Abstract
Inclusive fitness theory predicts that parental care will vary with relatedness between potentially caring parents and offspring, potentially shaping mating system evolution. Systems with extra-pair paternity (EPP), and hence variable parent–brood relatedness, provide valuable opportunities to test this prediction. However, existing theoretical and empirical studies assume that a focal male is either an offspring's father with no inbreeding, or is completely unrelated. We highlight that this simple dichotomy does not hold given reproductive interactions among relatives, complicating the effect of EPP on parent–brood relatedness yet providing new opportunities to test inclusive fitness theory. Accordingly, we tested hierarchical hypotheses relating parental feeding rate to parent–brood relatedness, parent kinship and inbreeding, using song sparrows (Melospiza melodia) experiencing natural variation in relatedness. As predicted, male and female feeding rates increased with relatedness to a dependent brood, even controlling for brood size. Male feeding rate tended to decrease as paternity loss increased, and increased with increasing kinship and hence inbreeding between socially paired mates. We thereby demonstrate that variation in a key component of parental care concurs with subtle predictions from inclusive fitness theory. We additionally highlight that such effects can depend on the underlying social mating system, potentially generating status-specific costs of extra-pair reproduction.
Keywords: extra-pair paternity, inbreeding, inclusive fitness, kinship, parental care, parent–offspring relatedness
1. Introduction
A central ambition in evolutionary ecology is to understand how ‘altruistic' behaviours, which cost actors but benefit recipients, evolve as functions of interactions among relatives [1–4]. Parental care provided to dependent offspring represents one critically important altruistic behaviour that simultaneously emerges from, and can shape ongoing evolution of, complex reproductive strategies and mating systems. Variable parental care, therefore, provides one long-standing focus for developing and testing inclusive fitness theory [5].
Parental or alloparental care is typically predicted to increase with a focal adult's relatedness to dependent offspring, following the basic principle of Hamilton's rule [1,3,4,6]. Systems where relatedness between potentially caring adults and dependent offspring varies among family groups offer interesting opportunities to test this prediction, and to examine the degree to which adaptive plastic responses in parental care can arise and potentially shape mating system evolution. Such variation in adult–offspring relatedness is commonplace in socially monogamous systems with variable extra-pair paternity (EPP) [7–11]. Here, potentially caring males might not sire all offspring produced by their socially paired female [12]. All else being equal, paternal care is then predicted to increase with a male's paternity success and resulting male–brood relatedness, defined as the total number of copies of an allele that is present in focal male i that is expected to be present in the brood (hereafter ‘total allelic value', TAVi) [5,12–15]. Decreased paternal care following paternity loss can then create a cost of female extra-pair reproduction that could be sufficient to constrain the evolution of underlying polyandry [16]. Systems characterized by social monogamy, biparental care and EPP are consequently interesting systems where evolutionary dynamics of parental care and mating system are directly intertwined, attracting substantial theory development [13,14,17–19] and empirical tests [6,20–24].
Yet existing theoretical and empirical studies typically assume that the relatedness between a potentially caring male and a dependent offspring is either ½ or 0, meaning the male is either the offspring's father with no inbreeding, or is completely unrelated [13,14,19,25,26]. The male's total ‘relatedness' to a dependent brood, or TAVi, is then simply ½BS • PWPO where BS is brood size and PWPO is the proportion of the brood that are genetic offspring of the focal male (i.e. within-pair offspring, WPO). This expression reduces to ½NWPO, where NWPO is the number of WPO [12]. Similarly, the decrease in TAVi to a potentially caring male resulting from EPP is simply ½BS • PEPO, where PEPO is the proportion of the brood that are extra-pair offspring (EPO, hence PEPO = 1 − PWPO). However, these basic premises may not hold in reality, complicating the effect of EPP on parent–brood relatedness and associated optimal allocations of parental care.
Specifically, many populations and mating systems foster reproductive interactions among multiple relatives, including active or passive inbreeding and different forms of kin-structured reproductive groups or neighbourhoods [9–11,27,28]. Such systems can generate more subtle forms of variation in adult–offspring relatedness than a simple ‘parent or not’ dichotomy. Specifically, a focal male i that fails to sire an offspring of his socially paired female j could still be related to that EPO, and hence accrue some inclusive fitness benefit of paternal care, if he is related to the EPO's mother (i.e. his socially paired female) by coefficient of kinship kij > 0, and/or to the EPO's genetic father (i.e. his socially paired female's extra-pair mate q) by kiq > 0 [12,29] (electronic supplementary material, appendix S1). Quantitatively, a male's relatedness to an EPO that he did not sire but could rear is riEPO = kij + kiq [12]. Furthermore, the general expressions for relatedness between a focal male and female and their WPO are riWPO = ½ + kij + ½fi and rjWPO = ½ + kij + ½fj, respectively, where fi and fj are these parents' own coefficients of inbreeding [12,30] (electronic supplementary material, appendix S1). Similarly, a female's relatedness to its EPO is rjEPO = ½ + kjq + ½fj, where kjq is the coefficient of kinship between j and q. These expressions show that a focal parent can be considerably more closely related to its offspring than the basic value of ½ when it is related to its mate (kij > 0 or kjq > 0) and/or is inbred itself (fi > 0 or fj > 0), and hence given inbreeding in the current and/or previous generation [12,31–33]. Consequently, the total relatedness between a potentially caring male and a focal dependent brood, TAVi, is most generally calculated as the sum of riWPO or riEPO across all WPO and EPO within the brood, respectively, whereas TAVj for a female is the sum of rjWPO and rjEPO across all these offspring [12] (electronic supplementary material, appendix S1). TAV for a focal brood can, therefore, differ between paired males and females, and can substantially exceed the typically assumed basic values of ½NWPO and ½BS, respectively [12]. Furthermore, because a potentially caring male's relatedness to an EPO may not be zero, the decrease in TAVi resulting from EPP no longer simply equals ½BS • PEPO. Rather, this difference (hereafter ‘lost allelic value', LAV) can be calculated as LAV = PAV − TAVi, where PAV is the ‘potential allelic value' of the brood to the male if he had sired the entire brood (electronic supplementary material, appendix S1). Subtle patterns of adaptive variation in the degree of parental care might then be predicted, such that paternal care might increase more tightly with increasing TAV than with BS, and decrease with increasing LAV (and PEPO, table 1), reflecting the fundamental premise that care should be adjusted in proportion to relatedness to dependent offspring. Such subtle modulation of parental care might then further affect mating system dynamics emerging among interacting relatives [13,14,18,19].
Table 1.
