Abstract
Early-onset hypertension confers increased risk for cardiovascular mortality in the community. Whether early-onset hypertension also promotes the development of target end-organ damage (TOD), even by mid-life, has remained unknown. We studied 2680 middle-aged CARDIA Study participants (mean age 50±4 years, 57% women) who underwent up to eight serial blood pressure (BP) measurements between 1985–2011 (age range at baseline 18–30 years) in addition to assessments of echocardiographic left ventricular hypertrophy, coronary calcification, albuminuria, and diastolic dysfunction in 2010–2011. Age of hypertension onset was defined as the age at first of two consecutively attended examinations with BP ≥140/90 mmHg or use of antihypertensive medication. Participants were divided in groups by hypertension onset age (<35 years, 35–44 years, ≥45 years, or no hypertension). While adjusting for TOD risk factors, including systolic BP, we used logistic regression to calculate odds ratios for cases (participants with TOD) versus controls (participants without TOD) to examine the relation of hypertension onset age and hypertensive TOD. Compared with normotensive individuals, hypertension onset at age <35 years was related to odds ratios of 2.29 (95% confidence interval [CI], 1.36-3.86), 2.94 (95% CI, 1.57-5.49), 1.12 (95% CI, 0.55-2.29), and 2.06 (95% CI, 1.04-4.05) for left ventricular hypertrophy, coronary calcification, albuminuria, and diastolic dysfunction, respectively. In contrast, hypertension onset at age ≥45 years was not related to increased odds of TOD. Our findings emphasize the importance of assessing age of hypertension onset in hypertensive patients in order to identify high-risk individuals for preventing hypertensive complications.
Keywords: Blood pressure, Follow-up studies, Early onset hypertension, Organ damage, Risk factors, Cardiovascular disease, Hypertension
Graphical Abstract
Introduction
Although hypertension is a well-known risk factor for cardiovascular disease (CVD), the impact of age of hypertension onset on CVD risk has not been widely studied. Recent data from the Framingham Heart Study suggest that early onset hypertension is associated with a considerably greater risk for CVD mortality compared to hypertension that begins later in life.1,2,3 In addition, early onset, and not late onset hypertension, in parents is also strongly associated with hypertension in offspring.1
Target end-organ damage (TOD) is a common complication of hypertension which considerably increases the risk of incident overt CVD, even in well-controlled hypertension.4-10 Furthermore, regression of TOD has been associated with lower likelihoods of CVD morbidity and mortality, independent of blood pressure (BP) lowering in hypertensive persons.11 Although early onset hypertension has been shown to increase the risk of CVD death,1 it remains unclear if early onset hypertension also predisposes to TOD already by mid-life. This information could be useful for understanding the disease mechanisms through which early onset hypertension leads to manifest CVD. In addition, these data could be used to improve CVD risk prediction and guidance on antihypertensive therapy in patients with early onset hypertension.
Our objective was to determine the relation of hypertension onset age with hypertensive TOD in middle-aged individuals. We assessed the association of hypertension onset age with presence of left ventricular hypertrophy (LVH), coronary calcification, albuminuria, and left ventricle diastolic dysfunction in 2680 CARDIA study participants aged 43–55 years. CARDIA is a large cohort study drawn from the general population that includes individuals with varying ethnic backgrounds. The participants underwent eight follow-up examinations over a timespan of up to 31 years. A particular strength of this cohort for exploring the association between age of hypertension onset and TOD is that nearly all participants were free from hypertension at baseline which enabled accurate determination of hypertension onset age. We hypothesized that early onset hypertension associates more strongly than late onset hypertension with hypertensive TOD.
Methods
Study sample
Our study sample included participants of the prospective Coronary Artery Risk Development in Young Adults (CARDIA) cohort study. The CARDIA study was designed to examine the risk factors and development of CVD in young adults who were aged 18–30 years (mean age 25±4 years) at baseline.12 All data and materials of the CARDIA study have been made publicly available at the National Institutes of Health (NIH)’s Biologic Specimen and Data Repository Information Coordinating Center (BioLINCC) and can be accessed at https://biolincc.nhlbi.nih.gov/studies/cardia/.
The CARDIA cohort consists of 5115 participants who were recruited between 1985 and 1986 in four centers in Birmingham, AL; Chicago, IL; Minneapolis, MN; and Oakland, CA. The participants were chosen as evenly as possible regarding to age, sex, race, and education. Further details of the CARDIA study and its sampling have been published previously.12 We considered the 3499 CARDIA participants who attended examination cycle 8 in 2010–2011. Participants who had missing covariates or TOD measurements at cycle 8 (n=819) were excluded, resulting in a final sample of 2680. Informed consent was obtained from all participants, and the study was approved by all institutional committees of each participating center.
Clinical evaluations and measurements
Follow-up examinations were carried out eight times in 1985–86, 1987–1988, 1990–91, 1992–93,1995–96, 2000–01, 2005–06 and 2010–2011. At examinations one through six, BP was measured at each visit three times using a random zero mercury sphygmomanometer between the first and sixth examinations. All personnel collecting BP data were trained and certified centrally at each examination cycle, including pretraining and post-training tests. For quality control, BP measurements were carried out in duplicates. No distinct differences were observed between centers when comparing technician-specific single-point histograms13 A validated Omron model HEM907XL oscillometric monitor14 was used at the seventh and eighth examinations for BP measurements. BP was three times measured from the right arm of sitting participants after five minutes of quiet rest by a trained technician at all examinations. Differences between auscultatory and oscillometric measurements were corrected by calibrating the oscillometric values to sphygmomanometer measures as previously described.15 We used the average of the second and third measurements as the BP at each cycle.
