Abstract
Objectives
To examine the association of protein intake with frailty progression in very old adults.
Design
The Newcastle 85+ study, a prospective longitudinal study of people aged 85 years old in Northeast England and followed over 5 years.
Setting and Participants
668 community-dwelling older adults (59% women) at baseline, with complete dietary assessment and Fried frailty status (FFS).
Measures
Dietary intake was estimated with 2 × 24-h multiple pass recalls at baseline. FFS was based on five criteria: shrinking, physical endurance/energy, low physical activity, weakness and slow walking speed and was available at baseline and 1.5, 3 and 5 years. The contribution of protein intake (g/kg adjusted body weight/day [g/kg aBW/d]) to transitions to and from FFS (robust, pre-frail and frail) and to death over 5 years was examined by multi-state models.
Results
Increase in one unit of protein intake (g/kg aBW/d) decreased the likelihood of transitioning from pre-frail to frail after adjusting for age, sex, education and multimorbidity (hazard ratios [HR]: 0.44, 95% confidence interval [CI]: 0.25–0.77) but not for the other transitions. Reductions in incident frailty were equally present in individuals with protein intake ≥0.8 (HR: 0.60, 95% CI: 0.43–0.84) and ≥1 g/kg aBW/d (HR: 0.63, 95% CI: 0.44–0.90) from 85 to 90 years. This relationship was attenuated after adjustment for energy intake, but the direction of the association remained the same (e.g. g/kg aBW/d model: HR: 0.71, 95% CI: 0.36–1.41).
Conclusion
High protein intake, partly mediated by energy intake, may delay incident frailty in very old adults. Frailty prevention strategies in this age group should consider adequate provision of protein and energy.
Keywords: malnutrition, aged 80 and over, multi-state model, PROMISS, fried, protein, frailty, older people
Key points
Most of the observed transitions were in the forward direction (i.e. robust to pre-frail and pre-frail to frail), but there were still some recoveries.
Higher protein intake decreased the likelihood of incident frailty after adjusting for key socioeconomic and health factors, but not for other transitions (e.g. recovery from frailty).
This relationship was attenuated after adjusting for energy intake but the direction of the association remained the same.
Introduction
Frailty is a clinical syndrome defined as an increased vulnerability or failure to return to homeostatic equilibrium after a stressor event that increases the risk of dependency, hospitalisation and death [1]. Pre-frailty and frailty are estimated to be present in 42 and 11% of community-dwelling older adults, respectively, and both increase with age [2]. Moreover, frail older adults are at increased risk of disability, hospitalisation, care home admission and death [1]. Two popular frailty models include the cumulative deficits model [3] and the frailty phenotype [4], the latter using five criteria: muscle weakness, slow walking speed, low physical activity, exhaustion and unintentional weight loss [4]. Malnutrition is central to all the criteria proposed in the frailty phenotype [4]. Provision of adequate dietary protein could therefore be a viable strategy to modulate the progression of frailty in older adults [1] as it may slow down the progressive loss of muscle mass and physical function [5]. A recent systematic review concluded that older adults with higher protein intake were less likely to be frail but the studies included were mostly cross-sectional (prevalent frailty at baseline) as prospective studies with several time points were scarce and seldom included very old adults [6]. We have previously shown that low protein intake was associated with lower muscle strength and physical performance [7], worse disability trajectories [8] and incident disability [9] in very old adults. We therefore aimed to determine whether transitions between frailty states (robust, pre-frail and frail) and to death varied by protein intake in very old adults as they aged further. Our hypothesis was that higher protein intake was protective against frailty incidence between the age of 85 and 90 years but not impactful enough to promote recovery to either a pre-frail or robust state at this age.
Methods
Newcastle 85+ study
The Newcastle 85+ study is a longitudinal population-based study that approached all people turning 85 in 2006/2007 (born in 1921) in Newcastle and North Tyneside, UK. At baseline, the analytic sample comprised 668 very old adults living in the community, with complete protein intake assessment, height, weight and Fried frailty status (FFS). Full details of the Newcastle 85+ study have been published elsewhere [10].