Summary of key focal variables and predictions based on underlying kin selection and inclusive fitness theory. Subscripts i and j refer to a socially paired male and female respectively, and q refers to the female's extra-pair mate. Individuals i and j could produce within-pair offspring (WPO), while individuals j and q could produce extra-pair offspring (EPO) through extra-pair paternity (EPP). Full details of metric calculations are in electronic supplementary material, appendix S1.
| hypothesis set | focal variables | predicted response by males | predicted response by females |
|---|---|---|---|
| 1A | brood total allelic value (TAV) | paternal feeding rate will increase with increasing TAV more tightly than with increasing BS | maternal feeding rate will increase with increasing TAV more tightly than with increasing BS |
| 1B | brood total allelic value (TAV) controlling for brood size (BS) | paternal feeding rate will increase with increasing TAV after controlling for BS | maternal feeding rate will increase with increasing TAV after controlling for BS |
| 2 | lost allelic value (LAV) and paternity loss (PEPO) | paternal feeding rate will decrease with increasing LAV and PEPO | maternal feeding rate will not vary directly with LAV or PEPO |
| 3A | coefficient of kinship between mates (kij) | paternal feeding rate will increase with increasing kij | maternal feeding rate will increase with increasing kij |
| 3B | individual's own coefficient of inbreeding (f) | paternal feeding rate will not vary with fi | maternal feeding rate will not vary with fj |
Additionally, recent advances in inclusive fitness theory predict that the kinship kij between paired parents will directly influence optimal parental investment [31]. Specifically, if parental care, which forms a component of parental investment, can ameliorate inbreeding depression in offspring viability, then the optimal degree of care is predicted to increase with increasing kij [31]. By contrast, under these circumstances, optimal care is not predicted to vary directly and adaptively with fi or fj [31], but could potentially show inbreeding depression if inbred parents are resource-constrained. Consequently, kij and fi or fj, which constitute the fundamental underlying elements that determine parent–offspring relatedness and hence shape TAV and LAV, are predicted to have different direct effects on parental care [31]. However, the resulting suite of predictions regarding variation in parental care in relation to TAV, LAV, kij, fi and fj (table 1) has not been tested in any system.
We recorded rates at which adults provisioned broods of dependent offspring (hereafter ‘feeding rates’) as a measure of parental care in a song sparrow (Melospiza melodia) population where BS, EPP, kij, fi and fj and hence TAV and LAV vary substantially among individuals and breeding attempts [12,31], and tested three sets of hypotheses and associated predictions (table 1). First, we tested whether female and male feeding rates increased with increasing total relatedness to their brood, measured as TAVi or TAVj. Since TAV is intrinsically positively correlated with BS overall but can vary within levels of BS (electronic supplementary material, appendix S1 and S4), we further tested whether feeding rates increased with TAV after controlling for BS. Second, we tested whether male feeding rates decreased with increasing LAV (or PEPO), and hence with the value of offspring lost though EPP. Third, we focussed on the fundamental underlying elements and tested whether male and female feeding rates increased with increasing kij but not with increasing fi or fj as predicted by inclusive fitness theory [31]. While the focal song sparrows are typically socially monogamous, some are socially polygynous (i.e. one male simultaneously socially paired with ≥ 2 females), and paternal care can be differentially allocated to offspring of different females [5,34–36]. We therefore additionally tested whether parental feeding rate varied with social status, and whether TAV, LAV, kij, fi and fj interacted with social status to shape patterns of parental care arising given complex reproductive interactions among relatives.
2. Methods
(a). Study system
Testing the focal predictions (table 1) requires quantifying the degree of parental care expressed across family groups comprising social parents and WPO and/or EPO with known parental kij, kiq, kjq, fi and fj. These data are available from a resident, pedigreed population of song sparrows on Mandarte Island, British Columbia, Canada [37].
On Mandarte, both song sparrow sexes can breed from age one year, and pairs typically rear 2–3 broods of 1–4 nestlings during April–July each year. Each year since 1975, all territories were mapped, all nests were monitored, and all nestlings and any immigrants were uniquely colour-ringed [35,36,38]. The socially paired adults attending each nest were identified and sexes were attributed from observed reproductive behaviour (male song, female incubation), allowing identification of socially monogamous and polygynous breeding pairs [35,36,38]. Genetic parentage analyses demonstrated 28% EPP (affecting 44% of broods), but no extra-pair maternity [39] (electronic supplementary material, appendix S2). Mandarte is part of a large meta-population [38] and the small local population size (mean 33.5 adult females, range 4–72), plus occasional immigrants (mean approx. 0.9/year) generates substantial variation in k and f [12].
(b). Parental feeding rates
As a measure of parental care, we recorded parental feeding rates defined as the number of provisioning visits made to a focal nest per hour by each socially paired parent (electronic supplementary material, appendix S2). The dataset totalled 337 1-hour observation ‘sessions' spanning the 12-day nestling period at 138 different nests (38, 46 and 44 in 2003, 2007 and 2008, respectively), with a median of 2 sessions per nest (range: 1–7). Nests attended by socially monogamous pairings were defined as ‘monogamous’ (n = 79). We defined each polygynous male's first hatched nest among broadly concurrent attempts as ‘primary polygynous' (n = 30), and his second or third concurrent nest as ‘secondary polygynous' (n = 29). Since females did not always pair with the same male across nesting attempts, and some females bred in multiple years, the 138 nests were attended by 65 and 54 different females and males, respectively (generating 75 different pairings).
(c). Statistical analyses
We used standard pedigree algorithms to compute each individual's fi or fj, and kij, kiq and kjq between individuals, and hence calculate relatedness between each focal parent and each nestling they reared. Male TAV (TAVi), female TAV (TAVj) and LAV were then calculated for each brood (electronic supplementary material, appendix S1). We fitted linear mixed-effects models (LMMs) to test specified hypotheses relating male and female feeding rates to the focal variables (table 1).
In general, feeding rates often vary with multiple non-focal variables, including nestling age [40,41], time of season and day [42], mate behaviour [43,44] and social status [35]. We therefore used a comparative modelling approach, and compared a null LMM that included baseline effects on feeding rate to LMMs that additionally included each focal variable. Baseline effects comprised nestling age (days after hatch, continuous variable), nest lay date (continuous variable), time of day (morning or afternoon, two-level factor) and nest social status (monogamous, primary polygynous or secondary polygynous three-level factor). Since effects on male and female feeding rates were modelled separately but experimental, empirical and theoretical studies suggest that a focal individual's behaviour might be influenced by its mate's behaviour [43,45,46], each null LMM also included the focal individual's mate's simultaneously observed feeding rate (as a continuous covariate) and interactions with social status. However, key model results remained quantitatively similar when mate feeding rate was removed.
First, to test the prediction that parental feeding rates increased with increasing TAV more than with BS (table 1) we compared support for LMMs that additionally included TAV or BS versus the null LMM. Here, TAV and BS were modelled as continuous covariates, therefore adding one parameter to the null model. We then z-standardized TAV within each level of BS (i.e. TAVz = (TAV − μTAV)/σTAV, where μTAV and σTAV are the mean and standard deviation of TAV within each BS) and compared LMMs that included additive and interactive effects of TAVz and BS (as a four-level factor) to models that did not include TAVz.