In addition to echocardiography, all participants underwent measurements for coronary artery calcification (CAC) and urine albumin-creatinine ratio (UACR) at examination cycle eight. Echocardiographic measurements were performed according to a standardized protocol across all study centers using two-dimensionally guided M-mode and Doppler echocardiography. All echocardiograms were read centrally by experienced echocardiographic sonographers. Left ventricular mass (LVM) and peak velocity flow in early and late diastole were calculated from the echocardiograms as previously described.16 LVM index was calculated by dividing LVM by body surface area (0.007184 × Weight (kg)0.425 × Height (cm)0.725). CAC levels were measured with a cardiac multi-detector computed tomography.17 All images were evaluated centrally and the Agatston score was calculated for each participant.18 Urinary creatinine and albumin levels were measured using a single, untimed spot urine sample and the samples were analyzed centrally, as described previously.19
Current smoking was obtained with self-administrated questionnaires. Height and weight were measured from participants with light clothing. Total cholesterol, high-density lipoprotein cholesterol (HDL-cholesterol) and serum glucose were measured from fasting samples by standard enzymatic methods.12,20 Diabetes was defined by serum fasting glucose ≥7 mmol/l or use of antihyperglycemic medication. Information on use of antihypertensive medication was collected by self-report and from medications brought on-site at each examination.
Exposure and outcome variables
We used age of hypertension onset as the exposure variable. Assessment of hypertension onset was based on all available BP measurements performed at eight serial examinations from 1985 through 2011. We defined hypertension onset as BP ≥140/90 mmHg or use of antihypertensive medication on two consecutively attended examinations. We used this definition to reduce variation between single observed high BP measurements to demonstrate a lasting change in BP. In accordance with previous studies, we defined the age of hypertension onset as the age at the first examination on which the criteria for hypertension was met.21,1 We divided the participants into 4 groups according to their age of hypertension onset: <35 years, 35–44 years, ≥45 years, or no hypertension.
We used LVH, left ventricle diastolic dysfunction, coronary calcification and albuminuria at examination eight as the outcome variables. We defined LVH as increased LVM index >115 g/m2 in men and >95 g/m2 in women.22 Left ventricle diastolic dysfunction was defined as a ratio between peak velocity flow in early and late diastole of >2.0 or <0.8.22 We defined CAC as an Agatston score ≥10023 and albuminuria as UACR >30 mg/g.24
Statistical analyses
We assessed the sample characteristics at examination eight in the whole sample and in subgroups by hypertension onset age. We compared the participants’ characteristics and measured TOD using a chi-squared test for categorical variables and analysis of variance for continuous variables. Due to a skewed distribution, we log-transformed UACR before analyses. We also compared the study sample baseline characteristics with CARDIA study participants who were excluded from the analyses. Using univariable and multivariable logistic regression models, we examined the relation of hypertension onset age groups and presence of TOD using a case (presence of TOD) versus control (no TOD) design, with those who did not develop hypertension as the referent group. We used case-control study design, instead of a prospective approach, which allowed for the exposure variable to consistently precede the outcome variable in timing. We performed a sensitivity analysis without individuals who were hypertensive at exam 8. In the main analysis, these individuals were classified non-hypertensive as a result of not having hypertension on two consecutive examinations. We also examined the association between age of hypertension onset and number of TODs (0, 1 or ≥ 2) using multinomial logistic regression model. We adjusted the multivariable analyses for age, sex, body mass index (BMI), total serum cholesterol, HDL-cholesterol, smoking status, use of antihypertensive medication, diabetes, race, and systolic BP. The covariates were drawn from examination eight. We also performed a supplementary analysis by adjusting for covariates drawn from the baseline examination instead of examination eight. Diabetes was not included as a covariate in this analysis due to the low number of diabetic individuals at baseline. We tested for a linear trend in odds ratios across categories of age at hypertension onset. We carried out all analyses using SAS software version 9.4 (SAS Institute, Cary, NC). A two-tailed p<0.05 was considered statistically significant.
Results
The characteristics of the whole study sample (mean age 50 years; 57% women) and in groups according to hypertension onset age are presented in Table 1. In general, the participants’ CVD risk factors, such as diabetes and smoking prevalence, BMI, and HDL-cholesterol, improved with increasing age of hypertension onset. Individuals with early onset hypertension were more likely to be men. Although some differences were statistically significant, we observed no major differences in the study sample baseline characteristics compared with CARDIA study participants who were excluded from the analyses (Table S1). A total of 2, 3, 4, 5, 6, 7 and 8 BP measurements were available for 14, 22, 59, 96, 182, 373, and 1934 participants, respectively.
Table 1.
Sample characteristics.
| Age of hypertension onset |
||||||
|---|---|---|---|---|---|---|
| Characteristic | All | < 35 y | 35-44 y | ≥45 y | No hypertension | P value |
| N | 2680 | 94 | 251 | 136 | 2199 | |
| Age, years (SD) | 50.1 (3.6) | 49.8 (3.4) | 49.9 (3.5) | 53.3 (1.8) | 49.9 (3.6) | <0.001 |
| No women (%) | 1525 (56.9) | 51 (54.3) | 150 (59.8) | 83 (61.0) | 1241 (56.4) | 0.52 |
| BMI, kg/m2 (SD) | 25.2 (5.5) | 28.1 (6.2) | 28.5 (6.2) | 27.4 (5.7) | 24.6 (5.2) | <0.001 |
| Current smoker (%) | 447 (16.7) | 18 (19.2) | 46 (18.3) | 24 (17.7) | 359 (16.3) | 0.75 |
| Cholesterol, mmol/l (SD) | 5.0 (0.9) | 4.8 (1.0) | 4.8 (1.0) | 4.8 (1.0) | 5.0 (0.9) | 0.0002 |
| HDL-cholesterol, mmol/l (SD) | 1.5 (0.5) | 1.4 (0.6) | 1.38 (0.4) | 1.43 (0.5) | 1.5 (0.5) | <0.001 |
| Systolic blood pressure, mmHg (SD) | 118 (15.3) | 128 (15.6) | 127 (18.7) | 126 (19.0) | 117 (13.8) | <0.001 |
| Diastolic blood pressure, mmHg (SD) | 73.8 (10.8) | 80.7 (9.9) | 79.7 (12.4) | 78.4 (11.8) | 72.5 (10.2) | <0.001 |
| Use of antihypertensive medication (%) | 686 (25.6) | 83 (88.3) | 226 (90.0) | 124 (91.2) | 253 (11.5) | <0.001 |
| Diabetes (%) | 240 (9.0) | 27 (28.7) | 51 (20.3) | 23 (16.9) | 139 (6.3) | <0.001 |
| Non-Hispanic black (%) | 1280 (47.8) | 71 (75.5) | 189 (75.3) | 74 (54.4) | 946 (43.0) | <0.001 |
Sample characteristics were drawn from examination eight. BMI, body mass index; HDL, high density lipoprotein.
