Abstract
SOX2 is essential for maintaining neurosensory stem cell properties, although its involvement in the early neurosensory development of cranial placodes remains unclear. To address this, we used Foxg1-Cre to conditionally delete Sox2 during eye, ear, and olfactory placode development. Foxg1-Cre mediated early deletion of Sox2 eradicates all olfactory placode development, and disrupts retinal development and invagination of the lens placode. In contrast to the lens and olfactory placodes, the ear placode invaginates and delaminates NEUROD1 positive neurons. Furthermore, we show that SOX2 is not necessary for early ear neurogenesis, since the early inner ear ganglion is formed with near normal central projections to the hindbrain and peripheral projections to the undifferentiated sensory epithelia of E11.5–12.5 ears. However, later stages of ear neurosensory development, in particular, the late forming auditory system, critically depend on the presence of SOX2. Our data establish distinct differences for SOX2 requirements among placodal sensory organs with similarities between olfactory and lens but not ear placode development, consistent with the unique neurosensory development and molecular properties of the ear.
Keywords: placode development, neuronal projections, inner ear, eye, olfactory system
1. Introduction
SOX2 is necessary for embryonic development, in particular the brain and various sensory organs (Kondoh and Lovell-Badge, 2015), including the olfactory (Panaliappan et al., 2018), visual (Taranova et al., 2006), and auditory systems (Dvorakova et al., 2016; Kempfle et al., 2016; Kiernan et al., 2005; Puligilla et al., 2010; Steevens et al., 2017). SOX2 acts by maintaining an undifferentiated pro-neurosensory cell state that allows for proliferation in various developmental systems (Hagey et al., 2018; Kageyama et al., 2019; Reiprich and Wegner, 2015; Wegner, 1999). In the ear, precursors with the ability to proliferate and differentiate as hair cells or neurons express SOX2 (Kempfle et al., 2016; Martinez-Monedero et al., 2008). SOX2 is needed to maintain hair cell precursors but is downregulated in differentiating hair cells upon upregulation of the basic Helix-Loop-Helix (bHLH) transcription factor ATOH1 (Dabdoub et al., 2008; Kiernan et al., 2005; Nishimura et al., 2017). In analogy to its role in brain development (Kageyama et al., 2019), it has been proposed that SOX2 is necessary in neuronal development for the upregulation of the neuronal bHLH genes Neurogenin1 [Neurog1; (Ma et al., 2000; Ma et al., 1998)], Neurod1 (Jahan et al., 2010; Kim et al., 2001; Liu et al., 2000), and possibly the Pou domain factor Pou4f1 downstream of NEUROD1 (Huang et al., 2001). It was suggested that SOX2 needs to be downregulated by these bHLH factors for the progression of neurosensory development (Dabdoub et al., 2008; Evsen et al., 2013). The notion that SOX2 is essential for neurogenesis of the inner ear was further investigated by overexpressing Sox2 from a constitutive β-actin promoter (Evsen et al., 2013) and by using nonsensory cells transfected with Sox2.nucEGFP in cochlear explants (Puligilla et al., 2010) to show the induction of the expression of the proneural gene, Neurog1. Additional compelling evidence exists that delayed deletion of Sox2 results in truncated neurogenesis in the cochlea (Dvorakova et al., 2016; Kempfle et al., 2016). Similarly, lack of Sox2 expression in Lcc mutants [light coat and circling; a mutant with an X-ray-irradiation-induced mutation in an enhancer of Sox2 expression in the developing inner ear] (Kiernan et al., 2005) has been correlated with a late absence of neurons at E15.5 (Puligilla et al., 2010), but no data on earlier stages were shown. More recent data show that Sox2-CreER can be used to eliminate Sox2 and block neurogenesis in inner ear development (Steevens et al., 2017).
To go beyond existing data and reveal the dependency of early neuronal development on the otocyst-expressed SOX2 protein, we chose Foxg1-Cre mutant mice that have been demonstrated to lead to earlier and more profound recombination compared to other cre lines expressed in the developing ear (Duncan and Fritzsch, 2013; Kersigo et al., 2011). Tissue-specific recombination has been shown at the 5–6-somite stage in the prospective otic ectoderm of Foxg1-Cre embryos (Barrionuevo et al., 2008). Foxg1-Cre is exclusively expressed in the ear, the olfactory placode, the forebrain and anterior part of the eye (Dastidar et al., 2011; Duggan et al., 2008; Garaffo et al., 2015; Hwang et al., 2009; Kawauchi et al., 2009; Pauley et al., 2006; Shen et al., 2018). Foxg1-Cre has been effectively used to study olfactory placode development that shows a loss of olfactory differentiation and olfactory neurogenesis following Foxg1-Cre mediated Sox2f/f deletion (Panaliappan et al., 2018). We confirm and extend these previous findings on effective recombination of Sox2 using Foxg1-Cre in olfactory development. Additionally, we show for the first time that lens development is nearly equally affected by Sox2 deletion. In contrast to the massive effects on neurosensory development in the olfactory system, ear placode invagination continues without SOX2 protein and proceeds to generate vestibular neurons with a transient near-normal development of central and peripheral processes. However, without SOX2 protein, no inner ear sensory epithelia develop and all early formed neurons perish when they become dependent on the neurotrophic support of sensory epithelia. Our data confirm that late forming spiral ganglion neurons depend on SOX2+ sensory epithelia and never fully develop, as previously suggested (Dvorakova et al., 2016; Kempfle et al., 2016).
2. Material and methods
2.1. Experimental animals
This study was performed in agreement with the Guide for the Care and Use of Laboratory Animals (National Research Council. Washington, DC. The National Academies Press, 1996.). The experimental design was approved by the Animal Care and Use Committee of the Institute of Molecular Genetics, Czech Academy of Sciences. The experimental mice were housed in a controlled environment (12-h light - 12-h dark cycles) with free access to food and water. All experiments were performed with the littermates (males and females) cross-bred from two transgenic mouse lines: Sox2flox (Sox2tm1.1Lan/J; stock #013093) and Foxg1-Cre (129(Cg)-Foxg1tm1(cre)Skm/J, stock #004337) from The Jackson Laboratory, maintained on B6/129 background. The Sox2-deficient embryos (Sox2cKO) were generated by crossing Sox2f/f to Foxg1-Cre, Sox2+/f. Heterozygous (Foxg1-Cre;Sox2+/f) animals were viable, born in appropriate Mendelian ratios, and were phenotypically indistinguishable from control mice (Sox2f/f or Sox2+/f). To determine the efficiency of CRE-mediated recombination, we used reporter Ai9 mice (RCL-tdTomato, stock #007909 Jackson Laboratory, provided by co-author M. Anderova). For genotyping, DNA was isolated from mouse tail and PCR was performed with specific primers for Cre and Sox2 as described previously (Dvorakova et al., 2016). Phenotyping and data analysis were performed by investigators who were blinded to the genotypes of the mice.
2.2. 3D reconstruction of the inner ear structure
3D-reconstruction of the inner ear was done as previously described (Dvorakova et al., 2016; Kopecky et al., 2012). Briefly, inner ears were dehydrated in ethanol series and stained with 0.5 μg/ml Rhodamine B isothiocyanate in 100% ethanol. Samples were then cleared by MSBB solution and mounted onto a glass slide prior to imaging. Images were taken by a Zeiss LSM 880 confocal microscope and processed in ImageJ. 3D structures of inner ears were reconstructed from confocal stacks in Amira software (ThermoFisher Scientific) by segmenting areas of interest.
2.3. Immunohistochemistry and morphological evaluations
Embryos or dissected ears were fixed in 4% paraformaldehyde (PFA) in 1x Phosphate Buffered Saline, pH 7.4 (PBS). For vibratome sections, dissected tissues were embedded in 4% agarose gel and sectioned at 80 μm on a Leica VT1000S vibratome. The primary antibodies used were: rabbit anti-NeuN (ab177487, Abcam, 1:500), rabbit anti-Ki67 (9129, Cell Signalling, 1:400), mouse anti-Isl1 (39.3F7, Developmental Hybridoma Bank,1:130), goat anti-Neurod1 (sc-1084, Santa Cruz Biotechnology, 1:100), rabbit anti-Cre (908001, BioLegend, 1:500), goat anti-Sox2 (sc-17320, Santa Cruz Biotechnology, 1:250), rabbit anti-Sox2 (AB5603, Millipore, 1:500), rabbit anti-Sox10 (ab155279, Abcam, 1:250), rabbit anti-cleaved Caspase-3 (9661, Cell Signaling Technology, 1:100), goat anti-GATA3 (AF2605, R&D Systems, 1:20), rabbit anti-Pax2 (PRB-276P, Covance, 1:100), goat anti-TrkC (AF1404, R&D Systems, 1:100), anti-Neurofilament 200 (N4142, Sigma-Aldrich,1:200), and mouse anti-acetylated α Tubulin (T6793, Sigma-Aldrich,1:400). The anti-Isl1 antibody, developed by T.M. Jessell and S. Brenner-Morton, was obtained from the NICHD Developmental Studies Hybridoma Bank at the University of Iowa. The secondary antibodies were used in dilution 1:400: Alexa Fluor 488 AffiniPure Goat Anti-Mouse (Jackson ImmunoResearch 115-545-146), Alexa Fluor 594 AffiniPure Goat Anti-Rabbit (Jackson ImmunoResearch 111-585-144), DyLight488-conjugated AffiniPure Mouse Anti-Goat (Jackson ImmunoResearch 205-485-108) and DyLight594-conjugated AffiniPure Mouse Anti-Goat (Jackson ImmunoResearch 205-515-108) The nuclei were counterstained with Hoechst 33342 (861405, Sigma-Aldrich, 1:2000). Image acquisition was completed using the Zeiss LSM 880 NLO scanning confocal with ZEN lite program. For neuron quantification, ISL1+ neurons were counted in all ISL1/SOX2 immunolabeled vibratome sections containing the vestibular ganglion, using the “Cell Counter” ImageJ plugin (n = 4 embryos/genotype and 2 ganglia/embryo), as described previously (Chumak et al., 2016). The VG area was determined using ImageJ. For statistics, multiple t-test with the Holm-Sidak method was used (GraphPad Prism). Caspase3+ areas were assessed using the thresholding tool in ImageJ. The Mann–Whitney U test was used, since Gaussian distribution could not be assumed (GraphPad Prism).
