Abstract
The programmed axon degeneration pathway has emerged as an important process contributing to the pathogenesis of several neurological diseases. The most crucial events in this pathway include activation of the central executioner SARM1 and NAD+ depletion, which leads to an energetic failure and ultimately axon destruction. Given the prevalence of this pathway, it is not surprising that inhibitory therapies are currently being developed in order to treat multiple neurological diseases with the same therapy. Charcot-Marie-Tooth disease (CMT) is a heterogeneous group of neurological diseases that may also benefit from this therapeutic approach. To evaluate the appropriateness of this strategy, the contribution of the programmed axon degeneration pathway to the pathogenesis of different CMT subtypes is being actively investigated. The subtypes CMT1A, CMT1B and CMT2D are the first to have been examined. Based on the results from these studies and advances in developing therapies to block the programmed axon degeneration pathway, promising therapeutics for CMT are now on the horizon.
Keywords: CMT, GARS, HSP, MPZ, NAD+, NMNAT, Peripheral neuropathy, PMP22, Programmed axon degeneration, SARM1, Therapy development
1. Introduction
Charcot-Marie-Tooth disease (CMT) is a diverse group of peripheral neuropathy disorders that result in symptoms of sensory and motor nerve impairment in patients. The >80 genes associated with different types of CMT generally either directly disrupt myelin or axon integrity of peripheral nerves (Stojkovic, 2016). However, secondary axon degeneration has been detected in all types of demyelinating CMT and has been suggested to be the primary driver of functional deficits in these patients (Krajewski et al., 2000). Therefore, inhibiting axon degeneration in all forms of CMT may potentially be beneficial. This approach has become feasible due to our growing understanding of injury-induced axon degeneration, which has been greatly facilitated by the discovery of the Wallerian degeneration slow (WldS) mouse. This naturally occurring mouse strain was first reported in 1989 and was demonstrated to have inhibited traumatic axon degeneration. Distal axons from transected sciatic nerves remained functional for up to two weeks whereas wildtype axons degenerated in less than two days (Lunn et al., 1989; Tsao et al., 1994). These initial studies revealed that axons possess an autonomous axon degeneration program and set the stage for uncovering the molecular mechanism for this process.
2. Programmed Axon Degeneration
The importance of Wallerian-like degeneration for neurological disease was revealed by demonstrating that WldS is neuroprotective in several disease model mice including, Chemotherapy Induced Peripheral Neuropathy (Wang et al., 2001; Wang et al., 2002), Parkinson’s disease (Cheng and Burke, 2010; Hasbani and O’Malley, 2006; Sajadi et al., 2004) and Multiple Sclerosis (Kaneko et al., 2006)[reviewed in (Conforti et al., 2014)]. Although the precise molecular mechanism regulating Wallerian-like axon degeneration remains unknown, significant advances have been made towards reaching this goal. Further characterization of the WldS mouse revealed that neuroprotection is genetically dominant and caused by expression of a chimeric gene containing an N-terminal coding region fragment of the ubiquitin factor E4B (UBE4B) gene fused to the full coding region of the nicotinamide nucleotide adenylyltransferase 1 (NMNAT1) gene (Conforti et al., 2000; Mack et al., 2001). The WldS mouse contains two copies of this gene in a tandem repeat on chromosome 4 leading to overexpression of this chimeric protein in both the CNS and PNS (Conforti et al., 2000; Lyon et al., 1993; Samsam et al., 2003). The NMNAT1 gene encodes an enzyme that catalyzes the synthesis of NAD+ from NMN and ATP and this function is required for delaying axon degeneration (Araki et al., 2004). Further studies have identified a number of other proteins and processes involved in this programmed axon degradation pathway including SARM1, MAP kinase signaling, Hiw (D. melanogaster), Axed (D. melanogaster), Peb (D. melanogaster), ZNRF1, DPYSL2, mitochondrial and endosome function, autophagy and sodium and potassium channel activity (Court and Coleman, 2012; Farley et al., 2018; Mishra et al., 2013; Neukomm et al., 2017; Shen et al., 2013; Wakatsuki et al., 2011; Wang et al., 2018; Xiong et al., 2012). Of these, NMNAT, SARM1 and MAP kinase signaling have been best characterized in mammals and therefore will be summarized as a lead in to the possibility of targeting this pathway as a therapeutic for CMT. Reference the following for a more extensive review of the programmed axon degeneration pathway (Carty and Bowie, 2019; Gerdts et al., 2016; Sapar and Han, 2019).
2.1. NMNAT/NAD+
Mammals have three paralogous NMNAT genes, NMNAT1, -2 and -3, all of which are highly conserved. The expression of the different paralogs is distinct: NMNAT1 is broadly expressed and localized to nuclei, NMNAT2 is highly expressed in brain and localized to cytoplasm and NMNAT3 is weakly expressed in brain and has been suggested to be localized to mitochondria (Di Stefano and Conforti, 2013). This differential expression indicates that the endogenous NMNAT paralogs cannot compensate for one another even though they are functionally redundant in delaying axon degeneration (Sasaki et al., 2009a; Yahata et al., 2009; Yan et al., 2010). NMNAT2 was predicted to function endogenously in axon degeneration given its expression pattern. Indeed NMNAT2 specific knockdown is sufficient to induce Wallerian-like degeneration of uninjured axons (Gilley and Coleman, 2010). Interestingly, NMNAT2 is a highly labile protein that is constantly replenished by axonal transport, suggesting that NMNAT2 depletion is likely a key initiation step for degeneration when axon trafficking is disrupted (Gilley and Coleman, 2010). Additionally, the WldS transgene encodes NMNAT1 as part of the chimeric protein, demonstrating that providing a source of stable NMNAT to axons is able to delay this initiation event (Araki et al., 2004). However, the mechanism responsible for the delay in NMNAT depletion initiated axon degeneration is less clear. NAD+ synthesis activity of both NMNAT2 and WldS is required to delay axon degeneration suggesting that regulation of NAD+ levels may contribute (Araki et al., 2004; Yan et al., 2010).
NAD+ is a cofactor involved in redox reactions that are critical for energy metabolism but also plays important roles in regulating cell signaling, DNA repair, chromatin structure and circadian rhythm (Kato and Lin, 2014; Nikiforov et al., 2015; Yoshino et al., 2018). Therefore, NMNAT2 depletion in axons likely leads to reduced NAD+ levels and consequently dysfunctional energy metabolism. NAD+ levels have been demonstrated to decline in Wallerian degenerating axons (Wang et al., 2005). However, increasing axonal NAD+ levels is not sufficient to reproduce the NMNAT protective effect. WldS mice have increased NMNAT activity but NAD+ levels remain unchanged likely due to an upstream rate-limiting reaction (Mack et al., 2001). Additionally, augmenting NAD+ axon levels by exogenous application of metabolites and modification of enzymes involved in NAD+ biosynthesis and consumption only modestly delays axon degeneration if at all (Araki et al., 2004; Sasaki et al., 2006; Sasaki et al., 2009b; Wang et al., 2005). These findings demonstrate that NMNAT-mediated axon protection involves a more complicated mechanism than simply preserving axonal NAD+ levels. Further insights pertaining to the role of NAD+ in axon degeneration have been revealed by studying the prodegenerative factor SARM1.
