Abstract
Incidence of endocrine cancers is rising every year. Over the last decade, evidence has accumulated that demonstrates the anti-cancer effects of an anti-diabetic drug - metformin - in endocrine malignancies. We performed a literature review utilizing the Pubmed, Medline and clinicaltrials.gov databases using the keyword “metformin” plus the following terms: “thyroid cancer,” “thyroid nodules,” “parathyroid,” “hyperparathyroidism,” “adrenal adenoma,” “Cushing syndrome,” “hyperaldosteronism,” “adrenocortical cancer,” “neuroendocrine tumor (NET),” “pancreatic NET (pNET),” “carcinoid,” “pituitary adenoma,” “pituitary neuroendocrine tumor (PitNET)”, “prolactinoma,” “pheochromocytoma/paraganglioma.” We found 37 studies describing the preclinical and clinical role of metformin in endocrine tumors. The available epidemiological data shows an association between exposure of metformin and lower incidence of thyroid cancer and pNETs in diabetic patients. Metformin treatment has been associated with better response to cancer therapy in thyroid cancer and pNETs. Preclinical evidence suggests that the primary direct mechanisms of metformin action include inhibition of mitochondrial oxidative phosphorylation via inhibition of both mitochondrial complex I and mitochondrial glycerophosphate dehydrogenase, leading to metabolic stress. Decreased ATP production leads to an activation of a cellular energy sensor, AMPK, and subsequent downregulation of mTOR signaling pathway, which is associated with decreased cellular proliferation. We also describe several AMPK-independent mechanisms of metformin action, as well as the indirect mechanisms targeting insulin resistance. Overall, repositioning of metformin has emerged as a promising strategy for adjuvant therapy of endocrine tumors. The mechanisms of synergy between metformin and other anti-cancer agents need to be elucidated further to guide well-designed prospective trials on combination therapies in endocrine malignancies.
Keywords: Endocrine tumors, Metformin, Mechanism of action, Preclinical evidence, Clinical trials
Introduction
Metformin is a biguanide that has been vastly known as an anti-hyperglycemic drug. Although metformin was initially identified as an anti-diabetic drug, its pleiotropic effects have been recognized over the past several years. Metformin is emerging as a drug with numerous beneficial effects that include: body weight control; reduction of the risk for cardiovascular and neuropsychiatric disorders; and treatment of nonalcoholic fatty liver disease, metabolic syndrome and cancer (Amin, et al. 2019; Zhou, et al. 2018). The anti-cancer potential of metformin was first recognized in diabetic patients. These patients treated with metformin were characterized by a lower cancer incidence in comparison to patients who were on other anti-diabetic medications (Schulten 2018). Subsequently, several epidemiological data documented the association between metformin therapy and a lower risk of developing breast, colon, pancreatic and liver cancers in diabetic patients (Andrzejewski, et al. 2018). The realization of the anti-cancer potential of metformin initiated a series of investigations to uncover the role of metformin as a potential drug for the prevention and treatment of a variety of cancers (Vancura, et al. 2018). To date, several clinical trials have been conducted, with many still ongoing and recruiting patients (https://clinicaltrials.gov/ct2/home) (Chae, et al. 2016). The results for some of these clinical studies are highly encouraging and demonstrate the potential of metformin in the prevention and treatment of various cancer types such as breast, pancreatic, gastric, colorectal, endometrial, prostate, and bladder, either alone or in combination with other drugs (Chae et al. 2016; Hawkes, et al. 2014; Lee, et al. 2016; Nayan, et al. 2015; Niraula, et al. 2012; Spillane, et al. 2013). Based on a meta-analysis that included 13,008 cancer patients with type 2 diabetes, an improvement in overall, and cancer-specific survival was observed in patients treated with metformin in comparison to other glucose-lowering drugs (Yin, et al. 2013). Another meta-analysis that included 65,540 cancer patients with diabetes from 47 independent studies, demonstrated an association of metformin use with the reduction in colon, liver, and lung cancer incidence and improved survival in all examined cancer types (Gandini, et al. 2014). While there is abundant information available showing the efficacy of metformin in multiple cancers, limited information is currently available regarding the effects of metformin on cancers of the endocrine system.
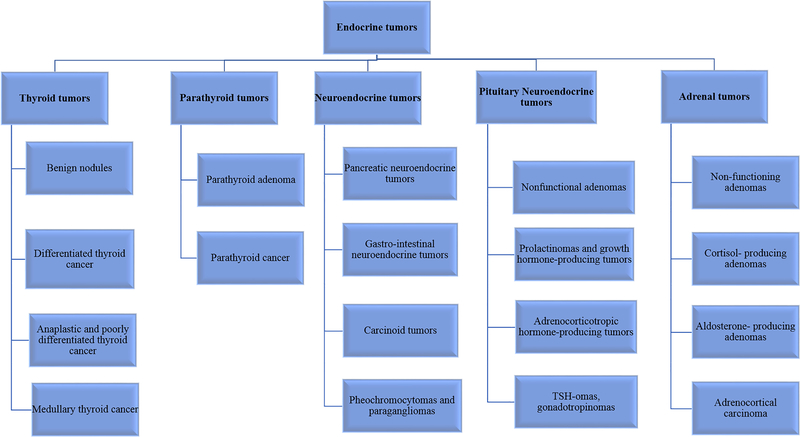
The endocrine system involves a number of hormone-producing glands that regulate various body functions (Asa and Mete 2018) (Figure 1). The most common endocrine tumors arising from endocrine glands include thyroid; parathyroid; pituitary; and neuroendocrine tumors of the gastrointestinal system (Zhang and Nose 2011). Development of cancer in any of these endocrine glands can affect the production of hormones and thus lead to severe consequences. Most of the endocrine tumors tend to be benign, however, some of them become malignant and require extensive therapy. Of note, the incidence of endocrine cancers is increasing every year worldwide (Asa and Mete 2018). The prognosis of patients with metastatic endocrine cancers is variable, with high mortality rates and suboptimal treatment response in poorly differentiated aggressive tumors of the endocrine system (Lima, et al. 2017). The standard of care therapy for patients with endocrine tumors varies depending on the origin of the tumor, its nature (benign or malignant), the presence of an over-production of hormones by the tumor and the tumor stage (metastatic or not). Over the last decade, the growth inhibitory effects of metformin have been observed in patients with endocrine tumors. Although these studies are limited, nonetheless, there is compelling evidence from in-vitro and in-vivo models that point towards the efficacy of metformin in the treatment of endocrine tumors.
Figure 1.
Different types of endocrine tumors.
Metformin exhibits its anti-cancer effects through multiple pathways that can be inter-connected or work independently (Vancura et al. 2018). The targets of metformin that were predominately studied include mitochondrial respiratory chain complex I, adenosine monophosphate-activated protein kinase (AMPK) and mechanistic target of rapamycin (mTOR) (Li, et al. 2018; Sosnicki, et al. 2016). These targets are components of biologically important pathways associated with cellular metabolism, proliferation, survival, and apoptosis. Besides these, numerous other targets of metformin have been identified in cancer cells such as cyclins (CCND1, CCNE2), microRNAs (miR-26a, miR-34a) and mitochondrial genes (mGPDH) (Schulten 2018). In this review, we discuss the anti-cancer potential of metformin in endocrine tumors and the underlying mechanisms of action.
Differentiated Thyroid Cancer (DTC)
Epidemiology
Thyroid cancer is the most common endocrine malignancy, accounting for more than 90% of the endocrine cancers. It’s the fifth most common cancer in women in the United States (2018; Cabanillas, et al. 2016). Thyroid cancer can be categorized into two different types based on its origin: epithelial-derived and neuroendocrine C-cell derived thyroid cancer. Epithelial-derived DTC variants include papillary thyroid cancer (PTC), follicular thyroid cancer (FTC) and Hurthle cell thyroid cancer (HTC), while de-differentiated variants include poorly differentiated thyroid cancer (PDTC) and anaplastic thyroid cancer (ATC). Medullary thyroid cancer (MTC) is derived from neuroendocrine C-cells producing calcitonin, which is responsible for calcium balance. Among these types, PTC is the most common subtype of thyroid cancer (Schmidbauer, et al. 2017).
In the last few years, the anti-cancer effects of metformin have been identified in thyroid cancer patients. It is particularly interesting in view of the fact that the incidence of thyroid cancer has been reported to be higher in type 2 diabetes mellitus (T2DM) patients (Li and Qian 2017). Epidemiological evidence has shown the association between T2DM, insulin resistance and cancer (Vigneri, et al. 2009). Obesity, which is a risk factor for the development of insulin resistance and T2DM, has also been linked with an increased risk of thyroid cancer (Kitahara, et al. 2011). The protective effects of metformin either alone or in combination with JQ1 (an inhibitor of bromodomain-containing protein 4) have been observed in an obesity-induced thyroid cancer mouse model (Park, et al. 2016; Park, et al. 2018). In a recent retrospective study based on the Korean population, metformin intake was associated with lower thyroid cancer incidence risk. These protective effects of metformin, however, were only observed in individuals who either were on metformin for longer periods of time or those with a higher cumulative dose of metformin (Cho, et al. 2018). Another study, based on a Taiwanese population that included more than 1.4 million T2DM patients who had been exposed to metformin, observed a significantly lower risk of developing thyroid cancer in comparison to those who had never been on metformin. Similar to the above-mentioned Korean study, the individuals who had a higher cumulative dose of metformin, or had been on metformin for a longer time duration, had a lower incidence of thyroid cancer (Tseng 2014). In contrast to the aforementioned studies, a case-control analysis based on a U.K. database showed no association between metformin and thyroid cancer incidence. This study was, however, based on a smaller number of patients on metformin (98 cases and 416 matched controls) (Becker, et al. 2015).
Besides lowering the thyroid cancer risk, metformin treatment has been associated with longer disease-free survival in T2DM patients with cervical lymph node metastasis of DTC (Jang, et al. 2015). In a retrospective study involving 240 DTC individuals, diabetic patients who were treated with metformin had smaller thyroid cancer size, higher complete response rate and longer progression-free survival in comparison to non-metformin treated diabetic patients and non-diabetic thyroid cancer patients matched by age, gender and BMI (Klubo-Gwiezdzinska, et al. 2013).
The potential mechanisms for the growth inhibitory effects of metformin have been elucidated in several preclinical studies.