2.4. EdU and BrdU labeling
The noon of the day when a vaginal plug was found was determined as embryonic day 0.5. 10 μg/g EdU and 50 μg/g BrdU in PBS was intraperitoneally injected into pregnant mice at E9.5 and E10.5, respectively. Embryos were collected at E11.5 and fixed in 4% PFA for 1 hour. Transverse 80 μm sections were prepared on a Leica VT1000S vibratome. Proliferated cells were stained using Click-iT EdU Alexa Fluor 647 Imaging Kit (C10340, ThermoFisher) and mouse anti-BrdU antibody (B2531, Sigma-Aldrich, 1:1000).
2.5. Lipophilic dye tracing
The pattern of innervation was evaluated in whole ears and brains using lipophilic dye tracing in 10% PFA with 0.3M sucrose fixed tissue as previously described (Fritzsch et al., 2016a). At least three mutants and similar numbers of control littermates were used for each stage examined (E11.5, E12.5, and E18.5). For dye application, heads were split along the midline to allow insertion of filter strips soaked with lipophilic dyes into specific areas of the brain or the ear. We inserted filter strips loaded with different colored lipophilic dyes into the trigeminal ganglion (to label the trigeminal nerve), into the jugular foramen of IX/X nerve (to label IX-XI nerves), into the vestibular nuclei of the brainstem around rhombomere 5 (to label vestibular afferents), into the alar plate of rhombomere 5/6 (to label intermediate nerve fibers and vestibular/cochlear afferents), into the basal plate of rhombomere 4 (to label vestibular afferents and vestibular/cochlear efferents), into rhombomere 4 near the midline (to label the facial nerve/vestibular and cochlear efferents), into the cochlear duct (to label cochlear afferents), and into vestibular end organs (to label vestibular afferents), as detailed in (Maklad et al., 2010; Simmons et al., 2011). Diagrams illustrating the location of the dye insertions are in Fig. 12. After diffusion of the lipophilic tracer from insertion to target, we prepared the ears or brains as whole mounts, mounted with glycerol on a glass slide, using appropriate spacers to avoid distortion, and imaged them using a Leica SP8 confocal microscope. We always checked the injection site to ensure that only central or peripheral projections were analyzed with proper insertions of the filter strips. Whole mounted brains and ears were imaged within an hour to avoid blurring of afferents due to the washing out effects of lipid and lipophilic dyes. Image stacks were collected and single images or sets of stacks were obtained to provide detailed information about the progressive development and loss of ear innervation over time. Images were compiled into plates to show the most pertinent details using Corel Draw.
Fig. 12. Schematic of the morphological ear defects and central projection changes in Sox2cKO.
In control mice (right), the inner ear is formed by a complex labyrinth of ducts and recesses with six sensory epithelia, five vestibular: the maculae of the utricle (U) and saccule (S), and the cristae of the three semicircular canal ampullae (PC, AC, HC), and the auditory sensory organ of the cochlea, the organ of Corti (OC). Neurons of two distinct vestibular ganglia (VG, green) and spiral ganglia (SG, red) form afferent projections from the ear to the brain. The ear is also innervated by the inner ear efferents (IEE, lilac) that end on the sensory cells. In Sox2cKO, the deletion of Sox2 results in inner ear dysmorphology with cochlear agenesis, a rudimental saccule, and with all other structures being unrecognizable (left) at E14.5. The absence of SOX2 does not affect the initial formation of the vestibular ganglion and neurite navigation to their central targets, the vestibular nuclei and cerebellum, (green line). Similarly, dye tracing from the trigeminal ganglion (magenta), and from the jugular foramen of the IX/X nerve (blue) show comparable central projections in control and Sox2cKO mutant littermates at E11.5 and E12.5. In contrast to control embryos, no afferent innervation (red) could be traced from the cochlea to the cochlear nucleus in Sox2cKO. The red dot in the mutant shows the possible transient formation of some SG neurons. Black arrowheads indicate applications of lipophilic fluorescent dyes. Trigeminal (V), vestibulocochlear (VIII), glossopharyngeal (IX), vagal (X) cranial nerves; AC, anterior canal crista; CC, semicircular canal; CB, cerebellum; CD, cochlear duct; ED, endolymphatic duct; HC, horizontal canal crista; PC, posterior canal crista; r2, r4, r6, rhombomere 2, 4, 6; S, saccule; SG, spiral ganglion; U, utricle.
2.6. Alazarin Red-Alcian Blue staining
Staining of skeletal preparations was performed with alizarin red and alcian blue as previously described (McLeod, 1980). Briefly, the heads of E17.5 embryos were skinned, eviscerated, and fixed for 1h in 4% PFA and stored in 100% methanol. Samples were defatted for 2–3 days in acetone, and stained sequentially with Alcian blue and alizarin red S in 2% KOH. The stained skeleton preparations were cleared with 1% KOH, 20% glycerol, and stored in 50% ethanol/50% glycerol.
3. Results
3.1. SOX2 elimination in the lens, olfactory, and ear placodes impairs early eye and olfactory development, whereas early inner ear development is not affected.
The Sox2-deficient embryos (Sox2cKO) were generated by crossing Sox2f/f to Foxg1-Cre. Heterozygous (Sox2+/f; Foxg1-Cre) animals were viable, born in appropriate Mendelian ratios, and were phenotypically indistinguishable from control mice (Sox2f/f or Sox2+/f; Fig. S1A–F). Thus, the decreased gene dose of Sox2 in heterozygous mutant mice did not affect normal development. However, homozygous Sox2 deletion (Sox2f/f; Foxg1-Cre) was lethal at birth, thus preventing analysis of these mutants at postnatal stages. Analysis of the gross external morphology of Sox2cKO embryos showed an abnormal eye phenotype, a shortened snout without olfactory pits, and flattened forehead (Fig. S1G–I).
First, to determine the relative onset of Sox2 deletion in Sox2-deficient embryos, we examined early SOX2 protein expression at pre-placodal lens stages, in the eye vesicle, and ear placode at the 11-somite stage (E8.5). Sox2cKO mice that showed a detectable Cre protein level showed no detectable SOX2 protein in the invaginating ear placode and in the presumptive lens ectoderm (Fig. 1D, F). Note that SOX2 protein was only eliminated in some cells of the eye vesicle in Sox2cKO consistent with the regional expression of Cre recombinase under Foxg1 control (Fig. 1F). At E9.0, SOX2 was detected in the temporal domain of the eye vesicle, but was completely missing in the otocyst, the epithelium of the olfactory and pre-lens placodes, and the nasal half of the eye vesicle in Sox2cKO mice (Fig. 2). TdTomato reporter expression, reflecting the pattern of Foxg1-Cre activity, correlated with Sox2 deletion, suggesting complete topologically restricted deletion of the Sox2 gene in areas of early Foxg1-Cre expression (Fig. 2C, F, I). These data indicate that Foxg1-Cre effectively recombines Sox2f/f during the early stages of olfactory, lens/eye and ear development consistent with previous reports of early effective recombination of the transcription factor Gata3 (Duncan and Fritzsch, 2013).
Fig. 1. SOX2 is eliminated in the ear and pre-lens placodes in early Sox2cKO embryos.
