Abstract
Vaso-occlusive crisis (VOC) is a hallmark of sickle cell disease (SCD) and occurs when deoxygenated sickled red blood cells occlude the microvasculature. Any stimulus, such as mental stress, which decreases microvascular blood flow will increase the likelihood of red cell entrapment resulting in local vaso-occlusion and progression to VOC. Neurally mediated vasoconstriction might be the physiological link between crisis triggers and vaso-occlusion. In this study, we determined the effect of mental stress on microvascular blood flow and autonomic nervous system reactivity. Sickle cell patients and controls performed mentally stressful tasks, including a memory task, conflict test and pain anticipation test. Blood flow was measured using photoplethysmography, autonomic reactivity was derived from electrocardiography and perceived stress was measured by the State-Trait Anxiety Inventory questionnaire. Stress tasks induced a significant decrease in microvascular blood flow, parasympathetic withdrawal and sympathetic activation in all subjects. Of the various tests, pain anticipation caused the highest degree of vasoconstriction. The magnitude of vasoconstriction, sympathetic activation and perceived stress was greater during the Stroop conflict test than during the N-back memory test, indicating the relationship between magnitude of experimental stress and degree of regional vasoconstriction. Baseline anxiety had a significant effect on the vasoconstrictive response in sickle cell subjects but not in controls. In conclusion, mental stress caused vasoconstriction and autonomic nervous system reactivity in all subjects. Although the pattern of responses was not significantly different between the two groups, the consequences of vasoconstriction can be quite significant in SCD because of the resultant entrapment of sickle cells in the microvasculature. This suggests that mental stress can precipitate a VOC in SCD by causing neural-mediated vasoconstriction.
Introduction
Sickle cell disease (SCD) is a genetic disorder in which polymerization of deoxygenated sickle hemoglobin (HbS) leads to decreased deformability of the normally flexible erythrocytes. These rigid sickle-shaped red blood cells (RBC) can occlude the microvasculature leading to the sudden onset of painful vaso-occlusive episodes (VOC).1,2 After HbS deoxygenates in the capillaries, it takes some time (seconds) for HbS polymerization and the subsequent flexible-to-rigid transformation. If the transit time of RBC through the microvasculature is longer than the polymerization time, sickled RBC will lodge in the microvasculature.3 Any trigger that decreases microvascular blood flow will prolong the transit time, promoting the entrapment of sickled RBC, resulting in vaso-occlusion. This physiology of SCD, described decades ago,4,5 is fundamental to understanding the triggering of VOC. Patients report that stress, cold, and pain itself can trigger the onset of VOC6 but the frequency of VOC is highly variable. To date, the mechanism of how such events might trigger regional vaso-occlusion has not been fully elucidated.
Psychological stress is an exacerbating factor in many chronic illnesses, such as SCD,7–10 coronary artery disease and myocardial ischemia.11,12 Stress is significantly associated with increased pain intensity, reductions in social and physical activities and greater health care utilization.8,13,14 Day-to-day stressors have been associated with onset of pain and the course of VOC in SCD.9,10 Stress is well-known to modulate autonomic nervous system (ANS) activity which in turn plays a major role in the regulation of regional blood flow.15 Interestingly, SCD children with greater mental-stress-induced autonomic reactivity had more severe clinical disease.16,17 SCD subjects also have augmented ANS-mediated vasoconstriction in response to sighing, hypoxia, and pain.18–20 Therefore, autonomic dysregulation in SCD represents a plausible physiological link between mental stress and sickle RBC retention in the microvasculature.16,18–21 Further understanding of this proposed mechanism of VOC triggering would not only help to predict disease manifestations, but would also open up opportunities for therapeutic intervention in disorders such as SCD in which preservation of microvascular blood flow is important.22
To address the role of mental stress in the physiology of SCD, we objectively quantified microvascular blood flow, measured by photoplethysmography, in response to standardized mental stress tasks in subjects with SCD and in controls. We also assessed cardiac ANS balance by analysis of heart rate variability in response to mental stress. We correlated photoplethysmogram-derived physiological indices with subjective indices of perceived stress assessed from standardized anxiety questionnaires. The aim of this study was to determine the relationship of peripheral and cardiac ANS responses with mental stress in SCD.
Methods
The study was conducted under an institutional review board-approved protocol at the Children’s Hospital Los Angeles with approved consent/assent. Twenty SCD subjects with Hb SS, S-β0, S-β+ or SC genotype and 16 age- and race-matched controls from the patients’ family and friends were recruited.
Experimental setup and study protocol
All studies were performed in an ANS laboratory under strictly controlled settings.18 Neuropsychological stress was assessed at baseline using the State-Trait Anxiety Inventory (STAI) questionnaire.23 The STAI Y-1 and Y-2 evaluate “anxiety at this moment, aka state anxiety” and “how people generally feel, aka trait anxiety”, respectively.
