regions derived from the lateroventral pallium such as the amygdala, claustrum and dorsal endopiriform nucleus might be homologous to the parts of the birds’ DVR in terms of developmental-based homology. For example, the whereabouts of the avian pallial amygdala remain uncertain, but there are several studies suggesting that a part of the caudal DVR, including at least the caudal nidopallium and the whole arcopallium of birds, may be avian pallial amygdala43–45. As for the claustrum, a recent study suggested that a part of the avian lateral mesopallium (superficial mesopallial cortical structure) is the strict homologous region of the mammalian claustrum25,44,46. A part of lateral nidopallium is the homologous region of mammalian dorsal endopiriform nucleus25,46,47.
We found that chick NR4A2 was majorly expressed in the arcopallium (Fig. 7) which was suggested to be avian pallial amygdala, whereas mouse Nr4a2 was not expressed in the pallial amygdala during embryonic development nor in adults24,25. This evidence showed that chick NR4A2 expression in the arcopallium did not reflect developmental-based homologies. Similarly, we also found that chick CTGF was majorly expressed in the arcopallium (Fig. 7) whereas mouse Ctgf was not expressed in the pallial amygdala (P8 and adult)24. This evidence showed that chick CTGF expression in the arcopallium did not reflect developmental-based homologies. One feature shared by the avian arcopallium and mammalian neocortex L5/6 consists of the neural connections of motor output neurons projecting to sub-pallial targets. We assume that the similarity in the expression pattern might better reflect function than homology and be the result of convergent evolution. Chick TOX were majorly expressed in the medial mesopallium (Fig. 7), which was not the avian homologous region of mouse Tox expressing domains (the hippocampus and claustrum, Table S1). Chick NEFH were expressed in the basorostralis (Fig. 4a,b) and entopallium (Fig. 4c,d). All of these NEFH-expressing domains were not the avian homologous region of mouse Nefh-expressing domains (hippocampus, piriform cortex and olfactory bulb, Table S1).
In this study, we only tested a small number of genes. However, our results suggested that avian hyperpallium, which share developmental-based homology and functional-based analogy between birds and mammals, displayed conserved expression patterns of neocortical deep layer genes (Fig. 7). In contrast, our results suggest that most pallial regions have undergone major reorganisation in terms of gene expression patterns between birds and mammals. This is consistent with the findings of recent comprehensive transcriptome analyses14,15, which have demonstrated that gene expression patterns in the adult mouse cortex are not compatible with those of the adult chick pallium. Over hundreds of millions of years, pallium of birds and mammals has become astonishingly diversified.
Conclusion
The avian arcopallium and mammalian neocortex L5/6 share the feature of neural connections of motor output neurons projecting to sub-pallial targets. We used chick orthologues of mammalian neocortical L5/6 markers and performed in situ hybridisation in the chick telencephalon. We found that NR4A2 and CTGF expression reflected the neural connection patterns between avian DVR and mammalian neocortex, but TOX, NEFH, CTIP2 and FOXP2 expression patterns did not. Thus, not all the expression patterns of chick orthologues of L5/6 genes support the cell-type homology hypothesis. This suggests that the cell types of brainstem projection neurons are not conserved between the avian arcopallium and the mammalian neocortex L5/6.
Methods
Animals and tissues
Fertilized eggs of domestic chicks (Gallus domesticus, the Cobb strain) were purchased from a local dealer (3-M, Aichi, Japan) and incubated at Teikyo University (Kaga, Itabashi-ku, Tokyo). Animal experiments were carried out as described previously48,49. Briefly, newly hatched chicks (P0) were captured and placed in dark plastic enclosures in a breeder at 30 °C for one day (P1). P1 chicks were deeply anesthetized with a 1:1 mixture solution of ketamine (10 mg/ml, ketalar-10, Sankyo Co., Tokyo, Japan) and xylazine (2 mg/ml, Sigma, St. Louis, Missouri, USA) by intraperitoneal injection (0.40 ml/individual) and perfused through the heart with 4% paraformaldehyde in 0.1 M phosphate buffered saline (pH 7.5) (PFA-PBS). In this study, we used 2 chicks for the NR4A2 condition, 3 for the CTGF condition, 4 for the NEFH condition, 3 for the TOX condition, and 4 for the CTIP2 and FOXP2 conditions (a total of 7 chicks). Dissected brains were immersed in PFA-PBS overnight at 4 °C and placed in an 18% sucrose/PFA-PBS solution for cryoprotection for two days at 4 °C. Next, brains were embedded in Tissue-Tek OCT compound (Sakura Finetechnical, Tokyo, Japan), frozen immediately on dry ice, and stored at −80 °C until use. All procedures were reviewed and approved by the committee on animal experiments of Teikyo University and conducted under the guidelines of the national regulations for animal welfare in Japan.
