Abstract
Ischemic vascular remodeling occurs in response to stenosis or arterial occlusion leading to a change in blood flow and tissue perfusion. Altered blood flow elicits a cascade of molecular and cellular physiological responses leading to vascular remodeling of the macro- and microcirculation. Although cellular mechanisms of vascular remodeling such as arteriogenesis and angiogenesis have been studied, therapeutic approaches in these areas have had limited success due to the complexity and heterogeneous constellation of molecular signaling events regulating these processes. Understanding central molecular players of vascular remodeling should lead to a deeper understanding of this response and aid in the development of novel therapeutic strategies. Hydrogen sulfide (H2S) and nitric oxide (NO) are gaseous signaling molecules that are critically involved in regulating fundamental biochemical and molecular responses necessary for vascular growth and remodeling. This review examines how NO and H2S regulate pathophysiological mechanisms of angiogenesis and arteriogenesis, along with important chemical and experimental considerations revealed thus far. The importance of NO and H2S bioavailability, their synthesis enzymes and cofactors, and genetic variations associated with cardiovascular risk factors suggest that they serve as pivotal regulators of vascular remodeling responses.
Introduction
Everywhere we look, complex magic of nature blazes before our eyes.
— Vincent van Gogh
While there are visible complexities to which van Gogh refers, far more complexity exists in the form of cellular signaling and small molecules that are involved in regulating various biological functions. The quote “The art of simplicity is a puzzle of complexity” may hold true with the gaseous signaling molecules otherwise known as “gasotransmitters” – nitric oxide (NO) and hydrogen sulfide (H2S). Nitrogen and sulfur are among the fundamental elements that play an essential part of life on Earth (261). Sulfides and different nitrogen oxides have been studied extensively in environmental, chemical, and various biological processes including their role in the origin of life itself. For quite some time, NO and H2S were simply considered toxic gases and environmental hazards, yet they have emerged as important regulatory signaling molecules for numerous physiological and pathological functions including vasorelaxation, anti-inflammation, antithrombotic effects, central nervous system function, and protection against ischemia-reperfusion (I/R) injury (166, 167). In this review, we discuss the roles of NO and H2S, and their respective metabolites in relation to vascular remodeling along with molecular signaling mechanisms. Importantly, we also discuss the synthesis, catabolism, chemical interactions, and detection methods associated with these gaseous signaling mediators.
Historical Perspective of NO and H2S—Overview
Nitric oxide (NO)
Joseph Priestley detailed many of his discoveries in his Volume 1: Experiments and Observations on Different Kinds of Air, where the mention of a colorless gas “nitrous air” (nitric oxide) was first observed. However, appreciation of the biological functions of NO was not fully realized until 1980 to 1990. Much before the discovery of NO, in 1847 an Italian chemist Ascanio Sobrero discovered glyceryl trinitrate, popularly called nitroglycerin for its dangerous properties that can cause severe headaches even with minimal exposure. In 1867, Swedish chemist and engineer Alfred Nobel patented his famous dynamite when he stabilized nitroglycerin with kieselguhr (207). Coincidentally, workers at the nitroglycerin factory with angina pectoris had their condition improve when exposed to this compound. However, it was not until 1879 when William Murrell, a British physician, identified the therapeutic effects of nitroglycerin for angina pectoris. Almost a century later, in 1977, Ferid Murad identified the beneficial effects of nitroglycerin to derive NO release in vascular smooth muscle. Seminal works also during the 1980s from Robert F. Furchgott and Louis J. Ignarro identified endothelium-derived relaxing factor (EDRF) as NO with physiological implications in regulating vascular tone (97, 115, 141, 256). Work from Murad further revealed that the nitrovasodilator function is mediated through sGC/cGMP pathway (229). Collectively these works established NO as a key-signaling molecule regulating vascular tone, which was recognized with the Nobel Prize in Physiology and Medicine in 1998. Currently, NO has been associated with many pathophysiological responses such as inflammation, cancer, tissue growth and development, and in the nervous and cardiovascular systems.
NO is enzymatically produced by three nitric oxide synthases (NOSs)—neuronal NOS (nNOS), inducible NOS (iNOS), and endothelial NOS (eNOS) that all use L-arginine as a substrate in conjunction with other cofactors including tetrahydrobiopterin, calcium, oxygen, NADPH, and others. NOS also contributes to the production of other NO metabolites such as nitrite (NO2−) and nitrate (NO3−), which are formed from by oxidation of NO (217). Nitrate and nitrite were originally considered to be inactive metabolic by-products of NO. This notion has changed following a series of publications from many researchers demonstrating that these metabolites have therapeutic potential through their conversion to NO or on their own via other pathways in pathophysiological states (198). The role of NO metabolites such as nitrite is especially emphasized under oxygen-deprived conditions such as hypoxia/ischemia where the NOS isoforms may be dysfunctional. There is increasing literature also identifying nitrate/nitrite as a biomarker and for therapeutic potential against ischemic/reperfusion injury including cardiovascular disease, which has been known since ancient times (157).
Hydrogen sulfide (H2S)
Hydrogen sulfide, a recent addition to the gasotransmitter family, was once known only for its serious health hazards. “Environmental toxin,” “sewer gas,” and “rotten egg gas” have been synonymous with H2S. However, only in the last two decades H2S has been identified for its role in many biological functions. A historical account of H2S has been reviewed recently (350). Identification of H2S as a toxic gas dates back to the observations of Italian physician Bernardino Ramazzini from sewage cleaners during the 1700s (350). In 1863, H2S was identified again for its negative qualities in the formation of sulfhemoglobin, which is responsible for cyanosis even at low levels. Earlier works between 1877 and 1884 identified H2S as a “bacterial product” with its roles in intestinal and periodontal diseases, and more recently in regulating bacterial resistance to antibiotics. It was not until the 1940’s that H2S was identified as an inhibitor of cytochrome c oxidase (169). Only in the last two decades, have positive effects of H2S as a regulator of physiological functions, including the cardiovascular and nervous system, been identified.
Like NO, H2S also regulates key physiological processes including vascular reactivity, inflammation, cardioprotection, and protection against tissue I/R injury (165, 400). Although there is considerable research in these areas, much uncertainty remains with respect to the signaling mechanisms that are involved in H2S chemical biology. For example, how can H2S be both pro- and anti-inflammatory, and both toxic and also cytoprotective? Clearly, concentration dependent responses are likely involved through activation of different signaling pathways, which requires further study to better appreciate its role in health and disease.
Vascular Growth and Remodeling Concepts
The vascular system plays a critical role in both functional and structural changes responsible for organ physiology. Blood vessels are the structures that supply oxygen and nutrients to various tissues that aid in their growth. Vascular remodeling is a dynamic process involving alterations in structural geometry of blood vessels in response to external stimuli, which can be mediated by hemodynamics, pressure changes, or injury. These changes initiated as an adaptive process can turn mal-adaptive long-term and lead to impaired vascular functions. Early development of the embryo occurs through simple diffusion of nutrients and growth factors. At this stage, there is little biological need for a capillary network. However, the circulatory system including the heart, blood, and blood vessels is the first physiological system to be functional, as organism development progresses in complexity. Early cardiovascular system development is essential for distribution of O2 to various developing parts of the embryo, as O2 can no longer simply diffuse. Thus, rapid formation of a complex vascular network occurs with the onset of events in vascular growth called “vascularization.” Vasculogenesis is a process of initiation of endothelial precursor cells eventually forming primitive tubule-like structures expanding to form a vascular network called “angiogenesis.” This process includes the formation of new blood vessels from existing ones, otherwise known as “sprouting,” and division of existing blood vessels termed ‘intussusception’ eventually branch into arterioles and capillaries.
Types of vascular growth
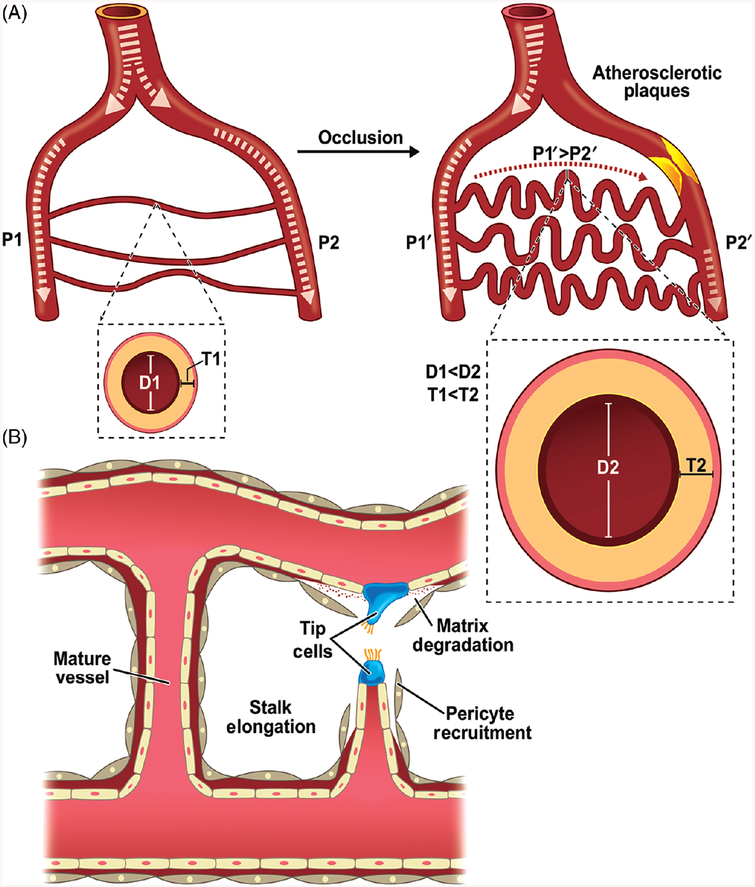
A robust and functional vascular network is fundamental for development, growth, and tissue maintenance. Vascular growth processes continue after birth and are necessary throughout the life of an organism, thus maintaining physiologic homeostasis including organ growth, wound healing, and the menstrual changes of the uterine mucosa (96). Based on the mechanism, occurrence, and result, vascular growth can be distinguished principally as three types (130). Firstly, during vasculogenesis differentiation of precursor cells or angioblasts into endothelial cells occurs eventually leading to the de novo formation of a primitive vascular network. Secondly, in angiogenesis, new capillaries form from pre-existing capillary vessels (288). Thirdly, in arteriogenesis, formerly regarded as a variant of angiogenesis, mature arteries undergo remodeling response affecting luminal diameter and wall thickness, thus distinguishing it from other mechanisms of vascular growth (36, 301). Stimulation of arteriogenesis can occur in response to differential blood flow shear rate via an occlusion in a main conduit artery that disrupts this balance and initiates blood flow from a connecting collateral artery to a downstream reentry zone, thereby stimulating arteriogenesis. The change in hemodynamics experienced by the collateral vessel stimulates outward vascular remodeling to increase lumen diameter and wall thickness (Fig. 1A).
Figure 1.
Vascular remodeling due to arteriogenesis or angiogenesis: remodeling of macro- and microvasculature occurs through arteriogenesis and angiogenesis, respectively. (A) In the absence of occlusion, blood flow across two arterial branches is minimal resulting in equivalent pressure (P1 = P2). Upon arterial occlusion of one branch, fluid shear stress due to altered blood flow causes differential pressure (P1 > P2), resulting in increased luminal diameter (D1 < D2) and vascular wall thickness (T1 < T2). (B) Angiogenesis is a multistep process involving endothelial cell activation and extracellular matrix degradation followed by tip cell sprouting and progressive vessel stalk elongation. Further structural support and vessel stabilization occur by pericyte recruitment.
Angiogenesis: Physiology
Angiogenesis is a fundamental process by which new blood vessels are formed from pre-existing capillaries that includes tip cell sprouting, stalk elongation and vessel maturation (87) (Fig. 1B). It also plays a central role in various physiological processes including embryonic development, wound repair and the menstrual cycle. Further upon angiogenic stimuli, proteolytic enzymes are produced leading to degradation of extracellular matrix thereby allowing endothelial cells (EC’s) to migrate. A fine balance of proteolytic enzymes and their inhibitors exist to prevent pathological angiogenesis. In addition to migration of EC’s, degradation of the extracellular matrix also leads to release of growth factors including VEGF, FGF, and IGF-1 which are otherwise sequestered within the matrix (59).
Angiogenesis is characterized by step-wise progression including: (i) increased endothelial solute permeability, (ii) proliferation and migration of endothelial cells (typically involving pericyte and matrix changes), (iii) assembly of ECs into sprouting vessels, (iv) differentiation of ECs to form new vessels, and (v) maturation of the new vasculature. The first step of this process is altered permeability of the existing vessel. A change in solute permeability may occur in response to several mediators with vascular endothelial growth factor (VEGF) binding to its receptor in the ECs leading to increased vascular permeability. As a result, movement of endothelial junction proteins occurs away from cell-cell junctions including, platelet endothelial cell adhesion molecule (PECAM-1), vascular endothelial cadherin (VE-cadherin), and occludin (81, 98, 158). Degradation of extracellular matrix facilitates cell migration beneath the basement membrane, involving a prominent role of the actin cytoskeleton (344).
Several potential regulators including vascular endothelial growth factor (VEGF), basic fibroblast growth factor (bFGF), platelet-derived growth factor (PDGF), and angiopoietin 1 (Ang-1) regulate EC migration and proliferation. Numerous reports suggest a pronounced angiogenic effect of VEGF and the loss of a single VEGF allele results in early lethality of mouse embryos (39, 84, 184, 240). Although compelling evidence suggests the importance of VEGF throughout the angiogenesis process, homologs of VEGF can provide some functional redundancy. For instance, VEGF-A is responsible for endothelial migration, MMP activity, and permeability. Whereas, PDGF is restricted to embryonic angiogenesis, but is involved during pathological angiogenesis during ischemia, inflammation, and cancer.
Conversely, overexpression of Ang-1 or of Ang-1 in combination with VEGF is characterized by the formation of large vessels. Studies showed that vasculature of mice lacking Ang-1 and Tie-2 remained at a primitive stage of development and failed to undergo further remodeling (76, 297, 349). Signaling through the PDGF-B and transforming growth factor β (TGF-β) pathways may provide a link in mediating shear stress and angiogenesis (38). Angiopoietins and their Tie receptors, as well as ephrins and Eph-B receptors or macrophage chemotactic protein-1, are also likely candidates for mediating such cell-cell interactions. Multiple cellular mechanisms and an increasing combination of genes and molecules are involved in building and adapting blood vessels throughout a lifetime. Although endothelial cells are early key players in angiogenesis and remodeling, the interaction of endothelial cells with inflammatory cells such as macrophages, pericytes, and smooth muscle cells is also important for capillary stabilization and large vessel remodeling.
Angiogenesis: Pathophysiology
Although physiological angiogenesis and pathological angiogenesis are different, several mechanisms mediating pathological blood vessel growth resemble those during embryogenesis. In atherosclerosis, an increase in proangiogenic factors and a decrease in antiangiogenic factors stimulate angiogenesis; thus known as the angiogenic switch.
The vasa vasorum, a small network of microvessels penetrating the adventitial and outer medial layers of the vessel wall, run longitudinally along the length of the vessel and form circumferential arches around the lumen. These vessels are responsible for the transport of oxygen and nutrients to the adventitial layer of vascular wall as well as removal of waste products (32–34). During early and late stages of atherosclerosis, vasa vasorum angiogenesis and ectopic neovascularization occurs (35). It is thought to be an adaptive response to hypoxia or endothelial damage as it increases the oxygen and nutrient supply to the arterial wall to prevent ischemia (36). Vasa vasorum may contribute to atherosclerosis and plaque instability through neovessels and the delivery of factors that enhance plaque progression (37), as the density of vasa vasorum is higher in patients with cardiovascular disease compared to patients with no complications of atherosclerosis in the early stages of the disease (36).
During pathological angiogenesis, inflammatory cells such as monocytes/macrophages, platelets, mast cells and other leukocytes are often chemoattracted to the sites of inflammation or wound (364) and arteriogenic factors (VEGF, bFGF, TGF β−1, interleukin-8, PDGF, IGF-1, MCP-1, TNF-α and proteinases) in turn, attract endothelial and smooth muscle cells, fibroblasts, leukocytes or platelets (19, 39). Chronic inflammation and angiogenesis are interrelated processes. Although angiogenesis has a major role in wound healing and tissue repair, it can support the events of inflammation via leukocyte rolling, supply of nutrients, cytokines, and adhesion molecules to the site of injury (52, 337). However, hypoxia can upregulate NO/nitrite and H2S signaling that may significantly decrease inflammatory responses while facilitating vascular growth (22, 24, 166, 262).
Arteriogenesis: Physiology
Arteriogenesis is a process where mature arteries undergo remodeling often from interconnecting collateral arterioles after arterial occlusion, thus distinguishing it from other mechanisms of vascular growth (35,364). Nevertheless, there exists another hypothesis of de novo growth of arteries that requires formation of new arterial conduits via arterialization of the capillary network (200). In the case of remodeling pre-existing arteries, among the various factors that trigger arteriogenesis, hemodynamic forces play a major role in stimulating remodeling responses of collateral arteries (299). Vessels adapt to flow to withstand the shear stress to which they are subjected (Fig. 1A). Studies have shown arterial occlusion can disrupt the balance in blood flow leading to increased flow through collaterals. This increase in blood flow alters shear stress along the collateral network acting as an inductive factor for changes in smooth muscle cell number and function. As a result, blood flows through collateral anastomoses, increasing lumen diameter and wall thickness of the vessel (263, 397). In addition to hemodynamic forces, the collateral vessel wall may also be exposed to circumferential, longitudinal, and radial stress (303). However, the extent of the influence of these forces on the vessel wall remains unclear. The change in mechanical forces including shear stress has been shown to increase MCP-1 (monocyte chemoattractant protein 1) secretion in cultured human endothelial cells, leading to increased monocyte chemotaxis and adhesion (143, 331, 372). Subsequent adhesion of monocytes and their transformation into macrophages leading to the production of various cytokines and growth factors is required for the remodeling of existing arteries. For instance, early growth response protein 1 (EGR-1) was upregulated in the endothelium of growing collateral vessels in mice within the first 12 h after arterial occlusion (294). Additionally, it is reported that VEGFR-1 and multiple VEGF isoforms improved ischemic limb perfusion and mediate collateral growth in ischemic disease by mobilizing leukocytes and recruiting them to the pericollateral space (57, 387, 396).
Arteriogenesis: Pathophysiology
Stimulation of arteriogenesis an attractive treatment target for arterial obstructive disease. Several strategies have been reported to stimulate the process of arteriogenesis including shear stress, MCP-1 expression, granulocyte-macrophage colony-stimulating factor (GM-CSF) infusion and use of other growth factors. Although these strategies have shown promise in animal models, more research has to be done in human subjects before arteriogenesis can be brought from bench to bedside.
Studies have shown that collateral arteries continue to remodel even after the end of shear stress, suggesting shear stress may be primarily required only for initiation of arteriogenesis (308). However, collateral vessels may also regress after shear stress ceases. It has been shown that collateral artery remodeling could restore blood flow to approximately 35% of distal perfusion levels, probably because the initially elevated shear stress progressively decreases. To check if this deficiency in blood flow restoration could be increased, artificial fluid shear stress was increased over the preexistent collateral vasculature in a rabbit femoral artery occlusion model (80). An increase in sustained artificial shear stress led to a maximal restoration of flow countering the idea that shear stress may only be important in early arteriogenesis.
Following occlusion of the main artery, collateral arteries are recruited at the site of occlusion. A decrease in the arterial pressure behind the occlusion, leads to an imbalance in blood flow, leading to redistribution of blood flow in collateral arterioles that balances high pressure with low-pressure regions. This causes an increased flow velocity and shear stress in the preexistent collateral arteries leading to activation of the endothelium and upregulation of MCP-1. A monocyte deficient mouse model clearly demonstrates the importance of monocytes and macrophage accumulation for collateral growth (15). Following arterial occlusion, macrophages derived from monocytes create an inflammatory environment leading to upregulation of TNFα. It has been shown that TNFα is a pivotal regulator of arteriogenesis and that knockout or inhibition of TNFα leads to significant reduction in collateral formation (116, 131). Activated lymphocytes also secrete cytokines and modulate cellular trafficking of monocytes/macrophages, thereby participating in arteriogenesis. Targeted genetic disruption of the T-cell receptor CD4, showed a reduction of blood flow recovery in the mouse hindlimb after femoral occlusion. Moreover, injection of isolated T-cells rescued blood flow recovery and increased macrophage accumulation highlighting an important role of lymphocyte/monocyte/macrophage interactions in arteriogenesis (341).
Lastly, GM-CSF, may also be important for arteriogenesis as a clinical study with patients who were not eligible for coronary artery bypass surgery showed that a short-term local and systemic application protocol augments collateral flow. Furthermore, it has been shown that infusion of GM-CSF following femoral artery occlusion in the rabbit stimulated collateral development (312). Additionally, combinational therapy of MCP-1 and GM-CSF increased blood flow perfusion to 40% after a week of arterial occlusion. The role of these regulators in arteriogenesis is evident, but highly complex. More research is needed to expand our knowledge of these molecules and how they are involved in collateral formation.
Molecular signaling in ischemic vascular remodeling
Ischemia is characterized by a decreased partial oxygen pressure (pO2) in the tissue and reduced tissue perfusion. This leads to conditions insufficient to meet metabolic needs and reduced nutrients to the tissue. The onset of ischemia triggers a cascade of events including molecular, and intra- and extracellular responses in the remodeling of the ischemic tissue (40, 333). This results in compulsory responses of vasodilation followed by new blood vessel formation and remodeling of the pre-existing vascular network. Vascular remodeling is essential for normal growth and development or tissue regeneration under pathological conditions or as a recovery process following an ischemic insult (193).
Many biological mediators, including growth factors, cytokines, matrix components, and hemodynamic changes are involved in effective vascular remodeling under physiological and pathological conditions (40, 333). Although endothelial cells take center-stage in this tightly regulated process, there are important contributions from and interaction with other cell types. Based on the type of the blood vessel, the organ vascular bed, and the cellular composition restructuring responses may vary. Nonetheless, some common molecular mechanisms work to stimulate vascular growth and remodeling.
Hypoxia
Oxygen is a key modulator of various cellular signaling mechanisms. It is a requirement for cellular respiration and survival resulting in tight control of the microenvironment to which cells are exposed. The levels of O2 concentrations can range from 10–20% in “Normoxia” or 1% to 10% under ‘Hypoxia”. Responsiveness to O2 concentrations also depends on the cell type and organ and the environment that prevails (156, 220, 273). Hypoxia typically results from insufficient blood supply to a tissue, which triggers cellular adaptations events including endothelial cell migration and proliferation (397). However, hyperoxia can also be detrimental to cells as it increases reactive oxygen formation leading to cell injury (45).
There are certain cellular enzymes that may not effectively function under hypoxia, while others are expressed and uniquely function to mediate compensatory effects in cell systems. NO and H2S are signaling molecules acting as sensors responsible for the acute vascular response to hypoxia (45, 164, 248). Hypoxia can destabilize eNOS by oxidizing its cofactor tetrahydrobiopterin (BH4). However, nitrite can be reduced to form NO, mediated by the enzymes xanthine oxidase (XO), deoxy-hemoglobin (Hb) and myoglobin (Mb). At the cellular level, hypoxia can also trigger CSE/H2S pathway that mediates reduction of nitrite to NO via XO or impacting NOS function (22). Importantly, these observations are not observed under normoxic conditions.
HIF and growth factors
Hypoxia plays a major role in regulating growth factor expression and/or function. Hypoxia-inducible factor-1 (HIF-1) is a key factor responsible for the cell adaptation during reduced O2 conditions (361). HIFα modulates responses to hypoxia by regulating vascular remodeling via VEGF and its receptor VEGFR, stromal cell-derived factor 1 (SDF-1), placental growth factor (PGF), PDGF, angiopoietin 2 (Ang-2) and stem cell factor (SCF) (285). bFGF is implicated in the regulation of both angiogenesis and arteriogenesis; however, it is believed to be induced by the pro-angiogenic monocytes mediating vascular remodeling (120). Importantly, therapeutic angiogenesis requires a combination of growth factors in the formation of stable and mature blood vessels (246). Apart from hypoxia, NO can upregulate the transcriptional activity and expression of HIF-1, but also stabilize it (251). Additionally, H2S has been shown to augment HIF-1 activity, subsequently stimulating ischemic vascular growth (22).
VEGF
The vascular endothelial cell growth factor family of proteins are crucial mediators of angiogenesis and vasculogenesis under pathophysiological conditions. Ischemic insult in the tissues induces VEGF expression in many cell types. HIF-1 regulates the expression of VEGF-A by binding to the hypoxia response element (HREs) in the promoter region of the VEGF gene (172). This in turn allows adaptation of the system to cope with decreased O2 partial pressure (pO2) by increasing vascular density. However, prolonged HIF activation can contribute to major structural alterations and can also lead to cell apoptosis (272).
VEGF-A is a dimeric glycoprotein that binds to both of its receptors VEGFR1 and VEGFR2 on the surface of endothelial and other cell types. Established literature suggests a strong affinity between VEGF-A and VEGFR-2 that increases its tyrosine kinase activity upon binding (371). VEGFR-2 is a crucial regulator of vascular development, and in the formation of the branched vasculature, whereas VEGFR-1 may act as a negative regulator of VEGFR-2 (79). In vitro studies have established that VEGFR-2 regulates endothelial cell proliferation, migration and survival mediated via phosphatidylinositol 3′-kinase (PI3K)/AKT pathway (102). Animal studies performed on specific gene knockouts have substantiated the function and physiological relevance of these relationships (293,318). Studies performed in mice deficient in Flk-1/VEGFR-2 have established its crucial role in the development of hematopoietic and endothelial cells. Genetic knockout of VEGFR-2 is embryonically lethal in mice and will not survive beyond 9.5 days postcoitum. Similar studies corroborate these findings suggesting that tyrosine residue 1175 in VEGFR-2/Flk-1 (Y1175, human; 1173 in mouse) is essential for embryogenesis (293), whereas VEGFR-1 has been involved in aberrant angioblast division and excessive proliferation (155).
Studies performed in ischemic animal models have suggested the essential role for endogenous VEGF in post-ischemic angiogenesis and limb perfusion (119, 145). Inhibition of growth factors such as VEGF can have severe implications on vascular tone and hemodynamics necessary for changes in vascular remodeling. Adenoviral inhibition of VEGF performed in a unilateral hindlimb ischemic mouse model showed significantly reduced ischemia-induced angiogenesis and collateral formation along with critically reduced blood flow impairing muscle tissue recovery (145). Endogenous upregulation of VEGF levels has been observed in several studies in models of ischemia. In the hindlimb ischemia model, VEGF levels were upregulated at days 1 and 3 postsurgery, which reflected an increase in the number of capillaries (63). Another study observed increased expression of VEGF in ischemic limbs on day 7, returning to normal after day 14 postsurgery (192). These results clearly demonstrate a correlation between enhanced VEGF levels and ischemic revascularization. Other studies have confirmed induction of VEGF and angiogenesis in animal models of ischemia including mice, rats, and rabbits (119, 199, 353). Defective VEGF ligand/receptor expression leads to impaired angiogenesis after ischemia as observed in a murine model of peripheral arterial disease (PAD) with diabetes mellitus (DM) (24, 119). Additionally, VEGF may lead to changes in vascular integrity leading to dysregulation of the angiogenesis-regulatory network (304). These observations have been confirmed in clinical studies where VEGF is compromised in ischemic vascular diseases, including PAD and diabetes, leading to impaired collateral formation (160, 253, 384). However, other factors along with a balance between pro- and antiangiogenic markers also play a role in pathophysiological regulation of vessel growth (152). Additionally, both NO and H2S signaling have been shown to play a critical role in VEGF-induced ischemic vascular remodeling (22, 92, 163, 396). Moreover, genetic loss of either eNOS or CSE in mice affects VEGF expression, and impairs VEGF-mediated vascular growth and blood flow recovery in ischemic hindlimbs (22, 163, 396).
Other growth factors
Several other growth factors and cytokines including angiopoietin-1, placental growth factor (PlGF), platelet-derived growth factor, granulocyte colony-stimulating factor (GCSF), hepatocyte growth factor (HGF), and the FGF family (such as acidic FGF and basic FGF) have beneficial effects on revascularization including angiogenesis and collateral growth response. Monocytes/macrophages and other inflammatory cells can release these growth factors. Furthermore, other factors including fibronectin and proteoglycans will aid in remodeling of the extracellular matrix, which serves as a biological reservoir for growth factor accumulation and action. There are a number of animal studies performed in ischemic models that have demonstrated the role of these factors in improving vessel growth (114, 330). Moreover, these growth factors and cytokines induce, enhance, and mobilize progenitor cells and recruit them to the site of injury, contributing to the process of neovascularization (163,252).
FGFs are potent angiogenic inducers and are involved in stimulating fibroblast maturity. Adenoviral treatments of FGF in a rabbit hindlimb ischemia model have increased collateral growth and muscle perfusion (289). FGF proteins may induce angiogenesis and arteriogenesis, suggesting a therapeutic utility for peripheral vascular disease (PVD), is a systemic disorder that involves the narrowing of peripheral blood vessels (vessels situated away from the heart or the brain) as a result of arteriosclerosis or atherosclerosis (vascular lipid plaque deposition) (154). Clinical studies were performed on patients with angina pectoris and coronary artery disease (CAD) through FGF therapy via gene transfer (112, 113, 125). The Angiogenic Gene Therapy (AGENT) trial targeted FGF delivery as a therapeutic modality for CAD with results in patients of improved regional myocardial perfusion and treadmill times (112, 113). However, it seems that the clinical treatment has gender-specific angiogenic responses with males responding better than females (125). Another study confirmed the role of bFGF role in regulating arteriogenesis. It was observed in a diabetic mouse hindlimb ischemia model that reduced levels of bFGF lead to impaired collateral growth and blood flow (20). However, this impaired collateral circulation in diabetic mouse hindlimbs can be restored by exogenous bFGF therapy. In one of the major randomized clinical trials conducted in patients with intermittent claudication, called the “TRAFFIC study,” patients were treated with bFGF to study its therapeutic potential for angiogenesis (180). Improvements in peak walking time and ankle-brachial pressure index were observed in bFGF treated group compared to the placebo group. However, positive effects of these growth factors were also coupled with increased proteinuria in patients treated with bFGF (179,180). Platelet activation upregulates platelet-derived microparticles (PMP) that induce revascularization following chronic ischemia (32). PMPs mediate upregulation of growth factors including VEGF and PDGF that induce angiogenesis in ischemic myocardium of mice. Another study performed in a diabetic mouse model of ischemia has suggested the role of PDGF-C as a critical regulator and a novel target for the treatment of ischemic cardiovascular diseases in diabetes (225). GCSF is a potent cytokine that influences the function of granulocytes and improves the function of cardiomyocytes, endothelial cells and mobilizes bone marrow-derived progenitor cells to the site of injury and enhances collateral and capillary development (70, 175, 352).