Second, to test the prediction that male but not female feeding rate decreased with increasing LAV (or PEPO; table 1) we compared LMMs that additionally included each of these covariates to the null LMM. Third, to test the predictions that parental feeding rates would increase with increasing kij, but not vary with fi and fj, we compared LMMs that included each of these three covariates to the null LMM. These LMMs additionally included BS (as a continuous covariate). Finally, we expected male feeding rates to be lower at secondary polygynous nests, while female feeding rates could be higher if they compensated [43,44], implying that both sexes' feeding rates might depend on social status. Consequently, we additionally fitted LMMs that included 2-way interactions between nest social status and each focal variable.
LMMs assumed Gaussian distributions for feeding rates. All continuous variables within interaction terms were centred to minimize multicollinearity and aid model convergence. To account for non-independence across multiple observation sessions of the same nest and parents, random individual identity, social mate identity, and nest identity effects were included in all LMMs. We used Akaike information criterion, corrected for small sample sizes (AICc), to assess whether LMMs that included each focal predictor variable were better supported than the null LMM and/or than their competing predictor (e.g. TAV versus BS), defined as a difference in AICc (ΔAICc) equalling or exceeding two units [47].
All models were fitted using R 3.1.1 [48] with packages lme4 [49], lmerTest [50] and MuMIn [51]. Raw means are presented ± 1 s.d. Full distributions of all variables, and relationships between feeding rate and null variables, are in electronic supplementary material, appendix S3. LMM results are presented as standardized estimates (regression slope β) ± 1 s.e.). Estimates and SEs for factor levels not in interactions (i.e. brood size, time of day, and social status) are presented as least square means. Full details of all LMMs are in electronic supplementary material, appendix S6. Data are available from the Dryad Digital Repository: https://doi.org/10.5061/dryad.1zcrdfnf [52].
3. Results
(a). Baseline effects of sex and social status
Across all observation sessions, mean female and male feeding rates were 6.4 ± 4.1 and 4.2 ± 3.4 trips per hour, respectively. Males had lower mean feeding rates at secondary polygynous nests (1.4 ± 2.3) than at primary polygynous (4.7 ± 3.8) or monogamous nests (5.1 ± 3.1), while females had higher mean feeding rates at secondary polygynous nests (9.1 ± 4.9) than at primary polygynous (6.4 ± 3.6) or monogamous nests (5.4 ± 3.4; electronic supplementary material, appendix S3 and S6). Male and female feeding rates were positively correlated at primary polygynous and monogamous nests (Pearson correlation coefficient: rp = 0.40, 0.44, respectively), but weakly negatively correlated at secondary polygynous nests (rp = −0.10; electronic supplementary material, appendix S3). Secondary females therefore partially compensated for lower feeding rates of their socially polygynous mates.
(b). Brood size and total allelic value
Models for sex-specific feeding rates that additionally included BS (continuous variable) were substantially better supported than the null LMM for females (ΔAICc = −11.1), but only slightly better supported for males (ΔAICc = −0.7). These LMMs showed that feeding rate increased with increasing BS in females (β = 0.87 ± 0.23), and tended to do so in males (β = 0.38 ± 0.21; electronic supplementary material, appendix S3 and S6).
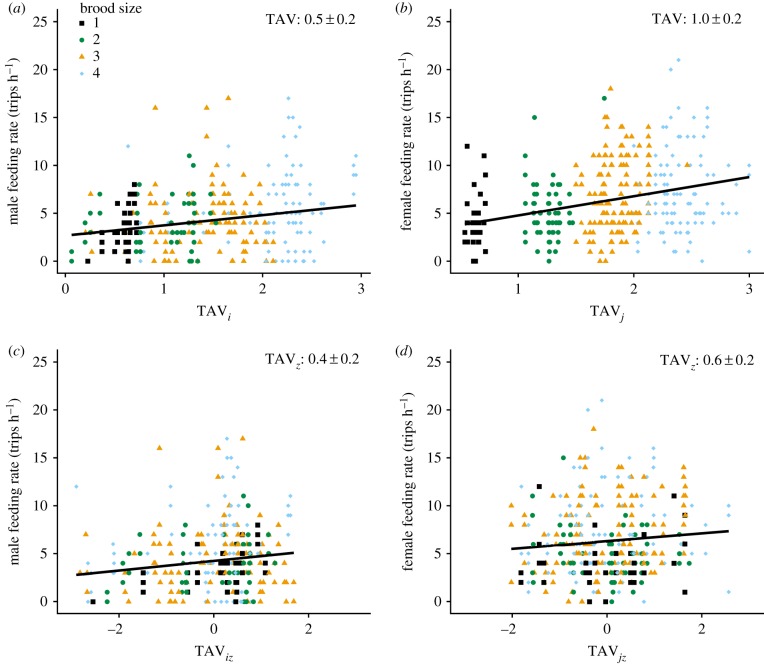
As expected, TAVi and TAVj were strongly but not perfectly positively correlated across the 139 observed broods (rp = 0.75), and TAV was positively correlated with BS in both sexes (males: rp = 0.54; females: rp = 0.76; electronic supplementary material, appendix S4 and S6). However, both TAVi and TAVj varied considerably within levels of BS, reflecting underlying variation in PEPO, kij, kiq, kjq, fi and fj (electronic supplementary material, appendix S4). LMMs that included brood TAV were much better supported than the null LMM for both sexes (males: ΔAICc = −5.1, females: ΔAICc = −14.9), showing that feeding rate increased with TAV in both sexes (figure 1). Importantly, LMMs that included TAV were better supported than competing LMMs that included BS for males (ΔAICc = −4.3) and females (ΔAICc = −3.8; electronic supplementary material, appendix S6). Consequently, as predicted, male and female feeding rates were better explained by increasing TAV than by increasing BS.
Figure 1.
Relationships between male and female song sparrow parental feeding rates and (a,b) brood total allelic value (TAVi and TAVj) and (c,d) standardized TAV within each level of brood size (TAViz and TAVjz). Points represent observation sessions. Colours and symbols denote different brood sizes (1: black, square; 2: green, large circle; 3: yellow, triangle; 4: blue, small circle). Lines show predicted regressions of feeding rates on TAV or TAVz. Regression slopes are presented as β estimates ± 1 s.e. (full details in electronic supplementary material, appendix S6). (Online version in colour.)