2.2. SARM1
Identification of prodegenerative factors was required to demonstrate that Wallerian degeneration is truly a programmed process. Therefore, a Drosophila genetic screen was performed to identify mutated genes that delayed degeneration of transected olfactory receptor neuron axons. Mutations in dSarm were identified that dramatically delay axon degeneration (Osterloh et al., 2012). These findings were supported by an RNAi screen in primary mouse DRG neurons that demonstrated SARM1 knockdown delayed degeneration of transected axons (Gerdts et al., 2013). Additionally, transected sciatic nerve axons from SARM1 knockout mice remained functional for at least two weeks confirming a role for SARM1 as a prodegenerative factor (Osterloh et al., 2012). Interestingly, SARM1 knockout was able to protect axons of NMNAT2 deficient mice into old age whereas WldS delayed but did not prevent axon degeneration demonstrating that SARM1 is required for axon degeneration (Gilley et al., 2017). SARM1 is highly expressed in brain and is localized to mitochondria, synapses and microtubules (Chen et al., 2011; Kim et al., 2007; Panneerselvam et al., 2012). It is a member of the Toll-interleukin receptor (TIR) domain-containing protein family but is unique in that it does not regulate Toll-like receptor signaling (Couillault et al., 2004; Kaiser and Offermann, 2005; Kim et al., 2007; Liberati et al., 2004). SARM1 is evolutionarily conserved and its C-terminal TIR domain is especially conserved more so than TIR domains from other proteins (Kim et al., 2007; Malapati et al., 2017). SARM1 also contains two tandem sterile alpha motif (SAM) domains and multiple armadillo repeat motifs near the N-terminus. Structure-function studies revealed that the N-terminal region of SARM1 is auto-inhibitory, the SAM domain is required for multimerization and the TIR domain triggers axon degeneration (Chuang and Bargmann, 2005; Gerdts et al., 2013; Panneerselvam et al., 2013).
In order to determine where SARM1 fits into the axon degeneration pathway, the epistatic relationship between SARM1, NMNAT and NAD+ was evaluated. SARM1 knockout is able to rescue the embryonic lethality and axon extension defects observed in NMNAT2 knockout mice demonstrating that SARM1 functions downstream of NMNAT2 (Gilley et al., 2015). Additionally, SARM1 knockout is able to partially preserve NAD+ levels in transected axons revealing that the majority of axonal NAD+ loss is downstream of SARM1 (Gerdts et al., 2015; Gilley et al., 2015). These findings suggest that depletion of axonal NAD+ levels would be sufficient to trigger axon degeneration. Indeed stimulating NAD+ consumption in SARM1 knockout cells by pharmacological activation of poly ADP-ribose polymerase triggers axon degeneration (Gerdts et al., 2015). Recent advances have expanded our understanding of how NMNAT2 depletion activates SARM1 and causes NAD+ loss. SARM1 was initially thought be a non-enzymatic protein and was suggested to function as a scaffold similar to the proposed function for other TIR family members (Watters et al., 2007). However, TIR domains, including the SARM1 TIR domain, have since been demonstrated to possess NADase activity and are able to cleave NAD+ into ADP-ribose, cyclic ADP-ribose and nicotinamide (Essuman et al., 2017; Essuman et al., 2018). Additionally, NMNAT1 has been shown to inhibit the NADase activity of SARM1 and NMNAT2 depletion has been suggested to activate SARM1 due to accumulation of the NAD+ precursor NMN (Gilley et al., 2015; Sasaki et al., 2016). These findings reveal that SARM1 activation is precisely regulated by NMNAT activity in order to control axonal NAD+ levels and establish SARM1 as the executioner of axon degeneration. The loss of axonal NAD+ levels likely results in an energetic failure and signals axon degeneration to occur but MAP kinase signaling has also been demonstrated to contribute to this process.
2.3. MAP Kinase Signaling
Genetic deletion of the prodegenerative factor Dual Leucine Zipper Kinase (DLK), a mitogen-activated protein kinase kinase kinase (MAPKKK), was demonstrated to delay degeneration of transected DRG neurons motivating a careful analysis of the MAP kinase signaling pathway in programmed axon degeneration (Miller et al., 2009). Results revealed that three MAPKKKs (DLK, MEKK4 and MLK2), two MAPKKs (MKK4 and MKK7) and three JNKs (JNK1, -2 and -3) are involved in the degeneration of transected DRG axons (Yang et al., 2015). Interestingly, SARM1 knockout abolished MKK4 activation upon transection and MKK4/MKK7 knockdown did not extend the delayed axon degeneration observed with SARM1 knockout (Yang et al., 2015). Additionally, inducing SARM1 activation leads to rapid activation of MKK4 and axon degeneration, which is prevented by depletion of MKK4/MKK7 or downstream JNK1/JNK2/JNK3 (Yang et al., 2015). These findings suggest that MAP kinase signaling is downstream of SARM1. However, these conclusions are controversial due to a conflicting report. MKK4/MKK7 were demonstrated to reduce axonal NAD+ and ATP levels and initiate degeneration of transected DRG neurons but not uninjured neurons with SARM1 activation (Walker et al., 2017). MKK4/MKK7 knockdown also resulted in increased expression of NMNAT2 and the microtubule regulating protein SCG10 (Walker et al., 2017). This effect was not dependent on SARM1 and the elevated NMNAT2 levels were demonstrated to be sufficient to delay axon degeneration (Walker et al., 2017). Therefore, a mechanism with MAP kinase signaling upstream of NMNAT2 was proposed; whereby MAP kinase activation reduces NMNAT2 expression to alleviate SARM1 inhibition and decrease NAD+ levels. Interestingly both studies conclude that MAP kinase signaling is required to achieve reduced ATP levels upon injury but do not elaborate on how this occurs (Walker et al., 2017; Yang et al., 2015). However, ATP loss was shown to be upstream of calpain activation, which facilitates cytoskeleton proteolysis and breakdown of the axon (Yang et al., 2013; Yang et al., 2015). Additional factors may also contribute to this process including SCG10, which accelerates axon degeneration when depleted (Shin et al., 2012). Although, the precise contribution of MAP kinase signaling to the programmed axon degeneration pathway is unclear, it is apparent that it plays an important role. Future studies are required to elucidate the molecular mechanism, determine if MAP kinase signaling influences multiple steps of the pathway and identify feedback regulatory loops.
3. Inhibiting Programmed Axon Degeneration as a Therapy for CMT
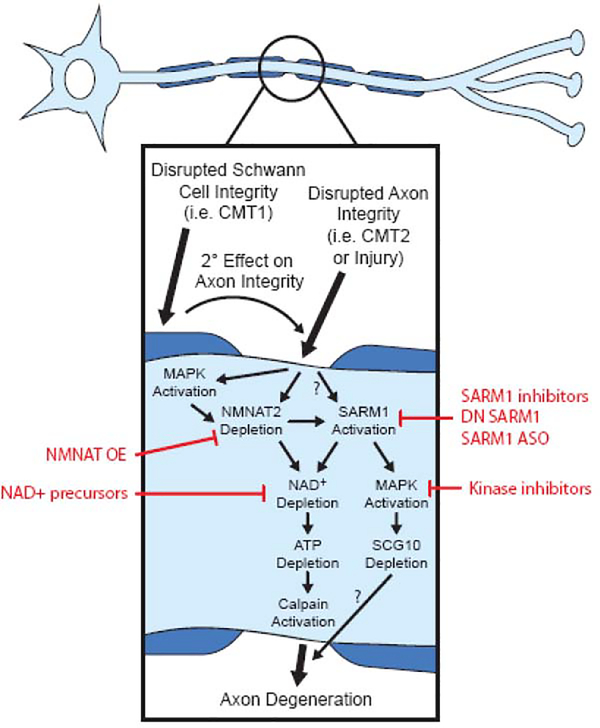
Given the occurrence of axon degeneration in all types of CMT, therapy development targeted at inhibiting this process may be a beneficial approach for the multiple types and subtypes of this disease. CMT Type 1 (CMT1) disrupts myelin integrity leading to secondary axon degeneration whereas CMT Type 2 (CMT2) directly affects axon integrity (Stojkovic, 2016). Therefore, blocking programmed axon degeneration holds the most therapeutic promise for CMT2 but may also help slow the progression of symptoms in CMT1 patients. In order to determine if this is a suitable treatment strategy, the role of programmed axon degeneration in CMT pathogenesis needs to be evaluated. Rodent models of the different CMT subtypes should be crossbred with inhibitors of this pathway, including WldS and SARM1 knockout rodents, to determine if they rescue disease pathology. These types of studies are starting to be pursued but are limited by the availability of disease-recapitulating rodent models of CMT. Progress made in these endeavors will be discussed below and a model for the proposed interaction between the programmed axonal degradation pathway and CMT is depicted in Fig. 1.
