Mantle cell lymphoma (MCL) is an incurable aggressive non-Hodgkin lymphoma (NHL), which accounts for approximately 5% of all NHL. Novel agents and rituximab maintenance therapy (RM) have greatly improved patient outcomes,1,2 but most patients still experience recurrent relapses. This highlights the need for risk-adapted therapies.1 The prognostic value of [(18)F]fluorodeoxyglucose positron-emission-tomography (FDG-PET) has already been demonstrated in various lymphoma entities,3 but its utility in MCL remains unclear.4–12 In the LyMa-PET project, we centrally reviewed PET results from patients enrolled in the LyMa trial, a prospective, multicenter, international, randomized phase III trial (NCT00921414) that investigated RM after autologous stem-cell transplantion (ASCT) in young previously untreated MCL patients.2 Our aim was to investigate the prognostic value of the image-derived FDG-PET quantitative indices.
In the LyMa trial, FDG-PET was optional at diagnosis, before ASCT (iPET) and after-ASCT (eotPET), and not used in the decision-making strategy. FDG-PET images were acquired in voluntary centers participating in the LyMa trial, according to the local protocol and following the rules of good practice.13 FDG-PET data were centrally collected and analyzed on a dedicated workstation (PLANET®Onco-Solution, Dosisoft, France) and evaluated by two experienced readers (CBM and CB) who were blinded to clinical information, treatment arm and follow-up.
For the initial staging, a positive FDG-PET signal was defined as an area of increased uptake thought to be lymphoma-related. Different quantitative metrics were extracted from the FDG-PET data set, measured as volume of interest (VOI) covering the entire nodal and extra-nodal lesions as visualized by increased FDG uptake: SUVmax, defined as the standard-uptake-value (SUV) of the maximum intensity voxel within the VOI; metabolic tumor volume (MTV), defined as the functional volume of the area with the highest uptake, using a 40% thresholding for the segmentation-step; total lesion glycolysis (TLG), defined as the product of SUVmean (average measure of SUV within the calculated boundaries of a lesion) and MTV of the area with the highest uptake. A SUVmax gradient was calculated at baseline for each patient as the difference between SUVmax and the pathological focus with minimal activity. For each metric, the baseline value (i.e. for SUVmax: SUVmax), the values before-ASCT (i.e. for SUVmax: SUVmaxiPET) and after-ASCT (i.e. for SUVmax: SUVmaxeotPET) were considered. The reduction between metrics at iPET, eotPET and PET at baseline were calculated (i.e. for SUVmax: ΔSUVmaxiPET and ΔSUVmaxeotPET). iPET and eotPET were also interpreted visually using the five-point Deauville scale (DS), as recommended.3 Details regarding the statistical methods are described in the Online Supplementary Data.
Among the 299 patients enrolled in the LyMa study, FDG-PET data from 104 patients were retrieved from 28 different centers (out of 81 centers). This included 104 examinations performed at diagnosis, 64 prior to ASCT and 44 after ASCT. The LyMa-PET population did not differ statistically from the entire LyMa population regarding the baseline characteristics, randomization arm, follow up and outcome (Online Supplementary Table S1). The four-year-PFS calculated from the time of inclusion for the 104 patients was 71.1 %, 95%CI (Comorbidity Index) [61.4%;78.8%]; four year overall survival (4y-OS) was 79.6%, 95%CI [70.5%;86.2%] and the estimated median follow-up was 56.5 months, 95%CI [52.6;64.1].