Furthermore, models that included standardized TAV within brood size (TAVz) were better supported than the null LMM (without BS) for females (ΔAICc = −2.9) and males (ΔAICc = −2.6). Models that included TAVz and BS were also better supported than the null with BS for females (ΔAICc = −3.0) and males (ΔAICc = −2.5; electronic supplementary material, appendix S6). Feeding rates, therefore, increased with TAVz within broods of each size (figure 1). LMMs that additionally included TAVz by BS interactions were marginally better supported than the null model (males: ΔAICc = −1.3, females: ΔAICc = −2.1), but less well supported than models without interactions (males: ΔAICc = 2.0, females: ΔAICc = 2.1). Meanwhile, LMMs that included TAVz by social status interactions were marginally better supported than the null model for males (ΔAICc = −1.7), and slightly less well supported for females (ΔAICc = +0.6). Overall, these results show that, in accordance with the prediction (table1), increased TAV was associated with increased parental feeding rates (figure 1).
(c). Lost allelic value
Across all nests, PEPO varied between 0.00 and 1.00 (mean: 0.27 ± 0.35), and LAV varied between 0.000 and 1.913 (mean: 0.208 ± 0.373). As expected, LAV was positively correlated with PEPO across all 138 focal broods (rp = 0.89; electronic supplementary material, appendix S5). Yet some broods had low LAV relative to PEPO, reflecting cases where cuckolded males were closely related to EPO (electronic supplementary material, appendix S5). PEPO, and hence LAV, varied with social status. Specifically, primary polygynous nests had higher PEPO than monogamous nests (0.33 ± 0.30 versus 0.21 ± 0.30; β = 0.12 ± 0.04, 85%CI: 0.06–0.19) and secondary polygynous nests (0.24 ± 0.30; β = -0.10 ± 0.45, 85%CI: −0.19–0.004), while monogamous and secondary polygynous nests were similar (β = 0.03 ± 0.45, 85%CI: −0.06–0.11; electronic supplementary material, appendix S6).
Models for sex-specific feeding rates that included LAV were slightly better supported than the null LMM (including BS as a covariate) for males (ΔAICc = −1.9), but less well supported for females (ΔAICc = +1.7). As predicted, male feeding rate tended to decrease with increasing LAV, but female feeding rate did not (figure 2). LMMs that additionally included LAV by social status interactions were slightly less well supported than the null LMM for males (ΔAICc = +1.0), but indicated that males at primary polygynous nests showed the greatest reduction in feeding rate with increasing LAV (figure 2; electronic supplementary material, appendix S6). There was no support for LMMs that included a LAV by social status interaction in females (ΔAICc = +7.9; figure 2; electronic supplementary material, appendix S6). Since LAV and PEPO were correlated, conclusions were very similar for models that included PEPO rather than LAV as the focal variable (electronic supplementary material, appendix S5 and S6).
Figure 2.
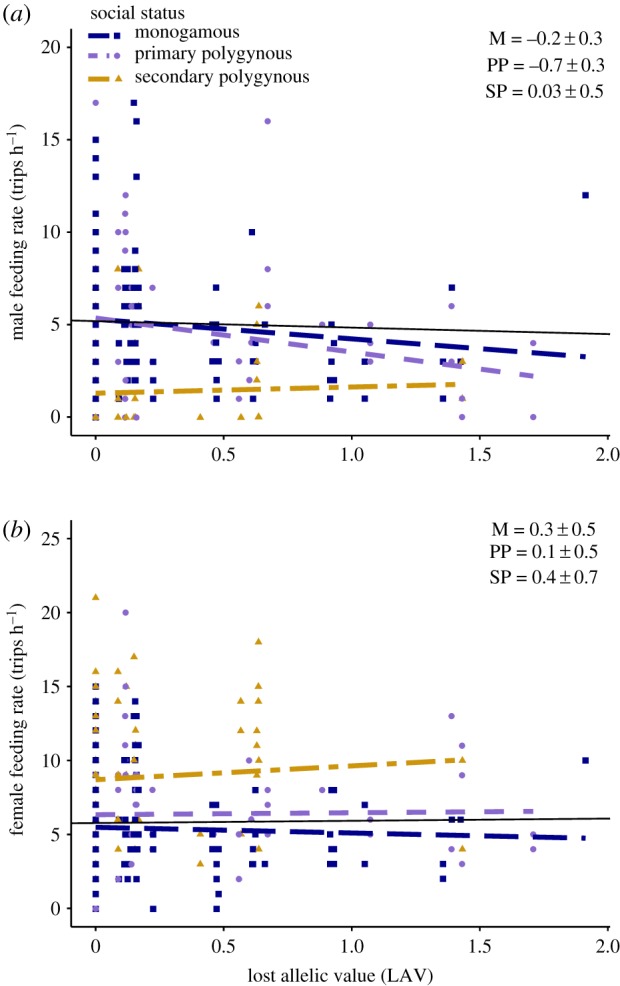
Relationships between (a) male and (b) female song sparrow parental feeding rates and lost allelic value (LAV). Colours and symbols indicate nest social status (monogamous (M): blue, square; primary polygynous (PP): purple, diamond; secondary polygynous (SP): yellow, triangle). Points represent observation sessions. Lines show predicted regressions of feeding rate on LAV overall (black), and for each social status. Regression slopes are presented as standardized β estimates ± 1 s.e. from models that included a standardized LAV by social status interaction and represent the absolute slope (non-contrast) of the relationship. Y-axes are on different scales for males and females. (Online version in colour.)
(d). Kinship (k) and inbreeding (f) coefficients
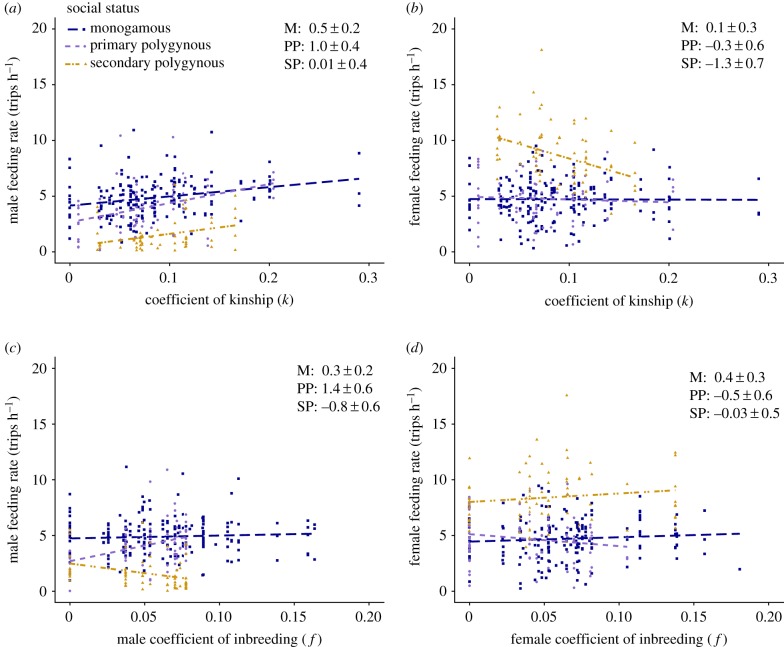
Individuals' coefficients of kinship with their social mates (kij) varied between 0.000 and 0.301 (mean: 0.087 ± 0.055). Models for sex-specific feeding rates that included kij were better supported than the null LMM (including BS) for males (ΔAICc = −4.4), but less well supported for females (ΔAICc = +2.0; electronic supplementary material, appendix S6). Males in pairs with higher kij had higher feeding rates but females did not (figure 3). LMMs that additionally included kij by social status interactions were similarly supported as the null LMM for males (ΔAICc = −0.7), but suggest that feeding rate tended to increase most markedly with increasing kij at primary polygynous nests (figure 3). Such models were less well supported for females (ΔAICc = +1.8), but suggest that females at secondary polygynous nests had lower feeding rates increasing kij (figure 3).
