Abstract
Objective:
Obesity is a major long-term concern in HIV-positive patients due to the pathogenic link between obesity and non-communicable chronic diseases (NCD). We aim to characterize changes in body mass index (BMI) over time on antiretroviral therapy (ART) and investigate the association between weight gain and survival in South Africa.
Design & Methods:
Prospective cohort study among HIV-positive adults on first-line ART between April 2004–2015 in Johannesburg, South Africa. We used latent-class growth modelling (adjusted for age, gender, and CD4 count) to identify groups of individuals with similar patterns of change in BMI over time.
Results:
11,263 patients were included. The best fit model involved two linear and two quadratic trajectories. 35% of patients categorized into group one (mean BMI at ART initiation, 20.4 kg/m2; mean BMI after 8 years of follow-up, 20.9 kg/m2), 38% into group two (24.5 to 26.2 kg/m2), 21% into group three(29.5 to 32.6 kg/m2)and 6% into group four (36.5 to 40.0 kg/m2). Over the 8 years of follow-up, 6% of our cohort went down in BMI standard category, while 45% went up. The largest increase occurred in the first 12 months on ART. In years two through eight, we saw a more gradual increase in BMI.
Conclusion:
The largest gain in BMI in HIV patients occurred in the first year on ART. During follow-up, over 50% of our population changed BMI categories putting them at increased risk for NCDs. Consistent counselling on nutritional and lifestyle changes could help improve ART patients’ long term health outcomes.
Keywords: antiretroviral therapy, body mass index, trajectory, HIV, middle-income country, South Africa, public sector
INTRODUCTION
Rates of obesity globally have nearly tripled since 1975. In 2016, an estimated 1.9 billion (39%) adults worldwide were overweight or obese[1]. The largest increase has been in low- and middle-income countries (LMICs) where demographic, social, and economic changes such as urbanization, expanded education, increased industrialization, and rising incomes have occurred rapidly[2]. These changes have resulted in substantial lifestyle modifications including a more highly processed diet and decreased physical activity[3–7]. All have contributed to the rise in obesity, resulting in an increased risk of metabolic disorders and cardiovascular diseases.
South Africa has been at the forefront of the obesity epidemic in sub-Saharan Africa. Between 1998 and 2008, the estimated proportion of the South African adult population who were overweight or obese increased from 29.1 to 31.1% among males (a relative increase of 6.9%), and from 56.2 to 59.5% among women (5.9%)[8]. Despite goals to reduce obesity by 10%, as proposed by the South African government’s 2013 strategic plan[9, 10], recent data suggest the overall proportion of adult South Africans who are overweight or obese is likely to increase further by 2020[11], resulting in higher mortality from non-communicable diseases (NCD).
Obesity has also become a major concern among HIV-positive individuals, given their increased cardiovascular risk due to both the HIV virus itself[12, 13] and to antiretroviral therapy (ART)[14–18]. After the introduction of ART, regional body fat distribution abnormalities due to the nucleoside reverse transcriptase inhibitor (NRTI) stavudine became important metabolic concerns for patients[14]. With the phasing out of stavudine use in first-line ART since 2010, when stavudine was replaced with tenofovir in most LMICs, lipoatrophy[16] has received less attention. Since then, concern regarding HIV patients has shifted towards obesity, attributable to improved health due to effective ART, normal aging, and obesity trends similar to those seen in the general population[17–20].
There is a lack of research on the effects of obesity on patient outcomes in LMICs and even less so within the HIV-infected population. In South Africa, statistics from HIV clinics show a high prevalence (33%) of obesity in HIV positive patients[21, 22], and a substantial proportion (58%) of those on ART become overweight or obese within one year of treatment initiation[22]. Since obesity contributes to the incidence of NCDs and those on ART are already more susceptible to a range of these conditions, the synergistic effect is potentially devastating[23–26]. Few studies have elucidated weight change patterns that are indicative of the future severity of the obesity epidemic in HIV positive patients on ART in South Africa. Previous studies of weight change in HIV-positive cohorts have focused only on the first 12 to 24 months on ART when weight gain may be considered beneficial[21, 22]. To address this research gap, we used latent-class growth modelling techniques to identify groups of individuals following similar progressions of BMI over time on ART in addition to assess BMI progression and associated attrition between 2004–2015 in a large, public-sector, HIV-positive, South African population.
METHODS
Cohort description
We conducted a prospective cohort study over eight years of follow-up among HIV-positive adults on standard first-line ART between April 2004–2015 at Themba Lethu Clinic (TLC) in Johannesburg, using the Themba Lethu Clinical Cohort[27]. TLC began initiating patients onto ART in 2004 under South Africa’s public-sector treatment program. Care and treatment at TLC is provided according to national treatment guidelines[28–31]. The standard first-line regimen until April 2010 was stavudine or zidovudine plus lamivudine and efavirenz; after April 2010, tenofovir or zidovudine replaced stavudine[28–31]. As of April 2017, the cohort included over 40,000 patients, of whom over 35,000 had initiated ART. Patient data, including demographic characteristics, clinical conditions, laboratory test results, and medications, are entered on site by a clinician or a data entry clerk into a live data capturing system called TherapyEdge-HIV™. Weight and height are routinely measured at medical visits. Mortality is recorded in the patient clinical record and confirmed via linkage with the National Vital Registration system[32, 33].
Eligible patients
All ART-naïve, non-pregnant, HIV-positive adult patients (ages 18–75 years) newly initiating standard first-line ART were eligible for the analysis. We excluded patients with missing date of ART initiation or baseline CD4 count and those without enough weight and height measurements to calculate at least 10 measures of BMI (kg/m2) at least one month apart.
Use of data was approved by the Human Research Ethics Committee of the University of the Witwatersrand (M140201). Approval for analysis of de-identified data was granted by the Institutional Review Board of Boston University (H-29768).
Body Mass Index (BMI)
Baseline BMI was calculated from weight and height measured at ART initiation or from an average of measurements taken between 30 days prior to or 7 days after initiation. Weight and height at visits occurring between 1 month and 8 years after ART initiation were used to calculate subsequent BMI observations. When multiple measurements were available for a single month, all available weight and height measurements were averaged before calculating the monthly BMI. Observations with implausible measurements (<25 or >200 kg for weight, <120 or >200 cm for height) were excluded as were measurements suggesting implausible changes between visits (>4 standard deviations from that patient’s average height and weight). Less than 1% of available measurements were considered implausible, suggesting that the measured height and weight observations appear accurate. For visits where height was unavailable, height from previous/subsequent visits was used. BMI was categorized as underweight (<18.5 kg/m2), normal (18.5–24.9 kg/m2), overweight (25.0–29.9 kg/m2), obese class I (30.0–34.9 kg/m2), obese class II (35.0–39.9 kg/m2), and obese class III (>40 kg/m2)[34]. Patients could change BMI categories over time, but once assigned to a trajectory group, as explained below, patients remained there throughout follow-up.