We first analyzed FDG-PET parameters at diagnosis and investigated their prognostic value. As shown in previous reports,11 FDG-PET was pathologic in all patients. The sensitivity of FDG-PET for the detection of splenic lesions was 100% (50/50). According to conventional assessment, 80.8% of patients (84/104) had extranodal locations at diagnosis (including bone marrow, digestive tract or ear/nose/throat sites). The sensitivity of FDG-PET was only 42 % for these extranodal lesions (40/104). Quantitative metrics were extracted in all patients but one, due to deviations on quality controls (n=103) (Online Supplementary Table S2). FDG avidity was heterogeneous and varied greatly from one patient to another, with SUVmax ranging from 1.8 to 33.8 (median=7.39, Online Supplementary Table S2), in line with reported data.4,5 A broad intra-individual heterogeneity was also observed with a SUVmax gradient >5 in 53 cases (51%) and >10 in 24 cases (23%). With the oncogenesis of MCL being a multistep process, progressing from a less to a more aggressive form,14 a low SUVmax value might be related to less aggressive MCL cells, while high SUVmax values might reflect a more aggressive tumor with a high proliferative index (as observed in Richter’s syndrome). Indeed, an elevated SUVmax (>10.3) was found to be associated with aggressive variants (Fisher-Exact P=0.004 and P=0.003, respectively) and Ki67>30% (n=70; Fisher-Exact P<0.001 and P<0.001). In contrast, SUVmax was not associated with the MIPI score (classified as low/intermediate/high) (Fisher-Exact P=0.529 and P=0.680). These results support the existence of a close relationship between tumor cell biology and SUV in MCL. In addition, they suggest that SUVmax calculation at diagnosis could be used as a prognostic parameter to assess tumor cell aggressiveness and in particular tumor cell proliferation. Unlike the measurement of Ki67 positivity in a tumor biopsy, FDG-PET has the advantage of being a whole-body non-invasive technique.
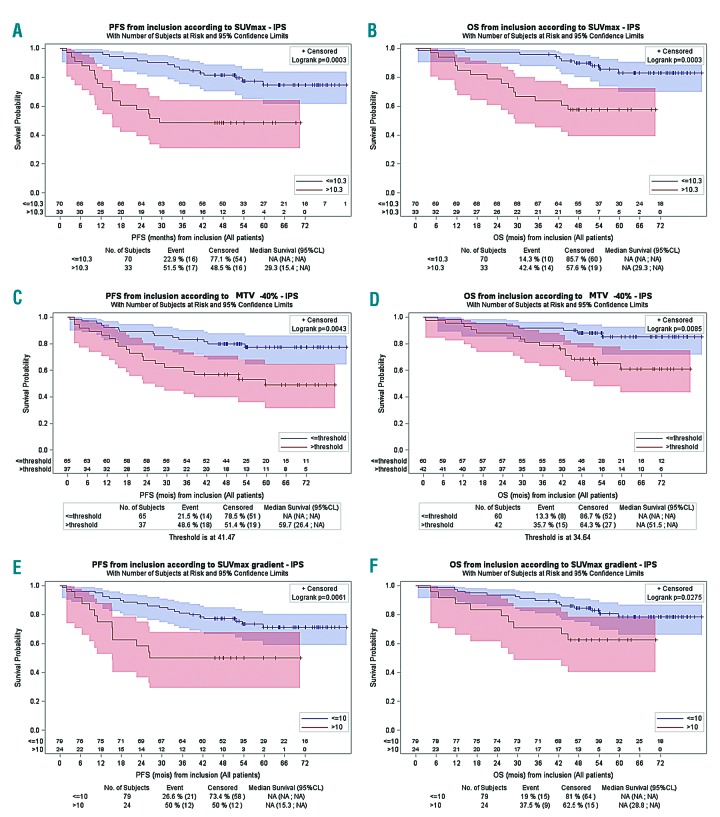
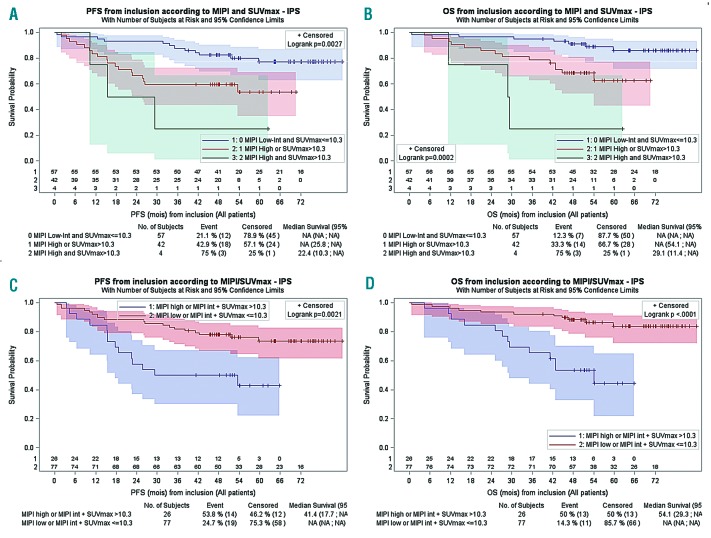
In terms of prognostic value, all FDG-PET metrics determined on the area with the highest uptake significantly impacted both on the overall survival (OS) and progression-free survival (PFS) in the univariate analyses. Patients with a high SUVmax (>10.3) or SUVmax gradient>10 or a high MTV (>41.47) had a shorter PFS (P=0.0003, P=0.0061 and P=0.0043, respectively) and OS (P=0.0003, P= 0.0275 and P=0.0085, respectively) (Figure 1). In the multivariate analysis, only SUVmax>10.3 (Table S3) was associated with shorter PFS (P<0.001, HR=5.41; 95% CI: 2.49–11.78) and OS (P<0.001, HR=6.32; 95% CI: 2.58–15.45). We then investigated the predictive value of a scoring system that combines MIPI (low-intermediate versus high) and SUVmax (<=10.3 versus >10.3), as previously described.4 Patients could be classified into three distinct survival groups (Figure 2). The difference in survival was consistent after adjusting for the treatment arm (PFS: Group 1 hazard rate (HR)=2.9, Group 2 HR=7.7; OS: Group 1 HR=3.5, Group 2 HR=18.8). Due to the small number of cases in the two risk-factor groups, these results should be interpreted with caution. When MIPI and SUVmax > 10.3 were combined only for intermediate risk patients, a better segregation of the two risk groups with significantly different PFS and OS profiles could be achieved (Figure 2). Therefore, patients presenting with a high MIPI or intermediate MIPI plus a SUVmax >10.3 at diagnosis might be candidates for an alternative therapy.