The mean level and prevalence of hypertensive TOD according to hypertension onset age are shown in Table 2. In general, the level and prevalence of LVH, coronary calcification, albuminuria and left ventricle diastolic dysfunction increased with decreasing age of hypertension onset. Odds of TOD increased linearly in unadjusted models (P<0.005 for all), but not in the multivariable adjusted models (P>0.11 for all). Table 3 shows the odds of TOD by age group of hypertension onset. In unadjusted models, individuals with hypertension onset at age < 35 years had odds ratios of 3.35 (95% confidence interval [95% CI], 2.16–5.20), 4.33 (95% CI 2.61–7.18), 3.49 (95% CI 1.91–6.36), and 2.17 (95% CI 1.22–3.85) for LVH, coronary calcification, albuminuria and left ventricle diastolic dysfunction compared to individuals without hypertension, respectively. In models adjusted for common TOD risk factors determined at examination eight, including systolic BP, the respective odds ratios were 2.29 (95% CI 1.36–3.86), 2.94 (95% CI 1.57–5.49), 1.12 (95% CI 0.55–2.29), and 2.06 (95% CI 1.04–4.05); Table 3. The results were similar when covariates were drawn from the baseline examination (Table S2). In fact, our findings on the adverse nature of early-onset hypertension were even more evident when participants with new-onset hypertension at the last examination, who were classified non-hypertensive, were excluded (Table S3).
Table 2.
Prevalence of hypertensive organ damage according to hypertension onset age.
| Hypertension onset age |
||||||
|---|---|---|---|---|---|---|
| All | <35 years | 35-44 years | ≥45 years | No hypertension | P value | |
| N | 2680 | 94 | 251 | 136 | 2199 | |
| LVMI, g/m2 (SD) | 85.2 (21.3) | 97 (27.2) | 94 (27.1) | 92 (22.3) | 83 (19.7) | <0.001 |
| LVH, n (%) | 444 (16.6) | 33 (35.1) | 73 (29.1) | 32 (23.5) | 306 (13.9) | <0.001 |
| CAC-score, AU (SD) | 40.2 (205) | 215 (743) | 62 (177) | 80 (210) | 28 (142) | <0.001 |
| Increased CAC-score, n (%) | 235 (8.8) | 22 (23.4) | 40 (15.9) | 28 (20.6) | 145 (6.6) | <0.001 |
| UACR, median (IQR) | 4.79 (3.31-8.38) | 6.67 (4.03-15.57) | 7.27 (4.51-16.95) | 5.09 (3.56-8.38) | 4.55 (3.19-7.68) | <0.001 |
| Increased UACR, n (%) | 165 (6.2) | 14 (14.9) | 36 (14.3) | 10 (7.4) | 105 (4.8) | <0.001 |
| E/A ratio (SD) | 1.3 (0.4) | 1.14 (0.4) | 1.18 (0.3) | 1.20 (0.4) | 1.33 (0.4) | <0.001 |
| Abnormal E/A ratio, n (%) | 239 (8.9) | 15 (16.0) | 31 (12.4) | 16 (11.8) | 177 (8.1) | 0.005 |
LVMI, Left ventricular mass index; LVH, Left ventricular hypertrophy; CAC, Coronary artery calcification; AU, Agatston units, UACR, urine albumin/creatinine ratio; E/A ratio, ratio between E wave peak velocity flow in early diastole and A wave peak velocity flow in late diastole, IQR, interquartile range.
Table 3.
Odds of hypertensive organ damage according to hypertension onset age.
| LVH | Coronary calcification | Albuminuria | Diastolic dysfunction | |||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Hypertension onset age |
n/N | OR (95% CI) | n/N | OR (95% CI) | n/N | OR (95% CI) | n/N | OR (95% CI) |
| Unadjusted model | ||||||||
| <35 | 33/94 | 3.35 (2.16-5.20)* | 22/94 | 4.33 (2.61-7.18)* | 14/94 | 3.49 (1.91-6.36)* | 15/94 | 2.17 (1.22-3.85)† |
| 35-44 | 73/251 | 2.54 (1.88-3.42)* | 40/251 | 2.69 (1.84 −3.92)* | 36/251 | 3.34 (2.23-5.00)* | 31/251 | 1.61 (1.07-2.42)‡ |
| ≥45 | 32/136 | 1.90 (1.26-2.88)† | 28/136 | 3.67 (2.35-5.75)* | 10/136 | 1.58 (0.81-3.10) | 16/136 | 1.52 (0.88-2.62) |
| No hypertension | 306/2199 | 1.00 | 145/2199 | 1.00 | 105/2199 | 1.00 | 177/2199 | 1.00 |
| Multivariable-adjusted model | ||||||||
| <35 | 33/94 | 2.82 (1.67-4.74)* | 22/94 | 3.15 (1.69-5.86)* | 14/94 | 1.49 (0.73-3.02) | 15/94 | 2.04 (1.04-3.99)‡ |
| 35-44 | 73/251 | 2.07 (1.40-3.08)* | 40/251 | 1.94 (1.17-3.23)‡ | 36/251 | 1.63 (0.96-2.76) | 31/251 | 1.58 (0.93-2.69) |
| ≥45 | 32/136 | 1.52 (0.92-2.51) | 28/136 | 1.49 (0.83-2.65) | 10/136 | 0.84 (0.39-1.81) | 16/136 | 1.43 (0.74-2.76) |
| No hypertension | 306/2199 | 1.00 | 145/2199 | 1.00 | 105/2199 | 1.00 | 177/2199 | 1.00 |
| Multivariable+SBP-adjusted model | ||||||||
| <35 | 33/94 | 2.29 (1.36-3.86)† | 22/94 | 2.94 (1.57-5.49)* | 14/94 | 1.12 (0.55-2.29) | 15/94 | 2.06 (1.04-4.05)‡ |
| 35-44 | 73/251 | 1.67 (1.12-2.48)‡ | 40/251 | 1.83 (1.10-3.05)‡ | 36/251 | 1.25 (0.74-2.09) | 31/251 | 1.59 (0.93-2.73) |
| ≥45 | 32/136 | 1.23 (0.74-2.03) | 28/136 | 1.41 (0.79-2.52) | 10/136 | 0.62 (0.29-1.34) | 16/136 | 1.44 (0.75-2.79) |
| No hypertension | 306/2199 | 1.00 | 145/2199 | 1.00 | 105/2199 | 1.00 | 177/2199 | 1.00 |
n/N indicates number of individuals with organ damage/number of individuals in category. LVH, Left ventricular hypertrophy; OR, odds ratio; CI, confidence interval; SBP, systolic blood pressure.