Monocytes
Monocytes play a key role in mediating arteriogenesis. Stimulation of monocyte recruitment has evidenced an increase in responses toward collateral vessel growth under ischemic conditions (90,96). Studies indicate that recruitment and transformation of monocytes into macrophages at the site of injury is crucial during arteriogenesis (90, 96, 307). During early stages of vessel remodeling, signaling factors such as NO stimulate monocyte recruitment through modulation of cytokines and growth factor expression to promote vascular remodeling (181, 385). Additionally, tissue macrophages can stimulate the fusion of endothelial tip cells, leading to the formation of a vascular network (82). Growth factors such as PlGF and VEGF and signaling molecules including NO and H2S play an important role in monocyte recruitment (163, 311). PlGF activates monocytes resulting in the generation of proinflammatory cytokines and chemokines via activation of VEGFR-1 (271).
Impairment of monocytes can lead to reduced ischemic vascular remodeling. Several animal studies have confirmed that accumulation of monocytes/macrophages was observed at the site of ischemia leading to the growth of collateral arteries following femoral artery ligation (5, 121, 309). Dysregulation of selectin signaling can impair monocyte recruitment under diabetic settings leading to reduced vascular remodeling (41). Treatment with MCP-1 has reduced P-selectin expression and induced monocyte recruitment to the ischemic site, thereby correcting the post-ischemic vascular deficits in remodeling and blood flow recovery in ischemic limbs (41). Another study performed using isolated human CD14 positive monocytes suggest that these cells can attain endothelial cell properties expressing their markers. The proliferation of these monocyte transformed endothelial cells is mediated by upregulation of VEGF, ICAM-1 and vascular cell adhesion molecule-1 (VCAM-1) that induce vWF expression (83). A separate study performed in ICAM-1 knockout mice showed that there is a significantly decreased number of monocytes that resulted in delayed arteriogenesis (132). Furthermore, studies performed in osteopetrotic (op/op) mice demonstrated that collateral artery growth is impaired following inhibition of monocyte differentiation by blocking Macrophage Colony-Stimulating Factor (M-CSF) (15, 174). These studies strongly suggest that monocytes/macrophages play a key role in mediating different growth factors and chemokines and facilitate vessel remodeling through arteriogenesis.
Shear stress
Changes in blood flow stimulate adaptive changes in the vasculature in terms of structure and size of the blood vessels. Mechanical forces such as shear stress trigger a cascade of biochemical signaling that mediates changes in biological events. These external forces also induce morphological and structural adaptations in the endothelium and the vessel wall. Established literature suggests that increased shear stress can demonstrate vasoprotective roles that are mediated via growth factors including VEGF, FGF, and PDGF, and signaling factors such as nitric oxide. Recently, we have revealed that CSE/H2S signaling is altered in response to flow and regulates vascular remodeling mediated by altered shear stress (401).
Shear stress plays a key regulatory role in the process of arteriogenesis. In vitro studies have clearly established the importance of laminar flow in regulating endothelial signaling and functions leading to vessel remodeling (33, 412). Studies performed by increasing the collateral blood flow through increased shear pressures demonstrate a direct role of shear stress in increased vessel growth (80,270). A study performed in a pig model of hindlimb ischemia demonstrated that there was an increased number and size of collateral vessels formed following an abrupt increase in collateral blood flow (270). However, this shear-mediated collateral formation can be completely inhibited by blocking NO/VEGF/Rho signaling pathways (300). Shear stress can upregulate signaling mechanisms that promote monocyte recruitment and factors that facilitate its adhesion to the endothelium including MCP-1, ICAM-1, and VCAM-1 (282, 331). Furthermore, studies performed in multiple animal models have confirmed that MCP-1, VEGF, and ephrinB2 can induce collateral vessel formation through monocyte/macrophage recruitment. Additionally, a few studies have suggested that these monocytes/macrophages can be differentiated into endothelial cells (307, 331).
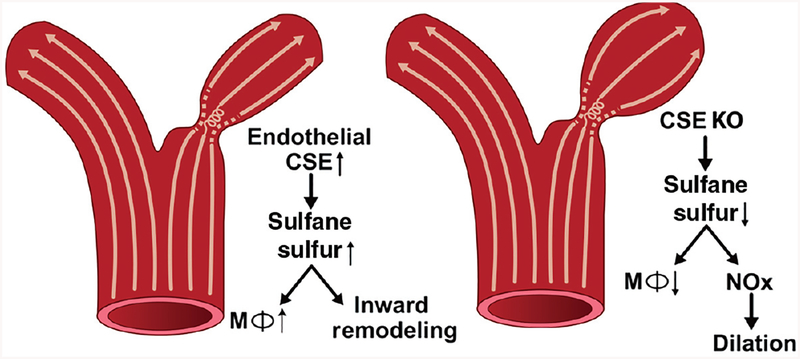
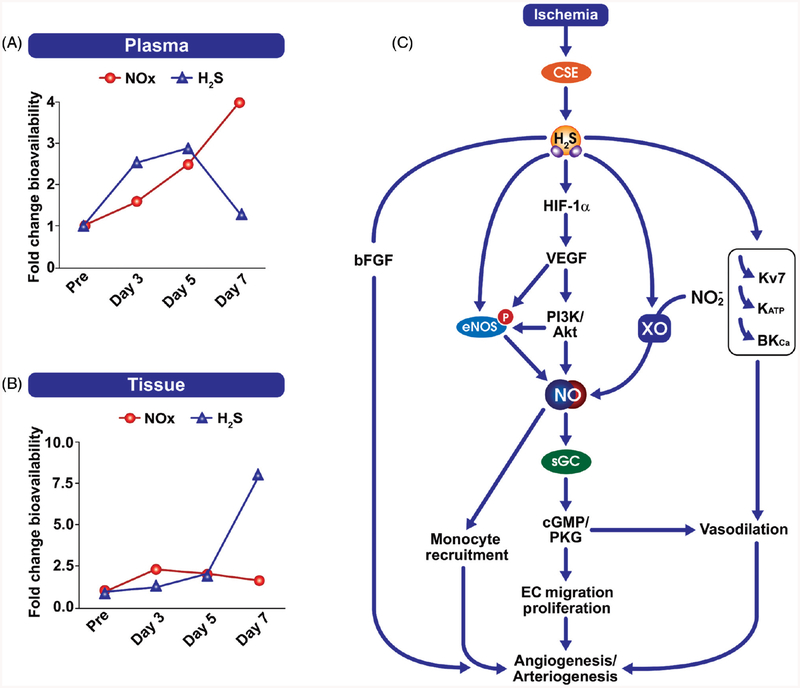
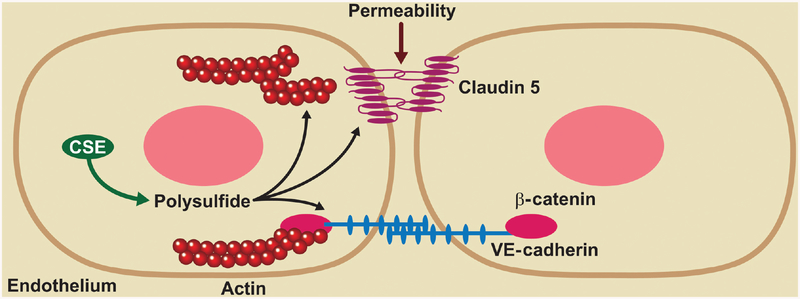
Unidirectional laminar flow is associated with elevated NO bioavailability, reduced oxidative stress, and suppression of proinflammatory genes. However, in disturbed flow regions, there is reduced NO production, increased reactive oxygen species and increased proinflammatory genes (402). Our lab has recently shown that CSE expression is regulated by shear stress (400). We found that oscillatory shear increased CSE protein expression, sulfane sulfur levels, and increased macrophage recruitment in regions of disturbed flow. Using the partial carotid ligation model to study inward vascular remodeling, genetic deficiency of CSE showed reduced medial thickening and a dilated arterial phenotype (Fig. 2). Interestingly, carotid vessel NO levels were increased in CSE knockout mice and that treatment with the NO scavenger cPTIO corrected the reduction in inward vascular remodeling (400). These findings reveal CSE expression to be important in shear dependent responses of atheroprone vascular regions involving both endothelial activation and flow-dependent vascular remodeling through altered NO bioavailability.
Figure 2.
CSE modulates flow induced vascular remodeling: CSE expression and sulfane sulfur production are enhanced by disturbed flow in conduit vessels. The enhanced CSE expression causes increased macrophage recruitment in these areas leading to flow induced vascular remodelling. In case of CSE knockout animals, they showed reduced sulfane sulfur levels following partial carotid artery ligation, defective inward remodeling, and a dilated vascular phenotype. The dilated phenotype is due to elevated NO bioavailability in CSE knockout carotid arteries.
Reactive oxygen species
Oxidative stress is a key player influencing many vascular functions under normal and diseased conditions (89, 110). Reactive oxygen species (ROS), including superoxide, hydrogen peroxide and the nitrogen derivative peroxynitrite, are reactive species that are associated with cardiovascular pathology (21, 71). Increased ROS production may also be associated with inactivation of NOS and reduced NO bioavailability. Sources of ROS formation may vary with studies reporting that NADPH-dependent oxidases (Noxs), superoxide dismutase (SOD), xanthine oxidase (XO), nitric oxide synthases (NOSs), and mitochondrial oxidases as all potent sources of ROS (68).
However, ROS may also serve role as a pro- angiogenic and arteriogenic mediators. Evidence from cardiovascular disease animal models and human studies suggests a significant role of NADPH oxidase activation in vascular remodeling and endothelial dysfunction. The isoforms of Nox including Nox1, Nox2, Nox4, and Nox5 may be expressed in endothelial cells. They vary in their subcellular localization and the type of ROS they produce, and can play important roles in regulating the production of VEGF and subsequent events of ischemic revascularization.
Studies performed in Nox2 deficient mice demonstrated the importance of the ROS mediated role in neovascularization under tissue ischemia (356). Reduction of Nox2-derived ROS production inhibited VEGF-A thereby affecting blood flow recovery and capillary density under ischemic conditions. Nox4 has also been implicated for its cytoprotective role and revascularization. Nox4 is highly expressed in vascular endothelial cells and is constitutively active and produces hydrogen peroxide. Protective roles of Nox4 were also observed by a reduction in oxidative and ER stress signaling. Animal studies performed in Nox4 knockout mice and transgenic overexpression reveal that endogenous Nox4 protects the vasculature during ischemic or inflammatory stress (64, 310). Similarly, upregulation of Nox4 in the cardiomyocytes plays a protective role post-myocardial infarction (MI). Nox4 modulates macrophage polarization toward an M2 phenotype, resulting in improved post-MI vascular remodeling by attenuating MMP-2 activity in cardiomyocytes (223).
Extracellular superoxide dismutase (ecSOD) is another ROS associated enzyme that plays a regulatory role in the maintenance of vascular functions (153). ecSOD is an endothelial expressing enzyme that regulates the NO bioavailability, blood pressure, and vascular O2•− levels. Studies performed in mice lacking ecSOD has demonstrated an impaired post-ischemic revascularization (153). Whereas, restoration of ecSOD in mice upregulates muscle H2O2 levels and accelerates vasculogenesis in ischemic muscles (365). Another study demonstrates that SOD reduces pulmonary hypertension, reduces ischemic injury, and induces vascular remodeling (326). Additionally, depletion of vascular ecSOD augments chronic hypoxic pulmonary hypertension and inhibits hypoxia-induced vascular remodeling (242). However, excessive and prolonged ROS generation inhibits cellular functions and induces apoptosis.
ROS and ER stress: Redox homeostasis in endoplasmic reticulum (ER) is quite important. A simple alteration in ER-regulated protein folding pathways may lead to enhanced ROS production, and enhanced ROS levels have a profound effect on the ER protein folding process, thus affecting cellular homeostasis. ER stress particularly plays an important role in the initial cellular adaptation of ischemia and angiogenesis. Mild ER stress upregulates VEGF expression and mediate appropriate tissue vascular supply under ischemia (103). Insufficient metabolites and oxygen supply and nutrient deprivation especially during the events of hypoxia/ischemia may upregulate ER stress signaling (19, 266). These stressors can be alleviated during neovascularization within the ischemic tissue. ER stress transcription factor, C/EBP homologous protein-10 (CHOP-10) can inhibit eNOS transcription subsequently inhibiting ischemic revascularization, whereas postischemic revascularization has been restored in CHOP-10 knockout mice (103). Accumulation of ROS leads to defective postischemic revascularization in mice models of diabetes and hypercholesterolemic mice (77, 370). It was observed that the inhibited vessel growth in diabetes is associated with an upregulation of ER stress markers under ischemia (53). However, the role of ER stress regulation is a complex and poorly understood phenomenon in relation to endothelial functions and vascular remodeling.
NO and H2S Pathophysiological Functions
Nitric oxide
Synthesis
NO, the first identified gasotransmitter, is generated in a variety of biological tissues and is an important regulator of key functions in the cardiovascular system (222, 417). NO is produced by enzymatic and non-enzymatic sources in and around vasculature. Enzymatic production of NO is primarily mediated by NOS family of proteins. As discussed in the introduction, NOS has three different isoforms namely neuronal NOS (nNOS), inducible NOS (iNOS), endothelial NOS (eNOS). Although these isoforms are genetically different, they all produce NO by catalyzing the oxidation of L-arginine to L-citrulline in the presence of various cofactors. All three NOS isoforms have been reported in the cardiovascular system; however, eNOS is the dominant NOS isoform in vascular endothelium (107).
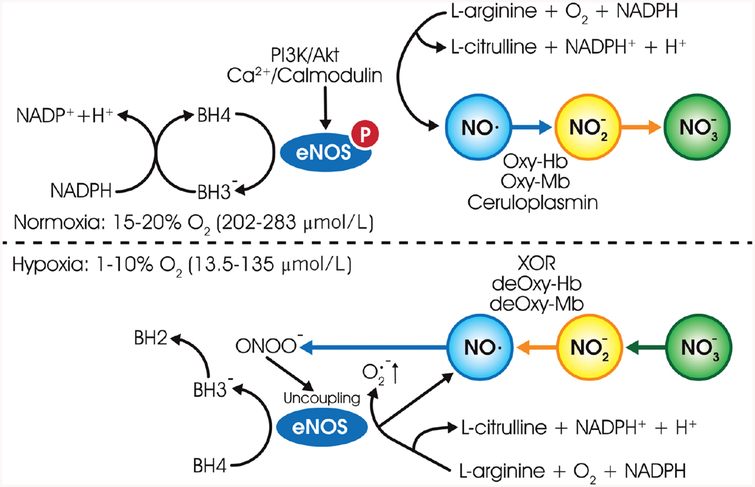
Although eNOS functions as a homodimer, it can be functionally divided into two major domains: a C-terminal reductase domain, and an N-terminal oxygenase domain (190). The dimer is stabilized by the binding of 5, 6, 7, 8-tetrahydrobiopterin (BH4) and zinc to the N-terminal oxygenase domain. Electron transfer starts when reduced NADPH binds to the C-terminal of reductase domain of the one of a monomer of NOS dimer. Within this reductase domain, electron transfers from NADPH to FAD followed by flavin mononucleotide (FMN). FMN further shuttles the electron from reductase domain to the heme of oxygenase domain of the NOS dimer. The reduced heme then catalyzes the conversion of L-arginine to L-citrulline with the NO being the by-product under normoxic conditions (Fig. 3). However, under hypoxic conditions, nitrite may be reduced by reductases, including xanthine oxidase, deoxyhemoglobin, deoxymyoglobin to produce NO. Further eNOS uncoupling occurs under hypoxic stress leading to the production of superoxide instead of NO. In addition, BH4 oxidation also occurs further leading to eNOS uncoupling and conversion of BH4 to BH2 (Fig. 3).
Figure 3.
The nitric oxide synthesis pathway: under normal oxygen parameters (normoxia), in the presence of NAPDH and cofactors such as BH4, FMN, FAD, and eNOS catalyze the conversion of L-arginine to L-citrulline and NO. Further, one electron oxidation of NO catalyzed by ceruloplasmin in plasma and cytochrome c oxidase in tissues yields nitrite, further with relatively faster two-electron oxidation yielding nitrate by heme proteins in blood and tissues. In the absence of oxygen (hypoxia), nitrite is reduced by reductases, including xanthine oxidase, deoxyhemoglobin, deoxymyoglobin to produce NO. Further, oxidative stress leads to BH4 oxidation and eNOS uncoupling leading to superoxide production.
While eNOS is the primary source of NO in the vasculature, enzyme-independent NO generation also occurs. It is reported that dietary nitrate enters the enterocirculatory system and gets absorbed and concentrated in salivary gland where it is readily converted to nitrite by facultative anaerobic bacteria on the surface of the tongue (295). This nitrite is swallowed and may be converted to nitrous acids and nitric oxide under acidic conditions (pH 3) in the lumen of the stomach (13). Nitrite may also re-enter the circulation and be reduced to NO during ischemia or hypoxic conditions, the reactions mediated by xanthine oxidase (XO) (381), deoxyhemoglobin (106), deoxymyoglobin (329), or aldehyde oxidase (AO) (Fig. 4).
Figure 4.
A schematic diagram of nitrate, nitrite, and nitric oxide (NO) pathway from exogenous (dietary) sources: dietary nitrate taken (1) is absorbed systemically (2) and is concentrated 10-fold in the salivary gland and enters the enterosalivary circulatory system where it is converted to nitrite by bacterial nitrite reductases on the dorsum of the tongue (3). When nitrite reaches the lumen of the stomach, acidic gastric juice converts nitrite to nitrosating species that can further react with ascorbic acid in gastric juice to yield NO (4). It can also reenter the circulation as nitrite and be reduced to NO by xanthine oxidase (XO) and aldehyde oxidase (AO) (4). Nitrite in the arterial circulation may also be reduced to nitric oxide due to hemoglobin deoxygenation causing vasodilation (5).
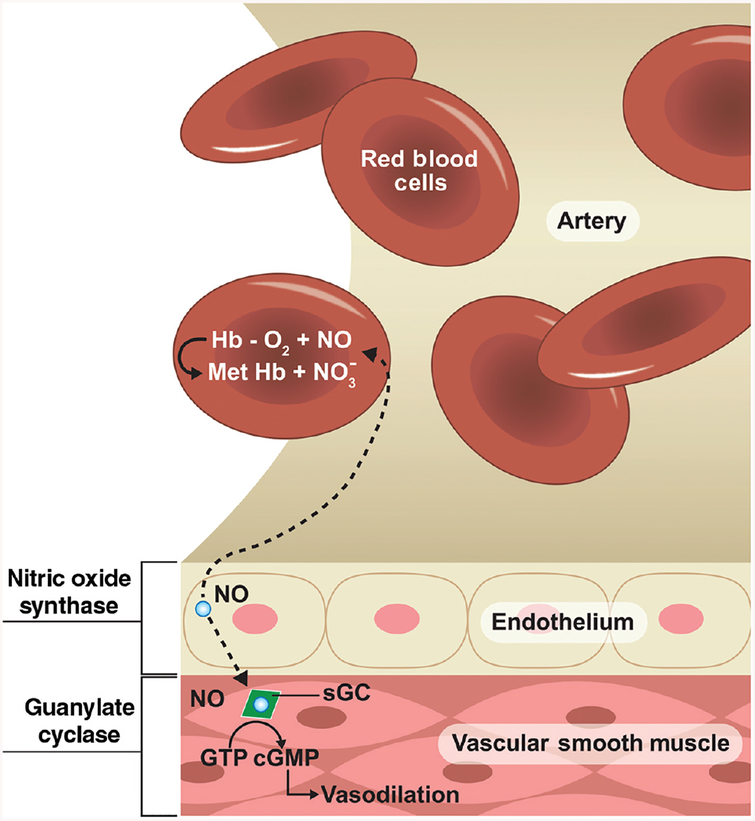
The rapid diffusion of nitric oxide between cells is crucial for understanding its biological activity. Primarily, nitric oxide produced in the endothelium activates soluble guanylate cyclase, which in turn initiates the production of the messenger cGMP (cyclic guanosine monophosphate). cGMP causes the smooth muscle cells in the vessel walls to relax leading to vasodilation. NO also diffuses into red blood cells and quickly reacts with oxyhemoglobin to form methemoglobin and nitrate (Fig. 5).
Figure 5.
Principal reactions of nitric oxide in tissues: nitric oxide produced in the endothelium diffuses into vascular smooth muscle cells and activates soluble guanylate cyclase, which in turn initiates the production of the messenger cGMP (cyclic guanosine monophosphate). cGMP relaxes smooth muscle cells in the vessel walls leading to vasodilation. NO can also diffuse into red blood cells and react with oxyhemoglobin to form methemoglobin and nitrate.
NO chemistry and measurement
Chemical properties
Nitric oxide (NO) has been established as a signaling molecule regulating several pathophysiological processes due to its unique chemical properties. It can easily diffuse across cellular membranes and into tissues with little reactivity (177, 386). It is important to understand the chemical properties of NO, which is a principal determinant of its biological action. NO is relatively unstable (half-life: 2~30 s) due to its unpaired electron, and it can gain or lose one electron to form the ions NO− or NO+ (196). However, nitric oxide is not highly reactive just because it is a free radical. This is due to the fact that the unpaired electron of NO is in the higher energy antibonding Pi orbital that can react only with another unpaired antibonding Pi electron from another free radical. However, most of the physiologically available free radicals do not have a unpaired antiboding Pi electron. Consequently, NO reacts only with a select few free radicals and transition metals like heme iron at a faster rate to have any physiological consequences.
NO has vastly different reaction rates with different molecules as a guide, the biological chemistry of nitric oxide can be simplified by reflecting its biological activity. NO can react with oxygen and water and be converted into nitrogen dioxide (NO2) and nitrous acid (HNO2), respectively. HNO2 is a weak and monobasic acid (pKa: 3.398). NO may also further oxidize to nitrate (NO3−). NO reacts with O2− (superoxide anion) to form peroxynitrite anion (OONO−), the deprotonated form of peroxynitrous acid. Peroxynitrite (ONOO−) is an unstable structural isomer of nitrate, which is generated from the reaction between NO and superoxide. Importantly, peroxynitrite is a not a free radical, but rather a powerful oxidant.
Decomposition of peroxynitrous acid involves the formation of HO and NO2 as intermediate and this accounts for peroxynitrite’s higher oxidizing capacity, as both the intermediates are potent oxidants. Whereas OONO- reacts with unsaturated fatty acids to form lipid peroxide, NO shows the opposite effect of terminating lipid peroxidation chain reaction. Consequently, NO per se is not toxic unless it gives rise to peroxynitrite, which is toxic. NO is involved in activation of guanylate cyclase (sGC), which is responsible for downstream signaling effects to regulate vascular tone. As NO is a small molecule that rapidly diffuses into capillary circulation and is consumed by oxyhemoglobin, its tissue level is determined by its rate of production and latter two factors.
NO also can be reduced to nitroxyl (HNO). Unlike most all other acid-base equilibriums, the chemistry of HNO is complex due to its electronic ground states: the protonated HNO species is a ground state singlet (1HNO) and the deprotonated anion is a ground state triplet (3NO−). Deprotonation of 1HNO or protonation of 3NO− requires a change in the electronic spin state, which make this process slow. Hence, numerous other reactions will occur before they deprotonate or protonate to set up an acid-base equilibrium (317).
Measurement
It is extremely important to specifically measure exact NO levels in biological samples to understand its role in physiological and pathological processes. To date, there are several commonly used methods to measure NO.
Griess assay
Nitrite (NO2−) is one of the primary, stable, and nonvolatile breakdown products of NO. The Griess assay is used to measure nitrite as a surrogate for NO bioavailability. This assay was originally described by Griess in 1879 (269), which uses sulfanilamide and N-1-napthylethylenediamine dihydrochloride (NED) under acidic (phosphoric acid) conditions. Through the years, many modifications to the original reaction have been described. This assay can be used to detect nitrite in a variety of biological and experimental liquid matrices such as plasma, serum, urine, and tissue culture medium. The nitrite sensitivity depends on the matrix. The limit of detection is 2.5 μmol/L nitrite (in ultrapure, deionized distilled water). This method has become one of the most popular assays for determining NO bioavailability. However, there are several issues that must be considered including: (i) this is an indirect assay of NO levels; (ii) the spectrophotometric plate reader-based assay is limited in its detection sensitivity; and (iii) simultaneous measurement of nitrite and nitrate often overestimates the amount of NO produced based on the fact that nitrate metabolism can be independent of NOS activity.
Chemiluminescence detection and electron paramagnetic resonance (EPR) spectrometry
Real-time NO analysis can be performed using a GE Sievers Nitric Oxide Analyzer (NOA 280i) or other similar NO analyzers. Using highly sensitive, ozone-chemiluminescence technology, it has unsurpassed versatility for liquid and exhaled breath nitric oxide (NO) measurement. This assay takes advantage of NO reaction with ozone to generate photons within a reaction chamber enabling highly sensitive detection of NO metabolites including nitrite, nitrate, and nitrosothiols in the biological samples. A reaction sparger is coupled to the detector that allows significant experimental flexibility to measure NO levels itself; the amount of NO contained within specific metabolites upon chemical liberation, and rates of NO production from chemical and cell models. Another method of direct NO detection is by using electron paramagnetic resonance (EPR) spectrometry, which can be used to detect formed stable NO adduct after endogenous NO reacted with several iron complexes, such as hydrophobic Fe/diethyldithiocarbamide or Fe2+-N-methyl-d-glucaminedithiocarbamate. Low sensitivity and trapping agent selectivity can pose challenges for this approach (126).
For liquid samples, chemiluminescent detection uses different workflow techniques to measure nitrate, nitrite, and nitrosothiols. Nitrate is the major oxidation production of NO in the presence of oxyhemoglobin or superoxide, and chemiluminescence can measure total nitric oxide by converting the nitrate and nitrite to NO using vanadium (III) chloride in hydrochloric acid at 90°C. Nitrite is the major oxidation production of NO in the absence of oxyhemoglobin or superoxide, and can measure nitrite by converting the nitrite to nitric oxide using iodide and acetic acid at room temperature. Measurements of low molecular weight compounds such as S-nitrosoglutathione are made using a Cu(I)/Cysteine reagent. Measurements of higher molecular weight species such as S-nitroso-albumin or hemoglobin are made using iodine, iodide, and acetic acid after chemical or chromatographic removal of nitrite.
Electrochemical detection
The NO electrochemical sensor allows the measurement of NO concentration in the biological samples ranging from subnanomolar to micromolar levels (409). NO can be either electrochemically reduced or oxidized, and the resulting transfer of electrons is measured as a current proportional to the NO concentration. One of the commercially available systems is amiNO, which allows consumers to choose the size, flexibility, durability, sensitivity, and response time for their system with a limit of NO detection (1.0 nmol/L).
Fluorescence probes
Fluorescent NO probes have been developed to facilitate real-time detection of NO. There are various fluorescent probes available for the detection of NO including the well-used diacetate based diaminofluorescein and rhodamine dyes (187,408). Additionally, recent developments have seen a flurry of fluorescent dyes with improved chemical detection, which includes o-diamino aromatic compounds (283, 346), luminescent lanthanide complexes (354), transition-metal complexes (48, 127), quantum dots (65, 379), and carbon nanotube sensors (105, 149). The sensitivity of these probes are <10 μmol/L, but these fluorescent probes may have issues with specificity (detection of other thiols vs. nitrosating species) and autofluorescence based on the biological substances.
Implications for vascular growth and remodeling
As discussed earlier, vascular remodeling is the process during which the vessel wall reorganizes its cellular and extracellular components in response to a chronic stimulus, for instance, arterial occlusion. This arterial occlusion initiates a signaling cascade that leads to ischemic vascular remodeling creating a smaller vascular lumen. This reduction in the artery function leads to changes in blood flow and oxygen tension. Both angiogenesis and arteriogenesis respond differently to these changes. Under pathological conditions, the change in hemodynamic forces acts as a primary stimulator of ischemic vascular remodeling. Previous reports have identified the essential molecules for vasculogenesis, angiogenesis, and remodeling, and that when deleted, cause embryonic lethality due to vascular defects (57, 231, 413). Among these molecules, NO is essential for endogenous collateral development and angiogenesis under ischemia.
Following occlusion of artery, as an initial response to the ischemic environment, NO-mediated vasodilation occurs temporarily leading to increased perfusion of collaterals. An increase in collateral recruitment leads to an increase in wall shear stress, which is a mechanical stimulus that activates eNOS expression and activity. Studies have shown that collateral artery diameter and blood perfusion within the ischemic hindlimb was significantly reduced in eNOS−/− and L-NAME treated mice subjected to femoral artery ligation (FAL) (260). Furthermore, eNOS−/− mice showed reduced VEGF-induced mobilization of endothelial progenitor cells (EPCs) and increased mortality after myelosuppression. However, infusion of wild-type progenitor cells, rescued the phenotype in a mouse model of hindlimb ischemia, suggesting the importance of eNOS in the mobilization of progenitor cells for the collateral growth (3). On the contrary, another group has shown that ischemic challenge in eNOS−/− showed defective arteriogenesis, but no impairment in endothelial progenitor cell recruitment. Additionally, they showed that the defects could be rescued by intramuscular delivery of an adenovirus encoding a constitutively active allele of eNOS, eNOS S1179D (395). A study in dogs showed a 6.2-fold increase in eNOS expression in the growing collaterals but not in mature collaterals following chronic occlusion of the left circumflex artery, suggesting the importance of eNOS in the development of collateral vessels (37). However, another study in mice suggested eNOS activity is not required for collateral growth (214). In this study using FAL in various eNOS mutant versus wild-type (WT) mice revealed a similar decrease in collateral-dependent blood flow until day 7, suggesting delayed collateral growth rather than inhibition. No difference in collateral arteries of eNOS mutant versus WT mice was observed at 1 and 3 weeks suggesting impaired recovery of blood flow in eNOS deficient mice may be due to insufficient vasodilation and not inhibition of collateral growth. In summary, eNOS is important for ischemic vascular remodeling and blood flow restoration.