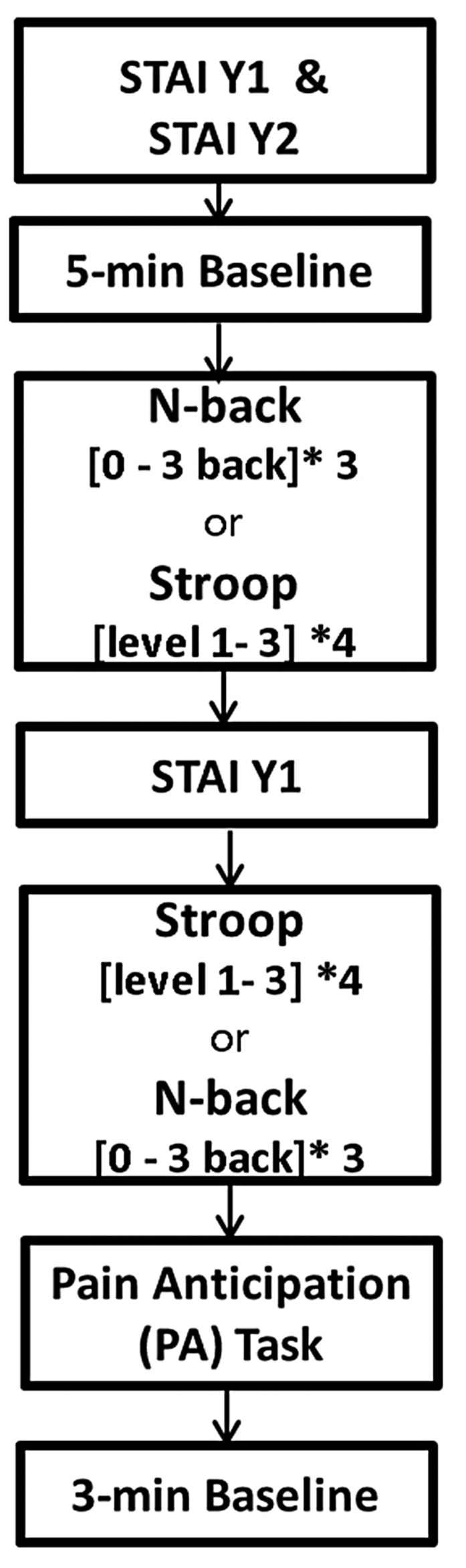
Following 5 minutes of baseline recording, the stress induction protocol was presented through psychological software (E-prime 2.0, Psychology Software Tools, USA). The protocol consisted of a memory task (N-back)24 and a conflict test (Stroop),25,26 presented in a randomized order, followed by a pain anticipation (PA) test (Figure 1). During the N-back task, the subjects were asked to respond when the current letter matched the letter from n steps (n=zero, one, two, or three back) earlier in the sequence. During the Stroop task, the participants were asked to identify the font color of a word, not the written name of the word. We measured state anxiety between tasks. During the PA task, subjects read the following sentence on their computer screen: “You will receive a maximum pain stimulus in one minute. When you cannot tolerate the pain any longer, say STOP and the device will cool down to normal level immediately”. However, no pain stimulus was actually applied.
Figure 1.
Time sequence of the study protocol. The subjects were randomly assigned to perform the N-back or Stroop test first. STAI: State-Trait Anxiety Inventory; Y-1: Sate questionnaire; Y-2 Trait questionnaire.
Physiological measurements and analysis parameters
All the physiological monitoring sensors were attached to the subjects’ left arm. Microvascular blood flow was measured using photoplethysmography (Nonin Medical Inc., USA) and laser Doppler flowmetry (Perimed, Sweden). Respiration (thoracic and abdominal bands, zRip DuraBelt, Philips), the electrocardiogram and continuous blood pressure (Nexfin, Amsterdam) were recorded.
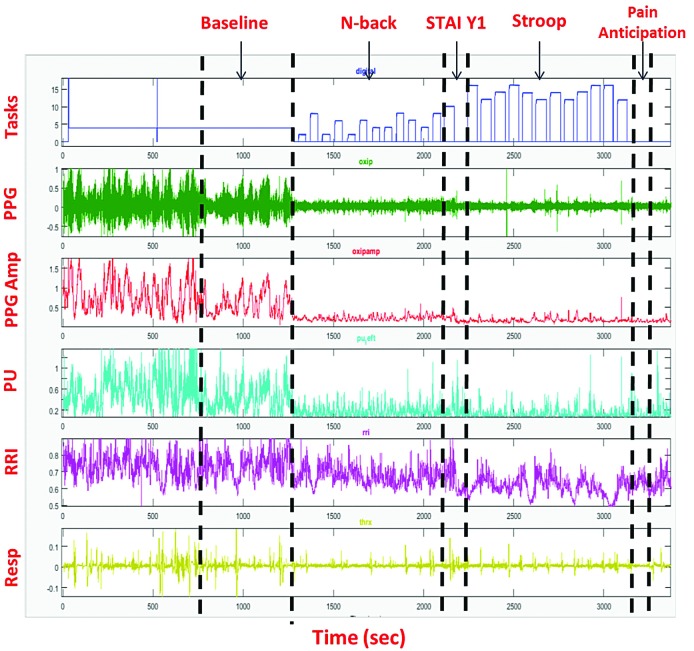
Recorded data from all devices were exported for processing and analysis in MATLAB. The photoplethysmogram amplitude was normalized to its own 95th percentile value during the full study. The average microvascular blood flow was calculated over the 5 min baseline period, the N-back, Stroop and PA tasks. The percent decrease in the amplitude of the photoplethysmogram or microvascular perfusion waveforms (Figure 2; 2nd and 3rd signals, respectively) from the baseline mean was interpreted as a vasoconstriction response.18,27
Figure 2.