P<0.001;
P<0.01;
P<0.05. Multivariable-adjusted model is adjusted for age, sex, body mass index, total serum cholesterol, HDL-cholesterol, smoking status, use of antihypertensive medication, race and diabetes. Trend test p-values for odds ratios across age groups for LVH, coronary calcification, albuminuria, and diastolic dysfunction were <0.0001, <0.0001, <0.0001, and 0.005, respectively. The corresponding p-values were 0.009, 0.068, 0.56, and 0.16 for the multivariable-adjusted models and 0.12, 0.11, 0.68, and 0.16 for the multivariable+SBP-adjusted models, respectively.
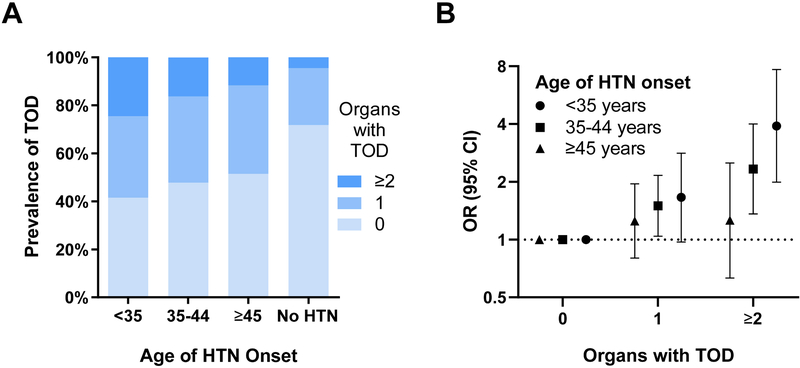
Prevalence of TOD (0,1 or ≥2 damaged organs) in subgroups by hypertension onset age is shown in Figure 1. Non-hypertensive participants had the lowest prevalence of TOD (23.7%; 4.6% with TOD in ≥2 organs). 59.5% of individuals with hypertension onset at age <35 years had hypertensive TOD and 24.5% had damage in multiple organs. The odds of having TOD in 1 or ≥2 organs by hypertension onset age is shown in Table 4. Early onset hypertension was particularly strongly related to having TOD in ≥2 organs (P<0.001, Table 4). Figure 1 also illustrates the adjusted odds of having TOD in 1 or ≥2 organs by hypertension onset age subgroups.
Figure 1.
Proportion of individuals with 0, 1 or ≥2 types of target organ damage by hypertension onset age (Panel A). Odds of having 0, 1 or ≥2 organs with damage by hypertension onset age (Panel B). TOD, target end-organ damage; HTN, hypertension; OR, odds ratio; CI, confidence interval.
Table 4.
Odds of having 0, 1 or ≥2 organs with organ damage by hypertension onset age.
| TOD in 0 organs | TOD in 1 organ | TOD in ≥2 organs | ||||
|---|---|---|---|---|---|---|
|
|
||||||
| Hypertension onset age | n | OR (95% CI) | n | OR (95% CI) | n | OR (95% CI) |
| <35 years (n=94) | 39 | 1.00 | 32 | 1.66 (0.97-2.82) | 23 | 3.91 (1.99-7.69)* |
| 35–44 years (n=251) | 120 | 1.00 | 90 | 1.50 (1.04-2.16)‡ | 41 | 2.33 (1.36-4.00)† |
| ≥45 years (n=136) | 70 | 1.00 | 50 | 1.25 (0.80-1.95) | 16 | 1.26 (0.63-2.51) |
TOD, target organ damage; OR, odds ratio; CI, confidence interval. Models are adjusted for age, sex, body mass index, total serum cholesterol, HDL-cholesterol, smoking status, systolic blood pressure, use of antihypertensive medication, race and diabetes. Organ damage sum (0, 1 or ≥2) was defined as the total number of organs with damage.
P<0.001;
P<0.01;
P<0.05.
Discussion
Hypertension onset at age <35 years of age was associated with significantly increased odds of LVH, coronary calcification, and left ventricular diastolic dysfunction in middle-aged individuals whereas onset at ≥45 years of age was not. The odds of TOD for all aforementioned organs were always greatest in the group with hypertension onset at under 35 years of age. 24.5% of the individuals in this group had concurrent TOD in two or more organs.
To our knowledge, no previous studies have examined the relation of hypertension onset age and conventional hypertensive TOD. Limited data also exist on the impact of hypertension onset age on risk of overt CVD. The first results on this domain are from a 1987 study which examined the risk of CVD events in patients drawn from 34 general practices.3 In this study, hypertension onset at age 40–49 years was related to a notable 5.2-fold odds of CVD events compared to normotensives of similar age. In contrast, the corresponding odds ratio was only 1.2 for individuals with hypertension onset at age 60–65 years. However, this study had some limitations, such as lack of adjustment for many conventional CVD risk factors, including cholesterol and diabetes. In addition, age at hypertension diagnosis, instead of objectively defined age of onset based on serial measurements, was used as the exposure variable. These limitations were addressed in a recent publication based on Framingham Heart Study Original cohort data.1,2 In this study, we observed that objectively diagnosed early onset hypertension (onset at age <55 years) was related to 2.2-fold odds of CVD death compared with individuals who never developed hypertension, as compared with only 1.5-fold odds in individuals who developed hypertension at age ≥65 years. Overall, these prior results demonstrate that early-onset hypertension is a strong predictor of CVD outcomes in later life. The results of the current study expand these previous findings by demonstrating that early onset hypertension is also related to a high risk of TOD already by mid-life. Given that TOD increases the risk of overt CVD several-fold in hypertensive patients,4-10 our findings could have considerable clinical importance as they highlight the need of adequate BP control particularly in young hypertensive patients.