Outcome
Patients were categorized as alive, deceased, lost to follow-up, transferred out, or down-referred (transfer of stable patients to a primary health care facility) based on their last available information. Our primary outcome, attrition was defined as a combination of all-cause mortality and loss to follow-up (defined as being at least three months late for the patient’s last scheduled visit to the clinic).
Statistical analysis
Group-based trajectory modeling, a semi-parametric statistical technique[35–37], was used to identify patients following similar patterns of change in BMI over time since ART initiation. Grouping individuals based on all available BMI measurements allows us to identify latent BMI trajectory groups rather than relying on apriori decisions to group patients based simply on their BMI at ART initiation or their absolute change in BMI over time. The group-based trajectory modeling approach classifies individuals into latent group-based trajectories and estimates model parameters using maximum likelihood[38]. A maximum of four trajectories were chosen a priori in order to maintain simplicity. Consistent with prior established norms for fitting group-based trajectory models[35, 36][1,2], we started with a single quadratic trajectory model and compared model fit statistics (Bayesian Information Criteria (BIC)) to two-group, three-group, and four-group models in a stepwise fashion, leaning towards parsimony in number of groups. Change in BIC was used to choose between more complex and simpler models[38]. We then fit the shape of the patterns of BMI over time comparing BIC values for 81 models consisting of linear, quadratic, and cubic trajectories. Time-fixed covariates, including gender, age (continuous), and CD4 count ART initiation (continuous) and the outcome of attrition were then incorporated into the chosen model.
Individual patients were assigned to a single trajectory group based on maximum posterior probability of measurement[35]. High average posterior probabilities indicate that the model discriminates between individuals with differential patterns of change and can serve as an approximation for the internal reliability of each trajectory[35]. After selecting the appropriate model, we calculated the average posterior probability of group membership for each BMI trajectory identified. We considered values of greater than 0.8 as an indication of adequate internal reliability[35]. We also assessed proportions of membership for each trajectory group to ensure membership of at least 5% of the sample[35].
Survival analysis
We used a Cox proportional hazard model to investigate the association between trajectory group membership and attrition. The model was adjusted for gender, age (18–24, 25–29, 30–39, 40–49 and ≥50), NRTI, non-nucleoside reverse transcriptase inhibitor (NNRTI), CD4 count (0–49, 50–99, 100–199, 200–349 and ≥350), WHO stage, and hemoglobin stage at ART initiation. A Kaplan-Meir survival curve was used to compare survival times for each trajectory group. As patients were not eligible unless they had at least 10 BMI measurements, person-time was calculated from the individual’s 10th BMI observation to either: 1) attrition, 2) transfer, or 3) close of the dataset (December 2015), whichever occurred first, in order to prevent immortal person-time bias.
Analyses were performed using STATA version 15 (StataCorp, College Station, Texas, USA). The “traj” plugin was used in the group-based trajectory modelling[37].
RESULTS
Cohort description
A total of 11,263 patients were eligible for the analysis (Supplemental Online Content 1, Figure 1). The cohort was predominately female (60.2%), with a median age of 37.6 years (interquartile range (IQR) 32.3–44.2), median CD4 count of 109 cells/mm3 (IQR: 42–184), and median BMI of 22.0 kg/m2 (19.5–25.4)(Table 1). At ART initiation, 13.1% of female patients and 2.8% of male patients were considered obese (BMI ≥ 30 kg/m2). Patients were predominately initiated on a standard first-line regimen containing either stavudine (63.0%) or tenofovir (34.6%) plus lamivudine and efavirenz (94.0%). Patients had a median follow-up time from ART initiation to last available visit of 96 months (IQR: 51–96). 61% of the sample was followed for 8 years after ART initiation.
Table 1.
Demographic and clinical characteristics at ART initiation stratified by BMI trajectory groups (N=11,263)
| Characteristics | Group 1 (n=3,882) | Group 2 (n=4,258) | Group 3 (n=2,401) | Group 4 (n=722) | Total (n=11,263) | |
|---|---|---|---|---|---|---|
| Gender | Female | 1652 (42.56%) | 2609 (61.27%) | 1869 (77.84%) | 645 (89.34%) | 6775 (60.15%) |
| Male | 2230 (57.44%) | 1649 (38.73%) | 532 (22.16%) | 77 (10.66%) | 4488 (39.85%) | |
| Age | median (range) | 37 (18.1–72.5) | 37.4 (32.1–71.5) | 38.7 (18.2–72.2) | 39.5 (18.5–66.8) | 37.6 (18.1–72.5) |
| CD4 count category | <50 | 1148 (29.57%) | 1172 (27.52%) | 659 (27.45%) | 172 (23.82%) | 3151 (27.98%) |
| 50–100 | 763 (19.65%) | 776 (18.22%) | 436 (18.16%) | 129 (17.87%) | 2104 (18.68%) | |
| 100–200 | 1276 (32.87%) | 1525 (35.81%) | 761 (31.70%) | 237 (32.83%) | 3799 (33.73%) | |
| 200–350 | 627 (16.15%) | 703 (16.51%) | 493 (20.53%) | 157 (21.75%) | 1980 (17.58%) | |
| >350 | 68 (1.75%) | 82 (1.93%) | 52 (2.17%) | 27 (3.74%) | 229 (2.03%) | |
| median (IQR) | 101.5 (40–177) | 112 (43–181) | 113 (43–192) | 127 (52–201) | 109 (42–184) | |