Raw waveform and wave amplitude signal output from the Biopac System. Example of a recording from a single subject. The top panel (Tasks) is the output of the E-prime software where the height of the bars represents the difficulty of the task. The second and third panels are the photoplethysmography (PPG) signal and PPG amplitude (PPG Amp), respectively. The fourth panel is microvascu-lar perfusion (PU) determinecd by laser-Doppler. Panel five is the R-to-R interval from the electrocardiogram and panel six is the respiratory signal.
Cardiac autonomic balance was assessed by analysis of the R-to-R interval and heart rate variability19,28,29 during baseline and mental stress tasks. The following power spectral indices were calculated: low frequency power, reflecting a combination of cardiac sympathetic and parasympathetic activity; high frequency power, reflecting parasympathetic activity;29,30 and the ratio of low frequency power to high frequency power, reflecting sympathovagal balance.30
Percent changes in mean microvascular blood flow and mean spectral indices from baseline to tasks were calculated. The Student t-test (or Wilcoxon sign rank) or χ2 test was used to test baseline group differences and task differences. Robust regression was used to correlate vasoconstriction response and state anxiety during the PA task. Repeated measures analysis of variance was used to test differences in N-back and Stroop sublevels and accuracy scores. All statistical analyses were performed using STATA/IC 14.1 (StataCorp LP, TX, USA) with nominal significance set at P≤0.05.
The methods are described in detail in the Online Supplementary Methods S1.
Results
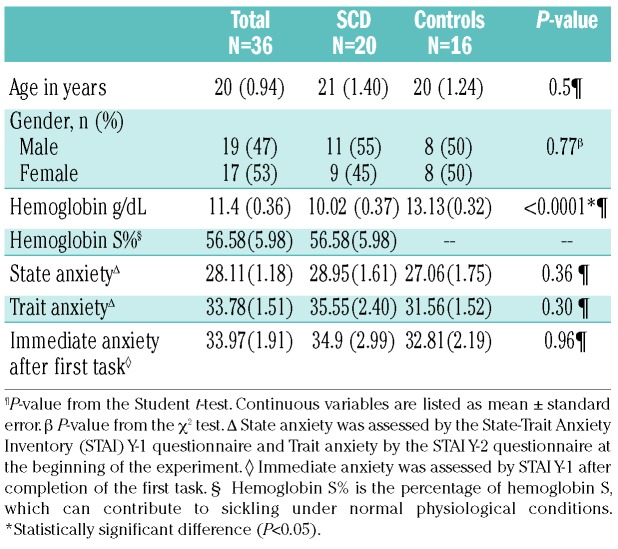
Data from a total of 20 SCD patients and 16 controls were analyzed. Transfused and non-transfused subjects with SCD were grouped together and healthy and sickle cell trait subjects (controls) were combined after it had been demonstrated that these factors were not statistically significant in the analyses. The percentage of HbS (HbS%) was considered to be zero in patients with sickle cell trait as the cellular distribution of HbS differs in sickle cell trait and does not contribute to sickling under the conditions of the experiments in this study, making the HbS% in sickle cell trait not comparable to that in transfused or non-trait sickle phenotypes. The subjects’ characteristics are summarized in Table 1. Nine (45%) SCD subjects were on chronic transfusion, nine (45%) were being treated with hydroxyurea and two (10%) were not receiving either treatment. The characteristics of both groups were balanced except for hemoglobin concentration on the study day. Sixty-one percent of subjects had a level of education equivalent to high school or superior. Seventy-two percent reported that they had a high level of competitiveness on the visit screening questionnaire.
Table 1.
Population characteristics.
Vasoconstriction due to mental stress
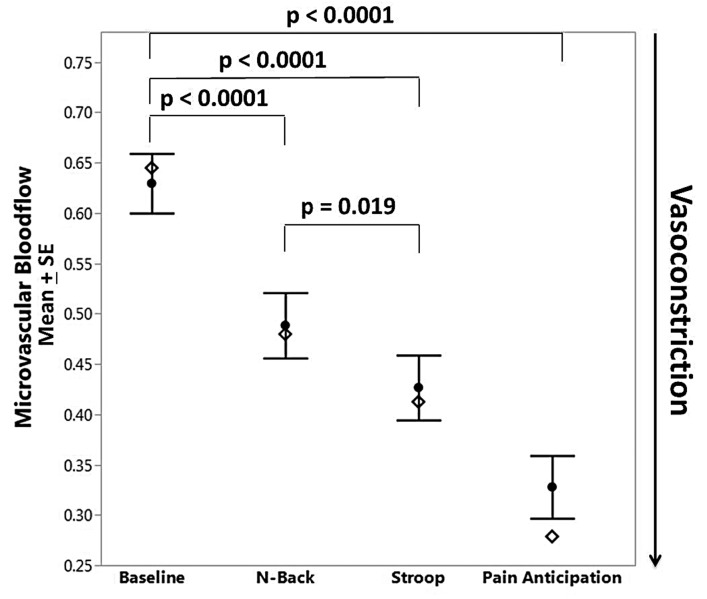
As determined from the photoplethysmogram, there was a significant drop in microvascular blood flow during both cognitive tasks (N-back and Stroop, P<0.0001) and the PA task (P<0.0001) (Figure 3). Figure 2 (signal 2) shows a typical response of vasoconstriction in one subject. The drop in microvascular blood flow from baseline was greater during the PA task than during the cognitive tasks. A similar vasoconstriction response was observed when the microvascular blood flow was assessed by laser-Doppler flowmetry. Subjects had higher anxiety scores immediately after completing the tasks than at baseline (STAI Y-1, mean difference=6; P=0.0007). Eighty-five percent of patients with SCD and 75% of controls showed vasoconstriction compared to baseline during at least one cognitive task. Eighty-five percent of SCD patients and 87.5% of controls had decreases in mean blood flow during the PA task. There was no difference in the magnitude of responses between individuals with SCD and controls. Demographic variables such as age, gender, race, number of days from last menstruation, and laboratory values were not associated with the magnitude of the vasoconstriction response.