Whole mount analysis of SOX2 expression pattern in E8.5 control, heterozygous Foxg1-Cre;Sox2+/f, and Sox2cKO embryos (11 somites), displayed in a dorsal view (control n = 9, Sox2cKO n = 5, Foxg1-Cre;Sox2+/f n = 5). Foxg1-Cre expression is visualized by using anti-Cre antibody in heterozygous Foxg1-Cre;Sox2+/f, and Sox2cKO embryos (C-F). Split channel images show SOX2 (C’-F’) and Cre immunolabeling (C”-F”). The dotted oval indicates the area of the otic pit (A, C-C”, D-D”). SOX2 expression is lost in the Sox2cKO ear invaginating placode (D, D’) compared to control (A), and heterozygous Foxg1-Cre;Sox2+/f embryos (C, C’). In the developing eye, SOX2 is eliminated in the presumptive lens ectoderm (PLE) and decreased SOX2 levels are evident in many cells of the optic vesicle (OV) in Sox2cKO (F, F’) compared to control embryos (B) and Foxg1-Cre;Sox2+/f (E, E’). Note Foxg1-Cre expression in the surface ectoderm in the area of the PLE (E, F, E”, F”). HS, Hoechst nuclear staining. Scale bars: 50 μm.
Fig. 2. Complete SOX2 deletion in the otocyst, pre-lens and olfactory placodes, and in the nasal part of the eye vesicle mirrors the tdTomato reporter pattern for Foxg1-cre at E9.0 embryos (18 somites).
(A, D, G) In the control embryo, SOX2 is expressed in the neural tube and olfactory placode (op, arrowhead in A, D), eye vesicle prior to eye cup formation as well as the pre-lens placode (p-lp, arrowhead in D), and ear vesicle (G). (B-I) In Sox2cKO, SOX2 protein is detectable in the neural tube (B), and in the temporal domain of the eye vesicle (dotted area indicates the nasal domain of the eye vesicle in E, F), and it is completely missing in the epithelium of the olfactory placode (B), pre-lens placode (E) and the ear epithelium (H). A reporter of Foxg1-cre expression, tdTomato, indicates that Foxg1-cre expression matches the SOX2 deletion pattern in Sox2cKO (C, F, I). Note the few tdTomato positive cells anteroventral to the ear (I). HS, Hoechst nuclear staining. Scale bars: A-C 200 μm; D-I 50 μm.
Olfactory placode effects:
As reported previously in the same Sox2cKO mutant (Sox2f/f; Foxg1-Cre) (Panaliappan et al., 2018), we confirmed that an early loss of SOX2 in the olfactory placode and the telencephalon affects olfactory development, as the entire neuronal lineage as well as olfactory placode invagination is abolished in Sox2cKO compared to control littermates (Fig. 3A–D). Thus, Sox2cKO mice show no morphological structure of the olfactory epithelium formation nor olfactory pit development, resulting in a shortened snout, flattened forehead, and absence of nasal bones (Fig. 3A–D, and Fig. S1G–I, L) that critically depends on ingrowing olfactory fibers for its formation (Fritzsch et al., 2019). Note that the facial whiskers were present on the snout, although the distribution of the follicles was altered in Sox2cKO compared to the littermate control, possibly due to abnormalities in the size of the snout and secondary effects on whisker patterning (Fig. S1J, K). Previous work has already demonstrated lack of the neurogenic marker expression, Neurog1 and Neurod1, in this Sox2cKO model (Panaliappan et al., 2018), which we confirmed (data not shown).
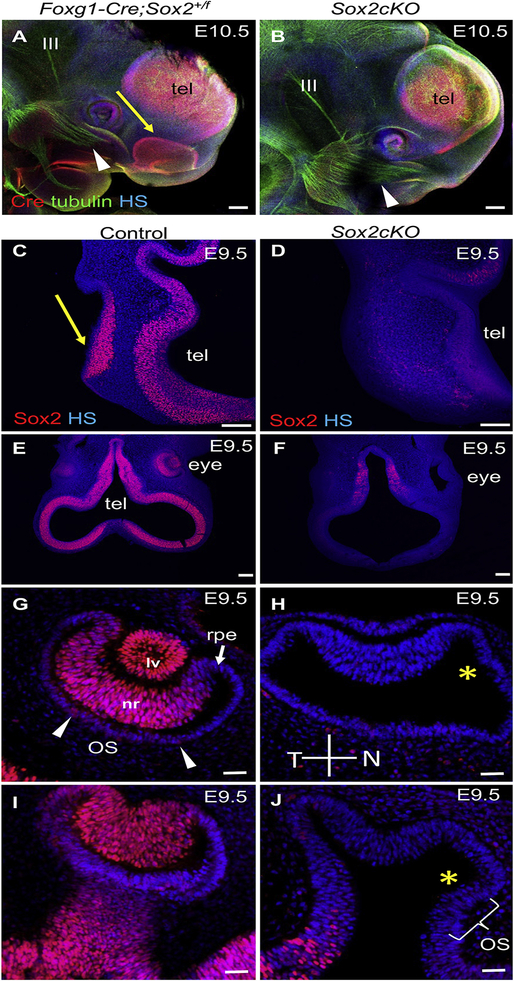
Fig. 3. The olfactory and lens placodes fail to invaginate in Sox2cKO.
(A, B) Whole-mount immunostaining shows the Cre expression pattern and innervation (tubulin) at E10.5. The Foxg1-Cre+ domain of the olfactory epithelium in Sox2cKO is missing (yellow arrow indicates the olfactory epithelium in Foxg1-Cre;Sox2+/f). The snout has an abnormal shape and a smaller eye with missing lens vesicle is apparent in Sox2cKO. Note, neuronal fibers from the trigeminal ganglion (arrowhead) innervate the presumptive olfactory area in Sox2cKO similar to controls, and the oculomotor nerve (III) extends from the midbrain in both control and mutant mice. (C, D) Formation of the olfactory placode with SOX2 expression is indicated in cross-sections by arrow in E9.5 controls (n = 2; arrow indicates the olfactory epithelium). In Sox2cKO, no SOX2 expression is detected in the area of presumptive olfactory placode (n = 2). Note the reduced SOX2 expression in the telencephalon (tel) of Sox2cKO (D), consistent with Foxg1-Cre expression in the telencephalon in (A, B). (E, F) Cross-sections show that SOX2 is undetectable in the developing Sox2cKO eye compared to the control at E9.5. (G-J) Higher magnification images show SOX2 expression in the neural retina (nr), lens vesicle (lv), and in the ventral area of the eye cup in the optic stalk (OS, arrowheads) in control embryos, whereas in Sox2cKO, no SOX2 expression is detectable in the eye. Note the failure of invagination of the lens placode to form the lens vesicle in Sox2cKO, the decreased number of neural retina cells, and lack of the U-shape formation of the optic cup with a large gap remaining between the neural retina and the retinal pigment epithelium (yellow asterisk in H, J). HS, Hoechst nuclear staining; N, nasal; T, temporal; tel, telencephalon vesicle; rpe, retinal pigment epithelium. Scale bars: A, B 200 μm; C, D, E, F 100 μm; G-J 50 μm
Eye development:
Conditional deletion of Sox2 in the lens placode prevented lens formation and altered eye development, in part due to loss of SOX2 in the nasal half of the retina (Fig. 2D–F). At E9.5, no SOX2 expression was detected in the retina and was significantly reduced in the telencephalon of Sox2cKO compared to the high levels of SOX2 in the lens vesicle and neural retina in the developing eye of control littermates (Fig. 3E–J). A noticeable failure of invagination of the lens placode to form the lens vesicle and abnormalities in the formation of the optic cup were observed in Sox2cKO at E9.5. By E11.5, Sox2cKO in comparison to heterozygous Sox2cKO or control littermates was missing a lens and displayed a reduced retina and overall reduced ocular size (Fig. 4A–C). Histological analyses at E14.5 and E17.5 revealed severe ocular deformities, including extremely reduced eye size and enlarged space between the neural retina and the retinal pigment cells in Sox2cKO (Fig. 4D–I). Note that extraocular muscles were present in the Sox2cKO mutant at E14.5 (arrows in Fig. 4F) and we could trace their innervation in whole mounted dye tracing (Fig. S2).
Fig. 4. Deletion of Sox2 results in a complete loss of lens formation and abnormal eye development.
(A, B) In E11.5 controls and heterozygous Sox2 mutants (Foxg1-Cre;Sox2+/f), the lens vesicle (lv), optic stalk (OS) and the two-layered optic cup with an outer layer of retinal pigment epithelium (rpe) and the inner neural retina (asterisk) are formed. (C) The Sox2cKO developing eye appears to be smaller without any detectable lens vesicle, with a reduced neural retina domain, and abnormal formation of the optic stalk at E11.5. (D-I) At E14.5 and E17.5, the size of the optic cup is significantly reduced without the optic lens in Sox2cKO compared to controls and heterozygous Sox2 mutants. The retinal pigment epithelium and neural retina are present, although significantly diminished in Sox2cKO (n = 3 per genotype and time point). Note the presence of extraocular muscles in the Sox2cKO mutant at E14.5 (arrows in F). tel, telencephalon. Scale bars: A-C 200 μm; D-I 100 μm.
