Abstract
The animal mitochondrial genome, although small, can have a big impact on health and disease. Non-pathogenic sequence variation among mitochondrial DNA (mtDNA) haplotypes influences traits including fertility, healthspan and lifespan, whereas pathogenic mutations are linked to incurable mitochondrial diseases and other complex conditions like ageing, diabetes, cancer and neurodegeneration. However, we know very little about how mtDNA genetic variation contributes to phenotypic differences. Infrequent recombination, the multicopy nature and nucleic acid-impenetrable membranes present significant challenges that hamper our ability to precisely map mtDNA variants responsible for traits, and to genetically modify mtDNA so that we can isolate specific mutants and characterize their biochemical and physiological consequences. Here, we summarize the past struggles and efforts in developing systems to map and edit mtDNA. We also assess the future of performing forward and reverse genetic studies on animal mitochondrial genomes.
This article is part of the theme issue ‘Linking the mitochondrial genotype to phenotype: a complex endeavour’.
Keywords: mitochondrial DNA, genetic engineering, linkage mapping, genotype to phenotype
1. Introduction
Understanding how a genome instructs the phenotypic characteristics of an organism is one of the major scientific endeavours of modern molecular genetics. This is largely achieved by a combination of forward genetic studies, which use phenotypic traits to unbiasedly map the genetic basis of defined biological phenomena, and reverse genetics studies, which analyse the phenotypic effects of modifying a given genetic element. For the nuclear genome, our ability to perform forward and reverse genetics has been established for several decades [1]. The recent advances in sequencing technologies and the CRISPR-Cas9 revolution have further enhanced our capacity for mapping and editing nuclear genomes with unprecedented efficiency in a multitude of organisms [2], leading to plentiful applications in research, industry, medicine and agriculture.
By contrast, the mitochondrial genome has been left behind in this genetic engineering era. Infrequent recombination and the multicopy nature of mtDNA present many challenges that prevent us from mapping the genetic underpinnings of phenotypic traits. Moreover, the lack of a robust method to genetically modify mtDNA in nearly all species leaves us little power to study various aspects of mtDNA biology and to model disease progression caused by pathogenic mutations. Given the essential functions of mtDNA and its link to incurable diseases, there is an increasing pressure for the development of genetic tools to dissect the complex roles mtDNA plays in development, ageing, disease and evolution. In this article, we discuss the motivation and challenges of mapping and editing animal mtDNA.
2. Why do we want to link mtDNA genotypes to phenotypes?
With a few exceptions, the mitochondrial genome is found in the matrix of the dynamic mitochondrial network of all eukaryotic cells in multiple copies. There is a vast diversity in mtDNA structure and composition among species of different kingdoms [3,4]. Generally speaking, yeast and plant mtDNA are large (e.g. approx. 76–86 kb in different Saccharomyces cerevisiae strains [5,6] and approx. 367 kb in Arabidopsis thaliana [7]), owing to an increased number of non-coding elements, such as introns and repeat sequences. The genome organization and gene content are also more variable [3,8]. By contrast, most bilaterian mitochondrial genomes tend to be a compact circular molecule of less than 20 kb, carrying 37 intron-less genes encoding 13 proteins, 2 ribosomal RNAs (rRNAs) and 22 transfer RNAs (tRNAs) [9,10]. Besides the coding region with all the genes aligned one after the other, there is also a non-coding region (or control region) that contains elements important for replication and transcription [11].
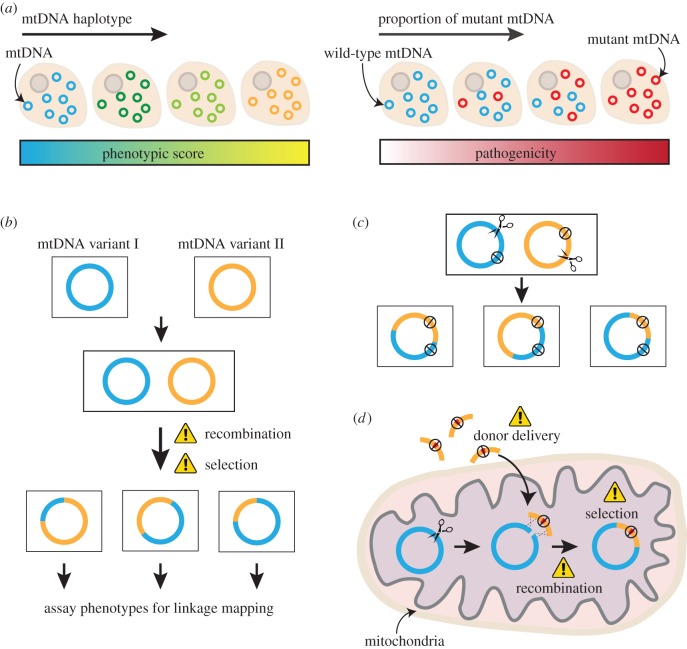
Despite the simple composition of animal mtDNA, variations in its sequence can have broad and significant phenotypic consequences (figure 1a). Within a species, multiple mtDNA variants, known as haplotypes, exist. This is largely a consequence of uniparental inheritance, as different genotypes isolated in individual lineages evolve independently based on the demands from the local environment and the paired nuclear genome. Human haplotypes often differ for less than 0.3% of the 16.5 kb genome—fewer than 50 single nucleotide polymorphisms (SNPs) [12]. However, these minor differences have been associated with differences in longevity [13–17], spermatozoa motility [18–20], risk of multiple sclerosis [21,22], type 2 diabetes [23] and certain cancers [24,25]. Some haplotypes can even impact a repertoire of phenotypes (i.e. pleiotropism) [26]. More direct evidence that mtDNA sequence variation affects complex traits comes from studies in which numerous backcrosses were performed to generate strains that have the same nuclear background but different mitochondrial genotypes. For example, 9.3% of nuclear genes showed differential expressions between males of five Drosophila lines that differed only by their mtDNA sequence, and one mtDNA genotype even led to male sterility [27]. In another study, two mouse strains with mitochondrial genotypes that differ for only 39 SNPs in the coding region showed differences in lifespan, insulin regulation, body weight and signs of ageing including telomere shortening, tumour incidence and ovarian function [28]. In some cases, mismatches between mitochondrial and nuclear genomes can even result in embryonic lethality [29–31].
Figure 1.
Motivation, challenges and opportunities for mapping and editing animal mtDNA. (a) Animal mtDNA can impact health and disease. Sequence variation in mtDNA haplotypes is linked to phenotypic variation. The level of pathogenic mtDNA mutations determines the pathogenicity and severity of symptoms. (b) Mapping of animal mtDNA can be achieved by mixing two mtDNA genotypes with defined phenotypic differences to generate heteroplasmic individuals. Homoplasmic recombinant genomes are then isolated based on spontaneous or induced recombination. Subsequently, individuals carrying different recombinant genomes are assayed for a given phenotype to define trait-associated SNP(s). Current challenges holding back our mapping capacity include the low rate of recombination in animal mitochondria and lack of a system to isolate and select individuals that are homoplasmic for only one type of recombinant genome. (c) Expression of mito-nucleases in heteroplasmic lines can be used to induce and isolate organisms that are homoplasmic for a certain recombinant mtDNA. Expression of chosen mito-nucleases (e.g. mitoRE, mitoTALEN and mitoZFN) introduces double-strand break(s) at different positions of the two parental genomes. The break in each genome will be repaired based on the homologous sequence in the other genome, resulting in the generation of recombinant genomes lacking recognition sites of the targeted nucleases. The mito-nucleases also select against the parental genomes to allow the recombinant mtDNA to take over. The black stop symbol indicates the lack of a recognition site for the expressed mito-nucleases. (d) Multiple challenges remain in order to transform animal mtDNA, including the delivery of external DNA into mitochondria, the low frequency of recombination and the inability to select for transformed genomes. The latter two challenges may be overcome by expression of mito-nucleases, which induces recombination to promote the incorporation of the desired modification(s) presented on the donor template, and selects against the parental genomes to allow the takeover by the transformant.