| HIV viral load | ≤100,000 | 376 (9.69%) | 425 (9.98%) | 248 (10.33%) | 73 (10.11%) | 1122 (9.96%) |
| >100,000 | 339 (8.73%) | 368 (8.64%) | 185 (7.71%) | 54 (7.48%) | 946 (8.40%) | |
| missing | 3167 (81.58%) | 3465 (81.38%) | 1968 (81.97%) | 595 (82.41%) | 9195 (81.64%) | |
| Hemoglobin | median (IQR) | 11.8 (10.2–13.4) | 11.8 (10.3–13.3) | 11.9 (10.5–13.1) | 12 (10.7–13.2) | 11.9 (10.4–13.3) |
| missing | 82 (2.11%) | 101 (2.37%) | 57 (2.37%) | 21 (2.91%) | 261 (2.32%) | |
| Tuberculosis | no | 3260 (83.98%) | 3755 (88.19%) | 2127 (88.59%) | 667 (92.38%) | 9809 (87.09%) |
| yes | 622 (16.02%) | 503 (11.81%) | 274 (11.41%) | 55 (7.62%) | 1454 (12.91%) | |
| NRTI | tenofovir | 1184 (30.50%) | 1424 (33.44%) | 941 (39.19%) | 353 (48.89%) | 3902 (34.64%) |
| zidovudine | 83 (2.14%) | 95 (2.23%) | 62 (2.58%) | 28 (3.88%) | 268 (2.38%) | |
| stavudine | 2615 (67.36%) | 2739 (64.33%) | 1398 (58.23%) | 341 (47.23%) | 7093 (62.98%) | |
| NNRTI | efavirenz | 3686 (94.95%) | 3985 (93.59%) | 2232 (92.96%) | 685 (94.88%) | 10588 (94.01%) |
| nevirapine | 196 (5.05%) | 273 (6.41%) | 169 (7.04%) | 37 (5.12%) | 675 (5.99%) | |
| ART start date | < April 2010 | 2524 (65.02%) | 2690 (63.18%) | 1387 (57.77%) | 349 (48.34%) | 6950 (61.71%) |
| ≥ April 2010 | 1358 (34.98%) | 1568 (36.82%) | 1014 (42.23%) | 373 (51.66%) | 4313 (38.29%) | |
| Baseline BMI | median (IQR) | 19.1 (17.7–20.5) | 22.6 (20.7–24.2) | 26.7 (24.3–29.1) | 33.2 (30.0–36.3) | 22.0 (19.5–25.4) |
| Standard BMI Category at baseline | underweight | 1523 (39.23%) | 297 (6.98%) | 29 (1.21%) | 3 (0.42%) | 1852 (16.4%) |
| normal weight | 2342 (60.33%) | 3225 (75.74%) | 717 (29.86%) | 45 (6.23%) | 6329 (56.19%) | |
| overweight | 16 (0.41%) | 704 (16.53%) | 1214 (50.56%) | 133 (18.42%) | 2067 (18.35%) | |
| obese I | 0 (0.00%) | 30 (0.70%) | 409 (17.03%) | 286 (39.61%) | 725 (6.44%) | |
| obese II | 1 (0.03%) | 1 (0.02%) | 29 (1.21%) | 180 (24.93%) | 211 (1.87%) | |
| obese III | 0 (0.00%) | 1 (0.02%) | 3 (0.12%) | 75 (10.39%) | 79 (0.70%) | |
| Number of BMI Measurements | median (IQR) | 22 (15–30) | 22 (15–30) | 21 (15–30) | 20 (14–27) | 22 (15–30) |
| Total follow-up time* | median (IQR) | 96 (49.5–96.0) | 96 (49.0–96.0) | 96 (56.2–96.0) | 96 (59.6–96.0) | 96 (51.1–96.0) |
| Follow-up time for survival analysis** | median (IQR) | 96.0 (28.4–96.0) | 96.0 (30.5–96.0) | 96.0 (36.5–96.0) | 96.0 (38.2–96.0) | 96.0 (30.8–96.0) |
| Patient outcome | alive | 2254 (58.06%) | 2584 (60.69%) | 1558 (64.89%) | 485 (67.17%) | 6881 (61.09%) |
| deceased | 267 (6.88%) | 183 (4.30%) | 74 (3.08%) | 26 (3.60%) | 550 (4.88%) | |
| lost to follow-up | 508 (13.09%) | 470 (11.04%) | 220 (9.16%) | 54 (7.48%) | 1252 (11.12%) | |
| transferred out | 519 (13.37%) | 565 (13.27%) | 350 (14.58%) | 102 (14.13%) | 1536 (13.64%) | |
| down referred | 334 (8.60%) | 456 (10.71%) | 199 (8.29%) | 55 (7.62%) | 1044 (9.27%) | |
IQR=Interquartile Range; BMI=body mass index; ART= Antiretroviral Therapy; NNRTI=Non-Nucleoside Reverse Transcriptase Inhibitor
Total follow-up time= months between ART initiation and last available BMI measurement
Follow-up time for survival analysis= months between 10th BMI observation and last available BMI measurement. 10th observation cutoff used to avoid immortal person-time bias because only patients with 10+ BMI measurements were included in our study.
Growth curve model
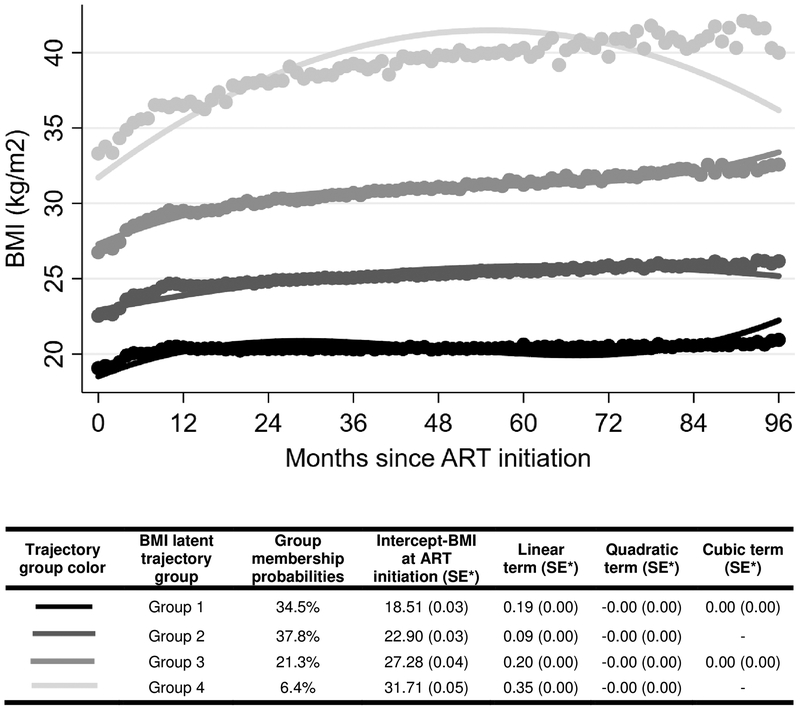
Four-group trajectory models were selected based on model fit statistics. The best fit of the four-group models involved two linear and two quadratic trajectories (Table 1 and Figure 1), with 34.5% patients categorized into group one (lowest baseline BMI, increasing), 37.8% into group two (second lowest baseline BMI, increasing), 21.3% into group three (second highest baseline BMI, increasing), and 6.4% into group four (highest baseline BMI, increasing). The average posterior probability of group membership was over 0.95 for all groups, suggesting the chosen model able to accurately categorize individuals with similar patterns of change. When stratified by the four BMI trajectory groups, there was a higher proportion of females in group two (61.3%), three (77.8%), and four (89.3%) than in group one (42.6%). Median baseline CD4 count increased as trajectory group increased, with a median CD4 count of 101.5 cells/mm3 (IQR: 40–177) in group one and 127 cells/mm3 (IQR: 52–201) for those in group four. Patients in group one (29.6%), two (27.5%), and three (27.5%) had a higher proportion of patients with CD4 counts <50 cells/mm3 than did group four (23.8%). Patients in group one had a higher proportion of patients with tuberculosis (16.0%).
Figure 1. Adult body mass trajectories after ART initiation at TLC 2004–2017. Based on 256,609 total BMI observations. (N=11,263).
*standard error
**dots on the graph are observed group means at each month.
Predictors of body mass index group membership
Males had slightly lower, odds than females of being a member of group two when compared to group one, but male gender was not a predictor of membership for group three or four. Increasing age decreased the odds of being in trajectory groups two, three, or four compared to group one, while increasing CD4 increased the odds of being in group two, three or four (i.e. the three highest BMI groups) compared to group one (Supplemental Online Content 1, Table 1).