Ear development:
Surprisingly, elimination of SOX2 in the ear placode (Fig.1D) did not affect ear vesicle formation, the delamination of neuroblasts from SOX2 negative ear epithelium, and the formation of the vestibular ganglion (VG) at E9.5 (Fig. 5A–D). In contrast to controls (Fig. 5A–A”), SOX2 was not expressed in ISL1+ neurons and all SOX2+ cells co-expressed SOX10 in Sox2cKO (Fig. 5B–B”), which is consistent with glial cell molecular characteristics. Delaminating NEUROD1+ neuroblasts were detected in the ear epithelium of Sox2cKO and were indistinguishable from control littermates (arrowheads Fig. 5C, D). Likewise, at E10.5, the number of ISL1+ neurons in the area of the VG were comparable between Sox2cKO and control littermates (Fig. 5E–G), suggesting that overall, early neurogenesis in the inner ear is not affected by SOX2 elimination at the level of the ear placode (Fig.1A, D). Although the number of neurons in the VG of Sox2cKO is not affected, neurite outgrowth towards the epithelium of the otocyst appeared somewhat reduced at E10.5, indicating abnormalities in inner ear development (Fig. 5I, J).
Fig. 5. Delamination of NEUROD1+ neuroblasts from the SOX2-deficient epithelium and formation of the vestibular ganglion in Sox2cKO is comparable to control embryos.
(A, B) In E9.5 controls, SOX2 is expressed in the otocyst (ot) and in developing neurons of the vestibular ganglion (VG), whereas SOX2 is not detectable in the ear epithelium or neurons in Sox2cKO (n = 5 embryos per genotype). The boundaries of the Sox2cKO ear vesicle (otocyst) are delineated by yellow lines (B). Note, in Sox2cKO, SOX2 is not expressed in ISL1+ VG neurons and all SOX2+ cells co-express SOX10, consistent with glial cell molecular characteristics. Split channel images show SOX2 (A’, B’) and SOX10 immunolabeling (A”, B”). (C, D) NEUROD1+ neuroblasts (arrowheads) delaminate from the SOX2+ otic epithelium in controls and SOX2 negative epithelium in Sox2cKO. (E-G) The quantification of neurons in the VG area show no significant differences between control and Sox2cKO embryos at E10.5 (ISL1+ neurons per 1000 μm2; mean ± s.d.; n = 4 embryos per genotype and 2 ganglia per embryo, multiple t test with the Holm-Sidak method, P = NS). Dotted lines indicate the boundaries of the VG in A-A”, B-B”, E, F. (H) Plane of sections A-F is displayed. (I, J) Whole-mount immunostaining with anti-acetylated α-tubulin (nerve fibers) and anti-CRE shows apparently diminished innervation of the ear epithelium in Sox2cKO compared to littermate heterozygous Foxg1-Cre;Sox2+/f (arrowheads), indicating abnormalities in inner ear development at E10.5 (n = 3 embryos per genotype). (K, L) Immunostaining for the proliferating nuclear antigen Ki67 shows proliferating ISL1+ neurons in the VG of Sox2cKO and control embryos at E9.5 (n = 2 embryos per genotype). (M, N) The proliferation pattern evaluated by double-immunolabeling of EdU (injected at E9.5) and BrdU (injected at E10.5) shows an overall similar proliferation pattern in the VG between control and Sox2cKO littermates at E11.5, although decreased BrdU-labeling (green) in the VG of Sox2cKO is apparent (n = 2 embryos per genotype). GG, geniculate ganglion; HS, Hoechst nuclear staining; ot, otocyst. Scale bars: 50 μm; M, N 100 μm.
3.2. Neuronal development is disrupted by massive apoptosis in the inner ear of Sox2cKO.
We next assessed the changes in cell proliferation by examining the expression patterns of cell cycle markers. Ki67 is a nuclear protein present in proliferating cells (in G1, S, G2 and M phase). The proliferation of VG neurons was unaffected at E9.5, as shown by Ki67 immunolabeling (Fig. 5K, L). To investigate cell cycle changes, we examined BrdU and EdU incorporation in S phase. Consistent with the Ki67 results suggesting that mitotic activity was maintain in VG neurons, the proliferation pattern in the VG was similar between control and Sox2cKO littermates, as assessed by EdU (injected at E9.5) and BrdU (injected at E10.5) labeling (Fig. 5M, N). However, the number of BrdU-labeled cells in the VG of Sox2cKO is decreased compared to controls at E11.5. Immunostaining for cleaved caspase 3 revealed significantly increased apoptosis in the VG of Sox2cKO compared to controls at E11.5 (Fig. 6A, B, in detail A’, B’, and C). Apoptosis seemingly results in a rapid reduction in size of the VG in mutant mice at E12.5, as shown by labeling with anti-NeuN, a marker for differentiated neurons (Fig. 6D, E).
Fig. 6. Neuronal development is arrested by apoptosis in Sox2cKO vestibular ganglion neurons at E11.5.
(A, B) Cleaved caspase-3 whole-mount immunostaining shows significantly increased apoptosis in the mutant vestibular ganglion (VG, demarcated by dotted line) compared to controls. Note the drastically reduced innervation of the spiral ganglion (SG, arrowhead) in Sox2cKO compared to control. A’, B’ display higher magnification images of the VG. (C) Cleaved caspase-3 immunostaining was quantified using ImageJ in the VG of E11.5 control (n = 6) and Sox2cKO embryos (n = 5). **P = 0.0043, Mann-Whitney U test. The data are presented as mean ± s.d. (D, E) Whole-mount immunostaining with anti-NeuN, a neuronal soma marker, shows a diminished VG domain at E12.5. GG, geniculate ganglion; HS, Hoechst nuclear staining; PC, posterior crista. Scale bars: 100 μm, 50 μm for A’ and B‘.
In contrast to the VG, the spiral ganglion in Sox2cKO mice was represented by a few neurons expressing the zinc finger transcription factor GATA3 (Fig. 7A, B). At E12.5, GATA3 is highly expressed in the pro-sensory domain of the cochlear duct and differentiating spiral ganglion neurons (Karis et al., 2001; Luo et al., 2013). Similarly, the Trk protein tyrosine kinase receptor (TrkC) for the nerve growth factor NTF3 was expressed in some neurons of Sox2cKO (Fig. 7E, F), which could also reflect saccular neuron formation (Fariñas et al., 2001; Fritzsch et al., 2016b). Note that the spatial expression domain of GATA3 is enlarged in the Sox2cKO cochlea compared to controls, indicating abnormalities in the establishment of the pro-sensory domain by the elimination of Sox2 (Fig. 7A–D).
Fig. 7. Abnormalities in the specification of the sensory epithelium of the cochlea are associated with a few residual spiral ganglion neurons in the Sox2cKO inner ear.
(A-D) Immunohistochemistry for the transcription factors GATA3 and PAX2 in cross sections reveals abnormalities in the formation of sensory epithelium with a broader expression of GATA3 and a lack of a clearly defined prosensory region in the epithelium of the cochlear duct (CD) in Sox2cKO (n = 3 embryos per genotype). The prosensory domain in the control cochlea is marked by co-expression of Pax2 and GATA3 and is indicated by the arrow in (A). Spiral ganglion (SG) neurons express GATA3 (arrowheads indicate the SG in the control and the residual SG in Sox2cKO). The inserts show GATA3 expression. (E, F) Representative vibratome sections show the expression of TrkC, tyrosine kinase receptor for the nerve growth factor NTF3, in SG neurons. HS, Hoechst nuclear staining. Scale bars: 100 μm.
3.3. Conditional deletion of Sox2 causes severe inner ear dysmorphology.
A computer-assisted 3D-reconstruction revealed profound differences in the morphogenesis of the inner ear between Sox2cKO and control embryos at E14.5 (Fig. 8, Supplementary movie). The Sox2cKO inner ears showed cochlear agenesis and rudimental saccule development, but all other structures were either absent or undiscernible at E14.5. Inner ear malformations in our Sox2cKO mutant were more pronounced compared to the previously described Lcc mutant, which seemingly develops a recognizable cochlear duct (Kiernan et al., 2005). Our data confirm that SOX2 is required for normal morphogenesis of the inner ear and that despite normal initial neuronal development, an early complete ablation of Sox2 in the otocyst results in abnormal morphology of the inner ear. Our results support the emerging idea that SOX2 plays an important role in the development of the non-sensory structures of the inner ear (Gu et al., 2016), and non-sensory growth and morphogenesis of the otocyst (Steevens et al., 2019). It has been suggested that early loss of SOX2 impacts otic epithelial volume and progenitor proliferation and results in non-sensory tissue dysmorphogenesis, including canal truncations and ablations (Steevens et al., 2019).
Fig. 8. Inner ear structural malformations in Sox2cKO are revealed by computer-assisted 3D-reconstruction.
The inner ear of Sox2cKO shows cochlear agenesis and a rudimental saccule but all other structures are missing or undiscernible compared to the control inner ear at E14.5 (n = 2 embryos per genotype). aa, anterior ampulla; asc, anterior semicircular canal; cd, cochlear duct; ed, endolymphatic duct; la, lateral ampulla; lsc, lateral semicircular canal; pa, posterior ampulla; psc, posterior semicircular canal; sac, saccule; ut, utricle. Note the enlargement of the apex.