Preclinical evidence
Metformin treatment inhibits the growth of thyroid cancer cell lines in-vitro (Chen, et al. 2012; Klubo-Gwiezdzinska et al. 2013). Treatment with metformin can induce cell cycle arrest in G1 phase and induce apoptosis, thus leading to a reduction in cellular viability (Chen et al. 2012). Metformin-mediated AMPK activation leading to downregulation of p70S6K/pS6 expression is one of the underlying mechanisms for the suppression of thyroid cancer cell growth (Klubo-Gwiezdzinska et al. 2013). AMPK is an energy sensor and a major regulator of cellular energy homeostasis. AMPK is activated under energy stress conditions when ATP levels drop within the cell. Under such circumstances, the major energy consuming processes, such as cellular proliferation, need to be inhibited (Motoshima, et al. 2006). Metformin, which is a well-known mitochondrial complex 1 inhibitor, increases the AMP/ADP ratio within the cell resulting in AMPK activation, which initiates a series of events to preserve energy within the cell (Choi and Park 2013). AMPK activation is believed to activate the downstream target tuberous sclerosis complex 2 (TSC2), which in turn inhibits the mammalian target of rapamycin (mTOR) signaling pathway (Plews, et al. 2015). Since p70S6K/pS6 is downstream of mTOR, inhibition of mTOR hampers their activation leading to reduced protein synthesis, which is necessary for cancer cell growth (Lei, et al. 2017). This metformin-induced reduction in p70S6K activation has also been demonstrated in a small set of thyroid tumor samples collected from the metformin-treated thyroid cancer patients (Klubo-Gwiezdzinska et al. 2013).
In an athymic mouse model, metformin treatment inhibited the growth of PTC and promoted necrosis of established tumors. These anti-tumorigenic effects of metformin were not only associated with activation of AMPK but also with suppression of the AKT signaling pathway (Cho, et al. 2014). Metformin-mediated AMPK activation has been shown to inhibit the phosphorylation of insulin receptor substrate-1 (IRS-1). IRS-1 is a downstream mediator of insulin-like growth factor 1 receptor (IGF-1R), which transmits signals from the IGF-1R to the PI3K/AKT pathway (Pierotti, et al. 2013). Metformin treatment phosphorylates an inhibitory serine residue on the IRS-1, which blocks its signaling to AKT, thus leading to the suppression of the AKT signaling pathway (Zakikhani, et al. 2010). mTOR is also a downstream target of AKT, which suggests its dual suppression by inhibition of AKT signaling and activation of the AMPK pathway (Pierotti et al. 2013). IRS-1 is also a substrate for insulin signaling and metformin mediated inhibition of IRS-1 blocks insulin/IGF1/AKT/mTOR signaling pathway and protects against insulin-associated cancer progression (Saini and Yang 2018).
In a recent study published by our group, mGPDH (mitochondrial glycerophosphate dehydrogenase) was identified as a metformin target in thyroid cancer cells (Thakur, et al. 2018). mGPDH is an enzyme present on the outer surface of the inner mitochondrial membrane and is part of the glycerol-3-phosphate shuttle. Originally, mGPDH was identified as a metformin target in the liver, inhibiting hepatic gluconeogenesis in a rat model (Madiraju, et al. 2014). The primary function of this enzyme is to transfer electrons generated during glycolysis in the cytosol to the mitochondrial electron transport chain. mGPDH, along with its cytosolic counterpart (cGPDH), forms an important link between glycolysis and oxidative phosphorylation (Mracek, et al. 2013). Our study revealed that inhibiting mGPDH activity and expression is associated with a reduction in oxidative phosphorylation, which has a negative impact on thyroid cancer cell growth (Thakur et al. 2018). The in-vivo experiments in thyroid cancer metastatic mouse models demonstrated that thyroid tumors characterized by higher mGPDH expression were much more sensitive to metformin effects in comparison to low-mGPDH expressing tumors. Moreover, mGPDH as a metformin target is overexpressed in patient-derived thyroid cancer samples compared with normal tissue (Thakur et al. 2018). These studies document that metformin is an inhibitor of mitochondrial function, which affects mitochondrial respiration through inhibition of mitochondrial complex 1 and mGPDH. In a separate study, metformin treatment inhibited glucose uptake by PTC cells both in-vitro and in-vivo. This reduction in glucose uptake was associated with decreased expression of HK2 and GLUT1 genes (Shen, et al. 2017). Altogether, these studies demonstrate that metformin can target cellular metabolism by multiple mechanisms in thyroid cancer cells. However, the effect size observed in-vitro with supra-therapeutic metformin concentrations might not be translated to a similar effect size in-vivo in humans (Table 2).
Table 2.
The table summarizes the effects of metformin on different endocrine tumors as reported in various preclinical studies.
| Endocrine tumors | Reference | Study design (cell lines) | Metformin dose | Study endpoint |
|---|---|---|---|---|
| Thyroid cancer | (Klubo-Gwiezdzinska et al. 2013) | In-vitro study (FTC133, TPC1, BCPAP) | Variable (0.5–5 mM) | Dose- and time-dependent reduction in cellular proliferation in DTC cells |
| Thyroid cancer | (Chen, et al. 2012) | In-vitro study (HTh74, HTh74Rdox, C643, SW1736, FTC133) | Variable (0.1–40 mM) | Metformin treatment inhibited cell proliferation, cell cycle progression and promoted apoptosis in thyroid cancer cells in a dose-dependent manner |
| PTC | (Cho, et al. 2014) | In-vitro study (BCPAP, BHP10–3SC) | Variable (0.1–20 mM) | Dose-dependent inhibition of cellular viability and induction of apoptosis in PTC cells |
| In-vivo study (BHP10–3SC) | Variable (10, 50, 100 mg/ml of metformin in drinking water for 30 days) | Dose-dependent reduction in tumor volume in PTC mouse model | ||
| DTC | (Thakur, et al. 2018) | In-vitro study (FTC133, BCPAP) | 5 mM | Inhibition of cellular proliferation as well as mitochondrial respiration in DTC cells |
| In-vivo study (FTC133) | 12.5 mg in water by oral gavage for 4 weeks | Inhibition of tumor growth in a DTC mouse model characterized by high mGPDH expression | ||
| PTC | (Shen, et al. 2017) | In-vitro study (BCPAP, KTC1) | Variable (2.5– 10 mM) | Reduction in cellular viability and glucose uptake |
| In-vivo study (BCPAP) | 300 mg/kg/day by intraperitoneal injections for a week | Reduction in glucose uptake | ||
| Thyroid cancer | (Kheder, et al. 2017) | In-vitro study (FTC133, K1E7, RO82-W-1, 8305C, TT, Nthy-ori 3–1) | Variable (0.03–20 mM) | Inhibition of cellular proliferation, migration and induction of apoptosis in a variety of thyroid cancer cell lines in a dose-dependent manner |
| Obesity-induced thyroid cancer | (Park, et al. 2016) | In-vivo study (ThrbPV/PVPten+/-mice) | 0.5 mg/ml in drinking water | Inhibition of tumor progression by blocking vascular invasion and anaplasia in obesity-induced thyroid cancer mice models |
| Obesity-induced thyroid cancer | (Park, et al. 2018) | In-vivo study (ThrbPV/PVPten+/- mice) | 0.5 mg/ml in drinking water | Metformin, in combination with JQ1, led to a reduction in thyroid cancer growth and improved survival in obesity-induced thyroid cancer mice models |
| MTC | (Klubo-Gwiezdzinska, et al. 2012) | In-vitro study (TT and MZ-CRC-1) | Variable (0.5–5 mM) | Inhibition of cellular growth in a dose- and time-dependent manner |
| ATC | (Nozhat et al. 2018) | In-vitro study (SW1736, C643, 8305C) | Variable (2.5–60 mM) | Dose-dependent reduction in cellular viability, migration and induction of apoptosis |
| ATC | (Chen, et al. 2015) | In-vitro study (HTh7, HTh74Rdox) | 5 mM | Metformin, in combination with sorafenib, resulted in increased apoptosis and cell cycle arrest |
| ATC | (Hanly, et al. 2015) | In-vitro study (Nthy-ori 3, BCPAP, 8505c) | 2 mM | Metformin, in combination with vemurafenib, resulted in increased loss of cellular viability and induction of apoptosis |
| NET | (Vlotides, et al. 2014) | In-vitro study (BON1, GOT1, NCI-H727) | Variable (0.1–10 mM) | Dose and time-dependent decrease in cell viability |
| pNET | (Herrera-Martinez, et al. 2019) | In-vitro study (BON1, QGP-1) | 10mM | Time-dependent decrease in cell viability |
| PCC | (Li, et al. 2017) | In-vitro study (PC12) | Variable (1–50 mM) | Dose-dependent reduction in cellular viability, inhibition of cell cycle progression and promotion of cell apoptosis |
| PGL | (Florio, et al. 2018) | In-vitro study (PTJ64i and PTJ86i) | Variable (5–40 mM) | Dose-dependent inhibition of cellular viability |
| ACC | (Poli, et al. 2016) | In-vitro study (H295R, SW13) | Variable (0.5– 250 mM) | Dose-dependent reduction in cellular viability, proliferation and induction of apoptosis |
| In-vivo study (H295R) | 3 mg/100 µL PBS for 40 days | Reduction in tumor volume | ||
| PitNETs | (An, et al. 2017) | In-vitro study (GH3) | Variable (2–50 mM) | Dose-dependent reduction in cellular proliferation, growth hormone secretion, and induction of apoptosis |
| In-vivo study (GH3) | Variable (100, 300, 500 mg/kg for 4 weeks) | Dose-dependent reduction in tumor size and growth hormone secretion | ||
| PitNETs | (Faggi, et al. 2018) | In-vitro study (GH3) | Variable (0.4– 5 mM) | Inhibition of cellular viability |
| PitNETs | (Vazquez-Borrego, et al. 2019) | In-vitro study (Primary culture on ACTH-secreting adenomas, non-functioning pituitary adenomas, GH-secreting adenomas, prolactinomas; cell lines- AtT20, GH3) | 5mM, 10mM | Inhibition of cellular viability in a cell type dependent manner. |
| Pituitary corticotroph tumor | (Jin, et al. 2018) | In-vitro study (AtT20) | Variable (2.5–40 mM) | Reduction in cellular proliferation, ACTH secretion and induction of apoptosis |
The effects of metformin on hormone overproduction in thyroid cancer
While DTC is not routinely associated with a significant over-production of hormones, metastatic follicular thyroid cancer may produce excess thyroid hormones leading to signs and symptoms of thyrotoxicosis (Sharma, et al. 2011). There are no data showing the role of metformin in the regulation of the production of thyroid hormones in thyroid cancer patients. In a prospective study performed on patients with insulin resistance, while the thyroid hormone levels were still within normal range, a small but significant increase in free triiodothyronine levels was observed in patients receiving metformin (Anil, et al. 2016). Also, in an in-vivo study performed on male rats, metformin treatment resulted in an increase in the levels of free triiodothyronine (FT3) and free thyroxine (FT4), as well as a slight reduction in thyrotropin (TSH) levels (An, et al. 2017).