Protein intake
Dietary intake was assessed by a 24 h multiple pass recall on two non-consecutive occasions at baseline and nutritional intake estimated using the McCance and Widdowson’s sixth edition food composition tables [11]; full details can be found elsewhere [12, 13]. Body weight (BW) was measured to the nearest 0.1 kg using a digital scale and adjusted to be within the desired body mass index for older adults of 22–27 kg/m2 as previously described [14, 15]. Protein intake was expressed in three ways: as a continuous variable (g/kg aBW/d) and as a binary variable using cut points of 0.8 and 1.0 g/kg aBW/d based on previously published results from this cohort [7, 8, 15].
Frailty
The FFS was derived for each time point based on approximations from the Cardiovascular Health Study methodology [4, 16] (Figure S1), by scoring (1) for every component that was present (shrinking, poor endurance/energy, low physical activity, weakness and slow walking speed) and (0) if absent (range 0–5). Further details of the individual components are given in the supplementary methods. Participants with a score of zero were defined as robust, with 1–2 as pre-frail and with 3 or more components as frail.
Figure 1.
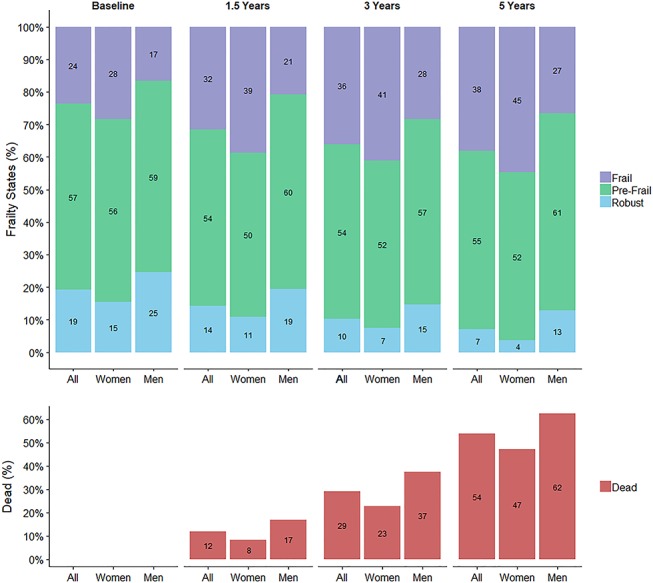
Fried frailty states and death (%), by follow-up and sex. Death is an absorbing state and is represented as a proportion of the total number of participants in each wave (cumulative).
Mortality and confounders
Information on date of death was obtained from National Health Service (NHS) Digital, UK [20]. The time to death was calculated as the time between age at baseline (2006–2007) and time of death (censored at 29 August 2012). Years of full-time education were categorised as 0–9 years, 10–11 years or 12 or more years in full-time education. Disease count was created by scoring eight chronic diseases (cardiac, respiratory and cerebrovascular disease, arthritis, hypertension, diabetes mellitus, cognitive impairment and cancer in the past 5 years) diagnosed by the General practitioner (GP) as either present (1) or absent (0) [17].
Statistical analysis
Normality was assessed by Q–Q plots. Non-Gaussian distributed variables are presented as medians and interquartile ranges, and categorical data are presented as percentages (with corresponding frequency). To determine the contribution of protein intake (g/kg aBW/d) to transitions between FFS and to death over 5 years, we fitted a multi-state model with four states: robust, pre-frail, frail and death (absorbing state) (the illness-death model with the allowed transitions is shown in Figure S2). Due to the limited number of transitions between non-adjacent states (e.g. robust to frail) and the consequent non-convergence of the final models, we assumed that transitions from robust to frail or vice versa had to go through pre-frail.
We fitted three models for protein intake (g/kg aBW/d) (continuous) or protein intake ≥ or < to 0.8 (binary) or ≥ or < to 1 g/kg aBW/d (binary) with increasing complexity: Model l included protein intake, age, sex and years of full time education, Model 2 was further adjusted for number of chronic diseases from GP record reviews at baseline, 1.5, 3 and at 5 years of follow-up and Model 3 was also adjusted for energy intake.