Besides eNOS, NO from other sources also plays a crucial role in ischemic vascular remodeling. Studies have shown that rabbits subjected to unilateral hindlimb ischemia showed defective collateral formation when treated with L-NAME thus interrupting NO production (34). In another study, targeted deletion of eNOS delayed collateral formation but finally, normal development was observed following FAL. However, iNOS deletion blunted collateral vessels formation but with only a mild reduction of blood flow following FAL. When they combined the inhibition of both isoforms of NOS, it completely abrogated arteriogenesis suggesting the importance of NO from different enzymatic sources in collateral formation with important roles of both iNOS and eNOS (357). In addition to NOS mediated NO production, bone marrow-derived mononuclear cells, especially monocytes produce NO as a result of increased shear stress following arterial occlusion facilitating collateral formation (305, 357).
Work from our group has demonstrated that exogenous nitrite therapy is an effective therapeutic intervention stimulating collateral formation by producing NO specifically at the site of ischemia. Chronic sodium nitrite therapy restored blood flow, vascular density, and endothelial cell proliferation at the ischemic site in a NO-dependent manner following FAL (176). Another study in humans showed dilation of arteries under hypoxia that is subjected to sodium nitrite infusion suggesting hypoxic augmentation of nitrite-mediated vasodilation in vivo (203). A similar study in humans also showed nitrite-induced vasodilation is associated with a reduction of nitrite to NO and showed that hemoglobin acts as a nitrite reductase, causing hypoxic vasodilation (61). Furthermore, in a canine study, collateral growth was induced by brief (2 min), repetitive episodes of myocardial ischemia. In this study, collateral blood flow was significantly hampered in L-NAME-treated animals compared to controls suggesting that the induction of coronary collateralization requires the production of NO (211).
In addition to exogenous addition of NO donors and NO metabolites, shear stress may act as a potential trigger for NO release during ischemic stress. As discussed above, shear stress, which is already highest at the arteriolar level, further increases following arterial occlusion. Studies have shown that endothelium-dependent relaxing factors, including NO, can increase as a result of shear stress (215). Hence, this could result in the local release of NO primarily at the site of ischemia to induce collateral development. Supporting this hypothesis, studies have shown that rabbits subjected to chronic elevation of shear stress by placing an arteriovenous shunt between the common carotid artery and the external jugular vein in the carotid arteries showed increased NO production. However, inhibition of NO synthesis by L-NAME inhibits adaptive shear stress regulation in vessels subjected to chronically increased blood flow (358).
Hydrogen sulfide
Synthesis
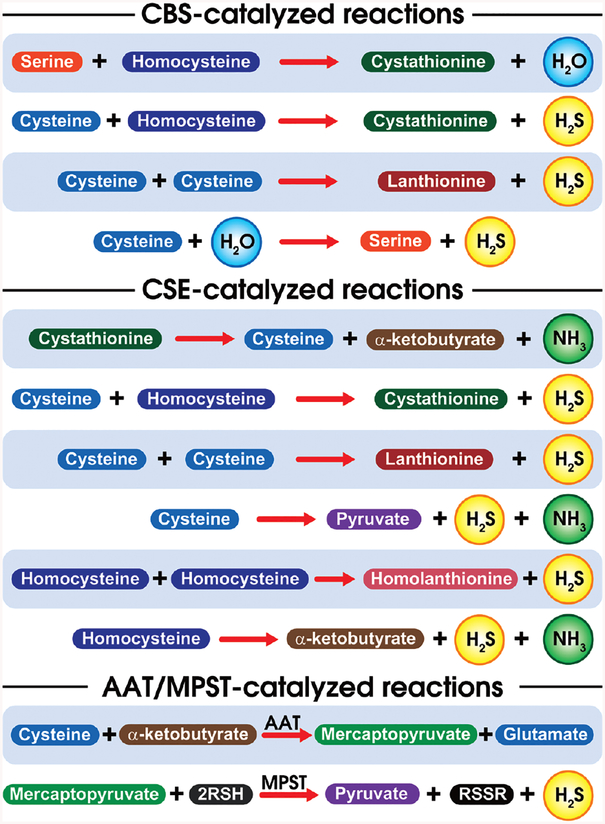
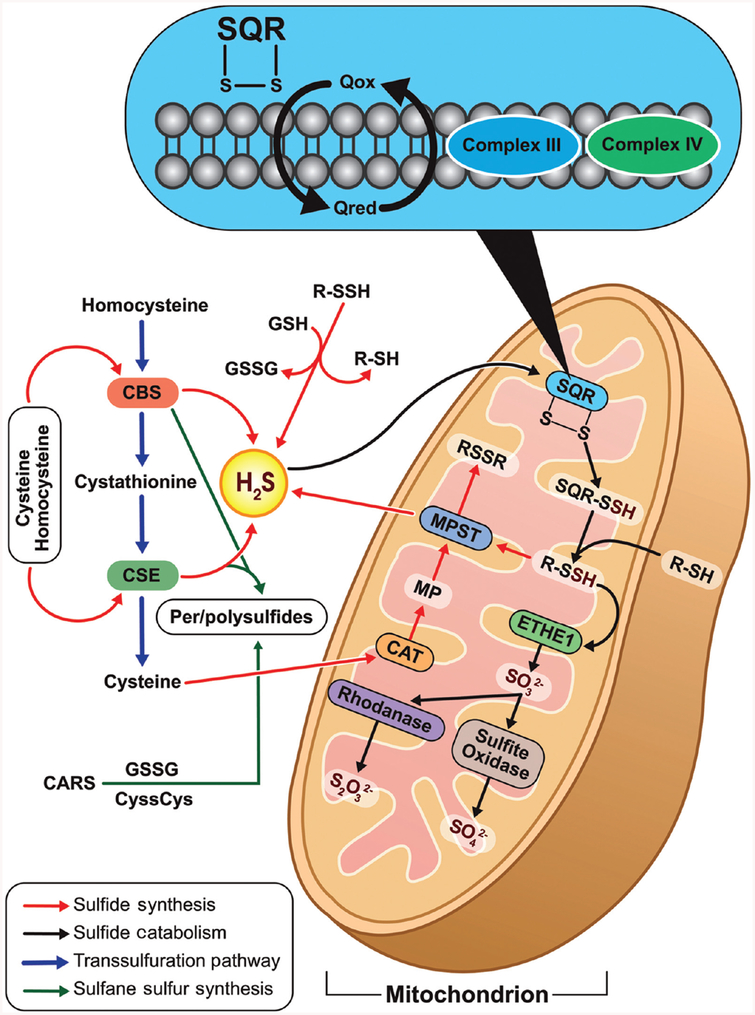
Hydrogen sulfide is the third gasotransmitter to be discovered alongside NO and CO. H2S is primarily produced from L-cysteine and homocysteine catalyzed by two pyridoxal-5′-phosphate (PLP)-dependent enzymes namely cystathionine β synthase (CBS) and cystathionine γ lyase (CSE) in the transsulfuration pathway (135, 235). CBS catalyzes the condensation of homocysteine with cysteine to yield cystathionine liberating H2S. This reaction is closely related to the canonical reaction catalyzed by CBS involving the condensation of homocysteine with serine liberating H2O (197). Although CBS has been identified in tissues throughout the body (275) it is predominantly present in the central nervous system and liver (1, 108). CSE catalyzes the conversion of cystathionine to L-cysteine and α-ketobutyrate (Fig. 6). Further, it catalyzes the conversion of L-cysteine to form thiocysteine, pyruvate, and ammonia. Thiocysteine in turn may nonenzymatically breakdown to form pyruvate and H2S (137, 197, 247). CSE has predominantly located within the vasculature (378). Besides CBS and CSE, 3-mercaptopyruvate sulfurtransferase (MPST), primarily located in the mitochondria, also enzymatically produces H2S. Cysteine aminotransferase (CAT) converts cysteine to 3-mercaptopyruvate, from which H2S is generated by MPST (235, 275, 399) (Fig. 7).
Figure 6.
Schematic representation of H2S generating reactions: this figure is a schematic representation of H2S generating reactions of CBS, CSE, and MPST. CBS and CSE, catalyze elimination/addition reactions at the β- and γ-positions of sulfur-containing amino acids, respectively.
Figure 7.
Regulation of H2S production: L-Cysteine can be converted to H2S via cystathionine β-synthase (CBS) or cystathionine γ-lyase (CSE), both of which require pyroxidal-5-phosphate for their activity. L-Cysteine can also be converted to 3-mercaptopyruvate via cysteine aminotransferase (CAT), which can, in turn, be metabolized by 3-mercaptopyruvate sulfurtransferase (MPST) to generate H2S. Further H2S is oxidized by sulfide quinone oxidoreductase (SQR) in mitochondria to produce SQR-persulfide. Sulfur dioxygenase oxidizes persulfide to sulfite (H2SO3), which is metabolized by rhodanese to produce thiosulfate (H2S2O3). The balance between H2S synthesis and catabolism determines its cellular concentration.
Intracellular H2S exists in various biochemical forms including free sulfide, acid labile sulfide, and bound sulfane sulfur. These sulfide pools are important in regulating the amount of bioavailable of sulfur, with the most relevant being acid labile and bound sulfane sulfur (281, 325). Acid-labile sulfur releases H2S in an acidic microenvironment (pH ~ 6.0 or less). This pool consists of sulfur present in iron-sulfur clusters such as rubredoxins and ferredoxins. Due to its highly unstable nature, H2S may be released when needed to regulate cellular functions. Bound sulfane sulfur containing divalent sulfur bond can release H2S in basic condition (pH 8.4) or be reduced by catalase generating H2S suggesting the cellular redox state is important in regulating sulfane sulfur bioavailability (235, 250, 281, 325).
H2S is also generated by enterobacteric microflora, mainly by colonic sulfate-reducing bacteria (321). In the gut, H2S regulates functions such as inflammation and ischemia/reperfusion injury. Its bioavailability is associated with gastrointestinal disorders such as ulcerative colitis, Crohn’s disease, and irritable bowel syndrome (336).
Chemistry and measurement of hydrogen sulfide
Chemistry of H2S
H2S is a colorless gas with the odor of rotten eggs, and is slightly soluble in water, resulting in the formation of a weak acid with an acid dissociation constant (pKa1) of 7.04 and pKa2 of 19 at 37°C (324). According to the acid dissociation constant, at physiological pH and 37°C, ~20% of sulfide is present as an H2S gas, whereas at physiological pH and 25°C, ~40% of sulfide is present as an H2S gas. Anionic deprotonation products of H2S are HS− and S2−. In vivo, pH favors sulfide existence primarily as H2S gas and its highly reactive anion, HS− (138, 324). Sulfur of H2S is in −2 oxidation state and can be readily oxidized. In fact, H2S gas does not react readily with oxygen, but in aqueous solutions H2S can readily undergo oxidation. Oxidation products include many sulfur compounds, such as polysulfide, polythionates, elemental sulfur, thiosulfate, sulfite, sulfate, sulfide radical, and disulfide. In addition, under neutral pH, sulfide can also form complexes with divalent metals and protein with metal center (i.e., heme) (93, 189).
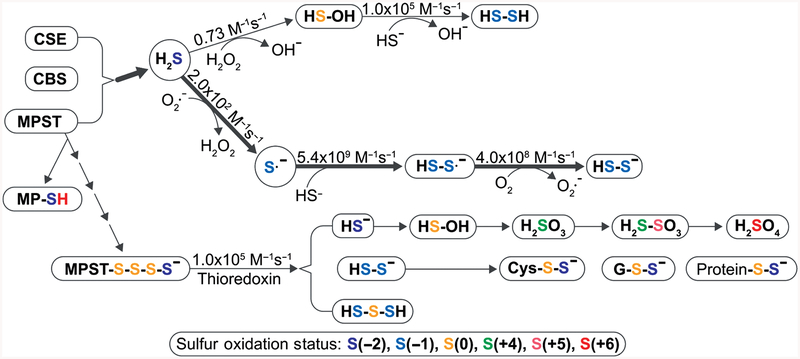
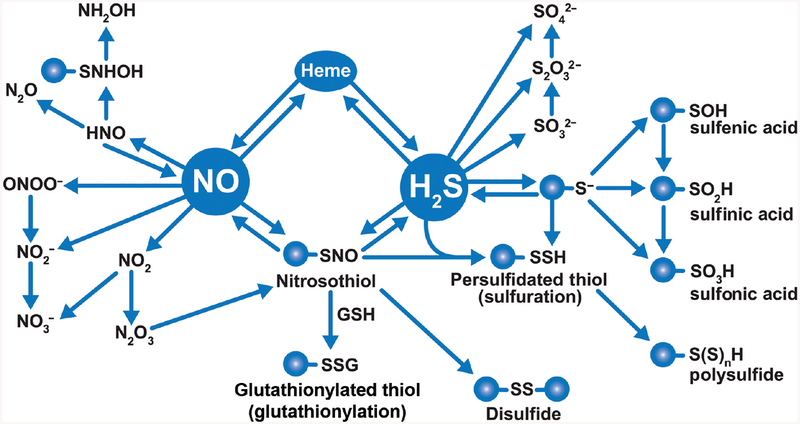
The terms thiol, sulfhydryl, and mercaptan are all used interchangeably to mean the same functional group (−SH). When two sulfur atoms form a single bond with each other, it is called disulfide (−S−S−). Depending upon the nonsulfur moieties attached to either sulfur atoms of a disulfide group, it can be named differently such as disulfide or dialkyl disulfide (RSSR), persulfide or alkyl hydrodisulfide (RSSH). The free radical form of persulfide or disulfide is called perthiyl (RSS·). If the R group in persulfide is a cysteine residue in a peptide (i.e., if persulfide was formed by transfer of a thiol to an existing thiol on a cysteine), the protein is considered to have undergone sulfuration (94). RSnH is similarly called (alkyl)hydropolysulfide and RSnR (alkyl)polysulfide (Fig. 8). Importantly, given the low Km of H2S reaction with hydrogen peroxide (H2O2) (0.73 M−1s−1) compared to superoxide (2.0 × 102 M−1s−1), it is likely that reactive oxygen species may be a major pathway for hydroper- and hydropolysulfide formation.
Figure 8.
Reactivity of H2S and H2Sn: H2S is generated by CSE, CBS, and MPST. H2S can undergo one-electron oxidation or two-electron oxidation to form HSOH or form S·-, respectively. Both of them can be oxidized to persulfide. According to the bimolecular rate constants for reaction of H2S oxidation, H2S is oxidized slower by H2O2 than that of superoxide. MPST receive sulfur from 3MP to generate MPST polysulfide chain, which can be reduced by thioredoxin (Trx) to release H2Sn, such as H2S, H2S2, H2S3, etc. Once H2S is produced, it can be oxidized to hydrogen thioperoxide (HSOH), sulfurous acid (H2SO3), thiosulfuric acid (H2S2O3), sulfuric acid (H2SO4), etc. In addition, it also reacts with intracellular thiols (cysteine, glutathione, and protein cysteine residue) to form their persulfide forms.
Measurement of hydrogen sulfide
Lack of understanding of the physiological concentrations of sulfide can be attributed to inaccurate and less-sensitive measurements. It is important to accurately measure biological concentrations of sulfide due to toxicity at higher concentrations. This problem has been overcome with recent developments in the detection of sulfide and its various pools with specific and sensitive techniques.
Many detection techniques have been developed, including colorimetric measurements, electrochemical assays, gas chromatography, etc. However, inconsistent results have been reported with these techniques due to sulfide volatilization, harsh treatment conditions, and high background activity (165). To address the physiological and pathological role of sulfide, accurate detection in biological samples remains an important consideration. Below we discuss commonly used techniques to measure H2S and associated considerations.
Methylene blue assay
The methylene blue assay was first described by Fisher in 1883 to measure hydrogen sulfide in natural waters. This assay is a simple spectrophotometric-based assay that employs zinc acetate to “trap” sulfide that is subsequently reacted with N, N-dimethyl-p-phenylenediamine under acidic conditions causing the formation of methylene blue. However, several issues have been noted by investigators including insensitive nature, harsh chemical treatments, and inability to obey Beer’s law contributing to high variability and often inaccurate measurements of biological sulfide (249, 324). Thus, use of the methylene blue assay to accurately measure biological sulfide levels may not be robust or reliable.
Electrode detection
There are many electrodes that have been developed to detect biological levels of H2S. A typical electrode consists of a sensing electrode (working electrode) and a reference electrode separated by an electrolyte, such as sulfide ion selective electrode (284, 367), gold electrodes (292), glassy carbon electrode (GCE) (319), inkjet-printed sulfide-selective electrode (IPSSE) (274) etc. Most electrode approaches have a detection range of 1~10 μmol/L. However, recent use of nanomaterials (such as carbon nanotubes and graphene) increase the sensitivity of H2S measurement, but its application to biological samples remains poorly understood due to strong chemical pretreatment.
Gas chromatographic analysis
Gas chromatographic analysis of H2S may be performed after its derivatization to bis (pentafluorobenzyl) sulfide (95), which is extracted into organic solvents followed by GC analysis. Measurement of H2S can also be performed by direct analysis of the headspace H2S gas (without derivatization) and subsequently analyzed through gas chromatography with a different detector, such as flame photometric detector (FPD) (230, 362), pulsed flame photometric detector (PFPD) (258), or mass spectrometery (MS) (129). These approaches are clearly sensitive and specific for gas H2S samples.
Fluorescent probes
In the past decade, a number of reaction-based fluorescent probes for sulfide have been developed, including Quantum Dot (QDs), dansylazide (DNS-Az), 7-azido-4-methylcoumarin (C-7Az), a dual-reacting probe based on 1,8-naphthalimide as fluorophore, and WSP1–5 7-glycidyloxy-9-(2-glycidyloxycarbonylphenyl)-2-xanthone (FEPO) (46, 227, 264, 265, 296, 375, 404). However, most of these probes exhibit some limitations such as slow response time, detection limit sensitivity, and interference from other biological thiols. Additionally, some of these fluorescent probes have excitation-emission wavelength (490–700 nm) near tissue autofluorescence wavelength and are affected by H2S levels that can be quickly scavenged by proteins or small molecular weight thiols (324). Fluorescent probes, such as SSP2 and SSP4, have been developed for sulfane sulfurs including persulfides, hydrogen persulfide, polysulfides, and protein-bound elemental sulfur. These probes work effectively under neutral to weak basic pH by releasing strong fluorescent molecules via fast intramolecular cyclization and are useful for measuring overall total sulfane bioavailability (17, 47).
Chemiluminescent detection
A unique method has been developed based on masking of fluorophores by azide. Upon exposure to H2S environment, the fluorophore gets reduced to release fluorescence. Ideally, azide-masked fluorophores function as selective probes for H2S detection. However, the inherent photosensitive azide functional group may reduce the accuracy to detect H2S and limit its application. To address the limitation of H2S-mediated azide probe, a chemiluminescent scaffold was developed to avoid unwanted photoactivation, such as azide-masked luminol compounds CLSS-1 and CLSS-2(340). Unmasking of the azide to release the free amine liberates luminol (for CLSS-1) or Isoluminol (for CLSS-2). Both of them can irreversibly react with sulfide to generate the luminescent signal. The limits of detection of CLSS-1 and CLSS-2 for H2S measurement are 0.7 and 4.6 μmol/L, respectively (7). CLSS-1 and CLSS-2 are not sufficiently soluble in neutral pH buffer, but another group has developed a water-soluble Mn-doped ZnS quantum dots (QDs) for chemiluminometric H2S measurement with a limit of detection of 0.1 nmol/L (6). These chemiluminescent detections of biological sulfide are highly specific and sensitive but do not resolve other sulfide metabolites.
Monobromobimane detection
Significant progress and improvements have been made regarding accurate hydrogen sulfide detection using monobromobimane (MBB) in biological samples. MBB can react with free hydrogen sulfide generating sulfide dibimane (SDB), which is quantified using high-performance liquid chromatography (HPLC) with fluorescence detection or mass spectrometry. MBB/HPLC assay is a much more sensitive and selective method than that of the methylene blue method (324). In addition, the MBB LC-MS/MS assay has been shown to both improve the assay’s sensitivity and also verifies the validity of the MBB/HPLC method (322). On this basis, our group developed novel workflow methods to measure acid-labile sulfide and bound sulfane sulfur by using an acidic releasing buffer (pH 2.6) containing Tris(2-carboxyethyl) phosphine hydrochloride (TCEP) followed by MBB/HPLC detection (323, 325). In this way, MBB-labeling protocols can be used to measure hydropersulfide/polysulfides and other small molecular weight sulfane sulfurs bound with significant precision and accuracy (140). However, given the progressive alkylation activity of MBB, it is important to be aware of reaction incubation times (27). Thus far, these analytical approaches have provided useful, accurate, and reliable ways to measure biological hydrogen sulfide across different studies (163, 268, 281).
Implications for vascular growth and remodeling
H2S can exert numerous biological functions ranging from cytoprotective to cytotoxic effects in a dose-dependent fashion (148, 161). Specifically, H2S exerts potent therapeutic effects in the setting of ischemia/reperfusion (I/R) injury in the heart, brain, lungs, and liver (275). A decade of studies on H2S biology has elucidated its role in the regulation of vascular homeostasis, neurological function, cytoprotection, anti-inflammation, revascularization, and therapeutic angiogenesis; along with modulation of cell survival responses, which is similar to many physiological roles of NO. It was presumed that H2S and NO exclusively worked through different signaling pathways; however, a decade of focus on H2S biology has revealed that H2S and NO are mutually required to elicit angiogenic responses (22,58), perhaps in a reciprocal manner (Fig. 9). Studies have shown that the NO scavenger cPTIO significantly blunted the effect of H2S therapy on ischemic tissue blood flow in wild-type mice (377). In addition, inhibition of endothelial NO biosynthesis with L-NAME abolished the H2S-induced microvascular endothelial cell proliferation. In addition, eNOS is required for postischemic blood flow recovery, because mice deficient in eNOS show a severe form of critical limb ischemia in mouse hindlimb models (3, 231). We also found that differential sources of NO formation (enzymatic vs. nitrite reduction) both participate during exogenous sulfide restoration of ischemic limb blood flow (22). Together, these findings indicate that sulfide serves as an important upstream activator to increase NO bioavailability during ischemic vascular remodeling.
Figure 9.
A schematic representation of H2S-mediated ischemic vascular signaling: (A and B) Plasma and tissue sulfide and NOx levels in WT mice followed by femoral artery ligation. (C) Ischemia leads to increased CSE expression, activity, and H2S generation. Further H2S increases VEGF production and phosphorylates eNOS leading to elevated NO bioavailability. H2S-dependent stimulation of NO activate the cGMP/PKG pathway by acting on sGC and regulates angiogenesis, arteriogenesis through cytokines and monocyte recruitment. H2S also leads to XO-mediated nitrite reduction to NO, under ischemic/hypoxic condition. Further, CSE-derived H2S causes vasodilation by activating ion channels and hyperpolarizing the vascular wall.
Consistent with this idea, aortic rings harvested from eNOS−/− mice exhibited no microvessel outgrowth in response to NaHS, compared with wild-type controls, demonstrating an important requirement of NO in the angiogenic effect of H2S (58). In contrast, chemical inhibition of CSE attenuated NO-mediated cGMP formation, vasodilation, and angiogenesis (58) (Fig. 9). In the rat aorta, NO donors increased CSE dependent H2S biogenesis in a cGMP-dependent manner. Furthermore, pre-incubating NO donors increased CSE mRNA and protein levels in smooth muscle cells, which ultimately result in increased sulfide production highlighting the mutual requirement of these molecules in eliciting angiogenic response (12, 49).
The regulation of endothelial permeability is important for vascular health. The vascular endothelial monolayer forms the innermost lining of all blood vessels performing a vital task of providing nutrients to the underlying tissue while regulating passage of molecules into extravascular tissue (75, 348, 369). Highly controlled passage of macromolecules between the blood and interstitial space is important for physiological homeostasis. While basal permeability involves normal solute exchange, vascular hyperpermeability precedes and accompanies a vast array of pathological and physiological events such as inflammation, tumorigenesis, ischemic injury, wound healing, and vascular growth and remodeling (397).
In late 2000, H2S importance on endothelial barrier function was initially examined. Multiple studies have demonstrated the cytoprotective effects of H2S at micromolar concentrations (212, 287, 383, 391) and cytotoxic effects (which are generally only apparent at millimolar concentrations). In experimental rats, inhalation of 80 p.p.m. of H2S decreased endothelial permeability during the early period of cardiac arrest and resuscitation, thereby diminishing brain edema at 24 h. Exogenous H2S also reduced vascular protein leak-age and leukocyte infiltration in a murine model of particulate matter-induced lung inflammation (380). H2S donors suppress leukocyte adherence to the vascular endothelium and can reduce leukocyte infiltration and thus edema formation. Suppression of endogenous H2S synthesis, through pharmacological blockade of CSE, resulted in enhanced leukocyte adhesion, leukocyte infiltration, and edema in the colon (403). We have recently reported that endothelial solute permeability is critically regulated via exogenous and endogenous sulfide bioavailability mainly via polysulfides. Importantly, knockdown or knockout of CSE resulted in increased endothelial barrier function and defective VEGF-mediated permeability, which would of course blunt physiological angiogenesis. Moreover, molecular inhibition of CSE expression was found to upregulate claudin-5 expression and enhanced tight junction arrangement which likely contributes to enhanced endothelial barrier function (398) (Fig. 10). Additional studies in this area are clearly needed to better understand how CSE production of sulfide regulates endothelial barrier responses in health and disease.
Figure 10.
Regulation of endothelial permeability by CSE-derived sulfur species: schematic overview showing the CSE-derived polysulfides increased endothelial solute permeability associated with disruption of endothelial junction proteins claudin 5 and VE-cadherin, along with enhanced actin stress fiber formation.
Experimental evidence indicates that H2S bioavailability is crucial for vascular remodeling under ischemia. Under ischemic conditions, H2S bioavailability is typically thought to increase due to decreased tissue O2 tension. However, this may also occur through altering H2S-producing enzymes (397). We found that upon femoral artery ligation in experimental mice models, CSE activity, and H2S levels in plasma and tissues goes up. However, the temporal relationship between H2S versus NO levels are different in plasma versus tissue, with early changes reflected in the plasma followed by changes in tissue levels (163) (Fig. 9). Furthermore, increase in plasma H2S level was completely blunted in CSE knockout mice and largely decreased in ischemic CSE deficient tissue, suggesting CSE as a prominent source of H2S under ischemia (163). Collateral arteriolar growth and remodeling are crucial during arteriogenesis (302). Genetic deficiency of CSE also abrogated the diameter and number of perfused gracilis arteries (163). Together these data suggest CSE critically regulates both endothelial permeability and ischemic vascular remodeling.
Other H2S-producing enzymes may also contribute vascular remodeling during ischemia. Homozygous CBS knockout (CBS−/−) animals showed high mortality rates after third and fourth postnatal weeks (376). Thus, studies on homozygous CBS knockout are limited. Nevertheless, heterozygous CBS mice (CBS+/−) serve as a model of hyperhomocysteinemia (HHcy) with significant impairment of hindlimb ischemic tissue perfusion on day 7 and day 28 compared to WT. Furthermore, dysregulation of VEGF and HIF-1α levels were observed in ischemic skeletal muscles of CBS+/−animals (365). HHcy also caused increased blood pressure and resistance, tachycardia, increased thickness and ECM accumulation in the aortic wall versus control groups. Aortic wall thickness was found to directly correlate with plasma Hcy levels. Dietary supplementation with 3-deazaadenosine (DZA), a Hcy scavenger rescued these effects (254). Similarly, a separate study showed reduced hind limb blood perfusion following FAL in CBS+/− animals compared to CBS+/+ (29). Together these results suggest that HHcy likely underlies vascular remodeling defects in CBS mutant mice versus deficiencies in sulfide bioavailability. Although homozygous and heterozygous 3MST knockout mice are available, no data have been published on their cardiovascular characteristics (234).
In addition to the genetic mutants of H2S producing enzymes, other H2S synthesis inhibitors were also used in several studies to understand ischemic vascular remodeling. Hypertensive rats showed reduced CSE activity in thoracic aorta and reduced plasma H2S levels. Wistar rats treated with proparglglycine (PAG) showed reduced CSE expression and activity, along with elevated blood pressure and impaired vascular remodeling. However, exogenous sulfide therapy rescued these effects in PAG treated rats and hypertensive rats (390). In summary, these studies highlight that H2S regulates numerous vascular function involved in growth and vascular remodeling.