Metformin also has an important role in the indirect regulation of insulin signaling, which is a growth stimulus of thyroid cancer. The presence of higher insulin levels, typically seen in insulin resistance, has been associated with higher thyroid cancer risk (Rezzonico, et al. 2009). DTC expresses IGF-1R which has a high similarity with the insulin receptor (IR) and responds to insulin stimulation. The presence of higher insulin levels can promote the growth of DTC through IGF-1R (Vella, et al. 2001). In addition to this, insulin can also enhance the growth promoting effects of thyroid stimulating hormone (TSH). The presence of higher TSH levels has been associated with the development and progression of thyroid cancer, but TSH alone has limited tumorigenic potential. The presence of growth factors, such as insulin or insulin-like growth factor (IGF) along with TSH, has been shown to augment the growth of thyroid cancer cells to a much greater extent (Boelaert 2009; Vella et al. 2001). Metformin treatment is associated with a reduction in circulating insulin levels, which suggests its role in the inhibition of thyroid cancer growth by antagonizing the growth-promoting action of insulin (Dowling, et al. 2012). This is also supported by an in-vitro study in which metformin treatment abolished the growth-promoting effects of insulin on anaplastic thyroid cancer cell lines (Chen et al. 2012).
Prospective clinical trials
There are currently two clinical trials utilizing metformin in the management of thyroid cancer. One study, currently open for enrollment, is focused on metformin therapy as an add-on to treatment with radioactive iodine (NCT03109847). This study is designed to assess if metformin can mitigate the myelosuppressive effects of radioactive iodine (RAI), which is used for the therapy of DTC. There is an evidence from retrospective studies that metformin attenuates the RAI-induced decrease in peripheral blood cells in patients with DTC (Bikas, et al. 2016).
The second study, which has been completed, was testing the role of metformin in suppressing TSH levels in thyroid cancer patients. Fifty patients, whose levothyroxine dose was reduced by 30%, were randomized to metformin 500 mg daily or placebo. After 3 months of therapy, there was no difference in TSH levels between the groups, suggesting no benefit of brief exposure to a minimal dose of metformin in the reduction of TSH concentration (Mousavi, et al. 2014).
Medullary Thyroid Cancer (MTC)
Epidemiology
MTC originates from the parafollicular C-cells of the thyroid gland. It is a rare type of cancer, with only 3–5% of thyroid cancer patients diagnosed with this neuroendocrine tumor. MTC is characterized by a high prevalence of central and lateral neck lymph node metastases, thus appropriate extensive surgery is the best therapeutic option to achieve remission. However, the locoregional recurrence rate is high and, once the disease presents with distant metastases, the routine management consists of therapy with tyrosine kinase inhibitors (TKIs) (Accardo, et al. 2017), which does not lead to complete remission but extends the time to progression. At present, there is no clinical information available regarding the use of metformin in these patients, mostly because of the rare occurrence of this disease.
Preclinical evidence
There are a few in-vitro studies that have determined the anti-cancer effects of metformin on the MTC derived cell lines. A study published in 2017 analyzed the growth inhibitory effects of metformin on a variety of thyroid cancer cell lines with different origins – FTC, PTC, ATC and MTC – and normal thyroid follicular cells. Irrespective of the origin of thyroid cancer cell lines, metformin suppressed cellular proliferation at concentrations that fall within the therapeutic range (Kheder, et al. 2017). In another study, the anti-cancer effects of metformin were tested in two MTC cell lines, TT and MZ-CRC-1. The authors reported inhibition in cancer cell growth as well as a reduction in the ability to form spheroids. This study also demonstrated metformin-induced inhibition of the mTOR-p70S6K signaling pathway, downregulation of p-ERK activation and decreased expression of cyclin-D1 in MTC cells (Klubo-Gwiezdzinska, et al. 2012). These in-vitro observations were also supported by human MTC samples characterized by higher expression of metformin targets - cyclin D1 and mTOR-p70S6K in comparison to the corresponding normal tissues (Klubo-Gwiezdzinska et al. 2012). The metformin-induced growth inhibitory effects in this study were found to be independent of AMPK activation.
mTOR is known to be activated by multiple pathways, among which the PI3K/AKT pathway is the most common. However, in the aforementioned study, metformin treatment did not inhibit AKT activation as is revealed by the analysis of p-AKT levels in the MTC cell lines. This indicates inhibition of mTOR by another pathway. Activation of ERK (p-ERK) has also been shown to activate the mTOR signaling pathway either by inhibiting TSC2 or by phosphorylation of RAPTOR protein (regulatory-associated protein of mTOR), both of which promotes mTOR signaling (Mendoza, et al. 2011). Metformin treatment has been shown to lower p-ERK levels in MTC as well as in certain DTC cell lines (Klubo-Gwiezdzinska et al. 2013; Klubo-Gwiezdzinska et al. 2012).
The role of metformin on hormone overproduction in MTC
MTC is known to produce calcitonin, which is used as a tumor biomarker and a prognostic factor in the surveillance of MTC patients. Significantly elevated calcitonin may lead to calcium imbalance as well as promote intestinal peristalsis leading to severe diarrhea. There are no studies looking at the effect of metformin on calcitonin production by MTC cells.
One of the most debilitating complications of metastatic MTC is ACTH-dependent Cushing syndrome due to the overproduction of ACTH by MTC cells, stimulating a cortisol release from the adrenal glands. At present, there is no information on the effects of metformin on ACTH production by MTC cells.
Clinical trials
There are currently no clinical trials investigating the role of metformin in MTC.
Anaplastic Thyroid Cancer (ATC)
Epidemiology
ATC is a rare type of thyroid malignancy but is associated with a very high mortality rate. It is one of the most lethal cancers and is associated with extremely poor prognosis (Limaiem and Giwa 2019). ATC accounts for 2–3% of all thyroid malignancies. The first-line treatment for ATC patients includes surgical resection followed by high-beam radiation therapy and chemotherapy. Patients with ATC have a very short median survival of around six months (Saini, et al. 2018). ATC is resistant to most standard treatments, which makes its management enormously difficult. However, recently, combination therapy with dabrafenib (BRAF inhibitor) and trametinib (MEK inhibitor) led to an objective response in 69% of patients with locally advanced or metastatic ATC, leading to the FDA approval of this combination therapy for ATC (Subbiah, et al. 2018).
At present, there is no clinical information available regarding the effects of metformin on the management of ATC.
Preclinical evidence
In the in-vitro studies performed on anaplastic thyroid cancer cell lines, metformin treatment resulted in the reduction of cellular proliferation and the induction of apoptosis (Chen et al. 2012; Nozhat, et al. 2018). Metformin treatment can also inhibit cell migration and affect the morphology of ATC cells (Nozhat et al. 2018). In another study, the addition of metformin improved the growth inhibitory effects of sorafenib (a multi-kinase inhibitor) on ATC cell lines. The combination of metformin with sorafenib resulted in increased apoptosis and cell cycle arrest in ATC cells in comparison to treatment with either metformin or sorafenib alone. The growth inhibition in these cells was associated with inhibition of the ERK signaling pathway (Chen, et al. 2015). In a similar study, the combination of metformin with vemurafenib (a BRAFV600E inhibitor) resulted in an increased loss of cell viability and apoptosis in ATC and PTC cells in comparison to treatment with either metformin or vemurafenib alone (Hanly, et al. 2015).
At present, there is no strong evidence elucidating the mechanism of metformin action in ATC cells. Moreover, the above observations need to be validated in animal models.
Clinical trials
There are currently no clinical trials investigating the role of metformin in ATC.
Benign Thyroid Nodules
Epidemiology
Thyroid nodules are very common and nearly 90% of thyroid nodules are benign in nature (Durante, et al. 2018). The most common risk factors for developing thyroid nodules include age, gender, family history of thyroid nodules, and radiation exposure. Besides these, the other important risk factors that have been associated with an increased incidence of thyroid nodules are obesity, diabetes, insulin resistance, and metabolic syndrome (Buscemi, et al. 2018; Duran, et al. 2014; Moon, et al. 2018; Tang, et al. 2017). One or more of these risk factors are usually associated with each other and the presence of one risk factor can promote the appearance of another. Metformin use has been associated with the management of all these risk factors (Fournier, et al. 2014; Zhou et al. 2018).
In a patient based prospective study that included 66 women with small benign thyroid nodules and insulin resistance, metformin treatment (500mg/twice a day/6 months) resulted in a significant (30%) reduction in nodule size in comparison to non-metformin treated women. A combination of levothyroxine with metformin had resulted in an even better outcome in these patients with a 55% reduction in their nodular size (Rezzonico, et al. 2011). In a prospective study that included 100 patients with insulin resistance, metformin therapy resulted in a significant reduction in body-mass index, insulin resistance, thyroid volume and thyroid nodule size (Anil et al. 2016).
In a recently published meta-analysis study that included 240 patients from three randomized control trials and four prospective studies, metformin treatment caused a decrease in nodular volume, TSH level and improvement in the insulin resistance in these patients (Sui, et al. 2018). Another very recent meta-analysis, including 189 patients fulfilling the analysis criteria, provided similar conclusions (He, et al. 2019).
Preclinical evidence
To the best of our knowledge, there is no preclinical information available regarding the effects of metformin on benign thyroid nodules or on the cells derived from them.
Clinical trials
In Brazil, a clinical trial is currently ongoing to examine the effects of insulin resistance and the use of metformin on the volume of thyroid nodules (NCT03183752). In a completed clinical trial performed on 89 prediabetic patients, metformin treatment resulted in a significant reduction in the volume of small solid nodules in comparison to placebo-treated patients. No significant effect of metformin was observed on the mixed solid-cystic nodules. In addition, metformin treatment resulted in a decrease in TSH levels in patients with TSH >2.5 μU/ml. Metformin treatment was also associated with lower thyroid gland volume compared with the placebo group (Karimifar, et al. 2014).
Parathyroid Tumors
Parathyroid tumors are relatively common, affecting around 30 people in 100,000 population (Thakker 2016). The most common type of parathyroid tumors are adenomas, which are benign in nature. Parathyroid tumors can cause hyperparathyroidism due to the overproduction of parathyroid hormone (PTH) leading to bone resorption, hypercalcemia, and its clinical consequences. The other type of parathyroid tumor is a carcinoma, a very aggressive malignancy, which luckily accounts for less than 1% of parathyroid tumors (DeLellis 2011). The most common treatment for PTH-hypersecreting carcinomas and adenomas is surgery. Interestingly, there are reports suggesting the association between hyperparathyroidism and insulin resistance and reversal of insulin resistance measured by HOMA-IR index post-curative parathyroidectomy (Duran, et al. 2017). Moreover, Tanaka et al. showed that parathyroid tumor tissues express IGF binding proteins and, based on in-vitro experiments, suggested that the IGF-1 and IGF binding proteins are involved in the growth regulation of parathyroid tumor cells (Tanaka, et al. 1994). The latter observation may form a basis for exploring the indirect effects of metformin on insulin signaling in these tumor models. However, at present, there is no epidemiological, preclinical or clinical data available on the role of metformin in parathyroid tumors.