Multi-state models describe the movement of an individual between a number of finite states in a continuous time stochastic process under the Markov assumption that the next state is only influenced by the current state [18, 19]. Multi-state models were fitted with the msm package in R v3.2.2 [20]. Point estimates and confidence intervals were used to assess statistical and clinical significance. The Broyden–Fletcher–Goldfarb–Shanno algorithm (quasi-Newton optimisation technique) was used to maximise the likelihood with results presented as hazard ratios (HR) and 95% confidence intervals (CI), alongside expected time spent in each state.
The Newcastle 85+ study was conducted according to the guidelines laid down by the 1964 Declaration of Helsinki, and all procedures involving human subjects were approved by the Newcastle and North Tyneside local research ethics committee (06/Q0905/2). Written informed consent was obtained from all participants, and when unable to do so, consent was obtained from a caregiver or a relative according to the UK Mental Capacity Act 2005.
Results
Missing and non-missing FFS
Compared to participants with a FFS, those with a missing FFS were in worse health and had lower protein intake. In addition, those without FFS were more likely to have missing data on protein intake and health variables (e.g. protein intake (g/kg aBW/d) was missing for 1% of the participants with a FFS and for 66% of those without) (Table S1).
Table 1.
Baseline health and sociodemographic characteristics of participants in robust, pre-frail and frail FFS
| Robust (n = 129) | Pre-frail (n = 386) | Frail (n = 159) | All (n = 674) | Missing | |
|---|---|---|---|---|---|
| Women | 47.3 (61) | 57.8 (223) | 71.1 (113) | 58.9 (397) | 0 (0) |
| Education | 0.3 (2) | ||||
| 0–9 years | 60.5 (78) | 62.8 (241) | 71.7 (114) | 64.4 (433) | |
| 10–11 years | 25.6 (33) | 22.7 (87) | 21.4 (34) | 22.9 (154) | |
| 12+ years | 14.0 (18) | 14.6 (56) | 6.9 (11) | 12.6 (85) | |
| Chronic diseases | 0 (0) | ||||
| 0–1 diseases | 35.7 (46) | 32.1 (124) | 13.2 (21) | 28.3 (191) | |
| 2–3 diseases | 55.8 (72) | 53.9 (208) | 56.6 (90) | 54.9 (370) | |
| 4+ diseases | 8.5 (11) | 14.0 (54) | 30.2 (48) | 16.8 (113) | |
| Energy (MJ/d) | 7.4 (6.2, 9.3) | 6.8 (5.6, 8.0) | 6.4 (5.2, 7.8) | 6.8 (5.6, 8.3) | 0.7 (5) |
| Total protein (g/d) | 66.7 (53.1, 82.0) | 61.8 (49.7, 75.2) | 55.3 (44.8, 69.8) | 61.2 (49.0, 76.0) | 0.7 (5) |
| Energy protein (%) | 15.5 (12.9, 17.4) | 15.6 (13.5, 18.3) | 14.9 (12.9, 17.4) | 15.4 (13.2, 17.8) | 0.7 (5) |
| Total protein (g/kg aBW/d) | 1.0 (0.8, 1.2) | 1.0 (0.8, 1.2) | 0.9 (0.7, 1.1) | 1.0 (0.8, 1.2) | 0.9 (6) |
| <0.8 g/kg aBW/d | 23.6 (30) | 27.2 (104) | 31.6 (50) | 27.5 (184) | 0.9 (6) |
| <1.0 g/kg aBW/d | 47.2 (60) | 54.0 (207) | 62.7 (99) | 54.8 (366) | 0.9 (6) |
| Carbohydrate (g) | 221 (172, 270) | 190 (154, 231) | 183 (153, 221) | 192 (158, 236) | 0.7 (5) |
| Energy carbohydrate (%) | 48.0 (43.9, 54.0) | 48.3 (43.3, 54.1) | 49.5 (45.2, 53.2) | 48.6 (43.9, 54.0) | 0.7 (5) |
| Fat (g) | 72.2 (57.7, 96.2) | 63.7 (49.8, 79.7) | 62.2 (47.2, 81.1) | 65.0 (50.4, 83.9) | 0.7 (5) |
| Energy fat (%) | 36.4 (31.1, 41.6) | 34.8 (30.5, 40.4) | 37.3 (31.0, 41.1) | 35.5 (30.8, 40.9) | 0.7 (5) |
Entries are percentages (%) and counts (n) for categorical variables and median (interquartile range) for non-normally distributed continuous variables. MJ, megajoules.