Figure 1: Working model of axon degeneration in CMT.
Axon degeneration in CMT occurs either as a primary axon-intrinsic process (CMT2) or secondary to defects extrinsic to axons (CMT1). Although it is unclear if these processes lead to activation of the molecular pathway that mediates Wallerian degeneration in CMT, there is ample evidence that NMNAT and SARM1 play a key role in acquired neuropathies where the primary process is slow distal axonal degeneration. An early event in this pathway is NMNAT2 depletion, which may be carried out by activation of MAPK signaling. Depletion of NMNAT2 leads to activation of SARM1, which may also occur independently of NMNAT2 depletion. SARM1 activation leads to a reduction in NAD+ levels due to the NADase activity of SARM1 and may also lead to additional MAPK signaling. Depletion of NAD+ leads to the loss of ATP, an energetic failure and Calpain activation. Calpain-mediated proteolysis then facilitates the structural breakdown of the axon. Activated MAPK signaling also depletes SCG10, a microtubule binding protein whose loss may contribute to axon degeneration. Intervention at several points along this pathway may offer potential therapeutic options (highlighted in red).
3.1. Type 1: Demyelinating CMT
CMT1 accounts for 50–60% of all CMT cases and is categorized into the following subtypes: CMT1A caused by duplication of the PMP22 gene accounts for 70–80% of all CMT1 cases, CMT1B caused by single nucleotide variants in the MPZ gene accounts for 6–10% of all CMT1 cases, CMT1C caused by single nucleotide variants in the LITAF gene accounts for 1–2% of all CMT1 cases, CMT1D caused by single nucleotide variants in the EGR2 gene accounts for <2% of all CMT1 cases, CMT1E caused by single nucleotide variants in the PMP22 gene accounts for <5% of all CMT1 cases and CMT1F caused by single nucleotide variants in the NEFL gene accounts for <5% of all CMT1 cases (Charcot-Marie-Tooth Neuropathy Type 1 GeneReviews: https://www.ncbi.nlm.nih.gov/books/NBK1205/) (Bassam, 2014; Murphy et al., 2012; Rudnik-Schoneborn et al., 2016). Given that CMT1A and CMT1B account for the vast majority of CMT1 cases, it is not surprising that these subtypes have been best characterized. Advances in our understanding of CMT1A and CMT1B pathomechanisms have been facilitated by the development of rodent models that recapitulate key disease phenotypes. This has also allowed the programmed axon degeneration pathway to be interrogated for its involvement in CMT1A and CMT1B pathogenesis.
Several CMT1A rodent models have been created including a rat model that contains additional copies of the mouse PMP22 gene and exhibits demyelination, onion blub formation, progressive muscle weakness and gait abnormalities similar to CMT1A patients (Grandis et al., 2004; Sereda et al., 1996). To determine if the programmed axon degeneration pathway contributes to CMT1A pathogenesis, the CMT1A rat model was crossed with WldS rats to generate heterozygous double transgenic rats. Rescue of compound muscle action potentials (CMAP) and nerve conduction velocities (NCV) at 10 weeks and axon number and diameter in tibial nerves and forelimb grip strength at 13 weeks were evaluated. CMT1A rats had dramatically reduced CMAP amplitudes and NCVs and both were increased by introduction of the WldS transgene but remained ≤50% of wildtype levels (Meyer zu Horste et al., 2011). Additionally, the CMT1A rats had a reduced number of tibial nerve axons and their diameter was decreased and both phenotypes were rescued in double transgenic rats (Meyer zu Horste et al., 2011). Forelimb grip strength was also assessed as a readout of muscle strength but the results were less clear. Wildtype, CMT1A and double transgenic rats had similar grip strengths at 13 weeks so the increase in grip strength from 5 to 13 weeks was quantified (Meyer zu Horste et al., 2011). The Wlds alone and double transgenic rats had much lower initial grip strengths likely due to their lower average body weight, which corresponded with a greater grip strength increase from 5 to 13 weeks as compared to wildtype and CMT1A rats (Meyer zu Horste et al., 2011). Taken together, these findings suggest that overexpressing WldS delays axon degeneration in CMT1A model rats, which results in moderate increases in nerve and potentially muscle function. WldS does not improve the myelination deficits in CMT1A rats, which likely explains the modest functional recovery due to impaired saltatory conduction of intact axons (Meyer zu Horste et al., 2011). Additionally, treating CMT1A rats with the NAD+ precursor NMN by intraperitoneal injections daily from 5 to 13 weeks did not rescue tibial nerve axon number or forelimb grip strength (Meyer zu Horste et al., 2011). However, this result is not surprising given the modest effect this approach has on delaying axon degeneration (Araki et al., 2004; Sasaki et al., 2006; Sasaki et al., 2009b; Wang et al., 2005). Nevertheless, the results of this study are promising and should be followed up by evaluating aged rodents for rescue given the degenerative nature of CMT1A and the much stronger inhibitor of programmed axon degeneration, SARM1 knockout, should be assessed.
CMT1B is caused by numerous different point mutations that are predominantly localized within the extracellular domain of the MPZ protein (Sanmaneechai et al., 2015). Several mutations have been suggested to be pathogenic due to loss of function, which is supported by the presence of CMT1B-like symptoms in MPZ knockout mice (Giese et al., 1992; Martini et al., 1995). These mice exhibit demyelination, impaired nerve conduction, axon degeneration and abnormal motor function similar to CMT1B patients (Giese et al., 1992; Martini et al., 1995; Martini, 1999). To evaluate the contribution of the programmed axon degeneration pathway to CMT1B pathogenesis, MPZ knockout mice were crossbred with WldS mice to generate MPZ homozygous knockout and WldS homozygous overexpression mice. Rescue of CMAPs, axon number in plantar and median nerves, intact motor unit number and forelimb grip strength at 12 weeks were assessed. MPZ knockout mice had dramatically reduced CMAP amplitudes and this value was increased by introducing two copies of the WldS transgene but remained <50% of wildtype levels (Samsam et al., 2003). Additionally, MPZ knockouts had a reduced number of plantar and median nerve axons and these measurements were increased by WldS overexpression (Samsam et al., 2003). The number of intact motor units was also quantified by retrograde labeling with Fluorogold. MPZ knockout mice had a reduced number of labeled motor neurons, which was rescued by WldS overexpression (Samsam et al., 2003). Furthermore, MPZ knockouts had reduced forelimb grip strength, which was moderately increased by introduction of WldS (Samsam et al., 2003). Similar to the findings for CMT1A, these findings suggest that overexpressing WldS delays axon degeneration in CMT1B model mice, which results in moderate increases in nerve and potentially muscle function. WldS does not improve the myelination deficits in MPZ knockout mice, which similarly may explain the modest functional recovery (Samsam et al., 2003). Additionally, CMAP amplitude, plantar nerve axon number and forelimb grip strength in 24 week old MPZ knockouts are not rescued by WldS overexpression and this effect is not due to diminished WldS expression in these aged mice (Samsam et al., 2003). Although the axon protective effects of WldS were transient, these findings indicate that the programmed axon degeneration pathway does contribute to CMT1B pathogenesis and suggest that the much stronger inhibitor of programmed axon degeneration, SARM1 knockout, should be evaluated for rescue. Additionally, conclusions from these studies would be strengthened by using rodent models that express common MPZ mutations rather than MPZ knockouts.
Rodent models for the remaining CMT1 subtypes (1C [LITAF T115N], 1D [EGR2 I268N], 1E [PMP22 L16P and G150D] and 1F [NEFL P22S and N98S]) are available but they have not been evaluated for the ability of inhibited programmed axon degeneration to improve disease phenotypes (Adebola et al., 2015; Baloh et al., 2009; Filali et al., 2011; Suter et al., 1992a; Suter et al., 1992b; Zhu et al., 2013). Given the promising results observed with CMT1A and CMT1B, these will likely be valuable experiments to pursue. However, this strategy does not improve myelination deficits suggesting that preserved axons will be less functional and the delay in axon degeneration will be somewhat transient. These predictions are supported by findings from the CMT1A and CMT1B studies but further analysis with SARM1 knockouts may yield more efficacious results (Meyer zu Horste et al., 2011; Samsam et al., 2003). Regardless, this approach may also be beneficial when used in combination with treatments that target the disease-causing gene, particularly in adult patients in order to prevent axon degeneration while myelin repair in underway.