cDNA cloning and RNA probe preparation
For preparation of probes, total RNA was extracted from the chick brain using TRIzol Reagent (Invitrogen, Carlsbad, CA, USA) and reverse-transcribed with Super-Script III (Invitrogen, Carlsbad, CA, USA) using an oligo (dT) primer, according to the manufacture’s protocol. RT-PCR was performed using the following gene specific primer pairs: 5′-atgaggctcccaagaaggat-3′ and 5′-aagcgatcggaacataccac-3′ for NEFH; 5′-tgcctggacccctactattg-3′ and 5′-tggactgaactggatggtga-3′ for TOX; 5′-ttccggttaagcagacgaag-3′ and 5′-ggaatgtggacggtgcttac-3′ for NR4A2; 5′-tttgtctactgaccccaaacagt-3′ and 5′-caaagcattacacataggcacaa-3′ for CTGF; 5′-agaccgtcttctcacgccta-3′ and 5′-gaactgtttcctgccagctc-3′ for CTIP2; 5′-gtctccccagcagctacaag-3′ and 5′-ggtggtgatgctttggaagt-3′ as forward and reverse primers for FOXP2, respectively. PCR products were subcloned into pGEM-T easy vector (Promega, Madison, WI, USA), Sanger sequenced, and confirmed. Plasmids containing the cDNA fragment for NR4A2, CTGF, NEFH, TOX, CTIP2, and FOXP2 were amplified by PCR with an M13 primer pair. The amplicons containing the T7 and SP6 promoter sites were purified using a PCR purification kit (Qiagen, Valencia, CA, USA). The digoxigenin (DIG)-labelled sense and antisense RNA probes were prepared by in vitro transcription using a DIG RNA labelling kit (Roche, NJ).
In situ hybridisation
The frozen brain blocks were cut into 18 µm-thick sections using a cryostat (Leica CM3050S or Leica CM1850, Leica Biosystems, Nußloch, Germany). Serial coronal sections were prepared from a level A 13.8 to A 7.0 of the Kuenzel and Masson’s atlas50. In situ hybridisation was performed as described previously with some modifications51. Briefly, brain sections were fixed in 4% PFA-PBS, pretreated, and hybridised with DIG-labelled riboprobes at 60 °C. After stringent washes, DIG-labelled riboprobes were detected immunocytochemically with alkaline phosphatase-conjugated anti-DIG antibody (1:1,000; Roche, NJ). To visualise the signals, chromogenic reaction with a nitro blue tetrazolium/5-bromo-4-chloro-3-indolyl phosphate were performed at room temperature for following hours: NR4A2, 24 hours; CTGF, 37–39 hours; NEFH, 12.5–19 hours; TOX, 37–39 hours; CTIP2, 36–38 hours and FOXP2, 46–48 hours. In every experiment, sense probes were used as negative controls.
Imaging
Digital images of sections were obtained with NanoZoomer 2.0HT or NanoZoomer XR systems (Hamamatsu Photonics, Shizuoka, Japan) and microscopic fields of interest were cropped using NDP.view2 software (Hamamatsu Photonics, Shizuoka, Japan). The images were then converted to 8-bit and the brightness and contrast of images was adjusted using ImageJ (https://imagej.nih.gov/ij/).
Supplementary information
Acknowledgements
This work was supported by the Grants-in-Aid for Scientific Research from the Japan Society for the Promotion of Science (S.Y., 24590096, 15K07945, 18K06667; N.A., 24790089; T.M., 25291071, 18K07351; and K.J.H., 26440182, 17K07492), the Uehara Memorial Foundation (S.Y.), the Sagawa Foundation for Promotion of Cancer Research (S.Y.), a Grant-in-Aid for Scientific Research on Innovative Areas “Memory dynamism” (26115522) and “Adaptive circuit shift” (15H01449) from the Ministry of Education, Culture, Sports, Science and Technology (K.J.H.), the Naito Foundation (K.J.H.), and the Japan Foundation for Applied Enzymology (K.J.H.). We thank Miss H. Isono (Teikyo University, Faculty of Pharmaceutical Sciences) for technical assistance.
Author contributions
S.Y. designed the study; T.F. and S.Y. performed the research; T.F., N.A., E.F., K.J.H. and S.Y. analysed the data; and T.F., N.A., T.M., K.J.H. and S.Y. wrote the paper. All authors reviewed the manuscript.