Baseline characteristics according to FFS
At baseline, women were more likely to be frail (e.g. 71.1% of those who were frail, and 47.3% of those who were robust were women) (Table 1) and this continued throughout the follow-up (Figure S1). Participants who were frailer at baseline had also more chronic diseases (e.g. 30.2% of frail and 8.5% of robust participants had four or more diseases). Those who were robust had, on average, higher energy intake and higher protein, carbohydrate and fat intake (but not percentage of energy from these macronutrients) than those who were pre-frail or frail (e.g. robust, pre-frail and frail participants had a protein intake of 66.7, 61.8 and 55.3 g/d, respectively) (Table 1).
Protein intake and transitions between frailty states and to death
There was a progressive decrease in robust FFS (19% of all participants at baseline, 7% by 5 years) and an increase in frail FFS (24% at baseline, 38% at 5 years) in men and women over the 5 years of follow-up (Figure 1). More than half (54%) of participants had died by 5 years. Table S2 shows the number of “transitions” between robust, pre-frail, frail and to death. On average, participants spent 1.43 years (95% CI: 1.15–1.77) robust, 3.01 years (95% CI: 2.63–3.44) pre-frail and 2.97 years (95% CI: 2.50–3.52) frail between age 85 and 90 years. An increase in one unit of protein intake (g/kg aBW/d) (continuous measure) decreased the likelihood of transitioning from pre-frail to frail in models adjusted for age, sex, education and number of chronic diseases (HR:0.44, 95% CI: 0.25–0.77) (Table 2). Significant reductions in incident frailty from pre-frailty were present in individuals with protein intake ≥0.8 (HR: 0.60, 95% CI: 0.43–0.84) and ≥1 g/kg aBW/d (HR: 0.63, 95% CI: 0.44–0.90) from 85 to 90 years (Table 2). These relationships were attenuated by further adjustment for total energy intake, but the direction of the associations remained the same (i.e. one unit increase g/kg aBW/d: HR: 0.71, 95% CI: 0.36–1.41; ≥0.8 g/kg aBW/d: HR: 0.67, 95% CI: 0.45–0.99; ≥1 g/kg aBW/d: HR: 0.78, 95% CI: 0.53–1.17) (Table 2). Other transition rates (robust to pre-frail, pre-frail to robust, pre-frail to dead and frail to pre-frail) did not vary by protein intake (Table 2), though there was a suggestion that participants with higher protein intake were less likely to die from a frailty state.
Table 2.