No prior studies have directly compared the differences of age of hypertension onset and age at hypertension diagnosis. For several other diseases, such as ankylosing spondylitis and dementia, a considerable delay exists between the time of first symptoms and time of diagnosis.25,26 However, these results may not be generalizable to hypertension which is usually a symptomless condition. In this study, we used objective BP measurements conducted at regular follow-ups to define age of hypertension, in contrast to self- or clinician-reported age of hypertension diagnosis. We believe this to be a more accurate method for determining the actual age of hypertension onset. However, additional studies are needed to assess the differences between self-reported and objectively assessed age of hypertension onset.
In previous studies, Franks et al. have shown that childhood hypertension increases the risk of premature death from endogenous causes.27 However, in this study, BP in childhood on a continuous scale did not significantly predict premature death. Several other studies have also observed an association for systolic BP, diastolic BP, and BP trajectories with left ventricular mass and LVH in children and young adults.28-31 Similar results have also been reported concerning carotid artery intima-media thickness. 30,32,33,34 However, these studies used repeatedly measured BP as the exposure variable instead of hypertension onset age.
Apart from classical markers of hypertensive TOD, such as LVH, the relation of hypertension onset age and cognitive decline has been assessed in the oldest-old. A single recent study by Corrada et al. suggested that hypertension onset at age >80 years could paradoxically be protective of incident dementia in a cohort of 559 individuals aged >90 years.35 This association remained even after evaluating the potential for survival bias. Although the main finding of this study was only borderline significant (p=0.04), it remains plausible that hypertension onset at a very high age could be protective against some adverse outcomes, in contrast to hypertension that begins in mid- or late life. However, this finding needs replication in an external population before any definitive conclusions can be made.
Age of hypertension onset has also been studied in the context of hypertension risk in the offspring. Family studies have previously shown that BP is a highly heritable trait.36 Furthermore, early onset hypertension in particular seems to have a strong genetic component.37-42 Apart from heritability estimates, recent epidemiological studies have also provided point estimates for the risk of hypertension in individuals categorized by age of parental hypertension onset. In the Johns Hopkins Precursor Study with a sample of 1160 male physicians, self-reported early-onset (at age ≤55 years) hypertension in both parents imparted a 6.2-fold higher adjusted risk for the development of hypertension throughout adult life, compared to individuals whose parents never developed hypertension.21 In the Framingham Heart Study Offspring cohort, objectively determined early-onset hypertension (at age ≤55 years) in both parents was related to 3.5-fold odds of hypertension.1 Interestingly, early-onset hypertension in grandparents also appears to raise the risk for hypertension in grandchildren, even after adjusting for early-onset hypertension in parents and lifestyle factors.1,43 These findings, together with the observed increased CVD risk related to early-onset, highlight the importance of assessing both parental and grandparental age of hypertension onset in hypertensive patients.
Our study has several strengths. Our large study sample was drawn from the general population. In addition, the participants were followed up for up to 31 years over eight follow-up examinations conducted at regular intervals. The participants were relatively young at baseline and therefore only a small number of participants had developed hypertension at the start of the study. Therefore, the study sample was optimal for assessing the relation of hypertension onset age with hypertensive TOD. In addition to strengths, we also recognize some limitations of this study. 68,4% of individuals who participated in the baseline examination took part in examination 8. However, there were no clinically significant differences between individuals who were included and excluded in this analysis (Table S1). We defined hypertension onset age by objectively measured elevated BP or use of antihypertensive medication on two consecutively attended examinations to reduce variation based on only one measurement and to represent a durable change in BP. However, we were not able to include the intensity of the antihypertensive therapy in the analyses despite recognizing that this could also affect the risk of hypertensive TOD. Lastly, the overall exposure time to hypertension is most likely a key contributing factor in the relation of hypertension onset age and hypertensive TOD. Notwithstanding potentially similar effects, we recognize that duration of exposure to hypertension is a distinct entity from age of hypertension onset, given that exposure from age 20 to 40 years is not the same as exposure from age 70 to 90 years. Accordingly, prior reports have shown that the effect of exposure time on CVD risk can vary depending on the patient’s age and that developing hypertension at older ages may even protect against dementia.35,44 In addition, hypertension onset age, but not hypertension duration, has a strong impact on the heritability of hypertension.1 Unfortunately, the differential effects of hypertension age of onset and hypertension exposure time on CVD risk cannot be assessed in this study sample as the CARDIA participants had a very narrow age range at baseline. As a result, exposure time is nearly equivalent to age of onset in CARDIA due to the narrow age range of the participants, precluding a meaningful analysis for comparing the effects of these two entities on TOD.
Perspectives
Our findings suggest that hypertensive TOD is robustly associated with early onset hypertension already by mid-life. Hypertensive TOD is a well-known and robust risk factor for CVD events such as myocardial infarction and stroke, independent of BP.7-10 Our findings further emphasize the importance of assessing age of hypertension onset in hypertensive patients in order to identify individuals at high risk of developing TOD and, as a result, to prevent TOD and overt CVD. Previous studies have shown that long-term cumulative BP levels are also associated with increased risk of CVD, independent of single occasion BP measurements.45,46 These results are in line with previous data on the impact of repeated BP measurements in childhood, adolescence and young adulthood, to some conventional TOD.28-34 However, in this study, we focus on the differences in odds of TOD and sum of TODs by subgroups according to age at hypertension onset. Additionally, determining age of hypertension onset could offer a simple method for clinicians to assess a patient’s overall risks associated with exposure to hypertension. Our results, in conjunction with previous research,1,2,3 suggest that physicians should be aggressive when diagnosing and treating young hypertensive patients who often remain undertreated.47,48 Physicians should consider initiating antihypertensive treatment in younger hypertensive patients when they do not respond to lifestyle changes, even if their estimated short-term CVD risk remains moderate. Further studies are still needed to elucidate the potential disease mechanisms of early onset hypertension.