Figure 3.
Microvascular blood flow under mental stress in all subjects. Significant vasoconstriction occurred during all mental stress tasks compared to baseline. Open diamonds represent group median values. SE: standard error of mean.
The Stroop test caused greater vasoconstriction than the N-back task, irrespective of the order in which the tests were presented (P=0.019) (Figure 3). Subjects who were randomized to perform the Stroop task first had greater anxiety responses than did the subjects who performed the N-back task first (mean difference=10; P=0.03). Overall the accuracy score was significantly lower for the Stroop task than for the N-back task in all subjects (mean score difference=25, P<0.001).
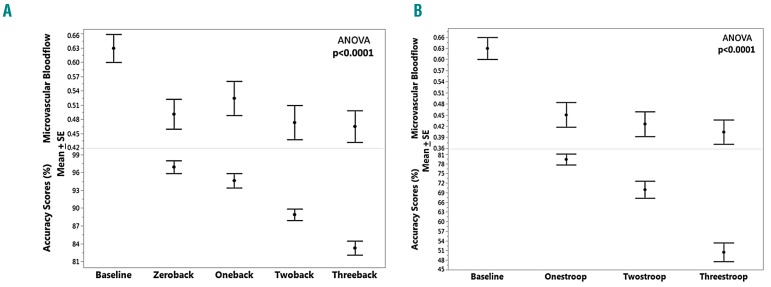
The accuracy score for the Stroop and N-back tasks decreased as the difficulty increased from zero-back to three-back in the N-back task and from level one to level three in the Stroop task (P<0.0001) (Figure 4) but there was no further change in blood flow with increasing difficulty. Once the subjects manifested vasoconstriction, in comparison with baseline vascular tone, the vasoconstriction remained throughout the whole task regardless of the difficulty of the tasks.
Figure 4.
Effect of error rate during mental stress tasks on blood flow. (A, B) Mean ± standard error (SE) of microvascular blood flow and accuracy scores in sublevels of the N-Back (zeroback, oneback, twoback and threeback) task (A) and Stroop (onestroop, twostroop and threestroop) (B).
Vasoconstriction response to perceived anxiety during pain anticipation
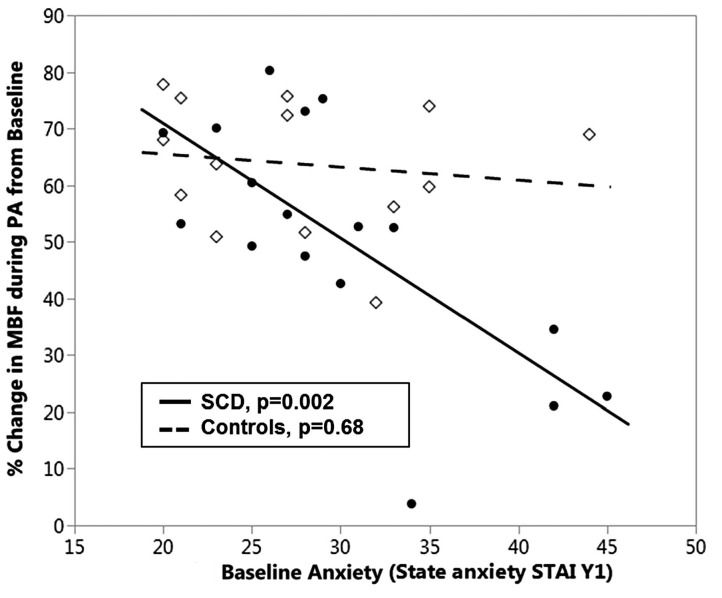
On robust regression, the effect of state anxiety on blood flow response was greater in SCD patients than in controls (P=0.03 for the interaction), suggesting that higher anxiety at baseline (STAI Y-1) in SCD subjects is associated with less change in blood flow (coefficient = −1.85, P=0.002) in response to pain anticipation (Figure 5). State anxiety had no effect on change in blood flow in control subjects. To understand why SCD subjects would have less response with high anxiety, we looked at the baseline blood flow. We found that highly anxious subjects tended to have a lower mean baseline blood flow (Online Supplementary Figure S1), meaning they were already vasoconstricted at baseline, limiting them from further vasoconstriction. This trend was not seen among controls. (Online Supplementary Figure S2A, B: high-anxiety SCD responder and low-anxiety SCD responder).