Differences in organismal traits and physiology caused by mtDNA sequence variation could simply be a result of differences in respiratory competence, or other factors that are more complex. For instance, impaired oogenesis and embryonic lethality result from an incompatibility between a tRNA polymorphism in Drosophila simulans (simw501) mtDNA and a polymorphism in the corresponding tRNA synthetase gene in Drosophila melanogaster strain Oregon-R nuclear DNA [30,31]. Mutations that do not directly affect the function of canonical mtDNA genes can also cause differences in organismal traits and physiology. Recently, over eight novel mitochondrial-derived peptides, encoded by small open-reading frames identified in human and rodent mitochondrial genomes, have been shown to have retrograde signalling functions that lead to systemic effects [32–35]. One such peptide is MOTS-c, which is encoded within the mitochondrial 12S rRNA gene and has been shown to protect against age- and diet-induced insulin resistance [35]. Many long and small non-coding RNAs have also been shown to be encoded in mammalian mtDNA, but their mode of action is currently unknown [36–39]. Furthermore, human mtDNA-coding sequences contain binding sites for nuclear transcription factors (e.g. c-Jun and CEBPb) and this may endow regulatory potential to these sequences [40]. Therefore, mtDNA can influence animal physiology, development and ageing in complex ways beyond our current understanding. Mitochondrial genotypes often differ for multiple SNPs, and a system to map the causative SNP(s) will help elucidate the underlying mechanism of how non-pathogenic mitochondrial genetic variations attribute to organismal traits.
In addition to linking non-pathogenic sequence variations to phenotypic differences, there is also a need to study pathogenic mutations and how they cause disease. Pathogenic mtDNA variants can be inherited or acquired owing to mutations or replication errors [41,42]. They often coexist with wild-type genomes within an individual (called heteroplasmy) and their abundance can change as the mtDNA divides and segregates during development and ageing. To date, over 350 mutations in mtDNA have been reported to cause a spectrum of incurable mitochondrial diseases that affect 1 in 5000 individuals in the UK [12,43]. Mitochondrial diseases caused by mtDNA mutations present diverse symptoms across individuals and tissues. Some pathogenic mtDNA mutations have pleiotropic effects, with different mutation loads causing different phenotypes (figure 1a). For instance, 3243A>G is a common pathogenic mutation in the tRNALEU gene that is associated with autism and diabetes when at low levels (approx. 10–30% of total mtDNA) [44,45], encephalomyopathies at medium levels (50–90% of total mtDNA [46]) and perinatal lethality at high levels (greater than 90% of total mtDNA). In addition, mtDNA mutations can have different biochemical and pathological consequences in different tissues [47] and individuals with different nuclear backgrounds or mitochondrial haplotypes. For instance, Leber hereditary optic neuropathy (LHON) is an inherited form of vision loss that has an acute onset of symptoms that usually begin in early adulthood. It is primarily owing to one of the three homoplasmic mtDNA mutations (3460G>A in ND1, 11778G>A in ND4 or 14484T>C in ND6 [48–50]) that affect complex I activity, but there is an increased risk of developing LOHN for males [51,52], for those with mtDNA haplotype J [53,54] or those who smoke or have excessive alcohol consumption [55]. Understanding how the nuclear genome and environmental conditions impact penetrance will help us gain more insights on disease prevention and treatment options. However, without being able to isolate specific mitochondrial mutants and model their effects under different conditions, our knowledge on these aspects is very limited.
To be able to fully understand how mtDNA influences health and disease, systems are required that can (i) genetically unlink mitochondrial SNPs, therefore allowing separation of neutral polymorphisms from the causative SNPs and (ii) isolate specific mitochondrial mutants for functional studies. However, multiple challenges hold us back from developing tools for mapping and editing animal mtDNA, which will be addressed in this prospective review.
3. What are the challenges to mapping mtDNA?
Many aspects of mitochondrial genetics and biology have made genetic mapping difficult. For instance, the effect of a mtDNA haplotype on a phenotype will depend strongly on the nuclear background [51,52,56–62]. However, the main challenges are infrequent recombination and the multicopy nature of mtDNA.
For nuclear genes, naturally existing variations or mutations induced by radiation, chemical or insertional mutagenesis (e.g. transposable elements) can be used for linkage mapping. SNPs are then genetically unlinked through recombination during meiosis. Subsequently, strains with different genotypes are assayed for a particular phenotype to link the phenotype to certain SNPs. A similar forward genetic approach could not be easily applied to study mitochondrial genes (figure 1b). First, recombination is rare in animal mitochondria, if occurring at all. Most observations of mtDNA recombination are one-off events in only a handful of species with few details of the parental genomes [63–71]. Second, as each cell contains many copies of mtDNA, it is difficult to create random mutations for linkage mapping. While mitochondrial mutations can be induced by chemicals, such as bleomycin [72–74], or in strains with reduced mtDNA polymerase proofreading capacity (known as mutator strains) [75–78], these approaches will generate heteroplasmic cells with individual genomes mutated at different loci and the genetic composition of each cell will be vastly different. Heteroplasmy prevents accurate linking of genotype to phenotype as the effects of individual variants are masked. This means that we can only use naturally existing mitochondrial genotypes with defined impacts on a given phenotype. We then need to mix them together to generate a heteroplasmic organism, so that they can recombine. Artificial heteroplasmy is often achieved by mitochondrial transfer between two homoplasmic eggs, which could be problematic for certain species. Third, even if recombination can be induced to occur at a relatively high frequency, different recombinants from the two defined parental genomes can be generated within the same cell or organism. In this case, the functional consequences of a recombinant genome will be masked by other recombinants or the parental genomes in the same cell. Hence, we have to find ways to select for organisms carrying only one type of recombinant.
4. Can we develop a system to map animal mtDNA?
Despite the above complications, linkage mapping has been made possible with Drosophila mtDNA. In a number of lineages of a heteroplasmic setting, we were able to isolate recombinant mitochondrial genomes owing to rare spontaneous recombination. Each recombinant had a strong selective advantage over the two parental genomes, and thus reached homoplasmy after a few generations. We used these to map mtDNA sequences that give one of the parental genomes a selfish transmission advantage [69]. What is more exciting, we also generated a system to induce recombination and select for individuals homoplasmic for recombinant genomes (figure 1c). In this set-up, cytoplasmic transfer was performed to generate heteroplasmic fruit flies containing two parental genomes. This was followed by the expression of mitochondrially targeted restriction enzyme(s) (mitoREs) to cut the parental genomes at different positions [69]. The double-strand break in one genome was efficiently repaired based on the homologous sequences presented in the other genome, and this generated recombinant mtDNA that lacks recognition sites for the mitoREs. The use of mitoREs also selects against the parental genomes as they are linearized and degraded, whereas the recombinant mtDNA will be resistant to cutting. This system is very efficient for isolating recombinants, even if the two parental genomes are highly diverged, including genomes from different species where the sequence homology is less than 92%. Isolation of homoplasmic recombinant genomes in this way opens up the exciting possibility of precisely defining trait-associated mtDNA SNPs.