3.2. Type 2: Axonal CMT
CMT2 accounts for 25–30% of all CMT cases and is categorized into numerous subtypes (2A-2W) of which the majority are very rare (Charcot-Marie-Tooth Neuropathy Type 2 GeneReviews: https://www.ncbi.nlm.nih.gov/books/NBK1285/) (Bassam, 2014; Murphy et al., 2012; Rudnik-Schoneborn et al., 2016). CMT2A is caused by single nucleotide variants in the MFN2 gene and accounts for ~30% of all CMT2 cases (Bassam, 2014; Rudnik-Schoneborn et al., 2016; Verhoeven et al., 2006). Unfortunately, the proportion of CMT2 accounted for by the remaining subtypes is not as clearly defined but estimates range anywhere up to 7% per subtype (Rossor et al., 2013). Given the infrequency of most of these, it is not surprising that disease models are not available for the majority of the subtypes. Rodent models have been generated for CMT2A, CMT2D, CMT2E, CMT2F, CMT2K, CMT2O and CMT2Q though (Achilli et al., 2009; Barbullushi et al., 2019; Barneo-Munoz et al., 2015; Sabblah et al., 2018; Seburn et al., 2006; Shen et al., 2016; Villalon et al., 2015; Xu et al., 2018). These developments have made it feasible to evaluate the programmed axon degeneration pathway in CMT2 pathogenesis but only CMT2D has been assessed to date.
CMT2D is caused by several different point mutations that are dispersed throughout the GARS protein (Yao and Fox, 2013). These mutations have been suggested to affect the aminoacylation activity of GARS given that mutations in five additional tRNA synthetase genes (YARS, AARS, HARS, MARS and WARS) have been demonstrated to cause CMT (Gonzalez et al., 2013; Jordanova et al., 2006; Latour et al., 2010; McLaughlin et al., 2012; Tsai et al., 2017; Vester et al., 2013). However, not all of the identified GARS mutations affect the enzymatic activity of the protein suggesting that the pathomechanism of CMT2D is more complex (Nangle et al., 2007; Seburn et al., 2006). The mouse model Nmf249 contains the known GARS mutation P278KY (corresponding to P234KY in humans) and exhibits axon diameter, nerve conduction and muscle function defects similar to CMT2D patients (Achilli et al., 2009). To determine if the programmed axon degeneration pathway contributes to CMT2D pathogenesis, Nmf249 mice were crossbred with WldS mice to generate double heterozygotes. Rescue of myelinated axon number in the motor branch of the femoral nerve and neuromuscular junction defects at 4 weeks were evaluated. Nmf249 heterozygotes had significantly less myelinated axons in the motor branch of the femoral nerve as compared to wildtype mice and this phenotype was not rescued in double heterozygous mice (Stum et al., 2011). Nmf249 heterozygotes and double heterozygotes also demonstrated a dramatic reduction in the diameter of the nerve suggesting that axon diameter and potentially total axon number are also affected (Stum et al., 2011). Additionally, neuromuscular junction defects including abnormal morphology and innervation and atrophic axons in Nmf249 heterozygotes was not rescued in double heterozygotes (Stum et al., 2011). These findings suggest that CMT2D pathogenesis does not depend upon the programmed axon degeneration pathway and instead initiates axon degeneration by an alternative mechanism. However, these conclusions would be strengthened by evaluating additional GARS mutations and by crossbreeding with the much stronger inhibitor of programmed axon degeneration, SARM1 knockout.
Although inhibition of the programmed axon degeneration pathway has yet to be demonstrated to rescue CMT2 pathogenesis, these are appealing experiments to pursue given that these diseases directly target axon viability by a variety of mechanisms. CMT2A should be given the highest priority because it accounts for the greatest proportion of CMT2 patients and rodent models of the disease are improving. The MFN2 T105M mouse exhibits muscle atrophy and impaired motor function and the MFN2 R364W rat has reduced CMAP amplitudes in the caudal nerve, reduced myelinated axon number in distal nerves and impaired motor function (Barbullushi et al., 2019). Additionally, mouse models for CMT2E (NEFL E397K), CMT2F (HSBP1 S315F), CMT2K (GDAP knockout), CMT2O (DHC H304R) and CMT2Q (DHTKD1 knockout) demonstrate phenotypes similar to CMT2 patients including muscle atrophy and impaired motor function (Barneo-Munoz et al., 2015; Sabblah et al., 2018; Shen et al., 2016; Villalon et al., 2015; Xu et al., 2018). It is likely that blocking the programmed axon degeneration pathway will be beneficial for multiple CMT2 subtypes given that inhibition of this pathway delays axon degeneration in several different neurological diseases (Conforti et al., 2014). If so, this approach may prove superior to subtype-specific treatments because these therapies are already being developed.
3.3. Other Types of CMT and Related Diseases
CMT1 and CMT2 are genetically autosomal dominant but CMT can also be autosomal recessive (CMT Type 4 [CMT4]) and X-linked (CMTX) (Hoyle et al., 2015). Additionally, some CMT mutations cause an intermediate form of the disease that directly affects both Schwann cells and axons (Berciano et al., 2017). Furthermore, mutations in the same gene can cause different forms of CMT, which make the categorization of the different types and subtypes complicated. Mouse models of CMT4 (subtypes 4B1 [MTMR2 knockout], 4B2 [SBF2 knockout], 4C [SH3TC2 knockout] and 4F [PRX knockout]) and CMTX (subtype X1 [CX32 knockout]) are available but the contribution of the programmed axon degeneration pathway to disease pathogenesis has not been assessed in any of them (Arnaud et al., 2009; Bolino et al., 2004; Gillespie et al., 2000; Nelles et al., 1996; Tersar et al., 2007). It will be of value to determine whether this pathway contributes to the pathogenesis of these diseases in order to identify potential therapeutic approaches and further our understanding of disease pathomechanisms. We would predict that inhibiting the programmed axon degeneration pathway in CMT subtypes where the axon is primarily affected would be most beneficial.
Hereditary spastic paraplegia (HSP) is a diverse group of neurological diseases that classically result in symptoms of leg muscle weakness and spasticity and in more severe cases peripheral neuropathy, intellectual disability, epilepsy, ataxia and/or vision loss (Boutry et al., 2019). The >60 genes associated with different types of HSP can be genetically autosomal dominant, autosomal recessive or X-linked (Boutry et al., 2019). Interestingly, some HSP genes are also known CMT-causing genes suggesting that these diseases are closely related (Boutry et al., 2019). Although HSP patients are genetically and symptomatically heterogeneous, axon degeneration that is more severe in distal regions of the spinal cord is a common pathological feature in postmortem studies (Fink, 2013). Therefore, inhibiting the programmed axon degeneration pathway in all forms of HSP may potentially be beneficial. To begin evaluating this possibility, a mouse model for X-linked HSP Type 2 (HSPX2; PLP1 knockout), which exhibits axon degeneration in the fasciculus gracilis of the rostral cervical region and axonal swellings in the optic nerve, was crossbred with WldS mice (Edgar et al., 2004; Garbern et al., 2002; Klugmann et al., 1997). Introducing the WldS transgene did not rescue the cervical fasciculus gracilis axon degeneration or the optic nerve axon swelling at 18 months by a qualitative analysis (Edgar et al., 2004). These findings suggest that HSPX2 pathogenesis does not depend on the programmed axon degeneration pathway but a quantitative analysis is required to substantiate this claim. Additionally, it will be valuable to assess rodent models for other subtypes of HSP, which are caused by mutations in genes involved in a variety of processes required for axon viability, ideally by breeding with SARM1 knockouts (Fink, 2013).