Figure 5.
Relation between vasoconstriction during pain anticipation and perceived stress (state anxiety) in sickle cell disease subjects and controls. State anxiety was determined at baseline by the State-Trait Anxiety Inventory Y-1 questionnaire (STAI Y-1) and assessed in response to change in microvascular blood-flow during pain anticipation (PA) in sickle cell disease (SCD) subjects (closed circles, —) and controls (open diamonds, - - -). SCD subjects who were highly anxious at baseline had a smaller vasoconstriction response during the PA task than the SCD subjects who were less anxious (P=0.002); this effect was not seen among controls. MBF: microvascular blood flow.
Cardiac autonomic response
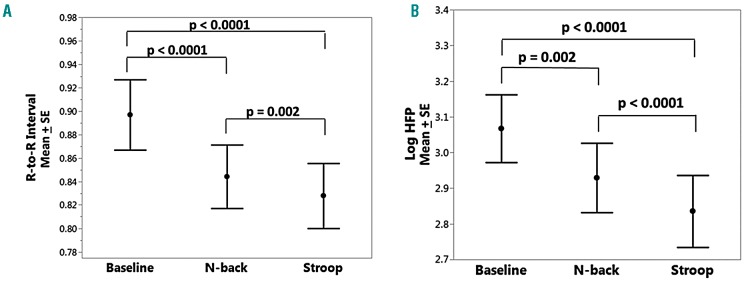
Since the ANS regulates blood flow and SCD subjects have dysautonomia,15,28,31,32 we explored the effect of mental stress responses on cardiac autonomic balance. In com parison to the value at baseline, there was a significant decrease in R-to-R interval, signifying an increase in heart rate, during all tasks (P<0.0001) (Figure 6A). As for the microvascular blood flow response, the R-to-R interval was less during the Stroop task than during the N-back task (P=0.002).
Figure 6.
Autonomic nervous system responses to mental stress. (A) R-to-R interval (sec) and (B) high frequency power (sec2/Hz, shown on a log scale) in response to the N-Back and Stroop tasks in all subjects. There is a significant decrease in R-to-R interval and parasympathetic withdrawal during mental stress tasks compared to baseline. SE: standard error of mean; HFP: high frequency power.
There was significant parasympathetic withdrawal during the N-back and Stroop tasks as reflected by the drop in high frequency power (P=0.002 and P<0.0001, respectively) (Figure 6B) The Stroop task caused stronger parasympathetic withdrawal than the N-back task (P<0.0001). There was more sympathetic activation during the Stroop test (low-to-high power ratio: P=0.03), but not during the N-back task. We did not analyze autonomic reactivity during the PA task because the 1-minute test period was not long enough to derive spectral indices.29
Discussion
VOC is a significant complication of SCD and a major cause of morbidity and mortality.33 The frequency of VOC is related in part to hemoglobin-F content, white blood cell count, inflammatory status and other factors.34–36 However, there is still significant variability in crisis frequency among SCD subjects with otherwise similar hematologic status. Pain crises can be promoted by preceding dehydration, infection, injury, exposure to cold or emotional stress.37,38 Much of the research in past decades has focused on adhesion and processes attributed to occlusion in the post-capillary venule, and to decreased flow due to nitric oxide depletion.39 While stress and cold are often mentioned, very little attention has been paid to decreased flow due to neurally induced vasoconstriction.32,40 SCD patients undergo a tremendous amount of stress not only due to environmental challenges but also the illness-related stress of painful episodes, repeated medical procedures and life-threatening complications. Stress causes ANS hyperreactivity by enhancing the sympathetic nervous system and dampening the parasympathetic system in SCD subjects compared to non-SCD individuals.16,17 Sympathetic and parasympathetic responses have been related to clinical vaso-occlusion in SCD, through ANS modulation of regional blood flow.15,17 SCD is probably the best example of a disorder in which decreased microvascular perfusion can be directly related to the pathology of the disorder, because the increase in transit time from decreased blood flow promotes entrapment of rigid red cells in small vessels.3,5 To our knowledge, this is the first study to quantify regional blood flow modulated by ANS reactivity under mental stress in SCD.
Our data show that experimental mental stress caused a decrease in regional blood flow in all participants. While we thought that SCD subjects would exhibit stronger vasoconstriction because of their hyperresponsiveness to sympathetically induced stimuli, such as sighing,28 we did not detect a difference in stress-induced vasoconstriction between SCD patients and controls. We did find a significantly higher anxiety response score (P=0.03) in subjects who were exposed to the more difficult mental stress test first (Stroop). We also found that the degree of vasoconstriction was proportional to the magnitude of the stress. Subjects reported that overall the Stroop task was more stressful: accuracy scores were lower and there was also a greater decrease in blood flow with this cognitive stressor task. However, different sublevels of difficulty within a task type did not correlate with levels of vasoconstriction. This finding suggests that consecutive stressful events could make SCD patients more vulnerable to vaso-occlusion. We think that variability in the vasoconstriction response to stress may account in part for differences in clinical severity among SCD patients who have the same hemoglobin phenotype. The frequency of VOC and intensity of pain are higher among patients found to have high anxiety and stress scores on standard psychological assessments.8,41,42 We tried to correlate the vasoconstrictive response with clinical severity. As our SCD patients were either on chronic transfusion or hydroxyurea, the number of VOC was too low to detect differences in this current relatively small sample.