NO-H2S Interactions and Intracellular Signaling
Chemical cross-reactivity (direct vs. indirect)
Both NO and H2S are chemically reactive gaseous molecules, and the nucleophilic properties of H2S and electrophilic characteristics of NO suggest a possible interaction to generate new intermediates in the biological processes. It is unlikely that direct reaction between NO and H2S would occur. However, nitric oxide can react with oxygen radicals to generate other nitrogen reactive species including nitrite, N2O3, and ONOO− (74). These reaction products of NO and other NO metabolites (e.g., electrophilic nitrated fatty acids) can directly react with hydrogen sulfide (such as hydrogen sulfide reaction with peroxynitrite to form sulfinyl nitrite (HSNO2)), which may dissociate into NO· and HSO. Reaction of NO with a sulfide radical can form nitrosothiol (RSNO), which is a reactive nitrogen species, and further reacts with glutathione (GSH) or thiol, resulting in the production of glutathionylated thiol (RSSG), disulfide bonds (RS-SR), or sulfurated thiol (PSSH, PS-(S)n-SH), respectively. Hydrogen sulfide by itself may react with RSNO to form thionitrous acid (HSNO), which further reacts with sulfide to form nitroxyl (HNO). HNO is a protonated form of NO and is highly reactive to nucleophiles such as free thiols. HNO can quickly dimerize to hyponitrous acid (H2N2O2), which is then dehydrated to nitrous oxide (N2O). HNO can also generate hydroxylamine ammonia (NH2OH). In addition, NO and H2S metabolites also can be oxidized to form dinitrogen trioxide (N2O3), nitrogen dioxide (NO2), nitrite (NO2−), peroxynitrite (ONOO−), nitroxyl (HNO), sulfenic acid (R-SOH), sulfinic acid (R-SO2H), sulfonic acid (R-SO3H), sulfite (SO32−), sulfate (SO42−), respectively. R-SOH can be directly reduced to free thiol by Trx, or further oxidized to generate R-SO2H or R-SO3H (Fig. 11). Though there are increasing studies demonstrating NO-H2S interaction, it is still unclear whether the kinetics of NO-H2S reaction and formation of these compounds are biologically significant.
Figure 11.
A schematic diagram of the interaction of NO and H2S: NO and H2S are directly involved in regulation of heme function. The reaction of NO and sulfide radical can form nitrosothiol (RSNO), which can also be produced from the reaction of dinitrogen trioxide (N2O3) and thiol. RSNO is reactive nitrogen species, and further reacts with glutathione (GSH) or thiol, resulting in the production of glutathionylated thiol (RSSG), disulfide bonds (RS-SR), or sulfhydrated thiol (PSSH, PS-(S) n-SH), respectively. NO and H2S also can be oxidized to form dinitrogen trioxide (N2O3), nitrogen dioxide (NO2), nitrite (NO2−), peroxynitrite (ONOO−), nitroxyl (HNO), sulfenic acid (R-SOH), sulfinic acid (R-SO2H), sulfonic acid (R-SO3H), sulfite (SO32−), sulfate (SO42−), respectively. R-SOH can be directly reduced to free thiol by Trx, or further oxidized to generate R-SO2H or R-SO3H. HNO can quickly dimerize to hyponitrous acid (H2N2O2), which is then dehydrated to nitrous oxide (N2O). HNO can also generate hydroxylamine ammonia (NH2OH).
Thiol posttranslational modifications
Cys residues contain a terminal thiol (-SH), which generally exists predominantly as thiolate anions (S−) that are strong nucleophiles (86). Generally, posttranslational modifications (PTMs) of cysteine residues included disulfide bonds (RS-SR), nitrosylation (RS-NO), glutathionylation (RS-SG), sulfuration (RS-SH), sulfenylation (RS-OH), sulfinic acid (R-SO2H) and sulfonic acid (R-SO3H) (54). A summary of these PTM and their formation is showed in (Fig. 11). Most of them can be induced by reactive oxygen or nitrogen species and be reduced back to a free thiol (-SH) by the antioxidant defense system. Though a given protein may contain numerous cysteine residues, only a minority of these cysteine residues will have availability and chemical properties to react with ROS/RNS due to the pKa of thiol. The lower the thiol’s pKa, the more likely it will be deprotonated and available for attack. In addition, redox stress, hydrophobicity, and molecular accessiblity (i.e., protein 3D structure) also affect individual cysteine’s PTM.
Recently, a greater number of studies have shown that various enzymes and proteins have per/polysulfide moieties at many cysteine residues (140, 393). To study the biological function of protein polysulfidation, there are several developed methods to detect protein polysulfidation, including tag-switch assays, MS-based methods, and a pull-down procedure (73, 101, 140, 393). However, these methods have different caveats due to the rather complex redox property of protein polysulfide, that is, individual sulfide residues of various polysulfides may be either nucleophilic or electrophilic depending on protonation state. Additionally, interference of protein persulfide is an important aspect for these developed approaches. Thus, confirmation with MS/MS is required to unequivocally identify protein per/polysulfidation.
Post-translational modification by NO
Reactive nitrogen species (RNS) can mediate several types of post-translational modifications of protein residues, which can potentially lead to alteration of protein function. However, this depends on the nature of modified proteins and the location of posttranslational modifications within the protein sequences. Some of these modifications meet the criteria that are generally used to be considered as signal mediators that correspond to other PTMs such as phosphorylation. Thus, it has been put forward that these PTMs, especially S-nitrosylation, may actually represent a new paradigm of signal transduction (208, 343). Here we discuss the characteristics of some of these modifications.
S-Nitrosothiol Formation: “Nitrosation,” is a specific chemical reaction involving addition of a nitrosonium ion (NO+) to a nucleophilic group, such as an amine or thiolate. The term “nitrosylation” is usually used to refer to direct addition of NO to a reactant like the coordination of NO to a metal center or thiyl radical. S-Nitrosylation (also called “S-nitrosation”) refers to the formation of a thionitrite bond (−S−N = O) or nitrosothiol in a thiol group (88, 146) as a consequence of either one electron oxidation of thiol to thiyl radical followed by addition of NO or nucleophilic attack by thiolate to the nitrogen of nitrosonium cation (NO+). Nitrosothiol can be formed by two different chemical mechanisms. S-nitrosothiols may have a combination of covalent, zwitterionic and ion pair resonance structures, R−S−N=O, R−S+=N−O−, RS−NO+, respectively (122). The R−S−N=O form is generally thought of as prevalent in the resonance hybrid. RSNO or HSNO can in turn generate NO+, NO•, NO−, and HNO which may have diverse physiological functions including cardioprotection, vasoregulation, and angiogenesis (85). HSNO can easily diffuse across membranes (210) and can potentially be used to transnitrosylate proteins at places remote from the original site of NO• synthesis.
Importantly, NO does not react directly with reduced thiols under biological conditions. However, prior oxidation of NO (to NO+) or of the thiol (to thiyl radical, RS·) can induce modification of thiols. Strong one-electron oxidants such as HOO· and HO· can oxidize thiols to thiyl radicals (122). Thus, the presence of other RNS like peroxynitrite, N2O3 is necessary for S-nitrosylation to take place. In this regard, N2O3 is the best candidate, as it can easily nitrosylate a free thiol or a thiolate.
Recent studies suggest that S-nitrosylation formation is related to localization of NOS enzymes (139, 144). It has indeed been found that proximity of NOS enzymes to membranes favors localized production of nitrosating species, as the reaction between NO and O2 is facilitated in the membrane hydrophobic compartments (195). This explains why a localized production of RNS is conducive to the formation of nitrosothiols, while generation of NO alone would predispose to a higher diffusion rate of NO, causing it to act at a longer distance by its classical signaling route. Thus, S-nitrosylation can be considered a short-range process and metal nitrosylation (such as in sGC or Cox proteins) a long-range effect for NO-mediated signaling. In addition to localization of NOS, another source of specificity for protein S-nitrosylation is the increased reactivity of the particular cysteine residues concerned. For this posttranslational modification, an acid-base aided mechanism has been postulated with more ionizable cysteines bearing low pKa (182) being more susceptible to S-nitrosylation.
Regulation of S-nitrosylation in proteins is mediated by its potential reversibility. The nitrosothiol is a relatively labile structure in physiological conditions. This points to its ability to act as a signaling modification, allowing easy “switch off” of the signals based on this modification. It reacts easily with other reduced thiols, like the highly abundant low molecular mass (LMM) molecule GSH, by transnitrosylation. Some enzymes with a role in denitrosylation have been described. One of them is “GSNO reductase” and breaks S-nitrosoglutathione (GSNO), which can control the overall levels of S-nitrosylation by reducing the GSNO levels (147,194). A role for thioredoxin has been described in directly denitrosylating certain protein thiols (345). Recently, it was reported to act as a specific denitrosylating enzyme regulating the activation of apoptosis by denitrosylating caspase-3.
Protein S-Thiolation
S-Thiolation is the reaction of incorporation of an LMM thiol to a protein thiol via a disulfide bridge, that is the formation of an LMM protein containing “mixed disulfide.” Different physiological thiols can be incorporated, although S-glutathionylation is probably the most likely modification in the intracellular environment as GSH is the most abundant intracellular LMM thiol (259). Related to NO signaling, the most significant reaction is thiolation by nitrosothiols, either in the LMM or in the protein thiol as shown in the following equation:
Thus, a nitrosothiol can be considered as an activated thiol that can lead to S-glutathionylation, a more stable modification. This mechanism can be considered in relation to the formation of sulfenic acid that can also cause S-glutathionylation (8, 60, 151). Another RNS, peroxynitrite, most probably acting as an oxidant, has been shown to produce S-glutathionylation (38, 56, 245, 368), showing that pathways induced by different RNS can lead to the same modification. In this regard, because it is reversible, S-glutathionylation may function as a mechanism for protection of reactive thiols from their oxidation to sulfinic and sulfonic acid, which are irreversible modifications (162, 277). Some enzymes have been shown to promote deglutathionylation such as sulfiredoxin, glutaredoxins, and the functioning pair thioredoxin-thioredoxin reductase (100).
Tyrosine nitration
Another posttranslational modification induced by RNS is the incorporation of a nitro (−NO2) group to the phenolic ring of tyrosine residues to form a 3-nitrotyrosine residue. It was earlier considered as a footprint of peroxynitrite (ONOO−) formation. However, some evidence indicates that ONOO− is not the direct nitrating agent and there may exist several pathways involving different reactive species (280, 339). These pathways need the concurrent presence of both RNS and oxidants. ONOO− has both characteristics (255). Tyrosine nitration is thought of as an irreversible modification. It can inhibit enzymes with essential tyrosine residues in the active center, like tyrosine 34 in mitochondrial manganese superoxide dismutase (MnSOD). This modification has been described in chronic renal allograft rejection (201, 202). MnSOD is an enzyme that accelerates the decomposition of O2−. Superoxide (O2·−) and NO react very rapidly yielding peroxynitrite (ONOO−). The latter reaction is of significance not only because of the consequences of ONOO− formation and tyrosine nitration but also because it can reduce the bioavailability of NO for the purposes of classical NO signaling. This modification has been identified in endothelial cells treated with the immunosuppressive drug cyclosporine A (CsA) (238, 239).
Post-translational modification by H2S
Protein persulfidation
Protein persulfidation has been suggested as a mode of H2S-based signaling (232). Persulfidation can occur by one of the following mechanisms: (i) sulfide anion mounts a nucleophilic attack on an oxidized cysteine residue like S-nitrosyl, sulfenic acid or disulfide, or (ii) transsulfuration mediated by GSS(n)H or a sulfhydrated (sulfuration) protein, or (iii) attack on H2S2 (236) or polysulfide by a cysteine thiol (111). Examples of persulfidation as a mode of protein function modification are well known despite our lack of clear understating of the reaction mechanism (30, 118, 209). Although protein sulfuration is considered as prevalent as phosphorylation (362), the modified biotin switch assay used to study “persulfuration” may not always be able to distinguish between persulfides and thiols (257). This raises substantial questions about the estimated 10% to 25% prevalence of persulfidation in the proteome (362). An example of the role of persulfidation in protein function modification is PTP1B whose persulfidation of the active site cysteine, has been confirmed by mass spectrometry (171). CSE-dependent persulfidation inactivates PTP1B leading to phosphorylated PERK accumulation in response to ER stress. Persulfidation of the p65 subunit of NF-κB after TNFα treatment has been demonstrated by LC-MS/MS to increase its antiapoptotic function (313). An opposite functional consequence (i.e., inhibition) is observed by S-nitrosylation of the same modified cysteine in p65. Persulfidation of ATP-sensitive K+ channels, mediated by CSE, results in channel opening and hyperpolarization of endothelial smooth muscle cells and vasodilation (233, 411).
Sulfuration of electrophiles by sulfide
Recently, direct sulfuration of some cellular electrophilic messenger molecules like 8-nitro-cGMP, nitro- or keto- derivatives of unsaturated fatty acids, and cyclopentenone prostaglandin by sulfide has been observed and may act as other mechanisms for H2S-mediated signaling (241). Electrophiles are endogenously generated byproducts of oxidation reactions and lipid peroxidation reactions in the presence of ROS and RNS species. They primarily react with cysteine residues for initiation of signal (88, 291, 342, 363). Electrophilic messenger molecules mentioned above can S-alkylate cysteine sulfhydryl groups to mediate redox-dependent signaling pathways (e.g., cGMP-dependent NO signaling) (31, 298). H2S can block iNOS-dependent S-guanylation (i.e., formation of a cysteine-cGMP adduct) of H-Ras, which would activate H-Ras to signal cell senescence in stress conditions (363). This phenomenon (increased levels of 8-SH-cGMP and the loss of H-Ras activation) may in part explain protective roles of H2S in myocardial infarction associated with heart failure. S-guanylation of H-Ras exemplifies opposing functions of H2S and NO. Examples of synergistic effects of H2S and NO can be observed in the endothelial system, where both induce vasorelaxation due to transnitrosylation mediated by H2S.
Clinical Implications and Therapeutic Approaches
NO bioavailability
As NO bioavailability is critical in regulating vascular reactivity, endothelial dysfunction in various pathologies often involves decreased NO bioavailability. Reduction in NO bioavailability may be due to several reasons including, reduced substrate or cofactors such as L-arginine or BH4, decreased expression of eNOS, eNOS inhibition by asymmetric dimethyl arginine (ADMA) or NO degradation by excessive ROS.
BH4 is an essential cofactor for eNOS catalyzed nitric oxide production. Oxidation of BH4 due to oxidative stress can lead to eNOS uncoupling (188). It is shown that BH4 oxidation by NADPH oxidase-mediated ROS production is an important abnormality found in hypertension (178, 188). Furthermore, it has been reported in various pathological conditions including ischemic reperfusion injuries, coronary artery disease, diabetes, and hypercholesterolemia that supplementation of BH4 has been shown to augment endothelium-dependent vasodilation and prevent endothelial dysfunction (91, 123, 133, 204, 315, 316, 347).
Reduced availability of the substrate, L-arginine for NO synthesis can also contribute to reducing NO bioavailability. Studies have shown that infusion of L-arginine improves endothelium-dependent vasodilation; however, it should also be noted that the intracellular concentration of L-arginine is far more than the Km of NOS enzymes. Hence, it is unlikely that substrate availability solely limits NO production (although increased arginase activity could increase this possibility). ADMA, an endogenous competitive inhibitor, competes with L-arginine for the active site of NOS. It is produced by endothelial cells and found in circulating plasma. An elevated level of ADMA has been observed in numerous cardiovascular pathologies (2,28, 159, 170, 183, 218, 306). Thus, it may suggest that administration of L-arginine may overcome a competitive inhibition of NOS by ADMA.
Furthermore, reactive oxygen species (ROS) may also necessarily limit NO bioavailability. NO can be inactivated in the presence of superoxide (26, 142, 221). This reaction leads to the formation of toxic by-products such as peroxynitrite. In addition to the toxic biochemical products, ROS-mediated NO deficiency itself will lead to cardiovascular complications.
Various clinical trials are currently ongoing in an attempt to increase nitric oxide bioavailability, although use of NO above as a drug, have been previously reported. For instance, nitroglycerin, which is an organic nitrate, was used to reduce angina and myocardial infarction. Various NO donors including NONOates (25, 205, 286, 332), sodium nitroprusside (SNP) (405), GSNO has been used to treat ischemic diseases. However, these donors have their own limitations including light sensitivity and route of administration. At present, use of nitrite or dietary nitrate reveals a differential therapeutic effect on ischemic diseases. Our lab has shown in several studies that sodium nitrite therapy restored blood flow and significantly increased vascular density in ischemic limbs of wild type and aged diabetic mice. We showed that restoration of angiogenesis and arteriogenesis was NO dependent, as it could be blocked by the NO scavenger cPTIO (23). In aged diabetic mice, restoration of vascular growth also involved xanthine oxidase mediated nitrite reduction to NO, as febuxostat blunted this effect (22). Moreover, a multicenter clinical trial was performed to study the safety and tolerability of multiple doses of oral sodium nitrite in patients with PAD, predominantly with diabetes, over a period of 10 weeks. Here we observed that sodium nitrite therapy increased flow mediated dilation of the brachial artery predominantly in subjects with diabetes suggesting a possible clinical benefit; however, these findings need to be further studied in a larger trial (219).
Currently, a study examining the effect of different vegetables having high and low inorganic nitrate contents on cardiovascular function in subjects with moderately increased blood pressure is ongoing. About 300 participants in the age group of 50 to 70 years are involved in this study. Another study is currently evaluating the effects of an acute dose of dietary nitrate in the form of beetroot juice on skeletal muscle blood flow in response to dynamic knee extension exercise in healthy older adults. All evaluations will be done with placebo controls. Previous reports with concentrated beetroot as a nitrate supplement demonstrated improved exercise tolerance in patients with HFpEF (78).
H2S bioavailability
In addition to NO bioavailability, the relationship of H2S bioavailability and cardiovascular pathology has received increasing attention over the past decade. Studies have shown that reduced H2S bioavailability due to CSE inhibition blunted cardioprotective effects in experimental models. In addition, plasma H2S levels had a direct correlation with infarct size, whereas increased H2S levels in plasma resulted in smaller infarct size and decreased H2S levels corresponds to increased infarct size and mortality (414). Furthermore, CSE knockout animal models were associated with impaired vasodilation, decreased blood perfusion affecting vascular remodeling; however, exogenous sulfide therapy corrected these defects (163).
In addition to the enzymatic regulation of H2S bioavailability, systemic microflora also significantly influence H2S bioavailability. The most prominent producers of H2S are sulfate-reducing bacteria (10, 237). It is reported that about 103 to 1011 sulfate-reducing bacteria per gm of human feces is present (104). Furthermore, the intestinal epithelium expresses specialized enzyme systems that efficiently degrade sulfide to thiosulphate and sulfate (351). We have reported the availability of various biochemical forms of sulfide through microbial regulation in the major organs. We observed that aorta and adipose tissue contained the highest amount of acid labile and bound sulfane sulfur using selective H2S pool liberation methods coupled with HPLC analysis (321). Our results are in agreement with another study reporting abundant H2S is available in the aortic tissues but in free and acid labile forms (185).
Controversies exist with regard to measurement of clinical sulfide levels, as methods such as methylene blue cannot reliably resolve physiological sulfide levels (237). Importantly, our group performed an observational study comparing the plasma levels of free hydrogen sulfide in patients with and without peripheral or coronary artery disease using the analytical monobromobiamine method. We found that plasma-free H2S levels were increased in vascular disease subjects with a surprising inverse relationship to NO bioavailability in these patients (268). We recently completed a follow up study measuring acid-labile, bound sulfane sulfur, and total sulfide levels that revealed a significant reduction in total sulfide levels in subjects with stable cardiovascular disease, which was also predictive of CVD (281). Interesting gender differences were observed as females with cardiovascular disease showed reduced acid labile sulfide compared to reduced bound sulfane sulfur in males with cardiovascular disease. These findings demonstrate that cardiovascular disease is significantly associated with decreased total sulfide but with differences in sulfide biochemical forms (free vs. acid labile vs. bound sulfane sulfur) in a gender specific manner. Additional studies are needed to better understand differences in discrete specific sulfide metabolites (e.g., hydroper/polysulfides, Fe-S clusters, or sulfo-heme proteins) and whether changes in CSE, CBS, or 3MPST expression or activity are involved.
Recently, a phase I clinical trial of a novel H2S prodrug (SG1002) was given to health subjects (n = 7) and heart failure (HF) patients (n = 8). Administration of SG1002 resulted in increased H2S and NO levels in healthy subjects. They also observed increased H2S levels and NO levels in HF subjects following 400 and 800 mg of SG1002 (Sulfzix), respectively. Further, they proposed this drug to be a potent stimulator of H2S levels in blood and NO bioavailability; however, a larger clinical study is required for further confirmation (276).
Lastly, in patients various types of shock including septic, cardiogenic, obstructive, and hypovolemic, total plasma H2S levels were elevated compared to control patients, suggesting plasma H2S levels as a potential biomarker for sepsis conditions (109). Further sodium thiosulfate, a medicine that releases H2S in the body, which is already used to treat other diseases such as renal failure, is shown to protect from myocardial damage following heart attack and delays the progression of coronary artery calcification in hemodialysis patients. Together, these data suggest that H2S donors could be potential therapeutics; however, further investigation and larger clinical studies are needed in these areas.
Cofactors and genetics: NO vs. H2S enzymes
Proper functioning of an enzyme depends on effective binding of the substrate and availability of the cofactors that can subsequently regulate various pathophysiological functions. Deficiency or overexpression, utility or lack of specific substrate can result in key differences between enzyme metabolic function and subsequent end-products. Both the signaling molecules NO and H2S can fall into the aforementioned categories and act as metabolic modulators. NO and H2S can have either inhibitory or stimulatory effects in the course of a pathophysiological event based on their biological levels and localization (166, 167). Enzymes involved with the production of both these gaseous molecules including NOS’s, CSE, CBS and 3-MPST, are clearly influenced by the presence or lack of substrates and cofactors that may vary in pathophysiological conditions of the vasculature. Genetic variation in the enzymes producing or consuming NO and H2S metabolites may also contribute to increased risk of CVD through alterations in their function.
NO/eNOS:
As discussed earlier, several cofactors regulate the activity of eNOS. L-arginine and tetrahydrobiopterin (BH4) are key regulatory factors that influence the production of NO via eNOS. L-arginine is a semi-essential amino acid and the only substrate of NOS that plays a critical role in diverse physiological functions. Additionally, arginine acts as a substrate in metabolic regulation of glutamate, polyamines, and creatinine (226). BH4 is a pteridine and an essential cofactor that plays a role for a set of enzymes including the three NOSs, aromatic amino acid hydroxylases (AAAH), and alkylglycerol mono-oxygenase (AGMO) that have key metabolic roles.
Deficiency of arginine can lead to severe pathological conditions and reduced NO bioavailability. Reduced arginine levels may have potential effects including cellular and organ dysfunction (366, 394). Arginine deficiency can cause elevated soluble adhesion molecules and cell-free hemoglobin that scavenges NO, and consequently increases oxidative stress and endothelial dysfunction. Pathologies involved with reduced arginine levels also involve elevated arginase levels that can contribute to increased ROS production (16, 244). In pre-eclampsia, excessive arginase, low SOD activity, and NO levels cause increased oxidative stress leading to endothelial dysfunction and microvascular damage. Furthermore, changes in arginine metabolism can have cardiovascular consequences including ventricular dysfunction (11). Additionally, factors such as low-density lipoprotein and cholesterol can reduce the activity of eNOS, which may be overcome by L-arginine supplementation (278, 314). This suggests the critical presence of L-arginine in regulating eNOS activity and NO bioavailability that eventually affects endovascular functions.
Tetrahydrobiopterin (BH4) is also involved in regulation of various biological processes and pathological states associated with endothelial dysfunction and cardiovascular disease. BH4 is involved in the production of the vasoactive substances NO and noradrenaline. Altered levels of BH4 can cause decreased NO bioavailability, elevated blood pressure, increased heart rate and endothelial dysfunction (382). This could be mediated by oxidation of BH4 causing BH4 radical formation, a heme-based oxidant that rapidly hydroxylates arginine. Association of oxidized BH4 and BH2 formation is observed in cardiovascular disease conditions including hypertension, aging, and diabetes (178, 328, 334). However, overexpression of GTP cyclohydrolase I (GCH) can help alleviate diabetes and atherosclerosis-induced endothelial dysfunction (388). Exogenous therapy with BH4 has reversed the effects of eNOS uncoupling, BH2 formation and restored endothelial function under ischemic vascular disease including diabetes (62, 213, 243).
Mutations in eNOS gene have been investigated for its influence on hypertension genetics, thus confirming its role in vascular homeostasis. Association of eNOS gene polymorphisms with Glu298Asp (rs1799983) and − 786T > C (rs2070744) has been identified with hypertension and coronary artery disease (43, 416) (Table 1). Interestingly, there is a significant association between these polymorphisms and heterogeneity was observed in a study including Caucasians and East Asians (416). In a separate study, distribution of Glu298Asp variants (within exon 7 of eNOS) have been studied in communities of North Eastern India (320). The frequency of eNOS with GG (75%), GT (22%), and TT (3%) genotypes have been observed in these groups. However, an ethnic association in these variants was observed revealing higher ‘T’ allele frequency in Caucasians, which was lower among Asian populations. These variations in the gene have functional implications for eNOS protein resulting in glutamate or aspartate, leading to cleavage susceptibility of 298 Asp eNOS (355). Furthermore, 894 G>T mutation has also been associated in patients with hypertension, cerebrovascular disease, and CAD (14, 42, 128, 281). More studies are needed to fully understand the pathophysiological importance of these various polymorphisms subsequently exploited for functional implications and association with disease.
Table 1.
Genetic Variations of Gasotransmitter Enzymes Associated with CVD
| SNPs in CBS | Model | Outcome | Reference |
|---|---|---|---|
| CBS T833C; G919A | Human |
|
(72, 359, 360) |
| CBS 844ins68 (rs234706) | Human |
|
(99,124,168, 407) |
| CBS C699T and the C1080T | Human; In vitro |
|
(4, 69) |
| CBS I278T and T353M | Human; Yeast |
|
(173) |
| CBS G919A | Human |
|
(338) |
| CBS T87N and D234N mutations | Human; In vitro |
|
(44) |
| CBS T833C (Ile278Thr), G1111A (Val371Met) mutations | Human; Mouse |
|
(55) |
| CBS SNP (rs11203172) | Human |
|
(134) |
| SNPs in MPST | Model | Outcome | Reference |
| MPST; IVS1-C110G; IVS2 + C39T | In vivo; In vitro |
|
(18) |
| SNPs in CTH | Model | Outcome | Reference |
| CTH G1364T; S403I | Human |
|
(282, 374) |
| CTH SNPs rs482843 | Human |
|
(228) |
| CTH SNPs (rs482843; rs1021737) | Human |
|
(191) |
| CTH IVS3–66 A > C, IVS10–430 C > T | Human |
|
(224) |
| CTH A476C; A477C | Mouse |
|
(117) |
| SNPs in eNOS | Model | Outcome | Reference |
| E298D (rs1799983); T786C (rs2070744) | Human |
|
(42, 394) |
| G894T; T-786C | Human |
|
(306, 340) |
| G894T | Human |
|
(15,41,118,270) |
| SNPs in MTHFR | Model | Outcome | Reference |
| C677T | Human |
|
(168,406) |
| rs1801131 | Human |
|
(327) |
H2S/CSE/CBS/MTHFR:
Alteration of transsulfuration pathways and H2S metabolism including bioavailability have been identified to influence cardiovascular function and health (9, 66, 165). Mechanisms of H2S generation including substrate utilization and its regulation can play a role in metabolic regulation. CSE is a proximal-5′-phosphate (PLP) dependent enzyme that predominantly produces H2S in the vasculature using cystathionine or L-cysteine as substrates (Table 1). However, there are other roles of substrates and enzymes that have recently been appreciated than previously thought. It has been observed that CSE can also use cystine (CysSSCys) as a substrate forming cysteine persulfide (CysSSH) that may be a biologically important bound sulfane sulfur (140). Moreover, another study suggests it is important to understand the enzyme source (CBS vs. CSE) for relative production of H2S to the degree of hyperhomocysteinemia (335). Homocysteine is metabolized to methionine by the action of 5, 10 methylenetetrahydrofolate reductase (MTHFR). Alternatively, homocysteine can be transformed to H2S via the transsulfuration pathway involving CBS and CSE. Although both CSE and CBS are involved in maintaining homeostasis of homocysteine levels, it is CSE that plays a central role in intracellular homocysteine balance in the peripheral vasculature, as CBS levels may be relatively low. Studies in murine models deficient in CSE have demonstrated that there is a ~18-fold increase in homocysteine levels in the aorta and heart of these mice (392). Lastly, little to no information is known regarding bioavailability of PLP that is a critical cofactor for both CSE and CBS in healthy, ischemic, or diseased vascular tissues. Clearly future studies are needed to advance our understanding of this area.
Genetic variations in the CTH (CSE) gene have been associated with hyperhomocysteinemia (Table 1). Polymorphic variants of CTH 1364 G>T (Rs1021737) in exon 12 of the CTH gene were associated with increased homocysteine levels (373, 374). We have observed from our recent study that patients with any form of cardiovascular disease (CVD), including CAD or PAD, have higher mutant 1364 T allele frequency than subjects without vascular disease (0.265, 0.256, and 0.273 vs. 0.107, respectively) (281). Moreover, there was a significant decrease in various pools of sulfide including acid-labile and bound sulfane sulfur (BSS), further corroborating increased risk of CVD. Notably, changes in sulfide levels have been associated in an ethnic and gender-specific manner. Male African American subjects have significantly low levels of BSS with PAD, which suggests it could potentially be a marker for increased risk for vascular disease. In another study, polymorphism of CTH (1364 G>T) has also been identified in women with preeclampsia, which also has implications for CVD (228). Specific studies implicating various gene polymorphisms and metabolic dysregulation in the transsulfuration pathway, and increased homocysteine levels that are associated with CVD are reported in Table 1. More studies are needed to understand the relationship between metabolic dysregulation of H2S bioavailability and gene variations of sulfide synthesis enzymes in association with CVD.
Future directions and unanswered questions
Regulatory roles of H2S and NO in various pathophysiological functions have been discussed throughout this review; however, an abundant number of unanswered questions remain regarding these molecules and how they participate in cardiovascular health and disease. As H2S signaling pathways are crucial in regulating cardiovascular responses and their deficiency leads to endothelial dysfunction and CVD, we largely do not understand how H2S synthesizing (e.g., CSE, CBS, and 3MPST) and catabolic (e.g., SQR1, ETHE1, and others) enzyme functions are regulated in vascular pathophysiological settings. A critical decrease in H2S levels with concomitant altered activities of these enzymes might provide further prognostic significance to predict pathological cardiovascular events. Preclinical studies and recent findings from ours and other groups have reported that metabolic dysfunctions including reduced bioavailability of H2S pools and NO metabolites are likely central factors in the progression of ischemic CVD that requires continued investigation (161, 163, 166, 281).