Neuroendocrine Tumors (NETs)
Epidemiology
Neuroendocrine tumors (NETs) represent a heterogeneous group of tumors that originate from cells with both neural and endocrine properties. NETs can be classified into different types based on their site of origin. The most common types of NETs are gastrointestinal NETs (GI-NETs), pancreatic neuroendocrine tumors (pNETs), and lung carcinoids (Oronsky, et al. 2017; Patel and Galoian 2018). The prevalence of NETs is on the rise (Dasari, et al. 2017). At present, the treatment options for these patients include curative surgery, treatment with both somatostatin analogs (SSA) and radiolabeled SSAs, as well as targeted treatment with tyrosine kinase inhibitors and mTOR pathway inhibitors (Young, et al. 2015).
Diabetes is an independent risk factor for the development of pNETs and GI-NETs (Halfdanarson, et al. 2014; Hassan, et al. 2008; Haugvik, et al. 2015). The therapeutic effects of metformin have been reported in patients with diabetes suffering from concurrent pNETs (Pusceddu, et al. 2016). In a recent retrospective study conducted in Italy, metformin treatment was associated with longer progression-free survival (PFS) in diabetic patients with pNETs in comparison to diabetic patients or non-diabetic patients who were not receiving metformin. This study also reported a significant improvement in PFS of pNET patients when metformin was implemented as an adjunct to combination therapy with everolimus (mTOR inhibitor) and SSAs (octreotide or lanreotide) or with SSAs alone (Pusceddu, et al. 2018). At present, there are no clinical studies analyzing the the effects of metformin in NETs other than pNETs.
Preclinical evidence
In a recent study performed on pNET cells (BON-1 and QGP-1), metformin treatment inhibited cellular viability in both cell lines in a time-dependent manner. The mechanism of growth inhibition was associated with reduction in p-AKT and p-ERK levels in BON-1 cells, but not in QGP-1 cells (Herrera-Martinez, et al. 2019). In another study, metformin treatment inhibited the cellular proliferation of neuroendocrine tumor cells of different origins in a dose-dependent manner (Vlotides, et al. 2014). Depending upon the origin of the cell lines (pancreatic neuroendocrine cells- BON1, midgut carcinoid cells- GOT1 and bronchopulmonary neuroendocrine cells- NCI-H727), the underlying mechanism for metformin-induced growth inhibition varied. However, regardless of their origin, metformin treatment suppressed mTOR signaling in all cell lines. Conversely, other metformin-induced effects, such as AMPK activation/inhibition and apoptosis induction, were dependent on cell line origin. This study also demonstrated Glycogen synthase kinase 3 (GSK) as a metformin target in BON1, GOT1 and NCI-H727 cells (Vlotides et al. 2014). GSK is a serine-threonine protein kinase that plays an important role in multiple processes such as cellular proliferation, differentiation, apoptosis, metabolism, immunity, and autophagy. GSK has two isoforms – GSK-3α and GSK-3β – that are constitutively active within the cells unless being inactivated by their phosphorylation. GSK-3 has multiple downstream targets by which it can regulate cell proliferation and survival processes. GSK-3 activity has been associated with tumorigenesis and has been investigated as a potential target in the treatment of cancer (Mancinelli, et al. 2017). Metformin treatment suppressed GSK-3 activity by promoting its phosphorylation in all three NET cell lines (BON1, GOT1, NCI-H727) (Vlotides et al. 2014). In another study, GSK-3α/β has been demonstrated as a therapeutic target in NETs. Treatment with a GSK-3 inhibitor led to a reduction in cellular viability, induction of apoptosis and inhibition of the migration potential in NET cell lines (BON1, QGP1, H727) (Aristizabal Prada, et al. 2018). Diminished oncogenic growth in these cell lines upon GSK-3 inhibition was associated with the inhibition of mTOR and EGFR signaling pathways. However, the detailed mechanism by which metformin inhibits GSK-3 is not understood and needs to be determined.
Clinical trials
To further confirm these observations and to establish the anti-tumorigenic effects of metformin, a prospective phase II trial (NCT02294006) is underway, testing the efficacy of metformin in combination with everolimus (mTOR inhibitor) and octreotide (SSA) in Fondazione IRCCS Istituto Tumori, Italy. A pilot phase II clinical trial (NCT02279758) is also being conducted in Instituto do Cancer do Estado de São Paulo, Brazil to test the treatment efficacy of metformin in patients with gastroenteropancreatic NETs. Besides this, a phase I trial (NCT02823691) is ongoing at the National Cancer Institute, Italy to assess the safety of metformin in combination with another SSA, lanreotide, in patients with gastro-intestinal or lung NET carcinoids.
Tumors of the Adrenal Medulla - Pheochromocytomas (PCC)/Paragangliomas (PGL)
Epidemiology
Pheochromocytomas and paragangliomas are rare neuroendocrine tumors with an incidence rate of approximately 2–8:1,000,000/year. While PCCs arise from the chromaffin cells of the adrenal medulla, PGLs originate from the ganglia located outside of the adrenal gland (Fishbein and Nathanson 2012). These tumors are mostly benign, but their malignant transformation does happen. Since the incidence of these tumors is so rare, at present, there is no patient-related information available on the efficacy of metformin treatment on these tumors.
Preclinical evidence
There are a few in-vitro studies performed on the cell lines derived from PCC/PGL tumors that demonstrate the anti-proliferative potential of metformin. As is reported in thyroid cancer and NET cell lines, metformin exposure inhibited cell proliferation, promoted cell cycle arrest and apoptosis of pheochromocytoma rat-derived cells (PC12 cell line) (Li, et al. 2017). This study demonstrated activation of the AMPK and inhibition of the mTOR and ERK signaling pathways upon metformin treatment. The inhibition of mTOR can occur through activation of the AMPK pathway. Moreover, there was a downregulation of Ccna2 and Ccnb2 expression, which plays an important role in the regulation of cell cycle progression (Li et al. 2017). A similar study was conducted on the adherent version of the PC12 cell line (PC12-Adh PHEO), which also demonstrated growth inhibition upon metformin treatment (Meireles, et al. 2017). Moreover, metformin-induced a reduction in oxygen consumption, which was most likely associated with the inhibition of mitochondrial respiration (Meireles et al. 2017). These metformin-associated effects need to be validated on human PCC derived cell lines.
Cell lines derived from head and neck PGLs (PTJ64i and PTJ86i) are also sensitive to metformin-mediated growth suppression. In combination with a pyruvate dehydrogenase kinase inhibitor, dichloroacetate, and a PPARα antagonist, GW6471, metformin had synergistic effects on the cellular viability and clonogenicity of these cell lines (Florio, et al. 2018). Such combination treatments might be effective in these tumors when the chemotherapy treatments are not effective and surgery rendering the patient as “no evidence of disease NED” is not feasible.
It will be interesting to establish if these in-vitro observations can be confirmed through animal models and human studies.
The role of metformin on hormone overproduction in PCCs/PGLs
To the best of our knowledge, there are currently no preclinical or clinical data on the association between metformin and overproduction of catecholamines by pheochromocytomas/paragangliomas.
Clinical trials
There are currently no clinical trials addressing the role of metformin in the management of metastatic pheochromocytomas/paragangliomas.
Tumors of the Adrenal Cortex – Adrenocortical Adenoma/Adrenocortical Carcinoma (ACC)
Epidemiology
Adrenocortical adenomas are the most common types of adrenal tumors and are benign in nature. These tumors can be functionally inactive or actively produce cortisol, aldosterone or androgens. The non-functional adenomas are usually left untreated unless they are large and growing, in which case surgery is indicated, whereas functional adenomas can be treated by their surgical removal and/or by medications that control the activity/production of hormones. In contrast to adenomas, ACCs are extremely rare in occurrence, affecting 1–2 people per million population. ACCs are malignant and lethal in nature and can be associated with a significant overproduction of hormones – particularly androgens – leading to signs and symptoms of virilization in affected women. The most common treatment for ACCs is surgery and chemotherapy (Else, et al. 2014).
Clinically, not much information is available related to the effects of metformin on the treatment of adenomas or ACCs. Nonetheless, there is a recent case study in which the anti-cancer effects of metformin were observed in a metastatic ACC patient. The patient was treated with mitotane in combination with other chemotherapeutic drugs, which resulted in stable disease. This was followed by metformin treatment (500mg/twice a day) in combination with mitotane, which resulted in the further reduction of the nodule size, with a continued response to this combination therapy for nine months (Peixoto, et al. 2018).
Preclinical evidence
To the best of our knowledge, there is only one preclinical study where the effects of metformin have been tested on ACCs through in-vitro and in-vivo experiments. The study analyzed the growth inhibitory effects of metformin on an adrenocortical cell line, H295R. Metformin treatment significantly reduced the cellular proliferation and viability of these cells by inhibiting phosphorylation of ERK½ and mTOR, along with activation of the AMPK pathway. In addition to this, metformin treatment promoted apoptosis of these cells by inhibiting the expression of anti-apoptotic genes that led to the activation of caspase 3, which promotes apoptosis. These in-vitro findings were also confirmed in-vivo, where metformin treatment resulted in the inhibition of tumor growth as confirmed by the reduction in Ki67 levels (Poli, et al. 2016).
The role of metformin on hormone overproduction in adrenocortical adenomas/ACCs
At present, there is no information related to the effects of metformin on the cortisol and aldosterone production by adenomas/ACCs.
Clinical trials
There are currently no clinical trials investigating the role of metformin in adenomas/ACCs.
Pituitary neuroendocrine tumors (PitNETs)
Epidemiology
PitNETs, also known as pituitary adenomas (PAs) are the most common tumors of the central nervous system. They are extremely diverse, depending on their cell of origin, and are categorized based on the hormone that they secrete. These PitNETs are usually benign in nature and can be classified as functional or non-functional, depending on whether or not they secrete hormones (Theodros, et al. 2015). Among the PitNETs, prolactinomas are the most common, comprising 32%–51% of all PitNETs. Other common PitNETs include non-functioning adenomas, growth hormone (GH)-secreting PitNETs, and adrenocorticotropic hormone (ACTH)-secreting PitNETs. Some rare occurring PitNETs, include thyroid-stimulating hormone (TSH)- and gonadotropin-secreting PitNETs (Mehta and Lonser 2017). The most common treatment for these patients is medical therapy (treatment of choice for prolactinomas) or surgical treatment, depending on the type of PitNETs. In cases where medical therapy fails, surgical resection is the only option. There are case reports suggesting that such patients may benefit from metformin treatment, as reported in a recent study where the anti-tumorigenic effects of metformin were observed in two patients with prolactinomas who were resistant to bromocriptine, a dopamine agonist. Combination treatment of bromocriptine and metformin in these patients lowered prolactin levels and reduced the tumor size, while bromocriptine alone was insufficient to reduce the tumor growth (Liu, et al. 2018).