Figure 3.
Relationships between male and female song sparrow parental feeding rates and (a,b) pair coefficient of kinship (kij) and (c,d) individual coefficient of inbreeding (fi or fj). Colours and symbols indicate nest social status (monogamous (M): blue, square; primary polygynous (PP): purple, diamond; secondary polygynous (SP): yellow, triangle). Points represent observation sessions. Lines show predicted regressions of feeding rate on kij, fi or fj. Regression slopes are presented as standardized β estimates ± 1 s.e. from LMMs that included a standardized focal variable by social status interaction and represent the absolute slope (non-contrast) of the relationship. y-axes are on different scales for males and female. (Online version in colour.)
Individuals' coefficients of inbreeding (f) varied between 0 and 0.164 (mean: 0.057 ± 0.035) for males and 0 and 0.181 (mean: 0.057 ± 0.039) for females. LMMs that included fi were marginally less well supported than the null LMM (including BS) for males (ΔAICc = +1.0), and females (ΔAICc = +1.5; electronic supplementary material, appendix S6). Overall, feeding rates did not vary markedly with fi in either sex (figure 3). However, LMMs that additionally included fi by social status interactions were slightly better supported than the null LMM in males (ΔAICc = −1.6) but not females (ΔAICc = +4.3). Male feeding rates tended to increase with increasing fi at primary polygynous nests, decrease with increasing fi at secondary polygynous nests, and did not vary with fi at monogamous nests (figure 3). Such patterns were not evident in females across social statuses (figure 3).
4. Discussion
Patterns of variation in parental feeding rates observed in song sparrows experiencing considerable natural variation in parent–brood relatedness, resulting from combinations of EPP, mate kinship and individual coefficient of inbreeding, broadly concurred with key predictions of inclusive fitness theory (table 1). A key result is that feeding rates of both sexes increased with increasing TAV of the dependent brood, even after controlling for brood size (TAVz; figure 1). Males and females consequently fed broods more often per hour as the expected number of identical-by-descent allele copies increased, to degrees that would generate notable increases in the total feeds received by highly related broods over the full nesting period (figure 1). These results support the central premise of existing models of optimal parental effort and investment that consider brood size [53] and relatedness [25], but provide conceptual and empirical advances by encompassing complex variation in relatedness arising from reproductive interactions among relatives [12].
Variation in brood TAV from the perspective of a potentially caring male partly reflects variation in paternity loss (proportion of offspring that are extra-pair; PEPO) and kinship with his socially paired female (kij) and her extra-pair male(s) (kiq) and resulting LAV. Our analyses provide some support for the prediction that male feeding rate will decrease with increasing LAV, and with increasing PEPO itself, and hence that males that lose relatedness to a dependent brood due to EPP provide less care. This concurs with some [6,13–15,23,54], but not all [6,15,54], previous empirical studies that tested whether paternal care decreases with increasing PEPO. However, our results highlight that key patterns of variation in paternal care might also depend on the social mating system. In particular, the negative effect of LAV on male feeding rate tended to be strongest at primary polygynous nests, perhaps reflecting the higher mean PEPO in these nests. Overall, these results support the hypothesis that female extra-pair reproduction can incur a cost in the form of reduced paternal care, potentially selecting against underlying polyandry [16,36], but imply that such costs might depend on social status [35].
Variation in TAV also reflects variation in kinship between socially paired mates (kij), and inclusive fitness theory predicts that optimal paternal care, interpreted as a component of parental investment, should increase with increasing kij [31]. Our results strongly support this prediction for males (figure 3), translating into substantial increases in the number of paternal feeds received by inbred broods. Such increases might yield an evolutionary benefit of inbreeding, or at least negate the underlying evolutionary cost [55]. This is because inbreeding increases parent–offspring relatedness and hence propagation of identical-by-descent allele copies (given no ‘opportunity cost' of lost outbred matings), but may also cause inbreeding depression in resulting offspring [32]. However, this cost can be negated if inbreeding parents can ameliorate inbreeding depression in resulting offspring through increased parental care [56]. This may be the case for song sparrows, since inbreeding depression in nestling survival from hatching to independence from parental care is weak [57], and inbreeding parents rear larger broods [58]. Consequently, there is weak selection against inbreeding despite strong inbreeding depression in individual fitness, and no evidence for active inbreeding avoidance through either social pairing or extra-pair reproduction [57,59]. By contrast, female song sparrows tended to decrease their feeding rate with increasing kij, perhaps reflecting a response to substantially increased male feeding rate. But, generally, it is unclear if increased levels of male care would be strong enough to substantially decrease inbreeding depression. Indeed, additional male care did not decrease inbreeding depression in burying beetles (Nicrophorus vespilloides) [60]. However, our results add to several recent experimental studies on diverse taxa suggesting parents mated to kin may adjust reproductive strategies to reduce inbreeding depression, for example by reducing their clutch size in burying beetles [61], gaining alloparental care from helpers in red-winged fairy-wrens (Malurus elegans) [62], providing increased levels of prenatal maternal provisioning in Japanese quail (Coturnix japonica) [63], adopting group living and maternal care in social spiders (Anelosimus cf. jucundus) [64], or more cooperative parental behaviour in an African cichlid (Pelvicachromis taeniatus) [28]. Yet, to our knowledge, no previous studies have directly examined how mate kinship influences parental care in wild non-cooperative breeding species. Since inbreeding occurs in many species (e.g. [27,56,63,65]) such effects warrant wider attention in the context of inclusive fitness theory [1]. In our system, and others, this could potentially include examining female responses to kinship with their extra-pair mates (kjq).