3.4. Pathfinding and innervation are altered in the inner ear of Sox2cKO.
We next investigated the innervation of the inner ear and formation of central projections in Sox2cKO. We wanted to understand how the absence of SOX2 in all delaminating neurons will affect their neurite navigation to the area they delaminated from as well as to their central targets (Fariñas et al., 2001; Fritzsch et al., 2002; Macova et al., 2019). At E11.5, ear vesicles allow safe insertion of small amounts of lipophilic dye tracer to reveal central projections and to compare them with adjacent cranial nerve projections. At this stage, inner ear vestibular afferents of Sox2cKO (green) were projecting to reach the cerebellum as in littermate control animals (Fig. 9A, B). Closer examination showed that inner ear afferents of Sox2cKO extend as far rostrally and caudally as in control animals but that overall tract size was reduced compared to littermate controls (green, Fig. 9A’–B”’). Dye injections into the vestibular nuclei (red) and facial branchial motor neurons (green) were used to label vestibular afferents and efferents. At E11.5, afferent and efferent fibers reached the area of the posterior canal crista of the vestibular system in both control and Sox2cKO mice (Fig. 9C, D). Together these data show that the initial development of inner ear sensory neurons from E8.5 to E11.5 is surprisingly near-normal, with proper peripheral and central targeting despite the lack of SOX2.
Fig. 9. The central and peripheral projections of the inner ear are comparable between controls and Sox2cKO at E11.5.
(A-B’”) Lateral views of whole-mounted hindbrains show analogous triple-lipophilic dye labeling (see Materials and Methods) from the ear (green), from the trigeminal ganglion (red), and from the jugular foramen of the IX/X nerve (magenta) in the control (A) and Sox2cKO mutant (B; n = 3 embryos per genotype). Vestibular afferents of the VIIIth cranial nerve (green), projecting to reach the cerebellum (CB) and the caudal part of the hindbrain, are comparable between the control (A) and mutant (B). Dye applications into the ear at this stage often in addition to labeling vestibular afferents also label the facial nerve (FN) extending around the developing ear and FN components (FBM, facial branchial motor neurons; IN, intermediate nerve; SSN, superior salivatory nucleus), as shown in control embryo (A). Trigeminal afferents (red) projecting in the descending tract of the trigeminal nerve (dV), glossopharyngeal (IX) and vagal (X) afferents (magenta) of the control are comparable to Sox2cKO afferent projections. (A’-A”’, B’-B”’) Details of vestibular afferent central projections (green) show fewer fibers in Sox2cKO (B’-B’”) compared to the control (A’-A”‘) but at the same time these fibers extend as far rostral (A’, B’) and caudal (A’“, B’”) as afferents in littermate controls. Inner ear projections are somewhat reduced near the VIIIth nerve root in the mutant (arrowheads in A” and B”). (C, D) Insertion of green dye into rhombomere 4 labels the FN and inner ear efferents extending to the posterior canal crista region (PC). Insertion of red colored dye into the vestibular nuclei labels vestibular ganglion neurons and vestibular afferents extending to the region of the PC in control and mutant mice as well as the saccule (S) in control. The same red dye application labeled the PC but not the saccula in Sox2cKO (D). Note that at this stage both inner ear afferents (red) and efferents (green) extend to the region of the PC despite the fact that no PC sensory epithelium ever forms in the Sox2cKO mice. Cranial nerves: V, trigeminal nerve; VII, facial nerve (FN); VIII, vestibulocochlear nerve; IX, glossopharyngeal nerve; X, vagus nerve; XI, accessory nerve; FBM, facial branchial motor neurons; IN, intermediate nerve (component of the facial nerve); SSN, superior salivatory nucleus (the visceromotor component of the facial nerve); VG, vestibular ganglion. Scale bars: 100 μm.
We next investigated how long these VG neurons and their projections remain in Sox2cKO, and whether spiral ganglion neurons also project to their peripheral and central targets. At E12.5, dye-labeling from the ear (green) showed almost identical central projections of VG neurons in control and Sox2cKO littermates (Fig. 10A, B). We next investigated the ability of spiral ganglion neurons to differentiate and reach the hindbrain at E12.5. E12.5 is the earliest embryonic day when it is possible to visualize spiral ganglion neurons and their projections by dye tracing in the mouse (Fritzsch et al., 2015; Karis et al., 2001). Targeted labeling of afferents by dye application into vestibular organs (green) and the cochlear duct (red) revealed the segregation of the central axons of spiral ganglion neurons to the cochlear nucleus from the vestibular nerve in control mice (Fig. 10C). In contrast, the same dye applications labeled inner ear afferents without any detectable segregated projection from the cochlea to the cochlear nucleus in Sox2cKO mice, indicating that the central projections of spiral ganglion neurons were missing (Fig. 10D). We next traced inner ear innervation from rhombomere 4 (green) and 5 (red, see Materials and Methods). Such applications label projections to the area of the posterior canal crista and the basal turn of the cochlea (red) in control animals (Fig. 10E and insert with a detail of red dye-labeled cochlear neurons and innervation). Comparable dye applications in Sox2cKO mice labeled only vestibular afferents and vestibular efferents and showed no cochlear innervation (Fig. 10F). Using the more sensitive detection method of immunolabeling, we showed multiple but significantly diminished inner ear efferent fibers extending across the Sox2cKO cochlea at E13.5 (Fig. S3).
Fig. 10. Vestibular projection remains near normal at E12.5 but no cochlear projection can be labeled in Sox2cKO.
(A, B) Representative images of the lateral views of whole-mounted hindbrains show analogous triple-dye labeling from the ear (green), from the trigeminal ganglion (magenta), and from the jugular foramen of the IX/X nerve (red) in control and mutant littermates at E12.5 (n = 3 embryos per genotype). Inner ear vestibular afferents (green) nearly identically project centrally in the control and Sox2cKO, although the number of afferents is reduced in Sox2cKO. (C, D) To evaluate the earliest central projections from spiral ganglion neurons to the cochlear nucleus at E12.5, lipophilic dye was inserted into the cochlear duct (red). Vestibular afferent fibers of the vestibular nerve (VN) are labeled by green dye inserted into the vestibular organs. Control animals show a profound cochlear projection (red) to the cochlear nucleus (CN), segregated from vestibular afferents (green, C). No cochlear projection to the CN is detectable in Sox2cKO only overlapping labeling of vestibular afferents (D). (E, F) Fillings from rhombomere 4 to label vestibular afferents (green), and from rhombomere 5 to label vestibular and cochlear afferents (red, see Materials and Methods) reveal basal turn spiral ganglia (SG) in the control (detail of red dye-labeled SG in insert). No cochlear innervation or neurons are labeled in mutant littermates (F). Note the larger vestibular ganglion (VG) in the control compared to Sox2cKO mice. VIII, vestibulocochlear nerve; IX, glossopharyngeal nerve; X, vagus nerve; CB, cerebellum; dV, the descending tract of trigeminal nerve; FN, facial nerve; PC, posterior crista. Scale bars: 100 μm.
After E13.5, the cartilage around the very small inner ear makes it nearly impossible to achieve selective dye tracing from the ear to the brain. We, therefore, opted to only dye the peripheral projections from the brain to the ear at later developmental stages. At E18.5, selective dye tracing of efferents and afferents showed profound differences between control and Sox2cKO mice (Fig. 11). Lipophilic dyes were applied to the solitary tract and facial branchial motor neurons (red), and to the contralateral efferents and cerebellum (green, Fig. 11A, B), or the facial branchial motor neurons/inner ear efferents (green) and the cochlear/vestibular/solitary tract (red, Fig. 11C, D). In control mice, efferents and afferents formed an interactive network of cells and fibers in the vestibular ganglion and the cochlea (Fig. 11A, C) with the facial sensory fibers and motor fibers of the intermediate nerve passing across the vestibular ganglion (red dye-labeled fibers in Fig. 11A), as previously described (Fritzsch et al., 1997). Similar applications of dyes labeled the efferents and afferents of the facial and intermediate nerve in Sox2cKO mice, including geniculate ganglion neurons (Fig. 11B). However, no vestibular or spiral ganglion neurons were labeled in the Sox2cKO mice. Inner ear efferents formed a meshwork of fibers, where the VG used to be in earlier stages, and reached to but did not innervate the residual ear vesicle (Fig. 11B, D). These data suggest that at least some inner ear efferents can survive past the loss of the VG neurons and remain in a projection pattern toward the residual ear, reflecting their earlier trajectory along vestibular afferents (Karis et al., 2001; Simmons et al., 2011).
Fig. 11. Only efferent fibers project to the Sox2cKO ear at E18.5.