Body mass index change over time on ART
Over the 8 years of follow-up, over 50% (n=5,736) of our population changed BMI categories: 6.2% (n=698) went down, while 44.7% (n=5,038) went up in BMI category. The scatterplot of BMI and time shows an even spread of the data and a positive relationship between months since ART initiation and BMI measured at that corresponding time point for all four BMI trajectory groups (see Supplemental Online Content 1, Figure 2).
Patients in group one started at a mean BMI of 19.1 kg/m2 (WHO normal weight) at ART initiation and remained in the WHO normal weight category with an increase to a mean BMI of 20.9 kg/m2; the mean BMI in group two shifted from 22.5 kg/m2 (WHO normal weight) to 26.2 kg/m2 (WHO overweight); the mean BMI in group three transitioned from 26.8 kg/m2 (WHO overweight) to 32.6 kg/m2 (WHO obesity class I); group four had the largest increase from an average BMI of 33.3 kg/m2 (WHO obesity class I) at the start of ART to 40.0 kg/m2 (WHO obesity class II) (Table 2).
Table 2.
Mean (SD**) body mass index (kg/m2) by group (N=11,263)
| ART Initiation | 1 year (12 months)* | 8 years (96 months)* | Last available | Change in BMI from ART to 1 year* | Change in BMI from 1 year to 8 years* | Change in BMI overall | |
|---|---|---|---|---|---|---|---|
| Group 1 | 19.06 (2.19) | 20.44 (1.92) | 20.92 (2.29) | 20.32 (2.23) | 1.41 (2.24) | 0.10 (2.11) | 1.26 (2.61) |
| Group 2 | 22.52 (2.77) | 24.54 (1.99) | 26.16 (2.41) | 25.48 (2.58) | 1.90 (2.98) | 1.09 (2.88) | 2.95 (3.77) |
| Group 3 | 26.76 (3.67) | 29.49 (2.69) | 32.63 (3.21) | 31.28 (3.05) | 2.59 (3.74) | 2.36 (4.63) | 4.52 (4.70) |
| Group 4 | 33.32 (5.77) | 36.48 (4.49) | 40.02 (4.88) | 39.46 (5.12) | 3.23 (4.63) | 3.86 (4.60) | 6.14 (6.27) |
| Total | 22.93 (4.98) | 25.01 (5.10) | 26.26 (5.93) | 25.83 (6.05) | 1.97 (3.13) | 1.15 (3.34) | 2.91 (4.13) |
Estimates are for those with available measurements at specified time points
Standard deviation
We also used transition matrices to assess patients shifting from BMI WHO category at ART initiation to last available BMI overall for the cohort (Table 3) and stratified by all four BMI trajectory groups (Supplemental Online Content 1, Tables 2–5) The majority of patients in group one were categorized as WHO normal weight at their last BMI measurement, while for the other three groups the majority of patients were overweight, obese I, obese II and obese III (Supplemental Online Content 1, Tables 3–5).
Table 3.
(A) Transition matrix for BMI at ART initiation and last available BMI for full sample (n=11,263).
| Underweight | Normal | Overweight | Obese I | Obese II | Obese III | Total | |
|---|---|---|---|---|---|---|---|
| Underweight | 527 (4.7%) |
1104 (9.8%) |
182 (1.6%) |
32 (0.3%) |
5 (0.0%) |
2 (0.0%) |
1852 (16.4%) |
| Normal | 233 (2.1%) |
3603 (32.0%) |
1876 (16.7%) |
482 (4.3%) |
112 (1.0%) |
23 (0.2%) |
6329 (56.2%) |
| Overweight | 8 (0.1%) |
255 (2.3%) |
935 (8.3%) |
632 (5.6%) |
189 (1.7%) |
48 (0.4%) |
2067 (18.4%) |
| Obese I | 1 (0.0%) |
22 (0.2%) |
109 (1.0%) |
321 (2.9%) |
195 (1.7%) |
77 (0.7%) |
725 (6.4%) |
| Obese II | - | 2 (0.0%) |
5 (0.0%) |
42 (0.4%) |
83 (0.7%) |
79 (0.7%) |
211 (1.9%) |
| Obese III | - | 1 (0.0%) |
1 (0.0%) |
4 (0.0%) |
15 (0.1%) |
58 (0.5%) |
79 (0.7%) |
| Total | 769 (6.8%) |
4987 (44.3%) |
3108 (27.6%) |
1513 (13.4%) |
599 (5.3%) |
287 (2.6%) |
N=11263 |
Survival analysis results
Overall, there were 1,802 attrition events (615 died, 1352 were lost to follow-up) in the cohort over 773,352 person-years. The incidence of attrition was 2.8 events per 100 person-years (95% CI, 2.7–2.9) (Table 4).
Table 4.
Crude and adjusted Cox proportional hazard models assessing the association between BMI trajectory groups and attrition (N=11,263)
| Variable | Number of events/person years | Incidence rate/100 person years (95% CI) | Crude hazard ratio (95% CI) | Adjusted* hazard ratio (95% CI) |
|---|---|---|---|---|
| Overall | 1802/64446 | 2.8 (2.7–2.9) | - | - |
| BMI trajectory groups | ||||
| Group 1 | 775/21534 | 3.60 (3.35–3.86) | 1.32 (1.19–1.47) | 1.22 (1.09–1.36) |
| Group 2 | 653/24255 | 2.69 (2.49–2.91) | Reference | Reference |
| Group 3 | 294/14293 | 2.06 (1.83–2.31) | 0.77 (0.67–0.89) | 0.85 (0.74–0.98) |
| Group 4 | 80/4364 | 1.83 (1.47–2.29) | 0.70 (0.55–0.88) | 0.85 (0.67–1.09) |
| Gender | ||||
| Female | 992/38653 | 2.57 (2.41–2.73) | Reference | Reference |
| Male | 810/25793 | 3.14 (2.93–3.36) | 1.23 (1.12–1.35) | 1.19 (1.07–1.31) |
| Age at ART initiation | ||||
| 18–24 | 95/2130 | 4.46 (3.65–5.45) | 1.62 (1.31–2.01) | 1.70 (1.37–2.11) |
| 25–29 | 272/7734 | 3.52 (3.12–3.96) | 1.30 (1.13–1.49) | 1.30 (1.13–1.50) |
| 30–39 | 766/28353 | 2.69 (2.51–2.89) | Reference | Reference |
| 40–49 | 460/18551 | 2.48 (2.26–2.72) | 0.93 (0.83–1.04) | 0.97 (0.86–1.09) |
| ≥50 | 209/7577 | 2.76 (2.41–3.16) | 1.04 (0.89–1.21) | 1.11 (0.95–1.29) |
| NRTI | ||||
| tenofovir | 328/26244 | 1.25 (1.12–1.39) | Reference | Reference |
| zidovudine | 41/1497 | 2.74 (2.02–3.72) | 2.08 (1.50–2.87) | 2.14 (1.54–2.97) |
| stavudine | 1433/36705 | 3.90 (3.71–4.11) | 2.85 (2.53–3.21) | 2.63 (2.32–2.98) |
| NNRTI | ||||
| efavirenz | 1690/60907 | 2.77 (2.65–2.91) | Reference | Reference |
| nevirapine | 112/3539 | 3.16 (2.63–3.81) | 1.12 (0.93–1.36) | 1.01 (0.84–1.23) |
| CD4 count at ART initiation (per 100 cells/μl) | ||||
| 0–49 | 556/17767 | 3.13 (2.88–3.40) | 1.97 (1.29–3.02) | 1.31 (0.83–2.05) |