Supplementary Material
Novelty and Significance.
What Is New?
Prior studies have demonstrated that hypertension onset at an early age is strongly related to cardiovascular mortality and risk of hypertension in offspring.
Whether early-onset hypertension also promotes the development of target end-organ damage, even by mid-life, has remained unknown.
What Is Relevant?
Early hypertension onset age is associated with increased risk of left ventricular hypertrophy, coronary calcification, and left ventricle diastolic dysfunction, compared to late onset hypertension.
Summary
Our findings emphasize the importance of assessing age of hypertension onset in hypertensive patients in order to identify high-risk individuals and to prevent hypertensive complications such as hypertensive target organ damage and overt cardiovascular disease.
Acknowledgements
We acknowledge the important contributions of the CARDIA participants and investigators. This study was conducted using CARDIA Research Materials obtained from the NHLBI Biologic Specimen and Data Repository Information Coordinating Center and does not necessarily reflect the opinions or views of the CARDIA study or the NHLBI.
Sources of funding
T.N. was funded by the Urmas Pekkala Foundation, the Paavo Nurmi Foundation, the Finnish Medical Foundation, and the Emil Aaltonen Foundation. K.S. was funded by the Aarne Koskelo Foundation.
Footnotes
Conflicts of Interest(s)/ Disclosure(s)
None.
References
- 1.Niiranen TJ, McCabe EL, Larson MG, Henglin M, Lakdawala NK, Vasan RS, Cheng S. Heritability and risks associated with early onset hypertension: multigenerational, prospective analysis in the Framingham Heart Study. BMJ. 2017; 357:j1949. doi: 10.1136/bmj.j1949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Niiranen TJ, Larson MG, McCabe EL, Xanthakis V, Vasan RS, Cheng S. Prognosis of prehypertension without progression to hypertension. Circulation. 2017; 136:1262–1264. doi: 10.1161/CIRCULATIONAHA.117.029317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Buck C, Baker P, Bass M, Donner A. The prognosis of hypertension according to age at onset. Hypertension. 1987; 9:204–208. doi: 10.1161/01.HYP.9.2.204 [DOI] [PubMed] [Google Scholar]
- 4.Bidani AK, Griffin KA. Basic science: Hypertensive target organ damage. J Am Soc Hypertens. 2015; 9:235–237. doi: 10.1016/j.jash.2015.01.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vernooij JWP, van der Graaf Y, Nathoe HM, Bemelmans RHH, Visseren FLJ, Spiering W. Hypertensive target organ damage and the risk for vascular events and all-cause mortality in patients with vascular disease. J Hypertens. 2013; 31:492–500. doi: 10.1097/HJH.0b013e32835cd3cd [DOI] [PubMed] [Google Scholar]
- 6.Tocci G, Figliuzzi I, Presta V, Attalla El Halabieh N, Citoni B, Coluccia R, Battistoni A, Ferrucci A, Volpe M. Adding markers of organ damage to risk score models improves cardiovascular risk assessment: Prospective analysis of a large cohort of adult outpatients. Int J Cardiol. 2017; 248:342–348. doi: 10.1016/j.ijcard.2017.07.078 [DOI] [PubMed] [Google Scholar]
- 7.Levy D, Garrison RJ, Savage DD, Kannel WB, Castelli WP. Prognostic Implications of Echocardiographically Determined Left Ventricular Mass in the Framingham Heart Study. N Engl J Med. 1990; 322:1561–1566. doi: 10.1056/NEJM199005313222203 [DOI] [PubMed] [Google Scholar]
- 8.Madhavan MV, Tarigopula M, Mintz GS, Maehara A, Stone GW, Généreux P. Coronary artery calcification: Pathogenesis and prognostic implications. J Am Coll Cardiol. 2014; 63:1703–1714. doi: 10.1016/j.jacc.2014.01.017 [DOI] [PubMed] [Google Scholar]
- 9.Viazzi F, Leoncini G, Conti N, Tomolillo C, Giachero G, Vercelli M, Deferrari G, Pontremoli R. Combined effect of albuminuria and estimated glomerular filtration rate on cardiovascular events and all-cause mortality in uncomplicated hypertensive patients. J Hypertens. 2010; 28:848–855. doi: 10.1097/HJH.0b013e328336ed09 [DOI] [PubMed] [Google Scholar]
- 10.Redfield MM, Jacobsen SJ, JCB Jr, Mahoney DW, Bailey KR, Rodeheffer RJ. Burden of systolic and diastolic ventricular dysfunction in the community: Appreciating the scope of the heart failure epidemic. JAMA. 2003; 289:194–202. doi: 10.1001/jama.289.2.194 [DOI] [PubMed] [Google Scholar]
- 11.Okin PM, Devereux RB, Jern S, Kjeldsen SE, Julius S, Nieminen MS, Snapinn S, Harris KE, Aurup P, Edelman JM, Wedel H, Lindholm LH, Dahlöf B. Regression of electrocardiographic left ventricular hypertrophy during antihypertensive treatment and the prediction of major cardiovascular events. JAMA. 2004; 292:2343–2349. doi: 10.1001/jama.292.19.2343 [DOI] [PubMed] [Google Scholar]
- 12.Friedman GD, Cutter GR, Donahue RP, Hughes GH, Hulley SB, Jacobs DR, Liu K, Savage PJ. Cardia: study design, recruitment, and some characteristics of the examined subjects. J Clin Epidemiol. 1988; 41:1105–1116. doi: 10.1016/0895-4356(88)90080-7 [DOI] [PubMed] [Google Scholar]
- 13.Kiefe CI, Williams OD, Bild DE, Lewis CE, Hilner JE, Oberman A. Regional disparities in the incidence of elevated blood pressure among young adults: The CARDIA study. Circulation. 1997; 96:1082–1088. doi: 10.1161/01.CIR.96.4.1082 [DOI] [PubMed] [Google Scholar]