Our results also broadly concur with the prediction that overall feeding rates should not vary with a parent's own fi, insofar as such null predictions can be rigorously tested. These results can also be interpreted to provide no overall evidence of direct inbreeding depression in parental feeding rates. The few previous studies quantifying inbreeding effects on parental care all compared highly inbred (e.g. f ≥ 0.25) to outbred parents in captivity. Inbred versus outbred prairie voles (Microtus ochrogaster) and burying beetles did not differ in multiple parental behaviours [66,67], whereas inbred female zebra finches (Taeniopygia guttata) incubated less than outbred females [68]. However, in song sparrows, the effects of fi on parental feeding rate appear to vary strongly with social status: male feeding rate increased markedly with increasing fi at primary polygynous nests but decreased at secondary polygynous nests, perhaps reflecting re-allocation of parental investment among broods by more inbred males. Future studies should further examine how effects of f on key parental behaviours are shaped by the social mating system.
Parental feeding rate is one key component of parental care that may be positively or negatively correlated with other components. Consequently, the degree to which variation in feeding rate captures variation in overall care, or in parental investment strictly defined [5,34], is unknown. Nevertheless, our results are striking in showing that one major component of care does vary with subtle variation in relatedness in accordance with inclusive fitness theory (table 1), especially in males. This raises interesting questions regarding how such outcomes could arise. Our results are inevitably correlative and hence cannot prove causal effects; but any experimental manipulation of such effects in free-living populations would be exceptionally challenging, and our analyses controlled for key potentially confounding variables that are known to affect feeding rates. The observed increases in parental feeding rates with increasing TAVz may therefore imply that song sparrows can respond to direct or indirect cues of relatedness. Some mechanisms by which this could be achieved have previously been identified in song sparrows. Specifically, preen wax composition, male song repertoire size and demographic status have been shown to indicate relatedness [69–71], but, of course, other mechanisms, such as differential offspring behaviour, might also be involved.
Supplementary Material
Supplementary Material
Supplementary Material
Acknowledgements
We thank the Tsawout and Tseycum First Nation bands for allowing access to Mandarte, numerous field assistants, graduate students and postdoctoral fellows who contributed to long-term data collection and Brad Duthie for insightful discussions regarding underlying concepts.
Ethics
This research was approved by the University of British Columbia's Animal Care Committee.
Data accessibility
Data available from the Dryad Digital Repository: https://doi.org/10.5061/dryad.1zcrjdfnf [52]. R code supporting this article has been uploaded as part of the electronic supplementary material.
Authors' contributions
E.A.G. and J.M.R. designed the research and wrote the manuscript. E.A.G. analysed the data. All other authors conducted key fieldwork and contributed to manuscript editing.
Competing interests
We declare no competing interests.
Funding
National Sciences and Engineering Research Council (P.A. and E.A.G); Izaak Walton Killam Memorial Fund for Advanced Studies (E.A.G. and J.M.R.), UK Natural Environment Research Council (R.J.S.) and the European Research Council (J.M.R.) provided funding.
References
- 1.Hamilton WD. 1964. The genetic evolution of social behavior I & II. J. Theor. Biol. 7, 1–52. ( 10.1016/0022-5193(64)90038-4) [DOI] [PubMed] [Google Scholar]
- 2.Trivers RL. 1971. The evolution of reciprocal altruism. Q Rev. Biol. 46, 35–57. ( 10.1086/406755) [DOI] [Google Scholar]
- 3.Frank SA. 2013. Natural selection. VII. History and interpretation of kin selection theory. J. Evol. Biol. 26, 1151–1184. ( 10.1111/jeb.12131) [DOI] [PubMed] [Google Scholar]
- 4.Bourke AFG. 2014. Hamilton's rule and the causes of social evolution. Phil. Trans. R. Soc. B 369, 20130362 ( 10.1098/rstb.2013.0362) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Clutton-Brock TH. 1991. The evolution of parental care. Princeton, NJ: Princeton University Press. [Google Scholar]
- 6.Remeš V, Freckleton J, Tökölyi J, Liker A, Székely T. 2015. The evolution of parental cooperation in birds. Proc. Natl Acad. Sci. USA 112, 13 603–13 608. ( 10.1073/pnas.1512599112) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Amos B, Barrott J, Dover GA. 1991. Breeding behaviour of pilot whales revealed by DNA fingerprinting. Heredity 67, 49–55. ( 10.1038/hdy.1991.64) [DOI] [PubMed] [Google Scholar]
- 8.Coltman DW, Pilkington JG, Pemberton JM. 2003. Fine-scale genetic structure in a free-living ungulate population. Mol. Ecol. 12, 733–742. ( 10.1046/j.1365-294X.2003.01762.x) [DOI] [PubMed] [Google Scholar]
- 9.Vidya TBC, Balmfort Z, Le Roux A, Cherry ML. 2009. Genetic structure, relatedness and helping behaviour in the yellow mongoose in farmland and a natural habitat. J. Zool. 278, 57–64. ( 10.1111/j.1469-7998.2009.00551.x) [DOI] [Google Scholar]
- 10.Hatchwell BJ. 2010. Cryptic kin selection: kin structure in vertebrate populations and opportunities for kin-directed cooperation. Ethology 116, 203–216. ( 10.1111/j.1439-0310.2009.01732.x) [DOI] [Google Scholar]
- 11.Sanderson JL, Wang J, Vitikainen EIK, Cant MA, Nichols HJ. 2015. Banded mongooses avoid inbreeding when mating with members of the same natal group. Mol. Ecol. 24, 3738–3751. ( 10.1111/mec.13253) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Reid JM, Bocedi G, Nietlisbach P, Duthie AB, Wolak ME, Gow EA, Arcese P. 2016. Variation in parent–offspring kinship in socially monogamous systems with extra-pair reproduction and inbreeding. Evolution 70, 1512–1529. ( 10.1111/evo.12953) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kokko H. 1999. Cuckoldry and the stability of biparental care. Ecol. Lett. 2, 247–255. ( 10.1046/j.1461-0248.1999.00075.x) [DOI] [Google Scholar]
- 14.Kokko H, Jennions MD. 2008. Parental investment, sexual selection and sex ratios. J. Evol. Biol. 21, 919–948. ( 10.1111/j.1420-9101.2008.01540.x) [DOI] [PubMed] [Google Scholar]
- 15.Griffith AS, Alonzo SH, Cornwallis CK. 2013. Why do cuckolded males provide parental care? PLoS Biol. 11, e1011520 ( 10.1371/journal.pbio.1001520) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Arnqvist G, Kirkpatrick M. 2005. The evolution of infidelity in socially monogamous passerines: the strength of direct and indirect selection on extra-pair copulation behavior in females. Am. Nat. 165, S26–S37. ( 10.1086/429350) [DOI] [PubMed] [Google Scholar]