Hazard ratios and 95% confidence intervals for the contribution of protein intake to transitions between FFS states and to death over 5 years
| Increase of 1 g/kg aBW/d | ≥0.8 g/kg aBW/d | ≥1 g/kg aBW/d | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | HR | 95% CI | HR | 95% CI | |
| Robust → pre-frail (n = 111) | ||||||
| Model 1a | 0.99 | 0.51–1.93 | 0.75 | 0.48–1.19 | 1.29 | 0.89–1.88 |
| Model 2b | 0.96 | 0.49–1.87 | 0.75 | 0.47–1.20 | 1.30 | 0.89–1.91 |
| Model 3c | 0.87 | 0.37–2.04 | 0.69 | 0.41–1.16 | 1.32 | 0.87–2.00 |
| Pre-frail → frail (n = 173) | ||||||
| Model 1a | 0.43 | 0.25–0.74 | 0.58 | 0.41–0.81 | 0.64 | 0.46–0.89 |
| Model 2b | 0.44 | 0.25–0.77 | 0.60 | 0.43–0.84 | 0.63 | 0.44–0.90 |
| Model 3c | 0.71 | 0.36–1.41 | 0.67 | 0.45–0.99 | 0.78 | 0.53–1.17 |
| Pre-frail → robust (n = 37) | ||||||
| Model 1a | 1.25 | 0.48–3.21 | 0.87 | 0.37–2.01 | 1.76 | 0.84–3.68 |
| Model 2b | 1.16 | 0.45–2.97 | 0.81 | 0.34–1.91 | 1.74 | 0.82–3.68 |
| Model 3c | 0.85 | 0.24–3.07 | 0.65 | 0.25–1.69 | 1.65 | 0.70–3.87 |
| Pre-frail → dead (n = 140) | ||||||
| Model 1a | 1.80 | 0.91–3.59 | 1.30 | 0.45–3.73 | 2.41 | 0.88–6.62 |
| Model 2b | 1.81 | 0.86–3.81 | 1.17 | 0.47–2.95 | 2.69 | 0.78–9.25 |
| Model 3c | 1.02 | 0.37–2.85 | 1.74 | 0.21–14.36 | 2.70 | 0.61–11.95 |
| Frail → pre-frail (n = 49) | ||||||
| Model 1a | 1.02 | 0.38–2.75 | 0.70 | 0.36–1.36 | 1.20 | 0.66–2.18 |
| Model 2b | 0.99 | 0.37–2.68 | 0.64 | 0.32–1.28 | 1.20 | 0.66–2.19 |
| Model 3c | 0.79 | 0.21–2.92 | 0.48 | 0.21–1.12 | 1.20 | 0.61–2.34 |
| Frail → dead (n = 142) | ||||||
| Model 1a | 0.60 | 0.34–1.06 | 0.79 | 0.57–1.10 | 0.63 | 0.43–0.93 |
| Model 2b | 0.62 | 0.34–1.11 | 0.84 | 0.60–1.17 | 0.61 | 0.41–0.91 |
| Model 3c | 0.85 | 0.41–1.77 | 0.90 | 0.61–1.34 | 0.65 | 0.41–1.02 |
Protein intake <0.8 or < 1.0 g/kg aBW/d was the reference category. N is the number of transitions.
aModel l included protein intake (g/kg aBW/d) or protein intake ≥ or < to 0.8 or ≥ or < to 1 g/kg aBW/d, age, sex and education.
bModel 2 was further adjusted for number of chronic diseases at baseline and follow-up.
cModel 3 was also further adjusted for energy intake.
Sensitivity analysis
We tested for interactions between protein intake and energy but none were significant apart from the transition from pre-frail to dead (one unit increase g/kg aBW/d: HR: 0.48, 95% CI: 0.30–0.78). Conclusions remained after further adjustment for protein intake distribution throughout the day, smoking, alcohol intake and other macronutrients and with protein intake per actual body weight instead of aBW.
Discussion
Main findings
Participants with higher protein intakes at baseline were less likely to transition from pre-frail to frail between age 85 and 90 years, though this relationship was attenuated after adjusting for energy intake, suggesting that energy intake partly mediates the relationship between protein intake and frailty progression. To the best of our knowledge, this is the first study to investigate the contribution of protein intake to frailty incidence, recovery and transition to death in the very old.
Protein intake and frailty incidence
Frailty is a complex construct, here operationalised by muscle weakness, slow walking speed, low physical activity, exhaustion and unintentional weight loss [4]. Nutrition is central to all these criteria [4], and higher protein is associated with a slower decline in grip strength [21, 22], muscle mass [23], walking speed [24] and weight-loss [25].