Preclinical evidence
Currently, there are limited studies suggesting the growth inhibitory effects of metformin on PitNETs. The anti-proliferative effects of metformin have been seen on an ACTH-secreting mouse corticotroph tumor cell line, AtT20, and growth hormone-secreting PitNET cell lines, GH3, and GH1 (An et al. 2017; Faggi, et al. 2018; Jin, et al. 2018). Besides growth inhibition, metformin-induced apoptosis in the cells by up-regulating the expression of pro-apoptotic genes and down-regulating the expression of anti-apoptotic genes. In ACTH-secreting AtT20 cells, metformin inhibited cell proliferation by activating the AMPK signaling pathway and inhibiting the IGF-1R/AKT/mTOR pathway. In a recently published study performed on primary cell cultures derived from PitNETs, metformin treatment inhibited cell viability in ACTH-secreting adenomas and non-functioning pituitary adenomas but not in GH-secreting adenomas and prolactinomas (Vazquez-Borrego, et al. 2019).
In a study performed by Faggi, et al. (2018) on GH-secreting PitNET cells, metformin treatment mediated the activation of AMPK, with inhibition of the mTOR-p70S6 kinase pathway suggested as the underlying mechanism. However, in the other study conducted by An, et al. (2017) on GH3 cells, the underlying mechanism for growth inhibition was associated with increased ATF3 signaling and inhibition of STAT3 activity. These in-vitro observations were also validated on a xenograft mouse model. ATF3 is a transcription factor that is induced by physiological stress. Metformin-mediated inhibition of mitochondrial respiration leads to oxidative stress, which in turn can stimulate ATF3 activity. ATF3 induction has been implicated in both the repression and activation of apoptosis in cancer cells, suggesting its dual role (Yin, et al. 2008). In GH3 cells, inhibition of ATF3 activity prevented metformin-induced apoptosis, which suggests its tumor suppressor role in PitNET cells. Similar to ATF3, STAT3 can also promote or inhibit cancer cell growth (Avalle, et al. 2017). Metformin-mediated STAT3 inhibition suggests its anti-oncogenic role in PitNETs, however further studies are required to understand the detailed mechanism by which metformin regulates the growth of these tumor cells.
The role of metformin in hormone production by PitNETs
At present, there are no clinical data available describing the role of metformin in the regulation of hormone production by functional PitNETs. However, in the above-mentioned in-vitro studies, metformin suppressed the production of ACTH and GH in pituitary tumor cell line models (An et al. 2017; Jin et al. 2018). On the contrary, metformin treatment did not affect hormone secretion in primary cells derived from either ACTH-secreting, GH-secreting or prolactin-secreting PitNets (Vazquez-Borrego et al. 2019).
Metformin treatment has been shown to lower TSH levels in diabetic patients, but the exact mechanism is not understood (Fournier et al. 2014).
Clinical trials
Currently, there is only one ongoing clinical trial (NCT02060383) focused predominantly on metformin as an agent ameliorating the side effects from the medical management of ACTH-secreting and GH-secreting pituitary adenomas. This is a phase IV, multi-center, randomized, open-label study of patients treated with the SST analog pasireotide for Cushing disease or acromegaly. Since SST analogs affect glucose metabolism, if previously normoglycemic patients experience increases in their fasting blood glucose and meet the criteria for diabetes while on pasireotide, they start anti-diabetic treatment using metformin. The efficacy of metformin on hyperglycemia management is assessed at a landmark of 16 weeks.
Conclusion and future prospects
Clinical trials, retrospective studies, and laboratory-based in-vitro and in-vivo studies suggest the efficacy of metformin as a growth inhibitory agent in endocrine tumors (Table 1, 2). Most clinical observations that demonstrate the usefulness of metformin treatment in endocrine tumors is derived from diabetic patients who were taking metformin. With regard to DTC and pNETs, there are substantial data suggesting the therapeutic effects of metformin in these cancers. This promising epidemiological and preclinical evidence led to the initiation of phase II (n=2) and phase 1 (n=1) clinical trials using metformin as monotherapy or adjuvant treatment in the management of NETs. Besides this, a small randomized clinical trial testing the efficacy of metformin use on the volume of benign thyroid nodules is also underway. For other endocrine malignancies, there is either limited or no clinical data demonstrating the efficacy of metformin. For MTC, ATC, PA, PCC/PGL and ACC, evidence for the potential anti-cancer role of metformin originate from a small number of cell line studies. For the remaining endocrine malignancies, the effects of metformin have never been investigated. To evaluate the effects of metformin on these rare forms of endocrine cancers, it is important to create good cell line and mouse models. These preclinical studies can form the basis for clinical trials, where the effects of metformin can be analyzed on the patient population.
Table 1.
The table summarizes the effects of metformin on different endocrine tumors as reported in various clinical studies.
| Endocrine tumors | Reference | Study design | Metformin dose | Study endpoint |
|---|---|---|---|---|
| Thyroid cancer | (Cho, et al. 2018) | Retrospective study including diabetic patients (Metformin users vs non-users) | Variable; Mean dose: 531 mg/daily | Reduction of thyroid cancer risk in metformin users |
| Thyroid cancer | (Tseng 2014) | Retrospective study including diabetic subjects (Metformin ever-users vs never-users) | Variable | Reduction of thyroid cancer risk in metformin users |
| Thyroid cancer | (Becker, et al. 2015) | Retrospective study including diabetic subjects (Metformin ever-users vs never-users) | Variable | No effect of metformin on thyroid cancer risk |
| Thyroid cancer | (Jang, et al. 2015) | Retrospective study including diabetic patients with DTC (Metformin users vs non-users) | Variable; Mean dose: 979 mg/day | Metformin use was associated with longer disease-free survival in patients with cervical lymph node metastasis of DTC |
| Thyroid cancer | (Klubo-Gwiezdzinska, et al. 2013) | Retrospective study (Diabetic patients with thyroid cancer vs control patients with thyroid cancer) | Variable | Diabetic patients who were on metformin had smaller tumors in comparison to non-metformin users and control thyroid cancer patients |
| Benign thyroid nodules | (Rezzonico, et al. 2011) | Prospective study in women with insulin resistance and thyroid nodular hyperplasia | Two 500 mg doses/day | Reduction in the size of the nodules in patients treated with either metformin alone or in combination with levothyroxine |
| Benign thyroid nodules | (Sui, et al. 2018) | Meta-analysis | Variable | Reduction in nodular size, improvement in thyroid function and insulin resistance |
| Benign thyroid nodules | NCT03183752 | Clinical trial evaluating the effects of metformin on the volume of benign thyroid nodules | 500 mg/three times a day | Undergoing |
| Thyroid nodules | (Cintosun, et al. 2017) | Prospective study on patients with insulin resistance | 1,700 mg/day for 6 months | Reduction in body-mass index, insulin resistance, thyroid volume and thyroid nodule size |
| Thyroid nodules | (He, et al. 2019) | Meta-analysis performed on patients with thyroid nodules and insulin resistance | Variable | Reduction in thyroid nodule size, TSH levels and insulin resistance |
| Thyroid nodules | (Karimifar, et al. 2014) | Clinical trial evaluating the effects of metformin on thyroid function of prediabetic patients | Starting dose- 500 mg/day to be increased to the maximum of 1500 mg/day | Decrease in TSH levels in patients with TSH >2.5 μU/ml; reduction in volume of small solid nodules only; inhibits an increase in thyroid volume |
| pNET | (Pusceddu, et al. 2018) | Retrospective study in patients with advanced pNETs (Diabetic patients on metformin vs diabetic patients on other treatment vs non-diabetic patients) | Variable | Improved progression-free survival in metformin-using diabetic patients in comparison to diabetic patients on other treatments and non-diabetic patients |
| NET | NCT02294006 | Clinical trial evaluating the effects of metformin in combination with everolimus and octreotide LAR in patients with advanced well-differentiated pNETs | Unknown | Unknown status |
| NET | NCT02823691 | Clinical trial evaluating the safety of lanreotide ATG in combination with metformin in gastro-intestinal or lung carcinoids patients. | Starting dose- 850 mg/day to be increased to the maximum of 2550 mg/day | Undergoing |
| NET | NCT02279758 | Clinical trial evaluating the effects of metformin on patients with well-differentiated NETs | 850 mg/twice a day | Unknown |
| ACC | (Peixoto, et al. 2018) | Case-study in a patient with metastatic ACC | 500 mg/twice a day | Reduction in the size of the adrenal tumor |
| Prolactinoma | (Liu, et al. 2018) | Retrospective analysis of two patients with prolactinomas treated with bromocriptine and metformin | 1.5 g/day | Reduction in tumor size and prolactin levels |
DTC - differentiated thyroid cancer; PTC - papillary thyroid cancer; MTC - medullary thyroid cancer; ATC - anaplastic thyroid cancer; pNET - pancreatic neuroendocrine tumors; NET - neuroendocrine tumors; PCC - pheochromocytoma; PGL - paraganglioma; ACC - adrenocortical carcinoma; TSH - thyroid stimulating hormone; ACTH - Adrenocorticotropic hormone
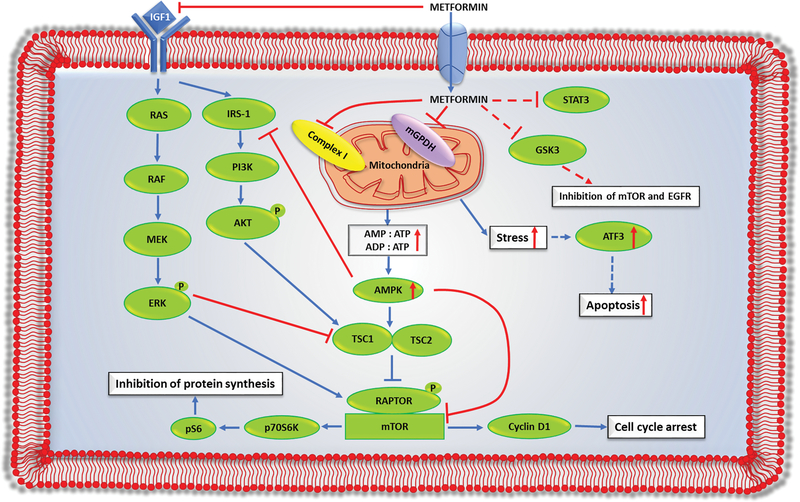
Another important question that must be addressed is the mechanism of action of metformin in different endocrine cancers. Current studies indicate multiple targets of metformin in cancer cells (Figure 2). The most common mechanism by which metformin controls tumorigenesis includes inhibition of mitochondrial function by hindering complex I activity and AMPK dependent and/or independent inhibition of the mTOR signaling pathway. Based on the available information from limited studies, the mechanism of action of metformin appears to be varied among different endocrine cancers. Besides the well-known targets, various additional metformin targets (e.g. mGPDH, ATF3, STAT3, GSK3, cyclins) have been identified in endocrine cancers. The exact mechanism by which metformin controls the action of these genes/proteins are not known and must be elucidated. Metformin treatment can also inhibit the insulin/IGF-1 signaling pathway, which is associated with cellular proliferation. Moreover, metformin can also decrease the production of certain growth-promoting hormones, such as TSH and GH, which could further contribute to the reduction of TSH- and GH-dependent tumors.