- 14.Ostchega Y, Nwankwo T, Sorlie PD, Wolz M, Zipf G. Assessing the validity of the omron HEM-907XL oscillometric blood pressure measurement device in a national survey environment. J Clin Hypertens. 2010; 12:22–28. doi: 10.1111/j.1751-7176.2009.00199.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jacobs DR, Yatsuya H, Hearst MO, Thyagarajan B, Kalhan R, Rosenberg S, Smith LJ, Barr RG, Duprez DA. Rate of decline of forced vital capacity predicts future arterial hypertension: The Coronary Artery Risk Development in Young Adults Study. Hypertension. 2012; 59:219–225. doi: 10.1161/HYPERTENSIONAHA.111.184101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kishi S, Teixido-Tura G, Ning H, Ambale Venkatesh B, Wu C, Almeida A, Choi E-YY, Gjesdal O, Jacobs DR, Schreiner PJ, Gidding SS, Liu K, Lima JACC. Cumulative blood pressure in early adulthood and cardiac dysfunction in middle age: The CARDIA study. J Am Coll Cardiol. 2015; 65:2679–2687. doi: 10.1016/j.jacc.2015.04.042 [DOI] [PubMed] [Google Scholar]
- 17.Carr JJ, Nelson JC, Wong ND, McNitt-Gray M, Arad Y, Jacobs DR, Sidney S, Bild DE, Williams OD, Detrano RC. Calcified Coronary Artery Plaque Measurement with Cardiac CT in Population-based Studies: Standardized Protocol of Multi-Ethnic Study of Atherosclerosis (MESA) and Coronary Artery Risk Development in Young Adults (CARDIA) Study. Radiology. 2005; 234:35–43. doi: 10.1148/radiol.2341040439 [DOI] [PubMed] [Google Scholar]
- 18.Agatston AS, Janowitz WR, Hildner FJ, Zusmer NR, Viamonte M, Detrano R. Quantification of coronary artery calcium using ultrafast computed tomography. J Am Coll Cardiol. 1990; 15:827–832. doi: 10.1016/0735-1097(90)90282-T [DOI] [PubMed] [Google Scholar]
- 19.Murtaugh MA, Jacobs DR Jr., Yu X, Gross MD, Steffes M, Coronary Artery Risk Development in Young Adults S. Correlates of urinary albumin excretion in young adult blacks and whites: the Coronary Artery Risk Development in Young Adults Study. Am J Epidemiol. 2003; 158:676–686. doi: 10.1093/aje/kwg208 [DOI] [PubMed] [Google Scholar]
- 20.Kim C, Siscovick DS, Sidney S, Lewis CE, Kiefe CL KT. Oral Contraceptive Use and Association With Glucose, Insulin, and Diabetes in young adult women: The CARDIA Study. Diabetes Care. 2002; 25:1027–1032. doi: 10.2337/diacare.25.6.1027 [DOI] [PubMed] [Google Scholar]
- 21.Wang N-YY, Young JH, Meoni LA, Ford DE, Erlinger TP, Klag MJ. Blood pressure change and risk of hypertension associated with parental hypertension: The Johns Hopkins precursors study. Arch Intern Med. 2008; 168:643–648. doi: 10.1001/archinte.168.6.643 [DOI] [PubMed] [Google Scholar]
- 22.Lang RM, Badano LP, Mor-Avi V et al. Recommendations for Cardiac Chamber Quantification by Echocardiography in Adults: An Update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr 2015; 28:1–39.e14. doi: 10.1016/j.echo.2014.10.003 [DOI] [PubMed] [Google Scholar]
- 23.Detrano R, Guerci AD, Carr JJ, Bild DE, Burke G, Folsom AR, Liu K, Shea S, Szklo M, Bluemke DA, O’Leary DH, Tracy R, Watson K, Wong ND, Kronmal RA. Coronary Calcium as a Predictor of Coronary Events in Four Racial or Ethnic Groups. N Engl J Med. 2008; 358:1336–1345. doi: 10.1056/NEJMoa072100 [DOI] [PubMed] [Google Scholar]
- 24.Chronic Kidney Disease (CKD): Clinical Practice Recommendations for Primary Care Physicians and Healthcare Providers. Div Nephrol Hypertens Gen Intern Med. 2011: 1–71. [Google Scholar]
- 25.Feldtkeller E, Khan M, van der Heijde D, van der Linden S, Braun J. Age at disease onset and diagnosis delay in HLA-B27 negative vs. positive patients with ankylosing spondylitis. Rheumatol Int. 2003; 23:61–66. doi: 10.1007/s00296-002-0237-4 [DOI] [PubMed] [Google Scholar]
- 26.Draper B, Cations M, White F, Trollor J, Loy C, Brodaty H, Sachdev P, Gonski P, Demirkol A, Cumming RG, Withall A. Time to diagnosis in young-onset dementia and its determinants: the INSPIRED study. Int J Geriatr Psychiatry. 2016; 31:1217–1224. doi: 10.1002/gps.4430 [DOI] [PubMed] [Google Scholar]
- 27.Franks PW, Hanson RL, Knowler WC, Sievers ML, Bennett PH, Looker HC. Childhood Obesity, Other Cardiovascular Risk Factors, and Premature Death. N Engl J Med. 2010; 362:485–493. doi: 10.1056/NEJMoa0904130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lai CC, Sun D, Cen R, Wang J, Li S, Fernandez-Alonso C, Chen W, Srinivasan SR, Berenson GS. Impact of long-term burden of excessive adiposity and elevated blood pressure from childhood on adulthood left ventricular remodeling patterns: The bogalusa heart study. J Am Coll Cardiol. 2014; 64:1580–1587. doi: 10.1016/j.jacc.2014.05.072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Urbina EM, Gidding SS, Bao W, Pickoff AS, Berdusis K, Berenson GS. Effect of body size, ponderosity, and blood pressure on left ventricular growth in children and young adults in the Bogalusa Heart Study. Circulation. 1995; 91:2400–2406. doi: 10.1161/01.CIR.91.9.2400 [DOI] [PubMed] [Google Scholar]