There is no doubt much will be learnt from mapping Drosophila mtDNA, and a similar mitoRE system could also be applied to other animals. However, much optimization is required to increase the flexibility and efficiency of mtDNA mapping. The availability and location of recognition sites presented in the two parental genomes limits the mitoRE system by constraining which genomes can be studied and where crossovers will occur. This shortfall may be rescued by using other mitochondrially targeted nucleases (mito-nucleases) that can be engineered to target more sequences of choice. Mitochondrially targeted zinc finger nucleases (mitoZFNs) and transcription activator-like effector nucleases (mitoTALENs) consist of a customizable DNA-binding domain fused with a nuclease domain and a mitochondrial localization signal. In mitoZFNs, each zinc finger domain recognizes a three or four nucleotide sequence and several domains can be engineered in an array to target longer sequences [79–81]. mitoTALENs provide increased sequence targeting flexibility as each transcription activator-like effector consists of an array of 34 amino acid repeats that each binds a single DNA base and can be engineered to target almost any sequence [82–88]. mitoZFNs and mitoTALENs have been used to eliminate pathogenic mtDNA that differs from the coexisting wild-type genome by only a single point mutation in rodent germ cells [86] and somatic tissues [89–91], and in patient-derived cells [87,92]. Therefore, it is feasible that they can replace mitoREs to achieve more flexible mapping in Drosophila and other animal models.
It should be noted that mitoZFNs/mitoTALENs can be difficult to implement. The importing efficiency of mitoTALENs/mitoZFNs into mitochondria can vary depending on the targeted sequence, which determines the protein properties of the DNA-binding domain [93]. Since mitoZFNs/mitoTALENs work as heterodimers, both monomers need to be present in a sufficient amount inside mitochondria to function. This can be challenging if the monomers have different importing efficiencies. Furthermore, being heterodimers requires the design of two independent DNA-binding modules to target a single sequence [93]. Hence, the plasmid constructs are usually large, which can impede their in vivo delivery. To overcome some of these shortfalls, ZF- and TALE-targeted monomeric nucleases have been recently developed [94,95] and tested in mitochondria [96]. With a smaller construct and simplification of importing only one type of DNA-binding domain into mitochondria, the monomeric versions present a promising alternative that allows more efficient delivery and increased flexibility of target sequences [96].
In addition, to achieve efficient mtDNA mapping, future research in species where there is active mitochondrial recombination may help us develop ways to increase the recombination frequency in animal mitochondria. For instance, key components for mtDNA recombination, including RecA homologues in A. thaliana and Rad52-like proteins in S. cerevisiae, have been identified [97–100]. These and other supplementary components can be targeted to animal mitochondria to induce recombination. Proteins mediating recombination in bacteria, bacteriophages and even metazoan nuclear genomes can also be targeted to achieve the same aim.
5. What are the challenges to editing animal mtDNA?
While mapping enables detangling of the functional consequences of individual SNPs, mtDNA editing is required to verify mapping by generating specific mutations. To date, a transformation system for mtDNA editing has been established in S. cerevisiae (baker's yeast) [101,102] and Chlamydomonas reinhardtii (green algae) [103]. Both are unicellular organisms with active recombination in mitochondria, allowing desired changes in a donor template to be introduced into the genome. The delivery of donor DNA was accomplished using biolistic bombardment into mitochondria of respiration-defective mutant strains, followed by the selection of successful transformants based on respiratory function. In yeast, integration of ARG8m has also been used to select for transformed cells with a mutated nuclear ARG8 [104]. ARG8 is required for arginine biosynthesis, so the selection is independent of respiratory function. Although the delivery of donor DNA is inefficient, and a large starting population is required to select transformants, the transformation system has made the two species very tractable models to study mitochondrial dynamics and the physiological consequences of mitochondrial mutations. For example, yeast mutants have been used to model human pathogenic mtDNA mutations [105–108]. The integration of ARG8m has been used to disrupt mitochondrial genes and to study mitochondrial gene expression [104,109,110]. Moreover, visible reporters have been inserted into the yeast mtDNA, including green fluorescent protein (GFP) added after the start of the Cox3 gene to study mitochondrial gene expression [111] and adaptation of the LacO–LacI-GFP system to visualize mtDNA [112]. Similarly, expression of GFP or the zeomycin resistance gene ble from mtDNA has been achieved in C. reinhardtii [113,114].
In animals, mitoREs have been expressed in the germline to isolate heritable homoplasmic mtDNA mutants in Drosophila [115]. This method relies on the selection of pre-existing mtDNA variants that lack the recognition site of the expressed mitoRE. The isolated ND2 and Cox1 mutants have been useful for disease modelling [116,117]. We and others have also used them to study how transmission of coexisting mitochondrial genomes is influenced by selection (summarized in [118]). However, this is the only approach that allows isolation of homoplasmic mitochondrial mutants in a metazoan, and the site of mutations is restricted to sequences proximal to or at the recognition site of the restriction enzyme used.
A transformation system to edit animal mtDNA in a more desired manner has not yet been established, and the challenges come in multiple ways (figure 1d). First, the transformation of the germline mtDNA is required to edit mitochondrial genomes of multicellular animals, which scales up the difficulty of this endeavour. Moreover, in yeast and algae, mtDNA loss is not lethal because this can be rescued by supplementary factors or compensatory cellular mechanisms (e.g. glycolysis or photosynthesis). However, in animals, we cannot isolate mtDNA mutants that are homoplasmic lethal at the organismal level. A system to isolate and maintain lethal mutations, for example, by expression of nuclear-encoded version of mitochondrial proteins, is far from being established in animals. Second, the rarity of recombination in animal mitochondria impedes the exchange between a donor DNA template and the endogenous genome, which is required to introduce specific mutations and precise tagging. Third, there is no efficient method to deliver donor DNA into mitochondria. There has been a great abundance of research on this topic with many exciting reports, including electroporation, protein–DNA conjugates, bacterial conjugation and nanocarriers like MITO-porter and adeno-associated virus-mediated transfer [119–130]. Nevertheless, few, if any, of these methods have been reproduced by independent laboratories, even at the cell level. One reason for this may be that it is very difficult to test for mitochondrial import definitively [131]. Most studies rely on sub-fractionation and the generation of mitoplasts (isolated mitochondria with outer membrane removed) to show uptake into the matrix. However, such methods are vulnerable to false positives caused by contamination. Success in the delivery of foreign genetic materials can be verified if there is a strong selection in favour of the transformed genome. Therefore, the fourth challenge is the lack of ways to select for transformants. Successful transformation often only occurs to a very small population, so the ability to select for homoplasmic transformants is incredibly important.
6. Can we edit animal mtDNA in the near future?
While we currently have no system to edit animal mtDNA, advances in mito-nucleases have helped us solve some of the challenges we mentioned above. For example, we have used mitoREs to induce recombination [69]. Importing recombination machinery from other species into animal mitochondria (see our earlier discussion in §4) might also increase the basal recombination frequency. Alternatively, in vitro-modified mtDNA could be directly delivered, so that recombination is not required to incorporate specific alterations. For instance, the first transformation of algae used mtDNA purified from C. reinhardtii or Chlamydomonas smithii [132].
The challenge of selection for transformants obtained by either recombination-based repair or delivery of in vitro-modified mtDNA could also be overcome by using mito-nucleases, which can be engineered to cut the endogenous genome to cause their subsequent degradation. In such systems, one can construct the donor template or modified genome to lack the recognition site, so that any transformants will be resistant to the cut and thus be selected for. For example, mitoREs have already been used to create D. melanogaster flies homoplasmic for very diverged Drosophila yakuba mtDNA by eliminating the endogenous genomes after mitochondrial transfer [133]. Similarly, engineered mitoTALENs and mitoZFNs are effective at selecting against mutant genomes that differ from the coexisting wild-type genome by just one nucleotide [86,87,89,90,92]. Therefore, mito-nucleases represent powerful tools to selectively eliminate untransformed genomes.
While the use of mito-nucleases enables screening of many embryos for successful mtDNA manipulation in species like Drosophila, it will be difficult and expensive to implement this in other animals like rodents. Editing mtDNA for these species can be first considered in cultured cell lines. A cell model will also be feasible for creating mutants that would otherwise be homoplasmic lethal at the organismal or tissue level. For instance, lethality, owing to reduced respiratory function, could be overcome by additional supplements in the cell culture medium. In this case, cells completely lacking mtDNA (ρ0 cells) can be used to protect the introduced in vitro-modified mtDNA from the competition with endogenous genomes.