(A, B) Lipophilic dye applied to rhombomere 4 (red) labels the facial and intermediate nerve, and dye injected into the cerebellum (green) labels vestibular neurons, vestibular afferents, and contralateral inner ear efferents (IEE) in controls. In Sox2cKO, facial and intermediate nerve fibers are labeled but no vestibular or spiral ganglion neurons are labeled/found (n = 3 embryos per genotype). Some IEE remain at least until this stage and form a meshwork of fibers where the vestibular ganglion used to be and occasionally extend toward but never reach the remaining rudimentary ear of Sox2cKO. (C) Dye applications show the distribution of afferents and efferents in the cochlea and spiral ganglion of controls with inner ear efferent fibers forming the intraganglionic bundle (IGSB, green). (D) The transmitted light image (blue background) shows a reduced residual ear vesicle in Sox2cKO. All vestibular afferents and the facial nerve are labeled by green dye injected into the ipsilateral rhombomere 4 and intermediate nerve afferents are labeled by red dye from rhombomere 5/6. Note that no vestibular neurons or any innervation in the cochlea are labeled in Sox2cKO. VIII, vestibulocochlear nerve; GG, geniculate ganglion; FN, facial nerve; IN, intermediate nerve; RF, radial fibers; OC, the organ of Corti; SG, spiral ganglion; VG, vestibular ganglion. Scale bars: 100 μm.
4. Discussion and conclusions
In this study, by analyzing Sox2 conditional knockout mice, we provide evidence that the absence of Sox2 differentially affects the development of the olfactory, lens, and ear (otic) placodes. We show that early ear but not olfactory or lens placode development can proceed without SOX2. The profound effects of Sox2 deletion on lens and olfactory placodal development correlate with the topological interconnection of olfactory and lens placodal development (Bhattacharyya et al., 2004; Couly and Le Douarin, 1985). Additionally, anterior lens and olfactory placodal cells differ from posterior ear placodal cells at molecular levels. While anterior lens-olfactory progenitors express Six3, Otx2, and Pax6, posterior placodal cells express Irx3, Gbx2, and Pax2/8 (Bouchard et al., 2010; Maier et al., 2014). Thus, the molecular action of SOX2 appears to be affected by the differing molecular signature of these placodes. In particular, the interdependency of SOX2 and PAX6 in the lens and olfactory induction process (Donner et al., 2007; Kamachi et al., 2001; Panaliappan et al., 2018; Quinn et al., 1996) supports the idea of a special requirement of SOX2 for this process, which is consistent with our results. A similar effect on the formation of these placodes was reported in Pax6 null mice (Ashery-Padan et al., 2000; Quinn et al., 1996). Although no direct evidence of SOX2 and PAX6 cooperation during the induction of the olfactory placode exists, disruption of olfactory placode development has been reported when Pax6 or Sox2 expression is lost in different mutants (Donner et al., 2007; Panaliappan et al., 2018; Quinn et al., 1996). Together these data indicate the necessity of SOX2- and PAX6-dependent transcriptional regulation in lens and olfactory placode development, regardless of whether this regulation is direct or indirect.
In contrast to the lens and olfactory placodes, which fail to invaginate, the ear (otic) placode invaginates and forms the ear (otic) vesicle in the absence of SOX2 in our Sox2cKO. Previous studies using the same Foxg1-Cre mouse line as we have (Barrionuevo et al., 2008) showed that conditional inactivation of Sox9 in the ear ectoderm results in the failure of ear placode invagination. Our data extend this finding and show that SOX2, in contrast to the overlapping expression of SOX9 (Mak et al., 2009), is not necessary for otic placode invagination and the formation of the otocyst. Additionally, our results provide evidence that lack of SOX2 protein has no effect on early ear neuronal delamination and NEUROD1 expression. This ability of the ear placode to undergo early neuronal development is in stark contrast to the olfactory system that never generates significant expression of Neurog1 and Neurod1 and thus olfactory neurogenesis is abolished in the absence of Sox2 (Panaliappan et al., 2018). Moreover, our Sox2cKO mutant forms the vestibular ganglion with proper peripheral and central targeting comparable to control littermates (Fig. 12, summary). In contrast to the initial formation of vestibular neurons and their central projections, we could only detect a few spiral ganglion neurons using molecular markers, but neither their peripheral nor central projections developed. It thus appears that some spiral ganglion neurons may only form transiently in the absence of Sox2 as previously reported using a different Cre line for delayed Sox2 deletion (Dvorakova et al., 2016).
Elimination of SOX2 may have a more prominent impact on spiral ganglion neurons in contrast to VG neurons because of their exclusive dependency on the sensory epithelium-produced neurotrophin 3 (NTF3) in the cochlea. Neurotrophins affect neuronal survival and initial wiring decisions during development. VG neurons are predominantly dependent on brain derived neurotrophic factor (BDNF) (Bianchi et al., 1996), whereas spiral ganglion neurons are mainly dependent on NTF3 (Fariñas et al., 2001). BDNF is not required for the initial survival of VG neurons and vestibular projections up to E12.0, as shown in Bdnf−/− mutants (Bianchi et al., 1996). In contrast to VG development, cochlear neurons differentiate in a base to apex progression, such that neurons at the base are the first to form projections (Matei et al., 2005). These neurons are fully dependent on NTF3 exclusively produced by the sensory epithelium. In the absence of NTF3, no spiral ganglion innervation is found at E12.5 with an overall loss of as many as 84% of spiral ganglion neurons between E13.5 and E15.5 in Ntf3−/− mutants (Fariñas et al., 2001). Accordingly, we found no innervation in the cochlea at E12.5 in contrast to the near-normal projections of VG neurons in Sox2cKO embryos. Despite this early development, all ear neurons of Sox2cKO eventually die by apoptosis. This cell death can be attributed to the sensory epithelia failing to differentiate and the resulting absence of neurotrophic support (Fritzsch et al., 2016b). Following apoptosis of these neurons, some efferent fibers remain for an unknown duration as signs of previous development (Fig. 11), consistent with previous reports (Ma et al., 2000). Thus we confirm that in the absence of SOX2 no sensory epithelia are established and overall ear morphogenesis is significantly reduced, as previously reported in Lcc mutants (Kiernan et al., 2005), and we newly show that early ear neuronal development is unaffected.
SOX2 belongs to Group B1, which has two other members, SOX1 and SOX3. In the chick, SOX3 is co-expressed with SOX2 in the neurogenic domain of the otic cup (Neves et al., 2007) and ectopic SOX3 expression in the preotic field is sufficient to promote the development of neuronal precursors (Abello et al., 2010). In contrast, in the mouse, Sox1 or Sox3 are not expressed in the otic placode (Wood and Episkopou, 1999), and they are not expressed together with NEUROG1 and NEUROD1 in the mouse otocyst (Puligilla et al., 2010). Moreover, in the absence of Sox1 in Sox1LacZ/LacZ mutants, ganglion cell morphology is unaffected, suggesting that SOX1 is not required for neuronal development and maintenance (Puligilla et al., 2010). SOX21 and SOX14 together make up the SoxB2 group of transcription factors, which are closely related to SoxB1 proteins (Wegner and Stolt, 2005). Only Sox21 has been shown to be expressed during ear development (Hosoya et al., 2011). Although Sox21 is expressed in the otocyst and delaminating neurons, absence of Sox21 in a knock-out mouse model causes only mild patterning defects in the inner ear (Hosoya et al., 2011). Together, these publications and our data indicate that neither SOX1–3 nor SOX21 seem to control the expression of proneural genes or early neurogenesis in the mouse inner ear. Therefore, different pathways involving factors other than SoxB2 or SoxB1 seem to initiate bHLH factor expression and neurogenesis.
Our analyses clearly show normal ear placode development and early neurogenesis without SOX2 protein. Previously, it has been suggested that the absence of innervation in older E15.5 Lcc embryos may indicate that no neurons ever form in these mutants (Kiernan et al., 2005; Puligilla et al., 2010). Notably, no analyses of early neurogenesis, neuronal molecular signature, and initial innervation in the inner ear of Lcc embryos were performed at the stages we identified here. The reported absence of innervation in late Lcc embryos can thus be reconciled with our observations of a delayed neuronal loss in our Sox2cKO mutant. A similar study, using a tamoxifen-inducible Sox2-CreERT2 (tamoxifen injected daily from E8.5 until E11.5), showed approximately a 70% volume reduction of the inner ear ganglion after Sox2 deletion at E11.75 (Steevens et al. 2017). The loss of SOX2 using the Sox2-CreER is only shown at E11.75 without any information from earlier stages, whereas we showed a complete elimination of SOX2 in the invaginating ear placode at E8.5. Moreover, using Foxg1-Cre, we showed delaminating NEUROD1+/SOX2− neurons, an unaffected number of neurons in the vestibular ganglion at E10.5, and comparable peripheral and central projections of vestibular neurons between control and mutant embryos at E11.5. Based on our data, a massive apoptosis of neurons of the Sox2-deficient inner ear starts before E11.75, and therefore, the authors may have completely missed the early stages of neurogenesis in the inner ear. Additionally, while the authors supported their conclusions with various techniques, they did not use the whole mount fiber tracing approach that we used here to effectively verify the presence of peripheral and central connections of differentiated inner ear neurons that may have been missed with their techniques.