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
is available for this paper at 10.1038/s41598-019-56960-4.
References
- 1.Kandel, E., Schwartz, J. & Jessell, T. Principles of neural science (New York: McGraw-hill, 2000).
- 2.Karten Harvey J. Vertebrate brains and evolutionary connectomics: on the origins of the mammalian ‘neocortex’. Philosophical Transactions of the Royal Society B: Biological Sciences. 2015;370(1684):20150060. doi: 10.1098/rstb.2015.0060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Reiner A, et al. Revised nomenclature for avian telencephalon and some related brainstem nuclei. J. Comp. Neurol. 2004;473:377–414. doi: 10.1002/cne.20118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jarvis ED, et al. Avian brains and a new understanding of vertebrate brain evolution. Nat. Rev. Neurosci. 2005;6:151–159. doi: 10.1038/nrn1606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Karten HJ. The organization of the avian telencephalon and some speculations on the phylogeny of the amniote telencephalon. Ann. NY Acad. Sci. 1969;167:164–179. doi: 10.1111/j.1749-6632.1969.tb20442.x. [DOI] [Google Scholar]
- 6.Dugas-Ford J, Ragsdale CW. Levels of homology and the problem of neocortex. Annu. Rev. Neurosci. 2015;38:351–368. doi: 10.1146/annurev-neuro-071714-033911. [DOI] [PubMed] [Google Scholar]
- 7.O’Leary DD, Koester SE. Development of projection neuron types, axon pathways, and patterned connections of the mammalian cortex. Neuron. 1993;10:991–1006. doi: 10.1016/0896-6273(93)90049-W. [DOI] [PubMed] [Google Scholar]
- 8.Zeier H, Karten HJ. The archistriatum of the pigeon: organization of afferent and efferent connections. Brain Res. 1971;31:313–326. doi: 10.1016/0006-8993(71)90185-5. [DOI] [PubMed] [Google Scholar]
- 9.Dubbeldam, J. L., DenBoerVisser, A. M. & Bout, R. G. Organization and efferent connections of the archistriatum of the mallard, Anas platyrhynchos L.: An anterograde and retrograde tracing study. J. Comp. Neurol. 388, 632–657, 10.1002/(Sici)1096-9861(19971201)388:4<632::Aid-Cne10>3.0.Co;2-N (1997). [DOI] [PubMed]
- 10.Sturdy CB, Wild JM, Mooney R. Respiratory and telencephalic modulation of vocal motor neurons in the zebra finch. J. Neurosci. 2003;23:1072–1086. doi: 10.1523/JNEUROSCI.23-03-01072.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xin Q, Ogura Y, Uno L, Matsushima T. Selective contribution of the telencephalic arcopallium to the social facilitation of foraging efforts in the domestic chick. Eur. J. Neurosci. 2017;45:365–380. doi: 10.1111/ejn.13475. [DOI] [PubMed] [Google Scholar]
- 12.Karten HJ. Neocortical Evolution: Neuronal Circuits Arise Independently of Lamination. Curr. Biol. 2013;23:R12–R15. doi: 10.1016/j.cub.2012.11.013. [DOI] [PubMed] [Google Scholar]
- 13.Dugas-Ford J, Rowell JJ, Ragsdale CW. Cell-type homologies and the origins of the neocortex. Proc. Natl Acad. Sci. USA. 2012;109:16974–16979. doi: 10.1073/pnas.1204773109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Belgard TG, et al. Adult pallium transcriptomes surprise in not reflecting predicted homologies across diverse chicken and mouse pallial sectors. Proc. Natl Acad. Sci. USA. 2013;110:13150–13155. doi: 10.1073/pnas.1307444110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Montiel JF, Vasistha NA, Garcia-Moreno F, Molnar Z. From sauropsids to mammals and back: New approaches to comparative cortical development. J. Comp. Neurol. 2016;524:630–645. doi: 10.1002/cne.23871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Molyneaux BJ, Arlotta P, Menezes JRL, Macklis JD. Neuronal subtype specification in the cerebral cortex. Nat. Rev. Neurosci. 2007;8:427–437. doi: 10.1038/nrn2151. [DOI] [PubMed] [Google Scholar]
- 17.Heuer H, et al. Connective tissue growth factor: a novel marker of layer VII neurons in the rat cerebral cortex. Neuroscience. 2003;119:43–52. doi: 10.1016/S0306-4522(03)00100-3. [DOI] [PubMed] [Google Scholar]