4. Programmed Axon Degeneration Targeted Therapeutics
Inhibiting programmed axon degeneration has emerged as an attractive therapeutic strategy because this pathway contributes to the pathogenesis of several neurological diseases including CMT (Conforti et al., 2014). Our growing understanding of this pathway also increases the appeal because multiple candidates within the pathway can be targeted to optimize treatment efficacy. The overall goal of increasing NMNAT activity, decreasing SARM1 activity and/or maintaining NAD+ levels within the axon can be accomplished by multiple approaches. Advances in developing these therapies will be discussed below.
4.1. Biologic Therapies
Some potential biologic therapies for blocking programmed axon degeneration include gene therapy to overexpress NMNAT or a SARM1 dominant negative and antisense oligonucleotides (ASO) to deplete SARM1. Gene therapies to regulate NMNAT expression and SARM1 activity are in their infancy but the results appear promising. Recombinant adeno-associated virus, serotype 6 was generated to express full-length NMNAT1 or NMNAT2 cDNA and IRES-EGFP (Ljungberg et al., 2012). Virus was injected into the CA1/dentate gyrus region of the hippocampus of tauopathy model mice at 6 weeks and neurodegeneration was evaluated at 5 months (Ljungberg et al., 2012). Both NMNAT1 and NMNAT2 overexpression protected against hippocampal neuron loss as demonstrated by the thickness of the stratum radiatum and stratum pyramidale (Ljungberg et al., 2012). Additionally, SARM1 dominant negatives were identified by introducing point mutations throughout the protein and testing their ability to delay axon degeneration. A combination of three point mutations (K193R, H194A and H685A; SARM1-CDN) produced the strongest dominant negative effect (Geisler et al., 2019). Adeno-associated virus, serotype 8 was created to express SARM1-CDN under the human Synapsin promoter and EGFP (Geisler et al., 2019). Virus was injected intrathecally at L6/S1 in postnatal day 11 or 12 mice and sciatic nerve transection was performed 5 weeks later. Sciatic nerves were dissected 5 and 10 days after transection and axons were quantified by toluidine blue staining of nerve cross sections. SARM1-CDN overexpression preserved axons to a same degree as SARM1 knockout at 5 days and to lesser degree at 10 days (Geisler et al., 2019). These promising gene therapy strategies should be further characterized and evaluated for their ability to rescue specific subtypes of CMT that depend on the programmed axon degeneration pathway. Additionally, ASO have become a very popular approach for treating neurological diseases but no reports on the development of SARM1 ASO are publically available yet (Wurster and Ludolph, 2018).
4.2. Small Molecule Therapies
Some potential small molecule therapies for inhibiting programmed axon degeneration include SARM1 and MAPK signaling inhibitors and NAD+ precursors. Several pharmaceutical companies are developing SARM1 inhibitors, but results from these endeavors are not available to the public yet. Additionally, several inhibitors are commercially available that target members of the MAPK signaling pathway and some have been extensively characterized like DLK and JNK inhibitors (Bogoyevitch et al., 2010; Siu et al., 2018). However, the contribution of MAPK signaling to the programmed axon degeneration pathway is less clear, which suggests that this may not be the best treatment strategy. There is currently a phase II clinical trial recruiting patients to test the JNK inhibitor CC-90001 in idiopathic pulmonary fibrosis though suggesting that these types of therapies may be clinically available in the near future (clinicaltrials.gov). Another popular approach is attempting to preserve NAD+ levels by supplying biosynthetic precursors. Exogenous application of the NAD+ precursor nicotinamide riboside (NR) has been demonstrated to delay axon degeneration (Sasaki et al., 2006; Vaur et al., 2017) and prevent features of diabetic and paclitaxel-induced peripheral neuropathy (Hamity et al., 2017; Trammell et al., 2016). There are numerous clinical trials that are using dietary supplements to provide NR in attempts to improve muscle, brain and cardiac function and to treat specific diseases like Parkinson’s disease and Li-Fraumeni syndrome (clinicaltrials.gov). However, a major pitfall of translating rodent studies on NR to human use is the fact that the effective doses of NR in rodents is much higher than what is proposed in the current clinical trials without any target validation to identify the correct dose of NR. Nevertheless, outcomes of the trials pertaining to neurological function will likely be informative and potentially influence future clinical trails on peripheral neuropathies like CMT.
5. Conclusion
CMT is a heterogeneous group of neurological diseases caused by >80 genes that typically directly disrupt myelin or axon integrity of peripheral nerves. Axon degeneration has been described in all types of CMT, which makes blocking this process an attractive treatment strategy. The programmed axon degeneration pathway has been demonstrated to contribute to the pathogenesis of several neurological diseases suggesting that this pathway may be involved in the CMT pathogenesis as well. Our understanding of the members participating in this pathway has significantly advanced recently and SARM1 has emerged as the central executioner of axon degeneration. Studies evaluating the involvement of the programmed axon degeneration pathway in the pathogenesis of different CMT subtypes are still in their infancy. However, some of the most common forms of the disease (CMT1A and CMT1B) have been assessed and blocking programmed axon degeneration by overexpressing WldS delays axon degeneration and modestly improves nerve and muscle function (Meyer zu Horste et al., 2011; Samsam et al., 2003). CMT2D and the CMT-related disease HSPX2 were also investigated but overexpressing WldS did not delay axon degeneration or improve other disease phenotypes (Edgar et al., 2004; Stum et al., 2011). Several other CMT subtypes are currently available to be studied given the existence of disease-recapitulating rodent models including the most common CMT2 subtype, CMT2A. These valuable experiments will identify which CMT subtypes may benefit from the programmed axon degeneration blocking treatments currently under development. Some of the most common therapeutic strategies include gene therapy to overexpress NMNAT or SARM1 dominant negatives and small molecules to inhibit SARM1 or supply NAD+ precursors. These treatments will likely rapidly progress in the upcoming years and it will be interesting to determine if these therapies will be beneficial for several neurological diseases including CMT.
Highlights.
Summary of the programmed axon degeneration pathway
Review of CMT subtypes evaluated for the contribution of the programmed axon degeneration pathway to their pathogenesis
Discussion of therapies being developed to inhibit the programmed axon degeneration pathway and treat neurological diseases like CMT
Acknowledgments
Dr. Kathryn Moss is supported by the Johns Hopkins University Provost’s Postdoctoral Diversity fellowship and Maryland Stem cell Research Fund Postdoctoral fellowship. Dr. Ahmet Hoke is supported by Dr. Miriam and Sheldon G. Adelson Medical Research Foundation and NIH R01 NS091260.