Overall, some of the challenges holding back animal mtDNA editing may be overcome using mito-nucleases. However, not being able to reliably deliver nucleic acids into animal mitochondria still presents as a huge barrier. This prevents us from importing not only donor template or in vitro-modified mtDNA for transformation, but also RNA for CRISPR-mediated mtDNA editing [134,135]. A mitochondria-adapted CRISPR-Cas9 platform, if established, could prompt a revolution in mitochondrial genome engineering and our biological understanding of mitochondria and mtDNA.
7. Concluding remarks
Significant obstacles must be overcome to achieve forward and reverse genetic studies for mitochondrial genomes. To date, there has been only one published case where homoplasmic recombinants were successfully isolated and used for functional mapping [69]. Moreover, no mtDNA transformation system has been established in any metazoan. These shortfalls hinder our understanding of how mtDNA impacts health and disease. However, there are exciting new possibilities to induce and select for mtDNA recombinants using mito-nucleases. As we learn more about mtDNA repair mechanisms and mitochondrial nucleic acid import strategies, other advances are yet to come to allow us to pass through the current technical bottlenecks and revolutionize mtDNA engineering.
Data accessibility
This article has no additional data.
Authors' contributions
A.K. and H.M. wrote the manuscript together.
Competing interests
We declare we have no competing interests.
Funding
This work is funded by Wellcome Trust grant 203767/Z/16/Z to A.K. and 202269/Z/16/Z to H.M.
References
- 1.Carroll D. 2017. Genome editing: past, present, and future. Yale J. Biol. Med. 90, 653–659. [PMC free article] [PubMed] [Google Scholar]
- 2.Gurumurthy CB, Grati M, Ohtsuka M, Schilit SLP, Quadros RM, Liu XZ. 2016. CRISPR: a versatile tool for both forward and reverse genetics research. Hum. Genet. 135, 971–976. ( 10.1007/s00439-016-1704-4) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Smith DR, Keeling PJ. 2015. Mitochondrial and plastid genome architecture: reoccurring themes, but significant differences at the extremes. Proc. Natl Acad. Sci. USA 112, 10 177–10 184. ( 10.1073/pnas.1422049112) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lavrov DV, Pett W. 2016. Animal mitochondrial DNA as we do not know it: mt-genome organization and evolution in nonbilaterian lineages. Genome. Biol. Evol. 8, 2896–2913. ( 10.1093/gbe/evw195) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Foury F, Roganti T, Lecrenier N, Purnelle B. 1998. The complete sequence of the mitochondrial genome of Saccharomyces cerevisiae. FEBS Lett. 440, 325–331. ( 10.1016/S0014-5793(98)01467-7) [DOI] [PubMed] [Google Scholar]
- 6.Wolters JF, Chiu K, Fiumera HL. 2015. Population structure of mitochondrial genomes in Saccharomyces cerevisiae. BMC Genomics 16, 451 ( 10.1186/s12864-015-1664-4) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Unseld M, Marienfeld JR, Brandt P, Brennicke A. 1997. The mitochondrial genome of Arabidopsis thaliana contains 57 genes in 366,924 nucleotides. Nat. Genet. 15, 57–61. ( 10.1038/ng0197-57) [DOI] [PubMed] [Google Scholar]
- 8.Burger G, Gray MW, Lang BF. 2003. Mitochondrial genomes: anything goes. Trends Genet. 19, 709–716. ( 10.1016/j.tig.2003.10.012) [DOI] [PubMed] [Google Scholar]
- 9.Anderson S, et al. 1981. Sequence and organization of the human mitochondrial genome. Nature 290, 457–465. ( 10.1038/290457a0) [DOI] [PubMed] [Google Scholar]
- 10.Boore JL. 1999. Animal mitochondrial genomes. Nucleic Acids Res. 27, 1767–1780. ( 10.1093/nar/27.8.1767) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Taanman JW. 1999. The mitochondrial genome: structure, transcription, translation and replication. Biochim. Biophys. Acta 1410, 103–123. ( 10.1016/S0005-2728(98)00161-3) [DOI] [PubMed] [Google Scholar]
- 12.2019. MITOMAP: a human mitochondrial genome database. See http://www.mitomap.org.
- 13.Bilal E, et al. 2008. Mitochondrial DNA haplogroup D4a is a marker for extreme longevity in Japan. PLoS ONE 3, e2421 ( 10.1371/journal.pone.0002421) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cai X-Y, et al. 2009. Association of mitochondrial DNA haplogroups with exceptional longevity in a Chinese population. PLoS ONE 4, e6423 ( 10.1371/journal.pone.0006423) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ross OA, McCormack R, Curran MD, Duguid RA, Barnett YA, Rea IM, Middleton D. 2001. Mitochondrial DNA polymorphism: its role in longevity of the Irish population. Exp. Gerontol. 36, 1161–1178. ( 10.1016/S0531-5565(01)00094-8) [DOI] [PubMed] [Google Scholar]
- 16.Niemi A-K, Hervonen A, Hurme M, Karhunen PJ, Jylhä M, Majamaa K. 2003. Mitochondrial DNA polymorphisms associated with longevity in a Finnish population. Hum. Genet. 112, 29–33. ( 10.1007/s00439-002-0843-y) [DOI] [PubMed] [Google Scholar]
- 17.De Benedictis G, et al. 1999. Mitochondrial DNA inherited variants are associated with successful aging and longevity in humans. FASEB J. 13, 1532–1536. ( 10.1096/fasebj.13.12.1532) [DOI] [PubMed] [Google Scholar]
- 18.Montiel-Sosa F, Ruiz-Pesini E, Enríquez JA, Marcuello A, Díez-Sánchez C, Montoya J, Wallace DC, López-Pérez MJ. 2006. Differences of sperm motility in mitochondrial DNA haplogroup U sublineages. Gene 368, 21–27. ( 10.1016/j.gene.2005.09.015) [DOI] [PubMed] [Google Scholar]
- 19.Ruiz-Pesini E, et al. 2000. Human mtDNA haplogroups associated with high or reduced spermatozoa motility. Am. J. Hum. Genet. 67, 682–696. ( 10.1086/303040) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Feng G-F, Zhang J, Feng L-M, Shen N-X, Li L-J, Zhu Y-M. 2013. Mitochondrial DNA haplogroup associated with sperm motility in the Han population. Asian J. Androl. 15, 630–633. ( 10.1038/aja.2013.83) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yu X, et al. 2008. mtDNA nt13708A variant increases the risk of multiple sclerosis. PLoS ONE 3, e1530 ( 10.1371/journal.pone.0001530) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ban M, Elson J, Walton A, Turnbull D, Compston A, Chinnery P, Sawcer S. 2008. Investigation of the role of mitochondrial DNA in multiple sclerosis susceptibility. PLoS ONE 3, e2891 ( 10.1371/journal.pone.0002891) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Poulton J, Luan J, Macaulay V, Hennings S, Mitchell J, Wareham NJ. 2002. Type 2 diabetes is associated with a common mitochondrial variant: evidence from a population-based case-control study. Hum. Mol. Genet. 11, 1581–1583. ( 10.1093/hmg/11.13.1581) [DOI] [PubMed] [Google Scholar]
- 24.Fang H, et al. 2010. Cancer type-specific modulation of mitochondrial haplogroups in breast, colorectal and thyroid cancer. BMC Cancer 10, 421 ( 10.1186/1471-2407-10-421) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ma L, et al. 2018. Breast cancer-associated mitochondrial DNA haplogroup promotes neoplastic growth via ROS-mediated AKT activation. Int. J. Cancer 142, 1786–1796. ( 10.1002/ijc.31207) [DOI] [PubMed] [Google Scholar]