- 18.Watakabe A, Ohsawa S, Hashikawa T, Yamamori T. Binding and complementary expression patterns of semaphorin 3E and plexin D1 in the mature neocortices of mice and monkeys. J. Comp. Neurol. 2006;499:258–273. doi: 10.1002/cne.21106. [DOI] [PubMed] [Google Scholar]
- 19.Watakabe A, et al. Comparative analysis of layer-specific genes in Mammalian neocortex. Cereb. Cortex. 2007;17:1918–1933. doi: 10.1093/cercor/bhl102. [DOI] [PubMed] [Google Scholar]
- 20.Artegiani B, et al. Tox: a multifunctional transcription factor and novel regulator of mammalian corticogenesis. EMBO J. 2015;34:896–910. doi: 10.15252/embj.201490061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lein ES, et al. Genome-wide atlas of gene expression in the adult mouse brain. Nature. 2007;445:168–176. doi: 10.1038/nature05453. [DOI] [PubMed] [Google Scholar]
- 22.Ng L, et al. An anatomic gene expression atlas of the adult mouse brain. Nat. Neurosci. 2009;12:356–362. doi: 10.1038/nn.2281. [DOI] [PubMed] [Google Scholar]
- 23.Ng L, et al. Surface-based mapping of gene expression and probabilistic expression maps in the mouse cortex. Methods. 2010;50:55–62. doi: 10.1016/j.ymeth.2009.10.001. [DOI] [PubMed] [Google Scholar]
- 24.Wang WZ, et al. Comparative aspects of subplate zone studied with gene expression in sauropsids and mammals. Cereb. Cortex. 2011;21:2187–2203. doi: 10.1093/cercor/bhq278. [DOI] [PubMed] [Google Scholar]
- 25.Puelles L, et al. Selective early expression of the orphan nuclear receptor Nr4a2 identifies the claustrum homolog in the avian mesopallium: Impact on sauropsidian/mammalian pallium comparisons. J. Comp. Neurol. 2016;524:665–703. doi: 10.1002/cne.23902. [DOI] [PubMed] [Google Scholar]
- 26.Jarvis ED, et al. Global view of the functional molecular organization of the avian cerebrum: mirror images and functional columns. J. Comp. Neurol. 2013;521:3614–3665. doi: 10.1002/cne.23404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wild JM, Arends JJ, Zeigler HP. Telencephalic connections of the trigeminal system in the pigeon (Columba livia): a trigeminal sensorimotor circuit. J. Comp. Neurol. 1985;234:441–464. doi: 10.1002/cne.902340404. [DOI] [PubMed] [Google Scholar]
- 28.Veenman CL, Wild JM, Reiner A. Organization of the avian “corticostriatal” projection system: a retrograde and anterograde pathway tracing study in pigeons. J. Comp. Neurol. 1995;354:87–126. doi: 10.1002/cne.903540108. [DOI] [PubMed] [Google Scholar]
- 29.Shanahan M, Bingman VP, Shimizu T, Wild M, Gunturkun O. Large-scale network organization in the avian forebrain: a connectivity matrix and theoretical analysis. Front. Comput. Neurosci. 2013;7:89. doi: 10.3389/fncom.2013.00089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lowndes M, Davies DC, Johnson MH. Archistriatal lesions impair the acquisition of filial preferences during imprinting in the domestic chick. Eur. J. Neurosci. 1994;6:1143–1148. doi: 10.1111/j.1460-9568.1994.tb00612.x. [DOI] [PubMed] [Google Scholar]
- 31.Yamaguchi S, et al. Thyroid hormone determines the start of the sensitive period of imprinting and primes later learning. Nat. Commun. 2012;3:1081. doi: 10.1038/ncomms2088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hawk JD, Abel T. The role of NR4A transcription factors in memory formation. Brain Res. Bull. 2011;85:21–29. doi: 10.1016/j.brainresbull.2011.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kim HS, et al. Identification of a family of low-affinity insulin-like growth factor binding proteins (IGFBPs): characterization of connective tissue growth factor as a member of the IGFBP superfamily. Proc. Natl Acad. Sci. USA. 1997;94:12981–12986. doi: 10.1073/pnas.94.24.12981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Abreu JG, Ketpura NI, Reversade B, De Robertis EM. Connective-tissue growth factor (CTGF) modulates cell signalling by BMP and TGF-beta. Nat. Cell Biol. 2002;4:599–604. doi: 10.1038/ncb826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Malik AR, Liszewska E, Jaworski J. Matricellular proteins of the Cyr61/CTGF/NOV (CCN) family and the nervous system. Front. Cell. Neurosci. 2015;9:237. doi: 10.3389/fncel.2015.00237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Khodosevich K, et al. Connective tissue growth factor regulates interneuron survival and information processing in the olfactory bulb. Neuron. 2013;79:1136–1151. doi: 10.1016/j.neuron.2013.07.011. [DOI] [PubMed] [Google Scholar]