In summary, our data using Foxg1-Cre mediated Sox2 deletion provide evidence of differential SOX2 requirements for the development of anterior lens and olfactory placodal cells and posterior ear placodal cells. Our findings indicate that SOX2 is not required for ear placode invagination and otocyst formation. In addition, our results provide comprehensive evidence that SOX2 is not necessary for early ear neurogenesis. We confirm previous work on the lack of sensory development in the ear in the absence of Sox2 but find that transient inner ear neuronal development does occur, with surprisingly well developed central and peripheral vestibular connections, indistinguishable from control littermates. The mechanism(s) through which SOX2 loss is compensated are likely complex, as we and others have established that SOX2 plays multiple roles in inner ear sensory and non-sensory development. Interactions of SOX2 with other transcription factors likely represent a potential mechanism allowing for different roles of SOX2 in development. Several transcription factors have been shown to cooperate with SOX2 in different otic lineages, including C-MYC, EYA1, SIX1, and CHD7 (Ahmed et al., 2012; Evsen et al., 2013; Kempfle et al., 2016; Kwan et al., 2015). Further work is required to fully mechanistically explain the different levels of Sox2 dependency and the initiation of neurogenesis in inner ear development.
Supplementary Material
Highlights.
Foxg1-Cre-mediated Sox2 deletion disrupts olfactory and lens placode development.
Ear placode invagination and otocyst formation proceed without SOX2.
Early vestibular ganglion formation with neuron projections advances without SOX2.
Loss of SOX2 blocks cochlear neuronal and sensory development.
Overall ear morphogenesis is significantly reduced in the absence of SOX2.
Acknowledgements
We thank A. Pavlinek for editing the MS. We thank the Imaging Methods Core Facility at BIOCEV supported by the MEYS CR (LM2015062 Czech-BioImaging).
Funding
Funding for this study was provided by a grant from the Czech Science Foundation (17-04719S to GP); by BIOCEV CZ.1.05/1.1.00/02.0109 from the ERDF; OP RDE CZ.1.05/2.1.00/19.0395; by the institutional support of the Czech Academy of Sciences RVO: 86652036; and by NIH (R01 AG060504 to BF).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Competing interests
The authors have no competing interests to declare.
References
- Abello G, Khatri S, Radosevic M, Scotting PJ, Giraldez F, Alsina B, 2010. Independent regulation of Sox3 and Lmx1b by FGF and BMP signaling influences the neurogenic and non-neurogenic domains in the chick otic placode. Dev Biol 339, 166–178. [DOI] [PubMed] [Google Scholar]
- Ahmed M, Xu J, Xu PX, 2012. EYA1 and SIX1 drive the neuronal developmental program in cooperation with the SWI/SNF chromatin-remodeling complex and SOX2 in the mammalian inner ear. Development 139, 1965–1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashery-Padan R, Marquardt T, Zhou X, Gruss P, 2000. Pax6 activity in the lens primordium is required for lens formation and for correct placement of a single retina in the eye. Genes Dev 14, 2701–2711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrionuevo F, Naumann A, Bagheri-Fam S, Speth V, Taketo MM, Scherer G, Neubuser A, 2008. Sox9 is required for invagination of the otic placode in mice. Dev Biol 317, 213–224. [DOI] [PubMed] [Google Scholar]
- Bhattacharyya S, Bailey AP, Bronner-Fraser M, Streit A, 2004. Segregation of lens and olfactory precursors from a common territory: cell sorting and reciprocity of Dlx5 and Pax6 expression. Dev Biol 271, 403–414. [DOI] [PubMed] [Google Scholar]
- Bianchi LM, Conover JC, Fritzsch B, DeChiara T, Lindsay RM, Yancopoulos GD, 1996. Degeneration of vestibular neurons in late embryogenesis of both heterozygous and homozygous BDNF null mutant mice. Development 122, 1965–1973. [DOI] [PubMed] [Google Scholar]
- Bouchard M, de Caprona D, Busslinger M, Xu P, Fritzsch B, 2010. Pax2 and Pax8 cooperate in mouse inner ear morphogenesis and innervation. BMC developmental biology 10, 89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chumak T, Bohuslavova R, Macova I, Dodd N, Buckiova D, Fritzsch B, Syka J, Pavlinkova G, 2016. Deterioration of the Medial Olivocochlear Efferent System Accelerates Age-Related Hearing Loss in Pax2-Isl1 Transgenic Mice. Mol Neurobiol 53, 2368–2383. [DOI] [PubMed] [Google Scholar]
- Couly GF, Le Douarin NM, 1985. Mapping of the early neural primordium in quail-chick chimeras. I. Developmental relationships between placodes, facial ectoderm, and prosencephalon. Dev Biol 110, 422–439. [DOI] [PubMed] [Google Scholar]
- Dabdoub A, Puligilla C, Jones JM, Fritzsch B, Cheah KS, Pevny LH, Kelley MW, 2008. Sox2 signaling in prosensory domain specification and subsequent hair cell differentiation in the developing cochlea. Proceedings of the National Academy of Sciences 105, 18396–18401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dastidar SG, Landrieu PMZ, D’Mello SR, 2011. FoxG1 promotes the survival of postmitotic neurons. Journal of Neuroscience 31, 402–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donner AL, Episkopou V, Maas RL, 2007. Sox2 and Pou2f1 interact to control lens and olfactory placode development. Dev Biol 303, 784–799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duggan CD, DeMaria S, Baudhuin A, Stafford D, Ngai J, 2008. Foxg1 is required for development of the vertebrate olfactory system. Journal of Neuroscience 28, 5229–5239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan JS, Fritzsch B, 2013. Continued expression of GATA3 is necessary for cochlear neurosensory development. PloS one 8, e62046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dvorakova M, Jahan I, Macova I, Chumak T, Bohuslavova R, Syka J, Fritzsch B, Pavlinkova G, 2016. Incomplete and delayed Sox2 deletion defines residual ear neurosensory development and maintenance. Scientific reports 6, 38253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evsen L, Sugahara S, Uchikawa M, Kondoh H, Wu DK, 2013. Progression of neurogenesis in the inner ear requires inhibition of Sox2 transcription by neurogenin1 and neurod1. Journal of Neuroscience 33, 3879–3890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fariñas I, Jones KR, Tessarollo L, Vigers AJ, Huang E, Kirstein M, De Caprona DC, Coppola V, Backus C, Reichardt LF, Fritzsch B, 2001. Spatial shaping of cochlear innervation by temporally regulated neurotrophin expression. Journal of Neuroscience 21, 6170–6180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritzsch B, Beisel K, Jones K, Farinas I, Maklad A, Lee J, Reichardt L, 2002. Development and evolution of inner ear sensory epithelia and their innervation. Journal of neurobiology 53, 143–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritzsch B, Duncan JS, Kersigo J, Gray B, Elliott KL, 2016a. Neuroanatomical Tracing Techniques in the Ear: History, State of the Art, and Future Developments, Sokolowski B, Ed: Auditory and Vestibular Research: Methods and Protocols. Springer Science+Business Media; New York, pp. 243–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritzsch B, Elliott KL, Pavlinkova G, 2019. Primary sensory map formations reflect unique needs and molecular cues specific to each sensory system. F1000Research 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritzsch B, Kersigo J, Yang T, Jahan I, Pan N, 2016b. Neurotrophic factor function during ear development: expression changes define critical phases for neuronal viability, The Primary Auditory Neurons of the Mammalian Cochlea. Springer, pp. 49–84. [Google Scholar]
- Fritzsch B, Pan N, Jahan I, Elliott KL, 2015. Inner ear development: building a spiral ganglion and an organ of Corti out of unspecified ectoderm. Cell and tissue research 361, 7–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritzsch B, Sarai P, Barbacid M, Silos-Santiago I, 1997. Mice with a targeted disruption of the neurotrophin receptor trkB lose their gustatory ganglion cells early but do develop taste buds. International Journal of Developmental Neuroscience 15, 563–576. [DOI] [PubMed] [Google Scholar]
- Garaffo G, Conte D, Provero P, Tomaiuolo D, Luo Z, Pinciroli P, Peano C, D’Atri I, Gitton Y, Etzion T, 2015. The Dlx5 and Foxg1 transcription factors, linked via miRNA-9 and-200, are required for the development of the olfactory and GnRH system. Molecular and Cellular Neuroscience 68, 103–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu R, Brown RM 2nd, Hsu CW, Cai T, Crowder AL, Piazza VG, Vadakkan TJ, Dickinson ME, Groves AK, 2016. Lineage tracing of Sox2-expressing progenitor cells in the mouse inner ear reveals a broad contribution to non-sensory tissues and insights into the origin of the organ of Corti. Dev Biol 414, 72–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagey DW, Klum S, Kurtsdotter I, Zaouter C, Topcic D, Andersson O, Bergsland M, Muhr J, 2018. SOX2 regulates common and specific stem cell features in the CNS and endoderm derived organs. PLoS genetics 14, e1007224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosoya M, Fujioka M, Matsuda S, Ohba H, Shibata S, Nakagawa F, Watabe T, Wakabayashi K, Saga Y, Ogawa K, Okano HJ, Okano H, 2011. Expression and function of Sox21 during mouse cochlea development. Neurochem Res 36, 1261–1269. [DOI] [PubMed] [Google Scholar]