- 26.Marom S, Friger M, Mishmar D. 2017. MtDNA meta-analysis reveals both phenotype specificity and allele heterogeneity: a model for differential association. Sci. Rep. 7, 43449 ( 10.1038/srep43449) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Innocenti P, Morrow EH, Dowling DK. 2011. Experimental evidence supports a sex-specific selective sieve in mitochondrial genome evolution. Science 332, 845–848. ( 10.1126/science.1201157) [DOI] [PubMed] [Google Scholar]
- 28.Latorre-Pellicer A, et al. 2016. Mitochondrial and nuclear DNA matching shapes metabolism and healthy ageing. Nature 535, 561–565. ( 10.1038/nature18618) [DOI] [PubMed] [Google Scholar]
- 29.Ma H, et al. 2016. Incompatibility between nuclear and mitochondrial genomes contributes to an interspecies reproductive barrier. Cell Metab. 24, 283–294. ( 10.1016/j.cmet.2016.06.012) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Meiklejohn CD, Holmbeck MA, Siddiq MA, Abt DN, Rand DM, Montooth KL. 2013. An incompatibility between a mitochondrial tRNA and its nuclear-encoded tRNA synthetase compromises development and fitness in Drosophila. PLoS Genet. 9, e1003238 ( 10.1371/journal.pgen.1003238) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang C, Montooth KL, Calvi BR. 2017. Incompatibility between mitochondrial and nuclear genomes during oogenesis results in ovarian failure and embryonic lethality. Development 144, 2490–2503. ( 10.1242/dev.151951) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hashimoto Y, et al. 2001. A rescue factor abolishing neuronal cell death by a wide spectrum of familial Alzheimer's disease genes and Aβ. Proc. Natl Acad. Sci. USA 98, 6336–6341. ( 10.1073/pnas.101133498) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Guo B, Zhai D, Cabezas E, Welsh K, Nouraini S, Satterthwait AC, Reed JC. 2003. Humanin peptide suppresses apoptosis by interfering with Bax activation. Nature 423, 456–461. ( 10.1038/nature01627) [DOI] [PubMed] [Google Scholar]
- 34.Cobb LJ, et al. 2016. Naturally occurring mitochondrial-derived peptides are age-dependent regulators of apoptosis, insulin sensitivity, and inflammatory markers. Aging (Albany NY) 8, 796–809. ( 10.18632/aging.100943) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lee C, et al. 2015. The mitochondrial-derived peptide MOTS-c promotes metabolic homeostasis and reduces obesity and insulin resistance. Cell Metab. 21, 443–454. ( 10.1016/j.cmet.2015.02.009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rackham O, Shearwood A-MJ, Mercer TR, Davies SM. K., Mattick JS, Filipovska A. 2011. Long noncoding RNAs are generated from the mitochondrial genome and regulated by nuclear-encoded proteins. RNA 17, 2085–2093. ( 10.1261/rna.029405.111) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Alessio E, et al. 2019. Single cell analysis reveals the involvement of the long non-coding RNA Pvt1 in the modulation of muscle atrophy and mitochondrial network. Nucleic Acids Res. 47, 1653–1670. ( 10.1093/nar/gkz007) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Riggs CL, Summers A, Warren DE, Nilsson GE, Lefevre S, Dowd WW, Milton S, Podrabsky JE. 2018. Small non-coding RNA expression and vertebrate anoxia tolerance. Front. Genet. 9, 230 ( 10.3389/fgene.2018.00230) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Burzio VA, et al. 2009. Expression of a family of noncoding mitochondrial RNAs distinguishes normal from cancer cells. Proc. Natl Acad. Sci. USA 106, 9430–9434. ( 10.1073/pnas.0903086106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Blumberg A, Sri Sailaja B, Kundaje A, Levin L, Dadon S, Shmorak S, Shaulian E, Meshorer E, Mishmar D. 2014. Transcription factors bind negatively selected sites within human mtDNA genes. Genome Biol. Evol. 6, 2634–2646. ( 10.1093/gbe/evu210) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zheng W, Khrapko K, Coller HA, Thilly WG, Copeland WC. 2006. Origins of human mitochondrial point mutations as DNA polymerase γ-mediated errors. Mutat. Res. 599, 11–20. ( 10.1016/j.mrfmmm.2005.12.012) [DOI] [PubMed] [Google Scholar]
- 42.Khrapko K, Coller HA, André PC, Li XC, Hanekamp JS, Thilly WG. 1997. Mitochondrial mutational spectra in human cells and tissues. Proc. Natl Acad. Sci. USA 94, 13 798–13 803. ( 10.1073/pnas.94.25.13798) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gorman GS, et al. 2015. Prevalence of nuclear and mitochondrial DNA mutations related to adult mitochondrial disease. Ann. Neurol. 77, 753–759. ( 10.1002/ana.24362) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pons R, et al. 2004. Mitochondrial DNA abnormalities and autistic spectrum disorders. J. Pediatr. 144, 81–85. ( 10.1016/j.jpeds.2003.10.023) [DOI] [PubMed] [Google Scholar]
- 45.van den Ouweland JM, Lemkes HH, Gerbitz KD, Maassen JA. 1995. Maternally inherited diabetes and deafness (MIDD): a distinct subtype of diabetes associated with a mitochondrial tRNA(Leu)(UUR) gene point mutation. Muscle Nerve Suppl. 3, S124–S130. ( 10.1002/mus.880181425) [DOI] [PubMed] [Google Scholar]
- 46.Goto Y, Nonaka I, Horai S. 1990. A mutation in the tRNA(Leu)(UUR) gene associated with the MELAS subgroup of mitochondrial encephalomyopathies. Nature 348, 651–653. ( 10.1038/348651a0) [DOI] [PubMed] [Google Scholar]
- 47.Hämäläinen RH, Manninen T, Koivumäki H, Kislin M, Otonkoski T, Suomalainen A. 2013. Tissue- and cell-type-specific manifestations of heteroplasmic mtDNA 3243A>G mutation in human induced pluripotent stem cell-derived disease model. Proc. Natl Acad. Sci. USA 110, E3622–E3630. ( 10.1073/pnas.1311660110) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Huoponen K, Vilkki J, Aula P, Nikoskelainen EK, Savontaus ML. 1991. A new mtDNA mutation associated with Leber hereditary optic neuroretinopathy. Am. J. Hum. Genet. 48, 1147–1153. ( 10.1038/eye.1991.45) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wallace DC, Singh G, Lott MT, Hodge JA, Schurr TG, Lezza AM, Elsas LJ, Nikoskelainen EK. 1988. Mitochondrial DNA mutation associated with Leber's hereditary optic neuropathy. Science 242, 1427–1430. ( 10.1126/science.3201231) [DOI] [PubMed] [Google Scholar]
- 50.Johns DR, Neufeld MJ, Park RD. 1992. An ND-6 mitochondrial DNA mutation associated with Leber hereditary optic neuropathy. Biochem. Biophys. Res. Commun. 187, 1551–1557. ( 10.1016/0006-291X(92)90479-5) [DOI] [PubMed] [Google Scholar]
- 51.Bu XD, Rotter JI. 1991. X chromosome-linked and mitochondrial gene control of Leber hereditary optic neuropathy: evidence from segregation analysis for dependence on X chromosome inactivation. Proc. Natl Acad. Sci. USA 88, 8198–8202. ( 10.1073/pnas.88.18.8198) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hudson G, et al. 2007. Clinical expression of Leber hereditary optic neuropathy is affected by the mitochondrial DNA-haplogroup background. Am. J. Hum. Genet. 81, 228–233. ( 10.1086/519394) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Torroni A, et al. 1997. Haplotype and phylogenetic analyses suggest that one European-specific mtDNA background plays a role in the expression of Leber hereditary optic neuropathy by increasing the penetrance of the primary mutations 11778 and 14484. Am. J. Hum. Genet. 60, 1107–1121. [PMC free article] [PubMed] [Google Scholar]