Abbreviations
- ATP
Adenosine triphosphate
- AARS
Alanyl-tRNA synthetase
- ASO
Antisense oligonucleotide
- Axed
Axundead
- JNK
C-Jun N-terminal Kinase
- CMT
Charcot-Marie-Tooth disease
- CMT1
CMT Type 1
- CMT2
CMT Type 2
- CMT4
CMT Type 4
- CMAP
Compound muscle action potentials
- CX32
Connexin 32
- DHTKD1
Dehydrogenase E1 and transketolase domain containing 1
- DPYSL2
Dihydropyrimidnase like 2
- DLK
Dual Leucine Zipper Kinase
- DHC
Dynein heavy chain
- EGR2
Early growth response
- EGFP
Enhanced green fluorescent protein
- GDAP
Ganglioside-induced differentiation-associated protein
- GARS
Glycyl-tRNA synthetase
- HSBP1
Heat shock factor binding protein 1
- HSP
Hereditary spastic paraplegia
- Hiw
Highwire
- HARS
Histidyl-tRNA synthetase
- IRES
Internal ribosome entry site
- LITAF
Lipopolysaccharide induced TNF factor
- MEKK4
MAP/ERK kinase kinase 4
- MARS
Methionyl-tRNA synthetase
- MFN2
Mitofusin 2
- MAPKK
Mitogen-activated protein kinase kinase
- MAPKKK
Mitogen-activated protein kinase kinase kinase
- MLK2
Mixed lineage kinase 2
- MPZ
Myelin protein zero
- MTMR2
Myotubularin related protein 2
- NCV
Nerve conduction velocities
- NAD+
Nicotinamide adenine dinucleotide
- NMN
Nicotinamide mononucleotide
- Nmnat/NMNAT
Nicotinamide nucleotide adenylyltransferase ()
- NR
Nicotinamide riboside
- NEFL
Nuerofilament light
- Peb
Pebbled
- PRX
Periaxin
- PMP22
Peripheral membrane protein
- PLP1
Proteolipid protein 1
- SARM1-CDN
SARM1 dominant negative containing K193R, H194A and H685A
- SBF2
SET binding factor 2
- SH3TC2
SH3 domain and tetratricopeptide repeats 2
- SARM1
Sterile alpha and TIR motif containing 1
- SAM
Sterile alpha motif
- SCG10
Superior cervical ganglia 10
- TIR
Toll-interleukin receptor
- YARS
Tyrosyl-tRNA synthetase
- Ube4b
Ubiquitin factor E4B
- WldS
Wallerian degeneration slow
- CMTX
X-linked CMT
- HSPX2
X-linked HSP Type 2
- ZNRF1
Zinc and ring finger 1
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Achilli F, et al. , 2009. An ENU-induced mutation in mouse glycyl-tRNA synthetase (GARS) causes peripheral sensory and motor phenotypes creating a model of Charcot-Marie-Tooth type 2D peripheral neuropathy. Dis Model Mech. 2, 359–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adebola AA, et al. , 2015. Neurofilament light polypeptide gene N98S mutation in mice leads to neurofilament network abnormalities and a Charcot-Marie-Tooth Type 2E phenotype. Hum Mol Genet. 24, 2163–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araki T, et al. , 2004. Increased nuclear NAD biosynthesis and SIRT1 activation prevent axonal degeneration. Science. 305, 1010–3. [DOI] [PubMed] [Google Scholar]
- Arnaud E, et al. , 2009. SH3TC2/KIAA1985 protein is required for proper myelination and the integrity of the node of Ranvier in the peripheral nervous system. Proc Natl Acad Sci U S A. 106, 17528–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baloh RH, et al. , 2009. Congenital hypomyelinating neuropathy with lethal conduction failure in mice carrying the Egr2 I268N mutation. J Neurosci. 29, 2312–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbullushi K, et al. , 2019. Disease Modeling and Therapeutic Strategies in CMT2A: State of the Art. Mol Neurobiol. [DOI] [PubMed] [Google Scholar]
- Barneo-Munoz M, et al. , 2015. Lack of GDAP1 induces neuronal calcium and mitochondrial defects in a knockout mouse model of charcot-marie-tooth neuropathy. PLoS Genet. 11, e1005115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassam BA, 2014. Charcot-Marie-Tooth disease variants-classification, clinical, and genetic features and rational diagnostic evaluation. J Clin Neuromuscul Dis. 15, 117–28. [DOI] [PubMed] [Google Scholar]
- Berciano J, et al. , 2017. Intermediate Charcot-Marie-Tooth disease: an electrophysiological reappraisal and systematic review. J Neurol. 264, 1655–1677. [DOI] [PubMed] [Google Scholar]
- Bogoyevitch MA, et al. , 2010. c-Jun N-terminal kinase (JNK) signaling: recent advances and challenges. Biochim Biophys Acta. 1804, 463–75. [DOI] [PubMed] [Google Scholar]
- Bolino A, et al. , 2004. Disruption of Mtmr2 produces CMT4B1-like neuropathy with myelin outfolding and impaired spermatogenesis. J Cell Biol. 167, 711–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boutry M, et al. , 2019. Update on the Genetics of Spastic Paraplegias. Curr Neurol Neurosci Rep. 19, 18. [DOI] [PubMed] [Google Scholar]
- Carty M, et al. , 2019. SARM: From immune regulator to cell executioner. Biochem Pharmacol. 161, 52–62. [DOI] [PubMed] [Google Scholar]
- Chen CY, et al. , 2011. Sarm1, a negative regulator of innate immunity, interacts with syndecan-2 and regulates neuronal morphology. J Cell Biol. 193, 769–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng HC, et al. , 2010. The Wld(S) mutation delays anterograde, but not retrograde, axonal degeneration of the dopaminergic nigro-striatal pathway in vivo. J Neurochem. 113, 683–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuang CF, et al. , 2005. A Toll-interleukin 1 repeat protein at the synapse specifies asymmetric odorant receptor expression via ASK1 MAPKKK signaling. Genes Dev. 19, 270–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conforti L, et al. , 2000. A Ufd2/D4Cole1e chimeric protein and overexpression of Rbp7 in the slow Wallerian degeneration (WldS) mouse. Proc Natl Acad Sci U S A. 97, 11377–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conforti L, et al. , 2014. Wallerian degeneration: an emerging axon death pathway linking injury and disease. Nat Rev Neurosci. 15, 394–409. [DOI] [PubMed] [Google Scholar]
- Couillault C, et al. , 2004. TLR-independent control of innate immunity in Caenorhabditis elegans by the TIR domain adaptor protein TIR-1, an ortholog of human SARM. Nat Immunol. 5, 488–94. [DOI] [PubMed] [Google Scholar]
- Court FA, et al. , 2012. Mitochondria as a central sensor for axonal degenerative stimuli. Trends Neurosci. 35, 364–72. [DOI] [PubMed] [Google Scholar]
- Di Stefano M, et al. , 2013. Diversification of NAD biological role: the importance of location. FEBS J. 280, 4711–28. [DOI] [PubMed] [Google Scholar]
- Edgar JM, et al. , 2004. Oligodendroglial modulation of fast axonal transport in a mouse model of hereditary spastic paraplegia. J Cell Biol. 166, 121–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Essuman K, et al. , 2017. The SARM1 Toll/Interleukin-1 Receptor Domain Possesses Intrinsic NAD(+) Cleavage Activity that Promotes Pathological Axonal Degeneration. Neuron. 93, 1334–1343 e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Essuman K, et al. , 2018. TIR Domain Proteins Are an Ancient Family of NAD(+)-Consuming Enzymes. Curr Biol. 28, 421–430 e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farley JE, et al. , 2018. Transcription factor Pebbled/RREB1 regulates injury-induced axon degeneration. Proc Natl Acad Sci U S A. 115, 1358–1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filali M, et al. , 2011. Sensorimotor and cognitive function of a NEFL(P22S) mutant model of Charcot-Marie-Tooth disease type 2E. Behav Brain Res. 219, 175–80. [DOI] [PubMed] [Google Scholar]
- Fink JK, 2013. Hereditary spastic paraplegia: clinico-pathologic features and emerging molecular mechanisms. Acta Neuropathol. 126, 307–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garbern JY, et al. , 2002. Patients lacking the major CNS myelin protein, proteolipid protein 1, develop length-dependent axonal degeneration in the absence of demyelination and inflammation. Brain. 125, 551–61. [DOI] [PubMed] [Google Scholar]
- Geisler S, et al. , 2019. Gene therapy targeting SARM1 blocks pathological axon degeneration in mice. J Exp Med. 216, 294–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerdts J, et al. , 2013. Sarm1-mediated axon degeneration requires both SAM and TIR interactions. J Neurosci. 33, 13569–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerdts J, et al. , 2015. SARM1 activation triggers axon degeneration locally via NAD(+) destruction. Science. 348, 453–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerdts J, et al. , 2016. Axon Self-Destruction: New Links among SARM1, MAPKs, and NAD+ Metabolism. Neuron. 89, 449–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giese KP, et al. , 1992. Mouse P0 gene disruption leads to hypomyelination, abnormal expression of recognition molecules, and degeneration of myelin and axons. Cell. 71, 565–76. [DOI] [PubMed] [Google Scholar]