We found that participants with higher protein intake were less likely to have incident frailty (from pre-frailty) over 5 years in models adjusted for key confounders. These findings confirm a recent review concluding that higher protein intake was inversely associated with frailty in older adults (OR: 0.67, 95% CI: 0.56–0.82) [6], though only cross-sectional studies were included. Nevertheless, the few existing longitudinal observational studies, albeit mainly in younger old, are in agreement [6, 26]; older women (65–79 years) in the Women’s Health Initiative Observational Study (n = 24,000) with higher percentage of energy from protein (measured by food frequency questionnaire (FFQ)) were at reduced risk of frailty incidence (modified FFS) [27], and Spanish older adults (n = 1,800, 60+ years) with higher protein intakes (assessed by diet history) were less likely to develop incident frailty (FFS) over 3.5 years [28]. Conversely, others reported that older men (n = 5,900, 65+ years) from the Osteoporotic Fractures in Men study with higher percentage of energy from protein (assessed by FFQ) were as likely to be frail (modified FFS) after 4.6 years [29].
Protein intake and other frailty transitions
Protein intake was not associated with other transition rates in our study (robust to pre-frail, pre-frail to robust, pre-frail to dead and frail to pre-frail) possibly because of insufficient transitions between each state. However, there was a trend in participants with higher protein intake to be less likely to die from a frailty state, confirming results from the Women’s Health Initiative where older women (65–85 years, n = 10,000) who were frail (modified FFS) and had higher biomarker-calibrated protein intakes (measured with FFQ and calibrated with recovery biomarkers of energy and protein in a subsample) were less likely to die over 12 years of follow-up (P-trend = 0.03) [30].
Relationship partly mediated by energy intake
The observed associations between protein intake and frailty incidence were attenuated by further adjustment for energy intake though the direction of the associations remained, suggesting that energy intake partly mediates the relationship between protein intake and the transition between pre-frailty to frailty. This reinforces the key structural, functional and energy-producing role of protein. However, it remains difficult to disentangle the benefits of dietary protein and energy on frailty progression from previous studies since protein intake has been expressed as a fraction of energy on a number of occasions [27, 29]. Sufficient dietary energy is required for protein to optimally stimulate muscle protein synthesis and reduce the loss of muscle mass [31], and it may be that our observations are also a reflection of this.
Strengths and weaknesses
FFS was assigned to participants at baseline, 1.5, 3 and 5 years, but unobserved incidence and recovery from frailty states may have occurred between these time points. However, sustained recovery (either from pre-frail or frail) was uncommon in the very old adults of the Newcastle 85+ study and therefore we can assume that most of these unobserved transitions were from either (i) robust to pre-frail or (ii) pre-frail to frail, or (iii) that recovery was transient and soon reversed. Protein intake was measured at baseline only and, therefore, intakes were assumed to be stable or have declined proportionally over 5 years. Furthermore, misreporting is a common limitation of self-reported dietary assessment methods. We estimated that 26% of the participants were possible misreporters (using an energy intake: basal metabolic rate cut-off of 1.05–2.00) but these were not excluded because of uncertainty surrounding this estimate and the small differences between excluding and including misreporters [11]. Healthy behaviours cluster together (non-smoking, higher physical activity, more balanced diet, etc.), and higher protein and energy intake could have served as a proxy for other behaviours negatively associated with frailty. Finally, although attempts were made to infer causality from our study (plausibility, temporality and consistency), this cannot be proved. However, our findings are supported by a small trial (87 frail older adults) where protein-calorie supplementation of 2 × 200 ml (400 kcal and 25 g of protein) liquid supplements per day over 12 weeks resulted in improved outcomes highly relevant to FFS (slower decrease in the short physical performance battery and usual gait speed and improved timed-up-and-go time) compared to the control group [32]. The large range of variables collected and the use of multi-state models to understand the contribution of protein to transitions between frailty states and to death over 5 years are major strengths of this study.
Conclusion
Higher protein intake may delay the incidence of frailty in very old adults and this effect seems to be partly mediated by energy intake. This suggests that protein modulates frailty transitions not only because it provides energy but also for its functional and structural role. These findings need to be replicated in younger cohorts with long follow-ups where there is a greater possibility of recovery and where most participants start in a robust FFS and not already pre-frail/frail. Frailty prevention strategies in older adults should consider adequate provision of protein and energy.