Figure 2.
Mechanism of action of metformin in endocrine tumors: metformin is a negatively charged molecule that enters the cell through organic cation transporters (OCTs). After entering the cells, it can inhibit tumorigenesis through the following pathways. First, metformin, through its direct action, can inhibit complex 1 or mGPDH, either alone or together, to inhibit oxidative phosphorylation (OXPHOS). This inhibition of OXPHOS lowers the energy production (ATP synthesis) within the cells, which creates cellular stress in cancer cells. The reduction in ATP production increases both the AMP:ATP and ADP:ATP ratios, which activates AMPK. Activation of AMPK, in turn, leads to activation of tuberous sclerosis complex ½ (TSC ½), which inhibits the mTOR pathway. Second, metformin can inhibit insulin or insulin-like growth factor-1 (IGF-1) mediated activation of the RAS-MEK-ERK and PI3K-AKT pathways, which results in the inhibition of the mTOR pathway. Third, metformin-mediated activation of AMPK can inhibit phosphorylation of insulin receptor substrate-1 (IRS-1). IRS-1 is downstream of the IGF-1 pathway and upstream of the PI3K-AKT pathway. Inhibition of IRS-1 activity hampers AKT phosphorylation, which causes activation of the TSC complex and inhibition of the mTOR pathway. Last, metformin has other targets including STAT3, GSK3, and ATF3 in endocrine cells, but the exact pathway by which metformin targets these genes/proteins are not known.
One of the most important points of consideration in the preclinical studies reporting the anti-cancer effects is the metformin-doses and their translational relevance in humans. The plasma levels of metformin in patients taking therapeutic doses range from 10 to 40 μM, however metformin concentration in the portal vein is much higher and ranges from 40 to70 μM (Song 2016). Considering the plasma and portal vein levels of metformin, the majority of the in-vitro studies utilized supra-therapeutic doses of metformin, and thus, the effect size seen in-vitro might not translate to the in-vivo findings in humans (Table 2). However, it is important to note here that metformin accumulates in the cells and tissues over time, leading to a significant, several folds increase in its concentration within the cells compared to plasma levels (Rena, et al. 2017). The accumulation of metformin within the cells depends on the surface expression of the transporters like organic cation transporters (OCTs), multidrug and toxin extruders (MATEs) and plasma membrane monoamine transporters (PMATs) (McCreight, et al. 2016). The presence of OCTs in the liver results in 3–5 times higher accumulation of metformin within the hepatocytes than the portal vein. The concentration of metformin in the gut has been observed to be 30–300 times higher than the plasma concentrations (Song 2016). That being said, the supra-physiological concentrations of metformin used for the in vitro studies cannot be achieved in humans without potential toxicity. To establish the role of metformin in the management of endocrine tumors, it is important to validate the in-vitro findings through preclinical studies at concentrations that can be translated into humans. Most of the in vivo studies were performed at doses leading to plasma concentration of metformin in animals comparable to the metformin levels in the plasma of the humans upon administration of therapeutic doses. These studies are, however, very limited in number and lack the information about metformin levels in the tumor tissues which is an important factor in determining the efficacy of metformin as an anti-cancer drug.
Another important point that draws attention in these preclinical studies is that the metformin-associated growth inhibitory effects are primarily due to inhibition of proliferation, rather than induction of massive apoptosis or necrosis, suggesting that monotherapy with metformin will not lead to complete remission of the tumor. Therefore, utilization of metformin as an adjunct to other treatment modalities has emerged as a promising strategy. Numerous studies have exploited metformin in synergy with standard treatment or in combination with other drugs for the treatment of different types of cancer and have observed a better response than the use of metformin alone. However, the mechanisms of synergy between metformin and other anti-cancer agents are not well understood and must be elucidated further to guide well-designed prospective trials on combination therapies in endocrine malignancies. Furthermore, additional studies are needed to identify biomarkers and patients’ characteristics that are associated with the highest likelihood of response to metformin treatment in endocrine tumors.
A number of retrospective studies reported reduction in cancer incidence and progression of endocrine cancer. However, a retrospective design of these studies was associated with a significant inherent selection, information and immortal-time bias, unavailability of relevant information of all potential confounders, relatively small sample size and highly variable dosing and duration of metformin treatment. To overcome these limitations, it is important to conduct well designed prospective clinical trials that can take into account all these factors.
Overall, although there are several limitations in studies investigating the role of metformin in endocrine cancers, metformin appears to be a promising adjuvant agent in the treatment of endocrine malignancies.
Acknowledgments
Funding:
NIDDK intramural funding program ZIA DK07514002.
Footnotes
Declaration of interest:
No conflict of interest.
References
- 2018 Cancer Facts and Figures 26–27
- Accardo G, Conzo G, Esposito D, Gambardella C, Mazzella M, Castaldo F, Di Donna C, Polistena A, Avenia N, Colantuoni V, et al. 2017. Genetics of medullary thyroid cancer: An overview. Int J Surg 41 Suppl 1 S2–S6. [DOI] [PubMed] [Google Scholar]
- Amin S, Lux A & O’Callaghan F 2019. The journey of metformin from glycaemic control to mTOR inhibition and the suppression of tumour growth. Br J Clin Pharmacol 85 37–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- An J, Pei X, Zang Z, Zhou Z, Hu J, Zheng X, Zhang Y, He J, Duan L, Shen R, et al. 2017. Metformin inhibits proliferation and growth hormone secretion of GH3 pituitary adenoma cells. Oncotarget 8 37538–37549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrzejewski S, Siegel PM & St-Pierre J 2018. Metabolic Profiles Associated With Metformin Efficacy in Cancer. Front Endocrinol (Lausanne) 9 372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anil C, Kut A, Atesagaoglu B, Nar A, Bascil Tutuncu N & Gursoy A 2016. Metformin Decreases Thyroid Volume and Nodule Size in Subjects with Insulin Resistance: A Preliminary Study. Med Princ Pract 25 233–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aristizabal Prada ET, Weis C, Orth M, Lauseker M, Spottl G, Maurer J, Grabowski P, Grossman A, Auernhammer CJ & Nolting S 2018. GSK3alpha/beta: A Novel Therapeutic Target for Neuroendocrine Tumors. Neuroendocrinology 106 335–351. [DOI] [PubMed] [Google Scholar]
- Asa SL & Mete O 2018. Endocrine pathology: past, present and future. Pathology 50 111–118. [DOI] [PubMed] [Google Scholar]
- Avalle L, Camporeale A, Camperi A & Poli V 2017. STAT3 in cancer: A double edged sword. Cytokine 98 42–50. [DOI] [PubMed] [Google Scholar]
- Becker C, Jick SS, Meier CR & Bodmer M 2015. No evidence for a decreased risk of thyroid cancer in association with use of metformin or other antidiabetic drugs: a case-control study. BMC Cancer 15 719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bikas A, Van Nostrand D, Jensen K, Desale S, Mete M, Patel A, Wartofsky L, Vasko V & Burman KD 2016. Metformin Attenuates 131I-Induced Decrease in Peripheral Blood Cells in Patients with Differentiated Thyroid Cancer. Thyroid 26 280–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boelaert K 2009. The association between serum TSH concentration and thyroid cancer. Endocr Relat Cancer 16 1065–1072. [DOI] [PubMed] [Google Scholar]
- Buscemi S, Massenti FM, Vasto S, Galvano F, Buscemi C, Corleo D, Barile AM, Rosafio G, Rini N & Giordano C 2018. Association of obesity and diabetes with thyroid nodules. Endocrine 60 339–347. [DOI] [PubMed] [Google Scholar]
- Cabanillas ME, McFadden DG & Durante C 2016. Thyroid cancer. Lancet 388 2783–2795. [DOI] [PubMed] [Google Scholar]
- Chae YK, Arya A, Malecek MK, Shin DS, Carneiro B, Chandra S, Kaplan J, Kalyan A, Altman JK, Platanias L, et al. 2016. Repurposing metformin for cancer treatment: current clinical studies. Oncotarget 7 40767–40780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen G, Nicula D, Renko K & Derwahl M 2015. Synergistic anti-proliferative effect of metformin and sorafenib on growth of anaplastic thyroid cancer cells and their stem cells. Oncol Rep 33 1994–2000. [DOI] [PubMed] [Google Scholar]
- Chen G, Xu S, Renko K & Derwahl M 2012. Metformin inhibits growth of thyroid carcinoma cells, suppresses self-renewal of derived cancer stem cells, and potentiates the effect of chemotherapeutic agents. J Clin Endocrinol Metab 97 E510–520. [DOI] [PubMed] [Google Scholar]
- Cho SW, Yi KH, Han SK, Sun HJ, Kim YA, Oh BC, Park YJ & Park DJ 2014. Therapeutic potential of metformin in papillary thyroid cancer in vitro and in vivo. Mol Cell Endocrinol 393 24–29. [DOI] [PubMed] [Google Scholar]
- Cho YY, Kang MJ, Kim SK, Jung JH, Hahm JR, Kim TH, Nam JY, Lee BW, Lee YH, Chung JH, et al. 2018. Protective Effect of Metformin Against Thyroid Cancer Development: A Population-Based Study in Korea. Thyroid 28 864–870. [DOI] [PubMed] [Google Scholar]
- Choi YK & Park KG 2013. Metabolic roles of AMPK and metformin in cancer cells. Mol Cells 36 279–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dasari A, Shen C, Halperin D, Zhao B, Zhou S, Xu Y, Shih T & Yao JC 2017. Trends in the Incidence, Prevalence, and Survival Outcomes in Patients With Neuroendocrine Tumors in the United States. JAMA Oncol 3 1335–1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeLellis RA 2011. Parathyroid tumors and related disorders. Mod Pathol 24 Suppl 2 S78–93. [DOI] [PubMed] [Google Scholar]