- Gillespie CS, et al. , 2000. Peripheral demyelination and neuropathic pain behavior in periaxin-deficient mice. Neuron. 26, 523–31. [DOI] [PubMed] [Google Scholar]
- Gilley J, et al. , 2010. Endogenous Nmnat2 is an essential survival factor for maintenance of healthy axons. PLoS Biol. 8, e1000300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilley J, et al. , 2015. Absence of SARM1 rescues development and survival of NMNAT2-deficient axons. Cell Rep. 10, 1974–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilley J, et al. , 2017. Sarm1 Deletion, but Not Wld(S), Confers Lifelong Rescue in a Mouse Model of Severe Axonopathy. Cell Rep. 21, 10–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez M, et al. , 2013. Exome sequencing identifies a significant variant in methionyl-tRNA synthetase (MARS) in a family with late-onset CMT2. J Neurol Neurosurg Psychiatry. 84, 1247–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grandis M, et al. , 2004. Early abnormalities in sciatic nerve function and structure in a rat model of Charcot-Marie-Tooth type 1A disease. Exp Neurol. 190, 213–23. [DOI] [PubMed] [Google Scholar]
- Hamity MV, et al. , 2017. Nicotinamide riboside, a form of vitamin B3 and NAD+ precursor, relieves the nociceptive and aversive dimensions of paclitaxel-induced peripheral neuropathy in female rats. Pain. 158, 962–972. [DOI] [PubMed] [Google Scholar]
- Hasbani DM, et al. , 2006. Wld(S) mice are protected against the Parkinsonian mimetic MPTP. Exp Neurol. 202, 93–9. [DOI] [PubMed] [Google Scholar]
- Hoyle JC, et al. , 2015. The genetics of Charcot-Marie-Tooth disease: current trends and future implications for diagnosis and management. Appl Clin Genet. 8, 235–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordanova A, et al. , 2006. Disrupted function and axonal distribution of mutant tyrosyl-tRNA synthetase in dominant intermediate Charcot-Marie-Tooth neuropathy. Nat Genet. 38, 197–202. [DOI] [PubMed] [Google Scholar]
- Kaiser WJ, et al. , 2005. Apoptosis induced by the toll-like receptor adaptor TRIF is dependent on its receptor interacting protein homotypic interaction motif. J Immunol. 174, 4942–52. [DOI] [PubMed] [Google Scholar]
- Kaneko S, et al. , 2006. Protecting axonal degeneration by increasing nicotinamide adenine dinucleotide levels in experimental autoimmune encephalomyelitis models. J Neurosci. 26, 9794–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato M, et al. , 2014. Regulation of NAD+ metabolism, signaling and compartmentalization in the yeast Saccharomyces cerevisiae. DNA Repair (Amst). 23, 49–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim Y, et al. , 2007. MyD88–5 links mitochondria, microtubules, and JNK3 in neurons and regulates neuronal survival. J Exp Med. 204, 2063–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klugmann M, et al. , 1997. Assembly of CNS myelin in the absence of proteolipid protein. Neuron. 18, 59–70. [DOI] [PubMed] [Google Scholar]
- Krajewski KM, et al. , 2000. Neurological dysfunction and axonal degeneration in Charcot-Marie-Tooth disease type 1A. Brain. 123 ( Pt 7), 1516–27. [DOI] [PubMed] [Google Scholar]
- Latour P, et al. , 2010. A major determinant for binding and aminoacylation of tRNA(Ala) in cytoplasmic Alanyl-tRNA synthetase is mutated in dominant axonal Charcot-Marie-Tooth disease. Am J Hum Genet. 86, 77–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liberati NT, et al. , 2004. Requirement for a conserved Toll/interleukin-1 resistance domain protein in the Caenorhabditis elegans immune response. Proc Natl Acad Sci U S A. 101, 6593–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ljungberg MC, et al. , 2012. CREB-activity and nmnat2 transcription are down-regulated prior to neurodegeneration, while NMNAT2 over-expression is neuroprotective, in a mouse model of human tauopathy. Hum Mol Genet. 21, 251–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lunn ER, et al. , 1989. Absence of Wallerian Degeneration does not Hinder Regeneration in Peripheral Nerve. Eur J Neurosci. 1, 27–33. [DOI] [PubMed] [Google Scholar]
- Lyon MF, et al. , 1993. A gene affecting Wallerian nerve degeneration maps distally on mouse chromosome 4. Proc Natl Acad Sci U S A. 90, 9717–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mack TG, et al. , 2001. Wallerian degeneration of injured axons and synapses is delayed by a Ube4b/Nmnat chimeric gene. Nat Neurosci. 4, 1199–206. [DOI] [PubMed] [Google Scholar]
- Malapati H, et al. , 2017. The axon degeneration gene SARM1 is evolutionarily distinct from other TIR domain-containing proteins. Mol Genet Genomics. 292, 909–922. [DOI] [PubMed] [Google Scholar]
- Martini R, et al. , 1995. Protein zero (P0)-deficient mice show myelin degeneration in peripheral nerves characteristic of inherited human neuropathies. Nat Genet. 11, 281–6. [DOI] [PubMed] [Google Scholar]
- Martini R, 1999. P0-deficient knockout mice as tools to understand pathomechanisms in Charcot-Marie-Tooth 1B and P0-related Dejerine-Sottas syndrome. Ann N Y Acad Sci. 883, 273–80. [PubMed] [Google Scholar]
- McLaughlin HM, et al. , 2012. A recurrent loss-of-function alanyl-tRNA synthetase (AARS) mutation in patients with Charcot-Marie-Tooth disease type 2N (CMT2N). Hum Mutat. 33, 244–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer zu Horste G, et al. , 2011. The Wlds transgene reduces axon loss in a Charcot-Marie-Tooth disease 1A rat model and nicotinamide delays post-traumatic axonal degeneration. Neurobiol Dis. 42, 1–8. [DOI] [PubMed] [Google Scholar]
- Miller BR, et al. , 2009. A dual leucine kinase-dependent axon self-destruction program promotes Wallerian degeneration. Nat Neurosci. 12, 387–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishra B, et al. , 2013. Sodium and potassium currents influence Wallerian degeneration of injured Drosophila axons. J Neurosci. 33, 18728–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy SM, et al. , 2012. Charcot-Marie-Tooth disease: frequency of genetic subtypes and guidelines for genetic testing. J Neurol Neurosurg Psychiatry. 83, 706–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nangle LA, et al. , 2007. Charcot-Marie-Tooth disease-associated mutant tRNA synthetases linked to altered dimer interface and neurite distribution defect. Proc Natl Acad Sci U S A. 104, 11239–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelles E, et al. , 1996. Defective propagation of signals generated by sympathetic nerve stimulation in the liver of connexin32-deficient mice. Proc Natl Acad Sci U S A. 93, 9565–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neukomm LJ, et al. , 2017. Axon Death Pathways Converge on Axundead to Promote Functional and Structural Axon Disassembly. Neuron. 95, 78–91 e5. [DOI] [PubMed] [Google Scholar]
- Nikiforov A, et al. , 2015. The human NAD metabolome: Functions, metabolism and compartmentalization. Crit Rev Biochem Mol Biol. 50, 284–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osterloh JM, et al. , 2012. dSarm/Sarm1 is required for activation of an injury-induced axon death pathway. Science. 337, 481–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panneerselvam P, et al. , 2012. Targeting of pro-apoptotic TLR adaptor SARM to mitochondria: definition of the critical region and residues in the signal sequence. Biochem J. 442, 263–71. [DOI] [PubMed] [Google Scholar]
- Panneerselvam P, et al. , 2013. T-cell death following immune activation is mediated by mitochondria-localized SARM. Cell Death Differ. 20, 478–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossor AM, et al. , 2013. Clinical implications of genetic advances in Charcot-Marie-Tooth disease. Nat Rev Neurol. 9, 562–71. [DOI] [PubMed] [Google Scholar]
- Rudnik-Schoneborn S, et al. , 2016. Diagnostic algorithms in Charcot-Marie-Tooth neuropathies: experiences from a German genetic laboratory on the basis of 1206 index patients. Clin Genet. 89, 34–43. [DOI] [PubMed] [Google Scholar]