- 37.Kirkcaldie MT, et al. Neurofilament triplet proteins are restricted to a subset of neurons in the rat neocortex. J. Chem. Neuroanat. 2002;24:163–171. doi: 10.1016/S0891-0618(02)00043-1. [DOI] [PubMed] [Google Scholar]
- 38.Aprea J, et al. Transcriptome sequencing during mouse brain development identifies long non-coding RNAs functionally involved in neurogenic commitment. EMBO J. 2013;32:3145–3160. doi: 10.1038/emboj.2013.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Aliahmad P, Seksenyan A, Kaye J. The many roles of TOX in the immune system. Curr. Opin. Immunol. 2012;24:173–177. doi: 10.1016/j.coi.2011.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wild JM, Karten HJ, Frost BJ. Connections of the Auditory Forebrain in the Pigeon (Columba-Livia) J. Comp. Neurol. 1993;337:32–62. doi: 10.1002/cne.903370103. [DOI] [PubMed] [Google Scholar]
- 41.Krutzfeldt NOE, Wild JM. Definition and novel connections of the entopallium in the pigeon (Columba livia) J. Comp. Neurol. 2005;490:40–56. doi: 10.1002/cne.20627. [DOI] [PubMed] [Google Scholar]
- 42.Atoji Y, Wild JM. Afferent and efferent projections of the mesopallium in the pigeon (Columba livia) J. Comp. Neurol. 2012;520:717–741. doi: 10.1002/cne.22763. [DOI] [PubMed] [Google Scholar]
- 43.Fernandez AS, Pieau C, Reperant J, Boncinelli E, Wassef M. Expression of the Emx-1 and Dlx-1 homeobox genes define three molecularly distinct domains in the telencephalon of mouse, chick, turtle and frog embryos: implications for the evolution of telencephalic subdivisions in amniotes. Development. 1998;125:2099–2111. doi: 10.1242/dev.125.11.2099. [DOI] [PubMed] [Google Scholar]
- 44.Puelles L, et al. Pallial and subpallial derivatives in the embryonic chick and mouse telencephalon, traced by the expression of the genes Dlx-2, Emx-1, Nkx-2.1, Pax-6, and Tbr-1. J. Comp. Neurol. 2000;424:409–438. doi: 10.1002/1096-9861(20000828)424:3<409::AID-CNE3>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 45.Martinez-Garcia F, Martinez-Marcos A, Lanuza E. The pallial amygdala of amniote vertebrates: evolution of the concept, evolution of the structure. Brain Res. Bull. 2002;57:463–469. doi: 10.1016/s0361-9230(01)00665-7. [DOI] [PubMed] [Google Scholar]
- 46.Puelles L. Comments on the Updated Tetrapartite Pallium Model in the Mouse and Chick, Featuring a Homologous Claustro-Insular Complex. Brain Behav. Evol. 2017;90:171–189. doi: 10.1159/000479782. [DOI] [PubMed] [Google Scholar]
- 47.Puelles, L. et al. The pallium in reptiles and birds in the light of the updated tetrapartite pallium model in Evolution of nervous systems. 2nd Edn Vol. 1, (ed. Kaas, J.) 519–555 (Oxford: Elsevier, 2017).
- 48.Yamaguchi S, et al. Gene expression profile in cerebrum in the filial imprinting of domestic chicks (Gallus gallus domesticus) Brain Res. Bull. 2008;76:275–281. doi: 10.1016/j.brainresbull.2008.02.002. [DOI] [PubMed] [Google Scholar]
- 49.Yamaguchi S, et al. Up-regulation of microtubule-associated protein 2 accompanying the filial imprinting of domestic chicks (Gallus gallus domesticus) Brain Res. Bull. 2008;76:282–288. doi: 10.1016/j.brainresbull.2008.02.010. [DOI] [PubMed] [Google Scholar]
- 50.Kuenzel, W. J., & Masson, M. A stereotaxic atlas of the brain of the chick (Gallus domesticus) (Baltimore: Johns Hopkins University Press, 1988).
- 51.Yamaguchi S, Aoki N, Matsushima T, Homma KJ. Wnt-2b in the intermediate hyperpallium apicale of the telencephalon is critical for the thyroid hormone-mediated opening of the sensitive period for filial imprinting in domestic chicks (Gallus gallus domesticus) Horm. Behav. 2018;102:120–128. doi: 10.1016/j.yhbeh.2018.05.011. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.