- Huang EJ, Liu W, Fritzsch B, Bianchi LM, Reichardt LF, Xiang M, 2001. Brn3a is a transcriptional regulator of soma size, target field innervation and axon pathfinding of inner ear sensory neurons. Development 128, 2421–2432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang CH, Simeone A, Lai E, Wu DK, 2009. Foxg1 is required for proper separation and formation of sensory cristae during inner ear development. Developmental dynamics: an official publication of the American Association of Anatomists 238, 2725–2734. [DOI] [PubMed] [Google Scholar]
- Jahan I, Kersigo J, Pan N, Fritzsch B, 2010. Neurod1 regulates survival and formation of connections in mouse ear and brain. Cell and tissue research 341, 95–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kageyama R, Shimojo H, Ohtsuka T, 2019. Dynamic control of neural stem cells by bHLH factors. Neuroscience research 138, 12–18. [DOI] [PubMed] [Google Scholar]
- Kamachi Y, Uchikawa M, Tanouchi A, Sekido R, Kondoh H, 2001. Pax6 and SOX2 form a co-DNA-binding partner complex that regulates initiation of lens development. Genes Dev 15, 1272–1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karis A, Pata I, van Doorninck JH, Grosveld F, de Zeeuw CI, de Caprona D, Fritzsch B, 2001. Transcription factor GATA-3 alters pathway selection of olivocochlear neurons and affects morphogenesis of the ear. J Comp Neurol 429, 615–630. [DOI] [PubMed] [Google Scholar]
- Kawauchi S, Kim J, Santos R, Wu H-H, Lander AD, Calof AL, 2009. Foxg1 promotes olfactory neurogenesis by antagonizing Gdf11. Development 136, 1453–1464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kempfle JS, Turban JL, Edge AS, 2016. Sox2 in the differentiation of cochlear progenitor cells. Scientific reports 6, 23293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kersigo J, D’Angelo A, Gray BD, Soukup GA, Fritzsch B, 2011. The role of sensory organs and the forebrain for the development of the craniofacial shape as revealed by Foxg1-cre-mediated microRNA loss. Genesis 49, 326–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiernan AE, Pelling AL, Leung KK, Tang AS, Bell DM, Tease C, Lovell-Badge R, Steel KP, Cheah KS, 2005. Sox2 is required for sensory organ development in the mammalian inner ear. Nature 434, 1031–1035. [DOI] [PubMed] [Google Scholar]
- Kim W-Y, Fritzsch B, Serls A, Bakel LA, Huang EJ, Reichardt LF, Barth DS, Lee JE, 2001. NeuroD-null mice are deaf due to a severe loss of the inner ear sensory neurons during development. Development 128, 417–426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondoh H, Lovell-Badge R, 2015. Sox2: biology and role in development and disease. Academic Press. [Google Scholar]
- Kopecky BJ, Duncan JS, Elliott KL, Fritzsch B, 2012. Three-dimensional reconstructions from optical sections of thick mouse inner ears using confocal microscopy. Journal of microscopy 248, 292–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwan KY, Shen J, Corey DP, 2015. C-MYC transcriptionally amplifies SOX2 target genes to regulate self-renewal in multipotent otic progenitor cells. Stem Cell Reports 4, 47–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu M, Pereira FA, Price SD, Chu MJ, Shope C, Himes D, Eatock RA, Brownell WE, Lysakowski A, Tsai MJ, 2000. Essential role of BETA2/NeuroD1 in development of the vestibular and auditory systems. Genes Dev 14, 2839–2854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo XJ, Deng M, Xie X, Huang L, Wang H, Jiang L, Liang G, Hu F, Tieu R, Chen R, Gan L, 2013. GATA3 controls the specification of prosensory domain and neuronal survival in the mouse cochlea. Hum Mol Genet 22, 3609–3623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Q, Anderson DJ, Fritzsch B, 2000. Neurogenin 1 null mutant ears develop fewer, morphologically normal hair cells in smaller sensory epithelia devoid of innervation. Journal of the Association for Research in Otolaryngology 1, 129–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Q, Chen Z, del Barco Barrantes I, de la Pompa JL, Anderson DJ, 1998. neurogenin1 is essential for the determination of neuronal precursors for proximal cranial sensory ganglia. Neuron 20, 469–482. [DOI] [PubMed] [Google Scholar]
- Macova I, Pysanenko K, Chumak T, Dvorakova M, Bohuslavova R, Syka J, Fritzsch B, Pavlinkova G, 2019. Neurod1 is essential for the primary tonotopic organization and related auditory information processing in the midbrain. Journal of Neuroscience 6, 984–1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maier EC, Saxena A, Alsina B, Bronner ME, Whitfield TT, 2014. Sensational placodes: Neurogenesis in the otic and olfactory systems. Developmental biology 389, 50–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mak AC, Szeto IY, Fritzsch B, Cheah KS, 2009. Differential and overlapping expression pattern of SOX2 and SOX9 in inner ear development. Gene Expression Patterns 9, 444–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maklad A, Kamel S, Wong E, Fritzsch B, 2010. Development and organization of polarity-specific segregation of primary vestibular afferent fibers in mice. Cell and tissue research 340, 303–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Monedero R, Yi E, Oshima K, Glowatzki E, Edge AS, 2008. Differentiation of inner ear stem cells to functional sensory neurons. Developmental neurobiology 68, 669–684. [DOI] [PubMed] [Google Scholar]
- Matei V, Pauley S, Kaing S, Rowitch D, Beisel KW, Morris K, Feng F, Jones K, Lee J, Fritzsch B, 2005. Smaller inner ear sensory epithelia in Neurog 1 null mice are related to earlier hair cell cycle exit. Developmental dynamics: an official publication of the American Association of Anatomists 234, 633–650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLeod MJ, 1980. Differential staining of cartilage and bone in whole mouse fetuses by alcian blue and alizarin red S. Teratology 22, 299–301. [DOI] [PubMed] [Google Scholar]
- Neves J, Kamaid A, Alsina B, Giraldez F, 2007. Differential expression of Sox2 and Sox3 in neuronal and sensory progenitors of the developing inner ear of the chick. J Comp Neurol 503, 487–500. [DOI] [PubMed] [Google Scholar]
- Nishimura K, Noda T, Dabdoub A, 2017. Dynamic expression of Sox2, Gata3, and Prox1 during primary auditory neuron development in the mammalian cochlea. PloS one 12, e0170568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panaliappan TK, Wittmann W, Jidigam VK, Mercurio S, Bertolini JA, Sghari S, Bose R, Patthey C, Nicolis SK, Gunhaga L, 2018. Sox2 is required for olfactory pit formation and olfactory neurogenesis through BMP restriction and Hes5 upregulation. Development 145, dev153791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pauley S, Lai E, Fritzsch B, 2006. Foxg1 is required for morphogenesis and histogenesis of the mammalian inner ear. Developmental dynamics: an official publication of the American Association of Anatomists 235, 2470–2482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puligilla C, Dabdoub A, Brenowitz SD, Kelley MW, 2010. Sox2 induces neuronal formation in the developing mammalian cochlea. Journal of Neuroscience 30, 714–722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinn JC, West JD, Hill RE, 1996. Multiple functions for Pax6 in mouse eye and nasal development. Genes Dev 10, 435–446. [DOI] [PubMed] [Google Scholar]
- Reiprich S, Wegner M, 2015. From CNS stem cells to neurons and glia: Sox for everyone. Cell and tissue research 359, 111–124. [DOI] [PubMed] [Google Scholar]
- Shen W, Ba R, Su Y, Ni Y, Chen D, Xie W, Pleasure SJ, Zhao C, 2018. Foxg1 Regulates the Postnatal Development of Cortical Interneurons. Cerebral Cortex. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmons D, Duncan J, de Caprona DC, Fritzsch B, 2011. Development of the inner ear efferent system, Auditory and vestibular efferents. Springer, pp. 187–216. [Google Scholar]
- Steevens AR, Glatzer JC, Kellogg CC, Low WC, Santi PA, Kiernan AE, 2019. SOX2 is required for inner ear growth and cochlear nonsensory formation before sensory development. Development 146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steevens AR, Sookiasian DL, Glatzer JC, Kiernan AE, 2017. SOX2 is required for inner ear neurogenesis. Scientific reports 7, 4086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taranova OV, Magness ST, Fagan BM, Wu Y, Surzenko N, Hutton SR, Pevny LH, 2006. SOX2 is a dose-dependent regulator of retinal neural progenitor competence. Genes Dev 20, 1187–1202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wegner M, 1999. From head to toes: the multiple facets of Sox proteins. Nucleic acids research 27, 1409–1420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wegner M, Stolt CC, 2005. From stem cells to neurons and glia: a Soxist’s view of neural development. Trends Neurosci 28, 583–588. [DOI] [PubMed] [Google Scholar]
- Wood HB, Episkopou V, 1999. Comparative expression of the mouse Sox1, Sox2 and Sox3 genes from pre-gastrulation to early somite stages. Mech Dev 86, 197–201. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.