Along with a strong vasoconstriction response, significant autonomic reactivity was seen in all subjects. The Stroop test was consistently more stressful, and induced greater vasoconstriction as well as greater autonomic reactivity. There was both sympathetic activation as well as parasympathetic withdrawal during this cognitive task. Mental stressors are known to influence autonomic function by sympathetic or parasympathetic tone alterations. Higher anxiety induces atherosclerosis via enhanced sympathetic modulation, increasing the risk of cardiovascular disease.43 In addition, mental stress and anxiety have been linked to impaired endothelial function via autonomic dysfunction.43–45 Endothelial function, quantified by flow mediated dilation, decreases as a result of mental stress tasks.46 Similarly, in SCD, a synergistic interaction between impaired local vascular function and the exaggerated neurally mediated vasoconstrictive response could further reduce peripheral blood flow, setting the stage for VOC.
Consistent with the findings of our previous study,18 anticipation of pain caused significant vasoconstriction and this response was quantitatively greater than that of the calibrated experimental stress tasks (Figure 3). We do not have strong evidence to conclude that the presence of SCD alone influences mental stress-induced vasoconstriction but anxiety seems to be a modifying factor. Interestingly unlike control subjects, SCD subjects who were highly anxious had less vasoconstriction during the PA task and vice versa. We think that this pattern of response occurred because highly anxious subjects were already vasoconstricted at baseline and this limited the magnitude of further vasoconstriction. So the fact that SCD subjects have less change in the vasoconstriction response to the stressors than controls actually reflects their chronically vasoconstricted state. Although not statistically significant, the trend of lower baseline blood flow with high anxiety in SCD can be seen in Online Supplementary Figure S1, which also shows the significant variability in baseline measures. Photoplethysmogram and microvascular perfusion signals from Perimed do not have absolute units, so measurements made as percent changes from baseline are more reliable, basically correcting for baseline variability and allowing detection of the differences seen in Figure 5. These findings may be related to pain catastrophization and increased psychophysical pain sensitivity due to frequent pain episodes.7,47,48 Over the years, pain catastrophization may increase the frequency of pain and severity of pain crises.47,49 From a standpoint of neural physiology, repeated acute pain creates a central neural pathological pain connectome50 that leads to baseline chronic pain and chronic vasoconstriction. Although baseline blood flow was not statistically significantly lower, probably due to insufficient study power, we suspect that the above-described phenomenon is the explanation for our findings and warrants further study.
We showed that neurally mediated vasoconstriction is a biophysical marker of mental stress in SCD patients and controls. Mental stress has been identified as a trigger for pain crises in SCD and its connection with a decrease in microvascular perfusion seems to make a causal link to VOC. The probability of vaso-occlusion is predicted to be related to the relation between time to polymerization of deoxy HbS and microvascular flow.3,5 Obviously, HbS is the major pathology in SCD. However, neurally mediated changes in microvascular flow certainly play a significant and unappreciated role. Individual variation in patterns of vasoconstriction with different ANS reactivity may offer a possible biological explanation for the variability in the frequency of VOC in SCD patients with similar hemoglobin phenotype. Identifying the high-risk individuals who show a phenotype of chronic vasoconstriction and repeated pain crises, and targeting them with neuro-modulatory cognitive-based therapies may improve vascular and neural physiology in SCD. In the primary stage of a crisis, implementing these learned cognitive-based therapies or distraction and relaxation techniques will help to improve the prognosis during acute pain. Microvascular flow in response to stress may also serve as an important surrogate endpoint for therapy in SCD and other diseases in which small vessel blood flow and reactivity are important.
Some limitations of this study should be acknowledged. One limitation was that the small sample size did not allow us to detect a difference in the magnitude of vasoconstriction between groups and correlate it with a clinical outcome such as VOC. Since the concept that mental stress causes vasoconstriction has not been studied in SCD, prior effect size was not known to permit sample size calculation. Another reason for lack of difference between groups is that over 90% of our patients are on hydroxyurea or chronic transfusion and thus clinical crises are relatively uncommon. Any real magnitude differences would be more likely to emerge in studies with larger samples and untreated patients. However, the primary aim of this study was to understand the changes in peripheral and cardiac responses to mental stress. The fundamental study design presented here was able to detect changes in physiological signals with millisecond accuracy and clearly showed vasoconstriction responses and ANS reactivity due to mental stress in all subjects. We think that the consequences of these findings are mechanistically related to the pathophysiology of sickle cell vaso-occlusion.
Acknowledgments
This work was supported by grants from the National Institutes of Health National Heart, Lung, and Blood Institute (U01 HL117718). The authors thank Justin Abbott for his contribution to the data collection.