Several risk factors such as aging have long been recognized as major determinants for CVD. Aging is a major risk factor of CVD with associated complications of endothelial cell dysfunction, oxidative stress, and inflammation. As such, there is increasing interest in the association of H2S in several pathologies related to aging. An association of H2S signaling with age-related complications has been suggested (267). Experiments in Caenorhabditis elegans treated with exogenous H2S demonstrated a prolonged lifespan of the nematode (216, 279). Furthermore, published evidence shows that H2S can regulate age-related effects through inhibition of free radicals mediated via SIRT1 activation and subsequent interaction with the age-related gene Klotho (410). A recent study by Das et al also revealed that the exogenous sulfide treatment augments nicotinamide mononucleotide-mediated Sirt1 levels that reversed aspects of vascular aging (67). However, the interrelationship between H2S, aging, and cardiovascular disease mechanisms requires further investigation.
CSE/H2S can also regulate blood pressure and lipid metabolism that can influence endothelial cell activation during experimental atherogenesis (186). Defective CSE/H2S signaling contributes to ox-LDL-induced inflammation. Genetic inhibition of CSE leads to an increase in LDL/cholesterol levels and hyperhomocysteinemia, whereas exogenous H2S/CSE can rectify these defects in atherosclerosis (51, 206). This demonstrates the role of CSE/H2S in the regulation of lipid metabolism. Defective regulation of H2S and its enzymes has been associated with metabolic disease condition such as diabetes (150). Decreased bioavailability of H2S likely potentiates the pathogenesis of diabetes under ischemic conditions and with age. However, this defect might be rectified by enhancing H2S/CSE signaling (22, 50); whereas simply restoring vessel growth alone may not be sufficient for severe metabolic defects such as diabetes and associated complications. Future studies examining the relationship between H2S and metabolic function in conjunction with NO are clearly needed.
Lastly, future studies focused on the relationship between NO production and bioavailability and its effects on H2S signaling and synthesizing enzyme functions (e.g., CSE, CBS, and 3MPST) must be explored in relation to cardiovascular disease. Potential influences of ethylmalonic encephalopathy protein 1 (ETHE1), mitochondrial sulfide-quinone oxidoreductase (SQR), and cysteine dioxygenase (CDO) are also important to consider in this context (136, 290). However, how genetic variants of H2S and NO metabolic enzymes contribute to gasotransmitter bioavailability and subsequent physiological implications remain completely unknown. The use of novel genetic manipulation technologies (e.g., CRISPR/Cas9), advanced analytical chemistry techniques, and chemical signaling methodologies should lead to a better understanding of these gaseous mediators during vascular health and disease.
Didactic Synopsis.
Major teaching points
A robust and functional vascular network is a fundamental requirement for development, growth, and tissue maintenance.
- Based on the mechanism, occurrence and result, vascular growth can be distinguished principally as three types
- Vasculogenesis—de novo formation of a primitive vascular network
- Angiogenesis—new capillaries form from pre-existing blood vessels
- Arteriogenesis—mature arteries form from interconnecting arterioles after an arterial occlusion
Ischemic vascular remodeling occurs as a result of stenosis or arterial occlusion
Nitric oxide and hydrogen sulfide are known to be essential for ischemic vascular remodeling
The pathophysiological events of angiogenesis and arteriogenesis regulated by NO and H2S involve multiple cellular responses suggesting central roles in mediating vascular remodeling
Acknowledgements
This publication was supported by HL113303 to C. G. Kevil and by an Institutional Development Award (IDeA) from the National Institutes of General Medical Sciences of the NIH under grant number P20GM121307.
Footnotes
Disclosures
C.G. Kevil has intellectual property regarding nitric oxide/nitrite and hydrogen sulfide chemistry and is a cofounder of Innolyzer LLC. X. Shen has intellectual property regarding hydrogen sulfide chemistry and is an advisor for Innolyzer LLC.
References
- 1.Abe K, Kimura H. The possible role of hydrogen sulfide as an endogenous neuromodulator. J Neurosci 16: 1066–1071, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Achan V, Broadhead M, Malaki M, Whitley G, Leiper J, MacAllister R, Vallance P. Asymmetric dimethylarginine causes hypertension and cardiac dysfunction in humans and is actively metabolized by dimethylarginine dimethylaminohydrolase. Arterioscler Thromb Vasc Biol 23: 1455–1459, 2003. [DOI] [PubMed] [Google Scholar]
- 3.Aicher A, Heeschen C, Mildner-Rihm C, Urbich C, Ihling C, Technau-Ihling K, Zeiher AM, Dimmeler S. Essential role of endothelial nitric oxide synthase for mobilization of stem and progenitor cells. Nat Med 9: 1370–1376, 2003. [DOI] [PubMed] [Google Scholar]
- 4.Aras O, Hanson NQ, Yang F, Tsai MY. Influence of 699C→T and 1080C→T polymorphisms of the cystathionine beta-synthase gene on plasma homocysteine levels. Clin Genet 58: 455–459, 2000. [DOI] [PubMed] [Google Scholar]
- 5.Arras M, Ito WD, Scholz D, Winkler B, Schaper J, Schaper W. Monocyte activation in angiogenesis and collateral growth in the rabbit hindlimb. J Clin Invest 101: 40–50, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Azizi SN, Shakeri P, Chaichi MJ, Bekhradnia A, Taghavi M, Ghaemy M. The use of imidazolium ionic liquid/copper complex as novel and green catalyst for chemiluminescent detection of folic acid by Mn-doped ZnS nanocrystals. Spectrochim Acta A Mol Biomol Spectrosc 122: 482–488, 2014. [DOI] [PubMed] [Google Scholar]
- 7.Bailey TS, Pluth MD. Chemiluminescent detection of enzymatically produced hydrogen sulfide: Substrate hydrogen bonding influences selectivity for H2S over biological thiols. J Am Chem Soc 135: 16697–16704, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Barrett WC, DeGnore JP, Konig S, Fales HM, Keng YF, Zhang ZY, Yim MB, Chock PB. Regulation of PTP1B via glutathionylation of the active site cysteine 215. Biochemistry 38: 6699–6705, 1999. [DOI] [PubMed] [Google Scholar]
- 9.Bearden SE, Beard RS Jr., Pfau JC. Extracellular transsulfuration generates hydrogen sulfide from homocysteine and protects endothelium from redox stress. Am J Physiol Heart Circ Physiol 299: H1568–H1576, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Beerens H, Romond C. Sulfate-reducing anaerobic bacteria in human feces. Am J Clin Nutr 30: 1770–1776, 1977. [DOI] [PubMed] [Google Scholar]
- 11.Bekpinar S, Gurdol F, Unlucerci Y, Develi S, Yilmaz A. Serum levels of arginase I are associated with left ventricular function after myocardial infarction. Clin Biochem 44: 1090–1093, 2011. [DOI] [PubMed] [Google Scholar]
- 12.Benavides GA, Squadrito GL, Mills RW, Patel HD, Isbell TS, Patel RP, Darley-Usmar VM, Doeller JE, Kraus DW. Hydrogen sulfide mediates the vasoactivity of garlic. Proc Natl Acad Sci U S A 104: 17977–17982, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Benjamin N, O’Driscoll F, Dougall H, Duncan C, Smith L, Golden M, McKenzie H. Stomach NO synthesis. Nature 368: 502, 1994. [DOI] [PubMed] [Google Scholar]
- 14.Berger K, Stogbauer F, Stoll M, Wellmann J, Huge A, Cheng S, Kessler C, John U, Assmann G, Ringelstein EB, Funke H. The glu298asp polymorphism in the nitric oxide synthase 3 gene is associated with the risk of ischemic stroke in two large independent case-control studies. Hum Genet 121: 169–178, 2007. [DOI] [PubMed] [Google Scholar]
- 15.Bergmann CE, Hoefer IE, Meder B, Roth H, van Royen N, Breit SM, Jost MM, Aharinejad S, Hartmann S, Buschmann IR. Arteriogenesis depends on circulating monocytes and macrophage accumulation and is severely depressed in op/op mice. J Leukoc Biol 80: 59–65, 2006. [DOI] [PubMed] [Google Scholar]
- 16.Bernardi F, Constantino L, Machado R, Petronilho F, Dal-Pizzol F. Plasma nitric oxide, endothelin-1, arginase and superoxide dismutase in pre-eclamptic women. J Obstet Gynaecol Res 34: 957–963, 2008. [DOI] [PubMed] [Google Scholar]
- 17.Bibli SI, Luck B, Zukunft S, Wittig J, Chen W, Xian M, Papapetropoulos A, Hu J, Fleming I. A selective and sensitive method for quantification of endogenous polysulfide production in biological samples. Redox Biol 18: 295–304, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Billaut-Laden I, Rat E, Allorge D, Crunelle-Thibaut A, Cauffiez C, Chevalier D, Lo-Guidice JM, Broly F. Evidence for a functional genetic polymorphism of the human mercaptopyruvate sulfurtransferase (MPST), a cyanide detoxification enzyme. Toxicol Lett 165: 101–111, 2006. [DOI] [PubMed] [Google Scholar]
- 19.Binet F, Mawambo G, Sitaras N, Tetreault N, Lapalme E, Favret S, Cerani A, Leboeuf D, Tremblay S, Rezende F, Juan AM, Stahl A, Joyal JS, Milot E, Kaufman RJ, Guimond M, Kennedy TE, Sapieha P. Neuronal ER stress impedes myeloid-cell-induced vascular regeneration through IRE1alpha degradation of netrin-1. Cell Metab 17: 353–371, 2013. [DOI] [PubMed] [Google Scholar]
- 20.Bir SC, Fujita M, Marui A, Hirose K, Arai Y, Sakaguchi H, Huang Y, Esaki J, Ikeda T, Tabata Y, Komeda M. New therapeutic approach for impaired arteriogenesis in diabetic mouse hindlimb ischemia. Circ J 72: 633–640, 2008. [DOI] [PubMed] [Google Scholar]
- 21.Bir SC, Kolluru GK, Fang K, Kevil CG. Redox balance dynamically regulates vascular growth and remodeling. Semin Cell Dev Biol 23: 745–757, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bir SC, Kolluru GK, McCarthy P, Shen X, Pardue S, Pattillo CB, Kevil CG. Hydrogen sulfide stimulates ischemic vascular remodeling through nitric oxide synthase and nitrite reduction activity regulating hypoxia-inducible factor-1alpha and vascular endothelial growth factor-dependent angiogenesis. J Am Heart Assoc 1: e004093, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bir SC, Pattillo CB, Pardue S, Kolluru GK, Docherty J, Goyette D, Dvorsky P, Kevil CG. Nitrite anion stimulates ischemic arteriogenesis involving NO metabolism. Am J Physiol Heart Circ Physiol 303: H178–H188, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bir SC, Pattillo CB, Pardue S, Kolluru GK, Shen X, Giordano T, Kevil CG. Nitrite anion therapy protects against chronic ischemic tissue injury in db/db diabetic mice in a NO/VEGF-dependent manner. Diabetes 63: 270–281, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Blecher K, Martinez LR, Tuckman-Vernon C, Nacharaju P, Schairer D, Chouake J, Friedman JM, Alfieri A, Guha C, Nosanchuk JD, Friedman AJ. Nitric oxide-releasing nanoparticles accelerate wound healing in NOD-SCID mice. Nanomedicine 8: 1364–1371, 2012. [DOI] [PubMed] [Google Scholar]
- 26.Blough NV, Zafiriou OC. Reaction of superoxide with nitric oxide to form peroxonitrite in alkaline aqueous solution. Inorg ChemInorganic Chemistry 24: 3502–3504, 1985. [Google Scholar]
- 27.Bogdandi V, Ida T, Sutton TR, Bianco C, Ditroi T, Koster G, Henthorn HA, Minnion M, Toscano JP, van der Vliet A, Pluth MD, Feelisch M, Fukuto JM, Akaike T, Nagy P. Speciation of reactive sulfur species and their reactions with alkylating agents: Do we have any clue about what is present inside the cell? Br J Pharmacol 176: 646–670, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Boger RH, Bode-Boger SM, Szuba A, Tsao PS, Chan JR, Tangphao O, Blaschke TF, Cooke JP. Asymmetric dimethylarginine (ADMA): a novel risk factor for endothelial dysfunction: Its role in hypercholesterolemia. Circulation 98: 1842–1847, 1998. [DOI] [PubMed] [Google Scholar]
- 29.Bosch-Marce M, Pola R, Wecker AB, Silver M, Weber A, Luede-mann C, Curry C, Murayama T, Kearney M, Yoon YS, Malinow MR, Asahara T, Isner JM, Losordo DW. Hyperhomocyst(e)inemia impairs angiogenesis in a murine model of limb ischemia. Vasc Med 10: 15–22, 2005. [DOI] [PubMed] [Google Scholar]
- 30.Branzoli U, Massey V. Evidence for an active site persulfide residue in rabbit liver aldehyde oxidase. J Biol Chem 249: 4346–4349, 1974. [PubMed] [Google Scholar]
- 31.Bredt DS, Hwang PM, Snyder SH. Localization of nitric oxide synthase indicating a neural role for nitric oxide. Nature 347: 768–770, 1990. [DOI] [PubMed] [Google Scholar]
- 32.Brill A, Dashevsky O, Rivo J, Gozal Y, Varon D. Platelet-derived microparticles induce angiogenesis and stimulate post-ischemic revascularization. Cardiovasc Res 67: 30–38, 2005. [DOI] [PubMed] [Google Scholar]
- 33.Buchanan CF, Verbridge SS, Vlachos PP, Rylander MN. Flow shear stress regulates endothelial barrier function and expression of angiogenic factors in a 3D microfluidic tumor vascular model. Cell Adh Migr 8: 517–524, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Buckwalter JB, Curtis VC, Valic Z, Ruble SB, Clifford PS. Endogenous vascular remodeling in ischemic skeletal muscle: A role for nitric oxide. J Appl Physiol 94: 935–940, 2003. [DOI] [PubMed] [Google Scholar]
- 35.Buschmann I, Schaper W. The pathophysiology of the collateral circulation (arteriogenesis). J Pathol 190: 338–342, 2000. [DOI] [PubMed] [Google Scholar]
- 36.Cai W, Schaper W. Mechanisms of arteriogenesis. Acta Biochim Biophys Sin (Shanghai) 40: 681–692, 2008. [PubMed] [Google Scholar]
- 37.Cai WJ, Kocsis E, Luo X, Schaper W, Schaper J. Expression of endothelial nitric oxide synthase in the vascular wall during arteriogenesis. Mol Cell Biochem 264: 193–200, 2004. [DOI] [PubMed] [Google Scholar]
- 38.Calvert JW, Elston M, Nicholson CK, Gundewar S, Jha S, Elrod JW, Ramachandran A, Lefer DJ. Genetic and pharmacologic hydrogen sulfide therapy attenuates ischemia-induced heart failure in mice. Circulation 122: 11–19, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Carmeliet P, Ferreira V, Breier G, Pollefeyt S, Kieckens L, Gertsenstein M, Fahrig M, Vandenhoeck A, Harpal K, Eberhardt C, Declercq C, Pawling J, Moons L, Collen D, Risau W, Nagy A. Abnormal blood vessel development and lethality in embryos lacking a single VEGF allele. Nature 380: 435–439, 1996. [DOI] [PubMed] [Google Scholar]
- 40.Carmeliet P, Jain RK. Molecular mechanisms and clinical applications of angiogenesis. Nature 473: 298–307, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Carr CL, Qi Y, Davidson B, Chadderdon S, Jayaweera AR, Belcik JT, Benner C, Xie A, Lindner JR. Dysregulated selectin expression and monocyte recruitment during ischemia-related vascular remodeling in diabetes mellitus. Arterioscler Thromb Vasc Biol 31: 2526–2533, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Casas JP, Bautista LE, Humphries SE, Hingorani AD. Endothelial nitric oxide synthase genotype and ischemic heart disease: Meta-analysis of 26 studies involving 23028 subjects. Circulation 109: 1359–1365, 2004. [DOI] [PubMed] [Google Scholar]
- 43.Casas JP, Cavalleri GL, Bautista LE, Smeeth L, Humphries SE, Hingorani AD. Endothelial nitric oxide synthase gene polymorphisms and cardiovascular disease: A HuGE review. Am J Epidemiol 164: 921–935, 2006. [DOI] [PubMed] [Google Scholar]
- 44.Casique L, Kabil O, Banerjee R, Martinez JC, De Lucca M. Characterization of two pathogenic mutations in cystathionine beta-synthase: Different intracellular locations for wild-type and mutant proteins. Gene 531: 117–124, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chan CK, Vanhoutte PM. Hypoxia, vascular smooth muscles and endothelium. Acta Pharmaceutica Sinica B 3: 1–7, 2013. [Google Scholar]
- 46.Chen B, Li W, Lv C, Zhao M, Jin H, Jin H, Du J, Zhang L, Tang X. Fluorescent probe for highly selective and sensitive detection of hydrogen sulfide in living cells and cardiac tissues. Analyst 138: 946–951, 2013. [DOI] [PubMed] [Google Scholar]
- 47.Chen W, Liu C, Peng B, Zhao Y, Pacheco A, Xian M. New fluorescent probes for sulfane sulfurs and the application in bioimaging. Chem Sci 4: 2892–2896, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chen X, Tian X, Shin I, Yoon J. Fluorescent and luminescent probes for detection of reactive oxygen and nitrogen species. Chem Soc Rev 40: 4783–4804, 2011. [DOI] [PubMed] [Google Scholar]
- 49.Cheng Y, Ndisang JF, Tang G, Cao K, Wang R. Hydrogen sulfide-induced relaxation of resistance mesenteric artery beds of rats. Am J Physiol Heart Circ Physiol 287: H2316–H2323, 2004. [DOI] [PubMed] [Google Scholar]
- 50.Cheng Z, Garikipati VN, Nickoloff E, Wang C, Polhemus DJ, Zhou J, Benedict C, Khan M, Verma SK, Rabinowitz JE, Lefer D, Kishore R. Restoration of hydrogen sulfide production in diabetic mice improves reparative function of bone marrow cells. Circulation 134: 1467–1483, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cheung SH, Kwok WK, To KF, Lau JY. Anti-atherogenic effect of hydrogen sulfide by over-expression of cystathionine gamma-lyase (CSE) gene. PLoS One 9: e113038, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chidlow JH, Jr., Shukla D, Grisham MB, Kevil CG. Pathogenic angiogenesis in IBD and experimental colitis: New ideas and therapeutic avenues. Am J Physiol Gastrointest Liver Physiol 293: G5–G18, 2007. [DOI] [PubMed] [Google Scholar]
- 53.Chistiakov DA, Sobenin IA, Orekhov AN, Bobryshev YV. Role of endoplasmic reticulum stress in atherosclerosis and diabetic macrovascular complications. Biomed Res Int 2014: 610140, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chung HS, Wang SB, Venkatraman V, Murray CI, Van Eyk JE. Cysteine oxidative posttranslational modifications: Emerging regulation in the cardiovascular system. Circ Res 112: 382–392, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chwatko G, Boers GH, Strauss KA, Shih DM, Jakubowski H. Mutations in methylenetetrahydrofolate reductase or cystathionine beta-synthase gene, or a high-methionine diet, increase homocysteine thiolactone levels in humans and mice. FASEB J 21: 1707–1713, 2007. [DOI] [PubMed] [Google Scholar]
- 56.Clavreul N, Adachi T, Pimental DR, Ido Y, Schoneich C, Cohen RA. S-glutathiolation by peroxynitrite of p21ras at cysteine-118 mediates its direct activation and downstream signaling in endothelial cells. FASEB J 20: 518–520, 2006. [DOI] [PubMed] [Google Scholar]
- 57.Clayton JA, Chalothorn D, Faber JE. Vascular endothelial growth factor-A specifies formation of native collaterals and regulates collateral growth in ischemia. Circ Res 103: 1027–1036, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Coletta C, Papapetropoulos A, Erdelyi K, Olah G, Modis K, Panopoulos P, Asimakopoulou A, Gero D, Sharina I, Martin E, Szabo C. Hydrogen sulfide and nitric oxide are mutually dependent in the regulation of angiogenesis and endothelium-dependent vasorelaxation. Proc Natl Acad Sci U S A 109: 9161–9166, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Conway EM, Collen D, Carmeliet P. Molecular mechanisms of blood vessel growth. Cardiovasc Res 49: 507–521, 2001. [DOI] [PubMed] [Google Scholar]
- 60.Conway ME, Coles SJ, Islam MM, Hutson SM. Regulatory control of human cytosolic branched-chain aminotransferase by oxidation and S-glutathionylation and its interactions with redox sensitive neuronal proteins. Biochemistry 47: 5465–5479, 2008. [DOI] [PubMed] [Google Scholar]
- 61.Cosby K, Partovi KS, Crawford JH, Patel RP, Reiter CD, Martyr S, Yang BK, Waclawiw MA, Zalos G, Xu X, Huang KT, Shields H, Kim-Shapiro DB, Schechter AN, Cannon Iii RO, Gladwin MT. Nitrite reduction to nitric oxide by deoxyhemoglobin vasodilates the human circulation. Nat Med 9: 1498, 2003. [DOI] [PubMed] [Google Scholar]
- 62.Cosentino F, Hurlimann D, Delli Gatti C, Chenevard R, Blau N, Alp NJ, Channon KM, Eto M, Lerch P, Enseleit F, Ruschitzka F, Volpe M, Luscher TF, Noll G. Chronic treatment with tetrahydrobiopterin reverses endothelial dysfunction and oxidative stress in hypercholesterolaemia. Heart 94: 487–492, 2008. [DOI] [PubMed] [Google Scholar]
- 63.Couffinhal T, Silver M, Kearney M, Sullivan A, Witzenbichler B, Magner M, Annex B, Peters K, Isner JM. Impaired collateral vessel development associated with reduced expression of vascular endothelial growth factor in ApoE−/− mice. Circulation 99: 3188–3198, 1999. [DOI] [PubMed] [Google Scholar]
- 64.Craige SM, Chen K, Pei Y, Li C, Huang X, Chen C, Shibata R, Sato K, Walsh K, Keaney JF Jr. NADPH oxidase 4 promotes endothelial angiogenesis through endothelial nitric oxide synthase activation. Circulation 124: 731–740, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Cunha CRA, Oliveira A, Firmino TVC, Tenorio D, Pereira G, Carvalho LB, Jr., Santos BS, Correia MTS, Fontes A. Biomedical applications of glyconanoparticles based on quantum dots. Biochim Biophys Acta 1862: 427–439, 2018. [DOI] [PubMed] [Google Scholar]
- 66.d’Emmanuele di Villa Bianca R, Mitidieri E, Di Minno MN, Kirkby NS, Warner TD, Di Minno G, Cirino G, Sorrentino R. Hydrogen sulphide pathway contributes to the enhanced human platelet aggregation in hyperhomocysteinemia. Proc Natl Acad Sci U S A 110: 15812–15817, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Das A, Huang GX, Bonkowski MS, Longchamp A, Li C, Schultz MB, Kim LJ, Osborne B, Joshi S, Lu Y, Trevino-Villarreal JH, Kang MJ, Hung TT, Lee B, Williams EO, Igarashi M, Mitchell JR, Wu LE, Turner N, Arany Z, Guarente L, Sinclair DA. Impairment of an endothelial NAD(+)-H2S signaling network is a reversible cause of vascular aging. Cell 173: 74–89 e20, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Datla SR, Griendling KK. Reactive oxygen species, NADPH oxidases, and hypertension. Hypertension 56: 325–330, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.De Lucca M, Casique L. Characterization of cystathionine beta-synthase gene mutations in homocystinuric Venezuelan patients: Identification of one novel mutation in exon 6. Mol Genet Metab 81: 209–215, 2004. [DOI] [PubMed] [Google Scholar]
- 70.Deindl E, Zaruba MM, Brunner S, Huber B, Mehl U, Assmann G, Hoefer IE, Mueller-Hoecker J, Franz WM. G-CSF administration after myocardial infarction in mice attenuates late ischemic cardiomyopathy by enhanced arteriogenesis. FASEB J 20: 956–958, 2006. [DOI] [PubMed] [Google Scholar]
- 71.Di Meo S, Reed TT, Venditti P, Victor VM. Role of ROS and RNS sources in physiological and pathological conditions. Oxid Med Cell Longev 2016: 1245049, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ding R, Lin S, Chen D. The association of cystathionine beta synthase (CBS) T833C polymorphism and the risk of stroke: A meta-analysis. J Neurol Sci 312: 26–30, 2012. [DOI] [PubMed] [Google Scholar]
- 73.Doka E, Pader I, Biro A, Johansson K, Cheng Q, Ballago K, Prigge JR, Pastor-Flores D, Dick TP, Schmidt EE, Arner ES, Nagy P. A novel persulfide detection method reveals protein persulfide- and polysulfide-reducing functions of thioredoxin and glutathione systems. Sci Adv 2: e1500968, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Drew B, Leeuwenburgh C. Aging and the role of reactive nitrogen species. Ann N Y Acad Sci 959: 66–81, 2002. [DOI] [PubMed] [Google Scholar]
- 75.Dudek SM, Garcia JG. Cytoskeletal regulation of pulmonary vascular permeability. J Appl Physiol (1985) 91: 1487–1500, 2001. [DOI] [PubMed] [Google Scholar]
- 76.Dumont DJ, Gradwohl G, Fong GH, Puri MC, Gertsenstein M, Auerbach A, Breitman ML. Dominant-negative and targeted null mutations in the endothelial receptor tyrosine kinase, tek, reveal a critical role in vasculogenesis of the embryo. Genes Dev 8: 1897–1909, 1994. [DOI] [PubMed] [Google Scholar]
- 77.Ebrahimian TG, Heymes C, You D, Blanc-Brude O, Mees B, Waeckel L, Duriez M, Vilar J, Brandes RP, Levy BI, Shah AM, Silvestre JS. NADPH oxidase-derived overproduction of reactive oxygen species impairs postischemic neovascularization in mice with type 1 diabetes. Am J Pathol 169: 719–728, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Eggebeen J, Kim-Shapiro DB, Haykowsky M, Morgan TM, Basu S, Brubaker P, Rejeski J, Kitzman DW. One week of daily dosing with beetroot juice improves submaximal endurance and blood pressure in older patients with heart failure and preserved ejection fraction. JACC Heart Fail 4: 428–437, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Eichmann A, Simons M. VEGF signaling inside vascular endothelial cells and beyond. Curr Opin Cell Biol 24: 188–193, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Eitenmuller I, Volger O, Kluge A, Troidl K, Barancik M, Cai WJ, Heil M, Pipp F, Fischer S, Horrevoets AJ, Schmitz-Rixen T, Schaper W. The range of adaptation by collateral vessels after femoral artery occlusion. Circ Res 99: 656–662, 2006. [DOI] [PubMed] [Google Scholar]
- 81.Eliceiri BP, Paul R, Schwartzberg PL, Hood JD, Leng J, Cheresh DA. Selective requirement for Src kinases during VEGF-induced angiogenesis and vascular permeability. Mol Cell 4: 915–924, 1999. [DOI] [PubMed] [Google Scholar]
- 82.Fantin A, Vieira JM, Gestri G, Denti L, Schwarz Q, Prykhozhij S, Peri F, Wilson SW, Ruhrberg C. Tissue macrophages act as cellular chaperones for vascular anastomosis downstream of VEGF-mediated endothelial tip cell induction. Blood 116: 829–840, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Fernandez Pujol B, Lucibello FC, Gehling UM, Lindemann K, Weidner N, Zuzarte ML, Adamkiewicz J, Elsasser HP, Muller R, Havemann K. Endothelial-like cells derived from human CD14 positive monocytes. Differentiation 65: 287–300, 2000. [DOI] [PubMed] [Google Scholar]
- 84.Ferrara N, Carver-Moore K, Chen H, Dowd M, Lu L, O’Shea KS, Powell-Braxton L, Hillan KJ, Moore MW. Heterozygous embryonic lethality induced by targeted inactivation of the VEGF gene. Nature 380: 439–442, 1996. [DOI] [PubMed] [Google Scholar]
- 85.Filipovic MR, Miljkovic J, Nauser T, Royzen M, Klos K, Shubina T, Koppenol WH, Lippard SJ, Ivanovic-Burmazovic I. Chemical characterization of the smallest S-nitrosothiol, HSNO; cellular cross-talk of H2S and S-nitrosothiols. J Am Chem Soc 134: 12016–12027, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Finkel T From sulfenylation to sulfhydration: What a thiolate needs to tolerate. Sci Signal 5: pe10, 2012. [DOI] [PubMed] [Google Scholar]
- 87.Folkman J, Shing Y. Angiogenesis. J Biol Chem 267: 10931–10934, 1992. [PubMed] [Google Scholar]
- 88.Forman HJ, Fukuto JM, Torres M. Redox signaling: Thiol chemistry defines which reactive oxygen and nitrogen species can act as second messengers. Am J Physiol Cell Physiol 287: C246–C256, 2004. [DOI] [PubMed] [Google Scholar]
- 89.Fortuno A, San Jose G, Moreno MU, Diez J, Zalba G. Oxidative stress and vascular remodelling. Exp Physiol 90: 457–462, 2005. [DOI] [PubMed] [Google Scholar]