Supplementary Material
Acknowledgements
We acknowledge the operational support of the North of England Commissioning Support Unit (formerly NHS North of Tyne) and of the local general practitioners and their staff. We also thank the research nurses, dietary coders and management and clerical team for outstanding work throughout, as well as many colleagues for their expert advice. Thanks are due especially to the study participants and, where appropriate, their families and carers.
Declaration of Conflicts of Interest
None declared.
Declaration of Funding
Funding for this research is provided by the European Horizon 2020 PROMISS Project “Prevention Of Malnutrition In Senior Subjects in the EU”, Grant agreement no. 678732 (NM, CJ). The content reflects the authors’ views only and the Commission is not responsible for any use that may be made of the information it contains. The Newcastle 85+ Study has been funded by a joint grant from the Medical Research Council and Biotechnology and Biological Sciences Research Council (G0500997) and the Dunhill Medical Trust (R124/0509). AK was supported by the National Institute for Health Research (NIHR) School for Primary Care Research and AG by the NIHR Newcastle Biomedical Research Centre.
References
- 1. Morley JE, Vellas B, van Kan GA et al. Frailty consensus: A call to action. J Am Med Dir Assoc 2013; 14: 392–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Collard RM, Boter H, Schoevers RA, Oude Voshaar RC. Prevalence of frailty in community-dwelling older persons: A systematic review. J Am Geriatr Soc 2012; 60: 1487–92. [DOI] [PubMed] [Google Scholar]
- 3. Rockwood K, Mitnitski A. Frailty defined by deficit accumulation and geriatric medicine defined by frailty. Clin Geriatr Med 2011; 27: 17–26. [DOI] [PubMed] [Google Scholar]
- 4. Fried LP, Tangen CM, Walston J et al. Frailty in older adults: Evidence for a phenotype. J Gerontol A Biol Sci Med Sci 2001; 56: M146–56. [DOI] [PubMed] [Google Scholar]
- 5. Goisser S, Guyonnet S, Volkert D. The role of nutrition in frailty: An overview. J Frailty Aging 2016; 5: 74–7. [DOI] [PubMed] [Google Scholar]
- 6. Coelho-Junior HJ, Milano-Teixeira L, Rodrigues B, Bacurau R, Marzetti E, Uchida M. Relative protein intake and physical function in older adults: A systematic review and meta-analysis of observational studies. Nutrients 2018; 10: 1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Granic A, Mendonca N, Sayer AA et al. Low protein intake, muscle strength and physical performance in the very old: The Newcastle 85+ study. Clin Nutr 2017; 37: 2260–2270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Mendonça N, Granic A, Hill TR et al. Protein intake and disability trajectories in very old adults: The Newcastle 85+ study. J Am Geriatr Soc 2018; 67: 50–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Mendonça N, Kingston A, Granic A et al. Contribution of protein intake and its interaction with physical activity to transitions between disability states and to death in very old adults: the Newcastle 85+ Study.. Eur J Nutr 2019. 10.1007/s00394-019-02041-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Collerton J, Davies K, Jagger C et al. Health and disease in 85 year olds: Baseline findings from the Newcastle 85+ cohort study. BMJ 2009; 339: b4904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Food Standards Agency McCance and Widdowson's The Composition of Foods. 6th Summary edition. Cambridge: Royal Society of Chemistry, 2002. [Google Scholar]
- 12. Mendonça N, Hill TR, Granic A et al. Macronutrient intake and food sources in the very old: Analysis of the Newcastle 85+ study. Br J Nutr 2016; 115: 2170–80. [DOI] [PubMed] [Google Scholar]
- 13. Mendonça N, Hill TR, Granic A et al. Micronutrient intake and food sources in the very old: Analysis of the Newcastle 85+ study. Br J Nutr 2016; 116: 751–61. [DOI] [PubMed] [Google Scholar]
- 14. Berner LA, Becker G, Wise M, Doi J. Characterization of dietary protein among older adults in the United States: Amount, animal sources, and meal patterns. J Acad Nutr Diet 2013; 113: 809–15. [DOI] [PubMed] [Google Scholar]