Figure 1.
Univariate survival analyses according to metrics threshold. Progression-free survival (PFS) (A) and overall survival (OS) (B) according to SUVmax. PFS (C) and OS (D) according to the metabolic tumor volume (MTV)-40 %. PFS (E) and OS (F) according to SUVmax gradient.
Figure 2.
Prognostic Index combining MIPI and SUVmax. Progression-free survival(PFS) (A) and overall survival (OS) (B) according to mantle cell international prognotiìc index (MIPI) and SUVmax. Combining MIPI and SUV max > 10.3 for intermediate patients defines two risk groups with significantly different PFS (C) and OS (D) survival profiles.
In contrast to previous reported findings in other lymphoma entities,3 no prognostic value of MTV measured on the whole body was found for PFS or OS (data not shown). These results were calculated in only 33 patients as part of a preliminary study. A large inter-individual variability was observed, with values ranging from 26.7cm3 to 3931cm3. This large difference and the lack of a predictive value for survival might be explained by the frequent splenic involvement in MCL, which increased the MTV while not generally being associated with a poor prognosis.15 However, the volumetric analyses performed on the lesion with the highest uptake showed a negative prognostic impact on both PFS and OS. This observation reinforces the hypothesis that the prognosis of MCL is linked to the most aggressive contingent within the lesion with the highest uptake.
We then investigated the response according to iPET and eotPET. It is interesting to note that the most recent update for the management of malignant lymphomas3 does not mandate FDG-PET-based response assessment in MCL outside the context of a clinical trial due to heterogeneous published data.5,7,12 Indeed, the present work is the first to explore the value of FDG-PET in a large group of homogeneously treated patients enrolled in a multicenter prospective study. Results are presented in the Online Supplementary Tables S4 and S5. We found that the visual analysis of iPET and eotPET were not associated with better survival regardless of the chosen positivity cut-off (DS=5, DS≥4 or DS≥3), while SUVmaxiPET and ΔSUVmaxeotPET were associated with improved OS and PFS, respectively. These analyses should be interpreted with caution. Nevertheless, they suggest that the magnitude of the residual metabolic activity at an interim time-point may hold a predictive value and that the tumor’s chemosensitivity at the end of the treatment with an objective of complete normalization as measured by ΔSUVmaxeotPET seems to be relevant.
In summary, SUVmax of the lesion with the highest uptake determined at diagnosis, has a strong prognostic value for both PFS and OS. A new scoring system combining MIPI and SUVmax might also help to predict patient outcomes. Further prospective investigations are warranted to explore the potential interest of these metrics for therapeutic evaluation. The prospective multicentric LyMa101 study (NCT02896582) will provide an opportunity to confirm these results.
Footnotes
Funding: this work was supported by Roche France. This work has been supported in part by grants from the French National Agency for Research, called “Investissements d’Avenir” IRON Labex n. ANR-11-LABX-0018-01, SIRIC ILIAD and ArronaxPlusEquipexn. ANR-11-EQPX-0004, and by grants from DHU Oncogreffe (Nantes -France).