- Dowling RJ, Niraula S, Stambolic V & Goodwin PJ 2012. Metformin in cancer: translational challenges. J Mol Endocrinol 48 R31–43. [DOI] [PubMed] [Google Scholar]
- Duran AO, Anil C, Gursoy A, Nar A, Inanc M, Bozkurt O & Tutuncu NB 2014. Thyroid volume in patients with glucose metabolism disorders. Arq Bras Endocrinol Metabol 58 824–827. [DOI] [PubMed] [Google Scholar]
- Duran C, Sevinc B, Kutlu O & Karahan O 2017. Parathyroidectomy Decreases Insulin Resistance Index in Patients with Primary Hyperparathyroidism. Indian J Surg 79 101–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durante C, Grani G, Lamartina L, Filetti S, Mandel SJ & Cooper DS 2018. The Diagnosis and Management of Thyroid Nodules: A Review. JAMA 319 914–924. [DOI] [PubMed] [Google Scholar]
- Else T, Kim AC, Sabolch A, Raymond VM, Kandathil A, Caoili EM, Jolly S, Miller BS, Giordano TJ & Hammer GD 2014. Adrenocortical carcinoma. Endocr Rev 35 282–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faggi L, Giustina A & Tulipano G 2018. Effects of metformin on cell growth and AMPK activity in pituitary adenoma cell cultures, focusing on the interaction with adenylyl cyclase activating signals. Mol Cell Endocrinol 470 60–74. [DOI] [PubMed] [Google Scholar]
- Fishbein L & Nathanson KL 2012. Pheochromocytoma and paraganglioma: understanding the complexities of the genetic background. Cancer Genet 205 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Florio R, De Lellis L, Veschi S, Verginelli F, di Giacomo V, Gallorini M, Perconti S, Sanna M, Mariani-Costantini R, Natale A, et al. 2018. Effects of dichloroacetate as single agent or in combination with GW6471 and metformin in paraganglioma cells. Sci Rep 8 13610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fournier JP, Yin H, Yu OH & Azoulay L 2014. Metformin and low levels of thyroid-stimulating hormone in patients with type 2 diabetes mellitus. CMAJ 186 1138–1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gandini S, Puntoni M, Heckman-Stoddard BM, Dunn BK, Ford L, DeCensi A & Szabo E 2014. Metformin and cancer risk and mortality: a systematic review and meta-analysis taking into account biases and confounders. Cancer Prev Res (Phila) 7 867–885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halfdanarson TR, Bamlet WR, McWilliams RR, Hobday TJ, Burch PA, Rabe KG & Petersen GM 2014. Risk factors for pancreatic neuroendocrine tumors: a clinic-based case-control study. Pancreas 43 1219–1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanly EK, Bednarczyk RB, Tuli NY, Moscatello AL, Halicka HD, Li J, Geliebter J, Darzynkiewicz Z & Tiwari RK 2015. mTOR inhibitors sensitize thyroid cancer cells to cytotoxic effect of vemurafenib. Oncotarget 6 39702–39713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassan MM, Phan A, Li D, Dagohoy CG, Leary C & Yao JC 2008. Risk factors associated with neuroendocrine tumors: A U.S.-based case-control study. Int J Cancer 123 867–873. [DOI] [PubMed] [Google Scholar]
- Haugvik SP, Hedenstrom P, Korsaeth E, Valente R, Hayes A, Siuka D, Maisonneuve P, Gladhaug IP, Lindkvist B & Capurso G 2015. Diabetes, smoking, alcohol use, and family history of cancer as risk factors for pancreatic neuroendocrine tumors: a systematic review and meta-analysis. Neuroendocrinology 101 133–142. [DOI] [PubMed] [Google Scholar]
- Hawkes AL, Quinn M, Gebski V, Armes J, Brennan D, Janda M, fe MMETC & Obermair A 2014. Improving treatment for obese women with early stage cancer of the uterus: rationale and design of the levonorgestrel intrauterine device +/− metformin +/− weight loss in endometrial cancer (feMME) trial. Contemp Clin Trials 39 14–21. [DOI] [PubMed] [Google Scholar]
- He X, Wu D, Hu C, Xu T, Liu Y, Liu C, Xu B & Tang W 2019. Role of Metformin in the Treatment of Patients with Thyroid Nodules and Insulin Resistance: A Systematic Review and Meta-Analysis. Thyroid 29 359–367. [DOI] [PubMed] [Google Scholar]
- Herrera-Martinez AD, Pedraza-Arevalo S, F LL, Gahete MD, Galvez-Moreno MA, Castano JP & Luque RM 2019. Type 2 Diabetes in Neuroendocrine Tumors: Are Biguanides and Statins Part of the Solution? J Clin Endocrinol Metab 104 57–73. [DOI] [PubMed] [Google Scholar]
- Jang EK, Kim WG, Kwon H, Choi YM, Jeon MJ, Kim TY, Shong YK, Kim WB & Kim EY 2015. Metformin Is Associated with a Favorable Outcome in Diabetic Patients with Cervical Lymph Node Metastasis of Differentiated Thyroid Cancer. Eur Thyroid J 4 181–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin K, Ruan L, Pu J, Zhong A, Wang F, Tan S, Huang H, Mu J & Yang G 2018. Metformin suppresses growth and adrenocorticotrophic hormone secretion in mouse pituitary corticotroph tumor AtT20cells. Mol Cell Endocrinol 478 53–61. [DOI] [PubMed] [Google Scholar]
- Karimifar M, Aminorroaya A, Amini M, Mirfendereski T, Iraj B, Feizi A & Norozi A 2014. Effect of metformin on thyroid stimulating hormone and thyroid volume in patients with prediabetes: A randomized placebo-controlled clinical trial. J Res Med Sci 19 1019–1026. [PMC free article] [PubMed] [Google Scholar]
- Kheder S, Sisley K, Hadad S & Balasubramanian SP 2017. Effects of prolonged exposure to low dose metformin in thyroid cancer cell lines. J Cancer 8 1053–1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitahara CM, Platz EA, Freeman LE, Hsing AW, Linet MS, Park Y, Schairer C, Schatzkin A, Shikany JM & Berrington de Gonzalez A 2011. Obesity and thyroid cancer risk among U.S. men and women: a pooled analysis of five prospective studies. Cancer Epidemiol Biomarkers Prev 20 464–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klubo-Gwiezdzinska J, Costello J Jr., Patel A, Bauer A, Jensen K, Mete M, Burman KD, Wartofsky L & Vasko V 2013. Treatment with metformin is associated with higher remission rate in diabetic patients with thyroid cancer. J Clin Endocrinol Metab 98 3269–3279. [DOI] [PubMed] [Google Scholar]
- Klubo-Gwiezdzinska J, Jensen K, Costello J, Patel A, Hoperia V, Bauer A, Burman KD, Wartofsky L & Vasko V 2012. Metformin inhibits growth and decreases resistance to anoikis in medullary thyroid cancer cells. Endocr Relat Cancer 19 447–456. [DOI] [PubMed] [Google Scholar]
- Lee CK, Jung M, Jung I, Heo SJ, Jeong YH, An JY, Kim HI, Cheong JH, Hyung WJ, Noh SH, et al. 2016. Cumulative Metformin Use and Its Impact on Survival in Gastric Cancer Patients After Gastrectomy. Ann Surg 263 96–102. [DOI] [PubMed] [Google Scholar]
- Lei Y, Yi Y, Liu Y, Liu X, Keller ET, Qian CN, Zhang J & Lu Y 2017. Metformin targets multiple signaling pathways in cancer. Chin J Cancer 36 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H & Qian J 2017. Association of diabetes mellitus with thyroid cancer risk: A meta-analysis of cohort studies. Medicine (Baltimore) 96 e8230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M, Jiang X, Su T, Jiang L, Zhou W & Wang W 2017. Metformin Suppresses Proliferation and Viability of Rat Pheochromocytoma Cells. Med Sci Monit 23 3253–3260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M, Li X, Zhang H & Lu Y 2018. Molecular Mechanisms of Metformin for Diabetes and Cancer Treatment. Front Physiol 9 1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lima CR, Gomes CC & Santos MF 2017. Role of microRNAs in endocrine cancer metastasis. Mol Cell Endocrinol 456 62–75. [DOI] [PubMed] [Google Scholar]
- Limaiem F & Giwa AO 2019. Cancer, Anaplastic Thyroid. In StatPearls; Treasure Island (FL). [Google Scholar]
- Liu X, Liu Y, Gao J, Feng M, Bao X, Deng K, Yao Y & Wang R 2018. Combination Treatment with Bromocriptine and Metformin in Patients with Bromocriptine-Resistant Prolactinomas: Pilot Study. World Neurosurg 115 94–98. [DOI] [PubMed] [Google Scholar]
- Madiraju AK, Erion DM, Rahimi Y, Zhang XM, Braddock DT, Albright RA, Prigaro BJ, Wood JL, Bhanot S, MacDonald MJ, et al. 2014. Metformin suppresses gluconeogenesis by inhibiting mitochondrial glycerophosphate dehydrogenase. Nature 510 542–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mancinelli R, Carpino G, Petrungaro S, Mammola CL, Tomaipitinca L, Filippini A, Facchiano A, Ziparo E & Giampietri C 2017. Multifaceted Roles of GSK-3 in Cancer and Autophagy-Related Diseases. Oxid Med Cell Longev 2017 4629495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCreight LJ, Bailey CJ & Pearson ER 2016. Metformin and the gastrointestinal tract. Diabetologia 59 426–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta GU & Lonser RR 2017. Management of hormone-secreting pituitary adenomas. Neuro Oncol 19 762–773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meireles C, Neves F, Simeoni L & Lofrano-Porto A 2017. Metformin reduces viability and proliferation of pheochromocytoma cells in vitro. Presented at Society for Endocrinology ECE 2017, Lisbon, Portugal Endocrine Abstracts 49 GP26. [Google Scholar]
- Mendoza MC, Er EE & Blenis J 2011. The Ras-ERK and PI3K-mTOR pathways: cross-talk and compensation. Trends Biochem Sci 36 320–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moon JH, Hyun MK, Lee JY, Shim JI, Kim TH, Choi HS, Ahn HY, Kim KW, Park DJ, Park YJ, et al. 2018. Prevalence of thyroid nodules and their associated clinical parameters: a large-scale, multicenter-based health checkup study. Korean J Intern Med 33 753–762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motoshima H, Goldstein BJ, Igata M & Araki E 2006. AMPK and cell proliferation--AMPK as a therapeutic target for atherosclerosis and cancer. J Physiol 574 63–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mousavi Z, Dourandish L, Rokni H, Sadeghi R & Rasoul Zakavi S 2014. Effects of short-term metformin therapy associated with levothyroxine dose decrement on TSH and thyroid hormone levels in patients with thyroid cancer. Minerva Endocrinol 39 59–65. [PubMed] [Google Scholar]