- 90.Francke A, Weinert S, Strasser RH, Braun-Dullaeus RC, Herold J. Transplantation of bone marrow derived monocytes: A novel approach for augmentation of arteriogenesis in a murine model of femoral artery ligation. Am J Transl Res 5: 155–169, 2013. [PMC free article] [PubMed] [Google Scholar]
- 91.Fukuda Y, Teragawa H, Matsuda K, Yamagata T, Matsuura H, Chayama K. Tetrahydrobiopterin restores endothelial function of coronary arteries in patients with hypercholesterolaemia. Heart 87: 264–269, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Fukumura D, Gohongi T, Kadambi A, Izumi Y, Ang J, Yun CO, Buerk DG, Huang PL, Jain RK. Predominant role of endothelial nitric oxide synthase in vascular endothelial growth factor-induced angiogenesis and vascular permeability. Proc Natl Acad Sci U S A 98: 2604–2609, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Fukuto JM, Carrington SJ, Tantillo DJ, Harrison JG, Ignarro LJ, Freeman BA, Chen A, Wink DA. Small molecule signaling agents: The integrated chemistry and biochemistry of nitrogen oxides, oxides of carbon, dioxygen, hydrogen sulfide, and their derived species. Chem Res Toxicol 25: 769–793, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Fukuto JM, Ignarro LJ, Nagy P, Wink DA, Kevil CG, Feelisch M, Cortese-Krott MM, Bianco CL, Kumagai Y, Hobbs AJ, Lin J, Ida T, Akaike T. Biological hydropersulfides and related polysulfides - a new concept and perspective in redox biology. FEBS Lett 592: 2140–2152, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Funazo K, Tanaka M, Morita K, Kamino M, Shono T, Wu H-L. Pentafluorobenzyl p-toluenesulphonate as a new derivatizing reagent for gas chromatographic determination of anions. J Chromatography A 346: 215–225, 1985. [Google Scholar]
- 96.Fung E, Helisch A. Macrophages in collateral arteriogenesis. Front Physiol 3: 353, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Furchgott RF, Zawadzki JV. The obligatory role of endothelial cells in the relaxation of arterial smooth muscle by acetylcholine. Nature 288: 373–376, 1980. [DOI] [PubMed] [Google Scholar]
- 98.Gale NW, Yancopoulos GD. Growth factors acting via endothelial cell-specific receptor tyrosine kinases: VEGFs, angiopoietins, and ephrins in vascular development. Genes Dev 13: 1055–1066, 1999. [DOI] [PubMed] [Google Scholar]
- 99.Gallegos-Arreola MP, Figuera-Villanueva LE, Ramos-Silva A, Salas-Gonzalez E, Puebla-Perez AM, Peralta-Leal V, Garcia-Ortiz JE, Davalos-Rodriguez IP, Zuniga-Gonzalez GM. The association between the 844ins68 polymorphism in the CBS gene and breast cancer. Arch Med Sci 10: 1214–1224, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Gallogly MM, Mieyal JJ. Mechanisms of reversible protein glutathionylation in redox signaling and oxidative stress. Curr Opin Pharmacol 7: 381–391, 2007. [DOI] [PubMed] [Google Scholar]
- 101.Gao XH, Krokowski D, Guan BJ, Bederman I, Majumder M, Parisien M, Diatchenko L, Kabil O, Willard B, Banerjee R, Wang B, Bebek G, Evans CR, Fox PL, Gerson SL, Hoppel CL, Liu M, Arvan P, Hatzoglou M. Quantitative H2S-mediated protein sulfhydration reveals metabolic reprogramming during the integrated stress response. Elife 4: e10067, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Gerber HP, McMurtrey A, Kowalski J, Yan M, Keyt BA, Dixit V, Ferrara N. Vascular endothelial growth factor regulates endothelial cell survival through the phosphatidylinositol 3′-kinase/Akt signal transduction pathway. Requirement for Flk-1/KDR activation. J Biol Chem 273: 30336–30343, 1998. [DOI] [PubMed] [Google Scholar]
- 103.Ghosh R, Lipson KL, Sargent KE, Mercurio AM, Hunt JS, Ron D, Urano F. Transcriptional regulation of VEGF-A by the unfolded protein response pathway. PLoS One 5: e9575, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Gibson GR, Macfarlane GT, Cummings JH. Occurrence of sulphate-reducing bacteria in human faeces and the relationship of dissimilatory sulphate reduction to methanogenesis in the large gut. J Appl Bacteriol 65: 103–111, 1988. [DOI] [PubMed] [Google Scholar]
- 105.Giraldo JP, Landry MP, Faltermeier SM, McNicholas TP, Iverson NM, Boghossian AA, Reuel NF, Hilmer AJ, Sen F, Brew JA, Strano MS. Plant nanobionics approach to augment photosynthesis and biochemical sensing. Nat Mater 13: 400–408, 2014. [DOI] [PubMed] [Google Scholar]
- 106.Gladwin MT, Schechter AN, Kim-Shapiro DB, Patel RP, Hogg N, Shiva S, Cannon RO III, Kelm M, Wink DA, Espey MG, Oldfield EH, Pluta RM, Freeman BA, Lancaster JR Jr., Feelisch M, Lundberg JO. The emerging biology of the nitrite anion. Nat Chem Biol 1: 308–314, 2005. [DOI] [PubMed] [Google Scholar]
- 107.Godo S, Shimokawa H. Divergent roles of endothelial nitric oxide synthases system in maintaining cardiovascular homeostasis. Free Radic Biol Med 109: 4–10, 2017. [DOI] [PubMed] [Google Scholar]
- 108.Gong QH, Wang Q, Pan LL, Liu XH, Xin H, Zhu YZ. S-propargyl-cysteine, a novel hydrogen sulfide-modulated agent, attenuates lipopolysaccharide-induced spatial learning and memory impairment: Involvement of TNF signaling and NF-kappaB pathway in rats. Brain Behav Immun 25: 110–119, 2011. [DOI] [PubMed] [Google Scholar]
- 109.Goslar T, Mars T, Podbregar M. Total plasma sulfide as a marker of shock severity in nonsurgical adult patients. Shock 36: 350–355, 2011. [DOI] [PubMed] [Google Scholar]
- 110.Granger DN, Kvietys PR. Reperfusion injury and reactive oxygen species: The evolution of a concept. Redox Biol 6: 524–551, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Greiner R, Palinkas Z, Basell K, Becher D, Antelmann H, Nagy P, Dick TP. Polysulfides link H2S to protein thiol oxidation. Antioxid Redox Signal 19: 1749–1765, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Grines C, Rubanyi GM, Kleiman NS, Marrott P, Watkins MW. Angiogenic gene therapy with adenovirus 5 fibroblast growth factor-4 (Ad5FGF-4): a new option for the treatment of coronary artery disease. Am J Cardiol 92: 24N–31N, 2003. [DOI] [PubMed] [Google Scholar]
- 113.Grines CL, Watkins MW, Helmer G, Penny W, Brinker J, Marmur JD, West A, Rade JJ, Marrott P, Hammond HK, Engler RL. Angiogenic Gene Therapy (AGENT) trial in patients with stable angina pectoris. Circulation 105: 1291–1297, 2002. [DOI] [PubMed] [Google Scholar]
- 114.Grochot-Przeczek A, Dulak J, Jozkowicz A. Therapeutic angiogenesis for revascularization in peripheral artery disease. Gene 525: 220–228, 2013. [DOI] [PubMed] [Google Scholar]
- 115.Gruetter CA, Barry BK, McNamara DB, Gruetter DY, Kadowitz PJ, Ignarro L. Relaxation of bovine coronary artery and activation of coronary arterial guanylate cyclase by nitric oxide, nitroprusside and a carcinogenic nitrosoamine. J Cyclic Nucleotide Res 5: 211–224, 1979. [PubMed] [Google Scholar]
- 116.Grundmann S, Hoefer I, Ulusans S, van Royen N, Schirmer SH, Ozaki CK, Bode C, Piek JJ, Buschmann I. Anti-tumor necrosis factor-{alpha} therapies attenuate adaptive arteriogenesis in the rabbit. Am J Physiol Heart Circ Physiol 289: H1497–H1505, 2005. [DOI] [PubMed] [Google Scholar]
- 117.Gupta V, Kapopara PR, Khan AA, Arige V, Subramanian L, Sonawane PJ, Sasi BK, Mahapatra NR. Functional promoter polymorphisms direct the expression of cystathionine gamma-lyase gene in mouse models of essential hypertension. J Mol Cell Cardiol 102: 61–73, 2017. [DOI] [PubMed] [Google Scholar]
- 118.Hargrove JL. Persulfide generated from L-cysteine inactivates tyrosine aminotransferase. Requirement for a protein with cysteine oxidase activity and gamma-cystathionase. J Biol Chem 263: 17262–17269, 1988. [PubMed] [Google Scholar]
- 119.Hazarika S, Dokun AO, Li Y, Popel AS, Kontos CD, Annex BH. Impaired angiogenesis after hindlimb ischemia in type 2 diabetes mellitus: Differential regulation of vascular endothelial growth factor receptor 1 and soluble vascular endothelial growth factor receptor 1. Circ Res 101: 948–956, 2007. [DOI] [PubMed] [Google Scholar]
- 120.Heil M, Schaper W. Influence of mechanical, cellular, and molecular factors on collateral artery growth (arteriogenesis). Circ Res 95: 449–458, 2004. [DOI] [PubMed] [Google Scholar]
- 121.Heil M, Ziegelhoeffer T, Pipp F, Kostin S, Martin S, Clauss M, Schaper W. Blood monocyte concentration is critical for enhancement of collateral artery growth. Am J Physiol Heart Circ Physiol 283: H2411–H2419, 2002. [DOI] [PubMed] [Google Scholar]
- 122.Heinrich TA, da Silva RS, Miranda KM, Switzer CH, Wink DA, Fukuto JM. Biological nitric oxide signalling: Chemistry and terminology. Br J Pharmacol 169: 1417–1429, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Heitzer T, Krohn K, Albers S, Meinertz T. Tetrahydrobiopterin improves endothelium-dependent vasodilation by increasing nitric oxide activity in patients with Type II diabetes mellitus. Diabetologia 43: 1435–1438, 2000. [DOI] [PubMed] [Google Scholar]
- 124.Hendrix P, Foreman PM, Harrigan MR, Fisher WS III, Vyas NA, Lipsky RH, Lin M, Walters BC, Tubbs RS, Shoja MM, Pittet JF, Mathru M, Griessenauer CJ. Association of cystathionine beta-synthase polymorphisms and aneurysmal subarachnoid hemorrhage. J Neurosurg 128: 1771–1777, 2018. [DOI] [PubMed] [Google Scholar]
- 125.Henry TD, Grines CL, Watkins MW, Dib N, Barbeau G, Moreadith R, Andrasfay T, Engler RL. Effects of Ad5FGF-4 in patients with angina: An analysis of pooled data from the AGENT-3 and AGENT-4 trials. J Am Coll Cardiol 50: 1038–1046, 2007. [DOI] [PubMed] [Google Scholar]
- 126.Henry Y, Lepoivre M, Drapier JC, Ducrocq C, Boucher JL, Guissani A. EPR characterization of molecular targets for NO in mammalian cells and organelles. FASEB J 7: 1124–1134, 1993. [DOI] [PubMed] [Google Scholar]
- 127.Hilderbrand SA, Lim MH, Lippard SJ. Dirhodium tetracarboxylate scaffolds as reversible fluorescence-based nitric oxide sensors. J Am Chem Soc 126: 4972–4978, 2004. [DOI] [PubMed] [Google Scholar]
- 128.Hingorani AD, Liang CF, Fatibene J, Lyon A, Monteith S, Parsons A, Haydock S, Hopper RV, Stephens NG, O’Shaughnessy KM, Brown MJ. A common variant of the endothelial nitric oxide synthase (Glu298→Asp) is a major risk factor for coronary artery disease in the UK. Circulation 100: 1515–1520, 1999. [DOI] [PubMed] [Google Scholar]
- 129.Hitzfeld KL, Gehre M, Richnow HH. A novel online approach to the determination of isotopic ratios for organically bound chlorine, bromine and sulphur. Rapid Commun Mass Spectrom 25: 3114–3122, 2011. [DOI] [PubMed] [Google Scholar]
- 130.Hoefer IE, den Adel B, Daemen MJ. Biomechanical factors as triggers of vascular growth. Cardiovasc Res 99: 276–283, 2013. [DOI] [PubMed] [Google Scholar]
- 131.Hoefer IE, van Royen N, Rectenwald JE, Bray EJ, Abouhamze Z, Moldawer LL, Voskuil M, Piek JJ, Buschmann IR, Ozaki CK. Direct evidence for tumor necrosis factor-alpha signaling in arteriogenesis. Circulation 105: 1639–1641, 2002. [DOI] [PubMed] [Google Scholar]
- 132.Hoefer IE, van Royen N, Rectenwald JE, Deindl E, Hua J, Jost M, Grundmann S, Voskuil M, Ozaki CK, Piek JJ, Buschmann IR. Arteriogenesis proceeds via ICAM-1/Mac-1-mediated mechanisms. Circ Res 94: 1179–1185, 2004. [DOI] [PubMed] [Google Scholar]
- 133.Holowatz LA, Kenney WL. Acute localized administration of tetrahydrobiopterin and chronic systemic atorvastatin treatment restore cutaneous microvascular function in hypercholesterolaemic humans. J Physiol 589: 4787–4797, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Holwerda KM, Weedon-Fekjaer MS, Staff AC, Nolte IM, van Goor H, Lely AT, Faas MM. The association of single nucleotide polymorphisms of the maternal cystathionine-beta-synthase gene with early-onset preeclampsia. Pregnancy Hypertens 6: 60–65, 2016. [DOI] [PubMed] [Google Scholar]
- 135.Hu LF, Lu M, Hon Wong PT, Bian JS. Hydrogen sulfide: Neurophysiology and neuropathology. Antioxid Redox Signal 15: 405–419, 2011. [DOI] [PubMed] [Google Scholar]
- 136.Huang CW, Moore PK. H2S synthesizing enzymes: Biochemistry and molecular aspects. Handb Exp Pharmacol 230: 3–25, 2015. [DOI] [PubMed] [Google Scholar]
- 137.Huang S, Li H, Ge J. A cardioprotective insight of the cystathionine gamma-lyase/hydrogen sulfide pathway. Int J Cardiol Heart Vasc 7: 51–57, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Hughes MN, Centelles MN, Moore KP. Making and working with hydrogen sulfide: The chemistry and generation of hydrogen sulfide in vitro and its measurement in vivo: A review. Free Radic Biol Med 47: 1346–1353, 2009. [DOI] [PubMed] [Google Scholar]
- 139.Ibiza S, Perez-Rodriguez A, Ortega A, Martinez-Ruiz A, Barreiro O, Garcia-Dominguez CA, Victor VM, Esplugues JV, Rojas JM, Sanchez-Madrid F, Serrador JM. Endothelial nitric oxide synthase regulates N-Ras activation on the Golgi complex of antigen-stimulated T cells. Proc Natl Acad Sci U S A 105: 10507–10512, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Ida T, Sawa T, Ihara H, Tsuchiya Y, Watanabe Y, Kumagai Y, Suematsu M, Motohashi H, Fujii S, Matsunaga T, Yamamoto M, Ono K, Devarie-Baez NO, Xian M, Fukuto JM, Akaike T. Reactive cysteine persulfides and S-polythiolation regulate oxidative stress and redox signaling. Proc Natl Acad Sci U S A 111: 7606–7611, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Ignarro LJ, Buga GM, Wood KS, Byrns RE, Chaudhuri G. Endothelium-derived relaxing factor produced and released from artery and vein is nitric oxide. Proc Natl Acad Sci U S A 84: 9265–9269, 1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Ignarro LJ, Byrns RE, Buga GM, Wood KS, Chaudhuri G. Pharmacological evidence that endothelium-derived relaxing factor is nitric oxide: Use of pyrogallol and superoxide dismutase to study endothelium-dependent and nitric oxide-elicited vascular smooth muscle relaxation. J Pharmacol Exp Ther 244: 181–189, 1988. [PubMed] [Google Scholar]
- 143.Ito WD, Arras M, Winkler B, Scholz D, Schaper J, Schaper W. Monocyte chemotactic protein-1 increases collateral and peripheral conductance after femoral artery occlusion. Circ Res 80: 829–837, 1997. [DOI] [PubMed] [Google Scholar]
- 144.Iwakiri Y, Satoh A, Chatterjee S, Toomre DK, Chalouni CM, Fulton D, Groszmann RJ, Shah VH, Sessa WC. Nitric oxide synthase generates nitric oxide locally to regulate compartmentalized protein S-nitrosylation and protein trafficking. Proc Natl Acad Sci U S A 103: 19777–19782, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Jacobi J, Tam BY, Wu G, Hoffman J, Cooke JP, Kuo CJ. Adenoviral gene transfer with soluble vascular endothelial growth factor receptors impairs angiogenesis and perfusion in a murine model of hindlimb ischemia. Circulation 110: 2424–2429, 2004. [DOI] [PubMed] [Google Scholar]
- 146.Janssen-Heininger YM, Mossman BT, Heintz NH, Forman HJ, Kalya-naraman B, Finkel T, Stamler JS, Rhee SG, van der Vliet A. Redox-based regulation of signal transduction: Principles, pitfalls, and promises. Free Radic Biol Med 45: 1–17, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Jensen DE, Belka GK, Du Bois GC. S-Nitrosoglutathione is a substrate for rat alcohol dehydrogenase class III isoenzyme. Biochem J 331(Pt 2): 659–668, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Jiang J, Chan A, Ali S, Saha A, Haushalter KJ, Lam W-LM, Glasheen M, Parker J, Brenner M, Mahon SB, Patel HH, Ambasudhan R, Lipton SA, Pilz RB, Boss GR. Hydrogen sulfide—mechanisms of toxicity and development of an antidote. Sci Rep 6: 20831, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Jin H, Heller DA, Kalbacova M, Kim JH, Zhang J, Boghossian AA, Maheshri N, Strano MS. Detection of single-molecule H2O2 signalling from epidermal growth factor receptor using fluorescent single-walled carbon nanotubes. Nat Nanotechnol 5: 302–309, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Jin S, Pu SX, Hou CL, Ma FF, Li N, Li XH, Tan B, Tao BB, Wang MJ, Zhu YC. Cardiac H2S generation is reduced in ageing diabetic mice. Oxid Med Cell Longev 2015: 758358, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Johansson M, Lundberg M. Glutathionylation of beta-actin via a cysteinyl sulfenic acid intermediary. BMC Biochem 8: 26, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Jones WS, Duscha BD, Robbins JL, Duggan NN, Regensteiner JG, Kraus WE, Hiatt WR, Dokun AO, Annex BH. Alteration in angiogenic and anti-angiogenic forms of vascular endothelial growth factor-A in skeletal muscle of patients with intermittent claudication following exercise training. Vasc Med 17: 94–100, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Jung O, Marklund SL, Geiger H, Pedrazzini T, Busse R, Brandes RP. Extracellular superoxide dismutase is a major determinant of nitric oxide bioavailability: In vivo and ex vivo evidence from ecSOD-deficient mice. Circ Res 93: 622–629, 2003. [DOI] [PubMed] [Google Scholar]
- 154.Kapur NK, Rade JJ. Fibroblast growth factor 4 gene therapy for chronic ischemic heart disease. Trends Cardiovasc Med 18: 133–141, 2008. [DOI] [PubMed] [Google Scholar]
- 155.Kearney JB, Ambler CA, Monaco KA, Johnson N, Rapoport RG, Bautch VL. Vascular endothelial growth factor receptor Flt-1 negatively regulates developmental blood vessel formation by modulating endothelial cell division. Blood 99: 2397–2407, 2002. [DOI] [PubMed] [Google Scholar]
- 156.Keeley TP, Mann GE. Defining physiological normoxia for improved translation of cell physiology to animal models and humans. Physiol Rev 99: 161–234, 2019. [DOI] [PubMed] [Google Scholar]
- 157.Kevil CG, Kolluru GK, Pattillo CB, Giordano T. Inorganic nitrite therapy: Historical perspective and future directions. Free Radic Biol Med 51: 576–593, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Kevil CG, Payne DK, Mire E, Alexander JS. Vascular permeability factor/vascular endothelial cell growth factor-mediated permeability occurs through disorganization of endothelial junctional proteins. J Biol Chem 273: 15099–15103, 1998. [DOI] [PubMed] [Google Scholar]
- 159.Kielstein JT, Impraim B, Simmel S, Bode-Boger SM, Tsikas D, Frolich JC, Hoeper MM, Haller H, Fliser D. Cardiovascular effects of systemic nitric oxide synthase inhibition with asymmetrical dimethylarginine in humans. Circulation 109: 172–177, 2004. [DOI] [PubMed] [Google Scholar]
- 160.Kikuchi R, Nakamura K, MacLauchlan S, Ngo DT, Shimizu I, Fuster JJ, Katanasaka Y, Yoshida S, Qiu Y, Yamaguchi TP, Matsushita T, Murohara T, Gokce N, Bates DO, Hamburg NM, Walsh K. An antiangiogenic isoform of VEGF-A contributes to impaired vascularization in peripheral artery disease. Nat Med 20: 1464–1471, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.King AL, Polhemus DJ, Bhushan S, Otsuka H, Kondo K, Nicholson CK, Bradley JM, Islam KN, Calvert JW, Tao YX, Dugas TR, Kelley EE, Elrod JW, Huang PL, Wang R, Lefer DJ. Hydrogen sulfide cytoprotective signaling is endothelial nitric oxide synthase-nitric oxide dependent. Proc Natl Acad Sci U S A 111: 3182–3187, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Klatt P, Lamas S. Regulation of protein function by S-glutathiolation in response to oxidative and nitrosative stress. Eur J Biochem 267: 4928–4944, 2000. [DOI] [PubMed] [Google Scholar]
- 163.Kolluru GK, Bir SC, Yuan S, Shen X, Pardue S, Wang R, Kevil CG. Cystathionine gamma-lyase regulates arteriogenesis through NO-dependent monocyte recruitment. Cardiovasc Res 107: 590–600, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Kolluru GK, Prasai PK, Kaskas AM, Letchuman V, Pattillo CB. Oxygen tension, H2S, and NO bioavailability: Is there an interaction? J Appl Physiol (1985) 120: 263–270, 2016. [DOI] [PubMed] [Google Scholar]
- 165.Kolluru GK, Shen X, Bir SC, Kevil CG. Hydrogen sulfide chemical biology: Pathophysiological roles and detection. Nitric Oxide 35: 5–20, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Kolluru GK, Shen X, Kevil CG. A tale of two gases: NO and H2S, foes or friends for life? Redox Biol 1: 313–318, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Kolluru GK, Shen X, Yuan S, Kevil CG. Gasotransmitter heterocellular signaling. Antioxid Redox Signal 26: 936–960, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Konrad C, Muller GA, Langer C, Kuhlenbaumer G, Berger K, Nabavi DG, Dziewas R, Stogbauer F, Ringelstein EB, Junker R. Plasma homocysteine, MTHFR C677T, CBS 844ins68bp, and MTHFD1 G1958A polymorphisms in spontaneous cervical artery dissections. J Neurol 251: 1242–1248, 2004. [DOI] [PubMed] [Google Scholar]
- 169.Krahl ME, Keltch AK, Neubeck CE, Clowes GH. Studies on cell metabolism and cell division: V. cytochrome oxidase activity in the eggs of Arbacia punctulata. J Gen Physiol 24: 597–617, 1941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Krempl TK, Maas R, Sydow K, Meinertz T, Boger RH, Kahler J. Elevation of asymmetric dimethylarginine in patients with unstable angina and recurrent cardiovascular events. Eur Heart J 26: 1846–1851, 2005. [DOI] [PubMed] [Google Scholar]
- 171.Krishnan N, Fu C, Pappin DJ, Tonks NK. H2S-Induced sulfhydration of the phosphatase PTP1B and its role in the endoplasmic reticulum stress response. Sci Signal 4: ra86, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Krock BL, Skuli N, Simon MC. Hypoxia-induced angiogenesis: Good and evil. Genes Cancer 2: 1117–1133, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Kruger WD, Wang L, Jhee KH, Singh RH, Elsas LJ III. Cystathionine beta-synthase deficiency in Georgia (USA): Correlation of clinical and biochemical phenotype with genotype. Human mutation 22: 434–441, 2003. [DOI] [PubMed] [Google Scholar]
- 174.Kubota Y, Takubo K, Shimizu T, Ohno H, Kishi K, Shibuya M, Saya H, Suda T. M-CSF inhibition selectively targets pathological angiogenesis and lymphangiogenesis. J Exp Med 206: 1089–1102, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Kuethe F, Figulla HR, Herzau M, Voth M, Fritzenwanger M, Opfermann T, Pachmann K, Krack A, Sayer HG, Gottschild D, Werner GS. Treatment with granulocyte colony-stimulating factor for mobilization of bone marrow cells in patients with acute myocardial infarction. Am Heart J 150: 115, 2005. [DOI] [PubMed] [Google Scholar]
- 176.Kumar D, Branch BG, Pattillo CB, Hood J, Thoma S, Simpson S, Illum S, Arora N, Chidlow JH Jr., Langston W, Teng X, Lefer DJ, Patel RP, Kevil CG. Chronic sodium nitrite therapy augments ischemia-induced angiogenesis and arteriogenesis. Proc Natl Acad Sci U S A 105: 7540–7545, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Lancaster JR. Simulation of the diffusion and reaction of endogenously produced nitric oxide. Proc Natl Acad Sci U S A 91: 8137–8141, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Landmesser U, Dikalov S, Price SR, McCann L, Fukai T, Holland SM, Mitch WE, Harrison DG. Oxidation of tetrahydrobiopterin leads to uncoupling of endothelial cell nitric oxide synthase in hypertension. J Clin Invest 111: 1201–1209, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Lazarous DF, Unger EF, Epstein SE, Stine A, Arevalo JL, Chew EY, Quyyumi AA. Basic fibroblast growth factor in patients with intermittent claudication: Results of a phase I trial. J Am Coll Cardiol 36: 1239–1244, 2000. [DOI] [PubMed] [Google Scholar]
- 180.Lederman RJ, Mendelsohn FO, Anderson RD, Saucedo JF, Tenaglia AN, Hermiller JB, Hillegass WB, Rocha-Singh K, Moon TE, Whitehouse MJ, Annex BH, Investigators T. Therapeutic angiogenesis with recombinant fibroblast growth factor-2 for intermittent claudication (the TRAFFIC study): a randomised trial. Lancet 359: 2053–2058, 2002. [DOI] [PubMed] [Google Scholar]
- 181.Leibovich SJ, Polverini PJ, Fong TW, Harlow LA, Koch AE. Production of angiogenic activity by human monocytes requires an L-arginine/nitric oxide-synthase-dependent effector mechanism. Proc Natl Acad Sci U S A 91: 4190–4194, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Leichert LI, Jakob U. Global methods to monitor the thiol-disulfide state of proteins in vivo. Antioxid Redox Signal 8: 763–772, 2006. [DOI] [PubMed] [Google Scholar]
- 183.Leong T, Zylberstein D, Graham I, Lissner L, Ward D, Fogarty J, Bengtsson C, Bjorkelund C, Thelle D, Swedish-Irish-Norwegian C. Asymmetric dimethylarginine independently predicts fatal and nonfatal myocardial infarction and stroke in women: 24-year follow-up of the population study of women in Gothenburg. Arterioscler Thromb Vasc Biol 28: 961–967, 2008. [DOI] [PubMed] [Google Scholar]
- 184.Leung DW, Cachianes G, Kuang WJ, Goeddel DV, Ferrara N. Vascular endothelial growth factor is a secreted angiogenic mitogen. Science 246: 1306–1309, 1989. [DOI] [PubMed] [Google Scholar]
- 185.Levitt MD, Abdel-Rehim MS, Furne J. Free and acid-labile hydrogen sulfide concentrations in mouse tissues: Anomalously high free hydrogen sulfide in aortic tissue. Antioxid Redox Signal 15: 373–378, 2011. [DOI] [PubMed] [Google Scholar]
- 186.Li H, Mani S, Wu L, Fu M, Shuang T, Xu C, Wang R. The interaction of estrogen and CSE/H2S pathway in the development of atherosclerosis. Am J Physiol Heart Circ Physiol 312: H406–H414, 2017. [DOI] [PubMed] [Google Scholar]
- 187.Li H, Wan A. Fluorescent probes for real-time measurement of nitric oxide in living cells. Analyst 140: 7129–7141, 2015. [DOI] [PubMed] [Google Scholar]
- 188.Li H, Witte K, August M, Brausch I, Godtel-Armbrust U, Habermeier A, Closs EI, Oelze M, Munzel T, Forstermann U. Reversal of endothelial nitric oxide synthase uncoupling and up-regulation of endothelial nitric oxide synthase expression lowers blood pressure in hypertensive rats. J Am Coll Cardiol 47: 2536–2544, 2006. [DOI] [PubMed] [Google Scholar]
- 189.Li Q, Lancaster JR Jr. Chemical foundations of hydrogen sulfide biology. Nitric Oxide 35: 21–34, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Li XA, Everson W, Smart EJ. Nitric oxide, caveolae, and vascular pathology. Cardiovasc Toxicol 6: 1–13, 2006. [DOI] [PubMed] [Google Scholar]
- 191.Li Y, Zhao Q, Liu XL, Wang LY, Lu XF, Li HF, Chen SF, Huang JF, Gu DF. Relationship between cystathionine gamma-lyase gene polymorphism and essential hypertension in Northern Chinese Han population. Chin Med J 121: 716–720, 2008. [PubMed] [Google Scholar]
- 192.Li YJ, Guan H, Hazarika S, Liu CW, Annex BH. Impaired angiogenesis following hind-limb ischemia in diabetes mellitus mice. Chin Med Sci J 22: 232–237, 2007. [PubMed] [Google Scholar]
- 193.Liu J, Wang Y, Akamatsu Y, Lee CC, Stetler RA, Lawton MT, Yang GY. Vascular remodeling after ischemic stroke: Mechanisms and therapeutic potentials. Prog Neurobiol 115: 138–156, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Liu L, Hausladen A, Zeng M, Que L, Heitman J, Stamler JS. A metabolic enzyme for S-nitrosothiol conserved from bacteria to humans. Nature 410: 490–494, 2001. [DOI] [PubMed] [Google Scholar]