| 50–99 | 362/11903 | 3.04 (2.74–3.37) | 1.92 (1.25–2.95) | 1.33 (0.84–2.09) |
| 100–199 | 663/20851 | 3.18 (2.95–3.43) | 2.00 (1.31–3.05) | 1.49 (0.95–2.33) |
| 200–349 | 199/12457 | 1.60 (1.39–1.84) | 1.05 (0.68–1.64) | 1.01 (0.64–1.61) |
| 350+ | 22/1467 | 1.50 (0.99–2.28) | Reference | Reference |
| WHO stage at ART initiation | ||||
| I/II | 1303/50631 | 2.57 (2.44–2.72) | Reference | Reference |
| III/IV | 499/13815 | 3.61 (3.31–3.94) | 1.37 (1.24–1.52) | 1.17 (1.04–1.30) |
| Hemoglobin at ART initiation (g/dL) | ||||
| <10 | 392/11941 | 3.28 (2.98–3.62) | 1.20 (1.07–1.34) | 1.06 (0.94–1.20) |
| ≥10 | 1375/50952 | 2.70 (2.56–2.85) | Reference | Reference |
| Change in BMI** | - | - | 0.92 (0.91–0.93) | 0.91 (0.89–0.92) |
CI=confidence interval; BMI=body mass index; NNRTI=Non-Nucleoside Reverse Transcriptase Inhibitor; WHO=World Health Organization
All predictors are measured at ART initiation. Model adjusted for gender, BMI, age, NRTI, NNRTI, WHO stage, CD4 count at ART initiation, hemoglobin levels at ART initiation.
HR shows the change in hazard for every one kg/m2 increase in BMI from ART initiation and last available measurement. Adjusted model includes gender, BMI, age, NRTI, NNRTI, WHO stage, CD4 count at ART initiation, hemoglobin levels at ART initiation. Adjusted model does not include BMI trajectory group due to collinearity with change in BMI variable.
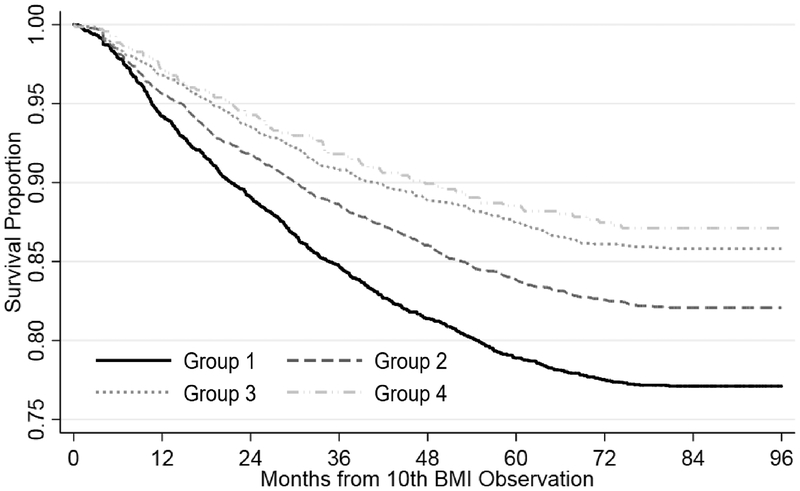
The probability of survival was 0.77 (95% CI: 0.76–0.79) in group one, 0.82 (95% CI: 0.81–0.83) in group two, 0.86 (95% CI: 0.84–0.87) in group three, and 0.87 (95% CI: 0.84–0.89) in group four (Figure 2). Patients in group one had a 22% increased hazards of attrition (hazard ratio (HR): 1.22; 95% CI: 1.09–1.36) over the follow-up period compared to those in group two, while group three and group four had approximately a 15% decreased hazards when compared to group two (Table 4).
Figure 2. Kaplan Meier curve displaying attrition stratified by BMI trajectory group, Themba Lethu Clinic, Johannesburg, South Africa (N=11,263).
As patients were not eligible unless they had at least 10 BMI measurements, person-time was calculated from the individual’s 10th BMI observation to either: 1) attrition (death and loss to follow-up), 2) transfer, or 3) close of the dataset (December 2015), whichever occurred first.
Looking at change in BMI (between ART initiation and last available measurement) instead of BMI trajectory group, we observe that every 1 kg/m2 increase in BMI is associated with 9% reduction in hazards of attrition (0.89–0.92). Additionally, males vs. females, patients <30 years of age vs. 30–39 years, those on stavudine or zidovudine vs. tenofovir, and patients with poorer health status at ART initiation (i.e. CD4 counts <200 cells/mm3 and WHO stage) had an increase in the hazards of attrition over the follow-up period.
DISCUSSION
We identified four BMI trajectory groups in a large cohort of HIV-positive adults on ART over an eight year observational period. The majority (38%) of patients in our cohort were categorized into BMI group two (second lowest baseline BMI), while 35% fell into group one (lowest BMI), 21% fell into group three (second highest BMI), and 6% fell into group four (highest BMI).
Although wasting was common early in the HIV epidemic, it has become less common with the introduction of ART in both high-income and LMICs[39–42]. With the exception of HIV-associated lipoatrophy linked to certain types of antiretroviral medications[43], there has been a large improvement in the health of HIV positive patients initiating ART, leading to a decline in wasting and an increase in obesity as HIV patients are living longer and gaining weight at rates similar to the general population[42, 44]. In our study, close to 30% of patients were categorized as overweight or obese at ART initiation, consistent with previous research on black African populations[45].
We found that increasing CD4 count increased the odds of being in BMI trajectory group two, three, or four compared to group one. Our results are comparable with previous research reporting that overweight and obese individuals have higher CD4 counts than their normal or underweight counterparts[46–48]. However, previous longitudinal studies assessing this association revealed contradicting results, with some studies reporting comparable immunological status between patients categorized as normal, overweight, and obese[49] and one study suggesting that obese individuals had significantly smaller increases in CD4 after initiation of ART compared to normal weight individuals[50]. One plausible explanation for our findings is that leptin, an adipocyte-derived hormone that influences body weight[51], may be involved in the pathway of overweight and obesity to immune functioning in HIV patients. Additionally, increased levels of leptin have been found to be related to improved immunological health[52]. An alternatively explanation could be related to a patients nadir CD4 and a patients time on ART, neither of which were assessed in our study. If normal weight patients were more immunosuppressed when they initiated ART, their ability to control viral replication and to restore immune function could have been compromised, limiting their ability to achieve higher CD4 counts compared to patients who started treatment at higher CD4 counts[46].