- 54.Brown MD, Sun F, Wallace DC. 1997. Clustering of Caucasian Leber hereditary optic neuropathy patients containing the 11778 or 14484 mutations on an mtDNA lineage. Am. J. Hum. Genet. 60, 381–387. ( 10.1086/515488) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kirkman MA, Yu-Wai-Man P, Korsten A, Leonhardt M, Dimitriadis K, De Coo IF, Klopstock T, Chinnery PF. 2009. Gene–environment interactions in Leber hereditary optic neuropathy. Brain 132, 2317–2326. ( 10.1093/brain/awp158) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hirose M, et al. 2016. Lifespan effects of mitochondrial mutations. Nature 540, E13–E14. ( 10.1038/nature20778) [DOI] [PubMed] [Google Scholar]
- 57.Zhu C-T, Ingelmo P, Rand DM. 2014. G×G×E for lifespan in Drosophila: mitochondrial, nuclear, and dietary interactions that modify longevity. PLoS Genet. 10, e1004354 ( 10.1371/journal.pgen.1004354) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rand DM, Mossman JA, Zhu L, Biancani LM, Ge JY. 2018. Mitonuclear epistasis, genotype-by-environment interactions, and personalized genomics of complex traits in Drosophila. IUBMB Life 70, 1275–1288. ( 10.1002/iub.1954) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mossman JA, Ge JY, Navarro F, Rand DM. 2019. Mitochondrial DNA fitness depends on nuclear genetic background in Drosophila. G3 9, 1175–1188. ( 10.1534/g3.119.400067) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mossman JA, Tross JG, Jourjine NA, Li N, Wu Z, Rand DM. 2017. Mitonuclear interactions mediate transcriptional responses to hypoxia in Drosophila. Mol. Biol. Evol. 34, 447–466. ( 10.1093/molbev/msw246) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wolff JN, Pichaud N, Camus MF, Côté G., Blier PU, Dowling DK. 2016. Evolutionary implications of mitochondrial genetic variation: mitochondrial genetic effects on OXPHOS respiration and mitochondrial quantity change with age and sex in fruit flies. J. Evol. Biol. 29, 736–747. ( 10.1111/jeb.12822) [DOI] [PubMed] [Google Scholar]
- 62.Zaidi AA, Makova KD. 2019. Investigating mitonuclear interactions in human admixed populations. Nat. Ecol. Evol. 3, 213–222. ( 10.1038/s41559-018-0766-1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ladoukakis ED, Zouros E. 2001. Direct evidence for homologous recombination in mussel (Mytilus galloprovincialis) mitochondrial DNA. Mol. Biol. Evol. 18, 1168–1175. ( 10.1093/oxfordjournals.molbev.a003904) [DOI] [PubMed] [Google Scholar]
- 64.Hoarau G, Holla S, Lescasse R, Stam WT, Olsen JL. 2002. Heteroplasmy and evidence for recombination in the mitochondrial control region of the flatfish Platichthys flesus. Mol. Biol. Evol. 19, 2261–2264. ( 10.1093/oxfordjournals.molbev.a004049) [DOI] [PubMed] [Google Scholar]
- 65.Kraytsberg Y. 2004. Recombination of human mitochondrial DNA. Science 304, 981 ( 10.1126/science.1096342) [DOI] [PubMed] [Google Scholar]
- 66.Guo X, Liu S, Liu Y. 2006. Evidence for recombination of mitochondrial DNA in triploid crucian carp. Genetics 172, 1745–1749. ( 10.1534/genetics.105.049841) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ciborowski KL, Consuegra S, García de Leániz C, Beaumont MA, Wang J, Jordan WC. 2007. Rare and fleeting: an example of interspecific recombination in animal mitochondrial DNA. Biol. Lett. 3, 554–557. ( 10.1098/rsbl.2007.0290) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ujvari B, Dowton M, Madsen T. 2007. Mitochondrial DNA recombination in a free-ranging Australian lizard. Biol. Lett. 3, 189–192. ( 10.1098/rsbl.2006.0587) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ma H, O'Farrell PH. 2015. Selections that isolate recombinant mitochondrial genomes in animals. eLife 4, e07247 ( 10.7554/eLife.07247) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Strakova A, et al. 2016. Mitochondrial genetic diversity, selection and recombination in a canine transmissible cancer. eLife 5, 415 ( 10.7554/eLife.14552) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.D'Aurelio M, Gajewski CD, Lin MT, Mauck WM, Shao LZ, Lenaz G, Moraes CT, Manfredi G. 2004. Heterologous mitochondrial DNA recombination in human cells. Hum. Mol. Genet. 13, 3171–3179. ( 10.1093/hmg/ddh326) [DOI] [PubMed] [Google Scholar]
- 72.Lim LO, Neims AH. 1987. Mitochondrial DNA damage by bleomycin. Biochem. Pharmacol. 36, 2769–2774. ( 10.1016/0006-2952(87)90263-2) [DOI] [PubMed] [Google Scholar]
- 73.Khaidakov M, Manjanatha MG, Aidoo A. 2002. Molecular analysis of mitochondrial DNA mutations from bleomycin-treated rats. Mutat. Res. 500, 1–8. ( 10.1016/S0027-5107(01)00270-6) [DOI] [PubMed] [Google Scholar]
- 74.Gazdhar A, Lebrecht D, Roth M, Tamm M, Venhoff N, Foocharoen C, Geiser T, Walker UA. 2014. Time-dependent and somatically acquired mitochondrial DNA mutagenesis and respiratory chain dysfunction in a scleroderma model of lung fibrosis. Sci. Rep. 4, 5336 ( 10.1038/srep05336) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Trifunovic A, et al. 2004. Premature ageing in mice expressing defective mitochondrial DNA polymerase. Nature 429, 417–423. ( 10.1038/nature02517) [DOI] [PubMed] [Google Scholar]
- 76.Stewart JB, Freyer C, Elson JL, Larsson N-G. 2008. Purifying selection of mtDNA and its implications for understanding evolution and mitochondrial disease. Nat. Rev. Genet. 9, 657–662. ( 10.1038/nrg2396) [DOI] [PubMed] [Google Scholar]
- 77.Ross JM, Coppotelli G, Hoffer BJ, Olson L. 2014. Maternally transmitted mitochondrial DNA mutations can reduce lifespan. Sci. Rep. 4, 6569 ( 10.1038/srep06569) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kauppila JHK, et al. 2016. A phenotype-driven approach to generate mouse models with pathogenic mtDNA mutations causing mitochondrial disease. Cell Rep. 16, 2980–2990. ( 10.1016/j.celrep.2016.08.037) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Beerli RR, Segal DJ, Dreier B, Barbas CF. 1998. Toward controlling gene expression at will: specific regulation of the erbB-2/HER-2 promoter by using polydactyl zinc finger proteins constructed from modular building blocks. Proc. Natl Acad. Sci. USA 95, 14 628–14 633. ( 10.1073/pnas.95.25.14628) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Gammage PA, Rorbach J, Vincent AI, Rebar EJ, Minczuk M. 2014. Mitochondrially targeted ZFNs for selective degradation of pathogenic mitochondrial genomes bearing large-scale deletions or point mutations. EMBO Mol. Med. 6, 458–466. ( 10.1002/emmm.201303672) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Minczuk M, Papworth MA, Miller JC, Murphy MP, Klug A. 2008. Development of a single-chain, quasi-dimeric zinc-finger nuclease for the selective degradation of mutated human mitochondrial DNA. Nucleic Acids Res. 36, 3926–3938. ( 10.1093/nar/gkn313) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Boch J, Scholze H, Schornack S, Landgraf A, Hahn S, Kay S, Lahaye T, Nickstadt A, Bonas U. 2009. Breaking the code of DNA binding specificity of TAL-type III effectors. Science 326, 1509–1512. ( 10.1126/science.1178811) [DOI] [PubMed] [Google Scholar]