Footnotes
Check the online version for the most updated information on this article, online supplements, and information on authorship & disclosures: www.haematologica.org/content/105/1/83
References
- 1.Rees DC, Williams TN, Gladwin MT. Sickle-cell disease. Lancet. 2010;376(9757): 2018–2031. [DOI] [PubMed] [Google Scholar]
- 2.Kassim AA, DeBaun MR. Sickle cell disease, vasculopathy, and therapeutics. Annu Rev Med. 2013;64(1):451–466. [DOI] [PubMed] [Google Scholar]
- 3.Christoph GW, Hofrichter J, Eaton WA. Understanding the shape of sickled red cells. Biophys J. 2005;88(2):1371–1376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Eaton WA, Hofrichter J. Sickle cell hemoglobin polymerization. In: Advances in Protein Chemistry. Elsevier; p63–279. [DOI] [PubMed] [Google Scholar]
- 5.Eaton WA, Hofrichter J, Ross PD. Editorial: Delay time of gelation: a possible determinant of clinical severity in sickle cell disease. Blood. 1976;47(4):621–627. [PubMed] [Google Scholar]
- 6.Murray N, May A. Painful crises in sickle cell disease--patients’ perspectives. BMJ. 1988;297(6646):452–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bhatt RR, Martin SR, Evans S, et al. The effect of hypnosis on pain and peripheral blood flow in sickle-cell disease: a pilot study. J Pain Res. 2017;10:1635–1644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gil KM, Carson JW, Porter LS, et al. Daily stress and mood and their association with pain, health-care use, and school activity in adolescents with sickle cell disease. J Pediatr Psychol. 2003;28(5):363–373. [DOI] [PubMed] [Google Scholar]
- 9.Levenson JL, McClish DK, Dahman BA, et al. Depression and anxiety in adults with sickle cell disease: the PiSCES project. Psychosom Med. 2008;70(2):192–196. [DOI] [PubMed] [Google Scholar]
- 10.Thomas LS, Stephenson N, Swanson M, Jesse DE, Brown S. A pilot study: the effect of healing touch on anxiety, stress, pain, pain medication usage, and physiological measures in hospitalized sickle cell disease adults experiencing a vaso-occlusive pain episode. J Holist Nurs. 2013;31(4):234–247. [DOI] [PubMed] [Google Scholar]
- 11.Jain D. Mental stress, a powerful provocateur of myocardial ischemia: Diagnostic, prognostic, and therapeutic implications. J Nucl Cardiol. 2008;15(4):491–493. [DOI] [PubMed] [Google Scholar]
- 12.Wei J, Rooks C, Ramadan R, et al. Meta-analysis of mental stress-induced myocardial ischemia and subsequent cardiac events in patients with coronary artery disease.. Am J Cardiol. 2014;114(2):187–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Porter LS, Gil KM, Sedway JA, Ready J, Workman E, Thompson RJ. Pain and stress in sickle cell disease: an analysis of daily pain records. Int J Behav Med. 1998;5(3):185–203. [DOI] [PubMed] [Google Scholar]
- 14.Porter LS, Gil KM, Carson JW, Anthony KK, Ready J. The role of stress and mood in sickle cell disease pain: an analysis of daily diary data. J Health Psychol. 2000;5(1):53–63. [DOI] [PubMed] [Google Scholar]
- 15.Connes P, Coates TD. Autonomic nervous system dysfunction: implication in sickle cell disease. C R Biol. 2013;336(3):142–147. [DOI] [PubMed] [Google Scholar]
- 16.Pearson SR, Alkon A, Treadwell M, Wolff B, Quirolo K, Boyce WT. Autonomic reactivity and clinical severity in children with sickle cell disease. Clin Auton Res. 2005;15(6):400–407. [DOI] [PubMed] [Google Scholar]
- 17.Treadwell MJ, Alkon A, Styles L, Boyce WT. Autonomic nervous system reactivity: children with and without sickle cell disease. Nurs Res. 2011;60(3):197–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Khaleel M, Puliyel M, Shah P, et al. Individuals with sickle cell disease have a significantly greater vasoconstriction response to thermal pain than controls and have significant vasoconstriction in response to anticipation of pain. Am J Hematol. 2017;92(11):1137–1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sangkatumvong S, Khoo MCK, Kato R, et al. Peripheral vasoconstriction and abnormal parasympathetic response to sighs and transient hypoxia in sickle cell disease. Am J Respir Crit Care Med. 2011;184(4):474–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chalacheva P, Khaleel M, Sunwoo J, et al. Biophysical markers of the peripheral vaso-constriction response to pain in sickle cell disease. PLoS One. 2017;12(5):e0178353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Connes P. Altered autonomic nervous system function in sickle cell disease. Am J Respir Crit Care Med. 2011;184(4):398–400. [DOI] [PubMed] [Google Scholar]
- 22.Goor DA, Sheffy J, Schnall RP, et al. Peripheral arterial tonometry: a diagnostic method for detection of myocardial ischemia induced during mental stress tests: a pilot study. Clin Cardiol. 2004;27(3):137–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.The State-Trait Anxiety Inventory (STAI). http://www.apa.org.http://www.apa.org/pi/about/publications/caregivers/practice-settings/assessment/tools/trait-state.aspx (accessed June 4, 2018).