We found that patients in trajectory group one, which has the lowest mean BMI at both baseline and after 8 years of follow-up, had increased hazards of attrition when compared to those in group two, while those in group three and group four had decreased the hazards of attrition. This could be due to the fact that 8 years of follow-up may not be long enough for obesity-related illness to lead to mortality/attrition. We could see the association between being overweight and obese and attrition decline as time on ART increases and patients in our population in the higher BMI categories begin to develop other NCDs, ultimately increasing their risk of death and loss from care. A recent meta-analysis containing data from nearly four million subjects showed a U-shaped relationship between BMI and mortality, with mortality increasing as soon as BMI exceeded 25 kg/m2, supporting this notion[53].
This observed association could also be explained by what is known as the “obesity paradox”, which suggests that being overweight or obese can be protective and associated with greater survival in patients with certain chronic diseases, like HIV[54]. Improved outcomes have been observed in overweight and obese patients with type 2 diabetes, chronic kidney disease, chronic heart failure, chronic obstructive pulmonary disease, and coronary artery disease compared with normal-weight and underweight counterparts[55–59]. As such, patients with chronic disease who are able to maintain weight may be healthier, while those losing weight may suffer from more advanced disease markers (e.g., underlying inflammation, increased cytokines)[60].
Alternative explanations could be misclassification bias[61,62], reverse causation[63], or a form of selection bias known as collider stratification bias[64]. First, the use of BMI may not be an as accurate a marker of body fat as waist circumference measurement, resulting in misclassification bias in our study[61,62]. Adjusting for waist circumference, if available, could have nullified or reversed the observed association between higher BMI trajectories and attrition in our study. Second, reverse causation could have underestimated the risk of mortality amongst patients that are classified as overweight or obesity in our cohort. The majority of HIV patients lose weight prior to death due to end stage consequences of dying. Thus, being overweight or obese would appear protective because impending death process is leading to a reduction in weight not that heavy weight in and of itself is protective[63]. Third, collider bias can result in the distortion of an association between BMI and death by conditioning on a common cause of weight change and death, such as HIV disease progression[64]. In our study advanced HIV disease could be a much stronger risk factor for death than being overweight or obese, resulting in higher BMI appearing protective because its presence indicates the absence of a more harmful risk factor[64].
Our findings should be considered alongside other potential study limitations. First, because our study reports data from large public sector government HIV clinics, our results may not be generalizable to the overall population. Second, lack of documentation of weight and height measurements could be causing us to incorrectly estimate the proportion of patients falling into the four separate BMI trajectory groups; however, we would anticipate the missingness to be random and not have a large impact on our estimates. Third, as our BMI trajectories are among HIV-positive patients that survive to initiate ART and have at least ten BMI measurements after treatment initiation, survivor bias could be an issue in our study. This could have resulted in a bias downwards in our estimate of the proportion of patients in BMI trajectory group one in addition to biasing our estimates of attrition in BMI trajectory groups three and four towards the null. Fifth, because the trajectory model uses logistic regression to investigate the association between risk factors and group membership, we may overestimate the strength of these associations. Finally, as we do not have access to an HIV-negative population for comparison we are unable to assess any association between HIV status and BMI trajectory.
Conclusion
The largest gain in BMI in HIV patients on ART in our cohort occurred in the first 12 months on ART. Over the 8 years of follow-up, over 50% of our population changed BMI categories: 6.2% went down in BMI category, while 44.7% went up in BMI category, putting them at increased risk for life threatening non-communicable chronic diseases. We also found that patients in group 3 and 4, which generally consist of those with higher BMIs, had a decrease in the hazards of attrition compared to patients with normal BMI. Further research in cohorts with potentially longer periods of follow-up is need to determine if this association between BMI and attrition is valid. Additionally, consistent counselling on nutritional and lifestyle changes could help improve ART patients long term health outcomes.
Supplementary Material
ACKNOWLEDGEMENTS
We express our gratitude to Right to Care, the nongovernmental organization supporting the study site through a partnership with the United States Agency for International Development, and to the staff of the participating clinics. We also thank the provincial and national Departments of Health for providing for the care of the patients under the Comprehensive Care Management and Treatment program and the HIV/AIDS conditional grant. Most of all we thank the patients attending the clinic for their continued trust in the treatment provided at the clinic.
Conflicts of Interest and Source of Funding: This study has been made possible by the generous support of the American People and the President’s Emergency Plan for AIDS Relief (PEPFAR) through USAID under the terms of Cooperative Agreements AID-674-A-12-00029 and 72067419CA00004 to HE2RO and NIH (5T32AI052074-13). The contents are the responsibility of the authors and do not necessarily reflect the views of PEPFAR, USAID, NIH or the United States Government.
Footnotes
Conflicts of Interest and Source of Funding
NIH (5T32AI052074-13) and USAID (AID-674-A-12-00029; 72067419CA00004) provided funding. The authors have no remaining conflicts of interest to declare.