- 195.Liu X, Miller MJ, Joshi MS, Thomas DD, Lancaster JR Jr. Accelerated reaction of nitric oxide with O2 within the hydrophobic interior of biological membranes. Proc Natl Acad Sci U S A 95: 2175–2179, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Lowenstein CJ, Dinerman JL, Snyder SH. Nitric oxide: A physiologic messenger. Ann Intern Med 120: 227–237, 1994. [DOI] [PubMed] [Google Scholar]
- 197.Lowicka E, Beltowski J. Hydrogen sulfide (H2S) - the third gas of interest for pharmacologists. Pharmacol Rep 59: 4–24, 2007. [PubMed] [Google Scholar]
- 198.Lundberg JO, Gladwin MT, Ahluwalia A, Benjamin N, Bryan NS, Butler A, Cabrales P, Fago A, Feelisch M, Ford PC, Freeman BA, Frenneaux M, Friedman J, Kelm M, Kevil CG, Kim-Shapiro DB, Kozlov AV, Lancaster JR Jr., Lefer DJ, McColl K, McCurry K, Patel RP, Petersson J, Rassaf T, Reutov VP, Richter-Addo GB, Schechter A, Shiva S, Tsuchiya K, van Faassen EE, Webb AJ, Zuckerbraun BS, Zweier JL, Weitzberg E. Nitrate and nitrite in biology, nutrition and therapeutics. Nat Chem Biol 5: 865–869, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Luo F, Wariaro D, Lundberg G, Blegen H, Wahlberg E. Vascular growth factor expression in a rat model of severe limb ischemia. J Surg Res 108: 258–267, 2002. [DOI] [PubMed] [Google Scholar]
- 200.Mac Gabhann F, Peirce SM. Collateral capillary arterialization following arteriolar ligation in murine skeletal muscle. Microcirculation 17: 333–347, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.MacMillan-Crow LA, Crow JP, Kerby JD, Beckman JS, Thompson JA. Nitration and inactivation of manganese superoxide dismutase in chronic rejection of human renal allografts. Proc Natl Acad Sci U S A 93: 11853–11858, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.MacMillan-Crow LA, Thompson JA. Tyrosine modifications and inactivation of active site manganese superoxide dismutase mutant (Y34F) by peroxynitrite. Arch Biochem Biophys 366: 82–88, 1999. [DOI] [PubMed] [Google Scholar]
- 203.Maher AR, Milsom AB, Gunaruwan P, Abozguia K, Ahmed I, Weaver RA, Thomas P, Ashrafian H, Born GVR, James PE, Frenneaux MP. Hypoxic Modulation of Exogenous Nitrite-Induced Vasodilation in Humans. Circulation 117: 670–677, 2008. [DOI] [PubMed] [Google Scholar]
- 204.Maier W, Cosentino F, Lutolf RB, Fleisch M, Seiler C, Hess OM, Meier B, Luscher TF. Tetrahydrobiopterin improves endothelial function in patients with coronary artery disease. J Cardiovasc Pharmacol 35: 173–178, 2000. [DOI] [PubMed] [Google Scholar]
- 205.Majumder S, Sinha S, Siamwala JH, Muley A, Reddy Seerapu H, Kolluru GK, Veeriah V, Nagarajan S, Sridhara SRC, Priya MK, Kuppusamy M, Srinivasan S, Konikkat S, Soundararajan G, Venkataraman S, Saran U, Chatterjee S. A comparative study of NONOate based NO donors: Spermine NONOate is the best suited NO donor for angiogenesis. Nitric Oxide 36: 76–86, 2014. [DOI] [PubMed] [Google Scholar]
- 206.Mani S, Li H, Untereiner A, Wu L, Yang G, Austin RC, Dickhout JG, Lhotak S, Meng QH, Wang R. Decreased endogenous production of hydrogen sulfide accelerates atherosclerosis. Circulation 127: 2523–2534, 2013. [DOI] [PubMed] [Google Scholar]
- 207.Marsh N, Marsh A. A short history of nitroglycerine and nitric oxide in pharmacology and physiology. Clin Exp Pharmacol Physiol 27: 313–319, 2000. [DOI] [PubMed] [Google Scholar]
- 208.Martinez-Ruiz A, Lamas S. S-nitrosylation: A potential new paradigm in signal transduction. Cardiovasc Res 62: 43–52, 2004. [DOI] [PubMed] [Google Scholar]
- 209.Massey V, Edmondson D. On the mechanism of inactivation of xanthine oxidase by cyanide. J Biol Chem 245: 6595–6598, 1970. [PubMed] [Google Scholar]
- 210.Mathai JC, Missner A, Kugler P, Saparov SM, Zeidel ML, Lee JK, Pohl P. No facilitator required for membrane transport of hydrogen sulfide. Proc Natl Acad Sci U S A 106: 16633–16638, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Matsunaga T, Warltier DC, Weihrauch DW, Moniz M, Tessmer J, Chilian WM. Ischemia-Induced Coronary Collateral Growth Is Dependent on Vascular Endothelial Growth Factor and Nitric Oxide. Circulation 102: 3098–3103, 2000. [DOI] [PubMed] [Google Scholar]
- 212.Matthew W, S. AJ, Hwa CS, Siau JL, Boon-Seng W, Sang CN, Barry H, K. MP. The novel neuromodulator hydrogen sulfide: An endogenous peroxynitrite ‘scavenger’? J Neurochem 90: 765–768, 2004. [DOI] [PubMed] [Google Scholar]
- 213.Mayahi L, Heales S, Owen D, Casas JP, Harris J, MacAllister RJ, Hingorani AD. (6R)-5,6,7,8-tetrahydro-L-biopterin and its stereoisomer prevent ischemia reperfusion injury in human forearm. Arterioscler Thromb Vasc Biol 27: 1334–1339, 2007. [DOI] [PubMed] [Google Scholar]
- 214.Mees B, Wagner S, Ninci E, Tribulova S, Martin S, van Haperen R, Kostin S, Heil M, de Crom R, Schaper W. Endothelial nitric oxide synthase activity is essential for vasodilation during blood flow recovery but not for arteriogenesis. Arterioscler Thromb Vasc Biol 27: 1926–1933, 2007. [DOI] [PubMed] [Google Scholar]
- 215.Melkumyants A, Balashov S, Khayutin V. Control of arterial lumen by shear stress on endothelium. Physiology 10: 204–210, 1995. [Google Scholar]
- 216.Miller DL, Roth MB. Hydrogen sulfide increases thermotolerance and lifespan in Caenorhabditis elegans. Proc Natl Acad Sci U S A 104: 20618–20622, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Milsom AB, Fernandez BO, Garcia-Saura MF, Rodriguez J, Feelisch M. Contributions of nitric oxide synthases, dietary nitrite/nitrate, and other sources to the formation of NO signaling products. Antioxid Redox Signal 17: 422–432, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Mittermayer F, Krzyzanowska K, Exner M, Mlekusch W, Amighi J, Sabeti S, Minar E, Muller M, Wolzt M, Schillinger M. Asymmetric dimethylarginine predicts major adverse cardiovascular events in patients with advanced peripheral artery disease. Arterioscler Thromb Vasc Biol 26: 2536–2540, 2006. [DOI] [PubMed] [Google Scholar]
- 219.Mohler ER III, Hiatt WR, Gornik HL, Kevil CG, Quyyumi A, Haynes WG, Annex BH. Sodium nitrite in patients with peripheral artery disease and diabetes mellitus: Safety, walking distance and endothelial function. Vasc Med 19: 9–17, 2014. [DOI] [PubMed] [Google Scholar]
- 220.Mohyeldin A, Garzon-Muvdi T, Quinones-Hinojosa A. Oxygen in stem cell biology: A critical component of the stem cell niche. Cell Stem Cell 7: 150–161, 2010. [DOI] [PubMed] [Google Scholar]
- 221.Moncada S, Palmer RM, Gryglewski RJ. Mechanism of action of some inhibitors of endothelium-derived relaxing factor. Proc Natl Acad Sci U S A 83: 9164–9168, 1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Moncada S, Palmer RM, Higgs EA. Nitric oxide: Physiology, patho-physiology, and pharmacology. Pharmacol Rev 43: 109–142, 1991. [PubMed] [Google Scholar]
- 223.Mongue-Din H, Patel AS, Looi YH, Grieve DJ, Anilkumar N, Sirker A, Dong X, Brewer AC, Zhang M, Smith A, Shah AM. NADPH oxidase-4 driven cardiac macrophage polarization protects against myocardial infarction-induced remodeling. JACC Basic Transl Sci 2: 688–698, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Moore LE, Malats N, Rothman N, Real FX, Kogevinas M, Karami S, Garcia-Closas R, Silverman D, Chanock S, Welch R, Tardon A, Serra C, Carrato A, Dosemeci M, Garcia-Closas M. Polymorphisms in one-carbon metabolism and trans-sulfuration pathway genes and susceptibility to bladder cancer. Int J Cancer 120: 2452–2458, 2007. [DOI] [PubMed] [Google Scholar]
- 225.Moriya J, Wu X, Zavala-Solorio J, Ross J, Liang XH, Ferrara N. Platelet-derived growth factor C promotes revascularization in ischemic limbs of diabetic mice. J Vasc Surg 59: 1402–1409, e1401–1404, 2014. [DOI] [PubMed] [Google Scholar]
- 226.Morris SM Jr. Arginine metabolism: Boundaries of our knowledge. J Nutr 137: 1602S–1609S, 2007. [DOI] [PubMed] [Google Scholar]
- 227.Moshe AB, Szwarcman D, Markovich G. Size dependence of chiroptical activity in colloidal quantum dots. ACS Nano 5: 9034–9043, 2011. [DOI] [PubMed] [Google Scholar]
- 228.Mrozikiewicz PM, Bogacz A, Omielanczyk M, Wolski HS, Bartkowiak-Wieczorek J, Grzeskowiak E, Czerny B, Drews K, Seremak-Mrozikiewicz A. The importance of rs1021737 and rs482843 polymorphisms of cystathionine gamma-lyase in the etiology of preeclampsia in the Caucasian population. Ginekol Pol 86: 119–125, 2015. [DOI] [PubMed] [Google Scholar]
- 229.Murad F, Waldman S, Molina C, Bennett B, Leitman D. Regulation and role of guanylate cyclase-cyclic GMP in vascular relaxation. Prog Clin Biol Res 249: 65–76, 1987. [PubMed] [Google Scholar]
- 230.Murata T, Rahardjo A, Fujiyama Y, Yamaga T, Hanada M, Yaegaki K, Miyazaki H. Development of a compact and simple gas chromatography for oral malodor measurement. J Periodontol 77: 1142–1147, 2006. [DOI] [PubMed] [Google Scholar]
- 231.Murohara T, Asahara T, Silver M, Bauters C, Masuda H, Kalka C, Kearney M, Chen D, Symes JF, Fishman MC, Huang PL, Isner JM. Nitric oxide synthase modulates angiogenesis in response to tissue ischemia. J Clin Invest 101: 2567–2578, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Mustafa AK, Gadalla MM, Sen N, Kim S, Mu W, Gazi SK, Barrow RK, Yang G, Wang R, Snyder SH. H2S signals through protein S-sulfhydration. Sci Signal 2: ra72, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Mustafa AK, Sikka G, Gazi SK, Steppan J, Jung SM, Bhunia AK, Barodka VM, Gazi FK, Barrow RK, Wang R, Amzel LM, Berkowitz DE, Snyder SH. Hydrogen sulfide as endothelium-derived hyperpolarizing factor sulfhydrates potassium channels. Circ Res 109: 1259–1268, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Nagahara N, Nagano M, Ito T, Shimamura K, Akimoto T, Suzuki H. Antioxidant enzyme, 3-mercaptopyruvate sulfurtransferase-knockout mice exhibit increased anxiety-like behaviors: A model for human mercaptolactate-cysteine disulfiduria. Sci Rep 3: 1986, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Nagpure BV, Bian JS. Interaction of hydrogen sulfide with nitric oxide in the cardiovascular system. Oxid Med Cell Longev 2016: 6904327, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Nagy P, Winterbourn CC. Rapid reaction of hydrogen sulfide with the neutrophil oxidant hypochlorous acid to generate polysulfides. Chem Res Toxicol 23: 1541–1543, 2010. [DOI] [PubMed] [Google Scholar]
- 237.Nava GM, Carbonero F, Croix JA, Greenberg E, Gaskins HR. Abundance and diversity of mucosa-associated hydrogenotrophic microbes in the healthy human colon. ISME J 6: 57–70, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Navarro-Antolin J, Lopez-Munoz MJ, Klatt P, Soria J, Michel T, Lamas S. Formation of peroxynitrite in vascular endothelial cells exposed to cyclosporine A. FASEB J 15: 1291–1293, 2001. [DOI] [PubMed] [Google Scholar]
- 239.Navarro-Antolin J, Redondo-Horcajo M, Zaragoza C, Alvarez-Barrientos A, Fernandez AP, Leon-Gomez E, Rodrigo J, Lamas S. Role of peroxynitrite in endothelial damage mediated by Cyclosporine A. Free Radic Biol Med 42: 394–403, 2007. [DOI] [PubMed] [Google Scholar]
- 240.Nicosia RF, Nicosia SV, Smith M. Vascular endothelial growth factor, platelet-derived growth factor, and insulin-like growth factor-1 promote rat aortic angiogenesis in vitro. Am J Pathol 145: 1023–1029, 1994. [PMC free article] [PubMed] [Google Scholar]
- 241.Nishida M, Sawa T, Kitajima N, Ono K, Inoue H, Ihara H, Motohashi H, Yamamoto M, Suematsu M, Kurose H, van der Vliet A, Freeman BA, Shibata T, Uchida K, Kumagai Y, Akaike T. Hydrogen sulfide anion regulates redox signaling via electrophile sulfhydration. Nat Chem Biol 8: 714–724, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Nozik-Grayck E, Woods C, Taylor JM, Benninger RK, Johnson RD, Villegas LR, Stenmark KR, Harrison DG, Majka SM, Irwin D, Farrow KN. Selective depletion of vascular EC-SOD augments chronic hypoxic pulmonary hypertension. Am J Physiol Lung Cell Mol Physiol 307: L868–876, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Nystrom T, Nygren A, Sjoholm A. Tetrahydrobiopterin increases insulin sensitivity in patients with type 2 diabetes and coronary heart disease. Am J Physiol Endocrinol Metab 287: E919–E925, 2004. [DOI] [PubMed] [Google Scholar]
- 244.Ogino K, Takahashi N, Takigawa T, Obase Y, Wang DH. Association of serum arginase I with oxidative stress in a healthy population. Free Radic Res 45: 147–155, 2011. [DOI] [PubMed] [Google Scholar]
- 245.Okamoto T, Akaike T, Sawa T, Miyamoto Y, van der Vliet A, Maeda H. Activation of matrix metalloproteinases by peroxynitrite-induced protein S-glutathiolation via disulfide S-oxide formation. J Biol Chem 276: 29596–29602, 2001. [DOI] [PubMed] [Google Scholar]
- 246.Oladipupo S, Hu S, Kovalski J, Yao J, Santeford A, Sohn RE, Shohet R, Maslov K, Wang LV, Arbeit JM. VEGF is essential for hypoxia-inducible factor-mediated neovascularization but dispensable for endothelial sprouting. Proc Natl Acad Sci U S A 108: 13264–13269, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Olas B Hydrogen sulfide in hemostasis: Friend or foe? Chem Biol Interact 217: 49–56, 2014. [DOI] [PubMed] [Google Scholar]
- 248.Olson KR. Hydrogen sulfide as an oxygen sensor. Antioxid Redox Signal 22: 377–397, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Olson KR, DeLeon ER, Liu F. Controversies and conundrums in hydrogen sulfide biology. Nitric Oxide 41: 11–26, 2014. [DOI] [PubMed] [Google Scholar]
- 250.Olson KR, Gao Y, DeLeon ER, Arif M, Arif F, Arora N, Straub KD. Catalase as a sulfide-sulfur oxido-reductase: An ancient (and modern?) regulator of reactive sulfur species (RSS). Redox Biol 12: 325–339, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Olson N, van der Vliet A. Interactions between nitric oxide and hypoxia-inducible factor signaling pathways in inflammatory disease. Nitric Oxide 25: 125–137, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Orlic D, Kajstura J, Chimenti S, Limana F, Jakoniuk I, Quaini F, Nadal-Ginard B, Bodine DM, Leri A, Anversa P. Mobilized bone marrow cells repair the infarcted heart, improving function and survival. Proc Natl Acad Sci U S A 98: 10344–10349, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Orrico C, Pasquinelli G, Foroni L, Muscara D, Tazzari PL, Ricci F, Buzzi M, Baldi E, Muccini N, Gargiulo M, Stella A. Dysfunctional vasa vasorum in diabetic peripheral artery obstructive disease with critical lower limb ischaemia. Eur J Vasc Endovasc Surg 40: 365–374, 2010. [DOI] [PubMed] [Google Scholar]
- 254.Ovechkin AV, Tyagi N, Sen U, Lominadze D, Steed MM, Moshal KS, Tyagi SC. 3-Deazaadenosine mitigates arterial remodeling and hypertension in hyperhomocysteinemic mice. Am J Physiol Lung Cell Mol Physiol 291: L905–L911, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Pacher P, Beckman JS, Liaudet L. Nitric oxide and peroxynitrite in health and disease. Physiol Rev 87: 315–424, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Palmer RM, Ferrige AG, Moncada S. Nitric oxide release accounts for the biological activity of endothelium-derived relaxing factor. Nature 327: 524–526, 1987. [DOI] [PubMed] [Google Scholar]
- 257.Pan J, Carroll KS. Persulfide reactivity in the detection of protein s-sulfhydration. ACS Chem Biol 8: 1110–1116, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Pandey SK, Kim KH. The fundamental properties of the direct injection method in the analysis of gaseous reduced sulfur by gas chromatography with a pulsed flame photometric detector. Anal Chim Acta 615: 165–173, 2008. [DOI] [PubMed] [Google Scholar]
- 259.Papapetropoulos A, Pyriochou A, Altaany Z, Yang G, Marazioti A, Zhou Z, Jeschke MG, Branski LK, Herndon DN, Wang R, Szabo C. Hydrogen sulfide is an endogenous stimulator of angiogenesis. Proc Natl Acad Sci U S A 106: 21972–21977, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Park B, Hoffman A, Yang Y, Yan J, Tie G, Bagshahi H, Nowicki PT, Messina LM. Endothelial nitric oxide synthase affects both early and late collateral arterial adaptation and blood flow recovery after induction of hind limb ischemia in mice. J Vasc Surg 51: 165–173, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Patel BH, Percivalle C, Ritson DJ, Duffy CD, Sutherland JD. Common origins of RNA, protein and lipid precursors in a cyanosulfidic protometabolism. Nat Chem 7: 301–307, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Pattillo CB, Fang K, Terracciano J, Kevil CG. Reperfusion of chronic tissue ischemia: Nitrite and dipyridamole regulation of innate immune responses. Ann N Y Acad Sci 1207: 83–88, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.PDF M The development in vitro of the blood of the early chick embryo. Proc R Soc Lond Ser B Biol Sci 111: 497–521, 1932. [Google Scholar]
- 264.Peng B, Chen W, Liu C, Rosser EW, Pacheco A, Zhao Y, Aguilar HC, Xian M. Fluorescent probes based on nucleophilic substitution-cyclization for hydrogen sulfide detection and bioimaging. Chemistry 20: 1010–1016, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Peng B, Xian M. Hydrogen sulfide detection using nucleophilic substitution-cyclization-based fluorescent probes. Methods Enzymol 554: 47–62, 2015. [DOI] [PubMed] [Google Scholar]
- 266.Pereira ER, Frudd K, Awad W, Hendershot LM. Endoplasmic reticulum (ER) stress and hypoxia response pathways interact to potentiate hypoxia-inducible factor 1 (HIF-1) transcriptional activity on targets like vascular endothelial growth factor (VEGF). J Biol Chem 289: 3352–3364, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Perridon BW, Leuvenink HG, Hillebrands JL, van Goor H, Bos EM. The role of hydrogen sulfide in aging and age-related pathologies. Aging 8: 2264–2289, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Peter EA, Shen X, Shah SH, Pardue S, Glawe JD, Zhang WW, Reddy P, Akkus NI, Varma J, Kevil CG. Plasma free H2S levels are elevated in patients with cardiovascular disease. J Am Heart Assoc 2: e000387, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Peter G Bemerkungen zu der Abhandlung der HH. Weselsky und Benedikt “Ueber einige Azoverbindungen”. Ber Dtsch Chem Ges 12: 426–428, 1879. [Google Scholar]
- 270.Pipp F, Boehm S, Cai WJ, Adili F, Ziegler B, Karanovic G, Ritter R, Balzer J, Scheler C, Schaper W, Schmitz-Rixen T. Elevated fluid shear stress enhances postocclusive collateral artery growth and gene expression in the pig hind limb. Arterioscler Thromb Vasc Biol 24: 1664–1668, 2004. [DOI] [PubMed] [Google Scholar]
- 271.Pipp F, Heil M, Issbrucker K, Ziegelhoeffer T, Martin S, van den Heuvel J, Weich H, Fernandez B, Golomb G, Carmeliet P, Schaper W, Clauss M. VEGFR-1-selective VEGF homologue PlGF is arteriogenic: Evidence for a monocyte-mediated mechanism. Circ Res 92: 378–385, 2003. [DOI] [PubMed] [Google Scholar]
- 272.Piret JP, Mottet D, Raes M, Michiels C. Is HIF-1alpha a pro- or an anti-apoptotic protein? Biochem Pharmacol 64: 889–892, 2002. [DOI] [PubMed] [Google Scholar]
- 273.Pittman RN. Regulation of tissue oxygenation In: Integrated Systems Physiology, from Molecule to Function to Disease #17. San Rafael, CA: Morgan & Claypool Life Sciences, 2011, p. ix, 89 p. [PubMed] [Google Scholar]
- 274.Pol R, Moya A, Gabriel G, Gabriel D, Cespedes F, Baeza M. Inkjet-printed sulfide-selective electrode. Anal Chem 89: 12231–12236, 2017. [DOI] [PubMed] [Google Scholar]
- 275.Polhemus DJ, Lefer DJ. Emergence of hydrogen sulfide as an endogenous gaseous signaling molecule in cardiovascular disease. Circ Res 114: 730–737, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Polhemus DJ, Li Z, Pattillo CB, Gojon G Sr., Gojon G Jr., Giordano T, Krum H. A novel hydrogen sulfide prodrug, SG1002, promotes hydrogen sulfide and nitric oxide bioavailability in heart failure patients. Cardiovasc Ther 33: 216–226, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Poole LB, Karplus PA, Claiborne A. Protein sulfenic acids in redox signaling. Annu Rev Pharmacol Toxicol 44: 325–347, 2004. [DOI] [PubMed] [Google Scholar]
- 278.Pritchard KA Jr., Groszek L, Smalley DM, Sessa WC, Wu M, Villalon P, Wolin MS, Stemerman MB. Native low-density lipoprotein increases endothelial cell nitric oxide synthase generation of superoxide anion. Circ Res 77: 510–518, 1995. [DOI] [PubMed] [Google Scholar]
- 279.Qabazard B, Li L, Gruber J, Peh MT, Ng LF, Kumar SD, Rose P, Tan CH, Dymock BW, Wei F, Swain SC, Halliwell B, Sturzenbaum SR, Moore PK. Hydrogen sulfide is an endogenous regulator of aging in Caenorhabditis elegans. Antioxid Redox Signal 20: 2621–2630, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Radi R Nitric oxide, oxidants, and protein tyrosine nitration. Proc Natl Acad Sci U S A 101: 4003–4008, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Rajpal S, Katikaneni P, Deshotels M, Pardue S, Glawe J, Shen X, Akkus N, Modi K, Bhandari R, Dominic P, Reddy P, Kolluru GK, Kevil CG. Total sulfane sulfur bioavailability reflects ethnic and gender disparities in cardiovascular disease. Redox Biol 15: 480–489, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Rao RM, Yang L, Garcia-Cardena G, Luscinskas FW. Endothelial-dependent mechanisms of leukocyte recruitment to the vascular wall. Circ Res 101: 234–247, 2007. [DOI] [PubMed] [Google Scholar]
- 283.Rathel TR, Leikert JJ, Vollmar AM, Dirsch VM. Application of 4,5-diaminofluorescein to reliably measure nitric oxide released from endothelial cells in vitro. Biol Proced Online 5: 136–142, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Rearick MS, Gilmour CC, Heyes A, Mason RP. Measuring sulfide accumulation in diffusive gradients in thin films by means of purge and trap followed by ion-selective electrode. Environ Toxicol Chem 24: 3043–3047, 2005. [DOI] [PubMed] [Google Scholar]
- 285.Rey S, Semenza GL. Hypoxia-inducible factor-1-dependent mechanisms of vascularization and vascular remodelling. Cardiovasc Res 86: 236–242, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Ridnour LA, Isenberg JS, Espey MG, Thomas DD, Roberts DD, Wink DA. Nitric oxide regulates angiogenesis through a functional switch involving thrombospondin-1. Proc Natl Acad Sci U S A 102: 13147–13152, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Rinaldi L, Gobbi G, Pambianco M, Micheloni C, Mirandola P, Vitale M. Hydrogen sulfide prevents apoptosis of human PMN via inhibition of p38 and caspase 3. Lab Invest 86: 391–397, 2006. [DOI] [PubMed] [Google Scholar]
- 288.Risau W Mechanisms of angiogenesis. Nature 386: 671–674, 1997. [DOI] [PubMed] [Google Scholar]
- 289.Rissanen TT, Markkanen JE, Arve K, Rutanen J, Kettunen MI, Vajanto I, Jauhiainen S, Cashion L, Gruchala M, Narvanen O, Taipale P, Kauppinen RA, Rubanyi GM, Yla-Herttuala S. Fibroblast growth factor 4 induces vascular permeability, angiogenesis and arteriogenesis in a rabbit hindlimb ischemia model. FASEB J 17: 100–102, 2003. [DOI] [PubMed] [Google Scholar]
- 290.Rose P, Moore PK, Zhu YZ. H2S biosynthesis and catabolism: New insights from molecular studies. Cell Mol Life Sci 74: 1391–1412, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Rudolph TK, Freeman BA. Transduction of redox signaling by electrophile-protein reactions. Sci Signal 2: re7, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Saiapina OY, Kharchenko SG, Vishnevskii SG, Pyeshkova VM, Kalchenko VI, Dzyadevych SV. Development of conductometric sensor based on 25,27-Di-(5-thio-octyloxy)calix[4]arene-crown-6 for determination of Ammonium. Nanoscale Res Lett 11: 105, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Sakurai Y, Ohgimoto K, Kataoka Y, Yoshida N, Shibuya M. Essential role of Flk-1 (VEGF receptor 2) tyrosine residue 1173 in vasculogenesis in mice. Proc Natl Acad Sci U S A 102: 1076–1081, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Sarateanu CS, Retuerto MA, Beckmann JT, McGregor L, Carbray J, Patejunas G, Nayak L, Milbrandt J, Rosengart TK. An Egr-1 master switch for arteriogenesis: Studies in Egr-1 homozygous negative and wild-type animals. J Thorac Cardiovasc Surg 131: 138–145, 2006. [DOI] [PubMed] [Google Scholar]
- 295.Sasaki T, Matano K. Formation of nitrite from nitrate at the dorsum linguae studies on nitrite formation in the human oral cavity (I). Food Hyg Safe Sci (Shokuhin Eiseigaku Zasshi) 20: 363–369, 1979. [Google Scholar]
- 296.Sathyadevi P, Chen YJ, Wu SC, Chen YH, Wang YM. Reaction-based epoxide fluorescent probe for in vivo visualization of hydrogen sulfide. Biosens Bioelectron 68: 681–687, 2015. [DOI] [PubMed] [Google Scholar]
- 297.Sato TN, Tozawa Y, Deutsch U, Wolburg-Buchholz K, Fujiwara Y, Gendron-Maguire M, Gridley T, Wolburg H, Risau W, Qin Y. Distinct roles of the receptor tyrosine kinases Tie-1 and Tie-2 in blood vessel formation. Nature 376: 70–74, 1995. [DOI] [PubMed] [Google Scholar]
- 298.Sawa T, Zaki MH, Okamoto T, Akuta T, Tokutomi Y, Kim-Mitsuyama S, Ihara H, Kobayashi A, Yamamoto M, Fujii S, Arimoto H, Akaike T. Protein S-guanylation by the biological signal 8-nitroguanosine 3′,5′-cyclic monophosphate. Nat Chem Biol 3: 727–735, 2007. [DOI] [PubMed] [Google Scholar]
- 299.Schaper W Tangential wall stress as a molding force in the development of collateral vessels in the canine heart. Experientia 23: 595–596, 1967. [DOI] [PubMed] [Google Scholar]
- 300.Schaper W Collateral circulation: Past and present. Basic Res Cardiol 104: 5–21, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Schaper W, Buschmann I. Arteriogenesis, the good and bad of it. Eur Heart J 20: 1297–1299, 1999. [DOI] [PubMed] [Google Scholar]
- 302.Schaper W, Scholz D. Factors regulating arteriogenesis. Arterioscler Thromb Vasc Biol 23: 1143–1151, 2003. [DOI] [PubMed] [Google Scholar]
- 303.Scheel K, Fitzgerald E, Martin R, Larsen R. The possible role of mechanical stresses on coronary collateral development during gradual coronary occlusion In: The Pathophysiology of Myocardial Perfusion. Amsterdam: Elsevier/North-Holland, 1979, pp. 489–518. [Google Scholar]
- 304.Schiekofer S, Galasso G, Sato K, Kraus BJ, Walsh K. Impaired revascularization in a mouse model of type 2 diabetes is associated with dysregulation of a complex angiogenic-regulatory network. Arterioscler Thromb Vasc Biol 25: 1603–1609, 2005. [DOI] [PubMed] [Google Scholar]
- 305.Schirmer SH, Millenaar DN, Werner C, Schuh L, Degen A, Bettink SI, Lipp P, van Rooijen N, Meyer T, Bohm M, Laufs U. Exercise promotes collateral artery growth mediated by monocytic nitric oxide. Arterioscler Thromb Vasc Biol 35: 1862–1871, 2015. [DOI] [PubMed] [Google Scholar]
- 306.Schnabel R, Blankenberg S, Lubos E, Lackner KJ, Rupprecht HJ, Espinola-Klein C, Jachmann N, Post F, Peetz D, Bickel C, Cambien F, Tiret L, Munzel T. Asymmetric dimethylarginine and the risk of cardiovascular events and death in patients with coronary artery disease: Results from the AtheroGene Study. Circ Res 97: e53–e59, 2005. [DOI] [PubMed] [Google Scholar]
- 307.Scholz D, Ito W, Fleming I, Deindl E, Sauer A, Wiesnet M, Busse R, Schaper J, Schaper W. Ultrastructure and molecular histology of rabbit hind-limb collateral artery growth (arteriogenesis). Virchows Arch 436: 257–270, 2000. [DOI] [PubMed] [Google Scholar]