- 15. Mendonça N, Granic A, Mathers JC et al. Prevalence and determinants of low protein intake in very old adults: Insights from the Newcastle 85+ study. Eur J Nutr 2017; 57: 2713–2722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Collerton J, Martin-Ruiz C, Davies K, Hilkens CM, Isaacs J, Kolenda C et al. Frailty and the role of inflammation, immunosenescence and cellular ageing in the very old: Cross-sectional findings from the Newcastle 85+ study. Mech Ageing Dev 2012; 133(6): 456–66. [DOI] [PubMed] [Google Scholar]
- 17. Kingston A, Davies K, Collerton J et al. The contribution of diseases to the male-female disability-survival paradox in the very old: Results from the Newcastle 85+ study. PLoS One 2014; 9: e88016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Cox DR, Miller HD. The Theory of Stochastic Processes. London: Chapman and Hall, 1965. [Google Scholar]
- 19. Meira-Machado L, de Uña-Álvarez J, Cadarso-Suárez C, Andersen PK. Multi-state models for the analysis of time-to-event data. Stat Methods Med Res 2009; 18: 195–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Jackson C. Multi-state models for panel data: The msm package for R. J Stat Softw 2011; 38: 28. [Google Scholar]
- 21. McLean RR, Mangano KM, Hannan MT, Kiel DP, Sahni S. Dietary protein intake is protective against loss of grip strength among older adults in the Framingham offspring cohort. J Gerontol A Biol Sci Med Sci 2016; 71: 356–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Beasley JM, Wertheim BC, LaCroix AZ et al. Biomarker-calibrated protein intake and physical function in the Women's Health Initiative. J Am Geriatr Soc 2013; 61: 1863–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Houston DK, Nicklas BJ, Ding J et al. Dietary protein intake is associated with lean mass change in older, community-dwelling adults: The health, aging, and body composition (health ABC) study. Am J Clin Nutr 2008; 87: 150–5. [DOI] [PubMed] [Google Scholar]
- 24. Isanejad M, Mursu J, Sirola J et al. Dietary protein intake is associated with better physical function and muscle strength among elderly women. Br J Nutr 2016; 115: 1281–91. [DOI] [PubMed] [Google Scholar]
- 25. Cawood AL, Elia M, Stratton RJ. Systematic review and meta-analysis of the effects of high protein oral nutritional supplements. Ageing Res Rev 2012; 11: 278–96. [DOI] [PubMed] [Google Scholar]
- 26. Otsuka R, Tange C, Tomida M et al. Dietary factors associated with the development of physical frailty in community-dwelling older adults. J Nutr Health Aging 2019; 23: 89–95. [DOI] [PubMed] [Google Scholar]
- 27. Beasley JM, LaCroix AZ, Neuhouser ML et al. Protein intake and incident frailty in the Women's Health Initiative observational study. J Am Geriatr Soc 2010; 58: 1063–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Sandoval-Insausti H, Pérez-Tasigchana RF, López-García E, García-Esquinas E, Rodríguez-Artalejo F, Guallar-Castillón P. Macronutrients intake and incident frailty in older adults: A prospective cohort study. J Gerontol A Biol Sci Med Sci 2016; 71: 1329–34. [DOI] [PubMed] [Google Scholar]
- 29. Shikany JM, Barrett-Connor E, Ensrud KE et al. Macronutrients, diet quality, and frailty in older men. J Gerontol A Biol Sci Med Sci 2014; 69: 695–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Zaslavsky O, Zelber-Sagi S, Hebert JR et al. Biomarker-calibrated nutrient intake and healthy diet index associations with mortality risks among older and frail women from the Women's Health Initiative. Am J Clin Nutr 2017; 105: 1399–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Carbone JW, McClung JP, Pasiakos SM. Recent advances in the characterization of skeletal muscle and whole-body protein responses to dietary protein and exercise during negative energy balance. Adv Nutr 2018; 10: 70–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kim CO, Lee KR. Preventive effect of protein-energy supplementation on the functional decline of frail older adults with low socioeconomic status: A community-based randomized controlled study. J Gerontol A Biol Sci Med Sci 2013; 68: 309–16. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