- 30.Hao G, Wang X, Treiber FA, Harshfield G, Kapuku G, Su S. Blood pressure trajectories from childhood to young adulthood associated with cardiovascular risk. Hypertension. 2017; 69:435–442. doi: 10.1161/HYPERTENSIONAHA.116.08312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sabo RT, Yen MS, Daniels S, Sun SS. Associations between childhood body size, composition, blood pressure and adult cardiac structure: The fels longitudinal study. PLoS One. 2014; 9:e106333. doi: 10.1371/journal.pone.0106333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Juhola J, Magnussen CG, Berenson GS et al. Combined effects of child and adult elevated blood pressure on subclinical atherosclerosis: The international childhood cardiovascular cohort consortium. Circulation. 2013; 128:217–224. doi: 10.1161/CIRCULATIONAHA.113.001614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Raitakari OT, Juonala M, Kähönen M, Taittonen L, Laitinen T, Mäki-Torkko N, Järvisalo MJ, Uhari M, Jokinen E, Rönnemaa T, Åkerblom HK, Viikari JSA. Cardiovascular Risk Factors in Childhood and Carotid Artery Intima-Media Thickness in Adulthood: The Cardiovascular Risk in Young Finns Study. JAMA. 2003; 290:2277–2283. doi: 10.1001/jama.290.17.2277 [DOI] [PubMed] [Google Scholar]
- 34.Koskinen J, Juonala M, Dwyer T et al. Utility of Different Blood Pressure Measurement Components in Childhood to Predict Adult Carotid Intima-Media Thickness. Hypertension. 2019; 73:335–341. doi: 10.1161/HYPERTENSIONAHA.118.12225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Corrada MM, Hayden KM, Paganini-Hill A, Bullain SS, DeMoss J, Aguirre C, Brookmeyer R, Kawas CH. Age of onset of hypertension and risk of dementia in the oldest-old: The 90+ Study. Alzheimer’s Dement. 2017; 13:103–110. doi: 10.1016/j.jalz.2016.09.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hottenga JJ, Whitfield JB, De Geus EJC, Boomsma DI, Martin NG. Heritability and stability of resting blood pressure in Australian twins. Twin Res Hum Genet. 2006; 9:205–209. doi: 10.1375/183242706776382455 [DOI] [PubMed] [Google Scholar]
- 37.Wilk JB, Djousse L, Arnett DK, Hunt SC, Province MA, Heiss G, Myers RH. Genome-wide linkage analyses for age at diagnosis of hypertension and early-onset hypertension in the HyperGEN study. Am J Hypertens. 2004; 17:839–844. doi: 10.1016/j.amjhyper.2004.06.003 [DOI] [PubMed] [Google Scholar]
- 38.Chiang KM, Yang HC, Liang YJ et al. A three-stage genome-wide association study combining multilocus test and gene expression analysis for young-onset hypertension in Taiwan Han Chinese. Am J Hypertens. 2014; 27:819–827. doi: 10.1093/ajh/hpt239 [DOI] [PubMed] [Google Scholar]
- 39.Lynn KS, Lu CH, Yang HY, Hsu WL, Pan WH. Construction of gene clusters resembling genetic causal mechanisms for common complex disease with an application to young-onset hypertension. BMC Genomics. 2013; 14:497. doi: 10.1186/1471-2164-14-497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Leu HB, Chung CM, Lin SJ et al. Association of circadian genes with diurnal blood pressure changes and non-dipper essential hypertension: A genetic association with young-onset hypertension. Hypertens Res. 2015; 38:155–162. doi: 10.1038/hr.2014.152 [DOI] [PubMed] [Google Scholar]
- 41.Shahin DS, Irshaid YM, Saleh AA. The A1166C polymorphism of the AT1R gene is associated with an early onset of hypertension and high waist circumference in Jordanian males attending the Jordan University Hospital. Clin Exp Hypertens. 2014; 36:333–339. doi: 10.3109/10641963.2013.827698 [DOI] [PubMed] [Google Scholar]
- 42.Chang T-J, Wang W-C, Hsiung CA, He C-T, Lin M-W, Sheu WH-H, Chang Y-C, Quertermous T, Chen I, Rotter J, Chuang L-M. Genetic Variation in the Human SORBS1 Gene is Associated With Blood Pressure Regulation and Age at Onset of Hypertension. Medicine (Baltimore). 2016; 95:e2970. doi: 10.1097/MD.0000000000002970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Niiranen TJ, McCabe EL, Larson MG, Henglin M, Lakdawala NK, Vasan RS, Cheng S. Risk for hypertension crosses generations in the community: a multi-generational cohort study. Eur Heart J. 2017; 38:2300–2308. doi: 10.1093/eurheartj/ehx134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vasan RS, Massaro JM, Wilson PWFF, Seshadri S, Wolf PA, Levy D, D’Agostino RB, Framingham Heart Study, Study FH. Antecedent blood pressure and risk of cardiovascular disease: The Framingham Heart Study. Circulation. 2002; 105:48–53. doi: 10.1161/hc0102.101774 [DOI] [PubMed] [Google Scholar]
- 45.Bonifonte A, Ayer T, Veledar E, Clark A, Wilson PWF. Antecedent blood pressure as a predictor of cardiovascular disease. J Am Soc Hypertens. 2015; 9:690–696. doi: 10.1016/j.jash.2015.06.013 [DOI] [PubMed] [Google Scholar]
- 46.Pool LR, Ning H, Wilkins J, Lloyd-Jones DM, Allen NB. Use of Long-term Cumulative Blood Pressure in Cardiovascular Risk Prediction Models. JAMA Cardiol. 2018; 3:1096–1100. doi: 10.1001/JAMACARDIO.2018.2763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chow CK, Teo KK, Rangarajan S et al. Prevalence, awareness, treatment, and control of hypertension in rural and urban communities in high-, middle-, and low-income countries. JAMA. 2013; 310:959–968. doi: 10.1001/jama.2013.184182 [DOI] [PubMed] [Google Scholar]
- 48.Irazola VE, Gutierrez L, Bloomfield G, Carrillo-Larco RM, Dorairaj P, Gaziano T, Levitt NS, Miranda JJ, Ortiz AB, Steyn K, Wu Y, Xavier D, Yan LL, He J, Rubinstein A. Hypertension Prevalence, Awareness, Treatment, and Control in Selected LMIC Communities Results from the NHLBI/UHG Network of Centers of Excellence for Chronic Diseases. Glob Heart. 2016; 11:47–59. doi: 10.1016/j.gheart.2015.12.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.