- 83.Moscou MJ, Bogdanove AJ. 2009. A simple cipher governs DNA recognition by TAL effectors. Science 326, 1501 ( 10.1126/science.1178817) [DOI] [PubMed] [Google Scholar]
- 84.Deng D, Yan C, Pan X, Mahfouz M, Wang J, Zhu J-K, Shi Y, Yan N. 2012. Structural basis for sequence-specific recognition of DNA by TAL effectors. Science 335, 720–723. ( 10.1126/science.1215670) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Mak AN.-S., Bradley P, Cernadas RA, Bogdanove AJ, Stoddard BL. 2012. The crystal structure of TAL effector PthXo1 bound to its DNA target. Science 335, 716–719. ( 10.1126/science.1216211) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Reddy P, et al. 2015. Selective elimination of mitochondrial mutations in the germline by genome editing. Cell 161, 459–469. ( 10.1016/j.cell.2015.03.051) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Bacman SR, Williams SL, Pinto M, Peralta S, Moraes CT. 2013. Specific elimination of mutant mitochondrial genomes in patient-derived cells by mitoTALENs. Nat. Med. 19, 1111–1113. ( 10.1038/nm.3261) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Hashimoto M, Bacman SR, Peralta S, Falk MJ, Chomyn A, Chan DC, Williams SL, Moraes CT. 2015. MitoTALEN: a general approach to reduce mutant mtDNA loads and restore oxidative phosphorylation function in mitochondrial diseases. Mol. Ther. 23, 1592–1599. ( 10.1038/mt.2015.126) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Bacman SR, et al. 2018. MitoTALEN reduces mutant mtDNA load and restores tRNAAla levels in a mouse model of heteroplasmic mtDNA mutation. Nat. Med. 24, 1696–1700. ( 10.1038/s41591-018-0166-8) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Gammage PA, et al. 2018. Genome editing in mitochondria corrects a pathogenic mtDNA mutation in vivo. Nat. Med. 24, 1691–1695. ( 10.1038/s41591-018-0165-9) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Bayona-Bafaluy MP, Blits B, Battersby BJ, Shoubridge EA, Moraes CT. 2005. Rapid directional shift of mitochondrial DNA heteroplasmy in animal tissues by a mitochondrially targeted restriction endonuclease. Proc. Natl Acad. Sci. USA 102, 14 392–14 397. ( 10.1073/pnas.0502896102) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Yang Y, et al. 2018. Targeted elimination of mutant mitochondrial DNA in MELAS-iPSCs by mitoTALENs. Protein Cell 9, 283–297. ( 10.1007/s13238-017-0499-y) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Bacman SR, Williams SL, Pinto M, Moraes CT. 2014. The use of mitochondria-targeted endonucleases to manipulate mtDNA. Meth. Enzymol. 547, 373–397. ( 10.1016/B978-0-12-801415-8.00018-7) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Kleinstiver BP, Wolfs JM, Kolaczyk T, Roberts AK, Hu SX, Edgell DR. 2012. Monomeric site-specific nucleases for genome editing. Proc. Natl Acad. Sci. USA 109, 8061–8066. ( 10.1073/pnas.1117984109) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Kleinstiver BP, Wolfs JM, Edgell DR. 2013. The monomeric GIY-YIG homing endonuclease I-BmoI uses a molecular anchor and a flexible tether to sequentially nick DNA. Nucleic Acids Res. 41, 5413–5427. ( 10.1093/nar/gkt186) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Pereira CV, Bacman SR, Arguello T, Zekonyte U, Williams SL, Edgell DR, Moraes CT. 2018. mitoTev-TALE: a monomeric DNA editing enzyme to reduce mutant mitochondrial DNA levels. EMBO Mol. Med. 10, e8084 ( 10.15252/emmm.201708084) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Miller-Messmer M, Kühn K, Bichara M, Le Ret M, Imbault P, Gualberto JM. 2012. RecA-dependent DNA repair results in increased heteroplasmy of the Arabidopsis mitochondrial genome. Plant Physiol. 159, 211–226. ( 10.1104/pp.112.194720) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Shedge V, Arrieta-Montiel M, Christensen AC, Mackenzie SA. 2007. Plant mitochondrial recombination surveillance requires unusual RecA and MutS homologs. Plant Cell 19, 1251–1264. ( 10.1105/tpc.106.048355) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Mbantenkhu M, Wang X, Nardozzi JD, Wilkens S, Hoffman E, Patel A, Cosgrove MS, Chen XJ. 2011. Mgm101 is a Rad52-related protein required for mitochondrial DNA recombination. J. Biol. Chem. 286, 42 360–42 370. ( 10.1074/jbc.M111.307512) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Stein A, Kalifa L, Sia EA. 2015. Members of the RAD52 epistasis group contribute to mitochondrial homologous recombination and double-strand break repair in Saccharomyces cerevisiae. PLoS Genet. 11, e1005664 ( 10.1371/journal.pgen.1005664) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Fox TD, Sanford JC, Mcmullin TW. 1988. Plasmids can stably transform yeast mitochondria lacking endogenous mtDNA. Proc. Natl Acd. Sci. USA 85, 7288–7292. ( 10.1073/pnas.85.19.7288) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Johnston SA, Anziano PQ, Shark K, Sanford JC, Butow RA. 1988. Mitochondrial transformation in yeast by bombardment with microprojectiles. Science 240, 1538–1541. ( 10.1126/science.2836954) [DOI] [PubMed] [Google Scholar]
- 103.Remacle C, Cardol P, Coosemans N, Gaisne M, Bonnefoy N. 2006. High-efficiency biolistic transformation of Chlamydomonas mitochondria can be used to insert mutations in complex I genes. Proc. Natl Acad. Sci. USA 103, 4771–4776. ( 10.1073/pnas.0509501103) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Steele DF, Butler CA, Fox TD. 1996. Expression of a recoded nuclear gene inserted into yeast mitochondrial DNA is limited by mRNA-specific translational activation. Proc. Natl Acad. Sci. USA 93, 5253–5257. ( 10.1073/pnas.93.11.5253) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Rinaldi T, Dallabona C, Ferrero I, Frontali L, Bolotin-Fukuhara M. 2010. Mitochondrial diseases and the role of the yeast models. FEMS Yeast Res. 10, 1006–1022. ( 10.1111/j.1567-1364.2010.00685.x) [DOI] [PubMed] [Google Scholar]
- 106.Rak M, Tetaud E, Duvezin-Caubet S, Ezkurdia N, Bietenhader M, Rytka J, di Rago J-P. 2007. A yeast model of the neurogenic ataxia retinitis pigmentosa (NARP) T8993G mutation in the mitochondrial ATP synthase-6 gene. J. Biol. Chem. 282, 34 039–34 047. ( 10.1074/jbc.M703053200) [DOI] [PubMed] [Google Scholar]
- 107.Kucharczyk R, Salin B, di Rago J.-P. 2009. Introducing the human Leigh syndrome mutation T9176G into Saccharomyces cerevisiae mitochondrial DNA leads to severe defects in the incorporation of Atp6p into the ATP synthase and in the mitochondrial morphology. Hum. Mol. Genet. 18, 2889–2898. ( 10.1093/hmg/ddp226) [DOI] [PubMed] [Google Scholar]