- 24.Smith KE, Schatz J. Working memory in children with neurocognitive effects from sickle cell disease: contributions of the central executive and processing speed. Dev Neuropsychol. 2016;41(4):231–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hoshikawa Y, Yamamoto Y. Effects of Stroop color-word conflict test on the autonomic nervous system responses. Am J Physiol. 1997;272(3 Pt 2):H1113–1121. [DOI] [PubMed] [Google Scholar]
- 26.Callister R, Suwarno NO, Seals DR. Sympathetic activity is influenced by task difficulty and stress perception during mental challenge in humans. J Physiol. 1992;454:373–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sunwoo J, Chalacheva P, Khaleel M, et al. A novel cross-correlation methodology for assessing biophysical responses associated with pain. J Pain Res. 2018;11:2207–2219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sangkatumvong S, Khoo MCK, Coates TD. Abnormal cardiac autonomic control in sickle cell disease following transient hypoxia. Conf Proc IEEE Eng Med Biol Soc. 2008;2008:1996–1999. [DOI] [PubMed] [Google Scholar]
- 29.[No authors listed]. Heart rate variability: standards of measurement, physiological interpretation and clinical use. Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Circulation. 1996;93(5): 1043–1065. [PubMed] [Google Scholar]
- 30.Eckberg DL. Sympathovagal balance: a critical appraisal. Circulation. 1997;96(9):3224–3232. [DOI] [PubMed] [Google Scholar]
- 31.Chalacheva P, Kato RM, Sangkatumvong S, et al. Autonomic responses to cold face stimulation in sickle cell disease: a time-varying model analysis. Physiol Rep. 2015;3(7). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Coates TD, Chalacheva P, Zeltzer L, Khoo MCK. Autonomic nervous system involvement in sickle cell disease. Clin Hemorheol Microcirc. 2018;68(2–3):251–262. [DOI] [PubMed] [Google Scholar]
- 33.Platt OS, Thorington BD, Brambilla DJ, et al. Pain in sickle cell disease. N Engl J Med. 1991;325(1):11–16. [DOI] [PubMed] [Google Scholar]
- 34.Hofstra TC, Kalra VK, Meiselman HJ, Coates TD. Sickle erythrocytes adhere to polymorphonuclear neutrophils and activate the neutrophil respiratory burst. Blood. 1996;87(10):4440–4447. [PubMed] [Google Scholar]
- 35.Turhan A, Weiss LA, Mohandas N, Coller BS, Frenette PS. Primary role for adherent leukocytes in sickle cell vascular occlusion: A new paradigm. Proc Natl Acad Sci U S A. 2002;99(5):3047–3051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hebbel RP, Boogaerts MAB, Eaton JW, Steinberg MH. Erythrocyte adherence to endothelium in sickle-cell anemia. N Engl J Med. 1980;302(18):992–995. [DOI] [PubMed] [Google Scholar]
- 37.Rees DC, Olujohungbe AD, Parker NE, Stephens AD, Telfer P, Wright J. Guidelines for the management of the acute painful crisis in sickle cell disease. Br J Haematol. 2003;120(5):744–752. [DOI] [PubMed] [Google Scholar]
- 38.Diggs LW. Sickle cell crises: Ward Burdick Award contribution. Am J Clin Pathol. 1965;44(1):1–19. [Google Scholar]
- 39.[No authors listed]. Sickle cell disease. Nat Rev Dis Primers. 2018;4:18011. [DOI] [PubMed] [Google Scholar]
- 40.Serjeant GR, Chalmers RM. Current concerns in haematology. 1. Is the painful crisis of sickle cell disease a “steal” syndrome? J Clin Pathol. 1990;43(10):789–791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mahdi N, Al-ola K, Khalek NA, Almawi WY. Depression, anxiety, and stress comorbidities in sickle cell anemia patients with vaso-occlusive crisis. J Pediatr Hematol Oncol. 2010;32(5):345–349. [DOI] [PubMed] [Google Scholar]
- 42.Gil KM, Abrams MR, Phillips G, Keefe FJ. Sickle cell disease pain: relation of coping strategies to adjustment. J Consult Clin Psychol. 1989;57(6):725–731. [DOI] [PubMed] [Google Scholar]
- 43.Narita K, Murata T, Hamada T, et al. Interactions among higher trait anxiety, sympathetic activity, and endothelial function in the elderly. J Psychiatr Res. 2007;41(5):418–427. [DOI] [PubMed] [Google Scholar]
- 44.Amiya E, Watanabe M, Komuro I. The relationship between vascular function and the autonomic nervous system. Ann Vasc Dis. 2014;7(2):109–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Toda N, Nakanishi-Toda M. How mental stress affects endothelial function. Pflugers Arch. 2011;462(6):779–794. [DOI] [PubMed] [Google Scholar]
- 46.Ghiadoni L, Donald AE, Cropley M, et al. Mental stress induces ttransient endothelial dysfunction in humans. Circulation. 2000;102(20):2473–2478. [DOI] [PubMed] [Google Scholar]
- 47.Mathur VA, Kiley KB, Carroll CP, et al. Disease related, non-disease related, and situational catastrophizing in sickle cell disease and its relationship with pain. J Pain. 2016;17(11):1227–1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Quartana PJ, Campbell CM, Edwards RR. Pain catastrophizing: a critical review. Expert Rev Neurother. 2009;9(5):745–758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Campbell CM, Moscou-Jackson G, Carroll CP, et al. An evaluation of central sensitization in patients with sickle cell disease. J Pain. 2016;17(5):617–627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mansour AR, Baliki MN, Huang L, et al. Brain white matter structural properties predict transition to chronic pain. Pain. 2013;154(10):2160–2168. [DOI] [PMC free article] [PubMed] [Google Scholar]