LIST OF SUPPLEMENTAL DIGITAL CONTENT
Supplemental Digital Content 1.pdf
REFERENCES
- 1.World Health Organization. Obesity and overweight. Available at http://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight. Accessed December 2018. [Google Scholar]
- 2.Pisa PT, Pisa NM. Economic growth and obesity in South African adults: an ecological analysis between 1994 and 2014. Eur J Public Health. 2017. June 1;27(3):404–409. [DOI] [PubMed] [Google Scholar]
- 3.GBD 2015 Risk Factors Collaborators. Global, regional, and national comparative risk assessment of 79 behavioural, environmental and occupational, and metabolic risks or clusters of risks, 1990–2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet. 2016. October 8;388(10053):1659–1724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Todes A, Kok P, Wentzel M, Van Zyl J, Cross C. Contemporary South African urbanization dynamics. Urban forum. 2010;21(3):331–48. [Google Scholar]
- 5.Peltzer K. Health behavior and protective factors among school children in four African countries. Int J Behav Med. 2009;16(2):172–80. [DOI] [PubMed] [Google Scholar]
- 6.Peltzer K. Tobacco use trends among adolescents and adults in South Africa. J Psychol Afr. 2008;18(2):339–45. [Google Scholar]
- 7.Steyn Krisela, Fourie Jean, Temple Norman (eds). Chronic Diseases of Lifestyle in South Africa: 1995 – 2005. Technical Report. Cape Town: South African Medical Research Council, 2006. Available at: http://www.mrc.ac.za/sites/default/files/files/2016-07-14/cdl1995-2005.pdf. Accessed December 2018. [Google Scholar]
- 8.Ardington C, Case A. Health: analysis of the NIDS wave 1 dataset. Discussion paper no. 2. Cape Town: Southern Africa Labour and Development Research Unit; 2009. Available at: http://www.nids.uct.ac.za/publications/discussion-papers/wave-1-papers/93-nids-discussion-paper-no02/file. Accessed December 2018. [Google Scholar]
- 9.National Department of Health. Strategic plan for the prevention and control of non-communicable diseases 2013–17. Pretoria: National Department of Health; 2013. Available at: http://www.hsrc.ac.za/uploads/pageContent/3893/NCDs%20STRAT%20PLAN%20%20CONTENT%208%20april%20proof.pdf. Accessed December 2018. [Google Scholar]
- 10.Massyn N, Day C, Peer N, Padarath A, Barron P, English R, editors.) District Health Barometer 2013/14. Durban, South Africa: Health Systems Trust; 2014. [Google Scholar]
- 11.Cois A, Day C. Obesity trends and risk factors in the South African adult population. BMC Obes. 2015. October 13;2:42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Freiberg MS, Chang CC, Kuller LH, Skanderson M, Lowy E, Kraemer KL, et al. HIV infection and the risk of acute myocardial infarction. JAMA Intern Med. 2013. April 22;173(8):614–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brown TT, Chu H, Wang Z, et al. Longitudinal increases in waist circumference are associated with HIV-serostatus, independent of antiretroviral therapy. AIDS 2007; 21:1731–8. [DOI] [PubMed] [Google Scholar]
- 14.Feinstein MJ, Bogorodskaya M, Bloomfield GS, Vedanthan R, Siedner MJ, Kwan GF, et al. Cardiovascular Complications of HIV in Endemic Countries. Curr Cardiol Rep. 2016. November;18(11):113 Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Aberg JA. Cardiovascular complications in HIV management: past, present, and future. J Acquir Immune Defic Syndr. 2009. January 1;50(1):54–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bernasconi E, Boubaker K, Junghans C, et al. Abnormalities of body fat distribution in HIV-infected persons treated with antiretroviral drugs: The Swiss HIV Cohort Study. J Acquir Immune Defic Syndr 2002; 31:50–5. [DOI] [PubMed] [Google Scholar]
- 17.Nguyen A, Calmy A, Schiffer V, et al. Lipodystrophy and weight changes: data from the Swiss HIV Cohort Study, 2000–2006. HIV Med 2008; 9:142–50. [DOI] [PubMed] [Google Scholar]
- 18.Crum-Cianflone N, Tejidor R, Medina S, et al. Obesity among patients with HIV: the latest epidemic. AIDS Patient Care STDS 2008; 22 (12):925–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Falutz J. HIV infection, body composition changes and related metabolic complications: contributing factors and evolving management strategies. Curr Opin Clin Nutr Metab Care 2011; 14:255–60. [DOI] [PubMed] [Google Scholar]
- 20.Flegal KM, Carroll MD, Kit BK, Ogden CL. Prevalence of obesity and trends in the distribution of body mass index among US adults, 1999–2010. JAMA 2012; 307:491–7. [DOI] [PubMed] [Google Scholar]
- 21.Huis In ‘t Veld D, Pengpid S, Colebunders R, Peltzer K. Body Mass Index and Waist Circumference in Patients with HIV in South Africa and Associated Socio-demographic, Health Related and Psychosocial Factors. AIDS Behav. 2018. June;22(6):1972–1986. [DOI] [PubMed] [Google Scholar]
- 22.Hurley E, Coutsoudis A, Giddy J, Knight SE, Loots E, Esterhuizen TM. Weight evolution and perceptions of adults living with HIV following initiation of antiretroviral therapy in a South African urban setting. S Afr Med J. 2011. September 5;101(9):645–50. [PubMed] [Google Scholar]
- 23.Hall V, Thomsen RW, Henriksen O, Lohse N. Diabetes in Sub Saharan Africa 1999–2011: epidemiology and public health implications. A systematic review. BMC Public Health. 2011. July 14;11:564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bradshaw D, Groenewald P, Laubscher R, Nannan N, Nojilana B, Norman R, et al. Initial burden of disease estimates for South Africa, 2000. S Afr Med J. 2003. September;93(9):682–8. [PubMed] [Google Scholar]
- 25.Losina E, Hyle EP, Borre ED, Linas BP, Sax PE, Weinstein MC, et al. Projecting 10-year, 20-year, and Lifetime Risks of Cardiovascular Disease in Persons Living With Human Immunodeficiency Virus in the United States. Clin Infect Dis. 2017. October 15;65(8):1266–1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Warriner AH, Burkholder GA, Overton ET. HIV-related metabolic comorbidities in the current ART era. Infect Dis Clin North Am. 2014. September;28(3):457–76. [DOI] [PubMed] [Google Scholar]
- 27.Fox MP, Maskew M, MacPhail AP, Long L, Brennan AT, Westreich D, MacLeod WB, Majuba P, Sanne IM. Cohort profile: the Themba Lethu Clinical Cohort, Johannesburg, South Africa. Int J Epidemiol. 2013. April;42(2):430–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.National Department of Health, Republic of South Africa (2004). The South African Antiretroviral Treatment Guidelines. Available at: http://southafrica.usembassy.gov/media/2004-doh-art-guidelines.pdf. Accessed December 2018.
- 29.National Department of Health, Republic of South Africa (2010). The South African Antiretroviral Treatment Guidelines. Available at: http://apps.who.int/medicinedocs/documents/s19153en/s19153en.pdf. Accessed December 2018.
- 30.National Department of Health, Republic of South Africa (2013). The South African Antiretroviral Treatment Guidelines. Available at: http://www.auruminstitute.org/phocadownload/guidelines-short.pdf. Accessed December 2018.
- 31.Pillay Y. Implementation of the Universal Test and Treat Strategy for HIV-positive patients and differentiated care for stable patients. August 2016. Available at: http://www.sahivsoc.org/Files/22%208%2016%20Circular%20UTT%20%20%20Decongestion%20CCMT%20Directorate%20(2).pdf. Accessed December 2018.