- Sabblah TT, et al. , 2018. A novel mouse model carrying a human cytoplasmic dynein mutation shows motor behavior deficits consistent with Charcot-Marie-Tooth type 2O disease. Sci Rep. 8, 1739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sajadi A, et al. , 2004. Wlds-mediated protection of dopaminergic fibers in an animal model of Parkinson disease. Curr Biol. 14, 326–30. [DOI] [PubMed] [Google Scholar]
- Samsam M, et al. , 2003. The Wlds mutation delays robust loss of motor and sensory axons in a genetic model for myelin-related axonopathy. J Neurosci. 23, 2833–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanmaneechai O, et al. , 2015. Genotype-phenotype characteristics and baseline natural history of heritable neuropathies caused by mutations in the MPZ gene. Brain. 138, 3180–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sapar ML, et al. , 2019. Die in pieces: How Drosophila sheds light on neurite degeneration and clearance. J Genet Genomics. 46, 187–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki Y, et al. , 2006. Stimulation of nicotinamide adenine dinucleotide biosynthetic pathways delays axonal degeneration after axotomy. J Neurosci. 26, 8484–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki Y, et al. , 2009a. Transgenic mice expressing the Nmnat1 protein manifest robust delay in axonal degeneration in vivo. J Neurosci. 29, 6526–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki Y, et al. , 2009b. Nicotinamide mononucleotide adenylyl transferase-mediated axonal protection requires enzymatic activity but not increased levels of neuronal nicotinamide adenine dinucleotide. J Neurosci. 29, 5525–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki Y, et al. , 2016. NMNAT1 inhibits axon degeneration via blockade of SARM1-mediated NAD(+) depletion. Elife. 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seburn KL, et al. , 2006. An active dominant mutation of glycyl-tRNA synthetase causes neuropathy in a Charcot-Marie-Tooth 2D mouse model. Neuron. 51, 715–26. [DOI] [PubMed] [Google Scholar]
- Sereda M, et al. , 1996. A transgenic rat model of Charcot-Marie-Tooth disease. Neuron. 16, 1049–60. [DOI] [PubMed] [Google Scholar]
- Shen H, et al. , 2013. Maintaining energy homeostasis is an essential component of Wld(S)-mediated axon protection. Neurobiol Dis. 59, 69–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen S, et al. , 2016. Bicyclic-Capped Histone Deacetylase 6 Inhibitors with Improved Activity in a Model of Axonal Charcot-Marie-Tooth Disease. ACS Chem Neurosci. 7, 240–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin JE, et al. , 2012. SCG10 is a JNK target in the axonal degeneration pathway. Proc Natl Acad Sci U S A. 109, E3696–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siu M, et al. , 2018. Dual Leucine Zipper Kinase Inhibitors for the Treatment of Neurodegeneration. J Med Chem. 61, 8078–8087. [DOI] [PubMed] [Google Scholar]
- Stojkovic T, 2016. Hereditary neuropathies: An update. Rev Neurol (Paris). 172, 775–778. [DOI] [PubMed] [Google Scholar]
- Stum M, et al. , 2011. An assessment of mechanisms underlying peripheral axonal degeneration caused by aminoacyl-tRNA synthetase mutations. Mol Cell Neurosci. 46, 432–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suter U, et al. , 1992a. A leucine-to-proline mutation in the putative first transmembrane domain of the 22-kDa peripheral myelin protein in the trembler-J mouse. Proc Natl Acad Sci U S A. 89, 4382–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suter U, et al. , 1992b. Trembler mouse carries a point mutation in a myelin gene. Nature. 356, 241–4. [DOI] [PubMed] [Google Scholar]
- Tersar K, et al. , 2007. Mtmr13/Sbf2-deficient mice: an animal model for CMT4B2. Hum Mol Genet. 16, 2991–3001. [DOI] [PubMed] [Google Scholar]
- Trammell SA, et al. , 2016. Nicotinamide Riboside Opposes Type 2 Diabetes and Neuropathy in Mice. Sci Rep. 6, 26933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai PC, et al. , 2017. A recurrent WARS mutation is a novel cause of autosomal dominant distal hereditary motor neuropathy. Brain. 140, 1252–1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsao JW, et al. , 1994. Loss of the compound action potential: an electrophysiological, biochemical and morphological study of early events in axonal degeneration in the C57BL/Ola mouse. Eur J Neurosci. 6, 516–24. [DOI] [PubMed] [Google Scholar]
- Vaur P, et al. , 2017. Nicotinamide riboside, a form of vitamin B3, protects against excitotoxicity-induced axonal degeneration. FASEB J. 31, 5440–5452. [DOI] [PubMed] [Google Scholar]
- Verhoeven K, et al. , 2006. MFN2 mutation distribution and genotype/phenotype correlation in Charcot-Marie-Tooth type 2. Brain. 129, 2093–102. [DOI] [PubMed] [Google Scholar]
- Vester A, et al. , 2013. A loss-of-function variant in the human histidyl-tRNA synthetase (HARS) gene is neurotoxic in vivo. Hum Mutat. 34, 191–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villalon E, et al. , 2015. Exacerbation of Charcot-Marie-Tooth type 2E neuropathy following traumatic nerve injury. Brain Res. 1627, 143–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakatsuki S, et al. , 2011. ZNRF1 promotes Wallerian degeneration by degrading AKT to induce GSK3B-dependent CRMP2 phosphorylation. Nat Cell Biol. 13, 1415–23. [DOI] [PubMed] [Google Scholar]
- Walker LJ, et al. , 2017. MAPK signaling promotes axonal degeneration by speeding the turnover of the axonal maintenance factor NMNAT2. Elife. 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, et al. , 2005. A local mechanism mediates NAD-dependent protection of axon degeneration. J Cell Biol. 170, 349–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang MS, et al. , 2001. The WldS protein protects against axonal degeneration: a model of gene therapy for peripheral neuropathy. Ann Neurol. 50, 773–9. [DOI] [PubMed] [Google Scholar]
- Wang MS, et al. , 2002. WldS mice are resistant to paclitaxel (taxol) neuropathy. Ann Neurol. 52, 442–7. [DOI] [PubMed] [Google Scholar]
- Wang Y, et al. , 2018. Neuronal autophagy and axon degeneration. Cell Mol Life Sci. 75, 2389–2406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watters TM, et al. , 2007. Structure, function and regulation of the Toll/IL-1 receptor adaptor proteins. Immunol Cell Biol. 85, 411–9. [DOI] [PubMed] [Google Scholar]
- Wurster CD, et al. , 2018. Antisense oligonucleotides in neurological disorders. Ther Adv Neurol Disord. 11, 1756286418776932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong X, et al. , 2012. The Highwire ubiquitin ligase promotes axonal degeneration by tuning levels of Nmnat protein. PLoS Biol. 10, e1001440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu WY, et al. , 2018. DHTKD1 Deficiency Causes Charcot-Marie-Tooth Disease in Mice. Mol Cell Biol. 38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yahata N, et al. , 2009. Nicotinamide mononucleotide adenylyltransferase expression in mitochondrial matrix delays Wallerian degeneration. J Neurosci. 29, 6276–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan T, et al. , 2010. Nmnat2 delays axon degeneration in superior cervical ganglia dependent on its NAD synthesis activity. Neurochem Int. 56, 101–6. [DOI] [PubMed] [Google Scholar]
- Yang J, et al. , 2013. Regulation of axon degeneration after injury and in development by the endogenous calpain inhibitor calpastatin. Neuron. 80, 1175–89. [DOI] [PubMed] [Google Scholar]
- Yang J, et al. , 2015. Pathological axonal death through a MAPK cascade that triggers a local energy deficit. Cell. 160, 161–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao P, et al. , 2013. Aminoacyl-tRNA synthetases in medicine and disease. EMBO Mol Med. 5, 332–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshino J, et al. , 2018. NAD(+) Intermediates: The Biology and Therapeutic Potential of NMN and NR. Cell Metab. 27, 513–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu H, et al. , 2013. Mutation of SIMPLE in Charcot-Marie-Tooth 1C alters production of exosomes. Mol Biol Cell. 24, 1619–37, S1–3. [DOI] [PMC free article] [PubMed] [Google Scholar]