Information on authorship, contributions, and financial & other disclosures was provided by the authors and is available with the online version of this article at www.haematologica.org.
References
- 1.Dreyling M, Campo E, Hermine O, et al. Newly diagnosed and relapsed mantle cell lymphoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2017;28(suppl_4):iv62–v71. [DOI] [PubMed] [Google Scholar]
- 2.Le Gouill S, Thieblemont C, Oberic L, et al. Rituximab after Autologous Stem-Cell Transplantation in Mantle-Cell Lymphoma. N Engl J Med. 2017;377(13):1250–1260. [DOI] [PubMed] [Google Scholar]
- 3.Barrington SF, Mikhaeel NG, Kostakoglu L, et al. Role of imaging in the staging and response assessment of lymphoma: consensus of the International Conference on Malignant Lymphomas Imaging Working Group. J Clin Oncol. 2014;32(27):3048–3058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bodet-Milin C, Touzeau C, Leux C, et al. Prognostic impact of 18F-fluoro-deoxyglucose positron emission tomography in untreated mantle cell lymphoma: a retrospective study from the GOELAMS group. Eur J Nucl Med Mol Imaging. 2010;37(9):1633–1642. [DOI] [PubMed] [Google Scholar]
- 5.Mato AR, Svoboda J, Feldman T, et al. Post-treatment (not interim) positron emission tomography-computed tomography scan status is highly predictive of outcome in mantle cell lymphoma patients treated with R-HyperCVAD. Cancer. 2012;118(14):3565–3570. [DOI] [PubMed] [Google Scholar]
- 6.Cohen JB, Hall NC, Ruppert AS, et al. Association of pre-transplantation positron emission tomography/computed tomography and outcome in mantle cell lymphoma. Bone Marrow Transplant. 2013; 48(9):1212–1217. [DOI] [PubMed] [Google Scholar]
- 7.Kedmi M, Avivi I, Ribakovsky E, et al. Is there a role for therapy response assessment with 2-[fluorine-18] fluoro-2-deoxy-D-glucose-positron emission tomography/computed tomography in mantle cell lymphoma¿ Leuk Lymphoma. 2014;55(11):2484–2489. [DOI] [PubMed] [Google Scholar]
- 8.Kolstad A, Laurell A, Jerkeman M, et al. Nordic MCL3 study: 90Y-ibritumomab-tiuxetan added to BEAM/C in non-CR patients before transplant in mantle cell lymphoma. Blood. 2014;123(19):2953–2959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bachanova V, Burns LJ, Ahn KW, et al. Impact of pretransplantation (18)F-fluorodeoxy glucose-positron emission tomography status on outcomes after allogeneic hematopoietic cell transplantation for non-Hodgkin lymphoma. Biol Blood Marrow Transplant. 2015; 21(9):1605–1611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lamonica D, Graf DA, Munteanu MC, Czuczman MS. 18F-FDG PET for measurement of response and prediction of outcome to relapsed or refractory mantle cell lymphoma therapy with ben-damustine-Rituximab. J Nucl Med. 2017;58(1):62–68. [DOI] [PubMed] [Google Scholar]
- 11.Bailly C, Carlier T, Touzeau C, et al. Interest of FDG-PET in the management of mantle cell lymphoma. Front Med (Lausanne). 2019;6:70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Albano D, Bosio G, Bianchetti N, et al. Prognostic role of baseline 18F-FDG PET/CT metabolic parameters in mantle cell lymphoma. Ann Nucl Med. 2019;33(7):449–458. [DOI] [PubMed] [Google Scholar]
- 13.Boellaard R, Delgado-Bolton R, Oyen WJG, et al. FDG PET/CT: EANM procedure guidelines for tumour imaging: version 2.0. Eur J Nucl Med Mol Imaging 2015;42(2):328–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vogt N, Dai B, Erdmann T, Berdel WE, Lenz G. The molecular pathogenesis of mantle cell lymphoma. Leuk Lymphoma. 2017;58(7):1530–1537. [DOI] [PubMed] [Google Scholar]
- 15.Hsi ED, Martin P. Indolent mantle cell lymphoma. Leuk Lymphoma. 2014;55(4):761–767. [DOI] [PubMed] [Google Scholar]