- 32.Garenne M, Collinson MA, Kabudula CW, Gómez-Olivé FX, Kahn K, Tollman S. Glob Health Action. Completeness of birth and death registration in a rural area of South Africa: the Agincourt health and demographic surveillance, 1992–2014 2016; 9: 10.3402/gha.v9.32795. Published online 2016 Oct 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Timaeus I, Dorrington R, Bradshaw D, and Nannan N (2002) Mortality trends in South Africa 1985–2000: From apartheid to AIDS. Cape Town, South African Medical Research Council. [Google Scholar]
- 34.World Health Organization. Body mass index – BMI. Available at: http://www.euro.who.int/en/health-topics/disease-prevention/nutrition/a-healthy-lifestyle/body-mass-index-bmi. Accessed December 2018. [Google Scholar]
- 35.Andruff H, Carraro N, Thompson A, Gaudreau P. Latent class growth modelling: A tutorial. Tutor Quant Methods Psychol. 2009;5(1):11–24. doi: 10.20982/tqmp.05.1.p011. [DOI] [Google Scholar]
- 36.Arrandale V, Koehoorn M, Macnab Y, et al. How to use SAS ® Proc Traj and SAS ® Proc Glimmix in Respiratory Epidemiology. 2006;24(3). doi: 10.1007/s10940-010-9113-7. [DOI] [Google Scholar]
- 37.Jones BL, Nagin DS. A Note on a Stata Plugin for Estimating Group-based Trajectory Models. Sociol Methods Res. 2013;42(4):608–613. doi: 10.1177/0049124113503141. [DOI] [Google Scholar]
- 38.Jones BL, Nagin DS, Roeder K. A SAS procedure based on mixture models for estimating developmental trajectories. Sociol Methods Res. 2001;29(3):374–393. [Google Scholar]
- 39.Hasse B, Iff M, Ledergerber B, et al. Obesity Trends and Body Mass Index Changes After Starting Antiretroviral Treatment: The Swiss HIV Cohort Study. Open Forum Infect Dis. 2014. July 1;1(2):ofu040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Smit E, Skolasky RL, Dobs AS, et al. Changes in the incidence and predictors of wasting syndrome related to human immunodeficiency virus infection, 1987–1999. Am J Epidemiol. 2002;156:211–218. [DOI] [PubMed] [Google Scholar]
- 41.Mocroft A, Sabin CA, Youle M, et al. Changes in AIDS-defining illnesses in a London clinic, 1987 1998. J Acquir Immune Defic Syndr. 1999;21:401–417. [PubMed] [Google Scholar]
- 42.Crum-Cianflone N, Roediger MP, Eberly L, Headd M, Marconi V, Ganesan A, Weintrob A, Barthel RV, Fraser S, Agan BK. Increasing rates of obesity among HIV-infected persons during the HIV epidemic. Infectious Disease Clinical Research Program HIV Working Group. PLoS One. 2010. April 9; 5(4):e10106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Treatment options for HIV-associated central fat accumulation. Cofrancesco J Jr, Freedland E, McComsey G. AIDS Patient Care STDS. 2009. January; 23(1):5–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Boodram B, Plankey MW, Cox C, et al. Prevalence and correlates of elevated body mass index among HIV-positive and HIV-negative women in the Women’s Interagency HIV Study, AIDS Patient Care STDS, 2009, vol. 23 (pg. 1009–16). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Guehi C, Badjé A, Gabillard D, Ouattara E, Koulé SO, Moh R, et al. High prevalence of being Overweight and Obese HIV-infected persons, before and after 24 months on early ART in the ANRS 12136 Temprano Trial. AIDS Res Ther. 2016. February 25;13:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Blashill AJ, Mayer KH, Crane HM, Grasso C, Safren SA. Body mass index, immune status, and virological control in HIV-infected men who have sex with men. J Int Assoc Provid AIDS Care. 2013. Sep-Oct;12(5):319–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shuter J, Chang CJ, Klein RS. Prevalence and predictive value of overweight in an urban HIV care clinic. J Acquir Immune Defic Syndr. 2001. March 1;26(3):291–7. [DOI] [PubMed] [Google Scholar]
- 48.Jones CY, Hogan JW, Snyder B, Klein RS, Rompalo A, Schuman P, Carpenter CC; HIV Epidemiology Research Study Group. Overweight and human immunodeficiency virus (HIV) progression in women: associations HIV disease progression and changes in body mass index in women in the HIV epidemiology research study cohort. Clin Infect Dis. 2003;37 Suppl 2:S69–80. [DOI] [PubMed] [Google Scholar]
- 49.Tedaldi EM, Brooks JT, Weidle PJ, Richardson JT, Baker RK, Buchacz K, et al. HOPS Investigators. Increased body mass index does not alter response to initial highly active antiretroviral therapy in HIV-1-infected patients. J Acquir Immune Defic Syndr. 2006. September;43(1):35–41. [DOI] [PubMed] [Google Scholar]
- 50.Crum-Cianflone NF, Roediger M, Eberly LE, Ganesan A, Weintrob A, Johnson E, et al. Infectious Disease Clinical Research Program HIV Working Group. Impact of weight on immune cell counts among HIV-infected persons. Clin Vaccine Immunol. 2011. June;18(6):940–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mantzoros CS. The role of leptin in human obesity and disease: a review of current evidence. Ann Intern Med. 1999. April 20;130(8):671–80. [DOI] [PubMed] [Google Scholar]
- 52.Kim SY, Lim JH, Choi SW, Kim M, Kim ST, Kim MS, et al. Preferential effects of leptin on CD4 T cells in central and peripheral immune system are critically linked to the expression of leptin receptor. Biochem Biophys Res Commun. 2010. April 9;394(3):562–8. [DOI] [PubMed] [Google Scholar]
- 53.Global BMI Mortality Collaboration, Di Angelantonio E, et al. Body-mass index and all-cause mortality: individual-participant-data meta-analysis of 239 prospective studies in four continents. Lancet. 2016. August 20;388(10046):776–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hainer V, Aldhoon-Hainerová I. Obesity paradox does exist. Diabetes Care. 2013. August;36 Suppl 2:S276–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Fleischmann E, Teal N, Dudley J, May W, Bower JD, Salahudeen AK. Influence of excess weight on mortality and hospital stay in 1,346 hemodialysis patients. Kidney Int 1999; 55:1560–7. [DOI] [PubMed] [Google Scholar]
- 56.Curtis JP, Selter JG, Wang Y, et al. The obesity paradox: body mass index and outcomes in patients with heart failure. Arch Intern Med 2005; 165:55–61. [DOI] [PubMed] [Google Scholar]
- 57.Fonarow GC, Srikanthan P, Costanzo MR, Cintron GB, Lopatin M. An obesity paradox in acute heart failure: analysis of body mass index and inhospital mortality for 108,927 patients in the Acute Decompensated Heart Failure National Registry. Am Heart J 2007; 153:74–81. [DOI] [PubMed] [Google Scholar]
- 58.Gruberg L, Weissman NJ, Waksman R, et al. The impact of obesity on the short-term and long-term outcomes after percutaneous coronary intervention: the obesity paradox? J Am Coll Cardiol 2002; 39:578–84. [DOI] [PubMed] [Google Scholar]
- 59.Uretsky S, Messerli FH, Bangalore S, et al. Obesity paradox in patients with hypertension and coronary artery disease. Am J Med 2007; 120:863–70. [DOI] [PubMed] [Google Scholar]
- 60.Yuh B, Tate J, Butt AA, Crothers K, Freiberg M, Leaf D, Logeais M, Rimland D, Rodriguez-Barradas MC, Ruser C, Justice AC. Weight change after antiretroviral therapy and mortality. Clin Infect Dis. 2015. June 15;60(12):1852–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wells JCK. Commentary: the paradox of body mass index in obesity assessment: not a good index of adiposity, but not a bad index of cardio-metabolic risk. Int J Epidemiol 2014; 43: 672–674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lennon H, Sperrin M, Badrick E, Renehan AG. The obesity paradox in cancer: a review. Curr Oncol Rep 2016; 18: 56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Preston SH, Stokes A. Obesity paradox: conditioning on disease enhances biases in estimating the mortality risks of obesity. Epidemiology 2014; 25: 454–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hernán MA, Hernández-Díaz S, Robins JM. A structural approach to selection bias. Epidemiology 2004; 15: 615–625. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.