- 17.Queller DC. 1997. Why do females care more than males? Proc. R. Soc. Biol. Sci. B 264, 1555–1557. ( 10.1098/rspb.1997.0216) [DOI] [Google Scholar]
- 18.Griffith SC, Owens IPF, Thuman KA. 2002. Extra-pair paternity in birds: a review of interspecific variation and adaptive function. Mol. Ecol. 11, 2195–2212. ( 10.1046/j.1365-294X.2002.01613.x) [DOI] [PubMed] [Google Scholar]
- 19.Houston AI, McNamara JM. 2002. A self-consistent approach to paternity and parental effort. Phil. Trans. R. Soc. Lond. B 237, 351–362. ( 10.1098/rstb.2001.0925) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dixon A, Ross D, O'Malley SLC, Burke T. 1994. Parental investment inversely related to degree of extra-pair paternity in the reed bunting. Nature 371, 698–700. ( 10.1038/371698a0) [DOI] [Google Scholar]
- 21.Wisenden BD. 1999. Alloparental care in fishes. Rev. Fish Biol. Fisheries 9, 45–70. ( 10.1023/A:1008865801329) [DOI] [Google Scholar]
- 22.Tallamy DW. 2000. Sexual selection and the evolution of exclusive paternal care in arthropods. Anim. Behav. 60, 559–567. ( 10.1006/anbe.2000.1507) [DOI] [PubMed] [Google Scholar]
- 23.Sheldon BC. 2002. Relating paternity to parental care. Phil. Trans. R. Soc. B 357, 341–350. ( 10.1098/rstb.2001.0931) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cornwallis CK, West SA, Davis KE, Griffith AS. 2010. Promiscuity and the evolution transition to complex societies. Nature 466, 969–974. ( 10.1038/nature09335) [DOI] [PubMed] [Google Scholar]
- 25.Westneat DF, Sherman PW. 1993. Parentage and the evolution of parental care. Behav. Ecol. 4, 66–77. ( 10.1093/beheco/4.1.66) [DOI] [Google Scholar]
- 26.Alonzo SH, Klug H. 2012. Maternity, paternity and parental care. In The evolution of parental care (eds Royle NJ, Smiseth PT, Kölliker M), pp. 189–205. Oxford, UK: Oxford University Press; ( 10.1093/acprof:oso/9780199692576.003.0011) [DOI] [Google Scholar]
- 27.Thünken T, Bakker TCM, Baldauf SA, Kullmann H. 2007. Active inbreeding in a chichlid fish and its adaptive significance. Curr. Biol. 17, 225–229. ( 10.1016/j.cub.2006.11.053) [DOI] [PubMed] [Google Scholar]
- 28.Hatchwell BJ. 2010. The evolution of cooperative breeding in birds: kinship, dispersal and life history. Phil. Trans. R. Soc. B 364, 3217–3227. ( 10.1098/rstb.2009.0109) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bose APH, Henshaw JM, Zimmermann H, Fritzsche K, Sefc KM. 2019. Inclusive fitness benefits mitigate costs of cuckoldry to socially paired males. BMC Biol. 17, 2 ( 10.1186/s12915-018-0620-6) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lynch M, Walsh B. 1998. Genetics and analysis of quantitative traits. Sunderland, MA: Sinauer. [Google Scholar]
- 31.Duthie AB, Lee AM, Reid JM. 2016. Inbreeding parents should invest more resources in fewer offspring. Proc. R. Soc. B 283, 20161845 ( 10.1098/rspb.2016.1845) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Duthie AB, Reid JM. 2015. Inbreeding by rejected relatives and the inclusive fitness benefit of inbreeding avoidance. PLoS ONE 10, e0125140 ( 10.1371/journal.pone.0125140) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Parker GA. 2006. Sexual conflict over mating and fertilization: an overview. Phil. Trans. R. Soc. B 361, 235–259. ( 10.1098/rstb.2005.1785) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Royle NJ, Smiseth PT, Kölliker M. 2012. The evolution of parental care. Oxford, UK: Oxford University Press; ( 10.1093/acprof:oso/9780199692576.003.0001) [DOI] [Google Scholar]
- 35.Smith JNM, Yom-Tov Y, Moses R. 1982. Polygyny, male parental care, and sex ratio in song sparrows: an experimental study. Auk 99, 555–564. [Google Scholar]
- 36.Arcese P. 1989. Intrasexual competition, mating system and natal dispersal in song sparrows. Anim. Behav. 38, 958–979. ( 10.1016/S0003-3472(89)80137-X) [DOI] [Google Scholar]
- 37.Smith JNM, Keller LF, Marr AB, Arcese P. 2006. Conservation and biology of small population: the song sparrows of Mandarte Island. New York, NY: Oxford University Press. [Google Scholar]
- 38.Smith JNM, Taitta MJ, Rogers CM, Arcese P, Keller LF, Cassidy ALE, Hochachka WM. 1996. A metapopulation approach to the population biology of the Song Sparrow Melospiza melodia. Ibis 138, 120–128. ( 10.1111/j.1474-919X.1996.tb04318.x) [DOI] [Google Scholar]
- 39.Sardell RJ, Keller LF, Arcese P, Bucher T, Reid JM. 2010. Comprehensive paternity assignment: genotype, spatial location and social status in song sparrows, Melospiza melodia. Mol. Ecol. 19, 4352–4364. ( 10.1111/j.1365-294X.2010.04805.x) [DOI] [PubMed] [Google Scholar]
- 40.Low M, Makan T, Castro I. 2012. Food availability and offspring demand influence sex-specific patterns and repeatability of parental provisioning. Behav. Ecol. 23, 25–34. ( 10.1093/beheco/arr145) [DOI] [Google Scholar]
- 41.Gow EA, Musgrove AB, Wiebe KL. 2013. Brood age and size influence sex-specific parental provisioning patterns in a sex-role reversed species. J. Ornithol. 154, 525–535. ( 10.1007/s10336-012-0923-2) [DOI] [Google Scholar]
- 42.Gow EA, Stutchbury BJM. 2013. Understanding sex differences in parental effort in a migratory songbird a sex-specific trade-off between reproduction and moult. Condor 115, 640–649. ( 10.1525/cond.2013.120091) [DOI] [Google Scholar]
- 43.McNamara JM, Gasson CE, Houston AI. 1999. Incorporating rules for responding into evolutionary games. Nature 401, 368–371. ( 10.1038/43869) [DOI] [PubMed] [Google Scholar]
- 44.Houston AI, Székely T, McNamara JM. 2005. Conflict between parents over care. Trends Ecol. Evol. 20, 33–38. ( 10.1016/j.tree.2004.10.008) [DOI] [PubMed] [Google Scholar]
- 45.Wright J, Cuthill I. 1990. Manipulation of sex differences in parental care: the effect of brood size. Anim. Behav. 40, 462–471. ( 10.1016/S0003-3472(05)80526-3) [DOI] [Google Scholar]
- 46.Sanz JJ, Kranenbarg S, Tinbergen JM. 2000. Differential response by males and females to manipulation of partner contribution in the Great Tit (Parus major). J. Anim. Ecol. 69, 74–84. ( 10.1046/j.1365-2656.2000.00373.x) [DOI] [Google Scholar]
- 47.Burnham KP, Anderson DR. 2002. Model selection and multi-model inference: a practical information-theoretic approach, 2nd edn Berlin, Germany: Springer. [Google Scholar]
- 48.R Core Development Team. 2018. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistial Computing; See http://www.R-project.org. [Google Scholar]
- 49.Bates D, Maechler M, Bolker B, Walker S. 2015. Fitting linear mixed-effects models using lme4. J. Stat. Softw. 67, 1–48. ( 10.18637/jss.v067.i01) [DOI] [Google Scholar]
- 50.Kuznetsova A, Brockhoff PB, Christensen RHB. 2016. lmerTest: tests in linear mixed effects models. R package. See https://CRAN.R-project.org/package=lmerTest.