- 108.Montanari A, Besagni C, De Luca C, Morea V, Oliva R, Tramontano A, Bolotin-Fukuhara M, Frontali L, Francisci S. 2008. Yeast as a model of human mitochondrial tRNA base substitutions: investigation of the molecular basis of respiratory defects. RNA 14, 275–283. ( 10.1261/rna.740108) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Sanchirico ME, Fox TD, Mason TL. 1998. Accumulation of mitochondrially synthesized Saccharomyces cerevisiae Cox2p and Cox3p depends on targeting information in untranslated portions of their mRNAs. EMBO J. 17, 5796–5804. ( 10.1093/emboj/17.19.5796) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Bonnefoy N, Fox TD. 2000. In vivo analysis of mutated initiation codons in the mitochondrial COX2 gene of Saccharomyces cerevisiae fused to the reporter gene ARG8m reveals lack of downstream reinitiation. Mol. Gen. Genet. 262, 1036–1046. ( 10.1007/PL00008646) [DOI] [PubMed] [Google Scholar]
- 111.Cohen JS, Fox TD. 2001. Expression of green fluorescent protein from a recoded gene inserted into Saccharomyces cerevisiae mitochondrial DNA. Mitochondrion 1, 181–189. ( 10.1016/S1567-7249(01)00012-5) [DOI] [PubMed] [Google Scholar]
- 112.Osman C, Noriega TR, Okreglak V, Fung JC, Walter P. 2015. Integrity of the yeast mitochondrial genome, but not its distribution and inheritance, relies on mitochondrial fission and fusion. Proc. Natl Acad. Sci. USA 112, E947–E956. ( 10.1073/pnas.1501737112) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Hu Z, Fan Z, Zhao Z, Chen J, Li J. 2012. Stable expression of antibiotic-resistant gene ble from Streptoalloteichus hindustanus in the mitochondria of Chlamydomonas reinhardtii. PLoS ONE 7, e35542 ( 10.1371/journal.pone.0035542) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Hu Z, Zhao Z, Wu Z, Fan Z, Chen J, Wu J, Li J. 2011. Successful expression of heterologous egfp gene in the mitochondria of a photosynthetic eukaryote Chlamydomonas reinhardtii. Mitochondrion 11, 716–721. ( 10.1016/j.mito.2011.05.012) [DOI] [PubMed] [Google Scholar]
- 115.Xu H, DeLuca SZ, O'Farrell PH. 2008. Manipulating the metazoan mitochondrial genome with targeted restriction enzymes. Science 321, 575–577. ( 10.1126/science.1160226) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Burman JL, Itsara LS, Kayser E.-B., Suthammarak W, Wang AM, Kaeberlein M, Sedensky MM, Morgan PG, Pallanck LJ. 2014. A Drosophila model of mitochondrial disease caused by a complex I mutation that uncouples proton pumping from electron transfer. Dis. Model. Mech. 7, 1165–1174. ( 10.1242/dmm.015321) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Chen Z, Qi Y, French S, Zhang G, Covian Garcia R, Balaban R, Xu H. 2015. Genetic mosaic analysis of a deleterious mitochondrial DNA mutation in Drosophila reveals novel aspects of mitochondrial regulation and function. Mol. Biol. Cell 26, 674–684. ( 10.1091/mbc.E14-11-1513) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Klucnika A, Ma H. 2019. A battle for transmission: the cooperative and selfish animal mitochondrial genomes. Open Biol. 9, 180267 ( 10.1098/rsob.180267) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Vestweber D, Schatz G. 1989. DNA–protein conjugates can enter mitochondria via the protein import pathway. Nature 338, 170–172. ( 10.1038/338170a0) [DOI] [PubMed] [Google Scholar]
- 120.Collombet JM, Wheeler VC, Vogel F, Coutelle C. 1997. Introduction of plasmid DNA into isolated mitochondria by electroporation. A novel approach toward gene correction for mitochondrial disorders. J. Biol. Chem. 272, 5342–5347. ( 10.1074/jbc.272.8.5342) [DOI] [PubMed] [Google Scholar]
- 121.Yoon YG, Koob MD. 2003. Efficient cloning and engineering of entire mitochondrial genomes in Escherichia coli and transfer into transcriptionally active mitochondria. Nucleic Acids Res. 31, 1407–1415. ( 10.1093/nar/gkg228) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Yoon YG, Koob MD. 2005. Transformation of isolated mammalian mitochondria by bacterial conjugation. Nucleic Acids Res. 33, e139 ( 10.1093/nar/gni140) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Khan SM, Bennett JP. 2004. Development of mitochondrial gene replacement therapy. J. Bioenerg. Biomembr. 36, 387–393. ( 10.1023/B:JOBB.0000041773.20072.9e) [DOI] [PubMed] [Google Scholar]
- 124.Keeney PM, et al. 2009. Mitochondrial gene therapy augments mitochondrial physiology in a Parkinson's disease cell model. Hum. Gene Ther. 20, 897–907. ( 10.1089/hum.2009.023) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Yu H, et al. 2012. Gene delivery to mitochondria by targeting modified adenoassociated virus suppresses Leber's hereditary optic neuropathy in a mouse model. Proc. Natl Acad. Sci. USA 109, E1238–E1247. ( 10.1073/pnas.1119577109) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Weissig V, Lasch J, Erdos G, Meyer HW, Rowe TC, Hughes J. 1998. DQAsomes: a novel potential drug and gene delivery system made from Dequalinium. Pharm. Res. 15, 334–337. ( 10.1023/A:1011991307631) [DOI] [PubMed] [Google Scholar]
- 127.D'Souza GGM, Rammohan R, Cheng S-M, Torchilin VP, Weissig V. 2003. DQAsome-mediated delivery of plasmid DNA toward mitochondria in living cells. J. Control Release 92, 189–197. ( 10.1016/S0168-3659(03)00297-9) [DOI] [PubMed] [Google Scholar]
- 128.Yasuzaki Y, Yamada Y, Harashima H. 2010. Mitochondrial matrix delivery using MITO-Porter, a liposome-based carrier that specifies fusion with mitochondrial membranes. Biochem. Biophys. Res. Commun. 397, 181–186. ( 10.1016/j.bbrc.2010.05.070) [DOI] [PubMed] [Google Scholar]
- 129.Ishikawa T, Somiya K, Munechika R, Harashima H, Yamada Y. 2018. Mitochondrial transgene expression via an artificial mitochondrial DNA vector in cells from a patient with a mitochondrial disease. J. Control Release 274, 109–117. ( 10.1016/j.jconrel.2018.02.005) [DOI] [PubMed] [Google Scholar]
- 130.Lightowlers RN. 2011. Mitochondrial transformation: time for concerted action. EMBO Rep. 12, 480–481. ( 10.1038/embor.2011.93) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Hoogewijs K, James AM, Murphy MP, Lightowlers RN. 2018. Signed-for delivery in the mitochondrial matrix: confirming uptake into mitochondria. Small Methods 2, 1700297 ( 10.1002/smtd.201700297) [DOI] [Google Scholar]
- 132.Randolph-Anderson BL, Boynton JE, Gillham NW, Harris EH, Johnson AM, Dorthu MP, Matagne RF. 1993. Further characterization of the respiratory deficient dum-1 mutation of Chlamydomonas reinhardtii and its use as a recipient for mitochondrial transformation. Mol. Gen. Genet. 236, 235–244. ( 10.1007/BF00277118) [DOI] [PubMed] [Google Scholar]
- 133.Ma H, O'Farrell PH. 2016. Selfish drive can trump function when animal mitochondrial genomes compete. Nat. Genet. 48, 798–802. ( 10.1038/ng.3587) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Gammage PA, Moraes CT, Minczuk M. 2017. Mitochondrial genome engineering: the revolution may not be CRISPR-Ized. Trends Genet. 34, 101–110. ( 10.1016/j.tig.2017.11.001) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Verechshagina N, Nikitchina N, Yamada Y, Harashima H, Tanaka M, Orishchenko K, Mazunin I. 2019. Future of human mitochondrial DNA editing technologies. Mitochondrial DNA A DNA Mapp. Seq. Anal. 30, 214–221. ( 10.1080/24701394.2018.1472773) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
This article has no additional data.