- 308.Scholz D, Schaper W. Preconditioning of arteriogenesis. Cardiovasc Res 65: 513–523, 2005. [DOI] [PubMed] [Google Scholar]
- 309.Scholz D, Ziegelhoeffer T, Helisch A, Wagner S, Friedrich C, Podzuweit T, Schaper W. Contribution of arteriogenesis and angiogenesis to postocclusive hindlimb perfusion in mice. J Mol Cell Cardiol 34: 775–787, 2002. [DOI] [PubMed] [Google Scholar]
- 310.Schroder K, Zhang M, Benkhoff S, Mieth A, Pliquett R, Kosowski J, Kruse C, Luedike P, Michaelis UR, Weissmann N, Dimmeler S, Shah AM, Brandes RP. Nox4 is a protective reactive oxygen species generating vascular NADPH oxidase. Circ Res 110: 1217–1225, 2012. [DOI] [PubMed] [Google Scholar]
- 311.Schwentker A, Vodovotz Y, Weller R, Billiar TR. Nitric oxide and wound repair: Role of cytokines? Nitric Oxide 7: 1–10, 2002. [DOI] [PubMed] [Google Scholar]
- 312.Seiler C, Pohl T, Wustmann K, Hutter D, Nicolet P-A, Windecker S, Eberli FR, Meier B. Promotion of collateral growth by granulocyte-macrophage colony-stimulating factor in patients with coronary artery disease. Circulation 104: 2012–2017, 2001. [DOI] [PubMed] [Google Scholar]
- 313.Sen N, Paul BD, Gadalla MM, Mustafa AK, Sen T, Xu R, Kim S, Snyder SH. Hydrogen sulfide-linked sulfhydration of NF-kappaB mediates its antiapoptotic actions. Mol Cell 45: 13–24, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314.Sessa WC, Harrison JK, Luthin DR, Pollock JS, Lynch KR. Genomic analysis and expression patterns reveal distinct genes for endothelial and brain nitric oxide synthase. Hypertension 21: 934–938, 1993. [DOI] [PubMed] [Google Scholar]
- 315.Setoguchi S, Mohri M, Shimokawa H, Takeshita A. Tetrahydrobiopterin improves endothelial dysfunction in coronary microcirculation in patients without epicardial coronary artery disease. J Am Coll Cardiol 38: 493–498, 2001. [DOI] [PubMed] [Google Scholar]
- 316.Settergren M, Bohm F, Malmstrom RE, Channon KM, Pernow J. L-arginine and tetrahydrobiopterin protects against ischemia/reperfusion-induced endothelial dysfunction in patients with type 2 diabetes mellitus and coronary artery disease. Atherosclerosis 204: 73–78, 2009. [DOI] [PubMed] [Google Scholar]
- 317.Shafirovich V, Lymar SV. Nitroxyl and its anion in aqueous solutions: Spin states, protic equilibria, and reactivities toward oxygen and nitric oxide. Proc Natl Acad Sci U S A 99: 7340–7345, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 318.Shalaby F, Rossant J, Yamaguchi TP, Gertsenstein M, Wu XF, Breitman ML, Schuh AC. Failure of blood-island formation and vasculogenesis in Flk-1-deficient mice. Nature 376: 62–66, 1995. [DOI] [PubMed] [Google Scholar]
- 319.Shalini Devi KS, Senthil Kumar A. A blood-serum sulfide selective electrochemical sensor based on a 9,10-phenanthrenequinone-tethered graphene oxide modified electrode. Analyst 143: 3114–3123, 2018. [DOI] [PubMed] [Google Scholar]
- 320.Shankarishan P, Borah PK, Ahmed G, Mahanta J. Prevalence of endothelial nitric oxide synthase (eNOS) gene exon 7 Glu298Asp variant in North Eastern India. Indian J Med Res 133: 487–491, 2011. [PMC free article] [PubMed] [Google Scholar]
- 321.Shen X, Carlstrom M, Borniquel S, Jadert C, Kevil CG, Lundberg JO. Microbial regulation of host hydrogen sulfide bioavailability and metabolism. Free Radic Biol Med 60: 195–200, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 322.Shen X, Chakraborty S, Dugas TR, Kevil CG. Hydrogen sulfide measurement using sulfide dibimane: Critical evaluation with electrospray ion trap mass spectrometry. Nitric Oxide 41: 97–104, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 323.Shen X, Kolluru GK, Yuan S, Kevil CG. Measurement of H2S in vivo and in vitro by the monobromobimane method. Methods Enzymol 554: 31–45, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 324.Shen X, Pattillo CB, Pardue S, Bir SC, Wang R, Kevil CG. Measurement of plasma hydrogen sulfide in vivo and in vitro. Free Radic Biol Med 50: 1021–1031, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 325.Shen X, Peter EA, Bir S, Wang R, Kevil CG. Analytical measurement of discrete hydrogen sulfide pools in biological specimens. Free Radic Biol Med 52: 2276–2283, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 326.Sherlock LG, Trumpie A, Hernandez-Lagunas L, McKenna S, Fisher S, Bowler R, Wright CJ, Delaney C, Nozik-Grayck E. Redistribution of extracellular superoxide dismutase causes neonatal pulmonary vascular remodeling and PH but protects Against experimental bronchopulmonary dysplasia. Antioxidants (Basel) 7: pii: E42, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 327.Shi H, Yang S, Liu Y, Huang P, Lin N, Sun X, Yu R, Zhang Y, Qin Y, Wang L. Study on environmental causes and SNPs of MTHFR, MS and CBS genes related to congenital heart disease. PLoS One 10: e0128646, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 328.Shinozaki K, Nishio Y, Okamura T, Yoshida Y, Maegawa H, Kojima H, Masada M, Toda N, Kikkawa R, Kashiwagi A. Oral administration of tetrahydrobiopterin prevents endothelial dysfunction and vascular oxidative stress in the aortas of insulin-resistant rats. Circ Res 87: 566–573, 2000. [DOI] [PubMed] [Google Scholar]
- 329.Shiva S, Huang Z, Grubina R, Sun J, Ringwood LA, MacArthur PH, Xu X, Murphy E, Darley-Usmar VM, Gladwin MT. Deoxymyoglobin is a nitrite reductase that generates nitric oxide and regulates mitochondrial respiration. Circ Res 100: 654–661, 2007. [DOI] [PubMed] [Google Scholar]
- 330.Shyu KG, Manor O, Magner M, Yancopoulos GD, Isner JM. Direct intramuscular injection of plasmid DNA encoding angiopoietin-1 but not angiopoietin-2 augments revascularization in the rabbit ischemic hindlimb. Circulation 98: 2081–2087, 1998. [DOI] [PubMed] [Google Scholar]
- 331.Shyy YJ, Hsieh HJ, Usami S, Chien S. Fluid shear stress induces a biphasic response of human monocyte chemotactic protein 1 gene expression in vascular endothelium. Proc Natl Acad Sci U S A 91: 4678–4682, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 332.Siamwala JH, Veeriah V, Priya MK, Rajendran S, Saran U, Sinha S, Nagarajan S, Pradeep T, Chatterjee S. Nitric oxide rescues thalidomide mediated teratogenicity. Sci Rep 2: 679, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 333.Silvestre JS, Smadja DM, Levy BI. Postischemic revascularization: From cellular and molecular mechanisms to clinical applications. Physiol Rev 93: 1743–1802, 2013. [DOI] [PubMed] [Google Scholar]
- 334.Sindler AL, Delp MD, Reyes R, Wu G, Muller-Delp JM. Effects of ageing and exercise training on eNOS uncoupling in skeletal muscle resistance arterioles. J Physiol 587: 3885–3897, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 335.Singh S, Padovani D, Leslie RA, Chiku T, Banerjee R. Relative contributions of cystathionine beta-synthase and gamma-cystathionase to H2S biogenesis via alternative trans-sulfuration reactions. J Biol Chem 284: 22457–22466, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 336.Singh SB, Lin HC. Hydrogen sulfide in physiology and diseases of the digestive tract. Microorganisms 3: 866–889, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 337.Siti HN, Kamisah Y, Kamsiah J. The role of oxidative stress, antioxidants and vascular inflammation in cardiovascular disease (a review). Vascul Pharmacol 71: 40–56, 2015. [DOI] [PubMed] [Google Scholar]
- 338.Song X-M, Zheng X-Y, Zhu W-L, Huang L, Li Y. Relationship between polymorphism of cystathionine beta synthase gene and congenital heart disease in Chinese nuclear families. Biomed Environ Sci 19: 452–456, 2006. [PubMed] [Google Scholar]
- 339.Souza JM, Peluffo G, Radi R. Protein tyrosine nitration–functional alteration or just a biomarker? Free Radic Biol Med 45: 357–366, 2008. [DOI] [PubMed] [Google Scholar]
- 340.Spencer Bailey T, Pluth MD. Chemiluminescent detection of enzymatically produced H2S. Methods Enzymol 554: 81–99, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 341.Stabile E, Burnett MS, Watkins C, Kinnaird T, Bachis A, la Sala A, Miller JM, Shou M, Epstein SE, Fuchs S. Impaired arteriogenic response to acute hindlimb ischemia in CD4-knockout mice. Circulation 108: 205–210, 2003. [DOI] [PubMed] [Google Scholar]
- 342.Stamatakis K, Perez-Sala D. Prostanoids with cyclopentenone structure as tools for the characterization of electrophilic lipid-protein interactomes. Ann NY Acad Sci 1091: 548–570, 2006. [DOI] [PubMed] [Google Scholar]
- 343.Stamler JS, Lamas S, Fang FC. Nitrosylation. the prototypic redox-based signaling mechanism. Cell 106: 675–683, 2001. [DOI] [PubMed] [Google Scholar]
- 344.Stossel TP. On the crawling of animal cells. Science 260: 1086–1094, 1993. [DOI] [PubMed] [Google Scholar]
- 345.Stoyanovsky DA, Tyurina YY, Tyurin VA, Anand D, Mandavia DN, Gius D, Ivanova J, Pitt B, Billiar TR, Kagan VE. Thioredoxin and lipoic acid catalyze the denitrosation of low molecular weight and protein S-nitrosothiols. J Am Chem Soc 127: 15815–15823, 2005. [DOI] [PubMed] [Google Scholar]
- 346.Strijdom H, Muller C, Lochner A. Direct intracellular nitric oxide detection in isolated adult cardiomyocytes: Flow cytometric analysis using the fluorescent probe, diaminofluorescein. J Mol Cell Cardiol 37: 897–902, 2004. [DOI] [PubMed] [Google Scholar]
- 347.Stroes E, Kastelein J, Cosentino F, Erkelens W, Wever R, Koomans H, Luscher T, Rabelink T. Tetrahydrobiopterin restores endothelial function in hypercholesterolemia. J Clin Invest 99: 41–46, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 348.Sukriti S, Tauseef M, Yazbeck P, Mehta D. Mechanisms regulating endothelial permeability. Pulm Circ 4: 535–551, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 349.Suri C, Jones PF, Patan S, Bartunkova S, Maisonpierre PC, Davis S, Sato TN, Yancopoulos GD. Requisite role of angiopoietin-1, a ligand for the TIE2 receptor, during embryonic angiogenesis. Cell 87: 1171–1180, 1996. [DOI] [PubMed] [Google Scholar]
- 350.Szabo C A timeline of hydrogen sulfide (H2S) research: From environmental toxin to biological mediator. Biochem Pharmacol 149: 5–19, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 351.Szabó C Hydrogen sulphide and its therapeutic potential. Nat Rev Drug Discov 6: 917, 2007. [DOI] [PubMed] [Google Scholar]
- 352.Takahashi T, Kalka C, Masuda H, Chen D, Silver M, Kearney M, Magner M, Isner JM, Asahara T. Ischemia- and cytokine-induced mobilization of bone marrow-derived endothelial progenitor cells for neovascularization. Nat Med 5: 434–438, 1999. [DOI] [PubMed] [Google Scholar]
- 353.Takeshita S, Zheng LP, Brogi E, Kearney M, Pu LQ, Bunting S, Ferrara N, Symes JF, Isner JM. Therapeutic angiogenesis. A single intraarterial bolus of vascular endothelial growth factor augments revascularization in a rabbit ischemic hind limb model. J Clin Invest 93: 662–670, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 354.Terai T, Urano Y, Izumi S, Kojima H, Nagano T. A practical strategy to create near-infrared luminescent probes: Conversion from fluorescein-based sensors. Chem Commun (Camb) 48: 2840–2842, 2012. [DOI] [PubMed] [Google Scholar]
- 355.Tesauro M, Thompson WC, Rogliani P, Qi L, Chaudhary PP, Moss J. Intracellular processing of endothelial nitric oxide synthase isoforms associated with differences in severity of cardiopulmonary diseases: Cleavage of proteins with aspartate vs. glutamate at position 298. Proc Natl Acad Sci U S A 97: 2832–2835, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 356.Tojo T, Ushio-Fukai M, Yamaoka-Tojo M, Ikeda S, Patrushev N, Alexander RW. Role of gp91phox (Nox2)-containing NAD(P)H oxidase in angiogenesis in response to hindlimb ischemia. Circulation 111: 2347–2355, 2005. [DOI] [PubMed] [Google Scholar]
- 357.Troidl K, Tribulova S, Cai WJ, Ruding I, Apfelbeck H, Schierling W, Troidl C, Schmitz-Rixen T, Schaper W. Effects of endogenous nitric oxide and of DETA NONOate in arteriogenesis. J Cardiovasc Pharmacol 55: 153–160, 2010. [DOI] [PubMed] [Google Scholar]
- 358.Tronc F, Wassef M, Esposito B, Henrion D, Glagov S, Tedgui A. Role of NO in flow-induced remodeling of the rabbit common Carotid artery. Arterioscler Thromb Vasc Biol 16: 1256–1262, 1996. [DOI] [PubMed] [Google Scholar]
- 359.Tsai MY, Hanson NQ, Bignell MK, Schwichtenberg KA. Simultaneous detection and screening of T833C and G919A mutations of the cystathionine beta-synthase gene by single-strand conformational polymorphism. Clin Biochem 29: 473–477, 1996. [DOI] [PubMed] [Google Scholar]
- 360.Tsai MY, Welge BG, Hanson NQ, Bignell MK, Vessey J, Schwichtenberg K, Yang F, Bullemer FE, Rasmussen R, Graham KJ. Genetic causes of mild hyperhomocysteinemia in patients with premature occlusive coronary artery diseases. Atherosclerosis 143: 163–170, 1999. [DOI] [PubMed] [Google Scholar]
- 361.Tsuzuki Y, Fukumura D, Oosthuyse B, Koike C, Carmeliet P, Jain RK. Vascular endothelial growth factor (VEGF) modulation by targeting hypoxia-inducible factor-1alpha→hypoxia response element→VEGF cascade differentially regulates vascular response and growth rate in tumors. Cancer Res 60: 6248–6252, 2000. [PubMed] [Google Scholar]
- 362.Ubuka T, Abe T, Kajikawa R, Morino K. Determination of hydrogen sulfide and acid-labile sulfur in animal tissues by gas chromatography and ion chromatography. J Chromatogr B Biomed Sci Appl 757: 31–37, 2001. [DOI] [PubMed] [Google Scholar]
- 363.Uchida K, Shibata T. 15-Deoxy-Delta(12,14)-prostaglandin J2: An electrophilic trigger of cellular responses. Chem Res Toxicol 21: 138–144, 2008. [DOI] [PubMed] [Google Scholar]
- 364.van Royen N, Piek JJ, Buschmann I, Hoefer I, Voskuil M, Schaper W. Stimulation of arteriogenesis; a new concept for the treatment of arterial occlusive disease. Cardiovasc Res 49: 543–553, 2001. [DOI] [PubMed] [Google Scholar]
- 365.Veeranki S, Givvimani S, Pushpakumar S, Tyagi SC. Hyperhomocysteinemia attenuates angiogenesis through reduction of HIF-1α and PGC-1α levels in muscle fibers during hindlimb ischemia. Am J Physiol Heart Circ Physiol 306: H1116–H1127, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 366.Vilas-Boas W, Cerqueira BA, Zanette AM, Reis MG, Barral-Netto M, Goncalves MS. Arginase levels and their association with Th17-related cytokines, soluble adhesion molecules (sICAM-1 and sVCAM-1) and hemolysis markers among steady-state sickle cell anemia patients. Ann Hematol 89: 877–882, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 367.Villa-Gomez DK, Cassidy J, Keesman KJ, Sampaio R, Lens PN. Sulfide response analysis for sulfide control using a pS electrode in sulfate reducing bioreactors. Water Res 50: 48–58, 2014. [DOI] [PubMed] [Google Scholar]
- 368.Viner RI, Williams TD, Schoneich C. Peroxynitrite modification of protein thiols: Oxidation, nitrosylation, and S-glutathiolation of functionally important cysteine residue(s) in the sarcoplasmic reticulum Ca-ATPase. Biochemistry 38: 12408–12415, 1999. [DOI] [PubMed] [Google Scholar]
- 369.Vogel SM, Malik AB. Cytoskeletal dynamics and lung fluid balance. Compr Physiol 2: 449–478, 2012. [DOI] [PubMed] [Google Scholar]
- 370.Volpe CMO, Villar-Delfino PH, Dos Anjos PMF, Nogueira-Machado JA. Cellular death, reactive oxygen species (ROS) and diabetic complications. Cell Death Dis 9: 119, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 371.Waltenberger J, Claesson-Welsh L, Siegbahn A, Shibuya M, Heldin CH. Different signal transduction properties of KDR and Flt1, two receptors for vascular endothelial growth factor. J Biol Chem 269: 26988–26995, 1994. [PubMed] [Google Scholar]
- 372.Wang DL, Wung B-S, Shyy Y-J, Lin C-F, Chao Y-J, Usami S, Chien S. Mechanical strain induces monocyte chemotactic protein-1 gene expression in endothelial cells. Circ Res 77: 294–302, 1995. [DOI] [PubMed] [Google Scholar]
- 373.Wang J, Hegele RA. Genomic basis of cystathioninuria (MIM 219500) revealed by multiple mutations in cystathionine gamma-lyase (CTH). Hum Genet 112: 404–408, 2003. [DOI] [PubMed] [Google Scholar]
- 374.Wang J, Huff AM, Spence JD, Hegele RA. Single nucleotide polymorphism in CTH associated with variation in plasma homocysteine concentration. Clin Genet 65: 483–486, 2004. [DOI] [PubMed] [Google Scholar]
- 375.Wang K, Peng H, Ni N, Dai C, Wang B. 2,6-dansyl azide as a fluorescent probe for hydrogen sulfide. J Fluoresc 24: 1–5, 2014. [DOI] [PubMed] [Google Scholar]
- 376.Wang L, Chen X, Tang B, Hua X, Klein-Szanto A, Kruger WD. Expression of mutant human cystathionine beta-synthase rescues neonatal lethality but not homocystinuria in a mouse model. Hum Mol Genet 14: 2201–2208, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 377.Wang MJ, Cai WJ, Li N, Ding YJ, Chen Y, Zhu YC. The hydrogen sulfide donor NaHS promotes angiogenesis in a rat model of hind limb ischemia. Antioxid Redox Signal 12: 1065–1077, 2010. [DOI] [PubMed] [Google Scholar]
- 378.Wang R Signaling pathways for the vascular effects of hydrogen sulfide. Curr Opin Nephrol Hypertens 20: 107–112, 2011. [DOI] [PubMed] [Google Scholar]
- 379.Wang S, Han MY, Huang D. Nitric oxide switches on the photoluminescence of molecularly engineered quantum dots. J Am Chem Soc 131: 11692–11694, 2009. [DOI] [PubMed] [Google Scholar]
- 380.Wang T, Wang L, Zaidi SR, Sammani S, Siegler J, Moreno-Vinasco L, Mathew B, Natarajan V, Garcia JG. Hydrogen sulfide attenuates particulate matter-induced human lung endothelial barrier disruption via combined reactive oxygen species scavenging and Akt activation. Am J Respir Cell Mol Biol 47: 491–496, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 381.Webb A, Bond R, McLean P, Uppal R, Benjamin N, Ahluwalia A. Reduction of nitrite to nitric oxide during ischemia protects against myocardial ischemia-reperfusion damage. Proc Natl Acad Sci U S A 101: 13683–13688, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 382.Wei CC, Wang ZQ, Wang Q, Meade AL, Hemann C, Hille R, Stuehr DJ. Rapid kinetic studies link tetrahydrobiopterin radical formation to heme-dioxy reduction and arginine hydroxylation in inducible nitricoxide synthase. J Biol Chem 276: 315–319, 2001. [DOI] [PubMed] [Google Scholar]
- 383.Whiteman M, Cheung NS, Zhu YZ, Chu SH, Siau JL, Wong BS, Armstrong JS, Moore PK. Hydrogen sulphide: A novel inhibitor of hypochlorous acid-mediated oxidative damage in the brain? Biochem Biophys Res Commun 326: 794–798, 2005. [DOI] [PubMed] [Google Scholar]
- 384.Wieczor R, Gadomska G, Ruszkowska-Ciastek B, Stankowska K, Budzynski J, Fabisiak J, Suppan K, Pulkowski G, Rosc D. Impact of type 2 diabetes on the plasma levels of vascular endothelial growth factor and its soluble receptors type 1 and type 2 in patients with peripheral arterial disease. J Zhejiang Univ Sci B 16: 948–956, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 385.Wiseman DM, Polverini PJ, Kamp DW, Leibovich SJ. Transforming growth factor-beta (TGF beta) is chemotactic for human monocytes and induces their expression of angiogenic activity. Biochem Biophys Res Commun 157: 793–800, 1988. [DOI] [PubMed] [Google Scholar]
- 386.Wood J, Garthwaite J. Models of the diffusional spread of nitric oxide: Implications for neural nitric oxide signalling and its pharmacological properties. Neuropharmacology 33: 1235–1244, 1994. [DOI] [PubMed] [Google Scholar]
- 387.Xie D, Li Y, Reed EA, Odronic SI, Kontos CD, Annex BH. An engineered vascular endothelial growth factor-activating transcription factor induces therapeutic angiogenesis in ApoE knockout mice with hindlimb ischemia. J Vasc Surg 44: 166–175, 2006. [DOI] [PubMed] [Google Scholar]
- 388.Xie HH, Zhou S, Chen DD, Channon KM, Su DF, Chen AF. GTP cyclohydrolase I/BH4 pathway protects EPCs via suppressing oxidative stress and thrombospondin-1 in salt-sensitive hypertension. Hypertension 56: 1137–1144, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 389.Xie X, Shi X, Xun X, Rao L. Endothelial nitric oxide synthase gene single nucleotide polymorphisms and the risk of hypertension: A meta-analysis involving 63,258 subjects. Clin Exp Hypertens 39: 175–182, 2017. [DOI] [PubMed] [Google Scholar]
- 390.Yan H, Du J, Tang C. The possible role of hydrogen sulfide on the pathogenesis of spontaneous hypertension in rats. Biochem Biophys Res Commun 313: 22–27, 2004. [DOI] [PubMed] [Google Scholar]
- 391.Yan SK, Chang T, Wang H, Wu L, Wang R, Meng QH. Effects of hydrogen sulfide on homocysteine-induced oxidative stress in vascular smooth muscle cells. Biochem Biophys Res Commun 351: 485–491, 2006. [DOI] [PubMed] [Google Scholar]
- 392.Yang G, Wu L, Jiang B, Yang W, Qi J, Cao K, Meng Q, Mustafa AK, Mu W, Zhang S, Snyder SH, Wang R. H2S as a physiologic vasorelaxant: Hypertension in mice with deletion of cystathionine gamma-lyase. Science 322: 587–590, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 393.Yang R, Qu C, Zhou Y, Konkel JE, Shi S, Liu Y, Chen C, Liu S, Liu D, Chen Y, Zandi E, Chen W, Zhou Y, Shi S. Hydrogen sulfide promotes Tet1- and Tet2-mediated Foxp3 demethylation to drive regulatory T cell differentiation and maintain immune homeostasis. Immunity 43: 251–263, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 394.Yeo TW, Lampah DA, Gitawati R, Tjitra E, Kenangalem E, McNeil YR, Darcy CJ, Granger DL, Weinberg JB, Lopansri BK, Price RN, Duffull SB, Celermajer DS, Anstey NM. Impaired nitric oxide bioavailability and L-arginine reversible endothelial dysfunction in adults with falciparum malaria. J Exp Med 204: 2693–2704, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 395.Yu J, deMuinck ED, Zhuang Z, Drinane M, Kauser K, Rubanyi GM, Qian HS, Murata T, Escalante B, Sessa WC. Endothelial nitric oxide synthase is critical for ischemic remodeling, mural cell recruitment, and blood flow reserve. Proc Natl Acad Sci U S A 102: 10999–11004, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 396.Yu J, Lei L, Liang Y, Hinh L, Hickey RP, Huang Y, Liu D, Yeh JL, Rebar E, Case C, Spratt K, Sessa WC, Giordano FJ. An engineered VEGF-activating zinc finger protein transcription factor improves blood flow and limb salvage in advanced-age mice. FASEB J 20: 479–481, 2006. [DOI] [PubMed] [Google Scholar]
- 397.Yuan S, Kevil CG. Nitric oxide and hydrogen sulfide regulation of ischemic vascular remodeling. Microcirculation 23: 134–145, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 398.Yuan S, Pardue S, Shen X, Alexander JS, Orr AW, Kevil CG. Hydrogen sulfide metabolism regulates endothelial solute barrier function. Redox Biology 9: 157–166, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 399.Yuan S, Patel RP, Kevil CG. Working with nitric oxide and hydrogen sulfide in biological systems. Am J Physiol Lung Cell Mol Physiol 308: L403–L415, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 400.Yuan S, Shen X, Kevil CG. Beyond a gasotransmitter: Hydrogen sulfide and polysulfide in cardiovascular health and immune response. Antioxid Redox Signal 27: 634–653, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 401.Yuan S, Yurdagul A Jr., Peretik JM, Alfaidi M, Al Yafeai Z, Pardue S, Kevil CG, Orr AW. Cystathionine gamma-lyase modulates flow-dependent vascular remodeling. Arterioscler Thromb Vasc Biol 38: 2126–2136, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 402.Yurdagul A, Finney AC, Woolard Matthew D, Orr AW. The arterial microenvironment: The where and why of atherosclerosis. Biochemical Journal 473: 1281–1295, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 403.Zanardo RC, Brancaleone V, Distrutti E, Fiorucci S, Cirino G, Wallace JL. Hydrogen sulfide is an endogenous modulator of leukocyte-mediated inflammation. FASEB J 20: 2118–2120, 2006. [DOI] [PubMed] [Google Scholar]
- 404.Zhang C, Wang R, Cheng L, Li B, Xi Z, Yi L. A redox-nucleophilic dual-reactable probe for highly selective and sensitive detection of H2S: Synthesis, spectra and bioimaging. Sci Rep 6: 30148, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 405.Zhang F, Iadecola C. Nitroprusside improves blood flow and reduces brain damage after focal ischemia. Neuroreport 4: 559–562, 1993. [DOI] [PubMed] [Google Scholar]
- 406.Zhang G, Dai C. [Correlation analysis between plasma homocysteine level and polymorphism of homocysteine metabolism related enzymes in ischemic cerebrovascular or cardiovascular diseases]. Zhonghua xue ye xue za zhi = Zhonghua xueyexue zazhi 23: 126–129, 2002. [PubMed] [Google Scholar]
- 407.Zhang G, Dai C. Gene polymorphisms of homocysteine metabolism-related enzymes in Chinese patients with occlusive coronary artery or cerebral vascular diseases. Thromb Res 104: 187–195, 2001. [DOI] [PubMed] [Google Scholar]
- 408.Zhang HX, Chen JB, Guo XF, Wang H, Zhang HS. Highly sensitive low-background fluorescent probes for imaging of nitric oxide in cells and tissues. Anal Chem 86: 3115–3123, 2014. [DOI] [PubMed] [Google Scholar]
- 409.Zhang X Real time and in vivo monitoring of nitric oxide by electrochemical sensors–from dream to reality. Front Biosci 9: 3434–3446, 2004. [DOI] [PubMed] [Google Scholar]
- 410.Zhang Y, Tang ZH, Ren Z, Qu SL, Liu MH, Liu LS, Jiang ZS. Hydrogen sulfide, the next potent preventive and therapeutic agent in aging and age-associated diseases. Mol Cell Biol 33: 1104–1113, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 411.Zhao W, Zhang J, Lu Y, Wang R. The vasorelaxant effect of H(2)S as a novel endogenous gaseous K(ATP) channel opener. EMBO J 20: 6008–6016, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 412.Zhou J, Li YS, Chien S. Shear stress-initiated signaling and its regulation of endothelial function. Arterioscler Thromb Vasc Biol 34: 2191–2198, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 413.Zhu H, Zhang M, Liu Z, Xing J, Moriasi C, Dai X, Zou MH. AMP-Activated protein kinase alpha1 in macrophages promotes collateral remodeling and arteriogenesis in mice in vivo. Arterioscler Thromb Vasc Biol 36: 1868–1878, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 414.Zhu YZ, Wang ZJ, Ho P, Loke YY, Zhu YC, Huang SH, Tan CS, Whiteman M, Lu J, Moore PK. Hydrogen sulfide and its possible roles in myocardial ischemia in experimental rats. J Appl Physiol (1985) 102: 261–268, 2007. [DOI] [PubMed] [Google Scholar]
- 415.Zintzaras E, Ioannidis JP. Heterogeneity testing in meta-analysis of genome searches. Genet Epidemiol 28: 123–137, 2005. [DOI] [PubMed] [Google Scholar]
- 416.Zintzaras E, Kitsios G, Stefanidis I. Endothelial NO synthase gene polymorphisms and hypertension: A meta-analysis. Hypertension 48: 700–710, 2006. [DOI] [PubMed] [Google Scholar]
- 417.Zweier JL, Samouilov A, Kuppusamy P. Non-enzymatic nitric oxide synthesis in biological systems. Biochim Biophys Acta 1411: 250–262, 1999. [DOI] [PubMed] [Google Scholar]