- 51.Zuur AF, Ieno EN, Walker N, Saveliev AA, Smith GM. 2010. Mixed effects models and extensions in ecology with R. New York, NY: Springer; ( 10.1007/978-0-387-87458-6) [DOI] [Google Scholar]
- 52.Gow EA, Arcese P, Dagenais D, Sardell RJ, Wilson S, Reid JM.2019. Data from: Testing predictions of inclusive fitness theory in inbreeding relatives with biparental care. Dryad Digital Repository. ( ) [DOI] [PMC free article] [PubMed]
- 53.Nur N. 1984. The consequences of brood size for breeding blue tits. II. Nestling weight, offpsring survival and optimal brood size. J. Anim. Ecol. 53, 497–517. ( 10.2307/4530) [DOI] [Google Scholar]
- 54.Alonzo SH. 2010. Social and coevolutionary feedbacks between mating and parental investment. Trends Ecol. Evol. 25, 99–108. ( 10.1016/j.tree.2009.07.012) [DOI] [PubMed] [Google Scholar]
- 55.Keller LF, Reid JA, Arcese P. 2008. Testing evoluntionary models of senescence in a natural population: age and inbreeding effects on fitness components in song sparrows. Proc. R. Soc. B 275, 597–604. ( 10.1098/rspb.2007.0961) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pilakouta N, Jamieson S, Moorad JA, Smiseth PT. 2015. Parental care buffers against inbreeding depression in burying beetles. Proc. Natl Acad. Sci. USA 112, 8031–8035. ( 10.1073/pnas.1500658112) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Reid JM, Arcese P, Bocedi G, Duthie AB, Wolak ME, Keller LF. 2015. Resolving the conundrum of inbreeding depression but no inbreeding avoidance: estimating sex-specific selection on inbreeding by song sparrows (Melospiza melodia). Evolution 69, 2846–2861. ( 10.1111/evo.12780) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Keller LF. 1998. Inbreeding and its fitness effects in an insular population of song sparrows (Meospiza melodia). Evolution 52, 240–250. ( 10.1111/j.1558-5646.1998.tb05157.x) [DOI] [PubMed] [Google Scholar]
- 59.Keller LF, Arcese P. 1998. No evidence for inbreeding avoidance in a natural population of song sparrows (Melospiza melodia). Am. Nat. 152, 380–392. ( 10.1086/286176) [DOI] [PubMed] [Google Scholar]
- 60.Ratz T, Castel E, Smiseth PT. 2018. Male assistance in parental care does not buffer against detrimental effects of maternal inbreeding on offspring. Front. Ecol. Evol. 6, 196 ( 10.3389/fevo.2018.00196) [DOI] [Google Scholar]
- 61.Ford LE, Henderson KJ, Smiseth PT. 2018. Differential effects of offspring and maternal inbreeding on egg laying and offspring performance in the burying beetle Nicrophorus vespilloides. J. Evol. Biol. 31, 1047–1057. ( 10.1111/jeb.13285) [DOI] [PubMed] [Google Scholar]
- 62.Lichtenauer W, van de Pol M, Cockburn A, Brouwer L. 2019. Indirect fitness benefits through extra-pair mating are large for an inbred minority, but cannot explain widespread infidelity among red-winged fairy-wrens. Evolution 73, 467–480. ( 10.1111/evo.13684) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ihle K, Hutter P, Tschirren B. 2017. Increased prenatal maternal investment reduces inbreeding depression in offspring. Proc. R. Soc. B 284, 20171347 ( 10.1098/rspb.2017.1347) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Avilés L, Bukowski TC. 2006. Group living and inbreeding depression in a subsocial spider. Proc. R. Soc. B 273, 157–163. ( 10.1098/rspb.2005.3308) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Cohen LB, Dearborn DC. 2004. Great frigatebirds, Fregata minor, choose mates that are genetically similar. Anim. Behav. 68, 1229–1236. ( 10.1016/j.anbehav.2003.12.021) [DOI] [Google Scholar]
- 66.Bixler A, Tang-Martinez Z. 2006. Reproductive performance as a function of inbreeding in prairie voles (Microtus ochrogaster). J. Mammalogy 87, 944–949. ( 10.1644/05-MAMM-A-353R2.1) [DOI] [Google Scholar]
- 67.Mattey SN, Smiseth PT. 2015. Complex effects of inbreeding on biparental cooperation. Am. Nat. 185, 1–12. ( 10.1086/679067) [DOI] [PubMed] [Google Scholar]
- 68.Pooley EL, Kennedy MW, Nager RG. 2014. Maternal inbreeding reduces parental care in zebra finch, Taeniopygia guttata. Anim. Behav. 97, 153–163. ( 10.1016/j.anbehav.2014.09.012) [DOI] [Google Scholar]
- 69.Slade JWG, Watson MJ, Kelly TR, Gloor GB, Bernards MA, MacDougall-Shackleton EA. 2016. Chemical composition of preen wax reflects major histocompatibility complex similarity in songbirds. Proc. R. Soc. B 283, 20161966 ( 10.1016/j.bbr.2008.08.036) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Reid JM, Duthie AB, Wolak ME, Arcese P. 2015. Demographic mechanisms of inbreeding adjustment through extra-pair reproduction. J. Anim. Ecol. 84, 1029–1040. ( 10.1111/1365-2656.12340) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Reid JM, Arcese P, Cassidy A, Marr A, Smith J, Keller LF. 2005. Hamilton and Zuk meet heterozygosity? Song repertoire size indicates inbreeding and immunity in song sparrows (Melospiza melodia). Proc. R. Soc. B 272, 481–487. ( 10.1098/rspb.2004.2983) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Gow EA, Arcese P, Dagenais D, Sardell RJ, Wilson S, Reid JM.2019. Data from: Testing predictions of inclusive fitness theory in inbreeding relatives with biparental care. Dryad Digital Repository. ( ) [DOI] [PMC free article] [PubMed]
Supplementary Materials
Data Availability Statement
Data available from the Dryad Digital Repository: https://doi.org/10.5061/dryad.1zcrjdfnf [52]. R code supporting this article has been uploaded as part of the electronic supplementary material.