- Mracek T, Drahota Z & Houstek J 2013. The function and the role of the mitochondrial glycerol-3-phosphate dehydrogenase in mammalian tissues. Biochim Biophys Acta 1827 401–410. [DOI] [PubMed] [Google Scholar]
- Nayan M, Bhindi B, Yu JL, Hermanns T, Mohammed A, Hamilton RJ, Finelli A, Jewett MA, Zlotta AR, Fleshner NE, et al. 2015. The effect of metformin on cancer-specific survival outcomes in diabetic patients undergoing radical cystectomy for urothelial carcinoma of the bladder. Urol Oncol 33 386 e387–313. [DOI] [PubMed] [Google Scholar]
- Niraula S, Dowling RJ, Ennis M, Chang MC, Done SJ, Hood N, Escallon J, Leong WL, McCready DR, Reedijk M, et al. 2012. Metformin in early breast cancer: a prospective window of opportunity neoadjuvant study. Breast Cancer Res Treat 135 821–830. [DOI] [PubMed] [Google Scholar]
- Nozhat Z, Mohammadi-Yeganeh S, Azizi F, Zarkesh M & Hedayati M 2018. Effects of metformin on the PI3K/AKT/FOXO1 pathway in anaplastic thyroid Cancer cell lines. Daru 26 93–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oronsky B, Ma PC, Morgensztern D & Carter CA 2017. Nothing But NET: A Review of Neuroendocrine Tumors and Carcinomas. Neoplasia 19 991–1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park J, Kim WG, Zhao L, Enomoto K, Willingham M & Cheng SY 2016. Metformin blocks progression of obesity-activated thyroid cancer in a mouse model. Oncotarget 7 34832–34844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park S, Willingham MC, Qi J & Cheng SY 2018. Metformin and JQ1 synergistically inhibit obesity-activated thyroid cancer. Endocr Relat Cancer 25 865–877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel P & Galoian K 2018. Molecular challenges of neuroendocrine tumors. Oncol Lett 15 2715–2725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peixoto RD, Gomes LM, Sousa TT, Racy DJ, Shigenaga M & Nagourney RA 2018. Efficacy of oral metformin in a patient with metastatic adrenocortical carcinoma: Examination of mechanisms and therapeutic implications. Rare Tumors 10 2036361317749645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierotti MA, Berrino F, Gariboldi M, Melani C, Mogavero A, Negri T, Pasanisi P & Pilotti S 2013. Targeting metabolism for cancer treatment and prevention: metformin, an old drug with multi-faceted effects. Oncogene 32 1475–1487. [DOI] [PubMed] [Google Scholar]
- Plews RL, Mohd Yusof A, Wang C, Saji M, Zhang X, Chen CS, Ringel MD & Phay JE 2015. A novel dual AMPK activator/mTOR inhibitor inhibits thyroid cancer cell growth. J Clin Endocrinol Metab 100 E748–756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poli G, Cantini G, Armignacco R, Fucci R, Santi R, Canu L, Nesi G, Mannelli M & Luconi M 2016. Metformin as a new anti-cancer drug in adrenocortical carcinoma. Oncotarget 7 49636–49648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pusceddu S, Buzzoni R, Vernieri C, Concas L, Marceglia S, Giacomelli L, Milione M, Leuzzi L, Femia D, Formisano B, et al. 2016. Metformin with everolimus and octreotide in pancreatic neuroendocrine tumor patients with diabetes. Future Oncol 12 1251–1260. [DOI] [PubMed] [Google Scholar]
- Pusceddu S, Vernieri C, Di Maio M, Marconcini R, Spada F, Massironi S, Ibrahim T, Brizzi MP, Campana D, Faggiano A, et al. 2018. Metformin Use Is Associated With Longer Progression-Free Survival of Patients With Diabetes and Pancreatic Neuroendocrine Tumors Receiving Everolimus and/or Somatostatin Analogues. Gastroenterology 155 479–489 e477. [DOI] [PubMed] [Google Scholar]
- Rena G, Hardie DG & Pearson ER 2017. The mechanisms of action of metformin. Diabetologia 60 1577–1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rezzonico J, Rezzonico M, Pusiol E, Pitoia F & Niepomniszcze H 2011. Metformin treatment for small benign thyroid nodules in patients with insulin resistance. Metab Syndr Relat Disord 9 69–75. [DOI] [PubMed] [Google Scholar]
- Rezzonico JN, Rezzonico M, Pusiol E, Pitoia F & Niepomniszcze H 2009. Increased prevalence of insulin resistance in patients with differentiated thyroid carcinoma. Metab Syndr Relat Disord 7 375–380. [DOI] [PubMed] [Google Scholar]
- Saini N & Yang X 2018. Metformin as an anti-cancer agent: actions and mechanisms targeting cancer stem cells. Acta Biochim Biophys Sin (Shanghai) 50 133–143. [DOI] [PubMed] [Google Scholar]
- Saini S, Tulla K, Maker AV, Burman KD & Prabhakar BS 2018. Therapeutic advances in anaplastic thyroid cancer: a current perspective. Mol Cancer 17 154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidbauer B, Menhart K, Hellwig D & Grosse J 2017. Differentiated Thyroid Cancer-Treatment: State of the Art. Int J Mol Sci 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulten HJ 2018. Pleiotropic Effects of Metformin on Cancer. Int J Mol Sci 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma M, Aronow WS, Patel L, Gandhi K & Desai H 2011. Hyperthyroidism. Med Sci Monit 17 RA85–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen CT, Wei WJ, Qiu ZL, Song HJ, Zhang XY, Sun ZK & Luo QY 2017. Metformin reduces glycometabolism of papillary thyroid carcinoma in vitro and in vivo. J Mol Endocrinol 58 15–23. [DOI] [PubMed] [Google Scholar]
- Song R 2016. Mechanism of Metformin: A Tale of Two Sites. Diabetes Care 39 187–189. [DOI] [PubMed] [Google Scholar]
- Sosnicki S, Kapral M & Weglarz L 2016. Molecular targets of metformin antitumor action. Pharmacol Rep 68 918–925. [DOI] [PubMed] [Google Scholar]
- Spillane S, Bennett K, Sharp L & Barron TI 2013. A cohort study of metformin exposure and survival in patients with stage I-III colorectal cancer. Cancer Epidemiol Biomarkers Prev 22 1364–1373. [DOI] [PubMed] [Google Scholar]
- Subbiah V, Kreitman RJ, Wainberg ZA, Cho JY, Schellens JHM, Soria JC, Wen PY, Zielinski C, Cabanillas ME, Urbanowitz G, et al. 2018. Dabrafenib and Trametinib Treatment in Patients With Locally Advanced or Metastatic BRAF V600-Mutant Anaplastic Thyroid Cancer. J Clin Oncol 36 7–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sui M, Yu Y, Zhang H, Di H, Liu C & Fan Y 2018. Efficacy of Metformin for Benign Thyroid Nodules in Subjects With Insulin Resistance: A Systematic Review and Meta-Analysis. Front Endocrinol (Lausanne) 9 494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka R, Tsushima T, Murakami H, Shizume K & Obara T 1994. Insulin-like growth factor I receptors and insulin-like growth factor-binding proteins in human parathyroid tumors. World J Surg 18 635–641; discussion 641–632. [DOI] [PubMed] [Google Scholar]
- Tang Y, Yan T, Wang G, Chen Y, Zhu Y, Jiang Z, Yang M, Li C, Li Z, Yu P, et al. 2017 Correlation between Insulin Resistance and Thyroid Nodule in Type 2 Diabetes Mellitus. Int J Endocrinol 2017. 1617458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thakker RV 2016. Genetics of parathyroid tumours. J Intern Med 280 574–583. [DOI] [PubMed] [Google Scholar]
- Thakur S, Daley B, Gaskins K, Vasko VV, Boufraqech M, Patel D, Sourbier C, Reece J, Cheng SY, Kebebew E, et al. 2018. Metformin Targets Mitochondrial Glycerophosphate Dehydrogenase to Control Rate of Oxidative Phosphorylation and Growth of Thyroid Cancer In Vitro and In Vivo. Clin Cancer Res 24 4030–4043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theodros D, Patel M, Ruzevick J, Lim M & Bettegowda C 2015. Pituitary adenomas: historical perspective, surgical management and future directions. CNS Oncol 4 411–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tseng CH 2014. Metformin reduces thyroid cancer risk in Taiwanese patients with type 2 diabetes. PLoS One 9 e109852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vancura A, Bu P, Bhagwat M, Zeng J & Vancurova I 2018. Metformin as an Anticancer Agent. Trends Pharmacol Sci 39 867–878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vazquez-Borrego MC, Fuentes-Fayos AC, Herrera Martinez AD, F LL, Ibanez-Costa A, Moreno Moreno P, Alhambra-Exposito MR, Barrera-Martin A, Blanco-Acevedo C, Dios E, et al. 2019. Biguanides exert antitumoral actions in pituitary tumor cells through AMPK-dependent and -independent mechanisms. J Clin Endocrinol Metab. [DOI] [PubMed] [Google Scholar]
- Vella V, Sciacca L, Pandini G, Mineo R, Squatrito S, Vigneri R & Belfiore A 2001. The IGF system in thyroid cancer: new concepts. Mol Pathol 54 121–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vigneri P, Frasca F, Sciacca L, Pandini G & Vigneri R 2009. Diabetes and cancer. Endocr Relat Cancer 16 1103–1123. [DOI] [PubMed] [Google Scholar]
- Vlotides G, Tanyeri A, Spampatti M, Zitzmann K, Chourdakis M, Spttl C, Maurer J, Nolting S, Goke B & Auernhammer CJ 2014. Anticancer effects of metformin on neuroendocrine tumor cells in vitro. Hormones (Athens) 13 498–508. [DOI] [PubMed] [Google Scholar]
- Yin M, Zhou J, Gorak EJ & Quddus F 2013. Metformin is associated with survival benefit in cancer patients with concurrent type 2 diabetes: a systematic review and meta-analysis. Oncologist 18 1248–1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin X, Dewille JW & Hai T 2008. A potential dichotomous role of ATF3, an adaptive-response gene, in cancer development. Oncogene 27 2118–2127. [DOI] [PubMed] [Google Scholar]
- Young K, Iyer R, Morganstein D, Chau I, Cunningham D & Starling N 2015. Pancreatic neuroendocrine tumors: a review. Future Oncol 11 853–864. [DOI] [PubMed] [Google Scholar]
- Zakikhani M, Blouin MJ, Piura E & Pollak MN 2010. Metformin and rapamycin have distinct effects on the AKT pathway and proliferation in breast cancer cells. Breast Cancer Res Treat 123 271–279. [DOI] [PubMed] [Google Scholar]
- Zhang Y & Nose V 2011. Endocrine tumors as part of inherited tumor syndromes. Adv Anat Pathol 18 206–218. [DOI] [PubMed] [Google Scholar]
- Zhou J, Massey S, Story D & Li L 2018. Metformin: An Old Drug with New Applications. Int J Mol Sci 19. [DOI] [PMC free article] [PubMed] [Google Scholar]